Abstract
Background
Intermittent catheterisation is a commonly recommended procedure for people with incomplete bladder emptying. There are now several designs of intermittent catheter (e.g. different lengths, 'ready to use' presentation) with different materials (e.g. PVC‐free) and coatings (e.g. hydrophilic). The most frequent complication of intermittent catheterisation is urinary tract infection (UTI), but satisfaction, preference and ease of use are also important to users. It is unclear which catheter designs, techniques or strategies affect the incidence of UTI, which are preferable to users and which are most cost effective.
Objectives
To compare one type of catheter design versus another, one type of catheter material versus another, aseptic catheterisation technique versus clean technique, single‐use (sterile) catheters versus multiple‐use (clean) catheters, self‐catheterisation versus catheterisation by others and any other strategies designed to reduce UTI and other complications or improve user‐reported outcomes (user satisfaction, preference, ease of use) and cost effectiveness in adults and children using intermittent catheterisation for incomplete bladder emptying.
Search methods
We searched the Cochrane Incontinence Group Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, and handsearching of journals and conference proceedings (searched 30 September 2013), the reference lists of relevant articles and conference proceedings, and we attempted to contact other investigators for unpublished data or for clarification.
Selection criteria
Randomised controlled trials (RCTs) or randomised cross‐over trials comparing at least two different catheter designs, catheterisation techniques or strategies.
Data collection and analysis
Two review authors assessed the methodological quality of trials and abstracted data. For dichotomous variables, risk ratios and 95% confidence intervals were derived for each outcome where possible. For continuous variables, mean differences and 95% confidence intervals were calculated for each outcome. Because of trial heterogeneity, it was not always possible to combine data to give an overall estimate of treatment effect.
Main results
Thirty‐one trials met the inclusion criteria, including 13 RCTs and 18 cross‐over trials. Most were small (less than 60 participants completed), although five trials had more than 100 participants. There was considerable variation in length of follow‐up and definitions of UTI. Participant dropout was a problem for several trials, particularly where there was long‐term follow‐up to measure incidence of UTI. Fifteen trials were more than 10 years old and focused mainly on comparing different catheterisation techniques (e.g. single versus multiple‐use) on clinical outcomes whereas, several more recent trials have focused on comparing different types of catheter designs or materials, especially coatings, and user preference. It was not possible to combine data from some trials owing to variations in the catheters tested and in particular the catheter coatings. Where there were data, confidence intervals around estimates were wide and hence clinically important differences in UTI and other outcomes could neither be identified nor reliably ruled out. No study assessed cost‐effectiveness.
Authors' conclusions
Despite a total of 31 trials, there is still no convincing evidence that the incidence of UTI is affected by use of aseptic or clean technique, coated or uncoated catheters, single (sterile) or multiple‐use (clean) catheters, self‐catheterisation or catheterisation by others, or by any other strategy. Results from user‐reported outcomes varied. The current research evidence is weak and design issues are significant. More well‐designed trials are strongly recommended. Such trials should include analysis of cost‐effectiveness because there are likely to be substantial differences associated with the use of different catheter designs, catheterisation techniques and strategies.
Keywords: Adult, Child, Female, Humans, Male, Urinary Catheters, Urinary Catheters/adverse effects, Equipment Reuse, Patient Dropouts, Urinary Catheterization, Urinary Catheterization/adverse effects, Urinary Catheterization/instrumentation, Urinary Catheterization/methods, Urinary Retention, Urinary Retention/therapy, Urinary Tract Infections, Urinary Tract Infections/etiology, Urinary Tract Infections/prevention & control
Intermittent catheterisation for long‐term bladder management
Background
Intermittent catheterisation is a common treatment used by people who have bladder emptying problems. A hollow tube (catheter) is passed through the channel to the bladder (urethra) or through a surgically made channel to the skin surface to regularly empty the bladder, usually several times every day. This treatment is used by people who have difficulty emptying their bladders themselves. However, this treatment often causes urine infections resulting in school or work absences or even hospitalisations.
Review question
There are different catheter designs and techniques which may affect urine infection. In this review we focused on urine infection in people who used different catheterisation techniques; different designs of catheter (coated [pre‐lubricated] or uncoated [separate or no lubricant]); sterile (single‐use) catheters or clean (multiple‐use) catheters; self‐catheterisation or catheterisation by others (such as parents or carers); and other strategies designed to reduce urine infection, including different ways of catheter cleaning for multiple‐use. We also focused on user satisfaction, preference and ease of use.
Study characteristics
Studies on urine infection and intermittent catheterisation are difficult for researchers to undertake because participants need to take part for many months and tend to drop out. Many of the reviewed trials were too small and had problems with participants not wanting to continue in the trial. Definitions of urine infection also varied considerably. Thus well‐designed and long‐term trials are strongly recommended.
Key results and quality of the evidence
Thirty‐one trials were included in the review. Currently, the evidence is weak and it is not possible to state that any catheter design, technique or strategy is better than another. Future research should consider cost as it is likely that there are big differences between different catheter designs and techniques.
Background
Intermittent catheterisation (IC) is the act of passing a catheter into the bladder to drain urine via the urethra or other catheterisable channel such as a Mitrofanoff continent urinary diversion (surgically constructed passage connecting bladder with abdominal surface). The catheter is removed immediately after urine drainage is complete. It is widely advocated as an effective bladder management strategy for incomplete bladder emptying.
IC can be undertaken by people of all ages, including the very elderly and children as young as four years old with parental supervision. Carers can also be taught the procedure where this is acceptable both to the IC user and carer. Even some people with disabilities such as blindness, lack of perineal sensation, tremor, mental disability and paraplegia can learn how to master the technique (Cottenden 2013).
Individualised care plans should identify appropriate catheterisation frequency based on user goals, impact on quality of life, frequency‐volume charts, functional bladder capacity and post voiding residual urine. The number of catheterisations per day varies; a general rule for adults is to catheterise frequently enough to avoid a bladder urine volume greater than 500 mL but clinical decisions are also provided by urodynamic findings, detrusor pressures on filling, presence of reflux and renal function.
Advantages of IC over indwelling catheterisation include:
greater opportunity for self‐care and independence;
reduced risk of common indwelling catheter‐associated complications, such as damage to the urethra or bladder neck, creation of false passages, persistent symptomatic urinary tract infection, stones in the bladder;
reduced need for equipment and appliances e.g. drainage bag;
greater freedom for expression of sexuality;
potential for reduced urinary symptoms (frequency, urgency, incontinence) between catheterisations.
Reviewing the topic, Wyndaele 2002 provides a full discussion of the benefits as well as potential adverse effects of IC. Urinary tract infection (UTI) is the most frequent complication and catheterisation frequency and the avoidance of bladder over‐filling are recognised as important prevention measures. It is recognised that prostatitis is a risk in men, but epididymitis and urethritis are relatively rare. Trauma from catheterisation, measured by haematuria, is reported but lasting effects appear limited. Estimates of the prevalence of urethral strictures and false passages increase with longer use of IC or with traumatic catheterisation. In a follow‐up of children with spina bifida who had used IC with an uncoated PVC catheter and added lubricant for at least five years, the incidence of urethritis, false passage, or epididymitis was very low and adherence to the protocol was excellent (Campbell 2004). Wyndaele and colleagues concluded that the most important preventative measures were good education of all involved in IC, adherence to the catheterisation protocol, use of an appropriate catheter material and good catheterisation technique, although the level of evidence for these clinical opinions is weak.
Designs and characteristics of catheters used in IC vary considerably so evaluation and selection of products is complex. Plain uncoated catheters (typically clear plastic PVC) are packed singly in sterile packaging. As per industry standards, all disposable catheters are labelled for one time use but PVC catheters are frequently reused because of cost or concern about the environment. Most are used with separate lubricant, although this is a matter of personal choice, some IC users preferring not to use lubricant or just to use water. Cleansing of the catheter varies from being washed with soap and water, boiled, soaked in disinfectant, or microwaved. Cleaned catheters are air dried and then stored in a convenient container (often plastic containers or bags). Coated catheters are single‐use only (they may not be cleaned and reused) and are designed to improve catheter lubrication and ease of insertion,which may (according to manufacturers) reduce trauma and UTI. The most common coating is hydrophilic, which is either pre‐activated, or requires the addition of water at the time of use to form a lubricious layer. Some non‐hydrophilic catheter designs are pre‐lubricated (whereby the catheter is supplied pre‐packed with a coating of water‐soluble gel). There are also several pre‐lubricated products with an integrated collection bag (all‐in‐one) which gives flexibility for the user and is efficient for hospital use. Finally, the design of catheters also varies, for example, length of catheter (standard versus more compact).
Definition of terms
1. Symptomatic UTI: a positive urine culture and the presence of symptoms, based on the UTI definition of the NIDRR 1992 (positive urine culture with pyuria and one or more systemic symptoms [fever, loin pain, dysuria, urgency, haematuria]). Some trials had varying definitions and we chose to accept symptomatic UTI as reported in the trials reviewed.
2. Asymptomatic bacteriuria: positive urine culture but absence of symptoms, again as reported in the trials.
3. Catheter design: different shapes, sizes, lengths and tips of catheter, different packaging
3a: Hydrophilic coated
i. Activated system, ready to use
ii. Activated closed system with integrated collection bag
iii. Not activated: sterile water provided in package for activation
iv. Not activated: water added by user
3b: Uncoated
i. Pre‐lubricated closed system with integrated collection bag
ii. Pre‐lubricated system with protective sleeve for no‐touch insertion
iii. Non‐lubricated: water‐soluble gel added by user.
4. Catheter materials: the base material of the catheter (e.g. PVC, PVC‐free, latex) and the presence or not of a bonded coating (e.g. hydrophilic).
5. Aseptic Technique (also referred to as ‘sterile’ technique in some older trials): sterile gloves, sterile single‐use catheter, sterile cleansing solution, sterile drainage tray and an aseptic technique for the catheterisation procedure.
6. Clean technique: clean gloves (or no gloves in the case of a user self‐catheterising), non‐sterile cleansing solution and a clean receptacle in which to drain urine. It should be noted that an aseptic technique always includes a sterile (single‐use) catheter whereas a clean technique may include a sterile catheter or a clean (multiple‐use) catheter.
7. Sterile catheters: catheters removed from sterile packaging and used once only. Coated catheters have a hydrophilic or other lubricated coating intended to replace the need for separate lubricant. Coated catheters are not intended for re‐use and are therefore defined as sterile.
8. Clean Catheters: Sterile catheters which are then re‐used after cleaning, typically with soap and water and air dried. The material is uncoated. Lubricant may be applied before insertion.
Objectives
To determine if certain designs of catheter, catheterisation technique, or other strategies (including re‐use) are better than others in terms of UTI, complications, user satisfaction, preference, ease of use and/or cost‐effectiveness for adults and/or children whose long‐term (with no predicted endpoint) bladder management is by IC.
The following specific comparisons were addressed.
Aseptic technique versus clean/other aseptic technique
Single‐use (sterile) catheter versus multiple‐use (clean) catheter
Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated)
One catheter length versus another catheter length
Any other techniques, strategies or designs that influence UTI, other complications or user‐reported outcomes
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials, including cross‐over trials, comparing catheterisation designs, techniques and other strategies for long‐term bladder management by IC.
Types of participants
Adults or children requiring IC for long‐term bladder management.
Types of interventions
Comparisons of intermittent catheter designs, catheterisation techniques and catheterisation strategies.
Types of outcome measures
1. Catheter‐associated infection (definition of infection as used in trial reports)
Symptomatic UTI (primary outcome variable)
Asymptomatic bacteriuria
2. Other complications/adverse effects
Urethral trauma/bleeding
Creation of false passages
Haematuria
Stricture formation
3. Participant‐assessed outcomes
Comfort, including ease of insertion and removal
Satisfaction
Preferences
Quality of life measures
4. Economic outcomes
Catheter and equipment costs
Frequency of catheterisation
Resource implications (personnel and other costs to services)
Formal economic analysis (cost‐effectiveness, cost‐utility)
Days missed from employment/school
5. Other outcomes
Microbiological culture of catheter surfaces
Additional outcomes judged to be important when performing the review
Search methods for identification of studies
We did not impose any language or other limits on the searches.
Electronic searches
The review strategy was that developed for the Cochrane Incontinence Review Group. Relevant trials were identified from the Cochrane Incontinence Group Specialised Register of controlled trials which is described, along with the search strategy, under the Incontinence Group's module in The Cochrane Library. The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, and handsearching of journals and conference proceedings. The Incontinence Group Specialised Register was searched using the Group's own keyword system. Search terms were:
topic.urine.incon* AND ({design.cct*} OR {design.rct*}) AND intvent.mech.cath* (All searches were of the keyword field of Reference Manager 2012).
The date of the most recent search of the Register for this version of the review was 30 September 2013.
Extra specific searches were also performed by the review authors details of which are given in Appendix 1.
Searching other resources
We searched the reference lists of relevant articles and conference proceedings for other possible trials. We also contacted one investigator for clarification, which was provided (Cardenas 2011).
Data collection and analysis
Trial selection
To be comprehensive in the initial reviews, three review authors (JP, MF and KM) assessed each title and abstract of trials identified by the search strategy and a final list was agreed on. Full reports were obtained of all potentially relevant randomised controlled trials based on defined inclusion criteria.
The agreed list of eligible trials was then assessed independently by two of the review authors (JP and MF). Cochrane 'Risk of bias' forms were completed for all trials so that the quality of random allocation, concealment, description of dropouts and withdrawals, analysis by intention‐to‐treat, and blinding during intervention and at outcome assessment or any other potential biases were consistently addressed. Any disagreements between primary review authors (JP and MF) were also discussed with KM and CM and consensus reached.
Methodological quality assessment
The quality of eligible trials was assessed independently by two of the review authors (JP, MF) using the Cochrane 'Risk of bias' tool, which includes quality of random allocation and concealment, blinding during intervention and at outcome assessment, dropouts and withdrawals, and source of funding. When trial quality was assessed any disagreements between review authors were discussed and consensus reached. 'Risk of bias' forms were completed for all trials.
Data abstraction
Relevant data regarding inclusion criteria (trial design, participants, interventions and outcomes), quality criteria (randomisation, blinding and control) and results were extracted independently by two review authors using a data abstraction form developed specifically for this review. Excluded trials and reasons for exclusion have been detailed in the 'Characteristics of excluded studies table.
Results
Description of studies
Results of the search
In the original review (Moore 2007), we included 14 trials. For the current review an additional 33 were identified, of which 17 met the inclusion criteria. Two trials (Fader 2001; Pascoe 2001) excluded from the original review met the revised inclusion criteria. In all, 18 studies were excluded: one was a review, 17 did not meet the review inclusion criteria. The final number was 31 trials addressing some aspect of sterile or clean intermittent catheterisation and using a measure of catheter‐associated infection, other complication, or participant satisfaction.
Trial participants were mainly adults with spinal cord injuries, but also included other groups such as children with neurogenic bladders due to myelomeningocoele, men with prostatic obstruction and women with multiple sclerosis. Those trials that used infection as the primary outcome variable were generally of longer duration (three to 12 months) and those that focused on participant preference/ease of use were generally of shorter duration (eight weeks or less). There were variations in definitions of UTI. Attrition was a problem for several trials and most were underpowered. Fifteen trials were between 10 and 20 years old. The flow of literature through the assessment process is shown in the PRISMA diagram (Figure 1).
Figure 1.

PRISMA study flow diagram
Included Studies
Thirty‐one trials met the inclusion criteria (Biering‐Sorensen 2007; Cardenas 2009; Cardenas 2011; Chartier‐Kastler 2011; Chartier‐Kastler 2013; Costa 2013; Day 2003; Denys 2012; De Ridder 2005; Domurath 2011; Duffy 1995; Fader 2001; Fera 2002; Giannantoni 2001; King 1992; Leek 2013; Leriche 2006; Mauroy 2001; Moore 1993; Moore 2006; Moore 2013; Pachler 1999; Pascoe 2001; Prieto‐Fingerhut 1999; Quigley 1993; Sarica 2010; Schlager 2001; Sutherland 1996; Taweesangsuksalul 2005; Vapnek 2003; Witjes 2009).
Eight of the 31 trials did not provide data in a format that could be used in meta‐analysis (Denys 2012; Fader 2001; Fera 2002; Giannantoni 2001; Mauroy 2001; Pascoe 2001, Sarica 2010; Taweesangsuksalul 2005).
Design
There were 13 parallel group randomised controlled trials (Cardenas 2009; Cardenas 2011; Day 2003; De Ridder 2005; Duffy 1995; Fera 2002; King 1992; Moore 2006; Prieto‐Fingerhut 1999; Quigley 1993; Sutherland 1996; Vapnek 2003; Witjes 2009).
The other 18 were cross‐over randomised controlled trials:
15 with two arms (Biering‐Sorensen 2007; Chartier‐Kastler 2013; Chartier‐Kastler 2011; Costa 2013; Denys 2012; Domurath 2011; Giannantoni 2001; Leek 2013; Leriche 2006; Moore 1993; Moore 2013; Pachler 1999; Pascoe 2001; Schlager 2001; Taweesangsuksalul 2005);
two with three arms (Mauroy 2001; Sarica 2010); and
one with four arms (Fader 2001).
As the number of randomised trials was adequate for the review, it was not deemed necessary to include non‐randomised trials. The inherent risk of bias in a non‐randomised design makes the results difficult to apply to outcomes such as urinary tract infection. In particular, the studies reviewed had design limitations; non‐randomisation would have compounded the limitations. However, further consideration could be given to the use of both non‐randomised and qualitative studies to assess user opinion or cost‐effectiveness.
Sample sizes
A total of 1737 participants were enrolled in the 31 trials with 1388 (80%) of all participants completing data collection. However, in two studies involving a total of 224 participants, the completion rate was not stated and we have assumed that all participants completed.
In most trials the sample size was small. Only five of the 31 trials had a sample size of 100 or more (Cardenas 2011; Chartier‐Kastler 2013; De Ridder 2005; Denys 2012; Witjes 2009).
Twelve trials included statistical power calculations (Biering‐Sorensen 2007; Cardenas 2009; Cardenas 2011; Chartier‐Kastler 2011; Chartier‐Kastler 2013; De Ridder 2005; Domurath 2011; Fader 2001; Leek 2013; Moore 2006; Moore 2013; Witjes 2009) only one (Chartier‐Kastler 2013) was able to achieve its predicted sample size.
At trial endpoint, sample sizes ranged from 10 (Schlager 2001; Taweesangsuksalul 2005) to 114 (Cardenas 2011) participants in total.
Participants
Trials included various types of patients using IC:
spinal cord injury (Cardenas 2011; Day 2003; De Ridder 2005; Denys 2012; Domurath 2011; Giannantoni 2001; King 1992; Moore 2006; Prieto‐Fingerhut 1999; Sarica 2010);
patents with spinal cord injury who had experienced more than one UTI (Cardenas 2009);
stroke (Quigley 1993);
spinal cord lesion (Biering‐Sorensen 2007; Chartier‐Kastler 2011);
prostatic obstruction from prostatic hyperplasia (Duffy 1995; Pachler 1999);
children with spina bifida (Moore 1993; Moore 2013; Schlager 2001);
spinal cord injury, Hinman syndrome, or spinal dysraphism (Sutherland 1996);
neurogenic bladder disorders (Costa 2013; Taweesangsuksalul 2005);
vesico‐sphincteric problem of neurological origin (Leriche 2006);
a variety of diagnoses (Chartier‐Kastler 2013; Fera 2002; Leek 2013; Mauroy 2001; Witjes 2009);
no stated aetiology of the bladder dysfunction (Fader 2001; Pascoe 2001; Vapnek 2003).
Age and gender also varied:
boys and girls with spina bifida (Moore 1993; Moore 2013; Schlager 2001);
boys with neurogenic bladders (Sutherland 1996);
adult men with prostatism (Duffy 1995; Pachler 1999);
adult men with a vesico‐sphincteric problem of neurological origin (Leriche 2006);
adult men and women with spinal cord injury or lesion (Biering‐Sorensen 2007; Cardenas 2009; Cardenas 2011; Chartier‐Kastler 2011; Chartier‐Kastler 2013; Day 2003; De Ridder 2005; Denys 2012,Domurath 2011; King 1992; Moore 2006, Prieto‐Fingerhut 1999; Sarica 2010);
adult males and females with non‐specified neurogenic bladder (Costa 2013; Leek 2013, Taweesangsuksalul 2005).
Twelve trials included only men as participants (Chartier‐Kastler 2011; Day 2003; De Ridder 2005; Domurath 2011; Duffy 1995; King 1992; Leriche 2006; Fader 2001; Pachler 1999; Sarica 2010; Sutherland 1996; Vapnek 2003); and one included women only (Biering‐Sorensen 2007). Age and gender were not stated in one (Quigley 1993), and gender was not stated in another (Pascoe 2001).
Setting
Settings ranged from:
acute care within an intensive care unit (Day 2003);
rehabilitation hospital (Biering‐Sorensen 2007; Giannantoni 2001; King 1992; Moore 2006; Prieto‐Fingerhut 1999; Quigley 1993);
spinal cord injury unit with follow‐up in the community (Cardenas 2011);
hospital outpatient clinic (Chartier‐Kastler 2011; Fera 2002; Leek 2013; Leriche 2006; Martins 2009; Mauroy 2001; Sarica 2010);
continuing or long‐term care (Duffy 1995);
paediatric clinic (Moore 2013);
community (Cardenas 2009; De Ridder 2005; Fader 2001; Moore 1993; Pachler 1999; Pascoe 2001; Schlager 2001; Sutherland 1996; Vapnek 2003).
The setting was not described in six trials (Chartier‐Kastler 2013; Costa 2013; Denys 2012; Domurath 2011; Taweesangsuksalul 2005; Witjes 2009).
Types of interventions
Interventions were separated into five main categories but in each of these there were variations in technique, catheter design and multiple‐use versus single‐use. In most cases there was no clear distinction made between self and caregiver/healthcare professional catheterisation. There were various comparisons made:
aseptic versus clean or other aseptic technique (Day 2003, Duffy 1995; King 1992; Moore 2006; Prieto‐Fingerhut 1999; Quigley 1993);
single‐use (sterile) catheters to multiple‐use (clean) catheters (Duffy 1995; King 1992; Leek 2013; Moore 1993; Moore 2013; Pachler 1999; Prieto‐Fingerhut 1999; Schlager 2001; Sutherland 1996; Vapnek 2003);
hydrophilic‐coated catheters or pre‐lubricated catheters with standard (coated or uncoated) catheters (Cardenas 2009; Cardenas 2011; De Ridder 2005; Fader 2001; Leriche 2006; Mauroy 2001; Moore 2013; Pachler 1999; Pascoe 2001; Sarica 2010; Sutherland 1996; Vapnek 2003; Witjes 2009);
uncoated catheter compared with a pre‐lubricated catheter (Giannantoni 2001);
one catheter length versus another length (Biering‐Sorensen 2007; Chartier‐Kastler 2011; Chartier‐Kastler 2013; Costa 2013; Domurath 2011);
one gel versus a standard gel (Fera 2002; Taweesangsuksalul 2005).
No trials were found comparing cleaning techniques or self‐catheterisation to catheterisation by others. The numbers add up to more than 31 since some trials fit into more than one category. Within these categories were various subcategories which are presented in detail in the Results section below.
Duration of intervention
In each arm of the cross‐over trials participants were catheterised for either one catheterisation (Taweesangsuksalul 2005); one to two days (Biering‐Sorensen 2007, Domurath 2011); one week (Fader 2001, Pascoe 2001); 12 to 14 days (Chartier‐Kastler 2011); three to four weeks (Pachler 1999, Denys 2012); six to seven weeks (Sarica 2010, Giannantoni 2001); 12 weeks (Chartier‐Kastler 2013), four months (Leek 2013, Schlager 2001); six months (Moore 1993), 48 weeks (Moore 2013); or for the time required to use 10 catheters (Costa 2013), 20 catheters (Leriche 2006), or 27 catheters (Mauroy 2001).
In the 13 parallel group trials, the duration of intervention varied:
24 hours (Day 2003);
four days (Quigley 1993);
one month (King 1992; Witjes 2009);
two months (Sutherland 1996);
three months (Duffy 1995);
four months (Fera 2002);
up to six months (Cardenas 2011);
up to 12 months (Cardenas 2009; De Ridder 2005; Moore 2006; Vapnek 2003);
or was of unclear duration (Prieto‐Fingerhut 1999).
Outcome measures
Twenty trials reported either symptomatic UTI or asymptomatic bacteriuria as the primary outcome measure (Cardenas 2009; Cardenas 2011; Day 2003; De Ridder 2005; Duffy 1995; Fera 2002; Giannantoni 2001; King 1992; Leek 2013; Mauroy 2001; Moore 1993; Moore 2006; Moore 2013; Pachler 1999; Prieto‐Fingerhut 1999; Quigley 1993; Sarica 2010; Schlager 2001; Sutherland 1996; Vapnek 2003).
The definition of symptomatic UTI varied between trials from 'clinical infection with symptoms of UTI and for which treatment was prescribed' to 10x5 CFU/mL (colony‐forming units/millilitre) plus at least one of the following symptoms of fever, pyuria, haematuria, chills, increased spasms or autonomic dysreflexia. (See the Characteristics of included studies table for a complete description provided in each report).
Six trials reported on either microscopic or macroscopic haematuria (De Ridder 2005; Mauroy 2001; Moore 2013; Pachler 1999; Sutherland 1996; Vapnek 2003).
16 trials included user‐reported outcomes (Biering‐Sorensen 2007; Cardenas 2011; Chartier‐Kastler 2011; Chartier‐Kastler 2013; Costa 2013; Domurath 2011; Fader 2001; Giannantoni 2001; Leriche 2006; Mauroy 2001; Moore 2013; Pachler 1999; Pascoe 2001; Sarica 2010; Taweesangsuksalul 2005; Witjes 2009). Some trials reported overall satisfaction, others reported mean satisfaction. These results could not be combined as they provided dichotomous and continuous data. Moreover, the tools used to measure user‐reported outcomes varied widely and only one trial used a validated tool (Chartier‐Kastler 2011).
Although some of the trials included calculations of the costs of one catheter versus another, none of the trials undertook a formal evaluation of cost‐effectiveness.
Excluded trials
Eighteen trials were excluded: one was a review, 17 did not meet the review inclusion criteria. (See the Characteristics of excluded studies table).
On‐going trials
None known.
Risk of bias in included studies
Details of the quality of each trial are given in the 'Risk of bias' tables in Characteristics of included studies. The findings are summarised in Figure 2; Figure 3.
Figure 2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
As with the review in 2007, the trials as a whole were methodologically weak. They were generally underpowered with variations in outcomes, particularly with respect to the definition of UTI and outcomes addressing participant preference. In the previous review, some studies included bacteriuria in the absence of symptoms (asymptomatic bacteriuria) as an outcome. The updated search of trials reflected current practice in which asymptomatic bacteriuria is not considered clinically relevant as a marker for UTI and only one trial included this measure (Leek 2013). Follow‐up ranged from 24 hours to 12 months; 15 of the 31 trials were more than 10 years old and were typically less rigorous in design and analysis. Attrition of participants was a particular problem (see below).
Allocation
The majority of the trial reports did not describe the method of allocation. In those that did, two (Moore 2006; Moore 2013) used opaque sealed envelopes opened by a third party who informed the research nurse of the assignment. De Ridder 2005 randomised participants in blocks of four and used opaque envelopes opened by the investigator but supplied from a central research office, and Quigley 1993 used 'blind selection of a marked piece of paper from a box'. Duffy 1995 did not describe method of randomisation but did indicate participants were stratified for research site or presence/absence of UTI. Eleven trials did not describe the method of randomisation, stating only that participants were 'randomised'.
Blinding
Mauroy 2001 masked all three test products. Other authors were unable to blind participants due to catheter packaging.
Data entry clerk and laboratory staff were blinded in six trials (Biering‐Sorensen 2007; Leek 2013; Moore 2006; Moore 2013; Sarica 2010; Witjes 2009).
Incomplete outcome data
In three studies (Cardenas 2011; De Ridder 2005; Moore 2013), attrition bias was assessed to be high risk. In each study the rate of dropout was higher in the hydrophilic‐coated arm compared to the uncoated arm.
In the De Ridder 2005 trial only 53% of the participants in the standard (uncoated) catheter group and 41% of the participants in the treatment (hydrophilic‐coated) catheter groups remained at the 12‐month study endpoint. Similar challenges were met by Cardenas 2011 with 60% of participants remaining in the standard (uncoated) arm and 43% in the treatment (hydrophilic‐coated) arm. Moore 2013 reported a completion rate of 75% in the standard (uncoated) arm and 62% in the treatment (coated) arm. Reasons for dropouts in all three trials included loss to follow‐up, preference for one study product, adverse events and withdrawing consent and improvement in voiding function as spinal cord injury healed. In the Cardenas 2011 and De Ridder 2005 trials, change in voiding status meant that some participants no longer required on‐going IC.
Other potential sources of bias
Intention‐to‐treat analysis
Three authors described intention‐to‐treat analysis (Chartier‐Kastler 2011; Moore 2006; Witjes 2009).
Source of funding
The source of funding was stated in 22 of the 31 studies, with 16 of those supported by industry (see 'Risk of bias' tables). In two of the 16 industry‐supported studies it was stated that only products were supplied for testing, whereas in the other 14 studies the extent of funding was unclear.
Effects of interventions
Comparison 01: Aseptic technique versus clean/other aseptic technique.
Six trials (Day 2003; Duffy 1995; King 1992; Moore 2006; Prieto‐Fingerhut 1999; Quigley 1993) reported on types of catheterisation technique. Three trials (Day 2003; Prieto‐Fingerhut 1999; Quigley 1993) compared an uncoated, pre‐lubricated catheter with an integrated bag versus an uncoated, non‐lubricated catheter to determine whether there was a difference in the incidence of UTI or asymptomatic bacteriuria. Whereas Day 2003 and Quigley 1993 used an aseptic technique in both arms, Prieto‐Fingerhut used aseptic technique with the pre‐lubricated catheter closed system and clean technique with the open system. Three trials (Duffy 1995; King 1992; Moore 2006) compared aseptic versus clean technique using uncoated, non‐lubricated catheters.
1.1 Asymptomatic bacteruria
Day 2003 and Moore 2006 both reported asymptomatic bacteriuria (Analysis 1.1). Day 2003 was a feasibility trial, which found that none of six participants in the closed system arm had asymptomatic bacteriuria compared to two out of five participants in the open system arm. For Moore 2006, the results were similar in the two groups (seven out of 16 for the aseptic arm versus nine out of 20 for the clean arm). The trials were too small to be reliable.
Analysis 1.1.

Comparison 1 Aseptic technique versus clean/other aseptic technique, Outcome 1 Number with asymptomatic bacteriuria.
1.2 Symptomatic UTI
Five out of the six trials reported symptomatic UTI (Duffy 1995; King 1992; Moore 2006; Prieto‐Fingerhut 1999; Quigley 1993) (Analysis 1.2). All had wide confidence intervals and there was no apparent trend favouring either technique.
Analysis 1.2.
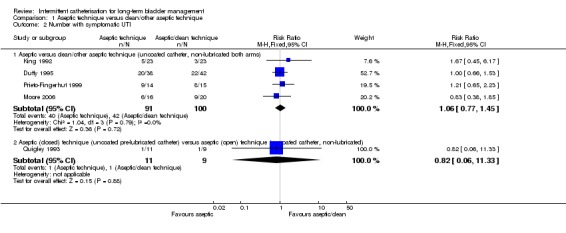
Comparison 1 Aseptic technique versus clean/other aseptic technique, Outcome 2 Number with symptomatic UTI.
1.3 Weeks to onset of UTI
Three trials (Duffy 1995; King 1992; Moore 2006) measured mean time to onset of UTI. None showed statistically significant results favouring either technique (Analysis 1.3). Mean onset ranged from 4.3 weeks (aseptic group) and 4.6 weeks (clean group) (Moore 2006); 3.11 weeks (aseptic group) and 3.48 weeks (clean group (Duffy 1995); and 1.25 weeks (aseptic group) and 1.12 weeks (clean group) (King 1992).
Analysis 1.3.

Comparison 1 Aseptic technique versus clean/other aseptic technique, Outcome 3 Weeks to onset of symptomatic UTI.
Comparison 02: Single‐use (sterile) catheter versus multiple‐use (clean) catheter
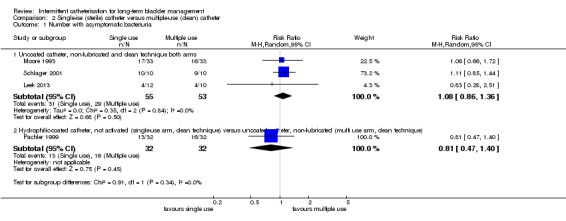
Five parallel trials (Duffy 1995; King 1992; Prieto‐Fingerhut 1999; Sutherland 1996; Vapnek 2003) and five two‐arm cross‐over trials (Leek 2013; Moore 1993; Moore 2013; Pachler 1999; Schlager 2001) compared single‐use (sterile) catheters with multiple‐use (clean) catheters. Six used clean technique in both arms (Leek 2013; Moore 2013; Pachler 1999; Schlager 2001; Sutherland 1996; Vapnek 2003) and four trials used aseptic technique with the single‐use catheter and clean technique with the multiple‐use catheter (Duffy 1995; King 1992; Moore 1993; Prieto‐Fingerhut 1999). Six compared one uncoated catheter used once with another uncoated catheter used multiple times (Duffy 1995; King 1992; Leek 2013; Moore 1993; Prieto‐Fingerhut 1999; Schlager 2001). In these trials the uncoated, non‐lubricated catheter used was the same in both arms, with the exception of Prieto‐Fingerhut 1999 who compared an uncoated, pre‐lubricated catheter with an integrated bag with an uncoated, non‐lubricated catheter. Four compared a hydrophilic‐coated (not activated) single‐use catheter with an uncoated, non‐lubricated (water added by user) multiple‐use catheter (Moore 2013; Pachler 1999; Sutherland 1996; Vapnek 2003).
Trial time frames ranged from three weeks to 12 months. Cleaning methods varied as did the number of times the multiple‐use catheter was re‐used. Pachler 1999, King 1992 and Vapnek 2003 instructed participants to use the cleaned catheter for 24 hours; in the Duffy 1995 and Moore 2013 trials clean catheters were re‐used for one week; Sutherland 1996, Moore 1993, Schlager 2001 and Leek 2013 do not describe the number of re‐uses of the non‐coated catheter.
2.1 Number with asymptomatic bacteriuria
Four cross‐over trials reported on the prevalence of asymptomatic bacteriuria (Leek 2013; Moore 1993; Pachler 1999; Schlager 2001) (Analysis 2.1). None reported a significant difference between the two arms.
Analysis 2.1.
Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 1 Number with asymptomatic bacteriuria.
2.2 Number with symptomatic UTI
Eight studies reported on number of symptomatic UTI, four parallel trials (Duffy 1995; King 1992; Prieto‐Fingerhut 1999; Sutherland 1996) and four cross‐over studies (Leek 2013; Moore 2013; Pachler 1999; Schlager 2001) (Analysis 2.2). All lay across the no‐difference line. We decided not to derive a summary estimate because of heterogeneity; however, there was no suggestion of a trend favouring either of the approaches.
Analysis 2.2.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 2 Number with symptomatic UTI.
2.3 Weeks to onset of symptomatic UTI
Two trials (Duffy 1995; King 1992) addressed weeks to onset of UTI and found no statistically significant differences (Analysis 2.3).
Analysis 2.3.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 3 Weeks to onset of symptomatic UTI.
2.4 Number with microscopic haematuria
One RCT (Sutherland 1996) reported no significant difference in microscopic haematuria between arms (Analysis 2.4).
Analysis 2.4.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 4 Number with microscopic haematuria.
2.5 Number with urethral trauma/bleeding/macroscopic bleeding
One cross‐over trial (Pachler 1999) reported on bleeding and no significant difference was found between arms (Analysis 2.5).
Analysis 2.5.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 5 Number with urethral trauma/bleeding/macroscopic haematuria.
2.6 Overall satisfaction
The only trial reporting overall satisfaction as an outcome was Moore 2013, who found a slightly higher number (42/48) of participants reporting satisfaction with the multiple‐use (uncoated) catheter compared to the single‐use (hydrophilic‐coated) catheter (35/48) (Analysis 2.6).
Analysis 2.6.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 6 Number reporting overall satisfaction.
2.7 Mean satisfaction
Sutherland 1996 reported on mean satisfaction level with no statistical difference found (Analysis 2.7).
Analysis 2.7.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 7 Mean satisfaction.
2.8 Ease of handling
The Pachler 1999 cross‐over trial found no significant difference between arms. The Moore 2013 cross‐over trial found in favour of the control arm (Analysis 2.8).
Analysis 2.8.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 8 Number reporting ease of handling.
2.9 Mean ease of handling
Sutherland 1996 reported a mean ease of handling with no statistical difference found (Analysis 2.9).
Analysis 2.9.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 9 Mean ease of handling.
2.10 Ease of insertion
Pachler 1999 found no significant difference between the two arms for ease of insertion (Analysis 2.10).
Analysis 2.10.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 10 Number reporting ease of insertion.
2.11 Mean ease of insertion
Sutherland 1996 found no significant difference in mean ease of insertion (Analysis 2.11).
Analysis 2.11.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 11 Mean ease of insertion.
2.12 Number reporting comfort
In the Moore 2013 trial there was no significant difference in reported comfort (Analysis 2.12).
Analysis 2.12.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 12 Number reporting comfort.
2.13 Convenience of product
No significant difference in reported convenience of use was found by Moore 2013 (Analysis 2.13).
Analysis 2.13.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 13 Number reporting convenience of product.
2.14 Mean convenience of product
Sutherland 1996 reported on mean convenience of product finding no significant difference between groups (Analysis 2.14).
Analysis 2.14.

Comparison 2 Single‐ise (sterile) catheter versus multiple‐use (clean) catheter, Outcome 14 Mean convenience of product.
Comparison 03: Hydrophilic‐coated or a pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated)
Fifteen trials compared a hydrophilic‐coated catheter (activated or not activated) or a pre‐lubricated catheter with one or more other catheters (pre‐lubricated, coated or uncoated) (Cardenas 2009; Cardenas 2011; De Ridder 2005; Denys 2012, Fader 2001; Giannantoni 2001; Leriche 2006; Mauroy 2001; Moore 2013; Pachler 1999; Pascoe 2001; Sarica 2010; Sutherland 1996; Vapnek 2003; Witjes 2009). (See Characteristics of included studies table for full description of catheters used).
3.1 Asymptomatic bacteruria
Only one trial reported asymptomatic bacteriuria (Pachler 1999). No significant difference was found between the arms (Analysis 3.1).
Analysis 3.1.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 1 Number with asymptomatic bacteriuria.
3.2 Number with symptomatic UTI
Six trials reported the number of symptomatic UTI (Cardenas 2009; De Ridder 2005; Moore 2013; Pachler 1999; Sutherland 1996; Vapnek 2003) (Analysis 3.2). The estimates from four of these trials (Cardenas 2011; Moore 2013; Pachler 1999; Sutherland 1996) had wide confidence intervals that straddled the no‐difference line.
Analysis 3.2.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 2 Number with symptomatic UTI.
In one study (Vapnek 2003), the risk ratio was not estimable due to the way the data were reported.
In the largest trial (De Ridder 2005), the authors reported on the incidence of UTI of all enrolled participants (N = 123), although the total number of UTIs was not reported. Only 57 participants (33/62 in the PVC arm; 25/61 in the hydrophilic arm) remained in the trial at the endpoint of 12 months and dropout was greater in the hydrophilic‐coated catheter arm. There were fewer patients with one or more UTI in the hydrophilic‐coated catheter group (i.e. results were better for the hydrophilic‐coated catheters) and this was marginally statistically significant (39 out of 61 versus 51 out of 62; RR 0.78; 95% CI 0.62 to 0.97).
We chose not to derive a summary estimate because of the heterogeneity amongst the trials and the problem of attrition bias.
3.3 Number of UTIs per month of use
One trial Cardenas 2011 reported the number of UTIs per month of use, with the hydrophilic arm showing 41/207 and the uncoated arm 76/349, which was not statistically significant (Analysis 3.3).
Analysis 3.3.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 3 Number of UTIs per month of use.
3.4 Number with urethral trauma/bleeding/macroscopic haematuria
Four trials reported on urethral trauma or visible bleeding. Cardenas 2011 noted a higher incidence in the hydrophilic cohort (14/105 versus 6/114) as did De Ridder 2005 (38/55 versus 32/59). One cross‐over trial reported 0/29 events in the hydrophilic arm and 5/29 in the standard arm (Leriche 2006). Pachler 1999 found no difference between groups. The combined data provided a confidence interval that straddled the no‐difference line (Analysis 3.4).
Analysis 3.4.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 4 Number with urethral trauma/bleeding/macroscopic haematuria.
3.5 Number with microscopic haematuria
Sutherland 1996 reported fewer cases of microscopic haematuria in the coated group (6/16 versus 11/14) (Analysis 3.5).
Analysis 3.5.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 5 Number with microscopic haematuria.
3.6 Mean microscopic haematuria
Moore 2013 reported on mean number of events of microscopic haematuria, finding no significant difference (Analysis 3.6).
Analysis 3.6.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 6 Mean microscopic haematuria.
3.7 Number with stricture formation
One event occurred in the coated group of the De Ridder trial (De Ridder 2005) (Analysis 3.7).
Analysis 3.7.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 7 Number with stricture formation.
3.8 Overall satisfaction
Three trials addressed overall satisfaction with a hydrophilic product compared with another (De Ridder 2005; Moore 2013; Witjes 2009) (Analysis 3.8). One study (De Ridder 2005) reported higher satisfaction with the hydrophilic catheter; whereas another (Moore 2013) reported slightly higher satisfaction with the uncoated catheter. One study (Witjes 2009) compared two hydrophilic products, one PVC‐based the other PVC‐free and found that satisfaction was higher (statistically significant P = 0.02) with the PVC‐based product.
Analysis 3.8.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 8 Number reporting overall satisfaction.
3.9 Mean overall satisfaction
Cardenas 2011 and Sutherland 1996 reported statistically significant differences for users of the hydrophilic product over the uncoated catheter; Leriche 2006 also reported statistically significant differences in favour of hydrophilic catheter over uncoated, pre‐lubricated catheter (Analysis 3.9). However, there was an imbalance in attrition in the Cardenas 2011 study, which meant that fewer patients have reported on preference in the hydrophilic arm. Those who did not remain in the study may have been less satisfied with the hydrophilic catheter than those who completed the study.
Analysis 3.9.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 9 Mean Overall satisfaction.
3.10 Number reporting preference
Three trials (De Ridder 2005; Leriche 2006; Pachler 1999) indicated that the hydrophilic product was preferred over the uncoated catheter. These differences were not statistically significant (Analysis 3.10).
Analysis 3.10.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 10 Number reporting preference.
3.11 Number reporting convenience of product
One cross‐over trial (Moore 2013) found no significant difference between the two arms (Analysis 3.11).
Analysis 3.11.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 11 Number reporting convenience of product.
3.12 Mean convenience of product
Sutherland 1996 reported on mean convenience of product, which did not vary significantly between products (Analysis 3.12).
Analysis 3.12.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 12 Mean convenience of product.
3.13 Number reporting ease of handling
Thirty‐one out of 32 participants in the Pachler 1999 two‐arm cross‐over trial reported handling the catheter to be either easy or tolerable in both arms (Analysis 3.13). In Moore 2013, ease of handling was acceptable in both groups with a trend favouring the uncoated catheter (29/49 hydrophilic group and 46/48 in the uncoated arm).
Analysis 3.13.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 13 Number reporting ease of handling.
3.14 Mean ease of handling
Sutherland 1996 reported no significant difference in mean ease of handling (Analysis 3.14).
Analysis 3.14.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 14 Mean ease of handling.
3.15 Number reporting ease of insertion
One RCT (Witjes 2009) and two cross‐over trials (Leriche 2006; Pachler 1999) reported on ease of insertion. No significant difference was found (Analysis 3.15).
Analysis 3.15.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 15 Number reporting ease of insertion.
3.16 Mean ease of insertion
Cardenas 2011 and Sutherland 1996 reported on mean ease of insertion; no significant difference was found (Analysis 3.16).
Analysis 3.16.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 16 Mean ease of insertion.
3.17 Mean ease of removal
Cardenas 2011 found that mean ease of removal did not vary significantly between groups (Analysis 3.17).
Analysis 3.17.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 17 Number reporting comfort.
3.18 Number reporting comfort
Moore 2013 found no significant difference in numbers reporting product comfort (Analysis 3.18).
Analysis 3.18.

Comparison 3 Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated), Outcome 18 Mean ease of removal.
Studies without data added
Six cross‐over trials (Fader 2001, Mauroy 2001, Pascoe 2001, Sarica 2010, Denys 2012, Giannantoni 2001) compared one hydrophilic‐coated catheter with one or more catheters (coated or uncoated), but did not provide data in a format that could be included in the data tables.
A key finding in Fader 2001, involving 61 participants, was that there were significant differences between four hydrophilic products (not activated, water added by user) with respect to user‐reported severity of sticking and discomfort on withdrawal, user‐reported satisfaction and ease of use.
Mauroy 2001 reported on infection, haematuria, user‐reported satisfaction and ease of use among 27 participants, but did not find any significant differences between the three hydrophilic (not activated, water added by user) products tested.
Pascoe 2001 randomised 27 participants to test two hydrophilic‐coated catheters, one activated and one not activated (water added by user), and found no significant differences in performance, but users expressed a preference for the activated catheter.
Sarica 2010 compared the use of three catheters, one uncoated (not lubricated), one pre‐lubricated with integrated bag and one hydrophilic‐coated (water added by user), and reported an advantage in the pre‐lubricated catheter for reduction in microscopic haematuria and improved comfort and handling. Only 10 of the 25 participants randomised completed the trial.
In Denys 2012, 97 participants completed a comparison of a new hydrophilic‐coated catheter (not activated, sterile water supplied) versus a hydrophilic catheter (water added by user). Although a cross‐over trial was undertaken, data on the control catheter were reported at baseline, but no further data on this group were reported.
Giannantoni 2001, a cross‐over trial with 18 participants, compared a pre‐lubricated catheter with protective sleeve versus an uncoated, non‐lubricated catheter (water added by user). The outcomes were UTI, asymptomatic bacteriuria and participant preference; however as UTI and asymptomatic bacteriuria findings were reported per sample not per participant, it was not possible to include the data. The pre‐lubricated catheter scored significantly better for comfort and ease of use.
Comparison 4. One catheter length versus another catheter length
Five two‐arm cross‐over trials compared a shorter catheter length with a standard catheter (Biering‐Sorensen 2007; Chartier‐Kastler 2011; Chartier‐Kastler 2013; Costa 2013; Domurath 2011). Chartier‐Kastler 2011 and Domurath 2011 compared hydrophilic‐coated catheters (activated) in both arms. In Biering‐Sorensen 2007 and Chartier‐Kastler 2013 the shorter catheter was hydrophilic‐coated (activated) and the standard catheters were various designs. Costa 2013 evaluated uncoated, pre‐lubricated catheters (closed system with integrated collection bag) in both arms, the only difference being standard (40 cm) versus shorter (30 cm) length. All but one trial tested the catheters on male participants, Biering‐Sorensen 2007 had female only participants. Participants in Biering‐Sorensen 2007, Chartier‐Kastler 2011; Chartier‐Kastler 2013 and Domurath 2011 had either spinal cord injuries or lesions, and those in Costa 2013 were paraplegics requiring wheelchairs for mobility.
One study (Chartier‐Kastler 2013) used a validated tool, the Intermittent Self‐Catheterisation Questionnaire (ISC‐Q) (Pinder 2012), reporting that more participants favoured the shorter catheter, however, only the difference in the total ISC‐Q scores was reported, not each of the four domain scores (see Discussion section).
4.1 Number reporting ease of handling
Chartier‐Kastler 2011, Costa 2013 and Domurath 2011 reported on ease of handling (Analysis 4.1). Results were mixed, with Chartier‐Kastler 2011 and Domurath 2011 reporting a preference for the shorter catheter, whereas participants in the Costa 2013 trial favoured the standard length catheter.
Analysis 4.1.

Comparison 4 Standard catheter versus shorter catheter length, Outcome 1 Number reporting ease of handling.
4.2 Number reporting ease of insertion
Both Chartier‐Kastler 2011 and Domurath 2011 found more participants reported either positively or neutrally for ease of insertion for the shorter catheter compared to the standard catheter (28 out of 30 versus 22 out of 30 and 33 out of 36 versus 30 out of 36 respectively) and that a higher number of participants rated the shorter catheter as discrete or neutral compared to the standard catheter (34 out of 36 compared to 28 out of 36 and 28 out of 30 compared to 17 out of 30 respectively) (Analysis 4.2).
Analysis 4.2.

Comparison 4 Standard catheter versus shorter catheter length, Outcome 2 Number reporting ease of insertion.
4.3 Number reporting product discretion
Chartier‐Kastler 2011 and Domurath 2011 reported on product discretion reporting that significantly more participants found the shorter product more discrete (Analysis 4.3).
Analysis 4.3.

Comparison 4 Standard catheter versus shorter catheter length, Outcome 3 Number reporting product discretion.
4.4 Number reporting preference
Domurath 2011, Costa 2013, Chartier‐Kastler 2013 and Biering‐Sorensen 2007 reported on user preference. Domurath 2011, Chartier‐Kastler 2013 and Biering‐Sorensen 2007 found in favour of the shorter catheter, but confidence intervals crossed the no‐difference line. However, Costa 2013 found that very few participants preferred the shorter catheter (seven out of 81) (Analysis 4.4).
Analysis 4.4.

Comparison 4 Standard catheter versus shorter catheter length, Outcome 4 Number reporting preference.
Comparison 5. Any other techniques, strategies or designs that influence UTI, other complications or satisfaction in adults and children using intermittent catheterisation for incomplete bladder emptying.
No randomised clinical trials that tested different cleaning methods and reported catheter‐associated infection were found.
One RCT (Fera 2002) and one cross‐over trial (Taweesangsuksalul 2005) were found which compared different gels used for lubricating catheters before insertion.
Fera 2002 compared gentamycin gel (0.1%) versus lidocaine gel (2%). There were 10 participants per arm and each used their allocated gel for four months. Urine analysis took place once every three weeks during the trial (a total of five samples for each participant). No significant difference was found in either the number of symptomatic UTIs or in occurrence of asymptomatic bacteriuria. Findings were potentially confounded due to four participants allocated to the lidocaine gel group receiving prophylactic antibiotics during the trial. Furthermore, two participants in the lidocaine group and one participant in the gentamycin group were treated for UTI during the trial and were taken out of the trial and returned following treatment.
Taweesangsuksalul 2005 compared participant satisfaction with Aloe vera gel versus an aqueous gel for lubricating the catheter before insertion. Ten participants used both lubricants and no significant difference was reported. It was not possible to include data as the satisfaction scale used was not reported.
Discussion
Summary of main results
The purpose of the current review was to compare catheter designs, materials, technique and any other strategies to reduce UTI in long‐term intermittent catheterisation (IC) users. A secondary aim was to compare user‐reported outcomes (user satisfaction, preference, ease of use) associated with various catheter designs, techniques, and materials. Despite an additional 17 trials added to the database for a current total of 31 trials, there remains an absence of robust evidence to support one product or technique over another with respect to control of clinical symptoms, particularly symptomatic UTI. There is some statistical support for user preference of hydrophilic catheters but the confidence intervals are wide and there is a risk of bias due to higher dropout in the intervention arms. Thus the available data do not provide sufficient guidance to prescribing clinicians or users on which technique (aseptic/clean); catheter type (coated/uncoated); method (single/multiple‐use); person catheterising (self/other); or any other strategy is better than another. Clinicians will need to base their decisions on clinical judgement in conjunction with users. Differential costs of catheters/techniques may also inform decision‐making.
Updates since the last review
The previous review (Moore 2007) found no convincing evidence that any specific technique, catheter design, method, person, or strategy was better than any other to minimise risk of UTI and other complications. The focus of that review was incidence of UTI related to IC. Our focus for the 2014 review expanded to include user‐reported outcome data. This meant that two studies that had user preference as an outcome and that did not meet the inclusion criteria for the 2007 review were added to this review. Moreover, in the 2007 review, data reported in three cross‐over trials were not included in the meta‐analysis. In the current review we have been able to include data from cross‐over trials, resulting in 12 additional trials (nine new and three from the previous review). However, data from the three cross‐over trials with more than two arms has been discussed in the narrative, not added to the meta‐analysis. Despite this number, there remains a lack of evidence that one catheter design or technique is superior to another in terms of control of symptomatic UTI.
Overall completeness and applicability
The number of potential permutations and combinations of techniques and catheters has led to problems with confounding, with several trials combining catheters and techniques such that it would not be possible to state the cause of any differences found. Large RCTs are needed to provide answers to each separate question (aseptic or clean technique; coated or uncoated catheter; single or multiple use, catheterisation by self or others). But because these trials are difficult to conduct and some combinations are much more commonly used than others, prioritisation is important. For example, aseptic versus clean technique question is of relatively low importance because in community settings (where most IC takes place) an aseptic technique is not practical. In hospital settings infection control policies indicate that an aseptic technique would be needed for safety.
A key clinical question remains the influence of catheters on UTI. The difficulty of establishing robust outcome measures of UTI remains problematic. Bacteriuria/positive culture is not clinically relevant unless accompanied by symptoms but the symptoms themselves may present in vague and imprecise ways, especially in adults with spinal cord injury where symptoms may be masked or unclear. However, despite these limitations symptomatic UTI remains the most clinically important primary outcome variable.
Men and women were not equally represented, limiting generalisability of results. Of the 29 trials that reported gender, there was a higher proportion of males (60%) enrolling in the trial, the majority of whom had spinal cord injury. Thirteen trials enrolled only men, whereas one trial enrolled only women.
In community settings there are two important questions: single versus multiple‐use; and coated versus uncoated catheter. In practice the most commonly used single‐use catheter is a coated catheter which would need to be compared with a single‐use uncoated catheter, to test if the coating is of importance. If coated catheters are not found to be superior then multiple‐use uncoated catheters need to be compared with single‐use uncoated catheters (to test if the sterility or single‐use of the catheter is of importance). The latter question is of highest importance because it has the most substantial cost implications. Although coated catheters are more expensive than uncoated catheters, it is the single‐use of the catheters (coated or uncoated) which makes this method of higher cost to individuals and health services.
There have been no RCTs comparing catheterisation by self compared with others. Moore (Moore 1993) presented descriptive data suggesting that there was no difference between the child self‐catheterising versus the parent. This question is of relatively low priority because catheterisation by others usually only takes place when the individual is not able to carry out the procedure themselves.
No RCTs comparing different methods of catheter cleaning were found when undertaking this review. We found a number of laboratory trials testing the sterility of catheters using different methods (cleaning with soap and water, chemical disinfection and microwave). Although most trials showed that pathogenic organisms were removed by cleaning, one trial testing the microwave method and one (incidentally) the soap and water method found residual pathogenic organisms. The clinical significance of these findings is unknown. The microwave method may be less practical than other methods due to the risk of catheter melting. No randomised controlled clinical trials of cleaning methods have been published and the comparative effectiveness of cleaning methods is therefore unknown.
Potential biases
1. Attrition: In three of the five larger trials of long duration (Cardenas 2011; De Ridder 2005; Moore 2013) dropouts occurred early and were more frequent in the treatment arm, thus resulting in an imbalance and a potential bias in favour of the treatment catheter because there were fewer long‐term data. In De Ridder 2005, only 53% of the standard (uncoated) catheter participants and 41% of the treatment (hydrophilic‐coated) catheter participants remained at the 12‐month study endpoint. Similar challenges were met by Cardenas 2011 with 60% of participants remaining in the standard (uncoated) arm and 43% in the treatment (hydrophilic‐coated) arm. Moore 2013 also reported a completion rate of 75% in the standard (uncoated) arm and 62% in the treatment (coated) arm. Reasons for dropouts in all three trials included loss to follow‐up, preference for one study product, adverse events and withdrawing consent and improvement in voiding function as spinal cord injury healed. In Cardenas 2011 and De Ridder 2005, change in voiding status meant that some participants no longer required on‐going IC.
It should be noted that in all three trials, the uncoated group had a higher proportion of participant completions at the final analysis. Future trialists will require funds to support long‐term and multicentre ventures that take into account the attrition challenges and the populations to be studied. Newly injured spinal cord individuals are attractive participants as their bladders are 'naive' to the long‐term consequences of catheterisation. However, their bladder/voiding status can be unpredictable and may change to spontaneous voiding as healing occurs. Community dwelling users of ICs such as those with myelomeningocoele or multiple sclerosis are an alternative population as their conditions are relatively stable but their catheterisation habits are less easy to control and follow‐up can be an issue.
2. Intention to treat (ITT): ITT analysis was declared by only two parallel arm trials (Moore 2006 and Witjes 2009).
3. Assessment of user‐reported outcomes: 15 trials had user‐reported outcomes, 14 of which used questions that had not undergone standard psychometric testing and validation. Chartier‐Kastler 2013 was the first trial to apply a newly developed and validated 24‐item Intermittent Self‐Catheterisation Questionnaire (ISC‐Q), which evaluates aspects of quality of life specific to the needs of individuals performing ISC (Pinder 2012). The tool has four domains (ease of use, discreetness, convenience and psychological well‐being) and a total score, although Chartier‐Kastler 2013 only reported the total score.
In this review the most frequently reported outcome measures related to ease of use, with nine studies reporting ease of insertion and seven reporting ease of handling. Fewer studies reported outcomes relating to discreetness and convenience. Future studies would benefit from adopting a more consistent approach to the measurement of user‐reported outcomes in assessing the benefit of one IC product over another.
4. There is a theoretical bias in cross‐over trials where individuals will score the first product more favourably than the second. Chartier‐Kastler 2013 was the only trial statistically addressing treatment sequence and reported no significant difference in scores.
Reporting standards
Reporting standards varied and not all trials followed the Consort guidelines, making it difficult to extract data. In those that followed good reporting standards, adverse events such as haematuria were clearly attributed to one of the trial arms.
Agreement or disagreement with other reviews
Two reviews have been published on the occurrence of UTI in IC users (Bermingham 2013; Li 2013). Bermingham et al created a Markov model to predict cost utility and QALY (quality‐adjusted life year), using data from the randomised trials on IC. The authors conclude that multiple use is the most cost‐effective type of IC. The authors also concluded, as we have, that there was limited evidence to support one catheter product over another with respect to symptomatic UTI. Of note is their conclusion that patients should be offered a choice between gel reservoir or hydrophilic catheters. Our review found no evidence to support this statement but we do agree with Bermingham et al that there is inadequate evidence to state that incidence of symptomatic UTI is affected by any one catheter design. The second review (Li 2013) also sought to examine the benefit of one catheter design (hydrophilic versus non‐hydrophilic) in the occurrence of UTI and haematuria. The authors concluded that both UTI and haematuria occurred less frequently with the use of hydrophilic‐coated catheters. The authors’ findings are not supported by our review. We suggest this may be related to the authors' errors of data extraction and interpretation including mistaking proportions for raw data in a forest plot, mixing laboratory trials (Stensballe 2005) with clinical trials, incorrect reporting of attrition leading to misleading conclusions, and finally, not reporting bleeding occurrences as well as microhaematuria which is worse in hydrophilic‐coated arms where reported. Thus we disagree with the findings of the Li et al review that hydrophilic catheters reduce symptomatic urinary tract infections and reduce haematuria.
Authors' conclusions
There is still no convincing evidence that incidence of UTI is affected by use of aseptic or clean technique, coated or uncoated catheters, single (sterile) or multiple‐use (clean) catheters or by any other strategy and user‐reported outcomes varied. The question of whether healthcare providers should cover the cost of single‐use products requires debate if no benefit over multiple‐use products can be demonstrated. The variability in user‐reported outcomes suggests patient choice could be important and there may be a benefit in combining single‐ and multiple‐use for an individual.
There is lack of evidence demonstrating the effectiveness of any particular catheter design, technique or strategy. Variations in clinical practice and growth in the use of single‐use catheters (particularly coated catheters) with associated increased costs mean that large well‐designed parallel group RCTs are needed. RCTs are difficult in this area and prioritisation is necessary.
The most important pragmatic question (both for clinical and cost‐effective reasons) is: Are multiple‐use catheters equivalent to single‐use catheters?
We recommend that the NIDRR 1992 definition of UTI is used as the primary outcome variable (positive urine culture with pyuria and one or more systemic symptoms (fever, loin pain, dysuria, urgency, haematuria). However, there is a need to validate these symptoms on IC users.
We recommend that a validated tool (e.g. Pinder 2012) is used to measure user acceptability.
Given the large differential costs for the methods, cost‐effectiveness will need to be assessed rigorously.
To assist cost‐effectiveness assessment, we recommend that user‐reported outcomes and health state utility are measured for different situations (e.g. home/out) as a secondary outcome variable. A validated outcome tool is required to allow comparisons across trials.
Feedback
Feedback from Professor Dr Andrei Krassioukov and colleagues, 8 August 2017
Summary
Our team that includes clinicians and scientists from Belgium, Canada, United States and Switzerland involved in rehabilitation and medical management of individuals with spinal cord injury (SCI) would like to ask your opinion and help to address the following issue.During the last few years, the international community engaged in strong debates on issues related to urinary tract infection and re‐use of catheters during the management of neurogenic bladder among individuals with SCI.
In this respect, this review became one of the leading documents that captured mind and attention of clinicians around the world. Although numerous countries completely switched to single‐use catheters as guidelines for management of individuals with SCI, the opinion that was expressed in the above‐mentioned review has the potential to make a significant negative impact on the future management of individuals with SCI.
Upon closer inspection of this review we have become concerned about data that has been presented as well as conclusions drawn by the authors. We are reaching out to you to make you aware of our findings and interpretation of the studies that authors of the ‘Intermittent catheterisation for long‐term bladder management” Prieto et al 2014 included and discussed in their review. We are seeking your advice and guidance on how we can proceed with this information.
We have identified three main concerns with this Cochrane review that was conducted by Prieto and co‐investigators (Prieto 2014):
First, upon close inspection it appears that there are discrepancies in data extraction. For example, in ‘Analysis 2.2 Comparison 2 Single‐use (sterile) catheter versus multiple‐use (clean) catheter, Outcome 2 Number with symptomatic UTI’ of eight data sets, six (Sutherland 1996, Leek 2013, Moore 2013, Duffy 1995, King 1992 and Prieto‐Fingerhut 1999) are inconsistent with data reported by original authors. Similar discrepancies are noted throughout analyses in the review. At times, it appears data has simply been displaced (for example King 1992: data for single‐use catheter and data for multiple‐use catheter must be swapped) and at times it appears data has been extracted differently than we would expect (for example Leek 2013: one arm of the cross over trial was reported instead of both arms). Additionally, an abstract referenced (Moore 2013) provided data in a form that could not be extracted (person‐weeks).
Secondly, although the review was published in 2014, the definition of symptomatic UTI was taken from the NIDRR 1992 statement in ‘The Prevention and Management of Urinary Tract Infections Among People with Spinal Cord Injuries’. At that time, there were other more current definitions available such as the Infectious Diseases Society of America (IDSA) 2009 Guidelines which comprehensively covers definitions of catheter associated urinary tract infections and is significantly different from the NIDRR 1992 definition. In addition, the authors of this Cochrane review chose to accept each study’s definition of symptomatic UTI, despite none of the studies using a definition consistent with current standards. As a result, heterogeneous and inappropriate definitions of symptomatic UTI were included in analysis.
Finally, we feel clarification regarding the Analyses 1.1 through 4.4 (30 in total) may be required. 20 of the 39 analyses consist of only 1 study, which is inconsistent with the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 which states “Meta‐analysis is the statistical combination of results from two or more separate studies”. There are also inconsistencies with providing subtotals and totals for a number of analyses. An example of such can be seen for ’Analysis 3.2 Comparison 3 Hydrophilic‐coated or a pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated)’. The authors “chose not derive a summary of estimate because of heterogeneity amongst the trials and the problem of attrition bias” despite having similar issues in other analyses and continuing to derive a summary in those instances. Alternatively, for ‘Analysis 2.2 Comparison 2 Single‐use (sterile) catheter versus multiple‐use (clean) catheter, Outcome 2 Number with symptomatic UTI’ the authors state “We decided not to derive a summary estimate because of heterogeneity; however, there was no suggestion of a trend favoring either of the approaches.” (p. 13). They in fact derived both subtotal and total summaries and provided them (p 73‐74). A number of other analyses that do not include subtotals or totals do not provide a reason why they were not completed.
Following careful re‐evaluation of the studies that were included in the review we have re‐analyzed the data and compared results between our analyses and previously published data by Prieto and co‐investigators. When discrepancies in data were revised, we found that in contrast to the 2014 review, Analysis 2.2 exhibits a trend (albeit small) toward single‐use (sterile) catheter, and Analysis 3.2 significantly favors hydrophilic catheters.
When a current definition of UTI (The IDSA 2009 Guidelines – Diagnosis, Prevention, and Treatment of Catheter‐Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America) is applied and data is examined through this lens, further changes to outcomes are visible. As it is not possible to attach or submit figures/tables with this comment, we can only offer to share our results in detail with you.
As Cochrane reviews are highly influential in the medical community we believe it is important others become aware of possible changes to the review. Would you be supportive of our group submitting a different perspective on the data published in the 2014 Cochrane review that was conducted by Prieto and co‐investigators to The Cochrane Collaboration, or should we consider other venues for this publication?
We look forward to hearing from you on this matter and welcome input for moving forward. If you have any questions or need further clarification, please feel free to contact us.
Reference:
IDSA 2009
Infectious Diseases Society of America (IDSA). Diagnosis, prevention, and treatment of catheter associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Information available here: http://www.idsociety.org/Guidelines/Patient_Care/IDSA_Practice_Guidelines/Infections_by_Organ_System/Genitourinary/Catheter‐Associated_Urinary_Tract_Infection/
Reply
This reply has been prepared jointly by the Cochrane Incontinence Group editorial office and the Cochrane Editorial Unit (CEU).
The Cochrane Incontinence editorial office and the CEU are grateful for the submission of the feedback from Professor Krassioukov and colleagues. Following an independent assessment of review by a statistician and an editor from the CEU, the decision to withdraw the review was agreed between the acting Editor in Chief, Karla Soares Weiser, and coordinating editor of the group, Luke Vale. The issues identified have been shared with the authors and the review will be republished following revision and update.
Contributors
Andrei V. Krassioukov, Kathleen Christison, Matthias Walter, Jean‐Jacques J.M. Wyndaele, Thomas M. Kessler, Michael Kennelly
Acknowledgements
The authors thank Professor Kathryn Getliffe (retired) formerly at the University of Southampton and Ms. Jaycee Commance, MN, BSc, RN at the time a student at the University of Alberta for their work on the 2007 Review as well as the Cochrane Incontinence review group for thier invaluable assistance in completing the details of the review.
Appendices
Appendix 1. Extra searches performed by the review authors for this update of the review
Extra specific searches were also performed by the review authors in two additional electronic bibliographic databases: EMBASE from January 1988 to May 2012, CINAHL (January 1982 to May 2012) and ERIC from January 1984 to May 2012. The search terms included individually or combined were: intermittent catheterisation/catheterization; randomised/randomized controlled trials; neurogenic bladder; incomplete emptying; catheter(s); hydrophilic coated, coated, uncoated, bacteriuria, symptomatic urinary tract infection, asymptomatic bacteriuria, asymptomatic urinary tract infection. In addition, to search for trials of catheter cleaning methods, the above terms were combined with the terms: microwaving, catheter cleaning, soap and water, antiseptic soak. However, no additional trials were identified and so no further searches of these databases were undertaken.
Data and analyses
Comparison 1.
Aseptic technique versus clean/other aseptic technique
Comparison 2.
Single‐ise (sterile) catheter versus multiple‐use (clean) catheter
Comparison 3.
Hydrophilic‐coated or other pre‐lubricated catheter versus other catheter (pre‐lubricated, coated or uncoated)
Comparison 4.
Standard catheter versus shorter catheter length
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number reporting ease of handling | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Hydrophilic‐coated versus hydrophilic‐coated | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.15, 1.12] |
| 1.2 Uncoated catheter versus uncoated catheter | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.10, 5.11] |
| 2 Number reporting ease of insertion | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Hydrophilic‐coated versus hydrophilic‐coated | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.02, 1.35] |
| 2.2 Uncoated catheter versus uncoated catheter | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.70, 0.96] |
| 3 Number reporting product discretion | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Hydrophilic‐coated versus hydrophilic‐coated | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.18, 1.69] |
| 4 Number reporting preference | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Hydrophylic‐coated versus hydrophilic‐coated | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.94, 1.42] |
| 4.2 Uncoated catheter versus uncoated catheter | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.05, 0.19] |
| 4.3 Hydrophilic‐coated versus various standard length catheters | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.19, 1.91] |
What's new
Last assessed as up‐to‐date: 30 September 2013.
| Date | Event | Description |
|---|---|---|
| 10 August 2017 | Amended | This review has been withdrawn following Feedback from a group of readers who have identified possible errors in the analysis of data in the review. These concern handling of cross‐over and multi‐arm trials as well as corrections and clarifications of data used. This review has been withdrawn whilst these issues are addressed. Changes are also required to reflect current methodological standards. The review will be re‐published following updating, revision and peer review. |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 9 August 2017 | Amended | This review has been withdrawn following Feedback from a group of readers who have identified possible errors in the analysis of data in the review. These concern handling of cross‐over and multi‐arm trials as well as corrections and clarifications of data used. This review has been withdrawn whilst these issues are addressed. Changes are also required to reflect current methodological standards. The review will be re‐published following updating, revision and peer review. |
| 8 August 2017 | Amended | This review has been withdrawn following correspondence from a reader who identified possible errors in the analysis of data in the review. These concern handling of cross‐over and multi‐arm trials as well as corrections and clarifications of data used. This review has been withdrawn whilst these issues are checked. Changes are also required to reflect current methodological standards. The review will be re‐published following updating, revision and peer review. |
| 10 September 2014 | New citation required but conclusions have not changed | Added 17 new studies in this update. Risk of bias was re‐assessed of all the included trials as per current recommendation. |
| 10 September 2014 | New search has been performed | Added 17 new studies in this update. Risk of bias was re‐assessed of all the included trlas as per current recommendation. |
| 22 August 2007 | New citation required and conclusions have changed | Substantive amendment |
Differences between protocol and review
The previous review (Moore 2007) used UTI and other complications for the primary outcomes. In the current review, incidence of UTI remains a key outcome, but we also added outcomes addressing user satisfaction, preference and ease of use for users who are exposed to different designs and materials (e.g. coatings), aseptic or clean technique, single‐use or multiple‐use catheters, catheterisation by self or others, or any other strategy (including catheter cleaning) designed to reduce UTI. Two trials excluded from the original review (Fader 2001; Pascoe 2001) met the inclusion criteria for the current review. In the current review the Cochrane 'Risk of bias' tool was used to assess all trials, including those in the original review.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: Single blind (to investigator) SAMPLE CALCULATION: Yes DURATION: 2 days WITHDRAWALS/DROPOUTS: Not stated ITT: Not applicable GEOGRAPHICAL LOCATION: Denmark SETTING: Outpatient clinic | |
| Participants | N = 24 DIAGNOSIS: Spinal cord lesion ELIGIBLE: Not stated ENROLLED: 24 COMPLETED: 24 AGE: Adults GENDER: Female | |
| Interventions | Short length female catheter (hydrophilic coated) versus standard length (various designs) female catheter | |
| Outcomes | Residual urine measured by ultrasound, satisfaction, handling, suitability of length | |
| Notes | No difference in residual urine outcomes, satisfaction or ease of handling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomised in blocks of four" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Single‐blind". Not possible to blind participants, but outcome assessment was blinded and the non‐blinding of participants was considered unlikely to introduce bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Study nurse (who carried out the ultrasound) was not present during the catheterization in order to remain blinded regarding the type of catheter used". participant‐reported handling and satisfaction of catheter and was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Source of Funding | Unclear risk | Coloplast A/S |
| Methods | DESIGN: Randomised controlled trial BLINDING PROCEDURES: Non‐blinded SAMPLE CALCULATION: Yes DURATION: 12 months WITHDRAWALS/DROPOUTS: 11 ITT: Not stated GEOGRAPHICAL LOCATION: North America SETTING: Community | |
| Participants | N = 56 DIAGNOSIS: Spinal cord injury for >6 months with >1 UTI ELIGIBLE: Not stated ENROLLED: 56 COMPLETED: 45 AGE: Adults GENDER: 29 male, 16 female | |
| Interventions | Hydrophilic‐coated catheter, not activated, water added by user versus uncoated catheter, non‐lubricated, single use both arms | |
| Outcomes | Symptomatic UTI, treatment with antibiotics | |
| Notes | No difference in incidence of bacteriuria or symptomatic UTIs between treatment and control groups. Significantly less UTIs treated with antibiotics in treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. More women in the control group, significantly more tetraplegic participants in the control group. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unblinded. Monthly self‐report of UTI symptoms together with urine sampling used to determine presence of UTI. Use of antibiotics determined by participant's clinician (independent of research trial). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers between intervention group (n = 6) and control group (n = 5), with similar reasons for missing data across groups. |
| Source of Funding | Low risk | National Institute on Disability and Rehabilitation Research US |
| Methods | DESIGN: Randomised controlled trial BLINDING PROCEDURES: Non‐blinded SAMPLE CALCULATION: Yes DURATION: Up to 6 months WITHDRAWALS/DROPOUTS: 110 ITT: Not stated GEOGRAPHICAL LOCATION: North America SETTING: Hospital and community | |
| Participants | N = 224 DIAGNOSIS: Spinal cord injury no longer than 3 months ELIGIBLE: Not stated ENROLLED: 224 COMPLETED: 114 AGE: Adults GENDER: 161 male, 39 female (enrolled ‐ did not state gender of numbers completing) | |
| Interventions | Hydrophilic‐coated catheter, activated versus uncoated catheter, non‐lubricated (single use both arms) | |
| Outcomes | Time to 1st UTI; UTI incidence; microhaematuria measured at weeks 3 and 4; satisfaction measured at 45 days | |
| Notes | Time to first antibiotic treated symptomatic UTI was significantly delayed in hydrophilic‐coated catheter group. 14 reports of bleeding in hydrophilic and 6 reports in uncoated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A centralized computer‐generated randomization list was generated" |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes were provided" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The inability to blind participants and clinicians to the catheter type is a potential limitation of the trial". Probably did not create performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The inability to blind participants and clinicians to the catheter type is a potential limitation of the trial". Outcome of UTI was participant‐ and clinician‐determined. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "In the hydrophilic catheter group... many of the investigational sites did not routinely use hydrophilic catheters. The trial protocol did not include a number of training catheterizations". A greater number of participants dropped out in the hydrophilic‐coated group, resulting in incomplete outcome data both for UTI, and participant satisfaction, which did not appear to have been measured in these participants. |
| Source of Funding | Unclear risk | Coloplast A/S |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: Not possible for participants. No blinding to investigator. SAMPLE CALCULATION: Yes DURATION: 14 days WITHDRAWALS/DROPOUTS: 9 (5 in control arm, 4 in other arm) ITT: Not applicable GEOGRAPHICAL LOCATION: France/Denmark SETTING: Hospital outpatient clinic | |
| Participants | N = 36 DIAGNOSIS: Spinal cord lesion and normal/impaired urethral sensation ELIGIBLE: 36 ENROLLED: 36 COMPLETED: 27 AGE: Adults GENDER: Male | |
| Interventions | Short length male hydrophilic‐coated (activated) catheter versus standard length male hydrophilic‐coated (activated) catheter | |
| Outcomes | Participant satisfaction and ease of use | |
| Notes | No infection outcome. Significant difference in favour of the compact catheter. No description of how the data were imputed, resulting in a potential risk of bias in favour of non‐inferiority. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised to one of two treatment groups by computer" |
| Allocation concealment (selection bias) | Low risk | "Sealed randomization envelopes were provided" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participant‐reported outcome ‐ risk of detection bias unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flowchart to report participant dropout presented, but no information to report how data handled in the analysis, particularly regarding imputation |
| Source of Funding | Unclear risk | Coloplast A/S |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: Non‐blinded SAMPLE CALCULATION: Yes DURATION: 12 weeks WITHDRAWALS/DROPOUTS: 6 ITT: Not applicable GEOGRAPHICAL LOCATION: France, Germany, Norway, Sweden, Denmark SETTING: Hospitals, clinics, research centres | |
| Participants | N = 106 DIAGNOSIS: Neurogenic bladder disfunction, various ELIGIBLE: 125 ENROLLED: 125 COMPLETED: 106 AGE: Adults GENDER: Male and female | |
| Interventions | Shorter versus longer catheter (both hydrophilic) | |
| Outcomes | ISC‐Q score and user preference | |
| Notes | Preference for shorter length catheter. Domain scores for ISC‐Q not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were allocated to the treatment sequence by randomization of numbers in sealed identical and non‐transparent envelopes" |
| Allocation concealment (selection bias) | Low risk | "patients were allocated to the treatment sequence by randomization of numbers in sealed identical and non‐transparent envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | User preference, so not possible to blind outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 19 enrolled participants did not complete all data collection |
| Source of Funding | Unclear risk | Coloplast A/S |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: None SAMPLE CALCULATION: No DURATION: Use of 10 catheter sets for each arm of trial FOLLOW‐UP: No WITHDRAWALS/DROPOUTS:9 ITT: Not applicable GEOGRAPHICAL LOCATION: 7 sites, US SETTING: Not stated | |
| Participants | N = 81 DIAGNOSIS: Paraplegic with neuropathic voiding dysfunction ELIGIBLE: Not stated ENROLLED: 91 COMPLETED: 82, although 23 of these did not fully complete test arm AGE: Adults GENDER: Male | |
| Interventions | Standard length catheter versus shorter length catheter (uncoated, pre‐lubricated closed system with integrated collection bag both arms) | |
| Outcomes | User preference for catheter length (standard versus shorter length), ease of use, sensation of emptying | |
| Notes | Preference for standard (longer length) catheter. Most common reason for preference was perception of complete bladder emptying. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomisation was balanced to site'. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | User preference, so not possible to blind outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 23 of 82 participants were unable to complete use of all 10 test catheters (shorter length) due to inadequate bladder drainage. |
| Source of Funding | Unclear risk | Hollister |
| Methods | DESIGN: Randomised controlled trial BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No (feasibility trial) DURATION: 24 hours FOLLOW‐UP: 3 urine samples for C&S in 24 hours WITHDRAWALS/DROPOUTS: No ITT: Not stated GEOGRAPHICAL LOCATION: Canada SETTING: ICU | |
| Participants | N =11 DIAGNOSIS: Neurogenic bladder due to recent spinal cord injury ELIGIBLE: 53 ENROLLED: 11 COMPLETED: 11 AGE: Adults GENDER: Male | |
| Interventions | Uncoated, pre‐lubricated catheter with an integrated bag with an uncoated, non‐lubricated catheter, aseptic technique both arms | |
| Outcomes | 3 urine samples for culture over a 24‐hour period + meatal swabs | |
| Notes | No difference between groups but sample too small and time frame too short to make any inferences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Source of Funding | Unclear risk | Not stated. |
| Methods | DESIGN: Randomised controlled trial BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: Yes DURATION: 12 months FOLLOW‐UP: Baseline, day 15 then monthly x 12 months WITHDRAWALS/DROPOUTS: 66 ITT: Not stated GEOGRAPHICAL LOCATION: Europe SETTING: Rehabilitation and community | |
| Participants | N =123 DIAGNOSIS: Neurogenic bladder due to spinal cord injury < 6 months ELIGIBLE: Unknown ENROLLED: 123 COMPLETED: 57 AGE: Adults GENDER: Male |
|
| Interventions | Hydrophilic‐coated catheter, activated versus uncoated catheter, non‐lubricated (single use both arms); assessed at Day 15 then monthly x 12 m. | |
| Outcomes | Primary: UTI Secondary: haematuria strictures, convenience; 82% PVC had UTI; 64% Speedicath; no difference in haematuria. | |
| Notes | UTI described as "clinical infection with Sx of UTI and for which treatment was prescribed", however, lab analyses did not differ between groups. significant challenges in retaining participants illustrating the difficulty of conducting trials in this group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised in two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but outcome not likely to be influenced by a lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data accounted for descriptively. However, larger numbers of dropouts in intervention group. This has not been accounted for in the analysis, which does not appear to be intention‐to‐treat. |
| Source of Funding | Unclear risk | Not stated. |
| Methods | DESIGN: Randomised cross‐over trial BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: 30 days WITHDRAWALS/DROPOUTS: 9 ITT: Not applicable GEOGRAPHICAL LOCATION: France SETTING: "11 French centres" | |
| Participants | N = 106 DIAGNOSIS: Spinal cord injury ELIGIBLE: 106 ENROLLED: 106 COMPLETED: 97 AGE: 18 to 65 years GENDER: Male (63) and female (34) | |
| Interventions | Novel "no‐touch" hydrophilic catheter versus participants' own hydrophilic catheter | |
| Outcomes | Participant preferences | |
| Notes | Reported on day 0 and then either day 15 or day 30 after use of test catheter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation was stratified per center" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers between intervention group (n = 4) and control group (n = 5), with similar reasons for dropout across groups. |
| Source of Funding | Unclear risk | Hollister France Inc. |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: Single blind (trial nurse) SAMPLE CALCULATION: Yes DURATION: 1 day WITHDRAWALS/DROPOUTS: 1 ITT: Not applicable GEOGRAPHICAL LOCATION: Three trial sites in Germany SETTING: Not stated | |
| Participants | N = 37 DIAGNOSIS: Spinal cord injury ELIGIBLE: 37 ENROLLED: 37 COMPLETED: 36 AGE: Adults GENDER: Male | |
| Interventions | Short length male hydrophilic‐coated catheter (activated) versus standard length male hydrophilic‐coated catheter (activated) | |
| Outcomes | Residual urine and user satisfaction | |
| Notes | Two observations of visible bleeding on test catheter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Crossover design, randomized in permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants, but risk of performance bias considered low as cross‐over trial. Blinding of trial nurse throughout all measurement procedures. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participant‐reported outcome ‐ risk of detection bias unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 withdrawal reported because the participant "did not consider the 'test' catheter material flexible enough" |
| Source of Funding | Unclear risk | Coloplast A/S. |
| Methods | DESIGN: Randomised controlled trial ALLOCATION: Not described but did stratify participants according to presence/ absence of UTI and trial site. BLINDING PROCEDURES: Unclear SAMPLE CALCULATION: Yes post hoc DURATION: 3 months FOLLOW‐UP: days 2, 4, 6, 10, 15, 60 & 90 WITHDRAWALS/DROPOUTS: 2 ITT: Not stated GEOGRAPHICAL LOCATION: USA SETTING: 3 long‐term care Veterans Administration Medical Centre Nursing Homes | |
| Participants | N = 80 DIAGNOSIS: Incomplete bladder emptying due to prostate obstruction ELIGIBLE: 203 ENROLLED: 82 COMPLETED: 80 to day 15; 39 completed to Day 90) AGE: Elderly GENDER: Male | |
| Interventions | Sterile technique vs clean technique (also single vs multiple use): sterile equipment and procedure, cleaning with betadine; Clean technique: catheter washed with soap and water and reused x 1 week (uncoated, non‐lubricated catheters) | |
| Outcomes | Number of treatment episodes for UTI + urinalysis. | |
| Notes | Some participants had indwelling catheters prior to enrolment in the trial (not stated how many); weeks to onset of symptomatic UTI was 3.11 (3.12) for treatment and 3.5 (3.02) for control. Dropout rate high after Day 15 with only 39 completing data collection to Day 90. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of outcome assessment ‐ this may have resulted in bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants had full dataset for Day 15, but missing outcome data at end of 90 days |
| Source of Funding | Low risk | Dept of Veterans Affairs US. |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: SAMPLE CALCULATION: Yes DURATION: 1 week for each of 4 arms WITHDRAWALS/DROPOUTS: None ITT: Not applicable GEOGRAPHICAL LOCATION: UK SETTING: 7 test centres run by continence nurse specialists | |
| Participants | N = 61 DIAGNOSIS: not stated ELIGIBLE: Not stated ENROLLED: 61 COMPLETED: 61 AGE: Adults GENDER: Male | |
| Interventions | Comparison of 4 hydrophilic‐coated catheters (not activated, water added by user) | |
| Outcomes | Smoothness of catheter removal, severity of sticking and user‐reported satisfaction and ease of use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation in Latin squares" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant‐reported outcome, but risk of detection bias considered low as cross‐over trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Source of Funding | Low risk | Continence Product Evaluation Network. |
| Methods | DESIGN: Randomised controlled trial BLINDING PROCEDURES: Unclear SAMPLE CALCULATION: No DURATION: 4 months FOLLOW‐UP: Urine culture every 3 weeks for 4 months (5 samples) WITHDRAWALS/DROPOUTS: 0 ITT: Not stated GEOGRAPHICAL LOCATION: Brazil SETTING: General School Hospital | |
| Participants | N = 20 DIAGNOSIS: Variable, mielomeningocele most common (25%) ELIGIBLE: Not stated ENROLLED: 20 COMPLETED: 20 AGE: Mixed adults and children 2‐79 years (mean not stated) GENDER: 12 male and 8 female | |
| Interventions | Other strategies designed to reduce infection: Gentamycin cream (0.1%) versus lidocaine jelly used as separate lubricant for IC | |
| Outcomes | Number of episodes of asymptomatic bacteriuria (>= 100,000 CFU/mL) , number of participants with symptomatic UTI | |
| Notes | Repeated measures of asymptomatic bacteriuria reported for each participant. Final measure used in table of results. Asymptomatic bacteriuria similar in both groups 8/10 in gentamycin group 6/10 in lidocaine group. 1/10 developed symptomatic UTI in gentamycin group, 2/10 in Lidocaine group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised in two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but outcome not likely to be influenced by a lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Source of Funding | Unclear risk | Not stated |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: Not clear SAMPLE CALCULATION: No DURATION: 7 weeks each arm FOLLOW‐UP: Urine for C&S at 2, 4, 7 weeks WITHDRAWALS/DROPOUTS: Not stated ITT: Not applicable GEOGRAPHICAL LOCATION: Italy SETTING: Rehabilitation Hospital | |
| Participants | N = 18 DIAGNOSIS: Neurogenic bladder due to recent spinal cord injury ELIGIBLE: Unknown ENROLLED: 18 COMPLETED: 18 AGE: Adults GENDER: 16 male & 2 female | |
| Interventions | Coated vs uncoated: Pre‐lubricated catheter with protective sleeve versus an uncoated, non‐lubricated catheter (water added by user); one catheter x 7 weeks then cross‐over to other group. | |
| Outcomes | UTI measured by C&S at 2, 4 & 7 weeks; Urethral wall trauma by counting cells on catheter surface; VAS re: satisfaction with catheters | |
| Notes | UTI defined as cloudy, odorous urine, onset of UI, increase autonomic dysreflexia, pyuria, bacteriuria; Sample size too small to draw conclusions. Attempted randomisation concealment; higher % of UTI in PVC group; no difference in urethral cell count; unable to use data in Table of Comparisons because of cross‐over design and no mid‐point data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "One of us organised randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Source of Funding | Unclear risk | Not stated |
| Methods | DESIGN: Randomised controlled trial BLINDING PROCEDURES: Not clear SAMPLE CALCULATION: No DURATION: 28 days FOLLOW‐UP: Daily urine dipslides WITHDRAWALS/DROPOUTS: 11 ITT: Not stated GEOGRAPHICAL LOCATION: USA SETTING: Rehabilitation Hospital | |
| Participants | N = 46 DIAGNOSIS: Neurogenic bladder due to recent spinal cord injury ELIGIBLE: 58 ENROLLED: 46 COMPLETED: 35 AGE: Adults GENDER: Male | |
| Interventions | Sterile vs clean technique (also Single Use vs Multiple Use) catheterisation kit and sterile single‐use catheter, meatus cleansed with povidone iodine. Clean technique: sterile catheter reused for one day after being washed with soap and water, non‐sterile gloves and container (uncoated, non‐lubricated catheters) | |
| Outcomes | Ddaily urine dipslides + symptomatic UTI | |
| Notes | No statistically significant differences between urine cultures or Sx UTI; weeks to onset of UTI was 1.1 (0.87) for treatment and 1.2 (1.0) for control. Number of days in trial varied from 1 to 28 with only nine participants completing more than twenty days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "46 patients were assigned randomly" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. UTI determined by lab results and participant symptoms |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were evenly balanced between groups |
| Source of Funding | Low risk | American Association of Spinal Cord Injury Nurses; Rehabilitation Institute Foundation US |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: 'Full blinding not possible, but microbiological data were entered by blinded staff' SAMPLE CALCULATION: Yes DURATION:16 weeks (8 weeks per arm) WITHDRAWALS/DROPOUTS: 3 participants dropped out ITT: Not applicable GEOGRAPHICAL LOCATION: Australia SETTING: Hospital outpatient pelvic floor clinic | |
| Participants | N = 23 DIAGNOSIS: Varied ELIGIBLE: 41 ENROLLED: 23 COMPLETED: 20 AGE: Adults GENDER: 6 male, 17 female | |
| Interventions | Uncoated catheter, non‐lubricated and clean technique both arms | |
| Outcomes | Asymptomatic and symptomatic UTI with microbiological confirmation | |
| Notes | No statistically significant difference in the rate of symptomatic UTI at week 8 or 16. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Computer‐generated randomisation code' |
| Allocation concealment (selection bias) | Low risk | 'Opaque sequentially numbered sealed envelopes' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Full blinding not possible, but microbiological data entered by blinded staff'. Risk of performance bias considered low as cross‐over trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Risk of detection bias considered low as cross‐over trial. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete data for 3 participants |
| Source of Funding | Unclear risk | Not stated |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: None SAMPLE CALCULATION: Not reported DURATION: Use of 20 sets of each product FOLLOW‐UP: WITHDRAWALS/DROPOUTS: 2 ITT: Not applicable GEOGRAPHICAL LOCATION: France SETTING: Hospital | |
| Participants | N = 31 DIAGNOSIS: Vesico‐sphincteric problem of neurological origin ELIGIBLE: Not stated ENROLLED: 31 COMPLETED: 29 AGE: Adults GENDER: Male | |
| Interventions | Hydrophilic‐coated activated catheter with integrated bag versus uncoated, pre‐lubricated catheter with integrated bag (aseptic technique, single use both arms) | |
| Outcomes | Product evaluation questionnaire and overall preference questionnaire | |
| Notes | Marked preference reported in favour of test catheter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant‐reported outcome, but risk of detection bias considered low as cross‐over trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data from 2 participants ‐ withdrawals described as not being related to the trial |
| Source of Funding | Unclear risk | Les Laboratoires Coloplast |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: Yes SAMPLE CALCULATION: No DURATION: Use of 27 sets of each product WITHDRAWALS/DROPOUTS: None ITT: Not applicable GEOGRAPHICAL LOCATION: France SETTING: Hospital outpatient clinic | |
| Participants | N = 27 DIAGNOSIS: Variable ELIGIBLE: Not stated ENROLLED:27 COMPLETED:27 AGE: Adults GENDER: 18 males, 9 females | |
| Interventions | Comparison of 3 hydrophilic‐coated (non‐activated, water added by user) catheters | |
| Outcomes | Self‐reported infection, haematuria, user‐reported satisfaction and ease of use | |
| Notes | Nursing staff as well as participants completed questionnaires | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'A random number was allocated to each subject' |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participant and nurse‐reported outcome ‐ risk of detection bias unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Source of Funding | Unclear risk | Not stated |
| Methods | DESIGN: Randomised controlled cross‐over trial with each arm 6 months BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: 6 months FOLLOW‐UP: Monthly WITHDRAWALS/DROPOUTS: Nil ITT: Not applicable GEOGRAPHICAL LOCATION: Canada SETTING: Community | |
| Participants | N = 30 DIAGNOSIS: Neurogenic bladder due to spina bifida ELIGIBLE: Unknown ENROLLED: 30 COMPLETED: 30 AGE: Children GENDER: 15 male, 15 female | |
| Interventions | SINGLE VS MULTI USE:sterile single‐use PVC or reused PVC (uncoated, non‐lubricated catheters) | |
| Outcomes | Bacteriuria > 10x3 CFU/mL obtained monthly; no difference between groups. | |
| Notes | Symptomatic UTI defined as + symptoms; catheters washed with liquid soap and water, air dried and reused (does not indicate length of reuse); several participants took prophylactic antibiotics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Both lab technologists were blind to the arm of the crossover design" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Source of Funding | Unclear risk | Spina Bifida Association of Northern & Southern Alberta and Canada, Baxter Corporation,Canadian Hospital Suppliers, Congdon's Aids to Daily Living, Glenrose Rehabilitation Hospital, Lever Soap, Mentor Corporation, Northern Alberta Urology Foundation, |
| Methods | DESIGN: Randomised controlled trial ALLOCATION: By third party using sealed opaque envelopes. BLINDING PROCEDURES: Data entry blinded SAMPLE CALCULATION: Yes DURATION: Up to 12 months FOLLOW‐UP: Weekly urinalysis WITHDRAWALS/DROPOUTS: None ITT: Yes GEOGRAPHICAL LOCATION: Canada SETTING: Rehabilitation Hospital | |
| Participants | N = 36 DIAGNOSIS: Neurogenic bladder due to recent high spinal cord injury; neurogenic bladder. ELIGIBLE: 50 ENROLLED: 36 COMPLETED: 36 AGE: Adults GENDER: 28 male, 8 female | |
| Interventions | Sterile technique vs clean technique Sterile single‐use PVC catheter with sterile technique or sterile single‐use PVC catheter with clean technique (clean gloves, clean container, non‐sterile wipes for cleansing pre catheterisation) (uncoated, non‐lubricated both arms) | |
| Outcomes | Days to onset of symptomatic UTI | |
| Notes | UTI defined as >= 10x5 CFU/mL, pyuria + accompanying symptoms; no difference between groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated random numbers" |
| Allocation concealment (selection bias) | Low risk | "Sealed brown envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "It was not possible to blind nursing staff" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "laboratory was blinded to subject allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Source of Funding | Low risk | Glenrose Rehabilitation Hospital; Small Faculties Grant University of Alberta |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: Not possible to blind participants. SAMPLE CALCULATION: Yes DURATION: 48 weeks (24 weeks each arm) WITHDRAWALS/DROPOUTS: 24 ITT: Not applicable GEOGRAPHICAL LOCATION: 4 sites in Western Canada SETTING: Paediatric clinic | |
| Participants | N = 46 DIAGNOSIS: Spina bifida ELIGIBLE: Not stated ENROLLED: 70 COMPLETED: 46 AGE: Children GENDER: 21 male, 25 female | |
| Interventions | Hydrophilic‐coated (not activated) single‐use catheter with an uncoated, non‐lubricated (water added by user) multiple‐use catheter | |
| Outcomes | Symptomatic UTI, antimicrobial use, haematuria, days of missed activities, user‐reported satisfaction | |
| Notes | Hydrophilic catheters do not appear to reduce febrile UTI or antibiotic use in community‐dwelling children using CIC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Computer generated list into random blocks of 8' |
| Allocation concealment (selection bias) | Low risk | 'Sealed opaque envelopes' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Not possible to blind subjects to product. All data was entered by an impartial technician.' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Risk of detection bias considered low as cross‐over trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24 participants dropped out |
| Source of Funding | Unclear risk | Glenrose Rehabilitation Hospital, Spina Bifida Association of Northern Alberta, Northern Alberta Urology Foundation Canada, Coloplast A/S (products) |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: 3 weeks each arm FOLLOW‐UP: 3 weeks WITHDRAWALS/DROPOUTS: Not clear ITT: Not applicable GEOGRAPHICAL LOCATION: Denmark SETTING: Community | |
| Participants | N = 32 DIAGNOSIS: Retention due to BPH ELIGIBLE: Not stated ENROLLED: 43 COMPLETED: 32 AGE: Adults GENDER: Male | |
| Interventions | Hydrophilic‐coated (not activated) (Lofric) single‐use catheter with an uncoated, non‐lubricated (water added by user) multiple‐use catheter x 3 weeks each | |
| Outcomes | Urine for C&S at baseline and each 3 week point; haematuria; responses to catheter use questionnaire | |
| Notes | UTI defined as > 10 x 4 CFU/mL. No differences between groups in questionnaire response, bacteriuria or haematuria but short follow‐up and small sample size. Unable to use data in Table of Comparisons because of cross‐over design and no mid‐point data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participant‐reported outcome ‐ risk of detection bias unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear number of participants included but not completed |
| Source of Funding | Unclear risk | Kirudan A/S (products) |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: No SAMPLE CALCULATION: No DURATION: 1 week WITHDRAWALS/DROPOUTS: 2 ITT: Not applicable GEOGRAPHICAL LOCATION: UK SETTING: Two test centres | |
| Participants | N = 27 DIAGNOSIS: Not stated. ELIGIBLE: Not stated ENROLLED: 27 COMPLETED: 25 AGE: Adults GENDER: Not stated | |
| Interventions | Hydrophilic‐coated catheters, one activated and one not activated (water added by user) | |
| Outcomes | User satisfaction and ease of use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant‐reported outcome, but risk of detection bias considered low as cross‐over trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data from 2 participants ‐ withdrawals described as catheter‐related reasons, but type of catheter not specified |
| Source of Funding | Unclear risk | Coloplast A/S |
| Methods | DESIGN: Randomised controlled trial BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: Unclear FOLLOW‐UP: Unclear WITHDRAWALS/DROPOUTS: Not stated ITT: Not applicable GEOGRAPHICAL LOCATION: USA SETTING: Rehabiltiation | |
| Participants | N = 29 DIAGNOSIS: Neurogenic bladder due to spinal cord injury ELIGIBLE: Unknown ENROLLED: 29 COMPLETED: Not stated AGE: Adults GENDER: 16 male, 13 female | |
| Interventions | Single‐use uncoated, pre‐lubricated catheter with an integrated bag (sterile) versus multiple‐use uncoated, non‐lubricated catheter (CLEAN) | |
| Outcomes | UTI Urine for C&S collected weekly ‐ unclear on trial time frame or endpoint. | |
| Notes | UTI as defined by NIDRR (1992); higher % of UTI in closed system (42% vs 29%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated, but does not appear to be any missing outcome data |
| Source of Funding | Unclear risk | Medical Marketing Group Inc. US |
| Methods | DESIGN: Randomised controlled trial BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: 4 days FOLLOW‐UP: WITHDRAWALS/DROPOUTS: 10 ITT: Not stated GEOGRAPHICAL LOCATION: SETTING: Rehabilitation | |
| Participants | N = 30 DIAGNOSIS: Neurogenic bladder due to recent spinal cord injury or stroke ELIGIBLE: Unknown ENROLLED: 30 COMPLETED: 20 AGE: Adults GENDER: Not stated | |
| Interventions | Uncoated, pre‐lubricated catheter with an integrated bag with an uncoated, non‐lubricated catheter, aseptic technique both arms | |
| Outcomes | UTI >10x5 CFU/mL + symptoms (fever, CV or SP tenderness) | |
| Notes | Data only collected for 4 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | Low risk | "blind selection of a marked piece of paper from a box prepared for the particular service" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data is not reported for the missing participants but there were the same number of participants with incomplete data in each arm. |
| Source of Funding | Unclear risk | Bard Inc. |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: No SAMPLE CALCULATION: No DURATION: 6 weeks WITHDRAWALS/DROPOUTS: 11 ITT: Not applicable GEOGRAPHICAL LOCATION: Turkey SETTING: Hospital clinic | |
| Participants | N = 25 DIAGNOSIS: Spinal cord injury ELIGIBLE: 25 ENROLLED: 21 COMPLETED: 10 (all three arms) AGE: Adults GENDER: Male | |
| Interventions | One uncoated (not lubricated), one pre‐lubricated with integrated bag and one hydrophilic coated (water added by user) | |
| Outcomes | Urethral cytology, urine culture, user satisfaction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sequence generated by table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Urethral cytology samples were taken by a blinded doctor" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data on one or more catheters from 15 participants ‐ 9 participants gave non‐approval of catheter as the reason (2 gel‐lubricated catheter, 5 PVC catheter, 2 hydrophilic catheter) |
| Source of Funding | Low risk | No funding |
| Methods | DESIGN: Randomised controlled cross‐over trial. BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: Each arm was 4 months FOLLOW‐UP: Weekly home visit for urine for C&S, catheter count, medication use and symptoms or signs of UTI WITHDRAWALS/DROPOUTS: None ITT: Not applicable GEOGRAPHICAL LOCATION: USA SETTING: Community | |
| Participants | N = 10 DIAGNOSIS: Neurogenic bladder due to spina bifida ELIGIBLE: 12 ENROLLED:10 COMPLETED: 10 AGE: Children GENDER: 4 male, 6 female | |
| Interventions | Single vs multiuse Uncoated catheter, non‐lubricated and clean technique both arms | |
| Outcomes | UTI weekly urine for C&S x 4 months | |
| Notes | UTI defined as + or > than 10x4 CFU/mL plus symptoms (fever, pain, change in continence, change in colour or odour of urine); No differences between groups (2 Sx UTI each). SS too small to draw any conclusions about effectiveness. Catheter cleaning: PVC rinsed with tap water, air dried. At end of day catheters were boiled x 3 minutes, air dried and stored in clean bag. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether laboratory technician was blinded to catheter |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal missing data |
| Source of Funding | Unclear risk | Mentor Corporation US |
| Methods | DESIGN: Randomised controlled trial BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: 8 weeks FOLLOW‐UP: Weekly urine C&S and microscopy x 8 weeks. WITHDRAWALS/DROPOUTS: 3 ITT: Not stated GEOGRAPHICAL LOCATION: USA SETTING: community | |
| Participants | N = 33 DIAGNOSIS: Neurogenic bladder due to spinal cord injury, Hinman syndrome, spinal dysraphism; ELIGIBLE: Not stated ENROLLED: 33 COMPLETED: 30 AGE: Children GENDER: Male |
|
| Interventions | Hydrophilic‐coated (not activated) (Lofric) single‐use catheter with an uncoated, non‐lubricated (water added by user) multiple‐use catheter. Method of cleaning catheter and length of reuse not described. | |
| Outcomes | UTI Haematuria > 3 RBC per HPF; VAS for satisfaction; | |
| Notes | UTI defined as 10x5 CFU/mL + Sx (not defined); participants with positive cultures were treated and re‐entered into the trial; no diff in bacteriuria b/w groups; haematuria lower in Lofric group but SS too small to draw conclusions and groups included gastric augmentation as well. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether laboratory technician was blinded to catheter |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants did not complete the trial, 2 in one arm and 1 in the other. Reasons for missing data unlikely to be related to outcome. |
| Source of Funding | Unclear risk | Not stated |
| Methods | DESIGN: Randomised controlled cross‐over trial BLINDING PROCEDURES: Double blind SAMPLE CALCULATIONS: NO DURATION: 1 procedure FOLLOW‐UP: None WITHDRAWLS/DROPOUTS: None ITT: Not applicable GEOGRAPHICAL LOCATION: Thailand SETTING: Not stated |
|
| Participants | N = 10 DIAGNOSIS: Neurogenic bladder disfunction ELIGIBLE: Not stated ENROLLED: 10 COMPLETED: 10 AGE: Adults GENDER: 4 male, 6 female | |
| Interventions | Aloe vera versus aqueous gel as lubricant | |
| Outcomes | Catheterisation time Adverse effect Satisfaction (nurse and participant) |
|
| Notes | Article written in Thai, abstract available in English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated in abstract |
| Allocation concealment (selection bias) | Unclear risk | Not stated in abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Source of Funding | Unclear risk | Not stated |
| Methods | DESIGN: Randomised controlled trial BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: 12 months FOLLOW‐UP: Urine C&S every 3 months WITHDRAWALS/DROPOUTS: 13 ITT: Not stated GEOGRAPHICAL LOCATION: 3 sites in USA SETTING: Community | |
| Participants | N = 62 DIAGNOSIS: Neurogenic bladder (cause not stated) ELIGIBLE: Not stated ENROLLED: 62 COMPLETED: 49 AGE: Adults GENDER: Male | |
| Interventions | Hydrophilic‐coated (not activated) (Lofric) single‐use catheter with an uncoated, non‐lubricated (water added by user) multiple‐use catheter. Reuse time 24 hours. | |
| Outcomes | UTI; pyuria; haematuria. | |
| Notes | UTI defined as 10x5 CFU/mL + at least one clinical symptom (fever, chills, malodorous urine, increased spasticity, malaise). Catheter cleaning not described; used 1 reused catheter per day. No statistically significant group differences were noted; unclear how long participants were using IC before entering trial; pre trial UTI based on participant recall. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised fashion" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether laboratory technician was blinded to catheter |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal rate greater in the experimental arm (8 vs 5). "Two patients per group related to the type of catheter used" |
| Source of Funding | Unclear risk | Astro Tech AB |
| Methods | DESIGN: Randomised controlled trial BLINDING PROCEDURES: Double blind SAMPLE CALCULATION: Yes DURATION: 4 weeks WITHDRAWALS/DROPOUTS: Unclear ITT: Yes and also per protocol analysis GEOGRAPHICAL LOCATION: 6 European countries (13 centres) SETTING: Community | |
| Participants | N = 195 DIAGNOSIS: Mixed ELIGIBLE: Not stated ENROLLED: 195 COMPLETED: Unclear AGE: Adults GENDER: 151 male; 44 female | |
| Interventions | Hydrophilic‐coated catheter, not activated, sterile water provided versus hydrophilic‐coated catheter, not activated, water added by user (aseptic technique both arms) | |
| Outcomes | Participant perception of handling | |
| Notes | Participant perception of handling was rated similarly in both groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Since most polymeric materials are visually similar, comparable materials could be produced and packaged in identical form". This was set up as a double‐blind trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This was set up as a double‐blind trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar incomplete outcome data in control (n = 6) and intervention (n = 10) groups |
| Source of Funding | Unclear risk | Not stated |
BPH: benign prostatic hyperplasia CFU/mL: colony‐forming unit per millilitre CIC: Clean Intermittent Catheterization C&S: culture and sensitivity IC: intermittent catheterisation ICU: intensive care unit ISC‐Q: Intermittent Self‐Catheterisation Questionnaire ITT: intention‐to‐treat NIDRR: National Institute on Disability and Rehabilitation Research RBC per HPF: red blood cell per RCT: randomised controlled trial SS: UI: urinary infection UTI: urinary tract infection VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bagi 2011 | Healthy volunteers used. |
| Bennett 1997 | Comparison of non‐hydrophilic gel lubricated catheter with or without an introducer tip to reduce UTI. Study design not clear. |
| Charbonneau 1993 | Retrospective chart review of incidence of UTI in participants using standard practice IC in rehabilitation from 1985‐1988 then evaluation of UTI in 18 participants of a closed catheter/bag system. Not a RCT. |
| Clarke 2005 | RCT comparing effect of prophylactic antimicrobial treatment versus discontinuation of antimicrobial treatment on UTI in IC users (children with neuropathic bladder). |
| Diokno 1995 | Evaluation of participant satisfaction with a pre‐lubricated hydrophilic catheter for IC. Not a RCT. |
| Edokpolo 2012 | Retrospective trial of UTI in long term IC users with spinal cord injury. Not a RCT. |
| Feng 2009 | Article in Chinese; abstract in English. RCT comparing effect of moxibustion and IC versus IC alone on bladder function in participants with neurogenic bladder dysfunction. |
| Grigoleit 2006 | Article in German; appears to be a review of catheterisation methods (based on short English abstract). |
| Hosseini 2008 | RCT comparing effect of clean IC with triamicinolone ointment versus water‐based lubricant on stricture formation in participants following internal urethrotomy due to obstruction/stricture. |
| Hudson 2005 | Laboratory trial evaluating the likelihood of catheter contamination based on catheter design; did not compare incidence of UTI. Not a RCT |
| Litherland 2007 | RCT comparing effect of two hydrophilic‐coated catheters on participant‐perceived discomfort. One procedure only. Non‐IC users undergoing gynaecology testing. |
| Martins 2009 | Cross‐over trial (non‐randomised) comparing hydrophilic‐coated catheter versus an uncoated catheter in children with a urostomy. |
| Nalinthip 1996 | Article in Thai; abstract translated. Comparison of UTI rate when IC performed by participant or nurse. |
| Sallami 2011 | RCT comparing effect of clean IC using a hydrophilic catheter versus a standard PVC nelaton catheter on participant satisfaction, complications and recurrence of urethral stricture following endoscopic urethrotomy. |
| Sherbondy 2002 | Survey of individuals using IC and reusing the catheter. Not a RCT. |
| Sims 1993 | Chart review of two different cleaning methods and comparison of UTI (wash with soap and water then soak in povidone iodine or allow to air dry). Not a RCT. |
| Stensballe 2005 | Laboratory evaluation (RCT) of friction force of 2 hydrophilic catheters and one non‐hydrophilic catheter. RCT. Healthy volunteers used. |
| Terpenning 1989 | Observational trial of the incidence and time to onset of UTI in elderly in a Veterans Administration Centre (USA). Not a RCT. |
IC: intermittent catheterisation RCT: randomised controlled trial UTI: urinary tract infection
Contributions of authors
JP and MF took the lead in article retrieval and summary of initial findings which were then reviewed and edited as necessary by JP, MF and KNM. All four review authors contributed towards the writing of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Incontinence Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. The NIHR is the largest single funder of the Cochrane Incontinence Group.
Declarations of interest
Katherine Moore was a co‐investigator on a trial sponsored by Coloplast (Cardenas 2011) and received product from Coloplast for another trial (Moore 2013). Mandy Fader has received intermittent catheter products for research purposes from Astra Tech AB.
Notes
This review has been withdrawn following Feedback from Andrei V. Krassioukov, Kathleen Christison, Matthias Walter, Jean‐Jacques J.M. Wyndaele, Thomas M. Kessler and Michael Kennelly who have identified possible errors in the analysis of data in the review. These concern handling of cross‐over and multi‐arm trials as well as corrections and clarifications of data used. This review has been withdrawn whilst these issues are addressed. Changes are also required to reflect current methodological standards. The review will be re‐published following updating, revision and peer review.
Withdrawn from publication for reasons stated in the review
References
References to studies included in this review
- Biering‐Sorensen F, Hansen HV, Nielsen PN, Looms D. Residual urine after intermittent catheterization in females using two different catheters. Scandinavian Journal of Urology and Nephrology 2007;41:341‐5. [DOI] [PubMed] [Google Scholar]
- Cardenas DD, Hoffman JM. Hydrophilic catheters versus noncoated catheters for reducing the Incidence of urinary tract infections: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2009;90(10):1668‐71. [DOI] [PubMed] [Google Scholar]
- Cardenas DD, Moore KN, Dannels‐McClure A, Scelza WM, Graves DE, Brooks M, et al. Intermittent catheterization with a hydrophilic‐coated catheter delays urinary tract infections in acute spinal injury: a prospective randomised, multicenter trial. Physical Medicine and Rehabilitation 2011;3:408‐17. [DOI] [PubMed] [Google Scholar]
- Chartier‐Kastler E, Lauge I, Ruffion A, Goossens D, Charvier K, Biering‐Sorensen F. Safety of a new compact catheter for men with neurogenic bladder dysfunction: a randomised, crossover and open‐labelled study. Spinal Cord 2011;49(7):844‐50. [DOI] [PubMed] [Google Scholar]
- Chartier‐Kastler E, Amarenco G, Lindbo L, Soljanik I, Andersen HL, Bagi P, et al. A prospective, randomized, crossover, multicenter study comparing quality of life using compact versus standard catheters for intermittent self‐catheterization. Journal of Urology 2013;190(3):942‐7. [DOI] [PubMed] [Google Scholar]
- Costa J, Menier M. Catheter length preference in wheelchair bound male patients who perform clean intermittent catheterisation (Abstract number 38). Neurourology and Urodynamics 2012;31(6):773‐4. [Google Scholar]; Costa J, Menier M, Doran T, Köhler T. Catheter length preference in wheelchair‐using men who perform routine clean intermittent catheterization. Spinal Cord 2013;51(10):772‐5. [DOI] [PubMed] [Google Scholar]
- Day RA, Moore KN, Alberts MK. A pilot trial comparing two methods of intermittent catheterization: Limitations and challenges. Urologic Nursing 2003;23(2):143‐7,158. [PubMed] [Google Scholar]
- Denys P, Previnaire J, Aegerter P, Seze M, Karsenty G, Amarenco G. Intermittent self‐catheterisation habits and opinion on aseptic VaPro catheter in French neurogenic bladder population. Spinal Cord 2012;50:853‐8. [DOI] [PubMed] [Google Scholar]
- Ridder DJMK, Everaert K, Fernandez LG, Valero JVF, Duran AB, Abrisqueta MLJ, et al. Intermittent catheterisation with hydrophilic‐coated catheters (Speedicath) reduces the risk of clinical urinary tract infection in spinal cord injured patients: a prospective randomzied parallel comparative trial. European Urology 2005;48(6):991‐5. [DOI] [PubMed] [Google Scholar]
- Domurath B, Kutzenberger J, Kurze I, Knoth H. Clinical evaluation of a newly developed catheter(SpeediCath Compact Male) in men with spinal cord injury:residual urine and user evaluation. Spinal Cord 2011;49:817–21. [DOI] [PubMed] [Google Scholar]
- Duffy LM, Cleary J, Ahern S, Kuskowski MA, West M, Wheeler L, et al. Clean intermittent catheterization: Safe, Cost‐effective bladder management for male residents of VA nursing homes. Journal of the American Geriatrics Society 1995;43(8):865‐70. [1058] [DOI] [PubMed] [Google Scholar]
- Fader M, Moore KN, Cottenden AM, Pettersson L, Brooks R, Malone‐Lee J. Coated catheters for intermittent catheterization: smooth or sticky?. British Journal of Urology International 2001;88 (4):373‐7. [DOI] [PubMed] [Google Scholar]
- Fera P. Lubricated urethral catheters with lidocaine versus gentamycin for clean intermittent catheterization. Brazilian Journal of Urology 2002;28(1):50‐6. [18009] [Google Scholar]
- Giannantoni A, Stasi SM, Scivoletto G, Virgili G, Dolci S, Porena M. Intermittent catheterization with a prelubricated catheter in spinal cord injured patients: a prospective randomized crossover trial. Journal of Urology 2001;166(1):130‐3. [15635] [PubMed] [Google Scholar]
- King RB, Carlson CE, Mervine J, Wu Y, Yarkony GM. Clean and sterile intermittent catheterization methods in hospitalized patients with spinal cord injury. Archives of Physical Medicine & Rehabilitation 1992;73(9):798‐802. [1360] [PubMed] [Google Scholar]
- Leek H, Stephenson Z, Reus A, Karantanis E, Moore KH. Clean intermittent self‐catheterisation: A randomised controlled crossover trial of single‐use versus multiple re‐use of non‐coated catheters; is cystitis rate altered? (Abstract number 170). Neurourology and Urodynamics 2012;32(6):759‐60. [Google Scholar]
- Leriche A, Charvier K, Bonniaud B, Peyrat L, N'Guyen P, Soler J, et al. An acceptability study: Speedicath set vs Actreen set in patients practicing self‐catheterisation. Progres en Urologie 2006;16:347‐51. [PubMed] [Google Scholar]
- Mauroy B, Soret J, Bonnal J, Fantoni J. Comparison between three intermittent catheters: a prospective study in a group of 27 patients. Annals of Urology 2001;35:223‐8. [DOI] [PubMed] [Google Scholar]
- Moore KN, Kelm M, Sinclair O, Cadrain G. Bacteriuria in intermittent catheterization users: the effect of sterile versus clean reused catheters. Rehabilitation Nursing 1993;18(5):306‐9. [1208] [DOI] [PubMed] [Google Scholar]
- Moore KN, Burt J, Voaklander D. Intermittent catheterization in the rehabilitation setting: a comparison of clean and sterile technique. Clinical Rehabilitation 2006;20(6):461‐8. [DOI] [PubMed] [Google Scholar]
- Moore KN, Kiddoo D, Sawatzky B, Afshar K, Dharamsi N, Bascu C, et al. Randomised crossover trial of hydrophilic single use versus PVC multiuse catheters for CIC in children with neural tube defects (spina bifida) (Abstract number 171). Neurourology and Urodynamics 2013;32(6):760‐1. [Google Scholar]
- Pachler J, Frimodt‐Moller C. A comparison of prelubricated hydrophilic and non‐hydrophilic polyvinyl chloride catheters for urethral catheterization. BJU International 1999;83(7):767‐9. [DOI] [PubMed] [Google Scholar]; Pachler J, Frimodt‐Moller C. Pre‐lubricated hydrophilic pvc urethral catheters compared to non‐hydrophilic pvc catheters. Proceedings of the International Continence Society (ICS), 28th Annual Meeting; 1998 Sept 14‐17; Jerusalem, Israel1998:325. [5702]
- Pascoe G, Clovis S. Evaluation of two coated catheters in intermittent self‐catheterisation. British Journal of Nursing 2001;10(5):325‐9. [DOI] [PubMed] [Google Scholar]
- Prieto‐Fingerhut T, Banovac K, Lynne CM. A trial comparing sterile and nonsterile urethral catheterization in patients with spinal cord injury. Rehabilitation Nursing 1997;22(6):299‐302. [5491] [DOI] [PubMed] [Google Scholar]
- Quigley PA, Riggin OZ. A comparison of open and closed catheterization techniques in rehabilitation patients. Rehabilitation Nursing 1993;18:26‐9,33. [2345] [DOI] [PubMed] [Google Scholar]
- Sarica S, Akkoc Y, Karapolat H, Aktug H. Comparison of the use of conventional, hydrophilic and gel‐lubricated catheters with regard to urethral micro trauma, urinary system infection, and patient satisfaction in patients with spinal cord injury: a randomized controlled study. European Journal of Physical and Rehabilitation Medicine 2010;46:473‐80. [PubMed] [Google Scholar]
- Schlager TA, Clark M, Anderson S. Effect of a single‐use sterile catheter for each void on the frequency of bacteriuria in children with neurogenic bladder on intermittent catheterization for bladder emptying. Pediatrics 2001;198(4):e71. [DOI] [PubMed] [Google Scholar]
- Sutherland RS, Kogan BA, Baskin LS, Mevorach RA. Clean intermittent catheterization in boys using the LoFric catheter. Journal of Urology 1996;156(6):2041‐3. [4867] [PubMed] [Google Scholar]
- Taweesangsuksakul R, Tamnanthong N, Hanpanich K, Chobchuen R, Kirdpon W. Effect of aloe vera for lubrication on urethral catheterisation. Journal of Thail Rehabilitation Medicine 2005;15(2):113‐8. [Google Scholar]
- Vapnek JM, Maynard FM, Kim J. A prospective randomized trial of the LoFric hydrophilic coated catheter versus conventional plastic catheter for clean intermittent catheterization. Journal of Urology 2003;169(3):994‐8. [15588] [DOI] [PubMed] [Google Scholar]
- Witjes JA, Popolo G, Marberger M, Jonsson O, Kapsand HP, Chapple CR. A multicenter, double‐blind, randomized, parallel group study comparing polyvinyl chloride and polyvinyl chloride‐free catheter materials. Journal of Urology 2009;182(6):2794‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Bagi P, Hannibalsen J, Permild R, Stilling S, Looms D. Safety of a new compact male intermittent catheter: randomized, cross‐over, single‐blind study in healthy male volunteers. Urology International 2011;86(2):179‐84. [DOI] [PubMed] [Google Scholar]
- Bennett C, Young M, Razi S, Adkins R, Diaz F, McCrary A. The effect of urethral introducer tip catheters on the incidence of urinarytract infection outcomes in spinal cord injured patients. Journal of Urology 1997;158(2):519‐21. [PubMed] [Google Scholar]
- Charbonneau‐Smith R. No‐touch catheterization and infection rates in a select spinal cord injured population. Rehabilitation Nursing 1993;18:296‐9,305,355‐6. [2282] [DOI] [PubMed] [Google Scholar]
- Clarke SA, Samuel M, Boddy S. Are prophylactic antibiotics necessary with clean intermittent catheterization? A randomised controlled trial. Journal of Pediatric Surgery 2005;40(3):568‐71. [DOI] [PubMed] [Google Scholar]
- Diokno AC, Mitchell BA, Nash AJ, Kimbrough JA. Patient satisfaction and the LoFric catheter for clean intermittent catheterization. Journal of Urology 1995;153(2):349‐51. [DOI] [PubMed] [Google Scholar]
- Edokpolo L, Stavris K, Foster H. Intermittent catheterization and recurrent urinary tract infection in spinal cord injury. Topics in Spinal Cord Rehabilitation 2012;18(2):187‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Ren Y, Wei D, Liu C. Clinical observation on moxibustion combined with intermittent urethral catheterization for treatment of neurogenic vesical dysfunction. Zhongguo Zhen Jiu 2009;28(2):21‐9. [PubMed] [Google Scholar]
- Grigoleit U, Pannek J, Stohrer M. Single‐use intermittent catheterisation][German] [Der Intermittierende Einmalkatheterismus]. Urologe 2006;45(2):175‐82. [DOI] [PubMed] [Google Scholar]
- Hosseini J, Kaviani A, Golshan AR. Clean intermittent catheterization with triamcinolone ointment following internal urethrotomy. Urology Journal 2008;5(4):265‐8. [PubMed] [Google Scholar]
- Hudson E, Murahata RI. The 'no‐touch' method of intermittent urinary catheter insertion: can it reduce the risk of bacteria entering the bladder?. Spina Cord 2005;43:611‐4. [DOI] [PubMed] [Google Scholar]
- Litherland A, Schlotz H. Patient‐perceived discomfort with two coated urinary catheters. British Journal of Nursing 2007;16(5):284‐7. [DOI] [PubMed] [Google Scholar]
- Martins M, Santos V, Secoli S, Mata M, Nogueira D, Souza D. A comparison between two catheters for clean intermittent catheterisation in continent children with a urostomy. Revista Da Escola de Enfermagem Da USP 2009;43(4):865‐71. [DOI] [PubMed] [Google Scholar]
- Nalinthip T. Urinary tract infection in self‐catheterisation compared to nurse‐catheterisation for bladder training in patients with spinal cord lesion (Abstract). Srinagarind Medical Journal 1996;11(4):345. [Google Scholar]
- Sallami S, Mouine Y, Rhouma S, Cherif K, Dahmani A, Horchani A. Clean intermittent catheterization following urethral stricture surgery using a low friction catheter versus conventional plastic catheter: a prospective, randomized trial. UroToday International Journal2011; Vol. 4, issue 2. [DOI: 10.3834/uij.1944-5784.2011.04.07] [DOI]
- Sherbondy AL, Cooper CS, Kalinowski SC, Boyt MA, Hawtrey CE. VAriability in catheter microwave sterilization techniques in a single clinic population. Journal of Urology 2002;168(2):562‐4. [PubMed] [Google Scholar]
- Sims L, Ballard N. A comparison of two methods of catheter cleansing and storage used with clean intermittent catheterization. Rehabilitation Nursing Research 1993;2:87‐92. [2279] [Google Scholar]
- Stensballe J, Looms D, Nielsen PN, Tvede M. Hydrophilic‐coated catheters for intermittent catheterisation reduce urethral micro trauma: a prospective, randomised, participant‐blinded, crossover trial of three different types of catheters. European Urology 2005;48(6):978‐83. [21523] [DOI] [PubMed] [Google Scholar]
- Terpenning MS, Allada R, Kauffman CA. Intermittent catheterization in the elderly. Journal of the American Geriatrics Society 1989;37(5):411‐6. [DOI] [PubMed] [Google Scholar]
Additional references
- Bermingham S, Hodgkinson S, Wright S, Hayter E, Spinks J, Pellowe C. Intermittent self catheterisation with hydrophilic, gel reservoir, and non‐coated catheters: a systematic review and cost effectiveness analysis. BMJ2013; Vol. 346, issue e8639:e1‐e16. [DOI: 10.1136/bmj.e8639] [DOI] [PMC free article] [PubMed]
- Campbell J, Moore KN, Voaklander D, Mix, L. Complications associated with clean intermittent catheterization in children with spina bifida. Journal of Urology 2004;171(6 Pt 1):2420‐2. [DOI] [PubMed] [Google Scholar]
- Cottenden A, Bliss DZ, Buckley B, Fader M, Gartley C, Hayder D, et al. Management using continence products. In: Abrams P, Cardozo L, Khoury S, Wein A editor(s). Incontinence: 5th International Consultation on Incontinence. Recommendations of the International Scientific Committee: Evaluation and Treatment of Urinary Incontinence, Pelvic Organ Prolapse and Faecal Incontinence; 2012 Feb 23‐25; Paris. 5th Edition. Belgium: International Consultation on Urological Diseases (ICUD), 2013:1651‐786. [Google Scholar]
- Li L, Ye W, Ruan H, Yang B, Zhang S, Li L. Impact of hydrophilic catheters on urinary tract infections in people with spinal cord injury: systematic review and meta‐analysis of randomized controlled trials. Archives of Physical Medicine and Rehabilitation 2013;94(4):782‐7. [DOI] [PubMed] [Google Scholar]
- National Institute on Disability and Rehabilitation Research (NIDRR). The prevention and management of urinary tract infections among people with spinal cord injuries. National Institute on Disability and Rehabilitation Research Consensus Statement. January 27‐29, 1992. Journal of the American Paraplegia Society 1992;15(3):194‐204. [DOI] [PubMed] [Google Scholar]
- Pinder B, Lloyd AJ, Elwick H, Denys P, Marley J, Bonniaud V. Development and psychometric validation of the intermittent self‐catheterization questionnaire. Clinical Therapeutics 2012;34(12):2302‐13. [DOI] [PubMed] [Google Scholar]
- Reference Manager Professional Edition Version 12. New York: Thomson Reuters2012.
- Wyndaele JJ. Complications of intermittent catheterization: their prevention and treatment. Spinal Cord 2002;40(10):536‐41. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Moore KN, Fader M, Getliffe K. Long‐term bladder management by intermittent catheterisation in adults and children. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006008.pub2] [DOI] [PubMed] [Google Scholar]
- Prieto J, Murphy CL, Moore KN, Fader M. Intermittent catheterisation for long‐term bladder management. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD006008.pub3] [DOI] [PubMed] [Google Scholar]


