Abstract
Rapid-start, immediate antiretroviral therapy (ART) initiation is a novel intervention that leads to earlier viral suppression. Longer-term data is essential before supporting this strategy more widely. CrescentCare, a federally qualified health center in New Orleans, followed 195 patients who received same-day ART; here we present a continuum of care for immediate ART intervention.
Keywords: care continuum, HIV, immediate ART, rapid ART
All persons living with HIV (PLWH) should be started on antiretroviral therapy (ART) [1]. Immediate ART, on the day of linkage, is a novel strategy with increasing data. Internationally, 3 trials have demonstrated higher rates of viral suppression and retention in care with immediate ART [2–4]. Domestically, San Francisco, our group, and a large academic center in Atlanta have demonstrated decreased time to ART initiation and viral suppression [5–7]. San Francisco has published on successful sustained viral suppression [8], but it is essential to demonstrate that rapid ART can be implemented in community-based clinics in the Deep South. Our study evaluates a rapid-start intervention for 2 groups over the same time period: (1) CrescentCare Start Initiative (CCSI): patients who were newly diagnosed, linked immediately, and offered same-day ART; and (2) Early Intervention Services (EIS): patients who were ART naïve, diagnosed >72 hours (range, 4 days–25 years), linked on the day of contact, and offered same-day ART. We present here a comparison of these 2 groups and outline a care continuum for both.
METHODS
On World AIDS Day, 2016, our health center commenced the CCSI program for all newly diagnosed PLWH. The first patient was seen on December 6, with the EIS cohort added on December 22. Methods for the implementation of CCSI at our agency have been published previously [7]. Highlights of the program include a community-based point-of-care HIV testing program, a dedicated linkage coordinator, transportation support, a 30-day dose pack of tenofovir alafenamide/emtricitabine and dolutegravir (TAF/FTC and DTG), and patient follow-up with a provider within 4 weeks of diagnosis.
For this analysis, we compared CCSI and EIS patients enrolled in the program from December 6, 2016, through February 28, 2018. CCSI patients were linked within 72 hours of diagnosis; any ART-naïve patient linked after that period was in the EIS cohort. Two patients missed their initial CCSI appointment and were then followed in the EIS cohort. Lab values and provider visits were captured for an additional 6 months through August 31, 2018. We created continua for both groups that compared linkage, immediate ART initiation, retention in care, and viral suppression. For retention in care, the HRSA definition (2 provider visits separated by 90 days within 12 months) was used. Viral suppression was defined as anyone with a viral load <200 c/mL within 6 months of the study period. Retention in care did not control for patients who transferred into another clinic. Viral suppression was confirmed in the Louisiana HIV surveillance database and included patients who transferred care.
Statistical significance was determined by Z-scores for population proportion and Kruskal-Wallis testing for 1-way analysis of variance. Logistic regression methods were used to assess differences between the EIS and CCSI cohorts after adjustment for potential covariates/confounders.
RESULTS
From December 2016 through February 2018, 130 patients were referred to the CCSI program. Ninety-seven percent (126/130) were linked within 72 hours of diagnosis. Four patients were lost to follow-up, and 126/126 (100%) were started on immediate ART. Please see Table 1 for demographics. Twenty percent (25/126) had a mental health diagnosis documented by ICD-10 code at the initial or second provider visit. The median CD4 count was 444 cells/mm3, and the median viral load was 42 600 copies/mL. Thirty-eight percent (48/126) had a gonorrhea, chlamydia, or syphilis infection diagnosed at the time of linkage.
Table 1.
Demographics and Outcomes
| CCSI (n = 126) | EIS (n = 69) | P | |
|---|---|---|---|
| Sex | |||
| Male | 94 (74.6) | 57 (82.6) | |
| Female | 27 (21.4) | 10 (14.5) | |
| Transfemale | 5 (4.0) | 2 (2.9) | .4533 |
| Race | |||
| African American | 81 (64.3) | 48 (69.6) | .7576 |
| White | 30 (23.8) | 14 (20.3) | |
| Latin/other | 15 (11.9) | 7 (10.1) | |
| HIV risk factor | |||
| MSM | 73 (57.9) | 42 (60.9) | .4775 |
| Heterosexual | 48 (38.1) | 22 (31.9) | |
| IDU | 5 (4.0) | 5 (7.2) | |
| Age <25 y | 35 (27.8) | 23 (33.3) | .4171 |
| Age, ya | 29 + 13 (24, 37) | 29 + 13 (24,37) | .9280 |
| Tobacco use | 50 (41.0) | 38 (55.1) | .0606 |
| Alcohol use | 73 (59.8) | 36 (52.2) | .3041 |
| Hypertension | 12 (9.5) | 11 (15.9) | .1840 |
| Diabetes | 4 (3.2) | 3 (4.3) | .6997 |
| Syphilis/GC/CT | 48 (38.1) | 32 (46.4) | .2609 |
| Mental health DX | 25 (20.0) | 23 (33.3) | .0394 |
| Baseline CD4a | 444 + 375 (265, 640) | 271 + 334.5 (124.5, 459) | .0003 |
| Baseline CD4,a % | 25.7 + 16.6 (15.6, 32.2) | 18 + 16 (11.7, 28.3) | .0022 |
| Baseline VLa | 42 600 + 147 000 (14 000, 161 000) | 70 150 + 187 700 (19 300, 207 000) | .2260 |
| Achieved VS | 125 (99.2) | 65 (94.2) | .0539 |
| Median time to VS | 29 + 33 (22, 55) | 28 + 30 (21, 51) | .6441 |
| Continued VS | 113 (89.7) | 53 (76.8) | .0157 b (.0456) c |
| Retained in care | 116 (92.1) | 55 (79.7) | .0120 b (.0365) c |
Bolded values demonstrate clinical significance.
Abbreviations: CCSI, CrescentCare Start Initiative; CT, chlamydia; DX, diagnosis; EIS, Early Intervention Services; GC, gonococcus; IDU, injection drug user; MSM, men who have sex with men; VL, viral load; VS, viral suppression.
aContinuous measures (age, CD4, VL, time to VL) are expressed as median + interquartile range (25th, 75th percentiles).
bUnadjusted.
cAdjusted for tobacco use.
Almost all (99.2%) achieved viral suppression. The median time from diagnosis to viral suppression was 29 days. One hundred sixteen of 126 (92%) met retention-in-care criteria. Ninety percent (113/126 continued to be virally suppressed [<200 copies/mL]) and had viral load testing within the past 6 months. The Louisiana HIV surveillance database confirmed 2 patients who transferred care.
Variations from the chosen medication regimen were rare. All but 2 patients were started on TAF/FTC and DTG, and no patient had their ART changed due to subsequent liver or kidney abnormalities. One hundred eighteen genotypes were reviewed, with 8 genotypes that failed or were not performed. Clinically significant transmitted resistance was seen in 22/118 (18.7%). Most commonly, patients had NNRTI mutations (18/22); 3/22 had NRTI mutations, and two of these had M184V/I mutations. All patients with transmitted resistance achieved viral suppression.
Seventy EIS patients were referred for immediate linkage and rapid access to ART between December 2016 and February 2018. Sixty-nine were linked into care (median time from diagnosis, 27.5 days; range, 4 days–25 years). All but 1 patient (98.6%) were prescribed ART on the day of linkage. Please see Table 1 for demographics. One-third (23/69, 33.3%) had a mental health diagnosis documented by ICD-10 code at the initial or second provider visit. The median CD4 count was 271 cells/mm3, and the median viral load was 70 150 copies/mL. Forty-six percent (32/69) had a gonorrhea, chlamydia, or syphilis infection diagnosed at the time of linkage.
The EIS cohort, similarly, achieved a high rate of viral suppression (94.2%), with a median time of 28 days from linkage to care. Eighty percent (55/69) met retention-in-care criteria. Seventy-seven percent (53/69) were virally suppressed (<200 copies/mL) and had viral load testing within the past 6 months. One patient was confirmed to have a viral load <200 copies/mL by the Louisiana HIV surveillance database. Controlling for the 6 patients who transferred care out of state, our retention in care for EIS patients was 87% and viral suppression was 84%.
All but 3 patients were started on TAF/FTC and DTG, and no medication changes occurred due to subsequent liver or kidney abnormalities. Sixty-three genotypes were reviewed, with 6 that failed or were not performed. Clinically significant transmitted resistance was seen in 6/63 (9.5%). NNRTI mutations were most common; 5/6 had NRTI mutation including 2/6 with M184V/I mutation, with 1 patient having both classes. All patients with transmitted resistance achieved viral suppression.
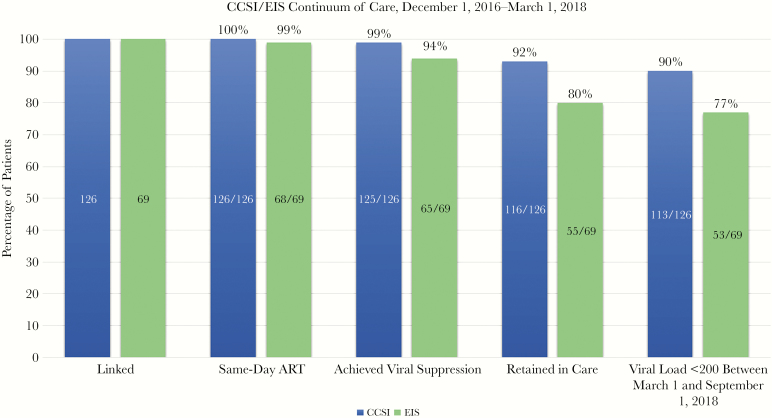
Comparing the CCSI and EIS groups, there were significant differences in retention in care and viral suppression: 92% vs 80% (P < .05) and 90% vs 77% (P < .05), respectively (Figure 1). The median CD4 count for CCSI was significantly higher than that of EIS (444 cells/mm3 vs 271 cells/mm3; P < .05), and there was a significantly higher proportion of mental health diagnoses in EIS (33.3%) vs CCSI (20%; P < .05). Logistic regression models were used to assess whether differences in retention in care and continued viral suppression between EIS and CCSI remained significant after adjustment for potential covariates. Tobacco use, hypertension, mental health diagnosis, and baseline CD4 were considered; the final models included only tobacco use and group (EIS or CCSI). EIS vs CCSI remained significant after adjustment for tobacco use.
Figure 1.
CCSI patients were linked within 72 hours of diagnosis. EIS patients were linked within 72 hours of contact to clinic. Retention in care criteria (2 visits separated by 3 months within the last 12 months) were significantly different between the two groups. Viral suppression last viral load <200 copies/ml and within the last six months were also significantly different between the two groups. Abbreviations: ART, antiretroviral therapy; CCSI, CrescentCare Start Initiative; EIS, Early Intervention Services.
CONCLUSIONS
This is the first published continuum of care for a US-based rapid-start model. Nearly all patients accepted treatment on their first visit in both cohorts. Viral suppression was reached in almost all patients, and transmitted viral resistance did not impact viral suppression.
The CCSI intervention group had significantly better outcomes than the EIS group. For newly diagnosed patients, the retention-in-care rate surpassed the 2017 rate of retention in care for Ryan White programming of 80.9% [10]. For patients who were ART naïve but were not immediately linked postdiagnosis, the rate of retention was consistent with the national standard. Those later to link had a significantly lower CD4 count and a higher rate of mental health diagnoses. The outcomes between CCSI and EIS can potentially be explained by differences in motivation or competing priorities and that the delay in linkage extends to lower rates of follow-up and medication adherence. The difference could also be explained by the profound relationship that develops between provider and patient on the day the patient is diagnosed with HIV, started on ART, counseled on improved health outcomes and U = U.
A rapid-start ART model is well suited for an FQHC, with its extended hours including weekends and same-day appointments. More than 50% of our patient population was enrolled in Medicaid at the time of linkage. It is imperative to ensure no break in ART coverage when developing a rapid ART model [11]. States that have expanded Medicaid are at a distinct advantage.
Our study had limitations. First, many rapid-start referrals were from our testing program and embedded sexual wellness center and were proactively seeking HIV testing. Second, our health center is not hospital affiliated, and most referrals were from the ambulatory setting and were lower acuity. Finally, limitations for scaling up this intervention in other settings include the necessity of a full-time linkage coordinator, incorporation of a 30-day ART dose pack, provider commitment to this model of care including flexible scheduling, and a pathway for guaranteed access to ART either through medical insurance enrollment or Ryan White services.
Innovations in HIV care are urgently needed in the Southern United States. Both cohorts demonstrate that starting patients on the day of diagnosis or linkage, before labs are obtained, is a safe, well-tolerated, and effective intervention. The International Antiviral Society-USA guidelines encourage rapid ART [12], whereas the Department of Health and Human Services guidelines label the practice investigational due to the lack of long-term clinical data in the United States. The success of this intervention demonstrates that community-based clinics can implement rapid ART in a real-world setting. These findings suggest that the benefits extend beyond increased rates of ART uptake and more rapid viral suppression to increased rates of retention in care and sustained viral suppression. This study further supports this model of care.
Acknowledgments
Financial support. Dr. Myers is supported in part by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services; http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 2 January 2019. [Google Scholar]
- 2. Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: the RapIT randomized controlled trial. PLoS Med 2016; 13:e1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Labhardt ND, Ringera I, Lejone TI, et al. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: the CASCADE randomized clinical trial. JAMA 2018; 319:1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koenig SP, Dorvil N, Dévieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLoS Med 2017; 14:e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bacon O, Chin JC, Hsu L, et al. The Rapid Art Program Initiative For HIV Diagnoses (RAPID) in San Francisco. Paper presented at: Conference on Retroviruses and Opportunistic Infections; March 4–7, 2018; Boston, MA. [Google Scholar]
- 6. Colasanti J, Sumitani J, Mehta CC, et al. Implementation of a rapid entry program decreases time to viral suppression among vulnerable persons living with HIV in the Southern United States. Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halperin J, Butler I, Conner K, et al. Linkage and antiretroviral therapy within 72 hours at a federally qualified health center in New Orleans. AIDS Patient Care STDS 2018; 32:39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coffey S, Bacchetti P, Sachdev D, et al. RAPID ART: high virologic suppression rates with immediate ART initiation in a vulnerable urban clinic population. AIDS. 2018 Dec 21. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Health Resources and Services Administration. Ryan White HIV/AIDS program annual client-level data report 2017 2018. http://hab.hrsa.gov/data/data-reports. Accessed 2 January 2019.
- 10. Ford N, Migone C, Calmy A, et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS 2018; 32:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society–USA Panel. JAMA 2018; 320:379–96. [DOI] [PMC free article] [PubMed] [Google Scholar]