Abstract
Our case report involves a 28-year-old man who was diagnosed with left elbow bursitis. After thorough macroscopic and microscopic examinations and serological and molecular tests, it was found that the inflammation had been caused by a Dirofilaria repens infection. This case report is the world’s first documented description.
Keywords: bursitis, diagnosis, Dirofilaria repens, dirofilariasis, elbow region
Dirofilariasis is a filarial nematode infection occurring in both humans and animals [1, 2]. The main hosts of these parasites are carnivorous animals, such as dogs, cats, and nondomesticated predators, whereas mosquitoes, such as Culex, Aedes, Anopheles, are vectors [3, 4]. Humans can become infected by the penetration of the larvae through the skin when a mosquito is collecting blood. Dirofilariasis in humans is widespread almost all over the world, including countries with a tropical, subtropical, or temperate climate, and is mainly caused by Dirofilaria repens and Dirofilaria immitis [3–5]. Filariasis caused by Dirofilaria repens is more likely to occur in Europe, Africa, and Asia, whereas filariasis caused by D immitis is more likely to occur in the Americas, Australia, and Japan [3, 6, 7]. Dirofilaria repens is a dioecious nematode; its adults are approximately 15-cm long and 0.3- to 0.6-mm thick. This nematode causes so-called subcutaneous dirofilariasis, which manifests itself, eg, in skin lesions in the form of nodules and edema, which can be accompanied by inflammation (adult organisms live inside the parasitic nodules). These nematodes can be found in various parts of the human body, including the chest, abdominal walls, on the scrotum, as well as under the conjunctiva and in the vitreous body of the eye [7–9]. The occurrence of microfilaremia in humans has also been described [10]. Dirofilaria immitis is larger than Dirofilaria repens, causes so-called pulmonary dirofilariasis, and causes human infection less frequent than Dirofilaria repens [3, 4, 11]. According to our knowledge, the presented case is the world’s first documented description of elbow bursitis caused by Dirofilaria repens in humans.
CASE REPORT
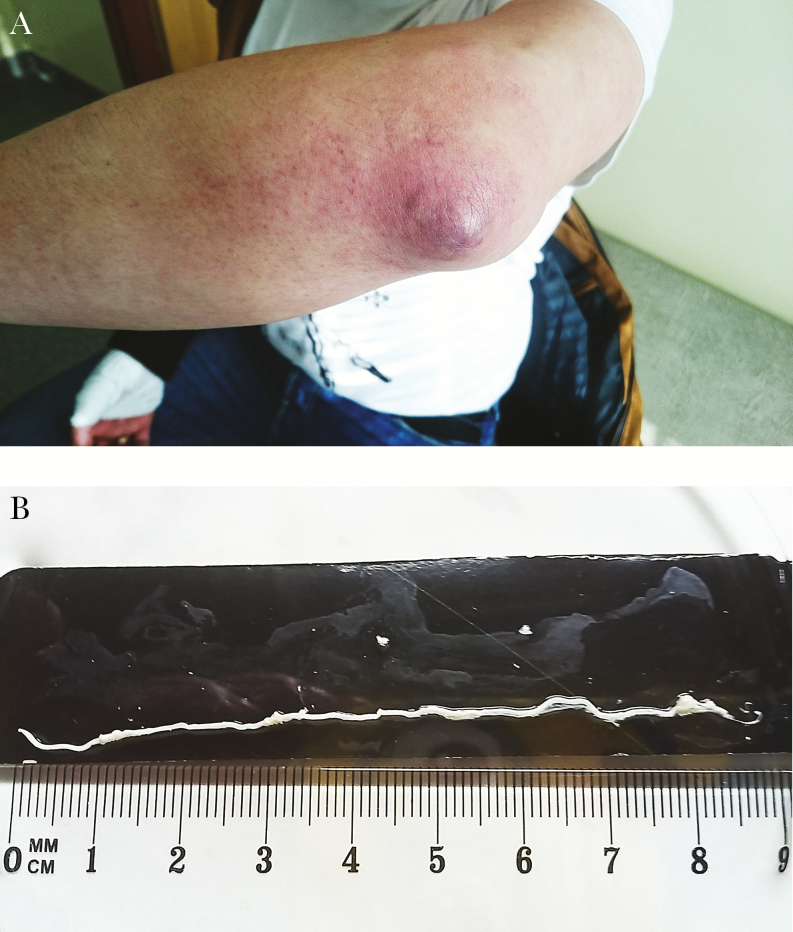
A 28-year-old man from the Lublin Voivodeship (Poland) came to the Orthopaedic Clinic with pain and swelling of the left elbow. The patient was diagnosed with left elbow bursitis (Figure 1A). The orthopedist performed a puncture and administered a steroid—diprophos (2 mL, 7 mg/mL in a single dose)—to the patient. After 1 week, the patient came to the Orthopaedic Clinic again with even more severe symptoms. The patient underwent another puncture of the left cubital bursa, during which the doctor aspirated the nematode. The patient was administered pyrantelum (250 mg once a day for 3 days), metronidazole (500 mg twice a day for 7 days), and zinnatt (500 mg twice a day for 7 days) and was referred to the Infectious Diseases Outpatient Clinic. In the medical history, the patient reported that he only traveled to the Netherlands (1 year before the onset of symptoms). One week after the removal of the nematode, the ailment ceased and the inflammation subsided. The nematode aspirated during the puncture was subjected to macroscopic, microscopic (direct preparation and hematoxylin, and eosin stain [H&E]), and immunohistochemical analysis. The genetic material (deoxyribonucleic acid [DNA]) was isolated from the nematode for the purpose of molecular analysis. In addition, blood was taken from the patient to carry out serological tests for antibodies. On the basis of these tests, elbow bursitis caused by Dirofilaria repens infection was diagnosed.
Figure 1.
Macroscopic image. (A) Left elbow bursitis in the examined patient; (B) Dirofilaria repens aspirated during the cubital bursa puncture (initially the object measured 12 cm, but 4 cm were cut off for microscopic examination).
The nematode obtained during the orthopedic procedure was subjected to macroscopic and microscopic analysis using a stereoscopic and optical microscope (Olympus BX41). During the macroscopic and microscopic observations, it was found that the tested nematode was 12-cm long and characteristic longitudinal ribs were visible on the surface of the cuticle (Figure 1B and Figure 2A–C).
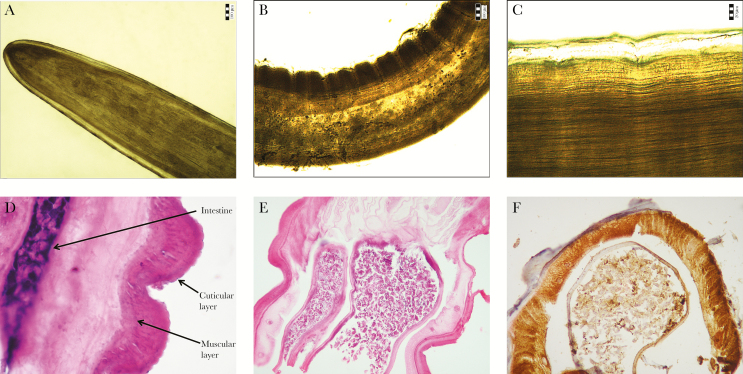
Figure 2.
Dirofilaria repens aspirated during the cubital bursa puncture—microscopic image. (A) Anterior part of the body; (B and C) characteristic longitudinal ribs are visible on the surface of the cuticle; (D) longitudinal section of the parasite with visible cuticular layer, muscular layer, and intestine (hematoxylin and eosin stain [H&E], ×600); (E) longitudinal section of the parasite with visible uterus (H&E, ×200); (F) positive immunoreactivity with antibody S-100 (S-100, ×200).
The adult nematode was fixed in 10% buffered formalin (pH 7.4) and processed routinely through dehydration with graded alcohol, acetone, and xylene and subsequently embedded in paraffin blocks. Four-micron-thick sections were stained with H&E. During the analysis of the preparations stained with H&E, a cuticular layer, a muscular layer, an intestine, and a double uterus were observed (Figure 2D and E). Sections for immunohistochemistry were placed on slides coated with organosilane (Dako Silanized slides, Code No. S3003) to prevent the specimen’s floating during the immunohistochemical procedure. These sections were immunohistochemically stained using the EnVisionTM Detection System (Dako Real EnVision Detection System, Peroxidase/DAB+, Rabbit/Mouse, Cat. No. K5007), with the following antibodies: S-100 protein (DAKO; code Z0311; dilution 1:400), Cytokeratin AE1/AE3 (DAKO; code M3515; dilution 1:50), and Vimentin (DAKO; code M0725, dilution 1:100). The immunohistochemical staining analysis showed that the reaction with antibodies AE1/AE3 and Vimentin was negative, which means the lack of a cross-reaction between antibodies used in human pathology and the antigens of the parasite. The reaction with S-100 was positive (Figure 2F).
Universal primers designed for one-step polymerase chain reaction (PCR) facilitating the detection and differentiation of filarial parasites were used: ITS1-F 5’-GGTGAACCTGCGGAAGGATC-3’ and ITS1-R 5’-CTCAATGCGTCTGCAAATCGC-3’ [12]. The PCR was carried out in a 50-µL reaction mixture containing 25 µL of PCR Mix Plus HGC (a ready-to-use PCR mixture containing recombinant Taq polymerase, a PCR buffer, magnesium chloride, nucleotides, stabilizers, and a gel loading buffer [A&A Biotechnology, Gdynia, Poland]), 2 µL of each primer (concentration 10 µM), 3 µL of DNA template, and 18 µL of distilled water. The time-temperature profile was similar to the original profile described by Nuchprayoon et al [12]: initial denaturation at 94oC for 5 minutes, followed by 35 cycles of denaturation at 94oC for 30 seconds, annealing at 58oC for 45 seconds, and extension at 72oC for 45 seconds. The final extension step was at 72oC for 10 minutes. The PCR product was sequenced using amplification primers and the standard procedure. The obtained sequence was analyzed using GeneStudio Pro Software (GeneStudio, Inc., Suwanee, GA). The results of the PCR molecular tests and the sequence reading showed that the parasite isolated from the patient was Dirofilaria repens. The sequence was deposited in GenBank under accession number MH059516.
The serological test with the patient’s serum was performed using Acanthocheilonema Viteae Enzyme immunoassay for the diagnosis of human filariasis (Bordier Affinity Products SA, Crissier, Switzerland). The test is devised for diagnosing filarial infections of lymphatique and African filariasis. The serological test result of the patient’s serum was positive (absorbance, 0.95; cutoff, 0.830).
DISCUSSION
Invasions caused by Dirofilaria repens have been observed worldwide, in countries with tropical, subtropical, and temperate climates. Most cases in Europe are diagnosed in Mediterranean countries, such as Italy, France, and Greece [7, 10, 13, 14]. In recent years, due to the warming climate, for example, the spread of infections with these parasites are observed in countries with a moderate climate (eg, the central-eastern part of Europe) [3–5, 7, 15]. In Poland, the prevalence of Dirofilaria repens infections in dogs ranges from 1.2% to 25.8% depending on the province (in central Poland it is 38%). In 2007–2012, 18 cases of Dirofilaria repens infections were described in humans [8, 15–18]. In the Netherlands, Dirofilaria repens infections in humans and dogs are sporadic. Few autochthonous humans and dogs cases have been reported [15, 19, 20].
In the course of subcutaneous dirofilariasis caused by Dirofilaria repens, a parasitic nodule forms with a diameter of approximately 0.5–2.5 cm. The resulting nodules can cause inflammation and migrate in the course of the disease. The treatment of subcutaneous dirofilariasis most often involves the surgical removal of the adult organism from the nodule [5–7]. In the international literature, many descriptions of unusual locations for this parasite in humans can also be found, for example, in the eyeball and the oral cavity [8, 13, 14].
Until recently, it was believed that in humans, only individual adult nematodes occurred, so no further development is observed. However, the occurrence of this nematode in the larval form—microfilariae—in the peripheral blood of humans, and in subcutaneous nodule aspirates, has also been described, which might indicate the complete development of this parasite in a human body [10, 11, 21].
According to our knowledge, our case is the first description of elbow bursitis caused by Dirofilaria repens in the world documented in such a manner. This case demonstrates that subcutaneous dirofilariasis is a growing epidemiological problem in Central and Eastern Europe among humans. Therefore, screening tests should be undertaken to assess the incidence of infections caused by this parasite in both humans and animals.
CONCLUSIONS
The increased incidence of filariasis caused by Dirofilaria nematodes, the unusual locations for parasites, and the occurrence of microfilaria in human peripheral blood require the development of the appropriate research algorithms for diagnostic procedures that could be used to achieve a correct diagnosis. The preparation of the appropriate diagnostic algorithm for suspected cases of invasions caused by this nematode will significantly raise the standards of laboratory operations in laboratory medicine. It is also important to draw the attention of physicians of various specialties, including orthopedists and surgeons, to the atypical clinical symptoms caused by the presence of Dirofilaria nematodes.
Acknowledgments
Financial support.This study was supported by the Statutory Funds of the Mecical University of Lublin No. DS43 (Anna Bogucka-Kocka), provided by the Polish Ministry of Science and Higher Education.
Potential conflicts of interest.All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Pampiglione S, Rivasi F. Human dirofilariasis due to Dirofilaria (Nochtiella) repens: an update of world literature from 1995 to 2000. Parasitologia 2000; 42:231–54. [PubMed] [Google Scholar]
- 2. Genchi C, Rinaldi L, Mortarino M, et al. Climate and Dirofilaria infection in Europe. Vet Parasitol 2009; 163:286–92. [DOI] [PubMed] [Google Scholar]
- 3. Genchi C, Kramer L. Subcutaneous dirofilariosis (Dirofilaria repens): an infection spreading throughout the old world. Parasit Vectors 2017; 10:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dantas-Torres F, Otranto D. Dirofilariosis in the Americas: a more virulent Dirofilaria immitis? Parasit Vectors 2013; 6:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simón F, Siles-Lucas M, Morchón R, et al. Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clin Microbiol Rev 2012; 25:507–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kartashev V, Simon F. Migrating Dirofilaria repens. N Engl J Med 2018; 378:e35. [DOI] [PubMed] [Google Scholar]
- 7. Zarnowska-Prymek H, Cielecka D, Salamatin R. Dirofilariasis–Dirofilaria repens–first time described in Polish patients. Przegl Epidemiol 2008; 62:547–51. [PubMed] [Google Scholar]
- 8. Wesolowska M, Kisza K, Szalinski M, et al. First case of heterochthonous subconjunctival dirofilariasis described in Poland. Am J Trop Med Hyg 2010; 83:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baptista-Fernandes T, Rodrigues M, Domingues D, et al. Dirofilariasis by Dirofilaria repens: an imported case and a brief review. Parasitol Int 2015; 64:261–3. [DOI] [PubMed] [Google Scholar]
- 10. Petrocheilou V, Theodorakis M, Williams J, et al. Microfilaremia from a Dirofilaria-like parasite in Greece. Case report. APMIS 1998; 106:315–8. [PubMed] [Google Scholar]
- 11. Tomazatos A, Cadar D, Török E, et al. Circulation of Dirofilaria immitis and Dirofilaria repens in the Danube Delta Biosphere Reserve, Romania. Parasit Vectors 2018; 11:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nuchprayoon S, Junpee A, Poovorawan Y, Scott AL. Detection and differentiation of filarial parasites by universal primers and polymerase chain reaction-restriction fragment length polymorphism analysis. Am J Trop Med Hyg 2005; 73:895–900. [PubMed] [Google Scholar]
- 13. Argy N, Sabou M, Billing A, et al. A first human case of ocular dirofilariosis due to Dirofilaria repens in Northeastern France. J Trop Med 2011; 2011:698647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jayasinghe RD, Gunawardane SR, Sitheeque MA, Wickramasinghe S. A case report on oral subcutaneous Dirofilariasis. Case Rep Infect Dis 2015; 2015:648278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Capelli G, Genchi C, Baneth G, et al. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasit Vectors 2018; 11:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demiaszkiewicz AW. Dirofilaria repens Railliet et Henry, 1911–a new parasite acclimatized in Poland. Ann Parasitol 2014; 60:31–5. [PubMed] [Google Scholar]
- 17. Bajer A, Rodo A, Mierzejewska EJ, et al. The prevalence of Dirofilaria repens in cats, healthy dogs and dogs with concurrent babesiosis in an expansion zone in central Europe. BMC Vet Res 2016; 12:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cielecka D, Żarnowska-Prymek H, Masny A, et al. Human dirofilariosis in Poland: the first cases of autochthonous infections with Dirofilaria repens. Ann Agric Environ Med 2012; 19:445–50. [PubMed] [Google Scholar]
- 19. Overgaauw P, van Dijk E. Autochthonous case of Dirofilaria repens in a dog in the Netherlands. Vet Rec 2009; 164:158. [DOI] [PubMed] [Google Scholar]
- 20. Overgaauw PA, van Dijk EP. A worm infection in the skin of a dog. First autochthonous Dirofilaria repens infection of a dog in the Netherlands. Tijdschr Diergeneeskd 2009; 134:936–8. [PubMed] [Google Scholar]
- 21. Potters I, Vanfraechem G, Bottieau E. Dirofilaria repens nematode infection with microfilaremia in traveler returning to Belgium from Senegal. Emerg Infect Dis 2018; 24:1761–3. [DOI] [PMC free article] [PubMed] [Google Scholar]