Abstract
Background
The impact of low-normal hemoglobin (Hb) levels and anemia on the risk of end-stage renal disease (ESRD) in general populations has rarely been examined.
Methods
510,620 Korean adults aged 40–80 years without known chronic kidney disease (CKD) underwent health examinations during 2002–2003 and were followed-up until 2013. Incidence of ESRD was identified by hospital discharge and clinical visit records.
Results
During a mean follow-up of 10.5, 575 women and 1047 men were diagnosed with ESRD. Lower Hb levels were associated with an increased risk of ESRD at given severity of albuminuria and at given estimated glomerular filtration rate (eGFR). Hb 13–13.9 g/dL in men, Hb 11–11.9 g/dL in women, and trace albuminuria assessed by dipstick urinalysis were associated with more than doubled risk. The risk associated with lower Hb was stronger in older (≥60 years) than younger women. Among 349,993 participants with information on eGFR, the multivariable-adjusted HRs associated with 1 g/dL lower Hb in participants with eGFR values ≥60, 30–59, and <30 mL/min/1.73 m2 were 1.34 (95% CI, 1.17–1.54), 1.55 (1.38–1.74), and 1.75 (1.47–2.09), respectively (Pinteraction between eGFR groups = .06).
Conclusions
Low-normal Hb levels and anemia are risk factors for ESRD incidence in person without CKD and for CKD progression to ESRD. Lower Hb increases the risk of ESRD through synergistic biological interactions with lower eGFR and albuminuria. The impacts of lower Hb may be stronger in older than younger women. Proper management and screening at earlier stage of Hb decline and anemia might reduce the burden of CKD.
Introduction
Approximately 1.2 million people died of chronic kidney disease (CKD) and subsequent end-stage renal disease (ESRD) worldwide in 2015, a 31.7% increase from 2005, and these diseases rose in rank to become the leading cause of premature death from 1990 and 2005 to 2015 [1]. Early detection and primary prevention in high-risk individuals are strategies used to reduce the burden of CKD. However, since most patients are asymptomatic until the advanced stages of CKD, determination of who should be screened and who is at high risk in asymptomatic adults has been a challenge [2].
Few studies examined the impact of low-normal hemoglobin (Hb) levels and anemia on development of ESRD, despite the fact that low Hb levels per se may contribute to the progression of CKD to ESRD [3,4], while Hb decline and anemia may occur as a consequence of CKD even at early stages [5]. Low-normal Hb levels, such as 13–13.9 g/dL in men, little attention has been paid to. Furthermore, it has been rarely examined and is unclear whether low-normal Hb levels and anemia increase ESRD incidence in individuals with normal kidney function, namely no albuminuria and normal glomerular filtration rate (GFR). A better understanding of the impact of low-normal Hb and anemia on ESRD may help identify high-risk groups and target Hb levels for the prevention, surveillance, and management of CKD in both general populations and CKD patients.
In this prospective cohort study, ESRD incidence was assessed according to Hb levels and albuminuria measured by the dipstick test, and the interaction between Hb levels and albuminuria was examined. In individuals with information on serum creatinine levels, these associations were examined after further consideration of the estimated GFR (eGFR).
Methods
Study population and follow-up
Ninety-seven percent of Koreans are insured through the National Health Insurance Service (NHIS) [6]. The study cohort (n = 514,795) was a random sample of the 5.15 million NHIS beneficiaries aged 40–79 years in 2002 who received a health examination during 2002–2003. From this sample, 3789 persons with missing information on cardiometabolic factors including fasting glucose and body mass index (BMI), an extreme BMI (<12 or ≥50 kg/m2), or extreme anemia (<7 g/dL) were excluded, as were 386 subjects with known prevalent CKD at baseline. We followed the remaining 510,620 individuals until December 31, 2013 via record linkage to the NHIS data. All patients who were discharged from the hospital due to ESRD or had at least 2 consecutive outpatient clinical visits for ESRD within a 1-year period for the first time during follow-up were considered incident cases. ESRD cases were identified using International Classification of Diseases, 10th revision (ICD-10) codes: N180 during 2002–2010, and N185 during 2011–2013. Due to the possibility of undiagnosed asymptomatic CKD at baseline, this study focused on incidence of ESRD rather than CKD. The authors were granted access to the anonymized data by the NHIS, according to Korean law [7]. This study was approved by the Institutional Review Board of Catholic Kwandong University (CKU-16-01-0301). Informed consent was waived because the data provided by the NHIS have been anonymized according to the strict confidentiality guidelines.
Data collection
Albuminuria was assessed by the dipstick test, generally using automated strip analysis, and it was reported as negative, trace, 1+, 2+, 3+, and 4+ [8] in baseline health examinations during 2002–2003. Dipstick urinalysis is highly sensitive to albumin and has reasonably good sensitivity and specificity for detecting albuminuria [9–12]. Serum Hb was measured using the cyanmethemoglobin method. Fasting serum glucose and total cholesterol were assayed using enzymatic methods [13]. Blood pressure was measured using a standard mercury sphygmomanometer, with the subject in a seated position. BMI was calculated as measured weight divided by the square of measured height (kg/m2)[6]. Smoking status, alcohol use, and history of cancer, heart disease, or stroke were assessed via a questionnaire. We considered individuals to have known prevalent CKD (N18), hypertension (I10-I15), and diabetes (E10-E14) at baseline if they visited a medical institution for those diagnosed conditions at least once within 6 months before or 2 months after the baseline health examination date.
The health examinations and data collection followed a standard protocol officially documented by government. The external quality assessment of clinical chemistry and urinalysis were regularly performed [14,15].
Statistical analysis
Poisson regression was performed to calculate sex- and age-adjusted ESRD incidence. The hazard ratios (HRs) for ESRD incidence were calculated using Cox proportional hazards models stratified by age (years) at baseline (40–44, 45–54, 55–64, 65–74, and 75–80). The multivariable analysis adjusted for age at baseline (continuous variable), sex, smoking status, alcohol use, BMI, physical activity (at least once a week; yes or no), income status (deciles; <4, 4–7, 8–10 [high income]), dipstick hematuria, and dipstick albuminuria, as well as comorbid diabetes, hypertension, cancer, and heart disease or stroke.
We considered eGFR in 349,993 participants with information on serum creatinine during 2009–2010 health examinations but without known CKD (N18) or extreme eGFR (<10 mL/min/1.73 m2). The CKD Epidemiology Collaboration (CKD-EPI) Creatinine equation was used to calculate eGFR [16].
Hb levels were primarily categorized into 5 sex-specific groups (<10, 10–10.9, 11–11.9, 12–12.9, and ≥13 g/dL in women; <12, 12–12.9, 13–13.9, 14–14.9, and ≥15 g/dL in men) in the main analyses, and into 3 groups (<11, 11–11.9, ≥13 g/dL in women; <13, 13–13.9, and ≥14 g/dL in men) in the analyses with consideration of eGFR. Hb levels were also used as a continuous variable. In the main analysis, men and women were analyzed separately, and a subgroup analysis by age (<60 and ≥60 years) was also performed.
The statistical interaction tests that examined the difference in effect size of Hb between subgroups according to sex, age, albuminuria, and eGFR categories used an inverse-variance weighted average method. Subgroup analyses were also used as a sensitivity test. All P values were 2-sided. All analyses used SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
General characteristics
During the mean follow-up of 10.5 years, among 233,497 women and 277,123 men, 575 women and 1072 men were diagnosed with ESRD. The mean±SD Hb level was 12.9±1.2 g/dL in women, and 14.8±1.1 g/dL in men. Individuals with lower Hb were more likely to have a low BMI and total cholesterol, as well as severe albuminuria (Table 1). People with severe albuminuria tended to have a high BMI, high total cholesterol, and comorbid diabetes, hypertension, and heart disease/stroke (Table A in S1 File). The individuals with the lowest Hb were both the youngest and the oldest age groups in women, while they were the oldest in men (Fig A in S1 File).
Table 1. Characteristics of participants according to sex and Hb categories.
| Women | Men | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hb group (g/dL) | Subtotal | ≥13 | 12–12.9 | 11–11.9 | 10–10.9 | <10 | Subtotal | ≥15 | 14–14.9 | 13–13.9 | 12–12.9 | <12 | |||
| Variables | Characteristics | N = 233,497 | n = 116,459 | n = 81,339 | n = 24,658 | n = 6,884 | n = 4,157 | N = 277,123 | n = 131,107 | n = 92,508 | n = 40,784 | n = 9,700 | n = 3,024 | ||
| Age | years | 54.0 | ±9.9 | 54.2±9.6 | 54.2±10.0 | 54.0±10.6 | 51.5±10.3 | 48.3±8.5 | 52.3 | ±9.5 | 50.7±8.7 | 52.4±9.4 | 54.9±10.2 | 58.4±10.8 | 60.2±11.0 |
| Body mass index | kg/m2 | 24.0 | ±3.1 | 24.3±3.1 | 23.8±3.0 | 23.4±3.0 | 23.3±3.0 | 23.4±3.0 | 24.0 | ±2.8 | 24.5±2.8 | 23.8±2.8 | 23.3±2.9 | 22.6±2.9 | 22.1±3.1 |
| Total cholesterol | mg/dL | 203 | ±39 | 207±40 | 201±38 | 195±38 | 191±39 | 181±35 | 199 | ±38 | 203±38 | 197±37 | 192±38 | 185±38 | 176±40 |
| Smoking status | Never smoker | 216,135 | (92.6) | 92.2 | 92.9 | 93.1 | 93.3 | 93.1 | 111,678 | (40.3) | 38.0 | 41.2 | 43.9 | 45.7 | 46.4 |
| Past smoker | 2,152 | (0.9) | 1.0 | 0.9 | 0.8 | 0.9 | 0.9 | 41,262 | (14.9) | 14.7 | 15.4 | 14.4 | 13.9 | 14.2 | |
| Current smoker | 6,398 | (2.7) | 3.1 | 2.5 | 2.3 | 2.1 | 1.8 | 111,450 | (40.2) | 42.7 | 38.8 | 37.0 | 35.4 | 34.4 | |
| Missing | 8,812 | (3.8) | 3.8 | 3.7 | 3.9 | 3.7 | 4.2 | 12,733 | (4.6) | 4.6 | 4.6 | 4.7 | 4.9 | 5.0 | |
| Alcohol use, | None | 187,901 | (80.5) | 80.0 | 80.9 | 81.5 | 80.9 | 79.9 | 95,540 | (34.5) | 31.4 | 35.1 | 39.6 | 44.8 | 49.2 |
| times/week | <2 | 35,687 | (15.3) | 15.7 | 15.0 | 14.3 | 15.2 | 15.9 | 124,181 | (44.8) | 47.5 | 44.8 | 39.8 | 34.6 | 30.0 |
| 3–7 | 4,231 | (1.8) | 2.0 | 1.7 | 1.5 | 1.7 | 1.5 | 53,497 | (19.3) | 19.8 | 18.8 | 19.1 | 18.6 | 18.7 | |
| Missing | 5,678 | (2.4) | 2.4 | 2.4 | 2.7 | 2.2 | 2.7 | 3,905 | (1.4) | 1.4 | 1.3 | 1.5 | 2.0 | 2.1 | |
| Physical activity | ≥1 times/week | 74,782 | (32.0) | 32.2 | 32.2 | 31.5 | 30.7 | 29.2 | 135,293 | (48.8) | 50.2 | 49.3 | 46.0 | 40.7 | 37.8 |
| Income status, | <4 (low income) | 67,202 | (28.8) | 28.6 | 28.7 | 29.8 | 28.9 | 29.4 | 50,306 | (18.2) | 16.2 | 18.4 | 21.6 | 24.8 | 26.0 |
| decile | 4–7 | 75,124 | (32.2) | 32.5 | 32.1 | 31.6 | 31.7 | 29.1 | 91,149 | (32.9) | 31.6 | 33.1 | 35.3 | 37.4 | 35.0 |
| >7 (high income) | 91,171 | (39.0) | 38.9 | 39.2 | 38.6 | 39.4 | 41.5 | 135,668 | (49.0) | 52.2 | 48.4 | 43.2 | 37.8 | 39.1 | |
| Age group | <60 years | 160,118 | (68.6) | 68.5 | 67.6 | 67.0 | 75.9 | 87.8 | 209,137 | (75.5) | 81.7 | 75.1 | 64.6 | 50.7 | 43.4 |
| ≥60 years | 73,379 | (31.4) | 31.5 | 32.4 | 33.0 | 24.1 | 12.2 | 67,986 | (24.5) | 18.3 | 24.9 | 35.4 | 49.3 | 56.6 | |
| Albuminuria | No | 225,173 | (96.4) | 96.3 | 96.7 | 96.6 | 95.7 | 95.8 | 267,344 | (96.5) | 96.2 | 97.0 | 96.6 | 95.2 | 93.4 |
| Trace | 3,688 | (1.6) | 1.6 | 1.5 | 1.5 | 2.0 | 1.3 | 4,294 | (1.5) | 1.6 | 1.4 | 1.5 | 1.8 | 1.8 | |
| 1+ | 3,172 | (1.4) | 1.5 | 1.2 | 1.2 | 1.5 | 1.6 | 3,603 | (1.3) | 1.5 | 1.0 | 1.2 | 1.9 | 2.1 | |
| 2+ | 1,157 | (0.5) | 0.5 | 0.5 | 0.4 | 0.6 | 1.0 | 1,460 | (0.5) | 0.6 | 0.4 | 0.5 | 0.8 | 2.0 | |
| ≥3+ | 307 | (0.1) | 0.1 | 0.1 | 0.1 | 0.2 | 0.4 | 422 | (0.2) | 0.1 | 0.1 | 0.2 | 0.3 | 0.7 | |
| Hematuria | No | 204,064 | (87.4) | 88.0 | 87.1 | 85.6 | 87.4 | 87.8 | 264,861 | (95.6) | 95.9 | 95.6 | 95.0 | 93.7 | 92.4 |
| Trace | 7,482 | (3.2) | 3.1 | 3.3 | 3.7 | 3.5 | 3.0 | 3,554 | (1.3) | 1.2 | 1.3 | 1.3 | 1.6 | 1.7 | |
| 1+ | 12,225 | (5.2) | 5.1 | 5.3 | 5.8 | 4.6 | 4.5 | 5,324 | (1.9) | 1.8 | 1.9 | 2.2 | 2.6 | 3.2 | |
| 2+ | 6,221 | (2.7) | 2.5 | 2.8 | 3.1 | 2.5 | 2.6 | 2,234 | (0.8) | 0.7 | 0.8 | 0.9 | 1.4 | 1.6 | |
| ≥3+ | 3,505 | (1.5) | 1.3 | 1.6 | 1.9 | 2.0 | 2.1 | 1,150 | (0.4) | 0.3 | 0.4 | 0.5 | 0.8 | 1.1 | |
| Comorbidity | Diabetesa | 22,906 | (9.8) | 11.0 | 8.9 | 8.4 | 7.9 | 6.4 | 34,362 | (12.4) | 12.4 | 11.6 | 12.9 | 15.5 | 19.7 |
| Hypertensionb | 75,715 | (32.4) | 35.9 | 30.0 | 28.1 | 24.9 | 20.1 | 96,350 | (34.8) | 35.6 | 33.5 | 34.2 | 37.4 | 39.2 | |
| Heart disease or strokec | 4,858 | (2.1) | 2.2 | 2.0 | 2.0 | 1.8 | 1.4 | 4,736 | (1.7) | 1.5 | 1.6 | 2.1 | 2.8 | 3.2 | |
| Cancerc | 1,604 | (0.7) | 0.6 | 0.7 | 0.8 | 0.8 | 0.8 | 1,258 | (0.5) | 0.2 | 0.4 | 0.7 | 1.8 | 3.8 | |
Hb, hemoglobin
Data are expressed as mean ± standard deviation, n(%), or %.
P values, which were calculated by the chi-square test and 1-way analysis of variance between Hb groups, were < .001 for each variable, except for comorbid cancer in women (P = .006)
aPersons with known diabetes by hospital visit records or fasting glucose level ≥126 mg/dL at baseline health screening.
bPersons with known hypertension by hospital visit records or systolic blood pressure ≥140 mm Hg at baseline health screening.
cPersons with a history of the diseases via questionnaire
Associations of Hb and albuminuria with ESRD incidence
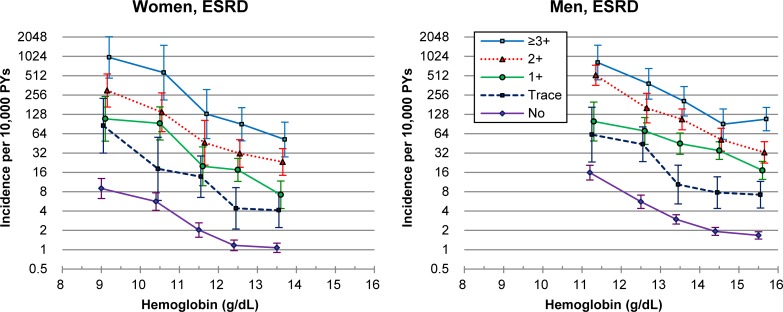
A similar reverse-J-curve association of Hb and a linear association of albuminuria with ESRD incidence were found in both men and women (Fig B in S1 File). The age-adjusted incidence of ESRD was generally higher at lower levels of Hb and with more severe albuminuria in each sex (Fig 1). The age-adjusted incidence per 10,000 person-years was 1.07 and 1.67 in men and women with no albuminuria and the highest Hb levels, respectively, while it was 986.1 and 820.6 in men and women with albuminuria ≥3+ and the lowest Hb levels, respectively.
Fig 1. Age-adjusted* incidence of end-stage renal disease (ESRD) in 25 combined hemoglobin and albuminuria groups in women and men.
*Poisson regression was used.
In the multivariable analysis, the pattern of HRs was generally similar to that of age-adjusted incidence. The risk associated with the lowest Hb category and no albuminuria was greater than the risk associated with the highest Hb category and albuminuria of 1+ (Table 2).
Table 2. HRsa for ESRD incidence in men and women in 25 combined Hb and albuminuria groups.
| Women | Men | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Albuminuria (dipstick) |
Hb group (g/dL) |
No. of subjects | No. of cases | P-value | HR | (95% CI) | Hb group (g/dL) |
No. of subjects | No. of cases | P-value | HR | (95% CI) |
| None | ≥13 | 112,142 | 145 | 1.00 | (Reference) | ≥15 | 126,138 | 223 | 1.00 | (Reference) | ||
| 12–12.9 | 78,635 | 112 | .084 | 1.24 | (0.97–1.59) | 14–14.9 | 89,734 | 197 | .042 | 1.22 | (1.01–1.48) | |
| 11–11.9 | 23,826 | 59 | < .001 | 2.29 | (1.69–3.11) | 13–13.9 | 39,412 | 147 | < .001 | 1.86 | (1.51–2.30) | |
| 10–10.9 | 6,589 | 39 | < .001 | 6.29 | (4.40–8.98) | 12–12.9 | 9,236 | 72 | < .001 | 3.47 | (2.64–4.55) | |
| <10 | 3,981 | 30 | < .001 | 11.16 | (7.46–16.70) | <12 | 2,824 | 60 | < .001 | 10.40 | (7.74–13.97) | |
| Trace | ≥13 | 1,879 | 10 | < .001 | 3.21 | (1.68–6.11) | ≥15 | 2,135 | 17 | < .001 | 3.04 | (1.85–4.99) |
| 12–12.9 | 1,238 | 7 | < .001 | 4.09 | (1.91–8.74) | 14–14.9 | 1,327 | 12 | < .001 | 3.78 | (2.11–6.76) | |
| 11–11.9 | 382 | 7 | < .001 | 12.91 | (6.03–27.62) | 13–13.9 | 607 | 8 | < .001 | 5.05 | (2.49–10.25) | |
| 10–10.9 | 136 | 3 | < .001 | 17.97 | (5.70–56.63) | 12–12.9 | 170 | 10 | < .001 | 17.20 | (9.06–32.64) | |
| <10 | 53 | 4 | < .001 | 139.09 | (50.98–379.53) | <12 | 55 | 4 | < .001 | 23.17 | (8.58–62.57) | |
| 1+ | ≥13 | 1,710 | 16 | < .001 | 4.96 | (2.95–8.34) | ≥15 | 1,914 | 37 | < .001 | 5.54 | (3.90–7.87) |
| 12–12.9 | 985 | 23 | < .001 | 14.17 | (9.08–22.11) | 14–14.9 | 962 | 39 | < .001 | 11.65 | (8.24–16.48) | |
| 11–11.9 | 308 | 8 | < .001 | 17.32 | (8.46–35.45) | 13–13.9 | 478 | 27 | < .001 | 15.86 | (10.57–23.78) | |
| 10–10.9 | 103 | 11 | < .001 | 54.91 | (29.50–102.21) | 12–12.9 | 186 | 17 | < .001 | 23.96 | (14.53–39.52) | |
| <10 | 66 | 6 | < .001 | 120.44 | (52.69–275.30) | <12 | 63 | 8 | < .001 | 24.73 | (12.09–50.56) | |
| 2+ | ≥13 | 577 | 17 | < .001 | 14.58 | (8.77–24.23) | ≥15 | 729 | 26 | < .001 | 9.39 | (6.22–14.16) |
| 12–12.9 | 384 | 16 | < .001 | 20.49 | (12.16–34.53) | 14–14.9 | 367 | 23 | < .001 | 16.45 | (10.61–25.50) | |
| 11–11.9 | 110 | 6 | < .001 | 31.50 | (13.79–71.95) | 13–13.9 | 224 | 28 | < .001 | 31.98 | (21.35–47.90) | |
| 10–10.9 | 44 | 8 | < .001 | 81.58 | (39.78–167.27) | 12–12.9 | 79 | 14 | < .001 | 40.33 | (23.16–70.25) | |
| <10 | 42 | 11 | < .001 | 272.59 | (145.61–510.29) | <12 | 61 | 31 | < .001 | 136.43 | (92.10–202.09) | |
| ≥3+ | ≥13 | 151 | 10 | < .001 | 28.84 | (15.10–55.10) | ≥15 | 191 | 22 | < .001 | 29.48 | (18.93–45.89) |
| 12–12.9 | 97 | 11 | < .001 | 51.39 | (27.46–96.18) | 14–14.9 | 118 | 13 | < .001 | 22.44 | (12.72–39.58) | |
| 11–11.9 | 32 | 5 | < .001 | 146.39 | (59.64–359.34) | 13–13.9 | 63 | 14 | < .001 | 46.88 | (27.00–81.38) | |
| 10–10.9 | 12 | 4 | < .001 | 435.83 | (157.67–1204.72) | 12–12.9 | 29 | 13 | < .001 | 88.17 | (49.61–156.70) | |
| <10 | 15 | 7 | < .001 | 810.56 | (375.01–1751.97) | <12 | 21 | 10 | < .001 | 163.25 | (85.07–313.29) | |
CI, confidence interval; ESRD, end-stage renal disease: Hb, hemoglobin; HR, hazard ratio
a Adjustment for age at baseline, smoking status, alcohol use, physical activity, income status, diabetes, hypertension, a history of heart disease or stroke, a history of cancer, dipstick hematuria, total cholesterol, and body mass index.
In the analysis of 15 combined Hb-albuminuria groups, the associations of lower Hb with ESRD generally did not weaken at ages ≥60 years in comparison to younger ages (Fig C in S1 File). Assuming a linear association, women had greater inverse associations with Hb than men (Table 3; HR per 1 g/dL lower Hb = 1.69 in women versus 1.52 in men, Pinteraction = 0.006). In women, but not in men, the HRs associated with lower Hb were stronger at ages ≥60 years than at ages <60 years. Additionally, the effect size of 1 g/dL lower Hb was not different according to 5 albuminuria categories in both men and women (Table B in S1 File)
Table 3. HRsa per 1 g/dL decrease in Hb or per 1 category severe albuminuria for ESRD incidence by sex and age.
| Women | Men | ||||||||||
| Age group | No. of cases | P-value | HR per 1 g/dL lower Hb (95% CI) |
PInteraction (age) |
No. of cases | P-value | HR per 1 g/dL lower Hb (95% CI) |
PInteraction (age) |
Pinteraction (sex) |
||
| All ages | 575 | < .001 | 1.69 | (1.59–1.79) | 1072 | < .001 | 1.52 | (1.46–1.59) | .006 | ||
| 40–59 years | 266 | < .001 | 1.51 | (1.39–1.64) | < .001 | 548 | < .001 | 1.52 | (1.43–1.61) | 0.977 | .902 |
| 60–80 years | 309 | < .001 | 1.99 | (1.83–2.16) | 524 | < .001 | 1.52 | (1.43–1.61) | < .001 | ||
| HR per 1 category severe albuminuria (95% CI) | HR per 1 category severe albuminuria (95% CI) | ||||||||||
| All ages | 575 | < .001 | 2.48 | (2.33–2.64) | 1072 | < .001 | 2.23 | (2.13–2.34) | .008 | ||
| 40–59 years | 266 | < .001 | 2.69 | (2.46–2.94) | 0.017 | 548 | < .001 | 2.30 | (2.15–2.46) | 0.226 | .005 |
| 60–80 years | 309 | < .001 | 2.31 | (2.11–2.53) | 524 | < .001 | 2.17 | (2.02–2.32) | .286 | ||
CI, confidence interval; ESRD, end stage renal disease; Hb, hemoglobin; HR, hazard ratio; Pinteraction (age), P-value for interaction between age groups; Pinteraction (sex), P-value for interaction between sexes.
a Adjustment for age at baseline, sex, smoking status, alcohol use, physical activity, income status, diabetes, hypertension, a history of heart disease or stroke, a history of cancer, dipstick hematuria, total cholesterol, and body mass index.
Analysis after further consideration of the eGFR
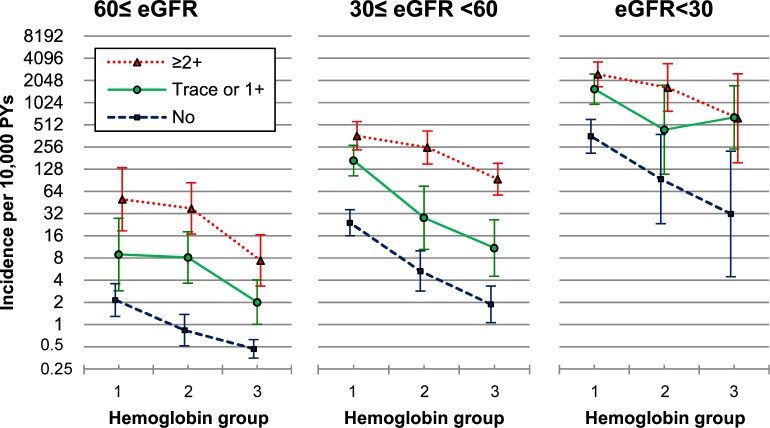
Among 349,993 participants with information on eGFR, during the mean follow-up of 4.0 years, 316 individuals were diagnosed with ESRD. Individuals with a lower eGFR tended to be older, female, and to have comorbid diabetes and hypertension (Table C in S1 File). The age-adjusted incidence and multivariable-adjusted HR of ESRD were generally higher at lower levels of Hb, with more severe albuminuria, and at a lower eGFR (Fig 2, Table D in S1 File).
Fig 2. Age-adjusted* incidence of ESRD in 27 combined hemoglobin, albuminuria, and eGFR groups.
ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation. Hemoglobin groups 1, 2, and 3 correspond to <11, 11–11.9 and ≥12 g/dL, respectively, in women, and <13, 13–13.9 and ≥14 g/dL, respectively, in men. *Poisson regression was used.
The multivariable-adjusted HRs associated with 1 g/dL lower Hb in participants with eGFR values ≥60, 30–59, and <30 mL/min/1.73 m2 were 1.34 (95% CI, 1.17–1.54), 1.55 (1.38–1.74), and 1.75 (1.47–2.09), respectively (Pinteraction between eGFR groups = .057). In the analysis of 9 combined albuminuria-eGFR subgroups, HRs associated with 1 g/dL lower Hb were generally lower in persons with eGFR ≥60 than with <60 mL/min/1.73 m2, regardless of albuminuria categories (Table E in S1 File).
When biological interaction between low Hb, albuminuria, and eGFR groups was assessed using multivariable-adjusted HRs (Table D in S1 File) [17], the synergy index was 45.5. These results suggest a strong synergistic interaction.
Discussion
Lower Hb levels were consistently associated with an increased risk of ESRD at a given severity of albuminuria and given levels of the eGFR. These results indicated that lower Hb interacts with albuminuria and a lower GFR to increase ESRD. Hb 13–13.9 g/dL in men, Hb 11–11.9 g/dL in women, and trace albuminuria by the dipstick test were associated with a more than doubled risk of ESRD after adjusting for confounders, including the eGFR. Women had stronger associations of low Hb and albuminuria with ESRD incidence than men. The HRs associated with lower Hb became stronger at ages ≥60 years than at ages <60 years in women.
Anemia as a risk factor for CKD incidence in the general population without CKD
Anemia has been generally considered a complication of CKD and a risk factor for the progression of CKD, but it has not been generally recognized as a risk factor for CKD incidence in the general population [2,18], despite some suggestive evidence [19,20]. In our study, lower Hb was associated with an increased risk of ESRD at each category of albuminuria severity and eGFR, including the lowest risk group with an eGFR ≥60 mL/min/1.73 m2 and no albuminuria. These results suggest that lower Hb is a risk factor both for CKD incidence in the general population, as well as for CKD progression in CKD patients, potentially through kidney damage by anemia-induced renal hypoxia [19], although at least a part of lower Hb could be consequence of CKD. The correction of anemia in persons with anemia but without CKD might prevent CKD development; however, the current study could not examine the impact of anemia of various etiologies, and specific recommendations (e.g., for iron replacement therapy) are therefore beyond our scope.
Stronger impact of anemia on CKD progression than on CKD incidence
In our study, people with albuminuria ≥trace or an eGFR <60 mL/min/1.73 m2 seemed to have stronger associations of lower Hb with ESRD incidence than people with no albuminuria or an eGFR ≥60 mL/min/1.73 m2. This may have been because the combined impacts of anemia and CKD in persons with CKD were stronger than the impact of anemia alone in persons without CKD.
Clinical relevance of our findings: Surveillance
Anemia has not been recognized as a risk factor for recommendation of CKD screening. The prevalence of anemia was 10.9% in women and 3.5% in men in Korea [21]. In the US, the prevalence of anemia nearly doubled from 2003–2004 to 2011–2012 (4.0% to 7.1%)[22]. Hb measurements are a routine part of global health assessment in most adults [5]. Since lower Hb was strongly associated with ESRD incidence, when anemia in both sexes and Hb 13–13.9 g/dL in men are detected in health checkups, kidney function tests may be additionally performed in order to detect potential CKD. Moreover, since lower Hb without CKD was also associated with a higher risk of ESRD, further studies should investigate whether it is beneficial to routinely monitor for CKD and ESRD development in individuals with lower Hb but normal kidney function at the time of Hb measurements.
Impact of Hb low-normal Hb levels and mild anemia
The current study demonstrated that men with Hb 13–13.9 g/dL had more than a doubled risk of ESRD compared with men with Hb ≥15 g/dL. Since Hb <13 g/dL is considered anemia in men, men with Hb 13–13.9 g/dL generally receive little attention in terms of the risk of CKD and ESRD. Furthermore, mild anemia in women (11–11.9 g/dL) and men (11–12.9 g/dL) is associated with over two-fold and four-fold increased risk of ESRD, compared with their respective counterparts. Proper management at earlier stage of anemia, for men with Hb 13–13.9 g/dL and individuals with mild anemia, might prevent CKD development in the general population and prevent progression to ESRD in CKD patients.
Importance of trace albuminuria
In our study, trace albuminuria increased the ESRD risk by more than 3-fold in both women and men. Due to its limited accuracy, especially sensitivity (proportion of true positives [albuminuria] correctly identified) for detecting albuminuria, dipstick urinalysis receives relatively little attention compared to blood tests, and trace proteinuria detected by the dipstick test is often ignored. For example, in Korea and Japan, the national population screening programs use urinalysis, but trace proteinuria is not officially considered proteinuria [8,23]. Our results clearly indicated that individuals with dipstick trace proteinuria should not be ignored, but should be further evaluated for kidney damage. The dipstick test for proteinuria is a simple, inexpensive, and instantaneous test that can be easily performed in most clinical settings [24]. Since it has a good specificity (proportion of true negatives [no albuminuria] correctly identified) and negative predictive value (proportion of true negatives among test negatives), given the increased risk of ESRD associated with even trace proteinuria, the dipstick proteinuria test has a role for risk stratification, especially in resource-limited settings.
Clinical relevance of our findings: Risk classification
The current study showed anemia (or, more broadly, Hb levels) to play a role in risk classification for the development and prognosis of CKD in the general population. As mentioned earlier, individuals with a low Hb (<14 g/dL in men or <12 g/dL in women), severe albuminuria, and GFR ≥60 mL/min/1.73 m2, who are considered a high-risk group, had a comparable risk to those with a GFR<30 mL/min/1.73 m2 but no albuminuria (the very high-risk group). Individuals with anemia (<13 g/dL in men or <11 g/dL in women), no albuminuria, and a GFR ≥60 mL/min/1.73 m2, who are considered a low-risk group by the KDIGO guidelines, had a comparable risk to those with a GFR 30–59 mL/min/1.73 m2, no albuminuria, and normal Hb (the moderate to high-risk group).
Impact of anemia and low Hb in the elderly population
Many risk factors of diseases have weaker associations with diseases in older ages, as exemplified by the associations between BMI and mortality [6], blood pressure and cardiovascular disease [25], gamma glutamyl transferase and cardiovascular disease [26], and albuminuria and ESRD incidence in the current study. For ESRD incidence, the relative risks associated with anemia or lower Hb were not weaker in individuals aged ≥60 years than those aged <60 years in our study. In women, the risks associated with lower Hb were greater in older adults than in younger adults. As the prevalence of anemia increases with age in adult men and postmenopausal women, both the absolute and relative risks associated with lower Hb could be greater in the elderly, especially in women. Since life expectancy has increased worldwide and the prevalence of anemia is higher in older adults [27], the importance of the management of anemia or lower Hb may increase with advancing age for the prevention of CKD and ESRD.
Strengths and limitations
The fact that Hb, rather than hematocrit, was used in the analysis is a strength of the study [5]. As a prospective cohort study, recall and selection biases related to the retrospective design were minimized. Nearly complete follow-up via record linkage to a national database is a further strength.
There are limitations in this study. First, the analysis of eGFR was limited to individuals who had a serum creatinine measurement during 2009–2010. Second, information on the etiology of anemia was unavailable. In Korea, more than half of anemias were iron deficiency anemia (IDA), while the proportion of IDA was markedly different according to sex and age [28]. The difference in the effect size of Hb by sex and age might be related to different etiology of anemia. Third, Hb and albuminuria assays were based on a single measurement. Although it is a limitation, using values that were measured once is less likely to overestimate the HRs due to regression dilution effects. Fourth, the study population comprised Koreans aged 40–80 years. Our results may not necessarily be generalized to younger people, or to other ethnic populations.
Conclusions
Low-normal Hb levels and anemia were risk factors for ESRD incidence in general population without CKD as well as for the progression of CKD to ESRD. Lower Hb had synergic biological interactions with lower eGFR and albuminuria to increase the risk of ESRD incidence. Hb of 13–13.9 g/dL in men, 11–11.9 g/dL in women, and trace albuminuria by dipstick urinalysis were associated with a more than doubled risk of ESRD. The impacts of lower Hb may be stronger in older than younger women. The inclusion of persons with anemia or low-normal Hb in surveillance and management programs for the primary and secondary prevention of CKD may reduce the burden of CKD.
Supporting information
(PDF)
Acknowledgments
The authors thank the staff at the Big Data Steering Department at the NHIS for providing the data and support.
Data Availability
The data underlying the results presented in the study are available from the National Health Insurance Data Sharing Service homepage of the NHIS (http://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do). Applications to use the data will be reviewed by the inquiry committee of NHIS and, once approved, access to data will be granted to the applicant. Applicants should contact the NHIS (Office of big data operation, +82-33-736-2469) for further information.
Funding Statement
The authors received no specific funding for this work.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qaseem A, Hopkins RH Jr, Sweet DE, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of P. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:835–847. 10.7326/0003-4819-159-12-201312170-00726 [DOI] [PubMed] [Google Scholar]
- 3.Iseki K, Ikemiya Y, Iseki C, Takishita S. Haematocrit and the risk of developing end-stage renal disease. Nephrol Dial Transplant. 2003;18:899–905. [DOI] [PubMed] [Google Scholar]
- 4.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17:1444–1452. 10.1681/ASN.2005091012 [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl (2011). 2012;2:279–335. [Google Scholar]
- 6.Yi SW, Ohrr H, Shin SA, Yi JJ. Sex-age-specific association of body mass index with all-cause mortality among 12.8 million Korean adults: a prospective cohort study. Int J Epidemiol. 2015;44:1696–1705. 10.1093/ije/dyv138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seong SC, Kim YY, Park SK, Khang YH, Kim HC, Park JH, et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7:e016640 10.1136/bmjopen-2017-016640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mok Y, Matsushita K, Sang Y, Ballew SH, Grams M, Shin SY, et al. Association of Kidney Disease Measures with Cause-Specific Mortality: The Korean Heart Study. PLoS One. 2016;11:e0153429 10.1371/journal.pone.0153429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem. 2009;46:205–217. 10.1258/acb.2009.009007 [DOI] [PubMed] [Google Scholar]
- 10.Lim D, Lee DY, Cho SH, Kim OZ, Cho SW, An SK, et al. Diagnostic accuracy of urine dipstick for proteinuria in older outpatients. Kidney Res Clin Pract. 2014;33:199–203. 10.1016/j.krcp.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JI, Baek H, Kim BR, Jung HH. Comparison of urine dipstick and albumin:creatinine ratio for chronic kidney disease screening: A population-based study. PLoS One. 2017;12:e0171106 10.1371/journal.pone.0171106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White SL, Yu R, Craig JC, Polkinghorne KR, Atkins RC, Chadban SJ. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am J Kidney Dis. 2011;58:19–28. 10.1053/j.ajkd.2010.12.026 [DOI] [PubMed] [Google Scholar]
- 13.Lee EY, Lee YH, Yi SW, Shin SA, Yi JJ. BMI and All-Cause Mortality in Normoglycemia, Impaired Fasting Glucose, Newly Diagnosed Diabetes, and Prevalent Diabetes: A Cohort Study. Diabetes Care. 2017;40:1026–1033. 10.2337/dc16-1458 [DOI] [PubMed] [Google Scholar]
- 14.Kim KD, Koo SH, Kim EC, Kim JM, Kim JH, Kim JQ, et al. Annual Report on External Quality Assessment in Urinalysis in Korea (2005). J Lab Med Qual Assur. 2006;28:63–89. [Google Scholar]
- 15.Min WK, Kim KD, Kim SS, Kim S-C, Kim YK, Kim JH, et al. Annual Report on External Quality Assessment in Clinical Chemistry in Korea (2003). J Lab Med Qual Assur. 2004;26:1–10. [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. [DOI] [PubMed] [Google Scholar]
- 18.Moyer VA, Force USPST. Screening for chronic kidney disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:567–570. 10.7326/0003-4819-157-8-201210160-00533 [DOI] [PubMed] [Google Scholar]
- 19.Fu Q, Colgan SP, Shelley CS. Hypoxia: The Force that Drives Chronic Kidney Disease. Clin Med Res. 2016;14:15–39. 10.3121/cmr.2015.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iseki K, Kohagura K. Anemia as a risk factor for chronic kidney disease. Kidney Int Suppl. 2007. 10.1038/sj.ki.5002481:S4–9. [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Health and Welfare. Korea Health Statistics 2015: Korea National Health and Nutrition Examination Survey (KNHANES VI-3). Sejong, Republic of Korea: Ministry of Health and Welfare; 2016. [Google Scholar]
- 22.Le CH. The Prevalence of Anemia and Moderate-Severe Anemia in the US Population (NHANES 2003–2012). PLoS One. 2016;11:e0166635 10.1371/journal.pone.0166635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagai K, Saito C, Watanabe F, Ohkubo R, Sato C, Kawamura T, et al. Annual incidence of persistent proteinuria in the general population from Ibaraki annual urinalysis study. Clin Exp Nephrol. 2013;17:255–260. 10.1007/s10157-012-0692-5 [DOI] [PubMed] [Google Scholar]
- 24.Wen CP, Yang YC, Tsai MK, Wen SF. Urine dipstick to detect trace proteinuria: an underused tool for an underappreciated risk marker. Am J Kidney Dis. 2011;58:1–3. 10.1053/j.ajkd.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 25.Yi SW, Mok Y, Ohrr H, Yi JJ, Yun YD, Park J, et al. Low Systolic Blood Pressure and Vascular Mortality Among More Than 1 Million Korean Adults. Circulation. 2016;133:2381–2390. 10.1161/CIRCULATIONAHA.115.020752 [DOI] [PubMed] [Google Scholar]
- 26.Yi SW, Lee SH, Hwang HJ, Yi JJ. Gamma-glutamyltransferase and cardiovascular mortality in Korean adults: A cohort study. Atherosclerosis. 2017;265:102–109. 10.1016/j.atherosclerosis.2017.08.028 [DOI] [PubMed] [Google Scholar]
- 27.Merchant AA, Roy CN. Not so benign haematology: anaemia of the elderly. Br J Haematol. 2012;156:173–185. 10.1111/j.1365-2141.2011.08920.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JO, Lee JH, Ahn S, Kim JW, Chang H, Kim YJ, et al. Prevalence and risk factors for iron deficiency anemia in the korean population: results of the fifth KoreaNational Health and Nutrition Examination Survey. J Korean Med Sci. 2014;29:224–229. 10.3346/jkms.2014.29.2.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
The data underlying the results presented in the study are available from the National Health Insurance Data Sharing Service homepage of the NHIS (http://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do). Applications to use the data will be reviewed by the inquiry committee of NHIS and, once approved, access to data will be granted to the applicant. Applicants should contact the NHIS (Office of big data operation, +82-33-736-2469) for further information.