Abstract
Background
Neonatal hyperbilirubinaemia is a common problem which carries a risk of neurotoxicity. Certain infants who have hyperbilirubinaemia develop bilirubin encephalopathy and kernicterus which may lead to long‐term disability. Phototherapy is currently the mainstay of treatment for neonatal hyperbilirubinaemia. Among the adjunctive measures to compliment the effects of phototherapy, fluid supplementation has been proposed to reduce serum bilirubin levels. The mechanism of action proposed includes direct dilutional effects of intravenous (IV) fluids, or enhancement of peristalsis to reduce enterohepatic circulation by oral fluid supplementation.
Objectives
To assess the risks and benefits of fluid supplementation compared to standard fluid management in term and preterm newborn infants with unconjugated hyperbilirubinaemia who require phototherapy.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5), MEDLINE via PubMed (1966 to 7 June 2017), Embase (1980 to 7 June 2017), and CINAHL (1982 to 7 June 2017). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
We included randomised controlled trials that compared fluid supplementation against no fluid supplementation, or one form of fluid supplementation against another.
Data collection and analysis
We extracted data using the standard methods of the Cochrane Neonatal Review Group using the Covidence platform. Two review authors independently assessed the eligibility and risk of bias of the retrieved records. We expressed our results using mean difference (MD), risk difference (RD), and risk ratio (RR) with 95% confidence intervals (CIs).
Main results
Out of 1449 articles screened, seven studies were included. Three articles were awaiting classification, among them, two completed trials identified from the trial registry appeared to be unpublished so far.
There were two major comparisons: IV fluid supplementation versus no fluid supplementation (six studies) and IV fluid supplementation versus oral fluid supplementation (one study). A total of 494 term, healthy newborn infants with unconjugated hyperbilirubinaemia were evaluated. All studies were at high risk of bias for blinding of care personnel, five studies had unclear risk of bias for blinding of outcome assessors, and most studies had unclear risk of bias in allocation concealment. There was low‐ to moderate‐quality evidence for all major outcomes.
In the comparison between IV fluid supplementation and no supplementation, no infant in either group developed bilirubin encephalopathy in the one study that reported this outcome. Serum bilirubin was lower at four hours postintervention for infants who received IV fluid supplementation (MD ‐34.00 μmol/L (‐1.99 mg/dL), 95% CI ‐52.29 (3.06) to ‐15.71 (0.92); participants = 67, study = 1) (low quality of evidence, downgraded one level for indirectness and one level for suspected publication bias). Beyond eight hours postintervention, serum bilirubin was similar between the two groups. Duration of phototherapy was significantly shorter for fluid‐supplemented infants, but the estimate was affected by heterogeneity which was not clearly explained (MD ‐10.70 hours, 95% CI ‐15.55 to ‐5.85; participants = 218; studies = 3; I² = 67%). Fluid‐supplemented infants were less likely to require exchange transfusion (RR 0.39, 95% CI 0.21 to 0.71; RD ‐0.01, 95% CI ‐0.04 to 0.02; participants = 462; studies = 6; I² = 72%) (low quality of evidence, downgraded one level due to inconsistency, and another level due to suspected publication bias), and the estimate was similarly affected by unexplained heterogeneity. The frequencies of breastfeeding were similar between the fluid‐supplemented and non‐supplemented infants in days one to three based on one study (estimate on day three: MD 0.90 feeds, 95% CI ‐0.40 to 2.20; participants = 60) (moderate quality of evidence, downgraded one level for imprecision).
One study contributed to all outcome data in the comparison of IV versus oral fluid supplementation. In this comparison, no infant in either group developed abnormal neurological signs. Serum bilirubin, as well as the rate of change of serum bilirubin, were similar between the two groups at four hours after phototherapy (serum bilirubin: MD 11.00 μmol/L (0.64 mg/dL), 95% CI ‐21.58 (‐1.26) to 43.58 (2.55); rate of change of serum bilirubin: MD 0.80 μmol/L/hour (0.05 mg/dL/hour), 95% CI ‐2.55 (‐0.15) to 4.15 (0.24); participants = 54 in both outcomes) (moderate quality of evidence for both outcomes, downgraded one level for indirectness). The number of infants who required exchange transfusion was similar between the two groups (RR 1.60, 95% CI 0.60 to 4.27; RD 0.11, 95% CI ‐0.12 to 0.34; participants = 54). No infant in either group developed adverse effects including vomiting or abdominal distension.
Authors' conclusions
There is no evidence that IV fluid supplementation affects important clinical outcomes such as bilirubin encephalopathy, kernicterus, or cerebral palsy in healthy, term newborn infants with unconjugated hyperbilirubinaemia requiring phototherapy. In this review, no infant developed these bilirubin‐associated clinical complications. Low‐ to moderate‐quality evidence shows that there are differences in total serum bilirubin levels between fluid‐supplemented and control groups at some time points but not at others, the clinical significance of which is uncertain. There is no evidence of a difference between the effectiveness of IV and oral fluid supplementations in reducing serum bilirubin. Similarly, no infant developed adverse events or complications from fluid supplementation such as vomiting or abdominal distension. This suggests a need for future research to focus on different population groups with possibly higher baseline risks of bilirubin‐related neurological complications, such as preterm or low birthweight infants, infants with haemolytic hyperbilirubinaemia, as well as infants with dehydration for comparison of different fluid supplementation regimen.
Keywords: Humans; Infant, Newborn; Phototherapy; Phototherapy/methods; Phototherapy/statistics & numerical data; Administration, Intravenous; Administration, Oral; Bilirubin; Bilirubin/blood; Breast Feeding; Breast Feeding/statistics & numerical data; Cerebral Palsy; Cerebral Palsy/prevention & control; Exchange Transfusion, Whole Blood; Exchange Transfusion, Whole Blood/statistics & numerical data; Fluid Therapy; Fluid Therapy/adverse effects; Fluid Therapy/methods; Hyperbilirubinemia, Neonatal; Hyperbilirubinemia, Neonatal/blood; Hyperbilirubinemia, Neonatal/therapy; Kernicterus; Kernicterus/prevention & control; Peristalsis; Time Factors
Plain language summary
Giving additional fluid to newborn infants having phototherapy for serious jaundice
Review question: does giving additional fluid improve outcomes in newborn infants with jaundice who require phototherapy?
Background: jaundice in newborn infants is common, because the infants' livers are unable to fully process bilirubin, the breakdown product of red blood cells. Some infants develop serious jaundice, and though uncommon, a small number suffer major complications as excessive bilirubin crosses from the blood to the brain. The complications include acute (short‐term or sudden onset) brain injuries and long‐term disability in the form of cerebral palsy (which affects movement and co‐ordination). The extent of jaundice is commonly assessed by looking at the infants' skin and eyes, and confirmed by checking the blood bilirubin level. Phototherapy (light treatment) is the main treatment, and if bilirubin remains very high after phototherapy, exchange transfusion (transfusing with new blood while removing blood containing high levels of bilirubin) is recommended. Several other treatments have also been evaluated. Among them, giving infants additional intravenous (into a vein) fluid to dilute the blood and increasing feeding to enhance bilirubin excretion in bowel movements have been practised. We examined whether fluid supplementation confers any additional benefit on top of phototherapy for infants with serious jaundice.
Search date: we searched medical databases in February 2016.
Study characteristics: we included seven studies (total participants = 494). All studies were on full‐term, healthy infants who were breastfeeding fully or partially. There were two main comparisons: fluid supplementation via intravenous route versus no fluid supplementation and fluid supplementation via intravenous route versus oral route (by increasing feeding by mouth). Most studies did not provide enough information on certain key aspects of the methods employed. Notably, in all studies, care personnel could not be masked from knowing whether or not the infants received additional fluid, and if so through which route, and this might have affected the interpretation of results, especially those that required a person to make a judgement.
Study funding sources: none of the included studies reported funding.
Key results: no infant in either the fluid supplementation or no fluid supplementation group developed clinical complications related to excessive bilirubin. Serum bilirubin was slightly lower at four and eight hours after treatment in fluid‐supplemented infants. Beyond eight hours, bilirubin levels were very similar whether or not additional fluid was given. Infants who received additional fluid appeared to have shorter duration of phototherapy (on average 10.70 hours shorter, participants = 218, studies = three) and lower risk of requiring exchange transfusion (on average 1% lower, participants = 462, studies = six), but in both analyses, inconsistent results among the included studies have weakened our confidence in the overall estimates. There were no differences in breastfeeding frequencies in the first three days between infants who received additional fluid and infants who did not.
In another comparison, one study showed that there were no clear differences between infants who received intravenous and oral fluid supplementation in all measurements (called outcomes), including blood bilirubin and the rate of change of bilirubin levels after four hours of study, as well as the number of infants who required exchange transfusion.
Quality of evidence: there was no evidence on the major clinical outcomes of bilirubin‐associated brain problems, as no infants in either group developed these problems. There was low‐ to moderate‐quality evidence for all major outcomes. Three main factors affected the quality of evidence: first, the use of bilirubin, a laboratory measurement, as the main outcome, rather than direct clinical outcomes that matter to patients; second, inconsistent study results; and third, unpublished studies that might change the review findings for the relevant outcomes.
Conclusions: there is no evidence that intravenous fluid supplementation affected major clinical outcomes such as acute‐ or long‐term brain problems associated with excessive bilirubin in healthy, full‐term newborn infants, mainly because the baseline risk of developing such problems was very low in this group of infants. Intravenous fluid supplementation may reduce serum bilirubin at certain time points but it is unclear whether this translates into important clinical benefits. Future research should focus on higher‐risk populations such as preterm infants or infants with haemolysis (increased red blood cell breakdown which causes a rapid rise in bilirubin).
Summary of findings
Summary of findings for the main comparison. Intravenous fluid supplementation versus no fluid supplementation for neonatal unconjugated hyperbilirubinaemia.
| Intravenous fluid supplementation versus no fluid supplementation for neonatal unconjugated hyperbilirubinaemia | ||||||
|
Patient or population: newborn infants with unconjugated hyperbilirubinaemia undergoing phototherapy Setting: neonatal intensive care unit Intervention: intravenous fluid supplementation Comparison: no fluid supplementation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no fluid supplementation | Risk with intravenous fluid supplementation | |||||
| Incidence of acute bilirubin encephalopathy | Study population | Not estimable | 74 (1 RCT) | ‐ | Effects not estimable as there were no reported cases of bilirubin encephalopathy in either group. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Bilirubin level (μmol/L): 4 hours postintervention | The mean serum bilirubin 4 hours postintervention was 344 μmol/L | The mean serum bilirubin 4 hours postintervention in the intervention group was 34 μmol/L lower (52.29 lower to 15.71 lower) | ‐ | 67 (1 RCT) | ⊕⊕⊝⊝ Low a,b | ‐ |
| Proportion of infants who required exchange transfusion | Study population | RR 0.39 (0.21 to 0.71) | 462 (6 RCTs) | ⊕⊕⊝⊝ Lowc,d | ‐ | |
| 147 per 1000 | 57 per 1000 (31 to 105) | |||||
| Frequency of breastfeeding per day: day 3 | The mean frequency of breastfeeding per day on day 3 was 9.5 times per day | The mean frequency of breastfeeding per day on day 3 in the intervention group was 0.9 more feeds (0.4 fewer to 2.2 more) | ‐ | 60 (1 RCT) | ⊕⊕⊕⊝ Moderate e | Although the study had high risk of bias in blinding of personnel, breastfeeding frequency on‐demand was considered unlikely to be affected. The study evaluated breastfeeding frequencies on days 1, 2, and 3. Data on day 3 were chosen because compared to the earlier period, it most closely reflected the breastfeeding frequency on discharge. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
a Although widely used, serum bilirubin is only a surrogate to the relevant clinical outcomes, namely, bilirubin encephalopathy or kernicterus. Quality of evidence downgraded one level on the basis of indirectness.
b There is at least one unpublished study that was registered in ClinicalTrials.gov that included similar outcomes (NCT01550627). Quality of evidence downgraded one level on the basis of suspected publication bias. This outcome was also assessed in a published study for which we are yet to acquire full‐text (Demirsoy 2011). Both of these studies are currently placed under Studies awaiting classification.
c Moderate degree of heterogeneity was present, as indicated by an I² statistic of 72%, which was mainly due to one included study with effect estimates in opposite direction to the other studies. Quality of evidence downgraded one level on the basis of inconsistency.
d There are two unpublished studies (NCT01550627; IRCT2013022711145N5) identified from ClinicalTrials.gov, and one published study that we are yet to acquire full‐text (Demirsoy 2011) that are likely to assess this outcome. All three studies are placed under Studies awaiting classification. Quality of evidence downgraded one level on the basis of suspected publication bias.
e Quality of evidence downgraded one level due to imprecision, as reflected by wide 95% CIs for the estimate.
Summary of findings 2. Intravenous fluid supplementation versus oral fluid supplementation for neonatal unconjugated hyperbilirubinaemia.
| Intravenous fluid versus oral fluid supplementation for neonatal unconjugated hyperbilirubinaemia | ||||||
|
Patient or population: newborn infants with unconjugated hyperbilirubinaemia undergoing phototherapy Setting: neonatal intensive care unit Intervention: intravenous fluid supplementation Comparison: oral fluid supplementation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with oral fluid supplementation | Risk with intravenous fluid supplementation | |||||
| Number of infants with abnormal neurological signs | Study population | Not estimable | 54 (1 RCT) | ‐ | Not estimable as there were no cases reported in either group. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Bilirubin level (μmol/L): 4 hours postphototherapy | The mean serum bilirubin 4 hours postphototherapy was 332 μmol/L | The mean serum bilirubin 4 hours postphototherapy in the intervention group was 11 μmol/L higher (21.58 lower to 43.58 higher) | ‐ | 54 (1 RCT) | ⊕⊕⊕⊝ Moderate a | ‐ |
| Rate of change in bilirubin (μmol/L/hour): during the first 4 hours of admission | The mean rate of decrease of serum bilirubin (μmol/L/hour) during the first 4 hours of admission was 10.4 μmol/L/hour | The mean rate of decrease of serum bilirubin (μmol/L/hour) during the first 4 hours of admission in the intervention group was 0.8 μmol/L/hour higher (2.55 lower to 4.15 higher) | ‐ | 54 (1 RCT) | ⊕⊕⊕⊝ Moderatea | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
a Although widely used, serum bilirubin is only a surrogate to the relevant clinical outcomes, namely, bilirubin encephalopathy or kernicterus. Quality of evidence downgraded one level on the basis of indirectness.
Background
Description of the condition
Most newborn infants develop jaundice soon after birth with elevated serum bilirubin levels(Maisels 1986; Dennery 2001). This is due to increased breakdown of red blood cells and decreased clearance of bilirubin, which in turn is due to immaturity of the conjugation process in the liver and increased enterohepatic circulation (Dennery 2001). The major concern for infants with hyperbilirubinaemia is the neurotoxicity of unconjugated bilirubin. If present in high enough concentration, unbound bilirubin, a subset of unconjugated bilirubin, crosses the blood‐brain barrier and deposits in the brain causing bilirubin encephalopathy and, in its most severe form, kernicterus (Dennery 2001; Ahlfors 2010).
The defining thresholds for significant neonatal hyperbilirubinaemia differ according to postnatal age and postmenstrual age of the infant at the time when serum bilirubin is measured. In addition, factors such as blood group incompatibility, glucose‐6‐phosphate dehydrogenase (G6PD) deficiency, ethnicity and sex, among others, would also place the infant in different risk zones where indication for treatment would vary. A widely used nomogram according to the hour of life, developed based on the data from a large cohort of infants of more than 35 weeks' gestation, has allowed clinicians to recognise infants at risk of severe hyperbilirubinaemia (Bhutani 1999). However, bilirubin level is only a surrogate marker for bilirubin encephalopathy and kernicterus and a consistent threshold level of bilirubin which predicts kernicterus has not been clearly demonstrated (Bhutani 2009; Trikalinos 2009).
A significant proportion of newborn infants who present to hospital with hyperbilirubinaemia have some degree of dehydration, due mainly to problems in establishing breastfeeding (Tudehope 1991; Seidman 1995; Gourley 2002; Manning 2007). Affected infants present with signs such as lethargy, fever, marked jaundice, excessive weight loss of more than 15% of birth weight, or hypernatraemic dehydration as typified by a serum sodium of above 150 mmol/L (Oddie 2001; Unal 2008; Boskabadi 2010). In the most severe cases, neurological manifestation, such as seizure, may occur with acute complications ranging from intraventricular haemorrhage and cerebral oedema to systemic complications, such as acute renal failure and disseminated intravascular coagulation (Unal 2008). Phototherapy may further increase the fluid deficits of these infants by increasing insensible water loss, especially in the preterm population (Kjartansson 1992; Maayan‐Metzger 2001; Grünhagen 2002).
It has been well‐documented that in general, breastfed infants have a higher bilirubin level than formula‐fed infants (Maisels 1986; Preutthipan 1993; Itoh 2001) and inadequate breastfeeding, rather than breast milk itself, has been reported as a risk factor for acute bilirubin encephalopathy and kernicterus (Geiger 2001; Gourley 2002; AAP 2004; Johnson 2009). However, some studies have reported otherwise, showing no difference in the levels of serum bilirubin between breastfed and formula‐fed infants (Rubaltelli 1993; Buiter 2008).
Phototherapy is the most widely used intervention to reduce serum bilirubin (Mills 2001; Springer 2001; Onyango 2009; Kumar 2011; Okwundu 2012; Malwade 2014; Bhola 2015). Other treatment modalities based on physiological assumptions of how bilirubin is cleared have been evaluated, including the use of clofibrate (Gholitabar 2012), metalloporphyrins (Suresh 2003), antenatal phenobarbital (Thomas 2007), and Chinese herbal medicine (Yu 2010). One other proposed mechanism to enhance the clearance of bilirubin is by facilitating bowel movement to decrease enterohepatic circulation, either by facilitating meconium or stool passage (Srinivasjois 2011) or by promoting peristalsis by means of increasing feed volume.
Description of the intervention
Fluid supplementation denotes any amount of fluid given in addition to the standard daily maintenance fluid intake of the infants, which may be administered either enterally or intravenously (IV). Daily maintenance fluid volume for infants changes with postnatal age, with increment over advancing days of life after birth with practice consensus ranging from 50 mL/kg/day to 90 mL/kg/day for first 24 hours of life, increasing by 20 mL/kg/day to 30 mL/kg/day each day after, reaching an upper limit of 150 mL/kg/day to 180 mL/kg/day (Bell 2014). However, in infants who are breastfeeding directly, accurate assessment of daily fluid intake is not possible. Whether the infant has achieved adequate daily fluid intake is mainly determined clinically via an assessment of hydration status, weight change, and stool and urine output (Brown 1986; Dewey 2003; Davanzo 2013).
How the intervention might work
Fluid supplementation for newborn infants with significant hyperbilirubinaemia, either through increased IV fluid or increased enteral feed, is practised in some neonatal units alongside standard treatment, such as phototherapy, based on the belief that an increase in the amount of body fluid alleviates the problem of neonatal hyperbilirubinaemia. Indirect evidence shows that dehydration in a newborn infant, reflected as significant weight loss, predisposes the infant to severe hyperbilirubinaemia (Chang 2012; Huang 2012; Yang 2013). While IV fluid supplementation is postulated to decrease bilirubin concentration directly through a reduction of haemoconcentration, increasing enteral feed volume is proposed to decrease bilirubin concentration through reduced enterohepatic circulation via an increased gut peristalsis (Dennery 2001).
It is believed that by decreasing the total serum bilirubin concentration, the risk of bilirubin‐induced neurotoxicity is decreased through a reduction in the amount of unbound bilirubin, the substance directly implicated in neurotoxicity (Watchko 2013). However, the level of total serum bilirubin bears only a modest correlation with that of unbound bilirubin, as this is determined by factors such as the concentration and bilirubin‐binding affinity of serum albumin (Amin 2011). Additionally, the concentration and duration of exposure to unbound bilirubin interact with other factors (e.g. the presence of systemic illness, such as sepsis and haemolysis), in determining the risk of neurotoxicity (Watchko 2013).
However, there are concerns of giving higher fluid volume, which include an increased risk of patent ductus arteriosus (PDA) and bronchopulmonary dysplasia (BPD) (Bell 2014), and there are uncertainties on the association between feed volume and gastrointestinal adverse effects, such as feed intolerance, gastro‐oesophageal reflux, and necrotising enterocolitis (Patole 2005). Other possible adverse effects of fluid supplementation include electrolyte imbalance such as hyponatraemia (Moritz 2004), and complications from the placement of IV catheters, such as thrombosis, phlebitis, and line‐associated systemic infections (Tagalakis 2002; Barría 2007; Wu 2012). Maintaining enteral feed for an infant with hyperbilirubinaemia poses a further concern when the infant has a rapid rise of serum bilirubin that is not responsive to phototherapy. This is because exchange transfusion, which represents the next step in management, usually requires a period of fasting.
Why it is important to do this review
Hyperbilirubinaemia in neonates is a common condition worldwide. Its management varies but includes fluid supplementation as a feasible intervention. Despite the availability of some clinical trials, there is no systematic review to date that assesses the findings of these trials and provides an overall estimate on the benefits and harms of this intervention to inform practice and research.
Objectives
To assess the risks and benefits of fluid supplementation compared to standard fluid management in term and preterm newborn infants with unconjugated hyperbilirubinaemia who require phototherapy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi‐RCTs, and cluster‐RCTs.
Types of participants
Newborn infants of any gestation, birthweight, or postnatal age who had unconjugated hyperbilirubinaemia detected via serum bilirubin or transcutaneous bilirubin level at or beyond a level at which in‐hospital treatment and monitoring were required as determined via a well‐established assessment tool, such as the Bhutani nomogram (Bhutani 1999), irrespective of their hydration status. All eligible infants had been treated with phototherapy, either before study entry or as part of the intervention in both treatment arms.
Types of interventions
Intervention
Fluid supplementation: additional amount of fluid on top of the standard fluid regimen, given enterally or IV, or both, aiming to restore normal hydration status in clinically dehydrated infants or provide additional circulating fluid in infants who are not clinically dehydrated, or both restoring and providing additional circulating fluid. See paragraph 3 under Description of the intervention for a description of a standard neonatal fluid regimen.
Comparison
Standard fluid regimen given enterally or IV or both, as practised routinely in the neonatal unit. We accepted various definitions of standard fluid regimen as long as the regimen was within the range of volume stated in our description of a standard fluid regimen under Description of the intervention.
The following were specific comparisons that we planned to include in the Cochrane Review:
comparison 1: IV fluid supplementation (IV infusion or bolus) with or without standard oral fluid regimen versus standard oral fluid regimen;
comparison 2: supplemented oral fluid regimen versus standard oral fluid regimen. Infants who were supplemented were most likely to be given additional expressed breast milk or formula milk on top of standard breast‐ or formula‐feeding regimen;
comparison 3: IV fluid supplementation (IV infusion or bolus) with or without standard oral fluid regimen versus supplemented oral fluid regimen;
comparison 4: in infants who were kept nil by mouth, IV fluid supplementation (IV infusion) in addition to standard maintenance IV fluid regimen versus standard maintenance IV fluid regimen;
comparison 5: IV fluid supplementation (IV infusion or bolus) with or without standard oral fluid regimen versus supplemented oral fluid regimen in addition to standard oral or IV fluid regimen;
comparison 6: IV fluid supplementation (IV infusion or bolus) using a specific type of fluid versus IV fluid supplementation (IV infusion or bolus) using another type of fluid.
All the above comparisons might apply for infants who were not clinically dehydrated, while comparisons 5 and 6 might apply for infants who were clinically dehydrated.
The type of fluid given (if not part of the comparison), the mode of measurement for bilirubin (serum or transcutaneous bilirubin level), the intensity and mode of phototherapy (conventional or fibreoptic), and concurrent treatment for hyperbilirubinaemia followed prespecified protocols for all infants. This is an example of a prespecified protocol of fluid regimen for a preterm infant: day 1: 60 mL/kg/day; day 2: 90 mL/kg/day; day 3: 120 mL/kg/day; day 4 onwards: 150 mL/kg/day, with adjustment of total fluid depending on clinical impression of possible fluid deficit or overload.
Except for fluid volume, all aspects of care, including the standard treatment for neonatal hyperbilirubinaemia such as the use of phototherapy, followed the same protocol in both the intervention and the control groups.
Types of outcome measures
Primary outcomes
Incidence of acute bilirubin encephalopathy (defined as acute clinical manifestations of bilirubin toxicity seen in the first weeks after birth, which was characterised by signs of irritability and hypertonia, with early retrocollis and opisthotonus, together with anyone of the following: drowsiness, poor feeding, alternating tone, high‐pitched cry, or a failed auditory brainstem response hearing screen).
Incidence of kernicterus (defined as the long‐term sequelae of bilirubin toxicity, which was characterised by extrapyramidal movement disorders, gaze abnormalities, auditory disturbances, intellectual deficits, and the posticteric sequelae of enamel dysplasia of the deciduous teeth) (Johnson 2002; AAP 2004).
Proportion of infants with moderate or severe cerebral palsy, defined as a non‐progressive disorder with abnormal muscle tone in at least one arm or leg that was associated with abnormal control of movement or posture and a modified Gross Motor Function Classification System (GMFCS) score (Palisano 2008) of 2 or greater (Rosenbaum 2007), measured at predefined intervals (e.g. at 6, 12, 18, and 24 months). We included results from individual assessments that measured other specific aspects of neurodevelopmental outcome, if available, as secondary outcomes (see eighth to 11th Secondary outcomes).
Secondary outcomes
-
Bilirubin level (serum bilirubin or transcutaneous bilirubin level) reported as follows:
absolute bilirubin level measured at specific time points and expressed in mmol/L or mg/dL, including the peak bilirubin value;
change in bilirubin level measured as the difference between bilirubin readings at two time points and expressed in mmol/L or mg/dL; and
rate of change in bilirubin expressed as mmol/L/hour or mg/dL/hour. We grouped absolute bilirubin level measured at the same time points (e.g. 6, 12, 24, 48, or 72 hours after initiation of phototherapy), and the difference in bilirubin levels between the same time points among the included trials under the same outcomes.
Duration of phototherapy (in hours).
Proportion of infants who required exchange transfusion.
Proportion of infants with clinical or echocardiographic evidence of significant PDA.
Proportion of infants with feed intolerance, either with documented diagnosis or with suggestive symptoms and/or signs such as vomiting, abdominal distension or discoloured gastric aspirate.
Proportion of infants with necrotising enterocolitis, classified using modified Bell's clinical staging (Bell 1978; Walsh 1986).
Proportion of infants with BPD. We accepted both the 'classical' and 'physiological' definitions of BPD. In the 'classical' definition, BPD was defined by a sustained need for any supplemental oxygen at 28 days of postnatal age (Northway 1967), or 36 weeks' postmenstrual age (Shennan 1988). In the 'physiological' definition, infants who required mechanical ventilation, continuous positive airway pressure, or supplemental oxygen exceeding 0.30 fraction were diagnosed with BPD without further testing. Infants who required lower supplemental oxygen who failed to sustain a desirable oxygen saturation during a timed stepwise reduction in oxygen concentration down to room air were also diagnosed with BPD (Walsh 2003).
Weight, either reported as changes in weight or number of infants with significant weight loss (greater than 10% of birth weight), measured at defined intervals such as weekly or twice weekly.
Proportion of infants with neurodevelopmental impairment, as indicated by a score of less than 70 in the Bayley Scales of Infant and Toddler Development, third edition (BSID‐III) (Albers 2007).
Proportion of infants with motor impairment, as indicated by a score of 2 or higher in the modified GMFCS evaluation (Palisano 2008).
Proportion of infants with bilateral visual impairment, defined as vision worse than 20/200 (Vohr 2012).
Proportion of infants with hearing impairment, defined as the inability to understand the oral directions of the examiner and to communicate, with or without hearing amplification (Vohr 2012).
Carer satisfaction, measured using a validated scale, such as Health‐Related Quality of Life Tool (HRQoL) or health outcome rating scales, or self‐reported parental dissatisfaction using scales such as any adapted version of the Parent Satisfaction Scale (Stallard 1996; Gerkensmeyer 2005).
Staff satisfaction, measured using a validated scale, such as HRQoL or health outcome rating scales, or self‐reported staff dissatisfaction.
Length of hospital stay (in days).
Exclusive breastfeeding during hospital stay or at discharge, or both.
The outcomes were measured at the following time points.
During hospital stay (acute bilirubin encephalopathy, bilirubin levels as specified above, duration of phototherapy, requirement for exchange transfusion, clinically important outcomes such as PDA, feed intolerance, necrotising enterocolitis, BPD, electrolyte imbalance, weight, and carer and staff satisfaction).
At discharge (weight, carer, and staff satisfaction).
For neurodevelopmental outcomes, at defined points beyond discharge (e.g. at 6, 12, 18, and 24 months).
Search methods for identification of studies
We followed the search strategy developed by Cochrane Neonatal.
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5).
MEDLINE (via PubMed (National Library of Medicine)) (1950 to 7 June 2017).
Embase (1980 to 7 June 2017).
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1982 to 7 June 2017).
We have outlined detailed search strategies for each of the above databases in Appendix 1, Appendix 2, Appendix 3, and Appendix 4.
We searched ongoing clinical trials and unpublished studies via the following sites:
ClinicalTrials.gov (www.clinicaltrials.gov/).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/).
ISRCTN registry (www.controlled‐trials.com).
The Australian and New Zealand Trial Registry (www.anzctr.org.au/).
We did not apply any language restrictions.
Searching other resources
We searched the references cited in Cochrane Reviews, guidelines, review articles, and conference proceedings for any additional trials.
Data collection and analysis
We followed standard Cochrane methods as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Two review authors (YMC and AAK) independently screened the articles based on the titles and abstracts and excluded studies that were clearly ineligible, leaving short‐listed articles for further assessment. Two other review authors (CFN and JYK) further assessed the short‐listed studies to determine whether these studies should be included in the meta‐analysis, excluded, or placed under the 'Studies awaiting assessment' section. We extracted the study‐related information on a dedicated proforma, and recorded the reasons for exclusion. Any differences in the decision between the two review authors in each of the two stages were discussed until a consensus was reached, with the involvement of an arbiter (NML) if necessary.
Selection of studies
We accepted published and unpublished studies, both in full article and abstract forms, as long as a complete 'Risk of bias' assessment was possible. We contacted the authors of studies identified to be relevant to obtain further information on study design, participant characteristics, cointerventions, and follow‐up data if the published data were insufficient.
Data extraction and management
Two review authors (CFN and JYK) independently extracted and coded all data for each included trial using a proforma designed specifically for this Cochrane Review. Duplicate entry of participants were screened for by matching the initial number of participants recruited against the total number along each step of the study. If a discrepancy was discovered, we attempted to look for an explanation in the article (multiple enrolment of the same participant in different hospital admissions). We contacted the trial authors for clarification if necessary. We resolved any disagreement among the review authors by discussions leading to a consensus.
Assessment of risk of bias in included studies
Two review authors (NML and YMC) independently assessed each included trial for risk of bias according to six major criteria as stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Sequence generation.
Allocation concealment.
Blinding of participant and personnel.
Blinding of outcome assessors.
Incomplete outcome data.
Selective outcome reporting.
Other issues (e.g. extreme baseline imbalance).
We provided a detailed description on each of the 'Risk of bias' criteria in Appendix 5.
Based on the information from the articles, we accorded a judgement of 'low', 'high,' or 'unclear' risk with justifications on each criterion and we completed a 'Risk of bias' table for each included trial. We discussed any disagreement among the review authors and involved a third review author (AAK) if necessary.
We presented our 'Risk of bias' assessment in a 'Risk of bias' graph and 'Risk of bias' summary.
Measures of treatment effect
We reported the outcome estimates for categorical data using risk ratio (RR), risk difference (RD), and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) if there was a statistically significant difference between the two groups. For continuous data, we used mean difference (MD) (when studies assessed the same outcome and measured it in the same way) or standardised mean difference (SMD) (when studies assessed the same outcome but measured it in different ways) values with their respective 95% confidence intervals (CI). We reported the results of the trials individually if pooled analyses were not possible.
Unit of analysis issues
There were no cluster‐RCTs included in this review. Should we have included a cluster‐RCT (e.g. trials in which assignment of intervention and control group was made at the neonatal intensive care unit (NICU) level), we would have adopted the following approach.
We would have assessed whether trial authors adjusted for the effects of clustering using the appropriate analysis methods, such as Generalized Estimating Equation (GEE) modelling. If no adjustment had been made, we would have performed adjustment by calculating the design effect based on a fairly large assumed intracluster correlation (ICC) of 0.10, which is a generally realistic estimate from studies on implementation research (Campbell 2001). If the unit of analysis was not stated in the trial, we would have inspected the width of the standard error (SE) or 95% CIs of the estimated treatment effects. If we had found an inappropriately small SE or a narrow 95% CI, we would have asked the trial authors to provide information on the unit of analysis. We would have followed the methods in the Cochrane Handbook for Systematic Reviews of Interventions for the calculations (Higgins 2011).
To address the issue of repeated measures in the included trials that arise from having multiple recording of serum bilirubin from the same infants, we followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) by setting up separate subgroups of outcomes for serum bilirubin recording obtained at different time points (e.g. at 6, 12, 24, 48, and 72 hours after initiating phototherapy), and not totalling up the participants among different subgroups to avoid multiple‐counting.
Dealing with missing data
We determined the dropout rates from each trial, and assessed the number of infants who were initially randomised against the total number analysed to determine whether each trial followed the intention‐to‐treat (ITT) principle. We considered a dropout rate of higher than 20% as significant. If we found a significant dropout rate with no reasonable explanation or a markedly different dropout rate between the assigned groups, we assigned the trial as having high risk of bias in the criterion of 'incomplete outcome data.' If we considered the extent of missing data to be critical to the final estimates in our meta‐analysis, we contacted the authors of the individual trials to request further information.
In this review, all infants recruited appeared to be included in the analyses, and thus all studies were judged to have low risk of attrition bias. Should there have been studies that were at high risk of attrition bias, we would have performed sensitivity analyses to assess how the overall results were affected with and without the inclusion of such studies.
Assessment of heterogeneity
First, we visually inspected the Forest plots for any gross evidence of heterogeneity of treatment effects. We used I² statistics to quantify the degree of inconsistency in the results (Higgins 2011), in accordance with the recommendations of the Cochrane Neonatal Review Group. We used the following cut‐offs for reporting heterogeneity: less than 25% no heterogeneity, 25% to 49% low heterogeneity, 50% to 74% moderate heterogeneity, and 75% or greater high heterogeneity. If we found moderate or high heterogeneity, we explored possible explanations using the following criteria, and determined whether the difference between study characteristics was too great for a meta‐analysis to be appropriate.
The criteria that we assessed included the following.
Baseline characteristics of the participants (gestational age, birth weight).
Clinical settings of the studies (e.g. tertiary or secondary neonatal unit).
Cointerventions.
Risk of bias (as detailed in the Assessment of risk of bias in included studies section).
Assessment of reporting biases
We used the funnel plot to screen for publication bias if there were sufficient numbers of included studies (greater than 10) reporting the same outcome, as the funnel plot is only useful with a minimum number of 10 studies included. If publication bias was suggested by a significant asymmetry of the funnel plot, we included a statement in our results with a corresponding note of caution in our discussion.
Data synthesis
We performed meta‐analysis using Review Manager 5 (RevMan 2014) with a fixed‐effect model, following the recommendations of the Cochrane Neonatal Review Group. Our primary data analyses followed the ITT principle, namely, the original number of participants allocated to each study arm was used as the denominator in subsequent analyses.
Where a single trial reported multiple trial arms, we included only the relevant arms. If there were more than two relevant arms (e.g. high IV fluid‐only regimen versus increased oral fluid‐only regimen versus standard oral fluid‐only regimen), we set up separate pair‐wise comparisons (e.g. high IV fluid‐only regimen versus standard oral fluid‐only regimen (comparison 1) and high oral fluid‐only regimen versus standard oral fluid‐only regimen (comparison 2)).
Quality of evidence
We used the GRADE approach, as outlined in the GRADE handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes.
For the comparison of IV fluid supplementation versus no fluid supplementation, we chose the number of infants with bilirubin encephalopathy (primary outcome of the review), serum bilirubin at four hours postinclusion, the number of infants who required exchange transfusion and frequency of breastfeeding in day three of admission (all secondary outcomes of the review).
For the comparison of IV fluid supplementation versus oral fluid supplementation, we chose the number of infants with abnormal neurological signs (primary outcome), serum bilirubin four hours postphototherapy and the rate of change of serum bilirubin during the first four hours of phototherapy (all secondary outcomes).
Two review authors independently assessed the quality of the evidence for each of the outcomes. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro Guideline Development Tool to create 'Summary of findings' tables to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned to undertake the following subgroup analyses if relevant data were available.
Within the comparison of IV fluid supplementation (IV infusion) with or without standard oral fluid regimen versus standard oral fluid regimen (comparison 1 in Types of interventions section), we separated infants who received IV supplementation with standard oral fluid regimen and infants without oral fluid regimen (nil by mouth).
Within the comparison of supplemental oral fluid regimen versus standard oral fluid regimen (comparison 2 in Types of interventions section), we separated infants who were breastfed versus infants who were formula‐fed.
Within the comparison of IV fluid supplementation (IV infusion) with or without standard oral fluid regimen versus supplemented oral fluid regimen (comparison 3 in Types of interventions section), we separated infants who received IV supplementation with standard oral fluid regimen versus infants without oral fluid regimen (nil by mouth). We separated IV supplementation through infusion versus bolus.
With the comparison of IV fluid supplementation with or without standard oral fluid regimen versus standard oral fluid regimen (comparison 4 in Types of interventions section), we separated IV supplement through infusion versus bolus.
-
Subgroups with different modes of phototherapy:
infants who received conventional phototherapy at the usual dose (single phototherapy);
infants who received intensive (double or beyond) conventional phototherapy;
infants who received fibreoptic phototherapy;
infants who received phototherapy via a light‐emitting diode (LED); and
infants under treatment via a combination of modes.
Infants who were preterm (less than 34 weeks of postmenstrual age), late preterm (34 to less than 37 weeks postmenstrual age), and term infants (postmenstrual age of 37 weeks and beyond).
Infants with proven haemolytic cause of hyperbilirubinaemia versus infants without a proven haemolytic cause.
Sensitivity analysis
Had relevant studies been available, we would have performed sensitivity analyses for the primary and secondary outcomes provided a sufficient number of trials were included to assess the impact of excluding trials at high risk of:
selection bias (in either one or both criteria of random sequence generation and allocation concealment);
attrition bias (incomplete outcome data).
Results
Description of studies
Results of the search
From the preliminary search through multiple databases, we identified 1616 records, including seven records that were identified from sources other than the major databases. Among the seven additional records, two were from clinicaltrials.gov registry (none from other trial registries) and five were from the reference list of the studies that were subsequently short‐listed. After removing duplicates, 1216 separate records were screened against titles and abstracts for relevance. Among these, 1191 articles were excluded outright and three records needed further information to determine their eligibility and so were placed under 'awaiting classification.' Twenty‐two articles were short‐listed for full‐text assessment, and seven studies were judged to be eligible by the review team, with the rest excluded with reasons. The PRISMA flow diagram of the studies from the initial search to the meta‐analysis is shown in Figure 1. A description of all the included studies is displayed in the Characteristics of included studies table, and the excluded studies with the reasons for exclusion are given in the Characteristics of excluded studies table.
1.
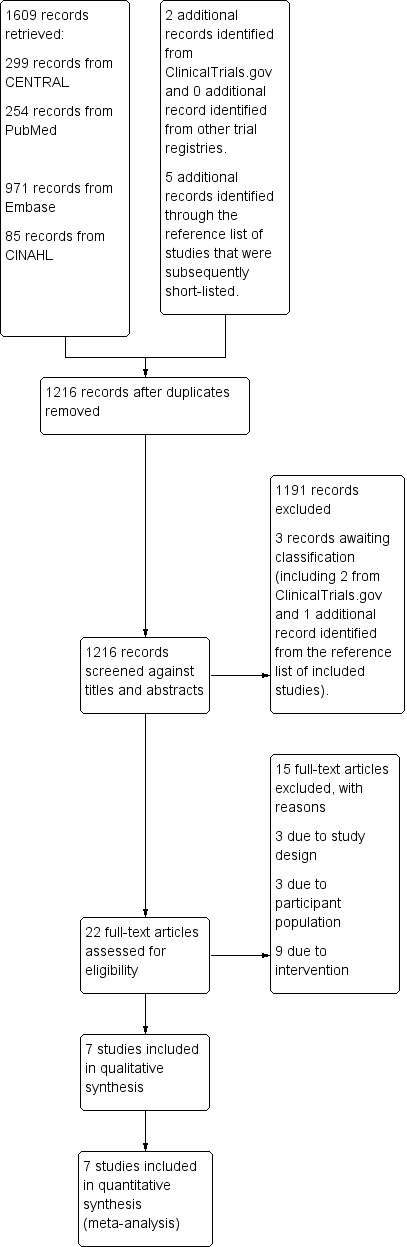
Study flow diagram.
Included studies
There were seven studies from five countries, including India (Mehta 2005; Patel 2014), Iran (Iranpour 2004; Saeidi 2009), Iraq (Easa 2013), Jordan (Al‐Masri 2012), and Malaysia (Boo 2002). Six studies were single‐centre RCTs and one was a two‐centre RCT (Al‐Masri 2012). The total number of participants in each study ranged from 54 (Boo 2002) to 100 (Saeidi 2009).
All studies enrolled term infant (37 weeks' gestation and beyond) of both sexes who were either breastfeeding exclusively (Iranpour 2004; Saeidi 2009; Al‐Masri 2012; Patel 2014), or were receiving mixed feeding (Boo 2002; Mehta 2005; Easa 2013). Serum bilirubin value was clearly stated as part of the inclusion criteria in all but one study (Al‐Masri 2012), and the threshold total serum bilirubin for inclusion was similar among the rest of the studies at approximately 300 μmol/L (18 mg/dL).
There were two major comparisons.
IV fluid supplementation versus no fluid supplementation (Iranpour 2004; Mehta 2005; Saeidi 2009; Al‐Masri 2012; Easa 2013; Patel 2014).
IV fluid supplementation versus oral fluid supplementation (Boo 2002).
In terms of the amount of IV fluid supplementation, four studies provided the supplementation in a certain percentage of the total maintenance fluid for the day (Boo 2002; Iranpour 2004; Al‐Masri 2012; Easa 2013), and this ranged from an additional 10% (Boo 2002) to 25% (Iranpour 2004; Easa 2013) of the maintenance fluid. One study provided additional 20 mL/kg/day (Patel 2014), one study administered an additional IV fluid regimen of 80 mL/kg/day on day one, and increasing by 10 mL/kg/day to a maximum of 120 mL/kg/day (Saeidi 2009), and the remaining study calculated the supplementation by presuming a fluid deficit of 50 mL/kg (mild dehydration), with an additional 50% of daily maintenance and an extra 20% as phototherapy allowance for the first eight hours of intervention (Mehta 2005). After eight hours, the infants were given an extra 30 mL/kg/day of oral feeds in the form of expressed breast milk or formula milk in additional of their usual feeding schedules.
In terms of the type of supplemented fluids, five studies used 1/5 (0.18%) normal saline and 5% dextrose (Boo 2002; Iranpour 2004; Mehta 2005; Saeidi 2009; Easa 2013), one study used 10% dextrose on days one and two and changed to 1/5 (0.18%) normal saline with 10% dextrose from day three onwards (Al‐Masri 2012), and the remaining study used normal saline (Patel 2014).
The duration of intervention varied from two to three hours (Patel 2014), eight hours (Mehta 2005), to 24 hours (Boo 2002; Iranpour 2004; Saeidi 2009) among the five studies with clear documentation. For the remaining two studies, the durations of intervention, as inferred by the supplemented fluid regimen, were at least three days (Al‐Masri 2012) and at least four days (Easa 2013).
All studies incorporated variable amount of details on the phototherapy device, including "double standard phototherapy" (Al‐Masri 2012), blue lights (Philips) (Boo 2002; Iranpour 2004; Mehta 2005; Easa 2013), LED light (Patel 2014), and fluorescent light (Saeidi 2009). Five studies stated the distance of the phototherapy source from the infants, including 20 cm (Iranpour 2004; Easa 2013) and 25 cm (Boo 2002; Saeidi 2009; Patel 2014).
In terms of outcomes, two studies evaluated the number of infants with bilirubin encephalopathy or infants with abnormal neurological signs (Boo 2002; Mehta 2005), which was the only predefined primary outcome of our review that was reported by the included studies. In the two studies that examined bilirubin encephalopathy, no study provided a clear definition of the outcome. One study stated the outcome as, "abnormal neurological signs suggestive of kernicterus" (Boo 2002). As the diagnostic criteria of kernicterus were not clearly stated, we categorised this under "infants with abnormal neurological signs." Another study did not provide a definition for the outcome (Mehta 2005). However, in both studies, no infant in either group developed this outcome. No studies included kernicterus or cerebral palsy as the outcomes in the methods or reported them in the results, probably because there was no incidence of kernicterus or cerebral palsy in the study population.
Among our secondary outcomes, five studies evaluated bilirubin level (Boo 2002; Iranpour 2004; Mehta 2005; Al‐Masri 2012; Easa 2013), five studies evaluated the rate of change of serum bilirubin (Boo 2002; Iranpour 2004; Mehta 2005; Saeidi 2009; Patel 2014), all seven studies examined the number of infants who required exchange transfusion (Boo 2002; Iranpour 2004; Mehta 2005; Saeidi 2009; Al‐Masri 2012; Easa 2013; Patel 2014), and three studies assessed the duration of phototherapy in hours (Iranpour 2004; Mehta 2005; Patel 2014). One study reported the frequency of breastfeeding during the first three days of hospital stay (Mehta 2005). The other secondary outcomes were either not examined, or not reported in sufficient details by any of the included studies. The only information related to the possible adverse effects of the intervention was provided in sufficient detail by Boo 2002, which reported the incidence of vomiting and abdominal distension. All studies provided bilirubin threshold levels for discontinuing phototherapy. Five studies used 239 μmol/L (14 mg/dL) as the threshold (Boo 2002; Iranpour 2004; Saeidi 2009; Easa 2013; Patel 2014), while Al‐Masri 2012 used 205 μmol/L (12 mg/dL), and Mehta 2005 used 259 μmol/L (15 mg/dL) as the thresholds. However, no studies stated whether the same thresholds applied for commencing phototherapy.
For the outcome 'number of infants who required exchange transfusion,' only two studies provided the criteria for exchange transfusion (Boo 2002; Mehta 2005). In Boo 2002, infants underwent exchange transfusion if their serum bilirubin remained at or above 340 μmol/L after commencement of intervention, although the duration of intervention before commencing exchange transfusion was not stated. Mehta 2005 used the increase in total serum bilirubin value in the first four hours of study (greater than 34 mmol/L (2 mg/dL)) and total serum bilirubin value at the eight‐hour mark of the study (342 mmol/L or greater (20 mg/dL or greater)) as thresholds for exchange transfusion.
Excluded studies
Fifteen studies were excluded predominantly on the basis of one of the following criteria:
study design (three studies): commentary, survey, or non‐randomised comparative trial;
population (three studies): hyperbilirubinaemia or phototherapy (or both) were not specified as inclusion criteria;
intervention (nine studies): studies examined different type of enteral or parenteral fluid administration, different timing of feed commencement, or different feed regimen without comparing different levels of fluid.
Studies awaiting classification
Three studies were placed under 'awaiting classification' (Demirsoy 2011; NCT01550627; IRCT2013022711145N5). All three studies were single‐centre RCTs that evaluated the effects of giving additional fluid to either term (Demirsoy 2011; IRCT2013022711145N5) or preterm infants (NCT01550627) who had hyperbilirubinaemia. The records of two studies were identified from ClinicalTrials.gov and both studies were stated to be completed (NCT01550627; IRCT2013022711145N5). The third study (Demirsoy 2011) was identified from the reference list of an included study (Al‐Masri 2012). We are awaiting further information from the study authors to enable appropriate determination of their eligibility.
Ongoing studies
We did not identify any ongoing studies.
Risk of bias in included studies
The overall risk of bias was mixed. There was either low or unclear risk of selection and detection biases, low risk of attrition bias, and high risk of performance bias across all included studies. Figure 2 shows the proportions of studies with different risks of bias according to each domain, and Figure 3 shows the risk of bias profile according to the study. Additionally, we have provided a detailed description of the risk of bias of each study in the Characteristics of included studies table. Our risk of bias assessments under each domain are summarised below.
2.
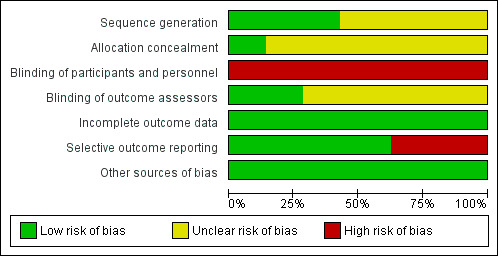
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
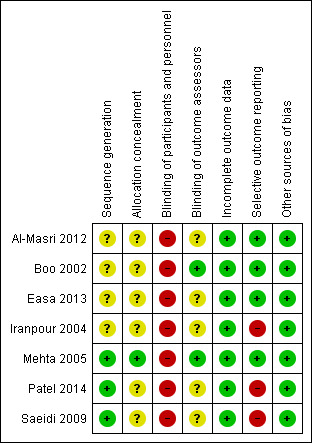
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For random sequence generation, three out of seven included studies were at low risk of bias as the authors provided clear description of random sequence generation methods, either using random number tables (Saeidi 2009; Patel 2014) or stratified block randomisation methods, which provided reassurance that the sequence generated was most likely random (Mehta 2005). The remaining four studies stated that infants were allocated randomly but did not provide further information on the methods of sequence generation. For allocation concealment, only one study provided sufficient information to enable an assessment of whether random sequence generation and allocation were carried out independently (Mehta 2005). Mehta 2005 stated that participants were allocated using serially numbered, opaque envelopes that were only opened after enrolment. Among the remaining studies, Boo 2002 mentioned the use of serially numbered and sealed envelopes without mentioning whether or not they were opaque, while the other studies did not provide any information specific to allocation.
Blinding
It was practically impossible to blind the care personnel on the fluid regimen that each infant received, and thus all seven studies were at high risk of bias in blinding of care personnel. Blinding of outcome assessors, which would have been possible for at least some outcomes such as serum bilirubin, was only reported as achieved in two studies (Boo 2002; Mehta 2005), with no specific information provided in the remaining five studies.
Incomplete outcome data
In all seven studies, all infants there were initially randomised appeared to have been included in the analysis where applicable.
Selective reporting
Four out of seven studies were judged at low risks of bias in selective outcome reporting as they reported major outcomes expected, including the clinical outcomes, with sufficient details (Boo 2002; Mehta 2005; Al‐Masri 2012; Easa 2013). In the remaining three studies, key clinical outcomes such as the incidence of bilirubin encephalopathy or the number of infants who required exchange transfusion (or both) were not reported (Iranpour 2004; Saeidi 2009; Patel 2014), or the outcomes were reported incompletely which precluded inclusion of the relevant data in the meta‐analysis (Saeidi 2009; Patel 2014).
Other potential sources of bias
We screened for other potential sources of bias including unit of analysis issue, extreme baseline imbalance, and any evidence of fraud and found no other obvious sources of bias.
Effects of interventions
In this review, a total of 494 infants were assessed in seven included studies.
Intravenous fluid supplementation versus no fluid supplementation (comparison 1)
See Table 1 (number of infants with bilirubin encephalopathy, serum bilirubin four hours postintervention, number of infants who required exchange transfusion, and frequency of breastfeeding per day on day three of admission). The quality of evidence, where applicable, was rated for these outcomes and reported alongside the corresponding results below.
Primary outcomes
1. Incidence of acute bilirubin encephalopathy
No infant developed bilirubin encephalopathy in either group, and so the results were not estimable (outcome displayed in the 'Summary of findings' table due to its importance, but quality of evidence not rated) (Analysis 1.1).
1.1. Analysis.
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 1 Number of infants with bilirubin encephalopathy.
2. Incidence of kernicterus
There were no data on incidence of kernicterus.
3. Proportion of infants with cerebral palsy
There were no data on proportion of infants with cerebral palsy.
Secondary outcomes
4. Bilirubin level
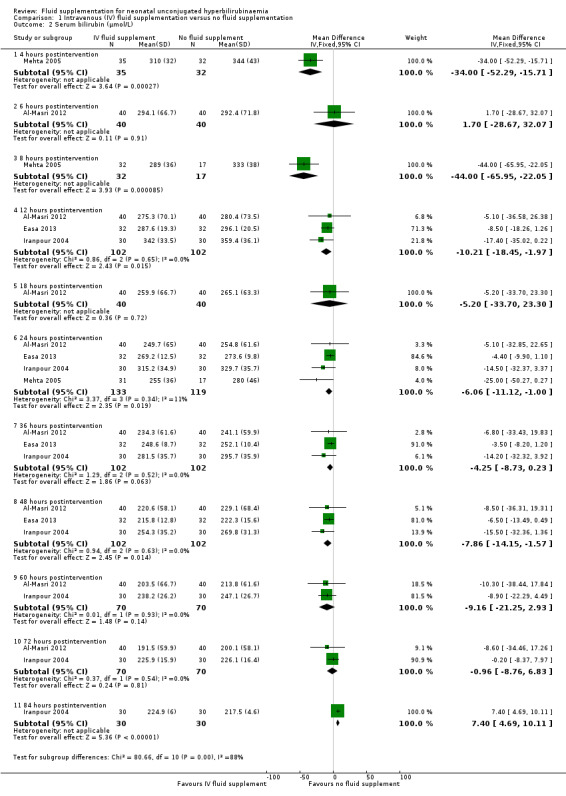
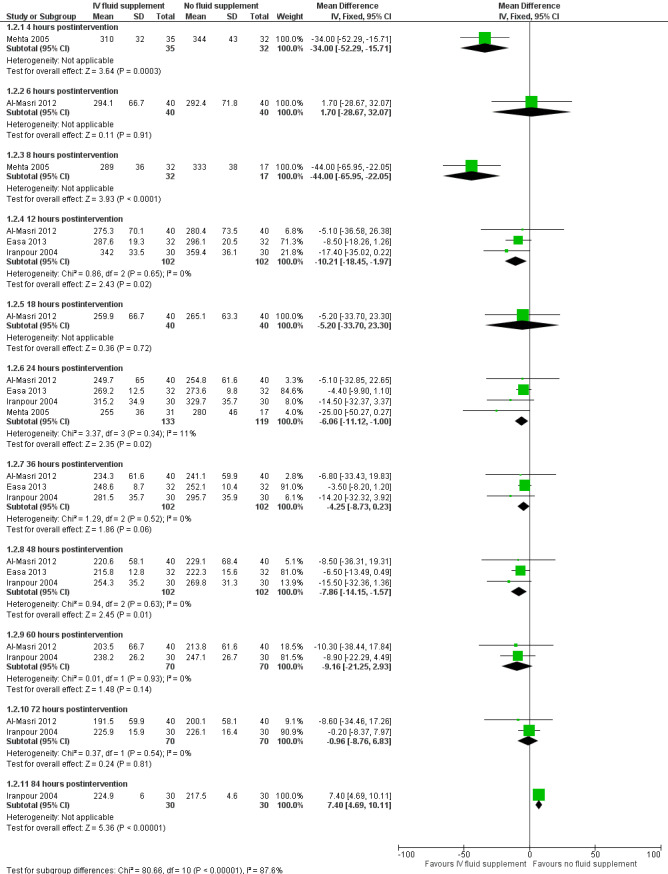
(Analysis 1.2; Figure 4).
1.2. Analysis.
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 2 Serum bilirubin (μmol/L).
4.
Forest plot of comparison: 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, outcome: 1.2 Serum bilirubin (μmol/L).
i. Four hours postintervention (Analysis 1.2.1)
Based on one study, infants who received IV fluid supplementation had a significantly lower serum bilirubin compared to infants without fluid supplementation (MD ‐34.00 μmol/L, 95% CI ‐52.29 to ‐15.71; participants = 67) (low quality of evidence, downgraded one level for indirectness as serum bilirubin was a surrogate for clinical outcomes, and another one level for suspected publication bias) (Mehta 2005).
ii. Six hours postintervention (Analysis 1.2.2)
In the one study that reported serum bilirubin at six hours, there was no significant difference in serum bilirubin between infants who received IV fluid supplementation and infants who were not supplemented (MD 1.70 μmol/L, 95% CI ‐28.67 to 32.07; participants = 80) (Al‐Masri 2012).
iii. Eight hours postintervention (Analysis 1.2.3)
In the one study that reported serum bilirubin at eight hours, infants who received IV fluid supplementation had a significantly lower serum bilirubin compared to infants without fluid supplementation (MD ‐44.00 μmol/L, 95% CI ‐65.95 to ‐22.05; participants = 49) (Mehta 2005).
iv. Twelve hours postintervention (Analysis 1.2.4)
Based on the results of three studies, infants who received IV fluid supplementation had a slightly lower serum bilirubin at 12 hours compared to infants who were not supplemented (MD ‐10.21 μmol/L, 95% CI ‐18.45 to ‐1.97; participants = 204; I² = 0%) (Iranpour 2004; Al‐Masri 2012; Easa 2013).
v. Eighteen hours postintervention (Analysis 1.2.5)
In the one study that reported serum bilirubin at 18 hours, there was no significant difference in serum bilirubin value between infants who received IV fluid supplementation and infants who were not supplemented (MD ‐5.20 μmol/L, 95% CI ‐33.70 to 23.30; participants = 80) (Al‐Masri 2012).
vi. Twenty‐four hours postintervention (Analysis 1.2.6)
Based on four studies, infants who received IV fluid supplementation had a slightly lower serum bilirubin at 24 hours compared to infants who were not supplemented (MD ‐6.06 μmol/L, 95% CI ‐11.12 to ‐1.00; participants = 252; I² = 11%) (Iranpour 2004; Mehta 2005; Al‐Masri 2012; Easa 2013).
vii. Thirty‐six hours postintervention (Analysis 1.2.7)
Based on three studies, there was no significant difference in serum bilirubin at 36 hours between infants who received IV fluid supplementation and infants who were not supplemented (MD ‐4.25 μmol/L, 95% CI ‐8.73 to 0.23; participants = 204; I² = 0%) (Iranpour 2004; Al‐Masri 2012; Easa 2013).
viii. Forty‐eight hours postintervention (Analysis 1.2.8)
Based on three studies, infants who received IV fluid supplementation had a slightly lower serum bilirubin at 48 hours compared to infants who were not supplemented (MD ‐7.86 μmol/L, 95% CI ‐14.15 to ‐1.57; participants = 204; I² = 0%) (Iranpour 2004; Al‐Masri 2012; Easa 2013).
ix. Sixty hours postintervention (Analysis 1.2.9)
Based on two studies, there was no significant difference in serum bilirubin at 60 hours between infants who received IV fluid supplementation and infants who were not supplemented (MD ‐9.16 μmol/L, 95% CI ‐21.25 to 2.93; participants = 140; I² = 0%) (Iranpour 2004; Al‐Masri 2012).
x. Seventy‐two hours postintervention (Analysis 1.2.10)
Based on two studies, there was no significant difference in serum bilirubin at 72 hours between infants who received IV fluid supplementation and infants who were not supplemented (MD ‐0.96 μmol/L, 95% CI ‐8.76 to 6.83; participants = 140; I² = 0%) (Iranpour 2004; Al‐Masri 2012).
xi. Eighty‐four hours postintervention (Analysis 1.2.11)
In the one study that reported serum bilirubin at 84 hours, infants who received IV fluid supplementation had a slightly higher serum bilirubin compared to infants who were not supplemented (MD 7.40 μmol/L, 95% CI 4.69 to 10.11; participants = 60) (Iranpour 2004).
5. Difference in serum bilirubin
One study reported the difference in serum bilirubin in percentage at the first four, eight and 24 hours of study (Mehta 2005). It found that infants who received IV fluid supplementation had a significantly greater percentage drop in serum bilirubin compared to non‐supplemented infants in all three periods of assessment (first four hours: MD 10.50%, 95% CI 6.66 to 14.34; participants = 67; first eight hours: MD 13.00%, 95% CI 7.49 to 18.51; participants = 49; first 24 hours: MD 8.00%, 95% CI 1.11 to 14.89; participants = 48) (Analysis 1.3).
1.3. Analysis.
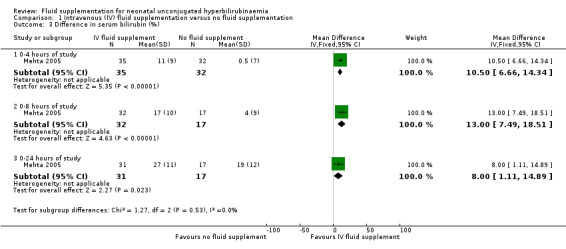
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 3 Difference in serum bilirubin (%).
6. Rate of change of serum bilirubin
There was one study that examined the rate of change of serum bilirubin for the first 12 hours. There was no significant difference between supplemented and non‐supplemented infants (MD 0.50 μmol/L/hour, 95% CI ‐0.21 to 1.21; participants = 60) (Analysis 1.4) (Iranpour 2004).
1.4. Analysis.
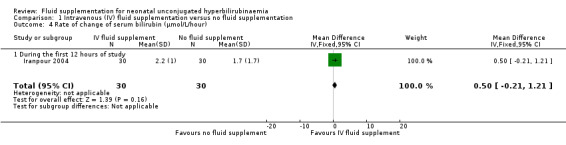
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 4 Rate of change of serum bilirubin (μmol/L/hour).
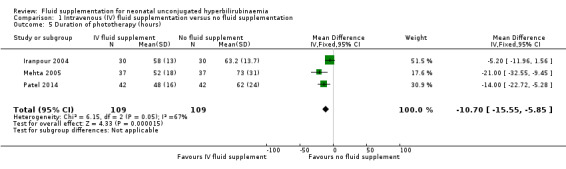
7. Duration of phototherapy
Based on three studies, infants who received IV fluid supplementation had a significantly shorter duration of phototherapy compared to non‐supplemented infants (MD ‐10.70 hours, 95% CI ‐15.55 to ‐5.85; participants = 218; I² = 67%) (Analysis 1.5) (Iranpour 2004; Mehta 2005; Patel 2014). There was a moderate degree of heterogeneity, as indicated by the I² statistic of 67%. The degree of heterogeneity appeared to be caused by one study (Iranpour 2004), as the I² statistic was reduced to 0% after removal of this study from the analysis. In terms of results, Iranpour 2004 showed no significant difference between the two groups in the duration of phototherapy, while the other two studies showed a significant reduction in the duration of phototherapy favouring the IV supplemented group.
1.5. Analysis.
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 5 Duration of phototherapy (hours).
We explored possible differences between Iranpour 2004 and two other studies that could have explained the heterogeneity observed. First, there were no major differences in the durations of phototherapy between Iranpour 2004 and the other two studies, discounting this as a possible contributory factor in the degree of heterogeneity. Next, in terms of setting, Iranpour 2004 was conducted in Iran, while Mehta 2005 and Patel 2014 were conducted in India. However, the difference in the regions where the studies were conducted did not provide a direct biological reason to explain the observed heterogeneity. One possible factor was the phototherapy thresholds in the NICU protocol, which might have differed between the two countries, although we were unable to confirm this as the detailed phototherapy protocol, including serum bilirubin thresholds, were not provided by the authors. Next, there was a difference in the risk of bias profile between Iranpour 2004 and the other two studies, as Iranpour 2004 had unclear risk of bias in both random sequence generation and allocation concealment, while Mehta 2005 and Patel 2014 had at least one low‐risk domain in either random sequence generation or allocation concealment (or both). However, we believed the difference in the risk of bias profile was by itself insufficient to explain the degree of heterogeneity observed. Next, in terms of fluid supplementation regimen, Iranpour 2004 administered an additional 25% of the infants' maintenance fluid using 1/5 saline and 5% dextrose for 24 hours, and this amounted to at least 20 mL/kg of additional fluid, while Mehta 2005 provided an additional 70 mL/kg/day IV 1/5 saline and 5% dextrose for eight hours, and Patel 2014 provided an additional 20 mL/kg of normal saline for two to three hours. The volume of additional fluid given (much higher in Mehta 2005 as compared to Iranpour 2004) and possibly type of fluid given (normal saline in Patel 2014 versus 1/5 saline and 5% dextrose in Iranpour 2004) might have contributed the difference in the effect estimates. Overall, there was no single factor that accounted for the degree of heterogeneity, although the volume or type (or both) of additional fluid might have played a role. Overall, we were unable to provide a clear explanation of the likely source of heterogeneity, but we considered pooling of studies still reasonable, as the direction of estimate between Iranpour 2004 and the other two studies did not differ substantially.
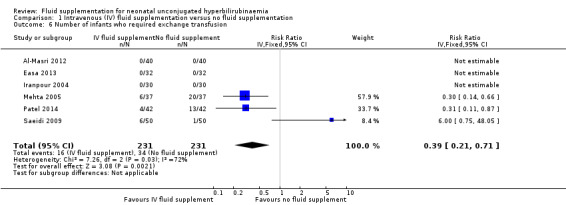
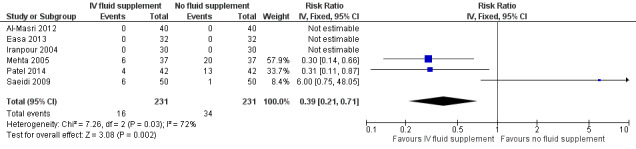
8. Proportion of infants who required exchange transfusion
(Analysis 1.6; Figure 5).
1.6. Analysis.
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 6 Number of infants who required exchange transfusion.
5.
Forest plot of comparison: 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, outcome: 1.6 Number of infants who required exchange transfusion.
There were six included studies for this outcome (Iranpour 2004; Mehta 2005; Saeidi 2009; Al‐Masri 2012; Easa 2013; Patel 2014), although three out of six studies contributed to the analysis (Mehta 2005; Saeidi 2009; Patel 2014), as there were no events in either group in the other three studies. Infants who received IV fluid supplementation were significantly less likely to require exchange transfusion compared to non‐supplemented infants, although the difference became non‐significant when the results were expressed as RD (RR 0.39, 95% CI 0.21 to 0.71; RD ‐0.01, 95% CI ‐0.04 to 0.02, participants = 462; studies = 6; I² = 72%) (low quality of evidence, downgraded one level due to inconsistency, and another level due to suspected publication bias).
There was a moderate degree of heterogeneity, as indicated by the I² statistic of 72%. The degree of heterogeneity appeared to be caused by one study (Saeidi 2009), as the I² statistic was reduced to 0% after removal of this study from the analysis. In terms of results, there was significantly lower number of fluid‐supplemented infants who required exchange transfusion in Mehta 2005 and Patel 2014, in contrast to Saeidi 2009, which reported higher number of fluid‐supplemented infants who required exchange transfusion, although the difference did not reach statistical significance. Saeidi 2009 was weighted the least among the three studies in view of its lowest control event rate.
We explored possible difference between Saeidi 2009 and the other studies that could have accounted for the degree of heterogeneity observed. Since the criteria for exchange transfusion were not stated in Saeidi 2009 and Patel 2014, we were unable to determine whether these accounted for the degree of heterogeneity. However, the rate of exchange transfusion in Saeidi 2009 was much lower at 7% (7/100 infants), as compared to Mehta 2005 (35.1%, 26/74 infants) and Patel 2014 (20.2%, 17/84 infants), raising the possibility that the bilirubin threshold for exchange transfusion in Saeidi 2009 was higher than that in Mehta 2005 and Patel 2014.
In terms of other possible factors, all three studies recruited healthy, term infants, and the amount and type of additional fluid, as detailed above under Included studies, could not explain the differences in effect estimates. Similarly, the delivery of cointerventions, including the phototherapy device and delivery as well as the bilirubin threshold for phototherapy (see Included studies) could not account for the observed heterogeneity.
Despite our inability to clearly identify a plausible factor that explained the degree of heterogeneity, we suspected that a difference in serum bilirubin threshold for exchange transfusion in Saeidi 2009 compared to others might have played a part in the observed difference in the rate of exchange transfusion, although this was only our deduction, having no clear information in the papers. Overall, we still considered pooling of the studies acceptable and presented the combined effect estimate of the three studies that contributed to this outcome, but we accepted the pooled results with lower confidence, as reflected by our downgrading on the quality of evidence due to inconsistency/heterogeneity.
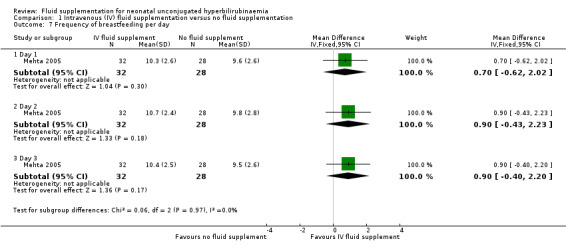
9. Frequency of breastfeeding per day
(Analysis 1.7; Figure 6).
1.7. Analysis.
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 7 Frequency of breastfeeding per day.
6.
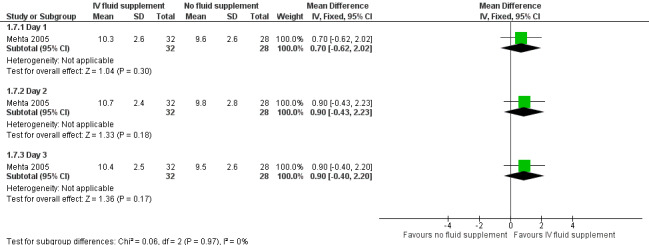
Forest plot of comparison: 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, outcome: 1.7 Frequency of breastfeeding per day.
In the one study that reported frequency of breastfeeding per day, there were no significant differences between the two groups on the frequencies of breastfeeding throughout the three days of evaluation (day 1: MD 0.70 feeds, 95% CI ‐0.62 to 2.02; participants = 60; day 2: MD 0.90 feeds, 95% CI ‐0.43 to 2.23; participants = 60; day 3: MD 0.90 feeds, 95% CI ‐0.40 to 2.20; participants = 60) (moderate quality of evidence, downgraded one level for imprecision) (Mehta 2005).
Intravenous fluid supplementation versus oral fluid supplementation (comparison 2)
One study provided results under this comparison (Boo 2002). See Table 2 (number of infants with abnormal neurological signs (classified under bilirubin encephalopathy for the purpose of this review), serum bilirubin value four hours postphototherapy, and rate of change of serum bilirubin during first four hours of admission). The quality of evidence, where applicable, was rated for these outcomes and reported alongside the corresponding results below. Serum bilirubin and the rate of change of serum bilirubin were chosen for Table 2 instead of the number of infants who required exchange transfusion because the criteria for exchange transfusion included both parameters.
Primary outcomes
1. Number of infants with abnormal neurological signs (bilirubin encephalopathy)
No infant developed bilirubin encephalopathy in either group, and so the results were not estimable (outcome displayed in the 'Summary of findings' table due to its importance, but quality of evidence not rated) (Analysis 2.1).
2.1. Analysis.
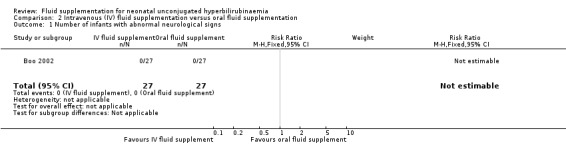
Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 1 Number of infants with abnormal neurological signs.
2. Incidence of kernicterus
There were no data on incidence of kernicterus.
3. Proportion of infants with cerebral palsy
There were no data on proportion of infants with cerebral palsy.
Secondary outcomes
4. Bilirubin level four hours after phototherapy
In the one study that reported this outcome, there was no significant difference between the two groups in serum bilirubin four hours after phototherapy (MD 11.00 μmol/L, 95% CI ‐21.58 to 43.58; participants = 54) (moderate quality of evidence, downgraded one level for indirectness as serum bilirubin was a surrogate for clinical outcomes) (Analysis 2.2) (Boo 2002).
2.2. Analysis.
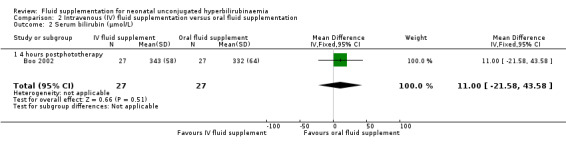
Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 2 Serum bilirubin (μmol/L).
5. Rate of change of serum bilirubin during the first four hours of admission
In the one study that reported this outcome, there was no significant difference between the two groups in the rate of change in serum bilirubin in the first four hours of phototherapy (MD 0.80 μmol/L/hour, 95% CI ‐2.55 to 4.15; participants = 54) (moderate quality of evidence, downgraded one level for indirectness as serum bilirubin was a surrogate for clinical outcomes) (Analysis 2.3) (Boo 2002).
2.3. Analysis.
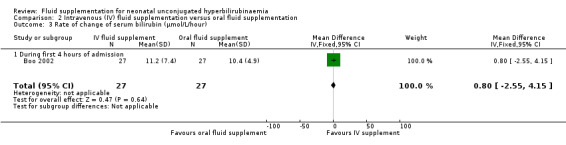
Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 3 Rate of change of serum bilirubin (μmol/L/hour).
6. Proportion of infants who required exchange transfusion
In the one study that reported this outcome, there was no significant difference between the two groups in the number of infants who required exchange transfusion (RR 1.60, 95% CI 0.60 to 4.27; RD 0.11, 95% CI ‐0.12 to 0.34, participants = 54) (Analysis 2.4) (Boo 2002).
2.4. Analysis.
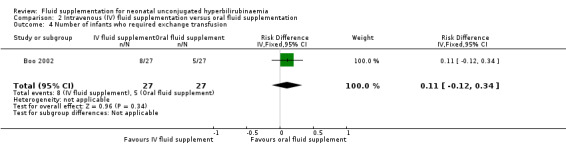
Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 4 Number of infants who required exchange transfusion.
7. Number of infants with feed intolerance and presented with vomiting
In the one study that reported this outcome, there was no infant in either group with vomiting (Analysis 2.5) (Boo 2002).
2.5. Analysis.
Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 5 Number of infants with feed intolerance: vomiting.
8. Number of infants with feed intolerance and presented with abdominal distension
In the one study that reported this outcome, there was no infant in either group with abdominal distension (Analysis 2.6) (Boo 2002).
2.6. Analysis.
Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 6 Number of infants with feed intolerance: abdominal distension.
Subgroup analyses
Due to insufficient studies, we did not perform any additional subgroup analysis as specified in our Methods.
Sensitivity analysis
We did not perform any sensitivity analysis because there was no study to be excluded based on our prespecified criteria in terms of selection and attrition biases.
Discussion
Summary of main results
This review shows that term, healthy newborn infants who received IV fluid supplementation while undergoing phototherapy had modestly lower serum bilirubin at four and eight hours after the commencement of intervention compared to infants who did not receive fluid supplementation. Beyond eight hours, serum bilirubin values between the two groups were very similar. Duration of phototherapy and the number of infants who required exchange transfusion appeared to be lower in IV fluid‐supplemented infants, although our confidence in the effect estimates was affected by moderate heterogeneity. Frequencies of breastfeeding were similar between supplemented and non‐supplemented infants based on the findings of one study. In another comparison, there was no evidence of differences between IV and oral fluid supplementation in all outcomes related to serum bilirubin, including the number of infants who required exchange transfusion.
Notably, all studies evaluated serum bilirubin as the major outcome. There were no cases of bilirubin‐related clinical morbidity or mortality such as bilirubin encephalopathy or kernicterus documented. As such, the reduction in serum bilirubin conferred by fluid supplementation at four and eight hours, as demonstrated in this review, did not translate into clinically important benefit in the population studied.
Overall completeness and applicability of evidence
Through a comprehensive search strategy, we identified seven eligible studies, including four studies that were not indexed in any major database. However, as all included studies were conducted in Asia, it is unclear whether our findings are generalisable to other parts of the world. A total of 494 newborn infants were assessed, although all were healthy, term infants, which limited the applicability of the findings in preterm infants, who might be at higher risk of developing bilirubin encephalopathy or kernicterus. Due to insufficient data, we were unable to undertake any subgroup analyses to further determine applicability of the findings to infants with different prognostic factors as laid out in the Subgroup analysis and investigation of heterogeneity section.
Quality of the evidence
There was overall low‐ to moderate‐quality evidence in the major outcomes which were reported by a small number of studies. The clearest risk of bias issue was the lack of blinding of care personnel in all studies, as blinding was impossible due to different fluid regimen employed. However, the lack of blinding of care personnel did not seriously affect the overall quality of evidence, as most included studies evaluated serum bilirubin, which was an objective parameter. Nonetheless, the use of serum bilirubin as the major outcome in all included studies has resulted in downgrading of the quality of evidence due to indirectness, as serum bilirubin was only a surrogate to the major clinical outcomes, namely, bilirubin encephalopathy and kernicterus. Besides indirectness, the other factors that resulted in downgrading of the quality of evidence included suspected publication bias, inconsistency (heterogeneity), and imprecision (see Table 1 and Table 2).
Potential biases in the review process
The evidence gathered in this review was the result of a comprehensive search from multiple databases with additional searches of non‐indexed articles and independent screening, selection, and assessment of eligible studies. However, there are three potentially eligible studies that were not included in our analyses as we are still awaiting their full‐texts. The inclusion of these studies might change the overall findings.
Agreements and disagreements with other studies or reviews
To‐date, there is no other review that examines the role of fluid supplementation in newborn infants with unconjugated hyperbilirubinaemia.
The American Academy of Pediatrics practice guideline recommends giving additional fluid, preferably breast or formula milk, only if the infant shows signs of dehydration. However, there is no systematic review or well‐conducted studies cited in support of this recommendation (AAP 2004).
Authors' conclusions
Implications for practice.
There is no evidence that intravenous fluid supplementation affects the primary outcomes of bilirubin encephalopathy, kernicterus, or cerebral palsy in healthy, term newborn infants with unconjugated hyperbilirubinaemia requiring phototherapy. This is mainly due to the very low risk of bilirubin‐associated clinical complications such as bilirubin encephalopathy or kernicterus in healthy, term infants, as shown in this review. Based on low‐ to moderate‐quality evidence, differences in total serum bilirubin levels between fluid‐supplemented and control groups (higher levels in the fluid‐supplemented group in one comparison) were detected at some time points but not at others. The clinical significance of this is uncertain. There is no evidence of adverse effects from fluid supplementation, and no evidence of a difference between the effectiveness of intravenous and oral fluid supplementations in reducing serum bilirubin or clinical complications.
Implications for research.
The findings of this review highlight the need to select appropriate population in studies that examine fluid supplementation in newborn infants with unconjugated hyperbilirubinaemia. Future randomised controlled trials should focus on the relevant population groups, for example, preterm or low birth weight infants, infants with haemolytic hyperbilirubinaemia, and infants with dehydration (for comparison of different fluid supplementation regimens), as these infants are at a higher risk of bilirubin‐associated clinical morbidities, such as bilirubin encephalopathy, kernicterus, and resultant cerebral palsy. These infants were excluded in all studies gathered in the present review. Future studies should include the aforementioned clinical outcomes as their primary outcomes alongside serum bilirubin.
Acknowledgements
We gratefully acknowledge the assistance provided by Ms Collen Ovelman, Managing Editor; Ms Yolanda Brosseau, Trial Search Co‐ordinator; and Dr Roger Soll, Co‐ordinating Editor of the Cochrane Neonatal Review Group in the development of this review.
We are grateful to Dr Praveen Kumar for providing additional information on his study (Mehta 2005).
Appendices
Appendix 1. CENTRAL search strategy
MeSH descriptor: [Infant, newborn]explode all trees
newborn*: ti,ab,kw
neonat*: ti,ab,tw
infant*: ti,ab,kw
#1 OR #2 OR #3 OR #4
MeSH descriptor: [Jaundice] explode all trees
jaundice: ti,ab,kw
Mesh descriptor: [hyperbilirubinemia, neonatal] explode all trees
hyperbilirubinaemia: ti,ab,kw
Mesh descriptor: [bilirubin] explode all trees
bilirubin: ti,ab,kw
Mesh descriptor: [icterus] explode all trees
icter*: ti,ab,kw
Mesh descriptor: [phototherapy] explode all trees
phototherapy:ti,ab,kw
#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
fluid: ti,ab,kw
Mesh descriptor: [feeding methods] explode all trees
feed*: ti,ab,kw
nutrition: ti,ab,kw
Search milk [TIAB]
Mesh descriptor: [rehydration] explode all trees
rehydrat*: ti,ab,kw
hydrat*: ti,ab,kw
volume: ti,ab,kw
supplement*: ti,ab,kw
#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26
#5 AND #16 AND #27
Limit to "trials"
Appendix 2. MEDLINE search strategy
Search "Infant, newborn"[Mesh]
Search newborn* [TIAB]
Search neonat* [TIAB]
Search infant* [TIAB]
Search #1 OR #2 OR #3 OR #4
Search Jaundice [Mesh]
Search jaundice [TIAB]
Search hyperbilirubinaemia, neonatal [Mesh]
Search hyperbilirubinaemia [TIAB]
Search bilirubin [Mesh]
Search bilirubin [TIAB]
Search icterus [Mesh]
Search icter* [TIAB]
Search phototherapy [Mesh]
Search phototherapy [TIAB]
Search #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
Search fluid [TIAB]
Search feeding methods [Mesh]
Search feed* [TIAB]
Search nutrition [TIAB]
Search milk [TIAB]
Search rehydration [Mesh]
Search rehydrat* [TIAB]
Search hydrat* [TIAB]
Search volume [TIAB]
Search supplement* [TIAB]
Search #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26
Search clinical trial [PT]
Search clinical trials [Mesh]
Search randomized [TIAB]
Search randomly [TIAB]
Search trial [TI]
Search #28 OR #29 OR #30 OR #31 OR #32
Search #5 AND #16 AND #27 AND #33
Appendix 3. Embase search strategy
Explode: "Infant, newborn"/all subheadings
(newborn*) in TI, AB
(neonat*) in TI, AB
(infant*) in TI, AB
Search #1 OR #2 OR #3 OR #4
Explode "Jaundice"/all subheadings
(jaundice) in TI, AB
Explode: "hyperbilirubinaemia, neonatal"/all subheadings
(hyperbilirubinaemia) in TI, AB
Explode: "bilirubin"/all subheadings
(bilirubin) in TI, AB
Explode: "icterus"/all subheadings
(icter*) in TI, AB
Explode: "phototherapy"/all subheadings
(phototherapy) in TI, AB
Search #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
(fluid) in TI, AB
Explode: "feeding methods"/all subheadings
(feed*) in TI, AB
(nutrition) in TI, AB
(milk) in TI, AB
Explode: "rehydration"/all subheadings
(rehydrat*) in TI, AB
(hydrat*) in TI, AB
(volume) in TI, AB
(supplement*) in TI, AB
Search #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26
Explode "RANDOMIZED‐CONTROLLED‐TRIAL"/ all subheadings
Explode "RANDOMIZATION"/ all subheadings
Explode "CONTROLLED‐STUDY"/ all subheadings
Explode "MULTICENTER‐STUDY"/ all subheadings
Explode "DOUBLE‐BLIND‐PROCEDURE"/ all subheadings
Explode "SINGLE‐BLIND‐PROCEDURE"/ all subheadings
(RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) in TI,AB
(SINGL* or DOUBL* or TREBL* or TRIPL*) near (BLIND* or MASK*) in TI,AB
Search #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35
Search #5 AND #16 AND #27 AND #36
Appendix 4. CINAHL search strategy
MH "Infant, newborn"/explode
TI newborn* or AB newborn*
TI neonat* or AB neonat*
TI infant* or AB infant*
#1 OR #2 OR #3 OR #4
MH "Jaundice"/explode
TI jaundice or AB jaundice
MH "hyperbilirubinaemia, neonatal"/all subheadings
TI hyperbilirubinaemia or AB hyperbilirubinaemia
MH "bilirubin"/explode
TI bilirubin or AB bilirubin
MH "icterus"/explode
TI icter* or AB icter*
MH "phototherapy"/all subheadings
TI phototherapy or AB phototherapy
#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
TI fluid or AB fluid
MH "feeding methods"/all subheadings
TI feed* or AB feed*
TI nutrition or AB nutrition
TI milk or AB milk
MH "rehydration"/ all subheadings
TI rehydrat* or AB rehydrat*
TI hydrat* or AB hydrat*
TI volume or AB volume
TI supplement* or AB supplement*
#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26
PT Clinical trial
AB randomised or AB randomised or AB random*
TI trial
MH "Clinical Trials"/explode
#28 OR #29 OR #30 OR #31
#5 AND #16 AND #27 AND #32
Appendix 5. Detailed description on the criteria for judging the risk of bias of included trials
Was the allocation sequence randomly generated?
-
Yes, low risk of bias:
a random (unpredictable) assignment sequence;
examples of adequate methods of sequence generation are computer‐generated random sequence, coin toss (for studies with two groups), rolling a dice (for studies with two or more groups), drawing of balls of different colours, dealing previously shuffled cards.
-
No, high risk of bias:
quasi‐randomised approach: examples of inadequate methods are: alternation, birth date, social insurance/security number, date in which they are invited to participate in the study, and hospital registration number.
non‐random approaches: allocation by judgement of clinician; by preference of participant; based on results of a laboratory test or a series of tests.
-
Unclear:
insufficient information about the sequence generation process to permit judgement.
2. Was the treatment allocation adequately concealed?
-
Yes, low risk of bias:
assignment must be generated independently by a person not responsible for determining the eligibility of participants. This person has no information about people included in trial and has no influence one assignment sequence or on decision about whether person is eligible to enter trial. Examples of adequate methods of allocation concealment are: central allocation, including telephone, web‐based and pharmacy‐controlled randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque sealed envelopes.
-
No, high risk of bias:
examples of inadequate methods of allocation concealment are: alternate medical record numbers, unsealed envelopes; date of birth; case record number; alternation or rotation; an open list of random numbers any information in study that indicated that investigators or participants could influence intervention group.
-
Unclear:
randomisation stated but no information on method of allocation used is available.
3. Blinding: was knowledge of the allocated interventions adequately prevented during the study?
Was the participant blinded to the intervention?
-
Yes, low risk of bias:
treatment and control groups are indistinguishable for participants or if participant was described as blinded and method of blinding was described.
-
No, high risk of bias:
blinding of study participants attempted, but likely that blinding could have been broken; participants were not blinded, and non‐blinding of others likely to introduce bias.
Unclear.
Was the care provider blinded to the intervention?
-
Yes, low risk of bias:
treatment and control groups are indistinguishable for the care/treatment providers or if care provider was described as blinded and method of blinding was described.
-
No, high risk of bias:
blinding of care/treatment providers attempted, but likely that blinding could have been broken; care/treatment providers were not blinded, and non‐blinding of others likely to introduce bias.
Unclear.
Was the outcome assessor blinded to the intervention?
-
Yes, low risk of bias:
adequacy of blinding should be assessed for primary outcomes. The outcome assessor described as blinded and method of blinding was described.
-
No, high risk of bias:
no blinding or incomplete blinding, and outcome or outcome measurement is likely to be influenced by lack of blinding.
Unclear.
4. Were incomplete outcome data adequately addressed?
Was the dropout rate described and acceptable?
The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given.
-
Yes, low risk of bias:
if percentage of withdrawals and dropouts does not exceed 20% (note: these percentages are arbitrary, not supported by literature);
no missing outcome data;
reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias);
missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups;
missing data have been imputed using appropriate methods.
-
No, high risk of bias:
absolute overall dropout rate higher than 20%;
reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
Unclear.
5. Are reports of the study free of suggestion of selective outcome reporting?
-
Yes, low risk of bias:
if all results from all prespecified outcomes have been adequately reported in published report of trial. This information is either obtained by comparing protocol and final trial report, or in absence of protocol, assessing that published report includes enough information to make this judgement. We identified trial protocols from trial registries as listed under Electronic searches. Alternatively, a judgement could be made if trial report lists outcomes of interest in methods of trial and then reports all these outcomes in results section of trial report.
-
No, high risk of bias:
not all of the study's prespecified primary outcomes have been reported;
one or more primary outcomes is reported using measurements, analysis methods, or subsets of data (e.g. subscales) that were not prespecified;
one or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect);
one or more outcomes of interest in review are reported incompletely so that they cannot be entered in a meta‐analysis;
study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear.
6. Other sources of potential bias
Were the groups similar at baseline regarding the most important prognostic indicators?
Groups have to be similar at baseline regarding demographic factors, and duration and severity of complaints. Alternatively if there were imbalances at baseline these have been accounted for in the analysis of study.
Were cointerventions avoided or similar?
There were no cointerventions or there were cointerventions but they were similar between treatment and control groups.
Was compliance acceptable in all groups?
The review author determines whether compliance with interventions is acceptable, based on reported intensity, duration, and number and frequency of sessions for both treatment intervention and control intervention(s). For example, ultrasound treatment is usually administered over several sessions; therefore, it is necessary to assess how many sessions each participant attended or if participants completed the course of an oral drug therapy. For single‐session interventions (e.g. surgery), this item is irrelevant.
Did the trials or trial authors receive financial support from agencies or organisations with a financial interest in the outcome of the trial?
Was the trial free of other issues, for instance, design‐specific risks of bias such as recruitment in cluster for cluster‐randomised controlled trial and block randomisation of unblinded trials?
Data and analyses
Comparison 1. Intravenous (IV) fluid supplementation versus no fluid supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of infants with bilirubin encephalopathy | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Serum bilirubin (μmol/L) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 4 hours postintervention | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐34.0 [‐52.29, ‐15.71] |
| 2.2 6 hours postintervention | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐28.67, 32.07] |
| 2.3 8 hours postintervention | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐44.0 [‐65.95, ‐22.05] |
| 2.4 12 hours postintervention | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐10.21 [‐18.45, ‐1.97] |
| 2.5 18 hours postintervention | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐5.20 [‐33.70, 23.30] |
| 2.6 24 hours postintervention | 4 | 252 | Mean Difference (IV, Fixed, 95% CI) | ‐6.06 [‐11.12, 1.00] |
| 2.7 36 hours postintervention | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐4.25 [‐8.73, 0.23] |
| 2.8 48 hours postintervention | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐7.86 [‐14.15, ‐1.57] |
| 2.9 60 hours postintervention | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐9.16 [‐21.25, 2.93] |
| 2.10 72 hours postintervention | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐8.76, 6.83] |
| 2.11 84 hours postintervention | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 7.40 [4.69, 10.11] |
| 3 Difference in serum bilirubin (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 0‐4 hours of study | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 10.5 [6.66, 14.34] |
| 3.2 0‐8 hours of study | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [7.49, 18.51] |
| 3.3 0‐24 hours of study | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [1.11, 14.89] |
| 4 Rate of change of serum bilirubin (μmol/L/hour) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.21, 1.21] |
| 4.1 During the first 12 hours of study | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.21, 1.21] |
| 5 Duration of phototherapy (hours) | 3 | 218 | Mean Difference (IV, Fixed, 95% CI) | ‐10.70 [‐15.55, ‐5.85] |
| 6 Number of infants who required exchange transfusion | 6 | 462 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.21, 0.71] |
| 7 Frequency of breastfeeding per day | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Day 1 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.62, 2.02] |
| 7.2 Day 2 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.43, 2.23] |
| 7.3 Day 3 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.40, 2.20] |
Comparison 2. Intravenous (IV) fluid supplementation versus oral fluid supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of infants with abnormal neurological signs | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serum bilirubin (μmol/L) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐21.58, 43.58] |
| 2.1 4 hours postphototherapy | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐21.58, 43.58] |
| 3 Rate of change of serum bilirubin (μmol/L/hour) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐2.55, 4.15] |
| 3.1 During first 4 hours of admission | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐2.55, 4.15] |
| 4 Number of infants who required exchange transfusion | 1 | 54 | Risk Difference (IV, Fixed, 95% CI) | 0.11 [‐0.12, 0.34] |
| 5 Number of infants with feed intolerance: vomiting | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number of infants with feed intolerance: abdominal distension | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Masri 2012.
| Methods |
Study design: randomised controlled trial. Study grouping: parallel group. |
|
| Participants |
Baseline characteristics IV fluid supplementation:
No fluid supplementation:
Inclusion criteria: healthy term breastfed infants with severe hyperbilirubinaemia. Exclusion criteria: "We excluded haemolytic disease (ABO or Rh incompatibility and a positive Coomb's test), G6PD deficiency, direct hyperbilirubinaemia, infection, dehydration, and prolonged jaundice persisting beyond 14 days of life." Pretreatment: "No major differences between the two groups." |
|
| Interventions |
IV fluid supplementation:
No fluid supplementation:
|
|
| Outcomes |
Serum bilirubin
Number of infants who required exchange transfusion (criteria not stated)
|
|
| Identification |
Sponsorship source: none stated. Country: Jordan. Setting: NICU in 2 medical centres. Comments: study conducted between September 2008 and October 2009. Author's name: Hazem A Al‐Masri. Institution: Royal Medical Services. Email: drhazem73@yahoo.com. Address: Department of Pediatrics, Royal Medical Services, Jordan. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Quote: "Infants were divided randomly into two groups." Authors did not state methods of sequence generation as they only described in a simple statement that "infants were divided randomly..." |
| Allocation concealment | Unclear risk | No statements on methods of randomisation or party involved sequence generation and allocation (or both) to enable an assessment of whether the 2 steps were performed independently from each other. |
| Blinding of participants and personnel All outcomes | High risk | Not stated if blinding occurred but due to nature of intervention (IV drip) as compared to oral feeding only in control group, it is highly unlikely that the researchers were blinded. |
| Blinding of outcome assessors All outcomes | Unclear risk | Not stated if the laboratory staff knew group that participants were allocated to. |
| Incomplete outcome data All outcomes | Low risk | Results for all 40 infants in each group analysed and presented in Table 2. |
| Selective outcome reporting | Low risk | Major outcomes specified in methods, namely serum bilirubin at different time points and number of infants who required exchange transfusion reported in sufficient detail in results. |
| Selective outcome reporting | Low risk | Major outcomes specified in methods, namely serum bilirubin at different time points and number of infants who required exchange transfusion reported in sufficient detail in results. |
| Other sources of bias | Low risk | None identified. |
Boo 2002.
| Methods |
Study design: randomised controlled trial. Study grouping: parallel group. |
|
| Participants |
Baseline characteristics IV fluid supplementation:
Oral fluid supplementation:
Inclusion criteria: healthy term infants (≥ 37 weeks' gestation) admitted to the NICU with TSB level ≥ 300 μmol/L and conjugated serum bilirubin levels ≤ 15% of the TSB. Exclusion criteria: unwell infants (e.g. septicaemia, feed intolerance, kernicterus), major congenital malformation, conjugated hyperbilirubinaemia > 15% of TSB levels, or prolonged jaundice persisting beyond 14 days of life. Pretreatment: no significant differences between groups in mean birth weight, mean gestational age, ethnic distribution, gender distribution, places of birth, modes of delivery, and types of feeding. No significant differences in proportion with birth trauma, ABO incompatibility, abnormal blood film, G6PD deficiency, herbal intake for breastfeeding mothers, and clinical dehydration. Mean total indirect serum bilirubin on admission were not significantly different. The infants in the enteral group were significantly older on admission than those in IV group (P = 0.02). |
|
| Interventions |
IV fluid supplementation:
Oral fluid supplementation:
|
|
| Outcomes |
Serum bilirubin
Rate of decrease of serum bilirubin
Number of infants who required exchange transfusion (when the serum bilirubin level remained > 340 µmol/L after commencement of phototherapy)
Number of infants with abnormal neurological signs
Number of infants with vomiting
Number of infants with abdominal distension
|
|
| Identification |
Sponsorship source: not stated. Country: Malaysia. Setting: NICU of a university hospital. Comments: study conducted between 1 October 1999 and 30 September 2000. Author's name: Boo NY. Institution: Universiti Kebangsaan Malaysia. Email: nyboo@mail.hukm.ukm.my. Address: Department of Paediatrics, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Yaacob Latif, Cheras, Kuala Lumpur, 56000, Malaysia. |
|
| Notes | Rate of decrease in bilirubin reported was indirect and not TSB. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Quote: "assignment into groups were based on information previously inserted randomly into sealed envelopes, which were then numbered sequentially." Unclear from the statements what method was used to generate the random sequence. |
| Allocation concealment | Unclear risk | Quote: "Assignment into groups was based on information previously inserted randomly into sealed envelopes, which were then numbered sequentially." Not stated if envelopes used were opaque (therefore, the contents will not be visible) although they were sealed and numbered sequentially. |
| Blinding of participants and personnel All outcomes | High risk | Although not clearly stated in paper, blinding appeared highly unlikely as the way of administering fluid differed between the 2 groups, 1 receiving IV supplementation while another receiving enteral supplementation. |
| Blinding of outcome assessors All outcomes | Low risk | Authors stated that the laboratory technicians had no knowledge of which fluid regimen was given to which infant. |
| Incomplete outcome data All outcomes | Low risk | All 54 randomised infants were included in analyses. |
| Selective outcome reporting | Low risk | Appeared that all major outcomes as specified in the methods were reported in sufficient detail in the results. We were unable to obtain trial protocol to assess whether there were any further outcomes that were not reported. |
| Other sources of bias | Low risk | None identified. |
Easa 2013.
| Methods |
Study design: randomised controlled trial. Study grouping: parallel group. |
|
| Participants |
Baseline characteristics IV fluid supplementation:
No fluid supplementation:
Inclusion criteria: delivered between 38 and 41 weeks' gestation following an uneventful pregnancy with TSB > 308 μmol/L (18 mg/dL) to < 375 μmol/L (22 mg/dL). Exclusion criteria: major congenital malformation, haemolytic disease (Rh or ABO incompatibility and a positive Coombs' test), infection (congenital or acquired), G6PD deficiency, dehydration, conjugated hyperbilirubinaemia > 15% of TSB levels, prolonged jaundice persisting beyond 14 days of life. Pretreatment: no significant differences between groups noted. |
|
| Interventions |
IV fluid supplementation:
No fluid supplementation:
|
|
| Outcomes |
Serum bilirubin
Number of infants who required exchange transfusion (criteria not stated)
|
|
| Identification |
Sponsorship source: none stated. Country: Iraq. Setting: neonatal unit of a teaching hospital. Comments: study conducted from 2 January 2010 to 31 December 2010. Author's name: Easa ZO. Institution: author's affiliation not stated in paper. Email: not provided in paper. Address: not provided in paper. |
|
| Notes | Interventions: text under Methods stated that, "neonates were assigned randomly to two groups, either the breast‐fed or formula‐fed with IV fluid (non‐supplemented group; N=32), or breast‐fed or formula fed in addition to IV fluid (supplemented group; N=32)". Based on abstract and whole manuscript, review author believed that there was a typographical error where the statement should have READ (mistake corrected in bold) "neonates were assigned randomly to two groups, either the breast‐fed or formula‐fed WITHOUT IV fluid (non‐supplemented group; N=32), or breast‐fed or formula fed in addition to IV fluid (supplemented group; N=32)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Quote: "assigned randomly to two groups." Unclear if allocation sequence was genuinely randomised as authors only stated that "neonates were assigned randomly to two groups." |
| Allocation concealment | Unclear risk | No information on how and where randomisation was performed to enable an assessment of whether random sequence was generated independently from allocation. |
| Blinding of participants and personnel All outcomes | High risk | No mention of blinding in study. As intervention group received IV drip compared to control group (no IV drip), it is highly unlikely that the research personnel were blinded to intervention. |
| Blinding of outcome assessors All outcomes | Unclear risk | Not stated if laboratory personnel who tested outcome measures (serum bilirubin) were blinded to the participant's allocation to either intervention or control. |
| Incomplete outcome data All outcomes | Low risk | All 64 infants initially randomised were analysed. |
| Selective outcome reporting | Low risk | Main outcomes which researchers stated in methodology (serum bilirubin levels) were presented adequately in mean and SD for an appropriate length of time (while infants still receiving phototherapy until TSB declined to < 14 mg/dL). Numbers of infants requiring exchange transfusion also described. |
| Other sources of bias | Low risk | None identified. |
Iranpour 2004.
| Methods |
Study design: randomised controlled trial. Study grouping: parallel group. |
|
| Participants |
Baseline characteristics IV fluid supplementation in addition to breastfeeding on‐demand:
Breastfeeding on‐demand without fluid supplementation:
Inclusion criteria: "These neonates were all Iranian race, healthy, breast‐fed, delivered between 38 and 41 weeks of gestation, following an uneventful pregnancy and had a total serum bilirubin (TSB) between 17 and 24.9 mg/dl (291 to 426 μmol/L)." Exclusion criteria: major congenital malformation, haemolytic disease (Rh or ABO incompatibility and a positive Coombs' test), infection (congenital or acquired), G6PD deficiency, dehydration, conjugated hyperbilirubinaemia > 15% of the TSB levels, and prolonged jaundice persisting beyond 14 days of life. Pretreatment: no statistical differences in baseline TSB at time of enrolment between groups (P = 0.17). |
|
| Interventions |
IV fluid supplementation in addition to breastfeeding on‐demand:
Breastfeeding on‐demand without fluid supplementation:
|
|
| Outcomes |
Serum bilirubin
Number of infants who required exchange transfusion (criteria not stated)
Duration of phototherapy
Rate of decrease in serum bilirubin
|
|
| Identification |
Sponsorship source: no information provided. Country: Iran. Setting: NICU. Comments: article identified via the reference list of an included article (Easa 2013). Study conducted between March and October 2003. Author's name: Iranpour R. Institution: Department of Pediatrics, Isfahan University of Medical Sciences, Isfahan, Iran. Email: not provided. Address: Correspondence to: Dr Iraj Haghshenas, Pediatric Department, Al‐Zahra Hospital, Isfahan, Iran. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Sequence generation not described as there was only a simple statement that, "neonates were assigned randomly" so it is unclear if allocation sequence was genuinely randomised. |
| Allocation concealment | Unclear risk | No information given to enable an assessment of whether random sequence was generated independently from allocation. |
| Blinding of participants and personnel All outcomes | High risk | Although not clearly stated, blinding appeared highly unlikely as 1 group received IV fluid while the other did not. |
| Blinding of outcome assessors All outcomes | Unclear risk | No mention if laboratory personnel who measured serum bilirubin levels were blinded to allocation received. |
| Incomplete outcome data All outcomes | Low risk | All 60 infants initially randomised were included in analyses. |
| Selective outcome reporting | High risk | Authors reported only serum bilirubin at different time points. Key clinical outcomes, such as infants with symptoms of bilirubin encephalopathy or kernicterus, infants who required exchange transfusion, or infants who developed adverse effects from IV infusion such as phlebitis were not reported. |
| Other sources of bias | Low risk | None identified. |
Mehta 2005.
| Methods |
Study design: randomised controlled trial. Study grouping: parallel group. |
|
| Participants |
Baseline characteristics Fluid supplementation:
No fluid supplementation:
Inclusion criteria: term (≥ 37 weeks' gestation) neonates presenting with severe non‐haemolytic hyperbilirubinemia (TSB > 308 mmol/L (18 mg/dL). Exclusion criteria: infants with TSB > 427 mmol/L (25 mg/dL), acute bilirubin encephalopathy (kernicterus), evidence of haemolysis, obvious signs of dehydration (i.e. sunken fontanel, reduced skin turgor, dry mucosa, tachycardia, delayed capillary refill, excessive weight loss), or major congenital malformations, and receiving IV fluids for any reason. Pretreatment: no major differences between groups in baseline characteristics. |
|
| Interventions |
Fluid supplementation:
No fluid supplementation:
|
|
| Outcomes |
Serum bilirubin
Rate of decrease of serum bilirubin
Number of infants who required exchange transfusion
Duration of phototherapy
Number of infants with bilirubin encephalopathy
Frequency of breastfeeding in the first 3 days of hospital stay
|
|
| Identification |
Sponsorship source: not stated. Country: India. Setting: tertiary level NICU. Comments: study period: September 2003 to June 2004. Author's name: Dr Praveen Kumar. Institution: Department of Pediatrics, Postgraduate Institute of Medical Education & Research, Chandigarh, India. Email: drpkumarpgi@rediffmail.com. Address: Department of Pediatrics, PGIMER, Chandigarh 160012, India. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "Assignment to the various groups was based on stratified block randomisation with variable block size." Methods of random sequence generation clearly stated. |
| Allocation concealment | Low risk | Researchers used serially numbered opaque envelopes to assign groups. Envelopes only opened after enrolment. |
| Blinding of participants and personnel All outcomes | High risk | In paragraph 2 under discussion, authors stated that, "they could not do complete blinding." It is noted that due to the study design, blinding would be impossible due to different management instituted to groups in which 1 group received IV drip in addition to oral feed as compared to the other group which only received oral feeding. |
| Blinding of outcome assessors All outcomes | Low risk | In paragraph 2 of discussion (page 783), authors stated that, "the laboratory personnel performing the serum bilirubin and other biochemical investigations were sent coded samples and were unaware of the group allocation." |
| Incomplete outcome data All outcomes | Low risk | All 54 infants enrolled were included in analyses. Number of infants with serum bilirubin levels at 4, 8, and 24 hours were lower than number at inclusion as some infants required exchange transfusion and their serum bilirubin readings were not included. |
| Selective outcome reporting | Low risk | All major outcomes, including predefined primary outcomes of the number of infants receiving exchange transfusion; duration of phototherapy; % drop in serum bilirubin at 4, 8, and 24 hours of study; and number of infants with bilirubin encephalopathy were reported in sufficient details. |
| Other sources of bias | Low risk | None identified. |
Patel 2014.
| Methods |
Study design: randomised controlled trial. Study grouping: parallel group. |
|
| Participants |
Baseline characteristics IV fluid supplementation:
No fluid supplementation:
Inclusion criteria: term neonates, well‐hydrated, aged 2‐10 days who had indirect non‐haemolytic jaundice with serum bilirubin ≥ 308 μmol/L (18 mg/dL). Exclusion criteria: jaundice in first day after birth, any manifestations of kernicterus, any form of haemolysis, dehydration, infants with symptoms of dehydration, congenital malformation, taking antibiotics, direct bilirubin > 15% of the total bilirubin, exchange transfusion if it was performed soon after admission (page 2525, column 1, paragraph 2). Pretreatment: no significant differences between groups in terms of gender, age on admission, weight, and serum bilirubin on admission. |
|
| Interventions |
IV fluid supplementation:
No fluid supplementation:
|
|
| Outcomes |
Rate of decrease of serum bilirubin
Number of infants who required exchange transfusion (criteria not stated)
Duration of phototherapy
|
|
| Identification |
Sponsorship source: no information provided. Country: India. Setting: NICU of a hospital (level unclear). Comments: conducted over 6‐month period but the authors did not state the month and year it was conducted. Author's name: Patel M (first author). Institution: Civil Hospital, Ahmedabad, India. B. J. Medical College. Email: not provided. Address: Department of Pediatrics, B. J. Medical College, Asarwa, Ahmedabad, 380016, Gujarat, India (address for all 3 study authors). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "By using a random number table, simple randomisation was performed." Methods of sequence generation clearly stated. |
| Allocation concealment | Unclear risk | No relevant information to enable an assessment of whether allocation was performed independently from random sequence generation. |
| Blinding of participants and personnel All outcomes | High risk | Although not mentioned in text, it is highly unlikely that research personnel were blinded due to nature of intervention which was IV fluid compared to enteral feeding only in control group. |
| Blinding of outcome assessors All outcomes | Unclear risk | Not stated if laboratory personnel were blinded to the group allocation. |
| Incomplete outcome data All outcomes | Low risk | All infants (42 in each group) appeared to have been analysed and their results presented. |
| Selective outcome reporting | High risk | Rates of decrement of bilirubin in first 6 and first 12 hours presented as means (without SD) as seen in Table 3. Anticipate difficulty obtaining this additional data from authors as corresponding author was not named and there was no email address included. Data for number of infants who required exchange transfusion were adequately reported. Several infants required exchange transfusion but, despite this, there was no report on numbers who developed bilirubin encephalopathy. |
| Other sources of bias | Low risk | None identified. |
Saeidi 2009.
| Methods |
Study design: randomised controlled trial. Study grouping: parallel group. |
|
| Participants |
Baseline characteristics IV fluid supplementation:
No fluid supplementation:
Inclusion criteria: term, healthy neonate, aged 2‐28 days, serum total bilirubin ≥ 308 μmol/L (18 mg/dL). Exclusion criteria: jaundice in first day after birth, any manifestations of kernicterus, any form of haemolysis, dehydration, any congenital malformation, treatment with antibiotics, direct bilirubin > 15% of total bilirubin, exchange transfusion if performed soon after admission. Pretreatment: no major differences in the baseline characteristics between groups. |
|
| Interventions |
IV fluid supplementation:
No fluid supplementation:
|
|
| Outcomes |
Rate of decrease of serum bilirubin
Number of infants who required exchange transfusion (criteria not stated)
|
|
| Identification |
Sponsorship source: not stated. Country: Iran. Setting: Paediatric ward of a hospital (level unclear). Comments: study period: October 2007 to April 2008. Some outcome data reported in a manner that was insufficient for pooling in meta‐analysis. Awaiting reply from study authors. Author's name: Associate Professor Farhad Heydarian. Institution: Paediatrics Ward, Ghaem Hospital, Ahmad Abad Ave, Mashhad, Iran. Email: heydarianf@mums.ac.ir. Address: Paediatrics Ward, Ghaem Hospital, Ahmad Abad Ave, Mashhad, Iran. |
|
| Notes | Outcome 'rate of decrease in serum bilirubin' was incompletely reported, as authors only reported median with a single figure given as "confidence interval." We are awaiting further information from study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "By using a random number table, simple randomisation was performed." |
| Allocation concealment | Unclear risk | Unclear how allocation carried out, in particular, whether allocation performed independently from sequence generation. |
| Blinding of participants and personnel All outcomes | High risk | Blinding to care personnel very unlikely as 1 group of infants received additional IV fluid while another group did not. |
| Blinding of outcome assessors All outcomes | Unclear risk | Unclear whether assessors of laboratory outcomes were blinded to allocation status of infants. |
| Incomplete outcome data All outcomes | Low risk | Appeared that all 100 infants randomised included in report for major outcomes of number of infants who required exchange transfusion and rate of decrease in serum bilirubin in first 24 hours. |
| Selective outcome reporting | High risk | The outcome 'Rate of decrease of serum bilirubin in the first 24 hours after admission' was incompletely reported, as authors only provided median figures with a single figure given for confidence interval (we are waiting study author's response to our request for these data). Study authors did not include any data on adverse events of IV fluids such as thrombophlebitis. Number of infants with bilirubin encephalopathy not reported. |
| Other sources of bias | Low risk | None identified. |
G6PD: glucose‐6‐phosphate dehydrogenase; NICU: neonatal intensive care unit; SD: standard deviation; TSB: total serum bilirubin.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Mendalawi 2010 | Letter to editor commenting on findings of an included study (Saeidi 2009). Excluded on basis of study design. |
| Balasubramanian 2012 | Evaluated 2 different types of intravenous fluid supplementation (hypotonic vs isotonic fluid), which was not within our prespecified comparison list. Excluded on basis of intervention. |
| Barone 2011 | A randomised controlled trial that evaluated 2 different levels of fluid in small‐for‐gestational age babies. Inclusion criteria of infants did not include hyperbilirubinaemia and phototherapy. Excluded on basis of participant population. |
| Brans 1987 | 3‐arm randomised controlled trial that compared effects of 3 regimen of fat emulsion in total parenteral nutrition for preterm infants. Excluded on basis of intervention. |
| Brown 1989 | Randomised controlled trial that compared 2 regimens of parenteral‐enteral nutrition combination for very‐low‐birth‐weight infants. Excluded on basis of intervention. |
| Carvalho 1981 | Non‐randomised trial that compared effects of water supplementation to no supplementation on bilirubin level for breastfed infants. Excluded on basis of study design. |
| Dunn 1988 | Randomised controlled trial that compared effects of early introduction of hypocaloric feeding versus no early feeding for preterm infants. Excluded on basis of intervention. |
| Gourley 1999 | Comparative trial that evaluated effects of 2 different milk formulae (whey‐predominant versus casein‐hydrolysate) in newborn infants. Trial did not compare 2 different levels of fluid. Excluded on basis of intervention. |
| Kavvadia 2000 | Randomised controlled trial that evaluated effects of 2 levels of fluid (1 starting with 60 mL/kg/day on day 1 and increasing to 150 mL/kg/day over the first week and the other receiving 20% less fluid over same period). Population enrolled were preterm infants in general and not infants with hyperbilirubinaemia or on phototherapy. Excluded on basis of participant population. |
| Maisels 1994 | Randomised controlled trial that compared frequent vs usual on‐demand breastfeeding regimen at reducing serum bilirubin of healthy term infants. Infants were healthy at birth and not infants with hyperbilirubinaemia who were undergoing phototherapy. Excluded on basis of population. |
| Makay 2007 | Randomised controlled trial that examined effects of early parenteral nutrition on prevention of jaundice in term and near‐term infants. Excluded on basis of intervention. |
| Martinez 1993 | 4‐arm randomised controlled trial that compared effects of 4 interventions for term newborn infants with severe hyperbilirubinaemia. Intervention involved a combination of continuing breastfeeding or starting formula feeding and starting or not starting phototherapy, and thus they were not related to fluid level. Excluded on basis of intervention. |
| Nicoll 1982 | Survey on practice of giving supplementary feeds to newborn infants and occurrence of jaundice. Excluded on basis of study design. |
| Rosegger 1986 | Randomised controlled trial comparing supplementation of formula or maltodextrin solution to breastfeeding term infants. Excluded on basis of intervention. |
| Wennberg 1966 | 4‐arm randomised controlled trial comparing feeding very‐low‐birth‐weight infants using 4 different feed regimens comprising different combinations of glucose solution, water, or saline and different timings of feed commencement. Excluded on basis of intervention. |
Characteristics of studies awaiting assessment [ordered by study ID]
Demirsoy 2011.
| Methods | Single‐centre, parallel randomised controlled trial (Turkey). |
| Participants | 250 Healthy term infants with hyperbilirubinaemia. |
| Interventions | Study group: intravenous fluid supplementation in addition to breastfeeding (125 infants) during phototherapy. Control group: breastfeeding without fluid supplementation during phototherapy. |
| Outcomes | Mean indirect serum bilirubin level at time of admission to neonatal intensive care unit and at 4, 8, 12, 24, and 48 hours after commencement of phototherapy, duration of phototherapy, duration of hospitalisation. |
| Notes | Insufficient information from the published abstract to merit inclusion, awaiting full‐text. |
IRCT2013022711145N5.
| Methods | Single‐centre, parallel randomised controlled trial (Iran). |
| Participants | Inclusion criteria: gestational age > 38 weeks, age > 24 hours on admission, serum level of total bilirubin > 15 mg/dL, indirect hyperbilirubinaemia. Exclusion criteria: fluid therapy required for conditions other than jaundice, preterm and low birth weight neonates, haemolytic jaundice, sepsis, prolonged jaundice. |
| Interventions | Study group: intravenous fluids containing dextrose 10% equal amounts of maintenance with sodium 30 mEq/L and potassium 20 mEq/L plus phototherapy. Control group: phototherapy with no intravenous fluid. |
| Outcomes | Serum bilirubin level on admission and at 6, 12, and 24 hours postadmission, duration of hospitalisation. |
| Notes | Study completed in 2012 according to the Iranian Registry of Clinical Trials. Study appeared not to have been published in full or in part according to our searches. Awaiting further information from the study author. |
NCT01550627.
| Methods | Single‐centre, parallel randomised controlled trial (Germany). |
| Participants | Preterm infants < 33 weeks' gestation undergoing phototherapy for hyperbilirubinaemia. |
| Interventions | Study group: extra intravenous fluid intake of 20% of total fluid demand per 24 hours of saline 0.9% during each 2‐hour period of phototherapy (12 hours total per day). Extra fluid intake was interrupted during 12‐hours break of phototherapy. Control group: previous fluid regimen, as intravenous fluid was given constantly, without a specific guideline according to extra fluid intake. |
| Outcomes | Highest TSB level within 1 week after the commencement of phototherapy. No other outcomes provided. |
| Notes | Study completed in 2009 according to the ClinicalTrials.gov. The study appeared not to have been published in full or in part according to our searches. Awaiting further information from the study author. |
Differences between protocol and review
Under Types of interventions, we added the purposes of fluid administration, as follows: "Fluid supplementation: additional amount of fluid on top of the standard fluid regimen, given enterally or IV, or both, aiming to restore normal hydration status in clinically dehydrated infants or provide additional circulating fluid in infants who are not clinically dehydrated, or both restoring and providing additional circulating fluid."
Under Types of interventions, we rearranged the comparisons and added an additional comparison of intravenous fluid supplementation using different types of fluid, with a statement explaining the applicability of different comparisons to infants who were clinically dehydrated and not dehydrated, as follows:
"The following were specific comparisons that we planned to include in the Cochrane Review:
comparison 1: IV fluid supplementation (IV infusion or bolus) with or without standard oral fluid regimen versus standard oral fluid regimen;
comparison 2: supplemented oral fluid regimen versus standard oral fluid regimen. Infants who were supplemented were most likely to be given additional expressed breast milk or formula milk on top of standard breast‐ or formula‐feeding regimen;
comparison 3: IV fluid supplementation (IV infusion or bolus) with or without standard oral fluid regimen versus supplemented oral fluid regimen;
comparison 4: in infants who were kept nil by mouth, IV fluid supplementation (IV infusion) in addition to standard maintenance IV fluid regimen versus standard maintenance IV fluid regimen;
comparison 5: IV fluid supplementation (IV infusion or bolus) with or without standard oral fluid regimen versus supplemented oral fluid regimen in addition to standard oral or IV fluid regimen;
comparison 6: IV fluid supplementation (IV infusion or bolus) using a specific type of fluid versus IV fluid supplementation (IV infusion or bolus) using another type of fluid.
All the above comparisons might apply for infants who were not clinically dehydrated, while comparisons 5 and 6 applied for infants who were clinically dehydrated."
We added peak bilirubin as a secondary outcome following suggestion from the editor. The first sentence of the first secondary outcome now reads as follows:
-
"Bilirubin level (serum bilirubin or transcutaneous bilirubin level) reported as follows:
absolute bilirubin level measured at specific time points and expressed in mmol/L or mg/dL, including the peak bilirubin value;"
We added 'Exclusive breastfeeding during hospital stay or at discharge, or both' posthoc following the identification of this review as relevant resource in the World Health Organization guideline development. Since this outcome was not reported in the included studies, we included the breastfeeding outcome which was reported by one study: frequency of breastfeeding per day.
We added 'Proportion of infants with feed intolerance, either with documented diagnosis or with suggestive symptoms and/or signs such as vomiting, abdominal distension or discoloured gastric aspirate' as secondary outcomes, as these outcomes were reported in one included study.
Under Assessment of risk of bias in included studies, we expanded the numbered of items seven from six (blinding from a single statement to two) to be consistent with the rest of the review.
Contributions of authors
NML: conceived the review title and developed the search strategy.
NML, YMC, and CFN: participated in screening, selection, data extraction, risk of bias assessment, and data entering via Covidence.
NML: developed the 'Summary of findings' tables using GRADEpro GDT.
NML: wrote the first draft of the review.
All authors participated in revising the draft review and approved the final version.
Sources of support
Internal sources
University of Malaya, Malaysia.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
National Institute for Health Research, UK.
UK Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
Declarations of interest
NML has no declaration of interest.
AAK has no declaration of interest.
YMC has no declaration of interest.
JYK has no declaration of interest.
CFG has no declaration of interest.
New
References
References to studies included in this review
Al‐Masri 2012 {published data only}
- Al‐Masri H. In healthy baby with severe jaundice do we need to give fluid supplementation during phototherapy?. Sudan Medical Journal 2012;48(3):176‐80. [Google Scholar]
Boo 2002 {published data only}
- Boo NY, Lee HT. Randomized controlled trial of oral versus intravenous fluid supplementation on serum bilirubin level during phototherapy of term infants with severe hyperbilirubinaemia. Journal of Paediatrics and Child Health 2002;38(2):151‐5. [DOI: 10.1046/j.1440-1754.2002.00746.x; PUBMED: 12030996] [DOI] [PubMed] [Google Scholar]
Easa 2013 {published data only}
- Easa ZO. Effect of intravenous fluid supplementation on serum bilirubin level during conventional phototherapy of term infants with severe hyperbilirubinemia. Al‐Qadisiah Medical Journal 2013;9(15):36‐45. [Google Scholar]
Iranpour 2004 {published data only}
- Iranpour R, Nohekhan R, Haghshenas I. Effect of intravenous fluid supplementation on serum bilirubin level in jaundiced healthy neonates during conventional phototherapy. Journal of Research in Medical Sciences 2004;9(4):186‐90. [Google Scholar]
Mehta 2005 {published data only}
- Mehta S, Kumar P, Narang A. A randomized controlled trial of fluid supplementation in term neonates with severe hyperbilirubinemia. Journal of Pediatrics 2005;147(6):781‐5. [DOI: 10.1016/j.jpeds.2005.07.026; PUBMED: 16356431] [DOI] [PubMed] [Google Scholar]
Patel 2014 {published data only}
- Patel M, Munshi S, Mehariya KM. Effect of fluid supplementation in severe neonatal hyperbilirubinemia. International Journal of Science and Research 2014;3(12):2524‐6. [www.ijsr.net/archive/v3i12/U1VCMTQxMDc5.pdf] [Google Scholar]
Saeidi 2009 {published data only}
- Saeidi R, Heydarian F, Fakehi V. Role of intravenous extra fluid therapy in icteric neonates receiving phototherapy. Saudi Medical Journal 2009;30(9):1176‐9. [PUBMED: 19750263] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Mendalawi 2010 {published data only}
- Al‐Mendalawi MD. Role of intravenous extra fluid therapy in icteric neonates receiving phototherapy. Saudi Medical Journal 2010;31(4):459‐60. [PUBMED: 20383431] [PubMed] [Google Scholar]
Balasubramanian 2012 {published data only}
- Balasubramanian K, Kumar P, Saini SS, Attri SV, Dutta S. Isotonic versus hypotonic fluid supplementation in term neonates with severe hyperbilirubinemia ‐ a double‐blind, randomized, controlled trial. Acta Paediatrica 2012;101(3):236‐41. [DOI: 10.1111/j.1651-2227.2011.02508.x; PUBMED: 22040311] [DOI] [PubMed] [Google Scholar]
Barone 2011 {published data only}
- Barone G, Giordano L, Zecca C, Priolo F, Zecca E, Maggio L. Proactive feeding regimen in preterm SGA infants: a randomized controlled trial. Journal of Maternal‐fetal & Neonatal Medicine 2011;24(Suppl 1):168. [DOI: 10.3109/14767058.2011.607964] [DOI] [Google Scholar]
Brans 1987 {published data only}
- Brans YW, Ritter DA, Kenny JD. Influence of intravenous fat emulsion on serum bilirubin in very low birthweight neonates. Archives of Disease in Childhood 1987;62(2):156‐60. [PUBMED: 3103546] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 1989 {published data only}
- Brown MR, Thunberg BJ, Golub L, Maniscalco W, Cox C, Shapiro DL. Decreased cholestasis with enteral instead of intravenous protein in the very low‐birth‐weight infant. Journal of Pediatric Gastroenterology and Nutrition 1989;9(1):21‐7. [PUBMED: 2506323] [PubMed] [Google Scholar]
Carvalho 1981 {published data only}
- Carvalho M, Hall M, Harvey D. Effects of water supplementation on physiological jaundice in breast fed babies. Archives of Disease in Childhood 1981;56(7):568‐9. [PUBMED: 7271293] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunn 1988 {published data only}
- Dunn L, Hulman S, Weiner J, Kliegman R. Beneficial effects of early hypocaloric enteral feeding on neonatal gastrointestinal function: preliminary report of a randomized trial. Journal of Pediatrics 1988;112(4):622‐9. [PUBMED: 2895173] [DOI] [PubMed] [Google Scholar]
Gourley 1999 {published data only}
- Gourley GR, Kreamer B, Cohnen M, Kosorok MR. Neonatal jaundice and diet. Archives of Pediatrics & Adolescent Medicine 1999;153(2):184‐8. [PUBMED: 9988249] [DOI] [PubMed] [Google Scholar]
Kavvadia 2000 {published data only}
- Kavvadia V, Greenough A, Dimitriou G, Forsling ML. Randomized trial of two levels of fluid input in the perinatal period ‐ effect on fluid balance, electrolyte and metabolic disturbances in ventilated VLBW infants. Acta Paediatrica 2000;89(2):237‐41. [PUBMED: 10709897] [DOI] [PubMed] [Google Scholar]
Maisels 1994 {published data only}
- Maisels MJ, Vain N, Acquavita AM, Blanco NV, Cohen A, DiGregorio J. The effect of breast‐feeding frequency on serum bilirubin levels. American Journal of Obstetrics and Gynecology 1994;170(3):880‐3. [PUBMED: 8141220] [DOI] [PubMed] [Google Scholar]
Makay 2007 {published data only}
- Makay B, Duman N, Ozer E, Kumral A, Yesilirmak D, Ozkan H. Randomized, controlled trial of early intravenous nutrition for prevention of neonatal jaundice in term and near‐term neonates. Journal of Pediatric Gastroenterology and Nutrition 2007;44(3):354‐8. [DOI: 10.1097/MPG.0b013e31802b31f2] [DOI] [PubMed] [Google Scholar]
Martinez 1993 {published data only}
- Martinez JC, Maisels MJ, Otheguy L, Garcia H, Savorani M, Mogni B, et al. Management of severe hyperbilirubinemia in fullterm newborns ‐ a controlled trial of 4 interventions. Pediatric Research 1993;33(6):662. [DOI: 10.1203/00006450-199306000-00042] [DOI] [PubMed] [Google Scholar]
Nicoll 1982 {published data only}
- Nicoll A, Ginsburg R, Tripp JH. Supplementary feeding and jaundice in newborns. Acta Paediatrica Scandinavica 1982;71(5):759‐61. [PUBMED: 6897478] [DOI] [PubMed] [Google Scholar]
Rosegger 1986 {published data only}
- Rosegger H. Maltodextrin in a 13% solution as a supplement in the first 4 days of life in breast‐fed mature newborn infants. Effect on drinking behavior, weight curve, blood picture, blood glucose and bilirubin. Wiener Klinische Wochenschrift 1986;98(10):310‐5. [PUBMED: 3727591] [PubMed] [Google Scholar]
Wennberg 1966 {published data only}
- Wennberg RP, Schwartz R, Sweet AY. Early versus delayed feeding of low birth weight infants: effects on physiologic jaundice. Journal of Pediatrics 1966;68(6):860‐6. [PUBMED: 5949016] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Demirsoy 2011 {published data only}
- Demirsoy U, Nalbantoğlu B, Nalbantoğlu A, Cakan M, Say A. Effect of fluid supplementation on serum bilirubin level during phototherapy of exclusively breastfed term Infants with hyperbilirubinemia. Breastfeeding Medicine 2011 Nov 2 [Epub ahead of print]:10.1089/bfm.2011.0085. [DOI: 10.1089/bfm.2011.0085; PUBMED: 22047110] [DOI] [PubMed] [Google Scholar]
IRCT2013022711145N5 {published data only}
- IRCT2013022711145N5. Effect of intravenous fluid therapy on serum level of bilirubin in icteric neonates during phototherapy. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013022711145N5 Date first received: 26 March 2013.
NCT01550627 {published data only}
- NCT01550627. Effect of intravenous fluid supplementation on serum bilirubin and cardiorespiratory parameters in preterm infants during phototherapy. clinicaltrials.gov/show/NCT01550627 Date first received: 7 February 2012.
Additional references
AAP 2004
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114(1):297‐316. [PUBMED: 15231951] [DOI] [PubMed] [Google Scholar]
Ahlfors 2010
- Ahlfors CE. Predicting bilirubin neurotoxicity in jaundiced newborns. Current Opinion in Pediatrics 2010;22(2):129‐33. [DOI: 10.1097/MOP.0b013e328336eb28; PUBMED: 20125026] [DOI] [PubMed] [Google Scholar]
Albers 2007
- Albers CA, Grieve AJ. Test Review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development– Third Edition. San Antonio, TX: Harcourt Assessment. Journal of Psychoeducational Assessment 2007;25(2):180‐98. [DOI: 10.1177/0734282906297199] [DOI] [Google Scholar]
Amin 2011
- Amin SB, Lamola AA. Newborn jaundice technologies: unbound bilirubin and bilirubin binding capacity in neonates. Seminars in Perinatology 2011;35(3):134‐40. [DOI: 10.1053/j.semperi.2011.02.007; PUBMED: 21641486] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barría 2007
- Barría RM, Lorca P, Muñoz S. Randomized controlled trial of vascular access in newborns in the neonatal intensive care unit. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2007;36(5):450‐6. [DOI: 10.1111/j.1552-6909.2007.00171.x; PUBMED: 17880315] [DOI] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [PUBMED: 413500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 2014
- Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD000503.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhola 2015
- Bhola K, Foster JP, Osborn DA. Chest shielding for prevention of a haemodynamically significant patent ductus arteriosus in preterm infants receiving phototherapy. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD009816.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutani 1999
- Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour‐specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near‐term newborns. Pediatrics 1999;103(1):6‐14. [PUBMED: 9917432] [DOI] [PubMed] [Google Scholar]
Bhutani 2009
- Bhutani VK, Johnson L. Kernicterus in the 21st century: frequently asked questions. Journal of Perinatology 2009;29(Suppl 1):S20‐4. [DOI: 10.1038/jp.2008.212; PUBMED: 19177056] [DOI] [PubMed] [Google Scholar]
Boskabadi 2010
- Boskabadi H, Maamouri G, Ebrahimi M, Ghayour‐Mobarhan M, Esmaeily H, Sahebkar A, et al. Neonatal hypernatremia and dehydration in infants receiving inadequate breastfeeding. Asia Pacific Journal of Clinical Nutrition 2010;19(3):301‐7. [PUBMED: 20805072] [PubMed] [Google Scholar]
Brown 1986
- Brown KH, Creed de Kanashiro H, Aguila R, Lopez de Romana G, Black RE. Milk consumption and hydration status of exclusively breast‐fed infants in a warm climate. Journal of Pediatrics 1986;108(5 Pt 1):677‐80. [PUBMED: 3701512] [DOI] [PubMed] [Google Scholar]
Buiter 2008
- Buiter HD, Dijkstra SS, Oude Elferink RF, Bijster P, Woltil HA, Verkade HJ. Neonatal jaundice and stool production in breast‐ or formula‐fed term infants. European Journal of Pediatrics 2008;167(5):501‐7. [DOI: 10.1007/s00431-007-0533-9; PUBMED: 17619902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2001
- Campbell MK, Mollison J, Grimshaw JM. Cluster trials in implementation research: estimation of intracluster correlation coefficients and sample size. Statistics in Medicine 2001;20(3):391‐9. [PUBMED: 11180309] [DOI] [PubMed] [Google Scholar]
Chang 2012
- Chang RJ, Chou HC, Chang YH, Chen MH, Chen CY, Hsieh WS, et al. Weight loss percentage prediction of subsequent neonatal hyperbilirubinemia in exclusively breastfed neonates. Pediatrics and Neonatology 2012;53(1):41‐4. [DOI: 10.1016/j.pedneo.2011.11.008; PUBMED: 22348493] [DOI] [PubMed] [Google Scholar]
Davanzo 2013
- Davanzo R, Cannioto Z, Ronfani L, Monasta L, Demarini S. Breastfeeding and neonatal weight loss in healthy term infants. Journal of Human Lactation 2013;29(1):45‐53. [DOI: 10.1177/0890334412444005; PUBMED: 22554678] [DOI] [PubMed] [Google Scholar]
Dennery 2001
- Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. New England Journal of Medicine 2001;344(8):581‐90. [DOI: 10.1056/NEJM200102223440807; PUBMED: 11207355] [DOI] [PubMed] [Google Scholar]
Dewey 2003
- Dewey KG, Nommsen‐Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics 2003;112(3 Pt 1):607‐19. [PUBMED: 12949292] [DOI] [PubMed] [Google Scholar]
Geiger 2001
- Geiger AM, Petitti DB, Yao JF. Rehospitalisation for neonatal jaundice: risk factors and outcomes. Paediatric and Perinatal Epidemiology 2001;15(4):352‐8. [PUBMED: 11703683] [DOI] [PubMed] [Google Scholar]
Gerkensmeyer 2005
- Gerkensmeyer JE, Austin JK. Development and testing of a scale measuring parent satisfaction with staff interactions. Journal of Behavioral Health Services & Research 2005;32(1):61‐73. [PUBMED: 15632798] [DOI] [PubMed] [Google Scholar]
Gholitabar 2012
- Gholitabar M, McGuire H, Rennie J, Manning D, Lai R. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD009017.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gourley 2002
- Gourley GR. Breast‐feeding, neonatal jaundice and kernicterus. Seminars in Neonatology 2002;7(2):135‐41. [PUBMED: 12208098] [DOI] [PubMed] [Google Scholar]
Grünhagen 2002
- Grünhagen DJ, Boer MG, Beaufort AJ, Walther FJ. Transepidermal water loss during halogen spotlight phototherapy in preterm infants. Pediatric Research 2002;51(3):402‐5. [DOI: 10.1203/00006450-200203000-00022; PUBMED: 11861949] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huang 2012
- Huang HC, Yang HI, Chang YH, Chang RJ, Chen MH, Chen CY, et al. Model to predict hyperbilirubinemia in healthy term and near‐term newborns with exclusive breast feeding. Pediatrics and Neonatology 2012;53(6):354‐8. [DOI: 10.1016/j.pedneo.2012.08.012; PUBMED: 23276439] [DOI] [PubMed] [Google Scholar]
Itoh 2001
- Itoh S, Kondo M, Kusaka T, Isobe K, Onishi S. Differences in transcutaneous bilirubin readings in Japanese term infants according to feeding method. Pediatrics International 2001;43(1):12‐5. [PUBMED: 11207992] [DOI] [PubMed] [Google Scholar]
Johnson 2002
- Johnson LH, Bhutani VK, Brown AK. System‐based approach to management of neonatal jaundice and prevention of kernicterus. Journal of Pediatrics 2002;140(4):396‐403. [DOI: 10.1067/mpd.2002.123098; PUBMED: 12006952] [DOI] [PubMed] [Google Scholar]
Johnson 2009
- Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM. Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). Journal of Perinatology 2009;29(Suppl 1):S25‐45. [DOI: 10.1038/jp.2008.211; PUBMED: 19177057] [DOI] [PubMed] [Google Scholar]
Kjartansson 1992
- Kjartansson S, Hammarlund K, Sedin G. Insensible water loss from the skin during phototherapy in term and preterm infants. Acta Paediatrica 1992;81(10):764‐8. [PUBMED: 1421879] [DOI] [PubMed] [Google Scholar]
Kumar 2011
- Kumar P, Chawla D, Deorari A. Light‐emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD007969.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maayan‐Metzger 2001
- Maayan‐Metzger A, Yosipovitch G, Hadad E, Sirota L. Transepidermal water loss and skin hydration in preterm infants during phototherapy. American Journal of Perinatology 2001;18(7):393‐6. [DOI: 10.1055/s-2001-18698; PUBMED: 11731893] [DOI] [PubMed] [Google Scholar]
Maisels 1986
- Maisels MJ, Gifford K. Normal serum bilirubin levels in the newborn and the effect of breast‐feeding. Pediatrics 1986;78(5):837‐43. [PUBMED: 3763296] [PubMed] [Google Scholar]
Malwade 2014
- Malwade US, Jardine LA. Home‐ versus hospital‐based phototherapy for the treatment of non‐haemolytic jaundice in infants at more than 37 weeks' gestation. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD010212.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manning 2007
- Manning D, Todd P, Maxwell M, Jane Platt M. Prospective surveillance study of severe hyperbilirubinaemia in the newborn in the UK and Ireland. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92(5):F342‐6. [DOI: 10.1136/adc.2006.105361; PUBMED: 17074786] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2001
- Mills JF, Tudehope D. Fibreoptic phototherapy for neonatal jaundice. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moritz 2004
- Moritz ML, Ayus JC. Hospital‐acquired hyponatremia: why are there still deaths?. Pediatrics 2004;113(5):1395‐6. [PUBMED: 15121959] [DOI] [PubMed] [Google Scholar]
Northway 1967
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline‐membrane disease. Bronchopulmonary dysplasia. New England Journal of Medicine 1967;276(7):357‐68. [DOI: 10.1056/NEJM196702162760701; PUBMED: 5334613] [DOI] [PubMed] [Google Scholar]
Oddie 2001
- Oddie S, Richmond S, Coulthard M. Hypernatraemic dehydration and breast feeding: a population study. Archives of Disease in Childhood 2001;85(4):318‐20. [PUBMED: 11567942] [DOI] [PMC free article] [PubMed] [Google Scholar]
Okwundu 2012
- Okwundu CI, Okoromah CA, Shah PS. Prophylactic phototherapy for preventing jaundice in preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD007966.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Onyango 2009
- Onyango AB, Suresh G, Were F. Intermittent phototherapy versus continuous phototherapy for neonatal jaundice. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008168] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palisano 2008
- Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Developmental Medicine and Child Neurology 2008;50(10):744‐50. [10.1111/j.1469‐8749.2008.03089.x; PUBMED: 18834387] [DOI] [PubMed] [Google Scholar]
Patole 2005
- Patole S. Strategies for prevention of feed intolerance in preterm neonates: a systematic review. Journal of Maternal‐fetal & Neonatal Medicine 2005;18(1):67‐76. [DOI: 10.1080/14767050500127724; PUBMED: 16105795] [DOI] [PubMed] [Google Scholar]
Preutthipan 1993
- Preutthipan A, Siripoonya P, Ruangkanchanasetr S. Serum bilirubin levels in breast‐fed vs formula‐fed infants during the first 3‐5 days of life. Journal of the Medical Association of Thailand 1993;76(4):217‐21. [PUBMED: 8113642] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenbaum 2007
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Developmental Medicine and Child Neurology. Supplement 2007;109:8‐14. [PUBMED: 17370477] [PubMed] [Google Scholar]
Rubaltelli 1993
- Rubaltelli FF. Unconjugated and conjugated bilirubin pigments during perinatal development. IV. The influence of breast‐feeding on neonatal hyperbilirubinemia. Biology of the Neonate 1993;64(2‐3):104‐9. [PUBMED: 8260541] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available from gdt.gradepro.org/app/handbook/handbook.html Updated October 2013.
Seidman 1995
- Seidman DS, Stevenson DK, Ergaz Z, Gale R. Hospital readmission due to neonatal hyperbilirubinemia. Pediatrics 1995;96(4 Pt 1):727‐9. [PUBMED: 7567338] [PubMed] [Google Scholar]
Shennan 1988
- Shennan A, Dunn M, Ohlsson A, Lennox K, Hoskins E. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527‐32. [PUBMED: 3174313] [PubMed] [Google Scholar]
Springer 2001
- Springer S, Soll R. High vs low dose conventional phototherapy for neonatal jaundice. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003308] [DOI] [Google Scholar]
Srinivasjois 2011
- Srinivasjois R, Sharma A, Shah P, Kava M. Effect of induction of meconium evacuation using per rectal laxatives on neonatal hyperbilirubinemia in term infants: a systematic review of randomized controlled trials. Indian Journal of Medical Sciences 2011;65(7):278‐85. [DOI: 10.4103/0019-5359.107388; PUBMED: 23422701] [DOI] [PubMed] [Google Scholar]
Stallard 1996
- Stallard P. Validity and reliability of the Parent Satisfaction Questionnaire. British Journal of Clinical Psychology 1996;35(Pt 2):311‐8. [PUBMED: 8773805] [DOI] [PubMed] [Google Scholar]
Suresh 2003
- Suresh G, Martin CL, Soll R. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004207] [DOI] [PubMed] [Google Scholar]
Tagalakis 2002
- Tagalakis V, Kahn SR, Libman M, Blostein M. The epidemiology of peripheral vein infusion thrombophlebitis: a critical review. American Journal of Medicine 2002;113(2):146‐51. [PUBMED: 12133753] [DOI] [PubMed] [Google Scholar]
Thomas 2007
- Thomas JT, Muller P, Wilkinson CS. Antenatal phenobarbital for reducing neonatal jaundice after red cell isoimmunization. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005541.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trikalinos 2009
- Trikalinos TA, Chung M, Lau J, Ip S. Systematic review of screening for bilirubin encephalopathy in neonates. Pediatrics 2009;124(4):1162‐71. [DOI: 10.1542/peds.2008-3545; PUBMED: 19786450] [DOI] [PubMed] [Google Scholar]
Tudehope 1991
- Tudehope D, Bayley G, Munro D, Townsend S. Breast feeding practices and severe hyperbilirubinaemia. Journal of Paediatrics and Child Health 1991;27(4):240‐4. [PUBMED: 1958424] [DOI] [PubMed] [Google Scholar]
Unal 2008
- Unal S, Arhan E, Kara N, Uncu N, Aliefendioglu D. Breast‐feeding‐associated hypernatremia: retrospective analysis of 169 term newborns. Pediatrics International 2008;50(1):29‐34. [DOI: 10.1111/j.1442-200X.2007.02507.x; PUBMED: 18279201] [DOI] [PubMed] [Google Scholar]
Vohr 2012
- Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. Journal of Pediatrics 2012;161(2):222‐8.e3. [DOI: 10.1016/j.jpeds.2012.01.057; PUBMED: 22421261 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179‐201. [PUBMED: 3081865] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2003
- Walsh MC, Wilson‐Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. Journal of Perinatology 2003;23(6):451‐6. [DOI: 10.1038/sj.jp.7210963; PUBMED: 13679930] [DOI] [PubMed] [Google Scholar]
Watchko 2013
- Watchko JF, Tiribelli C. Bilirubin‐induced neurologic damage ‐ mechanisms and management approaches. New England Journal of Medicine 2013;369(21):2021‐30. [DOI: 10.1056/NEJMra1308124; PUBMED: 24256380] [DOI] [PubMed] [Google Scholar]
Wu 2012
- Wu J, Mu D. Vascular catheter‐related complications in newborns. Journal of Paediatrics and Child Health 2012;48(2):E91‐5. [DOI: 10.1111/j.1440-1754.2010.01934.x; PUBMED: 21199061] [DOI] [PubMed] [Google Scholar]
Yang 2013
- Yang WC, Zhao LL, Li YC, Chen CH, Chang YJ, Fu YC, et al. Bodyweight loss in predicting neonatal hyperbilirubinemia 72 hours after birth in term newborn infants. BMC Pediatrics 2013;13:145. [DOI: 10.1186/1471-2431-13-145; PUBMED: 24053490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2010
- Yu Z, Han S, Guo X, Gao C, Ji C. Chinese herbal medicines for the treatment of neonatal jaundice. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD008434] [DOI] [Google Scholar]


