Abstract
Background
Immobilization of the lower limb is a risk factor for venous thromboembolism (VTE). Low molecular weight heparins (LMWHs) are anticoagulants, which might be used in adult patients with lower‐limb immobilization to prevent deep venous thrombosis (DVT) and its complications. This is an update of the review first published in 2008.
Objectives
To assess the effectiveness of low molecular weight heparin for the prevention of venous thromboembolism in patients with lower‐limb immobilization in an ambulatory setting.
Search methods
For this update, the Cochrane Vascular Information Specialist searched the Specialised Register, CENTRAL, and three trials registers (April 2017).
Selection criteria
Randomized controlled trials (RCTs) and controlled clinical trials (CCTs) that described thromboprophylaxis by means of LMWH compared with no prophylaxis or placebo in adult patients with lower‐limb immobilization. Immobilization was by means of a plaster cast or brace.
Data collection and analysis
Two review authors independently selected trials, assessed risk of bias and extracted data. The review authors contacted the trial authors for additional information if required. Statistical analysis was carried out using Review Manager 5.
Main results
We included eight RCTs that fulfilled our criteria, with a total of 3680 participants. The quality of evidence, according GRADE, varied by outcome and ranged from low to moderate. We found an incidence of DVT ranging from 4.3% to 40% in patients who had a leg injury that had been immobilized in a plaster cast or a brace for at least one week, and who received no prophylaxis, or placebo. This number was significantly lower in patients who received daily subcutaneous injections of LMWH during immobilization, with event rates ranging from 0% to 37% (odds ratio (OR) 0.45, 95% confidence interval (CI) 0.33 to 0.61; with minimal evidence of heterogeneity: I² = 26%, P = 0.23; seven studies; 1676 participants, moderate‐quality evidence). Comparable results were seen in the following groups of participants: patients with below‐knee casts, conservatively treated patients (non‐operated patients), operated patients, patients with fractures, patients with soft‐tissue injuries, and patients with distal or proximal thrombosis. No clear differences were found between the LMWH and control groups for pulmonary embolism (OR 0.50, 95% CI 0.17 to 1.47; with no evidence of heterogeneity: I² = 0%, P = 0.56; five studies, 2517 participants; low‐quality evidence). The studies also showed less symptomatic VTE in the LMWH groups compared with the control groups (OR 0.40, 95% CI 0.21 to 0.76; with minimal evidence of heterogeneity: I² = 16%, P = 0.31; six studies; 2924 participants; low‐quality evidence). One death was reported in the included studies, but no deaths due to pulmonary embolism were reported. Complications of major adverse events were rare, with minor bleeding the main adverse events reported.
Authors' conclusions
Moderate‐quality evidence showed that the use of LMWH in outpatients reduced DVT when immobilization of the lower limb was required, when compared with no prophylaxis or placebo. The quality of the evidence was reduced to moderate because of risk of selection and attrition bias in the included studies. Low‐quality evidence showed no clear differences in PE between the LMWH and control groups, but less symptomatic VTE in the LMWH groups. The quality of the evidence was downgraded due to risk of bias and imprecision.
Plain language summary
Low molecular weight heparin for prevention of venous thromboembolism in adults with lower‐limb immobilization in an outpatient setting
Background
Venous thromboembolism is a condition where a blood clot forms in the deep veins (DVT), most commonly of the leg. The concern is that it can travel up to block the arteries in the lungs (pulmonary embolism). In adult patients, immobilization of the lower limb with a plaster cast or brace is a risk factor for DVT and pulmonary embolism. To prevent this complication, preventive treatment with anticoagulants (medication that thins the blood) is often used, most commonly, low molecular weight heparin (LMWH). However, there is no agreement on this in existing national guidelines. Therefore, we searched the literature for trials on this topic, in order to assess the evidence.
Study characteristics and key results
We included eight studies in this review (current until April 2017). The studies included a total of 3680 participants. Participants received either LMWH subcutaneously once daily, or no preventive treatment or placebo. New cases of DVT ranged from 4.3% to 40% in the control groups and ranged from 0% to 37% in the LMWH groups. The risk of DVT was lower in participants who received LMWH. Further analysis also showed a reduction in the occurrence of DVT when the use of LMWH was compared to no treatment or placebo in the following groups of participants: patients with below‐knee casts, conservatively treated patients (patients not operated), operated patients, patients with fractures, patients with soft‐tissue injuries, patients with above‐knee thrombosis, and patients with below‐knee thrombosis. No clear differences were found between the LMWH and control groups for pulmonary embolism. The studies showed less symptomatic venous thromboembolism in the LMWH groups compared with the control groups. No cases of death due to pulmonary embolism were reported. One study reported one death in the control group.
There were few reported adverse effects in the treated patients. The main adverse events reported were cases of minor bleeding such as nose bleeds, blood in urine and dark stool.
Quality of evidence and conclusion
The use of LMWH in adult patients reduced DVT when immobilization of the lower limb was required, compared with no prevention or placebo. The quality of the evidence was downgraded to moderate due to risks of bias in some trials, such as lack of blinding of participants, or unclear reasons for excluding participants from the analyses. Low‐quality evidence showed no clear differences in pulmonary embolism between LMWH and the control groups, but fewer symptomatic venous thromboemboli in the LMWH groups. The quality of evidence was downgraded due to methodological issues and imprecision of the results.
Summary of findings
Summary of findings for the main comparison. Low molecular weight heparin compared to no prophylaxis or placebo in prevention of venous thromboembolism in patients with lower‐limb immobilization.
| Low molecular weight heparin compared to no prophylaxis or placebo in prevention of venous thromboembolism in patients with lower‐limb immobilization | ||||||
| Patient or population: prevention of venous thromboembolism in patients with lower‐limb immobilization Setting: ambulatory setting Intervention: low molecular weight heparin Comparison: no prophylaxis or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no prophylaxis or placebo | Risk with low molecular weight heparin | |||||
| Deep venous thrombosis | Study population | OR 0.45 (0.33 to 0.61) | 1676 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 174 per 1000 | 87 per 1000 (65 to 114) | |||||
| Pulmonary embolism | Study population | OR 0.50 (0.17 to 1.47) | 2517 (5 RCTs) | ⊕⊕⊝⊝ LOW 2 | ||
| 7 per 1000 | 4 per 1000 (1 to 10) | |||||
| Symptomatic venous thromboembolism | Study population | OR 0.40 (0.21 to 0.76) | 2924 (6 RCTs) | ⊕⊕⊝⊝ LOW 3 | ||
| 21 per 1000 | 9 per 1000 (5 to 16) | |||||
| Mortality due to pulmonary embolism | Study population | ‐ | 3111 (8 RCTs) | ‐ | No mortality due to pulmonary embolism was reported | |
| see comment | see comment | |||||
| Mortality due to other causes | Study population | OR 0.33 (0.01 to 8.15) |
3111 (8 RCTs) | ⊕⊕⊝⊝ LOW 4 | One death (in no prophylaxis/placebo group) was reported in the included studies | |
| 1 per 1000 | 0 per 1000 (0 to 5) |
|||||
| Adverse outcomes | Study population | OR 2.01 (0.83 to 4.86) | 3178 (8 RCTs) | ⊕⊕⊝⊝ LOW 5 | ||
| 40 per 1000 | 78 per 1000 (34 to 170) | |||||
| *We calculated the assumed risk of the no prophylaxis or placebo group from the average risk in the no prophylaxis or placebo groups (i.e. the number of participants with events divided by total number of participants of the no prophylaxis or placebo group included in the meta‐analysis). The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level as 3 out of 7 studies showed considerable risk of bias 2 Downgraded by two levels as 2 out of 5 studies showed considerable risk of bias, and imprecision of pooled results 3 Downgraded by two levels as 3 out of 6 studies showed considerable risk of bias, and imprecision of pooled results 4 Downgraded by two levels due to the low number of events, and imprecision of pooled results 5 Downgraded by two levels as 4 out of 8 studies showed considerable risk of bias, and imprecision of pooled results
Background
Description of the condition
Lower‐limb immobilization is associated with deep venous thrombosis (DVT) and pulmonary embolism (PE). Predisposing risk factors for venous thromboembolism (VTE) can be divided into individual patient factors, trauma, or surgery‐related factors. Patient‐related factors include: obesity, thrombophilia (a hereditary or acquired predisposition to thrombosis), a previous thrombosis, age over 40 years, or cardiac or respiratory failure (Anderson 2003; Clagett 1995; Zagrodnick 1990). Immobilization is considered a significant risk factor for the development of DVT and PE (Knudson 1996; Kudsk 1989; Kujath 1991). Other factors associated with an increased risk of VTE include: blood transfusion, surgery, fracture of the pelvis, femur, or tibia, spinal cord injury, head injury, shock on hospital admission, venous injury, more than three days on ventilation, the time from injury to operation, and operation time (Abelseth 1996; Knudson 2004).
In a group of 102 patients with lower‐limb fractures, Abelseth found a rate of DVT of 28% (Abelseth 1996). All patients underwent surgery and were mobilized without the use of a plaster cast. Proximal fractures were associated with a higher risk of DVT compared with more distal fractures (Abelseth 1996). Other reported incidences of venographically‐proven DVT in patients with lower‐limb fractures range from 27% to 78% (Breyer 1984; Geerts 1994; Hjelmstedt 1968; Kudsk 1989; Spieler 1972). The percentages in hospitalized patients are generally higher than in outpatients. In outpatients immobilized in plaster casts without LMWH, the incidence of DVT on ultrasonography ranges from 4.5% to 16.5% (Kock 1995; Kujath 1993; Reilmann 1993; Zagrodnick 1990). The incidence of PE in trauma patients with DVT without prophylaxis is 4.3%, with a high mortality rate (20% to 23.3%). In patients with DVT receiving thromboprophylaxis, this incidence can be lowered to 0.3% to 2.0% (Hill 2002).
Description of the intervention
The primary goal of administering thromboprophylaxis is to prevent PE and DVT and their sequelae. Oral anticoagulants, unfractionated heparin (UFH) and LMWH, have been studied as treatment options for this indication. In clinical guidelines, the recommendations for preventing venous thrombosis in patients with isolated lower‐limb injuries distal to the knee are sparse. The Italian Intersociety Consensus Statement states that most patients in orthopedic and traumatological fields, other than knee and hip replacements, should be considered for thromboprophylaxis after individual assessment of haemorrhagic risk (Della Rocca 2013). Guidelines in Emergency Medicine Network in the United Kingdom (GEMNet) only advises the use of thromboprophylaxis in patients with rigid cast immobilization and a permanent risk factor for VTE (Roberts 2013).
There remains substantial practice variation amongst surgeons regarding the use of anticoagulation measures (Batra 2006). In daily clinical practice, there remains a huge variation in the way thromboprophylaxis is used. A recent survey among Dutch orthopedic and trauma surgeons showed that 60% to 80% always prescribed thromboprophylaxis in patients with lower‐limb immobilization. Up to 8% of the participants never treated their patients with prophylaxis (van Adrichem 2015).
How the intervention might work
Low molecular weight heparin is proven to be effective in the prevention of venous thromboembolism (Weitz 1997). By treating patients with lower‐limb immobilization with LMWH, we expect to see less venous thromboembolism compared to patients who do not receive any protection.
Why it is important to do this review
This is the second update of the Cochrane review first published in 2008 (first update 2014). Since 2014, two additional studies on this subject have been published (Bruntink 2017; Van Adrichem 2017). Therefore, in order to include the most recent information, we updated this review.
Objectives
To assess the effectiveness of low molecular weight heparin for the prevention of venous thromboembolism in patients with lower‐limb immobilization in an ambulant setting.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomized controlled trials (RCTs) and controlled clinical trials (CCTs) that describe thromboprophylaxis, by means of low molecular weight heparin (LMWH), in adults with lower‐limb immobilization in an ambulatory setting. Treatment with LMWH could have started during hospital admission.
Types of participants
Adults treated with a device for lower‐limb immobilization, such as a leg cast or brace, in an ambulatory setting. Weight bearing and duration of leg cast use were not considered criteria for inclusion or exclusion.
Types of interventions
Studies comparing LMWH with no prophylaxis or placebo. Studies including oral anticoagulants, UFH, or aspirin were excluded.
Types of outcome measures
Primary outcomes
Morbidity
DVT ‐ confirmed by venography or ultrasonography
PE ‐ confirmed by a ventilation‐perfusion scan, a CT scan, or angiography
Symptomatic VTE ‐ symptomatic DVT, PE, or combination
Secondary outcomes
Mortality ‐ PE‐related
Mortality ‐ other causes
Adverse outcomes of treatment: bleeding, heparin induced thrombocytopenia (HIT), allergic reaction, others (definitions of adverse outcomes as reported by study authors).
Search methods for identification of studies
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
The Cochrane Vascular Specialised Register (19 April 2017).
The Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 3) via the Cochrane Register of Studies Online (searched 19 April 2017).
See Appendix 1 for details of the search strategy used for CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, and AMED, as well as through handsearching relevant journals. The full list of the databases, journals, and conference proceedings searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library.
In addition, the CIS searched the following trials registers for details of ongoing and unpublished studies (19 April 2017). See Appendix 2
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch).
ClinicalTrials.gov (clinicaltrials.gov)
International Standard Randomised Controlled Trial Number (ISRCTN) registry (www.isrctn.com).
Searching other resources
The review authors searched the reference lists of relevant studies.
Data collection and analysis
Selection of studies
Two review authors (KL and MH) independently assessed all studies identified by the literature searches, according to the inclusion criteria. Disagreements were resolved by discussion.
Data extraction and management
Two review authors (MH and AZ) independently extracted data to ensure objectivity and validity of findings. A third review author (HJ) cross checked the information, and disagreements were resolved by discussion. The review authors contacted trial authors for additional information if required.
Assessment of risk of bias in included studies
Two review authors (KL and AZ) independently assessed the risk of bias of the included studies by using Cochrane's 'Risk of bias' tool (Higgins 2011). Random sequence generation, allocation concealment, blinding of participants, personnel, and outcome assessors, incomplete outcome data, selective outcome reporting, and any other relevant biases were classified as 'low risk', 'high risk' or 'unclear risk'. They resolved any disagreement through discussion with review authors HJ and LJ.
Measures of treatment effect
We measured treatment effect by calculating odds ratios (OR) with 95% confidence interval (CI) for dichotomous data.
Unit of analysis issues
The individual participant was considered the unit of analysis.
Dealing with missing data
Where appropriate, we used all randomized participants for the analysis. However, many of the included studies had participants excluded after randomization, creating a disparity between the number of participants randomized and the number available for assessment of VTE outcomes. Therefore, we used the data from the populations as reported by the studies. These generally consisted of all participants who received treatment and had evaluable testing of VTE at the end of the study. If these values were not available, we used the reported per‐protocol data.
Assessment of heterogeneity
Statistical analysis was carried out using Review Manager 5 (RevMan 2014). Review author LJ coordinated the statistical analysis. We searched for clinical and statistical heterogeneity by visually inspecting the forest plots. We quantified statistical heterogeneity by means of an I² test (Deeks 2011; Higgins 2011). We interpreted an I² value higher than 50% as an indicator for substantial heterogeneity.
Assessment of reporting biases
We had planned to perform funnel plot analyses to assess reporting bias, when ten or more studies were included.
Data synthesis
We synthesized available data using Review Manager 5 (RevMan 2014). We investigated pooled estimates of the effects of treatment using a fixed‐effect model to calculate ORs with 95% CIs for dichotomous outcomes. When substantial heterogeneity was detected, we performed a random‐effects model analysis instead. If it was not possible to pool data, we planned to describe the results reported by the studies in the text.
Subgroup analysis and investigation of heterogeneity
We presented data by different groups of participants. These groups were selected, as these particular patient categories may influence the outcome.
DVT: regardless of type of plaster, whether operated or not
DVT: in below‐knee cast, whether operated or not
DVT: only non‐operated patients
DVT: only operated patients
DVT: fractures
DVT: soft‐tissue injuries
DVT: distal segment
DVT: proximal segment
Sensitivity analysis
If any trials were judged to be of high risk of bias, we planned to perform a sensitivity analysis to assess outcomes with and without trials with high risk of bias.
Summary of findings
We constructed a 'Summary of findings' table for the comparison LMWH compared to no prophylaxis or placebo in prevention of venous thromboembolism with lower‐limb immobilization' using the GRADEpro GDT software to present the main findings of the review (GRADEpro GDT 2015). We judged the outcomes deep venous thrombosis, pulmonary embolism, symptomatic venous thromboembolism, mortality due to pulmonary embolism, mortality due to other causes, and adverse outcomes, to be the most clinically relevant to healthcare professionals and patients. We calculated assumed control intervention risks from the mean number of events in the control groups of the selected studies for each outcome. We used the system developed by the GRADE Working Group to grade the quality of the evidence as high, moderate, low, or very low, based on within‐study risk of bias, directness of evidence, heterogeneity, precision of effects estimates, and risk of publication bias (Atkins 2004).
Results
Description of studies
Results of the search
See Figure 1.
1.
PRISMA study flow diagram
Included studies
We included eight RCTs (Bruntink 2017; Jorgensen 2002; Kock 1995; Kujath 1993; Lapidus 2007a; Lapidus 2007b; Lassen 2002; Van Adrichem 2017). The characteristics of these eight studies are summarized in the Characteristics of included studies table. All eight studies were reported as full papers and included a total of 3680 participants (range 105 to 1519). The participants included in the trials required lower‐limb immobilization for the treatment of leg injuries such as foot and ankle fractures and achilles ruptures. All studies included participants prospectively, with quite similar exclusion criteria. The most common exclusion criteria were: pregnancy, allergy to heparin or contrast media, uncontrolled hypertension, pre‐existing bleeding disorders, presence of malignancies, recent brain or gastrointestinal bleeding, previous DVT, and chronic venous insufficiency. Different LMWHs were used; they were administered once daily until removal of the plaster cast: nadroparin (2850 anti‐XA IU) (Bruntink 2017; Van Adrichem 2017), nadroparin (36 mg) (Kujath 1993), certoparin (Mono‐Embolex NM; 32 mg) (Kock 1995), tinzaparin (3500 anti‐Xa IU) (Jorgensen 2002), dalteparin (5000 IU) (Lapidus 2007a; Lapidus 2007b), dalteparin (2500 IU or 5000 IU depending on body weight) Van Adrichem 2017), and reviparin (1750 anti‐XA IU) (Lassen 2002). There were no relevant differences between treatment and control groups regarding demographics or risk factors.
In two studies, plaster cast fitted following surgery was used as an exclusion criterion (Kock 1995; Kujath 1993). In one study, patients who underwent surgery before randomization might have had heparin treatment for up to four days before randomization (Lassen 2002). Another study treated all patients for one week with LMWH before randomization (Lapidus 2007b). The included studies differed in the types of plaster cast (upper‐leg, lower‐leg, cylinder, or brace). There was also a variation in the duration of immobilization, ranging from 15 days (Kujath 1993), to 43 days (Lapidus 2007a).
In the included studies, the primary outcome parameter was DVT. Deep venous thrombosis, both symptomatic and asymptomatic, was diagnosed by means of ascending venography (Jorgensen 2002; Lapidus 2007b; Lassen 2002), or ultrasound (Bruntink 2017; Kock 1995; Kujath 1993; Lapidus 2007a). In these seven studies, every participant underwent a diagnostic exam. One study only performed ultrasound in participants who reported symptoms (Van Adrichem 2017).
Clinically‐suspected PE had to be confirmed by ventilation‐perfusion scintigraphy, angiography, or spiral CT‐scanning. Information concerning the number of patients with symptomatic and asymptomatic DVT and the extent of the DVT was collected. Secondary outcome parameters were mortality and side effects in both treatment and control groups.
Excluded studies
For this update, we excluded an additional nine studies, leading to a combined total of 47 excluded articles (Ayhan 2013; Cook 2011; Cvirn 2015; Garcia 2011; Horner 2014; Lim 2015; Samama 2013; Saragas 2014; Warot 2014). We stated the reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
2.
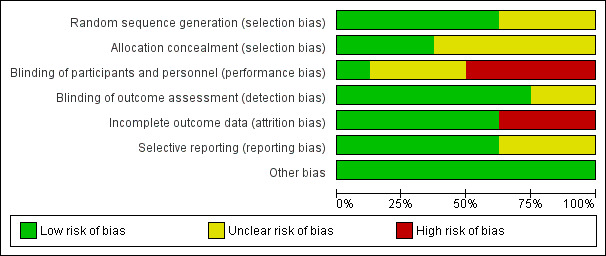
Risk of bias graph: review authors' judgements about each risk of bias domain, presented as percentages across all included studies
3.
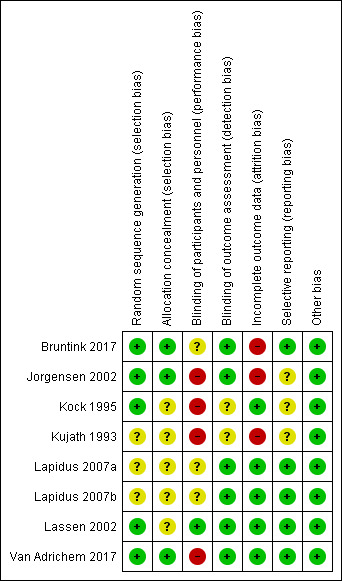
Risk of bias summary: review authors' judgements about each risk of bias domain for each included study
Allocation
We judged five studies at low risk of random sequence generation (Bruntink 2017; Jorgensen 2002; Kock 1995; Lassen 2002; Van Adrichem 2017), and three trials to be of unclear risk of bias, because insufficient information was provided (Kujath 1993; Lapidus 2007a; Lapidus 2007b). In two studies, participants were not recruited when study personnel were off duty (Lapidus 2007a; Lapidus 2007b).
We judged three studies at low risk of random sequence generation (Bruntink 2017; Jorgensen 2002; Van Adrichem 2017), and five trials to be of unclear risk of bias, because information on allocation concealment was not reported (Kock 1995; Kujath 1993; Lapidus 2007a; Lapidus 2007b; Lassen 2002).
Blinding
We judged four studies at high risk of performance bias, as they were open‐label studies, with the control group receiving no prophylaxis (Jorgensen 2002; Kock 1995; Kujath 1993; Van Adrichem 2017). We judged one study at low risk of performance bias, as participants and personnel were blinded to treatment assignment (Lassen 2002). We judged three studies to be of unclear risk of bias, as the blinding of study personnel was not explicitly reported (Bruntink 2017; Lapidus 2007a; Lapidus 2007b).
We judged two studies to be of unclear risk of detection bias, because no information on blinding of outcome assessors was provided (Kock 1995; Kujath 1993; Lassen 2002). We judged the remainder of the included studies to be at low risk of detection bias (Bruntink 2017; Jorgensen 2002; Lapidus 2007a; Lapidus 2007b; Van Adrichem 2017).
Incomplete outcome data
We judged three studies at high risk of attrition bias, because the number of participants excluded from analysis was either high (higher than 30%) or reasons were not clearly described per treatment assignment (Bruntink 2017; Jorgensen 2002; Kujath 1993). We judged the remainder of the included studies to be at low risk of attrition bias, as the number of participants excluded from analyses was either low or clearly described (Kock 1995; Lapidus 2007a; Lapidus 2007b; Lassen 2002; Van Adrichem 2017).
Selective reporting
We judged five studies to be at low risk of reporting bias, as all planned outcome measures, including adverse events, were reported (Bruntink 2017; Lapidus 2007a; Lapidus 2007b; Lassen 2002; Van Adrichem 2017). We judged three studies to be of unclear risk of bias, since the methods sections of the study reports did not describe planned primary and secondary outcomes, therefore, we were unable to judge if all outcomes were reported (Jorgensen 2002; Kock 1995). Kujath 1993 reported on primary outcomes, but not on possible adverse events, therefore, we also judged it to be of unclear risk of reporting bias.
Other potential sources of bias
We identified no other bias in the included studies and therefore, judged all included studies at low risk of other bias.
Effects of interventions
See: Table 1
Van Adrichem 2017 used a different study protocol, in which only patients with symptoms were examined using ultrasound. This can lead to an underestimation of the number of DVTs. Furthermore, primary asymptomatic DVT can still lead to late post‐thrombotic syndrome. Therefore, we considered asymptomatic DVT to be a relevant clinical outcome. However, due to the large number of included participants, we deemed this study valuable for this review. In order not to obscure the primary outcome, we only used the data from Van Adrichem 2017 in the pulmonary embolism and symptomatic VTE analyses.
Morbidity
Deep venous thrombosis (DVT)
Kujath and colleagues assessed 253 participants in their study, 126 of whom received a subcutaneous injection of Fraxiparin daily; 127 participants received no prophylaxis. Incidences of DVT were 16.5% (n = 21) in the control group and 4.8% (n = 6) in the LMWH group (odds ratio (OR) 0.25, 95% confidence interval (CI) 0.10 to 0.65; Kujath 1993). Kock and colleagues assessed 163 participants in the control group (no treatment), and 176 participants in the LMWH once daily group. The incidence of DVT in the prophylaxis group was 0% versus 4.3% (n = 7) in the control group (OR 0.06, 95% CI 0.00 to 1.04; Kock 1995). In 2002, Jorgensen and colleagues published the results of their venographic‐controlled study, and diagnosed DVT in 10 out of 99 participants in the treatment group and in 18 out of 106 participants in the control group. This difference was not significant (OR 0.55, 95% CI 0.24 to 1.26; Jorgensen 2002). In 2002, Lassen and colleagues found an incidence of 35 of 188 participants randomly assigned to receive placebo (18.6%) and in 17 of 183 participants (9%) in the LMWH group (OR 0.45, 95% CI 0.24 to 0.83; Lassen 2002). Lapidus and colleagues published two studies in 2007. The study on thromboprophylaxis after surgical treatment of Achilles tendon rupture revealed a high incidence of thromboembolic events: 37% (18/49) in the treatment group versus 40% (19/47) in the placebo group (OR 0.86, 95% CI 0.38 to 1.95; Lapidus 2007a). The study on prolonged thromboprophylaxis during immobilization after ankle surgery also showed a high incidences without a significant difference between groups: 21% in the treatment group versus 31% in the placebo group (OR 0.57, 95% CI 0.31 to 1.04; Lapidus 2007b). In the first study published following the most recent update of this review in 2014, 719 participants received either dalteparin or nadroparin, 716 participants did not receive prophylaxis (Van Adrichem 2017). Incidences of clinical relevant DVT were 1.0% (n = 7) in the treatment group and 1.3% (n = 9) in the control group (OR 0.77, 95% CI 0.29 to 2.09). The most recent study assessed 186 participants, 92 of whom received nadroparin, and 94 of whom received no prophylaxis. The treatment group showed a DVT incidence of 2.2% (n = 2), which was significantly lower than the DVT incidence of 11.7% (n = 11) in the control group (OR 0.17, 95% CI 0.04 to 0.78; Bruntink 2017).
Meta‐analysis
We conducted a meta‐analysis to establish whether there was evidence of a thromboprophylactic effect of LMWH, to estimate the size of this effect, and to investigate whether it was consistent across the included studies. We combined all participants, and subsequently assessed the effect for different groups of participants: surgically‐treated patients, patients with conservative treatment, patients with below‐knee casts, patients with cylinder or above‐knee casts, patients with fractures, patients with soft‐tissue injuries, PE, distal or proximal DVT, and finally, the number of patients with symptomatic VTE.
All participants, regardless of type of plaster, whether operated or not
Seven studies, with a total of 1676 participants, had injuries of the lower limb immobilized by a plaster cast or brace (Bruntink 2017; Jorgensen 2002; Kock 1995; Kujath 1993; Lapidus 2007a; Lapidus 2007b; Lassen 2002). The control group (N = 834) received no prophylaxis or placebo; the prophylaxis group received LMWH once daily (N = 842). The incidence of thromboembolic events ranged from 4.3% to 40% in the control group (145/834), and from 0% to 37% (77/842) in the prophylaxis group (OR 0.45, 95% CI 0.33 to 0.61, P < 0.001; Analysis 1.1).
1.1. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 1 Deep venous thrombosis: regardless of type of plaster, whether operated or not.
Participants with below‐knee casts, whether operated or not
We were able to obtain data on specific analyses of DVT in below‐knee casts or braces from six studies (Bruntink 2017; Jorgensen 2002; Kock 1995; Lapidus 2007a; Lapidus 2007b; Lassen 2002). Lassen 2002 and Kujath 1993 did not study the relationship between the type of cast and occurrence of thrombosis as part of their study designs. However, we could still add data from the group of participants with ruptured Achilles tendons from Lassen 2002. The incidences of DVT ranged from 0% to 37% in the LMWH groups, and from 3.6% to 40% in the control groups (OR 0.49, 95% CI 0.34 to 0.72; P < 0.001; Analysis 1.2; N = 1080).
1.2. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 2 Deep venous thrombosis: in below‐knee cast, whether operated or not.
Only Kock 1995 provided data on participants with cylinder or above‐knee casts, with a DVT incidence of 0/24 (0%) in the LMWH group and 2/24 (8.3%) in the control group.
Only participants with conservative treatment (i.e. non‐operated participants)
Five studies provided details of DVT in conservatively treated participants (i.e. non‐operated participants; Bruntink 2017; Jorgensen 2002; Kock 1995; Kujath 1993; Lassen 2002). When analyzed without consideration of type of cast or brace, the incidence ranged from 0% to 11.8% in the LMWH groups and from 4.3% to 17.3% in controls (OR 0.31, 95% CI 0.18 to 0.53, P < 0.001; Analysis 1.3; N = 974).
1.3. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 3 Deep venous thrombosis: conservative treatment (i.e. non‐operated patients).
Only surgically‐treated participants
We obtained information about surgically‐treated participants from four studies (Jorgensen 2002; Lapidus 2007a; Lapidus 2007b; Lassen 2002). The incidence of DVT in surgically‐treated participants ranged from 7.2% to 37% in the LMWH group, and from 18.0% to 40% in the control group (OR 0.54, 95% CI 0.37 to 0.80, P = 0.002; Analysis 1.4; N = 699).
1.4. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 4 Deep venous thrombosis: operated patients.
Fractures or soft‐tissue injuries
Six studies provided information on participants with fractures (Bruntink 2017; Jorgensen 2002; Kock 1995; Kujath 1993; Lapidus 2007b; Lassen 2002). These groups contained both surgically and conservatively treated participants. We found results in favour of the LMWH groups (OR 0.48, 95% CI 0.33 to 0.70, P < 0.001; Analysis 1.5; N = 1003). In participants with soft‐tissue injury, we again found results in favour of the LMWH groups (OR 0.39, 95% CI 0.22 to 0.68, P < 0.001; Analysis 1.6; N = 658; Jorgensen 2002; Kock 1995; Kujath 1993; Lapidus 2007a; Lassen 2002).
1.5. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 5 Deep venous thrombosis: fractures.
1.6. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 6 Deep venous thrombosis: soft‐tissue injuries.
Distal or proximal deep vein thrombosis
Five studies provided information on the segment in which the thrombus was located (Jorgensen 2002; Kock 1995; Lapidus 2007a; Lapidus 2007b; Lassen 2002). The incidence of distal segment DVT, defined as below‐knee DVT, ranged from 0% to 34.7% in participants who received LMWH, and from 2.5% to 34.0% in the control groups (OR 0.61, 95% CI 0.42 to 0.89, P = 0.009; Analysis 1.7; N = 1208). Proximal DVT (above knee) was rare; there were eight events in a total of 614 participants who received LMWH (incidence ranged from 0% to 4.0%) versus 20/603 events in the controls (incidence ranged from 0.9% to 6.4%, OR 0.41, 95% CI 0.19 to 0.91, P = 0.03; Analysis 1.8; N = 1217).
1.7. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 7 Deep venous thrombosis: distal segment.
1.8. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 8 Deep venous thrombosis: proximal segment.
Pulmonary embolism (PE)
In the studies under review, PE was a rare complication in immobilization of the lower extremity. Lassen 2002 reported that one participant in the treatment group and four participants in the control group showed clinical signs of PE; in two of them, both in the control group, PE was confirmed by a ventilation‐perfusion scan. Kujath 1993 reported that one participant in the group without prophylaxis showed clinical signs of a PE, but this diagnosis could not be proven by scintigraphic imaging. Van Adrichem 2017 reported that four participants in the treatment group and five in the control group developed a PE, diagnosed with a spiral CT scan. In the PROTECT study, two participants in the control group developed a pulmonary embolism (Bruntink 2017). Jorgensen 2002 reported no cases of pulmonary embolism. Overall, no clear differences were found between the treatment and control groups (OR 0.50, 95% CI 0.17 to 1.47, P = 0.21; Analysis 1.9; N = 2517).
1.9. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 9 Pulmonary embolism.
Symptomatic venous thromboembolism (VTE) ‐ symptomatic deep venous thrombosis (DVT), pulmonary embolism (PE), or combination
All studies but one reported on participants with symptomatic VTE. Lapidus 2007a did not report on participants with a symptomatic DVT, because it was not clinically possible to differentiate symptoms of a possible DVT from those of normal postoperative findings. Lapidus 2007b reported on two events in the LMWH group and six events in the placebo group. Lassen 2002 mentioned two participants with PE and four with symptomatic DVT, all of them in the placebo group. Kujath 1993 reported on nine symptomatic participants, however, it was not clear whether they were in the treatment group or not. For that reason, this study was not included in the pooled analysis. Kock 1995 did not report on symptomatic participants in their Lancet publication, but additional information from participants with thrombosis was found in an earlier publication. Four participants in the control group showed symptoms of DVT. Jorgensen 2002 did not find any symptomatic VTEs. Van Adrichem 2017 reported six participants with symptomatic DVT, three with PE and one with both DVT and PE in the LMWH group, and eight with DVT, four with PE, and one participant with both, in the control group. Bruntink 2017 reported two participants with a PE in the control group.
Symptomatic VTE was observed in 12 of 1469 (0.8%) participants receiving LMWH compared with 31 out of 1455 (2.1%) participants in the control group (OR 0.40, 95% CI 0.21 to 0.76; Analysis 1.10; six RCTs; N = 2924).
1.10. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 10 Symptomatic venous thromboembolism.
Mortality (Pulmonary embolism‐ (PE)‐related and other causes)
Van Adrichem 2017 reported one death in the no treatment group during their three‐month follow‐up after treatment; they reported that the death was assessed as possibly due to pulmonary embolism. However, a conclusive diagnosis could not be made because no autopsy was performed; the participant was over 90 years old and suffered from heart failure. The remaining seven studies reported no deaths due to PE (Analysis 1.11; N = 3111), or other causes (Analysis 1.12; N = 3111).
1.11. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 11 Mortality due to pulmonary embolism.
1.12. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 12 Mortality due to other causes.
Adverse outcomes of treatment
Major side effects, such as hematoma, acute major bleeding, allergic reaction, and thrombocytopenia were rare.
Lassen 2002 reported 14 participants in the LMWH group and 12 in the placebo group had a bleeding event. Lassen 2002 also reported major bleeding occurred in two participants in the LMWH group (retroperitoneal bleeding in one and permanent discontinuation of LMWH due to minor bleeding in another) and one in the placebo group (permanent discontinuation of study medication due to minor bleeding). Lassen 2002 reported no cases of heparin induced thrombocytopenia (HIT). Kock 1995 reported on five participants with minor complications (four small local hematomas, one facial eczema). Jorgensen 2002 reported no cases of HIT, hematomas, or severe bleeding. Kujath 1993 did not observe any side effects. Lapidus and colleagues did not report any cases of major bleeding (Lapidus 2007a; Lapidus 2007b). Lapidus 2007a reported one participant had a nosebleed after two days of dalteparin treatment. In Lapidus 2007b, two participants (one in each group) discontinued treatment due to minor bleeding. Van Adrichem 2017 reported no major bleeds, one clinically relevant non‐major bleed in the LMWH group, 55 minor bleeds in the treatment group, and 49 minor bleeds in the control group. Bruntink 2017 reported no major complications. Twenty‐two participants in the treatment group reported minor bleeding, hematuria, or dark stool (Bruntink 2017).
Combining all reported adverse events into a random‐effects model meta‐analysis showed an OR of 2.01, 95% CI 0.83 to 4.86, I² = 57%, 3178 participants; 8 studies; Analysis 1.13).
1.13. Analysis.
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 13 Adverse outcomes.
See also Table 1
Discussion
Summary of main results
We included eight studies in this updated review, with a total of 3680 participants. From these studies, we found that the incidence of deep venous thrombosis (DVT), diagnosed by compression ultrasound, venography, or both, in participants with a leg injury who were immobilized in a plaster cast or brace for at least one week and received no thromboprophylaxis (or placebo) was 4.3% to 40%. This was significantly higher than for participants who received daily subcutaneous injections of low molecular weight heparin (LMWH) during the entire period of immobilization (0% to 37%).
Comparable results were seen in the following groups of participants: patients with a below‐knee cast, surgically treated patients, conservatively treated patients (not surgically treated), patients with fractures, patients with soft‐tissue injuries, and patients with proximal or distal DVT. The odds ratios (OR) between the individual participant groups were similar, with an overlap of the confidence intervals (CI). Therefore, it was not possible to indicate a participant or patient group where prophylaxis was not indicated. No clear differences were found for PE between LMWH and the control groups. The studies found less symptomatic venous thromboembolism (VTE) in the LMWH groups compared with the control groups. One death (in the control group) was reported in the included studies. Major adverse events were rare; the main adverse events reported were cases of minor bleeding.
Overall completeness and applicability of evidence
The studies included in this review all assessed participants with lower‐limb immobilization. Some studies only included conservatively treated participants, whereas, others also included surgically treated participants. Studies included participants with both fractures and soft‐tissue injuries. These differences in inclusion criteria may have led to a lower external validity.
In addition to using Cochrane's 'Risk of bias' tool to assess methodological quality, we also assessed the validity and quality of the included randomized controlled trials using the scoring scheme provided on the website of Cochrane Netherlands (www.cochrane.nl). The overall quality of the eight included studies according to this scoring system, was rated 'good', although some remarks should be added.
The true thromboembolic rate in unprotected patients remains unknown, as high‐risk patients were excluded from participation in all eight studies, underestimating the incidence of thromboembolism in unprotected patients and the potentially beneficial effect of LMWHs. Although there is consensus among surgeons that thromboprophylaxis should be initiated in patients with a moderate to high risk for thromboembolism, there is no uniform definition of this patient group, leading to practice variation. Several attempts have been made to stratify the risk for VTE of immobilized patients (Gearhart 2000; Zagrodnick 1990). Knudson 2004 developed a scheme, based on analysis of 1602 episodes of VTE using the National Trauma Data Bank, to identify trauma patients with a high risk of thrombosis. Lower‐limb fracture and age over 40 years were among the factors used for selection. However, this study also stated that with this scheme, 90% of patients with VTE would be identified, and 10% would be missed. By contrast, in another study, patients under 40 years of age, with soft‐tissue injuries also developed DVT (Kock 1995). Up to now, no stratification method has proven its superiority or has gained general acceptance. In most studies in this review, investigators did not include patients with a high risk of DVT. So even in patients with a low to average risk of DVT, LMWH still provided significant protection.
In five studies, the included participants had a wide variety of trauma, ranging from fractures to soft‐tissue injuries, and tendon ruptures (Jorgensen 2002; Kock 1995; Kujath 1993; Lassen 2002; Van Adrichem 2017). Since the risk of DVT is found to be related to the presence and type of fracture, the extent of soft‐tissue injury, and type and duration of surgery, heterogeneity might have been introduced. However, randomization should have minimized this effect. Three studies focused on participants with a specific trauma (Bruntink 2017; Lapidus 2007a; Lapidus 2007b). We also observed a high drop‐out rate.
Five studies used ultrasound (Bruntink 2017; Kock 1995; Kujath 1993, Lapidus 2007a; Van Adrichem 2017), and three used venography (Jorgensen 2002; Lapidus 2007b; Lassen 2002), to diagnose DVT. The latter is considered the 'gold standard', but is rarely used in routine practice as the first line of investigation for DVT. Duplex ultrasonography and compression ultrasonography have a lower sensitivity and specificity compared to venography, especially for diagnosing calf vein thrombosis (CBO 2008; Lensing 1989). This might be the reason for the differences in the results between Kock 1995 and the studies that used venography (Jorgensen 2002; Lapidus 2007b; Lassen 2002), since there were no other major differences reported in participant characteristics or intervention. However, it does not explain the differences between the results of Kock 1995, Kujath 1993 and Lapidus 2007a. By only examining participants reporting symptoms, Van Adrichem 2017 only reported clinically‐relevant DVTs, therefore underestimating the number of DVTs in the study population. Due to this difference in study protocol, we only used the results of this study in the analyses for pulmonary embolism (Analysis 1.9) and symptomatic VTE (Analysis 1.10).
Lassen 2002 included participants who received up to four days of LMWH (32% of participants). Another study treated all participants with LMWH for one week prior to randomization (Lapidus 2007b). This might equalize the effects in the two groups and lead to an underestimation of the treatment effect. Therefore, Lapidus 2007b only focused on the duration of treatment, and not on the indication of treatment itself.
The dose of 3500 anti‐Xa IU of tinzaparin that Jorgensen 2002 used might have been too low, since another study showed equal antithrombotic effect using 4500 anti‐Xa IU of tinzaparin compared with 40 mg of enoxaparin, which is the standard dose in orthopedic surgery (Eriksson 2001). This possibly creates an underestimation of the effect of prophylaxis.
Quality of the evidence
The quality of evidence according GRADE varied by outcome and ranged from low to moderate with reasons for downgrading being risk of bias due to attrition and performance bias (DVT), imprecision and risk of bias due to attrition and performance bias (PE, symptomatic VTE and adverse outcomes) and low number of events and imprecision (mortality due to other causes).
See also Table 1
Potential biases in the review process
Two review authors independently carried out study selection, data extraction, and study quality assessment in order to reduce bias and subjectivity. We are confident that all potential studies are included in this review. However, the possibility remains that relevant data exist which have not been published or were not found in the search.
The total number of included studies in this review was eight. Since ten studies are required to perform a funnel plot analysis, reliable funnel plot analysis could not be performed.
By only examining patient reporting symptoms Van Adrichem 2016 only reported clinical relevant DVTs, therefore underestimating the number of DVTs in the study population. Due to this difference in study protocol the results of this study were only used in the analyses for 'pulmonary embolism' (Analysis 1.9) and 'symptomatic VTE' (Analysis 1.10).
Agreements and disagreements with other studies or reviews
As early as 1944, the first study on deep venous thrombosis (DVT) following leg injuries was published, reporting incidence rates of 7% to 18% (Bauer 1944). Nonfatal and fatal pulmonary embolism (PE) complicate DVT of the lower extremities. Fatal PE used to be a common cause of death in hospitals, because of the often clinically occult nature of DVT.
The discussion on the use of LMWH in immobilization of the lower limb focuses on the following issues: the reduction of symptomatic VTE, the reduction of asymptomatic DVT, the relevance of asymptomatic DVT, and the incidence of complications. With meta‐analyses, we were able to show that the use of LMWH in immobilization of the lower limb following leg injury results in a reduction of symptomatic VTE and of (asymptomatic) DVT.
Over 80% of DVTs diagnosed were located distally. Calf vein thrombosis propagates and becomes proximal in between 0% and 25% of patients, accounting for a mean of 10%. Up to 10% of proximal DVTs embolize massively, and are potentially fatal (Anonymous 1986; Schellong 2007). Opinions differ about the risk of post‐thrombotic syndrome (PTS) after distal DVT. It is stated that the risk is considerably lower than in proximal DVT. Results are inconsistent for the relationship between the location of the initial thrombus and the subsequent development of PTS. Some prospective studies reported rates of PTS after distal DVT that were as high as 20% to 80% (McLafferty 1998; Schulman 1986). Hence, distal DVT appears to be associated with a substantial risk of subsequent PTS. Further research is indicated to elicit the exact role of distal DVT in the development of PTS (Kahn 2006).
The incidence of complications in the review seemed to be low compared with data in the literature. Major bleeding was reported in 0.27% (two out of 1469 participants) and minor bleeding in up to 7.8% of participants (Van Adrichem 2017). In contrast, Reilmann 1993 reported up to 14% of participants with hematomas due to injections. Another study even described it up to 28%, although some of the participants were not treated with LMWH, but with unfractionated heparin (Zagrodnick 1990). In our seven included studies, no cases of heparin‐induced thrombocytopenia were described, which is in concordance with the observation that this condition is seldom seen in combination with LMWH (Bloemen 2012).
The eight studies used different types of LMWH. The total number of participants was insufficient to evaluate which type of LMWH to choose. Evidence published so far indicates that any differences between LMWH preparations, if they exist, must be extremely small (Geerts 2004). It is unlikely that a properly sized and designed study comparing the various LMWHs will ever take place. Sample size calculations quickly reach over 10,000 if one considers appropriate definitions of 'non‐inferiority' when comparing the various antithrombotic regimens (Vaitkus 2004).
Venography is considered the most accurate method of diagnosing DVT but is now rarely used in clinical practice (Abelseth 1996; Bergqvist 2002). It is an invasive procedure, and there is a reported incidence of serious adverse reactions to the contrast media in the range of 0.4% to 2% (Lensing 1990). Duplex ultrasound is the most common non‐invasive test used to diagnose venous thrombosis of the extremities. Compared with venography, ultrasound has been shown to be reliable in the diagnosis of proximal symptomatic DVT, with a sensitivity and specificity of over 90%. However, for distal thrombosis, a sensitivity of 73% can be reached when combining compression ultrasound with color‐doppler ultrasound (CBO 2008). Considering the properties of the imaging methods used to diagnose PE, a recent systematic review showed a sensitivity of 86% for the ventilation‐perfusion scintigraphy and a specificity of 46% compared with pulmonary angiography. For the CT scan, the percentages were 85% sensitivity and 94% specificity (Hayashino 2005). Consequently, the number of instances of missed DVT and PE seems to be acceptable when including studies with different diagnostic procedures, and should not influence our conclusions to a major extent. However, an underestimation of the incidence of VTE can occur when using ultrasound, CT‐scan, or ventilation‐perfusion scanning.
Increased incidence of DVT in an untreated population calls for measures, and thromboprophylaxis seems necessary if immobilization in a cast or brace is needed. Post‐traumatic DVT can lead to PE or long‐term damage in the form of PTS (Kakkar 1994). Low molecular weight heparin has been shown to be effective in reducing the incidence of VTE. The possible complications of major bleeding events (2/750 participants or 0.3%), and heparin‐induced thrombopenia (none in this review) have been shown to be extremely rare, and do not outweigh the beneficial effect of the reduction of thromboses.
Authors' conclusions
Implications for practice.
Moderate‐quality evidence showed that the use of low molecular weight heparin (LMWH) in outpatients reduced the number of venous thromboembolic events when a plaster cast or brace was required, when compared with no prophylaxis or placebo.The quality of evidence was downgraded to 'moderate' due to risk of selection and attrition bias. Low‐quality evidence showed no clear differences in PE between the LMWH and control groups, but less symptomatic VTE in the LMWH group. The quality of evidence was downgraded due to risk of bias and imprecision of results.
Implications for research.
Even with LMWH as a prophylactic measure, incidence rates of DVT, ranging from 0% to 10%, indicate a high absolute rate of morbidity in the population. In order to reduce the number of venous thromboembolic events, we encourage research to develop less immobilizing treatment options, and investigate further the use of other drugs such as the new oral anticoagulants (NOACs).
This review did not focus on the ongoing discussion of the clinical relevance of calf vein thrombosis. It simply confirmed its more frequent occurrence in immobilized patients. Future research might bring more clarity to the discussion on the significance of calf vein thrombosis.
Follow‐up studies on long‐term effects of DVT after immobilization, and the incidence of post‐thrombotic syndrome in these patients, could yield valuable information on the clinical relevance of primary asymptomatic DVT.
Low molecular weight heparin treatment in all included studies was administered once daily until removal of the plaster cast. Nevertheless, the optimal period of treatment is unclear, and further research should be conducted to gain insight into this matter.
We did not focus on scoring systems and individual risk factors in this analysis. Future research might give more directives on specific advice for different patients or patient groups, based on patient and trauma characteristics.
What's new
| Date | Event | Description |
|---|---|---|
| 7 August 2017 | Amended | In abstract incorrect I² for PE displayed and P values relating to heterogeneity incorrectly displayed as P values of effect estimate; text revised |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 20 June 2017 | New citation required but conclusions have not changed | Searches were rerun. Two new studies were included. Nine additional studies were excluded. New authors joined review team. 'Risk of bias' and 'Summary of findings' tables added. Conclusions not changed. |
| 20 June 2017 | New search has been performed | Searches were rerun. Two new studies were included. Nine additional studies were excluded. |
| 4 September 2013 | New search has been performed | Searches were rerun. No new studies were included. Five additional studies were excluded. One ongoing study added. |
| 4 September 2013 | New citation required but conclusions have not changed | Searches were rerun. No new studies were included. Five additional studies were excluded. One ongoing study added. Minor edits made. One new author joined review team. Conclusions not changed. |
| 14 February 2011 | Amended | Link to anticoagulant feedback added |
| 10 November 2008 | Amended | Amendment to Plain language summary at the request of the authors. Amendments to contact details of two authors. |
| 18 July 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors wish to acknowledge Dr M Testroote and Dr WAH Stigter for their contribution to previous versions of this review.
Appendices
Appendix 1. CENTRAL search strategy
| Search run on Wed Apr 19 2017 | ||
| #1 | MESH DESCRIPTOR Thrombosis | 1267 |
| #2 | MESH DESCRIPTOR Thromboembolism | 921 |
| #3 | MESH DESCRIPTOR Venous Thromboembolism | 258 |
| #4 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES | 2041 |
| #5 | (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*):TI,AB,KY | 19731 |
| #6 | MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES | 748 |
| #7 | (PE or DVT or VTE):TI,AB,KY | 5178 |
| #8 | ((vein* or ven*) near thromb*):TI,AB,KY | 6943 |
| #9 | (blood near3 clot*):TI,AB,KY | 3178 |
| #10 | (pulmonary near3 clot*):TI,AB,KY | 5 |
| #11 | (lung near3 clot*):TI,AB,KY | 5 |
| #12 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 | 25606 |
| #13 | MESH DESCRIPTOR Immobilization EXPLODE ALL TREES | 630 |
| #14 | MESH DESCRIPTOR Mobility Limitation EXPLODE ALL TREES | 255 |
| #15 | MESH DESCRIPTOR Splints EXPLODE ALL TREES | 363 |
| #16 | MESH DESCRIPTOR Orthopedic Fixation Devices | 61 |
| #17 | MESH DESCRIPTOR Casts, Surgical | 390 |
| #18 | MESH DESCRIPTOR Orthotic Devices | 466 |
| #19 | MESH DESCRIPTOR Foot Orthoses | 79 |
| #20 | immobili*:TI,AB,KY | 1885 |
| #21 | brace*:TI,AB,KY | 894 |
| #22 | splint*:TI,AB,KY | 1350 |
| #23 | plaster*:TI,AB,KY | 738 |
| #24 | cast*:TI,AB,KY | 3420 |
| #25 | boot:TI,AB,KY | 132 |
| #26 | stirrup:TI,AB,KY | 29 |
| #27 | bracing:TI,AB,KY | 290 |
| #28 | aircast:TI,AB,KY | 53 |
| #29 | #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 | 8235 |
| #30 | MESH DESCRIPTOR Lower Extremity EXPLODE ALL TREES | 5947 |
| #31 | MESH DESCRIPTOR Leg Injuries EXPLODE ALL TREES | 2736 |
| #32 | MESH DESCRIPTOR Achilles Tendon | 240 |
| #33 | leg:TI,AB,KY | 12176 |
| #34 | (lower extremity):TI,AB,KY | 2916 |
| #35 | (lower limb):TI,AB,KY | 3036 |
| #36 | ankle:TI,AB,KY | 5156 |
| #37 | achilles:TI,AB,KY | 604 |
| #38 | #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 | 23101 |
| #39 | MESH DESCRIPTOR Heparin, Low‐Molecular‐Weight EXPLODE ALL TREES | 1522 |
| #40 | *parin*:TI,AB,KY | 58379 |
| #41 | LMWH:TI,AB,KY | 866 |
| #42 | LMH:TI,AB,KY | 7 |
| #43 | (Clexane or klexane or lovenox):TI,AB,KY | 46 |
| #44 | (Fragmin or normiflo or clivarin* or danaproid or danaparoid ):TI,AB,KY | 245 |
| #45 | (antixarin or Zibor):TI,AB,KY | 2 |
| #46 | (cy 222):TI,AB,KY | 14 |
| #47 | (embolex or monoembolex or Mono‐embolex):TI,AB,KY | 24 |
| #48 | Kabi‐2165:TI,AB,KY | 39 |
| #49 | (pk‐10169 or pk10169):TI,AB,KY | 8 |
| #50 | (cy‐216 or cy216):TI,AB,KY | 46 |
| #51 | (Boxol or Liquemine):TI,AB,KY | 2 |
| #52 | fr‐860:TI,AB,KY | 5 |
| #53 | (kb‐101 or kb101):TI,AB,KY | 3 |
| #54 | (fluxum or lohepa or lowhepa ):TI,AB,KY | 11 |
| #55 | (op 2123 or op2123):TI,AB,KY | 1 |
| #56 | AVE5026 :TI,AB,KY | 2 |
| #57 | M118:TI,AB,KY | 3 |
| #58 | (RO‐14 or RO14):TI,AB,KY | 3 |
| #59 | #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 | 58468 |
| #60 | #38 AND #59 | 1865 |
| #61 | #12 AND #38 | 1551 |
| #62 | #12 AND #29 | 276 |
| #63 | #29 AND #59 | 655 |
| #64 | #60 OR #61 OR #62 OR #63 | 3438 |
Appendix 2. Trials registers searches
Clinicaltrials.gov
73 studies found for: heparin AND leg
WHO ICTRP
16 records for 14 trials found for: heparin AND leg
ISRCTN
20 results found for: heparin AND leg
Data and analyses
Comparison 1. Low molecular weight heparin versus no prophylaxis or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deep venous thrombosis: regardless of type of plaster, whether operated or not | 7 | 1676 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.33, 0.61] |
| 2 Deep venous thrombosis: in below‐knee cast, whether operated or not | 6 | 1080 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.34, 0.72] |
| 3 Deep venous thrombosis: conservative treatment (i.e. non‐operated patients) | 5 | 974 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.18, 0.53] |
| 4 Deep venous thrombosis: operated patients | 4 | 699 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.37, 0.80] |
| 5 Deep venous thrombosis: fractures | 6 | 1003 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.33, 0.70] |
| 6 Deep venous thrombosis: soft‐tissue injuries | 5 | 658 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.22, 0.68] |
| 7 Deep venous thrombosis: distal segment | 5 | 1208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.42, 0.89] |
| 8 Deep venous thrombosis: proximal segment | 5 | 1217 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.91] |
| 9 Pulmonary embolism | 5 | 2517 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.47] |
| 10 Symptomatic venous thromboembolism | 6 | 2924 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.21, 0.76] |
| 11 Mortality due to pulmonary embolism | 8 | 3111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Mortality due to other causes | 8 | 3111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 13 Adverse outcomes | 8 | 3178 | Odds Ratio (M‐H, Random, 95% CI) | 2.01 [0.83, 4.86] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bruntink 2017.
| Methods | Study design: prospective, randomized, controlled, single‐blind, multicenter study Method of randomization: sealed, numbered envelopes at a ratio of 1:1:1 in blocks of 15, stratified according to centre, to one of the three study groups, by the treating physician at the ED Concealment of allocation: sealed, numbered envelopes by treating physician at the ED, who was not involved in the remainder of the trial Losses to follow‐up: 124 (62 treatment group, 62 control group). Reasons for withdrawal: no fracture, no plaster cast, immobilization < 4 weeks, indication for surgery, no duplex sonography, withdrawal of consent |
|
| Participants | Country: The Netherlands Number randomized: 310 (treatment group 154; control group 156) Number completed study and used in analysis, reported in study publication: 186 (treatment group 92; control group 94) Age mean (SD): treatment group 47.7 (16.4); control group 44.5 (17.2) Sex (male/female): treatment group 39/53; control group 38/56 Inclusion criteria: a fracture of the ankle or foot, non‐surgical treatment with immobilization in a below‐knee plaster cast for a minimum of four weeks Exclusion criteria: a delay between injury and the emergency department visit of more than 72 h, a known hypersensitivity to nadroparin or fondaparinux, a history of venous thromboembolism, continuous anticoagulant therapy, hypercoagulability, a bleeding tendency or disorder, pregnancy or lactation, ‘active’ malignancy, a severe hepatic or renal impairment (deficiency of clotting factors or creatinine clearance < 30 mL/min), retinopathy, previous or active bleeding from the digestive tract, a hemorrhagic stroke within the previous two months, major surgery within the previous two months, intraocular, spinal, or brain surgery within the previous year, and severe hypertension (systolic blood pressure above 180 mmHg or diastolic blood pressure above 110 mmHg) |
|
| Interventions | Treatment group: Nadroparin 2850 IE anti‐Xa = 0.3 mL, given once daily Control group: no prophylaxis |
|
| Outcomes | A venous duplex sonography of the affected leg after removal of the cast on the final day of medication administration, or earlier if thrombosis was suspected | |
| Notes | A second treatment group receiving Fondaparinux was not included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..patients were enrolled and randomly assigned (by use of sealed, numbered envelopes, at a ratio of 1:1:1 in blocks of 15, stratified according to centre) to one of the three study groups, by the treating physician at the ED." |
| Allocation concealment (selection bias) | Low risk | Sealed, numbered envelopes from treating physician at the ED, who was not involved in the remainder of the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as a single‐blind study. Blinding of participants was not reported, blinding of personnel other than the ultrasound technician was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The ultrasound technician who assessed the primary outcome was blinded to the treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 124/310 participants were excluded from the analysis after randomization, 62 in both treatment and control group. Reasons for withdrawal: no fracture, no plaster cast, immobilization < 4 weeks, indication for surgery, no duplex sonography, withdrawal of consent |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported |
| Other bias | Low risk | No other bias was detected |
Jorgensen 2002.
| Methods | Study design: randomized, controlled, assessor‐blinded, open, multicenter trial Method of randomization: random numbers Concealment of allocation: sealed envelopes Losses to follow‐up: 95; treatment group 49; control group 46. (discomfort with self‐injection 18, methrorrhagia 1, refused phlebography 12, not possible to perform venography 26, miscellaneous 38 |
|
| Participants | Country: Denmark Number randomized: 300 (treatment group 148; control group 152) Number reported, included in analysis, presented in study publication: 205 (treatment group 99; control group 106) Age: adult patients > 18 years (range 18 to 93) Sex (male/female): treatment group 79/69; control group 93/59 Inclusion criteria: planned plaster immobilization of the lower leg for at least 3 weeks Exclusion criteria: pregnancy, allergy to heparin or contrast media, known liver or renal impairment, uncontrolled hypertension, bleeding disorders, recent GI bleeding, or inability to perform self injection |
|
| Interventions | Treatment group: LMWH 3500 IU anti‐Xa of tinzaparin (Innohep) once daily Control group: no prophylaxis |
|
| Outcomes | At cast removal, unilateral venography was performed | |
| Notes | Dose of tinzaparin relatively low, contained both operated and non‐operated patients, previous DVT was not excluded, 205/300 were included in final assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients were either treated with Tinzaparin, or received no treatment. A placebo was not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ' assessor‐blinded'; two radiologists, unaware of treatment, independently assessed the venograms |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 95 out of 300 patients were lost to follow‐up. They were evenly divided between groups (treatment group = 49, no treatment group = 46). Reasons for losses to follow‐up were discomfort with self‐injection (18), metrorrhagia (1), refusal of phlebography (12), not possible to perform venography (26), and miscellaneous (38). Reasons varied between the two groups. |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes were not described in methods. Therefore, it was unclear whether all assessed outcomes were reported. |
| Other bias | Low risk | No other bias was detected. |
Kock 1995.
| Methods | Study design: randomized, controlled, open trial Method of randomization: randomization list stratified for varicose veins and obesity Concealment of allocation: not reported Losses to follow‐up: 5 refused to take part, 32 excluded due to exclusion criteria, data not evaluated from 52: treatment group 21; control group 31 |
|
| Participants | Country: Germany Number randomized: 428; 5 refused to take part, 32 excluded due to exclusion criteria, data not evaluated from 52: treatment group 21; control group 31 (no final examination (12 treatment; 16 control), surgery performed before final examination (6 treatment, 12 control), changed groups (3 treatment, 3 control)) Number reported, included in analysis, presented in study publication: 339 (treatment group 176; control group 163) Age mean (range): treatment group 34.1 years (18 to 63); control group 33.5 years (18 to 64) Sex (male/female): treatment group 104/72; control group 104/59 Inclusion criteria: age 18 to 65, conservative treatment of injury with below‐knee cast or cylinder cast Exclusion criteria: previous DVT, pregnancy, clotting disorders or anticoagulation medication, bleeding, chronic venous insufficiency, contraindications for heparin prophylaxis, plaster cast after surgery |
|
| Interventions | Treatment group: LMWH 32 mg (certoparin; Mono‐Embolex NM) once daily Control group: no prophylaxis |
|
| Outcomes | At randomization and at plaster removal, compression ultrasound and duplex scanning were performed; suspected positive findings were confirmed by phlebography. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization with lists stratified for varicose veins and obesity |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study format, in which no placebo was used. The treatment group received injections of LMWH, the control group received none. Blinding of personnel was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data of 52 out of 428 randomized participants could not be evaluated, reasons provided |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes were not described. Therefore, it was unclear whether all assessed outcomes were reported. |
| Other bias | Low risk | No other bias was detected. |
Kujath 1993.
| Methods | Study design: randomized, controlled, open trial Method of randomization: randomization plan "after Sachs" Concealment of allocation: not reported Losses to follow‐up: 53 excluded post randomization (12 in treatment group interrupted prophylaxis without permission, 14 control group patients received prophylaxis, 18 lost to follow‐up, 6 participants operated on before 7th day, and 3 participants had cast removed before 7th day |
|
| Participants | Country: Germany Number randomized: 306, 53 excluded (12 in treatment group interrupted prophylaxis without permission, 14 control group patients received prophylaxis, 18 lost to follow‐up, 6 participants operated on before 7th day, and 3 participants had cast removed before 7th day Number included in analysis: 253; treatment group 126; control group 127 Age mean (range): treatment group 32.9 years (16 to 70); control group 35.6 years (16 to 76) Sex (male/female): treatment group 69/57; control group 77/50 Inclusion criteria: age over 16 years, injury of the lower limb being treated conservatively, immobilization by a plaster cast applied for at least 7 days Exclusion criteria: known thrombopathy, oral anticoagulation, recent brain or GI bleeding, acute pancreatitis, inflammatory heart disease |
|
| Interventions | Treatment group: LMWH 36 mg heparin fraction calcium (nadroparin; Fraxiparin) once daily Control group: no prophylaxis |
|
| Outcomes | After plaster removal, or at occurrence of symptoms, compression ultrasound to diagnose DVT; in case of doubtful or positive findings, a phlebography was carried out. In case of suspected PE, scintigraphic analysis was performed. | |
| Notes | None of the patients were operated on. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were allocated to two groups according to a random plan after Sachs". Unclear method of randomization |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of personnel was not reported. "Patients of group II did not receive heparin". A placebo was not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 53 out of 306 patients were excluded for various reasons. It is unclear how those were divided over the two groups. Reasons for exclusion were patient interruption of prophylaxis, patients receiving prophylaxis from co‐treating practitioner, change of treating physician, surgery before 7th day. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes were reported. However, nothing was reported on possible adverse events. |
| Other bias | Low risk | No other bias was detected |
Lapidus 2007a.
| Methods | Study design: randomized, controlled, double‐blind trial Method of randomization: by computer Concealment of allocation: not specifically reported Losses to follow‐up: 4 (withdrawal of consent treatment group 2, control group 2) and excluded from efficacy analysis |
|
| Participants | Country: Sweden Number randomized: 105; treatment group 52; control group 53 Number reported, included in analysis 1 (all participants with negative color duplex sonography, and all participants with DVT verified by phlebography): 91; treatment group 47; control group 44 Number reported, included in analysis 2 (all participants with color duplex sonography for patients with multiple distal DVT or proximal DVT, and all participants with DVT verified by phlebography): 96; treatment group 49; control group 47 Age mean (SD): treatment group 37 years (8); control group 42 years (9) Sex (male/female): treatment group 41/11; control group 42/11 Inclusion criteria: age 18 to 75 years, admitted for an acute (0 to 72 hours) Achilles tendon rupture, accepted for surgery Exclusion criteria: inability or refusal to give informed consent, ongoing treatment with anticoagulant therapy, known allergy for contrast media, kidney disorder, recent thromboembolic event, recent surgery, known malignancy, current bleeding disorder, pregnancy, treatment with platelet inhibitors |
|
| Interventions | Treatment group: LMWH dalteparin 5000 units sc once daily until removal of the plaster cast Control group: placebo |
|
| Outcomes | Diagnosis of DVT by means of ultrasound and confirmation by venography | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization by computer, no further information provided. However, patients were not included when study personnel were off duty |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as double‐blind. Each patient received a box containing 45 prefilled syringes with either Dalteparin or placebo. Syringes of both groups were identical. Blinding of personnel was not explicitly described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "evaluation was carried out by an experienced independent radiologist blinded to the randomization and previous phlebographic findings" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 patients were lost to follow‐up, evenly divided between the two groups. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were reported, as well as any adverse event. |
| Other bias | Low risk | No other bias was detected. |
Lapidus 2007b.
| Methods | Study design: randomized, controlled, double‐blind trial Method of randomization: not reported Concealment of allocation: not reported Losses to follow‐up: 75 considered non‐evaluable for primary analysis (35 treatment group and 40 in control group), due to: withdrawal of consent (38), technical failure of phlebography (27), refracture or resurgery (4), failure of protocol compliance (3), minor bleeding (1), inconclusive phlebography (1), never received allocated treatment due to DVT before start of treatment (1). Exclusions were evenly divided over the two groups |
|
| Participants | Country: Sweden Number randomized: 272; treatment group 136; control group 136 Number reported, included in analysis 1 (assessment using phlebography): 197; treatment group 101; control group 96 Number reported, included in analysis 2 (assessment using phlebography plus color duplex sonography): 226; treatment group 117; control group 109 Age mean (SD): treatment group 49 years (14); control group 48 years (14) Sex (male/female): treatment group 62/74; control group 62/74 Inclusion criteria: age 18 to 75 years, admitted for an acute (0 to 72 hours) ankle fracture and accepted for surgery Exclusion criteria: inability or refusal to give informed consent, ongoing treatment with anticoagulant therapy, known allergy for contrast media, kidney disorder, recent thromboembolic event, recent surgery, known malignancy, current bleeding disorder, pregnancy, treatment with platelet inhibitors, multi‐trauma |
|
| Interventions | Treatment group: LMWH dalteparin 5000 units sc once daily until removal of the plaster cast Control group: placebo |
|
| Outcomes | Diagnosis of DVT by means of ascending venography | |
| Notes | All patients treated with LMWH one week before randomization | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not described. Patients were not included when study personnel were off duty. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as double‐blind. Patients were blinded by using identical prefilled syringes. Blinding of personnel was not explicitly described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were assessed by a radiologist blinded to randomization and previous imaging findings. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 75 out of 272 patients were considered non‐evaluable for primary analysis due to withdrawal of consent (38), technical failure of phlebography (27), refracture or resurgery (4), failure of protocol compliance (3), minor bleeding (1), inconclusive phlebography (1), never received allocated treatment due to DVT before start of treatment (1). Exclusions were evenly divided over the two groups. |
| Selective reporting (reporting bias) | Low risk | Primary efficacy, secondary efficacy and any adverse events were assessed and reported |
| Other bias | Low risk | No other bias was detected |
Lassen 2002.
| Methods | Study design: randomized, controlled, double‐blind trial Method of randomization: computer (blocks of four) Concealment of allocation: not specifically reported Losses to follow‐up: 69 excluded from efficacy analysis: 2 received no injections (control group), 2 withdrew consent (treatment group), 4 withdrew because of adverse events (1 treatment, 3 control group), 61 did not have venograms that could be evaluated (31 treatment, 30 control group) |
|
| Participants | Country: Denmark Number randomized: 440; treatment group 217; control group 223. Number reported, included in analysis, reported in study publication: 371; treatment group 183; control group 188) Age median (interquartile range): treatment group 47 years (37 to 55); control group 47 years (37 to 56). Sex (male/female): treatment group 112/105; control group 114/108. Inclusion criteria: age 18 years or older, fracture of the leg or rupture of the Achilles tendon requiring at least 5 weeks of immobilization in a plaster cast or brace within 4 days of the injury Exclusion criteria: body weight < 35 kg, pre‐existing VTE, systolic BP > 200 mmHg or diastolic BP > 110 mm Hg, cerebral vascular aneurysm, cerebral vascular accident within the preceding 3 weeks, active gastroduodenal ulcer, bacterial endocarditis, platelet count 1000,000/cu mm, previous treatment with UFH or LMWH, fibrinolytic agents, or oral anticoagulants, known hypersensitivity to contrast media, kidney disorder, MI within the preceding 3 months, multiple myeloma, pregnancy, history of drug or alcohol abuse |
|
| Interventions | Treatment group: LMWH reviparin (1750 anti‐Xa units) once daily Control group: placebo |
|
| Outcomes | To diagnose DVT, venography was performed within one week after removal of the plaster. In cases of suspected PE, ventilation‐perfusion lung scanning or pulmonary angiography was performed. | |
| Notes | Study contained both operated and non‐operated patients; up to four days of LMWH prophylaxis was allowed before randomization. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | " randomization was performed by computer in blocks of four" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind study. Patients received identical prefilled syringes in both groups. Personnel were blinded until database was locked and results were revealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Venograms were evaluated by experienced radiologists, blinded to the treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 69/440 participants were excluded, divided evenly over both groups. Exclusion was mainly due to the technical impossibility to perform a venogram in the patient (61 participants). |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | No other bias was detected. |
Van Adrichem 2017.
| Methods | Study design: multicenter, controlled, randomized, open‐label with blinded outcome Method of randomization: computer‐generated block randomization with variable block sizes Concealment of allocation: data management unit, physicians, and researchers were unaware of the randomization scheme and block sizes Losses to follow‐up: after randomization, 33 excluded as either failed inclusion or met exclusion criteria, 23 withdrew consent, and 28 lost to follow‐up |
|
| Participants | Country: The Netherlands Number randomized: 1519; treatment 761; control group 758 Number reported ,included in analysis, reported in study publication: 1435; treatment group 719; control group 716 Age mean (SD): treatment group 46.5 (16.5); control group 45.6 (16.4) Sex (male/female): treatment group 347/372; control group 369/347 Inclusion criteria: patients 18 years of age or older who presented to the emergency department, and were treated for at least 1 week with casting of the lower leg (with or without surgery, before or after casting, but without multiple traumatic injuries) Exclusion criteria: history of venous thromboembolism, contraindications to low molecular weight heparin therapy, pregnancy, current use of anticoagulant therapy for other indications (use of antiplatelet drugs was allowed) |
|
| Interventions | Treatment group: LMWH once daily SC injection of 2850 IU nadroparin or 2500 IU dalteparin for participants ≤ 100 kg, or a double dose for participants weighing > 100 kg (LMWH (nadroparin or dalteparin) chosen according to preference at the hospital) Control group: no prophylaxis |
|
| Outcomes | Cumulative incidence of venous thromboembolism within 3 months of the procedure Safety outcome: cumulative incidence of major bleeding |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization with variable block sizes |
| Allocation concealment (selection bias) | Low risk | "To ensure concealment of treatment assignment the data management unit, physicians, and researchers were unaware of the randomization scheme and block sizes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial, participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes were assessed by an independent committee whose members were unaware of assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 84/1519 participants lost to follow‐up, withdrew consent, or failed inclusion criteria and were not included in the analysis. 93/719 patients did not adhere to the LMWH trial regimen. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary effective. as well as safety outcomes were reported. |
| Other bias | Low risk | No other bias was detected. |
BP: blood pressure DVT: deep vein thrombosis ED: emergency department GI: gastrointestinal Hg: mercury IU: international units LMWH: low molecular weight heparin MI: myocardial infarction PE: pulmonary embolism sc: subcutaneous UFH: unfractionated heparin VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abelseth 1996 | Prospective incidence study, not a randomized or controlled clinical trial, operated patients without plaster immobilization and LMWH, clinical and not outpatients |
| Ageno 2004 | Survey on thrombosis prophylaxis by Italian orthopedic surgeons |
| Anonymous 1991 | Not a randomized or controlled clinical trial |
| Anonymous 1995 | Not a randomized or controlled clinical trial |
| Armbrecht 1993 | Patients operated on for tendon rupture, one group treated with plaster and LMWH, the other group with early functional mobilization without prophylaxis. No clinical DVTs seen. |
| Ayhan 2013 | No LMWH used; trial was on compression stockings. |
| Bauer 1944 | Not a randomized or controlled clinical trial |
| Breyer 1984 | Not a randomized or controlled clinical trial |
| Bridges 2003 | LEAP‐study (LMWH expedited Anticoagulation Program) to decrease number of inpatient days on warfarin, and total hospital days for trauma patients requiring DVT; case‐control study |
| Cook 2011 | Trial protocol |
| Cvirn 2015 | Patients included did not meet inclusion criteria; no lower extremity trauma or immobilization, only healthy patients |
| Eriksson 2001 | Randomized controlled trial; comparison of fondaparinux and enoxaparin after hip‐fracture surgery |
| Freeark 1967 | Not a randomized or controlled clinical trial |
| Garcia 2011 | Patients included did not meet inclusion criteria: no lower extremity trauma or /immobilization, only ICU patients |
| Geerts 1994 | Prospective study of VTE after major trauma; no prophylaxis against VTE |
| Geerts 1996 | Randomized controlled clinical trial of LMWH and low‐dose heparin; focus on major trauma, not outpatients |
| Gehling 1994 | Prospective clinical study to determine incidence of DVT, no antithrombotic treatment |
| Gehling 1998 | Randomized controlled clinical trial; comparison of acetylsalicylic acid with LMWH in plaster immobilized trauma patients |
| Giannadakis 2000 | Prospective clinical study to determine incidence of DVT in selected patients, no antithrombotic treatment |
| Goel 2009 | A prospective randomized double‐blind controlled trial using LMWH with saline injection as placebo in adults who had sustained an isolated fracture below the knee that required operative fixation. Study authors did not focus on immobilization of the lower leg in plaster‐cast, so this study did not meet our inclusion criteria. The study authors included 238 patients, and all underwent bilateral venography for diagnosis of DVT. There was no statistically significant difference in the incidence of DVT between the patients treated with LMWH or placebo (P = 0.22). However, owing to a cessation of funding, recruitment had to be ended before the necessary sample size was established (another reason for exclusion). The study results could not categorically exclude a potentially beneficial role of LMWH treatment, and the authors recommended a further randomized controlled trial be undertaken. |
| Greenfield 1997 | Randomized controlled clinical trial. Clinical trauma patients were randomized to low‐dose UFH, LMWH, pneumatic compression devices, or foot pumps with or without vena caval filters. |
| Haas 1989 | Observational study on the use and tolerance of LMWH in ambulatory patients; not focused on trauma |
| Harenberg 1998 | Prospective cohort study to determine the clinical incidence of VTE and the tolerance to LMWH in operated and not operated surgical and orthopedic patients |
| Hjelmstedt 1968 | Not a randomized or controlled clinical trial |
| Horner 2014 | Patients included did not meet inclusion criteria; no lower extremity trauma or immobilization, only patients with DVT |
| Kannus 1991 | Review on treatment for acute tears of the lateral ligaments of the ankle; not focused on subject of thrombosis prophylaxis |
| Knudson 1996 | Randomized controlled clinical trial; LMWH in high‐risk trauma patients, compared with mechanical methods of prophylaxis |
| Knudson 2004 | Retrospective study to identify VTE incidence and risk factors in trauma patients using the American College of Surgeons National Trauma Data Bank |
| Kudsk 1989 | Not a randomized or controlled clinical trial |
| Lassen 2000 | Not a randomized or controlled clinical trial |
| Lim 2015 | Patients included did not meet inclusion criteria; no lower extremity trauma or immobilization, only ICU patients |
| Lippert 1995 | Not a randomized or controlled clinical trial |
| Marlovits 2007 | This study focused on prolonged thrombosis prophylaxis after arthroscopic surgery rather than immobilization of the lower leg after trauma, and was excluded for that reason. |
| Martinole 2003 | Not a randomized or controlled clinical trial |
| Micheli 1975 | Case report, expert opinion |
| NCT00843492 | The purpose of this ongoing study sponsored by GlaxoSmithKline is to evaluate the efficacy and safety of fondaparinux in comparison with a LMWH (nadroparin) in preventing VTE in patients with leg injuries below the knee that require a cast or other type of immobilization, but not surgery. This study does not meet our inclusion criteria (LMWH versus placebo or no prophylaxis) |
| Nesheiwat 1996 | Case report and literature review |
| Reilmann 1988 | Not a randomized or controlled clinical trial |
| Reilmann 1993 | Not a randomized or controlled clinical trial |
| Samama 2013 | Treatment protocol did not meet inclusion criteria; comparing fondaparinux with LMWH |
| Saragas 2014 | Not a randomized or controlled clinical trial |
| Schultz 2004 | Focus on multiple trauma patients. |
| Selby 2010 | In this study (known as the D‐KAF trial), consecutive patients with isolated fractures of the distal leg requiring surgery were randomized to dalteparin 5000 IU or placebo once daily SC. Patients were screened using proximal ultrasound (only of the upper leg, not the calf) at day 14. The researchers were interested in clinically important venous thromboembolism (CIVTE). The study authors found that the overall incidence of CIVTE was so low (1.9%; 95% CI 0.7 to 4.7%), with no observed differences between dalteparin and placebo, that recruitment was stopped early. For this reason, we did not include this study in our meta‐analysis. However, the study demonstrated the large discrepancy between trials that use venographic outcomes (all DVTs) and CIVTE. |
| Spieler 1972 | Not a randomized or controlled clinical trial |
| Warot 2014 | Not a randomized or controlled clinical trial |
| Wolf 1992 | Cohort of 515 patients with plaster immobilization of the lower leg treated with LMWH; no comparison |
| Zagrodnick 1990 | Retrospective data and prospective study to evaluate self‐injection of UFH and LMWH. |
CIVTE: clinically important venous thromboembolism DVT: deep vein thrombosis HIT: heparin‐induced thrombocytopenia ICU: intensive care unit LMWH: low molecular weight heparin SC: subcutaneous UFH: unfractionated heparin VTE: venous thromboembolism
Differences between protocol and review
'Risk of bias' and 'Summary of findings' tables added
Contributions of authors
AZ analyzed data, writing KvL assessed trial quality, extracted data. MvdH assessed trial quality, extracted data. LJ co‐ordinated and advised on statistical methods, and redirected writing. HMJJ cross‐checked information, supervised and redirected writing
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Incentive Award funding (16/72/06) to Cochrane Vascular. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Declarations of interest
AZ: none known KvL: none known MvdH: none known LJ: none known HMJJ: none known
Edited (no change to conclusions)
References
References to studies included in this review
Bruntink 2017 {published data only}
- Bruntink MM, Groutars YM, Schipper IB, Breederveld RS, Tuinebreijer WE, Derksen RJ, PROTECT Study Group. Nadroparin or fondaparinux versus no thromboprophylaxis in patients immobilised in a below‐knee plaster cast (PROTECT): a randomised controlled trial. Injury 2017;376:936‐40. [PUBMED: 28279428] [DOI] [PubMed] [Google Scholar]
- Derksen RJ, Groutars YME, Brand JG. Trial setup and preliminary results of PROTECT: prophylaxis of thromboembolic complications trial. European Journal of Trauma and Emergency Surgery 2011;37(Suppl 1):S141. [Google Scholar]
- NTR1733. Prevention of thrombosis in patients who are treated with a plaster cast for a fracture of the ankle or foot. www.trialregister.nl/trialreg/admin/rctview.asp?TC=1733 (Date registered 23 March 2009).
Jorgensen 2002 {published data only}
- Jorgensen PS, Warming, Hansen K, Paltred C, Berg H, Jensen R. Low molecular weight heparin (Innohep) thromboprophylaxis in outpatients with plaster casts. A phlebografic controlled study. Danish Orthopaedic Society Autumn Meeting. Copenhagen, Denmark, October 26‐27 2000.
- Jorgensen PS, Warming T, Hansen K, Paltved C, Berg HV, Jensen R, et al. Low molecular weight heparin (Innohep) as thromboprophylaxis in outpatients with a plaster cast; a venography controlled study. Thrombosis Research 2002;105(6):477‐80. [DOI] [PubMed] [Google Scholar]
Kock 1995 {published data only}
- Kock HJ, Schmit‐Neuerburg KP, Hanke J, Hakmann A, Althoff M, Rudofski G. Ambulatory prevention of thrombosis with low molecular weight heparin in plaster immobilization of the lower extremity. Vasa Supplementum 1992;35:105‐6. [PubMed] [Google Scholar]
- Kock HJ, Schmit‐Neuerburg KP, Hanke J, Hakmann A, Althoff M, Rudofsky G. Ambulant thrombosis prophylaxis with low‐molecular heparin in plaster immobilisation of the lower extremities. Hefte zur der Unfallchirurg 1993;232:245‐6. [Article in German] [Google Scholar]
- Kock HJ, Schmit‐Neuerburg KP, Hanke J, Hakmann A, Althoff M, Rudofsky G, et al. Ambulatory prevention of thrombosis with low molecular weight heparin in plaster immobilization of the lower extremity. Der Chirurg 1993;64(6):483‐91. [PubMed] [Google Scholar]
- Kock HJ, Schmit‐Neuerburg KP, Hanke J, Rudofsky G, Hirche H. Thromboprophylaxis with low‐molecular‐weight heparin in outpatients with plaster‐cast immobilisation of the leg. Lancet 1995 Aug 19;346(8973):459‐61. [DOI] [PubMed] [Google Scholar]
- Kock HJ, Schmit‐Neuerburg KP, Hanke J, Terwort A, Rudofsky G, Hirche H. Patient compliance during low molecular weight heparin thromboprophylaxis in outpatients with plaster cast immobilisation of the leg. Unfallchirurgie 1994;20(6):319‐28. [DOI] [PubMed] [Google Scholar]
- Kock HJ, Schmitt‐Neuerburg KP, Hanke J, Hakmann A, Althoff M, Rudofsky G. Prophylaxis of venous thromboembolism with low molecular weight heparin in surgical outpatients with immobilization of the lower limb by plaster cast. Haemostaseologie 1993;13 (suppl):S36‐S39. [Google Scholar]
Kujath 1993 {published data only}
- Habscheid W, Spannagel U, Kujath P, Schindler G. Prevention of thrombosis with low molecular weight heparin in ambulatory patients with injury of the lower extremity: an ultrasound study. Vasa Supplementum 1991;33:222‐3. [PubMed] [Google Scholar]
- Kujath P, Eckmann C. Thromboembolism prophylaxis in ambulatory surgery. Aktuelle Chirurgie 1995;30(6):372‐5. [Google Scholar]
- Kujath P, Spannagel U, Habscheid W. Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis 1993;23(suppl 1):20‐6. [DOI] [PubMed] [Google Scholar]
- Kujath P, Spannagel U, Habscheid W, Schindler G, Weckbach A. Thrombosis prevention in outpatients with lower limb injuries. Deutsche Medizinische Wochenschrift 1992;117(1):6‐10. [DOI] [PubMed] [Google Scholar]
Lapidus 2007a {published data only}
- Lapidus L, Rosfors S, Ponzer S, Levander C, Elvin A, Elvin G, et al. Prolonged thromboprophylaxis with dalteparin after surgical treatment of Achilles tendon rupture ‐ a randomized, placebo‐controlled study. Journal of Bone and Joint Surgery ‐ British Volume 2009;91‐B(Suppl 1):165. [DOI] [PubMed] [Google Scholar]
- Lapidus LJ, Rosfors S, Ponzer S, Levander C, Elvin A, Laerfars G, et al. Prolonged thromboprophylaxis with Dalteparin after surgical treatment of achilles tendon rupture: a randomized, placebo‐controlled study. Journal of Orthopaedic Trauma 2007;21(1):52‐7. [DOI] [PubMed] [Google Scholar]
Lapidus 2007b {published data only}
- Lapidus LJ, Ponzer S, Elvin A, Levander C, Laerfars G, Rosfors S, et al. Prolonged thromboprophylaxis with Dalteparin during immobilization after ankle fracture surgery: a randomized, placebo‐controlled, double‐blind study. Acta Orthopaedica 2007;78(4):528‐35. [DOI] [PubMed] [Google Scholar]
Lassen 2002 {published data only}
- Ahmad S, Bacher HP, Lassen MR, Hoppensteadt DA, Leitz H, Misselwitz F, et al. Investigations of the immunoglobulin subtype transformation of anti‐heparin‐platelet factor 4 antibodies during treatment with a low‐molecular‐weight heparin (clivarin) in orthopaedic patients. Archives of Pathology and Laboratory Medicine 2003;127(5):584‐8. [DOI] [PubMed] [Google Scholar]
- Lassen MR, Borris LC, Nakov RL. Use of the low‐molecular‐weight heparin reviparin to prevent deep‐vein thrombosis after leg injury requiring immobilization. New England Journal of Medicine 2002;347(10):726‐30. [DOI] [PubMed] [Google Scholar]
Van Adrichem 2017 {published and unpublished data}
- NCT01542762. Pot‐Cast: thrombosis prophylaxis during plaster cast lower leg immobilisation. clinicaltrials.gov/ct2/show/NCT01542762?term=nct01542762&rank=1 (Date first received 27 February 2012).
- Adrichem RA, Nemeth B, Algra A, Cessie S, Rosendaal FR, Schipper IB, et al. Thromboprophylaxis after knee arthroscopy and lower‐leg casting. New England Journal of Medicine 2017;376(6):515‐25. [PUBMED: 27959702] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abelseth 1996 {published data only}
- Abelseth G, Buckley RE, Pineo GE, Hull R, Rose MS. Incidence of deep‐vein thrombosis in patients with fractures of the lower extremity distal to the hip. Journal of Orthopaedic Trauma 1996;10(4):230‐5. [DOI] [PubMed] [Google Scholar]
Ageno 2004 {published data only}
- Ageno W, Dentali F, Imberti D. A survey of thrombosis prophylaxis use in patients with lower limb fractures. Thrombosis and Haemostasis 2004;92(5):1166‐7. [PubMed] [Google Scholar]
Anonymous 1991 {published data only}
- Anonymous. Low molecular weight heparin in the prevention of thrombosis during plaster cast immobilization. Therapiewoche 1991;41(43):2774. [Google Scholar]
Anonymous 1995 {published data only}
- Anonymous. Plaster cast immobilization and deep vein thrombosis [Immobilisation platree et thrombose veineuse profonde]. Sang Thrombose Vaisseaux 1995;7(9):641‐2. [Google Scholar]
Armbrecht 1993 {published data only}
- Armbrecht A, Zenker W, Egbers HJ, Havemann D. Plaster‐free, early functional after‐care of surgically managed Achilles tendon rupture. Der Chirurg; Zeitschrift für alle Gebiete der operativen Medizen 1993;64(11):926‐30. [Article in German] [PubMed] [Google Scholar]
Ayhan 2013 {published and unpublished data}
- Ayhan H, Iyigun E, Ince S, Can MF, Hatipoglu S, Saglam M. Comparison of three different protocols in the prevention of postoperative deep vein thrombosis in patients at high‐risk: randomized clinical study. European Surgical Research 2013;50:64‐5. [PUBMED: 23736377]23736377 [Google Scholar]
Bauer 1944 {published data only}
- Bauer G. Thrombosis following leg injuries. Acta Chirurgica Scandinavica 1944;90:229‐49. [Google Scholar]
Breyer 1984 {published data only}
- Breyer H, Koppenhagen K, Mabiki M, Rahmanzadeh R. The risk of thromboembolism due to plaster cast immobilisation and operation of the lower extremity [Thromboserisko durch Gipsimmobilisation und Operation an der unteren Extremitaet]. Hefte zur Unfallheilkunde 1984;164:423‐4. [Google Scholar]
Bridges 2003 {published data only}
- Bridges GG, Lee MD, Jenkins JK, Stephens MA, Croce MA, Fabian TC. Expedited discharge in trauma patients requiring anticoagulation for deep venous thrombosis prophylaxis: the LEAP Program. Journal of Trauma: Injury Infection and Critical Care 2003;54(2):232‐5. [DOI] [PubMed] [Google Scholar]
Cook 2011 {published and unpublished data}
- Cook D, Meade M, Guyatt G, Walter SD, Heels‐Ansdell D, Geerts W, et al. PROphylaxis for ThromboEmbolism in Critical Care Trial protocol and analysis plan. Journal of Critical Care 2011;26(2):223.e1‐9. [PUBMED: 21482348 ] [DOI] [PubMed] [Google Scholar]
Cvirn 2015 {published and unpublished data}
- Cvirn G, Waha JE, Ledinski G, Schlagenhauf A, Leschnik B, Koestenberger M, et al. Bed rest does not induce hypercoagulability. European Journal of Clinical Investigation 2015 Jan;45(1):63‐9. [PUBMED: 25413567 ] [DOI] [PubMed] [Google Scholar]
Eriksson 2001 {published data only}
- Eriksson BI, Bauer KA, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip‐fracture surgery. Steering Committee of the Pentasaccharide in Hip‐Fracture Surgery Study. New England Journal of Medicine 2001;345(18):1298‐304. [DOI] [PubMed] [Google Scholar]
Freeark 1967 {published data only}
- Freeark RJ, Boswick J, Fardin R. Posttraumatic venous thrombosis. Archives of Surgery 1967;95(4):567‐75. [DOI] [PubMed] [Google Scholar]
Garcia 2011 {published and unpublished data}
- Garcia DA. ACP Journal Club. Dalteparin did not differ from unfractionated heparin for reducing proximal DVT in critically ill patients. New England Journal of Medicine 2011;155(2):JC1‐7. [PUBMED: 21768573] [DOI] [PubMed] [Google Scholar]
Geerts 1994 {published data only}
- Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. New England Journal of Medicine 1994;331(24):1601‐6. [DOI] [PubMed] [Google Scholar]
Geerts 1996 {published data only}
- Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, et al. A comparison of low‐dose heparin with low‐molecular‐weight heparin as prophylaxis against venous thromboembolism after major trauma. New England Journal of Medicine 1996;335(10):701‐7. [DOI] [PubMed] [Google Scholar]
Gehling 1994 {published data only}
- Gehling H, Leppek R, Kunneke M, Gotzen L, Giannadakis K, Henkel J. Is prevention of thromboembolism in ambulatory and conservative therapy of rupture of the fibular ligament of the upper ankle joint necessary?. Unfallchirurg 1994;97(7):362‐5. [PubMed] [Google Scholar]
Gehling 1998 {published data only}
- Gehling H, Giannadakis K, Lefering R, Hessmann M, Achenbach S, Gotzen L. A comparison of acetylsalicylic acid with low‐molecular‐weight heparin for prophylaxis of deep‐vein thrombosis in outpatients with injuries and immobilizing bandages of the lower limb. Unfallchirurg 1998;101(1):42‐9. [Google Scholar]
Giannadakis 2000 {published data only}
- Giannadakis K, Gehling H, Sitter H, Achenbach S, Hahne H, Gotzen L. Is a general pharmacologic thromboembolism prophylaxis necessary in ambulatory treatment by plaster cast immobilization in lower limb injuries?. Unfallchirurg 2000;103(6):475‐8. [DOI] [PubMed] [Google Scholar]
Goel 2009 {published data only}
- Goel DP, Buckley R, Vries G, Abelseth G, Ni A, Gray R. Prophylaxis of deep‐vein thrombosis in fractures below the knee: a prospective randomised controlled trial. Journal of Bone and Joint Surgery ‐ British Volume 2009;91‐B:388‐94. [DOI] [PubMed] [Google Scholar]
Greenfield 1997 {published data only}
- Greenfield LJ, Proctor MC, Rodriguez JL, Luchette FA, Cipolle MD, Cho J. Posttrauma thromboembolism prophylaxis. Journal of Trauma: Injury Infection and Critical Care 1997;42(1):100‐3. [DOI] [PubMed] [Google Scholar]
Haas 1989 {published data only}
- Haas S, Biegholdt M. Prevention of thromboembolism with low molecular weight heparin [Ambulante thromboembolieprophylaxe mit niedermolekularem heparin]. Hamostaseologie 1989;9(5):237‐43. [Google Scholar]
Harenberg 1998 {published data only}
- Harenberg J, Piazolo L, Misselwitz F. Prevention of thromboembolism with low‐molecular‐weight heparin in ambulatory surgery and unoperated surgical and orthopedic patients. Zentralblatt Chirurgie 1998;123(11):1284‐7. [Article in German] [PubMed] [Google Scholar]
Hjelmstedt 1968 {published data only}
- Hjelmstedt A, Bergvall U. Incidence of thrombosis in patients with tibial fractures. Acta Chirurgica Scandinavica 1968;134(3):209‐18. [PubMed] [Google Scholar]
Horner 2014 {published and unpublished data}
- Horner D, Hogg K, Body R, Nash MJ, Baglin T, Mackway‐Jones K. The anticoagulation of calf thrombosis (ACT) project: results from the randomized controlled external pilot trial. Chest 2014 Dec;146(6):1468‐77. [PUBMED: 25010443 ] [DOI] [PubMed] [Google Scholar]
Kannus 1991 {published data only}
- Kannus P, Renström P. Treatment for acute tears of the lateral ligaments of the ankle. Journal of Bone and Joint Surgery ‐ American Volume 1991;73‐A(2):305‐12. [PubMed] [Google Scholar]
Knudson 1996 {published data only}
- Knudson MM, Morabito D, Paiement GD, Shackleford S. Use of low molecular weight heparin in preventing thromboembolism in trauma patients. Journal of Trauma: Injury Infection and Critical Care 1996;41(3):446‐59. [DOI] [PubMed] [Google Scholar]
Knudson 2004 {published data only}
- Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen L. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Annals of Surgery 2004;240(3):490–6, discussion 496‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kudsk 1989 {published data only}
- Kudsk KA, Fabian TC, Baum S, Gold RE, Mangiante E, Voeller G. Silent deep vein thrombosis in immobilized multiple trauma patients. American Journal of Surgery 1989;158(6):515‐9. [DOI] [PubMed] [Google Scholar]
Lassen 2000 {published data only}
- Lassen M, Boris L, Backer P, Nakov R, Fareed J. Efficacy and safety of low molecular weight heparin (Elivarine) in the prophylaxis of venous thromboembolism in patients with brace immobilization after injury of the lower extremity. Blood 2000;96:491A. [Google Scholar]
Lim 2015 {published and unpublished data}
- Lim W, Meade M, Lauzier F, Zarychanski R, Mehta S, Lamontagne F, et al. Failure of anticoagulant thromboprophylaxis: risk factors in medical‐surgical critically ill patients. Critical Care Medicine 2015;43(2):401‐10. [PUBMED: 25474533 ] [DOI] [PubMed] [Google Scholar]
Lippert 1995 {published data only}
- Lippert H. Prevention of venous thromboembolism beyond the period of hospitalisation and during out‐of‐hospital surgery. Hamostaseologie 1995;15(3):167‐8. [Google Scholar]
Marlovits 2007 {published data only}
- Marlovits S, Striessnig G, Schuster R, Stocker R, Luxl M, Trattnig S, et al. A prospective, randomized, placebo‐controlled study of extended‐duration post‐discharge thromboprophylaxis with enoxaparin following arthroscopic reconstruction of the anterior cruciate ligament. Blood 2004;104(11):1764. [DOI] [PubMed] [Google Scholar]
- Marlovits S, Striessnig G, Schuster R, Stocker R, Luxl M, Trattnig S, et al. Extended‐duration thromboprophylaxis with enoxaparin after arthroscopic surgery of the anterior cruciate ligament: a prospective, randomized, placebo‐controlled study. Arthroscopy 2007;23(7):696‐702. [DOI] [PubMed] [Google Scholar]
Martinole 2003 {published data only}
- Martinole A. Heparin prophylaxis for a leg cast?. Prescrire International 2003;12(66):159. [PubMed] [Google Scholar]
Micheli 1975 {published data only}
- Micheli LJ. Thromboembolic complications of cast immobilization for injuries of the lower extremities. Clinical Orthopaedics and Related Research 1975;108:191‐5. [DOI] [PubMed] [Google Scholar]
NCT00843492 {published data only}
- NCT00843492. A study to evaluate the efficacy and safety of fondaparinux for the prevention of venous blood clots in patients with a plaster cast or other type of immobilization for a below‐knee injury not needing surgery (FONDACAST). clinicaltrials.gov/ct2/show/NCT00843492 (First received 11 December 2008).
Nesheiwat 1996 {published data only}
- Nesheiwat F, Sergi AR. Deep venous thrombosis and pulmonary embolism following cast immobilization of the lower extremity. Journal of Foot and Ankle Surgery 1996;35(6):590‐4. [DOI] [PubMed] [Google Scholar]
Reilmann 1988 {published data only}
- Reilmann H, Bosch U, Barthels M. Prevention of thromboembolism in surgery. Orthopade 1988;17(1):110‐7. [PubMed] [Google Scholar]
Reilmann 1993 {published data only}
- Reilmann H, Weinberg AM, Forster EE, Happe B. Prevention of thrombosis in ambulatory patients. Orthopade 1993;22(2):117‐20. [Article in German] [PubMed] [Google Scholar]
Samama 2013 {published and unpublished data}
- Samama CM, Lecoules N, Kierzek G, Claessens YE, Riou B, Rosencher N, et al. Comparison of fondaparinux with low molecular weight heparin for venous thromboembolism prevention in patients requiring rigid or semi‐rigid immobilization for isolated non‐surgical below‐knee injury. Journal of Thrombosis and Haemostasis 2013;11(10):1833‐43. [PUBMED: 23965181] [DOI] [PubMed] [Google Scholar]
- Samama CM, Lecoules N, Kierzek G, Claessens YE, Riou B, Rosencher N, et al. Comparison of fondaparinux with low‐molecular‐weight heparin for venous thromboembolism prevention in patients requiring rigid or semi‐rigid Immobilization for isolated non‐surgical below‐knee injury [Étude comparant le fondaparinux à une héparine de bas poidsmoléculaire dans la prévention de la maladie thromboembolique veineuseen cas d’immobilisation rigide ou semi‐rigide après traumatisme isolénon chirurgical du membre inférieur au‐dessous du genou]. Annales Francaises de Medecine d'Urgence 2014;4(3):153‐66. [Google Scholar]
Saragas 2014 {published and unpublished data}
- Saragas NP, Ferrao PN, Saragas E, Jacobson BF. The impact of risk assessment on the implementation of venous thromboembolism prophylaxis in foot and ankle surgery. Foot and Ankle Surgery 2014 Jun;20(2):85‐9. [PUBMED: 24796824 ] [DOI] [PubMed] [Google Scholar]
Schultz 2004 {published data only}
- Schultz DJ, Brasel KJ, Washington L, Goodman LR, Quickel RR, Lipchik RJ, et al. Incidence of asymptomatic pulmonary embolus in moderately to severely injured trauma patients. Journal of Trauma: Injury Infection and Critical Care 2004;56(4):727‐33. [DOI] [PubMed] [Google Scholar]
Selby 2010 {published data only}
- Selby R, Geerts WH, Kreder HJ, Crowther MA, Kaus L, Sealey F. A double‐blind, randomized controlled trial of the prevention of clinically important venous thromboembolism after isolated lower leg fractures. Journal of Orthopaedic Trauma 2015;29(5):224‐30. [DOI] [PubMed] [Google Scholar]
- Selby R, Geerts WH, Kreder HJ, Crowther MA, Kaus L, Sealey F. Clinically important venous thromboembolism (CIVTE) following isolated leg fractures distal to the knee: epidemiology & prevention: the D‐KAF (dalteparin in knee‐to‐ankle fracture) trial. Journal of Bone and Joint Surgery ‐ British Volume 2010;92‐B(Suppl 1):7. [Google Scholar]
Spieler 1972 {published data only}
- Spieler U, Preter B, Brunner U. Traumatic thromboses of the deep venous system in recent tibial fractures [Traumatische thrombosen im tiefen venensystem bei frischen unterschenkelfrakturen]. Schweizerische Medizinische Wochenschrift 1972;102(43):1535‐40. [PubMed] [Google Scholar]
Warot 2014 {published and unpublished data}
- Warot M, Synowiec T, Wencel‐Warot A, Daroszewski P, Bojar I, Micker M, et al. Can deep vein thrombosis be predicted after varicose vein operation in women in rural areas?. Annals of Agricultural and Environmental Medicine 2014;21(3):601‐5. [PUBMED: 25292137 ] [DOI] [PubMed] [Google Scholar]
Wolf 1992 {published data only}
- Wolf N, Meiser H, Berg U, Goedicke M, Simonis G. Ambulatory prevention of thrombosis in a traumatologic patient sample. Vasa Supplementum 1992;35:109. [PubMed] [Google Scholar]
Zagrodnick 1990 {published data only}
- Zagrodnick J, Kaufner HK. Ambulatory thromboembolism prevention in traumatology using self‐injection of heparin. Unfallchirurg 1990;93(7):331‐3. [Article in German] [PubMed] [Google Scholar]
Additional references
Anderson 2003
- Anderson FA, Spencer F A. Risk factors for venous thromboembolism. Circulation 2003;107(23 suppl 1):I‐9. [DOI] [PubMed] [Google Scholar]
Anonymous 1986
- Anonymous. Consensus conference: prevention of venous thrombosis and pulmonary embolism. JAMA 1986;256(6):744‐9. [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batra 2006
- Batra S, Kurup H, Gul A, Andrew JG. Thromboprophylaxis following cast immobilisation for lower limb injuries‐survey of current practice in United Kingdom. Injury 2006;37(9):813–7. [DOI] [PubMed] [Google Scholar]
Bergqvist 2002
- Bergqvist D, Lowe G. Venous thromboembolism in patients undergoing laparoscopic and arthroscopic surgery and in leg casts. Archives of Internal Medicine 2002;162(19):2173‐6. [DOI] [PubMed] [Google Scholar]
Bloemen 2012
- Bloemen A, Testroote MJ, Janssen‐Heijnen ML, Janzing HM. Incidence and diagnosis of heparin‐induced thrombocytopenia (HIT) in patients with traumatic injuries treated with unfractioned or low‐molecular‐weight heparin: a literature review. Injury 2012;43:548‐52. [DOI] [PubMed] [Google Scholar]
CBO 2008
- CBO (Centraal Begeleidingsorgaan voor de intercollegiale toetsing). Concept directive diagnostics, prevention and treatment of venous thromboembolism and secondary prevention of arterial thrombosis [Conceptrichtlijn diagnostiek, preventie en behandeling van veneuze tromboembolie en secundaire preventie arteriële trombose]. Quality Institute for Health Care (Kwaliteitsinstituut voor de Gezondheidszorg), the Netherlands (http://www.cvgk.nl/legacy/bestanden/CBO‐richtlijn‐Stolling‐2009.pdf) 2008.
Clagett 1995
- Clagett GP, Anderson FA Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest 1995;108(4 Suppl):312S–34S. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Della Rocca 2013
- Della Rocca G, Danelli G, Randelli F, Romanini E, Biggi F, Laurora NR, et al. II Italian intersociety consensus statement on antithrombotic prophylaxis in orthopedics and traumatology. Minerva Anestesiologica 2013;79(7):778‐92. [PubMed] [Google Scholar]
Gearhart 2000
- Gearhart MM, Luchette FA, Proctor MC, Lutomski DM, Witsken C, James L, et al. The risk assessment profile score identifies trauma patients at risk for deep vein thrombosis. Surgery 2000;128(4):631‐40. [DOI] [PubMed] [Google Scholar]
Geerts 2004
- Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126 (3 Suppl):338S‐400S. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc). GRADEpro Guideline Development Tool (GRADEpro GDT). Version 2015. McMaster University (developed by Evidence Prime, Inc), Available from www.gradepro.org.
Hayashino 2005
- Hayashino Y, Goto M, Noguchi Y, Fukui T. Ventilaton‐perfusion scanning and helical CT in suspected pulmonary embolism: Meta‐analysis of diagnostic performance. Radiology 2005;234(3):740‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hill 2002
- Hill AB, Garber B, Dervin G, Howard A. Heparin prophylaxis for deep venous thrombosis in a patient with multiple injuries: an evidence‐based approach to a clinical problem. Canadian Journal of Surgery 2002;45(4):282–7. [PMC free article] [PubMed] [Google Scholar]
Kahn 2006
- Kahn SR. The post‐thrombotic syndrome: progress and pitfalls. British Journal of Haematology 2006;134(4):357–65. [DOI] [PubMed] [Google Scholar]
Kakkar 1994
- Kakkar VV. Prevention of venous thromboembolism. In: Bloom AL, Forbes CD, Thomas DP, Tuddenham EGD editor(s). Haemostasis and thrombosis. 3rd Edition. Edinburgh: Churchill Livingstone, 1994:1361‐79. [Google Scholar]
Kujath 1991
- Kujath P, Spannagel U, Habscheid W. Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis 1991;23(suppl 1):20–6. [DOI] [PubMed] [Google Scholar]
Lensing 1989
- Lensing AWA, Prandoni P, Brandjes D, Huisman PM, Vigo M, Tomasella G, et al. Detection of deep‐vein thrombosis by real‐time B‐mode ultrasonography. New England Journal of Medicine 1989;320(6):342‐5. [DOI] [PubMed] [Google Scholar]
Lensing 1990
- Lensing AW, Prandoni P, Buller HR, Casara D, Cogo A, Cate JW. Lower extremity venography with iohexol: results and complications. Radiology 1990;177(2):503‐5. [DOI] [PubMed] [Google Scholar]
McLafferty 1998
- McLafferty RB, Moneta GL, Passman MA, Brant BB, Taylor LM Jr, Porter JM. Late clinical and hemodynamic sequelae of isolated calf vein thrombosis. Journal of Vascular Surgery 1998;27(1):50–6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2013
- Roberts C, Horner D, Coleman G, Maitland L, Curl‐Roper Th, Smith R, Wood E, Mackway‐Jones K. Guidelines in Emergency Medicine Network (GEMNet): guideline for the use of thromboprophylaxis in ambulatory trauma patients requiring temporary limb immobilisation. Emergency Medicine Journal 2013;30(11):968‐82. [DOI] [PubMed] [Google Scholar]
Schellong 2007
- Schellong SM. Distal DVT: worth diagnosing? Yes. Journal of Thrombosis and Haemostasis 2007;5(Suppl.1):51‐4. [DOI] [PubMed] [Google Scholar]
Schulman 1986
- Schulman S, Granqvist S, Juhlin‐Dannfelt A, Lockner D. Long‐term sequelae of calf vein thrombosis treated with heparin or low‐dose streptokinase. Acta Medica Scandinavica 1986;219(4):349–57. [DOI] [PubMed] [Google Scholar]
Vaitkus 2004
- Vaitkus PT. Thromboprophylaxis in immobilized medical patients. European Journal of Medical Research 2004;9(3):131‐4. [PubMed] [Google Scholar]
van Adrichem 2015
- Adrichem RA, Oosten JP, Cannegieter SC, Schipper IB, Nelissen RGHH. Thromboprophylaxis for lower leg cast immobilisation and knee arthroscopy: a survey study. Netherlands Journal of Medicine 2015;73(1):23‐9. [PubMed] [Google Scholar]
Weitz 1997
- Weitz J. Low‐molecular‐weight heparins. New England Journal of Medicine September 1997;Volume 337(10):688‐98. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Testroote 2008
- Testroote M, Stigter WAH, Visser DC, Janzing HMJ. Low molecular weight heparin for prevention of venous thromboembolism in patients with lower‐leg immobilization. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006681.pub2] [DOI] [PubMed] [Google Scholar]
Testroote 2014
- Testroote M, Stigter WAH, Janssen L, Janzing HMJ. Low molecular weight heparin for prevention of venous thromboembolism in patients with lower‐leg immobilization. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD006681.pub3] [DOI] [PubMed] [Google Scholar]


