Abstract
Background
Sickle cell trait (HbAS) confers partial protection against malaria by reducing the adhesion of Plasmodium falciparum-infected erythrocytes to host receptors, but little is known about its potential protection against placental malaria.
Methods
Using flow cytometry, we assessed the recognition of HbAA and HbAS VAR2CSA-expressing infected erythrocytes, by plasma from 159 Beninese pregnant women with either HbAA (normal) or HbAS. Using multivariate linear models adjusted for gravidity, parasite infection at delivery, glucose-6-phosphate dehydrogenase deficiency, and α-thalassemia carriage, we observed significantly reduced cell surface antibody binding of HbAS-infected erythrocytes by plasma from HbAS compared with HbAA women (P < 10–3).
Results
The difference in cell surface antibody binding was only observed when infected erythrocytes and plasma were associated according to the same hemoglobin genotype. Similar levels of VAR2CSA-specific antibody were measured by enzyme-linked immunosorbent assay in the 2 groups, suggesting that the altered interaction between VAR2CSA and HbAS women’s antibodies could reflect abnormal display of VAR2CSA on HbAS erythrocytes.
Conclusions
Our data stress the need for assessments of erythrocyte disorders such as the sickle cell trait in a population group when studying immunological responses to P falciparum.
Keywords: Cell surface antibody binding, hemoglobin S, pregnancy-associated malaria, VAR2CSA
Each year, in sub-Saharan Africa, more than 30 million women are exposed to the risk of developing pregnancy-associated malaria (PAM) [1]. Pregnancy-associated malaria is a major cause of maternal and fetal anemia, fetal growth restriction, preterm birth, and low birth weight (LBW) [2]. Pregnancy-associated malaria is caused by the adhesion and the sequestration of Plasmodium falciparum-infected red blood cells (iRBCs) in placental intervillous spaces [3]. Adhesion of iRBCs in the placenta is mediated by a single var gene, VAR2CSA [4, 5], the PfEMP1 variant that interacts with chondroitin sulfate A (CSA) on syncytiotrophoblasts [6–8].
Primigravid women are the most susceptible to PAM because they lack specific protective immunity. Indeed, previous studies have shown that the level of VAR2CSA-specific antibodies increases with gravidity [9–11], and that the presence of these antibodies in the placenta is related to better pregnancy outcomes [12]. These antibodies inhibit the binding of iRBCs to CSA [13]. Women with higher plasma binding inhibition ability at delivery are less susceptible to PAM and to give birth to a LBW baby [11]. The genetic background of mothers may have an impact on the ability of surface-expressed VAR2CSA to mediate interaction with endothelial receptors.
Hemoglobin (Hb) S, responsible for sickle cell disease, results from a genetic mutation (Glu6Val) (rs334) at the sixth position within the β-globin chain (HBB, chromosome 11) of normal Hb (HbA) [14]. Hemoglobin S is highly prevalent in human populations living in malaria-endemic areas. The highest frequencies of HbS are observed in sub-Saharan Africa, the Middle East, and India, up to 18% [15], because of the protection afforded by HbS heterozygous carriage (HbAS, sickle cell trait) against severe P falciparum malaria-related symptoms and death, without serious hematological disadvantage [15]. HbAS is indeed associated with a 90% reduced risk of severe malaria [16].
It has been shown that HbAS iRBCs display lower amounts of VAR2CSA, which are aberrantly presented because they are anchored in enlarged and dispersed knobs, correlating with reduced cytoadherence capacity to CSA [17, 18]. However, little is known about the potential protection of HbS against PAM, despite some studies having reported that there was no significant interaction between sickle cell trait and P falciparum infection during pregnancy [19, 20]. Therefore, the influence of the sickle cell trait genotype needs to be investigated in the context of VAR2CSA in the PAM.
Other red blood cell (RBC) disorders, such as glucose-6-phosphate dehydrogenase (G6PD) deficiency and α-thalassemia, coexist in populations affected by P falciparum malaria and by abnormal Hb, and they also confer protection against malaria [21, 22]. The influence of G6PD deficiency on iRBCs cytoadherence has not yet been determined, but a previous study highlighted the abnormal display of PfEMP1 at the surface of α-thalassemia erythrocytes [23].
In addition, α-thalassemia results from 1 or several deletions of the alpha gene(s) (HBA1 and HBA2) located in the α-globin cluster on chromosome 16 [24]. The most common deletion encountered in sub-Saharan Africa is a deletion of 3.7 kb (α−3.7 deletion). The heterozygous carriage of this deletion (α−3.7 thalassemia trait) is not or only slightly symptomatic, but homozygous deletion can cause hematological alterations or be lethal [25].
In order to analyze the influence of HbS heterozygous carriage in the cell surface antibody binding during pregnancy, we assessed the ability of Beninese pregnant women’s plasma to recognize HbAS and HbAA VAR2CSA-expressing iRBCs. With HbAA iRBCs, the cell surface antibody binding did not change according to maternal HbS carriage, but when iRBCs and plasma were associated according to the same Hb genotype, we observed significantly lower cell surface antibody binding by plasma from HbAS compared with HbAA women. We were able to show that the level of cell surface antibody binding was associated with the anti-VAR2CSA antibody levels in these plasmas, although we did not observe a significant difference between these antibody levels in HbAA compared with HbAS women.
METHODS
Ethics Statement
The study was approved by the Comité Consultatif de Déontologie et d’Éthique of the Institut de Recherche pour le Développement ([IRD] Marseille, France) and the Comité d’Éthique de la Faculté des Sciences de la Santé (Université d’Abomey Calavi, Benin; FSS 026/2007/CE/FSS/UAC). All study procedures were performed in accordance with the institutional policies, guidelines, and regulations pertaining to research involving human subjects. Written informed consent was obtained for all participants.
Study Site and Population
The STOPPAM study, conducted in Benin between 2008 and 2011, enrolled 1037 pregnant women early in pregnancy and observed them every month until delivery [26]. Recent data identified 15.8% of women in the cohort as carriers of the HbAS genotype [19]. In the present work, for each woman, we considered some of the data and samples collected during the STOPPAM study, such as peripheral venous blood collected at delivery (plasma was purified from these samples) and thick and thin blood smears prepared to detect P falciparum infection. Thus, we performed a case-control study with 159 plasmas collected at delivery. The cases and controls were matched (1 case for 2 controls) according to the gravidity and P falciparum placental infection, because both are known to increase anti-P falciparum antibody levels [11] (34 primigravidae, 25 secundigravidae, and 100 multigravidae all distributed equivalently into 26 infected and 133 noninfected mothers). Cases comprised women with abnormal HbS (52 HbAS), and controls were women with normal erythrocytes (107 HbAA).
Blood Samples for Erythrocyte Use
Human HbAA erythrocytes were supplied from the “Etablissement Français du Sang”. After informed written consent was obtained in accordance with the Declaration of Helsinki, HbAS RBCs were obtained from voluntary donors. Red blood cells were separated from plasma and leucocytes and stored at 4°C before use. All HbAA and HbAS RBCs came from blood group O donors.
Plasmodium falciparum-Infected Erythrocytes Culture
Plasmodium falciparum FCR3 parasites expressing VAR2CSA (selected for their CSA) (Sigma-Aldrich, Saint-Quentin Fallavier, France) adhesion phenotype [27]) were grown in vitro in human HbAA and HbAS RBCs according to adapted procedures from “Methods in Malaria Research” (https://www.beiresources.org/portals/2/MR4/Methods_In_Malaria_Research-6th_edition.pdf) adapted from Trager and Jensen [28]. In brief, HbAA iRBCs were cultured in RNase protection assay (Roswell Park Memorial Institute [RPMI] 1640 medium (Gibco, Fisher Scientific, Illkirch, France) supplemented with 25 mM HEPES (Gibco), 2 mM L-glutamine (Gibco), 0.05 mg/mL gentamicin (Gibco), 2% AB human serum, and 0.5% AlbuMAX (Gibco). Cultures were maintained at 5% hematocrit in a gas mixture of 5% O2, 5.5% CO2, and 89.5% N2 and incubated at 37°C. HbAS RBCs were infected through coculture with late trophozoite and schizont-infected HbAA erythrocytes obtained after magnetic-activated cell sorting (MACS) (Miltenyi Biotec, Paris, France). HbAA and HbAS IRBs were cultured in the same medium described above.
Cell Surface Antibody Binding of Plasmodium falciparum-Infected Red Blood Cells by Plasmas Using Flow Cytometry
Cell surface antibody binding of iRBCs by plasma was conducted using a method adapted from Tuikue Ndam et al [29]. Infected red blood cells collected after MACS were resuspended in phosphate-buffered saline ([PBS] Gibco)-1% bovine serum albumin ([BSA] Roche Diagnostics, Meylan, France) and distributed in 96-well plates (500 000 iRBCs per well). After centrifugation (300 ×g for 2 minutes at room temperature), iRBCs were resuspended in PBS-1% BSA containing plasma (diluted 1:50) and mixed at 1000 rpm for 30 seconds. After 1-hour incubation at room temperature, the cells were washed twice with PBS-1% BSA. Then, antihuman immunoglobulin (Ig)G antibody phycoerythrin (PE)-conjugated (Jackson ImmunoResearch [Interchim], Montluçon, France) (dilution 1:100 in PBS-1% BSA) was added and mixed with the cells at 1000 rpm for 30 seconds. After 1-hour incubation at room temperature and 3 washes with PBS-1% BSA, the cells were resuspended in PBS-2% paraformaldehyde and kept in darkness at 4°C overnight. The following day, the iRBCs were washed 2 times with PBS-1% BSA and resuspended with TO-PRO-3 (Thermo Fisher Scientific) (dilution 1:10 000 in PBS) just before acquisition and analysis by flow cytometry (FACS Canto II BD). Using FlowJo software, the geomean values of PE fluorescence (gated according to TO-PRO-3 fluorescence) were used to characterize the level of cell surface antibody binding of iRBCs. To normalize data between plates, each value was divided by that of a pool of plasmas from pregnant Europeans who had never been infected by P. falciparum.
Molecular Determination of the Glucose-6-Phosphate Dehydrogenase Deficiency and the α-Thalassemia 3.7 Deletion
For each woman, deoxyribonucleic acid (DNA) was extracted primarily from the blood leucocyte fraction (QIAGEN, Courtaboeuf, France). When leucocytes were not available, DNA was extracted by the Chelex method [30] from blood spotted and dried onto Whatman 3 paper.
Glucose-6-phosphate dehydrogenase deficiency results from different mutations in the G6PD gene [31]. Normal G6PD activity is ensured by the G6PD B enzyme proteoform. An A376G mutation (rs1050829) is responsible for the G6PD A variant associated with a minimal loss in enzyme activity [32]. An additional mutation (mainly G202A or T968C in sub-Saharan populations) is responsible for the G6PD A− variant associated with enzyme deficiency. The G6PD A and A− variants were detected using polymerase chain reaction-restriction fragments length polymorphisms (PCR-RFLP), with a method adapted from Carter et al [33], and according to the GoTaqFlexi DNA Polymerase (Promega, Charbonnières-les-Bains, France) requirements. For G6PD A determination, Fok I (New England Biolabs, Evry, France) digestion was realized after PCR amplification. When G6PD A variant was identified, the additional mutations G6PD-G202A and G6PD-T968C were sought by PCR-RFLP, using the restriction enzymes NlaIII and NciI (New England Biolabs), respectively.
For α-thalassemia, the most common deletion encountered in sub-Saharan Africa is a deletion of 3.7 kb (α−3.7 deletion) resulting in an α-globin synthesis defect [25]. The gene deletion of 3.7 kb was detected by multiplex PCR with a method adapted from Liu et al [34] and according to the GoTaqFlexi DNA Polymerase (Promega) requirements.
Statistical Analysis
A χ2 test was used to compare the proportions of G6PD A− variant and α−3.7 deletion carriers among HbAA and HbAS women. Fisher’s exact test was performed to analyze the associations between carriage of HbS, G6PD A− variant, and α−3.7 deletion. Multivariate linear regression models were used to analyze the associations between the cell surface antibody binding of iRBCs (quantitative dependent variable) and different explanatory variables of interest: maternal genotypes (binary variable HbS vs HbA), G6PD A− variant (binary variable), and α-thalassemia−3.7 deletion carriage (binary variable). Our analyses were adjusted for gravidity and placental P falciparum infection at delivery, and the robust variance method was used to derive the confidence interval and P values of the regression coefficients. The analyses were carried out with STATA software, version 13.1 (STATA Corporation). The significance of deviation from Hardy-Weinberg equilibrium was tested using the χ2 goodness-of-fit test.
RESULTS
Characterization of Hemoglobin S, Glucose-6-Phosphate Dehydrogenase, and α-Thalassemia Maternal Genotypes
Of the 1037 women from the STOPPAM study [26], 635 were already genotyped for HbS carriage [19]. From these 635, we selected 159 individuals to constitute our subcohort for the case-control study.
We were then able to determine both G6PD and α-thalassemia genotypes for 79 HbAA and 37 HbAS women (Supplementary Table 1). Globally, 26.7% and 51.7% of women from our population group carried, respectively, the A− variant responsible for G6PD deficiency or the α-thalassemia−3.7 deletion. For the G6PD A− variant, only the additional mutation G6PD-G202A was found. No statistically significant deviation from Hardy-Weinberg equilibrium was observed for any polymorphism: G6PD A and A− variants ([χ2 test] χ2 = 2.98, P = .57, based on 10 000 permutations) and α-thalassemia−3.7 deletion ([χ2 test] χ2 = 0.11, P = .66, df = 1). Hemoglobin S was not tested because of lethality and therefore absence of HbSS individuals. The G6PD A− variant and α−3.7 deletion were equally distributed between HbAA and HbAS women (χ2 test, both P > .53, n = 116). We defined 2 groups for G6PD according to the enzyme deficiency resulting from the presence or absence of the variant A−. The “G6PD A−variant” group includes the women carrying the G6PD A− variant in either homozygous or heterozygous forms (A−B, A−A and A− A− genotypes), the second one BB, AB, and AA genotypes women. Regarding α-thalassemia, we also separated women into 2 groups according to the presence of the α−3.7 deletion. Therefore, we considered an “α−3.7 deletion” group, including women with the α−3.7 deletion in either homozygous (α−3.7/α−3.7) or heterozygous forms (αα/α−3.7), and a “no α−3.7 deletion” group, comprising women with normal α genes (αα/αα). We checked whether certain combinations of genetic variants were more frequently observed than expected in our sample with a χ2 test. Because G6PD, HBB, and HBA1/HBA2 are located on 3 different chromosomes, we would expect these RBC disorders to be independent of each other. We first compared the proportions of women carrying none, 1, or both RBC disorders amongst the HbAA and HbAS groups, but we did not see any significant differences between the different groups (Table 1). We then tested the association between the inheritance of HbS, G6PD deficiency, and α-thalassemia genotypes in our cohort (data not shown). We only noticed a significant link between α-thalassemia and HbS genotypes (Fisher’s exact test, P = 0.007, n = 116) consisting of more αα/α−3.7 genotypes and less α−3.7/α−3.7 genotypes in the HbAS compared with the HbAA group.
Table 1.
Proportion of Women Carrying G6PD Deficiency and/or α-Thalassemia Among HbAA and HbAS Groupsa
| Combined RBC Genetic Defects | HbAA | HbAS | P Value |
|---|---|---|---|
| n (%) | n = 79 | n = 37 | |
| No G6PDA− variant and no α−3.7 deletion | 28 (35.4%) | 14 (37.8%) | .96 |
| Only G6PD A− variant | 10 (12.7%) | 4 (10.8%) | .99 |
| Only α−3.7 deletion | 28 (35.4%) | 15 (40.6%) | .66 |
| Both G6PD A− variant and α−3.7 deletion | 13 (16.5%) | 4 (10.8%) | .60 |
Abbreviations: G6PDA, glucose-6-phosphate dehydrogenase A; HbAA, ; HbAS, sickle cell trait; RBC, red blood cells.
aWomen were separated into 4 groups according to their carriage of the G6PD A− variant and the α−3.7 deletion. P value was based on a χ2 test to analyze the distribution of women according to their G6PD A− variant and α−3.7 deletion carriages among HbAA and HbAS groups.
The Cell Surface Antibody Binding of HbAA- or HbAS-Infected Red Blood Cells Does Not Change According to Maternal Genotypes
We determined the level of cell surface antibody binding of normal HbAA and HbAS P falciparum-iRBCs and analyzed the impact of Hb, G6PD deficiency, or α-thalassemia maternal genotypes. Using a multivariate analysis, we did not observe a statistically significant difference for the cell surface antibody binding of HbAA iRBCs according to the RBC genotypes of the mothers from whom plasmas were obtained (multivariable linear regression, n = 111) (Table 2). We repeated the experiment, using HbAS iRBCs. As was the case for HbAA iRBCs, we did not observe any significant difference in the cell surface antibody binding of HbAS iRBCs according to the RBC genotypes of the mothers from whom plasmas were obtained (multivariable linear regression, n = 110) (Table 2).
Table 2.
Factors Tested for the Association With the Cell Surface Antibody Binding of HbAA iRBCs or HbAS iRBCs Using a Linear Multivariate Regressiona
| Cell surface binding (MFI/MFI0) | Covariates | Coefficient | 95% CI | P Value |
|---|---|---|---|---|
| HbAA iRBCs n = 111 (nHbAA = 75; nHbAS = 36) |
HbS carriage G6PD A− variant carriage α−3.7 deletion carriage Multigravidity Infection at delivery |
0.04 −0.08 −0.24 4.48 1.76 |
−1.68 to 1.76 −1.91 to 1.76 −1.84 to 1.36 3.04–5.92 −0.67 to 4.19 |
.96 .93 .77 <10–3 .15 |
| HbAS iRBCs n = 110 (nHbAA = 74; nHbAS = 36) |
HbS carriage G6PD A− variant carriage α−3.7 deletion carriage Multigravidity Infection at delivery |
−0.05 −0.16 0.02 0.75 0.49 |
−0.36 to 0.26 −0.43 to 0.12 −0.26 to 0.30 0.48–1.03 0.03–0.94 |
.74 .27 .88 <10–3 .04 |
Abbreviations: CI, confidence interval; G6PD, glucose-6-phosphate dehydrogenase; HbS, hemoglobin S; iRBCs, infected red blood cells; MFI, mean fluorescent intensity.
aCell surface antibody binding fluorescent intensities (MFI) were normalized to the value (MFI0) obtained by a pool of plasmas derived from European pregnant women. Covariates included in linear multivariate model regression were maternal genotypes: HbS carriage, G6PD A− variant carriage, and α−3.7 deletion carriage, as well as multigravidity and infection at delivery. A positive regression coefficient shows a positive association with the factor, and a negative regression coefficient shows a negative association.
Separately, we assessed the difference in cell surface antibody binding to HbAA and to HbAS iRBCs for each individual plasma sample. Our results showed that binding to HbAA iRBC was higher than to HbAS iRBC (Wilcoxon paired-test, P < 10–3, n = 140; data not shown).
Cell Surface Antibody Binding of Infected Red Blood Cells Is Lower for HbAS Mothers When Matching Infected Red Blood Cells to Plasmas From Mothers With the Same Hemoglobin Genotype
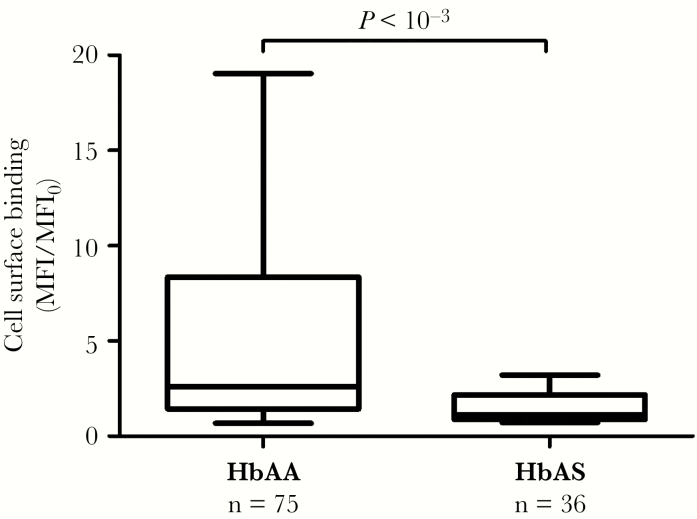
To get closer to in vivo biological conditions, we matched iRBCs to plasma samples from mothers with the same Hb genotype. Thus, we compared the cell surface antibody binding to HbAA iRBCs of plasma from HbAA mothers and similarly the cell surface antibody binding to HbAS iRBCs of plasma from HbAS mothers. We observed significantly lower cell surface antibody binding by plasma from HbAS mothers (multivariable linear regression, P < 10–3, n = 111, median value of HbAA group = 2.60; median value of HbAS group = 1.13) (Figure 1). The G6PD A− variant and α−3.7 deletion carriages were also included in the multivariate analysis as covariates but did not impact this observation (Table 3). All subsequent tests were conducted considering cell surface antibody binding when iRBCs and plasmas were matched by Hb genotype.
Figure 1.
Association between cell surface antibody binding and maternal hemoglobin (Hb) genotype when infected red blood cells and plasmas are matched by Hb genotype. Cell surface antibody binding fluorescent intensities (mean fluorescent intensity [MFI]) were normalized to the value (MFI0) obtained by a pool of plasmas derived from European pregnant women. P value was based on a multivariate linear regression to determine the association between cell surface antibody binding and maternal Hb genotype (HbAA or HbAS), adjusted for gravidity, malaria infection, glucose-6-phosphate dehydrogenase A− variant, and α−3.7 deletion carriages (n = 111). Box and whisker plots illustrate medians with 75th and 25th percentiles (boxes) and 90th and 10th percentiles (whiskers).
Table 3.
Factors Tested for the Association With the Cell Surface Antibody Binding of iRBCs (When iRBCs and Plasmas Are Matched by HbS Genotype) Using a Linear Multivariate Regressiona
| Covariates | Cell Surface Binding of iRBCs (MFI/MFI0) | ||
|---|---|---|---|
| n = 111 (nHbAA = 75; nHbAS = 36) | |||
| Coefficient | 95% CI | P Value | |
| HbS carriage | −3.25 | −4.28 to −2.22 | <10–3 |
| G6PD A− variant carriage | 0.30 | −1.35 to 1.96 | .72 |
| α−3.7 deletion carriage | −0.23 | −1.57 to 1.11 | .74 |
| Multigravidity | 3.32 | 2.12–4.52 | <10–3 |
| Infection at delivery | 1.99 | −0.07 to 4.04 | .06 |
Abbreviations: CI, confidence interval; G6PD, glucose-6-phosphate dehydrogenase; HbS, hemoglobin S; iRBCs, infected red blood cells; MFI, mean fluorescent intensity.
aCell surface antibody binding fluorescent intensities (MFI) were normalized to the value (MFI0) obtained by a pool of plasmas derived from European pregnant women. Covariates included in the linear multivariate model regression were maternal genotypes: HbS carriage, G6PD A− variant carriage, and α−3.7 deletion carriage, as well as multigravidity and infection at delivery. A positive regression coefficient shows a positive association with the factor, and a negative regression coefficient shows a negative association.
The Cell Surface Antibody Binding of Infected Red Blood Cells Is Associated With the Anti-VAR2CSA Antibody Level
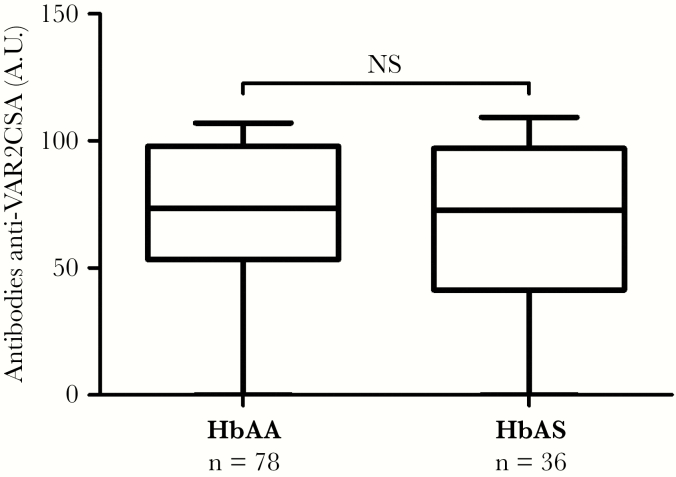
VAR2CSA is the antigen involved in the cytoadherence of iRBCs to CSA, causing their sequestration in the placenta, and is a promising placental malaria vaccine candidate [35]. The cell surface antibody binding of VAR2CSA-expressing iRBCs that we assessed could plausibly reflect multiple antibody-antigen interactions. Previous work measured the anti-VAR2CSA antibody levels for all plasma samples of the STOPPAM cohort taken at delivery. In brief, levels of anti-VAR2CSA antibodies were determined in plasma samples (at dilution 1:100) using an enzyme-linked immunosorbent assay (ELISA) assay targeting 0.5 μg/mL full-length ectodomain of VAR2CSA [11, 36]. We used these data to test the association of the iRBCs cell surface antibody binding and the corresponding antibody levels, when RBC and plasma Hb genotypes were matched (Table 4). We observed a significant positive association between the anti-VAR2CSA antibody levels and the iRBCs cell surface antibody binding (multivariable linear regression, P < 10–3, n = 140). We then assessed the association between this antibody level and maternal Hb genotype, but we found no significant difference in the level of anti-VAR2CSA antibodies between HbAA and HbAS women (multivariable linear regression, n = 114) (Figure 2). In addition, these antibody levels did not vary according to G6PD A− variant or α−3.7 deletion carriages (Table 5).
Table 4.
Association Between the iRBCs Surface Cell Surface Antibody Binding (When iRBCs and Plasmas Are Matched by Hb Genotype) and the Level of Anti-VAR2CSA Antibody Using a Linear Multivariate Regressiona
| Covariates | Cell Surface Binding of iRBCs (MFI/MFI0) | ||
|---|---|---|---|
| Coefficient | 95% CI | P Value | |
| Anti-VAR2CSA antibody levels (A.U.) | 0.05 | 0.03–0.7 | <10–3 |
| HbS carriage | −3.67 | −4.62 to −2.73 | <10–3 |
| Multigravidity | 3.05 | 2.02–4.09 | <10–3 |
| Infection at delivery | 1.19 | −0.37 to 2.75 | .13 |
Abbreviations: A.U., arbitrary units; CI, confidence interval; HbS, hemoglobin S; iRBCs, infected red blood cells; MFI, mean fluorescent intensity.
aCell surface antibody binding fluorescent intensities (MFI) were normalized to the value (MFI0) obtained by a pool of plasmas derived from European pregnant women. Covariates included in linear multivariate model regression were the anti-VAR2CSA antibody levels and maternal HbS carriage, as well as multigravidity and infection at delivery. A positive regression coefficient shows a positive association with the factor, and a negative regression coefficient shows a negative association.
Figure 2.
Association between the level of anti-VAR2CSA antibody and maternal hemoglobin (Hb) genotype. The antibody levels data come from a previously published work. The antibody levels were measured by enzyme-linked immunosorbent assay [11], and values were converted to arbitrary units (A.U.) [36]. P value was based on a multivariate linear regression to determine the association between the level of anti-VAR2CSA antibody and maternal Hb genotype (HbAA or HbAS), adjusted for gravidity, malaria infection, glucose-6-phosphate dehydrogenase A− variant, and α−3.7 deletion carriages (n = 114). Box and whisker plots illustrate medians with 75th and 25th percentiles (boxes) and 90th and 10th percentiles (whiskers).
Table 5.
Factors Tested for the Association With the Anti-VAR2CSA Antibody Level Using Linear Multivariate Regressiona
| Covariates | Anti-VAR2CSA Antibody Levels (A.U.) | ||
|---|---|---|---|
| n = 114 (nHbAA = 78; nHbAS = 36) | |||
| Coefficient | 95% CI | P Value | |
| HbS carriage | −4.15 | −17.62 to 9.31 | .54 |
| G6PD A− variant carriage | −6.04 | −19.47 to 7.39 | .37 |
| α−3.7 deletion carriage | −1.09 | −13.09 to 10.92 | .86 |
| Multigravidity | 11.09 | −2.81 to 25 | .12 |
| Infection at delivery | 3.95 | −10.94 to 18.83 | .60 |
Abbreviations: A.U., arbitrary units; CI, confidence interval; G6PD, glucose-6-phosphate dehydrogenase; HbS, hemoglobin S.
aCovariates included in linear multivariate model regression were maternal genotypes: HbS carriage, G6PD A− variant carriage, and α−3.7 deletion carriage, as well as multigravidity and infection at delivery. A positive regression coefficient shows a positive association with the factor, and a negative regression coefficient shows a negative association.
DISCUSSION
It is well known that HbS heterozygous carriage affords relative protection against severe forms of malaria, but whether the HbAS genotype also confers a selective protective advantage against PAM remains to be determined. In this study, using plasma samples from a cohort of Beninese pregnant women, we assessed the impact of maternal HbAS genotype on the cell surface antibody binding of plasma antibodies to iRBCs during PAM. Our findings are consistent with previous studies, showing a significant increase in the level of surface recognition of iRBCs by plasma as a function of gravidity, independently of maternal genotypes [11]. However, we observed significantly lower cell surface antibody binding of iRBCs by plasma from HbAS mothers than by plasmas from HbAA mothers when iRBCs and plasmas were matched according to Hb genotype. This lower cell surface antibody binding suggests modifications in the interactions between antigens and antibodies and could be explained by 2 main hypotheses: (1) HbAS women have a lower amount of antibodies recognizing the different antigens present on the iRBC surface, and (2) HbAS iRBCs display lower amounts of the recognized antigens and/or abnormally presented forms of these antigens, altering the capacity for antibody-mediated recognition. In previous work, we measured the level of anti-VAR2CSA antibody in women in the STOPPAM cohort [11], offering us a way to test the first hypothesis. Because VAR2CSA is the parasite ligand involved in the cytoadherence of iRBCs to CSA in PAM, and because women develop anti-VAR2CSA antibodies during successive pregnancies, it is plausible to imagine that the cell surface antibody binding of VAR2CSA is a major component of the overall cell surface antibody binding of iRBCs. To confirm this hypothesis, we first tested the association of the cell surface binding with the level of anti-VAR2CSA antibodies, and we revealed a positive association between these 2 factors. The levels of the antibodies measured by ELISA were nevertheless not significantly different between HbAA and HbAS mothers. However, cell surface antibody binding for each individual sample was shown by paired analyses to be significantly higher to HbAA compared with HbAS iRBC. All of these results suggest that the lower level of cell surface antibody binding of iRBCs by plasma from HbAS women, when RBC and plasma Hb genotypes were matched, is not due to lower amounts of antibodies recognizing VAR2CSA. Thus, our findings suggest that the expression of VAR2CSA is decreased and/or abnormal on HbAS iRBCs. Previous studies have documented lower levels of display of VAR2CSA [17] or PfEMP1 [37] at the HbAS iRBC surface. These studies also highlighted the abnormally shaped, heterogeneously distributed knobs on HbAS RBCs [37]. In this study, we cannot exclude the possibility that the cell surface antibody binding of other iRBC surface antigens may account for the lower total surface recognition of iRBCs by plasma of HbAS mothers, but the study by Chan et al [38] suggests that it is indeed PfEMP1 on iRBCs that is the principal target of anti-VSA antibodies. However, we could not exclude the possibility that the IgG affinity for the recognized antigens at the iRBC surface may be stronger for HbAA women.
CONCLUSIONS
Immunity during pregnancy, notably antibody-mediated recognition of VAR2CSA-expressing iRBCs, has been investigated in previous studies [11, 39]. Nevertheless, this study is the first to investigate the impact of abnormal HbS in the development of humoral immunity to malaria during pregnancy. It is also important to note that over half of our study group carried the α−3.7 deletion, in line with what has been previously found in neighboring Togo [40]. Likewise, over one quarter of investigated women presented the A− variant responsible for G6PD deficiency, consistent with already observed values in locations from coastal West Africa [41]. The G6PD A− variant and α−3.7 deletion were equally distributed between HbAA and HbAS women. Only 35.4% and 37.8%, respectively, carried neither the G6PD A− variant nor the α−3.7 deletion. Furthermore, we observed a significant link between α-thalassemia and HbS genotypes, with more αα/α−3.7 genotypes and less α−3.7/α−3.7 genotypes among the HbAS compared with the HbAA women. Malaria-protective effects of the combination of both polymorphisms have been studied, and a negative epistasis between sickle cell trait and heterozygous α-thalassemia carriage in relation to protection against malaria has already been described [42, 43]. However, according to our findings, it is important to match plasma and erythrocyte genotypes. To characterize the influence of G6PD deficiency and α-thalassemia, which do not show here any impact on cell surface antibody binding of HbAS erythrocytes, it would be necessary to study first these genetic defects separately and to consider their cocarriage with HbS thereafter, matching ideally in each experiment the plasma and the erythrocyte genotypes to better reproduce in vivo conditions, as we did here with HbAS iRBCs. Our results highlight the importance (1) of taking into account the Hb genotype of the mothers from whom plasmas are derived and (2) of the erythrocytes used for the in vitro assay of cell surface antibody binding, because RBC disorders could influence the export and/or the presentation of antigens. Furthermore, this study reinforces the hypothesis of VAR2CSA abnormal display at the HbAS erythrocyte surface, illustrating the consequence on HbAS iRBC surface recognition by HbAS plasmas. Despite the fact that sickle cell trait is the most widespread erythrocyte disorder in Africa, on the basis of our biological data we could not draw definitive conclusions on the impact of the HbAS genotype on PAM. Nevertheless, the same scientific strategy needs to be applied with respect to all the different genetic disorders, such as HbAC that is known to have an impact on LBW for example [19], as well as G6PD deficiency and α-thalassemia.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the voluntary blood donors for their participation to this project; and the Universities Paris Diderot and Paris Descartes for the doctoral scholarships awarded to Marilou Tétard and Margaux Chauvet. This study was part of the STOPPAM collaborative project. We are grateful to the women who participated in the study.
Author contributions.B. G., A. J. F .L., A. M., and F. M.-N. conceived and designed the project. M. C., M. L., A. M., M. T., and N. T. N. performed the experiments. M. C., G. C., A. M., F. M.-N., and J. M. performed the analysis. F. A., E. B., M. C., L. D., M. H., A. M., F. M.-N., and D. P. performed the genotyping. M. C., A. J. F. L., A. M., F. M. N., and M. T. wrote the paper. All authors reviewed the manuscript before submission.
Financial support. This work was funded by the Laboratoire d’Excellence GR-Ex, Paris, France, reference ANR-11-LABX-0051, that is funded by the program Investissements d’Avenir of the French National Research Agency, reference ANR-11-IDEX-0005-02.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: 29th Molecular Parasitology Meeting, September 2018, Woods Hole, MA.
References
- 1. World Health Organization. World Malaria Report. Geneva; World Health Organization; 2018. [Google Scholar]
- 2. Rogerson SJ, Desai M, Mayor A, et al. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis 2018; 18:e107–18. [DOI] [PubMed] [Google Scholar]
- 3. Andrews KT, Lanzer M. Maternal malaria: Plasmodium falciparum sequestration in the placenta. Parasitol Res 2002; 88:715–23. [DOI] [PubMed] [Google Scholar]
- 4. Salanti A, Dahlbäck M, Turner L, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med 2004; 200:1197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Viebig NK, Gamain B, Scheidig C, et al. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Rep 2005; 6:775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Viebig NK, Nunes MC, Scherf A, Gamain B. The human placental derived BeWo cell line: a useful model for selecting Plasmodium falciparum CSA-binding parasites. Exp Parasitol 2006; 112:121–5. [DOI] [PubMed] [Google Scholar]
- 7. Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 1996; 272:1502–4. [DOI] [PubMed] [Google Scholar]
- 8. Srivastava A, Gangnard S, Round A, et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc Natl Acad Sci U S A 2010; 107:4884–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duffy PE. Plasmodium in the placenta: parasites, parity, protection, prevention and possibly preeclampsia. Parasitology 2007; 134:1877–81. [DOI] [PubMed] [Google Scholar]
- 10. O’Neil-Dunne I, Achur RN, Agbor-Enoh ST, et al. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect Immun 2001; 69:7487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ndam NT, Denoeud-Ndam L, Doritchamou J, et al. Protective antibodies against placental malaria and poor outcomes during pregnancy, Benin. Emerg Infect Dis 2015; 21:813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuikue Ndam NG, Salanti A, Le-Hesran JY, et al. Dynamics of anti-VAR2CSA immunoglobulin G response in a cohort of Senegalese pregnant women. J Infect Dis 2006; 193:713–20. [DOI] [PubMed] [Google Scholar]
- 13. Fried M, Nosten F, Brockman A, et al. Maternal antibodies block malaria. Nature 1998; 395:851–2. [DOI] [PubMed] [Google Scholar]
- 14. Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol 1998; 11:1–51. [DOI] [PubMed] [Google Scholar]
- 15. Piel FB, Patil AP, Howes RE, et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Commun 2010; 1:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams TN, Mwangi TW, Wambua S, et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis 2005; 192:178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cyrklaff M, Srismith S, Nyboer B, et al. Oxidative insult can induce malaria-protective trait of sickle and fetal erythrocytes. Nat Commun 2016; 7:13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kilian N, Srismith S, Dittmer M, et al. Hemoglobin S and C affect protein export in Plasmodium falciparum-infected erythrocytes. Biol Open 2015; 4:400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tétard M, Milet J, Dechavanne S, et al. Heterozygous HbAC but not HbAS is associated with higher newborn birthweight among women with pregnancy-associated malaria. Sci Rep 2017; 7:1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel JC, Mwapasa V, Kalilani L, et al. Absence of association between sickle trait hemoglobin and placental malaria outcomes. Am J Trop Med Hyg 2016; 94:1002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mbanefo EC, Ahmed AM, Titouna A, et al. Association of glucose-6-phosphate dehydrogenase deficiency and malaria: a systematic review and meta-analysis. Sci Rep 2017; 7:45963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krause MA, Diakite SA, Lopera-Mesa TM, et al. α-Thalassemia impairs the cytoadherence of Plasmodium falciparum-infected erythrocytes. PLoS One 2012; 7:e37214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piel FB, Weatherall DJ. The α-thalassemias. N Engl J Med 2014; 371:1908–16. [DOI] [PubMed] [Google Scholar]
- 25. Medeiros Alcoforado GH de, Bezerra CM, Araújo Moura Lemos TM, et al. Prevalence of α-thalassemia 3.7 kb deletion in the adult population of Rio Grande do Norte, Brazil. Genet Mol Biol. 2012; 35:594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huynh BT, Fievet N, Gbaguidi G, et al. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am J Trop Med Hyg 2011; 85:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pouvelle B, Meyer P, Robert C, et al. Chondroitin-4-sulfate impairs in vitro and in vivo cytoadherence of Plasmodium falciparum infected erythrocytes. Mol Med 1997; 3:508–18. [PMC free article] [PubMed] [Google Scholar]
- 28. Trager W, Jensen JB. Human malaria parasites in continuous culture. Science 1976; 193:673–5. [DOI] [PubMed] [Google Scholar]
- 29. Tuikue Ndam NG, Fievet N, Bertin G, et al. Variable adhesion abilities and overlapping antigenic properties in placental Plasmodium falciparum isolates. J Infect Dis 2004; 190:2001–9. [DOI] [PubMed] [Google Scholar]
- 30. Wooden J, Kyes S, Sibley CH. PCR and strain identification in Plasmodium falciparum. Parasitol Today Pers Ed 1993; 9:303–5. [DOI] [PubMed] [Google Scholar]
- 31. Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008; 371:64–74. [DOI] [PubMed] [Google Scholar]
- 32. Howes RE, Dewi M, Piel FB, et al. Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malar J 2013; 12:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carter N, Pamba A, Duparc S, Waitumbi JN. Frequency of glucose-6-phosphate dehydrogenase deficiency in malaria patients from six African countries enrolled in two randomized anti-malarial clinical trials. Malar J 2011; 10:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu YT, Old JM, Miles K, et al. Rapid detection of alpha-thalassaemia deletions and alpha-globin gene triplication by multiplex polymerase chain reactions. Br J Haematol 2000; 108:295–9. [DOI] [PubMed] [Google Scholar]
- 35. Chêne A, Houard S, Nielsen MA, et al. Clinical development of placental malaria vaccines and immunoassays harmonization: a workshop report. Malar J 2016; 15:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guitard J, Cottrell G, Magnouha NM, et al. Differential evolution of anti-VAR2CSA- IgG3 in primigravidae and multigravidae pregnant women infected by Plasmodium falciparum. Malar J 2008; 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cholera R, Brittain NJ, Gillrie MR, et al. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci U S A 2008; 105:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chan JA, Howell KB, Reiling L, et al. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J Clin Invest 2012; 122:3227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chandrasiri UP, Randall LM, Saad AA, et al. Low antibody levels to pregnancy-specific malaria antigens and heightened cytokine responses associated with severe malaria in pregnancy. J Infect Dis 2014; 209:1408–17. [DOI] [PubMed] [Google Scholar]
- 40. Segbena AY, Kueviakoe I, Messie AK, et al. Hemoglobin anomalies at the university hospital center in Lome, Togo. Med Trop (Mars) 2002; 62:51–4. [PubMed] [Google Scholar]
- 41. Howes RE, Piel FB, Patil AP, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med 2012; 9:e1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams TN, Mwangi TW, Wambua S, et al. Negative epistasis between the malaria-protective effects of alpha+-thalassemia and the sickle cell trait. Nat Genet 2005; 37:1253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mpimbaza A, Walakira A, Ndeezi G, et al. Associations between erythrocyte polymorphisms and risks of uncomplicated and severe malaria in Ugandan children: A case control study. PLoS One 2018; 13:e0203229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.