Abstract
Background
Delayed motor development may occur in children with Down syndrome, cerebral palsy, general developmental delay or children born preterm. It limits the child's exploration of the environment and can hinder cognitive and social‐emotional development. Literature suggests that task‐specific training, such as locomotor treadmill training, facilitates motor development.
Objectives
To assess the effectiveness of treadmill interventions on locomotor development in children with delayed ambulation or in pre‐ambulatory children (or both), who are under six years of age and who are at risk for neuromotor delay.
Search methods
In May 2017, we searched CENTRAL, MEDLINE, Embase, six other databases and a number of trials registers. We also searched the reference lists of relevant studies and systematic reviews.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs that evaluated the effect of treadmill intervention in the target population.
Data collection and analysis
Four authors independently extracted the data. Outcome parameters were structured according to the International Classification of Functioning, Disability and Health model.
Main results
This is an update of a Cochrane review from 2011, which included five trials. This update includes seven studies on treadmill intervention in 175 children: 104 were allocated to treadmill groups, and 71 were controls. The studies varied in population (children with Down syndrome, cerebral palsy, developmental delay or at moderate risk for neuromotor delay); comparison type (treadmill versus no treadmill; treadmill with versus without orthoses; high‐ versus low‐intensity training); study duration, and assessed outcomes. Due to the diversity of the studies, only data from five studies were used in meta‐analyses for five outcomes: age of independent walking onset, overall gross motor function, gross motor function related to standing and walking, and gait velocity. GRADE assessments of quality of the evidence ranged from high to very low.
The effects of treadmill intervention on independent walking onset compared to no treadmill intervention was population dependent, but showed no overall effect (mean difference (MD) ‐2.08, 95% confidence intervals (CI) ‐5.38 to 1.22, 2 studies, 58 children; moderate‐quality evidence): 30 children with Down syndrome benefited from treadmill training (MD ‐4.00, 95% CI ‐6.96 to ‐1.04), but 28 children at moderate risk of developmental delay did not (MD ‐0.60, 95% CI ‐2.34 to 1.14). We found no evidence regarding walking onset in two studies that compared treadmill intervention with and without orthotics in 17 children (MD 0.10, 95% CI ‐5.96 to 6.16), and high‐ versus low‐intensity treadmill interventions in 30 children with Down syndrome (MD ‐2.13, 95% ‐4.96 to 0.70).
Treadmill intervention did not improve overall gross motor function (MD 0.88, 95% CI ‐4.54 to 6.30, 2 studies, 36 children; moderate‐quality evidence) or gross motor skills related to standing (MD 5.41, 95% CI ‐1.64 to 12.43, 2 studies, 32 children; low‐quality evidence), and had a negligible improvement in gross motor skills related to walking (MD 4.51, 95% CI 0.29 to 8.73, 2 studies, 32 children; low‐quality evidence). It led to improved walking skills in 20 ambulatory children with developmental delay (MD 7.60, 95% CI 0.88 to 14.32, 1 study) and favourable gross motor skills in 12 children with cerebral palsy (MD 8.00, 95% CI 3.18 to 12.82). A study which compared treadmill intervention with and without orthotics in 17 children with Down syndrome suggested that adding orthotics might hinder overall gross motor progress (MD ‐8.40, 95% CI ‐14.55 to ‐2.25).
Overall, treadmill intervention showed a very small increase in walking speed compared to no treadmill intervention (MD 0.23, 95% CI 0.08 to 0.37, 2 studies, 32 children; high‐quality evidence). Treadmill intervention increased walking speed in 20 ambulatory children with developmental delay (MD 0.25, 95% CI 0.08 to 0.42), but not in 12 children with cerebral palsy (MD 0.18, 95% CI ‐0.09 to 0.45).
Authors' conclusions
This update of the review from 2011 provides additional evidence of the efficacy of treadmill intervention for certain groups of children up to six years of age, but power to find significant results still remains limited. The current findings indicate that treadmill intervention may accelerate the development of independent walking in children with Down syndrome and may accelerate motor skill attainment in children with cerebral palsy and general developmental delay. Future research should first confirm these findings with larger and better designed studies, especially for infants with cerebral palsy and developmental delay. Once efficacy is established, research should examine the optimal dosage of treadmill intervention in these populations.
Keywords: Child, Preschool; Humans; Infant; Body Weight; Walking; Cerebral Palsy; Cerebral Palsy/complications; Cerebral Palsy/rehabilitation; Child Development; Child Development/physiology; Dependent Ambulation; Down Syndrome; Down Syndrome/complications; Down Syndrome/rehabilitation; Exercise Movement Techniques; Exercise Movement Techniques/instrumentation; Exercise Movement Techniques/methods; Locomotion; Locomotion/physiology; Motor Skills; Motor Skills/physiology; Motor Skills Disorders; Motor Skills Disorders/prevention & control; Motor Skills Disorders/rehabilitation; Randomized Controlled Trials as Topic
Plain language summary
Treadmill interventions in children under six years of age at risk of delay in motor skills
Review question
This is an update of the review published in 2011, which examined the effect of treadmill interventions on children below six years of age at risk of delay in motor skills.
Background
Helping children with motor delays to walk is often the focus of therapeutic intervention. Some literature suggests that treadmill training could provide an opportunity for children to walk with support for sufficient periods of time to enhance motor learning. This review examined existing evidence about treadmill interventions in young children with neuromotor impairment.
Search date
The evidence is current to May 2017.
Study characteristics
We included seven studies on treadmill intervention on 175 children with Down syndrome, cerebral palsy, general developmental delay or children with moderate risk for delay. Studies used home‐based or clinic‐based treadmill protocols, ranging in duration from six weeks to several months, or until the children walked independently.
Treadmill training versus no treadmill training was compared in five studies, including 117 children with one of the above mentioned risks. Treadmill training with or without orthotics (braces) was examined in 22 children with Down syndrome. High‐intensity versus low‐intensity treadmill training was compared in 36 children with Down syndrome.
Key results
Compared to no treadmill intervention, treadmill training helped 30 children with Down syndrome to walk earlier, but did not help 28 infants at moderate risk for developmental delay.
Overall, treadmill intervention did not improve overall gross motor function or gross motor skills related to standing. One study, which compared treadmill intervention with and without orthotics in 17 children with Down syndrome, suggested that adding orthotics might hinder gross motor progress. However, 20 ambulatory children with developmental delay, who engaged in treadmill training at preschool, improved walking skills. Twelve children with cerebral palsy, who received intensive treadmill training, showed faster achievement of motor milestones than children without treadmill training.
None of the studies reported problems or injuries from the treadmill training.
Overall, support for the intervention is limited. Confirmation from larger studies is necessary. Once efficacy of the intervention is established, optimal dosage research is needed.
Use of statistics
Statistical analysis was only performed on similar outcomes across studies.
Quality of the evidence
Standardized assessment for quality of evidence ranged from high to very low. Quality of evidence was determined by the number of children studied, completeness of the data, and random group assignment.
Summary of findings
Summary of findings for the main comparison. Summary of Finding Tables.
| Treadmill compared with no treadmill for children under six years of age at risk of neuromotor delay | |||
|
Patient or population: children under six years with cerebral palsy or Down syndrome or at risk of neuromotor delay Intervention: treadmill Comparison: no treadmill | |||
| Outcomes |
Absolute effects Mean difference (95% CI)* |
Number of participants (studies) | Quality of the evidence (GRADE) |
| Age of onset of independent walking (months) | MD ‐2.08 (‐5.38 to 1.22) | 58 (2 RCTs) |
⊕⊕⊕⊝
Moderate 1,2,3,4,5 |
| Age of onset of walking with assistance (days in study) | MD ‐38.54 (‐106.13 to 29.05) | 58 (2 RCTs) | ⊕⊝⊝⊝
Very low 2,3,5,6,7,8 |
| Gross motor function (GMFM) (%) | MD 0.88 (‐4.54 to 6.30) | 36 (2 RCTs) | ⊕⊕⊕⊝
Moderate 2,5,6,8,9 |
| Gross motor function related to standing (GMFM) ‐ Dimension D (%) | MD 5.41 (‐1.64 to 12.43) | 32 (1 RCT & 1 quasi‐RCT) | ⊕⊕⊝⊝ Low 2,5 |
| Gross motor function related to walking, running and jumping (GMFM) ‐ Dimension E (%) | MD 4.51 (0.29 to 8.73) | 32 (1 RCT & 1 quasi‐RCT) | ⊕⊕⊝⊝ Low 2,5,10 |
| Velocity (m/s) | MD 0.23 (0.08 to 0.37) | 32 (1 RCT & 1 quasi‐RCT) | ⊕⊕⊕⊕ High 2 |
|
*treadmill versus no treadmill CI: Confidence interval; MD: Mean difference; RCT: Randomised controlled trial | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1. Randomization took place through ID numbers provided by a computer program that a statistician assigned to participants after considering the three stratification factors of age, sex and birth weight. 2. Allocation concealment is unclear and there was no blinding of participants and personnel. 3. Substantial heterogeneity. 4. The estimate effect was different between meta‐analysed studies. 5. Small number of participants. 6. Randomization was used to allocate participants to the intervention or the control groups. 7. The included studies had different magnitudes of estimation effects. The wide range of the 95% CI was different between studies and was always large. 8. The 95% CI around the estimate of effect of all studies included in the meta‐analysis was very wide. 9. All included studies indicated no effect. 10. Heterogeneity was low.
Background
Description of the condition
Typical gross motor development
The World Health Organization (WHO) describes the gross motor development of infants as the attainment of six gross motor milestones. These are: (1) sitting without support; (2) crawling on hands and knees; (3) standing with assistance; (4) walking with assistance; (5) standing alone; and (6) walking alone. Approximately 86% of children with typical development attain all six milestones, though the sequence of attainment may vary. For instance, crawling on hands and knees is the most variable milestone; it is observed at different ages during the infant’s development and is sometimes even skipped. While infants are learning these temporary means of locomotion, they are gradually becoming able to support increasing amounts of weight while in a standing position until they eventually begin to walk at around 12 months of age. Attainment of this ultimate milestone has the widest age range, at between 8 and 18 months of age (WHO 2006), and may depend on various environmental factors, such as sensory or motor stimulation.
Developmental delay
The International Classification of Functioning, Disability and Health for Children and Youth (ICFCY; now integrated with ICF; WHO 2005) describes developmental delay as retardation in the achievement of developmental milestones. The most plausible cause of the motor delay is an alteration in the typical development and function of the central nervous system. Motor delays in locomotor abilities are defined by standards used in clinical paediatric settings. For example, the onset of independent walking should occur prior to 18 months of corrected age, so the presence of a motor delay would not be considered before this age. Developmental delay in infants is usually diagnosed via routine screening (Case‐Smith 1998), the use of norm‐referenced tests or criterion‐referenced tests, or both. Kinetic and kinematic analysis using force plates and video motion analysis may be used to further specify the delay; brain imaging techniques may be used to elucidate the aetiology of the delay. Although used for both research and clinical purposes, the tests are typically not good predictors for later outcomes and generally lack sensitivity in detecting small changes in motor development (Heineman 2008). In addition, in the paediatric population, the reliability of some of these tests may be affected by the child's emotional state, by daily fluctuations in performance or by the experience of the tester. Due to the continuous developmental changes occurring in the young brain, early diagnostic tests are relatively limited in predicting developmental outcomes (De Graaf‐Peters 2006), and the high level of variation in motor developmental trajectories in healthy children means that care has to be taken when interpreting results from motor assessments (Roze 2010).
Consequences of motor developmental delay
One of the major tasks in gross motor development is locomotion, the ability to move from one place to another (Bly 1995). The failure to attain walking, or the late attainment of walking, has consequences for the musculoskeletal system. The anatomy of the hip, for instance, needs weight bearing for proper bone growth and correct orientation of the femoral head (top part of the thigh bone), as well as for a correct alignment of the spine (Campbell 2006). As well as its importance for subsequent motor skill development, acquiring the ability to locomote is important for infants because of its impact on cognitive, social, and emotional skills. Researchers have demonstrated that, for infants with typical development, experience with locomotion is associated with the development of a broad array of cognitive skills, including the onset of wariness of heights; the concept of object permanence (objects hidden from sight still exist); a shift from self‐centred to landmark‐based spatial coding strategies; the ability to follow the pointing gestures and gaze of another person, and aspects of social referencing, social interactions, detour reaching, spatial memory, and language development (Bertenthal 1984; Bertenthal 1990; Campos 1989; Clearfield 2004; Clearfield 2011; Kermoian 1988; Walle 2014). This suggests that infants are better able to develop spatial cognition and learn about the world around them as they become able to locomote independently. Children who can walk independently show improved active exploration of their environment, as opposed to children who passively observe the environment when being held or carried through space. Anderson 2013 and Rosenbloom 1971 further suggest that the quality of movement may affect subsequent development. They propose that inefficient locomotion may hamper development by limiting the attention and energy that infants spend on exploration of the environment. Moreover, early locomotor experiences may have a larger impact on the developing brain than similar experiences at a later age, due to the brain's high plasticity during the first few postnatal years (De Graaf‐Peters 2006; Webb 2001). Earlier achievement of developmental milestones, in particular independent walking, have also been associated with better intellectual performance in adulthood (Murray 2007). In summary, independent locomotion at early age not only facilitates the infant's motor development, but also impacts other developmental domains and affects quality of life for the child and his/her family (Lepage 1998).
Population affected
There are various reasons for delays in typical motor development. Disorders affecting motor development during infancy include Down syndrome, cerebral palsy, spina bifida and a broad range of other neuromuscular disorders (Campbell 2006).
In addition, preterm birth, defined as childbirth occurring at less than 37 weeks or 259 days gestation (Beck 2010), is associated with a series of risk factors that make children vulnerable to delays in their developmental process (Formiga 2011). For instance, children who are born prematurely have higher rates of cerebral palsy, sensory deficits and learning disabilities compared with children born at term (Beck 2010).
The incidence of preterm birth rate is 6.2% in Europe, 6.4% in Australia and 11% to 12% in North America (excluding Mexico) (Beck 2010; Frey 2016), and the incidence of cerebral palsy is 1.5 to 2 per 1000 live births (Surveillance CP Europe). However, more epidemiological studies are needed to reliably assess the incidence of cerebral palsy, as its causes are not fully understood (Lie 2010). Approximately one in 800 children in the USA are born with Down syndrome, while the incidence in the UK is one in 1000 (Down's Syndrome Association).
Description of the intervention
According to some authors, high levels of motor activity are the key to motor development (Adolph 1998; Cunha 2016; Damiano 2006). In order to best influence neural plasticity (changes in the structure and function of the nervous system), it is important that any training is performed early in development and that it is specific to the task the child needs to master (Blackman 2002; Hodgson 1994; Morgan 2016). Intervention studies examining infants developing in a typical and atypical way show that task‐specific training may best facilitate the development of postural control (De Graaf‐Peters 2007; Hadders‐Algra 1996; Sveistrup 1997). This concept of task‐specificity can be considered an evidence‐based concept based on neuro‐scientific principles (Hodgson 1994).
Although the optimal window of intervention within the motor domain is not clear (Nelson 2000), it is reasonable to think of independent walking as a motor task that needs to be achieved by six years of age if long‐term negative effects are to be minimised. Locomotor treadmill interventions, with or without partial weight support, have been used to promote the acquisition of independent walking in children with Down Syndrome (Cherng 2007; Looper 2006) and cerebral palsy (Begnoche 2007; Mattern‐Baxter 2009a, Mattern‐Baxter 2013; Richards 1997).
Protocols of treadmill interventions described in the literature vary with regard to training speeds, support provided, manual assistance with stepping, and frequency and duration of the intervention. In studies of infants, the majority had training speeds ranging from 0.1 m/s to 0.22 m/s (Davis 1994); whereas older children were trained at higher speeds of 1.8 m/s (Begnoche 2007). The percentage of body weight used as partial weight support varied across studies and was provided either manually (the infant is supported under the arms, with the feet resting on the treadmill surface, bearing as much weight as comfortable) (Ulrich 2001), or with a commercially available pelvic harness or trunk harness, or both (Dodd 2007; Provost 2007). The support can also be provided by the children holding onto handle bars mounted on the treadmill (Mattern‐Baxter 2013). Only a few studies quantified the amount of body‐weight support provided during training (Mattern‐Baxter 2009a; Meyer‐Heim 2007; Provost 2007; Schindl 2000). Training duration ranged between two weeks (De Bode 2007; Phillips 2007; Provost 2007) and 57 weeks (Ulrich 2001), with some studies including breaks during the training programme (Cernak 2008; Day 2004; Prosser 2007). Frequency of the training sessions varied between studies, from two to six training sessions per week (Damiano 2009; Mattern‐Baxter 2009b). Manual facilitation of gait varied from no assistance with leg advancement to assistance from up to three physical therapists or assistants (Mattern‐Baxter 2009b).
In summary, the existing scientific literature exhibits wide variation in the parameters of treadmill interventions, indicating a need for systematic establishment of intervention protocols. Furthermore, research found in paediatric populations has used the treadmill for both prevention and rehabilitation purposes. Its use as a preventive tool mainly relates to infants who have no prior walking experience; whereas training in rehabilitation would be directed towards infants or children who, having walked independently, need to retrain that skill after injury/physical dysfunction or who need to improve their walking parameters, or both.
How the intervention might work
It is well established that brain plasticity exists and is particularly pronounced in the young nervous system (Kolb 2013; Stiles 2000; Stiles 2005). Experience‐dependent or activity‐dependent plasticity, or both, have been demonstrated in the human nervous system (Edgerton 1997; Eyre 2003). Similarly, plasticity has been demonstrated in postural control intervention studies (Hadders‐Algra 1996; Harbourne 2003). The capacity for the nervous system to reorganise is one of the fundamental mechanisms by which therapeutic interventions may be effective.
The treadmill is one form of intervention used in physical therapy to enhance the locomotor capabilities of patients (Eng 2007; Verschuren 2008); however, most of the scientific knowledge related to this topic comes from animal models (Sherrington 1910) or interventions in adult human populations (Sullivan 2007). In fact, the use of treadmill interventions for people with neurological disorders has its roots in animal studies (Barbeau 1987; Eidelberg 1980), where adult cats were able to regain stepping skills after a complete lesion of the spinal cord. The underlying mechanism by which this technique is effective is thought to reside in the regenerating capacity (plasticity) of the central nervous system when task‐specific motor practice is provided. Voluntary exercise and treadmill interventions specifically have been utilised in humans and in animal models to promote central nervous system (including spinal cord) plasticity and functional change (Cotman 2002a; Cotman 2002b; Jones 1999). The underlying neuronal mechanisms (e.g. neurons (nerve cells), neural circuits) responsible for such change are thought to be upregulation (activation) of trophic factors (molecules that sustain the health of a neuron), neurogenesis (formation of neurons), synaptogenesis (formation of new synapses/junctions between neurons), pre‐ and post‐synaptic modulation (changes in the strength of the signal from a sender (presynatic) to a receiver (post‐synaptic) neuron) and angiogenesis (formation of new blood vessels), among others. Such plasticity mechanisms are particularly active during early development. These neuroscience principles are the basis of the current motor learning theories (Kleim 2008; Newell 1991).
Plausible positive outcomes from treadmill interventions via central nervous system plasticity have been proposed in infants with Down syndrome and premature infants. Evidence from studies with children who have Down syndrome indicate statistically significant improvements in a variety of outcome measures, including obstacle negotiation and onset of walking. For this population, two main benefits from treadmill interventions implemented during early development have been described. First, it promotes the transition to continuous alternating steps in infants (including typically developing infants; Thelen 1986; Thelen 1991), which is an important precursor to walking (Ulrich 1992; Ulrich 1995; Ulrich 2001). Second, it leads to an acceleration of the onset of independent walking and an improvement of the quality of gait (Ulrich 2001).
Observational studies suggest that infants born prematurely follow similar developmental trajectories to their full‐term peers, although frequently with some delay (Angulo‐Barroso 2010; Luo 2009). The neonatal period of preterm infants is stressful, as the immaturity of vital physiological functions, such as respiration, blood pressure control and autoregulation of cerebral blood flow (the brain's ability to maintain constant blood flow despite variations in blood pressure), makes it difficult for the infant to adapt to the extrauterine (outside of the womb) situation. This results in vulnerability to delay in motor development and to developmental disorders (Formiga 2011; Goyen 2002; Pin 2010; Prins 2010), a vulnerability which, in part, is mediated by detectable lesions of the brain (Volpe 2009). The evidence available on the effect of treadmill interventions for this population is almost nonexistent. A case study of a premature infant showed an increase in the number of steps, of which almost 100% were exclusively alternating steps, during the post‐training phase (Bodkin 2003). However, encouraging as these results may seem, evidence of the effectiveness of treadmill interventions remains inconclusive. A recently published observational study investigated treadmill stepping behavior in healthy at‐term newborn infants. The authors suggested that the treadmill interventions that are used to promote the development of independent locomotion in infants at risk of delay could begin at birth (Teulier 2015).
Why it is important to do this review
The importance of children attaining independent walking has been well documented. A range of interventions to improve motor development in children is currently used in practice (Riethmuller 2009). However, there is a paucity of research on early interventions for children with physical disabilities, and most studies have methodological limitations (Hadders‐Algra 2014; Morgan 2016; Ziviani 2010).
Treadmill interventions are now being used in rehabilitation to prevent walking problems with children under six years of age. This intervention could have significant benefits in terms of preventing gross motor delays, promoting cognitive and social development, and promoting correct biomechanical function and efficiency during gait. It is important to evaluate the effectiveness of treadmill training as an early intervention method designed to improve motor function and to prevent neuromotor (related to or affecting the brain, nerves, muscles and movements) delays in children.
Diagnoses that may result in a delay in the acquisition of walking (Down syndrome, cerebral palsy, among others) have different intrinsic characteristics. Because of this, a differentiation of interventions or parameters specific to the diagnosis may be required, indicating the need to perform subgroup analyses.
There are several existing systematic reviews on treadmill interventions in paediatric populations (Damiano 2009; Mattern‐Baxter 2009b; Molina‐Rueda 2010; Morgan 2016; Mutlu 2009; Willoughby 2009). However, these reviews evaluated published reports from 1980 to 2008 on treadmill training for children aged up to 21 years. In addition to their reliance on published reports in English, their search strategy did not include terms of specific diagnoses that are known to cause gross motor delay in childhood, and some were limited to children with cerebral palsy (Mattern‐Baxter 2009b; Molina‐Rueda 2010; Mutlu 2009; Willoughby 2009).
To date, there is no systematic review of treadmill intervention that examines its effectiveness in children before or during the acquisition of independent walking, and that encompasses both prevention and rehabilitation. A systematic review of the literature is needed in order to define the extent of the preventive and rehabilitative effectiveness of treadmill training, and to define optimal training parameters for this intervention.
This review aims to fill this gap and to review all relevant studies, irrespective of publication status or language.
Objectives
To assess the effectiveness of treadmill interventions on locomotor development in children with delayed ambulation or in pre‐ambulatory children (or both), who are under six years of age and who are at risk of neuromotor delay.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTS (where participants are allocated in a way that is not strictly random, such as by alternation or date of birth).
Types of participants
Children up to six years of age with delays in gait development or the attainment of independent walking (children who cannot walk independently by the age of 18 months), or who are at risk of neuromotor delay (primarily with nonprogressive neurological disorder), however diagnosed.
We excluded studies that included children both older and younger than six years of age, and children diagnosed with a condition for which physical activity is contraindicated (for example, infants with genetic degenerative diseases, such as neuromuscular dystrophy, and those with diagnoses that preclude independent walking).
Types of interventions
Treadmill intervention of any type, frequency or intensity, aimed at (1) improving gait parameters such as walking speed, endurance, quality of step or (2) facilitating onset of independent walking or walking with assistance.
Comparison groups received no treatment or another treatment. Control group treatments could include physical therapy or another intervention designed to improve gait. We included studies with treadmill intervention as an adjunctive treatment. We also reported on studies comparing different types of treadmill interventions, for example, low versus high intensity.
Types of outcome measures
We accepted five types of outcome measures: standardised measures, questionnaires, self‐report data, data from motion analysis systems and coded‐video observations. We assessed the following outcomes, which are based on the ICFCY (now merged into ICF) (WHO 2005).
Primary outcomes
-
Body structure and functions (neuro‐musculoskeletal and movement‐related functions ‐ gait pattern functions):
Step frequency (number of alternating treadmill steps per minute, cadence during independent walking); and
Step quality (foot doing toe versus flat contact during treadmill stepping).
-
Activity and participation functions:
Age of onset of independent walking;
Age of onset of walking with assistance;
Gross motor function; and
Adverse events (such as falls and injuries due to falls).
Examples for measuring gross motor function are: Gross Motor Function Measure (GMFM; Russell 2002), Bayley Scales of Infant and Toddlers Development (BSID; Bayley 1993); Peabody Developmental Motor Scales ‐ 2 (PDMS‐2; Van Hartingsveldt 2005), among others.
Secondary outcomes
-
Body structure and functions (neuro‐musculoskeletal and movement‐related functions ‐ gait pattern functions):
Inter‐ and intra‐limb co‐ordination; and
Other gait parameters, for example, speed, step width, etc.
-
Activity and participation functions:
Infant or child quality of life.
Examples of measuring secondary outcomes are distance in meters/second, and Pediatric Quality of Life Inventory (Varni 2003).
Primary outcomes regarding 'body structure and functions' are measured during the whole length of the study (different timings depending on each study), whereas those under 'activity and participation functions' are measured at the end of the study (gross motor function), which coincides with 'age of onset of independent walking' or 'age of onset of walking with assistance'.
We excluded studies on the basis of outcome measures that were not the focus of our review.
Search methods for identification of studies
Electronic searches
We ran the searches for the original review in March 2011 and re‐ran them for this update in July 2014, May 2016, and May 2017 (see Appendix 1). We searched the following list of databases using the search strategies in Appendix 2. No date or language restrictions were applied.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, current issue) and which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 10 May 2017).
MEDLINE Ovid (1946 to April Week 4, 2017).
MEDLINE In‐Process and Other Non‐Indexed Citations Ovid (searched 5 May 2017).
MEDLINE Epub Ahead of Print Ovid (searched 5 May 2017).
Embase Ovid (1980 to 2017 Week 19).
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 10 May 2017).
PsycINFO Ovid (1967 to May Week 1 2017).
Science Citation Index Web of Science (SCI; 1970 to 9 May 2017).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1990 to 9 May 2017).
PEDro (www.pedro.org.au; searched 10 May 2017).
LILACS (Latin American and Caribbean Health Sciences Literature; lilacs.bvsalud.org/en; searched 10 May 2017).
ClinicalTrials.gov (clinicaltrials.gov; searched 10 May 2017).
WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/search/en; searched 10 May 2017).
CenterWatch (www.centerwatch.com; searched 10 May 2017).
metaRegister of Controlled Trials (mRCT; all years up to 9 July 2014). Not available after 2014 as service is under review.
Searching other resources
We checked whether studies incorporated in previous systematic reviews and other reviews of the subject fulfilled our inclusion criteria (see Criteria for considering studies for this review).
We checked whether bibliographies of reports identified through the search strategy contained other potential studies for inclusion.
Data collection and analysis
Selection of studies
In the original review (Valentin‐Gudiol 2011r), we divided the titles and abstracts yielded by the search strategy into two blocks. Two authors (KMB and CB) independently screened the first block of references, while two other authors (RA and MV) did the same with the second block, using the inclusion criteria described above (Criteria for considering studies for this review). RA functioned as the arbiter for KMB and CB, while KMB fulfilled this role for RA and MV, in case of discrepancies. The selected titles were read in full to determine their relevance for the review. We resolved disagreement about eligibility through discussion with the whole team.
For this update, CB, KMB and MV independently screened all references. MHA, and RA participated to resolve discrepancies.
We recorded our decisions in a PRISMA diagram (Moher 2009).
Data extraction and management
In the original review (Valentin‐Gudiol 2011r), four authors (MV, RA, CB and MG) independently extracted data from each trial using a data extraction form to collect information about the population, intervention, randomisation methods, blinding, sample size, outcome measures, follow‐up duration, attrition and handling of missing data, and methods of analysis. Disagreements were dealt by MHA and KMB.
For this update, CB, KMB, MG and MV extracted data from included studies.
Assessment of risk of bias in included studies
In this update, two review authors (CB and MV) independently assessed the risk of bias of each included study using Cochrane’s tool for assessing risk of bias (Higgins 2011a). Both review authors independently assessed each included study as low risk of bias, high risk of bias or unclear risk of bias in relation to the following seven domains: sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data (including data on attrition and exclusions); selective outcome reporting, and other risks of bias. We entered these judgements into a 'Risk of bias' table in Review Manager (RevMan), version 5 (Review Manager 2014), the latest version of Cochrane's meta‐analysis software, with a brief rationale for the judgements. Details on the seven possible sources of bias are described below.
Sequence generation: we described the method used to generate the allocation sequence in sufficient detail to assess whether or not the sequence was adequately generated and whether it should have produced comparable groups.
Allocation concealment: we described the method used to conceal allocation sequence in sufficient detail to assess whether intervention schedules could have been foreseen before, or during, recruitment. We judged whether or not there was adequate allocation concealment.
Blinding of participants and personnel: it is not possible to blind either those who deliver the therapy (treadmill training) or those infants who receive it, due to the nature of the intervention. Our assessment of risk of bias took into account the likely bias attributable to the inability to blind participants or personnel in such interventions.
Blinding of outcome assessment: we described any measures used to blind outcome assessors to assess whether knowledge of the allocated intervention was adequately prevented.
Incomplete outcome data: we extracted and reported data on attrition and exclusions, as well as the numbers involved (compared with the total randomised), reasons for attrition or exclusion (where reported or obtained from authors) and any re‐inclusions in analyses performed by review authors. For each included study, we assessed whether incomplete outcome data were adequately addressed.
Selective reporting: we attempted to assess the possibility of selective outcome reporting by investigators. We evaluated if each study was free from selective outcome reporting by considering whether or not all collected data were reported.
Other risks of bias: we assessed the extent to which each study was apparently free of other problems that could put it at high risk of bias, by describing important concerns not addressed in the other domains of Cochrane's 'Risk of bias' tool. We assessed other threats to validity as low risk of bias if the study appeared to be free of other sources of bias. For example, in general terms, when the treadmill intervention is home‐based and performed by parents, it is difficult to control aspects of how each parent motivates the child to keep walking on the treadmill. If there were important differences in this aspect, the overall performance of the children could have been different.
See also Differences between protocol and review.
Measures of treatment effect
We used Review Manager 2014 to calculate the adjustments to measures of treatment effects.
Continuous data
We analysed continuous data if means and standard deviations (SD) had been reported, could be obtained from primary investigators or could be calculated from the available data (Deeks 1997a; Deeks 1997b). As continuous outcomes were measured identically across studies, we calculated the mean difference (MD) with 95% confidence intervals (CI).
Dichotomous data
As the studies did not use identical dichotomous data, we were unable to calculate summary statistics on these data.
Please refer to Valentin‐Gudiol 2011p and Appendix 3 for methods archived for use in future updates of this review.
Unit of analysis issues
The only unit‐of‐analysis issue relevant for the analyses in this review pertained to cross‐over trials. We combined the results from the one cross‐over trial with those of the parallel‐group trials, including only the first phase before the point of cross‐over in the analyses (Criteria for considering studies for this review).
Please see Valentin‐Gudiol 2011p and Appendix 3 for additional methods archived for use in future updates of this review.
Dealing with missing data
We assessed missing data and dropouts in the included studies. We investigated and reported the reasons, numbers and characteristics of dropouts (see Characteristics of included studies tables).
We analysed missing continuous data either on an endpoint basis, including only participants with a final assessment, or using last observation carried forward to the final assessment, if these data were reported by trial authors. When the values for SD were not detailed in the publication, we contacted the authors, or else, if possible, calculated the values using the available data. We contacted the author of one study (through a co‐author) and successfully obtained the unpublished data (Chen 2008). For further details, see Characteristics of included studies tables.
Regarding dichotomous data, it was not necessary to contact any author. Please refer to Valentin‐Gudiol 2011p and Appendix 3 for methods to manage missing dichotomous data archived for use in future updates of this review.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important participant factors among trials (for example, age, diagnosis), and methodological heterogeneity by comparing trial factors (for example, randomisation concealment, blinding of outcome assessment, form of treadmill training, losses to follow‐up). Please refer to Valentin‐Gudiol 2011p or Appendix 3, or both, for information on additional methods archived for use in future updates of this review.
Assessment of reporting biases
We could not assess reporting biases due to the low number of studies. Please see Appendix 3, and our protocol (Valentin‐Gudiol 2011p), for methods to assess reporting bias archived for use in future updates of this review.
Data synthesis
We synthesised the data using Review Manager 2014, the latest version of Cochrane's meta‐analysis software. We performed the meta‐analysis using the random‐effects model programmed in Review Manager 2014 (Deeks 2011), and the inverse variance weighting method, and we reported statistical heterogeneity. Please refer to Valentin‐Gudiol 2011p, Differences between protocol and review and Appendix 3 for methods archived for use in future updates of this review.
Summary of findings
We exported data from Review Manager 2014 to GRADEprofiler (GRADEproGDT 2015), and produced a 'Summary of findings' table for the main comparison: treadmill compared with no treadmill for children under six years of age at risk of neuromotor delay. We included the following outcomes in the table: age of onset of independent walking (primary outcome), age of onset of walking with assistance (primary outcome), gross motor function (primary outcome) and velocity (secondary outcome).
We used the GRADE approach to assess the quality of evidence for each outcome pooled in the meta‐analysis (Schünemann 2011a; Schünemann 2011b). CB, KMB and MG independently evaluated the quality of evidence for each outcome according to the following criteria: risk of bias, consistency, directness, precision and publication bias, and assigned ratings of high‐quality, moderate‐quality, low‐quality or very low‐quality evidence.
Subgroup analysis and investigation of heterogeneity
Due to the data, and the variables given in the included studies, we were unable to perform all the subgroup analyses we had planned. We did, where possible, conduct subgroup analysis by diagnosis: cerebral palsy, Down syndrome, and risk of developmental delay. Please see Appendix 3 and our protocol (Valentin‐Gudiol 2011p) for additional subgroup analyses archived for use in future updates of this review.
Sensitivity analysis
Due to having such a small number of studies, and conducting only two meta‐analyses, we considered sensitivity analysis inappropriate. Please see Appendix 3 for sensitivity analyses archived for use in future updates of this review, and also refer to the protocol of the review (Valentin‐Gudiol 2011p).
Results
Description of studies
Results of the search
We identified a total of 3044 records for the original review and removed 892 duplicates. We examined the titles and abstracts of the remaining 2152 records, and excluded 2093 irrelevant records. When we examined the full texts of the remaining 59 reports, we excluded 50 that did not meet the inclusion criteria, and included five studies (from nine reports) in the review (see Valentin‐Gudiol 2011r).
For this update, we retrieved a total of 3017 records and removed 862 duplicates. We excluded 2130 irrelevant records on the basis of their title and abstract and retrieved the full text of the remaining 25 records for further examination. Of these, we excluded 21 full‐text reports that did not meet the inclusion criteria (see Criteria for considering studies for this review); see Excluded studies. We identified two new included studies and an additional report of a previously included study. We also found one ongoing study for which no data was available at the time of this review (NCT02424526). Figure 1 shows the flow of studies through the selection process.
1.

Study flow diagram.
Most of the unpublished data from one of the included studies in the original review (Chen 2008) has since been published, therefore the Chen 2008 data is presented in this updated review as an additional report of a new included study (Angulo‐Barroso 2013).
Included studies
In this review update, we included three new trials: two RCTs (Angulo‐Barroso 2013; Lowe 2015) and one quasi‐RCT (Mattern‐Baxter 2013). Angulo‐Barroso 2013 contained the data of Chen 2008 (an included study in the original review (Valentin‐Gudiol 2011r)). This review now includes seven published studies (12 reports) of treadmill interventions in children under six years of age at risk for neurodevelopmental delay (Angulo‐Barroso 2013;Cherng 2007; Looper 2010; Lowe 2015; Mattern‐Baxter 2013; Ulrich 2001; Ulrich 2008). Please refer to Table 2 for a summary of interventions and outcome measures.
1. Summary of interventions and outcome measures.
| Outcome or Subgroup | Disorder | Studies |
Comparsion groups (G1 versus G2) |
Sample size (G1/G2) |
Result of comparison | |
| Nº | ID | |||||
| 1.1. Step frequency (16 months) | Risk | 1 | Angulo‐Barroso 2013 | NTM versus TM | 15/13 | G1 = G2 |
| 1.2. Step quality (11 months) | Risk | 1 | Angulo‐Barroso 2013 | NTM versus TM | 15/13 | G1 < G2 |
| 1.3. Step quality (16 months) | Risk | 1 | Angulo‐Barroso 2013 | NTM versus TM | 15/13 | G1 < G2 |
| 1.4. Age of onset of independent walking [months] | DS and Risk | 2 | Angulo‐Barroso 2013; Ulrich 2001 | NTM versus TM | 30/28 | G1 < G2 |
| 1.5. Age of onset of walking with assistance [days in study] | DS and Risk | 2 | Angulo‐Barroso 2013; Ulrich 2001 | NTM versus TM | 30/28 | G1 = G2 |
| 1.6. Gross motor function measure (GMFM) [%] | CP and Risk | 2 | Cherng 2007; Chen 2008 | NTM versus TM | 19/17 | G1 = G2 |
| 1.7. GMFM related to standing, Dimension D [%] | Risk and CP | 2 | Lowe 2015; Mattern‐Baxter 2013 | NTM versus TM | 14/18 | G1 = G2 |
| 1.8. GMFM related to walking, running and jumping, Dimension E [%] | Risk and CP | 2 | Lowe 2015; Mattern‐Baxter 2013 | NTM versus TM | 14/18 | G1 = G2 |
| 1.9. Peabody Developmental Motor Scales ‐ 2 [raw scores] | CP | 1 | Mattern‐Baxter 2013 | NTM versus TM | 6/6 | G1 < G2 |
| 1.10. Pediatric Evaluation of Disability Inventory ‐ Mobility Scale scores | CP | 1 | Mattern‐Baxter 2013 | NTM versus TM | 6/6 | G1 < G2 |
| 1.11. Other gait parameters: velocity [m/s] | CP and Risk | 1 | Lowe 2015; Mattern‐Baxter 2013 | NTM versus TM | 4/4 | G1 < G2 |
| 1.12. Other gait parameters: velocity (follow‐up when walking independent) | Risk | 1 | Angulo‐Barroso 2013 | NTM versus TM | 15/13 | G1 = G2 |
| 1.13. Other gait parameters: step length [cm] | CP | 1 | Cherng 2007 | NTM versus TM | 4/4 | G1 = G2 |
| 1.14. Other gait parameters: step length (follow‐up when walking independently) | Risk | 1 | Angulo‐Barroso 2013 | NTM versus TM | 15/13 | G1 = G2 |
| 1.15. Other gait parameters: gait double‐limb support [%] | CP | 1 | Cherng 2007 | NTM versus TM | 4/4 | G1 = G2 |
| 1.16. Other gait parameters: gait double‐limb support (follow‐up when walking independently) [%] | Risk | 1 | Angulo‐Barroso 2013 | NTM versus TM | 15/13 | G1 = G2 |
| 2.1. Walking independent (1‐month follow‐up) [months] | DS | 1 | Looper 2010 | TM&O versus TM | 10/7 | G1 = G2 |
| 2.2. GMFM (1‐month follow‐up) [%] | DS | 1 | Looper 2010 | TM&O versus TM | 10/7 | G1 > G2 |
| 3.1. Step frequency [steps/min] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 16/14 | G1 > G2 |
| 3.2. Age of onset of independent walking [months] | DS | 1 | Wu 2007 | HI TM versus LG TM | 16/14 | G1 = G2 |
| 3.3. Age of onset of walking with assistance [months] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 16/14 | G1 = G2 |
| 3.4. Other gait parameters: velocity (follow‐up visit 1) [m/s] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.5. Other gait parameters: velocity (follow‐up visit 2) [m/s] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 13/12 | G1 < G2 |
| 3.6. Other gait parameters: velocity (follow‐up visit 3) [m/s] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.7. Other gait parameters: velocity (follow‐up visit 4) [m/s] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.8. Other gait parameters: gait double‐limb support (follow‐up visit 1) [%] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.9. Other gait parameters: gait double‐limb support (follow‐up visit 2) [%] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 13/12 | G1 > G2 |
| 3.10. Other gait parameters: gait double‐limb support (follow‐up visit 3) [%] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.11. Other gait parameters: gait double‐limb support (follow‐up visit 4) [%] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.12. Other gait parameters: gait ankle plantar flexion (follow‐up visit 1) [%] | DS | 1 | Wu 2010 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.13. Other gait parameters: gait ankle plantar flexion (follow‐up visit 2) [%] | DS | 1 | Wu 2010 | HI TM versus LG TM | 13/12 | G1 > G2 |
| 3.14. Other gait parameters: gait ankle plantar flexion (follow‐up visit 3) [%] | DS | 1 | Wu 2010 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.15. Other gait parameters: gait ankle plantar flexion (follow‐up visit 4) [%] | DS | 1 | Wu 2010 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.16. Other gait parameters: step length (follow‐up) [cm] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.17. Other gait parameters: step width (follow‐up) [cm] | DS | 1 | Ulrich 2008 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.18. Other gait parameters: gait ankle dorsiflexion (follow‐up) [%] | DS | 1 | Wu 2010 | HI TM versus LG TM | 13/12 | G1 = G2 |
| 3.19. Other gait parameters: toe‐off (follow‐up) [%] | DS | 1 | Wu 2010 | HI TM versus LG TM | 13/12 | G1 = G2 |
CP = Cerebral palsy; DS = Down syndrome; G1 = Group 1; G2 = Group 2; HI TM = high‐intensity treadmill; LG TM = low‐intensity treadmill; Na = total participants, number of analysed participants; Nº = number of studies included; NTM = no treadmill; TM = treadmill; TM&O = treadmill and orthoses; Risk = risk of developmental delay.
Location
All but one study were conducted in the USA; Cherng 2007 was conducted in Taiwan.
Design
One study had a cross‐over design (Cherng 2007), two were quasi‐RCTs (Mattern‐Baxter 2013; Looper 2010) and the other four were reported as parallel group RCTs, two of them without additional information about the randomisation process (Ulrich 2001; Ulrich 2008) and two with detailed information of how the randomisation process took place (Angulo‐Barroso 2013; Lowe 2015).
Sample sizes
The seven studies included 175 children. Sample sizes ranged from 8 (Cherng 2007) to 41 children (Angulo‐Barroso 2013), with the remaining five studies comprising 12, 22, 24, 32, and 36 children (Looper 2010; Lowe 2015; Mattern‐Baxter 2013; Ulrich 2001; Ulrich 2008, respectively).
According to diagnosis, there were 41 infants at moderate risk for developmental delay (in Angulo‐Barroso 2013); 20 with cerebral palsy (8 in Cherng 2007 and 12 in Mattern‐Baxter 2013), 24 with general developmental delay (Lowe 2015) and 90 children with Down syndrome (22 in Looper 2010; 32 in Ulrich 2001; 36 in Ulrich 2008).
Participants
Further details as regards participant characteristics can be found in the Characteristics of included studies tables.
Infants at moderate risk for developmental delay
Angulo‐Barroso 2013 examined the effects of treadmill intervention on 41 preterm infants at moderate risk for neuromotor delays. The children ranged from a corrected age of 6.2 months to 12.7 months at study onset. As an inclusion criterion, infants entered into the study when they were able to take 10 steps on the treadmill in one minute. No information on ethnicity was reported.
Cerebral palsy
Two studies examined the effects of treadmill training on 20 children with cerebral palsy (Cherng 2007; Mattern‐Baxter 2013).
Cherng 2007 focused on eight children diagnosed with cerebral palsy. Participants were between 42 and 75.6 months old at study onset and were diagnosed with spastic diplegic cerebral palsy. Two of the children were ambulatory without assistive devices; the remaining six children ambulated with assistive devices at study onset. No information on ethnicity was reported.
Mattern‐Baxter 2013 examined the effects of home‐based treadmill training on gross motor function in children with cerebral palsy. Participants were between 13.5 and 30.5 months of age at study onset. Four children were classified as level I of the Gross Motor Functional Classifications System (GMFCS) (Palisano 1997) and eight were classified as level II. Five of the children had hypotonia; the remaining seven had spasticity. Two children were African American, two were Asian, two were Hispanic and six were white. Eight children were nonambulatory at study onset, and four were able to walk with assistive devices.
Down syndrome
Three studies examined the effects of treadmill intervention on 90, non‐ambulatory children with Down syndrome (Looper 2010; Ulrich 2001; Ulrich 2008).
Ulrich 2001 included 32 children with Down syndrome who had a mean age of 10.1 months (standard deviation (SD) 1.94) at study onset. Participants were admitted into the study when they were able to sit for 30 seconds. Two infants were of mixed race, with the remaining infants being white. Nine of the 32 infants (28.1%) had received surgery for congenital heart disease.
Ulrich 2008 examined a different group of children with Down syndrome (36 children); ages ranged from 9.6 to 10.4 months. Two of the children were African American, two were bi‐racial and the remaining children were white. Fourteen of the 36 children (38.9%) had congenital heart defects. An eligibility criterion for commencing treadmill intervention was the ability to take a minimum of six steps in one minute on a moving treadmill while supported under the arms by a parent.
Looper 2010 examined 22 children with Down syndrome; ages ranged from 18.9 to 21.1 months at study onset. There was no information on ethnicity or medical conditions. Children entered the study when they were able to pull to stand but unable to cruise.
General developmental delay
Lowe 2015 examined 24 children with developmental delay. Children were admitted to the study if they showed developmental delay indicated by a Z score of ‐1.5 or more on a standardized developmental test. Of the 21 children who completed the trial, ages ranged from 26 to 51 months at study onset. Fifteen children were white, three were African American and three were classified as 'other', with 17 males and 4 females. All children were ambulatory without assistive device.
Intervention and comparisons
Treadmill intervention versus no treadmill intervention
This comparison was examined in a total of 117 children across three diagnoses: children at moderate risk for neuromotor delays (Angulo‐Barroso 2013), children with cerebral palsy (Cherng 2007; Mattern‐Baxter 2013), children with general developmental delay (Lowe 2015) and children with Down syndrome (Ulrich 2001).
Angulo‐Barroso 2013 randomised 41 moderate risk infants into two groups, however only 28 infants completed the study (13 infants in the control group; 15 infants in the treadmill intervention group; see Characteristics of included studies tables). Infants assigned to the control group did not receive treadmill training but continued with the standard physical therapy intervention prescribed by the local Early Intervention programme, as did infants in the experimental group. Infants in the treadmill intervention group engaged in home‐based intervention for up to eight minutes a day, five days a week. The belt speed used in the intervention was 0.2 m/s. These training parameters were similar to those applied in the study of Ulrich 2001. Treadmill intervention was discontinued once the infant was observed walking three independent steps over ground.
Cherng 2007 randomised eight children with cerebral palsy into two groups, each of whom received three 12‐week blocks of intervention with varying intervention schedules. Intervention A in the cross‐over design was a regular therapeutic intervention without use of a treadmill, while intervention B consisted of treadmill intervention in addition to a traditional therapeutic intervention. Interventions were carried out in 12‐week blocks for two to three sessions per week, and for 30 minutes per session, with one group receiving intervention schedule AAB and the other group receiving intervention schedule ABA. Assessments were conducted at study entry and subsequently in 12‐week increments.
Lowe 2015 quasi‐randomised 24 children with general developmental delay into two groups: a control group (no treadmill training) and an intervention group (treadmill training). Both groups continued their regularly scheduled physical therapy. The treadmill group received up to 15 minutes of treadmill training up to three times per week for six weeks in addition to their regular physical therapy, whereas the children in the control group received physical therapy only. The intervention took place at the children's preschool. The initial treadmill speed was based on the child’s overground walking speed and ranged between 0.54 to 0.80 m/s with a grade (incline) of zero to one. Treadmill speed was increased based on the child’s tolerance to 0.80 to 1.07 m/s and a grade of one to three. The children were placed in a harness, were not holding on and were encouraged to swing their arms. Weight support from the harness was provided, as necessary, to maintain optimal gait without deviations and was decreased progressively over time to no weight support. The decision to increase the speed and decrease weight support was based on the child’s ability to walk without increased gait deviations or anxiety.
Mattern‐Baxter 2013 quasi‐randomised 12 children with cerebral palsy into two groups: a control group (no treadmill training) and an intervention group (treadmill training). Both groups continued their regularly scheduled physical therapy. Twelve children completed the study with six children in each group (see Characteristics of included studies tables). The children in the intervention group were encouraged to walk as many minutes as possible, from a minimum of five minutes to a maximum of 20 minutes. Training sessions took place two times a day (six days per week) for a period of six weeks. The intervention was carried out by the children's parents with weekly supervision by a physical therapist. All children used the bilateral side bars mounted to the treadmill for holding on. The treadmill was stopped if a child stopped walking for more than five seconds. The treadmill speed was increased for each child, as tolerated, and was determined at the weekly visits and maintained throughout that week.
Ulrich 2001 randomised 32 children with Down syndrome to a treadmill training intervention (16 children) or a control group (16 children). The intervention group received treadmill intervention five days per week, at a speed of 0.2 m/s for up to eight minutes, as tolerated. The intervention was carried out in the children’s homes by the children’s families on portable treadmills. Children were held under the arms over the moving treadmill by a parent. The control group received physical therapy intervention without treadmill intervention at least every other week.
Treadmill intervention with the use of orthotics versus treadmill intervention without orthotic use
Looper 2010 allocated 22 children with Down syndrome to a treadmill intervention, with and without use of orthotics. Both the intervention and control groups engaged in home‐based treadmill intervention at a speed of 0.2 m/s, for up to eight minutes a day, five days a week. This was carried out by the parents and the children were held over the moving treadmill. Treadmill intervention was discontinued when the children could take three independent steps. The difference in the intervention group was the use of orthotics. The children were measured for these on the first visit and received them on their second, thereafter wearing them for eight hours a day, five days a week, for the study duration. The control group received orthotics after the end of the intervention and wore them prior to the final developmental assessment.
High‐intensity treadmill intervention versus a low‐intensity treadmill intervention
Ulrich 2008 randomised 36 children with Down syndrome to two groups to compare the effects of high‐intensity versus low‐intensity treadmill intervention. The low‐intensity group (18 children) received a home‐based treadmill intervention for five days a week, eight minutes per day, at a speed of 0.15 m/s until walking onset. The high‐intensity group (18 children) received an individualised treadmill intervention protocol in which the speed of the treadmill was increased depending on the child’s performance, and additional ankle weights were added during treadmill intervention. Treadmill intervention was terminated in both groups when the children achieved independent walking for three steps. In addition to the information provided in Ulrich 2008, information about this study came from four other publications: Angulo‐Barroso 2008, Wu 2007, Wu 2008 and Wu 2010. Wu 2007 also included comparisons of the high‐intensity and low‐intensity group data to no treatment using an historical control group from another included study (Ulrich 2001). We did not use data from these comparisons due to their being non‐randomised.
Outcomes
The included studies presented data on most of the outcomes identified in the protocol for this review (see Valentin‐Gudiol 2011p), with the exception of falls and injuries due to falls, inter‐ and intra‐limb co‐ordination and child quality of life. Below, we have listed all outcomes measured in the studies, including those that were not relevant for this review.
Angulo‐Barroso 2013, Ulrich 2001 and Ulrich 2008 used the BSID‐II to assess onset of assisted and independent walking. Angulo‐Barroso 2013 and Cherng 2007 used the GMFM, to assess gross motor function. Lowe 2015 and Mattern‐Baxter 2013 used Dimensions D and E of the GMFM, to assess gross motor function related to standing and walking. Mattern‐Baxter 2013 also used PDMS‐2 to assess the children's gross motor skills. Video coding was used to count frequency of alternating steps in two studies (Angulo‐Barroso 2013; Ulrich 2008). An instrumented gait mat (GaitRite mat, CIR systems) (Bilney 2003; Menz 2004) was used to compute the spatial‐temporal gait parameters in gait both with and without an obstacle in three studies (Angulo‐Barroso 2013; Ulrich 2001; Ulrich 2008), and a 3D motion analysis system (Vicon Motion Analysis System) (Bilney 2003; Webster 2005) was used to obtain the gait kinematics variables in one study (Ulrich 2008) .
Outcomes were presented separately by diagnosis because the effects of the treadmill intervention could vary given the different nature of each population. For instance, infants with Down syndrome are characterised by laxity, while children with cerebral palsy tend to have high tone. Therefore, repetition of the same movement (treadmill step) could have different neuromuscular consequences in a more compliant system versus a stiffer system.
Infants at moderate risk for developmental delay
Angulo‐Barroso 2013 examined children each month during the intervention period to monitor adherence to the treadmill protocol (experimental group), to videotape five one‐minute trials of the infants' stepping while being supported on the treadmill (both groups), and to administer the modified Ashworth scale (Bohannon & Smith 1987). The GMFM was administered at study entry and at walking onset. Chen 2008 provided the follow‐up information of the same sample at three and six months postintervention. During the treadmill period, the frequency of alternating steps on the treadmill, type of foot contact (step quality) and GMFM were examined. After independent walking onset, spatio‐temporal gait parameters measured by the GAITRite system (Bilney 2003; Menz 2004), and gait speed were assessed during the follow‐up.
Cerebral palsy
Cherng 2007 used all dimensions of the GMFM, muscle tone, selective motor control and gait velocity and gait parameters, such as stride length and double‐limb support, as outcome measures.
Mattern‐Baxter 2013 measured gross motor development with various outcome measures: Dimensions D (standing) and E (walking, running and jumping) of the GMFM, the locomotion subscale of the PDMS‐2, the timed 10‐minute walk test (Boyd 1999), the Functional Mobility Scales (FMS) (Graham 2004), and the number of alternating steps in 10 seconds (used as a measure of walking function). In addition, the mobility subscale of the Pediatric Evaluation of Disability Inventory (PEDI) (Feldman 1990) was administered via parent interview.
Down syndrome
Ulrich 2001 assessed the effectiveness of treadmill training using the number of days lapsed between entry into the study and the attainment of three developmental milestones as outcome measures: raising to stand, walking with help, and walking independently for three steps. In addition, follow‐up data for gait spatio‐temporal parameters were measured in the control and experimental groups, but were not reported.
Looper 2010 examined the average time in study until the infants achieved independent walking, and the infant's motor skill development after one‐month follow‐up using the GMFM.
Ulrich 2008 compared high‐intensity with low‐intensity treadmill training and examined the onset of several gross motor milestones from items of the motor subscale of BSID‐II. These were as follows: moving forward using pre‐walking methods (item 43), raising self to sitting position (item 47), raising self to standing position (item 52), walking sideways/cruising (item 54), walking with help (item 60), standing alone (item 61), walking alone (item 62) and walking alone with good co‐ordination (item 63). In addition, videotape analysis was performed on the frequency of alternating steps per minute on the treadmill every two months until onset of independent walking. Additional data from the children in this study were reported in four other publications (Angulo‐Barroso 2008; Wu 2007; Wu 2008; Wu 2010).
Wu 2007 presented data for age of walking onset, average velocity, stride length, step width, stride time, stance time and dynamic base. In a follow‐up article, Wu 2008 examined the ability and methods of obstacle clearance at walking onset, and at 3, 6, and 12 months after walking onset in 26 of the 30 children from the original high‐intensity versus low‐intensity treadmill intervention by Ulrich 2008. The ability to clear an obstacle was categorised as 'refusal, crawl, fall, and walk'. The five steps taken by the children leading up to the obstacle were analysed with the GAITRite system (Bilney 2003; Menz 2004).
The long‐term effects of high‐intensity treadmill and low‐intensity treadmill intervention in the same group of children with Down syndrome at 3, 6, 9 and 12 months postintervention were reported in an article by Angulo‐Barroso 2008. Six basic gait parameters were examined in a principal component analysis (normalised velocity, cadence, step length, step width, double support percentage and dynamic base).
Additionally, gait laboratory analysis was conducted during the one‐year follow‐up after walking onset following high‐intensity and low‐intensity treadmill intervention on 26 of the 30 analysed children with Down syndrome (Wu 2010). Timing and magnitude of peak extension and flexion at the hip, knee and ankle joints, as well as peak adduction and abduction at the hip joint, were compared in the high‐intensity and low‐intensity intervention groups.
General developmental delay
Lowe 2015 measured gross motor development via Dimensions D (standing) and E (walking, running and jumping) of the GMFM and measured self‐selected walking speed (Boyd 1999) with the timed 10‐minute walk test.
Excluded studies
Overall, we excluded 34 studies that appeared eligible for inclusion in this review update after examining the full‐text reports; we excluded 13 studies in the original review and 21 studies in this review update.
Of the 21 studies excluded in this update, we excluded 10 studies on the basis of the age of the participants, that is, the participants were older than six years of age (El‐Shamy 2017; Grecco 2013a; Grecco 2013c; Hilderley 2016; Johnston 2011; Kurz 2011; Romei 2012; Scholtes 2012; Sherief 2015; Su 2013); five studies because although they used treadmill training, they measured other outcomes that are outside the scope of interest of this review (Campbell 2012; Duarte 2014; Grecco 2013b; Jung 2016; Sarhan 2014); four studies because there was no control group (Pantall 2011; Schroeder 2014; Siekerman 2015; Willerslev‐Olsen 2014), one study because it was a case series (Lowe 2013), and one study because it was a case report (Christensen 2014).
In the original review (Valentin‐Gudiol 2011r), studies were excluded because participants were not randomly assigned (one study: Schlittler 2011); participants were older children (eight studies: Borggraefe 2007;Dodd 2007;Maltais 2003;Matsuno 2010; Meyer‐Heim 2007; Phillips 2007;Schindl 2000;Smania 2011); the studies used treadmill without training (three studies: Mussleman 2007; Pang 2003;Teulier 2009); or did not have a control group (one study: Borggraefe 2010).
Reasons for exclusion are detailed in the Characteristics of excluded studies tables.
Ongoing studies
We identified one ongoing study (NCT02424526) with an estimated completion date of June 2017, which we will report on in future updates. For more information, see Characteristics of ongoing studies tables.
Risk of bias in included studies
A comprehensive description of the risk of bias for each study can be found in the Characteristics of included studies tables. This information is summarised in Figure 2 and Figure 3.
2.
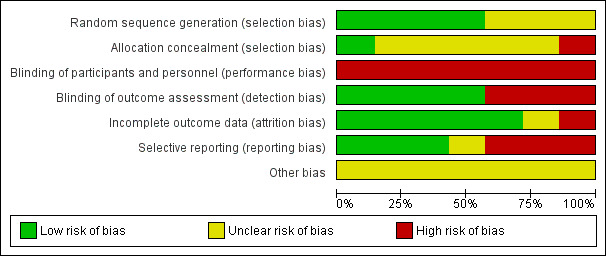
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
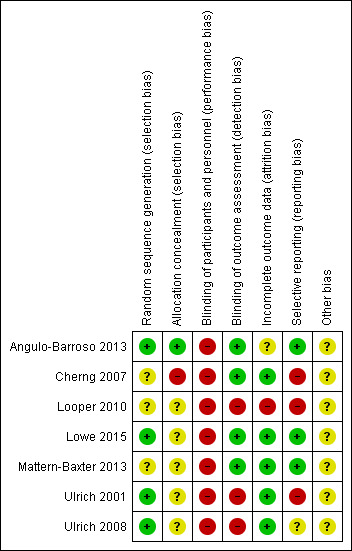
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. + = low risk, ‐ = high risk, ? = unclear risk
Allocation
Random sequence generation
We judged four studies to be at low risk of bias on this domain. The studies by Lowe 2015, Ulrich 2001 and Ulrich 2008 used a random numbers table to assign participants to the intervention or control group. In Angulo‐Barroso 2013, the randomisation took place through ID numbers provided by a computer programme, which a statistician assigned to participants after considering three stratification factors (age, sex and birth weight). Information on how the random sequence was generated was lacking in the other three studies, which we therefore assessed to be at unclear risk of bias for this domain (Cherng 2007; Looper 2010; Mattern‐Baxter 2013).
Allocation concealment
We rated one study at low risk of bias on this domain: Angulo‐Barroso 2013 used a computer programme for group allocation through a statistician, who assigned an ID to all participants. This ID was provided to the project coordinator and home assessment personnel, but the laboratory assessors were maintained blind to group allocation. Five studies had unclear risk of bias. In Ulrich 2001 and Ulrich 2008, one of the investigators used a table of random numbers to assign allocation, but this is not an acceptable method to ensure allocation concealment (Higgins 2011a). In the absence of other information, we assessed these studies to be at unclear risk of bias. Lowe 2015 was also rated as at unclear risk of bias due to use of a computer‐generated randomisation chart. In addition, two children from the intervention group were excluded from data analysis because they were considered outliers due to test results that approached those of children with typical development. Looper 2010 and Mattern‐Baxter 2013 were also at unclear risk of bias as they did not report how the allocation process took place. We rated one study, Cherng 2007, at high risk of bias because it was a cross‐sectional study; therefore, all children received training under two different conditions.
Blinding
Blinding of participants and personnel
For all studies, we rated the risk of performance bias as high, as parents, infants and personnel were aware of group allocation in all studies (Angulo‐Barroso 2013; Cherng 2007; Looper 2010; Lowe 2015; Mattern‐Baxter 2013; Ulrich 2001; Ulrich 2008).
Blinding of outcome assessment
Three studies suffered from a high risk of detection bias as the assessors were aware of group allocation (Looper 2010; Ulrich 2001; Ulrich 2008). In four studies, the risk of bias was considered to be low. In Cherng 2007, an independent therapist, who was unaware of the therapy the children had received, performed the gait parameter measurements. In Angulo‐Barroso 2013, the laboratory assessors were blinded to group allocation and, in Mattern‐Baxter 2013, performance on the two outcome measures, GMFM and PDMS‐2, was videotaped and thereafter reviewed by a therapist who was blinded to group allocation. Finally, in Lowe 2015, one of the outcomes assessors was blinded to group allocation.
Incomplete outcome data
In the five studies that assessed outcomes during or immediately after the intervention, or both, attrition and bias due to attrition was low (Cherng 2007; Lowe 2015; Mattern‐Baxter 2013; Ulrich 2001; Ulrich 2008). One study, Looper 2010, had a high risk of attrition bias. The remaining study, Angulo‐Barroso 2013, had an unclear risk related to intervention attrition bias since 14.6% of infants were excluded from the study due to noncompliance with the research protocol. Low compliance when implementing a demanding intervention (time and discipline wise) in a population at risk (low socioeconomic status) is rather common.
Selective reporting
In three studies, we judged the risk of reporting bias to be high, as not all data were reported (Cherng 2007; Looper 2010; Ulrich 2001). It was unclear whether all data had been reported in one study (Ulrich 2008). In the other three studies, we rated the risk of reporting bias as low since there was no evidence of reporting bias (Angulo‐Barroso 2013; Lowe 2015; Mattern‐Baxter 2013) .
Other potential sources of bias
In all studies, the risk of other sources of bias was unclear because of insufficient information (Angulo‐Barroso 2013; Cherng 2007; Looper 2010; Lowe 2015; Mattern‐Baxter 2013; Ulrich 2001; Ulrich 2008).
Effects of interventions
See: Table 1
We could only perform limited quantitative analysis due to the heterogeneous nature of the types of interventions used, the distinct nature of the diagnostic subgroups studied, and differences in outcome measures or time periods or both when data were collected. Because all studies had continuous outcome measures and they were all measured using the same scale, we calculated MDs to determine the effect estimate of treadmill intervention on the various outcome measures in the different subgroups of children.
In the original review (Valentin‐Gudiol 2011r), we could only conduct a meta‐analysis on the effects of treadmill intervention versus no treadmill intervention in children with different diagnoses for the total GMFM percentage scores and the onset of independent walking in days. In this update, we added a meta‐analysis on the GMFM Dimension D and E per cent scores and walking velocity in children with different diagnoses. We reported analyses from individual studies on the effects of treadmill training as well. We reported the effects of the intervention by type of treadmill intervention and outcomes.
Comparison 1. Treadmill intervention versus no treadmill intervention
This comparison was evaluated by five studies (Angulo‐Barroso 2013; Cherng 2007; Lowe 2015; Mattern‐Baxter 2013; Ulrich 2001). The outcomes are presented according to the levels of the ICF‐CY (WHO 2005), starting with the outcomes on the level of body structure and functions, such as step frequency and step quality, followed by the outcomes at the level of activities and participation, such as age of onset of independent walking and gross motor function. In the text below, we described the main outcomes from the meta‐analysis first, followed by findings from individual studies that were considered important, but could not be included in the meta‐analysis. We referred to the results by analysis number. Figure 4, Figure 5, and Figure 6 further illustrate the results from the meta‐analysis. In Table 1, we reported on outcome measures that were analysed for individual studies as well as for meta‐analysis, when possible.
4.
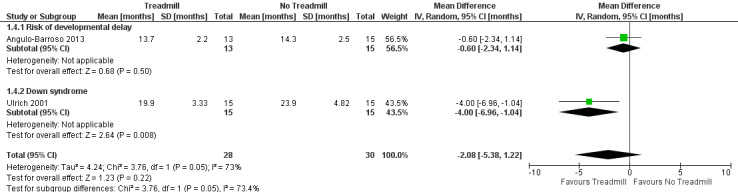
Forest plot of comparison: 1 No Treadmill vs Treadmill: Walking independently (months).
5.
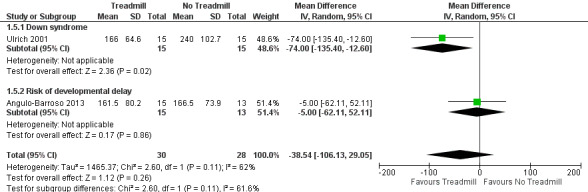
Forest plot of comparison: 1 Treadmill vs No Treadmill, outcome: 1.20 Age of onset of walking with assistance [days in study].
6.
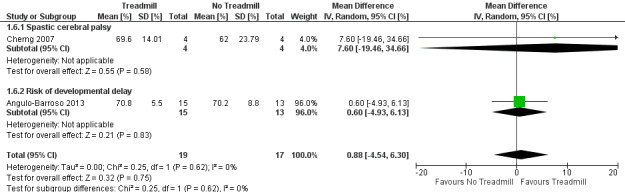
Forest plot of comparison: 1 No Treadmill vs Treadmill: Gross motor function (GMFM as %).
Primary outcomes
Body structure and functions
Step frequency (treadmill alternating steps)
Angulo‐Barroso 2013 (28 children at risk for motor delays) found no difference in the step frequency of experimental and control children at 16 months of age, suggesting that treadmill training did not help to increase step frequency in children at moderate risk for motor delays (MD 4.36, 95% CI ‐2.63 to 11.35, Analysis 1.1).
1.1. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 1 Step frequency (16 months): Risk of developmental delay (% alternate steps).
Step quality
Angulo‐Barroso 2013 found that treadmill training helped improve step quality for 28 children at risk of neuromotor disabilities. In the experimental group, from 11 to 16 months of age, there was a significant decrease of foot toe contact during treadmill stepping (at 11 months of age: MD ‐20.98, 95% CI ‐26.87 to ‐15.08, Analysis 1.2; at 16 months of age: MD ‐15.61, 95% CI ‐23.96 to ‐7.26, Analysis 1.3); thus, an increase of flat foot contact steps occurred.
1.2. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 2 Step quality (11 months): Risk of developmental delay (% toe contact).
1.3. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 3 Step quality (16 months): Risk of developmental delay [% toe contact].
Activity and participation functions
Age of onset of independent walking
The onset of independent walking was characterised across studies as the ability to take three to 10 independent steps. We conducted a meta‐analysis of two studies on a total of 58 children who had Down syndrome (Ulrich 2001) or infants at moderate risk of developmental delay (Angulo‐Barroso 2013). Overall, we found no evidence to suggest that the treadmill intervention was effective in promoting earlier independent walking (in months of age) (MD ‐2.08 (95% CI ‐5.38 to 1.22), Analysis 1.4; Figure 4). However, heterogeneity across studies was substantial (tau² = 4.24; I² = 73%), and it must be noted that the studies examined children with different diagnoses.
1.4. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 4 Age of onset of independent walking.
In children at risk of motor delays, Angulo‐Barroso 2013 found that children, both in the control and the experimental group, attained independent walking at similar corrected ages and did not find support for an effect of treadmill intervention on age of onset of independent walking (MD ‐0.60, 95% CI ‐2.34, 1.14, 28 children; Analysis 1.4; Figure 4).
In contrast, when 30 children with Down syndrome were randomised to receive treadmill intervention or serve as controls (Ulrich 2001), those in the treadmill intervention group learned to walk independently much faster than those in the control group (MD ‐4.00, 95% CI ‐6.96 to ‐1.04; Analysis 1.4; Figure 4).
Age of onset of walking with assistance
We included two studies, Angulo‐Barroso 2013 and Ulrich 2001, in a meta‐analysis on the effects of treadmill versus no treadmill intervention, and found that treadmill intervention did not affect the age of onset of walking with assistance (MD ‐38.54, 95% CI ‐106.13 to 29.05, 58 children; Analysis 1.5; Figure 5). We considered this evidence to be of very low quality due to the fact that there was large heterogeneity across studies (tau² = 1465.37; I² = 62%). The two studies were conducted on infants with different diagnoses: infants at moderate risk of developmental delay (Angulo‐Barroso 2013) and children with Down syndrome (Ulrich 2001). If we were to consider only the study with 30 infants with Down syndrome, we could say that treadmill training had positive effects on this outcome when compared to non‐training (MD ‐74.00, 95% CI ‐135.40 to ‐12.60, Analysis 1.5; Figure 5). In the group of 28 infants at moderate risk of developmental delay, a similar effect could not be demonstrated: MD ‐5.00, 95% CI ‐62.11 to 52.11 (Analysis 1.5; Figure 5).
1.5. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 5 Age of onset of walking with assistance (days in study).
Gross motor function
We conducted a meta‐analysis of two studies (Angulo‐Barroso 2013; Cherng 2007) on the effects of treadmill versus no treadmill intervention, and found moderate evidence that treadmill intervention did not affect total GMFM per cent scores (MD 0.88, 95% CI ‐4.54 to 6.30, 36 children (Analysis 1.6; Figure 6)). Heterogeneity across studies was low (tau² = 0%; I² = 0%). The two studies were conducted on infants with different diagnoses: spastic cerebral palsy (Cherng 2007) and infants at moderate risk of developmental delay (Angulo‐Barroso 2013). The absence of evidence of an effect of treadmill intervention on total GMFM per cent scores was reported in both groups of infants: those with cerebral palsy (MD 7.60, 95% CI ‐19.46 to 34.66, 8 children; Analysis 1.6; Figure 6) and those at moderate risk of developmental delay (MD 0.60, 95% CI ‐4.93 to 6.13, 28 children; Figure 6). We did not include Lowe 2015 and Mattern‐Baxter 2013 in this meta‐analysis because the authors measured only Dimensions D and E of the GMFM. Separate meta‐analyses of the outcomes on these two dimensions indicated that there was low‐quality evidence that treadmill training was not associated with a significant improvement in Dimension D per cent scores (MD 5.41, 95% CI ‐1.61 to 12.43; Analysis 1.7) and had a negligible effect on Dimension E per cent scores (MD 4.51, 95% CI 0.29 to 8.73; Analysis 1.8). The individual studies showed the following: Mattern‐Baxter 2013 demonstrated statistically significant improvements at the one‐month postintervention follow‐up in GMFM Dimension D per cent scores in the treadmill group in children with cerebral palsy (MD 11.57, 95% CI 0.05 to 23.09; Analysis 1.7), and Lowe 2015 showed favourable Dimension D per cent scores in the treadmill group in children with general developmental delay (MD 3.33, 95% CI 1.43 to 5.23; Analysis 1.7). Lowe 2015 further showed improvements in children with general developmental delay favouring the treadmill group in GMFM Dimension E per cent scores (MD 7.60, 95% CI 0.88 to 14.32; Analysis 1.8), but this was not true for children with cerebral palsy (MD 3.01, 95% CI ‐1.11 to 7.13; Analysis 1.8).
1.6. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 6 Gross motor function measure (GMFM).
1.7. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 7 Gross motor function related to standing (GMFM) ‐ Dimension D.
1.8. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 8 Gross motor function related to walking, running and jumping (GMFM) ‐ Dimension E.
Analysis of one study (Mattern‐Baxter 2013) on the effects of treadmill training versus no treadmill training on gross motor function as measured with the PDMS‐2, revealed that the treadmill intervention improved developmental scores (MD 8.00 , 90% CI 3.18 to 12.82, 12 children; Analysis 1.9). Similarly, after intervention, PEDI scores were better in the treadmill group than in the non‐treadmill group (MD 9.50, 95% CI 4.61 to 14.39, 12 children; Analysis 1.10).
1.9. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 9 Peabody Developmental Motor Scales ‐ 2: Spastic cerebral palsy.
1.10. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 10 Pediatric Evaluation of Disability Inventory ‐ Mobility Scale scores: Spastic cerebral palsy.
Falls and injuries due to falls
No study provided data on this outcome.
Secondary outcomes
Body structure and functions
Inter‐ and intra‐limb co‐ordination
No study provided data on this outcome.
Other gait parameters
We conducted a meta‐analysis of two studies (Lowe 2015; Mattern‐Baxter 2013) on the effect of treadmill versus no treadmill intervention on gait velocity in children with general developmental delay and cerebral palsy. The analysis showed evidence, which suggested that the treadmill intervention had a minimal effect in promoting a higher gait velocity in metres/second (MD 0.23, 95% CI 0.08 to 0.37; Analysis 1.11). However, it should be noted that these were two different populations (tau² = 0.00; I² = 0%), suggesting that the difference could be due to differences in the populations and not to the effect of the intervention. When examining the studies individually, more pronounced improvements in gait speed favouring the treadmill group were found in children with general developmental delay (Lowe 2015: MD 0.25, 95% CI 0.08 to 0.42; Analysis 1.11), but not in children with spastic cerebral palsy (Mattern‐Baxter 2013: MD 0.18, 95% CI ‐0.09 to 0.45; Analysis 1.11). One study measured velocity at follow‐up in 28 infants at moderate risk of developmental delay after independent walking onset (Angulo‐Barroso 2013). There was no effect with respect to walking velocity (MD 1.32, 95% CI ‐0.53 to 3.17; Analysis 1.12). Step length in centimetres and double‐limb support were measured in two studies that examined treadmill versus no treadmill intervention in eight children with spastic cerebral palsy (Cherng 2007) and 28 infants at moderate risk of developmental delay (Angulo‐Barroso 2013). No effect was found for step length (children with spastic cerebral palsy: MD 0.37, 95% CI ‐25.04 to 25.78; Analysis 1.13; infants at risk of developmental delay: MD 8.00, 95% CI ‐1.60 to 17.60; Analysis 1.14), or double‐limb support (children with spastic cerebral palsy: MD 3.80, 95% CI ‐21.52 to 29.12; Analysis 1.15; infants at risk of developmental delay: MD ‐4.19; 95% CI ‐10.02 to 1.64; Analysis 1.16) at the time of walking onset.
1.11. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 11 Other gait parameters ‐ velocity.
1.12. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 12 Other gait parameters ‐ velocity (follow‐up when walking independently): Risk of developmental delay.
1.13. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 13 Other gait parameters ‐ step length: Spastic cerebral palsy.
1.14. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 14 Other gait parameters ‐ step length (follow‐up when walking independently): Risk of developmental delay.
1.15. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 15 Other gait parameters ‐ gait double‐limb support: Spastic cerebral palsy.
1.16. Analysis.

Comparison 1 Treadmill versus no treadmill, Outcome 16 Other gait parameters ‐ gait double‐limb support (follow‐up when walking independently): Risk of developmental delay.
Activity and participation functions
Infant or child quality of life
No study provided data on this outcome.
Comparison 2. Treadmill intervention without orthotics versus treadmill intervention with orthotics
Only one study (Looper 2010), involving 17 children with Down syndrome, evaluated this comparison. This study measured only two of our outcomes: age of onset of independent walking and gross motor function. These were both primary outcomes. The study provided no data on the remaining primary outcomes of step frequency, step quality or age of onset of walking with assistance, or on any of the secondary outcomes (i.e. inter‐ and intra‐limb co‐ordination, other gait parameters or infant or child quality of life).
Primary outcomes
Activity and participation functions
Age of onset of independent walking
There was no difference in the age of onset of independent walking between the two intervention groups (MD 0.10, 95% CI ‐5.96 to 6.16; Analysis 2.1).
2.1. Analysis.

Comparison 2 Treadmill without orthoses versus treadmill with orthoses, Outcome 1 Walking independently (1‐month follow‐up): Down syndrome.
Gross motor function
The use of orthotics was associated with lower total scores on the GMFM one month after completion of the treadmill intervention (MD ‐8.40, 95% CI ‐14.55 to ‐2.25; Analysis 2.2). The lower total scores were mainly brought about by lower scores on dimensions D and E. The results suggested that early use of orthoses might hinder gross motor progress.
2.2. Analysis.

Comparison 2 Treadmill without orthoses versus treadmill with orthoses, Outcome 2 Gross motor function (GMFM 1‐month follow‐up): Down syndrome.
Comparison 3. High‐intensity treadmill intervention versus low‐intensity treadmill intervention
Ulrich 2008 was the only study to evaluate this comparison in their study of 30 children with Down syndrome. This study measured three of our primary outcomes (step frequency, age of onset of independent walking and age of onset of walking with assistance) and one of our secondary outcomes (other gait parameters). The study provided no data on our other outcomes.
Primary outcomes
Body structure and functions
Step frequency (treadmill alternating steps)
Ulrich 2008 calculated the values for frequency of alternating steps in both the high‐intensity and the low‐intensity groups. No differences in frequency of stepping were found prior to the training. After the intervention, those infants who received the high‐intensity training protocol took a greater number of steps than those who belonged to the low‐intensity group (MD ‐11.00, 95% CI ‐15.90 to ‐6.10; Analysis 3.1).
3.1. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 1 Step frequency: Down syndrome.
Activity and participation functions
Age of onset of independent walking
No clear evidence of a differential effect was observed on independent walking (MD ‐2.13, 95% CI ‐4.96 to 0.70; Analysis 3.2).
3.2. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 2 Age of onset of independent walking: Down syndrome.
Age of onset of independent walking or walking with assistance
No clear evidence of a differential effect was observed on supported walking (MD ‐1.86, 95% CI ‐4.09 to 0.37; Analysis 3.3).
3.3. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 3 Age of onset of walking with assistance: Down syndrome.
Secondary outcomes
Body structure and functions
Other gait parameters
Various gait parameters were examined in Ulrich 2008, and three additional publications of the same sample of 25 children with Down syndrome at 3, 6, 9 and 12 months (follow‐up visits one, two, three and four, respectively) after walking onset (Angulo‐Barroso 2013; Wu 2008; Wu 2010).
There was a positive effect of high‐intensity treadmill intervention compared to low‐intensity treadmill intervention on gait velocity at six months follow‐up (MD 0.16, 95% CI 0.01 to 0.31; Analysis 3.5), but not at three months (MD 0.05; 95% CI ‐0.06 to 0.16; Analysis 3.4), nine months (MD 0.10; 95% CI ‐0.07 to 0.27; Analysis 3.6), or 12 months (MD 0.16; 95% CI ‐0.07 to 0.39; Analysis 3.7).
3.5. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 5 Other gait parameters ‐ velocity (follow‐up visit 2): Down syndrome.
3.4. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 4 Other gait parameters ‐ velocity (follow‐up visit 1): Down syndrome.
3.6. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 6 Other gait parameters ‐ velocity (follow‐up visit 3): Down syndrome.
3.7. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 7 Other gait parameters ‐ velocity (follow‐up visit 4): Down syndrome.
Similarly, children in the high‐intensity group decreased double‐limb support at six months (follow‐up visit two) after walking onset (MD ‐4.00; 95% CI ‐7.91 to ‐0.09; Analysis 3.9), but not at three months (MD ‐2.90; 95% ‐8.07 to 2.27; Analysis 3.8), nine months (MD ‐2.00; 95% CI ‐6.29 to 2.29; Analysis 3.10), or 12 months (MD ‐0.80; 95% CI ‐3.27 to 1.67; Analysis 3.11).
3.9. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 9 Other gait parameters ‐ gait double‐limb support (follow‐up visit 2): Down syndrome.
3.8. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 8 Other gait parameters ‐ gait double‐limb support (follow‐up visit 1): Down syndrome.
3.10. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 10 Other gait parameters ‐ gait double‐limb support (follow‐up visit 3): Down syndrome.
3.11. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 11 Other gait parameters ‐ gait double‐limb support (follow‐up visit 4): Down syndrome.
Similarly, the high‐intensity treadmill intervention resulted in better timing of maximum ankle plantar flexion during gait compared to the low‐intensity group at six months (MD ‐4.80, 95% CI ‐8.76 to ‐0.84; Analysis 3.13), but not at three months (MD ‐3.10; 95% CI ‐7.34 to 1.14; Analysis 3.12), nine months (MD ‐2.90; 95% ‐6.28 to 0.48; Analysis 3.14), or 12 months (MD ‐3.40; 95% CI ‐8.98 to 2.18; Analysis 3.15). There was no difference between the high‐intensity and low‐intensity treadmill intervention groups on other gait parameters at the 12‐month follow‐up assessment such as step width (MD ‐0.58, 95% CI ‐2.11 to 0.95, 25 children; Analysis 3.16), step length (MD 2.68, 95% CI ‐0.99 to 6.35; Analysis 3.17), toe‐off (MD ‐0.90, 95% CI ‐5.49 to 3.69; Analysis 3.18), and gait ankle dorsiflexion (MD ‐2.80, 95% CI ‐5.96 to 0.36; Analysis 3.19).
3.13. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 13 Other gait parameters ‐ gait ankle plantar flexion (follow‐up visit 2): Down syndrome.
3.12. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 12 Other gait parameters ‐ gait ankle plantar flexion (follow‐up visit 1): Down syndrome.
3.14. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 14 Other gait parameters ‐ gait ankle plantar flexion (follow‐up visit 3): Down syndrome.
3.15. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 15 Other gait parameters ‐ gait ankle plantar flexion (follow‐up visit 4): Down syndrome.
3.16. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 16 Other gait parameters ‐ step width (follow‐up): Down syndrome.
3.17. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 17 Other gait parameters ‐ step length (follow‐up): Down syndrome.
3.18. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 18 Other gait parameters ‐ toe‐off (follow‐up): Down syndrome.
3.19. Analysis.

Comparison 3 High‐intensity treadmill versus low‐intensity treadmill, Outcome 19 Other gait parameters ‐ gait ankle dorsiflexion (follow‐up): Down syndrome.
Discussion
In this review, we included data from five RCTs and two quasi‐RCTs in which 175 children (97 of whom received the treadmill intervention with the remainder acting as controls), below the age of six years participated. One trial was reported in multiple publications (Ulrich 2008).
The unpublished data of Chen 2008, included in the original review were retained and included within Angulo‐Barroso 2013 in data analysis tables.
Summary of main results
The studies varied in the type of population studied (children with Down syndrome, cerebral palsy, general developmental delay or at risk for developmental delay), in time of evaluation (during the intervention, immediately after the intervention or during follow‐up after three to 12 months after intervention), the walking status of the children (pre‐ambulatory and already ambulating) and in the parameters assessed. The latter varied from primary outcomes of motor milestones, such as the onset of independent walking, to detailed gait parameters, which were secondary outcomes. Due to the heterogeneity of the studies, the meta‐analyses were restricted to few studies and limited to scores on the GMFM (total score and scores in Dimensions D and E, a primary outcome), the onset of independent walking in days (a primary outcome) and gait velocity (a secondary outcome). Also, given the small sample size of most included studies, power to find significant results was limited, implying that most studies provided moderate evidence that should be interpreted with caution.
Body structure and functions
The reported effect of treadmill intervention on gait parameters varied across studies, which makes it difficult to draw conclusions. For pre‐ambulatory children with cerebral palsy or children with moderate risk for developmental delay, no effect of treadmill intervention on step frequency (primary outcome), gait velocity, step length and double‐limb support (secondary outcomes) could be established. However, for children with developmental delay who were ambulatory, a positive effect on gait velocity was found after six weeks of treadmill training. There was a positive effect of treadmill training in regard to step quality for children with moderate risk for motor delay. The studies on the effect of high‐intensity individualised treadmill intervention in comparison to low‐intensity generalised treadmill intervention in children with Down syndrome suggested that the high‐intensity intervention was associated with a better ability to take alternating steps and an improved ability to clear obstacles during the year postintervention. Evidence of an effect on gait velocity and decreased double‐limb support was mixed in this population. There was no evidence of a different effect of low‐ and high‐intensity interventions on step length, step width or toe‐off.
Activity and participation functions
The results of this review indicate that treadmill intervention may be associated with an earlier onset of independent walking and supported walking in children with Down syndrome (both primary outcomes). In these children, both a high‐intensity individualised treadmill intervention and a low‐intensity generalised treadmill intervention had a similar effect on onset of independent walking. The effect of treadmill intervention on GMFM scores (primary outcome) in children with Down syndrome was not studied. However, it seemed that the early application of supramalleolar orthoses during treadmill training in children with Down syndrome may have a negative effect on GMFM scores.
Angulo‐Barroso 2013 did not find an effect of 40 minutes per week of treadmill training in infants at risk for developmental delay on the total score of the GMFM. On the other hand, Lowe 2015 did find a positive effect of a comparable amount of treadmill training on Dimenstion D (standing) and E (walking, running and jumping) of the GMFM. The two studies differed in the groups studied: developmental risk was higher in the participants of Lowe 2015 than in those of Angulo‐Barroso 2013, and the participants in Lowe 2015 were older than those of Angulo‐Barroso 2013. An additional main difference was that the children in Lowe 2015 were ambulatory at study onset whereas the children in Angulo‐Barroso 2013 were pre‐ambulatory. On the basis of these group characteristics, however, one would have expected an effect in the Angulo‐Barroso 2013 study rather than in Lowe 2015. Two explanations may be offered for the difference in outcome between the two studies. First, it is possible that the Dimensions D and E are more sensitive in measuring developmental changes induced by treadmill training than the total GMFM scores. Second, the better outcome in the Lowe 2015 study may be due to the fact that the treadmill training could be applied at a more intense dosage than in the Angulo‐Barroso 2013 study: in Lowe 2015, treadmill velocity ranged from 0.54 to 0.80 m/s and was part of the time combined with inclination of the treadmill surface, whereas Angulo‐Barroso 2013 used a treadmill velocity of 0.20 m/s. Interestingly, Lowe 2015 also reported that treadmill training was associated with a higher gait velocity. In addition, it should be realized that the ambulatory children with developmental delay (in Lowe 2015) were building on an already acquired skill and could improve their walking velocity, whereas the at‐risk children (Angulo‐Barroso 2013) were still in the process of attaining independent walking.
In children with cerebral palsy, we found that treadmill intervention applied for 60 to 90 minutes per week (Cherng 2007) was not associated with improved gross motor development measured with the total GMFM. However, treadmill training applied for 120 to 240 minutes per week in children with cerebral palsy was associated with a marginally faster improvement of Dimension D (standing) and Dimension E (walking, running and jumping) of the GMFM (Mattern‐Baxter 2013). This intensive treadmill training was however associated with a significant improvement of gross motor function as measured with the PDMS‐2 and function in daily life as measured with the PEDI (Feldman 1990), but not of walking velocity. This might be explained by the fact that the children were still acquiring the skill of walking.
Overall completeness and applicability of evidence
Overall, there were few studies assessing the effect of treadmill interventions in young children with or at high risk for motor developmental delay. Three of the seven studies examined treadmill interventions in children with Down syndrome (Looper 2010; Ulrich 2001; Ulrich 2008). One study assessed a treadmill intervention in infants at moderate risk for developmental delay (Angulo‐Barroso 2013), one study examined children with general developmental delay (Lowe 2015), and two studies assessed treadmill interventions in children with cerebral palsy (Cherng 2007; Mattern‐Baxter 2013). Two of the seven studies did not evaluate the effect of treadmill intervention versus no treadmill intervention, but assessed two modifications of the treadmill intervention (high‐ versus low‐intensity, with orthosis versus without orthosis) (Looper 2010; Ulrich 2008). This means that the evidence on the effect of a treadmill intervention alone is limited. The effect has been most extensively studied in children with Down syndrome.
Quality of the evidence
Most studies were designed as RCTs, a design which is associated with a high standard of evidence, all things being equal (Sacket level I) (Butler 2001; Sackett 1996). However, the studies in this review suffered from methodological limitations, in particular from a high risk of bias due to the absence of blinding. Performance bias is inevitable in studies on treadmill interventions, but detection bias, from which three of the seven studies suffered (the three studies on children with Down syndrome), may be prevented. Another important methodological limitation was the risk of attrition bias. Attrition occurred especially during follow‐up after the treadmill intervention. In general, the extent of attrition was moderate, but it was unclear whether or not attrition was selective.
'Summary of findings' table
We conducted GRADE (GRADEproGDT 2015) assessments of the quality of evidence for six outcomes, i.e. the outcomes that were included in a meta‐analysis. We judged the quality of evidence for the outcome ‘gait velocity’ as high for the comparison treadmill training versus no treadmill training. Quality was considered moderate for the outcomes 'age of onset of independent walking' and 'gross motor function'. We deemed the evidence to be of low or very low‐quality for the outcomes ‘GMFM Dimension D’ (low), GMFM Dimension E (low) and 'age of onset of walking with assistance' (very low). The strengths and weaknesses are discussed in detail in the footnotes of Table 1. The main reasons for downgrading from high‐quality evidence were: inconsistency for the outcomes, 'age of onset of independent walking' and 'age of onset of walking with assistance', and imprecision for the outcomes, 'age of onset of walking with assistance' and 'gross motor function'.
Potential biases in the review process
One of the authors of the review (Angulo‐Barroso) participated in the series of studies on children with Down syndrome. Three of the authors of this review update (RA, KM and MV) are also authors of studies included in this update, and therefore were not involved in selecting studies and assessing risk of bias. Instead, CB and MG selected studies, extracted data and assessed risk of bias. In case of disputes, MH first acted as arbiter, and the rest of the authors were contacted afterwards to find agreement. Other potential biases have not been identified.
Agreements and disagreements with other studies or reviews
The effects of treadmill intervention have been examined in previous systematic reviews (Damiano 2009; Molina‐Rueda 2010; Morgan 2016; Mutlu 2009; Novak 2013; Willoughby 2009), in children of all ages with or at risk of a motor developmental disorder, but only one of them was done specifically in children with cerebral palsy under six years of age (Morgan 2016).
All of these reviews concluded that there was insufficient evidence to support or condemn treadmill interventions in children with cerebral palsy (Damiano 2009; Molina‐Rueda 2010; Morgan 2016; Mutlu 2009; Novak 2013; Willoughby 2009); and that treadmill interventions in children with Down syndrome may accelerate development of walking (Damiano 2009). Interestingly, the Dodd 2007 study, in which children with cerebral palsy received maximally 60 minutes of treadmill training per week, did not show an effect of treadmill training on walking parameters such as walking speed and the distance walked in 10 minutes. On the other hand, individual studies did show an effect of treadmill training in children with cerebral palsy on the scores on Dimensions D and E of the GMFM (Lowe 2015) and on the PEDI (Mattern‐Baxter 2013).
These findings suggest that dosing of the training may matter. Dosing may be altered by means of the intensity of the training (e.g. speed of the treadmill, addition of an inclination) and by the duration of the training. Our review findings in children at risk of or with developmental delay, in children with Down syndrome and children with cerebral palsy are in line with this dosing hypothesis. The studies that applied either treadmill training for a substantial number of minutes per week (120 to 140 rather than 40 to 90 minutes per week, Mattern‐Baxter 2013) or a challenging type of treadmill exercise (relatively high treadmill velocity with inclination (Lowe 2015) or relatively high treadmill velocity in combination with the application of ankle weights (Ulrich 2008) were those studies that were associated with a significant effect on outcome (step frequency, gross motor function or function in daily life). Even though sample sizes in these studies were small, these findings are in line with other emerging evidence that dosing is important in the success of an intervention in children with cerebral palsy (Gordon 2011; Hadders‐Algra 2017; Kolobe 2014; Morgan 2013). This also suggests that a relatively short but intensive period of treadmill intervention might lead to accelerated improvements compared to the same amount of intervention spread out over a longer period of time.
Authors' conclusions
Implications for practice.
Regular frequent practice of motor activity is the cornerstone of motor development. This is, for instance, reflected by the fact that during typical development novice walkers spontaneously produce about 14,000 steps and about a hundred falls per day (Adolph 2012). Evidence is accumulating that task‐specific training is a useful tool to promote motor development in children with or at risk for delayed motor development (Morgan 2016). The current review assessed the evidence for the effectiveness of treadmill intervention in young children under six years of age with or at risk for motor developmental delay. Given the limited number of studies, and their heterogeneity, this review can provide no firm evidence for the clinical application of treadmill interventions. With some caution, the review indicates that treadmill intervention in children with Down syndrome may assist in facilitating an earlier onset of walking. Limited data suggest that children with Down syndrome who received a more intensive treadmill intervention may be more accomplished in their gait parameters when compared to children who received a less intensive treadmill intervention. Based on this review, an intensive treadmill intervention may consist of two to four hours per week or a challenging treadmill training that uses a relatively high velocity and an inclined surface. Although dosage may be a critical component of early intervention, efficacy of the treadmill intervention at this early stage in development needs to be demonstrated first.
The limited evidence in this review also suggests that, in children with Down syndrome, application of orthoses during treadmill interventions, and before walking onset, may have a negative effect on gross motor development.
Home‐based protocols, where the intervention is carried out by parents or caregivers with instruction/supervision by a physical therapist, appears to be a feasible intervention for children with Down syndrome and cerebral palsy. This type of home‐based approach can more easily provide the necessary intensity of intervention for task‐specific ambulation training. However, the effectiveness of a home‐based model of intensive treadmill training has only been established in the literature for children with cerebral palsy and moderate‐risk infants, involving small sample sizes. An alternative feasible and effective approach may be the application of a challenging treadmill intervention for a couple of times a week in the preschool setting. From a clinical perspective, it is also important to consider the intrinsic differences of the studied populations. It is generally accepted that infants with Down syndrome are hypotonic and their neuromusculoskeletal systems may benefit from heavy repetition of a highly patterned movement. In contrast, infants at risk for neuromotor delay and children with cerebral palsy may present variable levels of muscle tone and frequently hypertonicity. Although a home‐based treadmill intervention seems to be valuable for this population, an additional intervention with more variability of movement in individuals with less compliant neuromuscular systems would perhaps be needed to trigger optimal results.
Implications for research.
Both neurophysiologic and early intervention literature suggests that task‐specific training facilitates motor development. Treadmill interventions are a good example of task‐specific training. Although some more studies have emerged on this topic since the original review in 2011 (Valentin‐Gudiol 2011r), this updated review highlights the need for more RCTs on the effect of treadmill intervention on larger sample sizes. After establishing the efficacy of the treadmill intervention, an important question to consider is that of the optimal dosing of treadmill interventions. Some of this work has already been completed for children with Down syndrome. However, studies that examine the optimal dosage of treadmill interventions for children at risk for developmental motor delay and cerebral palsy are currently lacking. Given the results in Down syndrome, and because the literature suggests that high‐intensity intervention has a larger effect on motor development than low‐intensity intervention in children with cerebral palsy (Gordon 2011; Hadders‐Algra 2017), it would be worthwhile to investigate the effect of treadmill intervention applied at higher dosages versus lower dosages. An important methodological issue that future studies need to take into account is masking of group identity. Masking of participants and personnel applying the treadmill intervention for group status is impossible. However, masking of group identity of persons assessing outcomes is perfectly feasible.
What's new
| Date | Event | Description |
|---|---|---|
| 3 December 2015 | New citation required but conclusions have not changed | One new study included in the review. |
| 28 September 2015 | New search has been performed | This review was updated following a new search in July 2014, May 2016, and May 2017. |
Acknowledgements
We are grateful for the feedback of Geraldine Macdonald, Co‐ordinating Editor of the Cochrane Developmental, Psychosocial and Learning Problems Group (CDPLPG), which helped us to improve this review. We are grateful for the guidance of Laura MacDonald, former Managing Editor of CDPLPG, during our process of writing the original review, and to Joanne Wilson, current Managing Editor of CDPLPG, and Dr Kathy Wolfe for assisting during our review update. Also, thanks to Marta Roqué from Cochrane Iberoamericana Centre for advice on the development of specific sections of the protocol and also to the peer referees and statistician who commented on the review. We would like to thank Claire Kerr, Lecturer in Rehabilitation Sciences at Queen's University, for her useful feedback. Many thanks to Margaret Anderson of the CDPLPG for invaluable assistance with refining the search strategy. The authors would also like to acknowledge the National Institute for Health Research who provided funding for the original review.
Appendices
Appendix 1. Record of searches 2011 to 2017
| Database | Search Date | Date range/Issue | Number of records | Limits applied |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | 7 July 2014 | 2014 Issue 6 | 177 | Year=2011‐2014 |
| 4 May 2016 | 2016 Issue 4 | 139 | Year= 2014‐2016 | |
| CENTRAL via Cochrane Register of Studies Online (CRSO) | 10 May 2017 | Current issue | 146 | Deduplicated with 2016 records |
| Ovid MEDLINE | 7 July 2014 | 1948 to June Week 4 2014 | 221 | ED=20110301‐20140707 |
| 4 May 2016 | 1946 to April Week 3 2016 | 142 | ED=20140623‐20160421 | |
| 8 May 2017 | 1946 to April Week 4 2017 | 98 | ED=20160421‐20170426 | |
| Embase Ovid | 7 July 2014 | 1980 to 2014 Week 26 | 312 | Year=2011‐current |
| 4 May 2016 | 1980 to 2016 Week 18 | 336 | Year= 2014‐current | |
| 8 May 2017 | 1980 to 2017 Week 19 | 123 | Year= 2016‐current | |
| CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature) | 8 July 2014 | 1937 to 8 July 2014 | 247 | EM=20110301‐ |
| 4 May 2016 | 1937 to 4 May 2016 | 98 | EM = 20140623‐ | |
| 10 May 2017 | 1937 to 10 May 2017 | 53 | EM = 20160501‐ | |
| PsycINFO Ovid | 7 July 2014 | 1967 to July Week 1 2014 | 36 | UP=20110321‐2014060707 |
| 4 May 2016 | 1967 to April Week 4 2016 | 30 | UP=2014630‐20160425 | |
| 10 May 2017 | 1967 to May Week 1 2017 | 9 | UP=20160425‐20170501 | |
| Science Citation Index Web of Science (SCI) | 8 July 2014 | 1970 to 4 July 2014 | 254 | Year=2011‐2014 |
| 4 May 2016 | 1970 to 3 May 2016 | 202 | Year =2014‐2016 | |
| 10 May 2017 | 1970 to 9 May 2017 | 119 | Year =2016‐2017 | |
| Conference Proceedings Index ‐ Science Web of Science (CPCI‐S) | 8 July 2014 | 1990 to 4 July 2014 | 2 | Year=2011‐2014 |
| 4 May 2016 | 1990 to 3 May 2016 | 5 | Year =2014‐2016 | |
| 10 May 2017 | 1990 to 9 May 2017 | 0 | Year =2016‐2017 | |
| PEDro (www.pedro.org.au) | 8 July 2014 | all available years | 21 | Records added since 21 March 2011 |
| 4 May 2016 | All available years | 9 | Records added since 1 July 2014 | |
| 10 May 2017 | All available years | 5 | Records added since 1 May 2016 | |
| LILACS (Latin American and Caribbean Health Service; lilacs.bvsalud.org/en) | 9 July 2014 | All available years | 41 | Year=2011‐2014 |
| 4 May 2016 | All available years | 28 | Year =2014‐2016 | |
| 10 May 2017 | All available years | 4 | Year =2016‐2017 | |
| ClinicalTrials.gov (clinicaltrials.gov) | 9 July 2014 | All available years | 35 | Records added since 1 March 2011 |
| 4 May 2016 | All available years | 30 | Records added since 1 July 2014 | |
| 10 May 2017 | All available years | |||
| World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/search/en) | 9 July 2014 | All available years | 15 | Records added since 1 March 2011 |
| 4 May 2016 | All available years | 9 | Records added since 1 July 2014 | |
| 10 May 2017 | All available years | 6 | Records added since 1 May 2016 | |
| CenterWatch (www.centerwatch.com) | 9 July 2014 | All available years | 0 | No limits |
| 4 May 2016 | All available years | 0 | No limits | |
| 10 May 2017 | Not searched after 2016 | 0 | No records found in previous searches | |
| metaRegister of Controlled Trials (mRCT) | 9 July 2014 | All available years | 6 | Deduplicated with 2011 records |
| 4 May 2016 | Not searched after 2014 | ‐ | Service no longer available | |
| 10 May 2017 | Not searched after 2014 | ‐ | Service no longer available | |
| Total number of records 2011 to 2017 | 2017 | 2395 + 622 | ||
Appendix 2. Search strategies (2011 to 2017)
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
#1 MeSH descriptor Physical Therapy Modalities, this term only #2 MeSH descriptor Physical Therapy (Specialty), this term only #3 physiotherap* or physio NEXT therap* or physical NEXT therap* #4 MeSH descriptor Exercise Therapy, this term only #5 treadmill* or tread‐mill* #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Motor Skills, this term only #8 MeSH descriptor Motor Skills Disorders, this term only #9 MeSH descriptor Psychomotor Disorders, this term only #10 MeSH descriptor Psychomotor Performance, this term only #11 MeSH descriptor Movement Disorders, this term only #12 MeSH descriptor Developmental Disabilities, this term only #13 ((motor or neuromotor or neuro‐motor or psychomotor or psycho motor or development*) NEAR/3 (impair* or skill* or disorder* or deficit* or delay* or disabilit* or dysfunc*)) #14 MeSH descriptor Walking explode tree 1 #15 MeSH descriptor Gait, this term only #16 MeSH descriptor Gait Disorders, Neurologic, this term only #17 MeSH descriptor Gait Ataxia, this term only #18 gait* #19 walk or walking #20 MeSH descriptor Locomotion, this term only #21 locomotor* or locomotion* #22 (ambulation or ambulatory or nonambulation or nonambulatory or non‐ambulation or non‐ambulatory) #23 stepping #24 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23) #25 MeSH descriptor Disabled Children, this term only #26 MeSH descriptor Down syndrome, this term only #27 MeSH descriptor Cerebral Palsy, this term only #28 MeSH descriptor Spinal Dysraphism, this term only #29 (down* NEXT syndrome or cerebral NEXT pals* or (spin* NEAR/3 injur*) or spina NEXT bifida) #30 MeSH descriptor Infant, Low Birth Weight explode all trees #31 MeSH descriptor Infant, Premature, this term only #32 low NEXT birth NEXT weight #33 preterm* or pre NEXT term* or prematur* #34 (#25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 or #33) #35 baby or babies or infant* or toddler* or child* or preschool* or pre‐school* or schoolchild* #36 MeSH descriptor Child explode all trees #37 MeSH descriptor Infant, this term only #38 (#35 OR #36 OR #37) #39 (#24 OR #34) #40 (#6 AND #38 AND #39)
CENTRAL via Cochrane Register of Studies Online (CRSO)
#1MESH DESCRIPTOR Physical Therapy Modalities #2MESH DESCRIPTOR Physical Therapy Specialty #3((physiotherap* or physio therap* or physical therap*)):TI,AB #4tread‐mill*:ti,ab #5treadmill*:ti,ab #6MESH DESCRIPTOR Exercise Therapy EXPLODE ALL TREES #7#1 OR #2 OR #3 OR #4 OR #5 OR #6 #8MESH DESCRIPTOR Motor Skills #9MESH DESCRIPTOR Motor Skills Disorders #10MESH DESCRIPTOR Psychomotor Disorders #11MESH DESCRIPTOR Psychomotor Performance #12MESH DESCRIPTOR Movement Disorders #13MESH DESCRIPTOR Developmental Disabilities #14(((motor or neuromotor or neuro‐motor or psychomotor or psycho motor or development*) adj3 (impair* or skill* or disorder* or deficit* or delay* or disabilit* or dysfunc*))):TI,AB #15(((impair* or skill* or disorder* or deficit* or delay* or disabilit* or dysfunc* ) adj3 (motor or neuromotor or neuro‐motor or psychomotor or psycho motor or development*))):TI,AB #16MESH DESCRIPTOR Walking EXPLODE ALL TREES #17MESH DESCRIPTOR Gait #18MESH DESCRIPTOR Gait Disorders, Neurologic #19MESH DESCRIPTOR Gait Ataxia #20gait:ti,ab #21MESH DESCRIPTOR locomotion #22(walk or walking):ti,ab #23(locomotor* or locomotion*):ti,ab #24(ambulation or ambulatory or nonambulation or nonambulatory or non‐ambulation or non‐ambulatory):ti,ab #25stepping:ti,ab485 #26#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 #27MESH DESCRIPTOR Disabled Children #28MESH DESCRIPTOR Down Syndrome #29MESH DESCRIPTOR Cerebral Palsy #30MESH DESCRIPTOR Spinal Dysraphism #31(down* syndrome or cerebral pals* or (spin* adj3 injur*) or (injur* adj3 spin*) or spina bifida):ti,ab #32MESH DESCRIPTOR Infant, Low Birth Weight EXPLODE ALL TREES #33MESH DESCRIPTOR Infant, Premature EXPLODE ALL TREES #34(low birth weight or pre‐term* or preterm* or prematur*):ti,ab #35#27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 #36#26 AND #35 #37(2016 or 2017):YR #38#36 AND #37
Ovid MEDLINE
1 Physical Therapy Modalities/ 2 "Physical Therapy (Specialty)"/ 3 (physiotherap$ or physio therap$ or physical therap$).tw. 4 Exercise Therapy/ 5 tread‐mill$.tw. 6 treadmill$.tw. 7 or/1‐6 8 Motor Skills/ 9 Motor Skills Disorders/ 10 Psychomotor Disorders/ 11 Psychomotor Performance/ 12 Movement Disorders/ 13 Developmental Disabilities/ 14 ((motor or neuromotor or neuro‐motor or psychomotor or psycho motor or development$) adj3 (impair$ or skill$ or disorder$ or deficit$ or delay$ or disabilit$ or dysfunc$)).tw. 15 exp Walking/ 16 Gait/ 17 Gait Disorders, Neurologic/ 18 Gait Ataxia/ 19 gait.tw. 20 locomotion/ 21 (walk or walking).tw. 22 (locomotor$ or locomotion$).tw. 23 (ambulation or ambulatory or nonambulation or nonambulatory or non‐ambulation or non‐ambulatory).tw. 24 stepping.tw. 25 or/8‐24 26 Disabled Children/ 27 down syndrome/ 28 cerebral palsy/ 29 spinal dysraphism/ 30 (down$ syndrome or cerebral pals$ or (spin$ adj3 injur$) or spina bifida).tw. 31 exp infant, low birth weight/ or infant, premature/ 32 (low birth weight or pre‐term$ or preterm$ or prematur$).tw. 33 or/26‐32 34 Infant/ 35 exp child/ 36 (baby or babies or infant$ or child$ or toddler$ or pre‐school$ or preschool$ or schoolchild$).tw. 37 34 or 35 or 36 38 randomised controlled trial.pt. 39 controlled clinical trial.pt. 40 randomi#ed.ab. 41 placebo$.ab. 42 drug therapy.fs. 43 randomly.ab. 44 trial.ab. 45 groups.ab. 46 or/38‐45 47 exp animals/ not humans.sh. 48 46 not 47 49 25 or 33 50 7 and 37 and 48 and 49
Embase Ovid
1 physiotherapy/ 2 pediatric physiotherapy/ 3 (physiotherap$ or physio therap$ or physical therap$).tw. 4 treadmill/ 5 tread‐mill$.tw. 6 treadmill.tw. 7 kinesiotherapy/ 8 or/1‐7 9 motor performance/ 10 psychomotor performance/ 11 motor dysfunction/ 12 developmental disorder/ 13 motor development/ 14 ((motor or neuromotor or neuro‐motor or psychomotor or psycho motor or development$) adj3 (impair$ or skill$ or disorder$ or deficit$ or delay$ or disabilit$ or dysfunc$)).tw. 15 locomotion/ 16 walking/ 17 gait/ 18 GAIT DISORDER/ 19 ataxia/ 20 gait.tw. 21 (walk or walking).tw. 22 (ambulation or ambulatory or nonambulation or nonambulatory or non‐ambulation or non‐ambulatory).tw. 23 (locomotor$ or locomotion$).tw. 24 stepping.tw. 25 handicapped child/ 26 Down syndrome/ (21539) 27 cerebral palsy/ (18656) 28 spina bifida/ (4734) 29 (down$ syndrome or cerebral pals$ or (spin$ adj3 injur$) or spina bifida).tw. 30 prematurity/ 31 exp low birth weight/ 32 (low birth weight or pre‐term$ or preterm$ or prematur$).tw. 33 or/9‐24 34 or/25‐32 35 or/33‐34 36 exp child/ 37 infant/ 38 (baby or babies or infant$ or child$ or toddler$ or pre‐school$ or preschool$ or schoolchild$).tw. 39 or/36‐38 40 Clinical trial/ 41 Randomized controlled trial/ 42 Randomization/ 43 Single blind procedure/ 44 Double blind procedure/ 45 Crossover procedure/ 46 Placebo/ 47 Randomi#ed.tw. 48 RCT.tw. 49 (random$ adj3 (allocat$ or assign$)).tw. 50 randomly.ab. 51 groups.ab. 52 trial.ab. 53 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 54 Placebo$.tw. 55 Prospective study/ 56 (crossover or cross‐over).tw. 57 prospective.tw. 58 or/40‐57 59 8 and 35 and 39 and 58
CINAHLPlus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S50 S31 and S34 and S49 S49 S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 S48 TI (evaluat* study or evaluat* research) or AB (evaluate* study or evaluat* research) or TI (effectiv* study or effectiv* research) or AB(effectiv* study or effectiv* research) OR TI (prospectiv* study or prospectiv* research) or AB(prospectiv* study or prospectiv* research) orTI (follow‐up study or follow‐up research) or AB (follow‐up study or follow‐up research) S47 "cross over*" S46 crossover* S45 (MH "Crossover Design") S44 (tripl* N3 mask*) or (tripl* N3 blind*) S43 (trebl* N3 mask*) or (trebl* N3 blind S42 (doubl* N3 mask*) or (doubl* N3 blind S41 (singl* N3 mask*) or (singl* N3 blind S40 (clinic* N3 trial*) or (control* N3 trial*) S39 (random* N3 allocat* ) or (random* N3 assign*) S38 randomis* or randomiz* S37 (MH "Meta Analysis") S36 (MH "Clinical Trials+") S35 MH random assignment S34 S32 or S33 S33 TI(baby or babies or infant* or child* or toddler* or pre‐school* or preschool* or schoolchild*) or AB(baby or babies or infant* or child* or toddler* or pre‐school* or preschool* or schoolchild*) S32 (MH "Child") OR (MH "Infant") OR (MH "Child, Preschool S31 S29 or S30 S30 S6 and S28 S29 S6 and S19 S28 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 S27 TI(low birth weight or pre‐term* or preterm* or prematur*) or AB(low birth weight or pre‐term* or preterm* or prematur*) S26 (MH "Infant, Low Birth Weight+") S25 (MH "Infant, Premature") S24 TI (down* syndrome or cerebral pals* or (spin* N3 injur*) or spina bifida) or AB (down* syndrome or cerebral pals* or (spin* N3 injur*) or spina bifida) S23 (MH "Down syndrome") S22 (MH "Spina Bifida") S21 (MH "Cerebral Palsy") S20 (MH "Child, Disabled") S19 S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S18 AB((motor or neuromotor or neuro‐motor or psychomotor or psycho motor or development*) and (impair* or skill* or disorder* or deficit* or delay* or disabilit* or dysfunc*)) S17 TI((motor or neuromotor or neuro‐motor or psychomotor or psycho motor or development*) and (impair* or skill* or disorder* or deficit* or delay* or disabilit* or dysfunc*)) S16 TI(ambulation or ambulatory or nonambulation or nonambulatory or non‐ambulation or non‐ambulatory) or AB(ambulation or ambulatory or nonambulation or nonambulatory or non‐ambulation or non‐ambulatory) S15 TI(gait* or locomotor* or locomotion* or step or stepping or walk* or walking) or AB(gait* or locomotor* or locomotion* or step or stepping or walk* or walking) S14 (MH "Locomotion") S13 (MH "Gait") OR (MH "Gait Disorders, Neurologic") OR (MH "Gait Apraxia") OR (MH "Step") S12 (MH "Walking") S11 (MH "Infant Development Disorders") S10 (MH "Child Development Disorders") S9 (MH "Developmental Disabilities S8 (MH "Psychomotor Disorders") S7 (MH "Motor Skills") OR (MH "Motor Skills Disorders") OR (MH "Psychomotor Performance") S6 S1 or S2 or S3 or S4 or S5 S5 TI(physiotherap* or physio therap* or physical therap*) or AB(physiotherap* or physio therap* or physical therap*) S4 TI (treadmill* or tread‐mill*) or AB(treadmill* or tread‐mill*) S3 TI (treadmill* or tread‐mill*) or AB(treadmill* or tread‐mill*) S2 (MH "Treadmills") S1 (MH "Physical Therapy") OR (MH "Gait Training") OR (MH "Pediatric Physical Therapy") OR (MH "Therapeutic Exercise")
PsycINFO OVID
1. physical therapy/ 2. (physiotherap$ or physio therap$ or physical therap$).tw. 3. (treadmill$ or tread‐mill$).tw. 4. or/1‐3 5. Psychomotor Development/ 6. Motor Development/ 7. Motor Performance/ 8. Motor Coordination/ 9. Walking/ 10. Motor Skills/ 11. Locomotion/ 12. (gait$ or locomotor$ or locomotion$ or step or stepping or walk$).tw. 13. (ambulation or ambulatory or nonambulation or nonambulatory or non‐ambulation or non‐ambulatory).tw. 14. ((motor or neuromotor or neuro‐motor or psychomotor or psycho motor or development$) adj3 (impair$ or skill$ or disorder$ or deficit$ or delay$ or disabilit$ or dysfunct$)).tw. 15. developmental disabilities/ 16. cerebral palsy/ 17. Down's Syndrome/ 18. Spina Bifida/ 19. (down$ syndrome or cerebral pals$ or (spin$ adj3 injur$) or spina bifida).tw. 20. premature birth/ 21. birth weight/ 22. (low birth weight or pre‐term$ or preterm$ or prematur$).tw. 23. or/5‐22 24. (baby or babies or infant$ or child$ or toddler$ or pre‐school$ or preschool$ or schoolchild$).tw. 25. (infancy 2 23 mo or preschool age 2 5 yrs).ag. 26. 24 or 25 27. 4 and 23 and 26 28. clinical trials/ 29. (randomis$ or randomiz$).tw. 30. (random$ adj3 (allocat$ or assign$)).tw. 31. ((clinic$ or control$) adj trial$).tw. 32. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 33. (crossover$ or "cross over$").tw. 34. random sampling/ 35. Experiment Controls/ 36. Placebo/ 37. placebo$.tw. 38. exp program evaluation/ 39. treatment effectiveness evaluation/ 40. ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. 41. exp experimental methods/ 42. or/28‐41 43. 27 and 42
Science Citation Index Web of Science (SCI)
#13 #12 AND #11 #12 TS=(random* or trial* or intervention* ) #11 #10 AND #9 AND #3 #10 TS=(baby or babies or infant* or child* or toddler* or pre‐school* or preschool* or schoolchild*) #9 #8 OR #7 OR #6 OR #5 OR #4 #8 TS=(low birth weight or pre‐term* or preterm* or prematur*) #7 TS=(down* syndrome or cerebral pals* or spin* injur* or spina bifida) #6 TS=((motor or neuromotor or neuro‐motor or psychomotor or psycho‐motor or development*) SAME (impair* or skill* or disorder* or deficit* or delay* or disabilit* or dysfunc*) ) #5 TS=(ambulation or ambulatory or nonambulation or nonambulatory or non‐ambulation or non‐ambulatory) #4 TS=(gait* or locomotor* or locomotion* or step or stepping or walk* or walking) #3 #2 OR #1 #2 TS=(treadmill* or tread mill*) #1 TS=(physical therap* or physiotherap* or physio therap*)
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S)
#13 #12 AND #11 #12 TS=(random* or trial* or intervention* ) #11 #10 AND #9 AND #3 #10 TS=(baby or babies or infant* or child* or toddler* or pre‐school* or preschool* or schoolchild*) #9 #8 OR #7 OR #6 OR #5 OR #4 #8 TS=(low birth weight or pre‐term* or preterm* or prematur*) #7 TS=(down* syndrome or cerebral pals* or spin* injur* or spina bifida) #6 TS=((motor or neuromotor or neuro‐motor or psychomotor or psycho‐motor or development*) SAME (impair* or skill* or disorder* or deficit* or delay* or disabilit* or dysfunc*) ) #5 TS=(ambulation or ambulatory or nonambulation or nonambulatory or non‐ambulation or non‐ambulatory) #4 TS=(gait* or locomotor* or locomotion* or step or stepping or walk* or walking) #3 #2 OR #1 #2 TS=(treadmill* or tread mill*) #1 TS=(physical therap* or physiotherap* or physio therap*)
PEDro
Using Advanced Search
Abstract &Title| child* treadmill* (with Match all search terms (AND) selected)
LILACS (Latin American and Caribbean Health Sciences Literature)
(bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=A)
("WALKing" or "GAIT" or "GAIT ataxia" or "GAIT disorders, neurologic" or gait$ or walk or walking or "DOWN SYNDROME" or "CEREBRAL PALSY" or "SPINA BIFIDA" or "infant, LOW BIRTH WEIGHT" or "infant, extremely LOW BIRTH WEIGHT" or "infant, very LOW BIRTH WEIGHT" or "infant, PREMATURE" or "MOTOR SKILLS" or "MOTOR SKILLS disorders" or "PSYCHOMOTOR disorders" or "PSYCHOMOTOR performance" or "LOCOMOTION" or step or stepping or ambulation or ambulatory or neuromotor or neuro‐motor [Words] ) and ( "PHYSIOTHERAPY (specialty)" OR "PHYSIOTHERAPY (techniques)" or "PHYSICAL THERAPY (specialty)" or "PHYSICAL THERAPY modalities" or physiotherap$ or treadmill$ or tread‐mill$ [Words] ) and (baby or babies or toddler$ or infant$ or child$ or preschool$ or pre‐school$ or schoolchild$ or "INFANT" or "CHILD, preschool" or "CHILD" [Words])
ClinicalTrials.gov
(clinicaltrials.gov)
Using Advanced Search
Search Terms| treadmill AND Study Type| Interventional Studies AND Age Group| Child (birth to 17)
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)
Using Advanced search: Intervention| Treadmill AND limit by Search for clinical trials in children AND Recruitment status = all
CenterWatch
treadmill limited to Clinical trial Listings
metaRegister of Controlled Trials (mRCT)
treadmill and children.
Appendix 3. Table of unused methods
| Measures of treatment effect |
Continuous data If the same continuous outcome (for example, infant's gross motor development level) is measured differently across studies, we will compare standardised mean differences (SMD) with 95% CI across studies (Deeks 2011). Where necessary, we will use formulas to convert F ratios, t‐values and Chi² values into SMDs (Higgins 2011b; Lipsey 2001), using Hedges g to correct for small sample bias. |
|
Dichotomous data We will analyse the outcomes of any study reporting binary/dichotomous data by calculation of the risk ratio (RR) for the occurrence of an event (rather than a non‐event) for its consistency as a summary statistic and ease of interpretation. | |
| Unit of analysis issues | We will take into account the unit of analysis and determine whether: (1) individuals were randomised in groups (i.e. cluster‐randomised trials); (2) results were reported at multiple time points; and (3) individuals simultaneously received multiple interventions. Cluster‐randomised trials For trials that use clustered randomisation, we will present results with proper controls for clustering (robust standard errors or hierarchical linear model). If appropriate controls are not used and it is not possible to obtain the full set of each individual participant's data, we will control the data for clustering using the procedures outlined by Higgins 2011c. For dichotomous outcome measures, we will divide the number of events and the number of participants per trial arm by the design effect (1 + (1 ‐ m)*r), where m is the average cluster size andr is the intracluster correlation coefficient (ICC). For continuous outcome measures, we will divide the number of participants per trial arm by the design effect, with the mean values unchanged. To determine the ICC, we will use estimates in the primary trials on a study‐by‐study basis. In the case of these values not being reported, we will use external estimates of the ICC that are appropriate to the context of each trial and average cluster size. If they were still not available, we will then use statistical procedures outlined by Higgins 2011c. Multiple time points When the results are measured at multiple time points, we will only consider baseline measurements and the last time point measurements. Multiple interventions per individual If it is found that participants in some trials receive multiple treatments, we will conduct meta‐analysis on those studies separately. |
| Dealing with missing data | For dichotomous data, we will report the missing data and dropouts for included studies along with the number of participants who are included in the final analyses as a proportion of all participants in each study. We will provide reasons for missing data in a narrative summary. The extent to which the results of the review could be altered by the missing data can be assessed based on consideration of best‐case and worst‐case scenarios (Gamble 2005). The best‐case scenario is the one where all participants with missing outcomes in the experimental condition had good outcomes and all those with the missing outcomes in the control condition had poor outcomes, and the worst‐case scenario is vice versa (Higgins 2011c). However, as the best‐case and worst‐case scenarios method is too extreme, a more plausible approach is needed. We will use the method suggested by Higgins 2011c, which can incorporate specific reasons for missing data and considers plausible event risks among missing participants in relation to risks among those observed. |
| Assessment of heterogeneity | We will describe statistical heterogeneity using I² (Deeks 2011), a quantity that describes approximately the proportion of variation in point estimates that is due to heterogeneity rather than sampling error). In addition, we will employ a chi² test of homogeneity to determine the strength of evidence that heterogeneity is genuine. If an individual study appears to be an outlier, we may carry out a sensitivity analysis with and without the study. If the primary studies are judged to be substantially heterogeneous (i.e.I² > 50%, Deeks 2011 ) even within these subgroups, we will only give a descriptive analysis, particularly if there is variation in direction of effect. |
| Assessment of reporting biases | In order to investigate the relationship between effect size and standard error, we will draw funnel plots if sufficient studies are available (that is, 10 or more individual studies). Asymmetry could be attributable to publication bias, but might also reflect a real relationship between trial size and effect size. If we find such a relationship, we will examine clinical variation of the studies (Sterne 2011). As a direct test for publication bias, we will compare results extracted from published journal reports with results obtained from other sources, including correspondence. |
| Data synthesis | For dichotomous outcomes, we will also calculate the number needed to treat for an additional beneficial outcome. |
| Subgroup analysis | We will undertake subgroup analysis if clinically different interventions are identified or there are clinically relevant differences between participant groups. We will thus investigate any subgroup differences in order to establish whether there is a single intervention effect, specifically:
|
| Sensitivity analysis | We will conduct sensitivity analysis, where data permit, to determine whether findings are sensitive to restricting inclusion to studies judged to be at low risk of bias. In these analyses, we will re‐evaluate the findings, limiting the inclusion to published studies or to those studies that have a low risk of:
|
Data and analyses
Comparison 1. Treadmill versus no treadmill.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Step frequency (16 months): Risk of developmental delay (% alternate steps) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Step quality (11 months): Risk of developmental delay (% toe contact) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Step quality (16 months): Risk of developmental delay [% toe contact] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Age of onset of independent walking | 2 | 58 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐5.38, 1.22] |
| 4.1 Risk of developmental delay | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.34, 1.14] |
| 4.2 Down syndrome | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐6.96, ‐1.04] |
| 5 Age of onset of walking with assistance (days in study) | 2 | 58 | Mean Difference (IV, Random, 95% CI) | ‐38.54 [‐106.13, 29.05] |
| 5.1 Down syndrome | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐74.0 [‐135.40, ‐12.60] |
| 5.2 Risk of developmental delay | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐62.11, 52.11] |
| 6 Gross motor function measure (GMFM) | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 0.88 [‐4.54, 6.30] |
| 6.1 Spastic cerebral palsy | 1 | 8 | Mean Difference (IV, Random, 95% CI) | 7.60 [‐19.46, 34.66] |
| 6.2 Risk of developmental delay | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐4.93, 6.13] |
| 7 Gross motor function related to standing (GMFM) ‐ Dimension D | 2 | 32 | Mean Difference (IV, Random, 95% CI) | 5.41 [‐1.61, 12.43] |
| 7.1 Spastic cerebral palsy | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 11.57 [0.05, 23.09] |
| 7.2 Developmental delay | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 3.33 [1.43, 5.23] |
| 8 Gross motor function related to walking, running and jumping (GMFM) ‐ Dimension E | 2 | 32 | Mean Difference (IV, Random, 95% CI) | 4.51 [0.29, 8.73] |
| 8.1 Spastic cerebral palsy | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 3.01 [‐1.11, 7.13] |
| 8.2 Developmental delay | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 7.60 [0.88, 14.32] |
| 9 Peabody Developmental Motor Scales ‐ 2: Spastic cerebral palsy | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Pediatric Evaluation of Disability Inventory ‐ Mobility Scale scores: Spastic cerebral palsy | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Other gait parameters ‐ velocity | 2 | 32 | Mean Difference (IV, Random, 95% CI) | 0.23 [0.08, 0.37] |
| 11.1 Spastic cerebral palsy | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.09, 0.45] |
| 11.2 Developmental delay | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.25 [0.08, 0.42] |
| 12 Other gait parameters ‐ velocity (follow‐up when walking independently): Risk of developmental delay | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Other gait parameters ‐ step length: Spastic cerebral palsy | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14 Other gait parameters ‐ step length (follow‐up when walking independently): Risk of developmental delay | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15 Other gait parameters ‐ gait double‐limb support: Spastic cerebral palsy | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16 Other gait parameters ‐ gait double‐limb support (follow‐up when walking independently): Risk of developmental delay | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Treadmill without orthoses versus treadmill with orthoses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Walking independently (1‐month follow‐up): Down syndrome | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Gross motor function (GMFM 1‐month follow‐up): Down syndrome | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. High‐intensity treadmill versus low‐intensity treadmill.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Angulo‐Barroso 2013.
| Methods | Randomised controlled trial | |
| Participants |
Number randomised: 41 infants with moderate risk for neuromotor disabilities were initially randomised (25 intervention, 16 control). Number analysed: 28 were analysed (13 control: 9 male, 4 female; 15 intervention: 9 male, 6 female). Dropouts/withdrawals: 10 intervention (6 did not follow protocol, 3 voluntarily withdrew, 1 diagnosed with genetic disorder), 3 control (1 unable to schedule data collection, 1 diagnosed with genetic disorder, 1 received Botox injections on multiple occasions). All participants entered the study when they were able to take 10 steps on the treadmill in 1 minute (minimum age: 6 months; maximum age: 13 months, to guarantee minimum length of TM training). Of the included infants, 18 were low‐birth‐weight (< 1500 g), 21 had low gestational age (< 32 weeks), 22 had a brain insult, 15 received prolonged ventilator use, 11 were from multiple births. Mean age (SD): control 9.0 (1.4) months; intervention 9.7 (1.3) months. Ethnicity: no information available. |
|
| Interventions |
Control:
Intervention:
|
|
| Outcomes |
From unpublished data obtained from Chen 2008:
|
|
| Notes |
Country: USA. Funding source: This work was funded by a research grant from the U.S. Office of Special Education & Rehabilitative Services (H324C040016) awarded to the first author. Comment: This study was initially recorded as Chen 2008 since, at the time of the original review, data were unpublished but obtained from the author. For the update, the trial was published and, at this point, for Angulo‐Barroso 2013, we included unpublished data from Chen and published data on the actual paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Eligible participants were randomised to either treadmill training group or the control group by a statistician using a computer programme for group allocation, considering 3 stratification factors: age, gender, and birth weight. All participants were assigned an ID, which was entered into the computer by a statistician to conduct the participant's allocation. |
| Allocation concealment (selection bias) | Low risk | Comment: The information (see support for judgement above) was provided to the project coordinator and home assessment personnel but maintained the laboratory assessors blind to group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: The laboratory assessors were blind to group allocation. |
| Incomplete outcome data (attrition bias) Experimental group 1 | Unclear risk |
Treadmill training:
Control:
|
| Selective reporting (reporting bias) | Low risk | Comment: No evidence of reporting bias. |
| Other bias | Unclear risk | Comment: None noted. |
Cherng 2007.
| Methods | Randomised controlled trial (cross‐over design: AAB, ABA). | |
| Participants |
Number randomised: 20 children were screened and 12 children met the inclusion criteria, but only 8 children joined the study program; they were control and crossed over to intervention. They all had a diagnosis of spastic cerebral palsy (two females and six males). Number analysed: 8 children (2 female, 6 male). Dropouts/withdrawals: none. Mean age (SD): not reported; age range from 3.5 to 6.3 years. Ethnicity: not reported. |
|
| Interventions |
Control (A):
Intervention (B):
|
|
| Outcomes |
|
|
| Notes |
Country: Taiwan. Funding source: This study was supported by NSC 92‐2218‐E‐006‐003 and through a collaboration of National Cheng Kung University and Chi Mei Medical Center. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The children were divided equally into 2 groups and randomly assigned to the schedules. |
| Allocation concealment (selection bias) | High risk | Comment: Cross‐sectional trial. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: One independent therapist, who was not aware of any child's grouping or stage within the study, took all the measurements on gait parameters. |
| Incomplete outcome data (attrition bias) Experimental group 1 | Low risk |
Regular therapeutic treatment
Treadmill training
|
| Selective reporting (reporting bias) | High risk |
Quote: "Outcomes measures included muscle tone..." Comment: no data about muscle tone provided. |
| Other bias | Unclear risk | Comment: We did not have enough information to make a judgement. |
Looper 2010.
| Methods | Quasi‐randomised controlled trial. | |
| Participants |
Number randomised: 22 infants with Down syndrome randomised (10 intervention; 12 control). Number of males and females not reported. Number analysed: 22 infants. Dropouts/withdrawals: five infants discontinued the intervention in the control group. Mean age (SD): 21.4 (4.0) months. Ethnicity: not reported. |
|
| Interventions |
Control group:
Intervention:
|
|
| Outcomes |
|
|
| Notes |
Country: USA. Funding source: Funds provided by the Foundation for Physical Therapy PODS II awards to Dr Looper, a grant from the Michigan Physical Therapy Association, and a grant from the Rackham Graduate School, University of Michigan. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The participants were randomly assigned to groups based on a random list of 1 (treadmill) and 2 (treadmill plus orthoses) from random.org. The first participant who entered the study (convenience sample) was assigned to the first number on the list, the second participant to the second number, the third to the third, etc. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided as regards how the allocation process took place. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Neither personnel nor participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: No blinding. |
| Incomplete outcome data (attrition bias) Experimental group 1 | High risk |
Orthosis and treadmill training:
Treadmill training alone:
|
| Selective reporting (reporting bias) | High risk | Comment: Anthropometric measurements were taken at each monthly visit, and treadmill training was videotaped. No information on either measurement or video assessment was reported. Also, age of onset of independent walking was not directly reported and the authors provided information about study duration only. |
| Other bias | Unclear risk | Comment: We did not have enough information to make a judgement. |
Lowe 2015.
| Methods | Randomised controlled trial. | |
| Participants |
Number randomised: 24 infants with developmental delay met the inclusion criteria and were randomised (12 intervention; 12 control). Number of males and females not reported at this point. Number analysed: 21 infants (12 intervention, 9 males and 3 females; 9 control, 8 males, 1 female). Dropouts/withdrawals: three infants discontinued the intervention in the control group. Mean age (SD): not reported; age ranged from 2 to 5 years (participants were aged 26 to 51 months in intervention group; participants were aged 27 to 48 months in control group). Ethnicity: intervention group: 58.33% white, 25% black, 16.67% other; Control group: 90% white, 0% black, 10% other. |
|
| Interventions |
Control:
Intervention:
|
|
| Outcomes |
|
|
| Notes |
Country: USA. Funding source: Grant support: NIGMS IDEA Program award P30 GM110702. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Participants were randomised to the control or treatment group using a computer‐generated randomisation chart. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: The testers established inter‐rater reliability: ICC more than 0.90 for each test. |
| Incomplete outcome data (attrition bias) Experimental group 1 | Low risk |
Treadmill training:
Control:
|
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of reporting bias. |
| Other bias | Unclear risk | Comment: none noted. |
Mattern‐Baxter 2013.
| Methods | Controlled clinical trial. | |
| Participants |
Number randomised: 15 children with diagnosis of cerebral palsy (GMFCS levels I and II), who were able to sit for at least 30 seconds unsupported and demonstrated the ability to take 10 consecutive steps when held on hands or torso, were included. 12 children completed the study. Number analysed: 12 children were quasi‐randomised and matched by GMFCS levels and age. 6 intervention (3 boys, 3 girls), 6 control (5 boys, 1 girl). Dropouts/withdrawals: 3 (1 child became ill and had to be hospitalised, 1 child dropped out because family reasons, 1 child received genetic diagnosis therefore had to be excluded). Mean age (SD): intervention 21.7 (6.5, range 15.5 to 32) months, control 21.3 (6.07, range 13.5 to 30.5) months. Ethnicity: 2 African‐American, 2 Asian, 2 Hispanic, 6 white. |
|
| Interventions |
Control:
Intervention
|
|
| Outcomes |
|
|
| Notes |
Country: USA. Funding source: Supported by a paediatric section research grant of the American Physical Therapy Association (grant number: 527109). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Explained in CONSORT diagram, but no information in the text as regards how the randomisation took place. |
| Allocation concealment (selection bias) | Unclear risk | Comment: The children were quasi‐randomised by the principal investigators and matched by GMFCS levels and age. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No blinding. All participants aware of group allocation. No blinding of personnel, but blinding for some outcome measures. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Blinding for the GMFM‐66 and PDMS‐2 was achieved by videotaping the children's gross motor skills in their homes. The videotapes were subsequently reviewed by a physical therapist who was blinded to group allocation. |
| Incomplete outcome data (attrition bias) Experimental group 1 | Low risk | Comment: All outcomes were reported. |
| Selective reporting (reporting bias) | Low risk | Comment: No evidence of reporting bias. |
| Other bias | Unclear risk | Comment: None noted. |
Ulrich 2001.
| Methods | Randomised controlled trial. | |
| Participants |
Number randomised: 32 infants with Down syndrome, randomised into 2 groups (16 intervention; 16 control); total number of males and females was not provided. Enrolled when able to sit for 30 seconds. Number analysed: 30 (15 intervention (no breakdown by sex was provided for this group), 15 control (8 male, 7 female)). Dropouts/withdrawals: 2 infants discontinued the intervention (one in each group) and 2 more were lost to gait follow‐up (one in each group), as reported in Wu 2007. Any discrepancies in the paper were resolved through oral discussion between MV and RA who was one of the authors involved in both this study and in Ulrich 2008, and who was also a review author. Average age at entry: mean 10.1 months (SD 1.94). Mean age (SD): control 10.2 (2.2) months, intervention 9.9 (1.7) months. Ethnicity: 2 mixed race; remaining participants were white. |
|
| Interventions |
Control:
Intervention:
|
|
| Outcomes |
|
|
| Notes |
Country: USA (Indiana, Tennesse, Ohio). Funding sources: Grants from the National Institute for Disability and Rehabilitation Research and from the March of Dimes Birth Defects Foundation. Other comments: The control group from this study was also used in another paper (Wu 2007) that relates to Ulrich 2008. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Infants were randomised into two groups. In addition, Wu 2007 reported on the use of a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk |
Comment: We obtained the following information from another publication of the same study (Wu 2007): Quote: "The randomisation procedure was conducted by the fourth investigator for the two cohorts separately via a table of random numbers". Comment: This means that each randomisation was conducted separately with the involvement of only the 4th author and with the use of a table of random numbers. This does not give us enough information to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Neither participants nor personnel were blinded. Infants in the treadmill intervention group had treadmills placed in their homes. Parents were trained to implement the training. A team of researchers visited all participants bi‐weekly throughout the study. Infants were videoed on the treadmill and their growth was assessed and parents maintained a log book that was read by a research staff member during each visit. Parents were asked to include information regarding the dates and length of their paediatric physical therapy sessions, the general activities that the therapist prescribed for parent implementation, and an estimate of the amount of time the parent spent implementing physical therapy activities at home. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Assessors were aware of infant's group assignment. |
| Incomplete outcome data (attrition bias) Experimental group 1 | Low risk | Comment: Treadmill training. One dropout was not reported in this paper but was reported in Wu 2007 (the same control group was used for this paper). |
| Selective reporting (reporting bias) | High risk | Comment: Not all outcomes were reported. |
| Other bias | Unclear risk |
Comment: All parents were asked to keep a log book, including information regarding treadmill training (for those in the experimental group) and any other relevant information regarding the infant's health state and daily activities, including any therapeutic session administered other than treadmill training. Quote: "Given that there were no group differences on the 11 anthropometric measures at entry, it appears that randomisation process resulted in producing comparable treatment groups". |
Ulrich 2008.
| Methods | Randomised controlled trial. | |
| Participants |
Number randomised: 36 infants with Down syndrome were randomised into two groups: low‐intensity (15 children) and high‐intensity (19 children). There were another two infants with unknown initial group allocation who withdrew from the study for emerging medical conditions. All participants were included when they were able to take 6 steps per minute on a treadmill while being supported. Number analysed: 30 children were analysed in the final sample (16 high‐intensity training (12 males, 4 females), 14 low‐intensity training (6 males, 8 females); (28 with trisomy 21; two with mosaic type). Dropouts/withdrawals: 6 infants discontinued the intervention (4 low‐intensity, 2 high‐intensity). An additional 5 infants were lost to gait follow‐up (2 low‐intensity, 3 high‐intensity). Any discrepancies in the paper were resolved through oral discussion between MV and RA who was one of the authors involved in both Ulrich 2001 and this study, and who was also a review author. Corrected age at entry; mean (SD): higher‐intensity group 9.65 (1.61) months, lower‐intensity group 10.40 (2.14) months. Ethnicity: 2 African American, 2 bi‐racial, and remaining infants were white. |
|
| Interventions |
Control group (low‐intensity treadmill training):
Experimental group (high‐intensity treadmill training):
Four additional publications (Wu 2007; Angulo‐Barroso 2008; Wu 2008; Wu 2010) dealt with the follow‐up from this intervention including assessments from 1 to 15 months postwalking onset (i.e. after termination of the intervention). |
|
| Outcomes | The study reported frequency of alternating treadmill steps and onset of assisted and independent walking. The follow‐up publications reported on spatio‐temporal variables, joint kinematics, and gait adaptation parameters. In addition, Wu 2007 presented follow‐up on spatio‐temporal gait variables, including a historical control group from Ulrich 2001 (we did not use these data as the study was not randomised). Publication Wu 2007
Publication Angulo‐Barroso 2008
Publication Wu 2008
Publication Wu 2010
|
|
| Notes |
Country: USA (Michigan, Ohio, Indiana). Funding sources: Research grant from the US Office of Special Education and Rehabilitative Services (H324C010067), a US Office of Special Education Programs Leadership Training Grant (H325D020028), and the Steelcase Foundation in Michigan. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Used a random numbers table to assign to either low‐intensity training group or high‐intensity training group (described in Wu 2007). |
| Allocation concealment (selection bias) | Unclear risk |
Comment: We obtained the following information from another publication of the same study (Wu 2007): Quote: "The randomisation procedure was conducted by the fourth investigator for the two cohorts separately via a table of random numbers." Comment: This means that each randomisation was conducted separately with the involvement of only the 4th author and with the use of a table of random numbers. This did not give us enough information to make a judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) Experimental group 1 | Low risk |
High‐intensity treadmill training:
Low‐intensity treadmill training:
|
| Selective reporting (reporting bias) | Unclear risk | Comment: It is not clear if all data are reported. |
| Other bias | Unclear risk | Comment: We did not have enough information to make a judgement. |
BWSTT: Body weight supported treadmill training. CONSORT: Consolidated Standards of Reporting Trials. FMS: Functional Mobility Scale. Gait‐Keeper: Light treadmill used for gait training. GMFCS: Gross Motor Function Classification System. GMFM: Gross Motor Function Measure. GMFM‐66: 66‐item Gross Motor Function Measure. ICC: Interclass coefficient. LiteGait: Gait training device that simultaneously controls weight bearing, posture, and balance over a treadmill. NDT: Neurodevelopmental Treatment. PDMS‐2: Peabody Developmental Motor Scales, Second Edition. PEDI: Pediatric Evaluation of Disability Inventory. SD: Standard deviation. SMO: Supra malleolar orthosis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Borggraefe 2007 | The participants were older children. |
| Borggraefe 2010 | The participants were older children. There was no control group. |
| Campbell 2012 | Treadmill used simultaneously with kicking exercises in the experimental group. Outcome measures were motor development only, none included the outcome measures that are the focus of this review. |
| Christensen 2014 | Case report. |
| Dodd 2007 | The participants were older children. |
| Duarte 2014 | None of the included outcome measures were the focus of this review. In addition, most children were older. |
| El‐Shamy 2017 | The participants were older children. |
| Grecco 2013a | None of the outcome measures were the same as in this review. This report assessed only functional balance. Most of the participants were older. |
| Grecco 2013b | Outcome measures were not relevant to the review. They evaluated stabilometry. |
| Grecco 2013c | Most of the participants were older (mean age 6 to 6.8 years old). |
| Hilderley 2016 | The participants were older children. |
| Johnston 2011 | The participants were older children. |
| Jung 2016 | Treadmill not used for training but to describe biomechanics of walking and compare parameters to overground walking. |
| Kurz 2011 | The participants were older children. |
| Lowe 2013 | Case series (part of a dissertation). |
| Maltais 2003 | The participants were older children. |
| Matsuno 2010 | The participants were older children. |
| Meyer‐Heim 2007 | The participants were older children. |
| Mussleman 2007 | No training with the treadmill, it was used for investigation purposes. |
| Pang 2003 | No training with the treadmill, it was used for investigation purposes. |
| Pantall 2011 | No control group. |
| Phillips 2007 | The participants were older children. |
| Romei 2012 | The participants were older children. |
| Sarhan 2014 | They did not use an outcome measure to look for motor skills other than balance. The parameters tested were not exactly in line with our main outcomes: age of independent walking and gross motor function. These authors really asked an equipment question, not a developmental/functional question. |
| Schindl 2000 | The participants were older children. |
| Schlittler 2011 | Allocation to groups not random nor quasi‐random. |
| Scholtes 2012 | The participants were older children. |
| Schroeder 2014 | Observational study. No control group. |
| Sherief 2015 | The participants were older children. |
| Siekerman 2015 | Report where all infants were placed on the treadmill. |
| Smania 2011 | The participants were older children. |
| Su 2013 | The participants were older children. |
| Teulier 2009 | No training with the treadmill, it was used for investigation purposes. |
| Willerslev‐Olsen 2014 | No control group. |
Characteristics of ongoing studies [ordered by study ID]
NCT02424526.
| Trial name or title | NCT02424526 Official title: Intensive Home‐based Treadmill Training and Walking Attainment in Young Children With Cerebral Palsy |
| Methods | Randomised controlled trial |
| Participants | Number to recruit: 24 infants (12 per group) aged between 1 and 3 years old, who show signs of walking readiness as demonstrated by the ability to sit for 30 seconds when placed and to take 5 to 7 steps when supported at the trunk or arms; and who show bilateral impairment (i.e. diplegia and quadriplegia, but not hemiplegia), who demonstrate upper motor neuron signs (i.e. spasticity and/or hyperreflexia), and who have been identified as high‐risk for a motor disability by a physician. |
| Interventions |
Control group (low‐intensity treadmill training): Home‐based treadmill training
Experimental group (high‐intensity treadmill training): Home‐based treadmill training
The children will be assessed before, immediately after, at 1‐month and at 4‐months following the intervention via standardized outcome measures. |
| Outcomes |
|
| Starting date |
Start date: July 2015 Estimated end date: June 2018 |
| Contact information |
Contact 1 Name: Katrin Mattern‐Baxter, PT, DPT, PCS Telephone: 916‐278‐5766. Email:kbaxter@csus.edu Contact 2 Name: Leah Vargas Email: leah.vargas@csus.edu |
| Notes | Country: USA |
Differences between protocol and review
Title: we have deleted 'with partial body weight support' from the title since it is not mentioned in our inclusion criteria (Criteria for considering studies for this review) or anywhere else in the review.
Background: minor modifications to ensure that references were up‐to‐date.
Objectives: the objectives of the updated version have been broaden by adding "delayed ambulation" children to the rest of the included population, and by specifying that we are looking at the effectiveness of treadmill interventions on locomotor development.
Types of participants: we specified that we excluded studies that included children both older and younger than six years of age.
-
under primary outcomes, for clarity, we defined 'step frequency' and replaced 'walking with assistive devices' with 'walking with assistance';
we added 'gait parameters' to our list of secondary outcomes, as we had assumed this under 'gait pattern functions' but not explicitly expressed it; and
we specified that we excluded studies on the basis of outcome measures that were not the focus of our review.
Electronic searches: we did not search for dissertations in WorldCat.
Data collection and analysis: we removed any methods not used due to type or amount of data and placed these in Appendix 3.
Assessment of risk of bias in included studies: since the publication of our protocol (Valentin‐Gudiol 2011p), the 'blinding' domain has been split into two (blinding of participants and personnel; blinding of outcome assessment). Also, we did not contact the authors for additional information when the risk of bias was unclear.
Measures of treatment effect: none of the continuous outcomes were measured differently across studies, and therefore we did not compute standardised mean differences (SMD) with 95% CIs.
Data synthesis: we used Review Manager 2014 (the latest version of Cochrane's meta‐analysis software) to synthesise the data, instead of the 2011 version. Similarly, we used the random‐effects model to perform the meta‐analysis instead of the default fixed‐effect method in Review Manager 2011.
Summary of findings: beneath the Data synthesis section, we added information about how we have conducted the Table 1 using GRADEproGDT 2015.
Contributions of authors
In the original review (Valentin‐Gudiol 2011r), CB, KM, MV and RA screened all results obtained and selected studies to be included. RA functioned as the arbiter for KM and CB, while KM fulfilled this role for RA and MV, in case of discrepancies. CB, MG, MV and KM extracted data from the trials. CB, MV and RA assessed the risk of bias of each included study. MV entered data into RevMan. MG carried out data analysis. MV, KM, MG and MH interpreted the analysis. MV wrote the results and KM and MH wrote the discussion, conclusions and abstracts with inputs from MV and RA. CB and MG also edited the final document.
In this update, CB, KM and MV screened all references and selected studies to be included. MH and RA resolved discrepancies. CB, KM, MG and MV extracted data. CB and MV assessed the risk of bias of each included study. MV entered data into RevMan. MG carried out data analysis. MV, KM, MG and MH interpreted the analysis. CB, KM and MG assessed the quality of evidence for each outcome using the GRADE approach. MV wrote the results and KM and MH wrote the discussion, conclusions and abstracts, with inputs from MV and RA. CB and MG also edited the final document.
MV and RA have overall responsibility for the review.
Sources of support
Internal sources
None, Other.
External sources
-
National Institute for Health Research, UK.
Cochrane Incentive Award
Declarations of interest
Marta Valentin Gudiol is an author on Angulo‐Barroso 2013 and did not extract data from this study. Katrin Mattern‐Baxter (KMB) is an author on Mattern‐Baxter 2013 and was not involved in extracting data from this study. KMB is employed as an Associate Professor at California State University, Sacramento, and is paid as a Consultant for local school‐based services for children with developmental disability and as a Physical Therapist at Physical Edge. KMB and her institution receive funds from the Thrasher Fund grant for an unrelated project. KMB received fees from Kaiser Community Benefit grants to develop continuing education courses for physical therapists in 2013 and 2014. Classes were held free for physical therapists. KMB received travel and accommodation expenses from The Douglas Education Service District, Oregon, to hold a continuing education class at the Therapy in Educational Settings conference in Oregon in 2014. KMB presented an educational session at the American Physical Therapy Association’s (APTA) Combined Sections Meeting in 2015 and 2016, and KMB's expenses were covered by the APTA. Montserat Girabent Farrés ‐ none known. Caritat Bagur‐Calafat ‐ none known. Mijna Hadders‐Algra (MHA) is employed as a Professor of developmental neurology and receives payment for lectures carried out across the world on the subject. MHA's institution receives grants for work on early intervention. MHA received royalties for two books at Mac Keith Press ('Postural control: a key issue in developmental disorders' and 'The examination of the child with minor neurological dysfunction'), and one Dutch book on the general principles of infant motor development. MHA declares that none of these books address the issue of intervention by means of treadmill locomotion. Rosa Maria Angulo‐Barroso is an author on the Angulo‐Barroso 2013, Ulrich 2001 and Ulrich 2008 studies, and was not involved in extracting data from these studies.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Angulo‐Barroso 2013 {published and unpublished data}
- Angulo‐Barroso RM. Walking patterns in infants at moderate risk for neuromotor disabilities with or without treadmill training [personal communication]. Email to: M Valentín‐Gudiol 20 July 2011.
- Angulo‐Barroso RM, Tiernan C, Chen LC, Valentin‐Gudiol M, Ulrich D. Treadmill training in moderate risk preterm infants promotes stepping quality ‐ results of a small randomised controlled trial. Research in Developmental Disabilities 2013;34(11):3629‐38. [DOI: 10.1016/j.ridd.2013.07.037] [DOI] [PubMed] [Google Scholar]
- Chen L, Looper J, Neary H, Ulrich D, Angulo‐Barroso R. Walking patterns in infants at moderate risk for neuromotor disabilities with or without treadmill training. Journal of Sport and Exercise Psychology 2008;30 Suppl:S40‐S41. [Google Scholar]
- Valentín‐Gudiol M. Walking patterns in infants at moderate risk for neuromotor disabilities with or without treadmill training [personal communication]. Email to: RM Angulo‐Barroso 17 June 2011.
Cherng 2007 {published data only}
- Cherng R, Liu C, Lau T, Hong R. Effects of treadmill training with body weight support on gait and gross motor function in children with spastic cerebral palsy. American Journal of Physical Medicine and Rehabilitation 2007;86(7):548‐55. [PUBMED: 17581289] [DOI] [PubMed] [Google Scholar]
Looper 2010 {published data only}
- Looper J, Ulrich DA. Effect of treadmill training and supramalleolar orthosis use on motor skill development in infants with Down syndrome: a randomized clinical trial. Physical Therapy 2010;90(3):382‐90. [DOI: 10.2522/%E2%80%8Bptj.20090021] [DOI] [PubMed] [Google Scholar]
Lowe 2015 {published data only}
- Lowe L, McMillan AG, Yates C. Body weight support treadmill training for children with developmental delay who are ambulatory. Pediatric Physical Therapy 2015;27:386‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattern‐Baxter 2013 {published data only (unpublished sought but not used)}
- Mattern‐Baxter K, McNeil S, Mansoor JK. Effects of home‐based locomotor treadmill training on gross motor function in young children with cerebral palsy: a quasi‐randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2013;94(11):2061‐7. [DOI: 10.1016/j.apmr.2013.05.012; PUBMED: 23747646] [DOI] [PubMed] [Google Scholar]
Ulrich 2001 {published data only}
- Ulrich DA, Ulrich BD, Angulo‐Kinzler RM, Yun J. Treadmill training of infants with Down syndrome: evidence‐based developmental outcomes. Pediatrics 2001;108(5):e84. [DOI: 10.1542/peds.108.5.e84] [DOI] [PubMed] [Google Scholar]
Ulrich 2008 {published data only}
- Angulo‐Barroso RM, Wu J, Ulrich DA. Long‐term effects of different treadmill interventions on gait development in new walkers with Down syndrome. Gait and Posture 2008;27(2):231‐8. [PUBMED: 17499993] [DOI] [PubMed] [Google Scholar]
- Ulrich DA, Lloyd MC, Tiernan CW, Looper JE, Angulo‐Barroso RM. Effects of intensity of treadmill training on developmental outcomes and stepping in infants with Down syndrome: a randomized trial. Physical Therapy 2008;88(1):114‐22. [DOI: 10.2522/%E2%80%8Bptj.20070139] [DOI] [PubMed] [Google Scholar]
- Wu J, Looper J, Ulrich BD, Ulrich DA, Angulo‐Barroso RM. Exploring effects of different treadmill interventions on walking onset and gait patterns in infants with Down syndrome. Developmental Medicine & Child Neurology 2007;49(11):839–45. [PUBMED: 17979862] [DOI] [PubMed] [Google Scholar]
- Wu J, Looper J, Ulrich DA, Angulo‐Barroso RM. Effects of various treadmill interventions on the development of joint kinematics in infants with Down syndrome. Physical Therapy 2010;90(9):1265‐76. [PUBMED: 20651010] [DOI] [PubMed] [Google Scholar]
- Wu J, Ulrich DA, Looper J, Tiernan CW, Angulo‐Barroso RM. Strategy adoption and locomotor adjustment in obstacle clearance of newly walking toddlers with down syndrome after different treadmill interventions. Experimental Brain Research 2008;186(2):261‐72. [DOI: 10.1007/s00221-007-1230-7] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Borggraefe 2007 {published data only}
- Borggraefe I, Kumar A, Schaefer JS, Berweck S, Meyer‐Heim A, Hufschmidt A, et al. Robotic assisted treadmill therapy for children with a central gait impairment. Monatsschrift Kinderheilkunde 2007;155(6):529‐34. [Google Scholar]
Borggraefe 2010 {published data only}
- Borggraefe I, Kiwull L, Schaefer JS, Koerte I, Blaschek A, Meyer‐Heim A, et al. Sustainability of motor performance after robotic‐assisted treadmill therapy in children: an open, non‐randomized baseline‐treatment study. European Journal of Physical and Rehabilitation Medicine 2010;46(2):125‐31. [PUBMED: 20485217] [PubMed] [Google Scholar]
Campbell 2012 {published data only}
- Campbell SK, Gaebler‐Spira D, Zawacki L, Clark A, Boynewicz K, Regnier RA, et al. Effects on motor development of kicking and stepping exercise in preterm infants with periventricular brain injury: a pilot study. Journal of Pediatric Rehabilitation Medicine 2012;5(1):15‐27. [DOI: 10.3233/PRM-2011-0185; PMC3584696; PUBMED: 22543889] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 2014 {published data only}
- Christensen C, Lowes LP. Treadmill training for a child with spina bifida without functional ambulation. Pediatric Physical Therapy 2014;26(2):265‐73. [DOI: 10.1097/PEP.0000000000000029; PUBMED: 24675133] [DOI] [PubMed] [Google Scholar]
Dodd 2007 {published data only}
- Dodd KJ, Foley S. Partial body‐weight‐supported treadmill training can improve walking in children with cerebral palsy: a clinical controlled trial. Developmental Medicine and Child Neurology 2007;49(2):101‐5. [DOI: 10.1111/j.1469-8749.2007.00101.x; PUBMED: 17253995] [DOI] [PubMed] [Google Scholar]
Duarte 2014 {published data only}
- Duarte ND, Grecco LAC, Galli M, Fregni F, Santos Oliveira C. Effect of transcranial direct‐current stimulation combined with treadmill training on balance and functional performance in children with cerebral palsy: a double‐blind randomized controlled trial. PLOS One 2014;9(8):e105777. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Shamy 2017 {published data only}
Grecco 2013a {published data only}
- Grecco LA, Freitas TB, Satie J, Bagne E, Oliveira CS, Souza DR. Treadmill training following orthopedic surgery in lower limbs of children with cerebral palsy. Pediatric Physical Therapy 2013;25(2):187‐92. [DOI: 10.1097/PEP.0b013e3182888495; PUBMED: 23542199] [DOI] [PubMed] [Google Scholar]
Grecco 2013b {published data only}
- Grecco LAC, Tomita SM, Christovão TC, Pasini H, Sampaio LM, Oliveira CS. Effect of treadmill gait training on static and functional balance in children with cerebral palsy: a randomized controlled trial. Brazilian Journal of Physical Therapy 2013;17(1):17‐23. [PUBMED: 23538455] [DOI] [PubMed] [Google Scholar]
Grecco 2013c {published data only}
- Grecco LAC, Zanon N, Sampaio LMM, Oliveira CS. A comparison of treadmill training and overground walking in ambulant children with cerebral palsy: randomized controlled clinical trial. Clinical Rehabilitation 2013;27(8):686‐96. [DOI] [PubMed] [Google Scholar]
Hilderley 2016 {published data only}
- Hilderley AJ, Fehlings D, Lee GW, Wright FV. Comparison of a robotic‐assisted gait training program with a program of functional gait training for children with cerebral palsy: design and methods of a two group randomized controlled cross‐over trial. Springerplus 2016;5(1):1886. [DOI: 10.1186/s40064-016-3535-0; PMC5084143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2011 {published data only}
- Johnston TE, Watson KE, Ross SA, Gates PE, Gaughan JP, Lauer RT, et al. Effects of a supported speed treadmill training exercise program on impairment and function for children with cerebral palsy. Developmental Medicine and Child Neurology 2011;53(8):742‐50. [DOI: 10.1111/j.1469-8749.2011.03990.x; PUBMED: 21679357] [DOI] [PubMed] [Google Scholar]
Jung 2016 {published data only}
- Jung TY, Kim Y, Kelly LE, Abel MF. Biomechanical and perceived differences between overground and treadmill walking in children with cerebral palsy. Gait Posture 2016;45:1‐6. [DOI: 10.1016/j.gaitpost.2015.12.004; PUBMED: 26979874] [DOI] [PubMed] [Google Scholar]
Kurz 2011 {published data only}
- Kurz MJ, Stuberg W, DeJong SL. Body weight supported treadmill training improves the regularity of the stepping kinematics in children with cerebral palsy. Developmental Neurorehabilitation 2011;14(2):87‐93. [DOI: 10.3109/17518423.2011.552459; PUBMED: 21410400] [DOI] [PubMed] [Google Scholar]
Lowe 2013 {published data only}
- Lowe LM. Treadmill Training With Partial Body Weight Support In Ambulatory Children With Developmental Delay [dissertation]. University of Central Arkansas, 2013. [Google Scholar]
Maltais 2003 {published data only}
- Maltais D, Bar‐Or O, Pierrynowski M, Galea V. Repeated treadmill walks affect physiologic responses in children with cerebral palsy. Medicine & Science in Sports & Exercise 2003;35(10):1653‐61. [DOI: 10.1249/01.MSS.0000089343.67237.50; PUBMED: 14523301] [DOI] [PubMed] [Google Scholar]
Matsuno 2010 {published data only}
- Matsuno VM, Camargo MR, Palma GC, Alveno D, Barela AM. Analysis of partial body weight support during treadmill and overground walking of children with cerebral palsy. Revista Brasileira De Fisioterapia 2010;14(5):404‐10. [PUBMED: 21180866] [PubMed] [Google Scholar]
Meyer‐Heim 2007 {published data only}
- Meyer‐Heim A, Borggraefe I, Ammann‐Reiffer C, Berweck S, Sennhauser FH, Colombo G, et al. Feasibility of robotic‐assisted locomotor training in children with central gait impairment. Developmental Medicine and Child Neurology 2007;49(12):900‐6. [DOI: 10.1111/j.1469-8749.2007.00900.x; PUBMED: 18039236] [DOI] [PubMed] [Google Scholar]
Mussleman 2007 {published data only}
- Musselman KE, Yang JF. Loading the limb during rhythmic leg movements lengthens the duration of both flexion and extension in human infants. Journal of Neurophysiology 2007;97(2):1247‐57. [DOI: 10.1152/jn.00891.2006; PUBMED: 17151226] [DOI] [PubMed] [Google Scholar]
Pang 2003 {published data only}
- Pang MYC, Lam T, Yang JF. Infants adapt their stepping to repeated trip‐inducing stimuli. Journal of Neurophysiology 2003;90(4):2731‐40. [DOI: 10.1152/jn.00407.2003; PUBMED: 12826655] [DOI] [PubMed] [Google Scholar]
Pantall 2011 {published data only}
- Pantall A, Teulier C, Smith BA, Moerchen V, Ulrich BD. Impact of enhanced sensory input on treadmill step frequency: infants born with myelomeningocele. Pediatric Physical Therapy 2011;23(1):42‐52. [DOI: 10.1097/PEP.0b013e318206eefa; PMC3461189; PUBMED: 21266940] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2007 {published data only}
- Phillips JP, Sullivan KJ, Burtner PA, Caprihan A, Provost B, Bernitsky‐Beddingfield A. Ankle dorsiflexion fMRI in children with cerebral palsy undergoing intensive body‐weight‐supported treadmill training: a pilot study. Developmental Medicine and Child Neurology 2007;49(1):39‐44. [DOI: 10.1111/j.1469-8749.2007.0102a.x; PUBMED: 17209975] [DOI] [PubMed] [Google Scholar]
Romei 2012 {published data only}
- Romei M, Montinaro A, Piccinini L, Maghini C, Germiniasi C, Bo I, et al. Efficacy of robotic‐assisted gait training compared with intensive task‐oriented physiotherapy for children with cerebral palsy. The Fourth IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics; 2012 June 24‐27; Rome, Italy. 2012:1890‐4. [DOI: 10.1109/BioRob.2012.6290748] [DOI]
Sarhan 2014 {published data only}
- Sarhan RS, Chevidikunnan MF, Gaowgzeh RA. Locomotor treadmill training program using driven gait orthosis versus manual treadmill therapy on motor output in spastic diplegic cerebral palsy children. Nitte University Journal of Health Science 2014;4(4):10‐17. [Google Scholar]
Schindl 2000 {published data only}
- Schindl MR, Forstner C, Kern H, Hesse S. Treadmill training with partial body weight support in nonambulatory patients with cerebral palsy. Archives of Physical Medicine and Rehabilitation 2000;81(3):301‐6. [PUBMED: 10724074] [DOI] [PubMed] [Google Scholar]
Schlittler 2011 {published data only}
- Schlittler CX, Lopes TF, Raniero EP, Barela JA. Treadmill training effects on walking acquisition and motor development in infants at risk of developmental delay. Revista Paulista de Pediatria 2011;29(1):91‐9. [DOI: 10.1590/S0103-05822011000100015] [DOI] [Google Scholar]
Scholtes 2012 {published data only}
- Scholtes VA, Becher JG, Janssen‐Potten Y, Dekkers H, Smallenbroek L, Dallmeijer AJ. Effectiveness of functional progressive resistance exercise training on walking ability in children with cerebral palsy: a randomized controlled trial.. Research in Developmental Disabilities 2012;33(1):181‐8. [DOI: 10.1016/j.ridd.2011.08.026; PUBMED: 22093663] [DOI] [PubMed] [Google Scholar]
Schroeder 2014 {published data only}
- Schroeder AS, Kries R, Riedel C, Homburg M, Auffermann H, Blaschek A, et al. Patient‐specific determinants of responsiveness to robot‐enhanced treadmill therapy in children and adolescents with cerebral palsy. Developmental Medicine & Child Neurology 2014;56(12):1172‐9. [DOI: 10.1111/dmcn.12564; PUBMED: 25154424] [DOI] [PubMed] [Google Scholar]
Sherief 2015 {published data only}
- Sherief AEAA, Abo Gazya AA, Gafaar AE. Integrated effect of treadmill training combined with dynamic ankle foot orthosis on balance in children with hemiplegic cerebral palsy. Egyptian Journal of Medical Human Genetics 2015;16(2):173‐9. [DOI: 10.1016/j.ejmhg.2014.11.002] [DOI] [Google Scholar]
Siekerman 2015 {published data only}
- Siekerman K, Barbu‐Roth M, Anderson DI, Donnelly A, Goffinet F, Teulier C. Treadmill stimulation improves newborn stepping. Developmental Psychobiology 2015;57(2):247‐54. [DOI: 10.1002/dev.21270; PUBMED: 25644966] [DOI] [PubMed] [Google Scholar]
Smania 2011 {published data only}
- Smania N, Bonetti P, Gandolfi M, Cosentino A, Waldner A, Hesse S, et al. Improved gait after repetitive locomotor training in children with cerebral palsy. American Journal of Physical Therapy and Rehabilitation 2011;90(2):137‐49. [DOI: 10.1097/PHM.0b013e318201741e; PUBMED: 21217461] [DOI] [PubMed] [Google Scholar]
Su 2013 {published data only}
- Su IY, Chung KK, Chow DH. Treadmill training with partial body weight support compared with conventional gait training for low‐functioning children and adolescents with nonspastic cerebral palsy: a two‐period crossover study. Prosthetics & Orthotics International 2013;37(6):445‐53. [DOI: 10.1177/0309364613476532; PUBMED: 23436693] [DOI] [PubMed] [Google Scholar]
Teulier 2009 {published data only}
- Teulier C, Smith BA, Kubo M, Chang C, Moerchen V, Murazko K, et al. Stepping responses of infants with myelomeningocele when supported on a motorized treadmill. Physical Therapy 2009;89(1):60‐72. [PMC2614450] [DOI] [PMC free article] [PubMed] [Google Scholar]
Willerslev‐Olsen 2014 {published data only}
- Willerslev‐Olsen M, Lorentzen J, Nielsen JB. Gait training reduces ankle joint stiffness and facilitates heel strike in children with cerebral palsy. NeuroRehabilitation 2014;35(4):643‐55. [DOI: 10.3233/NRE-141180; PUBMED: 25318785] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02424526 {unpublished data only}
- NCT02424526. Intensive home‐based treadmill training and walking attainment in young children with cerebral palsy. clinicaltrials.gov/ct2/show/NCT02424526 (first received 16 April 2015). [NCT02424526]
Additional references
Adolph 1998
- Adolph KE, Vereijken B, Denny MA. Learning to crawl. Child Development 1998;69(5):1299‐312. [PUBMED: 9839417] [PubMed] [Google Scholar]
Adolph 2012
- Adolph KE, Cole WG, Komati M, Garciaguirre JS, Badaly D, Lingeman JM, et al. How do you learn to walk? Thousands of steps and dozens of falls per day. Psychological Science 2012;23(11):1387‐94. [DOI: 10.1177/0956797612446346; PMC3591461; PUBMED: 23085640] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2013
- Anderson DI, Campos JJ, Witherington DC, Dahl A, Rivera M, He MX, et al. The role of locomotion in psychological development. Frontiers in Psychology 2013;4:1‐17. [DOI: 10.3389/fpsyg.2013.00440; PMC3719016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Angulo‐Barroso 2008
- Angulo‐Barroso M, Wu J, Ulrich D. Long‐term effect of different treadmill interventions on gait development in new walkers with Down syndrome. Gait Posture 2008;27(2):231‐8. [DOI: 10.1016/j.gaitpost.2007.03.014; PUBMED: 17499993] [DOI] [PubMed] [Google Scholar]
Angulo‐Barroso 2010
- Angulo‐Barroso RM, Tiernan CW, Chen LC, Ulrich D, Neary H. Treadmill responses and physical activity levels of infants at risk for neuromotor delay. Pediatric Physical Therapy 2010;22(1):61‐8. [DOI: 10.1097/PEP.0b013e3181ccc8c6; PUBMED: 20142707] [DOI] [PubMed] [Google Scholar]
Barbeau 1987
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Research 1987;412(1):84‐95. [PUBMED: 3607464] [DOI] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Manual for the Bayley Scales of Infant Development II. 2nd Edition. San Antonio (TX): The Psychological Corporation, 1993. [Google Scholar]
Beck 2010
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization 2010;88(1):31‐8. [DOI: 10.1590/S0042-96862010000100012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Begnoche 2007
- Begnoche DM, Pitetti KH. Effects of traditional treatment and partial body weight treadmill training on the motor skills of children with spastic cerebral palsy. A pilot study. Pediatric Physical Therapy 2007;19(1):11‐9. [DOI: 10.1097/01.pep.0000250023.06672.b6; PUBMED: 17304093] [DOI] [PubMed] [Google Scholar]
Bertenthal 1984
- Bertenthal BI, Campos JJ, Barrett KC. Self‐produced locomotion: an organizer of emotional, cognitive, and social development in infancy. In: Emde RN, Harmon RJ editor(s). Continuities and Discontinuities in Development. New York: Plenum, 1984:175–210. [DOI: 10.1007/978-1-4613-2725-7_8] [DOI] [Google Scholar]
Bertenthal 1990
- Bertenthal BI, Campos JJ. A systems approach to the organizing effects of self‐produced locomotion during infancy. Advances in Infancy Research 1990;6:1‐60. [Google Scholar]
Bilney 2003
- Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture 2003;17(1):68‐74. [PUBMED: 12535728] [DOI] [PubMed] [Google Scholar]
Blackman 2002
- Blackman JA. Early intervention: a global perspective. Infants and Young Children 2002;15(2):11‐9. [Google Scholar]
Bly 1995
- Bly L, Ariz TN. Motor Skills Acquisition in the First Year, An Illustrated Guide to Normal Development. Therapy Skills Builders, 1995. [Google Scholar]
Bodkin 2003
- Bodkin AW, Baxter RS, Heriza CB. Treadmill training for an infant born preterm with a grade III intraventricular hemorrhage. Physical Therapy 2003;83(12):1107‐18. [PUBMED: 14640869] [PubMed] [Google Scholar]
Bohannon & Smith 1987
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Physical Therapy 1987;67(2):206‐7. [PUBMED: 3809245] [DOI] [PubMed] [Google Scholar]
Boyd 1999
- Boyd R, Fatone S, Rodda J, Olesch C, Starr R, Cullis E, et al. High‐ or low‐technology measurements of energy expenditure in clinical gait analysis?. Developmental Medicine and Child Neurology 1999;41(10):676‐82. [PUBMED: 10587044] [DOI] [PubMed] [Google Scholar]
Butler 2001
- Butler C, Darrah J. Effects of neurodevelopmental treatment (NDT) for cerebral palsy: an AACPDM evidence report. Developmental Medicine & Child Neurology 2001;43(11):778‐90. [PUBMED: 11730153] [DOI] [PubMed] [Google Scholar]
Campbell 2006
- Campbell SK, Palisano RJ, Vander Linden DW. Physical Therapy for Children. St Louis (MO): Elsevier Saunders, 2006. [Google Scholar]
Campos 1989
- Campos JJ, Bertenthal BI. Locomotion and psychological development in infancy. In: Morrison F, Lord C, Keating D editor(s). Applied Developmental Psychology. New York: Academic Press, 1989:230–58. [Google Scholar]
Case‐Smith 1998
- Case‐Smith J, Heaphy T, Marr D, Galvin B, Koch V, Ellis MG, et al. Fine motor and functional performance outcomes in preschool children. American Journal of Occupational Therapy 1998;52:788‐96. [DOI: 10.5014/ajot.52.10.788] [DOI] [Google Scholar]
Cernak 2008
- Cernak K, Stevens V, Price R, Shumway‐Cook A. Locomotor training using body‐weight support on a treadmill in conjunction with ongoing physical therapy in a child with severe cerebellar ataxia. Physical Therapy 2008;88(1):88‐97. [DOI: 10.2522/ptj.20070134; PUBMED: 17940104] [DOI] [PubMed] [Google Scholar]
Chen 2008
- Chen L, Looper J, Neary H, Ulrich D, Angulo‐Barroso R. Walking patterns in infants at moderate risk for neuromotor disabilities with or without treadmill training. Journal of Sport and Exercise Psychology 2008;30 Suppl:S40‐S41. [Google Scholar]
Clearfield 2004
- Clearfield MW. The role of crawling and walking experience in infant spatial memory. Journal of Experimental Child Psychology 2004;89(3):214‐41. [DOI: 10.1016/j.jecp.2004.07.003; PUBMED: 15501452] [DOI] [PubMed] [Google Scholar]
Clearfield 2011
- Clearfield MW. Learning to walk changes infants' social interactions. Infant Behavior and Development 2011;34(1):15‐25. [DOI: 10.1016/j.infbeh.2010.04.008; PUBMED: 20478619] [DOI] [PubMed] [Google Scholar]
Cotman 2002a
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences 2002;25(6):295‐301. [PUBMED: 12086747] [DOI] [PubMed] [Google Scholar]
Cotman 2002b
- Cotman CW, Engesser‐Cesar C. Exercise enhances and protects brain function. Exercise and Sport Sciences Reviews 2002;30(2):75‐9. [PUBMED: 11991541] [DOI] [PubMed] [Google Scholar]
Cunha 2016
- Cunha AB, Lobo MA, Kokkoni E, Galloway JC, Tudella E. Effect of short‐term training on reaching behavior in infants: a randomized controlled clinical trial. Journal of Motor Behavior 2016;48(2):132‐42. [DOI] [PubMed] [Google Scholar]
Damiano 2006
- Damiano DL. Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Physical Therapy 2006;86(11):1534‐40. [PUBMED: 17094192] [DOI] [PubMed] [Google Scholar]
Damiano 2009
- Damiano DL, DeJong SL. A systematic review of the effectiveness of treadmill training and body weight support in paediatric rehabilitation. Journal of Neurologic Physical Therapy 2009;33(1):27‐44. [DOI: 10.1097/NPT.0b013e31819800e2; PMC2982788; PUBMED: 19265768] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 1994
- Davis DW, Thelen E, Keck J. Treadmill stepping in infants born prematurely. Early Human Development 1994;39(3):211‐23. [PUBMED: 7712955] [DOI] [PubMed] [Google Scholar]
Day 2004
- Day JA, Fox EJ, Lowe J, Swales HB, Behrman AL. Locomotor training with partial body weight support on a treadmill in a nonambulatory child with spastic tetraplegic cerebral palsy: a case report. Pediatric Physical Therapy 2004;16(2):106‐13. [DOI: 10.1097/01.PEP.0000127569.83372.C8; PUBMED: 17057535] [DOI] [PubMed] [Google Scholar]
De Bode 2007
- Bode S, Mathern GW, Bookheimer S, Dobkin B. Locomotor training remodels fMRI sensorimotor cortical activations in children after cerebral hemispherectomy. Neurorehabilitation and Neural Repair 2007;21(6):497‐508. [DOI: 10.1177/1545968307299523; PMC4080925; PUBMED: 17369509] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Graaf‐Peters 2006
- Graaf‐Peters VB, Hadders‐Algra M. Ontogeny of the human central nervous system: what is happening when?. Early Human Development 2006;82(4):257‐66. [DOI: 10.1016/j.earlhumdev.2005.10.013; PUBMED: 16360292] [DOI] [PubMed] [Google Scholar]
De Graaf‐Peters 2007
- Graaf‐Peters VB, Blauw‐Hospers CH, Dirks T, Bakker H, Bos AF, Hadders‐Algra M. Development of postural control in typically developing children and children with cerebral palsy: possibilities for intervention?. Neuroscience and Biobehavioral Reviews 2007;31(8):1191‐200. [DOI: 10.1016/j.neubiorev.2007.04.008; PUBMED: 17568673] [DOI] [PubMed] [Google Scholar]
Deeks 1997a
- Deeks J. Are you sure that's a standard deviation? (part 1). Cochrane News 1997; Vol. 10, issue 11‐12.
Deeks 1997b
- Deeks J. Are you sure that's a standard deviation? (part 2). Cochrane News 1997; Vol. 11, issue 11‐2.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Down's Syndrome Association
- What is the incidence of Down's Syndrome?. www.downs‐syndrome.org.uk/for‐new‐parents/faqs/general/ (accessed 19 April 2011).
Edgerton 1997
- Edgerton VR, Leon RD, Tillakaratne N, Recktenwald MR, Hodgson JA, Roy RR. Use‐dependent plasticity in spinal stepping and standing. Advances in Neurology 1997;72:233‐47. [PUBMED: 8993702] [PubMed] [Google Scholar]
Eidelberg 1980
- Eidelberg E, Story JL, Meyer BL, Nystel J. Stepping by chronic spinal cats. Experimental Brain Research 1980;40(3):241‐6. [PUBMED: 7428879] [DOI] [PubMed] [Google Scholar]
Eng 2007
- Eng JJ, Tang PF. Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidence. Expert Review of Neurotherapeutics 2007;7(10):1417‐36. [DOI: 10.1586/14737175.7.10.1417; PMC3196659; PUBMED: 17939776] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eyre 2003
- Eyre JA. Development and plasticity of the corticospinal system in man. Neural Plasticity 2003;10(1‐2):93‐106. [DOI: 10.1155/NP.2003.93; PMC2565418] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldman 1990
- Feldman AB, Haley SM, Coryell J. Concurrent and construct validity of the Pediactric Evaluation of DIsability Inventory. Physical Therapy 1990;70(10):602‐10. [PUBMED: 2217539] [DOI] [PubMed] [Google Scholar]
Formiga 2011
- Formiga CKMR, Linhares MB. Motor development curve from 0 to 12 months in infants born preterm. Acta Paediatrica 2011;100(3):379‐84. [DOI: 10.1111/j.1651-2227.2010.02002.x; PUBMED: 20825603] [DOI] [PubMed] [Google Scholar]
Frey 2016
- Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Seminars in Fetal and Neonatal Medicine 2016;21(2):68‐73. [DOI: 10.1016/j.siny.2015.12.011; PUBMED: 26794420] [DOI] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [DOI: 10.1016/j.jclinepi.2004.09.013; PUBMED: 15878471] [DOI] [PubMed] [Google Scholar]
Gordon 2011
- Gordon AM. To constrain or not to constrain, and other stories of intensive upper extremity training for children with unilateral cerebral palsy. Developmental Medicine and Child Neurology. 2011;53 Suppl 4:56‐61. [DOI: 10.1111/j.1469-8749.2011.04066.x; PUBMED: 21950396] [DOI] [PubMed] [Google Scholar]
Goyen 2002
- Goyen TA. Longitudinal motor development of "apparently normal" high‐risk infants at 18 months, 3 and 5 years. Early Human Development 2002;70(1‐2):103‐15. [PUBMED: 12441208] [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed prior to 16 April 2017. Hamilton, ON: GRADE Working Group, McMaster University, 2014.
Graham 2004
- Graham HK, Harvey A, Rodda J, Nattrass GR, Pirpiris M. The Functional Mobility Scale (FMS). Journal of Pediatric Orthopaedics 2004;24(5):514‐20. [PUBMED: 15308901] [DOI] [PubMed] [Google Scholar]
Hadders‐Algra 1996
- Hadders‐Algra M, Brogren E, Forssberg H. Training affects the development of postural adjustments in sitting infants. Journal of Physiology 1996;493(Pt 1):289‐98. [PMC1158969] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadders‐Algra 2014
- Hadders‐Algra M. Early diagnosis and early intervention in cerebral palsy. Frontiers in Neurology 2014;4(5):185. [DOI: 10.3389/fneur.2014.00185; PMC4173665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadders‐Algra 2017
- Hadders‐Algra M, Boxum AG, Hielkema T, Hamer EG. Effect of early intervention in infants at very high risk of cerebral palsy: a systematic review. Developmental Medicine and Child Neurology 2017;59:246‐58. [DOI] [PubMed] [Google Scholar]
Harbourne 2003
- Harbourne RT, Stergiou N. Nonlinear analysis of the development of sitting postural control. Developmental Psychobiology 2003;42(4):368‐77. [DOI: 10.1002/dev.10110; PUBMED: 12672087] [DOI] [PubMed] [Google Scholar]
Heineman 2008
- Heineman KR, Hadders‐Algra M. Evaluation of neuromotor function in infancy ‐ a systematic review of available methods. Journal of Developmental and Behavioral Pediatrics 2008;29(4):315‐23. [DOI: 10.1097/DBP.0b013e318182a4ea; PUBMED: 18698195] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hodgson 1994
- Hodgson JA, Roy RR, Leon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task?. Medicine and Science in Sports and Exercise 1994;26(12):1491‐7. [PUBMED: 7869884] [PubMed] [Google Scholar]
Jones 1999
- Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion‐induced structural plasticity in the motor cortex of adult rats. Journal of Neuroscience 1999;19(22):10153‐63. [PUBMED: 10559423] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kermoian 1988
- Kermoian R, Campos JJ. Locomotor experience: a facilitator of spatial cognitive development. Child Development 1988;59(4):908‐17. [PUBMED: 3168629] [PubMed] [Google Scholar]
Kleim 2008
- Kleim JA, Jones TA. Principles of experience‐dependent neural plasticity: implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research 2008;51(1):S225‐39. [DOI: 10.1044/1092-4388(2008/018); PUBMED: 18230848] [DOI] [PubMed] [Google Scholar]
Kolb 2013
- Kolb B, Mychasiuk R, Muhammad A, Gibb R. Brain plasticity in the developing brain. Progress in Brain Research 2013;207:35‐64. [DOI: 10.1016/B978-0-444-63327-9.00005-9; PUBMED: 24309250] [DOI] [PubMed] [Google Scholar]
Kolobe 2014
- Kolobe TH, Christy JB, Gannotti ME, Heathcock JC, Damiano DL, Taub E, et al. Research summit III proceedings on dosing in children with an injured brain or cerebral palsy: executive summary. Physical Therapy 2014;94(7):907‐20. [DOI: 10.2522/ptj.20130024; PMC4078265; PUBMED: 24525862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lepage 1998
- Lepage C, Noreau L, Bernard PM. Association between characteristics of locomotion and accomplishment of life habits in children with cerebral palsy. Physical Therapy 1998;78(5):458‐69. [PUBMED: 9597060] [DOI] [PubMed] [Google Scholar]
Lie 2010
- Lie KK, Grøholt EK, Eskild A. Association of cerebral palsy with Apgar score in low and normal birthweight infants: population based cohort study. BMJ 2010;341:c4990. [DOI: 10.1136/bmj.c4990] [DOI] [PMC free article] [PubMed] [Google Scholar]
Looper 2006
- Looper J, Wu J, Angulo‐Barroso R, Ulrich D, Ulrich BD. Changes in step variability of new walkers with typical development and with Down syndrome. Journal of Motor Behavior 2006;38(5):367‐72. [DOI: 10.3200/JMBR.38.5.367-372; PMC2254170; PUBMED: 16968682] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luo 2009
- Luo H, Chen P, Hsieh W, Lin K, Lu T, Chen WJ, et al. Associations of supported treadmill stepping with walking attainment in preterm and full‐term infants. Physical Therapy 2009;89(11):1215‐25. [DOI: 10.2522/ptj.20080369; PUBMED: 19762484] [DOI] [PubMed] [Google Scholar]
Mattern‐Baxter 2009a
- Mattern‐Baxter K, Bellamy S, Mansoor JK. Effects of intensive locomotor treadmill training on young children with cerebral palsy. Pediatric Physical Therapy 2009;21(4):308‐18. [DOI: 10.1097/PEP.0b013e3181bf53d9; PUBMED: 19923970] [DOI] [PubMed] [Google Scholar]
Mattern‐Baxter 2009b
- Mattern‐Baxter K. Effects of partial body weight supported treadmill training on children with cerebral palsy. Pediatric Physical Therapy 2009;21(1):12‐22. [DOI: 10.1097/PEP.0b013e318196ef42; PUBMED: 19214072] [DOI] [PubMed] [Google Scholar]
Menz 2004
- Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the GAITRite walkway system for the quantification of temporo‐spatial parameters of gait in young and older people. Gait Posture 2004;20(1):20‐5. [DOI: 10.1016/S0966-6362(03)00068-7; PUBMED: 15196515] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molina‐Rueda 2010
- Molina‐Rueda F, Aguila‐Maturana AM, Molina‐Rueda MJ, Miangolarra‐Page JC. Treadmill training with or without partial body weight support in children with cerebral palsy: systematic review and meta‐analysis [Pasarela rodante con o sin sistema de suspensión del peso corporal en niños con parálisis cerebral infantil: revisión sistemática y metaanálisis]. Revista de Neurologia 2010;51(3):135‐45. [PUBMED: 20645264] [PubMed] [Google Scholar]
Morgan 2013
- Morgan C, Novak I, Badawi N. Enriched environments and motor outcomes in cerebral palsy: systematic review and meta‐analysis. Pediatrics 2013;132(3):e735‐46. [DOI: 10.1542/peds.2012-3985; PUBMED: 23958771] [DOI] [PubMed] [Google Scholar]
Morgan 2016
- Morgan C, Darrah J, Gordon AM, Harbourne R, Spittle A, Johnson R, et al. Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Developmental Medicine & Child Neurology 2016;58(9):900‐9. [DOI: 10.1111/dmcn.13105; PUBMED: 27027732] [DOI] [PubMed] [Google Scholar]
Murray 2007
- Murray GK, Jones PB, Kuh D, Richards M. Infant developmental milestones and subsequent cognitive function. Annals of Neurology 2007;62(2):128‐36. [DOI: 10.1002/ana.21120; PMC3465788; PUBMED: 17487877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutlu 2009
- Mutlu A, Krosschell K, Spira DG. Treadmill training with partial body‐weight support in children with cerebral palsy: a systematic review. Developmental Medicine and Child Neurology 2009;51(4):268‐75. [DOI: 10.1111/j.1469-8749.2008.03221.x; PUBMED: 19207302] [DOI] [PubMed] [Google Scholar]
Nelson 2000
- Nelson CA. Neural plasticity and human development: the role of early experience in sculpting memory systems. Developmental Science 2000;3(2):115‐36. [DOI: 10.1111/1467-7687.00104] [DOI] [Google Scholar]
Newell 1991
- Newell KM. Motor skill acquisition. Annual Review of Psychology 1991;42:213‐37. [DOI: 10.1146/annurev.ps.42.020191.001241; PUBMED: 2018394] [DOI] [PubMed] [Google Scholar]
Novak 2013
- Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Developmental Medicine and Child Neurology 2013;55(10):885‐910. [DOI: 10.1111/dmcn.12246; PUBMED: 23962350] [DOI] [PubMed] [Google Scholar]
Palisano 1997
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine and Child Neurology 1997;39:214‐23. [DOI: 10.1111/j.1469-8749.1997.tb07414.x] [DOI] [PubMed] [Google Scholar]
Pin 2010
- Pin TW, Eldridge B, Galea MP. Motor trajectories from 4 to 18 months corrected age in infants born at less than 30 weeks of gestation. Early Human Development 2010;86(9):573‐80. [DOI: 10.1016/j.earlhumdev.2010.07.008; PUBMED: 20709474] [DOI] [PubMed] [Google Scholar]
Prins 2010
- Prins SA, Lindern JS, Dijk S, Versteegh FGA. Motor development of premature infants born between 32 and 34 weeks. International Journal of Pediatrics 2010 Sept 7 [Epub ahead of print]. [DOI: 10.1155/2010/462048; PMC2946567; PUBMED: 20885965] [DOI] [PMC free article] [PubMed]
Prosser 2007
- Prosser LA. Locomotor training within an inpatient rehabilitation program after pediatric incomplete spinal cord injury. Physical Therapy 2007;87(9):1224‐32. [DOI: 10.2522/ptj.20060252; PUBMED: 17636156] [DOI] [PubMed] [Google Scholar]
Provost 2007
- Provost B, Dieruf K, Burtner PA, Phillips JP, Bernitsky‐Beddingfield A, Sullivan K, et al. Endurance and gait in children with cerebral palsy after intensive body weight‐supported treadmill training. Pediatric Physical Therapy 2007;19(1):2‐10. [DOI: 10.1097/01.pep.0000249418.25913.a3; PUBMED: 17304092] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 1997
- Richards CL, Malouin F, Dumas F, Marcoux S, Lepage C, Menier C. Early and intensive treadmill locomotor training for young children with cerebral palsy: a feasibility study. Pediatric Physical Therapy 1997;9(4):158‐65. [Google Scholar]
Riethmuller 2009
- Riethmuller AM, Jones RA, Okely AD. Efficacy of interventions to improve motor development in young children: a systematic review. Pediatrics 2009;124(4):e782‐92. [DOI] [PubMed] [Google Scholar]
Rosenbloom 1971
- Rosenbloom L. The contribution of motor behaviour to child development. Physiotherapy 1971;57(4):159‐62. [PUBMED: 4252512] [PubMed] [Google Scholar]
Roze 2010
- Roze E, Meijer L, Braeckel KN, Ruiter SA, Bruggink JL, Bos AF. Developmental trajectories from birth to school age in healthy term‐born children. Pediatrics 2010;126(5):e1134‐42. [DOI: 10.1542/peds.2010-0698] [DOI] [PubMed] [Google Scholar]
Russell 2002
- Russell DJ, Rosenbaum PL, Avery LM, Lane M. Gross Motor Function Measure (GMFM 66 & GMFM 88) User's Manual. Clinics in Developmental Medicine N0. 159. London: McKeith Press, 2002. [Google Scholar]
Sackett 1996
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. British Medical Journal 1996;312(7023):71‐2. [PMC2349778; PUBMED: 8555924] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ. Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sherrington 1910
- Sherrington CS. Flexion‐reflex of the limb, crossed extension‐reflex, and reflex stepping and standing. Journal of Physiology 1910;40(1‐2):28‐121. [PMC1533734] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stiles 2000
- Stiles J. Neural plasticity and cognitive development. Developmental Neuropsychology 2000;18(2):237‐72. [DOI: 10.1207/S15326942DN1802_5; PUBMED: 11280966] [DOI] [PubMed] [Google Scholar]
Stiles 2005
- Stiles J, Reilly J, Paul B, Moses P. Cognitive development following early brain injury: evidence for neural adaptation. Trends in Cognitive Sciences 2005;9(3):136‐43. [DOI: 10.1016/j.tics.2005.01.002; PUBMED: 15737822] [DOI] [PubMed] [Google Scholar]
Sullivan 2007
- Sullivan KJ, Brown DA, Klasses T, Mulroy S, Ge T, Azen SP, et al. Effects of task‐specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Physical Therapy 2007;87(12):1580‐602. [DOI: 10.2522/ptj.20060310; PUBMED: 17895349] [DOI] [PubMed] [Google Scholar]
Surveillance CP Europe
- Surveillance of Cerebral Palsy in Europe. www‐rheop.ujf‐grenoble.fr/scpe2/site_scpe/index.php (accessed 5 March 2011).
Sveistrup 1997
- Sveistrup H, Woollacott MH. Practice modifies the developing automatic postural response. Experimental Brain Research 1997;114(1):33‐43. [PUBMED: 9125449] [DOI] [PubMed] [Google Scholar]
Teulier 2015
- Teulier C, Lee DK, Ulrich BD. Early gait development in human infants: plasticity and clinical applications. Developmental Psychobiology 2015;57(4):447‐58. [DOI: 10.1002/dev.21291; PUBMED: 25782975] [DOI] [PubMed] [Google Scholar]
Thelen 1986
- Thelen E. Treadmill‐elicited stepping in 7‐month‐old infants. Child Development 1986;57(6):1498‐506. [PUBMED: 3802974] [PubMed] [Google Scholar]
Thelen 1991
- Thelen E, Ulrich BD. Hidden skills: a dynamic systems analysis of treadmill stepping during the first year. Monographs of the Society for Research in Child Development 1991;56(1):1‐98. [PUBMED: 1922136] [PubMed] [Google Scholar]
Ulrich 1992
- Ulrich BD, Ulrich DA, Collier DH. Alternating stepping patterns: hidden abilities of 11‐month‐old infants with Down syndrome. Developmental Medicine and Child Neurology 1992;34(3):233‐9. [PUBMED: 1532783] [DOI] [PubMed] [Google Scholar]
Ulrich 1995
- Ulrich BD, Ulrich DA, Collier DH, Cole EL. Developmental shifts in the ability of infants with Down syndrome to produce treadmill steps. Physical Therapy 1995;75(1):14‐23. [PUBMED: 7809193] [DOI] [PubMed] [Google Scholar]
Van Hartingsveldt 2005
- Hartingsveldt MJ, Cup E, Oostendorp RA. Reliability and validity of the fine motor scale of the Peabody Developmental Motor Scales–2. Occupational Therapy International 2005;12(1):1‐13. [PUBMED: 15962696] [DOI] [PubMed] [Google Scholar]
Varni 2003
- Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a paediatric population health measure: feasibility, reliability, and validity. Ambulatory Pediatrics 2003;3(6):329‐41. [PUBMED: 14616041] [DOI] [PubMed] [Google Scholar]
Verschuren 2008
- Verschuren O, Ketelaar M, Takken T, Helders PJ, Gorter JW. Exercise programs for children with cerebral palsy: a systematic review of the literature. American Journal of Physical Medicine and Rehabilitation 2008;87(5):404‐17. [DOI: 10.1097/PHM.0b013e31815b2675; PUBMED: 17993987] [DOI] [PubMed] [Google Scholar]
Volpe 2009
- Volpe JJ. The encephalopathy of prematurity ‐ brain injury and impaired brain development inextricably intertwined. Seminars in Pediatric Neurology 2009;16(4):167‐78. [DOI: 10.1016/j.spen.2009.09.005; PMC2799246; PUBMED: 19945651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walle 2014
- Walle EA, Campos JJ. Infant language development is related to the acquisition of walking. Developmental Psychology 2014;50(2):336‐48. [DOI: 10.1037/a0033238; PUBMED: 23750505] [DOI] [PubMed] [Google Scholar]
Webb 2001
- Webb SJ, Monk CS, Nelson CA. Mechanisms of postnatal neurobiological development: implications for human development. Developmental Neuropsychology 2001;19(2):147‐71. [DOI: 10.1207/S15326942DN1902_2; PUBMED: 11530973] [DOI] [PubMed] [Google Scholar]
Webster 2005
- Webster KE, Wittwer JE, Feller JA. Validity of GAITRite walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture 2005;22(4):317‐21. [DOI: 10.1016/j.gaitpost.2004.10.005; PUBMED: 16274913] [DOI] [PubMed] [Google Scholar]
WHO 2005
- International Classification of Functioning, Disability and Health for Children and Youth. apps.who.int/classifications/icfbrowser/ (accessed 10 February 2011).
WHO 2006
- WHO Multicentre Growth Reference Study Group. Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatrica 2006;95 Suppl 450:86‐95. [DOI: 10.1080/08035320500495563] [DOI] [PubMed] [Google Scholar]
Willoughby 2009
- Willoughby KL, Dodd KJ, Shields N. A systematic review of the effectiveness of treadmill training for children with cerebral palsy. Disability and Rehabilitation 2009;31(24):1971‐9. [DOI: 10.3109/09638280902874204; PUBMED: 19874075] [DOI] [PubMed] [Google Scholar]
Wu 2007
- Wu J, Looper J, Ulrich BD, Ulrich D, Angulo‐Barroso R. Exploring effects of different treadmill interventions on walking onset and gait patterns in infants with Down syndrome. Developmental Medicine and Child Neurology 2007;49(11):839‐45. [DOI: 10.1111/j.1469-8749.2007.00839.x; PUBMED: 17979862] [DOI] [PubMed] [Google Scholar]
Wu 2008
- Wu J, Ulrich D, Looper J, Tiernan C, Angulo‐Barroso R. Strategy adoption and locomotor adjustment in obstacle clearance of newly walking toddlers with down syndrome after different treadmill interventions. Experimental Brain Research 2008;186(2):261‐72. [DOI: 10.1007/s00221-007-1230-7; PUBMED: 18064443] [DOI] [PubMed] [Google Scholar]
Wu 2010
- Wu J, Looper J, Ulrich D, Angulo‐Barroso R. Effects of various treadmill interventions on the development of joint kinematics in infants With Down syndrome. Physical Therapy 2010;9(90):1265‐76. [DOI: 10.2522/ptj.20090281; PUBMED: 20651010] [DOI] [PubMed] [Google Scholar]
Ziviani 2010
- Ziviani J, Feeney R, Rodger S, Watter P. Systematic review of early intervention programmes for children from birth to nine years who have a physical disability. Australian Occupational Therapy Journal 2010;57(4):210‐23. [DOI: 10.1111/j.1440-1630.2010.00850.x; PUBMED: 20854595] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Valentin‐Gudiol 2011p
- Valentin‐Gudiol M, Mattern‐Baxter K, Girabent‐Farrés M, Bagur‐Calafat C, Hadders‐Algra M, Angulo‐Barroso R. Treadmill interventions with partial body weight support in children under 6 years of age at risk of neuromotor delay. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009242.pub2] [DOI] [PubMed] [Google Scholar]
Valentin‐Gudiol 2011r
- Valentin‐Gudiol M, Mattern‐Baxter K, Girabent‐Farrés M, Bagur‐Calafat C, Hadders‐Algra M, Angulo‐Barroso R. Treadmill interventions with partial body weight support in children under six years of age at risk of neuromotor delay. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009242] [DOI] [PubMed] [Google Scholar]


