Abstract
Phospholipase A2 (PLA2) enzymes are the upstream regulators of the eicosanoid pathway liberating free arachidonic acid from the sn−2 position of membrane phospholipids. Increased levels of intracellular arachidonic acid serve as a substrate for the eicosanoid biosynthetic pathway enzymes including cyclooxygenases, lipoxygenases and cytochrome P450s that lead to inflammation. The Group IVA cytosolic (cPLA2), Group VIA calcium-independent (iPLA2), and Group V secreted (sPLA2) are three well-characterized human enzymes that have been implicated in eicosanoid formation. In this review, we will introduce and summarize the regulation of catalytic activity and cellular localization, structural characteristics, interfacial activation and kinetics, substrate specificity, inhibitor binding and interactions, and the downstream implications for eicosanoid biosynthesis of these three important PLA2 enzymes.
Keywords: Phospholipase, Catalytic Mechanism, HD-XMS, Molecular Dynamics, LC/MS Assays, Substrate Specificity
Introduction
Phospholipases A2 (PLA2) are lipolytic enzymes that play a central role in cellular lipid metabolism and signaling [1]. When PLA2 enzymes are activated in cells, they catalyze the hydrolysis of the ester bond at the sn−2 position of membrane phospholipids which are generally enriched in arachidonic acid (AA) and other polyunsaturated fatty acids (PUFA) [2]. The release of AA and other PUFAs triggers a cascade of cellular processes that involve cyclooxygenases and lipoxygenases that are key in the biosynthesis of eicosanoids including leukotrienes, prostaglandins and thromboxanes [3]. Eicosanoids are lipid mediators that regulate a variety of cellular responses, and they are especially important in immunity and inflammation [4]. Lysophospholipids, which constitutes the other products of PLA2 catalysis, can lead to a variety of cellular metabolites such as lysophosphatidic acid (LPA) which can bind to G protein-coupled receptors [5, 6]. They are also precursors of platelet-activating factor (PAF) which is a potent inflammatory lipid mediator [7, 8].
The PLA2 superfamily consists of 16 groups of structurally and functionally diverse enzymes [9]. The six main types of PLA2 enzymes include the secreted (sPLA2), cytosolic (cPLA2), calcium-independent (iPLA2), platelet-activating factor acetylhydrolase (PAF-AH), also known as lipoprotein-associated PLA2 (Lp-PLA2), lysosomal PLA2 (LPLA2), and adipose-PLA2 (AdPLA) [9]. Our recent studies have focused on three human recombinant enzymes, namely the Group IVA cytosolic (cPLA2), Group VIA calcium-independent (iPLA2), and Group V secreted (sPLA2), which are all water-soluble, membrane-associated enzymes with distinct structures and biological functions [10, 11]. The structure of each enzyme contains a unique active site where the substrate binds and an interfacial surface that mediates association with cellular membranes [12]. Hydrogen/deuterium exchange mass spectrometry (HD-XMS) and molecular dynamics simulations were successfully employed by our laboratory to study the interactions of these enzymes with membranes, substrates, and inhibitors [13–15]. In this review, we discuss what we have now learned about the regulation of activity and cellular localization, structural characteristics, interfacial activation and kinetics, substrate specificity, inhibitor binding and interactions, and implications for the biosynthesis of eicosanoids by these three PLA2 enzymes under physiological conditions.
Regulation of activity and cellular localization
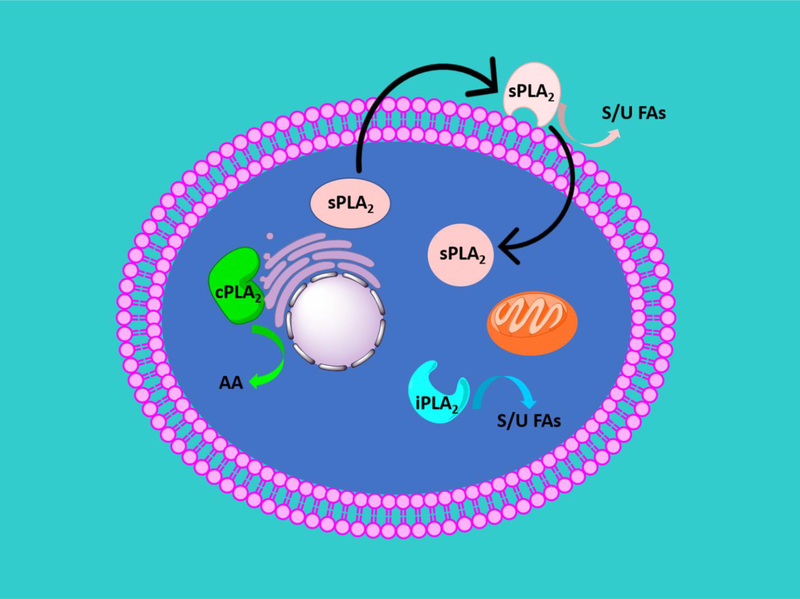
PLA2 activity is regulated by complex mechanisms of activation that cause translocation of the PLA2 enzymes to cellular membranes. cPLA2 activity is regulated by calcium and phosphorylation [16]. The C2 domain contains a calcium binding site that aids in the translocation of cPLA2 to the membrane upon increases in intracellular calcium [17, 18]. Several studies demonstrated a calcium-mediated translocation of cPLA2 to the nuclear envelop and endoplasmic reticulum [19–22]. Phosphorylation of Ser505 has been also found to contribute to cPLA2 activation [23]. In addition to activation by calcium and phosphorylation, phosphatidylinositol 4,5-bisphosphate (PIP2) has been shown to enhance the enzymatic activity of cPLA2 [24, 25]. iPLA2 is a calcium-independent enzyme whose activity is regulated, stabilized, and increased by ATP [9, 26, 27]. It has been shown that iPLA2 is also regulated through other mechanisms of activation including caspase cleavage, calmodulin, and ankyrin repeat mediated oligomerization [9]. Individual sPLA2 enzymes are expressed in specific cell types such as immune cells, epithelial cells, and others [28–30]. It has been shown that sPLA2 hydrolyzes the plasma membrane to release lysophospholipids and free fatty acids which may cause an increase in intracellular calcium concentration that activates cPLA2 [31, 32]. It has been reported that sPLA2 also hydrolyzes oxidized phospholipids in LDL and HDL contributing to atherosclerosis [33, 34]. Figure 1 is a cartoon representation that depicts some of the reported cellular localizations of cPLA2, iPLA2, and sPLA2.
Figure 1.
Cartoon representation that depicts reported cellular localizations of cPLA2, iPLA2, and sPLA2. cPLA2 translocates to the perinuclear membranes including the Golgi. iPLA2 was found in the cytosol and was also found associated with mitochondria and may have different functions/localizations in different cells. sPLA2 was found to be secreted where it may act on cells or may be internalized and act intracellularly. Extracellularly, sPLA2 was found to act on extracellular phospholipids such as microvesicles/exosomes, surfactants, lipoproteins, and bacterial membranes. AA is an abbreviation for arachidonic acid. S/U FAs is an abbreviation for saturated and unsaturated fatty acids.
Structural Characteristics
cPLA2 and iPLA2 share a common catalytic Ser/Asp dyad and they have a similar molecular weight of approximately 85 to 90 kDa. In both enzymes, the catalytic Ser lies in a lipase consensus motif of GXSXG/S [9]. They also contain a very similar sequence motif called the “dual signature nucleotide” (GXGXXG) that contains two important features for catalysis: first an oxyanion hole which stabilizes the tetrahedral intermediate after the attack of the catalytic Ser at the sn−2 carbonyl group, and second a positively charged residue of Arg (cPLA2) or Lys (iPLA2) that stabilizes the binding of the phosphate group [14, 35]. The crystal structure of cPLA2 identified the C2 domain and an α/β hydrolase catalytic domain [18]. Human iPLA2 is expressed in two active splice variants that differ by a 54 amino acid insert. Sequence alignment studies revealed an ankyrin repeat region, a linker region, and an α/β hydrolase catalytic domain [36, 37]. The crystal structure of the short splice variant was recently published and showed a dimer [38]. For the long splice variant, a homology model was previously published [13, 14], and the catalytic domain was consistent with the crystal structure of the short form where applicable [38]. sPLA2 is a small 14 kDa protein that contains six disulfide bonds [9]. The crystal structure of the Group V sPLA2 has not been reported, but a homology model was previously published [13]. sPLA2 utilizes a His/Asp catalytic dyad. It has been suggested that sPLA2 activity may be affected by cPLA2 or vice versa [22, 39].
Interfacial activation and kinetics
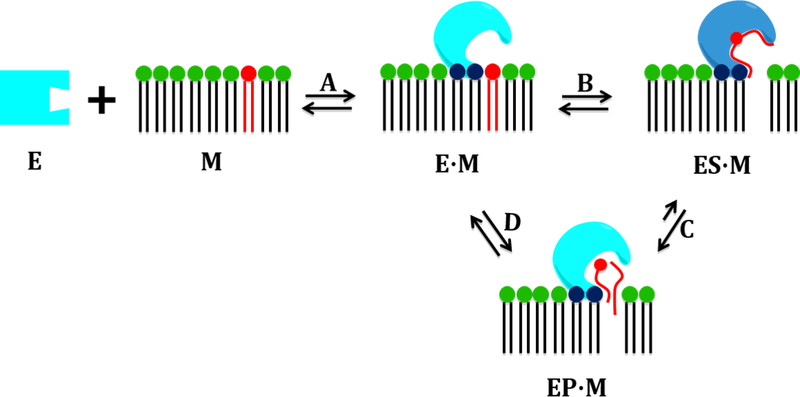
PLA2 enzymes can exist in a water-soluble state (E), but their water-insoluble phospholipid substrates are part of cellular membranes (M). The first step of the PLA2 catalytic cycle is the association with the surface of the membrane (EM) through their interfacial surface (Figure 2). By using HD-XMS and molecular dynamics, we showed that the membrane acts as an allosteric ligand, shifting the conformation of a PLA2 from the closed form in water to the open form on the surface of the membrane [13, 14]. This process enables the enzyme to extract and bind a phospholipid molecule in the active site (ES·M), where it is converted into product (EP·M). Figure 3 shows the mechanism for the hydrolysis of a phospholipid molecule by cPLA2 or iPLA2 [14]. The interfacial activation of PLA2 enzymes can best be explained by the “surface dilution kinetics” model [40, 41]. These studies were conducted by developing interaction models of cPLA2, iPLA2, and sPLA2 with the membrane (Figure 4). Several peptide regions, which are part of the interfacial surface of each enzyme, showed decreased in H/D exchange rates upon association with phospholipid vesicles [13, 14]. These peptide regions were used to place each enzyme on the surface of the membrane. It is worth noting that the membrane interaction models only consider a monomeric association of the enzyme with the membrane, although these enzymes may be aggregated, and iPLA2 was reported to exist as a dimer or a tetramer [42].
Figure 2.
Cartoon representation of the catalytic cycle of PLA2 enzymes (reprinted from reference 13).
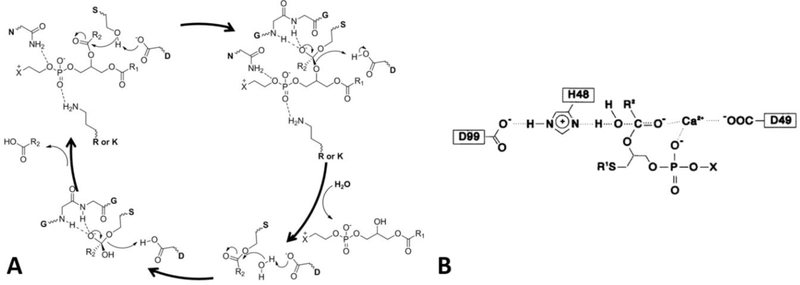
Figure 3.
Mechanism for the hydrolysis of a phospholipid molecule by cPLA2 or iPLA2 (reprinted from reference 14).
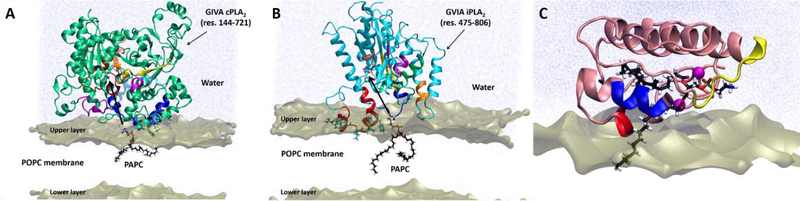
Figure 4.
Interaction models for (A) cPLA2, (B) iPLA2, and (C) sPLA2 with the membrane based on HD-XMS data (adapted form references 13 and 14). The colored peptide regions on each enzyme showed decreased deuteration levels upon association with phospholipid vesicles [43–45].
Substrate specificity
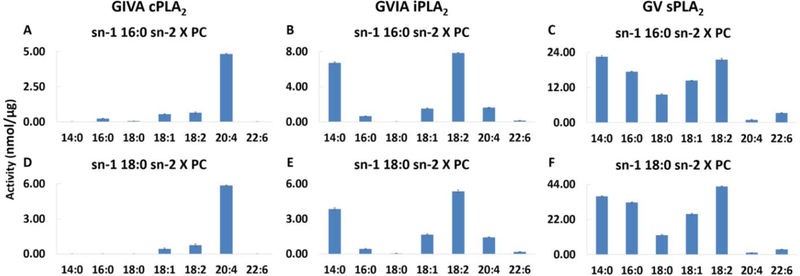
Determining the activity of PLA2 enzymes toward a wide variety of phospholipid substrates has always been a very challenging task using traditional radioactive assays. We have recently developed a novel lipidomics-based PLA2 assay that enabled us to measure the activity of cPLA2, iPLA2, and sPLA2 toward a variety of commercially available synthetic and natural phospholipids [13]. cPLA2 showed distinct specificity for arachidonic acid at the sn−2 position, while iPLA2 and GV sPLA2 are more permissive with preference for linoleic and myristic acid (Figure 5). No significant differences in specificity for the fatty acid at the sn−2 position were observed between palmitic and stearic acid at the sn−1 position. cPLA2 and sPLA2 activities are slightly better toward phospholipids containing stearic acid at the sn−1 position, whereas iPLA2 activity is somewhat better toward phospholipids containing palmitic acid at the sn−1 position [13]. Previously published studies suggested that, although both cPLA2 and iPLA2 contribute to LPC accumulation during stimulation of macrophages, 18:0 LPC appears to be produced primarily by cPLA2 whereas 16:0 LPC appears to be produced primarily by iPLA2 [46, 47]. Molecular dynamics simulations guided by HD-XMS revealed that cPLA2 contains a deep channel-like active site which accommodates a phospholipid molecule in its entirety (see Movie S1 from ref 14: http://movieusa.glencoesoftware.com/video/10.1073/pnas.1424651112/video-1). The active site of cPLA2 is enriched with aromatic residues that interact with the four double bonds of the arachidonic acids through pi-pi stacking (see Movie 1 from ref 13: http://pubs.acs.org/doi/suppl/10.1021/jacs.7b12045/suppl_file/ja7b12045_si_002.avi). In contrast, iPLA2 and sPLA2 contain a more flexible active site that allows them to tightly bind phospholipids containing a larger variety of fatty acids in the sn−2 position by recruiting different binding pockets. Linoleic acid containing a single bis-allylic position binds to an aromatic pocket while myristic acid binds to an aliphatic pocket in both iPLA2 and sPLA2 [13, 14].
Figure 5.
Enzymatic activity of (A, D) cPLA2, (B, E) iPLA2, and (C, F) sPLA2 toward a variety of phospholipid substrates (reprinted from reference 13).
Inhibitor binding and interactions
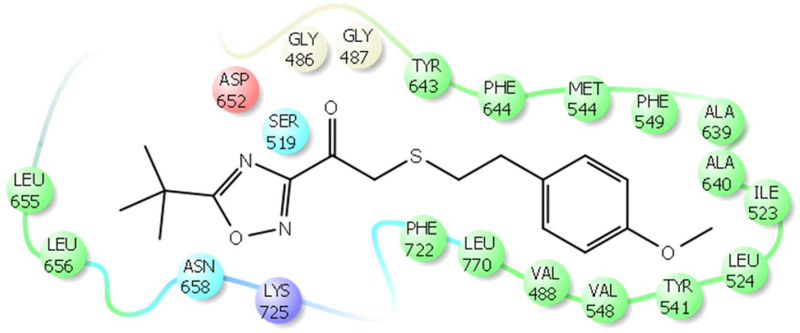
The implication of cPLA2, iPLA2, and sPLA2 in chronic inflammatory diseases makes them attractive targets for the development of potent and selective inhibitors [9, 48]. HD-XMS and molecular dynamics simulations were used to understand the binding and interactions of cPLA2 and iPLA2 inhibitors [49–56]. Fluoroketone inhibitors were identified as potent iPLA2 inhibitors in 2010 [57]. In our effort to improve their potency and selectivity, we have developed structureactivity relationships using computer-aided drug design [51, 52]. Using these models, we were able to design, synthesize, and test new fluoroketone compounds. These studies led to the development of new more potent and selective fluoroketone inhibitors. We were also able to identify a novel class of iPLA2 inhibitors that contain a heterocyclic ring instead of the fluoromethyl group [51]. Molecular dynamics simulations showed that the carbonyl group of the inhibitor forms hydrogen bonding with the oxyanion hole (Gly486/Gly487) and the heterocyclic ring with Asn658 (Figure 6). The hydrophobic tail of the inhibitor binds in a pocket where the sn2 fatty acid normally binds.
Figure 6.
Binding and interactions of an iPLA2 inhibitor containing a heterocyclic ring.
Eicosanoid biosynthesis
cPLA2, iPLA2, and sPLA2 have all been implicated in cellular eicosanoid biosynthesis. cPLA2 is activated by various stimuli that mobilize intracellular calcium and/or phosphorylation, such as Toll-like and purinergic receptors that initiate signaling during an inflammatory response. When activated, cPLA2 translocates to the perinuclear and endoplasmic reticulum membranes, releasing the free arachidonic acid from the phospholipid to which it is esterified. The release of arachidonic acid triggers a cascade of molecular events that involves cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) enzymes leading to inflammation [3]. iPLA2 is involved in homeostatic cellular functions, primarily membrane homeostasis and remodeling [58]. Since iPLA2 does not show strong specificity for the esterified fatty acid, it may contribute to the release of arachidonic acid. sPLA2 enzymes are induced in several if not all situations [59].
Eicosanoids are products of the oxidation of arachidonic acid by downstream enzymes including COX [60], LOX [61], and CYP [62]. Oxidation of arachidonic acid can also be caused via non-enzymatic free radical mechanisms [3]. Prostaglandins and thromboxanes are produced by the downstream enzymes of the COX pathway. In particular, functional coupling of thromboxane A synthase 1 (TBXAS1) and PGD synthase (PGDS) with COX1 has been shown to produce the eicosanoids thromboxane A2 (TXA2) and PGD2 during stimulation of macrophages [63]. In addition, PGE synthase 1 (mPGES1; also known as PTGES) and PGI2 synthase (PGIS) are coupled with COX2 to produce PGE2 and PGI2 [63, 64]. Leukotrienes are produced by the downstream enzymes of the LOX pathway. An example is the 5-LOX pathway that involves coupling of cPLA2 to 5-LOX, which are both calcium dependent, and formation of leukotrienes B4 (LTB4) and C4 (LTC4) by LTA4 hydrolase (LTA4H) and LTC4 synthase (LTC4S), respectively [65, 66]. Advances in the lipidomics field now allows routine monitoring of eicosanoid production in stimulated macrophages. This information can be used to develop computational models that predict the outcomes of drug candidates [67].
Supplementary Material
Acknowledgements
This work was supported by NIH grant GM20501–42 (E.A.D.). We would like to thank Carol Mu for helping with figures and editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dennis EA, Introduction to Thematic Review Series: Phospholipases: Central Role in Lipid Signaling and Disease, J. Lipid Res, 56 (2015) 1245–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Buczynski M, Dumlao D, Dennis E, Thematic review series: proteomics. an integrated omics analysis of eicosanoid biology, J. Lipid Res, 50 (2009) 1015–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dennis EA, Norris PC, Eicosanoid storm in infection and inflammation, Nat. Rev. Immunol, 15 (2015) 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Harizi H, Corcuff JB, Gualde N, Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology, Trends Mol. Med, 14 (2008) 461–469. [DOI] [PubMed] [Google Scholar]
- [5].Riaz A, Huang Y, Johansson S, G-Protein-Coupled Lysophosphatidic Acid Receptors and Their Regulation of AKT Signaling, Int. J. Mol. Sci, 17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moolenaar WH, Kranenburg O, Postma FR, Zondag GCM, Lysophosphatidic acid: G-protein signalling and cellular responses, Curr. Opin. Cell Biol, 9 (1997) 168–173. [DOI] [PubMed] [Google Scholar]
- [7].Min JH, Wilder C, Aoki J, Arai H, Inoue K, Paul L, Gelb MH, Platelet-activating factor acetylhydrolases: broad substrate specificity and lipoprotein binding does not modulate the catalytic properties of the plasma enzyme, Biochemistry, 40 (2001) 4539–4549. [DOI] [PubMed] [Google Scholar]
- [8].Venable ME, Zimmerman GA, McIntyre TM, Prescott SM, Platelet-activating factor: a phospholipid autacoid with diverse actions, J. Lipid Res, 34 (1993) 691–702. [PubMed] [Google Scholar]
- [9].Dennis E, Cao J, Hsu Y-H, Magrioti V, Kokotos G, Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention, Chem. Rev, 111 (2011) 6130–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mouchlis VD, Dennis EA, Membrane and inhibitor interactions of intracellular phospholipases A2, Adv. Biol. Regul, 61 (2016) 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vasquez AM, Mouchlis VD, Dennis EA, Review of four major distinct types of human phospholipase A2, Adv. Biol. Regul, 67 (2018) 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cao J, Burke J, Dennis E, Using hydrogen/deuterium exchange mass spectrometry to define the specific interactions of the phospholipase A2 superfamily with lipid substrates, inhibitors, and membranes, J. Biol. Chem, 288 (2013) 1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mouchlis VD, Chen Y, McCammon JA, Dennis EA, Membrane Allostery and Unique Hydrophobic Sites Promote Enzyme Substrate Specificity, J. Am. Chem. Soc, 140 (2018) 3285–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mouchlis VD, Bucher D, McCammon JA, Dennis EA, Membranes serve as allosteric activators of phospholipase A2, enabling it to extract, bind, and hydrolyze phospholipid substrates, PNAS, 112 (2015) E516–E525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bucher D, Hsu YH, Mouchlis VD, Dennis EA, McCammon JA, Insertion of the Ca2+-independent phospholipase A2 into a phospholipid bilayer via coarse-grained and atomistic molecular dynamics simulations, PLoS Comput. Biol, 9 (2013) e1003156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gijon MA, Leslie CC, Regulation of arachidonic acid release and cytosolic phospholipase A2 activation, J. Leukoc. Biol, 65 (1999) 330–336. [DOI] [PubMed] [Google Scholar]
- [17].Nalefski EA, Sultzman LA, Martin DM, Kriz RW, Towler PS, Knopf JL, Clark JD, Delineation of two functionally distinct domains of cytosolic phospholipase A2, a regulatory Ca2+-dependent lipidbinding domain and a Ca2+-independent catalytic domain, J. Biol. Chem, 269 (1994) 18239–18249. [PubMed] [Google Scholar]
- [18].Dessen A, Tang J, Schmidt H, Stahl M, Clark J, Seehra J, Somers W, Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism, Cell, 97 (1999) 349–360. [DOI] [PubMed] [Google Scholar]
- [19].Schievella AR, Regier MK, Smith WL, Lin LL, Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum, J. Biol. Chem, 270 (1995) 30749–30754. [DOI] [PubMed] [Google Scholar]
- [20].Glover S, de Carvalho MS, Bayburt T, Jonas M, Chi E, Leslie CC, Gelb MH, Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen, J. Biol. Chem, 270 (1995) 15359–15367. [DOI] [PubMed] [Google Scholar]
- [21].Sierra-Honigmann MR, Bradley JR, Pober JS, “Cytosolic” phospholipase A2 is in the nucleus of subconfluent endothelial cells but confined to the cytoplasm of confluent endothelial cells and redistributes to the nuclear envelope and cell junctions upon histamine stimulation, Lab. Invest, 74 (1996) 684–695. [PubMed] [Google Scholar]
- [22].Shirai Y, Balsinde J, Dennis EA, Localization and functional interrelationships among cytosolic group IV, secreted group V, and Ca2+-independent group VI phospholipase A2s in P388D 1 macrophages using GFP/RFP constructs, Biochim. Biophys. Acta, 1735 (2005) 119–129. [DOI] [PubMed] [Google Scholar]
- [23].Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ, cPLA2 is phosphorylated and activated by MAP kinase, Cell, 72 (1993) 269–278. [DOI] [PubMed] [Google Scholar]
- [24].Mosior M, Six DA, Dennis EA, Group IV cytosolic phospholipase A2 binds with high affinity and specificity to phosphatidylinositol 4,5-bisphosphate resulting in dramatic increases in activity, J. Biol. Chem, 273 (1998) 2184–2191. [DOI] [PubMed] [Google Scholar]
- [25].Leslie CC, Channon JY, Anionic phospholipids stimulate an arachidonoyl-hydrolyzing phospholipase A2 from macrophages and reduce the calcium requirement for activity, Biochim. Biophys. Acta, 1045 (1990) 261–270. [DOI] [PubMed] [Google Scholar]
- [26].Ramanadham S, Ali T, Ashley JW, Bone RN, Hancock WD, Lei X, Calcium-independent phospholipases A2 (iPLA2s) and their roles in biological processes and diseases, J. Lipid Res, 56 (2015) 1643–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hazen SL, Gross RW, Human myocardial cytosolic Ca2+-independent phospholipase A2 is modulated by ATP. Concordant ATP-induced alterations in enzyme kinetics and mechanism-based inhibition, Biochem. J, 280 ( Pt 3) (1991) 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Triggiani M, Granata F, Frattini A, Marone G, Activation of human inflammatory cells by secreted phospholipases A2, Biochim. Biophys. Acta, 1761 (2006) 1289–1300. [DOI] [PubMed] [Google Scholar]
- [29].Lambeau G, Gelb MH, Biochemistry and physiology of mammalian secreted phospholipases A2, Annu. Rev. Biochem, 77 (2008) 495–520. [DOI] [PubMed] [Google Scholar]
- [30].Balboa MA, Shirai Y, Gaietta G, Ellisman MH, Balsinde J, Dennis EA, Localization of group V phospholipase A2 in caveolin-enriched granules in activated P388D1 macrophage-like cells, J. Biol. Chem, 278 (2003) 48059–48065. [DOI] [PubMed] [Google Scholar]
- [31].Kim YJ, Kim KP, Han SK, Munoz NM, Zhu X, Sano H, Leff AR, Cho W, Group V phospholipase A2 induces leukotriene biosynthesis in human neutrophils through the activation of group IVA phospholipase A2, J. Biol. Chem, 277 (2002) 36479–36488. [DOI] [PubMed] [Google Scholar]
- [32].Rubio JM, Rodriguez JP, Gil-de-Gomez L, Guijas C, Balboa MA, Balsinde J, Group V secreted phospholipase A2 is upregulated by IL-4 in human macrophages and mediates phagocytosis via hydrolysis of ethanolamine phospholipids, J. Immunol, 194 (2015) 3327–3339. [DOI] [PubMed] [Google Scholar]
- [33].Rosenson RS, Hurt-Camejo E, Phospholipase A2 enzymes and the risk of atherosclerosis, Eur. Heart J, 33 (2012) 2899–2909. [DOI] [PubMed] [Google Scholar]
- [34].Sartipy P, Camejo G, Svensson L, Hurt-Camejo E, Phospholipase A2 modification of low density lipoproteins forms small high density particles with increased affinity for proteoglycans and glycosaminoglycans, J. Biol. Chem, 274 (1999) 25913–25920. [DOI] [PubMed] [Google Scholar]
- [35].Jenkins C, Mancuso D, Yan W, Sims H, Gibson B, Gross R, Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities, J. Biol. Chem, 279 (2004) 48968–48975. [DOI] [PubMed] [Google Scholar]
- [36].Larsson Forsell PK, Kennedy BP, Claesson HE, The human calcium-independent phospholipase A2 gene multiple enzymes with distinct properties from a single gene, Eur. J. Biochem, 262 (1999) 575–585. [DOI] [PubMed] [Google Scholar]
- [37].Larsson PK, Claesson HE, Kennedy BP, Multiple splice variants of the human calciumindependent phospholipase A2 and their effect on enzyme activity, J. Biol. Chem, 273 (1998) 207–214. [DOI] [PubMed] [Google Scholar]
- [38].Malley KR, Koroleva O, Miller I, Sanishvili R, Jenkins CM, Gross RW, Korolev S, The structure of iPLA2β reveals dimeric active sites and suggests mechanisms of regulation and localization, Nat. Commun., 9 (2018) 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Masuda S, Murakami M, Komiyama K, Ishihara M, Ishikawa Y, Ishii T, Kudo I, Various secretory phospholipase A2 enzymes are expressed in rheumatoid arthritis and augment prostaglandin production in cultured synovial cells, FEBS J, 272 (2005) 655–672. [DOI] [PubMed] [Google Scholar]
- [40].Carman G, Deems R, Dennis E, Lipid signaling enzymes and surface dilution kinetics, J. Biol. Chem, 270 (1995) 18711–18714. [DOI] [PubMed] [Google Scholar]
- [41].Roberts MF, Deems RA, Dennis EA, Dual role of interfacial phospholipid in phospholipase A2 catalysis, PNAS, 74 (1977) 1950–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ackermann EJ, Kempner ES, Dennis EA, Ca2+-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization, J. Biol. Chem, 269 (1994) 9227–9233. [PubMed] [Google Scholar]
- [43].Hsu Y-H, Burke J, Li S, Woods V, Dennis E, Localizing the membrane binding region of Group VIA Ca2+-independent phospholipase A2 using peptide amide hydrogen/deuterium exchange mass spectrometry, J. Biol. Chem, 284 (2009) 23652–23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Burke J, Hsu Y-H, Deems R, Li S, Woods V, Dennis E, A phospholipid substrate molecule residing in the membrane surface mediates opening of the lid region in group IVA cytosolic phospholipase A2, J. Biol. Chem, 283 (2008) 31227–31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Burke JE, Karbarz MJ, Deems RA, Li S, Woods VL Jr., Dennis EA, Interaction of group IA phospholipase A2 with metal ions and phospholipid vesicles probed with deuterium exchange mass spectrometry, Biochemistry, 47 (2008) 6451–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gil-de-Gomez L, Astudillo AM, Guijas C, Magrioti V, Kokotos G, Balboa MA, Balsinde J, Cytosolic group IVA and calcium-independent group VIA phospholipase A2s act on distinct phospholipid pools in zymosan-stimulated mouse peritoneal macrophages, J. Immunol, 192 (2014) 752–762. [DOI] [PubMed] [Google Scholar]
- [47].Murakami M, Masuda S, Ueda-Semmyo K, Yoda E, Kuwata H, Takanezawa Y, Aoki J, Arai H, Sumimoto H, Ishikawa Y, Ishii T, Nakatani Y, Kudo I, Group VIB Ca2+-independent phospholipase A2γ promotes cellular membrane hydrolysis and prostaglandin production in a manner distinct from other intracellular phospholipases A2, J. Biol. Chem, 280 (2005) 14028–14041. [DOI] [PubMed] [Google Scholar]
- [48].Mouchlis VD, Barbayianni E, Mavromoustakos TM, Kokotos G, The application of rational design on phospholipase A2 inhibitors, Curr. Med. Chem, 18 (2011) 2566–2582. [DOI] [PubMed] [Google Scholar]
- [49].Burke J, Babakhani A, Gorfe A, Kokotos G, Li S, Woods V, McCammon J, Dennis E, Location of inhibitors bound to group IVA phospholipase A2 determined by molecular dynamics and deuterium exchange mass spectrometry, J. Am. Chem. Soc, 131 (2009) 8083–8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hsu Y-H, Bucher D, Cao J, Li S, Yang S-W, Kokotos G, Woods V, McCammon J, Dennis E, Fluoroketone inhibition of Ca2+-independent phospholipase A2 through pinding pocket association defined by hydrogen/deuterium exchange and molecular dynamics, J. Am. Chem. Soc, 135 (2013) 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mouchlis VD, Limnios D, Kokotou MG, Barbayianni E, Kokotos G, McCammon JA, Dennis EA, Development of potent and selective inhibitors for group VIA calcium-independent phospholipase A2 guided by molecular dynamics and structure-activity relationships, J. Med. Chem, 59 (2016) 4403–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mouchlis VD, Morisseau C, Hammock BD, Li S, McCammon JA, Dennis EA, Computer-aided drug design guided by hydrogen/deuterium exchange mass spectrometry: A powerful combination for the development of potent and selective inhibitors of Group VIA calcium-independent phospholipase A2, Bioorg. Med. Chem, 24 (2016) 4801–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mouchlis VD, Michopoulou V, Constantinou-Kokotou V, Mavromoustakos T, Dennis EA, Kokotos G, Binding conformation of 2-oxoamide inhibitors to group IVA cytosolic phospholipase A2 determined by molecular docking combined with molecular dynamics, J. Chem. Inf. Model, 52 (2012) 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mouchlis VD, Magrioti V, Barbayianni E, Cermak N, Oslund RC, Mavromoustakos TM, Gelb MH, Kokotos G, Inhibition of secreted phospholipases A2 by 2-oxoamides based on alpha-amino acids: Synthesis, in vitro evaluation and molecular docking calculations, Bioorg. Med. Chem, 19 (2011) 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mouchlis VD, Mavromoustakos TM, Kokotos G, Molecular docking and 3D-QSAR CoMFA studies on indole inhibitors of GIIA secreted phospholipase A2, J. Chem. Inf. Model, 50 (2010) 1589–1601. [DOI] [PubMed] [Google Scholar]
- [56].Mouchlis VD, Mavromoustakos TM, Kokotos G, Design of new secreted phospholipase A2 inhibitors based on docking calculations by modifying the pharmacophore segments of the FPL67047XX inhibitor, J. Comput.-Aided Mol. Des, 24 (2010) 107–115. [DOI] [PubMed] [Google Scholar]
- [57].Kokotos G, Hsu Y-H, Burke J, Baskakis C, Kokotos C, Magrioti V, Dennis E, Potent and selective fluoroketone inhibitors of group VIA calcium-independent phospholipase A2, J. Med. Chem, 53 (2010) 3602–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Balsinde J, Balboa MA, Dennis EA, Antisense inhibition of group VI Ca2+-independent phospholipase A2 blocks phospholipid fatty acid remodeling in murine P388D1 macrophages, J. Biol. Chem, 272 (1997) 29317–29321. [DOI] [PubMed] [Google Scholar]
- [59].Fitzpatrick FA, Soberman R, Regulated formation of eicosanoids, J. Clin. Invest, 107 (2001) 1347–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Smith WL, DeWitt DL, Garavito RM, Cyclooxygenases: structural, cellular, and molecular biology, Annu. Rev. Biochem, 69 (2000) 145–182. [DOI] [PubMed] [Google Scholar]
- [61].Kuhn H, O’Donnell VB, Inflammation and immune regulation by 12/15-lipoxygenases, Prog. Lipid Res, 45 (2006) 334–356. [DOI] [PubMed] [Google Scholar]
- [62].Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL, Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha, Drug Metab. Dispos, 35 (2007) 1126–1134. [DOI] [PubMed] [Google Scholar]
- [63].Brock TG, McNish RW, Peters-Golden M, Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2, J. Biol. Chem, 274 (1999) 11660–11666. [DOI] [PubMed] [Google Scholar]
- [64].Naraba H, Murakami M, Matsumoto H, Shimbara S, Ueno A, Kudo I, Oh-ishi S, Segregated coupling of phospholipases A2, cyclooxygenases, and terminal prostanoid synthases in different phases of prostanoid biosynthesis in rat peritoneal macrophages, J. Immunol, 160 (1998) 2974–2982. [PubMed] [Google Scholar]
- [65].Mandal AK, Jones PB, Bair AM, Christmas P, Miller D, Yamin TT, Wisniewski D, Menke J, Evans JF, Hyman BT, Bacskai B, Chen M, Lee DM, Nikolic B, Soberman RJ, The nuclear membrane organization of leukotriene synthesis, Proc. Natl. Acad. Sci. USA, 105 (2008) 20434–20439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mandal AK, Skoch J, Bacskai BJ, Hyman BT, Christmas P, Miller D, Yamin TT, Xu S, Wisniewski D, Evans JF, Soberman RJ, The membrane organization of leukotriene synthesis, Proc. Natl. Acad. Sci. USA, 101 (2004) 6587–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kihara Y, Gupta S, Maurya MR, Armando A, Shah I, Quehenberger O, Glass CK, Dennis EA, Subramaniam S, Modeling of eicosanoid fluxes reveals functional coupling between cyclooxygenases and terminal synthases, Biophysical journal, 106 (2014) 966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.