Abstract
Background
Low cardiac output syndrome remains a serious complication, and accounts for substantial morbidity and mortality in the postoperative course of paediatric patients undergoing surgery for congenital heart disease. Standard prophylactic and therapeutic strategies for low cardiac output syndrome are based mainly on catecholamines, which are effective drugs, but have considerable side effects. Levosimendan, a calcium sensitiser, enhances the myocardial function by generating more energy‐efficient myocardial contractility than achieved via adrenergic stimulation with catecholamines. Thus potentially, levosimendan is a beneficial alternative to standard medication for the prevention of low cardiac output syndrome in paediatric patients after open heart surgery.
Objectives
To review the efficacy and safety of the postoperative prophylactic use of levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease.
Search methods
We identified trials via systematic searches of CENTRAL, MEDLINE, Embase, and Web of Science, as well as clinical trial registries, in June 2016. Reference lists from primary studies and review articles were checked for additional references.
Selection criteria
We only included randomised controlled trials (RCT) in our analysis that compared prophylactic levosimendan with standard medication or placebo, in infants and children up to 18 years of age, who were undergoing surgery for congenital heart disease.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias according to a pre‐defined protocol. We obtained additional information from all but one of the study authors of the included studies. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of evidence from the studies that contributed data to the meta‐analyses for the prespecified outcomes. We created a 'Summary of findings' table to summarise the results and the quality of evidence for each outcome.
Main results
We included five randomised controlled trials with a total of 212 participants in the analyses. All included participants were under five years of age. Using GRADE, we assessed there was low‐quality evidence for all analysed outcomes. We assessed high risk of performance and detection bias for two studies due to their unblinded setting. Levosimendan showed no clear effect on risk of mortality (risk ratio (RR) 0.47, 95% confidence interval (CI) 0.12 to 1.82; participants = 123; studies = 3) and no clear effect on low cardiac output syndrome (RR 0.64, 95% CI 0.39 to 1.04; participants = 83; studies = 2) compared to standard treatments. Data on time‐to‐death were not available from any of the included studies.
There was no conclusive evidence on the effect of levosimendan on the secondary outcomes. The length of intensive care unit stays (mean difference (MD) 0.33 days, 95% CI ‐1.16 to 1.82; participants = 188; studies = 4), length of hospital stays (MD 0.26 days, 95% CI ‐3.50 to 4.03; participants = 75; studies = 2), duration of mechanical ventilation (MD ‐0.04 days, 95% CI ‐0.08 to 0.00; participants = 208; studies = 5), and the risk of mechanical circulatory support or cardiac transplantation (RR 1.49, 95% CI 0.19 to 11.37; participants = 60; studies = 2) did not clearly differ between the groups. Published data about adverse effects of levosimendan were limited. A meta‐analysis of hypotension, one of the most feared side effects of levosimendan, was not feasible because of the heterogeneous expression of blood pressure values.
Authors' conclusions
The current level of evidence is insufficient to judge whether prophylactic levosimendan prevents low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease. So far, no significant differences have been detected between levosimendan and standard inotrope treatments in this setting.
The authors evaluated the quality of evidence as low, using the GRADE approach. Reasons for downgrading were serious risk of bias (performance and detection bias due to unblinded setting of two RCTs), serious risk of inconsistency, and serious to very serious risk of imprecision (small number of included patients, low event rates).
Plain language summary
Levosimendan to prevent reduced heart function and death after heart surgery in infants and children with congenital heart disease
Background
Reduced heart function is a potentially fatal complication after heart surgery in infants and children. There are different drugs available for prevention and treatment, but they can trigger serious side effects. Levosimendan is a calcium sensitiser that enhances the heart's pump function. It potentially triggers fewer side effects than conventional medication.
Review question
In this review, we assessed whether the prophylactic use of levosimendan prevented reduced heart function and death in infants and children after surgery for congenital heart disease. We searched different medical literature databases and trial registers that collect information about planned, ongoing, and completed studies. We considered trials in which one group had received levosimendan and a second had received another drug instead, after heart surgery. Two review authors independently screened and collected the data.
Study characteristics
We identified five studies that had a total of 212 patients. All patients were under five years of age. The patients were given levosimendan during or immediately after heart surgery for a duration of 20 to 72 hours. They were monitored for 20 hours to six days. We asked all of the study authors for additional information about their trials. All but one author responded. The evidence is current to June 2016.
Quality of evidence
We found low‐quality evidence for all outcomes. This was mainly due to the small number of included patients (high imprecision of results). Thus, all results of the meta‐analysis must be viewed with caution.
Key results
The available data revealed no clear difference between levosimendan and conventional medication in preventing reduced heart function and death after heart surgery in the studied population. We also found no clear difference in the length of stay in the intensive care unit. The available data did not allow us to judge whether one of the treatment arms was superior to the other for three secondary outcomes: length of hospital stay, time on mechanical ventilation, need to implant circulatory support devices or the need for cardiac transplantation. Overall, few side effects were reported in any of the groups. We were unable to pool data to generate useful information about the safety of levosimendan.
Summary of findings
Summary of findings for the main comparison. Prophylactic levosimendan compared to standard treatment in children having undergone heart surgery.
| Prophylactic levosimendan compared to standard treatment in children following heart surgery | ||||||
| Patient or population: paediatric patients following heart surgery Setting: institutions providing postoperative care after heart surgery for congenital heart disease Intervention: levosimendan Comparison: standard inotropes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with standard inotropes | Risk with levosimendan | |||||
| Mortality follow‐up: range 1 day to 6 days | Study population | RR 0.47 (0.12 to 1.82) | 123 (3 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | ||
| 57 per 1000 | 27 per 1000 (7 to 104) | |||||
| Low cardiac output syndrome (LCOS) follow‐up: range 1 day to 6 days | Study population | RR 0.64 (0.39 to 1.04) | 83 (2 RCTs) | ⊕⊕⊝⊝ LOW 1, 3 | ||
| 367 per 1000 | 235 per 1000 (143 to 381) | |||||
| Length of intensive care unit stay (Length of ICU stay) follow‐up: range 1 day to 6 days | The mean length of stay in the ICU in the control groups ranged from 2.05 to 11 days | The mean length of stay in ICU in the intervention groups was 0.33 days higher (1.16 lower to 1.82 higher) | ‐ | 188 (4 RCTs) | ⊕⊕⊝⊝ LOW 1, 4 | |
| Length of hospital stay follow‐up: range 1 day to 6 days | The mean length of hospital stay in the control groups ranged from 15 to 15.8 days | The mean length of hospital stay in the intervention groups was 0.26 days higher (3.50 lower to 4.03 higher) | ‐ | 75 (2 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Duration of mechanical ventilation follow‐up: range 1 day to 6 days | The mean duration of mechanical ventilation in the control groups ranged from 0.29 to 6.5 days | The mean duration of mechanical ventilation in the intervention groups was 0.04 days lower (0.08 lower to 0.00 higher) | ‐ | 208 (5 RCTs) | ⊕⊕⊝⊝ LOW 1, 4 | |
| Mechanical circulatory support or cardiac transplantation follow‐up: range 1 day to 6 days | Study population | RR 1.49 (0.19 to 11.37) | 60 (2 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | ||
| 10 per 1000 | 14 per 1000 (2 to 108) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded for imprecision due to small sample size.
2 Downgraded for imprecision due to few number of events (less than 300).
3 Detection of outcome potentially dependent on study personnel: risk of detection bias in two studies due to unblinded setting.
4 Considerable inconsistency between study results.
Background
The possibilities of modern cardiac surgery have dramatically changed the management of congenital heart lesions in the last 50 years (Verheugt 2008). Despite the enormous decrease in morbidity and mortality after congenital cardiac surgery, low cardiac output syndrome (LCOS) remains a serious complication after surgery for congenital heart disease (Hoffman 2003). In 1975, Parr and colleagues reported that approximately 25% of American children with congenital heart disease postoperatively develop LCOS (Parr 1975). Wernovsky and colleagues found similar high rates in a study of American neonates and infants after the arterial switch operation in 1995 (Wernovsky 1995). Despite the fact that the reported incidence of LCOS varies between different studies, which is partly due to the lack of a standardized definition of LCOS, recent studies from around the world confirm the high incidence of this critical condition after cardiac surgery in children (Baysal 2010; Butts 2012; Froese 2009; Samadi 2012). Thus, the prevention and early treatment of LCOS in patients with congenital heart disease remains a major task of postoperative intensive care professionals (Stocker 2007).
Description of the condition
Low cardiac output syndrome is a potentially life‐threatening condition in which the pump function of the heart is not sufficient to maintain adequate circulation and oxygen delivery to all organs. An unambiguous definition of LCOS is lacking, and the literature offers different terms to describe this situation, such as postoperative myocardial dysfunction, postoperative cardiocirculatory dysfunction, acute cardiovascular dysfunction, postsurgery heart failure, heart failure, or postcardiotomy shock. A recent definition of LCOS in adult patients after cardiac surgery has been given in a consensus statement in 2012 (Vela 2012):
measured cardiac index below 2.2 L/min/m², without associated relative hypovolaemia or
presence of cardiogenic shock (defined by cardiac index below 2.0 L/min/m², with systolic blood pressure (SBP) below 90 mmHg, with oliguria, without relative hypovolaemia)
patients in whom the clinical manifestations are consistent with low cardiac output, i.e. oliguria (defined by diuresis less than 0.5 mL/kg/h), central venous saturation less than 60% (with normal arterial saturation), lactate higher than 3 mmol/L (without relative hypovolaemia), or both, or patients with postoperative inotropic medication, intra‐aortic counterpulsation balloon pump (IABP), or both.
As invasive measurement of the cardiac index in infants and children is not routinely performed, the constellation of clinical signs and abnormal parameters, i.e. the association of tachycardia, low SBP, poor perfusion with increasing core‐peripheral temperature gap, oliguria, low central venous saturation, and metabolic acidosis is mainly used to define LCOS (Hoffman 2003).
Causes of LCOS after cardiac surgery are multifactorial. A combination of the underlying heart disease and negative consequences of the cardiac surgery contribute to the development of LCOS. In particular, inadequate myocardial protection during aortic cross clamping, negative effects of cardioplegia, reperfusion myocardial injury, ventriculotomy, perioperative arrhythmias, activation of the inflammatory and complement cascades, and alterations in systemic and pulmonary vascular resistances are held responsible (Bailey 2004; Wernovsky 1995).
Low cardiac output syndrome accounts substantially for morbidity and mortality after surgery for congenital heart disease. In addition the need for longer mechanical ventilation (Shi 2008), and a prolonged hospital stay (Hoffman 2003), it has been identified as the main cause of death in children after open heart surgery (Ma 2007).
Given these fatal consequences, defining the best therapy for LCOS is an important challenge of paediatric cardiology and intensive care. Different vasoactive drugs are routinely used to treat it. All of them are given to increase the cardiac output. Therapeutic drug classes include inodilators, inotropes, inovasopressors, and vasodilators. To be more precise, dobutamine, dopamine, epinephrine, norepinephrine, milrinone, inhaled nitric oxide, and prostacyclin derivatives are mainly used ‐ as monotherapy or in combination (Vogt 2011). Catecholamines are often useful in the short term, but are limited by down‐regulation of adrenergic receptors, increased myocardial oxygen consumption, and excessive chronotropy at escalating dosing strategies (Namachivayam 2006). Phosphodiesterase inhibitors (e.g. milrinone) do not increase myocardial oxygen consumption as catecholamines do, but may have other side effects, such as thrombocytopenia and arrhythmia (Overgaard 2008).
Description of the intervention
Levosimendan is the first compound of the new drug class of calcium sensitisers. It generates more energy‐efficient myocardial contractility and induces peripheral and coronary vasodilatation without an increase of myocardial oxygen consumption (Endoh 2002; Turanlahti 2004). Levosimendan has been shown to be effective and safe for the treatment of heart failure, including LCOS, after cardiac surgery in adults. It was associated with reduced mortality compared to other drugs such as dobutamine in patients with low‐output heart failure (6% postoperative patients included) in the LIDO study (after 31 days: hazard ratio (HR) 0.43, 95% confidence intervals (CI) 0.18 to 1.00); P = 0.063; after 180 days: HR 0.57, 95% CI 0.34 to 0.95; P = 0.029; Follath 2002), or placebo in patients after acute myocardial infarction, left ventricular failure, or both (no postoperative patients included) in the RUSSLAN study (after 14 days: HR 0.56, 95% CI 0.33 to 0.95; P = 0.031; after 180 days: HR 0.67, 95% CI 0.45 to 1.00; P = 0.053; Moiseyev 2002). Evidence from meta‐analyses about beneficial effects of levosimendan in adults is extensive, e.g. a recently published meta‐analysis of studies that included adult patients after cardiac surgery showed reduced mortality among patients with reduced ejection fraction (odds ratio (OR) 0.48, 95% CI 0.23 to 0.76; P = 0.004), and a reduced incidence of LCOS (OR 0.24, 95% CI 0.15 to 0.36; P < 0.001; Lim 2015).
In paediatric patients, levosimendan is used for the treatment of acute heart failure in primary myocardial disease, or after sepsis, as well as for the treatment (Braun 2004; Magliola 2009; Namachivayam 2006; Vogt 2011), and prevention of LCOS (Egan 2006; Ebade 2013; Lechner 2012; Momeni 2011; Pellicer 2013; Ricci 2012), or in case of increased pulmonary artery pressure after surgery for congenital heart disease (Luca 2006). Since 2015, the German Society for Pediatric Cardiology (Deutsche Gesellschaft für Pädiatrische Kardiologie) recommends levosimendan as second line treatment for acute heart failure (Miera 2015). In an international guideline for septic shock, levosimendan is recommended for paediatric patients as a treatment for persistent low cardiac output state with high systemic vascular resistance and normal blood pressure (Dellinger 2013). A systematic review on levosimendan use in paediatric populations (surgical and medical patients) was published in 2015 (Silvetti 2015). Most of the included studies reported an improvement of ventricular function, central venous oxygen saturation, serum lactate levels and cardiac index. However, the meta‐analysis of randomised controlled trials showed no effect of levosimendan regarding the three analysed outcomes of mortality, ICU stay, and hospital stay.
Levosimendan is administered intravenously as either a loading dose followed by continuous infusion, or by continuous infusion. Typical loading doses range from 6 µg/kg (Magliola 2009; Namachivayam 2006) to 24 µg/kg (Braun 2004), over ten minutes (Namachivayam 2006) to 60 minutes (Egan 2006). Continuous infusion rates vary between 0.05 µg/kg/min (Momeni 2011; Namachivayam 2006) and 0.2 µg/kg/min (Braun 2004), and administration intervals range from 24 hours (Namachivayam 2006) to 72 hours (Ricci 2012). An often used dose regime is 12.5 µg/kg over ten minutes as a bolus dose, followed by 0.2 µg/kg/min over 24 hours (Shann 2003). If given prophylactically, the medication is started before, while on, or immediately after, separation from cardiopulmonary bypass (CPB). The bolus dose might be omitted, depending on the individual case.
Levosimendan has a half‐life of approximately one hour. It is reduced to an amine metabolite and further acetylated to the active metabolite OR‐1896. The half‐life of OR‐1896 is approximately 80 hours. OR‐1896 has a similar haemodynamic profile to levosimendan, which explains the long‐lasting haemodynamic effects after levosimendan infusion. Age‐dependant variables, such as composition and size of body water compartments, and different maturation of metabolic pathways, have a significant effect on drug pharmacokinetics. Because of the proportionately larger distribution volume of the drug in children, levosimendan dosing based on body surface area instead of weight is discussed to be more appropriate (Turanlahti 2004).
Adverse effects of levosimendan have been described as arrhythmia, hypotension, dizziness, headache, insomnia, hypocalcaemia, anaemia, and gastrointestinal disorders (Orion Pharma 2009). There is limited information about the proportion of paediatric patients who suffer side effects. Where available, reported proportions vary between 0% and 54% (Egan 2006; Lechner 2012; Momeni 2011; Ricci 2012; Turanlahti 2004). Hypotension, one of the most feared side effects, is reported to occur in 11% to 30% in retrospective studies (Fernández 2012; Lobacheva 2010). Fatal adverse effects are not reported.
How the intervention might work
Experience in adults with heart failure has shown that survival is adversely impacted by inotrope use (Elkayam 2007). As inotropy means the modification of muscular contractility, drugs that enhance myocardial contractility are generally called inotropes. Conventional inotropic agents increase myocardial concentrations of cyclic adenosine monophosphate (cAMP) that produce an increase in intracellular calcium, which as a side effect, possibly leads to myocardial cell death or lethal arrhythmia (Packer 1993).
However, calcium sensitisers, like levosimendan, act on the responsiveness of myofilaments to calcium. The effect of these pharmacological agents is based on a unique dual mechanism of action: calcium sensitisation through binding to troponin C in the myocardium, and the opening of adenosine triphosphate (ATP)‐sensitive potassium channels in systemic, pulmonary, and coronary vascular smooth muscle, which leads to vascular relaxation. These mechanisms evoke positive inotropy and vasodilation (Papp 2005). Through stabilization of the conformational change that occurs when levosimendan binds to calcium‐saturated cardiac troponin C, the calcium binding in the myofilaments is prolonged for a short period of time, which enhances myocardial contractility without elevated intracellular calcium requirements and without increased oxygen consumption. In the absence of calcium (e.g. during diastole), the levosimendan binding pocket of cardiac troponin C is not exposed, which inhibits binding of the drug. As a consequence, levosimendan does not impair myocardial relaxation (Luca 2006). In addition, the activation of ATP‐sensitive potassium channels, both on sarcolemma and mitochondria, may protect against myocardial ischaemia, and decreased levels of cytokines may prevent the development of further myocardial remodeling (Papp 2005).
In addition to its own positive inotropic effect, the use of levosimendan may, through its non‐ß‐adrenergic actions, allow for interruption of catecholamine infusions, which may mitigate the tolerance or tachyphylaxis associated with these drugs (Namachivayam 2006).
Why it is important to do this review
Evidence‐based medicine in paediatric cardiology is a particular challenge. No single institution caring for patients with congenital heart disease is capable of conducting an appropriately powered drug trial in a short enough period of time to allow for practice evolution. Furthermore, no international clinical database exists (Hoffman 2011). As a consequence, cardiovascular drugs in paediatric patients are commonly used on the basis of adult trials. Specific guidelines on the safe and effective use of drugs for the prevention and treatment of LCOS in infants and children are lacking. Consequently, prevention and treatment for LCOS is characterized by high variability in drug use and drug dosage. Levosimendan, being one drug for this indication, is not used routinely in paediatric patients in Europe. This may be due to limited study data, high treatment costs, market authorisation only in selected European countries (Vogt 2011), and missing approval for use in infants and children (Orion Pharma 2009).
The promising mode of action of levosimendan and its successful use in the adult population justifies further investigation into its effect in paediatric patients. This review is an essential step to provide further information about its potential to prevent LCOS and mortality in infants and children undergoing surgery for congenital heart disease.
Objectives
To review the efficacy and safety of the postoperative prophylactic use of levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomised controlled trials (RCTs) in parallel design, reported as full text. We also tried to identify possibly relevant studies reported in conference abstracts, and asked the authors for further information.
Types of participants
We included infants and children from birth to 18 years of age who were undergoing corrective or palliative heart surgery for any type of congenital heart disease. Stratification of the study population into three age‐groups, as described in the protocol, was not feasible due to the small number of included studies and participants.
Types of interventions
Intervention
Prophylactic levosimendan intravenous infusion alone or combined with other inotrope medications, i.e. milrinone, vasopressin, or nitric oxide, alone or in combination, started during or immediately after surgery for congenital heart disease, and regardless of the administration protocol, provided that levosimendan infusion rates were at least 0.05 µg/kg/min, and that levosimendan was administered for a duration of at least six hours.
Comparative intervention
Placebo or inotrope medication except levosimendan or other calcium sensitisers. Depending on the surgical intervention, infants and children would rarely be weaned from cardiopulmonary bypass without inotropic medication. Any existing trials comparing levosimendan with placebo were included in this review. We regarded other inotrope medication (such as epinephrine, norepinephrine, dopamine, dobutamine, or a combination of them), milrinone, vasopressin, inhaled nitric oxide, intravenous nitroprusside, or combination regimes as eligible comparators, as long as they did not contain levosimendan or other calcium sensitisers. We excluded studies using medication regimes that contained levosimendan or any other calcium sensitizer.
Types of outcome measures
Primary outcomes
All‐cause mortality within 30 days.
Time to death (censored after three months).
LCOS, defined as two or more of the following: (a) blood lactate higher than 3 mmol/L (27 mg/dL) or increase in blood lactate of at least 2 mmol/L (18 mg/dL) from baseline, prior to administration of levosimendan, (b) central venous oxygen saturation less than 50% in biventricular physiology without shunts, (c) increase in arterial to central venous oxygen saturation difference by at least 20% from baseline, prior to administration of levosimendan, (d) urine output less than 1 mL/kg/h, (e) peripheral skin temperature to core body temperature difference of more than 7°C, (f) cardiac index less than 2.2 L/min/m².
Secondary outcomes
Length of intensive care unit (ICU) stay.
Length of hospital stay.
Duration of mechanical ventilation.
Inotrope score.
Number of patients requiring mechanical circulatory support (e.g. extracorporeal membrane oxygenation (ECMO), pulsatile assist devices) or cardiac transplantation.
Number or proportion of adverse events (adverse events include: arrhythmia; hypotension, defined as blood pressures below blood pressure appropriate for age or body surface area; headache; intraventricular haemorrhage; hypocalcaemia; hyperpotassaemia; bronchospasm; thrombocytopenia, defined as platelet count less than 50/nL, or drop in platelet count of more than 100% from baseline, prior to administration of levosimendan; anaemia, defined as haemoglobin value below the age‐appropriate normal value; elevated serum levels of liver enzymes, defined as serum enzymatic activities more than two‐fold the age‐appropriate normal values; left ventricular ejection fraction (LVEF) less than 50%, or left ventricular fraction of shortening (LVFS) less than 28%, as assessed by biplane or M‐mode echocardiography).
If there had been any data on patient quality of life and economic costs available in the analysed studies, we would have commented on these outcomes in the discussion section, in a narrative form.
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 6) in the Cochrane Library (searched 14 June 2016);
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE Ovid (1946 to 14 June 2016);
Embase Classic and Embase Ovid (1947 to 13 June 2016);
Web of Science Core Collection, Thomson Reuters (1900 to 14 June 2016).
We adapted the preliminary search strategy for MEDLINE Ovid for use in the other databases (Appendix 1). We applied the Cochrane sensitivity‐maximising RCT filter to MEDLINE Ovid and adaptations of it to the other databases, except CENTRAL (Lefebvre 2011).
For trials that were completed or nearing completion, but had not yet been published, we also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov), Current Controlled Trials (www.controlled‐trials.com), and the WHO International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/) on 29 September 2016, using the search term levosimendan.
We searched all databases from their inception to the present, and imposed no restriction on the language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. If there were missing data, we contacted authors of published trials. We contacted expert colleagues from scientific medical societies of Great Britain, Germany, Italy, and Switzerland to obtain information about possible unpublished data. Furthermore, we searched the manufacturer's website for additional trial information in November 2016 (www.simdax.com).
Data collection and analysis
We carried out data analysis using RevMan 5 software (RevMan 5 2014). We performed additional analyses using the R package meta 4.7‐1 (R Development Core Team 2016).
Selection of studies
Two authors (JH and BS) independently screened the titles and abstracts of all of the reports we identified with the search. We retrieved the full‐text study reports and publications of those that seemed to meet our inclusion criteria, and two authors (JH and BS) independently screened these to make a final decision for inclusion. We identified and recorded reasons for exclusion of the ineligible studies following review of the full text. Disagreements would have been resolved by discussion with the third author (GR) if needed. We identified and excluded duplicates, and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009; Figure 1), and Characteristics of excluded studies table.
1.
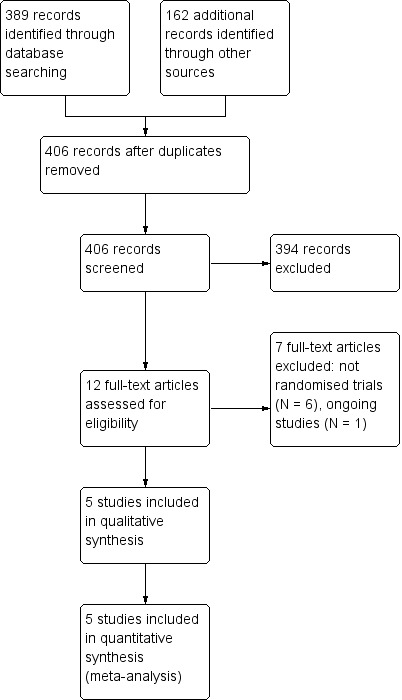
Study flow diagram.
Data extraction and management
We used a data collection form for study characteristics and outcome data. Two review authors (JH and BS) extracted the following study characteristics from included studies:
Methods: study design, total duration of study, details of any run‐in period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: N, mean age, age range, gender, inclusion and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
One review author (JH) transferred data into the Review Manager 5 file (RevMan 5 2014). We double‐checked that data were entered correctly by comparing the data presented in RevMan 5 with the study reports. A second review author (BS) spot‐checked study characteristics for accuracy against the trial reports.
Assessment of risk of bias in included studies
Two authors (JH and BS) independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving the third author (GR). We assessed risk of bias according to the following domains:
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias (e.g. industry funding).
We graded each potential source of bias as high, low ,or unclear, and provided a quote from the study report with a justification for our judgment in the 'Risk of bias' table. For each of the domains listed, we summarized the 'Risk of bias' judgements across different studies. Where information on the risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the Differences between protocol and review section of this review.
Measures of treatment effect
We assessed binary outcomes (mortality, LCOS) with risk ratio (RR) as an effect measure. For continuous variables, we used the mean difference (MD), or if more appropriate, the standardised mean difference (SMD). As information to calculate hazard ratios for time‐to‐event variables (time‐to‐death, duration of mechanical ventilation) was not available, we used MD and SMD as an effect measure.
Unit of analysis issues
As we only included RCTs in parallel design, unit of analysis issues did not occur.
Dealing with missing data
We contacted investigators in order to obtain missing data. Some unpublished data were added as a result.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis.
Assessment of reporting biases
We obtained additional information from all but one study author. Funnel plots would have been applied to assess reporting bias if the number of studies had allowed it (Harbord 2006), and results would have been adjusted for an additional sensitivity analysis (Rücker 2011).
Data synthesis
We undertook meta‐analyses only where this was meaningful, i.e. if the treatments, participants, outcomes, and the underlying clinical question were similar enough for pooling to make sense. We used a random‐effects model to pool data and illustrated data using forest plots with 95% CI.
Subgroup analysis and investigation of heterogeneity
Due to the small number of infants and children studied in clinical trials, and owing to the fact that most surgical interventions for congenital heart disease are carried out in the first year of life, we planned an age‐based subgroup analysis of two age groups: infants younger than 12 months of age, and children and adolescents from one to 18 years of age. Furthermore, we defined two subgroups based on cardiovascular physiology: (1) infants and children after biventricular surgical repair of a congenital heart disease versus (2) infants and children after univentricular palliation of a congenital heart disease. Neither subgroup analyses were feasible, due to the small number of studies and participants.
Sensitivity analysis
We expected to find studies with different levels of risk of bias. For a sensitivity analysis, studies with high risk of bias would have been excluded.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies. We avoided making recommendations for practice; our implications for research suggest priorities for future research, and outline the remaining uncertainties in the area.
Summary of findings table
We created a 'Summary of findings' table using the following outcomes: mortality, LCOS, length of intensive care unit stay, length of hospital stay, duration of mechanical ventilation, mechanical circulatory support, and cardiac transplantation. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and GRADEpro software (GRADEpro GDT 2014). We justified all decisions to down‐ or up‐grade the quality of studies in the footnotes, and we made comments to aid reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
We searched the electronic databases on 14 June 2016. The search yielded 65 records from CENTRAL, 205 records from MEDLINE, 55 records from Embase, and 64 records from Web of Science. For details about the applied search strategy, see Appendix 1. After de‐duplication, we had 272 records left for screening.
We ran registry searches on ClinicalTrials.gov, Current Controlled trials, and the WHO ICTRP Search Portal on 29 September 2016, which yielded another 134 records after de‐duplication. In the case of records that seemed to meet the inclusion criteria of the review but had not yet published any results, we contacted the authors about the current status of the study. See Characteristics of ongoing studies for more details.
Our searches of other resources yielded no additional studies.
From all sources, we assessed 12 records as eligible for full‐text screening, five of which were included in the qualitative and quantitative synthesis, and seven were excluded with reasons.
See Figure 1 for the study flow diagram.
Included studies
We included five RCTs in parallel design, with a total of 212 infants and children.
Sample sizes varied between 20 (Pellicer 2013) and 63 (Ricci 2012). In two studies, the authors excluded one patient each after randomisation (Lechner 2012; Momeni 2011). As the authors had stopped collecting information on the outcome after exclusion, these patients were not included in the meta‐analysis. Two studies included only neonates (Pellicer 2013; Ricci 2012), one study focused on infants aged up to one year (Lechner 2012), one study included children aged up to 38 months (Ebade 2013), and one study up to 5 years (Momeni 2011). None examined subgroups of patients by age or by type of cardiac disease. Patients with single ventricle physiology did not participate in any of the studies.
Four of the studies were conducted in tertiary care children's hospitals in Europe (Lechner 2012; Momeni 2011; Pellicer 2013, Ricci 2012), and one in the department of anaesthesia of a university hospital in Egypt (Ebade 2013).
Of the included studies, one compared levosimendan with loading dose versus dobutamine infusion (Ebade 2013), three compared levosimendan without loading dose versus milrinone (Lechner 2012; Momeni 2011; Pellicer 2013), and one compared levosimendan without loading dose versus standard inotrope infusion (milrinone and dopamine (Ricci 2012)). Levosimendan was not compared to placebo in any of the studies. Follow‐up intervals varied between 20 hours and six days.
None of the studies evaluated time‐to‐death or adverse events as defined endpoints. Low cardiac output syndrome was a defined outcome in only one study (Ricci 2012). In addition, we were able to reconstruct the number of infants and children with LCOS according to our definition, from unpublished data from two authors (Lechner 2012; Pellicer 2013). Two studies reported on length of hospital stay (Lechner 2012; Momeni 2011), three studies reported on inotropic scores (Lechner 2012; Pellicer 2013; Ricci 2012), and four studies on length of ICU stay (Ebade 2013; Lechner 2012; Momeni 2011; Ricci 2012). All five studies evaluated mortality, duration of mechanical ventilation, and circulatory support or cardiac transplantation.
Three study authors did not comment on conflicts of interest or funding (Ebade 2013; Momeni 2011; Ricci 2012). One study author stated there were no conflicts of interest (Lechner 2012). One study author disclosed financial support for pharmacokinetic studies from Orion Pharma Spanish Division (Pellicer 2013).
For further details about the included studies see the Characteristics of included studies table.
Excluded studies
After full‐text screening, we excluded six records because they were not RCTs. For further details about the excluded studies, see the Characteristics of excluded studies table.
Ongoing studies
Registry searches yielded one ongoing study, which is still recruiting participants (EUCTR 2012‐005310‐19‐ES). For further details see the Characteristics of ongoing studies table.
Risk of bias in included studies
Generally the risk of bias in the included studies was low.
Allocation
In one study, sealed envelopes were used for randomisation and allocation concealment (Ebade 2013). As no further details about the exact method of randomisation via sealed envelopes were given, risk of selection bias was judged as unclear. In the other studies, computer‐generated random numbers or codes were used for allocation of patients, and sealed envelopes ensured allocation concealment (Lechner 2012; Momeni 2011; Pellicer 2013; Ricci 2012).
Blinding
Two studies were not blinded at all (Ebade 2013; Ricci 2012). As study participants in the two studies were younger than 38 months of age, unblinded participants may not represent a high risk, but unblinded personnel does. One study was unblinded after 48 hours (Pellicer 2013). As outcome assessment in the study was performed over six days, there was a potential risk of detection bias after unblinding.
Incomplete outcome data
In Momeni 2011, 41 patients were randomised to one of the study groups. After initiation of the study medication, two patients died and two patients required extracorporeal membrane oxygenation. These four patients were excluded from the study. We judged this to be a high risk of attrition bias because these were serious events of major interest. In this meta‐analysis, the aforementioned events were re‐included for mortality and mechanical circulatory support, and excluded for the other outcomes because there were no further data available.
Selective reporting
In the reports of the included studies, all outcomes listed in the methods section were reported.
Other potential sources of bias
We found no other potential sources of bias.
For further details about our rating of risk of bias for each criterion of the included studies, see the 'Risk of bias' tables in the Characteristics of included studies section, Figure 2, and Figure 3.
2.
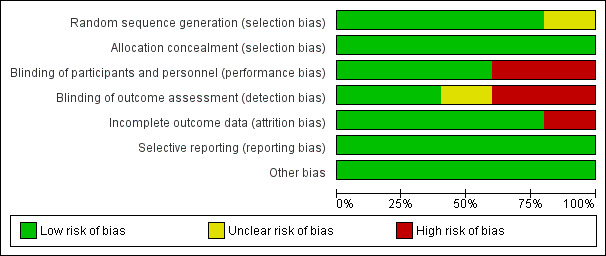
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
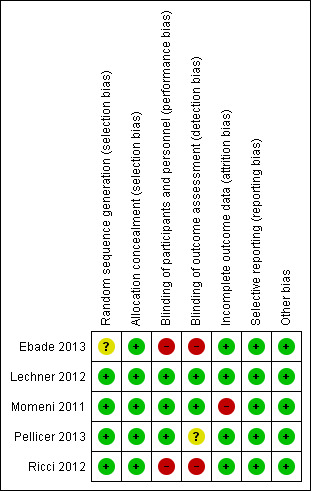
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Primary outcomes
We pooled data for mortality and LCOS using the Mantel‐Haenzel method and a random‐effects model. We assessed the outcomes with risk ratio as the effect measure.
All‐cause mortality within 30 days
We only assessed mortality up to six days after initiation of the study medication. In the study of Ebade 2013 and Lechner 2012 no events concerning mortality were reported. Overall, the number of events was small (levosimendan 3/63, control 6/60). Prophylactic administration of levosimendan did not show a reduction of mortality in infants and children undergoing surgery for congenital heart disease compared to the administration of milrinone, dopamine, or a combination of dobutamine and milrinone (RR 0.47, 95% CI 0.12 to 1.82; participants = 123; studies = 3; I² = 0%; low‐quality evidence). To assess for heterogeneity, we re‐analysed the data with R (R Development Core Team 2016), which provided a 95% confidence interval for I² = 0% (95% CI 0% to 55.3%). This broad confidence interval showed the large uncertainty in the I² value, and thus, in assessing heterogeneity among only a few studies. The interpretation is not that homogeneity was proven, but that there was no reason to assume considerable heterogeneity. For further details and illustration of data see Analysis 1.1 and Figure 4. GRADE assessment revealed low‐quality evidence, due to considerable imprecision (small number of included patients, low event rate). For details of the quality of evidence assessment see Table 1.
1.1. Analysis.
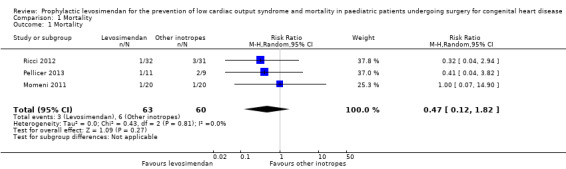
Comparison 1 Mortality, Outcome 1 Mortality.
4.
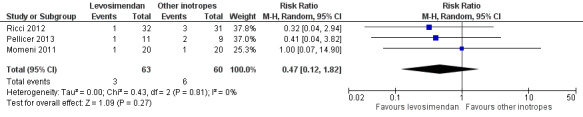
Forest plot of comparison: 1 Mortality, outcome: 1.1 Mortality.
Time‐to‐death
Detailed time‐to‐death data were not available from any of the studies to generate hazard ratio (HR) and Kaplan‐Meier estimates.
Low cardiac output syndrome (LCOS)
As expected, the definition of LCOS varied between the studies and did not always exactly meet the definition we stated in the protocol of this review. Three studies measured this outcome (Lechner 2012; Pellicer 2013; Ricci 2012), one of which reported no LCOS events (Lechner 2012).
Low cardiac output syndrome was reported to occur in 15/62 patients in the levosimendan group, and in 22/60 patients in the control group. Prophylactic administration of levosimendan did not show a reduction of LCOS compared to other inotropes (RR 0.64, 95% CI 0.39 to 1.04; participants = 83; studies = 2; I² = 0%; low‐quality evidence). The outcome was based mainly on the results of the largest study (Ricci 2012, participants = 63). For further details and illustration of data see Analysis 2.1 and Figure 5. GRADE assessment showed low‐quality evidence, due to the risk of detection bias (largest contributing study was not blinded), and on the small number of included patients (imprecision). For details of the quality of evidence assessment, see Table 1.
2.1. Analysis.
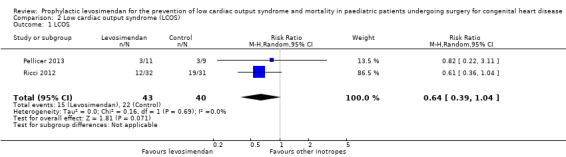
Comparison 2 Low cardiac output syndrome (LCOS), Outcome 1 LCOS.
5.
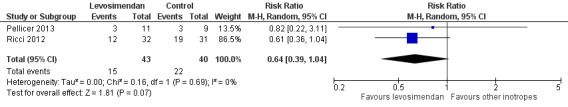
Forest plot of comparison: 2 Low cardiac output syndrome (LCOS), outcome: 2.1 LCOS.
Secondary outcomes
We used the inverse variance method and a random‐effects model to pool data and calculate the mean difference for length of ICU stay, length of hospital stay, and duration of mechanical ventilation. We used the Mantel‐Haenzel method and a random‐effects model to pool data and calculate the risk ratio for mechanical circulatory support or cardiac transplantation.
We were unable to create funnel plots to assess reporting biases due to the small number of studies.
Length of ICU stay
Four studies reported data on length of ICU stay (Ebade 2013; Lechner 2012; Momeni 2011; Ricci 2012). Results varied between the studies, leading to conflicting point estimates. Overall, there was inconclusive evidence for the outcome, length of ICU stay (MD 0.33 days, 95% CI ‐1.16 to 1.82; participants = 188; studies = 4; I² = 35%; low‐quality evidence). To adjust for the very differing time frames, which led to an inappropriately high weight for Ebade 2013, due to the small standard deviation in this study, we also performed a sensitivity analysis, using standardised mean difference as an effect measure. Again there was no difference between the two treatment arms (SMD ‐0.05, 95% CI ‐0.54 to 0.44; low‐quality evidence). Heterogeneity of the results between the studies based on the I² statistic was higher than in the primary outcome analyses. For further details, see Analysis 3.1 and Analysis 3.2. GRADE assessment revealed low‐quality evidence, due to conflicting point estimates between the studies (inconsistency) and the small number of included patients (imprecision). For details of the quality of evidence assessment see Table 1.
3.1. Analysis.
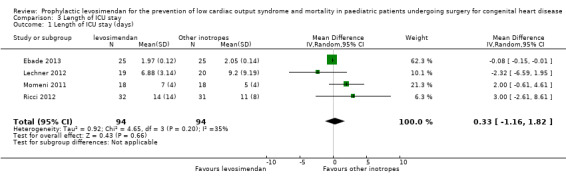
Comparison 3 Length of ICU stay, Outcome 1 Length of ICU stay (days).
3.2. Analysis.
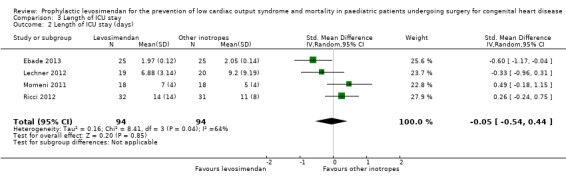
Comparison 3 Length of ICU stay, Outcome 2 Length of ICU stay (days).
Length of hospital stay
Only two studies contributed to the analysis of length of hospital stay (Lechner 2012; Momeni 2011). Confidence intervals of the outcome in the two studies were very similar. Overall, there was inconclusive evidence regarding the question of whether levosimendan or conventional inotrope treatment resulted in a longer hospital stay (MD 0.26 days, 95% CI ‐3.50 to 4.03; participants = 75; studies = 2; I² = 0%; low‐quality evidence). For further details see Analysis 4.1. Using GRADE, we assessed the quality of the evidence to be low, due to the very small number of included patients. For details of the quality of evidence assessment see Table 1.
4.1. Analysis.
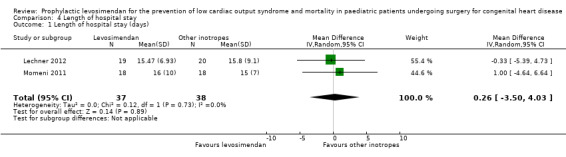
Comparison 4 Length of hospital stay, Outcome 1 Length of hospital stay (days).
Duration of mechanical ventilation
All studies evaluated the duration of mechanical ventilation. Results varied widely between the studies, leading to inconclusive evidence for the outcome (MD ‐0.04 days, 95% CI ‐0.08 to 0.00; participants = 208; studies = 5; I² = 0%; low‐quality evidence). When using standardised mean difference as an effect measure to balance the weight between the studies, again, there was no difference between levosimendan and the control groups (SMD ‐0.08, 95% CI ‐0.43 to 0.27: low‐quality evidence). To assess for heterogeneity, we re‐analysed the data with R, which provided a 95% confidence interval for I² = 0% (95% CI 0% to 76.4%). This broad confidence interval again showed the large uncertainty in the I² value, and thus, in assessing heterogeneity with only few studies. The interpretation is not that homogeneity was proven, but that there was no reason to assume considerable heterogeneity. For further details, see Analysis 5.1 and Analysis 5.2. GRADE assessment revealed low‐quality of evidence, due to conflicting point estimates between the studies (inconsistency), and the small number of included patients (imprecision). For details of the quality of evidence assessment see Table 1.
5.1. Analysis.
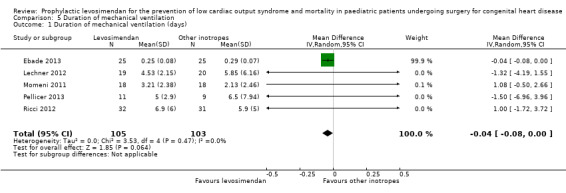
Comparison 5 Duration of mechanical ventilation, Outcome 1 Duration of mechanical ventilation (days).
5.2. Analysis.
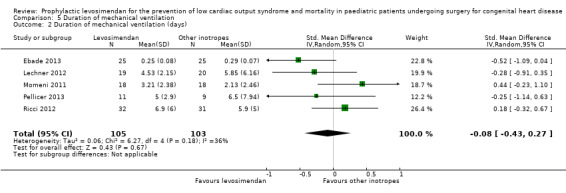
Comparison 5 Duration of mechanical ventilation, Outcome 2 Duration of mechanical ventilation (days).
Inotrope score
Three studies evaluated the postoperative level of inotrope medication using inotrope scores (Lechner 2012; Pellicer 2013; Ricci 2012). Meta‐analysis of the outcome was not feasible due to inconsistently defined scores.
Mechanical circulatory support or cardiac transplantation
All five studies reported on the number of patients who required mechanical circulatory support or cardiac transplantation. Three studies did not show any events for this outcome (Ebade 2013; Lechner 2012; Ricci 2012). As the event occurred extremely rarely (overall three cases, no case of cardiac transplantation), the significance of the analysed result was very limited (RR 1.49, 95% CI 0.19 to 11.37; participants = 60; studies = 2; I² = 0%; low‐quality evidence). For further details see Analysis 6.1. Used GRADE to assess the quality of the evidence, we determined that it was low, due to the small number of included patients and the very small number of events. For details of the quality of evidence assessment see Table 1.
6.1. Analysis.
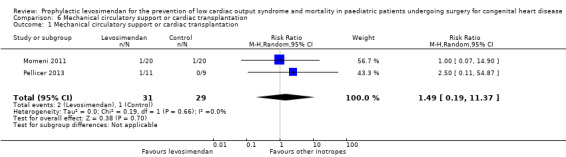
Comparison 6 Mechanical circulatory support or cardiac transplantation, Outcome 1 Mechanical circulatory support or cardiac transplantation.
Adverse events
The authors of two studies stated explicitly that serious adverse events (Lechner 2012), or side effects (Ricci 2012), did not occur. Overall data regarding adverse events were very limited.
Arrhythmia
Lechner 2012 and Momeni 2011 stated they did not see any arrhythmias in their study population. Pellicer 2013 reported atrioventricular asynchrony in one case in the levosimendan group (1/11) and no arrhythmias in the control group (0/9). Ebade 2013 and Ricci 2012 did not clearly state if they saw arrhythmias in their study population or not.
Hypotension
Meta‐analysis was not feasible for hypotension because different types of blood pressures were recorded and there were different manners of reporting. Ebade 2013 reported systolic and diastolic blood pressure as means and standard deviations with P values that indicated no significant difference between the study groups. Lechner 2012 reported systolic arterial pressure as means and standard errors of mean in a figure, with no significant difference between the study groups. Momeni 2011 and Ricci 2012 published data of mean arterial pressure as means and standard deviations, and found no significant differences between the study groups. Pellicer 2013 reported diastolic blood pressure as means and standard errors of mean, which did not show a group‐dependent treatment effect.
Headache
As in four of the five included studies participants were younger than 38 months, data about headache could not be collected. The remaining study, Momeni 2011, did not state whether headaches occurred in the study population.
Intraventricular haemorrhage
Intraventricular haemorrhage was reported in one of nine patients in the levosimendan group (grade III intraventricular haemorrhage), and in two of nine patients in the control group (one grade I and one grade III intraventricular haemorrhage) in one of the included studies (Pellicer 2013). The other four studies did not mention this adverse event.
Hypocalcaemia and hyperpotassaemia
Pellicer 2013 found no incidence of hyperpotassaemia; the other four trials did not assess hyperpotassaemia. None of the trials assessed hypocalcaemia.
Bronchospasm
Pellicer 2013 reported that they saw no cases of bronchospasm. The other studies did not address bronchospasm.
Thrombocytopenia and anaemia
Pellicer 2013 reported that they saw no cases of thrombocytopenia. The other studies did not report assessment of thrombocytopenia. None of the studies reported assessment of anaemia.
Elevated liver enzymes
In Momeni 2011, aspartate‐aminotransferase (synonym: glutamate‐oxalacetate‐transaminase) was elevated from 1 hour to 48 hours (end of study) after PICU admission in both treatment groups, with no significant difference between the groups. In Pellicer 2013, liver enzymes were measured and showed not to be elevated, according to the definition of our review. Ricci 2012 assessed the impact of levosimendan on different organs using the paediatric multiple organ dysfunction score (P‐MODS; Graciano 2005), showing that average scores were low and not significantly different between the two treatment groups. However, the score did include bilirubin or liver enzymes as measures of liver function. Ebade 2013 and Lechner 2012 did not report on elevated liver enzymes.
Left ventricular ejection fraction (LVEF) less than 50%, or left ventricular fraction of shortening (LVFS) less than 28%
None of the included studies specified measurement of LVEF less than 50%. Three of the included studies did not specify measurement of LVFS (Ebade 2013; Momeni 2011; Ricci 2012). Lechner 2012 reported LVFS values in a figure, showing no significant difference between the treatment groups. None of the displayed values was less than 28%. Pellicer 2013 found LVFS less than 28% in 3/11 patients in the levosimendan group and in 3/9 patients in the control group, which represented no significant difference between the treatment arms.
Discussion
Summary of main results
The search for studies of prophylactic levosimendan to prevent LCOS and reduce mortality in paediatric patients undergoing surgery for congenital heart disease, yielded documentation from five studies that met our inclusion criteria, with a total of 212 patients. The studies were published between 2011 and 2013. They were heterogeneous in the study population’s congenital heart disease severity, in the medication the control group received (dobutamine, milrinone, and milrinone plus dopamine), in dosages of intervention and control medication, and recorded outcomes. None of the studies compared levosimendan to placebo. Two studies included only neonates, in two other studies, the study population was younger than 38 months of age; just one study included children as old as five years, which limited the evidence mainly to neonates and young infants.
Using the GRADE approach, there was low‐quality evidence for each of the analysed outcomes.
Follow‐up for mortality, one of our primary outcomes, only lasted six days, which yielded very few events. Meta‐analysis showed no clear differences between the treatment groups. Time‐to‐death was not recorded in any of the studies. Prophylactic administration of levosimendan did not show a significant reduction in LCOS in the meta‐analysis of the three studies (122 patients) that recorded LCOS as an outcome, although this result was based mainly on the input of one of the included studies. In addition, the definition for LCOS varied among the studies.
Meta‐analysis of secondary outcomes, such as length of ICU stay, length of hospital stay, duration of mechanical ventilation, and the need for mechanical circulatory support or cardiac transplantation, revealed no clear differences between the levosimendan and control groups. The secondary outcomes of length of ICU stay, length of hospital stay, and duration of mechanical ventilation might not necessarily reflect the true effect of the interventions, because they were also dependant on the clinical practices in the different institutions. Meta‐analysis of inotrope scores was not feasible, due to inconsistently defined scores across the studies.
Data on adverse events were very limited. Two studies claimed not to have recorded any adverse events. Safety outcomes were too seldom and inconsistently reported to validate any comparisons between studies. None of the studies demonstrated a significant difference between levosimendan and their particular control group for hypotension.
Overall completeness and applicability of evidence
The current level of evidence was insufficient to judge whether prophylactic levosimendan prevents LCOS and lowers mortality in paediatric patients undergoing surgery for congenital heart disease. Overall, the number of RCTs and included participants was too low to draw definitive conclusions regarding the primary outcomes of this review (mortality, time‐to‐death, or LCOS). The following findings also limited the applicability of the evidence:
Study populations in the available studies included no children over five years of age, or with univentricular cardiac anatomy ‐ important patient groups in daily practice.
Given that there was only one study in which an initial loading dose was administered, conclusions about whether a loading dose was better, was impossible (Ebade 2013).
Application rates of levosimendan infusion varied between 0.05 µg/kg/min and 0.2 µg/kg/min, over an infusion period that ranged between 20 and 72 hours. The limited number of studies did not enable comparisons to judge the effect of different dosages.
Follow‐up periods were too short to yield robust evidence on mortality and time‐to‐death.
Inconsistency in LCOS definitions affected conclusions about this key outcome. Furthermore, differentiating between clinical signs of LCOS and side effects from the study medication (e.g. hypotension) is sometimes difficult, and misinterpretation can lead to faulty outcome data.
Data on side effects from the study medication were very limited, and again, short follow‐up periods limited conclusions about safety outcomes.
Quality of the evidence
Our search yielded five RCTs with a total of 212 patients.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of evidence.
Study limitations and risk of bias: as detection of LCOS was potentially dependant on the study personnel, there was a certain risk of detection bias in unblinded settings. Since the largest study that contributed to LCOS was not blinded, quality of the evidence was downgraded.
Consistency: for length of ICU stay and duration of mechanical ventilation, the quality of the evidence was downgraded because of conflicting point estimates of the studies that contributed to the outcome (high inconsistency).
Imprecision: the quality of the evidence for imprecision was downgraded for all outcomes, because of the small number of included patients, which resulted in wide confidence intervals that included a risk ratio of 1.0. For mortality and mechanical circulatory support or cardiac transplantation, we downgraded the quality of the evidence two levels because of the very low event rate. For length of hospital stay, the quality of the evidence was downgraded two levels because of the very small number of included patients.
Indirectness: quality of the evidence was not downgraded for indirectness in any of the assessed outcomes.
Publication bias: quality of the evidence was not downgraded for concerns regarding publication bias.
In summary, the quality of the evidence for each of the analysed outcomes of this review was low. Therefore, we could not draw a robust conclusion on the effect of prophylactic levosimendan in paediatric patients undergoing surgery for congenital heart disease.
For more details on the quality of evidence and the GRADE assessment see Table 1.
Potential biases in the review process
We identified relevant studies through an updated electronic search in June 2016. The identified RCTs were identical to a current published systematic review about levosimendan in paediatric patients (Silvetti 2015).
To obtain all the relevant data, we contacted all the study authors of the included studies. Four of the five authors responded, which could represent a reporting bias because we did not obtain information beyond that reported in the literature from one of the trials. Furthermore, some of the outcomes sought for this review were unavailable from all included studies, due to fewer outcomes or a somewhat different focus in the study protocols than that in this review’s protocol.
Agreements and disagreements with other studies or reviews
A systematic review published in 2015 focused on the effect of levosimendan in paediatric patients in general (Silvetti 2015). The electronic search was conducted in the Cochrane Central Register of Clinical Trials, MedCentral, PubMed, and Embase databases, and the authors identified the same RCTs as those included in our review. In their final analysis, the authors included another 19 articles that were either case reports or case series about levosimendan in paediatric patients. Of the 623 included patients, 94% were undergoing cardiac surgery. Side effects reported in case series and retrospective studies involved persistent hypotension and tachycardia. For meta‐analysis, the authors included the five RCTs also included in this review, focusing on only three outcomes (mortality, ICU stay, hospital stay). In agreement with our results, they detected no clear difference in mortality, length of ICU stay, or length of hospital stay, according to the published analysis results. The authors did not summarize methodological details of the included studies or give any comments on the risk of bias or the quality of the evidence, e.g. with a GRADE assessment.
A systematic review of the prophylactic effect of milrinone in children in the setting of cardiac surgery was published in 2015 (Burkhardt 2015). Three of the five included studies compared milrinone to levosimendan, and were therefore, also included in our review (Lechner 2012; Momeni 2011; Pellicer 2013). For the endpoints LCOS, duration of ICU stay, duration of hospital stay, and duration of mechanical ventilation, the authors performed subgroup analyses, pooling data of the three trials that examined milrinone versus levosimendan, which showed no significant difference between the two treatment arms.
We are not aware of any other studies addressing our review question that might have been overlooked, and should have been included in this review.
Authors' conclusions
Implications for practice.
The current level of low‐quality evidence was insufficient to judge whether prophylactic levosimendan prevents low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease. So far, no clear differences in effects were detected between levosimendan and standard inotrope treatments in this setting.
The authors evaluated the quality of evidence as low, using the GRADE approach. Reasons for downgrading were serious risk of bias (performance and detection bias due to unblinded setting of two RCTs), serious risk of inconsistency, and serious to very serious risk of imprecision (small number of included patients, low event rates).
Implications for research.
The number of RCTs evaluating the effect of prophylactic levosimendan in paediatric patients after surgery for congenital heart disease is limited, the number of included patients too low, and follow‐up periods too short, to yield results with sufficient significance.
According to our sample size calculation, 652 participants were needed to prove superiority of levosimendan for the outcome mortality. We started from an assumed event rate in the control group of p0 = 10%, and assumed a risk ratio of 0.5 (as observed in the meta‐analysis). Assuming equal group sizes, alpha = 0.05, and a power 1‐beta = 0.80, we used the formula given by Schulz and Grimes (Schulz 2005): N per group = c*((RR + 1) ‐ p0*(RR^2 + 1))/(p0*(1 ‐ RR)^2). For alpha = 0.05 and 1‐beta = 0.80, we obtained c = 7.85. This provided 432 per group, for a total of 864 for both groups. As the current sample size was 212, this meant that 652 new patients were needed to see superiority of levosimendan in mortality.
Future RCTs should be multicentric to address the inherent problem of small patient numbers per center, and greater effort should be made to contribute data from infants and children from different age groups, with diverse cardiac anatomies (including univentricular physiology), while paying attention to the specific pharmacokinetic profile of levosimendan. To allow comparisons, a standard definition of LCOS, with criteria as objective as possible, is essential. Follow‐up periods should be extended to detect higher numbers of important events and obtain better evidence on long‐term outcomes.
Network meta‐analysis of all interventions, including milrinone, levosimendan, and all comparators listed in Types of outcome measures would be desirable, as including all comparators in a joint analysis is likely to increase the precision of the treatment effect estimates for all comparisons.
What's new
| Date | Event | Description |
|---|---|---|
| 26 July 2017 | New citation required but conclusions have not changed | Review amendment for publication with a new citation as parts of the abstract were edited in May 2017. |
| 11 May 2017 | Amended | Parts of the abstract edited. |
Notes
26 July 2017: The abstract was edited and this amendment is being published as a new citation.
Acknowledgements
We thank Nicole Martin for her valuable help with the design of the search strategy and the electronic literature searches across databases, as well as her helpful editorial comments. We would also like to thank the authors of the included studies who provided additional data about their trials.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor: [Heart Defects, Congenital] explode all trees #2 MeSH descriptor: [Heart Diseases] explode all trees and with qualifier(s): [Congenital ‐ CN] #3 (heart near/2 (defect* or abnormal* or malform*)) #4 (congenital near/2 (heart or cardiac or cardio*)) #5 deletion next syndrome* #6 (alagille* near/2 syndrome*) #7 (watson* near/2 syndrome*) #8 (hepatofacioneurocardiovertebral near/2 syndrome*) #9 cardiovertebral near/2 syndrome* #10 (arteriohepatic near/2 dysplasia*) #11 "hepatic ductular hypoplasia*" #12 aort* near/3 coarctation* #13 (arrhythmogenic near/3 ventricular) #14 Barth next Syndrome #15 ((heart* or cor) 2 triatria*) #16 (subdivided near/3 atrium*) #17 "coronary vessel anomal*" #18 myocardial next bridging* #19 "criss‐cross heart*" #20 "crisscross heart*" #21 "criss cross heart*" #22 dextrocardia* #23 (kartagener* near/2 (syndrome* or triad)) #24 siewert next syndrome* #25 "polynesian bronchiectas?s" #26 "primary ciliary dyskinesia" #27 (paten* near/2 ductus near/2 arteriosus) #28 (ebstein* near/2 (anomal* or malform*)) #29 ectopia next cordis #30 (Eisenmenger* near/2 (complex or syndrome)) #31 ((heart or cardiac) near/2 "septal defect*") #32 heterotaxy next syndrome* #33 polysplenia next syndrome* #34 asplenia next syndrome* #35 ambiguus near/3 situs #36 isomerism #37 ivemark next syndrome #38 visceral next heterotax* #39 "hypoplastic left heart syndrome" #40 isolated next noncompaction #41 isolated next non‐compaction #42 leopard next syndrome* #43 cardiomyopathic* next lentiginosis #44 lentigines next syndrome* #45 levocardia #46 marfan* next syndrome #47 noonan next syndrome #48 turner* next syndrome #49 fallot* near/2 tetralogy #50 (transpos* near/4 (arter* or vessel*)) #51 (tricuspid near/2 atresias) #52 (fallot* near/2 trilogy) #53 turner* next syndrome #54 bonnevie‐ullrich next syndrome #55 "bonnevie ullrich syndrome" #56 "bonnevie‐ullrich status" #57 "bonnevie ullrich status" #58 "ullrich‐turner syndrome" #59 (gonadal near/2 dysgenesis) #60 "univentricular heart" #61 "arterial switch" #62 norwood* #63 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 #64 levosimendan or levosimedan or or1259 or "or 1259" or simdax #65 MeSH descriptor: [Cardiotonic Agents] this term only #66 (inotropic near/2 (agent* or drug* or medicat* or act*)) #67 inodilator* #68 (calcium near/2 sensiti*) #69 #64 or #65 or #66 or #67 or #68 #70 #63 and #69
MEDLINE
1. exp Heart Defects, Congenital/ 2. exp Heart Diseases/cn [Congenital] 3. (heart adj2 (defect* or abnormal* or malform*)).tw. 4. (congenital adj2 (heart or cardiac or cardio*)).tw. 5. deletion syndrome*.tw. 6. (alagille* adj2 syndrome*).tw. 7. (watson* adj2 syndrome*).tw. 8. (hepatofacioneurocardiovertebral adj2 syndrome*).tw. 9. (cardiovertebral adj2 syndrome*).tw. 10. (arteriohepatic adj2 dysplasia*).tw. 11. hepatic ductular hypoplasia*.tw. 12. (aort* adj3 coarctation*).tw. 13. (arrhythmogenic adj3 ventricular).tw. 14. Barth Syndrome.tw. 15. ((heart* or cor) adj2 triatria*).tw. 16. (subdivided adj3 atrium*).tw. 17. coronary vessel anomal*.tw. 18. myocardial bridging*.tw. 19. criss‐cross heart*.tw. 20. crisscross heart*.tw. 21. criss cross heart*.tw. 22. dextrocardia*.tw. 23. (kartagener* adj2 (syndrome* or triad)).tw. 24. siewert syndrome*.tw. 25. polynesian bronchiectas?s.tw. 26. primary ciliary dyskinesia.tw. 27. (paten* adj2 ductus adj2 arteriosus).tw. 28. (ebstein* adj2 (anomal* or malform*)).tw. 29. ectopia cordis.tw. 30. (Eisenmenger* adj2 (complex or syndrome)).tw. 31. ((heart or cardiac) adj2 septal defect*).tw. 32. heterotaxy syndrome*.tw. 33. polysplenia syndrome*.tw. 34. asplenia syndrome*.tw. 35. (ambiguus adj3 situs).tw. 36. isomerism.tw. 37. ivemark syndrome.tw. 38. visceral heterotax*.tw. 39. hypoplastic left heart syndrome.tw. 40. isolated noncompaction.tw. 41. isolated non‐compaction.tw. 42. leopard syndrome*.tw. 43. cardiomyopathic* lentiginosis.tw. 44. lentigines syndrome*.tw. 45. levocardia.tw. 46. marfan* syndrome.tw. 47. noonan syndrome.tw. 48. turner* syndrome.tw. 49. (fallot* adj2 tetralogy).tw. 50. (transpos* adj4 (arter* or vessel*)).tw. 51. (tricuspid adj2 atresias).tw. 52. (fallot* adj2 trilogy).tw. 53. turner* syndrome.tw. 54. bonnevie‐ullrich syndrome.tw. 55. bonnevie ullrich syndrome.tw. 56. bonnevie‐ullrich status.tw. 57. bonnevie ullrich status.tw. 58. ullrich‐turner syndrome.tw. 59. (gonadal adj2 dysgenesis).tw. 60. univentricular heart.tw. 61. arterial switch.tw. 62. norwood*.tw. 63. or/1‐62 64. levosimendan.tw. 65. levosimedan.tw. 66. or1259.tw. 67. "or 1259".tw. 68. simdax.tw. 69. Cardiotonic Agents/ 70. (inotropic adj2 (agent* or drug* or medicat* or act*)).tw. 71. inodilator*.tw. 72. (calcium adj2 sensiti*).tw. 73. or/64‐72 74. 63 and 73 75. randomized controlled trial.pt. 76. controlled clinical trial.pt. 77. randomized.ab. 78. placebo.ab. 79. drug therapy.fs. 80. randomly.ab. 81. trial.ab. 82. groups.ab. 83. 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 84. exp animals/ not humans.sh. 85. 83 not 84 86. 74 and 85
Embase
1. exp congenital heart malformation/ 2. exp heart disease/cn [Congenital Disorder] 3. (heart adj2 (defect* or abnormal* or malform*)).tw. 4. (congenital adj2 (heart or cardiac or cardio*)).tw. 5. deletion syndrome*.tw. 6. (alagille* adj2 syndrome*).tw. 7. (watson* adj2 syndrome*).tw. 8. (hepatofacioneurocardiovertebral adj2 syndrome*).tw. 9. (cardiovertebral adj2 syndrome*).tw. 10. (arteriohepatic adj2 dysplasia*).tw. 11. hepatic ductular hypoplasia*.tw. 12. (aort* adj3 coarctation*).tw. 13. (arrhythmogenic adj3 ventricular).tw. 14. Barth Syndrome.tw. 15. ((heart* or cor) adj2 triatria*).tw. 16. (subdivided adj3 atrium*).tw. 17. coronary vessel anomal*.tw. 18. myocardial bridging*.tw. 19. criss‐cross heart*.tw. 20. crisscross heart*.tw. 21. criss cross heart*.tw. 22. dextrocardia*.tw. 23. (kartagener* adj2 (syndrome* or triad)).tw. 24. siewert syndrome*.tw. 25. polynesian bronchiectas?s.tw. 26. primary ciliary dyskinesia.tw. 27. (paten* adj2 ductus adj2 arteriosus).tw. 28. (ebstein* adj2 (anomal* or malform*)).tw. 29. ectopia cordis.tw. 30. (Eisenmenger* adj2 (complex or syndrome)).tw. 31. ((heart or cardiac) adj2 septal defect*).tw. 32. heterotaxy syndrome*.tw. 33. polysplenia syndrome*.tw. 34. asplenia syndrome*.tw. 35. (ambiguus adj3 situs).tw. 36. isomerism.tw. 37. ivemark syndrome.tw. 38. visceral heterotax*.tw. 39. hypoplastic left heart syndrome.tw. 40. isolated noncompaction.tw. 41. isolated non‐compaction.tw. 42. leopard syndrome*.tw. 43. cardiomyopathic* lentiginosis.tw. 44. lentigines syndrome*.tw. 45. levocardia.tw. 46. marfan* syndrome.tw. 47. noonan syndrome.tw. 48. turner* syndrome.tw. 49. (fallot* adj2 tetralogy).tw. 50. (transpos* adj4 (arter* or vessel*)).tw. 51. (tricuspid adj2 atresias).tw. 52. (fallot* adj2 trilogy).tw. 53. turner* syndrome.tw. 54. bonnevie‐ullrich syndrome.tw. 55. bonnevie ullrich syndrome.tw. 56. bonnevie‐ullrich status.tw. 57. bonnevie ullrich status.tw. 58. ullrich‐turner syndrome.tw. 59. (gonadal adj2 dysgenesis).tw. 60. univentricular heart.tw. 61. arterial switch.tw. 62. norwood*.tw. 63. or/1‐62 64. levosimendan/ 65. levosimendan.tw. 66. levosimedan.tw. 67. or1259.tw. 68. "or 1259".tw. 69. simdax.tw. 70. cardiotonic agent/ 71. (inotropic adj2 (agent* or drug* or medicat* or act*)).tw. 72. inodilator*.tw. 73. (calcium adj2 sensiti*).tw. 74. or/64‐73 75. 63 and 74 76. random$.tw. 77. factorial$.tw. 78. crossover$.tw. 79. cross over$.tw. 80. cross‐over$.tw. 81. placebo$.tw. 82. (doubl$ adj blind$).tw. 83. (singl$ adj blind$).tw. 84. assign$.tw. 85. allocat$.tw. 86. volunteer$.tw. 87. crossover procedure/ 88. double blind procedure/ 89. randomized controlled trial/ 90. single blind procedure/ 91. 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 92. (animal/ or nonhuman/) not human/ 93. 91 not 92 94. 75 and 93
Web of Science
# 15 #14 AND #13 # 14 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) # 13 #12 AND #9 # 12 #11 OR #10 # 11 TS=("inotropic agent*" or "inotropic drug*" or "inotropic medicat*" or "inotropic act*" or inodilator* or "calcium sensiti*") # 10 TS=(levosimendan or levosimedan or or1259 or "or 1259" or simdax) # 9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 8 TS=("turner* syndrome" or "bonnevie‐ullrich syndrome" or "bonnevie ullrich syndrome" or "bonnevie‐ullrich status" or "bonnevie ullrich status" or "ullrich‐turner syndrome" or "gonadal dysgenesis" or "univentricular heart" or "arterial switch" or norwood*) # 7 TS=("leopard syndrome*" or "cardiomyopathic* lentiginosis" or "lentigines syndrome*" or levocardia or "marfan* syndrome" or "noonan syndrome" or "turner* syndrome" or "fallot* tetralogy" or "transpos* arter*" or "transpos* vessel*" or "tricuspid atresias" or "fallot* trilogy") # 6 TS=("heart septal defect*" or "cardiac septal defect*" or "heterotaxy syndrome*" or "polysplenia syndrome*" or "asplenia syndrome*" or "ambiguus situs" or isomerism or "ivemark syndrome" or "visceral heterotax*" or "hypoplastic left heart syndrome" or "isolated noncompaction" or "isolated non‐compaction") # 5 TS=(dextrocardia* or "kartagener* syndrome*" or "kartagener* triad" or "siewert syndrome*" or "polynesian bronchiectas?s" or "primary ciliary dyskinesia" or "paten* ductus arteriosus" or "ebstein* anomal*" or "ebstein* malform*" or "ectopia cordis" or "Eisenmenger* complex" or "Eisenmenger* syndrome") # 4 TS=("hepatic ductular hypoplasia*" or "aort* coarctation*" or "arrhythmogenic ventricular" or "Barth Syndrome" or "heart* triatria*" or "cor triatria*" or "subdivided atrium*" or "coronary vessel anomal*" or "myocardial bridging*" or "criss‐cross heart*" or "crisscross heart*" or "criss cross heart*") # 3 TS=("deletion syndrome*" or "alagille* syndrome*" or "watson* syndrome*" or hepatofacioneurocardiovertebral or "cardiovertebral syndrome*" or "arteriohepatic dysplasia*" or "hepatic ductular hypoplasia*" or "cardiovertebral syndrome" or "arteriohepatic dysplasia*") # 2 TS=(congenital heart or congenital cardiac or congenital cardio*) # 1 TS=(heart defect* or heart abnormal* or heart malform*)
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.12, 1.82] |
Comparison 2. Low cardiac output syndrome (LCOS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LCOS | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.39, 1.04] |
Comparison 3. Length of ICU stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of ICU stay (days) | 4 | 188 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐1.16, 1.82] |
| 2 Length of ICU stay (days) | 4 | 188 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.54, 0.44] |
Comparison 4. Length of hospital stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay (days) | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐3.50, 4.03] |
Comparison 5. Duration of mechanical ventilation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of mechanical ventilation (days) | 5 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.08, 0.00] |
| 2 Duration of mechanical ventilation (days) | 5 | 208 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.43, 0.27] |
Comparison 6. Mechanical circulatory support or cardiac transplantation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mechanical circulatory support or cardiac transplantation | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.19, 11.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ebade 2013.
| Methods | Randomised controlled trial. | |
| Participants | 50 (25 intervention, 25 control), aged 7 to 38 months, with atrial or ventricular septal defects with high systolic pulmonary artery pressure (PAP) exceeding 50% of systemic systolic pressure, assigned for surgical correction of the defect by cardiopulmonary bypass (CPB). | |
| Interventions | Intervention: levosimendan infusion started immediately after declamping of the aorta; an initial loading dose of 15 µg/kg given over a ten‐minute period, followed by infusion at 0.1 to 0.2 µg/kg/min. Control: dobutamine infusion started immediately after declamping of the aorta, at 4 to 10 µg/kg/min. |
|
| Outcomes | Recorded until 20 hours after ICU admission: 1. mean PAP recorded preoperatively by transthoracic echocardiography, intraoperatively by pulmonary artery catheter, postoperatively by transoesophageal echocardiography; assessment until 20 hours after ICU admission (lower score = improvement) 2. mean cardiac index recorded by transoesophageal 4‐MHz Doppler probe (Cardio Q, Deltex Medical), assessment until 20 hours after ICU admission (higher score = improvement) |
|
| Notes | Exact rate of infusion for intervention and control drug was titrated according to haemodynamic data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized using sealed envelopes and allocated to two equal groups". No further details of random sequence generation stated. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was performed using sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes listed in the methods section reported. |
| Selective reporting (reporting bias) | Low risk | No incomplete reporting suspected. |
| Other bias | Low risk | Note: Measurement of cardiac index by transoesophageal Doppler is not considered to be gold standard. Especially in the setting of an unblinded study, there is a certain risk of detection bias. |
Lechner 2012.
| Methods | Randomised controlled trial. | |
| Participants | 40 (20 intervention, 20 control), aged under one year, undergoing corrective open‐heart surgery for their congenital heart defects, children with tetralogy of Fallot excluded | |
| Interventions | Intervention: levosimendan continuous infusion of 0.1 µg/kg/min, started at the time of weaning from CPB, for the first 24 hours postoperative. Control: milrinone continuous infusion of 0.5 µg/kg/min, started at the time of weaning from CPB, for the first 24 hours postoperative. |
|
| Outcomes | Recorded until 48 hours after initiation of the study drug: 1. cardiac index calculated from cardiac output and the patient's body surface area, cardiac output measurement using transoesophageal Doppler technique (Cardio Q, Deltex Medical) (higher score = improvement). 2. heart rate, systemic arterial pressure, left atrial pressure, mixed venous saturation, lactate concentrations, inotrope score (dopamine + dobutamine + epinephrine x 100 + norepinephrine x 100 (dosages in µg/kg/min)), cerebral near infrared spectroscopy, occurrence of LCOS (defined as tachycardia, acidosis, oliguria, a widened arterial‐mixed venous oxygen saturation difference, need for mechanical circulatory support). |
|
| Notes | 1 patient (intervention group) had to be excluded from the intention‐to‐treat analysis (did not receive the intervention because of immediate reoperation), 2 additional patients (intervention group) had to be excluded from the per‐protocol analysis (because of serious violation of the study protocol) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drugs prepared and labelled blinded by the local pharmacy, both drugs administered at the same infusion rates, opaque black syringes and catheters used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study drugs labelled blinded, both drugs administered at same infusion rates, opaque black syringes and catheters used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis available for N = 39, per‐protocol analysis available for N = 37. |
| Selective reporting (reporting bias) | Low risk | No incomplete reporting suspected. |
| Other bias | Low risk | Note: measurement of CI by transoesophageal Doppler is not considered to be gold standard. Furthermore it was not possible to obtain reliable standard deviations for inotropic score, as the information extracted from a figure (Figure 4 in this source) deviated from information provided by personal communication with the study author. |
Momeni 2011.
| Methods | Randomised controlled trial. | |
| Participants | 41 (20 intervention, 21 control), aged under five years, undergoing corrective surgery for congenital heart defects using CPB and in need of inotropic support. | |
| Interventions | Intervention: 0.05 µg/kg/min of levosimendan, started at onset of CPB, allowed to be doubled, for a maximum of 48 hours. Control: 0.4 µg/kg/min of milrinone, started at onset of CPB, allowed to be doubled, for a maximum of 48 hours. |
|
| Outcomes | Recorded until 48 hours after admission to paediatric ICU: 1. Lactate level at four hours postoperatively. 2. Biologic markers and haemodynamic data (heart rate, mean arterial pressure, lactate, difference between arterial and mixed venous oxygen saturations, troponin, creatinine, alanine aminotransferase, aspartate aminotransferase). |
|
| Notes | One patient (control group) excluded who did not receive study medication because of modification of the surgical plan, two patients (1 intervention group, 1 control group) excluded because they required extracorporeal membrane oxygenation at the end of CPB, two patients (1 intervention group, 1 control group) excluded because of intraoperative death. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random code. |
| Allocation concealment (selection bias) | Low risk | Allocation code was concealed in an envelope opened by the study nurse in charge of preparing the medication. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Syringes and tubing system covered with aluminium foil, both drugs administered at same infusion rates. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Syringes and tubing system covered with aluminium foil, both drugs administered at same infusion rates. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two patients (1 intervention group, 1 control group) excluded because they required extracorporeal membrane oxygenation at the end of CPB, two patients (1 intervention group, 1 control group) excluded because of intraoperative death. |
| Selective reporting (reporting bias) | Low risk | No incomplete reporting suspected. |
| Other bias | Low risk | No other bias suspected. |
Pellicer 2013.
| Methods | Randomised controlled trial. | |
| Participants | 20 (11 intervention, 9 control) neonates undergoing cardiovascular surgery with CPB. | |
| Interventions | Intervention: continuous intravenous infusion of levosimendan started intraoperatively and increased stepwise: dose 1 (0.1 µg/kg/min) starting immediately after central line placed and maintained for duration of surgical procedure, dose 2 (0.15 µg/kg/min) upon admission to neonatal ICU, dose 3 (0.2 µg/kg/min) starting after two hours of stability with dose 2; infusion stopped after 48 hours. Control: continuous intravenous infusion of milrinone started intraoperatively and increased stepwise: dose 1 (0.5 µg/kg/min) starting immediately after central line placed and maintained for duration of surgical procedure, dose 2 (0.75 µg/kg/min) upon admission to neonatal ICU, dose 3 (1 µg/kg/min) starting after two hours of stability with dose 2, until 48 hours after infusion started, afterwards infusion rate tapered according to attending physician. |
|
| Outcomes | Recorded until day 6 postsurgery: 1. Heart rate, breathing rate, central and peripheral temperature, arterial blood pressure, SaO2, cerebral and peripheral perfusion‐oxygenation (using NIRS instrument NIRO‐3000). 2. Blood gases, acid‐base status, CO‐oximetry, lactate, glucose, haemoglobin‐concentration, creatinine, N‐terminal probrain natriuretic peptide, troponine I, pro‐inflammatory and anti‐inflammatory factors. |
|
| Notes | Beyond 48 hours open study. Financial support of Orion Pharma Spanish Division for the pharmacokinetic studies (not part of outcome measures). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. Randomisation stratified by type of congenital heart defects and risk adjustment (using the congenital heart surgery method) |
| Allocation concealment (selection bias) | Low risk | Study nurse not involved in the clinical care of the infants was custodian of the allocation code. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study medications prepared in identical opaque syringes, infusion tubes covered by aluminium foil, same infusion rates. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear risk due to unblinding of study after 48 hours, potential risk of detection bias after unblinding (study time point T3 and T4). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes listed in the methods section reported. |
| Selective reporting (reporting bias) | Low risk | No incomplete reporting suspected. |
| Other bias | Low risk | Financial support of Orion Pharma Spanish Division for the pharmacokinetic studies (not part of outcome measures). Not deemed an important source of bias. |
Ricci 2012.
| Methods | Randomised controlled trial. | |
| Participants | 63 (32 intervention, 31 control) neonates undergoing risk‐adjusted classification for congenital heart surgery (RACHS) 3 and 4 procedures | |
| Interventions | Intervention: continuous infusion of 0.1 µg/kg/min levosimendan for 72 hours added to standard inotropic support, started while weaning from CPB. Control: standard post‐CPB inotrope infusion (milrinone 0.75 µg/kg/min and dopamine 5 to 10 µg/kg/min, adrenaline 0.05 to 0.3 µg/kg/min if necessary). |
|
| Outcomes | Recorded until 72 hours postoperatively: 1. Incidence of LCOS (defined as tachycardia (heart rate > 170 beats/min), oliguria (urine output < 0.5 mL/kg/h), cold extremities (peripheral temperature < 27°C), with or without at least 30% difference in arterial to mixed venous oxygen saturation or metabolic acidosis (an increase in base deficit of greater than 4 or an increase in lactate of more than 2 mg/dL) on two successive blood gas measurements, cardiac arrest, need for extracorporeal membrane oxygenation 2. Lactate, heart rate, mean arterial pressure, inotropic score, diuresis, need for peritoneal dialysis, mixed venous oxygen saturation, brain natriuretic peptide (BNP), number of ventilation days, paediatric cardiac ICU length of stay and survival, side effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐generation program. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing the allocation group opened by a nurse in charge of preparing the infusions. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes listed in the methods section reported. |
| Selective reporting (reporting bias) | Low risk | No incomplete reporting suspected. |
| Other bias | Low risk | No other bias suspected. |
ICU = intensive care unit
LCOS = low cardiac output syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bravo 2011 | Uncontrolled case series study, not RCT. Patients with LCOS of any origin, not exclusively postoperative LCOS. |
| Di Chiara 2010 | Preoperative administration of levosimendan for a cohort of selected neonates with hypoplastic left heart syndrome, not RCT. |
| Egan 2006 | Retrospective observational study, not RCT. |
| Giordano 2013 | Case‐control retrospective study, not RCT. |
| Osthaus 2009 | Retrospective study, not RCT. |
| Turanlahti 2004 | Single group phase II study, not RCT. Patients in preoperative setting without LCOS. |
LCOS = low cardiac output syndrome
Characteristics of ongoing studies [ordered by study ID]
EUCTR 2012‐005310‐19‐ES.
| Trial name or title | Double‐blind randomised clinical trial to evaluate the efficacy and safety of levosimendan as pre‐ischaemic myocardial conditioner in paediatric cardiac surgery |
| Methods | RCT |
| Participants | 36 patients of both genders, aged one month to 14 years, who are to undergo cardiac surgery, with high risk factors of postoperative acute heart failure |
| Interventions | Intervention: levosimendan; control: placebo. |
| Outcomes | HR, MAP, central venous pressure, thermal gradient, capillary refill time, diuresis, inotropic score, inotropic medication, atrial natriuretic peptide, troponine‐I, myoglobine, lactate, central venous oxygen saturation, oxygen transport, changes in the neurohormonal profile, adverse events, all‐cause mortality, analysis of economic impact of levosimendan, days in ICU, days of mechanical ventilation, survival at 30 days |
| Starting date | 05 June 2013 |
| Contact information | Hospital Universitario Virgen de las Nieves, Granada, Spain |
| Notes | Anticipated date of completion: unknown |
HR = heart rate
MAP = mean arterial pressure
ICU = intensive care unit
Differences between protocol and review
'Children' was replaced by 'paediatric patients' in the review title, since neonates were a relevant part of the included study population.
Definitions of LCOS in the included studies varied and did not necessarily meet the definition stated in the protocol.
The data extraction form that was used was not piloted as mentioned in the protocol, as the number of included studies was very small. We returned to the reports that had already undergone data extraction whenever data were missing from the data extraction form.
Subgroup analyses, as mentioned in the protocol (age‐based and cardiovascular‐physiology‐based), were not feasible due to the small number of events.
In addition to the planned methods stated in the protocol, we provided a ‘Summary of findings’ table in the review to present the main findings in a transparent and simple tabular format. In addition, we conducted a GRADE assessment of the quality of evidence which we also presented in the ‘Summary of findings’ table.
Contributions of authors
JH, BS and GR: conceived and designed the review.
JH and BS: collected, screened, extracted, and interpreted data.
JH: co‐ordinated the review, wrote authors for additional data, wrote the protocol and the review.
BS: revised the review critically for important intellectual content.
GR: provided methodological input, analysed data, revised the review critically for important intellectual content.
All authors read and approved the final version of the review.
Sources of support
Internal sources
-
Department of Congenital Heart Defects and Pediatric Cardiology, Heart Center, University of Freiburg, Freiburg, Germany.
Consists of providing free access to computers, data bases, full‐text literature, collaboration with experts of different faculties and partial release from clinical work to the authors. No additional financial grants.
External sources
No sources of support supplied
Declarations of interest
JH: None known.
GR: received payment for a one‐day course on statistical methods in meta‐analysis by Grünenthal Group, Aachen, Germany.
BS: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Ebade 2013 {published data only}
- Ebade A, Khalil M, Mohamed A. Levosimendan is superior to dobutamine as an inodilator in the treatment of pulmonary hypertension for children undergoing cardiac surgery. Journal of Anesthesia 2013;27(3):334–9. [DOI: 10.1007/s00540-012-1537-9] [DOI] [PubMed] [Google Scholar]
Lechner 2012 {published data only}
- Lechner E, Hofer A, Leitner‐Peneder G, Freynschlag R, Mair R, Weinzettel R, et al. Levosimendan versus milrinone in neonates and infants after corrective open‐heart surgery. Pediatric Critical Care Medicine 2012;13(5):542‐8. [DOI: 10.1097/PCC.0b013e3182455571] [DOI] [PubMed] [Google Scholar]
Momeni 2011 {published data only}
- Momeni M, Rubay J, Matta A, Rennotte M‐T, Veyckemans F, Poncelet A, et al. Levosimendan in congenital cardiac surgery: a randomized, double‐blind clinical trial. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(3):419‐24. [DOI: 10.1053/j.jvca.2010.07.004] [DOI] [PubMed] [Google Scholar]
Pellicer 2013 {published data only}
- Pellicer A, Riera J, Lopez‐Ortego P, Bravo M, Madero R, Perez‐Rodriguez J, et al. Phase 1 study of two inodilators in neonates undergoing cardiovascular surgery. Pediatric Research 2013;73(1):95‐103. [DOI: 10.1038/pr.2012.154] [DOI] [PubMed] [Google Scholar]
Ricci 2012 {published data only}
- Ricci Z, Garisto C, Favia I, Vitale V, Chiara L, Cogo P. Levosimendan infusion in newborns after corrective surgery for congenital heart disease: randomized controlled trial. Intensive Care Medicine 2012;38(7):1198‐204. [DOI: 10.1007/s00134-012-2564-6] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bravo 2011 {published data only}
- Bravo MC, López P, Cabañas F, Perez‐Rodriguez J, Pérez‐Fernández E, Pellicer A. Acute effects of levosimendan on cerebral and systemic perfusion and oxygenation in newborns: an observational study. Neonatology 2011;99(3):217‐23. [DOI: 10.1159/000314955] [DOI] [PubMed] [Google Scholar]
Di Chiara 2010 {published data only}
- Chiara L, Ricci Z, Garisto C, Morelli S, Giorni C, Vitale V, et al. Initial experience with levosimendan infusion for preoperative management of hypoplastic left heart syndrome. Pediatric Cardiology 2010;31(1):166‐7. [DOI: 10.1007/s00246-009-9571-6] [DOI] [PubMed] [Google Scholar]
Egan 2006 {published data only}
- Egan J, Clarke A, Williams S, Cole A, Ayer J, Jacobe S, et al. Levosimendan for low cardiac output: a pediatric experience. Journal of Intensive Care Medicine 2006;21(3):183‐7. [DOI: 10.1177/0885066606287039] [DOI] [PubMed] [Google Scholar]
Giordano 2013 {published data only}
- Giordano R, Palma G, Palumbo S, Cioffi S, Russolillo V, Mucerino M, et al. Single center experience with levosimendan administration after pediatric cardiac surgery. Giornale Italiano di Cardiologia / Abstract del XLIII Congresso Nazionale SICP ‐ Sezione Pediatrica e delle Cardiopatie Congenite SICCH 2013;14(Suppl 1 AL N 10):35S‐6S. [Google Scholar]
Osthaus 2009 {published data only}
- Osthaus W, Boethig D, Winterhalter M, Huber D, Goerler H, Sasse M, et al. First experiences with intraoperative Levosimendan in pediatric cardiac surgery. European Journal of Pediatrics 2009;168(6):735‐40. [DOI: 10.1007/s00431-008-0834-7] [DOI] [PubMed] [Google Scholar]
Turanlahti 2004 {published data only}
- Turanlahti M, Boldt T, Palkama T, Antila S, Lehtonen L, Pesonen E. Pharmacokinetics of levosimendan in pediatric patients evaluated for cardiac surgery. Pediatric Critical Care Medicine: A Journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2004;5(5):457‐62. [DOI: 10.1097/01.PCC.0000137355.01277.9C] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
EUCTR 2012‐005310‐19‐ES {unpublished data only}
- EUCTR 2012‐005310‐19‐ES. Double‐blind randomized clinical trial to evaluate the efficacy and safety of levosimendan as preischemic myocardial conditioner in pediatric cardiac surgery. www.clinicaltrialsregister.eu/ctr‐search/search?query=EUCTR2012‐005310‐19‐ES (accessed 26 November 2014).
Additional references
Bailey 2004
- Bailey J, Hoffman T, Wessel D, Nelson D, Atz A, Chang A, et al. A population pharmacokinetic analysis of milrinone in pediatric patients after cardiac surgery. Journal of Pharmacokinetics and Pharmacodynamics 2004;31(1):43‐59. [DOI] [PubMed] [Google Scholar]
Baysal 2010
- Baysal A, Sasmazel A, Yildirim A, Kocak Tu, Sunar H, Zeybek R. The effects of thyroid hormones and interleukin‐8 levels on prognosis after congenital heart surgery. Archives of the Turkish Society of Cardiology 2010;38(8):537‐43. [PubMed] [Google Scholar]
Braun 2004
- Braun J‐P, Schneider M, Kastrup M, Liu J. Treatment of acute heart failure in an infant after cardiac surgery using levosimendan. European Journal of Cardio‐thoracic Surgery 2004;26(1):228‐30. [DOI] [PubMed] [Google Scholar]
Burkhardt 2015
- Burkhardt B, Rücker G, Stiller B. Prophylactic milrinone for the prevention of low cardiac output syndrome and mortality in children undergoing surgery for congenital heart disease. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD009515.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Butts 2012
- Butts R, Scheurer M, Atz A, Zyblewski S, Hulsey T, Bradley S. Comparison of maximum vasoactive inotropic score and low cardiac output syndrome as markers of early postoperative outcomes after neonatal cardiac surgery. Pediatric Cardiology 2012;33(4):633‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dellinger 2013
- Dellinger R, Levy M, Rhodes A, Annane D, Gerlach H, Opal S. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Intensive Care Medicine 2013;39(2):165‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elkayam 2007
- Elkayam U, Tasissa G, Binanay C, Stevenson L, Gheorghiade M, Warnica J, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. American Heart Journal 2007;153(1):98‐104. [DOI] [PubMed] [Google Scholar]
Endoh 2002
- Endoh M. Mechanisms of action of novel cardiotonic agents. Journal of Cardiovascular Pharmacology 2002;40(3):323‐38. [DOI] [PubMed] [Google Scholar]
Fernández 2012
- Fernández de Palencia‐Espinosa MA, Cárceles‐Barón MD, Blázquez‐Álvarez MJ, Arocas‐Casañ V, Rubia‐Nieto A. [Retrospective descriptive study about the use of levosimendan in children undergoing surgical correction for congenital heart disease]. Revista Española de Anestesiología y Reanimación 2012;59(9):489‐96. [DOI] [PubMed] [Google Scholar]
Follath 2002
- Follath F, Cleland J, Just H, Papp J, Scholz H, Peuhkurinen K, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low‐output heart failure (the LIDO study): a randomised double‐blind trial. Lancet 2002;360(9328):196‐202. [DOI] [PubMed] [Google Scholar]
Froese 2009
- Froese N, Sett S, Mock T, Krahn G. Does troponin‐I measurement predict low cardiac output syndrome following cardiac surgery in children?. Critical Care and Resuscitation 2009;11(2):116‐21. [PubMed] [Google Scholar]
Graciano 2005
- Graciano A, Balko J, Rahn D, Ahmad N, Giroir B. The Pediatric Multiple Organ Dysfunction Score (P‐MODS): development and validation of an objective scale to measure the severity of multiple organ dysfunction in critically ill children. Critical Care Medicine 2005;33(7):1484‐91. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 16 June 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Harbord 2006
- Harbord R, Egger M, Sterne J. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffman 2003
- Hoffman T, Wernovsky G, Atz A, Kulik T, Nelson D, Chang A, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003;107(7):996‐1002. [DOI] [PubMed] [Google Scholar]
Hoffman 2011
- Hoffman T. Newer inotropes in pediatric heart failure. Journal of Cardiovascular Pharmacology 2011;58(2):121‐5. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lim 2015
- Lim J, Deo S, Rababa'h A, Altarabsheh S, Cho Y. Levosimendan reduces mortality in adults with left ventricular dysfunction undergoing cardiac surgery: a systematic review and meta‐analysis. Journal of Cardiac Surgery 2015;30(7):547‐54. [DOI] [PubMed] [Google Scholar]
Lobacheva 2010
- Lobacheva G, Khar'kin A, Manerova A, Dzhobava E. Intensive care for newborns and babies of the first year of life with acute heart failure after cardiosurgical interventions. Anesteziologiia i Reanimatologiia 2010;5:23‐7. [PubMed] [Google Scholar]
Luca 2006
- Luca L, Colucci W, Nieminen M, Massie B, Gheorghiade M. Evidence‐based use of levosimendan in different clinical settings. European Heart Journal 2006;27(16):1908‐20. [DOI] [PubMed] [Google Scholar]
Ma 2007
- Ma M, Gauvreau K, Allan C, Mayer J, Jenkins K. Causes of death after congenital heart surgery. Annals of Thoracic Surgery 2007;83(4):1438‐45. [DOI] [PubMed] [Google Scholar]
Magliola 2009
- Magliola R, Moreno G, Vassallo J, Landry L, Althabe M, Balestrini M, et al. Levosimendan, a new inotropic drug: experience in children with acute heart failure [Levosimendán, un nuevo agente inotrópico:experiencia en niños con fallo cardíaco agudo]. Archivos Argentinos de Pediatria 2009;107(2):139‐45. [DOI] [PubMed] [Google Scholar]
Miera 2015
- Miera O, Daehnert I, Haas N, Hirt M, Thul J. Acute heart failure and ventricular assist device (VAD)/extracorporeal membrane oxygenation (ECMO) [Akute herzinsuffizienz und ventrikulärer assist device (VAD)/extrakorporale membranoxygenierung (ECMO)]. Available from www.kinderkardiologie.org/leitlinien/ (accessed 18 February 2015).
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ (Clinical research ed.) 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moiseyev 2002
- Moiseyev V, Poder P, Andrejevs N, Ruda M, Golikov A, Lazebnik L, et al. Safety and efficacy of a novel calcium sensitiser, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo‐controlled, double‐blind study (RUSSLAN). European Heart Journal 2002;23(18):1422‐32. [DOI] [PubMed] [Google Scholar]
Namachivayam 2006
- Namachivayam P, Crossland D, Butt W, Shekerdemian L. Early experience with levosimendan in children with ventricular dysfunction. Pediatric Critical Care Medicine 2006;7(5):445‐8. [DOI] [PubMed] [Google Scholar]
Orion Pharma 2009
- Orion Pharma. Simdax ‐ Summary of product characteristics. Available from www.simdax.com/About‐Simdax/SPC/ (accessed 16 September 2014).
Overgaard 2008
- Overgaard C, Dzavik V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation 2008;118(10):1047‐56. [DOI] [PubMed] [Google Scholar]
Packer 1993
- Packer M. The search for the ideal positive inotropic agent. New England Journal of Medicine 1993;329(3):201‐2. [DOI] [PubMed] [Google Scholar]
Papp 2005
- Papp Z, Csapo K, Pollesello P, Haikala H, Edes I. Pharmacological mechanisms contributing to the clinical efficacy of levosimendan. Cardiovascular Drug Reviews 2005;23(1):71‐98. [DOI] [PubMed] [Google Scholar]
Parr 1975
- Parr G, Blackstone E, Kirklin J. Cardiac performance and mortality early after intracardiac surgery in infants and young children. Circulation 1975;51(5):867‐74. [DOI] [PubMed] [Google Scholar]
R Development Core Team 2016 [Computer program]
- R Development Core Team. R: a language and environment for statistical computing. Version 3.3.2. Vienna, Austria: The R Foundation for Statistical Computing, 2016.
RevMan 5 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rücker 2011
- Rücker G, Schwarzer G, Carpenter J, Binder H, Schumacher M. Treatment‐effect estimates adjusted for small‐study effects via a limit meta‐analysis. Biostatistics 2011;12(1):122‐42. [DOI] [PubMed] [Google Scholar]
Samadi 2012
- Samadi M, Malaki M, Ghaffari S, Khalili R. Correlation between pediatric open heart surgery outcomes and arterial‐mixed venous oxygen saturation differences. Journal of Cardiovascular and Thoracic Research 2012;4(2):41‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2005
- Schulz K, Grimes D. Sample size calculations in randomised trials: mandatory and mystical. Lancet 2005;365(9467):1348‐53. [DOI] [PubMed] [Google Scholar]
Shann 2003
- Shann F. Drug Doses. 12th Edition. Victoria: Royal Children’s Hospital, 2003. [Google Scholar]
Shi 2008
- Shi S, Zhao Z, Liu X, Shu Q, Tan L, Lin R, et al. Perioperative risk factors for prolonged mechanical ventilation following cardiac surgery in neonates and young infants. Chest 2008;134(4):768‐74. [DOI] [PubMed] [Google Scholar]
Silvetti 2015
- Silvetti S, Silvani P, Azzolini M, Dossi R, Landoni G, Zangrillo A. A systematic review on levosimendan in paediatric patients. Current Vascular Pharmacology 2015;13(1):128‐33. [DOI] [PubMed] [Google Scholar]
Stocker 2007
- Stocker C, Shekerdemian L, Norgaard M, Brizard C, Mynard J, Horton S, et al. Mechanisms of a reduced cardiac output and the effects of milrinone and levosimendan in a model of infant cardiopulmonary bypass. Critical Care Medicine 2007;35(1):252‐9. [DOI] [PubMed] [Google Scholar]
Vela 2012
- Vela J, Benitez J, Gonzalez M, Lopez M, Perez R, Meneses V, et al. Summary of the consensus document: clinical practice guide for the management of low cardiac output syndrome in the postoperative period of heart surgery. Medicina Intensiva/Sociedad Espanola de Medicina Intensiva y Unidades Coronarias 2012;36(4):277‐87. [DOI] [PubMed] [Google Scholar]
Verheugt 2008
- Verheugt C, Uiterwaal C, Grobbee D, Mulder B. Long‐term prognosis of congenital heart defects: a systematic review. International Journal of Cardiology 2008;131(1):25‐32. [DOI] [PubMed] [Google Scholar]
Vogt 2011
- Vogt W, Läer S. Treatment for paediatric low cardiac output syndrome: results from the European EuLoCOS‐Paed survey. Archives of Disease in Childhood 2011;96(12):1180‐6. [DOI] [PubMed] [Google Scholar]
Wernovsky 1995
- Wernovsky G, Wypij D, Jonas R, Mayer J, Hanley F, Hickey P, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low‐flow cardiopulmonary bypass and circulatory arrest. Circulation 1995;92(8):2226‐35. [DOI] [PubMed] [Google Scholar]


