Abstract
Homeostasis is a founding principle of integrative physiology. In current systems biology, however, homeostasis seems almost invisible. Is homeostasis a key goal driving body processes, or is it an emergent mechanistic fact? In this perspective piece, I propose that the integrative physiological and systems biological viewpoints about homeostasis reflect different epistemologies, different philosophies of knowledge. Integrative physiology is concept driven. It attempts to explain biological phenomena by continuous formation of theories that experimentation or observation can test. In integrative physiology, “function” refers to goals or purposes. Systems biology is data driven. It explains biological phenomena in terms of “omics”–i.e., genomics, gene expression, epigenomics, proteomics, and metabolomics–it depicts the data in computer models of complex cascades or networks, and it makes predictions from the models. In systems biology, “function” refers more to mechanisms than to goals. The integrative physiologist emphasizes homeostasis of internal variables such as Pco2 and blood pressure. The systems biologist views these emphases as teleological and unparsimonious in that the “regulated variable” (e.g., arterial Pco2 and blood pressure) and the “regulator” (e.g., the “carbistat” and “barostat”) are unobservable constructs. The integrative physiologist views systems biological explanations as not really explanations but descriptions that cannot account for phenomena we humans believe exist, although they cannot be observed directly, such as feelings and, ultimately, the conscious mind. This essay reviews the history of the two epistemologies, emphasizing autonomic neuroscience. I predict rapprochement of integrative physiology with systems biology. The resolution will avoid teleological purposiveness, transcend pure mechanism, and incorporate adaptiveness in evolution, i.e., “Darwinian medicine.”
Keywords: allostasis, allostatic load, autonomic nervous system, catecholamines, homeostasis, homeostat, sympathetic noradrenergic system, systems biology
INTRODUCTION
What Happened at Harvard?
All physiologists know well that Walter B. Cannon, the most prominent American physiologist of the early 20th century, coined the term homeostasis. He spent his entire scientific career at Harvard Medical School, where he was the chair of the Department of Physiology from 1906 to 1942.
The Harvard Medical School no longer has a Department of Physiology. It has a Department of Systems Biology. The website for the Department of Systems Biology at Harvard (https://sysbio.med.harvard.edu) describes the subject matter as follows.
“Systems biology is the study of systems of biological components, which may be molecules, cells, organisms or entire species. Living systems are dynamic and complex, and their behavior may be hard to predict from the properties of individual parts. To study them, we use quantitative measurements of the behavior of groups of interacting components, systematic measurement technologies such as genomics, bioinformatics and proteomics, and mathematical and computational models to describe and predict dynamical behavior. Systems problems are emerging as central to all areas of biology and medicine.”
This description does not mention homeostasis or the scientific tradition that Cannon began at the same institution. What happened?
Bernard’s Purpose of Bodily Processes and Cannon’s “Useful End”
In the late 1800s, Claude Bernard, widely regarded as the father of modern physiology (164), promulgated the founding statements of integrative physiology. He is well known for introducing the notion of an apparently constant inner world (milieu intérieur), the fluid environment that bathes body cells, thereby insulating them from vicissitudes of the external environment. Even more meaningful, he proposed a purpose for body processes. “The constancy of the internal environment is the condition for free and independent life…All the vital mechanisms, however varied they might be, always have one purpose, that of maintaining the integrity of the conditions of life within the internal environment (17).”
Cannon took up the same theme in his book The Way of an Investigator (28). He wrote, “My first article of belief is based on the observation, almost universally confirmed in present knowledge, that what happens in our bodies is directed toward a useful end.” Cannon devotes a chapter of his book Bodily Changes in Pain, Hunger, Fear and Rage, to the “Utility of Bodily Changes,” which includes the following statement:
“It has long been recognized that the most characteristic feature of reflexes is their “purposive” nature, or their utility either in preserving the welfare of the organism or in safeguarding it against injury (27).”
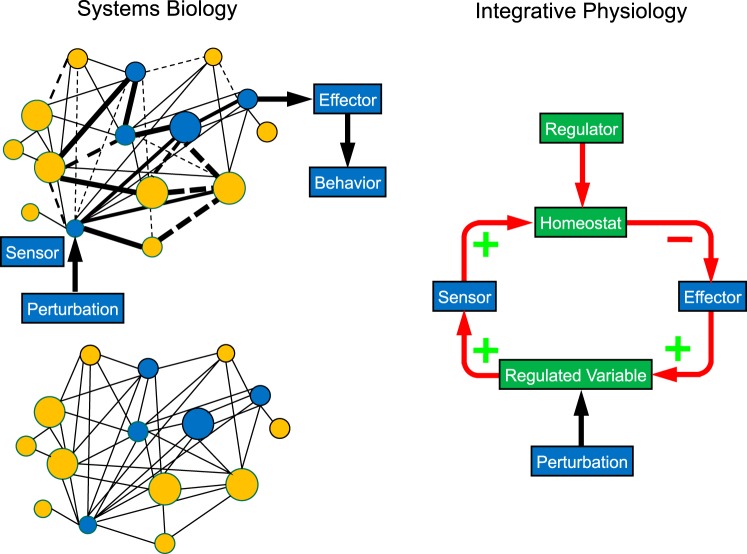
Note Cannon’s use of quotation marks here. “Purposive,” with the quotation marks, summarizes in a single word the essence of the challenge at the interface of systems biology with integrative physiology (Fig. 1).
Fig. 1.
Concept diagrams illustrating systems biological (left) and integrative physiological views (right) about organismic responses to an environmental or internal perturbation. Objects in blue are measurable, and objects in green are conceptual. In the integrative physiology model, there is a negative feedback loop that keeps levels of the monitored variable within bounds. In the systems physiology model, because positive (solid lines) and negative relationships (dashed lines) are embedded in the network, homeostasis is an emergent phenomenon resulting from a pattern of activation (thick solid lines) and inhibition (thick dashed lines).
Bernard’s concept of the milieu intérieur and Cannon’s of homeostasis were revolutionary in the history of medical ideas, yet to a systems biologist Bernard’s assertion about the purpose of body processes and Cannon’s about the “useful end” might seem awkward or unnecessary. This is because these statements are teleological.
TELEOLOGY
Teleology refers to a purpose or goal as the reason or explanation for something. Understanding the positions of integrative physiology and systems biology requires consideration of the history and meaning of teleology.
Aristotle’s telos referred to intrinsic purposes or ends; e.g., the telos of an acorn is to become an oak tree. Atomists such as Democritus and Titus Lucretius Carus opposed the notion of the driving “final cause” inherent in Aristotle’s telos. In his epic poem On the Nature of Things, Lucretius states that “nothing in the body is made in order that we may use it. What happens to exist is the cause of its use” (Book IV, lines 811–842).
Teleology has had a checkered past in science, and it remains an unsettled aspect of biology. Physics and chemistry discarded teleological explanations as these scientific disciplines matured; when an apple falls from a tree, there is no reason to conceptualize that the telos of the apple drives its motion. In biology, however, the debate continues.
It is a debate that has garnered substantial popular and media attention because of the conflict between creationism and Darwinian evolution. The philosopher Thomas Nagel, in his 2012 book Mind and Cosmos: Why the Materialist Neo-Darwinian Conception of Nature is Almost Certainly False (117), argued that evolutionary theory cannot account for existence as experienced by the human mind, and since the mind is a basic aspect of nature, evolutionary explanations must be inadequate.
Nagel’s book was rapidly adopted by creationists and condemned by evolutionary biologists. Daniel Dennett stated, “It’s cute and it’s clever and it’s not worth a damn (139).” Dennett has offered his own quite different conception of consciousness (118). He takes the extreme opposite position, that consciousness is essentially an illusion. According to his “multiple drafts” idea, the brain contains semi-independent “agencies,” and when “content fixation” occurs in any one of them, the effects are propagated such that parallel processing gives the appearance of a serial account, and that serial account is the “self” (44). The “computational theory of mind” asserts that consciousness is an emergent property from operations of a complex information-processing system (3, 99, 105, 149).
A key argument against teleological explanations in physiology has to do with timing. In considering Nagel’s Mind and Cosmos, Michael Ruse wrote, “What does one mean by “teleology,” or more specifically, what is a “teleological explanation”? It is a form of explanation that makes reference to causes that can be understood only in terms of the future (139).”
As explained below, however, instinct, imprinting, and Pavlovian and operant conditioning can readily explain how neurobehavioral, autonomic, and physiological changes can and often do occur before an anticipated event. Indeed, “feedforward” adjustments are probably far more prevalent in human daily life and more efficient in maintaining homeostasis than are reactive internal reflexes.
Mayr’s Teleonomic Processes and Programs
The evolutionary biologist and philosopher Ernst Mayr (1904–2005) proposed “teleonomic” forms of end-directed processes. To Mayr, a teleonomic process or behavior is goal-directed, and the goal-directedness depends on the operation of a program (108), “coded or prearranged information that controls a process (or behavior) leading it toward a goal. The program contains not only the blueprint of the goal but also the instructions of how to use the information of the blueprint. A program is not a description of a given situation but a set of instructions persisting toward an end point under varying conditions, where the end state of the process is determined by its properties at the beginning.” Mayr emphasizes that “the goal of a teleonomic activity does not lie in the future, but is coded in the program” (108). Programs enabling organisms to make and respond to predicted events or situations could provide key survival advantages, although natural selection itself of course operates only in the present, without anticipation.
Mayr’s teleonomic processes address “why” questions. In Mayr’s words,
“To be sure, questions that begin with “What?” and “How?” are sufficient for explanation in the physical sciences. However, since 1859, no explanation in the biological sciences had been complete until a third kind of question was asked and answered: “Why?” It is the evolutionary causation and its explanation that is asked for in this question. Anyone who eliminates evolutionary “Why?” questions closes the door on a large area of biological research…“Why?” questions do not introduce a metaphysical element into the analysis, and…there is no conflict between causal and teleological analysis, provided it is precisely specified what is meant by “teleological.”
Systems biology avoids “why” questions. The essence of systems biology is description and prediction from complexity. “Function” in systems neurobiology refers to neuronal networks, processes, and behaviors that at least theoretically can be observed, not to purposes or goals that cannot be observed.
The Marvelous Mesh
The remarkable history of the rete mirabile, to which this essay will return repeatedly, demonstrates the risk of conceptualizing purposes. The second century Greek physician Galen taught that the arterial blood containing the vital spirit would be filtered by a network of blood vessels at the base of the brain, the rete mirabile, which would produce the animal spirit responsible for cognitive functions. The animal spirit would then be distributed by tubes to the body organs, enabling them to function in concert, or sympathy, with each other. This is the origin of the phrase “sympathetic nervous system.”
Galen’s concept was scientific in that it generated predictions that observation or experimentation could test (167), although testing it did take 13 centuries. He depended on dissections of farm animals such as oxen, since human autopsies were forbidden in his time. Although ruminants (and sea mammals, discussed below) possess retia mirabilia, humans do not. This fact of course vitiated Galen’s theory about the purpose of the rete mirabile.
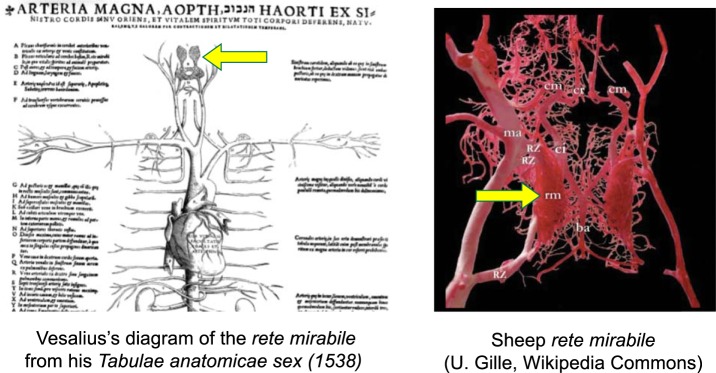
Andreas Vesalius (1514–1564) was the first to recognize Galen’s mistake. Initially Vesalius supported Galen’s notions. For instance, in 1538 he drew and wrote in his book Tabulae Anatomicae Sex (163) about “a reticular plexus at the base of the brain, the rete mirabile…wherein the vital is elaborated into the animal spirit.” (Fig. 2) (140). Five years later, however, in his De humani corporis fabrica libri septem, Vesalius wrote, “I cannot sufficiently marvel at my own stupidity; I who have so labored in my love for Galen that I have never demonstrated the human head without that of a lamb or ox, to show in the latter what I could not in the former….” (148).
Fig. 2.
The rete mirabile (“marvelous meshwork”). Left: Vesalius’s 1538 diagram, including the rete mirabile (yellow arrow). Right: the rete mirabile (rm) of a sheep. ba, Basilar artery; ci, internal carotid artery; cr, rostral cerebral artery; cm, middle cerebral artery; ma, maximillary artery; RZ, Retezuflüsse (rete tributaries).
No one knows the purposes of the rete mirabile. The same anatomic substrate might have been the basis for evolution to serve different physiological functions in different ecological niches. For instance, a vascular countercurrent system may have evolved to cool the brain (150), especially during exercise (11). Heat transfer occurs when relatively cool venous blood from the nasopharyngeal mucosa flows into the pterygoid plexus surrounding the carotid rete mirabile at the base of the brain. Delivery of blood that is cooler than the core temperature could also result in decreased sweating in a hot environment and consequently provide a mechanism for water preservation (124, 153). The rete mirabile dampens blood pressure oscillations at the level of the brain, and this could be important in the giraffe when the animal stoops to drink and the brain is below the level of the heart. In sea mammals, the large surface area of the rete mirabile and the damping of pressure oscillations may prevent dissolved nitrogen coming out of solution during rapid ascent to the sea surface.
These explanations reflect the approach of an integrative physiologist. The purposes of the rete mirabile in ruminants, giraffes, or sea mammals would not be the concern of a systems biologist.
From Teleology to Biocybernetics
In the early 1940s, the Mexican physician Arturo Rosenblueth became acquainted with Norbert Wiener and Julian Bigelow at the Massachusetts Institute of Technology during Rosenblueth’s stay in Cannon’s laboratory at Harvard. The three had the idea that control systems could enable machines to self-regulate based on feedback.
In an essay published in 1943, “Behavior, purpose and teleology,” Rosenblueth et al. (138) classified behavior in terms of 1) active or nonactive; if active then 2) purposeful or nonpurposeful; if purposeful then 3) feedback-regulated, i.e., “teleological,” or non-feedback regulated; and if teleological then 4) predictive (extrapolative) or nonpredictive. Teleology was not taken to imply “final causes” in the sense of Aristotle’s telos but was viewed mechanistically as synonymous with purpose controlled by feedback. Rosenblueth et al. (138) also argued that the “broad classes of behavior are the same in machines as in living organisms,” regardless of the complexity of the behavior.
This was several years before Wiener’s book appeared in which he first used the term, “cybernetics” (165). He and his colleagues were attempting to develop a theory of control systems that would be applicable to a wide variety of disciplines, including biology–“biocybernetics.” Wiener and Schade (166) distinguished two forms of biocybernetics, medical biocybernetics, and neurocybernetics, as follows.
“[N]eurocybernetics…is concerned with the pathways of action via sense-organs, neurons and effectors because of the fact that cybernetics is primarily concerned with the construction of theories and models. The symbols and hardware in neurocybernetics resemble more closely the elements of the nervous system and the sense-organs. Medical cybernetics is where homeostasis or the maintenance of the internal constant environment is the main consideration. There is no sharp distinction between these two fields.... (166)”
It is not difficult to conceptualize correspondences of integrative physiology with medical cybernetics and of systems neurobiology with neurocybernetics.
MODELS
Both integrative physiology and systems biology depend on explanatory models (137).
Ashby and Good Regulators
W. Ross Ashby was a founder of the field of cybernetics (6). Ashby’s “good regulator” theorem, which Conant and Ashby (35) proved mathematically in 1970, states that a good regulator models well the system it regulates. The verb “models” here means that each variable of the regulator corresponds to one and only one of the variables being regulated, like the teeth of a good key correspond well to the lock. “Good” means that the regulator is maximally efficient and simple. Conant and Ashby (35) proposed that the living brain learns to model its environment. Ashby (5) viewed the occurrence of good regulation as the product of eons during which natural selection has acted on requisite variety of control systems. Wiener agreed (165) when he wrote that both “phylogenetic learning” and “ontogenetic learning” are modes by which an animal can adjust itself to its environment.
Damasio’s Somatic Marker Hypothesis and Rejection of Mind-Body Duality
The philosopher Rene Descartes (1596–1650) followed a long religious tradition separating the mind from the body. The central tenet of Cartesian mind-body dualism is that humans possess a material body and an immaterial mind. Reality consists of the res extensa, physical matter, and the res cogitans, the conscious mind.
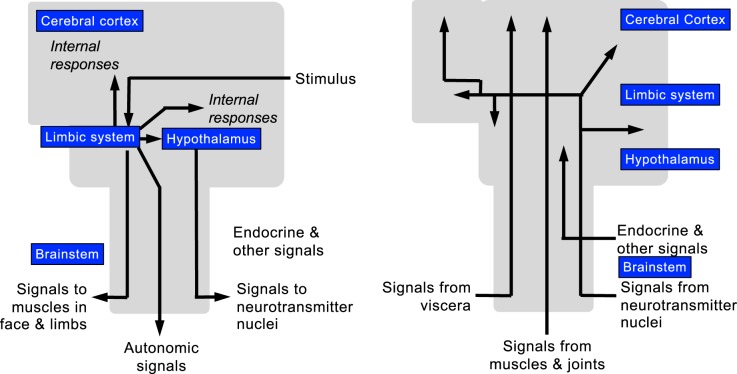
In his book, Descartes’ Error, Antonio Damasio takes the opposite position (38), according to which the brain and body interact complexly to generate the mind. Damasio conceptualizes that afferent information to the brain comes from sense organs conveying signals from outside the body and also comes from inside the body in the form of signals ascending in the central nervous system from brainstem neurotransmitter nuclei, muscles and joints, visceral organs, and the circulation (e.g., hormones, cytokines) (Fig. 3). Stimuli reaching limbic/hypothalamic centers result not only in ascending signals to the outer cortex but also in descending signals to muscles in the face and limbs, the autonomic nervous system, brainstem neurotransmitter nuclei, and the endocrine and immune systems.
Fig. 3.
Central neural levels and pathways mediating responses to an external signal (stimulus) (left) and internal signals (right). Adapted with permission (38).
Damasio (38) draws a distinction between emotions and feelings, defining feelings as mental experiences of body states that arise from interpretation of emotions by the brain. Hypothesized “somatic markers” are the feelings associated with emotions. The experience of an emotion in the sense described originally by Schachter and Singer (144) depends on cognitions appropriate for such an emotion coupled with physiological arousal. The latter aspect is where the autonomic nervous system enters the picture. For instance, Hohmann (83) conducted structured interviews about experienced emotional feelings of patients with spinal cord transections. The patients reported decreased feelings of sexual urge, fear, and anger, presumably because of decreased afferent information ascending via the spinal cord to the brain and decreased sympathetic efferent outflows descending via the spinal cord from the brain.
Damasio’s model (38) resolves a long-standing debate in physiological psychology about the James-Lange versus Cannon-Bard theories of emotion (26). According to the James-Lange theory, emotion is the product of bodily sensations about physiological arousal reaching the brain, whereas according to the Cannon-Bard theory, emotions drive physiological changes. The somatic markers hypothesis incorporates both processes.
To Damasio (38), consciousness may be primitive “core consciousness,” as in subhuman animals, or “extended consciousness,” as in humans. Extended consciousness requires a concept of the self and memory of one’s own emotions and feelings. That is, as humans we include in our modeling the consequences of what we do or feel in terms of their effects on us. By Damasio’s definition, Hal, the mutinous computer in the science fiction book by Arthur C. Clarke, 2001: A Space Odyssey, had extended consciousness:
“He might have handled it—as most men handle their own neuroses—if he had not been faced with a crisis that challenged his very existence. He had been threatened with disconnection; he would be deprived of all his inputs, and thrown into an unimaginable state of unconsciousness (34).”
In Damasio’s recent book, The Strange Order of Things (40), he presents the view that homeostasis is a kind of force ensuring that “life is regulated within a range that is not just compatible with survival but also conducive to flourishing, to a projection of life into the future of an organism or a species.” Damasio (40) seems to be returning here to Aristotle’s telos, with homeostasis a driving factor and not merely an emergent outcome. About feelings and homeostasis he writes, “…feelings…[are] the subjective experiences of the momentary state of homeostasis within a living body…” Systems biology may be insufficient to explain the feeling of what happens, as Damasio entitled another one of his books (39).
What is Missing is Ourselves
The evolutionary biologist Richard Dawkins states near the beginning of The Blind Watchmaker that biology is “the study of complicated things that give the appearance of having been designed for a purpose” (42). As humans, we seem to be continually seeking out purposes for what we experience.
Daniel Kahneman (Nobel Prize, 2002) has described two types of thinking (89), System 1 and System 2. System 1 thinking is fast and intuitive, uses little energy, and enables multitasking but incorporates preconceptions and biases, and therefore, it is prone to erroneous decision making. System 2 thinking is slow, analytical, unbiased, and more likely to lead to correct decision making but is slower, uses more energy, and obviates multitasking.
An example of System 1 teleological thinking is that the body contains a thermostat that has the “purpose” of regulating core temperature. System 2 thinking leads one to the conclusion that not only does the body’s thermostat not have a purpose, there is no thermostat at all. The thermostat is unnecessary for describing the complex and dynamic mechanisms determining core temperature.
An article published in Science in September 2018 reveals both teleological thinking and the human urge to see purposes in phenomena (158). A caterpillar chews on a leafy plant. Rapidly within the plant there is long-distance transmission of ionized intracellular calcium mediated by the amino acid glutamate (a prominent neurotransmitter in animals). The first sentence of the report reads, “Plants respond within minutes to stresses such as wounding with both local and system-wide reactions that prime nondamaged regions to mount defenses.” (italics added). The statement is teleological and reveals the investigators’ purpose-seeking mindset. Stressors and stress responses are externally observable, but stress is an unobserved condition or state aroused by a stressor that “drives” a stress response. Stresses as states or forces are not observable, but we believe they exist anyway. Similarly, another article published in Science in January 2019 about mechanisms of baroreceptor mechanotransduction begins, “Blood pressure (BP) is tightly regulated to ensure that the body is prepared to meet varied daily activity demands.” (italics added)
HIERARCHIES
Rather than conceptualize the presence of homeostats located in specific brain areas, it seems more appropriate to theorize that there are hierarchies of input-output relationships at several levels in the brain, with modulation of those relationships by ascending and descending signals (Fig. 4). Homeostasis would then be the product of activities in these hierarchies, which are neuroanatomic and neurochemical at the same time.
Fig. 4.
The central autonomic network (CAN). Red arrow indicates neuronal input from a variety of sensors to the nucleus of the solitary tract (NTS). Magenta arrow indicates humoral input via circumventricular organs such as the area postrema (AP). A5, A5 area; AMY, amygdala; ANS, autonomic nervous system; CING, cingulate cortex; CVLM, caudal ventrolateral medulla; DMNX, dorsal motor nucleus of the vagus nerve; HACER, hypothalamic area mediating conditioned emotional responses; Hippo, hippocampus; insula, insular cortex; LC, locus ceruleus; PAG, periaquaductal gray region; PBN, parabrachial nucleus; Pre-Bötz, pre-Bötzinger complex; PVN, paraventricular nucleus; raphe, raphe nuclei; RPG, respiratory pattern generator; RTN, retrotrapezoid nucleus; RVLM, rostral ventrolateral medulla; VTA, ventral tegmental area. The anatomic connections in the CAN are complex, but this underestimates the actual complexity because of subnuclei, chemical pathways with different neurotransmitters and cotransmitters, and receptor subtypes and locations.
Cannon recognized the key roles the brain plays both in elaborating “emergency” responses and in coordinating body systems to keep values for internal variables within physiological bounds (29). Cannon and Britton (30) reported that cerebral decortication evokes rage behavior accompanied by hyperglycemia; decorticated adrenalectomized animals exhibit the same behavior but without hyperglycemia. These findings were consistent with cortical restraint of primitive emotional behaviors and of emotion-associated adrenomedullary secretion. Cannon’s student Philip Bard used serial brain sectioning in decorticate animals to obtain evidence that physiological concomitants of primitive emotions originate in the hypothalamus (12). The phenomenon of “sham rage” in decorticate animals fits with the view that overall the cerebral cortex exerts inhibitory restraint on the hypothalamic expression of primitive emotional behaviors. On the other hand, stimulation of baroreceptor afferents inhibits sham rage (13).
In the 1920s to 1930s, W. R. Hess focused on hypothalamic regulation of parasympathetic and sympathetic outflows and their behavioral concomitants. He showed that stimulation of posterior hypothalamic sites that altered functions of internal organs via sympathetic outflows also evoked appropriate behaviors directed toward the environment (“ergotropic” effects). Stimulation of anterior sites evoked signs consistent with generalized parasympathetic activation that were also associated with characteristic behaviors (e.g., postural change associated with defecation), which Hess viewed as protective against overloading (“trophotropic”). The sympathetic-ergotropic and parasympathetic-trophotropic areas seemed to operate in a state of dynamic equilibrium (82). These findings, for which Hess received a Nobel Prize in 1949, reinforced the view that autonomic functions depend crucially on the central nervous system and that higher centers modulate autonomic outflows in coordinated neuroendocrine and behavioral patterns.
The “central autonomic network” (Fig. 4) conceptualized by the neurologist Eduardo Benarroch (16) includes (in ascending order in the neuraxis) the caudal ventrolateral medulla (CVLM), nucleus of the solitary tract (NTS), dorsal motor nucleus of the vagus, the nucleus ambiguus (NA), rostral ventrolateral medulla (RVLM), midline raphe nuclei, locus ceruleus (LC), periaqueductal gray matter (PAG), parabrachial nuclear complex (PBN), paraventricular nucleus of the hypothalamus (PVN), hypothalamic area mediating conditioned emotional responses (HACER), amygdala (AMY), hippocampus (Hippo.), and insular, anterior cingulate (CING), and retro-orbital or prefrontal cortex. Tract tracing with pseudorabies virus has confirmed that five cell groups in the brain determine sympathetic outflows (152): the PVN, A5 noradrenergic cell group, raphe nuclei, ventromedial medulla, and RVLM. Other brain areas transneuronally labeled after infection of the superior cervical and stellate ganglia are the PAG and lateral hypothalamus. The retrotrapezoid nucleus (RTN), pre-Bötzinger nucleus, and respiratory pattern generator (RPG) have been added to the network, as respiration may be considered to be an autonomic function. The ventral tegmental area (VTA) has also been added because of its role in neurobehavioral phenomena such as mood and reinforcement.
One can theorize that hierarchical networks involving input-output relationships continuously orchestrate and learn adaptive patterns of observable behaviors, cognition, memory, mood, and autonomic systems. Taken together, these networks function as “good regulators” determining levels of internal variables and act as if there were homeostatic comparators–i.e., “homeostats” (1, 70, 91).
Regulation of different variables involve shared brainstem centers (90). For instance, a key common site of initial synapse formation for internal reflexes is the NTS, and a key common site of output to the sympathetic preganglionic neurons is the RVLM. Neurons in the RVLM mediate sympathoneural and adrenomedullary responses to a variety of stressors, such as glucoprivation and systemic hypotension. Whether RVLM neurons constitute part of a final common pathway regardless of the stressor [in line with Selye’s doctrine of nonspecificity (66, 146)] or have a degree of stressor specificity (129) remains unsettled.
Combinations of functional magnetic resonance imaging with laboratory psychological challenges in healthy volunteers and in patients with particular brain or autonomic lesions have indicated specific roles of nodes in this hierarchy in maintaining homeostasis. For instance, Craig (35a) has argued that the parabrachial nucleus (PBN) is the main integration site for all homeostatic afferent activity and that the anterior insular cortex is the site for the subjective image of oneself as a sentient entity, i.e., emotional awareness. The latter seems to fit with Damasio’s somatic marker hypothesis (33).
Classically conditioned behaviors involve the limbic system and medial prefrontal cortex. Consistent with Hess’s view (82), hypothalamic activity patterns are modulated by the limbic centers, which reflect emotions, habituation, sensitization, imprinting, and classical conditioning. Expression of conditioning-related neural activity in amygdala and insula depends on both cognitions and representations of bodily states of autonomic arousal (36), such as via input from cardiac baroreceptors (73). This interpretation provides a neuroanatomic substrate for the proposal by Schachter and Singer (144) about a half century ago of cognitive and physiological determinants of emotional experience.
Executive functions and simulations of future events are mediated at the level of the outer frontal cortex. Higher cortical centers are responsible for cognition, instrumentally conditioned learned behaviors, predictions of future events, and interpretations of environmental and social stimuli.
Benarroch’s (16) central autonomic network does not incorporate coordination with movement, but both the sympathetic noradrenergic neuronal system and the sympathetic adrenergic hormonal system are hardwired to portions of the cerebral cortex responsible for motor outflows (recall the etymology of emotion, from the Latin ēmoveō, “to move”). Dum et al. (47) used transneuronal retrograde transport of a nonpathogenic form of the rabies virus to identify cortical areas that communicate through multisynaptic connections with the adrenal medulla. They identified two general cortical sources of output: a broad network of lateral cortical motor areas that are involved with movement selection, preparation, and execution and a smaller medial network in multiple cingulate cortical areas that are involved with cognition and emotion. The association between movement and autonomic outflows fits well with Cannon’s concept of “fight or flight,” according to which sympathoadrenal stimulation prepares the organism for potentially life-saving extreme physical exertion (29).
HOW HOMEOSTASIS HAPPENS
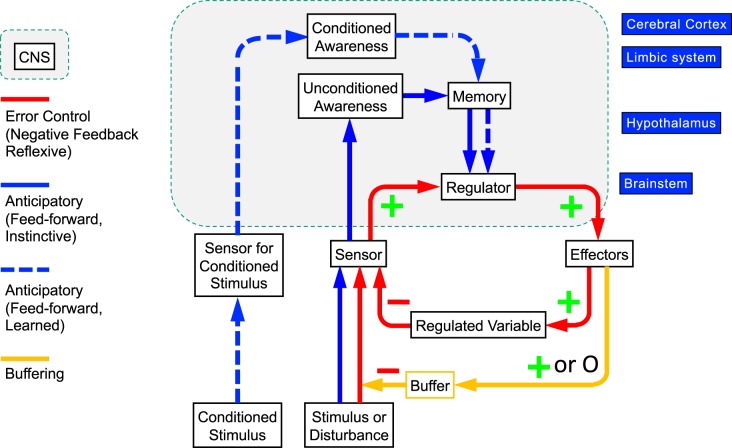
Irwin J. (“Irv”) Kopin near the end of his life proposed with me that three types of processes maintain homeostasis (Fig. 5) (67). The first and most well-known is error control by negative feedback regulation, which applies to the internal reflexes described below. The second is anticipatory (“feedforward”) regulation. The third is buffering. Figure 5 shows the relationships of reflexive error control via negative feedback (red), buffering (tan), and anticipatory regulation (blue). The anticipatory control mechanisms can be instinctive (solid lines) or conditioned (dashed lines). A disturbance can arouse anticipatory instinctive responses by pathways involving awareness (conscious or unconscious), and an associated conditioned stimulus can arouse anticipatory responses by pathways involving awareness and conditioned learning. A disturbance is sensed by interoceptors (e.g., gastrointestinal hemorrhage) or exteroceptors (e.g., touching a hot iron).
Fig. 5.
Concept diagram illustrating 3 ways homeostasis happens: negative feedback regulation via internal reflexes, anticipatory regulation via conditioning or instinct, and buffering. In the model, the effectors are both autonomic and nonautonomic. Effector responses to a disturbance are determined by 3 forms of regulation. First, effector activities are determined from input-output (afferent-efferent) curves relating sensory input to effector output (error control by negative feedback). Second, via exteroceptive or interoceptive input, effector activities are altered by instinct or imprinting in advance of a change in the level of the regulated variable. Third, via exteroceptive input, effector activities are altered by associative learning (classical or instrumental conditioning), also in advance of a change in the level of the regulated variable. The extent of sensor activation in response to a disturbance is modulated by buffering. Buffering is a means of diminishing the intensity of an external disturbance, thereby reducing the required use of reflexive homeostatic mechanisms. Effector responses can also modify buffering in advance of a disturbance, via instinct or learned behaviors (e.g., piloerection during cold exposure, donning a jacket before entering a cold environment).
Internal Reflexes
Baroreflexes, chemoreflexes, glucose regulation, and temperature regulation have received extensive research attention in integrative physiology for many years. By the names of these reflexes, one can recognize their presumed purposes. The purpose of baroreflexes is homeostasis of blood pressure (173); the purpose of chemoreflexes is homeostasis of the Po2, Pco2, and pH of the arterial blood; the purpose of glucose regulation is homeostasis of this vital fuel, and the purpose of temperature regulation is homeostasis of core temperature.
Barostasis.
In the early 1920s, the German physiologist Heinrich Hering demonstrated in dogs that electrical stimulation of the carotid sinus nerve (later termed Heing’s nerve) decreased heart rate and blood pressure and that cutting this nerve produced opposite cardiovascular changes. Hering (80) was the first to show that bilateral transection of the carotid sinus nerve produces hypertension.
The carotid sinus baroreceptors are distortion receptors, not pressure receptors. In fact, no biological system has been discovered in which blood pressure is sensed directly. Stimulation of the carotid sinus baroreceptors by local stretch evokes the reflexive changes in blood pressure and heart rate. All the baroreceptor afferent nerve traffic enters the central nervous system at the nucleus of the NTS in the medulla. Destruction of the NTS also produces labile hypertension (119) and excessive responses to conditioned stimuli (120).
An integrative physiologist could claim that the NTS is the site of the “barostat” determining the autonomic responses, depending on the sensed discrepancy between afferent information and a set point for responding (52, 53). Interestingly, the current use of the term barostat refers to a device for regulating gastrointestinal tone by monitoring gut volume changes and delivering controlled distensions to maintain a set pressure (160), but the idea is essentially the same.
At hypothalamic and limbic system levels there are yet other input-output relationships for emotional, behavioral, or arousal states that impact barostatic function. Thus, stimulation of the PVN attenuates responses of neurons of the medullary NTS that are activated when phenylephrine is injected to increase blood pressure (46). Increased activity in limbic system centers such as the amygdala and hippocampus as part of classically conditioned fear alters the relationship between PVN activity and blood pressure. Both elicitation of the “defense” reaction and sciatic nerve electrical stimulation modulate the carotid sinus baroreflex (93, 94).
Finally, at higher cortical levels there are yet other input-output relationships for executive functions, psychosocial restraint, operantly conditioned behaviors, and simulations, all of which modulate the relationship between baroreceptor afferent and autonomic efferent traffic. Exercise is well known to alter baroreflex-cardiovagal functions, although researchers have disagreed about the exact changes in baroreflex function curves (18, 21, 88). Carotid sinus baroreceptors continue to regulate arterial pressure and heart rate during exercise, but due to “central command” they are reset to regulate blood pressure around an exercise-induced increased setpoint (88). Baroreflex function can also be classically conditioned (48). Consistent with classical conditioning of baroreflex-cardiovagal gain, in baboons trained by operant conditioning to increase diastolic blood pressure in 12-h sessions beginning at 12 noon, baroreflex-cardiovagal gain was found to decrease in advance of the sessions and to increase before the sessions ended, without concurrent alterations in blood pressure itself (65).
The pathways and interactions are highly complex and have not yet been defined specifically in humans; overall the system operates as if there were feedforward regulation of the “barostat.” That the baroreflex has the “purpose” of regulating blood pressure and that the NTS is the barostat are teleological concepts. The barostat is a kind of metaphor for a complex neuronal network involving multiple centers at multiple levels in the neuraxis.
One can even argue that the purpose of baroreceptors is not to regulate blood pressure at all but to regulate delivery of oxygenated blood to the brain. Thus, in aquatic animals the body is surrounded by about the same pressure much of the time, and whales, dolphins, and porpoises do not have carotid sinus baroreceptors, because they do not have carotid sinuses. They have retia mirabilia.
Chemoreflexes.
“Chemoreflexes” involve sensory information about pO2, pCO2, and pH at carotid body chemoreceptors and at sensors in the brainstem (76). Afferents from the carotid bodies synapse in the NTS, which relay to the “respiratory pattern generator” (RPG, Fig. 4) (77).
During exercise, central command and metaboreflexes from exercising muscles stimulate ventilation to maintain arterial pCO2 and pH; however, the neural circuits underlying central command and metaboreflexes affecting breathing remain incompletely understood (76). Consistent with the view that breathing is in part controlled by feed-forward Pavlovian conditioning, adding resistive loads to consecutive inspirations results in anticipatory increases in inspiratory motor drive (171).
No amount of description of chemoreflex pathways maintaining blood oxygen and carbon dioxide levels and pH can convey the sense of breathlessness, air hunger, or distress experienced during asphyxiation or the daytime fatigue and drowsiness attending sleep apnea.
Glucostasis.
A variety of neuroendocrine factors determine glucose levels, including glucose-regulatory hormones, insulin, glucagon, adrenaline, cortisol, and growth hormone. The fact that blood glucose concentrations are unchanged by exercise despite very high rates of glucose flux suggests a form of “integral control” (91).
If glucose levels are regulated by so many factors, why is there type 2 diabetes mellitus or “metabolic syndrome?” One concept proposes a “flip-flop” insulin/glucagon controller. In metabolic syndrome, disruption of the flip-flop mechanism by amyloid accumulation in the pancreatic islets in type 2 diabetes mellitus could lead to hyperglucagonemia, hyperinsulinemia, insulin resistance, glucose intolerance, and impaired insulin responsiveness to hyperglycemia (91). Another potential explanation is altered negative feedback inhibition exerted by pancreatic islet δ-cells on nearby insulin-secreting β-cells (84).
These explanations are far removed from the feelings of hunger or satiety or the “sweet tooth” that so importantly influence glucose ingestion by humans. Glucose is sensed in the brain (131), and glucose-sensing cells determine eating responses to glucoprivation (136). Hyperglycemia can also be classically conditioned (170) and is a well-known correlate of experienced distress (79).
An experiment done by Walter B. Cannon more than a century ago illustrates the role of gastric contractions in the feeling of hunger (27, 31). A colleague accustomed himself to having a tube ending in a rubber balloon in his stomach so that intragastric pressure could be monitored over time by inflating the balloon and recording its pressure using a water manometer and float recorder. The subject fasted and reported when he felt hunger pangs, which were noted on the pressure recording. The report of feeling pangs consistently followed the onset of the phasic increases in pressure, in accordance with Damasio’s somatic marker hypothesis (and ironically, in conflict with the Cannon-Bard theory). Cannon and Washburn (31) concluded the following:
“Hunger…is normally the signal that the stomach is contracted for action; the unpleasantness of hunger leads to eating; eating starts gastric secretion, distends the contracted organ, initiates the movements of gastric digestion, and abolishes the sensation…The periodic activity of the alimentary canal in fasting…is…an exhibition in the digestive organs of readiness for prompt attack on the food swallowed by the hungry animal.”
Systems biology has not yet incorporated the feeling of hunger, much less the role of stomach contractions in the feeling of being hungry, in models of glucose regulation.
Thermostasis.
Even in the primitive brain of the fruit fly, there is a form of coding of temperature (54, 57). The antennae contain hot-sensing and cold-sensing neurons that project to distinct but adjacent central glomeruli, and second-order neurons can respond to both heat and cold (54). Because the flies are not poikilothermic, they must sense and react rapidly to altered external temperature to survive. Silencing the hot- or cold-sensing neurons results in distinct deficits in avoidance locomotor responses to reach places with appropriate temperature. In the words of the first author, “The role of our senses is to create an internal representation of the physical and chemical features of the external world,” and by implication the region of these glomeruli is the site of the modeling.
Mammals have a brainstem network of neural pathways for thermoregulation (116). These pathways are associated with behaviors such as sweating, panting, saliva spreading, avoiding heat, and seeking shade in response to heat and sun exposure, shivering, piloerection, stomping, and blowing on the fists in response to cold. Some of these adjustments are instinctive and some conditioned (37).
It has been proposed that four types of effectors mediate thermoregulatory responses to cold: cutaneous vasoconstriction limiting heat loss, shivering, sympathetic noradrenergic outflow to brown adipose tissue (BAT) for thermogenesis (115), and, at least when core temperature actually declines, adrenaline (55, 56). Cooling of the preoptic area increases sympathetic outflow to BAT and augments BAT thermogenesis (114), whereas activation of vagal afferent traffic decreases sympathetic outflow to BAT and inhibits BAT thermogenesis (104). Tupone et al. (159) described “thermoregulatory inversion,” in which BAT thermogenesis decreases in response to skin cooling. This phenomenon occurs after transection of the neuraxis rostral to the dorsomedial hypothalamus in anesthetized rats.
These concepts about temperature regulation do not consider that in humans anticipatory regulation and buffering largely determine how temperature is actually controlled; if you are planning to venture out into the cold, you put on a jacket. Even looking out the window at the frozen outdoors could arouse a variety of conditioned responses before you go outside (51, 98, 145). Descriptions of thermoregulatory pathways and mechanisms do not explain the feeling of being hot or cold.
Anticipatory Regulation
Patterned stress responses may be instinctive, imprinted, or learned. Instinct is a genetically determined response that is independent of adaptability. An autonomically mediated reflex at the level of the lower brainstem can be considered instinctive. Imprinting refers to an environmentally elicited, largely but not exclusively genetically determined behavior (e.g., newly hatched ducklings follow the first moving object they see; see Ref. 101). Pavlovian (classical) conditioning is a form of associative learning. Instrumental (operant) conditioning, which affords the greatest amount of adaptability, involves acquisition or extinction of behaviors based on their consequences (reward or positive reinforcement and punishment or negative reinforcement).
Modeling is the basis for anticipatory regulation, which is far more efficient and less prone to system failure than negative feedback regulation. Feedforward regulation occurs by instinct, imprinting, Pavlovian conditioning, instrumental conditioning, and (especially in humans) cognitive simulations. This counters one of the classic arguments against teleological explanations, that responses cannot precede the stimuli that elicit those responses.
Buffering
Buffering is a means of diminishing the intensity of an external disturbance. Many mammals have fur, which creates a layer of motionless air as an insulator above the skin, whales have blubber, and birds have closely packed feathers. The barrier to heat loss can be enhanced by reflexive bristling of the hair, which is mediated by arrector pili muscle supplied by sympathetic noradrenergic nerves (32); this increases the depth of the layer of motionless air. Behavioral responses to buffer the cold include huddling, seeking shelter, hibernation, and migration. Humans can also don appropriate clothing, which is inserting a buffer. Thus, buffering can be energy dependent or energy independent. In Fig. 5, the + or 0 refers to buffering that is dependent on or independent of effector activation.
DARWINIAN MEDICINE: HOMEOSTASIS IN EVOLUTIONARY PERSPECTIVE
Suppose you are eating when something “goes down the wrong pipe.” Immediately, you reflexively cough. The mechanisms of sensation, the spinal cord and brainstem networks and neurotransmitters, and the efferent outflows to skeletal, visceral, and cardiac muscle have been described (22). The idea of a cough reflex, however, is a teleological concept, and the designated name for the reflex indicates the physiologist’s urge to seek out purposes. In this case, the “purpose” of the behavior seems straightforward, because choking is an immediate life threat, and an open airway is mandatory for homeostasis. Thus, lack of airway protection in multiple system atrophy, a rare form of chronic autonomic failure, increases the risk of death from aspiration pneumonia or from asphyxia after vomiting (147). No amount of analysis of the purpose (a province of integrative physiology) or mechanism (a province of systems biology) of the cough reflex explains why we humans have a risk of aspirating when we eat. This requires understanding of the phylogenetic steps that led to the confluence of the respiratory and gastrointestinal tracts at the back of the human pharynx (22), the province of “Darwinian medicine” (also called evolutionary medicine).
In their book, Why We Get Sick, Randolph M. Nesse and George C. Williams (123) considered the problem of aspiration from an evolutionary perspective. In the primordial fish that were our ancestors, lungs arose as an extension of gastrointestinal tract tissue (23). Dry land offered ecological niches that favored survival of animals that could take in oxygen by gulping air. With the evolution of land creatures, lungs replaced gills as the source of oxygen. The intersection between the feeding and breathing systems that causes our tendency to aspirate and the strong survival advantage of clearing the airway by coughing are part of our evolutionary heritage. Neither integrative physiology nor systems biology takes into account the natural selective pressures that were operational at the time of the ecological opportunities and genetic variations.
A recent Delphi study enumerated several core concepts of evolutionary medicine (75). Among these, the following seem especially relevant to homeostasis.
Evolutionary Tradeoffs
Evolutionary changes in one trait that improve fitness can be linked to changes in other traits that decrease fitness. Life history traits, such as age at first reproduction, reproductive lifespan, and rate of senescence, are shaped by evolution and have implications for health and disease.
Plasticity
Environmental factors can shift developmental trajectories in ways that influence health, and the plasticity of these trajectories can be the product of evolved adaptive mechanisms.
Defenses
Many signs and symptoms of disease (e.g., fever) are useful defenses, which can be pathological if dysregulated.
Mismatch
Disease risks can be altered for organisms living in environments that differ from those in which their ancestors evolved.
Internal Reflexes in Evolutionary Perspective
Let us now reconsider baroreflexes, chemoreflexes, glucostasis, and temperature regulation with a Darwinian medical perspective.
The integrative physiologist would explain sympathetically mediated hypertension (69, 102) in terms of barostatic resetting, perhaps related to stress (50, 155, 156, 169); the systems biologist would explain this form of hypertension in terms of multiple alterations of functional relationships within the central autonomic network (41, 125). Neither approach accounts satisfactorily for why, given the multiplicity and redundancy of blood pressure regulatory mechanisms, hypertension exists at all. The Darwinian medical approach would consider survival advantages of maintaining blood flow to vital organs in response to hemorrhage, preserving cerebral blood flow during orthostasis, ingesting salt and calories whenever they become available, and even behavioral effects of high baroreceptor afferent traffic (49). All of these could have afforded survival advantages at the time new ecological niches opened up adaptive opportunities.
Integrative physiological and systems biological explanations also do not take into account the natural selective factors that were operative during the evolution of chemoreflexes. For instance, migrants to high-altitude regions in the western and eastern hemispheres encountered new ecological niches where genetic changes enhancing adaptation to hypoxic environments offered clear selective advantages. Andean high-altitude dwellers have high hemoglobin levels, which increases the delivery of oxygenated blood to body organs; however, this comes at the risk of chronic mountain sickness (4). Tibetans living at even higher altitudes do not have elevated hemoglobin levels. Numerous single-nucleotide polymorphisms have been located near the gene encoding hemoglobin production that correlates with relatively low hemoglobin levels (15). Instead, high-altitude-dwelling Tibetans have accelerated ventilatory responses to additional hypoxia, higher levels of nitric oxide (possibly increasing oxygen diffusion capacity), and more rapid blood flow to larger capillary beds in skeletal muscle (14).
Type 2 diabetes, obesity, and metabolic syndrome are well-known health burdens of modern humanity. Evolutionary pressures in operation over millions of years have resulted in humans ingesting too much of all foodstuffs. As Randolph Nesse has written (122),
“…one would think we would be designed to eat what is good for us. The system would work fine if we lived on the African savanna. In the natural environment, fat, salt, and sugar are in such short supply that when they are encountered, the useful response is to consume them. Fat provides twice as many calories per gram as carbohydrates. Sugar is often associated with ripe fruits, and seeking it out was usually beneficial. Now that we can choose our foods, we prefer what was in short supply on the African savanna.”
Bariatric surgery is the most successful known treatment for morbid obesity, and there are ancillary benefits in terms of amelioration of insulin resistance and hypertension (72, 112, 141). Nevertheless, even this type of treatment involves a disappointingly high recidivism rate (157, 161). One factor influencing the likelihood of weight regain after bariatric surgery may be geographic origin; there is more weight regain in African-Americans than Caucasian-Americans (157). Differential survival advantages of adaptation to different ecological niches during human evolution might explain this phenomenon.
Regarding thermostasis, neither systems biological nor integrative physiological explanations incorporate the natural selective factors that were at play when humans migrated to regions with different temperatures, humidity, barometric pressures, and seasonal light/dark cycles. The marked expansion of the brain during human evolution posed a challenge in terms of thermoregulation within cerebral tissue (24). Although humans rarely if ever have a carotid rete mirabile, it has been hypothesized that the diversity in human craniofacial features (e.g., nostril width) in different geographical regions reflects survival advantages of mechanisms for selective brain cooling in hot environments (85). Regulation of sympathetically mediated vasoconstrictor tone in the nasopharynx could also contribute to selective brain cooling (8). In humans, the segment of internal carotid artery passing through the cavernous sinus may be too short for countercurrent heat exchange. It is much more likely that in humans brain and core temperatures are regulated together via evaporative heat loss from the naked skin (20).
Pleiotropy, Senescence, and Homeostatic Capacity
Both Bernard (17) and Cannon (29) emphasized that the capacity to maintain the constancy of the inner world of the body decreases with aging. They paid little attention to diseases and none to chronic, multisystem disorders of senescence; however, modern medicine is increasingly concerned with the management of aging-related disorders of regulation. By allowing larger fluctuations of key internal variables, there is more allostatic load and a greater likelihood of induction of positive feedback loops.
In Why We Get Sick, Nesse and Williams (123) ask, “If senescence so devastates our fitness, why hasn’t natural selection eliminated it?...Our bodies do have some capacity to repair damage and replace worn-out parts; it is just that this capacity is limited. The body can’t maintain itself indefinitely. Why not?” They answer with an application of Williams’s pleiotropic theory. Pleiotropy is a situation where one gene produces more than one phenotypic trait. In the context of senescence, the pleiotropic theory states that genes that give a benefit in youth impose a cost with age. In Williams’s words, “…senescence results from genes that increase youthful vigor at the price of vigor later on…” (168).
Williams’s pleiotropy concept may apply to disorders associated with aging. Briefly, at least some of these conditions may exist because of an evolutionary tradeoff, i.e., enhanced survival in the young reproducers at the expense of degeneration of homeostatic systems in the elderly.
Allostasis, Allostatic Load, and Catecholaminergic Neurodegeneration
The concept of allostasis incorporates alterations in the tolerated steady-state level, i.e., “stability through change” (151). For example, a respiratory viral infection can be associated with a new apparent steady state involving low-grade fever, elevated heart rate, and malaise. These allostatic adjustments keep the regulated variables at altered levels.
Although allostatic adjustments are both compensatory and adaptive, with repetition and aging they might come at a cost over time, i.e., allostatic load, corresponding to long-term “wear and tear” (1). Many examples of allostatic load in experimental animals as well as in humans involve the effects of repeated episodes or chronic stress in which brain activation of neuroendocrine functions plays a role (110, 111). In systems biological terms, allostasis is manifested by shifts in input-output relationships. In integrative physiological terms, allostasis is manifested by adjustment of set points in reaction to and anticipation of changes in internal and external demands (1).
Multisystem diseases and disorders might reflect disturbances of regulation that lead to adverse effects of compensatory activation or of declining efficiency of homeostatic negative feedback loops. Factors such as stress, maladaptation, allostatic load, and diminished resilience may not only alter the manifestations but also determine the outcomes of acute pathophysiological phenomena such as tilt-induced neurally mediated hypotension and senescence-related, neurodegenerative disorders of catecholaminergic systems.
The autonomic nervous system operates exactly at the interface of the body and mind (38) and is a major determinant of the neurobiology of homeostasis (86). In the nascent clinical discipline of autonomic medicine (63, 103, 109, 142), dysregulation of homeostatic systems figures prominently in degenerative diseases in the elderly.
A group of senescence-related disorders involve degeneration of catecholaminergic neurons that use norepinephrine or dopamine as their chemical messengers. Individuals with relatively efficient central and autonomic catecholamine systems might have had survival advantages during their reproductive years in terms of being able to increase rapidly and massively the delivery of catecholamines to their receptors as part of vigilance (7), remembering distressing events (19, 43, 58, 132), initiating behaviors (2, 92), experiencing emotions and mood states (59, 144), learning classically conditioned responses (113, 143), acquiring and retaining appetitive or avoidance behaviors (25, 74, 130), feeling pain (9, 45, 71, 95, 134, 135), and temporarily reversing muscle fatigue (27).
The advantages associated with these adjustments, however, could have come at the cost of accelerated senescence of catecholaminergic neurons, a form of pleiotropy. The catecholamine “autotoxicity” theory (68) is based on inherent cytotoxicity of products of enzymatic or spontaneous oxidation of catecholamines in the cytoplasm of cells in which the catecholamines are produced. Most of the released catecholamine is recycled by neuronal reuptake. Stressors that evoke release of catecholamines in effect shift intraneuronal catecholamines from vesicular to cytoplasmic pools (64). Accordingly, repeated episodes of stress could cause neuronal injury via autotoxicity, and the more substantial the catecholamine release, the greater the amount of autotoxicity and the more likely the manifestations of aging-related catecholaminergic neurodegeneration. Thus, in rats, chronic restraint, which evokes activation of catecholaminergic neurons inside and outside the brain (96, 97, 126, 127), reduces the numbers of substantia nigra dopaminergic and locus ceruleus noradrenergic neurons (154), as is found in Parkinson’s disease (PD) (172).
Specifically, the catecholaldehyde hypothesis states that accumulation of the toxic catecholaldehyde 3,4-dihydroxyphenylacetaldehyde (DOPAL) is central to the degenerative process in PD. When sequestered in storage vesicles catecholamines are harmless, but in the cytoplasm they can undergo spontaneous or enzymatic oxidation to form toxic reaction products (81). Most of cytoplasmic dopamine undergoes enzymatic oxidation catalyzed by monoamine oxidase (MAO) to form hydrogen peroxide and DOPAL. DOPAL is highly toxic (107, 128) via both increased oxidative stress (100) and modifications of numerous intracellular proteins that are important for catecholamine neuronal homeostasis (87, 162) such as α-synuclein (AS). DOPAL oligomerizes, aggregates, and forms quinone protein adducts with AS (87), and because DOPAL-induced AS oligomers impede vesicular functions (133), DOPAL-AS interactions could challenge neuronal homeostasis and eventually kill off catecholaminergic neurons, all because of pleiotropy.
What Good are Homeostats?
If homeostats are unnecessary and simplistic, what good are they? First, lumping the complex networks that make up the metaphorical regulators and conceptualizing purposes in controlling the regulated variables enable definitions of otherwise difficult ideas, such as stress. In stress, a homeostat senses a discrepancy between afferent information about the regulated variable and the set point for arousing a response. Stress is then the condition, and the error signal is the measure of the extent of stress. The integrated error signal could correspond to a kind of “memory” that would more be more efficient than the instantaneous error signal in returning the regulated variable to the set point value (61). The homeostat theory lends itself straightforwardly to computer models that define homeostatic resetting (allostasis), compensatory activation of alternative effectors, effector sharing, allostatic load, and induction of pathophysiological positive feedback loops (60–62).
A second reason for thinking in terms of homeostats is that it seems fruitful for bringing worthwhile conceptual questions to mind. To illustrate this, we return a last time to the rete mirabile. If the rete mirabile enabled selective brain cooling, then during exercise the temperature of the blood bathing the thermosensitive brainstem neurons should be less than the temperature of the blood in the carotid artery (10, 11). Doubt has been raised about whether the rete mirabile actually does enable selective brain cooling (106), but suppose it does. How would the brain’s thermostat then be able to sense and correctly regulate the core temperature? And why do humans lack a rete mirabile? In human evolution, were genetic changes fostering evaporative heat loss via the naked skin more adaptive than those fostering countercurrent temperature regulation via a rete mirabile (20)? These are questions an integrative or evolutionary physiologist might ask but a systems biologist would not.
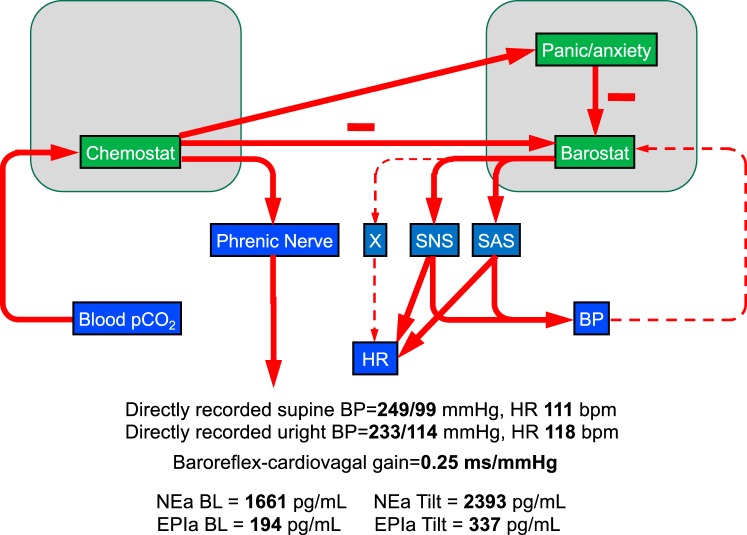
Third, the homeostat idea helps explain clinical phenomena in autonomic medicine. Consider the following case of a patient evaluated at the National Institutes of Health (NIH) Clinical Center for autonomically mediated hypertension (69, 102). He had “complex” sleep apnea, meaning that his condition worsened rather than improved with continuous positive airway pressure (CPAP). The complex sleep apnea included Cheyne-Stokes respiration (rhythmic cycles of hyperventilation and apnea during sleep), and he had learned that in patients with heart failure CO2 inhalation abolishes Cheyne-Stokes respiration (121). He brought with him and used during his hospitalization a modified CPAP device that administered CO2 via the mask. He had also been diagnosed with a form of renal tubular acidosis and panic/anxiety, and for these he took large doses of sodium bicarbonate and was on a clonidine patch (33). For autonomic function testing, the clonidine was tapered and eventually stopped after ∼2 wk. The directly recorded brachial systolic blood pressure was dangerously high at ∼250 mmHg. There were very high arterial plasma levels of norepinephrine and adrenaline, and baroreflex-cardiovagal gain was near zero (Fig. 6).
Fig. 6.
Baroreflex-chemoreflex interactions in a patient with self-induced hypercarbia. The patient slept each night with a positive airway pressure device into which carbon dioxide was pumped to treat Cheyne-Stokes respiration. Chemoreflex activation released the sympathetic noradrenergic system (SNS) and sympathetic adrenergic system (SAS) from barostatic restraint, blocked baroreflex-cardiovagal outflow (X), and evoked panic/anxiety, resulting in extreme hypertension and tachycardia and high arterial plasma levels of norepinephrine (NEa) and epinephrine (EPIa). BL, baseline; BP, blood pressure; HR, heart rate.
Analysis of the case was as follows. Inhaling CO2 produces metabolic acidosis by reacting with body water to generate carbonic acid. The patient’s chemostat sensed the hypercarbia and metabolic acidosis, and this released the sympathetic noradrenergic and adrenergic systems from barostatic restraint, blocked the cardiac baroreflex, and evoked a panicky feeling. High sodium bicarbonate intake exacerbated the hypertension.
The most effective therapy for this patient’s profound dysautonomia was education (63). The patient was counseled not to inhale CO2 via his CPAP device and to stop taking sodium bicarbonate.
Conclusions
In conclusion, just as internal models of reality in organisms have evolved, reaching the level of sophistication and complexity of our conscious minds, theories about such models seem to have evolved, from Aristotle’s telos to Galen’s rete mirabile; from Bernard’s milieu intérieur (17) and Cannon’s “useful end” (28) to Conent and Ashby’s good regulators (35), Wiener’s neurocybernetics (165), and Mayr’s teleonomic programming (108); from Descartes’s mind-body dualism to Damasio’s (39) somatic markers hypothesis; and from concept-driven integrative physiology and data-driven systems biology to Nesse and Williams’s evolutionary medicine (123). Rapprochement of integrative physiology with systems biology will require recognition that each of us has a System 1-thinking, intuitive, feeling mind that seeks out purposes and asks “why” questions and a System 2-thinking, rational, unfeeling mind that does not.
Perspectives and Significance
Homeostasis is a founding principle of integrative physiology but seems almost invisible in current systems biology. Is homeostasis a key goal driving body processes, or is it an emergent mechanistic fact? I propose that the integrative physiological and systems biological viewpoints about homeostasis reflect different epistemologies, i.e., different philosophies of knowledge. Integrative physiology explains biological phenomena by theories that experimentation or observation can test. In integrative physiology, “function” refers to goals or purposes. Systems biology explains biological phenomena in terms of “omics” data and depicts those data in complex computer models. In systems biology, “function” refers to mechanisms rather than goals. This essay reviews the history of the two epistemologies especially as they pertain to autonomic neuroscience. Physiology in the future will avoid teleological purposiveness, transcend pure mechanism, and incorporate adaptiveness in evolution, i.e.,“Darwinian medicine.”
GRANTS
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S.G. interpreted results of experiments; D.S.G. prepared figures; D.S.G. drafted manuscript; D.S.G. edited and revised manuscript; D.S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
I acknowledge with fondness and appreciation the mentorship I received from the late Dr. Irwin J. (“Irv”) Kopin.
REFERENCES
- 1.Acevedo A, Androulakis IP. Allostatic breakdown of cascading homeostat systems: A computational approach. Heliyon 3: e00355, 2017. doi: 10.1016/j.heliyon.2017.e00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albares M, Thobois S, Favre E, Broussolle E, Polo G, Domenech P, Boulinguez P, Ballanger B. Interaction of noradrenergic pharmacological manipulation and subthalamic stimulation on movement initiation control in Parkinson’s disease. Brain Stimul 8: 27–35, 2015. doi: 10.1016/j.brs.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Allen M, Friston KJ. From cognitivism to autopoiesis: towards a computational framework for the embodied mind. Synthese 195: 2459–2482, 2018. doi: 10.1007/s11229-016-1288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appenzeller O, Minko T, Pozharov V, Bonfichi M, Malcovati L, Gamboa J, Bernardi L. Gene expression in the Andes; relevance to neurology at sea level. J Neurol Sci 207: 37–41, 2003. doi: 10.1016/S0022-510X(02)00356-8. [DOI] [PubMed] [Google Scholar]
- 5.Ashby WR. Design for a Brain. London: Chapman & Hall, 1960. [Google Scholar]
- 6.Ashby WR. An Introduction to Cybernetics. London: Chapman & Hall, 1956. [Google Scholar]
- 7.Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res 88: 501–520, 1991. doi: 10.1016/S0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- 8.Azzaroni A, Parmeggiani PL. Postural and sympathetic influences on brain cooling during the ultradian wake-sleep cycle. Brain Res 671: 78–82, 1995. doi: 10.1016/0006-8993(94)01323-A. [DOI] [PubMed] [Google Scholar]
- 9.Bajic D, Proudfit HK. Projections of neurons in the periaqueductal gray to pontine and medullary catecholamine cell groups involved in the modulation of nociception. J Comp Neurol 405: 359–379, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Baker MA. A brain-cooling system in mammals. Sci Am 240: 130–139, 1979. doi: 10.1038/scientificamerican0579-130. [DOI] [PubMed] [Google Scholar]
- 11.Baker MA, Chapman LW. Rapid brain cooling in exercising dogs. Science 195: 781–783, 1977. doi: 10.1126/science.836587. [DOI] [PubMed] [Google Scholar]
- 12.Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. Am J Physiol 84: 490–515, 1928. doi: 10.1152/ajplegacy.1928.84.3.490. [DOI] [Google Scholar]
- 13.Bartorelli C, Bizzi E, Libretti A, Zanchetti A. Inhibitory control of sinocarotid pressoceptive afferents on hypothalamic autonomic activity and sham rage behavior. Arch Ital Biol 98: 308–326, 1960. [Google Scholar]
- 14.Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA 104, Suppl 1: 8655–8660, 2007. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P, Zheng YT. Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA 107: 11459–11464, 2010. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 68: 988–1001, 1993. doi: 10.1016/S0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 17.Bernard C. Lectures on the Phenomena of Life Common to Animals and Vegetables. Springfield, IL: Charles C. Thomas, 1974. [Google Scholar]
- 18.Bevegård BS, Shepherd JT. Regulation of the circulation during exercise in man. Physiol Rev 47: 178–213, 1967. doi: 10.1152/physrev.1967.47.2.178. [DOI] [PubMed] [Google Scholar]
- 19.Borodovitsyna O, Flamini MD, Chandler DJ. Acute stress persistently alters locus coeruleus function and anxiety-like behavior in adolescent rats. Neuroscience 373: 7–19, 2018. doi: 10.1016/j.neuroscience.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Brengelmann GL. Specialized brain cooling in humans? FASEB J 7: 1148–1152, 1993. doi: 10.1096/fasebj.7.12.8375613. [DOI] [PubMed] [Google Scholar]
- 21.Bristow JD, Brown EB Jr, Cunningham DJ, Goode RC, Howson MG, Pickering TG, Sleight P. Changes in baroreflex sensitivity at the transitions between rest and exercise. J Physiol 202: 84P–85P, 1969. [PubMed] [Google Scholar]
- 22.Brooks SM. Perspective on the human cough reflex. Cough 7: 10, 2011. doi: 10.1186/1745-9974-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown E, James K. The lung primordium an outpouching from the foregut! Evidence-based dogma or myth? J Pediatr Surg 44: 607–615, 2009. doi: 10.1016/j.jpedsurg.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Bruner E, De La Cuétara JM, Musso F. Quantifying patterns of endocranial heat distribution: brain geometry and thermoregulation. Am J Hum Biol 24: 753–762, 2012. doi: 10.1002/ajhb.22312. [DOI] [PubMed] [Google Scholar]
- 25.Campese VD, Soroeta JM, Vazey EM, Aston-Jones G, LeDoux JE, Sears RM. Noradrenergic regulation of central amygdala in aversive pavlovian-to-instrumental transfer. eNeuro 4: 4, 2017. doi: 10.1523/ENEURO.0224-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannon WB. Again the James-Lange and thalamic theories of emotion. Psychol Rev 38: 281–295, 1931. doi: 10.1037/h0072957. [DOI] [Google Scholar]
- 27.Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage. New York: D. Appleton, 1919. [Google Scholar]
- 28.Cannon WB. The Way of an Investigator. New York: W. W. Norton, 1945. [Google Scholar]
- 29.Cannon WB. The Wisdom of the Body. New York: W.W. Norton, 1939. [Google Scholar]
- 30.Cannon WB, Britton SW. Studies on the conditions of activity in endocrine glands. XV. Pseudoaffective medulliadrenal secretion. Am J Physiol 72: 283–294, 1925. doi: 10.1152/ajplegacy.1925.72.2.283. [DOI] [Google Scholar]
- 31.Cannon WB, Washburn AL. An explanation of hunger. Am J Physiol 29: 441–454, 1912. doi: 10.1152/ajplegacy.1912.29.5.441. [DOI] [Google Scholar]
- 32.Chaplin G, Jablonski NG, Sussman RW, Kelley EA. The role of piloerection in primate thermoregulation. Folia Primatol (Basel) 85: 1–17, 2014. doi: 10.1159/000355007. [DOI] [PubMed] [Google Scholar]
- 33.Charney DS, Heninger GR, Redmond DE Jr. Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci 33: 19–29, 1983. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- 34.Clarke AC. 2001: A Space Odyssey. New York: Penguin Books, 1993. [Google Scholar]
- 35.Conant RC, Ashby WR. Every good regulator of a system must be a model of that system. Int J Syst Sci 1: 89–97, 1970. doi: 10.1080/00207727008920220. [DOI] [Google Scholar]
- 35a.Craig AD. Cooling, pain, and other feelings from the body in relation to the autonomic nervous system. Handb Clin Neurol 117: 103–109, 2013. doi: 10.1016/B978-0-444-53491-0.00009-2. [DOI] [PubMed] [Google Scholar]
- 36.Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33: 653–663, 2002. doi: 10.1016/S0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham CL, Crabbe JC, Rigter H. Pavlovian conditioning of drug-induced changes in body temperature. Pharmacol Ther 23: 365–391, 1983. doi: 10.1016/0163-7258(83)90019-0. [DOI] [PubMed] [Google Scholar]
- 38.Damasio A. Descartes’ Error. Emotion, Reason, and the Human Brain. New York: Avon Books, 1994. [Google Scholar]
- 39.Damasio A. The Feeling of What Happens. New York: Harcourt Brace, 1999. [Google Scholar]
- 40.Damasio AR. The Strange Order of Things. Life, Feeling, and the Making of Cultures. New York: Pantheon Books, 2018. [Google Scholar]
- 41.Dampney RA, Michelini LC, Li DP, Pan HL. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am J Physiol Heart Circ Physiol 315: H1200–H1214, 2018. doi: 10.1152/ajpheart.00216.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawkins R. The Blind Watchmaker. New York: Norton, 1987. [Google Scholar]
- 43.Dębiec J, Bush DE, LeDoux JE. Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fear in rats—a possible mechanism for the persistence of traumatic memories in PTSD. Depress Anxiety 28: 186–193, 2011. doi: 10.1002/da.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dennett DC. Consciousness Explained. Boston, MA: Little, Brown, 1991. [Google Scholar]
- 45.Drummond PD, Finch PM, Skipworth S, Blockey P. Pain increases during sympathetic arousal in patients with complex regional pain syndrome. Neurology 57: 1296–1303, 2001. doi: 10.1212/WNL.57.7.1296. [DOI] [PubMed] [Google Scholar]
- 46.Duan YF, Kopin IJ, Goldstein DS. Stimulation of the paraventricular nucleus modulates firing of neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 277: R403–R411, 1999. doi: 10.1152/ajpregu.1999.277.2.R403. [DOI] [PubMed] [Google Scholar]
- 47.Dum RP, Levinthal DJ, Strick PL. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proc Natl Acad Sci USA 113: 9922–9927, 2016. doi: 10.1073/pnas.1605044113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dworkin BR, Dworkin S. Learning of physiological responses: II. Classical conditioning of the baroreflex. Behav Neurosci 109: 1119–1136, 1995. doi: 10.1037/0735-7044.109.6.1119. [DOI] [PubMed] [Google Scholar]
- 49.Dworkin BR, Filewich RJ, Miller NE, Craigmyle N, Pickering TG. Baroreceptor activation reduces reactivity to noxious stimulation: implications for hypertension. Science 205: 1299–1301, 1979. doi: 10.1126/science.472749. [DOI] [PubMed] [Google Scholar]
- 50.Esler M. Mental stress and human cardiovascular disease. Neurosci Biobehav Rev 74: 269–276, 2017. doi: 10.1016/j.neubiorev.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Flory GS, Langley CM, Pfister JF, Alberts JR. Instrumental learning for a thermal reinforcer in 1-day-old rats. Dev Psychobiol 30: 41–47, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 52.Folkow B. The pathophysiology of hypertension. Differences between young and elderly patients. Drugs 46, Suppl 2: 3–7, 1993. doi: 10.2165/00003495-199300462-00003. [DOI] [PubMed] [Google Scholar]
- 53.Folkow B. “Structural factor” in primary and secondary hypertension. Hypertension 16: 89–101, 1990. doi: 10.1161/01.HYP.16.1.89. [DOI] [PubMed] [Google Scholar]
- 54.Frank DD, Jouandet GC, Kearney PJ, Macpherson LJ, Gallio M. Temperature representation in the Drosophila brain. Nature 519: 358–361, 2015. doi: 10.1038/nature14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frank SM, Cattaneo CG, Wieneke-Brady MB, El-Rahmany H, Gupta N, Lima JA, Goldstein DS. Threshold for adrenomedullary activation and increased cardiac work during mild core hypothermia. Clin Sci (Lond) 102: 119–125, 2002. doi: 10.1042/cs1020119. [DOI] [PubMed] [Google Scholar]
- 56.Frank SM, Satitpunwaycha P, Bruce SR, Herscovitch P, Goldstein DS. Increased myocardial perfusion and sympathoadrenal activation during mild core hypothermia in awake humans. Clin Sci (Lond) 104: 503–508, 2003. doi: 10.1042/CS20020256. [DOI] [PubMed] [Google Scholar]
- 57.Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell 144: 614–624, 2011. doi: 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geracioti TD Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE Jr, Kasckow JW. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry 158: 1227–1230, 2001. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- 59.Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, Shekhar A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety 27: 339–350, 2010. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- 60.Goldstein DS. Adrenaline and the Inner World: An Introduction to Scientific Integrative Medicine. Baltimore, MD: Johns Hopkins University, 2006. [Google Scholar]
- 61.Goldstein DS. Computer models of stress, allostasis, and acute and chronic diseases. Ann N Y Acad Sci 1148: 223–231, 2008. doi: 10.1196/annals.1410.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldstein DS. Concepts of scientific integrative medicine applied to the physiology and pathophysiology of catecholamine systems. Compr Physiol 3: 1569–1610, 2013. doi: 10.1002/cphy.c130006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldstein DS. Principles of Autonomic Medicine. http://www.dysautonomiainternational.org/pdf/PAM_1_Intro.pdf, 2017.
- 64.Goldstein DS. Stress, allostatic load, catecholamines, and other neurotransmitters in neurodegenerative diseases. Endocr Regul 45: 91–98, 2011. doi: 10.4149/endo_2011_02_91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldstein DS, Harris AH, Brady JV. Baroreflex sensitivity during operant blood pressure conditioning. Biofeedback Self Regul 2: 127–138, 1977. doi: 10.1007/BF00998663. [DOI] [PubMed] [Google Scholar]
- 66.Goldstein DS, Kopin IJ. Evolution of concepts of stress. Stress 10: 109–120, 2007. doi: 10.1080/10253890701288935. [DOI] [PubMed] [Google Scholar]
- 67.Goldstein DS, Kopin IJ. Homeostatic systems, biocybernetics, and autonomic neuroscience. Auton Neurosci 208: 15–28, 2017. doi: 10.1016/j.autneu.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldstein DS, Kopin IJ, Sharabi Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol Ther 144: 268–282, 2014. doi: 10.1016/j.pharmthera.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldstein DS, Levinson PD, Zimlichman R, Pitterman A, Stull R, Keiser HR. Clonidine suppression testing in essential hypertension. Ann Intern Med 102: 42–49, 1985. doi: 10.7326/0003-4819-102-1-42. [DOI] [PubMed] [Google Scholar]
- 70.Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress 5: 55–58, 2002. doi: 10.1080/102538902900012345. [DOI] [PubMed] [Google Scholar]
- 71.Goldstein DS, Tack C, Li ST. Sympathetic innervation and function in reflex sympathetic dystrophy. Ann Neurol 48: 49–59, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 72.Graham C, Switzer N, Reso A, Armstrong C, Church N, Mitchell P, Debru E, Gill R. Sleeve gastrectomy and hypertension: a systematic review of long-term outcomes. Surg Endosc. In press. doi: 10.1007/s00464-018-6566-5. [DOI] [PubMed] [Google Scholar]
- 73.Gray MA, Beacher FD, Minati L, Nagai Y, Kemp AH, Harrison NA, Critchley HD. Emotional appraisal is influenced by cardiac afferent information. Emotion 12: 180–191, 2012. doi: 10.1037/a0025083. [DOI] [PubMed] [Google Scholar]
- 74.Groessl F, Munsch T, Meis S, Griessner J, Kaczanowska J, Pliota P, Kargl D, Badurek S, Kraitsy K, Rassoulpour A, Zuber J, Lessmann V, Haubensak W. Dorsal tegmental dopamine neurons gate associative learning of fear. Nat Neurosci 21: 952–962, 2018. doi: 10.1038/s41593-018-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grunspan DZ, Nesse RM, Barnes ME, Brownell SE. Core principles of evolutionary medicine: A Delphi study. Evol Med Public Health 2018: 13–23, 2017. doi: 10.1093/emph/eox025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron 87: 946–961, 2015. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Halter JB, Beard JC, Porte D Jr. Islet function and stress hyperglycemia: plasma glucose and epinephrine interaction. Am J Physio Endocrinol Physioll 247: E47–E52, 1984. doi: 10.1152/ajpendo.1984.247.1.E47. [DOI] [PubMed] [Google Scholar]
- 80.Hering HE. Die Karotissinusreflexe auf Herz und Gefasse. Dresden, Germany: Steinkopff, 1927. [Google Scholar]
- 81.Herrera A, Muñoz P, Steinbusch HWM, Segura-Aguilar J. Are dopamine oxidation metabolites involved in the loss of dopaminergic neurons in the nigrostriatal system in Parkinson’s disease? ACS Chem Neurosci 8: 702–711, 2017. doi: 10.1021/acschemneuro.7b00034. [DOI] [PubMed] [Google Scholar]
- 82.Hess WR. Das Zwischenhirn. Basel: Schwabe, 1949. [Google Scholar]
- 83.Hohmann GW. Some effects of spinal cord lesions on experienced emotional feelings. Psychophysiology 3: 143–156, 1966. doi: 10.1111/j.1469-8986.1966.tb02690.x. [DOI] [PubMed] [Google Scholar]
- 84.Huising MO, van der Meulen T, Huang JL, Pourhosseinzadeh MS, Noguchi GM. The different δ-cells make in glucose control. Physiology (Bethesda) 33: 403–411, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Irmak MK, Korkmaz A, Erogul O. Selective brain cooling seems to be a mechanism leading to human craniofacial diversity observed in different geographical regions. Med Hypotheses 63: 974–979, 2004. doi: 10.1016/j.mehy.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Jänig W. The Integative Action of the Autonomic Nervous System. Neurobiology of Homeostasis. New York: Cambridge Univeristy, 2006. [Google Scholar]
- 87.Jinsmaa Y, Sharabi Y, Sullivan P, Isonaka R, Goldstein DS. 3,4-Dihydroxyphenylacetaldehyde-induced protein modifications and their mitigation by N-acetylcysteine. J Pharmacol Exp Ther 366: 113–124, 2018. doi: 10.1124/jpet.118.248492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol 91: 27–36, 2006. doi: 10.1113/expphysiol.2005.032102. [DOI] [PubMed] [Google Scholar]
- 89.Kahneman D. Thinking, Fast and Slow. New York: Farrar, Strauss and Giroux, 2011. [Google Scholar]
- 90.Kakall ZM, Cohen EM, Farnham MM, Kim SJ, Nedoboy PE, Pilowsky PM. Integration of hindbrain and carotid body mechanisms that control the autonomic response to cardiorespiratory and glucoprivic insults. Respir Physiol Neurobiol. In press. doi: 10.1016/j.resp.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 91.Koeslag JH, Saunders PT, Terblanche E. A reappraisal of the blood glucose homeostat which comprehensively explains the type 2 diabetes mellitus-syndrome X complex. J Physiol 549: 333–346, 2003. doi: 10.1113/jphysiol.2002.037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumada M, Sagawa K. Modulation of carotid sinus baroreceptor reflex by central gray stimulation. Nihon Seirigaku Zasshi 36: 147–148, 1974. [PubMed] [Google Scholar]
- 94.Kumada M, Schramm LP, Altmansberger RA, Sagawa K. Modulation of carotid sinus baroreceptor reflex by hypothalamic defense response. Am J Physiol 228: 34–45, 1975. doi: 10.1152/ajplegacy.1975.228.1.34. [DOI] [PubMed] [Google Scholar]
- 95.Kuraishi Y, Harada Y, Takagi H. Noradrenaline regulation of pain-transmission in the spinal cord mediated by alpha-adrenoceptors. Brain Res 174: 333–336, 1979. doi: 10.1016/0006-8993(79)90857-6. [DOI] [PubMed] [Google Scholar]
- 96.Kvetnansky R, Armando I, Weise VK, Holmes C, Fukuhara K, Deka-Starosta A, Kopin IJ, Goldstein DS. Plasma dopa responses during stress: dependence on sympathoneural activity and tyrosine hydroxylation. J Pharmacol Exp Ther 261: 899–909, 1992. [PubMed] [Google Scholar]
- 97.Kvetňansk R, Goldstein DS, Weise VK, Holmes C, Szemeredi K, Bagdy G, Kopin IJ. Effects of handling or immobilization on plasma levels of 3,4-dihydroxyphenylalanine, catecholamines, and metabolites in rats. J Neurochem 58: 2296–2302, 1992. doi: 10.1111/j.1471-4159.1992.tb10977.x. [DOI] [PubMed] [Google Scholar]
- 98.Lê AD, Khanna JM, Kalant H. Role of Pavlovian conditioning in the development of tolerance and cross-tolerance to the hypothermic effect of ethanol and hydralazine. Psychopharmacology (Berl) 92: 210–214, 1987. doi: 10.1007/BF00177917. [DOI] [PubMed] [Google Scholar]
- 99.Leisman G, Koch P. Networks of conscious experience: computational neuroscience in understanding life, death, and consciousness. Rev Neurosci 20: 151–176, 2009. doi: 10.1515/REVNEURO.2009.20.3-4.151. [DOI] [PubMed] [Google Scholar]
- 100.Li SW, Lin TS, Minteer S, Burke WJ. 3,4-Dihydroxyphenylacetaldehyde and hydrogen peroxide generate a hydroxyl radical: possible role in Parkinson’s disease pathogenesis. Brain Res Mol Brain Res 93: 1–7, 2001. doi: 10.1016/S0169-328X(01)00120-6. [DOI] [PubMed] [Google Scholar]
- 101.Lorenz K. King Solomon’s Ring. New York: Crowell, 1952. [Google Scholar]
- 102.Louis WJ, Doyle AE, Anavekar S. Plasma norepinephrine levels in essential hyoertension. N Engl J Med 288: 599–601, 1973. doi: 10.1056/NEJM197303222881203. [DOI] [PubMed] [Google Scholar]
- 103.Low PA, Benarroach EE. Clinical Autonomic Disorders: Evaluation and Management. Philadelphia, PA: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 104.Madden CJ, Santos da Conceicao EP, Morrison SF. Vagal afferent activation decreases brown adipose tissue (BAT) sympathetic nerve activity and BAT thermogenesis. Temperature (Austin) 4: 89–96, 2017. doi: 10.1080/23328940.2016.1257407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mainzer K. The emergence of mind and brain: an evolutionary, computational, and philosophical approach. Prog Brain Res 168: 115–132, 2007. doi: 10.1016/S0079-6123(07)68010-8. [DOI] [PubMed] [Google Scholar]
- 106.Maloney SK, Fuller A, Mitchell G, Mitchell D. Brain and arterial blood temperatures of free-ranging oryx ( Oryx gazella). Pflugers Arch 443: 437–445, 2002. doi: 10.1007/s004240100704. [DOI] [PubMed] [Google Scholar]
- 107.Mattammal MB, Haring JH, Chung HD, Raghu G, Strong R. An endogenous dopaminergic neurotoxin: implication for Parkinson’s disease. Neurodegeneration 4: 271–281, 1995. doi: 10.1016/1055-8330(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 108.Mayr E. The idea of teleology. J Hist Ideas 53: 117–135, 1992. doi: 10.2307/2709913. [DOI] [Google Scholar]
- 109.McDougall SJ, Münzberg H, Derbenev AV, Zsombok A. Central control of autonomic functions in health and disease. Front Neurosci 8: 440, 2015. doi: 10.3389/fnins.2014.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McEwen BS. Allostasis and the epigenetics of brain and body health over the life course: the brain on stress. JAMA Psychiatry 74: 551–552, 2017. doi: 10.1001/jamapsychiatry.2017.0270. [DOI] [PubMed] [Google Scholar]
- 111.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav 43: 2–15, 2003. doi: 10.1016/S0018-506X(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 112.Mingrone G, Cummings DE. Changes of insulin sensitivity and secretion after bariatric/metabolic surgery. Surg Obes Relat Dis 12: 1199–1205, 2016. doi: 10.1016/j.soard.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 113.Mizunami M, Terao K, Alvarez B. Application of a prediction error theory to pavlovian conditioning in an insect. Front Psychol 9: 1272, 2018. doi: 10.3389/fpsyg.2018.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mohammed M, Madden CJ, Burchiel KJ, Morrison SF. Preoptic area cooling increases the sympathetic outflow to brown adipose tissue and brown adipose tissue thermogenesis. Am J Physiol Regul Integr Comp Physiol 315: R609–R618, 2018. doi: 10.1152/ajpregu.00113.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morrison SF. Central control of body temperature. F1000 Res 5: 880, 2016. doi: 10.12688/f1000research.7958.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci 16: 74–104, 2011. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nagel T. Mind and Cosmos: Why the Materialist Neo-Darwinian Conception of Nature is Almost Certainly False. New York: Oxford University Press, 2012. [Google Scholar]
- 118.Nagel T. What is it like to be a bat? Philos Rev 83: 435–450, 1974. doi: 10.2307/2183914. [DOI] [Google Scholar]
- 119.Nathan MA, Reis DJ. Chronic labile hypertension produced by lesions of the nucleus tractus solitarii in the cat. Circ Res 40: 72–81, 1977. doi: 10.1161/01.RES.40.1.72. [DOI] [PubMed] [Google Scholar]
- 120.Nathan MA, Tucker LW, Serverini WH, Reis DJ. Enhancement of conditioned arterial pressure responses in cats after brainstem lesions. Science 201: 71–73, 1978. doi: 10.1126/science.663640. [DOI] [PubMed] [Google Scholar]
- 121.Naughton MT. Pathophysiology and treatment of Cheyne-Stokes respiration. Thorax 53: 514–518, 1998. doi: 10.1136/thx.53.6.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nesse RM. How is Darwinian medicine useful? West J Med 174: 358–360, 2001. doi: 10.1136/ewjm.174.5.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nesse RM, Williams GC. Why We Get Sick. The New Science of Darwinian Medicine. New York: Times Books, 1994. [Google Scholar]
- 124.O’Brien HD. Augmenting trait-dependent diversification estimations with fossil evidence: a case study using osmoregulatory neurovasculature. Brain Behav Evol 91: 148–157, 2018. doi: 10.1159/000488887. [DOI] [PubMed] [Google Scholar]
- 125.Oyarce MP, Iturriaga R. Contribution of oxidative stress and inflammation to the neurogenic hypertension induced by intermittent hypoxia. Front Physiol 9: 893, 2018. doi: 10.3389/fphys.2018.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pacak K, Armando I, Fukuhara K, Kvetnatsky R, Palkovits M, Kopin IJ, Goldstein DS. Noradrenergic activation in the paraventricular nucleus during acute and chronic immobilization stress in rats: an in vivo microdialysis study. Brain Res 589: 91–96, 1992. doi: 10.1016/0006-8993(92)91165-B. [DOI] [PubMed] [Google Scholar]
- 127.Pacák K, Palkovits M, Kvetnanský R, Fukuhara K, Armando I, Kopin IJ, Goldstein DS. Effects of single or repeated immobilization on release of norepinephrine and its metabolites in the central nucleus of the amygdala in conscious rats. Neuroendocrinology 57: 626–633, 1993. doi: 10.1159/000126417. [DOI] [PubMed] [Google Scholar]
- 128.Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE. The neurotoxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS One 5: e15251, 2010. [Erratum in: PLoS One 6: 2011.] doi: 10.1371/journal.pone.0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Parker LM, Le S, Wearne TA, Hardwick K, Kumar NN, Robinson KJ, McMullan S, Goodchild AK. Neurochemistry of neurons in the ventrolateral medulla activated by hypotension: Are the same neurons activated by glucoprivation? J Comp Neurol 525: 2249–2264, 2017. doi: 10.1002/cne.24203. [DOI] [PubMed] [Google Scholar]
- 130.Pauli WM, Larsen T, Collette S, Tyszka JM, Seymour B, O’Doherty JP. Distinct Contributions of Ventromedial and Dorsolateral Subregions of the Human Substantia Nigra to Appetitive and Aversive Learning. J Neurosci 35: 14220–14233, 2015. doi: 10.1523/JNEUROSCI.2277-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pénicaud L, Leloup C, Lorsignol A, Alquier T, Guillod E. Brain glucose sensing mechanism and glucose homeostasis. Curr Opin Clin Nutr Metab Care 5: 539–543, 2002. doi: 10.1097/00075197-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 132.Pietrzak RH, Gallezot JD, Ding YS, Henry S, Potenza MN, Southwick SM, Krystal JH, Carson RE, Neumeister A. Association of posttraumatic stress disorder with reduced in vivo norepinephrine transporter availability in the locus coeruleus. JAMA Psychiatry 70: 1199–1205, 2013. doi: 10.1001/jamapsychiatry.2013.399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 133.Plotegher N, Berti G, Ferrari E, Tessari I, Zanetti M, Lunelli L, Greggio E, Bisaglia M, Veronesi M, Girotto S, Dalla Serra M, Perego C, Casella L, Bubacco L. DOPAL derived alpha-synuclein oligomers impair synaptic vesicles physiological function. Sci Rep 7: 40699, 2017. doi: 10.1038/srep40699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Proudfit HK. Pharmacologic evidence for the modulation of nociception by noradrenergic neurons. Prog Brain Res 77: 357–370, 1988. doi: 10.1016/S0079-6123(08)62802-2. [DOI] [PubMed] [Google Scholar]
- 135.Randich A, Maixner W. The role of sinoaortic and cardiopulmonary baroreceptor reflex arcs in nociception and stress-induced analgesia. Ann NY Acad Sci 467: 385–401, 1986. doi: 10.1111/j.1749-6632.1986.tb14642.x. [DOI] [PubMed] [Google Scholar]
- 136.Ritter S, Dinh TT, Li AJ. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav 89: 490–500, 2006. doi: 10.1016/j.physbeh.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 137.Rosenblueth A, Wiener N. The role of models in science. Philos Sci 12: 316–321, 1945. doi: 10.1086/286874. [DOI] [Google Scholar]
- 138.Rosenblueth A, Wiener N, Bigelow J. Behavior, purpose and teleology. Philos Sci 10: 18–24, 1943. doi: 10.1086/286788. [DOI] [Google Scholar]
- 139.Ruse M. Evolutionary biology and the question of teleology. Stud Hist Philos Biol Biomed Sci 58: 100–106, 2016. doi: 10.1016/j.shpsc.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 140.Russell GA. Vesalius and the emergence of veridical representation in Renaissance anatomy. Prog Brain Res 203: 3–32, 2013. doi: 10.1016/B978-0-444-62730-8.00001-3. [DOI] [PubMed] [Google Scholar]
- 141.Samson R, Milligan G, Lewine E, Sindi F, Garagliano J, Fernandez C, Moore R, DuCoin C, Oparil S, LE Jemtel TH. Effect of sleeve gastrectomy on hypertension. J Am Soc Hypertens 12: e19–e25, 2018. doi: 10.1016/j.jash.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 142.Sandroni P, Low PA. Autonomic Disorders: A Case-Based Approach. Cambridge, UK: Cambridge University Press, 2015. [Google Scholar]
- 143.Saunders BT, Richard JM, Margolis EB, Janak PH. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat Neurosci 21: 1072–1083, 2018. doi: 10.1038/s41593-018-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schachter S, Singer JE. Cognitive, social, and physiological determinants of emotional state. Psychol Rev 69: 379–399, 1962. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- 145.Schwarz-Stevens KS, Cunningham CL. Pavlovian conditioning of heart rate and body temperature with morphine: effects of CS duration. Behav Neurosci 107: 1039–1048, 1993. doi: 10.1037/0735-7044.107.6.1039. [DOI] [PubMed] [Google Scholar]
- 146.Selye H. The Stress of Life. New York: McGraw-Hill, 1956. [Google Scholar]
- 147.Shimohata T, Ozawa T, Nakayama H, Tomita M, Shinoda H, Nishizawa M. Frequency of nocturnal sudden death in patients with multiple system atrophy. J Neurol 255: 1483–1485, 2008. doi: 10.1007/s00415-008-0941-4. [DOI] [PubMed] [Google Scholar]
- 148.Singer C, Rabin C. A Prelude to Modern Science. New York: Cambridge University, 1946. [Google Scholar]
- 149.Smith R, Lane RD. The neural basis of one’s own conscious and unconscious emotional states. Neurosci Biobehav Rev 57: 1–29, 2015. doi: 10.1016/j.neubiorev.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 150.Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man. A quantitative method of assessing baroreflex sensitivity. Circ Res 24: 109–121, 1969. doi: 10.1161/01.RES.24.1.109. [DOI] [PubMed] [Google Scholar]
- 151.Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Handbook of Life Stress, Cognition, and Health, edited by Fisher J and Reason J. New York: Wiley, 1988, p. 629–649. [Google Scholar]
- 152.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 491: 156–162, 1989. doi: 10.1016/0006-8993(89)90098-X. [DOI] [PubMed] [Google Scholar]
- 153.Strauss WM, Hetem RS, Mitchell D, Maloney SK, O’Brien HD, Meyer LCR, Fuller A. Body water conservation through selective brain cooling by the carotid rete: a physiological feature for surviving climate change? Conserv Physiol 5: cow078, 2017. doi: 10.1093/conphys/cow078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sugama S, Sekiyama K, Kodama T, Takamatsu Y, Takenouchi T, Hashimoto M, Bruno C, Kakinuma K. Chronic restraint stress triggers dopaminergic and noradrenergic: Possible role of chronic stress in the onset of Parkinson’s disease. Brain Behav Immun 51: 39–46, 2016. [Erratum in: Brain Behav Immun 61: 389, 2017. 10.1016/j.bbi.2016.12.009. 28040434.] doi: 10.1016/j.bbi.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sullivan PA, Procci WR, DeQuattro V, Schoentgen S, Levine D, van der Meulen J, Bornheimer JF. Anger, anxiety, guilt and increased basal and stress-induced neurogenic tone: causes or effects in primary hypertension? Clin Sci (Lond) 61, Suppl 7: 389s–392s, 1981. doi: 10.1042/cs061389s. [DOI] [PubMed] [Google Scholar]
- 156.Sullivan P, Schoentgen S, DeQuattro V, Procci W, Levine D, Van der Meulen J, Bornheimer J. Anxiety, anger, and neurogenic tone at rest and in stress in patients with primary hypertension. Hypertension 3: II-119–II-123, 1981. doi: 10.1161/01.HYP.3.6_Pt_2.II-119. [DOI] [PubMed] [Google Scholar]
- 157.Thomas DD, Anderson WA, Apovian CM, Hess DT, Yu L, Velazquez A, Carmine B, Istfan NW. Weight recidivism after roux-en-y gastric bypass surgery: an 11-year experience in a multiethnic medical center. Obesity (Silver Spring), 27: 217–225, 2019. doi: 10.1002/oby.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Toyota M, Spencer D, Sawai-Toyota S, Jiaqi W, Zhang T, Koo AJ, Howe GA, Gilroy S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361: 1112–1115, 2018. doi: 10.1126/science.aat7744. [DOI] [PubMed] [Google Scholar]
- 159.Tupone D, Cano G, Morrison SF. Thermoregulatory inversion: a novel thermoregulatory paradigm. Am J Physiol Regul Integr Comp Physiol 312: R779–R786, 2017. doi: 10.1152/ajpregu.00022.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van der Schaar PJ, Lamers CB, Masclee AA. The role of the barostat in human research and clinical practice. Scand J Gastroenterol Suppl 230: 52–63, 1999. doi: 10.1080/003655299750025552. [DOI] [PubMed] [Google Scholar]
- 161.Velapati SR, Shah M, Kuchkuntla AR, Abu-Dayyeh B, Grothe K, Hurt RT, Mundi MS. Weight regain after bariatric surgery: prevalence, etiology, and treatment. Curr Nutr Rep 7: 329–334, 2018. doi: 10.1007/s13668-018-0243-0. [DOI] [PubMed] [Google Scholar]
- 162.Vermeer LM, Florang VR, Doorn JA. Catechol and aldehyde moieties of 3,4-dihydroxyphenylacetaldehyde contribute to tyrosine hydroxylase inhibition and neurotoxicity. Brain Res 1474: 100–109, 2012. doi: 10.1016/j.brainres.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vesalius A. Tabulae Anatomicae Sex. Venice: Ioannis Stephani Calcarensis, 1538. [Google Scholar]
- 164.Virtanen R. Claude Bernard and His Place in the History of Ideas. Lincoln, NE: University of Nebraska, 1960. [Google Scholar]
- 165.Wiener N. Cybernetics: or Control and Communication in the Animal and the Machine. Cambridge, MA: M.I.T., 1948. [Google Scholar]
- 166.Wiener N, Schade JP. Introduction to neurocybernetics. In: Progress in Brain Research 2 Nerve, Brain and Memory Models, edited by Wiener N and Schade JP. Amsterdam: Elsevier, 1963, p. 1–6. [Google Scholar]
- 167.Wilkinson M. Testing the null hypothesis: the forgotten legacy of Karl Popper? J Sports Sci 31: 919–920, 2013. doi: 10.1080/02640414.2012.753636. [DOI] [PubMed] [Google Scholar]
- 168.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11: 398–411, 1957. doi: 10.1111/j.1558-5646.1957.tb02911.x. [DOI] [Google Scholar]
- 169.Wolf SG, Cardon PV, Shepard EM, Wolff HG. Life Stress and Essential Hypertension. Baltimore, MD: Williams & Wilkins, 1955. [Google Scholar]
- 170.Woods SC, Kuskosky PJ. Classically conditioned changes of blood glucose level. Psychosom Med 38: 201–219, 1976. doi: 10.1097/00006842-197605000-00006. [DOI] [PubMed] [Google Scholar]
- 171.Zaman J, Van den Bergh O, Fannes S, Van Diest I. Learning to breathe? Feedforward regulation of the inspiratory motor drive. Respir Physiol Neurobiol 201: 1–6, 2014. doi: 10.1016/j.resp.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 172.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 60: 337–341, 2003. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 173.Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, Patapoutian A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362: 464–467, 2018. doi: 10.1126/science.aau6324. [DOI] [PMC free article] [PubMed] [Google Scholar]