Abstract
Background
Historically, women have generally been attended and supported by other women during labour. However, in hospitals worldwide, continuous support during labour has often become the exception rather than the routine.
Objectives
The primary objective was to assess the effects, on women and their babies, of continuous, one‐to‐one intrapartum support compared with usual care, in any setting. Secondary objectives were to determine whether the effects of continuous support are influenced by:
1. Routine practices and policies in the birth environment that may affect a woman's autonomy, freedom of movement and ability to cope with labour, including: policies about the presence of support people of the woman's own choosing; epidural analgesia; and continuous electronic fetal monitoring.
2. The provider's relationship to the woman and to the facility: staff member of the facility (and thus has additional loyalties or responsibilities); not a staff member and not part of the woman's social network (present solely for the purpose of providing continuous support, e.g. a doula); or a person chosen by the woman from family members and friends;
3. Timing of onset (early or later in labour);
4. Model of support (support provided only around the time of childbirth or extended to include support during the antenatal and postpartum periods);
5. Country income level (high‐income compared to low‐ and middle‐income).
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 October 2016), ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (1 June 2017) and reference lists of retrieved studies.
Selection criteria
All published and unpublished randomised controlled trials, cluster‐randomised trials comparing continuous support during labour with usual care. Quasi‐randomised and cross‐over designs were not eligible for inclusion.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We sought additional information from the trial authors. The quality of the evidence was assessed using the GRADE approach.
Main results
We included a total of 27 trials, and 26 trials involving 15,858 women provided usable outcome data for analysis. These trials were conducted in 17 different countries: 13 trials were conducted in high‐income settings; 13 trials in middle‐income settings; and no studies in low‐income settings. Women allocated to continuous support were more likely to have a spontaneous vaginal birth (average RR 1.08, 95% confidence interval (CI) 1.04 to 1.12; 21 trials, 14,369 women; low‐quality evidence) and less likely to report negative ratings of or feelings about their childbirth experience (average RR 0.69, 95% CI 0.59 to 0.79; 11 trials, 11,133 women; low‐quality evidence) and to use any intrapartum analgesia (average RR 0.90, 95% CI 0.84 to 0.96; 15 trials, 12,433 women). In addition, their labours were shorter (MD ‐0.69 hours, 95% CI ‐1.04 to ‐0.34; 13 trials, 5429 women; low‐quality evidence), they were less likely to have a caesarean birth (average RR 0.75, 95% CI 0.64 to 0.88; 24 trials, 15,347 women; low‐quality evidence) or instrumental vaginal birth (RR 0.90, 95% CI 0.85 to 0.96; 19 trials, 14,118 women), regional analgesia (average RR 0.93, 95% CI 0.88 to 0.99; 9 trials, 11,444 women), or a baby with a low five‐minute Apgar score (RR 0.62, 95% CI 0.46 to 0.85; 14 trials, 12,615 women). Data from two trials for postpartum depression were not combined due to differences in women, hospitals and care providers included; both trials found fewer women developed depressive symptomatology if they had been supported in birth, although this may have been a chance result in one of the studies (low‐quality evidence). There was no apparent impact on other intrapartum interventions, maternal or neonatal complications, such as admission to special care nursery (average RR 0.97, 95% CI 0.76 to 1.25; 7 trials, 8897 women; low‐quality evidence), and exclusive or any breastfeeding at any time point (average RR 1.05, 95% CI 0.96 to 1.16; 4 trials, 5584 women; low‐quality evidence).
Subgroup analyses suggested that continuous support was most effective at reducing caesarean birth, when the provider was present in a doula role, and in settings in which epidural analgesia was not routinely available. Continuous labour support in settings where women were not permitted to have companions of their choosing with them in labour, was associated with greater likelihood of spontaneous vaginal birth and lower likelihood of a caesarean birth. Subgroup analysis of trials conducted in high‐income compared with trials in middle‐income countries suggests that continuous labour support offers similar benefits to women and babies for most outcomes, with the exception of caesarean birth, where studies from middle‐income countries showed a larger reduction in caesarean birth. No conclusions could be drawn about low‐income settings, electronic fetal monitoring, the timing of onset of continuous support or model of support.
Risk of bias varied in included studies: no study clearly blinded women and personnel; only one study sufficiently blinded outcome assessors. All other domains were of varying degrees of risk of bias. The quality of evidence was downgraded for lack of blinding in studies and other limitations in study designs, inconsistency, or imprecision of effect estimates.
Authors' conclusions
Continuous support during labour may improve outcomes for women and infants, including increased spontaneous vaginal birth, shorter duration of labour, and decreased caesarean birth, instrumental vaginal birth, use of any analgesia, use of regional analgesia, low five‐minute Apgar score and negative feelings about childbirth experiences. We found no evidence of harms of continuous labour support. Subgroup analyses should be interpreted with caution, and considered as exploratory and hypothesis‐generating, but evidence suggests continuous support with certain provider characteristics, in settings where epidural analgesia was not routinely available, in settings where women were not permitted to have companions of their choosing in labour, and in middle‐income country settings, may have a favourable impact on outcomes such as caesarean birth. Future research on continuous support during labour could focus on longer‐term outcomes (breastfeeding, mother‐infant interactions, postpartum depression, self‐esteem, difficulty mothering) and include more woman‐centred outcomes in low‐income settings.
Keywords: Female; Humans; Pregnancy; Delivery, Obstetric; Labor, Obstetric; Personal Autonomy; Cesarean Section; Doulas; Pregnancy Outcome; Professional-Patient Relations
Plain language summary
Continuous support for women during childbirth
What is the issue?
In the past, women have been cared for and supported by other women during labour and birth, and have had someone with them throughout, which we call ‘continuous support’. However, in many countries more women are giving birth in hospital rather than at home. This has meant continuous support during labour has become the exception rather than the norm. The aim of this Cochrane Review was to understand the effect of continuous support on a woman during labour and childbirth, and on her baby. We collected and analysed all relevant studies to answer this question (search date: October 2016).
Why is this important?
Research shows that women value and benefit from the presence of a support person during labour and childbirth. This support may include emotional support (continuous presence, reassurance and praise) and information about labour progress. It may also include advice about coping techniques, comfort measures (comforting touch, massage, warm baths/showers, encouraging mobility, promoting adequate fluid intake and output) and speaking up when needed on behalf of the woman. Lack of continuous support during childbirth has led to concerns that the experience of labour and birth may have become dehumanised.
Modern obstetric care frequently means women are required to experience institutional routines. These may have adverse effects on the quality, outcomes and experience of care during labour and childbirth. Supportive care during labour may enhance physiological labour processes, as well as women's feelings of control and confidence in their own strength and ability to give birth. This may reduce the need for obstetric intervention and also improve women's experiences.
What evidence did we find?
We found 26 studies that provided data from 17 countries, involving more than 15,000 women in a wide range of settings and circumstances. The continuous support was provided either by hospital staff (such as nurses or midwives), or women who were not hospital employees and had no personal relationship to the labouring woman (such as doulas or women who were provided with a modest amount of guidance on providing support). In other cases, the support came from companions of the woman's choice from her own network (such as her partner, mother, or friend).
Women who received continuous labour support may be more likely to give birth 'spontaneously', i.e. give birth vaginally with neither ventouse nor forceps nor caesarean. In addition, women may be less likely to use pain medications or to have a caesarean birth, and may be more likely to be satisfied and have shorter labours. Postpartum depression could be lower in women who were supported in labour, but we cannot be sure of this due to the studies being difficult to compare (they were in different settings, with different people giving support). The babies of women who received continuous support may be less likely to have low five‐minute Apgar scores (the score used when babies’ health and well‐being are assessed at birth and shortly afterwards). We did not find any difference in the numbers of babies admitted to special care, and there was no difference found in whether the babies were breastfed at age eight weeks. No adverse effects of support were identified. Overall, the quality of the evidence was all low due to limitations in study design and differences between studies.
What does this mean?
Continuous support in labour may improve a number of outcomes for both mother and baby, and no adverse outcomes have been identified. Continuous support from a person who is present solely to provide support, is not a member of the woman's own network, is experienced in providing labour support, and has at least a modest amount of training (such as a doula), appears beneficial. In comparison with having no companion during labour, support from a chosen family member or friend appears to increase women's satisfaction with their experience. Future research should explore how continuous support can be best provided in different contexts.
Summary of findings
Summary of findings for the main comparison. Continuous support compared to usual care (all trials) for women during childbirth.
| Continuous support compared to usual care (all trials) for women during childbirth | ||||||
| Patient or population: women during childbirth Setting: Hospital settings in Australia, Belgium, Botswana, Brazil, Canada, Chile, Finland, France, Greece, Guatamala, Iran, Mexico, Nigeria, South Africa, Thailand, Turkey, USA Intervention: continuous support Comparison: usual care (all trials) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care (all trials) | Risk with Continuous support | |||||
| Spontaneous vaginal birth | Study population | Average RR 1.08 (1.04 to 1.12) | 14369 (21 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 679 per 1000 | 733 per 1000 (706 to 760) | |||||
| Negative rating of/negative feelings about birth experience | Study population | Average RR 0.69 (0.59 to 0.79) | 11133 (11 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 177 per 1000 | 122 per 1000 (104 to 140) | |||||
| Postpartum depression | Study population | ‐ | 5716 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | Both trials (Hodnett 2002; Hofmeyr 1991) were widely disparate in populations, the hospital conditions where they were conducted, and the type of support provider. We concluded that combining the trials data would not yield meaningful information. In both trials the direction of effect was the same. Hodnett 2002 used the Edinburgh Postnatal Depression Inventory and reported the frequencies of scores greater than 12. Hofmeyr 1991 used the Pitt Depression Inventory and reported scores indicating mild (less than 20), moderate (20 to 34), and severe (> 34) depressive symptomatology. We combined the frequencies of moderate and severe depressive symptomatology, since Pitt scores > 19 have been considered indicative of postpartum depression (Avan 2010). Continuous support resulted in a large reduction in depressive symptomology in Hofmeyr 1991 (RR 0.18, 95% CI 0.09 to 0.36). There was little or no difference in depressive symptomatology in Hodnett 2002 (RR 0.86, 95% CI 0.73 to 1.02) |
|
| see comment | see comment | |||||
| Admission to special care nursery | Study population | Average RR 0.97 (0.76 to 1.25) | 8897 (7 RCTs) | ⊕⊕⊝⊝ LOW 4 5 | ||
| 81 per 1000 | 79 per 1000 (62 to 101) | |||||
| Exclusive or any breastfeeding at any time point, as defined by trial authors | Study population | Average RR 1.05 (0.96 to 1.16) | 5584 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 6 | ||
| 601 per 1000 | 631 per 1000 (577 to 697) | |||||
| Labour length | The mean length of labour in the usual care group ranged from 5.3 to 12.7 hours. | The mean length of labour in the continuous support group was on average 0.69 hours (1.04 to 0.34 hours) shorter |
5429 (13 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| Caesarean birth | Study population | Average RR 0.75 (0.64 to 0.88) | 15347 (24 RCTs) | ⊕⊕⊝⊝ LOW 1 7 | ||
| 146 per 1000 | 109 per 1000 (93 to 128) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Most studies contributing data had design limitations. (‐1)
2 Statistical heterogeneity (I² > 60%). Variation in size of effect. (‐1)
3 The two trials were widely disparate in populations, the hospital conditions within which they were conducted, and the type of support provider. (‐1)
4 Most studies contributing data had design limitations and two studies contributing 37.6% weight had serious design limitations. (‐1)
5 Wide confidence interval crossing the line of no effect. (‐1)
6 Statistical heterogeneity (I² > 60%). Variation in direction of effect. (‐1)
7 Heterogeneity I² = 58%. Variation in effect size. (‐1)
Background
This is an update of a review last published in 2013 (Hodnett 2013).
Description of the condition
Historically and cross‐culturally, women have been attended and supported by other women during labour and birth. However, since the middle of the twentieth century, in many countries most women gave birth in hospital rather than at home, and continuous support during labour has become the exception rather than the routine. Concerns about dehumanisation of women's birth experiences (in high‐, middle‐, and low‐income countries) have led to calls for a return to continuous, one‐to‐one support by women for women during labour (Klaus 2002). Research has demonstrated that women benefit from and value the presence of a support person during labour, to provide psychological, physical, emotional, informational and practical support (Kabakian‐Khasholian 2015). This support person may act as an advocate for the woman, for example by helping to communicate her preferences to a health worker, and also provides encouragement, reassurance, and physical comfort. A support person may also help to communicate to the woman about her progress through labour, suggest coping techniques, and support her decision‐making. Two World Health Organization (WHO) guidelines recommend a companion of the woman's choice during labour and childbirth, to improve labour outcomes and women's satisfaction with services (World Health Organization 2015; World Health Organization 2016).
Description of the intervention
Common elements of continuous support during childbirth include emotional support (e.g. continuous presence, reassurance and praise), information about labour progress and advice regarding coping techniques, comfort measures (e.g. comforting touch, massage, warm baths/showers, encouraging mobility, promoting adequate fluid intake and output) and advocacy (e.g. helping the woman to articulate her wishes to others). The period of support for this intervention varies greatly across studies and contexts. For example, some doula programs may initiate support during the pregnancy, provide continuous support during labour and childbirth, and provide support through three months postpartum. Other programs focus specifically on facility‐based care, and continuous support is provided from around the time of admission through the birth. Definitions for what constitutes "continuous" support vary across trials and contexts. For example, "continuous" is defined as "no interruption" (Langer 1998), "minimum of 80% of the time" (Hodnett 2002), and "as continuously as possible" (Hofmeyr 1991) across three large trials in this review.
For the purposes of this review, we have defined continuous support as some combination of comfort measures, emotional support, provision of information, and advocacy on behalf of the woman, provided from at least early labour (before 6 cm dilation) or within one hour of hospital admission (for admission with greater than or equal to 6 cm dilation), through until at least the birth, and provided by a person whose sole responsibility is to provide support to the woman, as continuously as practical in a given context.
How the intervention might work
Two complementary theoretical explanations have been offered for the effects of labour support on childbirth outcomes. Both explanations hypothesise that labour support enhances labour physiology and mothers' feelings of control and competence, reducing reliance on medical interventions. The first theoretical explanation considers possible mechanisms when companionship during labour is used in stressful, threatening and disempowering clinical birth environments (Hofmeyr 1991). During labour, women may be uniquely vulnerable to environmental influences; modern obstetric care frequently subjects women to institutional routines, high rates of intervention, unfamiliar personnel, lack of privacy and other conditions that may be experienced as harsh. These conditions may have an adverse effect on the progress of labour and on the development of feelings of competence and confidence; this may in turn impair adjustment to parenthood and establishment of breastfeeding, and increase the risk of postpartum depression. The provision of support and companionship during labour may to some extent buffer such stressors.
The second theoretical explanation does not focus on a particular type of birth environment. Rather, it describes two pathways ‐ enhanced passage of the fetus through the pelvis and soft tissues, as well as decreased stress response ‐ by which labour support may reduce the likelihood of operative birth and subsequent complications, and enhance women's feelings of control and satisfaction with their childbirth experiences (Hodnett 2002a). Enhanced fetopelvic relationships may be accomplished by encouraging mobility and effective use of gravity, supporting women to assume their preferred positions and recommending specific positions for specific situations. Studies of the relationships among fear and anxiety, the stress response and pregnancy complications have shown that anxiety during labour is associated with high levels of the stress hormone epinephrine in the blood, which may in turn lead to abnormal fetal heart rate patterns in labour, decreased uterine contractility, a longer active labour phase with regular well‐established contractions and low Apgar scores (Lederman 1978; Lederman 1981). Furthermore, individual interventions (e.g. labour induction, epidural anaesthesia, caesarean birth) and a cascade of interventions throughout labour may disrupt hormonal physiology and introduce risks to the woman or her baby, both in the short and long term (Buckley 2015). Emotional support, information and advice, comfort measures and advocacy may reduce anxiety and fear and associated adverse effects during labour.
Continuous support has been viewed by some as a form of pain relief, specifically, as an alternative to epidural analgesia (Dickinson 2002), because of concerns about the deleterious effects of epidural analgesia, including on labour progress (Anim‐Somuah 2011). Many labour and birth interventions routinely involve, or increase the likelihood of, co‐interventions to monitor, prevent or treat adverse effects, in a "cascade of interventions". Continuous, one‐to‐one support has the potential to limit this cascade and therefore, to have a broad range of different effects, in comparison to usual care. For example, if continuous support leads to reduced use of epidural analgesia, it may in turn involve less use of electronic fetal monitoring, intravenous drips, synthetic oxytocin, drugs to combat hypotension, bladder catheterisation, vacuum extraction or forceps, episiotomy and less morbidity associated with these, and may increase mobility during labour and spontaneous birth (Caton 2002; Anim‐Somuah 2011) and impact the experience of giving birth.
Why it is important to do this review
A systematic review examining factors associated with women's satisfaction with the childbirth experience suggests that continuous support can make a substantial contribution to women's satisfaction. When women evaluate their experience, four factors predominate: the amount of support from caregivers, the quality of relationships with caregivers, being involved with decision‐making and having high expectations or having experiences that exceed expectations (Hodnett 2002a).
Clarification of the effects of continuous support during labour, overall and within specific circumstances, is important in light of public and social policies and programs that encourage this type of care. For example, the Congress in Uruguay passed a law in 2001 decreeing that all women have the right to companionship during labour. In several low‐ and middle‐income countries (including China, South Africa, Tanzania and Zimbabwe), the Better Births Initiative promotes labour companionship as a core element of care for improving maternal and infant health (World Health Organization 2016a). In many low‐income countries, women are not permitted to have anyone with them during labour and birth. Efforts to change policies in these settings have led to questions about the effectiveness of support from spouses/partners or other support people of the woman's own choosing, particularly in settings where the cost of paid companions (e.g. doulas) would be prohibitive.
In North America, and increasingly in many other areas of the world, the services of women with special training in labour support have become available. Most commonly known as doula (a Greek word for 'handmaiden'), this new member of the caregiver team may also be called a labour companion, birth companion, labour support specialist, labour assistant or birth assistant. A number of North American organisations offer doula training, certification and professional support; according to one estimate more than 50,000 people have received this training to date (P Simkin, personal communication). Some North American hospitals have begun to sponsor doula services. In a recent national survey of childbearing women in the United States, 6% of respondents indicated that they had used doula services during their most recent labours (Declercq 2013). Many associations for doulas have been established in high‐income countries, including DONA International, Doula UK, NCT Doula, British Doula, Childbirth International, Australian Doulas, Australian Doula College and Europoean Doula Network, among others. Doula services are usually paid for out‐of‐pocket, and therefore affordable to affluent, higher‐educated women only. However, a meta‐analysis conducted by Zhang 1996a showed that socially disadvantaged populations, such as low‐income women, could benefit more from doula support. Maternal healthcare systems in dozens of high‐ and low‐ to middle‐income countries throughout the world are developing new traditions for supportive female companionship during labour (Pascali‐Bonaro 2010).
Questions have arisen about the ability of employees (such as nurses or midwives) to provide effective labour support, in the context of modern institutional birth environments (Hodnett 1997). For example, nurses and midwives often have simultaneous responsibility for more than one labouring woman, spend a large proportion of time managing technology and keeping records, ensure adherence to institutional practices and protocols, and begin or end work shifts in the middle of women's labours. They may work in short‐staffed environments or lack labour support skills.
Companions chosen by a woman from her own network, such as spouses/partners and female relatives, usually have little experience in providing labour support and are often themselves in need of support when with a loved one during labour and birth. As they are frequently available to assume the role, often without extra cost to families or health systems, it is important to understand their effectiveness as providers of continuous labour support.
In addition to questions about the impact of the type of provider of labour support, there are other questions about the effectiveness of support, including its impact under a variety of environmental conditions, and whether its effects are mediated by when continuous support begins (early versus active labour).
There are also questions about the relative impact of different models of labour support; specifically, effects of support provided only during the intrapartum period versus effects of an extended model with support during the antenatal, intrapartum and postpartum periods.
Childbearing women, policy‐makers, payers of health services, health professionals and facilities and those who provide labour support all need evidence about the effects of continuous support, overall and under specific conditions.
Objectives
The primary objective was to assess the effects, on women and their babies, of continuous, one‐to‐one intrapartum support compared with usual care, in any setting. Secondary objectives were to determine whether the effects of continuous support are influenced by the following:
-
Routine practices and policies in the birth environment that may affect a woman's autonomy, freedom of movement and ability to cope with labour, including:
policies about the presence of support people of the woman's own choosing;
epidural analgesia; and
continuous electronic fetal monitoring.
-
The provider's relationship to the woman and to the facility:
staff member of the facility (and thus may have additional loyalties or responsibilities);
not a staff member and not part of the woman's social network (present solely for the purpose of providing continuous support, e.g. a doula); or
a person chosen by the woman from family members and friends.
Timing of onset (early or later in labour).
Model of support (support provided only around the time of childbirth or extended to include support during the antenatal and postpartum periods).
Country income level (high‐income compared to low‐ and middle‐income)
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs), including cluster‐RCTs, comparing continuous labour support by either a familiar or unfamiliar person (with or without healthcare professional qualifications) with usual care, in which there was random allocation to treatment and control groups, were considered for inclusion in the review. RCTs published in abstract form only were not eligible for inclusion (unless additional information could be obtained from the authors). Quasi‐RCTs and RCTs using a cross‐over design were not eligible for inclusion in the review.
Types of participants
Pregnant women, in labour.
Types of interventions
We evaluated continuous presence and support during labour and birth. The person providing the support could have qualifications as a healthcare professional (nurse, midwife) or training as a doula or childbirth educator, or be a family member, a spouse/partner, a friend or a stranger with some or no special training in labour support. The control group received usual care, as defined by the trialists. In all cases, 'usual care' did not involve continuous intrapartum support, but it could involve other measures, such as routine epidural analgesia, to help women to cope with labour.
Types of outcome measures
Theoretically, continuous support can have many diverse physiological and psychosocial effects (both short‐ and long‐term), and therefore, a larger than usual number of outcomes were considered.
Primary outcomes
Woman
Spontaneous vaginal birth.
Negative rating of/negative feelings about the birth experience, as defined by trial authors.
Postpartum depression (defined using a pre‐specified cutoff score on a validated instrument).
Baby
Admission to special care nursery.
Exclusive or any breastfeeding at any time point, as defined by trial authors.
Secondary outcomes
Woman
Any analgesia/anaesthesia (pain medication).
Regional analgesia/anaesthesia.
Synthetic oxytocin during labour.
Labour length.
Severe labour pain (postpartum report).
Caesarean birth.
Instrumental vaginal birth.
Perineal trauma (defined as episiotomy or laceration requiring suturing).
Delayed skin‐to‐skin contact (defined as immediately following birth), not pre‐specified.
Delayed initiation of breastfeeding (more than one hour after birth, or as defined by trial authors), not pre‐specified.
Time from birth to initiation of breastfeeding, not pre‐specified.
Unlikely to recommend birth in that institution, not pre‐specified.
Restricted mobility during labour, as defined by trial authors, not pre‐specified.
Baby
Low five‐minute Apgar score (≤7, or as defined by trial authors).
Prolonged newborn hospital stay, as defined by trial authors.
Longer‐term outcomes
Difficulty mothering, as defined by trial authors (including low confidence as mother).
Low self‐esteem in the postpartum period.
Unsatisfactory mother‐infant interactions, not pre‐specified.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting their Information Specialist (31 October 2016).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences; and
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (1 June 2017) for unpublished, planned and ongoing trial reports using the search terms detailed in Appendix 1
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Hodnett 2013. For this update, the following methods were used for assessing the 27 reports that were identified as a result of the updated search. The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted another review author.
Data extraction and management
We adapted the recommended Cochrane Pregnancy and Childbirth data extraction form for this review. For eligible studies, two review authors independently extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted another review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third review author.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants; and
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); or
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update, the quality of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook to assess the quality of the body of evidence relating to the following outcomes for the main comparison (comparison 1: continuous support versus usual care ‐ all trials).
Spontaneous vaginal birth.
Caesarean birth.
Negative rating of/negative feelings about the birth experience, as defined by trial authors.
Postpartum depression, (defined using a pre‐specified cutoff score on a validated instrument).
Admission to special care nursery.
Exclusive or any breastfeeding at any time point, as defined by trial authors.
Labour length.
GRADEpro Guideline Development Tool was used to import data from Review Manager 5.3 (RevMan 2014) to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals (CI).
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. If trials measured the same outcome but used different methods, we would have used the standardised mean difference.
Unit of analysis issues
Cluster‐randomised trials
Had we found cluster‐randomised trials, we would have included them in the analyses along with individually‐randomised trials. Our plan was: we would adjust their sample sizes or standard errors using the methods described in the Handbook (Section 16.3.4 or 16.3.6 as appropriate) (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we had used ICCs from other sources, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. In future updates of this review, if we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
We noted levels of attrition for included studies. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (< 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (> 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate the source.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged to be sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary is treated as the average range of possible treatment effects and we discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we did not combine trials. When we used random‐effects analyses, the results were presented as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated the source using subgroup analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce the effect. We added two new subgroup analyses on the model of support received (D) and country income level where trials were conducted (E).
We planned the following subgroup analyses.
(A) Three subgroup analyses that concern characteristics of the childbirth environment
Trials in settings in which women were permitted to be accompanied by one or more support persons of their own choosing compared with trials in which accompaniment was not permitted.
Trials conducted in settings in which epidural analgesia was available compared with trials in settings in which it was unavailable.
Trials in which there was a policy of routine electronic fetal heart rate monitoring compared with trials in settings in which continuous electronic fetal monitoring was not routine.
(B) One subgroup analysis that concerns characteristics of the providers of labour support
Trials in which the caregivers were employees of the institution, compared with trials in which the caregivers were not employees and were not members of the woman's social network, compared with trials in which the providers were not employees and were lay people chosen by the participants (e.g. spouse/partner, friend, close relative).
(C) One subgroup analysis that concerns differences in the timing of onset of continuous support
Trials in which continuous labour support began prior to or during early labour (as defined by trial authors), compared with trials in which continuous support began in active labour.
(D) One subgroup analysis that concerns the model of support received
Trials in which support was provided solely during the intrapartum period, compared with trials in which extended support was provided during the antenatal and postpartum periods, in addition to continuously during the intrapartum period.
(E) One subgroup analysis that concerns the country income level
Trials conducted in high‐income settings, compared with trials conducted in low‐ or middle‐income settings.
The following outcomes were used in subgroup analyses:
spontaneous vaginal birth;
negative ratings of the birth experience;
postpartum depression;
admission to special care nursery;
exclusive or any breastfeeding at any time point, as defined by trial authors;
any analgesia/anaesthesia;
synthetic oxytocin during labour; and
caesarean birth.
The five primary outcomes and three common labour intervention outcomes were used in the subgroup analyses. While normally subgroup analyses are restricted to primary outcomes, we also included the outcome of caesarean birth, because there is widespread concern about escalating caesarean rates worldwide, and subgroup analyses could be helpful to policy makers in decisions about the provision of continuous labour support.
We assessed subgroup differences by interaction tests available in RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Because few of the trial reports contained all of the information needed for the above subgroup analyses, we contacted the trial authors in an attempt to verify the presence/absence of routine electronic fetal monitoring (EFM), hospital policy regarding the presence of a support person, the presence/absence of epidural analgesia and timing of onset of continuous support. We excluded some studies included in the primary comparisons from the subgroup analyses concerning the use of EFM, presence/absence of epidural analgesia, hospital policy regarding the presence of a support person, because their status was unknown. For tests of differences between these subgroups, we recalculated the overall analysis by including only the studies in which these characteristics were known.
We were unable to carry out subgroup analysis for subgroup (C) timing of onset of continuous support because we could not sufficiently categorise trials according to this subgroup, and in subgroup (D) there were only data for one outcome.
Sensitivity analysis
We performed sensitivity analyses, for the primary outcomes, in instances where there was a high risk of bias associated with selection bias (allocation concealment). We also performed sensitivity analyses for any outcomes where reciprocal data had to be calculated to include data in an analysis (exclusive breastfeeding; negative rating of/negative feelings about the birth experience).
Results
Description of studies
Results of the search
In the previous version of this review (Hodnett 2013), 23 trials met the inclusion criteria, but one trial (Thomassen 2003) provided no usable outcome data. Eight trial reports were awaiting further classification and one was ongoing.
For this update, we searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 October 2016), ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP) (1 June 2017). We assessed 27 new reports and re‐assessed eight that were awaiting classification and one ongoing trial reported in Hodnett 2013 (see Figure 1).
1.
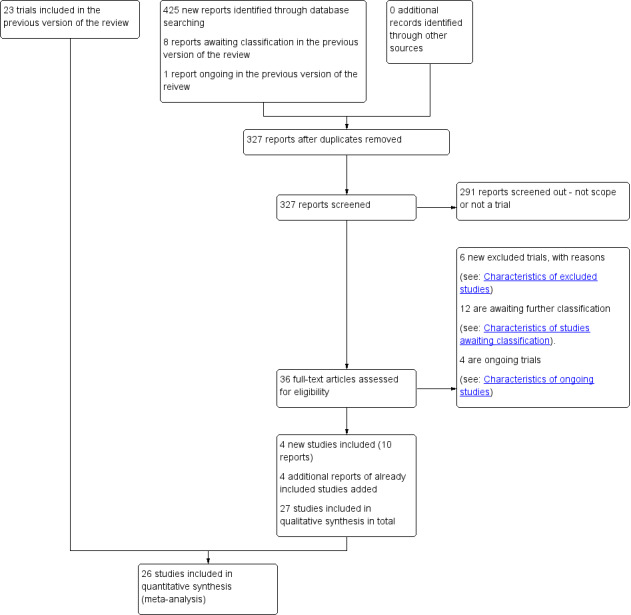
Study flow diagram
We included four new studies (10 reports) (involving 570 women) (Akbarzadeh 2014; Hans 2013; Isbir 2015; Safarzadeh 2012) and four new reports of four already included studies (Bruggemann 2007; Campbell 2006; Kashanian 2010; Morhason‐Bello 2009). We excluded six studies because: the intervention was not continuous support during labour (Dong 2009; ISRCTN33728802; Orbach‐Zinger 2012; U1111‐1175‐8408; Wan 2011); and participants were not randomly assigned to study groups (Senanayake 2013). Twelve studies are awaiting translation and classification (Aghdam 2015; Bakhshi 2015; Farahani 2005; Huang 2003; IRCT2013111710297N3; McGrath 1999; NCT00664118; Pinheiro 1996; Rahimiyan 2015; Samieizadeh 2011; Sangestani 2013; Shahshahan 2014). Of these studies, five are awaiting translation (Aghdam 2015; Bakhshi 2015; Farahani 2005; Samieizadeh 2011; Sangestani 2013), we are awaiting further information from authors for six (Huang 2003; IRCT2013111710297N3; McGrath 1999; Pinheiro 1996; Rahimiyan 2015; Shahshahan 2014), and were unable to locate contact details for the authors of NCT00664118. Four trials are ongoing (IRCT2015083123837N1; NCT01216098; NCT01947244; NCT02550730).
Included studies
We included a total of 27 studies, and provided full details in the Characteristics of included studies tables. Of these, 26 studies involving 15,858 women contributed data to the analyses for primary and secondary outcomes; one included study (Thomassen 2003) met our inclusion criteria but did not report data on any of our pre‐specified outcomes. Thomassen 2003 is not described in this section, but details are provided in the Characteristics of included studies tables.
Methods
We included 27 randomised controlled trials (RCTs).
Settings
All 26 trials (n = 15,858) that provided usable outcome data were conducted in hospital settings. The 26 trials were conducted in 17 countries: Australia (Dickinson 2002), Belgium (Bréart ‐ Belgium 1992), Botswana (Madi 1999), Brazil (Bruggemann 2007), Canada (3 studies: Gagnon 1997; Hodnett 1989; Hodnett 2002), Chile (Torres 1999), Finland (2 studies: Hemminki 1990a; Hemminki 1990b), France (Bréart ‐ France 1992), Greece (Bréart ‐ Greece 1992), Guatemala (Klaus 1986), Iran (3 studies: Akbarzadeh 2014; Kashanian 2010; Safarzadeh 2012), Mexico (Langer 1998), Nigeria (Morhason‐Bello 2009), South Africa (Hofmeyr 1991), Thailand (Yuenyong 2012), Turkey (Isbir 2015) and USA (6 studies: Campbell 2006; Cogan 1988; Hans 2013; Hodnett 2002; Kennell 1991; McGrath 2008). The trials were conducted under widely disparate hospital conditions, regulations and routines.
Based on World Bank Development Indicators (World Bank 2017), at the time of study publication: 13 trials were conducted in high‐income settings (Bréart ‐ Belgium 1992; Bréart ‐ France 1992; Campbell 2006; Cogan 1988; Dickinson 2002; Gagnon 1997; Hans 2013; Hemminki 1990a; Hemminki 1990b; Hodnett 1989; Hodnett 2002; Kennell 1991; McGrath 2008), 13 trials were conducted in middle‐income settings (Akbarzadeh 2014; Bréart ‐ Greece 1992; Bruggemann 2007; Hofmeyr 1991; Isbir 2015; Kashanian 2010; Klaus 1986; Langer 1998; Madi 1999; Morhason‐Bello 2009; Safarzadeh 2012; Torres 1999; Yuenyong 2012), and no studies were conducted in low‐income settings. Chile (Torres 1999) and Greece (Bréart ‐ Greece 1992) are classified as high‐income settings in 2017; however, they were classified as middle‐income settings at the time of the study publications and were treated as middle‐income settings in this analysis. Two studies were conducted in lower‐middle income countries (Guatemala (Klaus 1986), and Nigeria (Morhason‐Bello 2009)).
There was remarkable consistency in the descriptions of continuous support across all trials. In most instances the intervention included continuous or nearly continuous presence, at least during active labour. One trial (Hans 2013) was a community doula intervention throughout pregnancy, labour, childbirth and three months postpartum, including continuous support during childbirth. Twenty‐four of the 26 trials that provided usable outcome data (all except Cogan 1988 and Dickinson 2002) also included specific mention of comforting touch and words of praise and encouragement.
Seventeen trials reported funding sources. The majority of these trials were funded by government or charitable grants. One study (Campbell 2006) reported to have received a "small stipend" from Johnson and Johnson to complete data analysis, though authors reported that; "Johnson & Johnson did not influence the design and conduct of the study or the analysis and interpretation of the data". The remaining nine trials (Bréart ‐ Belgium 1992; Bréart ‐ France 1992; Bréart ‐ Greece 1992; Cogan 1988; Isbir 2015; Kashanian 2010; Safarzadeh 2012; Thomassen 2003; Torres 1999) did not clearly report funding sources. Five trials declared no conflicts of interest (Akbarzadeh 2014; Bruggemann 2007; Isbir 2015; Kashanian 2010; Yuenyong 2012); this was not reported in the remaining trials.
Participants
In 20 trials, pregnant women were recruited around the time of admission to the hospital for childbirth or during the active stage of labour; whereas in six trials, women were recruited during antenatal visits, ranging from 12 to 38 weeks (Campbell 2006; Hans 2013; Hodnett 1989; McGrath 2008; Morhason‐Bello 2009; Torres 1999).
Interventions and comparisons
The interventions included continuous presence and support for women during childbirth by a member of hospital staff, a woman in a doula role, or a trained or untrained member of the woman's social network (e.g. spouse or partner, family member, or friend).
In 11 trials (Bréart ‐ Belgium 1992; Bréart ‐ France 1992; Campbell 2006; Cogan 1988; Dickinson 2002; Gagnon 1997; Hemminki 1990a; Hemminki 1990b; Hodnett 1989; Hodnett 2002; McGrath 2008), hospital policy permitted women to be accompanied by their spouses/partners or other family members during labour, while in the other 15 trials, no additional support people were allowed, or it was unclear if other support people were allowed. Epidural analgesia was not routinely available in eight trials (Bréart ‐ Greece 1992; Hofmeyr 1991; Isbir 2015; Kashanian 2010; Klaus 1986; Madi 1999; Morhason‐Bello 2009; Yuenyong 2012). We were unsuccessful in obtaining information about the availability of epidural analgesia in four trials (Akbarzadeh 2014; Cogan 1988, Hans 2013; Safarzadeh 2012). Epidural analgesia was routinely available in the other 14 trials. Electronic fetal heart rate monitoring was not routine in nine trials (Bruggemann 2007; Hofmeyr 1991; Isbir 2015; Kashanian 2010; Klaus 1986; Langer 1998; Madi 1999; Morhason‐Bello 2009; Yuenyong 2012). In nine trials (Campbell 2006; Dickinson 2002; Gagnon 1997; Hemminki 1990a; Hemminki 1990b; Hodnett 1989; Hodnett 2002; Kennell 1991; McGrath 2008) electronic fetal monitoring (EFM) was used routinely. We were unsuccessful in obtaining information about the use of EFM in eight trials (Akbarzadeh 2014; Bréart ‐ Greece 1992; Bréart ‐ Belgium 1992; Bréart ‐ France 1992; Cogan 1988; Hans 2013; Safarzadeh 2012; Torres 1999).
It was not possible to categorise most of the trials according to the pre‐specified subgroups of early versus active labour. In four trials (Cogan 1988; Hodnett 1989; Klaus 1986; Madi 1999), the support began in early labour. In the other 22 trials, the timing of onset of support was much more heterogenous, as were definitions of early and active labour, in instances in which these were defined. Women were in varying phases of labour, from elective induction to active labour.
In addition, the people providing the support intervention varied in their experience, qualifications and relationship to the labouring women. In nine trials (Bréart ‐ Belgium 1992; Bréart ‐ France 1992; Bréart ‐ Greece 1992; Dickinson 2002; Gagnon 1997; Hemminki 1990a; Hemminki 1990b; Hodnett 2002; Kashanian 2010), the support was provided by a member of the hospital staff, for example, a midwife, student midwife or nurse. In 10 trials the providers were not members of the hospital staff and were not part of the woman's social network; they were women with or without special training, such as doulas or women who had given birth before (Akbarzadeh 2014; Hans 2013; Hodnett 1989; Hofmeyr 1991; Isbir 2015; Kennell 1991; Klaus 1986; McGrath 2008); a childbirth educator (Cogan 1988), or retired nurses (Langer 1998). In seven trials they were companions of the woman's choice from her social network, with or without brief training ‐ a female relative or friend or the woman's spouse/partner (Bruggemann 2007; Campbell 2006; Madi 1999; Morhason‐Bello 2009; Safarzadeh 2012; Torres 1999; Yuenyong 2012).
The comparisons were usual care in the same setting. Usual care did not involve continuous support during childbirth, but in some cases included other coping measures, such as routine epidural analgesia.
Excluded studies
We excluded a total of 19 trials (Bender 1968; Bochain 2000; Brown 2007; Dalal 2006; Dong 2009; Gordon 1999; Hemminki 1990c; Lindow 1998; Manning‐Orenstein 1998; Orbach‐Zinger 2012; Ran 2005; Riley 2012; Scott 1999; Senanayake 2013; Sosa 1980; Trueba 2000; Tryon 1966; Wan 2011; Zhang 1996b). Eight trials were excluded as they were not randomised trials (Bender 1968; Dalal 2006; Ran 2005; Scott 1999; Senanayake 2013; Sosa 1980; Trueba 2000; Tryon 1966). Eight trials were excluded because the intervention was not continuous support during childbirth (Bochain 2000; Brown 2007; Dong 2009; Lindow 1998; Manning‐Orenstein 1998; Orbach‐Zinger 2012; Wan 2011; Zhang 1996b). One trial reported as an abstract provided insufficient information to assess eligibility (Riley 2012). Two further trials were excluded because they did not provide any usable data, due to post‐randomisation exclusions (Gordon 1999) and data not separated by treatment group (Hemminki 1990c). Please refer to table Characteristics of excluded studies for details.
Risk of bias in included studies
We provided details of the risk of bias in each study in the Characteristics of included studies tables and the methodological quality summary (Figure 2) and methodological quality graph (Figure 3).
2.
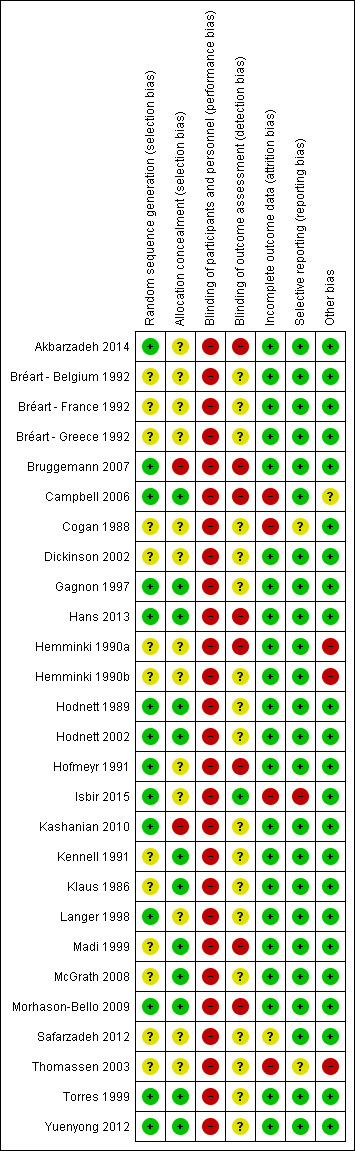
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
3.
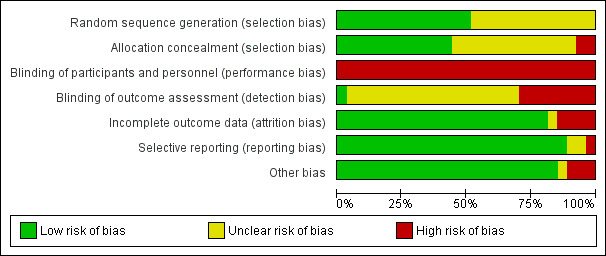
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
Allocation
Random sequence generation: 13 trials were at unclear risk of bias (Bréart ‐ Belgium 1992; Bréart ‐ France 1992; Bréart ‐ Greece 1992; Cogan 1988; Dickinson 2002; Hemminki 1990a; Hemminki 1990b; Kennell 1991; Klaus 1986; Madi 1999; McGrath 2008; Safarzadeh 2012; Thomassen 2003) because they did not describe the method of random assignment. Fourteen trials described using a computer random number generator or referred to a random number table (Akbarzadeh 2014; Bruggemann 2007; Campbell 2006; Gagnon 1997; Hans 2013; Hodnett 1989; Hodnett 2002; Hofmeyr 1991; Isbir 2015; Kashanian 2010; Langer 1998; Morhason‐Bello 2009; Torres 1999; Yuenyong 2012) and were assessed as low risk of bias.
Allocation concealment: the risk of selection bias was high in two small trials (Bruggemann 2007; Kashanian 2010). In Bruggemann 2007, women picked their treatment allocation from an opaque container; Kashanian 2010 used block randomisation under which allocation could have been easily predicted. In 12 trials (Campbell 2006; Gagnon 1997; Hans 2013; Hodnett 1989; Hodnett 2002; Kennell 1991; Klaus 1986; Madi 1999; McGrath 2008; Morhason‐Bello 2009; Torres 1999; Yuenyong 2012), risk of selection bias was low with allocation described as either using central allocation, e.g. Hodnett 2002 used a central, computerised randomisation service accessed by telephone or other trials described using sequentially numbered, opaque, sealed envelopes. In the remaining trials (Akbarzadeh 2014; Bréart ‐ Belgium 1992; Bréart ‐ France 1992; Bréart ‐ Greece 1992; Cogan 1988; Dickinson 2002; Hemminki 1990a; Hemminki 1990b; Hofmeyr 1991; Isbir 2015; Langer 1998; Safarzadeh 2012; Thomassen 2003), risk of selection bias was unclear, e.g. one trial used methods that were centrally controlled but not concealed (Cogan 1988).
Blinding
Blinding of participants and personnel (performance bias): neither those providing nor receiving care could be blinded to the presence/absence of a person providing continuous support. Hodnett 2002 provided evidence to discount contamination and co‐intervention as serious threats to validity. All trials therefore were assessed as having high risk of bias for blinding of participants and personnel.
Blinding of outcome assessment (detection bias): In eight trials group assignment was known and no attempt to blind outcome assessment was apparent. These trials were assessed as being at high risk of bias (Akbarzadeh 2014, Bruggemann 2007; Campbell 2006; Hans 2013; Hemminki 1990a; Hofmeyr 1991; Madi 1999; Morhason‐Bello 2009). One trial was assessed as being at low risk of bias because some blinding of outcome assessment was performed (Isbir 2015). In the remaining 18 trials, risk of bias for blinding of outcome assessment was unclear, often because it was not reported who assessed outcomes and whether or not they were blinded (Bréart ‐ Belgium 1992; Bréart ‐ France 1992; Bréart ‐ Greece 1992; Cogan 1988; Dickinson 2002; Gagnon 1997; Hemminki 1990b; Hodnett 1989; Hodnett 2002; Kashanian 2010; Kennell 1991; Klaus 1986; Langer 1998; McGrath 2008; Safarzadeh 2012; Thomassen 2003; Torres 1999; Yuenyong 2012).
Incomplete outcome data
Attrition bias: we did not include data for outcomes assessed in hospital in a comparison if there was more than 20% loss to follow‐up; we did not include longer‐term outcome data if there was more than 25% loss to follow‐up. Based on these criteria, one trial (Thomassen 2003) provided no usable outcome data. Three further trials were assessed as being at high risk of bias for attrition bias (Campbell 2006; Cogan 1988, Isbir 2015). Isbir 2015 had a total of nine post‐randomisation exclusions, due to emergency caesarean sections. One trial (Safarzadeh 2012) had unclear risk of attrition bias.
Selective reporting
All outcomes appear to have been reported on in most trials. In two trials, it was unclear whether selective reporting had taken place (Cogan 1988; Thomassen 2003). In Cogan 1988 the outcomes had not been specified a priori. In Thomassen 2003 the sample size was based on caesarean section rate, but it is unclear why only emergency caesarean section was reported. One trial (Isbir 2015) had high risk of reporting bias, because women who underwent emergency caesarean section were excluded from the analysis of other outcomes, and caesarean section is a frequently reported outcome for continuous support during childbirth.
Other potential sources of bias
Three trials were assessed as being at high risk of other bias: in two trials the women had been told the purpose of the study differentially (Hemminki 1990a; Hemminki 1990b) and one trial was stopped early for "a range of largely organizational issues" when only a quarter of the original sample size had been enrolled (Thomassen 2003). Risk of bias was unclear in one study (Campbell 2006) and no other sources of bias were apparent in the remaining trials.
Effects of interventions
See: Table 1
Main comparison: continuous support versus usual care ‐ all trials
We considered 23 outcomes. Between one and 24 trials contributed to the analysis of each outcome. Sensitivity analyses, conducted by removing the trials (all of which were small) with a high likelihood of selection bias (Bruggemann 2007; Kashanian 2010) did not alter the conclusions. According to our pre‐specified criteria, there was statistical heterogeneity in all but three outcomes (instrumental vaginal birth, low five‐minute Apgar score, and low postpartum self‐esteem). Inspection of the forest plots did not suggest sources of heterogeneity. For the three outcomes postpartum depression, delayed initiation of breastfeeding and difficulty mothering, this statistical heterogeneity confirmed our conclusion that based on clinical heterogeneity a summary statistic would not yield meaningful results (discussed further below). We report the results of fixed‐effect analyses for instrumental vaginal birth, low five‐minute Apgar score, and low postpartum self‐esteem (the latter only contained one trial), and random‐effects analyses for all other outcomes in which summary statistics were computed.
Primary outcomes
Women who had continuous, one‐to‐one support during labour were:
more likely to have
-
a spontaneous vaginal birth (21 trials, 14,369 women, average risk ratio (RR) 1.08, 95% confidence interval (CI) 1.04 to 1.12, I² = 61%, Tau² = 0.00, low‐quality evidence), Analysis 1.1;
We included 21 studies that reported spontaneous vaginal birth that we assessed for small‐study effect (publication bias). For spontaneous vaginal birth, we observed that most studies clustered around the effect estimate without any obvious asymmetry, indicating a low risk of publication bias (Figure 4).
1.1. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 1 Spontaneous vaginal birth.
4.
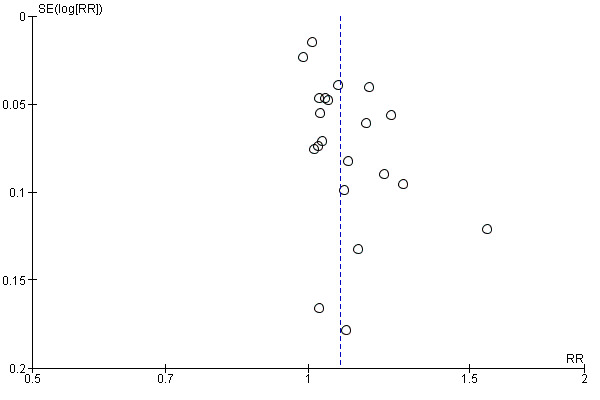
Funnel plot of comparison: 1 Continuous support versus usual care ‐ all trials, outcome: 1.1 Spontaneous vaginal birth
less likely to have
-
reported negative rating of or negative feelings about childbirth experience (11 trials, 11,133 women, average RR 0.69, 95% CI 0.59 to 0.79, I² = 63%, Tau² = 0.03, low‐quality evidence), Analysis 1.2;
we included 11 studies that reported negative rating of or negative feelings about birth experience that we assessed for small‐study effect (publication bias). For negative rating of or negative feelings about birth experience, we observed that most studies clustered around the effect estimate without any obvious asymmetry, indicating a low risk of publication bias (Figure 5). Two trials reported negative ratings or negative feelings about childbirth experience as satisfaction with care received (Bruggemann 2007) and overall rating of birth experience (Campbell 2006). We acknowledge that this is not ideal, and so carried out a sensitivity analysis to account for this. We removed Bruggemann 2007 and Campbell 2006 from the overall analysis to see if this made any difference to the result. The overall result was unchanged (9 trials, 10,427 women, average RR 0.72, 95% CI 0.61 to 0.84, I² = 62%,Tau² = 0.03).
1.2. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 2 Negative rating of/negative feelings about birth experience.
5.
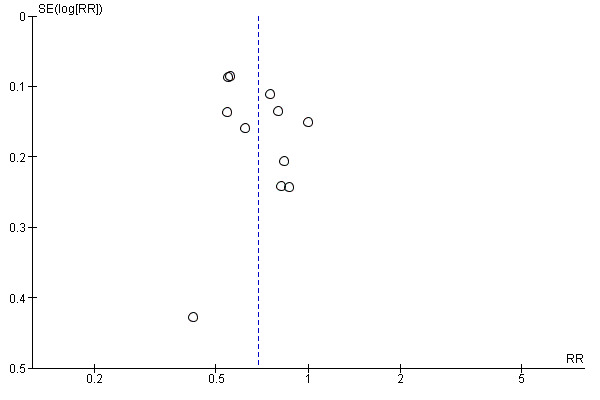
Funnel plot of comparison: 1 Continuous support versus usual care ‐ all trials, outcome: 1.2 Negative rating of/negative feelings about birth experience
and there was no apparent impact of continuous support on
admission to the special care nursery (7 trials; 8897 infants, average RR 0.97, 95% CI 0.76 to 1.25, I² 37%, Tau² = 0.03, low‐quality evidence), Analysis 1.4; and
-
exclusive or any breastfeeding at any time point, as defined by trial authors (4 trials, 5584 women, average RR 1.05, 95% CI 0.96 to 1.16, I² = 66%, Tau² = 0.01, low‐quality evidence), Analysis 1.5;
trials reported exclusive or any breastfeeding at any time point as: self‐reported breastfeeding duration at one month postpartum (Langer 1998), at six weeks postpartum (Hofmeyr 1991), and at four months postpartum (Hans 2013). Hodnett 2002 reported "not breastfeeding at all" at six weeks postpartum and we calculated the reciprocal for this outcome for this analysis (Analysis 1.5). We acknowledge that this is not ideal, and so carried out a sensitivity analysis to account for this. We removed Hodnett 2002 from the overall analysis to see if this made any difference to the result. The overall result was unchanged (3 trials, 1025 women, average RR 1.11, 95% CI 0.98 to 1.27, I² = 48%,Tau² = 0.01).
1.4. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 4 Admission to special care nursery.
1.5. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 5 Exclusive or any breastfeeding at any time point, as defined by trial authors.
Evidence of postpartum depression was a reported outcome in just two trials (Hodnett 2002; Hofmeyr 1991). Hodnett 2002 used the Edinburgh Postnatal Depression Inventory and reported the frequencies of scores greater than 12. Hofmeyr 1991 used the Pitt Depression Inventory and reported scores indicating mild (< 20), moderate (20 to 34), and severe (> 34) depressive symptomatology. We combined the frequencies of moderate and severe depressive symptomatology, since Pitt scores greater than 19 have been considered indicative of postpartum depression (Avan 2010). The two trials were widely disparate in populations, the hospital conditions within which they were conducted, and the type of support provider (Hodnett 2002 conducted in 13 tertiary and community hospitals in the USA and Canada, and Hofmeyr 1991 conducted in one community hospital in South Africa). We concluded that combining the studies would not yield meaningful information. In both trials the direction of effect was the same. In Hofmeyr 1991, eight out of 74 women (10.8%) in the group receiving continuous support had depressive symptomatology compared to 44 out of 75 women (58.6%) in the control group (RR 0.18, 95% CI 0.09 to 0.36). In Hodnett 2002, 245 out of 2816 (8.7%) in the supported group had depressive symptomatology, compared to 277 out of 2751 (10.0%) in the control group (RR 0.86, 95% CI 0.73 to 1.02; Analysis 1.3, low‐quality evidence).
1.3. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 3 Postpartum depression.
Secondary outcomes
Women who had continuous, one‐to‐one support during labour were:
more likely to have
-
shorter labours (13 trials, 5429 women, mean difference (MD) ‐0.69 hours, 95% CI ‐1.04 to ‐0.34, I² = 66%, Tau² = 0.20, low‐quality evidence), Analysis 1.9;
we included 13 studies that reported duration of labour that we assessed for small‐study effect (publication bias). For duration of labour, we observed that most studies clustered around the effect estimate, without any obvious asymmetry, indicating a low risk of publication bias (Figure 6).
shorter time from birth to initiation of breastfeeding (1 trial, 585 women, MD ‐44.60 minutes, 95% CI ‐47.63 to ‐41.57), Analysis 1.16;
1.9. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 9 Labour length.
6.
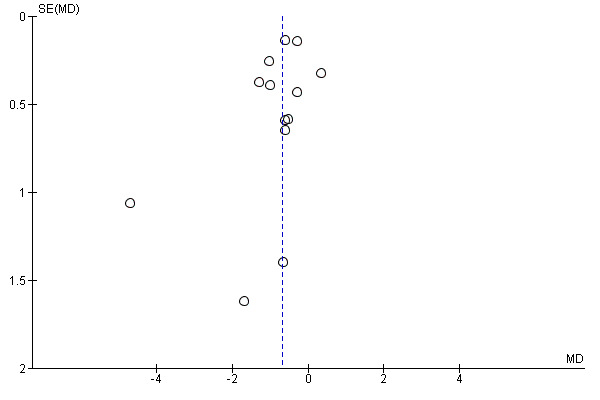
Funnel plot of comparison: 1 Continuous support versus usual care ‐ all trials, outcome: 1.9 Labour length
1.16. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 16 Time from birth to initiation of breastfeeding.
and less likely to have
-
any intrapartum analgesia or anaesthesia (15 trials, 12,433 women, average RR 0.90, 95% CI 0.84 to 0.96, I² = 73%, Tau² = 0.01), Analysis 1.6;
we included 15 studies that reported use of any analgesia or anaesthesia that we assessed for small‐study effect (publication bias). For use of any analgesia or anaesthesia, we observed that most studies fell at the top around the effect estimate without any obvious asymmetry, suggesting a low risk of publication bias (Figure 7).
regional analgesia or anaesthesia (9 trials, 11,444 women, average RR 0.93, 95% CI 0.88 to 0.99, I² = 81%, Tau² = 0.01), Analysis 1.7; the effect should be interpreted with caution because the forest plot suggests that the apparent small effect was caused by a large effect in one study.
-
an instrumental vaginal birth (19 trials, 14,118 women, RR 0.90, 95% CI 0.85 to 0.96), Analysis 1.12;
we included 19 studies that reported instrumental vaginal birth that we assessed for small‐study effect (publication bias). For instrumental vaginal birth, we observed that most studies clustered around the effect estimate without any obvious asymmetry, indicating a low risk of publication bias (Figure 8).
-
a caesarean birth (24 trials, 15,347 women, average RR 0.75, 95% CI 0.64 to 0.88, I² = 58%, Tau² = 0.07, low‐quality evidence), Analysis 1.11;
we included 24 studies that reported caesarean birth that we assessed for small‐study effect (publication bias). For caesarean birth, we observed that most studies clustered around the top of the effect estimate without any obvious asymmetry, indicating a low risk of publication bias (Figure 9).
unsatisfactory mother‐infant interactions (defined as not managing well with baby at 8 weeks postpartum, or as defined by trial authors); no trial reported this outcome. Hofmeyr 1991 reported the prevalence of women who self‐reported that they were managing well with their baby at six weeks postpartum and this was found to be higher in the continuous support group (149 women, 90.5% in support group versus 65.3% in control group, P < 0.001).
-
a baby with a low five‐minute Apgar score (14 trials, 12,615 infants, RR 0.62, 95% CI 0.46 to 0.85), Analysis 1.18;
we included 14 studies that reported low five‐minute Apgar score that we assessed for small‐study effect (publication bias). For low five‐minute Apgar score, we observed that most studies clustered around the effect estimate, with a cluster to the left side, indicating a slight risk of publication bias (Figure 10).
1.6. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 6 Any analgesia/anaesthesia.
7.
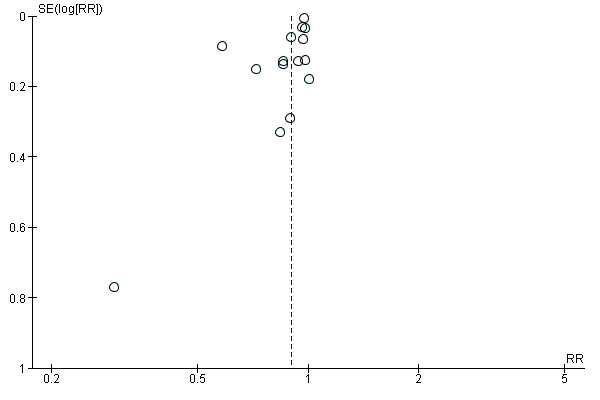
Funnel plot of comparison: 1 Continuous support versus usual care ‐ all trials, outcome: 1.6 Any analgesia/anaesthesia
1.7. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 7 Regional analgesia/anaesthesia.
1.12. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 12 Instrumental vaginal birth.
8.
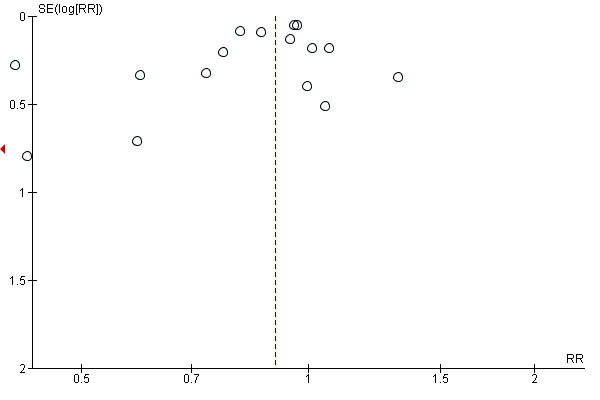
Funnel plot of comparison: 1 Continuous support versus usual care ‐ all trials, outcome: 1.12 Instrumental vaginal birth
1.11. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 11 Caesarean birth.
9.
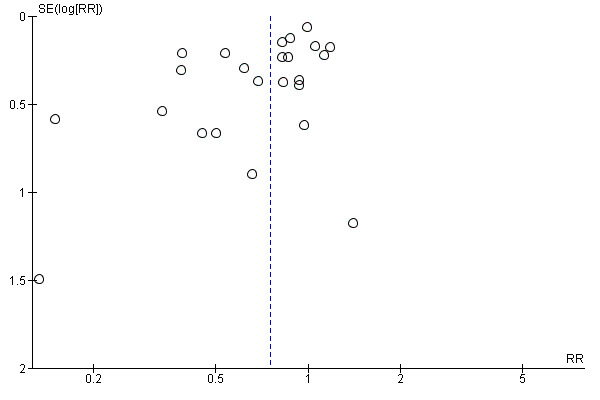
Funnel plot of comparison: 1 Continuous support versus usual care ‐ all trials, outcome: 1.11 Caesarean birth
1.18. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 18 Low 5‐minute Apgar score.
10.
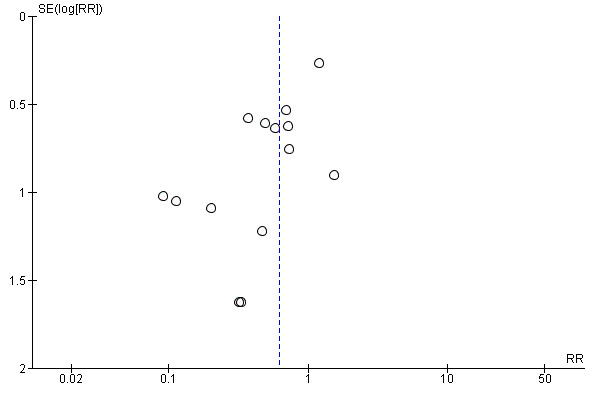
Funnel plot of comparison: 1 Continuous support versus usual care ‐ all trials, outcome: 1.18 Low 5‐minute Apgar score
and there was no apparent impact of continuous labour support on
the likelihood of serious perineal trauma (4 trials, 8120 women, average RR 0.97, 95% CI 0.92 to 1.01, I² = 44%, Tau² = 0.00), Analysis 1.13;
postpartum report of severe labour pain (4 trials; 2456 women, average RR 1.00, 95% CI 0.83 to 1.21, I² = 78%, Tau² = 0.03), Analysis 1.10;
trials reported labour pain as: perception of pain (high) during labour based on a validated visual analogue pain rating scale (Langer 1998), self‐reported intensity of pain (Bréart ‐ Belgium 1992; Bréart ‐ France 1992), and the McGill pain rating index for labour pain (Hofmeyr 1991).
low postpartum self‐esteem (1 trial, 652 women, RR 1.00, 95% CI 0.77 to 1.30), Analysis 1.21, (using the Coopersmith self‐esteem inventory, where 'low' was calculated as a score of 0 to 16; timing of administration of self‐esteem assessment not reported);
Hofmeyr 1991 reported self‐esteem scores at day 1, 6 weeks, and 1 year postpartum using the Coopersmith self‐esteem inventory (using mean and either standard deviation or standard error). Hofmeyr 1991 reported no clear difference in mean self‐esteem scores between the support and control groups at day 1 or 1 year postpartum. However, mean self‐esteem scores were higher in the support group at six weeks postpartum, compared to the control group (mean 74.5, SE 2.0 versus mean 58.8, SE 2.8, P = 0.0001). A score above 46 is indicative of high self‐esteem (Ryden 1978).
prolonged neonatal hospital stay (3 trials, 1098 infants, average RR 0.83, 95% CI 0.42 to 1.65, I² = 62%, Tau² = 0.15), Analysis 1.19;
-
difficulty mothering, Analysis 1.20;
three trials reported results related to difficulty in mothering (Campbell 2006; Hodnett 2002; Hofmeyr 1991). As was the case with postpartum depression, the trials were widely disparate in populations, the hospital conditions where they were conducted, the type of support provider. Inspection of the forest plot supported our conclusion that combining the trials would not yield meaningful information. In Hodnett 2002, 873 out of 2836 (30.8%) women in the continuous support group reported difficulty mothering, compared to 853 out of 2765 (30.8%) in the control group (RR 1.00, 95% CI 0.92, 1.08). In Campbell 2006, 11 out of 229 (3.8%) women in the continuous support group reported difficulty mothering, compared to 38 out of 265 (14.3%) in the control group (RR 0.33, 95% CI 0.18, 0.64). In Hofmeyr 1991, 33 out of 74 (44.6%) in the continuous support group found becoming a mother easy, compared to 8 out of 75 (10.7%) in the control group. The Hofmeyr 1991 data are not shown on our forest plot as we did not feel that reciprocal data were appropriate to use in this outcome.
-
use of synthetic oxytocin during labour (17 trials, 12,833 women, average RR 0.97, 95% CI 0.91 to 1.03, I² = 61%, Tau² = 0.01), Analysis 1.8;
we included 17 studies that reported use of synthetic oxytocin during labour that we assessed for small‐study effect (publication bias). For use of synthetic oxytocin during labour, we observed that most studies fell at the top around the effect estimate and clustered on the left, indicating a slight risk of publication bias (Figure 11);
delayed skin‐to‐skin contact (1 trial, 212 infants, RR 0.82, 95% CI 0.64 to 1.04), Analysis 1.14, (more than one hour after delivery, or as defined by trial authors); and
restricted mobility during labour (1 trial, 6915 women, RR 1.02, 95% CI 1.00 to 1.05), Analysis 1.17, (defined as not able to move throughout labour).
1.13. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 13 Perineal trauma.
1.10. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 10 Postpartum report of severe labour pain.
1.21. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 21 Low postpartum self‐esteem.
1.19. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 19 Prolonged neonatal hospital stay.
1.20. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 20 Difficulty mothering.
1.8. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 8 Synthetic oxytocin during labour.
11.
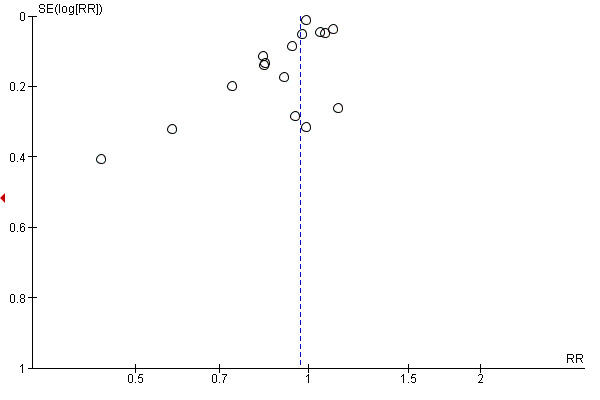
Funnel plot of comparison: 1 Continuous support versus usual care ‐ all trials, outcome: 1.8 Synthetic oxytocin during labour
1.14. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 14 Delayed skin‐to‐skin contact, as defined by trial authors.
1.17. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 17 Restricted mobility during labour, as defined by trial authors.
Reported outcomes that were based on few trials, a small number of women, or both, should be interpreted with caution. These outcomes are: unsatisfactory mother‐infant interactions, delayed initiation of breastfeeding, low postpartum self‐esteem, delayed skin‐to‐skin contact, and restricted mobility during labour.
Two trials reported results related to delayed initiation of breastfeeding (Campbell 2006; Morhason‐Bello 2009). As was the case with postpartum depression and difficulty in mothering, the trials were widely disparate in populations, hospital conditions where they were conducted, the type of support provider. Inspection of the forest plot supported our conclusion that combining the trials would not yield meaningful information. Delayed initiation of breastfeeding was reduced in both studies that reported this outcome, but data were not aggregated due to extreme heterogeneity. Campbell 2006 reported the number of mothers who started breastfeeding within the first hour, which we recalculated to the reciprocal, and calculated to 112 out of 229 (48.9%) in the continuous support group reported delayed initiation of breastfeeding, compared to 172 out of 265 (64.9%) in the control group (RR 0.75, 95% CI 0.64, 0.88; Analysis 1.15). In Morhason‐Bello 2009, no women in the continuous support group (N = 94) reported delayed initiation of breastfeeding, compared to 68 out of 115 (59.1%) in the control group (RR 0.01, 95% CI 0.00, 0.14).
1.15. Analysis.

Comparison 1 Continuous support versus usual care ‐ all trials, Outcome 15 Delayed initiation of breastfeeding (> 1 hour after birth, or as defined by trial authors).
No trials reported results related to women who were unlikely to recommend birth in that institution.
Subgroup comparisons
We present the results of the subgroup analyses below. Although we made every effort to obtain required information from trial authors, none of the subgroup comparisons were based on the total number of included trials for which usable data were available. Thus, results must be interpreted with caution. We did not report results for postpartum depression or exclusive or any breastfeeding at any time point, because too few trials provided data. Only two trials contributed data about postpartum depression (Hodnett 2002; Hofmeyr 1991) and four about exclusive or any breastfeeding at any time point (Hans 2013; Hodnett 2002; Hofmeyr 1991; Langer 1998).
We were unable to conduct the planned subgroup comparison based on timing of onset of labour support. It was not possible to categorise most of the trials according to the pre‐specified subgroups of early versus active labour. In four trials (Cogan 1988; Hodnett 1989; Klaus 1986; Madi 1999), the support began in early labour. In the other 22 trials, both the definitions of early and active labour and the timing of onset of support were much more heterogenous, where they were defined. Women were in varying phases of labour, from elective induction to active labour.
As noted in Subgroup analysis and investigation of heterogeneity, totals in the subgroup analysis figures may differ slightly from those in the main comparisons.
Subgroup A.1: Policies about the presence of companions during labour and birth
Spontaneous vaginal birth: In nine trials (10,889 women) companions were permitted (average RR 1.03, 95% CI 1.00 to 1.06). In 10 trials (3329 women) companions were not permitted (average RR 1.11, 95% CI 1.06 to 1.17). Chi² for the subgroup comparison = 7.19, P = 0.007, Analysis 2.1. There was a modest but clear difference between the subgroups. Although the group in which companions were permitted was borderline, both favoured support over usual care. Women who were not able to have companions with them in labour benefited more from having continuous support in labour than those who were not permitted to have a companion.
Negative ratings of or negative views about the birth experience: In five trials (8639 women) companions were permitted (average RR 0.77, 95% CI 0.60 to 1.00). In six trials (2539 women) companions were not permitted (average RR 0.63, 95% CI 0.53 to 0.74). Chi² for the subgroup comparison = 1.74, P = 0.19, Analysis 2.2. There was no clear difference between the subgroups, which both favoured support regardless of whether or not a labour companion was permitted. It should be noted that when the two trials that reported reciprocal data were omitted from the analysis there was evidence of a subgroup difference (test for subgroup differences: Chi² = 6.53, P = 0.01.
Postpartum depression: too few trials contributed to this outcome to produce a meaningful subgroup analysis, Analysis 2.3.
Admission to special care nursery: in two trials (7328 women), companions were permitted (average RR 0.99, 95% CI 0.82 to 1.20). In five trials (1569 women), companions were not permitted (average RR 0.87, 95% CI 0.47 to 1.61). Chi² for the subgroup comparison = 0.17, P = 0.68, Analysis 2.4. No clear difference was observed between the subgroups. Labour companions do not appear to affect the number of babies admitted to special care nursery.
Exclusive or any breastfeeding at any time point: too few trials contributed to this outcome to produce a meaningful subgroup analysis, Analysis 2.5.
Any intrapartum analgesia or anaesthesia: In seven trials (9752 women) companions were permitted (average RR 0.97, 95% CI 0.96 to 0.99), and in seven trials (2598 women) companions were not permitted (average RR 0.78, 95% CI 0.61 to 1.00). Chi² for the subgroup comparison = 3.12, P = 0.08, Analysis 2.6. There may be a small difference between subgroups but we cannot be certain of this.
Synthetic oxytocin during labour: in five trials (9495 women) companions were permitted (average RR 0.99, 95% CI 0.90 to 1.10). In 10 trials (3125 women) companions were not permitted (average RR 0.94, 95% CI 0.85 to 1.05). Chi² for the subgroup comparison = 0.45, P = 0.50, Analysis 2.7. There was no clear difference between these subgroups.
Caesarean birth: in 11 trials (11,326 women) companions were permitted (average RR 0.91, 95% CI 0.79 to 1.04). In 11 trials (3849 women) companions were not permitted (average RR 0.68, 95% CI 0.52 to 0.88). Chi² for the subgroup comparison = 3.73, P = 0.05, Analysis 2.8. A clear difference was observed between the subgroups, although the confidence intervals just cross. It appears that women not permitted to have other support benefited most from having continuous support. The effect was not clear for those women who were permitted to have other companions present.
2.1. Analysis.

Comparison 2 Continuous support versus usual care ‐ policy regarding presence of companion, Outcome 1 Spontaneous vaginal birth.
2.2. Analysis.

Comparison 2 Continuous support versus usual care ‐ policy regarding presence of companion, Outcome 2 Negative rating of/negative feelings about birth experience.
2.3. Analysis.

Comparison 2 Continuous support versus usual care ‐ policy regarding presence of companion, Outcome 3 Postpartum depression.
2.4. Analysis.

Comparison 2 Continuous support versus usual care ‐ policy regarding presence of companion, Outcome 4 Admission to special care nursery.
2.5. Analysis.

Comparison 2 Continuous support versus usual care ‐ policy regarding presence of companion, Outcome 5 Exclusive or any breastfeeding at any time point, as defined by trial authors.
2.6. Analysis.

Comparison 2 Continuous support versus usual care ‐ policy regarding presence of companion, Outcome 6 Any analgesia/anaesthesia.
2.7. Analysis.

Comparison 2 Continuous support versus usual care ‐ policy regarding presence of companion, Outcome 7 Synthetic oxytocin during labour.
2.8. Analysis.

Comparison 2 Continuous support versus usual care ‐ policy regarding presence of companion, Outcome 8 Caesarean birth.
Subgroup A.2: Availability of epidural analgesia
Spontaneous vaginal birth: In 13 trials (12,672 women), epidural analgesia was routinely available (average RR 1.06, 95% CI 1.02 to 1.10). In six trials (1546 women) epidural analgesia was not routinely available (average RR 1.11, 95% CI 1.04 to 1.19). Chi² for the subgroup comparison = 1.38, P = 0.24, Analysis 3.1. There was no evidence of a difference between subgroups.
Negative ratings of or negative views about the birth experience: In nine trials (10,404 women) epidural analgesia was routinely available (average RR 0.73, 95% CI 0.63 to 0.86). In two trials (774 women) epidural analgesia was not routinely available (RR 0.55, 95% CI 0.48 to 0.63). Chi² for the subgroup comparison = 7.10, P = 0.008, Analysis 3.2. Continuous support produced more positive feelings about birth experience regardless of epidural availability; however, the difference was clearly greater in women unable to access epidural anaesthesia. The subgroup difference was still apparent even when we omitted the two trials where we had analysed the data as reciprocals (Bruggemann 2007; Campbell 2006), test for subgroup differences: Chi² = 15.32, P < 0.0001.
Postpartum depression: too few trials contributed to this outcome to produce a meaningful subgroup analysis, Analysis 3.3.
Admission to special care nursery: In five trials (8380 women) epidural analgesia was routinely available (average RR 0.98, 95% CI 0.85 to 1.13). In two trials (517 women) epidural analgesia was not routinely available (average RR 0.26, 95% CI 0.08 to 0.88). Chi² for the subgroup comparison = 4.51, P = 0.03, Analysis 3.4. There was a modest subgroup difference for this outcome. Babies of women who did not have access to epidurals and had continuous support were less likely to be admitted to the special care nursery. Similar numbers of babies were admitted to special care in the continuous support and usual care groups when the women had access to epidurals.
Exclusive or any breastfeeding at any time point: too few trials contributed to this outcome to produce a meaningful subgroup analysis, Analysis 3.5.
Any intrapartum analgesia or anaesthesia: In nine trials (10,888 women), epidural analgesia was routinely available (average RR 0.91, 95% CI 0.84 to 0.97). In five trials (1462 women) epidural analgesia was not routinely available (average RR 0.83, 95% CI 0.69 to 0.99). Chi² for the subgroup comparison = 0.89, P = 0.35, Analysis 3.6. There was no clear difference between the subgroups for this outcome.
Synthetic oxytocin during labour: in eight trials (10,568 women) epidural analgesia was routinely available; (average RR 0.99, 95% CI 0.94 to 1.05). In eight trials (2129 women), epidural analgesia was not routinely available; (average RR 0.80, 95% CI 0.62 to 1.03). Chi² for the subgroup comparison = 2.56, P = 0.11, Analysis 3.7. There was no clear difference between the subgroups for this outcome.
Caesarean birth: in 14 trials (13,064 women), epidural analgesia was routinely available (average RR 0.91, 95% CI 0.81 to 1.02). In eight trials (2149 women), epidural analgesia was not routinely available (average RR 0.54, 95% CI 0.41 to 0.72). In two very small trials (34 women and 100 women), we were unable to determine if epidural analgesia was routinely available (average RR 0.36, 95% CI 0.04 to 3.09). Chi² for the subgroup comparison = 12.03, P = 0.002, Analysis 3.8. Continuous support did not make a clear difference on rates of caesarean section compared with usual care in women who had epidurals available to them. However, there was a clear difference between this subgroup and women not able to have an epidural. Continuous support had a greater effect in reducing the rate of caesarean sections when women were not able to have epidurals.
3.1. Analysis.

Comparison 3 Continuous support versus usual care ‐ availability of epidural analgesia, Outcome 1 Spontaneous vaginal birth.
3.2. Analysis.

Comparison 3 Continuous support versus usual care ‐ availability of epidural analgesia, Outcome 2 Negative rating of/negative feelings about birth experience.
3.3. Analysis.

Comparison 3 Continuous support versus usual care ‐ availability of epidural analgesia, Outcome 3 Postpartum depression.
3.4. Analysis.

Comparison 3 Continuous support versus usual care ‐ availability of epidural analgesia, Outcome 4 Admission to special care nursery.
3.5. Analysis.

Comparison 3 Continuous support versus usual care ‐ availability of epidural analgesia, Outcome 5 Exclusive or any breastfeeding at any time point, as defined by trial authors.
3.6. Analysis.

Comparison 3 Continuous support versus usual care ‐ availability of epidural analgesia, Outcome 6 Any analgesia/anaesthesia.
3.7. Analysis.

Comparison 3 Continuous support versus usual care ‐ availability of epidural analgesia, Outcome 7 Synthetic oxytocin during labour.
3.8. Analysis.

Comparison 3 Continuous support versus usual care ‐ availability of epidural analgesia, Outcome 8 Caesarean birth.
Subgroup A.3: Routine use of electronic fetal monitoring (EFM)
Spontaneous vaginal birth: In eight trials (9717 women) EFM was routine (average RR 1.07, 95% CI 1.01 to 1.13). In seven trials (1913 women) EFM was not routine (average RR 1.11, 95% CI 1.06 to 1.17). In six trials (2811 women), the policy about routine EFM is not known (average RR 1.09, 95% CI 1.00 to 1.18). Chi² for the subgroup comparison = 1.27, P = 0.53, Analysis 4.1. This subgroup analysis is difficult to interpret but it appears that continuous support probably increases the likelihood of women having a spontaneous vaginal birth regardless of EFM. There was no evidence of a subgroup difference. The effect was greater in women who did not have EFM although the lower CI touches the upper CI of those who had EFM. Where it was not clear whether women had EFM or not, the CIs cross the line of no effect.
Negative ratings of or negative views about the birth experience: four trials (7467 women) were conducted in settings with routine EFM (average RR 0.72, 95% CI 0.55 to 0.94). Four trials (1710 women) were conducted in settings in which EFM was not routine (average RR 0.60, 95% CI 0.49 to 0.74). Three trials (1977 women) were conducted in settings in which the use of routine EFM is not known (average RR 0.84, 95% CI 0.65 to 1.08). Chi² for the subgroup comparison = 4.02, P = 0.13, Analysis 4.2. The use of EFM made no clear difference to the number of women with negative feelings about their birth experience. It should be noted that there was also no subgroup difference when we omitted the two trials where we had analysed the data as reciprocals (Bruggemann 2007; Campbell 2006), test for subgroup differences: Chi² = 4.01, P = 0.13.
Postpartum depression: too few trials contributed to this outcome to produce a meaningful subgroup analysis, Analysis 4.3.
Admission to special care nursery: in three trials (7740 women) EFM was routine (average RR 0.97, 95% CI 0.84 to 1.11). In three trials (729 women) EFM was not routine (average RR 0.48, 95% CI 0.15 to 1.52). In one trial (428 women), it is not known whether EFM was routine (RR 1.98, 95% CI 0.76 to 5.18). Chi² for the subgroup comparison = 4.76, P = 0.09, Analysis 4.4. The use of EFM made no clear difference to the number of women who had babies admitted to special care.
Exclusive or any breastfeeding at any time point: too few trials contributed to this outcome to produce a meaningful subgroup analysis, Analysis 4.5.
Any intrapartum analgesia or anaesthesia: in six trials (8580 women), EFM was routine (average RR 0.88, 95% CI 0.79 to 0.99). In six trials (2186 women), EFM was not routine (average RR 0.90, 95% CI 0.79 to 1.04). In two trials (1579 women), the policy about routine EFM was unknown (average RR 0.89, 95% CI 0.80 to 0.99). Chi² for the subgroup comparison = 0.07, P = 0.97, Analysis 4.6. There is no clear difference in numbers of women receiving any analgesia or anaesthesia between the subgroups. Women who had routine EFM, and those where it was not clear if they had EFM, who had continuous support were less likely to require analgesia although this result is borderline. The effect was not clear for the women who did not have routine EFM.
Synthetic oxytocin during labour: In four trials (8340 women) EFM was routine (average RR 0.93, 95% CI 0.78 to 1.11). In eight trials (1789 women) EFM was not routine (average RR 0.80, 95% CI 0.64 to 1.01). In five trials (2718 women) it is not known whether EFM was routine (average RR 1.02, 95% CI 0.97 to 1.08). Chi² for the subgroup comparison = 4.62, P = 0.10, Analysis 4.7. There was no evidence of a subgroup difference for this outcome.
Caesarean birth: In nine trials (10,123 women), EFM was routine (average RR 0.84, 95% CI 0.71 to 1.00). In nine trials (2529 women) EFM was not routine (average RR 0.59, 95% CI 0.43 to 0.81). In six trials (2695 women), it is not known whether EFM was routine (average RR 0.86, 95% CI 0.56 to 1.32). Chi² for the subgroup comparison = 3.95, P = 0.14, Analysis 4.8. There was no evidence of a subgroup difference for this outcome. Women who had continuous support and did not have routine EFM were less likely to have a caesarean section. The subgroup of women who did have routine EFM and continuous care probably had fewer caesarean sections then those with usual care but the result is borderline.
4.1. Analysis.

Comparison 4 Continuous support versus usual care ‐ policy about routine EFM, Outcome 1 Spontaneous vaginal birth.
4.2. Analysis.

Comparison 4 Continuous support versus usual care ‐ policy about routine EFM, Outcome 2 Negative rating of/negative views about birth experience.
4.3. Analysis.

Comparison 4 Continuous support versus usual care ‐ policy about routine EFM, Outcome 3 Postpartum depression.
4.4. Analysis.

Comparison 4 Continuous support versus usual care ‐ policy about routine EFM, Outcome 4 Admission to special care nursery.
4.5. Analysis.

Comparison 4 Continuous support versus usual care ‐ policy about routine EFM, Outcome 5 Exclusive or any breastfeeding at any time point, as defined by trial authors.
4.6. Analysis.

Comparison 4 Continuous support versus usual care ‐ policy about routine EFM, Outcome 6 Any analgesia/anaesthesia.
4.7. Analysis.

Comparison 4 Continuous support versus usual care ‐ policy about routine EFM, Outcome 7 Synthetic oxytocin during labour.
4.8. Analysis.

Comparison 4 Continuous support versus usual care ‐ policy about routine EFM, Outcome 8 Caesarean birth.
Subgroup B: Provider characteristics
Spontaneous vaginal birth: In nine trials (10,813 women) the support was provided by a member of the hospital staff (average RR 1.05, 95% CI 1.01 to 1.09). In six trials (2035 women) the support was provided by a woman who was not part of the hospital staff nor part of the woman's social network (average RR 1.15, 95% CI 1.05 to 1.26). In six trials (1620 women), the support was provided by a member of the woman's social network (average RR 1.04, 95% CI 0.97 to 1.11). Chi² for the subgroup comparison = 3.99, P = 0.14, Analysis 5.1. There is no evidence of a difference between subgroups. The largest effect, favouring continuous support, was observed in the group of women supported by people not employed by the hospital or chosen by the women, for example, doulas, although heterogeneity is high within this outcome. A smaller effect size was seen with support given by hospital staff. The difference in spontaneous vaginal birth numbers between usual care and continuous care for women who chose their companion was not clear.
Negative ratings of or negative views about the birth experience: in four trials (8145 women) support providers were hospital staff (average RR 0.87, 95% CI 0.73 to 1.03). In three trials (1325 women) the providers were not hospital staff and not part of the woman's social network (average RR 0.65, 95% CI 0.53 to 0.80). In four trials (1708 women), providers were part of the woman's social network (average RR 0.58, 95% CI 0.50 to 0.67). Chi² for the subgroup comparison = 12.65, P = 0.002, Analysis 5.2. The effect size of negative views about the birth experience, favouring continuous support, was similar for women who were supported by someone not employed by the hospital, if they knew the person giving the support or not. The effect was less clear with those women who were supported by hospital staff with the CI crossing the line of no effect. The subgroup difference was still apparent even when we omitted the two trials where we had analysed the data as reciprocals (Bruggemann 2007; Campbell 2006), test for subgroup differences: Chi² = 5.24, P = 0.07.
Postpartum depression: too few trials contributed to this outcome to produce a meaningful subgroup analysis, Analysis 5.3.
Admission to special care nursery: in three trials (7428 women), the support was provided by a member of the hospital staff (average RR 0.99, 95% CI 0.82 to 1.20). In two trials (829 women), the support was provided by a woman who was not a member of the hospital staff and not part of the woman's social network (average RR 0.57, 95% CI 0.17 to 1.87). In two trials (640 women) the support was provided by a member of the woman's social network (average RR 1.38, 95% CI 0.61 to 3.14). Chi² for the subgroup comparison = 1.47, P = 0.48, Analysis 5.4. There were only seven trials in this subgroup analysis and there was no evidence of a difference between them. All subgroup meta‐analysis crossed the line of no effect.
Exclusive or any breastfeeding at any time point: no trials contributed to this outcome, Analysis 5.5.
Any intrapartum analgesia or anaesthesia: In six trials (9152 women) the support was provided by a member of the hospital staff (average RR 0.97, 95% CI 0.96 to 0.99). In four trials (1790 women), the support was provided by a woman who was not a member of the staff and was not part of the woman's social network (average RR 0.72, 95% CI 0.47 to 1.10). In four trials (1408 women) the support was provided by a member of the woman's social network (average RR 0.93, 95% CI 0.86 to 1.01). Chi² for the subgroup comparison = 3.16, P = 0.21, Analysis 5.6. There was no evidence of a difference between subgroups for this outcome, CIs overlapped, and were either very close to, or touching the line of no effect.
Synthetic oxytocin during labour: In six trials (9561 women), the support was provided by a member of the hospital staff (average RR 1.01, 95% CI 0.93 to 1.11). In four trials (1081 women), the support was provided by a woman who was not a member of the staff and was not part of the woman's social network (average RR 0.67, 95% CI 0.43 to 1.06). In seven trials (2191 women), the support was provided by a member of the woman's social network (average RR 0.99, 95% CI 0.96 to 1.01). Chi² for the subgroup comparison = 3.04, P = 0.22, Analysis 5.7. There was no evidence of a subgroup difference.
Caesarean birth: in nine trials (10,786 women), the support was provided by a member of the hospital staff (average RR 0.94, 95% CI 0.84 to 1.05). In nine trials (2502 women), the support was provided by a woman who was not a member of the hospital staff and not part of the woman's social network (average RR 0.61, 95% CI 0.45 to 0.83). In six trials (2059 women), the support was provided by a member of the woman's social network (average RR 0.76, 95% CI 0.50 to 1.17). Chi² for the subgroup comparison = 7.02, P = 0.03, Analysis 5.8. There appears to be evidence of a subgroup difference between the groups of women supported by hospital staff, and those supported by non‐staff, not chosen by the women herself. For reducing caesarean section rates, the effect was greater for women being supported by a companion who they did not choose and were not hospital staff. Continuous support by hospital staff did not make a clear difference. Similarly, there was not a clear difference for women with support given by a chosen companion although the meta‐analysis appears to favour continuous support over usual care.
5.1. Analysis.

Comparison 5 Continuous support versus usual care ‐ variations in provider characteristics, Outcome 1 Spontaneous vaginal birth.
5.2. Analysis.

Comparison 5 Continuous support versus usual care ‐ variations in provider characteristics, Outcome 2 Negative rating of/negative feelings about birth experience.
5.3. Analysis.

Comparison 5 Continuous support versus usual care ‐ variations in provider characteristics, Outcome 3 Postpartum depression.
5.4. Analysis.

Comparison 5 Continuous support versus usual care ‐ variations in provider characteristics, Outcome 4 Admission to special care nursery.
5.5. Analysis.

Comparison 5 Continuous support versus usual care ‐ variations in provider characteristics, Outcome 5 Exclusive or any breastfeeding at any time point, as defined by trial authors.
5.6. Analysis.

Comparison 5 Continuous support versus usual care ‐ variations in provider characteristics, Outcome 6 Any analgesia/anaesthesia.
5.7. Analysis.

Comparison 5 Continuous support versus usual care ‐ variations in provider characteristics, Outcome 7 Synthetic oxytocin during labour.
5.8. Analysis.

Comparison 5 Continuous support versus usual care ‐ variations in provider characteristics, Outcome 8 Caesarean birth.
Subgroup C: Timing of onset of continuous support
We were unable to conduct the planned subgroup comparison based on timing of onset of labour support as it was not possible to categorise most of the trials according to this pre‐specified subgroup.
Subgroup D: Model of support
Spontaneous vaginal birth: no trials contributed to this outcome.
Negative ratings of or negative views about the birth experience: no trials contributed to this outcome.
Postpartum depression: no trials contributed to this outcome.
Admission to special care nursery: no trials contributed to this outcome.
Exclusive or any breastfeeding at any time point: too few trials contributed to this outcome to produce a meaningful subgroup analysis, Analysis 6.1.
Any intrapartum analgesia or anaesthesia: no trials contributed to this outcome.
Synthetic oxytocin during labour: no trials contributed to this outcome.
Caesarean birth: no trials contributed to this outcome.
6.1. Analysis.

Comparison 6 Continuous support versus usual care ‐ variations in model of support, Outcome 1 Exclusive or any breastfeeding at any time point, as defined by trial authors.
Subgroup E: Country income level
Spontaneous vaginal birth: 10 trials (11,284 women) were conducted in high‐income countries (HIC) (average RR 1.07, 95% CI 1.02 to 1.12). Eleven trials (3085 women) were conducted in middle‐income countries (MIC) (average RR 1.11, 95% CI 1.03 to 1.20). Chi² for subgroup comparison = 0.72, P = 0.39, Analysis 7.1. There was no evidence of a subgroup difference between the groups, but both had more spontaneous vaginal births in the continuous support groups though the effect was greater in MIC.
Negative ratings of or negative views about the birth experience: six trials (9021 women) took place in HIC (average RR 0.74, 95% CI 0.60 to 0.93). Five trials took place in middle‐income countries (MIC) (average RR 0.63, 95% CI 0.52 to 0.76; participants = 2112). Chi² for the subgroup comparison = 1.34, P = 0.25, Analysis 7.2. There was no clear difference among groups from different countries. All women reported less negative feeling about their childbirth experience if they had continuous support in labour. It should be noted, that when the two trials that reported reciprocal data were omitted from the analysis (Bruggemann 2007; Campbell 2006), there was evidence of a small subgroup difference, test for subgroup differences: Chi² = 3.09, P = 0.08.
Postpartum depression: too few trials contributed to this outcome to produce a meaningful subgroup analysis, Analysis 7.3.
Admission to special care nursery: three trials (7740 women) took place in HIC (average RR 0.97, 95% CI 0.84 to 1.11). Four trials (1157 women) took place in MIC (average RR 0.80, 95% CI 0.25 to 2.56). Chi² for subgroup comparison = 0.11, P = 0.74, Analysis 7.4. There were no clear differences among trials conducted in HIC and MIC. Trials in all countries did not find a clear difference between continuous support and usual care in numbers of babies admitted to special care nurseries.
Exclusive or any breastfeeding at any time point: too few trials contributed to this outcome to produce a meaningful subgroup analysis, Analysis 7.5.
Any analgesia or anaesthesia: eight trials (10,145 women) were conducted in HIC (average RR 0.89, 95% CI 0.81 to 0.97). Seven trials (2288 women) took place in MIC (average RR 0.93, 95% CI 0.84 to 1.04). Chi² for subgroup differences = 0.52, P = 0.47, Analysis 7.6. There was no evidence of a subgroup difference. Both subgroups appeared to favour continuous support although the results were unclear.
Synthetic oxytocin during labour: six trials (9907 women) took place in HIC (average RR 0.97, 95% CI 0.87 to 1.08). Eleven trials (2926 women) took place in MIC (average RR 0.92, 95% CI 0.81 to 1.05). Chi² for subgroup differences = 0.38, P = 0.54, Analysis 7.7. There was no difference between subgroups for this outcome. The CIs of both groups touch the line of no effect.
Caesarean birth: twelve trials (11,738 women) took place in HIC (average RR 0.89, 95% CI 0.77 to 1.02). Twelve trials (3609 women) took place in MIC (RR 0.62, 95% CI 0.46 to 0.84). Chi² for subgroup differences = 4.30, P = 0.04, Analysis 7.8. There appears to be evidence of a subgroup difference with trials in MIC reporting a larger effect size in the form of fewer caesarean births taking place in the continuous support group. Whilst trials in HIC report results in the same direction, the effect size was smaller and CIs just cross the line of no effect.
7.1. Analysis.

Comparison 7 Continuous support versus usual care ‐ country income level, Outcome 1 Spontaneous vaginal birth.
7.2. Analysis.

Comparison 7 Continuous support versus usual care ‐ country income level, Outcome 2 Negative rating of/negative feelings about birth experience.
7.3. Analysis.

Comparison 7 Continuous support versus usual care ‐ country income level, Outcome 3 Postpartum depression.
7.4. Analysis.

Comparison 7 Continuous support versus usual care ‐ country income level, Outcome 4 Admission to special care nursery.
7.5. Analysis.

Comparison 7 Continuous support versus usual care ‐ country income level, Outcome 5 Exclusive or any breastfeeding at any time point, as defined by trial authors.
7.6. Analysis.

Comparison 7 Continuous support versus usual care ‐ country income level, Outcome 6 Any analgesia/anaesthesia.
7.7. Analysis.

Comparison 7 Continuous support versus usual care ‐ country income level, Outcome 7 Synthetic oxytocin during labour.
7.8. Analysis.

Comparison 7 Continuous support versus usual care ‐ country income level, Outcome 8 Caesarean birth.
Discussion
Summary of main results
This review included a total of 27 trials and summarises data from 26 trials involving 15,858 women, conducted in hospital settings in 17 countries under a wide variety of circumstances. Continuous one‐to‐one support was given by providers with a variety of experiences, through having given birth themselves or education or both, and practice as nurses, midwives, doulas or childbirth educators, or by the woman's spouse or partner, female relative or close friend.
In the primary comparison, women who were allocated to continuous one‐to‐one support were more likely to have a spontaneous vaginal birth (low‐quality evidence) and less likely to have any intrapartum analgesia or to report negative ratings of or negative feelings about the birth experience (low‐quality evidence). In addition, these women had shorter labours (low‐quality evidence), were less likely to have a caesarean birth (low‐quality evidence) or instrumental vaginal birth, regional analgesia, or a baby with a low five‐minute Apgar score. Data from two trials for postpartum depression were not combined due to differences in women, hospitals and care providers included; both trials found fewer women developed depressive symptomatology if they had been supported in birth, although this may have been a chance result in one of the studies (low‐quality evidence). There was no apparent impact on other intrapartum interventions, maternal or neonatal complications, or breastfeeding, such as admission to special care nursery (low‐quality evidence), and exclusive or any breastfeeding at any time point, as defined by trial authors (low‐quality evidence). This review did not identify any adverse effects of continuous labour support. This form of care appears to confer important benefits without attendant risks. The results of earlier versions of this review prompted organisations in Canada, the UK and the USA to issue practice guidelines, advocating continuous support (ACOG 2017; ACOG 2016; AWHONN 2002; MIDIRS 2008; NICE Intrapartum Care 2007; Lee [SOGC] 2016; World Health Organization 2015; World Health Organization 2016). The results of the primary comparison in the current review offer continued justification for such practice guidelines.
The subgroup analyses should be interpreted with caution. Individually, each should be considered exploratory and hypothesis‐generating, particularly when the sample size in one subgroup was much smaller than in another. However, taken in their totality, the consistency of the patterns suggests that the effectiveness of continuous intrapartum support may be enhanced or reduced by policies and practices in the birth setting and by the nature of the relationship between the provider of continuous support and labouring woman.
We chose three aspects of the birth environment ‐ routine use of electronic fetal monitoring (EFM), availability of epidural analgesia and policies about the presence of additional support people of the woman's own choosing ‐ as proxies for environmental conditions that may mediate the effectiveness of labour support. This review cannot answer questions about the mechanisms whereby settings with epidural analgesia limit the effectiveness of labour support. The impact of epidural analgesia may be direct (Anim‐Somuah 2011) or indirect, as part of the 'cascade of interventions' described in the Background. The effects of a policy of routine EFM are less clear, possibly because we were unable to obtain information about EFM policies for several of the trials. Women who were not permitted to have companions with them in labour, benefited more from continuous support than those in settings that permitted their own companions, in the form of fewer caesarean sections and more spontaneous vaginal births. Labour support appears to be effective in reducing the adverse consequences of the fear and distress associated with labouring alone in an unfamiliar environment. A report of a qualitative component of one of the included trials (Langer 1998), aptly titled "Alone, I wouldn't have known what to do", provides further justification for this argument.
Effects of continuous labour support may vary by provider characteristics. Divided loyalties, additional duties besides labour support, self‐selection and the constraints of institutional policies and routine practices may all have played a role in the apparently limited effectiveness of members of the hospital staff. Childbirth environments influence the healthcare professionals who work in them as well as labouring women and their support people. Furthermore, while women often want and benefit from the presence of selected members of their social network, the support of partners and others with whom they have a longstanding relationship is qualitatively different and more complex than that of a woman who is experienced and often trained to provide labour support and who has no other role other than to provide support. Members of a woman's social network may be less experienced with childbirth and have their own needs relating to the woman, baby and childbirth process, compared to someone in a doula role. An early trial of labour support with partners present found that women received more support from their partners when a doula was present to guide them, and the partners themselves reported more support (Hodnett 1989). While continuous labour support appears to be more effective in achieving desirable clinical outcomes and fewer negative experiences when it is provided by someone in a doula role (e.g. caregivers who are not employees of an institution and thus have no obligation to anyone other than the labouring woman) and who have an exclusive focus on this task, support from a member of the woman's social network is effective in reducing women's negative birth experiences.
Subgroup analysis of trials conducted in high‐income countries compared with trials conducted in middle‐income countries suggests that continuous labour support offers similar benefits to women and babies for most outcomes, with the exception of caesarean birth, where studies from middle‐income countries showed a larger reduction in caesarean birth. It should be noted that only two included studies were conducted in lower‐middle income countries (Guatemala (Klaus 1986), and Nigeria (Morhason‐Bello 2009)), so conclusions about the effect of continuous support for women and babies in the poorest countries is limited but worthy of further exploration.
There remains relatively little information about the effects of continuous intrapartum support on mothers' and babies' health and well‐being in the postpartum period, and none of the included studies contributed to the proposed subgroup analysis to compare extended to intrapartum‐only models of continuous support.
Overall completeness and applicability of evidence
The studies included in this review are from a diverse range of geographical locations (Oceania, Europe, Africa, South America, North America, Middle East and Asia) and country‐income levels, ranging from low‐ to high‐income countries. However, only two trials were conducted in lower‐middle income countries. Although we included data from 26 studies, there were diverse interventions among these studies, ranging from providing support only during active labour to providing support across the pregnancy, labour, childbirth and postpartum periods.
Many of the studies in this review included only women with uncomplicated pregnancies, nulliparous women or both. Nulliparous women are an important group to target as they are by definition less experienced with the childbirth process compared to multiparous women, but there is a gap in the evidence related to multiparous women and women with complicated pregnancies.
Quality of the evidence
We assessed included studies at varying levels of risk of bias (see Figure 2; Figure 3). None of the studies blinded women or staff due to the nature of the intervention and only one study (Isbir 2015) attempted to fully blind the outcome assessors. We used GRADEpro software to assess the quality of evidence contributing to GRADE outcomes for the main review comparison (see Table 1). Evidence contributing to all GRADE outcomes (spontaneous vaginal birth, negative rating of/negative feelings about birth experience, postpartum depression, admission to special care nursery, exclusive or any breastfeeding at any time point, as defined by trial authors, labour length, and caesarean birth) were graded low quality. Evidence was downgraded for lack of blinding in studies (it is not possible to blind study participants or personnel to a continuous support intervention) and other limitations in study designs, inconsistency, or imprecision of effect estimates.
Potential biases in the review process
We followed the review process recommended by Cochrane. We obtained and reviewed all potentially relevant studies identified from the search results, resolving any disagreements by discussion. Potential bias in the review process should be minimal: two review authors independently assessed studies for inclusion and extracted the data. We resolved discrepancies through discussion or, if required, we consulted another review author. Review author GJ Hofmeyr was the author of a study included in this review. He was not involved in extracting data from the trials which he had authored. For some outcomes, such as exclusive breastfeeding and negative rating of or negative feelings about the birth experience, some trials reported the outcome in the opposite way. For example, Hodnett 2002 reported "not breastfeeding at all" at six weeks postpartum and Bruggemann 2007 reported satisfaction with care received and Campbell 2006 overall rating of birth experience. We calculated the reciprocals for these two outcomes so that they could be included in analyses. We acknowledge that this is not ideal and so performed additional sensitivity analyses to omit studies with reciprocal data to ensure that this made no difference to the overall result. Sensitivity analyses excluding reciprocal data for these two outcomes made no difference to the overall results, although some differences were observed in terms of subgroup analyses, as reported in the results section.
Agreements and disagreements with other studies or reviews
There are no other Cochrane Reviews that assess continuous support for women during childbirth.
A non‐Cochrane review (Steel 2015) used critical integrative review methods to explore professional doulas supporting and caring for women. This review provided a descriptive analysis of workforce issues in professional doula care, role and skills of professional doulas, physical outcomes of professional doula care, and social outcomes of professional doula care, while noting several methodological weaknesses across the body of evidence.
Authors' conclusions
Implications for practice.
Continuous support during labour may improve outcomes for women and infants, including increased spontaneous vaginal birth, shorter duration of labour, and decreased caesarean birth, instrumental vaginal birth, use of any analgesia, use of regional analgesia, low‐ five‐minute Apgar score and negative feelings about childbirth experiences. We found no evidence of harms of continuous labour support. Subgroup analyses should be interpreted with caution, and considered as exploratory and hypothesis generating, but evidence suggests continuous labour support with certain provider characteristics (support from someone with a doula role), in settings in which epidural analgesia was not routinely available, in settings where women were not permitted to have companions of their choosing with them in labour (e.g., spouse/partner, mother, friend), and in middle‐income country settings, may have a favourable impact on outcomes such as caesarean birth.
Implications for research.
There remains relatively little information about the effects of continuous intrapartum support on mothers' and babies' health and well‐being in the postpartum period, and thus trials across all types of settings, which include a focus on longer‐term outcomes for the woman and baby, would be helpful. In particular, exploring longer‐term outcomes related to exclusive or any breastfeeding, difficulty mothering, low‐self esteem, and unsatisfactory mother‐infant interactions would help to better understand the impact of continuous support outside the time period of labour, childbirth and the immediate postpartum period. Relatedly, given the rising rates of caesarean birth globally, future research could consider exploring targeted continuous support interventions for nulliparous women, to reduce the risk of first caesarean birth.
This review update included six new outcomes not previously reported. These outcomes were designed to be woman‐centric, promote the inclusion of women's experiences of care as important aspects of quality of care, and ensure that women's choices, autonomy and control are respected. Woman‐reported measures of the experience and outcomes of maternal and newborn care are essential for a full evaluation of maternity care practices and systems. The inclusion of these outcomes is aligned with the World Health Organization (WHO) vision for quality of care for pregnant women and newborns (Tunçalp 2015). We hope that the inclusion of these outcomes in this review will encourage researchers, policy‐makers, clinicians and other key stakeholders to consider the importance of measuring and promoting woman‐centred care. A mixed‐methods systematic review concluded that women across the world may experience mistreatment during childbirth, including physical abuse (slapping, hitting, pinching), verbal abuse, stigma and discrimination, lack of supportive care, neglect and denial of autonomy (Bohren 2015). It is possible that continuous support for women during childbirth could help reduce mistreatment during childbirth and promote respectful maternity care, as a labour companion could act as an advocate for the woman and potentially safeguard against mistreatment. Further research could explore the impact of labour companionship on mistreatment and respectful care.
The trials in resource‐constrained countries were relatively small compared to the trials in higher‐income countries. Additional, larger trials may be required in resource‐constrained settings, where the cost of providing continuous support may compete with other resource priorities. In these settings, implementation research could explore how to effectively implement labour companionship to assuage provider and health system barriers to intervention. Outcomes that have been under‐researched in resource‐poor settings, but are causes of significant morbidity and poor experiences of care may be worthy of further exploration, including urinary and faecal incontinence, pain during intercourse, prolonged perineal pain, postpartum depression, respect for women's choices and preferences, and promoting dignified and respectful maternity care.
Trials of different models of training providers of labour support would help to inform decision makers about the most effective models in the context of their settings. Trials are also needed to understand whether an extended model of care across the antenatal, intrapartum and postpartum periods adds value to the intrapartum‐only continuous support, which has been the primary focus of trials to date. Similarly, future research could compare the effectiveness of models of one‐to‐one midwifery care with continuous support by an additional person, as it is unclear whether routine midwifery tasks such as charting and managing equipment, impact the provision of supportive care.
Economic analyses of the relative costs and benefits, as well as sufficient details about the training and/or orientation materials provided to the support person (whether they are a doula or a lay person) would be an important contribution to the current evidence base.
What's new
| Date | Event | Description |
|---|---|---|
| 4 August 2017 | Amended | Amendment to the text describing the results for the subgroup analysis of synthetic oxytocin (Analysis 5.7). The text incorrectly described results for a fixed‐effect model and not a random‐effects model. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 31 October 2016 | New search has been performed | Search updated and four new trials incorporated (Akbarzadeh 2014; Hans 2013; Isbir 2015; Safarzadeh 2012). A 'Summary of findings' table has been added for this update. Six new outcomes have been added. |
| 31 October 2016 | New citation required but conclusions have not changed | Four new trials included, but conclusions remain unchanged. |
| 29 June 2013 | New citation required but conclusions have not changed | Review updated. No new trials or data included. |
| 29 June 2013 | New search has been performed | Search updated. One new abstract identified and added to Characteristics of studies awaiting classification because it does not contain sufficient details to permit classification (Safarzadeh 2013). |
| 12 July 2012 | New citation required but conclusions have not changed | One new trial added (Yuenyong 2012). Data inadvertently omitted for one outcome (postpartum depression) from a prior trial (Hofmeyr 1991) have now been included. Minor clarifications to the text and changes to Results which did not substantively alter Conclusions. |
| 14 June 2012 | New search has been performed | Search updated and one new trial met inclusion criteria. One other trial report and seven new abstracts were found, one of which describes an ongoing study, and none of which contain sufficient details to permit classification. |
| 31 December 2010 | New search has been performed | Search updated. We evaluated and added new trials. We obtained additional information from trial authors. Other revisions included numerous changes to bring the entire Review up‐to‐date in terms of current methodological guidelines. We altered the acceptable follow‐up rate for long term outcomes, and we expanded the number of outcomes to be included in the planned subgroup analyses. |
| 25 October 2010 | New citation required but conclusions have not changed | New author joined the review team to update the review. |
| 12 May 2008 | Amended | Converted to new review format. |
| 18 April 2007 | New search has been performed | Search updated in February 2007. Two new trials identified. We excluded one (Dalal 2006) and included the other (Campbell 2006). The Results section was updated accordingly. With the exception of the outcome of labour length, there were no substantive changes in results or conclusions of the Review. Minor edits were made throughout. Additional text was added to the Discussion. |
| 30 October 2006 | New search has been performed | Search updated. One 'awaiting assessment' trial was assessed and included (Thomassen 2003). |
Acknowledgements
We thank Ellen Hodnett for her important contributions as lead author of this review across many updates and Simon Gates, who wrote the initial draft of the statistical methods and provided statistical advice for the protocol and several updates of the review (Hodnett 2003; Hodnett 2011; Hodnett 2012; Hodnett 2013).
We are very grateful to the investigators who provided additional information: O Bruggemann, D Campbell, R Cogan, A Gagnon, E Hemminki, M Kashanian, J Kennell, M Klaus, A Langer, B Madi, S McGrath, G Trueba, E Kopplin, G Isbir, and S Yuengong. We thank Agnes Cho, Qian Xu and Jiang Huangye for translation of Chinese publications, and Qian Xu for contacting the trial author for additional details. Ellen Hodnett and Justus Hofmeyr also provided additional information about their trials. Tanya Webb performed the second data entry on the earlier version of the review, contacted trial authors for additional information and provided secretarial support. The Consumer Panel of Cochrane Pregnancy and Childbirth (of which Carol Sakala is a member) provided many helpful suggestions for both the protocol and earlier versions of the review.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
The World Health Organization, Justus Hofmeyr, Carol Sakala, Rieko Fukuzawa and Anna Cuthbert retain copyright and all other rights in their respective contributions to the manuscript of this Review as submitted for publication.
As part of the pre‐publication editorial process, this review has been commented on by six peers (an editor and five referees who are external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
labour or labor or birth or childbirth AND
support OR doula OR companion OR companionship OR husband OR partner
Data and analyses
Comparison 1. Continuous support versus usual care ‐ all trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous vaginal birth | 21 | 14369 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.04, 1.12] |
| 2 Negative rating of/negative feelings about birth experience | 11 | 11133 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.59, 0.79] |
| 3 Postpartum depression | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Admission to special care nursery | 7 | 8897 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.25] |
| 5 Exclusive or any breastfeeding at any time point, as defined by trial authors | 4 | 5584 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.96, 1.16] |
| 6 Any analgesia/anaesthesia | 15 | 12433 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 7 Regional analgesia/anaesthesia | 9 | 11444 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.99] |
| 8 Synthetic oxytocin during labour | 17 | 12833 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 9 Labour length | 13 | 5429 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.04, ‐0.34] |
| 10 Postpartum report of severe labour pain | 4 | 2456 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.21] |
| 11 Caesarean birth | 24 | 15347 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.64, 0.88] |
| 12 Instrumental vaginal birth | 19 | 14118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.85, 0.96] |
| 13 Perineal trauma | 4 | 8120 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.92, 1.01] |
| 14 Delayed skin‐to‐skin contact, as defined by trial authors | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.64, 1.04] |
| 15 Delayed initiation of breastfeeding (> 1 hour after birth, or as defined by trial authors) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 16 Time from birth to initiation of breastfeeding | 1 | 585 | Mean Difference (IV, Fixed, 95% CI) | ‐44.60 [‐47.63, ‐41.57] |
| 17 Restricted mobility during labour, as defined by trial authors | 1 | 6915 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [1.00, 1.05] |
| 18 Low 5‐minute Apgar score | 14 | 12615 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.46, 0.85] |
| 19 Prolonged neonatal hospital stay | 3 | 1098 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.42, 1.65] |
| 20 Difficulty mothering | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21 Low postpartum self‐esteem | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
Comparison 2. Continuous support versus usual care ‐ policy regarding presence of companion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous vaginal birth | 19 | 14218 | Risk Ratio (IV, Random, 95% CI) | 1.08 [1.04, 1.12] |
| 1.1 Other support permitted | 9 | 10889 | Risk Ratio (IV, Random, 95% CI) | 1.03 [1.00, 1.06] |
| 1.2 Other support not permitted | 10 | 3329 | Risk Ratio (IV, Random, 95% CI) | 1.11 [1.06, 1.17] |
| 2 Negative rating of/negative feelings about birth experience | 11 | 11178 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.60, 0.79] |
| 2.1 Other support permitted | 5 | 8639 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.60, 1.00] |
| 2.2 Other support not permitted | 6 | 2539 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.53, 0.74] |
| 3 Postpartum depression | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Other support permitted | 1 | 5567 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.73, 1.02] |
| 3.2 Other support not permitted | 1 | 149 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.09, 0.36] |
| 4 Admission to special care nursery | 7 | 8897 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.76, 1.25] |
| 4.1 Other support permitted | 2 | 7328 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.82, 1.20] |
| 4.2 Other support not permitted | 5 | 1569 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.47, 1.61] |
| 5 Exclusive or any breastfeeding at any time point, as defined by trial authors | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Other support permitted | 1 | 4559 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.92, 1.02] |
| 5.2 Other support not permitted | 2 | 804 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.98, 1.13] |
| 6 Any analgesia/anaesthesia | 14 | 12350 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 6.1 Other support permitted | 7 | 9752 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.96, 0.99] |
| 6.2 Other support not permitted | 7 | 2598 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.61, 1.00] |
| 7 Synthetic oxytocin during labour | 15 | 12620 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.91, 1.04] |
| 7.1 Other support permitted | 5 | 9495 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.90, 1.10] |
| 7.2 Other support not permitted | 10 | 3125 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.85, 1.05] |
| 8 Caesarean birth | 22 | 15175 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.67, 0.91] |
| 8.1 Other support permitted | 11 | 11326 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.79, 1.04] |
| 8.2 Other support not permitted | 11 | 3849 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.52, 0.88] |
Comparison 3. Continuous support versus usual care ‐ availability of epidural analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous vaginal birth | 19 | 14218 | Risk Ratio (IV, Random, 95% CI) | 1.08 [1.04, 1.12] |
| 1.1 Epidural analgesia routinely available | 13 | 12672 | Risk Ratio (IV, Random, 95% CI) | 1.06 [1.02, 1.10] |
| 1.2 Epidural analgesia not routinely available | 6 | 1546 | Risk Ratio (IV, Random, 95% CI) | 1.11 [1.04, 1.19] |
| 2 Negative rating of/negative feelings about birth experience | 11 | 11178 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.60, 0.79] |
| 2.1 Epidural analgesia routinely available | 9 | 10404 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.63, 0.86] |
| 2.2 Epidural analgesia not routinely available | 2 | 774 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.48, 0.63] |
| 3 Postpartum depression | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Epidural analgesia routinely available | 1 | 6915 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.75, 1.05] |
| 3.2 Epidural analgesia not routinely available | 1 | 149 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.09, 0.36] |
| 4 Admission to special care nursery | 7 | 8897 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.76, 1.25] |
| 4.1 Epidural analgesia routinely available | 5 | 8380 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.85, 1.13] |
| 4.2 Epidural analgesia not routinely available | 2 | 517 | Risk Ratio (IV, Random, 95% CI) | 0.26 [0.08, 0.88] |
| 5 Exclusive or any breastfeeding at any time point, as defined by trial authors | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Epidural analgesia routinely available | 2 | 5214 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.94, 1.06] |
| 5.2 Epidural analgesia not routinely available | 1 | 149 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.95, 1.40] |
| 6 Any analgesia/anaesthesia | 14 | 12350 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 6.1 Epidural analgesia routinely available | 9 | 10888 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.84, 0.97] |
| 6.2 Epidural analgesia not routinely available | 5 | 1462 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.69, 0.99] |
| 7 Synthetic oxytocin during labour | 16 | 12697 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 7.1 Epidural analgesia routinely available | 8 | 10568 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.94, 1.05] |
| 7.2 Epidural analgesia not routinely available | 8 | 2129 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.62, 1.03] |
| 8 Caesarean birth | 24 | 15347 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.64, 0.88] |
| 8.1 Epidural analgesia routinely available | 14 | 13064 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.81, 1.02] |
| 8.2 Epidural analgesia not routinely available | 8 | 2149 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.41, 0.72] |
| 8.3 Unknown availability of epidural analgesia | 2 | 134 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.04, 3.09] |
Comparison 4. Continuous support versus usual care ‐ policy about routine EFM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous vaginal birth | 21 | 14441 | Risk Ratio (IV, Random, 95% CI) | 1.08 [1.04, 1.12] |
| 1.1 Setting had routine EFM | 8 | 9717 | Risk Ratio (IV, Random, 95% CI) | 1.07 [1.01, 1.13] |
| 1.2 Setting did not have routine EFM | 7 | 1913 | Risk Ratio (IV, Random, 95% CI) | 1.11 [1.06, 1.17] |
| 1.3 Policy about routine EFM not known | 6 | 2811 | Risk Ratio (IV, Random, 95% CI) | 1.09 [1.00, 1.18] |
| 2 Negative rating of/negative views about birth experience | 11 | 11154 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.60, 0.79] |
| 2.1 Setting had routine EFM | 4 | 7467 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.55, 0.94] |
| 2.2 Setting did not have routine EFM | 4 | 1710 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.49, 0.74] |
| 2.3 Policy about routine EFM not known | 3 | 1977 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.65, 1.08] |
| 3 Postpartum depression | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Setting had routine EFM | 1 | 6915 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.75, 1.05] |
| 3.2 Setting did not have routine EFM | 1 | 149 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.09, 0.36] |
| 4 Admission to special care nursery | 7 | 8897 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.76, 1.25] |
| 4.1 Setting had routine EFM | 3 | 7740 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.84, 1.11] |
| 4.2 Setting did not have routine EFM | 3 | 729 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.15, 1.52] |
| 4.3 Policy about routine EFM not known | 1 | 428 | Risk Ratio (IV, Random, 95% CI) | 1.98 [0.76, 5.18] |
| 5 Exclusive or any breastfeeding at any time point, as defined by trial authors | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Setting had routine EFM | 1 | 4559 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.92, 1.02] |
| 5.2 Setting did not have routine EFM | 2 | 804 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.98, 1.13] |
| 5.3 Policy about routine EFM not known | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 1.28 [1.02, 1.60] |
| 6 Any analgesia/anaesthesia | 14 | 12345 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 6.1 Setting had routine EFM | 6 | 8580 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.79, 0.99] |
| 6.2 Setting did not have routine EFM | 6 | 2186 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.79, 1.04] |
| 6.3 Policy about routine EFM not known | 2 | 1579 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.80, 0.99] |
| 7 Synthetic oxytocin during labour | 17 | 12847 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 7.1 Setting had routine EFM | 4 | 8340 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.78, 1.11] |
| 7.2 Setting did not have routine EFM | 8 | 1789 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.64, 1.01] |
| 7.3 Policy about routine EFM not known | 5 | 2718 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.97, 1.08] |
| 8 Caesarean birth | 24 | 15347 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.64, 0.88] |
| 8.1 Setting had routine EFM | 9 | 10123 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.71, 1.00] |
| 8.2 Setting did not have routine EFM | 9 | 2529 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.43, 0.81] |
| 8.3 Policy about routine EFM not known | 6 | 2695 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.56, 1.32] |
Comparison 5. Continuous support versus usual care ‐ variations in provider characteristics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous vaginal birth | 21 | 14468 | Risk Ratio (IV, Random, 95% CI) | 1.08 [1.04, 1.12] |
| 1.1 Support people were hospital staff | 9 | 10813 | Risk Ratio (IV, Random, 95% CI) | 1.05 [1.01, 1.09] |
| 1.2 Support people were not hospital staff and were chosen by woman | 6 | 1620 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.97, 1.11] |
| 1.3 Support people were not hospital staff and not chosen by woman | 6 | 2035 | Risk Ratio (IV, Random, 95% CI) | 1.15 [1.05, 1.26] |
| 2 Negative rating of/negative feelings about birth experience | 11 | 11178 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.60, 0.79] |
| 2.1 Support people were hospital staff | 4 | 8145 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.73, 1.03] |
| 2.2 Support people were not hospital staff and not chosen by woman | 3 | 1325 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.53, 0.80] |
| 2.3 Support people were not hospital staff and were chosen by woman | 4 | 1708 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.50, 0.67] |
| 3 Postpartum depression | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Support people were hospital staff | 1 | 5567 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.73, 1.02] |
| 3.2 Support people were not hospital staff and not chosen by woman | 1 | 149 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.09, 0.33] |
| 3.3 Support people were not hospital staff and were chosen by woman | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Admission to special care nursery | 7 | 8897 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.76, 1.25] |
| 4.1 Support people were hospital staff | 3 | 7428 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.82, 1.20] |
| 4.2 Support people were not hospital staff and not chosen by woman | 2 | 829 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.17, 1.87] |
| 4.3 Support people were not hospital staff and were chosen by woman | 2 | 640 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.61, 3.14] |
| 5 Exclusive or any breastfeeding at any time point, as defined by trial authors | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Support people were hospital staff | 1 | 4559 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.92, 1.02] |
| 5.2 Support people were not hospital staff and not chosen by woman | 3 | 1025 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.98, 1.26] |
| 5.3 Support people were not hospital staff and were chosen by woman | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Any analgesia/anaesthesia | 14 | 12350 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 6.1 Support people were hospital staff | 6 | 9152 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.96, 0.99] |
| 6.2 Support people were not hospital staff and not chosen by woman | 4 | 1790 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.47, 1.10] |
| 6.3 Support people were not hospital staff and were chosen by woman | 4 | 1408 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 7 Synthetic oxytocin during labour | 17 | 12833 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 7.1 Support people were hospital staff | 6 | 9561 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.93, 1.11] |
| 7.2 Support people were not hospital staff and not chosen by woman | 4 | 1081 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.43, 1.06] |
| 7.3 Support people were not hospital staff and were chosen by woman | 7 | 2191 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.96, 1.01] |
| 8 Caesarean birth | 24 | 15347 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.64, 0.88] |
| 8.1 Support people were hospital staff | 9 | 10786 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 8.2 Support people were not hospital staff and not chosen by woman | 9 | 2502 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.45, 0.83] |
| 8.3 Support people were not hospital staff and were chosen by woman | 6 | 2059 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.50, 1.17] |
Comparison 6. Continuous support versus usual care ‐ variations in model of support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive or any breastfeeding at any time point, as defined by trial authors | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Intrapartum period only | 3 | 5363 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 1.2 Extended model (antenatal, intrapartum, postnatal) | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.02, 1.60] |
Comparison 7. Continuous support versus usual care ‐ country income level.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous vaginal birth | 21 | 14369 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.04, 1.12] |
| 1.1 High‐income country | 10 | 11284 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [1.02, 1.12] |
| 1.2 Middle‐income country | 11 | 3085 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.03, 1.20] |
| 2 Negative rating of/negative feelings about birth experience | 11 | 11133 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.59, 0.79] |
| 2.1 High‐income country | 6 | 9021 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.60, 0.93] |
| 2.2 Middle‐income country | 5 | 2112 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.52, 0.76] |
| 3 Postpartum depression | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 High‐income country | 1 | 5567 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.73, 1.02] |
| 3.2 Middle‐income country | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.09, 0.36] |
| 4 Admission to special care nursery | 7 | 8897 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.25] |
| 4.1 High‐income country | 3 | 7740 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.11] |
| 4.2 Middle‐income country | 4 | 1157 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.25, 2.56] |
| 5 Exclusive or any breastfeeding at any time point, as defined by trial authors | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 High‐income country | 2 | 4780 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.83, 1.43] |
| 5.2 Middle‐income country | 2 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.13] |
| 6 Any analgesia/anaesthesia | 15 | 12433 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 6.1 High‐income country | 8 | 10145 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.97] |
| 6.2 Middle‐income country | 7 | 2288 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.04] |
| 7 Synthetic oxytocin during labour | 17 | 12833 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 7.1 High‐income country | 6 | 9907 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.08] |
| 7.2 Middle‐income country | 11 | 2926 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
| 8 Caesarean birth | 24 | 15347 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.64, 0.88] |
| 8.1 High‐income country | 12 | 11738 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.77, 1.02] |
| 8.2 Middle‐income country | 12 | 3609 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.46, 0.84] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akbarzadeh 2014.
| Methods | RCT | |
| Participants | Pregnant women aged 18 to 35 years, with a singleton, term pregnancy, and healthy fetal membranes. No history of medical, surgical, or mental problems and no special problems during pregnancy. The participants’ uterine contractions started spontaneously and, at admission, the contractions occurred every 5 to 10 minutes and cervical dilatation was 3 to 4 cm | |
| Interventions | The study was conducted in Shoushtari Hospital, Iran in 2012. In the supportive care group (N = 50), the doula was constantly beside the mother from the beginning of the mother’s maternity ward admission (beginning of the active phase of labour at 3 to 4 cm cervical dilatation) to the end of the second stage of labour. Supportive measures classified into psychological and emotional, educational, and physical categories were offered to the mother. Psychological and emotional support included touching, empathy, compassion, encouraging the mother to continue cooperation in the labour process, reassurance, taking mother’s hands, maintaining eye contact, creating a sense of trust and confidence, continuous talking, and reduction of fear during labour. Educational support included informing the mother about the natural process of childbirth and answering her questions. Finally, physical support included cooling the mother, satisfying her hunger and thirst, and helping her change the positions in various stages of labour. The control group (N = 50) received the department’s routine care and underwent no interventions |
|
| Outcomes | Pain assessment (before and after intervention) using the VAS. VAS is a scale numerated from 0 to 10 with 0, 1 to 3, 4 to 6, 7 to 9, and 10 representing no, mild, moderate, severe, and the worst possible pain. Mode of delivery (CS, VD) |
|
| Notes | For all women who received doula care in the intervention group, the doula was one researcher. Unknown if companions were typically permitted on the ward, if continuous EFM was used routinely, or if epidural anaesthesia was available Dates of study: 2012 Funding: The study was financially supported by the Research Center for Traditional Medicine and History of Medicine and the Research Vice‐Chancellor of Shiraz University of Medical Sciences, Shiraz, Iran. Conflicts of interest: The authors declare that they have no known conflict of interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Akbarzadeh 2015 and Masoudi 2014 specify the process of selecting blocks, "the subjects were selected through simple random sampling and were divided into supportive care, acupressure, and control groups using stratified block randomisation. In doing so, a number was randomly selected from the table of random numbers and the researcher moved toward the right or left column or row and wrote the 5 digit numbers down. Since the participants were divided into 3 groups in this study, 3‐therapy method was used and classification was performed as follows: A: supportive care group, B: acupressure group, and C: control group. Accordingly, ABC: 1, ACB: 2, BAC: 3, BCA: 4, CAB: 5, and CBA: 6. It should be noted that numbers 0, 7, and 9 were ignored". |
| Allocation concealment (selection bias) | Unclear risk | Unclear if central randomisation, sequentially numbered/opaque containers were used during allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There is no mention of blinding of participants or personnel throughout this study, but blinding of participants and personnel is not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study data were collected using interview form (including demographic information, history of pregnancy, familial status, and pregnancy information), observation form (including evaluation of uterine contractions, fetal heart rate, labour progress, and delivery outcome), and VAS. VAS is a scale numerated from 0 to 10 with 0, 1 to 3, 4 to 6, 7 to 9, and 10 representing no, mild, moderate, severe, and the worst possible pain. High risk of bias for pain reporting in the intervention‐supportive care group, as pain is a subjective and self‐reported outcome and women would know whether they were in the intervention or control group. Low risk of bias for mode of delivery, as was assessed using observation and mode of delivery is an objective outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From tables 1 and 2, it appears that there was no missing data from either outcome in the intervention or control group |
| Selective reporting (reporting bias) | Low risk | Important outcomes were included across the 3 references for this study |
| Other bias | Low risk | Low risk of other bias |
Bruggemann 2007.
| Methods | RCT | |
| Participants | 212 nulliparous women in active labour at term (105 support group, 107 control group) at a University‐affiliated hospital in São Paulo, Brazil. To be eligible a companion of the woman's choosing had to be available. 49.5% of the companions were present at enrolment and the others were phoned and asked to come to the hospital (4 failed to make it before delivery) | |
| Interventions | Support was 'presence of a chosen companion during labour and delivery'. 'The companions received verbal and written information on the activities involved in providing support, expected behaviour when confronted with signs of tiredness, anxiety, concern, crying, screaming and/or the woman's feelings of inability to cope, compliance with regulations and the possibility of requesting information from staff'. in 47.6% of the sample the woman's companion was her partner, for 29.5% it was her mother. The control group received usual care where a companion during labour and delivery was not permitted. For both groups labour and delivery care was provided 'according to the routine protocol including active management of labour (early amniotomy, use of oxytocin, intermittent EFM and systematic analgesia)' |
|
| Outcomes | Satisfaction with labour and delivery, perinatal and breastfeeding outcome in the 12 hours post delivery | |
| Notes | All women in labour at this hospital received epidural analgesia as a routine practice. Therefore, we did not include epidural analgesia data in the review. EFM was not used routinely Dates of study: February 2004 ‐ March 2005 Funding: financial support of CAPES (Coordination of improvement for graduated personnel), Brazil. Conflicts of interest: The author(s) declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Computer generated sequence of random numbers.' |
| Allocation concealment (selection bias) | High risk | 'Individual assignment numbers were all placed in an opaque container to assure the concealment. The eligible women who had agreed to participate selected one of the numbers once, and were therefore allocated to either intervention group or control according to the list.' This process was open to selection bias as women could have re‐picked another number from the container. No audit process is possible with this system of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, clinicians and researchers were aware of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Data collection by the author, who knew group allocation. Higher risk of bias for the "satisfaction" outcomes, compared to the clinical outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record data were collected and in‐hospital questionnaires were completed for 100% of sample |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Bréart ‐ Belgium 1992.
| Methods | RCT | |
| Participants | 3 trials are reported separately in 1 publication. Participants were nulliparous, healthy, in spontaneous labour, term, with singleton vertex presentations. Trial in Belgium: N = 264 (133 permanent support; 131 control) | |
| Interventions | Permanent presence of a midwife compared to varying degrees of presence. Fathers were allowed to be present | |
| Outcomes | Oxytocin, epidural analgesia, labour length, mode of birth, Apgar scores, mothers' views of their experiences | |
| Notes | Epidural analgesia was available and it is not known whether EFM was used routinely Dates of study: not clear, trials ended in 1992 Funding: not clear ‐ "European Community concerted action". Conflicts of interest: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were 'randomly assigned'. The envelopes were prepared by the co‐ordinating centre. No mention of the process of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. No mention if they were opaque or consecutively numbered. The process of how the envelopes were opened was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided about blinding of participants or personnel, but blinding of participants and personnel is not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided about blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completion rate for medical record data and in‐hospital questionnaire were 99.2% and 91.0% respectively |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Bréart ‐ France 1992.
| Methods | See Bréart ‐ Belgium 1992 | |
| Participants | See Bréart ‐ Belgium 1992 Trial in France: N = 1320 (656 continuous support; 664 control) | |
| Interventions | See Bréart ‐ Belgium 1992. Fathers were allowed to be present | |
| Outcomes | See Bréart ‐ Belgium 1992 | |
| Notes | Epidural analgesia was available and it is unknown whether EFM was routine Dates of study: not clear, trials ended in 1992 Funding: not clear ‐ "European Community concerted action". Conflicts of interest: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were 'randomly assigned'. The envelopes were prepared by the co‐ordinating centre. No mention of the process of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. No mention if they were opaque or consecutively numbered. The process of how the envelopes were opened was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided about blinding of participants or personnel, but blinding of participants and personnel is not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided about blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completion rate for medical record data and in‐hospital questionnaire was > 95%. There were some discrepancies in the total number enrolled. Two reports show 656 in the permanent support group and 664 in the control group for a total of 1320. The table of results in 1 report shows 654 in the permanent support and 666 in control. The in‐hospital questionnaire results are shown for 654 and 664 women (total 1318) but the authors state this is 95% of the sample, meaning the total is 1386. The N reported for each outcome were used in the data tables in this review |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Bréart ‐ Greece 1992.
| Methods | See Bréart ‐ Belgium 1992 | |
| Participants | See Bréart ‐ Belgium 1992. Trial in Greece: N = 569 (295 permanent support; 274 control) | |
| Interventions | See Bréart ‐ Belgium 1992. Fathers/family members were not permitted to be present | |
| Outcomes | See Bréart ‐ Belgium 1992, except that mothers' views were not reported | |
| Notes | Epidural analgesia was not available. Not stated if EFM was used routinely Dates of study: not clear, trials ended in 1992 Funding: not clear ‐ "European Community concerted action". Conflicts of interest: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were 'randomly assigned'. The envelopes were prepared by the co‐ordinating centre. No mention of the process of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. No mention if they were opaque or consecutively numbered. The process of how the envelopes were opened was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided about blinding of participants or personnel, but blinding of participants and personnel is not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided about blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completion rate for medical record data were 97%. No in‐hospital questionnaire data were available |
| Selective reporting (reporting bias) | Low risk | All medical record outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Campbell 2006.
| Methods | RCT | |
| Participants | 600 nulliparous, low‐income, under‐insured pregnant women (300 doula group, 300 control group) booked for delivery at a hospital in New Jersey, USA were enrolled between 12 and 38 weeks' gestation. They were considered low risk, with no contraindications to labour and had a female friend or relative willing to act as their lay doula. The doula was in addition to support people of their own choosing | |
| Interventions | Intervention: continuous support by a female friend or relative who had 2, 2‐hour sessions about labour support. The training sessions were conducted for nearly all of the lay caregivers when the participants were 34 to 36 weeks' gestation. Control group: support people of their own choosing |
|
| Outcomes | Labour length, epidural analgesia, oxytocin augmentation, cervical dilation at epidural insertion, length of second stage labour, caesarean birth, 1‐min Apgar score > 6, 5‐min Apgar score > 6, delayed initiation of breastfeeding | |
| Notes | Epidural analgesia was available and EFM was used routinely Dates of study: enrolment took place between 1998 and 2002 Funding: Johnson & Johnson provided a small stipend to complete the data analysis. "Johnson & Johnson did not influence the design and conduct of the study or the analysis and interpretation of the data." Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomisation scheme" |
| Allocation concealment (selection bias) | Low risk | Consecutively‐numbered, sealed opaque envelopes contained treatment assignments. After obtaining consent, a research assistant opened the next envelope. It was unclear whether the research assistant enrolling the woman was the same one that opened the envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Labour room staff, participants, and the participant's caregivers were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Medical record abstraction was done by the author who was not blinded. The 6‐week questionnaire data collection was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Medical record information was completed for 97.7% of the sample (82.3% in the intervention group and 94.3% in the control group). The differential rates are due to withdrawals from the intervention group for doula related reasons (incomplete training and not being present during labour). The 6‐week questionnaire was completed for 82.3% of the sample. Only those women included in the study at delivery had the opportunity to complete the questionnaire and thus the differential completion rate between groups remained (76.3% in the intervention group and 88.3% in the control group). The differential withdrawals could introduce selection bias |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | The training of the doulas giving the intervention was done by the research assistant, who was herself a doula. This same research assistant enrolled all study participants |
Cogan 1988.
| Methods | RCT | |
| Participants | 34 women (primigravidas and multigravidas) at 26 to 37 weeks' gestation in 2 Texas hospitals (20 to supported group and 14 to usual care). They were in early, uncomplicated preterm labour | |
| Interventions | Intervention: support provided by a Lamaze childbirth preparation instructor. Support included continuous presence, acting as a liaison with hospital staff, providing information, and teaching relaxation and breathing measures. Usual care: intermittent nursing care. Family members allowed to be present |
|
| Outcomes | Fetal distress, caesarean birth, artificial oxytocin, labour length, Apgar scores, neonatal intensive care | |
| Notes | Not stated if epidural analgesia was available or if EFM was used routinely Dates of study: accepted to journal 1987. Exact dates not reported Funding: not reported. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned." No further details provided |
| Allocation concealment (selection bias) | Unclear risk | Admitting nurse telephoned research assistant to obtain treatment allocation. No details about whether the research assistant had foreknowledge of the treatment allocation scheme |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, participant's family members and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Medical record information collected by 'research assistants who did not know the group membership of the women'. However, medical records written by staff who were not blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals occurred before analysis (6 (30%) in support group and 3 (21%) in control). This resulted in a follow‐up rate of 73.5%. The withdrawals were done differentially in the support group, i.e. some women were withdrawn because of an event that occurred before the support person arrived. Women in the control group with the same event were not withdrawn. We were able to re‐create the original study groups for 1 outcome only, caesarean birth, and therefore it was included in the analysis table |
| Selective reporting (reporting bias) | Unclear risk | No outcomes were stated a priori |
| Other bias | Low risk | No other sources of bias noted |
Dickinson 2002.
| Methods | RCT, stratified by induced or spontaneous labour at trial entry | |
| Participants | 992 nulliparous women at term (499 to continuous support and 493 to control), cephalic fetal presentation, cervical dilatation < 5 cm, in a hospital in Perth, Western Australia | |
| Interventions | Group 1: continuous physical and emotional support by midwifery staff, and women were encouraged to use pharmacologic and nonpharmacologic alternatives to epidural analgesia. Group 2: continuous midwifery support was not provided and women were encouraged to have epidural analgesia as their primary method of pain relief in labour | |
| Outcomes | Labour length (expressed as median and interquartile range), epidural analgesia, mode of delivery, 5 min Apgar score < 7, arterial cord pH | |
| Notes | The stated purpose was to compare the effects of intrapartum analgesic techniques on labour outcomes. Continuous midwifery support was conceptualised as an analgesic technique. Both groups had access to opioids and nitrous oxide. No data were presented about the number of women who used no pharmacologic analgesia. Because the type of analgesia used was a measure of compliance rather than an outcome, no data on analgesic outcomes are included in this review. It was not stated if other support person was allowed. epidural analgesia was available and EFM was used routinely Dates of study: May 1997 ‐ October 1999 Funding: The conduct of this research was supported by NH&MRC Grant 970076. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details about how the blocks of treatment allocations were produced |
| Allocation concealment (selection bias) | Unclear risk | Randomisation on presentation in the labour and delivery unit, "by selection from a blocked group of eight sealed opaque envelopes, replenished from blocks of 12". No further details about process |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel is not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if the outcome assessor was blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was 100% follow‐up for medical record data and in‐hospital survey. A 6‐month questionnaire was completed by 64.7% of the sample and these data were not used |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported. Effects on breastfeeding were not analysed by treatment group and thus the results could not be included in the review |
| Other bias | Low risk | No other sources of bias noted |
Gagnon 1997.
| Methods | RCT | |
| Participants | 413 women admitted to an intrapartum unit at a tertiary care teaching hospital in Montreal, Canada, were randomly allocated to experimental (N = 209) or control (N = 204) groups. All but 3 in the experimental group and 6 in the control group were accompanied by a spouse, relative or friend during labour. All participants were nulliparous, with singleton fetuses, > 37 weeks' gestation, and in labour | |
| Interventions | Experimental: One‐to‐one nursing care from randomisation until 1 hour post birth. Care was provided by on‐call nurses who were hired specifically for the study and had received a 30‐hour training program and quarterly refresher workshops. The training program included critical reviews of the literature concerning the effects of intrapartum medical and nursing practices, as well as discussions of stress and pain management techniques. The nurse provided the usual nursing care plus physical comfort, emotional support, and instruction on relaxation and coping techniques. The nurse took meal breaks and brief rest breaks. Women in the comparison group received usual nursing care by the regular unit staff, consisting of intermittent support and monitoring |
|
| Outcomes | Caesarean birth, caesarean birth for cephalopelvic disproportion or failure to progress, post‐randomisation artificial oxytocin augmentation, post‐randomisation analgesia/anaesthesia, instrumental vaginal delivery (forceps or vacuum extraction), NICU admission, perineal trauma, mean duration of labour post‐randomisation, postpartum urinary catheterisation | |
| Notes | The participants had been admitted to the unit for an average of 5 hours (SD = 4 hours) prior to randomisation. 36 women in the experimental group and 41 in the control group had epidural analgesia prior to randomisation. 55 women in the experimental group and 45 in the control group had intravenous oxytocin augmentation of labour prior to randomisation. Mean duration of labour post‐randomisation was 9.2 hours (SD = 4.3). Epidural analgesia was available but it was not stated if EFM was used routinely Dates of study: not clear Funding: This project was supported by the Fonds de la recherche en sante' du Que'bec (FRSQ), a research funding agency of the government of Quebec, Montreal, Quebec, Canada. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized using a list of computer generated random numbers." |
| Allocation concealment (selection bias) | Low risk | "Randomized in blocks of eight... Group assignments were placed in sequentially numbered, sealed, opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, participants' family members, and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome data not collected by clinical staff, self‐administered questionnaires were used, and data collectors reviewed nurses' notes after "most" other data from medical records was collected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Hans 2013.
| Methods | RCT | |
| Participants | 248 pregnant women (124 control group, 124 doula group) attending 2 affiliated prenatal clinics, who were aged under 22 years, less than 34 weeks' gestation, and not planning to move from the area or give their baby up for adoption | |
| Interventions | Intervention: doula support from a community doula service during pregnancy through 3 months postpartum, as well as usual care services. 4 doulas were trained as part of the program, from the communities surrounding the hospital, and participated in a 10 week training session. Doulas initiated contact with participants, and scheduled weekly visits throughout her pregnancy through 3 months postpartum, and participants were encouraged to call the doula when they went into labour. Control: usual prenatal healthcare and social services |
|
| Outcomes | Parent‐child interactions: reported through 2 parenting constructs (maternal sensitive responsiveness and maternal encouragement and guidance) and 1 child construct (positive involvement with parent), as measured through video recordings of parent‐child interactions at 4, 12 and 24 months of age using the Parent‐Child Observation Guide. Parenting attitudes: reported through the Adult‐Adolescent Parenting Inventory at 4 months of age. Parenting stress: reported through the Parenting Stress Inventory‐Short Form at 4, 12 and 24 months of age. Breastfeeding duration |
|
| Notes | For all women who received doula care in the intervention group, the doula was 1 of 4 study doulas. Unknown if companions were typically permitted on the ward, if continuous EFM was used routinely, or if epidural anaesthesia was available. Women in the doula group were encouraged to contact their doula at the start of their labour, but only 101 women (81.5%) in the doula group had the doula in attendance at the birth. The authors report that the most common reasons for doula absence at the birth were: (1) short labours; and (2) failed communication between the woman and the doula. In the analysis, it is not possible to determine which women in the doula group had a doula present with them during birth Dates of study: 3 year period, dates not clear Funding: This research was supported by Grant R40MC 00203 from the United States Maternal and Child Health Bureau and by a grant from the Irving B. Harris Foundation. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation took place in blocks of 4, 6 or 8 (with equal numbers to intervention and comparison group within a block |
| Allocation concealment (selection bias) | Low risk | Randomisation done from a series of sealed opaque envelopes, labelled with a sequential subject identification number, and containing an assignment to intervention or control group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for the participants or study personnel to be blinded to this intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Standardised validated scales used to assess outcome, but participants and assessors knew to which group participants were randomised |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Good study retention over period up to 24 months; all outcomes reported in methods reported in results; all analyses were by intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Hemminki 1990a.
| Methods | 2 RCTs reported in the same publication. The Zelen method was used: only those participants randomised to the experimental group were told the true purpose of the trial and asked for consent. The participants in the control group were told about the study in the introduction letter for the postpartum questionnaire and they were told it was "a study on factors influencing birth". | |
| Participants | Healthy nulliparous and parous women in labour at a hospital in Finland. 86 women were enrolled in Trial A. The actual number enrolled to each group was not noted but medical record data were collected for 79 women (41 in the support group and 38 in the control group). These 79 women represented 91.9% of the total sample | |
| Interventions | Trial A: in 1987, the intervention was 1:1 support by midwifery students from enrolment until transfer to the postpartum ward. The midwifery students volunteered, were not specially trained in support and responsible for the other routine intrapartum care. The control group 'was cared for according to the normal routine of the midwife and by a medical student, if s(he) was on duty'. Over 70% of fathers were present |
|
| Outcomes | Labour length, medical interventions, complications (mother and baby), pharmacologic pain relief, method of birth, mothers' evaluations of their experiences | |
| Notes | Not stated if epidural analgesia was available or if EFM was used routinely Dates of study: "spring 1987" Funding: Study supported by a grant from the Finnish Academy of Sciences. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how the allocation sequence was produced |
| Allocation concealment (selection bias) | Unclear risk | "Randomization coding was done in blocks of 6 and put into non‐transparent envelopes. The envelope was opened at the reception ward when it was decided to transfer mother to labour ward." It was not stated if the envelopes were consecutively numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, participant's family, and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were collected from medical records by a researcher who was not blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record data were collected on 91.9% of the sample. A questionnaire was administered at 2‐3 days postpartum. This was completed by only 70% of the sample and thus the data were not used |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | Mothers were told the purpose of the study differentially (see methods for Trial A above) |
Hemminki 1990b.
| Methods | See Hemminki 1990a. | |
| Participants | See Hemminki 1990a. 161 women were enrolled in Trial B (81 in the support group and 80 in control). | |
| Interventions | Trial B: in 1988, the intervention was support by a new group of midwifery students. All students were involved in the trial, not just volunteers. The students were permitted to leave their participants to witness other interventions and deliveries. The control group "was cared for according to the normal routine of the midwife" and by a medical student as enrolment was limited to days when medical students were on duty. Slightly fewer than 70% of fathers were present |
|
| Outcomes | See Hemminki 1990a | |
| Notes | Not stated if epidural analgesia was available or if EFM was used routinely Dates of study: "autumn 1988" Funding: Study supported by a grant from the Finnish Academy of Sciences. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | The block size was reduced from the first study. "To lessen the frustration resulting from opening a code for a control mother, randomisation envelopes contained a maximum of two similar codes in sequence (not told in advance)". "Put into non‐transparent envelopes". The envelope was opened in the labour ward. It was not stated if the envelopes were consecutively numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding of participants and personnel, but blinding of participants and personnel is not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record data were collected on 100% of the sample. A questionnaire was administered at 2‐3 days postpartum and completed by 93.7% of the sample |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | Mothers were told the purpose of the study differentially (see methods for Trial A above) |
Hodnett 1989.
| Methods | RCT, stratified by type of prenatal classes (Lamaze versus general) | |
| Participants | 145 nulliparous women (72 to support group and 73 to control) in the last trimester of a healthy pregnancy, booked for delivery at a Toronto, Canada, hospital | |
| Interventions | Support provided by a monitrice (community 'lay' midwife or midwifery apprentice) compared with usual hospital care, defined as the intermittent presence of a nurse. Support described as including physical comfort measures, continuous presence, information, emotional support, and advocacy. The monitrice met with the woman twice in the latter weeks of pregnancy, to discuss her birth plans. Comparable prenatal attention was provided to the controls. All but 1 woman also had husbands or partners present during labour. Support began in early labour at home or in hospital and continued through delivery |
|
| Outcomes | Intrapartum interventions, perceived control, method of delivery | |
| Notes | Epidural analgesia was available and EFM was used routinely Dates of study: recruitment and follow‐up complete by 1986 Funding: supported by a grant from the National Health Research and Development Program, Health and Welfare Canada. Project no. 6606‐2939‐43. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation done over the phone by a third party who had no knowledge of the participant, but used the open table of random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Initial collection of medical record data were not blinded. "Duplicate abstraction was done by a second research assistant blind to the subject's study group assignment, on a random sample of 20 records. Interrater agreement of over 95% was obtained for all categories of intervention and physical outcomes." In‐home interview at 2 to 4 weeks postpartum was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Method of delivery outcome available on 88.3% of sample. Other outcomes collected on only 71% of the sample and thus not used |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Hodnett 2002.
| Methods | Multicentre RCT with prognostic stratification for parity and hospital | |
| Participants | 6915 nulliparous and parous women in labour at 13 hospitals in the USA and Canada (3454 to continuous labour support and 3461 to usual care). Eligibility criteria: live singleton fetus or twins, no contraindications to labour, in labour. Women were excluded if gestational age was < 34 weeks or if they were so high risk that a 1:1 patient‐nurse ratio was medically necessary | |
| Interventions | Experimental: continuous support from staff labour and delivery nurses who had volunteered for and received a 2‐day training workshop in labour support. Prior to the trial, the support nurses had opportunities to practice their skills. They also had opportunities to continue learning from each other and the labour support trainer, throughout the trial. The nurses with training were part of the regular staffing complement of the unit and they provided care to the continuous support group but not to the usual care group. Usual care: intermittent support from a nurse who had not received labour support training |
|
| Outcomes | Intrapartum interventions, method of birth, immediate complications (mother or baby), complications (mother or baby) in the first 6‐8 weeks postpartum, perceived control, postpartum depression, breastfeeding at 6‐8 weeks, relationship with partner and with baby, likes and dislikes about birth experience and future preferences for labour support | |
| Notes | Other support person(s) were allowed, epidural analgesia was available and EFM was used routinely Dates of study: enrolment between 19 May 1999, and 25 May 2001. Funding: supported by US PHS grant 5R01NR04684 from the National Institutes of Health, National Institute for Nursing Research. The Data Coordinating Centre in the Maternal, Infant, and Reproductive Health Research Unit is supported by grants from the Centre for Research in Women’s Health, Sunnybrook and Women’s College Health Sciences Centre, and the Department of Obstetrics and Gynaecology at the University of Toronto. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation program |
| Allocation concealment (selection bias) | Low risk | "Randomization was centrally controlled with the use of a computerized randomisation program at the data co‐ordinating centre, accessible by means of a touch‐tone telephone." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, participants' families, and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collectors were not blinded as they read nurses' notes to collect data about type of nursing care provided. However random chart audits yielded no errors in reporting study outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record data were collected on 100% of the sample. In‐hospital questionnaires were completed by 96.4% and 6 to 8 week questionnaires by 81% of the sample |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Hofmeyr 1991.
| Methods | RCT | |
| Participants | 189 nulliparous women (92 to support and 97 to control) in active labour at a community hospital serving low‐income women in South Africa | |
| Interventions | Intervention group: support by carefully trained, volunteer lay women, for at least several hours (supporters not expected to remain after dark). Control group: intermittent care on a busy ward. Spouses/family members were not permitted |
|
| Outcomes | Intrapartum interventions, method of birth, complications (mother and baby), anxiety, pain, mothers' perceptions of labour, breastfeeding | |
| Notes | Epidural analgesia was not available and EFM was not used routinely. While scores on an instrument measuring postpartum depression were reported in categories of "low", "moderate," and 'high", the authors stated that categorisation was not appropriate as a clinical diagnostic definition of depression. To achieve the latter, the change in score must be reported, and these data were not collected Dates of study: not clear, received by journal 1990 Funding: South African Medical Research Council. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random |
| Allocation concealment (selection bias) | Unclear risk | "Randomly ordered cards in sealed opaque envelopes". Not stated if consecutively numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It was not possible to keep the interviewer blind to the group assignment, as sometimes participants volunteered information which identified them as belonging to 1 group or another |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record data were collected on 100% of the sample and questionnaires within 24 hours postpartum were completed by 99%. The 6‐week follow‐up interviews were completed by 78.8% of the sample, no imbalances existed between groups and thus the data were included in the analysis. At 1‐year interviews were complete for 46% of the sample and data from these were not used. Nikodem reported on a larger sample of women with 1‐year follow‐ups but the completion rate was still only 50% of the original number enrolled |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Isbir 2015.
| Methods | RCT | |
| Participants | This study took place in the obstetrics clinic of a state hospital in a city located in the Middle Anatolia Region in Turkey between April and August 2014. Pregnant women at least 18 years of age, who were literate, with at least a primary school education, gestational age ≥ 37 weeks, conscious, able to communicate in Turkish, and ≥ 3 cm dilated | |
| Interventions | The intervention group (N = 36) was given routine care and continuous supportive care during labour and delivery. Supportive care was provided by 5 midwifery students from the School of Health who had taken an obstetrics course in their third year and had expressed willingness to join the study. The students were provided 24 hours of skills training directed toward continuous supportive care and practices during labour and a 2‐hour theoretical course for the research procedures that was taught by the researchers. The interventions began upon hospital admission and finished at the end of the third stage of labour. Participants in the control group (N = 36) received the routine care that was normally provided at the hospital only. At the participating hospital, pregnant women are monitored, and midwives perform the deliveries; doctors are consulted when necessary. The physical support normally offered by midwives during the first phase of labour included controlling room temperature and odours, patient positioning, and hygiene and urinary elimination | |
| Outcomes | Delivery fear (10 item scale to measure fear during delivery) during latent, active and transitional phases of labour; pain score (assessed on a 10 cm VAS ranging from 0 (no pain) to 10 (worst pain)) during latent, active and transitional phases of labour; perceived control and support in birth (SCIB subscales include internal control, external control, and support, with a total of 33 items and a 5‐point Likert scale to score responses, scale range 33 to 165, higher score associated with higher degree of perceived support and control during birth); duration of labour (hours); use of oxytocin, and caesarean section (see note) | |
| Notes | EFM not used routinely and epidural anaesthesia not available in the study hospital. Women who had in labour emergency caesarean section were excluded from both the intervention group (N = 3 excluded) and the control group (N = 6 excluded) of the study, as the investigators felt that the emergency situation interfered with supportive care, and control passed from the research and midwife team to the doctor. Caesarean section is included in this review as an outcome, calculated from the number of participants randomised to the intervention group (N = 36) and control group (N = 36), less the number of in labour emergency caesarean sections for each group Dates of study: April ‐ August 2014 Funding: not reported. Conflicts of interest: The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised block assignment was used to assign 72 women to the intervention group (N = 36) and the control group (N = 36). A randomised block procedure (Vickers 2006) was performed as follows: (a) a block size of 4 was selected; (b) subjects were calculated as having 6 conditions (TTCC, TCTC, CCTT, TCCT, CTCT, and CTTC); and (c) blocks were randomly selected to determine the assignment of all 72 participants, with an allocation ratio of 1:1. Predictable block size |
| Allocation concealment (selection bias) | Unclear risk | No mention of central assignment, sequential numbering or opaque envelopes or containers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for the woman/provider to be blinded due to the intervention, although the woman was blinded to differences between control and intervention. No mention of data analysis blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded to the group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data reported for 33/36 women in intervention and 30/36 women in control. All drop outs due to emergency caesarean section. Post randomisation exclusion |
| Selective reporting (reporting bias) | High risk | Women who underwent emergency CS were excluded from the analysis, but mode of delivery is a frequently reported outcome for labour companionship |
| Other bias | Low risk | No other bias identified |
Kashanian 2010.
| Methods | RCT | |
| Participants | 100 nulliparous women at term (50 to support and 50 to routine care) in active labour at a university hospital in Tehran, Iran from March to September 2003 | |
| Interventions | "Women allocated to the intervention group were shown to an isolated room and were supported by an experienced midwife. The women were free to choose their position, and able to eat and walk about freely. During labour, the midwife explained the process of labour and the importance of body relaxation. Midwife‐led support included close physical proximity, touch, and eye contact with the labouring women, and teaching, reassurance, and encouragement. The midwife remained with the woman throughout labour and delivery, and applied warm or cold packs to the woman's back, abdomen, or other parts of the body, as well as performing massage according to each woman's request." "Women allocated to the routine care group were admitted to the labour ward (where 5‐7 women labour in the same room), did not receive continuous support, and followed the routine orders of the ward. They did not have a private room, did not receive one‐to‐one care,were not permitted food, and did not receive education and explanation about the labour process. The only persons allowed in the delivery room were nurses, midwives, and doctors." |
|
| Outcomes | Duration of labour, caesarean delivery, oxytocin use, Apgar score at 5 minutes | |
| Notes | EFM was not used routinely and epidural analgesia was not available Dates of study: March ‐ September 2003 Funding: not reported. Conflicts of interest: The authors declare that they have no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From personal communication ‐ equal numbers of envelopes were produced for each letter (see below) and put into a box. No list of treatment allocations was created |
| Allocation concealment (selection bias) | High risk | "Allocated to one of two groups using 4‐part, block randomisation". Used "sealed envelopes labelled A, B, C, and D: envelopes A and C (intervention group) and B and D (routine care group). Patients then chose an envelope, which was opened by the investigator". Further details from personal communication ‐ the women picked from all the envelopes produced. Once an envelope was picked it was discarded. This process was open to selection bias as women previously in the trial may have shared knowledge of which envelope contained which group with women not yet enrolled in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | From personal communication "The co worker of investigator collected the outcome data and she was blind for the study group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record information was collected on 100% of the sample |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Kennell 1991.
| Methods | RCT of continuous support versus usual care with an 'inconspicuous observer' plus a retrospective non‐random control group. This review is restricted to comparisons of the outcomes of the participants who were randomly assigned | |
| Participants | 412 nulliparous women (212 in support group and 200 in observed group) were part of the RCT. They were aged 13 to 34 years, with singleton, term, healthy pregnancies, many not English‐speaking, in active labour at a public hospital in Texas which provides care for low‐income patients | |
| Interventions | The description of the setting, the participants, and the type of care echo developing world conditions. All women laboured in a large 12‐bed room. For the women in the support group a doula stayed by their bedside and gave continuous support. For those in the observed group they had the routine intermittent presence of a nurse and continuous presence of an "inconspicuous observer" who "kept a record of staff contact, interaction and procedures". The observer was away from the beside and never spoke to the labouring woman |
|
| Outcomes | Analgesia/anaesthesia, labour length, artificial oxytocin use, method of birth, complications (mother and baby), neonatal health, number of women who rated their experience as negative | |
| Notes | In instances in which outcome data (such as analgesia/anaesthesia use) in the published report were only provided for subgroups, the primary author was contacted and he provided complete outcome data for all women who were originally randomised. Family members were not allowed to be present. Epidural analgesia was available and EFM was used routinely Dates of study: not clear, accepted by journal 1991 Funding: grant HD 16915 awarded by the National Institute of Child Health and Human Development, Bethesda, Md; by the Arthur Vining Davis Foundations, Jacksonville, and by the Pittway Corporation Charitable Foundations, Chicago. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as random, however participants were randomised to the control group if they were eligible, but admitted to the hospital on days that doulas were already assigned to other patients |
| Allocation concealment (selection bias) | Low risk | "Randomly assigned" is stated in the report. In the protocol for the trial it states "numbered opaque envelopes" would be used. The envelopes "would contain the random assignments of the women to control or treatment groups and would be numbered sequentially" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and labour ward staff not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who assessed the outcome data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is some discrepancy in the number of women enrolled in the study. The report states 412 were enrolled and reports outcome data on all 412 women. But it also states that "14 women that agreed to participate were not included in the study." The reasons for not including them seem to be events that would happen after randomisation ‐ e.g. transferred due to staffing limitations, withdrew, undetected breech, interrupted observations, etc., and thus the sample appears to have numbered 426. Data are reported for 412 women (96.7% of 426) |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Klaus 1986.
| Methods | RCT. Purposefully enrolled more women to the control group. See 'Risk of bias' table below | |
| Participants | 465 healthy nulliparous women (186 to support group and 279 to control) in labour at the Social Security Hospital in Guatemala | |
| Interventions | Support group: continuous emotional and physical support by a doula. Control group: usual hospital routines (described as no consistent support) |
|
| Outcomes | Labour length, use of artificial oxytocin, method of birth, problems during labour and birth, fetal distress, Apgar scores, transfer to neonatal intensive care nursery | |
| Notes | No family members permitted to be present. epidural analgesia was not available and EFM was not used routinely Dates of study: not clear, accepted by journal June 1986 Funding: grants from the Thraher Fund, the Pitway Corporation Charitable Foundation, and the Maternal and Child Health Research (MC‐R‐390430). Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Enrolled using randomised design". "Pool of envelopes contained more control group to ensure similar sized groups with uncomplicated labours and deliveries." They anticipated more complications in control group based on an earlier study (Sosa 1980). No information on how allocation sequence was generated |
| Allocation concealment (selection bias) | Low risk | "Randomly assigned according to contents of a sealed opaque envelope. Each envelope was numbered sequentially." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who assessed the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Mother‐infant pairs were excluded when the mother developed a complication during labour, delivery, or post partum that required special care, if the baby's weight was below 5.5 lbs or above 8 lbs, if there were twins or congenital malformations." This occurred for about 10% of cases in both groups resulting in reported outcomes for 89.6% of those randomised. Unpublished data on the excluded women were provided by the author. Labour length data were only available for 48.4% of the sample (225 of 465) and thus not included |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Langer 1998.
| Methods | RCT | |
| Participants | 724 women (361 to support and 363 to control) admitted for delivery at a large social security hospital in Mexico City, who met the following criteria: singleton fetus, no previous vaginal delivery, < 6 cm cervical dilatation, and no indications for an elective caesarean delivery | |
| Interventions | Support group: continuous support from 1 of 10 women who had received doula training (6 were retired nurses), throughout labour, birth, and the immediate postpartum period. Support included: emotional support, information, physical comfort measures, social communication, ensuring immediate contact between mother and baby after birth, and offering advice about breastfeeding during a single brief session postnatally. Control group: women received "routine care" |
|
| Outcomes | The main outcomes were exclusive and full breastfeeding at 1 month postpartum. Other outcomes included labour length, epidural anaesthesia, forceps birth, caesarean birth, meconium staining, and Apgar scores, as well as mothers' perceived control during childbirth, anxiety, pain, satisfaction, and self‐esteem | |
| Notes | Partners and family members were not permitted. Epidural analgesia was available but it was not stated if EFM was used routinely Dates of study: not clear, received 9 July 1997 Funding: Wellstart International provided financial and technical support. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated random number list". "The treatment sequence was kept at a central level." |
| Allocation concealment (selection bias) | Unclear risk | "Opaque envelopes with the assignment were locked in a cabinet to which only a social worker exclusively in charge of randomisation and the principal investigator had access. An envelope with a paper inside showing to which group each woman was assigned was opened by the social worker immediately after recruitment in the labour and delivery unit". Not stated if envelopes were sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data were collected by 2 "blinded social workers" who reviewed clinical records, but records were written by staff aware of group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record data and in‐hospital interview data were collected for 100% of the sample. A in‐home interview was completed at 1 month postpartum for 92.2% of the sample |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Madi 1999.
| Methods | RCT | |
| Participants | 109 Black women from Botswana (53 in support group and 56 in usual care group), mean age 19 years, 80% unmarried, mostly students, who had met the following criteria: nulliparous, in labour, pregnancy at term, no history of pregnancy complications, cephalic presentation, normal spontaneous labour with cervical dilation 1 to 6 cm, female relative present who was willing to remain with the woman for the duration of labour | |
| Interventions | Support group: continuous presence of female relative (usually her mother) in addition to usual hospital care. Congrol group: usual hospital care, which involved staff:patient ratios of 1:4, and no companions permitted during labour |
|
| Outcomes | Spontaneous vaginal birth, vacuum extraction, caesarean birth, analgesia, amniotomy, artificial oxytocin during labour, Apgar scores (1‐ and 5‐min) | |
| Notes | Epidural analgesia was not available and it was not stated whether EFM was used routinely Dates of study: October 1994 ‐ January 1995 Funding: part of Master's degree of Banyana Cecilia Madi, sponsored by Government of Botswana. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated." No other details provided |
| Allocation concealment (selection bias) | Low risk | "Selection of an opaque, numbered, sealed envelope from a box of envelopes that were shuffled in the woman's presence. When opened the envelope revealed a code indicating her group." An assistant that was not involved in the recruitment process shuffled the envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, participants' families, and labour room staff were not blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The researcher, who was involved in the recruitment of participants, collected the medical record data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record data were collected on 100% of the sample |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
McGrath 2008.
| Methods | RCT. Enrollment occurred at childbirth education classes and randomisation occurred when the woman arrived at hospital in labour | |
| Participants | 420 nulliparous middle and upper class women (224 on doula group and 196 in control group) were enrolled in the third trimester of an uncomplicated pregnancy in Cleveland, Ohio. All women expected to be accompanied during labour by their male partner | |
| Interventions | Experimental group: a doula met the couple at the hospital as soon as possible after random assignment (typically within an hour of their arrival at the hospital) and remained with them throughout labour and delivery. The central component of doula support was the doula’s continuous bedside presence during labour and delivery, although her specific activities were individualised to the needs of the labouring woman. Doula support included close physical proximity, touch, and eye contact with the labouring woman, and teaching, reassurance, and encouragement of the woman and her male partner. All doulas completed training requirements that were equivalent to the DONA international doula certification. Control group: routine obstetric and nursing care which included the presence of a male partner or other support person |
|
| Outcomes | Caesarean delivery, epidural anaesthesia, oxytocin use, labour length, mode of delivery, fever during labour, satisfaction at 6 weeks postpartum | |
| Notes | Epidural analgesia was available and EFM was used routinely. The author has been contacted for data split by study group and questionnaire data for the control group Dates of study: enrolment from October 1988 through October 1992. Funding: supported in full by Grant HD 16915 awarded by the National Institute of Child Health and Human Development, Bethesda, Maryland, USA. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details stated |
| Allocation concealment (selection bias) | Low risk | "When the research co‐ordinator was informed that an enrolled woman had arrived at the hospital in early active labour, she opened the next sequentially numbered opaque envelope to determine random assignment to the doula or control group". The research co‐ordinator was off‐site and called by the staff or the study participant |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, participants' family members, and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Research assistants had access to the medical records, including information about procedures and interventions. Unclear if this includes group assignment. Questionnaires were completed by the participant and her partner, before hospital discharge |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record data were collected on 100% of the sample. The in‐hospital and 6‐week questionnaires were completed by 87.9% and 87.5% of the doula group. No information was provided for the control group |
| Selective reporting (reporting bias) | Low risk | The primary outcomes of caesarean birth and epidural anaesthesia were reported for each study group. Other labour and delivery outcomes were reported for the full sample only (not split by group). The in‐hospital and 6‐week questionnaire data were only reported for the doula group. The author has been contacted for these missing details |
| Other bias | Low risk | No other sources of bias noted |
Morhason‐Bello 2009.
| Methods | RCT | |
| Participants | 603 women from Ibadan, Nigeria with anticipated vaginal delivery were enrolled between 30 and 32 weeks' gestation at an antenatal clinic (305 to intervention and 298 to control) from November 2006 to March 2007 | |
| Interventions | Those in the experimental group were informed to bring someone of their choice to act as a companion during labour. On arrival in labour the accompanying companions were provided with an information leaflet that explained their responsibilities. These included: gentle massage of the woman’s back during contraction, reassuring words, spiritual support inform of prayers and also acting as intermediary between the woman and healthcare team. After studying the leaflets, they were allowed to seek clarifications. The information leaflet was also interpreted for those that are not literate. The attending midwife allowed and ensured companions performed their expected duties throughout. The companions were told to offer continuous support – they were to be by the patient’s side except for feeding and use of toilet until 2 hours after childbirth. Husbands were the most common support person (65.4%). The women in the control group had only routine care where relatives of patients are usually barred from the labour ward |
|
| Outcomes | Caesarean section rate, active phase of labour duration, pain score, need for analgesia, need for oxytocin augmentation, time from delivery to initiation of breastfeeding and the emotional experience during labour. | |
| Notes | Epidural analgesia was not available and it was not stated whether EFM was used routinely. We have requested further details from the authors. The randomisation process was well done, but resulted in an imbalance in socioeconomic status between the groups. Women in the experimental group tended to be more educated (82% versus 48% with tertiary level) and skilled workers (78% versus 39%). This imbalance was noted and discussed by the authors Dates of study: November 2006 ‐ 30 March 2007 Funding: received financial support from the Gates Institute, Bloomberg School of Public Health Johns Hopkins University through the Center for Population and Reproductive Health, College of Medicine University of Ibadan, Nigeria. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation sequence was generated using a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | "Random permuted blocks of size four were used to ensure a balanced design." "Based on the sequence of treatments generated using this method, treatment groups (A and B) were written on pieces of cardboard paper and put into sealed opaque envelopes. Each of the opaque envelopes had a serial number on it." "Two trained research assistants (RAs) non‐medical staff, supervised the randomisation procedure at every clinic. On each clinic day, consented women that met the inclusion criteria were given serial numbers with allotted treatment group based on their arrival time. Only the statistician and RAs had access to the list of numbers used to prevent clinicians’ influence on the randomisation. Each participant opened the opaque envelope in the presence of an RA, and the assigned treatment group was recorded on the woman’s medical record file." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, participants' families, and labour room staff were not blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The treatment group was noted in the chart so it is likely that the data collectors were unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up was completed for 97% of the sample |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Safarzadeh 2012.
| Methods | RCT | |
| Participants | 150 primiparous women with a single foetus, who arrived at the labour ward in active labour (4 cm cervical dilatation). The age range of the women was 18 to 34 years, and gestational age was 38 to 42 weeks. None of the women had evidence of any severe obstetric disease | |
| Interventions | This study was carried out in maternity wards of Zahedan and Mirjaveh, Iran from July 2007 to May 2008. Women in the intervention group received doula support during active labour from an untrained woman such as a female friend or relative (mother‐in‐law, mother, sister‐in‐law, sister) who had been selected by the mother. Women in the control group (without doula support) received routine care. To avoid contamination between the intervention and control groups, separate labour rooms, screens between the beds or beds at opposite ends of the same room were used |
|
| Outcomes | Severity of pain: using a VAS at the beginning of active labour (4 cm cervical dilation) and at the end of the second active phase of labour (10 cm cervical dilatation). Duration of the active phases of labour Mode of delivery Use of medication (oxytocin/promethazine/hyosin) |
|
| Notes | Unknown if companions were typically permitted on the labour ward, if continuous EFM was used routinely, or if epidural anaesthesia was available Dates of study: July 2007 to May 2008 Funding: not clear. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There is no mention of how the sequence was generated, e.g. “Subjects were selected using simple random sampling and were randomly divided into two groups: one group with doula support (n=75) and one control group (n=75)." |
| Allocation concealment (selection bias) | Unclear risk | There is no mention of how allocation was concealed, e.g. “Subjects were selected using simple random sampling and were randomly divided into two groups: one group with doula support (n=75) and one control group (n=75)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There is no mention of how participants and/or personnel were blinded, but blinding of participants and personnel is not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no mention of blinding of outcome assessment, e.g. "The severity of pain was measured in both groups using a Visual Analogue Scale at the beginning of active labour (4 cm cervical dilation) and at the end of the second active phase of labour (10 cm cervical dilatation). Duration of the active phases of labour, the type of delivery and the use of medication (oxytocin/promethazine/hyosin) in both groups were recorded." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study states that 75 women were randomised to control and 75 randomised to intervention. However, neither the results section nor table 2 (relevant outcome data), report the total number of women for each outcome assessed |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in the method section are reported in the results section |
| Other bias | Low risk | No evidence of other bias |
Thomassen 2003.
| Methods | RCT, no details regarding method of random assignment | |
| Participants | 144 "healthy" women having their first baby booked for delivery at a Swedish hospital (72 to doula group and 72 to usual care). Participants were enrolled at 36 weeks' gestation | |
| Interventions | Continuous presence by a doula who had met the woman during pregnancy, compared to usual care | |
| Outcomes | Emergency caesarean birth and epidural analgesia | |
| Notes | The trial author reported that the information about randomisation method and outcomes of those lost to follow‐up are no longer available. Epidural analgesia was available. It was not stated if other support person(s) were allowed or if EFM was used routinely Dates of study: March 1998 ‐ March 2000 Funding: not reported in translation. Conflicts of interest: not reported in translation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized' ‐ no further details provided or available |
| Allocation concealment (selection bias) | Unclear risk | No details provided or obtained |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome data were collected by researches blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Medical record data collected on 70.1% of sample. No usable outcome data, due to serious risk of attrition bias. Outcomes are reported for 55/72 (76%) of the intervention group and 46/72 (64%) of the control group. Reason for the 41 “dropouts” were preterm birth, induction, or caesarean section “for medical reasons”, and participant withdrawal. No numbers are given for individual reasons, or by group, but it is clear that some “dropouts” were prior to labour and others were during labour. Numbers in the report show the number of dropouts was actually 43 |
| Selective reporting (reporting bias) | Unclear risk | Sample size was based on caesarean section rate. The only outcome reported was emergency caesarean |
| Other bias | High risk | Trial was stopped early for "a range of largely organizational issues" when only 1/4 of the original sample size had been enrolled |
Torres 1999.
| Methods | RCT | |
| Participants | 435 women (217 in companion group, 218 in control group) with a singleton pregnancy and considered to be low‐risk at University Hospital in Santiago, Chile. Enrolled at 34 to 36 weeks' gestation | |
| Interventions | Intervention group: psychosocial support during labour from a companion chosen by the pregnant woman. The companions were trained by trial staff to provide emotional support, promote physical comfort and encourage progress of labour, without interfering with the activities of the obstetricians or midwives. They were with the labouring woman continuously from admission to delivery. Women were encouraged to pick a companion who had experienced a vaginal birth. Control group did not have companion. Both groups laboured in a room with other women where curtains were pulled for privacy |
|
| Outcomes | Caesarean section, exclusive breastfeeding, duration of labour, mode of delivery, use of oxytocics, presence of meconium, regional anaesthesia, birth asphyxia, Apgar scores, level of neonatal care, maternal satisfaction | |
| Notes | Epidural analgesia was available. It was not stated if EFM was used routinely. Authors have been contacted for further details Dates of study: 1997 ‐ 1999 Funding: not reported in translation. Conflicts of interest: not reported in translation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Used blocks of 6. Group assignment used sealed opaque envelopes numbered consecutively. A member of the trial team enrolled women and did not know in advance the content of each envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who assessed the outcomes and whether they were blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record data were collected for 100% of the sample and in‐hospital surveys were completed by 95.8%. A 6‐week phone interview was completed for 71.2% of the sample and thus these data were not used |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias noted |
Yuenyong 2012.
| Methods | RCT | |
| Participants | 120 nulliparous women, aged 18 to 30 years, at least 36 weeks' gestation, singleton fetus with cephalic presentation, able and willing to have a close female relative with them during labour and birth, booked to give birth at a regional teaching hospital in Thailand | |
| Interventions | Experimental group: close female relative who attended a 2‐hour preparation class on labour routines and supportive actions, and provided continuous support during the active portion of hospital labour. The institution required that the researcher remain in order to monitor the relative's activities. Control group: usual care by health professionals, which included intermittent support. Family members were not permitted to stay with the woman | |
| Outcomes | Oxytocin during labour, analgesia, labour length, spontaneous birth, assisted vaginal birth, caesarean birth, Apgar scores, perceived control | |
| Notes | Epidural analgesia was not available and continuous EFM was not used Dates of study: November 2006 ‐ May 2007 Funding: Supported by the 90th Anniversary of Chulalongkorn University and Thailand Nursing Council Fund. Conflicts of interest: The authors report no conflict of interest or relevant financial relationships. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence generated by a software program |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were used. Envelopes were consecutively‐numbered on the outside |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, participants' families, and labour room staff were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Research assistant blinded to group assignment collected data on satisfaction. Unclear if clinical outcome data were assessed by a researcher blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% lost to follow‐up: 2 in the experimental group and 4 in the control group |
| Selective reporting (reporting bias) | Low risk | Appears complete |
| Other bias | Low risk | 6 women (10%) in experimental group did not receive continuous support |
CS: caesarean section DONA: EFM: electronic fetal monitoring min: minutes NICU: neonatal intensive care unit RCT: randomised controlled trial SD: standard deviation VAS: visual analogue scale VD: vaginal delivery
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bender 1968 | Two studies were reported, N = 12 in the first study and N = 30 in the second. Neither was an RCT. Both employed alternate allocation that was neither centrally controlled nor concealed. The researcher delivered the intervention and collected outcome data. In the first study the researcher also enrolled participants. No usable outcome data were reported |
| Bochain 2000 | The intervention was not continuous labour support. It was a short nursing intervention (taking approximately 1 hour) administered in early labour for women undergoing Misoprostol induction |
| Brown 2007 | The intervention was not continuous labour support. It was an educational intervention to promote childbirth companions in hospital deliveries. A cluster‐RCT was undertaken at 10 South African state maternity hospitals |
| Dalal 2006 | Not an RCT. 100 randomly‐selected mothers who had a birth companion were compared with 50 randomly‐selected mothers who did not have one. Mothers were matched for age and socioeconomic status |
| Dong 2009 | Intervention is the effects of air sac combined with nitrous oxide gas and doula care; Not a clean comparison with the control group who have no access to nitrous oxide gas |
| Gordon 1999 | 30% of those enrolled were excluded post‐randomisation, 73/232 in the doula group and 69/246 in the control group. A letter was sent to the first author, asking for data on the excluded participants that would permit an intent‐to‐treat analysis. If and when a response is received, we will evaluate the trial report again |
| Hemminki 1990c | Third study in the same report as Hemminki 1990a and Hemminki 1990b. This was a small pilot RCT of support by laywomen that was 'stopped for economic and other practical reasons'. 31 women were enrolled but 7 dropped out (all from the intervention group). Very little data were reported and it was not separated by treatment group and thus unusable |
| ISRCTN33728802 | Intervention was midwifery education for either support in labour or evidence‐based care |
| Lindow 1998 | Support was not continuous, and was quite brief in duration. 16 women in active labour were randomised to either 1 hour with a supportive companion or 1 hour without. The only outcome was maternal oxytocin level for 16 minutes post‐support or control period |
| Manning‐Orenstein 1998 | Not a randomised trial. Women chose to either have a doula or have Lamaze preparation for childbirth |
| Orbach‐Zinger 2012 | Intervention is the presence of a partner during epidural insertion, not during labour |
| Ran 2005 | Not an RCT. Translated personal communication from the author stated "I randomly sampling allocated the patient, did not use any random tool" |
| Riley 2012 | Published in abstract form ‐ authors contacted but unable to provide additional information |
| Scott 1999 | Not a trial. A review of selected studies of intrapartum support |
| Senanayake 2013 | Control group not randomised |
| Sosa 1980 | Strong evidence of selection bias. "A woman was removed from the study if labour was false or prolonged; if fetal distress necessitated an intervention such as oxytocin, caesarean delivery, or forceps"; or if the infant was asphyxiated or ill at birth, etc. "If a woman was removed, her group assignment was inserted at random into the pool of unused assignments. Women were enrolled in the study until there were 20 in the control group and 20 in the experimental group." The total study sample of 127 mothers includes 95 in the control group and 32 in the experimental group. Thus assignment was not random |
| Trueba 2000 | Direct contact with investigator revealed that randomisation was not used. On arrival at the hospital, women were asked if they wanted to have a doula. If they accepted, a doula was assigned to them. Also support was not continuous throughout active labour for most women, since admission to the labour ward (and assignment of a doula) did not usually occur until 8 cm |
| Tryon 1966 | Not an RCT. "After a random start, the matched groups were alternately assigned to experimental and control groups." Women who developed severe complications in labour (number not specified), such as fetal distress, were dropped from the study |
| U1111‐1175‐8408 | Intervention is an educational booklet for birth companions, not continuous support |
| Wan 2011 | The study compared 2 types of nursing care: intervention is continuous primary nursing care, compared to task‐centred primary nursing care |
| Zhang 1996b | Not a trial of continuous 1‐to‐1 support. On admission to the labour ward, women received instruction about normal labour, non‐pharmacological methods to ease pain, and how to push in second stage, from a team of physicians and nurses. Support was continuous, depending on the women's needs, but not 1‐to‐1 |
EFM: electronic fetal monitoring RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Aghdam 2015.
| Methods | Clinical randomised trial |
| Participants | 80 primigravida women referred to Alavi Hospital of Ardabil for delivery. |
| Interventions | Intervention group: 40 women were supported by a trained midwife in addition to routine care of delivery room. Control group: 40 women received routine obstetrics care in the delivery room without an attendant |
| Outcomes | Duration of active stage and second stage of labour |
| Notes | Information from abstract only ‐ insufficient details to permit classification. Persian translation needed |
Bakhshi 2015.
| Methods | Randomised clinical trial |
| Participants | 80 primigravida women |
| Interventions | Intervention group: supportive care Control group: routine care |
| Outcomes | Onset of lactogenesis |
| Notes | Abstract only ‐ insufficient details to permit classification. Persian translation needed |
Farahani 2005.
| Methods | Randomised controlled trial |
| Participants | 290 low‐risk pregnant women |
| Interventions | Intervention group: unclear intervention. Appears to be one‐to‐one support with non‐pharmacologic interventions to relief pain (such as breathing technique, relaxation, massage). Control group: unclear ‐ possibly no support |
| Outcomes | Mode of birth |
| Notes | Information from abstract only ‐ insufficient details to permit classification. Persian translation needed |
Huang 2003.
| Methods | RCT |
| Participants | 6758 low‐risk women in labour |
| Interventions | Intervention group: women accompanied by trained personnel who provided physiological and psychological support in the whole process of birth until 2 hours after birth. Control group: unclear. Intervention compared with "conventional therapies" |
| Outcomes | Incidence of postpartum haemorrhage, puerperal infection, neonatal morbidity, neonatal aspiration pneumonia, fetal distress, maternal satisfaction/anxiety |
| Notes | Communication sent to author regarding details of randomisation process, the nature of the intervention, and information to allow classification for analysis subgroups. Awaiting response |
IRCT2013111710297N3.
| Methods | Interventional trial |
| Participants | 100 women, singleton, term pregnancy, expecting a vaginal birth, with spontaneous onset of labour |
| Interventions | Intervention: routine care with supportive person with women through labour (family member or friend chosen by the women) Control: routine care |
| Outcomes | Duration of labour Cervical laceration Pain Satisfaction |
| Notes | Could be same study as Shahshahan 2014. Awaiting response from authors |
McGrath 1999.
| Methods | Randomised controlled trial to compare the effects of epidural analgesia and continuous doula support. 531 low risk primigravidas randomised |
| Participants | Low‐risk primigravidas |
| Interventions | Intervention: continuous doula support with narcotic and/or epidural analgesia given when necessary. Control: narcotic medication followed by epidural analgesia if necessary (the hospital's standard protocol for pain relief) |
| Outcomes | Epidural analgesia, narcotic, pitocin, maternal fever, forceps/vacuum delivery, caesarean section |
| Notes | Awaiting additional information |
NCT00664118.
| Methods | Insufficient details |
| Participants | 500 nulliparous women, aged 18 to 45 years, in spontaneous labour, requesting analgesia in the latent phase of first stage of labour |
| Interventions | Intervention group: "Doula combined analgesia". Control group: analgesia without doula |
| Outcomes | Mode of birth, maternal VAS, side effects, lower back pain at 3 months, use of oxytocin after analgesia, duration of analgesia, breastfeeding success at 6 weeks, Apgar scores at 1 and 5 minutes, cord gases, neonatal sepsis and antibiotics |
| Notes | Not enough information available to classify. Unable to find contact details of authors |
Pinheiro 1996.
| Methods | "Intervention study", 510 deliveries, analysis for 110 deliveries |
| Participants | Primigravida women |
| Interventions | Intervention 1: psychosocial support from female doula during labour starting on arrival to delivery room Intervention 2: psychosocial support from male doula during labour starting on arrival to delivery room Control: "without intervention" |
| Outcomes | Caesarean section, duration of labour, incidence of breastfeeding |
| Notes | Awaiting additional information |
Rahimiyan 2015.
| Methods | RCT. 2 treatment arms. Block randomisation used. 200 women randomised |
| Participants | All pregnant women with low‐risk pregnancies aged 18 to 35 years, gestational age of 42‐38 weeks, “with singleton fetuses who had been hospitalised for pregnancy and the doctor ‐ had a natural labor.”, cervix 3 to 4 cm dilated |
| Interventions | Intervention group: one‐to‐one support by research midwife throughout labour and delivery. Control group: not clear. “Control group, according to the usual training centres ‐ medical assistants and interns, women were jointly care. Careful person, the various intervals, been replaced, as well as samples often bed rest, restricted, and no association and non‐pharmacologic methods of labor was not used.” |
| Outcomes | "Progress of labor (the time of examination, the cervix changes in time to get home (sort of) and oxytocin)", "delivery outcomes (including type of delivery, duration of labour, the degree perineal laceration, rupture of the water bag, notes the main reasons for caesarean delivery)" |
| Notes | We are unclear on aspects of reporting in this trial. AC contacted authors 25/10/2016 for further clarification. Awaiting response |
Samieizadeh 2011.
| Methods | RCT |
| Participants | “210 primiparous mothers at the age range of 18‐35 years old who were at 37 weeks of gestational age or grater at onset of labor” |
| Interventions | Intervention group: women had a female companion experienced at birth to provide continuous physical and emotional support. Control group: women were alone in labour |
| Outcomes | Duration of labour, time of delivery, Apgar scores, breastfeeding intent and early breastfeeding initiation 1 hour after birth and demographic factors of women |
| Notes | Abstract only ‐ insufficient details to permit classification. Persian translation needed |
Sangestani 2013.
| Methods | RCT |
| Participants | 64 parturient women |
| Interventions | Intervention group: presence of Doula. Control: usual care without Doula |
| Outcomes | State of anxiety |
| Notes | Abstract and trial registration only ‐ insufficient details to permit classification. Persian translation needed |
Shahshahan 2014.
| Methods | RCT |
| Participants | Women aged 18 to 35 years, with 38 to 42 week gestation singleton pregnancy and intention to have vaginal birth |
| Interventions | Intervention 1: women received routine intervention with a support person. Intervention 2: women received routine intervention without a support person. Intervention 3: women did not receive routine intervention with a support person. Intervention 4: women did not receive routine intervention or support person |
| Outcomes | Length (min) of first and second stage of labour, instrumental delivery, the cervical laceration (yes/no), perineal tear (into 4 categories), pain before and after labour using the numeric rating scale, between 0 (no pain) and 10 (worst pain), satisfied with labour experience |
| Notes | Unclear of details of this trial. MB contacted authors 25/10/2017. Awaiting response |
VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
IRCT2015083123837N1.
| Trial name or title | Studying the impacts of the combination of effective methods compared with doula on reducing anxiety and pain of mothers in childbirth in the Jam Tohid Hospital in 2015 |
| Methods | RCT |
| Participants | Inclusion criteria: Iranian; age between 16 and 44; first pregnancy; at least 32 weeks of gestational age; lack of pregnancy complications; having a single, live fetus; spontaneous contractions; intact sac; no pelvic restraint; no history of hospitalisation due to complications of pregnancy; no history of diseases such as thyroid, and diseases related to the kidney, heart, liver, diabetes, and lack of psychiatric diseases. Exclusion criteria: fetal macrosomia; the studied pregnant women's unwillingness to continue their cooperation with the researcher and participation in the intervention; fetal anomaly detection during the study; onset of labour before or after term; fetal distress; premature rupture of membranes; the use of pain relievers; the occurrence of any complications during labour and delivery and the need for caesarean section. Target sample size: 150 |
| Interventions | Insufficient details |
| Outcomes | Anxiety |
| Starting date | 21/04/2015 |
| Contact information | ra_ravangard@yahoo.com; ravangard@sums.ac.ir |
| Notes | Study ongoing |
NCT01216098.
| Trial name or title | Impact of Doula support on childbirth outcomes for women undergoing a vaginal birth after cesarean (VBAC) |
| Methods | 2‐armed randomised controlled trial conducted at British Columbia Women's Hospital |
| Participants | Women who have had at least 1 prior caesarean birth, are eligible for VBAC, and plan to attempt a VBAC after counselling at the Best Birth Clinic. Singleton gestation, cephalic presentation, term gestation (37 to 42 weeks at time of delivery). Estimated enrolment: 534 |
| Interventions | Intervention group: women will receive doula support alongside standard care. Control group: no intervention ‐ women will receive standard care alone |
| Outcomes | Primary outcomes: use of epidural analgesia and cervical dilation at time of epidural administration. Secondary outcomes: use of nitrous oxide analgesia during labour, use of narcotic analgesia during labour (type and amount), number of visits to the assessment room before admission, mode of delivery (caesarean section, spontaneous vaginal, or forceps/vacuum), indication(s) for repeat caesarean (if applicable), length of time between admission and the start of active pushing, length of time between the start of active pushing and delivery, length of time between delivery and discharge |
| Starting date | October 2010 |
| Contact information | Patricia Janssen: pjanssen@interchange.ubc.ca |
| Notes | Study completion date February 2015. Contacted Patricia Janssen 07/02/2017 ‐ trial not yet published. Will assess eligibility following publication in next update |
NCT01947244.
| Trial name or title | Doula home visiting randomised trial |
| Methods | RCT |
| Participants | Estimated enrolment: 300 Pregnant women between 12 and 34 weeks' gestation, aged 14 to 24 years |
| Interventions | Intervention: Doula home visiting antenatally and postnatally, including support in labour Control: Case managed, low‐intensity care |
| Outcomes | Breastfeeding duration Long term infant and women outcomes |
| Starting date | September 2013 |
| Contact information | Sydney L Hans, University of Chicago |
| Notes |
NCT02550730.
| Trial name or title | Best Beginnings for Babies Birth Sister Program Evaluation |
| Methods | Parallel RCT |
| Participants | Primiparous women, 16‐24 weeks' gestation with singleton pregnancy |
| Interventions | Intervention: Doula care antenatally, and postnatally, and including continuous care in labour Control: Routine care |
| Outcomes | Caesarean section rate Preterm births Low birthweight babies Breastfeeding initiation and duration |
| Starting date | June 2015 |
| Contact information | Julie Mottl‐Santiago, Boston Medical Centre |
| Notes |
Differences between protocol and review
In this review update, we re‐organised the outcome list and added six new outcomes from the previous version of the review (Hodnett 2013). These include:
delayed skin‐to‐skin contact, as defined by trial authors;
delayed initiation of breastfeeding (more than one hour after birth, or as defined by trial authors);
time from birth to initiation of breastfeeding;
unlikely to recommend birth in that institution;
restricted mobility during labour, as defined by trial authors; and
unsatisfactory mother‐infant interactions.
We included these outcomes based on the rationale that women's experiences of care are an important component of quality of care, as noted in the World Health Organization (WHO) quality of care vision for pregnant women and newborns (Tunçalp 2015). We based these outcomes on available evidence, including the WHO Standards for improving quality of maternal and newborn care in health facilities (World Health Organization 2016), and other Cochrane Reviews that explored similar topics. In this update, we assessed all new trials for these outcomes, and returned to all previously included trials to identify and include any data contributing to these new outcomes.
We also modified one review outcome to "Exclusive or any breastfeeding at any time point as defined by trial authors" (from “Breastfeeding at one to two months postpartum”), to be more inclusive of trials that had postpartum follow‐up at a time point outside of the specified range.
We have added two new subgroup analyses on the model of support received (support solely during the intrapartum period, compared with extended support during antenatal and postpartum periods, in addition to continuous support during the intrapartum period), and country income level where trials were conducted (high‐income countries compared to low‐ and middle‐income countries).
This review update also includes an assessment of the quality of the evidence using the GRADE approach.
We also performed sensitivity analyses for any outcomes where reciprocal data had to be calculated in order to include data in an analysis (exclusive breastfeeding; negative rating of/negative feelings about the birth experience).
In 2017, we added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
Contributions of authors
Meghan Bohren led the current review update. Carol Sakala wrote the initial draft of the Discussion in a previous version of the review (Hodnett 2012). Anna Cuthbert conducted the GRADE assessments. All review authors participated in all aspects of the preparation of the protocol and in writing the text of the review. All authors participated in the update of the review.
Sources of support
Internal sources
University of Toronto, Canada.
University of the Witwatersrand, South Africa.
Fort Hare University, South Africa.
East London Hospital Complex, South Africa.
National Perinatal Epidemiology Unit, Oxford, UK.
Childbirth Connection (formerly Maternity Center Association), USA.
Warwick Clinical Trials Unit, University of Warwick, UK.
Department of Reproductive Health and Research including UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction, World Health Organization, Switzerland.
External sources
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Meghan Bohren: is conducting a related Cochrane qualitative evidence synthesis on labour companionship (Bohren 2016).
Justus Hofmeyr: is an author of one study included in this review and did not participate in decisions, assessment or data extraction related to this study (Hofmeyr 1991).
Carol Sakala: none known.
Rieko Fukuzawa: I am a nurse‐midwife by background. I have received lecture fees provided by Child Research Net (http://www.childresearch.net/overview.html), a web‐based non‐profit research institution in Tokyo. I have also been a guest researcher for Child Research Net since 2005 and have received 15000 yen (about $130) per article as an author, but other than that, no salary. As part of the Child Research Net, I run a ‘Doula Laboratory’(http://www.blog.crn.or.jp/lab/03/). I was a volunteer advisor for the Ippan Shadan Houjin Doula Kyoukai (incorporated association: https://www.doulajapan.com/) from April 2012 to July 2015. Currently I am conducting a government‐funded study on the development and evaluation of a non‐medical support program for women during childbirth in Japan (total 4,810,000 yen (about US$42,000) from April 2016 through March 2019. I received a grant from University of Tsukuba to cover the professional translation fee for Japanese translation of this review in February 2017 (268,964 yen (about US$2,400)).
Anna Cuthbert: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Akbarzadeh 2014 {published data only}
- Akbarzadeh M, Masoudi Z, Hadianfard MJ, Kasraeian M, Zare N. Comparison of the effects of maternal supportive care and acupressure (BL32 acupoint) on pregnant women's pain intensity and delivery outcome. Journal of Pregnancy 2014;2014:129208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarzadeh M, Masoudi Z, Zare N, Kasraeian M. Comparison of the effects of maternal supportive care and accupressure (at BL32 acupoint) on labor length and infant's Apgar score. Global Journal of Health Science 2015;8(2):236‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRCT2014021211706N6. Comparison the effect of supportive care for the mother and acupressure (point bl32) on the length of labor and neonate APGAR. http://en.search.irct.ir/view/17019 (first received 14 February 2014).
- Masoudi Z, Akbarzadeh M, Vaziri F, Zare N, Ramzi M. The effects of decreasing maternal anxiety on fetal oxygenation and nucleated red blood cells count in the cord blood. Iranian Journal of Pediatrics 2014;24(3):285‐92. [PMC free article] [PubMed] [Google Scholar]
Bréart ‐ Belgium 1992 {published data only}
- Bréart G, Garel M, Mlika‐Cabanne N. Evaluation of different policies of management of labour for primiparous women. Trial B: Results of the continuous professional support trial. In: Kaminski M editor(s). Evaluation in Pre‐, Peri‐, and Post‐Natal Care Delivery Systems. Paris, France: INSERM, 1992:57‐68. [Google Scholar]
- Bréart G, Mlika‐Cabane N, Kaminski M. The evaluation of different policies for the management of labour. Proceedings of 3rd European Health Services Research Meeting; 1991 Dec 13‐14; London, UK. 1991.
- Bréart G, Mlika‐Cabane N, Kaminski M, Alexander S, Herruzo‐Nalda A, Mandruzzato P, et al. Evaluation of different policies for the management of labour. Early Human Development 1992;29(1‐3):309‐12. [DOI] [PubMed] [Google Scholar]
- Bréart G, Mlika‐Cabane N, Thornton J, Trakas D, Alexander S, Mandruzzato P, et al. European trials on artificial rupture of membranes and professional support during labour. Journal of Perinatal Medicine 1992;20(Suppl 1):37. [Google Scholar]
Bréart ‐ France 1992 {published data only}
- Bréart G, Garel M, Mlika‐Cabanne N. Evaluation of different policies of management of labour for primiparous women. Trial B: Results of the continuous professional support trial. In: Kaminski M editor(s). Evaluation in Pre‐, Peri‐, and Post‐natal Care Delivery Systems. Paris, France: INSERM, 1992:57‐68. [Google Scholar]
- Bréart G, Mlika‐Cabane N, Kaminski M. The evaluation of different policies for the management of labour. Proceedings of 3rd European Health Services Research Meeting; 1991 Dec 13‐14; London, UK. 1991.
- Bréart G, Mlika‐Cabane N, Kaminski M, Alexander S, Herruzo‐Nalda A, Mandruzzato P, et al. Evaluation of different policies for the management of labour. Early Human Development 1992;29(1‐3):309‐12. [DOI] [PubMed] [Google Scholar]
- Bréart G, Mlika‐Cabane N, Thornton J, Trakas D, Alexander S, et al. European trials on artificial rupture of membranes and professional support during labour. Journal of Perinatal Medicine 1992;20(Suppl 1):37. [Google Scholar]
Bréart ‐ Greece 1992 {published data only}
- Bréart G, Garel M, Mlika‐Cabanne N. Evaluation of different policies of management of labour for primiparous women. Trial B: Results of the continuous professional support trial. In: Kaminski M editor(s). Evaluation in Pre‐, Peri‐, and Post‐Natal Care Delivery Systems. Paris, France: INSERM, 1992:57‐68. [Google Scholar]
- Bréart G, Mlika‐Cabane N, Kaminski M. The evaluation of different policies for the management of labour. Proceedings of 3rd European Health Services Research Meeting; 1991 Dec 13‐14; London, UK. 1991.
- Bréart G, Mlika‐Cabane N, Kaminski M, Alexander S, Herruzo‐Nalda A, Mandruzzato P, et al. Evaluation of different policies for the management of labour. Early Human Development 1992;29(1‐3):309‐12. [DOI] [PubMed] [Google Scholar]
- Bréart G, Mlika‐Cabane N, Thornton J, Trakas D, Alexander S, et al. European trials on artificial rupture of membranes and professional support during labour. Journal of Perinatal Medicine 1992;20(Suppl 1):37. [Google Scholar]
Bruggemann 2007 {published and unpublished data}
- Bruggemann OM, Parpinelli MA, Osis MJD, Cecatti JG, Neto ASC. Support to a woman by a companion of her choice during childbirth: a randomized controlled trial. Neonatal Intensive Care 2008;21(1):44‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggemann OM, Parpinelli MA, Osis MJD, Cecatti JG, Neto ASC. Support to woman by a companion of her choice during childbirth: a randomized controlled trial. Reproductive Health 2007;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2006 {published and unpublished data}
- Campbell D, Scott KD, Klaus MH, Falk M. Female relatives or friends trained as labor doulas: outcomes at 6 to 8 weeks postpartum. Birth 2007;34(3):220‐7. [DOI] [PubMed] [Google Scholar]
- Campbell DA, Lake MF, Falk M, Backstrand JR. A randomized control trial of continuous support in labor by a lay doula. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2006;35(4):456‐64. [DOI] [PubMed] [Google Scholar]
- NCT00127361. Evaluation of continuous support in labor [Study of having a female friend as labor support]. clinicaltrials.gov/ct2/show/record/NCT00127361 (first received 4 August 2005).
Cogan 1988 {published and unpublished data}
- Cogan R, Spinnato JA. Social support during premature labor: effects on labor and the newborn. Journal of Psychosomatic Obstetrics and Gynaecology 1988;8(3):209‐16. [Google Scholar]
Dickinson 2002 {published and unpublished data}
- Dickinson JE, Paech MJ, McDonald SJ, Evans SF. Maternal satisfaction with childbirth and intrapartum analgesia in nulliparous labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43(6):463‐8. [DOI] [PubMed] [Google Scholar]
- Dickinson JE, Paech MJ, McDonald SJ, Evans SF. The impact of intrapartum analgesia on labour and delivery outcomes in nulliparous women. Australian and New Zealand Journal of Obstetrics and Gynaecology 2002;42(1):59‐66. [DOI] [PubMed] [Google Scholar]
- Henderson JJ, Dickinson JE, Evans SF, McDonald SJ, Paech MJ. Impact of intrapartum epidural analgesia on breast‐feeding duration. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43(5):372‐7. [DOI] [PubMed] [Google Scholar]
Gagnon 1997 {published and unpublished data}
- Gagnon A, Waghorn K. One‐to‐one nurse labor support of nulliparous women stimulated with oxytocin. Journal of Obstetric, Gynecologic and Neonatal Nursing 1999;28(4):371‐6. [DOI] [PubMed] [Google Scholar]
- Gagnon A, Waghorn K, Covell C. A randomized trial of one‐to‐one nurse support of women in labor. Birth 1997;24(2):71‐7. [PubMed] [Google Scholar]
Hans 2013 {published data only}
- Edwards C, Thullen J, Korfmacher J, Lantos D, Henson G, Hans L. Breastfeeding and complementary food: randomized trial of community doula home visiting. Pediatrics 2013;132(Suppl 2):S160‐6. [DOI] [PubMed] [Google Scholar]
- Hans SL, Thullen M, Henson LG, Lee H, Edwards RC, Bernstein VJ. Promoting positive mother‐infant relationships: a randomized trial of community doula support for young mothers. Infant Mental Health Journal 2013;34(5):446‐57. [Google Scholar]
Hemminki 1990a {published data only}
- Hemminki E, Virta AL, Koponen P, Malin M, Kojo‐Austin H, Tuimala R. A trial on continuous human support during labor: feasibility, interventions and mothers' satisfaction. Journal of Psychosomatic Obstetrics and Gynaecology 1990;11(4):239‐50. [Google Scholar]
Hemminki 1990b {published data only}
- Hemminki E, Virta AL, Koponen P, Malin M, Kojo‐Austin H, Tuimala R. A trial on continuous human support during labor: feasibility, interventions and mothers' satisfaction. Journal of Psychosomatic Obstetrics and Gynaecology 1990;11(4):239‐50. [Google Scholar]
Hodnett 1989 {published and unpublished data}
- Hodnett ED, Osborn RW. A randomized trial of the effects of monitrice support during labor: mothers' views two to four weeks postpartum. Birth 1989;16(4):177‐83. [DOI] [PubMed] [Google Scholar]
- Hodnett ED, Osborn RW. Effects of continuous intrapartum professional support on childbirth outcomes. Research in Nursing and Health 1989;12(5):289‐97. [DOI] [PubMed] [Google Scholar]
Hodnett 2002 {published and unpublished data}
- Hodnett ED, Lowe NK, Hannah ME, Willan AR, Stevens B, Weston JA, et al. Effectiveness of nurses as providers of birth labor support in North American hospitals. A randomized controlled trial. JAMA 2002;288(11):1373‐81. [DOI] [PubMed] [Google Scholar]
- Muir HA, Hodnett ED, Hannah ME, Lowe NK, Willan AR, Stevens B, et al. The influence of continuous labor support on the choice of analgesia, ambulation and obstetric outcome. Anesthesiology 2002;96(Suppl 1):P44. [Google Scholar]
Hofmeyr 1991 {published and unpublished data}
- Chalmers B, Wolman WL, Hofmeyr GJ, Nikodem C. Companionship in labour and the mother‐infant relationship: preliminary report of a randomised trial. Proceedings of the 9th Conference on Priorities in Perinatal Care; 1990 March; Johannesburg, South Africa. 1990:139‐41.
- Chalmers B, Wolman WL, Hofmeyr GJ, Nikodem C. Companionship in labour: effect on the mother‐infant relationship. Proceedings of the International Conference on Primary Care Obstetrics and Perinatal Health; 1991; Utrecht, The Netherlands. 1991:50.
- Gulmezoglu AM, Chalmers BE, Nikodem VC, Wolman WL, Hofmeyr GJ. Companionship in labour: do the personality characteristics of labour supporters influence effectiveness?. Proceedings of the 11th Conference on Priorities in Perinatal Care in South Africa; 1992 March; Caledon, South Africa. 1992:115‐6.
- Hofmeyr GJ, Nikodem C, Gulmezoglu M, Wolman WL. Companionship to modify the clinical birth environment: long‐term effects on mother and child. Proceedings of the 11th Conference on Priorities in Perinatal Care in South Africa; 1992 March; Caledon, South Africa. 1992:113‐4.
- Hofmeyr GJ, Nikodem C, Gulmezoglu M, Wolman WL. Companionship to modify the clinical birth environment: long‐term effects on mother and child. Proceedings of the 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:34.
- Hofmeyr GJ, Nikodem VC, Chalmers BE, Kramer T. Clinically orientated care during labour reduces the chance of successful breastfeeding. Proceedings of the 10th Conference on Priorities in Perinatal Care in South Africa; 1991 March 12‐15; Eastern Transvaal, South Africa. 1991:107‐9.
- Hofmeyr GJ, Nikodem VC, Mahomed K, Gulmezoglu AM, Lawson M, Walt LA, et al. Companionship to modify the clinical birth environment: no measurable effect on stress hormone levels. Journal of Obstetrics and Gynaecology 1995;15(3):178‐81. [Google Scholar]
- Hofmeyr GJ, Nikodem VC, Wolman WL, Chalmers BE, Kramer T. Companionship to modify the clinical birth environment: effects on progress and perceptions of labour, and breastfeeding. British Journal of Obstetrics and Gynaecology 1991;98(8):756‐64. [DOI] [PubMed] [Google Scholar]
- Nikodem VC, Nolte AG, Wolman W, Gulmezoglu AM, Hofmeyr GJ. Companionship by a lay labour supporter to modify the clinical birth environment: long‐term effects on mother and child. Curationis 1998;21(1):8‐12. [DOI] [PubMed] [Google Scholar]
- Trotter C, Wolman WL, Hofmeyr J, Nikodem C, Turton R. The effect of social support during labour on postpartum depression. South African Journal of Psychology 1992;22(3):134‐9. [Google Scholar]
- Wolman WL, Chalmers B, Hofmeyr J, Nikodem VC. Postpartum depression and companionship in the clinical birth environment: a randomized, controlled study. American Journal of Obstetrics and Gynecology 1993;168(5):1388‐93. [DOI] [PubMed] [Google Scholar]
Isbir 2015 {published data only}
Kashanian 2010 {published data only}
- Kashanian M, Javadi F, Haghighi MM. Effect of continuous support during labor on duration of labor and rate of cesarean delivery. International Journal of Gynaecology and Obstetrics 2010;109(3):198‐200. [DOI] [PubMed] [Google Scholar]
- Kashanian M, Javadi F, Haghighi MM. Evaluation of how continuous support during labour affects the duration of labour and rate of caesarean delivery. Annals of the Academy of Medicine Singapore 2012;41(9 Suppl 1):S90. [Google Scholar]
Kennell 1991 {published and unpublished data}
- Kennell J, Klaus M, McGrath S, Robertson S, Hinkley C. Continuous emotional support during labor in a US hospital. JAMA 1991;265(17):2197‐201. [PubMed] [Google Scholar]
- Kennell J, Klaus M, McGrath S, Robertson S, Hinkley C. Medical intervention: the effect of social support during labour. Proceedings of Annual Meeting of the American Pediatric Society/Society for Pediatric Research; 1988 May; USA. 1988.
- Kennell J, McGrath S, Klaus M, Robertson S, Hinkley C. Labor support: what's good for the mother is good for the baby. Pediatric Research 1989;25:15A. [Google Scholar]
- Martin S, Landry S, Stellman L, Kennell J, McGrath S. The effect of doula support during labor on mother‐infant interaction at 2 months. Infant Behaviour and Development 1998;21(Suppl 1):556. [Google Scholar]
Klaus 1986 {published and unpublished data}
- Klaus MH, Kennell JH, Robertson SS, Sosa R. Effects of social support during parturition on maternal and infant morbidity. British Medical Journal (Clinical Research Ed.) 1986;293(6547):585‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 1998 {published and unpublished data}
- Campero L, García C, Díaz C, Ortiz O, Reynoso S, Langer A. "Alone, I wouldn't have known what to do": a qualitative study on social support during labor and delivery in Mexico. Social Science & Medicine 1998;47(3):395‐403. [DOI] [PubMed] [Google Scholar]
- Langer A, Campero L, García C, Reynoso S. Effects of psychosocial support during labour and childbirth on breastfeeding, medical interventions, and mothers' wellbeing in a Mexican public hospital: a randomised clinical trial. British Journal of Obstetrics and Gynaecology 1998;105(10):1056‐63. [DOI] [PubMed] [Google Scholar]
Madi 1999 {published and unpublished data}
- Madi BC, Sandall J, Bennett R, MacLeod C. Effects of female relative support in labor: a randomized controlled trial. Birth 1999;26(1):4‐8. [DOI] [PubMed] [Google Scholar]
McGrath 2008 {published data only}
- McGrath SK, Kennell JH. A randomized controlled trial of continuous labor support for middle‐class couples: effect on cesarean delivery rates. Birth 2008;35(2):92‐7. [DOI] [PubMed] [Google Scholar]
Morhason‐Bello 2009 {published data only}
- ACTRN12609000994280. Assessment of the effect of social support in labour: a randomised controlled trial [Assessment of the effect of psycho‐social support in labour on caesarean section rate: a randomised controlled trial]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000994280 (first received 17 November 2009).
- Morhason‐Bello IO, Adedokun BO, Ojengbede OA. Social support during childbirth as a catalyst for early breastfeeding initiation for first‐time Nigerian mothers. International Breastfeeding Journal 2009;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morhason‐Bello IO, Adedokun BO, Ojengbede OA, Olayemi O, Oladokun A, Fabamwo AO. Assessment of the effect of psychosocial support during childbirth in Ibadan, south‐west Nigeria: a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2009;49(2):145‐50. [DOI] [PubMed] [Google Scholar]
- Ojengbede O, Morhason‐Bello I, Adedokun B, Becker S, Oni G, Tsui A. Psycho‐social support in labour, as a catalyst for contraceptive uptake in Nigeria: preliminary analysis of a randomised controlled trial. International Journal of Gynaecology and Obstetrics 2009;107(Suppl 2):S623‐4. [Google Scholar]
Safarzadeh 2012 {published data only}
- IRCT201111156242N2. The effect of doula supporting on the severity of pain and duration of active phase of labor in primiparous women. en.search.irct.ir/view/7818 (first received 13 March 2012).
- Safarzadeh A, Beigi M, Salehian T, Khojasteh F, Burayri T, Dokht Navabirigi S, et al. Effect of doula support on labour pain and outcomes in primiparous women in Zahedan, southeastern Iran: a randomized controlled trial. Journal of Pain & Relief 2012;1:5. [DOI: 10.4172/2167-0846.1000112] [DOI] [Google Scholar]
- Safarzadeh A, Beigi M, Salehian T, Khojasteh F, Rigi SN, Buryri T, et al. Effect of doula support on labour pain and outcomes in primiparous women in Zahedan, southeastern Iran: a randomized controlled trial. Evidence Live Evidence Live; 2013 March 25‐26; Oxford, UK. Oxford UK, 2013.
Thomassen 2003 {published data only}
- Thomassen P, Lundwall M, Wiger E, Wollin L, Uvnäs‐Moberg K. Doula ‐ a new concept in obstetrics [Doula ‐ ett nytt begrepp inom forlossningsvarden]. Lakartidningen 2003;100(51‐2):4268‐71. [PubMed] [Google Scholar]
Torres 1999 {published data only}
- Kopplin E, Torres‐Pereyra J, Peña V, Salinas R. Impact of psychosocial supports during childbirth: the decrease of caesarean and bonuses of the process. Pediatric Research 2000;47:834. [Google Scholar]
- Torres J, Kopplin E, Peña V, Klaus M, Salinas R, Herrera M. Impact of emotional support during labour in the decrease of caesarean sections and satisfaction with the process [Impacto del apoyo emocional durante el parto en la disminucion de cesareas y gratificacion del proceso]. Revista Chilena de Obstetricia y Ginecologia 1999;64(5):405‐12. [Google Scholar]
Yuenyong 2012 {published and unpublished data}
- Yuenyong S, O'Brien B, Jirapeet V. Effects of labor support from close female relative on labor and maternal satisfaction in a Thai setting. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2012;41(1):45‐56. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bender 1968 {published data only}
- Bender B. A test of the effects of nursing support on mothers in labor. ANA Regional Clinical Conferences 1967 Philadelphia/Kansas City. New York: Appleton‐Century‐Crofts, 1968:171‐9. [PubMed] [Google Scholar]
Bochain 2000 {published data only}
- Bochain SS. Induced Labor Intervention for Self‐efficacy: a Nursing Intervention for Women Prone to an Unpredictable Labor Pattern [PhD thesis]. Kingston, Rhode Island: University of Rhode Island, 2000. [Google Scholar]
Brown 2007 {published data only}
- Brown H, Hofmeyr GJ, Nikodem VC, Smith H, Garner P. Promoting childbirth companions in South Africa: a randomised pilot study. BMC Medicine 2007;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalal 2006 {published data only}
- Dalal R, Rathnakumar S. Birth companion and mother ‐ a preliminary report. 49th All India Congress of Obstetrics and Gynaecology; 2006 Jan 6‐9; Kerala State, India. 2006:140.
Dong 2009 {published data only}
- Dong X, Qi H, Hu L, Luo X, Zhu Y, Cai J. The effects of air sac combined with nitrous oxide and Doula accompanying delivery in vaginal delivery. International Journal of Gynaecology and Obstetrics 2009;107:S158‐9. [Google Scholar]
Gordon 1999 {published data only}
- Gordon NP, Walton D, McAdam E, Derman J, Gallitero G, Garrett L. Effects of providing hospital‐based doulas in health maintenance organization hospitals. Obstetrics and Gynecology 1999;93(3):422‐6. [DOI] [PubMed] [Google Scholar]
Hemminki 1990c {published data only}
- Hemminki E, Virta AL, Koponen P, Malin M, Kojo‐Austin H, Tuimala R. A trial on continuous human support during labor: feasibility, interventions and mothers' satisfaction. Journal of Psychosomatic Obstetrics and Gynaecology 1990;11(4):239‐50. [Google Scholar]
ISRCTN33728802 {published data only}
- ISRCTN33728802. Labour support and evidence based medicine: a randomised controlled trial of implementation. isrctn.com/ISRCTN33728802 (first received 5 July 2006).
Lindow 1998 {published data only}
- Lindow SW, Hendricks MW, Thompson JW, Spuy ZM. The effect of emotional support on maternal oxytocin levels in labouring women. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1998;79(2):127‐9. [DOI] [PubMed] [Google Scholar]
Manning‐Orenstein 1998 {published data only}
- Manning‐Orenstein G. A birth intervention: the therapeutic effects of doula support versus Lamaze preparation on first‐time mothers' working models of caregiving. Alternative Therapies in Health and Medicine 1998;4(4):73‐81. [PubMed] [Google Scholar]
Orbach‐Zinger 2012 {published data only}
- Orbach‐Zinger S, Ginosar Y, Sverdlik J, Treitel C, Mackersey K, Bardin R, et al. Partner's presence during initiation of epidural labor analgesia does not decrease maternal stress: a prospective randomized controlled trial. Anesthesia and Analgesia 2012;114(3):654‐60. [DOI] [PubMed] [Google Scholar]
Ran 2005 {published data only}
- Ran KQ, He AM, Han QR, Tang ZL, Lei FH. Comfortable nursing and the mental state in parturient women. Chinese Journal of Clinical Rehabilitation 2005;9(32):62‐3. [Google Scholar]
Riley 2012 {published data only}
- Riley L, Hutchings Y, Lieberman E. The effect of doula support on epidural use among nulliparous women in tertiary care centers. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S70‐1. [Google Scholar]
Scott 1999 {published data only}
- Scott KD, Klaus PH, Klaus MH. The obstetrical and postpartum benefits of continuous support during childbirth. Journal of Women's Health & Gender‐Based Medicine 1999;8(10):1257‐64. [DOI] [PubMed] [Google Scholar]
Senanayake 2013 {published data only}
- Senanayake HM, Somawardana UABP, Samarasinghe M. Effect of a female labour companion and of educating her regarding support during labour on perinatal and labour outcomes. Sri Lanka Journal of Obstetrics and Gynaecology 2013;35(4):112‐5. [Google Scholar]
Sosa 1980 {published data only}
- Sosa R, Kennell J, Klaus M, Urrutia J. The effect of a supportive woman on mothering behavior and the duration and complications of labor. Pediatric Research 1979;13:338. [Google Scholar]
- Sosa R, Kennell JH, Klaus MH, Robertson S, Urrutia J. The effect of a supportive companion on perinatal problems, length of labor, and mother‐infant interaction. New England Journal of Medicine 1980;303:597‐600. [DOI] [PubMed] [Google Scholar]
Trueba 2000 {published and unpublished data}
- Trueba G, Contreras C, Velazco MT, Lara EG, Martinez HB. Alternative strategy to decrease cesarean section: support by doulas during labor. Journal of Perinatal Education 2000;9(2):8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tryon 1966 {published data only}
- Tryon PA. Use of comfort measures as support during labor. Nursing Research 1966;15(2):109‐18. [PubMed] [Google Scholar]
U1111‐1175‐8408 {published data only}
- U1111‐1175‐8408. RBR‐776d9s Effects of an educational manual on the support provided by companion during delivery [Efeitos de um manual educativo no apoio prestado por acompanhantes durante o parto]. ensaiosclinicos.gov.br/rg/RBR‐776d9s (first received 26 October 2015).
Wan 2011 {published data only (unpublished sought but not used)}
- Wan H, Hu S, Thobaben M, Hou Y, Yin T. Continuous primary nursing care increases satisfaction with nursing care and reduces postpartum problems for hospitalized pregnant women. Contemporary Nurse 2011;37(2):149‐59. [DOI] [PubMed] [Google Scholar]
Zhang 1996b {published data only}
- Zhang CL, Yu ZJ, Feng AH. Study of psychological nursing to ease pain during labour. Chinese Journal of Nursing 1996;31(6):311‐3. [PubMed] [Google Scholar]
References to studies awaiting assessment
Aghdam 2015 {published data only}
- Aghdam SK, Kazemzadeh F, Nikjoo R. The effect of the doula support during labor on delivery length in primigravida women. Iranian Journal of Obstetrics, Gynecology and Infertility 2015;18(150):8‐13. [Google Scholar]
Bakhshi 2015 {published data only}
- Bakhshi M, Kordi M, Esmaeeli H. The effect of continuous support during labor on the onset of lactogenesis stage II in primiparas. Journal of Mazandaran University of Medical Sciences 2015;25(130):153‐8. [Google Scholar]
Farahani 2005 {published data only}
- Farahani SHM, Malekzadegan A, Mohammadi R, Hosseini F. Effect of the one to one midwifery care during labor on modes of delivery. Iran Journal of Nursing 2005;18(43):71‐82. [Google Scholar]
Huang 2003 {published data only}
- Huang XH, Xiang XY, Shen RG, Shang Q, Zhu LP, Qian X, et al. Study on intrapartum service model during normal labor. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics and Gynecology] 2003;38(7):385‐7. [PubMed] [Google Scholar]
IRCT2013111710297N3 {published data only}
- IRCT2013111710297N3. Effect of the presence of support person and routine intervention for women during childbirth in Isfahan, Iran: A randomized controlled trial. en.search.irct.ir/view/15847 (first received 25 November 2013). [DOI] [PMC free article] [PubMed]
McGrath 1999 {published data only}
- McGrath S, Kennell J, Suresh M, Moise K, Hinkley C. Doula support vs epidural analgesia: impact on cesarean rates. Pediatric Research 1999; Vol. 45, issue 4:16A.
NCT00664118 {published data only}
- NCT00664118. Doula combined latent phrase epidural analgesia in primiparous women (DCLEAP). clinicaltrials.gov/ct2/show/NCT00664118 (first received 18 April 2008).
Pinheiro 1996 {published data only}
- Pinheiro RT, Sousa PLR, Horta B, Souza RM, Sousa MGR, Sousa FR. Male and female young doulas ‐ effective on labour? [abstract]. Xth World Congress of Psychiatry; 1996 August 23‐28; Madrid, Spain. 1996.
Rahimiyan 2015 {published data only}
- Rahimiyan MN, Rahnavard T, Lari MZ. Effect of the one to one midwifery care during labor on modes of delivery and duration of labor and increase satisfaction with childbirth. Biosciences Biotechnology Research Asia 2015; Vol. 12.
Samieizadeh 2011 {published data only}
- Samieizadeh T, Sereshti M, Dashipur A R, Mohammadinia N, Arzani A. The effect of supportive companionship on length of labor and desire to breastfeed in primiparous women. Journal of Urmia Nursing and Midwifery Faculty 2011;9(4):1‐9. [Google Scholar]
Sangestani 2013 {published data only}
- IRCT201112106827N2. The influence of doula on the parturient anxiety in delivery ward, 2011. en.search.irct.ir/view/8079 (first received 17 January 2012).
- Sangestani G, Khatiban M, Pourolajal J, Oshvandi K. Influence of doula on the primiparous parturients' anxiety in the delivery ward. Hayat 2013;19(4):48‐60. [Google Scholar]
Shahshahan 2014 {published data only}
- Shahshahan Z, Mehrabian F, Mashoori S. Effect of the presence of support person and routine intervention for women during childbirth in Isfahan, Iran: A randomized controlled trial. Advanced Biomedical Research 2014;3:155, 1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
IRCT2015083123837N1 {published data only}
- IRCT2015083123837N1. Studying the impacts of the combination of effective methods compared with doula on reducing anxiety and pain of mothers in childbirth in the Jam Tohid Hospital in 2015. en.search.irct.ir/view/25405 (first received 26 October 2015).
NCT01216098 {published data only}
- NCT01216098. Impact of doula support on intrapartum outcomes for women undergoing a vaginal birth after cesarean (vbac). clinicaltrials.gov/ct2/show/NCT01216098 (first received 5 October 2010).
NCT01947244 {published data only}
- NCT01947244. Illinois maternal, infant, and early childhood home visiting (miechv): doula randomized controlled trial. clinicaltrials.gov/show/NCT01947244 (first received 13 September 2013).
NCT02550730 {published data only}
- NCT02550730. Birth sisters best beginnings evaluation. clinicaltrials.gov/ct2/show/record/NCT02550730 (first received 23 June 2015).
Additional references
ACOG 2016
- American College of Obstetricians and Gynecologists and Society for Maternal‐Fetal Medicine. Safe prevention of the primary cesarean delivery. www.acog.org/Resources‐And‐Publications/Obstetric‐Care‐Consensus‐Series/Safe‐Prevention‐of‐the‐Primary‐Cesarean‐Delivery (accessed 20 June 2017).
ACOG 2017
- American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Approaches to limit intervention during labor and birth; Committee Opinion Number 687. www.acog.org/Resources‐And‐Publications/Committee‐Opinions/Committee‐on‐Obstetric‐Practice/Approaches‐to‐Limit‐Intervention‐During‐Labor‐and‐Birth (accessed 20 June 2017).
Anim‐Somuah 2011
- Anim‐Somuah M, Smyth RMD, Jones L. Epidural versus non‐epidural or no analgesia in labour. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD000331.pub3] [DOI] [PubMed] [Google Scholar]
Avan 2010
- Avan B, Richter LM, Ramchandani PG, Norris SA, Stein A. Maternal postnatal depression and children's growth and behaviour during the early years of life: exploring the interaction between physical and mental health. Archives of Disease in Childhood 2010;95(9):690‐5. [DOI] [PubMed] [Google Scholar]
AWHONN 2002
- Association of Women's Health, Obstetric, Neonatal Nurses. Professional nursing support of laboring women. www.awhonn.org (accessed 2002).
Bohren 2015
- Bohren MA, Vogel JP, Hunter EC, Lutsiv O, Makh SK, Souza JP, et al. The mistreatment of women during childbirth in health facilities globally: a mixed‐methods systematic review. PLOS Medicine 2015;12(6):1‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohren 2016
- Bohren MA, Munthe‐Kaas H, Berger BO, Allanson EE, Tuncalp O. Perceptions and experiences of labour companionship: a qualitative evidence synthesis. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD012449] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buckley 2015
- Buckley SJ. Hormonal Physiology of Childbearing: Evidence and Implications for Women, Babies, and Maternity Care. www.nationalpartnership.org/research‐library/maternal‐health/hormonal‐physiology‐of‐childbearing.pdf (accessed prior to 17 June 2017).
Caton 2002
- Caton D, Corry MP, Frigoletto FD, Hopkins DP, Lieberman E, Mayberry L, et al. The nature and management of labor pain: executive summary. American Journal of Obstetrics and Gynecology 2002;186(5):S1‐15. [DOI] [PubMed] [Google Scholar]
Declercq 2013
- Declercq ER, Sakala C, Corry MP, Applebaum S, Herrlich A. Listening to Mothers III: Pregnancy and Birth. Report of the Third National U.S. Survey of Women’s Childbearing Experiences. transform.childbirthconnection.org/wp‐content/uploads/2013/06/LTM‐III_Pregnancy‐and‐Birth.pdf (accessed prior to 17 June 2017).
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodnett 1997
- Hodnett E. Are nurses effective providers of labor support? Should they be? Can they be?. Birth 1997;24(2):78‐80. [PubMed] [Google Scholar]
Hodnett 2002a
- Hodnett ED. Pain and women's satisfaction with the experience of childbirth: a systematic review. American Journal of Obstetrics and Gynecology 2002;186(5):S160‐72. [DOI] [PubMed] [Google Scholar]
Kabakian‐Khasholian 2015
- Kabakian‐Khasholian T, El‐Nemer A, Bashour H. Perceptions about labour companionship at public teaching hospitals in three Arab countries. International Journal of Gynaecol and Obstetrics 2015;129(3):223‐6. [DOI] [PubMed] [Google Scholar]
Klaus 2002
- Klaus MH, Kennell JH, Klaus PH. The doula book: how a trained labor companion can help you have a shorter, easier and healthier birth. 2nd Edition. Cambridge, MA: Perseus Books, 2002. [Google Scholar]
Lederman 1978
- Lederman RP, Lederman E, Work BA, Jr, McCann DS. The relationship of maternal anxiety, plasma catecholamines, and plasma cortisol to progress in labor. American Journal of Obstetrics and Gynecology 1978;132(5):495‐500. [DOI] [PubMed] [Google Scholar]
Lederman 1981
- Lederman E, Lederman RP, Work BA Jr, McCann DS. Maternal psychological and physiologic correlates of fetal‐newborn health status. American Journal of Obstetrics and Gynecology 1981;139(8):956‐8. [DOI] [PubMed] [Google Scholar]
Lee [SOGC] 2016
- Lee L, Dy J, Azzam H [Society of Obstetricians and Gynaecologists of Canada Clinical Practice Guideline No. 336]. Management of spontaneous labour at term in healthy women. JOGC: Journal of Obstetrics and Gynaecology Canada 2016;38(9):843‐65. [DOI] [PubMed] [Google Scholar]
MIDIRS 2008
- MIDIRS. Support in labour: informed choice for women leaflet. Midwives Information and Resource Service. Bristol, England, 2008.
NICE Intrapartum Care 2007
- National Collaborating Centre for Women’s and Children’s Health. Section 4.3. Support in labour. Intrapartum Care: Care of Healthy Women and Their Babies During Childbirth. London, UK: RCOG Press, September 2007:73‐5. [PubMed] [Google Scholar]
Pascali‐Bonaro 2010
- Pascali‐Bonaro D. Global labor support trends. Personal communication 5 June 2010.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryden 1978
- Ryden MB. An adult version of the Coopersmith Self‐Esteem Inventory: Test‐retest reliability and social desirability. Psychological Reports 1978;43(3):1189‐90. [Google Scholar]
Steel 2015
- Steel A, Frawley J, Adams J, Diezel H. Trained or professional doulas in the support and care of pregnant and birthing women: a critical integrative review. Health & Social Care in the Community 2015;23(3):225‐41. [DOI] [PubMed] [Google Scholar]
Tunçalp 2015
- Tunçalp Ö, Were WM, MacLennan C, Oladapo OT, Gülmezoglu AM, Bahl R, et al. Quality of care for pregnant women and newborns – the WHO vision. British Journal of Obstetrics and Gynaecology 2015;122(8):1045‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
World Bank 2017
- World Bank. World Bank Country and Lending Groups: Historical classification by income. datahelpdesk.worldbank.org/knowledgebase/articles/906519‐world‐bank‐country‐and‐lending‐groups (accessed 13 March 2017).
World Health Organization 2015
- World Health Organization. WHO recommendations for augmentation of labour. http://apps.who.int/iris/bitstream/10665/112825/1/9789241507363_eng.pdf 2014. [PubMed]
World Health Organization 2016
- World Health Organization. Standards for improving quality of maternal and newborn care in health facilities. www.who.int/maternal_child_adolescent/documents/improving‐maternal‐newborn‐care‐quality/en/ (accessed prior to 17 June 2017).
World Health Organization 2016a
- WHO Reproductive Health Library. Labour companionship: every woman's choice. extranet.who.int/rhl/resources/videos/labour‐companionship‐every‐womans‐choice (accessed prior to 17 June 2017).
Zhang 1996a
- Zhang J, Bernasko JW, Leybovich E, Fahs M, Hatch MC. Continuous labor support from labor attendant for primiparous women: a meta‐analysis. Obstetrics and Gynecology 1996;88(4 pt 2):739‐44. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hodnett 2003
- Hodnett ED, Gates S, Hofmeyr G J, Sakala. Continuous support for women during childbirth. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003766] [DOI] [PubMed] [Google Scholar]
Hodnett 2007
- Hodnett ED, Gates S, Hofmeyr GJ, Sakala C. Continuous support for women during childbirthCochrane. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003766.pub2] [DOI] [PubMed] [Google Scholar]
Hodnett 2011
- Hodnett ED, Gates S, Hofmeyr GJ, Sakala C, Weston J. Continuous support for women during childbirth. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD003766.pub3] [DOI] [PubMed] [Google Scholar]
Hodnett 2012
- Hodnett ED, Gates S, Hofmeyr GJ, Sakala C. Continuous support for women during childbirth. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD003766.pub4] [DOI] [PubMed] [Google Scholar]
Hodnett 2013
- Hodnett ED, Gates S, Hofmeyr GJ, Sakala C. Continuous support for women during childbirth. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003766.pub5] [DOI] [PubMed] [Google Scholar]


