Abstract
Background
Endometriosis is defined as the presence of endometrial tissue (glands and stroma) outside the uterine cavity. This condition is oestrogen‐dependent and thus is seen primarily during the reproductive years. Owing to their antiproliferative effects in the endometrium, progesterone receptor modulators (PRMs) have been advocated for treatment of endometriosis.
Objectives
To assess the effectiveness and safety of PRMs primarily in terms of pain relief as compared with other treatments or placebo or no treatment in women of reproductive age with endometriosis.
Search methods
We searched the following electronic databases, trial registers, and websites: the Cochrane Gynaecology and Fertility Group (CGFG) Specialised Register of Controlled Trials, the Central Register of Studies Online (CRSO), MEDLINE, Embase, PsycINFO, clinicaltrials.gov, and the World Health Organization (WHO) platform, from inception to 28 November 2016. We handsearched reference lists of articles retrieved by the search.
Selection criteria
We included randomised controlled trials (RCTs) published in all languages that examined effects of PRMs for treatment of symptomatic endometriosis.
Data collection and analysis
We used standard methodological procedures as expected by the Cochrane Collaboration. Primary outcomes included measures of pain and side effects.
Main results
We included 10 randomised controlled trials (RCTs) with 960 women. Two RCTs compared mifepristone versus placebo or versus a different dose of mifepristone, one RCT compared asoprisnil versus placebo, one compared ulipristal versus leuprolide acetate, and four compared gestrinone versus danazol, gonadotropin‐releasing hormone (GnRH) analogues, or a different dose of gestrinone. The quality of evidence ranged from high to very low. The main limitations were serious risk of bias (associated with poor reporting of methods and high or unclear rates of attrition in most studies), very serious imprecision (associated with low event rates and wide confidence intervals), and indirectness (outcome assessed in a select subgroup of participants).
Mifepristone versus placebo
One study made this comparison and reported rates of painful symptoms among women who reported symptoms at baseline.
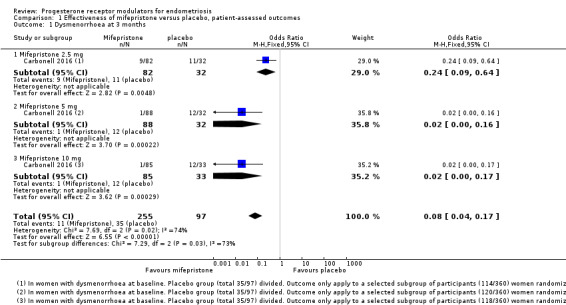
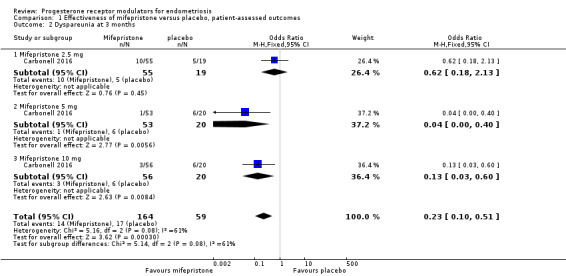
At three months, the mifepristone group had lower rates of dysmenorrhoea (odds ratio (OR) 0.08, 95% confidence interval (CI) 0.04 to 0.17; one RCT, n =352; moderate‐quality evidence), suggesting that if 40% of women taking placebo experience dysmenorrhoea, then between 3% and 10% of women taking mifepristone will do so. The mifepristone group also had lower rates of dyspareunia (OR 0.23, 95% CI 0.11 to 0.51; one RCT, n = 223; low‐quality evidence). However, the mifepristone group had higher rates of side effects: Nearly 90% had amenorrhoea and 24% had hot flushes, although the placebo group reported only one event of each (1%) (high‐quality evidence). Evidence was insufficient to show differences in rates of nausea, vomiting, or fatigue, if present.
Mifepristone dose comparisons
Two studies compared doses of mifepristone and found insufficient evidence to show differences between different doses in terms of effectiveness or safety, if present. However, subgroup analysis of comparisons between mifepristone and placebo suggest that the 2.5 mg dose may be less effective than 5 mg or 10 mg for treating dysmenorrhoea or dyspareunia.
Gestrinone comparisons
Ons study compared gestrinone with danazol, and another study compared gestrinone with leuprolin.
Evidence was insufficient to show differences, if present, between gestrinone and danazol in rate of pain relief (those reporting no or mild pelvic pain) (OR 0.71, 95% CI 0.33 to 1.56; two RCTs, n = 230; very low‐quality evidence), dysmenorrhoea (OR 0.72, 95% CI 0.39 to 1.33; two RCTs, n = 214; very low‐quality evidence), or dyspareunia (OR 0.83, 95% CI 0.37 to 1.86; two RCTs, n = 222; very low‐quality evidence). The gestrinone group had a higher rate of hirsutism (OR 2.63, 95% CI 1.60 to 4.32; two RCTs, n = 302; very low‐quality evidence) and a lower rate of decreased breast size (OR 0.62, 95% CI 0.38 to 0.98; two RCTs, n = 302; low‐quality evidence). Evidence was insufficient to show differences between groups, if present, in rate of hot flushes (OR 0.79, 95% CI 0.50 to 1.26; two RCTs, n = 302; very low‐quality evidence) or acne (OR 1.45, 95% CI 0.90 to 2.33; two RCTs, n = 302; low‐quality evidence).
When researchers compared gestrinone versus leuprolin through measurements on the 1 to 3 verbal rating scale (lower score denotes benefit), the mean dysmenorrhoea score was higher in the gestrinone group (MD 0.35 points, 95% CI 0.12 to 0.58; one RCT, n = 55; low‐quality evidence), but the mean dyspareunia score was lower in this group (MD 0.33 points, 95% CI 0.62 to 0.04; low‐quality evidence). The gestrinone group had lower rates of amenorrhoea (OR 0.04, 95% CI 0.01 to 0.38; one RCT, n = 49; low‐quality evidence) and hot flushes (OR 0.20, 95% CI 0.06 to 0.63; one study, n = 55; low quality evidence) but higher rates of spotting or bleeding (OR 22.92, 95% CI 2.64 to 198.66; one RCT, n = 49; low‐quality evidence).
Evidence was insufficient to show differences in effectiveness or safety between different doses of gestrinone, if present.
Asoprisnil versus placebo
One study (n = 130) made this comparison but did not report data suitable for analysis.
Ulipristal versus leuprolide acetate
One study (n = 38) made this comparison but did not report data suitable for analysis.
Authors' conclusions
Among women with endometriosis, moderate‐quality evidence shows that mifepristone relieves dysmenorrhoea, and low‐quality evidence suggests that this agent relieves dyspareunia, although amenorrhoea and hot flushes are common side effects. Data on dosage were inconclusive, although they suggest that the 2.5 mg dose of mifepristone may be less effective than higher doses. We found insufficient evidence to permit firm conclusions about the safety and effectiveness of other progesterone receptor modulators.
Keywords: Female; Humans; Danazol; Danazol/therapeutic use; Dysmenorrhea; Dysmenorrhea/drug therapy; Dysmenorrhea/epidemiology; Dyspareunia; Dyspareunia/drug therapy; Dyspareunia/epidemiology; Endometriosis; Endometriosis/drug therapy; Estrenes; Estrenes/therapeutic use; Gestrinone; Gestrinone/adverse effects; Gestrinone/therapeutic use; Gonadotropin‐Releasing Hormone; Gonadotropin‐Releasing Hormone/analogs & derivatives; Hormone Antagonists; Hormone Antagonists/administration & dosage; Hormone Antagonists/adverse effects; Hormone Antagonists/therapeutic use; Leuprolide; Leuprolide/adverse effects; Leuprolide/therapeutic use; Mifepristone; Mifepristone/administration & dosage; Mifepristone/adverse effects; Mifepristone/therapeutic use; Norpregnadienes; Norpregnadienes/therapeutic use; Oximes; Oximes/therapeutic use; Prevalence; Randomized Controlled Trials as Topic; Receptors, Progesterone; Receptors, Progesterone/antagonists & inhibitors
Plain language summary
Progesterone receptor modulators for endometriosis
Review question
Researchers in the Cochrane Collaboration reviewed evidence on the effectiveness and safety of progesterone receptor modulators for women with endometriosis.
Background
Endometriosis is a disease of endometrial tissue (glands and stroma) outside the uterine cavity. It is oestrogen‐dependent and thus is seen primarily during the reproductive years. It can cause pain in the abdomen, generally during periods (menstruation) or associated with sexual intercourse. Progesterone receptor modulators have been advocated as one of the hormonal treatments for endometriosis.
Study characteristics
We found 10 randomised controlled trials including 960 women; the evidence is current to November 2016.
Key results
Three studies assessed mifepristone. Moderate‐quality evidence shows that mifepristone relieves dysmenorrhoea (painful periods) in women with endometriosis. Evidence suggests that if 40% of women taking placebo experience dysmenorrhoea, then between 3% and 10% of women taking mifepristone will do so. Low‐quality evidence suggests that mifepristone also relieves dyspareunia (pain during sexual intercourse). However, amenorrhoea (absence of menstrual periods) and hot flushes were common side effects of mifepristone. Nearly 90% of the mifepristone group had amenorrhoea, and 24% had hot flushes, although researchers reported only one event of each (1%) among women taking placebo. Evidence was insufficient to show differences in rates of nausea, vomiting, or fatigue, if present.
Comparisons of different doses of mifepristone were inconclusive, although evidence suggests that the 2.5 mg dose may be less effective than higher doses.
Other studies assessed other progesterone receptor modulators. Researchers compared gestrinone versus other treatments (danazol or leuprolin), ulipristal versus leuprolide acetate, and asoprisnil verus placebo. However, evidence was insufficient to allow firm conclusions regarding the safety and effectiveness of these interventions.
Quality of the evidence
The quality of the evidence ranged from moderate to low. The main limitations were serious risk of bias (associated with poor reporting of methods and high or unclear rates of attrition in most studies), very serious imprecision (associated with low event rates and wide confidence intervals), and indirectness (outcome assessed in a select subgroup of participants).
Summary of findings
Summary of findings for the main comparison. Mifepristone versus placebo for endometriosis.
| Mifepristone versus placebo for endometriosis | ||||||
|
Patient or population: women with symptomatic endometriosis Settings: gynaecology clinic Intervention: progesterone receptor modulator (mifepristone) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Mifepristone | |||||
|
Prevalence of dysmenorrhoea Follow‐up: 3 months |
402 per 1000 | 51 per 1000 (26 to 103) | OR 0.08 (0.04 to 0.17) | 352 (1) | ⊕⊕⊕⊝ Moderatea,b | |
|
Prevalence of dyspareunia Follow‐up: 3 months |
288 per 1000 | 85 per 1000 (43 to 171) | OR 0.23 (0.10 to 0.51) | 223 (1) | ⊕⊕⊝⊝ Lowa,c | |
|
Side effects: amenorrhoea Follow‐up: 3 months |
11 per 1000 | 884 per 1000 (507 to 983) | OR 686.16 (92.29 to 5101.33) | 360 (1) | ⊕⊕⊕⊝ High |
239/270 events in the mifepristone group vs 1/90 in the placebo group |
|
Side effects: hot flushes Follow‐up: 3 months |
11 per 1000 | 243 per 1000 (42 to 701) | OR 28.79 (3.93 to 210.73) | 360 (1) | ⊕⊕⊕⊝ High |
66/270 events in the mifepristone group vs 1/90 in the placebo group |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aDowngraded one level for serious imprecision (wide confidence intervals and/or very few events)
bOutcome applied only to women with dysmenorrhoea at baseline, but this was 352/360 women randomised, so not downgraded for indirectness
cOutcome applied only to women with dyspareunia at baseline, which was 223/360 women randomised. Downgraded one level for serious indirectness
Summary of findings 2. Gestrinone versus danazol for endometriosis.
| Gestrinone versus danazol for endometriosis | ||||||
|
Patient or population: women with symptomatic endometriosis Settings: gynaecology clinic Intervention: progesterone receptor modulator (gestrinone) Comparison: danazol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Danazol | Gestrinone | |||||
| Pelvic pain: none or mild | 890 per 1000 | 852 per 1000 (727 to 927) |
OR 0.71 (0.33 to 1.56) |
230 (2) |
⊕⊕⊝⊝ Very lowa,b,c | |
|
Dysmenorrhoea: none or mild Follow‐up: 6 months |
721 per 1000 | 650 per 1000 (502 to 775) | OR 0.72 (0.39 to 1.33) | 214 (2) | ⊕⊝⊝⊝ Very lowa,b,c | |
|
Dyspareunia: none or mild Follow‐up: 6 months |
889 per 1000 | 869 per 1000 (748 to 937) | OR 0.83 (0.37 to 1.86) | 222 (2) | ⊕⊝⊝⊝ Very lowa,b,c | |
|
Side effects: hirsutism Follow‐up: 6 months |
248 per 1000 | 464 per 1000 (345 to 587) | OR 2.63 (1.60 to 4.32) | 302 (2) | ⊕⊝⊝⊝ Very lowb,c,d | I2 = 68% |
|
Decreased breast size Follow‐up: 6 months |
477 per 1000 | 360 per 1000 (257 to 472) | OR 0.62 (0.38 to 0.98) | 302 (2) | ⊕⊕⊝⊝ Lowb,c | |
|
Side effects: hot flushes Follow‐up: 6 months |
425 per 1000 | 368 per 1000 (270 to 482) |
OR 0.79 (0.50 to 1.26) |
302 (2 studies) |
⊕⊝⊝⊝ Very lowb,c,d | I2 = 72% |
|
Side effects: acne Follow‐up: 6 months |
556 per 1000 | 644 per 1000 (529 to 744) |
OR 1.45 (0.90 to 2.33) |
302 (2 studies) |
⊕⊕⊝⊝ Lowb,c | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aAssessed in all randomised participants. Not all were symptomatic at baseline (although results show no significant differences in baseline symptoms between groups). Outcome therefore applies only to a select subgroup of participants: downgraded one level for serious indirectness
bDowngraded one level for serious risk of bias associated with poor reporting of study methods, high attrition in one study, and high risk of other bias in both studies
cImprecision of results (wide confidence intervals and/or few events), downgraded one level for serious imprecision
dDowngraded one level for serious inconsistency
Summary of findings 3. Gestrinone versus leuprolin for endometriosis.
| Gestrinone versus leuprolin for endometriosis | ||||||
|
Patient or population: women with symptomatic endometriosis Settings: gynaecology clinic Intervention: progesterone receptor modulator (gestrinone) Comparison: leuprolin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Leuprolin | Gestrinone | |||||
|
Dysmenorrhoea, verbal rating scale Follow‐up: 6 months |
In control group, mean score for dysmenorrhoea on verbal rating scale was 0.04 points | Mean score in gestrinone group was 0.35 points higher (0.12 to 0.58 higher) | 55 (1) | ⊕⊕⊝⊝ Lowa | Verbal rating scale defines dysmenorrhoea according to limitation of ability to work (mild = 1, moderate = 2, incapacitated = 3) | |
|
Dyspareunia, verbal rating scale Follow‐up: 6 months |
In control group, mean score for dyspareunia on verbal rating scale was 0.43 points | Mean score in gestrinone group was 0.33 points lower (0.62 to 0.04 lower) | 52 (1) | ⊕⊕⊝⊝ Lowa | Verbal rating scale defines dyspareunia according to limitation of sexual activity (discomfort tolerated = 1; pain interrupts intercourse = 2, intercourse avoided owing to pain = 3) | |
|
Amenorrhoea Follow‐up: 6 months |
962 per 1000 | 500 per 1000 (26 to 908) | OR 0.04 (0.01 to 0.38) | 49 (1) | ⊕⊕⊝⊝ Lowb | Only 55 events overall |
|
Spotting or bleeding Follow‐up: 6 months |
38 per 1000 | 475 per 1000 (94 to 887) | OR 22.92 (2.64 to 198.66) | 49 (1) | ⊕⊕⊝⊝ Lowb | Only 12 events overall |
|
Side effects: hot flushes Follow‐up: 6 months |
679 per 1000 | 297 per 1000 (112 to 571) |
OR 0.20 (0.06 to 0.63) |
55 (1) | ⊕⊕⊝⊝ Lowb | Only 27 events overall |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aDowngraded two levels for very serious imprecision: confidence intervals were compatible with no clinically meaningful difference between groups, or with small benefit in one group
bDowngraded two levels for very serious imprecision: small overall sample size (n = 55) and low event rates
Background
Description of the condition
Endometriosis is defined as the presence of endometrial tissue (glands and stroma) outside the uterine cavity. It is a condition that is oestrogen‐dependent and thus is seen primarily during the reproductive period (Berek 2007; Giudice 2010). The prevalence of endometriosis ranges from 6% to 10% in women of reproductive age, from 50% to 60% in women and teenage girls with pelvic pain, and has been reported as up to 50% among women with infertility (Cramer 2002; Eskenazi 1997; Goldstein 1980); endometriosis can also be found in asymptomatic women during surgical procedures such as laparoscopy or sterilisation. Endometriosis constitutes a substantial burden on the health‐related quality of life of women and on the finite healthcare resources of national health systems worldwide (Vercellini 2014).
Rokitansky reported this disease for the first time in 1860. Although considerable progress has been made, the pathogenesis remains unclear. The 'retrograde menstruation theory' proposed in the 1920s (Sampson 1927) speculated that endometriosis derives from reflux of endometrial fragments regurgitated through the fallopian tubes during menstruation, with subsequent implantation on the peritoneum and the ovary. Hormonal imbalance involved in development of endometriosis includes increased oestrogen synthesis and metabolism and progesterone resistance (Tosti 2016).
Minimally invasive surgical treatments play an important role in the diagnosis and removal of endometriosis. Unfortunately, this approach is limited by recurrences of endometriosis. Goals of management are to provide pain relief, to limit recurrences, and to restore or preserve fertility when needed. Current medical treatment (Brown 2014) has focused on blocking ovarian oestrogen secretion (with gonadotropin‐releasing hormone analogues or antagonists (GnRH‐a or GnRH antagonists)) or halting oestrogen‐induced growth of ectopic endometrium (with oral contraceptives and androgens). The downside of these approaches includes a significant decrease in bone mass, along with hot flushes, vaginal dryness, acne, and hirsutism, which impair quality of life for many women. Furthermore, the mean length of time before recurrence of pain after completion of medical therapy is between 6 and 18 months (Mahutte 2003). The ideal medical therapy for endometriosis should relieve pain and inhibit endometrial proliferation while avoiding a hypo‐oestrogenic state.
Description of the intervention
Progesterone, acting via its cognate receptors, plays a central role in regulation of uterine function, making progesterone receptor an attractive therapeutic target. Progesterone receptor modulators (PRMs) represent a class of synthetic ligands that can exert agonist, antagonist, or mixed effects on various progesterone target tissues. Mifepristone (MFP, RU486) is a progesterone and glucocorticoid receptor antagonist that was first synthesised in 1980. Since that time, numerous related compounds of progesterone receptor ligands have been synthesised, exhibiting a spectrum of activity ranging from pure progesterone receptor antagonists (PRAs) to mixed agonists/antagonists. PRAs and partial agonist antagonists ‐ selective progesterone receptor modulators (SPRMs) ‐ belong to the large progesterone receptor ligand family.
Owing to their antiproliferative effects in the endometrium, PRMs have been advocated for treatment of symptomatic endometriosis but may not provide appropriate treatment for asymptomatic endometriosis‐associated infertility. Unlike long‐acting GnRH‐a, PRMs are not associated with a decrease in bone mineral density, and their use is accompanied by an increase in oestradiol and progesterone receptors (Neulen 1996), suggesting that the endometrial antiproliferative effect is due to progesterone antagonism. This offers a new alternative: suppression of endometrial proliferation in the midst of an oestrogenic environment. To date, compared with the widely used steroidal progesterone receptor agonists, only three PRMs have been approved by the FDA as emergency contraceptives: RU486, ulipristal acetate (UPA or CDB 2914), and asoprisnil (J867) (Li 2016). Few studies of PRMs for endometriosis have been published, and some show clinical benefit (Chwalisz 2004; Kettel 1996; Kettel 1998). In an uncontrolled study, mifepristone relieved pelvic pain without producing significant side effects (Koide 1998). However, it is reported that mifepristone caused downregulation of progesterone receptors (PRs) and oestrogen receptors (ERs) and upregulation of androgen receptors (ARs) (Narvekar 2004), and in low daily doses, it inhibited ovulation and induced amenorrhoea in most women (Brown 2002). Administration of PRMs also has been found to be accompanied by morphological changes within the endometrium, described as PRM‐associated endometrial changes (PAECs) (Williams 2007). These histological changes are recognised as a distinct histological entity, and the mechanisms by which they develop are poorly understood. The clinical effectiveness of PRMs remains to be evaluated.
How the intervention might work
Progesterone receptor antagonists block the action of progesterone. Therefore, it is not surprising that they have clinical application in the medical termination of pregnancy (Brenner 2002; Brenner 2005). PRAs work as antiprogestins ‐ substances that prevent cells from making or using progesterone. SPRMs represent a new class of progesterone receptor ligands that exert clinically relevant tissue‐selective progesterone agonist, antagonist, or partial (mixed) agonist/antagonist effects on various progesterone target tissues in an in vivo situation, depending on the biological action studied (Spitz 2003). In human cell lines, mifepristone, asoprisnil, ulipristal acetate, lonaprisan, and telapristone acetate suppress endometrial proliferation, resulting in endometrial atrophy. Additional studies on animal models show that mifepristone, onapristone, and ZK136799 suppressed endometrial growth and reduced the production of prostaglandins with possible benefits for pain (Bouchard 2011). As PRMs have a relatively minor effect on serum oestradiol and androgen levels, application of PRMs may have greater efficacy and flexibility than traditional treatments for endometriosis as the result of selective inhibition of endometrial proliferation without systemic effects of oestrogen deprivation. PRMs also have the potential to suppress endometrial prostaglandin and decrease menstruation; this mechanism is proposed to reduce endometriosis‐related pain (Tosti 2016).
Why it is important to do this review
Until now, no systematic review has examined this topic. Doctors considering the use of PRMs in clinical practice are uncertain about the balance between benefit in terms of pain relief and unacceptable side effects. In this review, we evaluated the clinical effectiveness and safety of PRMs, primarily in terms of pain relief, and collated the best evidence for their use as single agents in women with endometriosis.
Objectives
To assess the effectiveness and safety of PRMs primarily in terms of pain relief as compared with other treatments or placebo or no treatment in women of reproductive age with endometriosis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) published in all languages that examined effects of PRMs for treatment of symptomatic endometriosis. We excluded quasi‐RCTs from this review. We also excluded cross‐over trials, as the design is not valid in this context.
Types of participants
Women of reproductive age who have symptoms ascribed to the diagnosis of endometriosis. The diagnosis must have been established during a surgical procedure performed before the start of treatment. We did not consider trials that included women with asymptomatic disease or infertility alone.
Types of interventions
We included trials comparing PRMs versus any other drug therapy or placebo or no treatment. We also included studies that compared doses or regimens of PRMs.
We did not consider studies involving surgical removal of pelvic organs, nor studies exploring the use of hormonal treatment as an adjunct to therapeutic surgery or other medical treatment for endometriosis.
Types of outcome measures
Primary outcomes
Pain measures: relief of endometriosis‐related pain at completion of therapy (decrease in pain scores, dysmenorrhoea, and dyspareunia) or other pain‐related outcomes, as reported by included studies
Side effects that occur during therapy (Including fatigue, nausea, anorexia, vomiting, weight loss, skin rash, amenorrhoea, endometrial hyperplasia, or hypoadrenalism)
Secondary outcomes
Quality of life (QOL). If studies report use of more than one scale, preference is given to the Short Form (SF)‐36, then to other validated generic scales, and finally to condition‐specific scales
Change in size and extent of endometrial cysts
Change in cancer antigen 125 (CA125)
Recurrence rates (percentage of women with recurrence)
Side effects that persist after treatment
Search methods for identification of studies
Electronic searches
We developed a comprehensive literature search strategy in consultation with the Information Specialist of the Cochrane Gynaecology and Fertility Group (CGFG). Two review authors (JF and HS) independently conducted a systematic search of the published and unpublished literature from inception of the databases to 28 November 2016 with no restrictions on language or publication status.
We searched the following databases.
CGFG Specialised Register (Appendix 1).
Central Register of Studies (CRS Online) (Appendix 2).
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily, and Ovid MEDLINE(R) (Appendix 3).
Ovid Embase (Appendix 4).
Ovid PsycINFO (Appendix 5).
Searching other resources
We scanned the references of all included studies and relevant reviews to identify additional relevant trials, and we handsearched relevant journals and articles that may not be included in the electronic databases. We also scanned the following databases from inception to 28 November 2016.
Trial registers for ongoing and registered trials.
http://www.clinicaltrials.gov (a service of the US National Institutes of Health).
http://www.who.int/trialsearch/Default.aspx (World Health Organization International Trials Registry Platform search portal).
DARE (Database of Abstracts of Reviews of Effects) in the Cochrane Library (http://onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html) for reference lists from relevant non‐Cochrane reviews.
Web of Knowledge (http://wokinfo.com/) (another source of trials and conference abstracts).
OpenGrey (http://www.opengrey.eu/) for unpublished literature from Europe.
LILACS (Latin American Caribbean Health Sciences Literature) (http://regional.bvsalud.org/php/index.php?lang=en).
PubMed and Google for recent trials not yet indexed on MEDLINE.
Data collection and analysis
Selection of studies
We downloaded to a reference management database (Endnote) all titles and abstracts retrieved by electronic searching and removed duplicates; two review authors (JF and YW) independently examined the remaining references. We excluded studies that clearly do not meet the inclusion criteria and obtained copies of the full text of potentially relevant references. Two review authors (MZ and HZ) independently assessed the eligibility of retrieved papers. Review authors resolved differences by discussion or by appeal to a third review author (WH), if required. We documented reasons for exclusion and presented the selection process in a PRISMA flow chart.
Data extraction and management
Two review authors (JFand HZ) independently extracted data from eligible studies using a data extraction form that they designed and pilot‐tested.
Data extracted include:
participant characteristics;
number of participants in each arm of the trial;
number excluded from analysis;
type of intervention;
proportion of participants who received all/part/none of the intended treatment;
methods of randomization, blinding, and allocation concealment applied;
length of follow‐up; and
outcome measurements.
We resolved differences between review authors by discussion or by appeal to a third review author (WH), if necessary. Review authors were not blinded to article, author, or journal title, and tried to contact trial authors for further information and updated data.
Assessment of risk of bias in included studies
Two review authors (JF and HS) independently assessed the methodological risk of bias of each trial according to the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions, Section 8.5 (Higgins 2011). According to the Cochrane 'Risk of bias' assessment tool, assessment for risk of bias in included studies consists of six domains ‐ random sequence generation and allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias); and other sources of bias (other bias) ‐ and yields a judgement of low risk, high risk, or unclear risk. We resolved differences by discussion among review authors or by consultation with the CGFG.
Measures of treatment effect
For dichotomous data (e.g. recurrence rates), we used numbers of events in control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios. If similar outcomes were reported on different scales, we calculated standardized mean differences. We treated ordinal data (e.g. pain scores) as continuous data and presented 95% confidence intervals for all outcomes.
Unit of analysis issues
We conducted the primary analysis per woman randomised.
Dealing with missing data
We analyzed data on an intention‐to‐treat basis as far as possible and attempted to obtain missing data from the original investigators. If studies reported sufficient detail for calculation of mean differences but no information on associated standard deviation (SD), we planned to assume that outcomes had a standard deviation equal to the highest standard deviation used for other studies within the same analysis. Otherwise, we analyzed only available data. We found that no imputation was necessary.
Assessment of heterogeneity
We assessed heterogeneity between studies by visually inspecting forest plots and by estimating the I2 value, which summarises the percentage of heterogeneity between trials that cannot be ascribed to sampling variation. We will consider an I2 < 25% to show heterogeneity of low level, 25% to 50% moderate level, and > 50% high level. If we found evidence of substantial heterogeneity in later updates, we considered possible reasons for it. We did not combine results of trials using different comparator drugs.
Assessment of reporting biases
In view of the difficulty involved in detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by staying alert for duplication of data. If we included 10 or more studies in an analysis, we planned to use a funnel plot to explore the possibility of a small‐study effect (tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We considered the following comparisons.
We combined data from primary studies using a fixed‐effect model for the following.
PRMs versus placebo, stratified by dose.
PRMs versus no treatment, stratified by dose.
PRMs versus other medical therapies, stratified by dose (danazol, GnRH analogue, combined oral contraceptive pill (OCP), levonorgestrel‐releasing intrauterine system, each in a separate analysis, not stratified).
Dose or regimen comparison of PRMs.
In the meta‐analyses, we will display graphically to the right of the centre line an increase in the odds of a particular outcome that may be beneficial (e.g. pain relief) or detrimental (e.g. adverse effects), and we will display to the left of the centre line a decrease in the odds of a particular outcome.
For Comparison 1 (PRMs vs placebo), two analyses showed that the event rate was too low in control groups to allow review authors to split the group for the purpose of stratification. Therefore, we pooled all data in a single analysis and reported a separate analysis stratified by dose but not pooled (this applied to the outcomes 'amenorrhoea at 3 months' and 'hot flushes at 3 months').
Subgroup analysis and investigation of heterogeneity
We planned to explore the following potential sources of heterogeneity by conducting subgroup analyses: different mode of administration and different course.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcomes to determine whether conclusions were robust to arbitrary decisions made regarding trial eligibility and analysis of results. Through these analyses, we planned to consider whether conclusions would have differed if:
eligibility were restricted to studies without high risk of bias;
studies with outlying results had been excluded; or
a random‐effects model had been adopted.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro and Cochrane methods. This table presents our evaluation of the overall quality of the body of evidence for the main review outcomes ('Relief in endometriosis‐related pain', 'Side effects occurring during therapy') for the main review comparison ('Mifeprostone vs placebo'). We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. Two review authors independently judged evidence quality (as high, moderate, low, or very low) and resolved disagreements by discussion. We justified judgements and documented and incorporated them into reporting of results for each outcome.
Results
Description of studies
Results of the search
Through the search, we retrieved 353 articles. We retrieved in full text 19 that were potentially eligible. Ten articles met our inclusion criteria. We excluded nine studies. (See Characteristics of included studies, Characteristics of excluded studies, and the PRISMA flow chart – Figure 1.) We tried to contact trial authors to request information including missing data. We reported in Table 4 data that were unsuitable for analysis.
1.
Study flow diagram.
1. Data unsuitable for analysis.
| Outcome | Study | Comparison | Measure | Int group | Control group | P value | |
| Combined non‐pelvic pain, dysmenorrhoea, and dyspareunia |
Chwalisz 2004 | Asoprisnil: 5 mg (n = 31), 10 mg (n = 33), 25 mg (n = 32) Placebo (n = 34) |
Mean reduction at 3 months on 0‐4 pain scale |
0.5 points for each dose | < 0.1 points | < 0.05 |
Included studies
Study design and setting
We included in the review eight RCTs (Bromham 1995; Carbonell 2012; Carbonell 2016; Chwalisz 2004; Fedele 1989; GISG 1996; Hornstein 1990; Spitz 2009). We included two other RCTs (Dawood 1997; Worthington 1993) that did not report the primary and secondary outcomes of our review.
Participants
We included 960 women with endometriosis confirmed by laparoscopy.
Interventions
Carbonell 2016 and Carbonell 2012 compared different dosages of mifepristone for laparoscopically confirmed endometriosis, Chwalisz 2004 compared three doses of asoprisnil with placebo, and Spitz 2009 compared three doses of ulipristal with leuprolide acetate. Bromham 1995 and Fedele 1989 compared gestrinone with danazol, GISG 1996 compared gestrinone with leuprolin depot, and Hornstein 1990 compared different dosages of gestrinone.
Outcomes
Eight studies reported one or more of our primary outcomes. Investigators reported some specific outcomes only in women who were symptomatic at baseline.
Excluded studies
We excluded nine studies from the review. Bulun 2016,Kettel 1996, and Kettel 1998 are not RCTs; Mettler 1987, Nieto 1996,Yang 2006, and Zhang 2016 provided interventions that were a mixture of surgical and medical therapy; Nobel 1980 compared danazol with an OCP; and Thomas 1987 concentrated on asymptomatic endometriosis and its effects on fertility.
Risk of bias in included studies
Refer to the 'Risk of bias' tables and Figure 2 and Figure 3.
2.
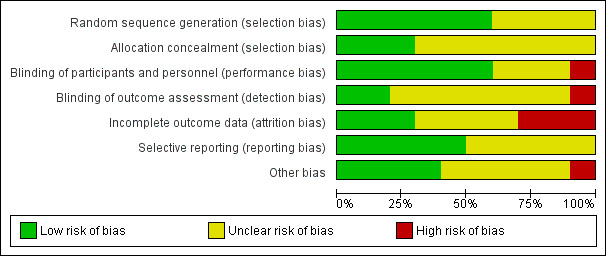
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
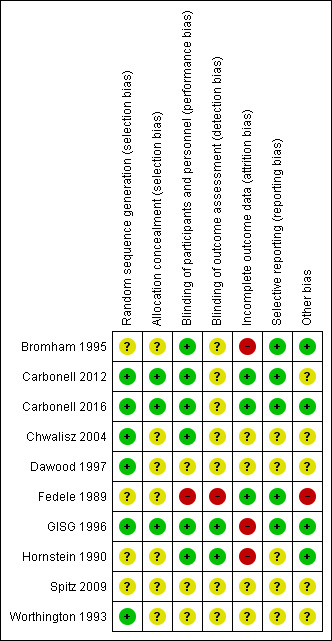
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Carbonell 2016 used a random list obtained from the MEDSTAT 2.1 programme (low risk of bias). Carbonell 2012 used a computer‐generated randomization process (low risk of bias); Chwalisz 2004 and GISG 1996 included details of their randomization process and were at low risk of this bias. Bromham 1995, Fedele 1989, Hornstein 1990, and Spitz 2009 did not describe the method used and were at unclear risk of bias for this domain.
Allocation concealment
Carbonell 2016 applied opaque sealed envelopes that contained a card indicating the treatment group to which the participant was assigned (low risk of bias). Carbonell 2012 applied opaque sealed envelopes that contained a card indicating the treatment group to which the participant was assigned (low risk of bias). GISG 1996 also included allocation concealment in the study design and was at low risk of selection bias. The other seven studies did not describe the method used and were at unclear risk of bias for this domain.
Blinding
We rated six studies as having low risk of performance bias (Bromham 1995; Carbonell 2012; Carbonell 2016; Chwalisz 2004; GISG 1996; Hornstein 1990) and two as having low risk of detection bias (GISG 1996; Hornstein 1990). Fedele 1989 was unblinded, and we rated it as having high risk of performance bias and detection bias. Other studies did not clearly describe blinding procedures, and we rated them as having unclear risk of bias in one or both of these domains.
Incomplete outcome data
Carbonell 2016 and Carbonell 2012 analyzed all women randomised, and we judged them to be at low risk of attrition bias. Three studies reported large losses to follow‐up (> 10%), and we rated them as having high risk of attrition bias (Bromham 1995;GISG 1996;Hornstein 1990). Other studies did not clearly state attrition rates, and we rated them as having unclear risk.
Selective reporting
Five studies reported our main review outcomes, and we rated them as having low risk of selective reporting (Bromham 1995; Carbonell 2012, Carbonell 2016; Fedele 1989; GISG 1996). We rated other studies as having unclear risk of selective reporting, as they reported insufficient data for review authors to make a judgement.
Other potential sources of bias
We rated four studies as having low risk of other bias (Bromham 1995; Carbonell 2016; GISG 1996; Hornstein 1990). We rated one study (Fedele 1989) as having high risk because researchers amended drug doses during the trial, and only seven participants given gestrinone and nine taking danazol had repeat laparoscopy. We rated the other studies as having unclear risk of bias in this domain because information was insufficient for review authors to make a judgement as to other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
1. Mifepristone
Carbonell 2016 compared three doses of mifepristone (2.5 mg, 5 mg, 10 mg) versus placebo for three months and as a dose comparison (without placebo) for six months. Carbonell 2012 compared 5 mg versus 25 mg of mifepristone for six months.
1.1 Mifepristone versus placebo
Primary outcomes
1.1.1 Pain measures
One study (Carbonell 2016 ) reported rates of patient‐assessed dysmenorrhoea or dyspareunia (respectively) at three months among women who reported these symptoms at baseline.
Symptoms were less common in the mifepristone group for both dysmenorrhoea (OR 0.08, 95% CI 0.04 to 0.17; one RCT, n = 352; moderate‐quality evidence; Figure 4) and dyspareunia (OR 0.23, 95% CI 0.10 to 0.51; one RCT, n = 223; low‐quality evidence).
4.
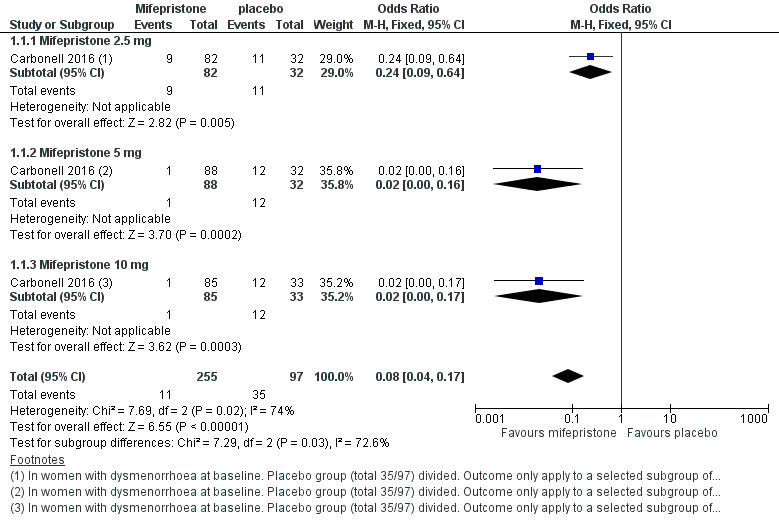
Forest plot of comparison: 1 Effectiveness of mifepristone versus placebo, patient‐assessed outcomes, outcome: 1.1 Dysmenorrhoea at three months.
Subgroup analysis
We stratified the data by dose of mifepristone (2.5 mg, 5 mg, 10 mg).
With respect to dysmenorrhoea, all three doses were more effective than placebo, but the test for subgroup differences indicated a significant difference in effectiveness between doses (Chi2 = 7.29, df = 2 (P = 0.03), I2 = 72.6%). When we excluded the 2.5 mg dose from the analysis, the effect estimate showed increased benefit for mifepristone (OR 0.02, 95% CI 0.00 to 0.09), and the test for subgroup differences showed no evidence of a difference between groups (Chi2 = 0.00, df = 1 (P = 0.96), I2 = 0%). This suggests that 5 mg and 10 mg doses are more effective than the 2.5 mg dose, and that results show no clear difference in effectiveness between 5 mg and 10 mg doses.
With respect to dyspareunia, all three doses were more effective than placebo, but the test for subgroup differences indicated a significant difference in effectiveness between doses (Chi2 = 3.68, df = 2 (P = 0.16), I2 = 45.6%). When we exclude the 2.5 mg dose from the analysis, the effect estimate shows increased benefit for mifepristone (OR 0.09, 95% CI 0.03 to 0.30), and the test for subgroup differences shows no evidence of a difference between groups (Chi2 = 0.63, df = 1 (P = 0.43), I2 = 0%). This suggests that 5 mg and 10 mg doses are more effective than the 2.5 mg dose, and that results show no clear difference in effectiveness between 5 mg and 10 mg doses.
1.1.2 Side effects at three months
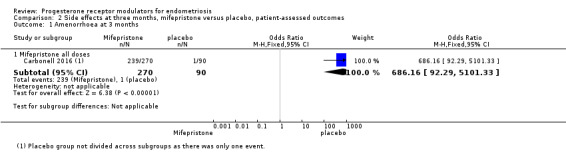
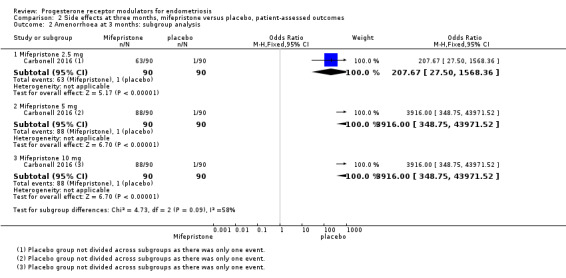
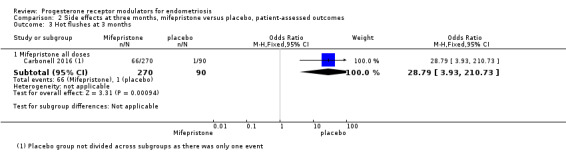
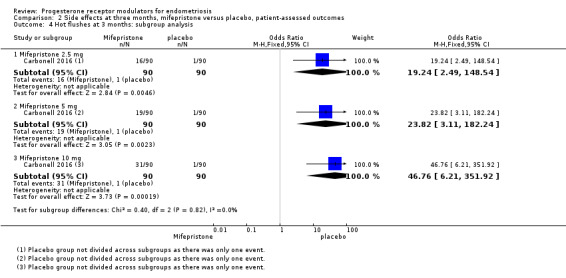
The mifepristone group had higher rates of amenorrhoea (OR 686.16, 95% CI 92.29 to 5101.33; one RCT, n = 360; high‐quality evidence) and hot flushes (OR 28.79, 95% CI 3.93 to 210.73; one RCT, n = 360; high‐quality evidence) at three months of treatment. These CIs are extremely wide because nearly 90% of the mifepristone group had amenorrhoea and 24% had hot flushes, and investigators reported only one event of each (1%) in the placebo group.
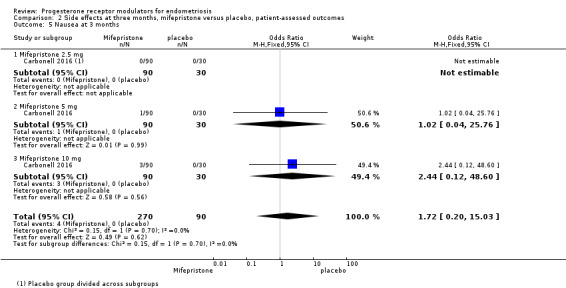
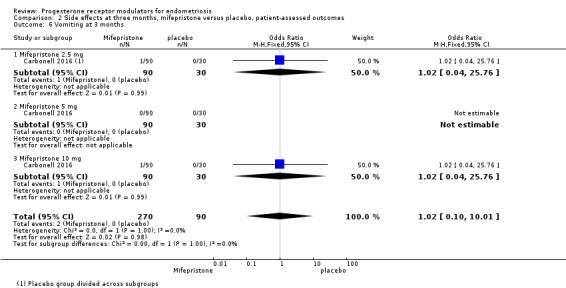
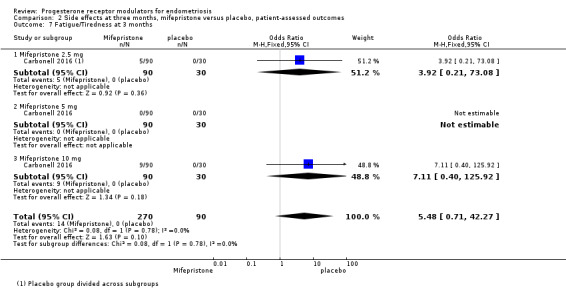
Evidence was insufficient to show differences between groups in rates of nausea (OR 1.72 , 95% CI 0.20 to 15.03; one RCT, n = 360), vomiting (OR 1.02, 95% CI 0.10 to 10.01), or fatigue/tiredness (OR 5.48, 95% CI 0.71 to 42.27; one RCT, n = 360; very low‐quality evidence) at three months, if present. No studies reported endometrial hyperplasia or hypoadrenalism. See Figure 5 and Figure 6.
5.
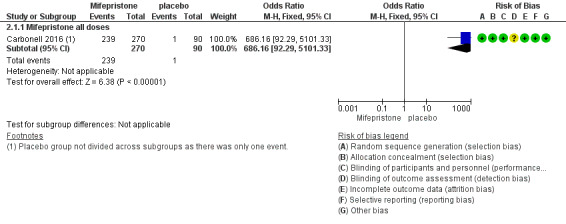
Forest plot of comparison.
2 Mifepristone versus placebo, patient‐assessed outcomes, outcome: 2.1 Amenorrhoea at three months.
6.
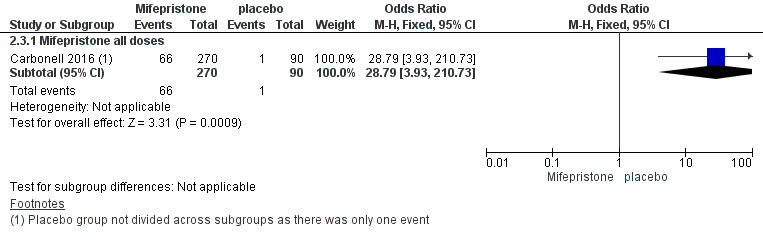
Forest plot of comparison: side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, outcome: 2.2 Hot flushes at three months.
Subgroup analysis
We subgrouped data by dose of mifepristone (2.5 mg, 5 mg, 10 mg).
All three doses were associated with higher rates of amenorrhoea than occurred with placebo, but the test for subgroup differences indicated a significant difference in effectiveness between doses (test for subgroup differences: Chi2 = 4.73, df = 2 (P = 0.09), I2 = 57.7%). When we excluded the 2.5 mg dose from the analysis, the test for subgroup differences showed no evidence of a difference between groups (test for subgroup differences: Chi2 = 0.00, df = 1 (P = 1.00), I2 = 0%). This suggests that 5 mg and 10 mg doses are more likely than the 2.5 mg dose to be associated with amenorrhoea.
For other reported side effects (nausea, vomiting, and fatigue or tiredness), results show no indication of a difference between subgroups (test for subgroup differences, I2 = 0%).
Secondary outcomes
No studies reported on our secondary outcomes.
1.2 Mifepristone dose comparison
Two studies compared different doses of mifepristone at six months of follow‐up. One trial (Carbonell 2016) compared 2.5 mg versus 5 mg and 10 mg, and the other (Carbonell 2012) compared 5 mg versus 25 mg. Carbonell 2016 reported data on effectiveness as well as side effects. Carbonell 2012 provided a graph showing pain scores on a 0 to 10 visual analogue scale (VAS). As investigators reported the standard deviation for only one group, we assumed equal variances in the two groups. Carbonell 2012 also reported data on selected side effects.
Primary outcomes
1.2.1 Pain measures at six months
When we compared lower doses versus higher doses of mifepristone, the data were too imprecise to reveal differences between groups in rates of dysmenorrhoea, dyspareunia, or pelvic pain, as follows.
Dysmenorrhoea (Analysis 3.1;Analysis 3.2).
3.1. Analysis.
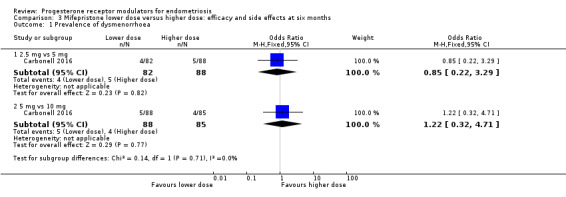
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 1 Prevalence of dysmenorrhoea.
3.2. Analysis.
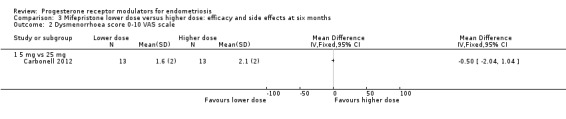
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 2 Dysmenorrhoea score 0‐10 VAS scale.
2.5 mg vs 5 mg: OR 0.85, 95% CI 0.22 to 3.29; one RCT, n = 170; very low‐quality evidence.
5 mg vs 10 mg: OR 1.22, 95% CI 0.32 to 4.71; one RCT, n = 173; very low‐quality evidence.
5 mg vs 25 mg: MD on VAS 0 to 10 scale ‐0.50 points, 96% CI ‐2.04 to 1.04; one RCT, n = 26; very low‐quality evidence).
Dyspareunia (Analysis 3.3;Analysis 3.4).
3.3. Analysis.
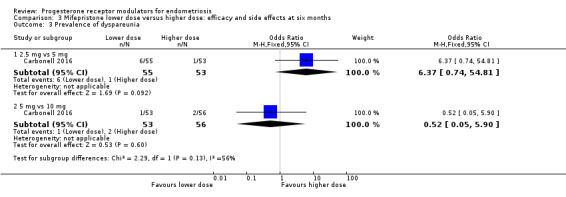
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 3 Prevalence of dyspareunia.
3.4. Analysis.
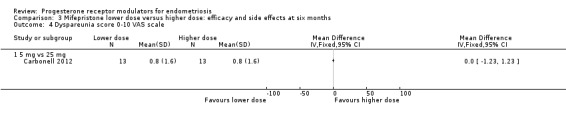
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 4 Dyspareunia score 0‐10 VAS scale.
2.5 mg vs 5 mg: OR 6.37, 95% CI 0.74 to 54.81; one RCT, n = 108; very low‐quality evidence.
5 mg vs 10 mg: OR 0.52, 95% CI 0.05 to 5.90; one RCT, n = 109; very low‐quality evidence.
5 mg vs 25 mg: MD on VAS 0 to 10 scale 0.00 points, 95% CI ‐1.23 to 1.23; n = 26; very low‐quality evidence.
Pelvic pain (Analysis 3.5).
3.5. Analysis.
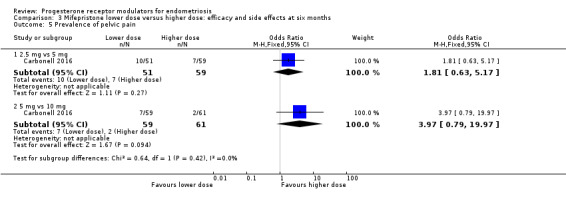
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 5 Prevalence of pelvic pain.
2.5 mg vs 5 mg: OR 1.81, 95% CI 0.63 to 5.17; one RCT, n = 110; very low‐quality evidence.
5 mg vs 10 mg: OR 3.97, 95% CI 0.79 to 19.97; one RCT, n = 120; very low‐quality evidence.
1.2.2 Side effects at six months
When we compared lower doses versus higher doses of mifepristone, amenorrhoea was less common among women taking 2.5 mg than in those taking 5 mg, and fatigue or tiredness was less common among women taking 5 mg than in those taking 10 mg. For other outcomes, the data were too imprecise to show whether differences between groups occurred. Effect estimates were as follows.
Amenorrhoea (Analysis 3.6).
3.6. Analysis.
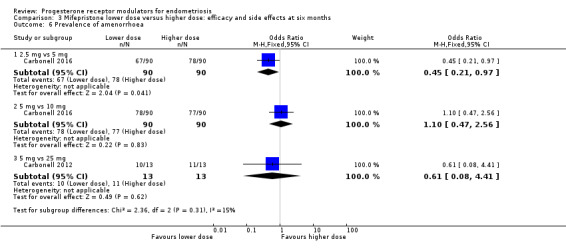
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 6 Prevalence of amenorrhoea.
2.5 mg vs 5 mg: OR 0.45, 95% CI 0.21 to 0.97; n = 180; very low‐quality evidence.
5 mg vs 10 mg: OR 1.10, 95% CI 0.47 to 2.56; n = 180; very low‐quality evidence.
5 mg vs 25 mg: OR 0.61, 95% CI 0.08 to 4.41; n = 52; very low‐quality evidence.
Hot flushes (Analysis 3.7).
3.7. Analysis.
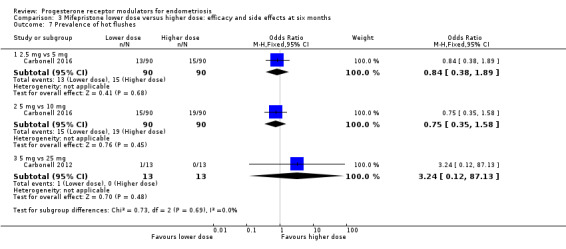
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 7 Prevalence of hot flushes.
2.5 mg vs 5 mg: OR 0.84, 95% CI 0.38 to 1.89; n = 180; very low‐quality evidence.
5 mg vs 10 mg: OR 0.75, 95% CI 0.35 to 1.58; n = 180; very low‐quality evidence.
5 mg vs 25 mg: OR 3.24, 95% CI 0.12 to 87.13; n = 52; very low‐quality evidence.
Nausea (Analysis 3.8).
3.8. Analysis.
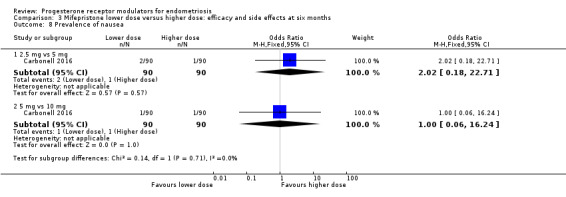
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 8 Prevalence of nausea.
2.5 mg vs 5 mg: OR 2.02, 95% CI 0.18 to 22.71; n = 180; very low‐quality evidence.
5 mg vs 10 mg: OR 1.00, 95% CI 0.06 to 16.24; n = 180; very low‐quality evidence.
Vomiting (Analysis 3.9).
3.9. Analysis.
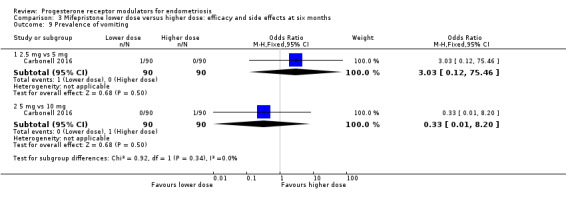
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 9 Prevalence of vomiting.
2.5 mg vs 5 mg: OR 3.03, 95% CI 0.12 to 75.46; n = 180; very low‐quality evidence.
5 mg vs 10 mg: OR 0.33, 95% CI 0.01 to 8.20; n = 180; very low‐quality evidence.
Fatigue/tiredness (Analysis 3.10).
3.10. Analysis.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 10 Prevalence of fatigue/tiredness.
2.5 mg vs 5 mg: OR 13.92, 95% CI 0.77 to 250.94; n = 180; very low‐quality evidence.
5 mg vs 10 mg: OR 0.03, 95% CI 0.00 to 0.54; n = 180; very low‐quality evidence.
Endometrial thickness > 20 mm (Analysis 3.11).
3.11. Analysis.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 11 Prevalence of endometrial thickness > 20 mm.
2.5 mg vs 25 mg: OR 1.41, 95% CI 0.28 to 1.86; n = 52; very low‐quality evidence.
Secondary outcomes
No studies reported on secondary outcomes.
2. Gestrinone
Gestrinone was another PRM assessed in the included trials. No RCTs compared gestrinone versus no treatment or placebo.
2.1 Gestrinone versus danazol
Primary outcomes
2.1.1 Pain measures
Two studies reported this outcome (Bromham 1995; Fedele 1989).
Data were insufficient to show differences between groups, if present, in the rate of pain relief (those reporting no or mild symptoms), with respect to pelvic pain (OR 0.71, 95% CI 0.33 to 1.56; two RCTs, n = 214; I2 = 0%; very low‐quality evidence), dysmenorrhoea (OR 0.72, 95% CI 0.39 to 1.33; two RCTs, n = 214; I2 = N/A; very low‐quality evidence), and dyspareunia (OR 0.83, 95% CI 0.37 to 1.86; two RCTs, n = 222; I2 = 0%; very low‐quality evidence).
2.1.2 Side effects
The gestrinone group had lower rates of decreased breast size (OR 0.62, 95% CI 0.38 to 0.98; two RCTs, n = 302; I2 = 0%; low‐quality evidence), muscle cramps (OR 0.49, 95% CI 0.30 to 0.78; two RCTs, n = 302; I2 = 0%; low‐quality evidence), and hunger (OR 0.59, 95% CI 0.36 to 0.97; one RCT, n = 264; very low‐quality evidence), but higher rates of hirsutism (OR 2.73, 95% CI 1.67 to 4.46; two RCTs, n = 302; I2 = 68%; very low‐quality evidence) and seborrhoea (greasy skin) (OR 2.74, 95% CI 1.69 to 4.46; two RCTs, n = 302; I2 = 65%; low‐quality evidence, respectively).
Evidence was insufficient to show differences between groups, if present, in rates of acne, voice problems, swelling of hands or feet, hot flushes, headache, nausea, vomiting, loss of appetite, dizziness, tiredness, faintness, skin rash, weight gain, vaginal dryness, and raised liver transaminase.
Evidence shows substantial statistical heterogeneity for the outcomes of hirsutism, seborrhoea, and hot flushes (I2= 68%, 65%, 72%). We considered that this was likely due to clinical heterogeneity related to variations in study location and patient population.
No studies reported endometrial hyperplasia or hypoadrenalism.
Secondary outcomes
No studies reported on our secondary outcomes.
2.2 Gestrinone versus leuprolin
Primary outcomes
2.2.1 Pain measures
One study compared gestrinone versus the GnRH analogue leuprolin (GISG 1996). This study reported symptom scores at six months.
When researchers compared gestrinone versus leuprolin, measurements on the 1 to 3 verbal rating scale (lower score denotes benefit) show that the mean dysmenorrhoea score was higher in the gestrinone group (MD 0.35 points, 95% CI 0.12 to 0.58; one RCT, n = 55; low‐quality evidence), but the mean dyspareunia score was lower in the gestrinone group (MD 0.33 points, 95% CI 0.62 to 0.04; low‐quality evidence).
Outcomes measured on a 0 to 10 VAS scale showed a lower mean dysmenorrhoea score in the leuprolin group (MD 0.82 points, 95% CI 0.15 to 1.49) and a higher mean dyspareunia score in the leuprolin group (MD 1.16 points, 95% CI 2.08 to 0.24). (See Figure 7 and Figure 8.)
7.
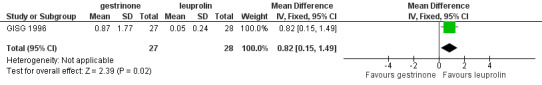
Forest plot of comparison: 7 Gestrinone versus leuprolin for six months: efficacy and side effects, outcome: 7.1 Painful periods, visual analogue scale.
8.
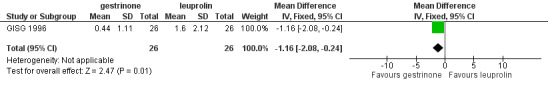
Forest plot of comparison: 7 Gestrinone versus leuprolin for six months: efficacy and side effects, outcome: 7.3 Pain on intercourse, visual analogue scale.
2.2.2 Side effects
The gestrinone group had lower rates of hot flushes (OR 0.20, 95% CI 0.06 to 0.63; one RCT, n = 55; very low‐quality evidence), amenorrhoea (OR 0.04, 95% CI 0.01 to 0.38; one RCT, n = 49; very low‐quality evidence), and headache (OR 0.20, 95% CI 0.06 to 0.63; one RCT, n = 55; very low‐quality evidence), but higher rates of spotting or bleeding (OR 22.92, 95% CI 2.64 to 198.66; one RCT, n = 55; very low‐quality evidence). Evidence was insufficient to show differences between groups, if present, in rates of seborrhoea, swelling hands/feet, leg or muscle cramps, nausea, dizziness, skin rash, vaginal dryness, mood changes, joint pain, drowsiness, and tachycardia. Investigators reported no data on endometrial hyperplasia or hypoadrenalism.
Secondary outcomes
No studies reported on secondary outcomes.
2.3 Gestrinone dose comparison
Primary outcomes
2.2.1 Pain measures
Hornstein 1990 compared two doses of gestrinone (1.25 mg vs 2.5 mg). Evidence was insufficient to show differences between groups, if present, in rates of pain relief (OR 0.27, 95% CI 0.01 to 8.46; one RCT, n = 10; very low‐quality evidence). .
2.2.2 Side effects
Evidence was insufficient to show differences between groups, if present, in rates of any adverse effect (OR 0.04, 95% CI 0.00 to 1.12; one RCT, n = 12; very low‐quality evidence), discontinuation due to headaches (OR 0.28, 95% CI 0.01 to 8.42; one RCT, n = 12), or irregular bleeding (OR 2.5, 95% CI 0.16 to 38.60; one RCT, n = 12). Reported adverse effects included weight gain, headache, palpitations, peripheral oedema, and hirsutism. No studies reported on endometrial hyperplasia or hypoadrenalism.
Secondary outcomes
No studies reported on our secondary outcomes.
3. SPRMs
Two studies compared SPRMs versus placebo (Chwalisz 2004) or leuprolide acetate (Spitz 2009). These studies were presented as abstracts and did not report data suitable for analysis.
3.1 Asoprisnil versus placebo
Chwalisz 2004 (n = 130) compared three different doses of asoprisnil versus placebo.
Primary outcome
3.1.1 Pain measures
Study authors reported that asoprisnil reduced average daily combined non‐menstrual pelvic pain/dysmenorrhoea scores during all treatment months compared with placebo, and that this treatment induced amenorrhoea during the entire treatment period in a dose‐dependent manner (placebo: 0%; 5 mg: 50%; 10 mg: 71%; and 25 mg: 93%).
3.1.2 Side effects
The included study did not report these effects.
Secondary outcomes
The included study did not report these outcomes.
3.2 Uliprisnil versus leuprolide acetate
Spitz 2009 compared six months of treatment with three doses of ulipristal (12.5 mg, 25 mg, and 50 mg) versus leuprolide acetate depot in 38 women.
Primary outcome
3.2.1 Pain measures
Study authors reported that the 50 mg dose of ulipristal was associated with fewer days of pain, less severe pain, and accompanying distress when compared with lower doses, and that it was as effective as leuprolide acetate.
3.1.2 Side effects
The included study did not report these effects.
Secondary outcomes
The included study did not report these outcomes.
Other analyses
Data were too few for review authors to conduct planned subgroup and sensitivity analyses, or to construct a funnel plot to assess reporting bias.
Discussion
Summary of main results
Mifepristone represents relief of dysmenorrhoea and dyspareunia with side effects of amenorrhoea and hot flushes. We found no evidence of benefit of gestrinone compared with other treatments. Data on asoprisnil and ulipristal are limited. Results were based on very limited evidence and thus should be interpreted with caution.
Overall completeness and applicability of evidence
The included studies partially answered the review question. Studies investigated relevant participants and interventions, but future studies must be more unified and specific about study outcomes. Methods of outcome evaluation differed among studies. Progesterone receptor modulators (PRMs) showed effects of relieving pain, but more evidence is needed. Evidence is limited by the small number of comparisons reported and by the small sample size included for each comparison.
Quality of the evidence
Five of eight trials adequately described randomization and allocation concealment, and seven trials reported blinding. Five studies reported on a priori outcomes; two were at unclear risk and one at high risk of reporting bias. The quality of the body of evidence ranged from very low to high.
The main limitations were serious risk of bias (associated with poor reporting of methods and high or unclear rates of attrition in most studies), very serious imprecision with low event rates, small sample sizes, and very wide confidence intervals for most comparisons, as well as indirectness (outcomes assessed in a selected subgroup of participants). Only two studies were at low risk of attrition bias, which is a matter of concern because attrition may well have been related to participant response. The main limitation remains the issue of multiple comparisons with a small number of trials. Results show lack of consistency in outcome measures used, which leads to difficulties in combining data in a suitable meta‐analysis, thus making it difficult for review authors to draw clinically relevant conclusions.
Potential biases in the review process
We aimed to identify all relevant studies by performing comprehensive database searches. Rare side effects of voice problems found in randomised controlled trials (RCTs) examining gestrinone versus danazol may introduce bias. Our choice of multiple measures for primary outcomes (e.g. dysmenorrhoea, dyspareunia, amenorrhoea, hot flushes) could be another potential source of bias, especially as selective reporting is apparent in at least one of the included studies.
Agreements and disagreements with other studies or reviews
The conclusions of other non‐Cochrane reviews (Bouchard 2011; Giudice 2010; Tosti 2016) and of studies that were excluded from this review were substantially the same as the conclusions of this review, and more studies are needed to evaluate the effectiveness and safety of PRMs.
Authors' conclusions
Implications for practice.
Among women with endometriosis, moderate‐quality evidence shows that mifepristone relieves dysmenorrhoea, and low‐quality evidence suggests that this agent also relieves dyspareunia; however, amenorrhoea and hot flushes are common side effects. Data on dosage, although inconclusive, suggest that the 2.5 mg dose may be less effective than higher doses. We found insufficient evidence to permit firm conclusions about the safety and effectiveness of other progesterone receptor modulators.
Implications for research.
Effects of PRM‐associated endometrial change on endometriotic lesions are unclear, and long‐term effects remain to be determined. This systematic review has identified the need for well‐designed, larger RCTs with minimal attrition, to confirm and extend the findings of the trials reviewed here. Studies comparing the relative efficacy and safety of different PRMs including mifepristone, gestrinone, ulipristal, and asoprisnil would provide important clinical evidence. Future studies should report quality of life and pain relief scores (dysmenorrhoea and dyspareunia scores measured on a 0 to 10 visual analogue scale (VAS)).
Acknowledgements
Review authors thank the Cochrane Gynaecology and Fertility Group for its support.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group specialised register search strategy
From inception to 28 November 2016
Procite platform
Keywords CONTAINS "endometriosis" or "Endometriosis‐Symptoms" or "pelvic pain" or "dyspareunia" or Title CONTAINS "endometriosis" or "Endometriosis‐Symptoms" or "pelvic pain" or "dyspareunia"
AND
Keywords CONTAINS "mifepristone"or"RU486"or"gestrinone"or"Onapristone" or "asoprisnil" or "CBD 2914" or "CDB‐2914" or "CDB‐4124" or "Ulipristal" or "ulipristal acetate" or "progestagen receptor levels" or" progesterone receptor agonist" or "Progesterone Receptor Modulator" or "selective progesterone receptor modulator" or Title CONTAINS "mifepristone"or"RU486"or"gestrinone"or"Onapristone" or "asoprisnil" or "CBD 2914" or "CDB‐2914" or "CDB‐4124" or "Ulipristal" or "ulipristal acetate" or "progestagen receptor levels" or" progesterone receptor agonist" or "Progesterone Receptor Modulator" or "selective progesterone receptor modulator" (44 hits)
Appendix 2. CENTRAL CRSO search strategy
Searched 28 November 2016
Web platform
#1 MESH DESCRIPTOR Endometriosis EXPLODE ALL TREES 513
#2 Endometrio*:TI,AB,KY 1289
#3 (pelvi* pain):TI,AB,KY 878
#4 #1 OR #2 OR #3 1934
#5 MESH DESCRIPTOR Mifepristone EXPLODE ALL TREES 376
#6 mifepristone:TI,AB,KY 714
#7 RU486:TI,AB,KY 47
#8 (progest* adj1 antagonist*):TI,AB,KY 68
#9 (progest* adj1 modulator*):TI,AB,KY 33
#10 (ru 486 or ru486):TI,AB,KY 143
#11 ru38486:TI,AB,KY 5
#12 ru‐38486:TI,AB,KY 1
#13 MESH DESCRIPTOR Gestrinone EXPLODE ALL TREES 29
#14 Gestrinone:TI,AB,KY 58
#15 Onapristone:TI,AB,KY 9
#16 Aglepristone:TI,AB,KY 2
#17 anti‐progestin*:TI,AB,KY 2
#18 antiprogestin*:TI,AB,KY 47
#19 Asoprisnil:TI,AB,KY 10
#20 J867:TI,AB,KY 1
#21 Telapristone:TI,AB,KY 1
#22 CDB‐2914:TI,AB,KY 9
#23 Ulipristal:TI,AB,KY 37
#24 (SPRM* or PRAs or PRA):TI,AB,KY 788
#25 MESH DESCRIPTOR Receptors, Progesterone EXPLODE ALL TREES WITH QUALIFIERS AD,AG,AI,ME,TU 159
#26 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 1782
#27 #4 AND #26 65
Appendix 3. MEDLINE search strategy
From 1946 to 28 November 2016
Ovid platform
1 exp Endometriosis/ (19876) 2 Endometri$.tw. (84071) 3 exp Dyspareunia/ (1809) 4 Dyspareunia.tw. (3194) 5 pelvi$ pain.tw. (7650) 6 or/1‐5 (95322) 7 exp Mifepristone/ (5981) 8 mifepristone.tw. (3252) 9 RU486.tw. (2312) 10 (progest$ adj1 antagonist$).tw. (512) 11 (progest$ adj1 modulator$).tw. (11) 12 ru 486.tw. (1780) 13 mifegyne.tw. (13) 14 ru38486.tw. (409) 15 ru‐38486.tw. (465) 16 mifeprex.tw. (12) 17 r38486.tw. (1) 18 exp Gestrinone/ (198) 19 Gestrinone.tw. (192) 20 Lilopristone.tw. (20) 21 Onapristone.tw. (187) 22 Aglepristone.tw. (82) 23 (dimetriose or dimetrose).tw. (1) 24 Nemestran.tw. (4) 25 anti‐progestin$.tw. (151) 26 antiprogestin$.tw. (935) 27 Asoprisnil.tw. (47) 28 J867.tw. (13) 29 Telapristone.tw. (9) 30 CDB‐2914.tw. (48) 31 CDB‐4124.tw. (19) 32 Proellex.tw. (8) 33 Ulipristal.tw. (254) 34 (SPRM$ or PRAs).tw. (340) 35 exp Receptors, Progesterone/me, tu [Metabolism, Therapeutic Use] (9329) 36 or/7‐35 (18327) 37 6 and 36 (2106) 38 randomized controlled trial.pt. (469810) 39 controlled clinical trial.pt. (95074) 40 randomized.ab. (404455) 41 randomised.ab. (81254) 42 placebo.tw. (197050) 43 clinical trials as topic.sh. (189502) 44 randomly.ab. (285441) 45 trial.ti. (178951) 46 (crossover or cross‐over or cross over).tw. (76010) 47 or/38‐46 (1208254) 48 exp animals/ not humans.sh. (4669479) 49 47 not 48 (1114154) 50 37 and 49 (212)
Appendix 4. Embase search strategy
From 1980 to 28 November 2016
Ovid platform
1 exp endometriosis/ (30876) 2 Endometri$.tw. (104349) 3 Dyspareunia.tw. (5475) 4 pelvi$ pain.tw. (11229) 5 or/1‐4 (120810) 6 exp mifepristone/ (11579) 7 mifepristone.tw. (3888) 8 RU486.tw. (2483) 9 (progest$ adj1 antagonist$).tw. (521) 10 exp antigestagen/ (12402) 11 (progest$ adj1 modulator$).tw. (18) 12 exp progesterone receptor modulator/ (586) 13 ru 486.tw. (4148) 14 mifegyne.tw. (181) 15 ru38486.tw. (406) 16 ru‐38486.tw. (913) 17 mifeprex.tw. (111) 18 zk 98296.tw. (3) 19 r38486.tw. (1) 20 exp GESTRINONE/ (621) 21 Gestrinone.tw. (204) 22 Lilopristone.tw. (22) 23 Onapristone.tw. (216) 24 Aglepristone.tw. (80) 25 (dimetriose or dimetrose).tw. (14) 26 Nemestran.tw. (11) 27 anti‐progestin$.tw. (178) 28 antiprogestin$.tw. (968) 29 Asoprisnil.tw. (63) 30 J867.tw. (15) 31 Telapristone.tw. (16) 32 CDB‐2914.tw. (106) 33 CDB‐4124.tw. (47) 34 Proellex.tw. (22) 35 Ulipristal.tw. (466) 36 (SPRM$ or PRAs).tw. (596) 37 exp progesterone receptor/dt, pd [Drug Therapy, Pharmacology] (65) 38 or/6‐37 (14686) 39 5 and 38 (1698) 40 Clinical Trial/ (995452) 41 Randomized Controlled Trial/ (461742) 42 exp randomization/ (83639) 43 Single Blind Procedure/ (27251) 44 Double Blind Procedure/ (136941) 45 Crossover Procedure/ (53825) 46 Placebo/ (321968) 47 Randomi?ed controlled trial$.tw. (149359) 48 Rct.tw. (22353) 49 random allocation.tw. (1629) 50 randomly.tw. (338581) 51 randomly allocated.tw. (26583) 52 allocated randomly.tw. (2208) 53 (allocated adj2 random).tw. (843) 54 Single blind$.tw. (18663) 55 Double blind$.tw. (172862) 56 ((treble or triple) adj blind$).tw. (647) 57 placebo$.tw. (247461) 58 prospective study/ (386445) 59 or/40‐58 (1967839) 60 case study/ (92866) 61 case report.tw. (323319) 62 abstract report/ or letter/ (986309) 63 or/60‐62 (1393358) 64 59 not 63 (1916879) 65 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5725014) 66 64 not 65 (1793800) 67 39 and 66 (434)
Appendix 5. PsycINFO search strategy
From 1806 to 28 November 2016
Ovid platform
1 exp Dyspareunia/ (240) 2 Dyspareunia.tw. (528) 3 Endometriosis.tw. (209) 4 pelvi$ pain.tw. (489) 5 or/1‐4 (1140) 6 mifepristone.tw. (226) 7 RU486.tw. (159) 8 ru 486.tw. (88) 9 Gestrinone.tw. (0) 10 anti‐progestin$.tw. (4) 11 antiprogestin$.tw. (18) 12 (progest$ adj1 antagonist$).tw. (19) 13 Ulipristal.tw. (3) 14 SPRM$.tw. (3) 15 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 (434) 16 5 and 15 (3) 17 random.tw. (48413) 18 control.tw. (375365) 19 double‐blind.tw. (20296) 20 clinical trials/ (10020) 21 placebo/ (4732) 22 exp Treatment/ (669135) 23 or/17‐22 (1033445) 24 16 and 23 (1)
Data and analyses
Comparison 1. Effectiveness of mifepristone versus placebo, patient‐assessed outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dysmenorrhoea at 3 months | 1 | 352 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.04, 0.17] |
| 1.1 Mifepristone 2.5 mg | 1 | 114 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.09, 0.64] |
| 1.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.16] |
| 1.3 Mifepristone 10 mg | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.17] |
| 2 Dyspareunia at 3 months | 1 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.10, 0.51] |
| 2.1 Mifepristone 2.5 mg | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.18, 2.13] |
| 2.2 Mifepristone 5 mg | 1 | 73 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.40] |
| 2.3 Mifepristone 10 mg | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.60] |
1.1. Analysis.
Comparison 1 Effectiveness of mifepristone versus placebo, patient‐assessed outcomes, Outcome 1 Dysmenorrhoea at 3 months.
1.2. Analysis.
Comparison 1 Effectiveness of mifepristone versus placebo, patient‐assessed outcomes, Outcome 2 Dyspareunia at 3 months.
Comparison 2. Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amenorrhoea at 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Mifepristone all doses | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 686.16 [92.29, 5101.33] |
| 2 Amenorrhoea at 3 months: subgroup analysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Mifepristone 2.5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 207.67 [27.50, 1568.36] |
| 2.2 Mifepristone 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3916.0 [348.75, 43971.52] |
| 2.3 Mifepristone 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3916.0 [348.75, 43971.52] |
| 3 Hot flushes at 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Mifepristone all doses | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 28.79 [3.93, 210.73] |
| 4 Hot flushes at 3 months: subgroup analysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Mifepristone 2.5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.24 [2.49, 148.54] |
| 4.2 Mifepristone 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 23.82 [3.11, 182.24] |
| 4.3 Mifepristone 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 46.76 [6.21, 351.92] |
| 5 Nausea at 3 months | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.20, 15.03] |
| 5.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 5.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.12, 48.60] |
| 6 Vomiting at 3 months | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.10, 10.01] |
| 6.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 6.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 7 Fatigue/Tiredness at 3 months | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.48 [0.71, 42.27] |
| 7.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.92 [0.21, 73.08] |
| 7.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.11 [0.40, 125.92] |
2.1. Analysis.
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 1 Amenorrhoea at 3 months.
2.2. Analysis.
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 2 Amenorrhoea at 3 months: subgroup analysis.
2.3. Analysis.
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 3 Hot flushes at 3 months.
2.4. Analysis.
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 4 Hot flushes at 3 months: subgroup analysis.
2.5. Analysis.
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 5 Nausea at 3 months.
2.6. Analysis.
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 6 Vomiting at 3 months.
2.7. Analysis.
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 7 Fatigue/Tiredness at 3 months.
Comparison 3. Mifepristone lower dose versus higher dose: efficacy and side effects at six months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prevalence of dysmenorrhoea | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 2.5 mg vs 5 mg | 1 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.22, 3.29] |
| 1.2 5 mg vs 10 mg | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.32, 4.71] |
| 2 Dysmenorrhoea score 0‐10 VAS scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 5 mg vs 25 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Prevalence of dyspareunia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 2.5 mg vs 5 mg | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.37 [0.74, 54.81] |
| 3.2 5 mg vs 10 mg | 1 | 109 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.90] |
| 4 Dyspareunia score 0‐10 VAS scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 5 mg vs 25 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Prevalence of pelvic pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 2.5 mg vs 5 mg | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.63, 5.17] |
| 5.2 5 mg vs 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.97 [0.79, 19.97] |
| 6 Prevalence of amenorrhoea | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.97] |
| 6.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.56] |
| 6.3 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.08, 4.41] |
| 7 Prevalence of hot flushes | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.89] |
| 7.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.35, 1.58] |
| 7.3 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.12, 87.13] |
| 8 Prevalence of nausea | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.71] |
| 8.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.24] |
| 9 Prevalence of vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.46] |
| 9.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.20] |
| 10 Prevalence of fatigue/tiredness | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.92 [0.77, 250.94] |
| 10.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.54] |
| 11 Prevalence of endometrial thickness > 20 mm | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.28, 7.13] |
Comparison 4. Gestrinone versus danazol for six months: efficacy and side effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 None or mild pelvic pain | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.56] |
| 2 None or mild dysmenorrhoea | 2 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.39, 1.33] |
| 3 None or mild dyspareunia | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.86] |
| 4 Adverse effects | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Acne | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.90, 2.33] |
| 4.2 Seborrhoea | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.67, 4.46] |
| 4.3 Hirsutism | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.60, 4.32] |
| 4.4 Voice problems | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.34, 1.43] |
| 4.5 Swelling hands/feet | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.82, 2.38] |
| 4.6 Hot flushes | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.50, 1.26] |
| 4.7 Decreased breast size | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 0.98] |
| 4.8 Leg or muscle cramps | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.78] |
| 4.9 Headache | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.84, 2.21] |
| 4.10 Nausea | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.84, 2.19] |
| 4.11 Vomiting | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.32, 1.43] |
| 4.12 Loss of appetite | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.72, 2.37] |
| 4.13 Hunger | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.36, 0.97] |
| 4.14 Dizziness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.75, 2.05] |
| 4.15 Tiredness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.84, 2.45] |
| 4.16 Faintness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.54, 2.76] |
| 4.17 Skin rash | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.91, 3.20] |
| 4.18 Weight gain | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.27] |
| 4.19 Vaginal dryness | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.00] |
| 4.20 Raised liver transaminases | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.00] |
4.1. Analysis.

Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 1 None or mild pelvic pain.
4.2. Analysis.
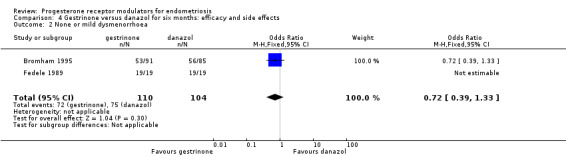
Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 2 None or mild dysmenorrhoea.
4.3. Analysis.
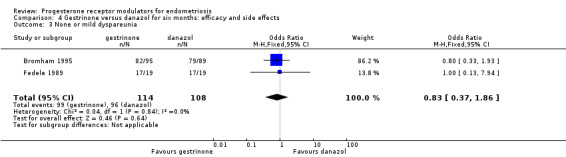
Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 3 None or mild dyspareunia.
4.4. Analysis.
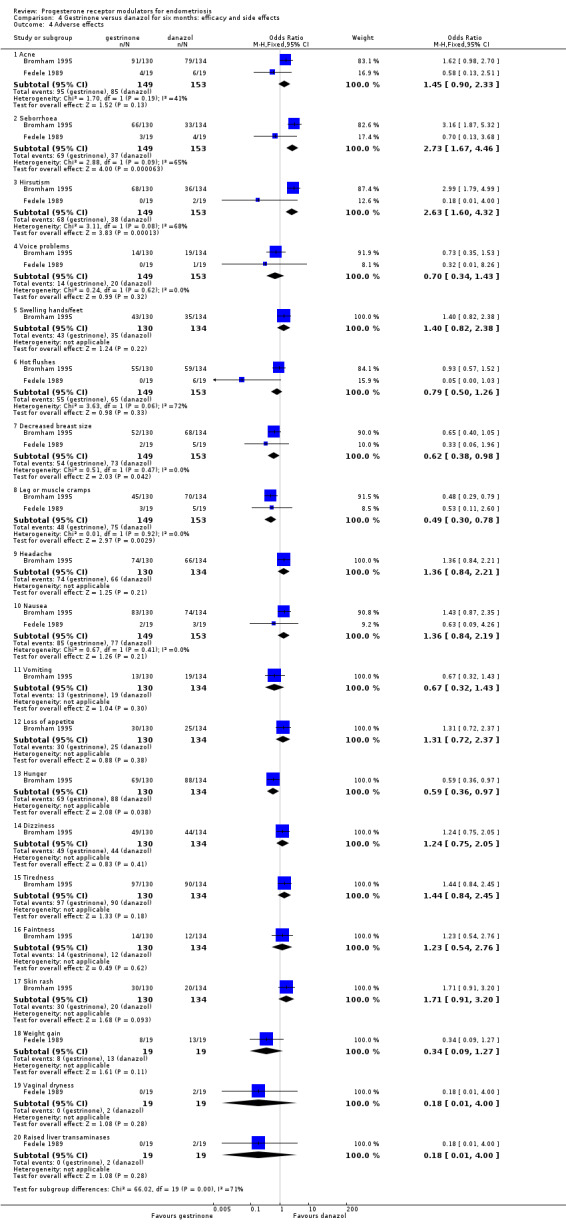
Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 4 Adverse effects.
Comparison 5. Gestrinone versus leuprolin for six months: efficacy and side effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Painful periods, visual analogue scale | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.82 [0.15, 1.49] |
| 2 Painful periods, verbal rating scale | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.12, 0.58] |
| 3 Pain on intercourse, visual analogue scale | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐2.08, ‐0.24] |
| 4 Pain on intercourse, verbal rating scale | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.62, ‐0.04] |
| 5 Non‐menstrual pain, visual analogue scale | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.76, 0.94] |
| 6 Side effects | 1 | 813 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.42, 1.01] |
| 6.1 Seborrhoea | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.13, 82.71] |
| 6.2 Swelling hands/feet | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.59 [0.26, 121.96] |
| 6.3 Hot flushes | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.63] |
| 6.4 Leg or muscle cramps | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.55] |
| 6.5 Headache | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.63] |
| 6.6 Nausea | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.49] |
| 6.7 Dizziness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.8 Skin rash | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.13, 82.71] |
| 6.9 Vaginal dryness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.21] |
| 6.10 Mood changes | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.10, 4.34] |
| 6.11 Joint pain | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.12 Drowsiness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.13 Tachycardia | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.49] |
| 6.14 Amenorrhoea | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.38] |
| 6.15 Spotting or bleeding | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 22.92 [2.64, 198.66] |
5.1. Analysis.
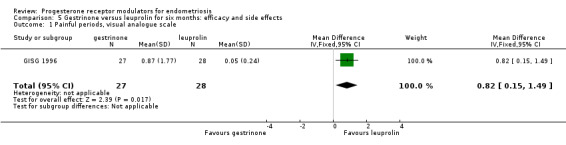
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 1 Painful periods, visual analogue scale.
5.2. Analysis.
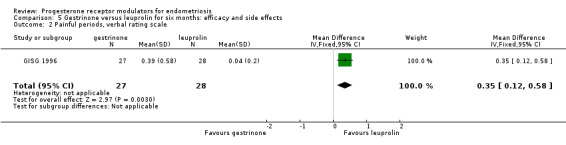
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 2 Painful periods, verbal rating scale.
5.3. Analysis.
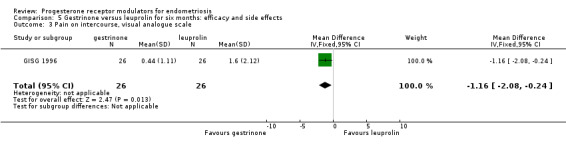
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 3 Pain on intercourse, visual analogue scale.
5.4. Analysis.
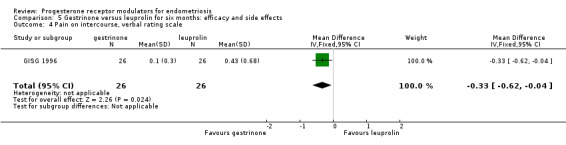
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 4 Pain on intercourse, verbal rating scale.
5.5. Analysis.
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 5 Non‐menstrual pain, visual analogue scale.
5.6. Analysis.
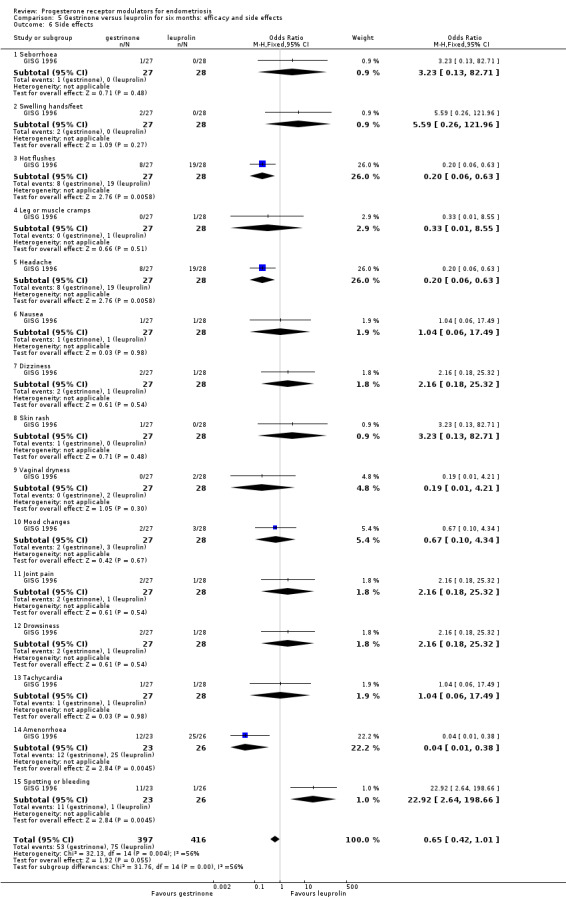
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 6 Side effects.
Comparison 6. Gestrinone 1.25 mg versus gestrinone 2.5 mg: efficacy and side effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 improvement in pain | 1 | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.01, 8.46] |
| 2 Side effect | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Noted any side effect | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 1.12] |
| 2.2 Discontinued because of headaches | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 8.42] |
| 2.3 Irregular bleeding | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.16, 38.60] |
6.1. Analysis.
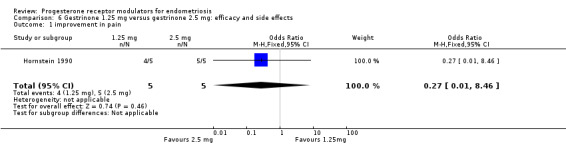
Comparison 6 Gestrinone 1.25 mg versus gestrinone 2.5 mg: efficacy and side effects, Outcome 1 improvement in pain.
6.2. Analysis.
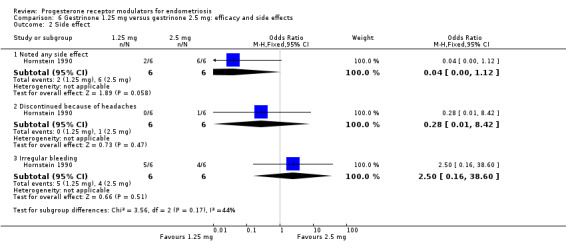
Comparison 6 Gestrinone 1.25 mg versus gestrinone 2.5 mg: efficacy and side effects, Outcome 2 Side effect.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bromham 1995.
| Methods | Randomised double‐blind multi‐centre study Method of randomization not described Pharmaceutical company stated | |
| Participants | 269 British women aged 18 to 45 years Inclusion criteria: endometriosis confirmed by laparoscopy or laparotomy. Exclusion criteria: requiring surgical excision, serious systemic disease, requiring long‐term treatment, previous failure of danazol treatment, other hormonal treatment within 2 months, unwillingness to use mechanical contraception | |
| Interventions | Gestrinone 2.5 mg twice weekly plus 'dummy' danazol for 6 months (132 women) Danazol 200 mg bd plus 'dummy' gestrinone for 6 months (137 women) Duration of treatment: 6 months | |
| Outcomes | AFS scores at laparoscopy after 6 months of treatment Pain scores during treatment and at 1 year follow‐up Side effects Fertility | |
| Notes | Repeat laparoscopy 23 days (median) after cessation of treatment Follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated at random, no other details |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind', 'double‐dummy'. Participants received 2 identical tablets Study authors state that participants were blinded but do not reveal who else was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 15 participants from the gestrinone group, including 4 with hirsutism, and 17 participants from the danazol group, including 6 with headache, withdrew because of adverse symptoms. An additional 22 participants, including 10 from the gestrinone group and 12 from the danazol group, withdrew because of lack of efficacy, pregnancy, elevated hepatic function tests, or reasons unrelated to the trial (19% attrition rate for efficacy outcomes) |
| Selective reporting (reporting bias) | Low risk | Includes main outcomes and side effects |
| Other bias | Low risk | No other specific source of bias detected |
Carbonell 2012.
| Methods | Randomised double‐blind study Method of randomization described Pharmaceutical company stated | |
| Participants | 26 women Inclusion criteria: endometriosis confirmed by laparoscopy | |
| Interventions | Group I received 1 tablet of 25 mg mifepristone daily Group II received 1 tablet of 5 mg mifepristone daily Duration of treatment: 6 months |
|
| Outcomes | AFS scores at laparoscopy after 6 months of treatment
Pain scores during treatment Side effects |
|
| Notes | Laparoscopy and endometrial biopsy were performed before and after treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a random list |
| Allocation concealment (selection bias) | Low risk | Applied opaque sealed envelopes that contained a card indicating the treatment group to which the participant was assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women completed 6 months of treatment |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No other specific source of bias detected |
Carbonell 2016.
| Methods | Randomised double‐blind study Method of randomization described Pharmaceutical company stated | |
| Participants | 360 women Inclusion criteria: endometriosis confirmed by laparoscopy | |
| Interventions | Group I received 1 tablet of 2.5 mg mifepristone daily for 6 months (90 women) Group II received 1 tablet of 5 mg mifepristone daily for 6 months (90 women) Group III received 1 tablet of 10 mg mifepristone daily for 6 months (90 women) Group IV received 1 tablet of placebo daily for 3 months (90 women) |
|
| Outcomes | AFS scores at laparoscopy after 6 months of treatment
Pain scores during treatment Side effects |
|
| Notes | Laparoscopy and endometrial biopsy were performed before and after treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a random list obtained from the MEDSTAT 2.1 programme |
| Allocation concealment (selection bias) | Low risk | Applied opaque sealed envelopes that contained a card indicating the treatment group to which the participant was assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women in treatment group completed 6 months of treatment, and placebo group finished 3 months of treatment |
| Selective reporting (reporting bias) | Low risk | Includes main outcomes |
| Other bias | Low risk | No other specific source of bias detected |
Chwalisz 2004.
| Methods | Multi‐centre double‐blind placebo‐controlled parallel‐group study | |
| Participants | 130 women | |
| Interventions | Asoprisnil 5 mg (n = 31), 10 mg (n = 33), 25 mg (n = 32), or placebo (n = 34) was administered orally once daily for 12 weeks | |
| Outcomes | Pain scores during treatment and at 1 year follow‐up Side effects, serum E2 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised double‐blind study Pharmaceutical company stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, no details |
| Selective reporting (reporting bias) | Unclear risk | Unclear, no details |
| Other bias | Unclear risk | Unclear, insufficient details to permit a judgement |
Dawood 1997.
| Methods | Phase II prospective randomised double‐blind study | |
| Participants | 11 patients | |
| Interventions | Gestrinone 1.25 mg (5 participants) or 2.5 mg (6 participants) orally twice a week for 24 weeks | |
| Outcomes | Revised AFS scores before and at the end of treatment Serum hormone, sex hormone binding globulin, and lipid concentrations were measured Quantitated computerised tomography of thoracic 12 through lumbar 4 vertebral bodies |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to 1 of 2 treatment groups according to a computer‐generated order |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear, no details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, no details |
| Selective reporting (reporting bias) | Unclear risk | Unclear, insufficient details to allow a judgement |
| Other bias | Unclear risk | Unclear, insufficient details to allow a judgement |
Fedele 1989.
| Methods | Open randomised trial No source of funding stated | |
| Participants | 39 Italian women aged 23 to 35 years Inclusion criteria: infertility, laparoscopic diagnosis of endometriosis in preceding 3 months Exclusion criteria: bilateral tubal occlusion, severe dyspermia in partner, use of danazol or other sex steroids in preceding 6 months, severe systemic or endocrine disease | |
| Interventions | Gestrinone 2.5 mg twice weekly (20 women) increasing to 3 times a week if no amenorrhoea by 1 month (7 of the 20) Danazol 600 mg per day (19 women) increasing to 800 mg per day if no amenorrhoea by 1 month (2 of the 19) Duration of treatment: 6 months | |
| Outcomes | rAFS scores at laparoscopy 1 month after cessation of treatment Pain scores during treatment and at 18 month follow‐up Plasma hormone levels before and during treatment Pregnancy rates post treatment Side effects | |
| Notes | Follow‐up: 12 months Losses to follow‐up: 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'patients were randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women accounted for |
| Selective reporting (reporting bias) | Low risk | Includes main outcomes |
| Other bias | High risk | Only 7 participants given gestrinone and 9 participants taking danazol had repeat laparoscopy. If amenorrhoea was not obtained after 1 month of treatment, the gestrinone dose was increased to 2.5 mg 3 times a week (7 participants) and the danazol dose to 800 mg a day (2 participants) |
GISG 1996.
| Methods | Randomised double‐blind double‐dummy multi‐centre trial Method of randomization described Pharmaceutical company stated | |
| Participants | 55 Italian women aged 18 to 40 years Inclusion criteria: chronic pelvic pain, laparoscopic diagnosis of endometriosis with no attempts at endometriosis reduction other than biopsy up to 3 months before study entry, no medical or surgical treatment for endometriosis between laparoscopy and study entry, not wanting pregnancy in the immediate future Exclusion criteria: treatment for endometriosis other than non‐steroidal anti‐inflammatory drugs in the previous 6 months, concomitant pelvic pain causing disorder, contraindications to the use of gestrinone or GnRH analogues, abnormal baseline bone density values, unwillingness to use barrier contraception | |
| Interventions | Gestrinone 2.5 mg twice weekly plus placebo injections (27 women) Intramuscular (IM) leuprolide acetate 3.75 mg once a month plus placebo tablets (28 women) Duration of treatment: 6 months | |
| Outcomes | Pain symptoms Bone mineral density Lipid profile | |
| Notes | Follow‐up: 6 months 6 withdrawals during treatment period 7 lost to follow‐up 8 pregnancies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomized', 'allocating consecutively numbered anonymous packages' |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing randomization codes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'double blind, double dummy'. Each participant received an active drug and a dummy placebo. Participants and clinicians were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'double blind, double dummy'. Each participant received an active drug and a dummy placebo. Participants and clinicians were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up detailed, 6 withdrawals during treatment period, 7 lost to follow‐up (11%) |
| Selective reporting (reporting bias) | Low risk | Includes main outcomes |
| Other bias | Low risk | No other specific source of bias detected |
Hornstein 1990.
| Methods | Randomised double‐blind trial Pharmaceutical company stated | |
| Participants | 12 American women Inclusion criteria: endometriosis (stage 2 to 3 disease according to rAFS classification) diagnosed on videotaped laparoscopy within previous 6 weeks Exclusion criteria: none specified | |
| Interventions | Gestrinone 1.25 mg twice weekly (6 women) Gestrinone 2.5 mg twice weekly (6 women) Duration of treatment: 6 months | |
| Outcomes | rAFS scores for endometriosis at laparoscopy following treatment Symptom scores during treatment and follow‐up Side effects Bone densitometry Hormonal, lipoprotein, haematological, and biochemical measurements | |
| Notes | Second laparoscopy within 4 weeks of treatment completion Follow‐up: 6 months Losses to follow‐up: 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial, method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 2 (17%) |
| Selective reporting (reporting bias) | Unclear risk | Reports main outcomes. No details on pelvic pain scores after treatment |
| Other bias | Low risk | No other specific source of bias detected |
Spitz 2009.
| Methods | Randomised, double‐blind | |
| Participants | 38 women | |
| Interventions | 6 months of treatment with 3 doses of ulipristal, CDB‐4124 (12.5 mg, 25 mg, and 50 mg) compared with leuprolide acetate depot | |
| Outcomes | Pain scores during treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated at random, no other details |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear, no details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, no details |
| Selective reporting (reporting bias) | Unclear risk | Unclear, insufficient details to allow a judgement |
| Other bias | Unclear risk | Unclear, insufficient details to allow a judgement |
Worthington 1993.
| Methods | Randomised double‐blind | |
| Participants | 20 premenopausal women with mild to moderate endometriosis | |
| Interventions | 1.25 mg or 2.5 mg gestrinone 2 times per week for 6 months | |
| Outcomes | Metabolic effects of oral gestrinone on plasma lipoprotein risk markers for cardiovascular disease and on bone density Median total endometriosis scores |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear, no details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, no details |
| Selective reporting (reporting bias) | Unclear risk | Unclear, insufficient details to allow a judgement |
| Other bias | Unclear risk | Unclear, insufficient details to allow a judgement |
AFS: American Fertility Society
GnRH: gonadotropin‐releasing hormone
rAFS: retrospective American Fertility Society score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bulun 2016 | Not an RCT; single‐arm study |
| Kettel 1996 | Not an RCT |
| Kettel 1998 | Not an RCT |
| Mettler 1987 | The “three step” therapy discussed in this study is a mixture of surgical and medical therapy |
| Nieto 1996 | 23/25 participants taking gestrinone and 18/18 participants given danazol had surgery before receiving medical treatment |
| Nobel 1980 | Comparison of danazol vs oral contraceptive pill |
| Thomas 1987 | This study concentrates on effects on asymptomatic endometriosis and fertility |
| Yang 2006 | Conservative or half‐conservative surgery combined with drug therapy |
| Zhang 2016 | Using laparoscopic minimally invasive combined drug therapy |
RCT: randomised controlled trial
Differences between protocol and review
Change to title
Upon consultation with the Cochrane Gynaecology and Fertility Group, we changed the title of this review from "Progesterone receptor antagonists and progesterone receptor modulators for endometriosis" to "Progesterone receptor modulators for endometriosis" as PRMs include PRA and SPRM. We added "asoprisnil" or "CBD 2914" or "CDB‐2914" or "CDB‐4124" or "Ulipristal" or "ulipristal acetate" to the search strategies.
Change to objectives
We edited the objectives to make it clear that comparisons with no treatment or with placebo were eligible for the review, as this was unclear from objectives stated in the protocol.
Change to types of interventions in the methods
We edited the types of interventions section to make it clear that surgical interventions were not eligible.
We added decreases in dysmenorrhoea and dyspareunia to the primary outcomes, as these are the types of endometriosis pain reported in most reports.
We added dose or regimen comparisons of PRMs. This was previously a subgroup analysis, but we wished to allow inclusion of studies in which this was the main comparison.
Change to data synthesis
We removed progesterone receptor antagonists because they are a type of progesterone receptor modulator. We added dose or regimen comparisons of PRMs as a main comparison.
We edited the data synthesis section to make it clear that surgical interventions were not eligible.
Change to subgroup analysis and investigation of heterogeneity
As the data synthesis section states that different comparisons will be made for different drugs, we kept 'different course or dosage' in the subgroup and deleted 'different drug'.
Change to types of outcome measures
The protocol stated that pain scores would be used to measure the primary outcome, and this was our preferred measure. However, we also included other pain‐related data if reported by the included studies because we determined that this type of measure would be informative.
Change to measures of treatment effect
The protocol stated that Peto odds ratios would be calculated. Instead, we used Mantel‐Haenszel odds ratios, as Peto odds ratios are not recommended as a default approach for meta‐analysis (Higgins 2011).
Contributions of authors
Selection of studies: JF, YW, MZ, HZ.
Data extraction and management: JF, HZ.
Assessment of risk of bias in included studies: JF, HS.
Consultation: WH.
Review writing: JF, HC.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
JF, HS, MZ, HZ, YW, HC and WH have no interests to declare.
New
References
References to studies included in this review
Bromham 1995 {published data only}
- Bromham DR, Booker MW, Rose GL, Wardle PG, Newton JR. A multicentre comparative study of gestrinone and danazol in the treatment of endometriosis. Journal of Obstetrics and Gynaecology 1995;15:188‐94. [Google Scholar]
- Bromham DR, Booker MW, Rose GL, Wardle PG, Newton JR. Updating the clinical experience in endometriosis ‐ the European perspective. British Journal of Obstetrics and Gynaecology 1995;102 Suppl 12:12‐6. [DOI] [PubMed] [Google Scholar]
Carbonell 2012 {published data only}
- Carbonell JL, Perera O, Riveron AM, Giuseppe JG. Treatment with 5 mg or 25 mg of mifepristone daily for six months. A randomized double‐blind study [Tratamiento de la endometriosis con 5 mg o 25 mg diariosde mifepristona durante 6 meses. Ensayo clı ´nico aleatorizado,doble ciego]. Progresos de Obstetricia y Ginecologia 2012;55(2):51‐9. [Google Scholar]
Carbonell 2016 {published data only}
- Carbonell JL, Riveron AM, Leonard Y, González J, Heredia B, Sánchez C. Mifepristone 2.5, 5, 10 mg versus placebo in the treatment of endometriosis. Journal of Reproductive Health and Medicine 2016;2:17‐25. [Google Scholar]
Chwalisz 2004 {published data only}
- Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Treatment of endometriosis with the novel selective progesterone receptor modulator (SPRM) asoprisnil. Fertility and Sterility 2004;82(Suppl 2):S83‐4. [Google Scholar]
Dawood 1997 {published data only}
- Dawood MY, Obasiolu CW, Ramos J, Khan‐Dawood FS. Clinical, endocrine, and metabolic effects of two doses of gestrinone in treatment of pelvic endometriosis. American Journal of Obstetrics and Gynecology 1997;176(2):387‐94. [DOI] [PubMed] [Google Scholar]
Fedele 1989 {published data only}
- Fedele L, Arcaini L, Bianchi S, Viezzoli T, Arcaini L, Candiani GB. Gestrinone versus danazol in the treatment of endometriosis. Fertility and Sterility 1989;51:781‐5. [DOI] [PubMed] [Google Scholar]
GISG 1996 {published data only}
- Gestrinone Italian Study Group. Gestrinone versus a gonadotropin releasing hormone agonist for the treatment of pelvic pain associated with endometriosis: a multicenter, randomised, double‐blind study. Fertility and Sterility 1996;66:911‐9. [DOI] [PubMed] [Google Scholar]
Hornstein 1990 {published data only}
- Hornstein MD, Glaeson RE, Barbieri RL. A randomised, double‐blind prospective trial of two doses of gestrinone in the treatment of endometriosis. Fertility and Sterility 1990;53:237‐41. [DOI] [PubMed] [Google Scholar]
Spitz 2009 {published data only}
- Spitz IM. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Current Opinion in Obstetrics and Gynecology 2009;21:318‐24. [DOI] [PubMed] [Google Scholar]
- Spitz IM, Wiehle RD, Van AA. Progesterone receptor modulators in endometriosis: a new therapeutic option. In: Garcia‐Velasco J, Rizk B editor(s). Textbook of Endometriosis. India: Jaypee Brothers Medical Publishers Ltd, 2009. [Google Scholar]
Worthington 1993 {published data only}
- Worthington M, Irvine LM, Crook D, Lees B, Shaw RW, Stevenson JC. A randomized comparative study of the metabolic effects of two regimens of gestrinone in the treatment of endometriosis. Fertility and Sterility 1993;59(3):522‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bulun 2016 {unpublished data only}
- Bulun S. Ulipristal for endometriosis‐related pelvic pain. ClinicalTrials.gov Identifier:NCT02213081 This study is ongoing but is not recruiting participants.
Kettel 1996 {published data only}
- Kettel L, Murphy A, Morales A, Ulmann A, Baulieu EE, Yen SS. Treatment of endometriosis with the antiprogesterone mifepristone (RU486). Fertility and Sterility 1996;65(1):23‐8. [DOI] [PubMed] [Google Scholar]
Kettel 1998 {published data only}
- Kettel LM, Murphy AA, Morales AJ, Yen SS. Preliminary report on the treatment of endometriosis with low dose mifepristone (RU 486). American Journal of Obstetrics and Gynecology 1998;178:1151‐6.. [DOI] [PubMed] [Google Scholar]
Mettler 1987 {published data only}
- Mettler L, Semm K. Three‐step therapy of genital endometriosis in cases of human infertility with lynestrenol, danazol or gestrinone administration in the second step. In: Raynaud JP editor(s). Medical Management of Endometriosis. New York: Raven Press, 1984:33‐47. [Google Scholar]
Nieto 1996 {published data only}
- Nieto A, Tacuri C, Serra M, Keller J, Cortes‐Prieto J. Long term follow‐up of endometriosis after two different therapies (gestrinone and buserelin). Clinical & Experimental Obstetrics and Gynecology 1996;23:199‐203. [PubMed] [Google Scholar]
Nobel 1980 {published data only}
- Noble AD, Letchworth AT. Treatment of endometriosis: a study of medical management. British Journal of Obstetrics and Gynaecology 1980;87:726‐8. [DOI] [PubMed] [Google Scholar]
Thomas 1987 {published data only}
- Thomas EJ, Cooke ID. Impact of gestrinone on the course of asymptomatic endometriosis. British Medical Journal 1987;294:272‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2006 {published data only}
- Yang D, Ma W, Qu F, Ma B. Comparative study of Yiweining and gestrinone for post‐operational treatment of stage 3 endometriosis. Chinese Journal of Integrative Medicine 2006;12(3):218‐20. [DOI] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang YX. Effect of mifepristone in the different treatments of endometriosis. Clinical & Experimental Obstetrics and Gynecology 2016;43(3):350‐3. [PubMed] [Google Scholar]
Additional references
Berek 2007
- Berek JS. Endometriosis. Berek & Novak's Gynecology. 14. Baltimore, MD: Lippincott Williams & Wilkins, 2007. [Google Scholar]
Bouchard 2011
- Bouchard P, Chabbert BN, Fauser BC. Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertility and Sterility 2011;96:1175‐89. [DOI] [PubMed] [Google Scholar]
Brenner 2002
- Brenner RM, Slayden OD, Critchley HO. Anti‐proliferative effects of progesterone antagonists in the primate endometrium: a potential role for the androgen receptor. Reproduction 2002;124(2):167‐72. [DOI] [PubMed] [Google Scholar]
Brenner 2005
- Brenner RM, Slayden OD. Progesterone receptor antagonists and the endometrial antiproliferative effect. Seminars in Reproductive Medicine 2005;23(1):74‐81. [DOI] [PubMed] [Google Scholar]
Brown 2002
- Brown A, Cheng L, Lin S, Baird DT. Daily low‐dose mifepristone has contraceptive potential by suppressing ovulation and menstruation: a double‐blind randomized control trial of 2 and 5 mg per day for 120 days. Journal of Clinical Endocrinology and Metabolism 2002;87(1):63‐70. [DOI] [PubMed] [Google Scholar]
Brown 2014
- Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2014;3:Art. No.: CD009590.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cramer 2002
- Cramer D, Missmer S. The epidemiology of endometriosis. Annals of the New York Academy of Sciences 2002;955:11‐22. [DOI] [PubMed] [Google Scholar]
Eskenazi 1997
- Eskenazi B, Warner M. Epidemiology of endometriosis. Obstetrics and Gynecology Clinics of North America 1997;24:235‐58. [DOI] [PubMed] [Google Scholar]
Giudice 2010
- Giudice LC. Clinical practice: endometriosis. The New England Journal of Medicine 2010;362(25):2389‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein 1980
- Goldstein DP, deCholnoky C, Emans SJ, Leventhal JM. Laparoscopy in the diagnosis and management of pelvic pain in adolescents. Journal of Reproductive Medicine 1980;24:251‐6. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org. Wiley.
Koide 1998
- Koide S. Mifepristone: auxiliary therapeutic use in cancer and related disorders. Journal of Reproductive Medicine 1998;43:551‐60. [PubMed] [Google Scholar]
Li 2016
- Li W, Li X, Zhang B, Gao C, Chen Y, Jiang Y. Current progresses and trends in the development of progesterone receptor modulators. Current Medicinal Chemistry 2016;23:2507‐54. [DOI] [PubMed] [Google Scholar]
Mahutte 2003
- Mahutte NG, Arici A. Medical management of endometriosis‐associated pain. Obstetrics & Gynecology Clinics of North America 2003;30:133‐50. [DOI] [PubMed] [Google Scholar]
Narvekar 2004
- Narvekar N, Cameron S, Critchley HO, Lin S, Cheng L, Baird DT. Low‐dose mifepristone inhibits endometrial proliferation and up‐regulates androgen receptor. Journal of Clinical Endocrinology & Metabolism 2004;89(5):2491‐7. [DOI] [PubMed] [Google Scholar]
Neulen 1996
- Neulen J, Williams RF, Breckwoldt M, Chwalisz K, Baulieu EE, Hodgen GD. Non‐competitive anti‐oestrogenic actions of progesterone antagonists in primate endometrium: enhancement of oestrogen and progesterone receptors with blockade of post‐receptor proliferative mechanisms. Human Reproduction 1996;11:1533‐7. [DOI] [PubMed] [Google Scholar]
Sampson 1927
- Sampson J. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the pelvic cavity. American Journal of Obstetrics and Gynecology 1927;14:422‐69. [Google Scholar]
Spitz 2003
- Spitz IM. Progesterone antagonists and progesterone receptor modulators: an overview. Steroids 2003;68:981‐93. [DOI] [PubMed] [Google Scholar]
Tosti 2016
- Tosti C, Biscione A, Morgante G, Bifulco G, Luisi S, Petraglia F. Hormonal therapy for endometriosis: from molecular research to bedside. European Journal of Obstetrics & Gynecology and Reproductive Biology 2016;5:32. [DOI] [PubMed] [Google Scholar]
Vercellini 2014
- Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nature Reviews Endocrinology 2014;10:261‐75. [DOI] [PubMed] [Google Scholar]
Williams 2007
- Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Human Reproduction 2007;22(6):1696‐704. [DOI] [PubMed] [Google Scholar]


