Abstract
Increasingly variable, extreme, and nonpredictable weather events are predicted to accompany climate change, and such weather events will especially affect temperate, terrestrial environments. Yet, typical protocols in comparative physiology that examine environmental change typically employ simple step-wise changes in the experimental stressor of interest (e.g., temperature, water availability, oxygen, nutrition). Such protocols fall short of mimicking actual natural environments and may be inadequate for fully exploring the physiological effects of stochastic, extreme weather events. Indeed, numerous studies from the field of thermal biology, especially, indicate nonlinear and sometimes counterintuitive findings associated with variable and fluctuating (but rarely truly stochastic) protocols for temperature change. This Perspective article suggests that alternative experimental protocols should be employed that go beyond step-wise protocols and even beyond variable protocols employing circadian rhythms, for example, to those that actually embrace nonpredictable elements. Such protocols, though admittedly more difficult to implement, are more likely to reveal the capabilities (and, importantly, the limitations) of animals experiencing weather, as distinct from climate. While some possible protocols involving stochasticity are described as examples to stimulate additional thought on experimental design, the overall goal of this Perspective article is to encourage comparative physiologists to entertain incorporation of nonpredictable experimental conditions as they design future experimental protocols.
WEATHER, CLIMATE, AND COMPARATIVE PHYSIOLOGY
As the American humorist Mark Twain quipped, “Climate is what we expect. Weather is what we get.” Long before “climate change” became a household phrase, Mark Twain recognized the important difference between the relatively stable nature of “climate” (measurable environmental characteristics that are consistently observed year after year) and the typically less predictable nature of much shorter-term environmental changes that we call “weather” (events measured in hours, days, and weeks). Predictions of ever more extreme weather are associated with climate change (10, 13). Long-standing theoretical predictions, some accompanied by experimental data, indicate that extreme weather events decrease biodiversity and shift geographic species distribution through population eradication, if not actual species extinction (12, 15–18, 20, 23, 27, 28, 30, 31, 33–38, 40). As such, weather (not climate) may be the most challenging characteristic of climate change facing both developing and mature terrestrial animals (3, 21, 36), or indeed animals in any environment that is not buffered from weather-induced changes (e.g., large lakes, oceans).
Many of the studies associated with the effects of weather “correlate” variable environments with species survival, diversity, and reproduction. Comparative physiologists are in a position to actually create experimental environmental stressors and so move beyond correlation to causation by contributing vital data and even testing hypotheses emerging from ecological and population biology. In particular, we can play more prominent roles in assessing how nonpredictable weather events (especially temperature change) can lead to decreased fitness, and even ascribe mechanisms (behavioral, physiological, molecular, biochemical) for fitness reduction. Moreover, stochastic environmental variation could lead to as-yet-unexplored longer-term individual and population effects, potentially acting through epigenetic inheritance (4).
TRADITIONAL PROTOCOLS FOR INVESTIGATING ENVIRONMENTAL EFFECTS
Loosely paraphrasing Mark Twain, “Responses to climate change are what comparative physiologists study. Weather is what animals get.” Indeed, most of us control our experimental temperatures to fractions of a degree Celsius, gas partial pressures within a millimeter of mercury, and salinities within a part per thousand. Moreover, when we do induce ambient changes, we are often slaves to powers of 10 or neat and tidy fractions thereof. Thus, there are hundreds of comparative physiological articles that have induced a step-wise, ramp-up, or (rarely) a sine wave form of environmental temperature change (as an example) of 3.0°, 5.0°, or 10.0°C in the laboratory (Fig. 1, A–F), followed by determination the animal’s physiological responses to the new temperature steady state. Mea culpa—e.g., (5, 29, 39). Yet, steady-state, mean temperatures typical of experiments rarely reflect natural conditions, where temperature variations occur on multiple complex timescales ranging from a few hours to years (8).
Fig. 1.
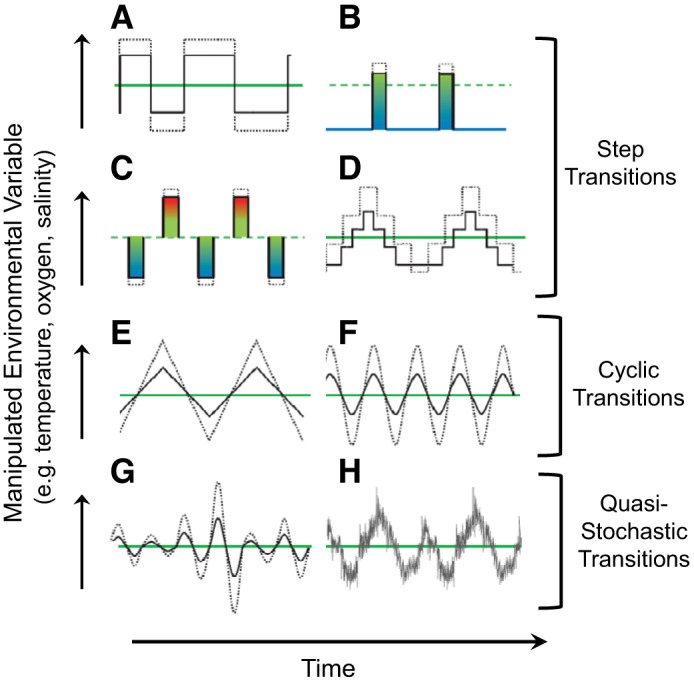
Experimental protocols typically used for inducing temperature or other changes in comparative physiological experiments exploring animals’ responses to climate change. A–D: the most frequently used step transition protocols. E and F: cyclic transitions (spike and especially sine wave protocols), which are much less frequently employed. G and H: semistochastic transitions that are more realistic, but less easy to induce. Modified from Colinet et al. (6) with permission. Further details of these protocols are provided in Ref. 6.
The carefully controlled, traditional experimental protocols described above have certainly been useful in revealing mechanisms. However, they may be inadequate for investigating the full repertoire of animals’ natural physiological regulatory responses associated with nonpredictable, extreme weather events attending climate change. How animals subjected to such stochasticity actually cope (or equally importantly, fail to cope) will be critical to research into future climate change. Yet, as numerous authors have observed, the typically carefully controlled laboratory conditions rarely represent those occurring in the field (e.g., 8, 9, 24). Of course, matching laboratory to field conditions is not a new concept by any means. Consequently, limitations of steady-state protocols in physiological studies have been pointed out many times, often by researchers in the field of thermal biology, which is increasingly emphasizing the importance of using non-steady-state experimental protocols to reveal the more realistic responses of animals to non-steady-state changes (e.g., 1, 2, 6–9, 19, 26).
WHY STOCHASTIC ELEMENTS IN EXPERIMENTAL PROTOCOLS?
Why are experimental protocols employing nonpredictable environmental conditions of importance to comparative physiologists? In fact, nonlinear and sometimes counterintuitive effects can emerge from non-steady-state protocols. For example, fluctuating temperatures can have generally positive effects at low temperatures, but often have negative effects at high temperatures (for an introduction to that literature, see Ref. 19). Some animals have been shown to develop more rapidly or show differences in physiological performance when exposed to stochastically varying environments compared with those in steady-state or cycling in predictable fashions (6, 11, 32). Yet, a close examination of that literature reveals that the terms “variable” and “fluctuating” are often used to describe step-wise changes of differing magnitudes, or to describe predictable, rhythmic cyclic changes, examples of which are schematically indicated in Fig. 1, E–G. Even the protocols shown in Fig. 1, G and H, while quite different from the other protocols and including stochastic elements, nonetheless employ an element of predictable cycling and so are only “quasi-stochastic”. Of course, even nonpredictable extreme environmental changes often show some predictable changes associated with diurnal or seasonal cycles or El Niño/La Niña events, as examples. Thus, quasi-stochastic protocols imposing nonpredictability upon basic cyclicity will certainly be informative. Yet, our knowledge of the effects of truly nonpredictable environmental changes on animal physiology is very limited.
Unfortunately, the message of the potential importance of employing variable (however that be defined) rather than steady-state conditions in our protocols appears not to be heavily penetrating into comparative physiological laboratories investigating implications of long-term climate change. Only infrequently are non-steady-state protocols employed, and these only rarely involve semistochastic or fully stochastic protocols. Indeed, such protocols may be likely to be questioned by contemporary referees of journal articles (just as some reviewers of this Perspective article questioned the approach).
CHALLENGES OF EXPERIMENTAL STOCHASTICITY
To be fair, there are reasonable concerns surrounding protocols with nonpredictable changes in variables. Let me raise some of these concerns and comment upon each in turn.
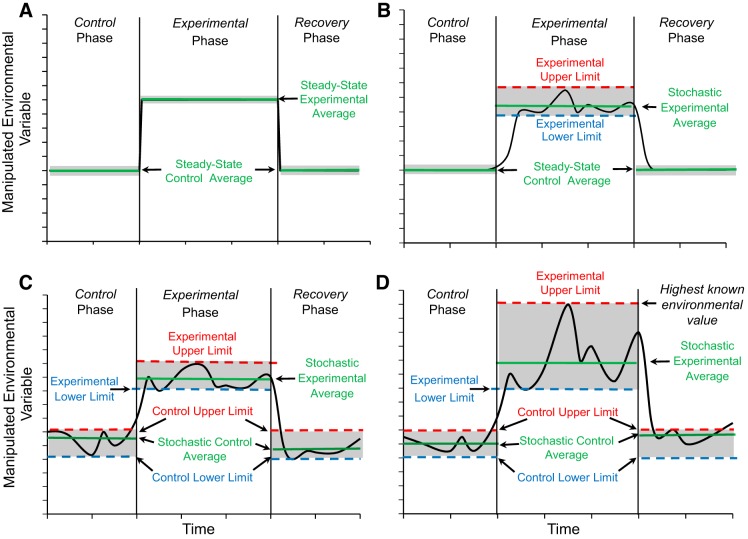
Defining a “control condition” comprises a significant concern in protocols employing nonpredictable experimental conditions. Establishing a constant acclimation period for a control is straightforward in constant condition protocols (Fig. 2A), or even those with an experimental phase with nonpredictable elements (Fig. 2B). Moving beyond these approaches, a “stochastic control” (is that very phrase oxymoronic?) could also be employed, which actually might be the most realistic for mimicking weather events. Thus, limited nonpredictable variation around a control set point could be combined with a subsequent elevated (or decreased) set point designated as the experimental level, around which the same degree of nonpredictable variation continues (Fig. 2C). A recovery period mirroring the initial control could then follow. In this scenario, stochasticity confined within upper and lower bounds equally applies to control, experimental, and recovery phases. Another example of a potential stochastic protocol would not artificially limit the nonpredictable variation of the environmental variable as in Fig. 2C, but would actually allow changes that incorporate extremes in the form of the highest (and/or lowest, not illustrated) previously measured environmental value (Fig. 2D).
Fig. 2.
Suggested experimental protocols that could be used individually or, better still, in combination, for studying physiological responses to changes in environmental variables associated with weather and climate. A: an initial control phase at a constant level is followed by an experimental phase of elevated constant level. B: an initial control phase at a constant level is followed by an experimental phase where levels fluctuate stochastically around the same constant level shown in A, with upper and lower values established to limit possible level fluctuations. C: an expansion of the protocol in B, in which the control phase, itself, is characterized by stochastic fluctuation similarly bound by upper and lower limits. Note that these protocols could be reversed in direction to examine low rather than the illustrated high values. D: modification of the protocol from C, allowing greater excursion limits coinciding with the highest known environmental values.
Standardization across emerging studies is one of the more vexing concerns of stochastic protocols, for by its very nature, a protocol employing unpredictable environmental variation should be … well … unpredictable. Yet, there must be some form of standardization, such that other researchers can attempt to replicate the results. The answer to standardization of experiments involving nonpredictable changes could involve ensuring that all changes in manipulated variables within the control, experimental, and recovery phases of the protocols are truly random. Thus, nonpredictable changes are induced in the rate, magnitude, and duration of the increase in the environmental variable, the rate of subsequent variable decrease to control levels and perhaps even the time interval between multiple tests. The “standardization,” thus, lies in random, bias-free temperature increases, which another researcher should be able to replicate by employing similar randomization.
Statistical analysis comprises another significant concern involving data emerging from stochastic protocols. Concurrently employing multiple combinations of protocols (e.g., Fig. 2, A–D), each with multiple replicates, would allow differentiation of the specific effects of nonpredictable changes. Within any given protocol, time series averaging by any one of a variety of different models could be employed to assess physiological changes within the nonpredictable phases (25). Another possible approach may involve decomposing stochastic environmental variable time series using Fourier transformation, which has recently been used to examine the biological effects of climate variability across multiple timescale effects (8). Yet another approach, which has been employed to test effects of nonpredictably varying environmental conditions, is an “ecomechanical” approach for analyzing data over time (see Ref. 9). Clearly, standardized statistical approaches will need to emerge in parallel with increased use of protocols, including stochasticity.
Induction of nonpredictable variability is another concern. Inducing true stochasticity is obviously more complex than just a one-time adjustment to a controller to create a new, constant steady-state condition. However, many regulation devices (especially for temperature) now accept computer-programmed input, and a random number generator could be employed to input truly random changes within fixed ranges (Fig. 2, B–D). Alternatively, experimental protocols could be built around so-called “stochastic weather generators” that have emerged from ecology, agriculture, and climate science to develop and test models for experimental environments mimicking various weather scenarios (14, 22). For the electronics-capable investigator, a small investment in electronic components can enable the programing of nonvariable changes in the variable of interest.
Comparability of physiological studies based on stochastic environmental changes with existing traditional studies is a final concern (additional concerns could be argued, of course). This is where inclusion of traditional step-wise protocols alongside stochastic ones (i.e., deploying all of the multiple protocols depicted in Fig. 2, each in a different experimental population) could be particularly illuminating. Indeed, of the relatively few comparative physiological studies employing quasi-stochastic elements, most also carry out more conventional step-wise protocols in parallel, creating what might be considered a “meta-control” (6, 11, 32). That data emerging from a single laboratory using experimental protocols with nonpredictable elements may differ from data from more conventional step-wise or ramp protocols used in that laboratory is highly informative per se, and it should stimulate additional thought and experimentation.
Perspectives and Significance
The comparative physiological community can contribute to the contemporary study of climate change by exploring the physiological effects of extreme weather events associated with global climate change. In doing so, we should be encouraged to increase the use of stochastic protocols that more realistically mimic short-term weather events to supplement protocols based on statistical calculations of long-term climate change.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.W.B. prepared figures; W.W.B. drafted manuscript; W.W.B. edited and revised manuscript; W.W.B. approved final version of manuscript.
ACKNOWLEDGMENTS
I am grateful to Drs. Ed Dzialowski and Gerald Kerth for highly insightful suggestions for improvement of this manuscript.
REFERENCES
- 1.Angilletta MJ. Thermal Adaption: A Theoretical and Empirical Synthesis. Oxford, UK: Oxford University, 2009. [Google Scholar]
- 2.Bozinovic F, Bastías DA, Boher F, Clavijo-Baquet S, Estay SA, Angilletta MJ Jr. The mean and variance of environmental temperature interact to determine physiological tolerance and fitness. Physiol Biochem Zool 84: 543–552, 2011. doi: 10.1086/662551. [DOI] [PubMed] [Google Scholar]
- 3.Burggren W. Developmental phenotypic plasticity helps bridge stochastic weather events associated with climate change. J Exp Biol 221: jeb161984, 2018. doi: 10.1242/jeb.161984. [DOI] [PubMed] [Google Scholar]
- 4.Burggren W. Epigenetic inheritance and its role in evolutionary biology: Re-evaluation and new perspectives. Biology (Basel) 5: 24, 2016. doi: 10.3390/biology5020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burggren WW, Bautista GM, Coop SC, Couturier GM, Delgadillo SP, García RM, González CAA. Developmental cardiorespiratory physiology of the air-breathing tropical gar, Atractosteus tropicus. Am J Physiol Regul Integr Comp Physiol 311: R689–R701, 2016. doi: 10.1152/ajpregu.00022.2016. [DOI] [PubMed] [Google Scholar]
- 6.Colinet H, Sinclair BJ, Vernon P, Renault D. Insects in fluctuating thermal environments. Annu Rev Entomol 60: 123–140, 2015. doi: 10.1146/annurev-ento-010814-021017. [DOI] [PubMed] [Google Scholar]
- 7.Czarnoleski M, Dragosz-Kluska D, Angilletta MJ Jr. Flies developed smaller cells when temperature fluctuated more frequently. J Therm Biol 54: 106–110, 2015. doi: 10.1016/j.jtherbio.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Dillon ME, Woods HA, Wang G, Fey SB, Vasseur DA, Telemeco RS, Marshall K, Pincebourde S. Life in the frequency domain: The biological impacts of changes in climate variability at multiple time scales. Integr Comp Biol 56: 14–30, 2016. doi: 10.1093/icb/icw024. [DOI] [PubMed] [Google Scholar]
- 9.Dowd WW, King FA, Denny MW. Thermal variation, thermal extremes and the physiological performance of individuals. J Exp Biol 218: 1956–1967, 2015. doi: 10.1242/jeb.114926. [DOI] [PubMed] [Google Scholar]
- 10.Drake JM. Population effects of increased climate variation. Proc Biol Sci 272: 1823–1827, 2005. doi: 10.1098/rspb.2005.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake MJ, Miller NA, Todgham AE. The role of stochastic thermal environments in modulating the thermal physiology of an intertidal limpet, Lottia digitalis. J Exp Biol 220: 3072–3083, 2017. doi: 10.1242/jeb.159020. [DOI] [PubMed] [Google Scholar]
- 12.Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. Climate extremes: observations, modeling, and impacts. Science 289: 2068–2074, 2000. doi: 10.1126/science.289.5487.2068. [DOI] [PubMed] [Google Scholar]
- 13.Fischer EM, Beyerle U, Knutti R. Robust spatially aggregated projections of climate extremes. Nat Clim Chang 3: 1033–1038, 2013. doi: 10.1038/nclimate2051. [DOI] [Google Scholar]
- 14.Fodor N, Dobi I, Mika J, Szeidl L. Applications of the MVWG multivariable stochastic weather generator. Scientific World Journal 2013: 571367, 2013. doi: 10.1155/2013/571367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frederiksen M, Daunt F, Harris MP, Wanless S. The demographic impact of extreme events: stochastic weather drives survival and population dynamics in a long-lived seabird. J Anim Ecol 77: 1020–1029, 2008. doi: 10.1111/j.1365-2656.2008.01422.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanski I, Meyke E. Large-scale dynamics of the glanville fritillary butterfly: landscape structure, population processes, and weather. Ann Zool Fenn 42: 379–395, 2005. [Google Scholar]
- 17.Jonas JL, Buhl DA, Symstad AJ. Impacts of weather on long-term patterns of plant richness and diversity vary with location and management. Ecology 96: 2417–2432, 2015. doi: 10.1890/14-1989.1. [DOI] [PubMed] [Google Scholar]
- 18.Kindvall O. The impact of extreme weather on habitat preference and survival in a metapopulation of the bush cricket Metrioptera bicolor in Sweden. Biol Conserv 73: 51–58, 1995. doi: 10.1016/0006-3207(95)90063-2. [DOI] [Google Scholar]
- 19.Kingsolver JG, Higgins JK, Augustine KE. Fluctuating temperatures and ectotherm growth: distinguishing non-linear and time-dependent effects. J Exp Biol 218: 2218–2225, 2015. doi: 10.1242/jeb.120733. [DOI] [PubMed] [Google Scholar]
- 20.Kingsolver JG, Woods HA, Buckley LB, Potter KA, MacLean HJ, Higgins JK. Complex life cycles and the responses of insects to climate change. Integr Comp Biol 51: 719–732, 2011. doi: 10.1093/icb/icr015. [DOI] [PubMed] [Google Scholar]
- 21.Levy O, Buckley LB, Keitt TH, Smith CD, Boateng KO, Kumar DS, Angilletta MJ Jr. Resolving the life cycle alters expected impacts of climate change. Proc Biol Sci 282: 20150837, 2015. doi: 10.1098/rspb.2015.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehan S, Guo T, Gitau MW, Flanagan DC. Comparative study of different stochastic weather generators for long-term climate data simulation. Climate (Basel) 5: 26, 2017. doi: 10.3390/cli5020026. [DOI] [Google Scholar]
- 23.Menezes-Oliveira VB, Scott-Fordsmand JJ, Soares AM, Amorim MJ. Effects of temperature and copper pollution on soil community—extreme temperature events can lead to community extinction. Environ Toxicol Chem 32: 2678–2685, 2013. doi: 10.1002/etc.2345. [DOI] [PubMed] [Google Scholar]
- 24.Morash AJ, Neufeld C, MacCormack TJ, Currie S. The importance of incorporating natural thermal variation when evaluating physiological performance in wild species. J Exp Biol 221: jeb16467, 2018. doi: 10.1242/jeb.164673. [DOI] [PubMed] [Google Scholar]
- 25.Morel M, Achard C, Kulpa R, Dubuisson S. Time-series averaging using constrained dynamic time warping with tolerance. Pattern Recognit 74: 77–89, 2018. doi: 10.1016/j.patcog.2017.08.015. [DOI] [Google Scholar]
- 26.Niehaus AC, Angilletta MJ Jr, Sears MW, Franklin CE, Wilson RS. Predicting the physiological performance of ectotherms in fluctuating thermal environments. J Exp Biol 215: 694–701, 2012. doi: 10.1242/jeb.058032. [DOI] [PubMed] [Google Scholar]
- 27.Oliver TH, Brereton T, Roy DB. Population resilience to an extreme drought is influenced by habitat area and fragmentation in the local landscape. Ecography 36: 579–586, 2013. doi: 10.1111/j.1600-0587.2012.07665.x. [DOI] [Google Scholar]
- 28.Parmesan C, Root TL, Willig MR. Impacts of extreme weather and climate on terrestrial biota. Bull Am Meteorol Soc 81: 443–450, 2000. doi:. [DOI] [Google Scholar]
- 29.Perrichon P, Pasparakis C, Mager EM, Stieglitz JD, Benetti DD, Grosell M, Burggren WW. Interactive effect of elevated temperature and Deepwater Horizon oil exposure on the cardiac performance of the larval mahi-mahi, Coryphaena hippurus. Biol Open 2018. doi: 10.1371/journal.pone.0203949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piessens K, Adriaens D, Jacquemyn H, Honnay O. Synergistic effects of an extreme weather event and habitat fragmentation on a specialised insect herbivore. Oecologia 159: 117–126, 2009. doi: 10.1007/s00442-008-1204-x. [DOI] [PubMed] [Google Scholar]
- 31.Saltz D, Rubenstein DI, White GC. The impact of increased environmental stochasticity due to climate change on the dynamics of asiatic wild ass. Conserv Biol 20: 1402–1409, 2006. doi: 10.1111/j.1523-1739.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- 32.Saunders LM, Tompkins DM, Hudson PJ. Stochasticity accelerates nematode egg development. J Parasitol 88: 1271–1272, 2002. doi: 10.1645/0022-3395(2002)088[1271:SANED]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Schippers P, Verboom J, Vos CC, Jochem R. Metapopulation shift and survival of woodland birds under climate change: will species be able to track? Ecography 34: 909–919, 2011. doi: 10.1111/j.1600-0587.2011.06712.x. [DOI] [Google Scholar]
- 34.Smith MD. The ecological role of climate extremes: current understanding and future prospects. J Ecol 99: 651–655, 2011. doi: 10.1111/j.1365-2745.2011.01833.x. [DOI] [Google Scholar]
- 35.Tinsley RC, Stott LC, Viney ME, Mable BK, Tinsley MC. Extinction of an introduced warm-climate alien species, Xenopus laevis, by extreme weather events. Biol Invasions 17: 3183–3195, 2015. doi: 10.1007/s10530-015-0944-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasseur DA, DeLong JP, Gilbert B, Greig HS, Harley CD, McCann KS, Savage V, Tunney TD, O’Connor MI. Increased temperature variation poses a greater risk to species than climate warming. Proc Biol Sci 281: 20132612, 2014. doi: 10.1098/rspb.2013.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verboom J, Schippers P, Cormont A, Sterk M, Vos CC, Opdam PFM. Population dynamics under increasing environmental variability: implications of climate change for ecological network design criteri. Landsc Ecol 25: 1289–1298, 2010. doi: 10.1007/s10980-010-9497-7. [DOI] [Google Scholar]
- 38.WallisDeVries MF, Baxter W, Van Vliet AJ. Beyond climate envelopes: effects of weather on regional population trends in butterflies. Oecologia 167: 559–571, 2011. doi: 10.1007/s00442-011-2007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson CM, Burggren WW. Interspecific differences in metabolic rate and metabolic temperature sensitivity create distinct thermal ecological niches in lizards (Plestiodon). PLoS One 11: e0164713, 2016. doi: 10.1371/journal.pone.0164713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Loreau M, He N, Wang J, Pan Q, Bai Y, Han X. Climate variability decreases species richness and community stability in a temperate grassland. Oecologia 188: 183–192, 2018. doi: 10.1007/s00442-018-4208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]