Abstract
Background
Fungal infection of the toenails, also called onychomycosis, is a common problem that causes damage to the nail's structure and physical appearance. For those severely affected, it can interfere with normal daily activities. Treatment is taken orally or applied topically; however, traditionally topical treatments have low success rates due to the nail's physical properties. Oral treatments also appear to have shorter treatment times and better cure rates. Our review will assist those needing to make an evidence‐based choice for treatment.
Objectives
To assess the effects of oral antifungal treatments for toenail onychomycosis.
Search methods
We searched the following databases up to October 2016: the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We also searched five trials registers and checked the reference lists of included and excluded studies for further references to relevant randomised controlled trials (RCTs). We sought to identify unpublished and ongoing trials by correspondence with authors and by contacting relevant pharmaceutical companies.
Selection criteria
RCTs comparing oral antifungal treatment to placebo or another oral antifungal treatment in participants with toenail onychomycosis, confirmed by one or more positive cultures, direct microscopy of fungal elements, or histological examination of the nail.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 48 studies involving 10,200 participants. Half the studies took place in more than one centre and were conducted in outpatient dermatology settings. The participants mainly had subungual fungal infection of the toenails. Study duration ranged from 4 months to 2 years.
We assessed one study as being at low risk of bias in all domains and 18 studies as being at high risk of bias in at least one domain. The most common high‐risk domain was 'blinding of personnel and participants'.
We found high‐quality evidence that terbinafine is more effective than placebo for achieving clinical cure (risk ratio (RR) 6.00, 95% confidence interval (CI) 3.96 to 9.08, 8 studies, 1006 participants) and mycological cure (RR 4.53, 95% CI 2.47 to 8.33, 8 studies, 1006 participants). Adverse events amongst terbinafine‐treated participants included gastrointestinal symptoms, infections, and headache, but there was probably no significant difference in their risk between the groups (RR 1.13, 95% CI 0.87 to 1.47, 4 studies, 399 participants, moderate‐quality evidence).
There was high‐quality evidence that azoles were more effective than placebo for achieving clinical cure (RR 22.18, 95% CI 12.63 to 38.95, 9 studies, 3440 participants) and mycological cure (RR 5.86, 95% CI 3.23 to 10.62, 9 studies, 3440 participants). There were slightly more adverse events in the azole group (the most common being headache, flu‐like symptoms, and nausea), but the difference was probably not significant (RR 1.04, 95% CI 0.97 to 1.12; 9 studies, 3441 participants, moderate‐quality evidence).
Terbinafine and azoles may lower the recurrence rate when compared, individually, to placebo (RR 0.05, 95% CI 0.01 to 0.38, 1 study, 35 participants; RR 0.55, 95% CI 0.29 to 1.07, 1 study, 26 participants, respectively; both low‐quality evidence).
There is moderate‐quality evidence that terbinafine was probably more effective than azoles for achieving clinical cure (RR 0.82, 95% CI 0.72 to 0.95, 15 studies, 2168 participants) and mycological cure (RR 0.77, 95% CI 0.68 to 0.88, 17 studies, 2544 participants). There was probably no difference in the risk of adverse events (RR 1.00, 95% CI 0.86 to 1.17; 9 studies, 1762 participants, moderate‐quality evidence) between the two groups, and there may be no difference in recurrence rate (RR 1.11, 95% CI 0.68 to 1.79, 5 studies, 282 participants, low‐quality evidence). Common adverse events in both groups included headache, viral infection, and nausea.
Moderate‐quality evidence shows that azoles and griseofulvin probably had similar efficacy for achieving clinical cure (RR 0.94, 95% CI 0.45 to 1.96, 5 studies, 222 participants) and mycological cure (RR 0.87, 95% CI 0.50 to 1.51, 5 studies, 222 participants). However, the risk of adverse events was probably higher in the griseofulvin group (RR 2.41, 95% CI 1.56 to 3.73, 2 studies, 143 participants, moderate‐quality evidence), with the most common being gastrointestinal disturbance and allergic reaction (in griseofulvin‐treated participants) along with nausea and vomiting (in azole‐treated participants). Very low‐quality evidence means we are uncertain about this comparison's impact on recurrence rate (RR 4.00, 0.26 to 61.76, 1 study, 7 participants).
There is low‐quality evidence that terbinafine may be more effective than griseofulvin in terms of clinical cure (RR 0.32, 95% CI 0.14 to 0.72, 4 studies, 270 participants) and mycological cure (RR 0.64, 95% CI 0.46 to 0.90, 5 studies, 465 participants), and griseofulvin was associated with a higher risk of adverse events, although this was based on low‐quality evidence (RR 2.09, 95% CI 1.15 to 3.82, 2 studies, 100 participants). Common adverse events included headache and stomach problems (in griseofulvin‐treated participants) as well as taste loss and nausea (in terbinafine‐treated participants). No studies addressed recurrence rate for this comparison.
No study addressed quality of life.
Authors' conclusions
We found high‐quality evidence that compared to placebo, terbinafine and azoles are effective treatments for the mycological and clinical cure of onychomycosis, with moderate‐quality evidence of excess harm. However, terbinafine probably leads to better cure rates than azoles with the same risk of adverse events (moderate‐quality evidence).
Azole and griseofulvin were shown to probably have a similar effect on cure, but more adverse events appeared to occur with the latter (moderate‐quality evidence). Terbinafine may improve cure and be associated with fewer adverse effects when compared to griseofulvin (low‐quality evidence).
Only four comparisons assessed recurrence rate: low‐quality evidence found that terbinafine or azoles may lower the recurrence rate when compared to placebo, but there may be no difference between them.
Only a limited number of studies reported adverse events, and the severity of the events was not taken into account.
Overall, the quality of the evidence varied widely from high to very low depending on the outcome and comparison. The main reasons to downgrade evidence were limitations in study design, such as unclear allocation concealment and randomisation as well as lack of blinding.
Plain language summary
What is the best medication for a fungal infection of the toenail?
Review question
We aimed to find out which medications, taken by mouth for at least six weeks, are the most effective at curing fungal infection of the toenail, a condition that is known as onychomycosis, in people of any age. We compared these medications to each other or placebo (an inactive drug or treatment).
Background
Fungal infection of the toenails is a common condition, which has a low risk of complications and associated health risks. However, for those severely affected, it might affect normal daily activities.
Medication taken by mouth appears to cure the condition more quickly and effectively than topical treatment. There are three main antifungal medications: griseofulvin, different medications in the azole group (itraconazole, fluconazole, albaconazole, posaconazole, ravuconazole), and terbinafine.
We wanted to assess the following two main outcomes.
1. Does the nail look normal after treatment (clinical cure)? 2. Is the nail free from fungus at a microscopic level (mycological cure)?
Study characteristics
We identified 48 studies with 10,200 participants of both sexes. The average age of the participants across studies ranged from 36 to 68; most studies included participants aged 18 and over. Our included studies compared the three main groups of medication against each other or to placebo. Most studies took place in outpatient dermatology settings in the USA and Europe. The participants mainly had fungal infection under the toenails. A small number of studies included a specific group of participants, such as those with diabetes. All but one study looked at fungal infections caused by dermatophyte, which are fungi that digest keratin. Study duration ranged from 4 months to 2 years, with most lasting 12 to 15 months.
Key results
The evidence is current to October 2016.
We found high‐quality evidence that compared with placebo, both terbinafine and azoles are more effective for achieving a normal‐looking nail and curing the toenail infection (i.e. looking at the microscopic level to see if the fungus is gone). Terbinafine or azoles may also prevent the infection reoccurring more than placebo (low‐quality evidence). There was probably no significant difference in the risk of adverse events reported when comparing either azoles or terbinafine with placebo (moderate‐quality evidence). The most common adverse events amongst terbinafine‐treated and azole‐treated participants included stomach problems and headache.
We found that compared to azoles, terbinafine was probably more effective in curing the nails in terms of appearance and infection (moderate‐quality evidence). The risk of side effects was probably the same for both treatments (moderate‐quality evidence), and the most common adverse events in both groups were headache, viral infection, and rash. There may be no difference in recurrence rate (low‐quality evidence).
A third type of treatment, griseofulvin, was probably as effective as the azole medications in curing the nails in terms of appearance and infection (moderate‐quality evidence), but it may be less effective than terbinafine when assessing the same outcomes (low‐quality evidence). Griseofulvin caused more side effects than the other two treatments, although the quality of the evidence was moderate (compared to azole) to low (compared to terbinafine). The most common adverse events in both groups included stomach problems and feeling sick. We are uncertain about the effect of griseofulvin compared to azoles on the rate of recurrence, and studies comparing terbinafine and griseofulvin did not assess this outcome.
Quality of the evidence
The evidence for the primary outcomes of cure (in terms of appearance and infection) was high to moderate quality except for the comparisons of griseofulvin versus terbinafine (low quality) and combination terbinafine plus azole versus terbinafine alone (very low quality). The evidence quality for side effects was mainly moderate, but two comparisons had low evidence for this outcome. Not all comparisons measured recurrence rate, and the available evidence was based on low‐ to very low‐quality evidence. No studies reported on participants' quality of life. Many studies had problems in the study design: it was often unclear how they decided which participants would receive which treatment or ensured that participants weren't aware of the treatment allocation. Many studies also did not use a placebo.
Summary of findings
Summary of findings for the main comparison. Azole compared to terbinafine for toenail onychomycosis.
| Azole compared to terbinafine for toenail onychomycosis | |||||
| Patient or population: participants with confirmed toenail onychomycosis Setting: outpatients clinics Intervention: azole Comparison: terbinafine | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with terbinafine | Risk with azole | ||||
| Clinical cure | Study population | RR 0.82 (0.72 to 0.95) | 2168 (15 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| 575 per 1000 | 471 per 1000 (414 to 546) | ||||
| Mycological cure | Study population | RR 0.77 (0.68 to 0.88) | 2544 (17 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| 682 per 1000 | 525 per 1000 (464 to 600) | ||||
| Adverse events | Study population | RR 1.00 (0.86 to 1.17) | 1762 (9 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| 346 per 1000 | 346 per 1000 (298 to 405) | ||||
| Recurrence rate | Study population | RR 1.11 (0.68 to 1.79) | 282 (5 RCTs) | ⊕⊕⊝⊝ Lowc | |
| 333 per 1000 | 370 per 1000 (227 to 597) | ||||
| Quality of life | None of the studies addressed quality of life. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded by one level for risk of bias because of large number of unblinded studies, lack of description of randomisation process and allocation concealment for most studies. bDowngraded by one level for risk of bias (large number of unblinded studies, lack of description of randomisation process and allocation concealment for most studies). cDowngraded by two levels for risk of bias (large number of unblinded studies, lack of description of randomisation process and allocation concealment for most studies) and imprecision (small numbers of participants in this comparison).
Summary of findings 2. Terbinafine compared to placebo for toenail onychomycosis.
| Terbinafine compared to placebo for toenail onychomycosis | |||||
| Patient or population: patients with confirmed toenail onychomycosis Setting: outpatient clinics Intervention: terbinafine Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with placebo | Risk with terbinafine | ||||
| Clinical cure | Study population | RR 6.00 (3.96 to 9.08) | 1006 (8 RCTs) | ⊕⊕⊕⊕ Higha | |
| 62 per 1000 | 370 per 1000 (244 to 560) | ||||
| Mycological cure | Study population | RR 4.53 (2.47 to 8.33) | 1006 (8 RCTs) | ⊕⊕⊕⊕ Higha | |
| 167 per 1000 | 755 per 1000 (412 to 1000) | ||||
| Adverse events | Study population | RR 1.13 (0.87 to 1.47) | 399 (4 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| 429 per 1000 | 484 per 1000 (373 to 630) | ||||
| Recurrence rate | 667 per 1000 | 33 per 1000 (7 to 253) |
RR 0.05 (0.01 to 0.38) |
35 (1 RCT) | ⊕⊕⊝⊝ Lowc |
| Quality of life | Not addressed by any of the trials | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aLarge number of unblinded studies and studies with poor description of blinding and randomisation but large effect estimate; therefore, this outcome was not downgraded for risk of bias as the quality of evidence was considered to be high because of the large effect observed. bDowngraded by one level due to risk of bias (randomisation and blinding was poorly described in most studies). cDowngraded by two levels due to poor description of randomisation and blinding as well as due to selective follow‐up and only single study with small number of participants.
Summary of findings 3. Azole compared to placebo for toenail onychomycosis.
| Azole compared to placebo for toenail onychomycosis | |||||
| Patient or population: participants with confirmed toenail onychomycosis Setting: outpatient clinics Intervention: azole Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with placebo | Risk with azole | ||||
| Clinical cure | Study population | RR 22.18 (12.63 to 38.95) |
3440 (9 studies) | ⊕⊕⊕⊕ Higha | |
| 14 per 1000 | 309 per 1000 (176 to 543) |
||||
| Mycological cure | Study population | RR 5.86 (3.23 to 10.62) | 3440 (9 RCTs) | ⊕⊕⊕⊕ Higha | |
| 74 per 1000 | 431 per 1000 (237 to 781) | ||||
| Adverse events | Study population | RR 1.04 (0.97 to 1.12) | 3441 (9 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| 537 per 1000 | 559 per 1000 (521 to 602) | ||||
| Recurrence rate | Study population | RR 0.55 (0.29 to 1.07) |
26 (1 RCT) |
⊕⊕⊝⊝ Lowc | |
| 1000 per 1000 | 550 per 1000 (290 to 1000) |
||||
| Quality of life | None of the studies addressed quality of life. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aLarge number of unblinded studies and studies with poor description of blinding and randomisation, but large effect estimate; therefore, this outcome was not downgraded for risk of bias as the quality of evidence was considered to be high because of the large effect observed. bDowngraded by one level because of risk of bias (high number of unblinded studies and studies with poor description of blinding and randomisation). cDowngraded by two levels due to poor description of randomisation and blinding as well as selective follow‐up and only single study with small number of participants.
Summary of findings 4. Griseofulvin compared to azole for toenail onychomycosis.
| Griseofulvin compared to azole for toenail onychomycosis | |||||
| Patient or population: participants with confirmed toenail onychomycosis Setting: outpatient clinics Intervention: griseofulvin Comparison: azole | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with azole | Risk with griseofulvin | ||||
| Clinical cure | Study population | RR 0.94 (0.45 to 1.96) | 222 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| 144 per 1000 | 136 per 1000 (65 to 283) | ||||
| Mycological cure | Study population | RR 0.87 (0.50 to 1.51) | 222 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| 186 per 1000 | 161 per 1000 (93 to 280) | ||||
| Adverse events | Study population | RR 2.41 (1.56 to 3.73) | 143 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| 276 per 1000 | 665 per 1000 (430 to 1000) | ||||
| Recurrence rate | Study population | RR 4.00 (0.26 to 61.76) | 7 (1 RCT) | ⊕⊝⊝⊝ Very lowc | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Quality of life | None of the studies addressed quality of life. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded by one level due to risk of bias (about half of the studies were not blinded). bDowngraded by one level due to risk of bias (two unblinded studies; neither participants nor outcome assessors were blinded). cDowngraded by three levels due to risk of bias (single study; neither participants nor outcome assessors were blinded) and imprecision (two levels due to single study, low number of participants and wide confidence intervals).
Summary of findings 5. Griseofulvin compared to terbinafine for toenail onychomycosis.
| Griseofulvin compared to terbinafine for toenail onychomycosis | |||||
| Patient or population: participants with confirmed toenail onychomycosis Setting: outpatient clinics Intervention: griseofulvin Comparison: terbinafine | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with terbinafine | Risk with Griseofulvin | ||||
| Clinical cure | Study population | RR 0.32 (0.14 to 0.72) | 270 (4 RCTs) | ⊕⊕⊝⊝ Lowa | |
| 561 per 1000 | 179 per 1000 (78 to 404) | ||||
| Mycological cure | Study population | RR 0.64 (0.46 to 0.90) | 465 (5 RCTs) | ⊕⊕⊝⊝ Lowa | |
| 716 per 1000 | 458 per 1000 (329 to 645) | ||||
| Adverse events | Study population | RR 2.09 (1.15 to 3.82) | 100 (2 RCTs) | ⊕⊕⊝⊝ Lowb | |
| 160 per 1000 | 334 per 1000 (184 to 611) | ||||
| Recurrence rate | No studies addressed recurrence rate. | ||||
| Quality of life | No studies addressed quality of life. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded by two levels due to risk of bias (two studies not blinded; other studies at unclear risk for blinding of participant and outcome assessor). bDowngraded by two levels due to risk of bias (two levels: one unblinded study; one study at unclear risk of bias for blinding or participants and outcome assessor).
Summary of findings 6. Combination terbinafine plus azole compared to terbinafine monotherapy for toenail onychomycosis.
| Combination terbinafine plus azole compared to terbinafine monotherapy for toenail onychomycosis | |||||
| Patient or population: participants with confirmed toenail onychomycosis Setting: outpatient clinics Intervention: combination terbinafine plus azole Comparison: terbinafine monotherapy | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with terbinafine monotherapy | Risk with combination terbinafine plus azole | ||||
| Clinical cure | Study population | RR 1.41 (1.01 to 1.97) |
176 (1 RCT) | ⊕⊝⊝⊝ Very lowa | |
| 368 per 1000 | 519 per 1000 (732 to 726) |
||||
| Mycological cure | Study population | RR 1.41 (1.08 to 1.83) |
176 (1 RCT) | ⊕⊝⊝⊝ Very lowa | |
| 474 per 1000 | 668 per 1000 (512 to 867) | ||||
| Adverse events | Study population | RR 0.64 (0.34 to 1.21) | 176 (1 RCT) | ⊕⊕⊝⊝ Lowb | |
| 232 per 1000 | 148 per 1000 (79 to 280) | ||||
| Recurrence rate | No studies addressed recurrence rate. | ||||
| Quality of life | No studies addressed quality of life. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded by three levels due to risk of bias (two levels: single non‐blinded study) and imprecision (single study). bDowngraded by two levels due to risk of bias (single non‐blinded study) and imprecision (single study)
Background
Please see Appendix 1 for a glossary of the medical terms used throughout the text.
Description of the condition
Fungal infection is a common problem that can affect both the skin and nails of the foot. Fungal infection of the nail is also known as 'onychomycosis' or 'tinea unguium'. Onychomycosis is a chronic disorder affecting the structure of the nail (Baran 1999). While distressing (Drake 1999), for most people the condition has a low risk of complications or associated health risks (ETG Dermatology 2009). Exceptions are those with peripheral vascular disease and the immunosuppressed, where complications associated with the infection are more common (Gupta 1998). A particular and common example of both of these circumstances is diabetes. Onychomycosis is common in people with diabetes (Gupta 1998), and complications of onychomycosis in people with diabetes can be limb‐threatening (Cathcart 2009).
There are several clinical forms of onychomycosis, and Hay 2011 has proposed a new classification based on current understanding of the underlying pathophysiology.
Distal lateral subungual onychomycosis (DLSO) – this is the most common form of onychomycosis, where the fungus invades from the distal or lateral undersurface of the nail plate. Clinical features include hyperkeratosis and a range of dyschromias (discolouration) including melanonychia (brown or black pigmentation of the nail), onycholysis (detachment of the nail from the nail bed), and streaking (coloured bands) of the nail. Streaking appears in other forms of onychomycosis but is most common in DLSO.
Superficial onychomycosis (SO) – this is where the nail plate itself can be white or black and present with a wide range of dyschromias. The nail surface is infected, whereas the rest of the nail plate, the nail bed, and the matrix remain unaltered. It can present as superficial patches or striae (groove‐like marks on the nail).
Endonyx onychomycosis (EO) – the nail plate is invaded through direct penetration of the fungal hyphae in the distal nail plate. It presents as lamellar (or length‐wise) splitting of the nail and discolouration in the nail plate without nail bed invasion.
Proximal subungual onychomycosis (PSO) – this classically originates from the proximal nail and nailfold, slowly extending distally. This form of onychomycosis is difficult to treat successfully.
Mixed pattern onychomycosis (MPO) ‐ different patterns of nail plate invasion often appear in the same person, sometimes even in the same nail. Proximal subungual onychomycosis and SO regularly occur together as well as DLSO with SO.
Total dystrophic onychomycosis (TDO) – this presents at the end stage of different forms of nail plate invasion, and it is caused by different organisms. The nail is completely damaged and crumbles away, while the nailbed is thickened and ridged.
Onychomycosis can present as a secondary complication of other conditions, such as psoriasis or trauma to the nail (Elewski 2015).
Please refer to De Berker 2013 or Fleckman 2001 for information on normal nail anatomy.
Prevalence
The prevalence of onychomycosis is estimated to be 2% to 14% (Ghannoum 2000; Watanabe 2010). Approximately a third of people with diabetes have onychomycosis (Cathcart 2009).
Infecting organism
Onychomycosis can be caused by dermatophytes (fungi that digest keratin), yeasts (microscopic fungi) and non‐dermatophyte moulds (fungi) (Bombace 2016). Most cases of onychomycosis are caused by dermatophytes, which are classified in three genera: Trichophyton,Microsporum, and Epidermophyton (Weitzman 1995). In onychomycosis, Trichophyton rubrum and Trichophyton mentagrophytes are the most common pathogens (Weitzman 1995). The Candida genus is the most common yeast involved in onychomycosis, and the non‐dermatophyte moulds include Scopulariopsis brevicaulis, Aspergillus, and Fusarium spp as well as others (Bombace 2016). The causative organisms vary by type of infection. DLSO can be caused by a wide variety of fungi; the most commonly encountered species in this form are dermatophytes, butCandida albicans (yeasts) and Fusarium spp (non‐dermatophyte moulds) are not uncommon. The most common cause of SO is the dermatophyte T mentagrophytes orT rubrum, but it can also be caused by Fusarium or Acremonium, while a wide array of fungi can cause PSO, including T rubrum, Fusarium, Candida, and Aspergillus (Weitzman 1995). In EO, the nail plate is most commonly invaded by Trichophyton soudanense or Trichophyton violaceum (Hay 2011).
Diagnosis of the fungal infection
Onychomycosis is the most prevalent nail disease, accounting for approximately 50% of all onychopathies (Wolff 2007). An accurate diagnosis is important, and it is desirable to confirm the presence of fungi by culture or of hyphae (branching filamentous structures) by microscopy (ETG Dermatology 2009), as some dermatological conditions can produce changes to the nail and skin that mimic fungal infection (e.g. trauma or psoriasis) (Andre 1987), and the causative fungus will inform treatment (De Berker 2009). At present, clinicians rely on clinical examination and a combination of direct microscopic (potassium hydroxide (KOH)) examination and fungal culture to establish a diagnosis (Scher 2007).
If both microscopy and culture are performed, one of the two will be positive in approximately 80% of cases of onychomycosis (ETG Dermatology 2009; Gupta 2013; Weinberg 2003). However, a direct microscopy assessment is negative in up 20% of cases, while culture may yield a false negative result in up to 40% of cases that are positive for microscopy (Brillowska‐Dabrowska 2007). The results of the culture will vary with the methods used as well as the method of collecting the nail sample, and some studies have reported even lower diagnostic accuracy (Shenoy 2008; Weinberg 2003). Nail infections caused by non‐dermatophytes such as Scopulariopsis andScystalidium may require repeated microscopy or culture, as non‐dermatophytes can be both contaminants as well as causative organisms (Bombace 2016). More recently, studies have suggested that at least two positive tests (microscopy, culture, histological sample, etc.) are required to confirm diagnoses (Gupta 2013). Also, it is time consuming to conduct cultures due to the slow growth of the fungus (Brillowska‐Dabrowska 2007). If direct microscopic examination by potassium hydroxide preparation and fungal culture are negative, histological examination of the nail plate may be advisable (Brillowska‐Dabrowska 2007). More recently, polymerase chain reaction techniques have been developed to aid the diagnosis and identification of the causative agent (Verrier 2012); this might become more important in the future.
Quality of life
Although onychomycosis is not a life‐threatening condition, it can alter many important nail functions and have adverse effects on the person's quality of life. The impact is greater on psychosocial functioning than on physical functioning (Shaw 2002). Whilst it is dismissed by many as a purely cosmetic problem, relegated to causing no more distress to the person than a crinkly nail (Stone 2000), to those severely affected, it can interfere with normal daily activities, such as walking and standing. It can cause shoes to fit poorly and may affect the productivity of those whose work requires them to stand all day (Drake 1998). In those with diabetes mellitus, onychomycosis has been linked to more severe complications, such as foot ulcers and cellulitis (Mayser 2009).
Description of the intervention
Drug therapy and its history
Prior to 1958, when griseofulvin was introduced as the first significant oral antifungal agent (Gupta 1994), only topical drugs existed for fungal infection (De Berker 2009). While the use of topical treatments may avoid the risk of adverse effects associated with systemic treatments, the response rate is poor, especially with multiple nail involvement or with involvement of more than the distal two‐thirds of the nail plate (i.e. thick nails) (Grover 2012), although the more recently developed topical treatments tavaborole and efinaconazole have shown promising results (Poulakos 2016).
Griseofulvin is produced by various species of Penicillium and is effective against dermatophyte infection but not against C albicans (yeasts) (Blank 1959). In 1944, benzimidazole was the first azole discovered to have antifungal activity, and 1969 saw the introduction of clotrimazole and miconazole, followed by econazole in 1974 and ketoconazole in 1977 (Gupta 1994). No oral form of miconazole nitrate or econazole was ever marketed, as they are poorly absorbed from the gastrointestinal tract (Gupta 1994). Although clotrimazole is a broad‐spectrum azole, it is not used when oral treatment is required because orally or parenterally (intravenously) administered clotrimazole induces an enzyme reaction that results in the accelerated degradation of the drug with loss of antifungal activity (Gupta 1994). Ketoconazole has been available since 1977, but it is associated with hepatotoxicity (Jones 1982). Although this appears to be a rare adverse effect, it has significantly reduced its popularity as an oral antifungal agent (Jones 1982).
The development of azoles continued with the introduction of itraconazole and fluconazole in the 1980s (Gupta 1994a). The absorption of itraconazole is rapid and can be maximised if taken with food. Fluconazole was discovered in 1982 and can be given intravenously as well as in oral form. Fluconazole is indicated for candidiasis as well as fungal skin infection; it has been used in the past to treat fungal nail infections and is still used for this indication in some countries. The allylamine group of antifungal drugs is the most recent development, with naftifine becoming the first commercially available allylamine in 1985 (but only in topical form) (Gupta 1994a). The next significant event was the introduction of oral terbinafine; terbinafine is an allylamine with a broad spectrum of antifungal activity. Its mechanism of action is fungicidal (i.e. it kills fungi directly), as opposed to fungistatic agents such as azoles, which simply halt new fungal growth (Gupta 1994a). Because terbinafine is currently the only allylamine for oral treatment, we use the term 'terbinafine' rather than 'allylamines' throughout the review.
Currently, terbinafine (continuous dosing) and itraconazole (pulse dosing one week per month) are the mainstays of oral treatments for onychomycosis (De Berker 2009). The cure rates reported are around 50%, although they vary widely (De Berker 2009). The elderly and those with nondermatophyte infections are less likely to respond to treatment (De Berker 2009).
Side effects
The most common side effects of oral antifungal agents include headaches, gastrointestinal side effects, and rashes (De Berker 2009). Severe adverse reactions, including fatal hepatotoxicity, are seen in fewer than 1% of cases (Greenblatt 2014; Kao 2014; Yan 2014). Drug interactions can cause serious problems during oral treatment therapy, and the azole drugs can inhibit hepatic drug metabolism (Back 1992). Women who are pregnant or may become pregnant should not use oral antifungals. Ketoconazole, fluconazole, and terbinafine may be excreted in breast milk; therefore, it is not advisable to breastfeed whilst being treated (ETG Dermatology 2009).
How the intervention might work
The antifungal agents either halt the growth of the fungus (fungistatic) or actually kill the fungus (fungicidal) (Gupta 1994). The azoles (e.g. ketoconazole) impair the synthesis of ergosterol in fungal cell membranes, which leads to the breakdown of the cell, while griseofulvin disrupts the cell microtubule function (Gupta 1994). Both are fungistatic, while terbinafine, which is fungicidal, interacts with ergosterol synthesis at an earlier stage, causing cell death (Gupta 1994a). Different dosing regimens have been used, both continuous daily dosing as well as pulse dosing (e.g. 1 week of treatment followed by 3 weeks with no treatment, with a minimum treatment duration of 12 weeks) (Gupta 2015). Given that the condition is caused by infestation of the nail by different fungi, most commonly Trichophytum, antifungal agents should eliminate the cause of the nail changes, namely the fungal infection, and allow for the return of the normal nail (Gupta 2015).
Why it is important to do this review
Onychomycosis is a common complaint. It can be treated either orally or with topical agents. Topical treatments have traditionally been more readily available as over‐the‐counter preparations, and they are the first‐line treatment for fungal skin conditions (El‐Gohary 2014). However, topical treatments have very low success rates due to the physical properties of the nail (Crawford 2007; Ghannoum 2014), even if the more recently developed topical treatments tavaborole and efinaconazole have shown more promising results (Poulakos 2016). Oral treatments are more commonly prescribed for onychomycosis, and they appear to have the benefit of shorter treatment times and better cure rates than topical preparations (Gupta 2015). There have been several published reviews and overviews of oral treatments, but no recent systematic review of the evidence has been produced (Bandolier 1996; Crawford 2002; Epstein 1998; Trepanier 1998). A systematic review of the evidence for oral treatments for toenail onychomycosis will assist clinicians and people with the condition in making an evidence‐based choice for treatment.
The plans for this review were published as a protocol 'Oral antifungal medication for toenail onychomycosis' (Kreijkamp‐Kaspers 2012).
Objectives
To assess the effects of oral antifungal treatments for toenail onychomycosis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with a parallel group design. We also included cross‐over trials.
Types of participants
Participants of all ages with toenail onychomycosis confirmed by at least one positive culture or confirmed fungal elements on direct microscopy or histological examination of the nail.
Types of interventions
We considered all oral antifungal interventions for treating toenail onychomycosis with treatment durations from a minimum of six weeks. Comparisons were as follows.
Oral active treatment versus another oral active treatment (we did not consider dose‐finding studies of the same drug unless they also contained a placebo group).
Oral active treatment versus placebo.
Types of outcome measures
Primary outcomes
Clinical cure, i.e. the proportion of participants that on clinical examination are 'cured'. We followed the definition of 'clinical cure' as given by the authors of the included studies. The timeframes for clinical cure may vary by study and might be as long as 6 to 24 months post‐treatment.
Mycological cure demonstrated by negative results on microscopy, no growth of dermatophyte in culture, or both. This outcome is distinct from the disease‐free nail in that it does not require the demonstration of the normal‐appearing nail and requires shorter participant follow‐up.
When studies recorded measurements at multiple time points during the intervention, we consider the measurement at the predefined endpoint of the study as our primary outcome.
Secondary outcomes
Quality of life
Adverse events
Recurrence rate
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 12 October 2016.
The Cochrane Skin Group Specialised Register using the search strategy in Appendix 2.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 10) in the Cochrane Library using the strategy in Appendix 3.
MEDLINE via Ovid (from 1946) using the strategy in Appendix 4.
Embase via Ovid (from 1974) using the strategy in Appendix 5.
LILACS (Latin American and Caribbean Health Science Information database from 1982) using the strategy in Appendix 6.
Trials registers
We searched the following trials registers on 22 May 2016. See Appendix 7 for search strategies.
The ISRCTN registry (www.isrctn.com).
ClinicalTrials.gov (www.clinicaltrials.gov).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/).
The EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
References from published studies
We checked the bibliographies of included and excluded studies for further references to relevant trials.
Unpublished literature
We sought to identify unpublished and ongoing trials by correspondence with authors and by contacting the pharmaceutical companies that produce relevant products. We contacted the following drug companies.
AstraZeneca.
GlaxoSmithKline.
Janssen‐Cilag Ltd.
Pfizer Ltd.
Novartis (Sandoz, the generic pharmaceuticals division of Novartis).
We did not identify further companies producing other products identified from trials.
Adverse effects
We did not perform a separate search for adverse effects of the target interventions. However, we examined data on adverse effects from the included studies we identified.
Data collection and analysis
We included six 'Summary of findings' tables for six comparisons, which included all of our primary and secondary outcomes. We also used the GRADE approach to assess the quality of all outcomes using the following five domains: risk of bias, inconsistency, imprecision, indirectness, and publication bias. Quality of evidence could be either high, moderate, low, or very low (Higgins 2011; Schünemann 2013).
Selection of studies
Two review authors (SKK and KH) independently checked titles and abstracts identified from the searches. We set aside studies where it was clear that they were not relevant; we retrieved, for further independent assessment, the full text of those citations for which it was not possible to make a decision. Two review authors independently decided which trials met the inclusion criteria and resolved any disagreements by discussion or referral to a third review author (MvD). We detailed excluded studies and reasons for exclusion in the 'Characteristics of excluded studies' tables in the review.
Data extraction and management
Four review authors (SKK, LG, GK, KH) independently extracted data using a data extraction form. We resolved discrepancies by discussion or through consultation with a third review author (MvD). We requested missing data from trial authors where relevant. One review author (SKK) checked and entered all data. The review authors were not blinded to the names of study authors, journals, or institutions.
Assessment of risk of bias in included studies
Two review authors (SKK and LG, GK or KH) independently assessed each included study using the Cochrane Collaboration's tool for assessing risk of bias, described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other issues (e.g. extreme baseline imbalance). We assessed blinding and completeness of outcome data for each outcome separately. We completed a 'Risk of bias' table for each eligible study. We discussed any disagreement amongst all review authors to achieve a consensus.
We reported the 'Risk of bias' assessment using a 'Risk of bias' summary figure, which presents all of the judgements for every study. This may guide readers to the weight they should give to results of each study.
Measures of treatment effect
We entered data into Cochrane Review Manager 5 (RevMan 5) software for data analysis (RevMan 2014). We reported estimates for dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CI).
Unit of analysis issues
In RCTs the unit of analysis was the individual participant, not the individual nail(s) affected. If we had identified cross‐over RCTs, we would have only extracted and analysed data from the first period due to the likely carry‐over effect from the first treatment episode in the cross‐over period. In the case of multiple treatment trials, we created pair‐wise comparisons as set out in Chapter 16.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
Where possible, we extracted data to allow an intention‐to‐treat (ITT) analysis including all randomised participants according to the groups to which they were originally assigned. We calculated the percentage lost to follow‐up in each group and reported this information. When there was a discrepancy in the number randomised and the number analysed in each treatment group, we attempted to obtain missing data or further information from trial authors when needed. We did not make any assumptions about loss to follow‐up for dichotomous or continuous data, and we analysed results for those who completed the trial.
Assessment of heterogeneity
We examined heterogeneity in a two‐step process. First, we assessed clinical heterogeneity (e.g. age, severity of disease, different populations). Second, we examined statistical heterogeneity using the I² statistic (Higgins 2003). Values of I² statistic under 25% indicate a low level of heterogeneity and would justify use of a fixed‐effect model for meta‐analysis. I² values between 25% and 75% are considered moderate, while values higher than 75% indicate high levels of heterogeneity. We used a random‐effects model for all analyses, as in the absence of heterogeneity the estimates would be similar to a fixed‐effect analysis. We did not pool studies if important 'face value' heterogeneity or substantial statistical heterogeneity were present. We used the I² statistic as a guide in the interpretation of the evidence, not as an absolute measure to make major decisions (Ioannidis 2007).
Assessment of reporting biases
When we identified more than 10 RCTs in a single comparison, we drew funnel plots to test for reporting bias as discussed in chapter 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We pooled data using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
Methods of synthesising the studies depended on quality, design, and heterogeneity. We explored both clinical and statistical heterogeneity as described above. We first investigated 'face value' heterogeneity (which includes participants' age and severity of the condition). If there were no obvious clinical reasons for important heterogeneity that may impact on the outcome of pooling, we proceeded to assessing statistical heterogeneity. In the presence of statistical heterogeneity, we explored the cause of this by means of a sensitivity analysis (removing or adding studies one by one in order to identify the source of heterogeneity).
The studies did not allow for the planned subgroups analyses, which were based on the following.
Subtype of onychomycosis.
Participants with underlying health conditions, such as diabetes mellitus, peripheral vascular disease, and immunosuppression.
We did perform subgroup analysis based on the duration of follow‐up as a toenail will need at least 12 months to grow out completely (Geyer 2004).
Sensitivity analysis
We included all eligible trials in the initial analysis and carried out sensitivity analyses to evaluate the effect of trials at risk of bias. This was done by excluding trials most susceptible to bias based on the 'Risk of bias' assessment: those with inadequate allocation concealment; high levels of postrandomisation losses or exclusions; and uncertain or unblinded outcome assessments. By the same method, we also assessed the impact of heterogeneity on the overall estimate.
Results
Description of studies
Results of the search
The primary database searches described in Electronic searches yielded 444 records, and we identified an additional 136 records through the trial registry searches; after removing duplicates, there were a total of 534 unique records, none of which pertained to ongoing trials.
We excluded 439 records based on titles and/or abstracts, leaving 95 full‐text records. We excluded 27 (see Characteristics of excluded studies), leaving 68 included papers reporting on 48 studies and involving 10,200 participants (see Characteristics of included studies).
Five studies did not contribute to the pooled analyses. One excluded and replaced participants that did not show response to treatment and did not account for these participants in analysis (Arenas 1991). Hay 1985 included a wide range of dermatophyte infections and conducted analyses based on number of toenails rather than affected participants. Furthermore, three studies included both fingernail and toenail onychomycosis but did not separate the fingernail and toenail data (Al Rubaie 1997; Mishra 2002; Piepponen 1992). We have contacted the authors to obtain further data and will include them in the quantitative analyses when data become available.
We did not identify any cross‐over trials.
We included 43 studies in the pooled data analyses. Please see Figure 1 for our study flow diagram.
1.
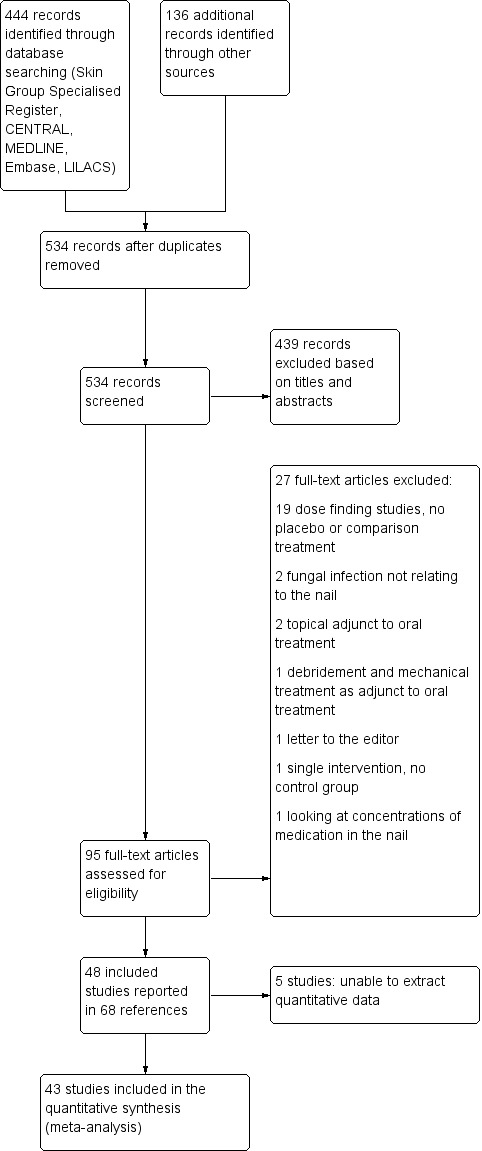
Study flow diagram.
Included studies
The pooled analyses included 43 studies with 9730 participants (see the Characteristics of included studies section).
Trial settings
All studies were RCTs, and 16 had a placebo arm. Twenty‐six were published in 2000 or earlier. Authors described more than half (24 studies) as multicentre, and most were conducted in outpatient dermatology settings in Western countries: 17 studies had at least one trial site in the USA, and 16 studies had a European trial site.
Participants
Sample size varied from 20 to 1381 participants (median 120). The average age of the participants across studies ranged from 36 to 68 years, and most studies included participants aged 18 and over, with only three studies accepting participants aged 14 to 16 years (La Placa 1994; Maddin 2013; Svejgaard 1985). All studies included participants of both sexes. Most were open to general dermatology outpatients with subungual onychomycosis of the toenail, but a small number of studies included only a specific patient group such as people with diabetes in Gupta 2006 or black participants (term used by study authors) in Billstein 1999. One study looked specifically at non‐dermatophyte nail infections (Ranawaka 2016).
Interventions
Trials evaluated several oral antifungal interventions, including terbinafine, azoles (itraconazole, fluconazole, albaconazole, posaconazole, ravuconazole) and griseofulvin in continuous or intermittent pulse therapy. Eight studies compared terbinafine monotherapy with placebo (Billstein 1999; Drake 1997; Elewski 2002; Elewski 2012; Goodfield 1992; Lebwohl 2001; Svejgaard 1997; Watson 1995), and nine studies compared azole monotherapy with placebo (Elewski 1997; Elewski 2002; Gupta 2000; Gupta 2005; Jones 1996; Ling 1998; Maddin 2013; Scher 1998; Sigurgeirsson 2013). Seventeen studies compared terbinafine monotherapy with azole monotherapy (Arca 2002; Brautigam 1995; De Backer 1998; Degreef 1999; Elewski 2012; Gupta 2001a; Gupta 2001b; Gupta 2006; Gupta 2009; Havu 2000; Honeyman 1997; Kejda 1999; Kouznetsov 2002; Ranawaka 2016; Sigurgeirsson 1999; Tosti 1996; Won 2007), one study compared two different azoles (Arca 2002), and one study compared terbinafine monotherapy with combination terbinafine plus azole therapy (Gupta 2001c). Seven studies compared griseofulvin with either an azole or terbinafine (Cullen 1987; Faergemann 1995; Gupta 2001b; Hofmann 1995; Korting 1993; La Placa 1994; Svejgaard 1985; Walsoe 1990). Study duration ranged from 4 months to 2 years, with most lasting 12 to 15 months.
Outcome measures
All studies addressed one or both of our two primary outcomes of clinical and mycological cure. Most studies addressed adverse events. Only nine studies addressed recurrence rate (Brautigam 1995; Drake 1997; Gupta 2009; Jones 1996; Korting 1993; Ranawaka 2016; Sigurgeirsson 1999; Tosti 1996; Watson 1995), and none addressed quality of life.
Excluded studies
We excluded 27 studies from the review (see Characteristics of excluded studies table).
The most common reason for exclusion was that the study assessed the efficacy of different regimens of a single drug, without comparing different drugs or drugs and placebo. This applied to 19 of the excluded studies (Alpsoy 1996; Avner 2006a; Avner 2006b; Chen 1999; De Cuyper 1996; De Doncker 1996; Finlay 1994; Havu 1997; Havu 1999; Pollak 2001; Schatz 1995; Shemer 1999; Sommer 2003; Tausch 1997; van der Schroeff 1992; Warshaw 2001; Warshaw 2005; Watanabe 2004; Yadav 2015).
Two studies examined infections other than toenail onychomycosis; namely, tinea pedis in Gomez 1996 and fungal skin infections in Zaias 1983. Two studies examined the efficacy of adjuncts to oral anti‐fungal therapy, such as topical treatment (Hay 1987; Maleszka 2001), and one study compared oral anti‐fungal therapy to 'palliative care', which consisted of trimming, soaking, and cleaning (Albreski 1999).
There was also one study that measured drug concentration in healthy nails (Faergemann 1996), one letter to the editor that did not report a trial (Safer 2000), and one study with no control group (Goodfield 1990).
Risk of bias in included studies
Two review authors (SKK and LG, GK or KH) independently assessed each of the 48 included studies for risk of bias across six specific domains, using the Cochrane 'Risk of bias' assessment tool (Higgins 2011), described in the Methods (see Assessment of risk of bias in included studies).
We report these assessments in the 'Risk of bias' table associated with each study, as well as the 'Risk of bias' summary (Figure 2). We only assessed one study as being at low risk of bias in all domains (Gupta 2005), while we judged 18 studies to be at high risk of bias in at least one domain; 11 of these were at high risk in two or more domains (Arca 2002; Arenas 1991; Arenas 1995; Gupta 2001b; Kejda 1999; Korting 1993; La Placa 1994; Mishra 2002; Piepponen 1992; Tosti 1996; Won 2007). The most common high risk domain was 'blinding of personnel and participants', for which 14 studies were deemed at high risk of bias.
2.
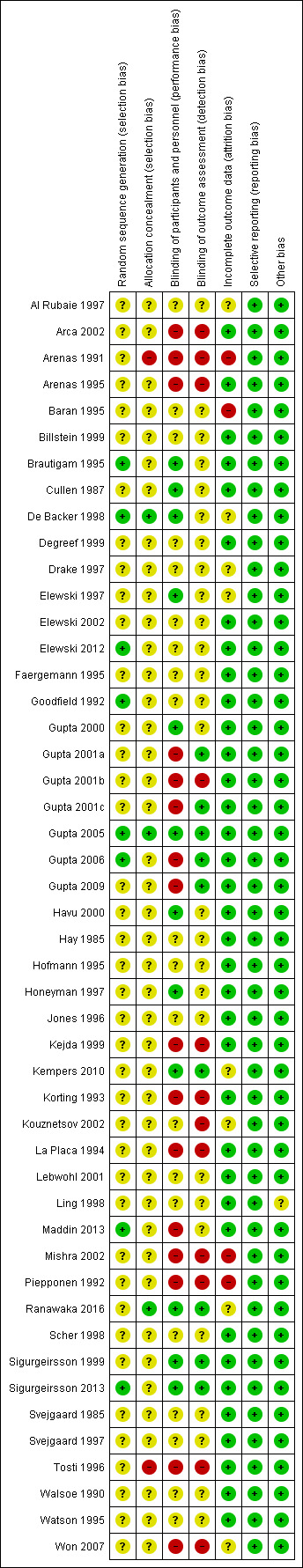
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We judged eight studies to be at low risk for this domain (Brautigam 1995; De Backer 1998; Elewski 2012; Goodfield 1992; Gupta 2005; Gupta 2006; Maddin 2013; Sigurgeirsson 2013). All clearly stated the method of sequence generation. For example, a "computer generated randomisation schedule in order of obtaining informed consent" in Brautigam 1995 or "random tables of Fisher and Yates" in Goodfield 1992. We assessed 40 studies as being at unclear risk, as there was no mention of the method of sequence generation.
Allocation concealment
We assessed three studies as being at low risk with regard to allocation concealment, as they had a clear description of their allocation concealment method (De Backer 1998; Gupta 2005; Ranawaka 2016). Forty‐three studies were at unclear risk because they provided no information regarding the method of allocation concealment. We assessed two studies as being at high risk (Arenas 1991; Tosti 1996), as participants were "assigned sequentially to treatment".
Blinding
Performance bias
There were 12 low‐risk studies for this domain (Brautigam 1995; Cullen 1987; De Backer 1998; Elewski 1997; Gupta 2000; Gupta 2005; Havu 2000; Honeyman 1997; Kempers 2010; Ranawaka 2016; Sigurgeirsson 1999; Sigurgeirsson 2013); these studies explicitly described the technique used for blinding, for instance the double‐dummy technique or "the active and placebo formulations were packaged so that both the participant and the investigator were blinded" (Gupta 2000). We assessed 20 studies as being at unclear risk; of these, 19 studies did not describe a method of blinding, and the one remaining study stated that some of the treatment groups were blinded while others were not (Elewski 2012). We deemed 16 studies to be at high risk; these studies were predominantly open or single‐blind studies, and in one study there was no mention of blinding (Kouznetsov 2002).
Detection bias
In terms of detection bias, there were nine studies we deemed to be at low risk, either because the authors specified that the outcome assessors were blinded (Gupta 2001a; Gupta 2001c; Gupta 2005; Gupta 2006; Kempers 2010; Ranawaka 2016; Sigurgeirsson 1999; Sigurgeirsson 2013), or they described the method of blinding of the outcome assessors (Gupta 2009).
We assessed 27 studies as being at unclear risk, 26 of which did not specify whether the outcome assessors were blinded or how they were blinded. In the one remaining study (Maddin 2013), there was a dedicated person to look after medication, but medications differed in appearance.
We judged 12 studies to be at high risk: seven were open‐label studies (Arca 2002; Arenas 1991; Arenas 1995; Korting 1993; Tosti 1996; Won 2007; Kejda 1999), while five gave no information on blinding in the text (La Placa 1994; Kouznetsov 2002; Mishra 2002; Piepponen 1992; Gupta 2001b).
Incomplete outcome data
We judged 36 studies to be at low risk because they accounted for all participants in the analysis (Arca 2002; Elewski 2012; Gupta 2001a; Gupta 2001b; Gupta 2006; Gupta 2009; Havu 2000; Maddin 2013; Sigurgeirsson 1999; Tosti 1996; Walsoe 1990), all study dropouts were accounted for (Arenas 1995; Billstein 1999; Cullen 1987; Degreef 1999; Elewski 2002; Faergemann 1995; Goodfield 1992; Gupta 2000; Gupta 2001c; Gupta 2005; Hofmann 1995; Honeyman 1997; Jones 1996; Kejda 1999; La Placa 1994; Ling 1998; Korting 1993; Sigurgeirsson 2013; Scher 1998; Svejgaard 1985; Watson 1995) or the number of participants unaccounted for was very low (Brautigam 1995 (two participants), Hay 1985 (six participants), Lebwohl 2001 (four participants), and Svejgaard 1997 (one participant)).
We deemed eight studies to be at unclear risk. In four of these studies, there were unexplained dropouts, but the numbers of missing participants were similar across treatment groups (Al Rubaie 1997; De Backer 1998; Drake 1997; Won 2007). In two studies the number of discontinuations was not clear from the text (Kempers 2010; Kouznetsov 2002), and in two studies the number of dropouts was dissimilar between the treatment arms (Elewski 1997; Ranawaka 2016).
We assessed four studies as being at high risk (Arenas 1991; Baran 1995; Mishra 2002; Piepponen 1992).
Selective reporting
We judged all 48 studies included in the review as being at low risk of reporting bias, as they reported all the outcomes they described in the Methods section, and all studies also had at least one of our primary outcomes (clinical cure and/or mycological cure) as their prespecified primary trial outcome. None of the trials used surrogate markers, and there was no indication of selective reporting of outcomes.
Other potential sources of bias
We deemed 47 studies to be at low risk of other potential sources of bias. Other bias was unclear in the remaining study because of pharmaceutical sponsorship and heavy involvement in the study (Ling 1998): "For the evaluation of efficacy at the end of treatment and at the six‐month follow‐up, clinical success was arbitrarily defined by the sponsor of the study".
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
The following comparisons address our prespecified outcomes.
Azole versus terbinafine.
Terbinafine versus placebo.
Azole versus placebo.
Griseofulvin versus azole.
Griseofulvin versus terbinafine.
Terbinafine plus azole versus terbinafine monotherapy.
For the clinical and mycological cure outcomes, we established subgroups based on duration of follow‐up (52 weeks and under or over 52 weeks of follow‐up including treatment duration).
None of the studies addressed quality of life.
Comparison 1: azole versus terbinafine
See Table 1 for quality assessments for this comparison.
Seventeen studies (1317 participants) compared azole with terbinafine (Arca 2002; Arenas 1995; Brautigam 1995; De Backer 1998; Degreef 1999; Elewski 2012; Gupta 2001a; Gupta 2001b; Gupta 2006; Gupta 2009; Havu 2000; Honeyman 1997; Kejda 1999; Kouznetsov 2002; Sigurgeirsson 1999; Tosti 1996; Won 2007). Azoles included fluconazole (Havu 2000); posaconazole (Elewski 2012); fluconazole and itraconazole in two arms (Arca 2002); and itraconazole, ketoconazole and fluconazole in three arms (Gupta 2001b). All other studies used itraconazole as the only azole.
This is the main comparison for our review, and we present the results in the Table 1, which includes a detailed discussion of the quality of the evidence using the GRADE framework as described in Quality of the evidence section.
Primary outcomes
Clinical cure
See Analysis 1.1.
1.1. Analysis.
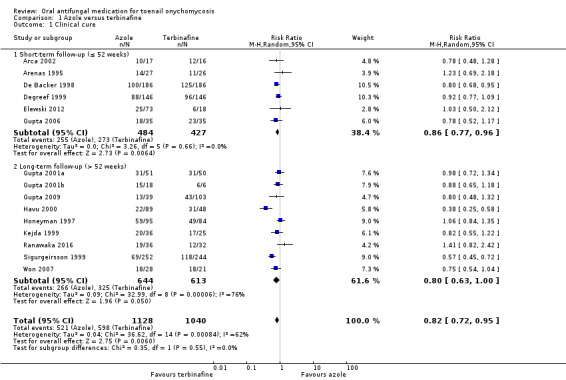
Comparison 1 Azole versus terbinafine, Outcome 1 Clinical cure.
Fifteen studies reported clinical cure as an outcome (Arca 2002; Arenas 1995; De Backer 1998; Degreef 1999; Elewski 2012; Gupta 2001a; Gupta 2001b; Gupta 2006; Gupta 2009; Havu 2000; Honeyman 1997Kejda 1999; Ranawaka 2016; Sigurgeirsson 1999; Won 2007).
In the pooled azole group, 521 (46%) participants achieved clinical cure compared to 598 (58%) participants in the combined terbinafine group. There was moderate‐quality evidence that participants in the azole group were 18% less likely to achieve clinical cure compared to participants receiving terbinafine (RR 0.82, 95% CI 0.72 to 0.95, 15 studies, 2168 participants; I² = 62%).
Two studies caused statistical heterogeneity (Havu 2000; Sigurgeirsson 1999), and removing them from the analyses reduced the statistical heterogeneity to 0%. This did not change the direction of the effect but did reduce its magnitude (RR 0.89, 95% CI 0.82 to 0.97). We could not explain the statistical heterogeneity based on clinical differences between these studies and the rest of the studies. We suspect the outlier effect is due to the size of Sigurgeirsson 1999 and the size of the effect estimate in Havu 2000.
Because there were more than 10 RCTs in this comparison, we drew funnel plots (Figure 3) to test for reporting bias as discussed in Chapter 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
3.
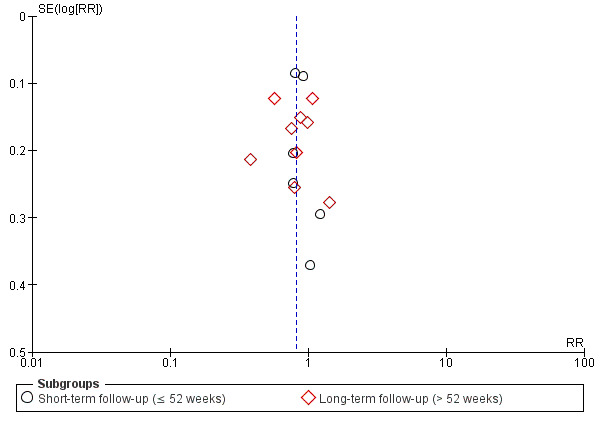
Funnel plot of comparison: 3 Azole versus terbinafine, outcome: 3.1 Clinical cure.
When only including studies using itraconazole (Arenas 1995; De Backer 1998; Degreef 1999; Gupta 2001a; Gupta 2006; Gupta 2009; Honeyman 1997Kejda 1999; Sigurgeirsson 1999; Won 2007), the effect estimate remained similar (RR 0.85, 95% CI 0.74 to 0.96).
Ranawaka 2016 looked at onychomycosis caused by non‐dermatophyte moulds only and found no difference when comparing azole to terbinafine (RR 1.41, 95% CI 0.82 to 2.42). When removing this study from the meta‐analysis, the overall results did not change (RR 0.81, 95% CI 0.70 to 0.92).
When comparing subgroups based on short‐ or long‐term follow‐up, we observed low statistical heterogeneity (I² = 3.3%, P value for subgroup differences = 0.55). In studies with short‐term follow‐up, the azole group was 14% less likely to achieve clinical cure (RR 0.86, 95% CI 0.77 to 0.96), and with long‐term follow‐up the azole group was 20% less likely to achieve clinical cure (RR 0.80, 95% CI 0.63 to 1.00) compared to the terbinafine group. However, this difference was not statistically significant.
Mycological cure
See Analysis 1.2.
1.2. Analysis.
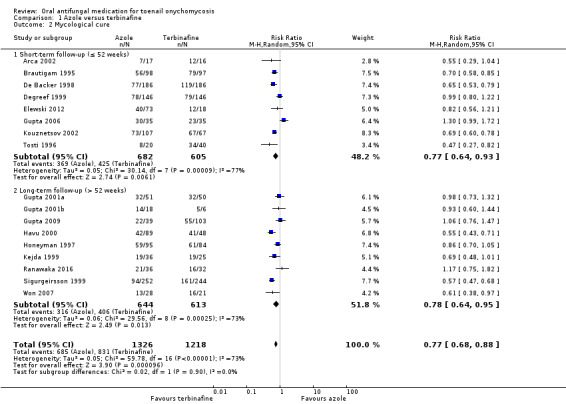
Comparison 1 Azole versus terbinafine, Outcome 2 Mycological cure.
Seventeen studies reported mycological cure as an outcome (Arca 2002; Brautigam 1995; De Backer 1998; Degreef 1999; Elewski 2012; Gupta 2001a; Gupta 2001b; Gupta 2006; Gupta 2009; Havu 2000; Honeyman 1997; Kejda 1999; Kouznetsov 2002; Ranawaka 2016; Sigurgeirsson 1999; Tosti 1996; Won 2007).
In the pooled azole group, 685 (52%) participants achieved mycological cure, compared to 831 (68%) participants in the pooled terbinafine group. There was moderate‐quality evidence that participants in the azole group were 23% less likely to achieve mycological cure compared to participants receiving terbinafine (RR 0.77, 95% CI 0.68 to 0.88, 17 studies, 2544 participants) (I² = 73%).
We could not attribute statistical heterogeneity to specific studies.
Because there were more than 10 RCTs, we drew funnel plots (Figure 4) to test for reporting bias as discussed in chapter 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
4.
Funnel plot of comparison: 3 Azole versus terbinafine, outcome: 3.2 Mycological cure.
When only including studies using the azole itraconazole (De Backer 1998; Degreef 1999; Gupta 2001a; Gupta 2006; Gupta 2009; Honeyman 1997Kejda 1999; Sigurgeirsson 1999; Won 2007), the effect estimate remained similar (RR 0.78, 95% CI 0.67 to 0.90).
One study looked at onychomycosis caused by non‐dermatophyte moulds only and found no difference when comparing azole to terbinafine (RR 1.17, 95% CI 0.75 to 1.82; Ranawaka 2016). When removing this study from the meta‐analysis the overall results did not change (RR 0.76, 95% CI 0.67 to 0.86).
When comparing subgroups based on short‐ or long‐term follow‐up, we saw no statistical heterogeneity (I² = 0%; P value for subgroup differences = 0.90). In studies with short‐term follow‐up, the azole group was 23% less likely to achieve clinical cure (RR 0.77, 95% CI 0.64 to 0.93), and with long‐term follow‐up the azole group was 23% less likely to achieve clinical cure (RR 0.78, 95% CI 0.64 to 0.95) compared to the terbinafine group.
Secondary outcomes
Adverse events
See Analysis 1.3.
1.3. Analysis.
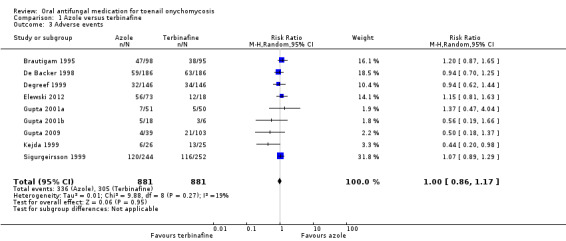
Comparison 1 Azole versus terbinafine, Outcome 3 Adverse events.
Nine studies compared terbinafine therapy with azole therapy for adverse events (Brautigam 1995; De Backer 1998; Degreef 1999; Elewski 2012; Gupta 2001a; Gupta 2001b; Gupta 2009; Kejda 1999; Sigurgeirsson 1999). There were 881 participants in the combined terbinafine groups and 881 participants in the combined azole groups.
In the combined terbinafine group, 305 (35%) participants experienced an adverse event compared to 336 (38%) in the terbinafine group. This difference was not statistically significant (RR 1.00, 95% CI 0.86 to 1.17, 9 studies, 1762 participants; I² = 19%; moderate‐quality evidence).
The most common adverse events amongst terbinafine‐treated participants included headache, viral infection, dyspepsia, taste disorders, flu‐like symptoms, nausea, fatigue, and rash/urticaria. The most common adverse events amongst azole‐treated participants included headache, viral infection, diarrhoea, constipation, nausea, abdominal pain, abnormal liver function tests, dizziness, and rash.
Two studies reported only adverse event data for events serious enough to cause discontinuation (Havu 2000; Honeyman 1997), so we excluded them from the above analysis. In Honeyman 1997, none of the 84 terbinafine participants and 6 of 95 itraconazole participants (6%) dropped out due to adverse events. In Havu 2000, 1 of 48 terbinafine participants (2%) and 3 of 89 azole participants (3%) dropped out due to adverse events.
Recurrence rate
See Analysis 1.4.
1.4. Analysis.
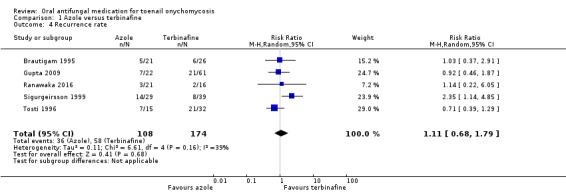
Comparison 1 Azole versus terbinafine, Outcome 4 Recurrence rate.
Five studies comparing terbinafine and azole therapies assessed the recurrence rate (Brautigam 1995;Gupta 2009;Ranawaka 2016; Sigurgeirsson 1999;Tosti 1996). In terms of clinical heterogeneity, the inclusion criteria for these studies are similar, and all studies compared terbinafine and itraconazole therapy, albeit in varying doses.
There was no statistically significant difference in the recurrence rate between participants receiving terbinafine or azole (RR 1.11, 95% CI 0.68 to 1.79, 5 studies, 282 participants; I² = 39%; low‐quality evidence).
One study looked at onychomycosis caused only by non‐dermatophyte moulds and found no difference in recurrence rate when comparing azole to terbinafine (RR 1.14, 95% CI 0.22 to 6.05; Ranawaka 2016). When removing this study from the meta‐analysis the overall results did not change (RR 1.11, 95% CI 0.64 to 1.92).
Comparison 2: terbinafine versus placebo
See Table 2 for quality assessments for this comparison.
Eight studies (N = 1006) comparing terbinafine (N = 682) with placebo (N = 324) provided data for this comparison (Billstein 1999; Drake 1997; Elewski 2002; Elewski 2012; Goodfield 1992; Lebwohl 2001; Svejgaard 1997; Watson 1995). All studies used terbinafine 250 mg daily for 12 to 24 weeks.
Primary outcomes
Clinical cure
See Analysis 2.1.
2.1. Analysis.
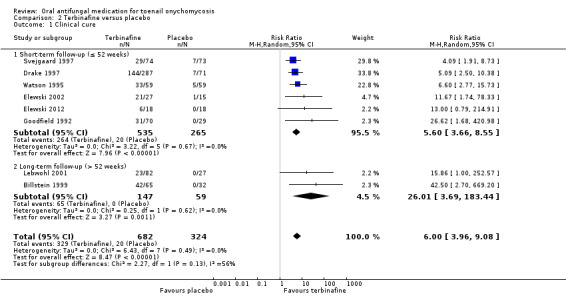
Comparison 2 Terbinafine versus placebo, Outcome 1 Clinical cure.
All eight studies reported clinical cure as an outcome. Six studies assessed the nail for clinical cure at 52 weeks or less from start of treatment (Drake 1997; Elewski 2002; Elewski 2012; Goodfield 1992; Svejgaard 1997; Watson 1995), and two studies assessed nails at 72 weeks and 78 weeks, respectively (Billstein 1999; Lebwohl 2001).
In the pooled placebo group, 20 (6%) participants achieved clinical cure compared to 329 participants in the pooled terbinafine group (48%). People treated with terbinafine were six times more likely achieve clinical cure compared with people receiving placebo (RR 6.00, 95% CI 3.96 to 9.08, 8 studies, 1006 participants; I² = 0%; high‐quality evidence).
When comparing subgroups based on short‐ or long‐term follow‐up, we identified some heterogeneity (I² = 56%, P value for subgroup differences = 0.13) due to the differences in placebo cure rate (8.5% in the short‐term follow‐up and 0% in the long‐term follow‐up). The estimated effect for short‐term follow‐up was RR 5.60 (95% CI 3.66 to 8.55) and for long‐term follow‐up, RR 26.01 (95% CI 3.69 to 183.44).
We did not assess any studies as being at high risk of bias, so we did not perform sensitivity analysis based on that consideration.
Mycological cure
See Analysis 2.2.
2.2. Analysis.
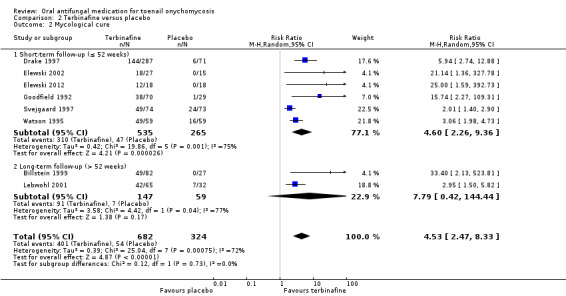
Comparison 2 Terbinafine versus placebo, Outcome 2 Mycological cure.
All eight studies reported mycological cure.
In the pooled placebo group, 54 (16.7%) participants achieved mycological cure compared to 401 participants in the intervention group (58.8%). There was moderate statistical heterogeneity as confirmed by an I² of 72%. There was no obvious clinical heterogeneity: the interventions were similar across studies, and no study examined a particular subset of the population. There was high‐quality evidence that participants in the terbinafine group were 4.5 times more likely to achieve mycological cure compared to participants receiving placebo (RR 4.53, 95% CI 2.47 to 8.33, 8 studies, 1006 participants; I² = 72%).
When comparing subgroups based on short‐ or long‐term follow‐up, we did not identify any heterogeneity (I² = 0%, P value for subgroup differences = 0.73). In studies with short‐term follow‐up the intervention group was 4.6 times as likely to achieve mycological cure (RR 4.60, 95% CI 2.26 to 9.36), and with long‐term follow‐up the intervention group was 7.79 times as likely to achieve mycological cure (RR 7.79, 95% CI 0.42 to 144.44).
We did not assess any studies as being at high risk of bias, so we did not perform sensitivity analysis based on that consideration.
Secondary outcomes
Adverse events
See Analysis 2.3.
2.3. Analysis.
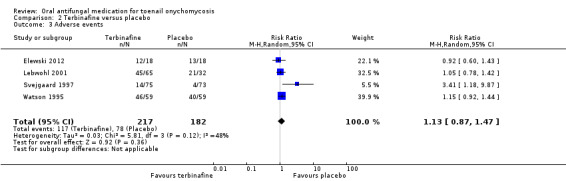
Comparison 2 Terbinafine versus placebo, Outcome 3 Adverse events.
Four studies compared terbinafine therapy with placebo for adverse events (Elewski 2012; Lebwohl 2001; Svejgaard 1997; Watson 1995). There were 217 participants in the pooled terbinafine groups and 182 participants in the pooled placebo groups.
In the pooled terbinafine group 117 (54%) participants experienced an adverse event compared to 78 (43%) in the placebo group. This difference was not statistically significant (RR 1.13, 95% CI 0.87 to 1.47, 4 studies, 399 participants; I² = 48%; moderate‐quality evidence).
The most common adverse events amongst terbinafine‐treated participants included gastrointestinal symptoms (diarrhoea, dyspepsia, abdominal pain, flatulence), infections (e.g. upper respiratory tract infection), headache, fatigue and disturbance of taste/smell.
One other study reported adverse event data for events serious enough to cause discontinuation; however, authors provided no information for the placebo group, limiting the interpretation and precluding inclusion of the data in the analysis (Drake 1997). Of 287 participants receiving terbinafine, nine experienced 'severe' adverse events (rash, diarrhoea, abdominal pain), with five withdrew from the study as a result.
Although we found no evidence of increased adverse events when comparing terbinafine with placebo, readers should interpret this result with caution due to the low number of studies.
Recurrence rate
See Analysis 2.4.
2.4. Analysis.
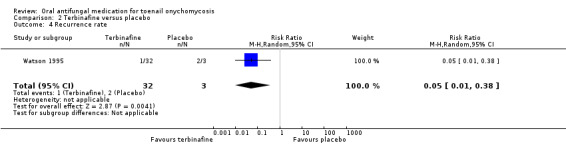
Comparison 2 Terbinafine versus placebo, Outcome 4 Recurrence rate.
Two studies compared the recurrence rate between those treated with terbinafine and placebo (Drake 1997, Watson 1995). Drake 1997 did not report the recurrence rate for the placebo group; therefore, we could not calculate relative risk for this study or pool the outcome data. Of the 157 participants who achieved cure and were followed up after the primary endpoint of the study, 11% had recurrence. In Watson 1995, participants receiving terbinafine therapy had a recurrence rate of 3.1% (1 of 32 participants that achieved cure had a recurrence), compared to 67% (2 of 3 participants) recurrence in the placebo group (RR 0.05, 95% CI 0.01 to 0.38, 1 study, 35 participants, low‐quality evidence).
Comparison 3: azole versus placebo
See Table 3 for quality assessments for this comparison.
Nine studies (N = 3440) compared azole (N = 2651) with placebo (N = 789), including four studies with itraconazole, (Elewski 1997; Gupta 2000; Jones 1996; Maddin 2013), two studies with fluconazole (Ling 1998; Scher 1998), and one study each for posaconazole (Elewski 2012), albaconazole (Sigurgeirsson 2013), and ravuconazole (Gupta 2005). Albaconazole and ravuconazole are drugs under development which are not commercially available at present. Therefore, we present the results with and without these two studies.
Primary outcomes
Clinical cure
See Analysis 3.1.
3.1. Analysis.
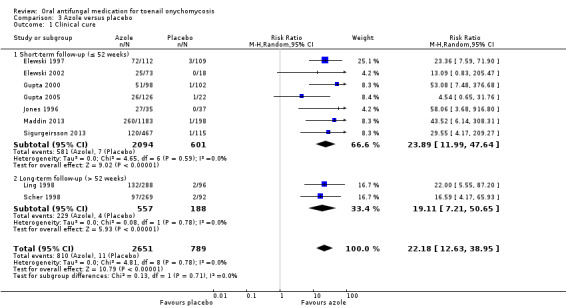
Comparison 3 Azole versus placebo, Outcome 1 Clinical cure.
All nine studies reported clinical cure as outcome (Elewski 1997; Elewski 2012; Gupta 2000; Gupta 2005; Jones 1996; Ling 1998; Maddin 2013; Scher 1998; Sigurgeirsson 2013).
In the pooled placebo group, 11 (13.9%) participants achieved clinical cure compared to 810 (30.5%) participants in the pooled azole group. There was high‐quality evidence that participants in the azole group were 22 times more likely to achieve clinical cure compared to participants receiving placebo (RR 22.18, 95% CI 12.63 to 38.95, 9 studies, 3440 participants; I² = 0%).
When excluding the studies using unregistered medications (Gupta 2005; Sigurgeirsson 2013), participants in the azole group were 25 times more likely to achieve clinical cure compared to participants receiving placebo (RR 25.28, 95% CI 13.64 to 46.85).
When comparing subgroups based on short‐ or long‐term follow‐up, we did not observe any statistical heterogeneity (I² = 0%, P value for subgroup differences = 0.71). In studies with short‐term follow‐up the intervention group was 23 times as likely to achieve clinical cure (RR 23.89, 95% CI 11.99 to 47.64), and with long‐term follow‐up the intervention group was 19 times as likely to achieve clinical cure (RR 19.11, 95% CI 7.21 to 50.65).
We assessed two studies as being at high risk of bias in at least one domain (Ling 1998; Maddin 2013); however, sensitivity analyses excluding these two studies did not change the direction or magnitude of the treatment effect (RR 20.62, 95% CI 10.76 to 39.52).
Mycological cure
See Analysis 3.2.
3.2. Analysis.
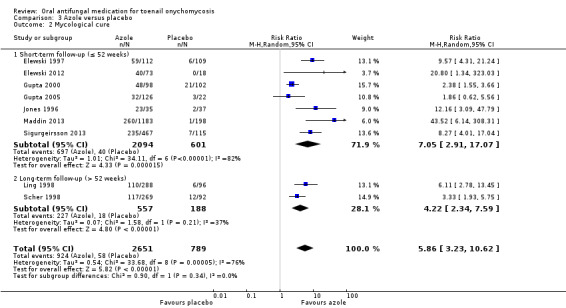
Comparison 3 Azole versus placebo, Outcome 2 Mycological cure.
All nine studies reported mycological cure as an outcome (Elewski 1997; Elewski 2012; Gupta 2000; Gupta 2005; Jones 1996; Ling 1998; Maddin 2013; Scher 1998; Sigurgeirsson 2013).
In the pooled placebo group, 58 (7.4%) participants achieved mycological cure compared to 924 (34.8%) participants in the intervention groups.
Statistical heterogeneity was high as confirmed by an I² of 76%. The main clinical difference was the use of different subtypes of azole medications, although these were similar in pharmacological action. However, subgroup analyses for itraconazole‐only studies did not reduce statistical heterogeneity (I² = 89%).
There was high‐quality evidence that participants in the azole group were almost six times more likely to achieve mycological cure compared to participants receiving placebo (RR 5.86, 95% CI 3.23 to 10.62, 9 studies, 3440 participants; I² = 76%).
Three studies seemed to contribute to statistical heterogeneity (Gupta 2000; Gupta 2005; Scher 1998). Removing these from the analyses reduced the statistical heterogeneity to 0%. This did not change the direction of the effect but did increase the effect size (RR 8.99, 95% CI 5.98 to 13.52).
When excluding the studies using unregistered medication (Gupta 2005; Sigurgeirsson 2013), participants in the azole group were 6.6 times more likely to achieve mycological cure compared to participants receiving placebo (RR 6.62, 95% CI 3.23 to 13.57).
When comparing subgroups based on short‐ or long‐term follow‐up, we saw low statistical heterogeneity (I² = 0%, P value for subgroup differences = 0.34). In studies with short‐term follow‐up the intervention group was seven times as likely to achieve mycological cure (RR 7.05, 95% CI 2.91 to 17.07), and with long‐term follow‐up the intervention group was four times as likely to achieve mycological cure (RR 4.22, 95% CI 2.34 to 7.59).
We assessed two studies as being at high risk of bias in at least one domain (Ling 1998; Maddin 2013); however, sensitivity analyses excluding these two studies did not change the direction or magnitude of the treatment effect (RR 4.93, 95% CI 2.68 to 9.07).
Secondary outcomes
Adverse events
See Analysis 3.3.
3.3. Analysis.
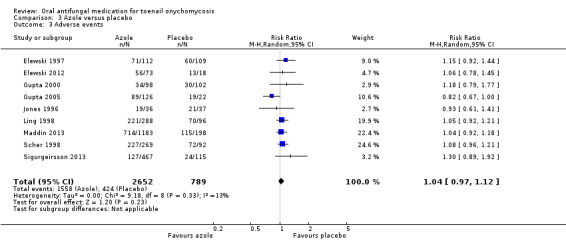
Comparison 3 Azole versus placebo, Outcome 3 Adverse events.
Nine studies compared azole therapy with placebo for adverse events (Elewski 1997; Elewski 2012; Gupta 2000; Gupta 2005; Jones 1996; Maddin 2013; Sigurgeirsson 2013; Ling 1998; Scher 1998). There were 2652 participants in the pooled azole groups and 789 participants in the pooled placebo groups.
In the pooled azole group, 1558 (59%) participants experienced an adverse event compared to 424 (54%) in the placebo group. This difference was not statistically significant (RR 1.04, 95% CI 0.97 to 1.12; 9 studies, 3441 participants, I² = 13%; moderate‐quality evidence). The most common adverse events amongst azole‐treated participants included headache, upper respiratory tract infections (URTI)/rhinitis/sinusitis, flu‐like symptoms, nausea, fatigue, abdominal pain, diarrhoea, dizziness, rash, and elevated liver function tests.
Recurrence rate
See Analysis 3.4.
3.4. Analysis.
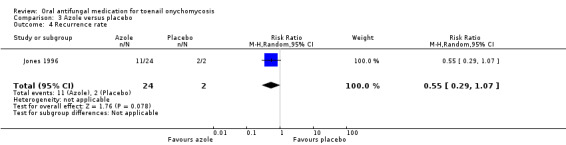
Comparison 3 Azole versus placebo, Outcome 4 Recurrence rate.
One study compared the recurrence rate between azole therapy and placebo, showing that 46% (11 of 24 participants) of participants receiving azole therapy had a recurrence, while 100% (2 of 2 participants) receiving placebo had a recurrence (RR 0.55, 95% CI 0.29 to 1.07, 26 participants; low‐quality evidence; Jones 1996).
Comparison 4: griseofulvin versus azole
See Table 4 for quality assessments for this comparison.
Five studies (222 participants) compared griseofulvin (N = 125) with an azole (N = 97) (Cullen 1987; Gupta 2001b; Korting 1993; Svejgaard 1985; Walsoe 1990). All studies used itraconazole, with one study using two azole arms, ketoconazole and itraconazole (Gupta 2001b). Griseofulvin doses ranged from 500 mg to 1200 mg per day.
Primary outcomes
Clinical cure
See Analysis 4.1.
4.1. Analysis.
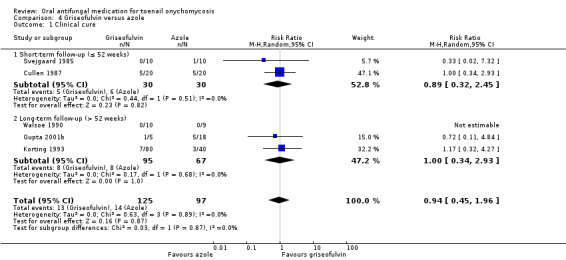
Comparison 4 Griseofulvin versus azole, Outcome 1 Clinical cure.
Five studies reported clinical cure as an outcome (Cullen 1987; Gupta 2001b; Korting 1993; Svejgaard 1985; Walsoe 1990).
In the pooled griseofulvin group, 13 (10%) participants achieved clinical cure compared to 14 (10.4%) participants in the azole group. There was no statistically significant difference in the chance of achieving clinical cure between the griseofulvin and the azole group (RR 0.94, 95% CI 0.45 to 1.96, 5 studies, 222 participants; I² = 0%; moderate‐quality evidence).
When comparing subgroups based on short‐ or long‐term follow‐up, we observed no statistical heterogeneity between groups (I² = 0%, P value for subgroup differences = 0.87). In studies with both short‐ and long‐term follow‐up, we saw no statistically significant difference between the two treatment groups (RR 0.89, 95% CI 0.32 to 2.35 and RR 1.00, 95% CI 0.34 to 2.91, respectively).
Mycological cure
See Analysis 4.2.
4.2. Analysis.
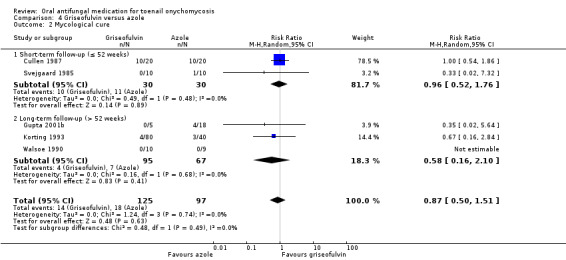
Comparison 4 Griseofulvin versus azole, Outcome 2 Mycological cure.
Five studies reported mycological cure as an outcome (Cullen 1987; Gupta 2001b; Korting 1993; Svejgaard 1985; Walsoe 1990).
In the pooled griseofulvin group, 14 (11.2%) participants achieved mycological cure compared to 18 (18.6%) participants in the azole group. There was no statistically significant difference in the chance of achieving mycological cure between the griseofulvin and the azole group (RR 0.87, 95% CI 0.50 to 1.51, 5 studies, 222 participants; I² = 0%; moderate‐quality evidence).
When comparing subgroups based on short‐ or long‐term follow‐up, we saw no statistical heterogeneity between groups (I² = 0%, P value for subgroup differences = 0.49). In both studies with short‐ and long‐term follow‐up, we saw no statistically significant difference between the two treatment groups (RR 0.96, 95% CI 0.52 to 1.76 and RR 0.58, 95% CI 0.16 to 2.10, respectively).
Secondary outcomes
Adverse events
See Analysis 4.3.
4.3. Analysis.
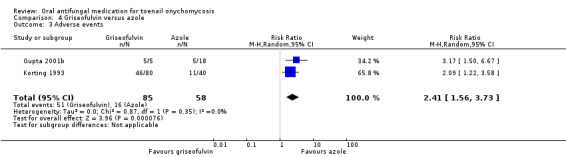
Comparison 4 Griseofulvin versus azole, Outcome 3 Adverse events.
Two studies compared griseofulvin with azole treatment for adverse events (Gupta 2001b; Korting 1993). There were 85 participants in the pooled griseofulvin group and 58 participants in the pooled azole group.
In the pooled griseofulvin group, 51 (60%) participants experienced an adverse event compared to 16 (28%) in the azole group. Participants receiving griseofulvin were more than twice as likely to experience an adverse event compared to participants receiving azole (RR 2.41, 95% CI 1.56 to 3.73, 2 studies, 143 participants; I² = 0%; moderate‐quality evidence). The most common adverse events amongst griseofulvin‐treated participants included gastrointestinal disturbance, changes in hepatic and renal function, allergic reaction, and photodermatitis. The most common adverse events amongst azole‐treated participants included nausea, vomiting, and changes in liver function.
Two studies only reported adverse event data for events serious enough to cause discontinuation (Cullen 1987; Walsoe 1990), precluding their inclusion in the above analysis. In Cullen 1987, 8 of 20 (40%) participants treated with griseofulvin discontinued due to adverse events compared to 4 of 20 (20%) participants treated with azole. In Walsoe 1990, 1 of 10 (10%) participants in the griseofulvin group discontinued due to adverse events compared to 2 of 9 (22%) participants treated with azoles.
Recurrence rate
See Analysis 4.4.
4.4. Analysis.
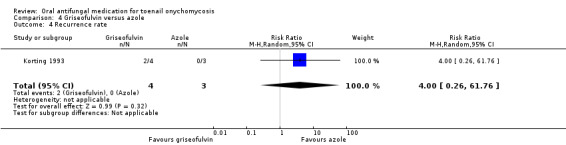
Comparison 4 Griseofulvin versus azole, Outcome 4 Recurrence rate.
One study compared the recurrence rate between azole therapies and griseofulvin (Korting 1993). In the griseofulvin group two of the four participants that achieved cure had a recurrence, while none of the three participants that achieved cure on azole treatment had a recurrence. This difference was not statistically significant (RR 4.00, 95% CI 0.26 to 61.76, 1 study, 7 participants; very low‐quality evidence).
Comparison 5: griseofulvin versus terbinafine
See Table 5 for quality assessments for this comparison.
Five studies (465 participants) compared griseofulvin (N = 236) with terbinafine (N = 229) (Baran 1995; Faergemann 1995; Gupta 2001b; Hofmann 1995; La Placa 1994). All studies used terbinafine 250 mg daily, and the daily dose of griseofulvin varied from 500 mg to 1200 mg.
Primary outcomes
Clinical cure
See Analysis 5.1.
5.1. Analysis.
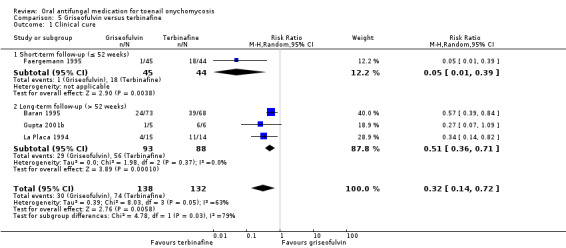
Comparison 5 Griseofulvin versus terbinafine, Outcome 1 Clinical cure.
Four studies reported clinical cure as an outcome (Baran 1995; Faergemann 1995; Gupta 2001b; La Placa 1994).
In the pooled griseofulvin group, 30 (21.7%) participants achieved clinical cure compared to 74 (56%) participants in the terbinafine group. The chance of achieving clinical cure was 68% lower in the griseofulvin group when compared to the terbinafine group (RR 0.32, 95% CI 0.14 to 0.72, 4 studies, 270 participants; I² = 63%; low‐quality evidence).
One study caused statistical heterogeneity (Faergemann 1995). Removing this study from the analyses reduced the statistical heterogeneity to 0%. This did not change the direction of the effect but did reduce the size of the effect (RR 0.50, 95% CI 0.35 to 0.70).
Only one study had short‐term follow‐up (Faergemann 1995), and only one participant in this study achieved clinical cure, resulting in an RR of 0.05 (95% CI 0.01 to 0.39).
In the long‐term follow‐up the griseofulvin group had a 49% lower chance of achieving clinical cure when compared to the terbinafine group (RR 0.51, 95% CI 0.36 to 0.71).
Mycological cure
See Analysis 5.2.
5.2. Analysis.
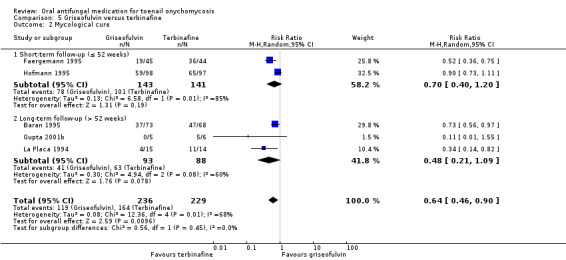
Comparison 5 Griseofulvin versus terbinafine, Outcome 2 Mycological cure.
Five studies reported mycological cure as an outcome (Baran 1995; Faergemann 1995; Gupta 2001b; Hofmann 1995; La Placa 1994).
In the pooled griseofulvin group, 119 (50.4%) participants achieved mycological cure, compared to 164 (71.6%) participants in the terbinafine group. The chance of achieving mycological cure was 36% lower in the griseofulvin group when compared to the terbinafine group (RR 0.64, 95% CI 0.46 to 0.90, 5 studies, 465 participants; I² = 68%; low‐quality evidence).
Two studies caused statistical heterogeneity (Baran 1995; Hofmann 1995). Removing these two studies from the analyses reduced the statistical heterogeneity to 7%. This did not change the direction of the effect but did increase the magnitude of the effect (RR 0.46, 95% CI 0.31 to 0.68).
When comparing subgroups based on short‐ or long‐term follow‐up, we saw no statistical heterogeneity between groups (I² = 0%, P value for subgroup differences = 0.45). In studies with both short‐ and long‐term follow‐up, there were no statistical differences between the griseofulvin and the terbinafine groups (RR 0.70, 95% CI 0.40 to 1.20 and RR 0.48, 95% CI 0.21 to 1.09, respectively).
Secondary outcomes
Adverse events
See Analysis 5.3.
5.3. Analysis.
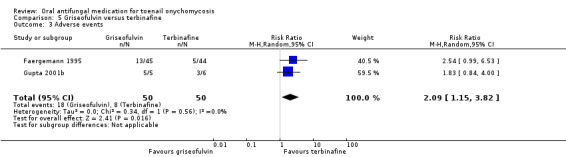
Comparison 5 Griseofulvin versus terbinafine, Outcome 3 Adverse events.
Two studies comparing griseofulvin therapy with terbinafine therapy reported adverse events (Faergemann 1995; Gupta 2001b). There were 50 participants in the pooled griseofulvin groups and 50 participants in the pooled terbinafine groups.
In the pooled griseofulvin group, 18 (36%) participants experienced an adverse event compared to 8 (16%) in the terbinafine group. Participants receiving griseofulvin were 2.3 times more likely to experience an adverse event compared to participants receiving terbinafine (RR 2.09, 95% CI 1.15 to 3.82, 2 studies, 100 participants; I² = 0%; low‐quality evidence). The most common adverse events amongst griseofulvin‐treated participants included gastrointestinal disturbance, headache, changes in hepatic and renal function, nausea, URTI, allergic reaction, urticaria, and photodermatitis. The most common adverse events amongst terbinafine‐treated participants included gastrointestinal disturbance, loss of taste, nausea, vertigo, and increased sweating.
One study only reported adverse event data for events serious enough to cause discontinuation, precluding its inclusion in the above analysis (Hofmann 1995). Of 98 participants receiving griseofulvin, 16 (16%) experienced adverse events that necessitated withdrawal from the study, compared to 10 of 97 (10%) participants treated with terbinafine who withdrew due to adverse events.
Recurrence rate
We did not identify any studies that addressed the recurrence rate.
Comparison 6: terbinafine plus azole versus terbinafine monotherapy
See Table 6 for quality assessments for this comparison.
One study in 176 participants compared terbinafine plus azole (N = 81) versus terbinafine monotherapy (N = 95) (Gupta 2001c), using repeat pulses of itraconazole (200 mg twice daily for a week) followed by one pulse of terbinafine (250 mg twice daily for a week) versus one week of 250 mg terbinafine twice a day, followed by a three‐week, treatment‐free interval.
Primary outcomes
Clinical cure
See Analysis 6.1.
6.1. Analysis.
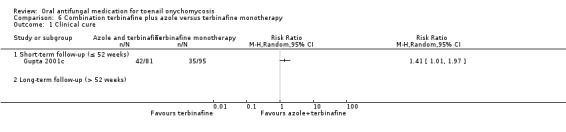
Comparison 6 Combination terbinafine plus azole versus terbinafine monotherapy, Outcome 1 Clinical cure.
In the pooled azole plus terbinafine group, 42 (51.9%) participants achieved clinical cure compared to 35 (36.8%) in the terbinafine‐only group.
Therefore, the combination group had a 41% higher chance of achieving clinical cure (RR 1.41, 95% CI 1.01 to 1.97, 1 study, 176 participants, very low‐quality evidence).
Mycological cure
See Analysis 6.2.
6.2. Analysis.
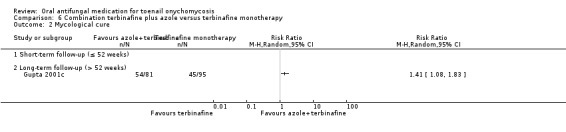
Comparison 6 Combination terbinafine plus azole versus terbinafine monotherapy, Outcome 2 Mycological cure.
In the pooled azole plus terbinafine group, 54 (66.7%) achieved mycological cure compared to 45 (47.4%) in the terbinafine‐only group.
Therefore, the combination group had a 41% higher chance of achieving mycological cure (RR 1.41, 95% CI 1.08 to 1.83, 1 study, 176 participants, very low‐quality evidence).
Secondary outcomes
Adverse events
See Analysis 6.3.
6.3. Analysis.
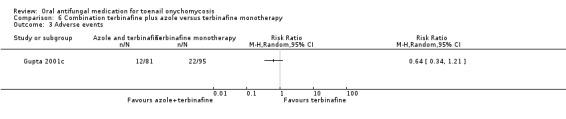
Comparison 6 Combination terbinafine plus azole versus terbinafine monotherapy, Outcome 3 Adverse events.
In the combination therapy group, 12 (15%) participants experienced an adverse event compared to 22 (23%) in the terbinafine monotherapy group. This difference was not statistically significant (RR 0.64, 95% CI 0.34 to 1.21, 1 study, 176 participants; low‐quality evidence).
The most common adverse events in the combination group were gastrointestinal complaints, headache, fatigue, cutaneous eruption, drowsiness, and elevated liver function tests. The most common adverse events in the terbinafine monotherapy group were gastrointestinal, taste loss/disturbance, headache, and elevated liver function tests.
Recurrence rate
The trial did not report the recurrence rate.
Discussion
Summary of main results
This review included 48 studies with 10,200 participants, and we could extract data from 43 studies with 9730 participants. Most of the studies were published in 2000 or earlier, and half were multicentre studies in a dermatology outpatient setting.
Azoles versus terbinafine
See Table 1.
When comparing azoles and terbinafine directly, there was moderate‐quality evidence that terbinafine was probably more effective in achieving mycological (15 studies) and clinical cure (17 studies). We performed sensitivity analyses: excluding one study with non‐dermatophyte onychomycosis, excluding studies that contributed to significant statistical heterogeneity, and including studies using itraconazole only; these analyses confirmed the robustness of our estimates. Comparing the two treatments, there was probably no difference in the risk of adverse events (moderate‐quality evidence), and there may be no difference in the recurrence rate (low‐quality evidence). Common adverse events in both groups included headache, viral infection, and nausea.
Terbinafine versus placebo
See Table 2.
Eight studies in just over 1000 participants provided high‐quality evidence that terbinafine was more effective for achieving clinical and mycological cure in onychomycosis when compared to placebo. Moderate‐quality evidence found that there was probably no difference in the number of adverse events reported. Common adverse events amongst terbinafine‐treated participants included gastrointestinal symptoms, infections, and headache. Low‐quality evidence suggested that terbinafine may lower the recurrence rate when compared to placebo.
Azoles versus placebo
See Table 3.
We found azoles to be more effective than placebo in achieving mycological and clinical cure (nine studies provided high‐quality evidence). We performed sensitivity analyses excluding studies at high risk of bias, confirming the robustness of our estimate. Participants in the azole group experienced slightly more adverse events, but the difference was probably not significant (moderate‐quality evidence). The most common adverse events amongst azole‐treated participants included headache, flu‐like symptoms, and nausea. Azoles may lower the recurrence rate when compared to placebo (low‐quality evidence).
When looking at the estimates for azoles versus placebo and terbinafine versus placebo, the effect estimate for azoles is higher than the effect estimate for terbinafine (clinical cure: RR 22.18 for azoles versus RR 6.00 for terbinafine, and mycological cure: RR 5.86 for azoles versus RR 4.53 for terbinafine). However, differences in the study populations or other factors might influence an indirect comparison. Hence, we have more confidence in the above direct comparison between terbinafine and azoles.
Other comparisons included azole versus griseofulvin (comparison 4) and terbinafine versus griseofulvin (comparison 5).
Azole versus griseofulvin
See Table 4.
Two hundred twenty‐two participants from five studies provided moderate‐quality evidence that azole and griseofulvin probably had the same effect on clinical and mycological cure. However, the risk of adverse events was probably much higher in the griseofulvin group (moderate‐quality evidence). The most common adverse events amongst griseofulvin‐treated participants included gastrointestinal disturbance and allergic reaction, and amongst azole‐treated participants, they included nausea and vomiting. Very low‐quality evidence means we are uncertain how griseofulvin impacts the recurrence rate compared to azole.
Terbinafine versus griseofulvin
See Table 5.
Compared to griseofulvin, low‐quality evidence showed that terbinafine may improve the outcomes of clinical cure (four studies provided data) and mycological cure (five studies provided data). We performed sensitivity analyses removing studies that contributed to significant statistical heterogeneity and confirmed the robustness of our estimates. Griseofulvin was also associated with a higher risk of adverse events, although this result was based on low‐quality evidence. Common adverse events in the griseofulvin group included headache and gastrointestinal issues, whereas common adverse events in the terbinafine group included taste loss and nausea. None of our included studies addressed recurrence rate for this comparison.
Compared with placebo, we found a clear treatment benefit to using the oral antifungal treatments terbinafine and azole, without excess harm (as measured by the adverse events rate). However, slight uncertainty remains around the possible harms of treatment, because even though there was no difference in the number of adverse events for azoles or terbinafine, the quality of the evidence for this outcome was moderate. For griseofulvin, there were indications of excess harm as the number of adverse events was considerably higher when compared to terbinafine or azole treatment, although the quality of the evidence for these treatments was low to moderate, respectively.
None of the included studies addressed quality of life, so this remains an outstanding uncertainty. Not all of the comparisons addressed the outcome of recurrence rate, and those that did provided quite uncertain to very uncertain evidence.
Please see Table 6 for details of our sixth comparison.
Overall completeness and applicability of evidence
We were able to look at all relevant and currently used oral antifungal treatments, as a large number of the included studies used them all. Many studies addressed our primary outcomes, which resulted in an effect estimate with narrow confidence margins. The study population was representative of the general population, and the results are applicable to clinical practice. Most studies included participants with T rubrum and T mentagrophytes. Therefore, the results might not be applicable to onychomycosis caused by non‐dermatophyte moulds and Candida.
However, there were major limitations related to the assessment of secondary outcomes. Firstly, none of the studies addressed quality of life as an outcome, which indicates a major gap in the research. Furthermore, the data for adverse events were very heterogenous and not all trials reported adverse events. In addition, we were unable to assess the severity of adverse events, as only data related to the prevalence of adverse events were available.
The limited data available suggest that adverse events were more prevalent with griseofulvin treatment. When comparing azoles with terbinafine, there was no statistically significant difference in the occurrence of adverse events. In addition, we were unable to assess the severity of adverse events, as only data related to the prevalence of adverse events were available.
Recurrence rate was reported in a limited number of studies only, so the reported differences between treatment arms were underpowered and not statistically significant.
Quality of the evidence
We summarise the quality of evidence for all our comparisons in Table 2; Table 3; Table 1; Table 4; Table 5; and Table 6. Overall, the quality of the evidence varied widely from high to very low depending on the outcome and comparison. The main reason for downgrading evidence was limitations in study design such as lack of blinding.
The quality of the evidence for the primary outcomes was high for azole versus placebo (comparison 3) as well as terbinafine versus placebo (comparison 2), and it was moderate for azole versus terbinafine directly (comparison 1). For griseofulvin versus azole (comparison 4), the quality of the evidence was moderate; for griseofulvin versus terbinafine (comparison 5), it was low, and for terbinafine plus azole versus terbinafine monotherapy (comparison 6), quality was very low.
For our secondary outcome, adverse events, the quality of the evidence was moderate (comparisons 1, 2, 3, 4) or low (comparisons 5 and 6). The quality of the evidence for recurrence rate was low (comparisons 1, 2, 3) or very low (comparison 4).
Limitations in study design or execution
Many of the studies had methodological limitations; often information on allocation concealment and details about randomisation was unclear. Because of this, we downgraded the level of evidence by one level. In the comparisons where in addition to the first problem at least half of the studies were non‐blinded studies, we downgraded a further level.
Inconsistency of results
We found many studies (4 to 16, depending on the comparison) reporting on our primary and secondary outcomes, with minimal inconsistency of the results between studies. We have not downgraded the evidence for any of the comparisons for inconsistency.
Indirectness of evidence
All included studies report on our primary outcomes, clinical and mycological cure; none of the studies used surrogate or intermediate outcome markers.
Imprecision
For our primary outcomes and the secondary outcome adverse events, the sample size was sufficiently large to detect a statistically significant difference between the treatment groups for most comparisons. However, for recurrence rate for comparison one (azole versus terbinafine), we may have failed to detect a meaningful difference due the small sample size. Therefore, we have downgraded the evidence by one level for this outcome. For recurrence rate in comparison four (griseofulvin versus azole), we included only one study with seven participants; therefore, we reduced the evidence by two levels for imprecision. Comparison six (combination terbinafine plus azole versus terbinafine monotherapy) only included one study, which again increased the risk of imprecision, so we downgraded the evidence by one level.
Publication bias
We conducted a comprehensive search that would have reduced the risk of publication bias. For the primary outcomes, where we found more than 10 studies, funnel plots (Figure 3; Figure 4) confirmed a low risk of publication bias.
Large effect
For the comparisons including placebo (comparisons two and three), we upgraded the level of evidence by one or two levels due to the large effect seen for the primary outcomes.
Potential biases in the review process
For this review we searched a wide range of databases with no restrictions based on language. We contacted pharmaceutical companies for additional unpublished trials and searched trials registers. We may have missed other relevant studies, especially ones with a negative result, and the fact that five studies have not yet been incorporated may be a source of potential bias. However, funnel plots for the primary outcomes do not show evidence of publication bias. Even in its presence, we believe that given the large number of studies included in this review, additional negative studies are unlikely to change the overall conclusion.
Two authors independently selected studies for inclusion, extracted data, and assessed risk of bias, with a third author acting as arbiter in order to minimise the risk of bias in the review process. In addition, we attempted to contact study authors for additional data.
None of the review authors had a conflict of interest regarding any of the medications in this review.
Agreements and disagreements with other studies or reviews
Recent systematic reviews found that both azoles and terbinafine are effective treatments (De Sa 2014; Ferrari 2011; Gupta 2015), which is in agreement with our findings. Ferrari 2011 states that terbinafine is more effective for achieving cure than azoles (itraconazole), which is also consistent with our findings. Gupta 2015 suggests that a specific dosing of itraconazole was as effective as the terbinafine treatment; however, the number of studies included in this review was considerably lower than in ours, which might have affected the power to detect such a difference. De Sa 2014 suggested that for non‐dermatophyte infections, azoles are the most effective treatment. This advice is partly based on treatment trials that did not include a control group. In our review we did not analyse subgroups based on causative organism.
While we set out to assess risks of the treatment, we could only assess the frequency of reported adverse events for the different treatments, not the severity. We found low‐quality evidence that griseofulvin is associated with an increased rate of adverse events compared to azoles, and we are uncertain about adverse events for the comparison of griseofulvin versus terbinafine, as the quality of the evidence was very low. In the literature, case reports have been published on serious adverse events (Kao 2014); however, systematic reviews looking specifically at liver injury caused by azoles and terbinafine found that the absolute numbers of patients affected are low (Greenblatt 2014; Yan 2014).
Authors' conclusions
Implications for practice.
There is high‐quality evidence to support the conclusion that oral azole and terbinafine treatments are more effective for achieving mycological cure and clinical cure for onychomycosis compared to placebo. When compared directly, terbinafine is probably more effective than azoles and likely not associated with excess adverse events (both moderate‐quality evidence).
For adverse events overall, the quality of the evidence was moderate to low; only a limited number of studies reported adverse events, and the severity of the events was not taken into account, which limits the direct application to clinical practice.
Low‐certainty evidence showed griseofulvin to be less effective than terbinafine in terms of both mycological and clinical cure, while griseofulvin and azole probably had similar efficacy (moderate‐quality evidence). Griseofulvin was associated with more adverse reactions than azoles (moderate‐quality) and terbinafine (low‐quality).
The evidence in this review applies for treatments of least 12 weeks in duration, as all included studies had the typical treatment duration of at least 12 weeks.
In terms of limitations of the current evidence, reporting of the secondary outcomes was limited. None of the included studies assessed quality of life, and only a few studies reported recurrence rate, mainly leading to non‐significant results. Also, most studies included participants with T rubrum and T mentagrophytes, so the results might not be applicable to onychomycosis caused by non‐dermatophyte moulds and Candida.
Implications for research.
This review found a large evidence base for the efficacy and effectiveness of the different oral treatments for onychomycosis. The quality of the evidence was high when comparing terbinafine or azole with placebo for the primary outcomes; however, the direct comparison for terbinafine and azole was only of moderate quality, mostly due to limitations in the study designs such as the lack of blinding. This could be consolidated with further well‐designed, double‐blind randomised controlled trials with enough follow‐up, possibly up to 12 months, to fully judge the effect of treatment. Furthermore, none of the included studies addressed the impact on quality of life. Further research could concentrate on the effect of treatment on quality of life as well as the adverse events associated with these treatments, including the severity of the adverse events associated with oral azoles and oral terbinafine, to further strengthen our confidence when prescribing these medications.
Acknowledgements
The Cochrane Skin Group editorial base wishes to thank Sue Jessop who was the Key Editor for this review; Ben Carter and Esther van Zuuren, who were the Statistical and Methods Editors, respectively; and the clinical referees, Chinmanat Tangjaturonrusamee and Shari Lipner. We also thank Meggan Harris, who copy‐edited the review.
Some parts of the Background and Methods sections of this review use text that was originally written by author Sally Bell‐Syer for use in the original review protocol (Bell‐Syer 2004), which has now been withdrawn, and in other Cochrane reviews, in her role as Cochrane Review author or Managing Editor of the Cochrane Wounds Group.
Appendices
Appendix 1. Glossary
| Medical term | Explanation |
| Distal | Top |
| Fungal hyphae | Cylindrical thread‐like structures |
| Hyperkeratosis | Thickening of the nail |
| Lateral | Side |
| Lamellar | Length‐wise |
| Nailfold | Where the nail meets the skin |
| Proximal | Base |
| Striae | Groove‐like marks on the nail |
| Subungual | Under the nail |
Appendix 2. Specialised Register search strategy
(onychomycos* and (toe* or toenail* or foot or feet)) or ("tinea unguium” and (toenail* or toe*)) or ((fungal or fungus) and (toenail* or toe*)) or (ringworm and (toenail* or toe*))
Appendix 3. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor Onychomycosis explode all trees #2 MeSH descriptor Foot Dermatoses explode all trees #3 (#1 AND #2) #4 (fungal or fungus) near/4 (toenail* or toe*) #5 (ringworm near/4 (toenail* or toe*)) #6 (onychomycos*) #7 (tinea next unguium) #8 (toenail* or toe* or foot or feet) #9 (#6 AND #8) #10 (#7 AND #8) #11 (#3 OR #4 OR #5 OR #9 OR #10)
Appendix 4. MEDLINE (Ovid) search strategy
1. exp Onychomycosis/ 2. exp Foot Dermatoses/ 3. 1 and 2 4. ((fungal or fungus) adj4 (toenail$ or toe$)).mp. 5. (ringworm adj4 (toenail$ or toe$)).mp. 6. Onychomycos$.mp. 7. "tinea unguium".mp. 8. (toenail$ or toe$).mp. 9. (foot or feet).mp. 10. 8 or 9 11. 6 and 10 12. 7 and 10 13. 3 or 4 or 5 or 11 or 12 14. randomised controlled trial.pt. 15. controlled clinical trial.pt. 16. randomized.ab. 17. placebo.ab. 18. clinical trials as topic.sh. 19. randomly.ab. 20. trial.ti. 21. 14 or 15 or 16 or 17 or 18 or 19 or 20 22. (animals not (humans and animals)).sh. 23. 21 not 22 24. 13 and 23
[Lines 14‐23: Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 5. Embase (Ovid) search strategy
1. exp toenail onychomycosis/ 2. ((fungal or fungus) adj4 (toenail$ or toe$)).mp. 3. (ringworm adj4 (toenail$ or toe$)).mp. 4. Onychomycos$.mp. 5. "tinea unguium".mp. 6. (toenail$ or toe$).mp. 7. (foot or feet).mp. 8. 6 or 7 9. 4 and 8 10. 5 and 8 11. 1 or 2 or 3 or 9 or 10 12. crossover procedure.sh. 13. double‐blind procedure.sh. 14. single‐blind procedure.sh. 15. (crossover$ or cross over$).tw. 16. placebo$.tw. 17. (doubl$ adj blind$).tw. 18. allocat$.tw. 19. trial.ti. 20. randomised controlled trial.sh. 21. random$.tw. 22. or/12‐21 23. (ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/) and HUMAN/ 24. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 25. 24 not 23 26. 22 not 25 27. 11 and 26
Appendix 6. LILACS search strategy
((onychomycos$ or onicomicos$) and (pie$ or toe$)) or (("tinea unguium” or "tina ungeal") and (pie$ or toe$)) or ((fungal or fungus or hongo$ or fungico) and (pie$ or toe$)) or ((ringworm or tina) and (pie$ or toe$))
These terms were combined with the Controlled clinical trials topic‐specific query filter.
Appendix 7. Search stategies for trials registers
Searches done on 22‐05‐2016.
The metaRegister of Controlled Trials www.controlled‐trials.com/
1) onychomycosis or "fungal toenail" or "toenail fungus" or" nail fungus" or "fungal nail"
2 results: not relevant/topical treatment
The US National Institutes of Health Ongoing Trials Register
1. onychomycoses OR onychomycosis OR toenail fungus OR toenail mycosis | Interventional Studies | Phase 3, 4
96 results: three already included (Maddin 2013; Sigurgeirsson 2013; Elewski 2012), others not relevant (topical treatments, experimental unregistered drugs)
The Australian New Zealand Clinical Trials Registry www.anzctr.org.au/
1. "fungal" 2. "onychomycosis" 3. "nail" and "fungal" In category "drug treatment"
Five results, nil relevant
The World Health Organization International Clinical Trials Registry platform apps.who.int/trialsearch/default.aspx
1) onychomycosis or "fungal toenail" or "toenail fungus" or" nail fungus" or "fungal nail"
126 results, two already included (Maddin 2013; Sigurgeirsson 2013), others not relevant (topical treatments, experimental unregistered drugs)
The EU Clinical Trials Register www.clinicaltrialsregister.eu
1. onychomycosis or fungal toenail or toenail fungus or nail fungus or fungal nail
61 results, one study already included (Sigurgeirsson 2013), others not relevant (topical treatments, experimental unregistered drugs)
Data and analyses
Comparison 1. Azole versus terbinafine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 15 | 2168 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.72, 0.95] |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 6 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.96] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 9 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.00] |
| 2 Mycological cure | 17 | 2544 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.68, 0.88] |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 8 | 1287 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.64, 0.93] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 9 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.64, 0.95] |
| 3 Adverse events | 9 | 1762 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.86, 1.17] |
| 4 Recurrence rate | 5 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.68, 1.79] |
Comparison 2. Terbinafine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 8 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 6.00 [3.96, 9.08] |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 6 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 5.60 [3.66, 8.55] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 26.01 [3.69, 183.44] |
| 2 Mycological cure | 8 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 4.53 [2.47, 8.33] |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 6 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 4.60 [2.26, 9.36] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 7.79 [0.42, 144.44] |
| 3 Adverse events | 4 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.87, 1.47] |
| 4 Recurrence rate | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.38] |
Comparison 3. Azole versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 9 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 22.18 [12.63, 38.95] |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 7 | 2695 | Risk Ratio (M‐H, Random, 95% CI) | 23.89 [11.99, 47.64] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 2 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 19.11 [7.21, 50.65] |
| 2 Mycological cure | 9 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 5.86 [3.23, 10.62] |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 7 | 2695 | Risk Ratio (M‐H, Random, 95% CI) | 7.05 [2.91, 17.07] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 2 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 4.22 [2.34, 7.59] |
| 3 Adverse events | 9 | 3441 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.97, 1.12] |
| 4 Recurrence rate | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.29, 1.07] |
Comparison 4. Griseofulvin versus azole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 5 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.45, 1.96] |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.32, 2.45] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 3 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.34, 2.93] |
| 2 Mycological cure | 5 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.50, 1.51] |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.52, 1.76] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 3 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.16, 2.10] |
| 3 Adverse events | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.56, 3.73] |
| 4 Recurrence rate | 1 | 7 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.26, 61.76] |
Comparison 5. Griseofulvin versus terbinafine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 4 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.14, 0.72] |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.39] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 3 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.36, 0.71] |
| 2 Mycological cure | 5 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.46, 0.90] |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 2 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.40, 1.20] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 3 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.21, 1.09] |
| 3 Adverse events | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.15, 3.82] |
Comparison 6. Combination terbinafine plus azole versus terbinafine monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Long‐term follow‐up (> 52 weeks) | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mycological cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Long‐term follow‐up (> 52 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al Rubaie 1997.
| Methods | Design: double‐blind randomised controlled trial | |
| Participants | Number of participants randomised: not stated Number included in analysis: 45 Sex: not stated (both sexes included) Age: not stated Number completing treatment: 2 discontinuations, not clear if included in analyses. Inclusion criteria: toenail or fingernail onychomycosis Type/location/characteristics of infection: toenails and finger nails Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: Dubai Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Clinical cure and mycological cure | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | Abstract only, results not separated for finger or toenails, not included in our quantitative meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[m]ulticentre, randomised, double‐blind study" Comment: no information on random sequence generation provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[m]ulticentre, randomised, double‐blind study" Comment: no information on allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "250 mg of terbinafine was administered daily for 16 weeks, followed by 8 weeks of placebo. Griseofulvin in a dose of 1000 mg daily was administered for 24 weeks." Comment: extension of terbinafine treatment with duration with 8 weeks of placebo implied an attempt at making treatments seem identical to participants. However, no information is provided regarding blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[t]he overall clinical and mycological evaluation of week 48 …" Comment: no specific information regarding blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "45 patients were analysed" Comment: no info on number of participants that were initially randomised or if discontinuations are included in the analyses |
| Selective reporting (reporting bias) | Low risk | Quote: "[s]uccess was defined as a mycological cure (i.e. negative KOH preparation and culture) and the absence of clinical symptoms (paronychial inflammation, hyperkeratosis, onychomycosis" Comment: all results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Arca 2002.
| Methods | Design: open‐label randomised controlled trial | |
| Participants | Number of participants randomised: 50 Number included in analysis: 50 Number completing treatment: 50 Sex (M/F): 24/26 Age: 16‐67 years, mean age 43 years Inclusion criteria: distal subungual toenail onychomycosis Type/location/characteristics of infection: distal subungual toenail onychomycosis Duration of infection: not stated Exclusion criteria: pregnancy or breastfeeding; psoriasis; severe liver/renal/endocrinologic impairment; concomitant therapy with rifampin, phenytoin, digoxin, oral anticoagulants and cyclosporine; hypersensitivity to azoles and terbinafine Washout period: 4 months for oral and 4 weeks for topical antifungals Setting: Turkey Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 6 months post‐treatment (9 months total) Outcomes measured: clinical outcome (on a 6‐category scale ranging from deterioration to complete improvement); mycological cure (KOH preparation and culture negative) Safety and tolerability assessed by: drug tolerability assessed every 4 weeks during 3 month |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned" Comment: method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned" Comment: method of allocation concealment not stated, unclear if any selection bias in allocating patients to particular treatments. No evidence to suggest that a robust method was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[a] comparative, open, prospective study" Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[a] comparative, open, prospective study" Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For all 50 patients that were randomised outcome data are available |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
Arenas 1991.
| Methods | Design: open‐label RCT | |
| Participants | Number of participants randomised: unclear, initially stated 90, but participants that did not show a response or did not attend appointments where eliminated from the studies and replaced Number included in analysis: 83 Sex: not stated but M/F ratio 3:1 Mean age: 40.5 years (range 20‐60) Number completing treatment: unclear, see above Inclusion criteria: onychomycosis in the first toe, diagnosed by clinical examination or laboratory findings Type/location/characteristics of infection: first toe only Duration of infection: not stated Exclusion criteria: pregnancy or breastfeeding, treatment in the last month prior to the study Washout period: 1 month Setting: hospital dermatology department in Mexico Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Monthly millimetric measurements of the nail | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | Not included in the meta‐analysis due to methodological issues, namely the exclusion and replacement of participants that did not respond to treatment; total number of participants unclear, we have written to the author, but he has not been able to provide the needed data yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomly selected to enter the study and were divided into two groups of 45 patients each." "Patients were assigned a number according to the order in which they came to the clinic and were randomly included in any of the following groups." Comment: no evidence provided of any method of random sequence generation |
| Allocation concealment (selection bias) | High risk | Quote: "[p]atients were assigned a number according to the order in which they came to the clinic and were randomly included in any of the following groups." Comment: given that order of presentation to clinic was used to assign a number and it was an open‐label study, there is no suggestion of any allocation concealment being done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[a] comparative, open, prospective, longitudinal study" Comment: open‐label study, no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[a] comparative, open, prospective, longitudinal study with a blind evaluator was undertaken" Comment: no method of blinding the outcome assessor was detailed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotes: "[p]atients were eliminated from the study if they did not keep up their appointments, became pregnant during the study, did not show improvement of clinical symptoms or mycosis after 3 months of treatment, or reported side effects." "Side effects were recorded and most of the patients who were eliminated from the study were substituted with other patients." "In subgroup I/U (14 patients) one patient dropped out and in subgroup I/P (12 patients) one patient dropped out and two patients were eliminated; and in subgroup G/Is (13 patients) two patients experienced side effects and in subgroup G/U (14 patients) one patient experienced side effects. In subgroups I/Is and G/P all patients finished treatment." Comment: participants that did not respond to treatment were replaced. risk of incomplete outcome data given the replacement of participants who were eliminated or dropped out. 7 of an original 90 patients were not included in results. |
| Selective reporting (reporting bias) | Low risk | Quote: "[m]ethods: monthly millimetrical measurements of the nail were taken according to the Zaias method." Comment: results described 'cure' rates. No mention of millimetrical measurements in Results. All outcome measures presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other sources of bias seen |
Arenas 1995.
| Methods | Design: open‐label comparative randomised trial | |
| Participants | Number of participants randomised: 53 Sex: not stated (both sexes included) Mean age: 39 years Number included in analysis: 43 Number completing treatment: 40 Inclusion criteria: age > 18 years, onychomycosis Type/location/characteristics of infection: distal or lateral subungual onychomycosis diagnosed by physical examination, KOH smear and culture Duration of infection: 3 months to 26 years Exclusion criteria: abnormal haematology, blood chemistries, urinalysis, liver tests; onychomycosis caused by resistant fungi; pregnancy or lactation; treatment for gastric hyperacidity; psoriasis, other serious conditions. Withdrawal criteria: serious adverse effects, development of severe disease not associated with the treatment, non‐cooperative participants, voluntary withdrawal Washout period: 3 months for antimycotic treatment Setting: hospital dermatology department in Mexico Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 9 months Outcomes measured: % of nail involvement, nail abnormalities, nail changes, nail growth, participant's evaluation of treatment, doctor's evaluation of treatment (cure vs improvement) Safety and tolerability assessed by: reporting of adverse events, LFTs |
|
| Source of funding | Janssen Pharmaceutical assisted in data management and statistical analyses | |
| Conflict of interest | Clear disclosure of pharmaceutical industry involvement. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned to one of the two treatment groups." Comment; method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[c]omparative, open, and prospective study" Comment: participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[c]omparative, open and prospective study" Comment: no mention that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[i]n the itraconazole treatment group 3 patients dropped out for unknown reasons and 1 patient withdrew because of headache; 23 [of 27 randomised] finished the follow‐up period." "In the terbinafine treatment group 7 patients dropped out for unknown reasons and 2 patients because of … adverse events; 17 patients [of 26 randomised] finished the follow‐up period." Comment: all participants that entered the study are accounted for. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Baran 1995.
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 143 Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: 120 (141 for tolerability) Number completing treatment: not stated Inclusion criteria: age > 18 years, onychomycosis of feet alone or of the feet and hands, use of contraception in women of childbearing age Type/location/characteristics of infection: toenail onychomycosis, confirmed Duration of infection: 0‐71 years Exclusion criteria: pregnancy, breastfeeding Washout period: 1 month for topical or systemic therapy Setting: multicentre Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 2 and 6 months post‐treatment cessation Outcomes measured: complete cure (clinical disappearance of pathological zone of nail + mycological cure), concentration of terbinafine in toenail Safety and tolerability assessed by: basic labs, side effects |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]his study was a multicenter double‐blind study with two parallel groups" Comment: method of sequence generation not stated. Groups similar for age, sex, duration of disease, nails affected and pathogenic agent |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]his study was a multicenter double‐blind study with two parallel groups" Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[t]his study was a multicenter double‐blind study with two parallel groups" "The dosages were 250 mg/day for terbinafine and 1 g/day for griseofulvin. The duration of treatment in both groups was up to the longest recommended for griseofulvin i.e. a maximum of 12 months." Comment: study states that it is blinded, no mention of method, unclear if treatments appeared identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: mycological and clinical assessment occurred "1 month after the start of treatment, then every 2 months". Comment: blinded, but no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "[o]f a total of 143 patient recruited by 15 centres, 120 were assessed for effectiveness and 141 for tolerability. Two patients never took the treatment, 13 did not return after inclusion and 8 recorded negative mycology on inclusion." "As big toenails are the most difficult to cure, we calculated their data separately." Comment: adequate explanation of dropouts. However, results reported number of toenails cured, not number of participants cured. Stated what the ITT numbers of participants were, but did not state the number of participants cured |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Billstein 1999.
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 109 Sex: 73% male Mean age: 42.3‐46.9 years in the different treatment groups (range 18‐75) Number included in analysis: 71 Number completing treatment: not stated Inclusion criteria: age 18‐75 years Type/location/characteristics of infection: dermatophyte infection of the toenail Duration of infection: average in groups 94.2‐137.8 weeks Exclusion criteria: white superficial toenail onychomycosis, immunosuppression or immunodeficiency, hepatic enzymes > 1.5 times the upper limit of normal, marked lab abnormalities Washout period: 3 months for systemic, 1 month for topical Setting: USA multicentre Ethnicity: black participants Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 72 weeks Outcomes measured: mycologic efficacy, clinical cure (0% involvement of target toenail) Safety and tolerability assessed by: not stated |
|
| Source of funding | Novartis Pharmaceuticals Corporation | |
| Conflict of interest | Clear disclosure of pharmaceutical industry involvement (Novartis). No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised into four treatment groups" Comment: method of random sequence generation not stated. States groups have no statistically significant differences in age, sex, percentage of target toenails infected, duration of current episode of onychomycosis. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomised into four treatment groups" Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Both patient and physician were blinded to the treatment for the 24‐week treatment period. Blinding was maintained through the end of the study." Comment: study is blinded, but no method stated for how terbinafine tablets and placebo tablets were made indistinguishable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[a]ssessments were done at … weeks 4, 8, 12, and 24 during the treatment period. Follow‐up assessments were scheduled for weeks 30, 36, 42, 48, 60, and 72." Comment: Methods note that physicians were blinded for the 24‐week study period, but there is no mention that outcome assessor blinding was maintained for the follow‐up period. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[t]he efficacy analysis included all patient who were randomised to therapy, fulfilled the mycologic criteria at screening, took at least one dose of the study drug, and had at least one post‐baseline assessment." Comment: numbers of participants screened and included in the study are shown. Enough information provided to conduct an ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Brautigam 1995.
| Methods | Design: double‐blind, parallel group study | |
| Participants | Number of participants randomised: 195 Sex: 64% male Mean age: 49 years Number included in analysis: 170 Number completing treatment: not stated Inclusion criteria: men and women 18 or over Type/location/characteristics of infection: clinical diagnosis of distal subungual or proximal onychomycosis and growth of dermatophytes in a mycological culture up to 12 weeks after start of treatment Duration of infection: not stated Exclusion criteria: pregnant or breastfeeding women, participants with pre‐existing renal, hepatic or gastrointestinal disease, bacterial or yeast infections of the nails or the periungual area, or psoriasis or psoriatic changes of the toenails Washout period: 3 months for systemic, 1 month for topical Setting: Germany multicentre Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 40 weeks Outcomes measured: mycological cure, area and length of the affected nail Safety and tolerability assessed by: number of adverse events, packed cell volume, haemoglobin concentration, erythrocyte and leucocyte counts, erythrocyte indices and concentration of creatinine, cholesterol, triglyceride, gamma glutamyltransferase, glutamic‐oxoacetic transaminase, glutamic‐pyruvic transaminase, alkaline phosphatase, bilirubin and potassium |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[t]he patients were randomly assigned to treatment with either terbinafine or itraconazole according to a computer generated randomisation schedule in the order of obtaining informed consent. A restricted form of randomisation was used to provide blocking over time. All centres received multiple complete blocks of length four, thus the assignments of each treatment were balanced after each block." Comment: random sequence was computer generated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "[t]o keep the treatment double‐blind the patients additionally took a placebo of the comparative drug." Comment: adequate participant blinding likely to have occurred with this method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[a]t each visit nail clippings were taken and sent to a central laboratory", "Clinical response to treatment was monitored by observing the movement of a scratch at the border between infected and normal areas on the patient's most affected nail". Comment: method blinding of outcome assessment not stated for these assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[a]ll the patients except two who did not have any follow up visits were included in the analyses of drug tolerance. All patients who completed the study as planned and all those who stopped treatment because of adverse events or ineffectiveness of treatment were included in the analysis of efficacy. One last result was used in patients who withdrew from the study." Comment: there is no outcome data for 25 of the original 195 participants entered into the study. 11 were excluded due to not following protocol, 3 were excluded due to concomitant disease, 7 due to adverse events. Therefore most attrition is appropriately accounted for. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Cullen 1987.
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 40 Sex: not stated (both sexes included) Mean age: 55.5 years and 51.6 years in the treatment groups (range 28‐80) Number included in analysis: 26 (40 for adverse events) Number completing treatment: 26 Inclusion criteria: toenail onychomycosis Type/location/characteristics of infection: lateral or distal subungual onychomycosis of the toenails positive for fungal elements on KOH and culture Duration of infection: not stated Exclusion criteria: superficial white onychomycosis, systemic mycotic disease, significant systemic illness, pregnancy and lactation, porphyria, hepatic disorders Washout period: 1 month for systemic, 2 weeks for topical Setting: USA Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 6 months Outcomes measured: clinical cure, mycological cure Safety and tolerability assessed by: side effects, monthly blood tests including LFTs and CBC |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were assigned on a randomised schedule in a double‐blind manner to one of two groups." Comment: method of sequence generation not stated. No demographic table provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were assigned on a randomised schedule in a double‐blind manner to one of two groups." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "[o]ne group received ketoconazole in a dosage of 200 mg/day and placebo griseofulvin. The other group was given placebo ketoconazole and active micro size griseofulvin in a dosage of 1 gm/day." Comment: adequate blinding likely to have occurred with this double‐dummy method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[p]atients were examined every four weeks for six months by clinical observation, photography and measurement of involved nails …" Comment: states double‐blinded, but no method of outcome assessor blinding stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[t]wo individuals were lost to follow‐up in the ketoconazole group", "adverse reactions severe enough to necessitate discontinuance of the trial medication occurred in four of the ketoconazole‐treated patients and in eight of the griseofulvin‐treated patients" Comment: high dropout rate, (of 40 randomised patients, 26 completed treatment and 26 were included in efficacy analysis), but all dropouts are explained and accounted for, and all 40 patients were included in adverse events analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
De Backer 1998.
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 372 Sex: not stated (both sexes included) Mean age: not stated, but over 18 years to enter study Number included in analysis: varies for each outcome. Article states there are 372 in ITT analysis. 331 participants in mycology data; 336 participants in clinical data. Number completing treatment: 331 Inclusion criteria: age 18 or older, subungual dermatophyte infection confirmed by direct microscopy and dermatophyte culture Type/location/characteristics of infection: not stated Duration of infection: not stated Exclusion criteria: non‐dermatophytic onychomycosis, pregnancy or breastfeeding, concomitant disease or conditions interfering with absorption from the GI tract, significant kidney or liver disease, hypersensitivity to the antifungals being used, alcohol abuse, radiotherapy, clinically relevant abnormalities in values for creatinine, ALT, AST, GGT, ALP, bilirubin Washout period ‐ 4 months for oral antifungal treatment, 1 month for topical antifungal treatment Setting: Belgium multicentre Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 48 weeks Outcomes measured: mycological and clinical cure for target nail selected at start of study. Overall efficacy, overall tolerability as rated by participants and investigators. Safety and tolerability assessed by: adverse events, LFTs, kidney function tests |
|
| Source of funding | Supported by an educational grant from Novartis Pharmaceuticals Corporation | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding (Novartis). No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[t]he randomization list was computer‐generated in balanced blocks of four" Comment: low risk of selection bias as robust method used for random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "[e]ligible patients received a numbered box containing the study medication." Comment: low risk of selection bias as allocation concealment carried out |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "[a] double‐dummy technique was used: each daily dose was either a 250 mg terbinafine tablet (250 mg/day dose) and placebo capsules or two 100 mg itraconazole capsules (200 mg/day dose) and a placebo tablet. Patients were instructed to take two capsules and one tablet daily after the evening meal for 12 weeks. These instructions were printed on each medication blister pack" Comment: low risk of performance bias as robust double‐dummy method used for blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: states investigators were blinded, but details of method not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "[a] total of 372 patients (186 in each group) with dermatophyte infection confirmed by microscopy and culture were included in the intent‐to‐treat analysis." "At week 48 the percentage of patients with negative microscopy was statistically significantly higher in the terbinafine group than in the itraconazole group (77.9% [127 of 163] vs 55.4% [93 of 168])." Comment: article states there were 372 participants in the ITT analysis but 331 and 336 participants were included in mycological and clinical cure data, respectively. Reasons for non‐inclusions not clear. Number of missing participants similar across treatment groups |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Degreef 1999.
| Methods | Design: randomised double‐blind controlled trial | |
| Participants | Number of participants randomised: 297 Sex: not stated (both sexes included) Mean age: not stated, range 18‐65 years Number included in analysis: ITT population of 292 (146 in each group); 289 were available for efficacy (145 in itraconazole group and 144 in terbinafine group) Number completed treatment: 258 started the follow‐up period Inclusion criteria: age 18‐65 years, microscopically and culturally proven onychomycosis of the toenail Type/location/characteristics of infection: onychomycosis of toenail caused by dermatophyte with no evidence of a superimposed Candida infection, more than 50% of surface of at least 1 nail affected (or if lunula involved, at least 25% of surface of 1 nail affected) Duration of infection: not stated Exclusion criteria: abnormal LFTs, pregnancy/lactation, psoriasis, concurrent use of rifampicin, phenytoin, digoxin, oral anticoagulants or H2‐receptor antagonists, serious disease, previous hypersensitivity to azoles or terbinafine Washout period: 3 months for systemic antifungals, 1 month for topical antifungals Setting: Europe multicentre Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 48 weeks Outcomes measured: mycological cure, clinical evaluation, clinical response Safety and tolerability assessed by: adverse events, blood samples for haematology and biochemistry, LFTs |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients, who gave written informed consent to participate, were randomised to 12 weeks' treatment with itraconazole 200 mg daily or terbinafine 250 mg daily." Comment: method of sequence generation not stated. Demographics ‐ no significant differences between groups |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[r]andomized double‐blind comparison" Comment: study states that it is blinded, but no method stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[r]andomized double‐blind comparison", "secondary efficacy variables were investigator's global clinical evaluation of response to treatment, performed at the end of treatment and at each visit during follow‐up" Comment: study states it is blinded, but no method of assessor blinding stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[a] total of 299 patients were recruited into the trial, of whom 297 were randomised to treatment. The intention‐to‐treat population comprised 292 patients, 146 in each group; 289 patients were considered evaluable for efficacy." "The intention‐to‐treat worse‐case analysis was the primary analysis for safety." Comment: dropouts accounted for (except for the 5 participants in the original 297 randomised who were not included in the 292 ITT population). Low dropout rate between ITT group and "those considered evaluable for efficacy". ITT analysis carried out for some outcomes |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Drake 1997.
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 358 Sex: 77% male Mean age: 45 years Number included in analysis: 238 Number completing treatment: not stated Inclusion criteria: mycological confirmation of onychomycosis and evidence of ability of nail to grow Type/location/characteristics of infection: distal subungual onychomycosis of at least 1 great toenail (if both great toenails affected, more severe was selected for observation and testing) Duration of infection: not stated Exclusion criteria: psoriasis, proximal subungual onychomycosis, superficial white onychomycosis Washout period: 3 months for oral antifungals, 1 month for topical antifungals Setting: multicentre, USA and Canada Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 24 weeks follow‐up without treatment. Those with negative mycology and > 5 mm unaffected nail growth were followed up for an additional 48 weeks Outcomes measured: negative mycology (negative culture and KOH), length of unaffected nail,% nail involvement, participant‐ranked response to therapy (excellent, very good, slight improvement, fair, poor/no effect), recurrence rate Safety and tolerability assessed by ‐ physical exam, vital signs, lab evaluation (haematology, LFTs, bilirubin), reports of adverse events |
|
| Source of funding | Supported by Novartis pharmaceuticals cooperation | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding (Novartis), several authors work for Novartis | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomised" Comment: method of random sequence generation not stated. Baseline characteristics similar for different groups |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[t]he 96‐week study included a double‐blind treatment phase of 24 weeks in which patients were randomly assigned to one of three parallel groups: oral terbinafine for 12 weeks then placebo for 12 weeks, oral terbinafine for 24 weeks, or placebo for 24 weeks." "This phase was followed by 24 weeks of blinded follow‐up without treatment (weeks 24 to 48)" Comment: study states participants were blinded, and all treatment groups were given therapy for the same duration; however it is unclear if placebo and terbinafine were indistinguishable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[m]ycologic and clinical assessments were performed on the 'target' nail" Comment: study states evaluators were blinded, but no detail on method of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "[a] total of 358 patients … were enrolled." "Patients were randomly assigned to one of three parallel groups in a 2:2:1 ratio: oral terbinafine for 12 weeks (N = 142), oral terbinafine for 24 weeks (N = 145), or placebo (N = 71)." "At week 48, 70% of patients treated with terbinafine for 12 weeks and 87% of patients treated for 24 weeks exhibited both negative microscopy and negative culture versus 9% for placebo‐treated patients." No patient numbers provided for these percentages Comment: number of participants reported in outcome data is not always clear 358 participants were randomised (287 into terbinafine groups and 71 into placebo group). The number included in short‐term follow‐up data is unclear as only percentages are reported. For long‐term follow‐up, where actual patient numbers are reported, 238 participants were included in long‐term follow‐up analysis; those with new unaffected nail by week 48 were designated by protocol to be followed up for an additional 48 weeks. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Elewski 1997.
| Methods | Design: multicentre, randomised, double‐blind placebo‐controlled study | |
| Participants | Number of participants randomised: 221 Sex: not stated (both sexes included) Mean age: not stated, range 18‐70 years Number included in analysis: 214 Number completing treatment: not stated Inclusion criteria: 18‐70 years old with onychomycosis of the toenail confirmed by positive results of KOH and positive culture for a dermatophyte; AND at least 25% involvement of nail bed of a great toe. Persons with total dystrophic nail bed disease were also included. Type/location/characteristics of infection: global evaluation of cleared or marked improvement Duration of infection: not stated Exclusion criteria: onychomycosis due to moulds/Candida spp without dermatophyte, psoriasis or history thereof, baseline LFTs > 2 upper limit of normal, H2‐R blockers, antacids, rifampin, phenobarbital, phenytoin, carbamazepine, terfenadine, digoxin, hypersensitivity to azole compounds, investigational drug use within 1 month prior to screening visit, systemic antifungal therapy within 2 months or topical antifungal therapy within 2 weeks before screening visit, participation in a clinical trial for onychomycosis treatment within 6 months before screening visit, pregnant, nursing mothers. If of childbearing potential, were required to use contraception Washout period: 6 months Setting: USA (multicentre) Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 9 months after treatment Outcomes measured: global evaluation based on reduction in extent of nail involvement and improvement in clinical signs as compared to baseline, KOH Ex, culture findings Safety and tolerability: well tolerated, adverse events similar to placebo |
|
| Source of funding | Janssen Research Foundation | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]he study participants were randomly assigned to twelve weeks of treatment with either two 100 mg capsules of itraconazole once a day or matching placebo." Method not stated. Baseline characteristics of groups similar |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he study participants were randomly assigned to twelve weeks of treatment with either two 100 mg capsules of itraconazole once a day or matching placebo." Comment: method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "[t]he study participants were randomly assigned to twelve weeks of treatment with either two 100 mg capsules of itraconazole once a day or matching placebo." Comment: matching placebo method used to blind participants. Likely adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[d]ouble‐blind", "Clinical success was defined as a global evaluation of cleared or markedly improved any time during the trial for the first time." Comment: no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "[t]he primary statistical analysis was for the intent‐to‐treat population, which included all patients who … received at least one dose of double‐blind medication and had at least one visit after baseline." "The proportion of placebo patients who did not complete was significantly greater in the placebo group than that in the itraconazole treatment group: 54 of 104 placebo patients discontinued the study while only 19 of 110 itraconazole‐treated patients discontinued before trial completion." Comment: unexplained discontinuations considerably higher in placebo group than treatment group, but this does not affect our analysis. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All presspecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Elewski 2002.
| Methods | Design: parallel group RCT. | |
| Participants | Number of participants randomised: 42 Sex: 75% male Mean age: 57.3 years and 55.5 years (range 23‐83) Number included in analysis: 39 Number completing treatment: 39 Inclusion criteria: mycological diagnosis (KOH test and culture for dermatophyte) of onychomycosis Type/location/characteristics of infection: distal subungual onychomycosis affecting at least 1 great toenail Duration of infection: at least 1 year Exclusion criteria: not stated Washout period ‐ not stated Setting: USA (multicentre) Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 24 weeks after treatment Outcomes measured: mycology, nail growth measurement, skin tests (TRIPA‐reactivity) Safety and tolerability: not mentioned |
|
| Source of funding | Supported in part by Novartis as an unrestricted, educational grant to the department. "No authors have received personal financial support for this study." | |
| Conflict of interest | "Conflict of interest: [3 authors] are or have been consultants for Novartis". | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]wo of 3 patients in each group (TRIPA‐reactive and TRIPA‐nonractive) were randomised to receive terbinafine, 1 of 3 to receive placebo." Comment: method of random sequence generation not stated. Baseline characteristics of groups similar |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[t]he study design was a double‐blind comparison of the effects of terbinafine with placebo tablets." Comment: states double‐blinded, but no method (e.g. double‐dummy or indistinguishable tablets) stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[m]ycologic assessment, nail growth measurements, and skin tests were repeated at week 12 (end of treatment), week 24, and week 36." Comment: states double‐blinded, but no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Table I. No of patients discontinued (before receiving treatment): 2 in terbinafine group, 1 in placebo group." Comment: number of participants discontinuing in each group is shown; low dropout number. Based on tabulated data, 39 of the 42 randomised participants were included in the analysis. ITT analysis not used |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Elewski 2012.
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 218 Sex: 75% male Mean age: 50.8 years (range 18‐75) Number treated: 216 Number completed: 178 Inclusion criteria: clinical and mycological diagnosis of onychomycosis and evidence of ability of nail to grow Type/location/characteristics of infection: approx 25‐75% of at least 1 great toenail (if both great toenails affected, more severe was selected for observation and testing) Duration of infection: not stated Exclusion criteria: pregnancy, lactation, nail abnormalities other than onychomycosis, liver disease, family history of long QT syndrome, clinically significant condition Washout period: 3 months for oral antifungals, 1 month for topical antifungals Setting: 23 centres in USA Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 24 or 36 weeks (up until 48 weeks after 1st dose) Outcomes measured: complete cure = negative mycology (negative culture and KOH) and clinical cure (0% nail involvement, i.e. absence of onycholysis/subungual hyperkeratosis) Treatment success = negative mycology and < 10% nail involvement Safety and tolerability assessed by: reports of adverse events, ECG, vital signs, physical exam, lab tests (LFTs, haematology) |
|
| Source of funding | This study was funded by Schering‐Plough Corporation, now Merck and Co, Inc, Whitehouse Station, NJ, USA | |
| Conflict of interest | Author conflicts of interest disclosed include employee of Merck and Co | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[p]atients were randomized according to a computer‐generated randomization schedule using a central interactive voice‐ response system." Comment: robust random sequence generation method used |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[p]atients in the 24‐week treatment regimens were blinded with respect to whether they received posaconazole or identical (in terms of taste and appearance) placebo. Patients in the 12‐week posaconazole treatment regimen were blinded to treatment until week 12, when they ended therapy. Patients in the 12‐week terbinafine treatment regimen were aware of their treatment assignment." Comment: not all treatment groups blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[t]his was an investigator‐blinded study. The investigator or qualified designee performed the clinical assessments at each visit and remained blinded to all treatment arms." Comment: no mention of method of blinding, study itself was partially unblinded (see above) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[t]he primary efficacy analysis was based on the modified intent‐to‐treat population (defined as randomised subjects who had a baseline assessment and at least one post baseline assessment available, and who had been exposed to at least one dose of study medication." Comment: low risk of attrition bias as clear subject completion numbers and intent‐to‐treat analysis all provided |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Faergemann 1995.
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 89 Sex (M/F): 65/24 Mean age: 45 years (range 18‐71) Number included in analysis: 84 Number completed treatment: NA because there was an open component to the study after the double‐blinded comparison Inclusion criteria: culture‐proven dermatophyte infection Type/location/characteristics of infection: involving at least 1 of digits 2‐4 Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: Sweden (multicentre) Comorbidities: not stated |
|
| Interventions |
Participants who did not improve after 16 weeks were entered into an open‐label study and given 250 mg/day terbinafine for 16 weeks with the study code still blinded. |
|
| Outcomes | Duration of follow‐up: NA because there was an open component to the study after the double‐blinded comparison. Considered 16 weeks as this is when complete randomisation was lost Outcomes measured: mycological cure, clinical cure Safety and tolerability assessed by: side effects, LFTs |
|
| Source of funding | No information given | |
| Conflict of interest | One author is an employee of the research and development laboratories from Pfizer inc | |
| Notes | There was an open‐label phase after the double‐blinded comparison, but the 'results of treatment: end point analysis' appears to describe the double‐blinded phase only, before randomisation was lost. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]he patients were randomly assigned." Method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he patients were randomly assigned." Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[d]ouble‐blind", "Patients who did not improve after 16 weeks were entered into an open study and given 250 mg/day terbinafine for 16 weeks, with the study code still blinded, and then followed up for 20 weeks." States double‐blinded, but no method of blinding stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[d]ouble‐blind" States double‐blinded, no mention of blinding method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 89 patients were randomised, 84 were included in analysis efficacy analysis and 89 in adverse events analysis. Comment: dropouts are explained, low dropout rate. Enough data given to allow ITT analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Goodfield 1992.
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 112 (99 toenail) Number included in analysis: 85 Sex (M/F): 55/30 Mean age: 44 years (range 19‐78) Number completing treatment: not clear Inclusion criteria: mycological and clinical evidence of dermatophyte infection of fingernails and toenails Type/location/characteristics of infection: toenail and fingernail infections (worst affected nail selected for assessment) Duration of infection: not stated Exclusion criteria: renal/hepatic/GI disease, psoriasis, yeast infection of nails, pregnancy, lactation Washout period: not stated Setting: 8 dermatology centres in UK Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 36 weeks after treatment Outcomes measured: mycological cure = negative microscopy and culture, clinical cure Safety and tolerability assessed by: adverse event reporting, biochemical and haematological variables |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[p]atients … were randomised (with random tables of Fisher and Yates)" Comment: method of random sequence generation adequate to minimise selection bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomised in a double‐blind, placebo controlled parallel group comparison." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[p]atients were randomised in a double‐blind, placebo controlled parallel group comparison." Comment: states double‐blinded, but no method stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[p]atients were seen at monthly intervals throughout the treatment period. At each visit the mycological, biochemical and haematological investigations were repeated; compliance and the occurrence of side effects were ascertained, and the target nail was examined clinically." Comment: no mention of outcome assessor blinding method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[o]ne hundred and twelve patients were enrolled into the study, 99 with toenail infection." Comment: data provided for both ITT analysis and per protocol analysis (Table II) |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Gupta 2000.
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 200 Number included in analysis: 152 Mean age: 45 years Sex (M/F): 117/35 Number completing treatment: 152 Inclusion criteria: onychomycosis confirmed by microscopy and positive culture Type/location/characteristics of infection: 'target' great toenail was the more severely affected, with > 25% involvement of nail surface and < 75% nail plate involvement Duration of infection: not stated Exclusion criteria: hypersensitivity to imidazole derivatives, onychomycosis associated with moulds without dermatophytes or Candida, elevated LFTs, serious illness, psoriasis, taking drugs that interact with itraconazole Washout period: 6 months for oral antifungals, 2 weeks for topical antifungals Setting: Canada, outpatients Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 36 weeks after treatment Outcomes measured: clinical appearance relative to baseline visit (cleared, markedly improved, slightly/moderately improved, unchanged, deterioration), mycological success = KOH and culture both negative Safety and tolerability assessed by: adverse event reports |
|
| Source of funding | This study was supported by a grant from Janssen‐Ortho Inc, Canada | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding (Janssen‐Ortho). No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned to treatment with either two 100‐mg capsules of itraconazole or matching placebo capsules twice daily (i.e. 400 mg daily)." Comment: method of random sequence generation not stated. Baseline characteristics not different between groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned to treatment with either two 100‐mg capsules of itraconazole or matching placebo capsules twice daily (i.e. 400 mg daily)." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "[t]he active and placebo formulations were packaged so that both the patient and the investigator were blinded." Comment: adequate blinding likely occurred using this method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[d]ouble‐blind", "At the end of treatment at week 9 and at weeks 12, 24, 36 and 48, the investigator categorized the disease state of the target toenail relative to the baseline visit" Comment: no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[a] total of 20 and 28 patients were excluded from the itraconazole and placebo groups, respectively, when evaluating efficacy. The reasons were (itraconazole vs placebo groups): culture negative at prescreen (0 vs 3 patients), KOH negative at prescreen (12 vs 17), culture and KOH negative at prescreen (0 vs 2), patient discontinued before week 9 (7 vs 5) and no KOH or culture data available at week 9 (1 vs 1)." Comment: reasons for dropouts explained in the text. Similar number of dropouts between groups. Sufficient data provided to complete ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Gupta 2001a.
| Methods | Design: single‐blind RCT | |
| Participants | Number of participants randomised: 101 Sex (M/F): 52/49 Mean age: 53.1 years; elderly only Number included in analysis: 101 Number completing treatment: 101 Inclusion criteria: onychomycosis caused by a dermatophyte involving at least 1 big toe, in a participant aged > 60 Type/location/characteristics of infection: not stated Duration of infection: not stated Exclusion criteria: history of hypersensitivity or allergic reaction to terbinafine or azoles; intake of any medications known to interact with terbinafine or itraconazole Washout period: not stated Setting:USA and Canada (multicentre) Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 18 months Outcomes measured: clinical evaluation (estimation of the nail plate area involved). Mycologic examination (microscopy, culture). Clinical efficacy (mycologic cure + either clinical cure or reduction of clinically involved nail plate to < 10%). Safety and tolerability assessed by: bloodwork (LFTs, CBC), adverse events reported by participant at each visit (investigator asked to determine potential relationship of the AE to the study drug) |
|
| Source of funding | Study is stated to be non‐industry‐sponsored | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a]ll consenting, consecutive patients were randomly assigned to blocks of 6 to receive terbinafine (continuous) or itraconazole (pulse) therapy." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]erbinafine (continuous) therapy was 250 mg/day administered for 12 weeks. Itraconazole pulse therapy, 200 mg twice daily given for 1 week with 3 weeks off between successive pulses, was administered for 3 pulses. Subjects were asked to take the medications after a meal." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[s]ingle‐blind", "the clinical evaluation was performed in a single‐blinded manner so that the evaluator was not aware of the randomization order or therapy being received by the patient." Comment: high risk of performance bias as participants and personnel likely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[t]he clinical evaluation was performed in a single‐blinded manner so that the evaluator was not aware of the randomisation order or therapy being received by the patient." Comment: likely adequate blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[t]here were a total of 101 patients with 50 receiving terbinafine continuous treatment and 51 patients administered itraconazole (pulse) therapy" Comment: low risk of attrition bias as outcomes of all patients included in analysis, with no exclusions |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Gupta 2001b.
| Methods | Design: single‐blinded RCT | |
| Participants | Number of participants randomised: 59 Sex (M/F): 48/11 Mean age: 68 years Number included in analysis: 59 Number completing treatment: 56 Inclusion criteria: age > 18 years Type/location/characteristics of infection: toenail onychomycosis caused by S brevicaulis spp Distal and lateral onychomycosis, moderate‐severe disease of target nail Duration of infection: not stated Exclusion criteria: allergy to any of the drugs in the study, medications known to interact with study medications, immunocompromise, pregnancy and lactation Washout period: 6 months for oral and 2 weeks for topical Setting: USA and Canada (multicentre) Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 12 months Outcomes measured: clinical cure, mycological cure Safety and tolerability assessed by: side effects, LFTs, CBC, RFTs |
|
| Source of funding | Study is stated to be non‐industry‐sponsored | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[e]ligible patients with onychomycosis due to S. brevicaulis were randomly divided to receive treatment with griseofulvin, ketoconazole, itraconazole, terbinafine and fluconazole". "At baseline, for the 5 treatment groups, there was no significant difference in the mean age of the patients or mean severity of disease". Comment: no method of random sequence generation stated. Baseline characteristics between groups appear similar from the text, but no tabulated data provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[e]ach consecutive patient who fulfilled the inclusion criteria was considered for the study". Comment: no method of allocation concealment stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[s]ingle‐blinded", "Treatment with the oral agents was given as follows: griseofulvin 600 mg twice daily for 12 months, ketoconazole 200 mg daily for 4 months, itraconazole 3 pulses with each pulse consisting of 200 mg twice daily for 1 week on, 3 weeks off, terbinafine 250 mg daily for 12 weeks, and fluconazole 150 mg/day for 12 weeks." Comment: study states that it is single‐blinded, but does not state any methods for blinding participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[s]ingle‐blinded", "When the patients were seen at the follow‐up, at month 12 from the start of treatment, the efficacy parameters were CC (clinical cure) and MC (mycological cure)". Comment: study states that it is single‐blinded, but does not state any methods for blinding outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[i]n this study a total of 59 patients with S. brevicaulis onychomycosis of the toes were evaluated". "Patients were randomised to the following groups: griseofulvin (11 patients), ketoconazole (12 patients), itraconazole (12 patients), terbinafine (12 patients) and fluconazole (12 patients)." Comment: low risk of attrition bias as outcomes for all randomised participants reported, with no exclusions |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Gupta 2001c.
| Methods | Design: single‐blind, randomised, prospective study | |
| Participants | Number of participants randomised: 190 (IIT: 93, TTT: 97). 14 participants who did not meet inclusion/exclusion criteria did not commence the study ‐ 176 started treatment (IIT: 81, TTT: 95) Number included in analysis: 165 (IIT: 75, TTT: 90) Number completing treatment: 165 (IIT: 75, TTT: 90) Sex (M/F): IIT 33/42, TTT 60/30 Mean age: 35.6 years (range 25‐53) Inclusion criteria: at least 18 years old, clinical diagnosis of distal and lateral onychomycosis of the toes. Dermatophyte had to be aetiologic organism Type/location/characteristics of infection: distal and lateral onychomycosis of the toes Duration of infection: not stated Exclusion criteria: those who had received oral antifungal therapy within the previous 3 months or applied topical antifungal to the feet during the previous 1 month, proximal subungual or white superficial onychomycosis, onychomycosis caused by Candida or nondermatophyte moulds, participants taking medications known to interact with itraconazole or terbinafine, individuals with concomitant nail disease such as psoriasis or lichen planus. Prengnancy, lactation, inadequate contraception, history of renal disease, participants with baseline liver function tests (ALT, AST, alkaline phosphatase, total bilirubin) elevated to more than twice the upper limit of normal Washout period: not stated Setting: 3 outpatient dermatology offices in North America Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 72 weeks Outcomes measured: mycological cure rate (negative light microscopy and culture), clinical cure (nail plate appeared completely normal), effective therapy (mycological cure and outgrowth of at least 5mm new clinically unaffected nail plate) and complete cure (mycological and clinical cure simultaneously), recurrence rate Safety and tolerability assessed by: adverse effect reporting; liver enzymes changes are stated but whether they were tested for routinely is unclear |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[r]andomised", "Patients were assigned to one arm of the study or the other in balanced blocks of 6 at each centre". "The baseline characteristics of age, race, duration of onychomycosis, causative organism, and percentage of toenail involved were similar, with no statistically significant difference between the 2 treatment groups". Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he nature of the treatment was discussed with each patients, and informed consent was obtained". Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[t]he study was single‐blinded with the evaluator of the primary and secondary outcome measures not being aware of the randomization order or the treatment being administered to the patient". Comment: high risk of performance bias as participants and personnel likely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[t]he study was single‐blinded with the evaluator of the primary and secondary outcome measures not being aware of the randomization order or the treatment being administered to the patient". Comment: likely adequate blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[a] total of 190 patients were found to have dermatophyte toe onychomycosis after initial screening for the study and were randomised (IIT: 93, TTT: 97). Fourteen patients were found to violate inclusion/exclusion criteria or decided not to start therapy after randomization but before start of treatment. The number of patients who received intervention therapy were IIT 81 and TTT 95. At the end of week 72, there were 75 patients in the IIT group who were regarded as having completed the study with 6 withdrawals. In the TTT group, the corresponding numbers were 90 and 5 patients, respectively." Comment: low number of exclusions with reasons for exclusion stated in the text. Enough data provided to perform an ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Gupta 2005.
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 151 Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: 135 Number completing treatment: 135 Inclusion criteria: onychomycosis confirmed by direct microscopy and/or fungal culture Type/location/characteristics of infection: distal subungual onychomycosis of 1 great toenail (min area 25%), and at least 2 mm proximal nail clear Duration of infection: not stated Exclusion criteria: conditions known to produce abnormal‐appearing nails (psoriasis), proximal subungual onychomycosis, white superficial onychomycosis, allergy to azole drugs, use of drugs that prolong QT interval, abnormal LFTs Washout period: 3 months for oral antifungals, 2 weeks for topical antifungals Setting: 10 dermatology practices in USA, Canada, France Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 36 weeks after treatment Outcomes measured: effective cure (mycological and clinical cure or > 30% improvement), percentage of nail plate infected, length of unaffected nail, mycological examination of cultures, concentrations of ravuconazole in plasma and in toenails Safety and tolerability assessed by: adverse event reports, haematology, serum chemistry, urinalysis |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[u]pon subject enrolment, subject number and treatment assignment were obtained from a central call‐in randomization system". "Age, sex and race distribution was similar between the four treatment groups." Comment: likely robust randomisation using this method. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Low risk | Quote: "[u]pon subject enrolment, subject number and treatment assignment were obtained from a central call‐in randomization system". Comment: allocation concealment likely achieved using the method above, as patients very unlikely unable to preempt treatment allocation prior to study enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "[m]edication was administered in a double‐dummy fashion. Placebo tablets were identical to the treatment drug tablets. Neither subject nor evaluating physician was aware of which treatment group the subject had been assigned to." Comment: blinding of participants and personnel likely adequate using this double‐dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[n]either subject nor evaluating physician was unaware of which treatment group the subject had been assigned to". Comment: outcome assessment likely blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[o]ne hundred and fifty‐one subjects were randomised by 10 investigators in three countries … Of these 151 subjects, 148 received at least one dose of the study medication, and 135 were evaluable at the end of the study." "Of the 16 subjects who were randomised to treatment but were not evaluable at the test of cure visit, three were not treated because of withdrawal of consent or inability to comply with protocol requirements. Of the 13 treated subjects who were randomised to treatment but not evaluable at the test of cure visit, three had no test of cure evaluation performed, and 10 subjects did not complete the study". Comment: low number of dropouts, with reasons for exclusion explained Sufficient data provided to complete ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Gupta 2006.
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 70 Sex: not stated (both sexes included) Mean age: not stated Number treated: 70 Number completed: 64 Inclusion criteria: clinical and mycological diagnosis of onychomycosis with type I or type II diabetes Type/location/characteristics of infection: dermatophyte infection of at least 1 great toenail with 10% or more involvement Duration of infection: not stated Exclusion criteria: pregnancy, breastfeeding, malignancy other than basal cell carcinoma or squamous cell carcinoma, abnormal liver function tests, uncontrolled renal/hepatic disease, immunosuppressant treatment Washout period: 12 months for oral antifungals, 4 weeks for topical antifungals Setting: USA (2 private practices) Comorbidities: type 1 or type 2 diabetes |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 48 weeks Outcomes measured: comple/e cure = negative mycology (negative culture and KOH) and effective cure (0% nail involvement, i.e. absence of onycholysis/subungual hyperkeratosis). Treatment success = negative mycology and <10% nail involvement Safety and tolerability assessed by: reports of adverse events and LFTs |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[p]atients meeting inclusion/exclusion criteria with dermatophyte onychomycosis of the target great toenails were allocated through computer‐generated block randomization in blocks of 10 in a ratio of 1:1 to one of the two treatment groups". Comment: robust randomisation likely achieved with the above method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[r]andomization was concealed and performed by someone other than the investigators assessing the outcome measures." Comment: no method of allocation concealment stated and unclear whether allocation was concealed from participants as well as investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[s]ingle‐blind", "evaluator‐blind", "Randomisation was concealed and performed by someone other than the investigators assessing the outcome measures. During the treatment period, the designated evaluators remained blinded." Comment: high risk of performance bias as participants likely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[d]uring the treatment period, the designated evaluators remained blinded." Comment: outcome assessor blinding likely adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[s]eventy patients were enrolled; this was the intention‐to‐treat population. Six patients withdrew consent, one from the itraconazole and five from the terbinafine treatment arms. The patient from the itraconazole group withdrew because of gastric side effects. The remaining five patients withdrew for reasons unrelated to the study medication". "Missing data was entered with the last observation carried forward method". "All subjects who received at least one dose of the treatment medication were included in the analysis." Comment: small number of dropouts, with reasons for exclusion detailed in the text. Anlysis was performed using the last observation carried forward method. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Gupta 2009.
| Methods | Design: randomised, evaluator‐blind, comparator‐controlled trial | |
| Participants | Number of participants randomised: 142 Sex: not stated (both sexes included) Mean age: 51 years (range 23‐98) Number treated: 142 Number completed: 105 Inclusion criteria: clinical and mycological (positive potassium hydroxide (KOH] and culture) diagnosis of a dermatophyte infection Type/location/characteristics of infection: dermatophyte infection of at least 1 great toenail with 20% or more involvement Duration of infection: not stated Exclusion criteria: pregnancy, breastfeeding, malignancy other than basal cell carcinoma or squamous cell carcinoma, abnormal liver function tests, uncontrolled renal/hepatic disease, immunosuppressant treatment Washout period: 12 months for oral antifungals, 4 weeks for topical antifungals Setting: Canada (2 outpatient clinics) Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 72 weeks Outcomes measured: mycological cure rates (negative KOH and culture) and effective cure rate (simultaneous mycological cure and ≤ 10% nail plate involvement) Safety and tolerability assessed by: reports of adverse events and LFTs |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[d]ata from a Canadian study of continuous terbinafine and intermittent itraconazole was compared to an intermittent terbinafine regimen using a similar protocol to the randomised study." "Patients attending one of two Southwestern Ontario (Canada) dermatology clinics who met the treatment criteria were provided with an intermittent terbinafine regimen (TOT)". Comment: patients not randomised as all patients received the same treatment. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[d]ata from a Canadian study of continuous terbinafine and intermittent itraconazole was compared to an intermittent terbinafine regimen using a similar protocol to the randomised study." "Patients attending one of two Southwestern Ontario (Canada) dermatology clinics who met the treatment criteria were provided with an intermittent terbinafine regimen (TOT)". Comment: all patients received the same treatment, so allocation concealment likely not performed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[t]he study medications were obtained by subjects from a local pharmacy by prescription and self‐administered." Comment: high risk of performance bias as participants unlikely to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[a]ll nail samples were processed by a local mycology laboratory with the laboratory staff blinded to the treatment arm and the treatment time‐point." Comment: outcome assessment blinding likely adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[i]n the TOT group, one patient did not continue follow‐up to week 48 (reason unknown). Another patient completed treatment despite an AE of restlessness, but was then lost to follow‐up. An additional 12 patients were lost to follow‐up between weeks 48 and 72 for reasons related to therapy." Comment: reasons for dropouts explained. Sufficient data to perform ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other clear bias seen |
Havu 2000.
| Methods | Design: RCT | |
| Participants | Number of participants randomised: 137 Sex (M/F): 60/77 Mean age: 48.8 years Number included in analysis: 137 Number completing treatment: 130 Inclusion criteria: age 18‐65 years, clinical diagnosis of onychomycosis affecting at least ⅓ of a nail, confirmed by positive potassium hydroxide (KOH) wet mount and culture for a dermatophyte Type/location/characteristics of infection: affecting at least ⅓ of the fingernail or toenail Duration of infection: not stated Exclusion criteria: systemic antifungal therapy within the previous 6 months, topical antifungal therapy within the previous month, pregnancy, lactation, any concomitant disease that could affect study outcome, participants on medications known to interact with either study agent, history of allergy to terbinafine or azole drug, discontinuation of previous therapy with terbinafine or an azole drug due to adverse effects Washout period: not stated Setting: Finland; 6 centres Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 60 weeks Outcomes measured: primary efficacy endpoint was mycological cure (negative direct microscopy of KOH wet mount and negative culture for a dermatophyte), clinical evaluation based on 4‐point rating scare (complete cure, minimal symptoms, slight improvement or failure) Safety and tolerability assessed by: reporting of adverse events, physical examination, tolerability of treatment rated on a 5‐point scale (very good, good, moderate, poor, or very poor) by both participant and investigator |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomly divided into three groups to receive active treatment with either terbinafine 250 mg daily for 12 weeks, fluconazole 150 mg once weekly for 12 weeks, or fluconazole 150 mg once weekly for 24 weeks". "Patients in all three treatment groups were well matched for age, duration of infection and species of dermatophyte." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomly divided into three groups to receive active treatment with either terbinafine 250 mg daily for 12 weeks, fluconazole 150 mg once weekly for 12 weeks, or fluconazole 150 mg once weekly for 24 weeks." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "[d]ouble‐blind, double‐placebo", "To maintain blinding, patients in group A also received placebo capsules once weekly for weeks 1‐24; patients in group B also received a placebo tablet daily for weeks 1‐12 and a placebo capsule once weekly for weeks 13‐24; patients in group C also received a placebo tablet daily for weeks 1‐12." Comment: likely adequate blinding of participants achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[d]ouble‐blind". "Mycological evaluation comprised of direct microscopy of a KOH wet mount and culture for a dermatophyte." "Clinical outcome was evaluated separately by the patient and physician." Comment: study states that it is double‐blind; no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[a] total of 137 patients with mycologically confirmed toenail or fingernail onychomycosis were included in the study. Of these, 130 were evaluable at the 60‐week follow‐up visit." "Seven patients withdrew from the study. Of these, one withdrew due to taste disturbance with terbinafine; one for constipation and depression; one for raised serum alanine aminotransferase level; and one for nausea and diarrhoea. In addition, one subject from each group was withdrawn because of protocol violations." Comment: low number of dropouts, with reasons for exclusion explained. Sufficient data for ITT analysis provided |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Hay 1985.
| Methods | Design: double‐blind study | |
| Participants | Number of participants randomised: 90 Number included in analysis: 74 Sex (M/F): 53/21 Mean age: not stated Number completing treatment: 64 Inclusion criteria: dermatophyte infection, no further criteria given Type/location/characteristics of infection: any location including 20 infected toenails Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: UK Comorbidities: not stated |
|
| Interventions |
Both doses were doubled if not sufficient effect after 3 months of treatment |
|
| Outcomes | Cure, no further info available | |
| Source of funding | Janssen Pharmaceutical Ltd, UK, supplied the medication ketoconazole | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | This study is not included in the quantitative meta‐analysis as there are a number of different dermatophyte infections included, and extraction on participant level for onychomycosis only is not possible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]here were no significant differences in the age, sex, weight or height distribution of either group." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[a] total of 90 patients entered the study. Of these, 16 subsequently failed to attend and so results of treatment in 74 were available for assessment. Thirty‐seven patients were receiving ketoconazole or griseofulvin respectively." Comment: no method of allocation concealment stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[t]he merits of oral ketoconazole and griseofulvin in dermatophytosis have been compared in a double‐blind study on 74 patients with 152 infected sites." Comment: study title states that it is double‐blind, but no method of blinding of participants and personnel described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[d]ouble‐blind". "Patients' symptoms and clinical improvement were analysed using the Wilcoxon test to compare successive time points for each treatment and using the Mann‐Whitney U‐test to compare results of treatments at each visit." Comment: study claims to be double‐blind, no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[a] total of 90 patients entered the study. Of these 16 subsequently failed to attend and so results of treatment in 74 were available for assessment". "Ten patients dropped out of the trial during the study period, seven in the ketoconazole and three in the griseofulvin group. In only one case was this due to a side effect, diarrhoea, attributed to ketoconazole. A further patient taking ketoconazole needed cimetidine for a duodenal ulcer and antifungal therapy was withdrawn." Comment: of the 16 patients who were enrolled but excluded from analysis, 10 were accounted for. There is low risk of attrition bias as the majority of attrition is explained. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Hofmann 1995.
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 195 Number treated: 195 Sex: 56% male Mean age: 50 years (range 19‐93) Number completed: 171 (24 lost due to protocol violations and no show at follow‐up visits) Inclusion criteria: distal subungual onychomycosis confirmed by culture and wet mount Duration of infection: not described Exclusion criteria: < 18 years, pregnant, breastfeeding, preexisting renal, hepatic or gastrointestinal disease, psoriasis or psoriatic nail changes, bacterial or yeast infections nail Washout period: 3 months oral medication and 1 month topical medication Setting: 22 centres in Germany Comorbidities: no |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up:48 weeks and 72 weeks Outcomes measured: negative culture, growth of healthy nail, nail score Safety and tolerability assessed by: adverse event monitoring, transaminase monitoring |
|
| Source of funding | This study was supported in part by Sandoz AG, Nurnberg, Germany | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a] total of 195 patients, from 22 centres, were included in the study and were randomised to receive either 250 mg/d of terbinafine (N = 97) or 1000 mg/d of micronized griseofulvin (N = 98)." "Patients' characteristics were comparable for both treatment groups." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomised and assigned to one of two treatment groups". Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[t]he study consisted of a 48‐week double‐blind treatment phase and a 24‐week double‐blind follow‐up phase." Comment: study claims that it is double‐blind; no method of participant or personnel blinding stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[d]ouble‐blind". "Examination for fungi included identification by microscopic evaluation of potassium hydroxide preparation and mycological culture. The clinical response to treatment was monitored by observance of the outgrowth of a scratch mark placed at the border between infected and normal area on each patient's most involved nail, excluding that of the little toe." Comment: study claims that it is double‐blind, no method of outcome assessment blinding stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[a] total of 195 patients, from 22 centres, were included in the study and were randomised … Fourteen patients in the terbinafine group and 10 patients in the griseofulvin group were excluded from the evaluation of drug efficacy, mainly because of protocol violations in terms of intake of study drugs or non‐appearance for control visits." Comment: small number of dropouts with all exclusions accounted for |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Honeyman 1997.
| Methods | Design: double‐blind parallel group RCT | |
| Participants | Number of participants randomised: 179 Sex: not stated (both sexes included) Mean age: 40.4 years Number treated: 174 Number completed: 135 Inclusion criteria: clinical and mycological diagnosis of onychomycosis Type/location/characteristics of infection: toe with distal subungual onychomycosis Duration of infection: not stated Exclusion criteria: pregnancy, breastfeeding, systemic diseases/conditions that might affect the study and therapy with concomitant drugs that might interfere with the metabolism of the drugs being studied Washout period: 3 months for oral antifungals, 1 month for topical antifungals Setting: South America (multicentre) Comorbidities: none |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 52 weeks Outcomes measured: efficacy = negative mycology (negative culture and KOH) and clinical cure (level of onycholysis / subungual hyperkeratosis/paronychial inflammation). Treatment success = effectively cured participant (> 50% improvement) + mycological cure Safety and tolerability assessed by: reports of adverse events, LFTs, haematology |
|
| Source of funding | The study drugs were supplied by Sandoz Basle | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]wo randomised groups of patients with toe distal sub‐ungual onychomycosis received ..." Comment: study claims to be randomised, no method of random sequence generation stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]wo randomised groups of patients with toe distal sub‐ungual onychomycosis received either one tablet (250 mg) of terbinafine pulse itraconazole placebo or two tablets (100 mg each) of itraconazole plus terbinafine placebo, once a day for 4 months." Comment: unclear whether patients or personnel could anticipate patient allocation prior to enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "[t]wo randomised groups of patients with toe distal sub‐ungual onychomycosis received either one tablet (250 mg) of terbinafine pulse itraconazole placebo or two tablets (100 mg each) of itraconazole plus terbinafine placebo, once a day for 4 months." Comment: likely adequate blinding of participants and personnel achieved using this double‐dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[c]linical assessments at entry and during the visits scheduled in the treatment and post‐treatment phases were performed for onycholysis, hyperkeratosis and paronychial inflammation." "Mycological evaluation … was performed at entry and after the 2nd, 4th, 6th, 8th, 10th and 12th months." Comment: no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "from 179 recruited patients … 6 were excluded from the efficacy analysis as they were only examined at the entry visit and dropped from the study. In the itraconazole group, 2 patients discontinued for unknown reasons. In the terbinafine group, 1 patient discontinued as the nail fell off. Thirty‐nine patients … did not complete the study and were excluded from the final analysis of efficacy at the 12th month. The reasons were protocol violation (18 patients), loss of follow‐up after the 4th month (17 patients) and side effects before the 4th month (4 patients, all in the itraconazole group." Comment: of the initial 179 patients, there were 39 dropouts. These exclusions were accounted for, and most (175 patients) were evaluated for adverse effects. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Jones 1996.
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 73 Number included in analysis: 68 Sex (M/F): 50/18 Mean age: 48 years (range 18‐70) Number completing treatment: not stated. 68 had data beyond the baseline visit Inclusion criteria: T rubrum‐positive onychomycosis of the great toenail. Type/location/characteristics of infection: positive onychomycosis of the great toenail Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: USA Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 12 weeks, then monitoring for relapse Outcomes measured: healthy nail growth,% of nail area involved, signs of onychomycosis, investigator's global evaluation, mycological evaluation Safety and tolerability assessed by: LFTs, urinalysis, urine pregnancy tests |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]hirty‐six of the patients were randomised to receive itraconazole 200 mg daily for 12 weeks; and 37 patients received placebo." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned to treatment with 100‐mg capsules once daily of itraconazole or placebo". Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[d]ouble‐blind". "Patients were randomly assigned to treatment with 100‐mg capsules once daily of itraconazole or placebo to be taken with a meal at the same time each day for 12 weeks". Comment: states study was double‐blinded, no method of binding participants or personnel (e.g. double‐dummy or matching placebo) stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[d]ouble‐blind". "[Patients] were … evaluated by investigators at wk 4, 8, and 12 for healthy nail growth, percent of nail area involved, signs of onychomycosis, the investigator's global evaluation, and mycologically." Comment: states study was double‐blinded, no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[o]f the 76 patients enrolled in the trial, 68 had data beyond the baseline visit and were included in the results of initial effectiveness". "All 73 patients were included in the safety analysis". Comment: all dropouts accounted for. Sufficient information provided to complete intention‐to‐treat analysis. Safety data reported for most patients enrolled in study |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Kejda 1999.
| Methods | Design: open‐label, randomised, parallel‐group study | |
| Participants | Number of participants randomised: 61 Sex (M/F): 27/34 Mean age: 47 years Number included in analysis: 51 Number completing treatment: 61 (10 in the itraconazole group received an additional pulse and were not included in efficacy analysis) Inclusion criteria: distal and lateral subungual onychomycosis with ≥ 50% nail plate involvement; positive KOH test or positive culture; mycologic examination anonymous for the clinician; mycologic evaluation at baseline then every 3rd mo for 9mo after treatment; and lab blood parameters within normal limits. Type/location/characteristics of infection: positive onychomycosis of the toenails Duration of infection: not stated Exclusion criteria: systemic antimycotic therapy within 3 months prior; use of any antifungal agent during trial (incl top); pregnancy/breastfeeding/lack of reliable contraception; use of cisapride, astemizole, terfenadine, simvastatin, lovastatin, phenytoin, triazolam, or rifampin; serious diseases affecting the liver or kidneys; or psoriasis Washout period: not stated Setting: Czech Republic Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: every 3rd month for 9 months after treatment Outcomes measured: affected nails/participants, global clinical parameter, KOH test, culture Safety and tolerability assessed by: reported adverse effects (cephalgia, exanthem, urticaria, diarrhoea, fatigue, dyspepsia, bloating, gryphosis of mycotic nails, obstipation, weight gain, flatulence, myositis) |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a] total of 61 patients were randomly assigned treatment and entered into the study." "Patients were similar in gender and age." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[a] total of 61 patients were randomly assigned treatment and entered into the study." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[t]his open, randomised, parallel‐group study represents a comparative clinical evaluation of therapeutic efficacy and tolerability of oral itraconazole pulse therapy (400 mg twice daily, 1 week/month, 3 pulses) and continuous terbinafine therapy (250 mg/day, 98 days)." Comment: participants and personnel likely unblinded as study states that it was an open study. Hence, there is a high risk of performance bias, especially as one treatment was pulse therapy and the other was continuous. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[o]pen". "Patients were evaluated every third month for 9 months after treatment". Comment: outcome assessors likely unblinded as study states that it was an open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[a] total of 61 patients were randomly assigned treatment and entered into the study. In the itraconazole arm, 10 patients received an additional pulse and were not included in the efficacy analysis." Comment: all dropouts explained and accounted for. Sufficient data provided for ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Kempers 2010.
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 1381 (3:3:1 for intervention 1, 2, and 3, respectively) Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: not stated, only percentages given Number completing treatment: not stated Inclusion criteria: no details given, conference abstract only Type/location/characteristics of infection: no details given, conference abstract only Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: USA and Canada (multicentre) Comorbidities: not stated |
|
| Interventions |
Follow‐up 40 weeks after treatment, 52 weeks in total |
|
| Outcomes | Complete cure of the big toenail (clinical cure and mycological cure) | |
| Source of funding | Commercial support: 100% is sponsored by Stiefel Laboratories | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | Conference abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a] total of 1381 subjects were randomised (3:3:1) to treatment and received the study drug." Comment: no method of random sequence generation stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[a] total of 1381 subjects were randomised (3:3:1) to treatment and received the study drug." Comment: no method of allocation concealment stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "[t]his randomised, multicenter, parallel group, placebo‐controlled, evaluator‐blinded study was designed to compare the efficacy of itraconazole given as one 200‐mg tablet QD with itraconazole given in two 100‐mg capsules QD for 12 weeks of treatment and 40 weeks of follow‐up." Comment: study states that it was placebo controlled. Likely adequate blinding of participants and personnel achieved with this method. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[t]his randomised, multicenter, parallel group, placebo‐controlled, evaluator‐blinded study was designed to compare the efficacy of itraconazole given as one 200‐mg tablet QD [4 times daily] with itraconazole given in two 100‐mg capsules QD for 12 weeks of treatment and 40 weeks of follow‐up." Comment: study states that it was evaluator‐blinded. Likely adequate blinding of outcome assessors achieved with this method |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "[a] total of 1381 subjects were randomised ..." "The proportions of subjects (intent to treat population) with complete cure at week 52 were greater in the active treatment groups (22.3% in the itraconazole 200‐mg tablet group and 21.7% in the itraconazole 100‐mg capsule group) compared with the placebo groups (1.0%). Comment: study states that intent to treat population was used for analysis. Outcome data presented as percentages only, with number of participants per group not stated in the text. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Korting 1993.
| Methods | Design: controlled open‐label trial | |
| Participants | Number of participants randomised: 120 Number included in analysis: 108; 1 participant suffered exclusively from fingernail involvement Number completing treatment: 109 Sex (M/F): 56/53 Mean age: 46 years Inclusion criteria: suggestive clinical appearance, positive KOH preparation, and a dermatophyte cultured on Kimmig's agar within 3 months of commencing treatment Type/location/characteristics of infection: positive toenail onychomycosis Duration of infection: not stated Exclusion criteria: severe additional diseases (e.g. impaired liver and kidney function, lupus erythematosus, porphyria), systemic antifungal treatment within the previous 4 weeks, age < 18 years, pregnant/lactating women, and those not using suitable contraceptive measures Washout period: not stated Setting: Germany Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 4 week interval evaluation with treatment for up to 18 months Outcomes measured: clinical status, mycological status, adverse reactions Safety and tolerability assessed by: AEs, glutamic‐pyruvic transaminase, GGT, total and low‐density lipoprotein cholesterol levels |
|
| Source of funding | Janssen GmbH, Neuss, Germany, supplied the study medication | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | No clear other bias seen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]he patients were assigned a study number in the order of their agreement to take part in the study". "The study number served for the random assignment of the patients to the three treatment groups (1:1:1 ratio)." Comment: method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he patients were assigned a study number in the order of their agreement to take part in the study". "The study number served for the random assignment of the patients to the three treatment groups (1:1:1 ratio)." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[t]he study described here was a controlled open trial" Comment: study states that it was an open trial with no mention of blinding in the Methods; therefore, there is a high risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[t]he study described here was a controlled open trial" Comment: study states that it was an open‐label trial with no mention of blinding in the Methods; therefore, there is a high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[a] total of 120 patients … were asked to take part in the study." "Discontinuation of treatment because of side effects was necessary in different proportions of the various treatment groups." "Results are reported for all subjects enrolled in the study except for those who dropped out before the completion of baseline parameters (intention‐to‐treat analysis)." Comment: outcome data provided for 108 of the original 120 randomised participants. Small number of dropouts, with reasons for exclusions explained. ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Kouznetsov 2002.
| Methods | Design: controlled trial | |
| Participants | Number of participants randomised: 174 Sex (M/F): 137/37 Mean age: not stated Number included in analysis: 174 Number completing treatment: 174 Inclusion criteria: 5y history of toenail dermatomycosis Type/location/characteristics of infection: positive toenail onychomycosis Duration of infection: minimum 5 years Exclusion criteria: not stated Washout period: not stated Setting: not stated Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 3 years after treatment Outcomes measured: mycological culture and microscopy Safety and tolerability assessed by: not stated |
|
| Source of funding | No informations available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | Conference abstract only, not a full article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]he patients … were randomly divided into two groups" Comment: no method of random sequence generation stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he patients … were randomly divided into two groups" Comment: no method of allocation concealment stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[t]he mycological efficacy and recurrences of the onychomycosis … were invested during three years of observation." Comment: no mention of blinding of participants or personnel; therefore, there is a unclear risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[t]he mycological efficacy and recurrences of the onychomycosis … were invested during three years of observation." Comment: no mention of blinding of outcome assessment; therefore, there is a high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "[t]he patients (n = 174) … were randomly divided into two groups; the first group (N = 67) treated by terbinafine …; the second group (N = 107) received itraconazole". Comment: numbers of patients randomised into each group provided. Outcome data likely presented as a percentage of the number of randomised patients (i.e. ITT analysis); however, no details regarding presence or absences of dropouts provided. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
La Placa 1994.
| Methods | Design: double‐blinded study | |
| Participants | Number of participants randomised: 29 Sex (M/F): 17/12 Mean age: 36.3 years (range 14‐76) Number included in analysis: 29 Number completed treatment: 28 Inclusion criteria: unclear Type/location/characteristics of infection: dermatophyte infection of the toenail Duration of infection: not stated Exclusion criteria: other systemic illnesses or on other therapy Washout period: not stated Setting: Italy Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 6 months post‐treatment (10 months for intervention 1, 15 months for intervention 2) Outcomes measured: mycological (positive Sabourad culture) and clinical cure (method of evaluation not stated) Safety and tolerability assessed by: drug tolerability, full blood count, and liver enzymes assessed every 4 weeks during treatment |
|
| Source of funding | Help from pharmaceutical company was provided (Sandoz) | |
| Conflict of interest | Clear disclosure of pharmaceutical industry involvement. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of method given |
| Allocation concealment (selection bias) | Unclear risk | No details of method given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[i]n a comparative double‐blind study...". No other details given. Comment: the differing duration of treatment and follow‐up ("14 were treated with terbinafine 250 mg/day for 4 months and 15 with griseofulvin 1 g/day for 9 months") adds confusion as to how the study could be double‐blinded despite authors describing it as such. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[i]n a comparative double‐blind study..." Comment: apart from stating that the study is double‐blind, there is no detail of method or explicit statement about blinding in the text. The differing duration of follow‐up adds confusion as to how the study could be double‐blinded despite authors describing it as such. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[o]f 29 patients affected by toenail onychomycosis ... 11 of the 14 patients with toenail onychomycosis (78.5%) treated with terbinafine with completely cured." Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Lebwohl 2001.
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 97 Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: 96 Number completing treatment: not stated Inclusion criteria: not stated Type/location/characteristics of infection: target toenail, not specifically stated Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: USA (multicentre) Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 72 weeks after treatment Outcomes measured: negative mycology (culture and KOH microscopy), zero nail involvement, overall efficacy according to participant and investigator (5‐point scale of excellent, very good, good, fair, poor) Safety and tolerability assessed by: adverse event reporting |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | Paper has no Methods section | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind, placebo‐controlled, multicenter study". Comment: states double‐blinded, but no method stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "clinical and mycologic evaluations of a target toenail were performed " Comment: method not stated other than as outlined in quote above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "97 subjects were randomised." Comment: attrition not accounted for in data analysis, but only 1 participant (of 96 total in that group) appears to have been lost to follow‐up for the outcome of clinical cure, and 3 participants (of 94 total in that group) appear to have been lost to follow‐up for mycology. Overall, attrition represents very small proportion of total subjects. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias reported |
Ling 1998.
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 386 Sex: not stated (both sexes included) Mean age: not stated, range 18‐70 years Number included in analysis: 331 (from table II) Number completing treatment: 245 (141 dropouts) Inclusion criteria: mycologically confirmed (KOH test and positive culture) onychomycosis of toenail Type/location/characteristics of infection: distal subungual onychomycosis of toenail, with a target toenail being a large toenail with > 25% involvement and > 2 mm healthy nail at nail fold Duration of infection Exclusion criteria: pregnancy, lactation, hypersensitivity to azoles, significant systemic disease, diabetes mellitus requiring medication, concomitant use of interfering drugs, HIV positivity, liver disease, positive fungal culture for non‐dermatophytes, psoriasis, lichen planus, anatomical abnormalities of toe, regular heavy alcohol intake Washout period: 3 months for oral antifungals, 2 weeks for topical antifungals Setting: USA (multicentre); 24 centres Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 9 months total treatment plus 6 month additional blinded follow‐up for those with clinical cure or improvement Outcomes measured: clinical response compared to baseline (classified as cure, improvement or failure), mycologic evaluation (KOH, fungal culture). Clinical success = clinical cure or area involved < 25%. Post‐treatment cure and relapse. Quality of life questionnaire. Safety and tolerability assessed by: adverse event reporting, lab tests, vital signs, weight |
|
| Source of funding | Sponsored by pharmaceutical industry | |
| Conflict of interest | "For the evaluation of efficacy at the end of treatment and at the 6‐month follow‐up, clinical success was arbitrarily defined by the sponsor of the study." Comment: industry sponsored and input unclear |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomised to four double‐blind treat‐ment groups" Comment: no further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[m]ulticenter, randomised, double‐blind, parallel, placebo‐controlled trial" Comment: no further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[o]f the 384 subjects, 243 (221 fluconazole, 22placebo) entered the posttherapy follow‐up phase. Fifty‐two of the 386 randomised subjects were excluded from the efficacy analysis for non‐efficacy–related reasons, including protocol violations,withdrawal of consent, adverse events leading to early discontinuation, or loss to follow‐up" Comment: all participants are accounted for, although large number of dropouts in placebo group |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Unclear risk | Quote: "[t]he evaluation of efficacy at the end of treatment and at the 6‐month follow‐up, clinical success was arbitrarily defined by the sponsor of the study" |
Maddin 2013.
| Methods | Design: randomised, multicentre, parallel group, placebo‐controlled study | |
| Participants | Number of participants randomised: 1381 Sex (M/F): 1034/347 Mean age: 47.4 years (range 16‐75) Number included in analysis: 1381 Number completing treatment: 1169 Inclusion criteria: clinical diagnosis of distal and/or lateral subungual onychomycosis affecting > 1 great toenail. > 25% to 75% nail involvement and > 2 mm of nail length uninvolved. Positive potassium hydroxide (KOH) microscopic examination and a culture positive for a dermatophyte from the target toenail Duration of infection: not specified Exclusion criteria: currently or within the previous 24 weeks participated in an investigational trial involving systemic treatment of onychomycosis of the fingernail or toenail, those having used any topical onychomycosis treatments in the 2 weeks prior to screening, and those with onychomycosis due to a Candida sp without the presence of a dermatophyte Setting: 62 sites in 7 countries (USA, Canada, South Africa, the Dominican Republic, Ecuador, Honduras and Panama) Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 52 weeks Outcomes measured: clinical and mycological cure, % nail involvement, total number of fingernail and toenails with onychomycosis over time, proportion of participants with no signs or symptoms of tinea pedis over time Safety and tolerability assessed by: recordings of adverse events and concomitant medications, clinical laboratory tests, electrocardiograms and audiology assessments |
|
| Source of funding | Funding for this research was provided by Stifel, a GSK company | |
| Conflict of interest | Authors have served as consultants for Stiefel, a GSK company and L Bulger is an employee of Stiefel | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization schedule was generated by QST consultations, stratified by investigational site and utilized a block size of 7" Comment: clear description of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote "schedule was generated by QST consultations" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[b]ecause the tablets and capsules differed obviously in appearance and dosing regimen, a designated individual at each site who was not involved with the evaluation of the patients dispensed, collected and accounted for the study drugs and thus was unblinded... Patients were instructed not to discuss the appearance or dosing regiment of their assigned study drugs with the investigator/evaluators." Comment: no ability to verify this actually happened |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[b]ecause the tablets and capsules differed obviously in appearance and dosing regimen, a designated individual at each site who was not involved with the evaluation of the patients dispensed, collected and accounted for the study drugs and thus was unblinded ... Patients were instructed not to discuss the appearance or dosing regiment of their assigned study drugs with the investigator/evaluators." Comment: unclear degree to which investigators/evaluators would be blinded given that patients and a single individual could inform them of details that could introduce detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "of the 118 patients 111 completed part 1, 56 in the terbinafine and 55 placebo" |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Mishra 2002.
| Methods | Design: open‐label parallel group trial | |
| Participants | Number of participants: 200 Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: 140 Number completing treatment: 140 Inclusion criteria: finger or toenail onychomycosis confirmed on culture Duration of infection: not specified Exclusion criteria: not specified Setting: SCB Medical College, Cuttack, India Comorbidities: not stated |
|
| Interventions |
Both for a period of 4 pulses |
|
| Outcomes | Cure (not further defined) | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | Unable to separate toenail from fingernail results, not included in pooled analysis, only abstract available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a]ll patients were assigned individual identification numbers and were divided randomly and equally into two groups (A and B) using a table of random numbers" Comment: no clear information given on method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "using a table of random numbers" Comment: no further information given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "randomised, single‐blind, longitudinal, clinical comparative study ... The drugs were bought by the physician and dispensed to the patients in unmarked packets " Comment: personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[t]he drugs were bought by the physician and dispensed to the patients in unmarked packets" Comment: personnel/physicians were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "10 (16%) patients in Group A and 12 (20%) patients in Group B could not complete the one‐year follow up period and were excluded from the analysis of the results." Comment: large proportion of patients unable to complete follow‐up; reason for being unable to complete 1‐year follow‐up not given |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other sources of bias seen |
Piepponen 1992.
| Methods | Design: parallel group single blind RCT | |
| Participants | Number of participants: 61 Sex (M/F): 29/32 Mean age: 44 years (range 18‐70) Number included in analysis: 36 Number completing treatment: 51 (some dropped out in follow‐up periods after completing treatment) Inclusion criteria: outpatients with a clinical diagnosis of onychomycosis of finger or toenails caused by dermatophytes and proven by culture Duration of infection: not specified Exclusion criteria: participants were excluded from the study if they were under 18 or over 70 years of age (although a patient that was "seven years old" was included), pregnant or lactating, had advanced liver disease, or used concomitantly rifampicin, the contraceptive pill, anticoagulant agents or antacid treatment Setting: 5 dermatological centres in Finland Comorbidities: not stated |
|
| Interventions |
Treatment duration 6‐9 months depending on clinical condition of the nail(s) |
|
| Outcomes | Mycological cure, negative culture and clinical assessment of the nail | |
| Source of funding | Orion Pharmaceutics | |
| Conflict of interest | Lead author works at Orion Pharmaceutics | |
| Notes | Unable to extract data on participant level for toenails, not included in pooled analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[m]edication was delivered in identical‐looking sealed plastic containers" Quote: "[s]ingle‐blind study" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[s]ingle blind study" Comment: unclear if outcome assessor ("investigator") was the one that was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "84% completed the treatment ... 10% ..." and "24% discontinued the study. " Comment: large number of patients discontinued without clear reasons given |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other sources of bias seen |
Ranawaka 2016.
| Methods | Design: randomised, double‐blind, comparative study | |
| Participants | Number of participants: 90 Sex: not stated (both sexes included) Mean age: 47.6 years (range 20‐80) Number included in analysis: 57 Number completing treatment: 57 Inclusion criteria: outpatients with a clinical diagnosis of onychomycosis of finger or toenails caused by non‐dermatophytes and proven by culture Duration of infection: 1 month‐20years Exclusion criteria: pregnant women, breastfeeding mothers, patients with known renal or liver impairment, congestive cardiac failure Setting: dermatology clinic at the General Hospital Chillaw, Sri Lanka and Base Hospital Homagama, Sri Lanka Comorbidities: not stated |
|
| Interventions |
In divided doses for 7 days per month (1 week on and 3 weeks off monthly pulses). 2 pulses were prescribed for fingernails and 3 pulses for toenails |
|
| Outcomes | Clinical and mycological cure. Clinical cure was defined as complete absence of all the clinical signs of onychomycosis. Mycological cure was defined as negative direct microscopy and culture | |
| Source of funding | No information available | |
| Conflict of interest | Authors did not declare any conflict of interest | |
| Notes | Data for toenails only provided after communication with the lead author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the treatment options were documented separately and packed in covered opaque envelopes consecutively numbered according to the randomisation schedule as to have a ratio of 1:1 |
| Allocation concealment (selection bias) | Low risk | Quote: "[t]he allocation sequence was concealed from the researcher enrolling and assessing the participants" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "[t]he participants and the investigator (outcome assessor) were blind to the type of therapy " Comment: participants and personnel were blinded to therapy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[t]he participants and the investigator (outcome assessor) were blind to the type of therapy. Same investigator performed clinical assessment on all the participants at each visit until cure" Comment: outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some differences in loss to follow‐up (7/43 in itraconazole and 14/47 in terbinafine defaulted to other treatments before the end of the trial) |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other biases identified |
Scher 1998.
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 362 Sex: not stated (both sexes included) Mean age: not stated; range 18‐70 years Number included in analysis: 355 ITT Number completing treatment: 313 Inclusion criteria: mycological diagnosis and a positive culture for dermatophytes Type/location/characteristics of infection: 25% involvement of the target nail with at least 2 mm of healthy nail from the nail fold to the proximal onychomycotic border Duration of infection: not stated Exclusion criteria: pregnancy, lactation, hypersensitivity to azoles, significant systemic disease, diabetes mellitus, immunosuppression, renal or hepatic dysfunction, fungal culture positive for non‐dermatophytes, drugs that may interfere with azoles Washout period: 3 months for oral antifungals, 2 weeks for topical antifungals Setting: USA (multicentre) Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 6 months after treatment Outcomes measured: clinical (visual; % of nail involved, distance from nail fold, signs/symptoms of onychomycosis) and mycologic (microscopic and microbiologic) evaluations Safety and tolerability: adverse event reporting, blood and urine specimens (haematology, blood chemistry, urinalysis), vital signs, body weight, use of concomitant medications |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]his study followed a randomised, double‐blind, fixed‐dose, parallel‐group, placebo‐controlled multi‐center design ... Patients were randomly assigned to one of the following four treatment regimens" Comment: not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[t]his study followed a randomised, double‐blind,fixed‐dose, parallel‐group, placebo‐controlled multi‐center design". Comment: study claims to be double‐blind, no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[t]his study followed a randomised, double‐blind,fixed‐dose, parallel‐group, placebo‐controlled multi‐center design " Comment: stated multiple times that remained double‐blinded on follow‐up visits, no details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis included. All participants are accounted for. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
Sigurgeirsson 1999.
| Methods | Design: prospective, randomised, double‐blind, double‐dummy, multicentre, parallel‐group study | |
| Participants | Number of participants randomised: 843 Sex: 58% male Mean age: 50.1 years (range 18‐75) Number included in analysis: 496 (this is the ITT population) Number completing treatment: not stated Inclusion criteria: men and women aged 18‐75 years with clinical diagnosis of distal subungual or total dystrophic onychomycosis of the toenails, confirmed by positive mycological culture and microscopy Type/location/characteristics of infection: only participants with dermatophyte infections were included, all were required to have involvement of a great toenail Duration of infection: not stated Exclusion criteria: pregnant and lactating women, people receiving drugs known or believed to interact with either of the study agents, people with conditions that might lead to altered absorption, metabolism or excretion of study agents, systemic antifungal therapy within 12 months prior to screening visit, or topical antifungal therapy within the 4 weeks prior to screening visit, diagnosis of immunodeficiency disorder, psoriasis or mucocutaneous candidiasis, radiotherapy, chemotherapy or immunosuppressive therapy within 12 weeks of the start of study, alanine transaminase and/or aspartate transaminase levels more than 1‐5 times the upper limit of the normal range and/or serum creatinine level above 300 μmol/L Washout period: 12 months for systemic, 4 weeks for topical Setting: participants were recruited from 35 centres in Finland, Germany, Iceland, Italy, the Netherlands and the UK Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 56 weeks (treatment phase till week 16, follow‐up phase till week 72) Outcomes measured: mycological cure, clinical cure, % nail involvement, efficacy as rated by participants Safety and tolerability assessed by: number and type of adverse events |
|
| Source of funding | Novartis provided support and funding | |
| Conflict of interest | Dr Sigurgeirsson has received funds for research and fees for speaking and organising educational meetings from several pharmaceutical companies, including Novartis Pharma. Professor Evans has received funds for research and also fees for speaking and consulting from a number of pharmaceutical companies, including Novartis Pharma and Janssen Pharmaceuticals. Dr Billstein is an employee of Novartis Pharmaceuticals Corporation, USA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "[b]oth the investigators and the participants remained blinded throughout the entire 72‐week study" Comment: participants and personnel were blinded to study group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[b]oth the investigators and the participants remained blinded throughout the entire 72‐week study". Comment: it is clearly stated that investigators, including those assessing clinical cure, were blinded for the entire 72‐week study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[e]fficacy results are based on the number of observed cases in the ITT population at 72 weeks". Comment: ITT performed |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Sigurgeirsson 2013.
| Methods | Design: randomised, double‐blind, placebo‐controlled, parallel group study | |
| Participants | Number of participants randomised: 582 Sex (M/F): 441/141 Mean age: 48.6 years (range 19‐74) Number included in analysis: 582 Number completing treatment: 482 Inclusion criteria: distal subungual onychomycosis affecting at least 1 great toenail (target toenail) with > 25% nail involvement, > 2 mm of unaffected toenail at the proximal end, and microscopic (KOH, calcofluor) and culture confirmation of dermatophytes. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and total bilirubin levels that were < 1.5 times the upper normal limit. Baseline ECG normal or clinically insignificant. Duration of infection: not specified Exclusion criteria: women who were pregnant, trying to become pregnant or breastfeeding, receipt of an investigational drug within 4 weeks before the first dose of the study product, an investigational drug treatment for onychomycosis within 6 months before the first dose of the study product; schedule receipt of any other investigational drug during the study; receipt of any known substrate of the 3A4 isozyme of cytochrome P450 (CYP3A4) with QT prolongation potential; concomitant use of prohibited medications, a history of any condition that could possibly affect drug absorption (e.g. gastrectomy), uncontrolled diabetes, clinically significant peripheral vascular disease or circulatory impairment, any major illness within 30 days before screening, ECG abnormalities deemed clinically significant. Washout period: 4 weeks Setting: 26 centres in the USA, 3 in Canada and 1 in Iceland Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 52 weeks Outcomes measured: mycological and clinical cure, adverse events Safety and tolerability assessed by: clinical laboratory indicators, vital signs and physical examination results and ECG measurements |
|
| Source of funding | Supported by Stiefel, a GSK company | |
| Conflict of interest | Dr Sigurgeirsson was a sponsored investigator on this study and a member of an advisory board that assisted in the planning and design of the study. He also has served as a consultant and investigator for and received honoraria from Arpedia, Celtic, deCode, Galderma, Novartis, Prostrakan, Stiefel, TLT, Topica, and Vertex. Dr van Rossem, Mr Malahias, and Ms Raterink are employees of Stiefel, a GSK company | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "independently randomized (with a 1:1:1:1:1 schedule) using a computer‐generated schedule to 1 of the 5 study groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "Investigators, study centre personnel, patients, study monitors, and statisticians were unaware of the assigned study treatment", "placebo‐matched capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[i]nvestigators, study centre personnel, participants, study monitors, and statisticians were unaware of the assigned study treatment." Comment: outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High completion rate (82%‐98%) and very low loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Svejgaard 1985.
| Methods | Design: double‐blind study | |
| Participants | Number of participants randomised: 20 Sex (M/F): 18/2 Mean age: 40.5 years (range 14‐65) Number included in analysis: 17 Number completing treatment: the study defines 'completing treatment' as treatment till cure, rather than stipulating a timeframe. 9 participants in the ketoconazole group were treated for 8‐12 months (mean 10.6), 5 participants in the griseofulvin group were treated for 12 months Inclusion criteria: culturally proven onychomycosis caused by dermatophytes severe enough to indicate systemic treatment Duration of infection: 1‐30 years (mean 9.3) Exclusion criteria: not specified Washout period: not specified, but 15 participants in the study had received prior treatment with griseofulvin for less than 3 months without side effects Setting: not explicitly stated, but the author is based at Rigshospital, Copenhagen, Denmark, and acknowledges technical assistance from the Dermatological Department of this hospital. Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 12 months Outcomes measured: 'cure' defined as clinical and mycological cure Safety and tolerability assessed by: laboratory tests, including haemoglobin, leucocyte count, platelet estimate, creatinine, cholesterol and alanine‐aminotransferase |
|
| Source of funding | Ketoconazle tablets were supplied by Janssen Pharmaceutica, Beerse, Belgium and griseofulvin tablets by Leo, Haelsingborg, Sweden | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | This a 2‐part study. The first part assesses responsiveness of infection of various body parts to ketoconazole. The details above apply to the second part of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[i]n the double‐blind study ... on a randomised basis" Comment: study claims to be randomised, but does not state method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[i]n the double‐blind study ... on a randomised basis" Comment: not stated how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[i]n the double‐blind study ... on a randomised basis" Comment: study claims to be double‐blind, but no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[i]n the double‐blind study ... on a randomised basis" Comment: study claims to be double‐blind, but no further details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Svejgaard 1997.
| Methods | Design: double‐blind, controlled, multicentre study | |
| Participants | Number of participants randomised: 148 Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: 147 Number completing treatment: 127 Inclusion criteria: age of 18 years or older Type/location/characteristics of infection: proven modest to severe dermatophyte infection of 1 or both great toenails (positive microscopy and culture) Duration of infection: not stated Exclusion criteria: impaired liver and kidney function, pregnant or lactating women Washout period: 1 month for both topical and systemic treatment Setting: Denmark (multicentre) 15 dermatology clinics and 5 hospital departments Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 12 months Outcomes measured: mycological cure, clinical cure, % of nail unaffected, degree of subungual keratosis Safety and tolerability assessed by: number and type of side effects |
|
| Source of funding | Supported by Sandoz Ag Basle | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | Those who had no improvement or deterioration (terbinafine or placebo group) were treated with further 3 months of terbinafine ‐ these participants are not included in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a]fter 1‐month wash‐out period with no topical or systemic treatment the patients were randomised to receive ..." Comment: study stated to be randomised, but method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he investigation was carried out as a double‐blind, controlled, multi‐centre ..." Comment: method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[t]he investigation was carried out as a double‐blind, controlled, multi‐centre ..." Comments: no further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[t]he investigation was carried out as a double‐blind, controlled, multi‐centre ..." Comments: no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other clear bias seen |
Tosti 1996.
| Methods | Design: open‐label, randomised study | |
| Participants | Number of participants randomised: 63 Sex (M/F): 31/32 Mean age: 47.3 years (range 27‐60) Number included in analysis: 60 (57 with toenail infection) Number completing treatment: 60 (57 with toenail infection) Inclusion criteria: not stated Type/location/characteristics of infection: toenails, fingernails or both Duration of infection: not stated Exclusion criteria: systemic antifungal agents in previous 6 weeks, participant with severe liver, renal or cardiovascular disease and pregnant women Washout period: not stated, but participants who has systemic antifungal therapy in previous 6 weeks were excluded Setting: Italy Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 10 months (for toenail infection) Outcomes measured: mycological cure, presence of nail deformity Safety and tolerability: number of participants who reported adverse side effects |
|
| Source of funding | This study was partially supported by Novartis Farma SpA Italy and by the University of Bologna ‐ funds for selected research topics | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]he experimental design was open and randomised. Patients were assigned sequentially to treatment." Comment: unclear if random sequence generation was adequate |
| Allocation concealment (selection bias) | High risk | Quote: "[t]he experimental design was open and randomised. Patients were assigned sequentially to treatment." Comment: likely allocation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "[t]he experimental design was open" Comment: study was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[t]he experimental design was open" Comment: study was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[a]ll participants who started treatment were considered able to be evaluated even if they withdrew the first day because of adverse events (intention to treat)." Comment: ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
Walsoe 1990.
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 20 Sex: not stated for toenail subgroup (both sexes included) Mean age: not stated Number included in analysis: 20 Number completing treatment: 20 Inclusion criteria Type/location/characteristics of infection: toenail or fingernail onychomycosis caused by T rubrum or T mentagrophytes Duration of infection: not stated Exclusion criteria: antimycotic therapy within 1 month of start of study, pregnancy or serious concurrent disease Washout period: not explicitly stated, by participants with antimycotic therapy within 1 month of start of study were excluded Setting: not stated, study authors are all from Copenhagen Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 12 months Outcomes measured: cure (defined as clinical and mycological cure), marked improvement (defined as positive microscopy and negative culture), and improvement (50% clinical improvement compared to baseline and positive mycology) Safety and tolerability: side effects reported |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double‐blind study ... randomised basis" Comment: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "double‐blind study ... randomised basis" Comment: method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[f]or each patient, 12 boxes were prepared, each containing blister packs" Comment: blister packs were used, but it was not clear whether any visual differences remained between treatments |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind study ... randomised basis" Comment: method not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other risks of bias identified |
Watson 1995.
| Methods | Design: RCT | |
| Participants | Number of participants randomised: 118 Sex: 58% male Mean age: not stated Number included in analysis: 118 ITT Number completing treatment: 111 Inclusion criteria: distal or total dermatophyte onychomycosis of at least 1 toenail confirmed by mycological culture Type/location/characteristics of infection: distal or total dermatophyte onychomycosis, at least 1 toenail Duration of infection: not stated Exclusion criteria: renal, hepatic, cardiovascular or gastrointestinal disease, psoriasis, pregnancy, lactation, inadequate contraception, if non‐dermatophyte was considered to be primary pathogen, if participant used topical or oral antifungal agent within 2 or 6 weeks, respectively Washout period: not stated Setting: 13 centres in Australia and New Zealand Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 48 weeks from start of treatment Outcomes measured: clinical assessment (no signs of infection or considerable, minor or no improvement) and mycology (microscopy and mycological culture) Safety and tolerability: adverse event reporting, biochemical, haematologic studies, urinalysis and clinical examination |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]his was a randomised, double‐blind, 48‐week study." Comment: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]his was a randomised, double‐blind, 48‐week study." Comment: method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "[t]his was a randomised, double‐blind, 48‐week study." Comment: study claims to be double‐blind, but no method stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[t]his was a randomised, double‐blind, 48‐week study." Comment: no mention of method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other risks of bias identified |
Won 2007.
| Methods | Design: open‐label, randomised study | |
| Participants | Number of participants randomised: 72 Sex: 50% male Mean age: 45.8 years (range 17‐70) Number included in analysis: 49 Number completing treatment: assumed 49 (no discontinuations reported) Inclusion criteria: unclear Type/location/characteristics of infection: distal or distolateral subungual toenail onychomycosis, not more than 75% involvement of nail plate, confirmed by microscopy or culture Duration of infection: not specified Exclusion criteria: any systemic disease Washout period: 1 month for topical antifungal therapies or topical steroids, 2 months for systemic antifungal therapy Setting: 2 research centres in Seoul, Korea Comorbidities: not stated |
|
| Interventions |
|
|
| Outcomes | Duration of follow‐up: 96 weeks Outcomes measured: mycological cure, clinical cure, adverse events, subject acceptance Safety and tolerability: adverse events reporting, measurement of alanine aminotransferase, aspartate aminotransferase and gamma‐glutamyl transpeptidase |
|
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]articipants were randomly selected" to their treatment group Comment: no method is given |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]articipants were randomly selected" to their treatment group Comment: method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding is not mentioned in this study, and participants were given 300 mg of itraconazole daily for 1 week every 4 weeks or 250 mg terbinafine daily for 12 weeks. Because of these factors, it is possible that blinding could have been broken. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding is not mentioned in this study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: because the nail involvement was statistically different between groups, 21 of the initial 70 randomised participants were excluded, and the outcome data are unavailable. No systemic differences between withdrawals between groups |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other risks of bias identified |
AE: adverse event; ALP: alkaline phosphatase; ALT: alanine transaminase; AST: aspartate transaminase; CBC: complete blood count; ECG: electrocardiogram; GGT: gammaglutamyl transferase; GI: gastrointestinal; IIT: itraconazole‐itraconazole‐terbinafine (3 pulses total); ITT: intention‐to‐treat; KOH: potassium hydroxide; LFT: liver function test; NA: not applicable;RCT: randomised controlled trial; RFT: renal function test; TRIPA: trichophytin antigen; TTT: terbinafine × 3 pulses.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albreski 1999 | Study compares itraconazole to 'palliative care': trimming, soaking and cleaning. No placebo group |
| Alpsoy 1996 | This is a dose‐finding study with no comparisons between different drugs or between drug and placebo |
| Avner 2006a | This is a dose‐finding study with no comparisons between different drugs or between drug and placebo |
| Avner 2006b | This is a dose‐finding study with no comparisons between different drugs or between drug and placebo |
| Chen 1999 | This is a dose‐finding study with no comparisons between different drugs or between drug and placebo |
| De Cuyper 1996 | This is a dose‐finding study with no comparisons between different drugs or between drug and placebo |
| De Doncker 1996 | This is a dose‐finding study with no comparisons between different drugs or between drug and placebo |
| Faergemann 1996 | Pharmacokinetic study of drug concentrations in healthy nails |
| Finlay 1994 | Dose‐finding with no comparison between different drugs or a placebo. Also, focuses on pharmacokinetics and nail plate concentrations of drug (not clinical or mycological cure) |
| Gomez 1996 | Looked at tinea pedis |
| Goodfield 1990 | Not an RCT: no comparison group, only a treatment group |
| Havu 1997 | This is a dose‐finding study (continuous vs pulse) with no comparisons between different drugs or between drug and placebo |
| Havu 1999 | This is a dose‐finding study (continuous vs pulse) with no comparisons between different drugs or between drug and placebo |
| Hay 1987 | Looked at efficacy of topical adjunct to griseofulvin |
| Maleszka 2001 | Study assesses efficacy of adjuncts (amorolfine and pentoxifylline) to itraconazole and does not compare 2 different anti‐fungal agents |
| Pollak 2001 | Dose finding for terbinafine, no placebo group |
| Safer 2000 | Letter to the editor, not an RCT |
| Schatz 1995 | Dose finding for terbinafine, no placebo group |
| Shemer 1999 | This is a dose‐finding study for itraconazole with no comparisons between different drugs or between drug and placebo |
| Sommer 2003 | Dose finding for terbinafine, no placebo group |
| Tausch 1997 | Dose finding for terbinafine, no placebo group |
| van der Schroeff 1992 | Dose finding for terbinafine, no placebo group |
| Warshaw 2001 | This is a dose‐finding study for terbinafine (continuous vs intermittent) with no placebo group |
| Warshaw 2005 | Dose finding (continuous vs pulse) for terbinafine, no placebo group |
| Watanabe 2004 | This is a dose‐finding study for itraconazole pulse therapy |
| Yadav 2015 | Dose finding for terbinafine, no placebo group |
| Zaias 1983 | Fungal skin infections, not nail infections |
RCT: randomised controlled trial.
Differences between protocol and review
The objectives in the Abstract and Main text have been changed from "To compare the benefits and harms of oral antifungal treatments for toenail onychomycosis'" to "To assess the effects of oral antifungal treatments for toenail onychomycosis" according to the Cochrane recommended format.
Types of studies: we clarified that we would included cross‐over trials in this review; however, we did not identify any.
Types of participants: we edited this from "Participants of all ages with toenail onychomycosis confirmed by positive cultures or confirmed fungal elements on direct microscopy or histological examination of the nail" to "Participants of all ages with toenail onychomycosis confirmed by at least one positive culture or confirmed fungal elements on direct microscopy or histological examination of the nail" to make the number of positive cultures needed clear.
Types of interventions: we added, "we did not consider dose‐finding studies of the same drug unless they also contained a placebo group" to clarify that we aimed to compare different medications, not different doses of the same medication.
Types of outcome measures: when measurements took place at multiple time points during the intervention, we consider the measurement at the predefined endpoint of the study as our primary outcome.
The secondary outcome measure "time to recurrence" was changed to recurrence rate. This is because none of the studies reported time to recurrence, and the review authors agreed to report a recurrence rate instead.
Searching other resources, 'Unpublished literature': in the protocol, we planned to contact further companies producing other products identified from trials, but we did not identify any.
Compared with the published protocol, there were some alterations in the tasks completed by review authors: the third review author acting as arbiter was MvD rather than SaBS; four review authors (SKK, LG, GK, KH) independently extracted data using a data extraction form rather than SKK and PM. Two review authors (SKK plus LG, GK or KH) independently assessed each included study using Cochrane's tool for assessing risk of bias rather than SKK and PM (Higgins 2011). We added authors to the review team after the publication of the protocol to reduce to workload in view of the large number of included studies. To ensure consistency, SKK was involved in the data extraction and 'Risk of bias' assessment of all included studies.
Data collection and analysis: we included six 'Summary of findings' tables for six comparisons, which included all of our primary and secondary outcomes. We also used the GRADE approach to assess the quality of all outcomes using the following five domains: risk of bias, inconsistency, imprecision, indirectness and publication bias. Quality of evidence could be either high, moderate, low, or very low (Higgins 2011; Schünemann 2013).
Measures of treatment effect: we were not able to present continuous data as mean difference (MD) or standardised mean difference or overall effect size with standard deviations (SD) as planned in the protocol, because all data were presented as dichotomous.
Assessment of heterogeneity: we used a random‐effects model for all analyses instead of a fixed‐effect model when statistical heterogeneity was low, as in the absence of heterogeneity the random‐effects model would have similar results as a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity: we conducted subgroup analyses based on short‐ and long‐term follow‐up, based on the notion that a toenail will need at least 12 months to fully grow out (Geyer 2004); this affects the assessment of clinical cure in particular. We could not perform the planned subgroups based on subtype of onychomycosis or underlying health conditions, as we did not identify trials looking at these subgroups specifically.
Contributions of authors
SKK was the contact person with the editorial base. SKK coordinated contributions from the co‐authors and wrote the final draft of the review. SKK and KH screened papers against eligibility criteria. SKK obtained data on ongoing and unpublished studies. SKK, KH, GK, and LG appraised the quality of papers. SKK, KH, GK, and LG extracted data for the review and sought additional information about papers. SKK, KH, GK, and LG entered data into RevMan. SKK and MVD analysed and interpreted data. SKK, SB‐S and MVD wrote the Methods section. SB‐S edited the protocol and the review. SB‐S and MVD commented on all drafts and advised on methods and interpretation. SKK drafted the clinical sections of the Background and responded to the clinical comments of the referees. SKK and MVD responded to the methodology and statistics comments of the referees. SVB‐S was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. SKK is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Sanne Kreijkamp‐Kaspers: none known. Kate Hawke: none known. Linda Guo: none known. George Kerin: none known. Sally EM Bell‐Syer: none known. Parker Magin: none known. Sophie V Bell‐Syer: none known. Mieke L van Driel: none known.
New
References
References to studies included in this review
Al Rubaie 1997 {published data only}
- Al Rubaie SM. Double blind comparison between terbinafine & griseofulvin in the treatment of fingernail and toe nail onychomycosis. Australasian Journal of Dermatology 1997;38(Suppl 2):289. [CENTRAL: CN‐00499326] [Google Scholar]
Arca 2002 {published data only}
- Arca E, Tastan HB, Akar A, Kurumlu Z, Gur AR. An open, randomized, comparative study of oral fluconazole, itraconazole and terbinafine therapy in onychomycosis. Journal of Dermatological Treatment 2002;13(1):3‐9. [PUBMED: 12006131] [DOI] [PubMed] [Google Scholar]
Arenas 1991 {published data only}
- Arenas R, Fernandez G, Dominguez L. Onychomycosis treated with itraconazole or griseofulvin alone with and without a topical antimycotic or keratolytic agent. International Journal of Dermatology 1991;30(8):586‐9. [PUBMED: 1657803] [DOI] [PubMed] [Google Scholar]
Arenas 1995 {published data only}
- Arenas R, Dominguez‐Cherit J, Fernandez LM. Open randomized comparison of itraconazole versus terbinafine in onychomycosis. International Journal of Dermatology 1995;34(2):138‐43. [PUBMED: 7737776] [DOI] [PubMed] [Google Scholar]
Baran 1995 {published data only}
- Baran R. A multicentre double‐blind parallel group study comparing the efficacy, safety and tolerability of terbinafine (250 mg/day) with griseofulvin (1g/day) in the treatment of dermatophytic onychomycosis. Journal of the European Academy of Dermatology & Venereology 1995;5(Suppl 1):S79. [CENTRAL: CN‐00454083] [Google Scholar]
- Baran R, Belaich S, Beylot C, Bonnetblanc J, Cribier B, Daniel F, et al. Comparative multicentre double‐blind study of terbinafine (250 mg per day) versus griseofulvin (1 g per day) in the treatment of dermatophyte onychomycosis. Journal of Dermatological Treatment 1997;8(2):93‐97. [EMBASE: 1997242786] [Google Scholar]
Billstein 1999 {published data only}
- Billstein S, Kianifard F, Justice A. Terbinafine vs. placebo for onychomycosis in black patients. International Journal of Dermatology 1999;38(5):377‐379. [PUBMED: 10369551] [DOI] [PubMed] [Google Scholar]
Brautigam 1995 {published data only}
- Brautigam M. Terbinafine versus itraconazole: a controlled clinical comparison in onychomycosis of the toenails. Journal of the American Academy of Dermatology 1998;38(5 Pt 3):S53‐6. [PUBMED: 9594938] [DOI] [PubMed] [Google Scholar]
- Brautigam M, Nolting S, Schopf RE, Weidinger G. German randomized double‐blind multicentre comparison of terbinafine and itraconazole for the treatment of toenail tinea infection. British Journal of Dermatology 1996;134(Suppl 46):18‐21. [PUBMED: 8763463] [DOI] [PubMed] [Google Scholar]
- Brautigam M, Nolting S, Schopf RE, Weidinger G. Randomised double blind comparison of terbinafine and itraconazole for treatment of toenail tinea infection. Seventh Lamisil German Onychomycosis Study Group. BMJ 1995;311(7010):919‐22. [PUBMED: 7580551] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigam M, Weidinger G, Nolting S. Successful treatment of toenail mycosis with terbinafine and itraconazole gives long term benefits. BMJ 1998;317(7165):1084. [PUBMED: 9774312] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cullen 1987 {published data only}
- Cullen SI, Cullen MK. Ketoconazole and griseofulvin in the treatment of toenail dermatophyte onychomycosis. Current Therapeutic Research, Clinical and Experimental 1987;41(1):24‐29. [EMBASE: 1987096720] [Google Scholar]
De Backer 1998 {published data only}
- Backer M, Vroey C, Lesaffre E, Scheys I, Keyser P. Twelve weeks of continuous oral therapy for toenail onychomycosis caused by dermatophytes: a double‐blind comparative trial of terbinafine 250 mg/day versus itraconazole 200 mg/day. Journal of the American Academy of Dermatology 1998;38(5 Pt 3):S57‐63. [PUBMED: 9594939] [DOI] [PubMed] [Google Scholar]
- Cuyper C, Hindryckx PH. Long‐term outcomes in the treatment of toenail onychomycosis. British Journal of Dermatology 1999;141(Suppl 56):15‐20. [PUBMED: 10730909] [DOI] [PubMed] [Google Scholar]
Degreef 1999 {published data only}
- Degreef H, Palacio A, Mygind S, Ginter G, Pinto Soares A, Zuluaga de Cadena A. Randomized double‐blind comparison of short‐term itraconazole and terbinafine therapy for toenail onychomycosis. Acta Dermato‐Venereologica 1999;79(3):221‐3. [PUBMED: 10384922] [DOI] [PubMed] [Google Scholar]
Drake 1997 {published data only}
- Drake LA, Shear NH, Arlette JP, Cloutier R, Danby FW, Elewski BE, et al. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. Journal of the American Academy of Dermatology 1997;37(5 Pt 1):740‐5. [PUBMED: 9366820] [DOI] [PubMed] [Google Scholar]
Elewski 1997 {published data only}
- Elewski BE, Scher RK, Aly R, Daniel R 3rd, Jones HE, Odom RB, et al. Double‐blind, randomized comparison of itraconazole capsules vs. placebo in the treatment of toenail onychomycosis. Cutis 1997;59(4):217‐20. [PUBMED: 9104548] [PubMed] [Google Scholar]
- Odom R, Daniel CR, Aly R. A double‐blind, randomized comparison of itraconazole capsules and placebo in the treatment of onychomycosis of the toenail. Journal of the American Academy of Dermatology 1996;35(1):110‐1. [PUBMED: 8682945] [DOI] [PubMed] [Google Scholar]
Elewski 2002 {published data only}
- Elewski BE, Charif M, Cooper KD, Ghannoum M, Birnbaum JE. Reactivity to trichophytin antigen in patients with onychomycosis: effect of terbinafine. Journal of the American Academy of Dermatology 2002;46(3):371‐5. [PUBMED: 11862171] [DOI] [PubMed] [Google Scholar]
Elewski 2012 {published data only}
- Elewski B, Pollak R, Ashton S, Rich P, Schlessinger J, Tavakkol A. A randomized, placebo‐ and active‐controlled, parallel‐group, multicentre, investigator‐blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. British Journal of Dermatology 2012;166(2):389‐98. [PUBMED: 21967490] [DOI] [PubMed] [Google Scholar]
- Elewski B, Tavakkol A, Pollak R, Ashton S. A randomized, placebo‐ and active‐controlled, parallel‐group, multicenter,investigator‐blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Journal of American Academy of Dermatology 2010;62(3 Suppl 1):AB76. [EMBASE: 70142781] [DOI] [PubMed] [Google Scholar]
Faergemann 1995 {published data only}
- Faergemann J, Anderson C, Hersle K, Hradil E, Nordin P, Kaaman T, et al. Double‐blind, parallel‐group comparison of terbinafine and griseofulvin in the treatment of toenail onychomycosis. Journal of the American Academy of Dermatology 1995;32(5 Pt 1):750‐3. [PUBMED: 7722020] [DOI] [PubMed] [Google Scholar]
Goodfield 1992 {published data only}
- Goodfield MJ. Short‐duration therapy with terbinafine for dermatophyte onychomycosis: a multicentre trial. British Journal of Dermatology 1992;126(Suppl 39):33‐5. [PUBMED: 1531926] [DOI] [PubMed] [Google Scholar]
- Goodfield MJ, Andrew L, Evans EG. Short term treatment of dermatophyte onychomycosis with terbinafine. BMJ 1992;304(6835):1151‐4. [PUBMED: 1392793] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2000 {published data only}
- Gupta AK, Maddin S, Arlette J, Giroux J‐M, Shear NH. Itraconazole pulse therapy is effective in dermatophyte onychomycosis of the toenail: a double‐blind placebo‐controlled study. Journal of Dermatological Treatment 2000;11(1):33‐7. [EMBASE: 2000114935] [Google Scholar]
Gupta 2001a {published data only}
- Gupta AK, Konnikov N, Lynde CW. Single‐blind, randomized, prospective study on terbinafine and itraconazole for treatment of dermatophyte toenail onychomycosis in the elderly. Journal of the American Academy of Dermatology 2001;44(3):479‐84. [PUBMED: 11209118] [DOI] [PubMed] [Google Scholar]
Gupta 2001b {published data only}
- Gupta AK, Gregurek‐Novak T. Efficacy of itraconazole, terbinafine, fluconazole, griseofulvin and ketoconazole in the treatment of Scopulariopsis brevicaulis causing onychomycosis of the toes. Dermatology 2001;202(3):235‐8. [PUBMED: 11385230] [DOI] [PubMed] [Google Scholar]
Gupta 2001c {published data only}
- Gupta AK, Lynde CW, Konnikov N. Single‐blind, randomized, prospective study of sequential itraconazole and terbinafine pulse compared with terbinafine pulse for the treatment of toenail onychomycosis. Journal of the American Academy of Dermatology 2001;44(3):485‐91. [PUBMED: 11209119] [DOI] [PubMed] [Google Scholar]
Gupta 2005 {published data only}
- Gupta AK, Leonardi C, Stoltz RR, Pierce PF, Conetta B. A phase I/II randomized, double‐blind, placebo‐controlled, dose‐ranging study evaluating the efficacy, safety and pharmacokinetics of ravuconazole in the treatment of onychomycosis. Journal of the European Academy of Dermatology and Venereology : JEADV 2005;19(4):437‐43. [PUBMED: 15987289] [DOI] [PubMed] [Google Scholar]
Gupta 2006 {published data only}
- Gupta A, Gover M. Pulse itraconazole versus continuous terbinafine for the treatment of dermatophyte toenail onychomycosis in patients with diabetes mellitus. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB151. [CENTRAL: CN‐00602562] [DOI] [PubMed] [Google Scholar]
- Gupta AK, Gover MD, Lynde CW. Pulse itraconazole vs. continuous terbinafine for the treatment of dermatophyte toenail onychomycosis in patients with diabetes mellitus. Journal of the European Academy of Dermatology and Venereology : JEADV 2006;20(10):1188‐93. [PUBMED: 17062029] [DOI] [PubMed] [Google Scholar]
Gupta 2009 {published data only}
- Gupta AK, Cooper EA, Paquet M. Recurrences of dermatophyte toenail onychomycosis during long‐term follow‐up after successful treatments with mono‐ and combined therapy of terbinafine and itraconazole. Journal of Cutaneous Medicine and Surgery 2013;17(3):201‐6. [PUBMED: 23673304] [DOI] [PubMed] [Google Scholar]
- Gupta AK, Lynch LE, Kogan N, Cooper EA. The use of an intermittent terbinafine regimen for the treatment of dermatophyte toenail onychomycosis. Journal of the European Academy of Dermatology and Venereology 2009;23(3):256‐62. [PUBMED: 19438818] [DOI] [PubMed] [Google Scholar]
Havu 2000 {published data only}
- Havu V, Heikkila H, Kuokkanen K, Nuutinen M, Rantanen T, Saari S, et al. A double‐blind, randomized study to compare the efficacy and safety of terbinafine (Lamisil) with fluconazole (Diflucan) in the treatment of onychomycosis. British Journal of Dermatology 2000;142(1):97‐102. [PUBMED: 10651701] [DOI] [PubMed] [Google Scholar]
- Salo H, Pekurinen M. Cost effectiveness of oral terbinafine (Lamisil) compared with oral fluconazole (Diflucan) in the treatment of patients with toenail onychomycosis. PharmacoEconomics 2002;20(5):319‐24. [PUBMED: 11994041] [DOI] [PubMed] [Google Scholar]
Hay 1985 {published data only}
- Hay RJ, Clayton YM, Griffiths WA, Dowd PM. A comparative double blind study of ketoconazole and griseofulvin in dermatophytosis. British Journal of Dermatology 1985;112(6):691‐6. [PUBMED: 3890924] [DOI] [PubMed] [Google Scholar]
Hofmann 1995 {published data only}
- Hofmann H, Brautigam M, Weidinger G, Zaun H. Treatment of toenail onychomycosis. A randomized, double‐blind study with terbinafine and griseofulvin. LAGOS II Study Group. Archives of Dermatology 1995;131(8):919‐22. [PUBMED: 7632064] [DOI] [PubMed] [Google Scholar]
Honeyman 1997 {published data only}
- Honeyman JF, Talarico Filho S, Arruda LHF, Pereira Jr AC, Santamaria JR, Souza EM, et al. Itraconazole versus terbinafine (Lamisil): which is better for the treatment of onychomycosis?. Journal of the European Academy of Dermatology and Venereology 1997;9(3):215‐21. [EMBASE: 1998017627] [Google Scholar]
Jones 1996 {published data only}
- Jones HE, Zaias N. Double‐blind, randomized comparison of itraconazole capsules and placebo in onychomycosis of toenail. International Journal of Dermatology 1996;35(8):589‐90. [PUBMED: 8854164] [DOI] [PubMed] [Google Scholar]
Kejda 1999 {published data only}
- Kejda J. Itraconazole pulse therapy vs continuous terbinafine dosing for toenail onychomycosis. Postgraduate Medicine 1999;Spec No:12‐5. [PUBMED: 10492661] [PubMed] [Google Scholar]
Kempers 2010 {published data only}
- Kempers S, Quiring J, Bulger L, Scher R, Bissonnette R. A novel itraconazole tablet for the treatment of onychomycosis. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB87. [EMBASE: 70142824] [Google Scholar]
Korting 1993 {published data only}
- Korting HC, Schafer‐Korting M, Zienicke H, Georgii A, Ollert MW. Treatment of tinea unguium with medium and high doses of ultramicrosize griseofulvin compared with that with itraconazole. Antimicrobial Agents and Chemotherapy 1993;37(10):2064‐8. [PUBMED: 8257124] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kouznetsov 2002 {published data only}
- Kouznetsov AV, Potekaev NN, Belenko OV. Terbinafine and itraconazol in the treatment of onychomycosis: results of 3 years observation. Journal of the European Academy of Dermatology and Venereology 2002;16(S1):242. [CENTRAL: CN‐00478618; DOI: 10.1046/j.1468-3083.16.s1.1.x] [DOI] [Google Scholar]
La Placa 1994 {published data only}
- Placa M, Stinchi C, Venturo N, Morelli R, Tosti A. Terbinafine vs griseofulvin in the treatment of dermatophyte onychomycosis [Terbinafina vs griseofulvina nella terapia delle onicomicosi da dermatofiti]. Giornale Italiano di Dermatologia e Venereologia 1994;129(4):19‐22. [EMBASE: 1994255170] [Google Scholar]
Lebwohl 2001 {published data only}
- Lebwohl MG, Daniel CR, Leyden J, Mormon M, Shavin JS, Tschen E, et al. Efficacy and safety of terbinafine for nondermatophyte and mixed nondermatophyte and dermatophyte toenail onychomycosis. International Journal of Dermatology 2001;40(5):358‐60. [PUBMED: 11555003] [DOI] [PubMed] [Google Scholar]
Ling 1998 {published data only}
- Ling MR, Swinyer LJ, Jarratt MT, Falo L, Monroe EW, Tharp M, et al. Once‐weekly fluconazole (450 mg) for 4, 6, or 9 months of treatment for distal subungual onychomycosis of the toenail. Journal of the American Academy of Dermatology 1998;38(6 Pt 2):S95‐102. [PUBMED: 9631991] [DOI] [PubMed] [Google Scholar]
Maddin 2013 {published data only}
- Maddin S, Quiring J, Bulger L. Randomised, placebo‐controlled, phase 3 study of itraconazole for the treatment of onychomycosis. Journal of Drugs in Dermatology 2013;12(7):758‐763. [PUBMED: 23884486] [PubMed] [Google Scholar]
Mishra 2002 {published data only}
- Mishra M, Panda PL. A comparative study of efficacy of oral itraconazole and terbinafine pulse therapy in the treatment of onychomycosis. Annales de Dermatologie et de Venereologie 2002;129:IC1753. [CENTRAL: CN‐00454549] [Google Scholar]
Piepponen 1992 {published data only}
- Piepponen T, Blomqvist K, Brandt H, Havu V, Hollmen A, Kohtamaki K, et al. Efficacy and safety of itraconazole in the long‐term treatment of onychomycosis. Journal of Antimicrobial Chemotherapy 1992;29(2):195‐205. [PUBMED: 1324238] [DOI] [PubMed] [Google Scholar]
Ranawaka 2016 {published data only}
- Ranawaka RR, Nagahawatte A, Gunasekara TA, Weerakoon HS, Silva SH. Randomized, double‐blind, comparative study on efficacy and safety of itraconazole pulse therapy and terbinafine pulse therapy on nondermatophyte mold onychomycosis: A study with 90 patients. The Journal of dermatological treatment 2016;27(4):364‐72. [PUBMED: 26651495] [DOI] [PubMed] [Google Scholar]
Scher 1998 {published data only}
- Elewski BE. US experience with fluconazole in nail infections. Journal of the European Academy of Dermatology and Venereology 1998;11(Suppl 2):S106. [CENTRAL: CN‐00478523; DOI: 10.1111/j.1468-3083.1998.tb00994.x] [DOI] [Google Scholar]
- Rich P, Scher RK, Breneman D, Savin RC, Feingold DS, Konnikov N, et al. Pharmacokinetics of three doses of once‐weekly fluconazole (150, 300, and 450 mg) in distal subungual onychomycosis of the toenail. Journal of the American Academy of Dermatology 1998;38(6 Pt 2):S103‐9. [PUBMED: 9631992] [DOI] [PubMed] [Google Scholar]
- Scher RK, Breneman D, Rich P, Savin RC, Feingold DS, Konnikov N, et al. Once‐weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. Journal of the American Academy of Dermatology 1998;38(6 Pt 2):S77‐86. [PUBMED: 9631989] [DOI] [PubMed] [Google Scholar]
Sigurgeirsson 1999 {published data only}
- Evans EG, Sigurgeirsson B. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. The LION Study Group. BMJ 1999;318(7190):1031‐5. [PUBMED: 10205099] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila H, Stubb S. Long‐term results in patients with onychomycosis treated with terbinafine or itraconazole. British Journal of Dermatology 2002;146(2):250‐3. [PUBMED: 11903235] [DOI] [PubMed] [Google Scholar]
- Sigurgeirsson B, Billstein S, Rantanen T, Ruzicka T, Fonzo E, Vermeer BJ, et al. L.I.O.N. Study: efficacy and tolerability of continuous terbinafine (Lamisil) compared to intermittent itraconazole in the treatment of toenail onychomycosis. Lamisil vs. Itraconazole in Onychomycosis. British Journal of Dermatology 1999;141(Suppl 56):5‐14. [PUBMED: 10730908] [DOI] [PubMed] [Google Scholar]
- Sigurgeirsson B, Olafsson JH, Steinsson JB, Paul C, Billstein S, Evans EG. Long‐term effectiveness of treatment with terbinafine vs itraconazole in onychomycosis: a 5‐year blinded prospective follow‐up study. Archives of Dermatology 2002;138(3):353‐7. [PUBMED: 11902986] [DOI] [PubMed] [Google Scholar]
- Warrick D, Church L. Continuous terbinafine versus intermittent itraconazole for toenail onychomycosis. Journal of Family Practice 1999;48(7):492‐3. [PUBMED: 10428238] [PubMed] [Google Scholar]
Sigurgeirsson 2013 {published data only}
- Sigurgeirsson B, Rossem K, Malahias S, Raterink K. A phase II, randomized, double‐blind, placebo‐controlled, parallel group, dose‐ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. Journal of the American Academy of Dermatology 2013;69(3):416‐25. [PUBMED: 23706639] [DOI] [PubMed] [Google Scholar]
Svejgaard 1985 {published data only}
- Svejgaard E. Oral ketoconazole as an alternative to griseofulvin in recalcitrant dermatophyte infections and onychomycosis. Acta Dermato‐venereologica 1985;65(2):143‐9. [PUBMED: 2408417] [PubMed] [Google Scholar]
Svejgaard 1997 {published data only}
- Brandrup F, Larsen PO. Long‐term follow‐up of toe‐nail onychomycosis treated with terbinafine. Acta Dermato‐venereologica 1997;77(3):238. [PUBMED: 9188883] [DOI] [PubMed] [Google Scholar]
- Svejgaard EL, Brandrup F, Kragballe K, Larsen PO, Veien NK, Holst M, et al. Oral terbinafine in toenail dermatophytosis. A double‐blind, placebo‐controlled multicenter study with 12 months' follow‐up. Acta Dermato‐venereologica 1997;77(1):66‐9. [PUBMED: 9059684] [DOI] [PubMed] [Google Scholar]
Tosti 1996 {published data only}
- Tosti A, Piraccini BM, Stinchi C, Colombo MD. Relapses of onychomycosis after successful treatment with systemic antifungals: a three‐year follow‐up. Dermatology (Basel, Switzerland) 1998;197(2):162‐6. [PUBMED: 9732167] [DOI] [PubMed] [Google Scholar]
- Tosti A, Piraccini BM, Stinchi C, Venturo N, Bardazzi F, Colombo MD. Treatment of dermatophyte nail infections: an open randomized study comparing intermittent terbinafine therapy with continuous terbinafine treatment and intermittent itraconazole therapy. Journal of the American Academy of Dermatology 1996;34(4):595‐600. [PUBMED: 8601647] [DOI] [PubMed] [Google Scholar]
Walsoe 1990 {published data only}
- Walsoe I, Stangerup M, Svejgaard E. Itraconazole in onychomycosis. Open and double‐blind studies. Acta Dermato‐venereologica 1990;70(2):137‐40. [PUBMED: 1969198] [PubMed] [Google Scholar]
Watson 1995 {published data only}
- Ellis DH, Watson AB, Marley JE, Williams TG. Non‐dermatophytes in onychomycosis of the toenails. British Journal of Dermatology 1997;136(4):490‐3. [PUBMED: 9155945] [PubMed] [Google Scholar]
- Watson A, Marley J, Ellis D, Williams T. Terbinafine in onychomycosis of the toenail: a novel treatment protocol. Journal of the American Academy of Dermatology 1995;33(5 Pt 1):775‐9. [PUBMED: 7593777] [DOI] [PubMed] [Google Scholar]
Won 2007 {published data only}
- Won CH, Lee J, Li K, Mi RC, Kim BJ, An JS, et al. The long term efficacy and relapse rate of itraconazole pulse therapy versus terbinafine continuous therapy for toenail onychomycosis ‐ a 96‐week follow‐up study. Korean Journal of Medical Mycology 2007;12(3):139‐47. [CENTRAL: CN‐00708731; EMBASE: 2008006956] [Google Scholar]
References to studies excluded from this review
Albreski 1999 {published data only}
- Albreski DA, Gross EG. The safety of itraconazole in the diabetic population. Journal of the American Podiatric Medical Association 1999;89(7):339‐45. [PUBMED: 10423939] [DOI] [PubMed] [Google Scholar]
Alpsoy 1996 {published data only}
- Alpsoy E, Yilmaz E, Basaran E. Intermittent therapy with terbinafine for dermatophyte toe‐onychomycosis: a new approach. Journal of Dermatology 1996;23(4):259‐62. [PUBMED: 8935341] [DOI] [PubMed] [Google Scholar]
Avner 2006a {published data only}
- Avner S, Nir N, Baruch K, Henri T. Two novel itraconazole pulse therapies for onychomycosis: a 2‐year follow‐up. Journal of Dermatological Treatment 2006;17(2):117‐20. [PUBMED: 16766337] [DOI] [PubMed] [Google Scholar]
Avner 2006b {published data only}
- Avner S, Nathansohn N Trau H. Onychomycosis recurrence: Long term follow‐up of 2 oral terbinafine regimens and the effect of topical bifonazole solution. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB133. [Google Scholar]
Chen 1999 {published data only}
- Chen J, Liao W, Wen H, Wu J, Yao Z. A comparison among four regimens of itraconazole treatment in onychomycosis. Mycoses 1999;42(1‐2):93‐6. [PUBMED: 10394855] [DOI] [PubMed] [Google Scholar]
De Cuyper 1996 {published data only}
- Cuyper C. Long‐term evaluation of terbinafine 250 and 500 mg daily in a 16‐week oral treatment for toenail onychomycosis. British Journal of dermatology 1996; Vol. 135, issue 1:156‐7. [PUBMED: 8776393] [PubMed]
De Doncker 1996 {published data only}
- Doncker P, Decroix J, Pierard GE, Roelant D, Woestenborghs R, Jacqmin P, et al. Antifungal pulse therapy for onychomycosis. A pharmacokinetic and pharmacodynamic investigation of monthly cycles of 1‐week pulse therapy with itraconazole. Archives of Dermatology 1996;132(1):34‐41. [PUBMED: 8546481] [DOI] [PubMed] [Google Scholar]
Faergemann 1996 {published data only}
- Faergemann J, Laufen H. Levels of fluconazole in normal and diseased nails during and after treatment of onychomycosis in toe‐nails with fluconazole 150mg once weekly. Acta Dermato‐venereologica 1996;76(3):219‐21. [PUBMED: 8800303] [DOI] [PubMed] [Google Scholar]
Finlay 1994 {published data only}
- Finlay AY, Thomas R, Dykes PJ, Smith SG, Jones TC. Descriptive correlations between various doses of oral terbinafine and concentrations in nail. Journal of Dermatological Treatment 1994;5(4):193‐7. [EMBASE: 1995029315] [Google Scholar]
Gomez 1996 {published data only}
- Gomez M, Arenas R, Salazar JJ, Gonzalez A, Moncado B, Rodriguez G. Tinea pedis. A multicenter trial to evaluate the efficacy and tolerance of a weekly dose of fluconazole [Tina de los pies. Estudio multicentrico para valorar la eficacia y tolerancia de una dosis semanal de fluconazol]. Dermatologia Revista Mexicana 1996;40(4):251‐5. [EMBASE: 1996238297] [Google Scholar]
Goodfield 1990 {published data only}
- Goodfield MJD. Clinical results with terbinafine in onychomycosis. Journal of Dermatological Treatment 1990;1(Suppl 2):55‐7. [EMBASE: 1990354009] [Google Scholar]
Havu 1997 {published data only}
- Havu V, Brandt H, Heikkila H, Hollmen A, Oksman R, Rantanen T, et al. A double‐blind, randomized study comparing itraconazole pulse therapy with continuous dosing for the treatment of toe‐nail onychomycosis. British Journal of Dermatology 1997;136(2):230‐4. [PUBMED: 9068738] [PubMed] [Google Scholar]
Havu 1999 {published data only}
- Havu V, Brandt H, Heikkila H, Hollmen A, Oksman R, Rantanen T, et al. Continuous and intermittent itraconazole dosing schedules for the treatment of onychomycosis: a pharmacokinetic comparison. British Journal of Dermatology 1999;140(1):96‐101. [PUBMED: 10215775] [DOI] [PubMed] [Google Scholar]
Hay 1987 {published data only}
- Hay RJ, Clayton YM, Moore MK. A comparison of tioconazole 28% nail solution versus base as an adjunct to oral griseofulvin in patients with onychomycosis. Clinical and Experimental Dermatology 1987;12:175‐7. [EMBASE: 1987131271] [DOI] [PubMed] [Google Scholar]
Maleszka 2001 {published data only}
- Maleszka R, Adamski Z. The treatment of extensive onychomycosis in aged patients [Leczenie Rozleglej Grzybicy Dermatofitowej Paznokci U Pacjentow W Starszym Wieku]. Wiadomosci Parazytologiczne 2001;47(4):817‐22. [EMBASE: 16886433] [PubMed] [Google Scholar]
Pollak 2001 {published data only}
- Pollak R, Billstein SA. Efficacy of terbinafine for toenail onychomycosis. A multicenter trial of various treatment durations. Journal of the American Podiatric Medical Association 2001;91(3):127‐31. [PUBMED: 11266494] [DOI] [PubMed] [Google Scholar]
Safer 2000 {published data only}
- Safer LF. Randomized double‐blind comparison of short‐term itraconazole and terbinafine therapy for toenail onychomycosis. Acta Dermato‐venereologica 2000;80(4):317. [EMBASE: 2000350111] [DOI] [PubMed] [Google Scholar]
Schatz 1995 {published data only}
- Schatz F, Brautigam M, Dobrowolski E, Effendy I, Haberl H, Mensing H, et al. Nail incorporation kinetics of terbinafine in onychomycosis patients. Clinical and Experimental Dermatology 1995;20(5):377‐83. [PUBMED: 8593713] [DOI] [PubMed] [Google Scholar]
Shemer 1999 {published data only}
- Shemer A, Nathansohn N, Kaplan B, Gilat D, Newman N, Trau H. Open randomized comparison of different itraconazole regimens for the treatment of onychomycosis. Journal of Dermatological Treatment 1999;10(4):245‐9. [EMBASE: 2000044216] [Google Scholar]
Sommer 2003 {published data only}
- Sommer S, Sheehan‐Dare RA, Goodfield MJ, Evans EG. Prediction of outcome in the treatment of onychomycosis. Clinical and Experimental Dermatology 2003;28(4):425‐8. [PUBMED: 12823307] [DOI] [PubMed] [Google Scholar]
Tausch 1997 {published data only}
- Tausch I, Brautigam M, Weidinger G, Jones TC. Evaluation of 6 weeks treatment of terbinafine in tinea unguium in a double‐blind trial comparing 6 and 12 weeks therapy. The Lagos V Study Group. British Journal of Dermatology 1997;136(5):737‐42. [PUBMED: 9205509] [PubMed] [Google Scholar]
van der Schroeff 1992 {published data only}
- Schroeff JG, Cirkel PK, Crijns MB, Dijk TJ, Govaert FJ, Groeneweg DA, et al. A randomized treatment duration‐finding study of terbinafine in onychomycosis. British Journal of Dermatology 1992;126(Suppl 39):36‐9. [PUBMED: 1531927] [DOI] [PubMed] [Google Scholar]
Warshaw 2001 {published data only}
- Warshaw EM, Carver SM, Zielke GR, Ahmed DD. Intermittent terbinafine for toenail onychomycosis: is it effective? Results of a randomized pilot trial. Archives of Dermatology 2001; Vol. 137, issue 9:1253. [PUBMED: 11559235] [PubMed]
Warshaw 2005 {published data only}
- Warshaw EM, Fett DD, Bloomfield HE, Grill JP, Nelson DB, Quintero V, et al. Pulse versus continuous terbinafine for onychomycosis: a randomized, double‐blind, controlled trial. Journal of the American Academy of Dermatology 2005;53(4):578‐84. [PUBMED: 16198776] [DOI] [PubMed] [Google Scholar]
Watanabe 2004 {published data only}
- Watanabe S. Optimal dosages and cycles of itraconazole pulse therapy for onychomycosis. Japanese Journal of Medical Mycology 2004;45(3):143‐7. [PUBMED: 15284828] [DOI] [PubMed] [Google Scholar]
Yadav 2015 {published data only}
- Yadav P, Singal A, Pandhi D, Das S. Comparative efficacy of continuous and pulse dose terbinafine regimes in toenail dermatophytosis: A randomized double‐blind trial. Indian Journal of Dermatology, Venereology and Leprology 2015;81(4):363‐9. [PUBMED: 26087080] [DOI] [PubMed] [Google Scholar]
Zaias 1983 {published data only}
- Zaias N, Drachman D. A method for the determination of drug effectiveness in onychomycosis. Trials with ketoconazole and griseofulvin ultramicrosize. Journal of the American Academy of Dermatology 1983;9(6):912‐9. [PUBMED: 6315789] [DOI] [PubMed] [Google Scholar]
Additional references
Bell‐Syer 2004
- Bell‐Syer SEM, Hall J, Bigby M. Oral treatments for toenail onychomycosis. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004766] [DOI] [Google Scholar]
Bombace 2016
- Bombace F, Iovene MR, Galdiero M, Martora F, Nicoletti GF, D'Andrea M, et al. Non‐dermatophytic onychomycosis diagnostic criteria: an unresolved question. Mycoses 2016;59(9):558‐65. [PUBMED: 27061613] [DOI] [PubMed] [Google Scholar]
Brillowska‐Dabrowska 2007
- Brillowska‐Dabrowska A, Saunte DM, Arendrup MC. Five‐hour diagnosis of dermatophyte nail infections with specific detection of Trichophyton rubrum. Journal of Clinical Microbiology 2007;45(4):1200‐4. [PUBMED: 17267633] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cathcart 2009
- Cathcart S, Cantrell W, Elewski B. Onychomycosis and diabetes. Journal of the European Academy of Dermatology and Venereology: JEADV 2009;23(10):1119‐22. [PUBMED: 19309423] [DOI] [PubMed] [Google Scholar]
Crawford 2007
- Crawford F, Hollis S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD001434.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Berker 2009
- Berker D. Clinical practice. Fungal nail disease. New England Journal of Medicine 2009;360(20):2108‐16. [PUBMED: 19439745] [DOI] [PubMed] [Google Scholar]
De Berker 2013
- Berker D. Nail anatomy. Clinics in Dermatology 2013;31(5):509‐15. [PUBMED: 24079579] [DOI] [PubMed] [Google Scholar]
De Sa 2014
- Sa DC, Lamas AP, Tosti A. Oral therapy for onychomycosis: an evidence‐based review. American Journal of Clinical Dermatology 2014;15(1):17‐36. [PUBMED: 24352873] [DOI] [PubMed] [Google Scholar]
Drake 1998
- Drake LA, Scher RK, Smith EB, Faich GA, Smith SL, Hong JJ, et al. Effect of onychomycosis on quality of life. Journal of the American Academy of Dermatology 1998;38(5 Pt 1):702‐4. [PUBMED: 9591814] [DOI] [PubMed] [Google Scholar]
Drake 1999
- Drake LA, Patrick DL, Fleckman P, Andr J, Baran R, Haneke E, et al. The impact of onychomycosis on quality of life: development of an international onychomycosis‐specific questionnaire to measure patient quality of life. Journal of the American Academy of Dermatology 1999;41(2 Pt 1):189‐96. [PUBMED: 10426887] [DOI] [PubMed] [Google Scholar]
El‐Gohary 2014
- El‐Gohary M, Zuuren EJ, Fedorowicz Z, Burgess H, Doney L, Stuart B, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD009992.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elewski 2015
- Elewski BE, Tosti A. Risk Factors and Comorbidities for Onychomycosis: Implications for Treatment with Topical Therapy. Journal of Clinical and Aesthetic Dermatology 2015;8(11):38‐42. [PUBMED: 26705439] [PMC free article] [PubMed] [Google Scholar]
ETG Dermatology 2009
- Dermatology Expert Group, Skin Infections Expert Group. Therapeutic Guidelines: Dermatology. Version 3. Melbourne: Therapeutic Guidelines Limited, 2009. [ISBN: 9780980476439] [Google Scholar]
Ferrari 2011
- Ferrari J. Fungal toenail infections. BMJ Clinical Evidence 2011:1715. [PUBMED: 21846413] [PMC free article] [PubMed]
Fleckman 2001
- Fleckman P, Allan C. Surgical anatomy of the nail unit. Dermatologic Surgery 2001;27(3):257‐60. [PUBMED: 11277893] [PubMed] [Google Scholar]
Geyer 2004
- Geyer AS, Onumah N, Uyttendaele H, Scher RK. Modulation of linear nail growth to treat diseases of the nail. Journal of the American Academy of Dermatology 2004;50(2):229‐34. [PUBMED: 14726877] [DOI] [PubMed] [Google Scholar]
Ghannoum 2000
- Ghannoum MA, Hajjeh RA, Scher R, Konnikov N, Gupta AK, Summerbell R, et al. A large‐scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. Journal of the American Academy of Dermatology 2000;43(4):641‐8. [PUBMED: 11004620] [DOI] [PubMed] [Google Scholar]
Ghannoum 2014
- Ghannoum M, Isham N. Fungal nail infections (onychomycosis): a never‐ending story?. PLoS Pathogens 2014;10(6):e1004105. [PUBMED: 24901242] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenblatt 2014
- Greenblatt HK, Greenblatt DJ. Liver injury associated with ketoconazole: review of the published evidence. Journal of Clinical Pharmacology 2014;54(12):1321‐9. [PUBMED: 25216238] [DOI] [PubMed] [Google Scholar]
Grover 2012
- Grover C, Khurana A. An update on treatment of onychomycosis. Mycoses 2012;55(6):541‐51. [PUBMED: 22540995] [DOI] [PubMed] [Google Scholar]
Gupta 1994
- Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. Part I. Journal of the American Academy of Dermatology 1994;30(5 Pt 1):677‐98; quiz 698‐700. [PUBMED: 8176006] [PubMed] [Google Scholar]
Gupta 1994a
- Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. Part II. Journal of the American Academy of Dermatology 1994;30(6):911‐33; quiz 934‐6. [PUBMED: 7619094] [DOI] [PubMed] [Google Scholar]
Gupta 1998
- Gupta AK, Konnikov N, MacDonald P, Rich P, Rodger NW, Edmonds MW, et al. Prevalence and epidemiology of toenail onychomycosis in diabetic subjects: a multicentre survey. British Journal of Dermatology 1998;139(4):665‐71. [PUBMED: 9892911] [DOI] [PubMed] [Google Scholar]
Gupta 2013
- Gupta AK, Simpson FC. Diagnosing onychomycosis. Clinics in dermatology 2013;31(5):540‐3. [PUBMED: 24079582] [DOI] [PubMed] [Google Scholar]
Gupta 2015
- Gupta AK, Daigle D, Paquet M. Therapies for Onychomycosis A Systematic Review and Network Meta‐analysis of Mycological Cure. Journal of the American Podiatric Medical Association 2015;105(4):357‐66. [PUBMED: 25032982] [DOI] [PubMed] [Google Scholar]
Hay 2011
Higgins 2003
- Higgins JPT, Thompson, SG, Deeks, JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7417):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ioannidis 2007
- Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta‐analyses. BMJ 2007;335(7626):914‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kao 2014
- Kao WY, Su CW, Huang YS, Chou YC, Chen YC, Chung WH, et al. Risk of oral antifungal agent‐induced liver injury in Taiwanese. British Journal of Clinical Pharmacology 2014;77(1):180‐9. [PUBMED: 23750489] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayser 2009
- Mayser P, Freund V, Budihardja D. Toenail onychomycosis in diabetic patients: issues and management. American Journal of Clinical Dermatology 2009;10(4):211‐20. [PUBMED: 19489654] [DOI] [PubMed] [Google Scholar]
Poulakos 2016
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scher 2007
- Scher RK, Tavakkol A, Sigurgeirsson B, Hay RJ, Joseph WS, Tosti A, et al. Onychomycosis: diagnosis and definition of cure. Journal of the American Academy of Dermatology 2007;56(6):939‐44. [PUBMED: 17307276] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. The GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. www.guidelinedevelopment.org/handbook 2013.
Shaw 2002
- Shaw JW, Joish VN, Coons SJ. Onychomycosis: health‐related quality of life considerations. PharmacoEconomics 2002;20(1):23‐36. [PUBMED: 11817990] [DOI] [PubMed] [Google Scholar]
Shenoy 2008
- Shenoy MM, Teerthanath S, Karnaker VK, Girisha BS, Krishna Prasad MS, Pinto J. Comparison of potassium hydroxide mount and mycological culture with histopathologic examination using periodic acid‐Schiff staining of the nail clippings in the diagnosis of onychomycosis. Indian journal of dermatology, venereology and leprology 2008;74(3):226‐9. [PUBMED: 18583788] [DOI] [PubMed] [Google Scholar]
Verrier 2012
- Verrier J, Pronina M, Peter C, Bontems O, Fratti M, Salamin K, et al. Identification of infectious agents in onychomycoses by Polymerase Chain Reaction‐Terminal Restriction Fragment Length Polymorphism. Journal of Clinical Microbiology 2012;50(3):553‐61. [PUBMED: 22170903] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watanabe 2010
- Watanabe S, Harada T, Hiruma M, Iozumi K, Katoh T, Mochizuki T, et al. Epidemiological survey of foot diseases in Japan: results of 30,000 foot checks by dermatologists. Journal of Dermatology 2010;37(5):397‐406. [PUBMED: 20536644] [DOI] [PubMed] [Google Scholar]
Weinberg 2003
- Weinberg JM, Koestenblatt EK, Tutrone WD, Tishler HR, Najarian L. Comparison of diagnostic methods in the evaluation of onychomycosis. Journal of the American Academy of Dermatology 2003;49(2):193‐7. [PUBMED: 12894064] [DOI] [PubMed] [Google Scholar]
Wolff 2007
- Wolff K, Lowell AG, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Fitzpatrick's Dermatology in General Medicine. 7. McGraw‐Hill Professional, 2007. [ISBN: 0071466908] [Google Scholar]
Yan 2014
- Yan J, Wang X, Chen S. Systematic review of severe acute liver injury caused by terbinafine. International Journal of Clinical Pharmacy 2014;36(4):679‐83. [PUBMED: 24986266] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kreijkamp‐Kaspers 2012
- Kreijkamp‐Kaspers S, Bell‐Syer Sally EM, Magin P, Bell‐Syer Sophie V, Driel Mieke L. Oral antifungal medication for toenail onychomycosis. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD010031] [DOI] [PMC free article] [PubMed] [Google Scholar]


