Abstract
Background
Given very limited data, we assessed the long-term outcomes among patients with extensively drug-resistant (XDR) tuberculosis (TB).
Methods
A retrospective population-based cohort study was performed in patients with XDR-TB diagnosed during 2011–2013 in the country of Georgia. Data were abstracted from the National TB Program, medical charts, interviews, and the national Georgian death registry.
Results
Among 111 patients starting treatment for XDR-TB, 59 (53.2%) had newly diagnosed tuberculosis, and 3 (2.9%) had human immunodeficiency virus (HIV) coinfection. The median length of follow-up from diagnosis of XDR-TB to death or the end of study was 53.9 months (interquartile range, 27.2–66.3 months). End-of-treatment outcomes were available for 106 patients; 35 (33.0%) had a favorable outcome, and 71 (67.0%) had an unfavorable outcome, including death in 16 (15.1%). An additional 20 patients died after cessation of initial treatment, increasing the overall mortality rate to 34.0%. In multivariable analysis, an unfavorable initial end-of-treatment outcome was associated with posttreatment death (adjusted odds ratio, 14.41; 95% confidence interval, 1.78–117.13).
Conclusions
The overall mortality rate and specifically the posttreatment mortality rate were high among patients with XDR-TB. Patients with an unfavorable end-of-treatment outcome had an increased risk of death during follow-up. Our findings highlight the need for improved adherence, better-tolerated and shorter therapies, and enhanced posttreatment surveillance among patients treated for XDR-TB.
Keywords: extensively drug-resistant tuberculosis (XDR-TB), former soviet Republic, posttreatment mortality
In the country of Georgia, long-term outcomes among patients with extensively drug-resistant tuberculosis reveal significant rates of loss to follow-up and a high posttreatment mortality rate.
Drug-resistant tuberculosis (TB) is a major challenge to global TB control [1]. Treatment options for patients with TB resistant to isoniazid and rifampin (multidrug-resistant [MDR] TB), or furthermore to a fluoroquinolone and at least 1 injectable agent in addition to isoniazid and rifampin (extensively drug-resistant [XDR] TB) [1] are associated with significant toxicities, are costly, and require treatment durations up to 24 months [2]. Several countries have assessed end-of-treatment outcomes for MDR-TB and found significantly higher rates of poor outcomes than cases susceptible to first-line agents [3–10]. A 2010 meta-analysis of patients with XDR-TB found a favorable outcome rate (treatment outcome of cured or treatment completed as defined by the World Health Organization (WHO) [11]) of 44% (range, 18%–67%) [12], which is much lower than favorable outcome rates found for MDR-TB (62%) or drug-susceptible TB, where favorable outcomes generally exceed 90% [3, 13].
While initial end-of-treatment outcomes are available from a number of countries with a high burden of MDR-TB, there are very limited data and few studies that have assessed long-term outcomes (events that occur after cessation of treatment) among patients with XDR-TB. Additionally, little is known about what happens to patients with XDR-TB if they are lost to follow-up (LFU) or have treatment failure, and what proportion of these patients die or reenter care after leaving TB treatment. Only a very limited number of studies evaluating long-term outcomes of patients with XDR-TB have been carried out in South Africa, where many patients were coinfected with human immunodeficiency virus (HIV), and in South Korea [4, 14]. No studies have been conducted in former Soviet republics, which have the highest global rates of MDR/XDR TB [3].
The country of Georgia, a former Soviet republic, has a very high burden of drug-resistant tuberculosis, including MDR-TB and XDR-TB. From 2011 to 2013, between 9% and 11% of newly diagnosed TB cases and between 31% and 38% of retreatment cases in Georgia were found to be MDR-TB, and the proportion of XDR among MDR-TB during these years was 6%, 9%, and 17%, respectively [15–17]. A 2008 study analyzed end-of-treatment outcomes in 380 Georgian patients treated for MDR-TB and found a favorable outcome rate of 53% [6].
The purpose of our study was to assess end-of-treatment and long-term outcomes among patients with XDR-TB in the country of Georgia during a period just prior to the availability of new drugs. As Georgia and other countries have recently begun to roll out new and repurposed drugs for XDR-TB treatment (including bedaquiline, delamanid, linezolid, and imipenem-cisplatin) [18], this baseline knowledge of long-term outcomes will be valuable public health data for comparisons with outcomes achieved with treatment regimens including new and repurposed second-line drugs. We also aimed to evaluate risk factors for poor outcomes, as this information can advise practitioners and public health officials in Georgia and other countries on further effective interventions to improve drug-resistant TB management.
METHODS
A retrospective population-based cohort study of patients starting treatment for XDR-TB was performed from January 2011 through December 2013 in the country of Georgia. All patients had culture-confirmed Mycobacterium tuberculosis with XDR confirmed by first-line and second-line drug-susceptibility testing (DST). Patients with XDR tuberculosis received treatment through the Georgian National Center for Tuberculosis and Lung Disease (NCTLD) and associated National TB Program (NTP) treatment centers. All patients were initially hospitalized at the NCTLD (located in Tbilisi, Georgia) until either sputum culture conversion or substantial clinical improvement occurred. Subsequent outpatient care was administered at regional NTP centers. Institutional review board approval for this study was obtained from the NCTLD and Emory University.
Data were abstracted from both the NCTLD/NTP surveillance database and patient medical charts at the NCTLD and NTP treatment centers. Abstracted data included demographic data, medical history, chest radiographs, TB history (including previous diagnoses and treatment outcomes), treatment and hospitalization initiation and cessation dates, medication-related adverse events, and end-of-treatment outcomes. All treatment durations refer to initiation and use of second-line treatment.
Acid-fast bacilli smears (AFB) and cultures and DSTs were performed at the Georgian National TB Reference Laboratory in Tbilisi, Georgia. DSTs were performed in accordance with WHO-recommended methods [19] and as described elsewhere [20, 21]. DSTs were performed for the first-line agents: streptomycin, isoniazid, rifampicin, and ethambutol and the second-line agents: kanamycin, capreomycin, ofloxacin, ethionamide, and para-aminosalicylic acid (PAS). Pyrazinamide DST was not performed, but published data reveal that 90% of XDR tuberculosis strains in Georgia are resistant to pyrazinamide [22].
Patients were treated with directly observed therapy in accordance with the 2011 WHO treatment guidelines for drug-resistant TB [2]. Anti-TB medications available to patients during the study period included standard first-line agents as well as prothionamide, kanamycin, capreomycin, levofloxacin, moxifloxacin, cycloserine, PAS, amoxicillin-clavulanate, clarithromycin, and clofazamine. Bedaquiline was first introduced into use in Georgia in 2011 through a compassionate use program and was implemented into programmatic use in 2015 [23]. Delaminid was first used in early 2015, also through a compassionate use program, and in late 2015 was implemented into programmatic use [24]. Georgian NTP also implemented the use of linezolid and imipenem-cilastatin in July 2014 for the treatment of XDR-TB.
Because there were no available methods to rapidly detect resistance to second-line anti-TB agents, patients diagnosed with MDR-TB (by Hain MTBDRplus, Xpert MTB/RIF or previous 1st line DST) were started on empiric regimens that typically included pyrazinamide, a fluoroquinolone, an injectable agent, prothionamide, and either cycloserine or PAS. Once the results of the second-line DST were available (on average by the end of 2 months of MDR-TB empiric treatment), the clinical team would individualize the regimen using the limited additional drug options. Despite ofloxacin resistance, fluoroquinolones (either moxifloxacin or levofloxacin) were often still used in treatment regimens. An injectable agent (either kanamycin or capreomycin) was also used in nearly all treatment regimens, even if DSTs showed resistance to both agents. In addition, at time of XDR-TB diagnosis, amoxicillin-clavulanate, clarithromycin, and/or clofazamine were added to the regimen, with the overall aim of treating the patients with ≥4 second-line agents likely to be effective.
Initial end-of-treatment outcomes were assigned by the treating physician at the cessation of treatment, in accordance with 2013–2014 WHO-defined treatment outcomes [11]. A favorable outcome was defined as cure or completion of treatment. A poor or unfavorable treatment outcome was defined as loss to follow-up (LFU), treatment failure, or death [11]. We defined final sputum culture conversion as 2 negative sputum cultures ≥30 days apart, with the date being the first of the 2 negative cultures. For patients who had culture reversion during treatment and then went on to achieve conversion, the time to culture conversion was measured from the start of treatment until final culture conversion.
Long-term outcomes were assessed based on events after cessation of initial treatment. Specifically, we evaluated whether patients reentered TB treatment and if they had died by the end of the follow-up period. These long-term outcomes were collected by the following methods: review of medical records at the NCTLD and affiliated centers; contact of patients’ physicians at NCTLD and other TB treatment centers in Georgia; contact of patients with XDR-TB via telephone by a study team member from the NCTLD; search for all patients with a diagnosis of XDR-TB in the Georgian death registry, part of the State Department of Statistics, for all-cause mortality and date of death (last search date, 5 November 2017). Patients who did not have a death certificate confirming death were assumed to be alive if there was no other confirmation from the NCTLD or family that they had died outside Georgia. Patients initially labeled in the Georgian NTP database as “transferred out” were assigned a WHO end-of-treatment outcome and long-term outcome if follow-up data became available from the patient or NCTLD physician. Otherwise they were excluded from analyses.
Data Analysis
All data were abstracted onto a standardized data collection form and then entered into an online Research Electronic Data Capture (REDCap) database [25]. Data analyses were performed using IBM SPSS Statistics 24 and SAS 9.4 software. Univariate analysis was carried out using logistic regression for variables of interest to assess risk factors for poor initial end-of-treatment outcomes and posttreatment mortality. A multivariable logistic regression model was used to evaluate the independent association of potential risk factors with poor outcomes. Model building and selection was based on the purposeful selection of covariates strategy, as described elsewhere, based on epidemiological findings in the univariate analysis, biological plausibility, and variables previously found be associated with poor end-of-treatment outcomes [26]. Kaplan-Meier curves were used to estimate long-term survival based on initial end-of-treatment outcome, and log-rank tests were used to compare Kaplan-Meier curves. Differences were considered significant at P ≤ .05 .
RESULTS
Patient Characteristics
Between 2011 and 2013, a total of 111 patients started treatment for XDR-TB (2011, n = 28; 2012, n = 31; and 2013, n = 52) in Georgia. These patients received treatment at 21 NTP-affiliated treatment centers in 9 regions throughout Georgia. Patient demographics are presented in Table 1. More than half of patients with XDR-TB (59 [53.2%]) had no previous history of tuberculosis, and 52 (46.8%) were retreatment TB cases, including 23 (20.7%) who had previously been treated with second-line agents. Ninety-eight patients (88.3%) had pulmonary TB, 5 (4.5%) had isolated extrapulmonary TB (pleural in 4 and urogenital in 1), and 8 (7.2%) had a combination of pulmonary and extrapulmonary TB (pleural in 7 and an unknown extrapulmonary site in 1). Eighty-four patients (76.0%) had positive AFB microscopy smears at diagnosis. Baseline chest radiography shows bilateral disease in 48 patients (43.2%), cavitary disease in 48 (43.2%), and fibrosis in 19 (17.1%).
Table 1.
Baseline Characteristics in Patients Starting TreatmentforExtensively Drug-Resistant Tuberculosis in the Country of Georgia
| Characteristic | Patients, No. (%)a |
|---|---|
| Age, median (IQR), y | 34.0 (26.5–49.4) |
| Sex | |
| Male | 77 (69.4) |
| Female | 34 (30.6) |
| BMI, median (IQR), kg/m2 | 20.4 (18.5–22.6) |
| Family status | |
| Single | 42 (38.5) |
| Married | 54 (49.5) |
| Divorced/separated | 8 (7.3) |
| Widowed | 5 (4.6) |
| Employment status | |
| Employed | 16 (14.5) |
| Unemployed | 81 (73.6) |
| Pensioner | 9 (8.2) |
| Student | 4 (3.6) |
| History of incarceration | 34 (30.6) |
| Tobacco use | 48 (43.6) |
| Alcohol use | |
| Never | 63 (59.4) |
| Moderate | 34 (32.1) |
| Excessive (>5 drinks/d) | 9 (8.5) |
| History of intravenous drug use | 3 (3.2) |
| HCV antibody positive | 23 (20.7) |
| HIV disease | 3 (2.9) |
| Other comorbid conditions | |
| Cardiovascular disease | 3 (2.7) |
| Diabetes mellitus | 9 (8.1) |
| Renal disease | 1 (0.9) |
| Thyroid disease | 8 (7.2) |
| HBV core antibody positive | 4 (3.6) |
| History of malignancy | 2 (1.8) |
| Psychiatric disease | 9 (8.1) |
| Known MDR tuberculosis contact | 26 (23.6) |
| Tuberculosis location | |
| Pulmonary | 98 (88.3) |
| Pulmonary and extrapulmonary | 8 (7.2) |
| Extrapulmonaryb | 5 (4.5) |
| XDR tuberculosis case type | |
| Newly diagnosed | 59 (53.2) |
| Retreatment | 52 (46.8) |
| Previous treatment | |
| First-line drugs only | 29 (55.8) |
| First- and second-line drugs | 23 (44.2) |
Abbreviations: BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; MDR, multidrug-resistant; XDR, extensively drug-resistant.
aData represent no. (%) of patients unless otherwise specified.
bExtrapulmonary locations were pleural in 4 patients and urogenital in 1.
Treatment Characteristics and Initial End-of-Treatment Outcomes
The median number of drugs to which the 111 M. tuberculosis isolates were resistant was 7 (interquartile range [IQR], 7–8), including 12 isolates (10.8%) with resistance to all 9 anti-TB drugs tested. Patients with XDR-TB were treated with a median of 10 anti-TB medications (IQR, 8–11) during the course of their XDR-TB treatment. The median (IQR) duration of hospitalization was 1.7 (1.3–3.5) months, and the median duration of second-line treatment was 16.3 (6.3–23.5) months. The median (IQR) number of cultures obtained during treatment was 10 (4–17). Six patients (6%) acquired additional drug resistance during treatment (2 each to PAS, capreomycin, and ethionamide). Adjunctive lung resection surgery was performed in 15 patients (13.5%).
End-of-treatment outcomes were available for 106 (95.5%) of 111 patients. Among these 106 patients, 35 (33.0%) had a favorable outcome (22 [20.8%] were cured and13 [12.3%] completed treatment), and 71 (67.0%) had an unfavorable treatment outcome (15 [14.2%] had treatment failure, 40 [37.7%] were LFU, and 16 [15.1%] died). The remaining 5 patients had unknown treatment outcomes after transferring outside Georgia. Among the 40 patients LFU, the median (IQR) duration of treatment was 6.1 (4.4–12.1) months, and only 10 patients (25.0%) received ≥12 months of treatment. At time of LFU, 19 (47.5%) of 40 patients had a positive culture for M. tuberculosis. During treatment, 42 (41.6%) of 101 patients with pulmonary TB had a final culture conversion, with a median (IQR) time to conversion of 2 (1–4.75) months. For the 6 patients who acquired additional drug resistance during treatment, 4 (66.7%) had unfavorable outcomes, including 1 treatment failure and 3 deaths.
No patients received the new or repurposed agents as part of initial XDR-TB treatment, but 6 patients received ≥1 of these later on during the course of therapy. Specific uses included 3 linezolid, 4 bedaquiline, 1 delaminid and 1 imipenem/cilastatin. End-of-treatment outcomes for these patients included 5 (83.3%) with cured and 1 (16.7%) LFU.
In univariate analysis, risk factors for poor end-of-treatment outcomes included age, male sex, tobacco use, history of incarceration, receiving retreatment for tuberculosis, presence of bilateral disease on chest radiograph, absence of culture conversion, and fewer months of second-line treatment (Table 2). In multivariate analysis, risk factors for an unfavorable end-of-treatment outcome included history of incarceration (adjusted odds ratio [aOR], 5.66; 95% confidence interval, 1.18–27.19) and absence of culture conversion (0.19; .06–.54) (Table 3).
Table 2.
End-of-Treatment Outcomes Among Patients With Extensively Drug-Resistant Tuberculosis, With Univariate Analysis of Risk Factors for Unfavorable Outcomes
| Variable | End-of-Treatment Outcome, No. (%)a | Univariate Analysis | ||
|---|---|---|---|---|
| Favorable (n = 35) | Unfavorable (n = 71) | OR (95% CI) | P Valueb | |
| Patient characteristics | ||||
| Age median (IQR), y | 28.6 (24.3–39.7) | 39.0 (29.8–51.9) | 1.05 (1.01–1.08) | .006 |
| Male sex | 17 (48.6) | 56 (78.9) | 3.95 (1.65–9.47) | .002 |
| Unemployed | 23 (65.7) | 53 (75.7) | 1.63 (.67–3.95) | .28 |
| Tobacco use | 7 (20) | 38 (54.3) | 4.75 (1.83–12.31) | .001 |
| Alcohol use | 10 (28.6) | 32 (47.8) | 2.29 (.95–5.49) | .06 |
| History of injection drug use | 0 (0) | 3 (5.1) | .55 | |
| History of incarceration | 3 (8.6) | 31 (43.7) | 8.27 (2.32–29.52) | <.001 |
| Started treatment while incarcerated | 0 (0) | 15 (21.1) | … | .002c |
| HCV antibody positive | 4 (11.4) | 19 (26.8) | 2.83 (.88–9.09) | .08 |
| HIV positive | 1 (3.0) | 2 (2.9) | 0.97 (.09–11.09) | >.99c |
| Diabetes mellitus | 2 (5.7) | 6 (8.5) | 1.52 (.29–7.96) | .62 |
| BMI ≤18.5 kg/m2 | 6 (18.8) | 19 (28.4) | 1.72 (.61–4.83) | .31 |
| Retreatment tuberculosis case | 8 (22.9) | 41 (57.7) | 4.61 (1.84–11.56) | .001 |
| Bilateral disease on radiograph | 7 (20.6) | 38 (54.3) | 4.58 (1.76–11.90) | .001 |
| Extrapulmonary diseased | 5 (14.3) | 8 (11.3) | 0.58 (.20–1.72) | .33 |
| No. of resistant agents, median (IQR) | 7 (7–8) | 7 (7–8) | 1.14 (.70–1.86) | .59 |
| MDR tuberculosis contact | 8 (22.9) | 15 (21.4) | 0.92 (.35–2.44) | .87 |
| Treatment characteristics | ||||
| Adjunctive surgery | 9 (25.7) | 6 (8.5) | 0.27 (.09–.82) | .03c |
| Final culture conversione | 26 (83.9) | 16 (22.9) | 0.06 (.02–.17) | <.001 |
| Time to culture conversion, median (IQR), mo | 2.5 (1–4.75) | 1.5 (1–4.25) | 0.99 (.87–1.12) | .86 |
| Culture reversion | 5 (17.9) | 9 (39.1) | 2.76 (.77–9.85) | .11 |
| Significant adverse event reported | 8 (22.9) | 7 (9.9) | 0.37 (.12–1.12) | .08 |
| Additional drug resistance acquired during treatmentf | 2 (6.1) | 4 (6.6) | 1.09 (.19–6.28) | .93 |
| Duration of hospitalization, median (IQR), mo | 1.6 (1.2–3.1) | 2.2 (1.4–3.6) | 1.04 (.91–1.19) | .58 |
| Duration of second-line treatment, median (IQR), mo | 23.8 (21.4–24.8) | 7.6 (5.1–16.5) | 0.79 (.72–.87) | <.001 |
Abbreviations: BMI, body mass index; CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; MDR, multidrug-resistant; OR, odds ratio.
aFavorable and unfavorable end-of-treatment outcomes are defined in Methods. Data represent no. (%) of patients unless otherwise specified.
bSignificant at P ≤ .05, with P values obtained using χ2 tests unless noted.
cFisher exact test was used because the expected cell count was <5.
dIncluding patients with isolated extrapulmonary tuberculosis and those with pulmonary and extrapulmonary tuberculosis.
ePatients with extrapulmonary tuberculosis were excluded
fPatients who were already resistant to all 9 agents were excluded.
Table 3.
Multivariate Model of Risk Factors for Unfavorable End-of-Treatment Outcomes Among Patients With Extensively Drug-Resistant Tuberculosisa
| Variable | Multivariate Analysis | |
|---|---|---|
| aOR (95% CI) | P Valueb | |
| Age (median [IQR], y) | 1.04 (.99–1.07) | .06 |
| Male sex | 1.09 (.34–3.54) | .88 |
| Tobacco use | 2.10 (.62–7.13) | .24 |
| History of incarceration | 5.66 (1.18–27.19) | .03 |
| Retreatment tuberculosis case | 2.44 (.77–7.73) | .13 |
| Final culture conversionc | 0.19 (.06–.54) | .002 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; IQR, interquartile range.
aOther variables considered in the model included alcohol use, bilateral disease on chest radiograph, and adjunctive surgery.
bSignificant at P ≤ .05.
cExcluding patients with extrapulmonary tuberculosis.
Long-term Outcomes
The median (IQR) length of follow-up from date of tuberculosis diagnosis to death or the end of the study was 53.9 (27.2–66.3) months. After cessation of initial XDR-TB, an additional 20 (22.2%) of 90 patients alive at the end of treatment died. Overall, 36 (34.0%) of 106 patients with XDR-TB died during the study period.
A total of 24 (26.7%) of 90 patients alive at the end of initial treatment reentered TB care: 11 (45.8%) after being LFU, 9 (37.5%) after treatment failure, and 4 (16.7%) after cure or completed treatment (ie, relapse). For the 4 patients with relapse, the median (IQR) time to relapse from initial treatment cessation was 21.6 (15.7–26.0) months. Retreatment outcomes for patients initially LFU who reentered care 1 cured, 2 currently on treatment, 2 LFU, 1 treatment failure and 5 deaths. For those patients who initially failed treatment, retreatment outcomes were: 4 cured, 1 LFU, 4 died. For those patients who relapsed, retreatment outcomes were: 3 treatment completed, 1 currently on treatment.
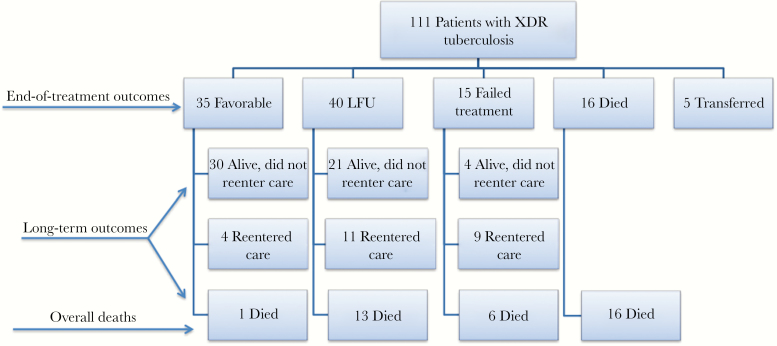
Among the 36 patients with XDR-TB who died during the study period, 16 died during the initial treatment regimen for XDR-TB, and 20 patients died after cessation of treatment. For the latter 20 additional patients, the median (IQR) time to death from the initial treatment cessation was 7.5 (4.8–22.9) months, and 19 (95%) had poor initial end-of-treatment outcomes (13 LFU and 6 with treatment failure). Nine (45%) of these patients died after reentering tuberculosis treatment. The 1 patient with a favorable end-of-treatment outcome who died was an 81-year-old woman with cardiovascular disease and a positive hepatitis C virus antibody who died 27 months after successfully completing treatment. A flowchart of long-term outcomes is displayed in Figure 1.
Figure 1.
Flowchart of end-of-treatment and long-term outcomes among patients with extensively drug-resistant (XDR) tuberculosis. Among 21 patients lost to follow-up (LFU) who were alive at end of the study period and did not reenter care, the median (interquartile range [IQR]) duration of initial treatment was 9.8 (5.4–14.6) months. The median (IQR) duration of initial treatment for 13 LFU patients who died during the study period was 5.3 (2.7–7.2) months; 5 of 13 deaths among patients LFU occurred after reentry to care, as did 4 of 6 deaths among patients with treatment failure.
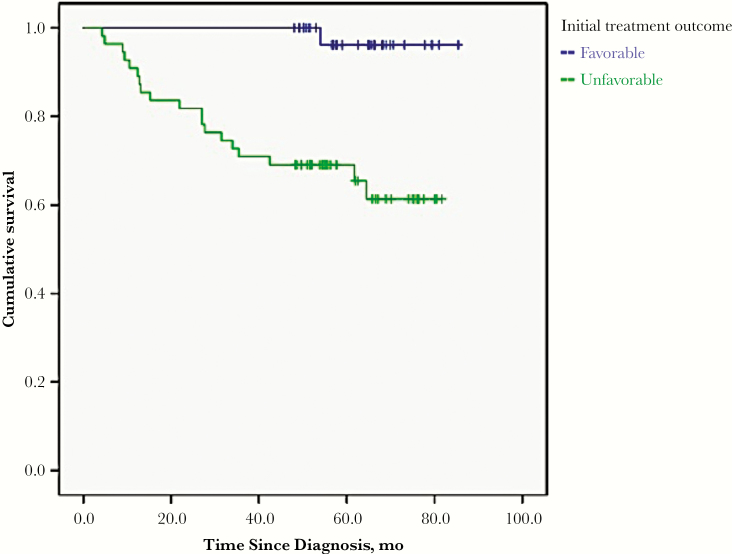
In univariate analysis, risk factors for posttreatment mortality included unfavorable end-of-treatment outcome, fewer months of treatment, absence of culture conversion, and increasing age (Table 4). In multivariable logistic regression, the risk factor for posttreatment mortality was an unfavorable initial end-of-treatment outcome (aOR, 14.41; 95% confidence interval, 1.78–117.13) (Table 5). As demonstrated by a Kaplan-Meier curve (Figure 2), patients with an initial unfavorable end-of-treatment outcome had a higher mortality rate than those with an initial favorable end-of-treatment outcome (log-rank P < .001).
Table 4.
Univariate Analysis of Risk Factors for All Cause Mortality Among Patients With Extensively Drug-Resistant Tuberculosis After Cessation of Initial Treatment
| Variable | Patients, No. (%)a | Univariate Analysis | ||
|---|---|---|---|---|
| Alive at End of Study (n = 70) | Posttreatment Death (n = 20) | OR (95% CI) | P Valueb | |
| Patient characteristics | ||||
| Age (years), median (IQR), y | 31 (25.2–44.3) | 41.2 (30–51.6) | 1.04 (1.01–1.07) | .05 |
| Male sex | 46 (65.7) | 16 (80) | 2.09 (.63–6.94) | .23 |
| Unemployed | 48 (69.6) | 16 (80) | 1.75 (.52–5.87) | .36 |
| Tobacco use | 29 (42) | 10 (50) | 1.38 (.51–3.74) | .53 |
| Alcohol use | 25 (37.3) | 10 (52.6) | 1.87 (.67–5.22) | .23 |
| History of injection drug use | 2 (3.3) | 1 (6.3) | 3.15 (.65–15.25) | .51c |
| History of incarceration | 21 (30) | 8 (40) | 1.56 (.56–4.36) | .40 |
| Started treatment while incarcerated | 12 (17.1) | 1 (5) | 0.25 (.03–2.09) | .28c |
| HCV antibody positive | 13 (18.6) | 6 (30) | 1.88 (.61–5.82) | .27 |
| HIV antibody positive | 2 (3) | 1 (5) | 1.71 (.15–19.91) | .55c |
| Diabetes mellitus | 4 (5.7) | 3 (15) | 2.91 (.59–14.27) | .18c |
| BMI ≤18.5 kg/m2 | 13 (20.3) | 4 (21.1) | 1.05 (.30–3.69) | >.99c |
| Retreatment tuberculosis case | 28 (40) | 12 (60) | 2.25 (.82–6.21) | .12 |
| Bilateral disease on radiograph | 25 (36.8) | 9 (45) | 1.41 (.51–3.86) | .51 |
| Extrapulmonary diseased | 9 (12.9) | 3 (15) | 1.20 (.29–4.91) | .73c |
| No. of resistant agents, median (IQR) | 7 (7–8) | 8 (7–8) | 1.23 (.68–2.22) | .49 |
| MDR tuberculosis contact | 14 (20.3) | 5 (25) | 1.31 (.41–4.22) | .76c |
| Treatment characteristics | ||||
| Adjunctive surgery | 12 (17.1) | 0 (0) | .06c | |
| Final culture conversione | 38 (58.5) | 3 (15) | 0.13 (.03–.47) | .005 |
| Time to culture conversion, median (IQR), mo | 2 (1–4.75) | 3 (2–4) | 0.95 (.70–1.28) | .73 |
| Culture reversion | 9 (21.4) | 1 (33.3) | 1.83 (.15–22.58) | .54c |
| Significant adverse event reported | 11 (15.7) | 2 (10) | 0.60 (.12–2.94) | .73c |
| Additional drug resistance acquired during treatmentf | 2 (3.1) | 3 (17.6) | 6.75 (1.03–44.26) | .06c |
| Duration of hospitalization, median (IQR), mo | 1.6 (1.2–3.1) | 2.2 (1.4–3.7) | 1.07 (.93–1.22) | .35 |
| Duration of second-line treatment, median (IQR), mo | 20.8 (10.3–24.3) | 7.3 (5–14.4) | 0.90 (.85–.96) | .002 |
| Unfavorable end-of-treatment outcomeg | 36 (51.4) | 19 (95) | 17.94 (2.28–141.47) | .006 |
Abbreviations: BMI, body mass index; CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; MDR, multidrug-resistant; OR, odds ratio.
aData represent no. (%) of patients unless otherwise specified.
bSignificant at P ≤ .05, with P values obtained using χ2 tests unless otherwise noted.
cFisher exact test was used because the expected cell count was <5.
dIncluding patients with isolated extrapulmonary tuberculosis and those with pulmonary and extrapulmonary tuberculosis.
eExcluding patients with extrapulmonary tuberculosis.
fPatients who were already resistant to all 9 agents were excluded.
gPatients who were lost to follow-up or in whom treatment failed.
Table 5.
Multivariate Model of Risk Factors for Death After Cessation of Initial Extensively Drug-Resistant Tuberculosis Treatment
| Variablea | Multivariate Analysis | |
|---|---|---|
| aOR (95% CI) | P Value | |
| Age (median [IQR], y) | 1.03 (.99–1.07) | .16 |
| Retreatment tuberculosis case | 1.40 (0.46–4.32) | .56 |
| Unfavorable end-of-treatment outcomeb | 14.41 (1.78–117.13) | .01 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; IQR, interquartile range.
aFinal culture conversion was the other variable considered in the model.
bUnfavorable outcomes included loss to follow-up or failed treatment.
Figure 2.
Long-term survival among patients with extensively drug-resistant tuberculosis stratified by favorable versus unfavorable initial end-of-treatment outcome. (Log-rank P < .001.)
DISCUSSION
There have been very limited data on posttreatment follow-up and long-term outcomes in patients with XDR-TB. Our study is the first, to our knowledge, to evaluate long-term outcomes in patients with XDR-TB in the former Soviet Union, an area of the world with the highest rates of drug-resistant TB [3]. The overall long-term mortality rate was high (34.0%), and an important finding in our study was that the majority of deaths among those with XDR-TB occurred after treatment. An independent risk factor for long-term mortality was a poor end of initial XDR-TB treatment outcome, and all but 1 posttreatment death occurred in patients who had unfavorable initial end-of-treatment outcomes, with 16 of 20 (80.0%) having a positive culture at the end of initial therapy. Given that many patients died after cessation of TB care, the mortality rate among persons with XDR-TB in Georgia and elsewhere has previously been underestimated.
Our study also found that more than half of the patients with XDR-TB in Georgia had no history of prior XDR-TB, indicating that primary transmission of XDR-TB is the main method of disease transmission in Georgia. We hypothesize that this may be related to prolonged infectiousness among patients with XDR-TB, which is due to delays in diagnosis as well as transmission from patients who are LFU and who have treatment failure and remain culture positive at cessation of treatment. Patients who have not completed treatment for highly drug-resistant TB pose a serious public health problem, given that many of them remain culture positive and thus infectious. In addition to our experience in Georgia, Dheda and colleagues [27] in South Africa also found a significant number of patients with XDR-TB who were not cured were alive at 12 months after end of treatment, and they were able to demonstrate direct downstream transmission of XDR-TB in the community as a result.
Long-term outcomes among patients with XDR-TB have previously been reported from only 2 sites. Our findings in Georgia reflect those from a study in South Korea [4], which found a LFU rate of 32% and an overall mortality rate of 49.3% among a cohort of XDR-TB patients with a 3–7-year follow-up period, with approximately 50% of deaths occurring during the follow-up period. In South Africa, Pietersen and colleagues [14] found a 73% mortality rate at 60 months of follow-up, with more than half of deaths occurring in the first 24 months. We believe that the lower long-term mortality rate among patients in Georgia with XDR tuberculosis (34%) is probably due to a much higher prevalence of HIV infection among patients with TB in the South Africa study (41.0% vs <3% in our Georgian study). In addition, 89.0% of patients in South Africa had a history of previous second-line treatment (retreatment cases), compared with 20.7% of patients with XDR XDR-TB in Georgia.
Our study is the first to assess risk factors for posttreatment mortality among persons with XDR-TB. In multivariate analysis, we found that an unfavorable outcome at the end of treatment (among patients alive at the end of treatment) was a major predictor for posttreatment death (aOR, 14.41). The high prevalence of LFU and poor prognosis among patients with XDR-TB emphasizes the need for further public health interventions to contact these patients, who frequently remain infectious and at risk for further transmission of XDR-TB. The use of new and repurposed drugs for the treatment of M/XDR-TB provides options for shorter treatment regimens and the possibility of future regimens that do not include an injectable agent [18, 28]. Further investigations for the high prevalence of LFU is also needed to help craft further interventions to decrease this prevalence, improve treatment outcomes, and reduce the risk of primary transmission of XDR-TB.
We were able to use several methods to assess for mortality, including patient and physician interviews and use of the Georgian national death registry. This registry is an electronic database where official death certificates are created and sent to the State Department of Statistics in 2 ways: from relatives of the deceased to the State Office for Statistics and healthcare institution data sent to the Centre for Medical Statistics and Information [29]. We believe that this system is up to date and accurate, and all patients who were listed as deceased in the NCTLD database and medical records had a corresponding death certificate. Death certificates are a valuable method of estimating mortality rate among patients with TB, particularly in settings where contacting patients may not be feasible and where death registries are well established, as in Georgia. This system was also used in the study performed in South Korea [4]. The public health importance and impact of having a well-functioning death registry in Georgia can provide an example for other low- and middle-income countries.
Our study is subject to several limitations. First, patients who were LFU or died of XDR-TB before treatment initiation would have been missed. A 2014 meta-analysis found pretreatment rates of loss to follow-up between 4% and 38% [30]. Second, alcohol, tobacco, and intravenous drug use were self-reported and thus may have been underreported in this study population. Next, about a third of patients with XDR-TB could not be contacted; however, we were able to use the country’s death registry as our final determinate of death, unless we had evidence that a patient died outside Georgia (which occurred in 1 patient). Finally, for some patients, the cause of death was unavailable, so reasons other than TB cannot be excluded. However, based on information we obtained from the database and medical charts as well as interviews with family members, we suspect that TB was associated with the vast majority of deaths.
In conclusion, in Georgia, long-term outcomes in patients with XDR-TB are poor, with low rates of initial treatment success, a significant proportion LFU, and a 34.0% long-term mortality rate. Many patients with XDR-TB remain alive and infectious after treatment cessation and thus pose a public health threat. The results of our study highlight the need for better surveillance during and after treatment for drug-resistant TB. Additional interventions are needed to prevent patients from dropping out of care, particularly when they are still culture positive, and more research is needed to assess why patients are LFU during treatment and the impact on disease transmission these patients may have. After treatment cessation, public health resources should focus on continual monitoring of patients, given a significant risk of death and relapse. With the availability of new and repurposed drugs and shorter treatment regimens, it is hoped that outcomes will be improved and LFU and treatment failure reduced. Our data provide important baseline data for assessing the impact of these new treatment regimens for XDR-TB, which continue to be rolled out in Georgia.
Acknowledgments
We thank the National Center for Tuberculosis and Lung Disease employees who helped us gain access to the Georgia national death registry.
Author contributions.M. F., N. L., R. R. K., and H. M. B. designed the study. N. L. and Z. A. oversaw data collection in the country of Georgia and obtained access to the death registry. M. F., N. A., and E. K. performed data collection. M. F. performed statistical analysis with assistance from R. R. K. and wrote the manuscript with assistance from R. R. K. and H. M. B. All authors contributed to the editing.
Financial support. This study was supported in part by the Fogarty International Center (grant D43TW007124), the National Institute of Allergy and Infectious Diseases (grants K23AI103044 and R21AI122001), the National Center for Advancing Translational Sciences (grants UL1TR000454 to the Atlanta Clinical and Translational Science Institute and UL1TR002378 to the Georgia Clinical and Translational Science Alliance fall under the grant agency National Center for Advancing Translational Sciences), Institute for Clinical and Translational Research (grant UL1TR000424), and the Emory University School of Medicine Dean’s Office.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 2. World Health Organization. Guidelines for the programmatic management of drug resistant tuberculosis: 2011 update. Geneva, Switzerland: World Health Organization; 2011. [PubMed] [Google Scholar]
- 3. World Health Organization. Global tuberculosis report 2018. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 4. Kim DH, Kim HJ, Park SK, et al. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med 2008; 178:1075–82. [DOI] [PubMed] [Google Scholar]
- 5. Kuksa L, Riekstina V, Leimane V, et al. Multi- and extensively drug-resistant tuberculosis in Latvia: trends, characteristics and treatment outcomes. Public Health Action. 2014; 4:S47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gegia M, Kalandadze I, Kempker RR, et al. Adjunctive surgery improves treatment outcomes among patients with multidrug-resistant and extensively drug-resistant tuberculosis. Int J Infect Dis 2012; 16:e391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Migliori GB, Besozzi G, Girardi E, et al. ; SMIRA/TBNET Study Group Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J 2007; 30:623–6. [DOI] [PubMed] [Google Scholar]
- 8. Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006; 368:1575–80. [DOI] [PubMed] [Google Scholar]
- 9. Alene KA, Yi H, Viney K, et al. Treatment outcomes of patients with multidrug-resistant and extensively drug resistant tuberculosis in Hunan Province, China. BMC Infect Dis 2017; 17:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kvasnovsky CL, Cegielski JP, van der Walt ML. Treatment outcomes for patients with extensively drug-resistant tuberculosis, KwaZulu-Natal and Eastern Cape Provinces, South Africa. Emerg Infect Dis 2016; 22:10.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Definitions and reporting framework for tuberculosis—2013 revision (updated December 2014). Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 12. Jacobson KR, Tierney DB, Jeon CY, et al. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2010; 51:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis 2009; 9:153–61. [DOI] [PubMed] [Google Scholar]
- 14. Pietersen E, Ignatius E, Streicher EM, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet 2014; 383:1230–9. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. Global tuberculosis report 2012. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 16. World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 17. World Health Organization. Global tuberculosis report 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 18. Rendon A, Tiberi S, Scardigli A, et al. Classification of drugs to treat multidrug-resistant tuberculosis (MDR-TB): evidence and perspectives. J Thorac Dis 2016; 8:2666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. Policy guidance on drug susceptibility testing (DST) of second-line antituberculosis drugs. Geneva, Switzerland: World Health Organization; 2008. [PubMed] [Google Scholar]
- 20. Parsons LM, Somoskövi A, Gutierrez C, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev 2011; 24:314–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tukvadze N, Kempker RR, Kalandadze I, et al. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLoS One. 2012; 7:e31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allana S, Shashkina E, Mathema B, et al. pncA gene mutations associated with pyrazinamide resistance in drug-resistant tuberculosis, South Africa and Georgia. Emerg Infect Dis 2017; 23:491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization. The use of bedaquiline in the treatment of multi-drug resistant tuberculosis: interim policy guidance. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 24. World Health Organization. The use of delamanid in the treatment of multi-drug resistant tuberculosis: interim policy guidance. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 25. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hosmer DW, Lemeshow S.. Applied logistic regression. 2nd ed.New York, NY: SAGE Publications, Inc; 2000. [Google Scholar]
- 27. Dheda K, Limberis JD, Pietersen E, et al. Outcomes, infectiousness, and transmission dynamics of patients with extensively drug-resistant tuberculosis and home-discharged patients with programmatically incurable tuberculosis: a prospective cohort study. Lancet Respir Med 2017; 5:269–81. [DOI] [PubMed] [Google Scholar]
- 28. Tiberi S, Scardigli A, Centis R, et al. Classifying new anti-tuberculosis drugs: rationale and future perspectives. Int J Infect Dis 2017; 56:181–4. [DOI] [PubMed] [Google Scholar]
- 29. Arax Hovhannesyan PM, Dadu A. Analysis of the epidemiological impact of tuberculosis in Georgia 2015. Available at: http://www.euro.who.int/__data/assets/pdf_file/0010/321949/Analysis-epidemiological-impact-TBC-Georgia.pdf. Accessed 1 October 2018.
- 30. MacPherson P, Houben RM, Glynn JR, et al. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ 2014; 92:126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]