Abstract
Background
Salivary gland dysfunction is an 'umbrella' term for the presence of either xerostomia (subjective sensation of dryness), or salivary gland hypofunction (reduction in saliva production). It is a predictable side effect of radiotherapy to the head and neck region, and is associated with a significant impairment of quality of life. A wide range of pharmacological interventions, with varying mechanisms of action, have been used for the prevention of radiation‐induced salivary gland dysfunction.
Objectives
To assess the effects of pharmacological interventions for the prevention of radiation‐induced salivary gland dysfunction.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 14 September 2016); the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library (searched 14 September 2016); MEDLINE Ovid (1946 to 14 September 2016); Embase Ovid (1980 to 14 September 2016); CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 14 September 2016); LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 to 14 September 2016); Zetoc Conference Proceedings (1993 to 14 September 2016); and OpenGrey (1997 to 14 September 2016). We searched the US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform for ongoing trials. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
We included randomised controlled trials, irrespective of their language of publication or publication status. Trials included participants of all ages, ethnic origin and gender, scheduled to receive radiotherapy on its own or in addition to chemotherapy to the head and neck region. Participants could be outpatients or inpatients. We included trials comparing any pharmacological agent regimen, prescribed prophylactically for salivary gland dysfunction prior to or during radiotherapy, with placebo, no intervention or an alternative pharmacological intervention. Comparisons of radiation techniques were excluded.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 39 studies that randomised 3520 participants; the number of participants analysed varied by outcome and time point. The studies were ordered into 14 separate comparisons with meta‐analysis only being possible in three of those.
We found low‐quality evidence to show that amifostine, when compared to a placebo or no treatment control, might reduce the risk of moderate to severe xerostomia (grade 2 or higher on a 0 to 4 scale) at the end of radiotherapy (risk ratio (RR) 0.35, 95% confidence interval (CI) 0.19 to 0.67; P = 0.001, 3 studies, 119 participants), and up to three months after radiotherapy (RR 0.66, 95% CI 0.48 to 0.92; P = 0.01, 5 studies, 687 participants), but there is insufficient evidence that the effect is sustained up to 12 months after radiotherapy (RR 0.70, 95% CI 0.40 to 1.23; P = 0.21, 7 studies, 682 participants). We found very low‐quality evidence that amifostine increased unstimulated salivary flow rate up to 12 months after radiotherapy, both in terms of mg of saliva per 5 minutes (mean difference (MD) 0.32, 95% CI 0.09 to 0.55; P = 0.006, 1 study, 27 participants), and incidence of producing greater than 0.1 g of saliva over 5 minutes (RR 1.45, 95% CI 1.13 to 1.86; P = 0.004, 1 study, 175 participants). However, there was insufficient evidence to show a difference when looking at stimulated salivary flow rates. There was insufficient (very low‐quality) evidence to show that amifostine compromised the effects of cancer treatment when looking at survival measures. There was some very low‐quality evidence of a small benefit for amifostine in terms of quality of life (10‐point scale) at 12 months after radiotherapy (MD 0.70, 95% CI 0.20 to 1.20; P = 0.006, 1 study, 180 participants), but insufficient evidence at the end of and up to three months postradiotherapy. A further study showed no evidence of a difference at 6, 12, 18 and 24 months postradiotherapy. There was low‐quality evidence that amifostine is associated with increases in: vomiting (RR 4.90, 95% CI 2.87 to 8.38; P < 0.00001, 5 studies, 601 participants); hypotension (RR 9.20, 95% CI 2.84 to 29.83; P = 0.0002, 3 studies, 376 participants); nausea (RR 2.60, 95% CI 1.81 to 3.74; P < 0.00001, 4 studies, 556 participants); and allergic response (RR 7.51, 95% CI 1.40 to 40.39; P = 0.02, 3 studies, 524 participants).
We found insufficient evidence (that was of very low quality) to determine whether or not pilocarpine performed better or worse than a placebo or no treatment control for the outcomes: xerostomia, salivary flow rate, survival, and quality of life. There was some low‐quality evidence that pilocarpine was associated with an increase in sweating (RR 2.98, 95% CI 1.43 to 6.22; P = 0.004, 5 studies, 389 participants).
We found insufficient evidence to determine whether or not palifermin performed better or worse than placebo for: xerostomia (low quality); survival (moderate quality); and any adverse effects.
There was also insufficient evidence to determine the effects of the following interventions: biperiden plus pilocarpine, Chinese medicines, bethanechol, artificial saliva, selenium, antiseptic mouthrinse, antimicrobial lozenge, polaprezinc, azulene rinse, and Venalot Depot (coumarin plus troxerutin).
Authors' conclusions
There is some low‐quality evidence to suggest that amifostine prevents the feeling of dry mouth in people receiving radiotherapy to the head and neck (with or without chemotherapy) in the short‐ (end of radiotherapy) to medium‐term (three months postradiotherapy). However, it is less clear whether or not this effect is sustained to 12 months postradiotherapy. The benefits of amifostine should be weighed against its high cost and side effects. There was insufficient evidence to show that any other intervention is beneficial.
Keywords: Female; Humans; Male; Amifostine; Amifostine/therapeutic use; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Fibroblast Growth Factor 7; Fibroblast Growth Factor 7/therapeutic use; Pilocarpine; Pilocarpine/therapeutic use; Quality of Life; Radiation‐Protective Agents; Radiation‐Protective Agents/adverse effects; Radiation‐Protective Agents/therapeutic use; Radiotherapy; Radiotherapy/adverse effects; Randomized Controlled Trials as Topic; Saliva, Artificial; Salivary Gland Diseases; Salivary Gland Diseases/etiology; Salivary Gland Diseases/prevention & control; Salivary Glands; Salivary Glands/radiation effects; Salivation; Salivation/drug effects; Salivation/radiation effects; Xerostomia; Xerostomia/etiology; Xerostomia/prevention & control
Plain language summary
Drugs for preventing dry mouth and problems with saliva after radiotherapy
Review question
To assess the effects of treatment with drugs in order to prevent damage to salivary glands following radiotherapy to the head and neck
Background
Problems with saliva production and salivary glands are a significant and mostly permanent side effect for people after radiotherapy treatment to the head and neck. When this occurs the condition is known as dry mouth or xerostomia. Dry mouth is not measurable and is a subjective or personal expression of how the mouth feels. It can have other causes and is a consequence of the production of less saliva or by the consistency of saliva. The rate of flow of saliva in an individual's mouth however can be measured. People who have dry mouth have a reduced quality of life. They can experience issues with taste and general discomfort, difficulties chewing, swallowing and speaking as well as tooth decay, thrush and other infections of the mouth. A wide range of drugs that work in different ways have been used to try and prevent problems with salivary glands caused by radiotherapy. Unfortunately there is currently not enough evidence to show which drugs or which type of drugs are most effective.
Study characteristics
The evidence in this review is current up to 14 September 2016. 39 studies were included with a total of 3520 participants. Participants were male and female, all ages and ethnic origins, out patients or in patients, who were scheduled to have radiation therapy with or without chemotherapy to the head and neck.
Drugs included were any prescribed to prevent salivary gland problems and given before or during radiotherapy. Information was collected from the end of radiotherapy except for that about adverse effects. Different techniques for giving radiation treatment that might reduce damage were not included.
The main outcomes measured were participant's own assessment of dry mouth and the measurement of salivary flow. Secondary outcomes measured included adverse or unwanted effects such as sweating, crying, watery discharge from the nose, diarrhoea and nausea.
Key results
There is some low‐quality evidence to suggest that the drug amifostine prevents the feeling of dry mouth in people receiving radiotherapy to the head and neck (with or without chemotherapy) in the short‐ (end of radiotherapy) to medium‐term (three months after radiotherapy). However it is less clear whether or not this effect is sustained to 12 months after radiotherapy. The benefits of amifostine should be weighed against its high costs and side effects. Adverse effects of vomiting, low blood pressure, feeling of sickness and allergic response were all more frequent in those receiving amifostine. There was insufficient evidence to show that any other treatment is beneficial.
Quality of the evidence
The quality of evidence for amifostine was found to be low because of risk of bias, inconsistency and imprecision caused by the small number of studies in the comparison or sample size. A standardized scale for measuring participant's experience of dry mouth would in future allow comparison and pooling together of results.
Summary of findings
Summary of findings for the main comparison. Pilocarpine compared to no treatment/placebo for preventing salivary gland dysfunction following radiotherapy.
| Pilocarpine compared to no treatment/placebo for preventing salivary gland dysfunction following radiotherapy | ||||||
| Patient or population: patients receiving radiotherapy on its own or in addition to chemotherapy to the head and neck region Intervention: pilocarpine Comparison: no treatment/placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment/placebo | Risk with pilocarpine | |||||
| Xerostomia ‐ Up to and including 6 months postRT Studies used different ways of measuring the outcome and therefore we combined the studies using SMD |
‐ | SMD 0.35 lower (1.04 lower to 0.33 higher) | ‐ | 126 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1 | Insufficient evidence of a difference at this time point and also at the end of RT and 3 months postRT 1 of the 2 studies in this assessment showed inconsistent results when using an alternative way of measuring this outcome at the 6‐month time point. 2 further studies showed insufficient evidence of a difference, 1 at the end of RT and the other at 3 months postRT |
| Salivary flow rate (unstimulated) ‐ Up to and including 3 months postRT Studies used different ways of measuring the outcome and therefore we combined the studies using SMD |
‐ | MD 0.06 lower (0.23 lower to 0.11 higher) | ‐ | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2 | Insufficient evidence of a difference at this time point and also at the end of RT Same results for stimulated salivary flow rates at end of RT, and 3, 6 and 12 months postRT Same results for a further study at the end of RT and 3 months postRT looking at whether or not stimulated and unstimulated salivary flow was > 0 g |
| Overall survival ‐ Up to and including 6 months postRT | 724 per 1000 | 775 per 1000 (579 to 1000) | RR 1.07 (0.80 to 1.43) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW3 | Insufficient evidence of a difference |
| Quality of life ‐ Up to and including 6 months postRT McMaster University Head and Neck Questionnaire (HNRQ). Score 1‐7, lower score = poorer quality of life |
Control group mean was 5.3 | MD 0.20 higher (0.19 lower to 0.59 higher) | ‐ | 90 (1 RCT) | ⊕⊝⊝⊝ VERY LOW3 | Insufficient evidence of a difference at this time point and also at the end of RT and 3 months postRT |
| Adverse effects | Insufficient evidence of a difference between groups for any reported adverse event, apart from for sweating where data from 5 studies showed an increased risk associated with pilocarpine (RR 2.98, 95% CI 1.43 to 6.22; P = 0.004; I2 = 0%; 389 participants; ⊕⊕⊝⊝ LOW4) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference; RT: radiotherapy | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by 1 level for risk of bias, 1 level for imprecision (small sample size and 95% CIs include both possibility of benefit and harm), and 1 level for inconsistency (I2 = 68%). 2Downgraded by 1 level for risk of bias, and 2 levels for imprecision (single study with 12 participants per group and 95% CIs include both possibility of benefit and harm). 3Downgraded by 1 level for risk of bias, and 2 levels for imprecision (single study and 95% CIs include both possibility of benefit and harm). 4Downgraded by 1 level for risk of bias, and 1 level for imprecision (very wide 95% CIs).
Summary of findings 2. Amifostine compared to no treatment/placebo for preventing salivary gland dysfunction following radiotherapy.
| Amifostine compared to no treatment/placebo for preventing salivary gland dysfunction following radiotherapy | ||||||
| Patient or population: patients receiving radiotherapy on its own or in addition to chemotherapy to the head and neck region Intervention: amifostine Comparison: no treatment/placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment/placebo | Risk with amifostine | |||||
| Xerostomia (0‐4 scale ‐ grade 2 or above) ‐ 12 months postRT | 418 per 1000 | 292 per 1000 (167 to 514) | RR 0.70 (0.40 to 1.23) | 682 (7 studies) | ⊕⊕⊝⊝ LOW1 | Insufficient evidence of a difference at this time point. However, both at the end of RT (RR 0.35, 95% CI 0.19 to 0.67; 3 studies, 119 participants) and up to 3 months postRT (RR 0.66, 95% CI 0.48 to 0.92; 5 studies, 687 participants), amifostine reduced the risk of developing grade ≥ 2 xerostomia |
| Salivary flow rate (mg/5 min) (unstimulated) ‐ 12 months postRT | Control group mean was 0.16 | MD 0.32 higher (0.09 higher to 0.55 higher) | ‐ | 27 (1 study) | ⊕⊝⊝⊝ VERY LOW2 | Amifostine led to increased unstimulated saliva flow both at 12 months postRT and at the end of RT, but there was insufficient evidence of a difference at 3 months postRT. This evidence was supported by a further study showing a benefit for amifostine at 12 months postRT when looking at incidence of producing > 0.1 g of saliva over 5 minutes (RR 1.45, 95% CI 1.13 to 1.86; 175 participants). A further study narratively reported no difference Insufficient evidence of a difference in stimulated saliva flow at any time point |
| Overall survival at 12 to 24 months postRT | 450 per 1000** | 531 per 1000 (383 to 747) | HR 1.18 (0.85 to 1.66) | 271 (2 studies) | ⊕⊝⊝⊝ VERY LOW3 | Insufficient evidence to determine whether or not amifostine reduces overall survival, progression‐free survival, disease‐free survival or locoregional tumour control up to 24 months postRT |
| Quality of life (Patient Benefit Questionnaire) ‐ 12 months postRT 8 items each on a 10‐point scale where higher = better QoL |
Control group mean was 6.66 | MD 0.7 higher (0.2 higher to 1.2 higher) | ‐ | 180 (1 study) | ⊕⊝⊝⊝ VERY LOW2 | Amifostine led to a small improvement in quality of life at 12 months postRT, but there was insufficient evidence of a difference at the end of RT and 3 months postRT A further study narratively reported no difference at end of RT and 6, 12, 18, and 24 months postRT |
| Adverse effects |
There was insufficient evidence of a difference between groups for any other adverse events |
|||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) **2014 5‐year overall survival rate of patients with head and neck squamous cell carcinoma (www.who.int/selection_medicines/committees/expert/20/applications/HeadNeck.pdf) CI: confidence interval; HR: hazard ratio; MD: mean difference; QoL: quality of life; RR: risk ratio; RT: radiotherapy | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by 1 level for risk of bias, and 1 level for inconsistency (I2 = 83%). 2Downgraded by 1 level for risk of bias, and 2 levels for imprecision (single study and small sample size). 3Downgraded by 1 level for risk of bias, and 2 levels for imprecision (small sample size and 95% CIs include both possibility of benefit and harm). 4Downgraded by 1 level for risk of bias, and 1 level for imprecision (very wide 95% CIs).
Summary of findings 3. Palifermin compared to placebo for preventing salivary gland dysfunction following radiotherapy.
| Palifermin compared to placebo for preventing salivary gland dysfunction following radiotherapy | ||||||
| Patient or population: patients receiving radiotherapy on its own or in addition to chemotherapy to the head and neck region Intervention: palifermin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with palifermin | |||||
| Xerostomia (0‐4 scale ‐ grade 2 or above) ‐ Up to and including 3 months postRT | 727 per 1000 | 705 per 1000 (560 to 887) | RR 0.97 (0.77 to 1.22) | 471 (3 studies) | ⊕⊕⊝⊝ LOW1 | Insufficient evidence of a difference at this time point |
| Overall survival at 42 to 72 months from baseline | 450 per 1000** | 450 per 1000 (324 to 626) | HR 1.00 (0.72 to 1.39) | (3 studies) | ⊕⊕⊕⊝ MODERATE2 | Insufficient evidence to determine whether or not amifostine reduces overall survival and progression‐free survival up to 72 months |
| Adverse effects | There was insufficient evidence of patients in either group experiencing more or less adverse events | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) **2014 5‐year overall survival rate of patients with head and neck squamous cell carcinoma (www.who.int/selection_medicines/committees/expert/20/applications/HeadNeck.pdf) CI: confidence interval; HR: hazard ratio; RR: risk ratio; RT: radiotherapy | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by 1 level for imprecision (95% CIs include both possibility of benefit and harm), and 1 level for inconsistency (I2 = 76%). 2Downgraded by 1 level for imprecision (95% CIs include both possibility of benefit and harm).
Background
Description of the condition
Xerostomia (dry mouth) has been defined as the "subjective sensation of dryness" (Sreebny 1996), whilst salivary gland hypofunction has been defined as "any objectively demonstrable reduction in either whole and/or individual salivary gland flow rates" (Navazesh 1992). Xerostomia is usually the result of a decrease in the volume of saliva secreted. Indeed, healthy individuals complain of a dry mouth when their unstimulated whole salivary flow rate falls below 50% of their normal level (Dawes 1987). However, xerostomia may also occur without a reduction in salivary flow (Porter 2004), possibly resulting from a change in composition of saliva secreted (Pankhurst 1996). Thus, xerostomia may, or may not be associated with salivary gland hypofunction. Salivary gland dysfunction is an 'umbrella' term for the presence of either xerostomia, or salivary gland hypofunction.
Salivary gland dysfunction is an extremely common side effect of radiotherapy to the head and neck region (Guchelaar 1997). The total dose for a course of radiotherapy for head and neck cancer is 50 Gy (gray) to 70 Gy (Shiboski 2007). However, doses over 52 Gy will cause severe salivary gland dysfunction (Porter 2004). A major decrease in saliva flow develops within one week of starting radiotherapy, and continues to deteriorate throughout treatment, culminating in permanent salivary gland dysfunction (Shiboski 2007). Indeed, even a dose of 20 Gy is enough to permanently damage salivary flow if it is given as a single dose (Porter 2004). Salivary gland hypofunction is associated with a variety of oral problems in this group of people (e.g. oral discomfort, taste disturbance, difficulty chewing, difficulty swallowing, speech problems, dental caries, oral candidiasis, and other oral infections). Certainly salivary gland dysfunction is associated with a significant impairment of quality of life in this group of patients.
Description of the intervention
The literature discusses a wide range of pharmacological interventions for preventing radiation‐induced salivary gland dysfunction. Examples of these include.
Parasympathomimetic drugs (choline esters, cholinesterase inhibitors)
Parasympathomimetic drugs stimulate salivary secretion by stimulating the parasympathetic nervous system. The parasympathetic nervous system increases bodily secretions such as tears, gastric juices, mucus and saliva to defend the body and help digestion. The most widely used parasympathomimetic drug in this clinical situation is pilocarpine hydrochloride (a choline ester) and has been licensed in many countries for the treatment of radiation‐induced salivary gland dysfunction (Wiseman 1995). Other indirectly acting parasympathomimetics, for example bethanecol, are much more widely used in other contexts, but have also been used 'off‐licence' to treat this condition (Epstein 1994).
Parasympatholytic drugs
Parasympatholytic drugs have the opposite effect to parasympathomimetic drugs, their action is anticholinergic, i.e. they inhibit the secretion of saliva. Results from animal tests (Ahlner 1994) and a study by Rode et al (Rode 1999; Rode 2001) suggest that the inhibition of salivary secretion during radiotherapy might actually protect later damage of the salivary glands and improve salivation following the treatment.
Cytoprotective agents
Cytoprotective agents can be administered before, with, or after cancer therapy to reduce or prevent damage or toxicity to the normal cells and tissues without compromising therapeutic efficacy. Amifostine is a cytoprotective agent and has been shown to accumulate in the salivary glands (Takahashi 1986); there are reports that this might lead to a reduction in parotid parenchymal damage due to radiotherapy (Bohuslavizki 1998), and reduce the incidence of radiation‐induced xerostomia (Brizel 2000).
Why it is important to do this review
Salivary gland dysfunction is a significant and mostly permanent side effect of radiotherapy to the head and neck region that has numerous knock‐on effects, negatively affecting quality of life. Unfortunately, the evidence for prevention using pharmacological agents is weak and some guideline statements do not currently recommend any (Buglione 2016). Although there is a recently published Cochrane Review looking at parasympathomimetic drugs for treating radiation‐induced salivary gland dysfunction (Davies 2015), other drugs with different modes of action have the potential to be effective in this situation, and a broader review of prophylactic measures was needed.
Objectives
To assess the effects of pharmacological interventions for the prevention of radiation‐induced salivary gland dysfunction.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials of parallel design. Trials were included irrespective of language of publication or publication status.
Types of participants
We included participants of all ages, ethnic origin and gender scheduled to receive radiotherapy on its own or in addition to chemotherapy to the head and neck region. Participants could be outpatients or inpatients.
Types of interventions
Active agents
Any pharmacological agent prescribed prophylactically for salivary gland dysfunction prior to or during radiotherapy, by any route, any dose, and for any length of time. Radiation techniques were excluded.
Control groups
No preventative intervention, placebo, or another pharmacological preventative measure for salivary gland dysfunction.
Types of outcome measures
As radiotherapy‐induced salivary gland dysfunction is considered to be permanent, we were interested in long‐term treatment effects and only collected data starting from the end of radiotherapy, except in the case of adverse effects.
Primary outcomes
The primary outcome measure for the review is salivary gland dysfunction as indicated by either:
xerostomia, i.e. the subjective sensation of dryness of the mouth. It was anticipated that different investigators would use different scales to assess xerostomia, e.g. visual analogue scales, verbal rating scales;
salivary flow rates (stimulated or unstimulated).
Secondary outcomes
The secondary outcome measures of the review are:
adverse effects, e.g. sweating, lacrimation (excess tears, crying), rhinorrhoea (watery discharge from the nose), diarrhoea, nausea;
survival data (overall, disease‐free, progression‐free, locoregional control);
other oral signs/symptoms, e.g. oral discomfort/pain, dysgeusia (taste disturbance), dysmasesia (difficulty in chewing), dysphagia (difficulty in swallowing), dysphonia (difficulty in speaking);
quality of life, e.g. ability to sleep, work, speak;
patient satisfaction;
cost data.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 14 September 2016) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library (searched 14 September 2016) (Appendix 2);
MEDLINE Ovid (1946 to 14 September 2016) (Appendix 3);
Embase Ovid (1980 to 14 September 2016) (Appendix 4);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 14 September 2016) (Appendix 5);
LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 to 14 September 2016) (Appendix 6);
Zetoc Conference Proceedings (1993 to 14 September 2016) (Appendix 7);
OpenGrey (1997 to 14 September 2016) (Appendix 8).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011). The Embase subject search was linked to an adapted version of the Cochrane Crowd Project filter for identifying randomised controlled trials in Embase Ovid (see www.cochranelibrary.com/help/central‐creation‐details.html for information).
Language
The search attempted to identify all relevant studies irrespective of language. Articles in Chinese (Han 2010; He 2004; Hu 2005; Wang 1998) were translated and included in the review. An article in Spanish (Fuertes 2004) was translated and subsequently excluded.
Searching other resources
Ongoing studies
We searched the following trial registries for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 14 September 2016) (Appendix 9);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 14 September 2016) (Appendix 10).
Reference list searching
The reference lists of review articles and standard clinical oncology textbooks were checked for additional studies. The reference lists of included studies were also checked for additional studies.
Handsearching
Only handsearching done as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL was included.
Unpublished studies
Requests for information about unpublished studies/studies published in the 'grey literature' were sent to relevant pharmaceutical companies, relevant investigators, editors of radiotherapy journals, and relevant professional organisations.
Data collection and analysis
Selection of studies
The titles and abstracts of all records identified by the search strategy were scanned independently and in duplicate by two review authors. For both studies that appeared to meet the inclusion criteria, and studies that contained insufficient information in the title and abstract to determine eligibility, we obtained the full‐text report and two review authors independently assessed them to establish whether they met the inclusion criteria. Studies excluded at this or subsequent stages were entered in the table of excluded studies with the reasons for exclusion recorded. All disagreements were resolved by discussion.
Data extraction and management
Two review authors independently and in duplicate extracted data using specially designed data extraction forms. The data extraction forms were piloted on several papers and modified as required before use. The data extracted included.
Citation details: including year of publication, country of origin, setting and source of funding.
Details of participants: including demographic characteristics, cancer details (type, stage, location), radiation therapy and criteria for inclusion.
Details of intervention: including type, duration and method of administration.
Details of outcomes reported: including method of assessment (if measurement scales were used, details of whether the scale was validated were recorded).
Sample size calculation and trial registration.
Authors were contacted where possible for clarification and missing information.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study using the Cochrane domain‐based, two‐part tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We contacted study authors for clarification or missing information where necessary and feasible. Disagreements were resolved through discussion, consulting a third review author to achieve consensus when necessary.
We completed a 'Risk of bias' table for each included study. For each domain of risk of bias, we described what was reported to have happened in the study. This information provided the rationale for our judgement of whether that domain was at low, high, or unclear risk of bias.
We assessed the following domains:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective outcome reporting (reporting bias);
other bias.
We categorised the overall risk of bias of individual studies as being at low, high, or unclear risk of bias according to the following criteria:
low risk of bias (plausible bias unlikely to seriously alter the results) if all domains were at low risk of bias;
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more domains were at high risk of bias; or
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more domains were at unclear risk of bias.
We also presented the 'Risk of bias' summary graphically.
Measures of treatment effect
For continuous outcomes (e.g. xerostomia on a visual analogue scale) where studies use the same scale, we used the mean values and standard deviations (SDs) reported in the studies in order to express the estimate of effect as mean difference (MD) with 95% confidence interval (CI). Where different scales were used to measure the same outcome, we expressed the treatment effect as standardised mean difference (SMD) with 95% CI.
For dichotomous outcomes, the estimate of effect of an intervention is expressed as risk ratios (RR) together with 95% CIs.
Unit of analysis issues
The participant is the unit of analysis.
Dealing with missing data
We contacted the author(s) of included studies, where feasible, to identify missing data and details of any other outcomes that may have been measured but not reported. We would have used the methods described in Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions to estimate missing SDs (Higgins 2011) if appropriate. We did not use any other statistical methods or perform any further imputation to account for missing data.
Assessment of heterogeneity
Before any pooling of data was conducted, for comparisons with two or more studies, clinical heterogeneity was assessed by examining the types of participants (e.g. cancer types), interventions (e.g. control group used, dose and mode of administration), and outcomes (e.g. stimulated salivary flow rates or quality of life questionnaires). Statistical heterogeneity was also assessed using a Chi2 test, where a P value < 0.1 indicated statistically significant heterogeneity. We also quantified the heterogeneity using the I2 statistic.
Assessment of reporting biases
Publication bias was to have been assessed for comparisons where at least 10 studies were included in a meta‐analysis. We would have used the recommendations on testing for funnel plot asymmetry (Egger 1997), as described in Section 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
Meta‐analyses were only undertaken where there were studies of similar comparisons reporting the same outcomes. We combined MDs for continuous data, and RRs for dichotomous data. Our general approach was to use a random‐effects model. Our preference for the more conservative random‐effects model is because statistical assessments can miss potentially important between‐study heterogeneity in small samples (Kontopantelis 2012).
We presented data not suitable for meta‐analysis in additional tables.
Subgroup analysis and investigation of heterogeneity
Where possible, subgroup analyses would have been performed according to cancer type and treatment plans for cancer, and age of participants (i.e. children under the age of 18 years).
Sensitivity analysis
Sensitivity analysis was to be undertaken on the primary outcomes by excluding studies at unclear and high risk of bias from the analyses and also excluding unpublished literature.
If any meta‐analyses had included studies with a large variation in sample size (for example several small studies and a single very large study), we would have undertaken a sensitivity analysis comparing the effect estimates from both random‐effects and fixed‐effect models. If these were different we would have reported on both analyses as part of the results section, and we would have considered possible interpretation.
Presentation of main results
We produced a 'Summary of findings' table for each comparison that included more than one study. We included data on: xerostomia, salivary flow rate, survival, quality of life and adverse events. We used GRADE methods (GRADE 2004), and the GRADEpro online tool for developing 'Summary of findings' tables (www.guidelinedevelopment.org). We assessed the quality of the body of evidence for each comparison and outcome by considering the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, and the risk of publication bias. We described our level of certainty in the overall findings for each comparison/outcome in terms of high, moderate, low, very low.
Results
Description of studies
Results of the search
Electronic searches identified a total of 3536 titles and abstracts. A further study was identified by one of the review authors' knowledge of the topic area. After removal of duplicates, 2284 records were identified for screening. Following screening of these titles and abstracts by two review authors, 87 were identified as potentially relevant. Full papers were retrieved and authors of abstracts were written to in order to gain the full papers. Following a second screening of these studies, 46 were excluded for reasons described in the Excluded studies section and in the table of Characteristics of excluded studies. One study is ongoing and a further study is awaiting classification. Therefore, 39 studies met our eligibility criteria and were included in this review. This process is presented graphically in Figure 1.
1.
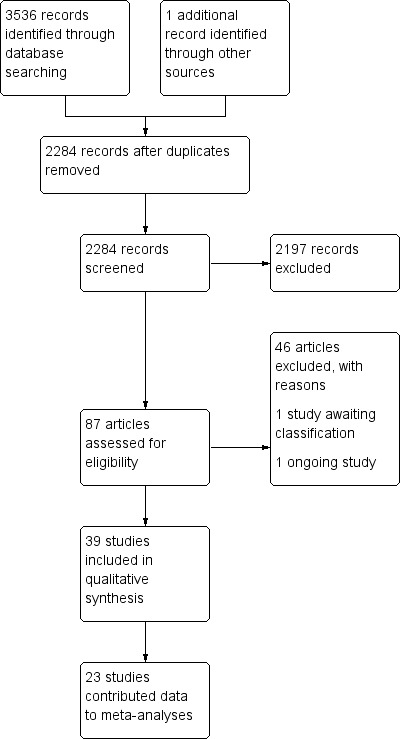
Study flow diagram.
Included studies
Characteristics of trial setting, publication status and funding
Thirty‐nine trials were included; five were multinational (Brizel 2000; Brizel 2008; Buentzel 2006; Henke 2011; Le 2011), six were conducted in China (Han 2010; He 2004; Hu 2005; Lin 2014; Peng 2006; Wang 1998), four were conducted in Germany (Büntzel 1998; Büntzel 2010; Grötz 2001; Vacha 2003), four in the USA (Fisher 2003; Haddad 2009; Lozada‐Nur 1998; Valdez 1993), three in Canada (Duncan 2005; Gornitsky 2004; Warde 2002), three in Brazil (Jaguar 2015; Jham 2007; Pimentel 2014), two in Thailand (Sangthawan 2001; Veerasarn 2006), two in India (Patni 2004; Reshma 2012), two in the Netherlands (Burlage 2008; Jellema 2006), one in Croatia (Lajtman 2000), one in Turkey (Abacioglu 1997), one in Greece (Antonadou 2002), one in Iran (Haddad 2002), one in France (Bardet 2011), one in Spain (Lanzós 2010), one in Japan (Watanabe 2010), and one in Slovenia (Rode 1999).
All trials had a parallel‐group design. Ten trials had more than one published paper, with Büntzel 1998 publishing seven papers relating to the one trial. Abacioglu 1997 is an unpublished dissertation and data were gained from the authors of two trials following publication of their results as conference abstracts (Lozada‐Nur 1998; Patni 2004). Eighteen of the trials received external funding, six trials received internal or no funding and the funding source was not stated in 15 trials.
One trial is ongoing (NCT02430298) and will be considered for future updates.
Characteristics of the participants
All of the trials recruited adults scheduled to receive radiotherapy to the salivary glands for cancer. The majority of participants were male. The type of cancer was head and neck at different sites in 36 trials and nasopharyngeal in 3 trials (Han 2010; He 2004; Lozada‐Nur 1998).
Ten of the trials explicitly stated that chemotherapy was given as part of the treatment regimen in addition to radiotherapy for all patients (Antonadou 2002; Brizel 2008; Buentzel 2006; Büntzel 1998; Han 2010; Henke 2011; Le 2011; Peng 2006; Vacha 2003; Watanabe 2010). Chemotherapy was given to some patients of the following four trials: Bardet 2011; Gornitsky 2004; Haddad 2009; Lozada‐Nur 1998. The other trials either undertook no chemotherapy, or were unclear about whether any chemotherapy was given.
Four studies explicitly referred to neck dissection but varied in the clarity of reporting: two clearly reported the proportions in each group that had their submandibular glands removed (Burlage 2008; Vacha 2003); one only reported the proportion that had neck dissection in each group, but did not refer to salivary gland removal (Haddad 2009); and one only stated that participants were stratified by submandibular gland removal, but numbers of participants affected were not reported (Jellema 2006).
The 39 included studies randomised 3520 participants, ranging from 10 to 291.
The percentage of participants lost to follow‐up ranged from 0% to 38%.
Characteristics of the intervention
All of the trials provided a detailed description of the intervention including the dose and method of administration for the test and control groups. Twenty‐one trials included a placebo control group and 14 a 'no intervention' control group, the remaining four trials making head‐to‐head comparisons (Bardet 2011; Jellema 2006; Jham 2007; Watanabe 2010).
Pilocarpine hydrochloride was assessed in 12 trials at various dosages: Abacioglu 1997; Burlage 2008; Fisher 2003; Gornitsky 2004; Haddad 2002; Lajtman 2000; Lozada‐Nur 1998; Pimentel 2014; Rode 1999; Sangthawan 2001; Valdez 1993; Warde 2002.
Biperiden plus pilocarpine was assessed in one trial: Rode 1999.
Amifostine was assessed in 12 trials at various dosages: Antonadou 2002; Bardet 2011; Brizel 2000; Buentzel 2006; Büntzel 1998; Haddad 2009; He 2004; Jellema 2006; Patni 2004; Peng 2006; Vacha 2003; Veerasarn 2006.
Chinese medicine was assessed in five trials, all comparing different herbs: Han 2010; Hu 2005; Lin 2014; Reshma 2012; Wang 1998.
Palifermin was assessed in three trials: Brizel 2008; Henke 2011; Le 2011.
Bethanechol was assessed in two trials: Jaguar 2015; Jham 2007.
Artificial saliva was assessed in one trial: Jham 2007.
Selenium was assessed in one trial: Büntzel 2010.
Antiseptic mouthrinse was assessed in one trial: Lanzós 2010.
Antimicrobial lozenge was assessed in one trial: Duncan 2005.
Polaprezinc was assessed in one trial: Watanabe 2010.
Azulene oral rinse assessed in one trial: Watanabe 2010.
Venalot Depot (coumarin/ troxerutin) was assessed in one trial: Grötz 2001.
The length of follow‐up ranged from day 28 of the radiotherapy (RT) to 34 months: day 28/29 of RT (Pimentel 2014; Reshma 2012), end of RT (Abacioglu 1997; Hu 2005; Wang 1998), four weeks from start of RT (Lanzós 2010), four weeks after RT (Lin 2014; Grötz 2001), five weeks after RT (Gornitsky 2004), six weeks after RT (Büntzel 2010; Vacha 2003), seven weeks after RT (He 2004), two months after RT (Jham 2007), three months after RT (Brizel 2008; Han 2010; Jaguar 2015; Lozada‐Nur 1998), six months after RT (Duncan 2005; Fisher 2003; Haddad 2002; Sangthawan 2001; Warde 2002), 12 months after RT (Bardet 2011; Buentzel 2006; Büntzel 1998; Burlage 2008; Lajtman 2000; Rode 1999; Valdez 1993), 18 months after RT (Antonadou 2002), 24 months after RT (Brizel 2000; Henke 2011; Jellema 2006; Le 2011; Patni 2004; Veerasarn 2006), and 34 months after RT (Haddad 2009). Duration of follow‐up/timing of assessment was unclear in two studies (Peng 2006; Watanabe 2010).
Characteristics of outcome measures
The trials used a variety of assessment measures for salivary gland dysfunction. Ten trials included a subjective measure of salivary gland dysfunction, i.e. the patient was involved in the assessment through visual analogue scales (VAS) (Gornitsky 2004; Haddad 2002; Sangthawan 2001; Wang 1998), linear analogue scale (LASA) (Warde 2002), and modified patient questionnaires (Abacioglu 1997; He 2004; Jellema 2006; Lajtman 2000; Veerasarn 2006). One study reported 'acute' or 'chronic' dry mouth only (Peng 2006). Fifteen trials reported a clinical assessment of salivary gland dysfunction using various scales: RTOG/EORTC (Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer) scoring (Antonadou 2002; Brizel 2000; Buentzel 2006; Fisher 2003; Grötz 2001; He 2004; Jellema 2006; Patni 2004; Veerasarn 2006), NCI CTCAE (National Cancer Institute Common Terminology Criteria for Adverse Events) (Brizel 2008; Henke 2011; Le 2011), WHO (World Health Organization) grading/classification (Büntzel 1998), and Late Effects Normal Tissue Task Force (LENT)‐Subjective, Objective, Management, Analytic (SOMA) scales (Burlage 2008; Haddad 2002). Unstimulated or stimulated whole saliva secretion data or both were collected in ten trials (Abacioglu 1997; Brizel 2000; Buentzel 2006; Gornitsky 2004; He 2004; Lajtman 2000; Rode 1999; Valdez 1993; Veerasarn 2006; Wang 1998), and salivary gland scintigraphy was used in five trials (Fisher 2003; Grötz 2001; Lozada‐Nur 1998; Patni 2004; Veerasarn 2006).
Secondary outcomes were sporadically reported, using various scales. However, the majority of studies reported adverse events.
Excluded studies
Of the 87 trials that were identified as potentially eligible, 46 were excluded, with the main reason being the publication of an abstract only (17 publications), with insufficient information to allow thorough assessment: Bagga 2007; Borg 2007; Chambers 2005; Goyal 2007; Gu 2014; Kumarchandra 2010; Manoor 2014; Mitine 2000; Mix 2013; Norberg‐Spaak 1996; Norberg‐Spaak 1997; Park 2012; Park 2012a; Resubal 2011; Rudat 2005; Strnad 1997; Zale 1993.
Other reasons for exclusion were: not a randomised controlled trial or unclear if a randomised controlled trial; prevention of salivary gland dysfunction not the aim of study/not reported; radioactive iodine used rather than radiotherapy; study did not include head and neck cancer patients; the intervention was not a pharmacological agent.
Risk of bias in included studies
Allocation
Random sequence generation
Twenty of the included studies described an adequate method of random sequence generation and were assessed as at low risk of bias for this domain (Abacioglu 1997; Brizel 2000; Buentzel 2006; Burlage 2008; Gornitsky 2004; Haddad 2002; Haddad 2009; Henke 2011; Jaguar 2015; Jellema 2006; Jham 2007; Lanzós 2010; Le 2011; Lin 2014; Lozada‐Nur 1998; Pimentel 2014; Rode 1999; Sangthawan 2001; Valdez 1993; Veerasarn 2006). The remaining 19 studies stated that allocation was random but did not describe their methods and were therefore assessed as at unclear risk of bias for this domain.
Allocation concealment
Allocation concealment was clearly described in 16 of the included studies and they were assessed as being at low risk of bias for this domain (Abacioglu 1997; Brizel 2000; Buentzel 2006; Burlage 2008; Gornitsky 2004; Haddad 2002; Haddad 2009; Henke 2011; Lanzós 2010; Le 2011; Lozada‐Nur 1998; Pimentel 2014; Rode 1999; Sangthawan 2001; Valdez 1993; Veerasarn 2006). The remaining 23 did not describe any methods used to conceal the random sequence, and so were assessed as being at unclear risk of bias.
Blinding
Blinding of participants and personnel (performance bias)
Twenty‐one studies were placebo‐controlled and double‐blind, and were assessed at low risk of performance bias. In the remaining 18 studies, blinding of the patients and their caregivers to the allocated treatment was not possible because the active and control treatments were administered differently, the control group had no intervention at all, or the personnel administering or patients were not blinded to the intervention (Abacioglu 1997; Antonadou 2002; Bardet 2011; Brizel 2000; Büntzel 1998; Büntzel 2010; Haddad 2009; Han 2010; He 2004; Hu 2005; Jellema 2006; Jham 2007; Patni 2004; Peng 2006; Rode 1999; Vacha 2003; Veerasarn 2006; Watanabe 2010).
Blinding of outcome assessment (detection bias)
Twenty‐one studies were assessing the effect of the intervention versus a placebo where the assessor was also blinded and these have been assessed as at low risk of bias. A further study which was not placebo‐controlled was assessed at low risk of bias because the outcome assessment for salivary gland dysfunction was objective (Rode 1999). The remaining 17 studies were assessed as being at high risk of detection bias, as the assessor was not blinded, the intervention was assessed against no intervention, the administration of the drug was different in the intervention and control groups or the assessment of xerostomia was subjective (Abacioglu 1997; Antonadou 2002; Bardet 2011; Brizel 2000; Büntzel 1998; Büntzel 2010; Haddad 2009; Han 2010; He 2004; Hu 2005; Jellema 2006; Jham 2007; Patni 2004; Peng 2006; Vacha 2003; Veerasarn 2006; Watanabe 2010).
Incomplete outcome data
Twenty‐one studies had no or negligible attrition and were assessed as being low risk. Twelve studies were assessed to be at high risk of attrition bias, due to high dropout rates, no reasons given for dropouts or differential attrition between the groups, which could be linked to the intervention (Bardet 2011; Brizel 2008; Burlage 2008; Grötz 2001; Haddad 2002; Jellema 2006; Jham 2007; Lanzós 2010; Pimentel 2014; Vacha 2003; Veerasarn 2006; Warde 2002). For the six remaining studies, there was insufficient information to determine risk of attrition bias (Fisher 2003; Haddad 2009; Lajtman 2000; Lozada‐Nur 1998; Peng 2006; Sangthawan 2001).
Selective reporting
Nineteen of the included studies reported the outcomes specified in the methods section in full, including information about xerostomia and adverse effects (Abacioglu 1997; Antonadou 2002; Bardet 2011; Brizel 2000; Brizel 2008; Buentzel 2006; Büntzel 1998; Haddad 2002; Han 2010; He 2004; Henke 2011; Hu 2005; Jaguar 2015; Jham 2007; Le 2011; Lin 2014; Lozada‐Nur 1998; Veerasarn 2006; Warde 2002). One study was assessed to be at unclear risk of reporting bias (Peng 2006). The remaining 19 studies were assessed as at high risk of reporting bias as they did not report on adverse effects or xerostomia, did not report on all outcomes, only significant data were reported or data on individuals were not reported, and grouped data did not have the standard deviations.
Other potential sources of bias
We did not consider there to be any other issues arising from other potential sources in any of the studies and we therefore assessed them all as being at low risk of bias for this domain.
Overall risk of bias
Overall, three of the included studies (8%) were assessed as at low risk of bias for all domains (Buentzel 2006; Henke 2011; Le 2011), and four studies (10%) were assessed as being at unclear risk of bias for at least one domain (Brizel 2008; Jaguar 2015; Lin 2014; Lozada‐Nur 1998). The remaining 32 studies (82%) were at high risk of bias for at least one domain. Risk of bias can be viewed graphically in Figure 2 and Figure 3.
2.
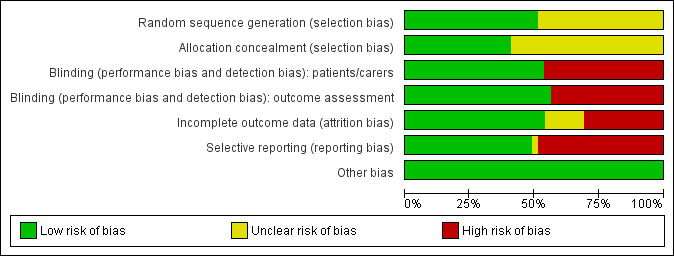
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
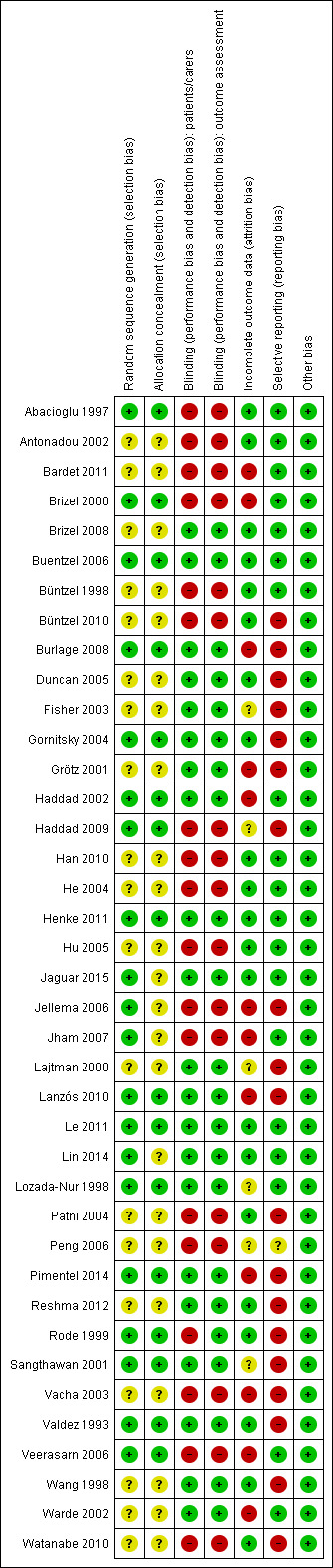
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3
Pilocarpine versus no treatment/placebo
Pilocarpine hydrochloride, at various dosages, was assessed in 12 trials: Abacioglu 1997; Burlage 2008; Fisher 2003; Gornitsky 2004; Haddad 2002; Lajtman 2000; Lozada‐Nur 1998; Pimentel 2014; Rode 1999; Sangthawan 2001; Valdez 1993; Warde 2002. Over 900 participants were randomised to either pilocarpine or no treatment/placebo; 698 were evaluated (although number varied by outcome/timing of assessment). Eleven of the trials were judged to be at high risk of bias; one was at unclear risk (Lozada‐Nur 1998).
Xerostomia
Nine trials evaluated xerostomia, however, the method of assessment varied across studies.
Seven trials presented continuous data on xerostomia obtained by simple VAS or a composite based on a number of xerostomia‐focused questions (Abacioglu 1997; Burlage 2008; Gornitsky 2004; Haddad 2002; Lozada‐Nur 1998; Sangthawan 2001; Warde 2002). The trial by Burlage 2008 was unable to be included in any statistical pooling as data were presented by Gy dose, but the number receiving each dose is unclear. There was no evidence of a difference between treatment groups at end of radiotherapy (standardised mean difference (SMD) 0.20, 95% confidence interval (CI) ‐0.16 to 0.56; P = 0.27; 122 participants), up to three months postradiotherapy (SMD 0.02, 95% CI ‐0.33 to 0.37; P = 0.92; 125 participants), or up to six months postradiotherapy (SMD ‐0.35, 95% CI ‐1.04 to 0.33; P = 0.31; 126 participants) (Analysis 1.1). There was substantial statistical heterogeneity present for the six‐month data (I2 = 68%, P = 0.08).
1.1. Analysis.
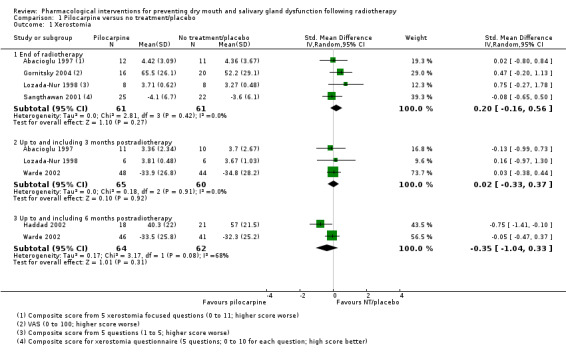
Comparison 1 Pilocarpine versus no treatment/placebo, Outcome 1 Xerostomia.
One trial used the LENT‐SOMA scale to provide an objective assessment of xerostomia (Haddad 2002). This single trial showed a statistically significant difference in favour of pilocarpine (mean difference (MD) ‐0.40, 95% CI ‐0.69 to ‐0.11; P = 0.006; 39 participants) at six months postradiotherapy (Analysis 1.2).
1.2. Analysis.
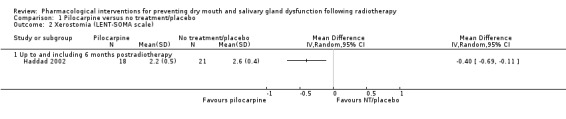
Comparison 1 Pilocarpine versus no treatment/placebo, Outcome 2 Xerostomia (LENT‐SOMA scale).
Two trials presented binary data on the number of participants with/without xerostomia (Lajtman 2000; Pimentel 2014). There was no evidence of a difference between treatment groups at the end of radiotherapy (risk ratio (RR) 0.60, 95% CI 0.18 to 2.02; P = 0.41; 11 participants) or at three months postradiotherapy (RR 1.00, 95% CI 0.92 to 1.08; P = 1.00; 48 participants) (Analysis 1.3).
1.3. Analysis.
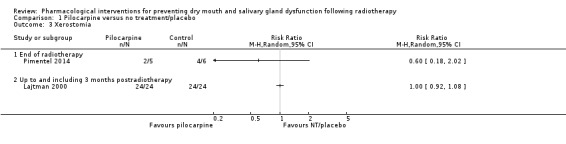
Comparison 1 Pilocarpine versus no treatment/placebo, Outcome 3 Xerostomia.
Salivary flow rates
Eight studies presented continuous data for either stimulated or unstimulated salivary flow (e.g. ml/min or g) (Abacioglu 1997; Burlage 2008; Gornitsky 2004; Lajtman 2000; Lozada‐Nur 1998; Pimentel 2014; Rode 1999; Valdez 1993). The studies by Burlage 2008; Lajtman 2000; Pimentel 2014 and Rode 1999 were unable to be included in any statistical pooling due to insufficient reporting of data. There was no evidence of a difference between treatment groups for unstimulated or stimulated flow rates at any time point (Analysis 1.4; Analysis 1.5).
1.4. Analysis.
Comparison 1 Pilocarpine versus no treatment/placebo, Outcome 4 Salivary flow rate (unstimulated).
1.5. Analysis.
Comparison 1 Pilocarpine versus no treatment/placebo, Outcome 5 Salivary flow rate (stimulated).
One study presented binary data on whether stimulated or unstimulated salivary flow was > 0 g (Fisher 2003). There is insufficient evidence to determine whether pilocarpine is beneficial for this outcome at any time point (Analysis 1.6; Analysis 1.7).
1.6. Analysis.
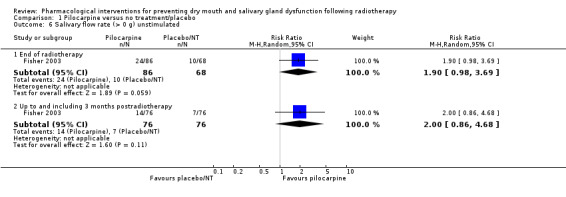
Comparison 1 Pilocarpine versus no treatment/placebo, Outcome 6 Salivary flow rate (> 0 g) unstimulated.
1.7. Analysis.
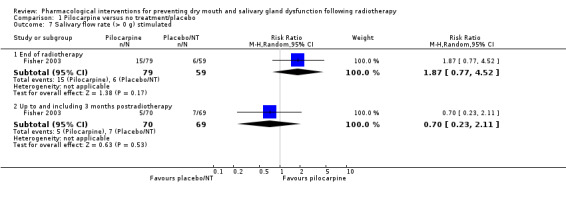
Comparison 1 Pilocarpine versus no treatment/placebo, Outcome 7 Salivary flow rate (> 0 g) stimulated.
Survival
Only one trial reported on overall survival within the trial period (six months) (Haddad 2002). There was no evidence of a difference between treatment groups (RR 1.07, 95% CI 0.80 to 1.43; P = 0.66; 60 participants) (Analysis 1.8).
1.8. Analysis.
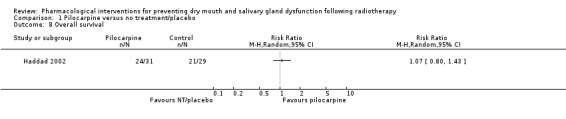
Comparison 1 Pilocarpine versus no treatment/placebo, Outcome 8 Overall survival.
Quality of life and other oral related symptoms
There was insufficient evidence to determine whether or not pilocarpine improved quality of life measurements for global quality of life, quality of life (HNRQ), oral discomfort, eating difficulties and sleeping problems at the end of radiotherapy (Analysis 1.9; Additional Table 4). One trial (Gornitsky 2004) found an increased risk in speech difficulties at the end of radiotherapy in the pilocarpine group (MD 20.20, 95% CI 1.93 to 38.47; P = 0.03; 34 participants) when assessed using a VAS scale (0 to 100 mm) (Additional Table 4).
1.9. Analysis.
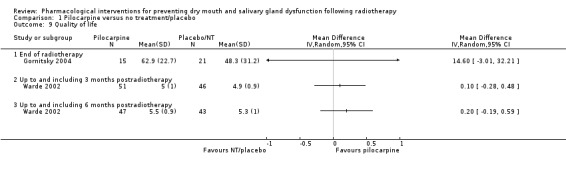
Comparison 1 Pilocarpine versus no treatment/placebo, Outcome 9 Quality of life.
1. Pilocarpine versus no treatment/placebo (other outcomes).
| Outcome | Study ID | Time point | Pilocarpine | Control | Results | Comments |
| Oral related symptoms (other than salivary gland dysfunction/xerostomia) | ||||||
| Oral discomfort | Gornitsky 2004 | End of radiotherapy | Mean 38.7 (SD 31.6) n = 16 |
Mean 56.7 (SD 26.7) n = 20 |
Mean difference ‐18.00 (95% CI ‐37.41 to 1.41), P = 0.07 | |
| Speech difficulties | Gornitsky 2004 | Mean 57.5 (SD 26.8) n = 16 |
Mean 37.3 (SD 27.5) n = 18 |
Mean difference 20.20 (95% CI 1.93 to 38.47), P = 0.03 | ||
| Eating difficulties | Gornitsky 2004 | Mean 47.4 (SD 33.9) n = 15 |
Mean 61.8 (SD 25.4) n = 17 |
Mean difference ‐14.40 (95% CI ‐35.38 to 6.58), P = 0.18 | ||
| Mucosal pain | Gornitsky 2004 | Mean 38.8 (SD 33.9) n = 17 |
Mean 53.6 (SD 34.2) n = 19 |
Mean difference ‐14.80 (95% CI ‐37.07 to 7.47), P = 0.19 | ||
| Oral complications | Pimentel 2014 | 1/5 | 4/6 | RR 0.30 (95% CI 0.05 to 1.89), P = 0.20 | ||
| Adverse events | ||||||
| Sweating | Abacioglu 1997 | 2/12 | 0/12 | Random‐effects meta‐analysis of 5 studies: RR 2.98 (95% CI 1.43 to 6.22), P = 0.004 Heterogeneity: I2 = 0%, P = 0.52 |
||
| Fisher 2003 | 18/118 | 5/114 | ||||
| Gornitsky 2004 | 3/28 | 1/28 | ||||
| Lozada‐Nur 1998 | 5/12 | 1/12 | ||||
| Sangthawan 2001 | 1/29 | 2/24 | ||||
| Chilling | Abacioglu 1997 | 1/12 | 0/12 | RR 3.00 (95% CI 0.13 to 67.06), P = 0.49 | ||
| Nausea | Gornitsky 2004 | 7/28 | 5/28 | Random‐effects meta‐analysis of 3 studies: RR 1.39 (95% CI 0.63 to 3.05), P = 0.41 Heterogeneity: I2 = 0%, P = 0.93 |
||
| Haddad 2002 | 3/18 | 3/21 | ||||
| Lozada‐Nur 1998 | 2/12 | 1/12 | ||||
| Vomiting | Fisher 2003 | 13/118 | 10/114 | Random‐effects meta‐analysis of 3 studies: RR 1.28 (95% CI 0.70 to 2.35), P = 0.43 Heterogeneity: I2 = 0%, P = 0.92 |
||
| Gornitsky 2004 | 6/28 | 5/28 | ||||
| Lozada‐Nur 1998 | 2/12 | 1/12 | ||||
| Headache | Gornitsky 2004 | 2/28 | 3/28 | RR 0.67 (95% CI 0.12 to 3.69), P = 0.64 | ||
| Excessive lacrimation (tears) | Fisher 2003 | 3/118 | 0/114 | Random‐effects meta‐analysis of 3 studies: RR 2.54 (95% CI 0.70 to 9.17), P = 0.15 Heterogeneity: I2 = 0%, P = 0.71 |
||
| Haddad 2002 | 1/18 | 0/21 | ||||
| Sangthawan 2001 | 4/25 | 2/22 | ||||
| Dysphasia | Lozada‐Nur 1998 | 3/12 | 2/12 | RR 1.50 (95% CI 0.30 to 7.43), P = 0.62 | ||
| Weakness | Fisher 2003 | 3/118 | 2/114 | RR 1.45 (95% CI 0.25 to 8.51), P = 0.68 | ||
| Nervous | Gornitsky 2004 | 0/28 | 1/28 | Random‐effects meta‐analysis of 2 studies: RR 1.02 (95% CI 0.11 to 9.33), P = 0.99 Heterogeneity: I2 = 0%, P = 0.33 |
||
| Lozada‐Nur 1998 | 1/12 | 0/12 | ||||
| Rhinitis | Fisher 2003 | 2/118 | 5/114 | Random‐effects meta‐analysis of 3 studies: RR 0.87 (95% CI 0.41 to 1.86), P = 0.72 Heterogeneity: I2 = 0%, P = 0.53 |
||
| Lozada‐Nur 1998 | 1/12 | 1/12 | ||||
| Sangthawan 2001 | 8/29 | 6/24 | ||||
| Blurred vision | Lozada‐Nur 1998 | 1/12 | 0/12 | RR 3.00 (95% CI 0.13 to 67.06), P = 0.49 | ||
| Urinary frequency | Fisher 2003 | 7/118 | 5/114 | Random‐effects meta‐analysis of 2 studies: RR 0.87 (95% CI 0.43 to 1.75), P = 0.70 Heterogeneity: I2 = 0%, P = 0.32 |
||
| Sangthawan 2001 | 6/25 | 8/22 | ||||
| Dizziness | Gornitsky 2004 | 0/28 | 2/28 | Random‐effects meta‐analysis of 2 studies: RR 0.80 (95% CI 0.18 to 3.45), P = 0.76 Heterogeneity: I2 = 13%, P = 0.28 |
||
| Sangthawan 2001 | 4/25 | 3/22 | ||||
| Palpitation | Sangthawan 2001 | 0/25 | 4/22 | RR 0.10 (95% CI 0.01 to 1.73), P = 0.11 | ||
| Skin flushing | Fisher 2003 | 1/118 | 0/114 | RR 2.90 (95% CI 0.12 to 70.44), P = 0.51 | ||
| Motor tremors | Fisher 2003 | 2/118 | 1/114 | RR 1.93 (95% CI 0.18 to 21.02), P = 0.59 | ||
| Sleep problems | Gornitsky 2004 | End of radiotherapy | Mean 37.3 (SD 36.4) n = 17 |
Mean 49.6 (SD 36.9) n = 19 |
Mean difference ‐12.30 (95% CI ‐36.27 to 11.67), P = 0.31 | |
| RTOG (grade 3; mucous membrane, pharynx and larynx) | Warde 2002 | No statistically significant difference between treatment groups | ||||
CI = confidence interval; RR = risk ratio; RTOG = Radiation Therapy Oncology Group; SD = standard deviation.
There was insufficient evidence from one study (Gornitsky 2004) to determine whether or not pilocarpine improved oral mucosal pain at the end of radiotherapy (MD ‐14.80, 95% CI ‐37.07 to 7.47; P = 0.19; 36 participants) (Additional Table 4). The effect of pilocarpine on the treatment and prevention of mucositis has been assessed in more detail in separate Cochrane Reviews (Clarkson 2010; Worthington 2011).
Side effects
No evidence of a difference was found between treatment groups for any reported adverse event, apart from for sweating where data from five studies showed an increased risk associated with pilocarpine (RR 2.98, 95% CI 1.43 to 6.22; P = 0.004; 389 participants) (Additional Table 4). There was no observed statistical heterogeneity (I2 = 0%, P = 0.52).
Cost
None of the included studies evaluating the effectiveness of pilocarpine reported cost data.
Biperiden plus pilocarpine versus no treatment
One trial, assessed at high risk of bias, compared biperiden and pilocarpine with no treatment (Rode 1999).
Xerostomia
No xerostomia data related to the effectiveness of biperiden and pilocarpine were reported.
Salivary flow rates
There was insufficient evidence, from a single trial of 60 participants (Rode 1999), to determine whether or not biperiden and pilocarpine reduced the unstimulated salivary flow rate between patients at the end of radiotherapy (Analysis 2.1).
2.1. Analysis.
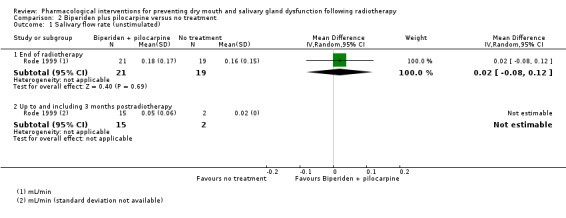
Comparison 2 Biperiden plus pilocarpine versus no treatment, Outcome 1 Salivary flow rate (unstimulated).
Survival
Not reported.
Quality of life and other oral related symptoms
There was insufficient evidence to determine whether or not biperiden and pilocarpine reduces the risk of WHO grade 3+ dysphagia up to one year after radiotherapy (Additional Table 5). No further data on quality of life or other oral related symptoms were reported.
2. Biperiden plus pilocarpine versus no treatment/placebo (other outcomes).
| Outcome | Study ID | Time point | Pilocarpine | Control | Results | Comments |
| Dysphagia (WHO grade 3+) | Rode 1999 | 12 months after RT | 1/30 | 4/30 | RR 0.25 (95% CI 0.03 to 2.11), P = 0.20 |
CI = confidence interval; RR = risk ratio; RT = radiotherapy; WHO = World Health Organization.
Side effects
No data were reported on side effects.
Cost
No cost data related to the effectiveness of biperiden and pilocarpine were reported.
Amifostine versus no treatment/placebo
Eleven trials, one at low risk of bias (Buentzel 2006) and ten at high risk of bias (Antonadou 2002; Brizel 2000; Büntzel 1998; Haddad 2009; He 2004; Jellema 2006; Patni 2004; Peng 2006; Vacha 2003; Veerasarn 2006), randomised 1036 participants (887 analysed, although the number varied by outcome/timing of assessment) to amifostine or no treatment group/placebo. The trial by Jellema 2006 had three comparison groups: two different doses of amifostine and a 'no treatment' group. For the purpose of this comparison, the two amifostine groups were combined.
Xerostomia
Three studies (Büntzel 1998; He 2004; Veerasarn 2006) were combined in a meta‐analysis showing that amifostine reduced the risk of developing grade ≥ 2 xerostomia (on a 0 to 4 scale) at the end of radiotherapy (RR 0.35, 95% CI 0.19 to 0.67; P = 0.001; 119 participants) (Analysis 3.1).
3.1. Analysis.
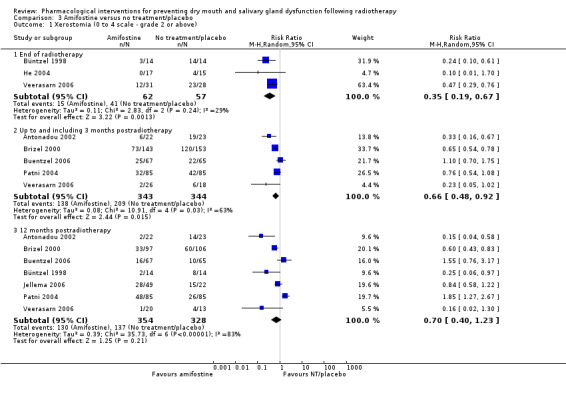
Comparison 3 Amifostine versus no treatment/placebo, Outcome 1 Xerostomia (0 to 4 scale ‐ grade 2 or above).
Up to and including three months postradiotherapy, a smaller effect was observed in favour of amifostine in a meta‐analysis of five studies (Antonadou 2002; Brizel 2000; Buentzel 2006; Patni 2004; Veerasarn 2006) (RR 0.66, 95% CI 0.48 to 0.92; P = 0.01; 687 participants) (Analysis 3.1). However, there was substantial heterogeneity present (I2 = 63%).
At 12 months postradiotherapy, there was insufficient evidence of a difference in the risk of grade ≥ 2 xerostomia (RR 0.70, 95% CI 0.40 to 1.23; P = 0.21; 682 participants analysed) (Antonadou 2002; Brizel 2000; Buentzel 2006; Büntzel 1998; Jellema 2006; Patni 2004; Veerasarn 2006) (Analysis 3.1). There was considerable heterogeneity present (I2 = 83%).
Three further studies had no usable data: one failed to report the data by study group and reported that "For the end point xerostomia we are not able to demonstrate that amifostine had a positive effect, and there was no difference detected between the arms in terms of xerostomia, with 41% of patients reporting xerostomia of grade ≥ 2" (Haddad 2009); one failed to report the timing of assessment (Peng 2006); and one only reported xerostomia during radiotherapy (i.e. not at any of the time points we were interested in) (Vacha 2003).
Salivary flow rates
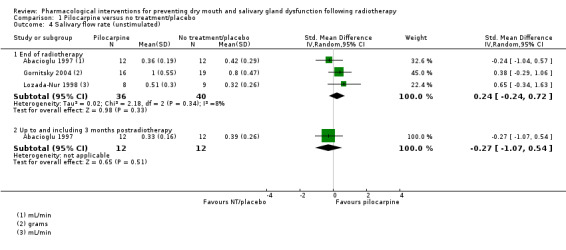
Unstimulated
There was inconsistent evidence regarding the effect of amifostine of unstimulated salivary flow rate. A greater salivary flow rate for those receiving amifostine was shown at the end of radiotherapy (MD 0.34, 95% CI 0.07 to 0.61; P = 0.01; 83 participants) (Analysis 3.2).
3.2. Analysis.
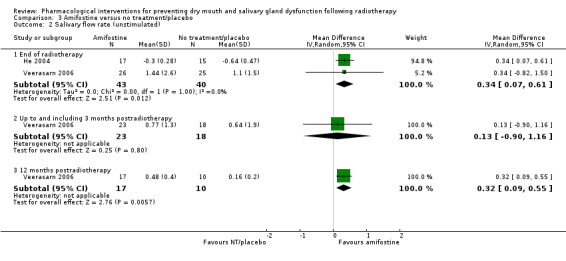
Comparison 3 Amifostine versus no treatment/placebo, Outcome 2 Salivary flow rate (unstimulated).
There was insufficient evidence of a difference from one study (Veerasarn 2006) up to and including three months postradiotherapy (MD 0.13, 95% CI ‐0.90 to 1.16; P = 0.8; 41 participants), but the same study showed a slight benefit in favour of amifostine at 12 months postradiotherapy (MD 0.32, 95% CI 0.09 to 0.55; P = 0.006; 27 participants) (Analysis 3.2). A further study (Brizel 2000) showed a benefit at 12 months postradiotherapy in favour of amifostine when looking at incidence of producing > 0.1 g of saliva over 5 minutes (RR 1.45, 95% CI 1.13 to 1.86; P = 0.004; 175 participants) (Analysis 3.3).
3.3. Analysis.
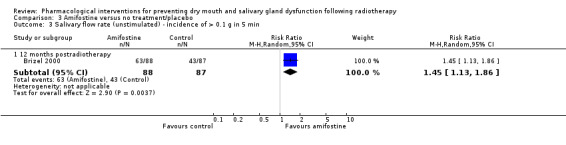
Comparison 3 Amifostine versus no treatment/placebo, Outcome 3 Salivary flow rate (unstimulated) ‐ incidence of > 0.1 g in 5 min.
Haddad 2009 failed to report the salivary flow data by study group and simply reported that "No difference was observed between the 2 treatment arms."
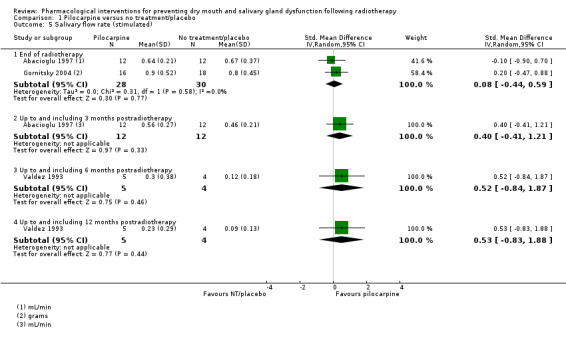
Stimulated
There was insufficient evidence of a difference from one study (Veerasarn 2006) at the end of radiotherapy (MD ‐0.09, 95% CI ‐1.48 to 1.30; P = 0.90; 47 participants), up to and including three months postradiotherapy (MD 0.38, 95% CI ‐1.43 to 2.19; P = 0.68; 41 participants), or 12 months postradiotherapy (MD 0.82, 95% CI ‐0.47 to 2.11; P = 0.21; 27 participants) (Analysis 3.4). There was also insufficient evidence of a difference from one study (Brizel 2000), analysing 173 participants, when looking at incidence of producing > 0.1 g of saliva over 5 minutes at 12 months postradiotherapy (RR 1.12, 95% CI 0.89 to 1.41; P = 0.32) (Analysis 3.5).
3.4. Analysis.
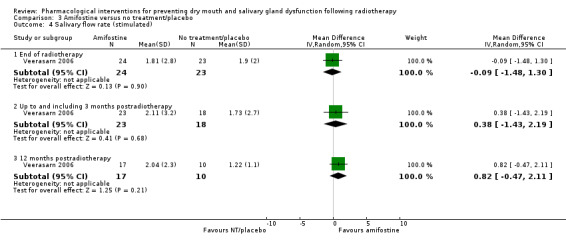
Comparison 3 Amifostine versus no treatment/placebo, Outcome 4 Salivary flow rate (stimulated).
3.5. Analysis.
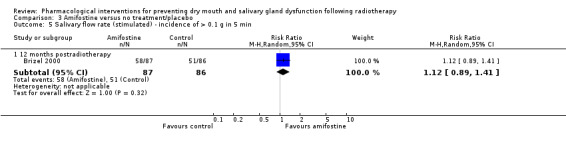
Comparison 3 Amifostine versus no treatment/placebo, Outcome 5 Salivary flow rate (stimulated) ‐ incidence of > 0.1 g in 5 min.
Haddad 2009 failed to report the salivary flow data by study group and simply reported that "No difference was observed between the 2 treatment arms."
Survival
There was insufficient evidence to determine whether or not amifostine reduces overall survival, progression‐free survival, disease‐free survival or locoregional tumour control up to 24 months postradiotherapy.
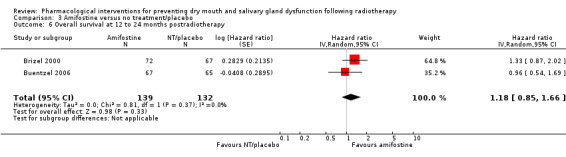
Overall survival
There was insufficient evidence from a meta‐analysis of two studies (Brizel 2000; Buentzel 2006) to determine whether or not amifostine reduces overall survival at 12 to 24 months postradiotherapy (hazard ratio (HR) 1.18, 95% CI 0.85 to 1.66; P = 0.33; 271 participants) (Analysis 3.6). Two further studies found no difference in overall survival at two years or more postradiotherapy (Haddad 2009; Jellema 2006) (Analysis 3.7).
3.6. Analysis.
Comparison 3 Amifostine versus no treatment/placebo, Outcome 6 Overall survival at 12 to 24 months postradiotherapy.
3.7. Analysis.
Comparison 3 Amifostine versus no treatment/placebo, Outcome 7 Overall survival ‐ narrative data.
| Overall survival ‐ narrative data | ||||
|---|---|---|---|---|
| Study | Time point | Amifostine | Control | Comments |
| Haddad 2009 | Median follow‐up 34 months after radiotherapy, minimum 26 months | "No differences noted" | ||
| Jellema 2006 | 24 months | 3 times weekly = 84% 5 times weekly = 58% |
70% | Reported narratively rather than as a risk ratio due to differing results in the amifostine arms |
Progression‐free survival
There was insufficient evidence from a meta‐analysis of two studies (Brizel 2000; Buentzel 2006) to determine whether or not amifostine reduces progression‐free survival at 12 to 24 months postradiotherapy (HR 0.94, 95% CI 0.70 to 1.27; P = 0.70; 247 participants) (Analysis 3.8). A further study (Antonadou 2002) found no difference at 18 months postradiotherapy (RR 1.11, 95% CI 0.81 to 1.51; P = 0.52; 45 participants) (Analysis 3.9). This was supported by Haddad 2009 who reported "no differences noted" (Analysis 3.10).
3.8. Analysis.
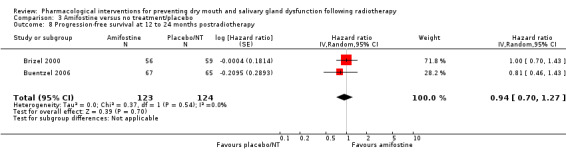
Comparison 3 Amifostine versus no treatment/placebo, Outcome 8 Progression‐free survival at 12 to 24 months postradiotherapy.
3.9. Analysis.
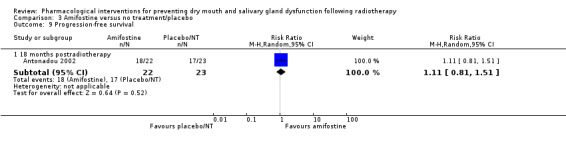
Comparison 3 Amifostine versus no treatment/placebo, Outcome 9 Progression‐free survival.
3.10. Analysis.
Comparison 3 Amifostine versus no treatment/placebo, Outcome 10 Progression‐free survival ‐ narrative data.
| Progression‐free survival ‐ narrative data | ||||
|---|---|---|---|---|
| Study | Time point | Amifostine | Control | Comments |
| Haddad 2009 | Median follow‐up 34 months after radiotherapy, minimum 26 months | "No differences noted" | ||
Locoregional tumour control
There was insufficient evidence from a meta‐analysis of two studies (Brizel 2000; Buentzel 2006) to determine whether or not amifostine reduces locoregional tumour control at 12 to 24 months postradiotherapy (HR 0.90, 95% CI 0.74 to 1.11; P = 0.33; 279 participants) (Analysis 3.11). Three further studies reported narrative evidence to support this result (Haddad 2009; Jellema 2006; Patni 2004) (Analysis 3.12).
3.11. Analysis.
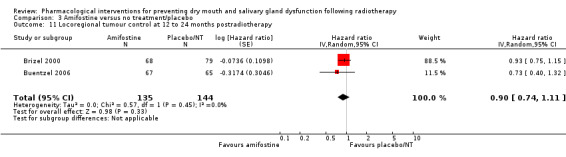
Comparison 3 Amifostine versus no treatment/placebo, Outcome 11 Locoregional tumour control at 12 to 24 months postradiotherapy.
3.12. Analysis.
Comparison 3 Amifostine versus no treatment/placebo, Outcome 12 Locoregional tumour control ‐ narrative data.
| Locoregional tumour control ‐ narrative data | ||||
|---|---|---|---|---|
| Study | Time point | Amifostine | Control | Comments |
| Haddad 2009 | Median follow‐up 34 months after radiotherapy, minimum 26 months | "No differences noted" | ||
| Jellema 2006 | 24 months | 3 times weekly = 67% 5 times weekly = 83% |
79% | Reported narratively rather than as a risk ratio due to differing results in the amifostine arms |
| Patni 2004 | 24 month | No data | No data | "Amifostine does not alter the response or the survival" |
Disease‐free survival
There was insufficient evidence from one study (Patni 2004) to determine whether or not amifostine reduces disease‐free survival at 24 months postradiotherapy (RR 0.94, 95% CI 0.73 to 1.21; P = 0.64; 170 participants) (Analysis 3.13). Two studies reported narrative evidence to support this result (Patni 2004; Veerasarn 2006) (Analysis 3.14).
3.13. Analysis.
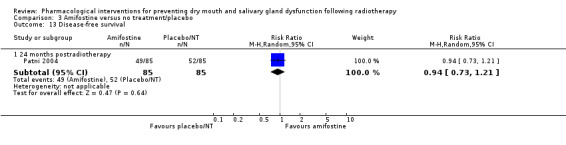
Comparison 3 Amifostine versus no treatment/placebo, Outcome 13 Disease‐free survival.
3.14. Analysis.
Comparison 3 Amifostine versus no treatment/placebo, Outcome 14 Disease‐free survival.
| Disease‐free survival | ||||
|---|---|---|---|---|
| Study | Time point | Amifostine | Control | Comments |
| Patni 2004 | 24 months | No data | No data | "Amifostine does not alter the response or the survival" |
| Veerasarn 2006 | 24 months | No data | No data | "There was no statistical difference in 2‐year disease‐free survival" |
Quality of life and other oral related symptoms
There was insufficient evidence of a difference in quality of life from one study (Brizel 2000), both at the end of radiotherapy (MD 0.38, 95% CI ‐0.07 to 0.83; P = 0.1; 298 participants), and up to and including three months postradiotherapy (MD 0.52, 95% CI ‐0.02 to 1.06; P = 0.06; 233 participants). The same study showed a benefit in favour of amifostine at 12 months postradiotherapy (MD 0.70, 95% CI 0.20 to 1.20; P = 0.006; 180 participants) (Analysis 3.15). A further study reported no differences in quality of life but did not present data (Jellema 2006) (Additional Table 6).
3.15. Analysis.
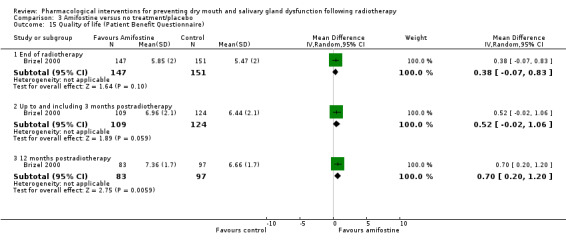
Comparison 3 Amifostine versus no treatment/placebo, Outcome 15 Quality of life (Patient Benefit Questionnaire).
3. Amifostine versus no treatment/placebo (other outcomes).
| Outcome | Study ID | Time point | Amifostine | Control | Results | Comments |
| Quality of life | Jellema 2006 | Assessed at end of RT and 6, 12, 18 and 24 months after RT | No data | No data | "No significant differences between the 3 treatment arms" | |
| Dysphagia (difficulty in swallowing) (0‐4 scale): grade 3 and above | Antonadou 2002 | End of RT | 14/22 | 23/23 | Random‐effects meta‐analysis of 2 studies: RR 0.50 (95% CI 0.17 to 1.48); P = 0.21 Heterogeneity: I2 = 40%, P = 0.20 |
|
| Büntzel 1998 | 1/14 | 5/14 | ||||
| Antonadou 2002 | 4 weeks after RT | 2/22 | 3/23 | RR 0.70 (95% CI 0.13 to 3.78); P = 0.68 | By 8 weeks after RT, no participants had grade 3 or above dysphagia | |
| Dysgeusia (taste disturbance) (0‐4 scale): grade 2 and above | Büntzel 1998 | End of RT | 3/14 | 14/14 | RR 0.24 (95% CI 0.10 to 0.61); P = 0.003 | |
| Cost data (mean per patient supportive care costs) | Büntzel 1998 | End of RT | USD 4401 | USD 5873 | P = 0.02 | |
| Vomiting | Antonadou 2002 | 1/22 | 0/23 | Random‐effects meta‐analysis of 5 studies: RR 4.90 (95% CI 2.87 to 8.38); P < 0.00001 Heterogeneity: I2 = 0%, P = 0.96 |
||
| Brizel 2000 | 55/150 | 11/153 | ||||
| Buentzel 2006 | 8/66 | 2/64 | ||||
| He 2004 | 1/17 | 0/15 | "1 patient left due to gastrointestinal tract reaction/side effect, all other patients completed the treatment" "At the beginning of treatment, nausea and vomiting was obvious for amifostine group, but after treating with metoclopramide, there was no significant difference between 2 groups in gastrointestinal tract reaction/side effect" |
|||
| Jellema 2006 | 10/60 | 0/31 | ||||
| Peng 2006 | 10/18 | Data not reported in control group. Unknown if this was due to 0 events | ||||
| Veerasarn 2006 | 18/32 | Data not reported in control group. Unknown if this was due to 0 events | ||||
| Hypotension | Antonadou 2002 | 3/22 | 0/23 | Random‐effects meta‐analysis of 3 studies: RR 9.20 (95% CI 2.84 to 29.83); P = 0.0002 Heterogeneity: I2 = 0%, P = 0.88 |
||
| Brizel 2000 | 22/150 | 2/153 | ||||
| Büntzel 1998 | 2/14 | 0/14 | ||||
| Veerasarn 2006 | 5/32 | Data not reported in control group. Unknown if this was due to 0 events | ||||
| Nausea | Brizel 2000 | 66/150 | 25/153 | Random‐effects meta‐analysis of 4 studies: RR 2.60 (95% CI 1.81 to 3.74); P < 0.00001 Heterogeneity: I2 = 0%, P = 0.45 |
||
| Buentzel 2006 | 4/66 | 4/64 | ||||
| He 2004 | 1/17 | 0/15 | "1 patient left due to gastrointestinal tract reaction/side effect, all other patients completed the treatment" "At the beginning of treatment, nausea and vomiting was obvious for amifostine group, but after treating with metoclopramide, there was no significant difference between 2 groups in gastrointestinal tract reaction/side effect" |
|||
| Jellema 2006 | 23/60 | 3/31 | ||||
| Peng 2006 | 10/18 | Data not reported in control group. Unknown if this was due to 0 events | ||||
| Veerasarn 2006 | 20/32 | Data not reported in control group. Unknown if this was due to 0 events | ||||
| Allergic response | Brizel 2000 | 8/150 | 0/153 | Random‐effects meta‐analysis of 3 studies: RR 7.51 (95% CI 1.40 to 40.39); P = 0.02 Heterogeneity: I2 = 0%, P = 0.77 |
||
| Buentzel 2006 | 2/66 | 0/64 | ||||
| Jellema 2006 | 4/60 | 0/31 | ||||
| Asthenia (weakness or lack of energy) | Buentzel 2006 | 3/66 | 1/64 | RR 2.91 (95% CI 0.31 to 27.24); P = 0.35 | ||
| Alopecia | Vacha 2003 | Similar in both groups and increased continuously during the treatment | ||||
| Skin toxicity | Vacha 2003 | Similar in both groups and increased continuously during the treatment | ||||
| Hot flush | Peng 2006 | "..dizziness, fatigue, hiccup, sneezing, facial flush all in less than 5% of the patients" | ||||
| Veerasarn 2006 | 17/32 | Data not reported in control group. Unknown if this was due to 0 events | ||||
| Somnolence (drowsiness) | Veerasarn 2006 | 18/32 | Data not reported in control group. Unknown if this was due to 0 events | |||
| Sneezing | Peng 2006 | "..dizziness, fatigue, hiccup, sneezing, facial flush all in less than 5% of the patients" | ||||
| Veerasarn 2006 | 13/32 | Data not reported in control group. Unknown if this was due to 0 events | ||||
| Hiccup | Peng 2006 | "..dizziness, fatigue, hiccup, sneezing, facial flush all in less than 5% of the patients" | ||||
| Veerasarn 2006 | 10/32 | Data not reported in control group. Unknown if this was due to 0 events | ||||
| Dizziness | Peng 2006 | "...dizziness, fatigue, hiccup, sneezing, facial flush all in less than 5% of the patients" | ||||
| Fatigue | Peng 2006 | "..dizziness, fatigue, hiccup, sneezing, facial flush all in less than 5% of the patients" | ||||
CI = confidence interval; RR = risk ratio; RT = radiotherapy; USD = US dollars.
Two of the 11 studies presented data on dysphagia (Antonadou 2002; Büntzel 1998). There was insufficient evidence of a difference in the risk of developing grade ≥ 3 dysphagia (on a 0 to 4 scale) at the end of radiotherapy (RR 0.50, 95% CI 0.17 to 1.48; P = 0.21; 73 participants) and up to and including three months postradiotherapy (four weeks after) (RR 0.70, 95% CI 0.13 to 3.78; P = 0.68; 45 participants) (Additional Table 6). In Antonadou 2002, no participants had grade 3 or above dysphagia by eight weeks after radiotherapy.
One study presented data on dysgeusia (Büntzel 1998). The study showed that amifostine reduced the risk of developing grade ≥ 2 dysgeusia (on a 0 to 4 scale) at the end of radiotherapy (RR 0.24, 95% CI 0.10 to 0.61; P = 0.003; 28 participants) (Additional Table 6).
Side effects
Adverse events were reported inconsistently across the 11 included trials. There was a higher risk of vomiting in the amifostine group (RR 4.90, 95% CI 2.87 to 8.38; P < 0.00001; five studies; 601 participants) (Antonadou 2002; Brizel 2000; Buentzel 2006; He 2004; Jellema 2006). Two further studies reported high rates of vomiting in the amifostine group but did not mention vomiting in the control group (Peng 2006; Veerasarn 2006). The risk of hypotension was higher in the amifostine group (RR 9.20, 95% CI 2.84 to 29.83; P = 0.0002; three studies; 376 participants) (Antonadou 2002; Brizel 2000; Büntzel 1998). Another study reported hypotension only in the amifostine group (Veerasarn 2006). The risk of nausea was higher in the amifostine group (RR 2.60, 95% CI 1.81 to 3.74; P < 0.00001; four studies; 556 participants) (Brizel 2000; Buentzel 2006; He 2004; Jellema 2006). Two further studies reported high rates of nausea in the amifostine group but did not mention nausea in the control group (Peng 2006; Veerasarn 2006). The risk of allergic response was higher in the amifostine group (RR 7.51, 95% CI 1.40 to 40.39; P = 0.02; three studies; 524 participants) (Brizel 2000; Buentzel 2006; Jellema 2006). There was insufficient evidence of a difference in asthenia (weakness/lack of energy) from one study (RR 2.91, 95% CI 0.31 to 27.24; P = 0.35; 130 participants) (Buentzel 2006). Other side effects (alopecia, skin toxicity, hot flush, drowsiness, sneezing, hiccupping, dizziness and fatigue) were reported either narratively or only for the amifostine group (Additional Table 6).
Cost
One study (Büntzel 1998), analysing 28 participants, reported economic cost data in a separate paper (Bennett 2001). In 2001, the mean per patient supportive care costs were lower in the amifostine group (USD 4401) than the control group (USD 5873) (P = 0.02) (Additional Table 6).
Amifostine (comparison of dosages)
One trial, at high risk of bias, compared two different amifostine regimens of 200 mg/m² either five or three times a week (Jellema 2006) (a third 'no treatment' group was not considered in this comparison).
Xerostomia
There was insufficient evidence to determine whether or not different amifostine dosages reduced the risk of developing grade ≥ 2 xerostomia (on a 0 to 4 scale) at 12 months postradiotherapy (RR 0.94, 95% CI 0.58 to 1.53; P = 0.80; 49 participants) (Analysis 4.1).
4.1. Analysis.
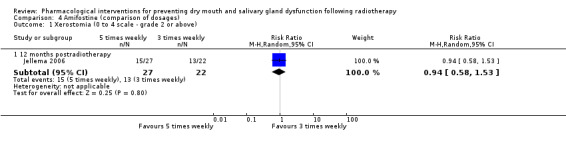
Comparison 4 Amifostine (comparison of dosages), Outcome 1 Xerostomia (0 to 4 scale ‐ grade 2 or above).
Salivary flow rates
No salivary flow rate data, related to the effectiveness of different doses of amifostine, were reported.
Survival
There is insufficient evidence reported on overall survival or locoregional tumour control (Analysis 4.2; Analysis 4.3).
4.2. Analysis.
Comparison 4 Amifostine (comparison of dosages), Outcome 2 Overall survival ‐ narrative data.
| Overall survival ‐ narrative data | ||||
|---|---|---|---|---|
| Study | Time point | Amifostine 3 times weekly | Amifostine 5 times weekly | Comments |
| Jellema 2006 | 24 months | 84% | 58% | |
4.3. Analysis.
Comparison 4 Amifostine (comparison of dosages), Outcome 3 Locoregional tumour control ‐ narrative data.
| Locoregional tumour control ‐ narrative data | ||||
|---|---|---|---|---|
| Study | Time point | Amifostine 3 times weekly | Amifostine 5 times weekly | Comments |
| Jellema 2006 | 24 months | 67% | 83% | |
Quality of life and other oral related symptoms
The paper reported "no significant differences between the three treatment arms" in quality of life assessed at the end of radiotherapy and 6, 12, 18 and 24 months after radiotherapy (Additional Table 7).
4. Amifostine: comparison of different doses (other outcomes).
| Outcome | Study ID | Time point | Amifostine 3 times weekly | Amifostine 5 times weekly | Results | Comments |
| Quality of life | Jellema 2006 | Assessed at end of RT and 6, 12, 18 and 24 months after RT | No data | No data | "No significant differences between the 3 treatment arms" | |
| Nausea | Jellema 2006 | 9/30 | 14/30 | RR 0.64 (95% CI 0.33 to 1.25); P = 0.19 | ||
| Vomiting | Jellema 2006 | 2/30 | 8/30 | RR 0.25 (95% CI 0.06 to 1.08); P = 0.06 | ||
| Allergic response | Jellema 2006 | 2/30 | 2/30 | RR 1.00 (95% CI 0.15 to 6.64); P = 1 |
CI = confidence interval; RR = risk ratio; RT = radiotherapy.
Side effects
There was insufficient evidence of a difference in nausea (RR 0.64, 95% CI 0.33 to 1.25; P = 0.19; 60 participants), vomiting (RR 0.25, 95% CI 0.06 to 1.08; P = 0.06; 60 participants), or allergic response (RR 1.00, 95% CI 0.15 to 6.64; P = 1; 60 participants) (Additional Table 7).
Cost
No cost data related to the effectiveness of different doses of amifostine were reported.
Amifostine (intravenous versus subcutaneous)
One study, at high risk of bias, compared intravenous and subcutaneous delivery of amifostine (Bardet 2011).
Xerostomia
There was insufficient evidence to determine whether or not different methods of amifostine delivery reduced the risk of developing grade ≥ 2 xerostomia (on a 0 to 4 scale) up to and including three months postradiotherapy (RR 1.03, 95% CI 0.76 to 1.40; P = 0.86; 263 participants). There was a benefit in favour of amifostine at 12 months postradiotherapy (RR 0.61, 95% CI 0.42 to 0.88; P = 0.008; 127 participants) (Analysis 5.1).
5.1. Analysis.
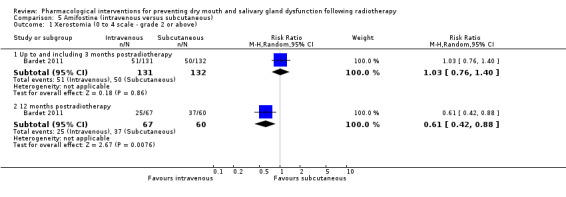
Comparison 5 Amifostine (intravenous versus subcutaneous), Outcome 1 Xerostomia (0 to 4 scale ‐ grade 2 or above).
Salivary flow rates
No salivary flow rate data, related to the effectiveness of different routes of administration of amifostine, were reported.
Survival
There was insufficient evidence of a difference in overall survival (HR 1.36, 95% CI 0.89 to 2.10; P = 0.16; Analysis 5.2) or locoregional tumour control (HR 1.34, 95% CI 0.76 to 2.36; P = 0.32; Analysis 5.3), both at 48 months postradiotherapy.
5.2. Analysis.
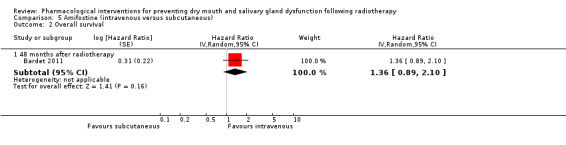
Comparison 5 Amifostine (intravenous versus subcutaneous), Outcome 2 Overall survival.
5.3. Analysis.
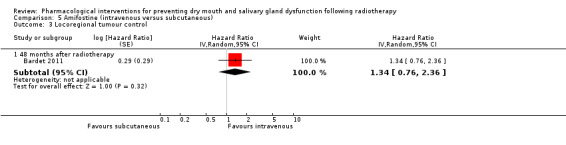
Comparison 5 Amifostine (intravenous versus subcutaneous), Outcome 3 Locoregional tumour control.
Quality of life and other oral related symptoms
No data on either quality of life or other oral related symptoms were reported.
Side effects
The single trial comparing intravenous and subcutaneous delivery of amifostine reported increased incidence of hypotension for intravenous delivery. Skin rash and local pain at injection site were worse for subcutaneous delivery (Additional Table 8). Results were inconclusive with regard to nausea/vomiting, fever, and asthenia (weakness/lack of energy).
5. Amifostine: different routes of administration (other outcomes).
| Outcome | Study ID | Time point | Intravenous | Subcutaneous | Results | Comments |
| Nausea/vomiting | Bardet 2011 | 29% | 36% | P = 0.267 | ||
| Hypotension | Bardet 2011 | 20% | 8% | P = 0.007 | ||
| Skin rash | Bardet 2011 | 10% | 22% | P = 0.012 | ||
| Local pain at injection site | Bardet 2011 | 0% | 8% | P = 0.001 | ||
| Fever | Bardet 2011 | 2% | 0% | P = 0.256 | ||
| Asthenia (weakness or lack of energy) | Bardet 2011 | 1% | 6% | P = 0.054 |
Cost
No cost data related to the effectiveness of different routes of administration of amifostine were reported.
Chinese medicine versus no treatment/placebo
Five studies compared some form of Chinese medicine with no treatment/placebo. Four studies were assessed as being at high risk of bias (Han 2010; Hu 2005; Reshma 2012; Wang 1998); one study was assessed as being at unclear risk of bias (Lin 2014).
Xerostomia
Hu 2005 found that patients who received Shenqi Fanghon recipe had a reduced risk of xerostomia at the end of radiotherapy compared to those in the no treatment control group (RR 0.39, 95% CI 0.28 to 0.55; P < 0.00001; 140 participants) (Analysis 6.1). The paper was translated from Chinese, the methods were unclear.
6.1. Analysis.
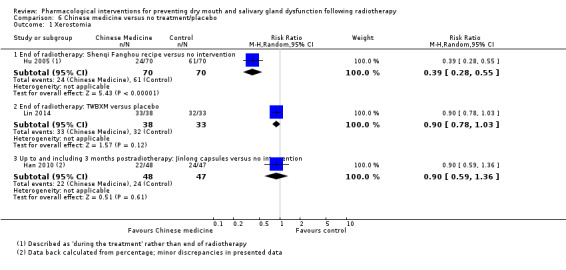
Comparison 6 Chinese medicine versus no treatment/placebo, Outcome 1 Xerostomia.
Lin 2014, a trial of 71 participants, however, found no evidence of a difference for Tianwang Buxin Mini‐pills when compared with placebo when xerostomia was evaluated using both dichotomous data (Analysis 6.1) or continuous data (Analysis 6.2). Similarly, Han 2010, a trial of 95 participants, found no evidence of a difference for Jinlong capsules when compared with no intervention (Analysis 6.1).
6.2. Analysis.
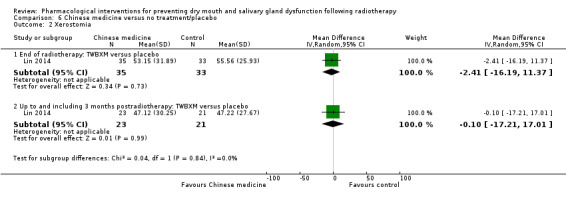
Comparison 6 Chinese medicine versus no treatment/placebo, Outcome 2 Xerostomia.
Wang 1998, a trial of 50 participants, found a difference in favour of Chinese medicine in an assessment of xerostomia (VAS) against a no treatment group at the end of radiotherapy (P < 0.05). The results were graphically represented and the standard deviations were not available from the paper.
Salivary flow rates
One study showed an increase in stimulated salivary flow rate in favour of Chinese medicine when compared with no treatment at the end of radiotherapy (MD 0.09, 95% CI 0.03 to 0.15; P = 0.001; 50 participants) (Analysis 6.3). The paper was translated from Chinese, with the standard deviations being estimated (Wang 1998). Reshma 2012 mentioned salivary status but provided no data.
6.3. Analysis.
Comparison 6 Chinese medicine versus no treatment/placebo, Outcome 3 Salivary flow rate (stimulated).
Survival
Hu 2005 evaluated overall survival (12 months postradiotherapy) but there was insufficient evidence to determine any effect (Analysis 6.4).
6.4. Analysis.
Comparison 6 Chinese medicine versus no treatment/placebo, Outcome 4 Overall survival (12 months postRT).
Quality of life and other oral related symptoms
Lin 2014 evaluated quality of life (at end of intervention and up to and including three months postradiotherapy) but there was insufficient evidence to determine any effect (Analysis 6.5). The same study showed insufficient evidence of a difference for other oral related symptoms (both at end of radiotherapy and one month after) (Additional Table 9). In Hu 2005 difficulty in mouth opening was worse in the control group (Additional Table 9).
6.5. Analysis.
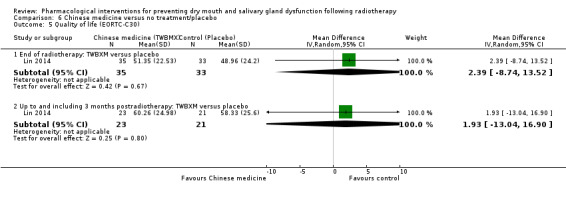
Comparison 6 Chinese medicine versus no treatment/placebo, Outcome 5 Quality of life (EORTC‐C30).
6. Chinese medicine (other outcomes).
| Outcome | Study ID | Intervention | Time point | Study | Control | Results | Comments |
| Dysphasia (difficulty in swallowing) (score for EORTC‐H&N35 questionnaire: mean (SD)) |
Lin 2014 | Chinese medicine (TWBXM) | End of RT | 50.2 (26.3); n = 35 | 38.9 (25.9); n = 33 | P = 0.07 | |
| Lin 2014 | Chinese medicine (TWBXM) | 1 month after RT | 30.2 (29.8); n = 23 | 26.7 (24.8); n = 21 | P = 0.65 | ||
| Dysgeusia (taste disturbance) (0 to 3 scale): grade 1 and above | Lin 2014 | Chinese medicine (TWBXM) | End of RT | 32/38 | 29/33 | RR 0.96 (95% CI 0.79 to 1.16); P = 0.13 | |
| Speech difficulty (mean (SD) score for EORTC‐H&N35 questionnaire) |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 36.3 (26.7); n = 35 | 28.6 (26.2); n = 33 | P = 0.23 | |
|
Lin 2014 |
Chinese medicine (TWBXM) | 1 month after RT | 27.4 (28.6); n = 23 | 22.7 (19.5); n = 21 | P = 0.50 | ||
| Difficulty in mouth opening (mean (SD) score for EORTC‐H&N35 questionnaire) |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 39.6 (28.2); n = 35 | 41.4 (27.7); n = 33 | P = 0.79 | |
|
Lin 2014 |
Chinese medicine (TWBXM) | 1 month after RT | 32.2 (31.5); n = 23 | 33.3 (24.1); n = 21 | P = 0.88 | ||
| Difficulty in mouth opening (0 to 2 scale): grade 1 and above |
Hu 2005 | Chinese medicine (Shenqi Fanghou recipe) | "During the treatment" | 22/70 | 52/70 | RR 0.42 (95% CI 0.29 to 0.61); P < 0.001 | |
| Skin toxicity (0 to 3 scale): grade 1 and above |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 35/38 | 30/33 | RR 1.01 (95% CI 0.88 to 1.17); P = 0.82 | |
| Skin toxicity (0 to 4 scale): grade 1 and above | Hu 2005 | Chinese medicine (Shenqi Fanghou recipe) | "During the treatment" | 57/70 | 68/70 | RR 0.84 (95% CI 0.74 to 0.94); P = 0.002 | |
| Skin toxicity (prevalence according to RTOG standards) | Han 2010 | Chinese medicine (Jinlong capsule) | 46.82% | 58.32% | Quote: "toxicities during and after treatment were assessed" Comment: time point for assessment unclear; minor discrepancies in presented data |
||
| Nausea/vomiting (0 to 3 scale): grade 1 and above |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 12/38 | 4/33 | RR 2.61 (95% CI 0.93 to 7.30); P = 0.183 | |
| Hoarseness |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 1/38 | 3/33 | RR 0.29 (95% CI 0.03 to 2.65); P = 0.26 | |
| Fatigue (mean (SD) score for EORTC‐C30 questionnaire) |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 43.2 (26.2); n = 35 | 42.4 (23.0); n = 33 | P = 0.88 | |
|
Lin 2014 |
Chinese medicine (TWBXM) | 1 month after RT | 31.2 (28.3); n = 23 | 36.4 (25.0); n = 21 | P = 0.51 | ||
| Pain (mean (SD) score for EORTC‐C30 questionnaire) |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 46.8 (23.2); n = 35 | 41.7 (27.4); n = 33 | P = 0.40 | |
|
Lin 2014 |
Chinese medicine (TWBXM) | 1 month after RT | 35.9 (27.0); n = 23 | 40.9 (29.9); n = 21 | P = 0.54 | ||
| Pain (mean (SD) score for EORTC‐H&N35 questionnaire) |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 55.4 (25.1); n = 35 | 42.4 (20.5); n = 33 | P = 0.02 | |
|
Lin 2014 |
Chinese medicine (TWBXM) | 1 month after RT | 31.6 (24.2); n = 23 | 37.8 (23.3); n = 21 | P = 0.35 | ||
| Dyspnea (mean (SD) score for EORTC‐C30 questionnaire) |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 17.1 (23.1); n = 35 | 16.7 (20.7); n = 33 | P = 0.93 | |
|
Lin 2014 |
Chinese medicine (TWBXM) | 1 month after RT | 20.5 (21.2); n = 23 | 13.6 (22.2); n = 21 | P = 0.28 | ||
| Insomnia (mean (SD) score for EORTC‐C30 questionnaire) |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 40.5 (25.0); n = 35 | 31.2 (25.3); n = 33 | P = 0.13 | |
|
Lin 2014 |
Chinese medicine (TWBXM) | 1 month after RT | 30.8 (24.8); n = 23 | 31.8 (28.1); n = 21 | P = 0.28 | ||
| Appetite loss (mean (SD) score for EORTC‐C30 questionnaire) |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 45.0 (30.7); n = 35 | 45.8 (29.0); n = 33 | P = 0.91 | |
|
Lin 2014 |
Chinese medicine (TWBXM) | 1 month after RT | 28.2 (26.1); n = 23 | 34.9 (30.0); n = 21 | P = 0.42 | ||
| Constipation (mean (SD) score for EORTC‐C30 questionnaire) |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 37.8 (27.4); n = 35 | 29.2 (20.3); n = 33 | P = 0.15 | |
|
Lin 2014 |
Chinese medicine (TWBXM) | 1 month after RT | 29.5 (30.3); n = 23 | 25.7 (20.4); n = 21 | P = 0.63 | ||
| Diarrhoea (mean (SD) score for EORTC‐C30 questionnaire) |
Lin 2014 |
Chinese medicine (TWBXM) | End of RT | 9.0 (15.0); n = 35 | 6.2 (13.2); n = 33 | P = 0.42 | |
|
Lin 2014 |
Chinese medicine (TWBXM) | 1 month after RT | 9.0 (15.1); n = 23 | 6.1 (16.7); n = 21 | P = 0.53 | ||
| Adverse effects | Han 2010 | Chinese medicine (Jinlong capsule) | Leukopenia, nausea, vomiting, 1 participant had dizziness and blood pressure drop, 1 participant had skin rash | Not reported | |||
| Adverse effects | Hu 2005 | Chinese medicine (Shenqi Fanghou recipe) | "During the treatment" | No adverse event | Not reported |
CI = confidence interval; EORTC = European Organisation for Research and Treatment of Cancer; H&N = head and neck; RR = risk ratio; RT = radiotherapy; RTOG = Radiation Therapy Oncology Group; SD = standard deviation; TWBXM = Tianwang Buxin Mini‐pills.
Side effects
There was insufficient evidence of a difference for any side effects (at end of intervention and one month after) (Additional Table 9).
Cost
No cost data related to the effectiveness of Chinese medicine were reported.
Palifermin versus placebo
Three trials, two at low (Henke 2011; Le 2011) and one at unclear risk of bias (Brizel 2008), evaluated palifermin versus placebo.
Xerostomia
In a meta‐analysis of all three trials, there was insufficient evidence of a difference in the incidence of > grade 2 xerostomia up to three months postradiotherapy (RR 0.97, 95% CI 0.77 to 1.22; P = 0.78; 471 participants). There was considerable heterogeneity present (I2 = 76%, P = 0.02) (Analysis 7.1). It should be noted that a large proportion of participants in Henke 2011 did not have assessments for xerostomia, but the intention‐to‐treat (ITT) rules stated that such participants would be assumed to have the outcome, and this may have had a substantial effect on the meta‐analysis result.
7.1. Analysis.
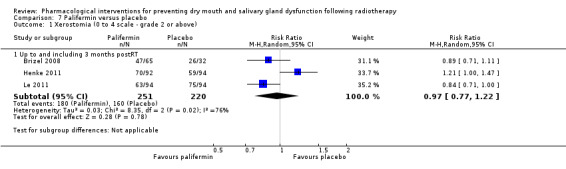
Comparison 7 Palifermin versus placebo, Outcome 1 Xerostomia (0 to 4 scale ‐ grade 2 or above).
Xerostomia was measured up to 12 months in two studies but no data were reported (Henke 2011; Le 2011).
Salivary flow rates
None of the trials evaluating palifermin provided data on salivary flow rates.
Survival
All three trials reported data on overall and progression‐free survival at 42 to 72 months from baseline. There was insufficient evidence of a difference in both overall survival (HR 1.00, 95% CI 0.72 to 1.39; P = 0.99; Analysis 7.2) and progression‐free survival (HR 1.06, 95% CI 0.80 to 1.42; P = 0.67; Analysis 7.3).
7.2. Analysis.
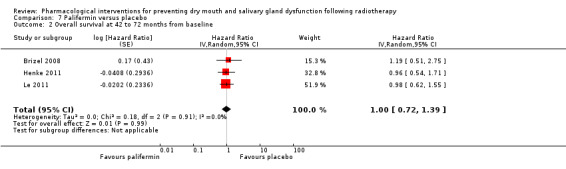
Comparison 7 Palifermin versus placebo, Outcome 2 Overall survival at 42 to 72 months from baseline.
7.3. Analysis.
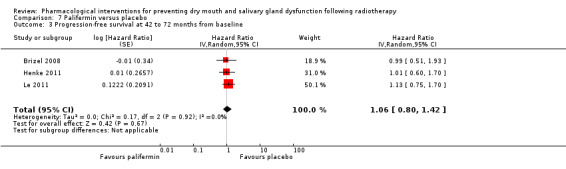
Comparison 7 Palifermin versus placebo, Outcome 3 Progression‐free survival at 42 to 72 months from baseline.
Quality of life and other oral related symptoms
All three trials provided data for a meta‐analysis of dysphagia at three months postradiotherapy, with insufficient evidence of a difference (RR 1.32, 95% CI 0.55 to 3.13; P = 0.54) (Additional Table 10). There was also insufficient evidence of a difference in mouth and throat soreness at three months postradiotherapy in a meta‐analysis of two trials (MD ‐0.12, 95% CI ‐0.27 to 0.02; P = 0.10) (Additional Table 10).
7. Palifermin versus placebo (other outcomes).
| Outcome | Study ID | Time point | Palifermin | Placebo | Results |
| Oral related symptoms (other than salivary gland dysfunction/xerostomia) | |||||
| Dysphagia | Le 2011 | 3 months postRT | 29/94 | 19/91 | Random‐effects meta‐analysis of 3 studies: RR 1.32 (95% CI 0.55 to 3.13); P = 0.54 Heterogeneity: I2 = 94%, P < 0.00001 |
| Brizel 2008 | 61/64 | 31/32 | |||
| Henke 2011 | 32/92 | 20/93 | |||
| Mouth and throat soreness ‐ 0 (no soreness) to 4 (extreme soreness) OMWQ‐HN scale | Le 2011 | 3 months postRT | n = 94, mean = 1.66, SD = 0.73 | n = 94, mean = 1.86, SD = 0.65 | Random‐effects meta‐analysis of 2 studies: mean difference ‐0.12 (95% CI ‐0.27 to 0.02); P = 0.10 Heterogeneity: I2 = 13%, P = 0.28 |
| Henke 2011 | n = 92, mean = 1.52, SD = 0.69 | n = 94, mean = 1.57, SD = 0.63 | |||
| Adverse events | |||||
| Nausea | Le 2011 | 47/94 | 42/91 | Random‐effects meta‐analysis of 2 studies: RR 0.96 (95% CI 0.77 to 1.19); P = 0.69 Heterogeneity: I2 = 28%, P = 0.24 |
|
| Brizel 2008 | 48/67 | 26/32 | |||
| Fever | Brizel 2008 | 30/67 | 13/32 | RR 1.10 (95% CI 0.67 to 1.81); P = 0.70 | |
| Constipation | Le 2011 | 31/94 | 24/91 | Random‐effects meta‐analysis of 2 studies: RR 1.15 (95% CI 0.82 to 1.60); P = 0.42 Heterogeneity: I2 = 0%, P = 0.57 |
|
| Brizel 2008 | 28/67 | 13/32 | |||
| Diarrhoea | Brizel 2008 | 14/67 | 8/32 | Random‐effects meta‐analysis of 2 studies: RR 1.28 (95% CI 0.49 to 3.36); P = 0.61 Heterogeneity: I2 = 57%, P = 0.13 |
|
| Henke 2011 | 11/92 | 5/93 | |||
| Insomnia | Brizel 2008 | 12/67 | 4/32 | Random‐effects meta‐analysis of 2 studies: RR 0.77 (95% CI 0.23 to 2.55); P = 0.67 Heterogeneity: I2 = 63%, P = 0.10 |
|
| Henke 2011 | 5/92 | 12/93 | |||
| Dyspnea | Brizel 2008 | 9/67 | 1/32 | RR 1.10 (95% CI 0.67 to 1.81); P = 0.70 | |
| Cough | Brizel 2008 | 8/67 | 5/32 | RR 0.76 (95% CI 0.27 to 2.15); P = 0.61 | |
| Headache | Brizel 2008 | 8/67 | 2/32 | Random‐effects meta‐analysis of 2 studies: RR 2.13 (95% CI 0.86 to 5.28); P = 0.10 Heterogeneity: I2 = 0%, P = 0.86 |
|
| Henke 2011 | 9/92 | 4/93 | |||
| Decreased weight | Le 2011 | 29/94 | 27/91 | Random‐effects meta‐analysis of 2 studies: RR 1.01 (95% CI 0.67 to 1.52); P = 0.96 Heterogeneity: I2 = 0%, P = 0.73 |
|
| Brizel 2008 | 7/67 | 4/32 | |||
| Dizziness | Brizel 2008 | 5/67 | 4/32 | RR 0.60 (95% CI 0.17 to 2.07); P = 0.42 | |
| Anxiety | Brizel 2008 | 4/67 | 5/32 | RR 0.38 (95% CI 0.11 to 1.33); P = 0.13 | |
| Hypomagnesemia | Brizel 2008 | 4/67 | 4/32 | RR 0.48 (95% CI 0.13 to 1.79); P = 0.27 | |
| Vomiting | Le 2011 | 26/94 | 26/91 | Random‐effects meta‐analysis of 2 studies: RR 0.98 (95% CI 0.72 to 1.33); P = 0.89 Heterogeneity: I2 = 0%, P = 0.96 |
|
| Brizel 2008 | 33/67 | 16/32 | |||
| Radiation skin injury | Le 2011 | 25/94 | 13/91 | RR 1.10 (95%CI 0.67 to 1.81); P = 0.70 | |
| Anaemia | Le 2011 | 21/94 | 34/91 | Random‐effects meta‐analysis of 2 studies: RR 0.83 (95% CI 0.33 to 2.05); P = 0.68 Heterogeneity: I2 = 54%, P = 0.14 |
|
| Brizel 2008 | 10/67 | 3/32 | |||
| Fatigue | Le 2011 | 21/94 | 20/91 | Random‐effects meta‐analysis of 3 studies: RR 0.88 (95% CI 0.60 to 1.30); P = 0.52 Heterogeneity: I2 = 2%, P = 0.36 |
|
| Henke 2011 | 7/92 | 14/93 | |||
| Brizel 2008 | 17/67 | 8/32 | |||
| Leukopenia | Le 2011 | 21/94 | 12/91 | Random‐effects meta‐analysis of 2 studies: RR 1.01 (95% CI 0.37 to 2.78); P = 0.98 Heterogeneity: I2 = 79%, P = 0.03 |
|
| Henke 2011 | 12/92 | 20/93 | |||
| Granulocytopenia | Brizel 2008 | 20/67 | 6/32 | RR 1.59 (95% CI 0.47 to 5.39); P = 0.45 | |
| Pharyngolaryngeal pain | Le 2011 | 20/94 | 23/91 | RR 0.84 (95% CI 0.50 to 1.42); P = 0.52 | |
| Hypokalemia | Le 2011 | 19/94 | 8/91 | RR 2.04 (95% CI 0.98 to 4.28); P = 0.06 | |
| Pyrexia | Le 2011 | 16/94 | 19/91 | RR 0.82 (95% CI 0.45 to 1.48); P = 0.50 | |
| Mucosal inflammation | Henke 2011 | 4/92 | 10/93 | RR 0.40 (95% CI 0.13 to 1.24); P = 0.11 | |
| Asthenia | Henke 2011 | 13/92 | 7/93 | RR 1.88 (95% CI 0.78 to 4.49); P = 0.16 | |
| Abdominal pain | Henke 2011 | 7/92 | 2/93 | RR 3.54 (95% CI 0.75 to 16.58); P = 0.11 | |
| Back pain | Henke 2011 | 6/92 | 1/93 | RR 6.07 (95% CI 0.74 to 49.40); P = 0.09 | |
| Febrile neutropenia | Henke 2011 | 1/92 Considered "serious adverse event" |
0/93 | RR 3.03 (95% CI 0.13 to 73.48); P = 0.50 | |
| Dehydration | Le 2011 | 13/94 | 19/91 | Random‐effects meta‐analysis of 3 studies: RR 0.75 (95% CI 0.45 to 1.25); P = 0.27 Heterogeneity: I2 = 30%, P = 0.24 |
|
| Henke 2011 | 6/92 | 13/93 | |||
| Brizel 2008 | 20/67 | 8/32 | |||
CI = confidence interval; OMWQ‐HN = Oral Mucositis Weekly Questionnaire ‐ Head and Neck Cancer; RR = risk ratio; RT = radiotherapy; SD = standard deviation.
Side effects
All three trials provided information on possible adverse events, sometimes reporting the same adverse event (Additional Table 10). There was no evidence of patients in either group experiencing more or less of these adverse events.
Cost
No cost data related to the effectiveness of palifermin were reported.
Bethanechol versus placebo
One study, at unclear risk of bias, compared bethanechol with placebo (Jaguar 2015).
Xerostomia
Bethanechol reduced the risk of developing grade ≥ 2 xerostomia (on a 0 to 3 scale) at the end of radiotherapy (RR 0.43, 95% CI 0.28 to 0.66; P = 0.0001; 84 participants). However, there was insufficient evidence of a difference up to and including three months postradiotherapy (RR 0.81, 95% CI 0.65 to 1.01; P = 0.06; 84 participants) (Analysis 8.1).
8.1. Analysis.
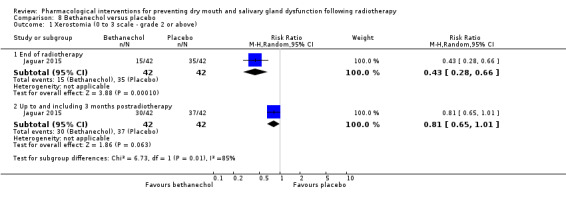
Comparison 8 Bethanechol versus placebo, Outcome 1 Xerostomia (0 to 3 scale ‐ grade 2 or above).
Salivary flow rates
Bethanechol increased unstimulated saliva flow (ml/min) at two months postradiotherapy (MD 0.19, 95% CI 0.06 to 0.32; P = 0.004; 97 participants) (Analysis 8.2).
8.2. Analysis.
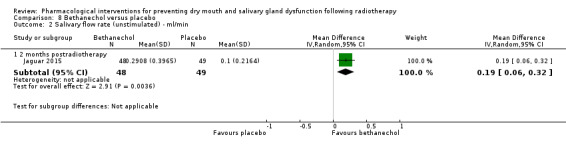
Comparison 8 Bethanechol versus placebo, Outcome 2 Salivary flow rate (unstimulated) ‐ ml/min.
There was insufficient evidence of a difference in stimulated saliva flow (ml/min) at two months postradiotherapy (MD 0.15, 95% CI ‐0.03 to 0.33; P = 0.11; 97 participants; Analysis 8.3).
8.3. Analysis.
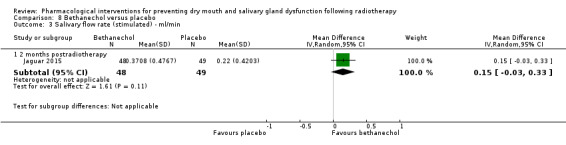
Comparison 8 Bethanechol versus placebo, Outcome 3 Salivary flow rate (stimulated) ‐ ml/min.
Survival
No survival data were reported.
Quality of life and other oral related symptoms
No data on either quality of life or other oral related symptoms were reported.
Side effects
The study reported narratively that there were no statistical differences between the groups in bethanechol‐related toxicity and that "no patient experienced severe (grade 3) toxicity and no one dropped out of the study due to adverse effects" (Additional Table 11).
8. Bethanechol versus placebo (other outcomes).
| Outcome | Study ID | Results |
| Adverse effects | Jaguar 2015 | No statistical difference between the groups in bethanechol‐related toxicity. Quote: "No patient experienced severe (grade 3) toxicity and no one dropped out of the study due to adverse effects" |
Cost
No cost data related to the effectiveness of bethanecol versus placebo were reported.
Bethanechol versus artificial saliva
One study, at high risk of bias, compared bethanechol with artificial saliva (Jham 2007).
Xerostomia
There was insufficient evidence of a difference in having a dry mouth (yes/no) either at the end of radiotherapy (RR 0.63, 95% CI 0.30 to 1.29; P = 0.2; 36 participants) or at 8 to 40 weeks postradiotherapy (RR 0.56, 95% CI 0.30 to 1.05; P = 0.07; 30 participants) (Analysis 9.1).
9.1. Analysis.
Comparison 9 Bethanechol versus artificial saliva, Outcome 1 Xerostomia (dry mouth? yes/no).
Salivary flow rates
Bethanechol increased unstimulated saliva flow (ml/min) at the end of radiotherapy (MD 0.12, 95% CI 0.01 to 0.23; P = 0.03; 36 participants), but there was insufficient evidence of a difference at 8 to 40 weeks postradiotherapy (MD 0.07, 95% CI ‐0.02 to 0.16; P = 0.13; 33 participants) (Analysis 9.2).
9.2. Analysis.
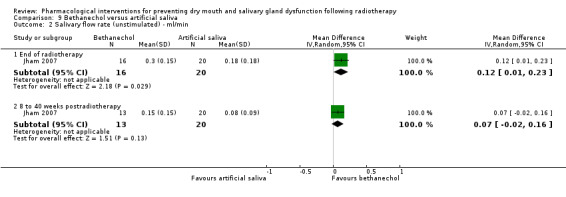
Comparison 9 Bethanechol versus artificial saliva, Outcome 2 Salivary flow rate (unstimulated) ‐ ml/min.
There was insufficient evidence of a difference in stimulated saliva flow (ml/min) at the end of radiotherapy (MD 0.13, 95% CI ‐0.03 to 0.29; P = 0.12; 32 participants), but there was a benefit in favour of bethanechol at 8 to 40 weeks postradiotherapy (MD 0.21, 95% CI 0.01 to 0.41; P = 0.04; 29 participants) (Analysis 9.3).
9.3. Analysis.
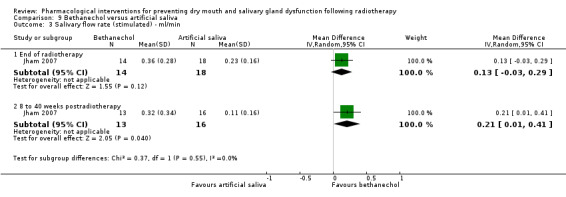
Comparison 9 Bethanechol versus artificial saliva, Outcome 3 Salivary flow rate (stimulated) ‐ ml/min.
Survival
There was insufficient evidence of a difference in overall survival at 40 weeks postradiotherapy (RR 1.59, 95% CI 0.43 to 5.84; P = 0.48; 43 participants; Analysis 9.4).
9.4. Analysis.
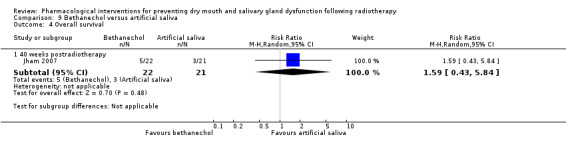
Comparison 9 Bethanechol versus artificial saliva, Outcome 4 Overall survival.
Quality of life and other oral related symptoms
No data on either quality of life or other oral related symptoms were reported.
Side effects
There were low rates of adverse effects (watering eyes, nervousness, frequent urination, sweating, warm face, cramps, diarrhoea, nausea) with insufficient evidence of any differences (Additional Table 12).
9. Bethanechol versus artificial saliva (other outcomes).
| Outcome | Study ID | Time point | Bethanechol | Artificial saliva | Results | Comments |
| Lacrimation (watering eyes) | Jham 2007 | End of RT | 3/22 | 0/21 | RR 6.70 (95% CI 0.37 to 122.29); P = 0.2 | |
| Nervousness | 3/22 | 0/21 | RR 6.70 (95% CI 0.37 to 122.29); P = 0.2 | |||
| Frequent urination | 3/22 | 0/21 | RR 6.70 (95% CI 0.37 to 122.29); P = 0.2 | |||
| Sweating | 2/22 | 0/21 | RR 4.78 (95% CI 0.24 to 94.12); P = 0.3 | "1 patient using bethanechol dropped out of the study due to excessive sweating (Grade 2 severity; National Cancer Institute Common Terminology Criteria for Adverse Events – NCI CTCAE, v 3" | ||
| Warm face | 2/22 | 0/21 | RR 4.78 (95% CI 0.24 to 94.12); P = 0.3 | |||
| Cramps | 1/22 | 0/21 | RR 2.87 (95% CI 0.12 to 66.75); P = 0.51 | |||
| Diarrhoea | 1/22 | 0/21 | RR 2.87 (95% CI 0.12 to 66.75); P = 0.51 | |||
| Nausea | 1/22 | 2/21 | RR 0.48 (95% CI 0.05 to 4.88); P = 0.53 |
CI = confidence interval; RR = risk ratio; RT = radiotherapy.
Cost
No cost data related to the effectiveness of bethanechol versus artifical saliva were reported.
Selenium versus no intervention
Selenium was compared to no intervention in one trial assessed as at high risk of bias (Büntzel 2010).
Xerostomia
We were unable to use any of the data as bar charts of mean scores (baseline to postradiotherapy) were presented with no standard deviations. The results reported in the text indicated that there was no evidence that selenium reduced xerostomia (Analysis 10.1).
10.1. Analysis.
Comparison 10 Selenium versus no selenium, Outcome 1 Xerostomia.
| Xerostomia | |
|---|---|
| Study | |
| Büntzel 2010 | "comparing the mean value of xerostomia, no statistically significant difference can be seen between the groups" |
Salivary flow rates
No data on salivary flow rates were reported.
Survival
No survival data were reported.
Quality of life and other oral related symptoms
We were unable to use any of the data for loss of taste or dysphagia as bar charts of mean scores were presented with no standard deviations, but some information was reported in the text (Additional Table 13). There was insufficient evidence of any differences.
10. Selenium versus no intervention (other outcomes).
| Outcome | Study ID | Time point | Reported in text |
| Loss of taste | Büntzel 2010 | 6 weeks after end RT | "Ageusia was milder in the selenium group. But the difference was not significant" |
| Dysphagia | Büntzel 2010 | 6 weeks after end RT | "The only significant difference was observed at week 7, when the selenium group had developed a mean value of 1.533 versus 2.167 in the control group (P = 0.05)" |
| Adverse events | Büntzel 2010 | 6 weeks after end RT | "23 serious adverse events (SAEs) were seen in the selenium group, compared to 22 in the control group (P = 0.476). No statistically significant differences in toxicities were found using the 2‐tailed Fisher's exact test" |
RT = radiotherapy.
Side effects
The text around adverse events implied that there was no evidence that selenium caused a higher number of events. We were unable to analyse the data as they were clustered (Additional Table 13).
Cost
No cost data related to the effectiveness of selenium were reported.
Antiseptic mouthrinse versus placebo
Antiseptic mouthrinse was assessed in one small trial at high risk of bias (Lanzós 2010).
Xerostomia
No data on xerostomia were reported.
Salivary flow rates
This outcome was only reported during radiotherapy (i.e. not at any of the time points we were interested in).
Survival
No survival data were reported.
Quality of life and other oral related symptoms
No quality of life data were reported, however, data on hyposialosis (drooling) were reported; there was insufficient evidence to determine whether or not antiseptic mouthrinse reduced or increased drooling in patients after four weeks (Additional Table 14).
11. Antiseptic mouthrinse versus placebo (other outcomes).
| Outcome | Study ID | Time point | Antiseptic rinse | Placebo | Results |
| Drooling | Lanzós 2010 | 4 weeks from baseline | Increased 6 No change or decreased 8 6/14 |
Increased 3 No change or decreased 7 3/10 |
RR 1.43 (95% CI 0.46 to 4.39); P = 0.53 |
| Adverse events | Lanzós 2010 | "No relevant adverse events were reported in any group" |
CI = confidence interval; RR = risk ratio.
Side effects
The study reported "no relevant adverse events were reported in any group" (Additional Table 14).
Cost
No cost data related to the effectiveness of antiseptic mouthrinses were reported.
Antimicrobial lozenge versus placebo
Antimicrobial lozenge was assessed in one trial assessed as at high risk of bias (Duncan 2005).
Xerostomia
There was insufficient evidence that the antimicrobial lozenges reduced xerostomia up to and including three months postradiotherapy (RR 1.16, 95% CI 0.97 to 1.40; P = 0.11; 133 participants) (Analysis 11.1).
11.1. Analysis.
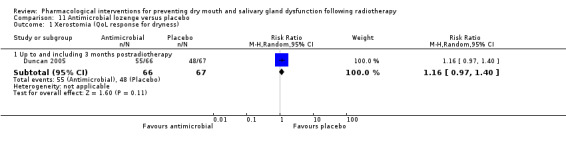
Comparison 11 Antimicrobial lozenge versus placebo, Outcome 1 Xerostomia (QoL response for dryness).
Salivary flow rates
No data on salivary flow rates were reported.
Survival
No survival data were reported.
Quality of life and other oral related symptoms
There was insufficient evidence of a difference between the groups for global quality of life (Analysis 11.2), mouth pain, sore/burning mouth, or throat pain (Additional Table 15).
11.2. Analysis.
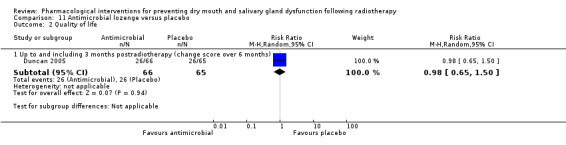
Comparison 11 Antimicrobial lozenge versus placebo, Outcome 2 Quality of life.
12. Antimicrobial lozenge versus placebo (other outcomes).
| Outcome | Study ID | Time point | Antimicrobial lozenge | Placebo | Results |
| Mouth pain | Duncan 2005 | Worse over 6 months | 32/66 | 32/62 | RR 0.94 (95% CI 0.66 to 1.33); P=0.72 |
| Sore/burning mouth | Duncan 2005 | Worse over 6 months | 32/65 | 32/62 | RR 0.95 (95% CI 0.68 to 1.35); P=0.79 |
| Throat pain | Duncan 2005 | Worse over 6 months | 29/66 | 36/65 | RR 0.79 (95% CI 0.56 to 1.12); P=0.19 |
| Dryness in mouth | Duncan 2005 | Worse over 6 months | 55/66 | 46/65 | RR 1.18 (95% CI 0.97 to 1.42); P=0.09 |
| Nausea | Duncan 2005 | Worse over 6 months | 27/66 | 14/65 | RR 1.90 (95% CI 1.10 to 3.28); P=0.02 |
| Diarrhoea | Duncan 2005 | Worse over 6 months | 6/66 | 3/65 | RR 1.97 (95% CI 0.51 to 7.54); P=0.32 |
| Constipation | Duncan 2005 | Worse over 6 months | 24/66 | 26/65 | RR 0.91 (95% CI 0.59 to 1.42); P=0.67 |
CI = confidence interval; RR = risk ratio.
Side effects
There is weak evidence that the antimicrobial lozenge may cause nausea, but insufficient evidence of a difference in dryness in the mouth, diarrhoea, or constipation (Additional Table 15).
Cost
No cost data related to the effectiveness of antimicrobial lozenges were reported.
Polaprezinc versus azulene oral rinse
One study at high risk of bias compared polaprezinc with azulene oral rinse (Watanabe 2010).
Xerostomia
There is some weak evidence that polaprezinc reduced severe xerostomia at the end of radiotherapy when compared with azulene rinse (RR 0.17, 95% CI 0.04 to 0.65; P = 0.009; 31 participants) (Analysis 12.1).
12.1. Analysis.
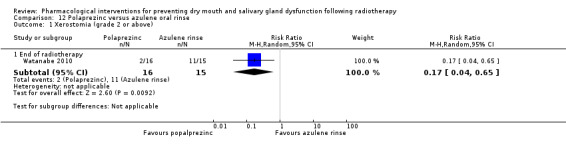
Comparison 12 Polaprezinc versus azulene oral rinse, Outcome 1 Xerostomia (grade 2 or above).
Salivary flow rates
No data on salivary flow rates were reported.
Survival
The study reported tumour response by RECIST (Response Evaluation Criteria In Solid Tumors) criteria for a specific group of patients only.
Quality of life and other oral related symptoms
There is some weak evidence that polaprezinc reduces severe oral pain and severe taste disturbance, however there is no evidence that polaprezinc helps patients to eat more when compared with azulene oral rinse (Additional Table 16).
13. Polaprezinc versus azulene rinse (other outcomes).
| Outcome | Study ID | Time point | Polaprezinc | Azulene rinse | Results |
| Pain > 2 (0‐3 scale) | Watanabe 2010 | Over RT period | 5/16 | 13/15 | RR 0.36 (95% CI 0.17 to 0.77); P = 0.008 |
| Taste disturbance > 2 (0‐3 scale) | Watanabe 2010 | Over RT period | 1/16 | 8/15 | RR 0.12 (95% CI 0.02 to 0.83); P = 0.03 |
| Disability of oral intake | Watanabe 2010 | Over RT period | 2/16 | 6/15 | RR 0.31 (95% CI 0.07 to 1.31); P = 0.11 |
CI = confidence interval; RR = risk ratio; RT = radiotherapy.
Side effects
No data on adverse events were reported.
Cost
No cost data related to the effectiveness of polaprezinc or azulene were reported.
Venalot Depot (coumarin/troxerutin) versus placebo
One small trial, assessed as at high risk of bias, compared Venalot Depot with placebo (Grötz 2001).
Xerostomia
RTOG scores are reported however these scores were a composite of radiation side effects on different sites, so could not be used for assessing xerostomia.
Salivary flow rates
The sialometric data showed the reading dropped to an unmeasurable level in both groups so this was abandoned as a primary marker of efficacy and the protocol changed.
Survival
We were unable to use the data on locoregional control.
Quality of life and other oral related symptoms
No data on either quality of life or other oral related symptoms were reported.
Side effects
The study reported that "no adverse events could be attributed to the experimental medication" (Additional Table 17).
14. Venalot Depot (coumarin/troxerutin) versus placebo.
| Outcome | Study ID | Results |
| Adverse events | Grötz 2001 | "No adverse events could be attributed to the experimental medication" |
Cost
No cost data related to the effectiveness of Venalot Depot were reported.
Sensitivity analysis
Risk of bias
There were too few studies at low risk of bias to carry out sensitivity analyses based on this factor.
Publication status
Four of the 39 studies were unpublished; Abacioglu 1997 was a dissertation, Lozada‐Nur 1998, Patni 2004 and Veerasarn 2006 were conference abstracts. The authors of these papers were contacted and their data were provided. Sensitivity analysis was undertaken to assess whether the inclusion of unpublished information had an effect on the results of the review.
Pilocarpine
Abacioglu 1997 and Lozada‐Nur 1998 assessed pilocarpine. Removing these unpublished trials from the results did not alter the findings of the review i.e. xerostomia and salivary flow rates at the end of radiotherapy and three months postradiotherapy were still not significant.
Amifostine
Patni 2004 and Veerasarn 2006 assessed amifostine.
Xerostomia
Removing Veerasarn 2006 from the analysis of xerostomia at the end of radiotherapy increases the effect estimate from RR 0.35 (95% CI 0.19 to 0.67; P = 0.001; 119 participants) to RR 0.22 (95% CI 0.09 to 0.53; P = 0.0008; 60 participants).
Removing Patni 2004 and Veerasarn 2006 from the analysis of xerostomia at three months postradiotherapy does not change the result but increases the uncertainty around the effect estimate from RR 0.66 (95% CI 0.48 to 0.92; P = 0.01; 687 participants) to RR 0.66 (95% CI 0.40 to 1.09; P = 0.1; 473 participants), thus including the possibility of harm associated with amifostine.
Removing Patni 2004 and Veerasarn 2006 from the analysis of xerostomia at 12 months postradiotherapy does not affect the result i.e. from RR 0.70 (95% CI 0.40 to 1.23; P = 0.21; 682 participants analysed) to RR 0.64 (95% CI 0.38 to 1.08; P = 0.09; 479 participants analysed).
Discussion
Summary of main results
A total of 39 trials were included in this review. We assessed the quality of the body of evidence for each outcome within a comparison (providing there was more than one study) using GRADE methodology (GRADE 2004).
Pilocarpine (Table 1), compared with no treatment/placebo, was evaluated in 12 trials. There was no evidence of a difference in xerostomia between treatment groups at end of radiotherapy, three or six months. Similarly, there was also no evidence of a difference between treatment groups for salivary flow rates (stimulated or unstimulated) at any time point. There was insufficient evidence to determine the benefit of pilocarpine with regard to improving quality of life or increasing survival. There was no difference in reported adverse events, apart from sweating, where data from five studies showed an increased risk with pilocarpine. The body of evidence for each outcome was rated as very low quality, except for the adverse event of sweating which was low quality.
Amifostine (Table 2), compared with no treatment/placebo, was evaluated in 11 studies. There is some (low‐quality) evidence that amifostine reduced the risk of developing grade ≥ 2 xerostomia (0 to 4 scale) at end of radiotherapy and, to a lesser extent, up to and including three months postradiotherapy. At 12 months postradiotherapy, there was insufficient evidence of a difference in the risk of grade ≥ 2 xerostomia. There was inconsistent (very low‐quality) evidence regarding the effect of amifostine on salivary flow rate. There was insufficient (very low‐quality) evidence to determine whether or not amifostine reduced overall survival, progression‐free survival, disease‐free survival or locoregional tumour control. Similarly, there was insufficient (very low‐quality) evidence to determine the benefit of amifostine in terms of quality of life. In general, adverse effects were poorly reported but there was (low‐quality) evidence that amifostine was associated with an increased risk of vomiting, hypotension, nausea and allergic response.
Palifermin (Table 3), compared with placebo, was evaluated in three trials. There is insufficient (low‐quality) evidence to determine whether or not palifermin reduced the incidence of grade ≥ 2 xerostomia (0 to 4 scale) up to three months postradiotherapy. There was insufficient (moderate‐quality) evidence to determine the effect of palifermin on overall or progression‐free survival. There was no evidence of a difference in reported adverse effects.
All evidence from any remaining comparisons did not undergo formal GRADE assessment but was considered to be of very low quality.
Five trials (four at high risk of bias and one at unclear risk of bias) evaluated different forms of Chinese medicine. There is some evidence to suggest a benefit from Shenqi Fanghon recipe and an unspecified Chinese medicine at reducing xerostomia. Similarly, the unspecified Chinese medicine improved salivary flow rates. However, these findings were from single studies at high risk of bias. There was insufficient evidence to determine if any of the Chinese medicines had any effect on quality of life and survival.
Other interventions evaluated, for which there is currently insufficient evidence to draw conclusions were:
amifostine ‐ comparison of doses (single trial, at high risk of bias);
amifostine ‐ different routes of administration (single trial, at high risk of bias);
biperiden (single trial, at high risk of bias);
bethanechol (single trial, at unclear risk of bias);
bethanecol versus artificial saliva (single trial, at high risk of bias);
selenium (single trial, at high risk of bias);
antiseptic mouthrinse verus placebo (single trial, at high risk of bias);
antimicrobial lozenge versus placebo (single trial, at high risk of bias);
polaprezinc versus azulene oral rinse (single trial, at high risk of bias);
Venalot Depot versus placebo (single trial, at high risk of bias).
Overall completeness and applicability of evidence
Although we found 39 eligible studies that covered a wide range of interventions, the evidence found is not sufficient to highlight much promise in terms of effective preventative treatments for salivary gland dysfunction. This is because, despite there being a reasonable number of studies for three of the interventions (amifostine: 12; pilocarpine: 12; palifermin: 3), there was inconsistency in the way outcomes were reported and in the timing of outcome measurement. Most comparisons included only a single small study, the large majority being at high risk of bias. The most complete body of evidence was for amifostine and the outcome of incidence of moderate to severe xerostomia. Guideline statements point out the lack of an established pharmacological prophylaxis for salivary gland dysfunction and highlight the potential of radiotherapeutic techniques/precautions in reducing damage to the salivary glands (for example parotid‐sparing plans) (Buglione 2016). Therefore, for completeness, it may be sensible to also carry out a Cochrane Review of non‐pharmacological interventions, although we are not aware of many randomised controlled trials.
As mentioned, a wide range of interventions were assessed, but the studies were also conducted in both middle‐income and high‐income countries with no exclusion criteria in terms of the population included. Unfortunately, many studies did not include an objective measure of saliva flow to go with the more subjective measure of xerostomia. Furthermore, xerostomia was often measured differently between comparisons making it difficult to get an overall picture of the comparative effectiveness of the different interventions.
It was interesting that two of the three interventions for which we were able to carry out meta‐analyses (amifostine and palifermin) have shown promise in a Cochrane Review on the prevention of oral mucositis, another major side effect of cancer treatment (Worthington 2011). As the evidence for amifostine in the prevention of salivary gland dysfunction is promising, this could be beneficial for patients as they may require fewer medications. However, amifostine is not currently recommended in clinical practice guidelines due to high costs and its side effects (Buglione 2016). Furthermore, the evidence for its long‐term benefit is weak. There was insufficient evidence to support the use of palifermin in this review, but it is possible that the intention‐to‐treat rules in one of the three studies in the xerostomia meta‐analysis may have influenced the result (Henke 2011). Therefore, further studies assessing palifermin may be of interest. Worthington 2011 reported that there was no evidence that pilocarpine prevents oral mucositis.
Quality of the evidence
We included 39 studies that randomised 3520 participants; the number of participants analysed varied by outcome and time point.
Pilocarpine
We have very little confidence in the effect estimates for the outcomes of xerostomia, salivary flow rate, survival and quality of life (none of which showed a difference), mainly due to concerns regarding the risk of bias of the studies and imprecision of the results, but also due to inconsistency in the case of xerostomia. Further studies are likely to change the results. We had a little more confidence (although still limited) in the effect estimate for the adverse effect of sweating, which occurred more frequently in those receiving pilocarpine. Again it was risk of bias and imprecision which limited our confidence, and further studies would probably change the effect estimate. For more details see Table 1.
Amifostine
Our confidence in the effect estimates for xerostomia was limited by concerns regarding the risk of bias and inconsistency in the results of the individual studies. New studies could change the results. We had a similar level of confidence in the results for the adverse effects of vomiting, hypotension, nausea and allergic response, which were all more frequent in those receiving amifostine. Risk of bias and imprecision were the factors affecting our confidence. We had very little confidence in the effect estimates for salivary flow rate, survival and quality of life due to risk of bias and imprecision. For more details see Table 2.
Palifermin
Our confidence in the effect estimate for xerostomia was limited by concerns regarding imprecision and inconsistency in the results of the individual studies. We were moderately confident that palifermin did not compromise survival. For more details see Table 3.
We did not formally assess the quality of the evidence for all other comparisons in this review, but it is all considered to be very low quality due to single small studies that are mostly at high risk of bias. Further studies would very likely change the effect estimates for all outcomes and time points within these comparisons.
Potential biases in the review process
Standard Cochrane methods were followed to avoid biases in the review process. However, we acknowledge that the decision to exclude data measured and reported during radiotherapy may be considered by some readers to be an arbitrary one. These data are potentially of interest and their exclusion may be thought of as a bias.
Agreements and disagreements with other studies or reviews
Although there are some systematic reviews on the treatment of salivary gland dysfunction caused by radiotherapy (Davies 2015; Mercadante 2017), we are not aware of any high quality systematic reviews on prevention.
Authors' conclusions
Implications for practice.
There is some low‐quality evidence to suggest that amifostine prevents the feeling of dry mouth in people receiving radiotherapy to the head and neck (with or without chemotherapy) in the short‐ (end of radiotherapy) to medium‐term (three months postradiotherapy). However, it is less clear whether or not this effect is sustained to 12 months postradiotherapy. The benefits of amifostine should be weighed against its high cost and side effects. There was insufficient evidence to show that any other intervention is beneficial.
Implications for research.
Further well conducted, well reported and adequately powered randomised controlled trials are needed to add to the evidence base for the interventions assessed in the single‐study comparisons of this systematic review. Amifostine should be assessed with longer term follow‐up to establish whether the promising shorter term effects are sustained. Palifermin should also be studied further and with longer follow‐up.
Trialists should endeavour to use similar scales to measure xerostomia i.e. one that can be dichotomised to report the incidence of moderate to severe or severe xerostomia or both. Buglione et al recommend several established standardised scales such as NCI CTCAE (National Cancer Institute Common Terminology Criteria for Adverse Events), RTOG (Radiation Therapy Oncology Group), and LENT‐SOMA (Late Effects Normal Tissue Task Force ‐ Subjective, Objective, Management, Analytic scale) (Buglione 2016). This should be reported alongside a more objective measure such as salivary flow rate. Adverse effects should also be clearly reported and quality of life would be a useful patient‐important outcome.
Notes
Survival data, locoregional control were included as secondary outcomes after the publication of the protocol as this information was deemed important and reported in the included studies.
Acknowledgements
We wish to thank Anne Littlewood, Information Specialist, Cochrane Oral Health, for developing the search strategy and conducting the database searches; and Luisa Fernandez Mauleffinch, Managing Editor and Copy Editor, Cochrane Oral Health, for her assistance with the editorial process and for translating articles in Spanish. We thank Martin McCabe, Chunjie Li, and Giovanni Lodi for their comments on the draft. We thank Saiba Ghafoor for her contribution to earlier versions. We would also like to thank Shi Zongdao for helping with Chinese translations and study authors for providing additional data and responding to our queries regarding their studies. We acknowledge the contribution of Andrew Davies and Emma Tavender to the protocol for this review.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
#1 ((radioth* or radiat* or irradiat* or radiochemo*):ti,ab) AND (INREGISTER) #2 ((xerostomi* or "dry mouth" or "salivation disorder*" or saliva* or hypersalivat* or hyposalivat* or xeroses or radioxerost* or "salivary gland hypofunction" or "salivary gland dysfunction" or "dysfunction of the salivary gland*" or "artificial saliva" or "saliva artificial"):ti,ab) AND (INREGISTER) #3 (#1 and #2) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 Exp RADIOTHERAPY/ #2 (radioth* OR radiat* OR irradiat* OR radiochemo*) #3 #1 OR #2 #4 Exp XEROSTOMIA/ #5 Exp SALIVARY GLANDS #6 ((parotid or sublingual or submandibular or salivary) AND gland*) #7 ((#5 or #6) AND (hypofunction or dysfunction* or disorder* or function*)) #8 (xerostomi* OR xeroses OR radioxerost* OR (dry* NEAR mouth*)) #9 (hyposalivat* OR hypersalivat* OR sialogogue* or sialagogue*) #10 saliva* #11 (#4 or #7 or #8 or #9 or #10) #12 #3 AND #11
Appendix 3. MEDLINE Ovid search strategy
1. exp RADIOTHERAPY/ 2. (radioth$ or radiat$ or irradiat$ or radiochemo$) 3. or/1‐2 4. exp XEROSTOMIA/ 5. exp SALIVARY GLANDS/ 6. ((parotid or sublingual or submandibular or salivary) AND gland$) 7. ((5 or 6) AND (hypofunction or diysfunction* or disorder* or function*)) 8. (xerostomi* OR xeroses OR radioxerost$ OR (dry$ adj5 mouth$)) 9. (hyposalivat$ OR hypersalivat$ OR sialogogue$ or sialagogue$) 10. saliva$ 11. 4 or 7 or 8 or 9 or 10 12. 3 AND 11
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in Box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. exp RADIOTHERAPY/ 2. (radioth$ or radiat$ or irradiat$ or radiochemo$) 3. or/1‐2 4. XEROSTOMIA/ 5. SALIVARY GLAND/ 6. ((parotid or sublingual or submandibular or salivary) AND gland$) 7. ((5 or 6) AND (hypofunction or diysfunction* or disorder* or function*)) 8. (xerostomi* OR xeroses OR radioxerost$ OR (dry$ adj5 mouth$)) 9. (hyposalivat$ OR hypersalivat$ OR sialogogue$ or sialagogue$) 10. saliva$ 11. 4 or 7 or 8 or 9 or 10 12. 3 AND 11
The above subject search was linked to adapted version of the Cochrane Embase Project filter for identifying RCTs in Embase Ovid (see www.cochranelibrary.com/help/central‐creation‐details.html for information).
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 5. CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) search strategy
S1 MH "Radiotherapy+" S2 (radioth* or radiat* or irradiat* or radiochemo*) S3 S1 or S2 S4 MH "Xerostomia+" S5 MH "Salivary Glands+" S6 ((parotid or sublingual or submandibular or salivary) AND gland*) S7 ((S5 or S6) AND (hypofunction or dysfunction* or disorder or function)) S8 ((xerostomi* or xeroses or radioxerost*) or (dry N5 mouth)) S9 (hyposalivat* or hypersalivat* or sialogogue* or sialagogue*) S10 saliva* S11 S4 or S7 or S8 or S9 or S10 S12 S3 and S11
The above subject search was linked to Cochrane Oral Health's filter for identifying RCTs in CINAHL EBSCO.
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S3 TI random* or AB random* S4 AB "latin square" or TI "latin square" S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S6 MH Placebos S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*) S8 TI blind* or AB mask* or AB blind* or TI mask* S9 S7 and S8 S10 TI Placebo* or AB Placebo* or SU Placebo* S11 MH Clinical Trials S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 6. LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database) search strategy
(Mh Radiotherapy or radiotherap$ or radioterapia or irradiat$ or radiochemo$) [Words] and (Mh Xerostomia or xerostom$ or "salivary gland$" or salivat$ or hypersalivat$ or hyposalivat$ or sialogogue$)
The above subject search was linked to the Brazilian Cochrane Center filter for LILACs via BIREME.
((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal)))and not (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL)))
Appendix 7. ZETOC Conference Proceedings search strategy
radiotherap* AND xerostomi* radiotherap* AND saliva* radiotherap* AND sialogog*
Appendix 8. OpenGrey search strategy
The search strategy for OpenSIGLE is below.
radiotherapy AND xerostomia radiotherapy AND saliva radiotherapy AND sialogogue
Appendix 9. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
(radiotherapy and salivary) or (radiotherapy and xerostomia)
Appendix 10. World Health Organization International Clinical Trials Registry Platform search strategy
radiotherapy and saliva or radiotherapy and salivary or radiotherapy and xerostomia
Data and analyses
Comparison 1. Pilocarpine versus no treatment/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Xerostomia | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 End of radiotherapy | 4 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.16, 0.56] |
| 1.2 Up to and including 3 months postradiotherapy | 3 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.33, 0.37] |
| 1.3 Up to and including 6 months postradiotherapy | 2 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.04, 0.33] |
| 2 Xerostomia (LENT‐SOMA scale) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Up to and including 6 months postradiotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Xerostomia | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 End of radiotherapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Up to and including 3 months postradiotherapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Salivary flow rate (unstimulated) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 End of radiotherapy | 3 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.24, 0.72] |
| 4.2 Up to and including 3 months postradiotherapy | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.07, 0.54] |
| 5 Salivary flow rate (stimulated) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 End of radiotherapy | 2 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.44, 0.59] |
| 5.2 Up to and including 3 months postradiotherapy | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.41, 1.21] |
| 5.3 Up to and including 6 months postradiotherapy | 1 | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.84, 1.87] |
| 5.4 Up to and including 12 months postradiotherapy | 1 | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.83, 1.88] |
| 6 Salivary flow rate (> 0 g) unstimulated | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 End of radiotherapy | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.98, 3.69] |
| 6.2 Up to and including 3 months postradiotherapy | 1 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.86, 4.68] |
| 7 Salivary flow rate (> 0 g) stimulated | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 End of radiotherapy | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.77, 4.52] |
| 7.2 Up to and including 3 months postradiotherapy | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.23, 2.11] |
| 8 Overall survival | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Quality of life | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 End of radiotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Up to and including 3 months postradiotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Up to and including 6 months postradiotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Biperiden plus pilocarpine versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Salivary flow rate (unstimulated) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 End of radiotherapy | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.08, 0.12] |
| 1.2 Up to and including 3 months postradiotherapy | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Salivary flow rate (> 0 g) unstimulated | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 End of radiotherapy | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.77, 1.58] |
| 2.2 Up to and including 3 months postradiotherapy | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 7.50 [1.88, 29.99] |
| 2.3 Up to and including 6 months postradiotherapy | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 29.00 [1.81, 465.07] |
| 2.4 Up to and including 12 months postradiotherapy | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 35.00 [2.20, 556.71] |
2.2. Analysis.
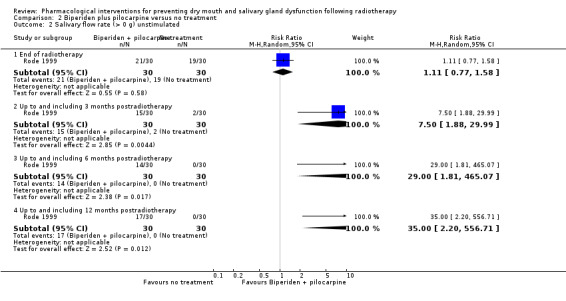
Comparison 2 Biperiden plus pilocarpine versus no treatment, Outcome 2 Salivary flow rate (> 0 g) unstimulated.
Comparison 3. Amifostine versus no treatment/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Xerostomia (0 to 4 scale ‐ grade 2 or above) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 End of radiotherapy | 3 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.19, 0.67] |
| 1.2 Up to and including 3 months postradiotherapy | 5 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.48, 0.92] |
| 1.3 12 months postradiotherapy | 7 | 682 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.40, 1.23] |
| 2 Salivary flow rate (unstimulated) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 End of radiotherapy | 2 | 83 | Mean Difference (IV, Random, 95% CI) | 0.34 [0.07, 0.61] |
| 2.2 Up to and including 3 months postradiotherapy | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.90, 1.16] |
| 2.3 12 months postradiotherapy | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.09, 0.55] |
| 3 Salivary flow rate (unstimulated) ‐ incidence of > 0.1 g in 5 min | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 12 months postradiotherapy | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.13, 1.86] |
| 4 Salivary flow rate (stimulated) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 End of radiotherapy | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐1.48, 1.30] |
| 4.2 Up to and including 3 months postradiotherapy | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐1.43, 2.19] |
| 4.3 12 months postradiotherapy | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐0.47, 2.11] |
| 5 Salivary flow rate (stimulated) ‐ incidence of > 0.1 g in 5 min | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 12 months postradiotherapy | 1 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.89, 1.41] |
| 6 Overall survival at 12 to 24 months postradiotherapy | 2 | 271 | Hazard ratio (Random, 95% CI) | 1.18 [0.85, 1.66] |
| 7 Overall survival ‐ narrative data | Other data | No numeric data | ||
| 8 Progression‐free survival at 12 to 24 months postradiotherapy | 2 | 247 | Hazard ratio (Random, 95% CI) | 0.94 [0.70, 1.27] |
| 9 Progression‐free survival | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 18 months postradiotherapy | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.81, 1.51] |
| 10 Progression‐free survival ‐ narrative data | Other data | No numeric data | ||
| 11 Locoregional tumour control at 12 to 24 months postradiotherapy | 2 | 279 | Hazard ratio (Random, 95% CI) | 0.90 [0.74, 1.11] |
| 12 Locoregional tumour control ‐ narrative data | Other data | No numeric data | ||
| 13 Disease‐free survival | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 24 months postradiotherapy | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.21] |
| 14 Disease‐free survival | Other data | No numeric data | ||
| 15 Quality of life (Patient Benefit Questionnaire) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 End of radiotherapy | 1 | 298 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.07, 0.83] |
| 15.2 Up to and including 3 months postradiotherapy | 1 | 233 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.02, 1.06] |
| 15.3 12 months postradiotherapy | 1 | 180 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.20, 1.20] |
Comparison 4. Amifostine (comparison of dosages).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Xerostomia (0 to 4 scale ‐ grade 2 or above) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 12 months postradiotherapy | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.58, 1.53] |
| 2 Overall survival ‐ narrative data | Other data | No numeric data | ||
| 3 Locoregional tumour control ‐ narrative data | Other data | No numeric data |
Comparison 5. Amifostine (intravenous versus subcutaneous).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Xerostomia (0 to 4 scale ‐ grade 2 or above) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Up to and including 3 months postradiotherapy | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.76, 1.40] |
| 1.2 12 months postradiotherapy | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.42, 0.88] |
| 2 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 48 months after radiotherapy | 1 | Hazard Ratio (Random, 95% CI) | 1.36 [0.89, 2.10] | |
| 3 Locoregional tumour control | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 48 months after radiotherapy | 1 | Hazard Ratio (Random, 95% CI) | 1.34 [0.76, 2.36] |
Comparison 6. Chinese medicine versus no treatment/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Xerostomia | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 End of radiotherapy: Shenqi Fanghou recipe versus no intervention | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.28, 0.55] |
| 1.2 End of radiotherapy: TWBXM versus placebo | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.03] |
| 1.3 Up to and including 3 months postradiotherapy: Jinlong capsules versus no intervention | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.36] |
| 2 Xerostomia | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 End of radiotherapy: TWBXM versus placebo | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐2.41 [‐16.19, 11.37] |
| 2.2 Up to and including 3 months postradiotherapy: TWBXM versus placebo | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐17.21, 17.01] |
| 3 Salivary flow rate (stimulated) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 End of radiotherapy: Chinese medicine versus no intervention | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.03, 0.15] |
| 4 Overall survival (12 months postRT) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.84, 1.30] |
| 5 Quality of life (EORTC‐C30) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 End of radiotherapy: TWBXM versus placebo | 1 | 68 | Mean Difference (IV, Random, 95% CI) | 2.39 [‐8.74, 13.52] |
| 5.2 Up to and including 3 months postradiotherapy: TWBXM versus placebo | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 1.93 [‐13.04, 16.90] |
Comparison 7. Palifermin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Xerostomia (0 to 4 scale ‐ grade 2 or above) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Up to and including 3 months postRT | 3 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 2 Overall survival at 42 to 72 months from baseline | 3 | Hazard Ratio (Random, 95% CI) | 1.00 [0.72, 1.39] | |
| 3 Progression‐free survival at 42 to 72 months from baseline | 3 | Hazard Ratio (Random, 95% CI) | 1.06 [0.80, 1.42] |
Comparison 8. Bethanechol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Xerostomia (0 to 3 scale ‐ grade 2 or above) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 End of radiotherapy | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.28, 0.66] |
| 1.2 Up to and including 3 months postradiotherapy | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.65, 1.01] |
| 2 Salivary flow rate (unstimulated) ‐ ml/min | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 2 months postradiotherapy | 1 | 97 | Mean Difference (IV, Random, 95% CI) | 0.19 [0.06, 0.32] |
| 3 Salivary flow rate (stimulated) ‐ ml/min | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 2 months postradiotherapy | 1 | 97 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.03, 0.33] |
Comparison 9. Bethanechol versus artificial saliva.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Xerostomia (dry mouth? yes/no) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 End of radiotherapy | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.30, 1.29] |
| 1.2 8 to 40 weeks postradiotherapy | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.30, 1.05] |
| 2 Salivary flow rate (unstimulated) ‐ ml/min | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 End of radiotherapy | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.01, 0.23] |
| 2.2 8 to 40 weeks postradiotherapy | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.02, 0.16] |
| 3 Salivary flow rate (stimulated) ‐ ml/min | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 End of radiotherapy | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.03, 0.29] |
| 3.2 8 to 40 weeks postradiotherapy | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.01, 0.41] |
| 4 Overall survival | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 40 weeks postradiotherapy | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.43, 5.84] |
Comparison 10. Selenium versus no selenium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Xerostomia | Other data | No numeric data |
Comparison 11. Antimicrobial lozenge versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Xerostomia (QoL response for dryness) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Up to and including 3 months postradiotherapy | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.97, 1.40] |
| 2 Quality of life | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Up to and including 3 months postradiotherapy (change score over 6 months) | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.65, 1.50] |
Comparison 12. Polaprezinc versus azulene oral rinse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Xerostomia (grade 2 or above) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 End of radiotherapy | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abacioglu 1997.
| Methods | Location: Turkey Number of centres: 1 Date of enrolment: July 1996 to January 1997 |
|
| Participants | Inclusion criteria: aged between 18 to 70 years. Histopathologic diagnosis of SCC of head and neck (nasopharynx, larynx, oropharynx, hypopharynx, oral cavity). WHO performance status 0 to 2. Patients to receive primary or postoperative radiation treatment for a minimum of 46 Gy totally and treatment fields to include at least the tail of parotis (1/3), submandibular glands and part of sublingual and minor salivary glands Exclusion criteria: patients with a histopathologic diagnosis other than SCC. Patients with an autoimmune disorder (e.g. Sjögren Syndrome) or diseases effecting saliva secretion. Difficulty in co‐operation for saliva collection, understanding the questionnaire and attending the follow‐up visits Age (years): pilocarpine: median 55 years, range 38 to 68 years; control: median 50 years, range 30 to 61 years Gender (M:F): pilocarpine 12:0; control 11:1 Cancer type: tumour location: pilocarpine: larynx = 8, nasopharynx = 2 and oral cavity = 2; control: larynx = 7, nasopharynx = 4 and oral cavity = 1 Radiotherapy: pilocarpine: mean dose = 60.2 Gy (range 48 to 70 Gy), number of fractions = 30.1 (mean), treatment time = 44.9 days (mean); control: mean dose = 63.8 Gy (range 50 to 70 Gy), number of fractions = 31.9 (mean), treatment time = 48.2 days (mean) Chemotherapy: none Number randomised: 24 (12 per group) Number evaluated: 24 (no dropouts, although not all participants available at all time points) |
|
| Interventions |
Pilocarpine versus no intervention Pilocarpine: 5 mg 3 times daily (4% solution) for 3 months from the beginning of RT Control: no treatment |
|
| Outcomes | Xerostomia: subjective evaluation scores for xerostomia (0 = no symptoms, 11 = severe xerostomia). Questionnaire included 5 questions Salivary flow rates: unstimulated and stimulated whole saliva secretion (unstimulated saliva pH measurements also recorded) Adverse effects: no serious toxicity Survival data: not reported Other oral symptoms: not reported Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: before RT, during RT, end of RT and 3 months after start of RT |
|
| Funding | None | |
| Trial registration | Not registered nor published | |
| Sample size calculation presented | Not included | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not explicit in trial report. Comment from author: "randomisation was performed with block randomisation with stratification of treatment fields" |
| Allocation concealment (selection bias) | Low risk | Comment from author: "sealed envelope were used for concealing" |
| Blinding (performance bias and detection bias) patients/carers | High risk | Pilocarpine versus no intervention. Blinding not possible |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Not possible due to 'no intervention' group and subjective assessment of xerostomia |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment from author: "no dropouts" Number of participants available for assessment varies by time point, however, those missing for assessment unlikely to influence results |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Antonadou 2002.
| Methods | Location: Greece Number of centres: 1 Date of enrolment: January 1997 to January 1998 |
|
| Participants | Inclusion criteria: histologically proven squamous cell carcinoma of the head and neck. A primary tumour greater than or = T2N0M0, expected survival time greater than or = 12 months, no evidence of metastasis, and no prior chemotherapy or RT. Normal liver and kidney function, adequate bone marrow reserve, no current or previous history of cardiovascular disease and no active systemic infection Exclusion criteria: not reported Age (years): amifostine: mean 53.3 (SD 6.9); control: mean 60.3 (SD 5.5) Gender: amifostine: 13 M, 9 F; control: 16 M, 7 F Cancer type: tumour location: (amifostine/control) nasopharynx = 2/3, oral cavity = 9/11, larynx = 6/6 and oropharynx = 5/3. TNM classification: (amifostine/control) T2 = 6/6, T3 = 13/16, T4 = 3/1, N0 = 12/14 and N1 to 3 = 10/9 Radiotherapy: mean total dose = amifostine: 66.8 Gy (SD 3.2); control: 66.4 Gy (SD 3.4). Treatment duration: mean = amifostine: 49.6 (SD 4.5) days; control: 55.9 (SD 8.9) Chemotherapy: carboplatin (90 mg/m²), once a week before RT in both groups Number randomised: 50 (amifostine 25, control 25) Number evaluated: 45 (amifostine 22, control 23) |
|
| Interventions |
Amifostine versus no intervention Amifostine (300 mg/m2), IV 30 minutes before RT on days 1 to 5 of each week. Antimetic treatment administered IV before the amifostine Control: nothing |
|
| Outcomes | Xerostomia: incidence of late xerostomia (RTOG grade 2 or more ‐ measured on a 0 to 4 scale) Salivary flow rates: not reported Adverse effects: haematologic toxicity, nausea, vomiting and transient hypotension Survival data: progression‐free survival at 18 months Other oral symptoms: incidence of grade 3 or greater acute mucositis and dysphagia Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: xerostomia: 3, 6, 9, 12 and 18 months after RT; haematologic toxicity and acute non‐haematological toxicities (mucositis and dysphagia): weekly for 7 weeks during RT, then 1, 2 and 3 months after RT |
|
| Funding | Not reported | |
| Trial registration | Not registered | |
| Sample size calculation presented | Yes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized (1:1)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) patients/carers | High risk | Amifostine versus no intervention. Blinding not possible |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Not possible due to 'no intervention' group and subjective assessment of xerostomia |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 45/50 participants evaluated (equal dropouts between groups). 3 participants dropped out of the amifostine arm. 2 denied further treatment (1 = week 2 and 1 = week 4) and 1 was lost to follow‐up. In the control arm, 1 participant died and 1 received palliative treatment because of disease progression |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Bardet 2011.
| Methods | Location: France Number of centres: 27 Date of enrolment: March 2001 to January 2006 |
|
| Participants | Inclusion criteria: newly diagnosed head and neck, eligible for radiotherapy. Over 75% of both parotid glands in field. Performance status ≤ 2, no distant metastases, neutrophils ≥ 2000/uL, platelets ≥ 1000,000/uL, creatine < 130 umol/L, aminotransferases ≤ 3 x the upper limit of normal, and ≥ 18 years Exclusion criteria: use of pilocarpine during RT and concomitant CT, second‐line treatment, incomplete assessment of salivary gland function Age: intravenous: mean 55.2 range 34 to 78; subcutaneous: mean 56.1 range 36 to 76 Gender: intravenous: 127 M, 16F; subcutaneous: 124 M, 24 F Cancer type: newly diagnosed squamous cell carcinoma of the head and neck, at all stages, and nodal status Radiotherapy: at least 40 Gy of radiation delivered postoperatively Chemotherapy: induction chemotherapy in 42 patients no concurrent chemotherapy Number randomised: 291 (intravenous 143, subcutaneous 148) Number evaluated: 127 (intravenous 67, subcutaneous 60) for xerostomia at 1 year |
|
| Interventions |
Intravenous versus subcutaneous amifostine Intravenous: 200 mg/m² daily, administered over 3 minutes, 15 to 30 minutes before RT Subcutaneous: 500 mg at 2 sites, 20 to 60 minutes before RT |
|
| Outcomes | Xerostomia: grade 2 or above (0 to 4 scale). Physician graded via RTOG before treatment, every 3 months for the 1st year and then every 6 months Salivary flow rates: unstimulated and stimulated saliva (mg/min) Adverse effects: nausea, vomiting, hypotension, skin rash, local pain at injection site, fever, asthenia Survival data: locoregional control, overall survival Other oral symptoms: dysgeusia (taste disturbance), dysphagia (difficulty in swallowing), dysphonia (difficulty in speaking) ‐ these 3 items were combined with the patients' sensation of mouth dryness and assessed using a patient benefit questionnaire (see QoL); grade 3+ acute mucositis Other oral signs: not reported Quality of life: patient benefit questionnaire Patient satisfaction: not reported Cost data: not reported Timing of assessment: acute xerostomia measured at 3 months; xerostomia, salivary flow rates and patient benefit questionnaire reported at 6 months, 1, 2 and 3 years; survival reported up to 4 years |
|
| Funding | Externally funded by pharmaceutical company; Schering‐Plough, France | |
| Trial registration | clinicaltrials.gov/show/NCT00158691ID ‐ 12 | |
| Sample size calculation presented | Yes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors claim "randomly assigned". No further details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) patients/carers | High risk | "Lack of double‐blind". Patients could not really be considered to be blinded as administration of amifostine differed |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Patient‐reported outcome see above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large loss to follow‐up. Attrition likely to be related to outcome |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Brizel 2000.
| Methods | Location: Europe, Canada, USA Number of centres: 35 to 40 (unclear) Date of enrolment: October 1995 to October 1997 33% dropout rate at 12 months |
|
| Participants | Inclusion criteria: patients with newly diagnosed, previously untreated squamous cell head and neck cancer. Inclusion of ≥ 75% of both parotid glands within radiation field and ≥ 40 Gy. Karnofsky Performance Status ≥ 60, granulocyte ≥ 2000 microL and platelet count ≥ 100,000 microL Exclusion criteria: patients with T1N0 or T2N0 carcinomas of the true vocal cords and tumours of the major or minor salivary glands or history of malignancy other than in situ cervix carcinoma within 5 years preceding diagnosis. Pregnant women Age: amifostine: 36 to 76, median = 55 years; control: 28 to 78, median = 56 years Gender: amifostine 123 M, 27 F; control 120 M, 33 F Cancer type: head and neck, various tumour sites, stages and node stages Radiotherapy: amifostine: definitive = 50, postoperative high risk = 70 and postoperative low risk = 28; control: definitive = 52, postoperative high risk = 65 and postoperative low risk = 36. 1.8 to 2.0 Gy , 5 days a week over 5 to 7 weeks for a total dose of 50 to 70 Gy Chemotherapy: none Number randomised: 315 randomised, but 12 never received any treatment (amifostine 150, control 153) Number evaluated: xerostomia at 12 months: 203 (amifostine 97, control 106), all included in analysis for locoregional control, all who received at least 1 dose of amifostine were assessed for toxicity |
|
| Interventions |
Amifostine versus no intervention Amifostine: (200 mg/m²) 3 minute intravenous 15‐30 minutes before RT Control: nothing |
|
| Outcomes | Xerostomia: incidence of grade 2+ acute (within 90 days of the start of RT) and chronic xerostomia (0 to 4 scale) Salivary flow rates: unstimulated and stimulated saliva production ‐ reported as median quantity (g) of saliva and also as number of participants producing > 0.1 g in 5 min ("a clinically relevant volume") Adverse effects: nausea, vomiting, hypotension, allergic response Survival data: locoregional control, progression‐free survival and overall survival at 24 months Other oral symptoms: oral discomfort, dysgeusia (taste disturbance), dysphagia (difficulty in swallowing), dysphonia (difficulty in speaking) ‐ all included in patient benefit questionnaire (see QoL); grade 3+ acute mucositis Other oral signs: not reported Quality of life: patient benefit questionnaire (8 items each on a 10‐point scale where higher = better QoL) Patient satisfaction: not reported Cost data: not reported Timing of assessment: xerostomia: within 3 months of start of RT, then at 12, 18 and 24 months; salivary flow rates: 12, 18 and 24 months after start of RT; quality of life: 12 months after start of RT |
|
| Funding | Source of funding: Medimmune Oncology | |
| Trial registration | Not registered | |
| Sample size calculation presented | Yes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "dynamic allocation process" (recognised methods referenced) |
| Allocation concealment (selection bias) | Low risk | Quote: "determined by a phone call from the enrolling institution to the protocol sponsor (US Bioscience)" Comment: it appears to be central/remote allocation |
| Blinding (performance bias and detection bias) patients/carers | High risk | Amifostine versus no intervention |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Open‐label, no blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 315 enrolled and randomised; 12 never received any treatment or follow‐up. Overall attrition 36% |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Brizel 2008.
| Methods | Location: Australia, Canada, USA Number of centres: 22 Date of conduct: September 1999 to May 2001 |
|
| Participants | Inclusion criteria: adults with newly diagnosed head and neck cancer. Patients with unknown primary and extensive neck disease also eligible. Karnofsky Performance Status > 60, haemoglobin > 10 g/dL, plus other similar criteria Exclusion criteria: prior head and neck radiation therapy, prior surgery for primary tumour beyond biopsy, prior chemotherapy, known allergy to Escherichia coli‐derived products, participation in another study within the previous 30 days, refusal to use adequate contraception during study, pregnant or breastfeeding Age: palifermin: mean 54 (range 25‐80); placebo: mean 56 (range 42‐75) Gender: palifermin 55 M, 12 F; placebo 27 M 5 F Cancer type: primary locations: oral cavity, oropharynx/nasopharynx, hypopharynx/larynx Radiotherapy: isocentric 4 to 6 MV photons either standard fractionation (once daily 2 Gy fractions 5 days/week: total primary tumour dose 70 Gy) or hyperfractionation (single 2 Gy fraction followed by a planned 1‐week treatment break. Then twice‐daily radiation: total dose of 72 Gy/6.5 weeks). Varied by centre Chemotherapy: cisplatin 20 mg/m² per day as IV bolus injection and fluorouracil 1000 mg/m² per day as continuous infusion, both on 1st 4 days of 1st and 5th weeks of RT Number randomised: 101 (69 palifermin, 32 placebo) Number evaluated: varies by outcome but 97 (65 palifermin, 32 placebo) analysed for our primary outcome of xerostomia |
|
| Interventions |
Palifermin versus placebo Palifermin: 60 µg/kg by IV bolus injection on study day 1 (Friday) before 1st week of CRT. Subsequent doses administered for 7 consecutive weeks, on each Friday after completion of weekly radiation treatment. 2 additional doses given on weeks 8 and 9 Placebo: as above Follow‐up: 5 weeks after end of RT |
|
| Outcomes | Xerostomia: incidence of grade 2 xerostomia using NCI CTC scale Salivary flow rates: not reported Adverse effects: nausea, vomiting, fever, constipation, dehydration, granulocytopenia, fatigue, diarrhoea, insomnia, anaemia, dysaphia, cough, headache, weight decrease, dizziness, anxiety, hypomagnesaemia Survival data: survival, progression‐free survival (up to 75 months) Other oral symptoms: mucositis (primary outcome of study), dysphagia Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported |
|
| Funding | Pharmaceutical trial (Amgen) | |
| Trial registration | Not registered | |
| Sample size calculation presented | Yes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double‐blind, randomized, placebo‐controlled study" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Quote: "double‐blind randomised placebo‐controlled study" |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Quote: "double‐blind randomised placebo‐controlled study". However there is a subjective element to the index |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Xerostomia data on 97 out of 101 enrolled. Quote: "3 patients in palifermin group and 1 in the placebo group discontinued study treatment with adverse events not considered related to study treatment". Comment: unclear if 3 of these were missing for xerostomia |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Buentzel 2006.
| Methods | Location: Europe, USA Number of centres: 18 (15 Europe, 3 USA) Date of recruitment: October 1996 to October 1998 |
|
| Participants | Inclusion criteria: at least 18 years of age scheduled for definitive or adjuvant chemoradiotherapy for histologically confirmed squamous cell carcinoma of the head and neck. Postsurgery the surgical wound must be healed but no later that 12 weeks after surgery. Inclusion of at least 75% of each parotid gland within radiation field. Life expectancy 12+ months, Karnofsky Performance Status 60+, adequate function of bone marrow, kidneys and the liver Exclusion criteria: evidence of distant metastatic disease, primary lesion of the parotid gland, or a history of prior malignancy within the past 5 years (other than non‐melanomatous skin cancers that are controlled or carcinoma in situ of the cervix). Scheduled to receive hyperfractionated or accelerated radiotherapy, previously treated with chemotherapy or other investigational therapies within 4 weeks of study entry. Pregnant women Age: amifostine: median 57 (range 29‐73); placebo: median 58 (range 23‐78) Gender: amifostine 54 M, 13 F; placebo 57 M, 8 F Cancer type: head and neck cancer, various primary sites and stages Radiotherapy: standard fractionation (1.8‐2.0 Gy per day, 5 days a week) over 6 to 7 weeks for a total dose of 60 to 70 Gy Chemotherapy: carboplatin 70 mg/m² IV over 30 minutes after amifostine and 30 minutes before RT Number enrolled: 132 Number randomised: 132 Number evaluated: 132 (ITT analysis) (67 amifostine; 65 placebo) |
|
| Interventions |
Amifostine versus placebo Amifostine: 300 mg/m² IV over 3 minutes (days 1‐5 and 21‐25 of treatment); 200 mg/m² IV over 3 minutes (days 6‐20 and 26‐30/35) Placebo (Mannitol): equivalent volume to amifostine |
|
| Outcomes | Xerostomia: RTOG acute and late radiation morbidity scoring criteria; incidence of grade 2 or higher acute or late xerostomia (0 to 4 scale) Salivary flow rates: stimulated and unstimulated saliva measurements (not assessed as less than a 3rd of participants had salivary function at 1 year) Adverse effects: nausea, vomiting, allergic response, asthenia Survival data: locoregional failure rate, progression‐free survival and overall survival Other oral symptoms: RTOG acute and late radiation morbidity scoring criteria: grade 3 or higher acute mucositis Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: acute xerostomia and mucositis measured up to 90 days after start of RT; late xerostomia measured up to 12 months after start of RT |
|
| Funding | Source of funding: MedImmune Oncology Inc grant | |
| Trial registration | Not registered | |
| Sample size calculation presented | Yes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assumed. Described as "dynamic allocation scheme", similar method to Brizel 2000 |
| Allocation concealment (selection bias) | Low risk | Fax of baseline data sent to central telephone number for randomisation number. Randomisation number identical to blinded drug container held at pharmacy |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Amifostine versus placebo |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Information provided by author: "blinded drug containers were kept at the pharmacy, treating physicians had no information about the randomization until the end of the follow‐up period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses carried out an ITT basis. Dropouts = 30 (23% dropout rate). Amifostine group = 21 (1 ‐ never treated, 16 ‐ toxicity, 1 ‐ patient request, 1 ‐ death, 2 ‐ other illness). Placebo group = 10 (1 ‐ never treated, 4 ‐ toxicity, 1 ‐ patient request, 1 ‐ death, 1 ‐ disease progression, 2 ‐ non‐compliance) |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Burlage 2008.
| Methods | Location: the Netherlands Number of centres: 2 Date of enrolment: April 1999 ‐ October 2003 |
|
| Participants | Inclusion criteria: biopsy confirmed HNSCC, initial 5% (wt/vol) citric acid‐stimulated parotid salivary flow > 0.1 mL/min Exclusion criteria: previous irradiation and/or previous or concurrent chemotherapy, patients with salivary gland tumours, severe cardiovascular disease or chronic obstructive pulmonary disease, pregnant women Age: pilocarpine: 18‐60 years 50 participants, > 60 years 35 participants; placebo: 18‐60 years 42 participants, > 60 years 42 participants Gender (M:F): pilocarpine 22:63; placebo 13:71 Cancer type: oral cavity (17%), oropharynx (18%), larynx (51%), hypopharynx (7%), nasopharynx (4%), unknown primary (1%) (equally distributed across groups) Submandibular gland removal: both removed: pilocarpine 2%, placebo 5%; 1 removed: pilocarpine 37%, placebo 38% Radiotherapy: clinical target volume of initial field encompassed the primary tumour site with 1.5 cm margin, neck node levels in which pathologic nodes were found and elective node areas on both sides. Conventional fractionation schedule. Received at least 40 Gy in daily 2 Gy fractions Chemotherapy: none Number randomised: 170 (85 per group) Number evaluated: 113 (pilocarpine 55, placebo 58) |
|
| Interventions |
Pilocarpine versus placebo Pilocarpine: 5 mg 4 times daily 2 days before start of RT until 14 days after RT Placebo: similar tablets, same schedule |
|
| Outcomes | Xerostomia: from validated head‐and‐neck symptom questionnaire on 5‐point scale; LENT SOMA Salivary flow rates: parotid salivary flow using Carlson‐Crittenden cups, from left and right hand parotid glands simultaneously under standardised conditions for 10 min. Flow stimulated with 5% (wt/vol) citric acid. Parotid flow complication probability also reported Adverse effects: not reported Survival data: locoregional control Other oral symptoms: eating, swallowing Other oral signs: not reported Quality of life: some covered in validated head‐and‐neck symptom questionnaire on 5‐point scale Patient satisfaction: not reported Cost data: not reported Timing of assessment: before RT, 6 weeks, 6 months and 12 months postRT |
|
| Funding | Not reported. Conflicts of interest: "none" reported | |
| Trial registration | Not registered | |
| Sample size calculation presented | Yes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was executed by the hospital pharmacist by computer, using random permuted blocks within strata. The randomisation key was opened after the last saliva collection (1 year after the last patient was included and after completion of all planned assessments)" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Quote: "Double‐blind randomised placebo‐controlled study". Intervention was tablets supplied by the pharmacy |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Quote: "Double‐blind randomised placebo‐controlled study". Intervention was tablets supplied by the pharmacy |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 32% missing at 12 months with no clear reasons given by study group |
| Selective reporting (reporting bias) | High risk | Adverse events and xerostomia data not fully reported |
| Other bias | Low risk | No other sources of bias are apparent |
Büntzel 1998.
| Methods | Location: Germany Number of centres: 1 Date of recruitment: not stated |
|
| Participants | Inclusion criteria: stage III or IV carcinoma of the head and neck, aged 16 to 80 and no evidence of systemic infection or liver or renal impairment. Tumour resected or excised before adjuvant RT Exclusion criteria: not reported Age: amifostine: median 61 (range 40‐77); control: median 58 (range 38‐75) Gender: amifostine 13 M, 1 F; control 12 M, 2 F Cancer type: tumour location (amifostine/control): larynx = 3/1, hypopharynx = 4/3, mesopharynx = 3/7, nose = 2/1, mouth = 2/2 Radiotherapy: 2 Gy fractions, 5 days a week for 6 weeks; maximum dose of 60 Gy (encompassing 75% of the major salivary glands) Chemotherapy: 20 min IV infusion of carboplatin (70 mg/m² days 1 to 5 and 21 to 25 of treatment) Number randomised: 28 (14 amifostine, 14 control) Number evaluated: 28 |
|
| Interventions |
Amifostine versus no intervention Amifostine: (500 mg) 15 min IV before carboplatin (days 1 to 5 and days 21 to 25). Followed by antiemetic regimen to control nausea/vomiting Control: nothing Use of supportive drugs reported: (amifostine/control): G‐CSF: 2/7; GM‐CSF: 0/7; antibiotics: 4/10 |
|
| Outcomes | Xerostomia: incidence and severity using WHO grading (0 to 4 scale ‐ we report grade 2 and above) Salivary flow rates: not reported Adverse effects: hypotension Survival data: not reported Other oral symptoms: dysgeusia (taste disturbance), dysphagia (difficulty in swallowing), mucositis (WHO) Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: economic evaluation (Bennett 2001) Timing of assessment: xerostomia at end of RT and 1 year; other oral symptoms at end of RT |
|
| Funding | US Bioscience who produce Ethyol‐amifostine | |
| Trial registration | Not registered | |
| Sample size calculation presented | Not reported | |
| Notes | Additional data presented but included extra 11 patients in amifostine group who were not entered in the study (not included in analyses) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) patients/carers | High risk | Amifostine versus no intervention |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Not blinded and xerostomia is a subjective outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Büntzel 2010.
| Methods | Location: Germany Number of centres: 6 Date of study: 2001 to 2007 |
|
| Participants | Inclusion criteria: SCCHN with deficiency in selenium and if radiation field included 75% of the major salivary glands Exclusion criteria: none reported Age: median 63.2 range 38.7‐83.0 Gender (M:F): selenium 16:6; control 15:2 Cancer type: head and neck cancer Radiotherapy: 1.8 to 2.0 Gy to primary tumour and lymphatic neck during daily radiation treatment; to total dose 60‐72 Gy Chemotherapy: unclear Number randomised: 40: 22 selenium, 18 control Number evaluated: 39: 22 selenium, 17 control |
|
| Interventions |
Selenium versus no intervention Selenium: 500 µg sodium selenite, 2 days before RT, 500 µg selenite and radiation days (300 µg if official holiday). Administrated as oral fluid 1 hour before RT Control: no intervention |
|
| Outcomes | Xerostomia: RTOG grade for xerostomia Salivary flow rates: not reported Adverse effects: serious adverse events reported Survival data: not reported Other oral symptoms: mucositis RTOG, dysgeusia (taste disturbance RTOG), dysphagia (difficulty in swallowing RTOG) Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: 1, 2, 3, 4, 5, 6, 7 weeks from start of RT and 6 weeks after RT |
|
| Funding | Externally funded by Arzneimittel, Germany | |
| Trial registration | Unclear | |
| Sample size calculation presented | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not given, stated "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Allocation after consent obtained |
| Blinding (performance bias and detection bias) patients/carers | High risk | Participants receive selenium oral fluid prior to radiotherapy or not. Blinding not possible |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Participants receive selenium oral fluid prior to radiotherapy or not, and their subjective assessment of xerostomia is included |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Initial study requiring 60 patients per arm stopped early due to slow accrual 113 screened. 93 selenium deficient. 40 consented. 1 withdrawal, 39 reported Selenium concentrations reported in other article elsewhere |
| Selective reporting (reporting bias) | High risk | Xerostomia but no standard deviations. Total adverse events reported but not per person |
| Other bias | Low risk | No other sources of bias are apparent |
Duncan 2005.
| Methods | Location: Canada Number of centres: multicentre (unclear how many) Date of enrolment: September 1997 to September 1999 |
|
| Participants | Inclusion criteria: SCCHN, non‐metastatic disease Exclusion criteria: none reported Age: lozenge median 59.7; placebo median 57.3 Gender (M:F) : lozenge (48:18), placebo (52:15) Cancer type: oral cavity, oropharynx, hypopharynx, nasopharynx, larynx Radiotherapy: conventional radical or postoperative radiotherapy to a dose of 50 Gy or greater delivered in once daily fractions (1.8 to 2.4 Gy) Chemotherapy: not mentioned, probably none Number randomised: 138 (69 per group) Number evaluated: 133 (lozenge 66; placebo 67) |
|
| Interventions |
Antimicrobial lozenge versus placebo Antimicrobial lozenge: BCoC, bacitracin, 6 mg; clotrimazole, 10 mg; gentamicin, 4 mg. Unclear how frequently taken or for how long Placebo: not described ‐ assumed similar |
|
| Outcomes | Xerostomia: item on trial specific checklist ‐ 'Did you have mouth dryness (1‐4 scale)?' and NCIC CTG ECTC physician‐rated (using patient diary) Salivary flow rates: not reported Adverse effects: not reported Survival data: not reported Other oral symptoms: mucositis using OMAS (primary outcome), mouth pain, chewing, numbness, mouth opening, burning mouth Other oral signs: not reported Quality of life: 2 tools ‐ European Organisation for Research and Treatment of Cancer Quality of Life questionnaire (EORT QLQ‐C30), trial specific checklist Patient satisfaction: not reported Cost data: not reported Timing of assessment: 2, 4, 6 during RT; 8‐9, 12‐14, 24 weeks on study |
|
| Funding | The National Cancer Institute of Canada, Clinical Trials Group | |
| Trial registration | Unclear | |
| Sample size calculation presented | No | |
| Notes | Primarily study to prevent mucositis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Quote: "double‐blind controlled trial". Placebo tablets given |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Quote: "double‐blind controlled trial". Placebo tablets given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Compliance with quality of life forms reported to be 93.3% but reasons for dropouts not reported. Similar low rates of attrition per group |
| Selective reporting (reporting bias) | High risk | Xerostomia reported, adverse events not reported for lozenge |
| Other bias | Low risk | No other sources of bias are apparent |
Fisher 2003.
| Methods | Location: USA Number of centres: unclear Date of randomisation: March 1998 to February 2000 |
|
| Participants | Inclusion criteria: oral and oropharyngeal squamous cell carcinoma, Karnofsky Performance Score ≥ 60, no prior radiotherapy to the head and neck, planned irradiation of the oral cavity or oropharynx in which at least 50% of the major salivary glands are to receive > 50 Gy Exclusion criteria: salivary gland malignancy; use of cholinergic, anticholinergic, and tricyclic drugs; and patients with uncontrolled asthma, acute iritis, or narrow‐angle glaucoma Age: pilocarpine 60.8 years; placebo 59.3 years Gender (M:F): pilocarpine 93:28, placebo 92:32 Cancer type: oral cavity 52; nasopharynx 3; oropharynx 104; hypopharynx 11; other 13; unknown 18 (evenly distributed across groups) Radiotherapy: 60‐70 Gy with 50% of volume of major salivary glands receiving 50 Gy Chemotherapy: not stated Number randomised: 249; 3 ineligible, all from pilocarpine arm (121 pilocarpine, 125 placebo) Number evaluated: 166 end of RT (pilocarpine 89, placebo 77); 166 at 3 months (pilocarpine 85, placebo 81); 137 at 6 months (pilocarpine 68, placebo 69) |
|
| Interventions |
Pilocarpine versus placebo Pilocarpine: 5 mg tablets 4 times daily starting 3 days before RT and continuing for 3 months Placebo: 5 mg tablets 4 times daily starting 3 days before RT and continuing for 3 months. 3 months after RT the placebo group were permitted to cross over to pilocarpine |
|
| Outcomes | Xerostomia: not reported Salivary flow rates: salivary gland scintigraphy (stimulated and unstimulated) Adverse effects: drug toxicities reported Survival data: not reported Other oral symptoms: RTOG acute mucositis, mouth pain, dysgeusia (taste disturbance), dysmasesia (difficulty in chewing), dysphagia (difficulty in swallowing), dysphonia (difficulty in speaking) Other oral signs: not reported Quality of life: University of Washington QoL scale Patient satisfaction: not reported Cost data: not reported Timing of assessment: pretreatment, end of RT, 3 months after end of RT, 6 months after end of RT |
|
| Funding | National Cancer Institute and MGI Pharma Inc. | |
| Trial registration | clinicaltrials.gov/show/NCT00003139ID ‐ 11 Protocol available |
|
| Sample size calculation presented | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Pilocarpine versus placebo |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Pilocarpine versus placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 213/249 available for analysis. Dropouts very high for salivary flow (33% end of RT and 3 months, 45% at 6 months) |
| Selective reporting (reporting bias) | High risk | Xerostomia not reported |
| Other bias | Low risk | No other sources of bias are apparent |
Gornitsky 2004.
| Methods | Location: Canada Number of centres: 1 Date of randomisation: March 1998 to September 2001 |
|
| Participants | Inclusion criteria: scheduled to receive external beam radiotherapy, using a bilateral radiation technique encompassing ≥ 2/3 of all major and minor salivary glands for a minimum of 5000 cGy (200 cGy per day) for 5‐7 weeks Exclusion criteria: clinically significant cardiovascular disease, chronic obstructive pulmonary disease, biliary tract disease, uncontrolled asthma, acute iritis, narrow angle glaucoma, participants who are pregnant or nursing. Hypersensitivity to pilocarpine, participants on tricyclic antidepressants, antihistamines with anticholinergic effects, beta blockers, or pilocarpine for ophthalmic indications were excluded Age (mean): pilocarpine 58 years; placebo 61 years Gender (M:F): pilocarpine 26:3, placebo 24:5 Cancer type: oral cavity 14; pharynx 13; tonsil 11; glottis 3; larynx 11; sinus 2; neck 1; unknown 1 (evenly distributed across groups) Radiotherapy: Mean dose = 64.7 Gy (pilocarpine group), 63.7 Gy (placebo group) Chemotherapy: pilocarpine 13 (45%); placebo 9 (32%) Number randomised: 58 Number evaluated: 58 (22 dropped out but ITT was used and missing data were calculated) |
|
| Interventions |
Pilocarpine versus placebo Phase 1 Pilocarpine: 5 mg tablets 5 times daily, half an hour before meals, before radiotherapy, and prior to sleep during the period of radiotherapy Placebo: identical tablets 5 times daily, half an hour before meals, before radiotherapy, and prior to sleep during the period of radiotherapy Phase 2 All received pilocarpine (5 mg) 4 times daily half an hour before meals and prior to sleep for 5 weeks |
|
| Outcomes | Xerostomia: subjective assessment of xerostomia: VAS (rated 0‐100) Salivary flow rates: whole saliva secretion (unstimulated and stimulated) using the SAXON test Adverse effects: not reported (data provided by author) Survival data: not reported Other oral symptoms: oral discomfort, difficulty with eating, dysphonia (difficulty in speaking), mucosal pain or burning (VAS, rated 0‐100) Other oral signs: not reported Quality of life: global quality of life, sleeping problems (VAS, rated 0‐100) Patient satisfaction: not reported Cost data: not reported Timing of assessment: prior to RT, end of RT, 5 weeks after end of RT |
|
| Funding | Pharmacia Canada | |
| Trial registration | Not registered | |
| Sample size calculation presented | Not reported | |
| Notes | Phase 2 data not included in the review Additional data provided by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment from author: "block of four using a random number table... allocation sequence prepared by pharmacy of Jewish General Hospital" |
| Allocation concealment (selection bias) | Low risk | Third party randomisation; coded bottles |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Bottles only distinguished by number allocated by pharmacy. Investigators, treating physicians and patients blinded |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Subjective outcomes self reported (patients unaware of treatment group) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis carried out on ITT basis. 38% dropout rate. 58 randomised, 22 dropped out (9 pilocarpine, 13 placebo) |
| Selective reporting (reporting bias) | High risk | Adverse events not reported |
| Other bias | Low risk | No other sources of bias are apparent |
Grötz 2001.
| Methods | Location: Germany Number of centres: 1 Date of randomisation: not stated |
|
| Participants | Inclusion criteria: scheduled to receive adjuvant or sole radiotherapy for head and neck cancer to a scheduled dose of 60 Gy. Cranial border of the field above the chin‐mastoid line so salivary glands are located in the core irradiation field Exclusion criteria: salivary gland disorders Age: mean age = 55 years Gender: 22 M, 1 F Cancer type: head and neck Radiotherapy: total dose = 60 Gy Chemotherapy: unclear Number randomised: 48 Number evaluated: 23 |
|
| Interventions |
Coumarin + troxerutin versus placebo Venalot Depot (coumarin 15 mg and troxerutin 90 mg) tablet: 2 tablets 3 times daily. Start 1 week before RT and 4 weeks after end of RT Control: placebo |
|
| Outcomes | Xerostomia: not reported, only as part of total RTOG Salivary flow rates: stimulated and unstimulated using sialoscintigraphy (sialometry abandoned as primary marker as not successfully collected). Acute radiation side effects RTOG score but for all organs Adverse effects: reddened skin, nausea Survival data: locoregional control Other oral symptoms: not reported Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: 4 weeks after RT |
|
| Funding | Not stated | |
| Trial registration | Unclear | |
| Sample size calculation presented | Not reported | |
| Notes | Unable to use data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Venalot Depot versus placebo |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Salivary flow rates objective outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 48 randomised, 25 dropped out. Dropouts per group not specified |
| Selective reporting (reporting bias) | High risk | Xerostomia not reported. Data for total RTOG score presented but no break down by condition or organ |
| Other bias | Low risk | No other sources of bias are apparent |
Haddad 2002.
| Methods | Location: Iran Number of centres: 1 Date of recruitment: 1998‐2000 |
|
| Participants | Inclusion criteria: 18‐70 year old patients, irradiated to the head and neck, both parotid glands in the radiation fields (minimum 40 Gy). No previous history of irradiation in this region
Exclusion criteria: asthma, chronic obstructive pulmonary disease, narrow‐angle glaucoma, biliary or renal lithiasis and hypertensive, heart or psychiatric disorders requiring medical treatment Age: mean across groups = 43 years (range 18 to 70 years) Gender (M:F): across groups 36:24 Cancer type: primary site of tumour. Pilocarpine group: nasopharynx (n = 17), neck adenopathy (n = 1). Placebo group: maxilla (n = 2), nasopharynx (n = 13), tongue (n = 1), tonsil (n = 5) Radiotherapy: standard fractionation (1.8 to 2 Gy per day, 5 days a week) and cobalt‐60 systems; mean parotid dose 58 Gy (pilocarpine 59 Gy; placebo 57 Gy) (range 45 to 70 Gy) Chemotherapy: none Number randomised: 60 Number evaluated: 39 (18 pilocarpine, 21 placebo) |
|
| Interventions |
Pilocarpine versus placebo Pilocarpine hydrochloride: 5 mg 3 times daily for 3 months starting from the beginning of RT Placebo: 5 mg 3 times daily for 3 months starting from the beginning of RT |
|
| Outcomes | Xerostomia: subjective evaluation score for xerostomia using 6 questions evaluated using VAS (0‐100 mm). Objective grading of xerostomia according to the Late Effects of Normal Tissues Subjective, Objective, Management and Analytic (LENT SOMA) scale Salivary flow rates: not reported Adverse effects: lacrimation (excess tears, crying), nausea Survival data: overall survival Other oral symptoms: not reported Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: 6 months postRT |
|
| Funding | Source of funding: Tehran University of Medical Sciences' research grant | |
| Trial registration | Not registered | |
| Sample size calculation presented | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomisation was performed at the start of radiotherapy by the sealed envelope method" Comment: although not clear the randomisation was probably done well as the pharmacy was involved in making and distributing the tables |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, pharmacy involvement |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Capsules only distinguished by a number recorded by the drug manufacturer. Investigators, treating physicians and patients blinded |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Capsules only distinguished by a number recorded by the drug manufacturer. Investigators, treating physicians and patients blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 60 enrolled. 13/31 (42%) dropouts in pilocarpine group; 8/29 (28%) dropouts in placebo group |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Haddad 2009.
| Methods | Location: USA Number of centres: 4 Date of enrolment: May 2003 to April 2006 |
|
| Participants | Inclusion criteria: with stage III or IV, previously untreated, locally advanced, SCCHN. Primary tumour types allowed: oropharynx, hyperpharynx, oral cavity, larynx, unknown primary Exclusion criteria: grade > 2 peripheral neuropathy other serious comorbid illness, involuntary weight loss of > 20% of body weight in 3 months preceding study Age: amifostine mean 55; control 57 Gender: amifostine: 27 M, 2 F; control: 23 M, 6 F Cancer type: (amifostine/control) oropharynx = 18/17, oral cavity = 5/6, larynx = 3/5, unknown primary = 2/0, other = 1/1 Neck dissection: amifostine 48%; control 38%; no details reported Radiotherapy: concomitant boost radiation, 72 Gy in 42 fractions over 6 weeks. Use of IMRT not allowed Chemotherapy: 4 weekly doses of carboplatin/paclitaxel. Induction chemotherapy was used in 29 of 58 patients overall with docetaxel, cisplatin, and 5‐fluorouracil Number randomised: 58 (29 per group) Number evaluated: unclear for xerostomia |
|
| Interventions |
Amifostine versus no intervention Subcutaneous daily amifostine at dose of 500 mg 30‐60 min before daily RT (before morning dose only, when schedule moved to twice daily radiotherapy at day 19). Average number of amifostine doses was 25 (median 28 doses). Amifostine withheld for skin toxicity |
|
| Outcomes | Xerostomia: Common Terminology Criteria for Adverse Events including xerostomia reported but not by group Salivary flow rates: saliva collection with and without citric acid simulation Adverse effects: not reported Survival data: overall survival, progression‐free survival, local control Other oral symptoms: Common Terminology Criteria for Adverse Events for mucositis, swallowing measured Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: xerostomia and mucositis assessed weekly throughout RT, then every 4 weeks after RT; salivary flow rate assessed at 12, 24 and 52 weeks after RT; dysphagia (swallowing) assessed at 8, 12, 24 and 52 weeks after RT; survival ‐ median follow‐up 34 months after RT, minimum 26 months |
|
| Funding | Medimmune Oncology | |
| Trial registration | Not registered | |
| Sample size calculation presented | Yes | |
| Notes | Quote: "Study stopped before completion of planned accrual because IMRT was becoming de facto standard technique in treating head and neck cancer" Study focuses on survival Not able to use data ‐ contacted authors for data 19 February 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation process was centralised and managed through the Dana‐Farber Cancer Institute protocol office" Comment: linked to Harvard University probably done well |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation process was centralised and managed through the Dana‐Farber Cancer Institute protocol office" |
| Blinding (performance bias and detection bias) patients/carers | High risk | No intervention group as comparator ‐ not blinded |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Subjective assessment of xerostomia |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many participants dropped out |
| Selective reporting (reporting bias) | High risk | Badly reported xerostomia and no adverse events |
| Other bias | Low risk | No other sources of bias are apparent |
Han 2010.
| Methods | Location: China Number of centres: 2 Date of conduct: 1 October 2007 to 31 July 2009 |
|
| Participants | Inclusion criteria: quote: "First‐visit patients; diagnosed as mid/moderate to advanced/terminal nasopharyngeal squamous carcinoma through pathological and radiographic examinations; Karnosfsky score ≥ 60; expected survival period > 6 months; without severe complications (e.g. hypertension, coronary heart disease, diabetes, history of mental illness)" Exclusion criteria: see above Age: Jinlong: mean 46.3 (SD 7.4), median 53; control: mean 47.4 (SD 6.8), median 52 Gender: Jinlong: 33 M, 16 F; control: 34 M, 14 F Cancer type: nasopharyngeal squamous carcinoma Radiotherapy: dose 60 to 76 Gy, 2 Gy per day, 5 times a week Chemotherapy: "concurrent chemoradiotherapy" (no further details) Number randomised: 97 (Jinlong: 49, control: 48) Number evaluated: 95 (Jinlong: 48, control: 47) |
|
| Interventions |
Jinlong capsules versus no intervention 4 tablets once, 3 tablets every day Duration: 3 months Follow‐up: 12 weeks after treatment Quote: "Jinlong capsule is a modern 'fresh medicine preparation' made of fresh gecko and fresh long‐noded pit vipers, using cryogenic modern biochemical extracting and separation techniques. It maintained to the greatest degree the activity of effective ingredients of organisms, and reasonable compatibility among the ingredients. Basic research has shown that Jinlong can directly damage cancer cells by blocking the mitosis and proliferation of cancer cells, fix the p21 small protein molecule, restore the regulation of cancer cells, and turn cancer cells to normal cells..." |
|
| Outcomes | Xerostomia: quote: "observe the patients for toxic and side effects during and after radiotherapy, assess the toxic and side effects according to RTOG's criteria" Salivary flow rates: not reported Adverse effects: leukopenia, nausea, vomiting, 1 participant had dizziness and blood pressure drop, 1 participant had skin rash Survival data: not reported Other oral symptoms: mucositis Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported |
|
| Funding | Not reported; conflicts of interest: not reported | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) patients/carers | High risk | Jinlong versus no intervention |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Jinlong versus no intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: "1 patient quit because of myocardial infarction (Tx Group)", "1 patient quit because of mucosa toxicity (control group)" |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
He 2004.
| Methods | Location: China Number of centres: 1 Date of conduct: not stated |
|
| Participants | Inclusion criteria: aged 20‐70 years; Karnofsky Performance Score > 70; Hb 90 to 150/L; blood pressure 12‐20/8‐15 kPa; normal kidney and liver function; no severe infection such as septicaemia; no heart disease; no medical history of low blood pressure, no other cancer and no history of radiotherapy Exclusion criteria: see above Age: aged 20 to 70 (no further details) Gender: not reported Cancer type: amifostine: nasopharyngeal squamous cell carcinoma stage 1 = 1, stage 2 = 7, stage 3 = 8 and stage 4 = 1. Control: nasopharyngeal squamous cell carcinoma stage 1 = 1, stage 2 = 5, stage 3 = 1 and stage 4 = 1 Radiotherapy: conventional with nasopharyngeal tumour dose (65‐74 Gy) Chemotherapy: none Number randomised: 32 (amifostine: 17; control: 15) Number evaluated: 32 (amifostine: 17; control: 15) ‐ 1 participant left amifostine group due to GI tract side effect but analysis states 17 in this group (possible ITT analysis) |
|
| Interventions |
Amifostine versus no intervention Amifostine (200 mg/m²), diluted with 'water for injection' at the concentration of 50 mg/mL, IV 15‐30 min before RT Control: nothing |
|
| Outcomes | Xerostomia: "mucositis and xerostomia according to RTOG's criteria" (0‐4 scale; we report grade 2 and above) Salivary flow rates: "method used to measure the amount of saliva: put a 0.2 g cotton ball under patient's tongue, after 3 minutes, use electronic balance to measure its weight", reported as decrease in saliva/change score (unstimulated) Adverse effects: GI tract reaction/side effects (nausea and vomiting) Survival data: not reported Other oral symptoms: mucositis (RTOG criteria) Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: 3, 5 and 7 weeks after start of RT |
|
| Funding | Not reported; conflicts of interest: not reported | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized into" Comment: no further details given |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) patients/carers | High risk | Amifostine versus no intervention |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Not possible due to no intervention group and subjective assessment of xerostomia |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/32 participants dropped out, however, appears to be included in analysis |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Henke 2011.
| Methods | Location: Australia, Canada and Europe Number of centres: 38 hospitals Date of conduct: January 2005 to August 2007 |
|
| Participants | Inclusion criteria: more than 18 years old; resected for pathohistologically documented high‐risk stage 2 to 4B SCC of the oral cavity, oropharynx, hypopharynx, or larynx; ECOG score of 0 to 2; at least 2 of 9 areas of the oral or oropharyngeal mucosa due to receive at least 50 Gy RT Exclusion criteria: tumours of the lips, paranasal sinuses, salivary glands, or unknown primary site; metastatic disease; history of chronic pancreatitis or acute pancreatitis within the last year; prior RT to the head and neck region or prior chemotherapy; previous treatment on this study or with other KGFs Age: palifermin: mean 56 (SD 8); placebo: mean 57 (SD 9) Gender: palifermin: 78 M, 14 F; placebo: 75 M, 19 F Cancer type: head and neck (oropharynx, oral cavity, larynx, hypopharynx, other) Radiotherapy: standard fractionation of once daily 2 Gy fractions, 5 days per week; total 60 Gy (for R0 resection) over 6 weeks, or 66 Gy (for R1 resection) over 7 weeks, both with allowable range of ± 15% Chemotherapy: cisplatin (100 mg/m²) IV after appropriate hydration on days 1 and 22 (for R0 resection), or days 1, 22 and 43 (for R1 resection) Number randomised: 186 (palifermin 92; placebo 94) Number evaluated: 186 (palifermin 92; placebo 94) |
|
| Interventions |
Palifermin versus placebo Palifermin: (120 µg/kg) 3 days prior to start of, and then once per week during radiochemotherapy, i.e. 7 doses for those with R0 resection, 8 doses for those with R1 resection (total dose = 840 or 960 µg/kg respectively) Placebo: same schedule with placebo |
|
| Outcomes | Xerostomia: incidence of grade ≥ 2 xerostomia (Common Terminology Criteria for Adverse Events (CTCAE) v 3.0, assessed at months 4, 6, 8, 10, 12, reported only at month 4 Salivary flow rates: not reported Adverse effects: assessed weekly during study treatment Survival data: overall and progression‐free survival, incidence of disease recurrence and death Other oral symptoms: incidence of dysphagia (difficulty in swallowing), OMWQ‐HN 0 (no soreness) to 4 (extreme soreness) scale for mouth and throat soreness assessed weekly and reported as mean score Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported |
|
| Funding | Quote: "This study was supported by Amgen" (Amgen also named as sponsor on trials registry ‐ pharmaceutical industry) | |
| Trial registration | NCT00131638 (clinicaltrials.gov/ct2/show/NCT00131638) | |
| Sample size calculation presented | Yes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment was made by a centralized interactive voice response system" Comment: large multicentre trial using high‐tech randomisation method ‐ likely to be done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "Random assignment was made by a centralized interactive voice response system" Comment: large multicentre trial using high‐tech randomisation method ‐ likely to be done properly |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Quote: "placebo‐controlled, double‐blind study" Comment: blinding feasible |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Quote: "placebo‐controlled, double‐blind study" Comment: blinding feasible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All cases accounted for. ITT analysis (participants having no assessment assumed to have event) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. Low risk for xerostomia at the time point used in meta‐analysis (4 months) ‐ however, it should be noted that xerostomia was measured up to 12 months but data not reported |
| Other bias | Low risk | No other sources of bias are apparent |
Hu 2005.
| Methods | Location: China Number of centres: 1 Date of conduct: January 2002 to June 2004 |
|
| Participants | Inclusion criteria: head and neck patients confirmed by pathological examination Exclusion criteria: not reported Age (years): treatment: mean 51 (SD 19); control: mean 49 (SD 18) Gender: treatment: 36 M 34 F; control: 38 M, 32 F Cancer type: treatment: nasopharyngeal (52), tonsil (11) and tongue (7); cancer stage: I = 6, II = 20, III = 28 and IV = 16. Control: nasopharyngeal (51), tonsil (11) and tongue (8); cancer stage: I = 6, II = 19, III = 29 and IV = 16 Radiotherapy: overall dose: 70 Gy for nasopharyngeal carcinoma, 55‐70 Gy for carcinoma of tonsil and tongue Chemotherapy: none Number randomised: 140 (treatment 70, control 70) Number evaluated: 140 (treatment 70, control 70) |
|
| Interventions |
Shenqi Fanghou recipe versus no intervention Shenqi Fanghou recipe: dangshen (30 g), astragalus root (30 g), tuckahoe (30 g), Chinese yam (30 g), hedyotic diffusa (30 g), barbated skullcup herb (30 g), pueraria root (30 g), fragrant solomonseal rhizome (10 g), glossy privet fruit (10 g), stiff silkorm (10 g), grassleaf sweetflag rhizome (10 g), atractylodes macrocephala (10 g), semen coicis (50 g), dried tangerine peel (6 g), paris root (20 g), figwort root (15 g), common anemarrhena rhizome (15 g), gambir plant (15 g), scorpion (5 g), radix notoginseng (5 g), radix glycyrrhizae (5 g) Dosage: solution of 400 ml (200 ml in the morning and 200 ml in the afternoon), starting the first day of RT for 35 to 38 days Control group: nothing Follow‐up: end of RT |
|
| Outcomes | Xerostomia: subjective assessment of dry mouth: 1) mild: can eat dry cooked rice, 2) moderate: have difficulty in eating dry cooked rice, or 3) severe: cannot eat dry cooked rice Salivary flow rates: not reported Adverse effects: none Survival data: survival after a follow‐up of more than 1 year Other oral symptoms: oropharyngeal mucosa reaction, difficulty in mouth opening Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported |
|
| Funding | Source of funding: government (The Bureau of Science and Technology of Shenzhen City); conflicts of interest: not reported | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided" Comment: no further details given |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the envelop method was used to randomise" Comment: insufficient information |
| Blinding (performance bias and detection bias) patients/carers | High risk | Shenqi Fanghon recipe versus no intervention |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Not possible due to no intervention group and subjective assessment of xerostomia |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported (quote: "no adverse events") |
| Other bias | Low risk | No other sources of bias are apparent |
Jaguar 2015.
| Methods | Location: Brazil Number of centres: 1 Date of conduct: January 2010 to March 2012 |
|
| Participants | Inclusion criteria: primary oral, oropharynx, or nasopharynx carcinomas (clinical stage ≥ II) scheduled to undergo 3‐D radiotherapy (RTC3D) or IMRT, ≤ 75 years of age Exclusion criteria: hypersensitivity to bethanechol, hypotension, hyperthyroidism, peptic ulcer disease, epilepsy, angina, parkinsonism, and patients using tricyclic antidepressants, and antihistamines Age: bethanechol: mean 55.9 (range 21 to 75); placebo: mean 55.8 (range 28 to 75) Gender: bethanechol: 37 M, 11 F; placebo: 39 M, 10 F Cancer type: oral cavity, oropharynx, nasopharynx Radiotherapy: once‐daily mega voltage (6 MV), given at 18 to 2.12 Gy per fraction, 5 days per week (duration unclear) for 7 weeks Chemotherapy: bethanechol 73%; placebo 71% (type of CT not reported) Number randomised: 97 (bethanechol 48, placebo 49) Number evaluated: 84 (bethanechol 42, placebo 42) |
|
| Interventions |
Bethanechol versus placebo Both groups: 1 tablet (25 mg) taken twice a day from beginning of RT and continued until 1 month after end of treatment (median 19 weeks) |
|
| Outcomes | Xerostomia: observer‐based grade and scored according to the subjective measures of Eisbruch (grade 0 to 3) ‐ reported as grade 2 and above Salivary flow rates: whole unstimulated and stimulated saliva flows collected over 5 min each and reported in ml/min (reported by RT‐type subgroups ‐ we combined the subgroups but numbers were not reported so we used the number randomised from table 1), also scintigraphy undertaken Adverse effects: bethanechol toxicities using National Cancer Institute Common Terminology Criteria for Adverse Events – NCI CTCAE, v 3.0 Survival data: not reported Other oral symptoms: not reported Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: xerostomia assessed weekly to 3 months postRT; saliva flow assessed during RT (range 30 to 35 Gy) and 2 months postRT |
|
| Funding | FAPESP (an independent public foundation) and CAPES (an organization of the Brazilian federal government under the Ministry of Education) Conflict of interest statement does not indicate whether there is conflict or not; quote: "All authors disclose any financial and personal relationships with other people or organizations" | |
| Trial registration | Not registered | |
| Sample size calculation presented | Yes (reported in supplementary data online) | |
| Notes | Supplementary data online dx.doi.org/10.1016/j.radonc.2015.03.017 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using the Epi‐Info® software version 6.04b, eight lists with a randomized sequence for patient allocation were generated, because a separate list was needed for each of the 8 strata defined by the 3 dichotomous stratification factors (randomization codes with block‐size of eight)" |
| Allocation concealment (selection bias) | Unclear risk | Authors do not state who randomised the participants and whether it was in a concealed manner |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Quote: "a placebo was manipulated identical in color, shape and weight. Both bethanechol and placebo therapies were coded as A and B. The clinician, patients as well as the statistician were unaware of the trial groups" |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Placebo trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 out of 97 dropped out with reasons for dropouts clearly stated by study group, but equal per group and similar reasons |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Jellema 2006.
| Methods | Location: the Netherlands Number of centres: 1 Date of recruitment: August 1999 to August 2003 |
|
| Participants | Inclusion criteria: stage III/IVB squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx and/or larynx or lymph node metastases in the head and neck area from an unknown primary. Treatment with bilateral primary or postoperative radiotherapy with curative intent. 75% of the parotid gland volume expected to receive a radiation dose of at least 40 Gy. Minimal life expectancy of 12 months and a WHO performance score from 0 to 2. Good understanding of the Dutch language Exclusion criteria: distant metastases (M1), previously irradiated patients, patients treated in combination with induction or concurrent chemotherapy, and patients with tumours that originated in the salivary glands. Pregnant patients, those participating in another investigational trial or in poor general health or psychological conditions. Patients who had severe cardiovascular disease, poor renal function or sustained hypotension not secondary to antihypertensive medication Age: mean age = 55 (24 to 73) Gender: AMI‐3: 20 M, 10 F; AMI‐5: 22 M, 8 F; control: 18 M, 13 F Cancer type: head and neck at various sites and stages and lymph node classifications Submandibular gland removal: participants were stratified by this factor but numbers of participants affected are not reported Radiotherapy: megavolt equipment using isocentre techniques after 3‐dimensional planning. 2 opposing lateral fields with an anterior field to cover the lower jugular and supraclavicular lymph node areas. All received 46 Gy to treated areas, boost doses varied from 56 Gy (in patients who had negative surgical margins) to 63.5 Gy (in patients who had lymph node metastasis with extranodal spread or positive margins). Patients treated primarily with radiotherapy received 70 Gy to macroscopic tumour Chemotherapy: none Number randomised: 91 (AMI‐3: 30; AMI‐5: 30; control: 31) Number evaluated: 71 (xerostomia at 12 months) (AMI‐3: 22; AMI‐5: 27; control: 22) |
|
| Interventions |
3 arms: Amifostine 1 versus amifostine 2 versus no intervention Group 1: amifostine 3 times weekly 200 mg/m² administered IV over 3 to 5 minutes 15 to 30 minutes before irradiation Group 2: amifostine 5 times weekly 200 mg/m² administered IV over 3 to 5 minutes 15 to 30 minutes before irradiation Control: nothing |
|
| Outcomes | Xerostomia: late and acute radiation‐induced xerostomia at grade 2 and above (0 to 4 scale ‐ RTOG/EORTC Late Radiation Morbidity Scoring); patient‐rated xerostomia and sticky saliva using QLQ‐H&N35 (1 to 4 scale converted linearly to a 0 to 100 mm scale where higher scores = worse symptoms) ‐ not used Salivary flow rates: not reported Adverse effects: vomiting (emesis), nausea, hypotension, allergic reaction Survival data: locoregional tumour control and overall survival Other oral symptoms: not reported Other oral signs: not reported Quality of life: QoL‐C30 version 3.0, the EORCT Core Questionnaire with supplemental head and neck specific module (QLQ‐H&N35) Patient satisfaction: not reported Cost data: not reported Timing of assessment: xerostomia and QoL assessed at end of RT and 6, 12, 18 and 24 months after RT; survival assessed to 60 months but reported in text at 2 years |
|
| Funding | Source of funding: not stated. Amifostine provided by Schering Plough | |
| Trial registration | Not registered | |
| Sample size calculation presented | Reported | |
| Notes | Have only reported locoregional tumour control and overall survival data narratively as it did not seem sensible to combine the 2 amifostine arms due to differing results. Numbers per group unclear for xerostomia at end of RT and therefore not able to use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment performed at university medical centre using a permuted block design |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) patients/carers | High risk | Blinding not possible |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Blinding not mentioned. Xerosomia is subjective measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22% dropouts at 12 months and difference in attrition between groups (i.e. no amifostine = 29%, amifostine3 = 27%, amifostine5 = 10%) |
| Selective reporting (reporting bias) | High risk | Quality of life was measured but not reported (only quote: "No significant differences") |
| Other bias | Low risk | No other sources of bias are apparent |
Jham 2007.
| Methods | Location: Brazil Number of centres: 1 Date of enrolment: October 2004 to July 2005 |
|
| Participants | Inclusion criteria: adults with biopsy‐proven malignant neoplasm of the head and neck who received external beam RT Exclusion criteria: conditions which may introduce adverse reaction to bethanechol: tricyclic antidepressants, antihistamines, betablockers, hypersensitivity Age: bethanechol: mean 57 (SD 15); control: 55 (SD 13) Gender: bethanechol: 17 M 5 F; control: 16 M 5 F Cancer type: malignant neoplasm of head and neck Radiotherapy: external beam RT, encompassing 1 or more salivary glands, minimum 45 Gy Chemotherapy: bethanechol 23%; control 48% (type of CT not reported) Number randomised: 43 (bethanechol 22; control 21) Number evaluated: range over outcomes (and time points). Xerostomia VAS at 08 to 40 weeks after RT: 30 (bethanechol 13; control 17) |
|
| Interventions |
Bethanechol versus artificial saliva Bethanechol: 25 mg 3 times daily (6 am, 2 pm, 10 pm) administered with RT and used until end of RT Control: artificial saliva (OralBalance) ‐ schedule not reported |
|
| Outcomes | Xerostomia: subjective VAS scale (length not mentioned ‐ not used), asking about dry mouth (yes/no) Salivary flow rates: whole resting saliva and whole stimulated saliva collected over 5 minutes and reported in ml/min Adverse effects: lacrimation, nervousness, frequent urination, sweating, warm face, cramps, diarrhoea, nausea Survival data: death Other oral: not reported Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: xerostomia and saliva flow assessed during RT (between 15th and 19th session), at end of RT and at least 2 months after RT (ranging from 8 to 40 weeks after) |
|
| Funding | CAPES (an organization of the Brazilian federal government under the Ministry of Education) gave financial support, Apsel Laboratories provided bethanechol, and Laclede provided artificial saliva | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using the Epi‐info software version 6.04b, 6 lists with randomized sequence for patient allocation were generated (random codes with block‐size of 8). Prior to allocation patients were stratified by RT treatment and age" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) patients/carers | High risk | Quote: "...for obvious reasons it was not possible for the study to be double‐blinded" |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Quote: "...for obvious reasons it was not possible for the study to be double‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Varies over outcomes. For xerostomia (VAS): 30% dropped out or died (bethanechol 41%; control 19%). Differential dropout and (apart from death) reasons for dropouts unclear |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Lajtman 2000.
| Methods | Location: Croatia Number of centres: unclear Date of conduct: unclear |
|
| Participants | Inclusion criteria: patients scheduled to receive external beam radiation therapy to the major salivary glands completely or partially included in the field Exclusion criteria: significant cardiovascular, pulmonary, hepatic or pancreatic disorders or gastroduodenal ulcers Age: not reported Gender: not reported Cancer type: not reported Radiotherapy: weekly external beam radiation therapy for 4 to 8 weeks, no further details Chemotherapy: not stated Number randomised: unclear Number evaluated: 48 |
|
| Interventions |
Pilocarpine versus placebo Pilocarpine: 5 mg capsules 4 times daily starting the day before RT and continuing for 3 months Placebo: 5 mg capsules 4 times daily starting the day before RT and continuing for 3 months |
|
| Outcomes | Xerostomia: standardised questionnaire (subjective assessment, administered by clinician) Salivary flow rates: stimulated salivary flow rate (parotid saliva by Carlson‐Crittenden cup; submandibular/sublingual saliva by standardised suction device) Adverse effects: not reported Survival data: not reported Other oral symptoms: not reported Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: 3 months (end of drug treatment), 6 months and 12 months |
|
| Funding | Unclear | |
| Trial registration | Unclear | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Double‐blind; pilocarpine versus placebo |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Double‐blind; pilocarpine versus placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear of number randomised to each group, therefore number of dropouts unclear |
| Selective reporting (reporting bias) | High risk | Adverse effects not fully reported |
| Other bias | Low risk | No other sources of bias are apparent |
Lanzós 2010.
| Methods | Location: Spain Number of centres: 1 Date of enrolment: May 2004 to May 2007 |
|
| Participants | Inclusion criteria: between 18 and 75 years of age. At least 10 teeth present in mouth. Willing to consent Exclusion criteria: presence of mucosal pathology, pregnant or undergoing orthodontic therapy Age: mouthwash: mean age 49.4 years (SD 15.4); control: mean age 54.3 years (SD 16.1) Gender (M:F): mouthwash 15:3, control 17:1 Cancer type: head and neck Radiotherapy: 50 to 80 Gy over 5 weeks Chemotherapy: probably none Number randomised: 36 (18 per group) Number evaluated: 16 at 4 weeks for stimulated saliva (mouthwash 9, control 7) |
|
| Interventions |
Antiseptic mouthrinse versus placebo Mouthwash: CHX 0.12% and 0.05% cetylpyridinium by oral rinse 15 ml twice daily (morning and night). From start of RT for 28 days Placebo: control without active ingredient |
|
| Outcomes | Xerostomia: not assessed Salivary flow rates: stimulated saliva (ml/min), pH saliva (0/1/2) Adverse effects: none reported Survival data: not reported Other oral symptoms: hiposialosis (drooling), mucositis, plaque, gingivitis, caries Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: 14, 28 days after RT started (i.e. no time points of interest) |
|
| Funding | Source of funding unclear; suspect pharmaceutical industry sponsored by intervention manufacture Perio‐Aid Tratamiento | |
| Trial registration | Unclear | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list assigned by random number |
| Allocation concealment (selection bias) | Low risk | Allocated after inclusion corresponding to numerically coded mouthrinse. Code only broken at end of study |
| Blinding (performance bias and detection bias) patients/carers | Low risk | List and numbered bottles provided by promoter. Participants and researchers blinded |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | 1 single assessor blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 56% attrition (antiseptic 50%, placebo 61%) for outcome of interest (salivary flow rates) at 4 weeks |
| Selective reporting (reporting bias) | High risk | Xerostomia not reported |
| Other bias | Low risk | No other sources of bias are apparent |
Le 2011.
| Methods | Location: North America and Europe Number of centres: 46 hospitals Date of conduct: August 2005 to September 2007 |
|
| Participants | Inclusion criteria: newly diagnosed unresected stage III to IV SCC of oral cavity, oropharynx, nasopharynx, hypopharynx or larynx, planned RT dose of more than 50 Gy to 2 subsites or oral cavity and oropharynx Exclusion criteria: evidence of secondary malignancy Age: mean 55.5 (SD 8.5) Gender: 159 M, 29 F Cancer type: SCC of oral cavity, oropharynx, nasopharynx, hypopharynx or larynx Radiotherapy: mean 68 Gy in both arms for 43 days Chemotherapy: cisplatin 100 mg/m² IV infusion on days 1, 22, and 43 of RT Number randomised: 188 (94 per group) Number evaluated: 188, 185 adverse events |
|
| Interventions |
Palifermin versus placebo Palifermin administered IV at 180 µg/Kg over a period of 30 to 60 seconds, in 8 weekly doses 3 days. Bolus injection before radiotherapy, then at weekend Placebo: matching as above (1.2 ml of sterile water +) Follow‐up: median follow‐up 25.9 months palifermin, 25.0 placebo |
|
| Outcomes | Xerostomia: incidence of grade ≥ 2 xerostomia (Common Terminology Criteria for Adverse Events (CTCAE) v 3.0 Dry Mouth/Xerostomia scale ‐ info from trials registry), assessed at months 4, 6, 8, 10, 12, reported only at month 4 Salivary flow rates: not reported Adverse effects: assessed by Common Terminology Criteria for Adverse Events: nausea, constipation, decreased weight, vomiting, anaemia, leukopenia, fatigue, dehydration Survival data: overall tumour response, time to locoregional tumour failure, incidence of secondary primary tumours, overall and progression‐free survival Other oral symptoms: dysphagia (difficulty in swallowing), OMWQ‐HN 0 (no soreness) to 4 (extreme soreness) scale for mouth and throat soreness (assessed twice weekly by trained evaluators during radiochemotherapy) Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported |
|
| Funding | Externally funded by Amgen GSK | |
| Trial registration | NCT00101582 (clinicaltrials.gov/show/NCT00101582) | |
| Sample size calculation presented | Reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation system. 1:1 allocation ratio |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation for all sites, probably allocation concealment |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Quote: "placebo‐controlled, double‐blind study" Comment: blinding feasible |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Quote: "placebo‐controlled, double‐blind study" Comment: blinding feasible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All cases accounted for. ITT analysis (patients having no assessment assumed to have event) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. Low risk for xerostomia at the time point used in meta‐analysis (4 months) ‐ however, it should be noted that xerostomia was measured up to 12 months but data not reported |
| Other bias | Low risk | No other sources of bias are apparent |
Lin 2014.
| Methods | Location: Taiwan, Republic of China Number of centres: 1 Date of enrolment: January 2003 to November 2004 |
|
| Participants | Inclusion criteria: histological evidence of carcinoma of head and neck, to receive RT, life expectancy ≥ 3 months, ECOG status ≤ 2. Criteria such as white blood cells, platelets, haemoglobin had to be within certain parameters Exclusion criteria: prior RT, presence of oral lesions, severe organ failure, brain metastasis Age: TWBXM: mean 51 (SD 15); placebo: 54 (SD 16) Gender: TWBXM: 29 M, 9 F; placebo: 32 M 3 F Cancer type: treatment group: head and neck cancer stage 0 = 4, I = 4, II = 10, III = 4, IVA = 12, IVB = 4. Control group: head and neck cancer stage 0 = 3, I = 5, II = 8, III = 4, IVA = 10, IVB = 5 Radiotherapy: TWBXM: mean dose 6944.9 cGy; placebo: mean dose 7098.4 cGy Chemotherapy: not mentioned probably not given Number randomised: 73 (TWBXM 38; placebo 35) Number evaluated: 71 (TWBXM 38; placebo 33) |
|
| Interventions |
Traditional Chinese medicine (TWBXM) versus placebo Tianwang Buxin Mini‐pills (TWBXM) ‐ 13 herbs listed in paper Placebo made of starch and designed to taste and look similar to intervention 3 g orally 3 times daily starting from initiation of RT until 1 month after RT completion Follow‐up: end of RT (end of RT for dichotomous data, 1 month postRT for continuous data) |
|
| Outcomes | Xerostomia: RTOG grades by evaluating physician ‐ none, slight, moderate mouth dryness Salivary flow rates: not reported Adverse effects: EORTC QLQ‐C30 ‐ skin, nausea, vomiting, leukopenia, fatigue, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhoea, body weight loss Survival data: not reported Other oral symptoms: EORTC QLQ‐C30 ‐ pain, swallowing, speech problems, mouth opening, teeth, mucositis, oral mucosa, loss of taste Other oral signs: not reported Quality of life: EORTC QLQ‐C30 and head and neck specific QLQ‐H & N35 Patient satisfaction: not reported Cost data: not reported |
|
| Funding | Government. Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan, Taiwan (grants CCMP92‐RD‐011 and CCMP93‐RD‐008). Quote: "All authors declare that there are no conflicts of interest" | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | Problematic data ‐ emailed corresponding author 22 February 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomized to study medicine according to a computer‐generated randomization schedule..." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Quote: "All patients, the study nurses and doctors were blinded to the group of the treatment group" |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Quote: "All patients, the study nurses and doctors were blinded to the group of the treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was 3% to 7% (data reported inconsistently in the report) at the end of RT, which is the preferred time point for this review. However, there were 29 (40%) dropouts at the time point of 1 month postRT |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Lozada‐Nur 1998.
| Methods | Location: USA Number of centres: 1 Date of conduct: not stated |
|
| Participants | Inclusion criteria: not reported Exclusion criteria: not reported Age: pilocarpine: mean 51.5 years (range 29 to 76); placebo: 54.8 years (range 47 to 68) Gender (M:F): pilocarpine 9:2, placebo 9:2 Cancer type: nasopharyngeal cancer Radiotherapy: total dose 60 to 70 Gy Chemotherapy: some Number randomised: 22 (11 per group) Number evaluated: 11 per group for incidence of dry mouth. There are discrepancies over the numbers |
|
| Interventions |
Pilocarpine versus placebo Pilocarpine: 5 mg tablets 3 times daily ‐ 4 times daily, 2 weeks before RT and concurrently with RT Placebo |
|
| Outcomes | Xerostomia: questionnaire Salivary flow rates: resting salivary flow Adverse effects: sweating, lacrimation (excess tears, crying), rhinorrhoea (watery discharge from the nose), diarrhoea, nausea, rhinitis, blurred vision, constipation, neuropathy Survival data: not reported Other oral symptoms: mucositis, dysphagia (difficulty in swallowing), dysgeusia (taste disturbance), pain Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: prior to radiation, weekly during treatment, end of RT, 3 months after RT |
|
| Funding | Supported by a grant from MGI Pharma Inc. | |
| Trial registration | Not registered or published | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Contact with authors confirmed that the random allocation was conducted by pharmaceutical company, producing coded containers |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation sequence was provided in a logo‐type format and kept by our dental assistant (in a locked cabinet)" Comment: further description of this from study authors implies allocation concealment |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Quote: "double‐blind" |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 dropped out because of adverse events (severe nausea and vomiting, and tumour growth). Unclear reasons for all dropouts for xerostomia outcome (6) |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Patni 2004.
| Methods | Location: India Number of centres: 1 Date of conduct: not reported |
|
| Participants | Inclusion criteria: locally advanced histologically proven squamous cell carcinoma of head and neck region. 75% or more of each parotid gland was included in the radiation portal. Age above 18 years. Expected survival > 12 months. Karnofsky Performance Status > 60. Normal haemogram, renal and liver functions, normal calcium levels Exclusion criteria: prior treatment for malignancy, associated hypotension or distant metastases Age: not reported Gender: 65% M Cancer type: head and neck Radiotherapy: external radiation therapy with gamma rays to a dose of 66 to 72 Gy with conventional fractionation Chemotherapy: 40 mg/m² cisplatin weekly Number randomised: 170 (85 per group) Number evaluated: 170 (85 per group) |
|
| Interventions |
Amifostine versus no intervention Amifostine: 250 mg IV over 3 minutes for 4 days a week from day 1 of radiotherapy until completion of treatment Control: nothing |
|
| Outcomes | Xerostomia: acute and late xerostomia grade 2 and above (RTOG 0‐4 scale) Salivary flow rates: parotid scintigraphy (no data) Adverse effects: not reported Survival data: disease‐free survival at 24 months and tumour response Other oral symptoms: mucositis (RTOG) Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessments: xerostomia at 3 and 12 months after RT; parotid scintigraphy at 3, 6, 12 and 24 months after RT; survival at 24 months |
|
| Funding | Not reported | |
| Trial registration | Not registered or published | |
| Sample size calculation presented | No | |
| Notes | Abstract with additional information provided by study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but unclear method |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) patients/carers | High risk | Not possible due to 'no treatment' group |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Not possible due to 'no treatment' group. Xerostomia is subjective outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | No information on adverse events |
| Other bias | Low risk | No other sources of bias are apparent |
Peng 2006.
| Methods | Location: China Number of centres: 1 Date of conduct: October 2003 to October 2005 |
|
| Participants | Inclusion criteria: quote: "1) H&N SC patients diagnosed with pathological examinations, who cannot endure surgery or cannot be treated with radical resection; 2) first‐visit patients who have not received cancer treatment, with no tumour metastasis; 3) WBC > 4.0*109/L, platelet count > 100.0*109/L, Hb > 10 g/L, normal function of heart, lungs, liver and kidneys; 4) Karnofsky score ≥ 60; 5) patients gave informed consent" Exclusion criteria: see above Age at baseline (years): amifostine: median 58; control: median 57 Gender: amifostine: 12 M, 6 F; control: 12 M, 6 F Cancer type: head and neck squamous carcinoma (amifostine/control): oral = 4/3, nasopharyngeal = 6/6, oropharyngeal = 5/5, hypopharyngeal = 1/1, laryngeal = 1/1, paranasalsinus = 1/1, glottis = 0/1 Radiotherapy: quote: "Primary site of tumour and cervical lymph nodes: 74.4 Gy overall, 1.2 Gy per time, 2 times per day (> 6 hours between these 2 times), for 4 to 5 weeks; separate routine fractionated RT for the neck area: 50 Gy overall, 25 times, 5 weeks" Chemotherapy: quote: "Continued IV infusion of 5 FU 750 mg/m² using pump, 24 hours per day for 3 days. On the 5th day after 5 FU use, intravenous infusion of cisplatin 50 mg/m² with 250 or 500 ml saline (2 to 4 hours). IV infusion of docetaxel 75 mg/m² with 250 ml saline (< 1.5 hours). Chemotherapy performed in rounds: 1st round – during RT, 2nd round – begin in the 5th week after RT, 3rd round – begin in the 9th week after RT" Number randomised: 37 (amifostine 18, control 19) Number evaluated: 36 (amifostine 18, control 18) |
|
| Interventions |
Amifostine versus no intervention Amifostine: quote: "400 mg amifostine each time, intravenous infusion 15 minutes before RT and chemotherapy (finish in 5 to 7 minutes)" Control: no intervention other than the same RT and chemotherapy |
|
| Outcomes | Xerostomia: only results for 'acute' and 'chronic' dry mouth reported; time and standards for assessments not described Salivary flow rates: not reported Adverse effects: hypotension, nausea, vomiting, dizziness, fatigue, hiccup, sneezing, facial flush Survival data: not reported Other oral symptoms: mucositis Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: not reported (therefore unable to use data) |
|
| Funding | Not reported; conflicts of interest: not reported | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized to" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) patients/carers | High risk | Amifostine versus no intervention. Blinding not possible |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Blinding not mentioned. Xerostomia is subjective measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "1 patient in the amifostine group quitted due to financial reasons" |
| Selective reporting (reporting bias) | Unclear risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Pimentel 2014.
| Methods | Location: Brazil Number of centres: 1 Date of conduct: not reported |
|
| Participants | Inclusion criteria: newly diagnosed head and neck cancer beginning treatment with RT Exclusion criteria: previous RT, concomitant chemotherapy, cardiopathy, hypertension, diabetes, allergy to pilocarpine, Sjögren syndrome, salivary gland tumours, chronic lung disease, glaucoma, peptic ulcer, taking betablockers or drugs that could alter salivary flow Age: mean 60 years (not given by group) Gender (M:F): 8:3 Cancer type: oral (n = 1); oropharynx (n = 3); mouth floor and tongue (n = 2); larynx (n = 4); pharynx (n = 1) Radiotherapy: 35 to 50 Gy, with daily doses about 2 Gy Chemotherapy: none Number randomised: unclear whether 29 or 11 (see attrition bias) Number evaluated: 11 (pilocarpine 5, placebo 6) |
|
| Interventions |
Pilocarpine versus placebo Pilocarpine: 5 mg 3 times daily for duration of RT Placebo: saline solution 3 times daily for duration of RT |
|
| Outcomes | Xerostomia: patient‐reported feeling of dry mouth Salivary flow rates: unstimulated (USF) and stimulated saliva (SSF) (ml/min) Adverse effects: reported narratively Survival data: locoregional control: not reported Other oral symptoms: oral mucositis, ulcers Other oral signs: difficulty in eating Quality of life: only eating Patient satisfaction: not reported Cost data: not reported Timing of assessment: weekly during RT (4 weeks) |
|
| Funding | The National Research Council (CNPq) | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...dispensing pharmacy held custody of the samples, separating those from the group who took pilocarpine solution from those which took the placebo. All patients were assigned a number corresponding to the medicine bottle. Researchers were not granted access to that information prior to the end of the survey" |
| Allocation concealment (selection bias) | Low risk | Quote: "...dispensing pharmacy held custody of the samples, separating those from the group who took pilocarpine solution from those which took the placebo. All patients were assigned a number corresponding to the medicine bottle. Researchers were not granted access to that information prior to the end of the survey" |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Double‐blind ‐ see above |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Double‐blind ‐ see above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "We pre‐selected 29 patients; however, the careful selection of the population was directly reflected in the number of enrolled patients and in the end, only 11 were included in the survey. We consider that this low number is a result not only of the exclusion stemming from previously established eligibility criteria but also from the breach of protocol" |
| Selective reporting (reporting bias) | High risk | Poorly reported data for xerostomia and no adverse events |
| Other bias | Low risk | No other sources of bias are apparent |
Reshma 2012.
| Methods | Location: India Number of centres: 1 Date of conduct: not stated |
|
| Participants | Inclusion criteria: carcinoma of the head and neck stage III and IV, aged between 30 and 70, to receive RT, normal haematology, biochemistry and Karnofsky Performance Index > 70% Exclusion criteria: poor general condition, associated co‐morbidities, psychiatric conditions Age: 30‐70 Gender: unclear Cancer type: SCC of head and neck Radiotherapy: 60 Gy on cobolt 60 for 30 days over 6 weeks Chemotherapy: not reported but probably none Number randomised: 20 or 40 unclear Number evaluated: no apparent dropouts but unclear how many started |
|
| Interventions |
Tulasi (Ocimum Sanctum) versus placebo Tulasi (Ocimum Sanctum) Placebo: vitamin B complex 2 capsules of 250 mg orally half an hour prior to RT *Healthy control group also included but not used (not randomised) Follow‐up: 29 days of RT |
|
| Outcomes | Xerostomia: unclear, quote: "patients were assessed at the end of every week for grade of mucositis, skin reaction and salivary status" Salivary flow rates: not reported Adverse effects: not reported Survival data: not reported Other oral symptoms: not reported Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported |
|
| Funding | Not reported | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | No useable data ‐ emailed corresponding author 27 January 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized into 2 arms" Comment: insufficient information on method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Placebo‐controlled |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | No adverse event data reported. Poorly reported xerostomia |
| Other bias | Low risk | No other sources of bias are apparent |
Rode 1999.
| Methods | Location: Slovenia Number of centres: 1 Dates and duration of recruitment period: not stated |
|
| Participants | Inclusion criteria: irradiated for head and neck cancer and salivary glands included in the irradiation field Exclusion criteria: not reported Age: aged 32 to 72, median 62 years Gender (M:F): 60:9 Cancer type: oral cavity (n = 14), oropharynx (n = 33), hypopharynx (n = 8), larynx (n = 11), other (n = 3) (evenly distributed across groups) Radiotherapy: 5 Gy per day, 5 days a week. Irradiated volume reduced twice during irradiation treatment: at 40 Gy for shielding the spinal cord and at 60 Gy for treating the area of original disease, up to 70 Gy. Postoperative patients received 50 Gy ‐ 56 Gy with parallel opposed portals only. 44 patients had postoperative RT and 25 were treated by RT alone Mean irradiation dose (Gy) delivered to area of salivary glands Chemotherapy: not stated Number randomised: 69 (A: 9, B: 30, C: 30) Number evaluated: 69 (A: 9, B: 30, C: 30) |
|
| Interventions |
Pilocarpine (postRT) + Biperiden (during RT) versus no intervention Group Aª: pilocarpine during RT and 6 weeks after. Pilocarpine hydrochloride (5 mg) perorally 3 times daily administered 1 hour before irradiation Group B: Biperiden during RT and pilocarpine after RT group. Biperidin chloride (2 mg tablets) 1 and a half hours before irradiation, and pilocarpine hydrochloride (5 mg 3 times daily) for 6 weeks after RT Group C: no intervention |
|
| Outcomes | Xerostomia: not reported Salivary flow rates: mean quantity of non‐stimulated saliva secretion (ml/min) Adverse effects: not reported Survival data: not reported Other oral symptoms: mucositis, swallowing WHO criteria Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: end of RT, 3 months, 6 months and 12 months after end of RT |
|
| Funding | Source of funding: none | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | ªGroup A data not used: randomisation to Group A was stopped after the first 9 patients for ethical reasons ‐ 3 months after RT total cessation of saliva secretion was observed in all except 1 patient Follow‐up: 12 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information provided by author: "Sequence centrally generated" |
| Allocation concealment (selection bias) | Low risk | Author included the following in an email "randomization with permuted blocs, participants were allocated to the treatment groups randomly. Allocation sequence was generated centrally, treating physician (radiologist) enrolled patient and participants were assigned to the groups by specialist in dental medicine" Comment: centralised random allocation and was probably adequately concealed |
| Blinding (performance bias and detection bias) patients/carers | High risk | No blinding undertaken |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Salivary flow objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients included in analysis |
| Selective reporting (reporting bias) | High risk | Xerostomia and adverse events not reported |
| Other bias | Low risk | No other sources of bias are apparent |
Sangthawan 2001.
| Methods | Location: Thailand Number of centres: 1 Date of recruitment: January 1998 to January 1999 |
|
| Participants | Inclusion criteria: histologically documented squamous cell carcinoma of head and neck who would receive definite or postoperative radiation Exclusion criteria: significant uncontrolled cardiac, pulmonary, renal or occular disease or required tricyclic antidepressants or antihistamine with anticholinergic effects, betablocker, pilocarpine for ophthalmic indications or chemotherapy Age: pilocarpine: 57 years; placebo: 58 years Gender (M:F): 49:11 Cancer type: oropharynx (n = 27); nasopharynx (n = 14); others (n = 19) Radiotherapy: Cobalt‐60 or 6 MV photon machine. Standard arrangement ‐ opposing lateral portals, loaded 1:1 and/or anterior low neck field. Both parotids treated to a dose of at least 50 Gy with an equal daily dose of 1.8‐2.0 Gy Chemotherapy: none Number randomised: 60 (30 per group) Number evaluated: 47 (25 pilocarpine; 22 placebo) |
|
| Interventions |
Pilocarpine versus placebo Pilocarpine jelly: self administered 5 mg 3 times daily at meal times for duration of RT (7 weeks) Placebo: self administered 3 times daily at meal times for duration of RT (7 weeks) Follow‐up: 6 months after RT |
|
| Outcomes | Xerostomia: subjective evaluation scores for xerostomia questionnaire (100 mm VAS for each of 5 questions) Salivary flow rates: not reported Adverse effects: reported ("non‐specific symptoms such as nausea, vomiting, dizziness, urinary frequency, palpitation, sweating and tearing") Survival data: not reported Other oral symptoms: not reported Other oral signs: disability to oral intake, amount of meals, use of analgesics Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: 6 months after RT |
|
| Funding | Faculty of Medicine, Prince of Songkla University | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information provided by author: "random number table" |
| Allocation concealment (selection bias) | Low risk | Information provided by author: "clinician not participating in study generated allocation sequence. Treatment codes concealed in sealed envelopes. Treatment coding not disclosed to investigator or patient" |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Quote: "identically appearing placebo... patients and investigators were unaware of which treatment was administered" |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Information provided by author: "treatment code was disclosed to the investigator only after completion of the analysis of the results of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 22% dropout rate |
| Selective reporting (reporting bias) | High risk | Poor reporting of xerostomia without SD |
| Other bias | Low risk | No other sources of bias are apparent |
Vacha 2003.
| Methods | Location: Germany Number of centres: 1 Date of conduct: October 1996 to February 1999 |
|
| Participants | Inclusion criteria: advanced tumours of larynx, oro or hypopharynx, all received surgery and were to receive RT plus CT, pathologically confirmed cancer, between 18 and 70 years, performance status WHO 0‐II, adequate bone marrow and liver and renal function Exclusion criteria: severe internal medical disorders, hyper or hypotension, history of RT or CT, women with inadequate contraception, secondary malignancies, recurrent tumours Age: amifostine: mean 53.5; control: mean 55.1 Gender: amifostine: M 21, F 4; control: M 19, F 6 Cancer type: amifostine: SSC (24) and adenocarcinoma (1); control: SSC (24) and lymphoepithelial cancer (1) Neck dissection involving removal of submandibular glands: radical bilateral (amifostine 32%, control 40%); radical unilateral + selective contralateral (amifostine 16%, control 12%); radical unilateral (amifostine 36%, control 16%) Radiotherapy: conventionally fractionated RT (5 x 2 Gy/week). Total dose 60 Gy for completely resected tumours, 70 Gy incomplete resected tumours Chemotherapy: individually planned. 70 mg/m² carboplatin on treatment days 1 to 5 and 29 to 33 just before RT session (all participants had this) Number randomised: 56 (not reported by group) Number evaluated: 50 (25 per group); 41 (amifostine 19, control 22) for xerostomia |
|
| Interventions |
Amifostine versus no intervention 250 mg amifostine given intravenously as short infusion over 10 to 15 minutes |
|
| Outcomes | Xerostomia: RTOG (not useable) Salivary flow rates: not reported Adverse effects: skin toxicity, loss of hair Survival data: not reported Other oral symptoms: oral mucositis Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: weekly during RT until week 6 (during RT; no usable data) |
|
| Funding | Not reported | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomized to receive..." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) patients/carers | High risk | No treatment control group |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Xerostomia is a subjective assessment by the patient |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for dropouts by study group unclear. 6 dropouts altogether: allergic skin reaction (1) after 5th application of amifostine, patient refusal (3), second malignancy (1), surgical complications (1). 27% attrition overall for xerostomia ‐ participants with larynx cancer excluded from analysis |
| Selective reporting (reporting bias) | High risk | Xerostomia selectively and poorly reported (only significant data reported) |
| Other bias | Low risk | No other sources of bias are apparent |
Valdez 1993.
| Methods | Location: USA Number of centres: multisite unclear how many Date of conduct and duration of recruitment period: not stated |
|
| Participants | Inclusion criteria: scheduled to receive external beam radiation therapy to the major salivary glands, completely or partially Exclusion criteria: significant cardiovascular, pulmonary, hepatic, or pancreatic disorders or gastroduodenal ulcers. Women with childbearing potential were required to have a pregnancy test with negative results before entry and to use contraception during the study Age: pilocarpine: 22 to 65 years, mean 42.6 years; placebo: 21 to 56 years, mean 40.2 years Gender (M:F): 6:4 Cancer type: mix of SCC, mucoepidermoid carcinoma, Hodgkin disease and malignant lymphoma Radiotherapy: mean dose 41.9 Gy Chemotherapy: not stated Number randomised: 10 (5 per group) Number evaluated: 9 (pilocarpine 5; placebo 4) |
|
| Interventions |
Pilocarpine versus placebo Pilocarpine: 5 mg capsules 4 times daily for 3 months starting the day before RT Placebo: 5 mg capsules 4 times daily for 3 months starting the day before RT All participants received a rigorous preventative oral hygiene regimen including topical fluoride applications |
|
| Outcomes | Xerostomia: subjective assessment using questionnaire Salivary flow rates: stimulated salivary flow rates (μl/min) Adverse effects: not reported Survival data: quote: "all tumour responded favourably and all were in complete remission for the remainder of the study" Other oral symptoms: not reported Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: 3, 4, 5, 6 and 12 months from start of RT |
|
| Funding | National Institute of Dental and Craniofacial Research (NIDCR). Randomisation sequence generation and allocation were undertaken by US National Institutes of Health (NIH) pharmaceutical service | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation not explicit but conducted by NIH Pharmaceutical Development Service |
| Allocation concealment (selection bias) | Low risk | Conducted by NIH Pharmaceutical Development Service; intervention dispensed in coded bottles |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Pilocarpine versus placebo |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Participants are blinded and providing saliva and questionnaire data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/5 participants in placebo group dropped out. No dropouts in pilocarpine group |
| Selective reporting (reporting bias) | High risk | No adverse event data reported |
| Other bias | Low risk | No other sources of bias are apparent |
Veerasarn 2006.
| Methods | Location: Thailand Number of centres: 5 Date of recruitment: February 1999 to September 2001 |
|
| Participants | Inclusion criteria: histological proven squamous cell carcinoma of head and neck region; ECOG performance statue 0‐2; adequate bone marrow, liver and renal functions; age 18‐70 years; no prior definite/radical surgery, chemotherapy, radiotherapy or biological response modifier; no evidence of distant metastasis; life expectancy ≥ 12 months; able to comply with a follow‐up schedule; weight loss ≤ 10% in previous 3 months Exclusion criteria: concomitant malignant disease in other parts of the body; active uncontrolled infection; pregnant or lactating women; medical or psychiatric illness that compromise the patient's ability to complete the study; concomitant use of chemotherapy Age: amifostine: mean 55 (23‐70); control: mean 52 (23 to 69) Gender: amifostine: 24 M, 8 F; control: 27 M, 8 F Cancer type: oral cavity, oropharynx, nasopharynx, larynx, hypopharynx Radiotherapy: standard fractionation (2 Gy, 5 days a week). Duration: 5 to 8 weeks. Definite RT = 70 Gy. Postoperative RT = 50 Gy. Amifostine group: definite RT = 15; postoperative RT = 17. Control group: definite RT = 18; postoperative RT = 17 Chemotherapy: none Number randomised: 67 (amifostine 32, control 35) Number evaluated: 62 (amifostine 32, control 30) |
|
| Interventions |
Amifostine versus no intervention Amifostine: (Ethyol) (200 mg/m²) IV (3 to 5 min), 30 min before RT. 5 consecutive days a week for 5 to 7 weeks (during RT) Control: nothing |
|
| Outcomes | Xerostomia: 1) questionnaire (6 questions) (RTOG/EORTC acute and late radiation morbidity scoring criteria) for xerostomia: dryness of mouth, oral comfort, quality of sleep, ability to speak, ability to chew and swallow and ability to wear dentures (average score 0 to 10: 0 = normal); 2) RTOG 0 to 4 scale ‐ grade 2 and above Salivary flow rates: unstimulated and stimulated whole saliva collection (mg/5 min) and scintigraphy Adverse effects: nausea, vomiting, hypotension Survival data: disease‐free survival Other oral symptoms: mucositis (RTOG grade 2‐3) Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessments: weekly during RT (for 6 weeks), then at end of RT and at 1, 2, 3, 6, 12, 18 and 24 months after RT; survival at 24 months after RT |
|
| Funding | Source of funding: unclear | |
| Trial registration | Not registered or published | |
| Sample size calculation presented | No | |
| Notes | Information on randomisation, and numbers and SDs from correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) patients/carers | High risk | Amifostine versus no intervention |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Xerostomia is subjective measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "We excluded 5 cases in the control as they did not have salivary gland function, or had severe salivary gland impairment" Comment: we are assuming that there were no other dropouts (assessment made on 32 in amifostine, 30 in control) |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Wang 1998.
| Methods | Location: China Number of centres: 1 Date of recruitment: May 1996 to October 1997 |
|
| Participants | Inclusion criteria: patients with head and neck cancer, pathology confirmed, aged 20 to 70 and radiation fields including the main salivary glands, total dose > 60 Gy Exclusion criteria: severe systemic disease or history of chronic diseases of the salivary glands Age (years): treatment: mean 46.8 (range 22 to 68); placebo: mean 45.5 (range 24 to 70) Gender: treatment: 20 M, 4 F; placebo: 21 M, 5 F Cancer type: treatment: nasopharyngeal = 17, throat = 4, tonsil = 3; placebo: nasopharyngeal = 18, throat = 4, tonsil = 3, hard palate = 1 Radiotherapy: 6 MV x‐ray with accelerator linear, SL‐75 produced by Philips. 2 Gy, 5 times per week for 6 to 7 weeks (total dose 60‐70 Gy) Chemotherapy: none Number randomised: 50 (treatment 24, placebo 26) Number evaluated: 50 (treatment 24, placebo 26) |
|
| Interventions |
Chinese medicine versus placebo (Dobell's solution) Chinese medicine and Dobell's solution (20 ml 3 times daily) rinsing and spray inhalation for the duration of the RT starting from the beginning of RT Chinese medicine: formulation of fragrant solomonseal rhizome (30 g), dwarf lilyturf tuber root (20 g), peach seed (24 g), dendrobium stem (30 g), wolfberry fruit (30 g), rehmannia dried root (40 g), prepared rehmannia root (40 g), American ginseng (30 g), safflower (20 g), chuanxiong rhizome (20 g). Mixture broken into a powder, soaked in 1000 ml of water, 40 degrees Centigrade for 6 hours, ultrasound oscillation for 10 minutes, centrifugation and the liquid part is used for clinical use Placebo: Dobell's solution Follow‐up: end of RT |
|
| Outcomes | Xerostomia: subjective evaluation score (VAS) for xerostomia, before and during the RT (at 10, 20, 30, 40, 50 and 60 Gy) Salivary flow rates: stimulated salivary flow rates, in morning around 9 am (at least 1 hour after breakfast) patients rinsed with water, then chewed gum and saliva collected after 5 minutes, before and during the RT (at 20, 40 and 60 Gy) Adverse effects: not reported Survival data: not reported Other oral symptoms: not reported Other oral signs: not reported Quality of life: not reported Patient satisfaction: not reported Cost data: not reported |
|
| Funding | Not reported; conflicts of interest: not reported | |
| Trial registration | Not registered | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided" Comment: no further details given |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Placebo used |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Adverse effects not reported. Data for xerostomia not usable (reported as a graph with no SDs) |
| Other bias | Low risk | No other sources of bias are apparent |
Warde 2002.
| Methods | Location: Canada Number of centres: 1 Date and duration of recruitment period: not stated |
|
| Participants | Inclusion criteria: squamous cell head and neck cancer, scheduled to receive RT with the inclusion of > 50% of both parotid glands in the radiation fields to doses > 50 Gy. Primary treatment and postoperative RT participants Exclusion criteria: previous RT or chemotherapy or pre‐existing xerostomia from other causes. Medical contraindication to pilocarpine Age: pilocarpine: mean 56.2 years; placebo: mean 57.8 years Gender (M:F): 94:36 Cancer type: SSC of head and neck Radiotherapy: 60‐70 Gy in 2 Gy daily fractions (68 participants), 60‐64 Gy in 40 fractions during 4 weeks using twice daily treatments (33 participants), 50 Gy in 25 daily fractions (7 participants), 60 Gy in 25 daily fractions (8 participants) and 51 Gy in 20 daily fractions (7 participants) Chemotherapy: not stated Number randomised: 130 (65 per group) Number evaluated: for xerostomia: 92 (pilocarpine 48, placebo 44) at 3 months postRT; 87 (pilocarpine 46, placebo 41) at 6 months postRT |
|
| Interventions |
Pilocarpine versus placebo Pilocarpine: 5 mg tablets 3 times daily starting day 1 of RT and continued until 1 month after completion of RT Placebo: tablets 3 times daily starting day 1 of RT and continued until 1 month after completion of RT |
|
| Outcomes | Xerostomia: VAS (patient‐completed) assessing patient's perception of dryness of their mouth (7 questions). Scores from 0 to 100, low scores = most difficulty Salivary flow rates: not reported Adverse effects: excessive sweating, acute toxicity of therapy (RTOG) Survival data: not reported Other oral symptoms: not reported Other oral signs: feeding tube inserted Quality of life: patients' quality of life (McMaster University Head and Neck Questionnaire (HNRQ)). Score 1‐7, lower score = poorer quality of life Patient satisfaction: not reported Cost data: not reported Timing of assessment: 1, 3 and 6 months after end of RT |
|
| Funding | Unrestricted educational grant from Pharmacia Canada | |
| Trial registration | Not registered | |
| Sample size calculation presented | Yes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) patients/carers | Low risk | Pilocarpine versus placebo, "double‐blind" |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Self reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 130 participants randomised. 19/65 dropouts in pilocarpine group; 24/65 dropouts in placebo group. 8 in pilocarpine and 4 in placebo dropped out for toxicity, otherwise reasons unclear |
| Selective reporting (reporting bias) | Low risk | Xerostomia and adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
Watanabe 2010.
| Methods | Location: Japan Number of centres: 1 Date of conduct: January and October 2009 |
|
| Participants | Inclusion criteria: head and neck cancer scheduled for RT or RT + CT Exclusion criteria: not reported Age: polaprezinc: 67.4 (range 53 to 78); control: 62.7 (range 35 to 86) Gender (M:F): polaprezinc 13:3, control 11:4 Cancer type: head and neck, mainly pharyngeal and laryngeal Radiotherapy (mean dose and duration): polaprezinc: 51 Gy (range 30‐70), 37 days (range 21‐55); control: 58 Gy (range 36‐70), 45 days (range 28‐79) Chemotherapy: some but unclear what, concomitantly carried out for 56% of polaprezinc and 80% of control Number randomised: 31 (polaprezinc 16, control 15) Number evaluated: 31 (polaprezinc 16, control 15) |
|
| Interventions |
Polaprezinc versus azulene oral rinse Polaprezinc granules (0.5 g) were dissolved in 20 ml of 5% sodium alginate Azulene oral rinse prepared by pouring 7 drops of 4% solution into 100 ml water Both groups administered via oral rinse. Rinse for 3 minutes, 4 times daily, from start to end RT. After rinsing, polaprezinc swallowed but azulene spat out. From start to end of RT |
|
| Outcomes | Xerostomia: CTCAE 0‐3 grade Salivary flow rates: not reported Adverse effects: not reported Survival data: tumour response by RECIST criteria for specific group of patients Other oral symptoms: pain, dysgeusia (taste disturbance), mucositis Other oral signs: amount of meals Quality of life: not reported Patient satisfaction: not reported Cost data: not reported Timing of assessment: over RT period (maximum score) |
|
| Funding | Source of funding unclear | |
| Trial registration | Unclear | |
| Sample size calculation presented | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomly assigned". No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) patients/carers | High risk | None. Polaprezinc swallowed after rinsing, while azulene is spat out. Quote: "open trial" |
| Blinding (performance bias and detection bias) outcome assessment | High risk | Xerostomia subjective measurement, and different interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | High risk | No adverse events reported |
| Other bias | Low risk | No other sources of bias are apparent |
CHX = chlorhexidine; CRT = chemoradiotherapy; CT = chemotherapy; CTCAE = Common Terminology Criteria for Adverse Events; ECOG = Eastern Cooperative Oncology Group score; EORTC = European Organisation for Research and Treatment of Cancer; F = female; G‐CSF = granulocyte‐colony stimulating factor; GI = gastrointestinal; GM‐CSF = granulocyte‐macrophage colony‐stimulating factor; Gy = gray; HNSCC = head and neck squamous cell carcinoma; IMRT = intensity‐modulated radiation therapy; ITT = intention‐to‐treat; IV = intravenous; KGFs = keratinocyte growth factors; M = male; min = minute; MV = megavolt; NCI CTC = National Cancer Institute Common Toxicity Criteria; NCIC CTG ECTC = National Cancer Institute of Canada Clinical Trials Group Expanded Common Toxicity Criteria; OMAS = Oral Mucositis Assessment Scale; QoL = quality of life; RECIST = Response Evaluation Criteria In Solid Tumours; RT = radiotherapy; RTOG = Radiation Therapy Oncology Group; SC = subcutaneous; SCC = squamous cell carcinoma; SCCHN = squamous cell carcinoma of the head and neck; SD = standard deviation; TNM = tumour, node and metastasis; VAS = visual analogue scale; WHO = World Health Organization; wt/vol = weight/volume.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anné 2002 | Not an RCT ‐ compares a subcutaneous amifostine group with the intravenous amifostine and no‐treatment arms from another study (Brizel 2000) |
| Bagga 2007 | Abstract; insufficient information |
| Bakowski 1978 | CCT |
| Belcaro 2008 | Not head and neck cancer patients |
| Bohuslavizki 1998 | Radioactive iodine not radiotherapy |
| Borg 2007 | Abstract; insufficient information |
| Bourhis 2000 | Salivary gland dysfunction was not a reported outcome |
| Braaksma 2002 | Data unavailable for the outcomes of interest to this review |
| Braaksma 2005 | Salivary gland dysfunction was not a reported outcome |
| Chambers 2005 | Abstract; insufficient information |
| Demiroz 2012 | Not an RCT |
| Fallahi 2013 | Radioactive iodine not radiotherapy |
| Fan 2011 | Unclear whether this is an RCT or not. Study authors contacted February 2016 but no reply received |
| Franzén 1995 | Salivary gland dysfunction was not a reported outcome |
| Fuertes 2004 | Quasi‐randomised trial (case history number) |
| Goyal 2007 | Abstract; insufficient information |
| Gu 2014 | Abstract; insufficient information |
| Johnson 2002 | Not an RCT |
| Karacetin 2004 | Quasi‐randomised trial |
| Koukourakis 2000 | No formal assessment of salivary gland dysfunction; data not presented for head and neck cancer patients |
| Kumarchandra 2010 | Abstract; insufficient information |
| Manoor 2014 | Abstract; insufficient information |
| Mateos 2001 | Quasi‐randomised trial (alternate assignment) |
| Mitine 2000 | Abstract; insufficient information |
| Mix 2013 | Abstract; insufficient information |
| Nicolatou‐Galitis 2003 | Not an RCT |
| Norberg‐Spaak 1996 | Abstract; insufficient information |
| Norberg‐Spaak 1997 | Abstract; insufficient information |
| Nyárády 2006 | Quasi‐randomised trial (alternate assignment) |
| Park 2012 | Abstract; insufficient information |
| Park 2012a | Abstract; insufficient information |
| Peters 1999 | Quasi‐randomised trial (day of birth) |
| Qian 2003 | Dissertation; unable to obtain a copy |
| Resubal 2011 | Abstract; insufficient information |
| Rieger 2012 | Control group not relevant for this review |
| Rischin 2010 | Tirapazamine is cancer treatment drug not a radiation protector |
| Rudat 2005 | Abstract; insufficient information |
| Schönekäs 1999 | Not an RCT |
| Sharma 2012 | Intervention not defined as a pharmacological agent |
| Strnad 1997 | Abstract; insufficient information |
| Su 2006 | Salivary gland dysfunction measured as an adverse event following administration of G‐CSF |
| Takahashi 1986 | Not an RCT |
| Thorstad 2003 | Not an RCT |
| Uchiyama 2005 | Unclear whether this is an RCT or not |
| Zale 1993 | Abstract; insufficient information |
| Zimmerman 1997 | Not an RCT |
CCT = controlled clinical trial; G‐CSF = granulocyte‐colony stimulating factor; RCT = randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Yu 2009.
| Methods | RCT |
| Participants | Nasopharynx cancer patients with poorly differentiated squamous carcinoma, at clinical phase III to VI, aged 25 to 69 years, first session of treatment, radiation field included > 75% of the area of salivary glands |
| Interventions | Amifostine versus no intervention |
| Outcomes | Xerostomia; salivary flow rates; adverse effects |
| Notes | Method/scale used to assess xerostomia unclear and further information required from study authors; the results do not have the potential to change any conclusions of the review |
RCT = randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT02430298.
| Trial name or title | Topical and oral melatonin for preventing concurrent radiochemotherapy induced oral mucositis and xerostomia in head and neck cancer patients |
| Methods | Parallel, double‐blind RCT |
| Participants | Head and neck cancer adult patients |
| Interventions | Topical and oral melatonin versus placebo |
| Outcomes | Xerostomia; oral mucositis; quality of life |
| Starting date | July 2013 |
| Contact information | Nutjaree Pratheepawanit Johns, Khon Kaen University |
| Notes | clinicaltrials.gov/show/NCT02430298 |
RCT = randomised controlled trial.
Differences between protocol and review
At least one measure of the primary outcome (xerostomia) or salivary flow (secondary outcome) had to be reported for potential inclusion within the review. The purpose of this minor amendment was to maintain the focus of the review on salivary gland dysfunction.
Sensitivity analysis, based on risk of bias, was originally to be done based on randomisation, allocation concealment and blinded outcome assessment. This was changed to eliminating high and unclear risk studies from the analyses so as not to rate the importance of any type of bias over another.
Contributions of authors
All authors contributed to all aspects of this review.
Sources of support
Internal sources
Cochrane Oral Health, UK.
The University of Manchester, Manchester Academic Health Sciences Centre (MAHSC), NIHR Manchester Biomedical Research Centre, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
Declarations of interest
Philip Riley: no interests to declare. Philip is an Editor with Cochrane Oral Health. Anne‐Marie Glenny: no interests to declare. Anne‐Marie is Deputy Co‐ordinating Editor of Cochrane Oral Health. Fang Hua: no interests to declare. Fang is an Editor with Cochrane Oral Health. Helen V Worthington: no interests to declare. Helen is Co‐ordinating Editor of Cochrane Oral Health.
New
References
References to studies included in this review
Abacioglu 1997 {unpublished data only}
- Abacioglu MU. Effect of Pilocarpine for the Prevention of Radiation‐Induced Xerostomia [Dissertation]. Marmara (Turkey): Marmara University Hospital, 1997.
Antonadou 2002 {published data only}
- Antonadou D, Pepelassi M, Synodinou M, Puglisi M, Throuvalas N. Prophylactic use of amifostine to prevent radiochemotherapy‐induced mucositis and xerostomia in head‐and‐neck cancer. International Journal of Radiation Oncology, Biology, Physics 2002;52(3):739‐47. [DOI] [PubMed] [Google Scholar]
Bardet 2011 {published data only}
- Bardet E, Martin L, Calais G, Alfonsi M, Feham N E, Tuchais C, et al. Subcutaneous compared with intravenous administration of amifostine in patients with head and neck cancer receiving radiotherapy: final results of the GORTEC2000‐02 phase III randomized trial. Journal of Clinical Oncology 2011;29(2):127‐33. [DOI] [PubMed] [Google Scholar]
- Bardet E, Martin L, Calais G, Tuchais C, Bourhis J, Rhein B, et al. Preliminary data of the GORTEC 2000‐02 phase III trial comparing intravenous and subcutaneous administration of amifostine for head and neck tumors treated by external radiotherapy. Seminars in Oncology 2002;29(6 Suppl 19):57‐60. [DOI] [PubMed] [Google Scholar]
Brizel 2000 {published data only}
- Brizel D, Sauer R, Wannenmacher M, Henke M, Eschwege F, Wasserman T. Randomized phase III trial of radiation amifostine in patients with head and neck cancer. 34th Annual Meeting of the American Society of Clinical Oncology; 1998 May 17‐19; Los Angeles (USA). Los Angeles (USA): American Society of Clinical Oncology, 1998. [Abs No 1487]
- Brizel DM, Wasserman T. The influence of intravenous amifostine on xerostomia and survival during radiotherapy for head and neck cancer: two year follow‐up of a prospective randomized trial. Journal of Clinical Oncology 2004;22(14 Suppl):5536. [DOI] [PubMed] [Google Scholar]
- Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. Journal of Clinical Oncology 2000;18(19):3339‐45. [DOI] [PubMed] [Google Scholar]
- Rudat V, Meyer J, Momm F, Bendel M, Henke M, Strnad V, et al. Protective effect of amifostine on dental health after radiotherapy of the head and neck. International Journal of Radiation Oncology, Biology, Physics 2000;48(5):1339‐43. [DOI] [PubMed] [Google Scholar]
- Strnad V, Sauer R. Radioprotection of head and neck tissue by amifostine. Frontiers of Radiation Therapy and Oncology 2002;37:101‐11. [DOI] [PubMed] [Google Scholar]
- Wasserman T, Mackowiak JI, Brizel DM, Oster W, Zhang J, Peeples PJ, et al. Effect of amifostine on patient assessed clinical benefit in irradiated head and neck cancer. International Journal of Radiation Oncology, Biology, Physics 2000;48(4):1035‐9. [DOI] [PubMed] [Google Scholar]
- Wasserman TH, Brizel DM, Henke M, Monnier A, Eschwege F, Sauer R, et al. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head‐and‐ neck cancer: 2‐year follow‐up of a prospective, randomized, phase III trial. International Journal of Radiation Oncology, Biology, Physics 2005;63(4):985‐90. [DOI] [PubMed] [Google Scholar]
Brizel 2008 {published data only}
- Brizel DM, Murphy BA, Rosenthal DI, Pandya KJ, Glück S, Brizel HE, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. Journal of Clinical Oncology 2008;26(15):2489‐96. [DOI] [PubMed] [Google Scholar]
Buentzel 2006 {published data only}
- Buentzel J, Micke O, Adamietz IA, Monnier A, Glatzel M, Vries A. Intravenous amifostine during chemoradiotherapy for head‐and‐neck cancer: a randomized placebo‐controlled phase III study. International Journal of Radiation Oncology, Biology, Physics 2006;64(3):684‐91. [DOI] [PubMed] [Google Scholar]
Büntzel 1998 {published data only}
- Bennett CL, Lane D, Stinson T, Glatzel M, Buntzel J. Economic analysis of amifostine as adjunctive support for patients with advanced head and neck cancer: preliminary results from a randomized phase II clinical trial from Germany. Cancer Investigation 2001;19(2):107‐13. [DOI] [PubMed] [Google Scholar]
- Buntzel J, Glatzel M, Schuth J, Frohlich D, Kuttner K. Selective cytoprotection with amifostine: a new strategy in supportive care of head and neck malignancies. Oto‐Rhino‐Laryngologia Nova 1997;7:276‐80. [Google Scholar]
- Buntzel J, Kuttner K, Russell L, Oster W, Schuth J, Glatzel M. Selective cytoprotection by amifostine (A) in the treatment of head and neck cancer with simultaneous radiochemotherapy (RCT). European Cancer Conference; 1997 Sep 14‐18; Hamburg (Germany). Hamburg (Germany): ECCO, 1997. [Abs No 852]
- Buntzel J, Kuttner K, Russell L, Oster W, Schuth J, Glatzel M. Selective cytoprotection by amifostine in the treatment of head and neck cancer with simultaneous radiochemotherapy. Proceedings of the American Society of Clinical Oncology 1997;16:393. [Abs No 1400] [Google Scholar]
- Büntzel J. Experiences with selenium in the treatment of acute and late toxicities due to radiochemotherapy of head and neck cancer [Erfahrungen mit Natriumselenit in der Behandlung von akuten und späten Nebenwirkungen der Radiochemotherapie von Kopf‐Hals‐Karzinomen]. Medizinische Klinik 1999;94 Suppl III:49‐53. [DOI] [PubMed] [Google Scholar]
- Büntzel J, Glatzel M, Kuttner K, Schuth J, Oster W, Frohlich F. Selective cytoprotection with amifostine in simultaneous radiochemotherapy of head neck cancer. Annals of Oncology 1996;7(81):381. [Google Scholar]
- Büntzel J, Glatzel M, Kuttner K, Weinaug R, Fröhlich D. Amifostine in simultaneous radiochemotherapy of advanced head and neck cancer. Seminars in Radiation Oncology 2002;12(1 Suppl 1):4‐13. [DOI] [PubMed] [Google Scholar]
- Büntzel J, Kuttner, Frohlich D, Schuth J, Glatzel M. Amifostine in simultaneous radiochemotherapy for head and neck cancer. Oto‐Rhino‐Laryngologia Nova 1997;7:204‐10. [Google Scholar]
- Büntzel J, Küttner K, Fröhlich D, Glatzel M. Selective cytoprotection with amifostine in concurrent radiochemotherapy for head and neck cancer. Annals of Oncology 1998;9(5):505‐9. [DOI] [PubMed] [Google Scholar]
- Büntzel J, Schuth J, Küttner K, Glatzel M. Radiochemotherapy with amifostine cytoprotection for head and neck cancer. Supportive Care in Cancer 1998;6(2):155‐60. [DOI] [PubMed] [Google Scholar]
Büntzel 2010 {published data only}
- Buentzel J, Micke O, Glatzel M, Bruns F, Kisters K, Muecke R. Evaluation of the effect of selenium on radiation‐induced toxicities in head neck cancer patients. Journal of Clinical Oncology 2009;27(15 Suppl):e20698. [Google Scholar]
- Büntzel J, Micke O, Glatzel M, Schäfer U, Riesenbeck D, Kisters K, et al. Selenium substitution during radiotherapy in head and neck cancer. Trace Elements and Electrolytes 2010;27:235‐9. [Google Scholar]
- Büntzel J, Riesenbeck D, Glatzel M, Berndt‐Skorka R, Riedel T, Mücke R, et al. Limited effects of selenium substitution in the prevention of radiation‐associated toxicities. Results of a randomized study in head and neck cancer patients. Anticancer Research 2010;30(5):1829‐32. [PubMed] [Google Scholar]
Burlage 2008 {published data only}
- Burlage FR, Roesink JM, Kampinga HH, Coppes RP, Terhaard C, Langendijk JA, et al. Protection of salivary function by concomitant pilocarpine during radiotherapy: a double‐blind, randomized, placebo‐controlled study. International Journal of Radiation Oncology, Biology, Physics 2008;70(1):14‐22. [DOI] [PubMed] [Google Scholar]
Duncan 2005 {published data only}
- Duncan GG, Epstein JB, Tu D, Sayed S, Bezjak A, Ottaway J, et al. Quality of life, mucositis, and xerostomia from radiotherapy for head and neck cancers: a report from the NCIC CTG HN2 randomized trial of an antimicrobial lozenge to prevent mucositis. Head & Neck 2005;27(5):421‐8. [DOI] [PubMed] [Google Scholar]
Fisher 2003 {published data only}
- Fisher J, Scott C, Scarantino CW, Leveque FG, White RL, Rotman M, et al. Phase III quality‐of‐life study results: impact on patients' quality of life to reducing xerostomia after radiotherapy for head‐and‐neck cancer ‐ RTOG 97‐09. International Journal of Radiation Oncology, Biology, Physics 2003;56(3):832‐6. [DOI] [PubMed] [Google Scholar]
- Scarantino C, LeVeque F, Swann RS, White R, Schulsinger A, Hodson I, et al. Effect of pilocarpine during radiation therapy: results of RTOG 97‐09, a phase III randomized study in head and neck cancer patients. Journal of Supportive Oncology 2006;4(5):252‐8. [PubMed] [Google Scholar]
- Scarantino CW, Leveque F, Scott C, White RL, Rotman M, Hodson DI, et al. A phase III study on the concurrent use of oral pilocarpine to reduce hyposalivation and mucositis associated with radiation therapy in head and neck cancer patients. Final results of RTOG 97‐09. International Journal of Radiation Oncology, Biology, Physics 2001;51(3 Suppl 1):85‐6. [Abs No 157] [Google Scholar]
- Scarantino CW, Leveque FG, Scott CB, White RL, Rotman M, Hodson DI. A phase III study of concomitant oral pilocarpine to reduce hypo‐salivation and mucositis associated with curative radiation therapy (RT) in head and neck (H&N) cancer patients. RTOG 9709. Proceedings of the American Society of Clinical Oncology 2001;20:225a. [Abs No 897] [Google Scholar]
Gornitsky 2004 {published and unpublished data}
- Gornitsky M, Shenouda G, Sultanem K, Katz H, Hier M, Black M, et al. Double‐blind randomized, placebo‐controlled study of pilocarpine to salvage salivary gland function during radiotherapy of patients with head and neck cancer. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2004;98(1):45‐52. [DOI] [PubMed] [Google Scholar]
Grötz 2001 {published data only}
- Grötz KA, Henneicke‐Von Zepelin HH, Kohnen R, Kutzner J, Belz GG. Prophylaxis of mucositis and dry mouth after head and neck radiotherapy. A new treatment. Krankenhauspharmazie 2002;23(5):193‐8. [Google Scholar]
- Grötz KA, Henneicke‐von Zepelin HH, Kohnen R, al‐Nawas B, Bockisch A, Kutzner J, et al. Prospective double‐blind study of prophylaxis of radioxerostomia with Coumarin/Troxerutine in patients with head and neck cancer [Prospektive, doppelblinde Therapiestudie zur Prophylaxe der Radioxerostomie durch Cumarin/Troxerutin bei Patienten mit Kopf‐Hals‐Karzinomen]. Strahlentherapie und Onkologie 1999;175(8):397‐404. [DOI] [PubMed] [Google Scholar]
- Grötz KA, Wüstenberg P, Kohnen R, Al‐Nawas B, Henneicke‐von Zepelin H, Bockisch A, et al. Prophylaxis of radiogenic sialadenitis and mucositis by coumarin/troxerutine in patients with head and neck cancer ‐ a prospective, randomized, placebo‐controlled, double‐blind study. British Journal of Oral and Maxillofacial Surgery 2001;39(1):34‐9. [DOI] [PubMed] [Google Scholar]
Haddad 2002 {published data only}
- Haddad P, Karimi M. A randomised, double‐blind, placebo‐controlled trial of concomitant pilocarpine with head and neck irradiation for prevention of radiation‐induced xerostomia. Radiotherapy and Oncology 2002;64(1):29‐32. [DOI] [PubMed] [Google Scholar]
Haddad 2009 {published data only}
- Haddad R, Sonis S, Posner M, Wirth L, Costello R, Braschayko P, et al. Randomized phase 2 study of concomitant chemoradiotherapy using weekly carboplatin/paclitaxel with or without daily subcutaneous amifostine in patients with locally advanced head and neck cancer. Cancer 2009;115(19):4514‐23. [DOI] [PubMed] [Google Scholar]
Han 2010 {published data only}
- Han F, Zhang JD, Shao ZY, Liu FJ. Effects of concurrent chemoradiotherapy combined with Jinlong capsules for patients with advanced nasopharyngeal squamous carcinoma. Chinese Journal of Cancer Prevention and Treatment 2010;(2):138‐9. [Google Scholar]
He 2004 {published data only}
- He X, Hu C, Wu Y. Radioprotective effect of amifostine in nasopharyngeal carcinoma. Fudan University Journal of Medical Sciences 2004;31(1):53‐8. [Google Scholar]
Henke 2011 {published data only}
- Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo‐controlled trial. Journal of Clinical Oncology 2011;29(20):2815‐20. [DOI] [PubMed] [Google Scholar]
Hu 2005 {published data only}
- Hu YR, Wu CQ, Liu YJ, Wang Y, Li X, Zhong H, et al. Clinical observation on effect of shenqi fanghou recipe in preventing and treating radiation injury in patients with head and neck tumour. Zhongguo Zhong Xi Yi Jie He Za Zhi 2005;25(7):623‐5. [PubMed] [Google Scholar]
Jaguar 2015 {published data only}
- Jaguar GC. A Prospective Study of Bethanechol in the Salivary Glands Physiology in Patients Following Head and Neck Radiotherapy [Estudo Prospectivo do Uso do Betanecol na Fisiologia de Glândulas Salivares em Pacientes Irradiados em Região de Cabeça e Pescoço] [Dissertation]. Sao Paulo (Brazil): Fundaçao Antonio Prudente, 2010. [Google Scholar]
- Jaguar GC, Campos L, Pellizzon AC, Lima EN, Kowalski LP, Alves FA. Double‐blind randomized prospective trial of bethanechol in the prevention of radiation‐induced salivary gland dysfunction in cancer patients. Supportive Care in Cancer 2012;20(1 Suppl):S252. [DOI] [PubMed] [Google Scholar]
- Jaguar GC, Lima EN, Kowalski LP, Pellizzon AC, Carvalho AL, Boccaletti KW, et al. Double blind randomized prospective trial of bethanechol in the prevention of radiation‐induced salivary gland dysfunction in head and neck cancer patients. Radiotherapy and Oncology 2015;115(2):253‐6. [DOI] [PubMed] [Google Scholar]
Jellema 2006 {published data only}
- Jellema AP, Slotman BJ, Muller MJ, Leemans CR, Smeele LE, Hoekman K, et al. Radiotherapy alone, versus radiotherapy with amifostine 3 times weekly, versus radiotherapy with amifostine 5 times weekly: a prospective randomized study in squamous cell head and neck cancer. Cancer 2006;107(3):544‐53. [DOI] [PubMed] [Google Scholar]
Jham 2007 {published data only}
- Jham BC, Chen H, Carvalho AL, Freire AR. A randomized phase III prospective trial of bethanechol to prevent mucositis, candidiasis, and taste loss in patients with head and neck cancer undergoing radiotherapy: a secondary analysis. Journal of Oral Science 2009;51(4):565‐72. [DOI] [PubMed] [Google Scholar]
- Jham BC, Teixeira IV, Aboud CG, Carvalho AL, Coelho Mde M, Freire AR. A randomized phase III prospective trial of bethanechol to prevent radiotherapy‐induced salivary gland damage in patients with head and neck cancer. Oral Oncology 2007;43(2):137‐42. [DOI] [PubMed] [Google Scholar]
Lajtman 2000 {published data only}
- Lajtman Z, Krajina Z, Krpan D, Vincelj J, Borcic V, Popovic‐Kovacic J. Pilocarpine in the prevention of postirradiation xerostomia. Acta Medica Croatica 2000;54(2):65‐7. [PubMed] [Google Scholar]
Lanzós 2010 {published data only}
- Lanzós I, Herrera D, Santos S, O'Connor A, Peña C, Lanzós E, et al. Mucositis in irradiated cancer patients: effects of an antiseptic mouthrinse. Medicina Oral, Patología Oral y Cirugía Bucal 2010;15(5):e732‐8. [DOI] [PubMed] [Google Scholar]
Le 2011 {published data only}
- Le QT, Kim HE, Schneider CJ, Murakozy G, Skladowski K, Reinisch S, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo‐controlled study. Journal of Clinical Oncology 2011;29(20):2808‐14. [DOI] [PubMed] [Google Scholar]
Lin 2014 {published data only}
- Lin KS, Jen YM, Chao TY, Lin YS, Wang LH, Lin CJ, et al. Prevention of acute radiation‐associated toxicity by traditional chinese medicine Tianwang Buxin mini‐pills in patients with head and neck cancer. Journal of Medical Sciences (Taipei, Taiwan) 2014;34:152‐60. [Google Scholar]
Lozada‐Nur 1998 {published and unpublished data}
- Lozada‐Nur F, Schoelch M, Fu K, Muscoplat C, Trivedi M, Smith C, et al. A pilot study to evaluate the effect of pilocarpine tablets on salivary flow and mucositis in head and neck cancer patients during radiotherapy. Proceedings of the American Society of Clinical Oncology 1998;17:399. [Abs No 1541] [Google Scholar]
Patni 2004 {published and unpublished data}
- Patni N, Patni S, Bapna A, Somani N, Gupta A, Ratnam BV. The role of amifostine in prophylaxis of radiotherapy induced mucositis and xerostomia in head and neck cancer. Journal of Clinical Oncology 2004;22(14 Suppl):5568. [Google Scholar]
Peng 2006 {published data only}
- Peng LH, Sun Y, Zhang XL, Zhang Q, Fu S. Efficacy of amifostine on patients with head and neck squamous cell carcinomas undergoing radiation therapy and chemotherapy and clinical observation on its hematologic toxicity. Journal of China Pharmacy 2006;17(5):1166‐8. [Google Scholar]
Pimentel 2014 {published data only}
- Pimentel MJ, Filho MM, Araújo M, Gomes DQ, Costa LJ. Evaluation of radioprotective effect of pilocarpine ingestion on salivary glands. Anticancer Research 2014;34(4):1993‐9. [PubMed] [Google Scholar]
Reshma 2012 {published data only}
- Reshma K, Kamalaksh S, Bindu YS, Pramod K, Asfa A, Amritha D, et al. Tulasi (Ocimum Sanctum) as radioprotector in head and neck cancer patients undergoing radiation therapy. Biomedicine 2012;32:39‐44. [Google Scholar]
Rode 1999 {published data only}
- Rode M, Smid L, Budihna M, Soba E, Rode M, Gaspersic D. The effect of pilocarpine and biperiden on salivary secretion during and after radiotherapy in head and neck cancer patients. International Journal of Radiation Oncology, Biology, Physics 1999;45(2):373‐8. [DOI] [PubMed] [Google Scholar]
Sangthawan 2001 {published data only}
- Sangthawan D, Watthanaarpornchai S, Phungrassami T. Randomized double blind, placebo‐controlled study of pilocarpine administered during head and neck irradiation to reduce xerostomia. Journal of the Medical Association of Thailand 2001;84(2):195‐203. [PubMed] [Google Scholar]
- Sangthawan D, Watthanaarpornchai S, Phungrassami T. Randomized double blind, placebo‐controlled study of pilocarpine administered during head and neck irradiation to reduce xerostomia. Radiation Oncology Association of Thailand 2002;7:242. [PubMed] [Google Scholar]
Vacha 2003 {published data only}
- Vacha P, Fehlauer F, Mahlmann B, Marx M, Hinke A, Sommer K, et al. Randomized phase III trial of postoperative radiochemotherapy +/‐ amifostine in head and neck cancer. Is there evidence for radioprotection?. Strahlentherapie und Onkologie 2003;179(6):385‐9. [DOI] [PubMed] [Google Scholar]
- Vacha P, Marx M, Engel A, Richter E, Feyerabend T. Side effects of postoperative radiochemotherapy with amifostine versus radiochemotherapy alone in head and neck tumors. Preliminary results of a prospective randomized trial. Strahlentherapie und Onkologie 1999;175 Suppl 4:18‐22. [PubMed] [Google Scholar]
Valdez 1993 {published data only}
- Valdez IH, Wolff A, Atkinson JC, Macynski AA, Fox PC. Use of pilocarpine during head and neck radiation therapy to reduce xerostomia and salivary dysfunction. Cancer 1993;71(5):1848‐51. [DOI] [PubMed] [Google Scholar]
- Wolff A, Atkinson JC, Macynski AA, Fox PC. Oral complications of cancer therapies. Pretherapy interventions to modify salivary dysfunction. NCI Monographs 1990;(9):87‐90. [PubMed] [Google Scholar]
Veerasarn 2006 {published and unpublished data}
- Veerasarn V, Phromratanapongse P, Suntornpong N, Lorvidhaya V, Chitapanarux I, Tesavibul C, et al. Effect of amifostine to prevent radiotherapy‐induced acute and late toxicity in head and neck cancer patients who had normal or mild impaired salivary gland dysfunction. European Journal of Cancer Supplements 2003;1(5):S273. [Abs No 908] [PubMed] [Google Scholar]
- Veerasarn V, Phromratanapongse P, Suntornpong N, Lorvidhaya V, Sukthomya V, Chitapanarux I, et al. Effect of amifostine to prevent radiotherapy‐induced acute and late toxicity in head and neck cancer patients who had normal or mild impaired salivary gland function. Journal of the Medical Association of Thailand 2006;89(12):2056‐67. [PubMed] [Google Scholar]
Wang 1998 {published data only}
- Wang Q, Liu H, Qiao N. Effects of traditional Chinese medicine on salivary glands in the patients with head and neck cancer during radiotherapy. Zhongguo Zhong Xi Yi Jie He Za Zhi 1998;18(11):662‐4. [PubMed] [Google Scholar]
Warde 2002 {published data only}
- Warde P, O'Sullivan B, Aslanidis J, Kroll B, Lockwood G, Math M, et al. A phase III placebo‐controlled trial of oral pilocarpine in patients undergoing radiotherapy for head and neck cancer. International Journal of Radiation Oncology, Biology, Physics 2002;54(1):9‐13. [DOI] [PubMed] [Google Scholar]
Watanabe 2010 {published data only}
- Watanabe T, Ishihara M, Matsuura K, Mizuta K, Itoh Y. Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. International Journal of Cancer 2010;127(8):1984‐90. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anné 2002 {published data only}
- Anné PR. Phase II trial of subcutaneous amifostine in patients undergoing radiation therapy for head and neck cancer. Seminars in Oncology 2002;29(6 Suppl 19):80‐3. [DOI] [PubMed] [Google Scholar]
Bagga 2007 {published data only}
- Bagga P, Anand AK, Raina A, Choudhary PS, Chaudhoory AR. Evaluation of pilocarpine for the prevention of radiation induced xerostomia in head and neck cancers. Annals of Oncology 2007;18(Suppl 9):ix177. [Abs No 62] [Google Scholar]
Bakowski 1978 {published data only}
- Bakowski MT, Macdonald E, Mould RF, Cawte P, Sloggem J, Barrett A, et al. Double blind controlled clinical trial of radiation plus razoxane (ICRF 159) versus radiation plus placebo in the treatment of head and neck cancer. International Journal of Radiation Oncology, Biology, Physics 1978;4(1‐2):115‐9. [DOI] [PubMed] [Google Scholar]
Belcaro 2008 {published data only}
- Belcaro G, Cesarone MR, Genovesi D, Ledda A, Vinciguerra G, Ricci A, et al. Pycnogenol may alleviate adverse effects in oncologic treatment. Panminerva Medica 2008;50(3):227‐34. [PubMed] [Google Scholar]
Bohuslavizki 1998 {published data only}
- Bohuslavizki KH, Klutmann S, Bleckmann C, Brenner W, Lassmann S, Mester J, et al. Salivary gland protection by amifostine in high‐dose radioiodine therapy of differentiated thyroid cancer. Strahlentherapie und Onkologie 1999;175(2):57‐61. [DOI] [PubMed] [Google Scholar]
- Bohuslavizki KH, Klutmann S, Brenner W, Kröger S, Buchert R, Bleckmann C, et al. Radioprotection of salivary glands by amifostine in high‐dose radioiodine treatment. Results of a double‐blinded, placebo‐controlled study in patients with differentiated thyroid cancer. Strahlentherapie und Onkologie 1999;175(Suppl 4):6‐12. [PubMed] [Google Scholar]
- Bohuslavizki KH, Klutmann S, Brenner W, Mester J, Henze E, Clausen M. Salivary gland protection by amifostine in high‐dose radioiodine treatment: results of a double‐blind placebo‐controlled study. Journal of Clinical Oncology 1998;16(11):3542‐9. [DOI] [PubMed] [Google Scholar]
- Bohuslavizki KH, Klutmann S, Jenicke L, Kröger S, Buchert R, Mester J, et al. Salivary gland protection by S‐2‐(3‐amino‐propylamino)‐ethylphosphorothioic acid (amifostine) in high‐dose radioiodine treatment: results obtained in a rabbit animal model and in a double‐blind multi‐arm trial. Cancer Biotherapy & Radiopharmaceuticals 1999;14(5):337‐47. [DOI] [PubMed] [Google Scholar]
Borg 2007 {published data only}
- Borg M, Krishnan S, Olver L, Stein B, Chatterton B, Coates E, et al. Randomised double‐blind trial of amifostine vs placebo for radiation‐induced xerostomia in patients with head and neck cancer. ANZ Journal of Surgery 2007;77(12):A3. [Google Scholar]
Bourhis 2000 {published data only}
- Bourhis J, Crevoisier R, Abdulkarim B, Deutsch E, Lusinchi A, Luboinski B, et al. A randomized study of very accelerated radiotherapy with and without amifostine in head and neck squamous cell carcinoma. International Journal of Radiation Oncology, Biology, Physics 2000;46(5):1105‐8. [DOI] [PubMed] [Google Scholar]
Braaksma 2002 {published data only}
- Braaksma M, Levendag P. Tools for optimal tissue sparing in concomitant chemoradiation of advanced head and neck cancer: subcutaneous amifostine and computed tomography‐based target delineation. Seminars in Oncology 2002;29(6 Suppl 19):63‐70. [DOI] [PubMed] [Google Scholar]
Braaksma 2005 {published data only (unpublished sought but not used)}
- Braaksma M, Agthoven M, Nijdam W, Uyl‐de Groot C, Levendag P. Costs of treatment intensification for head and neck cancer: concomitant chemoradiation randomised for radioprotection with amifostine. European Journal of Cancer 2005;41(14):2102‐11. [DOI] [PubMed] [Google Scholar]
Chambers 2005 {published data only}
- Chambers MS, Posner MR, Jones CU, Weber RS, Vitti R. Two phase III clinical studies of cevimeline for post‐radiation xerostomia in patients with head and neck cancer. Journal of Clinical Oncology 2005;23(16 Suppl):5503. [Google Scholar]
Demiroz 2012 {published data only}
- Demiroz C, Ozcan L, Cebelli G, Karadag O, Ozsahin EM. The effect of amifostine on acute and late radiation side effects in head and neck cancer patients. Turkiye Klinikleri Journal of Medical Sciences 2012;32:1207‐16. [Google Scholar]
Fallahi 2013 {published data only}
- Fallahi B, Beiki D, Abedi SM, Saghari M, Fard‐Esfahani A, Akhzari F, et al. Does vitamin E protect salivary glands from I‐131 radiation damage in patients with thyroid cancer?. Nuclear Medicine Communications 2013;34(8):777‐86. [DOI] [PubMed] [Google Scholar]
Fan 2011 {published data only}
- Fan Z, Lin H, Liu Z, He Q, Zhang S, Li W. Protective effect of amifostine in irradiation mucositis of the oral cavity and dry mouth after nasapharyngeal carcinoma. Military Medical Journal of Southeast China 2011;13(2):146‐8. [Google Scholar]
Franzén 1995 {published data only}
- Franzén L, Henriksson R, Littbrand B, Zackrisson B. Effects of sucralfate on mucositis during and following radiotherapy of malignancies in the head and neck region. A double‐blind placebo‐controlled study. Acta Oncologica (Stockholm, Sweden) 1995;34(2):219‐23. [DOI] [PubMed] [Google Scholar]
Fuertes 2004 {published data only}
- Fuertes Cabero S, Setoain Perego X, Rovirosa Casino A, Mateos Fernández JJ, Fuster Pelfort D, Ferre Jorge J, et al. Usefulness of pilocarpine in the prevention of xerostomia in patients with head and neck cancer treated with radiotherapy. Assessment with gammagraphy and salivary flow [Utilidad de la pilocarpina como profiláctico de xerostomia en pacientes con cáncer de cabeza y cuello tratados con radioterapia. Valoración mediante gammagrafía y flujo salivar]. Revista Española de Medicina Nuclear 2004;23(4):259‐66. [DOI] [PubMed] [Google Scholar]
Goyal 2007 {published data only}
- Goyal S, Sharma DN, Julka PK, Rath GK. Effect of oral pilocarpine on xerostomia and quality of life in patients receiving curative radiotherapy for head and neck cancers. Journal of Clinical Oncology 2007;25(18 Suppl):6088. [Google Scholar]
Gu 2014 {published data only}
- Gu J, Zhu S, Li X, Wu H, Li Y, Hua F. Effect of amifostine in head and neck cancer patients treated with radiotherapy: a systematic review and meta‐analysis based on randomized controlled trials. PloS One 2014;9(5):e95968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2002 {published data only}
- Johnson DJ, Scott CB, Marks JE, Seay TE, Atkins JN, Berk LB, et al. Assessment of quality of life and oral function of patients participating in a phase II study of radioprotection of oral and pharyngeal mucosa by the prostaglandin E(1) analog misoprostol (RTOG 96‐07). International Journal of Radiation Oncology, Biology, Physics 2002;54(5):1455‐9. [DOI] [PubMed] [Google Scholar]
Karacetin 2004 {published data only}
- Karacetin D, Yücel B, Leblebicioglu B, Aksakal O, Maral O, Incekara O. A randomized trial of amifostine as radioprotector in the radiotherapy of head and neck cancer. Journal of BUON 2004;9(1):23‐6. [PubMed] [Google Scholar]
Koukourakis 2000 {published data only}
- Koukourakis MI, Kyrias G, Kakolyris S, Kouroussis C, Frangiadaki C, Giatromanolaki A, et al. Subcutaneous administration of amifostine during fractionated radiotherapy: a randomized phase II study. Journal of Clinical Oncology 2000;18(11):2226‐33. [DOI] [PubMed] [Google Scholar]
Kumarchandra 2010 {published data only}
- Kumarchandra R, Shenoy K, Bindu YS, Kadaba P, Chandrashekar R. Ocimum Sanctum as a radioprotector in head and neck cancer patients undergoing radiation therapy. Free Radical Biology and Medicine 2010;49 Suppl:S64. [Google Scholar]
Manoor 2014 {published data only}
- Manoor Maiya V, Vaid N, Basu S, Vatyam S, Hegde S, Deshmukh S, et al. The use of xylitol for the prevention of xerostomia in patients receiving intensity modulated radiation therapy for head and neck cancers. International Journal of Radiation Oncology, Biology, Physics 2014;90(1 Suppl):S562. [Google Scholar]
Mateos 2001 {published data only}
- Mateos JJ, Setoain X, Ferre J, Rovirosa A, Navalpotro B, Martin F, et al. Salivary scintigraphy for assessing the protective effect of pilocarpine in head and neck irradiated tumours. Nuclear Medicine Communications 2001;22(6):651‐6. [DOI] [PubMed] [Google Scholar]
Mitine 2000 {published data only}
- Mitine C, Chaltin M, Salembier C, Merlo P, Beauduin M. Head and neck radiotherapy: does pilocarpine hydrochloride reduce radiation‐induced xerostomia?. Proceedings of the management of head and neck tumors: what are the challenges for the third millennium; 1999 Dec 3‐4; Brussels (Belgium). European Laryngological Society, 1999.
Mix 2013 {published data only}
- Mix MD, Jameson M, Tills M, Dibaj S, Groman A, Jaggernauth W, et al. Randomized double‐blind, placebo‐controlled, multicenter phase 2 trial of selenomethionine as a modulator of efficacy and toxicity of chemoradiation in locally‐advanced squamous cell carcinoma of the head and neck. International Journal of Radiation Oncology, Biology, Physics 2013;87(2 Suppl):S466‐7. [Google Scholar]
Nicolatou‐Galitis 2003 {published data only}
- Nicolatou‐Galitis O, Sotiropoulou‐Lontou A, Velegraki A, Pissakas G, Kolitsi G, Kyprianou K, et al. Oral candidiasis in head and neck cancer patients receiving radiotherapy with amifostine cytoprotection. Oral Oncology 2003;39:397‐401. [DOI] [PubMed] [Google Scholar]
Norberg‐Spaak 1996 {published data only}
- Norberg‐Spaak LE, Lundquist PG, Berndtson M, Klintenberg C. Can pilocarpine treatment during irradiation prevent salivary gland damage?. Swedish Dental Journal 1996;20:234. [Google Scholar]
Norberg‐Spaak 1997 {published data only}
- Norberg‐Spaak LE. Can pilocarpine treatment during irradiation prevent salivary gland damage. Clinical Otolaryngology and Allied Sciences 1997;22:88. [Google Scholar]
Nyárády 2006 {unpublished data only}
- Nyárády Z, Németh A, Bán A, Mukics A, Nyárády J, Ember I, et al. A randomized study to assess the effectiveness of orally administered pilocarpine during and after radiotherapy of head and neck cancer. Anticancer Research 2006;26(2B):1557‐62. [PubMed] [Google Scholar]
- Nyárády Z, Németh Á, Mukics A, Olasz L. Relieving symptoms of xerostomia with oral pilocarpine (Salagen) during irradiation in head‐and‐neck cancer. Journal of Cranio‐Maxillo‐Facial Surgery 2004;32 Suppl 1:230. [Abs No 459] [Google Scholar]
Park 2012 {published data only}
- Park J, McGuire DB, Kang H. Effects of cold sterile normal saline (CSNS) mouth care in head and neck cancer (HNC) patients undergoing concurrent chemoradiotherapy (CCRT). Supportive Care in Cancer 2012;20(1 Suppl):S246‐7. [Abs No 1028] [Google Scholar]
Park 2012a {published data only}
- Park K‐N, Son Y‐I, Baek CH. Protection of radiotherapy‐induced xerostomia with vitamin E+C complex. Otolaryngology ‐ Head and Neck Surgery 2012;147(2 Suppl):P174. [Google Scholar]
Peters 1999 {published data only}
- Peters K, Mücke R, Hamann D, Ziegler PG, Fietkau R. Supportive use of amifostine in patients with head and neck tumours undergoing radio‐chemotherapy. Is it possible to limit the duration of the application of amifostine?. Strahlentherapie und Onkologie 1999;175 Suppl 4:23‐6. [PubMed] [Google Scholar]
Qian 2003 {published data only}
- Qian Y. Effects of Traditional Chinese Medicine on Salivary Glands of Patients with Head and Neck Cancer During Radiotherapy [Dissertation]. China National Knowledge Infrastructure (CNKI), 2003. [Google Scholar]
Resubal 2011 {published data only}
- Resubal JR, Calaguas MJ. The effect of zilongjin(r) in patients with head and neck cancer needing radiotherapy with and without chemotherapy. Radiotherapy and Oncology 2011;99(Suppl 1):S336. [Google Scholar]
Rieger 2012 {published data only}
- Rieger JM, Jha N, Lam Tang JA, Harris J, Seikaly H. Functional outcomes related to the prevention of radiation‐induced xerostomia: oral pilocarpine versus submandibular salivary gland transfer. Head & Neck 2012;34(2):168‐74. [DOI] [PubMed] [Google Scholar]
Rischin 2010 {published data only}
- Rischin D, Peters LJ, O'Sullivan B, Giralt J, Fisher R, Yuen K, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans‐Tasman Radiation Oncology Group. Journal of Clinical Oncology 2010;28(18):2989‐95. [DOI] [PubMed] [Google Scholar]
Rudat 2005 {published data only}
- Rudat V, Münter M, Rades D, Grötz K, Haberkorn U, Brenner W. The effect of amifostine or IMRT to preserve the parotid function after radiotherapy of the head and neck region measured by quantitative salivary gland scintigraphy. Journal of Clinical Oncology 2005;23(16 Suppl):5502. [DOI] [PubMed] [Google Scholar]
Schönekäs 1999 {published data only}
- Schönekäs KG, Wagner W, Prott FJ. Amifostine ‐ a radioprotector in locally advanced head and neck tumours. Strahlentherapie und Onkologie 1999;175 Suppl 4:27‐9. [PubMed] [Google Scholar]
Sharma 2012 {published data only}
- Sharma A, Rath GK, Chaudhary SP, Thakar A, Mohanti BK, Bahadur S. Lactobacillus brevis CD2 lozenges reduce radiation‐ and chemotherapy‐induced mucositis in patients with head and neck cancer: a randomized double‐blind placebo‐controlled study. European Journal of Cancer 2012;48(6):875‐81. [DOI] [PubMed] [Google Scholar]
Strnad 1997 {published data only}
- Strnad V, Sauer R, Krafft T, Lerch S. Preliminary results of a randomised study using WR‐2721 in radiation therapy alone in patients with head and neck cancer. European Journal of Cancer 1997;33(Suppl 8):S189. [Google Scholar]
Su 2006 {published data only}
- Su YB, Vickers AJ, Zelefsky MJ, Kraus DH, Shaha AR, Shah JP, et al. Double‐blind, placebo‐controlled, randomized trial of granulocyte‐colony stimulating factor during postoperative radiotherapy for squamous head and neck cancer. Cancer Journal (Sudbury, Mass.) 2006;12(3):182‐8. [DOI] [PubMed] [Google Scholar]
Takahashi 1986 {published data only}
- Takahashi I, Nagai T, Miyaishi K, Maehara Y, Niibe H. Clinical study of the radioprotective effects of amifostine (YM‐08310, WR‐2721) on chronic radiation injury. International Journal of Radiation Oncology, Biology, Physics 1986;12(6):935‐8. [DOI] [PubMed] [Google Scholar]
Thorstad 2003 {published data only}
- Thorstad WL, Haughey B, Chao KS. Pilot study of subcutaneous amifostine in patients undergoing postoperative intensity modulated radiation therapy for head and neck cancer: preliminary data. Seminars in Oncology 2003;30(6 Suppl 18):96‐100. [DOI] [PubMed] [Google Scholar]
Uchiyama 2005 {published data only}
- Uchiyama Y, Murakami S, Kakimoto N, Nakatani A, Furukawa S. Effectiveness of cepharanthin in decreasing interruptions during radiation therapy for oral cancer. Oral Radiology 2005;21(1):41‐4. [Google Scholar]
Zale 1993 {published data only}
- Zale M, Chambers MS, Khan Z. Pilocarpine effects on xerostomia associated with radiation therapy. Journal of Dental Research 1993;72(Abs Spec Issue):375. [Abs No 2175] [Google Scholar]
Zimmerman 1997 {published data only}
- Zimmerman RP, Mark RJ, Juillard GF. Timing of pilocarpine treatment during head and neck radiotherapy: concomitant administration reduces xerostomia better than post‐radiation pilocarpine. International Journal of Radiation Oncology, Biology, Physics 1996;36 Suppl 1(1):236. [Abs No 155] [Google Scholar]
- Zimmerman RP, Mark RJ, Tran LM, Juillard GF. Concomitant pilocarpine during head and neck irradiation is associated with decreased posttreatment xerostomia. International Journal of Radiation Oncology, Biology, Physics 1997;37(3):571‐5. [DOI] [PubMed] [Google Scholar]
- Zimmerman RP, Rufus JM, Juillard GF. Concomitant pilocarpine during head and neck irradiation reduces xerostomia. Supportive Care in Cancer 1996;4(3):243. [Abs No 76] [Google Scholar]
References to studies awaiting assessment
Yu 2009 {published data only}
- Yu DS, Zhao W, Wu XL, Huang ZY, Huang RB. Amifostine in prevention of radiation‐induced xerostomia. Journal of Sun Yat‐Sen University (Medical Sciences) 2009;30(4S):86‐7, 95. [Google Scholar]
References to ongoing studies
NCT02430298 {published data only}
- NCT02430298. Topical/oral melatonin for preventing concurrent radiochemotherapy induced oral mucositis/xerostomia cancer patients [Topical and oral melatonin for preventing concurrent radiochemotherapy induced oral mucositis and xerostomia in head and neck cancer patients]. clinicaltrials.gov/show/NCT02430298 (first received 28 January 2014).
Additional references
Ahlner 1994
- Ahlner BH, Hagelqvist E, Lind MG. Influence on rabbit submandibular gland injury by stimulation or inhibition of gland function during irradiation. Histology and morphometry after 15 gray. Annals of Otology, Rhinology, and Laryngology 1994;103(2):125‐34. [DOI] [PubMed] [Google Scholar]
Andreassen 2003
- Andreassen CN, Grau C, Lindegaard JC. Chemical radioprotection: a critical review of amifostine as a cytoprotector in radiotherapy. Seminars in Radiation Oncology 2003;13(1):62‐72. [DOI] [PubMed] [Google Scholar]
Bennett 2001
- Bennett CL, Lane D, Stinson T, Glatzel M, Buntzel J. Economic analysis of amifostine as adjunctive support for patients with advanced head and neck cancer: preliminary results from a randomized phase II clinical trial from Germany. Cancer Investigations 2001;19(2):107‐13. [DOI] [PubMed] [Google Scholar]
Bohuslavizki 1998
- Bohuslavizki KH, Brenner W, Klutmann S, Hübner RH, Lassmann S, Feyerabend B, et al. Radioprotection of salivary glands by amifostine in high‐dose radioiodine therapy. Journal of Nuclear Medicine 1998;39(7):1237‐42. [PubMed] [Google Scholar]
Buglione 2016
- Buglione M, Cavagnini R, Rosario F, Maddalo M, Vassalli L, Grisanti S, et al. Oral toxicity management in head and neck cancer patients treated with chemotherapy and radiation: Xerostomia and trismus (Part 2). Literature review and consensus statement. Critical Reviews in Oncology/Hematology 2016;102:47‐54. [DOI] [PubMed] [Google Scholar]
Clarkson 2010
- Clarkson JE, Worthington HV, Furness S, McCabe M, Khalid T, Meyer S. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD001973.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2015
- Davies AN, Thompson J. Parasympathomimetic drugs for the treatment of salivary gland dysfunction due to radiotherapy. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD003782.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawes 1987
- Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. Journal of Dental Research 1987;66 Spec No:648‐53. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epstein 1994
- Epstein JB, Burchell JL, Emerton S, Le ND, Silverman S Jr. A clinical trial of bethanechol in patients with xerostomia after radiation therapy. A pilot study. Oral Surgery, Oral Medicine, and Oral Pathology 1994;77(6):610‐4. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guchelaar 1997
- Guchelaar HJ, Vermes A, Meerwaldt JH. Radiation‐induced xerostomia: pathophysiology, clinical course and supportive treatment. Supportive Care in Cancer 1997;5(4):281‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kontopantelis 2012
- Kontopantelis E, Reeves D. Performance of statistical methods for meta‐analysis when true study effects are non‐normally distributed: A simulation study. Statistical Methods in Medical Research 2012;21(4):409‐26. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Mercadante 2017
- Mercadante V, Al Hamad A, Lodi G, Porter S, Fedele S. Interventions for the management of radiotherapy‐induced xerostomia and hyposalivation: A systematic review and meta‐analysis. Oral Oncology 2017;66:64‐74. [DOI] [PubMed] [Google Scholar]
Navazesh 1992
- Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. Journal of Dental Research 1992;71(7):1363‐9. [DOI] [PubMed] [Google Scholar]
Pankhurst 1996
- Pankhurst CL, Smith EC, Rogers JO, Dunne SM, Jackson SH, Proctor G. Diagnosis and management of the dry mouth: Part 1. Dental Update 1996;23(2):56‐62. [PubMed] [Google Scholar]
Porter 2004
- Porter SR, Scully C, Hegarty AM. An update of the etiology and management of xerostomia. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2004;97(1):28‐46. [DOI] [PubMed] [Google Scholar]
Rades 2004
- Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiotherapy and Oncology 2004;70(3):261‐4. [DOI] [PubMed] [Google Scholar]
Rode 2001
- Rode M, Smid L, Budihna M, Gassperssic D, Rode M, Soba E. The influence of pilocarpine and biperiden on pH value and calcium, phosphate, and bicarbonate concentrations in saliva during and after radiotherapy for head and neck cancer. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2001;92(5):509‐14. [DOI] [PubMed] [Google Scholar]
Shiboski 2007
- Shiboski CH, Hodgson TA, Ship JA, Schiodt M. Management of salivary hypofunction during and after radiotherapy. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2007;103 Suppl(S66):e1‐19. [DOI] [PubMed] [Google Scholar]
Sreebny 1996
- Sreebny LM. Xerostomia: diagnosis, management and clinical complications. In: Edgar WM, O'Mullane DM editor(s). Saliva and Oral Health. 2nd Edition. London: British Dental Association, 1996:43‐66. [Google Scholar]
Takahashi 1986
- Takahashi T, Nagai K, Miyaishi K, Maehara Y, Niibe H. Clinical study of the radioprotective effects of amifostine (TM‐08310, WR‐2721) on chronic radiation injury. International Journal of Radiation Oncology, Biology, Physics 1986;12:935‐8. [DOI] [PubMed] [Google Scholar]
Wiseman 1995
- Wiseman LR, Faulds D. Oral pilocarpine: a review of its pharmacological properties and clinical potential in xerostomia. Drugs 1995;49(1):143‐55. [DOI] [PubMed] [Google Scholar]
Worthington 2011
- Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny A‐M, Littlewood A, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD000978.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimmerman 1997
- Zimmerman RP, Mark RJ, Tran LM, Juillard GF. Concomitant pilocarpine during head and neck irradiation is associated with decreased posttreatment xerostomia. International Journal of Radiation Oncology, Biology, Physics 1997;37(3):571‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Tavender 2004
- Tavender E, Davies AN, Glenny AM. Pharmacological interventions for preventing salivary gland dysfunction following radiotherapy. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004940] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tavender 2012
- Tavender E, Davies AN, Glenny AM. Pharmacological interventions for preventing salivary gland dysfunction following radiotherapy. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD004940.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


