Abstract
Recent clinical trials have yielded promising results suggesting that γδ T cell-based immunotherapies can be effective against hematological malignancies. Human T cells expressing Vγ9Vδ2+ receptors are particularly attractive candidates for this application, since they can be readily expanded in vitro in large quantities for adoptive transfer and do not require HLA-matching of donors and recipients. While it is well established that Vγ9Vδ2+ T cells are potently cytolytic against many human cancers and it has been shown that they can control transplanted human tumors in xenogeneic model systems, little is known about the parameters that determine the anti-tumor efficacy of adoptively transferred Vγ9Vδ2+ T cells in physiologically relevant scenarios. In particular, it may be important to separate their immunosurveillance functions from those employed in the context of an established tumor. Moreover, it is critical to understand how the presence of an immunosuppressive environment, such as one where tumor-infiltrating T cells are held in check by inhibitory ligands, affects the functions of Vγ9Vδ2+ T cells. This chapter describes how to establish Epstein-Barr Virus (EBV) infection of human umbilical cord blood mononuclear cells (CBMCs) within immunodeficient mice, so as to drive the in vivo formation of human B cell lymphomas that contain an immunosuppressive environment. Details are provided on how to expand human Vγ9Vδ2+ T cells from peripheral blood mononuclear cells (PBMCs), administer them to the mice, and evaluate tumors and other tissues.
Keywords: humanized mice, γδ T cells, Epstein-Barr virus, lymphoma, tumor immunosuppression, tumor immunosurveillance, tumor immunotherapy
1. Introduction
Anti-tumor functions of γδ T cells.
Since they were first identified about 30 years ago, it has been clear that human γδ T cells display potent cytotoxicity towards tumor target cells (Moingeon et al. 1986). Human γδ T cells lyse target cells in an MHC-unrestricted manner, and have been shown to efficiently kill a variety of neoplastic cell types, particularly those of hematological and epithelial origin (Ensslin and Formby 1991; Fisch et al. 1990; Sturm et al. 1990). Based on their ability to eradicate primary human tumor cells in vitro (Braza et al. 2011; Burjanadze et al. 2007; D’Asaro et al. 2010; Gertner-Dardenne et al. 2012; Kunzmann et al. 2000; Saitoh et al. 2008), and on studies showing that human γδ T cells can control xenografted human tumors in immune-deficient mice in vivo (Chen et al. 2001; Kabelitz et al. 2004; Lozupone et al. 2004; Malkovska et al. 1992; Xiang et al. 2014; Zheng et al. 2001), pilot clinical trials have been undertaken to investigate γδ T cell-based immunotherapies in cancer patients (for recent reviews see (Braza and Klein 2013; Fournie et al. 2013)). While the results of these studies have overall been promising (a recent meta-analysis of 13 clinical trials that used γδ T cell-based immunotherapies and involved patients with advanced or metastatic cancer found a total Effective Rate of 0.407 with a p value <0.014 (Buccheri et al. 2014)), the mechanistic pathways used by human γδ T cells to mediate anti-tumor effects in vivo remain poorly understood. For example, it is not clear whether their anti-tumor effects are necessarily due to their cytotoxic functions, since a number of studies have suggested that γδ T cells may also promote antigen-specific anti-tumor responses by acting as highly stimulatory antigen presenting cells (APCs) for HLA-restricted T cells (Altvater et al. 2012; Brandes et al. 2009; Brandes et al. 2005; Landmeier et al. 2009). Hence, methodologies that allow for investigation of mechanisms underlying the anti-tumor effects of human γδ T cells in vivo are of considerable interest.
EBV model system.
To generate a an experimental model for investigating the anti-tumor effects of human γδ T cell adoptive therapy, we have used Epstein-Barr virus (EBV) to drive the de novo formation of human B-lymphomas in vivo. EBV is a completely human-specific γ-herpesvirus that infects B lymphocytes, causing dysregulated proliferation (Moss et al. 2001). Primary EBV infection is typically controlled by cytolytic lymphocyte responses (Taylor et al. 2015). However, in situations where the initial viral infection is overwhelming or the cytolytic response is sub-optimal, failure to contain the virus leads to potentially fatal B cell proliferation. This is particularly common when pediatric patients who are naive to EBV are transplanted with organs or tissue from an EBV-infected individual; the ensuing pathology is thus termed post-transplant lymphoproliferative disease (PTLD) (Hopwood and Crawford 2000). PTLD is an increasingly common complication after allogeneic stem cell transplantation (Sanz and Andreu 2014).
We have taken advantage of the fact that human umbilical cord blood lymphocytes are naive to EBV to establish an experimental system resembling post-transplant lymphoproliferative disease. By transferring freshly isolated human umbilical cord blood mononuclear cells (CBMCs) and EBV into immunodeficient mice, the initially healthy human B cells become infected by EBV and undergo neoplastic transformation in vivo during the ensuing 2–3 weeks. Typically, about 80–90% of the mice will ultimately develop invasive lymphomas within the peritoneal cavity (Ma et al. 2015). The lymphomas are heavily infiltrated by autologous human CD4+ and CD8+ T cells derived from the umbilical cord blood sample (Ma et al. 2015). However, the B cells in the lymphomas express immunosuppressive ligands (e.g. PD-L1, PD-L2) that hold the anti-tumor functions of the T cells in check (Ma et al. 2016). Thus, this model provides the opportunity to evaluate both the immunosurveillance and tumor rejection functions of human γδ T cells. By adoptively transferring human γδ T cells within the first 1–2 weeks after the injection of CBMCs and EBV their impact on virally infected cells that are only nascently neoplastic can be evaluated (i.e. immunosurveillance). Alternatively, by waiting to administer the γδ T cells until 3–4 weeks, their effects can be evaluated in the context of established tumors containing an immunosuppressive environment (Zumwalde et al. 2017).
Expansion of Vδ 2+ T cells from human blood.
Human γδ T cells are divided into two main subsets based on their T cell receptor (TCR) usage of the Vδ1 chain or the Vδ2 chain. Most of the γδ T cells in human blood use the Vδ2 chain, which is typically paired with the Vγ9 chain. Nearly all Vδ2+ T cells can be potently activated in a TCR-dependent manner by small chemical compounds comprised of a hydrophobic alkyl moiety linked to a polar group that contains one or more phosphates (Bukowski et al. 1998; Bukowski et al. 1995; Morita et al. 1995; Tanaka et al. 1994). Compounds of this type include prenylpyrophosphate metabolites, such as isopentenylpyrophosphate (IPP). FDA-approved bisphosphonate drugs (e.g. Alendronate, Zoledronate) that are prescribed for the prevention and treatment of osteopenia lead to the potent activation of human Vδ2+ T cells, because they block the mevalonate biosynthesis pathway and cause the accumulation of IPP within cells. While the molecular recognition events are not yet fully resolved, it appears that IPP binds to the cytoplasmic tail of an immunoglobulin-superfamily molecule related to the B7-family, called butyrophilin 3A1 (BTN3A1) (Vavassori et al. 2013; Wang et al. 2013). The association of prenylpyrophosphate molecules with the cytoplasmic tail of BTN3A1 causes a change that is detected at the cell surface by Vδ2+ T cells, leading to their proliferation and induction of effector functions (Decaup et al. 2014; Rhodes et al. 2015; Riano et al. 2014; Sandstrom et al. 2014; Wang and Morita 2015). Exposing human peripheral blood mononuclear cells (PBMCs) to bisphosphonate drugs thus provides a simple means to selectively drive the proliferation and activation of Vδ2+ T cells, so as to produce an expanded population of cells that can be used for cellular immunotherapy.
The following sections provide detailed descriptions of methods for setting up the EBV-driven lymphoma model in immunodeficient mice, expanding Vδ2+ T cells from human PBMCs, and harvesting and analyzing lymphomas and other tissues from the mice.
2. Materials
2.1. Engraftment of NSG mice with EBV-exposed human umbilical cord blood cells
2.1.1. Preparation of EBV
A titered solution of the lytic M81 strain of EBV, stored in frozen aliquots at −80 oC or in LN2 (see Note 1). Virus is stored in 200 μl aliquots, at a concentration of 200 IU/μl. Thus, each vial contains a total of 40,000 IU of virus, which is sufficient for 20 mice (the average number of mice that can be generated from one cord blood sample).
2.1.2. Preparation of human umbilical cord blood cells and exposure to EBV
Human umbilical cord blood mononuclear cells (see Note 2).
Ficoll-Paque Premium (GE Healthcare; Pittsburgh, PA).
50 ml conical tubes (sterile).
RPMI 1640 medium (keep cold; 2–8°C).
Culture Medium: RPMI 1640, 10% bovine calf serum (heat inactivated), 3% human AB serum, 1% L-Glu, 1% Penicillin-Streptomycin.
Ammonium-Chloride-Potassium (ACK) lysis buffer (keep cold; 2–8°C).
Sterile PBS, tissue culture grade.
2.1.3. Injection of EBV-treated CBMCs into immunodeficient mice
6–8 week old NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice (Jackson Labs).
Tuberculin syringes and 28.5 gauge needles.
2.2. Expansion of Vδ 2+ T cells from adult peripheral blood and adoptive transfer into EBV-infected mice
2.2.1. Expansion of human Vδ2+ T cells
Anticoagulant treated peripheral blood obtained from healthy adult donors after informed consent, or purified peripheral blood mononuclear cells (PBMCs).
Ficoll-Paque PLUS (GE Healthcare; Pittsburgh, PA).
T cell culture medium: RPMI-1640, 15% bovine calf serum (heat inactivated), 3% human AB serum, 1% L-Glu, 1% Penicillin-Streptomycin, 200 U/mL interleukin-2 (IL-2).
Zometa (zoledronic acid; Novartis).
Antibodies to assess expansion and purity of γδ T cells, such as: anti-Vδ2 (clone B6; BioLegend), anti-Vγ9 (clone B3; BioLegend), anti-CD3 (clones OKT3 or HIT3a; BioLegend)
Flow cytometer for analysis of expanded γδ T cells.
Freezing solution (15% DMSO in bovine calf serum) and cryovials.
2.2.2. Adoptive transfer of human Vδ2+ T cells
Sterile PBS, tissue culture grade.
Tuberculin syringes and 28.5 gauge needles.
Isoflurane and isoflurane chamber for mouse anesthesia
2.3. Collection and analysis of tumors and spleen tissue
2.3.1. Harvesting tumor and spleen tissue
Forceps.
Surgical scissors.
Surgical foam board.
Pins.
2.3.2. Determining tumor burden and preparing tissues for further analysis
50 ml conical tubes, pre-weighed.
Scale.
10% Formalin solution, neutral buffered.
Filter mesh and plungers for manual dissociation of tissues, or gentleMACS dissociator (Miltenyi Biotec).
PBS
2.3.3. Histological analysis of tissues
Biopsy/embedding cassettes.
Absolute ethanol and solutions of 70% and 90% ethanol.
Xylene.
Forceps.
Scissors.
Base molds.
Heating block.
Paraffin wax.
2.3.4. Flow cytometric analysis of spleen or tumor tissues
Fc blocking solution consisting of 20% human AB serum in PBS.
Tubes appropriate for reading samples on flow cytometer.
Flow cytometry sample buffer consisting of a filtered solution of 1 mg/ml BSA in PBS.
Flow cytometer capable of detecting at least 4 channels (preferably 8 or 9).
Antibodies for human cell markers: anti-CD45 (clone HI30; BioLegend), anti-pan HLA-A,B,C (clone W6/32; BioLegend), anti-CD3 (clone OKT3 or HIT3a; BioLegend), anti-CD19 (clone SJ25C1; BioLegend) or anti-CD20 (clone 2H7; BioLegend), anti-Vδ2 (clone B6; BioLegend) or anti-Vγ9 (clone B3; BioLegend). Antibodies against CD19 and CD20 can be used in the same color improve detection of EBV-infected B cells.
3. Methods
3.1. Engraftment of NSG mice with EBV-exposed human umbilical cord blood cells
3.1.1. Preparation of EBV
Thaw sufficient virus for the experiment by briefly incubating one or more frozen aliquots at 37 oC. (Typically, 200 IU of virus is used per 1 × 106 CBMCs and each mouse is injected with 10 × 106 CBMCs, although these numbers may vary depending on the activity of the virus and the availability of CBMCs.)
3.1.2. Preparation of human umbilical cord blood cells and exposure to EBV
If frozen purified CBMCs are used, thaw the cells and wash them according to the supplier’s instructions, then resuspend at 1 × 108 cells/ml in culture medium. If whole cord blood is used, the CBMCs must first be isolated by density gradient centrifugation.
Dilute the whole cord blood with 3x the volume of cold RPMI, and place 35 ml each into 50 ml conical tubes. Slowly pipet 15ml of the Ficoll-Paque under 35 ml of the diluted cord blood.
Centrifuge at 400xg for 40–45 minutes in a centrifuge with a swinging bucket rotor without using brakes or acceleration.
After centrifugation, the blood will have separated into layers: plasma (clear top layer), CBMCs (cloudy or opaque layer at top of interface with Ficoll), dark red bottom layer containing granulocytes and erythrocytes. Aspirate the plasma layer without disturbing the CBMCs, and discard.
Gently aspirate the CBMCs and transfer into a fresh 50 ml conical tube.
Fill the CBMC-containing conical tube with RPMI, and centrifuge at 400xg for 15 min at 18°C with full acceleration and brake.
Discard the supernatant, resuspend the CBMC pellet in RPMI and spin at 300xg for 15 min at 18°C with full acceleration and brake.
Repeat step 7, but at 200xg. The spins described in steps 6–8 help remove platelets from CMBC.
If the CBMC appears red it likely has significant erythrocyte contamination. In this case, resuspend in 10–15 ml of cold ACK lysis buffer and incubate for 5 mins at room temperature. Lysis of erythrocytes should be evident by this time. Fill the tube with culture medium to dilute out the ACK lysis buffer, and spin the cells down at 300xg for 15 minutes.
Resuspend the CBMC pellet in 1.5–2ml culture medium, and count the cells. Dilute to 1 × 108 cells/ml in culture medium.
Add 2000 IU of M81 per 1 × 107 CBMC and incubate at 37°C, 5% CO2, for 2–4 hours, to promote viral attachment to the B lymphocytes in the CBMC sample.
Wash the CMBC with culture medium to remove non-attached virions. Resuspend in sterile PBS (room temperature) at a concentration of 5×107 cells per ml.
3.1.3. Injection of EBV-treated CBMCs into immunodeficient mice
Using sterile tuberculin syringes and 28.5 gauge needles, intraperitoneally inject 200 μl of the cell suspension (1 × 107 CBMC) per mouse.
Mice should be maintained in a specific pathogen-free facility using sterilized cages, bedding, food, and water. Tumors will form in the peritoneal cavity by about 21 days post-injection of EBV-exposed CBMCs; γδ T cell treatment can be performed at any time point depending on whether the goal is to investigate immunosurveillance functions (i.e. prevention of neoplastic cell outgrowth or tumor deposition), or effects that occur in the presence of established tumors (e.g. infiltration or eradication).
3.2. Expansion of Vδ 2+ T cells from adult peripheral blood and adoptive transfer into EBV-infected mice
3.2.1. Expansion of human Vδ2+ T cells
If frozen purified PBMCs are used, thaw the cells and wash them according to the supplier’s instructions, then resuspend at 2×106 cells/ml in T cell culture medium. If whole peripheral blood is used, the PBMCs must first be isolated by density gradient centrifugation using Ficoll-PLUS, as described for CBMCs in section 3.1.2.
Dilute Zometa to a concentration of 5 μM in T cell culture medium.
Add equal volumes of PBMCs (0.5 ml) and 5 μM Zometa (0.5 ml) to wells of a 24-well plate, resulting in a density of 1×106 cells/well and a final Zometa concentration of 2.5 μM (see Note 3).
Incubate the cells at 37 °C in a humidified incubator with 5% CO2.
Monitor the cultures to assess cell growth and acidification of the culture medium. Add 1 ml fresh T cell culture medium after 4–6 days, or sooner if the wells appear to be turning orange or yellow. As the cells expand (typically in the second week of culture), split each well into a second well and supplement with fresh T cell culture media.
Flow cytometric analyses should be performed to assess purity of γδ T cells and expression of ligands of interest (e.g. PD-1). (see Note 4).
Cultures containing expanded Vδ2+ T cells are typically harvested after 8–14 days, with the precise timing chosen according to the level of Vδ2+ T cell enrichment and total cell number. If necessary, contaminating cell types can be removed from the expanded Vδ2+ T cell culture by magnetic or flow cytometric sorting. (see Note 5).
The can also be stored frozen for up to 3 months prior to use. Chill a suspension of culture medium containing 2–10 × 107 cells/ml and mix with 2x volume of cold freezing solution (15% DMSO in bovine calf serum). Pipet into pre-chilled cryovials and immediately transfer to −80 °C.
3.2.2. Adoptive transfer of human Vδ2+ T cells
If the expanded γδ T cells have been stored frozen, thaw and wash cells several times with sterile PBS to remove DMSO.
Count cells and resuspend at 2.5×107 cells/ml in sterile PBS.
Anesthetize mice that were injected with EBV-exposed CBMCs.
Intravenously administer 2.5–5×107 cells in a volume of 100–200 μl by retro-orbital injection. Control mice should be injected with an equivalent volume of sterile PBS.
3.3. Collection and analysis of tumors and spleen tissue
3.3.1. Harvesting tumor and spleen tissue
Tumors are typically visible in the peritoneal cavity by 21 days post-injection of EBV-exposed CBMCs; mice typically become moribund by about 32–34 days post-injection. Thus, analyses can be done at any time during this window.
Euthanize the mice as specified by your institutional Animal Care and Use Committee.
Place murine carcasses with the ventral side up on a surgical foam board, and pin the limbs to the board.
Using a pair of forceps, pull the skin near the urethral opening upwards, and make an incision with a pair of surgical scissors. The incision should cut along the midline, and go from the urethral opening up to the chin.
Using the forceps carefully pinch the peritoneal sac pulling upwards and making an incision identical to the one in step 4 to expose the viscera.
Tumors are most commonly found initially in peri-pancreatic areas, and at later stages often invade pancreas, bile duct, liver, intestines, and (less commonly) other tissues. Locate the stomach on the left side of the peritoneal cavity, and with a pair of forceps, begin to separate the pancreas from the stomach and the intestines, delicately severing connections between the pancreas and the thorax using either another pair of forceps or a pair of surgical scissors.
Pull out the spleen and attached pancreas, and separate the spleen. Spleens should be weighed and then placed into cold culture medium or fixative solution for later analysis (e.g. flow cytometry or histology).
Place the pancreas and any associated tissue on the surgical foam board to examine for the presence of tumors. The mesenteric tissue wrapped around the pancreas can often be infiltrated with tumors or lymphoid aggregates giving it a bulbous, engorged appearance. Thus, while the pancreas itself is shaped like a flat pear with pink coloration (sometimes grey-ish when infiltrated by tumor cells), the tumors are usually lighter-colored masses with a firm texture. Carefully dissect the tumor tissue from the pancreas (see Figure 1A and B).
Using a pair of forceps, also examine the mesentery, stomach, intestine and liver lobes for tumors. These will appear as white/pinkish engorged masses resting on the tissue, and can be harvested using scissors.
Fig. 1.
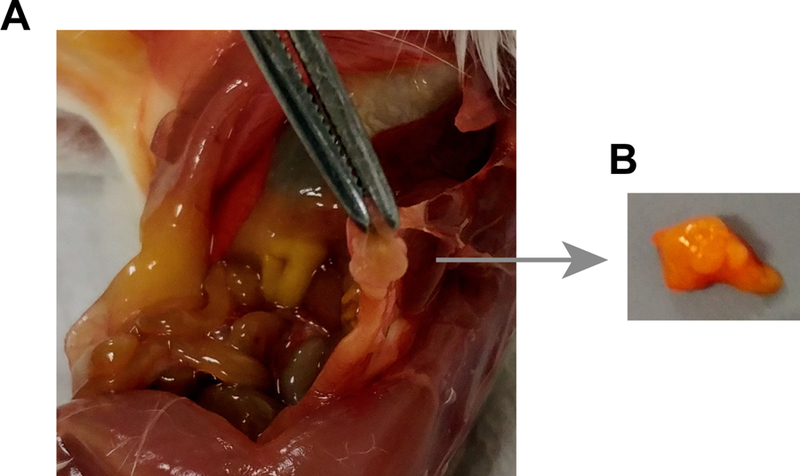
Removal of tumor tissue from EBV -infected mice. (a) View of peritoneal cavity containing a tumor mass (tissue held in forceps). (b) Excised tumor tissue
3.3.2. Determining tumor burden and preparing tissues for further analysis
Decide whether tumors will be analyzed by histology or flow cytometry, and prepare 50 ml conical tubes with 5–10 ml 10% neutral buffered formalin (histology) or cold culture medium (flow cytometry). Weigh the tubes containing the appropriate buffer, and record the weight on the side of the tube. To determine the tumor burden, place all of the excised tumor tissue from an individual mouse into a tube, and re-weigh using the same scale. Subtract the tube weight to obtain the weight of the tumor tissue.
Tissues collected for histology may be stored at room temperature in 10% neutral buffered formalin for 24–48h, before further processing.
Tissues that are to be analyzed by flow cytometry should be kept cold and processed immediately.
3.3.3. Histological analysis of fixed tissues
To prepare them for paraffin sectioning, cut the tissue into slices ~3mm thick. Tissue preparation is often performed by a core facility or a commercial service using an automated tissue processor. Alternatively, processing steps can be completed manually as described briefly in the following steps (Slaoui and Fiette 2011).
Place the tissues into appropriately labeled embedding cassettes. Put the cassettes in a beaker containing 70% ethanol, and incubate at room temperature for 60 min.
Transfer the cassettes to a beaker of 95% ethanol, and incubate for 60 min.
Transfer the cassettes to a beaker of absolute ethanol, and incubate for 60 min. Repeat twice.
Transfer the cassettes to a beaker containing xylene, and incubate for 20 min. Repeat twice, with the final incubation lasting 45 min to 24 hr.
Place cassettes in a container of molten paraffin wax and agitate gently for 30 min. Repeat twice.
Fill block molds with molten paraffin wax and position tissue within the wax as desired for sectioning; place cassette on top as backing.
Tissue can now be sectioned using a microtome and placed onto slides. For each tissue, it is helpful to perform hematoxylin and eosin staining on the first slide of a series of serial sections to identify areas of interest (e.g. tissues containing lymphocytes that likely correspond to tumor or lymphoid tissue). Further serial sections can then be chosen and immunohistochemistry performed using specific antibodies, as described (Lockridge et al. 2013; Ma et al. 2011; Ma et al. 2016; Ma et al. 2015; Ma et al. 2012; Zumwalde et al. 2017).
3.3.4. Flow cytometric analysis of spleen or tumor tissues
Prepare a single cell suspension from the tissue sample. Tissues can be dissociated manually by using the backside of a plunger from a plastic syringe to mash the tissue over filter mesh (40–100 μm), or automatically using a tissue dissociator. If desired, the single cell suspension can be stored frozen using the freezing procedure described in step 7 of section 3.2.1..
Centrifuge samples at 400xg for 5 minutes to pellet cells, and discard supernatant.
Resuspend in PBS and pass through a 40–100 μm filter mesh (see Note 6).
Suspend single cell suspension in 1 ml Fc-blocking buffer and incubate for 15 minutes at 4 oC.
Pellet cells by centrifugation and pour off supernatant.
Add cocktail of staining antibodies to the void volume in the tubes and incubate for 30 minutes at 4 °C.
Wash the samples with flow cytometry sample buffer, and resuspend in a volume of 0.3–0.5 ml.
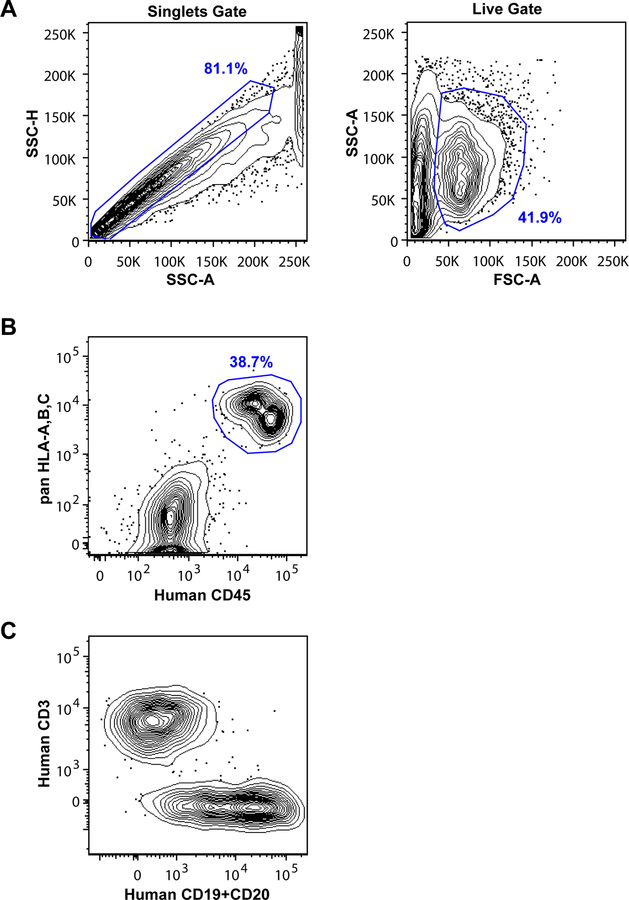
Fig. 2.
Flow cytometric gating for analysis of human cells from EBV -infected mice. (a) Examples of light scatter gating. Use height versus area for the same light scatter parameter (e.g., side scatter, or SSC) to identify singlet events; use the area of two different light scatter parameters (e.g., forward scatter versus side scatter) to exclude dead cells and debris from the analysis. (b) Identification of human cells using antibodies against HLA and human CD45. (c) Identification of human T cells and B cells using antibodies against CD3 and CD19 and/or CD20
Acknowledgements
Funding provided by a pilot grant from the University of Wisconsin Carbone Cancer Center that was supported in part by NIH grant NCI P30 CA014520, and by NIH grant R21 AI116007 and DoD CDMRP Award CA160396 to JEG; support for NAZ provided by T32 CA157322. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no conflicting financial interests.
Footnotes
4 Notes
We recommend using a lytic strain of EBV (e.g. M81, Akata) rather than the more commonly used B95–8 strain. B95–8 lacks EBV-encoded microRNAs and has an almost completely latent pattern of gene expression, whereas strains such as M81 contain the whole viral genome and show both lytic and latent gene expression. Since most of the EBV peptide epitopes recognized by CD8+ T cells derive from lytic genes, the use of a lytic strain produces better virally-driven CD8+ T cell expansion and thus also provides for better modeling of the immunosuppressive environment that can thwart anti-tumor T cell responses.
The titer of the virus particles must established prior to use. To do this, we make use of an EBV construct that expresses the green fluorescent protein (GFP) (Tsai et al. 2013). Infectious viral particles are produced from 293 cell lines that were stably infected with the M81 strain of EBV following transfection with EBV BZLF1 expression vector as described (Ma et al. 2011). The titer of infectious EBV is determined by assessing the number of fluorescent Raji cells derived from serial dilutions of the concentrated virus stock and calculating the green Raji unit (GRU) titer, as described (Ma et al. 2011).
Cord blood may be obtained as an anticoagulant-treated whole blood sample, or as purified cord blood mononuclear cells (CBMCs) that are supplied either fresh or frozen. The CD34+ cells are not needed for this protocol, and so if desired these may be removed from the CBMCs by magnetic sorting prior to EBV-exposure.
In Zometa expanded PBMC cultures, Vδ2+ T cells typically comprise 70–80% of the total CD3 population between days 7 and 11 post stimulation. However, the percentage of Vδ2+ T cells can vary depending on the initial frequency of γδ T cells in the starting PBMC sample and the potency of the Zometa preparation, among other variables. It is therefore advisable to monitor the Vδ2+ T cell expansion using flow cytometry, by testing the starting PBMCs at day 0, and on the day of harvest.
In the Zometa expanded Vδ2+ T cell cultures, we have observed that PD-1 becomes transiently upregulated, typically peaking at about day 4, then returns to baseline by day 6–8 and remains low for the subsequent days of culture. Remarkably, the Vδ2+ T cells maintain their low PD-1 expression after adoptive transfer (Zumwalde et al. 2017). Since low cell surface expression of PD-1 may be important for the anti-tumor functions of the adoptively transferred Vδ2+ T cells, we recommend that flow analyses be performed to confirm that the transient elevation of PD-1 expression has subsided, prior to adoptive transfer.
We have not seen evidence that contaminating cell types in cultures of Zometa-expanded Vδ2+ T cells have an impact on anti-tumor effects (Zumwalde et al. 2017). However, if desired, further sorting can be performed to increase Vδ2+ T cell purity. Since most of the contaminating cells are usually αβ T cells, negative selection can be performed using pan-αβ TCR antibodies. However, since other populations (e.g. NK cells) are sometimes also present at low frequencies in the culture, if an essentially 100% pure population of Vδ2+ T cells is desired, it may be necessary to positively select the Vδ2+ T cells using an antibody against Vδ2 or Vγ9. Importantly, we have not validated whether such positive selection using reagents that bind the TCR impacts the functioning of the Vδ2+ T cells after adoptive transfer.
The viability of cells from tumor samples is often much lower than that of spleen samples. Tumor samples typically also require additional filtering compared to the spleen.
Detection and analysis of human cells requires careful flow cytometric gating. It is helpful to set a singlets gate using side-scatter parameters, then gate on FSC vs. SSC to focus on live cells (see Figure 2A). It is best to use at least two different parameters to identify the total human cell population, such as pan-HLA-A,B,C staining and anti-human CD45. Murine cells can also be excluded using antibodies against H-2, CD45, or other widely distributed murine antigens. Human cells identified by pan-HLA-A,B,C and anti-CD45 typically distribute into two nodes (see Figure 2B), with the HLA-A,B,Cbright/CD45intermediate population corresponding to B lymphocytes and the HLA-A,B,Cintermediate/CD45bright subset corresponding to T lymphocytes. The EBV-infected B lymphocytes often down-regulate CD20, and to a lesser extent CD19, from the cell surface at later stages in the infection. Thus, it can be helpful to stain for both of these markers in the same channel to amplify the signal. Nevertheless, even though histological analyses of the same tissue sample typically reveal abundant CD20+ cells, flow cytometric staining often reveals a distribution of CD3-negative human cells that range from clearly CD19/CD20 positive to essentially negative (see Figure 2C).
To accurately gauge expression of critical markers or to identify potentially rare cell types (e.g. adoptively transferred γδ T cells), it is helpful to set up parallel “fluorescence-minus-one” negative control samples, that are stained with all of the antibodies except the one of interest.
When staining for the adoptively transferred Vγ9Vδ2 T cells, it is best to use just a single TCR-specific reagent, since staining is often sub-optimal if multiple TCR-binding reagents (e.g. anti-Vγ9, anti-Vδ2, and anti-pan γδ mAbs) are all used in the same staining panel.
If delineating the adoptively transferred γδ T cells from those of the cord blood sample is important, HLA-A2 mismatching is helpful (Zumwalde et al. 2017). This is accomplished by staining CBMC and PBMC samples in order to identify samples that are mismatched in regards to expression of HLA-A2 using an HLA-A2 specific antibody (clone BB7.2; BioLegend). After the HLA-A2 mis-matched γδ T cells are adoptively transferred, they can be readily distinguished from human cells that are derived from the cord blood sample by including the HLA-A2-specific antibody in the analysis panel.
References
- Altvater B, Pscherer S, Landmeier S, Kailayangiri S, Savoldo B, Juergens H, Rossig C (2012) Activated human gammadelta T cells induce peptide-specific CD8+ T-cell responses to tumor-associated self-antigens Cancer Immunol Immunother 61:385–396 doi: 10.1007/s00262-011-1111-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes M et al. (2009) Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses Proceedings of the National Academy of Sciences of the United States of America 106:2307–2312 doi: 10.1073/pnas.0810059106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Moser B (2005) Professional antigen-presentation function by human gammadelta T Cells Science 309:264–268 doi: 10.1126/science.1110267 [DOI] [PubMed] [Google Scholar]
- Braza MS, Klein B (2013) Anti-tumour immunotherapy with Vgamma9Vdelta2 T lymphocytes: from the bench to the bedside Br J Haematol 160:123–132 doi: 10.1111/bjh.12090 [DOI] [PubMed] [Google Scholar]
- Braza MS, Klein B, Fiol G, Rossi JF (2011) gammadelta T-cell killing of primary follicular lymphoma cells is dramatically potentiated by GA101, a type II glycoengineered anti-CD20 monoclonal antibody Haematologica 96:400–407 doi: 10.3324/haematol.2010.029520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccheri S, Guggino G, Caccamo N, Li Donni P, Dieli F (2014) Efficacy and safety of gammadeltaT cell-based tumor immunotherapy: a meta-analysis Journal of biological regulators and homeostatic agents 28:81–90 [PubMed] [Google Scholar]
- Bukowski JF, Morita CT, Band H, Brenner MB (1998) Crucial role of TCR gamma chain junctional region in prenyl pyrophosphate antigen recognition by gamma delta T cells J Immunol 161:286–293. [PubMed] [Google Scholar]
- Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H (1995) V gamma 2V delta 2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer J Immunol 154:998–1006 [PubMed] [Google Scholar]
- Burjanadze M et al. (2007) In vitro expansion of gamma delta T cells with anti-myeloma cell activity by Phosphostim and IL-2 in patients with multiple myeloma Br J Haematol 139:206–216 doi: 10.1111/j.1365-2141.2007.06754.x [DOI] [PubMed] [Google Scholar]
- Chen J, Niu H, He W, Ba D (2001) Antitumor activity of expanded human tumor-infiltrating gammadelta T lymphocytes Int Arch Allergy Immunol 125:256–263 doi:53824 [DOI] [PubMed] [Google Scholar]
- D’Asaro M et al. (2010) V gamma 9V delta 2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells J Immunol 184:3260–3268 doi: 10.4049/jimmunol.0903454 [DOI] [PubMed] [Google Scholar]
- Decaup E, Duault C, Bezombes C, Poupot M, Savina A, Olive D, Fournie JJ (2014) Phosphoantigens and butyrophilin 3A1 induce similar intracellular activation signaling in human TCRVgamma9+ gammadelta T lymphocytes Immunol Letters 161:133–137 doi: 10.1016/j.imlet.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Ensslin AS, Formby B (1991) Comparison of cytolytic and proliferative activities of human gamma delta and alpha beta T cells from peripheral blood against various human tumor cell lines Journal of the National Cancer Institute 83:1564–1569 [DOI] [PubMed] [Google Scholar]
- Fisch P et al. (1990) Gamma/delta T cell clones and natural killer cell clones mediate distinct patterns of non-major histocompatibility complex-restricted cytolysis J Exp Med 171:1567–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie JJ et al. (2013) What lessons can be learned from gammadelta T cell-based cancer immunotherapy trials? Cell Mol Immunol 10:35–41 doi: 10.1038/cmi.2012.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertner-Dardenne J et al. (2012) Human Vgamma9Vdelta2 T cells specifically recognize and kill acute myeloid leukemic blasts J Immunol 188:4701–4708 doi: 10.4049/jimmunol.1103710 [DOI] [PubMed] [Google Scholar]
- Hopwood P, Crawford DH (2000) The role of EBV in post-transplant malignancies: a review J Clin Pathol 53:248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, Pitters E, Zoller M (2004) Characterization of tumor reactivity of human V gamma 9V delta 2 gamma delta T cells in vitro and in SCID mice in vivo J Immunol 173:6767–6776 [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M (2000) Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma Blood 96:384–392 [PubMed] [Google Scholar]
- Landmeier S et al. (2009) Activated human gammadelta T cells as stimulators of specific CD8+ T-cell responses to subdominant Epstein Barr virus epitopes: potential for immunotherapy of cancer J Immunother 32:310–321 doi: 10.1097/CJI.0b013e31819b7c30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockridge JL et al. (2013) Mice Engrafted with Human Fetal Thymic Tissue and Hematopoietic Stem Cells Develop Pathology Resembling Chronic Graft-versus-Host Disease Biol Blood Marrow Transplant doi: 10.1016/j.bbmt.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone F et al. (2004) Effect of human natural killer and gammadelta T cells on the growth of human autologous melanoma xenografts in SCID mice Cancer Res 64:378–385 [DOI] [PubMed] [Google Scholar]
- Ma SD et al. (2011) A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas J Virol 85:165–177 doi: 10.1128/JVI.01512-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SD et al. (2016) PD-1/CTLA-4 Blockade Inhibits Epstein-Barr Virus-Induced Lymphoma Growth in a Cord Blood Humanized-Mouse Model PLoS Pathog 12:e1005642 doi: 10.1371/journal.ppat.1005642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SD et al. (2015) LMP1-deficient Epstein-Barr virus mutant requires T cells for lymphomagenesis J Clin Invest 125:304–315 doi: 10.1172/JCI76357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SD et al. (2012) An Epstein-Barr Virus (EBV) Mutant with Enhanced BZLF1 Expression Causes Lymphomas with Abortive Lytic EBV Infection in a Humanized Mouse Model J Virol 86:7976–7987 doi: 10.1128/JVI.00770-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkovska V, Cigel FK, Armstrong N, Storer BE, Hong R (1992) Antilymphoma activity of human gamma delta T-cells in mice with severe combined immune deficiency Cancer Res 52:5610–5616 [PubMed] [Google Scholar]
- Moingeon P et al. (1986) A unique T-cell receptor complex expressed on human fetal lymphocytes displaying natural-killer-like activity Nature 323:638–640 doi: 10.1038/323638a0 [DOI] [PubMed] [Google Scholar]
- Morita CT et al. (1995) Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells Immunity 3:495–507 [DOI] [PubMed] [Google Scholar]
- Moss DJ, Burrows SR, Silins SL, Misko I, Khanna R (2001) The immunology of Epstein-Barr virus infection Philos Trans R Soc Lond B Biol Sci 356:475–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DA et al. (2015) Activation of human gammadelta T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin J Immunol 194:2390–2398 doi: 10.4049/jimmunol.1401064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riano F et al. (2014) Vgamma9Vdelta2 TCR-activation by phosphorylated antigens requires butyrophilin 3 A1 (BTN3A1) and additional genes on human chromosome 6 Eur J Immunol 44:2571–2576 doi: 10.1002/eji.201444712 [DOI] [PubMed] [Google Scholar]
- Saitoh A et al. (2008) Anti-tumor cytotoxicity of gammadelta T cells expanded from peripheral blood cells of patients with myeloma and lymphoma Medical oncology 25:137–147 doi: 10.1007/s12032-007-9004-4 [DOI] [PubMed] [Google Scholar]
- Sandstrom A et al. (2014) The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells Immunity 40:490–500 doi: 10.1016/j.immuni.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz J, Andreu R (2014) Epstein-Barr virus-associated posttransplant lymphoproliferative disorder after allogeneic stem cell transplantation Curr Opin Oncol 26:677–683 doi: 10.1097/CCO.0000000000000119 [DOI] [PubMed] [Google Scholar]
- Slaoui M, Fiette L (2011) Histopathology procedures: from tissue sampling to histopathological evaluation Methods Mol Biol 691:69–82 doi: 10.1007/978-1-60761-849-2_4 [DOI] [PubMed] [Google Scholar]
- Sturm E, Braakman E, Fisch P, Vreugdenhil RJ, Sondel P, Bolhuis RL (1990) Human V gamma 9-V delta 2 T cell receptor-gamma delta lymphocytes show specificity to Daudi Burkitt’s lymphoma cells J Immunol 145:3202–3208 [PubMed] [Google Scholar]
- Tanaka Y et al. (1994) Nonpeptide ligands for human gamma delta T cells Proc Natl Acad Sci U S A 91:8175–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD (2015) The immunology of Epstein-Barr virus-induced disease Annu Rev Immunol 33:787–821 doi: 10.1146/annurev-immunol-032414-112326 [DOI] [PubMed] [Google Scholar]
- Tsai MH et al. (2013) Spontaneous lytic replication and epitheliotropism define an Epstein-Barr virus strain found in carcinomas Cell Rep 5:458–470 doi: 10.1016/j.celrep.2013.09.012 [DOI] [PubMed] [Google Scholar]
- Vavassori S et al. (2013) Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells Nat Immunol 14:908–916 doi: 10.1038/ni.2665 [DOI] [PubMed] [Google Scholar]
- Wang H et al. (2013) Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vgamma2Vdelta2 T cells J Immunol 191:1029–1042 doi: 10.4049/jimmunol.1300658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Morita CT (2015) Sensor Function for Butyrophilin 3A1 in Prenyl Pyrophosphate Stimulation of Human Vgamma2Vdelta2 T Cells J Immunol 195:4583–4594 doi: 10.4049/jimmunol.1500314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z et al. (2014) Targeted activation of human Vgamma9Vdelta2-T cells controls epstein-barr virus-induced B cell lymphoproliferative disease Cancer cell 26:565–576 doi: 10.1016/j.ccr.2014.07.026 [DOI] [PubMed] [Google Scholar]
- Zheng BJ et al. (2001) Anti-tumor effects of human peripheral gammadelta T cells in a mouse tumor model International journal of cancer Journal international du cancer 92:421–425 [DOI] [PubMed] [Google Scholar]
- Zumwalde NA et al. (2017) Adoptively transferred Vgamma9Vdelta2 T cells show potent antitumor effects in a preclinical B cell lymphomagenesis model JCI Insight 2 doi: 10.1172/jci.insight.93179 [DOI] [PMC free article] [PubMed] [Google Scholar]