Abstract
Background
Burn wounds cause high levels of morbidity and mortality worldwide. People with burns are particularly vulnerable to infections; over 75% of all burn deaths (after initial resuscitation) result from infection. Antiseptics are topical agents that act to prevent growth of micro‐organisms. A wide range are used with the intention of preventing infection and promoting healing of burn wounds.
Objectives
To assess the effects and safety of antiseptics for the treatment of burns in any care setting.
Search methods
In September 2016 we searched the Cochrane Wounds Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE, Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations), Ovid Embase, and EBSCO CINAHL. We also searched three clinical trials registries and references of included studies and relevant systematic reviews. There were no restrictions based on language, date of publication or study setting.
Selection criteria
We included randomised controlled trials (RCTs) that enrolled people with any burn wound and assessed the use of a topical treatment with antiseptic properties.
Data collection and analysis
Two review authors independently performed study selection, risk of bias assessment and data extraction.
Main results
We included 56 RCTs with 5807 randomised participants. Almost all trials had poorly reported methodology, meaning that it is unclear whether they were at high risk of bias. In many cases the primary review outcomes, wound healing and infection, were not reported, or were reported incompletely.
Most trials enrolled people with recent burns, described as second‐degree and less than 40% of total body surface area; most participants were adults. Antiseptic agents assessed were: silver‐based, honey, Aloe Vera, iodine‐based, chlorhexidine or polyhexanide (biguanides), sodium hypochlorite, merbromin, ethacridine lactate, cerium nitrate and Arnebia euchroma. Most studies compared antiseptic with a topical antibiotic, primarily silver sulfadiazine (SSD); others compared antiseptic with a non‐antibacterial treatment or another antiseptic. Most evidence was assessed as low or very low certainty, often because of imprecision resulting from few participants, low event rates, or both, often in single studies.
Antiseptics versus topical antibiotics
Compared with the topical antibiotic, SSD, there is low certainty evidence that, on average, there is no clear difference in the hazard of healing (chance of healing over time), between silver‐based antiseptics and SSD (HR 1.25, 95% CI 0.94 to 1.67; I2 = 0%; 3 studies; 259 participants); silver‐based antiseptics may, on average, increase the number of healing events over 21 or 28 days' follow‐up (RR 1.17 95% CI 1.00 to 1.37; I2 = 45%; 5 studies; 408 participants) and may, on average, reduce mean time to healing (difference in means ‐3.33 days; 95% CI ‐4.96 to ‐1.70; I2 = 87%; 10 studies; 979 participants).
There is moderate certainty evidence that, on average, burns treated with honey are probably more likely to heal over time compared with topical antibiotics (HR 2.45, 95% CI 1.71 to 3.52; I2 = 66%; 5 studies; 140 participants).
There is low certainty evidence from single trials that sodium hypochlorite may, on average, slightly reduce mean time to healing compared with SSD (difference in means ‐2.10 days, 95% CI ‐3.87 to ‐0.33, 10 participants (20 burns)) as may merbromin compared with zinc sulfadiazine (difference in means ‐3.48 days, 95% CI ‐6.85 to ‐0.11, 50 relevant participants). Other comparisons with low or very low certainty evidence did not find clear differences between groups.
Most comparisons did not report data on infection. Based on the available data we cannot be certain if antiseptic treatments increase or reduce the risk of infection compared with topical antibiotics (very low certainty evidence).
Antiseptics versus alternative antiseptics
There may be some reduction in mean time to healing for wounds treated with povidone iodine compared with chlorhexidine (MD ‐2.21 days, 95% CI 0.34 to 4.08). Other evidence showed no clear differences and is of low or very low certainty.
Antiseptics versus non‐antibacterial comparators
We found high certainty evidence that treating burns with honey, on average, reduced mean times to healing in comparison with non‐antibacterial treatments (difference in means ‐5.3 days, 95% CI ‐6.30 to ‐4.34; I2 = 71%; 4 studies; 1156 participants) but this comparison included some unconventional treatments such as amniotic membrane and potato peel. There is moderate certainty evidence that honey probably also increases the likelihood of wounds healing over time compared to unconventional anti‐bacterial treatments (HR 2.86, 95% C 1.60 to 5.11; I2 = 50%; 2 studies; 154 participants).
There is moderate certainty evidence that, on average, burns treated with nanocrystalline silver dressings probably have a slightly shorter mean time to healing than those treated with Vaseline gauze (difference in means ‐3.49 days, 95% CI ‐4.46 to ‐2.52; I2 = 0%; 2 studies, 204 participants), but low certainty evidence that there may be little or no difference in numbers of healing events at 14 days between burns treated with silver xenograft or paraffin gauze (RR 1.13, 95% CI 0.59 to 2.16 1 study; 32 participants). Other comparisons represented low or very low certainty evidence.
It is uncertain whether infection rates in burns treated with either silver‐based antiseptics or honey differ compared with non‐antimicrobial treatments (very low certainty evidence). There is probably no difference in infection rates between an iodine‐based treatment compared with moist exposed burn ointment (moderate certainty evidence). It is also uncertain whether infection rates differ for SSD plus cerium nitrate, compared with SSD alone (low certainty evidence).
Mortality was low where reported. Most comparisons provided low certainty evidence that there may be little or no difference between many treatments. There may be fewer deaths in groups treated with cerium nitrate plus SSD compared with SSD alone (RR 0.22, 95% CI 0.05 to 0.99; I2 = 0%, 2 studies, 214 participants) (low certainty evidence).
Authors' conclusions
It was often uncertain whether antiseptics were associated with any difference in healing, infections, or other outcomes. Where there is moderate or high certainty evidence, decision makers need to consider the applicability of the evidence from the comparison to their patients. Reporting was poor, to the extent that we are not confident that most trials are free from risk of bias.
Plain language summary
Antiseptics for Burns
Review question
We reviewed the evidence about whether antiseptics are safe and effective for treating burn wounds.
Background
Burn wounds cause many injuries and deaths worldwide. People with burn wounds are especially vulnerable to infections. Antiseptics prevent the growth of micro‐organisms such as bacteria. They can be applied to burn wounds in dressings or washes, which may help to prevent infection and encourage wound healing. We wanted to find out if antiseptics are more effective than other types of treatment, or whether one antiseptic may be more effective than others, in reducing infection and speeding up healing.
Study characteristics
In September 2016 we searched for randomised controlled trials (RCTs) involving antiseptic treatments for burn wounds. We included 56 studies with 5807 participants. Most participants were adults with recent second‐degree burns taking up less than 40% of their total body surface area. The antiseptics used included: silver‐based, honey, iodine‐based, chlorhexidine or polyhexanide (biguanides). Most studies compared antiseptics with a topical antibiotic (applied to the skin). A smaller number of studies compared antiseptics with a non‐antibacterial treatment, or with another antiseptic.
Key results
The majority of studies compared antiseptic treatments with silver sulfadiazine (SSD), a topical antibiotic used commonly in the treatment of burns. There is low certainty evidence that some antiseptics may speed up average times to healing compared with SSD. There is also moderate certainty evidence that burns treated with honey probably heal more quickly compared with those treated with topical antibiotics. Most other comparisons did not show a clear difference between antiseptics and antibiotics.
There is evidence that burns treated with honey heal more quickly (high certainty evidence) and are more likely to heal (moderate certainty evidence) compared with those given a range of non‐antibacterial treatments, some of which were unconventional. Burns treated with antiseptics such as nanocrystalline silver or merbromin may heal more quickly on average than those treated with Vaseline gauze or other non‐antibacterial treatments (moderate or low certainty evidence). Comparisons of two different antiseptics were limited but average time to healing may be slightly quicker for wounds treated with povidone iodine compared with chlorhexidine (low certainty evidence). Few participants in the studies experienced serious side effects, but this was not always reported. The results do not allow us to be certain about differences in infection rates. Mortality was low where reported.
Quality of the evidence
Most studies were not well reported and this makes it difficult to be sure if they were at risk of bias. In many cases a single (often small) study provides all the evidence for the comparative effects of the different treatments; and some similar studies provided conflicting results. Where there is moderate or high certainty evidence clinicians will need to consider whether the evidence from the comparison is relevant to their patients.
This plain language summary is up to date as of September 2016.
Summary of findings
Summary of findings for the main comparison. Silver‐based antiseptics versus topical antibiotics.
| Silver‐based antiseptics versus topical antibiotics | ||||||
|
Patient or population: people with burns Intervention: silver‐based antiseptics (primarily dressings) Comparison: topical antibiotics (SSD) Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with SSD | Risk with silver dressings | |||||
| Wound healing: time to complete healing (time‐to‐event data) | 739 per 1000 | 813 per 1000 (717 to 894) | HR 1.25 (0.94 to 1.67) | 259 (3 RCTs) | ⊕⊕⊝⊝ Low1 | Only three studies provided sufficient data for an HR; this showed that, on average, there is no clear difference in the 'chance' of healing in burns treated with silver‐based antiseptic dressings compared with SSD. HR calculated using standard methods for two trials |
| Risk difference: 74 more burns healed per 1000 with silver dressings than with SSD (22 fewer to 155 more) | ||||||
| Wound healing (mean time to healing) | The mean time to wound healing was 11.92 days | The mean time to wound healing in the intervention group was 3.33 days shorter (4.96 fewer to 1.70 fewer) | MD ‐3.33 days (‐4.96 to ‐1.70) | 1085 (10 RCTs) | ⊕⊕⊝⊝ Low2 | Silver may, on average, slightly improve mean time to healing compared with SSD |
| Wound healing (number of healing events) | 784 per 1000 | 917 per 1000 (784 to 1000) | RR 1.17 (1.00 to 1.37) | 408 (5 RCTs) |
⊕⊕⊝⊝ Low3 | There may be little difference in the number of healing events over short‐term follow‐up (up to 28 days) compared with SSD |
| Risk difference: 133 more burns healed per 1000 with silver dressings than with SSD (0 more to 290 more) | ||||||
| Infection | 151 per 1000 | 127 per 1000 (72 to 222) | RR 0.84 (0.48 to 1.49) | 309 (4 RCTs) | ⊕⊝⊝⊝ Very low4 | It is uncertain whether silver‐containing antiseptics increase or reduce the risk of infection compared with use of SSD as evidence is very low certainty |
| Risk difference: 24 fewer participants with adverse events per 1000 with silver dressings than with SSD (78 fewer to 71 more) | ||||||
| Adverse events | 227 per 1000 | 195 per 1000 (141 to 263) | RR 0.86 (0.63 to 1.18) | 440 (6 RCTs) | ⊕⊕⊝⊝ Low5 | There may be little or no difference in the number of adverse events in participants treated with silver dressings compared with SSD |
| Risk difference: 34 fewer participants with adverse events per 1000 with silver dressings than with SSD (86 fewer to 29 more). | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; MD: mean difference; RR: risk ratio; SSD: silver sulfadiazine | ||||||
| GRADE Working Group grades of evidence High: it is very likely that the effect will be close to what was found in the research. Moderate: it is likely that the effect will be close to what was found in the research, but there is a possibility that it will be substantially different. Low: it is likely that the effect will be substantially different from what was found in the research, but the research provides an indication of what might be expected. Very low: the anticipated effect is very uncertain and the research does not provide a reliable indication of what might be expected. | ||||||
1Not downgraded for risk of selection bias and detection bias because most participants were in a study at low risk of bias; downgraded twice for serious imprecision due to low numbers of participants and wide confidence intervals. 2Downgraded once for high risks of bias across varying domains (variously detection, selection, reporting and other sources of bias in 5 trials representing 31% of the analysis weight); downgraded once for inconsistency (I2 = 78%). A post‐hoc sensitivity analysis excluding studies with unit of analysis issues or intra‐individual designs did not materially effect result. 3Downgraded once due to risk of detection bias in two studies and selection bias in one study (representing in total 53% of the analysis weight); and once due to imprecision. 4Downgraded once for high risks of bias across varying domains (detection, selection and reporting bias affecting 51% of the analysis weight across 3 of 4 studies); downgraded once for indirectness from largest trial outcome (49% analysis weight), which related to inflammation and once due to imprecision. 5Downgraded once for high risks of detection bias affecting 2 studies contributing 93% of analysis weight; downgraded once for imprecision. Studies with intra‐individual design or unit of analysis issue contributed no weight to analysis due to zero events.
Summary of findings 2. Honey versus topical antibiotics.
| Honey versus topical antibiotics | ||||||
|
Patient or population: people with burns Intervention: honey Comparison: topical antibiotics (SSD or mafenide acetate) Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with topical antibiotics | Risk with honey | |||||
| Wound healing: time to complete healing (time‐to‐event data): honey versus SSD or mafenide acetate | 641 per 1000 | 919 per 1000 (827 to 973) | HR 2.45 (1.71 to 3.52) | 580 (5 RCTs) | ⊕⊕⊕⊝ Moderate1 | Burns treated with honey probably have a greater 'chance' of healing compared with SSD or mafenide acetate. HR calculated using standard methods for all trials |
| Risk difference: 278 more burns healed per 1000 with honey than with topical antibiotics (185 more to 332 more). | ||||||
| Wound healing (mean time to healing): honey versus SSD | The mean time to wound healing was 15.53 days | The mean time to wound healing was 3.79 days fewer (7.15 fewer to 0.43 fewer) | MD ‐3.79 (‐7.15 to ‐0.43) | 712 (6 RCTs) | ⊕⊝⊝⊝ Very low2 |
It is uncertain what the effect of honey is on mean time to wound healing compared with SSD |
| Wound healing (number of healing events): honey versus SSD | 434 per 1000 | 946 per 1000 (499 to 1000) | RR 2.18 (1.15 to 4.13) | 318 (4 RCTs) |
⊕⊕⊝⊝ Low3 | There may, on average, be more healing events in burns treated with honey compared with SSD over short‐term follow‐up (maximum 21 days) |
| Risk difference: 512 more burns healed per 1000 with honey than with SSD (65 more to 1358 more) | ||||||
| Incident infection: honey versus SSD or mafenide acetate | 135 per 1000 | 22 per 1000 (11 to 158) | RR 0.16 (0.08 to 0.34) | 480 (4 RCTs) | ⊕⊝⊝⊝ Very low4 |
It is uncertain if fewer burns treated with honey may become infected compared with those treated with SSD or mafenide acetate |
| Risk difference: 113 fewer infections (positive swabs in 3 RCTs) per 1000 with honey compared with topical antibiotics (124 fewer to 89 fewer) | ||||||
| Peristent infection: honey versus SSD | 964 per 1000 | 98 per 1000 (48 to 183) | RR 0.10 (0.05 to 0.19) | 170 (2 RCTs) |
||
| Risk difference: 867 fewer persistently positive swabs per 1000 with honey compared with topical antibiotics (961 to 781) | ||||||
| Adverse events: honey versus SSD | 16 per 1000 | 3 per 1000 (0 to 64) | RR 0.20 (0.01 to 3.97) | 250 (3 RCTs) | ⊕⊝⊝⊝ Very low5 | It is uncertain whether fewer participants treated with honey experience adverse events compared with those treated with SSD |
| Risk difference: 13 fewer participants with adverse events per 1000 with honey compared with SSD (16 fewer to 48 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; MD: mean difference; RR: risk ratio; SSD: silver sulfadiazine | ||||||
| GRADE Working Group grades of evidence High: it is very likely that the effect will be close to what was found in the research. Moderate: it is likely that the effect will be close to what was found in the research, but there is a possibility that it will be substantially different. Low: it is likely that the effect will be substantially different from what was found in the research, but the research provides an indication of what might be expected. Very low: the anticipated effect is very uncertain and the research does not provide a reliable indication of what might be expected. | ||||||
1Downgraded once for imprecision. A post‐hoc sensitivity analysis excluding a study with an intra‐individual design made no material difference to the analysis. 2Downgraded twice for imprecision and once for inconsistency; the downgrading for imprecision is based on the post‐hoc sensitivity analysis excluding a trial with an intra‐individual design. This is a conservative approach to the inclusion of this data. The result of the sensitivity analysis was to produce confidence intervals which included the possibility of both harm and benefit (MD ‐4.36; 95% CI ‐8.90 to 0.16). 3Downgraded once for imprecision and once for inconsistency. 4Downgraded twice for indirectness as the relationship between the surrogate outcome of positive swabs and clinical infection (used in all except one trial) is unclear, and once for imprecision due to low numbers of events. 5Downgraded once because of risks of detection bias in the trial which contributes all the weight in the analysis, and twice because of imprecision.
Summary of findings 3. Aloe vera versus topical antibiotics.
| Aloe Vera versus topical antibiotics | ||||||
|
Patient or population: people with burns Intervention: Aloe Vera Comparison: topical antibiotics (SSD or framycetin) Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with topical antibiotics | Risk with Aloe Vera | |||||
| Wound healing (number of healing events): Aloe Vera versus SSD | 389 per 1000 | 548 per 1000 (272 to 1000) | RR 1.41 (0.70 to 2.85) | 38 (1 RCT) | ⊕⊕⊝⊝ Low1 | It is unclear whether Aloe Vera may alter the number of healing events compared with SSD; confidence intervals are wide, spanning both benefits and harms so clear differences between treatments are not apparent |
| Risk difference: 159 more burns healed per 1000 with Aloe Vera than with SSD (117 fewer to 719 more) | ||||||
| Wound healing (mean time to healing): Aloe Vera versus SSD or framycetin | The mean time to wound healing was 21.25 days | The mean time to wound healing was 7.79 days shorter (17.96 shorter to 2.38 longer) | MD ‐7.79 (‐17.96 to 2.38) | 210 (3 RCTs) | ⊕⊝⊝⊝ Very low2 | It is uncertain whether there is a difference in mean time to healing between Aloe Vera and SSD or framycetin. No data were contributed by the trial using framycetin |
| Infection: Aloe Vera versus SSD | 36 per 1000 | 34 per 1000 (9 to 121) | RR 0.93 (0.26 to 3.34) | 221 (3 RCTs) | ⊕⊝⊝⊝ Very low3 | It is uncertain whether there is a difference in infection incidence between Aloe Vera and SSD |
| Risk difference: 3 fewer infections per 1000 with Aloe Vera than with SSD (27 fewer to 85 more) | ||||||
| Adverse events | No trial reported evaluable adverse event data for this comparison | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RR: Risk ratio; SSD: silver sulfadiazine | ||||||
| GRADE Working Group grades of evidence High: it is very likely that the effect will be close to what was found in the research. Moderate: it is likely that the effect will be close to what was found in the research, but there is a possibility that it will be substantially different. Low: it is likely that the effect will be substantially different from what was found in the research, but the research provides an indication of what might be expected. Very low: the anticipated effect is very uncertain and the research does not provide a reliable indication of what might be expected. | ||||||
1Downgraded twice for very serious imprecision. 2Downgraded once for risk of detection bias in a trial accounting for 47% of the analysis weight; once for inconsistency (I2 = 94%) and twice for imprecision. A post‐hoc sensitivity analysis excluding the trial with the intra‐individual design did not materially affect the result of the analysis. 3Downgraded once for risk of detection bias in a trial accounting for 84% of the analysis weight, and twice for imprecision. A post‐hoc sensitivity analysis excluding the trial with the intra‐individual design did not materially affect the result of the analysis.
Summary of findings 4. Iodine versus topical antibiotics.
| Iodine versus topical antibiotics | ||||||
|
Patient or population: people with burns Intervention: iodine‐based treatments Comparison: topical antibiotics (SSD) Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with topical antibiotics | Risk with iodine‐based treatments | |||||
| Wound healing (mean time to healing) | The mean time to wound healing was 20.07 days | The mean time to wound healing in the intervention group was 0.47 days shorter (2.76 shorter to 1.83 longer) | MD ‐0.47 (‐2.76 to 1.83) | 148 (2 RCTs) | ⊕⊝⊝⊝ Very low1 | It is uncertain whether there is a difference in mean time to wound healing between iodine‐based antiseptic treatments and SSD |
| Infection | No study reported evaluable data for infection | |||||
| Adverse events | 350 per 1000 | 301 per 1000 (122 to 735) | RR 0.86 (0.35 to 2.10) | 40 (1 RCT) | ⊕⊝⊝⊝ Very low2 | It is uncertain whether there is a difference in the proportion of participants with adverse events between iodine‐based antiseptic treatments and SSD |
| Risk difference: 48 fewer participants with adverse events per 1000 with iodine‐based treatments than with SSD (227 fewer to 385 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; SSD: silver sulfadiazine | ||||||
| GRADE Working Group grades of evidence High: it is very likely that the effect will be close to what was found in the research. Moderate: it is likely that the effect will be close to what was found in the research, but there is a possibility that it will be substantially different. Low: it is likely that the effect will be substantially different from what was found in the research, but the research provides an indication of what might be expected. Very low: the anticipated effect is very uncertain and the research does not provide a reliable indication of what might be expected. | ||||||
1Downgraded once for detection bias in one trial accounting for 61% of the analysis weight and twice for imprecision due to low participant numbers and confidence intervals that cross the line of no effect; one study also had an intra‐individual design, which may not have been accounted for in the analysis, this is taken account of in the double downgrading for imprecision. 2Downgraded once for detection bias in the single trial and twice for imprecision due to fragile confidence intervals, which cross the line of no effect.
Summary of findings 5. Silver versus non‐antibacterial.
| Silver versus non‐antibacterial | ||||||
|
Patient or population: people with burns Intervention: silver‐based interventions (dressings) Comparison: non‐antibacterial treatments (dressings and topical treatments) Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐antibacterial dressing | Risk with silver dressing | |||||
| Wound healing (number of healing events): silver xenograft vs petroleum gauze | 500 per 1000 | 565 per 1000 (295 to 1000) | RR 1.13 (0.59 to 2.16) | 32 (1 RCT) | ⊕⊕⊝⊝ Low1 | There may be little or no difference between silver xenograft and petroleum gauze |
| Risk difference: 65 more burns healed per 1000 with silver xenograft compared with petroleum gauze (205 fewer to 580 more) | ||||||
| Wound healing (mean time to healing): silver nanoparticle vs Vaseline gauze | The mean time to wound healing was 15.87 days | The mean time to wound healing in the silver nanoparticle group was 3.49 days shorter (4.46 shorter to 2.52 shorter) compared with gauze | MD ‐3.49 (‐4.46 to ‐2.52) | 204 (2 RCTs) | ⊕⊕⊕⊝ Moderate2 | The mean time to wound healing is probably slightly shorter in the group treated with silver nanoparticle dressing compared with Vaseline gauze |
| Infection | No study reported evaluable data for this comparison | |||||
| Adverse events | No study reported evaluable data for this comparison | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High: it is very likely that the effect will be close to what was found in the research. Moderate: it is likely that the effect will be close to what was found in the research, but there is a possibility that it will be substantially different. Low: it is likely that the effect will be substantially different from what was found in the research, but the research provides an indication of what might be expected. Very low: the anticipated effect is very uncertain and the research does not provide a reliable indication of what might be expected. | ||||||
1Downgraded twice for imprecision as fragile confidence intervals cross the line of no effect. 2Downgraded once for imprecision due to low numbers of participants.
Summary of findings 6. Honey versus non‐antibacterial.
| Honey versus non‐antibacterial | ||||||
|
Patient or population: people with burns Intervention: honey Comparison: non‐antibacterial treatments (dressings and topical treatments) Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐antibacterial dressing | Risk with honey | |||||
| Wound healing: time to complete healing (time‐to‐event data) | 1000 per 1000 | 1000 per 1000 (1000 to 1000) | HR 2.86 (1.60 to 5.11) | 164 (2 RCTs) | ⊕⊕⊕⊝ Moderate1 | The 'chance' of healing is probably somewhat greater in participants treated with honey compared with unconventional non‐antibacterial treatments |
| Risk difference: 0 difference burns healed per 1000 with honey compared with non‐antibacterial treatments (0 to 0) | ||||||
| Wound healing (mean time to healing) | The mean time to wound healing was 14.05 days | The mean time to wound healing in the intervention group was 5.32 days shorter (6.30 shorter to 4.34 shorter) | MD ‐5.32 (‐6.30 to ‐4.34) | 1156 (4 RCTs) | ⊕⊕⊕⊕ High | Participants treated with honey, on average, have a shorter mean time to healing compared with those treated with a range of treatments without antibacterial properties, including unconventional treatments |
| Infection (incident) | 370 per 1000 | 174 per 1000 (55 to 371) | RR 0.47 (0.23 to 0.98) | 92 (1 RCT) | ⊕⊝⊝⊝ Very low2 | It is uncertain whether there is a difference in the incidence or persistence of wound infection in participants treated with honey compared with a range of treatments without antimicrobial properties |
| Risk difference: 196 fewer incident infections (persistently positive swabs) per 1000 with honey compared with non‐antibacterial treatments (285 fewer to 7 fewer) | ||||||
| Infection (persistent) | 768 per 1000 | 115 per 1000 | RR 0.15 (0.06 to 0.40) | 147 of 164 randomised (2 RCTs) |
⊕⊝⊝⊝ Very low2 | |
| Risk difference: 653 fewer persistent infections (persistently positive swabs) per 1000 with honey compared with non‐antibacterial treatments (722 fewer to 461 fewer) | ||||||
| Adverse events | One study reported that there were no events in either intervention group; other studies did not report data that clearly related to the number of participants who experienced adverse events in each group | 239 (3 RCTs) |
⊕⊝⊝⊝ Very low3 | It is uncertain whether there is a difference in the incidence of adverse effects between participants treated with honey and those treated with a range of alternative non‐antimicrobial therapies | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High: it is very likely that the effect will be close to what was found in the research. Moderate: it is likely that the effect will be close to what was found in the research, but there is a possibility that it will be substantially different. Low: it is likely that the effect will be substantially different from what was found in the research, but the research provides an indication of what might be expected. Very low: the anticipated effect is very uncertain and the research does not provide a reliable indication of what might be expected. | ||||||
1Downgraded once for imprecision due to low numbers of participants. 2Downgraded twice for indirectness as swabs are a very surrogate measure of clinical infection and once for imprecision due to low numbers of participants 3Downgraded twice for imprecision and once for indirectness due to low numbers of events and participants and poor reporting of data with uncertainty around applicability to inclusion criteria.
Summary of findings 7. Chlorhexidine versus non‐antibacterial.
| Chlorhexidine versus non‐antibacterial | ||||||
|
Patient or population: people with burns Intervention: chlorhexidine Comparison: non‐antibacterial treatments (dressings) Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐antibacterial dressing | Risk with biguanides | |||||
| Wound healing: time to complete healing (time‐to‐event data): chlorhexidine versus polyurethane | 1000 per 1000 | 1000 per 1000 (1000 to 1000) | HR 0.71 (0.39 to 1.29) | 51 (1 RCT) | ⊕⊕⊝⊝ Low1 | There may be some difference in the 'chance' of healing for chlorhexidine compared with polyurethane but CIs span benefit and harm so a clear difference between treatments is not apparent |
| Risk Difference: 0 difference per 1000 for chlorhexidine compared with polyurethane (0 to 0) | ||||||
| Wound healing (mean time to healing): chlorhexidine versus non‐antibacterial | The mean time to wound healing ‐ chlorhexidine versus polyurethane was 10 days | The mean time to wound healing ‐ chlorhexidine versus polyurethane in the intervention group was 4.08 days longer (0.73 longer to 7.43 longer) | MD 4.08 (0.73 to 7.43) | 51
(1 RCT) 153 participants in 2 RCTs did not have evaluable data |
⊕⊕⊝⊝ Low2 | The mean time to wound healing may be slightly longer in burns treated with chlorhexidine compared with polyurethane; data from 2 additional RCTs comparing chlorhexidine with hydrocolloid lacked measures of variance |
| Infection: chlorhexidine versus no antimicrobial/no additional antimicrobial | 179 per 1000 | 184 per 1000 (86 to 396) | RR 1.11 (0.54 to 2.27) | 172 (2 RCT) | ⊕⊝⊝⊝ Very low3 | It is uncertain whether there is a difference in the incidence of infection between participants treated with chlorhexidine either alone or in addition to SSD and participants treated with no antimicrobial or SSD alone |
| Risk Difference: 15 more infections per 1000 with chlorhexidine compared with non‐antibacterial treatments (64 fewer to 178 more) | ||||||
| Adverse events: chlorhexidine versus hydrocolloid | 102 per 1000 | 20 per 1000 (2 to 168) | RR 0.20 (0.02 to 1.65) | 98 (1 RCT) | ⊕⊝⊝⊝ Very low4 | It is uncertain whether there is a difference in the number of participants with adverse effects between chlorhexidine and a hydrocolloid dressing |
| Risk Difference: 82 fewer participants with adverse events with chlorhexidine compared with hydrocolloid (100 fewer to 66 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High: It is very likely that the effect will be close to what was found in the research. Moderate: It is likely that the effect will be close to what was found in the research, but there is a possibility that it will be substantially different. Low: It is likely that the effect will be substantially different from what was found in the research, but the research provides an indication of what might be expected. Very low: The anticipated effect is very uncertain and the research does not provide a reliable indication of what might be expected. | ||||||
1Downgraded twice for imprecision due to wide confidence intervals, which cross the line of no effect, and fragility due to small numbers of participants. 2Downgraded twice for imprecision due to wide confidence intervals, which cross the line of no effect, and fragility due to small numbers of participants. The study with unit of analysis issues did not contribute to the analysis. 3Downgraded once due to risk of detection bias and once due to attrition bias in a trial with 90% of the analysis weight and twice due to imprecision. 4Downgraded once due to risk of detection bias and once due to attrition bias in the single trial; downgraded once for imprecision as confidence intervals cross line of no effect.
Summary of findings 8. Iodine versus non‐antibacterial.
| Iodine‐based treatments versus non‐antibacterial | ||||||
|
Patient or population: people with burns Intervention: iodine‐based treatments Comparison: non‐antibacterial treatments (dressings and topical treatments) Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐antibacterial treatments | Risk with iodine‐based treatments | |||||
| Wound healing (number of healing events): iodophor versus hydrogel | 700 per 1000 | 119 per 1000 (56 to 238) | RR 0.17 (0.08 to 0.34) | 120 (1 RCT) | ⊕⊕⊝⊝ Low1 | There may be a smaller number of healing events at 26 days in participants treated with iodophor compared with those treated with hydrogel |
| Risk difference: 581 fewer wounds healed per 1000 at 14 days with iodophor treatment compared with hydrogel (644 fewer to 462 fewer) | ||||||
| Wound healing (mean time to healing): iodine gauze versus carbon fibre | The mean time to wound healing) ‐ iodine gauze versus carbon fibre was 15.29 days | The mean time to wound healing) ‐ iodine gauze versus carbon fibre in the intervention group was 5.38 days longer (3.09 longer to 7.67 longer) | MD 5.38 (3.09 to 7.67) | 277 (1 RCT) | ⊕⊝⊝⊝ Very low2 | The clinical heterogeneity between these studies, both in terms of interventions and comparators, combined with the wide divergence in effects meant that they could not meaningfully be pooled. It is very uncertain what the effect of iodine compared with non‐antibacterial dressings/topical treatments is on mean time to wound healing |
| Wound healing (mean time to healing): iodophor versus MEBO | The mean time to wound healing) ‐ iodophor versus MEBO was 57 days | The mean time to wound healing) ‐ iodophor versus MEBO in the intervention group was 26 days shorter (30.48 shorter to 21.52 shorter) | MD ‐26.00 (‐30.48 to ‐21.52) | 55 (1 RCT) | ||
| Infection: iodine gauze versus MEBO | 58 per 1000 | 75 per 1000 (27 to 208) | RR 1.30 (0.47 to 3.61) | 211 (1 RCT) | ⊕⊕⊝⊝ Low3 | There may be little or no difference in the incidence of infection in participants treated with iodine gauze compared with those treated with MEBO |
| Risk difference: 17 more infections per 1000 with iodine gauze compared with MEBO (31 fewer to 151 more) | ||||||
| Adverse effects: iodine gauze versus MEBO | 106 per 1000 | 75 per 1000 (32 to 179) | RR 0.71 (0.30 to 1.69) | 211 (1 RCT) | ⊕⊕⊝⊝ Low3 | There may be little or no difference in the incidence of adverse effects in participants treated with iodine gauze compared with those treated with MEBO |
| Risk difference: 31 fewer participants with adverse events with iodine gauze compared with MEBO (74 fewer to 73 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; MEBO: moist exposed burn ointment; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High: it is very likely that the effect will be close to what was found in the research. Moderate: it is likely that the effect will be close to what was found in the research, but there is a possibility that it will be substantially different. Low: it is likely that the effect will be substantially different from what was found in the research, but the research provides an indication of what might be expected. Very low: the anticipated effect is very uncertain and the research does not provide a reliable indication of what might be expected | ||||||
1Downgraded twice for imprecision due to wide confidence intervals and fragility due to small numbers of participants and uncertainty about the analysis of an intra‐individual design. 2Downgraded twice for inconsistency and once for imprecision; there were different directions of effect in the two trials, which it is unclear can be reliably attributed to differences between the treatments although these were present; small numbers of participants in each trial also resulted in imprecision for individual estimates. 3Downgraded twice for imprecision due to wide confidence intervals, which include the possibility of both benefit and harm for the intervention.
Background
Description of the condition
A burn can be defined as an injury to the skin or other organic tissue caused by thermal trauma (Hendon 2002). Burns are caused by heat (including contact with flames, high temperature solids (contact burns) and liquids (scalds)), chemicals, electricity, friction or abrasion, and radiation (including sunburn and radioactivity). Respiratory damage, as a consequence of smoke inhalation, is also considered a type of burn (Hendon 2002).
Incidence and impact
Burn injuries are a considerable source of morbidity and mortality (Mock 2008). As outlined by the World Health Organization (WHO), the burden of injury falls predominantly on people living in low‐ and middle‐income countries; over 95% of the 300,000 annual deaths from fires occur in these countries (Mock 2008). Total burn mortality is inversely correlated with both national income and income inequality (Peck 2013). The much greater number of injuries resulting in disability and disfigurement are also disproportionately concentrated in low‐ and middle‐ income countries (Mock 2008). Fire‐related burns have been estimated to account for 10 million lost disability‐adjusted life years (DALYs) every year (WHO 2002), a figure that does not include the social and personal impact of non‐disabling disfigurement.
Additional mortality and morbidity are caused by other types of burns including scalding, and electrical and chemical burns (American Burn Association 2013). Globally, children and young people, and women are disproportionately affected by burn injuries, while the types and causes of injury in children differ somewhat from those seen in adults (Peck 2012).
Although, both incidence of burns and associated morbidity and mortality are much lower in high‐income countries, they are nevertheless significant. Annually in the UK around 250,000 people suffer a burn; 175,000 attend a hospital emergency department with a burn and, of these, approximately 13,000 are admitted to hospital and 300 die (National Burn Care Review 2001). In the USA, the figures for those receiving medical treatment were 450,000 with 40,000 hospitalisations and 3400 deaths (American Burn Association 2013). These data indicated that, in contrast to the global pattern, a majority of people with burns were male (69%), and while children aged under five years accounted for 20% of all cases, 12% were people aged 60 years or older (American Burn Association 2013).
Burn severity and extent
The severity of burns is categorised by the depth of the tissues affected; in the case of burns to the skin, this is the layers of cells in the skin (Demling 2005). Epidermal burns (sometimes known as first‐degree burns) are confined to the epidermis (outer surface of the skin), are not usually significant injuries, and heal rapidly and spontaneously. Partial‐thickness burns (sometimes known as second‐degree burns) involve varying amounts of the dermis (skin) and may become deeper and heal with varying amounts of scarring, which will be determined partly by the depth of the burn. Partial‐thickness burns are divided into superficial and deep partial‐thickness wounds: superficial partial‐thickness burns extend into the papillary or superficial upper layer of the dermis, whilst deep partial‐thickness burns extend downward into the reticular (lower) layer of the dermis. Full‐thickness burns (sometimes known as third‐degree burns) extend through all the layers of the skin. Where full‐thickness burns extend beneath the skin layers, into underlying structures (fat, muscle, bone); they are sometimes called full‐thickness and/or fourth‐degree burns) (Demling 2005; European Practice Guidelines 2002).
The age of people with burns affects their prognosis, with infants and older people having poorer outcomes (Alp 2012; DeSanti 2005). The area of a burn will also be key to the time taken to heal, and also to the risk of infection (Alp 2012). Burn size is determined by the percentage of the total body surface area that is burned; estimating this can be difficult, particularly in children; the most accurate method uses the Lund and Browder chart (Hettiaratchy 2004).
The depth of burn and its location may be predictors of psychological, social, and physical functioning following treatment (Baker 1996). Most extensive burns are a mixture of different depths, and burn depth can change and increase in the acute phase after the initial injury; the extent to which this occurs will depend on the effectiveness of the initial treatment (resuscitation) (Hettiaratchy 2004).
Burn wound infection
Infections are a potentially serious complication in people with burns. US data indicated that over a 10‐year period more than 19,000 complications in people with burns were reported. While 31% of these were recorded as pulmonary complications, 17% were wound infections, or cellulitis, or both, and 15% were recorded as septicaemia (a serious, life‐threatening illness caused by bacteria in the bloodstream) or other infectious complications (Latenser 2007). We were unable to locate other large‐scale international data for infection‐related complication rates.
Up to 75% of all burn deaths following initial resuscitation are a consequence of infection rather than more proximal causes such as osmotic shock and hypovolaemia (types of changes in the concentration of fluids in the body) (Bang 2002; Fitzwater 2003). Although this figure includes other types of hospital/healthcare‐acquired infections such as pneumonia, a substantial proportion follow an infection which would meet accepted criteria for infections of burn wounds (Alp 2012; Peck 1998). Burn wound infections also contribute to morbidity, lengthening recovery times, and increasing the extent of scarring (Church 2006; Oncul 2009), as well as the pain experienced by people with burns (Tengvall 2006).
All open wounds offer an ideal environment for microbial colonisation. Most wounds will contain some micro‐organisms but this will not necessarily lead to adverse events (AWMA 2011). Recently the view has developed that it is infection with sufficient or specific types of pathogenic micro‐organisms, or both, and possibly resulting biofilms (Percival 2004; Wolcott 2008) that may lead to negative outcomes and, potentially, delayed healing (Bowler 2003; Davies 2007; Madsen 1996; Trengove 1996). Biofilms are formed by bacteria that grow on a surface to form a film of cells. Growing in this way can make them more resistant to bactericidal agents. Previously it was thought that the critical factor was a threshold concentration of microbes (bioburden) (Robson 1968). However, the impact of microbial colonisation on wound healing is not independent of the host response. The ability of the host to provide adequate immune response is likely to be as critical, if not more so, in determining whether a wound becomes infected as the specifics of the flora in the wound.
People with burns have a particular vulnerability to infection, as a result of the loss of the physical barrier to infection, and the reduction in immunity mediated by the lost cells (Ninnemann 1982; Winkelstein 1984). Infections commonly occur in the acute period following the burn (Church 2006).
The spectrum of infective agents that can be present in the burn wounds varies. Nowadays, Gram‐positive bacteria such as Staphylococcus aureus (S. aureus), and Gram‐negative bacteria such as Pseudomona aeruginosa (P. aeruginosa) are the predominant pathogens (Wibbenmeyer 2006), although other micro‐organisms such as fungi, yeasts, and viruses can also be present (Church 2006; Polavarapu 2008). Multidrug‐resistant micro‐organisms, such as methicillin‐resistant S. aureus (MRSA), are frequently and increasingly identified in burns (Church 2006; DeSanti 2005; Keen 2010).
Description of the intervention
Standard care
The care for burn wounds is determined in part by their severity (depth), area, and location (National Network for Burn Care 2012). For significant injuries involving the lower layers of skin, standard care may involve a range of dressings or skin substitutes, or both, (Wasiak 2013) and more complex interventions such as hyperbaric oxygen therapy and negative pressure wound therapy (Dumville 2012; Villanueva 2004). The nature and extent of the burn wound, together with the type and amount of colonising micro‐organisms can also influence the risk of invasive infection (Bang 2002; Fitzwater 2003).
Antiseptics
Antiseptics are topical antimicrobial agents which are thought to prevent the growth of pathogenic micro‐organisms without damaging living tissue (Macpherson 2004). Applications broadly fall into two categories: lotions used for wound irrigation or cleaning, or both, with a brief contact time (unless used as a pack/soak), and products that are in prolonged contact with the wound such as creams, ointments, and impregnated dressings (BNF 2016).
Agents used primarily for wound irrigation/cleaning across wound types are commonly based on povidone‐iodine, chlorhexidine and peroxide agents. Less commonly used are traditional agents such as gentian violet and hypochlorites. Longer contact creams and ointments include fusidic acid, mupirocin, neomycin sulphate and iodine (often as cadexomer iodine). Some of these are rarely used in clinical practice. Silver‐based products such as silver sulfadiazine and silver‐impregnated dressings are increasingly used, as are honey‐based products. Aloe Vera is also sometimes used as an antiseptic although there is currently no available sterile source.
The British National Formulary (BNF) categorises antimicrobial dressings under honey‐based, iodine‐based, silver‐based, and other, which includes dressings impregnated with agents such as chlorhexidine or peroxides (BNF 2016). The choice of dressing for a burn wound is based on a number of factors including the need to accommodate movement, the minimisation of adherence to the wound surface, the prevention of infection, the ability to absorb wound fluid and maintain humidity, and the active promotion of healing (Wasiak 2013).
Antibiotics are substances that destroy or inhibit the growth of bacteria (Macpherson 2004) (normally by inhibiting deoxyribonucleic acid (DNA), protein synthesis or by disrupting the bacterial cell wall). Routine prophylaxis against infection with systemic antibiotics is not currently recommended. While it may reduce burn wound infections, or colonisation, or both, it does not decrease mortality, and may in fact increase the risk of selecting resistant micro‐organisms such as MRSA (Avni 2010; Barajas‐Nava 2013)
In contrast, antiseptics (the focus of this review) can be bactericidal (in that they kill micro‐organisms) or they can work by slowing the growth of organisms (bacteriostatic) (Macpherson 2004), but they usually work without damaging living tissue. Antiseptics can reduce the presence of other micro‐organisms such as viruses and fungi, as well as bacteria, and often work by damaging the surface of microbes (Macpherson 2004). According to the BNF (BNF 2016) antiseptics are used to reduce the presence of micro‐organisms on living tissue.
How the intervention might work
This review considers the use of antiseptics for both clinically infected and non‐infected burn wounds. The rationale for treating clinically infected wounds with antiseptic agents is to kill or slow the growth of the pathogenic micro‐organisms, thus preventing an infection from worsening and spreading (Kingsley 2004). In the case of burns, the prevention of infections, and systemic infections in particular, is especially important, as people with burns can have lowered immunity as a consequence of their injury (Church 2006). Improved healing may also result, although evidence on the association between wound healing and infection is limited (Jull 2015; O'Meara 2001; Storm‐Versloot 2010).
There is a widely held view that wounds that do not have clear signs of clinical infection, but that have characteristics such as retarded healing, may also benefit from a reduction in bacterial load (bioburden). Again, evidence for this is limited (AWMA 2011; Howell‐Jones 2005).
Why it is important to do this review
Burn wounds are a source of substantial morbidity and mortality; much of this results from the original wound becoming infected (Latenser 2007). While infections pose real risks to people with burns, the problem of antibiotic and multi‐drug resistance in bacteria continues to grow (Church 2006; DeSanti 2005; Keen 2010); alternatives to routine use of antibiotics for the minimisation of infection can be a key element of care.
There is a current published Cochrane review of antibiotics for the prevention (prophylaxis) of burn wound infection (Barajas‐Nava 2013), while a second Cochrane review of antibiotics for the treatment of infected burn wounds is now underway (Lu 2016). This review of antiseptics complements these reviews and will complete the assessment of evidence for agents with antimicrobial properties in the care of all burn wounds, whether infected or not. There will be some overlap between this review and other Cochrane and non‐Cochrane reviews of dressings for partial‐thickness burns (Wasiak 2013), and of individual agents with antiseptic properties for all types of wounds (Aziz 2012; Jull 2015; Storm‐Versloot 2010). However, this review will provide a single synthesis of the randomised evidence relating to all antiseptics for any type of burn wound.
Objectives
To assess the effects and safety of antiseptics for the treatment of burns in any care setting.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs), including cluster‐RCTs, irrespective of language of report. We planned to only include crossover trials if they reported outcome data at the end of the first treatment period, prior to crossover. We excluded quasi‐randomised studies.
Types of participants
We included studies enrolling participants of any age with burn wounds. We included burns of any type, severity, extent or current infection status, managed in any care setting. We accepted authors' definitions of the category of burn represented in included trials. We included trials of participants with burns, alongside people with other types of wounds where the participants with burns constituted at least 75% of the trial population.
Types of interventions
The interventions of interest were topical antiseptic agents. We included any RCT in which the use of a specific topical antiseptic was the only systematic difference between treatment groups; where the antiseptic agent was an integral part of the dressing we allowed for this. Control regimens could have included placebo, an alternative antiseptic, another therapy such as antibiotics or isolation of the patient, standard care or no treatment. We included studies that evaluated intervention schedules, including other therapies, provided that these treatments were delivered in a standardised way across the trial arms. We excluded trials in which the presence or absence of a specific antiseptic was not the only systematic difference. We also excluded evaluations of antiseptics used to prepare for the surgical treatment of burns (i.e. where antisepsis is part of the perioperative procedure).
We anticipated that likely comparisons would include use of different antiseptic agents, in particular, the use of different types of dressings impregnated with antiseptic agents; comparisons of impregnated dressings or other antiseptic preparations with standard care; and comparison of antiseptics with topical or systemic antibiotics. We anticipated that other elements of standard care may have been co‐interventions across trial arms.
Types of outcome measures
Primary outcomes
The primary effectiveness outcome for this review was wound healing. Trialists use a range of different methods of measuring and reporting this outcome. We considered that RCTs that reported one or more of the following provided the most relevant and rigorous measures of wound healing:
time to complete wound healing (correctly analysed using survival, time‐to‐event approaches). Ideally the outcome would be adjusted for appropriate covariates e.g. baseline wound area/degree/duration;
proportion of wounds completely healed during follow‐up (frequency of complete healing).
We used and reported the study authors’ definitions of complete wound healing where this was available. We reported outcome measures at the latest time point available (assumed to be length of follow‐up if not specified) and the time point specified in the methods as being of primary interest (if this was different from latest time point available).
Where both the outcomes above were reported, we presented all data in a summary outcome table for reference, but focused on reporting time to healing. When time to healing was analysed as a continuous measure, but it was not clear whether all wounds healed, we documented the use of the outcome in the study, but we did not extract, summarise or use the data in any meta‐analysis.
The primary safety outcome for the review was change in wound infection status (as defined by the study authors). In the case of wounds that were considered to be clinically infected at baseline, we assessed resolution of infections. In the case of wounds that were not considered to be clinically infected at baseline, we assessed the incidence of new infections. We also assessed the incidence of septicaemia, where data permitted. We did not extract data on microbiological assays not clearly linked to a diagnosis of infection.
Secondary outcomes
We included the following secondary outcomes:
-
Adverse events
Where reported, we extracted data on all serious adverse events and all non‐serious adverse events. We did not report individual types of adverse events other than pain (see below) or infection (see Primary outcomes).
-
Health‐related quality of life
We included quality of life where it was reported, using a validated scale such as the SF‐36 or EQ‐5D, or a validated disease‐specific questionnaire. Ideally, reported data were adjusted for the baseline score.
-
Pain (including pain at dressing change)
We included pain only where mean scores with a standard deviation were reported using a scale validated for the assessment of pain levels, such as a visual analogue scale (VAS).
-
Resource use (when presented as a mean with standard deviation)
We included measures of resource use such as number of dressing changes, number of nurse visits, length of hospital stay, and need for other interventions.
Costs associated with resource use (including estimates of cost‐effectiveness)
Mortality (overall and infection‐related).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify relevant RCTs:
the Cochrane Wounds Specialised Register (searched 26 September 2016);
the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2016, Issue 8, searched 26 September 2016);
Ovid MEDLINE (1946 to 26 September 2016);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 26 September 2016);
Ovid Embase (1974 to 26 September 2016);
EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 28 September 2016)
The search strategies are shown in Appendix 1.
We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries.
ClinicalTrials.gov (www.clinicaltrials.gov/).
WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch/Default.aspx).
EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses and health technology assessment reports.
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts of the citations retrieved by the searches for relevance. After this initial assessment, we obtained full‐text copies of all studies considered to be potentially relevant. Two review authors independently checked the full papers for eligibility; we resolved disagreements by discussion and, where required, the input of a third review author. We obtained translation support, where necessary, for non‐English language reports. Where the eligibility of a study was unclear, we attempted to contact study authors. We recorded all reasons for exclusion of studies for which we had obtained full copies. We completed a PRISMA flowchart to summarise this process (Liberati 2009).
Where studies were reported in multiple publications/reports, we attempted to obtain all publications. Whilst we included each study only once in the review, we extracted data from all reports to ensure that we obtained all available relevant data.
Data extraction and management
We extracted and summarised details of the eligible studies. Where possible we extracted data by treatment group for the prespecified interventions and outcomes in this review. Two review authors independently extracted data; discrepancies were resolved through discussion or by consultation with a third reviewer. Where data were missing from reports, we attempted to contact the study authors and request this information.
Where we included a study with more than two intervention arms, we only extracted data from intervention and control groups that met the eligibility criteria. Where the reported baseline data related to all participants, rather than to those in relevant treatment arms, we extracted the data for the whole trial and noted this. We collected outcome data for relevant time points as described in the Types of outcome measures.
Where possible, we extracted the following data:
bibliographic data, including date of completion/publication;
country of origin;
unit of randomisation (participant/wound);
unit of analysis;
trial design e.g. parallel, cluster;
care setting;
number of participants randomised to each trial arm and number included in final analysis;
eligibility criteria and key baseline participant data including cause, depth, extent (area/proportion of total body surface area (TBSA)) and location of burns; ages of participants, and whether they had a diagnosis of infection at baseline;
details of treatment regimen received by each group;
duration of treatment;
details of any co‐interventions;
primary and secondary outcome(s) (with definitions and, where applicable, time points);
outcome data for primary and secondary outcomes (by group);
duration of follow‐up;
number of withdrawals (by group) and number of withdrawals (by group) due to adverse events;
publication status of study;
source of funding for trial.
Assessment of risk of bias in included studies
Two review authors independently assessed included studies using the Cochrane tool for assessing risk of bias (Higgins 2011a). This tool addresses specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting and other issues. In this review we recorded issues with unit of analysis, for example where a cluster trial has been undertaken but analysed at the individual level in the study report.
We assessed blinding of outcome assessment and completeness of outcome data for each of the review outcomes separately. We presented our assessment of risk of bias using two 'Risk of bias' summary figures; one is a summary of bias for each item across all studies, and a second shows a cross‐tabulation of each trial by all of the risk of bias items.
We summarised a study’s risk of selection bias, detection bias, attrition bias, reporting bias and other bias. In many of the comparisons included in this review, we anticipated that blinding of participants and personnel may not be possible. For this reason the assessment of the risk of detection bias focused on whether blinded outcome assessment was reported (because wound healing can be a subjective outcome, it can be at high risk of measurement bias when outcome assessment is not blinded). For trials using cluster randomisation, we also planned to consider risk of bias for recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually‐randomised trials (Higgins 2011b) (Appendix 3).
Measures of treatment effect
We reported time‐to‐event data (e.g. time‐to‐complete wound healing) as hazard ratios (HRs) when possible, in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). If studies reporting time‐to‐event data (e.g. time to healing) did not report an HR, then, when feasible, we estimated this using other reported outcomes, such as numbers of events, through the application of available statistical methods (Parmar 1998; Tierney 2007). This included deriving an HR from data reported for multiple time points, where at least three time points were reported. Where no HR could be calculated, we reported dichotomous data at the latest time point. For dichotomous outcomes, we calculated the risk ratio (RR) with 95% confidence intervals (CIs). For continuous outcome data, we used the difference in means (MD) with 95% CIs for trials that used the same assessment scale. When trials used different assessment scales, we used the standardised difference in means (SMD) with 95% CIs.
Unit of analysis issues
Where studies were randomised at the participant level and outcomes measured at the wound level, for example for wound healing, we treated the participant as the unit of analysis when the number of wounds assessed appeared to be equal to the number of participants (e.g. one wound per person).
One unit of analysis issue that we anticipated was that randomisation may have been carried out at the participant level, with the allocated treatment used on multiple wounds per participant (or perhaps only on some participants), but data were presented and analysed per wound (clustered data).
In cases where included studies contained some or all clustered data, we reported this, noting whether data had been (incorrectly) treated as independent. We recorded this as part of the 'Risk of bias' assessment.
We also included studies with the split‐body design where either people with two similar burn wounds were enrolled and each burn wound was randomised to one of the interventions, or where one half of a wound was randomised to one treatment and the other half to a different treatment. These approaches are similar to the 'split‐mouth' approach (Lesaffre 2009). These studies should be analysed using paired data which reflects the reduced variation in evaluating different treatments on the same person. However, it was often not clear whether such analysis had been undertaken. This lack of clarity is noted in the 'Risk of bias' assessment and in the notes in the Characteristics of included studies table
We adopted a pragmatic but conservative post‐hoc approach to analyses including clustered and paired data. We included such studies in meta‐analyses where possible (where unadjusted clustered data would produce too‐narrow CIs and unadjusted paired data too‐wide CIs). We undertook a post‐hoc sensitivity analysis to explore the impact of including data that had been inappropriately unadjusted. Where the sensitivity analysis produced a materially different result to the primary analysis, we used this as the basis for the GRADE assessment and the 'Summary of findings' table. Where we pooled studies with paired data with one other trial, we also reported the results of both trials individually, and where a paired data study was the sole trial reporting outcome data, we noted the issues related to its design. We also noted where these trials were included in meta‐analyses but did not contribute weight to the analyses due to zero events or lack of measures of variance.
Dealing with missing data
It is common to have data missing from trial reports. Excluding participants from the analysis post randomisation, or ignoring participants who are lost to follow‐up compromises the randomisation and potentially introduces bias into the trial. If it was thought that study authors might be able to provide some missing data, we attempted to contact them; however, data are often missing because of loss to follow‐up. In individual studies, when data on the proportion of burns healed were presented, we assumed that randomly‐assigned participants not included in an analysis had an unhealed wound at the end of the follow‐up period (i.e. they were considered in the denominator but not in the numerator). When a trial did not specify participant group numbers before dropout, we presented only complete case data. For time‐to‐healing analysis using survival analysis methods, dropouts should be accounted for as censored data. Hence all participants will be contributing to the analysis. We acknowledge that such analysis assumes that dropouts are missing at random and there is no pattern of missingness. We presented data for all secondary outcomes as a complete case analysis.
For continuous variables (e.g. length of hospital stay) and for all secondary outcomes, we presented available data from the study reports/study authors and did not impute missing data. Where measures of variance were missing, we calculated these, wherever possible (Higgins 2011a). If calculation was not possible, we contacted the study authors. Where these measures of variation remained unavailable and we could not calculate them, we excluded the study from any relevant meta‐analyses that we conducted.
Assessment of heterogeneity
Assessment of heterogeneity can be a complex, multi‐faceted process. Firstly, we considered clinical and methodological heterogeneity; that is the degree to which the included studies varied in terms of participants, interventions, outcomes, and characteristics such as length of follow‐up. We supplemented this assessment of clinical and methodological heterogeneity by information regarding statistical heterogeneity ‐ assessed using the Chi² test (we considered a significance level of P < 0.10 to indicate statistically significant heterogeneity) in conjunction with the I² statistic (Higgins 2003). I² examines the percentage of total variation across RCTs that is due to heterogeneity rather than chance (Higgins 2003). Very broadly, we considered that I² values of 25%, or less, may mean a low level of heterogeneity (Higgins 2003), and values of 75% or more, indicated very high heterogeneity (Deeks 2011). Where there was evidence of high heterogeneity, we attempted to explore this further (see Data synthesis).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Publication bias is one of a number of possible causes of 'small study effects', that is, a tendency for estimates of the intervention effect to be more beneficial in smaller RCTs. Funnel plots allow a visual assessment of whether small study effects may be present in a meta‐analysis. A funnel plot is a simple scatter plot of the intervention effect estimates from individual RCTs against some measure of each trial’s size or precision (Sterne 2011). Funnel plots are only informative when there are a substantial number of studies included in an analysis; we had planned to present funnel plots for meta‐analyses that included at least 10 RCTs using Review Manager 5 (RevMan 5) (RevMan 2014) but there were no analyses with sufficient studies.
Data synthesis
We combined details of included studies in narrative review according to the comparison between intervention and comparator, the population and the time point of the outcome measurement. We considered clinical and methodological heterogeneity and undertook pooling when studies appeared appropriately similar in terms of burn type and severity, intervention type and antibacterial agent, duration of treatment and outcome assessment.
In terms of a meta‐analytical approach, in the presence of clinical heterogeneity (review author judgement), or evidence of statistical heterogeneity, or both, we used a random‐effects model. We planned to only use a fixed‐effect approach when clinical heterogeneity was thought to be minimal and statistical heterogeneity was estimated as non‐statistically significant for the Chi2 value and 0% for the I2 assessment (Kontopantelis 2013). We adopted this approach as it is recognised that statistical assessments can miss potentially important between‐study heterogeneity in small samples, hence the preference for the more conservative random‐effects model (Kontopantelis 2012). Where clinical heterogeneity was thought to be acceptable, or of interest, we considered conducting meta‐analysis even when statistical heterogeneity was high, but attempted to interpret the causes behind this heterogeneity and considered using meta‐regression for that purpose, if possible (Thompson 1999; Thompson 2002).
We presented data using forest plots, where possible. For dichotomous outcomes we presented the summary estimate as a RR with 95% CIs. Where continuous outcomes were measured in the same way across studies, we planned to present a pooled MD with 95% CIs; we pooled SMD estimates where studies measured the same outcome using different methods. For time‐to‐event data, we plotted (and, where appropriate, pooled) estimates of HRs and 95% CIs, as presented in the study reports, using the generic inverse variance method in RevMan 5 (RevMan 2014). Where time to healing was analysed as a continuous measure, but it was not clear if all wounds healed, we documented use of the outcome in the study, but did not summarise the data or use the data in any meta‐analysis.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined and the sum of available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach. The GRADE approach defines the 'certainty' of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables:
time‐to‐complete wound healing, when analysed using appropriate survival analysis methods;
proportion of wounds completely healing during the trial period;
mean time to healing when all wounds healed;
changes in clinical infection status;
adverse events.
Where comparisons had limited available data for specified outcomes we did not generate a 'Summary of findings' table for this comparison. Instead we decided to present these data together with GRADE judgements in an additional table, in order to keep the 'Summary of findings' tables section of the review manageable and improve readability.
In terms of the GRADE assessment, when making decisions for the risk of bias domain we downgraded only when studies had been classed at high risk of bias for one or more domains. We did not downgrade for unclear risk of bias assessments. In assessing the precision of effect estimates we assessed the size of confidence intervals, downgrading twice for imprecision when there were very few events and CIs around effects included both appreciable benefit and appreciable harm. We considered CI to be especially fragile where there were fewer than 50 participants; event rates were also considered in determining fragility.
Subgroup analysis and investigation of heterogeneity
When possible, we planned to perform subgroup analyses to explore the effect of interventions in children under the age of 18, in adults, and in older adults (aged over 65 years). When possible, we also planned to use subgroup analyses to assess the influence of burn size and depth on effect size. If there had been sufficient data these analyses would have assessed whether there were differences in effect sizes for burns of different depths.
When possible, we planned to perform subgroup analyses to explore the influence of risk of bias on effect size. We planned to assess the influence of removing from meta‐analyses studies classed as having high and unclear risk of bias. These analyses would have only included studies that were assessed as having low risk of bias in all key domains, namely, adequate generation of the randomisation sequence, adequate allocation concealment, and blinding of outcome assessor for the estimates of treatment effect.
Elements of this Methods section are based on the standard Cochrane Wounds protocol template.
Results
Description of studies
Results of the search
The search identified a total of 1565 records after duplicates were removed, of which we assessed 214 records as full texts (Figure 1).
1.
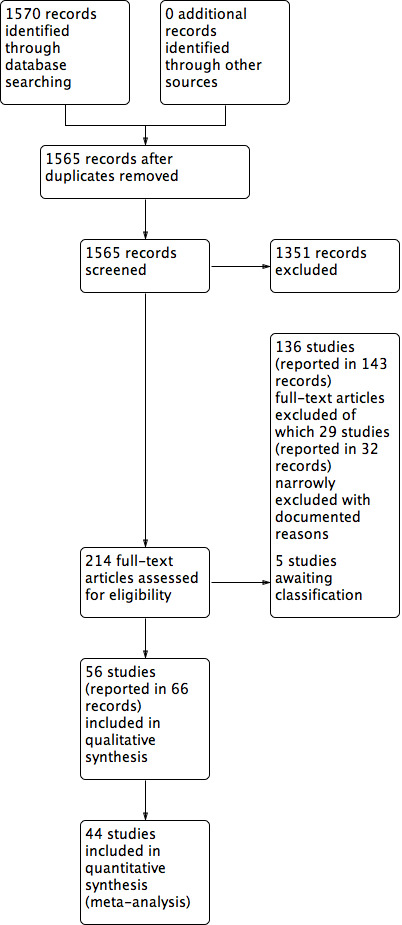
PRISMA flow diagram
Included studies
We included 56 studies reported in 66 publications, with a total of 5807 randomised participants. Most studies had two intervention groups but two studies had three arms, each study evaluating two relevant comparisons (Chen 2006; Thomas 1995), one study had four arms and evaluated five relevant comparisons (Li 1994) and one (Piccolo‐Daher 1990) had five arms and evaluated two relevant comparisons. A number of the studies enrolled participants with two comparable burn wounds and randomly assigned the wounds to the interventions (that is randomisation was at the wound rather than participant level).
Included studies assessed the following types of comparisons.
Comparisons between antiseptics and topical antibiotics
Comparisons between two antiseptics
Comparisons between antiseptics and treatments without antimicrobial properties.
The main groups of interventions and the direct comparisons between them are shown in Figure 2 and listed in Table 9.
2.
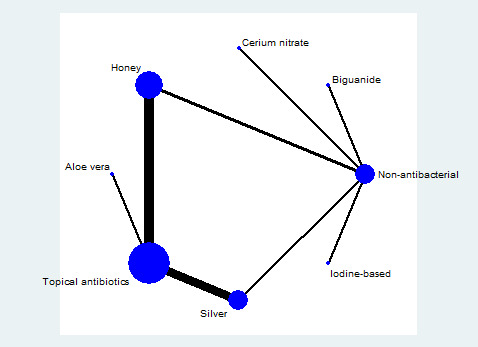
Network of included treatment types
1. Summary of comparisons.
| Comparison | Number of studies | Number of participants |
| Antiseptics versus topical antibiotics | ||
| Silver vs SSD | 16 | 1368 |
| Honey vs SSD or mafenide acetate | 11 | 856 |
| Aloe Vera vs SSD or framycetin | 5 | 338 |
| Iodine vs SSD | 2 | 158 |
| Sodium hypochlorite vs SSD | 1 | 20 |
| Chlorhexidine or polyhexanide (biguanides) vs SSD | 2 | 115 |
| Octenidine vs SSD | 1 | 30 |
| Ethacridine lactate (Rivanol) vs SSD | 1 | 115 |
| Merbromin vs zinc sulfadiazine | 1 | 125 |
| Arnebia euchroma vs SSD | 1 | 49 |
| Antiseptics versus alternative antiseptics | ||
| Chlorhexidine vs iodine | 1 | 213 |
| Iodine vs ethacridine lactate | 1 | 115 |
| Antiseptics versus non‐antibacterial | ||
| Silver vs non‐antibacterial | 3 | 299 |
| Honey vs non‐antibacterial | 3 | 256 |
| Chlorhexidine vs non‐antibacterial | 5 | 516 |
| Iodine vs non‐antibacterial | 4 | 663 |
| Ethacridine lactate vs non‐antibacterial | 1 | 115 |
| Cerium nitrate vs non‐antibacterial | 2 | 214 |
| Merbromin vs non‐antibacterial | 1 | 125 |
SSD: silver sulfadiazine
Studies awaiting classification
Five studies are awaiting classification (Gao 2016; Liu 2016; Rege 1999; Santi 2013; Wang 2015). We have been unable to obtain the full publication for Rege 1999 despite international search requests. Gao 2016 and Santi 2013 are published in abstract only and there is insufficient information available from these; we have so far been unable to contact the authors. Liu 2016 and Wang 2015 are very recent Chinese language studies for which we are awaiting both the full texts and a translation. See Studies awaiting classification for more details.
Excluded studies
We excluded 136 studies reported in 143 records after appraisal as full texts. We ordered many of these because the initial record contained so little information that it was not immediately obvious that they were not relevant. Upon obtaining the full texts it was clear that many studies were not eligible. More nuanced reasons for exclusion were noted for 29 studies reported in 32 records (see Characteristics of excluded studies). Eight trials were quasi‐randomised (Babb 1977; Bowser 1981; Cason 1966; Choudhary 2013; Daryabeigi 2010; Helvig 1979; Mohammadi 2013; Zhu 2006); eight assessed a comparison where use of an antiseptic was not the only difference between the groups (Afilalo 1992; Ang 2002; Ang 2003; Fisher 1968; Kumar 2004; Shoma 2010; Subrahmanyam 1999; Weng 2009); one assessed a mixture of antiseptic and non‐antiseptic agents within the same intervention group (Chokotho 2005); four assessed the same antiseptic in each arm (Brown 2016; Gee Kee 2015; Tredget 1998; Verbelen 2014) three assessed a population with a minority of people with burns (Colombo 1993; Madhusudhan 2015; Subrahmanyam 1993a), while three evaluated post‐surgical burns patients (Chmyrev 2011; Palombo 2011; Vehmeyer‐Heeman 2005); two trials were not designed to evaluate clinical efficacy, effectiveness or safety of interventions (Chen 2007; Xu 2009). We also identified one paper which was found to be a non‐randomised extension of an included study (Inman 1984); this is listed as an additional reference for this study.
Risk of bias in included studies
No studies had a low risk of bias for all domains. We judged only one study (Tang 2015) to be at low risk of bias across all except one domain, where there was an unclear risk of bias. All other studies had an unclear or high risk of bias for two or more domains. There were 17 studies with one domain classed at high risk of bias and we rated three of these studies as being at high risk of bias in more than one domain. Most studies had multiple domains which were at an unclear risk of bias. For only two domains (attrition bias and reporting bias) did we consider a majority of the studies to be at low risk of bias. Figure 3 and Figure 4 illustrate the predominance of unclear judgements across the domains.
3.
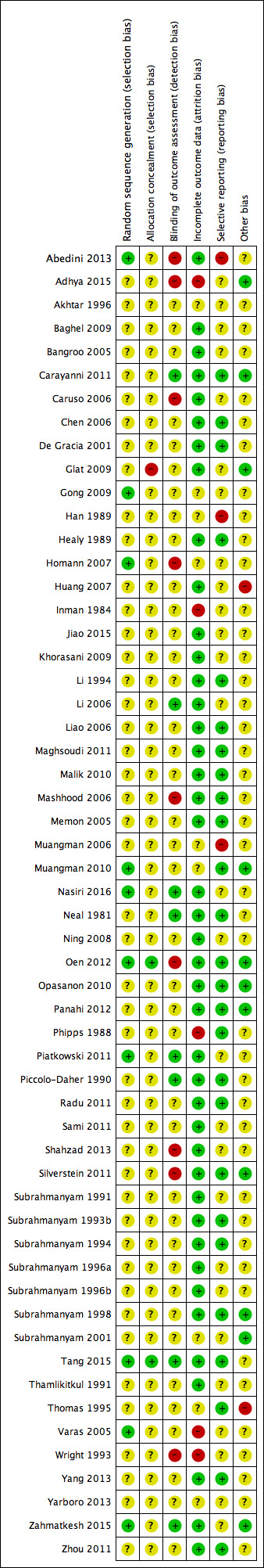
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
4.
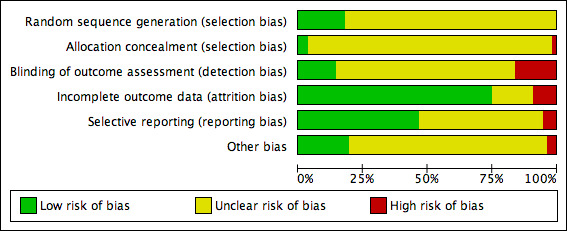
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Generation of randomisation sequence and concealment of allocation were not well reported. Most studies had an unclear risk of bias with allocation concealment, especially, poorly documented.
Blinding
Blinding of outcome assessment was largely unclear although several studies were clearly not blinded for their primary outcomes. We judged a smaller number to be at low risk of bias.
Incomplete outcome data
Most studies were at low risk of attrition bias with all participants accounted for or only a small number missing from analyses. Approximately 10% of studies were at high risk of bias and 20% were unclear.
Selective reporting
Just over half the studies were at low risk of bias, we judged three to be at high risk of bias and the remainder were unclear.
Other potential sources of bias
A minority of studies were sufficiently well‐reported and conducted for us to be confident that they were at low risk of other sources of bias. While we judged only two studies to be at high risk of bias due to unit of analysis issues (Huang 2007; Thomas 1995), over half the studies were too poorly reported for us to be clear that there were no other potential sources of bias. None of the ten studies which used intra‐individual designs for both randomisation and analysis made it clear whether they had used appropriate analytical methods for the paired data. We judged these studies to be unclear for this domain in the 'Risk of bias' assessment (Homann 2007; Khorasani 2009; Liao 2006; Malik 2010; Nasiri 2016; Piatkowski 2011; Radu 2011; Varas 2005; Yang 2013; Zhou 2011). The effect of failure to account for pairing would be to produce wider confidence intervals than the appropriate analysis. Zhou 2011 may be at particularly high risk of carry‐over effects from one intervention to another as it randomised burn areas rather than discrete burns.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
Individual study outcome data are shown in Table 10 (wound healing); Table 11 (wound infection); and Table 12 (secondary outcomes).
2. Summary of data for wound healing.
| Comparison | Study | Number participants /wounds | Duration | Time to wound healing (days) (mean (SD)) | Difference in means (days) (95% CI) | Proportion of wounds healed | Risk ratio (for longest time point) or Hazard Ratio (95% CI) |
| Antiseptic versus topical antibiotic | |||||||
| Silver hydrofibre Silver sulfadiazine (SSD) |
Abedini 2013 | Silver 35 SSD 34 |
Until healing | Silver 9.7 (7.2) SSD 15.7 (6.2) |
‐6.00 (‐9.17 to ‐2.83) | ‐ | ‐ |
| Silver hydrofibre Silver sulfadiazine |
Caruso 2006 | Silver 42 SSD 40 |
21 days | Median Silver 16 SSD 17 |
‐ | Silver 31 SSD 24 |
HR 1.67 (0.76 to 3.65) RR 1.23 (0.60 to 1.68) |
| Silver hydrofibre Silver sulfadiazine |
Muangman 2010 | Silver 35 SSD 35 |
NR | Silver 10 (3) SSD 13.7 (4) |
‐3.70 (‐5.36 to ‐2.04) | ‐ | ‐ |
| Silver hydrogel Silver sulfadiazine |
Adhya 2015 | Silver 84 SSD 79 (analysed silver 54, SSD 52) |
4 weeks/until healing | Silver 38.58 (26.27) SSD 32.58 (15.21) |
6.00 (‐2.14 to 14.14) | Deep dermal wounds only reported | ‐ |
| Silver hydrogel Silver sulfadiazine |
Glat 2009 | Silver 12 SSD 12 |
21 days+ | Silver 12.42 (3.58) SSD 12.75 (7.45) (participants followed up after 21 days when binary data reported) |
‐0.33 (‐5.01 to 4.35) | Silver 12 SSD 10 |
HR 1.03 (0.44 to 2.39) RR 1.19 (0.89 to 1.59) |
| Silver hydrogel Silver sulfadiazine |
Gong 2009 | Silver 52 SSD 52 |
21 days+ | Silver 12.85 (4.15) SSD 17.02 (4.86) (participants followed up after 21 days when binary data reported) |
‐4.17 (‐5.91 to ‐2.43) | Silver 52/52 (day 21) SSD 43/52 (day 21) |
RR 1.58 (1.16 to 2.16) |
| Silver foam Silver sulfadiazine |
Silverstein 2011 | Silver 50 SSD 51 |
21 days | Silver 13.44 (N = 47) SSD 17.11 (N = 51) Reported as NS |
‐ | 1 week Silver 16 SSD 10 3 weeks Silver 33 SSD 31 |
RR 1.09 (0.81 to 1.46) |
| Silver foam Silver sulfadiazine |
Tang 2015 | Silver 71 SSD 82 |
4 weeks | Silver 56/71 (median 15 days) SSD 65/82 (median 16 days) |
‐ | 28 days Silver 56 SSD 65 |
HR 1.22 (0.88 to 1.70) favouring silver RR 1.00 (0.84 to 1.17) |
| Silver foam Silver sulfadiazine |
Yarboro 2013 | 24 participants randomised; group allocation unclear | NR | ‐ | ‐ | ‐ | ‐ |
| Silver foam Silver sulfadiazine |
Zhou 2011 | 40 participants; part of each burn randomised to treatments |
14 days | Silver 12.53 (± 1.29) SSD 13.26 (± 1.62) |
‐0.73 (‐1.37 to 0.09) | ‐ | ‐ |
| Nanocrystalline silver Silver sulfadiazine Vaseline gauze |
Chen 2006a | Silver 65 SSD 63 Vaseline gauze 63 |
Until healing | Silver 14.57 (5.18) SSD 20.29 (2.75) Vaseline 18.03 (5.1) |
Silver vs SSD ‐5.72 (‐7.15 to ‐4.29) Silver vs Vaseline ‐3.49 (‐4.46 to ‐2.52) |
‐ | ‐ |
| Nanocrystalline silver Silver sulfadiazine |
Huang 2007 | 98 participants with 166 burns 83 burns in each group |
20 days | Silver 12.42 (5.40) SSD 15.79 (5.60) |
‐3.37 (‐4.49 to ‐1.75) | ‐ | ‐ |
| Nanocrystalline silver Silver sulfadiazine |
Muangman 2006 | Silver 25 SSD 25 |
NR | ‐ | ‐ | ‐ | ‐ |
| Nanocrystalline silver Silver sulfadiazine |
Varas 2005 | 14 participants with 2 burn areas; 14 burn areas in each group |
NR | ‐ | ‐ | ‐ | ‐ |
| Silver nitrate Silver sulfadiazine |
Liao 2006 | 120 participants with 2 burn areas; 120 burn areas in each group |
Until healing | Silver 13.5 (6.28) SSD 14.97 (6.89) |
‐1.47 (‐3.14 to 0.20) | ‐ | ‐ |
| Silver alginate Silver sulfadiazine |
Opasanon 2010 | Silver 30 SSD 35 |
NR | ‐ | ‐ | ‐ | ‐ |
| Honey Silver sulfadiazine |
Baghel 2009 | Honey 37 SSD 41 |
NR (2 months' follow‐up) | Honey 18.16 (SD ‐) SSD 32.68 (SD ‐) |
‐ | ‐ | ‐ |
| Honey Silver sulfadiazine |
Bangroo 2005 | Honey 32 SSD 32 |
21 days | ‐ | ‐ | Honey 10 days 26 ≥ 14 days 32 SSD ≥ 3 weeks 19 unclear if all healed |
RR 1.67 (1.25 to 2.22) |
| Honey Silver sulfadiazine |
Malik 2010 | 150 participants with 2 burns; 150 burns in each group |
24 days | Honey 13.47 (4.06) SSD 15.62 (4.40) |
‐2.15 (‐3.11 to ‐1.19) | 10 days Honey 30 SSD 13 14 days Honey 122 SSD 80 19 days Honey 140 SSD 90 21 days Honey 142 SSD 111 24 days Honey 142 SSD 121 |
HR 2.93 (2.23 to 3.86) |
| Honey Silver sulfadiazine |
Mashhood 2006 | Honey 25 SSD 25 |
6 weeks | ‐ | ‐ | 2 weeks Honey 13 SSD 5 4 weeks Honey 25 SSD 15 6 weeks Honey 25 SSD 25 |
HR 2.23 (1.19 to 4.19) |
| Honey Silver sulfadiazine |
Memon 2005 | Honey 40 SSD 40 |
46 days | Honey 15.3 (SD ‐) SSD 20.0 (SD ‐) |
‐ | Honey Day 16: 20 Day 26: 32 Day 30: 40 SSD Day 20: 16 Day 36: 34 Day 46: 40 |
HR 3.75 (2.18 to 6.45) |
| Honey Silver sulfadiazine |
Subrahmanyam 1991 | Honey 52 SSD 52 |
15 days | Honey 9.4 (2.3) SSD 17.2 (3.2)* *Jull 2015 author contact |
‐7.77 (‐8.84 to ‐6.70) | Honey 87% (42) SSD 10% (5) |
RR 8.40 (3.61 to 19.53) |
| Honey Silver sulfadiazine |
Subrahmanyam 1998 | Honey 25 SSD 25 |
21 days | Honey 4.92 (3.61) SSD 8.22 (8.31)* *Jull 2015 author contact |
‐3.30 (‐6.85 to 0.25) | Honey 25 SSD 21 |
RR 1.19 (0.99 to 1.43) |
| Honey Silver sulfadiazine |
Subrahmanyam 2001 | Honey 50 SSD 50 |
21 days | Honey 15.4 (3.2) SSD 17.2 (4.3)* *SD from Jull 2015 author contact |
‐1.80 (‐3.29 to 0.31) | Honey 50 SSD 24 |
RR 2.06 (1.55 to 2.75) |
| Honey Silver sulfadiazine |
Sami 2011 | Honey 25 SSD 25 |
60 days | ‐ | ‐ | Days 5‐10 Honey 14 SSD 3 Days 11‐15 Honey 6 SSD 2 Days 16‐20 Honey 3 SSD 7 Days 21‐30 Honey 1 SSD 8 Days 31‐40 Honey 1 SSD 3 Days 41‐50 Honey 0 SSD 1 Days 51‐60 Honey 0 SSD 1 |
HR 2.73 (1.43 to 5.24) |
| Honey Mafenide acetate |
Maghsoudi 2011 | Honey 50 Mafenide acetate 50 |
30 days | ‐ | ‐ | Day 7 Honey 42 Mafenide 36 Day 10 Honey 46 Mafenide 38 Day 15 Honey 48 Mafenide 40 Day 21 Honey 50 Mafenide 42 Day 30 Honey 50 Mafenide 50 |
HR 1.38 (0.91 to 2.09) |
| Honey (olea) Mafenide acetate |
Zahmatkesh 2015 | Honey 10 Mafenide acetate 20 |
20 days | Development of granulation tissue: median Honey: 12 (range 10.3‐13.6) Madenide: 17 (range 13.3‐20.6) Not all participants developed this |
‐ | Proportion of participants with granulation tissue at day 20 Honey 8/10 Mafenide 16/20 |
‐ |
| Aloe Vera Silver sulfadiazine |
Khorasani 2009 | Aloe Vera 30 SSD 30 |
24 days | Aloe Vera 15.9 (2) SSD 18.73 (2.65) |
‐2.85 (‐4.04 to ‐1.66) | ‐ | ‐ |
| Aloe Vera Silver sulfadiazine |
Panahi 2012 | Aloe Vera 60 SSD 60 |
14 days | ‐ | ‐ | ‐ | ‐ |
| Aloe Vera Silver sulfadiazine |
Shahzad 2013 | Aloe Vera 25 SSD 25 |
Until healing/ 2 months |
Aloe Vera 11 (4.18) SSD 24.24 (11.16) |
‐13.24 (‐17.91 to ‐8.57) | ‐ | ‐ |
| Aloe Vera Silver sulfadiazine |
Thamlikitkul 1991 | Aloe Vera 20 SSD 18 |
26 days | ‐ | Aloe Vera 55% (11) SSD 39% (7) |
RR 1.41 (0.70 to 2.85) | |
| Aloe Vera Framycetin |
Akhtar 1996 | Aloe Vera 50 Framycetin:50 |
NR | Aloe Vera 18 (SD ‐) Framycetin: 30.9 (SD ‐) |
‐ | ‐ | ‐ |
| Povidone iodine Silver sulfadiazine |
Homann 2007 | 43 participants each with 2 comparable burns; 43 burns in each group |
21 days | Povidone iodine 9.9 (4.5) SSD 11.3 (4.9) |
‐1.40 (‐3.39 to 0.59) | ‐ | ‐ |
| Iodophor Moist exposed burn ointment (MEBO) Ethacridine lactate Silver sulfadiazine |
Li 1994b | Iodophor 24 MEBO 31 Ethacridine lactate 22 SSD 38 |
Until healing | MEBO 57 (10.41) Iodophor 31 (6.43) Ethacridine lactate 32 (4.98) SSD 30 (4.72) |
Iodophor vs SSD: 1.00 (‐1.95 to 3.98) Ethacridine vs SSD 2.00 (‐0.57 to 4.57) Iodophor vs MEBO ‐26.0 (‐30.48 to ‐21.52) Ethacridine vs MEBO ‐25.00 (‐29.21 to ‐20.79) Iodophor vs ethacridine 2.00 (‐0.57 to 4.57) |
‐ | ‐ |
| Sodium hypochlorite Silver sulfadiazine |
Ning 2008 | 20 participants with 2 burns (20 burns/group) |
28 days | Sodium hypochlorite 20.0 (2.7) SSD 22.1 (3.0) |
‐2.10 (‐3.87 to 0.33) | ‐ | ‐ |
| Octenidine Silver sulfadiazine |
Radu 2011 | 30 participants with 2 burn areas; 30 burns in each group |
24 hours | ‐ | ‐ | ‐ | ‐ |
| Polyhexanide Silver sulfadiazine |
Piatkowski 2011 | Polyhexanide 30 with 38 burns SSD 30 with 34 |
NR | Polyhexanide 10 (‐) SSD 10 (‐) |
‐ | ‐ | ‐ |
|
Arnebia euchroma Silver sulfadiazine |
Nasiri 2016 | 49 participants with 2 burns (49 burns/group) | 36 days |
A euchroma 13.9 (5.3) SSD 17.5 (6.9) |
‐3.60 (‐6.41 to ‐1.06) | Day 7 A euchroma 3 SSD 0 Day 10 A euchroma 14 SSD 8 Day 13 A euchroma 24 SSD 13 Day 15 A euchroma 29 SSD 24 Day 20 A euchroma 41 SSD 35 Day 25 A euchroma 42 SSD 38 Day 30 A euchroma 45 SSD 43 Day 36 A euchroma 45 SSD 45 |
HR 1.42 (0.91 to 2.21) |
| Antiseptic versus alternative antiseptic | |||||||
| Iodine Chlorhexidine |
Han 1989 | Iodine 111 Chlorhexidine 102 |
NR | Iodine 9.48 (5.43) Chlorhexidine 11.69 (8.09) |
2.21 (0.34 to 4.08) | ‐ | ‐ |
| Antiseptic versus non‐antibacterial treatment | |||||||
| Nanocrystalline silver Vaseline gauze |
Jiao 2015 | Silver 38 Gauze 38 |
30 days | Silver 8.8 (2.3) Gauze 12.3 (2.8) |
‐3.50 (‐4.65 to 2.35) | ‐ | ‐ |
| Silver xenograft Petroleum gauze |
Healy 1989 | Silver 16 Gauze 16 |
14 days | Silver 12.9 (1.4) N = 9 Gauze 12.5 (2.7) N = 8 |
0.40 (‐1.68 to 2.48) | Silver 9/16 Gauze 8/16 |
RR 1.13 (0.59 to 2.16) |
| Honey Polyurethane film |
Subrahmanyam 1993b | Honey 46 Polyurethane 46 |
NR | Honey 10.8 (3.93) Polyurethane 15.3 (2.98)* *Jull 2015 author contact for SD |
‐4.50 (‐5.93 to ‐3.07) | ‐ | ‐ |
| Honey gauze Amniotic membrane |
Subrahmanyam 1994 | Honey gauze 40 Amniotic 24 |
30 days | Honey 9.4 (2.52) Amniotic 17.5 (6.66)* *Jull 2015 author contact for SD |
‐8.10 (‐10.88 to ‐5.32) | Day 10 Honey 23 Amniotic 4 Day 15 Honey 33 Amniotic 14 Day 20 Honey 38 Amniotic 20 Day 25 Honey 40 Amniotic 21 Day 30 Honey 40 Amniotic 24 |
HR 1.80 (1.09 to 2.98) |
| Honey Potato peel |
Subrahmanyam 1996a | Honey 50 Potato peel 50 |
21 days | Honey 10.4 (2.2) Potato peel 16.2 (2.3) *Jull 2015 author contact for SSD |
‐5.80 (‐6.88 to ‐4.92) | 7 days Honey 20 Potato peel 4 10 days Honey 36 Potato peel 12 15 days Honey 50 Potato peel 40 21 days Honey 50 Potato peel 50 |
HR 2.37 (1.53 to 3.67) |
| Honey "Conventional dressing" |
Subrahmanyam 1996b | Honey 450 "Conventional dressing" 450 |
NR | Honey: 8.8 (SD 2.1) "Conventional dressing": 13.5 (SD 4.1) *Jull 2015 author contact |
‐4.70 (‐5.13 to ‐4.27) | ‐ | ‐ |
| Silver sulfadiazine + chlorhexidine Silver sulfadiazine alone |
Inman 1984 | SSD + chlorhexidine 54 assessed SSD only 67 assessed Unclear if additional post‐randomisation exclusions |
Until healing (26 days) | ‐ | ‐ | ‐ | ‐ |
| Chlorhexidine Polyurethane |
Neal 1981 | Chlorhexidine 25 Polyurethane 26 |
30 days | Chlorhexidine 14.08 (7) Polyurethane 10 (5) |
4.08 (0.73 to 7.43) | Chlorhexidine Day 5: 1 Day 10: 8 Day 15: 19 Day 20: 21 Day 25: 22 Day 30: 25 Polyurethane Day 5: 4 Day 10: 17 Day 15: 22 Day 20: 23 Day 25: 23 Day 30: 26 |
HR 0.71 (0.39 to 1.29) |
| Chlorhexidine Hydrocolloid |
Phipps 1988 | Chlorhexidine 104 Hydrocolloid 92 |
NR | Chlorhexidine 69 analysed 11.83 (‐) Hydrocolloid 50 analysed 14.18 (‐) Not statistically significant |
‐ | ‐ | ‐ |
| Chlorhexidine tulle‐gras Hydrocolloid Hydrocolloid + SSD |
Thomas 1995c | Chlorhexidine tulle‐gras 18 Hydrocolloid 16 Hydrocolloid + SSD 16 |
NR | Chlorhexidine 11.1 (‐) Hydrocolloid 10.6 (‐) Hydrocolloid SSD 14.2 (‐) |
‐ | ‐ | ‐ |
| Chlorhexidine Hydrocolloid |
Wright 1993 | Chlorhexidine 49 Hydrocolloid 49 |
NR | Median Chlorhexidine 12 Hydrocolloid 12 P = 0.89; based on 67 participants |
‐ | ‐ | ‐ |
| Povidone iodine + Bepanthenol Moist exposed burn ointment (MEBO) |
Carayanni 2011 | Povidone iodine + Bepanthenol 107 MEBO 104 |
18 days | ‐ | ‐ | ‐ | ‐ |
| Iodine gauze Carbon‐fibre dressing |
Li 2006 | Iodine gauze 74 Carbon‐fibre dressing 203 |
NR | Calculated using method in Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c) Iodine 20.67 (9.7) Carbon 15.29 (4.24) |
5.38 (3.09 to 7.67) | ‐ | ‐ |
| Iodophor gauze Hydrogel |
Yang 2013 | 60 participants with burn wounds; 60 burn areas/group |
14 days | ‐ | ‐ | Day 7 Iodophor 4 Hydrogel 10 Day 14 Iodophor 7 Hydrogel 42 |
RR 0.17 (0.08 to 0.34) |
| Cerium nitrate + silver sulfadiazine Silver sulfadiazine alone |
De Gracia 2001 | CN + SSD 30 SSD 30 |
Until healing/ readiness for grafting |
CN + SSD 17.2 (8.3) N = 29 SSD 25.1 (19.4) N = 30 Partial‐thickness areas only; time to graft readiness reported for full‐thickness areas (CN + SSD 13.6 (11.3) SSD 24.6 (11.4)) |
‐ | ‐ | ‐ |
| Cerium nitrate + silver sulfadiazine Silver sulfadiazine alone |
Oen 2012 | CN + SSD 78 SSD 76 |
21 days | Median (IQR) for participants not requiring surgery CN + SSD 11.0 (7‐15) SSD 9.0 (5.0‐15.75) (13 vs 15 required surgery) |
‐ | ‐ | ‐ |
| Merbromin Sodium salicylate Zinc sulfadiazine Sodium salicylate + zinc sulfadiazine Collagenase + chloramphenicol |
Piccolo‐Daher 1990d | Merbromin 25 Sodium salicylate 25 Zinc sulfadiazine 25 Sodium salicylate + zinc sulfadiazine 25 Collagenase + chloramphenicol 25 |
NR | Merbromin 11.32 (3.99) Sodium salicylate 15.00 (8.00) Zinc sulfadiazine 11.08 (4.69) Sodium salicylate + zinc sulfadiazine 14.8 (7.61) Collagenase + chloramphenicol 12.32 (5.92) |
Merbromin vs sodium salicylate ‐3.68 (‐7.18 to ‐0.18) Merbromin vs zinc sulfadiazine ‐3.48 (‐6.85 to ‐0.11) |
‐ | ‐ |
CN: cerium nitrate; MEBO: moist exposed burn ointment; NR: not reported; NS: not significant; SD: standard deviation; SSD: silver sulfadiazine
aChen 2006 assessed the following relevant comparisons between antiseptic (silver) and non‐antibacterial (Vaseline gauze) and between silver and SSD
bLi 1994 assessed the following relevant comparisons between two antiseptics (ethacridine lactate and iodophor), between ethacridine lactate and a non‐antibacterial treatment (MEBO) and between iodophor and MEBO. cThomas 1995 the following relevant comparisons between antiseptic (chlorhexidine) and topical antibiotic (silver sulfadiazine) and between chlorhexidine and a non‐antibacterial treatment (hydrocolloid)
dPiccolo‐Daher 1990 assessed the following relevant comparisons: Merbromin vs sodium salicylate and Merbromin vs zinc sulfadiazine; other comparisons were not relevant to the review
3. Summary of reported data for infection.
| Comparison | Study | Number participants/burns | Duration | Measure reported | Reported data | RR (95% CI) |
| Antiseptic versus topical antibiotic | ||||||
| Silver hydrofibre Silver sulfadiazine |
Abedini 2013 | Silver 35 SSD 34 |
Until healing | ‐ | ‐ | ‐ |
| Silver hydrofibre Silver sulfadiazine |
Caruso 2006 | Silver 42 SSD 40 |
21 days | Participants with wound infection | Silver 8/42 SSD 6/40 |
1.27 (0.48 to 3.34) |
| Silver hydrofibre Silver sulfadiazine |
Muangman 2010 | Silver 35 SSD 35 |
NR | ‐ | ‐ | ‐ |
| Silver hydrogel Silver sulfadiazine |
Adhya 2015 | Silver 84 SSD 79 (analysed silver 54, SSD 52) |
4 weeks/until healing | ‐ | ‐ | ‐ |
| Silver hydrogel Silver sulfadiazine |
Glat 2009 | Silver 12 SSD 12 |
21 days+ | Participants with wound infection | Silver 0 SSD 0 |
‐ |
| Silver hydrogel Silver sulfadiazine |
Gong 2009 | Silver 52 SSD 52 |
21 days+ | ‐ | ‐ | ‐ |
| Silver foam Silver sulfadiazine |
Silverstein 2011 | Silver 50 SSD 51 |
21 days | ‐ | ‐ | ‐ |
| Silver foam Silver sulfadiazine |
Tang 2015 | Silver 71 SSD 82 |
4 weeks | Participants with new signs of inflammation | Silver 8/71 SSD 14/82 |
0.66 (0.29 to 1.48) |
| Silver foam Silver sulfadiazine |
Yarboro 2013 | 24 participants randomised; group allocation unclear | NR | ‐ | ‐ | ‐ |
| Silver foam Silver sulfadiazine |
Zhou 2011 | 40 participants; part of each burn randomised to treatments |
14 days | ‐ | ‐ | ‐ |
| Nanocrystalline silver Silver sulfadiazine |
Chen 2006a | Silver 65 SSD 63 Vaseline gauze 63 |
Until healing | ‐ | ‐ | ‐ |
| Nanocrystalline silver Silver sulfadiazine |
Huang 2007 | 98 participants with 166 burns 83 burns in each group |
20 days | Bacterial clearance rates | ‐ | ‐ |
| Nanocrystalline silver Silver sulfadiazine |
Muangman 2006 | Silver 25 SSD 25 |
NR | Participants with wound infection | Silver 3/25 SSD 4/25 |
0.75 (0.19 to 3.01) |
| Nanocrystalline silver Silver sulfadiazine |
Varas 2005 | 14 participants with 2 burn areas; 14 burn areas in each group | NR | ‐ | ‐ | ‐ |
| Silver nitrate Silver sulfadiazine |
Liao 2006 | 120 participants with 2 burns; 120 burns in each group | Until healing | ‐ | ‐ | ‐ |
| Silver alginate Silver sulfadiazine |
Opasanon 2010 | Silver 30 SSD 35 |
NR | ‐ | ‐ | ‐ |
| Honey Silver sulfadiazine |
Baghel 2009 | Honey 37 SSD 41 |
NR (2 months' follow‐up) | ‐ | ‐ | ‐ |
| Honey Silver sulfadiazine |
Bangroo 2005 | Honey 32 SSD 32 |
21 days | ‐ | ‐ | ‐ |
| Honey Silver sulfadiazine |
Malik 2010 | 150 participants with 2 burns; 150 burns in each group |
24 days | Burns with wound infection | Honey 6/150 SSD 29/150 |
0.21 (0.09 to 0.48) |
| Honey Silver sulfadiazine |
Mashhood 2006 | Honey 25 SSD 25 |
6 weeks | Time to achieve negative wound cultures | Honey 3 weeks SSD 5 weeks |
‐ |
| Honey Silver sulfadiazine |
Memon 2005 | Honey 40 SSD 40 |
46 days | ‐ | ‐ | ‐ |
| Honey Silver sulfadiazine |
Subrahmanyam 1991 | Honey 52 SSD 52 |
15 days | Persistent infections (positive cultures) | Honey 4/43 SSD 38/41 |
0.10 (0.04 to 0.26) |
| Honey Silver sulfadiazine |
Subrahmanyam 1998 | Honey 25 SSD 25 |
21 days | Participants with wound infection | Honey 0/25 SSD 4/25 |
0.11 (0.01 to 1.96) |
| Honey Silver sulfadiazine |
Subrahmanyam 2001 | Honey 50 SSD 50 |
21 days | Persistent infections (positive cultures) | Honey 4/44 SSD 42/42 |
0.10 (0.04 to 0.24) |
| Honey Silver sulfadiazine |
Sami 2011 | Honey 25 SSD 25 |
42 days | Persistent infections (positive cultures); participants becoming culture negative. Details of isolated organisms | Week 1 Honey 17/20 SSD 11/22 Week 2 Honey 20/20 SSD 16/22 Week 3 Honey 20/20 SSD 19/22 Week 4 Honey 20/20 SSD 21/22 Week 6 Honey 20/20 SSD 22/22 |
Not estimable at week 6 |
| Honey Mafenide acetate |
Maghsoudi 2011 | Honey 50 Mafenide acetate 50 |
30 days | New infections Day 7 New infections Day 21 |
Honey 2/50 Mafenide 2/50 Honey 0/50 Mafenide 10/50 |
0.05 (0.00 to 0.79) |
| Honey (olea) Mafenide acetate |
Zahmatkesh 2015 | Honey 10 Mafenide acetate 20 |
20 days | Infections (positive cultures) Day 7 | Honey 1/10 SSD 19/20 |
0.11 (0.02 to 0.68) |
| Aloe Vera Silver sulfadiazine |
Khorasani 2009 | Aloe Vera 30 SSD 30 |
24 days | Participants with wound infection | Aloe Vera 0 SSD 0 |
‐ |
| Aloe Vera Silver sulfadiazine |
Panahi 2012 | Aloe Vera 60 SSD 60 |
14 days | Participants with wound infection | Aloe Vera 1 SSD 0 |
2.95 (0.12 to 70.82) |
| Aloe Vera Silver sulfadiazine |
Shahzad 2013 | Aloe Vera 25 SSD 25 |
Until healing/ 2 months |
Participants with wound infection | Aloe Vera 3 SSD 4 |
0.75 (0.19 to 3.01) |
| Aloe Vera Silver sulfadiazine |
Thamlikitkul 1991 | Aloe Vera 20 SSD 18 |
26 days | ‐ | ‐ | ‐ |
| Aloe Vera Framycetin |
Akhtar 1996 | Aloe Vera 50 Framycetin 50 |
NR | Grade of infection | Lower in Aloe Vera | ‐ |
| Povidone iodine Silver sulfadiazine |
Homann 2007 | 43 participants each with 2 comparable burns; 43 burns in each group |
21 days | ‐ | ‐ | ‐ |
| Iodophor Moist exposed burn ointment (MEBO) Ethacridine lactate Silver sulfadiazine |
Li 1994b | Iodophor 24 MEBO 31 Ethacridine lactate 22 SSD 38 |
Until healing | ‐ | ‐ | ‐ |
| Sodium hypochlorite Silver sulfadiazine |
Ning 2008 | 20 participants with 2 burns (20 burns/group) | 28 days | ‐ | ‐ | ‐ |
| Octenidine Silver sulfadiazine |
Radu 2011 | 30 participants with 2 burn areas; 30 burns in each group |
24 hours | ‐ | ‐ | ‐ |
| Polyhexanide Silver sulfadiazine |
Piatkowski 2011 | Polyhexanide 30 with 38 burns SSD 30 with 34 burns |
NR | ‐ | ‐ | ‐ |
|
Arnebia euchroma Silver sulfadiazine |
Nasiri 2016 | 49 participants with 2 burns (49 burns/group) | 36 days | Infection score between 0 and 5; 1 point for each symptom of infection; 45 burns analysed/group |
A euchroma 0: 37/45 1: 7/45 2: 1/45 3: 0/45 SSD 0: 31/45 1: 11/45 2: 2/45 3: 1/45 |
‐ |
| Antispetic versus alternative antiseptic | ||||||
| Iodine Chlorhexidine |
Han 1989 | Iodine 111 Chlorhexidine 102 |
NR | Systemic antibiotics prescribed for clinical/bacteriological signs of infection | Iodine 4/111 Chlorhexidine 4/102 |
1.09 (0.28 to 4.24) |
| Antispetic versus non‐antibacterial treatment | ||||||
| Nanocrystalline silver Vaseline gauze |
Jiao 2015 | Nanocrystalline silver 38 Vaseline gauze 38 |
21 days | "Positive for bacteria" | Silver 1/38 Gauze 8/38 |
0.13 (0.02 to 0.95) |
| Silver xenograft Petroleum gauze |
Healy 1989 | Silver xenograft 16 Petroleum gauze 16 |
14 days | Rate of infection Bacterial colonisation |
"No difference" Details of organisms reported |
‐ |
| Honey Polyurethane film |
Subrahmanyam 1993b | Honey 46 Polyurethane film 46 |
NR | Infection on day 8 | Honey 8 Polyurethane 17 |
0.47 (0.23 to 0.98) |
| Honey gauze Amniotic membrane |
Subrahmanyam 1994 | Honey gauze 40 Amniotic membrane 24 |
30 days | Persistent infection at 7 days | Honey 4/28 Amniotic 11/19 |
0.25 (0.09 to 0.66) |
| Honey Potato peel |
Subrahmanyam 1996a | Honey 50 Potato peel 50 |
21 days | Persistent infection at 7 days | Honey 4/40 Potato 42/42 |
0.10 (0.04 to 0.25) |
| Honey "Conventional dressing" |
Subrahmanyam 1996b | Honey 450 "Conventional dressing" 450 |
NR | ‐ | ‐ | ‐ |
| Silver sulfadiazine + chlorhexidine Silver sulfadiazine alone |
Inman 1984 | SSD + chlorhexidine 54 assessed SSD 67 assessed Unclear if additional post‐randomisation exclusions |
Until healing (26 days) | Infection incidence | Chlorhexidine 10/54 SSD alone 12/67 |
1.03 (0.48 to 2.21) |
| Chlorhexidine Polyurethane |
Neal 1981 | Chlorhexidine 25 Polyurethane 26 |
30 days | Proven infection | Chlorhexidine 2/25 Polyurethane 1/26 |
2.08 (0.20 to 21.52) |
| Chlorhexidine Hydrocolloid |
Phipps 1988 | Chlorhexidine 104 Hydrocolloid 92 |
NR | ‐ | ‐ | ‐ |
| Chlorhexidine tulle‐gras Hydrocolloid Hydrocolloid + SSD |
Thomas 1995c | Chlorhexidine tulle‐gras 18 Hydrocolloid 16 Hydrocolloid + SSD 16 |
NR | Percentage of wounds with bacteria and pathogenic bacteria at baseline and post treatment | ‐ | ‐ |
| Chlorhexidine Hydrocolloid |
Wright 1993 | Chlorhexidine 49 Hydrocolloid 49 |
NR | ‐ | ‐ | ‐ |
| Povidone iodine + Bepanthenol Moist exposed burn ointment (MEBO) |
Carayanni 2011 | Povidone iodine + Bepanthenol f107 MEBO 104 |
18 days | New infections | Iodine 8/107 MEBO 6/104 |
1.30 (0.47 to 3.61) |
| Iodine gauze Carbon‐fibre dressing |
Li 2006 | Iodine gauze 74 Carbon‐fibre dressing 203 |
NR | ‐ | ‐ | ‐ |
| Iodophor gauze/ Hydrogel |
Yang 2013 | 60 participants with burns wounds; 60 burn areas/group |
14 days | Bacterial presence reported | No difference between groups | ‐ |
| Cerium nitrate + silver sulfadiazine Silver sulfadiazine alone |
De Gracia 2001 | CN + SSD 30 SSD 30 |
Until healing/ readiness for grafting |
Bacterial cultures at baseline Resolved New 2/13 Total post‐treatment Sepsis by day 5 Sepsis after day 5 |
CN + SSD 17/30 SSD 11/30 CN + SSD 16/17 SSD 8/11 CN + SSD 2/13 SSD 3/19 CN + SSD 3 SSD 6 CN + SSD 1 SSD 1 (both recovered) CN + SSD 0 SSD 3 (1 died) |
RR post‐treatment infection 0.50 (0.14 to 1.82) RR Sepsis 0.25 (0.03, 2.11) RR new infection 0.97 (0.19 to 5.04) RR resolution 1.29 (0.88 to 1.89) |
| Cerium nitrate + silver sulfadiazine Silver sulfadiazine alone |
Oen 2012 | CN +SSD 78 SSD 76 |
21 days | ‐ | ‐ | ‐ |
| Merbromin Sodium salicylate Zinc sulfadiazine Sodium salicylate + zinc sulfadiazine Collagenase + chloramphenicol |
Piccolo‐Daher 1990d | Merbromin 25 Sodium salicylate 25 Zinc sulfadiazine 25 Sodium salicylate + zinc sulfadiazine 25 Collagenase + chloramphenicol 25 |
NR | ‐ | ‐ | ‐ |
CN: cerium nitrate; MEBO: moist exposed burn ointment; NR: not reported; SSD: silver sulfadiazine
aChen 2006 also assessed a relevant comparison between antiseptic (silver) and non‐antibacterial (Vaseline gauze)
bLi 1994 also assessed relevant comparisons between two antiseptics (ethacridine lactate and iodophor), between ethacridine lactate and a non‐antibacterial treatment (MEBO) and between iodophor and MEBO. cThomas 1995 also assessed a relevant comparison between antiseptic (chlorhexidine) and topical antibiotic (silver sulfadiazine). dPiccolo‐Daher 1990 also assessed a relevant comparison between an antiseptic and topical antibiotic (zinc sulfadiazine).
4. Summary of secondary outcome data for comparisons.
| Study ID | Number participants/burns | Duration | Adverse events |
Pain Means (SD) |
Mortality | Quality of life |
Resource use Means (SD) |
Costs: Means (SD) Difference in means (95% CI) |
| Antiseptic versus topical antibiotic | ||||||||
| Silver versus SSD | ||||||||
| Abedini 2013 | Silver hydrofibre 35 SSD 34 |
Until healing | ‐ | Doses of fentanyl silver: 3.3 (1.9) SSD 10.3 (4.2) SD extrapolated from graph |
‐ | ‐ | ‐ | Costs of antibiotics, analgesics, dressings, accommodation, nursing/visiting (USD) Silver 26,000 (20,000) SSD 38,000 (30,000) Data extrapolated from graph MD ‐12,000 (‐24,065.99 to 65.99) |
| Caruso 2006 | Silver hydrofibre 42 SSD 40 |
21 days | All Silver 20 SSD 18 RR 1.06 (0.66 to 1.69) Serious Silver 8 SSD 8 RR 0.95 (0.40 to 2.29) |
Participants aged > 4 years (69%) VAS score during dressing changes Silver 3.63 SSD 4.77 P = 0.003 |
Silver 1 SSD 0 |
Dressing changes/day Silver 0.5 (0.1) SSD 1.2 (0.5) Total dressing changes Silver 7.7 (3.9) SSD 19.1 (13.2) MD ‐11.40 (‐15.66 to ‐7.14) |
Cost of nursing time (USD) Silver 14.30 SSD 21.90 Costs of study dressings Silver 684 SSD 398 Cost of all dressings (USD) Silver 845.5 SSD 759.6 Total care Silver 1040 (856.66) SSD 1180 (792.18) MD ‐140 (‐4.96.92 to 216.92) Cost effectiveness /patient healed (USD) Silver 1409.06 (1050.41‐1857.58) SSD 1967.95 (1483.06‐2690.22) MD ‐558.89 (‐1383.08 to 265.30) ICER ‐1019.21 (‐6320.59 to 4054.32) |
|
| Muangman 2010 | Silver hydrofibre 35 SSD 35 |
NR | ‐ | Pain during dressing Day 1: Silver 4.1 (2.1) SSD 6.1 (2.3) Day 3 Silver 2.1 ( 1.8) SSD 5.2 (2.1) Day 7 Silver 0.9 (1.4) SSD 3.3 (1.9) MD ‐1.42 (‐1.95 to ‐0.89) |
‐ | ‐ | ‐ | Total cost (USD) Silver 52 (29) SSD 93 (36) MD ‐ 41.00 (‐56.31 to ‐25.69) Hospital cost Silver 43 (28) SSD 57 (SD 25) Travel cost: Silver 9 (4) SSD 36 (SD 14) |
| Adhya 2015 | Silver hydrogel 84 SSD 79 analysed Silver 54 SSD 52 |
4 weeks/until healing | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Glat 2009 | Silver hydrogel 12 SSD 12 |
21 days+ | Silver 0 SSD 0 |
Pain during dressing changes (Wong‐Baker Faces Pain Scale observational pain assessment scale in infants or toddlers) Silver 2.33 (1.07) SSD 5.33 (1.44) ‐2.28 (‐3.35, ‐1.22) |
‐ | ‐ | Number of dressing changes (over 21 days) Silver 13.50 (4.70) SSD 13.42 (8.26) MD 0.08 (‐5.30 to 5.46) |
‐ |
| Gong 2009 | Silver hydrogel 52 SSD 52 |
21 days+ | During dressing: Silver no significant damage to granulation SSD damage to granulation |
Silver no pain during dressing SSD pain during dressing |
‐ | ‐ | ‐ | ‐ |
| Silverstein 2011 | Silver foam 50 SSD 51 |
21 days | 2 associated withdrawals in each group Other events reported Silver 16 SSD 10 RR 0.75 (0.48, 1.16) |
Dressing application (week 1) Silver 19.1 SSD 40.0 During wear silver 22.0 SSD 35.5 Dressing removal: reported as NS |
Silver 1 SSD 1 |
‐ | Mean number of dressing changes over 3 weeks (SD NR) Silver 2.24 (N = 47) SSD 12.4 Mean time to discharge Silver 5.62 d SSD 8.31 d |
Total costs (USD) Silver 309 (144) SSD 514 (282) Average C‐E Silver 395 (344‐450) SSD 776 (659‐892) ICER ‐1688 Based on 20 participants |
| Tang 2015 | Silver foam 71 SSD 82 |
4 weeks | Silver 4 participants with 5 events SSD 7 participants with 7 events RR 0.66 (0.20, 2.16) |
Baseline Silver 35.3 (22.4) SSD 42.9 (25.8) Week 4 Before dressing removal silver 6.78 (12.95) SSD 11.0 (17.3) MD ‐4.22 (‐9.03 to 0.59) During dressing removal silver 9.23 (13.61) SSD 19.1 (23.9) After dressing removal silver 9.41 (17.33) SSD 15.8 (19.7) MD ‐6.39 (‐12.26 to ‐0.52) |
‐ | ‐ | Total number of dressing changes silver 3.06 SSD 14.0 Per week silver 1.36 SSD 5.67 SD NR |
‐ |
| Yarboro 2013 | Silver foam SSD 24 participants randomised; group allocation unclear |
NR | ‐ | Mean after each treatment Silver 2.92 (1.12) SSD 4.70 (2.22) MD ‐0.98 (‐1.83 to ‐0.12) |
‐ | ‐ | Number of treatments required: Silver 4.10 (1.38) SSD 10.27 (7.46) MD ‐6.17 (‐10.46 to ‐1.88) |
‐ |
| Zhou 2011 | Silver foam SSD 40 participants; part of each burn randomised to each treatment |
14 days | No serious events | ‐ | ‐ | ‐ | ‐ | ‐ |
| Chen 2006a (nanoparticle) | Silver nanoparticle) 65 SSD 63 Vaseline gauze 63 |
Until healing | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Huang 2007 | 98 participants with 166 burns Nanocrystalline silver 83 burns SSD 83 burns |
20 days | No local allergic or systemic symptoms. No side effects related to silver dressing | ‐ | ‐ | ‐ | ‐ | ‐ |
| Muangman 2006 | Nanocrystalline silver 25 SSD 25 |
NR | ‐ | Silver 4 (± 0.6) SSD 5 (± 0.7) MD ‐1.51 (‐2.14 to ‐0.88) |
Silver 0 SSD 0 |
‐ | ‐ | ‐ |
| Varas 2005 | Nanocrystalline silver SSD 14 participants with 2 burn areas; 14 burn areas/group |
NR | Withdrawals due to pain/infection silver 0 SSD 5/10 after 4.8 d (0‐8) |
Silver 3.2 (2.68) SSD 7.9 (2.65) Paired data for 10 participants ‐1.69 (‐2.74 to ‐0.64) |
‐ | ‐ | ‐ | ‐ |
| Liao 2006 | Silver nitrate SSD 120 participants with 2 burns; 120 burns/ group |
Until healing | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Opasanon 2010 | Silver alginate 30 SSD 35 |
NR | ‐ | Silver 2.23 (1.87) SSD 6.08 (2.33) MD ‐1.79 (‐2.37 to ‐1.20) |
‐ | ‐ | Nursing time (min) Silver 8.47 (6.16) SSD 13.29 (4.19) Dressing changes Silver 2.93 (1.17) SSD 14.00 (4.18) MD ‐11.07 (‐12.52 to ‐9.62) |
‐ |
| Honey versus topical antibiotics | ||||||||
| Baghel 2009 | Honey 37 SSD 41 |
NR (2 months' follow‐up) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bangroo 2005 | Honey 32 SSD 32 |
21 days | Contractures or over‐granulation reported in 3 vs 5 cases | Pain reported as "worse" for honey group | ‐ | ‐ | ‐ | ‐ |
| Malik 2010 | Honey SSD 150 participants with 2 burns; 150 burns/group |
24 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mashhood 2006 | Honey 25 SSD 25 |
6 weeks | Honey no allergy or side effects SSD 2 participants irritation/burning (mild) |
Pain free 1 week honey 9 SSD 4 2 weeks honey 20 SSD 11 3 weeks honey 25 SSD 18 4 weeks honey 25 SSD 25 |
‐ | ‐ | ‐ | Cost per % of TBSA affected Honey 0.75 Rupees for 5 mL SSD 10 Rupees for 2 g ointment SD NR |
| Memon 2005 | Honey 40 SSD 40 |
46 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Subrahmanyam 1991 | Honey 52 SSD 52 |
15 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Subrahmanyam 1998 | Honey 25 SSD 25 |
21 days | SSD 4 participants required skin grafting | ‐ | ‐ | ‐ | ‐ | ‐ |
| Subrahmanyam 2001 | Honey 50 SSD 50 |
21 days | No irritation allergy or other side effects. Need for skin grafting Honey 4 SSD 11 |
Subjective relief of pain better in honey group | Hospital stay days Honey 22.0 (1.2) SSD 32.3 (2.0) |
|||
| Sami 2011 | Honey 25 SSD 25 |
60 days | ‐ | Time to complete relief of pain (mean) Honey 12 days SSD 16.8 days Up to 5 days Honey 9 SSD1 6‐12 days Honey 9 SSD 11 13‐21 days Honey 7 SSD 11 22‐26 days Honey 0 SSD 2 |
Amount used per dressing per % burn Honey 5 gm (sic) SSD 2 gm (sic) Based on adult participants |
Cost per dressing per % burn Honey 2.40 Rs SSD 4.92 Rs |
||
| Maghsoudi 2011 | Honey 50 Mafenide acetate 50 |
30 days | Honey 0 Mafenide 0 |
‐ | ‐ | ‐ | ‐ | ‐ |
| Zahmatkesh 2015 | Honey (olea) 10 Mafenide acetate 20 |
20 days | Need for surgical debridement Honey 0/10 Mafenide 13/20 |
‐ | ‐ | ‐ | ‐ | ‐ |
| Aloe Vera versus topical antibiotics | ||||||||
| Khorasani 2009 | Aloe Vera 30 SSD 30 |
24 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Panahi 2012 | Aloe Vera 60 SSD 60 |
14 days | ‐ | Changes from baseline Day 2 Aloe Vera 2.61 (1.55) SSD 1.19 (2.25) Day 7 Aloe Vera 5.13 (2.82) SSD 3.78 (2.83) Day 14 Aloe Vera 5.68 (3.2) SSD 4.54 (2.83) MD 1.14 (0.02 to 2.26) |
‐ | ‐ | ‐ | ‐ |
| Shahzad 2013 | Aloe Vera 25 SSD 25 |
Until healing/ 2 months |
‐ | Time to being pain free reported differently for groups | ‐ | ‐ | ‐ | Cost/%TBSA Aloe Vera 2.40 Rs SSD 4.92 Rs SD NR |
| Thamlikitkul 1991 | Aloe Vera 20 SSD 18 |
26 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Akhtar 1996 | Aloe Vera 50 Framycetin 50 |
NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Iodine versus SSD | ||||||||
| Homann 2007 | Povidone iodine Hydrogel SSD 43 participants each with 2 comparable burns; 43 burns in each group |
21 days | 20 participants with events. 6 systemic and considered unrelated to study interventions Iodine 6 (5 pain) SSD 7 (5 pain) |
‐ | ‐ | ‐ | ‐ | ‐ |
| Li 1994b | MEBO 31 Iodophor 24 Ethacridine lactate 22 SSD 38 |
Until healing | ‐ | ‐ | ‐ | ‐ | ‐ | All RMB (Chinese Yuan) MEBO 1836 (542.35) Iodophor 621 (130.83) Ethacridine 598 (125.43) SSD 674 (191.50) Ethacridine vs SSD MD ‐76.00 (‐1.56.34 to 4.34) Iodophor vs Ethacridine MD 23 (‐51.07 to 97.07) Ethacridine vs MEBO MD ‐1238 (‐1435.98 to ‐1040.02) Iodophor vs SSD MD ‐53.00 (‐133.29 to 27.29) Iodophor vs MEBO MD ‐1215 (‐1412.96 to ‐1017.04) |
| Other antiseptics versus topical antibiotics | ||||||||
| Ning 2008 | 20 participants with 2 burns (20 burns/group) | 28 days | No serious events in either group. | ‐ | ‐ | ‐ | ‐ | ‐ |
| Radu 2011 | Octenidine SSD 30 participants with 2 burn areas; 30 burns in each group |
24 hours | ‐ | Median VAS Octenidine 3 (1‐6) SSD 6 (3‐8) |
‐ | ‐ | ‐ | ‐ |
| Piatkowski 2011 | Polyhexanide 30 with 38 burns SSD 30 with 34 burns |
NR | ‐ | Graph data Baseline Polyhexanide Change 7.8 Between 1.2 SSD Change 8 Between 3 Day 1 Polyhexanide Change 4.2 Between 0.8 SSD Change 6 Between 2.6 Day 3 Polyhexanide Change 2.2 Between 0.2 SSD Change 5 Between 1.8 Day 5 Polyhexanide Change 1.4 Between 0.1 SSD Change 4 Between 1 Day 7 Polyhexanide Change 0.8 Between 0.1 SSD Change 3 Between 0.8 Day 10 Polyhexanide Change 0.2 Between 0.5 SSD Change 2 Between 0.5 Day 14 Polyhexanide Change 0 Between 0 SSD Change 1.4 Between 0 SD NR |
‐ | ‐ | ‐ | Costs/day (EUR) Materials Polyhexanide 5.14 SSD 6.96 Personnel Polyhexanide 9.63 SSD 9.63 Total Polyhexanide 14.77 SSD 16.59 SD NR |
| Nasiri 2016 |
Arnebia euchroma SSD 49 participants with 2 burns; 49 burns in each group |
36 days but up to 10 days for secondary outcomes | Specific complications such as burning, pain, itching, warming , allergic reactions and requiring skin graft. Scores reported for itching and warming. Skin graft risk A euchroma 2.2% (2.2 to 6.7) SSD 6.7% (0.9 to 14.3) |
Pain scores reported graphically for days 1, 3, 5 and 10 for minutes 1, 5 and 15 after dressing. Graphs appeared to show overlapping CI but P reported < 0.05 (CI could not be extracted) | ‐ | ‐ | ‐ | ‐ |
| Antiseptics versus alternative antiseptics | ||||||||
| Han 1989 | Iodine 111 Chlorhexidine 102 |
NR | ‐ | Pain at rest Iodine (N = 84) 9.18 (15.11) Chlorhexidine (N = 78) 11.44 (14.27) MD 2.26 (‐2.26 to 6.78) Pain on dressing removal Iodine (N = 92) 6.66 (11.06) Chlorhexidine (N = 84) 8.75 (15.84) MD 2.09 (‐2.00 to 6.18) |
‐ | ‐ | Number hospital visits (N unclear) Iodine 2.64 (1.45) Chlorhexidine 3.03 (1.62) MD 0.25 (‐.0.02 to 0.52) |
‐ |
| Antispetic versus non‐antibacterial treatment | ||||||||
| Jiao 2015 | Nanocrystalline silver 38 Vaseline gauze 38 |
30 days | Scar hyperplasia reported; no other data | ‐ | ‐ | ‐ | ‐ | ‐ |
| Healy 1989 | Silver xenograft 16 Petroleum gauze 16 |
14 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Subrahmanyam 1993b | Honey 46 Polyurethane film 46 |
NR | Honey 4 noted Polyurethane 6 noted Not clear all events were reported/basis of reported events |
‐ | ‐ | ‐ | ‐ | ‐ |
| Subrahmanyam 1994 | Honey gauze 40 Amniotic membrane 24 |
30 days | Honey 4/40 Amniotic 5/24 Not clear all events were reported/basis of reported events |
Numbers with pain evaluated with 4‐point scale None/mild Honey 33/40 Amniotic 13/24 Moderate/severe Honey 7/40 Amniotic 11/24 |
‐ | ‐ | ‐ | ‐ |
| Subrahmanyam 1996a | Honey 50 Potato peel 50 |
21 days | "Allergy or other side effects were not observed in any patient of either group" | "Subjective relief of pain was the same in both groups" | ‐ | ‐ | ‐ | ‐ |
| Subrahmanyam 1996b | Honey 450 "Conventional dressing" 450 |
NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Inman 1984 | SSD + chlorhexidine 54 assessed SSD only 67 assessed Unclear if additional post‐randomisation exclusions |
Until healing (26 days) | ‐ | Pain sufficient to stop treatment Chlorhexidine 1/54 SSD alone 0/67 |
Chlorhexidine 3/54 SSD alone 4/67 RR 0.93 (0.22 to 3.98) Infection‐related chlorhexidine 3/54 SSD alone 0/67 |
‐ | ‐ | ‐ |
| Neal 1981 | Chlorhexidine 25 Polyurethane 26 |
30 days | ‐ | Qualitative data only (chlorhexidine perceived as more painful) | ‐ | ‐ | ‐ | ‐ |
| Phipps 1988 | Chlorhexidine 104 Hydrocolloid 92 |
NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Thomas 1995c | Chlorhexidine tulle‐gras 18 Hydrocolloid 16 Hydrocolloid + SSD 16 |
NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Wright 1993 | Chlorhexidine 49 Hydrocolloid 49 |
NR | Chlorhexidine 1 Hydrocolloid 5 Denominator unclear |
VAS (summed for each visit) Chlorhexidine (N = 31) Hydrocolloid (N = 36) P = 0.284 |
‐ | ‐ | Number dressings Chlorhexidine 2.8 Hydrocolloid 2.61 SD NR |
‐ |
| Carayanni 2011 | Povidone iodine + Bepanthenol 107 MEBO 104 |
18 days | "Complications" Iodine 8 MEBO 11 RR 1.30 (0.47 to 3.61) |
Median pain scores reported graphically. Analgesia requirements also reported | ‐ | ‐ | Reduction of hospital stay (subtracted from a standard length of stay (10 days)) Iodine ‐3.01 (2.02) MEBO ‐3.63 (2.19) MD 0.62 (0.05 to 1.19) |
Costs of hospital stay including medicines and examinations and the visits and treatments after discharge 2006 (EUR) Total MEBO 529.66 (172.75) Total iodine 566.21 (151.45) MD 36.55 (‐7.33 to 80.43) ICERs reported per day of hospitalisation and per day of recovery gained. Total/hospitalisation day gained ‐58.95E (‐63.10, ‐55.09) (favours MEBO) |
| Li 2006 | Iodine gauze 74 Superficial 16 Deep 32 Residual 26 Carbon‐fibre dressing 203 Superficial 46 Deep 89 Residual 68 |
NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Yang 2013 | 60 participants with burn wounds; 60 burn areas/group (Iodophor gauze/ hydrogel) |
14 days | ‐ | Dressing change pain Iodophor 43 wounds caused evident pain (VAS score 3‐6) Hydrogel 37 wounds caused mild pain (VAS 1‐3) |
‐ | ‐ | ‐ | ‐ |
| De Gracia 2001 | Cerium nitrate + SSD 30 SSD 30 |
Until healing/ readiness for grafting |
‐ | ‐ | CN + SSD 1/30 SSD 4/30 RR 0.25 (0.03 to 2.11) |
‐ | Days of hospitalisation CN + SSD 23.3 (11.4) SSD 30.7 (22.7) MD ‐7.40 (‐16.49 to 1.69) |
‐ |
| Oen 2012 | Cerium nitrate + SSD 78 SSD 76 |
21 days | ‐ | Mean (SEM) CN + SSD 0.6 (0.2) SSD 1.2 (0.4) MD Procedural Mean (SEM) CN + SSD 1.3 (0.3) SSD 1.6 (0.5) MD ‐0.60 (‐0.70 to ‐0.50) |
CN SSD 1 SSD 5 RR 0.19 (0.02 to 1.63) |
‐ | ‐ | ‐ |
| Piccolo‐Daher 1990d | Multiple comparisons Merbromin 25 Sodium salicylate 25 Zinc sulfadiazine 25 Sodium salicylate + zinc sulfadiazine 25 Collagenase + chloramphenicol 25 |
NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
C‐E: cost‐effectiveness; CN: cerium nitrate; ICER: incremental cost‐effectiveness ratio; MEBO: moist exposed burn ointment; NR: not reported; NS: not significant; SD: standard deviation; SEM: standard error of mean; SSD: silver sulfadiazine; TBSA: total body surface area; VAS: visual analogue scale
aChen 2006 also assessed a relevant comparison between antiseptic (silver) and non‐antibacterial (Vaseline gauze)
bLi 1994 also assessed relevant comparisons between two antiseptics (ethacridine lactate and iodophor), between ethacridine lactate and a non‐antibacterial treatment (MEBO) and between iodophor and MEBO. cThomas 1995 also assessed a relevant comparison between antiseptic (chlorhexidine) and topical antibiotic (silver sulfadiazine). dPiccolo‐Daher 1990 also assessed a relevant comparison between an antiseptic and topical antibiotic (zinc sulfadiazine).
Comparison between antiseptics and topical antibiotics
1. Silver‐based antiseptic treatments versus topical antibiotics (16 studies, 1368 participants)
Silver‐based treatments included silver foam dressings, silver hydrogel dressings, silver alginate dressing, nanocrystalline silver dressing, silver hydrofibre dressing and silver nitrate. In each case the topical antibiotic used was silver sulfadiazine (SSD). Four studies randomised a total of 373 participants and assessed nanocrystalline silver (Chen 2006; Huang 2007; Muangman 2006; Varas 2005). Four studies with a total of 318 participants assessed silver foam dressings (Silverstein 2011; Tang 2015; Yarboro 2013; Zhou 2011). Silver hydrogel dressings were assessed in three studies (Adhya 2015; Glat 2009; Gong 2009; 191 participants) as were silver hydrofibre dressings (Abedini 2013; Caruso 2006; Muangman 2010; 201 participants). Single studies assessed a silver alginate dressing (Opasanon 2010, 65 participants) and silver nitrate treatment (Liao 2006, 120 participants).
Two studies included only children (Glat 2009; Zhou 2011) and three included only adults (Gong 2009, Huang 2007; Varas 2005). The remaining studies included both adults and children or did not report this. Most studies included recent burns described as second‐degree or partial‐thickness but one (Huang 2007) included only residual burns, unhealed despite previous treatment. The percentage of total body surface area affected (TBSA) was below 40% in all except one study (Adhya 2015 included burns up to 60% TBSA) and several studies imposed lower limits of 10%, 15% or 25%.
Four studies used burns rather than participants as the unit of analysis (Huang 2007; Liao 2006; Varas 2005; Zhou 2011). In Huang 2007 the randomisation was at the level of the participant but the analysis was conducted at the level of the burn wound, that is multiple burns on the same participants were treated with the same treatment and outcome data for the different wounds analysed (clustered data); the other studies employed an intra‐individual (split‐body) design for both randomisation and analysis; in each case it was not clear whether the analysis had adjusted appropriately. Although Tang 2015 enrolled participants with multiple burns, a single burn was selected at study enrolment and both randomisation and analysis were at the participant level.
Primary outcome: wound healing
Most studies reported some data on wound healing with this being presented in different ways. There were three studies (259 participants) with sufficient data to calculate a HR for healing and we pooled these data (Caruso 2006; Glat 2009; Tang 2015). In this analysis, on average, the use of silver‐containing antiseptics treatment (mainly dressings) showed no clear difference in time to healing compared with SSD; the estimate is imprecise, with CIs spanning benefits and harms (HR 1.25, 95% CI 0.94 to 1.67; I2 = 0%) Analysis 1.1 (low certainty evidence, downgraded twice for imprecision; confidence intervals included both the possibility of a 6% decrease and a 67% increase in the 'chance' of healing).
1.1. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 1 Wound healing (hazard ratio).
Mean time to wound healing was reported in ten studies where it seemed that all wounds had healed. On average, silver‐containing antiseptic treatments (mainly dressings) may decrease slightly the mean time to healing of burns compared with SSD (MD ‐3.33 days; 95% CI ‐4.96 to ‐1.70; I2 = 87%) (low certainty evidence downgraded once for risks of bias (variously detection, selection, reporting and other sources of bias across four of the studies and 30% of the analysis weight) and once for inconsistency due to high heterogeneity). Although statistical heterogeneity was high, all studies had the same direction of effect and favoured silver‐containing antiseptics Analysis 1.2. This was based on nine studies; Silverstein 2011 did not report measures of variance. We used a post‐hoc sensitivity analysis to explore the impact of including studies which may not have adjusted for clustered or intra‐individual designs. Excluding Huang 2007; Liao 2006; and Zhou 2011 from the analysis resulted in a lower level of heterogeneity (I2 = 36%) and a slightly larger estimate of effect (MD ‐4.53 days, 95% CI ‐5.74 to ‐3.32); excluding only trials with intra‐individual designs (Liao 2006; and Zhou 2011) or only the trial with unit of analysis issues (Huang 2007) also produced little difference.
1.2. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 2 Wound healing (mean time to healing).
The RR for short‐term follow‐up (maximum 28 days) suggested that on average the use of silver‐containing antiseptics may lead to a small difference in number of healing events over one month compared with SSD: RR 1.17 (95% CI 1.00 to 1.37; I2 = 45%) (Analysis 1.3) (low certainty evidence, downgraded once due to risk of detection bias in two studies and selection bias in one study; and once due to imprecision).
1.3. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 3 Wound healing (risk ratio) up to 28 days.
Primary outcome: infection
Incident infections were reported in three studies: Caruso 2006; Glat 2009; Muangman 2006. Tang 2015 reported new signs of wound inflammation which we grouped with the incident infections. It is uncertain whether use of silver‐containing antiseptics prevents infection compared with SSD because the certainty of the evidence is very low: RR 0.84 (95% 0.48 to 1.49; I2 = 0%) Analysis 1.4 (very low certainty evidence, downgraded once each for risk of bias (variously reporting, detection and selection), imprecision and indirectness). Huang 2007 reported bacterial clearance rates (including for specific strains including MRSA); these data are noted in Table 11.
1.4. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 4 Infection (up to 4 weeks or NR).
Secondary outcome: adverse events
Eight studies reported some data on adverse events (Caruso 2006; Glat 2009; Gong 2009; Huang 2007; Silverstein 2011; Tang 2015; Varas 2005; Zhou 2011); six studies reported the proportion of all participants with adverse events.
There was no clear difference in the incidence of adverse events between silver‐containing antiseptics and SSD in the number of participants with adverse events; the estimate is imprecise with wide CIs spanning benefits and harms: RR 0.86 (95% CI 0.63 to 1.18; I2 = 0%) (Analysis 1.5) (low certainty evidence, downgraded once for risks of bias (variously detection, selection, reporting and other sources of bias) across five of the trials, and once for imprecision). The analyses included six trials, three of which reported that there were no events. We considered a post‐hoc sensitivity analysis to explore the impact of including studies that may not have adjusted for clustered or intra‐individual designs, however, both Huang 2007 and Zhou 2011 reported zero events and therefore did not contribute weight to the analysis.
1.5. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 5 Adverse events (14‐28 days).
Other trials reported data relating to withdrawals or specific event types (Gong 2009) including serious adverse events and withdrawals due to adverse events: these data are not included in the main analysis but are reported separately (Analysis 1.6; Table 12). Because one of the two trials (Silverstein 2011; Varas 2005) reporting withdrawals due to adverse events had an intra‐individual design we both present pooled data for this analysis and report the results of the trials separately (Table 12).
1.6. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 6 Withdrawals due to adverse events (21 days or NR).
Secondary outcome: pain
Eleven trials reported some data on pain. The most commonly reported measures were pain in general (or at an unspecified time) and pain at dressing change. Caruso 2006; Glat 2009; Muangman 2010;Tang 2015; and Yarboro 2013 reported usable data on pain at dressing change. Gong 2009 reported only the presence of pain at dressing change in the SSD group and its absence in the silver‐based antiseptic group, and Silverstein 2011 only that there was no significant difference between the groups.
Silver‐based antiseptic treatments may on average slightly reduce pain at dressing change compared with SSD, SMD ‐1.20 (95% CI ‐1.92 to ‐0.49; I2 = 81%) (low certainty evidence, downgraded once for imprecision and once for inconsistency). There was significant statistical heterogeneity between the studies, but all of the trials reported lower pain levels in the silver antiseptic group than in the SSD group Analysis 1.7.
1.7. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 7 Pain at dressing change (up to 28 days or NR).
A general measure of pain was reported by three trials (Muangman 2006; Opasanon 2010; Varas 2005). Silver‐based antiseptic treatments may, on average, slightly reduce generally reported pain compared with SSD. Pain scores may on average be slightly lower in participants treated with silver dressings; the SMD was ‐1.66 (95% CI ‐2.06 to ‐1.27; I2 = 0%). Analysis 1.8 (low certainty evidence, downgraded once for risk of reporting bias or attrition bias affecting over half the participants and once for imprecision). We used a post‐hoc sensitivity analysis to explore the impact of including studies that may not have adjusted for clustered or intra‐individual designs. Excluding Varas 2005 resulted in no change to the estimate of effect but slightly wider confidence intervals (SMD ‐1.66, 95% CI ‐2.74 to ‐0.64; I2 = 0%). Further pain‐related measures, which could not be analysed here, are reported in Table 12.
1.8. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 8 Pain (time/follow‐up not specified).
Secondary outcome: mortality
Three trials reported mortality: Caruso 2006; Silverstein 2011 and Muangman 2006. It is uncertain whether silver‐containing antiseptic treatments have an effect on mortality. The RR was 1.59 (95% CI 0.20 to 12.64; I2 = 0%) Analysis 1.9. (very low certainty evidence, downgraded once for risks of detection bias and reporting bias and twice for imprecision; two trials at risk of detection bias, one at risk of reporting bias).
1.9. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 9 Mortality (21 days or NR).
Secondary outcome: resource use
Number of dressing changes was reported by six trials (Caruso 2006; Glat 2009; Opasanon 2010; Silverstein 2011; Tang 2015; Yarboro 2013). Participants treated with silver‐based antiseptics (dressings) may require fewer dressing changes compared with those treated with SSD. Data from four studies (Silverstein 2011 and Tang 2015 did not report measures of variance) suggests that, on average, silver‐containing antiseptics (dressings) may reduce the number of dressing changes, MD ‐7.56 dressing changes (95% CI ‐12.09 to ‐3.04; I2 = 84%) Analysis 1.10 (low certainty evidence, downgraded once for risks of detection and selection bias affecting three trials with 45% of analysis weight, and once for imprecision). The number of minutes of nursing time required was also reported by Opasanon 2010, this also showed that there may be a small benefit to silver‐based antiseptics (difference in means ‐4.82 minutes, 95% CI ‐7.42 to ‐2.22) (low certainty evidence, downgraded twice for imprecision) (Table 12). Silverstein 2011 reported mean time to discharge but without measures of variance; the data are shown in Table 12 but are not further analysed.
1.10. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 10 Resource use (number of dressings) (up to 28 days or NR).
Secondary outcome: costs
Four trials reported data for total costs of treatment (Abedini 2013; Caruso 2006; Muangman 2010; Silverstein 2011 (based on a subset of 20 participants' data)). It is uncertain whether or not silver‐based antiseptic dressings are cheaper overall than SSD. The pooled difference in means across the four trials was USD ‐117.18 (95% CI ‐280.02 to 45.67; I2 = 68%) Analysis 1.11. This is very low certainty evidence downgraded once for risk of detection bias in three of the four studies (accounting for over 50% of participants) and twice for imprecision (confidence intervals included both possibilities of cost reduction (USD 280) and increase (USD 46)). Cost‐effectiveness data from Caruso 2006 and Silverstein 2011 also showed uncertainty as to whether silver dressings were more cost‐effective than SSD (very low certainty evidence, downgraded twice for imprecision and once for risk of detection bias in both studies) (Table 12).
1.11. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 11 Costs (21 days or NR).
Summary of comparison
Low certainty evidence reporting the hazard or 'chance' of healing over time suggested that there may be a small benefit for burns treated with silver‐based antiseptics (mainly silver‐containing dressings) compared with SSD but confidence intervals were wide, spanning both benefits and harms so clear differences between treatments are not apparent. Low certainty evidence also showed that mean time to healing may be somewhat (3 days) shorter with silver‐based antiseptics compared to SSD. There is very low certainty evidence on infection incidence and mortality, meaning that it is unclear what the effect of the different interventions may be. There is low certainty evidence on adverse events suggesting that there may be little or no difference between the treatments. Pain scores may be slightly lower in participants treated with silver compared with SSD (low certainty evidence). Table 1
2. Honey or honey‐based dressings versus topical antibiotic (11 studies, 856 participants)
Nine studies used honey (variously described as pure, undiluted, unprocessed) (Baghel 2009; Malik 2010; Mashhood 2006; Memon 2005; Maghsoudi 2011; Sami 2011; Subrahmanyam 1991; Subrahmanyam 1998; Subrahmanyam 2001), one a honey dressing (Bangroo 2005) and one olea which contains honey and olive and sesame oils (Zahmatkesh 2015). Eight studies used SSD as the comparator and two used mafenide acetate (Maghsoudi 2011; Zahmatkesh 2015).
Nine studies included a mix of adults and children (Malik 2010; Mashhood 2006; Memon 2005; Maghsoudi 2011; Sami 2011; Subrahmanyam 1991; Subrahmanyam 1998; Subrahmanyam 2001; Zahmatkesh 2015), one included only adults (Baghel 2009), and one only children (Bangroo 2005). Six studies included participants with burns less than 40% TBSA (Malik 2010; Memon 2005; Maghsoudi 2011; Subrahmanyam 1991; Subrahmanyam 1998; Subrahmanyam 2001); two studies specified less than 50% TBSA (Baghel 2009; Bangroo 2005) and one less than 15% TBSA (Mashhood 2006). One study (Malik 2010) used an intra‐individual design and randomised burns on each participant to the treatments.
Primary outcome: wound healing
All studies reported some measure of wound healing. One study reported the mean time to healing of all wounds but with no measure of variance reported (Baghel 2009). A second study reported full data on only one intervention group (Bangroo 2005). Zahmatkesh 2015 reported the median time to formation of granulation tissue. These data are all presented in Table 10 but are not analysed further.
We could calculate HRs for healing for five studies (Maghsoudi 2011; Malik 2010; Mashhood 2006; Memon 2005; Sami 2011). Honey probably on average reduces time to healing compared with topical antibiotics: HR 2.45 (95% CI 1.71 to 3.52; I2 = 66%) Analysis 2.1 (moderate certainty evidence, downgraded once due to imprecision). This would correspond to an additional 278 (95% CI 185 to 332) more burns healed over time for every 1000 burns treated. We used a post‐hoc sensitivity analysis to explore the impact of including the study with an intra‐individual design (Malik 2010). The results of this sensitivity analysis differed little from the main analysis (HR 2.31, 95% CI 1.43 to 3.71; I2 = 67%).
2.1. Analysis.
Comparison 2 Honey versus topical antibiotics, Outcome 1 Wound healing (hazard ratio).
Six studies reported the mean time to healing where all wounds healed but for two studies (Baghel 2009; Memon 2005) no measure of variance was available. Honey may slightly shorten the mean number of days to wound healing compared with topical antibiotics). Based on analysis of four studies, the average mean time to healing was ‐3.79 days (95% CI ‐7.15 to ‐0.43; I2 = 96%) shorter in participants treated with honey compared with those treated with SSD, and all studies showed the same direction of effect despite high statistical heterogeneity Analysis 2.3. We used a post‐hoc sensitivity analysis to explore the impact of including the study with an intra‐individual design (Malik 2010). The estimate of effect was increased slightly, but wider confidence intervals included the possibility of a small increase in mean time to healing as well as a decrease (MD ‐4.36 days, 95% CI ‐8.90 to 0.17; I2 = 95%). This would be very low certainty evidence, downgraded twice for imprecision and once for inconsistency; in order to be conservative we have adopted the GRADE assessment based on the sensitivity analysis because it ascribes less certainty to the findings than that based on the main analysis.
2.3. Analysis.
Comparison 2 Honey versus topical antibiotics, Outcome 3 Wound healing (mean time to healing).
The RR for short‐term follow up (maximum 21 days) also suggested that, on average honey, probably leads to more short‐term healing events than topical antibiotic treatment: RR 2.18 (95% CI 1.15 to 4.13; I2 = 94%). Over a longer period of up to 60 days the RR was 1.65 (95% CI 0.99 to 2.76; I2 =99%), including the data from the last time points of Mashhood 2006 and Sami 2011. Data from a study which used different time points for the two groups were not included but contribute to the HR (Memon 2005). In each case this is low certainty evidence, downgraded once for imprecision and once for inconsistency.
Primary outcome: infection
Change in infection status
Eight studies comparing honey with topical antibiotics reported some measure of change in infection status. Four reported incident infection (Malik 2010; Maghsoudi 2011; Subrahmanyam 1998; Zahmatkesh 2015); three reported persistent infection (Sami 2011; Subrahmanyam 1991; Subrahmanyam 2001) and one reported time for swab cultures to become negative (Mashhood 2006) but with no measures of variance; these data are reported in Table 11 but are not further analysed. Most studies used a measure of infection based on swab cultures which is not a measure of clinical infection. Only Maghsoudi 2011, which compared honey with mafenide acetate, reported incidence of new clinical signs of infections (at 7 and 21 days).
Incident infections
It is uncertain if fewer burns treated with honey may become infected compared with those treated with topical antibiotics (SSD or mafenide acetate) when assessed at time points between seven and 24 days. The RR was 0.16 (95% 0.08 to 0.34; I2 = 0%) Analysis 2.4. This is very low certainty evidence, downgraded for twice for indirectness in all studies except Maghsoudi 2011 and once for imprecision due to low numbers of events. It was unclear if the analysis in Malik 2010 was adjusted for paired data. Excluding this study in a post‐hoc sensitivity analysis did not materially change the result; the RR was 0.09 (95% 0.02 to 0.35; I2 = 0%).
2.4. Analysis.
Comparison 2 Honey versus topical antibiotics, Outcome 4 Incident infection (up to 24 days).
Persistent infections
It is uncertain if wounds may be more likely to become infection free at 15 (Subrahmanyam 1991) or 21 days (Subrahmanyam 2001) in groups treated with honey compared with those treated with SSD. The RR was 0.10 (95% CI 0.05, 0.19; I2 = 0%) Analysis 2.5 (very low certainty evidence, downgraded once for imprecision and twice for indirectness in all studies except Maghsoudi 2011). Sami 2011 reported the proportion of participants with continuing positive cultures at multiple time points up to six weeks, at which point all were culture negative (Table 11).
2.5. Analysis.
Comparison 2 Honey versus topical antibiotics, Outcome 5 Persistent positive swabs (up to 21 days).
Secondary outcome: adverse events
Three studies comparing honey with topical antibiotics reported adverse events for all participants (Maghsoudi 2011; Mashhood 2006; Subrahmanyam 2001). Other trials reported only individual types of events but it was very unclear whether these data related to the number of participants experiencing adverse events or whether multiple events may have been reported for some individuals. These data are noted in Table 12 but are not analysed further. It is uncertain whether fewer participants treated with honey experience adverse events compared with those treated with SSD. There were no events in two trials and two events in the topical antibiotics group in the other trial; the RR was 0.20 (95% CI 0.01 to 3.97; I2 not calculable) Analysis 2.6. This is very low certainty evidence, downgraded once because of risks of detection bias in Mashhood 2006 and twice because of imprecision.
2.6. Analysis.
Comparison 2 Honey versus topical antibiotics, Outcome 6 Adverse events (time points between 21 days and 6 weeks).
Secondary outcome: pain
Four studies (Bangroo 2005; Mashhood 2006; Subrahmanyam 2001; Sami 2011) reported some data on pain. No study reported pain using a recognised scale and these data are presented in Table 12 but are not further analysed; no GRADE assessment was possible.
Secondary outcome: resource use
One study (Subrahmanyam 2001) reported on the length of hospital stay in participants treated with honey or SSD. There is probably a shorter length of stay in participants treated with honey compared with SSD (difference in means ‐10.30 days, 95% CI ‐10.95 to ‐9.65) (Table 12). This is moderate certainty evidence, downgraded once for imprecision. A second study (Sami 2011) reported the amounts of honey or SSD required per dressing per percentage area burned. No measures of variance were reported; these data are reported in Table 12 but are not further analysed; no GRADE assessment was possible.
Secondary outcome: costs
Two studies (Mashhood 2006; Sami 2011) reported on costs of treating burns with honey or SSD but did not report any measure of variance; these data are reported in Table 12 but are not further analysed; without an estimate of effect it is difficult to provide a GRADE assessment for the outcome.
Summary of comparison
Honey on average probably reduces the time to healing compared with topical antibiotics, assessed by evidence reporting the hazard or 'chance' of healing over time (moderate certainty evidence). The mean time to healing may, on average, be reduced in wounds treated with honey compared with topical antibiotics (low certainty evidence). Compared with topical antibiotics, honey may, on average, increase the number of healing events assessed over the short term (up to 3 weeks) but it is unclear whether this is still the case when studies with longer follow‐up are included (low certainty evidence). It is unclear if there are fewer infections in wounds treated with honey compared with topical antibiotics, and whether fewer initial infections persist (very low certainty evidence). It is uncertain whether the incidence of adverse events differs between groups (very low certainty evidence). Table 2
3. Aloe Vera versus topical antibiotics (5 studies, 338 participants)
Four studies compared Aloe Vera to SSD (Khorasani 2009; Panahi 2012; Shahzad 2013; Thamlikitkul 1991) and one compared it to framycetin cream (Akhtar 1996). The Aloe Vera was administered in a variety of creams, gel or dressings. The concentration of Aloe Vera was 0.5% in the one study that reported this (Khorasani 2009).
Three studies included mostly adults (Khorasani 2009; Panahi 2012; Shahzad 2013), one did not report participant age (Akhtar 1996) and in one the mean ages suggested a mix of adults and children (Thamlikitkul 1991). Inclusion criteria for TBSA of burns ranged from less than 5% (Panahi 2012) to less than 40% (Akhtar 1996). One study (Khorasani 2009) used an intra‐individual design.
Primary outcome: wound healing
Four studies reported data on wound healing. Three reported mean time to healing of all wounds (Akhtar 1996; Khorasani 2009; Shahzad 2013). Akhtar 1996 did not report a measure of variance, so these data are reported in Table 10 but are not further analysed. Based on the pooled data from the remaining two studies it is uncertain whether there is a difference in mean time to healing between treatments: average difference in means was ‐7.79 days (95% CI ‐17.96 to 2.38; I2 = 94%) Analysis 3.1. This is very low certainty evidence, downgraded once for risk of detection bias in a study accounting for 48% of the weight in the analysis (Shahzad 2013), once for inconsistency and twice for very high levels of imprecision. The confidence intervals included the possibility of healing time being shorter by almost 18 days or being two days longer. Because Khorasani 2009 used an intra‐individual design, we also note the separate MD for this study (MD ‐2.85 days, 95% CI ‐4.04 to ‐1.66) and Shahzad 2013 (MD ‐13.24 days, 95% CI ‐17.91 to ‐8.57).
3.1. Analysis.
Comparison 3 Aloe vera vs topical antibiotics, Outcome 1 Wound healing (mean time to healing).
One study reported the proportion of wounds healed at 26 days (Thamlikitkul 1991). It is unclear whether Aloe Vera may alter the number of healing events compared with SSD; confidence intervals were wide spanning both benefits and harms so clear differences between treatments are not apparent (RR 1.41, 95% CI 0.70 to 2.85) (Table 10). This is low certainty evidence, downgraded twice for serious imprecision; confidence intervals included the possibility of both a 30% reduction and a 285% increase in the chance of wound healing. None of the studies reported sufficient information for us to calculate an HR for wound healing.
Primary outcome: infection
Three studies reported data on the incidence of infections at different time points (Khorasani 2009 (24 days); Panahi 2012 (14 days); Shahzad 2013 (unclear time point)).
It is uncertain whether there is a difference between the groups (RR 0.93, 95% CI 0.26 to 3.34; I2 = 0%) Analysis 3.2 . This is very low certainty evidence, downgraded once for risk of detection bias because 84% of the analysis weight was represented by Shahzad 2013, which had a high risk of detection bias, and twice for imprecision. Very wide confidence intervals included both the possibility of lower (by 74%) or much higher (by over 300%) infection rates in the Aloe Vera groups. Khorasani 2009 which used an intra‐individual design reported zero events and therefore a sensitivity analysis to explore the impact of including it in the analysis is not required. Akhtar 1996 reported data on the grade of infection, which is reported in Table 11 but is not further analysed.
3.2. Analysis.
Comparison 3 Aloe vera vs topical antibiotics, Outcome 2 Infection (time points between 14 days and 2 months).
Secondary outcome: pain
One study reported the mean reductions in pain scores from baseline (Panahi 2012) and another reported time taken to achieve pain‐free status; data from this study were reported differently between the groups and are presented in Table 12 but not analysed further (Shahzad 2013). The data from Panahi 2012 suggest that there is probably a slightly greater decrease in pain in the Aloe Vera group (mean decrease from baseline 5.68) compared with the SSD group (mean decrease from baseline 4.54). The difference in means was 1.14 (95% CI 0.02 to 2.26) (Table 12). This is moderate certainty evidence downgraded once due to imprecision.
Secondary outcome: costs
One study reported data on the cost per percentage of TBSA healed but with no measures of variance (Shahzad 2013). These data are reported in Table 12 but are not further analysed; no GRADE assessment was possible.
Summary of comparison
It is uncertain whether there is a difference in the mean number of days to healing between Aloe Vera and topical antibiotics (very low certainty evidence, downgraded due to detection bias and imprecision). It is unclear whether Aloe Vera may change the proportion of burns healed at 26 days compared with SSD (low certainty evidence, downgraded twice for imprecision). It is uncertain whether there is a difference in the incidence of infection between the groups (very low certainty evidence, downgraded due to detection bias and imprecision). Table 3.
4. Iodine‐based treatments versus topical antibiotic (2 studies, 158 participants)
Two studies compared an iodine‐based treatment with SSD (Homann 2007; Li 1994). Li 1994 was a four‐armed study that compared 0.25% iodophor with SSD, and also included groups treated with ethacridine lactate (Rivanol) and moist burn ointment (see comparisons 8, 12, 16 and 17). There were 115 participants (aged over 16 years) with injuries described as deep second‐degree burns between 1% to 12% TBSA in the trial, of whom 62 were in groups relevant to this comparison. Homann 2007 used an intra‐individual study design and compared 3% pyrrolidone iodine liposome hydrogel (Repithel) with SSD (10 mg/g) in 43 participants with a mean TBSA of 11%; their age was not reported.
Primary outcome: wound healing
Both Homann 2007 and Li 1994 reported the mean time to healing of all wounds; this showed that the effect of iodine was very uncertain. The pooled difference in means was ‐0.47 days (95% CI ‐2.76 to 1.83; I2 = 42%) Analysis 4.1; this is very low certainty evidence, downgraded once due to risk of detection bias for the participants in Homann 2007 and twice due to imprecision; very wide confidence intervals included both the possibility of longer (by 2.8 days) or shorter (by 1.8 days) healing for participants in the iodine antiseptic group. It was not clear whether the analysis accounted for the paired data. Because of the intra‐individual design used by Homann 2007 we also report separately the effect estimate for this study (MD ‐1.40 days, 95% CI ‐3.39 to 0.59) and for Li 1994 (MD 1.00 days; 95% CI ‐1.98 to 3.98).
4.1. Analysis.
Comparison 4 Iodine‐based treatments versus topical antibiotics, Outcome 1 Wound healing (mean time to healing).
Primary outcome: infection
Neither study reported data on change in infection status.
Secondary outcome: adverse events
Homann 2007 reported data on adverse events in all participants and distinguished local events (which could be related to the different treatments given to the participants). It is uncertain whether there is a difference in incidence of adverse events between the groups; the RR was 0.86 (95% CI 0.35 to 2.10) (Table 12). This is very low certainty evidence, downgraded once due to risk of detection bias and twice due to high levels of imprecision; wide confidence intervals included both the possibility of both a 65% decrease and a 210% increase in events in the intervention group. It is also unclear whether the intra‐individual design was accounted for in the analysis.
Secondary outcome: costs
Li 1994 reported total treatment costs for each intervention group. There may be little or no difference in cost between the iodine and SSD treatments. The mean cost for the iodine group was RMB 621 compared with RMB 674 for the SSD group; the difference in means was RMB ‐53 (‐133.29 to 27.29) Table 12. This is low certainty evidence, downgraded twice due to high levels of imprecision; confidence intervals included both the possibility of substantially lower (RMB ‐133) and somewhat higher (RMB 27) costs.
Summary of comparison
The effect of iodine‐based products on would healing is very uncertain; the confidence intervals for the estimate included the possibility of both benefit and harm (very low certainty evidence, downgraded for risks of bias and imprecision). There were no evaluable data relating to infection Table 4.
5. Sodium hypochlorite versus topical antibiotics (1 study, 20 participants)
Ning 2008 compared sodium hypochlorite with SSD in 20 adult participants with deep partial‐thickness burns less than 60% TBSA. The study used an intra‐individual design and randomised comparable burns on the same person to each treatment. It was not clear whether the analysis was adjusted to take account of the paired data.
Primary outcome: wound healing
Ning 2008 reported the mean time to healing for burns treated with each intervention. Sodium hypochlorite may slightly decrease the mean time to healing. Mean time to healing for burns treated with sodium hypochlorite was 20 days compared with 22 days for burns treated with SSD (MD ‐2.10 days; 95% CI ‐3.87 to ‐0.33) Analysis 5.1. This is low certainty evidence because of the very high levels of imprecision (Table 10; Table 13). The confidence intervals were very fragile due to the small number of participants and uncertainty as to whether the paired data were correctly analysed.
5.1. Analysis.
Comparison 5 Silver‐based antiseptics versus non‐antimicrobial, Outcome 1 Wound healing (mean time to healing).
5. Summary of evidence and GRADE judgements for comparisons/outcomes with sparse data.
| Comparison | Number trials & study detail | Number participants | Wound healing evidence | Wound healing: certainty of the evidence | Infection evidence | Infection: certainty of the evidence | Adverse events evidence | Adverse events: certainty of the evidence |
| Sodium hypochlorite versus SSD | 1 trial Ning 2008 |
Trial N = 20 Intra‐individual design |
Mean time to healing MD 2.1 (3.87 to 0.33) |
Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Chlorhexidine or polyhexanide (biguanides) versus SSD | 2 trials Piatkowski 2011 Thomas 1995 |
Trial N = 110 participants with 126 burns; 106 burns relevant to comparison | ‐ | ‐ | ‐ | ‐ | ||
| Octenidine versus SSD | 1 trial Radu 2011 |
Trial N = 30 Intra‐individual design | ‐ | ‐ | ‐ | ‐ | ||
| Ethacridine lactate versus SSD | 1 trial Li 1994 |
Trial N = 115 Relevant to comparison: 60 |
Mean time to healing MD 2.0 (‐0.57 to 4.57) |
Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Merbromin versus zinc sulfadiazine | 1 trial Piccolo‐Daher 1990 |
Trial N = 125 Relevant to comparison: 50 |
Mean time to healing MD ‐3.48 (‐6.85 to ‐0.11) |
Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Arnebia euchroma versus SSD | 1 trial Nasiri 2016 |
Trial N = 49 Intra‐individual design |
HR 1.42 (0.91 to 2.21) Mean time to healing MD ‐3.60 (95% ‐6.41 to ‐1.06) |
Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Chlorhexidine versus Iodine‐based | 1 trial Han 1989 |
Trial N = 213 | Mean time to healing MD 2.21 (0.34 to 4.08) |
Low (Downgraded once for reporting bias and once for imprecision) | RR 1.09 (0.28 to 4.24) | Very low (downgraded once for risk of reporting bias and twice for imprecision) | ‐ | ‐ |
| Ethacridine lactate versus iodophor | 1 trial Li 1994 |
Trial N = 115 Relevant to comparison: 46 |
Mean time to healing MD ‐1.0 (‐4.31 to 2.31) |
Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Ethacridine lactate versus non‐antibacterial (MEBO) | 1 trial Li 1994 |
Trial N = 115 Relevant to comparison: 53 |
Mean time to healing MD ‐25.00 (‐29.1 to ‐20.79) |
Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Cerium nitrate versus non‐antibacterial | 2 trials Oen 2012 De Gracia 2001 |
Trial N = 214 Reporting wound healing: 214 Reporting infection: 60 |
No evaluable data | ‐ | RR 0.50 (0.14 to 1.82) | Low (downgraded twice for imprecision) | ‐ | ‐ |
| Merbromin versus sodium salicylate | 1 trial Piccolo‐Daher 1990 |
Trial N = 125 Relevant to comparison: 50 |
Mean time to healing MD ‐3.68 (‐7.18 to ‐0.18) |
Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
HR: hazard ratio; MD: mean difference; N: number; RR: risk ratio
Primary outcome: infection
Ning 2008 did not report data on change in infection status.
Secondary outcome: adverse events
Ning 2008 reported that there were no serious adverse events in either treatment group. Total adverse events were not reported. Low certainty evidence, downgraded twice for serious imprecision.
Summary of comparison
Sodium hypochlorite may slightly decrease the mean time to wound healing compared with SSD. This is low certainty evidence, downgraded twice due to imprecision. There were no analysable data for infection.
6. Chlorhexidine or polyhexanide (biguanides) versus topical antibiotics (2 studies, 115 participants)
Piatkowski 2011 randomised 72 burns from 60 adult participants to SSD or a polyhexanide‐containing dressing.
Thomas 1995 was a three‐armed study that compared chlorhexidine‐containing dressing with SSD. A third group were treated with a non‐antimicrobial dressing (see comparison 15). Fifty adults and children with a total of 54 burns were randomised; 34 of these burns were treated in groups relevant to this comparison; all burns were described as minor and the mean TBSA was less than 1% in all groups. In both studies it was unclear whether the analyses correctly adjusted for the design of the study with multiple burns from some participants.
Primary outcome: wound healing
Piatkowski 2011 and Thomas 1995 both reported the mean time to wound healing for each group but with no measure of variance. These data are reported in Table 10 but are not further analysed; no GRADE assessment was possible.
Primary outcome: infection
Neither study reported data on infection but Thomas 1995 reported the proportion of wounds with bacteria and pathogenic bacteria at baseline and post treatment; this is noted in Table 11 but the data are not extracted or analysed; no GRADE assessment was possible.
Secondary outcome: pain
Piatkowski 2011 reported pain at dressing change and between dressing changes at a number of time points from baseline up to 14 days. None of these data had any measure of variance so are reported in Table 12 but are not further analysed; no GRADE assessment was possible.
Secondary outcome: costs
Piatkowski 2011 reported costs per day for materials and personnel, and total costs, but without measures of variance. Again these data are shown in Table 12 but are not further analysed; no GRADE assessment was possible.
Summary of comparison
There were no analysable data for either of the primary outcomes or any secondary outcome.
7. Octenidine versus topical antibiotics (1 study, 30 participants)
Radu 2011 used an intra‐individual design with 30 adult participants with injuries described as second‐degree, partial‐thickness burns more than 3% TBSA to compare octenidine with SSD. It was unclear whether the analyses reported took the intra‐individual design into account.
Primary outcome: wound healing
Radu 2011 did not report wound healing.
Primary outcome: infection
Radu 2011 did not report change in infection status.
Secondary outcome: pain
Radu 2011 reported that the median VAS for the octenidine group was 3 (range 1 to 6) compared with 6 in the SSD group (range 3 to 8). Mean scores were not reported and these data were not analysed further (Table 12); no GRADE assessment was possible.
Summary of comparison
There were no analysable data for either of the primary outcomes or any secondary outcome.
8. Ethacridine lactate (Rivanol) versus topical antibiotics (1 study, 115 participants)
Li 1994 was a four‐armed study that compared ethacridine lactate (Rivanol) with SSD, and also included groups treated with iodophor and moist burn ointment (see comparisons 4, 12, 16 and 17). There were 115 participants (aged over 16 years) with injuries described as deep second‐degree burns between 1% to 12% TBSA in the trial, of whom 60 were in groups relevant to this comparison.
Primary outcome: wound healing
Li 1994 reported the mean time to healing of all wounds. There may be little or no difference between participants treated with ethacridine and those treated with SSD in mean time to healing. The difference in means was 2 days (95% CI ‐0.57 to 4.57) Analysis 6.1. This is low certainty evidence, downgraded twice due to high levels of imprecision; wide and fragile confidence intervals included both the possibility of healing being shorter by half a day or longer by over 4 days (Table 10; Table 13).
6.1. Analysis.
Comparison 6 Honey versus non‐antibacterial dressing, Outcome 1 Wound healing (hazard ratio).
Primary outcome: infection
Li 1994 did not report data on change in infection status.
Secondary outcome: costs
Li 1994 reported total treatment costs for each intervention group. There may be little or no difference in costs between the ethacridine lactate and SSD groups. The mean cost per participant was RMB 598 for ethacridine lactate versus RMB 674 for SSD. The difference in means was RMB ‐76 (95% CI ‐156.34 to 4.34) (Table 12). This is low certainty evidence, downgraded twice due to serious imprecision; wide and fragile confidence intervals included both a very considerable saving (RMB 156) and a small cost (RMB 4) for the antiseptic intervention.
Summary of comparison
There may be little or no difference in time to healing between the ethacridine lactate and the SSD groups. This is low certainty evidence, downgraded twice due to imprecision. There were no data reported on infections.
9. Merbromin versus topical antibiotic (1 study, 125 participants)
Piccolo‐Daher 1990 was a five‐armed trial with 125 participants of whom 50 were relevant to this comparison between merbromin and zinc sulphadiazine. Three arms with 75 participants in total were relevant to the review (see comparison 19). Although the unit of analysis was reported to be the burn rather than the participant, it appeared that participants only presented with one burn, therefore we do not believe that there is a unit of analysis issue.
Primary outcome: wound healing
Piccolo‐Daher 1990 reported the mean time to wound healing. Merbromin may slightly decrease the mean time to healing compared with zinc sulphadiazine. Mean time to healing was 11.32 days in the merbromin group compared with 14.8 days in the zinc sulfadiazine group. The difference in means was ‐3.48 (95% CI ‐6.85 to ‐0.11). This is low certainty evidence with wide, fragile confidence intervals, downgraded twice due to high levels of imprecision (Table 10; Table 13).
Primary outcome: infection
Piccolo‐Daher 1990 did not report data on change in infection status.
Secondary outcomes
Piccolo‐Daher 1990 did not report any secondary outcomes.
Summary of comparison
Merbromin may slightly decrease the mean time to healing compared with zinc sulphadiazine (low certainty evidence); there were no data reported on infections.
10. Arnebia euchroma versus topical antibiotic (1 study, 49 participants)
Nasiri 2016 was an intra‐individual design trial that randomised burns on 49 participants to the herbal extract of A euchroma or SSD.
Primary outcome: wound healing
Nasiri 2016 reported mean time to healing and the number of healing events at multiple time points, so we were able to calculate an HR. It is unclear whether there is a difference in the 'chance' of healing over time between treatment with A euchroma or SSD; this is uncertain as fragile confidence intervals spanned both benefit and harm. The HR was 1.42 (95% CI 0.91 to 2.21). There may be a small difference (3.6 days) in the mean time to healing (95% CI ‐6.41 to ‐1.06). In both cases this is low certainty evidence, downgraded twice for imprecision. In both analyses it was unclear whether correct adjustment for the intra‐individual design was undertaken; this increases uncertainty around the estimates of effect.
Primary outcome: infection
Nasiri 2016 reported the numbers of burns with an infection score between 0 and 5 for each treatment; one point was awarded for each symptom of infection. These data are reported in Table 11 but are not further analysed; no GRADE assessment was possible.
Secondary outcome: adverse events
Nasiri 2016 reported scores for specific complications such as burning, pain, itching, warming, and incidence of allergic reactions and requiring skin grafts. It was not clear that these represented data on the number of burns with associated adverse events in each group. The data are reported in Table 12 but are not further analysed; no GRADE assessment was possible.
Secondary outcome: pain
Nasiri 2016 reported pain scores graphically at multiple time points after injury (days) and at multiple time points after dressing (minutes). We could not extract confidence intervals from the graphs but all were reported by study authors to have differences between groups (P reported < 0.05). The data are noted in Table 12 but are not further analysed; no GRADE assessment was possible.
Summary of comparison:
It is unclear whether there is a difference in time to healing between treatment with A euchroma or SSD assessed by the hazard or 'chance' of healing over time. There may be a small reduction in the mean time to healing in burns treated with Aeuchroma compared with those treated with SSD. In both cases this is low certainty evidence. There were no evaluable data on the incidence of infection.
Comparisons between two antiseptics
11. Chlorhexidine versus povidone iodine (1 study, 213 participants)
Han 1989 enrolled 213 participants with burns less than 10% TBSA; approximately 20% were children. Participants were randomised to Bactigras (tulle‐gras wide‐meshed gauze dressing impregnated with 0.5 per cent chlorhexidine acetate BP) or Inadine (synthetic rayon dressing impregnated with 10 per cent povidone iodine ointment).
Primary outcome: wound healing
Han 1989 reported mean time to wound healing. There may be a slightly increased mean time to healing in the chlorhexidine group. Mean time to healing was 11.69 days in the chlorhexidine group compared with 9.48 in the iodine group. The difference in means was 2.21 days (95% CI 0.34 to 4.08) Analysis 8.1. This is low certainty evidence, downgraded once due to risk of reporting bias and once due to imprecision because of wide confidence intervals (Table 10; Table 13).
8.1. Analysis.
Comparison 8 Iodine‐based antiseptics versus non‐antibacterial treatments, Outcome 1 Wound healing (mean time to healing).
Primary outcome: infection
Han 1989 reported incident infections in each group. It is uncertain whether there is a difference in infection incidence between the treatments. There were 4/102 in the chlorhexidine and 4/111 in the povidone iodine groups. The RR was 1.09 (95% CI 0.28 to 4.24). This is very low certainty evidence, downgraded once due to risk of reporting bias and twice due to very serious imprecision (Table 11; Table 13).
Secondary outcome: pain
Han 1989 reported mean pain at rest and at dressing removal using a VAS. It is uncertain whether there is a difference between the chlorhexidine and povidone iodine groups on either measure of pain. The mean VAS score for pain at rest was 11.44 in the chlorhexidine group and 9.18 in the povidone iodine group. The difference in means was 2.26 (95% CI ‐2.26 to 6.78). The mean score for pain at dressing change was 8.75 in the chlorhexidine group and 6.66 in the povidone iodine group. The difference in means was 2.09 (95% CI ‐2.00 to 6.18) (Table 12). In both cases this is very low certainty evidence, downgraded once for risk of reporting bias and twice for very serious imprecision; wide confidence intervals included the possibility of both lower (‐2) and much greater pain scores (+6) in the chlorhexidine group.
Secondary outcome: resource use
Han 1989 reported the mean number of hospital visits for each participant in the two treatment groups. There may be little or no difference between the chlorhexidine (mean 2.64) and iodine (mean 3.03) groups. The difference in means was 0.25 visits (95% CI ‐ 0.02 to 0.52) (Table 12). This is low certainty evidence, downgraded once due to risk of reporting bias and once due to imprecision, as the confidence intervals included both the possibility of both slightly fewer and somewhat more visits in the intervention group.
Summary of comparison
Chlorhexidine‐based dressings may result in a slightly longer mean time to healing than povidone iodine dressings (low certainty evidence, downgraded due to risk of bias and imprecision). It is uncertain whether there is a difference between chlorhexidine and povidone iodine in the number of incident infections in burn wounds (very low certainty evidence, downgraded due to risk of bias and serious imprecision).
12. Iodophor versus ethacridine lactate (1 study, 115 participants)
Li 1994 was a four‐armed study that compared 0.25% iodophor with ethacridine lactate, and also included groups treated with SSD and moist burn ointment (see comparisons 4, 8, 16 and 17). There were 115 participants (aged over 16 years) with injuries described as deep second‐degree burns between 1% to 12% TBSA in the trial, of whom 53 were in groups relevant to this comparison.
Primary outcome: wound healing
Li 1994 reported mean time to wound healing. There may be little or no difference in healing time between participants treated with iodophor and those treated with ethacridine lactate. Mean time to healing was 31 days in the iodophor group compared with 32 days in the ethacridine lactate group (MD ‐1.00 day (95% CI ‐4.31 to 2.31) (Table 10; Table 13). This is low certainty evidence due to high levels of imprecision; wide confidence intervals included the possibility of both a shorter healing time by 4 days and a longer healing time by 2 days for the iodine group.
Primary outcome: infection
Li 1994 did not report data on change in infection status.
Secondary outcome: costs
Li 1994 reported total treatment costs for each intervention group. There may be little or no difference in costs between the iodine and ethacridine‐lactate groups. The cost per participant was RMB 621 in the iodine group compared with RMB 598 in the ethacridine‐lactate group. The difference in means was RMB 23.00 (95% CI ‐51.07 to 97.07) (Table 12). This is low certainty evidence, downgraded twice due to high levels of imprecision resulting from small numbers of participants; wide confidence intervals included both the possibilities of a saving of RMB 51and an additional cost of RMB 97.
Summary of comparison
There may be little or no difference in mean time to healing for participants treated with iodophor or ethacridine lactate (low certainty evidence). There were no data on infection.
Comparisons between antiseptics and treatments without antimicrobial properties
13. Silver dressings versus non‐antimicrobial treatments or no treatment (3 studies, 299 participants)
Chen 2006 was a three‐armed trial that randomised 191 participants with burns described as being second‐degree to a silver nanoparticle dressing or to Vaseline gauze. A third group of participants were treated with SSD (see comparison 1). The number of participants in groups relevant to this comparison was 128. Jiao 2015 randomised 76 participants with partial‐thickness burns to nanocrystalline silver or Vaseline gauze; in each case the dressing was applied over human epidermal growth factor. Healy 1989 randomised 32 participants (mostly children) to silver‐impregnated porcine xenograft or paraffin gauze.
Primary outcome: wound healing
Healy 1989 reported the proportion of wounds completely healed in each group by 14 days. There may be little or no difference between silver xenograft and paraffin gauze in proportion of wounds healed; 9/16 wounds healed in the silver group compared with 8/16 in the control group. The RR was 1.13 (95% CI 0.59 to 2.16) (low certainty evidence, downgraded twice because of serious imprecision) Table 10. The mean time to healing of these wounds was also reported but as not all wounds healed these data are reported in Table 10 but are not further analysed.
Chen 2006 and Jiao 2015 reported the mean time to healing for all wounds. The mean time to healing is probably slightly shorter in the silver group compared with the gauze group: ‐3.49 days (95% CI ‐4.46 to ‐2.52; I2 = 0%) a reduction from 15.87 days in the gauze group to 12.38 days. This is moderate certainty evidence downgraded because of imprecision.
Primary outcome: infection
It is very uncertain whether there is a difference in wound infections between silver and non‐antimicrobial treatments. Jiao 2015 reported the proportion of wounds testing positive for bacteria at 21 days as 1/38 in the silver group and 8/38 in the gauze group. The RR was 0.13 (95% CI 0.02 to 0.95). However this is not a measure of clinical infection. Healy 1989 reported data on specific bacteria colonisation but otherwise reported only that there was no difference in the infection rate between the groups; these data are noted in Table 11. This is very low certainty evidence, downgraded once for indirectness and twice for imprecision.
Secondary outcomes
Neither Chen 2006 nor Healy 1989 reported data on any secondary outcome while Jiao 2015 reported only one specific type of adverse event (scar hyperplasia); this is noted in Table 12 but is not further analysed.
Summary of comparison
Silver xenograft may make little or no difference to the proportion of burn wounds that heal by 14 days compared to a non‐antimicrobial (paraffin gauze) dressing (low certainty evidence downgraded twice for imprecision). Silver nanoparticle dressings probably result in burns healing in a slightly shorter mean time compared with Vaseline/petroleum gauze (moderate certainty evidence downgraded for imprecision). It is very uncertain whether there is any difference between the dressings in infection rates (very low certainty evidence downgraded for indirectness and imprecision) Table 5.
14. Honey or honey‐based dressings versus non‐antimicrobial treatments (3 studies, 256 participants)
Subrahmanyam 1993b randomised 92 participants to honey‐impregnated gauze or a bio‐occlusive, moisture‐permeable polyurethane dressing. Subrahmanyam 1994 randomised 64 participants to honey‐impregnated gauze or amniotic membrane; no other details of the comparison dressing were given. Subrahmanyam 1996a randomised 100 participants to honey plus dry guaze or an autoclaved potato peeling dressing plus dry gauze. All studies enrolled a mixture of adults and children, although most participants were adults where reported. All three studies enrolled participants with partial‐thickness burns less than 40% TBSA. Subrahmanyam 1996b compared honey with a mixed standard treatment group in which the following treatments were used: soframycin (90 participants), Vaseline‐impregnated gauze (90 participants), OpSite (90 participants), sterile gauze (90 participants) or left exposed (90 participants).
Primary outcome: wound healing
Two trials reported data at numerous time points allowing hazard ratios for healing to be calculated (Subrahmanyam 1994, Subrahmanyam 1996a). Honey probably somewhat increases the 'chance' of healing over time for partial‐thickness burns compared with non‐anti‐microbial treatments. The pooled HR was 2.86 (95% CI 1.60 to 5.11; I2 = 50%) Analysis 6.1. This is moderate certainty evidence downgraded once for imprecision. All wounds healed in both groups over the total time period assessed, but the hazard ratio corresponds to the increased likelihood of healing at earlier time points in the honey groups. As HRs could be calculated for all trials with dichotomous data, we have not reported RRs.
All four trials also reported mean times to healing, or data were available as a result of contact with the study author by a previous Cochrane Review (Jull 2015). On average, honey results in a somewhat shorter mean time to healing compared with the non‐antibacterial dressings evaluated. The pooled difference in means was ‐5.32 days (95% CI ‐6.30 to ‐4.34; I2 = 71%) Analysis 6.2. This is high certainty evidence although some of the comparators used are atypical.
6.2. Analysis.
Comparison 6 Honey versus non‐antibacterial dressing, Outcome 2 Wound healing (mean time to healing).
Primary outcome: infection
Three trials reported a measure of infection. However this was based on swab cultures which are only an indirect measure of infection and do not correspond to clinical infections. Subrahmanyam 1993b reported incidence of infection on day 8 in both groups. It is uncertain whether there may be fewer incidences of infection in wounds treated with honey compared with polyurethane dressing. There were 8/46 infections reported in the honey group compared with 17/46 in the polyurethane dressing group. The RR was 0.47 (95% CI 0.23 to 0.98) (very low certainty evidence, downgraded twice for imprecision and twice for indirectness).
Subrahmanyam 1994 and Subrahmanyam 1996a reported incidence of persistent positive swab cultures at day 7. It is uncertain whether persistent infections differ in participants treated with honey compared with participants treated with comparator topical treatments. The total number of participants considered to have a persistent infection was 8/78 in the honey groups compared with 53/69 in the non‐antibacterial groups.The pooled RR for persistent infection at day 7 was 0.15 (95% CI 0.06 to 0.40; I2 = 50%) Analysis 6.3. This is very low certainty evidence, downgraded twice for indirectness and once for imprecision.
6.3. Analysis.
Comparison 6 Honey versus non‐antibacterial dressing, Outcome 3 Persistent positive swabs (up to 30 days).
Secondary outcome: adverse events
Subrahmanyam 1993b and Subrahmanyam 1994 reported information on some adverse events but it was not clear that these represented all reported adverse events. Subrahmanyam 1996a reported that there were no adverse events in either the honey or the SSD group. This is very low certainty evidence, downgraded twice for serious imprecision and indirectness.
Secondary outcome: pain
Subrahmanyam 1994 measured pain using a four‐point scale and reported the number of participants in each group with no or mild pain and with moderate or severe pain. The mean scores were not reported and these data are not further analysed. Subrahmanyam 1996a reported only that subjective relief of pain was the same in both treatment groups. These data are reported in Table 12 but are not further analysed; no GRADE assessment was possible.
Summary of comparison
Based on the hazard or 'chance' of healing over time, honey probably, on average, somewhat shortens the time to healing for partial‐thickness burns compared with a range of non‐antibacterial alternatives, including treatments not commonly used in clinical practice. There is high certainty evidence of some reduction in the mean time to wound healing in the wounds treated with honey. It is uncertain if burns treated with honey may develop fewer infections than those treated with the comparison treatments. Table 6.
15. Chlorhexidine (biguanide) versus non‐antimicrobial treatments (5 studies, 516 participants)
Five studies compared chlorhexidine with no treatment or a non‐antimicrobial treatment. Inman 1984 randomised 121 participants to SSD plus chlorhexidine versus SSD alone in participants with full‐thickness burns; full‐thickness injuries were less than 15% TBSA. Other studies used chlorhexidine‐impregnated paraffin gauze or tulle‐gras. Neal 1981 (51 participants), Phipps 1988 (196 participants) and Thomas 1995 (50 participants) enrolled people with burns less than 5% TBSA while Wright 1993 (98 participants) required that burns be suitable for outpatient treatment. Comparators were plastic film dressing (Neal 1981) or hydrocolloid dressing (Phipps 1988; Thomas 1995; Wright 1993). Where reported, all studies enrolled a mix of adults and children. Thomas 1995 also assigned participants to a third arm treated with SSD (see comparison 6). As previously noted, some participants in Thomas 1995 had multiple burns analysed in the study, creating unit of analysis issues.
Primary outcome: wound healing
Neal 1981 reported the number of participants whose wounds healed at multiple time points and presented a Kaplan‐Meier curve. The trial did not show a clear difference between chlorhexidine and non‐antimicrobial film dressing in time to wound healing; wide and fragile CIs spanned both benefit and harm so a clear treatment effect is not apparent. The calculated HR, based on 25 participants in the chlorhexidine group and 26 in the film‐dressing group, was 0.71 (95% CI 0.39 to 1.29) (Table 10). Neal 1981 also reported the mean time to healing. This indicated that the mean time to healing may be slightly longer (14 days) in the chlorhexidine group (compared with 10 days in the film dressing group) with a difference in means of 4.08 days (95% CI 0.73 to 7.43); again the estimate was imprecise. Both Phipps 1988 and Thomas 1995 reported the mean time to healing in each group but did not report a measure of variance. Wright 1993 reported the median time to wound healing in each group. The data for these three trials are reported in Table 10 but are not further analysed. All the evidence is low certainty, downgraded twice because of serious imprecision due to low participant numbers, wide confidence intervals and poor reporting. The three trials that did not present analysable data were also all at high risk of bias across one or more domains. Because the study that had unit of analysis issues did not contribute to the analysis, (Thomas 1995) no sensitivity analysis was undertaken.
Primary outcome: infection
Inman 1984 reported the numbers of participants in each group with infection. It appeared that there were a number of post‐randomisation exclusions from the study, numbers are reported on a completed case basis. Neal 1981 reported the proportion of participants in each group with proven infections. It is uncertain whether there is a difference between the treatments. On average the RR for wound infection from these two studies was RR 1.11 (95% CI 0.54 to 2.27; I2 = 0%) Analysis 7.2. This is very low certainty evidence, downgraded twice due to high levels of imprecision and once due to attrition bias in Inman 1984: wide confidence intervals included the possibility of both substantial benefits and harms associated with the intervention.
7.2. Analysis.
Comparison 7 Chlorhexadine versus non‐antibacterial dressing, Outcome 2 Infection (up to 30 days).
Phipps 1988 reported proportions of participants with specific wound flora and Thomas 1995 reported percentages of wounds with bacteria and pathogenic bacteria; these data are noted in Table 11 but are not further analysed.
Secondary outcome: adverse events
Wright 1993 reported the number of participants with an adverse event in each group. It is uncertain whether chlorhexidine decreases the number of people experiencing adverse events. In the chlorhexidine group, 1/49 participants experienced an adverse event, compared with 5/49 in the comparison group. The RR was 0.20 (95% CI 0.02 to 1.65) (Table 12). This is very low certainty evidence, downgraded once due to risk of detection bias, once due to attrition bias and twice due to very high levels of imprecision as a result of very wide confidence intervals, which included a possible 98% reduction and also a 65% increase in events associated with the antiseptic intervention.
Secondary outcome: pain
Inman 1984 reported the numbers of participants in each group with pain sufficient to stop treatment. Neal 1981 reported qualitatively that chlorhexidine treatment was perceived as painful. Wright 1993 reported summing the VAS for each visit; the scores were not reported but a P value was given. All these data are reported in Table 12 but are not further analysed; no GRADE assessment was possible.
Secondary outcome: mortality
Inman 1984 reported total and infection‐related mortality in each treatment group. It is uncertain whether chlorhexidine in addition to SSD alters mortality. A total of 3/54 people died in the chlorhexidine group compared with 4/67 in the SSD‐alone group. The RR was 0.93 (95% CI 0.22 to 3.98) (Table 12). This is very low certainty evidence, downgraded once for attrition bias and twice for very serious imprecision due to wide confidence intervals, which included a possible 78% reduction and an almost 400% increase in deaths.
Secondary outcome: resource use
Wright 1993 reported the number of dressings used in each group as 2.8 in the chlorhexidine group and 2.61 in the hydrocolloid group. No measures of variance were reported and the data were not further analysed but are shown in Table 12; no GRADE assessment was possible.
Summary of comparison
Despite being evaluated in multiple trials the evaluable data were limited. There may be little or no difference in the time to wound healing between chlorhexidine and a film dressing (low certainty evidence downgraded twice for imprecision). It is uncertain whether the use of chlorhexidine reduces the incidence of infection compared with no additional antibacterial treatment (very low certainty evidence, downgraded twice due to imprecision and once due to attrition bias). It is also uncertain whether use of chlorhexidine plus SSD reduces mortality compared with SSD alone (very low certainty evidence, downgraded twice for imprecision and once for risk of attrition bias). Table 7
16. Iodine‐based treatments versus non‐antimicrobial treatments/no intervention (4 studies, 663 participants)
Carayanni 2011 randomised 217 participants with superficial or deep partial‐thickness thermal burns less than 15% TBSA to povidone iodine with a barrier of bepanthenol cream or MEBO. Randomisation was stratified by burn depth. Li 1994 was a four‐armed study that compared iodophor with moist burn ointment and also included groups treated with SSD and ethacridine lactate (see comparisons 4, 8, 12 and 17). There were 115 participants (aged over 16 years) with injuries described as deep second‐degree burns between 1% to 12% TBSA in the trial, of whom 55 were in groups relevant to this comparison. Li 2006 randomised 277 participants with superficial, deep or residual burn wounds to iodine gauze or to carbon fibre dressing. Yang 2013 enrolled 60 participants with residual burn wounds after one month of treatment and randomised burn areas to iodophor gauze or to a hydrogel dressing; this was an intra‐individual design.
Primary outcome: wound healing
Yang 2013 reported the proportion of wounds healed at seven and 14 days. Iodophor gauze may reduce the chances of residual burn wounds healing after 14 days: RR was 0.17 (95% CI 0.08 to 0.34) (Table 10). It was unclear whether the analysis adjusted for the paired data from the intra‐individual design.This is low certainty evidence, downgraded twice for imprecision due to uncertainties about the analysis and small numbers of participants.
Li 1994 reported mean time to wound healing as did Li 2006. It is unclear whether the use of iodine reduces the mean time to healing because the certainty of the evidence is very low. Clinical differences in the treatments used and very high levels of statistical heterogeneity (I2 = 99%) meant that pooling was unlikely to produce a meaningful answer. Li 1994 reported that mean time to healing for wounds was 31 days in the iodophor group and 57 days in the MEBO group (Li 1994), with a difference in means of ‐26 days (95% CI ‐30.48 to ‐21.52). Li 2006 reported that mean time to healing for wounds was 20.67 days in the iodine‐gauze group compared with 15.29 days in the carbon‐dressing group, with a difference in means of 5.3 days (95% CI 3.09 to 7.67) Analysis 8.1. This is very low certainty evidence, downgraded twice for inconsistency and twice for imprecision.
Primary outcome: infection
Carayanni 2011 reported the numbers of participants with infection. There may be little or no difference between iodine and MEBO in the incidence of infections. There were 8/107 participants with infections in the iodine group compared with 6/104 in the MEBO group. The RR was 1.30 (95% CI 0.47 to 3.61). This is low certainty evidence, downgraded twice for very high levels of imprecision with wide confidence intervals, which included the possibility of a both a reduction of 53% and an increase of 360% in infection rates for the iodine intervention (Table 11).
Yang 2013 reported bacterial presence in wounds and stated that there was no evidence of a difference between the groups (Table 11); these data were not further analysed.
Secondary outcome: adverse events
Carayanni 2011 reported adverse events including infections. There may be little or no difference between iodine and MEBO in the incidence of adverse events. There were 8/107 participants with reported events (all infections) in the iodine group and 11/104 in the MEBO group. The RR was 0.71 (95% CI 0.30 to 1.69). This is also low certainty evidence, downgraded twice due to the very high levels of imprecision with wide confidence intervals, which included the possibility of a 70% reduction or a 70% increase in events for the iodine intervention (Table 12).
Secondary outcome: pain
Yang 2013 reported pain at dressing change as the number of wounds and the level of pain. Carayanni 2011 reported median pain scores in graphical form only. In both cases these data are noted in Table 12 but are not extracted or analysed further; no GRADE judgement was possible.
Secondary outcome: resource use
Carayanni 2011 reported reduction in length of hospital stay from an expected duration based on burn characteristics. Hospital stay is probably reduced by slightly less time in participants treated with iodine compared with those treated with MEBO. There was a reduction of 3.01 days in the iodine group compared with 3.63 in the MEBO group; the difference in means was 0.62 days (95% CI 0.05 to 1.19) (Table 12). This is moderate certainty evidence, downgraded once for imprecision due to low numbers of participants.
Secondary outcome: costs
Li 1994 and Carayanni 2011 reported total treatment costs for each intervention group. It is uncertain whether iodine‐based treatments reduce costs compared with MEBO. Clinical differences in the treatments used and very high levels of statistical heterogeneity (I2 = 99%) meant that pooling was unlikely to produce a meaningful answer (Analysis 8.2). Li 1994 reported costs of RMB 621 for the iodophor group compared with RMB 1836 for the MEBO group (difference in means RMB ‐1215, 95% CI ‐1412.96 to ‐1017.04). Carayanni 2011 reported costs of EUR 566 for povidone iodine and EUR 529 for the MEBO group (difference in means EUR 36.55, 95% CI ‐7.33 to 80.43). This is very low certainty evidence, downgraded twice for high levels of inconsistency and twice for imprecision due to low participant numbers and wide confidence intervals.
8.2. Analysis.
Comparison 8 Iodine‐based antiseptics versus non‐antibacterial treatments, Outcome 2 Costs (duration 18 days +).
Summary of comparison
It is uncertain whether iodine‐based treatments decrease or increase the mean time to healing compared with treatments without antibacterial properties (very low certainty evidence downgraded twice due to inconsistency and twice for imprecision). Iodophor gauze may reduce the chances of residual burn wounds healing within 14 days compared with hydrogel treatment (low certainty evidence downgraded twice due to imprecision). There may be little or no difference in the occurrence of either infections or adverse events including infections, between povidone iodine and a non‐antibacterial comparator (low certainty evidence downgraded twice due to imprecision) Table 8.
17. Ethacridine lactate versus non‐antimicrobial treatments (1 study, 115 participants)
Li 1994 was a four‐armed study that compared ethacridine lactate with moist burn ointment and also included groups treated with SSD and iodophor (see comparisons 4, 8, 12 and 16). There were 115 participants (aged over 16 years) with injuries described as deep second‐degree burns between 1% to 12% TBSA in the trial, of whom 46 were in groups relevant to this comparison.
Primary outcome: wound healing
Li 1994 reported mean time to wound healing. Mean time to wound healing may be reduced in the ethacridine lactate group compared with the MEBO group. Mean times to heal were 32 days for the ethacridine group and 57 days for the MEBO group; the difference in means was ‐25 days (95% CI ‐29.21 to ‐20.79). This is low certainty evidence downgraded twice due to high levels of imprecision resulting from small numbers of participants (Table 10; Table 13).
Primary outcome: infection
Li 1994 did not report data on change in infection status.
Secondary outcome: costs
Li 1994 reported total mean treatment costs for each intervention group. Total costs may be lower in the ethacridine lactate group compared with the MEBO group. Costs in the ethacridine group were RMB 598 compared with RMB 1836 for people in the MEBO group. The difference in means was RMB ‐1238 (95% CI ‐1435.98 to ‐1040.22) Table 12. This is low certainty evidence downgraded twice due to high levels of imprecision resulting from small numbers of participants.
Summary of comparison
There may be a shorter mean time to healing in burns treated with ethacridine lactate compared with MEBO. This is low certainty evidence due to high levels of imprecision and fragile confidence intervals. There were no data on infection.
18. Cerium nitrate + topical antibiotic versus topical antibiotic alone (2 studies, 214 participants)
Oen 2012 randomised 154 adults with facial burns to cerium nitrate plus SSD or SSD alone while De Gracia 2001 randomised 60 participants with full or partial‐thickness burns to the same interventions.
Primary outcome: wound healing
Oen 2012 reported the median time to healing and interquartile range in each group for those participants who did not need to have surgery; data were therefore not included for all wounds and the data are not analysed further. De Gracia 2001 reported the mean time to healing for partial‐thickness burn areas (these made up part of the wound for all except one participant) but not data for whole wounds, as the full‐thickness burn areas were grafted when ready. These data are therefore not analysed further and no GRADE assessment was possible (Table 10).
Primary outcome: infection
De Gracia 2001 found that the effect of treatment with cerium nitrate in addition to SSD is unclear in terms of the number of participants with sepsis at up to five days and then subsequently compared with SSD alone. In total 1/30 participants in the cerium nitrate group had sepsis versus 4/30 in the control group. The RR was 0.25 (95% CI 0.03 to 2.11) so the wide confidence intervals included the possibility of both benefit and harm. This was also the case for the number of participants with post‐treatment infection compared with SSD alone; 3/30 participants developed an infection in the cerium nitrate group compared with 6/30 in the control group (RR 0.50, 95% CI 0.14 to 1.82). In both cases this is low certainty evidence, which was downgraded twice due to serious imprecision because of low numbers of events and participants (Table 11; Table 13).
Oen 2012 did not report data on change in infection status.
Secondary outcome: pain
Oen 2012 reported mean pain scores both generally and for procedures. Cerium nitrate plus SSD probably slightly reduces overall pain scores. In the cerium nitrate group the mean score was 0.6 compared with 1.2 in the control group. The difference in means was ‐0.60 (95% CI ‐0.70 to ‐0.50) Table 12. This is moderate certainty evidence downgraded once for imprecision due to the small number of participants.
Secondary outcome: mortality
Both De Gracia 2001 and Oen 2012 reported the number of participants who died in each treatment group. Cerium nitrate plus SSD may reduce mortality compared with SSD alone.There were 2/108 deaths in the cerium nitrate group compared with 9/106 in the SSD‐alone group. The RR was 0.22 (95% CI 0.05 to 0.99; I2 = 0%) Analysis 9.1. This is low certainty evidence downgraded twice due to imprecision because of wide confidence intervals, which were fragile due to low numbers of both events and participants. In one of the trials (Oen 2012) deaths occurred during the enrolment process so the effect of treatment group is unclear.
9.1. Analysis.
Comparison 9 Cerium nitrate versus non antibacterial treatment, Outcome 1 Mortality (short‐term or unclear).
Secondary outcome: resource use
De Gracia 2001 reported the mean length of hospital stay. It is unclear whether cerium nitrate in addition to SSD reduces hospital stay. The mean length of stay was 23.3 days in the cerium nitrate group versus 30.7 days in the control group. The difference in means was ‐7.4 days (95% CI ‐16.49 to 1.69) Table 12. This is low certainty evidence downgraded twice due to imprecision because of wide confidence intervals, which included both a substantial benefit (16.5 days) for the cerium nitrate group and a small benefit (1.7 days) for the comparison group.
Summary of comparison
There were no analysable data on wound healing. The effect of cerium nitrate in addition to SSD on rates of infection and of sepsis is unclear, compared with SSD alone (low certainty evidence with wide confidence intervals including both benefit and harm, downgraded twice for imprecision). There may be lower mortality rates in the cerium nitrate group compared with the group treated with SSD alone (low certainty evidence, downgraded twice due to serious imprecision).
19. Merbromin versus sodium salicylate (1 study, 125 participants)
This comparison was addressed by one trial. Piccolo‐Daher 1990 was a five‐armed trial with 125 participants of whom 50 were relevant to this comparison. Three arms with 75 participants in total were relevant to the review (see comparison 9). As above, although the unit of analysis was reported to be the burn rather than the participant it appeared that participants only presented with one burn, therefore we do not believe that there is a unit of analysis issue.
Primary outcome: wound healing
Piccolo‐Daher 1990 found that the mean time to wound healing may be slightly reduced in participants treated with merbromin (11.32 days) compared with those treated with sodium salicylate (15.0 days). The difference in means was ‐3.68 days (95% CI ‐7.18 to ‐0.18). This is low certainty evidence downgraded twice due to imprecision (Table 10; Table 13).
Primary outcome: infection
Piccolo‐Daher 1990 did not report data on change in infection status.
Secondary outcomes
Piccolo‐Daher 1990 did not report any secondary outcomes.
Summary of comparison
Burns treated with merbromin may have a slightly shorter mean time to healing than those treated with sodium salicylate (low certainty evidence downgraded twice due to serious imprecision). There were no data on infection.
Discussion
Summary of main results
We identified 56 eligible studies with 5807 randomised participants. The majority of these assessed treatments with antiseptic properties and compared them to the topical antibiotic silver sulfadiazine. Most participants appeared to be adults, although the majority of studies enrolled both adults and children. In most studies burns were required to correspond to a classification (by the studies' authors) of second degree and to be under 40% TBSA. Some studies focused on smaller and more superficial burns and a smaller number allowed some deeper burn areas. A minority of participants had residual burn wounds, but the great majority were enrolled in the period immediately after the injury.
Wound healing
Antiseptics compared with topical antibiotics
Evidence on wound healing is mixed and largely of low certainty due to small sample sizes and wide confidence intervals.
Measuring the hazard or 'chance' of healing over time using a HR suggested that there is no clear difference in time to healing between wounds treated with silver‐based antiseptics (mainly dressings) and those treated with topical antibiotics (all SSD); this is low certainty evidence as data came from 259 participants, and the 95% CIs spanned effects of both benefit and harm for the intervention. Low certainty evidence from a larger number of studies (979 participants), reporting mean time to healing of all wounds, suggested that there may be a modest benefit of healing time, approximately three days shorter in the silver‐based antiseptics arm; while studies that reported dichotomous healing data also suggested that there may be little difference in how many wounds treated with silver antiseptics may heal by three or four weeks compared with those treated with SSD.
Measuring the hazard or 'chance' of healing over time using an HR suggested that wounds treated with honey probably have a somewhat shorter time to healing than wounds treated with topical antibiotics (moderate certainty evidence based on 580 participants). There may, on average, be a greater number of healing events measured at short term (21‐day follow‐up) (low certainty evidence). It is uncertain whether there is a difference in mean time to healing (very low certainty evidence)
It is unclear whether there is a difference in the number of healing events over a 26‐day period in burns treated with Aloe Vera compared with SSD. It is uncertain if the overall average effect of mean time to healing differs between these treatments (very low certainty evidence). There is low certainty evidence that sodium hypochlorite may be associated with a mean time to healing that was lower by around two days than for SSD. Also with low certainty evidence, there may be a small benefit (around 3.5 days) in mean time to healing from merbromin compared with zinc sulfadiazine. There is low certainty evidence that there may be a similar small benefit of around 3.6 days for treatment with extract of the herb A euchroma, which has antiseptic properties, compared with SSD, but it was unclear whether there was a difference in the 'chance' of healing over time. There is low certainty evidence that there may be little or no treatment difference in wound healing for the comparisons of ethacridine lactate or iodine‐based treatments with silver sulfadiazine. There were no usable data for the primary outcomes from trials comparing chlorhexidine, polyhexanide or octenidine to silver sulfadiazine.
Antiseptics compared with alternative antiseptics
There were few comparisons between different antiseptics. Low evidence from a single trial indicated that there may be a small benefit of around two days in mean time to healing for wounds treated with povidone iodine compared with chlorhexidine. There may be little or no difference between iodophor and ethacridine lactate in wound healing times (low certainty evidence).
Antiseptics compared with non‐antibacterial alternative treatments
Several different antiseptic agents were compared with a range of dressings without antibacterial properties. The evidence from these comparisons is generally of low certainty.
There is moderate certainty evidence, based on 204 participants in two trials that, on average, burns treated with nanocrystalline silver dressings have a slightly shorter mean time to healing (by around 3.5 days) than those treated with Vaseline gauze. There is low certainty evidence that there may be little or no difference in the number of healing events at 14 days between burns treated with silver xenograft or paraffin gauze.
Measuring the hazard or 'chance' of healing over time using a HR suggested that wounds treated with honey probably, on average, have a somewhat shorter time to healing than wounds treated with unconventional non‐antibacterial treatments, based on 164 participants treated with honey compared with amniotic membrane or potato peelings (moderate certainty evidence). There is high certainty evidence for a shorter average mean time to healing in burns treated with honey compared with non‐antibacterial treatments, including the unconventional ones assessed using the HR. Burns healed, on average, in a mean time which was 5.3 days shorter in groups treated with honey.
Comparisons involving iodine‐based treatments produced contradictory results favouring both iodine and the comparator in terms of mean time to healing; it is uncertain where the true treatment effect may lie (very low certainty evidence). There may be fewer healing events over a short follow‐up period in wounds treated with iodophor gauze compared with hydrogel (low certainty evidence). Honey was compared with a range of treatments, some of which were unconventional. There is also low certainty evidence that both merbromin and ethacridine lactate may result in shorter mean times to healing compared with a non‐antibacterial treatment.
Infection
Antiseptics compared with topical antibiotics
Most comparisons did not report data on this key outcome but the comparisons of silver and Aloe Vera with SSD showed that there may be little or no difference between the treatment arms (low certainty evidence). There is uncertainty as to the effect of treatment with honey compared with SSD on infections (very low certainty evidence).
Antiseptics compared with alternative antiseptics
It is uncertain whether there was a difference in infections between chlorhexidine and povidone iodine (very low certainty evidence). There were no data on any other comparisons between antiseptics.
Antiseptics compared with non‐antibacterial alternative treatments
It is uncertain whether there were differences in burns treated with either silver‐based dressings or honey compared with a range of non‐antimicrobial treatments, some of which were unconventional; this is very low certainty evidence in both cases. There is moderate certainty evidence of no difference in infection rates for a comparison of an iodine‐based treatment with MEBO. The only comparison that showed any evidence of a benefit in infection reduction was the use of cerium nitrate in addition to silver sulfadiazine, compared with silver sulfadiazine alone, where there is some low certainty evidence of a reduced incidence of infections and sepsis. Other comparisons did not report usable data on infection rates.
Secondary outcomes
Adverse events were not reported for many comparisons, or they were reported in qualitative terms, which made it difficult to determine event rates for each intervention group, or they were reported only for specific types of event. There may be little or no difference in adverse events for any of the comparisons that did report the number of participants with an adverse event in each group; or the impact of treatments on adverse effects is very uncertain (low or very low certainty evidence).
Mortality was low where reported and there may be little or no difference between treatment groups in most comparisons; this was based on very small numbers of events and is low certainty evidence. The exception was the comparison of cerium nitrate plus SSD with SSD alone, where there may be fewer deaths in the cerium nitrate groups; again, event rates were low and this is low certainty evidence based on 214 participants in two trials.
Pain is of particular concern to people with burns and medical personnel: often this was not reported in sufficient detail for us to analyse but there was evidence that there may be lower levels of pain in participants treated with silver dressings compared with silver sulfadiazine (low certainty evidence). Pain probably decreases slightly more from baseline in those treated with Aloe Vera compared with SSD (moderate certainty evidence). There is also some low certainty evidence that participants treated with cerium nitrate plus SSD may have less pain than those treated with SSD alone. It was uncertain whether there was a difference in pain between participants treated with chlorhexidine and those treated with povidone iodine (very low certainty evidence).
Resource use was reported for a limited number of comparisons. Frequency of dressing changes and associated implications for nursing time and costs were the most commonly reported outcomes under this heading. There was some evidence that participants treated with silver dressings may require fewer dressing changes than those treated with SSD (low certainty evidence) and some evidence that participants who are treated with honey probably have a shorter hospital stay compared with those treated with SSD (moderate certainty evidence). Reduction from an expected length of stay in hospital is probably smaller in participants treated with iodine‐based dressings compared with MEBO (moderate certainty evidence). With a few exceptions, costs were not adequately reported or showed that there may be no differences between treatment groups. In some cases there may be cost differences between groups, but this is all low certainty evidence.
Overall completeness and applicability of evidence
Although we identified a large number of studies, many of these did not report, or did not fully report, the primary outcomes of this review: wound healing and infection. Usable data on key outcomes were therefore limited and often unavailable. Only a minority of studies reported enough data to enable us to calculate the most appropriate measure of time‐to‐event data ‐ a hazard ratio. Where this was not available we were in some cases able to report a mean time to healing or a relative risk of healing for a particular time point. Neither of these measures is ideal and both may give an impression of either an effect or a lack of effect which is not truly present, particularly where the event rate is high.
Usable evidence on infection was also limited, which is disappointing in an area in which infection control is so key. Although a number of studies reported microbiological data, the proportion reporting analysable data on clinical infection was much smaller. Much of the evidence is of low certainty or very low certainty because of indirectness and imprecision.
The geographical distribution of the studies reflected the concentration of disease burden outside of Western high‐income countries. Most studies included participants described as having second‐degree burns and there were no studies focusing on full‐thickness burns, although some studies allowed participants with some area of full‐thickness (described as third‐degree) injury. Therefore any conclusions that can be drawn from this review are likely to be directly relevant only to participants with second‐degree burns limited to TBSA of 40% or lower. Their reliability for other types of burns will be reduced by indirectness.
Quality of the evidence
For most of the comparisons assessed here the evidence relating to key outcomes was assessed as being of low or very low certainty. In some cases this was the result of evidence being at high risk of bias, but in more instances it was a consequence of serious imprecision or inconsistency, or both; in some cases indirectness was an issue due to the use of surrogate outcomes. Although we judged a minority of studies to be at high risk of bias, we judged most to be at unclear risk of bias on several or most domains. Often the fact that there was only a single study available ‐ or only a single study with analysable data ‐ meant that confidence intervals were very wide or fragile, or both, because of the small number of participants represented. A number of studies adopted an intra‐individual design (see Potential biases in the review process) and it was unclear whether this was taken into consideration in the analyses. There is, therefore, a high level of uncertainty around many of the findings. We note that this is the case although we adopted a conservative approach to downgrading for risk of bias in our GRADE assessments, and only downgraded where there was judged to be a high risk of bias: we did not downgrade for risk of bias where one or more domains had an unclear risk of bias.
Potential biases in the review process
Study design
A number of studies adopted an intra‐individual (split‐body) approach analogous to the 'split‐mouth' design (Lesaffre 2009). These studies have particular issues and, if incorrectly analysed, can produce inaccurate confidence intervals around the estimates of effect. Where there are a number of such studies for a given comparison there is a case for analysing them separately from parallel‐group designs. We had failed to anticipate the number of trials with these designs, which were eligible for inclusion in our review, and therefore our approach to handling them is necessarily post‐hoc. There were ten trials with this design and it was unclear whether they had accounted for the intra‐individual design in their analyses. In most of our analyses there were limited numbers of these studies as they were distributed across the large number of comparisons in the review. Therefore we have adopted a pragmatic and conservative approach: where these studies contributed data to a meta‐analysis with at least two other studies, we conducted a post‐hoc sensitivity analysis and used the results of that to inform the GRADE assessment if it differed substantively from the primary analysis. Where there was only one additional study in the analysis, we reported both the pooled results and the results of the two trials with different designs separately. Where these studies were present in an analysis but did not contribute weight to it (because of zero events or lack of measures of variance), we noted their presence. In all except one case the sensitivity analysis conducted did not materially affect the estimate of effect or the confidence intervals. In a single case we have downgraded twice rather than once for imprecision because, in the sensitivity analysis excluding a trial with an intra‐individual design, the confidence intervals differed from the main analysis in crossing the line of no effect. We are therefore confident that our post‐hoc approach to data from these trials is unlikely to have affected the findings of the review, and that fully including the data increases the comprehensiveness of the review.
Language and setting
Eleven of the included studies were reported in languages other than English, with ten in Chinese and one in Portuguese, as were many of the excluded studies (Chinese, German). We therefore do not believe that language bias is likely to be an issue. The included studies were conducted across a wide range of countries. Only around a third (17) of the studies were conducted in Western, high‐income countries. The majority were based in low‐ to middle‐income countries, almost all in Asia, where much of the mortality and morbidity burden from burns is concentrated. It therefore seems likely that in this respect participants in the included studies may reflect those with burns world wide.
Funding
The great majority of the included studies did not state how they were funded. Of those where the funding source was clear, five were funded by industry and six by other non‐commercial sources; two others reported both types of funding. Where funding sources are not clearly reported, it can be conservative to assume that this may be a source of bias. However, in this case many of the studies were small and of short duration, and it is therefore likely that they may not have received any external funding.
Publication bias
We did not find evidence of publication bias although it remains a possibility that undetected publication bias was present in some analyses. In some comparisons it was clear that the antiseptic treatment was intended as the comparator: the intervention that the trial was designed to evaluate was the non‐antibacterial comparator. If either funding or selective publication were leading to the introduction of bias or potential bias, this would mean that trials that favoured antiseptics would be disproportionately likely to be absent. This was not a pattern that we found evidence to support.
Agreements and disagreements with other studies or reviews
There is a current published Cochrane Review of antibiotics for the prevention (prophylaxis) of burn wound infection (Barajas‐Nava 2013), while a second Cochrane Review of antibiotics for the treatment of infected burn wounds is now underway (Lu 2016). This review of antiseptics complements these reviews and completes the assessment of evidence for agents with antimicrobial properties in the care of all burn wounds, whether infected or not.
There is some overlap between this review and other Cochrane and non‐Cochrane reviews of dressings for partial‐thickness burns (Wasiak 2013), and of individual agents with antiseptic properties for all types of wounds (Aziz 2012; Dat 2012; Jull 2015; Storm‐Versloot 2010; Vermuelen 2010), however, this review provides a single synthesis of the randomised evidence relating to all antiseptics for any type of burn wound as well as having a more recent search. This, together with differences in inclusion criteria mean that there are differences in the included studies. It is worth noting that over 30% of the studies in this review were published in 2010 or later. There are also differences in the approach to analysis, with this review deriving hazard ratios to allow evaluation of the 'chance' of healing over time for some of the comparisons; this is a more robust measure of the outcome than mean time to healing or the occurrence of healing events at a single time point.
Authors' conclusions
Implications for practice.
The effect of different treatments in many of the comparisons is unclear: it is often uncertain whether the antiseptics assessed in these (often single, small) trials are associated with any difference in healing, infections, adverse events or other outcomes. The certainty of this evidence is low or very low, primarily due to the high levels of imprecision around the estimates of effect.
In some cases (see Summary of main results) there is moderate or high evidence for the comparisons of honey to topical antibiotics or non‐antibacterial dressings. This suggested that there is an advantage to the use of honey over the alternative treatments in these comparisons in terms of wound healing. We note that there was very limited reporting of data on pain in the comparisons involving honey. Pain is particularly important in this patient group and has been reported to be a consideration in the use of honey. Practitioners may wish particularly to take the lack of data on this outcome into account, together with the evidence on healing and infection. There is, however, some moderate certainty evidence that pain may be reduced more from baseline in burns treated with Aloe Vera compared with silver sulfadiazine (SSD) and that there may be lower levels of pain in participants treated with cerium nitrate in addition to SSD compared with SSD alone.
Much of the evidence in this review will also need careful consideration by practitioners in order to determine whether it is relevant to their practice. There was a degree of heterogeneity in terms of the age of participants ‐ ranging from very young children to adults. However most of the studies ‐ with some notable exceptions ‐ focused on burns, which were described as, or corresponded to, 'second‐degree burns' and most were below 40% TBSA ‐ in some cases very much less than this. In addition some of the comparators used may not be considered by practitioners to be relevant to their clinical work. This is particularly the case for the comparisons involving honey and non‐antibacterial dressings. In many cases it is possible that the evidence may be only indirectly relevant to particular patient groups.
In many cases the methods used in the trials were not well described and we are unsure whether they were designed in a way that makes different types of bias unlikely; although we have not downgraded for an unclear risk of bias, we are not confident that it may not be present.
Implications for research.
There is a surprising paucity of randomised evidence assessing comparisons between some of the principal antiseptic agents ‐ both with each other and with either topical antibiotics or non‐antibacterial agents. Many comparisons were represented by a single trial and many trials did not report adequate data on key outcomes. The exception to this is that there are a large number of trials that assess (1) silver‐based treatment (mainly dressings) compared with the topical antibiotic SSD and (2) honey compared with alternative treatments including SSD. Very few of these trials, however, are sufficiently clearly reported for us to be completely confident that they were well‐conducted. This is also the case for the smaller number of trials available for other comparisons. Most trials were also small, meaning that there is necessarily a high level of imprecision and often inconsistency present in the comparisons to which they contribute. Nine comparisons included only a single small trial. Where more than one trial contributed to the comparison, it was still sometimes the case that primary outcomes were reported by only one trial ‐ this was particularly the case for infection. In some comparisons there was a large difference in the results of trials reporting an outcome; for example in the comparison of wound healing for iodine compared with non‐antibacterial treatments. For all these reasons the evidence for most outcomes for most comparisons was assessed as being of low or very low certainty. Even where there was evidence that was assessed as moderate or high certainty, the reporting of the trials was often insufficient for us to be very confident that bias was unlikely.
Given the key importance of infection control as well as wound healing, the lack of evidence on this outcome for many comparisons was particularly striking. In view of this uncertainty and the large number of treatment options with antiseptic properties, the design of future trials should be driven by high priority questions from patients and other decision makers. It is also important for research to ensure that the outcomes that are collected in research studies are those that matter to patients and health professionals; clinical infection and pain may be examples of such outcomes. Where trials are conducted, good practice guidelines must be followed in their design, implementation and reporting. Such trials should be adequately powered to detect differences in time to healing, should use appropriate statistical methods for time‐to‐event analyses and should include adequate follow‐up to allow all participants to heal. Consideration should also be given to enrolment criteria to ensure that trials are relevant to patients with differing levels of burn severity (depth) and extent (proportion of total body surface area).
What's new
| Date | Event | Description |
|---|---|---|
| 23 November 2017 | Amended | Minor edit made to 'Summary of Findings' table 1. |
Acknowledgements
The authors would like to acknowledge the contribution to the protocol of peer referees David Margolis, Heather Cleland, Camila Pino, Christine Fyfe and Abimbola Farinde and the copy editor Clare Dooley; and the contributions to the review of peer referees Andrew Jull, Mark Rodgers, Camila Pino and Caitlin Mitchell. They are also grateful to the copy editor, Denise Mitchell.
We would also like to acknowledge the translation assistance of Mario Cruciani, Irina Telegina, Jennifer Brown, Rachel Riera, Ana Luiza C Martimbianco and Debra Fayter.
Appendices
Appendix 1. Search strategies
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Anti‐Infective Agents] explode all trees #2 MeSH descriptor: [Penicillins] explode all trees #3 MeSH descriptor: [Cephalosporins] explode all trees #4 MeSH descriptor: [Aminoglycosides] explode all trees #5 MeSH descriptor: [Quinolones] explode all trees #6 MeSH descriptor: [Clindamycin] explode all trees #7 MeSH descriptor: [Metronidazole] explode all trees #8 MeSH descriptor: [Trimethoprim] explode all trees #9 MeSH descriptor: [Mupirocin] explode all trees #10 MeSH descriptor: [Neomycin] explode all trees #11 MeSH descriptor: [Fusidic Acid] explode all trees #12 MeSH descriptor: [Framycetin] explode all trees #13 MeSH descriptor: [Polymyxins] explode all trees #14 MeSH descriptor: [Chlortetracycline] explode all trees #15 (antibiotic* or antimicrobial* or antibacterial* or penicillin* or cephalosporin* or aminoglycoside* or quinolone* or clindamycin or metronidazole or trimethoprim or mupirocin or "pseudomonic acid" or neomycin or "fusidic acid" or framycetin or polymyxin* or chlortetracycline):ti,ab,kw #16 MeSH descriptor: [Antisepsis] explode all trees #17 antiseptic*:ti,ab,kw #18 MeSH descriptor: [Soaps] explode all trees #19 MeSH descriptor: [Iodophors] explode all trees #20 MeSH descriptor: [Chlorhexidine] explode all trees #21 MeSH descriptor: [Alcohols] explode all trees #22 MeSH descriptor: [Hydrogen Peroxide] explode all trees #23 MeSH descriptor: [Benzoyl Peroxide] explode all trees #24 MeSH descriptor: [Gentian Violet] explode all trees #25 MeSH descriptor: [Hypochlorous Acid] explode all trees #26 MeSH descriptor: [Hexachlorophene] explode all trees #27 MeSH descriptor: [Potassium Permanganate] explode all trees #28 MeSH descriptor: [Silver] explode all trees #29 MeSH descriptor: [Silver Sulfadiazine] explode all trees #30 MeSH descriptor: [Honey] explode all trees #31 ("soap" or "soaps" or iodophor* or povidone or iodine or chlorhexidine or betadine or "alcohol" or disinfectant* or "hydrogen peroxide" or "benzoyl peroxide" or "gentian violet" or hypochlorit* or eusol or dakin* or hexachlorophene or benzalkonium or "potassium permanganate" or "silver sulfadiazine" or "silver sulphadiazine" or honey*):ti,ab,kw #32 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 #33 MeSH descriptor: [Burns] explode all trees #34 ("burn" or "burns" or "burned" or scald*):ti,ab,kw #35 ("thermal" near injur*):ti,ab,kw #36 #33 or #34 or #35 #37 #32 and #36 in Trials
Ovid MEDLINE
1 exp Anti‐Infective Agents/ 2 exp Penicillins/ 3 exp Cephalosporins/ 4 exp Aminoglycosides/ 5 exp Quinolones/ 6 exp Clindamycin/ 7 exp Metronidazole/ 8 exp Trimethoprim/ 9 exp Mupirocin/ 10 exp Neomycin/ 11 exp Fusidic Acid/ 12 exp Framycetin/ 13 exp Polymyxins/ 14 exp Chlortetracycline/ 15 (antibiotic$ or antimicrobial$ or antibacterial$ or penicillin$ or cephalosporin$ or aminoglycoside$ or quinolone$ or clindamycin or metronidazole or trimethoprim or mupirocin or pseudomonic acid or neomycin or fusidic acid or framycetin or polymyxin$ or chlortetracycline).ti,ab. 16 exp Antisepsis/ 17 antiseptic$.ti,ab. 18 exp Soaps/ 19 exp Iodophors/ 20 exp Chlorhexidine/ 21 exp Alcohols/ 22 exp Hydrogen Peroxide/ 23 exp Benzoyl Peroxide/ 24 exp Gentian Violet/ 25 exp Hypochlorous Acid/ 26 exp Hexachlorophene/ 27 exp Potassium Permanganate/ 28 exp Silver/ 29 exp Silver Sulfadiazine/ 30 exp Honey/ 31 (soap$1 or iodophor$ or povidone or iodine or chlorhexidine or betadine or alcohol$1 or disinfectant$ or hydrogen peroxide or benzoyl peroxide or gentian violet or hypochlorit$ or eusol or dakin$ or hexachlorophene or benzalkonium or potassium permanganate or silver sulfadiazine or silver sulphadiazine or honey$).ti,ab. 32 or/1‐31 33 exp Burns/ 34 (burn or burns or burned or scald*).tw. 35 (thermal adj injur*).tw. 36 or/33‐35 37 and/32,36 38 randomized controlled trial.pt. 39 controlled clinical trial.pt. 40 randomi?ed.ab. 41 placebo.ab. 42 clinical trials as topic.sh. 43 randomly.ab. 44 trial.ti. 45 or/38‐44 (1006117) 46 exp animals/ not humans.sh. 47 45 not 46 48 and/37,47
Ovid Embase
1 exp Antiinfective Agent/ 2 exp Penicillin G/ 3 exp Cephalosporin/ 4 exp Aminoglycoside/ 5 exp Quinolone/ 6 exp Clindamycin/ 7 exp Metronidazole/ 8 exp Trimethoprim/ 9 exp Pseudomonic Acid/ 10 exp Neomycin/ 11 exp Fusidic Acid/ 12 exp Framycetin/ 13 exp Polymyxin/ 14 exp Chlortetracycline/ 15 (antibiotic$ or antimicrobial$ or antibacterial$ or penicillin$ or cephalosporin$ or aminoglycoside$ or quinolone$ or clindamycin or metronidazole or trimethoprim or mupirocin or neomycin or fusidic acid or framycetin or polymyxin$ or chlortetracycline).ti,ab. 16 exp antisepsis/ 17 antiseptic$.ti,ab. 18 exp Soap/ 19 exp Iodophor/ 20 exp Chlorhexidine/ 21 exp Alcohol/ 22 exp Hydrogen Peroxide/ 23 exp Benzoyl Peroxide/ 24 exp Gentian Violet/ 25 exp Hypochlorous Acid/ 26 exp Hexachlorophene/ 27 exp Potassium Permanganate/ 28 exp Silver/ 29 exp Silver Sulfadiazine/ 30 exp Honey/ 31 (soap$1 or iodophor$ or povidone or iodine or chlorhexidine or betadine or alcohol$1 or disinfectant$ or hydrogen peroxide or benzoyl peroxide or gentian violet or hypochlorit$ or eusol or dakin$ or hexachlorophene or benzalkonium or potassium permanganate or silver sulfadiazine or silver sulphadiazine or honey$).ti,ab. 32 or/1‐31 33 exp burn/ 34 (burn or burns or burned or scald*).tw. 35 (thermal adj injur*).tw. 36 or/33‐35 37 and/32,36 38 Randomized controlled trials/ 39 Single‐Blind Method/ 40 Double‐Blind Method/ 41 Crossover Procedure/ 42 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab. 43 (doubl* adj blind*).ti,ab. 44 (singl* adj blind*).ti,ab. 45 or/38‐44 46 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 47 human/ or human cell/ 48 and/46‐47 49 46 not 48 50 45 not 49 51 and/37,50
EBSCO CINAHL
S45 S31 AND S44 S44 S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 S43 TI allocat* random* or AB allocat* random* S42 MH "Quantitative Studies" S41 TI placebo* or AB placebo* S40 MH "Placebos" S39 TI random* allocat* or AB random* allocat* S38 MH "Random Assignment" S37 TI randomi?ed control* trial* or AB randomi?ed control* trial* S36 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S35 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S34 TI clinic* N1 trial* or AB clinic* N1 trial* S33 PT Clinical trial S32 MH "Clinical Trials+" S31 S26 AND S30 S30 S27 OR S28 OR S29 S29 TI thermal n1 injur* OR AB thermal n1 injur* S28 TI ( burn or burns or burned or scald* ) OR AB ( burn or burns or burned or scald* ) S27 (MH "Burns+") S26 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 S25 TI ( soap* or iodophor* or povidone or iodine or chlorhexidine or betadine or alcohol* or disinfectant* or hydrogen peroxide or benzoyl peroxide or gentian violet or hypochlorit* or eusol or dakin* or hexachlorophene or benzalkonium or potassium permanganate or silver sulfadiazine or silver sulphadiazine or honey*) or AB ( soap* or iodophor* or povidone or iodine or chlorhexidine or betadine or alcohol* or disinfectant* or hydrogen peroxide or benzoyl peroxide or gentian violet or or hypochlorit* or eusol or dakin* or hexachlorophene or benzalkonium or potassium permanganate or silver sulfadiazine or silver sulphadiazine or honey*) S24 (MH "Honey") S23 (MH "Silver Sulfadiazine") S22 (MH "Silver") S21 (MH "Hexachlorophene") S20 (MH "Gentian Violet") S19 (MH "Hydrogen Peroxide") S18 (MH "Alcohols+") S17 (MH "Chlorhexidine") S16 (MH "Povidone‐Iodine") S15 (MH "Iodine") S14 (MH "Soaps") S13 TI antiseptic* S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 S11 TI ( antibiotic* or antimicrobial* or antibacterial* or penicillin* or cephalosporin* or aminoglycoside* or quinolone* or clindamycin or metronidazole or trimethoprim or mupirocin or pseudomonic acid or neomycin or fusidic acid or framycetin or polymyxin* or chlortetracycline ) or AB ( antibiotic* or antimicrobial* or antibacterial* or penicillin* or cephalosporin* or aminoglycoside* or quinolone* or clindamycin or metronidazole or trimethoprim or mupirocin or pseudomonic acid or neomycin or fusidic acid or framycetin or polymyxin* or chlortetracycline) S10 (MH "Polymyxins+") S9 (MH "Neomycin") S8 (MH "Mupirocin") S7 (MH "Trimethoprim+") S6 (MH "Metronidazole") S5 (MH "Clindamycin") S4 (MH "Aminoglycosides+") S3 (MH "Cephalosporins+") S2 (MH "Penicillins+") S1 (MH "Antiinfective Agents+")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
[“antiseptic” OR “antibacterial”] AND “burn”
World Health Organization International Clinical Trials Registry Platform
[“antiseptic” OR “antibacterial”] AND “burn”
Appendix 2. Assessment of risk of bias
The Cochrane tool for assessing risk of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random‐number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque, or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Either of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on the observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reasons for missing outcome data are likely to be related to the true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce a clinically relevant bias in the observed effect size.
'As‐treated' analysis done with a substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes is/are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes was/were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review is/are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 3. Risk of bias in cluster‐randomised trials
In cluster‐randomised trials, particular biases to consider include:
recruitment bias;
baseline imbalance;
loss of clusters;
incorrect analysis; and
comparability with individually‐randomised trials.
Recruitment bias: can occur when individuals are recruited to the trial after the clusters have been randomised, as the knowledge of whether each cluster is an 'intervention' or 'control' cluster could affect the types of participants recruited.
Baseline imbalance: cluster‐randomised trials often randomise all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue. However, because small numbers of clusters are randomised, there is a possibility of chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals. Although this is not a form of bias as such, the risk of baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters. Reporting of the baseline comparability of clusters, or statistical adjustment for baseline characteristics, can help reduce concern about the effects of baseline imbalance.
Loss of clusters: occasionally complete clusters are lost from a trial, and have to be omitted from the analysis. Just as for missing outcome data in individually‐randomised trials, this may lead to bias. In addition, missing outcomes for individuals within clusters may also lead to a risk of bias in cluster‐randomised trials.
Incorrect analysis: many cluster‐randomised trials are analysed by incorrect statistical methods that do not take the clustering into account. Such analyses create a 'unit of analysis error' and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis.
Comparability with individually‐randomised trials: in a meta‐analysis that includes both cluster‐randomised and individually‐randomised trials, or includes cluster‐randomised trials with different types of clusters, possible differences between the intervention effects being estimated need to be considered. For example, in a vaccine trial of infectious diseases, a vaccine applied to all individuals in a community would be expected to be more effective than a vaccine applied to only half the people. Another example is provided by discussion of a Cochrane Review of hip protectors (Hahn 2005), where cluster trials showed a large positive effect, whereas individually‐randomised trials did not show any clear benefit. One possibility is that there was a 'herd effect' in the cluster‐randomised trials (which were often performed in nursing homes, where compliance with using the protectors may have been enhanced). In general, such 'contamination' would lead to underestimates of effect. Thus, if an intervention effect is still demonstrated despite contamination in those trials that were not cluster‐randomised, a confident conclusion about the presence of an effect can be drawn. However, the size of the effect is likely to be underestimated. Contamination and 'herd effects' may be different for different types of cluster.
Appendix 4. Extracted subgroup data for wound healing
| Study | Subgroups reported | Mean time to healing |
| Adhya 2015 | 20%‐40% superficial 20%‐40% deep dermal 40%‐60% superficial 40%‐60% deep dermal |
Silver: 15.7 (4.14) N = 15; SSD: 20.5 (8.75) N = 17 Silver: 38.6 (11.26) N = 17; SSD: 48.4 (14.11) N = 13 Silver: 26.0 (6.22) N = 6; SSD: 28.1 (12.76) N = 10 Silver: 45.4 (11.35); SSD: 58.9 (18.18) N = 14 |
| Chen 2006 | Superficial Deep |
Silver 9.6 (± 1.6) (N = 31); SSD 19.1 (± 2.6) (N = 33); Vaseline 13.5 (± 0.9) N = 32) Silver 19.1 (± 2.6) (N = 34); SSD 21.6 (± 2.9) (N = 30); Vaseline 22.7(± 2.9) (N = 31) |
| Gong 2009 | Superficial degree II Deep degree II |
Silver 9.8 (± 2.1); SSD 13.7 (± 2.8) (N = 28 in both groups) Silver 16.4 (± 2.8); SSD 20.9 (± 3.6) (N = 24 in both groups) |
| Li 2006 | Superficial Deep Residual |
Iodine 9.6 (2.4) (N = 16); carbon 7.4 (2.1) (N = 46) Iodine 19.6 (3.4) (N =32); carbon 16.2 (2.6) (N =89) Iodine 28.8 (10.4) (N =26); carbon 19.4 (6.2) (N = 68) |
| Liao 2006 | Superficial Deep |
N = 80: silver 9.5 (± 2.7); SSD 10.8 (± 3.4) N = 40: silver 21.5 (± 4.8); SSD 23.3 (± 6.4) |
Data and analyses
Comparison 1. Silver dressings versus topical antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound healing (hazard ratio) | 3 | 259 | Hazard Ratio (Random, 95% CI) | 1.25 [0.94, 1.67] |
| 2 Wound healing (mean time to healing) | 10 | 1085 | Mean Difference (IV, Random, 95% CI) | ‐3.33 [‐4.96, ‐1.70] |
| 3 Wound healing (risk ratio) up to 28 days | 5 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.00, 1.37] |
| 4 Infection (up to 4 weeks or NR) | 4 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.48, 1.49] |
| 5 Adverse events (14‐28 days) | 6 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.63, 1.18] |
| 6 Withdrawals due to adverse events (21 days or NR) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Pain at dressing change (up to 28 days or NR) | 5 | 353 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.92, ‐0.49] |
| 8 Pain (time/follow‐up not specified) | 3 | 135 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.06, ‐1.27] |
| 9 Mortality (21 days or NR) | 3 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.20, 12.64] |
| 10 Resource use (number of dressings) (up to 28 days or NR) | 6 | 446 | Mean Difference (IV, Random, 95% CI) | ‐7.56 [‐12.09, ‐3.04] |
| 11 Costs (21 days or NR) | 4 | 261 | Mean Difference (IV, Random, 95% CI) | ‐117.18 [‐280.02, 45.67] |
| 12 Cost‐effectiveness/wound healed (21 days) | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐384.71 [‐503.66, ‐265.75] |
1.12. Analysis.
Comparison 1 Silver dressings versus topical antibiotics, Outcome 12 Cost‐effectiveness/wound healed (21 days).
Comparison 2. Honey versus topical antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound healing (hazard ratio) | 5 | 580 | Hazard Ratio (Random, 95% CI) | 2.45 [1.71, 3.52] |
| 2 Wound healing (risk ratio) (up to 60 days) | 6 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.99, 2.76] |
| 3 Wound healing (mean time to healing) | 6 | 712 | Mean Difference (IV, Random, 95% CI) | ‐3.79 [‐7.15, ‐0.43] |
| 4 Incident infection (up to 24 days) | 4 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.08, 0.34] |
| 5 Persistent positive swabs (up to 21 days) | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.05, 0.19] |
| 6 Adverse events (time points between 21 days and 6 weeks) | 3 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 3.97] |
2.2. Analysis.
Comparison 2 Honey versus topical antibiotics, Outcome 2 Wound healing (risk ratio) (up to 60 days).
Comparison 3. Aloe vera vs topical antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound healing (mean time to healing) | 3 | 210 | Mean Difference (IV, Random, 95% CI) | ‐7.79 [‐17.96, 2.38] |
| 2 Infection (time points between 14 days and 2 months) | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.26, 3.34] |
Comparison 4. Iodine‐based treatments versus topical antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound healing (mean time to healing) | 2 | 148 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐2.76, 1.83] |
Comparison 5. Silver‐based antiseptics versus non‐antimicrobial.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound healing (mean time to healing) | 2 | 204 | Mean Difference (IV, Random, 95% CI) | ‐3.49 [‐4.46, ‐2.52] |
| 2 Positive swab (21 days) | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.95] |
5.2. Analysis.
Comparison 5 Silver‐based antiseptics versus non‐antimicrobial, Outcome 2 Positive swab (21 days).
Comparison 6. Honey versus non‐antibacterial dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound healing (hazard ratio) | 2 | 164 | Hazard Ratio (Fixed, 95% CI) | 2.86 [1.60, 5.11] |
| 2 Wound healing (mean time to healing) | 4 | 1156 | Mean Difference (IV, Random, 95% CI) | ‐5.32 [‐6.30, ‐4.34] |
| 3 Persistent positive swabs (up to 30 days) | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.06, 0.40] |
Comparison 7. Chlorhexadine versus non‐antibacterial dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound healing (mean time to healing) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Infection (up to 30 days) | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.54, 2.27] |
7.1. Analysis.
Comparison 7 Chlorhexadine versus non‐antibacterial dressing, Outcome 1 Wound healing (mean time to healing).
Comparison 8. Iodine‐based antiseptics versus non‐antibacterial treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound healing (mean time to healing) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Costs (duration 18 days +) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 9. Cerium nitrate versus non antibacterial treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (short‐term or unclear) | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.99] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abedini 2013.
| Methods | Country where data collected: Iran Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: not reported (until epithelialisation) |
|
| Participants | Inclusion criteria: partial‐thickness burn wounds < 24 h post‐injury with TBSA between 10%‐40% and aged 5‐60 years Exclusion criteria: chemical & electrical burns, multiple trauma and serious comorbidity Participants: 69 hospital patients Mean age (years): 27.9 vs 26.2 years Male participants: 67.6% vs 68.6% Burn type: fire 73.5% vs 74.3%; hot liquid 14.7% vs 20%; other 11.8% vs 5.7% Burn degree: NR Burn size (%TBSA): NR Burn location: NR |
|
| Interventions | Intervention arm 1: SSD cream, covered with cotton gauze, changed every other day. N = 34 Intervention arm 2: Silver nylon dressing (Agicoat) covered with cotton gauze, wetted regularly with sterile water, changed every 7 days. N = 35 Cointerventions: fentanyl analgesia as required |
|
| Outcomes | Primary outcome: wound healing rate (mean time to complete healing) Secondary outcome: resource use (total hospitalisation cost) |
|
| Notes | SD for wound healing and hospitalisation data extrapolated from graph Funding NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "sixty‐nine burn wounds patients were included and randomised (the random number generator was used) into two groups and given burn wound treatment with 1% AgSD or Agicoat®" Comment: unclear what random‐number generator was used but acceptable |
| Allocation concealment (selection bias) | Unclear risk | Quote: "sixty‐nine burn wounds patients were included and randomised (the random number generator was used) into two groups and given burn wound treatment with 1% AgSD or Agicoat®" Comment: no information on allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “both clinicians and patients or relatives were aware of the treatment procedure (open label design)” Comment: open label design and no mention of blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote “all patients remained in the study” Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | No specific quote Comment: no report of VAS or resource use, which were listed as assessed outcomes. Also many outcomes had to be extrapolated from graphs |
| Other bias | Unclear risk | No evidence of other sources of bias but reporting insufficient to be certain |
Adhya 2015.
| Methods | Country where data collected: India Parallel‐group RCT (stratified randomisation) Unit of randomisation: participant Unit of analysis: participant Duration: 4 weeks for most outcomes, until epithelialisation for wound healing |
|
| Participants | Inclusion criteria: second‐degree burns, 20% to 60% TBSA Exclusion criteria: superficial (first‐degree) or full‐thickness (third‐degree burns); pregnancy; "significant” comorbidities: pre‐existing heart disease; renal disease; diabetes Participants: 163 hospital patients (unclear if inpatient or outpatient) Mean age (years): 27.4 vs. 31.8 Male participants: 29/52 vs 25/52 Burn type: NR Burn degree and size (%TBSA): mix of 20% ‐40% TBSA (12 vs 15 superficial; 13 vs 17 deep dermal) and > 40%‐60% TBSA (10 vs 6 superficial; 14 vs 14 deep dermal) (also stratified in the analysis) Burn location: NR Note participant characteristic data refers to analysed participants not the total number randomised (substantial difference) |
|
| Interventions | Intervention arm 1: nano‐crystalline silver hydrogel (50 ppm), applied topically on alternate days. N = 52 Intervention arm 2; SSD cream (DISILVA, 1%), applied topically on alternate days. N = 52 Cointerventions: unspecified dressing |
|
| Outcomes | Primary outcome: wound healing ‐ proportion of wounds completely healed by 4 weeks (reported only for deep dermal burns) Primary outcome: wound healing ‐ time (days) to complete wound healing |
|
| Notes | Funding: Department of Science & Technology, West Bengal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Simple randomization sequence was generated by computer software” Comment: unclear what “simple” means in this context but computer‐generated randomisation sequences generally regarded as low risk |
| Allocation concealment (selection bias) | Unclear risk | Quote: “After allocation of patients into two different groups, SSD and AgNP gel were administered topically…” Comment: no detail on allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “this study was designed as an open‐label prospective, parallel group, randomized controlled trial.....Clinical assessments of burn wound were done on every week till 4th week and on completion of treatment.” Comment: open label trial with no mention of blinding assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Data for evaluation were obtained for 54 patients on SSD (2° deep‑dermal cases 27) and 52 (2° deep‑dermal cases 31) on AgNP treatment” Comment: 163 randomised, 57 lost to follow‐up. Similar numbers in each arm (30 vs. 27) but no reasons given |
| Selective reporting (reporting bias) | Unclear risk | Quote: “As shown in Table 12, considering deep‑dermal burn wounds only, the differences in treatment outcome at 4 weeks was statistically highly significant (P = 0.003) in favor of AgNP treatment. However, at 4 weeks, only 4 cases in AgNP arm had achieved complete wound healing compared to none in the SSD arm, and this was not a statistically significant difference [Table 13]. However, 25 had achieved 50% wound healing compared to 13 on SSD, and this was statistically significant (P = 0.001).” Comment: proportion of wounds healed completely by 4 weeks given for deep dermal wounds only. No explanation of why analysis would be stratified |
| Other bias | Low risk | Comment: no other issues identified, but reporting insufficient to be certain |
Akhtar 1996.
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: any age, TBSA >10% up to 40% Exclusion criteria: systemic diseases e.g. diabetes, or malignancy, vitamin deficient and immunosuppressed; electrical, chemical and radiation burns Participants: 100 patients from tertiary hospital Mean age (years): NR (comparable between groups) Male participants: NR (comparable between groups) Burn type: NR Burn degree NR (severity comparable between groups) Burn size (%TBSA): NR (severity comparable between groups, see inclusion criteria) Burn location: NR (comparable between groups) |
|
| Interventions | Intervention arm 1: Aloe vera cream every 3rd day. N = 50 Intervention arm 2: framycetin cream every 3rd day. N = 50 Cointerventions: NR |
|
| Outcomes | Wound healing (mean time to healing) | |
| Notes | Reported in abstract form only Funding NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Allocation to intervention was done by block randomization of 8 subjects." Comment: no information on how randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation to intervention was done by block randomization of 8 subjects." Comment: no information on whether allocation to treatment groups was concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote "Blinded randomized controlled trial." Comment: not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No specific quote Comment: reported in abstract only and unclear whether there was any or significant attrition |
| Selective reporting (reporting bias) | Unclear risk | No specific quote; reported in abstract only; unclear if all planned outcomes were reported |
| Other bias | Unclear risk | Abstract only, unclear if any additional sources of bias |
Baghel 2009.
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 2 months (follow‐up) |
|
| Participants | Inclusion criteria: 10‐50 years, 1st‐ or 2nd‐degree burn less than 50% TBSA Exclusion criteria: immunocompromised people; patients on chemotherapy, with renal or liver failure or with asthma Participants: 78 hospital patients Mean age (years): 34.5 vs 28.5 years Male participants: 21/37 vs 23/41 Burn type: NR Burn degree: 1st‐degree 21/37 vs 21/41; 2nd 16/37 vs 20/41 Burn size (%TBSA): < 10% 0 vs 2; 11%‐20% 7 vs 12; 21%‐30% 13 vs 10; 31%‐40% 8 vs 6; 41%‐50% 9 vs 11 Burn location: NR |
|
| Interventions | Intervention arm 1: pure undiluted honey; wounds dressed daily with sterile gauze and cotton dressing applied. N = 37 Intervention arm 2: SSD; wounds dressed daily with sterile gauze and cotton dressing applied. N = 41 Cointerventions: All stabilised and given IV antibiotics (ampicillin, gentamicin, metronidazole) for minimum 10 days in 2nd‐degree and 5 days in 1st‐degree, wounds cleaned |
|
| Outcomes | Primary outcome: wound healing (mean time to wound healing) | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote “after taking consent patients were randomly attributed to two study groups” Comment: no information on how randomisation sequence was derived |
| Allocation concealment (selection bias) | Unclear risk | Quote “after taking consent patients were randomly attributed to two study groups” Comment: no information on whether allocation of study treatment was concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Wound was assessed at third and seventh day and at the time of completion of study. Final outcome was measured after 2 months of follow‐up, in terms of complete and incomplete recovery." Comment: no information on whether assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no specific quote but outcome data on time to healing reported for all 78 randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but outcomes other than "complete recovery" were not prespecified so it is unclear whether all outcomes assessed were fully reported |
| Other bias | Unclear risk | Comment: no specific quote, no evidence of other bias but reporting insufficient to be certain |
Bangroo 2005.
| Methods | Country where data collected: India Parallel group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: < 12 years old, superficial thermal burn, < 50% TBSA Exclusion criteria: NR Participants: 64 hospital patients (children) Mean age (years): NR Male participants: 23/32 vs 25/32 Burn type: 56 wet burns, 8 dry burns Burn degree: NR/NA Burn size (%TBSA): < 10% 5 vs 3; 11%‐20% 2 vs 5; 21%‐30% 7 vs 8; 31%‐40% 16 vs 15; 41%‐50% 2 vs 1 Burn location: 12 facial, 20 extremities, 21 trunk and abdomen |
|
| Interventions | Intervention arm 1: honey dressing (changed twice daily) N = 32 Intervention arm 2: SSD (dressing changed twice daily) N = 32 Cointervention: Thorough bath, twice daily with tap water and soap; followed by sponging and peeling away dead skin. |
|
| Outcomes | Primary outcome: Wound healing (mean time to healing) Secondary outcome: Adverse events |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “two groups…. were formed and patients assigned to it randomly” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: “two groups were formed and patients assigned to it randomly” Comment: no information on whether the allocation of participants to interventions was concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Culture swabs were taken from the burnt surface on admission, before any treatment was instituted and repeated after 48 h and, thereafter, every 72 h until the wound healed" Comment: no information on whether assessment of healing was conducted by assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Wound healing took 10 days in 26 patients belonging to group A, while in 6 patients it took 2 weeks or more to heal.....Wound healing took 3 weeks or more in 19 patients belonging to group B." Comment: it appears that all participants (64 randomised) completed the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but the outcomes assessed were not prespecified so it is unclear whether all outcomes assessed were fully reported; the balance of probabilities is that they were. |
| Other bias | Unclear risk | No specific quote but no evidence of other source of bias, but reporting insufficient to be certain |
Carayanni 2011.
| Methods | Country where data collected: Greece Parallel‐group RCT (stratified by burn thickness) Unit of randomisation: participant Unit of analysis: participant Duration: 18 days |
|
| Participants | Inclusion criteria: thermal burns with TBSA < 15% and need for hospitalisation but no need of surgical operation Exclusion criteria: cancer or diabetes Participants: 217 randomised (3 excluded for needing surgery) hospital patients Mean age (years): 42.6 vs 42.7 Male participants: 60/104 vs 71/107 Burn type: flame 57 vs 56; scald 50 vs 48 Burn degree: deep partial‐thickness: 50 vs 52; superficial 54 vs 55 (stratified randomisation) Burn size (%TBSA): NR; surface area 10.26 (4.37) vs 9.89 (4.89) (cm2) Burn location: NR |
|
| Interventions | Intervention arm 1: moist exposed burn ointment (MEBO) applied twice per day. No dressings were used. N = 104 Intervention arms 2: povidone iodine applied twice per day plus bepanthenol cream applied twice daily after 3rd or 4th day (according to degree of epithelialisation). No dressings were used. N = 107 Cointerventions: burns were lightly debrided by antiseptic in the shower every second day |
|
| Outcomes | Primary outcome: infections Secondary outcome: adverse events Secondary outcome: resource use (length of hospital stay) Secondary outcome: cost associated with resource use Secondary outcome: pain (VAS) |
|
| Notes | Funding: most resources provided by Regional General Hospital of Athens "Georgios Gennimatas" (Greece) Department of Plastic Surgery, Microsurgery and Burn Center (equipment, stock medicines (except MEBO), and personnel) MEBO provided by MEBO International Group Company (MEBO medicines, China) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly, alteration [sic] was used of permuted 20 sub‐blocks of sizes from 1‐3 for deep partial thickness burns group and 25 sub‐blocs of the same size for the superficial partial burn groups." Comment: does not state how randomisation sequence was derived |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The allocation was carried out by the staff of outpatient reception desk of the Clinic. Patient Envelopes were provided for patients requiring treatment allocation in each group. These were numbered sequentially and a list was provided with the envelopes and completed with the trial number and patient name. The date when the envelope was opened (i.e., the date of randomization) was added." Comment: the envelopes were sequentially numbered but not said to be sealed or opaque, and it's not known what the reception staff knew about the participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinding was made only for persons evaluating treatment outcomes in order to eliminate classification bias." This was not the case for pain "Blinding the treatments was not possible because Povidone iodine has a characteristic color and odor" Comment: outcome assessors were blinded to treatment allocation except for pain outcomes where participants were the assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "211 (214 randomized) patients, aged between 18‐75 years were prospectively selected. Three patients were excluded because of violation of the inclusion criteria (need of surgical operation). The flow of the participants is described in Figure 1..... We did have loss of contact for the pain measurement (9th day and after) for 3 patients recovered earlier than 8th day (1 for the MEBO group and 2 for the old therapy group). These censored observations were imputed by the Method of Last Observation Carried Forward, with decreased risk of bias because the censoring occurred near the end of the follow‐up period" Comment: Figure one shows all randomised participants included in analysis; the number of participants affected by censoring was low. |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but primary outcomes and other outcomes specified and reported fully |
| Other bias | Low risk | Comment: no specific quote but no evidence of other sources of bias and detailed reporting of methods |
Caruso 2006.
| Methods | Country where data collected: USA Parallel‐group RCT (stratified by TBSA and age) Unit of randomisation: participant Unit of analysis: participant Duration: 21 days |
|
| Participants | Inclusion criteria: age ≥ 2 months; superficial, mid‐dermal, or mixed partial‐thickness burns, 5%‐40% TBSA, within 36 h of enrolment. Randomisation stratified by TBSA (5%‐20% or > 20% ‐40%) and age (0‐3 years or ≥ 4 years) Exclusion criteria: pregnancy; electrical, chemical, or frostbite burns; areas of burn likely to require excision/grafting; antibiotic use in 2 days prior to burn injury; evidence of inhalation injury; fractures and/or neurological injury. Participants: 84 hospital or clinic outpatients (unclear if some inpatients also included) Mean age (years): 29.4 vs 24.0 years Male participants: 27/42 vs 30/40 Burn type: scald 27/42 vs 18/40; flash 9/42 vs 13/40; flame 4/42 vs 8/40; contact, 0 vs 1; other 2 vs 0 Burn degree: superficial and mid‐dermal (N = NR) Burn size (%TBSA): 12.0% vs 10.8% (superficial 4.8% vs 4.2%; mid‐dermal BSA 8.8% vs. 8.1%) Burn location:NR |
|
| Interventions | Intervention arm 1: silver hydrofibre dressing (AQUACEL Ag Hydrofiber, 1.2% weight ionic silver). Dressing overlapped wounds by 4‐5 cm. Applied in hospital/clinic on day 1 and every 2‐3 days for 21 days. Dressing covered with gauze and retention dressings. (N = 42) Intervention arm 2: SSD cream (Silvadene, 1%). 1/16” (1.6 mm) thick application. Outer dressing and dressing changes per package insert but “at least once daily”. Home dressing changes permitted between clinic visits. (N = 42) Cointerventions: procedural medications & opiates where indicated |
|
| Outcomes | Primary outcome: wound healing (proportion of participants with full epithelialisation) Primary outcome: infection Secondary outcome: resource use (frequency of dressing changes) Secondary outcome: pain (VAS) |
|
| Notes | Patient characteristic data refers to participants included in analysis, not numbers randomised (2 participants from 1 group excluded) Funding: ConvaTec, a BristolMyers Squibb company (manufacturer of silver dressing) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were assigned randomly to a protocol of care that included either AQUACEL® Ag dressing or silver sulfadiazine. The randomization schedule was stratified by extent of burns (5% to 20% or _20% to 40% of TBSA) and age (0–3 years or 4 years and older)” Comment: no details on how randomisation schedule was produced |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were assigned randomly to a protocol of care that included either AQUACEL® Ag dressing or silver sulfadiazine” Comment: no information on allocation concealment is mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “Study treatment was not blinded”; “Outcomes were measured at every in‐clinic dressing change until study completion or premature study discontinuation” Comment: Blinding in relation to clinical outcome assessment was not mentioned. Healthcare cost analysis was performed by an independent group but no mention of blinding. Participants weren't blinded and outcomes were assessed at in‐clinic dressing change when group assignment would have been apparent based on the dressing. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “In the AQUACEL® Ag dressing group, all 42 patients were included in the safety and intent‐to‐treat analyses. In the silver sulfadiazine group, 40 of 42 patients were included in the safety and intent‐to‐treat analyses because 2 patients did not receive study treatment. Comment: although there was incomplete data for pain and long‐term follow‐up all participants were accounted for in the ITT wound healing analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: No direct quote but the outcomes to be assessed were not prespecified in the methods so it is unclear whether they were fully reported |
| Other bias | Unclear risk | Comment: No direct quote but no evidence of other sources of bias although high levels of manufacturer involvement were noted |
Chen 2006.
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR (until healing) |
|
| Participants | Inclusion criteria: second‐degree burn wounds (superficial or deep); in hospital within 0.5‐12 h Exclusion criteria: NR Participants: 191 hospital patients Mean age (years): (35 ± 12) vs (30 ± 9) vs (32 ± 11) Male participants: 42/65 vs 36/63 vs 35/63 Burn type: NR Burn degree: superficial 31 vs 33 vs 32; deep 34 vs 30 vs 31 Burn size (%TBSA): superficial: 38.3 ± 18.1 vs 22.5 ± 10.2 vs 28.3 ± 8.6; deep 10.1 ± 2.2 vs 6.3 ± 3.2 vs 8.2 ± 1.6) Burn location:NR |
|
| Interventions | Intervention arm 1: silver nanoparticle dressing, changed every day (N = 65) Intervention arm 2: 1% SSD cream, changed every day (N = 63) Intervention arm 3: Vaseline gauze, changed every day (N = 63) Cointerventions: wounds cleaned with 0. 5% iodophor |
|
| Outcomes | Primary outcome: wound healing (mean time to wound healing) | |
| Notes | Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author Funding NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: result section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | Comment: The whole process of conducting this RCT was not clear |
De Gracia 2001.
| Methods | Country where data collected: Phillipines Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR (until healing of partial‐thickness burns and readiness for skin grafting in full‐thickness burns) |
|
| Participants | Inclusion criteria: aged > 4 months with TBSA > 15%, admitted within 24 h of burn injury Exclusion criteria: inhalation injury, known hypersensitivity to sulfonamides, known methemoglobinemia during the pre‐burn period Participants: 60 participants with moderate or severe burns Mean age (years): 30 (11.5) vs 24 (14.6) Male participants: 16/30 vs 20/30 Burn type: NR Burn degree: partial and full‐thickness Burn size (%TBSA): partial‐thickness 22% vs 30%; full‐thickness 5.6% vs 2.1% Burn location: face, perineum, trunk, extremities (proportions not reported) |
|
| Interventions | Intervention arm 1: SSD (Flammazine) changed 2‐3 times daily for open dressings on face or perineum; daily on trunk and extremities (closed dressings) Intervention arm 2: SSD plus cerium nitrate (Flammacerium) changed 2‐3 times daily for open dressings on face or perineum; daily on trunk and extremities (closed dressings) Cointerventions: fluid and electrolyte resuscitation, wound cleansing with skin cleanser soap and water or normal saline |
|
| Outcomes | Primary outcome: wound healing (partial‐thickness burns only) Primary outcome: infection and septicaemia Secondary outcome: mortality Secondary outcome: adverse events |
|
| Notes | Data extraction and 'Risk of bias' assessment undertaken by translators from Portuguese Funding unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "20 patients.... were assigned consecutively to receive either SSD‐CN or SSD alone, according to a pre‐established randomized sequence" Comment: no information on how randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "20 patients.... were assigned consecutively to receive either SSD‐CN or SSD alone, according to a pre‐established randomized sequence" Comment: no information on whether allocation was concealed adequately |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The gross appearance of the burn wound was noted in all patients...... overall responses to therapy were rated in terms of wound bacterial count and time for epithelialization of partial thickness wounds or readiness of full thickness burns to accept skin grafts". Comment: no indication whether outcome assessment was performed in a blinded fashion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes were reported. |
| Other bias | Unclear risk | It is not clear if the groups were similar regarding relevant characteristics at baseline |
Glat 2009.
| Methods | Country where data collected: USA Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 21 days + |
|
| Participants | Inclusion criteria: age: 2 months–18 years; enrolment: < 36 h post‐injury; burn severity: superficial to mid‐dermal, TBSA 1%‐40% Exclusion criteria: electrical or chemical burns; deep or full‐thickness burns; previous antimicrobial or enzymatic debridement; death likely within study period; enrolment in a previous study; pregnancy Participants: 24 children attending a paediatric hospital; mixture of inpatients and outpatients Mean age (months): 22.8 vs 43.0 Burn size (%TBSA): TBSA 1%‐10% (stated as being “comparable” between treatment arms) All other characteristics NR |
|
| Interventions | Arm 1: SSD cream (Silvadene, 10 mg) 1/16” (1.6 mm) thickness every 2‐3 days Arm 2: silver hydrogel (SilvaSorb), 1/16” (1.6 mm) thickness every 2‐3 days Cointerventions: initial blister fluid drainage. Cream/gel covered with non‐adherent dressing, rolled gauze and Elasti‐net. Participants or parents were allowed to change wound dressings in outpatient cases. |
|
| Outcomes | Primary outcome: wound healing: time to complete wound healing (mean time to (full) re‐epithelialisation) Primary outcome: wound healing: proportion of wounds completely healed during follow‐up ((full) re‐epithelialisation at 21 days Secondary outcome: adverse events Secondary outcome: resource use (number of dressing chances) Secondary outcome: pain (during dressing changes, measured using the Wong‐Baker Faces Pain Scale/observational pain assessment scale in infants or toddlers) |
|
| Notes | Funding: Drexel University School of Medicine by Medline Industries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote “Patients were randomly assigned to a protocol of care that included either SSD cream or SilvaSorb Gel” Comment: no further details on method of randomisation |
| Allocation concealment (selection bias) | High risk | Quote: ”patients were randomly assigned to a protocol of care… without blinding of the physician investigator or other medical personnel to the type of treatment” Comment: states that physicians and other personnel were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: ”patients were randomly assigned to a protocol of care … without blinding of the physician investigator or other medical personnel to the type of treatment” Comment: mentions (unblinded) physicians as investigators, no mention of any independent assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 24 participants enrolled, mean/median/SD data for 4 stated outcomes reported for all participants |
| Selective reporting (reporting bias) | Unclear risk | Quote: “Study endpoints that were recorded included the following…” Comment: wording implies that there may have been other end points, though data are given for the stated endpoints |
| Other bias | Low risk | No direct quote. no evidence of other sources of bias and study methods reasonably well reported |
Gong 2009.
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 21 days + |
|
| Participants | Inclusion criteria: age 20‐40; fresh burn wound; total burn < 10% TBSA; no infection in wound; non‐joint site Exclusion criteria: NR Participants 104 hospital patients Burn degree and size: superficial 2nd‐degree 7.4 ± 1.6cm2; deep 2nd‐degree 7.7 ± 1.4cm2 vs superficial 2nd‐degree 7.1 ± 1.5cm2; deep 2nd‐degree 7.3 ± 1.3cm2 All other characteristics NR |
|
| Interventions | Intervention arm 1: ionic silver dressing combined with hydrogel, changed every other day to 7 days and then covered with hydrogel. N = 52 Intervention arm 2: 1% SSD, changed every other day. N = 52 Cointerventions: anti‐infection treatment and nutrition support |
|
| Outcomes | Primary outcome: wound healing (proportion completely healed) Primary outcome: infection (detection rate of wound bacteria) Secondary outcome: adverse events |
|
| Notes | Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author Funding NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“This prospective randomised trial was conducted according to the random number table” Comment: a random component in the sequence generation process was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no mention of blinding of key study personnel used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | No unit of analyses issues but reporting not sufficient to determine if other risks |
Han 1989.
| Methods | Country where data collected: UK Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: people attending ED with partial skin thickness burns Exclusion criteria: pregnancy, steroid or immunosuppressive therapy, diabetes, antibiotic therapy, iodine allergy; burns with more than 6 h between injury and admission, facial and perineal burns, burns > 10% TBSA; infected burns Participants: 213 people attending ED Mean age (years): NR; proportion children < 12 years 20.5 vs 20.7; detailed age breakdown also reported Male participants: NR distribution equal between groups; female:male ratio 1:1 vs 1.1.2 Burn type: steam/hot liquid 67 vs 80; flame/fumes 14 vs 10; hot object 15 vs 12; other 6 vs 9 Burn degree: NR Burn size (%TBSA): Mean NR. < 1%, 73 vs 87; 1%‐2%, 21 vs 15; 2%‐3%, 4 vs 4; 3%‐4%, 3 vs 3; 4%‐5%, 0 vs 2; > 5% 1 vs 0 Burn location: trunk and neck 11 vs 14; shoulder and proximal arms 5 vs 6; elbow and forearm 21 vs 19; wrists and hands 38 vs 42; thigh, knee and lower leg 19 vs 14; ankle and foot 8 vs 16 |
|
| Interventions | Intervention arm 1: 0.5% chlorhexidine acetate BP (N = 102) Intervention arm 2: lnadine (rayon dressing with 10% povidone iodine ointment) (n = III) as required; application of cold soaks using refrigerated sterile water/saline; cleansed with Hibidil (0.25 per cent chlorhexidine gluconate in sterile aqueous solution). Blisters deroofed only if large and tense. Dressings covered with gauze and crepe bandage. Upper limb injuries were elevated in a sling. |
|
| Outcomes | Primary outcome: infection (bacterial culture positive and clinical evidence) Secondary outcome: pain Secondary outcome: resource use (hospital visits) |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 213 patients who attended the Accident and Emergency Department, Royal Victoria Infirmary, Newcastle upon Tyne with partial skin thickness bums were entered into a prospective randomized (random permuted block allocation) single blind trial." Comment: insufficient information on how the randomisation sequence was derived. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A total of 213 patients who attended the Accident and Emergency Department, Royal Victoria Infirmary, Newcastle upon Tyne with partial skin thickness bums were entered into a prospective randomized (random permuted block allocation) single blind trial." Comment: no information on whether the allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All patients were reviewed in the clinic 3 days later in the first instance and subsequently every 5 days. A data sheet was prepared for each patient and data recorded during the change of dressing according to a predetermined grading system relating to the description of the wound and/or dressings and clinical parameters". Comment: no information on whether outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no specific quote but no information on whether all patients were involved in most analyses; children were specifically excluded from assessment of pain and a total of 24% of participants were not included for this outcome |
| Selective reporting (reporting bias) | High risk | Quote: "Mean scores for pain and wound characteristics were calculated for each patient." Comment: it was not clear whether these (and dressing performance) were planned as the only assessed outcomes; the outcomes that they planned to assess appear to be listed on the datasheet (fig 1) ‐ this includes healing, which is not properly reported (e.g. "there were no differences in the other parameters") |
| Other bias | Unclear risk | Comment: no specific quote, no evidence of other sources of bias but reporting insufficient to be certain |
Healy 1989.
| Methods | Country where data collected: UK Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: Up to 14 days |
|
| Participants | Inclusion criteria: people with partial‐thickness burns covering < 10% TBSA Exclusion criteria: burns to face and hands Participants: 32 individuals with burns (no further information) Mean age (years): 2.6 (includes 0 adults) versus 20.6 (includes 5 adults) Male participants: NR Burn type: scald 25, flame 6, contact 1 (numbers approximately equal between groups) Burn degree: partial‐thickness Burn size (%TBSA): 1.8 ± 0.8 vs 2.3 ± 0.6 Burn location: NR |
|
| Interventions | Intervention arm 1: silver‐impregnated porcine xenograft (E‐Z Derm) N = 16 Intervention arm 2: petroleum gauze (Jelonet) N = 16 Cointerventions: NR |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: adverse events (need for surgery) |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization to either the E‐Z Derm or Jelonet groups was by drawing a card from a sealed envelope." Comment: unclear how the randomisation process was designed and implemented so unclear if truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization to either the E‐Z Derm or Jelonet groups was by drawing a card from a sealed envelope." Comment: unclear whether allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All of the burns in both groups were assessed for the following: I. The need for surgical intervention to achieve healing............2. The time to spontaneous healing was noted in those patients not requiring surgical treatment. 3. Laboratory reports of significant growths of pathogenic microorganisms on culture of superficial wound swabs" Comment: no indication that assessment was carried out in a blinded manner |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no specific quote but all randomised participants appeared to be included in the analysis (based on tables) |
| Selective reporting (reporting bias) | Low risk | Quote: "All of the burns in both groups were assessed for the following: I. The need for surgical intervention to achieve healing, indicated by clinical evidence of an increase in burn depth and lack of evidence of spontaneous healing by 10‐14 days. 2. The time to spontaneous healing was noted in those patients not requiring surgical treatment. 3. Laboratory reports of significant growths of pathogenic microorganisms on culture of superficial wound swabs." Comment: specified outcomes were properly reported. |
| Other bias | Unclear risk | Comment: no specific quote but no evidence of other sources of bias, but reporting insufficient to be certain |
Homann 2007.
| Methods | Country where data collected: Germany RCT with intra‐individual design Unit of randomisation: burn Unit of analysis: burn Duration: 21 days |
|
| Participants | Inclusion criteria: 2 partial‐thickness burn wounds of comparable size, location and prior treatment, ≤ 3 days from injury; TBSA ≤ 50%; wound area between 36 cm2 ‐300 cm2; upper body injuries needed to both occur on wither ventral or dorsal side Exclusion criteria: infected wounds at study onset, wounds in the axillary or inguinal region, deep body folds or a distinctive adipose tissue region Participants: 43 participants with 2 comparable burns Mean age (years): NR Male participants: NR Burn type: NR Burn degree: partial‐thickness Burn size (%TBSA): 11.1 ± 7.7 (79.2 cm2 vs 77.3 cm2) Burn location: NR |
|
| Interventions | Intervention arm 1: polyvinylpyrrolidone iodine liposome hydrogel (Repithel) (3% PVP‐iodine, 3% phospholipin 90 H liposome). Applied once daily as 2 mm layer covered with paraffin gauze dressing. N = 43 Intervention arm 2: SSD (10 mg/g). Applied once daily as 2 mm layer covered with paraffin gauze dressing. N = 43 Cointerventions: no additional topical treatments |
|
| Outcomes | Primary outcome: wound healing Primary outcome: infection Secondary outcome: adverse events |
|
| Notes | Funding: Mundipharma GmbH (manufacturer) This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization list was prepared by the statistics department from Mundipharma GmbH, using the EDP program Rancode 3.6.” Comment: computer‐generated randomisation list is classed as low in terms of risk‐of‐bias. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “After written informed consent was obtained, patients were enrolled and the 2 burn wounds to be assessed were randomized to treatment with the liposome PVP‐I hydrogel Repithel or the silver‐sulfadiazine cream Flammazine.” Comment: no explicit mention of allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “A limitation to this study is the fact that, due to the characteristic coloring of PVP‐I, this was not a blinded study." Comment: unblinded study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Forty‐three patients comprised the full analysis set (intent‐to‐treat) and 39 patients completed the study per protocol. Protocol violations were wounds older than 3 days in 2 patients and lack of comparability of wounds or a full‐thickness (degree IIb/III) burn wound in 1 patient each.” Comment: no unexplained loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Quote: “The clinical assessment of study wounds included inflammation (secretion, reddening, coating) and healing tendency (very good, good, moderate, none).” Comment: some uncertainty about the above statement – the word “included” implies there may possibly have been more outcomes assessed. |
| Other bias | Unclear risk | Comment: it was unclear whether the analysis took account of the intra‐individual design |
Huang 2007.
| Methods | Country where data collected: China Parallel‐group RCT (multicentre) Unit of randomisation: participant Unit of analysis: burn Duration: 20 days |
|
| Participants | Inclusion criteria: men and women aged 18‐65 years with burn wounds unhealed 3 weeks after injury (residual burn wounds) Exclusion criteria: serious complications of heart, liver, kidney or blood system (blood production or bleeding issues); serious complications, shock or serious systemic infection; uncontrolled diabetes, pregnancy or breast feeding, allergy to solver ions; other reason unable to complete observation period Participants: 111 participants randomised, 98 analysed with 166 burns Mean age (years): NR Male participants: NR Burn type: NR (residual wound) Burn degree: NR Burn size (%TBSA): NR |
|
| Interventions | Intervention arm 1: nanocrystalline silver dressing (Acticoat) changed once daily where redness, swelling and high levels of exudate, otherwise every 3 days. Auxilliary dressing over intervention dressing. (83 burns analysed) Intervention arm 2: SSD (5 g per 80 cm2) changed once daily. (83 burns analysed) Cointerventions: washing/rinsing of wounds with sterile water |
|
| Outcomes | Primary outcome: wound healing Primary outcome: change in infection status Secondary outcome: adverse events |
|
| Notes | Data extracted from English language publication; 2 additional Chinese language publications Funding NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A multi‐center, randomized experimental design is adopted, with blinding and positive parallel control. The clinical trial was done in four burn centers throughout the country at the same time with the same experimental design. The observing doctor hands out the dressing to every patient according to the time that they come to the hospital and to a randomized serial number." Comment: no information on how the randomisation sequence was derived |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A multi‐center, randomized experimental design is adopted, with blinding and positive parallel control. The clinical trial was done in four burn centers throughout the country at the same time with the same experimental design. The observing doctor hands out the dressing to every patient according to the time that they come to the hospital and to a randomized serial number." Comment: insufficient information on whether the allocation sequence was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Standards for the healing of wound: the wound healed was determined by inspection by two doctors." Comment: no information on whether the outcome assessors were blinded (although the trial is described as blinded) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Altogether 111 patients were enrolled in this group, in the process of the trial, 13 patients were dropped out of the study. Among them two patients were dropped out because of silver allergy. Eight were removed because they left to their local clinic before the wound healed, therefore we do not have their related records. Three patients were dropped because of liver dysfunction. The remaining 98 patients who were included in the statistical analysis had altogether 166 residual wounds" Comment: 13/111 participants were not included in the analysis. The event rate was high so although there is potential for differential missing data the impact on the effect estimate was probably small. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "This study is to investigate the efficacy and safety of nanocrystalline silver (Acticoat) in the treatment of burn wounds, and to assess the clinical value of this dressing." Comment: no specification of how efficacy and safety was to be assessed so difficult to determine if all planned outcomes were reported. However a statistical analysis for wound healing rate was pre‐specified and presented |
| Other bias | High risk | Comment: unit of analysis issues arising from randomisation at the participant level and analysis at the level of the burn (multiple burns for some participants) |
Inman 1984.
| Methods | Country where data collected: Canada Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR (duration of healing up to mean 26 days) |
|
| Participants | Inclusion criteria: age > 1 year; full‐thickness burns; < 24 h post injury Exclusion criteria: prior topical antibiotic treatment, pregnant, allergic to sulfa drugs Participants: 121 analysed, N randomised unclear Mean age (years): 31 ± 21 vs 33 ± 25 Male participants: NR Burn type: flame 35 vs 38; scald 8 vs 20; electrical contact 3 vs 1; other 8 vs 8 Burn degree: full‐thickness Burn size (%TBSA): full‐thickness 13 ± 16 vs 10 ± 11 Burn location: perineal 10 vs 9 (9 vs 5 full‐thickness); inhalation injury 10 vs 16 (ventilator 7 vs 9) |
|
| Interventions | Intervention arm 1: SSD (1%) plus chlorhexidine digluconate (0.2%) cream (Silvazine); "buttered on to wound and/or wound dressed with "buttered" cotton gauze. 54 participants Intervention arm 2: SSD (1%) (Flamazine) buttered on to wound and/or wound dressed with "buttered" cotton gauze. 67 participants Cointerventions: antibiotics as appropriate; daily bathing with non‐antibacterial soap and wound debridement, wound excision as appropriate |
|
| Outcomes | Primary outcome: wound healing Primary outcome: infection Secondary outcome: mortality (overall, infection‐related) Secondary outcome: adverse events |
|
| Notes | A list of exclusions are presented that appear likely to account for post‐randomisation withdrawals, number randomised unclear Funding: British Columbia Professional Firefighters Association; Smith & Nephew Canada An additional paper (Snelling 1991) reported additional participants but it appeared that these participants were not randomised to the intervention groups and so are not reported here. The reference is provided as a secondary citation for the study. Funding: British Columbia Professional Firefighters Association and Smith and Nephew Canada |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomly assigned to receive either Silvazine or Flamazine”. Comment: no detail on randomisation methods |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomly assigned to receive either Silvazine or Flamazine”. Comment: no detail on allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Wounds were cultured with a swab once or twice weekly with twice weekly cultures being taken from most patients whose wounds involved more than 10 per cent of the body surface. Surface cultures were obtained at each culture session. Full‐thickness burn wound biopsies were also obtained, and examined for histological evidence of bacterial invasion into dermis or fat and quantitative bacterial counts determined.” Comment: no information on blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Patients who did not survive for 7 days, who had all eschar excised before day 7, who were discharged before day 7 or who went on to heal all of what was initially diagnosed as the full‐thickness component of the burn wound were excluded from the study group.” Comment: excluded participants would more usually be handled as part of an ITT population. As such, their exclusion is a potential source of bias. |
| Selective reporting (reporting bias) | Unclear risk | Quote: “The clinical assessment of study wounds included inflammation (secretion, reddening, coating) and healing tendency (very good, good, moderate, none).” Comment: some uncertainty about the above statement – the word “included” implies there may possibly have been more outcomes assessed |
| Other bias | Unclear risk | Comment: no direct quotes but no evidence of additional sources of bias, but reporting insufficient to be certain |
Jiao 2015.
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant (one wound per participant) Duration: until healed |
|
| Participants | Inclusion criteria: fresh burn wound; total burn 10%‐20% TBSA; no other serious injury; no other major diseases (including cancer, brain disease; heart disease; kidney disease; haematological system disease; and infection); admitted to hospital within 24 h of injury Exclusion criteria: NR Participants 76 hospital patients Male/female: 44/76 (24/38 vs 20/38) Age: 18‐58 (36.8 ± 14.2) (36.5 ± 11.8 vs 36.8 ± 13.2 %TBSA: 15.2 (4.3) Burn degree: superficial: 19 vs 22; deep 19 vs 16 All burns were located around knee areas |
|
| Interventions | Intervention arm 1: nano‐silver dressing (N =38) Intervention arm 2: ordinary sterile gauze (N = 38) Co‐interventions: human epidermal growth factor was coated on the surface of the wound; dressing was changed every other day |
|
| Outcomes | Primary outcome: wound completely healed Primary outcome: infection ‐ bacterial positive rate at different time points Secondary outcome: adverse events; scar hyperplasia |
|
| Notes | Paper in Chinese; data extraction and 'Risk of bias' assessment performed by one review author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a randomised table was used" Comment: not clear how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details to indicate whether allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details of outcome assessment were given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: result section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: unclear based on paper; protocol not obtained |
| Other bias | Unclear risk | Comment: no specific quote, no evidence of other sources of bias but reporting insufficient to be certain |
Khorasani 2009.
| Methods | Country where data collected: Iran RCT with intra‐individual design Unit of randomisation: burn Unit of analysis: burn Duration: 24 days |
|
| Participants | Inclusion criteria: 2 comparable second‐degree ("same site") burns e.g. on hands or feet with similar areas Exclusion criteria: electrical or chemical burns, diabetes, pregnancy, immunodeficiency, kidney disease Participants: 30 participants with 2 comparable burns Mean age (years): 33 (± 11) Male participants: 25/30 Burn type: NR Burn degree: 2nd degree Burn size (%TBSA): 19.8 ± 7.9 Burn location: 26 burns on right and left hand, 2 on right and left foot, 2 on right or left hand |
|
| Interventions | Intervention arm 1: 0.5% A vera cream produced from powder applied twice daily. 30 burns Intervention arm 2: SSD (concentration not explicitly stated) applied twice daily. 30 burns Cointerventions: wound cleaning with water and saline; dressings; fluid resuscitation; "other treatment protocols"; oral nutrition; occasional amino acid infusions; blood products |
|
| Outcomes | Primary outcome: wound healing Primary outcome: infection |
|
| Notes | Funding: Mazandaran University, Iran This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Each patient had one burn treated with topical SSD and one treated with aloe cream, randomly.” Comment: no further details on randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Each patient had one burn treated with topical SSD and one treated with aloe cream, randomly.” Comment: no further details on allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: ”At the time of each dressing, the wound was observed clinically for signs of infection, size, and rate and nature of epithelialization by an expert surgeon. In this study, the “B” part of the body was treated with SSD and the “A” part was treated with aloe cream. Patients and nursing staff were blinded to the procedure.” Comment: no mention of blinding of the surgeon/assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Finally, 30 patients were enrolled in this study.” Comment: 30 participants included in outcome reporting |
| Selective reporting (reporting bias) | Unclear risk | “At the time of each dressing, the wound was observed clinically for signs of infection, size, and rate and nature of epithelialization by an expert surgeon.” Comment: results of visual infection checks not reported (though the study does report on microbial swab contamination) |
| Other bias | Unclear risk | Comment: unclear whether analysis took into account the intra‐individual design |
Li 1994.
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR (until healing) |
|
| Participants | Inclusion criteria: people with deep second‐degree burn wounds 1%‐12% TBSA and aged 16‐70 Exclusion criteria: NR Participants: 115 hospital patients Mean age (years): NR Male participants: 84/115 Burn type: NR Burn degree: second‐degree Burn size (%TBSA): NR (about 100 cm2) Burn location: NR |
|
| Interventions | Intervention arm 1: Moist burn ointment (MEBO) every 6 h. N = 31 Intervention arm 2: 0.25% iodophor every 6 h. N = 24 Intervention arm 3: 1% Rivanol every 6 hs. N = 22 Intervention arm 4: SSD every 6 h. N = 38 Cointerventions: antibiotics for 3‐10 days |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: cost |
|
| Notes | Funding NR Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | The whole process of conducting this RCT was not clear |
Li 2006.
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: NR Exclusion criteria: NR Participants: 277 hospital patients with superficial, deep or residual burn wounds Mean age (years): 30.3 (range 5‐74) Male participants: NR Burn type: NR Burn degree: superficial 46 vs 16; deep 89 vs 32; residual 68 vs 26 Burn size (%TBSA): 3.4 ± 0.6 (range 0.1‐6.0) Burn location: trunk and limbs |
|
| Interventions | Intervention arm 1: carbon fibre dressing changed daily Intervention arm 2: 0.5% iodine gauze changed daily Cointerventions: NR |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: adverse events |
|
| Notes | Funding NR Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | The whole process of conducting this RCT was not clear |
Liao 2006.
| Methods | Country where data collected: China Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: NR (until healing) |
|
| Participants | Inclusion criteria: second‐degree burns (superficial or deep) within 72 h of injury; TBSA ≤ 60% Exclusion criteria: general infection, pregnancy, patients with serious heart, kidney or liver disease (AST > 1.5; ALT > 1.5); "mental disease" Participants: 120 hospital patients Mean age (years): NR Male participants: 99/120 Burn type: NR Burn degree: second‐degree; superficial/deep 80/40 Burn size (%TBSA): NR about 100 cm2 Burn location: NR |
|
| Interventions | Intervention arm 1: 0.1% silver nitrate changed every other day Intervention arm 2: 1% SSD changed every other day Cointerventions: wound cleansing with isotonic saline; treatment duration 14 days for superficial wounds, 28 days for deep wounds |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: adverse events |
|
| Notes | Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author Funding NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | The whole process of conducting this RCT was not clear including whether the analysis took account of the intra‐individual design |
Maghsoudi 2011.
| Methods | Country where data collected: Iran Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 3 months' follow‐up |
|
| Participants | Inclusion criteria: partial‐thickness (superficial thermal) burn, < 40% TBSA Exclusion criteria: NR Participants: 100 hospital patients Mean age (years): 25.2 vs 26.4 Male participants: 23 vs 25 Burn type: flame 43 vs 39; scald 7 vs 11 Burn degree: NR Burn size (%TBSA): 14.5 (10‐40) vs 15.6 (10.5‐40) Burn location: NR |
|
| Interventions | Intervention arm 1: honey applied in quantity 16 mL‐30 mL on alternate days after saline wash. Wound covered with dry gauze Intervention arm 2: mafenide acetate‐impregnated gauze over wound after saline wash. Changed daily. Cointerventions: wound cleansing with saline; 1% lidocaine before biopsy |
|
| Outcomes | Primary outcome: wound healing Primary outcome: infection |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were allocated at random” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “patients were allocated at random” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The wounds were inspected every two days until healing…..the amount of discharge, any foul smell, the type of granulation tissue and signs of healing, and the time taken for healing were noted. The wounds were observed for evidence of infection, excessive exudate, or leakage until healing…" Comment: no information on whether outcome assessors were blinded as to allocation; balance of probabilities based on quote is that assessment was unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “two groups of 50 randomly allocated patients” Comment: no withdrawals reported and Tables 2 and 3 suggest that all participants were accounted for |
| Selective reporting (reporting bias) | Low risk | Quote: “a clinical and histochemical comparison of burns treated with honey dressing and with mafenide acetate in order to assess their wound healing rates” Comment: all stated outcomes of interest were reported |
| Other bias | Unclear risk | Comment: no direct quotes but no evidence of additional sources of bias, but reporting insufficient to be certain |
Malik 2010.
| Methods | Country where data collected: Pakistan Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: NR |
|
| Participants | Inclusion criteria: partial‐thickness burns in 2 different parts of the body (same site, e.g. right and left abdomen) occurred within 24 h of treatment initiation. TBSA < 40% Exclusion criteria: diabetes, pregnancy, immunodeficiency, kidney diseases; electrical and chemical burns Participants: 150 hospital patients Mean age (years): 28 ± 16 Male participants: 67/150 Burn type: NR Burn degree: NR Burn size (%TBSA): 22.7 ± 8.5 (10‐38) Burn location: NR but same site/equivalent) |
|
| Interventions | Intervention arm 1: honey applied directly to wound twice daily; dressing changed twice daily Intervention arm 2: SSD applied daily Cointerventions: fluid resuscitation, oral nutrition, occasional IV infusion of amino acids and blood products |
|
| Outcomes | Primary outcome: wound healing Primary outcome: infection |
|
| Notes | Funding: NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Each patient had one burn site treated with honey and one treated with topical SSD, randomly” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Each patient had one burn site treated with honey and one treated with topical SSD, randomly” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “wound was observed clinically for signs of infection, size, and rate and nature of epithelialization by an expert surgeon…. Patients and nursing staff were blinded to the procedure” Comment: nursing staff were blinded but unsure whether the inspecting surgeon was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “150 patients were enrolled in this study” Comment: no withdrawals reported and Table 10 suggests that all participants were accounted for |
| Selective reporting (reporting bias) | Low risk | Quote: “rate of burn wound healing” Comment: all stated outcomes of interest were reported |
| Other bias | Unclear risk | Comment: it was unclear whether the analysis took account of the intra‐individual design of the study |
Mashhood 2006.
| Methods | Country where data collected: Pakistan Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 6 weeks' treatment; follow‐up at 6 months |
|
| Participants | Inclusion criteria: superficial and partial‐thickness burns, TBSA < 15% Exclusion criteria: deep burns; any medical illness beginning before or after injury Participants: 50 surgical hospital outpatients Mean age (years): 27.4 Male participants: NR (both men and women were included) Burn type: NR Burn degree: NR Burn size (%TBSA): NR Burn location: NR |
|
| Interventions | Intervention arm 1: pure honey applied once daily after wound cleansing with normal saline. N = 25 Intervention arm 2: 1% SSD cream once daily. N = 25 Cointerventions: wound cleansing with normal saline; sterile gauze dressings |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: pain Secondary outcome: costs Secondary outcome: adverse events |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... 50 patients were selected for the study. They were randomly assigned to two groups" Comment: no information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "... 50 patients were selected for the study. They were randomly assigned to two groups" Comment: no information on whether the allocations to treatment were adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "At the time of change of dressing details regarding the condition of the wound such as signs of wound infection, condition of surrounding unburned tissues, discharge, smell, necrotic tissue and state of epithelialization was noted. Swabs for bacterial density and cultures were also obtained regularly. Subjective factors such as pain and local irritation were recorded regularly. Allergies or other side effects were noted in both groups." Comment: appears that blinded assessment could not have occurred as observations were undertaken when dressings were changed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: " In group I treated with honey, 52% (n=13) of the patients had all the burns healed after 2 weeks and 100% (n=25) got cured after 4 weeks. In group II treated with 1% silver sulfadiazine, 20% (n=5) of the patients had their burns healed after 2 weeks, 60% (n=15) after 4 weeks and 100% (n=25) were cured by the end of 6 weeks of the treatment." Comment: results reported for all 50 randomised participants |
| Selective reporting (reporting bias) | Low risk | Quote: "The effectiveness of the two modalities of treatment was judged on the basis of three criteria: 1. Wound healing. 2. Pain relief. 3. Time taken for the wound to get sterile." Comment: all 3 prespecified outcomes were fully reported |
| Other bias | Unclear risk | Comment: no specific quote but no evidence of other sources of bias, but reporting insufficient to be certain |
Memon 2005.
| Methods | Country where data collected: Pakistan Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: aged 4‐62 years, superficial‐dermal, mid‐dermal or deep‐dermal burns 10%‐40% TBSA Exclusion criteria: people with chemical or electrical burns, superficial burns, full‐thickness burns or burns involving > 40% TBSA Participants: 80 Mean age (years): Male participants: 54/80 Burn type: NR (not chemical or electrical) Burn degree: superficial 18 vs 12, mid‐dermal 6/8, deep‐dermal 16/20 Burn size (%TBSA): 10%‐15% 18 vs 12; 16%‐25% 14 vs 20; 26%‐40% 8 vs 8 Burn location: NR |
|
| Interventions | Intervention arm 1: natural, unprocessed honey‐gauze dressings every other day Intervention arm 2: SSD dressings (SSD cream covered with occlusive dressing) every other day |
|
| Outcomes | Primary outcome: wound healing | |
| Notes | Funding source NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were allotted at random in two different groups” Comment: in addition, it was reported in the abstract that the design was “a quasiexperimental study” The method for generating the random sequence was not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The patients were allotted at random in two different groups”. Comment: there was no information on whether allocation sequence was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no quote but no information on blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Coment: ITT analysis was not reported, but since no drop‐outs were reported and all the randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results |
| Other bias | Unclear risk | Insufficient reporting to determine the risk of other sources of bias |
Muangman 2006.
| Methods | Country where data collected: Thailand Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: partial‐thickness burns < 25% TBSA Exclusion criteria: NR Participants: 50 people attending burns unit Mean age (years): 38 ± 25 vs 26 ± 27 Male participants: NR Burn type: flame 14 vs 12; scald 9 vs 12; electrical 1 vs 1; chemical 1 vs 0 Burn degree: NR Burn size (%TBSA): 15 ± 7 vs 15 ± 5 Burn location: NR |
|
| Interventions | Intervention arm 1: silver‐coated dressing moistened with sterile water (Acticoat), covered with dry dressing. Inner gauze moistened twice daily and silver dressing changed every 3 days Intervention arm 2: SSD and dry gauze dressing changed twice daily Cointerventions: 2 tabs of acetaminophen (paracetamol) (500 mg/tab) before dressing changes |
|
| Outcomes | Primary outcome: wound infection Secondary outcome: pain |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Fifty patients were identified and randomized into 2 groups” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Fifty patients were identified and randomized into 2 groups” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “A swab of wounds was sent for routine culture and sensitivity twice a week. Wounds were observed daily by an experienced burn surgeon for signs of infection such as erythema, induration, purulent discharge and malodor. Swabs were processed by the laboratory and returned results of 1+, 2+, or 3+ bacterial growth, corresponding to light, medium, or heavy growth on the culture plate ” Comment: no information on whether outcome assessors were blinded as to allocation; balance of probabilities based on quote is that assessment was unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Fifty patients were identified and randomized into 2 groups” Comment: no direct quotes on any withdrawals or whether outcome data was used for all 50 patients |
| Selective reporting (reporting bias) | High risk | Quote: “Patients were also reviewed for documentation of efficacy of treatment including day of burn wound closure, pain scores, type of cultured organisms, wound colonization and infection, surgical procedures and mortality between both groups” Comment: no information on day of wound burn closure |
| Other bias | Unclear risk | Comment: no direct quotes but no evidence of additional sources of bias, but reporting insufficient to be certain |
Muangman 2010.
| Methods | Country where data collected: Thailand Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: partial‐thickness burn (superficial second‐degree) within 24 h of enrolment and < 15% of TBSA Exclusion criteria: concomitant trauma, chemical and electrical burns, and serious comorbidity were excluded Participants: 70 people attending outpatient burns unit Mean age (years): 34.9 vs 42.3 years Male participants: 5 (42.9%) vs 17 (48.6%) Burn type: flame 8 vs 7/scalded 27 vs 28 Burn degree: 2nd‐degree Burn size (%TBSA): NR Burn location: NR |
|
| Interventions | Intervention arm 1: hydrofibre dressing coated with ionic silver (Aquacel Ag) with 1 cm overlap, covered with a layer of plain gauze, changed every 3 days. N = 35 Intervention arm 2: SSD and gauze dressing, changed daily. N = 35 Cointerventions: wound cleansing with saline, blisters removed |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: pain Secondary outcome: resource use |
|
| Notes | Funding: Faculty of Medicine Siriraj Hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomized by computer and assigned into two groups according to the burn wound treatment” Comment: computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomized by computer and assigned into two groups according to the burn wound treatment” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote “Dressings were evaluated …..on postburn day 1 and then every 3 days until the wound healed. At each evaluation after the dressing was removed, the burn wound was inspected for wound healing and change in depth and infection……Burn wounds were also observed daily by the experienced burn surgeon. After each burn dressing change in both groups, the performance characteristic photograph and questionnaire were recorded." Comment: no information on whether outcome assessors were blinded as to allocation; balance of probabilities based on quote is that assessment was unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Seventy patients were enrolled in the study and randomly assigned into two groups” Comment: no direct quotes on any withdrawals or whether outcome data was used for all 70 participants |
| Selective reporting (reporting bias) | Low risk | Quote: “The primary endpoint of this study was time‐to‐wound healing, defined as spelling [sic] of the wound. Secondary endpoints included pain assessment by patients’ pain scores during wound dressing…... Total dressing cost was divided into hospital charges including hospital fee, dressing cost and pain medication and transportation cost …for each hospital visit.” Comment: all stated outcomes of interest were reported |
| Other bias | Low risk | Comment: no direct quotes but no evidence of additional sources of bias with reasonable level of reporting |
Nasiri 2016.
| Methods | Country where data collected: Iran Intra‐individual RCT Unit of randomisation: burn Unit of analysis: burn Duration: 30 days |
|
| Participants | Inclusion criteria: aged 16–65 years, diagnosed by the same expert emergency burn physician based on the presentation of two same sites of second‐degree burns. The burn should have occurred within 24 h before the beginning of treatment, second‐degree burn on 2 sides of the same person's body, and with < 15% TBSA Exclusion criteria: People with epilepsy, diabetes, immunodeficiency disease, electrical and chemical burns, known allergy and sensitivity to either AEO or SSD, or pregnant women were excluded from the study Participants: 49 randomised; 45 analysed Mean age (years): 39.9 (SD 15.6) Male participants: NR but "most participants were women" Burn type: scalds 30; flame 14; contact 1 (analysed participants only) Burn degree: second‐degree Burn size (%TBSA): 3.7 (SD 2.4; range 1‐13) (analysed participants only) Burn location: 44% involved lower limbs (analysed participants only) |
|
| Interventions | Intervention arm 1: Arnebia euchroma ointment (AEO) Intervention arms 2: SSD Cointerventions: after admission and primary preparation, the wounds were washed with normal saline or sterile water and dried with sterile gases |
|
| Outcomes | Primary outcome: proportion of wounds healed at day 13 and mean time to wound healing (re‐epithelialisation) Primary outcome: signs of clinical infection rated on 6‐point scale from 0 = absent to 5 = all components present Secondary outcome: adverse events defined as erythema, edema, infection, inflammation, and general wound appearance Secondary outcome: pain and itching during first 15 minutes of dressing change measured using a 10‐point VAS |
|
| Notes | Funding: grant (118‐92) from Mazandaran University of Medical Sciences, Sari, Iran This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "These areas were randomly assigned to AEO treatment and the opposite site was treated with conventional treatment with SSD cream. A simple coin‐based randomization was performed for each patient after enrolment by the blinded staff nurse." Comment: the randomisation sequence was generated by an acceptable method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "These areas were randomly assigned to AEO treatment and the opposite site was treated with conventional treatment with SSD cream. A simple coin‐based randomization was performed for each patient after enrolment by the blinded staff nurse." Comment: not clear whether the allocation sequence was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The general condition of the wound areas were first observed and evaluated by the expert emergency burn physician and the Burn unit special nurse prior to utilization of topical agents. Thereafter, before each dressing, the wounds were assessed by same team who were unaware of the assigned treatment to each side and the ointment applied on the wounds for treatment." Comment: appears that outcome assessment was performed by individuals blinded to treatment allocation and separate from those applying dressings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "a total of 51 eligible patients were registered. Forty‐nine of them signed the consent form and were randomly allocated sequentially to the two sides and two treatment groups. Four patients were lost to follow up. Therefore, 45 patient's results were eligible for data analysis..... In addition, 1 patient in both groups needed bilateral skin graft on the day of 11th according to the plastic surgeon's decision. Furthermore, 2 patients in the SSD group needed skin graft from days 11–14, but their treatment area on the opposite area with AEO healed after 5 and 7 days, respectively" Comment: of the 49 randomised participants 4 were not included in the analysis; each participant was lost from both groups equally; all other participants' data were included in the analysis for each group |
| Selective reporting (reporting bias) | Unclear risk | Comment: the outcomes to be assessed were not defined in the methods section so it is not clear whether all planned outcomes were fully reported |
| Other bias | Unclear risk | Comment: there is no evidence of additional sources of bias; it is not clear whether the paired data were accounted for in the analysis |
Neal 1981.
| Methods | Country where data collected: UK Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: people with blistered burns Exclusion criteria: burns on face, hands or feet or injury > 12 h before attendance Participants: 51 people attending the ED Mean age (years): children 3.4 ± 3 vs 2.4 ± 3; adults 39 ± 20 vs 40 ± 18 Male participants: 10 vs 12 Burn type: scald 23 vs 22; other 2 vs 4 Burn degree: NR Burn size (%TBSA): 1.83 ± 1.5 vs 1.58 ± 1 Burn location: NR |
|
| Interventions | Intervention arm 1: paraffin gauze impregnated with chlorhexidine (Bactigras), covered by an absorbent dressing. N = 25 Intervention arm 2: plastic film (Opsite). N = 26 Cointerventions: removal of large blisters prior to treatment |
|
| Outcomes | Primary outcome: wound healing Primary outcome: infection Secondary outcome: pain |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A consecutive series of patients with blistered burns who attended the A/E Department were randomly selected to receive either a standard dressing or a plastic film." Comment: no information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A consecutive series of patients with blistered burns who attended the A/E Department were randomly selected to receive either a standard dressing or a plastic film." Comment: no information on whether the allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The endpoint taken was when the wounds were dry and epithelialised, needing only a dry protective dressing. Bias was minimised by having a specific endpoint and using the confirmatory judgement of assessors not directly involved in trial." Comment: it appears that the assessors were blinded to treatment allocation for the outcome of healing. However it is unclear whether the assessments of pain (by participants) and infection (by healthcare professionals) were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Fig. 1 shows that most of the patients' wounds had healed within sixteen or seventeen days" Comment: Figure 1 and the table which accompanies it show cumulative healing for all 51 randomised participants. |
| Selective reporting (reporting bias) | Low risk | Quote: ".....the following parameters were studied: the rate of healing, the rate of infection, and the degree of pain and social inconvenience." Comment: data were reported on all the prespecified parameters although it was not clear that planned methods for data management were followed |
| Other bias | Unclear risk | Comment: no specific quote but no evidence of other sources of bias, but reporting insufficient to be certain |
Ning 2008.
| Methods | Country where data collected: China Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: 28 days |
|
| Participants | Inclusion criteria: deep partial second‐degree burn wounds < 60% TBSA; age 18‐65; presented within 24 h of injury Exclusion criteria: complications; other disease; pregnancy; multiple trauma or serious comorbidity Participants: 20 participants with 2 comparable burns Mean age (years): NR Male participants: 12/20 Burn type: NR Burn degree: 2nd degree Burn size (%TBSA): 24.1 ± 0.2 Burn location: NR |
|
| Interventions | Intervention arm 1: sodium hypochlorite (Dermacyn) changed every other day. N = 20 burns Intervention arm 2: SSD changed every other day. N = 20 burns Cointerventions: NR |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: adverse events |
|
| Notes | Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author Funding NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it was not stated how the randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | The whole process of conducting this RCT was not clear, including whether the paired data were accounted for in the analysis |
Oen 2012.
| Methods | Country where data collected: Netherlands Parallel‐group RCT (multicentre) Unit of randomisation: participant Unit of analysis: participant Duration: 21 days; follow‐up to 12 months |
|
| Participants | Inclusion criteria: adults (aged > 18 years) with acute facial burns (thermal or electrical injuries involving face including scalp, ears and jaw line); neck included only if facial burn extended into it Exclusion criteria: facial burns < 0.25% TBSA; hospitalised for < 72 h; started with topical treatment before admission; unable to consent Participants: 154 (179 originally randomised) participants from 3 dedicated burns centres Mean age (years): 41.9 ± 16.9 vs 41.3 ± 14.5 Male participants: 64 vs 61 Burn type: scald 4 vs 3; flame 70 vs 60; contact 1 vs 2; electrical 2 vs 4; other 1 vs 7 Burn degree: NR Burn size (%TBSA): median 9.8 (IQR 5.0‐19.4) vs 9.3 (4.5‐17.0); facial 3.0 (2.0‐4.5) vs 3.0 (2.0‐4.5) Burn location: facial |
|
| Interventions | Intervention arm 1: SSD 10 mg/g plus cerium nitrate 22 mg/g (Flammacerium) at admission and once daily for 48‐72 h. Wounds were then washed daily with chlorhexidine, rinsed with water and left open. N = 78 Intervention arm 2: SSD 10 mg/g (Flammazine) once daily, covered with plain gauze dressing and a fixation dressing until healed. N = 76 Cointerventions: treatment protocols in clinical practice in Dutch burn centres. Washing with chlorhexidine gluconate (Hibiscrub and rinsing with water) |
|
| Outcomes | Primary outcome: wound healing secondary outcome: pain secondary outcome: mortality |
|
| Notes | Funding: Dutch Burns Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To this end, an allocation sequence was developed according to center using mixed randomization (M.N.). Prespecified inequality ranged from two to four, and block sizes varied from four to 11. Randomization sequences were generated with a random numbers table." Comment: an appropriate method was used to generate the randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was concealed from the physician enrolling patients, and subversion was prevented by using nontransparent envelopes." Comment: Does not specifically state sealed envelopes but appears to be appropriate allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "It was not possible to guarantee blinding of the observers to treatment allocation because of the presence and/or involvement in clinical care of most observers. The data analysts (I.O. and M.B.) were blinded." Comment: stated that assessors could not be guaranteed to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No specific quote but all randomised participants were accounted for in comprehensive flow diagram. There were 25 post‐randomisation exclusions for clearly documented reasons mostly related to protocol violations. These were balanced between the groups. 4 deaths occurred (3 vs 1) but these participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Quote: "Primary outcomes were number of patients requiring surgical intervention and time to complete wound healing......Secondary outcomes consisted of wound colonization, pain, and aesthetic and functional aspects." Comment: all specified outcomes were fully reported |
| Other bias | Low risk | Comment: no specific quote but no other sources of bias identified and good level of reporting |
Opasanon 2010.
| Methods | Country where data collected: Thailand Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: partial‐thickness burn, less than 24 h post‐burn injury, TBSA < 15% Exclusion criteria: pregnancy, immunocompromised patients and hypersensitivity to treatments used Participants: 65 Mean age (years): 42.31 ± 23.49 vs 31.03 ± 19.76 Male participants: 15 vs 21 Burn type: flame 8 (23%) vs 18 (60%)/dcald 27 (77%) vs 10 (33%)/other (chemical, contact burn 0 (0%) vs 2 (7%) Burn degree: NR Burn size (%TBSA): 2.77 ± 0.41 vs 7.93 ± 1.8 Burn location: upper limb 31% vs 53%/lower limb 46% vs 33%/hand 11% vs 3%/other 12% vs 11% |
|
| Interventions | Intervention arm 1: 1% SSD (1% AgSD) covered with dry gauze dressing changed every day until complete wound closure. N = 35 Intervention arm 2: Alginate silver dressing (Askina Calgitrol Ag) changed every 5 days until complete wound closure. N = 30 Cointerventions: none reported |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: pain |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Sixty‐five patients were identified and randomised into two groups” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Sixty‐five patients were identified and randomised into two groups” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “clinical assessment was evaluated by two experienced burn surgeons” Comment: no information on whether outcome assessors were blinded as to allocation of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Sixty‐five patients were identified and randomised into two groups” Comment: no withdrawals reported and Table 10 suggested that all participants were accounted for |
| Selective reporting (reporting bias) | Low risk | Quote: “pain scores, number of wound dressing change, nursing time and time of burn wound healing” Comment: all stated outcomes of interest were reported |
| Other bias | Low risk | Comment: no direct quotes but no evidence of additional sources of bias but reporting insufficient to be certain |
Panahi 2012.
| Methods | Country where data collected: Iran Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 14 days |
|
| Participants | Inclusion criteria: thermal second‐degree burns < 5% TBSA, which occurred in preceding 48 h with no other injuries Exclusion criteria: renal, hepatic, endocrine, cardiovascular or cerebrovascular disease, pregnancy, drug/alcohol abuse and concurrent use of antibiotics, steroids or immunosuppressive drugs Participants: 120 people with burns (setting NR) Mean age (years): 33.6 ± 13.4 vs 37.4 ± 12.7 Male participants: 21 (37.5) vs 25 (45.5) Burn type: hot water, steam 24 (42.9) vs 23 (41.8)/fire 22 (39.3) vs 18 (32.7)/hot liquid 5 (8.9) vs 10 (18.2)/hot object 2 (3.6) vs 3 (5.5)/chemical substance 3 (5.4) vs 1 (1.8) Burn degree: second Burn size (%TBSA): 2.48 ± 1.45 vs 2.38 ± 1.42 Burn location: NR |
|
| Interventions | Intervention arm 1: herbal cream (A vera gel, Lavandula stoechas essential oil, Pelargonium roseum essential oil), 5 g for each 10 cm² of burn area applied once daily. Sterile gauze used to cover wound and then bandaged. N = 60 Intervention arm 2: SSD 1% cream. Following cleansing and debridement with antimicrobial solution, 5 g for each 10 cm² of burn area applied once daily. Sterile gauze used to cover wound and then bandaged. N = 60 Cointerventions: cleansing and debridement with antimicrobial solution before randomised treatment period; analgesia |
|
| Outcomes | Primary outcome: infection Secondary outcome: pain |
|
| Notes | Funding: Baqiyatallah University of Medical Sciences, Iran. Herbal creams were provided by Barij Essence Pharmaceutical Co; 3 authors are described as members of this company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Participants were randomized in a double‐blind manner” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Participants were randomized in a double‐blind manner” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Patients were evaluated for the severity of pain, frequency of skin dryness and infection” Comment: no information on whether outcome assessors were blinded as to allocation; balance of probabilities based on quote is that assessment was unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “From the initial 120 patients…9 were excluded due to study protocol violation…Data from 111 completers (n=56 in the herbal cream and 55 in the SSD group) were included in the final analysis” Comment: reasons for withdrawals were reported ‐ study protocol violation; numbers excluded were not high |
| Selective reporting (reporting bias) | Low risk | Quote: “Patients were evaluated for the severity of pain, frequency of skin dryness and infection” Comment: all stated outcomes of interest were reported |
| Other bias | Low risk | Comment: no direct quotes but no evidence of additional sources of bias but reporting insufficient to be certain |
Phipps 1988.
| Methods | Country where data collected: UK Parallel group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: burns less than 5% TBSA (averaging under 1%) suitable for outpatient treatment Exclusion criteria: those needing inpatient treatment, facial burns, hand burns managed in bags and those whose treatment was to be continued elsewhere Participants: 196 outpatients Mean age (years): < 5 years: 21 vs 24; 5‐14 years: 7 vs 9; > 14 years: 64 vs 71 Male participants: 49 vs 64 Burn type: NR Burn degree: NR Burn size (%TBSA): < 1% Burn location: NR |
|
| Interventions | Intervention arm 1: hydrocolloid material covered with cotton gauze overlaid with cotton wool and secured with crepe bandage or adhesive tape. Dressing inspected on 3rd or 4th day and then changed weekly unless dressing contaminated or adverse symptoms developed Intervention arm 2: chlorhexidine‐impregnated tulle‐gras dressing covered with cotton gauze overlaid with cotton wool and secured with crepe bandage or adhesive tape. Dressing inspected on 3rd or 4th day and then changed weekly unless dressing contaminated or adverse symptoms developed Cointerventions: NR |
|
| Outcomes | Primary outcome: wound healing Primary outcome: infection |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were allocated randomly to one of two treatment groups” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “patients were allocated randomly to one of two treatment groups” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote "at each inspection of the wound, its progress towards healing was noted" Comment: no indication that outcome assessment was blinded but unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “119 of the 196 patients were followed to complete healing” Comment: details were given on why the excluded participants' data were not included |
| Selective reporting (reporting bias) | Low risk | Comment: no direct quotes but all stated outcomes of interest were reported |
| Other bias | Unclear risk | Comment: no direct quotes but no evidence of additional sources of bias but reporting insufficient to be certain |
Piatkowski 2011.
| Methods | Country where data collected: Netherlands Parallel‐group RCT Unit of randomisation: burns Unit of analysis: burns Duration: NR |
|
| Participants | Inclusion criteria: second‐degree burns up to 10% TBSA Exclusion criteria: Aged > 18 years; dermatological diseases and/or pre‐existent poly‐neuropathy Participants: 60 outpatients with 72 burns Mean age (years): 46.5 ± 15.6 vs 34 ± 14.2 Male participants: 19 vs 20 Burn type: scald 19 vs 19; contact 8 vs 2; flame 5 vs 7 Burn degree: all second‐degree Burn size (%TBSA): NR (cm² 151.2 ± 109.6 vs 134.7 ± 99) Burn location: hands 2 vs 6; arms 11 vs 13; thorax 2 vs 2; abdomen 4 vs 2; thighs 18 vs 8; feet 1 vs 3 |
|
| Interventions | Intervention arm 1: SSD cream (Flammazine) changed daily. N = 30 Intervention arm 2: polyhexanide‐containing bio‐cellulose dressing (Suprasorb X+PHMB) changed every 2nd or 3rd day. N = 30 Cointerventions: |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: pain Secondary outcome: costs |
|
| Notes | Funding NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Suitable patients were assigned to one of the treatment groups, using computer generated randomization." Comment: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A prospective, randomized, controlled single center study was designed to evaluate clinical efficacy of a polyhexanide containing bio‐cellulose dressing (group B) compared to a silver‐sulfadiazine cream (group A) in sixty partial‐thickness burn patients." Comment: no information on whether the allocations to treatment were adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Wound healing was documented using standardized digital photographs, which were assessed by two experienced wound specialists, that were blinded for the treatment." Comment: blinded outcome assessment documented although pain assessment probably not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no specific quote but all participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but although all planned outcomes were reported in some cases the data were only presented graphically |
| Other bias | Unclear risk | Comment: there is potential for unit of analysis issues as they analyse 72 wounds on 60 participants and 2 of the participants had more than one treatment. The data were not useful to our analysis. No other sources of bias were identified and methods were well reported |
Piccolo‐Daher 1990.
| Methods | Country where data collected: Brazil Parallel‐group RCT Unit of randomisation: participant Unit of analysis: burn Duration: NR |
|
| Participants | Inclusion criteria: second‐degree burns 1%‐20% TBSA Exclusion criteria: NR Participants: 125 Mean age (years): NR Male participants: NR Burn type: NR Burn degree: second‐degree Burn size (%TBSA): mean 4% Burn location: NR |
|
| Interventions | Intervention group 1: merbromin 2% N = 25 Intervention group 2: sodium salicylate 2% N = 25 Intervention group: zinc sulfadiazine 2% N = 25 Intervention group 4: sodium salicylate 2% + zinc sulfadiazine 2% N = 25 Intervention group 5: collagenase 0.6 μg/g + chloramphenicol 1% N = 25 Cointerventions: surgical debridement under general anaesthesia; occlusive dressings after topical application |
|
| Outcomes | Primary outcome: wound healing | |
| Notes | Funding NR. Study reported in Portuguese; data extraction and risk of bias provided by two translators. Although the unit of analysis is stated to be "burns" it appears that there was only one burn per participant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Time to wound healing was analysed by an observer who was blinded to the participant's treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | all proposed outcomes were reported |
| Other bias | Unclear risk | Unclear whether the groups had similar baseline characteristics |
Radu 2011.
| Methods | Country where data collected: Germany Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: 24 h |
|
| Participants | Inclusion criteria: aged 18‐80 years with 2nd‐degree partial‐thickness burn > 3% TBSA and at least two 10 cm² symmetrical or similar areas for comparison. Abbreviated Burn severity Index score no higher than 10 Exclusion criteria: NR Participants: 30 people with burns presenting at burn department of trauma centre Mean age (years): median 42 Male participants: 22/30 Burn type: scald 12, contact 7, flame 11 Burn degree: 2nd Burn size (%TBSA): median 18 (range 6‐36) Burn location: trunk 9, thigh 11, lower leg 5, arm 5 |
|
| Interventions | Intervention arm 1: SSD (Flammazine); gauze Intervention arm 2: octenidine gel, gauze Cointerventions: initial disinfection with Octinisept and removal of blisters; preparation for treatment with synthetic skin substitute |
|
| Outcomes | Secondary outcome: pain | |
| Notes | Funding NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective, randomized, non‐blinded, clinical study was conducted" Comment: no information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The prospective, randomized, clinical study was performed.... . Patients needed to have symmetrical or similar burned areas close to each other for comparability. Burns were randomly selected, one area was treated with Flammazine1/gauze, another area in the same patient was treated with Octenidine‐Gel1/ gauze as initial antiseptic treatment." Comment: no information on whether the treatment allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "the patient was instructed to mark his/her pain on a visual analogue scale". Comment: it was not clear if the participant was blinded. So unclear whether assessment was |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All enrolled participants completed the study." Comment: all randomised participants/burns included in the analysis |
| Selective reporting (reporting bias) | Low risk | Quote: "In this study we compared the feasibility and practicability, with focusing on pain scores, time of wound bed preparation and quality of the wound site" Comment: individual patient data were reported for the planned outcomes; a paired analysis is required to analyse these |
| Other bias | Unclear risk | Comment: it was unclear whether the analysis took into account the intra‐individual study design |
Sami 2011.
| Methods | Country where data collected: Pakistan Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 60 days |
|
| Participants | Inclusion criteria: partial‐thickness burns involving between 5% and 40% TBSA Exclusion criteria: NR Participants: 50 adults and children with partial‐thickness burns Mean age (years): range 18 months‐50 years) Male participants: 21/50 Burn type: NR Burn degree: second‐degree (partial‐thickness) Burn size (%TBSA): surface area Burn location: NR |
|
| Interventions | Intervention arm 1: pure unprocessed, undiluted honey applied once daily, covered with cotton sterilized gauze Intervention arms 2: layer of 1% SSD cream applied once daily Cointerventions: general management including initial debridement and wound excision were the same in both groups The wounds were cleansed with normal saline and thorough debridement done |
|
| Outcomes | Primary outcome: wound healing (epithelialisation) Primary outcome: infection (culture positive) Secondary outcome: pain (VAS 1‐10) and time to pain‐free status Secondary outcome: cost per dressing per %TBSA |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The cases were divided into two groups randomly by consecutive sampling method, in equal numbers." Comment: no information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The cases were divided into two groups randomly by consecutive sampling method, in equal numbers." Comment: no information on whether the allocation sequence was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "At the time of change of dressing, details regarding the condition of the wound such as signs of infection, condition of the surrounding tissue, discharge, smell, presence of necrotic tissue, and degree of epithilialisation were noted." Comment: unclear if this assessment was performed by personnel/assessors blinded to the allocation: since the interventions clearly differ then it may be unlikely that assessment could be blinded if it was performed by those changing the dressings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No direct quote but all participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | The primary and secondary outcomes were not defined in the methods section so it is difficult to assess if all planned outcomes were reported. |
| Other bias | Unclear risk | No evidence of other sources of bias but reporting insufficient to be certain |
Shahzad 2013.
| Methods | Country where data collected: Pakistan Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: treatment duration until healing (longest 60 days); 2 months' follow‐up |
|
| Participants | Inclusion criteria: 2nd‐degree burns presenting within 24 h of injury and TBSA < 25% Exclusion criteria: corrosive, electrical or chemical burns; history of diabetes, hypertension, epilepsy or kidney disease; pregnancy Participants: 50 people attending the ED and admitted to burns unit Mean age (years): 30.2 (15‐65); no significant difference between groups Male participants: 17 vs 9 Burn type: flame 16 vs 11; scald 9 vs 14 Burn degree: Burn size (%TBSA): 13.6 ± 4.7 (6‐25); no significant difference between groups Burn location: NR |
|
| Interventions | Intervention arm 1: A vera gel twice daily. N = 25 Intervention arm 2: 1% SSD twice daily. N = 25 Cointerventions: 3rd generation cephalosporins; fluid resuscitation, shock prevention/treatment; wound cleansing with Pyodine scrub and normal saline |
|
| Outcomes | Primary outcome: wound healing Primary outcome: infection Secondary outcome: pain |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Fifty patients with second degree burns were randomized (consecutive sampling method) into 2 groups." Comment: no information on how randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Fifty patients with second degree burns were randomized (consecutive sampling method) into 2 groups." Comment: no information on whether allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "At the time of change of dressing details regarding the condition of the wound such as signs of wound infection, condition of surrounding unburned tissues, discharge, smell, necrotic tissue and state of epithelialisation was noted by on every 3rd day. ...... The patients and attendants were given information regarding the Aloe Vera gel and SSD cream. Tape method was used to measure length and width of the wound and then these measurements were multiplied i.e. Area (in centimetre square) = length x width." Comment: outcome assessment done at time of dressing change making it unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Among 25 patients treated with Aloe dressing, 24 patients had complete recovery while 1 had incomplete. In the SSD group, out of 25 patients, 19 patients had complete recovery and 6 had hypertrophic scar formation or the development of contractures" Comment: all randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Patients were also reviewed for documentation of efficacy of treatment including time required for healing (epithelialization), pain scores, type of cultured organisms, wound colonization and infection, cost of treatment and mortality between both groups." Comment: all the planned outcomes were reported adequately |
| Other bias | Unclear risk | Comment: No evidence of other sources of bias, but reporting insufficient to be certain |
Silverstein 2011.
| Methods | Country where data collected: USA Parallel‐group RCT (multicentre) Unit of randomisation: participant Unit of analysis: participant Duration: 21 days + |
|
| Participants | Inclusion criteria: aged at least 5 years; had a thermal burn within 36 h of enrolment; 2.5%‐20% of TBSA (burns covering between 3% and 25% of TBSA, allowing for up to 10% of TBSA to be third‐degree burn); only second‐degree burn area treated as per study protocol Exclusion criteria: chemical or electrical burn; clinically‐infected burn; treatment of the burn with an active agent before study entry, and pregnancy; necrotising leukocytic vasculitis or pyoderma gangrenosa, diagnosed illness (e.g. HIV/AIDS, cancer, severe anaemia); corticosteroid use; other immunosuppressants/chemotherapy in past 30 days; known allergy/hypersensitivity to components; physical/mental condition meaning not expected to comply Participants: 101 participants at 10 centres Mean age (years) (SE): 37.0 (18.1) vs 39.2 (18.2) Male participants: 36/41 Burn type: scald n=17 vs 9, flash 17 vs 16, flame 13 vs 19, contact 2 vs 4; other 0 vs 3 Burn degree: second‐degree Burn size (%TBSA): mean partial‐thickness burn size values used within the analysis, 5.64% vs 4.93%, Burn location: NR |
|
| Interventions | Intervention arm 1: silver soft silicone foam (Mepilex Ag). Dressing changes every 5‐7 days (3–5 days during the acute phase) depending on the status of the burn. Additional light bandage as needed to ensure fixation Intervention arm 2: SSD cream applied once or twice daily to a thickness of approximately 2 mm, then covered with a gauze pad and gauze wrap or other fixation Cointerventions: wound cleansing; sharp debridement at baseline |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: adverse events Secondary outcome: pain Secondary outcome: costs |
|
| Notes | Funding: Molnlycke Health Care educational grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Enrolled subjects were assigned randomly to a treatment regimen that included either SSD or MAg. This was achieved through the use of sealed envelopes that were opened at the time of randomization. The randomization schedules were designed to ensure that equal numbers of patients were assigned to each treatment group at all participating centers.” Comment: no information on how randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "This was achieved through the use of sealed envelopes that were opened at the time of randomization." Comment: although use of sealed envelopes was reported there is insufficient information to determine if the allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The study treatment was not blinded. .... Observation of dressings in both groups continued until 21 days postburn or until full reepithelialization occurred, alternative therapy for infection was initiated, or significant change in burn depth required surgical intervention. Sharp debridement was carried out at baseline visit only. Outcomes were measured at every scheduled visit: ie, days 0 (at inclusion in study), 7, 14, 21, and 35 (1 day) until study discontinuation." Comment: the outcome assessment did not appear to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no specific quote but all except 2 randomised participants were included in analyses with the exception of cost assessment where analysis of fewer participants was prespecified |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but outcomes were specified in detail and all were reported adequately |
| Other bias | Low risk | Comment: no evidence of other sources of bias and well reported |
Subrahmanyam 1991.
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: superficial thermal burns < 40% TBSA Exclusion criteria: NR Participants: 104 participants attending burns unit Mean age (years): 28.5 (3.2) vs 26.7 (4.1) (information provided by author to Jull et al (Jull 2015). (range 1‐65 years) Male participants: 82/104 (42 vs 40) Burn type: thermal Burn degree: NR (superficial) Burn size (%TBSA): mean NR. most participants had 21%‐30% or 30%‐40%; mean 26.5 vs 27.2 Burn location: NR |
|
| Interventions | Intervention arm 1: 15 mL‐30 mL honey applied directly to wound, covered with gauze and bandaged, changed daily. N = 52 Intervention arm 2: SSD soaked gauze that was changed daily. N = 52 Cointerventions: washed with normal saline |
|
| Outcomes | Primary outcome: wound healing Secondary outcomes: pain and selected AE reported qualitatively |
|
| Notes | Funding NR. Information on allocation method, allocation concealment, blinding, mean TBSA, mean time to healing and standard deviation for mean time to healing were provided by the author to Jull et al. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “the cases were allotted at random to two groups” Comment: no further information to indicate how randomisation sequence was generated. Study author information that the sequence was generated by the "chit method", which is a method of drawing lots however the detail provided by the study authors was minimal and not sufficient to reassure us that the sequence was truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: “the cases were allotted at random to two groups” Comment: study author provided information to Jull et al that allocation concealment was by means of sequentially‐numbered, sealed envelopes but envelopes may not have been opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "In both groups culture and sensitivity determinations were performed on swabs taken from the surface at the time of admission. This was repeated on days 7 and 21 in all cases or untIl the wound healed. The time required for complete healing was noted in both groups." Comment: information provided by the author to Jull et al stated that outcomes assessors were blinded but data analysts were not. So still unclear. Additionally honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no specific quote but all randomised participants were included in the analysis (shown in tables) |
| Selective reporting (reporting bias) | Unclear risk | No specific quote but although the stated outcomes were all reported some were reported only qualitatively |
| Other bias | Unclear risk | Comment: no specific quote but no evidence of other sources of bias, but reporting insufficient to be certain |
Subrahmanyam 1993b.
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: partial‐thickness burns < 40% TBSA Exclusion criteria: Participants: 92 people attending a general hospital Mean age (years): 42.8 (3‐65) Male participants: 44 Burn type: NR Burn degree: NR (partial‐thickness) Burn size (%TBSA): 22.7 (15‐35) groups 22.8 vs 22.6 Burn location: NR |
|
| Interventions | Intervention arm 1: honey‐impregnated gauze prepared by dipping sterile gauze in unprocessed and undiluted honey, covered with pad and bandage, changed on alternate days unless signs of infection Intervention arm 2: bio‐occlusive, moisture‐permeable polyurethane dressing (OpSite) kept in place until day 8 if no sign of infection, leakage etc Cointerventions: washed with normal saline |
|
| Outcomes | Primary outcome: wound healing Primary outcome: infection |
|
| Notes | Funding NR; information on allocation method, allocation concealment, blinding, mean TBSA, mean time to healing and standard deviation for mean time to healing provided by author to Jull et al (Jull 2015) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After initial management, patients were allotted at random to two groups." Comment: no further information on methods of sequence generation; study author information that the sequence was generated by the "chit method", which is a method of drawing lots however the detail provided by the authors was minimal and not sufficient to reassure us that the sequence was truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After initial management, patients were allotted at random to two groups." Comment: study author provided information to Jull et al that allocation concealment was by means of sequentially‐numbered, sealed envelopes but not known whether these were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "In both groups bacterial culture and sensitivity determinations were performed from swabs taken from the surface of the wound.... until the wound healed. The time required for complete healing was noted in both groups." Study author provided a statement to Jull et al that outcome assessors were blinded Comment: despite author information that assessors were blinded, honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult; therefore judgement unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no specific quote but the outcomes cited were subsequently reported |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but all randomised participants were included in the analysis (shown in tables) |
| Other bias | Unclear risk | Comment: no specific quote but no evidence of other sources of bias but reporting insufficient to be certain |
Subrahmanyam 1994.
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: partial‐thickness burns less than 40% TBSA within 6 h of burn Exclusion criteria: NR Participants: 64 Mean age (years): 25 vs 24.6 (3‐62; 60 aged 21‐30) Male participants: 28 vs 15 Burn type: scald n = 25 vs 18, flame 12 vs 4, contact burn 3 vs 2 Burn degree: NR (partial‐thickness) Burn size (%TBSA): 18.5% vs 19.4% Burn location: NR |
|
| Interventions | Intervention arm 1: dry gauze dipped into unprocessed honey and applied to wound, covered with an absorbent dressing that was changed alternate days. Changed more often if signs of infection. N = 40 Intervention arm 2: amniotic membrane ‐ no other details of dressing given, after day 8 dressing was changed on alternate days, changed more often if signs of infection. N = 24 Cointerventions: washed with normal saline |
|
| Outcomes | Primary outcome: wound healing | |
| Notes | Funding NR Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by study author to Jull et al (Jull 2015) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After initial treatment, patients were allotted to the two groups at random." Comment: no further information on methods of sequence generation in study report but study author provided information that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After initial treatment, patients were allotted to the two groups at random." Comment: no further information on whether allocation was adequately concealed in study report but author provided information that allocation concealment was by means of sequentially‐numbered, sealed envelopes, although it is not clear whether the envelopes were opaque. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The following observations were recorded in all patients: leakage of exudate from the dressing, skin reactions, infection and time for wound healing. Pain was assessed during the change of dressing in both groups, by two separate observers." Comment: no indication as to whether the assessments were conducted by observers blinded to treatment allocation, author provided information to Jull et al (Jull 2015) that outcome assessors were blinded but honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult; therefore judgement unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no specific quote but all randomised participants included in analysis (based on table) |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but stated outcomes were all reported |
| Other bias | Unclear risk | Quote: "The honey‐impregnated gauze was prepared by dipping sterile gauze in unprocessed and undiluted honey. The gauze was applied to the wound and then covered with an absorbent dressing. These wounds were inspected every 2 days until healed. In contrast the patients treated with amniotic membrane had a first wound inspection on day 8, when the dressing was changed and then every second day until healed." Comment: unclear if differing observation times influenced outcomes |
Subrahmanyam 1996a.
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 21 days |
|
| Participants | Inclusion criteria: partial‐thickness burns < 40% TBSA, presenting within 6 h of injury Exclusion criteria: NR Participants: 100 Mean age (years): 28.2 vs 27.5 (range age 5‐59 years) Male participants: 29 vs 28 Burn type: scald n = 17 vs 15, flame 23 vs 22, contact 7 vs 12, explosives 2 vs 1, chemical 1 vs 0 Burn degree: NR (partial‐thickness) Burn size (%TBSA): 16.5 vs 17.2% (range 10‐40) Burn location: NR |
|
| Interventions | Intervention arm 1: 15 mL to 30 mL undiluted and unprocessed honey, dry gauze applied on top and covered with bandage, inspected on alternate days. N = 50 Intervention arm 2: autoclaved potato‐peel dressing, dry gauze and bandage applied, changed alternate days or earlier if signs of infection, or excessive exudate or leakage. N = 50 Cointerventions: washed with normal saline |
|
| Outcomes | Primary outcome: wound healing Primary outcome: infection |
|
| Notes | Funding NR Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by study author to Jull et al (Jull 2015) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the initial management, patients were allotted at random to two groups." Comment: no indication how the randomisation sequence was generated. Study author provided information to Jull et al (Jull 2015) that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After the initial management, patients were allotted at random to two groups." Comment: no further information on whether allocation was adequately concealed in study report but study author provided information to Jull et al that allocation concealment was by means of sequentially‐numbered, sealed envelopes but not known whether these were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The wounds were inspected every 2 days until healed." Comment: no indication as to whether outcome was determined by a blinded observer in study report; study author provided information to Jull et al that outcome assessors were blinded but honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult; therefore judgement unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no specific quote but all randomised participants were included in analysis (tables) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but outcomes cited in methods were all reported |
| Other bias | Unclear risk | No specific quote but no evidence of other sources of bias but reporting insufficient to be certain |
Subrahmanyam 1996b.
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: TBSA burnt < 40% | |
| Interventions | Intervention arm 1: pure, unprocessed, undiluted, honey, covered with gauze, changed every 2nd day Intervention arm 2: soframycin (90 participants), Vaseline‐impregnated gauze (90 participants), OpSite (90 participants), sterile gauze (90 participants) or left exposed (90 participants). “Dressings were replaced on alternative days, except in the case of OpSite, which was continued until the wounds healed... sterile linen changed at frequent intervals.” Frequency of dressing change is not mentioned with respect to the sterile gauze group |
|
| Outcomes | Primary outcome: wound healing | |
| Notes | Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by author to Jull et al (Jull 2015) Funding NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After initial treatment, the cases were divided at random into a study group treated with honey dressing and a control group treated with conventional dressing” Comment: method of generating the random sequence not reported. Study author provided information that the sequence was generated by the “chit method”, which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but study author provided information that allocation concealment was by means of sequentially‐numbered, sealed envelopes, although it is not clear whether the envelopes were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated in study report, but study author responded to request for further information from Jull et al by stating the investigators and outcome assessors were blinded. How blinding was achieved was not described in the response and honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult; therefore judgement unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears that all randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine whether there is a risk of outcomes being selectively reported |
| Other bias | Unclear risk | Comment: no specific quote but there was no evidence of other bias but reporting insufficient to be certain |
Subrahmanyam 1998.
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 30 days |
|
| Participants | Inclusion criteria: superficial thermal burns less than 40% TBSA within 6 h of burn Exclusion criteria: NR Participants: 50 people attending burns unit Mean age (years): 25.2 vs 26.4 Male participants: 14 vs 13 Burn type: flame 23/22, scalds 2/3, TBSA 14.5%/15.6% Burn degree: NR Burn size (%TBSA): 14.5 vs 15.6 Burn location: NR |
|
| Interventions | Intervention arm 1: 16 mL‐30 mL unprocessed honey, dry gauze applied on top and covered with bandage; honey changed alternate days Intervention arm 2: SSD impregnated gauze, changed daily Cointerventions: washed with normal saline |
|
| Outcomes | Primary outcome: wound healing | |
| Notes | Funding NR Information about allocation method, allocation concealment, blinding, mean time to healing and standard deviation for mean time to healing provided by author to Jull et al (Jull 2015) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the initial management, patients were allocated at random to two groups." Comment: no indication how the randomisation sequence was generated but study author provided information to Jull et al that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After the initial management, patients were allocated at random to two groups." Comment: no indication in study report whether the allocation was adequately concealed. Study author provided information to Jull et al that allocation concealment was by means of sequentially‐numbered sealed envelopes, although it is not clear whether the envelopes were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The wounds were observed for evidence of infection, excessive exudate or leakage until the wounds healed. The times taken for healing of the wounds were recorded in both groups." Comment: no indication if observers were blinded in study report; author provided information to Jull et al that outcome assessors were blinded but honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult; therefore judgement unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no specific quote but all randomised participants were included in the analysis (tables) |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but the specified outcomes of interest were all reported |
| Other bias | Low risk | Comment: no specific quote but there was no evidence of other bias but reporting insufficient to be certain |
Subrahmanyam 2001.
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 21 days |
|
| Participants | Inclusion criteria: less than 40% TBSA burn, hospitalised within 6 h post‐burn Exclusion criteria: Participants: 100 people attending burns unit Mean age (years): 26.5 ± 1 vs 25.2 ± 2 Male participants: 52 Burn type: NR Burn degree: NR Burn size (%TBSA): 22.5 ± 3 vs 23.4 ± 1; full‐thickness 3.2 +/‐2 vs 4.7 +/‐1% Burn location: NR |
|
| Interventions | Intervention arm 1: 15 mL‐30 mL unprocessed honey, dry gauze applied on top and covered with bandage, changed every 2 days. N = 50 Intervention arm 2: SSD impregnated gauze changed every 2 days. N = 50 Cointerventions: washed with normal saline |
|
| Outcomes | Primary outcome: wound healing Primary outcome: infection (resolution) Secondary outcome: resource use (hospital stay) |
|
| Notes | Funding NR Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by author to Jull et al (Jull 2015) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were allotted at random to two groups," Comment: no indication how the randomisation sequence was generated but author provided information to Jull et al that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were allotted at random to two groups," Comment: no indication in study report whether the allocation was adequately concealed. Study author provided information to Jull et al that allocation concealment was by means of sequentially‐numbered sealed envelopes, although it is not clear whether the envelopes were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The wounds were observed for evidence of infection, excessive exudate, or leakage until they healed." Comment: no indication that observers were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Thus, in all the patients in this group, the wounds healed by day 21..... In the group treated with sulphur sulphadiazine, the wounds healed in 4 patients by day 7, in 22 patients by 14 day, and in 24 patients by day 21 (mean, 17.2 days)." Comment: it is clear that all participants randomised to the honey group were included in the analysis but not that all of those in the SSD group were, although no attrition is reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but it was not clear which outcomes the authors planned to assess and therefore whether they were all reported fully |
| Other bias | Low risk | Comment: no specific quote but no evidence of other bias |
Tang 2015.
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: unclear (burn?) Duration: 4 weeks |
|
| Participants | Inclusion criteria: deep partial‐thickness thermal burn injury covering 2.5%‐25% TBSA (third‐degree areas were not to exceed 10% TBSA). aged 5‐65 years; at least one isolated burn area not on head or face with deep partial 2nd‐degree burn from 1%‐10% TBSA Exclusion criteria: burns older than 36 h, clinically infected; treated with active agent before study entry (SSD allowed up to 24 h before randomisation); dermatologic disorders or necrotising processes; underlying diseases such as HIV/AIDS, cancer, severe anaemia, insulin‐dependent diabetes, systemic glucocorticoid use except occasional prednisolone < 10 mg/d; immunosuppressive agents, radiation or chemotherapy in previous 30 days; known allergy/sensitivity to the products; pregnancy; previous participation in this (or other study within 1 month) Participants: 158 randomised participants (total number of burns > 200) Mean age (years): 36.2 (range 5.2‐65.5; only 5 < 12 years). No difference between groups Male participants: 55 vs 57 Burn type: scald 30 vs 41; flash 8 vs 7; flame 32 vs 31; contact 1 vs 3 Burn degree & TBSA: 2nd‐degree superficial partial 4.48% vs 4.29%; deep partial‐thickness 6.28% vs 5.18%; third‐degree 0.345% vs 0.317%. enrolled study site: 2.72 vs 2.64 Burn location: arm 52 vs 53, buttock 6 vs 7, hand 41 vs 42, leg 29 vs 35, thigh 24 vs 31, trunk 26 vs 27, other 43 vs 46 |
|
| Interventions | Intervention arm 1: absorbent foam silver dressing (Mepilex Ag) changed every 5‐7 days; gauze wrap as secondary dressing. N = 73 Intervention arm 2: SSD 1% cream; gauze pad and wrap as secondary dressing. N = 85 Cointerventions: debrided and/or cleansed according to standard practice |
|
| Outcomes | Primary outcome: wound healing primary outcome: infection Secondary outcome: adverse event Secondary outcome: pain |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Enrolled subjects were assigned randomly using a block design, with block sizes varying between 2.4 and 6 (in Viedoc, Pharma Consulting Group, Uppsala, Sweden) to either SSD or Mepilex Ag. Subjects were consecutively allocated to the treatment at each center and given a subject code, depending on which strata they belonged to." Comment: randomisation sequence computer‐generated using blocking design |
| Allocation concealment (selection bias) | Low risk | Quote: "Enrolled subjects were assigned randomly using a block design, with block sizes varying between 2.4 and 6 (in Viedoc, Pharma Consulting Group, Uppsala, Sweden) to either SSD or Mepilex Ag. Subjects were consecutively allocated to the treatment at each center and given a subject code, depending on which strata they belonged to." Comment: allocation conducted remotely by consecutive allocation of codes within stratified design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In addition, the investigator was required to make a subjective assessment of healing at each weekly assessment before cleansing and/or debridement. Percentage of the burn healed since baseline was to be performed by a blinded observer." Comment: assessment of healing was conducted by an assessor blinded to the treatment allocation; it's not clear whether assessment of other outcomes was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: ".....158 patients were randomized, and 153 patients were subjected to at least one treatment and were included in the ITT population, 71 (46%) of them randomized to Mepilex Ag and 82 (54%) randomized to SSD. Thirteen patients (8%) discontinued before the study ended, 5 (7%) of them from the Mepilex Ag group and 8 (10%) from the SSD group. One patient withdrew consent from the SSD group, while the other 12 discontinued because of other reasons (Fig. 1)." Comment: all participants were accounted for and the proportion who discontinued was low and low relative to the event rate for healing |
| Selective reporting (reporting bias) | Low risk | Quote: "The primary end point was time to healing (defined as 95% epithelialisation by visual inspection). The secondary end points were percentage of burns epithelialised/healed, numbers of burns healed or not at each visit (not at baseline), number of study burns requiring a skin graft, and number of dressing changes. Additional outcomes were measured assessing the tolerability and performance of the dressings on wound and periwound status, including pain and experience of use of the dressings." Comment: the defined outcomes were all fully reported |
| Other bias | Unclear risk | Comment: it was unclear how the designated burn was chosen in participants with multiple burns. However it was clear that there were no unit of analysis issues. |
Thamlikitkul 1991.
| Methods | Country where data collected: Singapore Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 26 days |
|
| Participants | Inclusion criteria: thermal 1st‐ or 2nd‐degree burns, < 30% TBSA, within 24 h of admission with no prior antibiotics or topical treatment for burn Exclusion criteria: diabetes mellitus and terminal patients Participants: 38 patients at 2 community hospitals Mean age (years): 18 vs 25.2 Male participants: 11 vs 11 Burn type: thermal 18 vs 17; electrical 2 vs 1 Burn degree: 1st 9 vs 5; 2nd 11 vs 13 Burn size (%TBSA): 8 vs 11.1 Burn location: NR |
|
| Interventions | Intervention arm 1: Aloe vera Linn. mucilage dressings changed twice daily Intervention arm 2: SSD dressings changed twice daily Cointerventions: intravenous fluid 6 vs 6; antibiotics 12 vs 12, analgesia 13 vs 13, tetanus 2 vs 1, sedatives 2 vs 2, other 2 vs 0 |
|
| Outcomes | Primary outcome: wound healing secondary outcome: adverse events |
|
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were designated to receive Aloe vera Linn., mucilage or silver sulfadiazine for topical treatment of their burns by stratified randomization selection based on two prognostic factors...." Comment: unclear how randomisation sequence was derived |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eligible patients were designated to receive Aloe vera Linn., mucilage or silver sulfadiazine for topical treatment of their burns by stratified randomization selection based on two prognostic factors...." Comment: unclear whether treatment allocations were adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Each patient was assessed daily for healing, side effects and satisfaction with the treatment" Comment: no information on whether assessment was conducted in a blinded fashion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no specific quote but all randomised participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote |
| Other bias | Unclear risk | Comment: no evidence of other sources of bias but reporting insufficient to be certain |
Thomas 1995.
| Methods | Country where data collected: UK Parallel‐group RCT Unit of randomisation: participant Unit of analysis: burn Duration: NR |
|
| Participants | Inclusion criteria: < 5% TBSA, presented up to 24 h post burn Exclusion criteria: burns to face, neck, axilla; chemical and electrical burns Participants: 50 participants with 54 burns Mean age (years): NR; children 10/18 vs 7/16 vs 7/16 Male participants: NR; ratios 2:1 vs 1:1.3 vs 1:1.3 no significant difference between groups Burn type: scalds 95% vs 56% vs 88%; no significant difference between groups Burn degree: NR (minor) Burn size (%TBSA): 0.84 vs 0.94 vs 0.79; no significant difference between groups Burn location: NR |
|
| Interventions | Intervention arm 1: chlorhexidine tulle‐gras. N = 18 Intervention arm 2: hydrocolloid (granuflex). N = 16 Intervention arm 3: hydrocolloid + SSD. N = 16 Cointerventions: NR |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: pain |
|
| Notes | Funding: Convatec/Squibb supplied granuflex | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to one of three treatment groups after obtaining informed consent" Comment: no information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to one of three treatment groups after obtaining informed consent". Comment: no information on whether allocation concealment was adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "During dressing changes the healing progress of the wound was noted..." Comment: no information on whether observers were blinded; balance of probabilities would be not |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no specific quote but unclear whether all randomised participants were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but outcomes mentioned in early part of text are reported in findings |
| Other bias | High risk | Comment: unit of analysis issues as randomisation was at the participant level whilst analysis was at the level of burn wounds (some participants had multiple burns) |
Varas 2005.
| Methods | Country where data collected: USA Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: completion of treatment (max 14 days) |
|
| Participants | Inclusion criteria: partial‐thickness burn injuries requiring topical wound care that, in the opinion of the observer, would not go on to require surgical excision and grafting. The wounds had to involve two areas far enough apart so as not to create interference of the treatments. Wounds of similar sizes were chosen, but not specifically measured. Exclusion criteria: NR Participants: 14 people attending a hospital/burn centre Mean age (years): 41 (25‐68) Male participants: 13/14 Burn type: 12 flame, 2 scalding (both arms same cause) Burn degree: NR partial‐thickness Burn size (%TBSA): 14.6% (4.5–27) Burn location: upper extremities 8 vs 8, lower extremities 4 vs 6, trunk 2 vs 0 |
|
| Interventions | Intervention arm 1: Acticoat‐ silver‐impregnated membrane applied wet and left in place; moistened and change of overlying dry gauze dressings every 6 h. 14 burns Intervention arm 2: SSD ‐ applied and removed then dressed with a dry gauze dressings twice daily. 14 burns Cointerventions: NR |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: pain Secondary outcome: adverse events |
|
| Notes | Funding NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by assignment using random drawing of sealed envelopes from a box with equal numbers of treatment and control envelopes. According to the protocol, the patient’s most left and/or upper‐most wound was labelled as wound #1, and the patient’s most right and/ or lower‐most wound was labelled as wound #2. Wound #1 was assigned randomly to one of the treatment algorithms, and wound #2 was assigned to the alternate algorithm." Comment: randomisation appeared adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed by assignment using random drawing of sealed envelopes from a box with equal numbers of treatment and control envelopes. According to the protocol, the patient’s most left and/or upper‐most wound was labelled as wound #1, and the patient’s most right and/ or lower‐most wound was labelled as wound #2. Wound #1 was assigned randomly to one of the treatment algorithms, and wound #2 was assigned to the alternate algorithm." Comment: it was unclear how well the allocation system was concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "endpoint for dressings both in the inpatient and outpatient setting was based on the clinical judgment of the attending physicians at the Burn Center" Comment: unclear whether the outcome assessor was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Fourteen patients were enrolled .....Four patients continued in the study until completion of treatment" Comment: very high proportion of participants did not complete treatment |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but outcomes were not clearly specified in methods section so difficult to determine if all assessed outcomes reported |
| Other bias | Unclear risk | Comment: unclear whether the analysis adjusted for intra‐individual design |
Wright 1993.
| Methods | Country where data collected: UK Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
|
| Participants | Inclusion criteria: partial‐thickness burns manageable through outpatients Exclusion criteria: burns requiring grafting, > 48 h post‐burn injury, sensitive to dressings, burn on face or hand joints, burn infected, receiving treatment other than first aid or more suited to alternative treatments Participants: 98 people presenting at ED/outpatient care. Other characteristics NR but "no statistically significant differences with regard to patient demographics and physical characteristics" (refers to participants included in analysis only) |
|
| Interventions | Intervention arm 1: hydrocolloid dressing (Granuflex) Intervention arm 2: paraffin gauze impregnated with 0.5% chlorhexidine acetate (Bactigras) Cointerventions: cleaned with sodium chloride solution and allowed to dry |
|
| Outcomes | Secondary outcome: pain Secondary outcome: resource use |
|
| Notes | Funded by ConvaTec Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Written, informed consent of the patients was obtained and witnessed, and the patients were randomly allocated to either Granuflex E or Bactigras" Comment: no further information on how the randomisation sequence was produced |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Written, informed consent of the patients was obtained and witnessed, and the patients were randomly allocated to either Granuflex E or Bactigras" Comment: no further information on whether allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "At each follow‐up attendance the following details were noted: 1. Reason for dressing change. 2. Ease of removal. 3. Wound appearance. 4. Pain while dressing was in situ. 5. Pain on dressing removal or application. 6. Analgesia or antibiotics administered. When the wound had completely healed a final evaluation was made, the quality of healing with regard to re‐epithelialization and cosmetic results was noted. The dressing was rated by both the investigator and the patient." Comment: it appeared that the investigator was not blinded to treatment allocation and also performed the assessment of outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Out of a total of 98 patients involved, 31 patients were withdrawn. Of these, 22 patients were lost to follow‐up, two patients requested withdrawal and there was one protocol violation." Comment: a large number of participants were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but outcomes were not fully prespecified so difficult to determine if all planned outcomes were reported |
| Other bias | Unclear risk | Comment: no evidence of other sources of bias but reporting insufficient to be certain |
Yang 2013.
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: burn Unit of analysis: burn Duration: 14 days |
|
| Participants | Inclusion criteria: total burn < 30% TBSA, deep partial second‐degree burn wounds, > one month treatment; residual wound < 10% TBSA, single wound < 5 cm x 5 cm Exclusion criteria: no general infection or complications Participants: 60 hospital patients each with 2 burns Mean age (years): 39 ± 13 (range 18‐65) Male participants: NR Burn type: NR Burn degree: NR Burn size (%TBSA): NR; size 18 ± 8 cm2 vs 15 ± 10 cm2 Burn location: NR |
|
| Interventions | Intervention arm 1: FLAMIGEL (hydrogel dressing) covered with cotton gauze, changed every day to 7 days, then every other day to 14 days. N = 60 burns Intervention arm 2: iodophor gauze covered with cotton gauze, changed every day to 7 days, then every other day to 14 days. N = 60 burns Cointerventions: |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: pain |
|
| Notes | Funding NR Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “This prospective randomised trial was conducted according to the random number table” Comment: a random component in the sequence generation process was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no mention of blinding of key study personnel used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | Reporting insufficient to determine whether the intra‐individual design was adjusted for or other risks |
Yarboro 2013.
| Methods | Country where data collected: USA Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: |
|
| Participants | Inclusion criteria: superficial partial‐thickness burns, 0‐4 days post thermal injury < 25% TBSA, aged 11‐80 years Exclusion criteria: burn on face, ears or scalp; allergic to silver Participants: 24 participants attending a wound management centre Mean age (years): 33.8 vs 33.9 Male participants: 18/24 Burn type: NR Burn degree: NR (superficial partial‐thickness) Burn size (%TBSA): NR (area burned 1103.10 ± 1086.10 cm² vs 753.70 ± 934.30 cm²) Burn location: NR |
|
| Interventions | Intervention arm 1: Aquacel Ag plus standard care Intervention arm 2: SSD plus standard care Cointerventions: whirlpool wound cleansing for 15 mins using hexaclorophene + selective debridement followed by wound dressing as per arm and 2nd dressing |
|
| Outcomes | Primary outcome: wound healing Secondary outcome: pain |
|
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty‐four subjects (18 men and 6 women) who sustained superficial partial‐thickness burns and who were between the ages of 19 and 53 years, and with time of injury from 0 to 4 days, were randomly assigned into a control group (silver sulfadiazine) and experimental group (Aquacel Ag)." Comment: no further information on how the randomisation sequence was produced |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Twenty‐four subjects (18 men and 6 women) who sustained superficial partial‐thickness burns and who were between the ages of 19 and 53 years, and with time of injury from 0 to 4 days, were randomly assigned into a control group (silver sulfadiazine) and experimental group (Aquacel Ag)." Comment: no information on whether treatment allocation concealment was adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Wound measurements were assessed at the time of the initial examination and every 4 days subsequently until the area was re‐epithelialized 100%. To ensure objectivity, the burn area was measured digitally with the software program Aspyra (AspyraLLC; Blue Springs, Missouri) in order to prevent discrepancies in wound measurements. Length and width of wounds were assessed based on a clock face with length from 12 to 6 o'clock and width 3 to 9 o'clock based on anatomic position......In addition, pain, utilizing the standard 0‐ to 10‐point scale, was assessed at the conclusion of each treatment session" Comment: digital methods were used to assess wound healing but it was unclear if the assessors were blinded to treatment allocation; assessment of pain was also unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote "Twenty‐four subjects (18 men and 6 women) who sustained superficial partial‐thickness burns and who were between the ages of 19 and 53 years, and with time of injury from 0 to 4 days, were randomly assigned into a control group (silver sulfadiazine) and experimental group (Aquacel Ag)." Comment: no withdrawals were reported but it was unclear whether all randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote, all outcomes mentioned in paper were reported in table |
| Other bias | Unclear risk | Comment: frequency of additional treatments and if they differed between groups is not reported |
Zahmatkesh 2015.
| Methods | Country where data collected: Iran Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 20 days |
|
| Participants | Inclusion criteria: participants with second‐degree burns (depth 0.2‐5.0 mm) up to 40% TBSA; aged 15‐55, referred during first 24 h following injury, negative culture on admission Exclusion criteria: participants with underlying conditions such as diabetes, chronic renal or hepatic diseases, and those with simultaneous burns, trauma, and skin lacerations were excluded Participants: 30 individuals with superficial or deep partial‐thickness burns Mean age (years): 24.8 (11.9) Male participants: 21/30 Burn type: direct fire or oil: 26 Burn degree: partial‐thickness burns; deep partial‐thickness 6/10 vs 11/20 Burn size (%TBSA): surface area Burn location: NR |
|
| Interventions | Intervention arm 1: olea ointment which contains 33.4% honey, 33.3% olive oil, and 33.3% sesame oil. After washing the wound with normal saline solution, 3–5 mm thick layer of Olea ointment was applied over the wound and closed dressing was performed every day Intervention arms 2: 1.5 mm‑thick layer of acetate mafenide ointment (8.5%) every 12 h, Cointerventions: debridement as required |
|
| Outcomes | Primary outcome: wound healing (development of granulation tissue) Primary outcome: infection (development of positive culture after 7 days) Secondary outcome: adverse events: need for surgical debridement |
|
| Notes | Funding: Kurdistan University of Medical Sciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "30 available patients .....who were divided into two groups using simple randomized method and table of random numbers" Comment: table of random numbers used to generate randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: "30 available patients .....who were divided into two groups using simple randomized method and table of random numbers" Comment: unclear whether allocation sequence was adequately concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the microbiologist and pathologist were blinded to the treatment groups. To assess the outcomes, the burn wounds were evaluated daily after a week of intervention by a pathologist and a microbiologist for the formation of granulation tissues, debridement (using scalpel), and wound culture results" Comment: blinded outcome assessment for all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "If they had positive culture, they were excluded from the study and treated by routine treatment for bacterial strains. However, the excluded patients were entered in the analysis." Comment: all participants appear to be included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but not clear that all the outcomes assessed were specified in the methods |
| Other bias | Low risk | Comment: does not appear to be any additional source of bias |
Zhou 2011.
| Methods | Country where data collected: China Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: 14 days |
|
| Participants | Inclusion criteria: paediatric superficial second‐degree burn wounds within 24 h Exclusion criteria: prior treatment; other disease Participants: 40 children with burns divided into 2 areas for treatment Mean age (years): 4.5 ± 2.2 (2‐6) Male participants: 22/40 Burn type: NR Burn degree: second‐degree Burn size (%TBSA): 3.85 ± 1.27 (3‐5) Burn location: neck or front trunk |
|
| Interventions | Intervention arm 1: Aquacel‐Ag, covered by 20 layers gauze; took off the outer layer at Day 3; changed new dressing at Day 7 with debridement Intervention arm 2: SD‐Ag, covered by 20 layers gauze; took off the outer layer at Day 3; changed new dressing at Day 7 with debridement Cointerventions: cleaned the wound with water; 5% chlorhexidine acetate for 2 min then cleaned with water again |
|
| Outcomes | Primary outcome: wound healing | |
| Notes | Funding NR Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | Note: the abstract stated that “two burn wound areas of similar size were selected from each patient”; in main text, it showed that these two burn areas actually was one wound, just divided to two same‐sized parts; this increases the chance of "carry‐over" from one test site to another. It was unclear whether the analysis adjusted for this intra‐individual design. |
AE: adverse event(s); AEO: Arnebia euchroma ointment; ALT amino alanine transferase; AST: aspartate amino transferase; A vera: Aloe vera; ED: Emergency Department; ITT: intention‐to‐treat; IV: intravenous; NR: not reported; SSD: silver sulfadiazine; TBSA: total body surface area; VAS: visual analogue scale;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afilalo 1992 | Ineligible intervention: antiseptic combined with SSD |
| Ang 2002 | Ineligible intervention: chlorhexidine rinse followed by SSD treatment |
| Ang 2003 | Ineligible intervention: chlorhexidine cleansing then SSD treatment for deeper burns |
| Babb 1977 | Ineligible study design: quasi‐randomised trial |
| Bowser 1981 | Ineligiblestudy design: quasi‐randomised trial |
| Brown 2016 | Antiseptic agent did not differ between groups |
| Cason 1966 | Ineligible study design: quasi‐randomised trial |
| Chen 2007 | Ineligible study design: study designed only to test moisture‐absorption properties of dressings over short time period |
| Chmyrev 2011 | Ineligible population ‐ post‐surgical burn wounds |
| Chokotho 2005 | Ineligible intervention ‐ comparison of non‐antiseptic with a mixture of antiseptic and non‐antiseptic agents |
| Choudhary 2013 | Ineligible study design: quasi‐randomised trial |
| Colombo 1993 | Ineligible population: minority burns patients |
| Daryabeigi 2010 | Ineligible study design: quasi‐randomised trial |
| Fisher 1968 | Ineligible was not the only systematic difference between the groups: other agents included in sprays |
| Gee Kee 2015 | Ineligible agent did not differ between groups |
| Helvig 1979 | Ineligible study design: quasi‐randomised trial |
| Kumar 2004 | Ineligible intervention: combination of SSD and chlorhexidine |
| Madhusudhan 2015 | Ineligiblepopulation ‐ residual burns were a minority of participants |
| Mohammadi 2013 | Ineligible study design: quasi‐randomised trial |
| Palombo 2011 | Ineligible population (post‐surgery burns) |
| Shoma 2010 | Ineligible intervention: additional treatments given in both comparison groups |
| Subrahmanyam 1993a | Ineligible population: burn wounds a minority of included participants |
| Subrahmanyam 1999 | Antiseptic was not only systematic difference between groups |
| Tredget 1998 | Antiseptic agent did not differ between groups |
| Vehmeyer‐Heeman 2005 | Wrong population: post‐surgical burn wounds |
| Verbelen 2014 | Antiseptic agent did not differ between groups |
| Weng 2009 | Antiseptic was not only systematic difference between groups |
| Xu 2009 | Ineligible study design: agents under investigation only used for short period to assess blood and urine levels |
| Zhu 2006 | Ineligible study design: quasi‐randomised trial |
RCT: randomised controlled trial; SSD: silver sulfadiazine
Characteristics of studies awaiting assessment [ordered by study ID]
Gao 2016.
| Methods | Country where data collected: NR Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: unclear |
| Participants | Inclusion criteria: irrespective of age with deep‐dermal burn wound; admitted to hospital less than 3 days after burn; the burn wounds were not to be operated on Exclusion criteria: burn wound involved the head and face region; history of allergy to dressing composed of ionic silver; serious infective wound; required emergency surgery Participants: 10 individuals; no details of participant characteristics were reported |
| Interventions | Intervention arm 1: silver‐impregnated antimicrobial dressing combined with a granulocyte macrophage colony‐stimulating factor (GM‐CSF) gel Intervention arms 2: gauze dressing combined with GM‐CSF gel Cointerventions: the GM‐CSF gel was applied in both arms |
| Outcomes | Primary outcome: no primary outcomes were reported Secondary outcome: pain Secondary outcome: resource use ‐ time to debridement complete; no outcomes currently have evaluable data |
| Notes | Funding: NR Reported as abstract only; study author contact not yet established Appears to be assessing time to debridement rather than the outcomes of this review |
Liu 2016.
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
| Participants | Inclusion criteria: participants with deep partial‐thickness burn wounds Exclusion criteria: NR Participants: 366 participants |
| Interventions | Intervention arm 1: gauze with iodophor Intervention arms 2: recombinant human granulocyte‐macrophage colony‐stimulating factor (rhGM‐CSF) gel Cointerventions: NR |
| Outcomes | Primary outcome: complete wound healing time Secondary outcome: adverse events |
| Notes | Paper in Chinese. Awaiting obtaining full text and translator assessment; details here based on English abstract |
Rege 1999.
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: unclear Duration: at least 2 weeks |
| Participants | Patients with burns (N = 17) |
| Interventions | Azadirachta indica (formulation unclear) SSD (formulation unclear) |
| Outcomes | Wound area? (ulcer score) Healing? (healing without deformity) Adverse events? (scar‐related events reported) |
| Notes | Abstract assessed; full text unobtainable |
Santi 2013.
| Methods | Country where data collected: NR Parallel‐group RCT Unit of randomisation: unclear Unit of analysis: unclear Duration: unclear |
| Participants | Inclusion criteria: children with second‐degree burns Exclusion criteria: NR Participants: 27 children in the intervention group, control group Mean age (years): 5 (range 4 months‐14 years) Male participants: 16 Burn type: NR Burn degree: second Burn size (%TBSA): NR Burn location: NR |
| Interventions | Intervention arm 1: alginate with embedded anti‐bacterial enzyme system Intervention arm 2: NR Cointerventions: NR |
| Outcomes | Primary outcome: wound healing Primary outcome: infection Secondary outcome: pain Secondary outcome: adverse events(?) |
| Notes | Reported in abstract form only, very limited information |
Wang 2015.
| Methods | Country where data collected: China Intra‐individual RCT Unit of randomisation: burn area Unit of analysis: burn area Duration: NR |
| Participants | Inclusion criteria: participants with deep or superficial partial‐thickness facial burn wounds Exclusion criteria: NR Participants: 25 participants, 10 with deep partial‐thickness and 15 with superficial partial‐thickness facial burns |
| Interventions | Intervention arm 1: silver hydrocolloid Intervention arms 2: biological dressing (porcine xenoderm [sic]) Cointerventions: NR |
| Outcomes | Primary outcome: wound healing time Primary outcome: infection Secondary outcome: resource use, possibly also pain |
| Notes | Paper in Chinese. Awaiting obtaining full text and translator assessment; details here based on English abstract |
NR: not reported; RCT; randomised controlled trial; SSD: silver sulfadiazine
Differences between protocol and review
The protocol did not address a particular study design which several of our included studies employed: the intra‐individual design where burns or burn areas were randomised to different treatments. The closest parallel to this is the 'split‐mouth' design. It was not clear that the analyses of these studies took the design into account. We have adopted the approach of including these studies in our meta‐analyses but undertaking post‐hoc sensitivity analyses to explore the impact of including them. Where there was a substantive difference between the results of the principal analysis and the sensitivity analysis we were conservative and used the results of the sensitivity analysis to inform the GRADE assessment.
Due to the large number of comparisons included in the review we did not produce a 'Summary of findings' table for every outcome for every comparison, in order to keep them to a manageable size. Instead, where comparisons had limited available data for prespecified outcomes we presented these data together with GRADE judgements in an additional table. Due to the large number of comparisons that only reported mean time to healing (where all wounds healed) as a measure of healing, we included this in both 'Summary of findings' tables and additional tables of GRADE judgements.
Contributions of authors
Gill Norman: designed and co‐ordinated the review; extracted data; checked the quality of data extraction; analysed and interpreted data; undertook and checked quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing and editing the review; made an intellectual contribution to the review; wrote to study authors; approved the final review prior to submission and is a guarantor of the review.
Janice Christie: extracted data; checked the quality of data extraction; analysed and interpreted data; undertook and checked quality assessment; made an intellectual contribution to the review wrote to study authors and approved the final review prior to submission.
Zhenmi Liu: extracted data; analysed and interpreted data; undertook quality assessment; made an intellectual contribution to the review; performed translations and approved the final review prior to submission.
Maggie Westby: checked the quality of data extraction; analysed and interpreted data; checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; contributed to writing or editing the review; made an intellectual contribution to the review; advised on the review and approved the final review prior to submission.
Jayne Jefferies: extracted data; undertook quality assessment; made an intellectual contribution to the review; and approved the final review prior to submission.
Thomas Hudson: extracted data; undertook quality assessment; made an intellectual contribution to the review and approved the final review prior to submission.
Jacky Edwards: contributed to writing or editing the review; made an intellectual contribution to the review and approved the final review prior to submission.
Devi Mohapatra: contributed to writing or editing the review; made an intellectual contribution to the review and approved the final review prior to submission.
Ibrahim Hassan: made an intellectual contribution to the review and approved the final review prior to submission..
Jo Dumville: contributed to writing and editing the review; made an intellectual contribution to the review; advised on the review; approved the final review prior to submission and is a guarantor of the review.
Contributions of editorial base:
Andrea Nelson (Editor): edited the protocol; advised on methodology, interpretation and protocol content; approved the final protocol prior to submission.
Tanya Walsh (Editor): edited the review; advised on methodology, interpretation and review content; approved the final review prior to submission.
Gill Rizzello (Managing Editor) co‐ordinated the editorial process; advised on interpretation and content; edited the protocol and the review.
Reetu Child and Naomi Shaw (Information Specialists): designed the search strategy; edited the search methods section and ran the searches.
Ursula Gonthier (Editorial Assistant) edited the references and the Plain Language Summary.
Sources of support
Internal sources
Division of Nursing, Midwifery and Social Work, School of Health Sciences, University of Manchester, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Cochrane Programme Grant funding (NIHR Cochrane Programme Grant 13/89/08 – High Priority Cochrane Reviews in Wound Prevention and Treatment) to Cochrane Wounds and by the NIHR Research Methods Programme Systematic Review Fellowship NIHR‐RMFI‐2015‐06‐52 (Zhenmi Liu). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester, UK.
Jo Dumville was partly funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester. The funder had no role in the design of the studies, data collection and analysis, decision to publish, or preparation of the manuscript. However, the review may be considered to be affiliated to the work of the NIHR CLAHRC Greater Manchester. The views expressed herein are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health.
Declarations of interest
Gill Norman: my employment at the University of Manchester is funded by the National Institute for Health Research (NIHR) UK and focuses on high priority Cochrane Reviews in the prevention and treatment of wounds.
Janice Christie: none known.
Zhenmi Lui: my employment at the University of Manchester is funded by the NIHR (NIHR Research Methods Programme Systematic Review Fellowship NIHR‐RMFI‐2015‐06‐52).
Maggie Westby: My employment at the University of Manchester is funded by the NIHR and focuses on high priority Cochrane Reviews in the prevention and treatment of wounds.
Jayne Jeffries: none known.
Thomas Hudson: none known.
Jacky Edwards: none known.
Devi Prasad Mohapatra: none known.
Ibrahim Hassan: none known.
Jo Dumville: I receive research funding from the NIHR for the production of systematic reviews focusing on high priority Cochrane Reviews in the prevention and treatment of wounds. This work was also partly funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester.
Andrew Jull (peer reviewer) is the author of a published Cochrane Review investigating the effect of honey on wounds Jull 2015, which was the source of some of the raw data for this review.
Edited (no change to conclusions)
References
References to studies included in this review
Abedini 2013 {published data only}
- Abedini F, Ahmadi A, Yavari A, Hosseini V, Mousavi S. Comparison of silver nylon wound dressing and silver sulfadiazine in partial burn wound therapy. International Wound Journal 2013;10(5):573‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adhya 2015 {published data only}
- Adhya A, Bain J, Ray O, Hazra A, Adhikari S, Dutta G, et al. Healing of burn wounds by topical treatment: a randomized controlled comparison between silver sulfadiazine and nano‐crystalline silver. Journal of Basic and Clinical Pharmacy 2015;6(1):29‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akhtar 1996 {published data only}
- Akhtar MA, Hatwar SK. Efficacy of Aloe vera extract cream in management of burn wound. Journal of Clinical Epidemiology 1996;49(Supp 1):24. [Google Scholar]
Baghel 2009 {published data only}
- Baghel PS, Shukla S, Mathur RK, Randa R. A comparative study to evaluate the effect of honey dressing and silver sulfadiazine dressing on wound healing in burn patients. Indian Journal of Plastic Surgery 2009;42(2):176‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bangroo 2005 {published data only}
- Bangroo AK, Katri R, Chauhan S. Honey dressing in pediatric burns. Journal of the Indian Association of Plastic Surgeons 2005;10(3):172‐5. [Google Scholar]
Carayanni 2011 {published data only}
- Carayanni VJ, Tsati EG, Spyropoulou GC, Antonopoulou FN, Ioannovich JD. Comparing oil based ointment versus standard practice for the treatment of moderate burns in Greece: a trial based cost effectiveness evaluation. BMC Complementary and Alternative Medicine 2011;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caruso 2006 {published data only}
- Caruso DM, Foster KN, Blome‐Eberwein SA, Twomey JA, Herndon DN, Luterman A, et al. Randomized clinical study of Hydrofiber dressing with silver or silver sulfadiazine in the management of partial‐thickness burns. Journal of Burn Care and Research 2006;27(3):298‐309. [DOI] [PubMed] [Google Scholar]
- Foster K, Caruso D, Antimarino J, Bauer G, Blome‐Eberwein S, Herndon D, et al. Randomized controlled study of silver dressing effects on partial‐thickness burn outcomes. Zeitschrift für Wasserrecht Sonderheft 2005;2:17. [Google Scholar]
Chen 2006 {published data only}
- Chen J, Han CM, Lin XW, Tang ZJ, Su SJ. Effect of silver nanoparticle dressing on second degree burn wound. Zhonghua wai ke za zhi [Chinese Journal of Surgery] 2006;44(1):50‐2. [PubMed] [Google Scholar]
De Gracia 2001 {published data only}
- Gracia CG. An open study comparing topical silver sulfadiazine and topical silver sulfadiazine‐cerium nitrate in the treatment of moderate and severe burns. Burns 2001;27(1):67‐74. [DOI: ] [DOI] [PubMed] [Google Scholar]
Glat 2009 {published data only}
- Glat PM, Kubat WD, Hsu JF, Copty T, Burkey BA, Davis W, et al. Randomized clinical study of SilvaSorb gel in comparison to Silvadene silver sulfadiazine cream in the management of partial‐thickness burns. Journal of Burn Care and Research 2009;30(2):262‐7. [DOI] [PubMed] [Google Scholar]
Gong 2009 {published data only}
- Gong Z‐H, Yao J, Ji J‐F, Yang J, Xiang T. Effect of ionic silver dressing combined with hydrogel on degree II burn wound healing. Journal of Clinical Rehabilitative Tissue Engineering 2009;13(42):8373‐6. [Google Scholar]
Han 1989 {published data only}
- Han KH, Maitra AK. Management of partial skin thickness burn wounds with Inadine dressings. Burns 1989;15(6):399‐402. [DOI] [PubMed] [Google Scholar]
Healy 1989 {published data only}
- Healy CM, Boorman JG. Comparison of E‐Z Derm and Jelonet dressings for partial skin thickness burns. Burns 1989;15(1):52‐4. [DOI] [PubMed] [Google Scholar]
Homann 2007 {published data only}
- Homann H‐H, Rosbach O, Moll W, Vogt PM, Germann G, Hopp M, et al. A liposome hydrogel with polyvinyl‐pyrrolidone iodine in the local treatment of partial‐thickness burn wounds. Annals of Plastic Surgery 2007;59(4):423‐7. [DOI] [PubMed] [Google Scholar]
- Rossbach O, Vogt P, Germann G. Repithel® in comparison with Flammazine® for IIa degree burns. European Wound Management Association Conference; 2005, 15‐17 September; Stuttgart, Germany. 2005:Poster abstract P45.
Huang 2007 {published data only}
- Huang Y, Li X, Liao Z, Zhang G, Liu Q, Tang J, et al. A randomized comparative trial between Acticoat and SD‐Ag in the treatment of residual burn wounds, including safety analysis. Burns 2007;33(2):161‐6. [DOI] [PubMed] [Google Scholar]
- Huang YS, Li XL, Liao ZJ, Zhang GA, Liu Q, Tang J, et al. Multi‐center clinical study of Acticoat (nanocrystalline silver dressing) for the management of residual burn wounds: summary report. Institute of Burn Research, Southwest Hospital, The Third Military Medical University 2004. [PubMed]
- Li XL, Huang YS, Peng YZ, Liao ZJ, Zhang GA, Liu Q, et al. Multi‐center clinical study of Acticoat (nanocrystalline silver dressing) for the management of residual burn wounds. Zhonghua Shao Shang za Zhi [Chinese Journal of Burns] 2006;22(1):15‐8. [PubMed] [Google Scholar]
Inman 1984 {published data only}
- Inman RJ, Snelling CF, Roberts FJ, Shaw K, Boyle JC. Prospective comparison of silver sulfadiazine 1 per cent plus chlorhexidine digluconate 0.2 per cent (Silvazine) and silver sulfadiazine 1 per cent (Flamazine) as prophylaxis against burn wound infection. Burns 1984;11(1):35‐40. [DOI] [PubMed] [Google Scholar]
- Snelling CF, Inman RJ, Germann E, Boyle JC, Foley B, Kester DA, et al. Comparison of silver sulfadiazine 1% with chlorhexidine digluconate 0.2% to silver sulfadiazine 1% alone in the prophylactic topical antibacterial treatment of burns. Journal of Burn Care and Rehabilitation 1991;12(1):13‐8. [DOI] [PubMed] [Google Scholar]
Jiao 2015 {published data only}
- Jiao J‐Q, Li Y, Huang Z, Hu W‐G. Effects of recombinant human epidermal growth factor gel combined with nano‐silver dressing for burn scar. Chinese Journal of Tissue Engineering Research/Journal of Clinical Rehabilitative Tissue Engineering Research 2015;19(25):4007‐11. [Google Scholar]
Khorasani 2009 {published data only}
- Khorasani G, Hosseinimehr SJ, Azadbakht M, Zamani A, Mahdavi MR. Aloe versus silver sulfadiazine creams for second‐degree burns: a randomised controlled study. Surgery Today 2009;39(7):587‐91. [DOI] [PubMed] [Google Scholar]
Li 1994 {published data only}
- Li Y, Wang N, Zhou C. A comparison between 'moist ointment' and 0.25% iodophor, silver sulfadiazine paste and 0.1% Rivanol in the treatment of deep II degree burn wounds. Chinese Journal of Plastic Surgery and Burns 1994;10(5):342‐5. [PubMed] [Google Scholar]
Li 2006 {published data only}
- Li LG, Chai JK, Guo ZR, Yang HM, Jia XM, Xu MH, et al. Application of carbon fiber dressing on burn wounds. Zhonhghua shao shang za zhi [Chinese Journal of Burns] 2006;44(15):1047‐9. [PubMed] [Google Scholar]
Liao 2006 {published data only}
- Liao ZJ, Huan JN, Lv GZ, Shou YM, Wang ZY. Multi‐center clinical study of the effect of silver nitrate ointment on the partial‐thickness burn wounds. Zhonghua wai ke za zhi [Chinese Journal of Surgery] 2006;22(5):359‐61. [PubMed] [Google Scholar]
Maghsoudi 2011 {published data only}
- Maghsoudi H, Salehi F, Khosrowshahi MK, Baghaei M, Nasirzadeh M, Shams R. Comparison between topical honey and mafenide acetate in treatment of burn wounds. Annals of Burns and Fire Disasters 2011;24(3):132‐7. [PMC free article] [PubMed] [Google Scholar]
Malik 2010 {published data only}
- Malik KI, Malik MA, Aslam A. Honey compared with silver sulphadiazine in the treatment of superficial partial‐thickness burns. International Wound Journal 2010;7(5):413‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mashhood 2006 {published data only}
- Mashhood AA, Khan TA, Sami AN. Honey compared with 1% silver sulfadiazine cream in the treatment of superficial and partial thickness burns. Journal of the Pakistan Association of Dermatologists 2006;16(1):14‐9. [Google Scholar]
Memon 2005 {published data only}
- Memon AR, Tahir SM, Khushk, Memon GA. Therapeutic effects of honey versus silver sulphadiazine in the management of burn injuries. Journal of Liaquat University of Medical and Health Sciences 2005;4(3):100‐4. [Google Scholar]
Muangman 2006 {published data only}
- Muangman P, Chuntrasakul C, Silthram S, Suvanchote S, Benjathanung R, Kittidacha S, et al. Comparison of efficacy of 1% silver sulfadiazine and Acticoat for treatment of partial‐thickness burn wounds. Chotmaihet thangphaet [Journal of the Medical Association of Thailand] 2006;89(7):953‐8. [PubMed] [Google Scholar]
Muangman 2010 {published data only}
- Muangman P, Pundee C, Opasanon S, Muangman S. A prospective, randomized trial of silver containing hydrofiber dressing versus 1% silver sulfadiazine for the treatment of partial thickness burns. International Wound Journal 2010;7(4):271‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nasiri 2016 {published data only}
- Nasiri E, Hosseinimehr SJ, Zaghi Hosseinzadeh A, Azadbakht M, Akbari J, Azadbakht M. The effects of Arnebia euchroma ointment on second‐degree burn wounds: a randomized clinical trial. Journal of Ethnopharmacology 2016;189:107‐16. [DOI] [PubMed] [Google Scholar]
Neal 1981 {published data only}
- Neal DE, Whalley PC, Flowers MW, Wilson DH. The effects of an adherent polyurethane film and conventional absorbent dressing in patients with small partial thickness burns. British Journal of Clinical Practice 1981;35(7‐8):254‐7. [PubMed] [Google Scholar]
Ning 2008 {published data only}
- Ning F‐G, Zhang G‐A. Efficacy of dermacyn on deep partial second‐degree burns. Chinese Journal of New Drugs 2008;17(8):691‐2. [Google Scholar]
Oen 2012 {published data only}
- Oen IM, Baar ME, Middelkoop E, Nieuwenhuis MK, Facial Burns Group. Effectiveness of cerium nitrate‐silver sulfadiazine in the treatment of facial burns: a multicenter, randomized, controlled trial. Plastic and Reconstructive Surgery 2012;130(2):274e‐83e. [DOI] [PubMed] [Google Scholar]
- Oen‐Coral IM, Baar M, Wal M, Beerthuizen G, Dokter J, Tempelman F, et al. Effectiveness of "two masks" of cerium‐silver sulphadiazine versus silver sulphadiazine in facial burns treatment: short‐term results of a randomised controlled trial. Burns 2009;35(Supp):S21. [Google Scholar]
Opasanon 2010 {published data only}
- Opasanon S, Muangman P, Namviriyachote N. Clinical effectiveness of alginate silver dressing in outpatient management of partial‐thickness burns. International Wounds Journal 2010;7(6):467‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opasanon S, Muangman P, Namviriyachote N, Chuntrasakul C. Clinical effectiveness of alginate silver dressing in outpatient management of partial‐thickness burns. EWMA Journal 2010;10(2):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Panahi 2012 {published data only}
- Moharamzad Y, Panahi Y, Beiraghdar F, Feizi I. Clinical efficacy of a topical herbal cream (Aloe vera, Geranium robertianum and Lavandula stoechas) in second‐degree burn wounds. Basic & Clinical Pharmacology & Toxicolocy 2010;107(Supp 1):466. [Google Scholar]
- Panahi Y, Beiraghdar F, Akbari H, Bekhradi H, Taghizadeh M, Sahebkar A. A herbal cream consisting of Aloe vera, Lavandula stoechas, and Pelargonium roseum as an alternative for silver sulfadiazine in burn management. Asian Biomedicine 2012;6(2):273‐8. [Google Scholar]
Phipps 1988 {published data only}
- Phipps A, Lawrence JB. Comparison of hydrocolloid dressings and medicated tulle‐gras in the treatment of outpatient burns. Beyond Occlusion: Wound Care Proceedings, Royal Society of Medicine Services International Congress and Symposium 1988;136:121‐5. [Google Scholar]
Piatkowski 2011 {published data only}
- Piatkowski A, Drummer N, Andriessen A, Ulrich D, Pallua N. RCT comparing a polihexanide containing bio‐cellulose dressing with silver sulfadiazine cream in partial thickness dermal burns. EWMA Journal 2011;2(Supp):63. [DOI] [PubMed] [Google Scholar]
- Piatkowski A, Drummer N, Andriessen A, Ulrich D, Pallua N. Randomized controlled single center study comparing a polyhexanide containing bio‐cellulose dressing with silver sulfadiazine cream in partial‐thickness dermal burns. Burns 2011;37(5):799‐803. [DOI] [PubMed] [Google Scholar]
Piccolo‐Daher 1990 {published data only}
- Piccolo‐Daher MT, Piccolo‐Lobo MS, Piccolo NS, Piccolo N. Efficacy of five topical agents in burn treatment: a comparative study [Estudo comparativo da eficacia de cinco medicamentos topicos no tratamento de queimaduros]. Revista Goiana de Medicina 1990;36(1):55‐9. [Google Scholar]
Radu 2011 {published data only}
- Radu CA, Gazyakan E, Germann G, Riedel K, Reichenberger M, Ryssel H. Optimizing Suprathel®‐therapy by the use of Octenidine‐Gel®. Burns 2011;37(2):294‐8. [DOI] [PubMed] [Google Scholar]
Sami 2011 {published data only}
- Sami AM, Qureshi N, Zeeshan M, HIrfan A, Khan M. Honey compared with silver sulfadiazine as burn wound dressing. Annals of the Pakistan Institute of Medical Science 2011;7(1):22‐5. [Google Scholar]
Shahzad 2013 {published data only}
- Shahzad MN, Ahmed N. Effectiveness of Aloe vera gel compared with 1% silver sulphadiazine cream as burn wound dressing in second degree burns. Journal of the Pakistan Medical Association 2013;63(2):225‐30. [PubMed] [Google Scholar]
Silverstein 2011 {published data only}
- Silverstein P, Heimbach D, Meites H, Latenser B, Mozingo D, Mullins F, et al. An open, parallel, randomized, comparative, multicenter study to evaluate the cost‐effectiveness, performance, tolerance, and safety of a silver‐containing soft silicone foam dressing (intervention) vs silver sulfadiazine cream. Journal of Burn Care and Research 2011;32(6):617‐26. [DOI] [PubMed] [Google Scholar]
- Silverstein P, Heimbach D, Meites H, Latenser B, Mozingo D, Mullins F, et al. Soft silicone dressing with silver versus silver sulfadiazine cream in the treatment of partial‐thickness burns: a randomised controlled trial. EWMA Journal 2010;10(2):149. [Google Scholar]
Subrahmanyam 1991 {published data only}
- Subrahmanyam M. Topical application of honey in treatment of burns. British Journal of Surgery 1991;78(4):497‐8. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1993b {published data only}
- Subrahmanyam M. Honey impregnated gauze versus polyurethane film (OpSite (R)) in the treatment of burns ‐ a prospective randomised study. British Journal of Plastic Surgery 1993;46(4):322‐3. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1994 {published data only}
- Subrahmanyam M. Honey‐impregnated gauze versus amniotic membrane in the treatment of burns. Burns 1994;20(4):331‐3. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1996a {published data only}
- Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns 1996;22(6):491‐3. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1996b {published data only}
- Subrahmanyam M. Addition of antioxidants and polyethylene glycol 4000 enhances the healing property of honey in burns. Annals of Burns and Fire Disasters 1996;9(2):93‐5. [Google Scholar]
Subrahmanyam 1998 {published data only}
- Subrahmanyam M. A prospective randomised clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns 1998;24(2):157‐61. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 2001 {published data only}
- Subrahmanyam M, Sahapure AG, Nagane NS, Bhagwat VR, Ganu JV. Effects of topical application of honey on burn wound healing. Annals of Burns and Fire Disasters 2001;14(3):143‐5. [Google Scholar]
Tang 2015 {published data only}
- Tang H, Lv G, Fu J, Niu X, Li Y, Zhang M, et al. An open, parallel, randomized, comparative, multicenter investigation evaluating the efficacy and tolerability of Mepilex Ag versus silver sulfadiazine in the treatment of deep partial‐thickness burn injuries. Journal of Trauma and Acute Care Surgery 2015;78(5):1000‐7. [DOI] [PubMed] [Google Scholar]
Thamlikitkul 1991 {published data only}
- Thamlikitkul V, Bunyapraphatsara N, Riewpaiboon W, Theerapong S, Chantrakul C, Thanaveerasuwan T, et al. Clinical trial of Aloe vera Linn. for treatment of minor burns. Siriraj Hospital Gazette 1991;43(5):31‐6. [Google Scholar]
Thomas 1995 {published data only}
- Thomas SS, Lawrence JC, Thomas A. Evaluation of hydrocolloids and topical medication in minor burns. Journal of Wound Care 1995;4(5):218‐20. [DOI] [PubMed] [Google Scholar]
Varas 2005 {published data only}
- Varas RP, O'Keeffe T, Namias N, Pizano LR, Quintana OD, Herrero Tellachea M, et al. A prospective, randomized trial of Acticoat versus silver sulfadiazine in the treatment of partial‐thickness burns: which method is less painful?. Journal of Burn Care and Rehabilitation 2005;26(4):344‐7. [DOI] [PubMed] [Google Scholar]
Wright 1993 {published data only}
- Wright A, MacKechnie DW, Paskins JR. Management of partial thickness burns with Granuflex 'E' dressings. Granuflex 'E' vs Bactigras. Burns 1993;19(2):128‐30. [DOI] [PubMed] [Google Scholar]
Yang 2013 {published data only}
- Yang H‐Z, Wang W‐K, Yuan L‐L, Wang S‐B, Luo G‐X, Wu J, et al. Multi‐center clinical trial of FLAMIGEL (hydrogel dressing) for the treatment of residual burn wound. Zhonhghua shao shang za zhi [Chinese Journal of Burns] 2013;29(2):177‐80. [PubMed] [Google Scholar]
Yarboro 2013 {published data only}
- Yarboro DD. A comparative study of the dressings silver sulfadiazine and Aquacel Ag in the management of superficial partial‐thickness burns. Advances in Skin and Wound Care 2013;26(6):259‐62 (20 ref). [DOI] [PubMed] [Google Scholar]
Zahmatkesh 2015 {published data only}
- Zahmatkesh M, Manesh MJ, Babashahabi R. Effect of olea ointment and acetate mafenide on burn wounds: randomized clinical trial. Iranian Journal of Nursing and Midwifery Research 2015;20(5):599‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2011 {published data only}
- Zhou P‐Y, Xia Z‐F, Ben D‐F, Ma B, Fang H, Feng P. Clinical application of Aquacel‐Ag dressing in treatment of pediatric superficial I burn wounds. Academic Journal of Second Military Medical University 2011;32(12):1321‐3. [Google Scholar]
References to studies excluded from this review
Afilalo 1992 {published data only}
- Afilalo M, Dankoff J, Guttman A, Lloyd J. DuoDERM hydroactive dressing vs silver sulphadiazine/Bactigras in the emergency treatment of partial skin thickness burns. Burns 1992;18(4):313‐6. [DOI] [PubMed] [Google Scholar]
Ang 2002 {published data only}
- Ang ES, Lee ST, Gan CS, See P, Chan YH, Ng LH, et al. The role of alternative therapy in the management of partial thickness burns of the face: experience with the use of moist exposed burn ointment (MEBO) compared with silver sulphadiazine. Annals of the Academy of Medicine 2002;29(1):7‐10. [PubMed] [Google Scholar]
- Ang ES, Lee ST, Gan CS, See PG, Chan YH, Ng LH, et al. Evaluating the role of alternative therapy in burn wound management: randomized trial comparing moist exposed burn ointment with conventional methods in the management of patients with second‐degree burns. Medscape General Medicine 2001;3(2):3. [PubMed] [Google Scholar]
Ang 2003 {published data only}
- Ang E, Lee S‐T, Gan CS‐G, Chan Y‐H, Cheung Y‐B, Machin D. Pain control in a randomized, controlled, clinical trial comparing moist exposed burn ointment and conventional methods in patients with partial‐thickness burns. Journal of Burn Care and Rehabilitation 2003;24(5):289‐96. [DOI] [PubMed] [Google Scholar]
Babb 1977 {published data only}
- Babb JR, Bridges K, Jackson DM, Lowbury EJ, Ricketts CR. Topical chemoprophylaxis: trials in silver phosphate chlorhexidine, silver sulphadiazine and povidone iodine preparations. Burns 1977;3(2):65‐71. [Google Scholar]
Bowser 1981 {published data only}
- Bowser BH, Caldwell FT, Cone JB, Eisenach KD, Thompson CH. A prospective analysis of silver sulfadiazine with and without cerium nitrate as a topical agent in the treatment of severely burned children. Journal of Trauma 1981;21(7):558‐63. [DOI] [PubMed] [Google Scholar]
Brown 2016 {published data only}
- Brown M, Dalziel SR, Herd E, Johnson K, Wong SR, Shepherd M. A randomized controlled study of silver‐based burns dressing in a pediatric emergency department. Journal of Burn Care and Research 2016;37(4):e340‐e347. [DOI] [PubMed] [Google Scholar]
Cason 1966 {published data only}
- Cason JS, Jackson DM, Lowbury EJ, Ricketts CR. Antiseptic and aseptic prophylaxis for burns: use of silver nitrate and of isolators. British Medical Journal 1966;2(5525):1288‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2007 {published data only}
- Chen J, Han C‐M, Su G‐L, Tang Z‐J, Su S‐J, Lin X‐W. Randomized controlled trial of the absorbency of four dressings and their effects on the evaporation of burn wounds. Chinese Medical Journal 2007;120(20):1788‐91. [PubMed] [Google Scholar]
Chmyrev 2011 {published data only}
- Chmyrev I. Use of silver containing wound dressings after late necrectomy in the patients with deep burns. EWMA Journal 2011;11(Supp 2):114. [Google Scholar]
Chokotho 2005 {published data only}
- Chokotho L, Hasselt E. The use of tannins in the local treatment of burn wounds: a pilot study. Malawi Medical Journal 2005;17(1):19‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choudhary 2013 {published data only}
- Choudhary KN, Soni PP, Sao DK, Murthy R, Deshkar AM, Nanda BR. Role of gentian violet paint in burn wound management: a prospective randomised control trial. Journal of the Indian Medical Association 2013;111(4):248‐50. [PubMed] [Google Scholar]
Colombo 1993 {published data only}
- Colombo P. Topical chloroxidating solution in wounds treatment: a controlled trial. Acta Toxicologica et Therapeutica 1993;14(2):65‐72. [Google Scholar]
Daryabeigi 2010 {published data only}
- Daryabeigi R, Heidari M, Hosseini SA, Omranifar M. Comparison of healing time of the 2 degree burn wounds with two dressing methods of fundermol herbal ointment and 1% silver sulfadiazine cream. Iranian Journal of Nursing and Midwifery Research 2010;15(3):97‐101. [PMC free article] [PubMed] [Google Scholar]
Fisher 1968 {published data only}
- Fisher AJ, Morrison G, Riet Rle S. A blind trial of two aerosol sprays in the exposure treatment of burns. Suid‐Afrikaanse Tydskrif Vir Geneeskunde [South African Medical Journal] 1968;42(34):903‐5. [PubMed] [Google Scholar]
Gee Kee 2015 {published data only}
- Gee Kee E, Kimble RM, Cuttle L, Stockton K. Comparison of three different dressings for partial thickness burns in children: study protocol for a randomised controlled trial. Trials 2013;14(403):Available from trialsjournal.biomedcentral.com/articles/10.1186/1745‐6215‐14‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee Kee EL, Kimble RM, Cuttle L, Khan A, Stockton KA. Randomized controlled trial of three burns dressings for partial thickness burns in children. Burns 2015;41(5):946‐55. [DOI] [PubMed] [Google Scholar]
Helvig 1979 {published data only}
- Helvig EI, Munster AM, Su CT, Oppel W. Cerium nitrate‐silver sulfadiazine cream in the treatment of burns: a prospective, randomized study. American Surgeon 1979;45(4):270‐2. [PubMed] [Google Scholar]
- Munster AM, Helvig E, Rowland S. Cerium nitrate‐silver sulfadiazine cream in the treatment of burns: a prospective evaluation. Surgery 1980;88(5):658‐60. [PubMed] [Google Scholar]
Kumar 2004 {published data only}
- Kumar RJ, Kimble RM, Boots R, Pegg SP. Treatment of partial‐thickness burns: a prospective, randomized trial using Transcyte. ANZ journal of surgery 2004;74(8):622‐6. [DOI] [PubMed] [Google Scholar]
Madhusudhan 2015 {published data only}
- Madhusudhan, V. Efficacy of 1% acetic acid in the treatment of chronic wounds infected with Pseudomonas aeruginosa: prospective randomised controlled clinical trial. International Wound Journal 2015;13(6):1129‐36. [DOI: 10.1111/iwj.12428] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammadi 2013 {published data only}
- Mohammadi AA, Seyed Jafari SM, Kiasat M, Pakyari MR, Ahrari I. Efficacy of debridement and wound cleansing with 2% hydrogen peroxide on graft take in the chronic‐colonized burn wounds: a randomized controlled clinical trial. Burns 2013;39(6):1131‐6. [DOI] [PubMed] [Google Scholar]
Palombo 2011 {published data only}
- Palombo M, Anniboletti T, Fasciani L, Delli Santi G, Schirosi M. Smart dressings for the reduction of burns infections. Burns 2011;37(Supp):S8‐9. [Google Scholar]
Shoma 2010 {published data only}
- Shoma A, Eldars W, Noman N, Saad M, Elzahaf E, Abdalla M, et al. Pentoxifylline and local honey for radiation‐induced burn following breast conservative surgery. Current Clinical Pharmacology 2010;5(4):251‐6. [DOI] [PubMed] [Google Scholar]
Subrahmanyam 1993a {published data only}
- Subrahmanyam M. Honey as a surgical dressing for burns and ulcers. Indian Journal of Surgery 1993;55(9):468‐73. [Google Scholar]
Subrahmanyam 1999 {published data only}
- Subrahmanyam M. Early tangential excision and skin grafting of moderate burns is superior to honey dressing: a prospective randomised trial. Burns 1999;25(8):729‐31. [DOI] [PubMed] [Google Scholar]
Tredget 1998 {published data only}
- Tredget EE, Shankowsky HA, Groeneveld A, Burrell R. A matched‐pair, randomized study evaluating the efficacy and safety of Acticoat silver‐coated dressing for the treatment of burn wounds. Journal of Burn Care and Rehabilitation 1998;19(6):531‐7. [DOI] [PubMed] [Google Scholar]
Vehmeyer‐Heeman 2005 {published data only}
- Vehmeyer‐Heeman M, Kerckhove E, Gorissen K, Boeckx W. Povidone‐iodine ointment: no effect of split skin graft healing time. Burns 2005;31(4):489‐94. [DOI] [PubMed] [Google Scholar]
Verbelen 2014 {published data only}
- Verbelen J, Hoeksema H, Heyneman A, Pirayesh A, Monstrey S. Aquacel Ag versus Acticoat: intermediate results of a prospective, randomized, controlled mono centre study in 100 patients. Burns 2011;37(Supp):S8. [Google Scholar]
- Verbelen J, Hoeksema H, Heyneman A, Pirayesh A, Monstrey S. Aquacel(®) Ag dressing versus ActicoatTM dressing in partial thickness burns: a prospective, randomized, controlled study in 100 patients. Part 1: burn wound healing. Burns 2014;40(3):416‐27. [DOI] [PubMed] [Google Scholar]
Weng 2009 {published data only}
- Weng Z‐Y, Ding R‐H, Han B, Chen Z‐H, Xie Z‐H, Tang J, et al. Nanometer silver dressing plus recombinant bovine basic fibroblast growth factor gel for residual burn wounds. Journal of Clinical Rehabilitative Tissue Engineering Research 2009;13(47):9357‐60. [Google Scholar]
Xu 2009 {published data only}
- Xu W. The curative effect and safety evaluation of nanometer silver used on II degree burn wound. Wound Repair and Regeneration 2009;17(4):A61. [Google Scholar]
Zhu 2006 {published data only}
- Zhu Z, Xu G, Zhao J. Clinical study on application of bovine amnion on burn wounds. Zhongguo xiu fu chong jian wai ke za zhi [Chinese Journal of Reparative and Reconstructive Surgery] 2006;20(7):735‐8. [PubMed] [Google Scholar]
References to studies awaiting assessment
Gao 2016 {published data only}
- Gao X, Jin X, Chen X, Yu J. Clinical study of debridement effect of the silver impregnated antimicrobial dressing combined with GM‐CSF gel. Journal of Burn Care and Research 2016;37:S66. [Google Scholar]
Liu 2016 {published data only}
- Liu J, Liao ZJ, Zhang Q. Phase IV clinical trial for external use of recombinant human granulocyte‐macrophage colony‐stimulating factor gel in treating deep partial‐thickness burn wounds. Zhonghua shao shang za zhi [Chinese Journal of Burns] 2016;32(9):542‐8. [DOI] [PubMed] [Google Scholar]
Rege 1999 {published data only}
- Rege NN, Dahanukar SA, Ginde VK, Thatte UM, Bapat RD. Safety and efficacy of Azadirachta indica in patients with second degree burns. Indian Practitioner 1999;52(4):240‐8. [Google Scholar]
Santi 2013 {published data only}
- Santi GD, Palombo M, Bruno A, Greca C, Palombo P. Anti‐bacterial enzyme system in the treatment of pediatric burn patient: our experience. EWMA Journal 2013;13(Supp 1):252. [Google Scholar]
Wang 2015 {published data only}
- Wang Q, Liu X‐W, Lv R‐R, Huo R. Biological dressing A versus Physiotulle Ag in the repair of degree ll facial burns. Chinese Journal of Tissue Engineering Research/Journal of Clinical Rehabilitative Tissue Engineering Research 2015;19(16):2573‐7. [Google Scholar]
Additional references
Alp 2012
- Alp E, Coruh A, Gunay GK, Yontar Y, Doganay M. Risk factors for nosocomial infection and mortality in burn patients: 10 years of experience at a university hospital. Journal of Burn Care and Research 2012;33(3):379‐85. [DOI] [PubMed] [Google Scholar]
American Burn Association 2013
- American Burn Association. National Burn Repository 2013: report of data from 2003‐2012. www.ameriburn.org/2013NBRAnnualReport.pdf (accessed 5 December 2016).
Avni 2010
- Avni T, Levcovich A, Ad‐El DD, Leibovici L, Paul M. Prophylactic antibiotics for burns patients: systematic review and meta‐analysis. BMJ 2010;340:241. [DOI: 10.1136/bmj.c241] [DOI] [PMC free article] [PubMed] [Google Scholar]
AWMA 2011
- Australian Wound Management Association (AWMA). Bacterial impact on wound healing: from contamination to infection. Version 1.5. October 2011. www.awma.com.au/publications/2011_bacterial_impact_position_1.5.pdf (accessed 5 December 2016).
Aziz 2012
- Aziz Z, Abu SF, Chong NJ. A systematic review of silver‐containing dressings and topical silver agents (used with dressings) for burn wounds. Burns 38;3:307‐18. [DOI] [PubMed] [Google Scholar]
Baker 1996
- Baker RA, Jones S, Sanders C, Sadinski C, Martin‐Duffy K, Berchin H, et al. Degree of burn, location of burn, and length of hospital stay as predictors of psychosocial status and physical functioning. Journal of Burn Care and Rehabilitation 1996;17(4):327‐33. [DOI] [PubMed] [Google Scholar]
Bang 2002
- Bang RL, Sharma PN, Sanyal SC, Al Naijadah I. Septicaemia after burn injury: a comparative study. Burns 2002;28(8):746‐51. [DOI] [PubMed] [Google Scholar]
Barajas‐Nava 2013
- Barajas‐Nava LA, Lopez‐Alcade J, Roque I, Figuls M, Sola I, Bonfill Cosp X. Antibiotic prophylaxis for preventing burn wound infection. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD008738.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2016
- British Medical Association, British Royal Pharmaceutical Society of Great Britain. British National Formulary. www.medicinescomplete.com/mc/bnf/current/ (accessed 5 December 2016).
Bowler 2003
- Bowler PG. The 105 bacterial growth guideline: reassessing its clinical relevance in wound healing. Ostomy Wound Management 2003;49(1):44‐53. [PubMed] [Google Scholar]
Church 2006
- Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clinical Microbiology Reviews 2006;19(2):403‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dat 2012
- Dat AD, Poon F, Pham KBT, Doust J. Aloe vera for treating acute and chronic wounds. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008762.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2007
- Davies CE, Hill KE, Newcombe RG, Stephens P, Wilson MJ, Harding KG, et al. A prospective study of the microbiology of chronic venous leg ulcers to reevaluate the clinical predictive value of tissue biopsies and swabs. Wound Repair and Regeneration 2007;15(1):17‐22. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Demling 2005
- Demling RH, DeSanti L. Managing the burn wound. eplasty.com/images/PDF/ManageBurnWound‐part1.pdf (accessed 5 December 2016).
DeSanti 2005
- Santi L. Pathophysiology and current management of burn injury. Advances in Skin and Wound Care 2005;18(6):323‐32. [DOI] [PubMed] [Google Scholar]
Dumville 2012
- Dumville JC, Munson C. Negative pressure wound therapy for partial‐thickness burns. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD006215.pub3] [DOI] [PubMed] [Google Scholar]
European Practice Guidelines 2002
- European Burn Association, British Burn Association. European Practice Guidelines for Burn Care. www.britishburnassociation.org/european‐standards (accessed 5 December 2016).
Fitzwater 2003
- Fitzwater J, Purdue GF, Hunt JL, O'Keefe GE. The risk factors and time course of sepsis and organ dysfunction after burn trauma. Journal of Trauma 2003;54(5):959‐66. [DOI] [PubMed] [Google Scholar]
Hahn 2005
- Hahn S, Puffer S, Torgerson DJ, Watson J. Methodological bias in cluster randomised trials. BMC Medical Research Methodology 2005;5:10. [DOI: 10.1186/1471-2288-5-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendon 2002
- Hendon D (editor). Total Burn Care. 4th Edition. Edinburgh: Saunders, 2002. [Google Scholar]
Hettiaratchy 2004
- Hettiaratchy S, Papini R. Initial management of a major burn II: assessment and resuscitation. BMJ 2004;329(7457):101‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. www.handbook.cochrane.org..
Howell‐Jones 2005
- Howell‐Jones RS, Wilson MJ, Hill KE, Price PE, Thomas DW. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. Journal of Antimicrobial Chemotherapy 2005;55(2):143‐9. [DOI] [PubMed] [Google Scholar]
Jull 2015
- Jull AB, Cullum N, Dumville JC, Westby M, Walker N, Deshpande S. Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD005083.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keen 2010
- Keen EF, Robinson BJ, Hospenthal DR, Aldous WK, Wolf SE, Chung KK, et al. Prevalence of multidrug‐resistant organisms recovered at a military burn center. Burns 2010;36(6):819‐25. [DOI] [PubMed] [Google Scholar]
Kingsley 2004
- Kingsley A, White R, Gray D. The wound infection continuum: a revised perspective. Wounds UK applied wound management supplement 2004. www.wounds‐uk.com/pdf/content_8975.pdf (accessed 5 December 2016).
Kontopantelis 2012
- Kontopantelis E, Reeves D. Performance of statistical methods for meta‐analysis when true study effects are non‐normally distributed: a simulation study. Statistical Methods in Medical Research 2012;21(4):409‐26. [DOI] [PubMed] [Google Scholar]
Kontopantelis 2013
- Kontopantelis E, Springate DA, Reeves D. A re‐analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta‐analyses. PLoS One 2013;26:e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Latenser 2007
- Latenser BA, Miller SF, Bessey PQ, Browning SM, Caruso DM, Gomez M, et al. National Burn Repository 2006: a ten‐year review. Journal of Burn Care and Research 2007;28(5):635‐8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lesaffre 2009
- Lesaffre E, Philstrom B, Needleman I, Worthington H. The design and analysis of split‐mouth studies: what statisticians and clinicians should know. Statistics in Medicine 2009;28:3470‐82. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2016
- Lu J, Yang M, Zhan M, Xuewen X, Yue J, Xu T. Antibiotics for treating infected burn wounds. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012084] [DOI] [Google Scholar]
Macpherson 2004
- Macpherson G (editor). Black's Student Medical Dictionary. London: A&C Black Publishers Limited, 2004. [Google Scholar]
Madsen 1996
- Madsen SM, Westh H, Danielsen L, Rosdahl VT. Bacterial colonisation and healing of venous leg ulcers. APMIS 1996;104(2):895‐9. [DOI] [PubMed] [Google Scholar]
Mock 2008
- Mock C, Peck M, Peden M, Krug E, WHO. A WHO plan for burn prevention and care. 2008. apps.who.int/iris/bitstream/10665/97852/1/9789241596299_eng.pdf (accessed 5 December 2016).
National Burn Care Review 2001
- National Burn Care Review: committee report. www.britishburnassociation.org/downloads/NBCR2001.pdf (accessed 5 December 2016).
National Network for Burn Care 2012
- National Network for Burn Care (NNBC). National Burn Care Referral Guidance. Version 1. February 2012. www.britishburnassociation.org/downloads/National_Burn_Care_Referral_Guidance_‐_5.2.12.pdf. (accessed 5 December 2016).
Ninnemann 1982
- Ninnemann JL. Immunologic defenses against infection: alterations following thermal injuries. Journal of Burn Care and Rehabilitation 1982;3(6):355‐66. [Google Scholar]
O'Meara 2001
- O'Meara S, Cullum NA, Majid M, Sheldon TA. Systematic review of antimicrobial agents used for chronic wounds. British Journal of Surgery 2001;88(1):4‐21. [DOI] [PubMed] [Google Scholar]
Oncul 2009
- Oncul O, Ulkur A, Acar V, Turhan E, Yeniz E, Karacaer Z, et al. Prospective analysis of nosocomial infections in a Burn Care Unit, Turkey. Indian Journal of Medical Research 2009;130:758‐64. [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Peck 1998
- Peck MD, Weber J, McManus A, Sheridan R, Heimbach D. Surveillance of burn wound infections: a proposal for definitions. Journal of Burn Care and Rehabilitation 1998;19(5):386‐9. [DOI] [PubMed] [Google Scholar]
Peck 2012
- Peck MD. Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns 2012;37(7):1087‐100. [DOI] [PubMed] [Google Scholar]
Peck 2013
- Peck M, Pressman MA. The correlation between burn mortality rates from fire and flame and economic status of countries. Burns 2013;39(6):1054‐9. [DOI] [PubMed] [Google Scholar]
Percival 2004
- Percival SL, Bowler PG. Biofilms and their potential role in wound healing. Wounds 2004;16(7):234‐40. [Google Scholar]
Polavarapu 2008
- Polavarapu N, Ogilvie MP, Panthaki ZJ. Microbiology of burn wound infections. Journal of Craniofacial Surgery 2008;19(4):899‐902. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robson 1968
- Robson MC, Lea CE, Dalton JB, Heggers JP. Quantitative bacteriology and delayed wound closure. Surgical Forum 1968;19:501. [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
SIGN 2015
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html#random (accessed 5 December 2016).
Sterne 2011
- Sterne JA, Egger M, Moher D, Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Storm‐Versloot 2010
- Storm‐Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD006478.pub2] [DOI] [PubMed] [Google Scholar]
Tengvall 2006
- Tengvall OM, Bjornhagen VC, Lindholm C, Jonsson C‐E, Wengstrom Y. Differences in pain patterns for infected and noninfected patients with burn injuries. Pain Management Nursing 2006;7(4):176‐82. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18:2693‐708. [DOI] [PubMed] [Google Scholar]
Thompson 2002
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21:1559‐74. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;7(8):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trengove 1996
- Trengove NJ, Stacey MC, McGechie DF, Stingemore NF, Mata S. Qualitative bacteriology and leg ulcer healing. Journal of Wound Care 1996;5:277‐80. [DOI] [PubMed] [Google Scholar]
Vermuelen 2010
- Vermeulen H, Westerbos SJ, Ubbink DT. Benefit and harm of iodine in wound care: a systematic review. Journal of Hospital Infection 2010;76(3):191‐9. [DOI] [PubMed] [Google Scholar]
Villanueva 2004
- Villanueva E, Bennett MH, Wasiak J, Lehm JP. Hyperbaric oxygen therapy for thermal burns. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004727.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wasiak 2013
- Wasiak J, Cleland H, Campbell F, Spinks A. Dressings for superficial and partial thickness burns. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD002106.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization (WHO) Dept. of Injuries and Violence Prevention. The injury chart book: a graphical overview of the global burden of injuries. 2002. apps.who.int/iris/handle/10665/42566 (accessed 5 December 2016).
Wibbenmeyer 2006
- Wibbenmeyer L, Danks R, Faucher L, Amelon M, Latenser B, Kealey GP, et al. Prospective analysis of nosocomial infection rates, antibiotic use and patterns of resistance in a burn population. Journal of Burn Care and Research 2006;27(2):152‐60. [DOI] [PubMed] [Google Scholar]
Winkelstein 1984
- Winkelstein AU. What are the immunological alterations induced by burn injury?. Journal of Trauma 1984;24:S72‐S83. [DOI] [PubMed] [Google Scholar]
Wolcott 2008
- Wolcott RD, Rhoads DD, Dowd SE. Biofilms and chronic wound inflammation. Journal of Wound Care 2008;17(8):333‐41. [DOI] [PubMed] [Google Scholar]


