Abstract
The migratory flights of birds are primarily fueled by fat; however, certain fatty acids may also enhance flight performance and the capacity to oxidize fat. The natural doping hypothesis posits that n–3 long-chain polyunsaturated fatty acids (PUFA) increase membrane fluidity and aerobic and fatty acid oxidative enzymes in the flight muscles, which enables prolonged endurance flight. Support for this hypothesis is mixed, and there is no empirical evidence for increased flight performance. We fed yellow-rumped warblers (Setophaga coronata coronata) diets enriched in either n–3 or n–6 long-chain PUFA or low in long-chain PUFA and evaluated flight muscle metabolism and endurance performance in a wind tunnel flights lasting up to 6 h. Fatty acid profiles of muscle phospholipids confirmed enrichment of the targeted dietary fatty acids, whereas less substantial differences were observed in adipose triacylglycerol. Contrary to the predictions, feeding n–3 PUFA decreased peroxisome proliferator-activated receptors-β mRNA abundance and muscle oxidative enzyme activities. However, changes in muscle metabolism were not reflected in whole animal performance. No differences were observed in flight performance among diet treatments in terms of endurance capacity, energy costs, or fuel composition. These measures of flight performance were more strongly influenced by body mass and flight duration. Overall, we found no support for the natural doping hypothesis in a songbird. Furthermore, we caution against extending changes in flight muscle metabolic enzymes or fatty acid composition to changes to migratory performance without empirical evidence.
Keywords: fatty acid nutrition, flight performance, migration, songbirds
INTRODUCTION
The migratory flights of birds are extreme feats of endurance exercise that last from several hours to many days (12, 18, 62). Similar to humans and other animal athletes, proper nutrition could be important to a bird’s success. Fatty acid nutrition has been highlighted for its potential to enhance flight performance, particularly in supporting the oxidation of fat, the principle fuel for flight (45, 48, 61). Mechanistically, there are several potential ways fatty acids can influence physiology and performance, including by altering the mobilization and oxidation rates of fatty acids, influencing membrane composition and fluidity, and serving as ligands for transcription factors [reviewed by Price (51)]. These mechanisms are not mutually exclusive and have been variously invoked to explain benefits of certain fatty acids found in the diets of birds (45, 48, 61). Yet, these mechanisms have been explored only superficially in migratory birds.
The natural doping hypothesis posits that long-chain n–3 long-chain polyunsaturated fatty acids (PUFA), eicosapentaenoic acid (EPA; 20:5n–3), and docosahexaenoic acid (DHA; 22:6n–3) help prime the flight muscles for endurance flight (36, 37, 61). Increasing the relative proportion of DHA and EPA in membranes increases membrane fluidity and may enhance metabolic protein activity (61). Furthermore, EPA and DHA are high-affinity ligands for peroxisome proliferator-activated receptors (PPARs), and increase the expression of genes supporting lipid metabolism (19). Combined, these mechanisms would increase the capacity to oxidize fatty acids in the flight muscle, which is needed for endurance flight (61). Support for the natural doping hypothesis was first observed in refueling migratory semipalmated sandpipers (Calidris pusilla) that consume a diet high in DHA and EPA (36, 37). Dietary EPA and DHA were incorporated into the flight muscle membranes and correlated with the oxidative enzyme activities (36, 37). In a follow up study of captive sedentary bobwhite quail (Collinus virginianus), EPA and DHA supplementation increased oxidative enzyme activities (42), providing evidence that dietary fatty acids can influence the regulation of metabolic enzyme activity.
The potential to enhance migratory performance may not be limited to n–3 PUFA. Other evidence suggests there are benefits of PUFA in general or of n–6 PUFA on peak metabolic rate (PMR) in red-eyed vireos (Vireo olivaceous; Ref. 46) and white-throated sparrows (Zonotrichia albicollis; Ref. 48). In addition, Price and Guglielmo (48) found no effect of dietary n–3 and n–6 PUFA on flight muscle oxidative capacity in sparrows but did observe an increase in PMR in birds fed n–6 PUFA. Using a carefully designed feeding protocol, Price and Guglielmo (48) were able to manipulate the phospholipid composition of the flight muscle independent of adipose fatty acid composition. This allowed them to attribute changes in PMR to the fatty acid composition of the fuel (48). However, the high proportion of DHA in the flight muscle phospholipids of the sparrows may limit conclusions about the effect of n–3 and n–6 PUFA on membrane phospholipids and PPAR signaling since DHA was enriched in the membranes of all the diet groups.
Studies from a wide variety of taxa suggest that both n–3 PUFA and n–6 PUFA alter exercise performance at the whole animal level. In rats, n–6 PUFA are associated with increased endurance (3) and long-chain n–3 PUFA increase resistance to fatigue (43). In mammals (53) and Atlantic salmon (Salmo salar, Refs. 9, 41), running or swimming speed is positively associated with the n–6 PUFA 18:2 n–6. On the other hand, elevated levels of DHA are found in the high-performance muscles such as hummingbird flight muscles and rattlesnake shakers (29). Additionally, in humans, endurance exercise training increases the proportion of DHA in muscle independent of diet (26). A key consideration in assessing performance benefits is the need to ensure that the performance test is relevant to the animal’s life history. In the case of avian migration, the best metrics of flight performance are arguably endurance capacity and energy efficiency, which could impact both the length of migratory flight and fuel requirements.
The goal of this study was to experimentally test the natural doping hypothesis from the tissue level to whole animal performance in a migratory bird, the yellow-rumped warbler (Setophaga coronata). We created diets specifically enriched in an n–3 long-chain PUFA (DHA), an n–6 long-chain PUFA [arachidonic acid (ARA); 20:4n–6 ], or monounsaturated fatty acids (MUFA) to alter the composition of flight muscle membranes in terms of type and total proportions of long-chain PUFA and limited differences in the adipose tissue. Based on the natural doping hypothesis, we predicted that birds supplemented with n–3 long-chain PUFA will have flight muscles with membranes enriched with DHA and increased oxidative capacity resulting in improved flight performance (lower costs, increased endurance, and reduced use of lean mass as fuel). This study is the first to fully integrate tissue to whole animal performance level tests of the influence of fatty acids on migratory birds. We assessed the following: 1) effects of diet on whole animal performance, 2) effects of diet on PPAR mRNA abundance and oxidative enzyme activity, and 3) changes in muscle metabolism during flight, and 4) the correlation between changes in flight muscle metabolism and whole animal performance.
MATERIALS AND METHODS
Animals and Experimental Design
Sixty yellow-rumped warblers were caught using mist nets in October 2013 at Long Point, ON, Canada and housed at the Advanced Facility for Avian Research at the University of Western Ontario (London, Ontario, Canada) in free-flight aviaries (3.7-m long × 2.1-m wide × 3-m tall and 3.6-m long × 2.4-m wide × 2.7-m tall). The birds were initially kept on a fall photoperiod of 12-h light and 12-h dark (12L:12D) and switched to a short-day winter photoperiod (9L:15D) in late November to allow the birds to enter a nonmigratory wintering condition. Decreasing day length was done gradually, shortening by an hour each day. The birds were fed a synthetic maintenance diet (Table 1), with canola oil used as the lipid source until the start of the study. This diet is modified from Guglielmo et al. (20) and was found to give the highest flight propensity in wind tunnels. Animal capture, care, and procedures followed Canadian Council on Animal Care guidelines and were approved by the University of Western Ontario Animal Use Subcommittee (protocol 2010-216) and by the Canadian Wildlife Service (permit CA-0256).
Table 1.
Composition of the synthetic diets fed to yellow-rumped warblers (Setophaga coronata) during the feeding trial
| Ingredients | Grams | Percent |
|---|---|---|
| Dextrosea | 450 | 16.3 |
| Caseinb | 100 | 3.6 |
| Agarb | 45 | 1.6 |
| Briggs-N salt mixc | 44 | 1.6 |
| AIN-76 vitamin mixc | 15 | 0.5 |
| Water | 2,000 | 72.4 |
| Oild | 85 | 3.1 |
| Cellulosee | 24 | 0.9 |
| Carb/protein/fat, %dry | 61.7/14.2/10.1 |
ADM Corn Processing, Decatur, IL.
Affeymetric USB, Cleveland, OH.
MP Biomedicals, Solon, OH.
Dietary oil is canola oil during maintenance phase or experimental oil blend during the experimental phase.
Sigma Aldrich, Oakville, OH.
During the experimental phase, the birds were housed in pairs in cages measuring 121-cm wide × 68-cm deep ×185-cm high for weeks 1 and 2, and when on the long-day photoperiod housed in cages measuring 70-cm wide × 50-cm deep × 60-cm high. We divided the birds into three experimental diet groups, with 20 birds per diet. To standardize the amount of time birds consumed their experimental diets and when they entered a spring migratory condition (time spent on long-day photoperiod), we also grouped the birds into 10 blocks, with 2 birds from each diet per block. Starting in late February, every Monday a new block of birds would begin the 6-wk experimental protocol. In week 1 the birds begin their experimental diet on their short-day photoperiod for 2 wk (see Supplemental Materials Figure S1 for experimental timeline; Supplemental Material for this article at https://figshare.com/articles/AJP_Supplemental_Materials_pdf/7492781/1). At the start of week 3, the birds were switched straight to a long-day photoperiod (16L:8D) to photo-stimulate them into a spring migratory condition. In week 5, basal metabolic rate (BMR) and PMR were measured (see below). In week 6, wind tunnel flight assessments and tissue sampling occurred. Our intention was for each diet group in a block to have one bird undergo endurance flight assessment (Flown; see below) and one sampled as an unflown Control from each diet. As flying in a wind tunnel is voluntary, if neither bird would fly, both birds would both be sampled as Controls after a 2- to 3-day recovery from the flight attempt.
The three experimental diets each had a unique dietary oil blend, which replaced the canola oil used in the maintenance diet. The MUFA diet oil blend was composed of olive and coconut oil (see Supplemental Materials Table S1; https://figshare.com/articles/AJP_Supplemental_Materials_pdf/7492781/1), which created a diet low in long-chain PUFA and higher in MUFA. The n–3 PUFA diet oil blend was composed of olive oil, coconut oil, and DHASCO (DSM Nutritional Products, Kaiseraugst, Switzerland), an algal oil high in DHA. The n–6 PUFA diet oil blend was composed of olive oil, coconut oil, and ARASCO (DSM Nutritional Products), a fungal oil high in ARA. Four weeks after the diet treatments were started with the first block of birds, it became apparent that the n–3 PUFA diet birds were having health problems. The birds were weaker than normal, had reduced flight capacity, and appeared to have larger than normal variation in daily food intake and body mass, and two died unexpectedly during this period. The n–3 and n–6 PUFA diets were both reformulated, reducing the long-chain PUFA intake by half (Table 2). After the reformulation, there were no further health issues. This problem only influenced the first four experimental blocks, with the first two blocks being the only birds to have detectable health issues and the two surviving individuals from these blocks were not included in the study. These birds were not replaced, and as such the n–3 PUFA group was composed of 16 birds total.
Table 2.
Composition of the reformulated dietary oil blends used in the experimental synthetic diets fed to the yellow-rumped warblers (Setophaga coronata)
| MUFA | n–3 PUFA | n–6 PUFA | |
|---|---|---|---|
| Percentage of dietary oil | |||
| Olive oil* | 72 | 57 | 58 |
| Coconut oil† | 28 | 15 | 30 |
| ARASCO (20:4n–6)‡ | 0 | 0 | 12 |
| DHASCO (22:6 n–3)‡ | 0 | 28 | 0 |
| Net fatty acid profile, g/100 g oil | |||
| SFA | 39.4 | 39.8 | 39.8 |
| MUFA | 53.8 | 49.1 | 47.1 |
| PUFA | 6.74 | 10.2 | 11.2 |
| n–3 PUFA | 0.50 | 5.02 | 0.42 |
| 18:3 n–3 | 0.50 | 0.40 | 0.42 |
| EPA (20:5 n–3) | 0 | 0 | 0 |
| DHA (22:6 n–3 | 0 | 4.62 | 0 |
| n–6 PUFA | 6.25 | 5.14 | 10.8 |
| 18:2 n–6 | 6.05 | 4.89 | 6.74 |
| ARA (20:4n–6) | 0.15 | 0.25 | 4.06 |
SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ARA, arachidonic acid.
Loblaws, Toronto, ON, Canada.
Spectrum Naturals, Delta, BC, Canada.
DSM Nutritional Products, Kaiseraugst, Switzerland.
Respirometry
To measure BMR, warblers were food-deprived for 2 h before the start of the dark period at 1900 to ensure they were in a postabsorptive state and placed into stainless steel chambers (1.12 liters) in an incubator at 31°C (Sanyo Incubator MIR-154, Sanyo Scientific). Inflow air into the chamber was dried using a gas drier (PC-4 Peltier Effect Dryer; Sable Systems International, Las Vegas, NV) and two desiccant columns (800-ml Drierite; W. A. Hammond Drierite, Zenia, OH). The dried air was split into 4 lines and passed individually through flow controllers (Flowbar 8; Sable Systems International) that regulated the flow to three 1.5-liter respirometry chambers and a background line at a rate of 1 l/min. Outflow air lines were attached to a multiplexer (MUX multiplexier, Sable Systems International), so that each line could be measured for 5 min before switching to the next line. The outflow air was subsampled at a rate of 200 ml/min before measuring water vapor (RH-300; Sable Systems International), CO2 (CA-2A; Sable Systems International), and O2 (FC-1B; Sable Systems International). The instruments were connected to an analog-to-digital converter (UI-2; Sable Systems International). With the use of Expedata software (Sable Systems International) for analysis, BMR was defined as the lowest rate oxygen consumption during a 5-min sampling period, which occurred 4–5 h into the dark period. V̇o2 and V̇co2 were lag corrected and calculated using Eqs. 11.7 and 11.8 from Lighton (33). The BMR V̇o2 measurements were converted into watts (W, J/s) adjusting for respiratory exchange ratio (RER) following Lighton (33).
Two to three days after BMR measurements, we used a hop/hover wheel to measure PMR (8, 48). The 24-cm diameter enclosed flight wheel (7.7 liters) was continuously supplied with dried air at a rate of 3 l/min. The air exiting the chamber was subsampled at 200 ml/min and water vapor, CO2, and O2 were measured as described above. The birds were deprived of food 3 h before to the PMR measurement. Each bird was given a 2-min adjustment period after it entered the flight wheel. The wheel was then manually spun, first slowly and then increasing in speed to maintain hovering flight. The birds were exercised until they began to pant and/or were unable to maintain hovering flight. PMR was calculated, similar to BMR, from the highest instantaneous V̇o2 averaged over a 1-min period (8). PMR measurements were not collected from all birds because a PMR measurement was stopped if the bird’s behavior in the flight wheel had the potential to damage their flight feathers or cause other bodily harm. Aerobic scope was calculated as PMR/BMR.
Endurance Flight Assessments and Sampling
Endurance flight performance was assessed using a wind tunnel designed for birds. Previous to the flight performance assessment, all birds (control and flown groups) had undergone flight testing during the fall (<20-min duration) and an additional flight (<15-min duration) the week before the flight assessment. For the flight performance assessments, the warblers were deprived of food 2 h before the start of the dark period to simulate the quiescent period that occurs before a migratory flight (51a). After the lights were turned off (1900), the birds were weighed and scanned using quantitative magnetic resonance (QMR, Echo-MRI-B; Echo Medical Systems, Houston, TX) to measure fat and wet lean mass (22) and placed in a cotton bag for 5 min. The birds were then flown under minimal light conditions, at 8 m/s, 70% relative humidity, and 15°C. Endurance flights lasted for up to 360 min. Flights ended when the bird voluntarily stopped three times within a 5-min period or reached the 360-min mark. The change in lean and fat mass was used to estimate the energy contribution from each fuel source using the conversion factors of 39.6 kJ/g for fat and 5.3 kJ/g for lean mass (16, 30). Power (W, J/s) was calculated from total energy expenditure divided by flight duration. Relative protein contribution was calculated as the proportion of total energy from lean mass catabolism.
The Flown birds were sampled immediately after flight (~2–7 h after lights off). During sampling, a 60- to 70-μl blood sample was collected and centrifuged (2,000 g for 10 min) to isolate the plasma. After this blood sampling was completed, the birds were weighed, and the body composition measured using QMR. The bird was then anesthetized using isoflurane (PrFlorane; Baxter, Mississaugua, ON, Canada) and killed by decapitation, and adipose and flight muscle samples were collected. The collected samples were flash frozen in liquid nitrogen and stored at −80°C. Control birds were deprived of food 2 h before sampling at the start of the dark period. Similar to the Flown birds, a 60- to 70-μl blood sample was collected, body mass and composition were measured, and muscle and adipose samples were collected and flash frozen. Sex was determined post mortem.
Fatty Acid Profiles
Fatty acid profiles of adipose, flight muscle phospholipids, and plasma were analyzed. Total lipids were extracted from 10 mg adipose, 50 mg flight muscle, and 10–15 μl of plasma, using methods modified from Price and Guglielmo (48) and Thomas et al. (59). Briefly, the tissue was homogenized in 2 ml chloroform-methanol-0.1% butylated hydroxytoluene (2:1:0.003 vol/vol/wt) and centrifuged at 3,750 g for 15 min. This was followed by the addition of 1 ml 0.25% potassium chloride. The samples were then incubated at 70°C for 10 min, and the bottom organic layer was removed, filtered through glass wool, and dried under a stream of nitrogen. We did not separate lipid fractions of adipose because the fatty acid content is almost completely reflective of neutral lipids (32). Because we did not separate lipid fractions of plasma, the fatty acid composition we report includes nonesterified fatty acid (NEFA), neutral lipids, and polar lipids. We were specifically interested in muscle phospholipids because muscle neutral lipids generally match adipose very closely (32). Thus, for the flight muscle, the phospholipid fraction was isolated using Supelclean solid phase extraction columns (Supelclean; Sigma-Aldrich, Oakville, ON) following Price and Guglielmo (48). Briefly, neutral lipids were eluted with 1.8 ml chloroform:isopropanol (2:1 vol/vol), followed by nonesterified fatty acids (NEFA) with 1.6 ml isopropyl ether:acetic acid (49:1 vol/vol), and the phospholipid fraction was eluted with 2 ml of methanol. The isolated fractions were dried under a nitrogen stream and resuspended in 100 μl chloroform. We added heptadecanoic acid (17:0, 200 μl, 3 mg/10 ml hexane; Sigma, St. Louis, MO) to the resuspended lipids as an internal standard. Before fatty acid methyl esters (FAMEs) generation, the resuspended lipid samples were dried under nitrogen. FAMEs were generated by the addition of 200 μl 0.5 N methanoic-HCl (Sigma-Aldrich) to the dried lipids and heated for 30 min at 90°C. Afterward, 800 μl water were added, and the FAMEs were extracted three times with 500 μl hexane. The hexane extract was dried under nitrogen and suspended in 80 μl chloroform. The FAMEs were separated on a 6890 N gas chromatograph (Agilent Technologies, Santa Clara, CA), with a flame ionization detector and a DB-225ms column (30-m long, 0.250 internal diameter, 0.25-μm film; Agilent Technologies, Palo Alto, CA) using N2 as the carrier gas. The temperature program was 80°C for 2 min, followed by a 5°C/min ramp to 180°C, which was held for 5 min, and then a 1°C/min to 200°C, followed by a 10°C/min ramp to 240°C, which was held for 3 min. Fatty acid peaks identities were determined by comparing retention times to commercial standards (Supelco 37 mix, Supelco PUFA No. 3 Menhaden Oil, Supelco FAME mix C8-C24; Sigma-Aldrich). To calculate fatty acid mass percent, the peak area for each fatty acid was divided by the total peak area of identified peaks and expressed as a proportion. With the exception of DHA, ARA, an EPA (20:5n–3), only fatty acids with at least one group with an average >1% were included for statistical analysis. The total proportion of each fatty acid class was calculated as follows: MUFA (14:1, 16:1 n–7, 18:1 n–9, and 18:1 n–7), PUFA (18:2n–3, 18:3 n–3, ARA, 22:5 n–3, and DHA), saturated fatty acids (SFA; 12:0, 14:0, 16:0, and 18:0), with n–3 and n–6 PUFA calculated as the sum of the n–3 and n–6 series, respectively, and long-chain PUFA as PUFA >18 carbons in length.
Flight Muscle Lipid Metabolism and PPAR Expression
Flight muscle mRNA abundance of PPAR-α, PPAR-β, PPAR-γ, heart-type fatty acid binding protein (H-FABP or FABP3), and a housekeeping gene (GAPDH) were measured using quantitative real-time PCR. RNA was isolated and DNAse treated from 30 mg of flight muscle tissue using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Mississauga, ON, Canada). cDNA synthesis was done using the High Capacity RNA-to-cDNA kit (Applied Biosystems, Burlington, ON, Canada), and the cDNA was then diluted 100-fold with water. Quantitative real-time PCR was run for each gene with a CFX384 Real-Time system instrument (Bio-Rad, Mississauga, ON, Canada). The PCR reaction conditions were 2 μl of the diluted cDNA, 6 μl 2× master mix (SensiFast SYBR and Fluorescein Mix, Bioline; Tauton, MA), 0.33 μM of the primers (Supplemental Table S2; https://figshare.com/articles/AJP_Supplemental_Materials_pdf/7492781/1) in a total reaction volume of 12 μl. The cycling conditions for all other genes were as follows: 95°C held for 10 min, followed by 45 cycles of 95°C for 20 s, 59°C for 20 s, and 72°C for 10 s. Primers and cycling conditions for H-FABP are as described by McFarlan et al. (40). All samples were run in triplicate. The mRNA expression was calculated using the 2−ΔΔCT method (34). For each gene, the arithmetic mean expression of the MUFA Control group was set to 1.
Maximum enzyme activity in the flight muscle of carnitine palmitoyl transferase (CPT; EC 2.3.1.21), citrate synthase (CS; EC 2.3.3.1), 3-hydroxyacyl-CoA dehydrogenase (HOAD; EC 1.1.1.35), and lactate dehydrogenase (LDH; EC 1.1.1.27) was measured following Price et al. (50).
Statistical Analysis
All statistics were performed using SAS (version 9.4; SAS Institute, Cary, NC), with significance set at α < 0.05, and trends in the data were considered at P < 0.1. By chance the sexes were evenly distributed across the diet treatments (MUFA: 11 males, 9 female; n–6 PUFA: 10 male, 10 female; and n–3 PUFA: 9 male, 7 female). Sex, tarsus length and wing cord were not significant factors or covariates (P > 0.05) and as such were not included in the statistical analysis. Body mass and the blocking factor were included as covariates when significant (P ≤ 0.05). Weekly body mass was analyzed using repeated measures to test for the effects of diet, time, and their interaction on body mass. Metabolic rates (BMR and PMR), and final body mass and body composition analyses were performed using one-way ANOVA testing for an effect of diet. For these analyses, the Flown and Control birds for each diet were combined as the birds were treated identically until the wind tunnel flight and there was no significant effect of flight (P > 0.05).
Fatty acid profiles were first analyzed by measures analysis of variance (MANOVA) using Wilks Lamda for each tissue to test for significant main effects and the diet and flight interaction. If the interaction was not significant in the MANOVA, it was dropped from the model to simplify the analysis. Significant effects or interactions were then evaluated by univariate analysis on individual fatty acids and fatty acid classes using ANOVA.
The Kruskal-Wallis nonparametric test was used to test for differences in flight duration. Other flight performance parameters tested included initial body mass and flight duration as covariates, and we initially tested for their interactions. Nonsignificant interactions were removed from the model (P > 0.05). Correlations between flight performance variables were determined using Pearson correlation coefficient.
Muscle metabolic enzyme activities and gene expression were analyzed using two-way ANOVA to test for the main effects and interaction of diet and flight treatment. Pearson correlation coefficients were used to test for relationships between muscle metabolic indicators and whole animal performance. For flight duration, all flights were included in the correlation analysis. Since flight duration was significantly and strongly correlated to energy costs and relative protein contribution, we used only the 360-min flights for correlations with energy costs and relative protein contribution as this would eliminate flight duration as a factor that would influence the correlations.
RESULTS
Fatty Acid Composition
The muscle phospholipids showed the greatest response to manipulation of dietary fatty acid composition (Table 3). The experimental diets altered the fatty acid composition of the muscle phospholipids (MANOVA: Wilks λ = 0.03: F14,80 = 31.86, P < 0.0001), but flight did not have any further effect (MANOVA: Wilks λ = 0.85: F6,41 = 0.47, P = 0.83) nor was there an interaction between diet and flight (MANOVA: Wilks λ = 0.87: F12,82 = 0.47, P = 0.93). The MUFA diet group had higher total MUFA and lower SFA in the muscle phospholipids compared with the n–3 and n–6 PUFA groups, and this was primarily the result of higher proportions 18:1n–9 and lower 16:0 and 18:0 in the MUFA group. No differences in total PUFA were observed among the diet groups. However, the MUFA group had less long-chain PUFA but with both DHA and ARA present. The n–6 PUFA and n–3 PUFA diet groups had higher ARA and DHA levels, respectively, in muscle phospholipids. The n–3 PUFA group had higher levels of EPA than the other diets but was substantially less than the proportions of DHA.
Table 3.
Fatty acid composition of the muscle phospholipids from yellow-rumped warblers (Setophaga coronata) fed different dietary oil blends
| Fatty Acid | MUFA | n–3 PUFA | n–6 PUFA | F Value | P Value |
|---|---|---|---|---|---|
| 16:0 | 27.86 ± 0.72b | 33.94 ± 0.70a | 29.50 ± 0.72b | F2,50 = 19.69 | <0.0001 |
| 18:0 | 25.71 ± 0.61b | 28.18 ± 0.60a | 29.61 ± 0.61a | F2,50 = 10.18 | 0.0002 |
| 18:1n–9 cis | 18.14 ± 0.57b | 7.14 ± 0.56c | 10.12 ± 0.57b | F2,50 = 99.16 | <0.0001 |
| 18:2n–6 cis | 6.58 ± 0.38b | 1.31 ± 0.37b | 1.65 ± 0.38b | F2,50 = 60.91 | <0.0001 |
| 20:4n–6 (ARA) | 10.34 ± 1.01b | 0.67 ± 0.98c | 18.95 ± 1.01a | F2,50 = 88.73 | <0.0001 |
| 20:5n–3 (EPA) | 0.06 ± 0.11b | 1.17 ± 0.11a | 0.09 ± 0.11b | F2,50 = 34.28 | <0.0001 |
| 22:6 n–3 (DHA) | 8.26 ± 1.40c | 25.00 ± 1.36a | 7.07 ± 1.39b | F2,50 = 53.16 | <0.0001 |
| SFA | 54.00 ± 1.04c | 62.70 ± 1.02a | 59.58 ± 1.04b | F2,50 = 18.43 | <0.0001 |
| MUFA | 19.72 ± 0.59a | 8.91 ± 0.57c | 11.67 ± 0.59b | F2,50 = 93.20 | <0.0001 |
| PUFA | 26.29 ± 1.21 | 28.38 ± 1.18 | 28.34 ± 1.21 | F2,50 = 0.98 | 0.38 |
| LCPUFA | 19.19 ± 1.30b | 27.03 ± 1.27a | 26.53 ± 1.30a | F2,50 = 11.50 | <0.0001 |
| n–3 PUFA | 8.90 ± 1.46b | 26.36 ± 1.42a | 7.60 ± 1.46b | F2,50 = 53.44 | <0.0001 |
| n–6 PUFA | 17.09 ± 0.90b | 2.01 ± 0.87c | 20.71 ± 0.89a | F2,50 = 128.08 | <0.0001 |
| n–6:n–3 | 2.13 ± 0.86b | 0.10 ± 0.85c | 4.69 ± 0.86a | F2,50 = 7.17 | 0.002 |
Data are %mean mass ± SE. Fatty acid groups include minor fatty acids not listed above. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ARA, arachidonic acid; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; LCPUFA, long-chain PUFA. Warblers per group: MUFA: 20; n–3 PUFA: 16; n–6 PUFA: 20.
P < 0.05, values with different superscripts differ between diet groups.
In adipose tissue, there was an overall effect of diet on fatty acid composition (MANVOA: Wilks λ < 0.001: F20,74 = 8.56, P < 0.0001); however, flight did not have any effect (MANOVA: Wilks λ = 0.09: F10,37 = 1.24, P = 0.29), and there was no interaction between flight and diet (MANOVA: Wilks λ = 0.80: F20,74 = 0.45, P = 0.97). When compared with the flight muscle, adipose had lower proportions of SFA and PUFA, higher proportions of MUFA. When the n–3 and n–6 diet groups were compared, the MUFA diet group had lower proportion of SFA but higher proportion of MUFA (Table 4). Differences in long-chain PUFA were present, but the levels were much lower compared with the muscle phospholipids, and none of the relative proportions of long-chain PUFA exceeded 1%.
Table 4.
Fatty acid composition of the adipose from yellow-rumped warblers (Setohaga coronata) fed different dietary oil blends
| Fatty Acid | MUFA | n–3 PUFA | n–6 PUFA | F Value | P Value |
|---|---|---|---|---|---|
| 12:0 | 3.88 ± 0.21a,b | 3.13 ± 0.21b | 4.39 ± 0.21a | F2,50 = 9.29 | 0.0004 |
| 14:0 | 3.09 ± 0.24b | 4.11 ± 0.23a | 3.73 ± 0.24b | F2,50 = 4.85 | 0.012 |
| 16:0 | 27.97 ± 0.86 | 29.09 ± 0.84 | 27.87 ± 0.86 | F2,50 = 0.64 | 0.53 |
| 16:1n–7 | 3.75 ± 0.29 | 3.32 ± 0.28 | 3.78 ± 0.29 | F2,50 = 0.81 | 0.45 |
| 18:0 | 6.32 ± 0.37b | 8.38 ± 0.36a | 8.45 ± 0.37a | F2,50 = 10.72 | 0.0002 |
| 18:1n–7 | 1.14 ± 0.08a | 0.93 ± 0.08a,b | 0.72 ± 0.08b | F2,47 = 6.99 | 0.002 |
| 18:1n–9 cis | 49.66 ± 1.05a | 44.06 ± 1.03b | 45.75 ± 1.05b | F2,50 = 7.71 | 0.001 |
| 18:2n–6 cis | 3.48 ± 0.37 | 3.80 ± 0.36 | 3.53 ± 0.37 | F2,50 = 0.22 | 0.80 |
| 20:4n–6 (ARA) | 0.04 ± 0.05b | 0.04 ± 0.05b | 0.63 ± 0.05a | F2,50 = 50.47 | <0.0001 |
| 20:5n–3 (EPA) | 0.002 ± 0.01b | 0.04 ± 0.01a | 0.002 ± 0.01b | F2,50 = 9.64 | 0.0003 |
| 22:6 n–3 (DHA) | 0.09 ± 0.12b | 0.98 ± 0.11a | 0.001 ± 0.12b | F2,50 = 20.24 | <0.0001 |
| SFA | 41.72 ± 0.87b | 45.14 ± 0.85a | 44.91 ± 0.87a | F2,50 = 4.87 | 0.012 |
| MUFA | 54.18 ± 0.85a | 49.41 ± 0.83b | 50.27 ± 0.85b | F2,50 = 9.11 | 0.0005 |
| PUFA | 3.85 ± 0.45 | 5.15 ± 0.44 | 4.58 ± 0.45 | F2,50 = 2.11 | 0.13 |
| LCPUFA | 0.15 ± 0.13c | 1.12 ± 0.13a | 0.65 ± 0.13b | F2,50 = 13.15 | <0.0001 |
| n–3 PUFA | 0.26 ± 0.13b | 1.26 ± 0.13a | 0.22 ± 0.13b | F2,50 = 19.46 | <0.0001 |
| n–6 PUFA | 3.54 ± 0.37 | 3.86 ± 0.36 | 4.29 ± 0.37 | F2,50 = 1.00 | 0.38 |
| n–6:n–3 | 20.07 ± 2.07a | 5.38 ± 2.02b | 20.34 ± 2.07a | F2,50 = 17.62 | <0.0001 |
Data are %mean mass ± SE. Fatty acid groups include minor fatty acids not listed above. Values with different superscripts differ (P < 0.05) between diet groups. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ARA, arachidonic acid; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; LCPUFA, long-chain PUFA. Warblers per group: MUFA: 20; n–3 PUFA: 16; n–6 PUFA: 20.
P < 0.05, values with different superscripts differ between diet groups.
In contrast to the flight muscle phospholipids and adipose, in the plasma there was an interaction between diet and flight (MANOVA: Wilks λ = 0.44: F20,78 = 1.92, P = 0.02), and main effects of flight (MANOVA: Wilks λ = 0.53: F10,39 = 3.45, P = 0.002) and diet (MANOVA: Wilks λ = 0.02: F20,78 = 22.06, P < 0.0001). The differences in the plasma fatty acids among diets were similar to those observed in the muscle phospholipid and adipose (Table 5). Additionally, the effect of flight on fatty acid composition was diet specific. In the MUFA group, 16:1n–7, 18:1n–9, and 18:2n–6 were higher in the Flown birds, and there was higher proportion of MUFA and lower proportion of SFA. In the n–3 PUFA group, MUFA were lower and SFA higher in the Flown birds. No significant effect of flight was observed in the n–6 PUFA group.
Table 5.
Fatty acid composition of total plasma lipids from Control and Flown yellow-rumped warblers (Setophaga coronata) fed different dietary oil blends
| Fatty Acid/Flight | MUFA | n–3 PUFA | n–6 PUFA | F Value | P Value |
|---|---|---|---|---|---|
| 12:0 | 1.07 ± 0.13a | 0.61 ± 0.13b | 0.79 ± 0.13b | F2,50 = 3.52 | 0.04 |
| 14:0 | 1.84 ± 0.21 | 1.42 ± 0.21 | 1.50 ± 0.22 | F2,50 = 1.11 | 0.34 |
| 15:0 | 1.57 ± 0.42 | 1.37 ± 0.42 | 0.53 ± 0.44 | F2,50 = 1.66 | 0.2 |
| 16:0 | 25.40 ± 0.50 | 26.24 ± 0.51 | 26.49 ± 0.50 | F2,50 = 1.27 | 0.29 |
| 16:1n–7 | |||||
| Control | 1.85 ± 0.16*a | 2.00 ± 0.16*a | 1.32 ± 0.17b | F2,50 = 5.12 | 0.01 |
| Flown | 2.37 ± 0.17a | 1.49 ± 0.18b | 1.56 ± 0.18b | ||
| 18:0 | 15.32 ± 1.41 | 13.54 ± 1.45 | 17.82 ± 1.47 | F2,50 = 2.12 | 0.13 |
| 18:1n–7 | 1.14 ± 0.08a | 0.93 ± 0.08a,b | 0.72 ± 0.08b | F2,47 = 6.99 | 0.002 |
| 18:1n–9 cis | |||||
| Control | 32.00 ± 2.64* | 37.46 ± 2.63 | 30.28 ± 2.78 | F2,50 = 4.62 | 0.01 |
| Flown | 41.34 ± 2.78a | 30.58 ± 2.94b | 28.21 ± 2.9b | ||
| 18:2n–6 cis | |||||
| Control | 4.32 ± 0.50* | 5.19 ± 0.37* | 3.83 ± 0.53 | F2,50 = 5.84 | 0.005 |
| Flown | 6.20 ± 0.50a | 3.59 ± 0.56b | 3.35 ± 0.56b | ||
| 20:3 n–6 | 0.57 ± 0.48 | 0.26 ± 0.49 | 1.28 ± 0.51 | F2,50 = 1.08 | 0.34 |
| 20:4n–6 (ARA) | 5.02 ± 0.80b | 0.71 ± 0.82c | 12.96 ± 0.80a | F2,50 = 55.06 | <0.0001 |
| 20:5n–3 (EPA) | 0.21 ± 0.25b | 4.32 ± 0.25a | 0.10 ± 0.26b | F2,50 = 89.76 | <0.0001 |
| 22:6 n–3 (DHA) | 0.48 ± 0.36b | 6.96 ± 0.37a | 0.36 ± 0.37b | F2,50 = 105.69 | <0.0001 |
| SFA | |||||
| Control | 47.91 ± 2.57*a | 39.08 ± 2.57b | 47.29 ± 2.71a | F2,50 = 3.33 | 0.04 |
| Flown | 40.25 ± 2.71 | 45.29 ± 2.88 | 46.57 ± 2.88 | ||
| MUFA | |||||
| Control | 37.13 ± 2.26*a | 41.64 ± 2.26*b | 32.88 ± 1.74a | F2,50 = 5.51 | 0.007 |
| Flown | 45.50 ± 2.38a | 34.35 ± 2.53b | 33.04 ± 2.53b | ||
| PUFA | 12.52 ± 1.07b | 17.66 ± 1.11a | 19.00 ± 1.13a | F2,50 = 9.82 | 0.0003 |
| LCPUFA | 5.82 ± 0.97b | 12.43 ± 1.00a | 13.59 ± 1.02a | F2,50 = 18.08 | <0.0001 |
| n–3 PUFA | 1.18 ± 0.58b | 11.92 ± 0.60a | 0.93 ± 0.61b | F2,50 = 109.80 | <0.0001 |
| n–6 PUFA | 10.90 ± 0.84b | 5.44 ± 0.87c | 17.88 ± 0.89a | F2,50 = 50.10 | <0.0001 |
| n–6 :n–3 | 10.11 ± 1.75b | 0.50 ± 1.80c | 23.94 ± 1.85a | F2,50 = 41.35 | <0.0001 |
Data are %mean mass ± SE. Fatty acid groups include minor fatty acids not listed above. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ARA, arachidonic acid; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; LCPUFA, long-chain PUFA. Warblers per group: n–3 PUFA: 7 Flown, 9 Control; n–6 PUFA: 8 Flown, 12 Control; MUFA: 9 Flown, 11 Control.
Significant effect of flight within a diet group.
P < 0.05, values with different superscripts differ between diet groups; for significant diet and flight interactions, letter comparisons are within Control and Flown groups.
Body Composition and Metabolic Rate
Warblers decreased in body mass during the first week of adjustment to the experimental diets and immediately following the switch to long-day photoperiod, but they increased in body mass as they entered into a migratory condition (Fig. 1; week: F6,371 = 13.14, P < 0.001). We also observed hyperphagia and migratory restlessness behavior, verifying the birds entered a migratory condition after switching to the long-day photoperiod. There was no significant main effect of diet or interaction between diet and time (diet: F2,371 = 2.70, P = 0.07; diet × time: F12,371 = 0.19, P = 0.99), and the blocking factor (treatment start date) was significant, with the last two blocks being slightly heavier overall (F9,371 = 5.09, P < 0.001). Final body composition at sampling in the Controls and preflight in the Flown birds did not differ among the diets for body mass (F2,53 <0.01, P = 0.99), wet lean mass (F2,53 = 0.23, P = 0.79), or fat mass (F2,53 = 0.48, P = 0.62), and there was no effect of the blocking factor (P > 0.05) (Table 6).
Fig. 1.
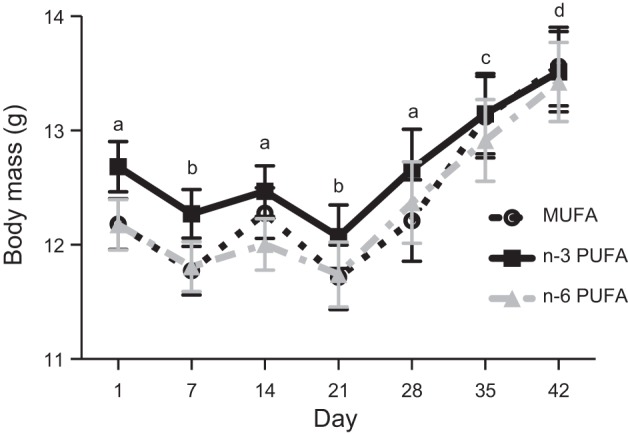
Body mass (means ± SE) of yellow-rumped warblers (Setophaga coronata) fed experimental diets over the first 42 days. Day 1 indicates the start of the experimental diets, and on day 14, birds shift from short day to long day photoperiod. Monounsaturated fatty acids (MUFA; n = 20): black circle and dotted line, n–3 long-chain polyunsaturated fatty acids (PUFA; n = 16): dark gray square and solid line, n–6 PUFA (n = 20): light gray triangle and dotted line. Letters indicate overall significant differences between days (P < 0.05).
Table 6.
Final body mass and composition at sampling (Controls) and preflight (Flown) and BMR and PMR of yellow-rumped warblers (Setophaga coronata) fed the different dietary oil blends
| MUFA | n–3 PUFA | n–6 PUFA | |
|---|---|---|---|
| Body mass, g | 13.55 ± 0.33 | 13.53 ± 0.36 | 13.54 ± 0.34 |
| Fat mass, g | 2.85 ± 0.23 | 2.54 ± 0.24 | 2.80 ± 0.23 |
| Lean mass, g | 8.53 ± 0.15 | 8.66 ± 0.16 | 8.52 ± 0.15 |
| BMR, W | 0.220 ± 0.004 | 0.226 ± 0.004 | 0.228 ± 0.004 |
| PMR, W | 2.00 ± 0.05 | 1.84 ± 0.05 | 1.92 ± 0.05 |
| Aerobic Scope | 9.00 ± 0.31 | 8.31 ± 0.32 | 8.29 ± 0.30 |
Body composition values reported are means ± SE. Basal metabolic rate (BMR), peak metabolic rate (PMR), and aerobic scope are least squared means ± SE with body mass included as a covariate. MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids. Warblers per group: MUFA: 20; n–3 PUFA: 16; n–6 PUFA: 20, with the exception of PMR and aerobic scope: MUFA: 15, n–6 PUFA: 12; n–3 PUFA: 12.
Diet did not significantly influence BMR (F2,52 = 1.24, P = 0.29), after we controlled for the positive effect of the covariate body mass (slope = 0.008; F1,52 = 34.79, P < 0.001). Unlike BMR, not all PMR measurements were successful, and only 31 successful PMR were measured (16 in the Control group, with 15 in the Flown group, 7 of which flew for 360 min). Similar to BMR, no diet effects were detected in PMR (F2,26 = 2.38, P = 0.11), after we controlled for the effects of the covariates body mass (slope = 0.003, F1,26 = 9.97, P = 0.04) and the blocking factor (F8,26 = 9.97, P < 0.001). Correspondingly, there were no significant differences among diets in aerobic scope (F2,27 = 2.02, P = 0.15), with the blocking factor included as a covariate (F8,27 = 4.48, P < 0.001).
Endurance Flight Performance
Twenty-four warblers successfully completed endurance flights (n–3 PUFA: 7 Flown, 9 Control; n–6 PUFA: 8 Flown, 12 Control; MUFA: 9 Flown, 11 Control). The majority of flights were 360 min in length (n = 15), and the remaining flights were typically 60–120 min in length (Fig. 2). We had 11 failed flights over the course of the study (n–3 PUFA: 4; n–6 PUFA: 4; MUFA: 3), and after the flight attempts these birds were sampled as Control birds. We were unable to determine any behavioral, physical factor (feather condition, body composition), or muscle biochemical marker that could differentiate these birds from either the Control or Flown groups.
Fig. 2.
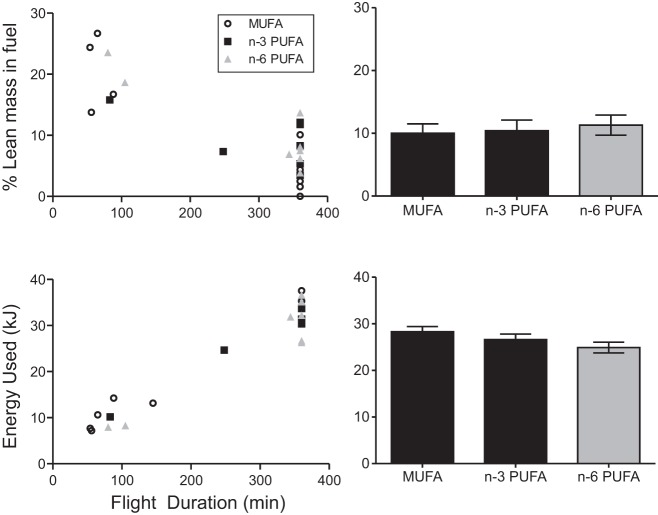
Effect of diet on relative protein contribution and total energy used during endurance flight of yellow rumped warblers (Setophaga coronata). Warblers were fed diets enriched in monounsaturated (MUFA: black open circles, n = 9), n–3 polyunsaturated (n–3 PUFA: gray squares, n = 7), or n–6 polyunsaturated (n–6 PUFA: light gray triangles, n = 8) acids. A: distribution of relative protein contribution with flight duration. B: relative protein contribution accounting for the effect of body mass and flight duration (least square means ± SE). C: distribution of total energy used and flight duration. D: total energy used accounting for flight duration and body mass (least square means ± SE).
Birds that flew longer had lower flight power (r = −0.56, P = 0.003) and a lower relative protein contribution used to fuel the flight (r = −0.80, P < 0.0001; see Supplemental Table S3; https://figshare.com/articles/AJP_Supplemental_Materials_pdf/7492781/1). The amount of fat catabolized increased with flight duration (r = 0.90, P < 0.0001), but lean mass catabolized did not correlate with flight duration (r = 0.04, P = 0.46). With the consideration that birds that flew the full 360 min, body mass was positively correlated with flight energy cost (r = 0.75, P = 0.0012) and negatively with relative protein contribution (r = −0.73, P = 0.0019; see Supplemental Table S4 and Supplemental Fig. S2: https://figshare.com/articles/AJP_Supplemental_Materials_pdf/7492781/1). The amount of fat catabolized was significantly correlated to body mass (r = 0.74, P = 0.001), and there was a trend for less lean mass catabolism with increasing body mass (r = −0.49, P = 0.06).
Flight duration did not vary among the diet groups (χ2 =0.8296, P = 0.67; Table 7). Additionally, diet did not alter the amount of mass catabolized during flight (F2,19 = 0.43, P = 0.65), with the included covariates: flight duration (F1,19 = 67.8, P < 0.0001) and preflight body mass (F1,19 = 0.82, P = 0.38). Similarly, no significant differences in lean mass catabolized (diet: F2,19 = 0.48, P = 0.62; duration: F1,19 = 0.15, P = 0.71; preflight body mass: F1,19 = 4.17, P = 0.55) or fat mass catabolized (diet: F2,19 = 1.88, P = 0.18; duration: F1,19 = 198, P < 0.0001; preflight body mass: F1,19 = 14.16, P = 0.001) were observed. Total energy used did not differ among diets (diet: F2,19 = 2.17, P = 0.14; duration: F1,19 = 270.14, P < 0.0001; preflight body mass: F1,19 = 13.89, P = 0.001). The relative protein contribution to the fuel mixture did not differ between diets (diet: F2,19 = 1.20, P = 0.33; duration: F1,19 = 28.79, P < 0.0001; preflight body mass: F1,19 = 4.18, P = 0.06). Similar results were observed when the 360-min flights only were tested (Table 7). Flight power (W) did not differ among diets when 360-min flights only were examined (diet: F2,11 = 1.11, P = 0.36; preflight body mass: F1,11 = 11.67; P = 0.006). Power is reported for the 360-min flights only because it was significantly correlated with flight duration, and flight power decreased with increasing flight duration. This could create an issue with autocorrelation and yield misleading conclusions about the effect of diet.
Table 7.
Flight performance metrics and body composition changes for yellow-rumped warblers (Setophaga coronata) fed the experimental diets for all flights or 360-min flights only
| MUFA | n–3 PUFA | n–6 PUFA | |
|---|---|---|---|
| All Flights | (n = 9) | (n = 7) | (n = 8) |
| Duration, min | 241.9 ± 70.7 | 304.4 ± 78.1 | 301.2 ± 75.3 |
| Mass loss, g | 1.06 ± 0.06 | 1.13 ± 0.06 | 1.05 ± 0.06 |
| Fat loss, g | 0.67 ± 0.03 | 0.61 ± 0.04 | 0.58 ± 0.03 |
| Lean loss, g | 0.34 ± 0.06 | 0.44 ± 0.07 | 0.39 ± 0.07 |
| 360-min Flights | (n = 5) | (n = 5) | (n = 5) |
| Mass loss, g | 1.23 ± 0.08 | 1.31 ± 0.08 | 1.27 ± 0.08 |
| Fat loss, g | 0.87 ± 0.04 | 0.79 ± 0.04 | 0.76 ± 0.04 |
| Lean loss, g | 0.28 ± 0.09 | 0.49 ± 0.09 | 0.42 ± 0.09 |
| Energy used, kJ | 36.1 ± 1.6 | 34.1 ± 1.6 | 32.5 ± 1.7 |
| Power, W | 1.67 ± 0.08 | 1.58 ± 0.08 | 1.51 ± 0.08 |
| RPC, % | 4.43 ± 1.6 | 8.01 ± 1.6 | 7.33 ± 1.6 |
Duration is means ± SE. All other values are least squared means ± SE, with body mass and flight duration included as covariates All Flights, and body mass included as a covariate for the 360-min Flights. MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; RPC, relative protein contribution.
PPAR mRNA Expression and Metabolic Enzymes
Neither diet nor flight significantly influenced PPAR-α mRNA abundance (diet: F2,50 = 1.5, P = 0.23; flight: F1,50 = 2.3, P = 0.14, diet × flight: F2,50 = 0.16, P = 0.85, Fig. 3). In contrast, PPAR-β differed significantly among diets (F2,50 = 3.77, P = 0.03) and was higher in Flown birds (F1,50 = 21.44, P < 0.001), but there was no significant diet × flight interaction (F2,50 = 1.43, P = 0.25). PPAR-β mRNA abundance was significantly lower in the n–3 PUFA diet group. For PPAR-γ, a significant interaction between diet and flight was observed (F2,50 = 3.26, P = 0.047), where expression was lower in both the MUFA and n–3 PUFA Flown groups but not in the n–6 PUFA group, which had intermediate mRNA levels.
Fig. 3.
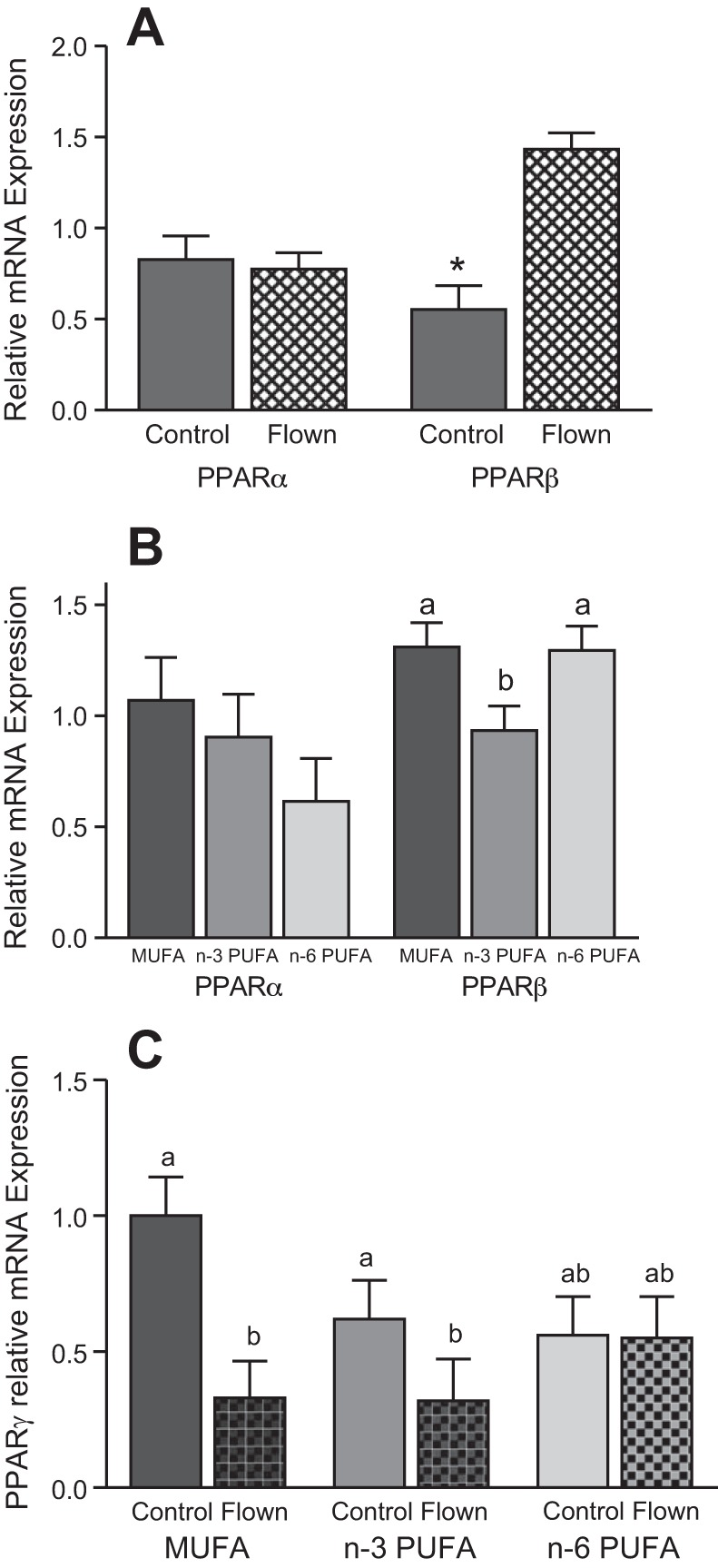
Effect of diet and flight on peroxisome proliferator-activated receptor (PPAR) mRNA expression in yellow-rumped warblers (Setophaga coronata). A: effect of flight on PPAR-α and PPAR-β mRNA expression. B: effect of diet on PPAR-α and PPAR-β expression. C: effect of diet and flight on PPAR-γ expression. Values are means ± SE. *A significant effect of flight on a PPAR isoform (A) and values that do not share a common letter differ within a PPAR isoform (B and C) (P < 0.05). Warblers per group: n–3 long-chain polyunsaturated fatty acid (PUFA): 7 Flown, 9 Control; n–6 PUFA: 8 Flown, 12 Control; MUFA: 9 Flown, 11 Control.
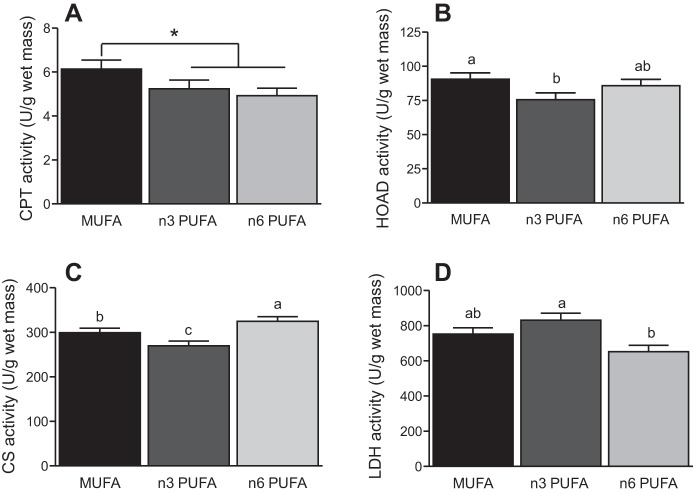
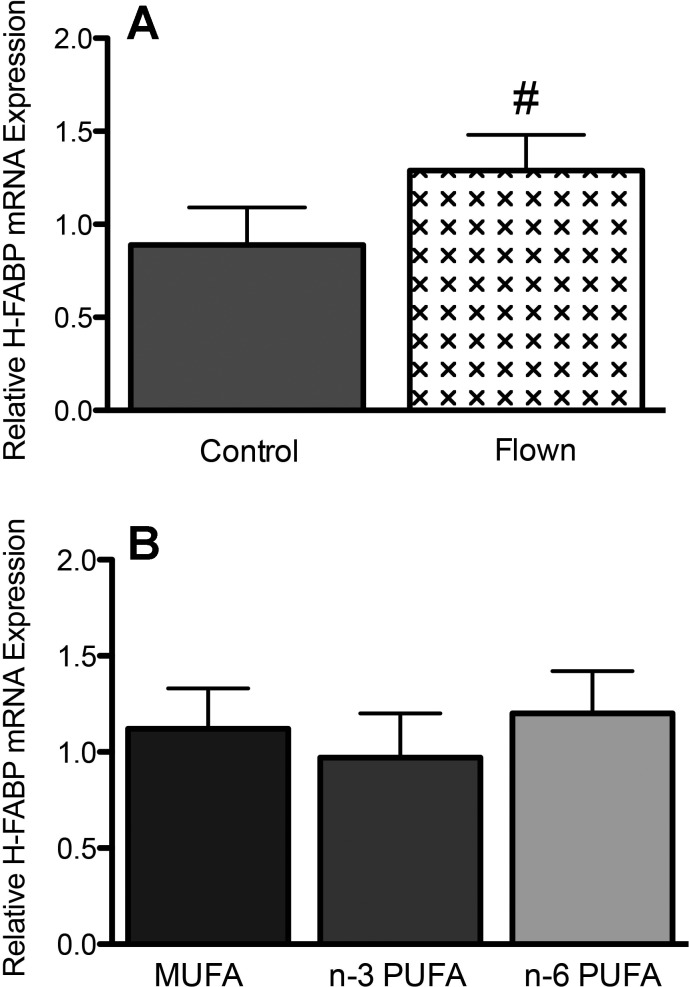
Metabolic enzyme activities were also influenced by the experimental diets, but flight, body mass, or the blocking factors were not significant (Fig. 4). The activity of CPT was not related to diet or flight (P > 0.05). However, when the two PUFA groups were combined they had lower CPT activity than the MUFA group (F1,50 = 4.58, P = 0.037). Activity of CS was significantly lower in the n–3 PUFA group compared with the n–6 PUFA group (diet: F2,50 = 6.81, P = 0.002; flight: F1,50 = 0.09, P = 0.76, diet × flight: F2,50 = 1.24, P = 0.30). The n–3 PUFA group also had lower HOAD activity compared with the n–6 PUFA and MUFA diet groups (diet: F2,50 = 3.28, P = 0.046; flight: F1,50 = 1.02, P = 0.32, diet × flight: F2,50 = 2.21, P = 0.12). In contrast, LDH activity was elevated in the n–3 PUFA group compared with the n–6 PUFA group (diet: F2,50 = 5.91, P = 0.008; flight: F1,50 < 0.01, P = 0.97, diet × flight: F2,50 = 0.64, P = 0.53). H-FABP mRNA expression was not influenced by diet, but a trend for increased expression during flight was observed (diet: F2,50 = 0.48, P = 0.62; flight: F1,50 = 3.63, P = 0.06, diet × flight: F2,50 = 0.15, P = 0.86, Fig. 5).
Fig. 4.
Flight muscle enzyme activity of carnitine palmitoyl transferase (CPT; A), 3-hydroxyacyl-CoA dehydrogenase (HOAD; B), citrate synthase (CS; C), and lactate dehydrogenase (LDH; D) of yellow-rumped warblers (Setophaga coronata) fed diets enriched in monounsaturated (MUFA; n = 20), n–3 polyunsaturated (n–3 PUFA; n = 16), or n–6 polyunsaturated (n–6 PUFA; n = 20) fatty acids. Values are means ± SE. Values that do not a share a letter differ (P < 0.05). *Significant difference between MUFA and the n–3 and n–6 PUFA diets combined.
Fig. 5.
Effect of flight (A) and diet (B) on heart type fatty acid binding protein (H-FABP) mRNA expression of yellow rumped warblers (Setophaga coronata). Warblers were fed diets enriched in monounsaturated (MUFA; n = 20), n–3 polyunsaturated (n–3 PUFA; n = 16), or n–6 polyunsaturated (n–6 PUFA; n = 20) fatty acids and Flown (n = 24) or unflown Control (n = 32) in a wind tunnel. #Significant trend (P < 0.1).
Correlations of Muscle Metabolism and Metabolic Rate with Flight Performance
There were no significant correlations between flight duration and muscle metabolism or metabolic rate (Supplemental Table S3; https://figshare.com/articles/AJP_Supplemental_Materials_pdf/7492781/1). Since flight performance (power and relative protein contribution) changed with duration, we focused solely on the 360-min flight for correlations between metabolic rate and muscle metabolism with flight performance (Supplemental Table S4). BMR was significantly correlated with flight power, likely as a result of body mass influencing both factors. Although neither BMR nor PMR was correlated with relative protein contribution, aerobic scope was positively correlated with the relative protein contribution. In terms of muscle metabolic indicators, the only significant correlation with flight performance was a negative correlation between HOAD and relative protein contribution.
DISCUSSION
The natural doping hypothesis proposes that DHA and EPA increase flight muscle oxidative capacity, thereby enabling prolonged endurance flight of migratory birds (36, 37, 61). In the current study, we found no support for the natural doping hypothesis. PUFA did influence muscle lipid metabolism, but feeding diets enriched with n–3 PUFA caused a coordinated decrease in oxidative capacity. However, dietary differences in muscle metabolism were not reflected in flight performance (energy cost, duration, relative protein contribution), and no treatment effects were evident at the whole animal level.
A similar, but distinct, hypothesis suggests that PUFA and/or 18:2 n–6 benefit migratory birds by increasing metabolic flight efficiency, PMR, and aerobic scope (45, 48). Unlike previous studies, we formulated experimental diets to manipulate only the intake of the DHA and ARA to avoid potential confusion in the interpretation of the effects of dietary fatty acid composition on exercise and metabolism (23). As a result, the proportion of 18:2 n–6 was similar among all diets. Other dietary differences were present but fairly minor, such as lower SFA in the MUFA diet and lower PUFA and 18:2 n–6 in the n–3 PUFA compared with n–6 PUFA. We found no overall beneficial effects of PUFA on muscle metabolic capacity or flight performance compared with MUFA, although our data cannot test for the effects of 18:2 n–6.
Effects of Dietary Fatty Acids and Flight on Tissue Fatty Acid Composition
The effect of our dietary manipulations of primarily long-chain PUFA was most apparent in the muscle phospholipids rather than adipose. Birds fed the n–3 and n–6 PUFA diets had the highest levels of long-chain PUFA in their muscles, which reflected the long-chain PUFA present in their diets. Birds fed the MUFA diet had the lowest proportion of long-chain PUFA in phospholipids, with a mix of ARA and DHA, presumably from elongation and desaturation of 18:2 n–6 and 18:3 n–3, respectively (56). Many birds have high levels of long-chain PUFA in their membranes, despite these fatty acids not being present in their diets (4, 32). This suggests that adult songbirds may be actively synthesize long-chain PUFA from precursor fatty acids for incorporation into muscle membranes and do so with any available precursor fatty acids. This also may indicate significant elongase and desaturase activity, which should be studied experimentally.
Differences among dietary treatments in the adipose fatty acid composition of birds were much smaller compared with the muscle phospholipids. Although significant differences were observed in the long-chain PUFA, they contributed <1.5% of the total fatty acids in adipose and as such would likely have only a small influence on overall fuel composition. The proportion of PUFA was lower than reported in other studies (39, 46, 48). Excess essential fatty acids, such as ARA and DHA, may be stored in the neutral lipids, including adipose (39). In our study, long-chain PUFA were likely not consumed in great enough quantities for this to occur at levels observed in other studies. Rather than being stored, ARA and DHA appeared to be preferentially incorporated into more metabolically active tissues such as the flight muscles. The greatest differences in fatty acid composition in the adipose were in the proportions of SFA and MUFA. The MUFA diet group had higher proportions of MUFA and lower proportions of SFA compared with birds fed PUFA diets. However, this difference was small (<5%). As such, it is unlikely that the fatty acid composition varied enough among diets to result in large differences in mobilization from the adipose during flight (49). Since the differences in the adipose fatty acid composition among diets were much smaller than in the muscle phospholipids, our study primarily tests the effects of phospholipids, which is most relevant to the natural doping hypothesis.
Similar to the adipose and muscle phospholipids, the expected differences in the plasma fatty acid composition from the dietary treatments were observed. Only a few fatty acids changed during flight (16:1n–7, 18:1 n–9, and 18:2 n–6), and these changes were only observed in the MUFA and n–3 PUFA diet groups. In the MUFA diet group, MUFA increased and SFA decreased in the plasma during flight, reflecting the major fatty acid components of adipose tissue. In the n–3 PUFA group, flown individuals had lower total MUFA during flight. Because of the small plasma volumes available, we were not able to measure fatty acid composition in the individual lipid classes (NEFA and polar and neutral lipids). This would enable a more direct examination of changes in plasma NEFA composition during flight for differential and preferential fatty acid mobilization, which was not observed in the adipose tissue.
Effect of Diet on Whole Animal Performance
Body composition and metabolic rate.
Overall, no significant differences in body mass or composition were observed among birds eating the experimental diets, suggesting that the chronic increased intake of DHA and ARA, at the levels used in the current study, does not cause overt systematic toxicity. This is in contrast to Andersson et al. (2) who found that higher plasma ARA concentration were negatively associated with body condition in urban great tits (Parus major). BMR was not affected by diet and was comparable with other measurements of migratory yellow-rumped warblers (20, 57). PMR was also not affected by diet. Both Pierce et al. (46) and Price and Guglielmo (48) found that increased 18:2 n–6 intake increased PMR. Our experimental diets contained similar proportions of 18:2 n–6 in each diet, which could explain why no difference was found. Furthermore, Price and Guglielmo (48) attributed the increase in PMR from high n–6 PUFA diet to changes in fuel composition, which differed more in their study than in our study.
Effect of individual variation and flight duration on flight performance.
Few studies have characterized how energy costs and fuel mixture changes during flight (17, 20). The relative protein contribution and flight cost decreased in warblers as flight duration increased and was similar to previous studies (17, 20). Flight duration did not influence the absolute amount of lean mass catabolized, unlike fat catabolism, which was tightly correlated with flight duration. Lean mass use during flight does not linearly increase over time, and its use during the initial stage of endurance flight is greater while the transition to fatty acid oxidation occurs (17).
The large number of 360-min flights (15 in total) obtained in our study allows for exploration of individual variation in flight performance traits. Flight fuel mixture was correlated to body mass and composition, with heavier and fatter individuals having an inferred lower relative protein contribution (see Supplemental Fig. S2; https://figshare.com/articles/AJP_Supplemental_Materials_pdf/7492781/1). This shift was driven by both an absolute increase in fat catabolism and a decrease in lean mass catabolism. A similar relationship was described with resting and active birds and mammals (30). This differs from other wind tunnel studies. A wind tunnel study of red knots (Calidris canutus) found that the relative protein contribution remained constant with increasing energy costs (31). However, this conclusion was based on body mass loss and plasma metabolite profiles to determine fuel mixture and not direct measurements. A recent wind tunnel study using yellow-rumped warblers found that body mass or composition was not significantly related to fuel mixture when examining flight of varying durations that also influence fuel mixture (20). Our study provides evidence that body mass and percent body fat alter fuel mixture independently of flight duration, supporting the hypothesis of Jenni and Jenni-Eiermann (30). The consequences or benefits of this fuel shift are unclear. All birds had a minimum of body fat of 16% at the start and 11% at the end of the flight. It is unlikely that the increase in relative protein contribution is related to insufficient fat stores (5–10% body fat) during flight (54).
Effect of Diet on Flight Performance
Voluntary flight duration was similar among all diets, suggesting that within a 360-min timeframe DHA and ARA do not influence endurance capacity or fatigue. This 360-min (6 h) duration is ecologically relevant for nocturnal migratory songbirds as the maximum nightly flight duration is likely ~8 h in the spring (14). The natural doping hypothesis focuses on priming muscle for increased fatty acid oxidation needed for flight, which could lower relative protein contribution. However, we found no difference in inferred fuel mixture among birds fed the experimental diets, suggesting that an abundance of long-chain n–3 PUFA is not critical for oxidizing fatty acids at high rates during flight.
In some animal studies, dietary PUFA can influence exercise performance, but others find no effect. For example in fish, long-chain n–3 PUFA may increase (60), decrease (9, 41) or not influence (52) exercise. Reduced fatigue resistance was observed in rats fed long-chain n–3 PUFA (10, 27). Conversely, diets higher in n–6 PUFA are associated with increased endurance capacity (3). Shei et al. (55) reviewed 13 human studies of the effects of DHA and EPA supplementation on exercise capacity, and found no overall enhancements in direct measures of athletic performance. Migratory birds may be a special case in terms of exercise performance, where endogenous upregulation of aerobic capacity during the migratory period increases performance (21, 40, 63), reducing any potential effect of diet.
Effects of Long-Chain PUFA on Muscle Metabolism
Generally, increasing n–3 long-chain PUFA increases PPAR activation, resulting in increased expression of fatty acid metabolism genes (19, 38). In our study the increase proportion of DHA in the membranes would serve as significant ligand source for muscle cells (6). This is beneficial for endurance exercise, as increased oxidative capacity in muscle increases fatigue resistance (10). On the other hand, there is evidence that n–3 long-chain PUFA increases anaerobic metabolism in skeletal muscles, rather than aerobic metabolism (28). PPAR-β mRNA abundance was lower in the n–3 PUFA diet treatment. This change was coordinated with overall lower oxidative enzyme activities (CPT, HOAD, and CS), and higher LDH activity. This is opposite to the predictions of the natural doping hypothesis (42), and differs from another study finding no effect of dietary PUFA on enzyme activity in songbirds (48). Both Nagahuedi et al. (42) and Price and Guglielmo (48) used similar manipulations, comparing n–3 long-chain PUFA with 18:2 n–6. Differences between the results of these studies and the current study hint at the potential of a species-specific response.
Lower CPT activity in the PUFA diets could suggest lower capacity to transfer fatty acids in the current study. Increasing the proportion of n–3 long-chain PUFA in the membranes can increase the activity of CPT (47). However, to observe this effect CPT activity would need to be assessed with intact membranes which our study’s assay does not do. Lower assayed CPT activity in our study could support the natural doping hypothesis’ premises of increasing n–3 long-chain PUFA enhancing the activity of membrane bound proteins in vivo (61).
Effect of Flight on Muscle Metabolism
PPAR-α mRNA abundance was not influenced by diet or flight. In contrast, PPAR-β mRNA abundance increased during flight, and the abundance was lower overall in the n–3 PUFA group. One exercise bout can increase the gene expression of PPAR-β, which then returns to baseline after recovery, while PPAR-α gene expression is not altered by exercise (44). These patterns suggest that while both PPARs show changes in preparation for migration in birds (13) and exercise training in humans (44), PPAR-β gene expression during flight may play an important role in the transition to and maintenance of fatty acid oxidation. PPAR-γ decreased in mRNA abundance in birds fed the MUFA and n–3 PUFA diets. The function of PPAR-γ in skeletal muscle is less understood than the other PPAR, but a role in maintaining insulin sensitivity, glucose homeostasis, and preventing lipid accumulation in skeletal muscle has been identified (1). Decreased mRNA abundance suggests that PPAR-γ mRNA abundance may be less important for sustaining muscle function during flight. The trend for increased H-FABP mRNA abundance during flight suggests that maintaining or increasing H-FABP may be important for maintaining adequate fatty acid transport to the mitochondria. Endurance flight did not alter metabolic enzyme activities but increased gene expression for these enzymes, via PPAR, may be needed to maintain their activity.
Correlations of Flight Performance with Metabolic Rate and Muscle Metabolism
Understanding the relationship between flight performance and muscle physiology and biochemistry is needed to grasp how alterations at the muscle level may be reflected in whole animal performance. The only significant factor affecting flight cost was body mass or related factors (fat and lean mass, and BMR). A positive correlation between flight power and BMR is likely driven by body mass, as heavier birds have higher BMR and higher flight costs. Two significant correlations were identified for fuel mixture. First, the inferred relative protein contribution was positively correlated to aerobic scope, but not to PMR and BMR or respective RER. Because of the difficulties in PMR measurements, the aerobic scope relationship was limited to only seven individuals. Potentially, birds that have a greater relative protein contribution during flight also have a greater protein contribution while exercising in a flight wheel. The exact contribution of protein, carbohydrates, and lipids to the fuel mixture during the PMR measurements cannot be determined from the RER measurements alone (~0.78). After 3 h of fasting, glucose supplies may be limited, and an increased ability to use amino acids as fuel could boost PMR. The second relationship was a negative correlation between relative protein contribution and HOAD activity, an enzyme involved in fatty acid β-oxidation. Lower dependence on fatty acids for fuel may be reflected by lower HOAD activity in these individuals.
Given the few significant correlations with flight performance, whether metabolic rate and metabolic enzymes are predictors of performance in a wind tunnel or migration is unclear. Similarly, Swanson et al. (58) found that muscle size but not metabolic enzyme activity could predict thermogenic capacity. The question of if and how modulating enzyme activity influences flight performance remains, but the answer is necessary to understanding how dietary fatty acids and other factors may influence migration.
Do Long-Chain PUFA Improve Migratory Performance?
Overall, our study does not provide support for the natural doping hypothesis, or for the benefits of PUFA on migratory performance in birds. Another controlled study also found no performance benefits of n–3 PUFA in migratory birds (48). However, other factors could alter the effect of dietary PUFA on muscle metabolism and performance and should be taken into consideration when studying the effects of PUFA on migration. The first is the possibility that migratory state influences the potential effect of PUFA. During migration the flight muscle has higher aerobic and fatty acid oxidative capacity (21, 40), and the photoperiod appears to be a sufficient cue for priming the flight muscle for migration (63). If the migratory state already primes birds for migration, further potential priming by fatty acids may be limited. Furthermore, it is important to note that the differences in metabolic enzymes between the diets in the current study are small (<15%) compared with the >90% increase during migration (40, 63). Additionally, the proportions of total PUFA increase during migration in white-throated sparrows, but this occurs with decreasing proportions of both n–3 and n–6 long-chain PUFA (32). This suggests that long-chain PUFA do not necessarily increase in the flight muscle membranes with increasing aerobic capacity.
Second, muscle fiber type may also influence the response to PUFA. Small birds, including many passerines and sandpipers, have exclusively fast-oxidative glycolytic fibers in their flights muscles and larger or more sedentary birds have additional fibers types (15, 35). Increasing oxidative capacity from n–3 PUFA could be the result of the shifting of muscle fibers to a more oxidative fiber type (25). If so, this may limit the scope or magnitude of the effect of n–3 PUFA in smaller passerines and sandpipers that already have highly oxidative muscle fibers and explain why quail responded dramatically to n–3 PUFA supplementation (42).
Third, there are large differences in the dietary intake and ratio of n–3 and n–6 PUFA among migratory birds. Sandpipers and other shorebirds may consume a diet high in n–3 long-chain PUFA, compared with the generally high n–6 PUFA diets of passerines (2, 11, 45, 61) including yellow-rumped warblers but with exceptions such as the Clinclidae and Hirundinidae families. Species may also differ in their tolerances. The warbler’s health was seriously affected when provided with the original n–3 PUFA diet, suggesting that there may be a safe upper limit for n–3 long-chain PUFA or DHASCO for warblers. The DHASCO inclusion level was originally chosen to be an estimated size-adjusted match to the intake of DHA used by Nagahuedi et al. (42) based on dose and body mass. It is not possible to determine if the effect was a result of high DHA intake or DHASCO itself. DHASCO is generally recognized as safe and the highest doses tested in toxicity studies find no observable adverse effects (24). Overall, the yellow-rumped warblers are well suited to dietary manipulations and wind tunnel studies; however, they likely do not naturally dope using n–3 PUFA in the wild. Future work should incorporate migratory species for which ecological or observational studies have suggested a direct role for n–3 PUFA on flight performance, such as sandpipers (37) and hummingbirds (29).
Finally, the inclusion of ARA did not appear to cause any detrimental effects to yellow-rumped warblers. Andersson et al. (2) proposed that because ARA is a precursor to proinflammatory prostaglandins (7), that increasing ARA could increase inflammation in birds. The effect of n–3 and n–6 PUFA intake and ratio on inflammation is still unclear, but the physiological effect of PUFA is more complex than simple categorizations of pro-inflammatory for ARA, and anti-inflammatory for EPA and DHA, and there are multiple levels of regulation for inflammation (7). We found no evidence for ARA negatively impacting health or performance, and higher proportions of ARA may not be a reliable indicator of poorer condition in songbirds.
Perspectives and Significance
We directly tested the natural doping hypothesis using carefully formulated diets to examine the effect of long-chain PUFA on animal physiology from the tissue to whole animal level. No direct support for the natural doping hypothesis or for any benefit or consequence of feeding diets low or high in n–3 or n–6 long-chain PUFA on endurance exercise performance was observed. Alterations in the flight muscle suggest decreased aerobic and oxidative capacity in the flight muscle with feeding of n–3 PUFA. However, since this was not associated with any flight performance parameter (endurance capacity, energy costs, and fuel mixture), conclusions about the direct effects of small modulations in flight muscle metabolism on exercise performance are limited. The potential effect of dietary PUFA on performance may be dependent on other factors such as species and their migratory strategy, migratory condition, and trophic base of the diet. In particular, our findings for a terrestrial songbird should be compared with experimental manipulations of shorebirds that typically feed on marine biofilm and invertebrates high in n–3 long-chain PUFA, which may influence the way they metabolize and respond to these fatty acids.
GRANTS
This research was funded by an Natural Sciences and Engineering Research Council Discovery Grant, the Canada Foundation for Innovation, and the Ontario Ministry of Research and Innovation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.F.D. and C.G.G. conceived and designed research; M.F.D. performed experiments; M.F.D. and C.G.G. analyzed data; M.F.D. and C.G.G. interpreted results of experiments; M.F.D. and C.G.G. prepared figures; M.F.D. drafted manuscript; M.F.D. and C.G.G. edited and revised manuscript; M.F.D. and C.G.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank A. Macmillan, L. Rooney, J. Hung, S. Wong, A. Diez, and L. Kennedy for assistance with bird care, wind tunnel flights, and sampling. Long Point Bird The observatory staff is thanked for assistance with bird capture. We also thank A. Macmillan for help with fatty acid analyses. L. Zhao provided guidance in laboratory PCR protocols. DSM Nutritional Products kindly provided the ARASCO and DHASCO experimental oils.
REFERENCES
- 1.Amin RH, Mathews ST, Camp HS, Ding L, Leff T. Selective activation of PPAR-γ in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am J Physiol Endocrinol Metab 298: E28–E37, 2010. doi: 10.1152/ajpendo.00446.2009. [DOI] [PubMed] [Google Scholar]
- 2.Andersson MN, Wang HL, Nord A, Salmón P, Isaksson C. Composition of physiologically important fatty acids in great tits differs between urban and rural populations on a seasonal basis. Front Ecol Evol 3: 522–613, 2015. doi: 10.3389/fevo.2015.00093. [DOI] [Google Scholar]
- 3.Ayre KJ, Hulbert AJ. Dietary fatty acid profile affects endurance in rats. Lipids 32: 1265–1270, 1997. doi: 10.1007/s11745-006-0162-5. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Hamo M, McCue MD, McWilliams SR, Pinshow B. Dietary fatty acid composition influences tissue lipid profiles and regulation of body temperature in Japanese quail. J Comp Physiol B 181: 807–816, 2011. doi: 10.1007/s00360-011-0558-2. [DOI] [PubMed] [Google Scholar]
- 6.Calder PC. Mechanisms of action of (n–3 ) fatty acids. J Nutr 142: 592S–599S, 2012. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 7.Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta 1851: 469–484, 2015. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Chappell MA, Bech C, Buttemer WA. The relationship of central and peripheral organ masses to aerobic performance variation in house sparrows. J Exp Biol 202: 2269–2279, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Chatelier A, McKenzie DJ, Prinet A, Galois R, Robin J, Zambonino J, Claireaux G. Associations between tissue fatty acid composition and physiological traits of performance and metabolism in the seabass (Dicentrarchus labrax). J Exp Biol 209: 3429–3439, 2006. doi: 10.1242/jeb.02347. [DOI] [PubMed] [Google Scholar]
- 10.Clavel S, Farout L, Briand M, Briand Y, Jouanel P. Effect of endurance training and/or fish oil supplemented diet on cytoplasmic fatty acid binding protein in rat skeletal muscles and heart. Eur J Appl Physiol 87: 193–201, 2002. doi: 10.1007/s00421-002-0612-6. [DOI] [PubMed] [Google Scholar]
- 11.Conway CJ, Eddleman WR, Simpson KL. Seasonal changes in fatty acid composition of the wood thrush. Condor 96: 791–794, 1994. doi: 10.2307/1369482. [DOI] [Google Scholar]
- 12.DeLuca WV, Woodworth BK, Rimmer CC, Marra PP, Taylor PD, McFarland KP, Mackenzie SA, Norris DR. Transoceanic migration by a 12 g songbird. Biol Lett 11: 20141045, 2015. doi: 10.1098/rsbl.2014.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeMoranville K. Characterization and Molecular Regulation of Metabolic and Muscle Flexibility in a Neotropical Migrant, Dumetella Carolinensis (Gray Catbird). (M.Sc. Thesis). Oxford, OH: Miami Univ., 2005. [Google Scholar]
- 14.Dossman BC, Mitchell GW, Norris DR, Taylor PD, Guglielmo CG, Matthews SN, Rodewald PG. The effects of wind and fuel stores on stopover departure behavior across a migratory barrier. Behav Ecol 27: 567–574, 2016. doi: 10.1093/beheco/arv189. [DOI] [Google Scholar]
- 15.Evans PR, Davidson NC, Uttley JD, Evans RD. Premigratory hypertrophy of flight muscles: an ultrastructural study. Ornis Scand 23: 238–243, 1992. doi: 10.2307/3676644. [DOI] [Google Scholar]
- 16.Gerson AR, Guglielmo CG. Flight at low ambient humidity increases protein catabolism in migratory birds. Science 333: 1434–1436, 2011. doi: 10.1126/science.1210449. [DOI] [PubMed] [Google Scholar]
- 17.Gerson AR, Guglielmo CG. Energetics and metabolite profiles during early flight in American robins (Turdus Migratorius). J Comp Physiol B 183: 983–991, 2013. doi: 10.1007/s00360-013-0767-y. [DOI] [PubMed] [Google Scholar]
- 18.Gill RE, Piersma T, Hufford G, Servranckx R, Riegen A. Crossing the ultimate ecological barrier: evidence for an 11 000-km-long nonstop flight from Alaska to New Zealand and Eastern Australia by bar-tailed godwits. Condor 107: 1–21, 2005. doi: 10.1650/7613. [DOI] [Google Scholar]
- 19.Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications—a review. Nutr J 13: 17, 2014. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guglielmo CG, Gerson AR, Price ER, Hays QR. The effects of dietary macronutrients on flight ability, energetics, and fuel metabolism of yellow-rumped warblers Setophaga coronata. J Avian Biol 48: 133–148, 2017. doi: 10.1111/jav.01351. [DOI] [Google Scholar]
- 21.Guglielmo CG, Haunerland NH, Hochachka PW, Williams TD. Seasonal dynamics of flight muscle fatty acid binding protein and catabolic enzymes in a migratory shorebird. Am J Physiol Regul Integr Comp Physiol 282: R1405–R1413, 2002. doi: 10.1152/ajpregu.00267.2001. [DOI] [PubMed] [Google Scholar]
- 22.Guglielmo CG, McGuire LP, Gerson AR, Seewagen CL. Simple, rapid, and non-invasive measurement of fat, lean, and total water masses of live birds using quantitative magnetic resonance. J Ornithol 152, Suppl 1: 75–85, 2011. doi: 10.1007/s10336-011-0724-z. [DOI] [Google Scholar]
- 23.Guglielmo CG. Move that fatty acid: fuel selection and transport in migratory birds and bats. Integr Comp Biol 50: 336–345, 2010. doi: 10.1093/icb/icq097. [DOI] [PubMed] [Google Scholar]
- 24.Hadley KB, Ryan AS, Nelson EB, Salem N Jr. Preclinical safety evaluation in rats using a highly purified ethyl ester of algal-docosahexaenoic acid. Food Chem Toxicol 48: 2778–2784, 2010. doi: 10.1016/j.fct.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto M, Inoue T, Katakura M, Hossain S, Mamun AA, Matsuzaki K, Arai H, Shido O. Differential effects of docoosahexaenoic and arachidonic acid on fatty acid composition and myosin heavy chain-related genes of slow- and fast-twitch skeletal muscle tissues. Mol Cell Biochem 415: 169–181, 2016. doi: 10.1007/s11010-016-2689-y. [DOI] [PubMed] [Google Scholar]
- 26.Helge JW, Wu BJ, Willer M, Daugaard JR, Storlien LH, Kiens B. Training affects muscle phospholipid fatty acid composition in humans. J Appl Physiol (1985) 90: 670–677, 2001. doi: 10.1152/jappl.2001.90.2.670. [DOI] [PubMed] [Google Scholar]
- 27.Henry R, Peoples GE, McLennan PL. Muscle fatigue resistance in the rat hindlimb in vivo from low dietary intakes of tuna fish oil that selectively increase phospholipid n–3 docosahexaenoic acid according to muscle fibre type. Br J Nutr 114: 873–884, 2015. doi: 10.1017/S0007114515002512. [DOI] [PubMed] [Google Scholar]
- 28.Higuchi T, Shirai N, Saito M, Suzuki H, Kagawa Y. Levels of plasma insulin, leptin and adiponectin, and activities of key enzymes in carbohydrate metabolism in skeletal muscle and liver in fasted ICR mice fed dietary n–3 polyunsaturated fatty acids. J Nutr Biochem 19: 577–586, 2008. doi: 10.1016/j.jnutbio.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Infante JP, Kirwan RC, Brenna JT. High levels of docosahexaenoic acid (22:6n–3 )-containing phospholipids in high-frequency contraction muscles of hummingbirds and rattlesnakes. Comp Biochem Physiol B Biochem Mol Biol 130: 291–298, 2001. doi: 10.1016/S1096-4959(01)00443-2. [DOI] [PubMed] [Google Scholar]
- 30.Jenni L, Jenni-Eiermann S. Fuel supply and metabolic constraints in migrating birds. J Avian Biol 29: 521–528, 1998. doi: 10.2307/3677171. [DOI] [Google Scholar]
- 31.Jenni-Eiermann S, Jenni L, Kvist A, Lindström A, Piersma T, Visser GH. Fuel use and metabolic response to endurance exercise: a wind tunnel study of a long-distance migrant shorebird. J Exp Biol 205: 2453–2460, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Klaiman JM, Price ER, Guglielmo CG. Fatty acid composition of pectoralis muscle membrane, intramuscular fat stores and adipose tissue of migrant and wintering white-throated sparrows (Zonotrichia albicollis). J Exp Biol 212: 3865–3872, 2009. doi: 10.1242/jeb.034967. [DOI] [PubMed] [Google Scholar]
- 33.Lighton JR. Measuring Metabolic Rates. Oxford, UK: Oxford University Press, 2008. [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔ C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Lundgren BO, Kiessling KH. Comparative aspects of fibre types, areas, and capillary supply in the pectoralis muscle of some passerine birds with differing migratory behaviour. J Comp Physiol B 158: 165–173, 1988. doi: 10.1007/BF01075830. [DOI] [Google Scholar]
- 36.Maillet D, Weber JM. Performance-enhancing role of dietary fatty acids in a long-distance migrant shorebird: the semipalmated sandpiper. J Exp Biol 209: 2686–2695, 2006. doi: 10.1242/jeb.02299. [DOI] [PubMed] [Google Scholar]
- 37.Maillet D, Weber JM. Relationship between n–3 PUFA content and energy metabolism in the flight muscles of a migrating shorebird: evidence for natural doping. J Exp Biol 210: 413–420, 2007. doi: 10.1242/jeb.02660. [DOI] [PubMed] [Google Scholar]
- 38.McClelland GB. Fat to the fire: the regulation of lipid oxidation with exercise and environmental stress. Comp Biochem Physiol B Biochem Mol Biol 139: 443–460, 2004. doi: 10.1016/j.cbpc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 39.McCue MD, Amitai O, Khozin-Goldberg I, McWilliams SR, Pinshow B. Effect of dietary fatty acid composition on fatty acid profiles of polar and neutral lipid tissue fractions in zebra finches, Taeniopygia guttata. Comp Biochem Physiol A Mol Integr Physiol 154: 165–172, 2009. doi: 10.1016/j.cbpa.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 40.McFarlan JT, Bonen A, Guglielmo CG. Seasonal upregulation of fatty acid transporters in flight muscles of migratory white-throated sparrows (Zonotrichia albicollis). J Exp Biol 212: 2934–2940, 2009. doi: 10.1242/jeb.031682. [DOI] [PubMed] [Google Scholar]
- 41.McKenzie DJ, Higgs DA, Dosanjh BS, Deacon G, Randall DJ. Dietary fatty acid composition influences swimming performance in Atlantic salmon (Salmo Salar) in seawater. Fish Physiol Biochem 19: 111–122, 1998. doi: 10.1023/A:1007779619087. [DOI] [Google Scholar]
- 42.Nagahuedi S, Popesku JT, Trudeau VL, Weber JM. Mimicking the natural doping of migrant sandpipers in sedentary quails: effects of dietary n–3 fatty acids on muscle membranes and PPAR expression. J Exp Biol 212: 1106–1114, 2009. doi: 10.1242/jeb.027888. [DOI] [PubMed] [Google Scholar]
- 43.Peoples GE, McLennan PL. Long-chain n–3 DHA reduces the extent of skeletal muscle fatigue in the rat in vivo hindlimb model. Br J Nutr 111: 996–1003, 2014. doi: 10.1017/S0007114513003449. [DOI] [PubMed] [Google Scholar]
- 44.Perry CGR, Lally J, Holloway GP, Heigenhauser GJF, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierce BJ, McWilliams SR. The fat of the matter: how dietary fatty acids can affect exercise performance. Integr Comp Biol 54: 903–912, 2014. doi: 10.1093/icb/icu098. [DOI] [PubMed] [Google Scholar]
- 46.Pierce BJ, McWilliams SR, O’Connor TP, Place AR, Guglielmo CG. Effect of dietary fatty acid composition on depot fat and exercise performance in a migrating songbird, the red-eyed vireo. J Exp Biol 208: 1277–1285, 2005. doi: 10.1242/jeb.01493. [DOI] [PubMed] [Google Scholar]
- 47.Power GW, Cake MH, Newsholme EA. Influence of diet on the kinetic behavior of hepatic carnitine palmitoyltransferase I toward different acyl CoA esters. Lipids 32: 31–37, 1997. doi: 10.1007/s11745-997-0005-4. [DOI] [PubMed] [Google Scholar]
- 48.Price ER, Guglielmo CG. The effect of muscle phospholipid fatty acid composition on exercise performance: a direct test in the migratory white-throated sparrow (Zonotrichia albicollis). Am J Physiol Regul Integr Comp Physiol 297: R775–R782, 2009. doi: 10.1152/ajpregu.00150.2009. [DOI] [PubMed] [Google Scholar]
- 49.Price ER, Krokfors A, Guglielmo CG. Selective mobilization of fatty acids from adipose tissue in migratory birds. J Exp Biol 211: 29–34, 2008. doi: 10.1242/jeb.009340. [DOI] [PubMed] [Google Scholar]
- 50.Price ER, McFarlan JT, Guglielmo CG. Preparing for migration? The effects of photoperiod and exercise on muscle oxidative enzymes, lipid transporters, and phospholipids in white-crowned sparrows. Physiol Biochem Zool 83: 252–262, 2010. doi: 10.1086/605394. [DOI] [PubMed] [Google Scholar]
- 51.Price ER. Dietary lipid composition and avian migratory flight performance: Development of a theoretical framework for avian fat storage. Comp Biochem Physiol A Mol Integr Physiol 157: 297–309, 2010. doi: 10.1016/j.cbpa.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 51a.Ramenofsky M, Agatsuma R. Migratory behaviour of captive white-crowned sparrows, Zonotrichia leucophrys gambelii, differs during autumn and spring migration. Behaviour 143: 1219–1240, 2006. doi: 10.1163/156853906778691586. [DOI] [Google Scholar]
- 52.Regan MD, Kuchel LJ, Huang SS, Higgs DA, Wang J, Schulte PM, Brauner CJ. The effect of dietary fish oil and poultry fat replacement with canola oil on swimming performance and metabolic response to hypoxia in stream type spring Chinook salmon parr. Aquaculture 308: 183–189, 2010. doi: 10.1016/j.aquaculture.2010.08.014. [DOI] [Google Scholar]
- 53.Ruf T, Valencak T, Tataruch F, Arnold W. Running speed in mammals increases with muscle n–6 polyunsaturated fatty acid content. PLoS One 1: e65, 2006. doi: 10.1371/journal.pone.0000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwilch R, Grattarola A, Spina F, Jenni L. Protein loss during long-distance migratory flight in passerine birds: adaptation and constraint. J Exp Biol 205: 687–695, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Shei RJ, Lindley MR, Mickleborough TD. Omega-3 polyunsaturated fatty acids in the optimization of physical performance. Mil Med 179, Suppl: 144–156, 2014. doi: 10.7205/MILMED-D-14-00160. [DOI] [PubMed] [Google Scholar]
- 56.Stevens L. Avian Biochemistry and Molecular Biology. Cambridge, UK: Cambridge Univ. Press, 2009. [Google Scholar]
- 57.Swanson DL, Dean KL. Migration-induced variation in thermogenic capacity in migratory passerines. J Avian Biol 30: 245, 1999. doi: 10.2307/3677350. [DOI] [Google Scholar]
- 58.Swanson DL, Zhang Y, King MO. Individual variation in thermogenic capacity is correlated with flight muscle size but not cellular metabolic capacity in American goldfinches (Spinus tristis). Physiol Biochem Zool 86: 421–431, 2013. doi: 10.1086/671447. [DOI] [PubMed] [Google Scholar]
- 59.Thomas RH, Meeking MM, Mepham JR, Tichenoff L, Possmayer F, Liu S, MacFabe DF. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflammation 9: 153, 2012. doi: 10.1186/1742-2094-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner GN, Balfry SK, Higgs DA, Lall SP, Farrell AP. Dietary fatty acid composition affects the repeat swimming performance of Atlantic salmon in seawater. Comp Biochem Physiol A Mol Integr Physiol 137: 567–576, 2004. doi: 10.1016/j.cbpb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Weber JM. The physiology of long-distance migration: extending the limits of endurance metabolism. J Exp Biol 212: 593–597, 2009. doi: 10.1242/jeb.015024. [DOI] [PubMed] [Google Scholar]
- 62.Wikelski M, Tarlow EM, Raim A, Diehl RH, Larkin RP, Visser GH. Avian metabolism: costs of migration in free-flying songbirds. Nature 423: 704, 2003. doi: 10.1038/423704a. [DOI] [PubMed] [Google Scholar]
- 63.Zajac DM, Cerasale DJ, Landman S, Guglielmo CG. Behavioral and physiological effects of photoperiod-induced migratory state and leptin on Zonotrichia albicollis: II. Effects on fatty acid metabolism. Gen Comp Endocrinol 174: 269–275, 2011. doi: 10.1016/j.ygcen.2011.08.024. [DOI] [PubMed] [Google Scholar]