Abstract
Background
At birth, infants' lungs are fluid‐filled. For newborns to have a successful transition, this fluid must be replaced by air to enable effective breathing. Some infants are judged to have inadequate breathing at birth and are resuscitated with positive pressure ventilation (PPV). Giving prolonged (sustained) inflations at the start of PPV may help clear lung fluid and establish gas volume within the lungs.
Objectives
To assess the efficacy of an initial sustained (> 1 second duration) lung inflation versus standard inflations (≤ 1 second) in newly born infants receiving resuscitation with intermittent PPV.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1), MEDLINE via PubMed (1966 to 17 February 2017), Embase (1980 to 17 February 2017), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 17 February 2017). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles to identify randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs comparing initial sustained lung inflation (SLI) versus standard inflations given to infants receiving resuscitation with PPV at birth.
Data collection and analysis
We assessed the methodological quality of included trials using Cochrane Effective Practice and Organisation of Care Group (EPOC) criteria (assessing randomisation, blinding, loss to follow‐up, and handling of outcome data). We evaluated treatment effects using a fixed‐effect model with risk ratio (RR) for categorical data and mean, standard deviation (SD), and weighted mean difference (WMD) for continuous data. We assessed the quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
Main results
Eight trials enrolling 941 infants met our inclusion criteria. Investigators in seven trials (932 infants) administered sustained inflation with no chest compressions. Use of sustained inflation had no impact on the primary outcomes of this review ‐ mortality in the delivery room (typical RR 2.66, 95% confidence interval (CI) 0.11 to 63.40; participants = 479; studies = 5; I² not applicable) and mortality during hospitalisation (typical RR 1.01, 95% CI 0.67 to 1.51; participants = 932; studies = 7; I² = 19%); the quality of the evidence was low for death in the delivery room (limitations in study design and imprecision of estimates) and was moderate for death before discharge (limitations in study design of most included trials). Amongst secondary outcomes, duration of mechanical ventilation was shorter in the SLI group (mean difference (MD) ‐5.37 days, 95% CI ‐6.31 to ‐4.43; participants = 524; studies = 5; I² = 95%; low‐quality evidence). Heterogeneity, statistical significance, and magnitude of effects of this outcome are largely influenced by a single study: When this study was removed from the analysis, the effect was largely reduced (MD ‐1.71 days, 95% CI ‐3.04 to ‐0.39, I² = 0%). Results revealed no differences in any of the other secondary outcomes (e.g. rate of endotracheal intubation outside the delivery room by 72 hours of age (typical RR 0.93, 95% CI 0.79 to 1.09; participants = 811; studies = 5; I² = 0%); need for surfactant administration during hospital admission (typical RR 0.97, 95% CI 0.86 to 1.10; participants = 932; studies = 7; I² = 0%); rate of chronic lung disease (typical RR 0.95, 95% CI 0.74 to 1.22; participants = 683; studies = 5; I² = 47%); pneumothorax (typical RR 1.44, 95% CI 0.76 to 2.72; studies = 6, 851 infants; I² = 26%); or rate of patent ductus arteriosus requiring pharmacological treatment (typical RR 1.08, 95% CI 0.90 to 1.30; studies = 6, 745 infants; I² = 36%). The quality of evidence for these secondary outcomes was moderate (limitations in study design of most included trials ‐ GRADE) except for pneumothorax (low quality: limitations in study design and imprecision of estimates ‐ GRADE).
Authors' conclusions
Sustained inflation was not better than intermittent ventilation for reducing mortality in the delivery room and during hospitalisation. The number of events across trials was limited, so differences cannot be excluded. When considering secondary outcomes, such as need for intubation, need for or duration of respiratory support, or bronchopulmonary dysplasia, we found no evidence of relevant benefit for sustained inflation over intermittent ventilation. The duration of mechanical ventilation was shortened in the SLI group. This result should be interpreted cautiously, as it can be influenced by study characteristics other than the intervention. Future RCTs should aim to enrol infants who are at higher risk of morbidity and mortality, should stratify participants by gestational age, and should provide more detailed monitoring of the procedure, including measurements of lung volume and presence of apnoea before or during the SLI.
Keywords: Humans; Infant, Newborn; Ductus Arteriosus, Patent; Ductus Arteriosus, Patent/epidemiology; Hospital Mortality; Intubation, Intratracheal; Intubation, Intratracheal/methods; Intubation, Intratracheal/mortality; Positive‐Pressure Respiration; Positive‐Pressure Respiration/methods; Positive‐Pressure Respiration/mortality; Pulmonary Surfactants; Pulmonary Surfactants/administration & dosage; Randomized Controlled Trials as Topic; Respiration, Artificial; Respiration, Artificial/utilization; Resuscitation; Resuscitation/methods; Time Factors
Prolonged lung inflation for neonatal resuscitation
Review question
Does the use of prolonged (or sustained, > 1 second duration) lung inflation rather than standard inflations (≤ 1 second) improve survival and other important outcomes among newly born babies receiving resuscitation at birth?
Background
At birth, the lungs are filled with fluid, which must be replaced by air for babies to breathe properly. Some babies have difficulty establishing effective breathing at birth, and 1 in every 20 to 30 babies receives help to do so. A variety of devices are used to help babies begin normal breathing. Some of these devices allow caregivers to give long (or sustained) inflations. These sustained inflations may help inflate the lungs and may keep the lungs inflated better than if they are not used.
Study characteristics
We collected and analysed all relevant studies to answer the review question and found eight studies enrolling 941 infants. In all studies, babies were born before the due date (from 23 to 36 weeks of gestational age). The sustained inflation lasted between 15 and 20 seconds at pressure between 20 and 30 cmH2O. Most studies provided one or more additional sustained inflations in cases of poor clinical response, for example, persistent low heart rate. We analysed one study (which included only nine babies) separately because researchers combined use of sustained or standard inflations with chest compressions.
Key results
The included studies showed no important differences among babies who received sustained versus standard inflations in terms of mortality, need for intubation during the first three days of life, or chronic lung disease. Babies receiving sustained inflation at birth may spend fewer days on mechanical ventilation. Several ongoing studies might help us to clarify whether differences between the two techniques may occur, as now we cannot exclude that small to moderate differences exist.
Quality of evidence
The quality of evidence is low to moderate because overall only a small number of studies have looked at this intervention; few babies were included in these studies; and some studies could have been better designed.
How up‐to‐date is this review?
We searched for studies that had been published up to February 2017.
Summary of findings
Summary of findings for the main comparison.
Use of initial sustained inflation compared with standard inflations in newborns receiving resuscitation with no chest compressions during resuscitation
| Use of initial sustained inflation compared with standard inflations in newborns receiving resuscitation with no chest compressions during resuscitation | ||||||
|
Patient or population: preterm infants resuscitated using PPV at birth Settings: delivery room in Europe (Austria, Germany, Italy), Canada, Egypt, Thailand Intervention: sustained inflation with no chest compressions Comparison: standard inflations with no chest compressions | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard inflations in newborns receiving resuscitation with no chest compressions | Use of initial sustained inflation | |||||
| Death ‐ death in the delivery room | Study population | RR 2.66 (0.11 to 63.4) | 479 (5 studies) | ⊕⊕⊝⊝ lowa,b | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Death ‐ death before discharge | Study population | RR 1.01 (0.67 to 1.51) | 932 (7 studies) | ⊕⊕⊕⊝ moderatea | ||
| 82 per 1000 | 83 per 1000 (55 to 124) | |||||
| Need for mechanical ventilation | Study population | RR 0.87 (0.74 to 1.03) | 484 (3 studies) | ⊕⊕⊕⊝ moderatea | ||
| 487 per 1000 | 424 per 1000 (360 to 502) | |||||
| Chronic lung disease ‐ BPD any grade | Study population | RR 0.9 (0.69 to 1.19) | 220 (2 studies) | ⊕⊕⊕⊝ moderatea | ||
| 483 per 1000 | 435 per 1000 (333 to 575) | |||||
| Chronic lung disease ‐ moderate to severe BPD | Study population | RR 0.95 (0.74 to 1.22) | 683 (5 studies) | ⊕⊕⊕⊝ moderatea | ||
| 257 per 1000 | 244 per 1000 (190 to 314) | |||||
| Pneumothorax ‐ any time | Study population | RR 1.44 (0.76 to 2.72) | 851 (6 studies) | ⊕⊕⊝⊝ lowa,c | ||
| 33 per 1000 | 48 per 1000 (25 to 90) | |||||
| Cranial ultrasound abnormalities ‐ intraventricular haemorrhage grade 3 to 4 | Study population | RR 0.89 (0.58 to 1.37) | 635 (5 studies) | ⊕⊕⊕⊝ moderatea | ||
| 120 per 1000 | 107 per 1000 (70 to 164) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
Assumed risk is the risk of the control arm.
aLimitations in study design: all studies at high or unclear risk of bias in at least one domain bImprecision: few events cImprecision: wide confidence intervals
Summary of findings 2.
Use of initial sustained inflation compared with standard inflations in newborns receiving resuscitation with chest compressions during resuscitation
| Use of initial sustained inflation compared with standard inflations in newborns receiving resuscitation with chest compressions during resuscitation | ||||||
|
Patient or population: preterm infants resuscitated by PPV at birth Settings: delivery room in Europe (Austria, Germany, Italy), Canada, Egypt, Thailand Intervention: sustained inflation with chest compressions Comparison: standard inflations with chest compressions | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard inflations in newborns receiving resuscitation with chest compressions | Use of initial sustained inflation | |||||
| Death ‐ death before discharge | See comment | See comment | Not estimable | 9 (1 study) | ⊕⊝⊝⊝ very lowa,b | Only 1 trial included |
| Chronic lung disease ‐ moderate to severe BPD | See comment | See comment | Not estimable | 7 (1 study) | ⊕⊝⊝⊝ very lowa,b | Only 1 trial included |
| Pneumothorax ‐ any time | See comment | See comment | Not estimable | 9 (1 study) | ⊕⊝⊝⊝ very lowa,b | Only 1 trial included |
| Cranial ultrasound abnormalities ‐ intraventricular haemorrhage grade 3 to 4 | See comment | See comment | Not estimable | 9 (1 study) | ⊕⊝⊝⊝ very lowa,b | Only 1 trial included |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
Assumed risk is the risk of the control arm.
aLimitations in study design: included study at high or unclear risk of bias in four domains bImprecision (downgraded by two levels): extremely low sample size, few events
Background
Description of the condition
At birth, infants' lungs are filled with fluid, which must be cleared for effective respiration to occur. Most newly born infants achieve this spontaneously and may use considerable negative pressure (up to ‐50 cmH2O) for initial inspirations (Karlberg 1962; Milner 1977). However, it is estimated that 3% to 5% of newly born infants receive some help to breathe at delivery (Saugstad 1998). Adequate ventilation is the key to successful neonatal resuscitation and stabilisation (Wyckoff 2015). Positive pressure ventilation (PPV) is recommended for infants who have absent or inadequate respiratory efforts, bradycardia, or both, at birth (Wyckoff 2015). Use of manual ventilation devices ‐ self‐inflating bags, flow‐inflating (or anaesthetic) bags, and T‐piece devices ‐ with a face mask or endotracheal tube (ETT) is advised. Although it is not included in the International Liaison Committee on Resuscitation (ILCOR) guidelines, respiratory support of infants in the delivery room with a mechanical ventilator and a nasopharyngeal tube has been described (Lindner 1999).
Description of the intervention
Devices recommended for PPV in the delivery room differ in terms of physical characteristics and ability to deliver sustained lung inflation (SLI). The most commonly used self‐inflating bag (O'Donnell 2004a; O'Donnell 2004b) may be of insufficient size to support sustained inflation (> 1 second). Both flow‐inflating bags and T‐pieces may be used to consistently deliver inflations > 1 second. Although target inflation pressures and long inspiratory times are achieved more consistently in mechanical models when T‐piece devices rather than bags are used, no recommendation can be made as to which device is preferable (Wyckoff 2015; Wyllie 2015). Positive end‐expiratory pressure (PEEP) is very important for aerating the lungs and improving oxygenation; SLI consists of prolonged high‐level PEEP.
How the intervention might work
When airways are liquid‐filled, it might be unnecessary to interrupt inflation pressures to allow the lung to deflate and exhale CO2 (Hooper 2016). Boon 1979 described a study of 20 term infants delivered by Caesarean section under general anaesthesia who were resuscitated with a T‐piece via an ETT. Trial authors reported that gas continued to flow through the flow sensor placed between the T‐piece and the ETT toward the infant at the end of a standard inflation of 1 second on respiratory traces obtained (Boon 1979). On the basis of this observation, this group performed a non‐randomised trial of sustained inflations given via a T‐piece and an ETT to nine term infants during delivery room resuscitation. Investigators reported that initial inflation with a T‐piece lasting 5 seconds produced a two‐fold increase in inflation volume compared with standard resuscitation techniques (Vyas 1981). Citing these findings, a retrospective cohort study described the effects of a change in management strategy for extremely low birth weight infants in the delivery room (Lindner 1999). The new management strategy included the introduction of an initial sustained inflation of 15 seconds obtained with a mechanical ventilator via a nasopharyngeal tube. This change in strategy was associated with a reduction in the proportion of infants intubated for ongoing respiratory support without an apparent increase in adverse outcomes. Pulmonary morbidity in very low birth weight infants was reported to be related directly to mortality in 50% of cases of death (Drew 1982). Moreover, multiple SLIs in very preterm infants improved both heart rate and cerebral tissue oxygen saturation, in the absence of any detrimental effects (Fuchs 2011). An observational study showed that sustained inflation of 10 seconds at 25 cmH2O in 70 very preterm infants at birth was not effective for infants who were not breathing, possibly owing to active glottic adduction (van Vonderen 2014). Newly born infants frequently take a breath and then prolong expiration via glottic closure and diaphragmatic braking, giving themselves prolonged end‐expiratory pressure.
Why it is important to do this review
Recommendations regarding use of sustained inflation at birth have varied between international bodies. Although European Resuscitation Council guidelines suggest giving five inflation breaths if the newborn is gasping or is not breathing (Wyllie 2015), the American Heart Association states that evidence is insufficient to recommend an optimum inflation time (Wyckoff 2015). Differences between these guidelines and their algorithms are intriguing (Klingenberg 2016). A narrative review reported that sustained inflation may reduce the need for mechanical ventilation among preterm infants at risk for respiratory distress syndrome (RDS) (Lista 2010). The same review showed that respiratory outcomes among infants receiving sustained inflation (25 cmH2O for 15 seconds) were improved over those reported for an historical group (Lista 2011).
This review updates the existing review "Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes", which was published in the Cochrane Database of Systematic Reviews in 2015 (O'Donnell 2015).
Objectives
To assess the efficacy of an initial sustained (> 1 second duration) lung inflation (SLI) versus standard inflations (≤ 1 second) in newborn infants receiving resuscitation with intermittent PPV.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs. We excluded observational studies (case‐control studies, case series) and cluster‐RCTs.
Types of participants
Term and preterm infants resuscitated via PPV at birth.
Types of interventions
Interventions included resuscitation with initial sustained (> 1 second) inflation versus resuscitation with regular (≤ 1 second) inflations:
with no chest compressions as part of the initial resuscitation (primary comparison); or
with chest compressions as part of the initial resuscitation (secondary comparison).
Types of outcome measures
Primary outcomes
Death in the delivery room
Death during hospitalisation
Death to latest follow‐up
Secondary outcomes
Apgar scores at 1 and 5 minutes
Heart rate at 5 minutes
Endotracheal intubation in the delivery room
Endotracheal intubation outside the delivery room during hospitalisation
Surfactant administration in the delivery room or during hospital admission
Need for mechanical ventilation
Duration in hours of respiratory support (i.e. nasal continuous airway pressure and ventilation via an ETT considered separately and in total)
Duration in days of supplemental oxygen requirement
Chronic lung disease: need for supplemental oxygen at 28 days of life; need for supplemental oxygen at 36 weeks of gestational age for infants born at or before 32 weeks of gestation
Air leaks (pneumothorax, pneumomediastinum, pneumopericardium, pulmonary interstitial emphysema) reported individually or as a composite outcome
Cranial ultrasound abnormalities: any intraventricular haemorrhage (IVH), grade 3 or 4 according to the Papile classification (Papile 1978), and cystic periventricular leukomalacia
Seizures including clinical and electroencephalographic
Hypoxic ischaemic encephalopathy for term and late preterm infants (grade 1 to 3 (Sarnat 1976))
Long‐term neurodevelopmental outcomes (rates of cerebral palsy on physician assessment, developmental delay (i.e. intelligence quotient (IQ) 2 standard deviations (SDs) < mean on validated assessment tool (e.g. Bayley's Mental Developmental Index))
Retinopathy of prematurity (ROP) (all stages and ≥ stage 3)
Patent ductus arteriosus (PDA) (pharmacological treatment and surgical ligation)
Search methods for identification of studies
See Cochrane Neonatal Review Group (CNRG) search strategy.
Electronic searches
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal search strategy for specialized register).
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) in the Cochrane Library; MEDLINE via PubMed (1966 to 17 February 2017); Embase (1980 to 17 February 2017); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 17 February 2017), using the following search terms: (sustained inflation) OR (sustained AND inflation) OR (sustained AND (inflat* AND (lung OR pulmonary))), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for full search strategy for each database). We did not apply language restrictions. We searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform ‐ www.whoint/ictrp/search/en/; and the ISRCTN Registry).
Searching other resources
We also searched abstracts of the Pediatric Academic Society (PAS) from 2000 to 2017, electronically through the PAS website (abstractsonline), using the following key words: "sustained inflation" AND "clinical trial".
Data collection and analysis
Selection of studies
For this update, two review authors (MB, MGC) independently screened all titles and abstracts to determine which trials met the inclusion criteria. We retrieved full‐text copies of all papers that were potentially relevant. We resolved disagreements by discussion between review authors.
Data extraction and management
Two review authors (MB, MGC) independently undertook data abstraction using a data extraction form developed ad hoc and integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group (EPOC) data collection checklist (EPOC 2015).
We extracted the following characteristics from each included trial.
Administrative details: study author(s); published or unpublished; year of publication; year in which trial was conducted; details of other relevant papers cited.
Trial details: study design; type, duration, and completeness of follow‐up; country and location of study; informed consent; ethics approval.
Details of participants: birth weight; gestational age; number of participants.
Details of intervention: type of ventilation device used; type of interface; duration and level of pressure of sustained lung inflation (SLI).
Details of outcomes: death during hospitalisation or to latest follow‐up; heart rate at 5 minutes; duration in hours of respiratory support; duration in days of supplemental oxygen requirement; long‐term neurodevelopmental outcomes; any adverse events.
We resolved disagreements by discussion between review authors. When available, we described ongoing trials identified by detailing primary trial author, research question(s) posed, and methods and outcome measures applied, together with an estimate of the reporting date.
When queries arose or additional data were required, we contacted trial authors.
Assessment of risk of bias in included studies
Two review authors (MB, SZ) independently assessed risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or via consultation with a third assessor. See Appendix 2 for a detailed description of risk of bias for each domain.
Selection bias (random sequence generation and allocation concealment)
Random sequence generation
For each included trial, we categorised risk of bias regarding random sequence generation as follows.
Low risk ‐ adequate (any truly random process, e.g. random number table; computer random number generator).
High risk ‐ inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number).
Unclear risk ‐ no or unclear information provided.
Allocation concealment
For each included trial, we categorised risk of bias regarding allocation concealment as follows.
Low risk ‐ adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes).
High risk ‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth).
Unclear risk ‐ no or unclear information provided.
Performance bias
Owing to the nature of the intervention, all trials were unblinded, leading to high risk of performance bias.
Detection bias
For each included trial, we categorised the methods used to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or different classes of outcomes.
Attrition bias
For each included trial and for each outcome, we described completeness of data including attrition and exclusions from analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes.
Reporting bias
For each included trial, we described how we investigated the risk of selective outcome reporting bias and what we found. We assessed methods as follows.
Low risk ‐ adequate (when it is clear that all of a trial's prespecified outcomes and all expected outcomes of interest to the review have been reported).
High risk ‐ inadequate (when not all of a trial's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; or the trial failed to include results of a key outcome that would have been expected to be reported).
Unclear risk ‐ no or unclear information provided (study protocol was not available).
Other bias
For each included trial, we described any important concerns that we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific trial design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each trial was free of other problems that could put it at risk of bias as follows.
Low risk ‐ no concerns of other bias raised.
High risk ‐ concerns raised about multiple looks at data with results made known to investigators, differences in numbers of participants enrolled in abstract, and final publications of the paper.
Unclear ‐ concerns raised about potential sources of bias that could not be verified by contacting trial authors.
We did not score blinding of the intervention because this was not applicable.
One review author entered data into RevMan 2014, and a second review author checked entered data for accuracy.
Measures of treatment effect
We conducted measures of treatment effect data analysis using RevMan 2014. We determined outcome measures for dichotomous data (e.g. death, endotracheal intubation in the delivery room, frequency of retinopathy) as risk ratios (RRs) with 95% confidence intervals (CIs). We calculated continuous data (e.g. duration of respiratory support, Apgar score) using mean differences (MDs) and SDs.
Unit of analysis issues
The unit of randomisation was the intended unit of analysis (individual neonate).
Dealing with missing data
We contacted trial authors to request missing data when needed.
Assessment of heterogeneity
As a measure of consistency, we used the I² statistic and the Q (Chi²) test (Deeks 2011). We judged statistical significance of the Q (Chi²) statistic by P < 0.10 because of the low statistical power of the test. We used the following cut‐offs for heterogeneity: < 25% no (none) heterogeneity; 25% to 49% low heterogeneity; 50% to 74% moderate heterogeneity; and ≥ 75% high heterogeneity (Higgins 2003). We combined trial results using the fixed‐effect model, regardless of statistical evidence of heterogeneity effect sizes.
Assessment of reporting biases
See Appendix 2.
Data synthesis
We performed statistical analyses using RevMan 2014. We used the standard methods of the Cochrane Neonatal Review Group. For categorical data, we used RRs, relative risk reductions, and absolute risk difference (RDs). We obtained means and SDs for continuous data and performed analyses using MDs and WMDs when appropriate. We calculated 95% CIs. We presented the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH), as appropriate. For each comparison reviewed, meta‐analysis could be feasible if we identified more than one eligible trial, and if homogeneity among trials was sufficient with respect to participants and interventions. We combined trials using the fixed‐effect model, regardless of statistical evidence of heterogeneity effect sizes. For estimates of RR and RD, we used the Mantel‐Haenszel method.
Quality of evidence
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: death in the delivery room or during hospitalisation; endotracheal intubation in the delivery room or outside the delivery room during hospitalisation; surfactant administration in the delivery room or during hospital admission; need for mechanical ventilation; chronic lung disease; air leaks; and cranial ultrasound abnormalities.
Two review authors independently assessed the quality of evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded evidence one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of evidence.
The GRADE approach yields an assessment of the quality of a body of evidence according to one of four grades.
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses of the safety and efficacy of sustained inflation during resuscitation in subgroups.
Term (≥ 37 weeks of gestation) and preterm (< 37 weeks of gestation) infants.
Type of ventilation device used (self‐inflating bag, flow‐inflating bag, T‐piece, mechanical ventilator).
Interface used (i.e. face mask, ETT, nasopharyngeal tube).
Duration of sustained lung inflation (i.e. > 1 second to 5 seconds, > 5 seconds).
Sensitivity analysis
We planned to conduct sensitivity analyses to explore effects of the methodological quality of trials and checked to ascertain whether studies with high risk of bias overestimated treatment effects.
Results
Description of studies
We have provided results of the search for this review update in the study flow diagram (Figure 1).
Figure 1.
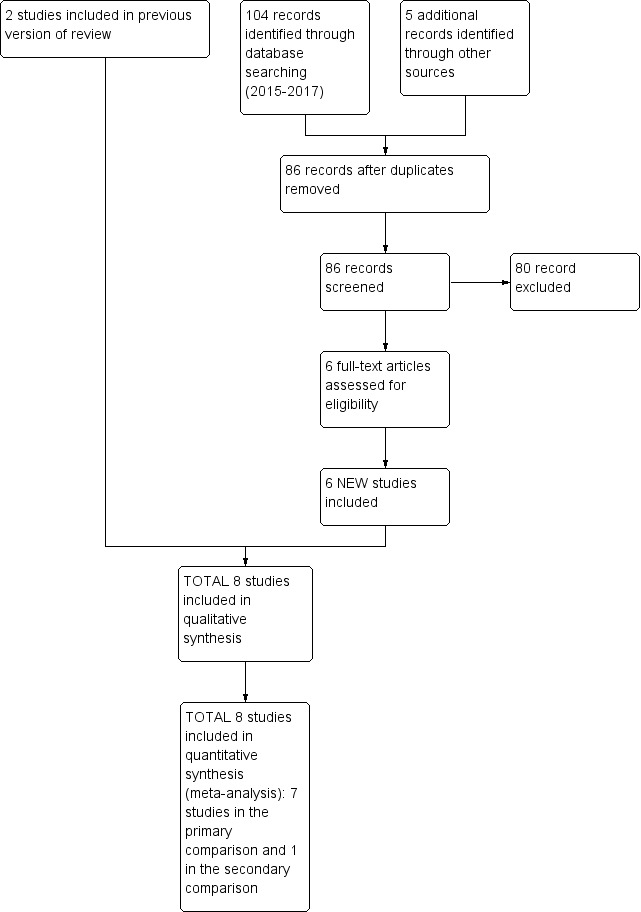
Study flow diagram: review update.
See Characteristics of included studies,Characteristics of excluded studies, and Characteristics of ongoing studies sections for details.
Included studies
Eight trials recruiting 941 infants (473 in SLI groups, 468 in control groups) met the inclusion criteria (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schmölzer 2015; Schwaberger 2015). We pooled seven trials (with 932 infants) in the primary comparison (i.e. use of sustained inflation with no chest compressions) (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schwaberger 2015). In contrast to other trials, Schwaberger 2015 sought to use near‐infrared spectroscopy (NIRS) to investigate whether SLI affected physiological changes in cerebral blood volume and oxygenation. We could not perform any meta‐analysis in the secondary comparison (intervention superimposed on uninterrupted chest compressions) because we included only one trial (a pilot study of nine preterm infants) (Schmölzer 2015).
We have listed characteristics of populations and interventions and comparisons of the eight trials under Characteristics of included studies and in Table 5.
Table 1.
Populations and interventions in included trials
|
Trial (no. infants) |
Antenatal steroids | Gestational age, weeks | Birth weight, grams | Device/Interface | Interventions/Controls | ||||
| SLI | Control | SLI | Control | SLI | Control | SLI and control | SLI | Control | |
| El‐Chimi 2017 (112) | 39% | 34.5% | mean 31.1 (SD 1.7) | mean 31.3 (SD 1.7) | mean 1561 (SD 326) | mean 1510 (SD 319) | Mask and T‐piece in SLI group Mask and self‐inflating bag with an oxygen reservoir in control group |
PIP of 20 cmH2O for 15 seconds, followed by PEEP of 5 cmH2O If needed: a second SLI of 15 seconds of 25 cmH2O for 15 seconds, followed by PEEP of 6 cmH2O; then a third SLI of 15 seconds of 30 cmH2O for 15 seconds, followed by PEEP of 7 cmH2O If still not satisfactory: intubated in delivery room |
PIP maximum 40 cmH2O, rate of 40 to 60 breaths/min for 30 seconds |
| Jiravisitkul 2017 (81) | 63% | 74% | 25 to 28 weeks: n = 17; 29 to 32 weeks: n = 26 |
25 to 28 weeks: n = 16; 29 to 32 weeks: n = 22 |
mean 1206 (SD 367) | mean 1160 (SD 411) | Mask and T‐piece | PIP of 25 cmH2O for 15 seconds If HR 60 to 100 beats/min and/or poor respiratory effort: a second SLI (25 cmH2O, 15 seconds) |
PIP 15 to 20 cmH2O, PEEP 5 cmH2O for 30 seconds, followed by resuscitation according to AHA guidelines |
| Lindner 2005 (61) | 81% | 80% | median 27.0 (IQR 25.0 to 28.9) | median 26.7 (IQR 25.0 to 28.9) | median 870 (IQR 410 to 1320) | median 830 (IQR 370 to 1370) | Nasopharyngeal tube (fixed at 4 to 5 cm) and mechanical ventilator | PIP of 20 cmH2O for 15 seconds If response was not satisfactory: 2 further SLIs of 15 seconds (25 and 30 cmH2O). Then PEEP at 4 to 6 cmH2O |
PIP 20 cmH2O, PEEP 4 to 6 cmH2O; inflation time 0.5 seconds; inflation rate 60 per min. Then, PEEP at 4 to 6 cmH2O |
| Lista 2015 (301) | 87% | 91% | mean 26.8 (SD 1.2); 25 to 26 weeks: n = 55 27 to 28 weeks: n = 88 |
mean 26.8 (SD 1.1); 25 to 26 weeks: n = 52; 27 to 28 weeks: n = 96 |
mean 894 (SD 247) | mean 893 (SD 241) | Mask and T‐piece | PIP 25 cmH2O for 15 seconds. Then reduced to PEEP of 5 cmH2O | PEEP 5 cmH2O, followed by resuscitation according to AHA guidelines |
| Mercadante 2016 (185) | 40% | 32% | mean 35.2 (SD 0.8) | mean 35.2 (SD 0.8) | mean 2345 (SD 397) | mean 2346 (SD 359) | Mask and T‐piece | PIP 25 cmH2O for 15 seconds, followed by PEEP of 5 cmH2O. In case of persistent heart failure (HR < 100 bpm): SLI repeated | PEEP 5 cmH2O, followed by resuscitation according to AAP guidelines |
| Ngan 2017 (162) | 78% | 70% | mean 28 (SD 2.5) | mean 28 (SD 2.5) | mean 1154 (SD 426) | mean 1140 (SD 406) | Mask and T‐piece | Two PIPs of 24 cmH2O. Duration of first SLI was 20 seconds. Duration of second SLI was 20 or 10 seconds, guided by ECO2 values. After SLIs, CPAP if breathing spontaneously or, if found to have apnoea or laboured breathing, mask IPPV at a rate of 40 to 60 bpm | IPPV, rate of 40 to 60 inflations/min until spontaneous breathing, at which time CPAP will be provided |
| Schmölzer 2015 (9) | 80%a | 100%a | mean 24.6 (SD 1.3)a | mean 25.6 (SD 2.3)a | mean 707 (SD 208)a | mean 808 (SD 192)a | Mask and T‐piecea | PIP for 20 + 20 secondsa during chest compressions | 3:1 compression:ventilation ratio according to resuscitation guidelines |
| Schwaberger 2015 (40) | not reported | not reported | mean 32.1 (SD 1.4) | mean 32.1 (SD 1.6) | mean 1692 (SD 297) | mean 1722 (SD 604) | Mask and T‐piece | PIP 30 cmH2O for 15 seconds, to be repeated once or twice with HR remaining < 100 bpm. Infants with HR > 100 bpm: PPV at 30 cmH2O PIP or CPAP at PEEP level of 5 cmH2O depending on respiratory rate | Resuscitation according to AHA guidelines PEEP 5 cmH2O if respiratory rate > 30 and signs of respiratory distress PPV at 30 cmH2O PIP if insufficient breathing efforts |
aInformation provided by study authors
Settings and populations
Researchers conducted the included studies on four different continents: two in Italy (Lista 2015; Mercadante 2016), two in Canada by the same contact author (Ngan 2017; Schmölzer 2015), one in Germany (Lindner 2005), one in Austria (Schwaberger 2015), one in Egypt (El‐Chimi 2017), and one in Thailand (Jiravisitkul 2017). Only one study was conducted at multiple centres (Lista 2015). Five of the six trials identified for this update included infants with mean birth weight > 1 kg (El‐Chimi 2017; Jiravisitkul 2017; Mercadante 2016; Ngan 2017; Schwaberger 2015), whereas the two previously included studies (Lindner 2005; Lista 2015) and the pilot trial (Schmölzer 2015) enrolled extremely low birth weight infants. Mercadante 2016 was the only trial conducted in late preterm infants. No trials enrolled full‐term infants. Table 5 shows additional information on populations.
Interventions
Trials pooled in the primary comparison (i.e. without chest compressions) reported that peak inspiratory pressure (PIP) was sustained for 15 seconds in six trials (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Lista 2015; Mercadante 2016; Schwaberger 2015) and for 20 seconds in Ngan 2017. However, levels of PIP ranged from 20 cmH2O (El‐Chimi 2017; Lindner 2005) to 24 (Ngan 2017), 25 (Jiravisitkul 2017; Lista 2015; Mercadante 2016), and 30 cmH2O (Schwaberger 2015). Investigators provided additional SLIs in cases of poor response, with the same (Jiravisitkul 2017; Mercadante 2016; Schwaberger 2015) or higher PIP (El‐Chimi 2017; Lindner 2005); researchers in Ngan 2017 based the duration of the second SLI on exhaled CO2 values. As regards interface and ventilation devices, most included trials used mask and T‐piece. However, Lindner 2005 used nasopharyngeal tube and ventilator, and El‐Chimi 2017 introduced a relevant bias into the study design by using a T‐piece ventilator in the SLI group and a self‐inflating bag in the control group (mask in both SLI and control groups). No trials reported whether prespecified levels of pressure for the SLI were actually delivered according to the protocol. Study authors did not monitor leaks at the mask and lung volumes during the manoeuvre. Whether the infant breathed before or during the SLI was not recorded: Apnoeic newborns at birth are known to show less gain in lung volume during an SLI than actively breathing infants (Lista 2017).
For the secondary comparison, in which infants in both SLI and control groups were resuscitated with chest compressions, duration of SLI was 20 + 20 seconds (Schmölzer 2015).
Table 5 shows additional information on interventions.
Excluded studies
We have summarised the reasons for exclusion of potentially eligible trials (Bouziri 2011; Harling 2005; te Pas 2007) in the Characteristics of excluded studies table.
In particular, we excluded te Pas 2007 because sustained inflation was only one element of the intervention, and because it is not possible to determine the relative contributions of various elements of this intervention to differences observed between groups. We excluded Harling 2005, as investigators randomised infants in this trial to receive inflation for 2 seconds or 5 seconds at initiation of PPV. All infants thus received sustained (> 1 second) inflations as defined in our protocol (O'Donnell 2004).
For the 2017 update, we excluded no eligible studies.
Risk of bias in included studies
We have presented a summary of the 'Risk of bias' assessment in Figure 2 and Figure 3. We have provided details of the methodological quality of included trials in the Characteristics of included studies section.
Figure 2.
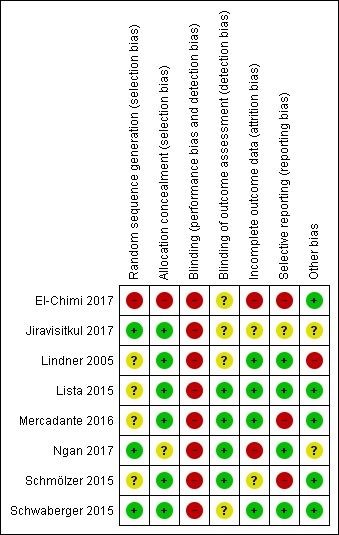
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Figure 3.
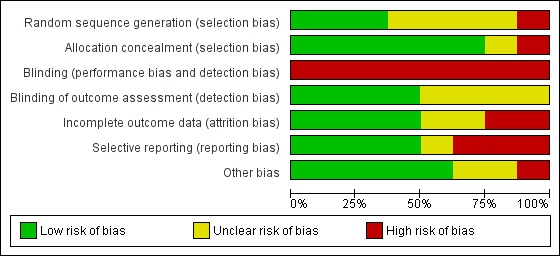
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
Allocation
One trial had high risk of selection bias: This quasi‐randomised trial (odd‐numbered sheets indicated allocation to the SLI group, and even‐numbered sheets to the control group) did not use opaque envelopes (information provided by study authors) (El‐Chimi 2017). In Jiravisitkul 2017 and Schwaberger 2015, risk of selection bias was low as regards random sequence generation and allocation concealment (opaque, numbered envelopes). In Ngan 2017, risk of selection bias was low as regards random sequence generation and was unclear for allocation concealment: Timing of randomisation resulted in many post‐randomisation exclusions, as results showed more post‐randomisation exclusions in the SLI group than in the control group. In the other four trials, risk of selection bias was unclear as regards random sequence generation and was low as regards allocation concealment (opaque, numbered envelopes) (Lindner 2005; Lista 2015; Mercadante 2016; Schmölzer 2015).
Blinding
Owing to the nature of the intervention, all trials were unblinded, leading to high risk of performance bias. However, four trials blinded researchers assessing trial endpoints to the nature of study treatments (Lista 2015; Mercadante 2016; Ngan 2017; Schmölzer 2015).
Incomplete outcome data
El‐Chimi 2017 referred almost half of enrolled infants to other NICUs; we excluded these studies from analysis owing to failure of follow‐up, although the primary outcome of the study (treatment failure/success within 72 hours) could have been determined and reported for these infants. In Ngan 2017, post‐randomisation exclusion (27%) resulted in fewer included infants in the SLI group. Most trials accounted for all outcomes (Lindner 2005; Lista 2015; Mercadante 2016; Schwaberger 2015).
Selective reporting
Four trials provided complete results for all reported outcomes (Lindner 2005; Lista 2015; Ngan 2017; Schwaberger 2015).
Other potential sources of bias
El‐Chimi 2017 and Schwaberger 2015 did not report sample size calculations. For Schwaberger 2015, investigators registered the protocol after study initiation. Jiravisitkul 2017 planned sample sizes of 40 infants for each group but allocated only 38 to the control group. Lindner 2005 was stopped after the interim analysis. It was unclear why study authors made this decision. Ngan 2017 did not achieve the planned sample size; in addition, the incidence of the primary outcome in the control group was less than that assumed for the sample size calculation, leading to lack of power to detect the chosen effect size. The other trials appear free of other bias.
We were unable to explore possible bias through generation of funnel plots because fewer than ten trials met the inclusion criteria of this Cochrane review.
Effects of interventions
Primary comparison: use of initial sustained inflation versus standard inflations in newborns receiving resuscitation with no chest compressions
Primary outcomes
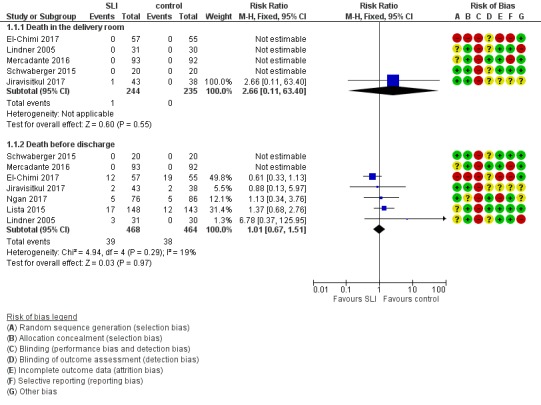
Death (Outcome 1.1)
Death in the delivery room (Outcome 1.1.1)
Five trials (N = 479) reported this outcome (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Mercadante 2016; Schwaberger 2015); one event occurred in the SLI group in Jiravisitkul 2017 (death in delivery room at 15 to 20 minutes of life, severe birth asphyxia as the result of a prolapsed cord), and none in the other four trials (typical RR 2.66, 95% CI 0.11 to 63.40; typical RD 0.00, 95% CI ‐0.02 to 0.02; participants = 479; studies = 5; I² not applicable for RR and I² = 0% for RD; Analysis 1.1 and Figure 4). We obtained data for this outcome directly from trial authors (Jiravisitkul 2017; Lindner 2005).
Analysis 1.1.
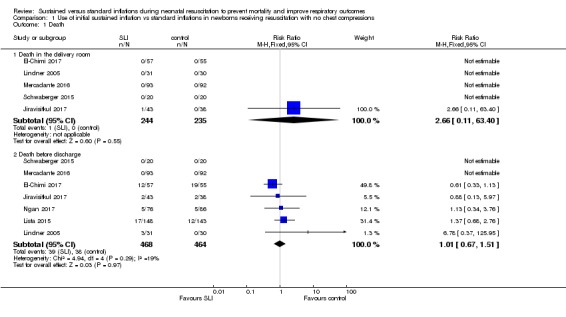
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 1 Death.
Figure 4.
Forest plot of comparison: 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, outcome: 1.1 Death.
Death during hospitalisation (Outcome 1.2.1)
All trials included in the primary comparison (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schwaberger 2015) reported mortality during hospitalisation (typical RR 1.01, 95% CI 0.67 to 1.51; typical RD 0.00, 95% CI ‐0.03 to 0.03; participants = 932; studies = 7; I² = 19% for RR and I² = 0% for RD; Analysis 1.1 and Figure 4).
We obtained data for this outcome directly from trial authors (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005).
In El‐Chimi 2017, 12 and 19 infants in SLI and control groups, respectively, died. In Jiravisitkul 2017, two infants in each group died: In the SLI group, one died of severe birth asphyxia as the result of a prolapsed cord, and the other died at 3 hours of life of suspected umbilical catheter migration with haemothorax; in the control group, one died of severe respiratory distress syndrome at 2 hours of life, and the other of septic shock at 168 days of life. In Lindner 2005, three deaths occurred in the sustained inflation group: at day 1 (respiratory failure), at day 36 (necrotising enterocolitis), and at day 107 (liver fibrosis of unknown origin). In Lista 2015, 12 infants in the control group and 17 in the sustained inflations group died during the trial. Mercadante 2016 and Schwaberger 2015 reported no events.
Secondary outcomes
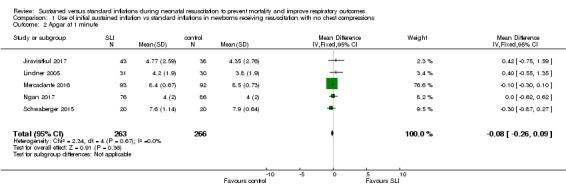
Apgar score at one minute (Outcome 1.2)
Five trials (N = 529) (Jiravisitkul 2017; Lindner 2005; Mercadante 2016; Ngan 2017; Schwaberger 2015) reported this outcome (MD ‐0.08, 95% CI ‐0.26 to 0.09; participants = 529; studies = 5; I² = 0%; Analysis 1.2). We obtained data for this outcome directly from trial authors (Jiravisitkul 2017; Lindner 2005; Mercadante 2016; Ngan 2017; Schwaberger 2015).
Analysis 1.2.
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 2 Apgar at 1 minute.
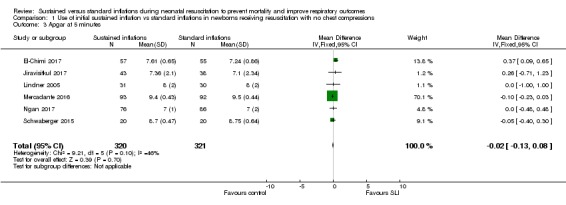
Apgar score at five minutes (Outcome 1.3)
Six trials (N = 641) (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Mercadante 2016; Ngan 2017; Schwaberger 2015) reported this outcome (MD ‐0.02, 95% CI ‐0.13 to 0.08; participants = 641; studies = 6; I² = 46%; Analysis 1.3). We obtained data for this outcome directly from trial authors (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Mercadante 2016; Ngan 2017; Schwaberger 2015).
Analysis 1.3.
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 3 Apgar at 5 minutes.
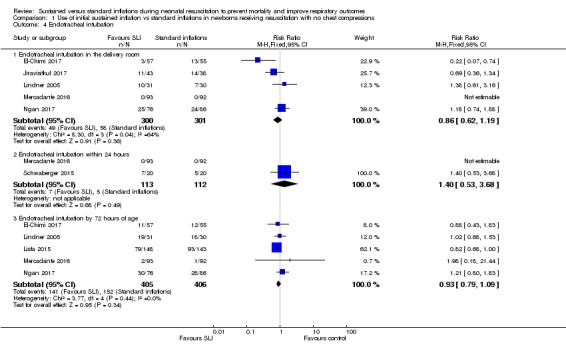
Endotracheal intubation (Outcome 1.4)
Endotracheal intubation in the delivery room (Outcome 1.4.1)
Five trials (N = 601) (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Mercadante 2016; Ngan 2017) reported this outcome (typical RR 0.86, 95% CI 0.62 to 1.19; typical RD ‐0.03, 95% CI ‐0.08 to 0.03; participants = 601; studies = 5; I² = 64% for RR and I² = 74% for RD; Analysis 1.4; Figure 5). We obtained data for this outcome directly from trial authors (Mercadante 2016).
Analysis 1.4.
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 4 Endotracheal intubation.
Figure 5.
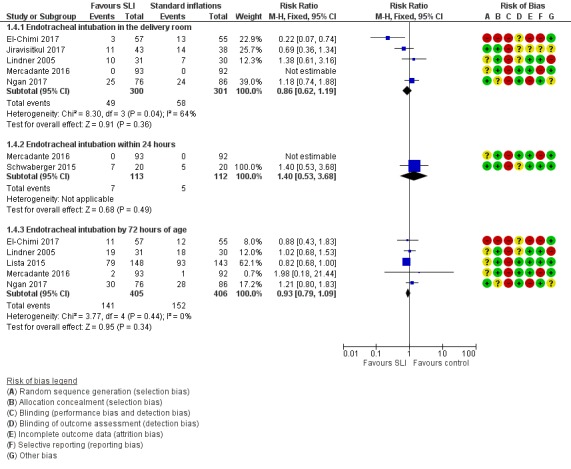
Forest plot of comparison: 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, outcome: 1.4 Endotracheal intubation.
Endotracheal intubation outside the delivery room within 24 hours (Outcome 1.4.2)
Two trials (N = 225) (Mercadante 2016; Schwaberger 2015) reported this outcome (RR 1.40, 95% CI 0.53 to 3.68; RD 0.02, 95% CI ‐0.04 to 0.07; participants = 225; studies = 2). The test for heterogeneity was not applicable because only one trial (Lindner 2005) reported events (Analysis 1.4; Figure 5). We obtained data for this outcome directly from trial authors (Mercadante 2016).
Endotracheal intubation outside the delivery room by 72 hours (Outcome 1.4.3)
Five included trials (N = 811) (El‐Chimi 2017; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017) reported this outcome (typical RR 0.93, 95% CI 0.79 to 1.09; typical RD ‐0.03, 95% CI ‐0.09 to 0.03; participants = 811; studies = 5; I² = 0% for RR and I² = 53% for RD) (Analysis 1.4;Figure 5). We obtained data for this outcome directly from trial authors (Mercadante 2016).
Surfactant administration (Outcome 1.5)
Surfactant administration in the delivery room (Outcome 1.5.1)
Three trials (N = 335) (El‐Chimi 2017; Lindner 2005; Ngan 2017) reported this outcome (typical RR 1.43, 95% CI 0.82 to 2.49; typical RD 0.04, 95% CI ‐0.02 to 0.11; participants = 335; studies = 3; I² = 0% for RR and I² = 72% for RD; Analysis 1.5;Figure 6). We obtained data for this outcome directly from trial authors (El‐Chimi 2017).
Analysis 1.5.
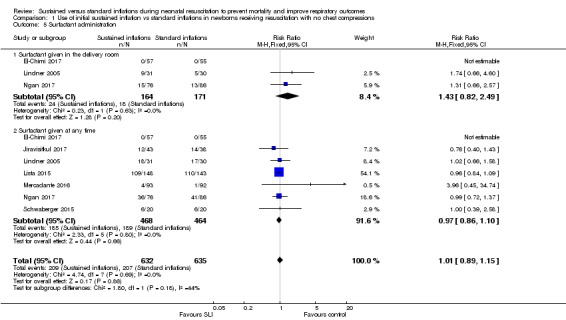
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 5 Surfactant administration.
Figure 6.
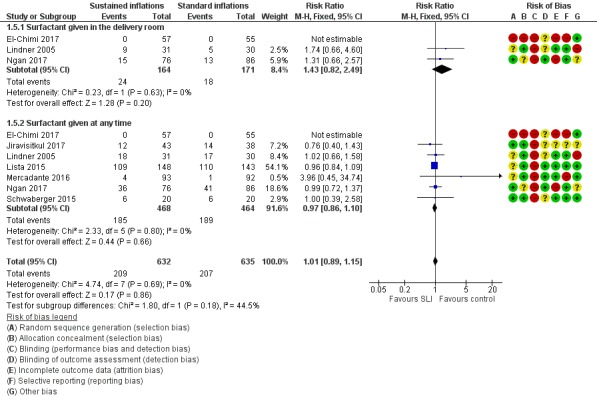
Forest plot of comparison: 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, outcome: 1.5 Surfactant administration.
Surfactant administration during hospital admission (Outcome 1.5.2)
All trials included in the primary comparison (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schwaberger 2015) (N = 932) reported this outcome (typical RR 0.97, 95% CI 0.86 to 1.10; typical RD ‐0.01, 95% CI ‐0.06 to 0.04; participants = 932; studies = 7; I² = 0% for RR and I² = 0% for RD; Analysis 1.5;Figure 6). We obtained data for this outcome directly from trial authors (El‐Chimi 2017; Lindner 2005;Mercadante 2016).
Need for mechanical ventilation (Outcome 1.6)
Three trials (N = 484) reported this outcome (El‐Chimi 2017; Jiravisitkul 2017; Lista 2015) (typical RR 0.87, 95% CI 0.74 to 1.03; typical RD ‐0.06, 95% CI ‐0.14 to 0.01; participants = 484; studies = 3; I² = 0% for RR and I² = 85% for RD) (Analysis 1.6). We obtained data for this outcome directly from trial authors (El‐Chimi 2017).
Analysis 1.6.
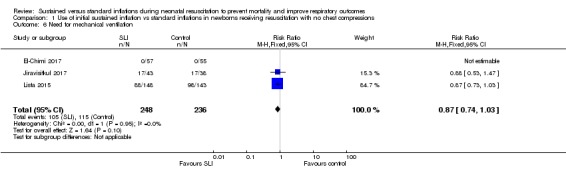
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 6 Need for mechanical ventilation.
Duration of nasal continuous airway pressure (Outcome 1.7)
Three trials (N = 355) reported this outcome (El‐Chimi 2017; Lindner 2005; Mercadante 2016) (MD 0.26 days, 95% CI ‐0.19 to 0.72; participants = 355; studies = 3; I² = 59%) (Analysis 1.7). We obtained data for this outcome directly from trial authors; data for this outcome refer to survivors at time of assessment (El‐Chimi 2017; Lindner 2005; Mercadante 2016).
Analysis 1.7.
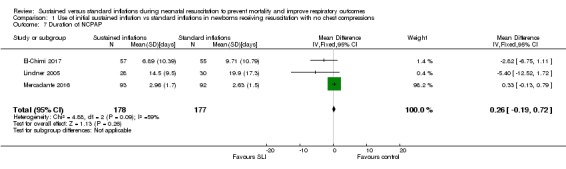
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 7 Duration of NCPAP.
Duration of ventilation via an ETT (Outcome 1.8)
Five trials (N = 524) reported this outcome (Jiravisitkul 2017; Lindner 2005; Mercadante 2016; Ngan 2017; Schwaberger 2015) (MD ‐5.37 days, 95% CI ‐6.31 to ‐4.43; participants = 524; studies = 5; I² = 95%; Analysis 1.8). Data for this outcome refer to survivors at time of assessment (Jiravisitkul 2017; Lindner 2005; Mercadante 2016). We obtained data for this outcome directly from trial authors (Jiravisitkul 2017; Mercadante 2016; Ngan 2017; Schwaberger 2015). Heterogeneity, statistical significance, and magnitude of effects of this outcome are largely influenced by a single study (Ngan 2017): when this study was removed from the analysis, the effect was largely reduced (MD ‐1.71 days, 95% CI ‐3.04 to ‐0.39, I² = 0%).
Analysis 1.8.
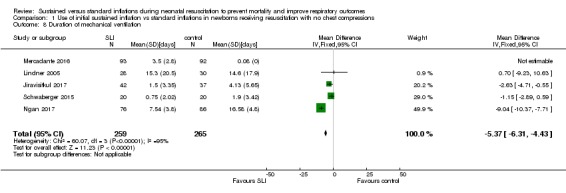
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 8 Duration of mechanical ventilation.
Duration of respiratory support (nasal continuous airway pressure and ventilation via an ETT, considered in total) (Outcome 1.9)
Two trials (N = 243) reported this outcome (Lindner 2005; Mercadante 2016) (MD 0.69 days, 95% CI 0.23 to 1.16; participants = 243; studies = 2; I² = 0%; Analysis 1.9). We obtained data for this outcome directly from trial authors; data refer to survivors at time of assessment (Lindner 2005; Mercadante 2016).
Analysis 1.9.
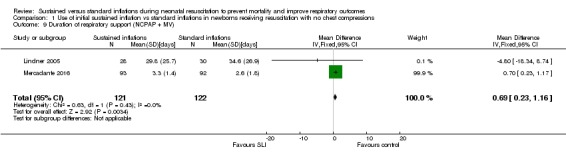
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 9 Duration of respiratory support (NCPAP + MV).
Duration of supplemental oxygen requirement (days) (Outcome 1.10)
One trial (N = 81) reported this outcome (Jiravisitkul 2017) (MD ‐9.73, 95% CI ‐25.06 to 5.60; participants = 81; studies = 1; Analysis 1.10). The test for heterogeneity was not applicable. We obtained data for this outcome directly from trial authors (Jiravisitkul 2017).
Analysis 1.10.
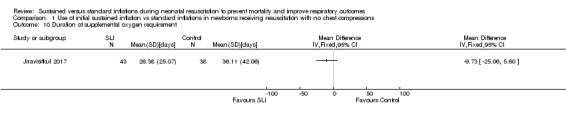
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 10 Duration of supplemental oxygen requirement.
Chronic lung disease (i.e. need for supplemental oxygen at 36 weeks of gestational age for infants born at or before 32 weeks of gestation) (Outcome 1.11)
Bronchopulmonary dysplasia (BPD) any grade (Outcome 1.11.1)
Two trials (N = 220) reported this outcome (Lindner 2005; Ngan 2017) (typical RR 0.90, 95% CI 0.69 to 1.19; typical RD ‐0.05, 95% CI ‐0.17 to 0.08; participants = 220; studies = 2; I² = 0% for RR and I² = 0% for RD). We obtained data for this outcome directly from trial authors; data refer to survivors at time of assessment (Lindner 2005; Analysis 1.11).
Analysis 1.11.
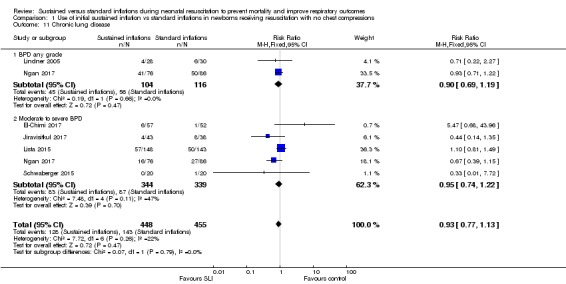
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 11 Chronic lung disease.
Moderate to severe BDP (Outcome 1.11.2)
Five included trials (N = 683) (El‐Chimi 2017; Jiravisitkul 2017; Lista 2015; Ngan 2017; Schwaberger 2015) reported this outcome (typical RR 0.95, 95% CI 0.74 to 1.22; typical RD ‐0.01, 95% CI ‐0.07 to 0.05; participants = 683; studies = 5; I² = 47% for RR and I² = 57% for RD; Analysis 1.11).
Air leaks (pneumothorax, pneumomediastinum, pneumopericardium, pulmonary interstitial emphysema) reported individually or as a composite outcome (Outcome 1.12)
Pneumothorax in first 48 hours of life (Outcome 1.12.1)
One trial (N = 81) (Jiravisitkul 2017) reported this outcome (RR 0.88, 95% CI 0.06 to 13.65; RD ‐0.00, 95% CI ‐0.07 to 0.06). The test for heterogeneity was not applicable (Analysis 1.12).
Analysis 1.12.
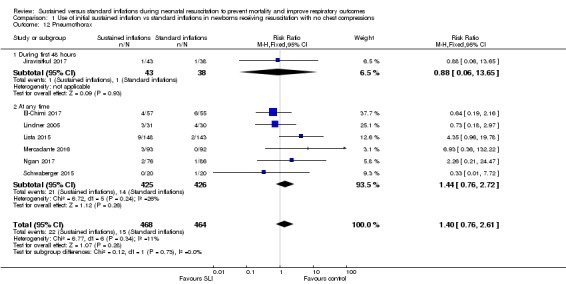
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 12 Pneumothorax.
Pneumothorax at any time (Outcome 1.12.2)
Six included studies (N = 851) (El‐Chimi 2017; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schwaberger 2015) reported this outcome (typical RR 1.44, 95% CI 0.76 to 2.72; typical RD 0.02, 95% CI ‐0.01 to 0.04; studies = 6; 851 infants; I² = 26% for RR and I² = 2% for RD; Analysis 1.12).
Cranial ultrasound abnormalities (Outcome 1.13)
Intraventricular haemorrhage (IVH), grade 3 or 4 according to the Papile classification (Papile 1978) (Outcome 1.13.1)
Five included trials (N = 635) (Jiravisitkul 2017; Lindner 2005; Lista 2015; Ngan 2017; Schwaberger 2015) reported this outcome (typical RR 0.89, 95% CI 0.58 to 1.37; typical RD ‐0.01, 95% CI ‐0.06 to 0.03; studies = 5; 635 infants; I2 = 4% for RR and I2 = 0% for RD; Analysis 1.13).
Analysis 1.13.
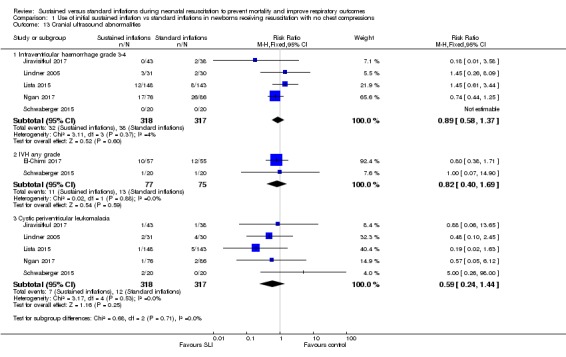
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 13 Cranial ultrasound abnormalities.
IVH any grade (Outcome 1.13.2)
Two included trials (N = 152) (El‐Chimi 2017; Schwaberger 2015) reported this outcome (typical RR 0.82, 95% CI 0.40 to 1.69; typical RD ‐0.03, 95% CI ‐0.15 to 0.08; studies = 3; 152 infants; I² = 0% for RR and I² = 0% for RD; Analysis 1.13).
Cystic periventricular leukomalacia (Outcome 1.13.3)
Five included trials (N = 635) (Jiravisitkul 2017; Lindner 2005; Lista 2015; Ngan 2017; Schwaberger 2015) reported this outcome (typical RR 0.59, 95% CI 0.24 to 1.44; typical RD ‐0.04, 95% CI ‐0.04 to 0.01; studies = 5; 635 infants; I² = 0% for RR and I² = 0% for RD; Analysis 1.13).
Retinopathy of prematurity (ROP) ≥ stage 3 (Outcome 1.14)
Five trials (N = 632) (Jiravisitkul 2017; Lindner 2005; Lista 2015; Ngan 2017; Schwaberger 2015) reported this outcome (typical RR 0.69, 95% CI 0.44 to 1.10; typical RD ‐0.04, 95% CI ‐0.08 to 0.01; studies = 5; 632 infants; I² = 42% for RR and I² = 40% for RD; Analysis 1.14). For Lindner 2005, data refer to survivors at time of assessment (Analysis 1.14).
Analysis 1.14.
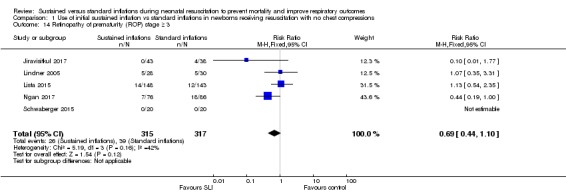
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 14 Retinopathy of prematurity (ROP) stage ≥ 3.
Patent ductus arteriosus (PDA) (Outcome 1.15)
Rate of PDA ‐ pharmacological treatment (Outcome 1.15.1)
Six included trials (N = 745) (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Lista 2015; Ngan 2017; Schwaberger 2015) reported this outcome (typical RR 1.08, 95% CI 0.90 to 1.30; typical RD 0.03, 95% CI ‐0.04 to 0.09; studies = 6; 745 infants; I² = 36% for RR and I² = 58% for RD; Analysis 1.15). We obtained data for this outcome directly from trial authors (Schwaberger 2015).
Analysis 1.15.
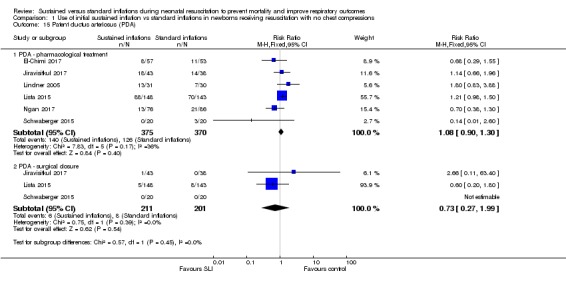
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 15 Patent ductus arteriosus (PDA).
Rate of PDA ‐ surgical closure (Outcome 1.15.2)
Three trials (N = 412) (Jiravisitkul 2017; Lista 2015; Schwaberger 2015) reported this outcome (typical RR 0.73, 95% CI 0.27 to 1.99; typical RD ‐0.01, 95% CI ‐0.05 to 0.03; studies = 3; 412 infants; I² = 0% for RR and I² = 26% for RD; Analysis 1.15). We obtained data for this outcome directly from trial authors (Schwaberger 2015).
The data refer to all randomised infants, unless otherwise specified.
No data were reported for the following outcomes: heart rate; need for supplemental oxygen at 28 days of life; seizures including clinical and electroencephalographic; hypoxic ischaemic encephalopathy in term and late preterm infants (grade 1 to 3; Sarnat 1976); and long‐term neurodevelopmental outcomes.
Death to latest follow‐up: No data were provided in addition to those already presented for death during hospitalisation (Analysis 1.1).
Subgroup analysis for the primary comparison
For the primary comparison, we were unable to conduct any of the four prespecified subgroup analyses because:
no term infants were included;
for ventilation devices, all trials used a T‐piece except Lindner 2005 (mechanical ventilator): We did not perform a separate analysis because of the very small sample size and the presence of high or unclear risk of bias in most GRADE domains. Moreover, El‐Chimi 2017 used a T‐piece ventilator in the SLI group and a self‐inflating bag in the control group; thus we could not include this as a subgroup;
for interface, all trials used a face mask, except Lindner 2005 (nasopharyngeal tube): As for ventilation devices, we did not perform a separate analysis for Lindner 2005; and
no trials used SLI < 5 seconds.
Secondary comparison: use of initial sustained inflation versus standard inflations in newborns receiving resuscitation with chest compressions
Primary outcomes
Death (Outcome 2.1)
Death in the delivery room (Outcome 2.1.1)
The included trial (N = 9) did not report this outcome (Schmölzer 2015).
Death during hospitalisation (Outcome 2.1.2)
One trial (N = 9) reported this outcome (RR 1.60, 95% CI 0.21 to 11.92; RD 0.15, 95% CI ‐0.45 to 0.75); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2015; Analysis 2.1). We obtained data for this outcome directly from trial authors (Schmölzer 2015).
Analysis 2.1.

Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 1 Death.
Secondary outcomes
Endotracheal intubation in the delivery room (Outcome 2.2)
One trial (N = 9) reported this outcome (RR 1.00, 95% CI 0.68 to 1.46; RD 0.00, 95% CI ‐0.34 to 0.34); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2015;Analysis 2.2). We obtained data for this outcome directly from trial authors (Schmölzer 2015).
Analysis 2.2.
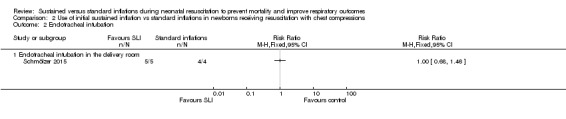
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 2 Endotracheal intubation.
Surfactant administration in the delivery room (Outcome 2.3)
One trial (N = 9) reported this outcome (RR 1.60, 95% CI 0.55 to 4.68; RD 0.30, 95% CI ‐0.30 to 0.90); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2015; Analysis 2.3). We obtained data for this outcome directly from trial authors (Schmölzer 2015).
Analysis 2.3.
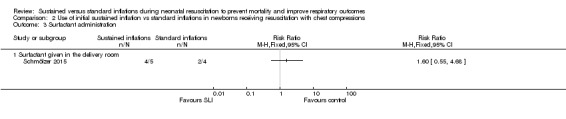
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 3 Surfactant administration.
Chronic lung disease (2.4, 2.5, 2.6)
Moderate to severe BDP (Outcome 2.4)
One trial (N = 9) reported this outcome (RR 0.89, 95% CI 0.33 to 2.37; RD ‐0.08, 95% CI ‐0.76 to 0.60); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2015; Analysis 2.3). We obtained data for this outcome directly from trial authors (Schmölzer 2015).
Pneumothorax at any time (Outcome 2.5)
One trial (N = 9) reported this outcome: No events occurred (Analysis 2.5). We obtained data for this outcome directly from trial authors (Schmölzer 2015).
Analysis 2.5.

Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 5 Pneumothorax.
Cranial ultrasound abnormalities (Outcome 2.6)
Intraventricular haemorrhage (IVH), grade 3 or 4 according to the Papile classification (Papile 1978) (Outcome 2.6.1)
One trial (N = 9) reported this outcome (RR 0.40, 95% CI 0.05 to 2.98; RD ‐0.30, 95% CI ‐0.90 to 0.30); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2015; Analysis 2.6). We obtained data for this outcome directly from trial authors (Schmölzer 2015).
Analysis 2.6.
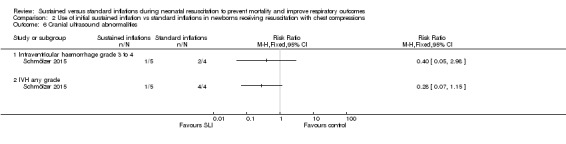
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 6 Cranial ultrasound abnormalities.
IVH any grade (Outcome 2.6.2)
One trial (N = 9) reported this outcome (RR 0.28, 95% CI 0.07 to 1.15; RD ‐0.80, 95% CI ‐1.23 to ‐0.37); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2015; Analysis 2.6). We obtained data for this outcome directly from trial authors (Schmölzer 2015).
Retinopathy of prematurity (ROP) ≥ stage 3 (Outcome 2.7)
One trial (N = 9) reported this outcome (RR 0.27, 95% CI 0.04 to 1.68; RD ‐0.55, 95% CI ‐1.10 to 0.00); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2015; Analysis 2.7). We obtained data for this outcome directly from trial authors (Schmölzer 2015).
Analysis 2.7.
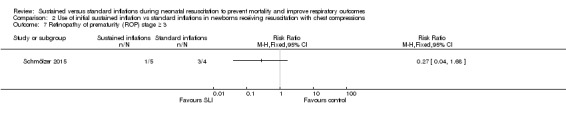
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 7 Retinopathy of prematurity (ROP) stage ≥ 3.
Rate of PDA ‐ pharmacological treatment (Outcome 2.8)
One trial (N = 9) reported this outcome (RR 0.46, 95% CI 0.17 to 1.25; RD ‐0.60, 95% CI ‐1.07 to ‐0.13); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2015; Analysis 2.8). We obtained data for this outcome directly from trial authors (Schmölzer 2015).
Analysis 2.8.
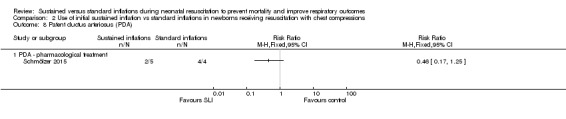
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 8 Patent ductus arteriosus (PDA).
For the secondary comparison, investigators provided no data on other prespecified outcomes.
Subgroup analysis for the secondary comparison
For the secondary comparison, we were unable to conduct any subgroup analysis, as we included only one trial.
Discussion
Summary of main results
We evaluated the merits of sustained lung inflation (SLI) versus intermittent ventilation in infants requiring resuscitation and stabilisation at birth. Eight trials enrolling 941 preterm infants met review inclusion criteria (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schmölzer 2015; Schwaberger 2015). Whereas the two trials included in the previous version of this review enrolled infants at 25+0 to 28+6 weeks (Lindner 2005; Lista 2015), the five more recent trials enrolled larger infants (El‐Chimi 2017; Jiravisitkul 2017; Mercadante 2016; Ngan 2017; Schwaberger 2015). One of the trials included in this update was not pooled with the other studies for analysis because investigators superimposed the intervention on chest compressions (Schmölzer 2015).
Sustained lung inflation was not better than intermittent ventilation for reducing mortality ‐ the primary outcome of this review. We rated the quality of evidence as moderate (GRADE) for death before discharge (limitations in study design of most included trials) and as low (GRADE) for death in the delivery room (limitations in study design and imprecision of estimates). When considering secondary outcomes, such as need for intubation, need for or duration of respiratory support, bronchopulmonary dysplasia, or pneumothorax, we found no benefit of SLI over intermittent ventilation. The quality of evidence for secondary outcomes was moderate (limitations in study design of most included trials ‐ GRADE), except for pneumothorax (low quality: limitations in study design and imprecision of estimates ‐ GRADE). Duration of mechanical ventilation was shorter in the SLI group (low quality: limitations in study design and imprecision of estimates ‐ GRADE). The first version of this review reported an increased rate of patent ductus arteriosus (PDA) in the sustained lung inflation group. However, this effect was not seen when the most recent trials were added to the analysis. We identified six ongoing trials.
Overall completeness and applicability of evidence
To date, seven trials comparing sustained versus standard inflations for initial resuscitation have enrolled 941 newborns. Available data were insufficient for assessment of clinically important outcomes, which were identified a priori. Study authors did not report outcomes such as duration of supplemental oxygen requirement and long‐term neurodevelopmental outcomes and did not enrol term infants. We could not perform an a priori subgroup analysis (gestational age, ventilation device, interface, duration of sustained inflation) to detect differential effects because of the paucity of included trials. Relevant questions such as the following remain unanswered: What is the optimal duration for an SLI? Which level of positive end‐expiratory pressure (PEEP) should follow? Which is the optimal interface/device? (McCall 2016) We were able to summarise available evidence in a comprehensive way, as we obtained additional information about study design and outcome data from all included trials (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schmölzer 2015; Schwaberger 2015) and from two excluded trials (Harling 2005; te Pas 2007). The five ongoing trials that we identified reported important differences in choice of gestational age (NCT02139800; NCT02493920; NCT02846597; NCT02858583; NCT02887924). NCT02139800 enrols infants at 23 to 26 weeks, NCT02493920 at 25 to 36 weeks, NCT02887924 at 26 to 29 weeks, and NCT02846597 at < 33 weeks, whereas NCT02858583 enrols term and preterm infants. These differences among study populations might prove to be important, as trials have reported that sustained inflation was more effective in infants at 28 to 30 weeks than at < 28 or > 30 weeks of gestation (te Pas 2007).
Quality of the evidence
According to the GRADE approach, we rated the overall quality of evidence for clinically relevant outcomes as low to moderate (see Table 1). We downgraded the overall quality of evidence for critical outcomes because of limitations in study design (i.e. selection bias due to lack of allocation concealment) and imprecision of results (few events for death in the delivery room and wide confidence intervals for pneumothorax). In addition, two trials did not report sample size calculations (El‐Chimi 2017; Schwaberger 2015), and the other three did not achieve them (Jiravisitkul 2017; Lindner 2005;Ngan 2017). Results of smaller studies are subject to greater sampling variation, and hence are less precise. Indeed, imprecision is reflected in the confidence interval around the intervention effect estimate from each study and in the weight given to the results of each study included in the meta‐analysis (Higgins 2011).
Potential biases in the review process
A major limitation of this Cochrane review is the definition of sustained lung inflation, as trials used different pressures, which may have impacted study results. No trials were blinded owing to the nature of the intervention. We excluded a potentially relevant trial (te Pas 2007) because sustained inflation was only one element of the intervention, and it is not possible to determine the relative contributions of various elements of this intervention to differences observed between groups. We excluded Harling 2005 because the control group received 2 seconds of inflation (5 seconds for the intervention group), whereas we defined sustained as > 1 second. For this update, we made a post hoc decision to add a comparison based on the presence of chest compressions during resuscitation.
Agreements and disagreements with other studies or reviews
Several systematic reviews of SLI have been recently published. Schmölzer 2014 conducted a systematic review of randomised clinical trials comparing SLI versus intermittent positive‐pressure ventilation (IPPV) as the primary respiratory intervention during respiratory support in preterm individuals at < 33 weeks of gestational age in the delivery room. This review included four trials, including two that we excluded from our systematic review (Harling 2005; te Pas 2007). Schmölzer 2014 reported a significant reduction in the need for mechanical ventilation within 72 hours after birth (typical risk ratio (RR) 0.87, 95% confidence interval (CI) 0.74 to 1.03). As in our analysis, significantly more infants treated with SLI received treatment for PDA (RR 1.27, 95% CI 1.05 to 1.54). Results showed no differences in bronchopulmonary dysplasia (BPD), death at latest follow‐up, or the combined outcome of death or BPD among survivors between groups. The findings of Schmölzer 2014 differ from the findings of this Cochrane review because of differences in the definition of duration of the intervention, and therefore in determination of included trials. A narrative review (Foglia 2016) including five trials (Harling 2005; Lindner 2005; Lista 2015; Mercadante 2016; te Pas 2007) concluded that at present, data are insufficient to support the use of SLI in clinical practice. An observational analytical cross‐sectional case–control study of 78 preterm infants showed that SLI resulted in lower rates of intubation in the delivery room, lower rates of invasive mechanical ventilation, and higher rates of intraventricular haemorrhage (Grasso 2015).
Authors' conclusions
Sustained lung inflation was not better than intermittent ventilation for reducing mortality in the delivery room (low‐quality evidence ‐ GRADE) and during hospitalisation (moderate‐quality evidence ‐ GRADE) ‐ primary outcomes of this review. When considering secondary outcomes, such as need for intubation, need for or duration of respiratory support, or bronchopulmonary dysplasia, we found no benefit of sustained inflation over intermittent ventilation (moderate‐quality evidence ‐ GRADE). Duration of mechanical ventilation was shortened in the SLI group (low‐quality evidence ‐ GRADE); however, this result should be interpreted cautiously, as it might have been influenced by study characteristics other than the intervention.
Additional studies of SLI for infants receiving respiratory support at birth should provide more detailed monitoring of the procedure, such as measurements of lung volume and presence of apnoea before or during SLI. Future randomised controlled trials should aim to enrol infants who are at higher risk of morbidity and mortality, and should stratify participants by gestational age. Researchers should also measure long‐term neurodevelopmental outcomes (e.g. Bayley Scales of Infant Development administered at two years of corrected age).
Acknowledgements
We thank Drs. Lindner, te Pas, Harling, El‐Farghali (for El‐Chimi 2017), Mercadante, Nuntnarumit (Jiravisitkul 2017), Schmolzer (for Ngan 2017 and Schmölzer 2015), and Schwaberger for their gracious assistance in providing extra data.
We acknowledge the help of Ms. Jennifer Spano and Ms. Colleen Ovelman in conducting literature searches for this update of the review.
Appendices
Appendix 1. Standard search method
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomisation, and the blinding and reporting of all outcomes of all infants enrolled in the trial. We assessed each criterion as low, high, or unclear risk. Two review authors separately assessed each study. We resolved any disagreement by discussion. We added this information to the table Characteristics of included studies. We evaluated the following issues and entered the findings into the risk of bias table. 1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated? For each included study, we categorised the method used to generate the allocation sequence as: a. low risk (any truly random process, e.g. random number table; computer random number generator); b. high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or c. unclear risk. 2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed? For each included study, we categorised the method used to conceal the allocation sequence as: a. low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); b. high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or c. unclear risk. 3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as: a. low risk, high risk, or unclear risk for participants; and b. low risk, high risk, or unclear risk for personnel. 4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment? For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as: a. low risk for outcome assessors; b. high risk for outcome assessors; or c. unclear risk for outcome assessors. 5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed? For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we re‐included missing data in the analyses. We categorised the methods as: a. low risk (< 20% missing data); b. high risk (≥ 20% missing data); or c. unclear risk. 6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting? For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as: a. low risk (when it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported); b. high risk (when not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or c. unclear risk. 7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias? For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: a. low risk; b. high risk; or c. unclear risk. If needed, we explored the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1.
Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death in the delivery room | 5 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.11, 63.40] |
| 1.2 Death before discharge | 7 | 932 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.51] |
| 2 Apgar at 1 minute | 5 | 529 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.26, 0.09] |
| 3 Apgar at 5 minutes | 6 | 641 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.13, 0.08] |
| 4 Endotracheal intubation | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Endotracheal intubation in the delivery room | 5 | 601 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.62, 1.19] |
| 4.2 Endotracheal intubation within 24 hours | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.53, 3.68] |
| 4.3 Endotracheal intubation by 72 hours of age | 5 | 811 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 5 Surfactant administration | 7 | 1267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |
| 5.1 Surfactant given in the delivery room | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.82, 2.49] |
| 5.2 Surfactant given at any time | 7 | 932 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.10] |
| 6 Need for mechanical ventilation | 3 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 7 Duration of NCPAP | 3 | 355 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.19, 0.72] |
| 8 Duration of mechanical ventilation | 5 | 524 | Mean Difference (IV, Fixed, 95% CI) | ‐5.37 [‐6.31, ‐4.43] |
| 9 Duration of respiratory support (NCPAP + MV) | 2 | 243 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [0.23, 1.16] |
| 10 Duration of supplemental oxygen requirement | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Chronic lung disease | 6 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.77, 1.13] |
| 11.1 BPD any grade | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.69, 1.19] |
| 11.2 Moderate to severe BPD | 5 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.74, 1.22] |
| 12 Pneumothorax | 7 | 932 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.76, 2.61] |
| 12.1 During first 48 hours | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.06, 13.65] |
| 12.2 At any time | 6 | 851 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.76, 2.72] |
| 13 Cranial ultrasound abnormalities | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Intraventricular haemorrhage grade 3‐4 | 5 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.37] |
| 13.2 IVH any grade | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.40, 1.69] |
| 13.3 Cystic periventricular leukomalacia | 5 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.44] |
| 14 Retinopathy of prematurity (ROP) stage ≥ 3 | 5 | 632 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.44, 1.10] |
| 15 Patent ductus arteriosus (PDA) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 PDA ‐ pharmacological treatment | 6 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.30] |
| 15.2 PDA ‐ surgical closure | 3 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.27, 1.99] |
Comparison 2.
Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Death before discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Endotracheal intubation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Endotracheal intubation in the delivery room | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Surfactant administration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Surfactant given in the delivery room | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Chronic lung disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Moderate to severe BPD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pneumothorax | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 At any time | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Cranial ultrasound abnormalities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Intraventricular haemorrhage grade 3 to 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 IVH any grade | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Retinopathy of prematurity (ROP) stage ≥ 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Patent ductus arteriosus (PDA) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 PDA ‐ pharmacological treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 2.4.
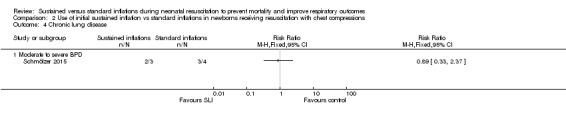
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 4 Chronic lung disease.
What's new
Last assessed as up‐to‐date: 17 February 2017.
| Date | Event | Description |
|---|---|---|
| 21 July 2017 | Amended | Typo corrected: Schwaberger 2015 used near‐infrared spectroscopy (NIRS) not a numerical rating scale (NRS). |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 7, 2015
| Date | Event | Description |
|---|---|---|
| 13 June 2017 | New citation required but conclusions have not changed | We included six new studies but made no changes to the main conclusions |
| 13 June 2017 | New search has been performed | We updated searches in 2017 and found six new eligible studies for inclusion |
| 6 July 2015 | Amended | We updated review author affiliation |
| 10 July 2008 | Amended | We converted the review to new review format |
Differences between protocol and review
We added clinically relevant outcomes (surfactant administration, need for mechanical ventilation, retinopathy of prematurity, and PDA).
We planned subgroup analyses according to gestational age (< 37 weeks, ≥ 37 weeks), ventilation device used (self‐inflating bag, flow‐inflating bag, T‐piece, mechanical ventilator), patient interface used (face mask, ETT, nasopharyngeal tube), and duration of sustained inflation (> 1 second to 5 seconds, > 5 seconds). We were unable to conduct any subgroup analyses as few trials met the inclusion criteria.
For this update, we made the post hoc decision to add a comparison based on use of chest compression during resuscitation. Moreover, we specified Unit of analysis issues and Sensitivity analysis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Prospective quasi‐randomised parallel controlled trial Setting: delivery room of Maternity Hospital, Ain Shams University, Cairo, Egypt Conducted: April 2012 to March 2014 |
|
| Participants | Inclusion criteria (as specified in the protocol): gestational age 26 to 33 weeks, birth weight > 750 grams Exclusion criteria (as specified in the protocol): major congenital anomalies; meconium aspiration syndrome, congenital diaphragmatic hernia, anterior abdominal wall defect, maternal chorioamnionitis |
|
| Interventions |
|
|
| Outcomes | Primary outcome was either "success" (defined as no need for further ventilatory support, need for exclusive NCPAP, or need for intubation beyond the first 72 hours after delivery) or "failure" (defined as need for intubation within first 72 hours of life, including DR intubation) Secondary outcomes were blood IL‐1b and TNF‐a levels, air leaks, BPD, IVH, PDA, and NEC |
|
| Notes | Study was registered at ClinicalTrials.gov (Identifier: NCT01255826) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | For randomisation, sequentially numbered sheets were used to assign eligible infants to resuscitation: Odd‐numbered sheets indicated those allocated to the SLI group, and even‐numbered to the control group |
| Allocation concealment (selection bias) | High risk | No opaque envelopes were used (information provided by study authors) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | After enrolment (n = 202), infants referred to other NICUs were excluded from analysis owing to failure of follow‐up. At study end, SLI group comprised 57 babies and CBMI group comprised 55 babies |
| Selective reporting (reporting bias) | High risk | Some outcomes were specified at https://clinicaltrials.gov/ct2/show/NCT01255826 but were not reported in the manuscript (e.g. duration of oxygen therapy, length of NICU stay) |
| Other bias | Low risk | Appears free of other bias |
| Methods | Prospective randomised parallel controlled trial Setting: delivery room of Ramathibodi Hospital, Mahidol University, Bangkok, Thailand Conducted: November 2013 to March 2015 |
|
| Participants | Included: 81 preterm infants (25 to 32 weeks of gestational age) requiring positive‐pressure ventilation or continuous positive airway pressure Exclusion criteria: major congenital anomalies, hydrops foetalis, prenatal diagnosis of upper airway obstruction, meconium‐stained amniotic fluid |
|
| Interventions |
All enrolled infants were resuscitated with an initial fraction of inspired oxygen (FiO2) of 0.3, which was adjusted by 0.1 every 30 seconds to achieve the target SpO2. Criteria for intubation included 1 of the following: remaining apnoeic after PPV, HR of 30 seconds before the start of chest compressions, or SpO2 < 80% despite CPAP via mask with FiO2 of 1.0 for 5 to 10 minutes Infants of multiple gestations were enrolled in the same intervention group |
|
| Outcomes | Primary outcomes: change in oxygen requirements, HR, and SpO2 during resuscitation; proportion of infants on room air during first 10 minutes after birth; need for intubation in the delivery room Secondary outcomes: survival at discharge, duration of hospitalisation, proportion of infants on MV within first 72 hours of life, duration of MV, duration of oxygen supplementation, need for surfactant, need for postnatal steroids, pneumothorax within first 48 hours after NICU admission, moderate to severe BPD as defined by Jobe and Bancalari, Apgar score at 5 minutes, PDA and need for surgical closure, grade 3 to 4 IVH, cystic periventricular leukomalacia, stage > 2 ROP, ROP requiring treatment |
|
| Notes | Study was registered in the Thai Clinical Trials Registry (TCTR20140418001) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block of 4 randomisation stratified by GA: 25 to 28 weeks and 29 to 32 weeks. Random sequence was generated by computer random number generator (information provided by study authors) |
| Allocation concealment (selection bias) | Low risk | Sequence numbers were kept in opaque sealed envelopes that were opened just before birth in the delivery room by a person not involved in resuscitation of infants |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear why 43 infants in SLI group and 38 in control group |
| Selective reporting (reporting bias) | Unclear risk | According to the Thai Clinical Trials Registry (TCTR20140418001), the only primary outcome was intubation in DR; only a key secondary outcome was specified: BPD |
| Other bias | Unclear risk | Planned sample size: 40 infants in each group; however, only 38 in control group |
| Methods | Prospective randomised parallel controlled trial Setting: Delivery Room, Ulm, Germany Conducted: August 1999 to February 2002 |
|
| Participants | Inclusion criteria: newly born infants at 25 to 28 weeks of gestation inclusive Exclusion criteria: severe malformations, oligo‐anhydramnios before 20 weeks of gestation, foeto‐foetal transfusion syndrome. A total of 61 infants were enrolled (31 in sustained inflation group and 30 in control group). |
|
| Interventions |
Infants received support from a mechanical ventilator via a nasopharyngeal tube Infants in both groups who had apnoea on NCPAP could be treated with NIMV (PIP 20 cmH2O; inflation time 0.3 seconds; inflation rate 60/min) for up to 4 minutes Treatment was deemed to have failed if infants had shown persistently poor respiratory effort, bradycardia, or cyanosis/low SpO2 in the delivery room; or if criteria combining clinical assessments of respiratory distress and evidence of impaired oxygenation, impaired ventilation (high CO2), or apnoea were met within 48 hours of birth |
|
| Outcomes | Primary outcome: rate of infants reaching criteria for intubation and mechanical ventilation at < 48 hours of life Secondary outcomes: mortality, Apgar score, endotracheal intubation, surfactant administration, duration of respiratory support, chronic lung disease, air leak, intraventricular haemorrhage, cystic periventricular leukomalacia, retinopathy of prematurity, PDA |
|
| Notes | Trial was stopped before target sample was recruited owing to slow enrolment. Clinical outcomes were reported for all randomised infants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomised, stratified for gestational age (25 to 26 weeks, 27 to 28 weeks) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants accounted for |
| Selective reporting (reporting bias) | Low risk | All reported outcomes provided with complete results |
| Other bias | High risk | Trial lacks power because only 61 infants were enrolled (instead of 110) |
| Methods | Multi‐centre prospective randomised parallel controlled trial Setting: Delivery Room, Italy Conducted: October 2011 to January 2013 Infants were assigned immediately after birth before the first breath to receive SLI manoeuvres and NCPAP or NCPAP alone in a 1:1 ratio in permuted blocks of variable size. Randomisation was stratified according to centre and gestational age (25 or 26 weeks and 27 or 28 weeks). Group assignment was contained in sequentially numbered, sealed, opaque envelopes that were prepared by an independent statistician. The trial was not blinded |
|
| Participants | Newly born infants at 25 to 28 weeks of gestation inclusive without major congenital malformations (i.e. congenital heart, cerebral, lung, abdominal malformations), foetal hydrops, and lack of parental consent. A total of 294 infants were enrolled (150 in the sustained lung inflation group and 144 in the control group) | |
| Interventions |
Infants in both groups who were not intubated in the delivery room were transferred to the NICU on NCPAP at 5 cmH2O with a fraction of inspired oxygen (FiO2) of 0.21 to 0.40 (in agreement with local protocols) |
|
| Outcomes | Primary outcome: rate of infants reaching mechanical ventilation within the first 72 hours of life Secondary outcomes: MV in the first 3 hours of life, highest FiO2, duration of NCPAP, need for and duration of bi‐level NCPAP, nasal IMV, conventional or high‐frequency ventilation, duration of hospitalisation, need for and number of doses of surfactant, occurrence of RDS, BPD, and mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomised (1:1 ratio), stratified for gestational age (25 to 26 weeks, and 27 to 28 weeks) |
| Allocation concealment (selection bias) | Low risk | Group assignment was contained in sequentially numbered, automatically generated, sealed, opaque envelopes that were prepared by an independent statistician and distributed to participating centres |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff performing the study also cared for infants later on. However, the decision to start MV was made by clinicians other than investigators involved in the study according to specific guidelines, and researchers assessing study endpoints were blinded to the nature of study treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 0.7% (control group) and 1.3% (SLI group) of participants were lost |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Appears free of other bias |
| Methods | Prospective randomised parallel controlled trial Setting: Delivery Room, Neonatal Intensive Care Unit (NICU) in Milan, Italy Conducted: September 2013 to June 2014 |
|
| Participants | Inclusion criteria: inborn infants with a gestational age of 34 to 36 weeks after parental consent is obtained Exclusion criteria: major congenital anomalies |
|
| Interventions |
In both groups, mask and T‐piece system were used |
|
| Outcomes | Primary outcome: need for respiratory support Secondary outcomes: air leak syndromes, NICU admission, NICU admission for respiratory disease, length of stay, exclusive breastfeeding at discharge |
|
| Notes | Sample size described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The decision to start respiratory support was made by clinicians other than investigators involved in the study according to specific guidelines, and researchers assessing study endpoints were blinded to the nature of study treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes accounted for |
| Selective reporting (reporting bias) | High risk | We could not ascertain whether deviations from the original protocol were evident in the final publication |
| Other bias | Low risk | |
| Methods | Prospective randomised parallel controlled trial Setting: Delivery Room, Neonatal Intensive Care Unit (NICU), Royal Alexandra Hospital (RAH), Edmonton, Canada Conducted: June 2013 to August 2014 |
|
| Participants | Inclusion criteria: infants between 23+0 and 32+6 weeks of gestation who require respiratory support for resuscitation in the delivery room Exclusion criteria: congenital abnormality or condition that might have an adverse effect on breathing or ventilation; absence of parents' consent for inclusion in the study |
|
| Interventions |
|
|
| Outcomes | Primary outcome: BPD (need for respiratory support or supplemental oxygen at corrected gestational age of 36 weeks) Secondary outcomes: rate of endotracheal intubation in the DR or the NICU, duration of MV and non‐invasive ventilation, neonatal death, air leak, PDA (medical or surgical), NEC, ROP, periventricular leukomalacia, abnormal cranial ultrasound (including IVH, parenchymal injury, and ventriculomegaly), surfactant administration, postnatal steroids, respiratory support or oxygen requirements at 28 days, neonatal death before discharge |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme (1:1 ratio). Randomisation stratified according to gestational age (to infants 23+0 to 27+6 and 28+0 to 32+6 weeks). Twins and/or triplets were randomised as individuals |
| Allocation concealment (selection bias) | Unclear risk | A sequentially numbered, brown, sealed envelope contained a folded card box with treatment allocation opened by the clinical team immediately before delivery. Timing of randomisation resulted in many post‐randomisation exclusions with the potential of inadequate allocation concealment, as more post‐randomisation exclusions occurred in the SLI group than in the control group |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After admission into the NICU, the clinical team was not made aware of treatment allocation. In addition, both data collector and outcome assessor were unaware of group allocation. The research team was not involved in clinical care of the infants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Post‐randomisation exclusion (27%) resulted in fewer included infants in the SLI group; this discrepancy might have yielded different results |
| Selective reporting (reporting bias) | Low risk | Protocol was registered at Clinicaltrials.gov (NCT01739114) |
| Other bias | Unclear risk | Planned sample size of 93 infants in each group was not achieved. Moreover, incidence of the primary outcome in the control group was lower than assumed for the sample size calculation, further underpowering the trial to detect the desired effect size |
| Methods | Prospective randomised parallel controlled trial Pilot (5 infants randomised to each group) Setting: Royal Alexandra Hospital, Edmonton, Alberta, Canada |
|
| Participants | Inclusion criteria: inborn infants between 23+0 and 32+6 weeks of postmenstrual age who required chest compressions in the delivery room Exclusion criteria: congenital abnormality or condition that might have an adverse effect on breathing or ventilation (e.g. congenital pulmonary or airway anomalies, congenital diaphragmatic hernia, congenital heart disease requiring intervention in neonatal period) |
|
| Interventions |
Default settings for airway pressures: PIP of 24 cmH2O and PEEP of 6 cmH2O |
|
| Outcomes | Primary outcome: return of spontaneous circulation Secondary outcomes (we obtained the following information directly from trial authors): all mortality before discharge from hospital, delivery room interventions (rate of intubation, use of epinephrine), mechanical ventilation, use of inotropic agents, NEC, moderate to severe BPD, ROP, brain injury as indicated by abnormal neuroimaging |
|
| Notes | Available as an abstract only (Canadian Paediatric Society 92nd Annual Conference) Trial was registered at ClinicalTrials.gov: NCT02083705 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Although not reported in the abstract, we obtained the following information directly from trial authors: A sequentially numbered, brown, sealed envelope contained a folded card box with treatment allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although not reported in the abstract, we obtained the following information directly from trial authors: Both data collector and outcome assessor were unaware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | Trial was registered at ClinicalTrials.gov: NCT02083705. However, secondary outcomes were not specified |
| Other bias | Low risk | Appears free of other bias |
| Methods | Prospective randomised parallel controlled trial Setting: Delivery Room, Graz, Austria Conducted: April 2012 to December 2013 |
|
| Participants | Inclusion criteria: preterm infants (28 weeks 0 days to 33 weeks 6 days) delivered by elective Caesarean section with HR < 100 or irregular breathing and/or pronounced signs of respiratory distress (grunting, tachypnoea, and increased work of breathing) Exclusion criteria: major congenital malformations, inherited disorders of metabolism and necessity of primary intubation within first 15 minutes after birth. In cases of multiple birth, only 1 of the infants was included |
|
| Interventions | Cord clamping within 30 seconds after delivery. Respiratory support with a T‐piece system in the delivery room
Initial fraction of inspired oxygen (FiO2) of 0.3 was adapted to achieve defined oxygen saturation targets (3‘: > 60%; 5‘: > 75%; 10‘: > 85%) |
|
| Outcomes | Primary outcome: changes in cerebral blood volume and cerebral tissue oxygenation index during immediate postnatal transition Secondary outcomes: SpO2, HR, VT, face mask leak, FiO2 within first 15 minutes after birth |
|
| Notes | Trial was registered at the German Clinical Trials Register (DRKS00005161) in July 2013, after study initiation (April 2012) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocked randomisation, 1:1 ratio, with a block size of 8 (www.randomizer.at) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. We obtained the following information directly from trial authors: Envelopes were opaque |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Cerebral ultrasound pictures were evaluated by a neonatologist blinded to participants. No information was provided for the other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes accounted for |
| Selective reporting (reporting bias) | Low risk | Protocol for this trial is available as supporting information. Reporting of the study conforms to Consolidated Standards of Reporting Trials (CONSORT) 2010 statement |
| Other bias | Low risk | Appears free of other bias. |
AAP: American Academy of Pediatrics AHA: American Heart Association BPD: bronchopulmonary dysplasia C:V: compression:ventilation CBMI: conventional bag/mask inflation CPAP: continuous positive airway pressure DR: delivery room ECO2: enzymatic carbonate (measure of carbon dioxide in the blood) FiO2: fraction of inspired oxygen HR: heart rate IL‐1b: interleukin‐1beta IMV: intermittent mandatory ventilation IPPV: intermittent positive pressure ventilation IVH: intraventricular haemorrhage MV: mandatory ventilation NCPAP: nasal continuous positive airway pressure NEC: necrotising enterocolitis NICU: neonatal intensive care unit NIMV: nasal intermittent mandatory ventilation PDA: patent ductus arteriosus PEEP: positive end‐expiratory pressure PIP: peak inspiratory pressure PPV: positive pressure ventilation RDS: respiratory distress syndrome ROP: retinopathy of prematurity SLI: sustained lung inflation SpO2: blood oxygen saturation level TNF‐a: tumour necrosis factor‐alpha VT: ventricular tachycardia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bouziri 2011 | Not a clinical trial. Does not investigate sustained lung inflation |
| Harling 2005 | Control group consisted of inflation for 2 seconds (5 seconds for intervention): As we defined sustained if > 1 second, this trial could not be included Infants in the SLI group were born more preterm and had lower median birth weight than those in the conventional group, although the P value was not provided. Median birth weight (range) was 885 (518 to 1460) grams in the SLI group and 1095 (560 to 1562) grams in the conventional group. Median gestational age (range) was 27 (23 to 30) weeks in the SLI group and 28 (23 to 31) weeks in the conventional group |
| te Pas 2007 | This RCT enrolled newly born infants born at < 33 weeks of gestation free of known major congenital anomalies with respiratory distress Infants were randomised to inflation of 10 seconds at 20 cmH2O with a T‐piece via a nasal tube, or to intermittent PPV with a self‐inflating bag via a face mask. Infants randomised to the T‐piece received inflation for 10 seconds at 20 cmH2O followed by NCPAP at 5 to 6 cmH2O. If the infant's clinical response was unsatisfactory, another inflation of 10 seconds at 25 cmH2O and NIMV (PIP 20 to 25 cmH2O, inflation rate 60 per minute) could be given. If the infants' condition improved (satisfactory heart rate and colour) but they had irregular breathing, they could receive NIMV for several minutes. Infants who were judged to have inadequate breathing, remain bradycardic, or remain cyanosed in the delivery room after these interventions were intubated and mechanically ventilated. Infants randomised to the self‐inflating bag received initial inflations of 30 to 40 cmH2O, followed by inflations not > 20 cmH2O (inflation time was not specified or recorded) for 30 seconds. Infants judged to have inadequate breathing, remain bradycardic, or remain cyanosed in the delivery room after this intervention were intubated and mechanically ventilated. Infants in the sustained lung inflation group who were not intubated were transferred to the neonatal intensive care unit (NICU) on NCPAP at 5 to 6 cmH2O; non‐intubated infants in the control group were transferred to the NICU with supplemental oxygen and were monitored with pulse oximetry The intervention in this trial was multi‐faceted. In addition to a sustained inflation, many other aspects of respiratory care provided at birth differed between groups (ventilation device used; interface used; whether PEEP was used; whether NIMV was used; time allowed for stabilisation before intubation was considered; time of starting NCPAP). It is not possible to determine the relative contribution (if any) of each element of this intervention to differences in outcomes observed between groups |
NCPAP: nasal continuous positive airway pressure NICU: neonatal intensive care unit NIMV: nasal intermittent mandatory ventilation PEEP: positive end‐expiratory pressure PIP: peak inspiratory pressure PPV: positive pressure ventilation RCT: randomised controlled trial SLI: sustained lung inflation
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Sustained aeration of infant lungs trial (SAIL) |
| Methods | Two‐arm randomised controlled multi‐centre clinical trial |
| Participants | Infants of 23 to 26 weeks of gestational age requiring respiratory support at birth. Sample size: 600 infants Inclusion criteria: gestational age at least 23 weeks but less than 27 completed weeks by best obstetrical estimate; requiring resuscitation/respiratory intervention at birth Exclusion criteria: considered non‐viable by attending neonatologist; refusal of antenatal informed consent; known major anomalies, pulmonary hypoplasia. Mothers unable to provide consent for medical care and who do not have a surrogate guardian will not be approached for consent |
| Interventions | SLI group: sustained inflation in the delivery room. The first sustained inflation will use inflation pressure of 20 cmH2O for 15 seconds Control group: CPAP of 5 to 7 cmH2O in the delivery room |
| Outcomes | Primary outcome: combined endpoint of death or BPD at 36 weeks of gestational age Secondary outcomes: oxygen profile over first 24 hours; oxygen profile with highest fraction of inspired oxygen (FiO2) level up to 48 hours; highest FiO2 level recorded during first 48 hours; heart rate in the delivery room (DR); detailed status on departure from DR; type of respiratory support (CPAP, PPV) and FiO2 on departure from DR; use of inotropic agents on arrival in NICU; circulatory support; need for intubation in DR or by 24 hours of age; pressure‐volume characteristics in DR; chest x‐ray reports showing pneumothorax or new chest drains in first 48 hours of life; duration of any chest drain in situ; head ultrasound and/or MRI findings of intraventricular haemorrhage; chest x‐ray between days 7 and 10; death or need for positive pressure ventilation at 7 days; highest FiO2 and area under the FiO2 curve for first week of life; pneumothorax and pulmonary interstitial emphysema (PIE); survival to discharge home without BPD, retinopathy of prematurity (grades 3 and 4), or significant brain abnormalities on head ultrasound; duration of respiratory support (ventilation, CPAP, supplemental oxygen); death in hospital; retinopathy of prematurity (ROP) stage 3 or greater requiring treatment; use of postnatal steroids for treatment of BPD; length of hospital stay; neurodevelopmental and respiratory outcome at 22 to 26 months of corrected gestational age |
| Starting date | August 2014. |
| Contact information | Haresh Kirpalani, BM, MSc; kirpalanih@email.chop.edu Sarah J Ratcliffe, PhD; sratclif@upenn.edu. |
| Notes | Estimated enrolment: 600 Estimated primary completion date: December 2017 |
| Trial name or title | Evaluation of pulmonary mechanics in preterm infant treated with sustained lung inflation at birth |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Preterm infants at 25 to 36 weeks |
| Interventions | SLI group: PIP of 25 cmH2O for 15 seconds followed by PEEP of 5 cmH2O; second SLI in case of poor response Control group: CPAP of 5 cmH2O with mask |
| Outcomes | Primary outcomes: change in reactance values measured by forced oscillation technique Secondary outcomes: need for intubation within first 72 hours of life; duration of respiratory support; death in hospital; number of surfactant doses; ROP stage 3 or greater requiring treatment; PDA requiring treatment; BPD; IVH |
| Starting date | July 2015 |
| Contact information | Mariarosa Colnaghi, MD; mariarosa.colnaghi@mangiagalli.it Domenica Mercadante, MD; domenica.mrc@hotmail.it |
| Notes | Estimated enrolment: 48 Estimated primary completion date: December 2015 |
| Trial name or title | Sustained lung inflation at birth for preterm infants at risk of respiratory distress syndrome: the proper pressure and duration: prospective randomised study |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Preterm infants ≤ 32 weeks of gestation with respiratory distress syndrome at birth |
| Interventions | Five arms: evaluating 2 different pressures ‐ 20 and 15 cmH2O, and for 2 different durations ‐ 10 and 20 seconds, during application of sustained lung inflation in resuscitation of preterm infants with respiratory distress in the delivery room, plus a control group without any SLI (CPAP 5 cmH2O) |
| Outcomes | Primary outcome: need for endotracheal intubation in the delivery room Secondary outcomes: need for mechanical ventilation; need for surfactant; neonatal mortality; death before hospital discharge; BPD; IVH; ROP; NEC; length of NICU and hospital stay; air leak syndrome |
| Starting date | March 2013 |
| Contact information | Nehad Nasef, Associate Professor Mansoura University Children Hospital, El Dakahlya, Egypt |
| Notes | Estimated enrolment: 100 Estimated primary completion date: October 2016 (for primary outcome) |
| Trial name or title | SURV1VE‐Trial ‐ Sustained inflation and chest compression versus 3:1 chest compression to ventilation ratio during cardiopulmonary resuscitation of asphyxiated newborns: a randomised controlled trial |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Infants (term or preterm infants) requiring chest compressions in the delivery room |
| Interventions | SLI group: PIP of 25 to 30 cmH2O for 45 seconds while receiving chest compression. This will be followed by PEEP of 5 to 8 cmH2O. If heart rate < 60/min, continue with chest compression + SLI for another 45 seconds. If heart rate remains < 60/min, continue with CC + SI Control group: chest compression at a rate of 90/min and 30 ventilations/min in a 3:1 C:V ratio |
| Outcomes | Primary outcomes: return of spontaneous circulation; duration of chest compression heart rate is > 60/min for 15 seconds |
| Starting date | January 2017 |
| Contact information | Georg Schmolzer, MD, PhD; georg.schmoelzer@me.com University of Alberta |
| Notes | Estimated enrolment: 118 Estimated primary completion date: December 2018 |
| Trial name or title | The effect of sustained lung inflation maneuver applied through nasal prong on early and late respiratory morbidities in preterm infants |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Preterm infants of 26 weeks 0 days and 29 weeks 6 days |
| Interventions | SLI group: PIP 25 cmH2O for 15 seconds with T‐piece and bi‐nasal prongs; second SLI in case of poor response Control group: CPAP |
| Outcomes | Primary outcome: surfactant need, intubation and mechanical ventilation needs within first 72 hours of life Secondary outcomes: heart rate, fractional inspiratory oxygen, CPAP pressure and oxygen saturation within first 72 hours of life in preterm infants; total non‐invasive, invasive respiratory support time; BPD; PDA; IVH, NEC; ROP; feeding intolerance, reaching birth weight; transition to full oral feeding time |
| Starting date | August 2016 |
| Contact information | Zekai Tahir Burak Women's Health Research and Education Hospital, Ankara, Turkey |
| Notes | Estimated enrolment: 250 Estimated primary completion date: September 2017 |
BPD: bronchopulmonary dysplasia CC: chest compression C:V: compression: ventilation CPAP: continuous positive airway pressure DR: delivery room FiO2: fraction of inspired oxygen IVH: intraventricular haemorrhage NICU: neonatal intensive care unit PEEP: positive end‐expiratory pressure PIE: pulmonary interstitial emphysema PIP: peak inspiratory pressure PPV: positive pressure ventilation ROP: retinopathy of prematurity SI: sustained inflation SLI: sustained lung inflation
Contributions of authors
Dr. Bruschettini and Dr. O'Donnell performed the literature search, extracted and analysed data, and wrote the manuscript. Prof. Davis performed the literature search, extracted data, checked the analysis, and reviewed the manuscript. Prof. Morley and Dr. Moja reviewed the manuscript. Dr. Zappettini performed the literature search, extracted data, and reviewed the manuscript. Dr. Calevo analysed data, checked the analysis, and reviewed the manuscript.
Sources of support
Internal sources
Institute for Clinical Sciences, Lund University; Research & Development, Section for HTA Analysis, Skåne University Hospital, Lund, Sweden.
Royal Women's Hospital, Melbourne, Australia.
University of Melbourne, Australia.
Istituto Giannina Gaslini, Genoa, Italy.
External sources
Murdoch Childrens Research Insitute, Australia.
National Health and Medical Research Council, Australia.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
- Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
MB, COD, PD, CM, LM, SZ, and MGC have no known conflicts of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
- El‐Chimi MS, Awad HA, El‐Gammasy TM, El‐Farghali OG, Sallam MT, Shinkar DM. Sustained versus intermittent lung inflation for resuscitation of preterm infants: a randomized controlled trial. Journal of Maternal‐fetal & Neonatal Medicine 2017;11:1273‐8. [DOI: 10.1080/14767058.2016.1210598; PUBMED: 27384245] [DOI] [PubMed] [Google Scholar]
- Jiravisitkul P, Rattanasiri S, Nuntnarumit P. Randomised controlled trial of sustained lung inflation for resuscitation of preterm infants in the delivery room. Resuscitation 2017;111:68‐73. [DOI: 10.1016/j.resuscitation.2016.12.003; PUBMED: 27987395] [DOI] [PubMed] [Google Scholar]
- Lindner W, Högel J, Pohlandt F. Sustained pressure‐controlled inflation or intermittent mandatory ventilation in preterm infants in the delivery room? A randomized, controlled trial on initial respiratory support via nasopharyngeal tube. Acta Paediatrica 2005;94(3):303‐9. [PUBMED: 16028648] [DOI] [PubMed] [Google Scholar]
- Lista G, Boni L, Scopesi F, Mosca F, Trevisanuto D, Messner H, et al. SLI Trial Investigators. Sustained lung inflation at birth for preterm infants: a randomized clinical trial. Pediatrics 2015;135(2):e457‐64. [DOI: 10.1542/peds.2014-1692; PUBMED: 25624390] [DOI] [PubMed] [Google Scholar]
- Mercadante D, Colnaghi M, Polimeni V, Ghezzi E, Fumagalli M, Consonni D, et al. Sustained lung inflation in late preterm infants: a randomized controlled trial. Journal of Perinatology 2016;36(6):443‐7. [DOI: 10.1038/jp.2015.222; PUBMED: 26820220] [DOI] [PubMed] [Google Scholar]
- Ngan AY, Cheung PY, Mason AH, O’Reilly M, Os S, Kumar M, et al. Using exhaled CO2 to guide initial respiratory support at birth: a randomised controlled trial. Archives of Disease in Childhood 2017. [DOI: 10.1136/archdischild-2016-312286] [DOI] [PubMed] [Google Scholar]
- Schmölzer G, O’Reilly M, Kushniruk K, Aziz K, Cheung PY. Sustained inflation and chest compression versus 3:1 chest compression: ventilation ratio during neonatal CPR – a randomized controlled trial. Paediatrics and Child Health 2015;20(5):e31. [EMBASE: 72223281] [Google Scholar]
- Schwaberger B, Pichler G, Avian A, Binder‐Heschl C, Baik N, Urlesberger B. Do sustained lung inflations during neonatal resuscitation affect cerebral blood volume in preterm infants? A randomized controlled pilot study. PloS One 2015;10(9):e0138964. [DOI: 10.1371/journal.pone.0138964; PUBMED: 26406467] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Bouziri A, Hamdi A, Khaldi A, Bel Hadj S, Menif K, Ben Jaballah N. Management of meconium aspiration syndrome with high frequency oscillatory ventilation. Tunisie Medicale 2011;89(7):632‐7. [PUBMED: 21780039] [PubMed] [Google Scholar]
- Harling AE, Beresford MW, Vince GS, Bates M, Yoxall CW. Does sustained lung inflation at resuscitation reduce lung injury in the preterm infant?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(5):F406‐10. [DOI: 10.1136/adc.2004.059303; PUBMED: 15863490 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pas AB, Walther FJ. A randomized, controlled trial of delivery‐room respiratory management in very preterm infants. Pediatrics 2007;120(2):322‐9. [DOI: 10.1542/peds.2007-0114; PUBMED: 17671058] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Foglia EE, Owen LS, Thio M, Ratcliffe SJ, Lista G, Pas A, et al. Sustained Aeration of Infant Lungs (SAIL) trial: study protocol for a randomized controlled trial. Trials 2015;16:95. [DOI: 10.1186/s13063-015-0601-9; PUBMED: 25872563] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT02139800. Sustained Aeration of Infant Lungs Trial (SAIL). clinicaltrials.gov/show/NCT02139800 (first received 08 May 2014).
- NCT02493920. Sustained Lung Inflation and Pulmonary Mechanics in Preterm Infant [Evaluation of pulmonary mechanics in preterm infant treated with sustained lung inflation at birth]. clinicaltrials.gov/show/NCT02493920 (first received 23 June 2015).
- NCT02846597. Proper pressure and duration of sustained lung inflation in preterm infants [Sustained lung inflation at birth for preterm infants at risk of respiratory distress syndrome: the proper pressure and duration: prospective randomized study]. clinicaltrials.gov/show/NCT02846597 (first received 22 July 2016).
- NCT02858583. SI + CC Versus 3:1 C:V Ratio During Neonatal CPR [SURV1VE‐Trial ‐ Sustained inflation and chest compression versus 3:1 chest compression to ventilation ratio during cardiopulmonary resuscitation of asphyxiated newborns: a randomized controlled trial]. clinicaltrials.gov/show/NCT02858583 (first received 30 July 2016).
- NCT02887924. SLI maneuver and respiratory morbidities [The effect of sustained lung inflation maneuver applied through nasal prong on early and late respiratory morbidities in preterm infants]. clinicaltrials.gov/show/NCT02887924 (first received 15 August 2016).
Additional references
- Boon AW, Milner AD, Hopkin IE. Lung expansion, tidal exchange, and formation of the functional residual capacity during resuscitation of asphyxiated neonates. Journal of Pediatrics 1979;95(6):1031‐6. [PUBMED: 387935] [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Drew JH. Immediate intubation at birth of the very‐low‐birth‐weight infant. Effect on survival. American Journal of Diseases of Children (1960) 1982;136(3):207‐10. [PUBMED: 7039303] [DOI] [PubMed] [Google Scholar]
- Effective Practice, Organisation of Care (EPOC). Cochrane EPOC Review Group data collection checklist. EPOC resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2015. Available at: http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/datacollectionchecklist.pdf.
- Foglia EE, Pas AB. Sustained lung Inflation: physiology and practice. Clinics in Perinatology 2016;43(4):633‐46. [DOI: 10.1016/j.clp.2016.07.002; PUBMED: 27837749] [DOI] [PubMed] [Google Scholar]
- Fuchs H, Lindner W, Buschko A, Trischberger T, Schmid M, Hummler HD. Cerebral oxygenation in very low birth weight infants supported with sustained lung inflations after birth. Pediatric Research 2011;70(2):176–80. [DOI: 10.1203/PDR.0b013e318220c1e0; PUBMED: 21522035] [DOI] [PubMed] [Google Scholar]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 17 August 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
- Grasso C, Sciacca P, Giacchi V, Carpinato C, Mattia C, Palano GM, et al. Effects of Sustained Lung Inflation, a lung recruitment maneuver in primary acute respiratory distress syndrome, in respiratory and cerebral outcomes in preterm infants. Early Human Development 2015;91(1):71‐5. [DOI: 10.1016/j.earlhumdev.2014.12.002; PUBMED: 25549915] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Hooper SB, Pas AB, Kitchen MJ. Respiratory transition in the newborn: a three‐phase process. Archives of Disease in Childhood. Fetal and Neonatal Edition 2016;101(3):F266‐71. [DOI: 10.1136/archdischild-2013-305704; PUBMED: 26542877] [DOI] [PubMed] [Google Scholar]
- Karlberg P, Cherry RB, Escardó FE, Koc G. Respiratory studies in newborn infants. II: Pulmonary ventilation and mechanics of breathing in the first minutes of life, including the onset of respiration. Acta Paediatrica 1962;51(2):121‐36. [DOI: 10.1111/j.1651-2227.1962.tb06521.x] [DOI] [Google Scholar]
- Klingenberg C, O'Donnell CP. Inflation breaths‐A transatlantic divide in guidelines for neonatal resuscitation. Resuscitation2016; Vol. 101:e19. [DOI: 10.1016/j.resuscitation.2015.12.021; PUBMED: 26855292] [DOI] [PubMed]
- Lindner W, Vossbeck S, Hummler H, Pohlandt F. Delivery room management of extremely low birth weight infants: spontaneous breathing or intubation. Pediatrics 1999;103(5 Pt 1):961‐7. [PUBMED: 10224173] [DOI] [PubMed] [Google Scholar]
- Lista G, Castoldi F. Alveolar recruitment in the delivery room: sustained lung inflation [Reclutamento alveolare in sala parto: la sustained lung inflation]. Minerva Pediatrica 2010;62(3 Suppl 1):17‐8. [PUBMED: 21089711] [PubMed] [Google Scholar]
- Lista G, Fontana P, Castoldi F, Cavigioli F, Dani C. Does sustained lung inflation at birth improve outcome of preterm infants at risk for respiratory distress syndrome?. Neonatology 2011;99(1):45‐50. [DOI: 10.1159/000298312; PUBMED: 20616570] [DOI] [PubMed] [Google Scholar]
- Lista G, Cavigioli F, Verde PA, Castoldi F, Bresesti I, Morley CJ. Effects of Breathing and Apnoea during Sustained Inflations in Resuscitation of Preterm Infants. Neonatology 2017;111(4):360‐6. [DOI: 10.1159/000454799; PUBMED: 28118641] [DOI] [PubMed] [Google Scholar]
- McCall KE, Davis PG, Owen LS, Tingay DG. Sustained lung inflation at birth: what do we know, and what do we need to know?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2016;101(2):F175‐80. [DOI: 10.1136/archdischild-2015-309611; PUBMED: 26527635] [DOI] [PubMed] [Google Scholar]
- Milner AD, Saunders RA. Pressure and volume changes during the first breath of human neonates. Archives of Disease in Childhood 1977;52(12):918‐24. [PUBMED: 606168] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell CPF, Davis PG, Morley CJ. Neonatal resuscitation: review of ventilation equipment and survey of practice in Australia and New Zealand. Journal of Paediatrics and Child Health 2004;40(4):208‐12. [PUBMED: 15009551] [DOI] [PubMed] [Google Scholar]
- O'Donnell CPF, Davis PG, Morley CJ. Positive pressure ventilation at neonatal resuscitation: review of equipment and international survey of practice. Acta Paediatrica 2004;93(5):583‐8. [PUBMED: 15174776] [DOI] [PubMed] [Google Scholar]
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of Neurology 1976;33(10):696–705. [PUBMED: 987769] [DOI] [PubMed] [Google Scholar]
- Saugstad OD. Practical aspects of resuscitating asphyxiated newborn infants. European Journal of Pediatrics 1998;157(Suppl 1):S11‐5. [PUBMED: 9462900] [DOI] [PubMed] [Google Scholar]
- Schmölzer GM, Kumar M, Aziz K, Pichler G, O'Reilly M, Lista G, et al. Sustained inflation versus positive pressure ventilation at birth: a systematic review and meta‐analysis. Archives of Disease in Childhood. Fetal and Neonatal Edition2015; Vol. 100, issue 4:F361‐8. [DOI: 10.1136/archdischild-2014-306836; PUBMED: 25550472] [DOI] [PubMed]
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Available from https://gdt.gradepro.org/app/handbook/handbook.html.Updated October 2013.
- Vonderen JJ, Hooper SB, Hummler HD, Lopriore E, Pas AB. Effects of a sustained inflation in preterm infants at birth. Journal of Pediatrics 2014;165(5):903‐8.e1. [DOI: 10.1016/j.jpeds.2014.06.007; PUBMED: 25039041] [DOI] [PubMed] [Google Scholar]
- Vyas H, Milner AD, Hopkin IE, Boon AW. Physiologic responses to prolonged and slow‐rise inflation in the resuscitation of the asphyxiated newborn infant. Journal of Pediatrics 1981;99(4):635‐9. [PUBMED: 7277110] [DOI] [PubMed] [Google Scholar]
- Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman JM, et al. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132(18 Suppl 2):S543‐60. [DOI: 10.1161/CIR.0000000000000267; PUBMED: 26473001] [DOI] [PubMed] [Google Scholar]
- Wyllie J, Bruinenberg J, Roehr CC, Rudiger M, Trevisanuto D, Urlesberger B. European Resuscitation Council Guidelines for Resuscitation 2015: Section 7. Resuscitation and support of transition of babies at birth. Resuscitation 2015;95:249‐63. [DOI: 10.1016/j.resuscitation.2015.07.029; PUBMED: 26477415] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- O'Donnell CPF, Davis PG, Morley CJ. Sustained inflations for neonatal resuscitation. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004953] [DOI] [Google Scholar]
- O'Donnell CP, Bruschettini M, Davis PG, Morley CJ, Moja L, Calevo MG, et al. Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD004953.pub2] [DOI] [PubMed] [Google Scholar]


