Abstract
Presence of mature gametocyte forms of malaria parasites in peripheral blood is a key requirement for malaria transmission. Yet, studies conducted in most malaria transmission zones report the absence of gametocyte in the majority of patients. We therefore sought to determine the risk factors of both all-stage and mature gametocyte carriage in an area with high stable transmission of Plasmodium falciparum in Cameroon. Gametocyte positivity was determined using three complementary methods: thick blood smear microscopy, RT-PCR and RT-LAMP, whereas exposure to the infection was assessed by enzyme-linked immunosorbent assay. Of 361 malaria endemic residents randomly included in the study (mean age: 28±23 years, age range: 2–100 years, male/female sex ratio: 1.1), 87.8% were diagnosed with P. falciparum infection, of whom 45.7% presented with fever (axillary body temperature ≥37.5°C). Mature gametocyte positivity was 1.9% by thick blood smear microscopy and 8.9% by RT-PCR targeting the mature gametocyte transcript, Pfs25. The gametocyte positivity rate was 24.1% and 36.3% by RT-PCR or RT-LAMP, respectively, when targeting the sexual stage marker, Pfs16. Multivariate analyses revealed anemia as a common independent risk factor for both mature and all-stage gametocyte carriage, whereas fever and low anti-gametocyte antibody levels were independently associated with all-stage gametocyte carriage only. Taken together, the data suggest important differences in risk factors of gametocyte carriage depending on stage analyzed, with anemia, fever and low antiplasmodial plasma antibody levels representing the major contributing risk factors.
Introduction
Despite considerable global efforts against malaria, the disease remains a major public health problem globally. In 2017, approximately 219 million malaria cases and 435,000 related deaths were recorded worldwide; the majority (92%) of which occurred in sub-Saharan Africa. In the same period, 390,130 of the cases were reported in Cameroon, where malaria remains highly endemic [1]. Malaria is caused by Plasmodium parasites, and 6 species (Plasmodium falciparum, Plasmodium malaria, Plasmodium ovale, Plasmodium vivax, Plasmodium knowlesi and Plasmodium cynomolgi) are responsible for malaria in humans [2,3]. Plasmodium parasites are transmitted through the bite of infected female Anopheles mosquitoes. Only carriers of the sexual parasite stages known as mature gametocytes are infectious to mosquitoes, and gametocyte carriage is dependent on host and parasite factors that may vary between individuals or geo-epidemiological transmission zones[4–6]. Gametocyte production in the human host is thought to initiate immediately after asexual division, resulting in the production and release of sexually committed rings that then develop into transmissible mature gametocyte also known as stage V gametocytes [7,8]. Indeed, only a small proportion (<5%) of the asexually multiplying parasite population often commit to sexual development, and only a small portion of the sexually committed parasites may develop into transmissible mature gametocyte forms [6]. The host and parasite factors that favor sexual commitment and maturation in malaria parasites are not fully understood but are believed to involve both parasite genetics and host immunological and/or stress-related responses [9]. Risk factors for mature gametocyte carriage in P. falciparum infected patients include patient age [10–12], gender [13], asexual parasite densities, [13–15], blood hemoglobin levels [15–17], infection duration [13], presence of a fever [5,11,14,16,18,19], as well as patient’s blood group [11,15]. Blood levels of Plasmodium gametocytes may also depend on the gametocytogenic potential of the infecting clones and the ability of the host environment to either promote or block gametocyte production in vivo [6,20]. Indeed, under certain stress conditions including antimalarial medication [21–24], anemia [15], and host immune activity [9], a strong gametocyte surge can be observed [25,26]. However, it is not known if all or some of the above factors constitute risk factors for sexual commitment and circulation of early gametocyte forms in infected persons. Understanding gametocyte production and dynamics as well as the associated risk factors from population-based studies is essential to effectively combating the disease in all transmission zones. Unfortunately, epidemiological studies on gametocyte carriage have been limited by lack of diagnostic tools capable of detecting all circulating gametocyte forms. With the advent of modern genomics technology, several molecular techniques including loop-mediated isothermal amplification and polymerase chain reaction based methods now exist for effective measurement of gametocyte carriage at endemic country levels. Amongst these, methods based on the early gametocytogenesis marker Pfs16 and the mature gametocyte stage marker Pfs25 are the most widely used [27–31].
In this study, both LAMP and PCR were used for high sensitivity detection of P. falciparum infection and gametocyte carriage, targeting the high abundance PfExp1 transcript as asexual stage marker and the Pfs16 and Pfs25 transcripts as all-stage or mature gametocyte markers, respectively. This allowed to separately assess the demographic, hematologic and immunological risk factors of all-stage and mature gametocyte carriage in a region with high stable transmission of P. falciparum in Cameroon. Taken together, the data suggest important differences in risk factors of gametocyte carriage that depend on the gametocyte stage investigated, with anemia, fever and low antiplasmodial plasma antibody levels representing major contributing factors.
Materials and methods
Study site and population
The study was conducted in 2016 from February to September in the Esse health district of the Mefou-and-Afamba Division, Centre Region of Cameroon with field sampling undertaken in late February to early March and a second in late August to early September. Esse is a rural community of approximately 166 200 inhabitants covering a surface area of about 3 358 km2, and characterized by high stable perennial transmission of P. falciparum parasites. However, the populations have limited access to the available health facilities. The climate is equatorial with two dry seasons (December to February and July to August) and two rainy seasons (March to June and September to November), and the relief is consisted of plains and hills.
Study design
This study was a cross-sectional study involving both children from two years of age and adults resident in two neighboring villages (Ongandi and Ngondibeles) in the Esse district in Cameroon. A non-probability sampling technique was adopted to recruit the maximum number of occupants of each household visited. Community sections with highest concentration of houses were targeted and the maximum number of consenting subjects in each household were enrolled for the study. Pregnant women were excluded from the study as they received intermittent preventive treatment comprising sulfadoxine and pyrimethamine known to increase gametocyte production in treated patients [21].The study was approved by the Cameroon National Ethics Committee (N°2014/05/458/L/CNERSH/SP), and administrative authorization was obtained from the Cameroon Ministry of Public Health. Written informed consent and assent forms were obtained from participants or their parents/tutors prior to inclusion in the study. Participation was voluntary, anonymous and without compensation. Structured questionnaires were used in obtaining clinical history (axillary body temperature, fever history, other known medical conditions, drug administration) as well as socio-demographic information (age, sex, place of residence including travel history). The presence of P. falciparum and other species of malaria parasites was detected by RDT testing (SD Bioline Malaria Ag P.f./Pan). Blood hemoglobin levels were determined using an automated hemoglobinometer (Hemoglobinometer Mission Hb, USA). All RDT positive persons were managed by a stand-by medical team and antimalarial treatments provided in accordance with national recommendations. P. falciparum parasitaemia was determined by microscopic examination of Giemsa-stained thick and thin blood smears. Parasite density was determined on the basis of the number of parasites per 200 leukocytes on a thick film, assuming total leukocyte counts of 8,000 cells/μL of whole blood.
Blood sampling and RNA isolation
Venous blood samples (3 ml) were collected into EDTA vacutainer tubes and used for total RNA isolation as previously described [32,33]. Briefly, freed parasites were obtained from 2 ml of whole blood following saponin lysis. Total RNA was then extracted from each parasite pellet using 500 μl of TRI reagent, and re-solubilized in 30 μl of DEPC-treated water. The extract were immediately stored at -80°C until used.
RT-LAMP and RT-PCR assays
P. falciparum gametocyte carriage was investigated by amplifying specific segments of the high-abundant P. falciparum sexual stage specific transcript, Pfs16 (PF3D7_0406200) or the mature gametocyte stage-specific transcript, Pfs25 (PF3D7_1031000). Pfs16 is expressed in all gametocyte stages, including sexually committed schizonts and merozoites following induction [28]. Primers for RT-LAMP assays included primers F3/B3, FIP/BIP and LoopF/LoopB whereas primers for RT-PCR assay included primers F3 as forward primer and primer B3 as the reverse primer (Table 1).
Table 1. RT-LAMP and RT-PCR primer sequences.
| Tests | Target gene | Primer name | Sequence (5’ to 3’) |
|---|---|---|---|
| RT-LAMP | Pfs16 | F3 | CTTCGCTTTTGCAAACCTG |
| B3 | CTAGCTGAGTTTCTAAAGGCAT | ||
| FIP (F1c-F2) | GGTTTGCAAAGTTGAAGGGGATCAGATGCAAATGACAAAGCA | ||
| BIP (B1c-B2) | CAGGAAGTTCTTCAGGTGCCTCACCTTGAGATAGTCCACCTT | ||
| Loop F | CTTTTCCAGCGGGCTTTT | ||
| Loop B | TCTTCATGCTGTTGGACCTAAT | ||
| RT-PCR | Pfs16 | F3 | CTTCGCTTTTGCAAACCTG |
| B3 | CATCTCCTTCGTCTCCTTCATC | ||
| Pfs25 | F3 | AAGTTACCGTGGATACTGTATG | |
| B3 | TGAGCATTTGGTTTCTCCAT |
Pfs16: Plasmodium falciparum sexual stage 16 kD; Pfs25: Plasmodium falciparum sexual stage 25 kD
Primers for the PfExp1-based RT-LAMP (infection detection) as well as all RT-LAMP procedures were essentially as described previously [33]. Briefly, each assay was done in a total reaction volume of 25 μL, including 2.5μL of RNA extract, 2μL of primer mixes, 15μL of reconstituted enzyme (ISO-DR001, OptiGene), and 5.5μL of DEPC treated water (Diethyl pyrocarbonate). All LAMP assays were run in 16-microtube formats using the Genie II isothermal amplifier (OptiGene, UK), and at 65μC isothermal temperature for 1 hr. The specificity of each amplification was verified by including an annealing step of 98–70°C following which a single peak at around 87°C was expected.
RT-PCR was done using the AgPath-ID One-Step RT-PCR reagent kit designed for robust amplification of RNA targets in purified samples. Briefly, 2.5 μl RNA sample was added to a reaction mix comprising 0.4 μM of each forward or reverse primer, and 12.5 μl of RT-PCR buffer and 1 μl of RT-PCR enzyme. The following cycling conditions were adopted for both the Pfs16 and Pfs25 RT-PCRs: 45°C for 15 min, 95°C for 15 min, and 40 cycles of 95°C for 10 seconds, 50°C for 10 seconds, and 60°C for 1 min. All amplification products were verified by electrophoresis on 1.2% agarose gel stained with GelGreen II and visualized under ultraviolet light.
Determination of anti-Plasmodium falciparum antibody responses
For each blood sample, plasma was collected and analyzed by direct ELISA to estimate total antibody levels against different antigen types, including a P. falciparum soluble protein extract and the sexual stage-specific recombinant proteins, Pfg27 (all gametocyte stages) and Pfs25 (mature gametocytes and ookinete stages). Total soluble antigens were extracted from P. falciparum strain 3D7 as previously described by freeze-thaw fractionation procedures [34,35]. Recombinant proteins corresponding to the gametocyte sexual stage antigens Pfs25 and Pfg27 were obtained from the BEI Resources (MRA-59 and MRA-1274, respectively) and used to determine host exposure to Plasmodium gametocytes.
For antibody ELISAs, 96-well microplates (F96 CERT-Maxisorp) were coated with each antigen (16 ng/well of antigen extract or 10 ng/well of recombinant proteins) in bicarbonate buffer (0.1 M, pH 8.5) by overnight incubation at 4°C. The plates were washed three times with 300 μL of wash buffer (PBS, pH 7.2) per well, and blocked for one hour at room temperature with 250 μl/well of 1% BSA in PBS. After washing three times with 300 μL/well of PBS buffer containing 0.05% Tween 20 (PBS/T), the plates were incubated with 100μL/well of diluted plasma (1:250 dilution in PBS/TB) for one hour at room temperature. The plates were then washed four times with 300 μL/well of wash buffer PBS/T. Human anti-IgG response was quantified using anti-human horseradish peroxidase (HRP)-conjugated IgG secondary Ab at 1:40 000 dilution (Thermo Fisher Scientific). Upon a 1 hour incubation at room temperature, the wells were washed four times with 300 μL/well of wash buffer PBS/T and subsequently incubated with tetramethylbenzidine (TMB) substrate solution for 10 minutes at room temperature. The developing color reaction was arrested by addition of 50 μl of 0.1 M sulphuric acid (H2SO4), and optical density (OD) was read at 450 nm. Plasma samples from European volunteer blood donors with no travel history to malaria endemic countries were used as negative controls. The OD was adjusted for background reactivity by including three blank wells in which wash buffer was used in place of plasma. The cut-off value was defined as a mean OD of negative control plus three times standard deviation (SD).
Statistical analyses
Qualitative variables were expressed as frequencies while numerical variables were presented as mean +/- SD or 95% CI (95% confidence interval) if they were normally distributed. The univariate analysis was performed with either the exact Fisher test or the Chi-square test for the qualitative variables, and the Wilcoxon test or the ANOVA test for the quantitative variables. All statistical analyses were performed using Stata software (version 11 SE). The OR and association of each variable was estimated using a logistic regression model to account for the potential confounding factors. Only p <0.05 values were considered significant.
Results
Prevalence of P. falciparum infection and gametocyte carriage
To determine possible risk factors of gametocyte carriage in an area of high stable transmission of P. falciparum, a total of 361 participants with a mean age of 28 ± 23 (min-max: 2–100) years were randomly enrolled in this study. P. falciparum infection and gametocyte carriage were determined by light microscopy (asexual forms and mature gametocytes), by RT-PCR (all gametocyte forms and mature gametocytes), and by RT-LAMP (asexual and all gametocyte forms). Of the 361 participants, 56.8% were positive for P. falciparum infection by light microscopy (mean parasite density = 2, 041 parasites/ μl), 62.3% by RT-LAMP, and 62.1% by RDT. 317 (87.8%) were positive for P. falciparum infection in at least one of the three diagnostic methods used (S1 Table). 35.3% of these infected subjects were identified as submicroscopic infections (negative by microscopy but positive by RDT or molecular testing). Mature gametocyte carriage was detected in 7 (1.9%) participants when using light microscopy (mean gametocytaemia = 23 gametocytes/μl, range: 16–32 gametocytes /μl), and in 32 (8.9%) individuals when using RT-PCR (Table 2).
Table 2. Prevalence of P.falciparum infection and gametocyte carriage.
| Tests | RDT | Microscopy | RT-LAMP | RT-PCR | |||
|---|---|---|---|---|---|---|---|
| Target | All stages | Asexual stages | Mature gametocytes |
PfExp1 (asexual stages) |
Pfs16 (all sexual stages) |
Pfs16 (all sexual stages) |
Pfs25 (mature gametocytes) |
| Results | 224 (62.1%) |
205 (56.8%) |
7 (1.9%) |
225 (62.3%) |
131 (36.3%) |
87 (24.1%) |
32 (8.9%) |
Interestingly, all stage gametocytes (mature and sexually committed rings) were detected in up to 87 participants (24.1%) when using RT-PCR, and in up to 131 individuals (36.3%) when using RT-LAMP (Table 2). Positivity for either all stage or mature gametocyte carriage was significantly higher amongst the microscopically positive patients than the submicroscopically infected individuals (45.9% vs 28.6% for all stage, and 13.7% vs 2.7% for mature gametocyte carriage, p = 0.003 and 0.001, respectively). All microscopy-based gametocyte positives were also positive by Pfs25 and Pfs16-based RT-PCR, and by Pfs16-based RT-LAMP. Furthermore, all Pfs16 and Pfs25-based RT-PCR positives were also positive by Pfs16-based RT-LAMP, indicating that RT-LAMP was more sensitive at detecting all-stage gametocytes than RT-PCR. Concordance between the different gametocyte detection methods were 93.1% for light microscopy and Pfs25-based RT-PCR, 77.8% for light microscopy and Pfs16-based RT-PCR, and 65.7% for light microscopy and Pfs16-based RT-LAMP. Of the 131 gametocyte positives, 8 (6.1%) were negative for P. falciparum asexual stage infection by both light microscopy and PfExp1-based RT-LAMP. Taken together, the data indicate a high prevalence of gametocyte carriage in the study population, albeit only a small proportion (24.4%) of the all stage gametocyte positives were also positive for the transmissible mature stages.
Demographic and clinical risk factors
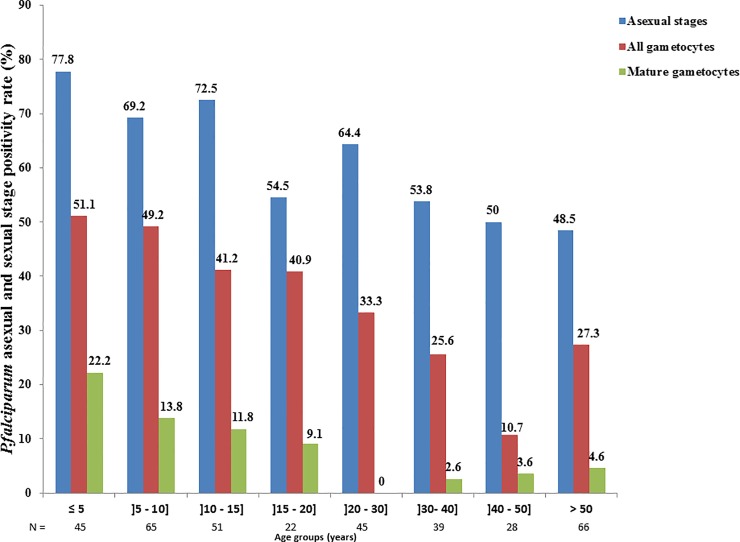
To determine if age, gender, and clinical status (presence or not of a fever) constitute risk factors for gametocyte carriage, univariate analyses were performed based on the proportion of gametocyte carriage in each analytic group. As shown in Fig 1, gametocyte carriage decreased with age with the highest prevalence observed among children below 5 years of age. Univariate analyses (Table 3) also revealed young age (children ≤ 18 years) as a major risk factor for both all stage and mature gametocyte carriage (OR = 2.311, 95% CI: [1.490–3.584], p<10−4 and OR = 5.365, 95%CI: [2.151–13.381], p<10−4, respectively) No association was found between gametocyte carriage and gender. On the other hand, gametocyte carriage increased with the onset of clinical symptoms. Indeed individuals with axiliary temperature ≥37.5°C are twice as likely to carry gametocytes (all stages or mature gametocytes) compared to non-febrile individuals (OR = 2.161, 95%CI: [1.396–3.345], p = 0.001 and OR = 2.395, 95%CI: [1.133–5.063], p = 0.022, respectively) (Table 3).
Fig 1. P. falciparum asexual and sexual stage positivity according to age.
Data showing declining gametocyte positivity (all stage and mature gametocytes) with age. All stage gametocyte positivity was determined by RT-LAMP targeting the pan-gametocyte marker, Pfs16, whereas mature gametocyte carriers were identified by Pfs25-based RT-PCR. Similarly, positivity for P. falciparum asexual stages was determined by RT-LAMP targeting the asexual stage-specific marker, PfExp1.
Table 3. Univariate analyses of risk factors for all stage and mature gametocyte carriage.
| All sexual stages* | Mature gametocytes only** | |||
|---|---|---|---|---|
| Variables | OR [95%CI] | P value | OR [95%CI] | P value |
| Gender | ||||
| Female, n = 174 | 1.390 [0.904–2.138] | 0.134 | 1.917. [0.908–4.049] | 0.088 |
| Male, n = 187 | 1 | 1 | ||
| Age group (years) | ||||
| ≤ 18 | 2.311 [1.490–3.584] | <10−4 | 5.365 [2.151–13.381] | <10−4 |
| > 18 | 1 | 1 | ||
| Fever (T≥37.5°C) | ||||
| Yes n = 155 | 2.161 [1.396–3.345] | 0.001 | 2.395 [1.133–5.063] | 0.022 |
| No n = 206 | 1 | 1 | ||
| Anaemia | ||||
| Yes n = 156 | 1.829 [1.185–2.824] | 0.006 | 2.743 [1.281–5.877] | 0.009 |
| No n = 205 | 1 | 1 | ||
| Parasitaemia (p/μl) | ||||
| High ≥ 100 n = 149 | 1.070 [0.577–1.983] | 0.831 | 3.562 [1.031–2.308] | 0.045 |
| Low < 100 n = 56 | 1 | 1 | ||
| ABO Group | ||||
| A, n = 93 | 1.059 [0.438–2.562] | 0.831 | 2.310 [0.689–7.735] | 0.175 |
| B, n = 75 | 1.268 [0.516–3.117] | 0.605 | 1.821 [0.541–6.127] | 0.333 |
| O, n = 164 | 1.015 [0.440–2.344] | 0.972 | 3.024 [0.963–9.496] | 0.058 |
| AB, n = 28 | 1 | 1 | ||
| Anti-P.f IgG | ||||
| Low reactivity n = 181 | 2.371 [1.525–3.686] | <10−4 | 6.136 [2.307–16.326] | <10−4 |
| High reactivity n = 180 | 1 | 1 | ||
| Anti-Pfs25 IgG | ||||
| Low reactivity n = 180 | 1.314 [0.854–2.020] | 0.214 | 1.325 [0.638–2.753] | 0.450 |
| High reactivity n = 181 | 1 | 1 | ||
| Anti-Pfg27 IgG | ||||
| Low reactivity n = 180 | 2.039 [1.317–3.158] | 0.001 | 2.381 [1.093–5.185] | 0.029 |
| High reactivity n = 181 | 1 | 1 | ||
* Positivity to Pfs16
** Positivity to Pfs25
Hematological and parasitological risk factors
As shown in Table 3, only anemia was associated with both all stage and mature gametocyte carriage in the study area (OR = 1.829, 95%CI: [1.185–2.824], p = 0.006). and OR = 2.743, 95%CI: [1.281–5.877], p = 0.009), respectively). No such significant associations were obtained between both all stage and mature gametocyte stage positivity and the ABO blood type (A, B, 0 and AB) or infection density (Table 3).
Immunological risk factors
To investigate if gametocyte carriage also depended on host pre-exposure to the infection and/or gametocyte carriage, we determined the association between specific plasma antibody levels and positivity for either all stage or mature gametocyte carriage. All tested antigens were differentially recognized by the different plasma samples, with the highest reactivity achieved by using protein extracts (ELISA median OD: 1.368, range: 0.101–2.889). A median OD of 0.405 (range: 0.0807–2.1057) and 0.2762 (range: 0.0499–1.8104) was obtained when using the gametocyte-specific protein, Pfg27 and the ookinete-specific surface protein Pfs25, respectively. As shown in Table 3, both all stage and mature gametocyte carriage were significantly associated with lower plasma antibody levels directed against the P. falciparum total antigen (OR = 2.371, 95%CI: [1.525–3.686], p<0.0001 and OR = 6.136, 95%CI: [2.307–16.326], p<0.0001). Positivity for all stage or mature gametocytes was also significantly associated with low antibody levels directed against the sexual stage-specific protein Pfg27 (OR = 2.039, 95%CI: [1.317–3.158], p = 0.001, and OR = 2.381, 95%CI: [1.093–5.185], p = 0.029, respectively) but not against the mature gametocyte/ookinete stage-specific protein Pfs25 (OR = 1.314, 95%CI: [0.854–2.020], p = 0.214 and OR = 1.325, 95%CI: [0.638–2.753], p = 0.450, respectively). Taken together, the data suggest a strong association between host pre-exposure to the infection (asexual and sexual stages) and gametocyte positivity, with weakly immune individuals more likely to develop P. falciparum gametocytaemia.
Independent risk factors of gametocyte carriage
To identify the demographic, hematological and serological risk factors that associated independently with gametocyte carriage in the population, multivariate analyses was done using young age, fever, anemia and low antibody responses against the P falciparum protein extract or sexual stage-specific Pfg27 antigen. As shown in Table 4 below, anemia, fever and low antibody responses against the sexual stage-specific Pfg27 protein were independently associated with all stage gametocyte positivity, whereas only anemia and low antibody responses against the P. falciparum protein extract were associated with mature gametocyte carriage. Together, the data suggest important differences between the risk factors for all stage and mature stage gametocyte carriage with anemia constituting the only common factor.
Table 4. Multivariate analysis of risk factors for all stage and mature gametocyte carriage.
| All sexual stages* | Mature gametocytes only** | |||
|---|---|---|---|---|
| Variables | OR [95%CI] | P value | OR [95%CI] | P value |
| Age (≤ 18 years) | 1.407 [0.8241–2.354] | 0.193 | 2.529 [0.920–6.952] | 0.072 |
| Anaemia | 1.675 [1.064–2.637] | 0.026 | 2.362 [1.068–5.226] | 0.034 |
| Fever (T°C≥37.5) | 1.738 [1.095–2.759] | 0.019 | 1.473 [0.662–3.277] | 0.343 |
| Low anti-P.f IgG | 1.612 [0.969–2.683] | 0.066 | 3.352 [1.144–9.827] | 0.027 |
| Low anti-Pfg27 IgG | 1.644 [1.030–2.623] | 0.037 | 1.532 [0.670–3.504] | 0.311 |
* Positivity to Pfs16
** Positivity to Pfs25
Discussion
This study aimed to determine the risk factors of gametocyte carriage in a high stable malaria transmission zone in Cameroon. We employed a recently developed RT-LAMP method [33] to include submicroscopically P. falciparum infected subjects in our analysis. Furthermore, we used a combination of three gametocyte detection techniques (RT-LAMP, RT-PCR, light microscopy) to identify active carriers of P. falciparum gametocytes in the study population. Exposure to gametocyte carriage was also investigated using an ELISA-based method. Together, our data show an extremely low prevalence (1.9%) of mature gametocyte carriage in the study area when using light microscopy. This finding is similar to those reported in other regions in Cameroon by Songue et al (0%), Van der Kolk et al (4.4%), and Sandeu et al (8.9%) using light microscopy as diagnostic method [36–38]. The mature gametocyte prevalence in our study population was only slightly increased to 8.9% when using RT-PCR as diagnostic method. We therefore sought to understand if such low prevalence rates also applied to total gametocytaemia that include both mature gametocytes and newly committed sexual stage parasites. Interestingly, the RT-PCR targeting the early gametocyte marker gene, Pfs16, revealed a gametocyte prevalence three-fold (24.1%) higher than the Pfs25 RT-PCR. Considering that the Pfs16 transcript is expressed at a two-fold higher level in mature gametocytes when compared to the Pfs25 transcript [39], it is arguable that the observed difference in gametocyte prevalence may be due to differences in assay sensitivities. However, considering the generally low densities of mature gametocytes in the peripheral blood, it was conceivable that the increased sensitivity of the Pfs16 target was due to the presence of early stage gametocytes (sexually committed rings) in the majority of infected blood samples. Indeed, Plasmodium gametocytogenesis is known to initiate in mid-trophozoite stage parasites leading to the release of sexually committed merozoites that infect and develop within circulating red blood cells as sexually committed rings before maturing through older gametocyte stages (stages I-V) [40]. However, only the sexually committed rings and mature stage V gametocytes appear in the circulation, as the remaining stages sequester in deep tissues, notably in the spleen and bone marrow [41,42]. Approximately 6% of the gametocyte positives (Pfs16-based RT-LAMP and Pfs25-based RT-PCR) were negative for P. falciparum infection as determined by light microscopy and PfExp1-based RT-LAMP. We interpreted such observations as corresponding to individuals who have recently cleared the infection either naturally or following antimalarial drug administration. This is consistent with several previous reports indicating that gametocyte positivity can proceed for several weeks after the clearance of asexual parasites, depending on the treatment or host immunity [43–45]
To determine possible risk factors of gametocyte carriage, we employed an RT-LAMP approach with previously demonstrated higher sensitivity when compared to RT-PCR [33] as main diagnostic method. Indeed, by RT-LAMP, we identified approximately 12% more gametocyte carriers compared to RT-PCR amplification of the same target gene. Additionally, all the RT-PCR gametocyte positives in our study were also positive by Pfs16-based RT-LAMP, justifying our use of RT-LAMP in the association studies. Consistent with findings elsewhere, young age, anemia [15–17] and fever [11,14,16,18,46,47] were identified as potential risk factors for both all stage and mature gametocyte carriage in the study area. In contrast to a previous study in Senegal [11] wherein various parasite density and the ABO blood groups (O and B) were identified as predictors of gametocyte positivity by light microscopy, we observed no such association with both all stage and mature gametocyte carriage based on molecular detection of the early gametocytogenesis Pfs16 marker or the mature gametocyte marker Pfs25 (cf Table 3). Interestingly, participants of age less than 18 were two times more likely to be positive for all stage gametocyte carriage, and five times more likely to be positive for the transmissible mature gametocyte stages than the adult participants. We therefore investigated the contribution of host immune factors such as anti-Plasmodial and anti-gametocyte antibody levels to gametocyte positivity using association analyses. Surprisingly, we observed a negative association between gametocyte carriage (all stage and mature gametocytes) and antibody levels against both the P. falciparum protein extract and the early gametocyte antigen, Pfg27. No such association was observed against antibody responses to the ookinete protein Pfs25 (Table 3). Considering that a similar negative association has previously been identified between gametocyte positivity and antibody levels against another early gametocyte antigen PfsEGXP [48], the above findings suggest the involvement of anti-Plasmodial/anti-gametocyte directed antibodies in protection against gametocyte production and/or survival in pre-exposed individuals.
Of the identified risk factors, only anemia and low IgG antibody response against the P. falciparum extract were independently associated with mature gametocyte carriage, and only anemia, fever and low antibody responses to the Pfg27 protein associated independently with all stage gametocyte carriage. Indeed, the role of red blood cell volume (hematocrit) in Plasmodium gametocytogenesis has previously been demonstrated and thought to be related to the release of stress factors following massive red blood cell lyses that may induce Plasmodium gametocytogenesis [49]. Similarly, the association between gametocyte carriage and fever could result from a stress-dependent process involving host inflammatory responses favoring gametocytogenesis [19]. Meanwhile, our observation of a negative association between gametocyte carriage and the anti-plasmodial or anti-gametocyte IgG antibody responses is consistent with the occurrence of some level of anti-gametocyte immunity in the study population.
Conclusion
In conclusion, gametocyte rates in the study population were comparatively high, and depended on test sensitivity and the gametocyte stage being targeted. On the basis of gametocyte positivity in the study population, we identified anemia as a common risk factor for both all stage and mature stage gametocyte carriage. Importantly, we identified low anti-Plasmodial antibody responses as major contributing factors to mature gametocyte carriage whereas low antibody responses to sexual stage antigens may associate strongly with gametocytogenesis and the initial release of early stage gametocytes into the peripheral blood. The observed negative association between the anti-gametocyte plasma antibody levels strongly support ongoing efforts to develop novel malaria transmission-blocking vaccines targeting Plasmodium gametocytogenesis.
Supporting information
(DOCX)
Acknowledgments
This work was funded through a Capacity Building and Training grant (G4 Award) from the Institute Pasteur International Division. We are particularly grateful to all participants and the parents who accepted their children to be included in this study. Our appreciations are also extended to the Malaria Research Unit staff of CPC (Nsango S, Matio S, Keumoe R, Ngou C, Feufack B and Bopda G) for their support and cooperation during the survey.
Data Availability
All relevant data are within the manuscript. Full individual-level dataset is also available at https://github.com/Essangui/Database.
Funding Statement
This work was funded through a Capacity Building and Training grant (G4 Award) from the Institute Pasteur International Division to LA. The funder played no role in the study design, data collection and analysis, as well as the decision to publish the obtained data.
References
- 1.WHO. World malaria report 2018. 20, avenue Appia CH-1211 Geneva 27; 2018 p. 240p.
- 2.Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SSG, Cox-Singh J, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet Lond Engl. 2004;363: 1017–1024. 10.1016/S0140-6736(04)15836-4 [DOI] [PubMed] [Google Scholar]
- 3.Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J. 2014;13: 68 10.1186/1475-2875-13-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider P, Schoone G, Schallig H, Verhage D, Telgt D, Eling W, et al. Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification. Mol Biochem Parasitol. 2004;137: 35–41. 10.1016/j.molbiopara.2004.03.018 [DOI] [PubMed] [Google Scholar]
- 5.Bousema T, Drakeley C. Epidemiology and Infectivity of Plasmodium falciparum and Plasmodium vivax Gametocytes in Relation to Malaria Control and Elimination. Clin Microbiol Rev. 2011;24: 377–410. 10.1128/CMR.00051-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meibalan E, Marti M. Biology of Malaria Transmission. Cold Spring Harb Perspect Med. 2017;7: a025452 10.1101/cshperspect.a025452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talman AM, Domarle O, McKenzie FE, Ariey F, Robert V. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar J. 2004; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nacher M. Does the shape of Plasmodium falciparum gametocytes have a function? Med Hypotheses. 2004;62: 618–619. 10.1016/j.mehy.2003.11.011 [DOI] [PubMed] [Google Scholar]
- 9.Bousema JT, Drakeley CJ, Sauerwein RW. Sexual-stage antibody responses to P. falciparum in endemic populations. Curr Mol Med. 2006;6: 223–229. [DOI] [PubMed] [Google Scholar]
- 10.Bousema JT, Gouagna LC, Drakeley CJ, Meutstege AM, Okech BA, Akim IN, et al. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar J. 2004;3: 1 10.1186/1475-2875-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grange L, Loucoubar C, Telle O, Tall A, Faye J, Sokhna C, et al. Risk Factors for Plasmodium falciparum Gametocyte Positivity in a Longitudinal Cohort. Nosten F, editor. PLOS ONE. 2015;10: e0123102 10.1371/journal.pone.0123102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koepfli C, Robinson LJ, Rarau P, Salib M, Sambale N, Wampfler R, et al. Blood-Stage Parasitaemia and Age Determine Plasmodium falciparum and P. vivax Gametocytaemia in Papua New Guinea. Carvalho LH, editor. PLOS ONE. 2015;10: e0126747 10.1371/journal.pone.0126747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sowunmi A, Fateye BA, Adedeji AA, Fehintola FA, Happi TC. Risk factors for gametocyte carriage in uncomplicated falciparum malaria in children. Parasitology. 2004;129: 255–262. 10.1017/S0031182004005669 [DOI] [PubMed] [Google Scholar]
- 14.von Seidlein L, Drakeley C, Greenwood B, Walraven G, Targett G. Risk factors for gametocyte carriage in Gambian children. Am J Trop Med Hyg. 2001;65: 523–527. [DOI] [PubMed] [Google Scholar]
- 15.Nacher M, Singhasivanon P, Silachamroon U, Treeprasertsuk S, Tosukhowong T, Vannaphan S, et al. Decreased hemoglobin concentrations, hyperparasitemia, and severe malaria are associated with increased plasmodium falciparum gametocyte carriage. J Parasitol. 2002;88: 97–101. 10.1645/0022-3395(2002)088[0097:DHCHAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 16.Price R, Nosten F, Simpson JA, Luxemburger C, Phaipun L, Ter Kuile F, et al. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am J Trop Med Hyg. 1999;60: 1019–1023. [DOI] [PubMed] [Google Scholar]
- 17.Stepniewska K, Price RN, Sutherland CJ, Drakeley CJ, von Seidlein L, Nosten F, et al. Plasmodium falciparum gametocyte dynamics in areas of different malaria endemicity. Malar J. 2008;7: 249 10.1186/1475-2875-7-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karl S, Laman M, Moore BR, Benjamin JM, Salib M, Lorry L, et al. Risk factors for Plasmodium falciparum and Plasmodium vivax gametocyte carriage in Papua New Guinean children with uncomplicated malaria. Acta Trop. 2016;160: 1–8. 10.1016/j.actatropica.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 19.Sumbele IUN, Bopda OSM, Kimbi HK, Ning TR, Nkuo-Akenji T. Influence of Plasmodium gametocyte carriage on the prevalence of fever, splenomegaly and cardiovascular parameters in children less than 15 years in the Mount Cameroon area: cross sectional study. BMC Infect Dis. 2015;15: 547 10.1186/s12879-015-1290-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouagna LC, Bancone G, Yao F, Yameogo B, Dabiré KR, Costantini C, et al. Genetic variation in human HBB is associated with Plasmodium falciparum transmission. Nat Genet. 2010;42: 328–331. 10.1038/ng.554 [DOI] [PubMed] [Google Scholar]
- 21.Barnes KI, Little F, Mabuza A, Mngomezulu N, Govere J, Durrheim D, et al. Increased Gametocytemia after Treatment: An Early Parasitological Indicator of Emerging Sulfadoxine‐Pyrimethamine Resistance in Falciparum Malaria. J Infect Dis. 2008;197: 1605–1613. 10.1086/587645 [DOI] [PubMed] [Google Scholar]
- 22.Shekalaghe SA, Drakeley C, van den Bosch S, ter Braak R, van den Bijllaardt W, Mwanziva C, et al. A cluster-randomized trial of mass drug administration with a gametocytocidal drug combination to interrupt malaria transmission in a low endemic area in Tanzania. Malar J. 2011;10: 247 10.1186/1475-2875-10-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karl S, Laman M, Moore BR, Benjamin J, Koleala T, Ibam C, et al. Gametocyte Clearance Kinetics Determined by Quantitative Magnetic Fractionation in Melanesian Children with Uncomplicated Malaria Treated with Artemisinin Combination Therapy. Antimicrob Agents Chemother. 2015;59: 4489–4496. 10.1128/AAC.00136-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WWARN (WorldWide Antimalarial Resistance Network). Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systematic review and meta-analysis of individual patient data. BMC Med. 2016;14 10.1186/s12916-016-0621-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandeu MM, Abate L, Tchioffo MT, Bayibéki AN, Awono-Ambéné PH, Nsango SE, et al. Impact of exposure to mosquito transmission-blocking antibodies on Plasmodium falciparum population genetic structure. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2016;45: 138–144. 10.1016/j.meegid.2016.08.025 [DOI] [PubMed] [Google Scholar]
- 26.Bonnet S, Gouagna C, Safeukui I, Meunier JY, Boudin C. Comparison of artificial membrane feeding with direct skin feeding to estimate infectiousness of Plasmodium falciparum gametocyte carriers to mosquitoes. Trans R Soc Trop Med Hyg. 2000;94: 103–106. [DOI] [PubMed] [Google Scholar]
- 27.Raj DK, Mishra S, Das BR, Dash AP. Plasmodium falciparum Pfs25 Gene Promoter has no Polymorphism in Natural Isolates of Eastern India. 2005; 4. [Google Scholar]
- 28.Buates S, Bantuchai S, Sattabongkot J, Han E-T, Tsuboi T, Udomsangpetch R, et al. Development of a reverse transcription-loop-mediated isothermal amplification (RT-LAMP) for clinical detection of Plasmodium falciparum gametocytes. Parasitol Int. 2010;59: 414–420. 10.1016/j.parint.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 29.Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, et al. Strategies for Detection of Plasmodium species Gametocytes. PLOS ONE. 2013;8: e76316 10.1371/journal.pone.0076316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waltmann A, Karl S, Chiu C, Mueller I. Limited Degradation of the Plasmodium falciparum Gametocyte Marker pfs25 mRNA Exposed to Tropical Temperatures: Considerations for Malaria Transmission Field Studies. Am J Trop Med Hyg. 2016;94: 886–889. 10.4269/ajtmh.15-0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamptey H, Ofori MF, Kusi KA, Adu B, Owusu-Yeboa E, Kyei-Baafour E, et al. The prevalence of submicroscopic Plasmodium falciparum gametocyte carriage and multiplicity of infection in children, pregnant women and adults in a low malaria transmission area in Southern Ghana. Malar J. 2018;17 10.1186/s12936-018-2479-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rio DC, Ares M, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc. 2010;2010: pdb.prot5439. 10.1101/pdb.prot5439 [DOI] [PubMed] [Google Scholar]
- 33.Kemleu S, Guelig D, Moukoko CE, Essangui E, Diesburg S, Mouliom A, et al. A Field-Tailored Reverse Transcription Loop-Mediated Isothermal Assay for High Sensitivity Detection of Plasmodium falciparum Infections. PloS One. 2016;11: e0165506 10.1371/journal.pone.0165506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rungsihirunrat K, Chaijaroenkul W, Siripoon N, Seugorn A, Thaithong S, Na-Bangchang K. Comparison of protein patterns between Plasmodium falciparum mutant clone T9/94-M1-1(b3) induced by pyrimethamine and the original parent clone T9/94. Asian Pac J Trop Biomed. 2012;2: 66–69. 10.1016/S2221-1691(11)60192-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heiber A, Spielmann T. Preparation of parasite protein extracts and western blot analysis. Bio-Protoc. 2014;4: e1136. [Google Scholar]
- 36.van der Kolk M, Tebo AE, Nimpaye H, Ndombol DN, Sauerwein RW, Eling WM. Transmission of Plasmodium falciparum in urban Yaounde, Cameroon, is seasonal and age-dependent. Trans R Soc Trop Med Hyg. 2003;97: 375–379. [DOI] [PubMed] [Google Scholar]
- 37.Songue E, Tagne C, Mbouyap P, Essomba P, Moyou–Somo R. Epidemiology of Malaria in three Geo-Ecological Zones along the Chad-Cameroon Pipeline. Am J Epidemiol Infect Dis. 2013;1: 27–33. 10.12691/ajeid-1-4-1 [DOI] [Google Scholar]
- 38.Sandeu MM, Bayibéki AN, Tchioffo MT, Abate L, Gimonneau G, Awono-Ambéné PH, et al. Do the venous blood samples replicate malaria parasite densities found in capillary blood? A field study performed in naturally-infected asymptomatic children in Cameroon. Malar J. 2017;16 10.1186/s12936-017-1978-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Barragán MJ, Lemieux J, Quiñones M, Williamson KC, Molina-Cruz A, Cui K, et al. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genomics. 2011;12 10.1186/1471-2164-12-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker DA. Malaria gametocytogenesis. Mol Biochem Parasitol. 2010;172: 57–65. 10.1016/j.molbiopara.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdulsalam AH, Sabeeh N, Bain BJ. Immature Plasmodium falciparum gametocytes in bone marrow. Am J Hematol. 2010;85: 943–943. 10.1002/ajh.21796 [DOI] [PubMed] [Google Scholar]
- 42.Aguilar R, Magallon-Tejada A, Achtman AH, Moraleda C, Joice R, Cisteró P, et al. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood. 2014;123: 959–966. 10.1182/blood-2013-08-520767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Targett G, Drakeley C, Jawara M, von Seidlein L, Coleman R, Deen J, et al. Artesunate Reduces but Does Not Prevent Posttreatment Transmission of Plasmodium falciparum to Anopheles gambiae. J Infect Dis. 2001;183: 1254–1259. 10.1086/319689 [DOI] [PubMed] [Google Scholar]
- 44.Bousema T, Roeffen W, Meijerink H, Mwerinde H, Mwakalinga S, van Gemert G-J, et al. The Dynamics of Naturally Acquired Immune Responses to Plasmodium falciparum Sexual Stage Antigens Pfs230 & Pfs48/45 in a Low Endemic Area in Tanzania. PLoS ONE. 2010;5 10.1371/journal.pone.0014114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The WorldWide Antimalarial Resistance Network (WWARN) DP Study Group. The Effect of Dosing Regimens on the Antimalarial Efficacy of Dihydroartemisinin-Piperaquine: A Pooled Analysis of Individual Patient Data. Garner P, editor. PLoS Med. 2013;10: e1001564 10.1371/journal.pmed.1001564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bousema T, Drakeley C. Epidemiology and Infectivity of Plasmodium falciparum and Plasmodium vivax Gametocytes in Relation to Malaria Control and Elimination. Clin Microbiol Rev. 2011;24: 377–410. 10.1128/CMR.00051-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sumbele IUN, Bopda OSM, Kimbi HK, Ning TR, Nkuo-Akenji T. Influence of Plasmodium gametocyte carriage on the prevalence of fever, splenomegaly and cardiovascular parameters in children less than 15 years in the Mount Cameroon area: cross sectional study. BMC Infect Dis. 2015;15 10.1186/s12879-015-1290-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nixon CP, Nixon CE, Michelow IC, Silva-Viera RA, Colantuono B, Obeidallah AS, et al. Antibodies to PfsEGXP, an Early Gametocyte-Enriched Phosphoprotein, Predict Decreased Plasmodium falciparum Gametocyte Density in Humans. J Infect Dis. 2018;218: 1792–1801. 10.1093/infdis/jiy416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demanga CG, Eng JWL, Gardiner DL, Roth A, Butterworth A, Adams JH, et al. The development of sexual stage malaria gametocytes in a Wave Bioreactor. Parasit Vectors. 2017;10 10.1186/s13071-017-2155-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the manuscript. Full individual-level dataset is also available at https://github.com/Essangui/Database.