Abstract
Background
Persistent pulmonary hypertension in the neonate (PPHN) is associated with high mortality. Currently, the therapeutic mainstay for PPHN consists of assisted ventilation and administration of inhaled nitric oxide (iNO). However, nitric oxide is costly, and its use may not be appropriate in resource‐poor settings. Approximately 30% of patients fail to respond to iNO. High concentrations of phosphodiesterases in the pulmonary vasculature have led to the use of phosphodiesterase inhibitors such as sildenafil or milrinone.
Objectives
To assess the efficacy and safety of sildenafil for treatment of pulmonary hypertension in neonates.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 3), MEDLINE via PubMed (1966 to 18 April 2017), Embase (1980 to 18 April 2017), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 18 April 2017). We searched clinical trials databases, conference proceedings, and reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
We included randomised and quasi‐randomised controlled trials of sildenafil compared with placebo or other pulmonary vasodilators, irrespective of dose, route, and duration of administration, in neonates with pulmonary hypertension, if investigators reported any of the prespecified outcomes.
Data collection and analysis
We assessed the methodological quality of trials regarding how bias was minimised at study entry, during study intervention, and at outcomes measurement. We extracted data on relevant outcomes; we estimated the effect size and reported it as risk ratio (RR), risk difference (RD), or mean difference (MD), as appropriate. We applied the I2 test of heterogeneity and used GRADE to assess the quality of evidence.
Main results
For this update, we identified two additional studies, for a total of five eligible trials that enrolled 166 infants. The methodological quality of these studies ranged from low to high risk of bias. Three studies were performed in resource‐limited settings, where iNO and high‐frequency ventilation were not available at the time of the study. One study compared sildenafil versus active controls, and another study evaluated sildenafil as adjuvant therapy to iNO. When comparing sildenafil with placebo, investigators noted significant reduction in mortality in the sildenafil alone group (three studies, 77 participants; typical RR 0.20, 95% confidence interval (CI) 0.07 to 0.56; I2 = 0% ‐ none; typical RR ‐0.36, 95% CI ‐0.53 to ‐0.18; number needed to treat for an additional beneficial outcome 3, 95% CI 2 to 6; I2 = 39% ‐ low). Trials reported no significant differences in mortality upon comparison of the sildenafil group versus the active control group (one study, 65 participants; typical RR 0.55, 95% CI 0.05 to 5.75), or when iNO was administered to both groups (one study, 24 participants; typical RR 1.27, 95% CI 0.26 to 6.28). Physiological parameters of oxygenation (oxygenation index, partial pressure of oxygen in arterial blood (PaO2)) suggested steady improvement after the first dose of sildenafil. None of the included trials identified any clinically important side effects. We rated the quality of evidence as low to very low owing to imprecision related to small sample size and unclear methodological features.
Authors' conclusions
Sildenafil used for treatment of pulmonary hypertension has potential for reducing mortality and improving oxygenation in neonates, especially in resource‐limited settings where iNO is not available. However, large‐scale randomised trials comparing sildenafil versus active controls (other pulmonary vasodilators) and providing follow‐up for survivors are needed to assess the comparative effectiveness and long‐term safety of sildenafil versus other pulmonary vasodilators.
Plain language summary
Sildenafil for pulmonary hypertension in neonates
Review question
Is sildenafil safe and effective in newborn babies with pulmonary hypertension?
Background
When a baby is born, pressure in the blood vessels of the lungs is high, and when normal breathing is established, this pressure starts to fall. In some babies, this transition does not occur and pressure remains high; this does not allow blood to go to the lungs to get adequate oxygen. This situation is called persistent pulmonary hypertension of the neonate (PPHN). Other events can lead to development of high pressure in lung blood vessels that can manifest within a few days after birth. Persistent high pressure in these vessels leads to delivery of less oxygen to all organs of the body. A medication called sildenafil may cause lung blood vessels to relax, allowing improved blood flow and improved delivery of oxygen to all organs.
Study characteristics
We identified five studies that evaluated effects of sildenafil: three studies that compared sildenafil with placebo (no sildenafil); one that compared sildenafil with other medication (magnesium sulphate); and one that used sildenafil in combination with another medicine (nitric oxide). These studies included 166 newborns and were conducted in Colombia, Mexico, Turkey, and Qatar.
Key results
Three studies that compared sildenafil and placebo (no sildenafil) reported that sildenafil reduced the number of deaths. Studies that compared sildenafil against another medication or that used another treatment with sildenafil described no significant reduction in the number of deaths. Sildenafil was more effective than placebo in improving oxygen levels. None of the five included studies reported safety concerns. However, these studies enrolled small numbers of infants, and most were conducted in settings where other treatments were not available. Sildenafil may be useful in settings where other treatment approaches are not available. However, additional studies are needed to compare sildenafil against existing treatment in a resourceful environment to assess its effectiveness and safety.
Quality of evidence
The quality of evidence for reducing mortality or improving respiratory parameters was low owing to the small number of included studies and the small number of babies evaluated. Some of the included studies have methodological issues, resulting in low to very low quality of evidence.
Summary of findings
Background
Description of the condition
Neonatal pulmonary hypertension and persistent pulmonary hypertension of the newborn (PPHN) are terms that can be used interchangeably to describe a neonate who has cyanosis in the first few days of life in the absence of a structural congenital cardiac lesion or haemoglobinopathy (Gersony 1984). The clinical diagnosis of pulmonary hypertension is considered when hypoxaemia is refractory to oxygen therapy or to lung recruitment strategies (partial pressure of oxygen in arterial blood (PaO2) < 55 mmHg despite fraction of inspired oxygen (FiO2) of 1.0) (Roberts 1997; Shah 2004) associated with a preductal to postductal oxygen gradient greater than 20 mmHg (Walsh‐Sukys 2000). Clinicians make the echocardiographic diagnosis of PPHN by demonstrating the presence of extrapulmonary right‐to‐left shunting at the ductal or atrial level in the absence of severe pulmonary parenchymal disease with Doppler evidence of tricuspid regurgitation (Shah 2004; Wessel 1997). During cardiac catheterisation, pulmonary hypertension is defined as pulmonary arterial pressure (PAP) greater than 25 to 30 mmHg (Adatia 2002). The incidence of pulmonary hypertension among newborns has been reported as approximately 2/1000 live births, with a reported mortality rate at various centres in the United States of 4% to 33% (Walsh‐Sukys 2000). Pulmonary hypertension in the neonate can be primary (idiopathic) or can occur secondary to pulmonary parenchymal disease (such as meconium aspiration syndrome, surfactant deficiency, or alveolocapillary dysplasia), severe pulmonary hypoplasia (Adatia 2002; Gersony 1984), polycythaemia, hypoglycaemia, sepsis, or maternal ingestion of prostaglandin inhibitors.
Description of the intervention
By virtue of its selective pulmonary vasodilator effects, inhaled nitric oxide (iNO) is considered the mainstay for treatment of pulmonary hypertension among term or near‐term neonates (Barrington 2010). Approximately 30% of neonates with PPHN fail to respond to iNO (Goldman 1996). For some patients, nitric oxide therapy is associated with rebound pulmonary hypertension when therapy is discontinued, as the result of suppression of endogenous nitric oxide production (Kinsella 2000). Other potential complications include development of methaemoglobinaemia. In addition, iNO is a costly intervention (Subhedar 2002). The potential role of iNO in the treatment of preterm neonates with respiratory insufficiency remains unclear (Finer 2017).
How the intervention might work
Advances in our understanding of the physiology of vasoactive mediators have revealed a high concentration of phosphodiesterases in the pulmonary vasculature (Rabe 1994). Inhibition of phosphodiesterase‐5 leads to increased concentrations of cyclic adenosine monophosphate (AMP) and guanosine monophosphate (GMP) locally, which in turn leads to relaxation of pulmonary vascular smooth muscles (Humbert 2004). Phosphodiesterase‐5 inhibitors include dipyridamole, zaprinast, pentoxifylline, and sildenafil (Travadi 2003). Dipyridamole has a significant systemic vasodilatory effect (Dukarm 1998). Zaprinast and pentoxifylline have not been adequately studied. Sildenafil has been studied in neonatal animal models. A neonatal pig model of pulmonary hypertension induced secondary to meconium aspiration (Shekerdemian 2002) demonstrated marked improvement in pulmonary vascular resistance and cardiac output (without deterioration in systemic oxygenation) one hour after intravenous infusion of sildenafil compared with control. In a separate experiment, Shekerdemian and co‐workers observed improvement in pulmonary vascular resistance; however, improvement was associated with systemic vasodilation and deterioration of oxygenation when sildenafil (0.5 mg/kg) was administered along with 20 ppm of iNO (Shekerdemian 2004). The interaction of sildenafil with other selective pulmonary vasodilators warrants further study.
Sildenafil has been used for treatment of pulmonary hypertension in adults (Kanthapillai 2004; Sastry 2004) in intravenous, oral (Ikeda 2005), and inhaled (Ichinose 2001) forms. Uncontrolled experiments in children showed that sildenafil reduced pulmonary vascular resistance (Abrams 2000; Erickson 2002; Carroll 2003) and improved exercise capacity. Uncontrolled studies on the use of sildenafil in neonates have reported improved pulmonary vascular resistance and survival (Erickson 2002; Kumar 2002). These reports have evoked a mixed reaction from the scientific community (Kumar 2002; Lewin 2002; Oliver 2002; Patole 2002). Marsh 2004 reported severe retinopathy of prematurity following use of sildenafil in a neonate with severe pulmonary hypertension; however, another study did not report this complication (Pierce 2005). Use of sildenafil in adults is suspected to worsen proliferative diabetic retinopathy (Burton 2000; Behn 2001). Therefore, retinal vascular growth must be carefully observed, especially in preterm neonates. Sastry 2004 reported a slightly higher incidence of backache, headache, numbness of feet and hands, and constipation among adults who received sildenafil versus placebo for primary pulmonary hypertension.
Why it is important to do this review
A systematic review of sildenafil for pulmonary hypertension in adults and children identified four eligible studies including 77 participants. Review authors concluded that more studies of adequate size are necessary (Kanthapillai 2004). This review did not include neonates. The disease is more prevalent among neonates and, in most cases, has a different pathophysiology (in neonates, characterised by failure of the natural decrease in pulmonary vascular resistance; in children and adults, occurring as primary disease or secondary to various chronic illnesses such as collagen vascular disease, left heart disease, chronic obstructive pulmonary disease, interstitial disease, or chronic thromboembolic disorders). This review systematically evaluates the use of sildenafil for treatment of pulmonary hypertension in neonates.
Objectives
To assess the efficacy and safety of sildenafil for treatment of pulmonary hypertension in neonates.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised controlled trials of sildenafil for treatment of pulmonary hypertension in neonates. We considered studies that used any route of administration (intravenous, inhaled, or oral), any dose of sildenafil, and any duration of administration. We did not include cross‐over studies owing to frequent resolution of the condition over a short time.
Types of participants
We included in the review both term and preterm infants (at postnatal age < 28 days after reaching 40 weeks' postmenstrual age (PMA)) with primary or secondary pulmonary hypertension. We included studies in which the diagnosis was based on clinical findings with or without echocardiographic confirmation. We excluded patients with known structural heart disease (other than patent foramen ovale or patent ductus arteriosus).
Types of interventions
We included the following interventions.
Sildenafil versus placebo or no treatment.
Sildenafil versus another pulmonary vasodilator.
Sildenafil and another pulmonary vasodilator versus another pulmonary vasodilator or placebo.
Types of outcome measures
Primary outcomes
-
Haemodynamic parameters (absolute values and change from baseline measured after the first dose, after 24 hours, after 30 hours, after 36 hours, after 42 hours, and at the end of treatment)
Pulmonary arterial pressure (PAP) in mmHg
Oxygenation (PaO2) or FiO2 requirement
Cardiac output in L/kg/min
Mean arterial blood pressure in mmHg
All‐cause mortality within the first 28 days of life (neonatal mortality)
Secondary outcomes
Changes in pulmonary vascular resistance index in Woods unit m2 (WUm2) (absolute values and change from baseline measured after first dose, after 24 hours, after 30 hours, after 36 hours, after 42 hours, and at the end of treatment)
Changes in systemic vascular resistance index in WUm2 (absolute values and change from baseline measured after first dose, after 24 hours, after 30 hours, after 36 hours, after 42 hours, and at the end of treatment)
Changes in oxygenation index (OI = PaO2 × FiO2/100) (absolute values and change from baseline measured after first dose, after 24 hours, after 30 hours, after 36 hours, after 42 hours, and at the end of treatment)
Rebound increase in PAP (dichotomous)
Decrease in cardiac output after weaning from sildenafil (dichotomous)
Extracorporeal membrane oxygenation (ECMO) treatment before discharge
All‐cause mortality before discharge
Length of hospitalisation (days)
Retinopathy of prematurity (among very preterm infants at < 32 weeks' gestation), any stage and stage 3 or greater
Intraventricular haemorrhage (any stage and grade 3 or greater)
Neurodevelopmental disability at 18 to 24 months (including cerebral palsy, cognitive impairment, deafness, and blindness)
Clinically important adverse effects reported by study authors (not prespecified)
-
Any other clinically important outcome reported by study authors (not prespecified)
Alveolar‐arterial oxygen difference (A‐a DO2)
Mean airway pressure
For all haemodynamic parameters, we planned to assess the change from baseline at 1, 2, 4, 6, 8, 12, 24, and 48 hours, or at nearest times reported by study authors.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (CNRG) (see the Cochrane Neonatal search strategy for specialized register).
Electronic searches
We applied standard search strategies of the CNRG as outlined in the Cochrane Library. We conducted a comprehensive electronic search including the Cochrane Central Register of Controlled Trials (CENTRAL 2017; Issue 3) in the Cochrane Library; MEDLINE via PubMed (1966 to 18 April 2017); Embase (1980 to 18 April 2017); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to18 April 2017), using the following search terms: (Sildenafil[MeSH] OR sildenafil OR tadalafil OR Viagra OR Phosphodiesterase Inhibitors[MeSH] OR Phosphodiesterase V[MeSH] OR pulmonary vasodilator) AND (hypertension, pulmonary[MeSH] OR PPHN OR hypertension OR persistent fetal circulation syndrome[MeSH] OR rebound OR persistent fetal circulation syndrome), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for full search strategies for each database). We did not apply language restrictions.
Searching other resources
We searched clinical trials registries for ongoing or recently completed trials ((clinicaltrials.gov); the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/); the ISRCTN Registry)). We excluded the following types of articles: letters, editorials/commentaries, reviews, lectures, and commentaries. We manually searched the reference lists of full‐text versions (RCTs and reviews) identified during the primary literature search.
Data collection and analysis
We used standard review methods of the CNRG to select studies for inclusion, to extract study data, and to assess the methodological quality of identified studies.
Selection of studies
We assessed for inclusion all published articles identified as potentially relevant by the literature search. We resolved discrepancies regarding inclusion/exclusion of studies by consensus.
Data extraction and management
Each review author extracted data separately using predesigned data abstraction forms. Review authors compared results and resolved differences. One review author entered data into RevMan 5.3 (RevMan 2014); the other review authors cross‐checked the printout against data entered into abstraction forms, and corrected errors by consensus.
If relevant articles were identified, review authors obtained data from study authors when published data provided inadequate information for the review, and when relevant data could not be abstracted.
Assessment of risk of bias in included studies
Three review authors (LEK, AO, PSS) used the Cochrane 'Risk of bias' tool (Higgins 2011) to independently assess risk of bias (low, high, or unclear) of all included trials for the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion. See Appendix 2 for a detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses in accordance with recommendations of the CNRG, using RevMan 5.3 software (RevMan 2014). Treatment effect estimates included typical risk ratio (RR), typical risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB), or number needed to treat for an additional harmful outcome (NNTH) for dichotomous outcomes, and mean difference (MD) for continuous outcomes. We reported all estimates of treatment effects with 95% confidence intervals (CIs).
Unit of analysis issues
The unit of analysis in included trials was the individual randomised neonate. We planned to include cluster‐RCTs, if available. We planned to analyse cluster‐RCTs using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), along with an estimate of the intracluster correlation coefficient.
Dealing with missing data
As needed, we requested from corresponding study authors additional information regarding study design or outcome measures. We planned to include in an intention‐to‐treat analysis all infants for whom included studies reported outcomes.
Assessment of heterogeneity
Heterogeneity testing including the I2 test, which we performed to assess the appropriateness of pooling data by using RevMan 5.3 software. We roughly categorised degree of heterogeneity according to the recommendations of Higgins and coworkers (Higgins 2011). We followed CNRG recommendations by using the following criteria to describe percentage of heterogeneity: < 25% no heterogeneity, ≥ 25% to 49% low heterogeneity, ≥ 50% to 74% moderate heterogeneity, and ≥ 75% high heterogeneity.
We performed planned subgroup analyses according to the criteria listed below.
Assessment of reporting biases
We assessed reporting and publication biases by examining the degree of asymmetry on a funnel plot, using RevMan 5.3 software, when a comparison includes at least 10 clinical trials.
Data synthesis
We performed meta‐analyses using Review Manager software (RevMan 2014) supplied by the Cochrane Collaboration. For estimates of typical RR and RD, we used the Mantel‐Haenszel method; for measured quantities, the inverse variance method; and for all meta‐analyses, we used the fixed‐effect model.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: respiratory parameters (PaO2, OI, mean PAP), mortality; and length of hospital stay.
Two review authors independently assessed the quality of evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded evidence one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of evidence.
The GRADE approach yields an assessment of the quality of a body of evidence according to one of four grades.
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned to perform a priori subgroup analyses based on the following.
Gestational age (term and preterm ‐ defined as < 37 weeks' gestation).
Method of diagnosis of pulmonary hypertension (clinical or echocardiographic).
Route of administration of sildenafil (oral vs intravenous vs inhaled).
Primary or secondary cause of pulmonary hypertension.
-
Comparison 1: sildenafil versus control
-
Category 1: type of control intervention
Subgroups: A. Sildenafil versus placebo. B. Sildenafil versus no treatment
-
Category 2: gestational age
Subgroups: 1. Preterm. 2. Term
-
-
Comparison 2: sildenafil versus other pulmonary vasodilator
-
Category 1: type of control intervention
Subgroups: A. Sildenafil versus inhaled nitric oxide. B. Sildenafil versus other pulmonary vasodilator
-
Category 2: gestational age
Subgroups 1. Preterm. 2. Term
-
-
Comparison 3: sildenafil and other pulmonary vasodilator versus other pulmonary vasodilator
-
Category 1: type of control intervention
Subgroups: A. Sildenafil and other pulmonary vasodilator versus inhaled nitric oxide. B. Sildenafil and nitric oxide versus other pulmonary vasodilator. C. Sildenafil and nitric oxide versus placebo/no treatment
-
Category 2: gestational age
Subgroups 1. Preterm. 2. Term
-
Post hoc comparison modification
After performing a revised literature search, we made a post hoc modification. Comparison 3 now includes "sildenafil plus other vasodilator (iNO) versus control (placebo) plus iNO" to include studies that used sildenafil as adjuvant therapy (both groups received nitric oxide) and compared treatment versus a placebo control.
Sensitivity analysis
If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses.
Results
Description of studies
In this update of our review, we evaluated 1674 citations (Figure 1). Review of 77 articles in full text yielded two additional studies that were eligible for inclusion (Al Omar 2016; Uslu 2011) and three studies (Baquero 2006; Herrera 2006; Vargas‐Origel 2010) that were included in a previous version of this review (Shah 2011). We excluded a total of six studies (Kahveci 2014; König 2014; Namachivayam 2006; Sayed 2015; Steinhorn 2009; Stocker 2003), we kept four studies (NCT01757782; NCT01373749; Soliz 2009; Alipour 2017) in awaiting assessment status, and we identified one ongoing study (NCT01720524).
1.
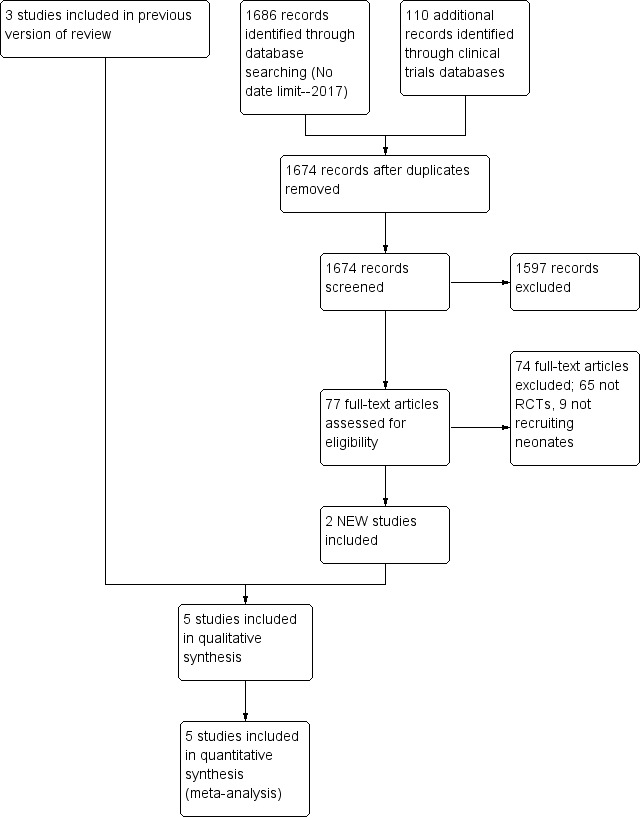
Study flow diagram: review update.
We excluded Kahveci 2014, as it was a retrospective comparison of sildenafil and iloprost. König 2014 compared sildenafil versus placebo, but we excluded this study because it included neonates with bronchopulmonary dysplasia, and some patients may not have received a diagnosis of pulmonary hypertension. We excluded Namachivayam 2006 because the study included patients from 0.1 year of age (potentially > 1 month), and most (> 80%) participants had congenital heart disease. Sayed 2015 performed a single‐arm uncontrolled evaluation of sildenafil. Steinhorn 2009 was an open‐label dose‐escalating study. Stocker 2003 studied infants following cardiac surgery. We identified NCT01757782 and NCT01373749 through ClinicalTrials.Gov and placed these studies in the awaiting status category, as published results were not yet available. We excluded Soliz 2009 because the abstract was presented at a scientific meeting but information was inadequate to distinguish it as a separate study. We placed Alipour 2017 in the awaiting further information classification, as review authors did not present clear details of when outcome measures were assessed.
Our previous report included Herrera 2006 in abstract form; in the current version of our review, we have included this study as a full report published in Spanish. For Baquero 2006, we requested data regarding haemodynamic measurements taken before and during the intervention period, as well as incidence of rebound hypoxaemia, incidence of intraventricular haemorrhage, length of stay, and number of infants needing ECMO. Study authors provided data on FiO2 before the start of therapy, mean arterial BP before the start of therapy and at 36 hours after therapy among survivors, and the number of infants with grade 3 or 4 intraventricular haemorrhage. In addition, study authors reported follow‐up data in abstract form from three infants (one neonatal death, one death at five months of age, and one loss to follow‐up) (Baquero 2006). Vargas‐Origel 2010 published data on a total of 53 study participants. At first, investigators enrolled 20 participants in the placebo group and 20 in the sildenafil group. After 40 participants had been enrolled, the institutional ethics board prohibited use of placebo, and investigators thereafter randomised study participants to sildenafil versus nitric oxide. The published manuscript provides data on 33 participants in the sildenafil group and 20 in the placebo group. We contacted study authors and requested only data from comparison of the first 40 participants (20 in the sildenafil group and 20 in the placebo group). Newly added studies include Al Omar 2016 and Uslu 2011. Study authors for Al Omar 2016 provided clarification regarding mortality outcomes data, demographic data (including birth weight standard deviation), and OI mean values.
For additional details, see Characteristics of included studies.
Baquero 2006
Setting: single‐centre pilot randomised double‐blind controlled trial at a regional neonatal intensive care unit (NICU) in Colombia. In this unit, iNO, high‐frequency ventilation, and ECMO were not available
Objective: to evaluate the feasibility of using oral sildenafil and to determine the effects of oral sildenafil on oxygenation in term and near‐term infants with PPHN
Population: term and near‐term (≥ 35.5 weeks' gestation) infants with severe hypoxaemia (need for mechanical ventilation with OI ≥ 40) and echocardiographically confirmed PPHN (presence of right‐to‐left shunt and estimated PAP ≥ 40 mmHg)
Intervention: oral sildenafil or placebo (diluent). The solution for sildenafil was prepared by crushing a 50 mg tablet of sildenafil in Orabase (diluent) to achieve a concentration of 2 mg/mL. The protocol for dosing included (1) first dose of 1 mg/kg (0.5 mL/kg) within 30 minutes of randomisation, (2) dosing every six hours, (3) potential doubling of the dose (1 mL/kg) if OI did not improve and blood pressure remained stable, and (4) discontinuation of treatment if OI was < 20, or if participant had received eight doses. Other aspects of care management remained the same in both study arms
Outcomes: mortality; changes in OI, mortality, PaO2, and mean arterial blood pressure. OI was not reported for two neonates who improved to meet study exit criteria
Recruitment: eligibility criteria met by 22 patients. Of these, two patients died (before enrolment in the study), four parents refused consent, and three parents were not approached for consent. Researchers enrolled a total of 13 participants (six in the placebo group and seven in the treatment group). The institutional review board terminated the study owing to the death of six participants enrolled in the study
Follow‐up: data reported in abstract form on four survivors in the sildenafil group assessed at 18 months of age
Herrera 2006
Setting: single‐centre randomised controlled study in Mexico. The centre did not have the facility needed to administer iNO
Objective: to compare the efficacy of oral sildenafil therapy versus conventional therapy in term neonates with PPHN at a centre without iNO
Population: term neonates with a diagnosis of PPHN and OI > 25
Intervention: participants randomised to sildenafil (n = 13) 2 mg/kg via orogastric tube or distilled water (n = 11). Total duration of therapy was 72 hours. Sildenafil was administered at 2 mg/kg/dose via orogastric tube every six hours
Outcomes: mortality; changes in OI, PaO2, mean arterial blood pressure, PaCO2, and intubation days were compared
Recruitment: 13 of 24 recruited neonates randomly selected to receive sildenafil. Outcomes data were reported on all 24 enrolled neonates
Vargas‐Origel 2010
Setting: single‐centre randomised controlled trial in Mexico. In this unit, iNO was not available at the start of the trial. Inhaled nitric oxide became available during the study, and the ethics board prohibited use of placebo after 40 neonates had been randomised
Objective: to evaluate the efficacy of oral sildenafil in newborns with PPHN
Population: term and post‐term infants with PPHN diagnosed within first 48 hours who had OI > 20
Intervention: participants given either oral sildenafil or placebo (normal saline). Solution for sildenafil was prepared by crushing a 50‐mg tablet of sildenafil in 20 mL of water. Protocol for dosing was 3 mg/kg/dose every six hours via nasogastric tube. Treatment was continued until OI was < 10. Other aspects of management of infant care remained the same in both arms of the study
Outcomes: data on OI, mean airway pressure, mean arterial pressure, PaO2, and mortality for first 40 participants randomised to sildenafil (20) and placebo (20) provided by study authors
Recruitment: 51 enrolled neonates. Following an ethics board decision, only the first 40 participants were randomised to placebo or sildenafil. Full‐text article presents data on all enrolled neonates; however, study authors provided data from the first 40 randomised neonates for inclusion in this review
Uslu 2011
Setting: single‐centre randomised double‐blind controlled trial at a regional referral NICU in Turkey. In this unit, iNO, high‐frequency ventilation, and ECMO were not available
Objective: to determine and compare clinical efficacy and side effects of intravenous MgSO4 and oral sildenafil therapy in newborns with PPHN
Population: term and near‐term (35 to 42 weeks' gestation) infants with hypoxaemic respiratory failure associated with PPHN. Inclusion criteria included PAP ≥ 40 mmHg, OI ≥ 30, and the need for mechanical ventilation. Researchers excluded neonates with congenital heart disease, suspicion of sepsis (and other anomalies), gastric intolerance, or gastric bleeding
Intervention: oral sildenafil (0.5 mg/kg every 6 hours) or IV MgSO4 (200 mg/kg loading dose, followed by a maintenance dose of 20 mg/kg/h)
Outcomes: primary outcome was time of adequate clinical response, defined as a decrease in PAP to < 20 mmHg and OI to < 15. Secondary outcomes included duration of mechanical ventilation, support of inotropic agent, mortality rate, and adverse events
Recruitment: eligibility criteria met by 77 patients, 72 of whom were randomised following parental consent. A total of 36 participants were given oral sildenafil, and 36 were treated with MgSO4. Complete data were collected on 31/36 infants in the sildenafil group (3 gastric bleeding, 2 incomplete records) and on 34/36 in the MgSO4 group (2 incomplete records)
Al Omar 2016
Setting: single‐centre randomised clinical trial conducted at an NICU in Qatar over three years (September 2011 to September 2014)
Objectives: to evaluate the feasibility and effectiveness of adding sildenafil as adjuvant therapy together with iNO when treating newborns with PPHN and/or hypoxaemic respiratory failure; to assess whether this approach could improve oxygenation, decrease time on mechanical ventilation, and prevent rebound hypoxaemic episodes
Population: newborn infants born at gestational age of 34 weeks or greater who at less than 48 hours of age had an OI greater than or equal to 20 mmHg; radiological, clinical, and biochemical evidence of acute hypoxaemic respiratory failure; surfactant therapy established where indicated and an arterial line
Intervention: participants given either oral sildenafil (2 mg/kg/dose every 6 hours) or placebo via nasogastric tube. Starting dose of iNO was 20ppm in both groups; weaning from iNO was carried out at 2% to 4% per hour
Outcomes: primary outcome was OI absolute values and change from baseline measured after first dose, every 6 hours for 7 days, or until the infant was extubated. Improvement in OI was defined as a decrease of 10% from the previously calculated OI value. Secondary outcome measures included haemodynamic parameters, PAP (measured by echocardiography), practicality of administration, gastric tolerance, hypotension, renal function, liver function, and length of stay. Study authors provided data on all‐cause mortality within the first 28 days of life. We were unable to obtain information regarding the variability of reported means for OI and mean PAP
Recruitment: eligibility criteria met by a total of 51 infants. Following exclusions for hypoxic‐ischaemic encephalopathy (HIE), congenital diaphragmatic hernia (CDH), and surfactant protein B deficiency, and four refusals to participate, 24 cases (13 sildenafil, 11 placebo) were included.
Results of the search
We have provided results of the search in Figure 1. We screened a total of 1674 publication records for inclusion, of which we retrieved 77 in full text.
Included studies
A previous version of this review (Shah 2011) included three studies (Baquero 2006; Herrera 2006; Vargas‐Origel 2010) that evaluated sildenafil in neonates with PPHN. We identified two new studies that met our inclusion criteria (Al Omar 2016; Uslu 2011).
Excluded studies
We excluded 74 full‐text articles that did not meet our inclusion criteria; 65 were not randomised clinical trials, and 9 did not recruit neonates.
Risk of bias in included studies
We have presented details on risk of bias of included studies in Figure 2. Overall, these studies were at low to high risk of bias, which we have summarised in Figure 3.
2.
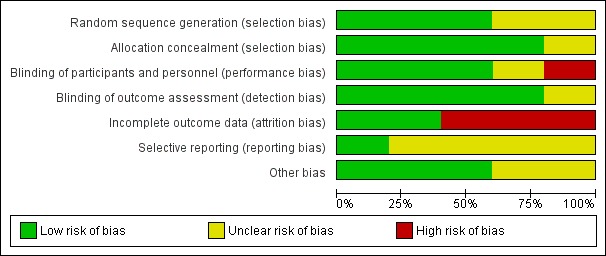
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
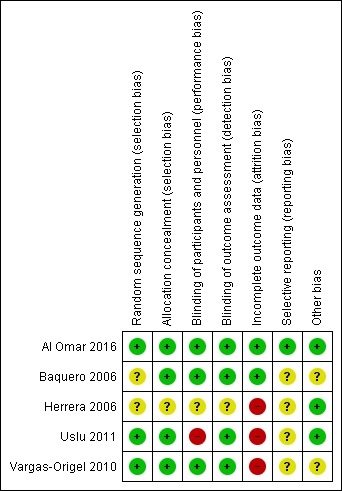
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Baquero 2006 was a randomised double‐blind placebo‐controlled trial. Investigators performed randomisation using presealed envelopes. The pharmacy prepared the solution in identical containers, and bedside clinicians were unaware of group assignment. Outcome assessment appears to be masked, as no one was aware of treatment allocation. Researchers terminated the study early on the basis of pre‐set criteria (which included hypotension, gastric intolerance or bleeding, renal failure, and death in six infants). Pre‐set criteria for discontinuation of dosing included OI < 20 or administration of a maximum of eight doses. Investigators used analysis of variance for repeated comparison of data on OI, blood pressure, and oxygen saturation. We assigned this study unclear risk of reporting bias, as we found no registration data to verify selective outcome reporting.
Herrera 2006 was a randomised placebo‐controlled trial that did not report the randomisation method used. Study authors described allocation as blinded. Investigators compared data using descriptive statistics and Student's t‐test. Data analysis excluded neonates who had been transferred. As we found no registration data from this trial to verify selective outcome reporting, we assessed risk of bias as unclear.
Vargas‐Origel 2010 was a randomised double‐blind placebo‐controlled trial. Two nurses who were not involved in the study generated randomisation using a random number table (information provided by study authors). The pharmacy prepared the solution, and bedside clinicians were unaware of group assignment. Outcome assessment was masked, as nurses were unaware of treatment allocation.
Uslu 2011 was a randomised controlled trial that evaluated sildenafil and magnesium sulphate (MgSO4; control). An independent researcher prepared a computer‐generated randomisation table and concealed allocation. Neonatologists could not be blinded owing to the nature of the interventions (orogastric vs intravenous). Study staff including nurses and data collectors were blinded. As this trial was not registered, we could not evaluate selective outcome reporting. We did not perform intention‐to‐treat analysis and noted that data were missing for seven neonates (five in the treatment group and two in the control group).
Al Omar 2016 was a randomised clinical trial that evaluated sildenafil and placebo in addition to "standard care", which included iNO. Investigators performed randomisation using an online sequence and concealed allocation using opaque envelopes distributed by the pharmacy (information provided by study authors). The treatment team was blinded to the study arm and reported outcomes data on all randomised neonates. Investigators reported all registered outcomes.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Sildenafil compared with placebo for pulmonary hypertension in neonates.
| Sildenafil compared with placebo for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates Setting: neonatal intensive care unit Intervention: sildenafil Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with sildenafil | |||||
| PaO2 in mmHg (absolute values) After 24‐25 hours | Mean PaO2 in mmHg (absolute values) After 24 to 25 hours = 0 | MD 15.31 higher (6.49 higher to 24.13 higher) | ‐ | 57 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) |
| Change in oxygenation index After 24 hours of treatment | Mean change in oxygenation index After 24 hours = 0 | MD 38.79 lower (56.97 lower to 20.61 lower) | ‐ | 12 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) |
| All‐cause mortality | Study population | RR 0.20 (0.07 to 0.56) | 77 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) | |
| 432 per 1000 | 77 per 1000 (22 to 238) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aImprecise due to small sample size
bRisk of bias due to unclear randomisation allocation and lack of clinical trial registration
Summary of findings 2. Sildenafil compared with active control for pulmonary hypertension in neonates.
| Sildenafil compared with active control for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates Setting: neonatal intensive care unit Intervention: sildenafil Comparison: magnesium sulphate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with magnesium sulphate | Risk with sildenafil | |||||
| All‐cause mortality | Study population | RR 0.55 (0.05 to 5.75) | 65 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b | Evidence was downgraded due to very serious imprecision, as results from this single study have not been replicated and risk of bias is evident in study design (missing data) | |
| 59 per 1000 | 32 per 1000 (3 to 338) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aImprecise due to small sample size; only one included study
bRisk of bias due to missing data (not analysed as intent to treat)
Summary of findings 3. Sildenafil plus iNO compared with placebo plus iNO for pulmonary hypertension in neonates.
| Sildenafil plus iNO compared with placebo plus iNO for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates Setting: neonatal intensive care unit Intervention: sildenafil plus iNO Comparison: placebo plus iNO | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo plus iNO | Risk with sildenafil plus iNO | |||||
| All‐cause mortality | Study population | RR 1.27 (0.26 to 6.28) | 24 (1 RCT) | ⊕⊕⊝⊝ LOWa | Evidence was downgraded due to imprecision . | |
| 182 per 1000 | 231 per 1000 (38 to 690) | |||||
| Length of stay in hospital (days) | Mean length of stay in hospital was 17.81 days. | MD 5.39 higher (5.31 lower to 16.09 higher) | ‐ | 24 (1 RCT) | ⊕⊕⊝⊝ LOWa | Evidence was downgraded due to imprecision . |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aImprecise due to very small sample size; only one included study
Sildenafil versus placebo (Comparison 1)
Three studies (Baquero 2006; Herrera 2006; Vargas‐Origel 2010) compared sildenafil versus placebo in neonates with persistent pulmonary hypertension.
Primary outcomes
Haemodynamic parameters (absolute values and change from baseline)
Pulmonary arterial pressure (in mmHg) (Outcome 1.1)
Only one study (Vargas‐Origel 2010) measured PAP at baseline and revealed no significant differences between sildenafil and control (mean difference (MD) 1.10, 95% confidence interval (CI) ‐7.68 to 9.88; heterogeneity ‐ not applicable). Changes in PAP were not reported at a later stage (Analysis 1.1).
1.1. Analysis.
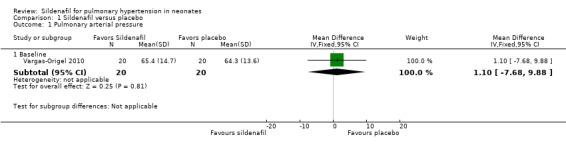
Comparison 1 Sildenafil versus placebo, Outcome 1 Pulmonary arterial pressure.
Oxygenation (PaO2 in mmHg) (Outcome 1.2)
Baquero 2006 reported that all participants in treatment and placebo groups required 100% oxygen before the start of therapy. Baquero 2006 reported that oxygen saturation (SaO2) steadily improved in the sildenafil group, was statistically significantly different from baseline in the sildenafil group at 12 hours (P < 0.03), and was significantly higher at 24 and 36 hours in the sildenafil group compared with the placebo group (P < 0.03).
The other two studies (Herrera 2006; Vargas‐Origel 2010) reported that PaO2 at baseline was higher in the sildenafil group than in the placebo group (MD 8.06 mmHg, 95% CI 1.58 to 14.54 mmHg; two studies, 64 participants; I2 = 43% ‐ low). This trend continued in both studies over the course of reporting: After the first dose (MD 11.09 mmHg, 95% CI 1.65 to 20.52 mmHg; two studies, 64 participants; I2 = 0% ‐ none), after 6 to 7 hours (MD 14.30 mmHg, 95% CI 5.25 to 23.34 mmHg; two studies, 63 participants; I2 = 0% ‐ none), and after 24 to 25 hours (MD 15.31 mmHg, 95% CI 6.49 to 24.13 mmHg; two studies, 58 participants; I2 = 0% ‐ none), PaO2 was higher in the sildenafil group than in the placebo group. Differences in PaO2 increased over time during the first 24 hours. Herrera 2006 reported results at 72 hours (MD 20.98, 95% CI 14.81 to 27.15; one study, 24 participants; I2 = not applicable), showing a significant increase in PaO2 in the sildenafil group.
Cardiac output in L/kg/min
No studies reported changes in cardiac output.
Mean arterial blood pressure (in mmHg) (Outcome 1.3)
One study (Baquero 2006) reported no statistically significant differences in mean arterial blood pressure between sildenafil and placebo groups and did not provide numerical data. Baquero 2006 and Vargas‐Origel 2010 provided data on mean arterial blood pressure before the start of therapy in all participants and at the end of therapy among survivors. Mean arterial blood pressure was higher at baseline in the sildenafil group than in the placebo group (MD 5.65, 95% CI 2.69 to 8.61 mmHg; two studies, 53 participants; I2 = 56% ‐ moderate). Values for mean arterial blood pressure after completion of therapy were available for only one of the survivors (Baquero 2006) in the placebo group (45.3 mmHg), whereas values were provided for six survivors in the sildenafil group: (mean + SD) 43.7 + 3.3 mmHg. Vargas‐Origel 2010 reported significantly higher mean arterial pressure in the sildenafil group than in the placebo group at completion of therapy (22.70 mmHg, 95% CI 1.23 to 44.17 mmHg; 40 participants; heterogeneity estimate ‐ not applicable) (Analysis 1.3).
1.3. Analysis.
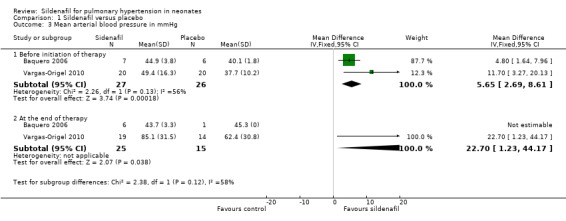
Comparison 1 Sildenafil versus placebo, Outcome 3 Mean arterial blood pressure in mmHg.
All‐cause mortality within first 28 days of life (Outcome 1.4)
All three studies that evaluated sildenafil alone reported data on mortality, showing a statistically significant reduction in mortality rate for the sildenafil group compared with the placebo group (RR 0.20, 95% CI 0.07 to 0.56; RD ‐0.36, 95% CI ‐0.53 to ‐0.18; number needed to treat for an additional beneficial outcome (NNTB) 3, 95% CI 2 to 6; three studies, 77 participants; I2 = 39% ‐ low) (Analysis 1.4).
1.4. Analysis.
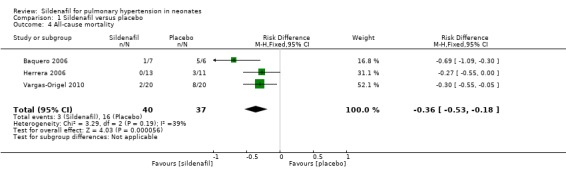
Comparison 1 Sildenafil versus placebo, Outcome 4 All‐cause mortality.
Secondary outcomes
Changes in pulmonary or systemic vascular resistance index in WUm2 (absolute values and change from baseline)
None of the three included studies that performed this comparison reported these data.
Changes in oxygenation index (absolute values and change from baseline)
Baquero 2006 reported these data for individual participants in a table format. We used these data to calculate OI for this study.
Oxygenation index (absolute values) (Outcome 1.5)
At baseline: Data show no statistically significant differences in OI at baseline between groups (MD ‐0.74, 95% CI ‐8.11 to 6.64; three studies, 77 participants; I2 = 28% ‐ low).
After administration of first dose: Results show a reduction in OI in the sildenafil alone group compared with the placebo group (MD ‐12.53, 95% CI ‐18.60 to ‐6.47; three studies, 77 participants; I2 = 27% ‐ low).
After 6 to 7 hours of treatment: Data show a statistically significant reduction in OI in the sildenafil alone group compared with the placebo group after 6 to 7 hours of treatment (MD ‐20.07, 95% CI ‐26.12 to ‐14.02; two studies, 63 participants; I2 = 0% ‐ none).
After 24 to 25 hours of treatment: Results show a statistically significant reduction in OI in the sildenafil alone group compared with the placebo group after 24 to 25 hours of treatment (MD ‐19.15, 95% CI ‐24.52 to ‐13.77; three studies, 69 participants; I2 = 0% ‐ none).
After administration of intervention for 30 hours: Data show a statistically significant reduction in OI in the sildenafil alone group compared with the placebo group after 30 hours of treatment (MD ‐45.46, 95% CI ‐61.87 to ‐29.05; one study, 11 participants; heterogeneity estimate ‐ not applicable).
After administration of intervention for 36 hours (completion of therapy): Results show a statistically significant reduction in OI in the sildenafil group compared with the placebo group after 36 hours of treatment (MD ‐32, 95% CI ‐45.74 to ‐17.76; one study, eight participants; heterogeneity estimates ‐ not applicable).
After 72 hours: Data show a statistically significant reduction in OI in the sildenafil alone group compared with the placebo group after 72 hours of treatment (MD ‐19, 95% CI ‐23.42 to ‐15.52; one study, 24 participants; heterogeneity estimates ‐ not applicable) (Analysis 1.5).
1.5. Analysis.
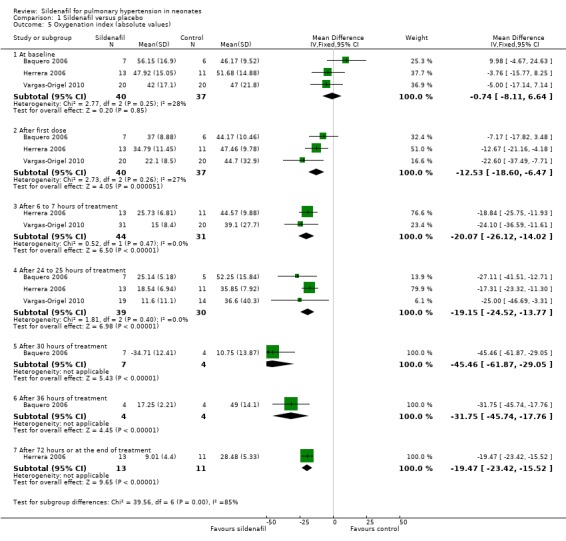
Comparison 1 Sildenafil versus placebo, Outcome 5 Oxygenation index (absolute values).
Changes in oxygenation index (Outcome 1.6)
We calculated these data from individual participant data provided in the original manuscript (Baquero 2006). After administration of first dose: Results show a statistically significant reduction in OI two hours after administration of the first dose of sildenafil alone compared with placebo (MD ‐17.14, 95% CI ‐27.75 to ‐6.53; one study, 13 participants; heterogeneity estimates ‐ not applicable).
After administration of intervention for 24 hours: Data show a statistically significant reduction in OI after administration of five doses of sildenafil alone (at 24 hours after administration) compared with placebo (MD ‐38.79, 95% CI ‐56.97 to ‐20.61; one study, 12 participants; heterogeneity estimates ‐ not applicable).
After administration of intervention for 30 hours: Results show a statistically significant reduction in OI after administration of six doses of sildenafil alone (at 30 hours after administration) compared with placebo (MD ‐33.08, 95% CI ‐50.85 to ‐15.31; one study, 11 participants; heterogeneity estimates ‐ not applicable).
After administration of intervention for 36 hours (completion of therapy): Data show a statistically significant reduction in OI after administration of seven doses of sildenafil alone compared with placebo (MD ‐44.75, 95% CI ‐65.55 to ‐23.95; one study, eight participants; heterogeneity estimates ‐ not applicable). By 42 hours, one participant in the sildenafil group and two participants in the control group had died. Two participants in the control group had significantly reduced OI before the last dose and were not given the last dose (according to prespecified criteria).
Herrera 2006 reported that OI improved within the first hour of administration (Analysis 1.6).
1.6. Analysis.
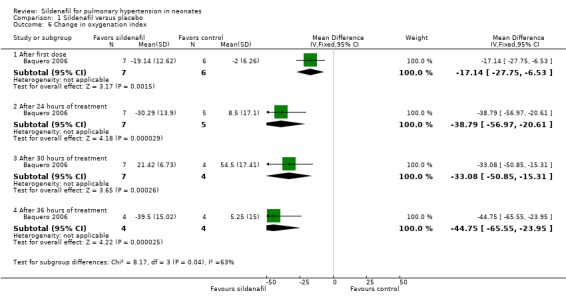
Comparison 1 Sildenafil versus placebo, Outcome 6 Change in oxygenation index.
Rebound increase in PAP or decrease in cardiac output after weaning from sildenafil (dichotomous)
Results show no evidence of rebound hypoxaemia in two participants for whom sildenafil was discontinued because of OI < 20 (Baquero 2006).
Decrease in cardiac output after weaning from sildenafil and the need for extracorporeal membrane oxygenation (ECMO) before discharge
No studies reported these outcomes.
Mortality before discharge
Investigators reported no additional mortality outside of the first 28 days.
Retinopathy of prematurity (among preterm infants at < 32 weeks' gestation)
None of the studies (Baquero 2006; Herrera 2006; Vargas‐Origel 2010) that compared sildenafil versus placebo enrolled preterm infants at risk for retinopathy of prematurity.
Intraventricular haemorrhage
Baquero 2006 reported no grade 3 or 4 intraventricular haemorrhage in any of the infants in either group.
Neurodevelopmental disability at 18 to 24 months (including cerebral palsy, cognitive impairment, deafness, and blindness)
Baquero 2006 reported data on neurodevelopmental follow‐up in abstract form. Investigators assessed only four out of six survivors in the sildenafil alone group at 18 months. One participant in the sildenafil group died during the neonatal period, one died at five months of age, and one was lost to follow‐up. All four participants had a normal neurological examination (Gessel scale of 100, 100, 100, and 111 points). All had normal magnetic resonance imaging (MRI), evoked potential, and electroencephalography (EEG) reading. Their growth parameters (weight, height, and head circumference) were within normal limits.
Any clinically important outcomes reported by study authors (not prespecified)
Alveolar‐arterial oxygen difference (A‐a DO2) (Outcome 1.7)
Two studies evaluating sildenafil alone reported on this outcome (Herrera 2006; Vargas‐Origel 2010).
At baseline: A‐a DO2 was not significantly different (MD 0.99, 95% CI ‐11.54 to 13.51; two studies, 64 participants; I2 = 0% ‐ none).
At 6 to 7 hours of age: A‐a DO2 was not significantly different (MD 0.01, 95% CI ‐27.72 to 27.74; one study, 24 participants; heterogeneity estimates ‐ not applicable).
At 24 to 25 hours of age: A‐a DO2 was not significantly different (MD 1.59, 95% CI ‐18.98 to 22.16; two studies, 57 participants; I2 = 74% ‐ moderate).
At 72 hours: A‐aDO2 was significantly lower in the sildenafil alone group (MD ‐18.34, 95% CI ‐26.59 to ‐10.09; one study, 24 participants; heterogeneity estimates ‐ not applicable) (Analysis 1.7).
1.7. Analysis.
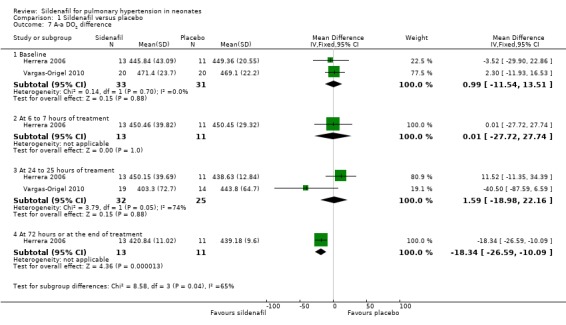
Comparison 1 Sildenafil versus placebo, Outcome 7 A‐a DO2 difference.
Mean airway pressure (Outcome 1.8)
Two studies evaluating sildenafil alone reported on this outcome (Herrera 2006; Vargas‐Origel 2010).
At baseline: Mean airway pressure was lower in the sildenafil alone group than in the placebo group (MD ‐2.09, 95% CI ‐3.30 to ‐0.88 cm of H2O; two studies, 64 participants; I2 = 0% ‐ none).
At 6 to 7 hours after administration: Mean airway pressure was significantly lower in the sildenafil alone group than in the placebo group (MD ‐5.94, 95% CI ‐7.36 to ‐4.52 cm of H2O; two studies, 64 participants; I2 = 35% ‐ low).
At 24 to 25 hours after administration: Mean airway pressure was significantly lower in the sildenafil alone group than in the placebo group (MD ‐6.64, 95% CI ‐8.49 to ‐4.80 cm of H2O; two studies, 57 participants; I2 = 0% ‐ none).
At 72 hours after administration: Mean airway pressure was significantly lower in the sildenafil group than in the placebo group (MD ‐8.58, 95% CI ‐10.37 to ‐6.79 cm of H2O; one study, 24 participants; heterogeneity estimate ‐ not applicable) (Analysis 1.8).
1.8. Analysis.
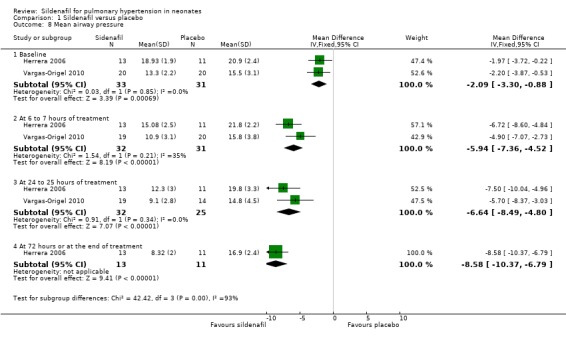
Comparison 1 Sildenafil versus placebo, Outcome 8 Mean airway pressure.
Sildenafil versus active control (Comparison 2)
One study (Uslu 2011) evaluated sildenafil alone against an active control (MgSO4).
Primary outcomes
Haemodynamic parameters (absolute values and change from baseline)
Pulmonary arterial pressure in mmHg
Investigators reported these data in graphical format and did not provide mean and variance measures. Uslu 2011 reported PAP absolute values that were significantly lower with sildenafil than with MgSO4 on day 1 (P = 0.001), on day 2 (P = 0.0001), and on the third day following treatment (P = 0.007).
Oxygenation (PaO2) or FiO2 requirement, cardiac output (L/kg/min), and mean arterial blood pressure (mmHg)
Uslu 2011 did not report changes in oxygenation, cardiac output, and mean arterial blood pressure.
All‐cause mortality (Outcome 2.1)
Data show no differences in mortality rate between sildenafil and MgSO4 (RR 0.55, 95% CI 0.05 to 5.75; RD ‐0.03; 95% CI ‐0.13 to 0.07; one study, 65 participants; heterogeneity estimate ‐ not applicable) (Analysis 2.1).
2.1. Analysis.
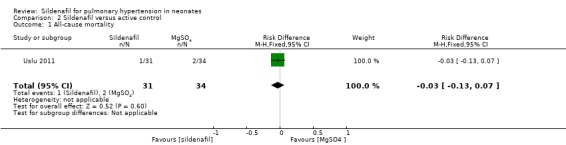
Comparison 2 Sildenafil versus active control, Outcome 1 All‐cause mortality.
Secondary outcomes
Oxygenation index
Researchers reported these data in graphical format and did not provide mean and variance measures. Uslu 2011 reported OI values that were significantly lower with sildenafil than with MgSO4 at 12 hours (P = 0.007), at 24 hours (P = 0.005), at 36 hours (P = 0.001), at 48 hours (P = 0.009), and at 60 hours (P = 0.01) after treatment. .
Time to adequate response (Outcome 2.2)
Data show no significant difference in the number of days to reach an adequate response between sildenafil and MgSO4 groups (MD ‐0.60 days, 95% CI ‐4.20 to 3.00; one study, 65 participants; heterogeneity estimate ‐ not applicable) (Analysis 2.2).
2.2. Analysis.
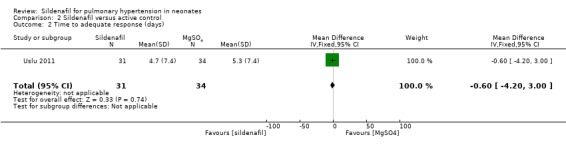
Comparison 2 Sildenafil versus active control, Outcome 2 Time to adequate response (days).
Duration of ventilation (Outcome 2.3)
Researchers reported no significant differences in the number of days on ventilation between sildenafil and MgSO4 groups (MD ‐1.30 days, 95% CI ‐4.54 to 1.94; one study, 65 participants; heterogeneity estimate ‐ not applicable) (Analysis 2.3).
2.3. Analysis.
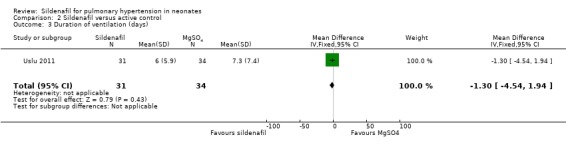
Comparison 2 Sildenafil versus active control, Outcome 3 Duration of ventilation (days).
Use of an inotropic agent (Outcome 2.4)
Despite similar numbers of neonates requiring inotropic support at baseline (sildenafil 9.1% (3/31) vs MgSO4 11.8% (4/34)), results show that significantly fewer neonates were receiving inotropic agents in the sildenafil group than in the MgSO4 group (RR 0.55, 95% CI 0.36 to 0.83; RD ‐0.37, 95% CI ‐0.59 to ‐0.15; NNTB 3, 95% CI 2 to 8; one study, 65 participants; heterogeneity estimate ‐ not applicable; P < 0.0008) (Analysis 2.4).
2.4. Analysis.
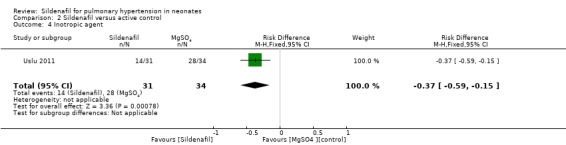
Comparison 2 Sildenafil versus active control, Outcome 4 Inotropic agent.
Retinopathy of prematurity (among preterm infants at < 32 weeks' gestation)
Uslu 2011 did not include extremely preterm infants at risk for retinopathy of prematurity.
Sildenafil plus iNO versus placebo plus iNO (Comparison 3)
One study (Al Omar 2016) evaluated sildenafil used as adjuvant therapy with nitric oxide against placebo with nitric oxide.
Primary outcomes
Haemodynamic parameters (absolute values and change from baseline) including PAP in mmHg, oxygenation (PaO2) or FiO2 requirement, cardiac output (L/kg/min), and mean arterial blood pressure (mmHg)
Study authors reported changes in mean PAP in graphical format and did not provide mean and variance measures for meta‐analysis. They did not report changes in PaO2 or FiO2, cardiac output, and mean arterial blood pressure.
All‐cause mortality (Outcome 3.1)
Al Omar 2016, which evaluated sildenafil as adjuvant therapy to iNo, reported no significant reduction in mortality (RR 1.27, 95% CI 0.26 to 6.28; RD 0.05, 95% CI ‐0.27 to 0.37; one study, 24 participants; heterogeneity estimate ‐ not applicable) (Analysis 3.1).
3.1. Analysis.
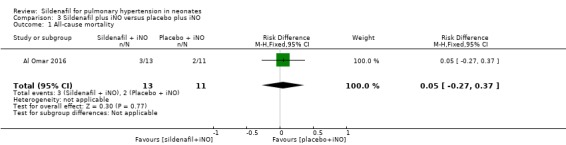
Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 1 All‐cause mortality.
Secondary outcomes
Oxygenation index
Al Omar 2016 reported mean changes in OI at six‐hourly intervals but did not provide mean or variance measures; therefore, we could not include this study in this meta‐analysis. Investigators presented data in graphical format, and we could not abstract absolute values.
Length of stay in hospital (Outcome 3.2)
Data show no significant differences in the number of days in hospital between groups given sildenafil versus placebo as adjuvant to iNO therapy (MD 5.39, 95% CI ‐5.31 to 16.09; one study, 24 participants; heterogeneity estimate ‐ not applicable) (Analysis 3.2).
3.2. Analysis.
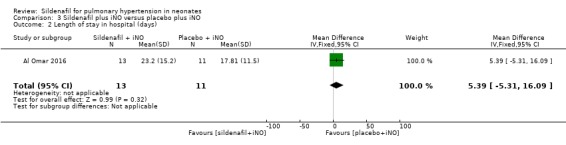
Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 2 Length of stay in hospital (days).
Use of an inotropic agent (Outcome 3.3)
Only one study included in this analysis (Al Omar 2016) evaluated use of an inotropic agent. Results show no significant differences in the need for an inotropic agent between groups given sildenafil or placebo adjuvant to iNO therapy (RR 1.06, 95% CI 0.37 to 3.00; RD 0.02, 95% CI ‐0.37 to 0.41; one study, 24 participants; heterogeneity estimate ‐ not applicable) (Analysis 3.3).
3.3. Analysis.
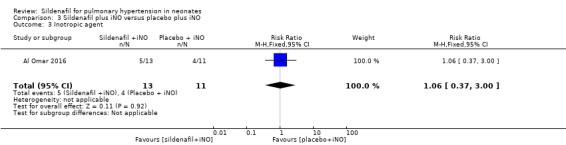
Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 3 Inotropic agent.
Duration of ventilation (Outcome 3.4)
Al Omar 2016 reported the duration of ventilation (days). Data show no significant differences in duration of ventilation among neonates treated with sildenafil or placebo given adjuvant to iNO therapy (MD 1.26, 95% CI ‐1.32 to 3.84; one study, 24 participants; heterogeneity estimate ‐ not applicable) (Analysis 3.4).
3.4. Analysis.
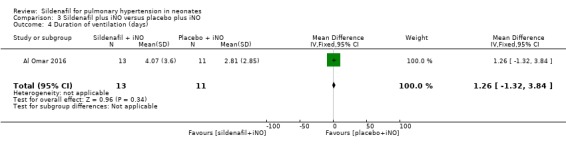
Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 4 Duration of ventilation (days).
Retinopathy of prematurity (among preterm infants at < 32 weeks' gestation) (Outcome 3.5)
Al Omar 2016 did not report any cases of retinopathy of prematurity in either group (Analysis 3.5).
3.5. Analysis.
Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 5 Retinopathy of prematurity.
Summary of findings
This review evaluated three comparisons against sildenafil: placebo alone, active control, and placebo with iNO. Overall, when compared with placebo alone, sildenafil significantly reduced mortality (Table 1). We graded the evidence as low owing to potential bias in outcome reporting/data analysis and imprecision related to a small number of participants.
When compared with an active control (Table 2) or when used as adjuvant therapy (Table 3), sildenafil did not show improved mortality among neonates with persistent pulmonary hypertension treated with sildenafil. We graded the evidence as low to very low owing to imprecision related to the small number of included studies.
Discussion
Summary of main results
Publications to date include five very small randomised controlled trials that evaluated sildenafil and included a total of 166 neonates. These trials were conducted in resource‐limited settings such as Columbia, Mexico, Qatar, and Turkey. The most commonly reported (40% to 45%) underlying diagnosis was meconium aspiration syndrome (MAS). All three studies that compared sildenafil versus placebo reported that intensive care units did not have facilities for providing high‐frequency ventilation or nitric oxide ‐ therapies that have shown promise in the treatment of persistent pulmonary hypertension in neonates (PPHN). Uslu 2011 compared sildenafil versus active control (magnesium sulphate; MgSO4). Al Omar 2016 evaluated sildenafil and placebo as adjuvant therapy to inhaled nitric oxide (iNO), as nitric oxide was routinely offered as standard of care.
Compared with placebo, sildenafil alone was associated with a significant reduction in mortality among neonates with PPHN. Respiratory parameters (oxygenation index (OI) and partial pressure of oxygen in arterial blood (PaO2)) showed significant improvement in the sildenafil group when compared with the placebo group. We did not identify a significant difference in mortality or respiratory parameters when sildenafil was compared with an active control or was used as adjuvant therapy with iNO. We noted heterogeneity in severity of illness at the time of entry in different studies; however, all enrolled participants who had moderate to severe hypoxaemic respiratory failure. Most studies reported steady improvement in oxygenation starting from the first dose of sildenafil. In one study of only 65 participants, sildenafil was effective in reducing the need for inotropes.
Overall completeness and applicability of evidence
Although studies comparing sildenafil versus placebo consistently reported improved respiratory outcomes with sildenafil, it must be noted that these studies had several limitations. The number of enrolled participants was small, reporting of various important outcomes was inadequate, and evidence showed heterogeneity between studies. Mortality was significantly reduced in our analyses when sildenafil alone was compared with placebo. This is clinically very significant and may influence treatment decisions made in these units, especially units in resource‐limited settings. Long‐term effects of sildenafil use remain unknown. Furthermore, data showed no significant improvement in mortality when sildenafil was compared with active control (MgSO4) or was used adjuvant to iNO therapy.
Included studies used heterogeneous sildenafil doses (range 0.5 to 3.0 mg/kg every 6 hours) and variable loading doses. Optimum dose, optimum route of administration, incidence of rebound pulmonary hypertension, and effectiveness in reducing rebound pulmonary hypertension remain unknown. Sildenafil may not be as effective in PPHN resulting from causes such as sepsis (where overproduction of nitric oxide leading to systemic vasodilation may be the major mechanism) but may be effective when PPHN has other causes such as chronic lung disease (Mesubi 2009; Mourani 2009). Concerns regarding retinal vascular growth (Kehat 2010) and lack of convincing data in preterm infants may preclude the use of sildenafil in preterm infants.
These five randomised clinical trials and several case reports, including recent reports from both resource‐limited settings (Juliana 2005; Simiyu 2006; Shivanna 2009; Sayed 2015) and resourceful settings (Steinhorn 2009), justify the call for a larger multi‐centre randomised controlled study. Studies of this type can be challenging. In addition to requiring multi‐centre collaboration, such studies would require that sildenafil be compared with other established therapies such as iNO and/or an optimal ventilatory strategy such as high‐frequency ventilation for PPHN. Resource‐limited settings could provide a platform for comparison of sildenafil versus placebo, and developed countries could provide settings for comparison with other management approaches. A large multi‐centre multi‐national trial in high‐income countries undertaken to evaluate the effectiveness of intravenous sildenafil versus placebo in terms of duration of nitric oxide treatment and treatment failure (additional treatment required for PPHN) is currently ongoing and may shed further light on this topic (NCT01720524). ClinicalTrials.gov (NCT01720524; updated 27 June 2016) reports an estimated enrolment of 64 and includes a plan for 12‐month and 24‐month safety and neurodevelopmental follow‐up. The estimated study completion date for this trial is December 2019. A pharmacokinetics study (NCT01670136; updated 17 March 2016) is currently recruiting preterm newborns receiving sildenafil as standard of care in the United States.
Quality of the evidence
We scored evidence quality using the GRADE PRO GDT online tool GRADEpro GDT. We graded the quality of evidence as low to very low, stemming from imprecision (small number of studies and small number of participants), lack of harmonised outcome measurement, and unclear methodological features due to inability to evaluate protocols because of lack of clinical trial registration. Without prospective registration, selective outcome reporting could not be addressed.
Potential biases in the review process
Variation in study design, including the addition of a post hoc adjuvant therapy comparison group, could have introduced a potential source of bias. After searching the literature, we believe that addition of this subgroup was warranted to account for future studies that are currently ongoing. Outcomes evaluated in the post hoc subgroup analysis did not influence our overall recommendations.
Agreements and disagreements with other studies or reviews
Case reports have described the efficacy of sildenafil for treatment of PPHN in neonates. A retrospective comparative assessment of iloprost versus sildenafil (Kahveci 2014) concluded that iloprost may be more effective than sildenafil in decreasing treatment time and improving ventilatory parameters for neonates with PPHN. As this review includes no published prospective randomised clinical trials of iloprost, we cannot confirm or disagree with these findings. Previous literature reviews (Spillers 2010; Iacovidou 2012) have presented information consistent with our finding that sildenafil may be effective in PPHN when other standard treatments such as iNO are not available.
Authors' conclusions
Implications for practice.
Sildenafil may have the potential to improve physiological parameters and to improve mortality among neonates with pulmonary hypertension in settings where iNO is not available. However, the safety and efficacy of sildenafil for treatment of PPHN have not yet been established in large randomised trials, and sildenafil should be used only within the context of randomised controlled trials.
Implications for research.
Additional studies are needed to evaluate the safety and efficacy of sildenafil for treatment of PPHN; some ongoing studies are currently being conducted in high‐resource settings. Additional research is needed to determine the optimum sildenafil dose and route of sildenafil administration. If found effective in achieving short‐term improvement in ongoing randomised controlled trials, long‐term data will be needed to demonstrate safety before treatment can be broadly implemented at national and individual clinical levels. Research studies should be conducted in both resource‐limited and high‐income settings to address questions such as effectiveness when used as stand‐alone therapy and as adjunctive therapy. A multi‐centre undertaking should include long‐term neurodevelopmental follow‐up. In resource‐limited settings, studies should enrol patients at high risk for mortality or needing extracorporeal membrane oxygenation (ECMO) owing to persistent hypoxaemia (OI > 25). Approximate rates for mortality or need for ECMO among these patients approaches 50%. A clinically significant effect size would be a 10% to 20% absolute risk reduction in rate of death and/or need for ECMO, with type 1 error of 5% and power of 80%; the required sample size for such a study would range from approximately 100 to 400 participants. This type of study would require a multi‐centre approach. In resource‐limited settings, mortality is higher and the required sample size could be lower. It would be crucial for such studies to include long‐term neurodevelopmental follow‐up, as studies to this point have not provided sufficient information on long‐term treatment effects.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 4, 2005 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 29 June 2017 | New citation required but conclusions have not changed | We added 2 new studies to this update and made no changes to the conclusions |
| 29 June 2017 | New search has been performed | We updated this review in June 2017 |
| 18 April 2011 | New citation required and conclusions have changed | We made some changes to the conclusions. A significant reduction in all‐cause mortality occurred within the first 28 days of life among the 77 recruited patients |
| 18 April 2011 | New search has been performed | We updated this review in April 2011 This update includes 1 new study We included as a full report 1 study that previously was available only in abstract form |
| 11 September 2008 | Amended | We converted this review to new review format |
| 27 April 2007 | New citation required and conclusions have changed | We made substantive amendments |
Acknowledgements
We would like to sincerely thank Drs. Augusta Sola, Jayendra Gohil, and Husam Salama for providing data on their studies. We would like to acknowledge the help of Dr. Cecilia Herbozo for translation of the Herrera 2006 study from Spanish. We acknowledge the valuable contributions made by Jennifer Spano and Collen Ovelman from the CNRG, who performed the literature searches.
Appendices
Appendix 1. Standard search methods
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of trials. For each trial, we sought information regarding the method of randomisation, and blinding and reporting of all outcomes of all infants enrolled in the trial. We assessed each criterion as having low, high, or unclear risk. Two review authors separately assessed each study. We resolved disagreements by discussion. We added this information to the Characteristics of included studies table. We evaluated the following issues and entered the findings into the risk of bias table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
a. Low risk (any truly random process, e.g. random number table; computer random number generator);
b. High risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
c. Unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
a. Low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
b. High risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
c. Unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
a. Low risk, high risk, or unclear risk for participants; and
b. Low risk, high risk, or unclear risk for personnel;
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
a. Low risk for outcome assessors;.
b. High risk for outcome assessors; or
c. Unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we re‐included missing data in the analyses. We categorised the methods as:
a. Low risk (< 20% missing data);
b. High risk (≥ 20% missing data); or
c. Unclear risk.
6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
a. Low risk (when it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
b. High risk (when not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
c. Unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
a. Low risk;
b. High risk; or
c. Unclear risk.
If needed, we explored the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. Sildenafil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pulmonary arterial pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Baseline | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐7.68, 9.88] |
| 2 PaO2 in mmHg (absolute values) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 8.06 [1.58, 14.54] |
| 2.2 After first dose | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 11.09 [1.65, 20.52] |
| 2.3 After 6 to 7 hours of treatment | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 14.30 [5.25, 23.34] |
| 2.4 After 24 to 25 hours of treatment | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | 15.31 [6.49, 24.13] |
| 2.5 After 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 20.98 [14.81, 27.15] |
| 3 Mean arterial blood pressure in mmHg | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Before initiation of therapy | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | 5.65 [2.69, 8.61] |
| 3.2 At the end of therapy | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | 22.70 [1.23, 44.17] |
| 4 All‐cause mortality | 3 | 77 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.36 [‐0.53, ‐0.18] |
| 5 Oxygenation index (absolute values) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At baseline | 3 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐8.11, 6.64] |
| 5.2 After first dose | 3 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐12.53 [‐18.60, ‐6.47] |
| 5.3 After 6 to 7 hours of treatment | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐20.07 [‐26.12, ‐14.02] |
| 5.4 After 24 to 25 hours of treatment | 3 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐19.15 [‐24.52, ‐13.77] |
| 5.5 After 30 hours of treatment | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐45.46 [‐61.87, ‐29.05] |
| 5.6 After 36 hours of treatment | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐31.75 [‐45.74, ‐17.76] |
| 5.7 After 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐19.47 [‐23.42, ‐15.52] |
| 6 Change in oxygenation index | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 After first dose | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐17.14 [‐27.75, ‐6.53] |
| 6.2 After 24 hours of treatment | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐38.79 [‐56.97, ‐20.61] |
| 6.3 After 30 hours of treatment | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐33.08 [‐50.85, ‐15.31] |
| 6.4 After 36 hours of treatment | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐44.75 [‐65.55, ‐23.95] |
| 7 A‐a DO2 difference | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.99 [‐11.54, 13.51] |
| 7.2 At 6 to 7 hours of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐27.72, 27.74] |
| 7.3 At 24 to 25 hours of treament | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [‐18.98, 22.16] |
| 7.4 At 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐18.34 [‐26.59, ‐10.09] |
| 8 Mean airway pressure | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐2.09 [‐3.30, ‐0.88] |
| 8.2 At 6 to 7 hours of treatment | 2 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐5.94 [‐7.36, ‐4.52] |
| 8.3 At 24 to 25 hours of treatment | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐6.64 [‐8.49, ‐4.80] |
| 8.4 At 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐8.58 [‐10.37, ‐6.79] |
1.2. Analysis.
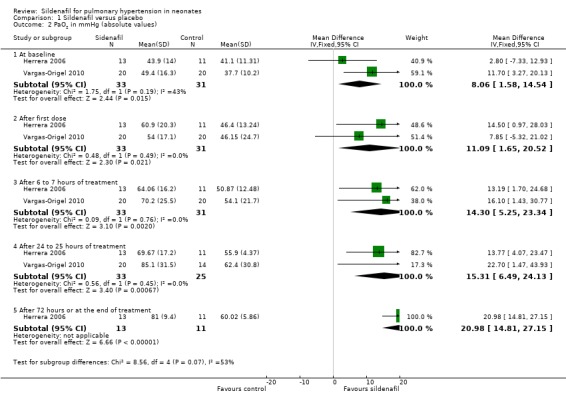
Comparison 1 Sildenafil versus placebo, Outcome 2 PaO2 in mmHg (absolute values).
Comparison 2. Sildenafil versus active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 1 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| 2 Time to adequate response (days) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐4.20, 3.00] |
| 3 Duration of ventilation (days) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐4.54, 1.94] |
| 4 Inotropic agent | 1 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.37 [‐0.59, ‐0.15] |
Comparison 3. Sildenafil plus iNO versus placebo plus iNO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.27, 0.37] |
| 2 Length of stay in hospital (days) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 5.39 [‐5.31, 16.09] |
| 3 Inotropic agent | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.37, 3.00] |
| 4 Duration of ventilation (days) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.26 [‐1.32, 3.84] |
| 5 Retinopathy of prematurity | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al Omar 2016.
| Methods | Randomised controlled trial Study location: neonatal intensive care unit at the Hamad Medical Corporation, in Doha, Qatar Study period: over 3 years from September 2011 to September 2014 | |
| Participants | 24 neonates born at 34 weeks or later with PPHN and OI > 20 Group 1: n = 13 Male: 7/13 (%) Mean GA: 38.1 (SD 2.3) weeks Mean birth weight: 3107 (SD 700) g Group 2: n = 11 Male: 8/11 (%) Mean GA: 39 (SD 1.61) weeks Mean birth weight: 3179 (SD 627) g |
|
| Interventions | iNO starting dose 20 ppm, weaning 2% to 4% every hour Group 1: iNO with 2 mg/kg sildenafil q6hours via orogastric tube (50 mg tablet crushed and diluted with 10 mL Orabase to prepare 5 mg/mL) Group 2: iNO with placebo (saline via orogastric tube) |
|
| Outcomes | Oxygen index (absolute values and change from baseline, after first dose and every 6 hours for 7 days; improvement in OI, defined as a decrease of 10% from previously calculated value) A‐a gradient Haemodynamic parameters Days of hospitalisation Mortality (all‐cause within 28 days of life) Inotropic agent ROP Length of stay |
|
| Notes | Registered with clinicaltrials.gov NCT01558466 Trial was funded by an investigator grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation (information obtained from author Salama) |
| Allocation concealment (selection bias) | Low risk | Randomisation by sealed envelope competed by pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treating physicians and nurses were unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Respiratory therapists were unaware of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data are reported on all 24 randomised neonates |
| Selective reporting (reporting bias) | Low risk | All registered outcomes were reported |
| Other bias | Low risk | Appears free of other bias |
Baquero 2006.
| Methods | Randomised controlled trial Study location: neonatal intensive care unit at the Hospital Niño Jesús in Barranquilla, Colombia Study period: 2003 to 2004 | |
| Participants | 13 neonates > 35.5 weeks' PMA with persistent hypoxaemia despite mechanical ventilation (OI ≥ 40) and echocardiographic diagnosis of PPHN were enrolled, at < 3 days old
Group 1: n = 7
Male: 4/7 (57%) Mean GA: 38.4 (SD 2.6) weeks Mean birth weight: 2803 (SD 617) grams Mean OI: 56 (SD 16.8) Group 2: n = 6 Male: 3/6 (50%) Mean GA: 37.2 (SD 1.9) weeks Mean birth weight: 2710 (SD 554) grams Mean OI: 46 (SD 9.5) |
|
| Interventions | Group 1: sildenafil (via orogastric tube) first dose of 1 mg/kg (0.5 mL/kg), subsequent doses every 6 hours; could be doubled to 2 mg/kg (1 mL/kg) if OI did not improve and BP remained stable until participant received a maximum of 8 doses, or until OI improved to < 20 Group 2: same volume of placebo (Orabase) first dose of 0.5 mL/kg, subsequent doses every 6 hours; could be doubled to 1 mL/kg if OI did not improve | |
| Outcomes | Mortality Changes in OI Changes in mean arterial blood pressure | |
| Notes | OI was determined for all 7 participants in sildenafil group for baseline and for first 6 doses. Oxygenation index was reported for only 4 participants for the seventh dose (2 met the pre‐set exit criteria for the study, and 1 died) OI was determined for all 6 participants in the placebo group at baseline, after dose 1, and after dose 2. One participant died before the third dose (measurement reported for 5 participants after third dose and after fourth dose); one participant died before fifth dose (measurements reported for 4 participants at doses 5, 6, and 7) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on sequence generation was reported |
| Allocation concealment (selection bias) | Low risk | Randomisation was by simple allocation of pre‐sealed numbers. No information is provided on whether envelopes were opaque or were sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Bedside clinicians were masked to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome measures are not subjective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all enrolled participants were reported |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration was not available to assess selective reporting |
| Other bias | Unclear risk | Small sample size; study was terminated prematurely |
Herrera 2006.
| Methods | Randomised controlled trial Study location: newborns treated in the intensive care unit of the Hospital Universitario de la Facultad de Medicina de la Universidad Autónoma de Nueco León, in San Andreas, Mexico Study period: May 2004 to October 2005 | |
| Participants | 24 term neonates with PPHN and OI > 25 Group 1: n = 13 Male: 5/13 (38%) Mean GA: 37 (SD 3.1) weeks Mean birth weight: 2741 (SD 66) grams Group 2: n = 11 Male: 6/11 (55%) Mean GA: 36.2 (SD 3.1) weeks Mean birth weight: 2651(SD 71) grams |
|
| Interventions | Group 1: sildenafil 2 mg/kg via orogastric tube (duration of administration ‐ 72 hours) Group 2: distilled water (2 mL/kg) | |
| Outcomes | Changes in OI, PaCO2, A‐a DO2, PaO2
Changes in mean arterial blood pressure
Duration of intubation Death |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported |
| Allocation concealment (selection bias) | Unclear risk | Reported as blinded study, but no information provided on how treatment allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was described as blinded, but no information was provided on how blinding was achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study was described as blinded, but no information was provided on how blinding was achieved |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Newborns whose data were incomplete or who were transferred to other hospitals were excluded |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration was not available for assessment of selective reporting |
| Other bias | Low risk | Appears free of other bias |
Uslu 2011.
| Methods | Randomised clinical trial Study location: neonatal intensive care unit of Diyarbakir Children's Hospital, Turkey Study period: 1 February 2007 to 30 April 2008 | |
| Participants | 65 term and near‐term neonates (35 to 42 weeks' GA) with persistent hypoxaemic respiratory failure (PAP ≥ 40 mmHg, OI ≥ 30, and need for mechanical ventilation) associated with PPHN were enrolled Group 1: sildenafil (n = 31) Male: 16/31 (52%) Mean PMA: 38.5 (SD 1.6) weeks Mean birth weight: 3229 (SD 364) grams Mean OI: 43.3 (SD 6.3) Group 2: MgSo4 (n = 34) Male: 20/34 (59%) Mean PMA: 38.3 (SD 1.7) weeks Mean birth weight: 3296 (SD 339) grams Mean OI: 44.0 (SD 8.8) |
|
| Interventions | Group 1: 25 mg tablet of sildenafil diluted with 25 mL sterile water for final concentration of 1 mg/mL. Dose of solution (0.5 mg/kg) was given via orogastric tube every 6 hours. Dose could be doubled (until maximum of 2 mg/kg) if OI did not improve. Sildenafil dose was tapered 50% after reaching OI level < 15 and PAP < 20 mmHg and was terminated in 1 day Group 2: MgSO4 loading dose of 200 mg/kg infused IV over 30 minutes followed by maintenance dose of 20 mg/kg/h. If OI did not improve sufficiently, MgSO4 infusion rate was increased slowly (10 mg/kg/h to maximum 100 mg/kg/h). When OI level was < 15 and PAP was < 20 mmHg, MgSO4 infusion was decreased gradually (10 mg/kg/h) and was terminated in 1 day |
|
| Outcomes | Primary outcome: time of adequate clinical response (defined as a decrease in PAP to < 20 mmHg (improvement in PAP) and in OI to < 15 (improvement in OI)) Secondary outcomes: Duration of mechanical ventilation Support of inotropic agent Mortality rate Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent researcher used a computer‐generated randomisation table |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As MgSO4 was given IV and sildenafil was given by orogastric tube, care providers could not be blinded to treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collector nurse, paediatric cardiologist, and ultrasonographer were blinded. Only the neonatologist who gave medical care to participants was not blinded to the type of treatment received |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were not analysed as intent to treat. Data were incomplete (missing) for 7 neonates (2 MgSO4, 5 sildenafil), 3 of whom had gastrointestinal bleeding |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration was not available for assessment of selective reporting |
| Other bias | Low risk | Appears free of other bias |
Vargas‐Origel 2010.
| Methods | Randomised controlled trial Study location: neonatal intensive care unit at the Unidad Medica de Alta Espexialidad No. 48, in Guanajuato, Mexico Study period: not reported | |
| Participants | 40 term neonates with PPHN and OI > 20 Group 1: n = 31 Male: 16/31(%) Mean GA: 37.8 (SD 1.6) weeks Mean birth weight: 2993 (SD 532) grams Group 2: n = 20 Male: 13/20 (%) Mean GA: 38.8 (SD 1.9) weeks Mean birth weight: 3043 (SD 563) grams |
|
| Interventions | Group 1: sildenafil 2 mg/kg via orogastric tube 3 mg/kg/dose until OI < 10 Group 2: normal saline | |
| Outcomes | Changes in OI, PaO2, A‐aDO2, PaCO2, mean airway pressure
Changes in mean arterial blood pressure Mortality Time on mechanical ventilation |
|
| Notes | Published manuscript described 31 participants in the sildenafil group (20 randomised to receive placebo, and another 11 randomised to receive nitric oxide). We contacted study authors and retrieved data for the first 40 infants (20 in each group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was generated by a random numbers table (information provided by study author). |
| Allocation concealment (selection bias) | Low risk | Allocation was generated by 2 nurses who were not involved with the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as blinded. "Pharmacy prepared the solution and bedside clinicians were unaware of group assignment" (information provided by study author). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as blinded. "Outcome assessment was masked as nurses were unaware of treatment allocation" (information provided by study author) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Published report had combined data on 51 participants and did not include information on when the change in randomisation occurred |
| Selective reporting (reporting bias) | Unclear risk | Protocol or trial registration was not available for assessment of selective reporting |
| Other bias | Unclear risk | Published data included subsequent participants; therefore, it is difficult to assess other bias |
A‐a DO2 = alveolar‐arterial oxygen difference BP = blood pressure GA = gestational age iNO = inhaled nitric oxide
MgSO4 = magnesium sulphate OI = oxygenation index PaCO2 = partial pressure of carbon dioxide in arterial blood PaO2 = partial pressure of oxygen in arterial blood
PAP = pulmonary artery pressure
PMA = postmenstrual age PPHN = persistent pulmonary hypertension of the newborn ROP = retinopathy of prematurity
SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kahveci 2014 | Retrospective study design |
| König 2014 | Unclear whether patients had persistent pulmonary hypertension in neonates (PPHN); newborns on nitric oxide (NO) were excluded |
| Namachivayam 2006 | Included patients whose age ranged from 0.1 years onwards Most participants had associated congenital heart disease |
| Sayed 2015 | Single‐arm study; no control arm |
| Steinhorn 2009 | Open‐label single‐arm study; no randomisation |
| Stocker 2003 | Only infants after cardiac surgery were included |
Characteristics of studies awaiting assessment [ordered by study ID]
Alipour 2017.
| Methods | Randomised controlled trial |
| Participants | 32 neonates born at 34 weeks or later with PPHN (cyanosis that continued 30 minutes after ventilation with 100% supplemental oxygen via hood) Group 1: n = 16 No participant demographics were reported Group 2: n = 16 No participant demographics were reported |
| Interventions | Tadalafil orally; 1 mg/kg/d Sildenafil orally; 1 mg/kg/dose 3 times daily |
| Outcomes | Tricuspid regurgitation (TR) severity assessed by echocardiography at baseline and after 6 months Mean pulmonary arterial pressure (MPAP) recorded via echocardiography based on gradient of pulmonary insufficiency or gradient TR on Doppler echocardiography Right ventricular end‐diastolic diameter (RVEDD) assessed by echocardiography at baseline and after 6 months Main pulmonary artery (MPA) diameter assessed by echocardiography at baseline and 6 months after treatment |
| Notes | Funding support, potential conflicts, and trial registration were not reported. This study was classified as awaiting classification, as the review authors required clarification of the time period of measurement. It is reported that echocardiography took place 6 months after treatment, which we believe could have taken hours. We have contacted the corresponding author multiple times |
NCT01373749.
| Methods | Randomised clinical trial |
| Participants | Inclusion criteria: Diagnosis of PPHN in the NICU, primary disease:neonate respiratory distress syndrome, meconium aspiration syndrome of newborn, severe neonatal infectious pneumonia. Pulmonary artery pressure > 50 mmHg, mechanical ventilation over 48 hours, primary OI (PO2/FiO2) < 300, difference of SpO2 between upper and lower limbs > 10%, high FiO2, oxygen inhalation test: positive Exclusion criteria: Congenital heart disease, diaphragmatic hernia |
| Interventions | Nitric oxide inhalation continued with sildenafil vs inhaled nitric oxide alone |
| Outcomes | Primary outcome measures: Persistent normal pulmonary artery pressure Pulmonary artery pressure returned to a normal level (< 30 mmHg) and lasting over 48 hours Secondary outcome measures: Recovery without complication (time frame: 1 month after therapy) Incidence of pulmonary disease (chronic lung disease) Incidence of brain injury (hypoxic‐ischaemic encephalopathy) Heart structure change (right ventricle enlarged) |
| Notes | Status verified as "recruiting" in January 2011 by Third Military Medical University |
NCT01757782.
| Methods | Randomised controlled trial |
| Participants | 40 neonates (GA > 34 weeks) admitted within 12 hours of delivery with diagnosis of MAS and development of PPHN Group 1: n = 20 Male: 9/20 (%) Mean birth weight: 2767 (SD 100) grams Group 2: n = 20 Male: 12/20 (%) Mean birth weight: 2691 (SD 90) grams |
| Interventions | Group 1: oral sildenafil (1 mg/kg/dose q6hours) administered through feeding tube for a total of 8 doses (treatment for 2 days) Group 2: placebo |
| Outcomes | Oxygen saturation Oxygenation index Length of hospitalisation Duration of mechanical ventilation Mortality |
| Notes | We identified study through ClinicalTrials.gov and contacted study authors, who stated that trial results are unavailable yet, but recruitment has been completed |
Soliz 2009.
| Methods | Randomised clinical trial |
| Participants | 49 term neonates with PPHN and OI > 25 were included |
| Interventions | Randomised to placebo (n = 20) vs sildenafil (n = 29) |
| Outcomes | OI, mean blood pressure and mean airway pressure |
| Notes | Difficult to differentiate from included studies, as some study authors overlap |
FiO2 = fraction of inspired oxygen GA = gestational age MAS = meconium aspiration syndrome MPA = mean pulmonary artery MPAP = mean pulmonary arterial pressure NICU = neonatal intensive care unit OI = oxygenation index PO2 = partial pressure of oxygen PPHN = persistent pulmonary hypertension in the neonate RVEDD = right ventricular end‐diastolic diameter SD = standard deviation SpO2 = peripheral capillary oxygen saturation TR = tricuspid regurgitation
Characteristics of ongoing studies [ordered by study ID]
NCT01720524.
| Trial name or title | A study to evaluate the safety and efficacy of IV sildenafil in the treatment of neonates with persistent pulmonary hypertension of the newborn |
| Methods | Multi‐centre randomised placebo‐controlled double‐blind 2‐armed parallel‐group study |
| Participants | Inclusion criteria: Neonates with persistent pulmonary hypertension of the newborn Age ≤ 96 hours and ≥ 34 weeks' gestational age Oxygenation index > 15 and < 60 Concurrent treatment with inhaled nitric oxide and ≥ 50% oxygen Exclusion criteria: Prior or immediate need for extracorporeal membrane oxygenation or cardiopulmonary resuscitation Expected duration of mechanical ventilation < 48 hours Profound hypoxaemia Life‐threatening or lethal congenital anomaly |
| Interventions | Treatment: intravenous (IV) sildenafil · Loading dose of 0.1 mg/kg over 30 minutes followed by maintenance dose of 0.03 mg/kg/h. To infuse minimum of 48 hours and maximum of 14 days Control: placebo IV placebo or 0.9% sodium chloride or 10% dextrose. Infusion rate based on weight |
| Outcomes | Primary outcome measures (at day 14 or until discharge): · Time on inhaled nitric oxide treatment after initiation of IV study drug · Treatment failure rate, defined as need for additional treatment targeting persistent pulmonary hypertension of the newborn Secondary outcome measures: · Time to final weaning off mechanical ventilation for persistent pulmonary hypertension of the newborn · Time from initiation of study drug to treatment failure · Change in oxygenation parameters at 6, 12, and 24 hours from baseline · Sildenafil plasma concentrations and corresponding pharmacokinetic (PK) parameters · Safety parameters: incidence and severity of adverse events and abnormal laboratory parameters Long‐term outcomes (12 and 24 months): · Developmental progress of participants as assessed by Bayley Scales of Infant Development and Behavior Questionnaire · Safety as assessed by adverse events and survival · Neurological progress of participants as assessed by the Neurology Optimality Score, also known as the Hammersmith Infant Neurological Examination · Visual status of participants as assessed by eye examination of anterior and posterior segments · Audiological status of participants as assessed by physiological and behavioural tests |
| Starting date | 17 September 2012 |
| Contact information | Pfizer CT.gov call centre: 1‐800‐718‐1021 |
| Notes | Includes a long‐term follow‐up investigation of developmental progress at 12 and 24 months |
Differences between protocol and review
An additional author (LEK) was added to this review team. We added to this update a post hoc comparison of sildenafil versus placebo as adjuvant therapy to inhaled nitric oxide.
Contributions of authors
LE Kelly reviewed the literature to identify trials, collected and entered data into RevMan, updated the review text, and revised the final version of this revision. A Ohlsson reviewed and edited the protocol, checked the search strategy and data entry, verified the data entered, and revised the final version of all versions of this review. PS Shah developed and edited the protocol, identified trials, wrote and edited the review, verified data entered into RevMan, and revised the final version of all versions of this review.
Sources of support
Internal sources
Department of Paediatrics, Mount Sinai Hospital, Toronto, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
Declarations of interest
The review authors have no conflicts of interest to declare.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Al Omar 2016 {published data only}
- Al Omar S, Salama H, Al Hail M, Al Rifai H, Bunahia M, Kasem W, et al. Effect of early adjunctive use of oral sildenafil and inhaled nitric oxide on the outcome of pulmonary hypertension in newborn infants. A feasibility study. Journal of Neonatal‐Perinatal Medicine 2016;9(3):251‐9. [DOI: 10.3233/NPM-16161; PUBMED: 27589542] [DOI] [PubMed] [Google Scholar]
Baquero 2006 {published data only}
- Baquero H, Neira F, Venegas ME, Sola A, Soliz A. Outcome at 18 months of age after sildenafil therapy for refractory neonatal hypoxemia. Pediatric Academic Societies' Annual Meeting; 2005 May 14‐17; Washington DC, United States 2005;57:2119. [PAS 2005: 57: 2119] [Google Scholar]
- Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics 2006;117(4):1077‐83. [DOI: 10.1542/peds.2005-0523; PUBMED: 16585301] [DOI] [PubMed] [Google Scholar]
Herrera 2006 {published data only}
- Herrea TR, Concha GP, Holberto CJ, Loera GRG, Rodríguez BI. Oral sildenafil as an alternative treatment in the persistent pulmonary hypertension in newborns [Sildenafil oral como alternativa en el tratamiento de recien nacidos con hipertension pulmonar persistente]. Revista Mexicana de Pediatria 2006;73(4):159‐63. [Google Scholar]
Uslu 2011 {published data only}
- Uslu S, Kumtepe S, Bulbul A, Comert S, Bolat F, Muhoglu A. A comparison of magnesium sulphate and sildenafil in the treatment of the newborns with persistent pulmonary hypertension; a randomized controlled trial. Journal of Tropical Pediatrics 2011;57(4):245‐50. [DOI: 10.1093/tropej/fmq091; PUBMED: 20923790] [DOI] [PubMed] [Google Scholar]
Vargas‐Origel 2010 {published and unpublished data}
- Vargas‐Origel A, Gomez‐Rodriguez G, Aldana‐Valenzuela C, Vela‐Huerta MM, Alarcon‐Santos SB, Amador‐Licona N. The use of sildenafil in persistent pulmonary hypertension of the newborn. American Journal of Perinatology 2010;27(3):225‐30. [DOI: 10.1055/s-0029-1239496; PUBMED: 19866403] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Kahveci 2014 {published data only}
- Khaveci H, Yilmaz O, Avsar UZ, Ciftel M, Kilic M, Laloglu F, et al. Oral sildenafil and inhaled iloprost in the treatment of pulmonary hypertension of the newborn. Pediatric Pulmonology 2014;49(12):1205‐13. [DOI: 10.1002/ppul.22985; PUBMED: 24420987] [DOI] [PubMed] [Google Scholar]
König 2014 {published data only}
- König K, Barfield CP, Guy KJ, Drew SM, Anderson CC. The effect of sildenafil on evolving bronchopulmonary dysplasia in extremely preterm infants: a randomised controlled pilot study. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(5):439‐44. [DOI: 10.3109/14767058.2013.818650; PUBMED: 23796045] [DOI] [PubMed] [Google Scholar]
Namachivayam 2006 {published data only}
- Namachivayam P, Theilen U, Butt WW, Cooper SM, Penny DJ, Shekerdemian LS. Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. American Journal of Respiratory and Critical Care Medicine 2006;174(9):1042‐7. [DOI: 10.1164/rccm.200605-694OC; PUBMED: 16917115] [DOI] [PubMed] [Google Scholar]
Sayed 2015 {published data only}
- Sayed A, Bisheer N. Outcome of oral sildenafil in neonatal persistent pulmonary hypertension of non‐cardiac causes. Journal of Neonatal‐Perinatal Medicine 2015;8(3):215‐20. [DOI: 10.3233/NPM-15814137; PUBMED: 26485555] [DOI] [PubMed] [Google Scholar]
Steinhorn 2009 {published data only}
- Steinhorn RH, Kinsella JP, Pierce C, Butrous G, Dilleen M, Oakes M, et al. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. Journal of Pediatrics 2009;155(6):841‐7. [DOI: 10.1016/j.jpeds.2009.06.012; PUBMED: 19836028] [DOI] [PubMed] [Google Scholar]
Stocker 2003 {published data only}
- Stocker C, Penny DJ, Brizard CP, Cochrane AD, Soto R, Shekerdemian LS. Intravenous sildenafil and inhaled nitric oxide: a randomised trial in infants after cardiac surgery. Intensive Care Medicine 2003;29(11):1996‐2003. [DOI: 10.1007/s00134-003-2016-4; PUBMED: 14530859] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Alipour 2017 {published data only}
- Alipour MR, Lookzadeh MH, Namayandeh SM, Pezeshkpour Z, Sarebanhassanabadi M. Comparison of tadalfil and sildenafil in controlling neonatal persistent pulmonary hypertension. Iran Journal of Pediatrics 2017;27(1):6385‐9. [DOI: 10.5812/ijp.6385] [DOI] [Google Scholar]
NCT01373749 {unpublished data only}
- NCT01373749. Nitro oxide inhalation continued with sildenafil on neonatal persistent pulmonary hypertension [Compare of continued nitro oxide inhalation and nitro oxide inhalation continued with oral sildenafil on treatment of neonatal persistent pulmonary hypertension]. clinicaltrials.gov/show/NCT01373749 (first received 31 May 2011).
NCT01757782 {unpublished data only}
- NCT01757782. Oral sildenafil in persistent pulmonary hypertension secondary to meconium aspiration syndrome in newborns [Oral sildenafil in persistent pulmonary hypertension secondary to meconium aspiration syndrome in newborns: a randomized placebo controlled trial]. clinicaltrials.gov/show/NCT01757782 (first received 18 December 2012). [ClinicalTrials.gov: NCT01757782]
Soliz 2009 {published data only}
- Soliz A, Concha E, Romero F. Oral sildenafil treatment as therapy for persistent pulmonary hypertension of the newborn: a multicenter randomized trial. Pediatric Academic Societies' Annual Meeting; 2009 May 2‐5; Baltimore MD, United States. 2009. [E‐PAS2009:5420.12]
References to ongoing studies
NCT01720524 {unpublished data only}
- NCT01720524. A study to evaluate safety and efficacy of IV sildenafil in the treatment of neonates with persistent pulmonary hypertension of the newborn. clinicaltrials.gov/show/NCT01720524 (first received 17 September 2012).
Additional references
Abrams 2000
- Abrams D, Schulze‐Neick I, Magee AG. Sildenafil as a selective pulmonary vasodilator in childhood primary pulmonary hypertension. Heart 2000;84(2):E4. [PUBMED: 10908271] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adatia 2002
- Adatia I. Recent advances in pulmonary vascular disease. Current Opinion in Pediatrics 2002;14(3):292‐7. [PUBMED: 12011667] [DOI] [PubMed] [Google Scholar]
Barrington 2010
- Barrington KJ, Finer NN. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD000509.pub4] [DOI] [PubMed] [Google Scholar]
Behn 2001
- Behn D, Potter MJ. Sildenafil‐mediated reduction in retinal function in heterozygous mice lacking the gamma‐subunit of phosphodiesterase. Investigative Ophthalmology and Visual Science 2001;42(2):523‐7. [PUBMED: 11157892] [PubMed] [Google Scholar]
Burton 2000
- Burton AJ, Reynolds A, O'Neill D. Sildenafil (Viagra) a cause of proliferative diabetic retinopathy?. Eye 2000;14(Pt 5):785‐6. [DOI: 10.1038/eye.2000.205; PUBMED: 11116706] [DOI] [PubMed] [Google Scholar]
Carroll 2003
- Carroll WD, Dhillon R. Sildenafil as a treatment for pulmonary hypertension. Archives of Disease in Childhood 2003;88(9):827‐8. [PUBMED: 12937112] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dukarm 1998
- Dukarm RC, Morin FC, Russell JA, Steinhorn RH. Pulmonary and systemic effects of the phosphodiesterase inhibitor dipyridamole in newborn lambs with persistent pulmonary hypertension. Pediatric Research 1998;44(6):831‐7. [DOI: 10.1203/00006450-199812000-00002; PUBMED: 9853914] [DOI] [PubMed] [Google Scholar]
Erickson 2002
- Erickson S, Reyes J, Bohn D, Adatia I. Sildenafil (Viagra) in childhood and neonatal pulmonary hypertension. Journal of the American College of Cardiology 2002;39:402. [Google Scholar]
Finer 2017
- Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD000399.pub3] [DOI] [Google Scholar]
Gersony 1984
- Gersony WM. Neonatal pulmonary hypertension: pathophysiology, classification, and etiology. Clinics in Perinatology 1984;11(3):517‐24. [PUBMED: 6488670] [PubMed] [Google Scholar]
Goldman 1996
- Goldman AP, Tasker RC, Haworth SG, Sigston PE, Macrae DJ. Four patterns of response to inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 1996;98(4 Pt 1):706‐13. [PUBMED: 8885950] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 19 April 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. handbook.cochrane.org.
Humbert 2004
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. New England Journal of Medicine 2004;351(14):1425‐36. [DOI: 10.1056/NEJMra040291; PUBMED: 15459304] [DOI] [PubMed] [Google Scholar]
Iacovidou 2012
- Iacovidou N, Syggelou A, Fanos V, Xanthos T. The use of sildenafil in the treatment of persistent pulmonary hypertension of the newborn: a review of the literature. Current Pharmaceutical Design 2012;12(21):3034‐45. [PUBMED: 22564297] [DOI] [PubMed] [Google Scholar]
Ichinose 2001
- Ichinose F, Erana‐Garcia J, Hromi J, Raveh Y, Jones R, Krim L, et al. Nebulized sildenafil is a selective pulmonary vasodilator in lambs with acute pulmonary hypertension. Critical Care Medicine 2001;29(5):1000‐5. [PUBMED: 11378612] [DOI] [PubMed] [Google Scholar]
Ikeda 2005
- Ikeda D, Tsujino I, Ohira H, Itoh N, Kamigaki M, Ishimaru S, et al. Addition of oral sildenafil to beraprost is a safe and effective therapeutic option for patients with pulmonary hypertension. Journal of Cardiovascular Pharmacology 2005;45(4):286‐9. [PUBMED: 15772514] [DOI] [PubMed] [Google Scholar]
Juliana 2005
- Juliana AE, Abbad FC. Severe persistent pulmonary hypertension of the newborn in a setting where limited resources exclude the use of inhaled nitric oxide: successful treatment with sildenafil. European Journal of Pediatrics 2005;164(10):626‐9. [DOI: 10.1007/s00431-005-1724-x; PUBMED: 16012855] [DOI] [PubMed] [Google Scholar]
Kanthapillai 2004
- Kanthapillai P, Lasserson T, Walters E. Sildenafil for pulmonary hypertension. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003562.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kehat 2010
- Kehat R, Bonsall DJ, North R, Connors B. Ocular findings of oral sildenafil use in term and near‐term neonates. Journal of AAPOS 2010;14(2):159‐62. [DOI: 10.1016/j.jaapos.2009.12.161; PUBMED: 20199882] [DOI] [PubMed] [Google Scholar]
Kinsella 2000
- Kinsella JP, Abman SH. Inhaled nitric oxide: current and future uses in neonates. Seminars in Perinatology 2000;24(6):387‐95. [PUBMED: 11153900] [DOI] [PubMed] [Google Scholar]
Kumar 2002
- Kumar S. Indian doctor in protest after using Viagra to save "blue babies". British Medical Journal 2002;325(7357):181. [PUBMED: 12142299 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewin 2002
- Lewin S. Viagra neonatal experimentation ‐ the Pandora's box!. Indian Pediatrics 2002;39(9):894‐5. [PUBMED: 12368555] [PubMed] [Google Scholar]
Marsh 2004
- Marsh CS, Marden B, Newsom R. Severe retinopathy of prematurity (ROP) in a premature baby treated with sildenafil acetate (Viagra) for pulmonary hypertension. British Journal of Ophthalmology 2004;88(2):306‐7. [PUBMED: 14736800] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mesubi 2009
- Mesubi OO, Ashwath R, Mhanna MJ. Oral sildenafil in premature infants with pulmonary arterial hypertension secondary to bronchopulmonary dysplasia. Pediatric Academic Societies Annual meeting; 2009 May 2‐5; Baltimore MD, United States. 2009. [E‐PAS2009:615]
Mourani 2009
- Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long‐term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. Journal of Pediatrics 2009;154(3):379‐84. [DOI: 10.1016/j.jpeds.2008.09.021; PUBMED: 18950791] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliver 2002
- Oliver J, Webb DJ. Sildenafil for "blue babies". Such unlicensed drug use might be justified as last resort. British Medical Journal 2002;325(373):1174. [PUBMED: 12433774] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patole 2002
- Patole S, Travadi J. Sildenafil for "blue babies". Ethics, conscience, and science have to be balanced against limited resources. British Medical Journal 2002;325(7373):1174. [PUBMED: 12434819] [PubMed] [Google Scholar]
Pierce 2005
- Pierce CM, Petros AJ, Fielder AR. No evidence for severe retinopathy of prematurity following sildenafil. British Journal of Ophthalmology 2005;89(2):250. [PUBMED: 15665374] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabe 1994
- Rabe KF, Tenor H, Dent G, Schudt C, Nakashima M, Magnussen H. Identification of PDE isozymes in human pulmonary artery and effect of selective PDE inhibitors. American Journal of Physiology 1994;266(5 Pt 1):L536‐43. [PUBMED: 7515580] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 1997
- Roberts JD Jr, Fineman JR, Morin FC 3rd, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. New England Journal of Medicine 1997;336(9):605‐10. [DOI: 10.1056/NEJM199702273360902; PUBMED: 9032045] [DOI] [PubMed] [Google Scholar]
Sastry 2004
- Sastry BKS, Narasimhan C, Reddy K, Raju BS. Clinical efficacy of sildenafil in primary pulmonary hypertension. Journal of the American College of Cardiology 2004;43(7):1149‐53. [DOI: 10.1016/j.jacc.2003.10.056; PUBMED: 15063421] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. https://gdt.gradepro.org/app/handbook/handbook.html Updated October 2013.
Shah 2004
- Shah PS, Hellmann J, Adatia I. Clinical characteristics and follow up of Down syndrome infants without congenital heart disease who presented with persistent pulmonary hypertension of newborn. Journal of Perinatal Medicine 2004;32(2):168‐70. [DOI: 10.1515/JPM.2004.030; PUBMED: 15085894] [DOI] [PubMed] [Google Scholar]
Shekerdemian 2002
- Shekerdemian LS, Ravn HB, Penny DJ. Intravenous sildenafil lowers pulmonary vascular resistance in a model of neonatal pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine 2002;165(8):1098‐102. [DOI: 10.1164/ajrccm.165.8.2107097; PUBMED: 11956051] [DOI] [PubMed] [Google Scholar]
Shekerdemian 2004
- Shekerdemian LS, Ravn HB, Penny DJ. Interaction between inhaled nitric oxide and intravenous sildenafil in a porcine model of meconium aspiration syndrome. Pediatric Research 2004;55(3):413‐8. [DOI: 10.1203/01.PDR.0000112033.81970.C2; PUBMED: 14711900] [DOI] [PubMed] [Google Scholar]
Shivanna 2009
- Shivanna B, Eichenwald EC, Stark AR. Sildenafil use in the newborn intensive care units is associated with improvement in pulmonary hypertension. Pediatric Academic Societies' Annual Meeting; 2009 May 2‐5; Baltimore MD, United States 2009. [E‐PAS2009:621]
Simiyu 2006
- Simiyu DE, Okello C, Nyakundi EG, Tawakal AH. Sildenafil in management of persistent pulmonary hypertension of the newborn: report of two cases. East African Medical Journal 2006;83(6):337‐40. [PUBMED: 16989380] [DOI] [PubMed] [Google Scholar]
Spillers 2010
- Spillers J. PPHN: is sildenafil the new nitric? A review of the literature. Advances in Neonatal Care 2010;10(2):69‐74. [DOI: 10.1097/ANC.0b013e3181d5c501; PUBMED: 20386371] [DOI] [PubMed] [Google Scholar]
Subhedar 2002
- Subhedar NV, Jauhari P, Natarajan R. Cost of inhaled nitric oxide therapy in neonates. Lancet 2002;359(9319):1781‐2. [DOI: 10.1016/S0140-6736(02)08632-4; PUBMED: 12049900] [DOI] [PubMed] [Google Scholar]
Travadi 2003
- Travadi JN, Patole SK. Phosphodiesterase inhibitors for persistent pulmonary hypertension of the newborn: a review. Pediatric Pulmonology 2003;36(6):529‐35. [DOI: 10.1002/ppul.10389; PUBMED: 14618646] [DOI] [PubMed] [Google Scholar]
Walsh‐Sukys 2000
- Walsh‐Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 2000;105:14‐20. [DOI] [PubMed] [Google Scholar]
Wessel 1997
- Wessel DL, Adatia I, Marter LJ, Thompson JE, Kane JW, Stark AR, et al. Improved oxygenation in a randomized trial of inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 1997;100:e7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Shah 2007
- Shah PS, Ohlsson A. Sildenafil for pulmonary hypertension in neonates. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005494.pub2; PUBMED: PMID: 17636802] [DOI] [PubMed] [Google Scholar]
Shah 2011
- Shah PS, Ohlsson A. Sildenafil for pulmonary hypertension in neonates. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD005494.pub3; PUBMED: PMID 21833954 ] [DOI] [PubMed] [Google Scholar]


