Abstract
Background
The randomised, double-blind, placebo-controlled Systolic Hypertension in Europe trial (Syst-Eur 1) proved that blood pressure (BP) lowering therapy starting with nitrendipine reduces the risk of cardiovascular complications in elderly patients with isolated systolic hypertension. In an attempt to confirm the safety of long-term antihypertensive therapy based on a dihydropyridine, the Syst-Eur patients remained in open follow-up after the end of Syst-Eur 1. This paper presents the second progress report of this follow-up study (Syst-Eur 2). It describes BP control and adherence to study medications.
Methods
After the end of Syst-Eur 1 all patients, treated either actively or with placebo, were invited either to continue or to start antihypertensive treatment with the same drugs as previously used in the active treatment arm. In order to reach the target BP (sitting SBP <150 mmHg), the first line agent, nitrendipine, could be associated with enalapril and/or hydrochlorothiazide.
Results
Of the 3787 eligible patients, 3516 (93%) entered Syst-Eur 2. At the last available visit, 72% of the patients were taking nitrendipine. SBP/DBP at entry in Syst-Eur 2 averaged 160/83 mmHg in the former placebo group and 151/80 mmHg in the former active-treatment group. At the last follow-up visit SBP/DBP in the patients previously randomised to placebo or active treatment had decreased by 16/5 mmHg and 7/5 mmHg, respectively. The target BP was reached by 74% of the patients.
Conclusion
Substantial reductions in systolic BP may be achieved in older patients with isolated systolic hypertension with a treatment strategy starting with the dihydropyridine calcium-channel blocker, nitrendipine, with the possible addition of enalapril and/or hydrochlorothiazide.
Keywords: calcium-channel blockers, elderly, isolated systolic hypertension
Introduction
The double-blind, placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial proved that antihypertensive treatment starting with nitrendipine reduced the risk of cardiovascular complications in older patients with isolated systolic hypertension [1,2]. Similar findings were obtained in two placebo-controlled trials in China [3,4], in which antihypertensive treatment was also initiated with a dihydropyridine calcium-channel blocker. For a variety of ethical and documentary reasons, it was decided to extend the Syst-Eur trial into an open-label, active treatment, follow-up study in the same population and based upon the original active trial medication. The vast majority of patients volunteered to participate in this open follow-up study, Systolic Hypertension in Europe Phase 2 (Syst-Eur 2), which will last until the end of 2001. In this article, which is the second progress report of Syst-Eur 2, we aim to describe blood pressure (BP) control and adherence to study medications during the first three years of follow-up and, also, to explore whether BP control was influenced by diabetic status, or smoking/drinking habits.
Methods
Design of the Syst-Eur 2 study
The protocols of the Syst-Eur 1 [1] and Syst-Eur 2 [5] studies were approved by the Ethics Committees of the University of Leuven and by the participating centres, and implemented according to the principles outlined in the Helsinki declaration [6]. Patients were eligible for the Syst-Eur 1 trial if they were at least 60 years of age and had a sitting systolic BP within the range 160–219 mmHg and a diastolic BP below 95 mmHg (with a systolic pressure of 140 mmHg or higher while they were standing). Patients were recruited from 198 centres in 23 countries across Western and Eastern Europe. Eligible patients were stratified by centre, sex and previous cardiovascular complications, and were randomised to double-blind treatment with either active medication or placebo. After the termination of Syst-Eur 1 [1] in Spring 1997, all the patients who were still in follow-up were requested to continue or to start antihypertensive therapy with the same drugs as previously used in the active-treatment arm. The goal of antihypertensive treatment during Syst-Eur 2 is to lower the sitting systolic BP (average of two readings, obtained after rest for five minutes) to less than 150 mmHg. The target pressure should be achieved by the stepwise titration of nitrendipine (10–40 mg/day), the first-line study medication, with the possible addition of either enalapril (5–20 mg/day) or hydrochlorothiazide (12.5–25 mg/day), or both of these drugs. If side effects occur during monotherapy with nitrendipine, the daily dose should first be back-titrated. If side effects persist at this lower dose, nitrendipine may be discontinued and enalapril started. Similarly, the second-line medication may be withdrawn because of side effects, and hydrochlorothiazide started. The open-label study medication may be associated with, or replaced by, any other antihypertensive or cardiovascular drug if a treatment-resistant patient requires it to reach the goal BP, or if a patient requires treatment for a cardiovascular disorder.
During the first year of Syst-Eur 2, clinic visits were scheduled every three months; from the second year onwards, reports are due every six months (i.e. supervised follow-up). Patients who withdraw from the study and who no longer participate in clinic visits, proceed to the non-supervised follow-up, during which the investigator has to collect, at annual intervals, information on vital status, occurrence of major events and the use of antihypertensive medications.
Data analysis
Database management and statistical analysis were performed using SAS software version 8.01 (Cary, NC, USA). The last available BP measurements before the end of Syst-Eur1 were taken as the baseline pressures in Syst-Eur2. Baseline blood and urine tests were those obtained nearest to 14 February 1997, the date on which Syst-Eur1 ended. The BP changes during follow-up were analysed using the difference between baseline and the last available measurements. Means were compared by the Student's t-test. Between-group proportions were compared by the chi-square test, and within-group proportions by McNemar's test.
Results
Study profile
A total of 4695 patients had been randomised in the Syst-Eur trial (Fig. 1). At the termination of Syst-Eur1 there had been 282 deaths (6.0%), while 124 patients (2.6%) without any report within the year before the trial stopped were counted as lost to follow-up [2]. These patients were not, therefore, eligible for further follow-up in Syst-Eur2. Furthermore, on February 14,1997, 502 patients (10.6%) had already proceeded to non-supervised follow-up. Of the remaining 3787 patients who were eligible for further follow-up in Syst-Eur2, 3516 (92.8%) participated. On June 15, 2000, 3021 of the 3516 patients enrolled in Syst-Eur2, were still in follow-up, 277 had proceeded to non-supervised follow-up and 158 had died. Sixty patients without any report within the last 18 months were counted as lost to follow-up (Fig. 1). The median follow-up in Syst-Eur2 was 37 months (range 0.3–40 months). The number of patient-years of follow-up in Syst-Eur2 totalled 9988.
Figure 1.
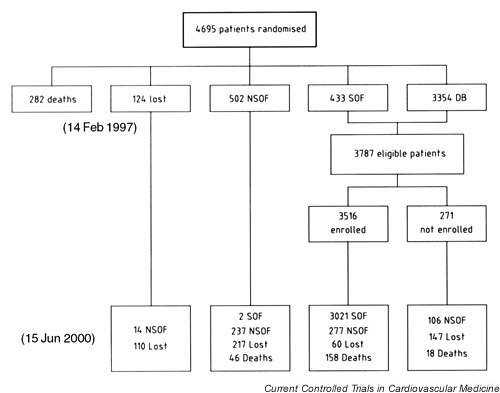
Profile of patients in the Syst-Eur study. Patients without any report within the last year were classified as lost-to-follow-up in Syst-Eur 1. Patients without any report within the last 18 months were counted as lost to follow-up in Syst-Eur 2. DB, double-blind; NSOF, non-supervised open follow-up; SOF, supervised open follow-up.
Patient characteristics
Table 1 presents the characteristics of the 3516 patients at entry into Syst-Eur1 and at baseline into Syst-Eur2. At randomisation, patients in the placebo and active-treatment groups were similar for the distribution of sex, age, BP, pulse rate, body-mass index, serum cholesterol, the use of tobacco and alcohol, and previous cardiovascular complications. Median follow-up in Syst-Eur1 was 1.7 years. At entry into Syst-Eur2 the 2340 women and 1176 men were, on average, 71.2 ± 6.3 years old. As expected, BP at entry into Syst-Eur2 was higher in the former placebo group as compared with the former active-treatment group. Body-mass index (0.23 ± 1.56 kg/m2) and total cholesterol (0.17 ± 0.91 mmol/l) had decreased (P < 0.001) during Syst-Eur1, but to a similar extent in the placebo and active-treatment groups. A total of 85 patients stopped smoking and 15 started smoking during Syst-Eur1. Only 38 patients had experienced a nonfatal stroke during Syst-Eur1 and 25 had a nonfatal myocardial infarction. A total of 359 (10.2%) patients had diabetes mellitus at randomisation and another 81 patients (2.3 %) developed diabetes during the Syst-Eur1 trial.
Table 1.
Patient characteristics at baseline and at the end of the Syst-Eur 1 trial
| Baseline Syst-Eur 1 | Baseline Syst-Eur 2 | ||||
| Characteristic | Placebo | Active | Placebo† | Active† | |
| Number | 1691 | 1825 | 1691 | 1825 | |
| Female sex | 1121 (66.3%) | 1219 (66.8%) | 1121 (66.3%) | 1219 (66.8%) | |
| Age (years) | 69.0 ± 6.0 | 69.0 ± 5.8 | 71.1 ± 6.4++ | 71.2 ± 6.3++ | |
| Sitting systolic blood pressure (mmHg) | 173.4 ± 9.5 | 173.3 ± 9.4 | 160.4 ± 16.2++ | *** | 151.0 ± 14.6++ |
| Sitting diastolic blood pressure (mmHg) | 85.6 ± 5.7 | 85.7 ± 5.7 | 83.4 ± 7.7++ | *** | 79.6 ± 7.8++ |
| Sitting heart rate (beats per minute) | 72.8 ± 8.0 | 72.8 ± 7.9 | 72.5 ± 9.1 | * | 73.1 ± 8.9 |
| Standing systolic blood pressure (mmHg) | 168.3 ± 11.5 | 167.9 ± 11.9 | 157.6 ± 16.6++ | *** | 148.2 ± 15.5++ |
| Standing diastolic blood pressure (mmHg) | 87.6 ± 7.6 | 87.6 ± 7.6 | 85.1 ± 9.2++ | *** | 81.6 ± 8.9++ |
| Body-mass index (kg/m2) | 27.3 ± 4.0 | 27.3 ± 4.2 | 27.0 ± 4.0++ | 27.1 ± 4.2++ | |
| Total cholesterol (mmol/l) | 6.0 ± 1.2 | 6.0 ± 1.2 | 5.9 ± 1.1++ | 5.8 ± 1.1++ | |
| High-density-lipoprotein cholesterol (mmol/l) | 1.40 ± 0.46 | 1.42 ± 0.48 | 1.36 ± 0.40+ | * | 1.40 ± 0.47 |
| History of stroke | 18 (1.1%) | 20 (1.1%) | 39 (2.3%)++ | 34 (1.9%)++ | |
| History of myocardial infarction | 61 (3.6%) | 62 (3.4%) | 73 (4.3%)++ | ** | 71 (3.9%)++ |
| Diabetes mellitus | 170 (10.1%) | 189 (10.4%) | 205 (12.1%)++ | 235 (12.9%)++ | |
| Current smokers | 106 (6.3%) | 121 (6.6%) | 71 (4.2%)++ | 86 (4.7%)++ | |
| Abstaining from alcohol | 1227 (72.6%) | 1311 (71.9%) | 1324 (78.3%)++ | 1388 (76.1%)++ | |
| <1 unit alcohol per day | 282 (16.7%) | 337 (18.4%) | 225 (13.3%)++ | 261 (14.3%)++ | |
| ≥ 1 unit alcohol per day | 181 (10.7%) | 176 (9.7%) | 142 (8.4%)++ | 175 (9.6%) | |
Values are given as mean ± SD or number of patients (%). † Indicates patients formerly randomised to placebo and active treatment. Significance of between-group differences: *P < 0.05; **P < 0.01; ***P < 0.001. Significance of within-group changes: +P < 0.01; ++P < 0.001.
Treatment
At the end of Syst-Eur1, significantly fewer patients (P < 0.001) in the active treatment group than in the control group had proceeded to combined treatment with various double-blind medications. Also, fewer patients (P < 0.001) randomised to active treatment were in open follow-up (Table 2). At the last visit in Syst-Eur1, 1514 (83.0%) patients of the active treatment group took nitrendipine, either in monotherapy (n = 1065; 58.4%) or in combination with enalapril and/or hydrochlorothiazide (n = 449; 24.6%). The average daily doses of the active double-blind medications were 28.1 ± 12.1 mg for nitrendipine (n = 1514), 13.6 ± 6.1 mg for enalapril (n = 557), and 21.4 ± 6.8 mg for hydrochlorothiazide (n = 220). Of the 235 diabetic patients randomised to active treatment, 197 (83.8%) took nitrendipine either in monotherapy (n = 136; 57.9%) or in combination with the second and/or the third line drug (n = 61; 26.0%).
Table 2.
Treatment status at the termination of the double-blind Syst-Eur 1 trial
| Active | ||
| Placebo* | treatment* | |
| Total number | 1691 | 1825 |
| Still in double-blind follow-up | 1487 (88%) | 1718 (94%) |
| No study drugs | 25 (1%) | 28 (2%) |
| Nitrendipine/placebo only | 665 (39%) | 1065 (58%) |
| Study medication other than | 797 (47%) | 625 (34%) |
| nitrendipine | ||
| Drugs taken† | ||
| Nitrendipine/placebo | 1396 (83%) | 1514 (83%) |
| Enalapril/placebo | 757 (45%) | 557 (31%) |
| Hydrochlorothiazide/placebo | 399 (24%) | 220 (12%) |
| Open-label antihypertensive | 20 (1%) | 13 (1%) |
| drugs‡ | ||
| Supervised open follow-up | 204 (12%) | 107 (6%) |
| No antihypertensive drugs | 43 (3%) | 25 (1%) |
| Open-label antihypertensive drugs | 142 (8%) | 74 (4%) |
| Treatment unknown | 19 (1%) | 8 (0%) |
*Indicates patients formerly randomised to placebo or active treatment. †Because many patients were on combined treatment, numbers do not add up. ‡To bridge medical emergencies without having to break the code, antihypertensive drugs could be prescribed during the double-blind trial for up to 3 consecutive months.
At the last visit in Syst-Eur2, the number of patients proceeding to combined treatment with the various study drugs was similar in the former placebo (40.6%) and active-treatment (43.8%) groups (Table 3). Of the 1691 patients previously randomised to placebo, 1194 (70.6%) took nitrendipine, either in monotherapy (n = 596; 35.2%) or in combination with enalapril and/or hydrochlorothiazide and/or other antihypertensive drugs (n = 598; 35.4%). Among the 1825 patients of the former active-treatment group, 1328 (72.8%) took nitrendipine, either alone (n = 676; 37.0%), or in combination with other drugs (n = 652; 35.7%) (Table 3). At the last available visit, the average daily doses of the study drugs in the patients formerly randomised to placebo were 31.0 ± 11.1 mg (n = 1194) for nitrendipine, 15.1 ± 5.7 mg for enalapril (n = 693), and 24.1 ± 9.7 mg (n = 326) for hydro-chlorothiazide. In the patients previously randomised to active treatment, these doses were 31.2 ± 11.2 mg (n = 1328), 15.3 ± 5.8 mg (n = 823), and 23.7 ± 8.2 mg (n = 424) respectively.
Table 3.
Antihypertensive drug treatment during Syst-Eur 2
| Formerly randomised to placebo | Formerly randomised to active treatment | |||||||
| Month 3 | Year 1 | Year 3 | Last visit | Month 3 | Year 1 | Year 3 | Last visit | |
| Total number of patients | 1086 | 1561 | 1210 | 1691 | 1206 | 1682 | 1291 | 1825 |
| On AH drugs | 966 (89%) | 1504 (96%) | 1177 (97%) | 1605 (95%) | 1186 (98%) | 1654 (98%) | 1267 (98%) | 1762 (97%) |
| Only nitrendipine | 713 (66%) | 732 (47%) | 403 (33%) | 596 (35%) | 651 (54%) | 747 (44%) | 475 (37%) | 676 (37%) |
| Study drugs other than nitrendipine | 127 (12%) | 547 (35%) | 546 (45%) | 687 (41%) | 466 (39%) | 738 (44%) | 599 (46%) | 799 (44%) |
| (no other AH drugs) | ||||||||
| Study drugs + other AH drugs | 19 (2%) | 82 (5%) | 118 (10%) | 145 (9%) | 14 (1%) | 69 (4%) | 120 (9%) | 155 (8%) |
| Other AH drugs only | 107 (10%) | 143 (9%) | 110 (9%) | 177 (10%) | 55 (5%) | 100 (6%) | 73 (5%) | 132 (7%) |
| Drugs taken† | ||||||||
| Nitrendipine | 821 (76%) | 1186 (76%) | 883 (73%) | 1194 (71%) | 998 (83%) | 1322 (79%) | 974 (75%) | 1328 (73%) |
| Enalapril | 132 (12%) | 552 (35%) | 565 (47%) | 693 (41%) | 426 (35%) | 714 (42%) | 618 (48%) | 823 (45%) |
| Hydrochlorothiazide | 22 (2%) | 157 (10%) | 272 (22%) | 326 (19%) | 168 (14%) | 335 (20%) | 334 (26%) | 424 (23%) |
| Other AH drugs | 126 (12%) | 225 (14%) | 228 (19%) | 322 (19%) | 69 (6%) | 169 (10%) | 193 (15%) | 287 (16%) |
| No AH drugs | 120 (11%) | 57 (4%) | 33 (3%) | 79 (5%) | 20 (2%) | 28 (2%) | 24 (2%) | 58 (3%) |
| Treatment unknown | NA | NA | NA | 7 (0%) | NA | NA | NA | 5 (0%) |
†Because many patients were on combined treatment, numbers do not add up. AH, antihypertensive; NA, not applicable.
At the last follow-up visit, the proportion of patients taking nitrendipine was 68.6% in the diabetic patient group, 72.4 % in the nondiabetic group, 78.3% in the patients who smoke, 71.7% in the nonsmokers group, 77.5% in the patients consuming at least 1 unit of alcohol per day, and 71.4% in the group of nondrinkers or very mild drinkers.
Blood pressure
At entry in Syst-Eur2, the mean sitting systolic BP in the patients formerly randomised to placebo was 160.4 ± 16.2 mmHg and in those of the former active-treatment group it was 151.0 ± 14.6 mmHg; the corresponding diastolic levels were 83.4 ± 7.7 mmHg and 79.6 ± 7.8 mmHg (Table 1). Of the 3516 patients, 1683 in the former placebo group and 1819 in the former active treatment group had their BP measured at least once during Syst-Eur2. At the last available visit in Syst-Eur2, in the patients of the former control group, the sitting blood pressure had fallen by 15.7 ± 18.7 mmHg systolic and by 5.1 ± 16.7 mmHg diastolic; in the patients previously randomised to active treatment, the corresponding BP reductions were 7.5 ± 16.7 mmHg systolic and 2.4 ± 11.8 mmHg diastolic, respectively (Fig. 2).
Figure 2.
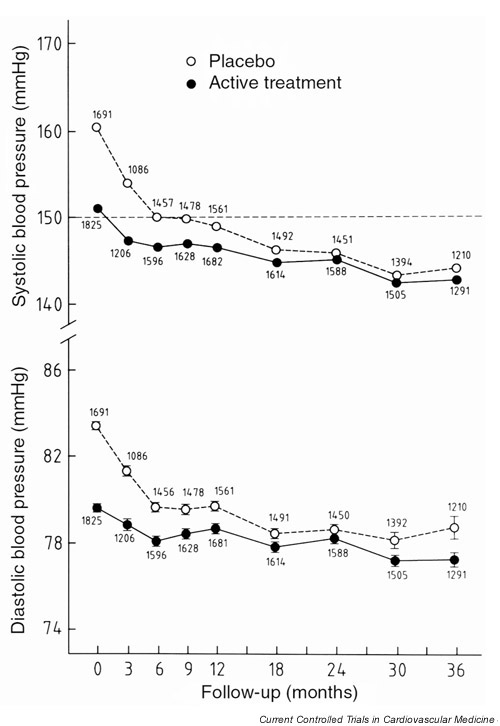
Average sitting systolic and diastolic blood pressures at baseline and during follow-up in Syst-Eur 2. Open and closed symbols indicate the patients formerly randomised to placebo or active treatment, respectively. The total number of patients at each follow-up visit is presented separately for the 2 previous arms of the trial.
The between-group differences in systolic and diastolic BP (placebo minus active treatment group) at entry in Syst-Eur 2 were 9.4 mmHg (95% confidence interval [CI] 8.4–10.4 mmHg) and 3.8 mmHg (95% CI 3.3–4.3 mmHg), respectively. At the last visit, these differences were 1.3 mmHg (95% CI 0.4–2.2 mmHg) and 1.2 mmHg (95% CI 0.3–2.1 mmHg) (Fig. 2).
At baseline in Syst-Eur2, 25.7 % of the patients randomised to placebo and 50.5% of those in the active-treatment group, had a sitting systolic BP less than 150 mmHg (P < 0.001). At the last visit in Syst-Eur2 these proportions were 71.9% and 76.0%, respectively (P = 0.006) (Fig. 3). The percentage of patients reaching the target BP was somewhat lower (P = 0.02) in the diabetic (69.3%) as compared to the nondiabetic patients (74.7%). By contrast, BP control was similar in patients consuming at least 1 unit of alcohol per day (74.6%) as compared to the other patients (74.0%). The proportion of patients reaching goal BP was similar in smokers (75.8%) and nonsmokers (73.9%).
Figure 3.
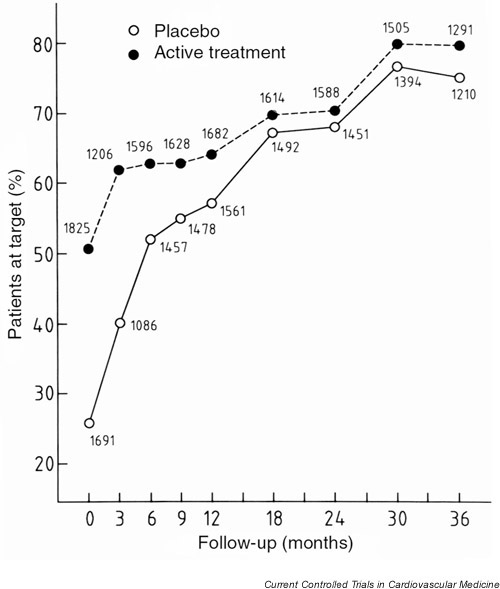
Proportion of patients reaching a systolic blood pressure below the target of 150 mmHg at baseline and during follow-up in Syst-Eur 2. Open and closed symbols indicate the patients formerly randomised to placebo or active treatment, respectively. The total number of patients at each follow-up visit is presented separately for the 2 previous arms of the trial.
In the former placebo group, 3.3% of the patients reaching the target BP were not taking any antihypertensive drugs, 36.9% were on nitrendipine only, 44.8% were taking enalapril and/or hydrochlorothiazide, and in 15.0% the study medication was associated with or replaced by other antihypertensive drugs. In the former active treatment group these percentages were 2.2%, 40.4%, 44.1% and 13.3%, respectively.
The distribution of the last available systolic BP was as follows: 2% <120 mmHg; 9% between 120 and 129 mmHg; 27% between 130 and 139 mmHg; 36% between 140 and 149 mmHg; 14% between 150 and 159 mmHg; 12% >160 mmHg (Fig. 4). Fourteen percent of the patients achieved a diastolic BP <70 mmHg, 47% between 70 and 79 mmHg, 33% between 80 and 89 mmHg and 6% >90 mmHg.
Figure 4.
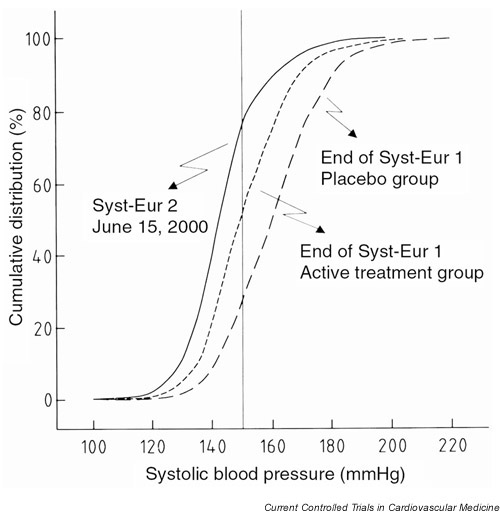
Cumulative distributions of systolic blood pressure at the end of Syst-Eur 1 in the placebo and active treatment group and at the last available visit in Syst-Eur 2. The vertical line indicates the goal systolic pressure of 150 mmHg.
Discussion
This paper describes BP control and compliance with study medications in 3516 older patients with isolated systolic hypertension who are being followed in Syst-Eur2 [5]. We found that substantial reductions in systolic BP could be achieved with a treatment strategy starting with the dihydropyridine calcium-channel blocker, nitrendipine, with the possible addition of enalapril and/or hydrochlorothiazide. At the last available follow-up visit, 74% of the patients had reached a systolic BP level below the target of 150 mmHg and an additional 14% achieved a systolic pressure below 160 mmHg. During Syst-Eur1, systolic BP decreased on average by 22 mmHg in the active-treatment group and by 13 mmHg in the placebo group. During Syst-Eur2, systolic BP further decreased by 7 mmHg and 15 mmHg, respectively. Part of the additional decrease in BP in the former active-treatment group might be due to the early termination of the Syst-Eur trial. Indeed, 261 (7.4%) of the patients participating in Syst-Eur2 had been randomised less than 6 months before the end of Syst-Eur1. In these patients, titration of study medication was probably not yet completed when the trial stopped in February 1997. Indeed, 85% of the active-treatment patients who were randomised less then 6 months before the end of Syst-Eur1 were still on monotherapy with nitrendipine. Another more likely explanation is that the evidence produced by the Syst-Eur trial [1,2] motivated the investigators to further up-titrate treatment to achieve optimal BP control and greater risk reduction in their patients.
From the start of the Syst-Eur trial, the Data Monitoring Committee carefully monitored BP control. At yearly intervals all centres received a list of patients who had not yet attained the target BP, together with information on the amount of study medications that these patients were taking. In addition, centres monitoring at least 10 patients received a graph showing the change of BP over time broken down by treatment group. BP control was personally discussed with the investigators at all of the 106 site visits and at the nine meetings for investigators that were held between 1989 and 2000. This quality control program probably contributed to the high rate of BP control currently achieved.
Syst-Eur2 is an open study that allows the use of antihypertensive treatment other than the study drugs [5]. Nonetheless, a high level of adherence to the study drugs was observed throughout the three initial years of follow-up. At the last available follow-up visit, 72% of the patients were taking nitrendipine and an additional 15% were taking study drugs other than nitrendipine. The withdrawal rate in Syst-Eur2 was also very low. Of the 3358 patients who were still alive on June15, 2000, 90% were still being followed in supervised, open follow-up and another 8.2% in non-supervised, open follow-up. Only 1.8% of the patients without any report within the last 18 months were counted as lost to follow-up.
Guidelines from the US Joint National Committee [7], the World Health Organization [8], the British Hypertension Society [9] and the Canadian Medical Association [10] all recommend an optimal target systolic BP of 140 mmHg in older hypertensive patients. These guidelines were published only after the protocol of Syst-Eur1 was written (1989). Syst-Eur2 was an extension of the Syst-Eur1 trial and it was decided that the protocols of these two studies should be as similar as possible. The World Health Organization and the British Hypertension Society based their advice mainly on the results of the Hypertension Optimal Treatment (HOT) trial [11]. Comparisons between the three randomised BP target groups (diastolic BP ≤ 90, ≤ 85 or ≤ 80 mmHg), however, showed no differences in cardiovascular outcomes in nondia-betic patients. Based on a Poisson model relating achieved BP to outcome, the optimal BP for reduction of major cardiovascular events was reported to be 139/83 mmHg. Nevertheless, patients whose BPs were below 150/90 mmHg were not apparently disadvantaged [9]. In the present population, the threshold of 140 mmHg proposed by the expert committees was reached by 38% of the patients. Because the target BP in the Syst-Eur trial was 150 mmHg, no efforts were undertaken to further lower the BP below 140 mmHg. Moreover, several experts had advised that the diastolic BP should not be lowered much below 70 mmHg [12,13,14]. In the present study, 14% of the patients had a diastolic BP below this threshold.
Comparison of BP control between various trials is difficult because of the large differences in the BP entry criteria, treatment targets, antihypertensive drugs used and definitions of achieved BP. In the HOT trial [11], systolic BP decreased on average by 26 mmHg, 28 mmHg and 30 mmHg in the diastolic BP target groups of ≤ 90 mmHg, ≤ 85 mmHg, and <80 mmHg, respectively. In our study the overall reduction in systolic BP of 29 mmHg was comparable to the changes obtained in the middle and low BP target groups of the HOT trial. Because systolic BP at randomisation was, on average, 3.5 mmHg lower in the HOT trial as compared with the Syst-Eur trial, however, the on-treatment systolic pressure in the Syst-Eur trial (144 mmHg) was similar to the systolic pressure achieved in the highest BP target group of the HOT trial (144 mmHg). In the Swedish Trial in Old Patients with Hypertension-2 (STOP-Hypertension-2) [15], patients aged 70–84 years, with moderate to severe hypertension (systolic BP >180 mmHg or diastolic BP >105 mmHg) were randomly assigned conventional antihypertensive drugs (diuretics or β-blockers) or newer drugs (calcium-channel blockers or angiotensin-converting enzyme inhibitors). Target BP was 160/95 mmHg. Systolic BP decreased from 194 mmHg to 158 mmHg in the conventional drug group, 159 mmHg in the angiotensin-converting enzyme inhibitor group and 159 mmHg in the group taking calcium channel blockers. In the Nordic Diltiazem (NORDIL) study [16] patients aged 50–74 years with a diastolic BP of at least 100 mmHg were randomised. The treatment target was a diastolic BP below 90 mmHg. Systolic BP was reduced from 173 mmHg to 155 mmHg in the diltiazem group and to 152 mmHg in the groups on older drugs. The percentages of patients reaching the target BP in the HOT, STOP-Hypertension-2 and NORDIL trials were not reported.
The double-blind International Nifedipine GITS study (INSIGHT) [17] recruited patients between 55 and 80 years old with hypertension (BP ≥ 150/95 mmHg, or systolic BP ≥ 160 mmHg) and with at least one additional cardiovascular risk factor. Patients were randomised to nifedipine GITS or co-amilozide. In both treatment groups, systolic BP fell from 173 mmHg to 138 mmHg. Between 54% and 59% of the patients reached the target BP, which was defined as a decrease in BP by at least 20/10 mmHg to a level below 140/90 mmHg. In the INSIGHT study, however, an intention-to-treat analysis of BP responses was not presented. Finally, in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [18], hypertensive patients (systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg) aged ≥ 55 years with at least one additional risk factor, were randomised to double-blind treatment with four types of antihypertensive drugs. Systolic BP fell from 145 mmHg at baseline to 136 mmHg at two years in the chlorthalidone group and to 138 mmHg in the doxazosin group. The percentages of patients reaching the target BP (<140 mmHg systolic and <90 mmHg diastolic) were 61% and 54% respectively.
Conclusion
On June 15, 2000, 90% of the patients entering Syst-Eur2 in 1997 were still being followed in supervised, open follow-up and 72% of the patients were still on nitrendipine as first-line treatment. 74% of the patients achieved a systolic BP below the target of 150 mmHg. The main results of the Syst-Eur2 study will be reported in the year 2002. Additional information can be found on the trial web site [20].
Competing interests
None declared.
Abbreviations
ALLHAT = Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; HOT = Hypertension Optimal Treatment trial; INSIGHT = International Nifedipine GITS study; NORDIL = Nordic Diltiazem study; STOP-Hypertension-2 = Swedish Trial in Old Patients with Hypertension-2; Syst-Eur = Systolic Hypertension in Europe trial; Syst-Eur 2 = Systolic Hypertension in Europe Phase 2. BP = blood pressure; CI = confidence interval.
Appendix
Trial coordinators
Robert Fagard, MD and Jan A Staessen, MD.
Regional coordinators
Guramy G Arabidze, MD (deceased) (Bellorussia and the Russian Federation); Willem H Birkenhäger, MD (the Netherlands); Christopher J Bulpitt, MD (United Kingdom); Manuel Carrageta, MD (Portugal); Hilde Celis, MD (Belgium); Françoise Forette, MD (France); Jozef Kocemba, MD (Poland); Gastone Leonetti, MD (Italy); Choudomir Nachev, MD (Bulgaria); Eoin T O'Brien, MD (Ireland); Eberhard Ritz, MD (Germany); José L Rodicio, MD (Spain); Joseph Rosen-feld, MD (Israel); Jaakko Tuomilehto (Finland, Estonia and Lithuania).
Steering committee
Guramy G Arabidze, MD (deceased); Paul De Cort, MD; Robert Fagard, MD; Françoise Forette, MD; Kalina Kawecka-Jaszcz, MD; Gastone Leonetti, MD; Choudomir Nachev, MD; Eoin T O'Brien, MD; José L Rodico, MD; Joseph Rosenfeld, MD; Jaakko Tuomilehto, MD; John Webster, MD and Yair Yodfat, MD.
Data monitoring committee
Christopher J Bulpitt, MD; Astrid E Fletcher, PhD; Jan A Staessen, MD and Lutgarde Thijs, BSc.
End-point committee
Peter W de Leeuw, MD; Robert Fagard, MD; Gastone Leonetti, MD and James C. Petrie, MD.
Ethics Committee
Willem H Birkenhäger, MD; Colin T Dollery, MD and Robert Fagard, MD;
Publication Committee
Willem H Birkenhäger, MD; Christopher J Bulpitt, MD; Jan A Staessen, MD and Alberto Zanchetti, MD.
Coordinating office
Nicole Ausseloos; Hilde Celis, MD; Elly Den Hond, DSc; Lut De Pauw, RN; Paul Drent; Robert Fagard, MD; Heng Fan; Tim Nawrot, BSc; Yvette Piccart; Jan A Staessen, MD; Yvette Toremans; Lutgarde Thijs, BSc; Sylvia Van Hulle, RN; Ji G Wang, MD and Renilde Wolfs.
Clinical centres
The clinical investigators are listed in Staessen et al. [19] and Gasowski et al. [5].
Acknowledgments
Acknowledgements
The Syst-Eur trial, initiated by the late Prof A Amery, was a concerted action of the BIOMED Research Program sponsored by the European Union. The trial was carried out in consultation with the World Health Organisation, the International Society of Hypertension, the European Society of Hypertension and the World Hypertension League. Syst-Eur 2 is sponsored by Bayer AG (Wuppertal, Germany). The study medication is donated by Bayer AG and Merck Sharpe and Dohme Inc (West Point, PA, USA).
References
- Staessen JA, Fagard R, Thijs L, Celis H, Arabidze G, Birkenhäger WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O'Brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A, for the Systolic Hypertension in Europe (Syst-Eur) Trial Investigators Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350:757–764. doi: 10.1016/S0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Thijs L, Birkenhäger WH, Bulpitt CJ, Fagard R, on behalf of the Syst-Eur investigators Update on the Systolic Hypertension in Europe (Syst-Eur) Trial. Hypertension. 1999;33:1476–1477. doi: 10.1161/01.hyp.33.6.1476. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang JG, Gong L, Liu G, Staessen JA, for the Systolic Hypertension in China (Syst-China) Collaborative Group Comparison of active treatment and placebo for older patients with isolated systolic hypertension. J Hypertens. 1998;16:1823–1829. doi: 10.1097/00004872-199816120-00016. [DOI] [PubMed] [Google Scholar]
- Gong L, Zhang W, Zhu Y, Zhu J, 11 collaborating centres in the Shangai area. Kong D, Page V, Ghadirian P, LaLorier J, Hamet P. Shanghai trial of nifedipine in the elderly (STONE). J Hypertens. 1996;14:1237–1245. doi: 10.1097/00004872-199610000-00013. [DOI] [PubMed] [Google Scholar]
- Gasowski J, Staessen JA, Celis H, Fagard RH, Thijs L, Birkenhäger WH, Bulpitt CJ, Fletcher AE, Arabidze GG, de Leeuw PW, Dollery CT, Duggan J, Kawecka-Jaszcz K, Leonetti G, Nachev C, Safar M, Rodicio JL, Rosenfeld J, Seux ML, Tuomilehto J, Webster J, Yodfat Y, on behalf of the Systolic Hypertension in Europe Investigators Systolic Hypertension in Europe (Syst-Eur) Trial Phase 2: objectives, protocol, and initial progress. J Hum Hypertens. 1999;13:135–145. doi: 10.1038/sj/jhh/1000769. [DOI] [PubMed] [Google Scholar]
- 41st World Medical Assembly Declaration of Helsinki: recommendations guiding physicians in biomedical research involving human subjects. Bull Pan Am Health Organ. 1990;24:606–609. [Google Scholar]
- The Joint National Committee on Detection Evaluation and Treatmentof Hypertension The sixth report of the Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure (JNC-VI). Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- International Society of Hypertension Guidelines Subcommittee 1999 World Health Organization – International Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens. 1999;17:151–183. doi: 10.1097/00004872-199917020-00001. [DOI] [PubMed] [Google Scholar]
- Ramsay LE, Williams B, Johnston GD, MacGregor GA, Poston L, Potter JF, Poulter NR, Russell G. British Hypertension Society guidelines for hypertension management 1999: summary. BMJ. 1999;319:630–635. doi: 10.1136/bmj.319.7210.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RD, Campbell N, Larochelle P, for the Task Force for the Development of the 1999 Canadian Recommendations for the Management of Hypertension Canadian Recommendations for the Management of Hypertension. Can Med Assoc J. 1999;161 (Suppl 12):S1–S17. [PMC free article] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, Ménard J, Rahn KH, Wedel H, Westerling S, for the HOT Study Group Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. doi: 10.1016/S0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- Kaplan N. New issues in the treatment of isolated systolic hypertension. Circulation. 2000;102:1079–1081. doi: 10.1161/01.cir.102.10.1079. [DOI] [PubMed] [Google Scholar]
- Somes GW, Pahor M, Shorr RI, Cushman WC, Applegate WB. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med. 1999;159:2004–2009. doi: 10.1001/archinte.159.17.2004. [DOI] [PubMed] [Google Scholar]
- Voko Z, Bots ML, Hofman A, Koudstaal PJ, Witteman JCM, Breteler MMB. J-shaped relation between blood pressure and stroke in treated hypertensives. Hypertension. 1999;34:1181–1185. doi: 10.1161/01.hyp.34.6.1181. [DOI] [PubMed] [Google Scholar]
- Hansson L, Lindholm L, Ekbom T, Dahlöf B, Lanke J, Scherstén B, Wester P-O, Hedner T, de Faire U, for the STOP-Hypertension-2 study group Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 2000;354:1751–1756. doi: 10.1016/S0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- Hansson L, Hedner T, Lund-Johansen P, Kjeldsen SE, Lindholm LH, Syvertsen JO, Lanke J, de Faire U, Dahlöf B, Karlberg BE, for the NORDIL Study Group Randomised trial of effects of calcium antagonists compared with diuretics and β-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet. 2000;356:359–365. doi: 10.1016/S0140-6736(00)02526-5. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, Ruilope LM. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet. 2000;356:366–372. doi: 10.1016/S0140-6736(00)02527-7. [DOI] [PubMed] [Google Scholar]
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2000;283:1967–1975. doi: 10.1001/jama.283.15.1967. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Fagard R, Thijs L, Celis H, Birkenhäger WH, Bulpitt CJ, de Leeuw PW, Fletcher AE, Babarskiene M-R, Forette F, Kocembe J, Laks T, Leonetti G, Nachev C, Petrie JC, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Zanchetti A, for the Systolic Hypertension in Europe Trial Investigators Subgroup and perprotocol analysis of the randomised European trial on isolated systolic hypertension in the elderly. Arch Intern Med. 1998;158:1681–1691. doi: 10.1001/archinte.158.15.1681. [DOI] [PubMed] [Google Scholar]
- Syst-Eur http://www.syst-eur.com


