Abstract
Background
Candidemia is one of the major causes of morbidity and mortality as a hospital acquired infection. Infectious diseases consultation (IDC) might be beneficial to improve candidemia outcomes; however, only limited data from short periods of time are available thus far.
Methods
An observational study of all candidemia patients at a large tertiary care hospital between 2002 and 2013 was conducted. A candidemia episode was defined as ≥ 1 positive result for Candida spp. in blood culture. Patients who died or transferred to another hospital within two days after their first positive blood culture were excluded. Independent risk factors for 30-day mortality were determined.
Results
Among 275 patients with 283 episodes of candidemia, 194 (68.6%) were male, and the mean age was 70.0 ± 15.8 years. Central line-associated bloodstream infections, peripheral line-associated bloodstream infections, intra-abdominal infection, and unknown source comprised 220 (77.7%), 35 (12.4%), 13 (4.7%), and 15 (5.3%) episodes, respectively. A total of 126 patients (44.5%) received IDC. Factors independently associated with 30-day mortality in patients with candidemia were urinary catheters use (adjusted hazard ratio [HR] = 2.94; 95% confidence interval [CI] = 1.48–5.87; P = 0.002) and severe sepsis/septic shock (adjusted HR = 2.10; 95% CI = 1.20–3.65; P = 0.009). IDC was associated with a 46% reduction in 30-day mortality (adjusted HR = 0.54; 95% CI = 0.32–0.90; P = 0.017).
Conclusion
IDC was independently associated with a reduction in 30-day mortality. Only 44.5% of patients with candidemia in this cohort received IDC. Routine IDC should be actively considered for patients with candidemia.
Introduction
Candidemia is one of the major causes of morbidity and mortality as hospital acquired infection, [1–5] and high overall mortality rate was reported with 25–60% [6–8]. Additionally, candidemia is related to the extended hospitalization and increased health care costs [6–8]. Clinical intervention by infectious diseases consultation (IDC) has been shown to reduce morbidity and/or mortality in several infections [9–12], including candidemia [13–16]. Underscoring the value of IDC as antifungal stewardships is important.
However, previous studies are limited by small patient sample sizes and short time periods of patient inclusion and/or follow up [13–16]. Moreover, studies of the survival outcome effects of IDC on candidemia in Asian countries, especially in Japan, are few.
In the past study [17], we reported that peripheral-line associated candidemia (PLAC) was an important cause of candidemia in the healthcare settings. Although IDC was associated with the predictors of PLAC, we did not evaluate the survival outcome of IDC on candidemia [17]. Thereby, we conducted a retrospective cohort study covering a 12-year period to evaluate the relationship between survival outcomes of candidemia and IDC in a tertiary care hospital in Japan as advances on previous work [17].
Materials and methods
Hospital setting and study design
A retrospective cohort study of all candidemia was conducted between January 2002 and December 2013 at the National Centre for Global Health and Medicine (NCGM), which has approximately 780 inpatient beds and serves as a tertiary referral hospital for metropolitan Tokyo. This study was approved by the ethics committee of the NCGM (approval no: NCGM-G-001589-00) and was implemented in accordance with the Declaration of Helsinki. Patient information was anonymized and deidentified prior to analysis. Due to the retrospective nature of the study, patient consent was waived.
Data source
We identified all cases of candidemia using the microbiological laboratory database. The parameters were collected from patient charts included the following: (i) demographics including time period variable which was divided 2002–2009 and 2010–2013 due to the establishment of the official infectious disease consultation service with five infectious disease specialists in 2010; (ii) immunosuppressive status; (iii) background and comorbid conditions; (iv) recent healthcare-associated exposures; (v) recent exposure to antibiotic and antifungal therapy; (vi) infection-related characteristics; (vii) the severity of illness (sepsis, severe sepsis, and septic shock) and haematogenous dissemination; (viii) antifungal therapy against candidemia; (ix) outcome (clinical failure, persistent candidemia, in-hospital and 30-day/180-day mortality, discharge to a long term care facility (LTCF), re-admission, length of hospital stay after candidemia (excluding those who died), and duration of candidemia, as well as past our study [17–19]. Persistent candidemia means the case with follow up-blood culture positive of Candida. spp., after 72hr with empirical therapy [17]. Duration of candidemia was calculated from the date when the initial blood culture positive of Candida. spp. was drawn, to the date when the follow-up blood culture negative of Candida. spp was drawn [17]. Additionally, we reviewed IDC for management of candidemia and whether or not recommended candidemia treatment including examination for endophthalmitis and endocarditis were performed.
Definitions of variables including candidemia episode
We defined an episode of candidemia as isolation of Candida spp. from at least one peripherally-taken blood culture in a patient with clinical signs and symptoms of infection [17, 20]. When caused by different Candida spp. or occurring at least 30 days apart, with improvement of clinical features of infection and at least one negative blood culture in the period [17, 21], we considered that episodes of candidemia were separated. We excluded the episodes identified within 48 hours of hospital admission, because these episodes were thought not to be hospital acquired, and determining important candidemia parameters, such as duration, would be difficult [17]. Additionally, episodes who died or transferred to another hospital within 2 days after their first positive blood culture were also excluded, due to the limited opportunity for evaluating IDC effects.
Central line-associated bloodstream infections (CLABSI) and intra-abdominal infection (IAI) were defined according to the National Healthcare Safety Network Surveillance definition and the guidelines of the Infectious Diseases Society of America (IDSA) [17, 20, 22, 23]. We defined peripheral line-associated bloodstream infections (PLABSI) as the presence of at least one of the following conditions: (i) the presence of phlebitis, and/or (ii) resolution of clinical symptoms after short-term peripheral line withdrawal with a careful exclusion of another focus of bacteraemia [17, 24]. We defined empiric and definitive therapy as administration of systemic antifungal drugs within 72 hours of the onset of candidemia, and based on the guideline of IDSA [17, 20]. The variables related therapy (the time to antifungal therapy, adequate source control, time to central or peripheral vein catheter removal, and clinical failure) were defined as past studies [17, 25]
Infectious disease consultation and appropriateness of an antifungal therapy or duration
IDC was recommended for patients with candidemia as per hospital policy, and was performed when requested by the primary physician in charge. Request for consultation was not mandatory. IDC comprised chart review, physical examination of the patient, a follow-up visit, and written recommendations for therapy based on published IDSA guidelines [20]. Individual case discussion was performed with the primary physician in charge. We evaluated whether antifungal therapy, including the duration, was in accordance with the published IDSA guidelines [20].
Microbiological data
Candida spp. from positive blood culture were identified using API 20 C AUX (Biomerieux Japan Co., Ltd., Japan), and ID 32 C (Biomerieux Japan Co., Ltd., Japan). Antifungal susceptibility testing was performed using the commercially prepared colorimetric microdilution panel (ASTY; Kyokuto Pharmaceutical Industrial Co., Ltd.). which was developed according to the CLSI recommendation. During the study period, there were no changes to the microbiological identification and susceptibility testing process.
Statistical analysis
Continuous variables were shown as the mean ± standard deviation (SD) or the median with interquartile range (IQR), and compared using Student’s t-test or Mann-Whitney U test. Categorical variables were shown as absolute and relative frequencies, and compared using the χ2 test or Fisher’s exact test.
We compared demographic characteristics, clinical factors, and outcomes between episodes with and without IDC, using logistic regression univariate analysis with odds ratios (OR) and 95% confidence intervals (CI). Multivariable survival analyses were performed and predictive models for 30-day and 31-180-day all-cause mortality were built.
For the 30-day and 31-180-day mortality models, Cox proportional hazards models were applied. We considered the potential predictive factors with a P-value of < 0.10 in the univariate analysis, or that were hypothesized a priori to be clinically or epidemiologically important, for inclusion in the multivariate model for predictive factors. The relationship of the variable of IDC and mortality during the 180-day follow up period was illustrated using a Kaplan-Meier estimator. Survival characteristics were compared between groups with and without IDC using the log-rank test. We defined statistical significance as a 2-sided P-value of < 0.05, and all statistical analyses were performed with SPSS Version 24 (IBM Corp., Armonk, NY, USA).
Results
Description of candidemia from 2002 to 2013
The 12-year study period included a total of 283 episodes of candidemia from 275 patients. The overall incidence of candidemia episodes from 2005–2013 was 0.10/1000 patient-days and 1.64/1000 hospital admissions. The mean age of this cohort was 70.0 ± 15.8 years and 194 (68.6%) were male. One hundred twenty-six patients (44.5%) received IDC. CLABSI, PLABSI, IAI, and unknown source of infection consisted of 220 (77.7%), 35 (12.4%), 13 (4.7%), and 15 (5.3%), respectively. From the 283 episodes of candidemia, 295 Candida spp. were collected, including 25 episodes of polymicrobial bacteraemia/fungaemia due to different species of Candida spp. (12 episodes) or to pathogens other than Candida spp. (13 episodes). Candida spp. were comprised of 131 (44.4%) isolates of C. albicans, 74 (25.1%) of C. glabrata, 45 (15.3%) of C. parapsilosis, 28 (9.5%) of C. tropicalis, and 17 (5.8%) of other Candida spp., including C. krusei (n = 4), C. guilliermondii (n = 3), C. lusitaniae (n = 2), C. dubliniensis (n = 1), and unclassified (n = 7).
Comparison of candidemia patients with and without IDC
Comparison of candidemia patients with and without IDC is summarized in Table 1.
Table 1. Comparison of candidemia patients with and without infectious disease consultation, n = 283.
| Category | Variable | With IDC | Without IDC | OR (95% CI) | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 126, 44.5%) | (n = 157, 55.1%) | ||||||||
| Demographics | Mean age (years) ± SD | 69.1 | ± 16.4 | 70.7 | ± 15.4 | 0.38 | |||
| Females | 35 | (27.8) | 54 | (34.4) | 0.73 | (0.44–1.22) | 0.23 | ||
| Candidemia from 2010 to 2013 | 70 | (55.6) | 33 | (21.0) | 4.70 | (2.79–7.90) | <0.001 | ||
| Immunocompromised status | HIV infection | 9 | (7.1) | 1 | (0.6) | 12.00 | (1.50–96.04) | 0.006 | |
| Neutropenia (< 0.5 × 109 cells/L) at onset | 2 | (1.6) | 5 | (3.2) | 0.49 | (0.094–2.57) | 0.47 | ||
| Chemotherapy in the past month | 29 | (15.9) | 29 | (18.5) | 0.83 | (0.45–1.56) | 0.57 | ||
| Steroid therapy in the past month | 26 | (20.6) | 25 | (15.9) | 1.37 | (0.75–2.52) | 0.31 | ||
| Radiation therapy in the past month | 6 | (4.8) | 12 | (7.6) | 0.60 | (0.22–1.66) | 0.32 | ||
| Transplantation in the past month | 3 | (2.4) | 1 | (0.6) | 3.81 | (0.39–37.03) | 0.33 | ||
| Background and comorbid conditions on admission | Dependent functional status | 52 | (41.3) | 69 | (43.9) | 0.90 | (0.56–1.44) | 0.65 | |
| Charlson's weighted index comorbity score (6), mean ± SD | 4.1 | ± 2.7 | 4.3 | ± 3.0 | 0.56 | ||||
| Diabetes mellitus | 36 | (28.6) | 40 | (25.5) | 1.17 | (0.69–1.98) | 0.56 | ||
| Solid-organ cancer within last 1 year | 42 | (33.3) | 69 | (43.9) | 0.64 | (0.39–1.04) | 0.069 | ||
| Hematological malignancy within last 1 year | 13 | (10.3) | 12 | (7.6) | 1.39 | (0.61–3.16) | 0.43 | ||
| Chronic kidney disease stage V | 10 | (7.9) | 8 | (5.1) | 1.61 | (0.61–4.20) | 0.33 | ||
| Liver diseases | 3 | (2.4) | 11 | (7.0) | 0.32 | (0.088–1.19) | 0.099 | ||
| Chronic heart disease | 28 | (22.0) | 31 | (19.7) | 1.16 | (0.65–2.06) | 0.61 | ||
| Chronic obstructive pulmonary disease | 11 | (8.7) | 22 | (14.0) | 0.59 | (0.27–1.26) | 0.17 | ||
| Cerebrovascular disease | 31 | (24.6) | 32 | (20.4) | 1.28 | (0.73–2.24) | 0.40 | ||
| Dementia | 6 | (4.8) | 14 | (8.9) | 0.51 | (0.19–1.37) | 0.18 | ||
| Connective tissue disease | 12 | (9.5) | 4 | (2.5) | 4.03 | (1.27–12.81) | 0.018 | ||
| Peptic ulcer disease | 9 | (7.1) | 17 | (10.8) | 0.63 | (0.27–1.47) | 0.29 | ||
| Peripheral vascular disease | 2 | (1.6) | 2 | (1.3) | 1.25 | (0.17–9.00) | 1.00 | ||
| Hemiplegia | 12 | (9.5) | 12 | (7.6) | 1.27 | (0.55–2.94) | 0.57 | ||
| Recent health care-associated exposures before onset of candidemia | Resided LTCF in the past 3 months | 8 | (6.3) | 6 | (3.8) | 1.71 | (0.58–5.05) | 0.33 | |
| Hospitalized in the past 3 months | 53 | (42.1) | 77 | (49.0) | 0.75 | (0.47–1.21) | 0.24 | ||
| Invasive procedure/surgery in the past 3 months | 46 | (36.5) | 54 | (34.4) | 1.10 | (0.67–1.79) | 0.71 | ||
| Tracheotomy in the past 3 months | 16 | (12.7) | 15 | (9.6) | 1.38 | (0.65–2.91) | 0.40 | ||
| Urinary catheters (for ≥ 2 days) at onset of candidemia | 86 | (68.3) | 93 | (59.2) | 1.48 | (0.91–2.42) | 0.12 | ||
| CVC (for ≥ 2 days) at same onset | 98 | (77.8) | 133 | (84.7) | 0.63 | (0.35–1.16) | 0.13 | ||
| Median days of CVC prior to onset of candidemia (IQR) | 11 | (4–24) | 14 | (6–23) | 0.19 | ||||
| Undergoing haemodialysis in the past month | 13 | (10.3) | 9 | (5.7) | 1.89 | (0.78–4.58) | 0.15 | ||
| Transfusion in the past month | 67 | (53.2) | 87 | (55.8) | 0.90 | (0.56–1.44) | 0.66 | ||
| TPN in the past month | 75 | (59.5) | 97 | (61.8) | 0.91 | (0.56–1.47) | 0.70 | ||
| Median days of TPN prior to onset of candidemia (IQR) | 6 | (0–15) | 8 | (0–19) | 0.35 | ||||
| ICU stay in current hospitalization before onset of candidemia | 24 | (19.0) | 12 | (7.6) | 2.84 | (1.36–5.95) | 0.004 | ||
| Median hospital days prior to the onset of candidemia (IQR) | 40 | (20–67) | 31 | (18–62) | 0.20 | ||||
| Exposure to antibiotic therapy (for ≥ 3 days) prior to isolation of Candida spp. | Over all | 120 | (95.2) | 147 | (93.6) | 1.36 | (0.48–3.85) | 0.56 | |
| Penicillinsa | 61 | (48.4) | 61 | (38.9) | 1.48 | (0.92–2.37) | 0.11 | ||
| Cephalosporinsb | 59 | (46.8) | 71 | (45.2) | 1.07 | (0.67–1.71) | 0.79 | ||
| Carbapenems | 73 | (57.9) | 68 | (43.3) | 1.80 | (1.12–2.90) | 0.014 | ||
| Fluoroquinolones | 36 | (28.6) | 31 | (19.7) | 1.63 | (0.94–2.82) | 0.083 | ||
| Aminoglycosides | 13 | (10.3) | 15 | (9.6) | 1.09 | (0.50–2.38) | 0.83 | ||
| Trimethoprim-sulfamethoxazole | 19 | (15.1) | 14 | (8.9) | 1.81 | (0.87–3.78) | 0.11 | ||
| Clindamycin | 14 | (11.1) | 17 | (10.8) | 1.03 | (0.49–2.18) | 0.94 | ||
| Metronidazole | 16 | (12.7) | 8 | (5.1) | 2.71 | (1.12–6.56) | 0.023 | ||
| Glycopeptide | 46 | (36.5) | 41 | (26.1) | 1.63 | (0.98–2.70) | 0.060 | ||
| Exposure to antifungal therapy (for ≥ 3 days) prior to isolation of Candida spp. | Overall | 20 | (15.9) | 18 | (11.5) | 1.46 | (0.73–2.89) | 0.28 | |
| Fluconazole | 4 | (3.2) | 8 | (5.1) | 0.61 | (0.18–2.08) | 0.56 | ||
| Micafungin | 15 | (11.9) | 7 | (4.5) | 2.90 | (1.14–7.34) | 0.020 | ||
| Voriconazole | 2 | (1.6) | 0 | (0.0) | 0.20 | ||||
| Liposomal amphotericin b | 4 | (3.2) | 0 | (0.0) | 0.038 | ||||
| Itraconazole | 3 | (2.4) | 5 | (3.2) | 0.74 | (0.17–3.16) | 0.74 | ||
| Microbiology | Candida species: | ||||||||
| C. albicans | 62 | (49.2) | 69 | (43.9) | 1.24 | (0.77–1.98) | 0.38 | ||
| C. glabrata | 29 | (23.0) | 45 | (28.7) | 0.74 | (0.43–1.28) | 0.28 | ||
| C. parapsilosis | 21 | (16.7) | 24 | (15.3) | 1.11 | (0.59–2.10) | 0.75 | ||
| C. tropicalis | 13 | (10.3) | 15 | (9.6) | 1.09 | (0.50–2.38) | 0.83 | ||
| Othersc | 7 | (5.6) | 10 | (6.4) | 0.87 | (0.32–2.34) | 0.78 | ||
| Polymicrobial bacteremia/fungemiad | 17 | (12.5) | 8 | (5.1) | 2.91 | (1.21–6.97) | 0.013 | ||
| Previous Candida colonization within a week before candidemia | 45 | (35.7) | 45 | (28.7) | 1.38 | (0.84–2.29) | 0.21 | ||
| Source of infection | CLABSI | 93 | (73.8) | 127 | (80.9) | 0.67 | (0.38–1.17) | 0.16 | |
| PLABSI | 22 | (17.5) | 13 | (8.3) | 2.34 | (1.13–4.87) | 0.020 | ||
| Intra-abdominal infection | 8 | (6.3) | 5 | (3.2) | 2.06 | (0.66–6.46) | 0.21 | ||
| Unknown source | 3 | (2.4) | 12 | (7.6) | 0.30 | (0.081–1.07) | 0.062 | ||
| Severity of illness indices at the time of candidemia | Sepsis | 48 | (38.1) | 44 | (28.0) | 1.58 | (0.96–2.61) | 0.072 | |
| Severe sepsis | 46 | (36.5) | 62 | (39.5) | 0.88 | (0.54–1.43) | 0.61 | ||
| Septic shock | 19 | (15.1) | 20 | (12.8) | 1.21 | (0.61–2.38) | 0.59 | ||
| Severe sepsis/septic shock | 65 | (51.6) | 82 | (55.2) | 0.98 | (0.61–1.56) | 0.91 | ||
| Reduced consciousness | 12 | (9.5) | 21 | (13.4) | 0.68 | (0.32–1.45) | 0.32 | ||
| Acute mechanical intubation/ventilation | 17 | (13.5) | 14 | (8.9) | 1.59 | (0.75–3.37) | 0.22 | ||
| Developed acute renal failure | 32 | (25.4) | 24 | (15.3) | 1.89 | (1.04–3.41) | 0.034 | ||
| Developed acute liver injury | 38 | (30.2) | 57 | (36.3) | 0.76 | (0.46–1.25) | 0.28 | ||
| Chorioretinitis | 18 | (14.3) | 15 | (9.6) | 1.58 | (0.76–3.27) | 0.22 | ||
| Therapy | Empirical antifungal therapy within 72 hours of the onset of candidemia | ||||||||
| Fluconazole | 25 | (19.8) | 59 | (37.6) | 0.41 | (0.24–0.71) | 0.001 | ||
| Micafungin | 92 | (73.0) | 77 | (49.0) | 2.81 | (1.70–4.65) | <0.001 | ||
| Voriconazole | 0 | (0.0) | 1 | (0.6) | 1.00 | ||||
| Liposomal amphotericin b | 7 | (5.6) | 4 | (2.5) | 2.25 | (0.64–7.87) | 0.23 | ||
| None | 1 | (0.8) | 16 | (10.2) | 0.071 | (0.009–0.54) | 0.001 | ||
| Change of antifungal drugs due to clinical failure | 18 | (14.3) | 14 | (8.9) | 1.70 | (0.81–3.57) | 0.16 | ||
| Complications | Overall | 26 | (20.6) | 20 | (12.7) | 1.78 | (0.94–3.37) | 0.07 | |
| Endophthalmitis | 18 | (14.3) | 15 | (9.6) | 1.58 | (0.76–3.27) | 0.22 | ||
| Endocarditis | 1 | (0.8) | 0 | (0.0) | 0.45 | ||||
| Pulmonary embolism | 2 | (1.6) | 0 | (0.0) | 0.20 | ||||
| Vertebral osteomyelitis | 1 | (0.8) | 0 | (0.0) | 0.45 | ||||
| Thrombosis | 4 | (3.2) | 5 | (3.2) | 1.00 | (0.26–3.79) | 1.00 | ||
| Outcome | Clinical failure | 26 | (19.8) | 43 | (27.4) | 0.66 | (0.37–1.15) | 0.14 | |
| Persistent candidemia for ≥ 72 hours of therapy | 122 | (96.8) | 127 | (80.9) | 7.21 | (2.47–21.06) | <0.001 | ||
| In-hospital mortality | 47 | (37.3) | 66 | (42.0) | 0.82 | (0.51–1.33) | 0.42 | ||
| 30-day mortality | 23 | (18.3) | 44 | (28.0) | 0.57 | (0.32–1.02) | 0.055 | ||
| Early (< 72 hours) | 0 | (0.0) | 1 | (0.6) | 1.00 | ||||
| Non-early (days 3–30) | 23 | (18.3) | 43 | (27.4) | 0.59 | (0.33–1.05) | 0.071 | ||
| 90-day mortality | 41 | (32.5) | 64 | (40.8) | 0.70 | (0.43–1.14) | 0.16 | ||
| 180-day mortality | 57 | (45.2) | 76 | (48.6) | 0.88 | (0.55–1.41) | 0.60 | ||
| Discharged to LTCF after being admitted from home | 40 | (31.7) | 45 | (28.7) | 1.16 | (0.70–1.93) | 0.57 | ||
| Additional hospitalizations in 6 months after completed candidemia therapy | 22 | (17.5) | 27 | (17.2) | 1.02 | (0.55–1.89) | 0.95 | ||
| Median total LOS days (IQR) | 97 | (68–148) | 71 | (46–110) | <0.001 | ||||
| Median LOS after candidemia day (IQR) | 52 | (26–83) | 31 | (15–54) | <0.001 | ||||
| Median LOS after candidemia day, excluding those who died (IQR) | 31 | (0–69) | 18 | (0–43) | 0.017 | ||||
| Median duration candidemia days (IQR) | 8 | (5–12) | 5 | (0–11) | <0.001 | ||||
Unless otherwise stated, data are presented as n (%)
IDC, infectious disease consultation; OR, odds ratio; CI, confidence interval; SD, standard deviation; LTCF, long term care facility; CVC, central venous catheter; IQR, interquartile range; TPN total parenteral nutrition; CLABSI, central line-associated blood stream infection; PLABSI, peripheral line-associated blood stream infection; LOS, length of hospital stay
aIncluded ampicilline, sulbactam/ampicilline, piperacillin, and tazobactam/piperacillin
bIncluded ceftriaxone, ceftazidime, and cefepime.
cOther Candida species were included C. gelliermondii (with IDC, 2; without IDC, 1), C. lusitaniae (2 in with IDC), C. krusei (with IDC, 1; without IDC, 3), C. dubliniesnsis (without IDC, 1) and unclassified (with IDC, 2; without IDC, 5).
dPolymicrobial bacteremia/fungemia were included due to different species of Candida spp. (with IDC, 6; without IDC, 6) and due to pathogens other than Candida spp. (with IDC, 11; without IDC, 2)
The IDC group (55.6% [70/126]) had significantly more patients with candidemia during 2010–2013 than non-IDC group (21.0% [33/157]) (P < 0.001). Although there was a similar profile of chronic conditions in both groups, patients in the IDC group were significantly more associated with HIV infection (P = 0.006) and connective tissue disease (P = 0.018). There were no differences of healthcare-associated exposures between the two groups. The IDC group had more previous exposure to carbapenems (P = 0.014), metronidazole (P = 0.023), micafungin (P = 0.020), and liposomal amphotericin b (P = 0.038). Although there was no difference of isolated Candida spp. between groups, the IDC group had significantly more episodes of polymicrobial bacteraemia/fungaemia than the non-IDC group (P = 0.013). The proportion of PLABSI was significantly higher in the IDC group (P = 0.020). No differences of severe sepsis/septic shock and chorioretinitis were observed between groups, but patients in the IDC group developed significantly more acute renal failure than did non-IDC group (P = 0.034). Regarding treatment, the IDC group more frequently received micafungin (P < 0.001) as empiric therapy. In contrast, the number of patients who received fluconazole or who did not receive any empiric therapy was higher in the non-IDC group (P = 0.001 for both). Patients with IDC were more likely to receive the appropriate definitive antifungal therapy (P < 0.001) and appropriate duration of antifungal therapy (P < 0.001). Although there was no difference in the overall adequate source control between groups, early CVC removal and early peripheral-line removal were more frequent in patients with IDC than patients without (P = 0.023, and P = 0.013, respectively). Patients with IDC received more consultations from an ophthalmologist to evaluate chorioretinitis (P < 0.001) (Table 2).
Table 2. Parameters associated with recommended candidemia treatment and results, n = 283.
| Variable | With IDC | Without IDC | OR (95% CI) | P value | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 126, 44.5%) | (n = 157, 55.1%) | |||||||
| Definitive antifungal therapy | Appropriate antifungal choice (n = 276) a | 120/124 | (96.8) | 125/152 | (82.2) | 6.48 | (2.20–19.07) | <0.001 |
| Liposomal amphotericin b | 4 | (3.2) | 3 | (2.0) | 1.66 | (0.36–7.54) | 0.70 | |
| Fluconazole | 51 | (41.1) | 19 | (12.5) | 4.89 | (2.69–8.90) | <0.001 | |
| Echinocandin | 9 | (7.3) | 12 | (7.9) | 0.91 | (0.37–2.24) | 0.84 | |
| Voriconazole | 0 | (0.0) | 3 | (2.0) | 0.26 | |||
| Appropriate planned duration of antifungal therapy (n = 280)b | 105/125 | (84.0) | 89/155 | (57.4) | 3.89 | (2.19–6.92) | <0.001 | |
| Median duration of antifungal therapyb | 19 | (15–31) | 14 | (6–20) | <0.001 | |||
| Intervention | Transthoracic echocardiogram | 22 | (17.5) | 1 | (0.6) | 33.00 | (4.38–248.59) | <0.001 |
| Adequate source control | 106 | (84.1) | 130 | (82.8) | 1.10 | (0.59–2.07) | 0.77 | |
| CVC removal | 86 | (68.3) | 120 | (76.4) | 0.66 | (0.39–1.12) | 0.12 | |
| Early CVC removal (≤ 48 hours) | 65 | (51.6) | 102 | (65.0) | 0.58 | (0.36–0.93) | 0.023 | |
| Peripheral-line removal | 17 | (13.5) | 8 | (5.1) | 2.91 | (1.21–6.97) | 0.013 | |
| Intra-abdominal drainage | 3 | (2.4) | 2 | (1.3) | 1.89 | (0.31–11.49) | 0.66 | |
| Consultation to Ophthalmologist | 98 | (77.8) | 54 | (34.4) | 6.68 | (3.92–11.38) | <0.001 | |
| Performed the ecocardiography | 22 | (17.5) | 1 | (0.6) | 33.00 | (4.38–248.59) | <0.001 | |
Unless otherwise stated, data are presented as n (%)
IDC, infectious disease consultation; OR, odds ratio; CI, confidence interval; IQR, interquartile range; CVC, central venous catheter
aAppropriate antifungal choice was unspecified for 7patients due to unclassified Candida species.
bDuration of therapy was unspecified for 3 patients.
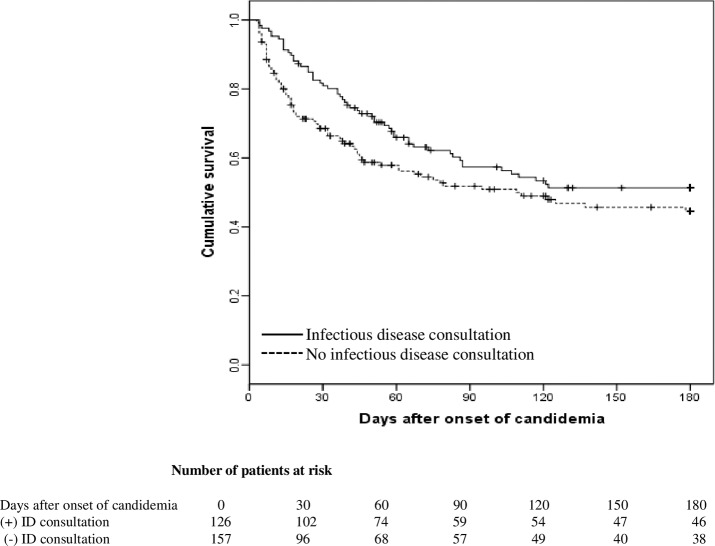
The overall 30-day and 180-day all-cause mortality were 23.7% (67/283) and 47.0% (133/283), respectively. The median length of time from diagnosis to death was 26 (IQR, 11–55) days. No difference in hospital mortality between two groups, but the IDC group had more episodes of persistent candidemia (P < 0.001) with longer total LOS (P < 0.001) and longer duration candidemia (P <0.001) (Table 1). The Kaplan-Meier survival curve for patients with candidemia stratified by IDC is shown in Fig 1.
Fig 1. Kaplan-Meier curve of candidemia based on infectious disease consultation.
Kaplan-Meier curve of candidemia based on infectious disease consultation. Adjusted hazard ratio for 30-day mortality was 0.54 (P = 0.017), and the crude hazard ratio for 31-180-day mortality was 0.98 (P = 0.92).
Predictive factors for mortality of candidemia
Predictive factors for mortality of candidemia are summarized in Table 3.
Table 3. Predictive factors for 30-day mortality of candidemia patients, n = 283.
| No. (%) of patients with: | Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Died ≤ 30 day after the onset | Survived > 30 day after the onset | Crude HR | P value | Adjusted HR | P value | |||||
| Variable | (n = 67, 23.7%) | (n = 216, 76.3%) | (95% CI) | (95% CI) | ||||||
| Mean age (years) ± SD | 73.6 | ± 12.0 | 68.9 | ± 16.7 | 1.02 | (1.00–1.04) | 0.035 | 1.01 | (1.00–1.03) | 0.15 |
| Females | 24 | (35.8) | 65 | (30.1) | 1.27 | (0.77–2.10) | 0.35 | |||
| Candidemia from 2010 to 2013 | 24 | (35.8) | 79 | (36.6) | 0.97 | (0.55–1.60) | 0.90 | |||
| HIV infection | 1 | (1.5) | 9 | (4.2) | 0.37 | (0.051–2.63) | 0.32 | |||
| Neutropenia (< 0.5 × 109 cells/L) at onset | 3 | (4.5) | 4 | (1.9) | 2.03 | (0.90–4.60) | 0.090 | |||
| Chemotherapy in the past month | 14 | (20.9) | 35 | (16.2) | 1.28 | (0.71–2.30) | 0.42 | |||
| Steroid therapy in the past month | 15 | (22.4) | 36 | (16.7) | 1.28 | (0.72–2.27) | 0.40 | |||
| Radiation therapy in the past month | 4 | (6.0) | 14 | (6.5) | 0.96 | (0.35–2.64) | 0.92 | |||
| Transplantation in the past month | 0 | (0.0) | 4 | (1.9) | 0.049 | (0.0–215.90) | 0.48 | |||
| Dependent functional status | 33 | (49.3) | 88 | (40.7) | 1.39 | (0.86–2.24) | 0.18 | |||
| Charlson's weighted index comorbity score (6), mean ± SD | 4.3 | ± 2.8 | 4.2 | ± 2.9 | 1.00 | (0.92–1.09) | 0.97 | 1.01 | (0.93–1.10) | 0.76 |
| Diabetes mellitus | 20 | (29.9) | 56 | (25.9) | 1.14 | (0.67–1.92) | 0.63 | |||
| Solid-organ cancer within last 1 year | 27 | (40.3) | 84 | (38.9) | 1.05 | (0.65–1.72) | 0.84 | |||
| Hematological malignancy within last 1 year | 7 | (10.4) | 18 | (8.3) | 1.18 | (0.54–2.58) | 0.68 | |||
| Chronic kidney disease stage V | 6 | (9.0) | 12 | (5.6) | 1.58 | (0.69–3.67) | 0.28 | |||
| Liver diseases | 1 | (1.5) | 13 | (6.0) | 0.28 | (0.039–2.00) | 0.20 | |||
| Chronic heart disease | 20 | (29.9) | 39 | (18.1) | 1.70 | (1.01–2.87) | 0.047 | |||
| Chronic obstructive pulmonary disease | 11 | (16.4) | 22 | (10.2) | 1.57 | (0.82–2.99) | 0.17 | |||
| Cerebrovascular disease | 17 | (25.4) | 46 | (21.3) | 1.22 | (0.70–2.11) | 0.48 | |||
| Dementia | 3 | (4.5) | 17 | (7.9) | 0.62 | (0.20–1.98) | 0.42 | |||
| Connective tissue disease | 4 | (6.0) | 12 | (5.6) | 0.99 | (0.36–2.72) | 0.98 | |||
| Peptic ulcer disease | 6 | (9.0) | 20 | (9.3) | 0.93 | (0.40–2.14) | 0.86 | |||
| Peripheral vascular disease | 2 | (3.0) | 2 | (0.9) | 2.98 | (0.73–12.19) | 0.13 | |||
| Hemiplegia | 3 | (4.5) | 21 | (9.7) | 0.47 | (0.15–1.48) | 0.20 | |||
| Resided in LTCF in the past 3 months | 1 | (1.5) | 13 | (6.0) | 0.28 | (0.038–2.00) | 0.20 | |||
| Hospitalized in the past 3 months | 37 | (55.2) | 93 | (43.1) | 1.61 | (0.99–2.60) | 0.054 | |||
| Invasive procedure/surgery in the past 3 months | 18 | (26.9) | 82 | (38.0) | 0.66 | (0.38–1.13) | 0.13 | |||
| Tracheotomy in the past 3 months | 8 | (11.9) | 23 | (10.6) | 1.06 | (0.51–2.21) | 0.88 | |||
| Urinary catheters (for ≥ 2 days) at onset of candidemia | 57 | (85.1) | 122 | (56.5) | 3.72 | (1.90–7.27) | < 0.001 | 2.94 | (1.48–5.87) | 0.002 |
| CVC (for ≥ 2 days) at same onset | 59 | (88.1) | 172 | (79.6) | 1.76 | (0.84–3.68) | 0.14 | |||
| Median days of CVC prior to onset of candiedemia (IQR) | 15 | (9–27) | 11 | (4–23) | 1.01 | (1.00–1.01) | < 0.001 | |||
| Undergoing hemodialysis in the past month | 6 | (9.0) | 16 | (7.4) | 1.16 | (0.50–2.69) | 0.72 | |||
| Transfusion in the past month | 41 | (61.2) | 113 | (52.6) | 1.29 | (0.79–2.10) | 0.32 | |||
| TPN in the past month | 43 | (64.2) | 129 | (59.7) | 1.21 | (0.73–1.99) | 0.47 | |||
| Median days of TPN prior to onset of candidemia (IQR) | 10 | (0–20) | 6 | (0–16) | 1.01 | (1.00–1.02) | 0.26 | |||
| ICU stay in current hospitalization before onset of candidemia | 10 | (14.9) | 26 | (12.0) | 1.16 | (0.59–2.27) | 0.67 | |||
| Median hospital days prior to the onset of candidemia (IQR) | 38 | (20–68) | 34 | (18–66) | 1.00 | (0.99–1.00) | 0.53 | |||
| Over all | 63 | (94.0) | 204 | (94.4) | 0.97 | (0.35–2.67) | 0.96 | |||
| Penicillinsa | 27 | (40.3) | 95 | (44.0 | 0.89 | (0.55–1.46) | 0.65 | |||
| Cephalosporinsb | 27 | (40.3 | 103 | (47.7 | 0.79 | (0.49–1.29) | 0.35 | |||
| Carbapenems | 35 | (52.2) | 106 | (49.1) | 1.08 | (0.67–1.75) | 0.74 | |||
| Fluoroquinolones | 18 | (26.9) | 49 | (22.7) | 1.18 | (0.69–2.03) | 0.54 | |||
| Aminoglycosides | 8 | (11.9) | 20 | (9.3) | 1.23 | (0.59–2.58) | 0.58 | |||
| Trimethoprim-sulfamethoxazole | 5 | (7.5) | 28 | (13.0) | 0.55 | (0.22–1.37) | 0.20 | |||
| Clindamycin | 9 | (13.4) | 22 | (10.2) | 1.25 | (0.62–2.52) | 0.54 | |||
| Metronidazole | 9 | (13.4) | 15 | (6.9) | 1.86 | (0.92–3.75) | 0.084 | |||
| Glycopeptide | 28 | (41.8) | 59 | (27.3) | 1.65 | (1.01–2.68) | 0.044 | |||
| Over all | 11 | (16.4) | 27 | (12.5) | 1.27 | (0.66–2.42) | 0.48 | |||
| Fluconazole | 0 | (0.0) | 12 | (5.6) | 0.046 | (0.0–6.19) | 0.22 | |||
| Micafungin | 10 | (14.9) | 12 | (5.6) | 2.32 | (1.18–4.54) | 0.014 | |||
| Voriconazole | 0 | (0.0) | 2 | (0.9) | 0.049 | (0.0–6882.16) | 0.62 | |||
| Liposomal amphotericin b | 0 | (0.0) | 4 | (1.9) | 0.049 | (0.0–215.90) | 0.48 | |||
| Itraconazole | 2 | (3.0) | 6 | (2.8) | 1.09 | (0.27–4.43) | 0.91 | |||
| C. albicans | 37 | (55.2) | 94 | (43.5) | 1.51 | (0.93–2.45) | 0.093 | |||
| C. glabrata | 16 | (23.9) | 58 | (26.9) | 0.89 | (0.51–1.56) | 0.68 | |||
| C. parapsilosis | 5 | (7.5) | 40 | (18.5) | 0.39 | (0.16–0.96) | 0.040 | 0.57 | (0.22–1.45) | 0.24 |
| C. tropicalis | 10 | (14.9) | 18 | (8.3) | 1.69 | (0.86–3.30) | 0.13 | |||
| Othersc | 3 | (4.5) | 14 | (6.5) | 0.69 | (0.22–2.20) | 0.53 | |||
| Polymicrobial bacteremia/fungemiad | 11 | (16.4) | 14 | (6.5) | 2.07 | (1.08–3.95) | 0.028 | 0.55 | (0.13–2.31) | 0.42 |
| Previous Candida colonization within one week before candidemia | 23 | (34.3) | 67 | (31.0) | 1.13 | (0.68–1.86) | 0.65 | |||
| CLABSI | 58 | (86.6) | 162 | (75.0) | 2.02 | (1.00–4.07) | 0.050 | 1.47 | (0.62–3.47) | 0.38 |
| PLABSI | 3 | (4.5) | 32 | (14.8) | 0.30 | (0.094–0.95) | 0.041 | 0.55 | (0.13–2.31) | 0.42 |
| Intra-abdominal infection | 2 | (3.0) | 11 | (5.1) | 0.57 | (0.14–2.31) | 0.43 | |||
| Unknown source | 4 | (6.0) | 11 | (5.1) | 1.18 | (0.43–3.23) | 0.75 | |||
| Sepsis | 15 | (22.4) | 77 | (35.6) | 0.57 | (0.32–1.01) | 0.053 | |||
| Severe sepsis | 33 | (49.3) | 75 | (34.7) | 1.68 | (1.04–2.71) | 0.034 | |||
| Septic shock | 16 | (24.2) | 23 | (10.6) | 2.16 | (1.23–3.79) | 0.007 | |||
| Sever sepsis/septic shock | 49 | (73.1) | 98 | (45.4) | 2.78 | (1.62–4.77) | < 0.001 | 2.10 | (1.20–3.65) | 0.009 |
| Reduced consciousness | 17 | (25.4) | 16 | (7.4) | 3.09 | (1.78–5.37) | < 0.001 | |||
| Acute mechanical intubation/ventilation | 14 | (20.9) | 17 | (7.9) | 2.29 | (1.27–4.13) | 0.006 | |||
| Developed acute renal failure | 23 | (34.3) | 33 | (15.3) | 2.38 | (1.44–3.94) | 0.001 | |||
| Developed acute liver injury | 27 | (40.3) | 68 | (31.5) | 1.36 | (0.83–2.21) | 0.22 | |||
| Chorioretinitis | 4 | (6.0) | 29 | (13.4) | 0.42 | (0.15–1.16) | 0.095 | |||
| Consultation to ID specialist | 23 | (34.3) | 103 | (47.7) | 0.56 | (0.34–0.93) | 0.025 | 0.54 | (0.32–0.90) | 0.017 |
Unless otherwise stated, data are presented as n (%)
HR, hazard ratio; CI, confidence interval; SD, standard deviation; LTCF, long term care facility; CVC, central venous catheter; IQR, interquartile range; TPN total parenteral nutrition; CLABSI, central line-associated blood stream infection; PLABSI, peripheral line-associated blood stream infection; ID, infectious disease
aIncluded ampicilline, sulbactam/ampicilline, piperacillin, and tazobactam/piperacillin
bIncluded ceftriaxone, ceftazidime, and cefepime.
cOther Candida species were included C. gelliermondii (survived, 3), C. lusitaniae (survived, 3), C. krusei (survived, 3; died, 1), C. dubliniesnsis (died, 1) and unclassified (survived, 6; died, 1).
dPolymicrobial bacteremia/fungemia were included due to different species of Candida spp. (survived, 8; died, 7) and due to pathogens other than Candida spp. (survived, 6; died, 4)
Patients with candidemia who died within 30 days of the first positive blood culture were more likely to be older (P = 0.035), to have chronic heart disease (P = 0.047), to have urinary catheters placed (P < 0.001), to have exposure to glycopeptide (P = 0.044), to have polymicrobial bacteremia/fungemia (P = 0.028), to have CLABSI (P = 0.050), and to be in severe sepsis/septic shock (P < 0.001). They were less likely to have candidemia due to C. parapsilosis (P = 0.040) and to have PLABSI (P = 0.041) than were candidemia patients who survived longer than 30 days after candidemia onset. In the multivariate model, factors independently associated with 30-day mortality among candidemia were urinary catheters use at onset (adjusted hazard ratio [HR] = 2.94; 95% CI = 1.48–5.87; P = 0.002) and severe sepsis/septic shock (adjusted HR = 2.10; 95% CI = 1.20–3.65; P = 0.009). IDC was associated with a decreased risk of 30-day mortality (adjusted HR = 0.54; 95% CI = 0.32–0.90; P = 0.017).
For the 216 patients who survived 30 days after initial positive culture, the effect of IDC from 31- to 180-day of the diagnosis of candidemia was also evaluated. There was no statistical difference in 31-180-day mortality after the diagnosis of candidemia between patients who did and did not receive IDC (crude HR = 0.98; 95% CI = 0.60–1.58; P = 0.92).
Discussion
We showed that IDC was associated with a 46% reduction in all-cause mortality among candidemia patients within 30 days of candidemia onset. Past studies have described the positive effect of IDC on mortality with candidemia; 18–24% and 39–56% in the IDC and the non-IDC groups, respectively [13–16]. Compared the data of rates of IDC, patient characteristics at baseline, and clinical outcomes of candidemia in a hospital in North America [16], the rate of IDC was lower (45% vs 77%), the median age and population of male were higher (69 years old vs 53 years old; 72% vs 55%), and 30 days mortality was slightly lower (18% vs 20%) among IDC group in our results. However, these studies were limited due to small sample sizes (50–171 patients) and short study periods (1–3 years). Although our study was a retrospective cohort study, our sample size was larger (283 patients) and study period was longer (12 years) than past studies [13–16]. Moreover, because previous studies did not use the cox proportional hazard model [13–16], the evaluation of the effect of IDC on mortality with candidemia was prone to confounding effects, and might thus be incorrect. In our study, the IDC group more frequently received appropriate choice (OR = 6.48; 95% CI = 2.20–19.07; P < 0.001) and appropriate duration (OR = 3.89; 95% CI = 2.19–6.92; P < 0.001) of antifungal therapy than the non-IDC group. The duration of antifungal therapy was significantly longer in the IDC group than the non-IDC group (19 days versus 14 days; P < 0.001). Past research has also revealed that IDC intervention for candidemia led to appropriate antifungal therapy [14–16].
Besides antifungal therapy, adequate source control is an important component of appropriate candidemia management. While previous research has reported that removal of CVC was more frequent in the IDC group than in the non-IDC group [13–16, 26], our results showed no statistical difference in numbers of removal of CVC between both groups. In our study, we found that the IDC group had a statistically higher proportion of the removal of peripheral-line than the non-IDC group (OR = 2.91; 95% CI = 1.21–6.97; P = 0.013), which, to the best of our knowledge, has not been reported previously. This might reflect the difficulty of diagnosing PLAC, and need for the consultation of an ID specialist [17]. In fact, the proportion of PLAC was higher in the IDC group than the non-IDC group (OR = 2.34; 95% CI = 1.13–4.87; P = 0.020). Similar to a past study [26], our study revealed that consultation with an ophthalmologist was conducted more often in the IDC group then in the non-IDC group (OR = 6.68; 95% CI = 3.92–11.38; P < 0.001). For candidemia, the evaluation of endophthalmitis is thought to be a predictive factor for outcome improvement of candidemia, and contributes to an appropriate choice and duration of antifungal therapy [20, 27]. While not statistically significant, the proportion of endophthalmitis was higher in the IDC group than the non-IDC group in our study (OR = 1.58; 95% CI = 0.76–3.27; P = 0.22), suggesting that IDC led to the detection of endophthalmitis in many cases.
On the other hand, urinary catheters placed (for ≥ 2 days) at onset of candidemia (adjusted HR, 2.94; 95% CI, 1.48–5.87, P = 0.002) and severe sepsis/septic shock (adjusted HR, 2.10; 95% CI, 1.20–3.65, P = 0.009) were independently associated with increased 30-day mortality. These results indicate that IDC might not significantly improve outcomes for patients with very severe conditions. Clinical severity such as severe sepsis/septic due to candidemia was previously reported as an independent risk factor for 30-day mortality [28].
The IDC group was associated with the following factors indicating the clinical severity of candidemia: ICU stay, development of acute renal failure, exposure to carbapenems/micafungin/liposomal amphotericin b, longer duration of candidemia, and longer length of hospital stay after candidemia [28]. In the IDC group, episodes of polymicrobial bacteremia/fungemia were statistically more frequently found than in the non-IDC group (OR = 2.91; 95% CI = 1.21–6.97; P = 0.013). This may be due to heavier contamination of peripheral lines than central lines, and better ability of ID specialists for diagnosing PLAC [17]. IDC as candidemia intervention may not improve the severity of the current disease status, however it did improve the mortality of candidemia, with improved management of the disease.
This study has several limitations. Due to the retrospective nature of the study, we were unable to collect information regarding the duration of time from onset of candidemia to IDC. This is a time-dependent variable, and might have been an unmeasured confounding factor. Similarly, unmeasured confounding factors such as other interventions might have affected the patients’ outcomes. Following the recommendation of IDC for candidemia was not mandatory. Although IDC consisted of chart review, physical examination of the patient, follow-up visits, and written recommendations for therapy based on published IDSA guidelines, final decision-making in each case depended on the primary team in charge.
In conclusion, this study was the first epidemiological clinical study with a large sample size and a long study period to evaluate the value of IDC in candidemia in Japan. IDC was associated with a 46% reduction in adjusted all-cause mortality among candidemia patients within 30 days of onset of candidemia. These results suggest that IDC should be actively considered to improve the frequently poor outcome of candidemia patients. Further studies, including evaluations of the outcome effects of time between IDC and onset of candidemia, are needed to further reduce the mortality of candidemia.
Supporting information
(XLSX)
Acknowledgments
We thank all the clinical staff at the NCGM for their dedicated clinical practice and patient care.
Data Availability
All relevant data are within the manuscript and Supporting Information files.
Funding Statement
This work was supported by Grants for International Health Research (28S-1106) from the Ministry of Health, Labor, and Welfare of Japan, and by Grants for International Medical Cooperation (26S-101) by the National Center for Global Health and Medicine, both to MI. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Centers for Disease Control and Prevention (CDC). Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60: 243–248. [PubMed] [Google Scholar]
- 2.Barchiesi F, Orsetti E, Gesuita R, Skrami E, Manso E; Candidemia Study Group. Epidemiology, clinical characteristics, and outcome of candidemia in a tertiary referral center in Italy from 2010 to 2014. Infection. 2016;44: 205–213. 10.1007/s15010-015-0845-z [DOI] [PubMed] [Google Scholar]
- 3.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;7: 19–37. [DOI] [PubMed] [Google Scholar]
- 4.Lionakis MS, Netea MG. Candida and host determinants of susceptibility to invasive candidiasis. PLoS Pathog. 2013;9: e1003079 10.1371/journal.ppat.1003079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39: 309–317. 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 6.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41: 1232–1239. 10.1086/496922 [DOI] [PubMed] [Google Scholar]
- 7.Tortorano AM, Kibbler C, Peman J, Bernhardt H, Klingspor L, Grillot R. Candidaemia in Europe: epidemiology and resistance. Int J Antimicrob Agents. 2006;27: 359–366. 10.1016/j.ijantimicag.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 8.Hassan I, Powell G, Sidhu M, Hart WM, Denning DW. Excess mortality, length of stay and cost attributable to candidaemia. J Infect. 2009;59: 360–365. 10.1016/j.jinf.2009.08.020 [DOI] [PubMed] [Google Scholar]
- 9.Bai AD, Showler A, Burry L, Steinberg M, Ricciuto DR, Fernandes T, et al. Impact of Infectious Disease Consultation on Quality of Care, Mortality, and Length of Stay in Staphylococcus aureus Bacteremia: Results From a Large Multicenter Cohort Study. Clin Infect Dis. 2015;60: 1451–1461. 10.1093/cid/civ120 [DOI] [PubMed] [Google Scholar]
- 10.Jain SR, Hosseini-Moghaddam SM, Dwek P, Gupta K, Elsayed S, Thompson GW, et al. Infectious diseases specialist management improves outcomes for outpatients diagnosed with cellulitis in the emergency department: a double cohort study. Diagn Microbiol Infect Dis. 2017;87: 371–375. 10.1016/j.diagmicrobio.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 11.Brennan MB, Allen GO, Ferguson PD, McBride JA, Crnich CJ, Smith MA. The Association Between Geographic Density of Infectious Disease Physicians and Limb Preservation in Patients With Diabetic Foot Ulcers. Open Forum Infect Dis. 2017;4: ofx015 10.1093/ofid/ofx015 eCollection 2017 Winter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spec A, Olsen MA, Raval K, Powderly WG. Impact of Infectious Diseases Consultation on Mortality of Cryptococcal infection in Patients without HIV. Clin Infect Dis. 2017;64:558–564. 10.1093/cid/ciw786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel M, Kunz DF, Trivedi VM, Jones MG, Moser SA, Baddley JW. Initial management of candidemia at an academic medical center: evaluation of the IDSA guidelines. Diagn Microbiol Infect Dis. 2005;52: 29–34. 10.1016/j.diagmicrobio.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 14.Takakura S, Fujihara N, Saito T, Kimoto T, Ito Y, Iinuma Y, et al. Improved clinical outcome of patients with Candida bloodstream infections through direct consultation by infectious diseases physicians in a Japanese university hospital. Infect Control Hosp Epidemiol. 2006;27: 964–968. 10.1086/504934 [DOI] [PubMed] [Google Scholar]
- 15.Moon C., Koo D.-W., Shin J.-H. Impact of Infectious Disease Consultation on Outcomes of Candidemia. ECCMID 2013, Berlin, Germany 27 April 2013. [Google Scholar]
- 16.Lee RA, Zurko J, Camins BC, Griffin RL, Rodriguez JM, McCarty TP, et al. Impact of Infectious Disease Consultation on Clinical Management and Mortality in Patients with Candidemia. Impact of Infectious Disease Consultation on Clinical Management and Mortality in Patients with Candidemia. Clin Infect Dis. 2018;3 10.1093/cid/ciy849 [DOI] [PubMed] [Google Scholar]
- 17.Ishikane M, Hayakawa K, Kutsuna S, Takeshita N, Ohmagari N. Epidemiology of Blood Stream Infection due to Candida Species in a Tertiary Care Hospital in Japan over 12 Years: Importance of Peripheral Line-Associated Candidemia. PLoS One. 2016;11: e0165346 10.1371/journal.pone.0165346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4: 94 10.1186/1471-2407-4-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41: 580–637. 10.1097/CCM.0b013e31827e83af [DOI] [PubMed] [Google Scholar]
- 20.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62: e1–50. 10.1093/cid/civ933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Ruiz M, Aguado JM, Almirante B, Lora-Pablos D, Padilla B, Puig-Asensio M, et al. Initial use of echinocandins does not negatively influence outcome in Candida parapsilosis bloodstream infection: a propensity score analysis. Clin Infect Dis. 2014;58: 1413–1421. 10.1093/cid/ciu158 [DOI] [PubMed] [Google Scholar]
- 22.National Healthcare Safety Network. Central Line-Associated Bloodstream Infection (CLABSI) and non-central line-associated Bloodstream Infection. Available at: http://www.cdc.gov/nhsn/acute-carehospital/clabsi/. Accessed 1 March 2018. [Google Scholar]
- 23.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50: 133–164. 10.1086/649554 [DOI] [PubMed] [Google Scholar]
- 24.Pujol M, Hornero A, Saballs M, Argerich MJ, Verdaguer R, Cisnal M, et al. Clinical epidemiology and outcomes of peripheral venous catheter-related bloodstream infections at a university-affiliated hospital. J Hosp Infect. 2007;67: 22–9. 10.1016/j.jhin.2007.06.017 [DOI] [PubMed] [Google Scholar]
- 25.Kontoyiannis DP, Vaziri I, Hanna HA, Boktour M, Thornby J, Hachem R, et al. Risk Factors for Candida tropicalis fungemia in patients with cancer. Clin Infect Dis. 2001;33: 1676–1681. 10.1086/323812 [DOI] [PubMed] [Google Scholar]
- 26.Mejia Carlos, Kronen Ryan, Lin Charlotte, Hsueh Kevin, Powderly William, Spec Andrej. Impact of Infectious Diseases Consultation on Mortality in Patients with Candidemia. Open Forum Infect Dis. 2017; 4: S52. [Google Scholar]
- 27.Shah CP, McKey J, Spirn MJ, Maguire J. Ocular candidiasis: a review. Br J Ophthalmol. 2008;92: 466–468. 10.1136/bjo.2007.133405 [DOI] [PubMed] [Google Scholar]
- 28.Murri R, Scoppettuolo G, Ventura G, Fabbiani M, Giovannenze F, Taccari F, et al. Initial antifungal strategy does not correlate with mortality in patients with candidemia. Eur J Clin Microbiol Infect Dis. 2016;35: 187–193. 10.1007/s10096-015-2527-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and Supporting Information files.