Abstract
Background
A couple may be considered to have fertility problems if they have been trying to conceive for over a year with no success. This may affect up to a quarter of all couples planning a child. It is estimated that for 40% to 50% of couples, subfertility may result from factors affecting women. Antioxidants are thought to reduce the oxidative stress brought on by these conditions. Currently, limited evidence suggests that antioxidants improve fertility, and trials have explored this area with varied results. This review assesses the evidence for the effectiveness of different antioxidants in female subfertility.
Objectives
To determine whether supplementary oral antioxidants compared with placebo, no treatment/standard treatment or another antioxidant improve fertility outcomes for subfertile women.
Search methods
We searched the following databases (from their inception to September 2016) with no language or date restriction: Cochrane Gynaecology and Fertility Group (CGFG) specialised register, the Cochrane Central Register of Studies (CENTRAL CRSO), MEDLINE, Embase, PsycINFO, CINAHL and AMED. We checked reference lists of appropriate studies and searched for ongoing trials in the clinical trials registers.
Selection criteria
We included randomised controlled trials (RCTs) that compared any type, dose or combination of oral antioxidant supplement with placebo, no treatment or treatment with another antioxidant, among women attending a reproductive clinic. We excluded trials comparing antioxidants with fertility drugs alone and trials that only included fertile women attending a fertility clinic because of male partner infertility.
Data collection and analysis
Two review authors independently selected eligible studies, extracted the data and assessed the risk of bias of the included studies. The primary review outcome was live birth; secondary outcomes included clinical pregnancy rates and adverse events. We pooled studies using a fixed‐effect model, and calculated odds ratios (ORs) with 95% confidence intervals (CIs) for the dichotomous outcomes of live birth, clinical pregnancy and adverse events. We assessed the overall quality of the evidence by applying GRADE criteria.
Main results
We included 50 trials involving 6510 women. Investigators compared oral antioxidants, including combinations of antioxidants, N‐acetyl‐cysteine, melatonin, L‐arginine, myo‐inositol, D‐chiro‐inositol, carnitine, selenium, vitamin E, vitamin B complex, vitamin C, vitamin D+calcium, CoQ10, pentoxifylline and omega‐3‐polyunsaturated fatty acids versus placebo, no treatment/standard treatment or another antioxidant.
Very low‐quality evidence suggests that antioxidants may be associated with an increased live birth rate compared with placebo or no treatment/standard treatment (OR 2.13, 95% CI 1.45 to 3.12, P > 0.001, 8 RCTs, 651 women, I2 = 47%). This suggests that among subfertile women with an expected live birth rate of 20%, the rate among women using antioxidants would be between 26% and 43%.
Very low‐quality evidence suggests that antioxidants may be associated with an increased clinical pregnancy rate compared with placebo or no treatment/standard treatment (OR 1.52, 95% CI 1.31 to 1.76, P < 0.001, 26 RCTs, 4271 women, I2 = 66%). This suggests that among subfertile women with an expected clinical pregnancy rate of 22%, the rate among women using antioxidants would be between 27% and 33%. Heterogeneity was moderately high.
There was insufficient evidence to determine whether there was a difference between the groups in rates of miscarriage (OR 0.79, 95% CI 0.58 to 1.08, P = 0.14, 18 RCTs, 2834 women, I2 = 23%, very low quality evidence). This suggests that, among subfertile women with an expected miscarriage rate of 7%, use of antioxidants would be expected to result in a miscarriage rate of between 4% and 7%. There was also insufficient evidence to determine whether there was a difference between the groups in rates of multiple pregnancy (OR 1.00, 95% CI 0.73 to 1.38, P = 0.98, 8 RCTs, 2163 women, I2 = 4%, very low quality evidence). This suggests that among subfertile women with an expected multiple pregnancy rate of 8%, use of antioxidants would be expected to result in a multiple pregnancy rate between 6% and 11%. Likewise, there was insufficient evidence to determine whether there was a difference between the groups in rates of gastrointestinal disturbances (OR 1.55, 95% CI 0.47 to 5.10, P = 0.47, 3 RCTs, 343 women, I2 = 0%, very low quality evidence). This suggests that among subfertile women with an expected gastrointestinal disturbance rate of 2%, use of antioxidants would be expected to result in a rate between 1% and 11%. Overall adverse events were reported by 35 trials in the meta‐analysis, but there was insufficient evidence to draw any conclusions.
Only one trial reported on live birth, clinical pregnancy or adverse effects in the antioxidant versus antioxidant comparison, and no conclusions could be drawn.
Very low‐quality evidence suggests that pentoxifylline may be associated with an increased clinical pregnancy rate compared with placebo or no treatment (OR 2.07, 95% CI 1.20 to 3.56, P = 0.009, 3 RCTs, 276 women, I2 = 0%). This suggests that among subfertile women with an expected clinical pregnancy rate of 25%, the rate among women using pentoxifylline would be between 28% and 53%.
There was insufficient evidence to determine whether there was a difference between the groups in rates of miscarriage (OR 1.34, 95% CI 0.46 to 3.90, P = 0.58, 3 RCTs, 276 women, I2 = 0%) or multiple pregnancy (OR 0.78, 95% CI 0.20 to 3.09, one RCT, 112 women, very low quality evidence). This suggests that among subfertile women with an expected miscarriage rate of 4%, the rate among women using pentoxifylline would be between 2% and 15%. For multiple pregnancy, the data suggest that among subfertile women with an expected multiple pregnancy rate of 9%, the rate among women using pentoxifylline would be between 2% and 23%.
The overall quality of evidence was limited by serious risk of bias associated with poor reporting of methods, imprecision and inconsistency.
Authors' conclusions
In this review, there was very low‐quality evidence to show that taking an antioxidant may provide benefit for subfertile women, but insufficient evidence to draw any conclusions about adverse events. At this time, there is limited evidence in support of supplemental oral antioxidants for subfertile women.
Keywords: Female; Humans; Pregnancy; Abortion, Spontaneous; Abortion, Spontaneous/epidemiology; Administration, Oral; Antioxidants; Antioxidants/administration & dosage; Antioxidants/adverse effects; Infertility, Female; Infertility, Female/drug therapy; Live Birth; Live Birth/epidemiology; Oxidative Stress; Pentoxifylline; Pentoxifylline/adverse effects; Pentoxifylline/therapeutic use; Pregnancy Rate; Pregnancy, Multiple; Randomized Controlled Trials as Topic
Vitamins and minerals for subfertility in women
Review question: Do supplementary oral antioxidants compared with placebo, no treatment/standard treatment or another antioxidant improve fertility outcomes for subfertile women (standard treatment includes less than 1 mg of folic acid).
Background: Many subfertile women undergoing fertility treatment also take dietary supplements in the hope of improving their fertility. This can be a very stressful time for women and their partners. It is important that these couples be given high‐quality evidence that will allow them to make informed decisions on whether taking a supplemental antioxidant when undergoing fertility treatment will improve their chances or cause any adverse effects. This is especially important, as most antioxidant supplements are uncontrolled by regulation. This review aimed to assess whether supplements with oral antioxidants increase a subfertile woman's chances of becoming pregnant and having a baby.
Search date: The evidence is current to September 2016.
Study characteristics: The review includes 50 randomised controlled trials that compare antioxidants with placebo or with no treatment/standard treatment, or with another antioxidant, in a total of 6510 women.
Funding sources: Funding sources were reported by only 14 of the 50 included trials.
Key results: Very low‐quality evidence suggests that antioxidants may be associated with an increased live birth and clinical pregnancy rate. Based on these results, we would expect that out of 100 subfertile women not taking antioxidants, 20 would have a baby, compared with between 26 and 43 women per 100 who would have a baby if taking antioxidants. There was insufficient evidence to draw any conclusions about the adverse effects of miscarriage, multiple births or gastrointestinal effects. Very low‐quality evidence suggests that pentoxifylline may also be associated with increased rates of clinical pregnancy, but there were only three trials in this analysis. In this case we would expect that out of 100 subfertile women not taking pentoxifylline, 25 would become pregnant, compared with between 28 and 53 women per 100 who would become pregnant if taking pentoxifylline to improve their chances of getting pregnant. There was also insufficient evidence to draw any conclusions about the adverse effects of pentoxifylline. Only one trial measured one antioxidant against another,so there was no evidence available to draw any conclusion from this comparison.
Quality of the evidence: The overall quality of evidence was limited by serious risk of bias associated with poor reporting of methods, imprecision and inconsistency.
Summary of findings
Summary of findings for the main comparison.
Antioxidant(s) compared to placebo or no treatment/standard treatment for female subfertility
| Antioxidant(s) compared to placebo or no treatment/standard treatment for female subfertility | ||||||
| Patient or population: subfertile women who had been referred to a fertility clinic and might or might not be undergoing assisted reproductive techniques Setting: fertility clinic Intervention: antioxidant(s) Comparison: placebo or no treatment/standard treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment/standard treatment | Risk with Antioxidant(s) | |||||
| Live birth; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 196 per 1,000 | 342 per 1,000 (262 to 433) | OR 2.13 (1.45 to 3.12) | 651 (8 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 2 | |
| Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 220 per 1,000 | 301 per 1,000 (270 to 332) | OR 1.52 (1.31 to 1.76) | 4271 (26 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 3, 5 | |
| Adverse events ‐ Miscarriage | 68 per 1,000 | 55 per 1,000 (41 to 73) | OR 0.79 (0.58 to 1.08) | 2834 (18 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 2 | |
| Adverse events ‐ Multiple pregnancy | 80 per 1,000 | 80 per 1,000 (60 to 107) | OR 1.00 (0.73 to 1.38) | 2163 (8 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 2 | |
| Adverse events ‐ Gastrointestinal disturbances | 24 per 1,000 | 37 per 1,000 (12 to 112) | OR 1.55 (0.47 to 5.10) | 343 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 4, 5 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded two levels due to very serious risk of bias; at high risk of bias in two domains.
2Downgraded one level due to serious imprecision; the event rate is low (< 300).
3Downgraded two levels due to very serious inconsistency (I2 = 81%) with differing directions of effect.
4Downgraded two levels due to very serious imprecision; the event rate is very low (n = 11).
5In practice, full downgrading not possible as evidence already graded as very low quality.
Summary of findings 2.
Pentoxifylline compared to placebo or no treatment/standard care for female subfertility
| Pentoxifylline compared to placebo or no treatment/standard care for female subfertility | ||||||
| Patient or population: subfertile women who had been referred to a fertility clinic and might or might not be undergoing assisted reproductive techniques Setting: fertility clinic Intervention: pentoxifylline Comparison: placebo or no treatment/standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment/standard care | Risk with Pentoxifylline | |||||
| Live birth; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 250 per 1,000 | 339 per 1,000 (185 to 538) | OR 1.54 (0.68 to 3.50) | 112 (1 study) | ⊕⊝⊝⊝ VERY LOW 1, 2 | |
| Clinical pregnancy; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 245 per 1,000 | 401 per 1,000 (280 to 535) | OR 2.07 (1.20 to 3.56) | 276 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 3, 4 | |
| Adverse events ‐ Miscarriage | 43 per 1,000 | 57 per 1,000 (20 to 150) | OR 1.34 (0.46 to 3.90) | 276 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2, 3 | |
| Adverse events ‐ Multiple pregnancy | 89 per 1,000 | 71 per 1,000 (19 to 233) |
OR 0.78 (0.20 to 3.09) |
112 (1 study) | ⊕⊝⊝⊝ VERY LOW 1, 2 | |
| Adverse events ‐ Gastrointestinal disturbances | Not reported in any included study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level due to questionable applicability: study table states that cause of infertility is male in 51 of 112 participants, although text states that the participants were 112 infertile women.
2Downgraded two levels due to very serious imprecision; the event rate is very low (n = 33 for live birth, n = 14 for miscarriage, n=9 for multiple pregnancy), wide confidence intervals.
3Downgraded two levels due to questionable applicability of one study (see footnote 1) and very serious risk of bias: all studies at unclear or high risk of bias in one or more domains.
4Downgraded one level due to serious imprecision; the event rate is low (n = 87).
Background
Description of the condition
A couple that has tried to conceive for a year or longer without success is considered to be subfertile (Evers 2002) or less fertile than a typical couple. The World Health Organization (WHO) (Zegers‐Hochschild 2009) defines infertility as the “failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse”. Levels of infertility in 2010 were similar to those in 1990 in most of the world, apart from declines in Sub‐Saharan Africa and in South Asia (Mascarenhas 2012). Forty to fifty per cent of cases of subfertility are due to causes in women. Influencing factors include ovulatory failure, tubal damage, endometriosis, poor egg quality and unexplained subfertility. It is suggested that up to 25% of couples who are planning a baby have difficulty (Boivin 2007; Hart 2003).
To overcome these fertility problems, many couples undergo assisted fertility techniques (assisted reproductive techniques (ART)). These include ovulation stimulation, intrauterine insemination (IUI), in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI).
Women use antioxidant supplements in preparation for ART and/or simultaneously with the treatment, and some women use supplements alone with no ART in an attempt to improve their fertility.
Description of the intervention
Antioxidants are biological and chemical compounds that reduce oxidative damage (imbalance between creation of reactive oxygen species and the body's ability to detoxify). They are a group of organic nutrients that include vitamins, minerals and polyunsaturated fatty acids (PUFAs). Some of the predominant antioxidants used in female subfertility are N‐acetyl‐cysteine; melatonin; vitamins A, C and E; folic acid; myo‐inositol; zinc and selenium. They may be administered as a single antioxidant or as combined therapy.
PUFAs are classified into omega‐3, omega‐6 and omega‐9. Omega‐9 is synthesised by animals, but omegas‐3 and ‐6 need to be supplemented in the diet. The main sources of omega‐6 are vegetable oils. Sources of omega‐3 are vegetable and fish oils. The ratio of omega‐6 to omega‐3 has risen in recent times (as a result of increased intake of vegetable oils) to the point where there is a reduced need for intake of omega‐6 and an increased need for intake of omega‐3 (Wathes 2007).
Pentoxifylline is a conventional medicine, a tri‐substituted xanthine derivative usually prescribed for intermittent claudication (cramping) (Drugs.com 2013). Pentoxifylline is also used in fertility treatment, as it is known to have a strong antioxidant effect by generating reactive oxygen species (Vircheva 2010). It has been shown to benefit men who have varicocoele‐associated infertility (engorged vein in the scrotum) (Oliva 2009).
The amino acid L‐arginine also has antioxidant properties that aid in the inflammatory response and act against oxidative damage (Ko 2012).
When oxidative damage occurs, toxins are produced as a consequence of all cells using oxygen to survive. Toxic end‐products may include molecules that have unpaired electrons, which may lead to the formation of free radicals. Free radicals may cause further harmful reactions with lipids in membranes, amino acids in proteins and carbohydrates within nucleic acids. An antioxidant molecule is thought to be capable of slowing or preventing the oxidation of other molecules and potentially of reducing the production of free radicals, which may cause this cellular damage.
Two major types of free radicals have been identified: reactive oxygen species (ROS) and reactive nitrogen species (RNS). Reactive oxygen species are products of normal cellular metabolism and consist of oxygen ions, free radicals and peroxides. The addition of one electron to oxygen forms the superoxide anion radical, which then can be converted to hydroxyl radical, peroxyl radical or hydrogen peroxide. Free radicals seek to participate in chemical reactions that relieve them of their unpaired electron, resulting in oxidation (Ruder 2008; Tremellen 2008). The presence of ROSs within the ovary and the endometrium has significant physiological and pathological implications for women when they try to conceive. Oxidative stress (OS) is a result of an imbalance between the amount of ROS and the quantity of natural antioxidants present within the body, and results in overwhelming the body’s natural defence mechanism. Both oxidative stress and ROS can attack lipids, proteins DNA and affect metabolic pathways (chemical transformations in the cells) (Agarwal 2012). Natural antioxidants present in the body include catalase, glutathione peroxidase, superoxide dismutase and glutathione reductase, vitamins C and E, ferritin and transferrin (Gupta 2007).
Indirect evidence from smoking and alcohol trials suggests that these factors have a negative impact on female fertility, potentially through the generation of excessive oxidative stress (Agarwal 2012; Ruder 2008). Other lifestyle factors such as diet, disease, pollution, stress and allergies also contribute to increased levels of free radicals (Agarwal 2012).
The global vitamin and supplement market has grown exponentially and has been reported in 2016 as being worth over USD 140 billion, growing from USD 96 billion in 2012 (Global Supplement report 2016; Reportlinker.com 2010). In 2009 sales of vitamins and dietary supplements in the United Kingdom "totalled £674.6 million, a growth of about 16% over the previous five years, with the two biggest selling areas being multivitamins (£138.6 million) and fish oils (£139.1 million)" (NHS News 2011). Multivitamin sales have increased steadily since 2009, with reported sales in 2015 of GBP 414 million, with sales to women accounting for the largest group (Mintel 2016). Vitamins and supplements are dispensed through various retail outlets, including health food shops, online retailers, health centres, fitness clubs, supermarkets and pharmacies.
In an effort to enhance fertility, couples are increasingly resorting to ART; however, these techniques do not cure the causes of subfertility, but rather overcome some of its barriers. Adjunct measures, including courses of dietary supplements such as oral antioxidants, may be beneficial (Ebisch 2007). However, most antioxidant supplements are uncontrolled by regulation, and thus their effects may be unpredictable in the population.
How the intervention might work
Antioxidants are said to have an important role in the regulation of all processes involved in the birth of a healthy baby (Gupta 2007). The local development of oxidative stress will have significant adverse effects on these processes. Conditions with which the adverse effects of oxidative stress may be associated in subfertile women include endometriosis, hydrosalpinges (dilated fallopian tubes), polycystic ovarian syndrome (PCOS), fetal malformations and potentially unexplained subfertility (Agarwal 2012; Ruder 2008; Zhao 2006).
At the time of conception, oxidative stress can lead to cell membrane lipid peroxidation, cellular protein oxidation and DNA damage, causing a negative effect upon the oocyte (immature egg cell), the embryo and implantation (Ruder 2008). Antioxidants would be expected to counteract the negative impact of oxygen‐free radicals by acting as free radical scavengers.
Supplementary antioxidants may have several methods of action. Fertility benefits of vitamin E include improvement in epithelial growth in blood vessels and in the endometrium (Ledee‐Bataille 2002). Higher vitamin D levels are associated with an increased likelihood of successful pregnancy and may be of particular benefit to women with PCOS in lowering hyperandrogenism (androgen excess) (Thomson 2012). Myo‐inositol helps ovarian function and decreases hyperandrogenism and insulin resistance (Nestler 1998); L‐arginine improves endometrial blood flow (Takasaki 2009); N‐acetyl‐cysteine is needed for fertile cervical mucus and ovulation (Badawy 2007); and PUFAs influence prostaglandin (lipid compounds with hormone‐like effects) synthesis and steroidogenesis (creation of steroid hormones), and also play a role in the composition of cell membranes of the sperm and oocyte, which is important during fertilisation (Wathes 2007). Cohort studies have shown some evidence suggesting that in some instances taking a multivitamin tablet may increase fertility (Haggarty 2006) or even regulate ovulation (Charvarro 2008).
Why it is important to do this review
There is currently limited evidence as to whether antioxidants improve fertility, and ongoing trials in this area show varied results. This review assesses the effectiveness of different antioxidants and different dosages. This is an update of a review first published in 2013 (Showell 2013)
Subfertile women are highly motivated to explore all avenues of treatment in their desire to have a healthy baby. Antioxidants are mostly unregulated and are readily available for purchase by consumers. Research has suggested that a significant number of women undergoing fertility treatment are taking oral supplements in the expectation that this will improve their chances of conception (O'Reilly 2014; Stankiewicz 2007). Consumer perception is that antioxidant therapy is not associated with harm and is associated only with benefit. It is important to establish whether or not this therapy does improve fertility and whether it is associated with any harm.
Objectives
To determine whether supplementary oral antioxidants compared with placebo, no treatment/standard treatment or another antioxidant improve fertility outcomes for subfertile women.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria
Randomised controlled trials (RCTs).
Cross‐over trials are included; however, we used only first‐phase data in the analysis. Achieving outcomes such as pregnancy and live birth would preclude entry of couples into the next trial phase (Dias 2006).
Exclusion criteria
Any quasi‐randomised trials.
Types of participants
Inclusion criteria
Trials that included subfertile women who had been referred to a fertility clinic and might or might not be undergoing assisted reproductive techniques (ART) such as in vitro fertilisation (IVF), intrauterine insemination (IUI) or intracytoplasmic sperm injection (ICSI).
Exclusion criteria
Trials enrolling only fertile women attending a fertility clinic exclusively as the result of male partner infertility.
Trials enrolling women exclusively with Vitamin D deficiency.
Types of interventions
Inclusion criteria
Any type of oral antioxidant supplementation versus control: placebo (plus or minus a co‐intervention) or no treatment/standard treatment (standard treatment includes folic acid < 1 mg);
Individual or combined oral antioxidants versus any antioxidant (head‐to‐head trials); or
Pentoxifylline versus control (placebo or no treatment/standard treatment).
On clinical advice, we analysed trials that used folic acid (standard treatment) and those that included a co‐intervention (a fertility drug such as clomiphene citrate or metformin) in both arms in the antioxidant versus placebo or no treatment/standard treatment comparison and not in the head‐to‐head comparison, as the controls were not considered to be active treatments. We analysed pentoxifylline trials as a separate comparison, as it was not possible to separate the antioxidant effects from the other medical effects of the drug.
Exclusion criteria
Interventions that included antioxidants alone versus fertility drugs as controls. These fertility drugs included metformin and clomiphene citrate.
Types of outcome measures
Primary outcomes
Live birth rate per woman randomly assigned: if live birth data were unavailable and the trial reported ongoing pregnancy, we reported ongoing pregnancy as live birth (footnoted in the forest plot). We define live birth as delivery of a live fetus after 20 completed weeks of gestation, and ongoing pregnancy as evidence of a gestational sac with fetal heart motion at 12 weeks, confirmed with ultrasound.
Secondary outcomes
Clinical pregnancy rate per woman (as confirmed by the identification of a gestational sac on ultrasound at seven or more weeks' gestation).
Any adverse effects reported by the trial. We subgrouped these events by the type of adverse event reported.
Search methods for identification of studies
We searched for all reports, published and unpublished, that described RCTs investigating oral antioxidant supplementation for subfertile women and its impact on live birth, pregnancy and adverse events rates. We used both indexed and free‐text terms, and applied no language or date restrictions.
Electronic searches
We searched the following databases:
The Cochrane Gynaecology and Fertility Group's (CGFG) specialised register of controlled trials from inception to September 2016 (Appendix 1). This register contains published and unpublished trials and conference abstracts;
Cochrane Central Register of Studies (CENTRAL CRSO) (from inception to September 2016) (Appendix 2);
MEDLINE (1946 to September 2016) (Appendix 3);
Embase (1980 to September 2016) (Appendix 4);
PsycINFO (from 1806 to September 2016) (Appendix 5);
AMED (Allied and Complementary Medicine) (1985 to September 2016) (Appendix 6);
CINAHL (1982 to September 2016) (Appendix 7).
The MEDLINE search was limited by the Cochrane highly sensitive search strategy filter for identifying randomised trials, which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.1.0, Chapter 6, 6.4.11) (Higgins 2011). We combined the Embase and CINAHL (OVID platform only) searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/mehodology/filters.html#random).
Searching other resources
(last searched September 2016)
International trial registers: the ClinicalTrials database, a service of the US National Institutes of Health (clinicaltrials.gov/ct2/home) and the World Health Organization International Trials Registry Platform search portal (www.who.int/trialsearch/Default.aspx);
Web of Knowledge for conference proceedings and published trials;
Google, using the keywords 'antioxidants female infertility' and 'antioxidants female subfertility';
Database for Abstracts of Reviews of Effects (DARE) for other reviews on this topic;
'Grey' literature (unpublished and unindexed), through the openGREY database (www.opengrey.eu/); (Appendix 8).
We also contacted known experts and personal contacts for information on any unpublished materials, and we checked the citation lists of appropriate papers for any relevant references.
Data collection and analysis
We conducted data collection and analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (MGS and RM‐P) independently reviewed titles and abstracts of trials for eligibility. We obtained the full texts of trials that we considered for inclusion. We sought further information from the authors of trials that did not contain sufficient information to make a decision about eligibility. We resolved any disagreements by reference to a third review author. We documented the selection process with a PRISMA flow chart (see Figure 1).
Data extraction and management
Two review authors (MGS and RM‐P) independently extracted data from the included trials using a data extraction form. We compared the two sets of extracted data and resolved discrepancies by discussion. The review authors screened the trials to ensure that there were no duplicate publications.
We designed the data extraction forms to extract information on study characteristics and outcomes. We have included this information and presented it in the Characteristics of included studies and the Characteristics of excluded studies tables, in keeping with the guidance provided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If any information on trial methodology or any trial data were missing, we contacted the study authors by email and by post. The predominant questions for trial authors concerned live birth data, clinical pregnancy, methods of randomisation and allocation concealment.
Assessment of risk of bias in included studies
We assessed the included studies for risks of bias using the Cochrane 'Risk of bias' tool, to assess selection bias (sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (completeness of outcome data); reporting bias (selective outcome reporting); and other potential sources of bias. Two review authors (MGS and RM‐P) assessed the included studies according to these six criteria, resolving any disagreements by discussion with a third review author. We sought published protocols.
We took care to search for within‐study selective reporting, for example trials failing to report outcomes such as live birth or reporting them in insufficient detail to allow inclusion. Where protocols were available, we assessed studies for differences between study protocols and published results.
In cases where included studies failed to identify the primary outcome of live birth but did report pregnancy rates, we carried out an informal assessment to determine whether pregnancy rates were similar to those in studies that reported live birth.
Measures of treatment effect
We expressed the dichotomous data for live birth, pregnancy rate, miscarriage and adverse events as Mantel‐Haenszel odds ratios (ORs) with 95% confidence intervals (95% CIs).
Unit of analysis issues
We analysed the outcomes of live birth, pregnancy and adverse events per woman randomly assigned, counting multiple births as one live birth event.
Dealing with missing data
In cases where trial data were missing, we first sought information from the original trial investigators. Details of authors contacted and the questions asked of them are contained in Characteristics of included studies. In addition, and where possible, we performed analyses on all outcomes on an intention‐to‐treat basis, i.e. to include in the analyses all women randomly assigned to each group and to analyse all women in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity according to the guidelines set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We examined heterogeneity between the results of different trials by visually examining the forest plots and the overlap of confidence intervals (poor overlap suggested heterogeneity), by considering the P value (a low P value or a large Chi2 statistic relative to the degree of freedom suggests heterogeneity), and by identifying the I2 statistic. If I2 was 50% or higher, we assumed high heterogeneity, and conducted a sensitivity analysis. A high I2 statistic suggests that variations in effect estimates were due to differences between trials rather than to chance alone.
Assessment of reporting biases
The search strategies covered multiple sources, without language or publication restrictions. We were alert to the possibility of duplication of data. We used a funnel plot to explore the possibility of small‐study effects in cases where estimates of intervention effect can be more beneficial in smaller studies (Higgins 2011).
Data synthesis
We conducted statistical analysis of the data using Review Manager 5 (RevMan 2014). We considered pregnancy outcomes to be positive, and higher numbers of pregnancy rates to be a benefit. We considered the outcomes of miscarriage and adverse events to be negative effects, and higher numbers harmful.
We combined data from primary studies using a fixed‐effect model in the following comparisons:
Antioxidants versus control (placebo or no treatment/standard treatment);
Antioxidants versus antioxidants or head‐to‐head stratification by type of antioxidant; and
Pentoxifylline versus control (placebo or no treatment/standard treatment).
We displayed increases in the odds of a particular outcome, which may be beneficial (e.g. live birth) or detrimental (e.g. adverse effects), graphically in meta‐analyses to the right of the centre line, and decreases in the odds of a particular outcome to the left of the centre line.
The aim was to define analyses that were comprehensive and mutually exclusive, so that we could slot all eligible study results into one stratum only. We specified comparisons so that any trials falling within each stratum could be pooled for meta‐analysis. Stratification allowed for consideration of effects within each stratum, as well as or instead of an overall estimate for comparison.
In trials with multiple arms, we pooled intervention groups versus the control group.
If individuals had been randomly re‐assigned after failed cycles, we did not pool the data in a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analyses:
Type of control, placebo or no treatment;
Type of antioxidant, whether individual or combined (three or more antioxidants combined);
Trials that enrolled women with different indications for infertility (i.e. PCOS, endometriosis, unexplained infertility or poor responders); and
Trials that enrolled women who were also undergoing IVF or ICSI.
If we detected substantial heterogeneity, we explored possible explanations by performing sensitivity analyses.
Sensitivity analysis
We conducted sensitivity analyses (using the random‐effects model in RevMan software) on the primary outcomes if we detected a high degree of heterogeneity (where the I2 statistic was 50% or more), excluding studies:
with a high risk of bias, or
that used antioxidants plus folic acid versus standard treatment (folic acid < 1 mg); or
that used antioxidants plus a fertility drug (a co‐intervention) versus placebo plus a fertility drug.
Overall quality of the body of evidence: 'Summary of findings' tables
We produced a 'Summary of findings' table, using GRADEpro GDT software (GRADEpro GDT 2015) and Cochrane methods (Higgins 2011) for the main review comparison (Antioxidant(s) compared to placebo or no treatment/standard treatment). This table evaluates the overall quality of the body of evidence for the main review outcomes (live birth, clinical pregnancy and adverse events), using GRADE criteria (study limitations, i.e. risk of bias, consistency of effect, imprecision, indirectness and publication bias). We have included an additional 'Summary of findings' table for the main review outcomes for the comparison of pentoxifylline compared to placebo or no treatment/standard care. Two review authors, working independently, made judgements about evidence quality ('high', 'moderate', 'low' or 'very low').
Results
Description of studies
Results of the search
2013 version of the review
The search retrieved 2127 abstracts and titles, which we screened to identify trials that met our inclusion criteria. We retrieved the full texts of 67 trials for appraisal. Only one study (Bonakdaran 2012) was not published in English, with the full text in Persian; however, the English abstract contained enough information to show that it did not meet the inclusion criteria, and we therefore excluded it. Of the 67 studies assessed, we included 27 and excluded 39. Please see Characteristics of included studies and Characteristics of excluded studies for study details. A repeat search in April 2013 revealed seven studies (Carlomagno 2012; Choi 2012; Mohammadbeigi 2012; Rosalbino 2012; Salehpour 2012; Schachter 2007; Salem 2012) that we placed into the 'Awaiting classification' section of the review. We found 12 ongoing trials in searches of the clinical trial registers (see Ongoing studies).
2017 Update
We assessed 926 abstracts (after 222 duplicates were removed) for inclusion from the title and abstract found in a search dated from April 2013 to September 2016. We assessed 39 of these papers in full text. One study was published in Persian (Mohammadbeigi 2012) and required translation (see Acknowledgements). We excluded 15 articles (14 studies) of the 39, and included 24 (23 studies). Of the latter, six were from the seven trials placed in 'Awaiting classification' in the original review, while Salem 2012 was excluded due to inappropriate intervention and control. See the PRISMA flow chart (Figure 1). For the current update four of the 12 previously ongoing trials are now included (Bentov 2014; Mohammadbeigi 2012; Unfer 2011; Youssef 2015). The conference abstract of the included study Aboulfoutouh 2011 in the original review became a secondary reference of Youssef 2015 in the update and Rezk 2004, formerly an excluded study, is now included as a secondary reference of Rizk 2005. Pourghassem 2010 was found to be the same trial as the excluded Ardabili 2012. We excluded Pasha 2011 due to an ineligible population. We added two trials (NCT03023514; NCT02058212) after the search in September 2016, so eight trials remain ongoing (Fernando 2014; NCT01019785; NCT03023514; NCT02058212; IRCT201112148408N1; CTRI/2012/08/002943; NCT01782911; NCT01267604).
Figure 1.
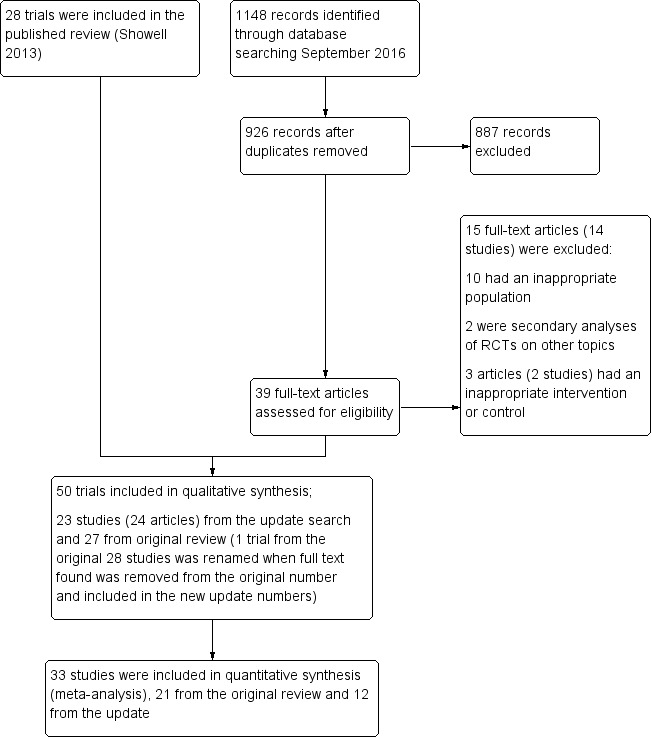
Study flow diagram.
We include 23 new trials in the 2017 update: Battaglia 1999; Bentov 2014; Brusco 2013; Carlomagno 2012; Cheraghi 2016; Choi 2012; Colazingari 2013; Daneshbodi 2013; Deeba 2015; El Refaeey 2014; Ismail 2014; Keikha 2010; Lesoine 2016; Maged 2015; Mohammadbeigi 2012; Pacchiarotti 2016; Panti Abubakar 2015; Polak de Fried 2013; Razavi 2015; Rosalbino 2012; Salehpour 2012; Schachter 2007; Valeri 2015.
Fifty trials are now included in this updated review (Characteristics of included studies) and 50 have been excluded (Characteristics of excluded studies).
Included studies
Fifty trials met the criteria for inclusion. Fourteen were based in Italy (Battaglia 1999; Battaglia 2002; Brusco 2013; Carlomagno 2012; Ciotta 2011; Colazingari 2013; Gerli 2007; Lisi 2012; Papaleo 2009; Pacchiarotti 2016; Rizzo 2010; Rosalbino 2012; Unfer 2011; Valeri 2015). Ten were based in Iran (Alborzi 2007; Aleyasin 2009; Cheraghi 2016; Daneshbodi 2013; Keikha 2010; Mohammadbeigi 2012; Rashidi 2009; Razavi 2015; Salehpour 2009; Salehpour 2012), seven in Egypt (Badawy 2006; El Refaeey 2014; Ismail 2014; Maged 2015; Rizk 2005; Nasr 2010; Youssef 2015), four in Turkey (Batioglu 2012; Cicek 2012; Eryilmaz 2011; Ozkaya 2011), three in Korea (Choi 2012; Kim 2006; Kim 2010), two in Spain (Creus 2008; Balasch 1997) and one each in the UK (Agrawal 2012), Hungary/Austria (Griesinger 2002), Mexico (Mier‐Cabrera 2008) USA (Westphal 2006), Canada (Bentov 2014), Bangladesh (Deeba 2015), Germany (Lesoine 2016), Nigeria (Panti Abubakar 2015) Israel (Schachter 2007) and Argentina (Polak de Fried 2013).
We tried to contact authors of all the included trials to obtain further details and clarification. However we could not obtain data for meta‐analysis from 14 trials (Carlomagno 2012; Choi 2012; Colazingari 2013; Daneshbodi 2013; Deeba 2015; Keikha 2010; Kim 2006; Kim 2010; Lesoine 2016; Mohammadbeigi 2012; Ozkaya 2011; Razavi 2015; Rosalbino 2012; Valeri 2015), and one did not report on the outcomes included in this review (Salehpour 2009). In one trial (Gerli 2007) (see Table 6), only half of the participants declared that they wanted to become pregnant before the study began; we have therefore included this trial, but have not used the data in the meta‐analysis (see Characteristics of included studies).
Table 1.
Gerli 2007‐ data not included in meta‐analysis
| Outcome | Data | Notes |
| Clinical pregnancy rate; myo‐inositol + folic acid | 4/23 | Only 42 of the 92 women enrolled in this trial declared a desire to become pregnant |
| Clinical pregnancy rate; folic acid + placebo | 1/19 | ‐ |
| Miscarriage rate; myo‐inositol + folic acid | Miscarriage reported, but unknown whether from treatment or control | 1 miscarriage occurred in the first trimester, but it is unknown from which group |
| Miscarriage rate; folic acid + placebo | Unknown | ‐ |
Duration of treatment ranged from 12 days (Battaglia 2002) to nearly two years (Alborzi 2007). One trial (Bentov 2014) was terminated before the end due to the publication of a paper (Levin 2012) describing the negative effects of polar body biopsy, an adjunctive treatment in this trial, on the development of the embryo. The trial began in 2010 and ran until 2012, enrolling 39 women.
Participants
The trials randomly assigned 6510 subfertile women who were attending a fertility clinic and might or might not be undergoing ART procedures such as IVF, IUI or ICSI. The age range of randomly‐assigned participants was 18 to 44 years; Battaglia 1999 enrolled women who were between 37 and 44 years.
Twenty‐one trials (Brusco 2013; Cheraghi 2016; Choi 2012; Colazingari 2013; Daneshbodi 2013; El Refaeey 2014; Ismail 2014; Keikha 2010; Lesoine 2016; Maged 2015; Mohammadbeigi 2012; Nasr 2010; Pacchiarotti 2016; Panti Abubakar 2015; Papaleo 2009; Razavi 2015; Rizk 2005; Rosalbino 2012; Salehpour 2012; Schachter 2007; Unfer 2011) included women with PCOS (four trials in the original review and 17 in the update). Other participants in the trials were enrolled for endometriosis, ovulation failure, tubal blockages and unexplained subfertility. One trial included women aged 35 to 42 years with poor oocyte quality and poor response (Rizzo 2010). Seven trials included women with more than one fertility problem: these reasons included a percentage of male partner subfertility, unexplained subfertility, ovulatory problems, poor responders, PCOS, tubal blockages and endometriosis (Agrawal 2012;Aleyasin 2009; Batioglu 2012;Battaglia 1999; Brusco 2013; Griesinger 2002; Westphal 2006). Four trials included a small percentage of women whose subfertility was caused by the male partner (Aleyasin 2009;Creus 2008;Balasch 1997; Griesinger 2002).
One trial enrolled only women who were aged over 40 (Valeri 2015) and one (Gerli 2007) included participants in whom "infertility was an ailment in only half of the participants in each group". The author of this trial states that there was "no difference in the proportions of infertile women in the groups".
Twenty‐seven studies included women undergoing IVF/ICSI (Aleyasin 2009; Batioglu 2012; Battaglia 1999; Battaglia 2002; Bentov 2014; Brusco 2013; Carlomagno 2012; Cheraghi 2016; Choi 2012; Ciotta 2011; Colazingari 2013; Eryilmaz 2011; Griesinger 2002; Kim 2006; Kim 2010; Lesoine 2016; Lisi 2012; Ozkaya 2011; Pacchiarotti 2016; Papaleo 2009; Polak de Fried 2013; Rizzo 2010; Rosalbino 2012; Salehpour 2009; Unfer 2011; Valeri 2015; Youssef 2015). Eleven studies included women undergoing natural intercourse or ovulation induction with timed intercourse or IUI (Agrawal 2012; Badawy 2006; Cicek 2012; Deeba 2015; El Refaeey 2014; Ismail 2014; Maged 2015; Mohammadbeigi 2012; Panti Abubakar 2015; Rizk 2005; Salehpour 2012). The remaining 12 studies enrolled women who were either having no adjunctive treatment or each trial included a number of differing treatments, i.e. some women having IVF while others were having IUI, and only one trial enrolled women undergoing laparoscopic ovarian drilling (Nasr 2010).
Further details of inclusion and exclusion criteria are available in the Characteristics of included studies table.
Interventions
A variety of antioxidants were used in the included trials. Comparisons covered antioxidants versus placebo, no treatment or standard treatment (folic acid < 1 mg), head‐to‐head comparisons (antioxidant versus antioxidant) and pentoxifylline versus placebo, no treatment or standard treatment.
Comparison antioxidants versus placebo, no treatment and standard treatment included the following: combinations of antioxidants; L‐arginine, vitamin E, myo‐inositol, D‐chiro‐inositol, carnitine, selenium, vitamin B complex, vitamin C, vitamin D+calcium, CoQ10, and omega‐3 polyunsaturated fatty acids. They were labelled as Octatron® (Youssef 2015), multiple micronutrients (Agrawal 2012; Deeba 2015; Ozkaya 2011; Panti Abubakar 2015) and Fertility Blend (Westphal 2006). The time that women received treatment or control in these trials ranged from two‐and‐a‐half menstrual cycles to six months. Four of these trials (Agrawal 2012; Deeba 2015; Panti Abubakar 2015; Westphal 2006) enrolled women undergoing ovulation induction with timed intercourse, and two (Ozkaya 2011; Youssef 2015) included women undergoing IVF/ICSI. More details of these combination antioxidants are given in the Characteristics of included studies. The remaining 44 trials gave single antioxidants, including two types of inositols and vitamin B complexes. The duration of treatment in these trials ranged from 12 days to two years.
The comparison 'antioxidants versus antioxidants' included only three trials (Colazingari 2013; Keikha 2010; Unfer 2011). Two of these three trials looked at the effects of myo‐inositol versus D‐chiro‐inositol, while Keikha 2010 looked at N‐acetyl‐ cysteine versus vitamin C. Only Unfer 2011 could be used in the meta‐analysis, as Colazingari 2013 and Keikha 2010 did not report on live birth, clinical pregnancy or adverse events. The head‐to‐head comparisons were included in an attempt to assess whether one antioxidant was more effective than another.
In summary:
22 included trials compared antioxidants versus placebo (Alborzi 2007; Badawy 2006; Battaglia 2002; Bentov 2014; Cheraghi 2016; Choi 2012; Daneshbodi 2013; Griesinger 2002; Ismail 2014; Kim 2006; Lesoine 2016; Mier‐Cabrera 2008; Mohammadbeigi 2012; Nasr 2010; Ozkaya 2011; Panti Abubakar 2015; Polak de Fried 2013; Rizk 2005; Rosalbino 2012; Salehpour 2009; Salehpour 2012; Westphal 2006);
21 trials compared antioxidants with 'no treatment' or standard treatment (Agrawal 2012; Batioglu 2012; Battaglia 1999; Brusco 2013; Carlomagno 2012; Cicek 2012; Ciotta 2011; Deeba 2015; El Refaeey 2014; Eryilmaz 2011; Gerli 2007; Lisi 2012; Maged 2015; Pacchiarotti 2016; Papaleo 2009; Rashidi 2009; Razavi 2015; Rizzo 2010; Schachter 2007; Valeri 2015; Youssef 2015);
three trials compared one antioxidant with another antioxidant (head‐to‐head comparisons) (Colazingari 2013; Keikha 2010; Unfer 2011);
two trials compared pentoxifylline with placebo (Balasch 1997; Creus 2008);
one trial compared pentoxifylline plus vitamin E with no treatment (Aleyasin 2009);
11 trials compared antioxidants plus a co‐intervention with a placebo or no treatment plus a co‐intervention at the same dosage (Badawy 2006; Cheraghi 2016; El Refaeey 2014; Maged 2015; Pacchiarotti 2016; Rashidi 2009; Razavi 2015; Rizk 2005; Rizzo 2010; Salehpour 2012; Schachter 2007). The co‐interventions used were clomiphene citrate and metformin;
In one trial, the control was unspecified (Kim 2010), and we tried unsuccessfully to contact this author by email and by post.
Seven trials (Cheraghi 2016; Griesinger 2002; Maged 2015; Pacchiarotti 2016; Rashidi 2009; Rosalbino 2012; Schachter 2007) were multi‐arm and fit into more than one of the above categories. In one trial (Cheraghi 2016) all women were prescribed the oral contraceptive pill as a pretreatment to ICSI.
Outcomes
Live birth
The primary outcome for this review was live birth. Ten trials reported on live birth (Agrawal 2012; Aleyasin 2009; Battaglia 2002; Bentov 2014; Cicek 2012; Nasr 2010; Panti Abubakar 2015; Polak de Fried 2013; Schachter 2007; Unfer 2011). We sent emails and letters to authors of all other included trials to ask whether they had any data on live birth. We received live birth data from Battaglia 2002, Panti Abubakar 2015, and Polak de Fried 2013 by email. Agrawal 2012, Cicek 2012 and Schachter 2007 reported on ongoing pregnancy, which we used as a surrogate for live birth.
Clinical pregnancy
Thirty‐six trials reported on clinical pregnancy rates in the text of the trial reports or through direct communication with the authors (Agrawal 2012; Aleyasin 2009; Badawy 2006; Balasch 1997; Batioglu 2012; Battaglia 1999; Battaglia 2002; Bentov 2014; Brusco 2013; Carlomagno 2012; Cheraghi 2016; Choi 2012; Cicek 2012; Creus 2008; Deeba 2015; El Refaeey 2014; Eryilmaz 2011; Gerli 2007; Griesinger 2002; Ismail 2014; Kim 2010; Lisi 2012; Maged 2015; Nasr 2010; Pacchiarotti 2016; Panti Abubakar 2015; Papaleo 2009; Polak de Fried 2013; Rashidi 2009; Rizk 2005; Rizzo 2010; Salehpour 2012; Schachter 2007; Unfer 2011; Westphal 2006; Youssef 2015). One trial reported only biochemical pregnancy or conception (Ciotta 2011) and another four trials reported only 'pregnancy rates' (Alborzi 2007; Mier‐Cabrera 2008; Mohammadbeigi 2012; Razavi 2015) (see data from these five trials in Table 7). Nine trials did not report any pregnancy outcomes (Colazingari 2013; Daneshbodi 2013; Keikha 2010; Kim 2006; Lesoine 2016; Ozkaya 2011; Rosalbino 2012; Salehpour 2009; Valeri 2015). We tried to contact authors of all the trials that did not report clinical pregnancy rates.
Table 2.
'Biochemical' and 'pregnancy' data for those trials that did not specifically report 'clinical pregnancy'
| Trial | Pregnancy in antioxidant group | Pregnancy in control group |
| Ciotta 2011 | 4/16 (myo‐inositol + folic acid) | 5/18 (folic acid) |
| Alborzi 2007 | 17/43 (pentoxifylline) | 16/45 (placebo) |
| Mier‐Cabrera 2008 | 0/16 (vitamins C + E), at follow‐up over 9 months 3/16 | 0/18 (placebo), at follow‐up over 9 months 2/18 |
| Mohammadbeigi 2012 | 9/22 (vitamin D) | 7/22 (placebo) |
| Razavi 2015 | 6/32 (selenium) | 1/32 (placebo) |
Adverse events
The following adverse events were reported:
Miscarriage: 24 trials either reported on miscarriage, or we calculated the numbers from the differences between live birth and clinical pregnancy rates (Agrawal 2012; Aleyasin 2009; Badawy 2006; Balasch 1997; Battaglia 1999; Battaglia 2002; Bentov 2014; Choi 2012; Cicek 2012; Creus 2008; El Refaeey 2014; Eryilmaz 2011; Ismail 2014; Nasr 2010; Pacchiarotti 2016; Papaleo 2009; Panti Abubakar 2015; Polak de Fried 2013; Rizzo 2010; Rizk 2005; Schachter 2007; Unfer 2011; Westphal 2006; Youssef 2015). We did not include the data from Rizk 2005 in the meta‐analysis for miscarriage, as no pregnancies were reported in the control group, and adding these miscarriage data would have skewed the analysis.
Multiple pregnancy: 10 trials reported on multiple pregnancy (Aleyasin 2009; Badawy 2006; El Refaeey 2014; Ismail 2014; Nasr 2010; Pacchiarotti 2016; Polak de Fried 2013; Rizk 2005; Salehpour 2012; Youssef 2015). We did not include Rizk 2005 in the meta‐analysis for multiple pregnancy, as no pregnancies occurred in the control group, and adding these data would have skewed the analysis. Nasr 2010 reported no multiple pregnancies in the antioxidant or placebo groups, so we did not include this study in the meta‐analysis;
Gastrointestinal disturbances: Three trials reported on nausea (Cicek 2012; Maged 2015; Westphal 2006). No cases of gastrointestinal disturbances were reported in treatment or control groups in Cicek 2012;
Ectopic pregnancy: Two trials reported ectopic pregnancies (Agrawal 2012; Aleyasin 2009);
Ovarian hyperstimulation syndrome (OHSS): three trials reported on OHSS (Kim 2006; Papaleo 2009; Rizk 2005).There were no cases of OHSS in treatment or control groups in Papaleo 2009 or Rizk 2005. Kim 2006 did not provide data for OHSS;
Preterm birth: One trial (Nasr 2010) reported on preterm birth.
We tried to contact authors of all the trials that did not report adverse events. We could not assume that there were no adverse events in trials where these were not reported.
Design
All 50 included trials were of parallel‐group design. One trial (Rosalbino 2012) was a five‐armed trial. Two trials (Griesinger 2002; Schachter 2007) were four‐armed, which used different dosages of vitamin C versus placebo and doses of vitamin B complex versus no treatment respectively, and four trials were three‐armed (Cheraghi 2016; Maged 2015; Pacchiarotti 2016; Rashidi 2009).
The sample size of the included trials ranged from 29 participants (Lesoine 2016) to 804 participants (Badawy 2006). Fourteen trials included in the meta‐analysis (Agrawal 2012; Battaglia 2002; Bentov 2014; Cicek 2012; Ciotta 2011; El Refaeey 2014; Eryilmaz 2011; Ismail 2014; Lisi 2012; Mier‐Cabrera 2008; Nasr 2010; Pacchiarotti 2016; Papaleo 2009; Salehpour 2012) reported carrying out a power calculation.
Funding
Funding sources were reported by only 14 of the 50 included trials. One study (Bentov 2014) reported the support of Ferring Pharmaceuticals and that one of the authors had a consultancy agreement with Fertility Neutraceuticals, responsible for manufacturing and distribution of the CoQ10 product, and is also on the Science Advisory Board for Ferring. Valeri 2015 reported funding by a pharmaceutical company and three studies (Carlomagno 2012; Lesoine 2016; Pacchiarotti 2016) included an author who was an employee of a pharmaceutical company. Schachter 2007 reported that laboratory costs were partially supported by a company producing vitamins and supplements. One trial reports self‐funding (Agrawal 2012), and eight reported gaining funding from their institutions (Aleyasin 2009; Carlomagno 2012; Cheraghi 2016; Creus 2008; Mier‐Cabrera 2008; Razavi 2015; Salehpour 2009; Westphal 2006). See details in Characteristics of included studies.
Excluded studies
We retrieved the full text of trials that were identified as potentially eligible for inclusion (see Figure 1). We excluded 54 trials; 33 of these were because the population did not meet criteria for inclusion in this review (Aflatoonian 2014; Ardabili 2012; Baillargeon 2004; Benelli 2016; Bonakdaran 2012; Cheang 2008; Ciotta 2012; Costantino 2009; Elgindy 2008; Elgindy 2010; Firouzabadi 2012; Genazzani 2008; Hebisha 2016; Hernández‐Yero 2012; Iuorno 2002; Jamilian 2016; Jamilian 2016a; Kamencic 2008; Kilicdag 2005; Le Donne 2012; Li 2013; Moosavifar 2010; Nestler 1999; Nestler 2001; Nordio 2012; Oner 2011; Pasha 2011; Pizzo 2014; Santanam 2003; Taheri 2015; Thiel 2006; Vargas 2011; Yoon 2010). Many of these trials recruited women with PCOS who were not attending a subfertility clinic and whose main concern was not pregnancy but rather ways to control their symptoms of PCOS. Seven were quasi‐controlled trials and therefore were not randomised (Aksoy 2010; Al‐Omari 2003; Crha 2003; Henmi 2003; Nazzaro 2011; Papaleo 2007; Tamura 2008). Nine had inappropriate treatment or control for inclusion (Asadi 2014; Elnashar 2007; Farzadi 2006; Hashim 2010; Immediata 2014; Papaleo 2008; Raffone 2010; Salem 2012; Twigt 2011). One (Elnashar 2005) was a conference abstract of another excluded trial (Elnashar 2007). Two were secondary analyses (Pal 2016; Ruder 2014). One was a duplicate study (Ghotbi 2007) of the included study Alborzi 2007 and we excluded Nichols 2010 after the lead investigator confirmed that this trial had been abandoned before recruitment because of lack of funding. One trial Rezk 2004, previously excluded, was now added as a sub‐study of the included study Rizk 2005.
Ongoing trials
Twelve trials were ongoing in the original review; five of these became included in the 2017 update (Agrawal 2012; Bentov 2014; Mohammadbeigi 2012; Unfer 2011; Youssef 2015); two became excluded trials: Ardabili 2012 (formerly known as Pourghassem 2010), and Pasha 2011. Five of the 12 trials remain ongoing (NCT01019785; IRCT201112148408N1; CTRI/2012/08/002943; NCT01782911; NCT01267604).
In addition we identified 3 further ongoing trials: NCT03023514; NCT02058212; Fernando 2014.
Risk of bias in included studies
See Figure 2 for a summary of risk of bias in individual trials, and Figure 3 for a summary of each risk of bias item across all included trials.
Figure 2.
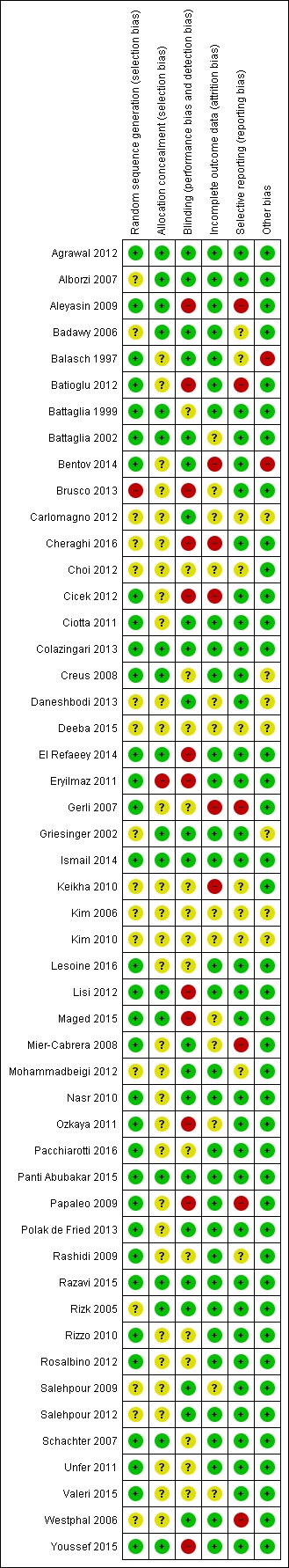
Methodological risk of bias summary: review authors' judgements about each methodological bias item for each included study.
Figure 3.
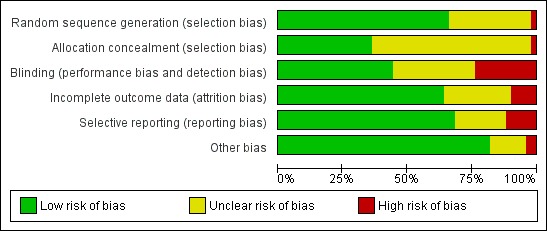
Methodological risk of bias graph: review authors' judgements about each methodological bias item presented as percentages across all included trials.
Sequence Generation
All of the 50 included trials were randomised with a parallel design. Thirty‐three trials described their methods of sequence generation, which typically were computer‐generated or used a random‐number table (Agrawal 2012; Aleyasin 2009; Balasch 1997; Batioglu 2012; Battaglia 2002; Battaglia 1999; Bentov 2014; Cicek 2012; Ciotta 2011; Colazingari 2013; Creus 2008; El Refaeey 2014; Eryilmaz 2011; Gerli 2007; Ismail 2014; Lesoine 2016; Lisi 2012; Maged 2015; Mier‐Cabrera 2008; Mohammadbeigi 2012; Nasr 2010; Ozkaya 2011; Pacchiarotti 2016; Papaleo 2009; Polak de Fried 2013; Rashidi 2009; Razavi 2015; Rizzo 2010; Rosalbino 2012; Schachter 2007; Unfer 2011; Valeri 2015; Youssef 2015). One trial (Panti Abubakar 2015) used a coin toss. Sixteen trials simply reported the trial as randomised with no description of method (Badawy 2006; Brusco 2013; Carlomagno 2012; Cheraghi 2016; Choi 2012; Daneshbodi 2013; Deeba 2015; Griesinger 2002; Keikha 2010; Kim 2006; Kim 2010; Mier‐Cabrera 2008; Rizk 2005; Salehpour 2009; Salehpour 2012; Westphal 2006). Alborzi 2007 reported the method, but it remained unclear whether randomisation was performed by coin flip or with the use of odd and even numbers. We rated only one trial (Brusco 2013) at high risk for this domain, due to lack of explanation of the methods of randomisation and the unbalanced numbers in the treatment and control groups. We conducted a sensitivity analysis on the exclusion of trials that we considered to be at high risk in any of the 'Risk of bias' domains.
Allocation
We judged 18 trials to be at low risk for allocation concealment (Agrawal 2012; Alborzi 2007; Aleyasin 2009; Badawy 2006; Battaglia 1999; Battaglia 2002; Bentov 2014; Colazingari 2013; Creus 2008; El Refaeey 2014; Griesinger 2002; Ismail 2014; Lisi 2012; Maged 2015; Razavi 2015; Rizk 2005; Schachter 2007; Youssef 2015). One trial (Eryilmaz 2011) replied through email correspondence that no allocation concealment was used. The remainder either did not describe any methods of allocation concealment or the description was not clear. We tried unsuccessfully to contact these authors regarding allocation concealment techniques.
Blinding
We considered that the blinding status of participants could influence findings for the outcomes of live birth, pregnancy and adverse effects, as antioxidants are easily available and it would be possible for participants to self‐medicate. Therefore if the participants were not blinded or the trial was not placebo‐controlled, or both, we considered the trial to be at high risk. Thirty‐two of the 50 included trials described some form of blinding of participants or investigators, or both. Four were triple‐blinded, with participants, clinicians/investigators and outcome assessors blinded (Agrawal 2012; Badawy 2006; Battaglia 2002; Mier‐Cabrera 2008). Eight were double‐blinded with blinding of participants and clinicians (Alborzi 2007; Bentov 2014; Ciotta 2011; Griesinger 2002; Razavi 2015; Rizk 2005; Salehpour 2009; Westphal 2006). Twelve stated that they were double‐blinded but did not declare who was blinded (Creus 2008; Cheraghi 2016; Carlomagno 2012; Colazingari 2013; Daneshbodi 2013; Gerli 2007; Ismail 2014; Keikha 2010; Pacchiarotti 2016; Polak de Fried 2013; Unfer 2011; Valeri 2015). Eight were single‐blinded: the participants were blinded in Balasch 1997, Panti Abubakar 2015 and Salehpour 2012; the embryologists were blinded in Papaleo 2009 and Lesoine 2016; and the outcome assessors were blinded in Lisi 2012, El Refaeey 2014 and Mohammadbeigi 2012. The remaining 18 trials did not report any blinding; however, nine of these used 'no treatment' as the control so blinding for these trials is problematic (Aleyasin 2009; Battaglia 1999; Batioglu 2012; Brusco 2013; Carlomagno 2012; Cicek 2012; Eryilmaz 2011; Maged 2015; Youssef 2015). Only Brusco 2013 stated that it was an open study. Valeri 2015 was also a no‐treatment trial but reported being double‐blinded. Nine trials did not report on blinding (Choi 2012; Deeba 2015; Kim 2006; Kim 2010; Ozkaya 2011; Rashidi 2009; Rizzo 2010; Rosalbino 2012; Schachter 2007).
Incomplete outcome data
Eighteen trials had no losses to follow‐up (Alborzi 2007; Aleyasin 2009; Badawy 2006; Batioglu 2012; Battaglia 1999; Brusco 2013; Ciotta 2011; Lesoine 2016; Lisi 2012; Maged 2015; Nasr 2010; Papaleo 2009; Polak de Fried 2013; Rashidi 2009; Rizk 2005; Rizzo 2010; Schachter 2007; Westphal 2006). Four trials reported losses but used intention‐to‐treat (ITT) analysis (Agrawal 2012; Ismail 2014; Unfer 2011; Youssef 2015). Nine trials had losses and described from which groups they were lost, but did not use ITT in the reporting of trials; however, we used ITT for them in the meta‐analysis (Balasch 1997; Battaglia 2002; Cheraghi 2016; Creus 2008; El Refaeey 2014; Mier‐Cabrera 2008; Pacchiarotti 2016; Panti Abubakar 2015; Salehpour 2012). Cheraghi 2016 explained the losses but was considered at high risk for attrition, as the losses were over 25% of the randomised women. Bentov 2014 had explained loss to follow‐up but reported data as percentages, so it is unclear if ITT was used. This trial was also terminated before finishing enrolment, and we therefore rated it at high risk for this domain. Salehpour 2009 had also explained losses, but because outcomes reported in the trial were different from outcomes in this review, we could not include this study in the meta‐analysis. Three trials (Cicek 2012, Eryilmaz 2011 and Griesinger 2002) had losses to follow‐up with no explanation of which groups were affected, however we took data from these trials as totals were given after dropouts, and we assumed that the groups were equal on allocation. The remaining 14 trials were not included in the meta‐analysis: Gerli 2007 had more than 30% dropouts from the treatment group, and data were unavailable for the 13 other trials (Carlomagno 2012; Choi 2012; Colazingari 2013; Daneshbodi 2013; Deeba 2015; Keikha 2010; Kim 2006; Kim 2010; Mohammadbeigi 2012; Ozkaya 2011; Razavi 2015; Rosalbino 2012; Valeri 2015). We tried to contact authors when the data were unavailable.
Selective reporting
Trial protocols were available for four trials (Bentov 2014; Mohammadbeigi 2012; Unfer 2011; Youssef 2015) through the clinical trials registries, but these were unavailable for the 46 remaining included trials. We therefore cannot confirm that on the basis of published reports alone the authors included all expected outcomes. However we considered a trial to be at low risk of selective reporting if the outcomes reported in the Methods were reported in the Results, and we rated 34 trials at low risk for this domain (Agrawal 2012; Alborzi 2007; Battaglia 1999; Battaglia 2002; Bentov 2014; Brusco 2013; Cheraghi 2016; Cicek 2012; Ciotta 2011; Colazingari 2013; Creus 2008; Daneshbodi 2013; El Refaeey 2014; Eryilmaz 2011; Griesinger 2002; Ismail 2014; Lesoine 2016; Lisi 2012; Maged 2015; Nasr 2010; Ozkaya 2011; Pacchiarotti 2016; Panti Abubakar 2015; Polak de Fried 2013; Razavi 2015; Rizk 2005; Rizzo 2010; Rosalbino 2012; Salehpour 2009; Salehpour 2012; Schachter 2007; Unfer 2011; Valeri 2015; Youssef 2015).
Failure to report live birth in subfertility trials is common, and is a major source of bias (Clarke 2010); it should be the default primary outcome in fertility trials. Only eight trials reported live birth (Aleyasin 2009; Battaglia 2002; Bentov 2014; Cicek 2012; Nasr 2010; Panti Abubakar 2015; Polak de Fried 2013; Unfer 2011). Two trials (Agrawal 2012; Schachter 2007) reported ongoing pregnancy, which we took to be live birth in the analysis. Mier‐Cabrera 2008 and Papaleo 2009 stated that they would report live birth, but reported only pregnancy. Adverse events were not well reported in most studies.
A funnel plot for clinical pregnancy (Figure 4) was symmetrical, except for an absence of studies in the lower left of the pyramid. This suggests a small‐study effect, indicating the potential for publication bias whereby small unpublished studies with negative results were not represented. Estimates of the intervention effect tend to be more beneficial in smaller studies and thus introduce the potential for selective reporting and publication bias.
Figure 4.
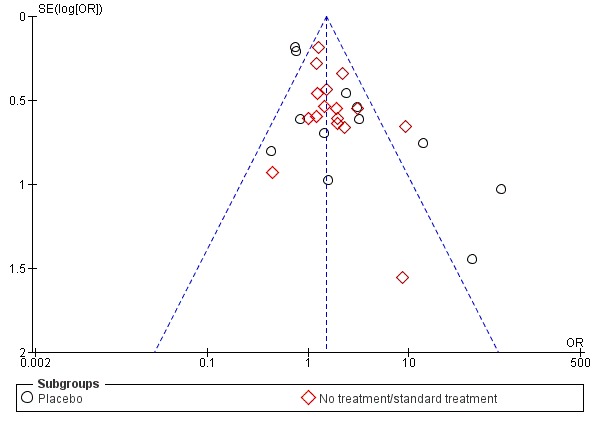
Funnel plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.5 Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).
Other potential sources of bias
We rated two trials (Balasch 1997; Bentov 2014) at high risk in this domain, for women receiving varying adjunctive treatments and early termination of the study, respectively. See details in Characteristics of included studies.
Reasons for studies with data included within the review but not in the analysis
Gerli 2007 (see Table 6) was not incorporated into the analysis, as only half the women randomly assigned reported a desire to become pregnant. Ninety‐two women were randomly assigned, 45 to the treatment group and 47 to the control group. Twenty‐three from the treatment group and 19 from the control wished to conceive; four from the treatment group and one from the control group became pregnant. This trial also had more than 30% dropouts from the treatment group.
Rashidi 2009 reported on clinical pregnancy, but there were no events in either the antioxidant or the no‐treatment arms of the trial.
Effects of interventions
1. Antioxidant supplement versus placebo, no treatment/standard treatment
Primary outcome: Live birth
1.1 Live birth; antioxidants versus placebo or no treatment/standard treatment
See Analysis 1.1.
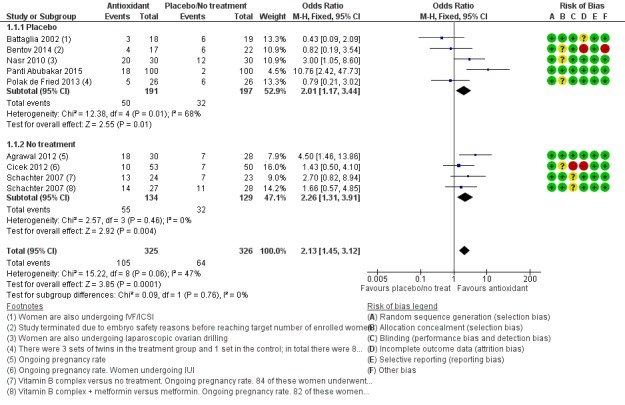
Analysis 1.1.
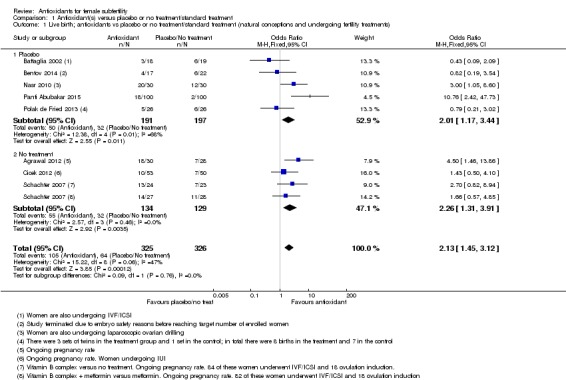
Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 1 Live birth; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).
Antioxidants were associated with an increased live birth rate compared with placebo or no treatment (odds ratio (OR) 2.13, 95% confidence interval (CI) 1.45 to 3.12, P > 0.001, 8 RCTs, 651 women, I2 = 47%, very low‐quality evidence) (Figure 5). This suggests that among subfertile women with an expected live birth rate of 20%, the rate among women using antioxidants would be between 26% and 43% (Table 1).
Figure 5.
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.1 Live birth; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).
In the eight trials that reported live birth (Agrawal 2012; Battaglia 2002; Bentov 2014; Cicek 2012; Nasr 2010; Panti Abubakar 2015; Polak de Fried 2013; Schachter 2007), the OR for live birth was 2.13 and for clinical pregnancy was 2.18. When we pooled all 26 studies that reported clinical pregnancy, the OR for clinical pregnancy was lower, at 1.52. This suggests that the clinical pregnancy rate in the eight trials that reported live birth may have been an overestimation of the effect of the antioxidants, and hence that the live birth rate in these trials is may also be an overestimate (Table 1).
The test for subgroup differences showed no evidence of a difference between the placebo and no‐treatment subgroups (Chi2 = 0.09, df = 1, P = 0.76, I2 = 0%).
Sensitivity analyses
1. We conducted a sensitivity analysis, restricted to trials without a high risk of bias in any domain. We removed two trials from the analysis: Bentov 2014, with a high risk of bias due to early termination of the trial, and Cicek 2012 due to the trial being unblinded and with unexplained group attrition. After removal there remained an association with increased live birth rate when compared to placebo or no treatment (OR 2.47, 95% CI 1.60 to 3.82, P < 0.001, 6 RCTs, 509 women, I2 = 54%).
2. When the two arms of Schachter 2007 were removed from the analysis, due to the use of folic acid or a fertility drug as a control (these were in both the intervention and control arms, with an antioxidant in addition in the intervention), there was still an association between increased live birth rate in the intervention arm, compared with placebo or no treatment (OR 2.15, 95% CI 1.38 to 3.32, P < 0.001, 7 RCTs, 549 women, I2 = 60%), although heterogeneity was moderately high.
1.2 Live birth; type of antioxidant
See Analysis 1.2.
Analysis 1.2.
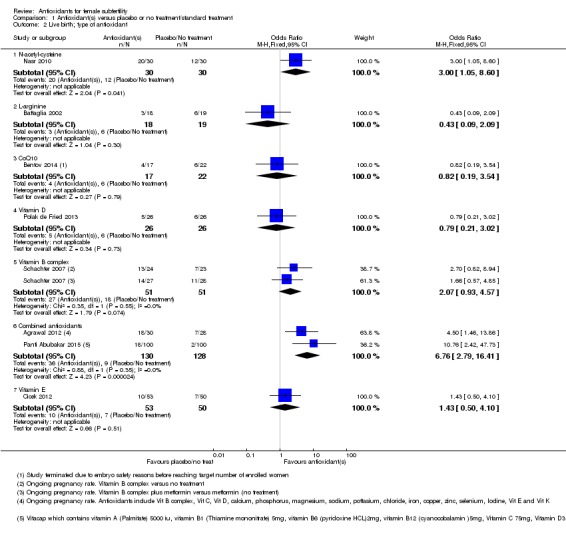
Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 2 Live birth; type of antioxidant.
We considered each type of antioxidant separately. Only two comparisons included more than one trial.
1.2.1Nasr 2010; compared N‐acetyl‐cysteine with placebo (OR 3.00, 95% CI 1.05 to 8.60, P = 0.04, 60 women).
1.2.2Battaglia 2002; compared L‐arginine with placebo (OR 0.43, 95% CI 0.09 to 2.09, P = 0.30, 37 women).
1.2.3Bentov 2014; compared CoQ10 with placebo (OR 0.82, 95% CI 0.19 to 3.54, P = 0.79, 39 women).
1.2.4Polak de Fried 2013; compared Vitamin D with placebo (OR 0.79, 95% CI 0.21 to 3.02, P = 0.73, 52 women).
1.2.5Schachter 2007, a four‐armed trial with two arms comparing a Vitamin B complex with no treatment and Vitamin B complex plus metformin versus metformin (also considered to be 'no treatment'), showing no association with increased live birth rate compared to no treatment (OR 2.07, 95% CI 0.93 to 4.57, P = 0.07, 102 women, I2 = 0%).
1.2.6Agrawal 2012 compared combined antioxidants with no treatment, and Panti Abubakar 2015 compared combined antioxidants with placebo. Combined antioxidants were associated with an increased live birth rate compared with placebo or no treatment (OR 6.76, 95% CI 2.79 to 16.41, P < 0.001, 2 RCTs, 258 women, I2 = 0%).
1.2.7 Cicek 2012 compared Vitamin E to no treatment (OR 1.43, 95% CI 0.50 to 4.10, P = 0.51, 103 women).
1.3 Live birth rate; indications for subfertility
See Analysis 1.3.
Analysis 1.3.
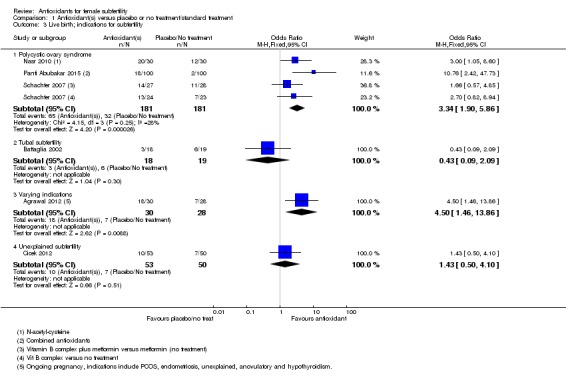
Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 3 Live birth; indications for subfertility.
1.3.1 Polycystic ovary syndrome
Three trials reported on women with PCOS: Panti Abubakar 2015; Nasr 2010; and Schachter 2007 (a four‐armed trial, which contributed to two comparisons in this analysis). Antioxidants were associated with an increased live birth rate compared with placebo or no treatment in women with PCOS (OR 3.34, 95% CI 1.90 to 5.86, P < 0.001, 3 RCTs, 362 women, I2 = 28%). Each trial included different antioxidants: 'N‐acetyl‐cysteine', combined antioxidants and Vitamin B complex.
1.3.2 Tubal subfertility
One trial (Battaglia 2002) enrolled women with tubal subfertility undergoing IVF (OR 0.43, 95% CI 0.09 to 2.09, P = 0.30, 37 women).
1.3.3 Varying indications
One trial (Agrawal 2012) enrolled women with various causes of subfertility (OR 4.50, 95% CI 1.46 to 13.86, P = 0.009, 58 women).
1.3.4 Unexplained subfertility
One trial (Cicek 2012) enrolled women with unexplained subfertility (OR 1.43, 95% CI 0.50 to 4.10, P = 0.51, 103 women)
1.4 Live birth; IVF/ICSI
See Analysis 1.4.
Analysis 1.4.
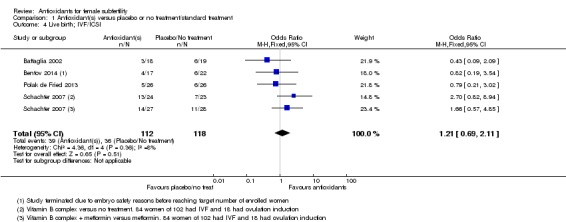
Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 4 Live birth; IVF/ICSI.
Four trials (Battaglia 2002; Bentov 2014; Polak de Fried 2013; Schachter 2007) compared antioxidants with placebo or no treatment in women having IVF/ICSI treatment and reporting live birth. Antioxidants were not associated with an increased live birth rate compared with placebo or no treatment in women undergoing IVF/ICSI (OR 1.21, 95% CI 0.69 to 2.11, P = 0.51, 4 RCTs, 230 women, I2= 8%).
Secondary outcome: Clinical pregnancy
Only 26 of the 50 included trials presented or provided data that could be used in this meta‐analysis. We have not included all data in the reports, as some were obtained through direct contact with the trialist (see Characteristics of included studies). We could not use the data for the remaining 24 trials in the meta‐analysis, as they provided either only 'pregnancy' or biochemical pregnancy data (see Table 7), only bio‐markers or embryo/oocyte numbers, or insufficient information in the reports, which were mainly conference abstracts. We tried to contact these authors to obtain the clinical pregnancy data and some responded saying that they did not have the data, while others did not respond at all.
1.5 Clinical pregnancy; antioxidants versus placebo or no treatment/standard treatment
See Analysis 1.5.
Analysis 1.5.
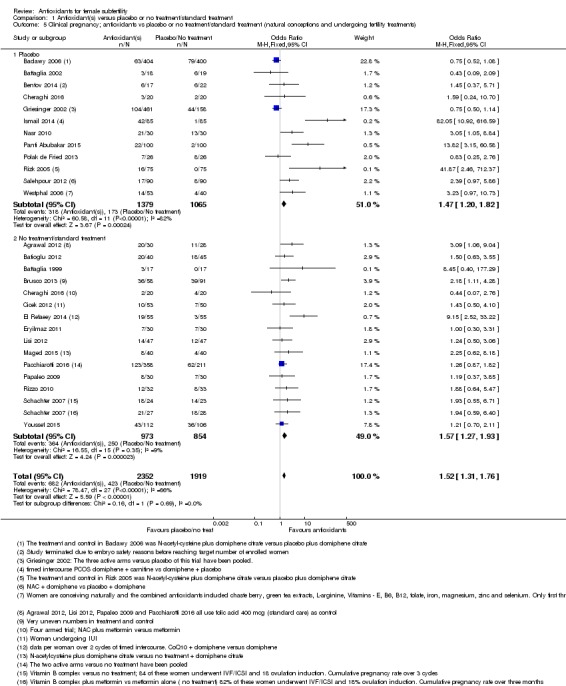
Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 5 Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).
Antioxidants were associated with an increased clinical pregnancy rate compared with placebo or no treatment (OR 1.52, 95% CI 1.31 to 1.76, P < 0.001, 26 RCTs, 4271 women, I2= 66%, very low‐quality evidence) (Figure 6). This suggests that among subfertile women with an expected clinical pregnancy rate of about 22%, the rate among women using antioxidants would be between 27% and 33% (Table 1). Heterogeneity was moderately high.
Figure 6.
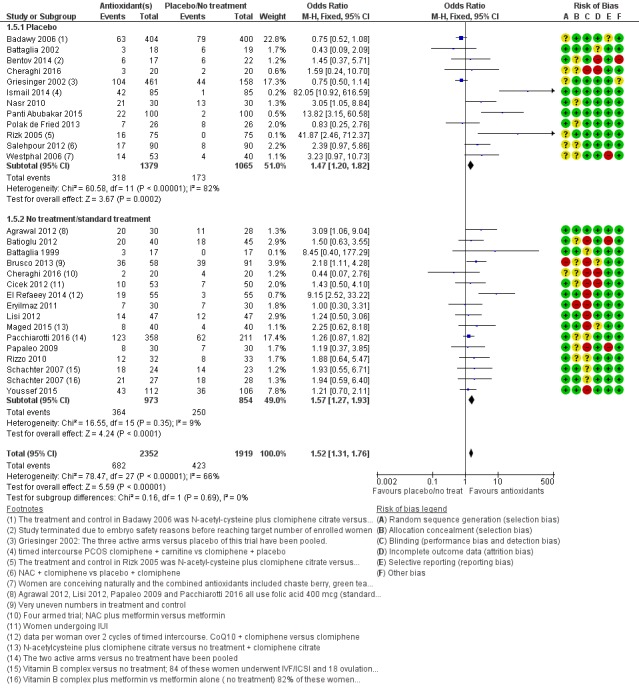
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.5 Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).
The test for subgroup differences showed no evidence of a difference between the placebo and no‐treatment subgroups (Chi2 = 0.16, df = 1, P = 0.69, I2 = 0%).
Sensitivity analyses
1. We conducted a sensitivity analysis, excluding trials with a high risk of bias in any domain.
Twelve trials (Batioglu 2012; Bentov 2014; Brusco 2013; Cheraghi 2016; Cicek 2012; El Refaeey 2014; Eryilmaz 2011; Lisi 2012; Maged 2015; Papaleo 2009; Youssef 2015; Westphal 2006) had a rating of high risk in any one or more of the 'Risk of bias' domains (see Characteristics of included studies). When these trials were removed in a sensitivity analysis there remained an association between antioxidants and an increased clinical pregnancy rate when compared to placebo (OR 1.46, 95% CI 1.23 to 1.74, P < 0.001, 14 RCTs, 3100 women, I2 = 78%); heterogeneity was very high.
2. We conducted a sensitivity analysis, excluding studies that used a fertility drug (metformin or clomiphene) as a control plus a placebo or no treatment (these agents were in both the intervention and control arms, with an antioxidant in addition in the intervention arm).
When these eight trials were removed from the analysis (Badawy 2006; Cheraghi 2016; El Refaeey 2014; Ismail 2014; Maged 2015; Rizk 2005; Salehpour 2012; Schachter 2007) there remained an association between antioxidants and an increased clinical pregnancy rate compared no treatment (OR 1.40, 95% CI 1.17 to 1.67, P < 0.001, 18 RCTS, 2682 women, I2 = 39%). Two trials (Cheraghi 2016; Schachter 2007) were multi‐armed, but only those arms with a fertility drug plus placebo/no treatment were removed in this analysis.
1.6 Clinical pregnancy; type of antioxidant
See Analysis 1.6.
Analysis 1.6.
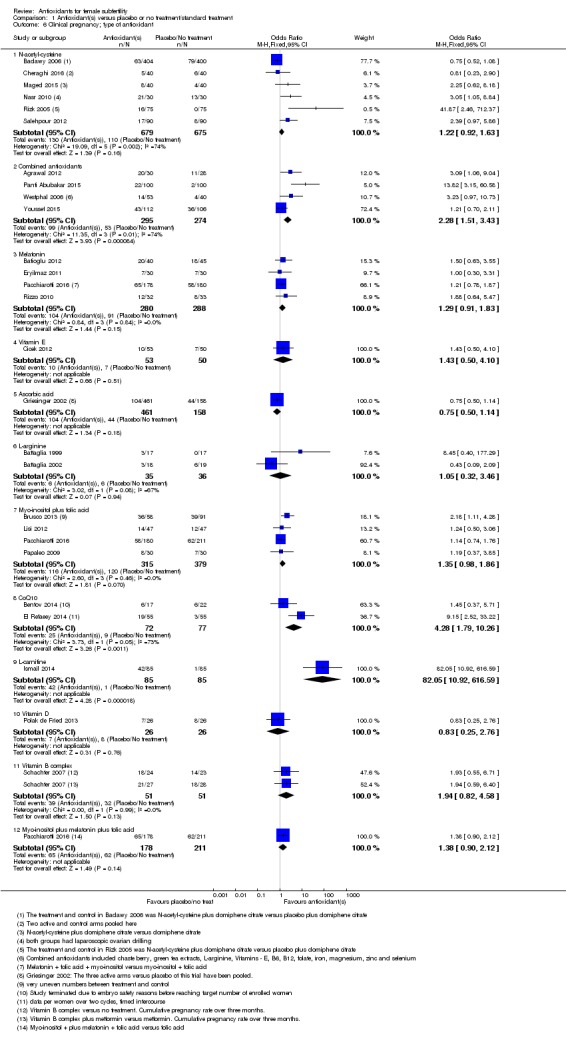
Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 6 Clinical pregnancy; type of antioxidant.
We considered each type of antioxidant separately (Figure 7).
Figure 7.
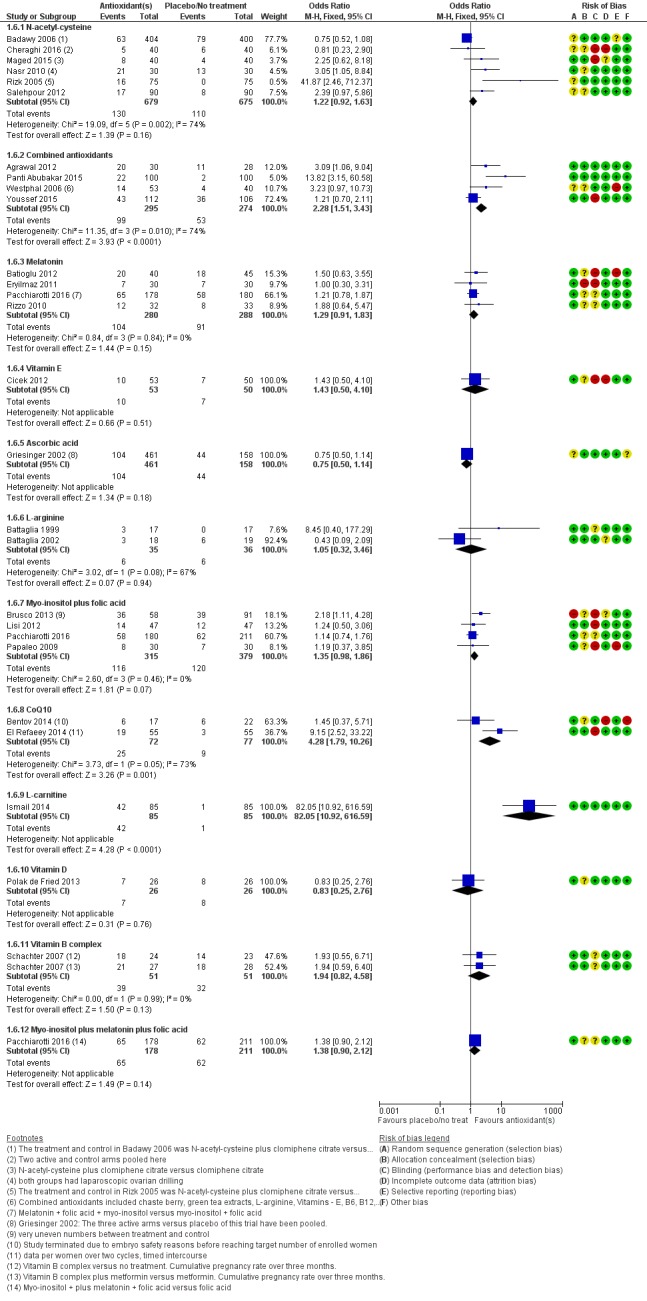
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.6 Clinical pregnancy; type of antioxidant.
1.6.1 N‐acetyl‐cysteine was not associated with an increased clinical pregnancy rate when compared with placebo, no treatment or standard treatment (OR 1.22, 95% CI 0.92 to 1.63, P = 0.16, 6 RCTs, 1354 women, I2 = 74%). Heterogeneity was very high, perhaps as a result of the high risk of bias in Badawy 2006 and Rizk 2005, the unclear risk of bias in Cheraghi 2016 and Salehpour 2012, or the additional treatment of laparoscopic drilling that women received in Nasr 2010.
1.6.2 Combined antioxidants (similar antioxidants were combined in each trial) were associated with an increased clinical pregnancy rate when compared to placebo or no treatment (OR 2.28, 95% CI 1.51 to 3.43, P < 0.001, 4 RCTs, 569 women, I2 = 74%). Heterogeneity was very high, and two of the trials enrolled small numbers of women.
1.6.3 There was no clear evidence of a difference in clinical pregnancy rates between melatonin and placebo or no treatment (OR 1.29, 95% CI 0.91 to 1.83, P = 0.15, 4 RCTs, 568 women, I2 = 0%).
1.6.4 There was no clear evidence of a difference in clinical pregnancy rates between Vitamin E and no treatment (OR 1.43, 95% CI 0.50 to 4.10, P = 0.51, 103 women).
1.6.5 There was no clear evidence of a difference in clinical pregnancy rates between ascorbic acid and placebo (OR 0.75, 95% CI 0.50 to 1.14, P = 0.18, 619 women).
1.6.6 There was no clear evidence of a difference in clinical pregnancy rates between L‐arginine and placebo or no treatment (OR 1.05, 95% CI 0.32 to 3.46, P = 0.94, 2 RCTs, 71 women, I2 = 67%).
1.6.7 There was no clear evidence of a difference in clinical pregnancy rates between myo‐inositol plus folic acid and placebo or no treatment (OR 1.35, 95% CI 0.98 to 1.86, P = 0.07, 4 RCTs, 694 women, I2 = 0%).
1.6.8 CoQ10 was associated with an increased clinical pregnancy rate when compared to placebo or no treatment (OR 4.28, 95% CI 1.79 to 10.26, P = 0.001, 2 RCTs, 149 women, I2 = 73%).
1.6.9 L‐carnitine was associated with an increased clinical pregnancy rate when compared to placebo (OR 82.05, 95% CI 10.92 to 616.59, P < 0.001, 170 women).
1.6.10 There was no clear evidence of a difference in clinical pregnancy rates between vitamin D and placebo (OR 0.83, 95% CI 0.25 to 2.76, P = 0.76, 52 women).
1.6.11 There was no clear evidence of a difference in clinical pregnancy rates between vitamin B complex in the two arms of Schachter 2007 and placebo or no treatment (OR 1.94, 95% CI 0.82 to 4.58, P = 0.13, 1 RCT, 102 women, I2 = 0%).
1.6.12 There was no clear evidence of a difference in clinical pregnancy rates between myo‐inositol plus melatonin plus folic acid and no treatment (OR 1.38, 95% CI 0.90 to 2.12, P = 0.14, 389 women).
1.7 Clinical pregnancy rate; indications for subfertility
See Analysis 1.7. (Figure 8).
Analysis 1.7.
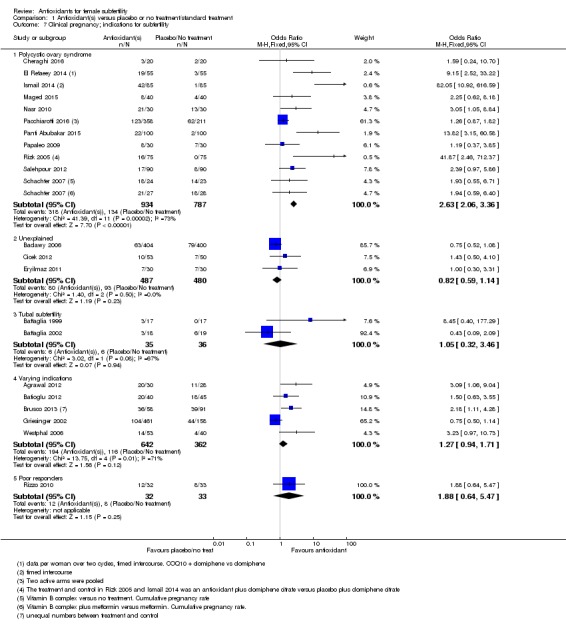
Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 7 Clinical pregnancy; indications for subfertility.
Figure 8.
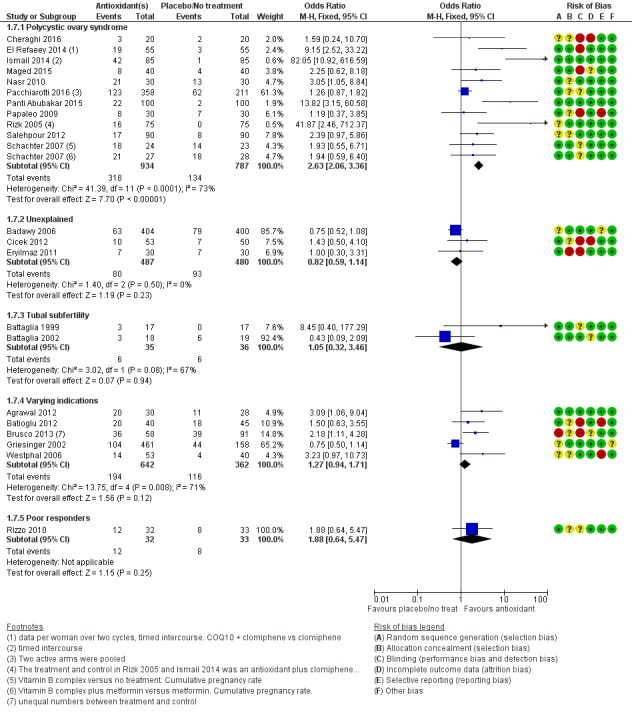
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.7 Clinical pregnancy; indications for subfertility.
1.7.1 Polycystic ovary syndrome
Antioxidants were associated with an increased clinical pregnancy rate when compared with placebo, no treatment or standard treatment in women with PCOS (OR 2.63, 95% CI 2.06 to 3.36, P < 0.001, 11 RCTs, 1721 women, I2 = 73%). Heterogeneity was high, probably due to the different antioxidants used.
1.7.2 Unexplained subfertility
There was no clear evidence of a difference in clinical pregnancy rates when antioxidants were compared with placebo, no treatment or standard treatment in women with unexplained subfertility (OR 0.82, 95% CI 0.59 to 1.14, P = 0.23, 3 RCTs, 967 women, I2 = 0%).
1.7.3 Tubal subfertility
There was no clear evidence of a difference in clinical pregnancy rates when antioxidants were compared with placebo, no treatment or standard treatment in women with tubal subfertility (OR 1.05, 95% CI 0.32 to 3.46, P = 0.94, 2 RCTs, 71 women, I2 = 67%).
1.7.4 Varying indications
There was no clear evidence of a difference in clinical pregnancy rates when antioxidants were compared with placebo, no treatment or standard treatment in women with varying indications (OR 1.27, 95% CI 0.94 to 1.71, P = 0.12, 5 RCTs, 1004 women, I2 = 71%).
1.7.5 Poor responders
There was no clear evidence of a difference in clinical pregnancy rates when antioxidants were compared with placebo, no treatment or standard treatment in women who were poor responders (OR 1.88, 95% CI 0.64 to 5.47, P = 0.25, 1 RCT, 65 women).
1.8 Clinical pregnancy rate; IVF/ICSI
See Analysis 1.8.
Analysis 1.8.
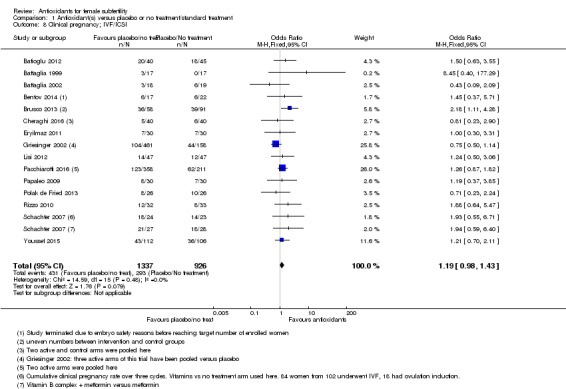
Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 8 Clinical pregnancy; IVF/ICSI.
There was no clear evidence of a difference in clinical pregnancy rates when antioxidants were compared with placebo, no treatment or standard treatment in women undergoing IVF/ICSI (OR 1.19, 95% CI 0.98 to 1.43, P = 0.08, 15 RCTs, 2263 women, I2 = 0%).
Secondary outcome: Adverse events
1.9 Adverse events
See Analysis 1.9.
Analysis 1.9.
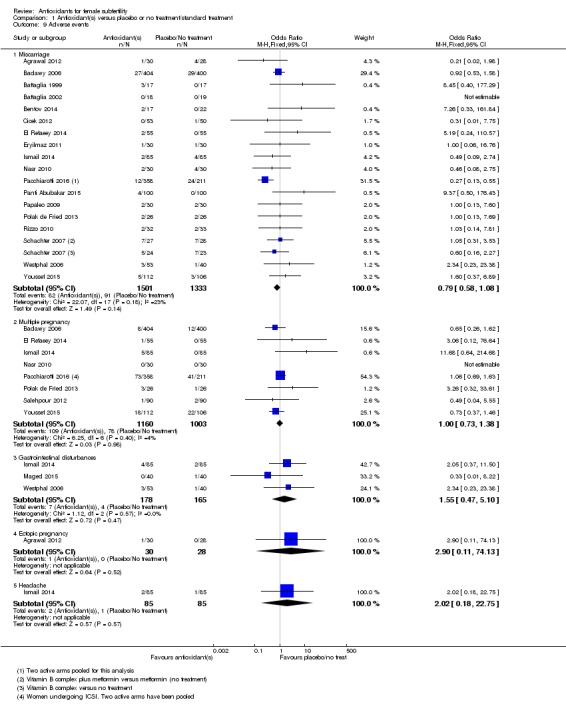
Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 9 Adverse events.
We subgrouped adverse event data according to the types of events that occurred, as reported by the trials. These included miscarriage, multiple pregnancy, gastrointestinal disturbances, ectopic pregnancy and headache. There was no evidence to suggest an association between antioxidants and adverse events, but data were limited, with 17 trials reporting on miscarriage, seven trials reporting on multiple pregnancy, and three reporting on gastrointestinal upsets, one reporting ectopic pregnancy and one reporting headache.
1.9.1 Miscarriage
There was no clear evidence of a difference in miscarriage rates when antioxidants were compared with placebo or no treatment (OR 0.79, 95% CI 0.58 to 1.08, P = 0.14, 18 RCTs, 2834 women, I2 = 23%, very low‐quality evidence) (Figure 9). This means that given the rate of 7% miscarriages in the control population, the use of antioxidants would be expected to result in a miscarriage rate of between 4% and 7% (Table 1). Most of the trials in this analysis were small, although one trial (Badawy 2006) enrolled 804 women. There were no events in one of the studies (Battaglia 2002).
Figure 9.
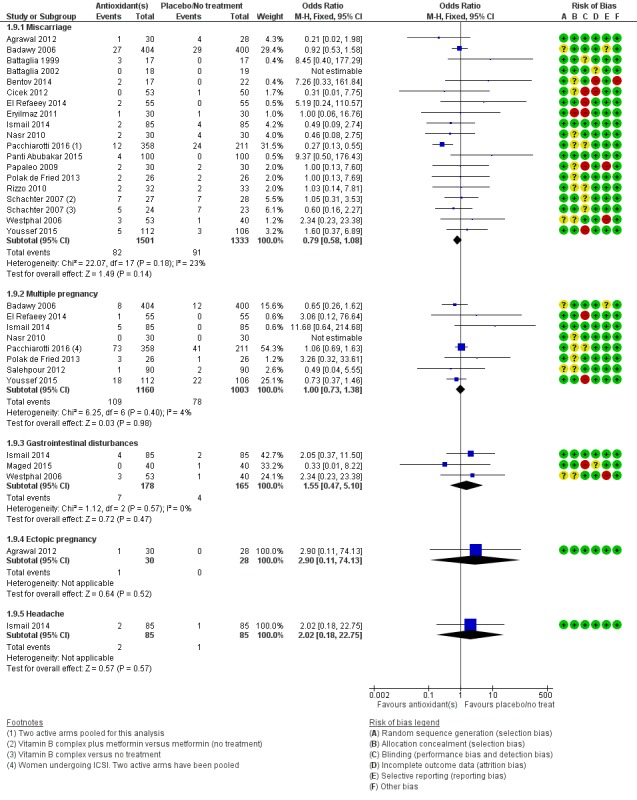
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.9 Adverse events.
1.9.2 Multiple pregnancy
There was no clear evidence of a difference in multiple pregnancy rates when antioxidants were compared with placebo or no treatment (OR 1.00, 95% CI 0.73 to 1.38, P = 0.98, 8 RCTs, 2163 women, I2 = 4%, very low‐quality evidence) (Figure 9). This means that if the multiple pregnancy rate in the control population is 8%, use of antioxidants instead would be expected to result in a multiple pregnancy rate between 6% and 11% (Table 1). There were no events in one of the studies (Nasr 2010)
1.9.3 Gastrointestinal disturbances
Three trials reported on gastrointestinal disturbances (Cicek 2012; Maged 2015; Westphal 2006). There was no clear evidence of a difference in gastrointestinal disturbances when antioxidants were compared with placebo or no treatment (OR 1.55, 95% CI 0.47 to 5.10, P = 0.47, 3 RCTs, 343 women, I2 = 0%, very low‐quality evidence) (Figure 9). This means that with a rate of 2% gastrointestinal disturbances in the control population, use of antioxidants instead would be expected to result in a gastrointestinal disturbances rate between 1% and 11% (Table 1).
1.9.4 Ectopic pregnancy
One trial (Agrawal 2012) reported on ectopic pregnancy. There was no clear evidence of a difference between the groups (OR 2.90, 95% CI 0.11 to 74.13, P = 0.52, 58 women).
1.9.5 Headache
One trial (Ismail 2014) reported on headache. There was no clear evidence of a difference between the groups (OR 2.02, 95% CI 0.18 to 22.75, P = 0.57, 170 women).
2. Head‐to‐head antioxidants
Only one trial (Unfer 2011) was included in the head‐to‐head comparison. Myo‐inositol was compared with D‐chiro‐inositol, among women with polycystic ovarian syndrome undergoing IVF.
Primary outcome: Live birth
See Analysis 2.1.
Analysis 2.1.
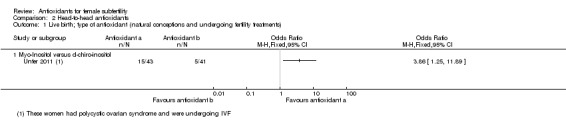
Comparison 2 Head‐to‐head antioxidants, Outcome 1 Live birth; type of antioxidant (natural conceptions and undergoing fertility treatments).
2.1 Live birth
Only one trial (Unfer 2011) reported on live birth. Live birth rates were higher in the myo‐inositol group than in the D‐chiro‐inositol group (OR 3.86, 95% CI 1.25 to 11.89, P = 0.02, 84 women).
Secondary outcome: Clinical pregnancy
See Analysis 2.2.
Analysis 2.2.
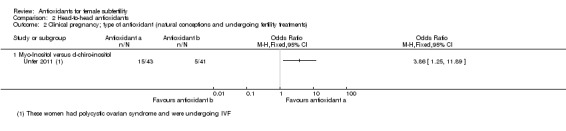
Comparison 2 Head‐to‐head antioxidants, Outcome 2 Clinical pregnancy; type of antioxidant (natural conceptions and undergoing fertility treatments).
2.2 Clinical pregnancy; type of antioxidant
One trial (Unfer 2011) reported on clinical pregnancy in this comparison. Clinical pregnancy rates were higher in the myo‐inositol group than in the D‐chiro‐inositol group (OR 3.86, 95% CI 1.25 to 11.89, P = 0.02, 84 women).
Secondary outcome: Adverse events
See Analysis 2.3.
Analysis 2.3.
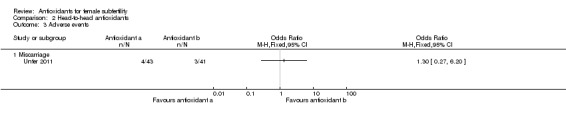
Comparison 2 Head‐to‐head antioxidants, Outcome 3 Adverse events.
2.3 Adverse events
2.3.1 Miscarriage
One trial (Unfer 2011) reported on the miscarriage rate in this comparison. There was no clear evidence of a difference between the myo‐inositol group and the D‐chiro‐inositol group (OR 1.30, 95% CI 0.27 to 6.20, P = 0.74, 84 women).
3. Pentoxifylline supplement versus placebo, no treatment/standard treatment
Primary outcome: Live birth
See Analysis 3.1.
Analysis 3.1.
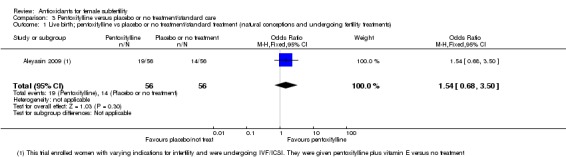
Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 1 Live birth; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).
3.1 Live birth; pentoxifylline versus placebo or no treatment/standard treatment
Only one trial (Aleyasin 2009) reporting live birth was included in the pentoxifylline comparison (OR 1.54, 95% CI 0.68 to 3.50, P = 0.30, 112 women, very low quality evidence). This trial enrolled women with varying indications for infertility, who were undergoing IVF/ICSI. They were given pentoxifylline plus vitamin E versus no treatment.
Secondary outcome: Clinical pregnancy
3.2 Clinical pregnancy; pentoxifylline versus placebo or no treatment/standard treatment
See Analysis 3.2.
Analysis 3.2.
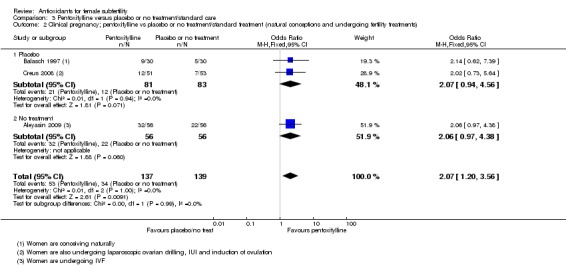
Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 2 Clinical pregnancy; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).
Three trials reported on pentoxifylline versus placebo or no treatment (Aleyasin 2009; Balasch 1997; Creus 2008). Pentoxifylline was associated with an increased clinical pregnancy rate compared with placebo or no treatment (OR 2.07, 95% CI 1.20 to 3.56, P = 0.009, 3 RCTs, 276 women, I2 = 0%, very low‐quality evidence) (Figure 10).This suggests that among subfertile women with an expected clinical pregnancy rate of 25%, the rate among women using pentoxifylline would be between 28% and 53% (Table 2).
Figure 10.
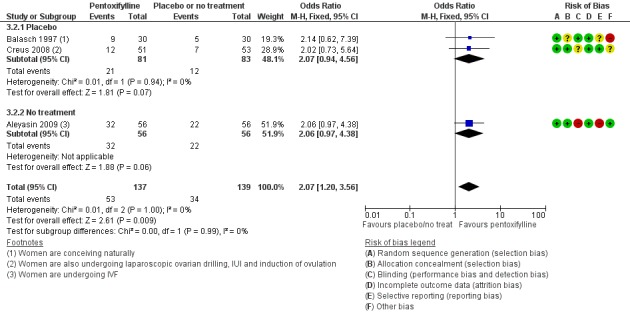
Forest plot of comparison: 3 Pentoxifylline versus placebo or no treatment/standard care, outcome: 3.2 Clinical pregnancy; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).
Sensitivity analysis for trials at high risk of bias
When Aleyasin 2009 was removed from the analysis (with a high risk of bias for blinding and selective reporting) there was no conclusive evidence of an association between pentoxifylline and increased clinical pregnancy rate (OR 2.07, 95% CI 0.94 to 4.56, P = 0.07, 2 RCTs, 164 women, I2 = 0%).
3.2.1 Clinical pregnancy; pentoxifylline versus placebo
Two trials (Balasch 1997; Creus 2008) reported on pentoxifylline versus placebo. There was no clear evidence of a difference between the pentoxifylline and placebo in clinical pregnancy rates (OR 2.07, 95% CI 0.94 to 4.56, P = 0.07, 2 RCTs, 164 women, I2 = 0%).
3.2.2 Clinical pregnancy rate; pentoxifylline versus no treatment
Aleyasin 2009 was the only trial in this subgroup. There was no clear evidence of a difference between the groups (OR 2.06, 95% CI 0.97 to 4.38, P = 0.06, 112 women).
3.3 Clinical pregnancy; type of antioxidant
See Analysis 3.3.
Analysis 3.3.
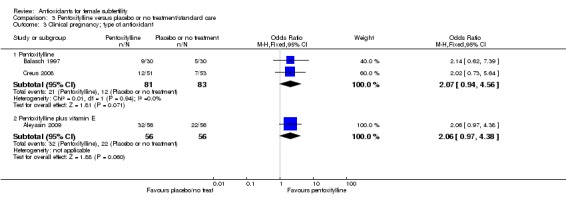
Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 3 Clinical pregnancy; type of antioxidant.
3.3.1 Pentoxifylline only
Two trials (Balasch 1997; Creus 2008) reported on pentoxifylline alone, with no clear evidence of a difference between the groups in clinical pregnancy rates (OR 2.07, 95% CI 0.94 to 4.56, P = 0.07, 2 RCTs, 164 women, I2 = 0%).
3.3.2 Pentoxifylline plus vitamin E
Only one trial (Aleyasin 2009) reported on pentoxifylline plus vitamin E, with no clear evidence of a difference between the groups in clinical pregnancy rates (OR 2.06, 95% CI 0.97 to 4.38, P = 0.06, 112 women).
3.4 Clinical pregnancy; indications for subfertility
See Analysis 3.4.
Analysis 3.4.
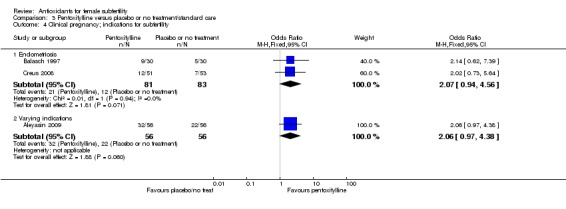
Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 4 Clinical pregnancy; indications for subfertility.
3.4.1 Clinical pregnancy rate; endometriosis
Pentoxifylline did not show an association with an increased clinical pregnancy rate (OR 2.07, 95% CI 0.94 to 4.56, P = 0.07, 2 RCTs, 164 women, I2 = 0%) in women with endometriosis.
3.4.2 Clinical pregnancy rate; varying indications
Only one trial (Aleyasin 2009) enrolled women who presented with different indications within the trial. There was no clear evidence of a difference between the groups (OR 2.06, 95% CI 0.97 to 4.38, P = 0.06, 112 women).
3.5 Clinical pregnancy rate; IVF/ICSI
See Analysis 3.5.
Analysis 3.5.
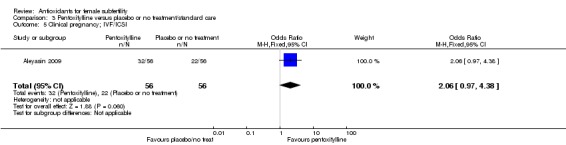
Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 5 Clinical pregnancy; IVF/ICSI.
Only one trial (Aleyasin 2009) enrolled women who were undergoing IVF/ICSI. There was no clear evidence of a difference between the groups (OR 2.06, 95% CI 0.97 to 4.38, P = 0.06, 112 women).
Secondary outcome: Adverse events
3.6 Adverse events
SeeAnalysis 3.6
Analysis 3.6.
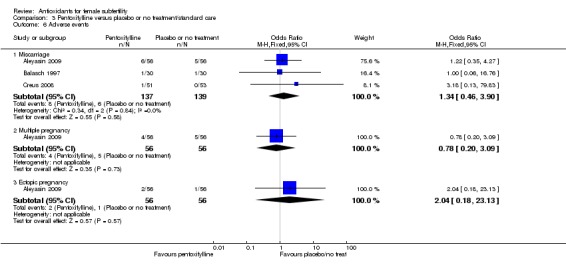
Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 6 Adverse events.
We subgrouped adverse event data according to the types of events that occurred, as reported by the trials. These included miscarriage, multiple pregnancy and ectopic pregnancy. No evidence suggested an association with antioxidants and adverse events, but data were limited, with three trials reporting on miscarriage, one on multiple pregnancy, and one on ectopic pregnancy.
3.6.1 Miscarriage
No evidence suggested a difference in miscarriage rates between antioxidants and placebo or no treatment, but data were limited with only three trials reporting on this outcome (OR 1.34, 95% CI 0.46 to 3.90, P = 0.58, 3 RCTs, 276 women, I2 = 0%, very low‐quality evidence) (Figure 11). This suggests that among subfertile women with an expected miscarriage rate of 4%, the rate among women using pentoxifylline would be between 2% and 15% (Table 2).
Figure 11.
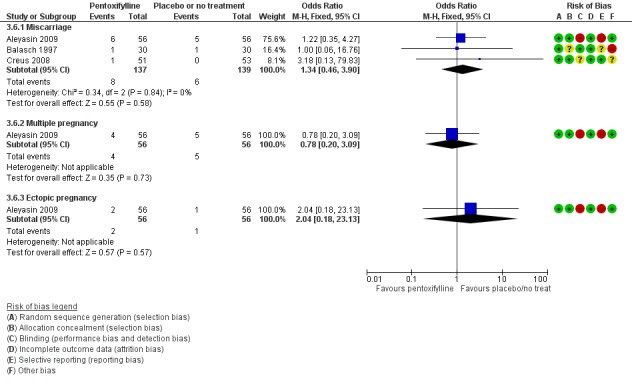
Forest plot of comparison: 3 Pentoxifylline versus placebo or no treatment/standard care, outcome: 3.6 Adverse events.
3.6.2 Multiple pregnancy
Only one trial (Aleyasin 2009) reported on multiple pregnancy. There was no clear evidence of a difference between the groups (OR 0.78, 95% CI 0.20 to 3.09, P = 0.73, 112 women, very low quality evidence).
3.6.3 Ectopic pregnancy
Only one trial (Aleyasin 2009) reported on ectopic pregnancy. There was no clear evidence of a difference between the groups (OR 2.04, 95% CI 0.18 to 23.13, P = 0.57, 112 women).
Discussion
Summary of main results
Effectiveness of antioxidants versus placebo or no treatment
Very low‐quality evidence indicates that for subfertile women the use of supplemental antioxidants may be effective in increasing rates of live birth. Eight trials with a total of 651 women reported on live birth (Table 1). The differences between the trials (heterogeneity) were moderate (I2 = 47% with a fixed‐effect model). The heterogeneity may be due to the trials enrolling women with differing indications for subfertility and varying types of antioxidants.
We conducted subgroup analyses, in accord with our protocol, by type of comparison and type of antioxidant. The association between antioxidants and an increased live birth rate persisted. When we considered specific indications for subfertility, there was an association between the use of antioxidants and increased live birth among women with polycystic ovary syndrome (PCOS). However we found no clear association between antioxidants and an increased live birth rate among women undergoing IVF or ICSI.
We performed a sensitivity analysis excluding trials at high risk of bias in any domain, and those that used folic acid or a fertility drug as a control (these were in both the intervention and control arms with an antioxidant in addition in the intervention, and classified as no treatment). When these trials were removed from the analysis there remained an association between antioxidants and an increased live birth rate, although heterogeneity was moderately high.
Antioxidants were associated with an increased clinical pregnancy rate when compared with either placebo or no treatment, although the quality of this evidence was assessed as 'very low' (Table 1). Heterogeneity was high, but there was no evidence that the effects differed by type of control (placebo or no treatment). We conducted sensitivity analyses excluding trials at high risk of bias and those using a standard or co‐intervention agent as their control. There remained an association between increased clinical pregnancy rates and antioxidants in the analysis when these trials were removed.
When we considered individual antioxidant interventions separately, both 'combined antioxidants' and CoQ10 showed an association between antioxidant and an increased clinical pregnancy rate, although heterogeneity in both groups was high. We found no association between melatonin, L‐arginine, myo‐inositol or vitamin B complex and clinical pregnancy rate, although these subgroups contained only three or fewer trials.
When we considered specific 'indications for subfertility', we found an association between antioxidants and increased clinical pregnancy in women with PCOS, but heterogeneity here was very high, probably due to varying antioxidants. We found no association between antioxidants and clinical pregnancy rates in women with unexplained subfertility, with tubal subfertility, with varying indications, or in trials that enrolled women with poor response.
There was no association between antioxidants and clinical pregnancy rates in women undergoing IVF or ICSI.
There was insufficient evidence to draw any conclusions about adverse events such as miscarriage, multiple pregnancy or gastrointestinal disturbances when comparing antioxidants with placebo or no treatment/standard treatment. The quality of evidence for miscarriage, multiple pregnancy and gastrointestinal disturbances was considered 'very low' (Table 1). The outcomes of ectopic pregnancy and headache were reported by only one trial in each group.
Effectiveness of antioxidants versus antioxidants-head‐to‐head
Only one trial reported on live birth, clinical pregnancy or adverse effects in this comparison, so meta‐analysis was not possible. The findings of this one trial suggested that myoinisitol may be associated with higher rates of live birth and clinical pregnancy than D‐chiro‐inositol. There was insufficient evidence to determine whether there was any difference between them in miscarriage rates.
Effectiveness of pentoxifylline versus placebo/no treatment
Only one trial reported on live birth for this comparison, so pooling of data was not possible. Very low‐quality evidence suggests that pentoxifylline may be associated with an increased clinical pregnancy rate; although, there were only three trials reporting on this outcome, two reported on pentoxifylline and one reported on pentoxifylline plus vitamin E. There was no evidence of an association between pentoxifylline and clinical pregnancy rates in women with endometriosis, and insufficient evidence to reach any conclusions about miscarriage rates. We deemed the evidence to be of 'very low quality' (Table 2).
Overall completeness and applicability of evidence
Of the 50 trials included in this review, 36 reported on clinical pregnancy, but only 10 trials reported on live birth. Miscarriage, harmful events and costs of the included trials generally were not well reported. Twenty‐four trials reported on miscarriage, 10 reported on multiple pregnancy, three trials discussed gastrointestinal disturbances, two ectopic pregnancy, one headache, three ovarian hyperstimulation syndrome and one preterm birth. The trials were generally quite small, and heterogeneity between them was moderately high overall.
We tried to assess which type of antioxidant might have a beneficial effect on the outcomes of interest in this review However, there were only one or two trials for most interventions. Similarly, the indications for subfertility within the trials were representative of the general subfertile population, but apart from trials on PCOS (with 12 trials across all comparisons), there were very few trials specific to one indication for subfertility (three for unexplained subfertility and two for tubal subfertility, two for endometriosis, one for male factor, one for poor responders), and when pooling was possible within these indications, we had to take into account that the women were also receiving different types of antioxidants and differing adjunctive interventions such as laparoscopic ovarian drilling, timed intercourse or IVF/ICSI. Apart from PCOS, it was therefore difficult to show any benefit or harm from antioxidants for a particular indication of subfertility.
Only one trial, with each arm using a different antioxidant, was included in the head‐to‐head analysis, so we could reach no conclusions about benefits or harms in this comparison.
In the pentoxifylline versus placebo/no treatment comparison there was evidence of association with clinical pregnancy, but as this agent is a medicine and has actions above and beyond the reactive oxygen species‐scavenging capabilities of antioxidants, it is difficult to say that this result is due to the antioxidant action of the drug.
Quality of the evidence
The quality of the evidence according to the 'Summary of findings' tables (Table 1; Table 2) was considered to be 'very low' for all outcomes in the antioxidant versus placebo/no treatment and in the pentoxifylline versus placebo/no treatment comparisons. Heterogeneity in many of the analyses was quite high; three of the main analyses had low heterogeneity, with an I2 value of 0%, but heterogeneity for the live birth outcome in the antioxidant versus placebo/no treatment comparison was 47%, and 66% for clinical pregnancy.
The overall quality of evidence was limited by serious risks of bias associated with poor reporting of methods, imprecision and inconsistency, leading to a downgrading of the evidence. The risk of bias within the evidence (because of methodological limitations) was moderately high (see Figure 2; Figure 3 and Characteristics of included studies). Not all trials described their sequence generation or allocation concealment methods, and most trials randomly assigned only small numbers of women.
The funnel plot for clinical pregnancy (Figure 4) was not symmetrical, which suggests that the high number of small studies may have had an excessively positive effect on the overall results. This high risk of bias in the included trials is also described in other antioxidant reviews (Lu 2012; Showell 2011) and seems to be common in this area of complementary medicine.
Potential biases in the review process
There may have been some potential for bias in the review process, as there were some changes to the protocol. These included additions and deletions to inclusion/exclusion criteria and to the subgroup analyses (see Differences between protocol and review). None of these changes was made as a result of the findings of included studies, but rather to improve the structure of the review. For this 2017 update we analysed trials that used an antioxidant plus an antioxidant versus the same antioxidants plus placebo/no treatment or standard treatment in the antioxidants versus no‐treatment comparison, whereas in the original review we treated them as head‐to‐head.
Agreements and disagreements with other studies or reviews
The results of our review are in agreement with those of other published reviews. Sekhon 2010 and Grajecki 2012 concluded that, despite numerous advances made in this area and possible positive effects of antioxidants, there is a need for further investigation using better‐quality randomised controlled trials within a larger population to determine the efficacy and safety of these supplements. A Cochrane Review Pentoxifylline versus medical therapies for subfertile women with endometriosis (Lu 2012) stated that evidence was still insufficient to support the use of pentoxifylline in the management of premenopausal women with endometriosis in terms of subfertility and relief of pain outcomes. Another Cochrane Review, Antioxidants for male subfertility (Showell 2014), found a small significant effect in favour of antioxidants for pregnancy and live birth and no apparent association with any reported adverse events; however, there were too few similar trials to provide conclusive evidence.
A systematic review by Pacis 2015 did not find any evidence to support the use of vitamin D in women undergoing ART. Another two systematic reviews (Irani 2014; Thomson 2012) looked at vitamin D for subfertile women with PCOS. They reported that there is some evidence for the beneficial effects of vitamin D supplementation on menstrual dysfunction, but the current evidence is limited and additional randomised controlled trials are required.
Another systematic review concentrating on women with PCOS was prepared by Unfer 2016. This review looked specifically at the effects of myo‐inositol for PCOS, and the review authors concluded that myo‐inositol provided a beneficial effect for PCOS; this was "mainly based on improving insulin sensitivity of target tissues, resulting in a positive effect on the reproductive axis...". Our current review found no evidence for this, but a Cochrane protocol (Showell 2016) has recently been published and will look specifically at the use of inositols for subfertile women with PCOS.
Two Cochrane Reviews (Bjelakovic 2008; Bjelakovic 2012) reported an increased risk of mortality associated with the use of supplemental antioxidants. Bjelakovic 2012 found this association with beta‐carotene and possibly vitamin E and vitamin A, but not with vitamin C or selenium. The review included healthy participants and participants with various stable diseases. The Cochrane Review Bjelakovic 2008 reported on the use of antioxidants (beta‐carotene, vitamin A, vitamin C and vitamin E) to prevent gastrointestinal cancers and found that there may be an increased risk of mortality for participants taking these antioxidants. The review authors found that selenium may have preventative effects on gastrointestinal cancers. Neither review supports the use of antioxidants as a preventative measure, and they call for tighter regulations. Bjelakovic 2008 suggests that antioxidants should be regulated as drugs.
Authors' conclusions
In this review, there is very low‐quality evidence to show that taking an antioxidant may provide benefit for subfertile women. There is insufficient evidence to draw any conclusions about adverse events. At this time, there is limited evidence in support of supplemental oral antioxidants for subfertile women.
Further appropriately‐powered and well‐designed randomised placebo‐controlled trials are needed to assess any evidence for benefits or harms or both of supplemental antioxidants for subfertile women. New trials should state a priori that they are going to report and follow up on the outcomes of live birth, clinical pregnancy and adverse events.
Acknowledgements
The authors wish to thank the following people for providing valuable information that assisted in the writing of this review:
Dr Mustafa Nazıroğlu for providing information on the trial Ozkaya 2011;
Aboubakr Elnashar for providing information on the trials Elnashar 2005 and Elnashar 2007;
Dr Rina Agrawal for providing information on the trial Agrawal 2012 and for informing me of her new ongoing trial;
Dr Mohamed Youssef for providing information on the trial Aboulfoutouh 2011 and for informing me that this trial is about to be published;
Dr Gianfranco Carlomagno for providing information on the trial Unfer 2011;
Dr Mariagrazia Stracquadanio for providing information on the trial Ciotta 2011;
Dr Badawy for providing information on the trial Badawy 2006;
Dr Balasch for providing information on the trial Balasch 1997;
Dr Papaleo for providing information on the trial Papaleo 2009;
Dr Lisi for providing information on the trial Lisi 2012;
Dr Battaglia for providing data on the trial Battaglia 2002; and
Dr Eryilmaz for providing information on the trial Eryilmaz 2011
Professor Joanne Barnes
Dr Vahid Seyfoddin for providing translation of the trial Mohammadbeigi 2012
The Cochrane Gynaecology and Fertility Group.
Jane Clarke, who initiated and conceptualised the review, extracted the initial pool of data and wrote the first draft of the review.
Dr Julie Brown assisted with assessing the trials for inclusion, extracted the data, assisted with the data analysis and helped with writing of the original review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility specialised register search strategy
PROCITE platform
Inception to 27 September 2016
Keywords CONTAINS "antioxidants"or "antioxidant" or "antioxidant levels" or "vitamin" or "vitamin A" or "vitamin B" or "Vitamin‐B‐12" or "Vitamin‐B‐12‐Therapeutic‐Use" or "vitamin B6" or "vitamin C" or "Vitamin D" or "vitamin E" or "vitamins" or "selenium" or "folic acid" or "glutathione" or "Menevit anti‐oxidant" or "carnitene" or "carnitine" or "ascorbic acid" or "zinc" or "fatty acids" or "oil" or "fish oils" or "plant extracts" or "tocopherol"or"ubiquinol "or"coenzyme Q10"or "multivitamins"or "N‐acetyl cysteine"or "L‐acetyl‐carnitine"or"acetyl L‐carnitine"or "acetylcysteine"or"pentoxifylline"or"alpha tocopherol"or"pycnogenol"or"Myo‐inositol"or "inositol"or "melatonin" or Title CONTAINS "antioxidants" or "antioxidant"or"antioxidant levels" or "vitamin" or "vitamin A" or "vitamin B" or "Vitamin‐B‐12" or "Vitamin‐B‐12‐Therapeutic‐Use" or "vitamin B6" or "vitamin C" or "Vitamin D" or "vitamin E" or "vitamins" or "selenium" or "folic acid" or "glutathione" or "Menevit anti‐oxidant" or "carnitene" or "carnitine" or "ascorbic acid" or "zinc"or "Myo‐inositol"or "inositol"or "melatonin"
AND
Keywords CONTAINS "IVF" or "ICSI" or "in‐vitro fertilisation " or "in‐vitro fertilisation procedure" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "intracytoplasmic morphologically selected sperm injection" or "superovulation" or "superovulation induction" or "IUI" or "insemination, intrauterine " or "Intrauterine Insemination" or "ART" or "artificial insemination" or "assisted reproduction techniques" or "subfertility‐Female" or "Polycystic Ovary Syndrome" or "PCOS" or "endometriosis"or "subfertility" or "unexplained and endometriosis related infertility" or"unexplained infertility" or"unexplained subfertility" or Title CONTAINS"IVF" or "ICSI" or "in‐vitro fertilisation " or "in‐vitro fertilisation procedure" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "intracytoplasmic morphologically selected sperm injection" or "superovulation" or "superovulation induction" or "IUI" or "insemination, intrauterine " or "Polycystic Ovary Syndrome" or "subfertility"
441 records found
Appendix 2. CENTRAL CRSO search strategy
Web platform
Inception to 27 September 2016
1 exp antioxidants/ or free radical scavengers/ (9281) 2 (antioxidant$ or radical scavengers).tw. (3411) 3 exp vitamins/ or exp ascorbic acid/ or exp dehydroascorbic acid/ or exp vitamin a/ or exp vitamin e/ or exp vitamin u/ or exp alpha‐tocopherol/ or exp beta carotene/ or exp beta‐tocopherol/ or exp gamma‐tocopherol/ (10370) 4 vitamin$.tw. (8540) 5 exp Zinc/ (1038) 6 (zinc or selenium).tw. (2819) 7 exp Selenium/ (385) 8 exp Glutathione Peroxidase/ or exp folic acid/ (2185) 9 (Glutathione$ or folate).tw. (2144) 10 exp Ubiquinone/ (244) 11 (ubiquin$ or folic acid).tw. (1262) 12 coenzyme q10.tw. (225) 13 exp Carnitine/ (409) 14 (carnitine$ or carotenoid$).tw. (1083) 15 (astaxanthin$ or lycopene$).tw. (280) 16 multivitamin$.tw. (449) 17 (betacarotene$ or beta carotene$).tw. (1032) 18 ascorbic acid.tw. (816) 19 n‐acetylcysteine.tw. (543) 20 exp Acetylcysteine/ (474) 21 alpha‐tocopherol$.tw. (902) 22 exp Pentoxifylline/ (386) 23 Pentoxifylline$.tw. (642) 24 (fish adj2 oil$).tw. (1134) 25 omega$.tw. (1103) 26 exp fatty acids/ or exp fish oils/ or exp cod liver oil/ or exp fatty acids, omega‐3/ or exp plant oils/ (14892) 27 fatty acid$.tw. (5704) 28 (plant adj4 oil$).tw. (52) 29 l‐arginine$.tw. (853) 30 flavonoid$.tw. (275) 31 riboflavin$.tw. (265) 32 pycnogenol$.tw. (59) 33 lutein$.tw. (1540) 34 lipoic acid$.tw. (154) 35 exp Inositol/ (234) 36 (Inositol or myoinositol).tw. (193) 37 mesoinositol.tw. (0) 38 myo inositol.tw. (65) 39 n acetyl cysteine.tw. (82) 40 d chiro inositol.tw. (15) 41 melatonin.tw. (858) 42 or/1‐41 (43448) 43 exp Infertility, Female/ (819) 44 female$ subfertil$.tw. (0) 45 female$ infertilit$.tw. (20) 46 subfertil$ women.tw. (13) 47 infertil$ women.tw. (326) 48 female$ fertility.tw. (3) 49 (in vitro fertilisation or intracytoplasmic sperm injection$).tw. (530) 50 intrauterine insemination$.tw. (399) 51 (ivf or icsi or iui).tw. (2495) 52 in vitro fertilization.tw. (1222) 53 ART.tw. (947) 54 Artificial reproduc$ technique$.tw. (0) 55 or/43‐54 (4722) 56 42 and 55 (372)
Appendix 3. MEDLINE search strategy
Ovid platform
From 1946 to 27 September 2016
1 exp antioxidants/ or free radical scavengers/ (330361) 2 (antioxidant$ or radical scavengers).tw. (101777) 3 exp vitamins/ or exp ascorbic acid/ or exp dehydroascorbic acid/ or exp vitamin a/ or exp vitamin e/ or exp vitamin u/ or exp alpha‐tocopherol/ or exp beta carotene/ or exp beta‐tocopherol/ or exp gamma‐tocopherol/ (264949) 4 vitamin$.tw. (139453) 5 exp Zinc/ (46748) 6 (zinc or selenium).tw. (92277) 7 exp Selenium/ (15844) 8 exp Glutathione Peroxidase/ or exp folic acid/ (41759) 9 (Glutathione$ or folate).tw. (102666) 10 exp Ubiquinone/ (6205) 11 (ubiquin$ or folic acid).tw. (19643) 12 coenzyme q10.tw. (1933) 13 exp Carnitine/ (7634) 14 (carnitine$ or carotenoid$).tw. (22030) 15 (astaxanthin$ or lycopene$).tw. (3855) 16 multivitamin$.tw. (2590) 17 (betacarotene$ or beta carotene$).tw. (8645) 18 ascorbic acid.tw. (22280) 19 n‐acetylcysteine.tw. (7320) 20 exp Acetylcysteine/ (9543) 21 alpha‐tocopherol$.tw. (11448) 22 exp Pentoxifylline/ (3622) 23 Pentoxifylline$.tw. (3534) 24 (fish adj2 oil$).tw. (7176) 25 omega$.tw. (29276) 26 exp fatty acids/ or exp fish oils/ or exp cod liver oil/ or exp fatty acids, omega‐3/ or exp plant oils/ (379560) 27 fatty acid$.tw. (138058) 28 (plant adj4 oil$).tw. (1435) 29 l‐arginine$.tw. (28928) 30 flavonoid$.tw. (18313) 31 riboflavin$.tw. (7206) 32 pycnogenol$.tw. (244) 33 lutein$.tw. (31314) 34 lipoic acid$.tw. (2734) 35 exp Inositol/ (20428) 36 (Inositol or myoinositol).tw. (30608) 37 mesoinositol.tw. (35) 38 myo inositol.tw. (4698) 39 n acetyl cysteine.tw. (1956) 40 d chiro inositol.tw. (122) 41 melatonin.tw. (16367) 42 or/1‐41 (1239820) 43 exp Infertility, Female/ (22690) 44 female$ subfertil$.tw. (35) 45 female$ infertilit$.tw. (991) 46 subfertil$ women.tw. (198) 47 infertil$ women.tw. (3052) 48 female$ fertility.tw. (1268) 49 (in vitro fertilisation or intracytoplasmic sperm injection$).tw. (5857) 50 intrauterine insemination$.tw. (1711) 51 (ivf or icsi or iui).tw. (18797) 52 in vitro fertilization.tw. (14872) 53 ART.tw. (42946) 54 Artificial reproduc$ technique$.tw. (69) 55 or/43‐54 (90627) 56 42 and 55 (4177) 57 randomized controlled trial.pt. (347097) 58 controlled clinical trial.pt. (85769) 59 randomized.ab. (265050) 60 placebo.tw. (147404) 61 clinical trials as topic.sh. (163996) 62 randomly.ab. (192945) 63 trial.ti. (113213) 64 (crossover or cross‐over or cross over).tw. (56490) 65 or/57‐64 (853363) 66 (animals not (humans and animals)).sh. (3711406) 67 65 not 66 (786854) 68 67 and 56 (463)
Appendix 4. Embase search strategy
Ovid platform
From 1980 to 27 September 2016
1 exp antioxidants/ or free radical scavengers/ (103607) 2 (antioxidant$ or radical scavengers).tw. (130000) 3 vitamin$.tw. (166429) 4 exp vitamin/ or exp ascorbic acid/ or exp carotenoid/ or exp tocopherol/ (432169) 5 exp Zinc/ (75273) 6 (zinc or selenium).tw. (106356) 7 exp Selenium/ (26722) 8 exp Glutathione Peroxidase/ or exp folic acid/ (62168) 9 (Glutathione$ or folate).tw. (116792) 10 exp Ubiquinone/ (6514) 11 (ubiquin$ or folic acid).tw. (22527) 12 coenzyme q10.tw. (3023) 13 exp Carnitine/ (10125) 14 (carnitine$ or carotenoid$).tw. (25360) 15 (astaxanthin$ or lycopene$).tw. (4669) 16 multivitamin$.tw. (3250) 17 (betacarotene$ or beta carotene$).tw. (11613) 18 ascorbic acid.tw. (25154) 19 n‐acetylcysteine.tw. (8893) 20 exp acetylcysteine/ (22243) 21 n‐acetyl‐cysteine.tw. (2448) 22 alpha‐tocopherol$.tw. (13936) 23 exp Pentoxifylline/ (10809) 24 Pentoxifylline$.tw. (4357) 25 (fish adj2 oil$).tw. (8913) 26 omega$.tw. (14166) 27 fatty acid$.tw. (154677) 28 exp edible oil/ or exp castor oil/ or exp cod liver oil/ or exp fish oil/ or exp lyprinol/ or exp olive oil/ or exp safflower oil/ or exp fatty acid/ or exp essential fatty acid/ or exp arachidonic acid/ or exp linoleic acid/ or exp linolenic acid/ or exp gamma linolenic acid/ or exp unsaturated fatty acid/ or exp omega 3 fatty acid/ or exp omega 6 fatty acid/ or exp polyunsaturated fatty acid/ (425301) 29 (plant adj4 oil$).tw. (2056) 30 l‐arginine$.tw. (32378) 31 flavonoid$.tw. (26182) 32 riboflavin$.tw. (7713) 33 pycnogenol$.tw. (352) 34 lipoic acid$.tw. (3265) 35 exp inositol/ (8444) 36 (Inositol or myoinositol).tw. (34393) 37 mesoinositol.tw. (36) 38 myo inositol.tw. (5416) 39 melatonin.tw. (19303) 40 d chiro inositol.tw. (142) 41 or/1‐40 (1344153) 42 exp Infertility, Female/ (34044) 43 (female$ adj2 subfertil$).tw. (94) 44 (female$ adj2 infertilit$).tw. (1554) 45 (subfertil$ adj2 women).tw. (348) 46 (infertil$ adj2 women).tw. (5126) 47 (female$ adj2 fertility).tw. (2010) 48 (vitro fertilisation or intracytoplasmic sperm injection$).tw. (7363) 49 (intrauterine adj3 insemination$).tw. (2295) 50 (ivf or icsi or iui).tw. (26704) 51 vitro fertilization.tw. (17633) 52 Artificial reproduc$ technique$.tw. (117) 53 exp artificial insemination/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ or exp intrauterine insemination/ (52724) 54 exp Superovulation/ (2032) 55 Superovulation.tw. (1744) 56 or/42‐55 (91018) 57 Clinical Trial/ (876796) 58 Randomized Controlled Trial/ (340260) 59 exp randomization/ (61167) 60 Single Blind Procedure/ (17227) 61 Double Blind Procedure/ (114019) 62 Crossover Procedure/ (36637) 63 Placebo/ (216063) 64 Randomi?ed controlled trial$.tw. (85514) 65 Rct.tw. (11229) 66 random allocation.tw. (1227) 67 randomly allocated.tw. (18541) 68 allocated randomly.tw. (1874) 69 (allocated adj2 random).tw. (717) 70 Single blind$.tw. (13168) 71 Double blind$.tw. (135429) 72 ((treble or triple) adj blind$).tw. (310) 73 placebo$.tw. (187097) 74 prospective study/ (230049) 75 or/57‐74 (1319736) 76 case study/ (19249) 77 case report.tw. (241847) 78 abstract report/ or letter/ (864231) 79 or/76‐78 (1120296) 80 75 not 79 (1283559) 81 41 and 56 and 80 (785)
Appendix 5. PsycINFO search strategy
Ovid platform
From 1806 to 27 September 2016
1 exp Antioxidants/ (1289) 2 (antioxidant$ or radical scavengers).tw. (2536) 3 exp Vitamins/ (2994) 4 vitamin$.tw. (4313) 5 exp Zinc/ (472) 6 (zinc or selenium).tw. (1360) 7 (Glutathione$ or folate).tw. (1988) 8 (ubiquin$ or folic acid).tw. (508) 9 coenzyme q10.tw. (101) 10 (carnitine$ or carotenoid$).tw. (439) 11 multivitamin$.tw. (148) 12 (betacarotene$ or beta carotene$).tw. (45) 13 ascorbic acid.tw. (330) 14 n‐acetylcysteine.tw. (141) 15 alpha‐tocopherol$.tw. (58) 16 Pentoxifylline$.tw. (53) 17 (fish adj2 oil$).tw. (138) 18 omega$.tw. (909) 19 exp Fatty Acids/ (2614) 20 fatty acid$.tw. (2322) 21 l‐arginine$.tw. (754) 22 or/1‐21 (15067) 23 exp Infertility/ (1531) 24 female$ subfertil$.tw. (2) 25 female$ infertilit$.tw. (40) 26 subfertil$ women.tw. (2) 27 infertil$ women.tw. (187) 28 female$ fertility.tw. (95) 29 (vitro fertilisation or intracytoplasmic sperm injection$).tw. (92) 30 intrauterine insemination$.tw. (13) 31 (ivf or icsi or iui).tw. (353) 32 vitro fertilization.tw. (435) 33 Artificial reproduc$ technique$.tw. (6) 34 or/23‐33 (2032) 35 22 and 34 (12)
Appendix 6. AMED search strategy
From 1982 to 27 September 2016
Ovid platform
1 exp Antioxidants/ or exp Free radicals/ (1454) 2 (antioxidant$ or radical scavengers).tw. (2082) 3 exp Vitamins/ or exp Dietary supplements/ (2999) 4 exp Ascorbic acid/ (252) 5 vitamin$.tw. (2113) 6 exp Zinc/ (100) 7 (zinc or selenium).tw. (421) 8 (Glutathione$ or folate).tw. (638) 9 exp Selenium/ (88) 10 (ubiquin$ or folic acid).tw. (149) 11 coenzyme q10.tw. (73) 12 exp Carnitine/ (16) 13 (carnitine$ or carotenoid$).tw. (171) 14 multivitamin$.tw. (54) 15 ascorbic acid.tw. (410) 16 n‐acetylcysteine.tw. (26) 17 Acetylcysteine.tw. (27) 18 alpha‐tocopherol$.tw. (80) 19 Pentoxifylline$.tw. (10) 20 (fish adj2 oil$).tw. (154) 21 omega$.tw. (211) 22 exp Fatty acids/ (432) 23 exp Fish oils/ (86) 24 fatty acid$.tw. (661) 25 (plant adj4 oil$).tw. (782) 26 l‐arginine$.tw. (109) 27 flavonoid$.tw. (1049) 28 riboflavin$.tw. (20) 29 (Inositol or myoinositol).tw. (45) 30 pycnogenol$.tw. (16) 31 or/1‐30 (7934) 32 exp Infertility female/ (150) 33 female$ subfertil$.tw. (0) 34 female$ infertilit$.tw. (18) 35 subfertil$ women.tw. (0) 36 infertil$ women.tw. (13) 37 female$ fertility.tw. (6) 38 (vitro fertilisation or intracytoplasmic sperm injection$).tw. (19) 39 intrauterine insemination$.tw. (5) 40 (ivf or icsi or iui).tw. (31) 41 in vitro fertilization.tw. (15) 42 Artificial reproduc$ technique$.tw. (0) 43 or/32‐42 (186) 44 31 and 43 (4)
Appendix 7. CINAHL search strategy
Ovid platform
From 1982 to 27 September 2016
| # | Query | Results |
| S63 | S50 AND S62 | 46 |
| S62 | S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 | 973,529 |
| S61 | TX allocat* random* | 4,373 |
| S60 | (MH "Quantitative Studies") | 13,575 |
| S59 | (MH "Placebos") | 9,311 |
| S58 | TX placebo* | 34,242 |
| S57 | TX random* allocat* | 4,373 |
| S56 | (MH "Random Assignment") | 39,396 |
| S55 | TX randomi* control* trial* | 89,855 |
| S54 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 777,926 |
| S53 | TX clinic* n1 trial* | 173,348 |
| S52 | PT Clinical trial | 78,187 |
| S51 | (MH "Clinical Trials+") | 189,346 |
| S50 | S26 AND S49 | 125 |
| S49 | S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 | 6,349 |
| S48 | TX intra‐uterine insemination | 10 |
| S47 | TX natural cycle* | 119 |
| S46 | TX timed intercourse | 22 |
| S45 | TX (ovari* N2 induction) | 12 |
| S44 | TX COH | 68 |
| S43 | TX ovarian hyperstimulation | 345 |
| S42 | TX superovulat* | 24 |
| S41 | TX ovulation induc* | 588 |
| S40 | TX intrauterine insemination | 152 |
| S39 | TX IUI | 83 |
| S38 | TX artificial insemination | 459 |
| S37 | TX assisted reproduct* | 1,331 |
| S36 | (MM "Insemination, Artificial") | 244 |
| S35 | (MM "Reproduction Techniques+") | 4,061 |
| S34 | TX intracytoplasmic sperm injection* | 246 |
| S33 | TX embryo* N3 transfer* | 809 |
| S32 | TX ovar* N3 hyperstimulat* | 348 |
| S31 | TX ovari* N3 stimulat* | 253 |
| S30 | TX IVF or TX ICSI | 1,289 |
| S29 | (MM "Fertilization in Vitro") | 1,487 |
| S28 | TX vitro fertilization | 2,919 |
| S27 | TX vitro fertilisation | 276 |
| S26 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 | 106,012 |
| S25 | TX Nutraceutical* | 738 |
| S24 | TX micronutrient* | 3,381 |
| S23 | TX nutritional supplement* | 1,710 |
| S22 | TX dietary supplement* | 28,026 |
| S21 | TX melatonin | 1,919 |
| S20 | TX n acetyl cysteine | 203 |
| S19 | TX l‐arginine | 938 |
| S18 | TX (fish N2 oil*) | 2,915 |
| S17 | (MH "Acetylcysteine") | 1,042 |
| S16 | TX n‐acetylcysteine | 691 |
| S15 | TX ascorbic acid | 4,277 |
| S14 | TX multivitamin* | 946 |
| S13 | TX(astaxanthin* or lycopene*) | 850 |
| S12 | TX (carnitine* or carotenoid*) | 2,960 |
| S11 | (MM "Carnitine") | 494 |
| S10 | TX coenzyme q10 | 415 |
| S9 | TX (ubiquin* or folic acid) | 6,007 |
| S8 | TX (zinc or selenium) | 6,881 |
| S7 | TX omega$ | 7,321 |
| S6 | TX fatty acid* | 17,157 |
| S5 | (MH "Fatty Acids, Omega 3") OR (MH "Fatty Acids, Unsaturated+") | 17,162 |
| S4 | TX vitamin* | 35,451 |
| S3 | (MH "Vitamins+") | 34,733 |
| S2 | TX antioxidant* | 16,804 |
| S1 | (MM "Antioxidants+") | 6,858 |
Appendix 8. Search strategies for The WHO portal (ICTRP), clinicaltrials.gov, Google, DARE, Web of Knowledge and OpenGrey
From inception to 27 September 2016
Web platforms
'antioxidants and subfertility', 'antioxidants and infertility', 'vitamin and subfertility', 'vitamin and infertility', 'N‐acetyl‐cysteine and subfertility', 'N‐acetyl‐cysteine and infertility', 'myo‐inositol and subfertility', 'myo‐inositol and subfertility', 'fatty acids and subfertility' and 'fatty acids and infertility';
Data and analyses
Comparison 1.
Antioxidant(s) versus placebo or no treatment/standard treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 8 | 651 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.45, 3.12] |
| 1.1 Placebo | 5 | 388 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.17, 3.44] |
| 1.2 No treatment | 3 | 263 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.31, 3.91] |
| 2 Live birth; type of antioxidant | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 N‐acetyl‐cysteine | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [1.05, 8.60] |
| 2.2 L‐arginine | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.09, 2.09] |
| 2.3 CoQ10 | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.19, 3.54] |
| 2.4 Vitamin D | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.21, 3.02] |
| 2.5 Vitamin B complex | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.93, 4.57] |
| 2.6 Combined antioxidants | 2 | 258 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.76 [2.79, 16.41] |
| 2.7 Vitamin E | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.50, 4.10] |
| 3 Live birth; indications for subfertility | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Polycystic ovary syndrome | 3 | 362 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [1.90, 5.86] |
| 3.2 Tubal subfertility | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.09, 2.09] |
| 3.3 Varying indications | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.5 [1.46, 13.86] |
| 3.4 Unexplained subfertility | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.50, 4.10] |
| 4 Live birth; IVF/ICSI | 4 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.69, 2.11] |
| 5 Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 26 | 4271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.31, 1.76] |
| 5.1 Placebo | 12 | 2444 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.20, 1.82] |
| 5.2 No treatment/standard treatment | 15 | 1827 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.27, 1.93] |
| 6 Clinical pregnancy; type of antioxidant | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 N‐acetyl‐cysteine | 6 | 1354 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.92, 1.63] |
| 6.2 Combined antioxidants | 4 | 569 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.51, 3.43] |
| 6.3 Melatonin | 4 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.91, 1.83] |
| 6.4 Vitamin E | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.50, 4.10] |
| 6.5 Ascorbic acid | 1 | 619 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.14] |
| 6.6 L‐arginine | 2 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.32, 3.46] |
| 6.7 Myo‐inositol plus folic acid | 4 | 694 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.98, 1.86] |
| 6.8 CoQ10 | 2 | 149 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.28 [1.79, 10.26] |
| 6.9 L‐carnitine | 1 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 82.05 [10.92, 616.59] |
| 6.10 Vitamin D | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.25, 2.76] |
| 6.11 Vitamin B complex | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.82, 4.58] |
| 6.12 Myo‐inositol plus melatonin plus folic acid | 1 | 389 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.90, 2.12] |
| 7 Clinical pregnancy; indications for subfertility | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Polycystic ovary syndrome | 11 | 1721 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.63 [2.06, 3.36] |
| 7.2 Unexplained | 3 | 967 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.14] |
| 7.3 Tubal subfertility | 2 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.32, 3.46] |
| 7.4 Varying indications | 5 | 1004 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.94, 1.71] |
| 7.5 Poor responders | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.64, 5.47] |
| 8 Clinical pregnancy; IVF/ICSI | 15 | 2263 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.98, 1.43] |
| 9 Adverse events | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Miscarriage | 18 | 2834 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.08] |
| 9.2 Multiple pregnancy | 8 | 2163 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.38] |
| 9.3 Gastrointestinal disturbances | 3 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.47, 5.10] |
| 9.4 Ectopic pregnancy | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.11, 74.13] |
| 9.5 Headache | 1 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.75] |
Comparison 2.
Head‐to‐head antioxidants
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth; type of antioxidant (natural conceptions and undergoing fertility treatments) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Myo‐Inositol versus d‐chiro‐inositol | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinical pregnancy; type of antioxidant (natural conceptions and undergoing fertility treatments) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Myo‐Inositol versus d‐chiro‐inositol | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Miscarriage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3.
Pentoxifylline versus placebo or no treatment/standard care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.68, 3.50] |
| 2 Clinical pregnancy; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 3 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.20, 3.56] |
| 2.1 Placebo | 2 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.94, 4.56] |
| 2.2 No treatment | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| 3 Clinical pregnancy; type of antioxidant | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pentoxifylline | 2 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.94, 4.56] |
| 3.2 Pentoxifylline plus vitamin E | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| 4 Clinical pregnancy; indications for subfertility | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Endometriosis | 2 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.94, 4.56] |
| 4.2 Varying indications | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| 5 Clinical pregnancy; IVF/ICSI | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| 6 Adverse events | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Miscarriage | 3 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.90] |
| 6.2 Multiple pregnancy | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.20, 3.09] |
| 6.3 Ectopic pregnancy | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.18, 23.13] |
What's new
Last assessed as up‐to‐date: 27 September 2016.
| Date | Event | Description |
|---|---|---|
| 16 October 2017 | Amended | Some references updated and corrected |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 8, 2013
| Date | Event | Description |
|---|---|---|
| 28 June 2017 | New search has been performed | Updated. Twenty‐three new trials added, making a total of 50 trials now included in this updated review. New studies added:Battaglia 1999; Bentov 2014; Brusco 2013; Carlomagno 2012; Cheraghi 2016; Choi 2012; Colazingari 2013; Daneshbodi 2013; Deeba 2015; El Refaeey 2014; Ismail 2014; Keikha 2010; Lesoine 2016; Maged 2015; Mohammadbeigi 2012; Pacchiarotti 2016; Panti Abubakar 2015; Polak de Fried 2013; Razavi 2015; Rosalbino 2012; Salehpour 2012; Schachter 2007; Valeri 2015. |
| 28 June 2017 | New citation required and conclusions have changed | With the addition of new studies data now show an association between the use of antioxidants and live birth and clinical pregnancy. |
| 22 April 2008 | Amended | Converted to new review format. |
| 9 August 2007 | New citation required and major changes | Substantive amendment |
Differences between protocol and review
After publication of the protocol:
two of the five protocol authors (Agarwal A, Gupta S) withdrew from involvement in the review.
we have removed the secondary outcome of stillbirth rate per woman.
we have removed the exclusion criterion 'Trials that exclusively reported on women who have previously had chemotherapy' as not clinically relevant to this review.
we have expanded the inclusion criteria for participants to include women undergoing ART. Exclusion criteria now cover trials that enrol exclusively fertile women attending a fertility clinic because of male partner infertility, and women who are Vitamin D‐deficient.
exclusion criteria for interventions now cover antioxidants versus fertility drugs alone as controls, as they are themselves active agents. They might include metformin or clomiphene citrate.
the review includes a subgroup analysis based on the type of subfertility problem, including women with PCOS, endometriosis, poor responders and tubal and unexplained subfertility, as well as a subgroup of women who are undergoing IVF or ICSI.
we have created a separate comparison for pentoxifylline, as we had concerns that this medicine does not have purely antioxidant capabilities.
we have updated the search strategy.
we have added two 'Summary of findings' tables.
where we had data from multi‐armed trials, we have pooled the intervention arms versus the control arm. This differs from the protocol, where we said that we would divide the intervention arms. This was done on the advice of a statistician.
we decided, with clinical advice, that we would treat trials using folic acid (< 1 mg) as a control as assessing standard treatment and would include them in the 'no treatment' subgroup.
For the 2017 update:
we have analysed trials that used an antioxidant plus an antioxidant versus the same antioxidants plus placebo/no treatment or standard treatment in the 'Antioxidants versus no treatment' comparison, whereas in the original review they were considered as head‐to‐head.
prior to the 2017 update, the effect estimate used was the Peto odds ratio. As this is not recommended as a default approach for meta‐analysis unless intervention effects are small (odds ratios close to one) and events are not particularly common (Higgins 2011), we have used the Mantel‐Haenszel odds ratio for the 2017 update.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Prospective randomised trial | |
| Participants | Women attending a teaching hospital fertility clinic undergoing ovulation induction for timed intercourse (N = 58). Mean age 32.2 years (range 19 to 40) Inclusion criteria: anovulatory infertility, at least 12 months of unexplained infertility, PCOS, hypothyroidism or minimal endometriosis Exclusion criteria: women whose partners had semen abnormalities and those who had been on multivitamins (except folate) 6 weeks before recruitment Women with tubal disease, moderate and severe endometriosis, medical disorders or haemoglobinopathies; smokers, those with excessive alcohol intake or BMI < 19 or > 34 kg/m2 |
|
| Interventions | 1. Multiple micronutrients (MMN): (n = 30) 1 tablet a day until completion of study (3 treatment cycles). Women who became pregnant could continue if they wished. These micronutrients consist of thiamine, riboflavin, niacin B3, vitamins B6 and B12, folate, vitamins C, A and D, calcium, phosphorus, magnesium, sodium, potassium, chloride, iron, zinc, copper, selenium, iodine, vitamin E, vitamin K, L‐arginine, inositol, N‐acetyl‐cysteine, biotin, pantothenic acid Mean age = 32.2 ± 0.65 2. Folic acid (n = 28): 1 tablet a day. Mean age = 32.5 ± 0.83 Women underwent ovulation induction with clomiphene citrate or human menopausal gonadotropin approximately 4 weeks after starting MMN or folic acid and continued until end of study, which was 3 cycles even if pregnancy was attained. |
|
| Outcomes | Clinical pregnancy Ongoing pregnancy Miscarriage Ectopic pregnancy |
|
| Notes | 2 women did not complete the study-1 from each group. Reasons given: 1 woman in the control group stopped because she wanted to take the micronutrients, and 1 in the treatment group stopped because of nausea Trial is self‐funded. Author stated in an email received 13th February that the trial was not funded. Recruited between Febuary and August 2009 Location: London UK Informed consent Ethical approval Sample size power calculation performed ITT performed Emailed author 12th January 2012about whether the women had IUI or timed intercourse. Author replied on 7th February 2012 saying that all women underwent timed intercourse, not IUI. This email also gave adverse event data (miscarriage and ectopic pregnancy data) for the first cycle. Dr Agrawal is also currently recruiting for a new trial. Emailed author on 9th August 2012 asking about any live birth data. Author replied saying that live birth data were unavailable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Third party randomization ... was carried out through the research and development department of the University College London and the Royal Free Hospitals using stratification..." "Participants were randomly allocated". Email sent 12th January 2012 asking for methods of randomisation. Author replied 13th February 2012 saying, "the subjects were randomised into 2 groups through computer randomisation". |
| Allocation concealment (selection bias) | Low risk | "Third party randomisation and allocation concealment was carried out through the research and development department of the University College London and the Royal Free Hospitals using stratification and numbered envelopes". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Women, caregivers and investigators were blinded to the treatment allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT was performed and explanations given for the 2 dropouts (1 from each group) |
| Selective reporting (reporting bias) | Low risk | Outcomes stated in the text are reported. |
| Other bias | Low risk | No other bias found |
| Methods | Randomised placebo‐controlled trial | |
| Participants | Women with infertility (n = 88) Mean age: Treatment: 29.7 years; Control: 28.3 years Inclusion criteria: Infertility for at least 12 months with endometriosis (different stages) diagnosed by laparoscopy Exclusion criteria: women with other infertility factors including tubal obstruction |
|
| Interventions | 1. Pentoxifylline 400 mg: 1 tablet twice a day for 12 months (n = 43) 2. Placebo (n = 45) Duration: 12 months, 1‐year follow‐up |
|
| Outcomes | Cumulative pregnancy rate Recurrence of endometriosis |
|
| Notes | Study approved by the Shiraz University of Medical Sciences Institutional Review Board Trial conducted in Shiraz, Iran, from January 2002 to December 2003 Funding source not reported Tried to contact the author regarding clinical pregnancy rate and live birth 12th February 2013, but no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were assigned into 1 of 2 groups by simple randomisation. An independent pharmacist generated the allocation and assigned the patients to their groups. To do so, he gave each patient a number on the basis of the order of her being referred to him. For example, the first patient was enlisted as number 1 and the second as number 2 and so on. He then assigned patients with odd numbers into one group and patients with even numbers into another. He decided which one should be the control group by flipping a coin". There is a query as to whether this trial is adequately randomised. It could be seen as block randomisation (cluster) or as alternate (in which case this study would have been excluded). After discussion with the statistician, we decided to include this study because of the double‐blind concealment-if double‐blinding was truly successful and nobody involved in recruitment affected the sequence, then it is a third‐party concealed allocation system that is protecting against selection bias, despite the lack of proper randomisation. |
| Allocation concealment (selection bias) | Low risk | An independent pharmacist generated the allocation and assigned the participants to their groups |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded: "During this period, neither the clinicians nor the patients knew who received the medication and who received the placebo. The only person who knew this was the pharmacist". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | Low risk | Both outcomes stated in Methods and reported on |
| Other bias | Low risk | No other bias found |
| Methods | Randomised clinical trial | |
| Participants | "Infertile women undergoing standardised controlled ovarian hyperstimulation for ICSI‐ZIFT [zygote intrafallopian transfer" (n = 112) Table 1 p. 177 mentions cause of infertility to be male in 51 of 112. Participants aged from 20 ‐ 39 years; mean age 29.69, (treatment group mean age: 29.96; control group mean age 29.41) with no previous history of IVF or ZIFT failure. Infertility duration from 1 ‐ 20 years Exclusion criteria: hypothalamic amenorrhoea, drug reactions, endometriosis and fibroids |
|
| Interventions | 1. Pentoxifylline 400 mg and vitamin E 400 mg: 1 tablet of each twice a day (n = 56). Administered for 2 cycles before ICSI‐ZIFT 2. No treatment (n = 56). Duration: 2 cycles. |
|
| Outcomes | Term delivery Clinical pregnancy rate confirmed by beta human chorionic gonadotropin (hCG) at 14 days after embryo transfer and transvaginal ultrasound 14 days after this Miscarriage rate Multiple pregnancy |
|
| Notes | Conducted in 1 centre in Tehran, Iran; ethical approval gained and written consents obtained Trial was carried out between April 2006 and April 2007 Funded by the institution For sensitivity analysis performed because more than half (41/56) of women had male subfertile partners or because both partners had fertility problems |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables were used |
| Allocation concealment (selection bias) | Low risk | Sealed opaque sequentially numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comparison group received no treatment. Authors stated "study not blinded" (p. 176) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | High risk | Cause of infertility is male in 51 of 112 participants (see Table 1 p. 177), although this is not mentioned in the text, where it says that the participants were 112 infertile women |
| Other bias | Low risk | No other bias found |
| Methods | Prospective randomised double‐blind controlled trial | |
| Participants | Women attending a fertility outpatient clinic for management of unexplained fertility problems (n = 804) Mean age: Treatment group: 27.9 years; Control group 28.1 years Inclusion criteria: All women had at least 1 year of marriage without conception, unexplained subfertility and normal ovulating cycles; tubes were patent Exclusion criteria: any known reason for subfertility Timed intercourse |
|
| Interventions | 1. N‐acetyl‐cysteine 1200 mg: 1 tablet a day plus clomiphene citrate 50 mg: 1 tablet twice a day for 5 days, starting on day 2 of the cycle (n = 404) 2. Placebo plus clomiphene citrate: 50 mg 1 tablet twice a day (n = 400) Duration of treatment: 1 cycle Timed intercourse |
|
| Outcomes | Number and size of follicles Hormonal profiles Endometrial thickness Clinical Pregnancy Miscarriage Multiple pregnancy No loss to follow‐up |
|
| Notes | Conducted in 1 centre in Mansoura, Egypt. Ethical approval and informed consents obtained. Trial ran from October 2003 to April 2005 Funding source not reported Contacted author 13th February 2013 regarding methods of randomisation, but no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation apart from: "Patients were allocated randomly to either the trial group". |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially‐numbered, identical envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, investigators, outcome assessor and clinicians were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | Outcomes stated in the text-multiple pregnancy and miscarriage-reported on, although not initially stated as outcomes of interest |
| Other bias | Low risk | No other bias found |
| Methods | Prospective randomised controlled trial. Pilot study | |
| Participants | Infertile women with asymptomatic minimal or mild endometriosis (n = 60). Mean age: Treatment: 31.2 ± 3.8; Control: 32.4 ± 3.1 Inclusion criteria: at least 12 months of primary infertility, no previous pelvic surgery, minimal or mild endometriosis confirmed by laparoscopy Exclusion criteria: any previous pelvic surgery, pelvic disorders such as adhesions and tubal obstructions, in addition to endometriosis |
|
| Interventions | 1. Pentoxifylline 400 mg: 1 tablet twice a day for 12 months (n = 30) 2. Placebo (n = 30). 12‐month duration and 12‐month follow‐up. During this time, participants received treatment for infertility problems (i.e. male problems, ovulatory problems, cervical mucus abnormalities, IUI, ovulation induction) |
|
| Outcomes | Pregnancy rates confirmed by ultrasound Miscarriage rate |
|
| Notes | 1 dropout from the treatment group and 3 from the control group-all due to refusal to start treatment after randomisation. Number reported is 56. ITT is used for meta‐analysis Trial held from November 1993 to December 1995 Single‐centre study conducted in Spain Ethical approval and all consents obtained Funding source not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process that was using a computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Allocation described as being "designated". Authors contacted regarding this and confirmed concealment "computerised allocation" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women are described as being blinded. Authors contacted regarding other blinded persons. They confirmed that participants were blinded, but investigators, outcome assessors and clinicians were not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only a small number of dropouts-4 participants lost; 1 in treatment, 3 in control. All explained-1 due to refusal and 3 due to failure to continue taking the medication. No ITT carried out |
| Selective reporting (reporting bias) | Unclear risk | Pregnancy rates were stated as the outcome of interest in the Methods section of the paper. However, miscarriage rates were given in the Results and were not mentioned in the Methods. 1 participant in each study group became pregnant, then miscarried, then became pregnant again. The first 2 pregnancies were not included in the analysis. Live births not reported |
| Other bias | High risk | Some women with other fertility issues apart from endometriosis were treated for these additional conditions (i.e. male factor (receiving bromocriptine), oligo‐ovulation (receiving ovulation induction and some additional IUI) poor post‐coital test, hyperprolactinaemia). Numbers of women in treatment and numbers of controls in each of these categories are given. However, these treatments may bias the results, as nearly double the control women in the additional treatment group received IUI compared with the treatment group |
| Methods | Randomised controlled trial | |
| Participants | Women with primary infertility between 20 and 40 years undergoing IVF (n = 85) Inclusion criteria: regular menstruation, no hormonal or non‐hormonal drug therapy for less than 3 months and no systemic illness Exclusion criteria: serious endometriosis, serious male factor (azoospermia) hypogonadism with an FSH level < 13. Also participants with cycles cancelled were excluded. |
|
| Interventions | 1. Melatonin 3 mg: 1 tablet a day (n = 40) 2. No treatment (n = 45). |
|
| Outcomes | Primary outcome: number of morphologically mature oocytes retrieved (Mll oocytes) Secondary outcome: fertilisation rate, embryo quality and pregnancy rate |
|
| Notes | No information on miscarriage numbers Funding sources not mentioned Clinical pregnancy data (not chemical) used in the meta‐analysis Trial held in Turkey, study dates not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation computer‐assisted 1:1. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Embyologist was the only person blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used. No dropouts were reported |
| Selective reporting (reporting bias) | High risk | Unclear why chemical pregnancy numbers are lower than clinical pregnancy numbers |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women attending fertility clinic having failed an IVF cycle (poor responders) (n = 34). Mean age: 40 ± 2.1 years, range 37 ‐ 44 years. Undergoing IVF Inclusion: Infertile women with tubal infertility who had not taken hormonal treatments 4 months prior to 1st IVF treatment Exclusion: Intercurrent illness, BMI > 30, endometriosis, ovarian functional cyst or ovariectomy, regular exercise, heavy smokers (> 10 a day), diastolic blood pressure > 90 mmHg |
|
| Interventions | 1. Oral L arginine 16 g: 1 tablet a day (n = 17) 2. No treatment (n = 17) Duration: from day 1 of the menstrual cycle to end of the IVF cycle |
|
| Outcomes | Hormonal and biochemical evaluation IVF cancellation rates Oocyte and embryo number Clinical pregnancy rates |
|
| Notes | Conducted in Italy, study dates not reported Funding source not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women attending Modena University Infertility Clinic (n = 37) Mean age (mean ± SD): 33.8 ± 3.1 years (range 28 ‐ 37 years), mean duration of infertility 6.8 ± 3.8 (range 4 ‐ 12 years). Inclusion criteria: All participants were selected from among women who suffered from tubal infertility. They had regular menstrual cycles (28 ± 4 days), and their partners were fertile according to World Health Organization standards Exclusion criteria: participants with intercurrent illness, BMI ≥ 30, endometriosis, ovarian functional cyst, PCOS, unilateral ovarian resection or ovariectomy, participants who took regular exercise, heavy smokers (> 10 cigarettes a day), those with hypertension (systolic blood pressure > 140 mm Hg and/or diastolic pressure > 90 mm Hg) and women who had received hormonal treatments in the 4 months before the first IVF attempt |
|
| Interventions | 1. L‐arginine 4 grams: 4 times a day (n = 18) 2. Placebo (n = 19). Both groups were undergoing IVF with long gonadotropin‐releasing hormone (GnRH) agonist protocol and pure FSH Duration: 10, 12 days |
|
| Outcomes | Clinical pregnancy rates Side effects Follicular number and diameter Endometrial thickness Live birth |
|
| Notes | Consent and ethical approval were obtained, and the trial was conducted in Modena, Italy, study dates not reported 32 participants completed the trial, with 5 dropouts due to poor response. Funding source not reported Author was emailed 16th August 2012 and 12th Febrary 2013 with request for the number of live births for each group. Author replied on 14th Febrary 2013, providing data for live birth and miscarriage |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random number table". |
| Allocation concealment (selection bias) | Low risk | "opening sequentially numbered sealed envelopes containing treatment allocation". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigtors, participants and outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 37 women were enrolled, and investigators stated "All 34 patients completed the trial". Numbers given for dropouts from each group. We contacted the authors regarding this ITT not used. Five were said to be cancelled because of "poor response". |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported, including live birth |
| Other bias | Low risk | No other bias found |
| Methods | Double‐blind placebo‐controlled randomised trial | |
| Participants | IVF/ICSI patients (n = 39) Inclusion criteria: infertility requiring IVF–ICSI and age 35 – 43, mean age; CoQ10 39.0 ± 0.79 and placebo 39.1 ± 0.52 Exclusion criteria: body mass index (BMI) >38 kg/m2; early follicular phase (day 2 – 4) serum FSH level 20 mIU/mL; abnormal uterine cavity as evidenced by sonohysterogram or hysterosalpingography; any current use of systemic steroid medication or any infertility treatment within 3 months of study enrolment; any contraindication to being pregnant and carrying a pregnancy to term; contraindication for the use of CoQ10, superfact, menopur, hCG, estrase, and progesterone suppositories; any ovarian or abdominal abnormality that may interfere with adequate TVS evaluation; absence of 1 or both ovaries; clinically relevant systemic disease (e.g. insulin‐dependent diabetes, adrenal dysfunction, organic intracranial lesion, PCOS, hyperprolactinemia, or hypothalamic tumor) or serious illness (neoplasia); history (within past 12 months) or current abuse of alcohol or drugs; administration of any investigational drugs within 3 months before the study enrolment; any medical condition that may interfere with the absorption, distribution, metabolism, or excretion of the study drugs; gastrointestinal diseases; malabsorption syndromes; and liver dysfunction; unexplained gynaecological bleeding; ejaculated sperm not sufficient for ICSI; abnormal controlled ovarian hyperstimulation (COH) screening blood done for both partners, including prolactin, thyroid stimulating hormone, HIV serology, hepatitis B and C serology, rubella, group and screen, and syphilis serology before participation in the study; the concurrent use of any of the following drugs: daunorubicin, doxorubicin, blood pressure medications, warfarin, timolol, atorvastatin, cerivastatin, lovastatin, pravastatin, simvastatin, gemfibrozil, tricyclic antidepressant medications (including amitriptyline, amoxapine, clomipramine, desipramine, doxepin, imipramine, nortriptyline, protriptyline, and trimipramine), multivitamins, or any vitamin supplementation except folic acid |
|
| Interventions | 1. CoQ10 600 mg: 1 tablet a day with breakfast (n = 17) 2. Placebo ‐ identical capsules containing rice oil and starch (n = 22) Duration of treatment up to 3 cycles if pregnancy did not occur. All participants took either CoQ10 or placebo for 2 months. On day 3 of the following cycle, they started ovarian stimulation for IVF while continuing the consumption of the supplements. |
|
| Outcomes | Primary outcome: number of euploid eggs per retrieval Secondary outcome: cumulative pregnancy rate per retrieval and cumulative livebirth rate per retrieval |
|
| Notes | 12 (5 dropouts) CoQ10 group and 15 (7 dropouts) in the placebo completed the study and 10 in the CoQ10 and 14 of the placebo group completed an IVF/ICSI cycle. Overall there were 15 dropouts from recruitment until the end of the study; 6 women withdrew from the study for personal reasons, 3 for conceiving spontaneously, 2 for poor compliance, 1 for failing to achieve the target BMI, and 3 because of poor ovarian response Participant enrolment to the study began in 2010 and was terminated in June 2012 before sample size reached, due to a paper published in May 2012 by Levin et al describing the negative effects of polar body biopsy on embryogenesis. In the CoQ10 group, 30.8% of the women were treated with the long luteal GnRH agonist protocol, compared with 7.7% in the placebo group. The rest of the participants in both groups were treated with the short microdose flare protocol 2 centres Toronto, Canada Trial registration no: NCT01048385 Informed consent Ethics approval Funding; Ferring Pharmaceuticals provided Menopur Conflict of interests; one of the authors has a consultancy role with Fertility Neutraceuticals involved in the manufacturing and distribution of CoQ10 Email sent to author regarding live birth, clinical pregnancy, dropouts and allocation concealment on 24th November 2015 'bentov@lunenfeld.ca', no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned in chronological order according to the day of study enrolment to a computer‐generated randomization" |
| Allocation concealment (selection bias) | Unclear risk | "Each enrolled participant received a pre‐assigned package containing either placebo or CoQ10 for the duration of the study". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The study was a double blind, placebo‐controlled, randomized trial". "Both the physician and the patient were blinded regarding assignment of the patients". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "At the point the study was terminated (June 2012), we had recruited a total of 39 patients who were evaluated and randomized (17 to the CoQ10 group and 22 to the placebo group). Only 27 had started the treatment with the supplements (12 of the CoQ10 group and 15 of the placebo group). In all, 24 patients completed the treatment cycle and had a polar body biopsy (PBBX) and embryo transfer done (10 of the CoQ10 group and 14 of the placebo group). Six patients withdrew from the study for personal reasons, three for conceiving spontaneously, two for poor compliance, one for failing to achieve the target BMI, and three because of poor ovarian response." |
| Selective reporting (reporting bias) | Low risk | Both primary and secondary outcomes reported in the Methods were reported in the results. Protocol available. |
| Other bias | High risk | "However, because of the premature termination of the study, the CoQ10 group had only one‐third and the control group half of the target number". Early termination of trial for embryo safety reasons may cause an overestimation of the effect of the intervention |
| Methods | Open‐label RCT Patients divided, according to a controlled randomized pattern, into two groups |
|
| Participants | Women undergoing ICSI (n = 149) Inclusion criteria: Age between 37 ‐ 40 years; "The recruitment criteria include being under 40 years old, at least one previous failed attempt with ICSI with low‐quality oocyte recovery, diagnosis of PCOS (i.e., with oligomenorrhea, hyperandrogenism and pelvic ultrasonographic appearance characterized by multiple anechoic areas) 8, diagnosis of “poor responders” (i.e., with poor ovarian response to hormonal stimulation, an age greater than 37 years and the need for high doses of FSH stimulation in previous cycles). Only ICSI treatments arrived to the transfer of embryos in the uterus (Embryo‐Transfer) and carried out on Day +2/3 are included in the study". Exclusion criteria: "Patients with a partner with a diagnosis of severe male infertility such as cryptozoospermia (i.e., retrieval of sperm in the semen after centrifugation) and azoospermia (i.e., eventual retrieval of sperm from the testicle or epididymis) were excluded from the study." |
|
| Interventions | 1. Myo‐inositol 2000 mg, D‐chiro‐inositol 400 mg, and folic acid 400 mg: 1 of each tablet a day (n = 58) 2. Folic acid 400 mg: 1 tablet a day (n = 91) Treatment duration 3 months before the ICSI cycle |
|
| Outcomes | Oocyte quality Embryo quality Biochemical pregnancy Clinical pregnancy "Each patient was included only once; therefore, the results for each patient refer to a single treatment cycle" |
|
| Notes | Conducted in Perugia Italy Trial duration between June 2012 and May 2013 Email sent to author regarding randomised pattern, allocation concealment and whether there were any dropouts; gianfrancesco.brusco@ospedale.perugia.it; no reply. No mention of ethics approval, consent or funding Authors state "no conflicts of interest" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "According to a randomized pattern, the total number of patients was divided into two groups". "The two groups were homogeneous within the parameters of inclusion adopted for the study". Numbers are very unequal between the groups, with 58 in the intervention group and 91 in control |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | "An open study" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts mentioned and groups are unequal |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in the Results |
| Other bias | Low risk | No other bias found |
| Methods | Double‐blind RCT | |
| Participants | Women undergoing ICSI (n not stated) | |
| Interventions | 1. Myo‐inostol 4 g and folic acid 400 μg: 1 tablet of each a day (n not stated) 2. Folic acid 400 μg: 1 tablet a day (n not stated) Taken for 3 months before ICSI and throughout pregnancy |
|
| Outcomes | Total rFSH units Number of stimulation days Fertilisation and cleavage rate Embryo quality Biochemical pregnancy rate Clinical pregnancy rate |
|
| Notes | Conducted in Italy, study dates not reported Conference abstract; percentages given but no total participant numbers available Funding by an institutional grant. An author was an employee of a pharmaceutical company Email sent to author 24th November 2015 Gianfranco.carlomagno@gmail.com; no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to two groups; MI treated or placebo" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Unknown |
| Other bias | Unclear risk | Unknown |
| Methods | Prospective randomised placebo‐controlled pilot trial | |
| Participants | Infertile Iranian women with PCOS, aged from 25 ‐ 35 years, undergoing ICSI treatment (N = 80) Inclusion criteria: Women who met the Rotterdam criteria for PCOS Exclusion criteria: Hypersensitivity to either MET (metformin) or NAC, infertility factors other than anovulation, male infertility, pelvic organic pathologies, congenital adrenal hyperplasia, thyroid dysfunction, Cushing’s syndrome, hyperprolactinaemia, androgen‐secreting neoplasia, diabetes mellitus, consumption of medications affecting carbohydrate metabolism and hormonal analogues other than progesterone 2 months prior to enrolment in the study and severe hepatic or kidney disease |
|
| Interventions | 4 groups (n = 20 in each, 5 dropouts from each) 1. Placebo oral rehydration salts; 3 times a day 2. Metformin 500 mg: 1 tablet 3 times a day 3. NAC 600 mg: 1 tablet 3 times a day 4. Metformin 500 mg: 1 tablet 3 times a day + NAC 600 mg: 1 tablet 3 times a day All treatments were administered for 6 weeks |
|
| Outcomes | Oocyte and embryo quality Endocrine parameters Clinical pregnancy Side effects |
|
| Notes | Iran, study ran from July 2012 to February 2013 Need to clarify primary and secondary outcomes Author emailed regarding RoB, live birth and miscarriage information; no reply Funded by institutional grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | State "random" but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Double‐blinded placebo‐controlled, but the placebo group received oral rehydration salts, which are usually in solution, while the treatments were tablets |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts accounted for, but > 25% dropout |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Prospective, randomised controlled trial | |
| Participants | Infertile women with PCOS (n = 100) undergoing IVF/ICSI | |
| Interventions | 1. Calcium 400 mg + vitamin D 1000 IU: 1 of each tablet a day (n = 50) 2. Placebo (n = 50) Given on the starting day of OC pretreatment, followed by controlled ovarian stimulation (COS) using GnRH antagonist for IVF/ICSI. Calcium 400 mg/day with vitamin D 1000 IU/d or placebo was administered once daily from the starting day of OC to the day of human chorionic gonadotropin (hCG) injection |
|
| Outcomes | Total dose and days of rhFSH administered Numbers of retrieved, mature and fertilised oocytes, and grade 1 or 2 embryos FF TNF‐a and IL‐6 concentrations at oocyte retrieval Embryo implantation rate Clinical pregnancy rate Miscarriage rate |
|
| Notes | Korea, study dates not reported Conference abstract Funding source not reported No data for clinical pregnancy or live birth stated in the conference abstract; emailed co‐author CH Kim; chnkim@amc.seoul.kr, asking about risk of bias, any full publication of the trial and whether they had any clinical pregnancy and miscarriage data. No reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "infertile patients with PCOS were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unknown |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unknown |
| Selective reporting (reporting bias) | Unclear risk | Unknown |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women with a diagnosis of unexplained infertility undergoing ovulation induction and IUI (n = 107) Inclusion criteria: no ovulatory problems, normal hysterosalpingography and laparoscopy. Normal semen sample Exclusion criteria: endometriosis, hypertension, diabetes, uterine myoma, ovarian cyst, excessive alcohol, caffeine, chronic illness and smoking |
|
| Interventions | 1. Vitamin E: 400 IU: one tablet per day from 3rd to 5th day of the menstrual cycle until the hCG injection. (n = 53) 2. No treatment (n = 50) 4 women were lost to follow‐up as a result of incorrect dose consumption (n = 3) and cycle cancellation (n = 1). ITT not used in the trial |
|
| Outcomes | Primary outcome: ongoing pregnancy rate Secondary outcomes: biochemical and clinical pregnancy rate, number of follicles, endometrium thickness, implantation rate |
|
| Notes | Study was conducted between June 2011 and December 2011 in Turkey Sample size calculated Ethics approved and written consent obtained Funding not reported, but authors say they have no conflict of interest Emailed author 9th August 2012 regarding the number of women lost from treatment and/or control group. Data added. Will perform sensitivity analysis for quality if we do not hear back from the author regarding ITT. No reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned according to a randomisation table |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded as the control was no treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons and numbers for attrition were given but unclear whether from treatment or control groups. ITT not used |
| Selective reporting (reporting bias) | Low risk | Nil known |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women with PCOS attending a fertility clinic-Gynaecological Endocrinology Clinics and Human Reproduction Pathophysiology Centre (n = 34) Inclusion criteria: women with PCOS younger than 40 years Exclusion criteria: concomitant endocrine and metabolic pathologies, such as hypothyroidism, hyperthyroidism, diabetes mellitus, androgen‐secreting cancers, adrenal hyperplasia, Cushing's syndrome Women received IVF or ICSI after evaluation of sperm analysis |
|
| Interventions | 1. Myo‐inisitol 2 g + folic acid 200 µg: 1 tablet of each twice a day (n = 16) 2. Folic acid 200 µg: 1 tablet twice a day (n = 18) Treatment was given over 3 months |
|
| Outcomes | Number of follicles Number of oocytes retrieved Number of embryos transferred Embryo quality Study states that there was "no difference in the total number of biochemical pregnancies detected", but no data were provided. Author replied, giving the data for chemical pregnancies and stating that no adverse events were reported |
|
| Notes | Trial held in Catania, Italy Contacted authors 21st November 2011 via letter and email regarding pregnancy data, allocation concealment and who was blinded. Author responded 28th November 2011 Emailed the author 5th February 2012 requesting data on clinical pregnancies and whether the sealed envelopes were numbered. No reply Funding, ethics approval and power calculation not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "According to a randomisation table, patients were divided into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Author states that allocation was in "white sealed envelopes". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "the investigation was performed in a double‐blind design". Author states, "clinicians and patients were blinded". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No women were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women with PCOS attending an IVF clinic (n = 100) Inclusion criteria: BMI <28 and FSH <10 IU/L with a diagnosis of PCOS according to Rotterdam 2003 and a normal uterine cavity Exclusion criteria: advanced stage (III or IV) endometriosis and those classified as poor responders or as suffering from premature ovarian failure |
|
| Interventions | 1. Myo‐inositol 550 mg and DCI 13.8 mg: 1 tablet of each twice a day (INOFOLIC combi, soft gel, Lo.Li. Pharma Roma, Italy; patented) (n = 47) 2. D‐chiro‐inositol 500 mg; 1 tablet twice a day (Interquim, s.a., Barcelona, Spain) (n = 53) Treatment was given for 12 weeks before rFSH administration and throughout pregnancy |
|
| Outcomes | Primary outcomes: number of morphologically mature oocytes, total IU of rFSH administered and the number of grade 1 embryos Secondary outcomes: E2 levels before hCG injection, the number of degenerated oocytes, maturation rate, fertilisation rate and the number of embryos transferred | |
| Notes | The trial was registered on clinicaltrials.org (NCT1338844) Conducted in Italy No reproductive outcomes given in the data but pregnancy referred to in the text; emailed author regarding pregnancy outcomes 14th September 2016. Author replied on the 27th September 2016, saying that there are no pregnancy data available but that the trial was double‐blinded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to a block of ten by a computer‐generated program" |
| Allocation concealment (selection bias) | Low risk | "The key to the coding of the treatments was kept by the Lo.Li. Pharma. Both the participants and the research team were blinded. The randomization code was not broken until the completion of the study" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One of the patients who was enrolled and assigned to the MI‐DCI treated group decided to quit the IVF procedure due to personal reasons" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile women with mild to moderate endometriosis (n = 104) post‐laparoscopic surgery Inclusion criteria: at least 12 months with asymptomatic primary infertility, regular menstruation, aged between 23 and 37 years with normal BMI. Women with other infertility factors were included if those factors were correctable and were non‐contributory Exclusion criteria: women with other pelvic disorders such as adhesions and tubal obstructions in addition to endometriosis |
|
| Interventions | 1. Pentoxifylline 400 mg: 1 tablet twice a day (n = 51) 2. Placebo (n = 53) Other procedures given post‐laparoscopy included biopsies, tubal dye perfusion and destruction of endometriotic implants by cautery Treatment was started with the first menses after laparoscopic surgery; then participants were observed for 6 months. During this time, participants with other infertility factors were treated (e.g. male problems or ovulatory defects). Treatments included IUI or ovulation induction, or both. Not all participants were treated or received the same treatment, thus the potential for bias. |
|
| Outcomes | Pregnancy Miscarriage |
|
| Notes | 6 women dropped out: 4 from the treatment group and 2 from the control group. Reasons explained. No ITT. Trial held in Barcelona, Spain Work supported in part by the Comissionat per Universitat i Recerca‐Generalitat de Catalunya Authors were also involved in Balasch 1997 Author emailed 23rd November 2011 regarding live birth data. No reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation list generated using the method of simple randomisation". |
| Allocation concealment (selection bias) | Low risk | "Concealment of treatment allocation was achieved with the use of sealed opaque envelopes, each containing a unique study number and prepared independently by a secretary". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Trial was blinded, not stated whether single, double or triple; "randomised controlled blind trial". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small numbers of dropouts and reasons explained, no ITT |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Unclear risk | Some women with other fertility issues apart from endometriosis were treated for these additional conditions (i.e. male factor (receiving bromocriptine), oligo‐ovulation (receiving ovulation induction and some additional IUI), poor post‐coital test, hyperprolactinaemia). Numbers of women in treatment and control in each of these categories are given. However, treatments may bias the results, as nearly double the control women in the additional treatment group received IUI compared with the treatment group |
| Methods | Randomised controlled trial | |
| Participants | Overweight and obese women with PCOS, aged 20 ‐ 40 years, (n = 84) diagnosed depending on the Rotterdam Criteria | |
| Interventions | 1. Oral omega 3, 3 g: 1 tablet a day 2. Placebo Duration of treatment 8 weeks |
|
| Outcomes | Biochemical markers (FSH, LH, Prolactin) | |
| Notes | Conference proceeding Conducted in Iran, study dates not reported Funding source not reported Author emailed regarding RoB and pregnancy data. No reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded (placebo control) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Unclear risk | Unknown |
| Methods | Randomised controlled trial | |
| Participants | Infertile women at Bangladesh fertility unit (n = 156) undergoing ovulation induction with clomiphene citrate | |
| Interventions | 1. Multinutrient supplementation; unknown included antioxidants and dosage 2. Folic acid; unknown dosage |
|
| Outcomes | Chemical pregnancy Clinical pregnancy Ovulation rate |
|
| Notes | Conference abstract only, limited details Set in Bangladesh, study dates not reported Funding source not reported Author emailed for RoB information and data. No reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Clinical pregnancy reported in Methods but not in the Results; this is a conference abstract, so they may not have the data yet |
| Other bias | Unclear risk | Unknown |
| Methods | Prospective randomised controlled trial | |
| Participants | Women with clomiphene‐citrate‐resistant PCOS attending a fertility outpatient clinic (n = 110) Inclusion criteria: diagnosis of PCOS. All women were previously treated with 150 mg clomiphene citrate daily for 5 days per cycle, for 2 or 3 cycles with persistent anovulation or ovulate with very thin endometrium (< 5 mm). All women had patent fallopian tubes Exclusion criteria: women with hyperprolactinaemia, hypercorticism and thyroid dysfunction and women receiving medications such as cholesterol‐lowering drugs, beta‐blockers and tricyclic antidepressants |
|
| Interventions | 1. CoQ10 60 mg: 3 capsules a day + clomiphene citrate 150 mg: 1 tablet a day, from cycle days 2 – 6 starting on cycle day 2 and until the day of hCG administration (n = 55) 2. Clomiphene citrate 150 mg: 1 tablet a day from cycle day 2 for 5 days (n = 55) The mean duration of CoQ10 treatment in the 1st cycle was 16.2 ± 2.1 days and in the 2nd cycle 17.1 ± 2.9 days |
|
| Outcomes | Primary outcomes: number of growing and mature follicles, serum oestradiol, serum progesterone, ovulation rate, endometrial thickness Secondary outcomes: clinical pregnancy (ultrasound visualisation of gestational sac with pulsating fetal pole) and miscarriage (spontaneous termination of a clinical pregnancy before 20 weeks of gestation) |
|
| Notes | Timed intercourse Sample size calculation done 4 dropouts from the intervention and 5 from the control group Egypt Trial duration January 2010 to January 2013 The study was approved by the departmental ethical committee and all participants gave informed consent before inclusion in the trial (committee reference no. 231, approved December, 12 2009) This trial is registered at ClinicalTrials.gov (ID NCT01910766) Email to author regarding live birth data and allocation concealment sent 26th November 2015. No reply. Endometrial thickness; significant difference in favour of the treatment group vs control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated using a computer generated random table" |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes" "Allocation process was done by a third party (nurse)" |
| Blinding (performance bias and detection bias) All outcomes | High risk | "The physicians monitoring the cycles were blinded to the protocol of each group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts accounted for from each arm |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as stated in the Methods |
| Other bias | Low risk | No other bias found |
| Methods | Randomised single‐centre controlled clinical trial | |
| Participants | Women undergoing IVF with sleep disturbances (n = 63) from 24 ‐ 38 years Inclusion criteria: unexplained infertility, no ovulatory problems, normal hysterosalpingogram or laparoscopy and normal semen sample Exclusion criteria: chronic drug usage, history of > 1 fertilisation failure, hypertension, diabetes, uterine myoma, ovarian cyst and smoking |
|
| Interventions | 1. Melatonin 3 mg; 1 tablet a day, taken at 22:00 to 23:00 from 3rd to 5th day of the menstrual cycle until the hCG injection (n = 30) 2. No treatment (n = 30) |
|
| Outcomes | Primary outcome: oocyte quality Secondary outcomes: fertilisation failure rate, number of follicles, number of oocytes retrieved, number of Mll oocytes, fertilisation rate, number of embryos transferred, embryo quality, implantation rate and clinical pregnancy rate |
|
| Notes | Trial held in Turkey Ethics approved, written informed consent gained. Authors declare no conflicts of interest Power calculation performed Emailed author 9th August 2012 regarding which group or groups lost the 3 women. Data added. Tried to contact the author again regarding live birth data 5th February 2013 Author replied on 7th February 2013 , saying that the 3 dropouts were from the treatment group, and that no allocation concealment was performed and no live birth data were available because participants were mainly from rural sites |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned according to a randomisation table |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding as control is no treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts explained |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Double‐blind randomised trial | |
| Participants | Women with oligomenorrhoea or amenorrhoea and PCOS were recruited from gynaecology, endocrine and fertility clinics. Women were < 35 years of age, mean age 29.7 (n = 92) "Infertility was an ailment in only half of the patients in each group. There was no difference in the proportions of infertile women with the groups". Exclsion criteria: hyperprolactinaemia, hormone treatment, abnormal thyroid function, congenital adrenal hyperplasia |
|
| Interventions | 1. InfolicⓇ, a combination of myo‐inositol 2g plus folic acid 400 μg: 1 tablet twice a day (n = 45). Mean age 29.0 2. Folic acid 400 μg: 1 tablet twice a day (n = 47). Mean age 29.7 Duration: 16 weeks |
|
| Outcomes | Ovulation frequency Hormonal levels Pregnancy |
|
| Notes | "Ethical committee approval was obtained before the study, and written informed consent was given by each patient". Trial carried out in Italy, study dates not reported Power calculation carried out High dropouts, > 30% in the treatment group. Included, but data not used, as half the participants did not want to conceive. Study is included on the basis that half the participants were from a subfertility clinic Funding source not reported Authors contacted (May 2010) to request any pregnancy outcomes considered and to ask whether the authors of the paper have the individual data on which women in each group were infertile. No reply as of 12th June 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was effected in a double blind fashion; patients received either MYO combined with folic acid (Inofolic®) or only folic acid as placebo, according to the code provided by computer‐generated randomization." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind fashion" ("placebo control" however control is folic acid therefore considered to be no treatment) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "The high dropout rate in the myo‐inositol arm (more than 30%) is notable". |
| Selective reporting (reporting bias) | High risk | Only half the participants declared before the study that they wanted to conceive. No ITT for the pregnancy outcome. 1 miscarriage was reported but no details of whether this occurred in the treatment or the control group. Miscarriage not prespecified as an outcome of interest |
| Other bias | Low risk | No other bias found |
| Methods | Prospective, randomised, placebo‐controlled, group comparative, double‐blind study | |
| Participants | Subfertile women having 1st IVF cycle aged < 40 years with mean age of 31.73 ± 4.4 years (n = 620) 10% described as male factor infertility, and associated data were not presented separately Inclusion criteria: tubal, idiopathic and male infertility were included Exclusion criteria: women with repeated IVF cycles and women with renal or gastrointestinal disease |
|
| Interventions | 1. Ascorbic acid 1 g: 1 tablet a day (n = 172) 2. Ascorbic acid 5 g: 1 tablet a day (n = 153) 3. Ascorbic acid 10 g: 1 tablet a day (n = 136) 4. Placebo (n = 158) Duration 1 cycle |
|
| Outcomes | Clinical pregnancy rate confirmed by fetal heartbeat at 8 weeks Implantation rate per embryo transfer |
|
| Notes | 1 woman lost to follow‐up-no explanation. Tried to contact author. No reply 10% of women had partners with male infertility Trial conducted in 2 clinics in Budapest, Hungary (n = 237) and Vienna, Austria (n = 383) No power calculation performed Pregnancies were confirmed at 8 weeks with no further follow‐up; authors contacted regarding this. No reply as of 12th June 2013 No clarity regarding the number of treatment cycles involved in this study Ethics approval not gained as "a study on vitamin supplementation is not subject to IRB approval". Consent forms were signed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "This prospective randomised double‐blind study". Method not described |
| Allocation concealment (selection bias) | Low risk | By an independent pharmacy in Vienna "prepared and coded by number". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women and clinicians were blinded: "double‐blind study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 set of participant data noted as missing but not explained; authors contacted regarding this |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Unclear risk | Unequal baseline group numbers |
| Methods | Randomised double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | Infertile women with PCOS. Mean age: Treatment group: 24.6 ± 3.2; Control group: 24.8 ± 2.7.Timed intercourse (n = 170) Inclusion criteria; < 35 years of age, presenting with primary or secondary infertility following regular intercourse for at least 1 year and diagnosed with PCOS with no other abnormalities Exclusion criteria; FSH values ≥ 10IU/ml |
|
| Interventions | 1. Clomiphene citrate 250 mg: 1 tablet a day from day 3 to day 7 of the cycle plus oral‐carnitine 3 g: 1 tablet a day from day 3 until the day of the first positive pregnancy test (n = 85) 2. Clomiphene citrate 250 mg: 1 tablet a day plus placebo (n = 85) All participants received clomiphene citrate from day 3 until day 7 of the cycle.Timed intercourse |
|
| Outcomes | Clinical pregnancy rate Miscarriage Multiple pregnancies Ovulation rate Days until hCG injection Endometrial thickness Number of follicles Number of pregnancies Laboratory parameters |
|
| Notes | All participants were counselled about their participation in the study. A signed informed consent was obtained. Participants had the right to refuse to participate or to withdraw from the study at any time without being denied their regular full clinical care. Personal information and medical data collected were subject to confidentiality and were not made available to a third party. Women’s Health Hospital, Assiut University, Assiut, Egypt The study was conducted between January 2010 and March 2012 Sample size calculation done "The authors have no conflict to disclose" Funding source not reported Email sent to author on 26th November 2015 regarding live birth data; author replied on 7th December 2015 saying there are no live birth data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated numbers" "randomized according to computer‐generated randomization tables to ensure an equal number of patients in each arm (1:1 ratio)". |
| Allocation concealment (selection bias) | Low risk | "using previously prepared sealed envelopes with computer‐generated numbers" "Throughout the trial, access to the randomization code was available only to the pharmacist who manufactured the placebo and packed the envelopes and was not available to any of the treating physicians or patients". "The capsules were placed in sacks and then stored in envelopes numbered from 1 to 170. The envelopes were numbered" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double blind" "The placebo capsules were specially manufactured to look identical to the L‐carnitine capsules". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18/170 dropouts with numbers per group and reasons given |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the Methods were reported |
| Other bias | Low risk | No other bias found |
| Methods | Double‐blinded randomised control trial | |
| Participants | Women aged 18 ‐ 41 with PCOS which was clomiphene‐resistant who attended fertility clinic in Iran (n = 93) | |
| Interventions | 1. Oral NAC 1.2 g: 1 tablet a day (n = 53) 2. Vitamin C (?dose) (n = 40) |
|
| Outcomes | Oestradiol levels Number of follicles > 18 mm Endometrial thickness |
|
| Notes | Conducted in Iran, study began in 2010 (end unknown) Unknown trial duration Funding source not reported Tried to contact author regarding pregnancy data, uneven number in each group. No reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded (another antioxidant, not placebo) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Uneven number in each group |
| Selective reporting (reporting bias) | Unclear risk | Unknown |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile women aged 25 ‐ 39 years with PCOS undergoing IVF (n = 58) | |
| Interventions | 1. NAC 400 mg: 1 tablet twice a day (n = unknown) 2. Placebo (n = unknown) Duration 13 ‐ 15 weeks. |
|
| Outcomes | Insulin sensitivity Endocrine levels Ovarian stimulation Number and size of follicles Number of retrieved oocytes Number and quality of embryos transferred Pregnancy rate Miscarriage Ovarian hyperstimulation syndrome rates |
|
| Notes | Conference abstract only Trial held in Korea, study dates not reported Funding source not reported The authors contacted to request pregnancy outcome data and study protocol to appraise risk of bias elements. No reply as of 14th September 2011 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients randomly assigned..." No description of method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unknown |
| Selective reporting (reporting bias) | Unclear risk | Unknown |
| Other bias | Unclear risk | Unknown |
| Methods | Prospective randomised controlled study | |
| Participants | Infertile women with a history of unexplained total fertilisation failure undergoing ICSI (n = 98). Ages not given Inclusion criteria: unknown Exclusion criteria: unknown |
|
| Interventions | 1. Omega‐3‐polyunsaturated fatty acids (o‐3 PUFAs) 1000 mg: 1 tablet a day (n = unknown) 2. Unknown control (n = unknown) |
|
| Outcomes | Total recombinant human (rh)FSH dose and days required Numbers of oocytes retrieved Number of oocytes fertilised Embryo quality Embryo implantation Clinical pregnancy rate |
|
| Notes | Conference abstract only Trial held in Korea, study dates not reported Funding source not reported Authors emailed 22nd November 2011regarding risk of bias, pregnancy data per woman, numbers in intervention and control groups and inclusion/exclusion criteria. No reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospective randomised controlled study"-no explanation given. |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unknownn. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unknown |
| Selective reporting (reporting bias) | Unclear risk | Unknown |
| Other bias | Unclear risk | Unknown |
| Methods | vRandomised study | |
| Participants | Women with PCOS indicated by oligomenorrhoea and/or hyperandrogenism and/or hyperandrogaenemia and/or typical features of ovaries on ultrasound scan were enrolled in this study. At least 2 of the above‐mentioned criteria were present in all the participants Women were undergoing IVF and aged < 40 years (n = 29) Exclusion criteria: any other medical conditions causing ovulatory disorders such as hyperprolactinaemia or thyroidal disorders or Cushing syndrome |
|
| Interventions | 1. Myo‐inositol 4000 mg plus folic acid 400ug: 1 tablet per day (n = 14) 2. Placebo (n = 15) Treatment was for 2 months prior to the IVF cycle and the trial ran for 4 months |
|
| Outcomes | Number of retrieved oocytes Ratio of follicles to retrieved oocytes Fertilisation rate Oocyte quality Amount of FSH units used Days of stimulation |
|
| Notes | Conducted in Germany, study dates not reported Has an author who was an employee of a pharmaceutical company Funding source not reported Contact details; Pedro‐Antonio Regidor (pedro‐antonio.regidor@exeltis.com) email sent on 13th October 2016 asking whether the placebo group received folic acid, methods of randomisation, allocation concealment, clinical pregnancy, live birth data and the length of the trial. Author replied 17th October 2016, no outcomes yet "but this is ongoing" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The method of randomization was a manual one. After fulfilling the including criteria the patients were allocated to the previously defined randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single‐blinded. "The biologist which carried out the fertilization was the blinded person. He did not know if the women were treated with myo‐Inositol or not" (placebo used) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed |
| Selective reporting (reporting bias) | Low risk | No apparent reporting bias |
| Other bias | Low risk | No other bias found |
| Methods | Randomised open‐label, multicentre pilot study | |
| Participants | Infertile women undergoing IVF/ICSI, mean age 34.4 ± 3.4 (n = 100) Exclusion criteria: women with PCOS, with any endocrine or metabolic disease, taking any hormonal treatment, with BMI > 30 kg/m2 |
|
| Interventions | 1. Myo‐inositiol 4000 mg: "into two administrations per day" + folic acid 400 µg: 1 tablet a day (n = 50) 2. Folic acid 400 µg: 1 tablet a day (n = 50) Duration of treatment 3 months, duration of trial 12 months |
|
| Outcomes | Length of stimulation Total quantity of gonadotropins required Number of oocytes retrieved Implantation rate Clinical pregnancy |
|
| Notes | Center for Reproductive Medicine Research, Clinica Villa Mafalda, Rome, Italy, study held from January 2011 to January 2012 Funding source not reported Emailed author 13th February 2013 regarding randomisation, allocation concealment and live birth data. Professor Lisi replied, clarifying these questions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomisation in a computer‐generated sequence" is written in the paper and in further correspondence from the author ..."About randomization, a computer software generated 100 numbers from 1 to 10,000, and the numbers were stored in sealed envelopes and opened on the day of preparation and explanation of the stimulation protocol to patients. Patients with odd number were assigned to folic acid, myo‐inositol and rhFSH; patients with even number were assigned to folic acid and rFSH". Unsure whether this may be quasi‐randomised. We sought further information from the author. Author replied, "The envelope outside had 100 numbers in order and opened in that order; numbers outside were different from numbers inside". |
| Allocation concealment (selection bias) | Low risk | Envelopes were numbered sequentially and were opaque |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label, although outcome assessors were blinded participants were not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised study | |
| Participants | Women with PCOS (based on Rotterdam criteria, ESHRE/ASRM 2004), the diagnosis of PCOS is determined by the presence of 2 of the following conditions: oligo‐ovulation or anovulation, hyperandrogenism and polycystic ovaries detected by ultrasonography with the presence of 12 or more follicles measuring 2 – 9mm in diameter, and/or at least 1 enlarged ovary (410 cm). None of the participants had history of clomiphene citrate resistance (n = 120) Timed intercourse Mean age was 26 years for all 3 groups Exclusion criteria: women with endocrinological abnormalities such as thyroid dysfunction or abnormal prolactin levels, those with hypothalamic or pituitary dysfunctions evaluated by low gonadotropin level, other causes of infertility such as tubal factor evaluated by HSG or laparoscopy, abnormal uterine cavity evaluated by sonohystrography or hysteroscopy and male factor, evaluated by semen analysis. Women with ovarian cysts and those with allergy to the study medications were also excluded from the study. Women who had received any hormonal medications (except progesterone for withdrawal bleeding) within the last 3e months before the study were also excluded |
|
| Interventions | 1. Clomiphene citrate 100 mg orally in 2 divided doses a day. No treatment (n = 40) 2. NAC 1200 mg in 2 divided doses a day (in the form of powder inserted in small pockets to be diluted into a standard glass of water from day 3 until day 7 of the menstrual cycle) (n = 40) 3. Metformin 500 mg: 1 tablet 3 times a day (n = 40) Treatment period; from day 3 to day 7 of the menstrual cycle, treatment was repeated in non‐pregnant cases for 3 successive cycles |
|
| Outcomes | Clinical pregnancy (defined as the presence of gestational sac containing fetal hearts on ultrasound scan) Occurrence and day of ovulation Endometrial thickness and pattern Number and size of follicles |
|
| Notes | Conducted in Egypt Trial period; September 2012 to March 2014. Funding source not reported Ahmed Mohamed Maged, Obstetrics and Gynecology Department, Kasr Aini Hospital Cairo University, 135 King Faisal Street Haram Giza, Cairo, Egypt. Tel: 0105227404. Fax:35873103. E‐mail prof.ahmedmaged@gmail.com. Email sent 13th October 2016 regarding live birth and any dropouts. No reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised at the beginning of each cycle by sealed opaque envelopes containing randomly generated numbers into 3 groups |
| Allocation concealment (selection bias) | Low risk | Patients were randomized at the beginning of each cycle by sealed opaque envelopes containing randomly generated numbers into 3 groups |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers analysed in each group are not given |
| Selective reporting (reporting bias) | Low risk | No known selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile women with peritoneal endometriosis stage 1 or 2 diagnosed by laparoscopy (n = 36). All participants had fulguration of endometrial implants. Mean age: Treatment group 32.7 ± 2.36; Placebo group 32.7 ± 2.36 Inclusion criteria: women between 25 and 35 years old who had been diagnosed as having peritoneal endometriosis on exploratory laparoscopy, with fertile male partner Exclusion criteria: women who reported having used nutritional supplements during the previous year; who had pelvic inflammatory disease or autoimmune, endocrine or metabolic disorders; or who did not agree to participate or missed a medical visit |
|
| Interventions | 1. Vitamins C 343 mg + Vitamin E 84 mg: in a bar form, 1 bar a day (n = 18) 2. Placebo (n = 18) Duration of trial was 6 months Follow‐up for up to 9 months after the trial |
|
| Outcomes | Live birth (no data available) Pregnancy (no explanation of whether pregnancies were biochemical, clinical or ongoing). "None of the patients became pregnant during the trial. Once the trial ended, patients were followed up for 9 months for a possible pregnancy". The pregnancy rate was 19% (3 of 16) in the supplementation group and 12% (2 of 18) in the placebo MDA, oxidative stress markers obtained during the exploratory laparoscopy |
|
| Notes | Consent signed Ethics was approved The study was conducted at the National Institute of Perinatology “Isidro Espinosa de los Reyes” in Mexico City, study dates not reported Funding given as a grant from Consejo Nacional de Ciencia Tecnologia Mexico. Power calculation done. Tried to contact author. Contacted author again 12th February 2013 to ask about clinical pregnancy and live birth. No reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reference was made to the use of 'randomisation codes', and investigators stated, "Thirty‐six participants were randomly assigned". Authors contacted regarding this |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the paper. Authors contacted regarding this. The response was, "women were randomly allocated depending on the colour of a ball they took out from a container" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women were blinded. The bars were "identical‐looking and tasting bars". Authors contacted regarding this and confirmed that investigators, outcome assessors and clinicians were blinded also. "Randomization codes were unlocked at the end of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 women in the treatment arm dropped out "for personal reasons". ITT not applied |
| Selective reporting (reporting bias) | High risk | Investigators stated they would collect live birth rates but reported only pregnancy rates |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile women with PCOS (n = 44). Natural or timed intercourse Mean age: Treatment group: 26.5 yr (20 ‐ 43); Control group: 29 yrs (23 ‐ 26) Inclusion criteria: primary or secondary infertility due to PCOS according to Rotterdam criteria including oligomenorrhoea, amenorrhoea, clinical or laboratory evidence of increase androgen level or polycystic ovaries in sonography Exclusion criteria: any definite gland disorders such as kaohsiung hypothyroid, hypothyroidism, diabetics and increase in blood prolactin levels |
|
| Interventions | 1. Clomiphene 50 mg: 1 tablet a day + 400 units of Vitamin D + 1000 mg calcium: 1 tablet a day (n = 22) 2. Clomiphene 50 mg + placebo: 1 tablet a day (n = 22) Duration: 3 menstruation cycles (3 months) |
|
| Outcomes | Follicle size Pregnancy (unknown whether this is clinical or biochemical ‐ sonography had been done for all participants up to 3 months but this could be to assess follicle size) |
|
| Notes | Conducted in Iran Trial was run between 2010 and 2011. Funding source not reported Email was sent to author on the 30th November 2015 regarding data and risk of bias robab20@yahoo.com ‐ no reply. Dr Vahid Seyfoddin helped translate key points from the paper. New email found nezhat79@gmail.com, email sent 27th September 2016 regarding block size, allocation concealment and clinical pregnancy data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were divided into two groups (22 Intervention and 22 controls) using block randomization method". Unknown process of selection of blocks |
| Allocation concealment (selection bias) | Unclear risk | Unknown block number |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Specialists did the randomisation only and the residents managed the study, the radiologists was blinded while using the same instrument and only one practitioner" (placebo control) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All participants completed the study" |
| Selective reporting (reporting bias) | Unclear risk | Unknown whether the reported pregnancies were biochemical or clinical. Protocol available |
| Other bias | Low risk | No other bias found |
| Methods | Randomised, double‐blind, placebo‐controlled pilot study | |
| Participants | Women undergoing unilateral laparoscopic ovarian drilling (LOD) for clomiphene‐resistant PCOS (n = 60) Aged 18 ‐ 38 years; mean age: treatment group 28.4 ± 4.2; placebo group 29.2 ± 3.7, with at least 2 years of infertility due to anovulation, patent fallopian tubes, normal semen analysis Exclusion criteria included no hormonal treatment for 3 months before enrolment and any contraindications to anaesthesia or laparoscopy |
|
| Interventions | 1. NAC 1.2 grams: 1 sachet a day for 5 days, starting at day 3 of the cycle (immediately after LOD) for 12 consecutive cycles (n = 30) 2. Placebo (n = 30) Both groups also had LOD Follow‐up by cycle monitoring and timed intercourse for a year. No women were lost to follow‐up. |
|
| Outcomes | Primary outcome: biochemical pregnancy Secondary outcomes: ovulation, number of follicles, endometrial thickness, clinical pregnancy, miscarriage, multiple pregnancies, ongoing pregnancy, number of preterm deliveries, live birth |
|
| Notes | Trial took place in Egypt between January 2005 and June 2007 Ethics obtained. Informed written consent. Endometrial thickness; significant difference in favour of the treatment group Funding source not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised double‐blind placebo‐controlled pilot study", "computer‐generated random numbers". |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelopes". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. "The placebo sachets were specially manufactured to look identical to the NAC sachets". "Throughout the study, access to the randomisation code was available only to the pharmacist and was not available to the treating gynaecologist or patients". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No women lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised trial | |
| Participants | Women undergoing IVF aged 22 ‐ 43 years. Mean age treatment group: 30.7 ± 4.5; placebo group: 28.8 ± 3.2) (n = 56) Inclusion criteria: non‐smokers, free from major illness including hypertension, all interested in becoming pregnant Exclusion criteria: myoma, adenomyosis, congenital abnormality, ovarian tumours, hormone or long‐term medication use |
|
| Interventions | 1. Multi‐vitamin/mineral (containing vitamins A, B, C, D, E and H, calcium, folic acid, nicotinic acid, iron, magnesium, phosphor copper, manganese and zinc): 1 tablet a day (n = 26) 2. Placebo (candy) (n = 30). for 45 days |
|
| Outcomes | Follicular fluid | |
| Notes | Conducted in Turkey 3 groups were used in the study. The 1st group consisted of age‐matched controls, so we did not use these data in this review. The 2nd and 3rd groups were randomly assigned Author emailed on 1st August 2012 to ask for any data on pregnancy, live birth or adverse events. Author replied on 13th August 2012. No outcomes appropriate to this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by a computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | High risk | Placebo used was candy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None mentioned |
| Selective reporting (reporting bias) | Low risk | None known |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled double‐blind trial | |
| Participants | Women with PCOS undergoing ICSI aged between 27 ‐ 38 years (n = 569) Inclusion criteria: absence of tubal, uterine, genetics and male causes of infertility; serum levels of FSH on day 3 of the ovarian cycle 512 IU/L; Rotterdam criteria for PCOS; normal uterine cavity; BMI of 20 to 26 kg/m2; first IVF treatment. Only women undergoing 1st‐time ICSI procedure fulfilling inclusion criteria were enrolled in the study in order to limit their heterogeneity |
|
| Interventions | 1. Myo‐inositol 4000 mg + folic acid 400 mcg (Inofolic®): 1 tablet twice a day and Melatonin 3 mg: 1 tablet twice a day (n = 178) 2. Myo‐inositol 4000 mg + folic acid 400 mcg (Inofolic®): 1 tablet twice a day (n = 180) 3. Folic acid 400 mcg: 1 tablet twice a day (n = 211) Treatment was from the first day of the cycle until 14 days after embryo transfer |
|
| Outcomes | Primary end points were: oocyte and embryo quality, clinical pregnancy (identified by the presence of a gestational sac on ultrasonography 5 weeks after oocyte retrieval) and implantation rates. Secondary outcomes were: gonadotropin IU administered, days of stimulation, serum estradiol(E2) levels and endometrial thickness on the day of human chorionic gonadotropin (hCG) administration. |
|
| Notes | Conducted in Italy Trial ran from July 2009 to December 2011 43 women dropped out, 16 from the control, 13 from the intervention group A and 14 from group B; reasons provided Clinical Trial registration Number: NCT01540747 (ClinicalTrials.gov registry) Has an author who was an employee of a pharmaceutical company Funding source not reported Emailed Dr Pacchiarotti 18th October 2016 to ask about allocation concealment and live birth data and asked about trial details from included study Valeri 2015. Contact details; arypac@gmail.com. Dr Pacchiarotti replied 20th March 2017 saying that the clinical pregnancy was per woman as they have 80% of the live birth data. We replied asking whether we could include these data. No reply yet. We will need to follow up on this for next review update. Power calculation performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a computer based random assignment schedule for each patient" |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double‐blinded" but not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout reasons were described and numbers given for each group |
| Selective reporting (reporting bias) | Low risk | Outcomes in the Methods were reported as per protocol |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women with PCOS having clomiphene citrate for ovulation induction (timed intercourse) (n = 200) | |
| Interventions | 1. Combined antioxidant supplementation; vitacap which contains vitamin A (Palmitate) 5000 iu, vitamin B1 (thiamine mononitrate) 5 mg, vitamin B6 (pyridoxine HCL) 2 mg, vitamin B12 (cyanocobalamin 5 mg, vitamin C 75 mg, vitamin D3 (cholecalciferol) 400 iu, Vitamin E (d‐alpha tecopheryl acetate ) 15 mg, nicotinamide 45 mg, folic acid 1000 mcg, ferrous fumerate 50 mg, dibasic calcium phosphate 70 mg, copper sulphate 0.1 mg, manganese sulphate 0.01 mg, zinc sulphate 50 mg, potassium iodide 0.025 mg and magnesium oxide 0.5 mg: 1 vitacap a day (n = 100) 2. Placebo; containing folic acid and fersolate. 1 tablet a day (n = 100) Treatment given for 6 months |
|
| Outcomes | Live birth Clinical pregnancy Menstrual regularisation |
|
| Notes | Conducted in Nigeria, study dates not reported Conference abstract Funding source not reported kapanti2002@yahoo.co.uk, email sent 18th October 2016. Author replied 20th October 201 with live birth data. Emailed author re allocation concealment of odd and even envelopes 25th January 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The women were randomized into two groups by picking one of the two closed envelops. within the envelop is written odd or even number. The odd number for intervention and even number for control. The selection of odd and even number for intervention and control group was done by toss of coin" |
| Allocation concealment (selection bias) | Low risk | "The women were randomized into two groups by picking one of the two closed envelopes, within the envelope is written odd or even number". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The trial was single blinded. The patient did not know" a placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts accounted for |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile women with PCOS undergoing ovulation induction for ICSI (n = 60) Mean age (years) treatment: 36.2 ± 2.4; non‐treatment 35.4 ± 2.5 Inclusion criteria: women aged < 40 years with PCOS, indicated by oligomenorrhoea (6 or fewer menstrual cycles during a period of 1 year), hyperandrogenism (hirsutism, acne or alopecia) or hyperandrogenaemia (elevated levels of total or free T) and typical features of ovaries on ultrasound scan. All women had been treated at the IVF clinic for > 12 months. Exclusion criteria: other medical conditions causing ovulatory disorders such as hyperinsulinaemia, hyperprolactinaemia, androgen excess such as adrenal hyperplasia or Cushing's syndrome Duration of infertility (months) treatment: 46.1 ± 18.5, non‐treatment: 37.7 ± 9.6 |
|
| Interventions | 1. Myo‐inositol 2 g + folic acid 400 µg (Inofolic®): 2 tablets a day (n = 30) 2. Folic acid 400 µg (n = 30) Duration: for 1 cycle of ICSI. Treatment starting on the day of GnRH administration |
|
| Outcomes | Number of mature oocytes retrieved Embryo quality Pregnancy Implantation rates Total number of days of FSH stimulation Total dose of gonadotropin administered Oestrogen levels Fertilisation rate Number of retrieved oocytes Embryo cleavage rate Live births Miscarriage rates Cancellation rate Incidence of moderate or severe ovarian hyperstimulation syndrome |
|
| Notes | The Institutional Review Board approved the protocol, and all participants gave written informed consent before entering into the trial Source of funding not stated Power calculation performed Authors contacted. Trial conducted in Italy, start date March 2004 (unknown end date) Authors could not supply live birth data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised according to "computerised allocation". Authors have since confirmed this |
| Allocation concealment (selection bias) | Unclear risk | "Computerised allocation" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Authors confirmed in correspondence that participants and investigators were not blinded; however, outcome assessors and clinicians were blinded. Not a placebo control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | High risk | Data on live birth were not reported in the paper, even though it was listed as an outcome in the abstract of the paper |
| Other bias | Low risk | No other bias found |
| Methods | Prospective, randomized, double‐blind placebo‐controlled trial | |
| Participants | Infertile women undergoing IVF/ICSI, 34 women with ICSI cycles and 18 oocyte donation (n = 52) | |
| Interventions | 1. Vitamin D 100.000 IU: 1 tablet per month (n = 26) 2. Placebo (n = 26) Trial duration; 5 consecutive months |
|
| Outcomes | Endometrial thickness Number of oocytes retrieved Cancellation rate Number of embryos transferred Implantation rate Clinical pregnancy rate Live birth |
|
| Notes | Conducted in Argentina,study dates not reported conference abstract Funding source not reported Author email; ester_polak@cermed.com. Author contacted on the 20th November 2015 regarding risk of bias factors and live birth data. Author replied 14th December 2015 regarding dropouts, miscarriage, adverse effects and live birth. Trial not yet published as a full text |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Fifty two patients were computer randomized" |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double blind" (placebo control) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "no dropouts" email from author |
| Selective reporting (reporting bias) | Low risk | No known selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Randomised clinical trial | |
| Participants | Infertile women with PCOS (n = 60). Mean age Treatment group: 24.95 ± 3.56, Control groups 25.8 ± 4.61 and 26.9 ± 4.44 respectively Inclusion criteria: oligomenorrhoea/amenorrhoea, hyperandrogenism, polycystic ovaries on transvaginal ultrasound. Exclusion criteria: women with systemic disease, coexisting male factor infertility or abnormal hysterosalpingography. Natural conception |
|
| Interventions | 1. Calcium 1000 mg + vitamin D 400 IU (n = 20) This arm is not used in the analysis. 2. Calcium 1000 mg + vitamin D 400 IU + metformin 1500 mg: 1 tablet of each a day (n = 20) 3. Metformin 1500 mg: 1 tablet a day (n = 20) Trial lasted 3 months with a 3‐month follow‐up. |
|
| Outcomes | Follicular response Frequency of menstrual cycle Chemical pregnancy Clinical pregnancy No pregnancy occurred in any of the groups |
|
| Notes | Trial held in Iran, study ran from February 2004 (unknown end date) Funding source not reported Tried to contact authors regarding allocation concealment and blinding 13th February 2013. No reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were divided into 3 groups with the use of a random number table |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts from the trial |
| Selective reporting (reporting bias) | Unclear risk | Reported only chemical pregnancy |
| Other bias | Low risk | No other bias found |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Infertile women with PCOS 18 ‐ 40 years old (n = 64) Mean age: 25.1 ± 4.5 vs 25.4 ± 4.9 years, P = 0.85 Inclusion criteria: age between 18 and 40 years with PCOS according to Rotterdam criteria Exclusion criteria: elevated levels of prolactin, thyroid disorder, or Type 2 diabetes and congenital adrenal hyperplasia. In addition, all PCOS women had normal baseline renal function tests, bilirubin, and aminotransferases |
|
| Interventions | 1. Selenium 200 ug: 1 tablet a day + metformin 500 mg: which was elevated in a stepwise manner during the first 3 weeks to incorporate the side effects until the participants were taking a total of 1500 mg a day (n = 32) 2. Placebo plus metformin: same dosage as above (n = 32) Treatment was for 8 weeks The trial ran from October 2014 to December 2014 |
|
| Outcomes | Pregnancy rates (biochemical) Hormone levels |
|
| Notes | Conducted in Iran Trial was supported by an institutional grant. Clinical trial number: IRCT201412295623N33 Contact details: Dr Z Asemi; asemi_r@yahoo.com. Email sent 18th October 2016 regarding clinical pregnancy data and block size. No reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients with PCOS were randomly divided into 2 groups" "Patient allocation and block size were obtained using random number tables". |
| Allocation concealment (selection bias) | Low risk | "At the time of randomization,sequentially numbered, sealed envelopes were opened. Allocation to study group was concealed until the main analyses were completed". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Supplements and placebos were in the same form of package and the participants and researcher were not conscious of the content of the pack until the end of trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons and numbers for dropouts from each group were provided. ITT used in the analysis |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | No other bias found |
| Methods | Placebo‐controlled, double‐blind, randomised trial | |
| Participants | Women diagnosed with clomiphene citrate‐resistant PCOS (n = 150) aged 18 ‐ 39 years, undergoing therapy for infertility. Timed intercourse. Mean age Treatment group: 28.9 ± 4.7, Placebo group 28.4 ± 5.7. Inclusion criteria: clomiphene citrate‐resistant, at least 1 patent tube, adequate semen analysis according to WHO guidelines, no hormonal treatment Exclusion criteria: hormonal treatment within 2 months of the study, no participants had taken medication to affect carbohydrate metabolism, hyperprolactinaemia, hypercorticism or thyroid dysfunction |
|
| Interventions | 1. NAC 1.2 g: 1 tablet a day + clomiphene citrate 100 mg: 1 tablet a day for 5 days, starting at day 3 of the cycle for 1 cycle (n = 75) 2. Placebo + clomiphene citrate 100 mg: 1 tablet a day (n = 75) |
|
| Outcomes | Ovulation rate Ongoing pregnancy rate, however only pregnancy rate reported Number of follicles of 18 mm Hormone levels Endometrial thickness Ovarian hyperstimulation syndrome (OHSS) Multiple gestations |
|
| Notes | Single‐centre university‐based hospital and private infertility practice in Eygpt Trial conducted from March 2002 to November 2003. Informed consent. No mention of funding source Data for miscarriage and multiple pregnancy not in meta‐analysis, as they appear to skew data because of the fact that there were no pregnancies or live birth events in the control group, so no miscarriages. The intervention appears worse in terms of miscarriage when it is simply due to the intervention group having pregnancy and live birth. Emailed author 7th September 2012 regarding the pregnancy rate in the control group and asking for live birth data. Author replied on 10.09.12, confirming that there were no pregnancies in the control group and no live birth data Endometrial thickness; significant difference in favour of the treatment group vs control; see conference abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to receive CC and either NAC or placebo". Method not described |
| Allocation concealment (selection bias) | Low risk | "Allocation was done by a third party (nurse)". "Using sealed envelopes". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The NAC and placebo were supplied in identical sachets. The patients and the physician monitoring the cycles were blinded to the identity of each medication". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts were reported |
| Selective reporting (reporting bias) | Low risk | No known selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Prospective, randomised clinical trial | |
| Participants | Women with low oocyte quality detected in previous IVF cycles (n = 65). Aged 35 ‐ 42 years. Mean age Treatment group 37.81 ± 2.61, Placebo 38.09 ± 1.97 IVF |
|
| Interventions | 1. Myo‐inositol 2 g + folic acid 200 mg + melatonin 3 mg: each tablet twice a day (n = 32) 2. Myo‐inositol 2 g + folic acid 200 mg: each tablet twice a day (n = 33) Administered continuously from the day of GnRH administration |
|
| Outcomes | Embryo quality Pregnancy rate, biochemical and clinical Total number of oocytes retrieved (immature and mature oocytes) Fertilisation rate per number of retrieved oocytes and embryo cleavage rate Spontaneous abortion defined as a pregnancy loss from 5 ‐ 12 weeks pregnancy |
|
| Notes | Setting: Messina, Italy, study dates not reported All participants gave written informed consent for the procedure, and the study was approved by the local ethics committee. Source of funding unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised: "According to a randomisation table, patients were assigned to receive either 2 g..." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Data given for all outcomes reported in the text |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled study | |
| Participants | Women aged < 40 years with PCOS undergoing ICSI (n = 54). Mean age Group 1: 36.8; Group 2: 36.9; Group 3 36.7; Group 4: 37.0; Placebo: 36.9 Exclusion criteria: Women with insulin resistance and/or hyperglycaemia |
|
| Interventions | 1. D‐chiro‐inositiol 300 mg: 1 tablet a day (n = 10) 2. D‐chiro‐inositol 600 mg: 1 tablet a day (n = 11) 3. D‐chiro‐inositol 1200 mg: 1 tablet a day (n = 10) 4. D‐chiro‐inositol 2400 mg: 1 tablet a day (n = 12) 5. Placebo (n = 11) Treatment given 8 weeks before ICSI |
|
| Outcomes | Number of oocytes retrieved Total rFSH 17B‐E2 levels on hCG administration Stimulation days Number of cycles cancelled |
|
| Notes | Conducted in Italy, study dates not reported Funding source not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization procedure was performed using a computer‐based program" |
| Allocation concealment (selection bias) | Unclear risk | Unknown allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised, controlled, double‐blind trial | |
| Participants | Women with PCOS attending IVF clinic (n = 46); Mean age Treatment group: 27; Control group 28 Exclusion criteria: infertility factors apart from anovulation, other pathologies, hormone consumption for less than 2 months before enrolment. |
|
| Interventions | 1. NAC 200 mg: 1 tablet 3 times a day (n = 23) 2. Placebo (n = 23) 7 women lost to follow‐up. Reasons described were intolerance to the smell of medications and blood samples inappropriate for the study Treatment 6 weeks' duration Follow‐up 6 weeks |
|
| Outcomes | Ovulation Weight Endocrine Metabolic and hormonal factors |
|
| Notes | Trial carried out in Teheran, Iran, from February 2007 and February 2008 Informed consent. Power calculation. Ethics approved. Funding source stated, "research is supported by Shahid Beheshti Medical University" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In order to minimise the effects of confounding factors through a randomised method". Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Medication was provided to patients by a midwife. Both patient and physician were blinded to the type of treatment regimen". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14 dropouts; 7 from each arm, reasons generally described but not for each woman |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the text were reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised placebo‐controlled double‐blind trial | |
| Participants | Infertile women with PCOS undergoing timed intercourse (n = 180) Women were aged 20 – 35 years Inclusion criteria: infertility duration < 10 years, BMI < 35 kg/m2, both participant tubes confirmed by hysterosalpingography or laparoscopy and with partner’s normal semen analysis results (total volume > 2 cc, concentration > 20 million/ml, total motility > 50%, normal morphology > 14%) Exclusion criteria: thyroid dysfunction, hyperprolactinaemia, hypercorticism, history of large ovarian cyst formation (> 6 cm), history of visual disturbance caused by clomiphene citrate and finally history of asthma and or allergy to medications. Women who had received any hormonal medications (except progesterone for withdrawal bleeding) or medications affecting glucose metabolism for at least 3 months before the study were also excluded; also no sexual dysfunction |
|
| Interventions | 1. NAC 1.2 g: 1 sachet a day (divided into 2 doses per day) + clomiphene citrate 100 mg: 1 tablet a day (n = 90) 2. Placebo + clomiphene citrate 100 mg/day divided and given in 2 doses a day given for 5 days starting at day 3 of the cycle. Timed intercourse occurred after an hCG trigger (n = 90) 8 women in the 1st group and 4 in the 2nd group left the study due to inappropriate drug intake or discontinued cycle; also 1 woman dropped out of the placebo group due to developing an ovarian cyst |
|
| Outcomes | Number of follicles > 18 mm Endometrial thickness Ovulation rates Pregnancy rates Adverse effects including multiple pregnancy Ovarian hyperstimulation syndrome |
|
| Notes | Trial carried out in the Shahid Beheshti University of Medical Sciences IVF Center, Taleghani Hospital, Iran between January 2008 and December 2009 Informed consent Power calculation Ethics approved Reprint request to: Dr Azadeh Akbari Sene, IVF Center, Infertility and Reproductive Health Research Center, Taleghani Hospital, Velenjak st, Tehran, Iran. Email: doctor_asturias@yahoo.com. RM‐P sent an email 12th December 2015. No reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Then patients were randomly divided into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. In the 2nd group in addition to 100 mg daily CC, participants received a placebo (oral rehydration solution powder) from day 3 until day 7. ORS powder was given to the participants in the same packets as NAC |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were explained |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the text were reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised trial | |
| Participants | Infertile women diagnosed with insulin‐resistant PCOS (n = 102) 18 women were scheduled for ovulation induction and 84 for IVF/ICSI Mean age: 28.8 ± 0.4 years |
|
| Interventions | 1. Folic acid 0.4 mg a day (n = 23) 2. Metformin 1700 mg a day (2 divided doses of 850 mg tablets) + folic acid 0.4 mg: 1 tablet a day (n = 28) 3. Vitamin B complex (50 mg B6, 400 ug folic acid, 500 ug B12, 1 g trimethylglycine and 6 mg pyridoxal‐5‐phosphate): 1 tablet a day (n = 24) 4. Metformin 1700 mg a day (2 divided doses of 850 mg tablets) + vitamin B complex: 1 tablet a day (n = 27) Women were recruited over 14 months and outcomes were measured over 3 cycles All groups were given folic acid |
|
| Outcomes | Homocysteine levels Cumulative clinical pregnancy rate over 3 cycles Ongoing pregnancy rate |
|
| Notes | Israel Tel Aviv, study dates not reported Dr Morey Schachter ivfdoc@asaf.health.gov.il. Email sent 8th December 2015, no reply Laboratory costs were partially funded by a company producing vitamins and supplements |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "These 102 patients were randomized before treatment, and after giving informed consent, assigned to one of four groups by opening sealed envelopes containing computer generated random assignation numbers" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing computer‐generated random assignation numbers" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unknown |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | No known selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Prospective randomised trial | |
| Participants | Euglycemic (normal levels of serum glucose) women with PCOS undergoing ovulation induction for ICSI (n = 84). Mean age Treatment group: 35.5 ± 3.2; D‐chiro‐inositol group: 36.5 ± 2.5 Inclusion criteria: women who have attended IVF department for > 12 months, younger than 40 years, diagnosed with PCOS according to the Rotterdam criteria Exclusion criteria: women with hyperglycaemia or insulin resistance, or both |
|
| Interventions | 1. Myo‐inositol 2 g: 1 tablet twice a day (n = 43) 2. D‐chiro‐inositol (member of vitamin B family) 0.6 g: 1 tablet twice a day (n = 41) Twice a day for 8 weeks |
|
| Outcomes | Total number of oocytes Number of mature oocytes Embryo quality Pregnancy, divided into biochemical and clinical pregnancies Miscarriage |
|
| Notes | Trial held in Messina, Italy, study dates not reported Funding source not mentioned. Emailed and posted letter to Dr Unfer 28th November 2011, requesting information on risk of bias. A colleague of the author, Gianfranco Carlomagno (gianfranco.carlomagno@agunco.it), replied 5th December 2011 with risk of bias information and offering to find data on live birth. Emailed back asking for live birth data 12th December 2011. Emailed again 10th August 2012 asking about live birth data. Author replied 16th August 2012. Live birth data added to the analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to receive..." In email correspondence, author replied "The randomisation was computer based". |
| Allocation concealment (selection bias) | Unclear risk | In email correspondence, author replied, "the treatments were provided in opaque envelopes identified by A or B". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | In email correspondence, author replied, "both patients and clinicians were blinded". However not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses were accounted for: 4 women in the control group had cancelled cycles and nil in the treatment group |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported, including live birth. Protocol available |
| Other bias | Low risk | No other bias found |
| Methods | Double‐blind randomised controlled trial | |
| Participants | Infertile women aged > 40 years undergoing IVF (n = 358) | |
| Interventions | 1. Melatonin 5 mg: 1 tablet a day (n = 178) 2. No treatment (n = 180) Trial ran from July 2009 to December 2013 |
|
| Outcomes | Oocyte maturity Oxidative stress Antioxidative capacity Progesterone concentration in follicular fluid Embryo grade |
|
| Notes | Conducted in Italy, trial ran from July 2009 to Decenber 2013 Trial funded by pharmaceutical company Trial registration no: NCT01540747 Email sent to Dr Pacchiarotti regarding the methods of this trial. Dr Pacchiarotti replied 20th March 2017 giving some methods information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind but control is no treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unknown |
| Selective reporting (reporting bias) | Low risk | No known selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Infertile women (n = 93). Mean age Treatment group: 35.4; Placebo group 34.8 Inclusion criteria: women aged 24 ‐ 42 years, unsuccessfully trying to conceive for 6 to 36 months Exclusion criteria: any woman taking any pharmacological treatment for infertility for 2 months before start of the trial. |
|
| Interventions | 1. Fertility blend: capsules containing chaste berry, green tea amino acid, L‐arginine, vitamins E, B6 and B12 and folate, iron, magnesium, zinc and selenium. 3 capsules a day for 3 menstrual cycles (n = 53) 2. Placebo (n = 40) Duration of treatment: 3 menstrual cycles, then women received an additional 3 months of open‐label fertility blend after completion of the study, with monitoring only of pregnancy and side effects Duration of trial: 4 months |
|
| Outcomes | Basal body temperature changes Length of menstrual cycle Pregnancy rates Side effects Mid‐luteal phase progesterone levels Miscarriage |
|
| Notes | No power calculation performed Institutional review board approval was obtained for the trial Conducted in the USA, study dates not reported Funding stated: David Sen Lin Foundation No loss to follow‐up. 14 pregnant in treatment group in first 3 months, then 17 in 6 months, but the second 3 months was unblinded; therefore, only first 3 months' data used. Not all women in the trial received the extra 3 months of treatment or placebo Miscarriage and side effect data cannot be used as they include data from the later 3 months when not all women received treatment or placebo in this phase. Tried to contact author 25th November 2009 with email, mail and fax, with no reply. Tried to contact author again regarding live birth, no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; mechanism not stated. "Fertilty Blend5 (FB), administered in a randomised, double‐blind, placebo‐controlled fashion". |
| Allocation concealment (selection bias) | Unclear risk | Mechanism not stated. Authors contacted May 2010 regarding this |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated as being double‐blinded, no clear explanation. Authors contacted regarding this. Placebo control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | High risk | Data on miscarriage and side effects cannot be used in analysis, as these data were combined with the extra open‐label 3‐month data. Not all women received treatment or placebo in this phase |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile couples with unexplained infertility seeking ICSI/IVF treatment following at least 3 failed previous IUI cycles (n = 218). Mean age Treatment group: 30.9 years; Control group: 30.6 Inclusion criteria: women aged < 40 years with normal ovulatory cycles, normal baseline; FSH 12 IU/l, thyroid‐stimulating hormone, prolactin levels, tubal patency at hysterosalpingography, normal transvaginal ultrasound scan, presence of both ovaries and normal findings at laparoscopy. All male partners had a normal semen analysis by WHO criteria Exclusion criteria: Couples who had received any form of vitamin supplementation for a period of 3 months before start of treatment |
|
| Interventions | Women in both groups received a daily dose of 2.5 mg of folic acid. 1. OCTATRON ® NERHADOU INTERNATIONAL (composition; vitamin A 3000 IU; d‐alpha tocopheryl acid; (vitamin E) 15 IU; ascorbic acid (vitamin C) 90 mg; Zinc (amino acid‐chelated) 11 mg; molybdenum (amino acid chelated) 45 μg; selenium (amino acid chelated) 55 μg, biotin 10 μg and mixed bioflavonoid 100 mg): 1 capsule a day (n= 112) 2. Folic acid 2.5 mg: 1 tablet a day (n = 106) Treatment was for 2½ months 7 women lost from each arm with explanation |
|
| Outcomes | The primary outcome was the number of mature oocytes Secondary outcomes were clinical pregnancy rate, defined as appearance of intrauterine gestational sac with fetal heart pulsation at 7 weeks Fertilisation rate Number of embryos transferred and cryopreserved Multiple pregnancy rate Early miscarriage rate Duration of stimulation Amount of FSH |
|
| Notes | Cairo Egypt Trial ran from February 2011 to March 2013 "On pregnancy confirmation, both groups received antioxidant and folic acid supplementation during the first trimester with follow‐up in accordance with this canter ’s policy. Participants ’ compliance with treatment, that is, the intake of supplements was confirmed and recorded on each visit by the caring physicians". This paper is the published version of Aboulfoutouh 2011 in first version of the review Email and letter sent to authors 9th August 2012asking about types of antioxidants used and ITT in the pregnancy outcome. Authors replied with data information. Participants were followed up only to clinical pregnancy, so no live birth data are provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We developed computer generated list for randomization" from an email received 12th December 2015 |
| Allocation concealment (selection bias) | Low risk | "used closed opaque envelops for concealment by third party nurse" from an email received 12th December 2015 |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding, control is no treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition explained |
| Selective reporting (reporting bias) | Low risk | Outcomes reported. Protocol available |
| Other bias | Low risk | No other bias found |
17B‐E2: 17B estradiol
ASRM: American Society for Reproductive Medicine
BMI: body mass index
CC: clomiphene citrate
ESHRE: European Society for Human Reproduction and Embryology
FSH: follicle stimulating hormone
GnRH: gonadotropin releasing hormone
hCG: human chorionic gonadotropin
HSG: hysterosalpingography
ICSI: intracytoplasmic sperm injection
ITT: intention‐to‐treat
IVF: in vitro fertilisation
MDA: malondialdehyde
NAC: N‐acetylcysteine
OC: oral contraceptive
OHSS: ovarian hyperstimulation syndrome
PCOS: poly cystic ovarian syndrome
rFSH: recombinant follicle stimulating hormone
SD: standard deviation
WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aflatoonian 2014 | Population were vitamin D‐deficient |
| Aksoy 2010 | Not a randomised study |
| Al‐Omari 2003 | Non‐randomised trial. "Forty‐two infertile PCOS were divided into three groups". |
| Ardabili 2012 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review. |
| Asadi 2014 | Vitamin D not given orally but by injection into the muscle |
| Baillargeon 2004 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review |
| Benelli 2016 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review |
| Bonakdaran 2012 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review |
| Cheang 2008 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review |
| Ciotta 2012 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review |
| Costantino 2009 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review |
| Crha 2003 | Not an RCT. "patients for the supplemented and control sets were selected by the case‐control method according to their age and smoking or non‐smoking habits." |
| Elgindy 2010 | Participants were fertile women with infertile male partners. |
| Elnashar 2007 | Interventions N‐acetyl‐cysteine versus metformin |
| Farzadi 2006 | The intervention versus control used in this trial was metformin versus placebo |
| Firouzabadi 2012 | 83% of the women enrolled were vitamin D‐deficient |
| Genazzani 2008 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review |
| Hashim 2010 | Interventions N‐acetyl‐cysteine plus clomiphene citrate versus metformin plus clomiphene citrate |
| Hebisha 2016 | Enrolled women who attended infertility clinic due to male factor issues |
| Henmi 2003 | Described as randomised, but authors confirmed the process of allocation as "alternative treatments". Additionally, 28/46 in the placebo arm withdrew because of travel difficulties and movement out of the study area. No withdrawals from the treatment arm were reported. There was no Intention‐to‐treat. |
| Hernández‐Yero 2012 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Immediata 2014 | Wrong comparison; Inositol vs metformin |
| Iuorno 2002 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Jamilian 2016 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review. |
| Jamilian 2016a | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Kamencic 2008 | This trial included women with endometriosis, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Kilicdag 2005 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Le Donne 2012 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Li 2013 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Moosavifar 2010 | Participants were not subfertile women; they were partners of subfertile men |
| Nazzaro 2011 | Not randomised. Attempted to contact authors regarding sequence allocation via email 10 November 2011 |
| Nestler 1999 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Nestler 2001 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Nichols 2010 | Lead investigator confirmed (May 2010). Stated that the trial was abandoned before recruitment because of lack of funding |
| Nordio 2012 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Oner 2011 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Pal 2016 | A secondary analysis of an RCT measuring ovulation induction (OI) outcomes in women with polycystic ovary syndrome (PCOS). |
| Papaleo 2007 | Not a randomised controlled trial |
| Papaleo 2008 | Interventions myo‐inositol plus folic acid versus clomiphene citrate |
| Pasha 2011 | Inappropriate population |
| Pizzo 2014 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Raffone 2010 | Interventions myo‐inositol plus folic acid versus metformin |
| Ruder 2014 | A secondary analysis of an RCT on the cost‐effectiveness of fast track to IVF |
| Salem 2012 | Different doses of clomiphene in each arm, i.e. L‐carnitine 3 gm plus clomiphene 100 mg (n = 85) versus clomiphene 150 mg (n = 85) |
| Santanam 2003 | The population included here were women with endometriosis, and the trial aimed to show differences in inflammatory markers. These women were not attending a fertility clinic |
| Taheri 2015 | Population is Vitamin D‐deficient |
| Tamura 2008 | A quasi‐randomised trial. "Patients were divided into two groups". Email sent asking about randomisation but undeliverable. Letter sent to University of Texas 12 January 2012. Letter returned to sender 17 February 2012. |
| Twigt 2011 | Participants were randomly assigned to different stimulation protocols and not to folic acid. All participants took folic acid |
| Vargas 2011 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
| Yoon 2010 | This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
PCOS: poly cystic ovarian syndrome
RCT: random controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Nutritional supplement for women With polycystic ovary syndrome or subfertilty |
| Methods | Randomised, parallel‐group, placebo‐controlled trial Method of generating randomisation sequence: computer‐generated randomisation Method of allocation concealment: pre‐numbered or coded identical Containers blinding and masking: participant‐ and investigator‐blinded |
| Participants | Inclusion criteria: Women between 18 and 38 years of age with PCOS Presence of any 2 of the following parameters: (Roterdam criteria 2003)
OR
|
| Interventions | Intervention 1: multiple micronutrients supplementation: 1 Formula A tablet + 1 Formula B tablet together after main meal twice a day for 4 months Ingredients Formula A: N‐acetyl L‐cysteine, elemental magnesium, zinc, iron, manganese, copper, selenium, iodine, chromium. Ingredients Formula B: inositol, vitamin C, para‐amino‐benzoic acid, vitamin E acetate, L‐arginine, D‐chiro‐inositol, vitamin B complex Control intervention 1: placebo tablets for Formula A: 1 tablet after main meal twice a day for 4 months Control intervention 2: placebo tablets for Formula B: 1 tablet after main meal twice a day for 4 months |
| Outcomes | Improvement in overall status of PCOS or infertility.
Improvement in different parameters defining the status of PCOS or infertility‐like hormonal levels, insulin resistance, weight and safety of the therapy
|
| Starting date | 31 August 2012 |
| Contact information | Dr Yashwant Mane Dr Yashwant Mane Atharva Infertilty and Test Tube Baby Center Jagir Complex Dwarka, Nasik, India 422011 Nashik, MAHARASHTRA India ph 02532598953 email drysmane7473@yahoo.co.in |
| Notes |
| Trial name or title | A pilot double‐blind randomised placebo‐controlled dose–response trial assessing the effects of melatonin on infertility treatment (MIART): study protocol |
| Methods | Pilot phase II cross‐over double‐blinded randomised placebo‐controlled dose–response trial Each treatment arm will be randomly allocated a letter (A, B, C or D) by way of opaque sealed envelope Randomisation will be computerised and participants will be randomised at a ratio of 1:1:1:1 to 1 of the groups, A–D, using the minimisation method. |
| Participants | We plan to recruit a total of 160 infertile women undergoing IVF/ICSI INCLUSION CRITERIA; 1. Undergoing first cycle of IVF or ICSI; 2. Age between 18 and 45; 3. Body mass index between 18 and 35; 4. Undergoing a gonadotrophin releasing hormone (GnRH) antagonist cycle (without oral contraceptive pill (OCP) scheduling). EXCLUSION CRITERIA 1. Current untreated pelvic pathology: moderate‐to‐severe endometriosis, submucosal uterine fibroids/polyps assessed by the treating specialist to affect fertility, pelvic inflammatory disease uterine malformations, Asherman’s syndrome and hydrosalpinx 2. Currently enrolled in another interventional clinical trial 3. Concurrent use of other adjuvant therapies (e.g. Chinese herbs, acupuncture) 4. Current pregnancy 5. Malignancy or other contraindication to IVF 6. Autoimmune disorders 7. Undergoing preimplantation genetic diagnosis 8. Hypersensitivity to melatonin or its metabolites 9. Concurrent use of any of the following medications: A. Fluvoxamine (e.g. luvox, movox, voxam); B. Cimetidine (e.g. magicul, tagamet); C. Quinolones and other CYP1A2 inhibitors (ciprofloxacin, avalox); D. Carbamazepine (e.g. tegretol), rifampicin (e.g. rifadin) and other CYP1A2 inducers; E. Zolpidem (e.g. stilnox), zopiclone (e.g. imovane) and other non‐benzodiazepine hypnotics. 10. Inability to comply with trial protocol |
| Interventions | 1. Placebo capsule taken twice a day; 2. 2 mg melatonin capsule twice a day (4 mg/day total); 3. 4 mg melatonin capsule twice per day (8 mg/day total); 4. 8 mg melatonin capsule twice per day (16 mg/day total). |
| Outcomes | Primary outcome: Clinical pregnancy rate, defined as the presence of a live pregnancy in the uterine cavity at a transvaginal ultrasound at 6 – 8 weeks’ gestation Secondary outcomes: Live birth, miscarriage, adverse events, melatonin serum levels |
| Starting date | Recruitment will start in July 2014. This will occur over 2 years. Analysis and dissemination will occur after this period of time. The study is expected to be completed by February 2017. |
| Contact information | Dr Shavi Fernando; shavif@hotmail.com |
| Notes | Participants will be recruited from Monash IVF infertility clinics in Melbourne, Australia over a period of 2 years Ethics and dissemination: Ethical approval has been obtained from Monash Health HREC (Ref:13402B), Monash University HREC (Ref: CF14/523‐2014000181) and Monash Surgical Private Hospital HREC (Ref: 14107). Data analysis, interpretation and conclusions will be presented at national and international conferences and published in peer reviewed journals. Trial registration number: ACTRN12613001317785 |
| Trial name or title | Evaluation of the effects of Calcium‐ Vitamine D supplementary on ovulation and fertility outcomes of patients with poly cystic ovary syndrome referring to Infertility Clinic of Imam Khomeini Hospital in 2011 and 2012 for in vitro fertilization |
| Methods | Randomisation: randomised. Blinding: double‐blind. Placebo: used |
| Participants | Abnormal menstrual cycles (oligomenorrhoea or amenorrhoea); sonographically‐confirmed polycystic ovary; hyperandrogenism |
| Interventions | Intervention 1: In control group, participants do not receive routine administration of calcium‐D combinations Intervention 2: In intervention group, supplementary tablets of 1000 mg calcium combined with 400 IU vitamin D are administered (orally) twice a day for 3 months |
| Outcomes | Pregnancy. Timepoint: 2 and 12 weeks. Method of measurement: sonography and biochemistry |
| Starting date | 21 January 2012 |
| Contact information | Azadeh Mahdian Department of Obstetrics and Gynecology, Vali‐e‐ Asr Hospital, Imam Khomeini Hospital Complex, Keshavarz Blv Tehran Iran, Islamic Republic of mahdian@razi.tums.ac.ir |
| Notes |
| Trial name or title | Vitamin D during In Vitro Fertilization (IVF) ‐ a prospective randomised trial delivery |
| Methods | Randomised double‐blind trial |
| Participants | Target sample size: 1000 women older than 18 years of age initiating IVF treatment in Sweden |
| Interventions | Dietary supplementation: ergocalciferol (vitamin D), either high 100,000 U once or low‐dose 500 U once |
| Outcomes | Biochemical pregnancy, live birth, take‐home baby rate, OHSS and pregnancy complication rate (pregnancy, hypertension, SGA, diabetes) |
| Starting date | November 2009 |
| Contact information | Pelle G. Lindqvist Karolinska University Hospital Huddinge ClinicalTrials.gov identifier NCT01019785. |
| Notes | clinicaltrials.gov/ct2/show/NCT01019785?term=NCT01019785&rank=1 |
| Trial name or title | Improving oocyte retrieval using a combined therapy of recombinant follicle‐stimulating hormone (rFSH) and inositol and melatonin |
| Methods | Randomised double‐blinded (participant, investigator) controlled trial. |
| Participants | Women 18 ‐ 39 years undergoing assisted reproductive techniques (ART) because of male infertility BMI 18 ‐ 30 kg/m2 Fewer than 3 prior oocyte retrievals No fertility problems |
| Interventions | Recombinant FSH: 225 IU rFSH Drug: recombinant FSH (rFSH) 225 IU Experimental: recombinant FSH inositol melatonin 225 IU rFSH, 4 g inositol and 3 mg melatonin dietary supplement: rFSH + inositol + melatonin 225 IU rFSH, 4 g inositol, 3 mg melatonin |
| Outcomes | Primary: total number of oocytes, number of clinical pregnancies, live birth rate |
| Starting date | December 2010 |
| Contact information | Vittorio Unfer, MD +39 0640500835 vunfer@gmail.com Gianfranco Carlomagno, PhD gianfranco.carlomagno@gmail.com University of Modena and Reggio Emilia Recruiting Reggio Emilia, Italy, 42100 Contact: Giovanni Battista La Sala, MD +39 0522 296464 giovanni.lasala@asmn.re.it Principal investigator: Giovanni Battista La Sala, MD Research Center for Reproductive Medicine Villa Mafalda Recruiting Roma, Italy, 00199 |
| Notes | May not become an included study because all women are fertile, but they have subfertile male partners ClinicalTrials.gov Identifier: NCT01267604. Recruiting. Trial found on clinicaltrials.gov on 7th August 2012 |
| Trial name or title | Effect of resveratrol on metabolic parameters and oocyte quality in PCOS patients (RES‐IVF) |
| Methods | Randomised |
| Participants | Women with PCOS |
| Interventions | Resveratrol versus placebo |
| Outcomes | Implantation Pregnancy rates |
| Starting date | February 2013 |
| Contact information | Israel Ortega, Medical doctor 91 180 2900 israel.ortega@ivi.es |
| Notes | Not yet recruiting. Madrid |
| Trial name or title | Use of antioxidant in endometriotic women to improve intracytoplasmic sperm injection (ICSI) (ROS) Official title: Does antioxidant supplementation to endometriotic women undergoing ICSI alter reactive oxygen species (ROS) levels and affect pregnancy outcome |
| Methods | Randomised, parallel‐assignment, single‐blind (participant) |
| Participants | Women with endometriosis undergoing IVF, 20 ‐ 40 years |
| Interventions | Drug: ascorbate 1000 mg, vitamin E 400, zinc and selenium |
| Outcomes | Pregnancy |
| Starting date | March 2013 |
| Contact information | Olfat Nouh Riad, Assisstant Professor, Cairo University Egyption centre for IVF Maadi, Egypt, 11451 |
| Notes | ClinicalTrials.gov Identifier: NCT02058212 |
| Trial name or title | Lipoic acid supplementation in IVF |
| Methods | Allocation: randomised |
| Participants | Women infertility ‐ 30 to 50 years |
| Interventions | Dietary supplement: oral lipoic acid|drug: vaginal progesterone |
| Outcomes | Number of implants per cycle Number of biochemical pregnancies per group Number of clinical pregnancies per group Number of live birth per group Number of miscarriage per group |
| Starting date | 2015 |
| Contact information | Lo.Li.Pharma s.r.l |
| Notes | ClinicalTrials.gov/show/NCT03023514 |
Contributions of authors
Marian Showell conducted the searches, assessed studies for inclusion, extracted data, analysed the data and wrote the review.
Rebecca Mackenzie‐Proctor assisted with assessing the trials for inclusion, extracted the data, assisted with the data analysis and helped with writing of the updated review.
Vanessa Jordan assisted with the methodology in the updated review and commented on the drafts.
Roger Hart helped with the writing of the review and provided clinical advice.
Sources of support
Internal sources
NZ GOVT MOH, New Zealand.
External sources
None, Other.
Declarations of interest
Roger Hart is the Medical Director of Fertility Specialists of WA and a shareholder in Western IVF. He has received educational sponsorship from Merck Serono and Ferring pharmaceuticals, and is on the medical advisory board of MSD and Ferring Pharmaceuticals.
Rebecca Mackenzie‐Proctor: no conflict of interest to declare
Vanessa Jordan: no conflict of interest to declare
Marian Showell: no conflict of interest to declare
Edited (no change to conclusions)
References
References to studies included in this review
- Agrawal R, Burt E, Gallagher AM, Butler L, Venkatakrishnan R, Peitsidis P. Prospective randomized trial of multiple micronutrients in subfertile women undergoing ovulation induction: a pilot study. Reproductive Biomedicine Online 2012;24(1):54‐60. [DOI] [PubMed] [Google Scholar]
- Alborzi S, Ghotbi S, Parsanezhad ME, Dehbashi S, Alborzi S, Alborzi M. Pentoxifylline therapy after laparoscopic surgery for different stages of endometriosis: a prospective, double‐blind, randomized, placebo‐controlled study. Journal of Minimally Invasive Gynecology 2007;14(1):54‐8. [DOI] [PubMed] [Google Scholar]; Ghotbi S, Parsanezhad ME, Dehbashi S, Alborzi S, Alborzi M. Pentoxifylline therapy after laparoscopic surgery for different stages of endometriosis: A prospective, double‐blind, randomized, placebo‐controlled study. Journal of Minimally Invasive Gynecology2007; Vol. 14, issue 1:54‐8. [DOI] [PubMed]
- Aleyasin A, Aghahosseini M, Mohseni M, Mahavi A. Effects of pentoxifylline and vitamin E on pregnancy rate in infertile women by ZIFT: a randomized clinical trial. Iranian Journal of Reproductive Medicine 2009;7:175‐9. [Google Scholar]
- Badawy A, Nashar AB, Totongy M. Clomiphene citrate plus N‐acetyl cysteine versus clomiphene citrate for augmenting ovulation in the management of unexplained infertility: a randomized double‐blind controlled trial. Fertility and Sterility 2006;86(3):647‐50. [DOI] [PubMed] [Google Scholar]
- Balasch J, Creus M, Fabregues F, Carmona F, Martinez‐Roman S, Manau D, et al. Pentoxifylline versus placebo in the treatment of infertility associated with minimal or mild endometriosis. Human Reproduction 1997;12(9):2046‐50. [DOI] [PubMed] [Google Scholar]
- Batioglu AS, Sahin U, Gurlek B, Ozturk N, Unsal E. The efficacy of melatonin administration on oocyte quality. Gynecological Endocrinology2012; Vol. 28, issue 2:91‐3. [DOI] [PubMed]
- Battaglia C, Salvatori M, Maxia N, Petraglia F, Facchinetti F, Volpe A. Adjuvant L‐arginine treatment for in‐vitro fertilization in poor responder patients. Human Reproduction1999; Vol. 14, issue 7:1690‐7. [DOI] [PubMed]
- Battaglia C, Regani G, Marsella T, Facchinetti F, Volpe A, Venturoli S. Adjuvant L‐arginine treatment in controlled ovarian hyperstimulation: a double‐blind randomized study. Human Reproduction 2002;17(3):659‐65. [DOI] [PubMed] [Google Scholar]
- Bentov Y, Hannam T, Jurisicova A, Esfandiari N, Casper RF. Coenzyme Q10 supplementation and oocyte aneuploidy in women undergoing IVF‐ICSI treatment. Clinical Medicine Insights. Reproductive Health 2014;8:31‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusco GF, Mariani M. Inositol: Effects on oocyte quality in patients undergoing ICSI. An open study. European Review for Medical and Pharmacological Sciences2013; Vol. 17, issue 22:3095‐102. [PubMed]
- Carlomagno G, Montanino Oliva M, Roseff SJ, Unfer V. Myo‐inositol: Ovarian stimulation and IVF outcomes. Fertility and Sterility2012; Vol. 98 Suppl 1, issue 3:S74‐S75 Abstract no:O‐251.
- Cheraghi E, Mehranjani MS, Shariatzadeh MA, Esfahani MHN, Ebrahimi Z. N‐Acetylcysteine improves oocyte and embryo quality in polycystic ovary syndrome patients undergoing intracytoplasmic sperm injection: An alternative to metformin. Reproduction, Fertility, and Development2016; Vol. 28, issue 6:723‐31. [DOI] [PubMed]; Cheraghi E, Shariatzadeh MA, Soleimani M, Nasr Esfahani MH, Ebrahimi Z. Association of follicular fluid endocrine changes with oocyte morphology and quality in PCOS patients undergoing metformin and N‐acetylcysteine treatment. International Journal of Fertility and Sterility 2013;7 Suppl 1:44. [Google Scholar]; Cheraghi E, Soleimani Mehranjani M, Ali Shariatzadeh M, Nasr Esfahani M, Ebrahimi Z. Co‐administration of metformin and N‐Acetyl Cysteine fails to improve clinical manifestations in PCOS individual undergoing ICSI. International Journal of Fertility and Sterility 2014;8:119‐28. [PMC free article] [PubMed] [Google Scholar]; Cheraghi E, Soleimani Mehranjani M, Shariatzadeh SM, Nasr Esfahani MH, Ebrahimi Z. Expression changes of GDF‐9 and BMP‐15 in the oocytes of PCOS patients undergoing treatment with N‐acetylcysteine. International Journal of Fertility and Sterility 2014;8 Suppl 1:201. [PMC free article] [PubMed] [Google Scholar]
- Choi S‐Y, Kim CH, Ahn J‐W, Kwon S‐K, Lee K‐H, Kang B‐M. Effect of calcium and vitamin D supplementation on follicular fluid tumor necrosis‐alpha and interleukin‐6 and IVF/ICSI outcomes in infertile patients with polycystic ovary syndrome. Fertility and Sterility2012; Vol. 98 Suppl 1, issue 3:S208‐S209 Abstract no:P‐326.
- Cicek N, Eryilmaz OG, Sarikaya E, Gulerman C, Genc Y. Vitamin E: Effect on controlled ovarian stimulation of unexplained infertile women. Journal of Assisted Reproduction and Genetics2012; Vol. 29, issue 4:325‐8. [DOI] [PMC free article] [PubMed]
- Ciotta L, Stracquadanio M, Pagano I, Carbonaro A, Palumbo M, Gulino F. Effects of myo‐inositol supplementation on oocyte's quality in PCOS patients: a double blind trial. European Review for Medical & Pharmacological Sciences 2011;15(5):509‐14. [PubMed] [Google Scholar]
- Colazingari S, Treglia M, Najjar R, Bevilacqua A. The combined therapy myo‐inositol plus D‐chiro‐inositol, rather than D‐chiro‐inositol, is able to improve IVF outcomes: results from a randomized controlled trial. Archives of Gynecology and Obstetrics 2013;288(6):1405‐11. [DOI] [PubMed] [Google Scholar]
- Creus M, Fabregues F, Carmona F, Pino M, Manau D, Balasch J. Combined laparoscopic surgery and pentoxifylline therapy for treatment of endometriosis‐associated infertility: a preliminary trial. Human Reproduction 2008;23:1910‐6. [DOI] [PubMed] [Google Scholar]
- Daneshbodi H, Vaziri N, Nadjarzadeh A, Lotfi MH, Mozaffari‐Khosravi H. Effect of omega‐3 supplementation on gonadotropins and prolactin levels in women with polycystic ovary syndrome: A double blinded randomized controlled trial. Iranian Journal of Reproductive Medicine. Research and Clinical Center for Infertitlity, 2013; Vol. 11:58‐9.
- Deeba F, Fatima P. Multiple micronutrients (MMN) in infertile women undergoing ovulation induction: A randomized control trial. Journal of Obstetrics and Gynaecology research. Blackwell Publishing, 2015; Vol. 41:170.
- Refaeey A, Selem A, Badawy A. Combined coenzyme Q10 and clomiphene citrate for ovulation induction in clomiphene‐citrate‐resistant polycystic ovary syndrome. Reproductive Biomedicine Online 2014;29(1):119‐24. [DOI] [PubMed] [Google Scholar]
- Eryilmaz OG, Devran A, Sarikaya E, Aksakal FN, Mollamahmutoglu L, Cicek N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. Journal of Assisted Reproduction & Genetics2011; Vol. 28, issue 9:815‐20. [DOI] [PMC free article] [PubMed]
- Gerli S, Mignosa M, Renzo GC. Effects of inositol on ovarian function and metabolic factors in women with PCOS: a randomized double blind placebo‐controlled trial. European Review for Medical & Pharmacological Sciences 2003;7(6):151‐9. [PubMed] [Google Scholar]; Gerli S, Papaleo E, Ferrari A, Renzo G. Randomized, double blind placebo‐controlled trial: effects of myo‐inositol on ovarian function and metabolic factors in women with PCOS. Eurpoean Review for Medical and Pharmacolgical Sciences 2007;11:347‐54. [PubMed] [Google Scholar]
- Griesinger G, Franke K, Kinast C, Kutzelnigg A, Riedinger S, Kulin S, et al. Ascorbic acid supplement during luteal phase in IVF. Journal of Assisted Reproduction and Genetics 2002;19(4):164‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AM, Hamed AH, Saso S, Thabet HH. Adding L‐carnitine to clomiphene resistant PCOS women improves the quality of ovulation and the pregnancy rate. A randomized clinical trial. European Journal of Obstetrics Gynecology and Reproductive Biology 2014;180:148‐52. [DOI] [PubMed] [Google Scholar]
- Keikha F. Combination N‐acetyl cysteine and clomiphene citrate in clomiphene citrate‐resistant polycystic ovary syndrome. Iranian Journal of Reproductive Medicine. Research and Clinical Center for Infertitlity, 2010; Vol. 8:12‐3.
- Kim C, Lee S, Koo Y, Lee H, Lee Y, Jeon I, et al. N‐acetyl‐cysteine treatment improves insulin sensitivity, ovarian response to gonadotrophin and IVF outcome in patients with polycystic ovary syndrome. ESHRE Conference Proceedings. 18 ‐ 21 June 2006; Vol. I:178. [Google Scholar]
- Kim CH, Yoon JW, Ahn JW, Kang HJ, Lee JW, Kang BM. The effect of supplementation with omega‐3‐polyunsaturated fatty acids in intracytoplasmic sperm injection cycles for infertile patients with a history of unexplained total fertilization failure.. Fertility and Sterility 2010;94(4):S242. Abstract no P‐518. [Google Scholar]
- Lesoine B, Regidor PA. Prospective randomized study on the influence of myoinositol in PCOS women undergoing IVF in the improvement of oocyte quality, fertilization rate, and embryo quality. International Journal of Endocrinology2016; Vol. 2016 (no pagination), issue 4378507. [DOI] [PMC free article] [PubMed]
- Lisi F, Carfagna P, Oliva MM, Rago R, Lisi R, Poverini R, et al. Pretreatment with myo‐inositol in non polycystic ovary syndrome patients undergoing multiple follicular stimulation for IVF: a pilot study. Reproductive Biology and Endocrinology2012; Vol. 10:52. [DOI: 10.1186/1477-7827-10-52] [DOI] [PMC free article] [PubMed]
- Maged AM, Elsawah H, Abdelhafez A, Bakry A, Mostafa WA. The adjuvant effect of metformin and N‐acetylcysteine to clomiphene citrate in induction of ovulation in patients with polycystic ovary syndrome. Gynecological Endocrinology2015; Vol. 31, issue 8:635‐8. [DOI] [PubMed]
- Mier‐Cabrera J, Geenera‐Garcia M, Jara‐Diaz, Perichart‐Perera Otilla, Vadillo‐Ortega F, Hernandez‐Guerrero C. Effect of vitamins C and E supplementation on peripheral oxidative stress markers and pregnancy rate in women with endometriosis. International Journal of Gynecology and Obstetrics 2008;100:252‐6. [DOI] [PubMed] [Google Scholar]
- Mohammadbeigi R, Afkhamzadeh A, Daneshpour NS. The effect of calcium‐vitamin D in efficacy of induction ovulation in infertile women with polycystic ovary syndrome. Iranian Journal of Obstetrics, Gynecology and Infertility 2012;15:7‐13. [Google Scholar]
- Nasr A. Effect of N‐acetyl‐cysteine after ovarian drilling in clomiphene citrate‐resistant PCOS women: a pilot study. Reproductive BioMedicine Online 2010;20(3):403‐9. [DOI] [PubMed] [Google Scholar]
- Ozkaya MO, NazIroglu M, Barak C, Berkkanoglu M. Effects of multivitamin/mineral supplementation on trace element levels in serum and follicular fluid of women undergoing in vitro fertilization (IVF). Biological Trace Element Research2011; Vol. 139, issue 1:1‐9. [DOI] [PubMed]; Ozkaya MO, Nazrolu M. Multivitamin and mineral supplementation modulates oxidative stress and antioxidant vitamin levels in serum and follicular fluid of women undergoing in vitro fertilization. Fertility and Sterility 2010;94(6):2465‐6. [DOI] [PubMed] [Google Scholar]
- Pacchiarotti A, Carlomagno G, Antonini G, Pacchiarotti A. Effect of myo‐inositol and melatonin versus myo‐inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecological Endocrinology2016; Vol. 32, issue 1:69‐73. [DOI] [PubMed]
- Panti Abubakar A, Egondu Shehu C, Saidu Y, Nwobodo EI, Tunau Abubakar K, Jimoh A, et al. Oxidative stress and antioxidant supplementation in patients with polycystic ovarian syndrome (PCOS). International Journal of Gynecology and Obstetrics2015; Vol. 131 Suppl 5:E505.
- Papaleo E, Unfer V, Baillargeon J, Fusi F, Occhi F, Santis L. Myo‐inositol may improve oocyte quality in intracytoplasmic sperm injection cycles: a prospective, controlled, randomized trial. Fertility and Sterility 2009;91(5):1750‐4. [DOI] [PubMed] [Google Scholar]
- Polak de Fried E, Bossi NM, Notrica JA, Vazquez Levin MH. Vitamin‐d treatment does not improve pregnancy rates in patients undergoing art: a prospective, randomized, double‐blind placebo‐controlled trial. Fertility and Sterility 2013;100(Suppl):S493. [Google Scholar]
- Rashidi B, Haghollahia F, Shariata M, Zayeriia F. The effects of calcium‐vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwanese Journal of Obstetrics and Gynecology 2009;48(2):142‐7. [DOI] [PubMed] [Google Scholar]
- Razavi M, Jamilian M, Kashan ZF, Heidar Z, Mohseni M, Ghandi Y, et al. Selenium supplementation and the effects on reproductive outcomes, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome. Hormone and Metabolic Research2015; Vol. 48, issue 3:185‐90. [DOI] [PubMed]
- Rezk A, Bedaiwy M, Enany H. N‐acetyl‐cysteine is a novel adjuvant to clomiphene citrate in clomiphene citrate–resistant patients with polycystic ovary syndrome. Human Reproduction; Abstracts of the 20th Annual Meeting of the ESHRE, June 27‐30. 2004 2004;19:i53. [DOI] [PubMed] [Google Scholar]; Rizk A, Bedaiwy M, Al‐Inany H. N‐acetyl‐cysteine is a novel adjuvant to clomiphene citrate in clomiphene citrate–resistant patients with polycystic ovary syndrome. Fertility and Sterility 2005;83(2):367‐70. [DOI] [PubMed] [Google Scholar]
- Rizzo P, Raffone E, Benedetto V. Effect of the treatment with myo‐inositol plus folic acid plus melatonin in comparison with a treatment with myo‐inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles: a prospective, clinical trial. European Review for Medical & Pharmacological Sciences 2010;14(6):555‐61. [PubMed] [Google Scholar]
- Rosalbino I, Raffone E. Does ovary need D‐chiro‐inositol?. Journal of Ovarian Research2012; Vol. 5, issue 1:14. [DOI] [PMC free article] [PubMed]
- Salehpour S, Tohidi M, Akhound M, Amirzargar N. N acetyl cysteine, a novel remedy for polycystic ovary syndrome. International Journal of Fertility and Sterility 2009;3(2):66‐73. [Google Scholar]
- Salehpour S, Akbari Sene A, Saharkhiz N, Sohrabi M, Moghimian F. N‐acetyl‐cysteine as an adjuvant to clomiphene citrate for successful induction of ovulation in infertile patients with polycystic ovary syndrome. Iranian Journal of Reproductive Medicine2012; Vol. 10 Suppl 1:18 Abstract no: O‐30. [DOI] [PubMed]; Salehpour S, Akbari Sene A, Saharkhiz N, Sohrabi M, Moghimian F. N‐acetyl‐cysteine as an adjuvant to clomiphene citrate for successful induction of ovulation in infertile patients with polycystic ovary syndrome. Iranian Journal of Reproductive Medicine. Research and Clinical Center for Infertitlity, 2012; Vol. 10:9. [DOI] [PubMed]; Salehpour S, Akbari Sene A, Saharkhiz N, Sohrabi MR, Moghimian F. N‐acetylcysteine as an adjuvant to clomiphene citrate for successful induction of ovulation in infertile patients with polycystic ovary syndrome. Journal of Obstetrics & Gynaecology Research 2012;38:1182‐6. [DOI] [PubMed] [Google Scholar]
- Schachter M, Raziel A, Strassburger D, Rotem C, Ron‐El R, Friedler S. Prospective, randomized trial of metformin and vitamins for the reduction of plasma homocysteine in insulin‐resistant polycystic ovary syndrome. Fertility & Sterility.2007; Vol. 88, issue 1:227‐30. [DOI] [PubMed]
- Unfer V, Carlomagno G, Rizzo P, Raffone E, Roseff S. Myo‐inositol rather than D‐chiro‐inositol is able to improve oocyte quality in intracytoplasmic sperm injection cycles: a prospective, controlled, randomized trial. European Review for Medical and Pharmacological Sciences 2011;15(4):452‐7. [PubMed] [Google Scholar]
- Valeri C, Sbracia G, Selman H, Antonini G, Pacchiarotti A. Beneficial effects of melatonin on oocytes and embryo quality in aged IVF patients. Human Reproduction 2015;30:i48‐9. [Google Scholar]
- Westphal LM, Polan ML, Trant AS. Double‐blind, placebo‐controlled study of Fertilityblend: a nutritional supplement for improving fertility in women. Clinical and Experimental Obstetrics & Gynecology 2006;33(4):205‐8. [PubMed] [Google Scholar]
- Aboulfoutouh I, Youssef M, Khattab S. Can antioxidants supplementation improve ICSI/IVF outcomes in women undergoing IVF/ICSI treatment cycles? Randomised controlled study. Fertility and Sterility 2011;96 Suppl 3:S242‐9. [Google Scholar]; Youssef MA, Abdelmoty HI, Elashmwi HA, Abduljawad EM, Elghamary N, Magdy A, et al. Oral antioxidants supplementation for women with unexplained infertility undergoing ICSI/IVF: randomized controlled trial. Human Fertility2015; Vol. 18, issue 1:38‐42. [DOI] [PubMed]
References to studies excluded from this review
- Aflatoonian A, Arabjahvani F, Eftekhar M, Sayadi M. Effect of vitamin D insufficiency treatment on fertility outcomes in frozen‐thawed embryo transfer cycles: A randomized clinical trial. Iranian Journal of Reproductive Medicine 2014;12(9):595‐600. [PMC free article] [PubMed] [Google Scholar]
- Aksoy AN, Kadanali S. Evaluating the effects of vitamin E addition to clomiphene citrate on endometrial receptivity: a prospective controlled study. Turkiye Klinikleri Jinekoloji Obstetrik 2010;20(2):71‐6. [Google Scholar]
- Al‐Omari W, Abdul‐Qadder A, Sulaiman W, Taher M. Antioxidants improve metabolic and ovarian response in polycsytic ovary syndrome. ESHRE. 2003; Vol. xviii:84. [Google Scholar]
- Ardabili HR, Gargari BP, Farzadi L. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Nutrition Research 2012;32(3):195‐201. [DOI] [PubMed] [Google Scholar]
- Asadi M, Matin N, Frootan M, Mohamadpour J, Qorbani M, Tanha FD. Vitamin D improves endometrial thickness in PCOS women who need intrauterine insemination: A randomized double‐blind placebo‐controlled trial. Archives of Gynecology and Obstetrics 2014;289(4):865‐870. [DOI] [PubMed] [Google Scholar]
- Baillargeon JP, Iuorno MJ, Jakubowicz DJ, Apridonidze T, He N, Nestler JE. Metformin therapy increases insulin‐stimulated release of D‐chiro‐inositol‐containing inositolphosphoglycan mediator in women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism2004; Vol. 89, issue 1:242‐9. [DOI] [PubMed]
- Benelli E, Ghianda S, Cosmo C, Tonacchera M. A combined therapy with myo‐inositol and d‐chiro‐inositol improves endocrine parameters and insulin resistance in PCOS young overweight women. International Journal of Endocrinology2016; Vol. 2016:3204083. [DOI] [PMC free article] [PubMed]
- Bonakdaran S, Khorasani ZM, Davachi B, Shakeri MT. Comparison of calcitriol and metformin effects on clinical and metabolic consequences of polycystic ovary syndrome. Iranian Journal of Obstetrics, Gynecology and Infertility 2012;14(8):16‐24. [Google Scholar]
- Cheang KI, Baillargeon J‐P, Essah PA, Ostlund RE Jr, Apridonize T, Islam L, et al. Insulin‐stimulated release of d‐chiro‐inositol‐containing inositol phosphoglycan mediator correlates with insulin sensitivity in women with polycystic ovary syndrome. Metabolism: Clinical and Experimental2008; Vol. 57, issue 10:1390‐7. [DOI] [PMC free article] [PubMed]
- Ciotta L, Stracquadanio M, Pagano I, Formuso C, Leo S, Cianci A. D‐chiro‐inositol treatment in patients with polycystic ovary syndrome. Giornale Italiano di Ostetricia e Ginecologia 2012;34(1):145‐8. [Google Scholar]
- Costantino D, Minozzi G, Minozzi F, Guaraldi C. Metabolic and hormonal effects of myo‐inositol in women with polycystic ovary syndrome: a double‐blind trial. European Review for Medical and Pharmacological Sciences 2009;13(2):105‐10. [PubMed] [Google Scholar]
- Crha I, Hruba D, Ventruba P, Fiala J, Totusek J, Visnova H. Ascorbic acid and infertility treatment. Central European Journal of Public Health 2003;11(2):63‐7. [PubMed] [Google Scholar]
- Elgindy EA, El‐Huseiny AM, Mostafa MI, Gaballah AM, Ahmed TA. N‐acetyl cysteine: could be an effective adjuvant therapy in ICSI cycles. ASRM. September 2008; Vol. 90:suppl 1. [DOI] [PubMed] [Google Scholar]; Elgindy EA, El‐Huseiny AM, Mostafa MI, Gaballah AM, Ahmed TA. N‐acetyl cysteine: could it be an effective adjuvant therapy in ICSI cycles? A preliminary study. Reproductive Biomedicine Online. 2010;20(6):789‐96. [DOI] [PubMed] [Google Scholar]
- Elnashar A, Fahmy M, Mansour A, Ibrahim K. N‐acetyl cysteine versus metformin in treatment of clomiphene citrate–resistant polycystic ovarysyndrome: a randomized study. 12th Annual Meeting of the Middle East Fertility Society. 2005; Vol. 10 Suppl 1. [Google Scholar]; Elnashar A, Fahmy M, Mansour A, Ibrahim K. N‐acetyl cysteine vs. metformin in treatment of clomiphene citrate–resistant polycystic ovarysyndrome: a prospective randomized controlled study. Fertility and Sterility 2007;88(2):407‐11. [DOI] [PubMed] [Google Scholar]; Elnashar A. Fahmy M. Mansour A. Ibrahim K. N‐acetyl cysteine vs. metformin in treatment of clomiphene citrate‐resistant polycystic ovary syndrome: a prospective randomized controlled study. Fertility and Sterility 2007;88(2):406‐9. [DOI] [PubMed] [Google Scholar]
- Farzadi L, Salman Zadeh S. Metformin‐therapy effects in 50 clomiphene citrate resistant PCOS patients. Journal of Medical Sciences 2006;6(5):765‐71. [Google Scholar]
- Firouzabadi RD, Aflatoonian A, Modarresi S, Sekhavat L, Mohammad Taheri S. Therapeutic effects of calcium & vitamin D supplementation in women with PCOS. Complementary Therapies in Clinical Practice 2012;18(2):85‐8. [DOI] [PubMed] [Google Scholar]
- Genazzani AD, Lanzoni C, Ricchieri F, Jasonni VM. Myo‐inositol administration positively affects hyperinsulinemia and hormonal parameters in overweight patients with polycystic ovary syndrome. Gynecological Endocrinology 2008;24(3):139‐44. [DOI] [PubMed] [Google Scholar]
- Hashim HA, Anwar K, El‐Fatah RA. N‐acetyl cysteine plus clomiphene citrate versus metformin and clomiphene citrate in treatment of clomiphene‐resistant polycystic ovary syndrome: a randomized controlled trial. Journal of Women's Health 2010;19(11):2043‐8. [DOI] [PubMed] [Google Scholar]
- Hebisha SA, Abdelhaleem BA, Salaam HN. Follicular fluid homocysteine with acetyl cysteine supplemented ovarian stimulation: Correlation with oocyte yield and ICSI cycle outcome. Reproductive BioMedicine Online2016; Vol. 32:S5. ; Hebisha SA, Mahmoud HM. Follicular fluid homocysteine levels with acetyl cysteine supplemented ovarian stimulation: correlation with oocyte yield and ICSI outcome. International Journal of Advanced Research 2016;4(9):1906‐9. [Google Scholar]; Hebisha SA, Omran MS, Sallam HN, Ahmed AI. Follicular fluid homocysteine levels with N‐acetyl cysteine supplemented controlled ovarian hyperstimulation, correlation with oocyte yield and ICSI cycle outcome. Fertility and Sterility2015; Vol. 104 Suppl, issue 3:e324.
- Henmi H, Endo T, Kitajima Y, Manase K, Hata H, Kudo R. Effects of ascorbic acid supplementation on serum progesterone levels in patients with a luteal phase defect. Fertility & Sterility 2003;80(2):459‐61. [DOI] [PubMed] [Google Scholar]
- Hernández‐Yero A, Santana Pérez F, Ovies Carballo G, Cabrera‐Rode E. Diamel therapy in polycystic ovary syndrome reduces hyperinsulinaemia, insulin resistance, and hyperandrogenaemia. International Journal of Endocrinology2012:382719. [DOI] [PMC free article] [PubMed]
- Immediata V, Romualdi D, Cicco S, Tagliaferri V, Florio C, Cirella E, et al. Metformin versus myoinositol: which one is better in obese PCOS patients ‐ a crossover study on clinical, endocrine and metabolic effects. Human Reproduction2014; Vol. 29 suppl 1:i334 Abstract no: P‐522.
- Iuorno MJ, Jakubowicz DJ, Baillargeon JP, Dillon P, Gunn RD, Allan G, et al. Effects of d‐chiro‐inositol in lean women with the polycystic ovary syndrome. Endocrine Practice 2002;8(6):417‐23. [DOI] [PubMed] [Google Scholar]
- Jamilian M, Bahmani F, Siavashani MA, Mazloomi M, Asemi Z, Esmaillzadeh A. The effects of chromium supplementation on endocrine profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: a randomized, double‐blind, placebo‐controlled trial. Biological Trace Element Research:2016; Vol. 172, issue 1:72‐8. [DOI] [PubMed]
- Jamilian M, Foroozanfard F, Bahmani F, Talaee R, Monavari M, Asemi Z. Effects of Zinc Supplementation on Endocrine Outcomes in Women with Polycystic Ovary Syndrome: a Randomized, Double‐Blind, Placebo‐Controlled Trial. Biological Trace Element Research2016; Vol. 170, issue 2:271‐8. [DOI] [PubMed]
- Kamencic H, Thiel JA. Pentoxifylline after conservative surgery for endometriosis: a randomized, controlled trial. Journal of Minimally Invasive Gynecology 2008;15(1):62‐6. [DOI] [PubMed] [Google Scholar]; Thiel JA, Kamencic H. Pentoxifylline (trental) after conservative surgery for endometriosis: a randomized control trial [abstract]. Journal of Minimally Invasive Gynecology 2006;(13 Suppl 5):S10. [DOI] [PubMed] [Google Scholar]
- Kilicdag EB, Bagis T, Tarim E, Aslan E, Erkanli S, Simsek E, et al. Administration of B‐group vitamins reduces circulating homocysteine in polycystic ovarian syndrome patients treated with metformin: a randomized trial. Human Reproduction2005; Vol. 20, issue 6:1521‐8. [DOI] [PubMed]
- Donne M, Alibrandi A, Giarrusso R, Lo Monaco I, Muraca U. Diet, metformin and inositol in overweight and obese women with polycystic ovary syndrome: effects on body composition. Minerva Ginecologica 2012;64(1):23‐9. [PubMed] [Google Scholar]
- Li Y, Ma H, Zhang Y, Kuang H, Ng EHY, Hou L, et al. Effect of berberine on insulin resistance in women with polycystic ovary syndrome: Study protocol for a randomized multicenter controlled trial. Trials2013; Vol. 14, issue 1:226. [DOI] [PMC free article] [PubMed]
- Moosavifar N, Mohammadpour AH, Jallali M, Karimiz G, Saberi H. Evaluation of effect of silymarin on granulosa cell apoptosis and follicular development in patients undergoing in vitro fertilization. Eastern Mediterranean Health Journal 2010;16(6):642‐5. [PubMed] [Google Scholar]
- Nazzaro A, Salerno A, Marino S, Granato C, Pastore E. The addition of melatonin to myo‐inositol plus folic acid improves oocyte quality and pregnancy outcome in IVF cycle: a prospective clinical trial. Human Reproduction 2011;26:i227‐8. [Google Scholar]
- Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G. Ovulatory and metabolic effects of D‐chiro‐inositol in the polycystic ovary syndrome. New England Journal of Medicine1999; Vol. 340, issue 17:1314‐20. [DOI] [PubMed]
- Nestler J, Gunn R, Bates S, Gregory J, Jacobson W, Rogol A. D‐chiro‐inositol (INS‐1) enhances ovulatory rate in hyperandrogenemic, oligomenorrheic women with the polycystic ovary syndrome. Fertility & Sterility2001; Vol. 76, issue 3 Suppl 1:S110‐1.
- Nichols J. A randomised controlled trial to compare conception rates for preconceptional folic acid 400 mg daily versus Pregnacare Plus in assisted conception. World Health Organization International Clinical Trials Registry Platform2010. [ISRCTN23488518]
- Nordio M, Proietti E. The combined therapy with myo‐inositol and D‐chiro‐inositol reduces the risk of metabolic disease in PCOS overweight patients compared to myo‐inositol supplementation alone. European Review for Medical & Pharmacological Sciences 2012;16(5):575‐81. [PubMed] [Google Scholar]
- Oner G, Muderris II. Clinical, endocrine and metabolic effects of metformin vs N‐acetyl‐cysteine in women with polycystic ovary syndrome. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011;159(1):127‐31. [DOI] [PubMed] [Google Scholar]
- Pal L, Zhang H, Williams J, Santoro NF, Diamond MP, Schlaff WD, et al. Vitamin D status relates to reproductive outcome in women with polycystic ovary syndrome: Secondary analysis of a multicenter randomized controlled trial. Journal of Clinical Endocrinology and Metabolism2016; Vol. 101, issue 8:3027‐35. [DOI] [PMC free article] [PubMed]
- Papaleo E, Unfer V, Baillargeon JP, Santis L, Fusi F, Brigante C, et al. Myo‐inositol in patients with polycystic ovary syndrome: a novel method for ovulation induction. Gynecological Endocrinology 2007;23(12):700‐3. [DOI] [PubMed] [Google Scholar]
- Papaleo E, De Santis, Baillargeon JP, Zacchè M, Fusi FM, Brigante C, et al. Comparison of myo‐inositol plus folic acid vs clomiphene citrate for first‐line treatment in women with polycystic ovary syndrome. Human Reproduction ESHRE 24th Annual Meeting, Barcelona, 6‐9 July 2008. 2008; Vol. 23 Suppl 1 Abstract No: O‐251 Oral. [Google Scholar]
- Pasha NG. Assessment of the effect of Ca‐vitamin D and metformin on PCOS. WHO International Clinical Trials Registry Platform Search Portal. [IRCT: IRCT201009131760N9]
- Pizzo A, Lagana AS, Barbaro L. Comparison between effects of myo‐inositol and D‐chiro‐inositol on ovarian function and metabolic factors in women with PCOS. Gynecological Endocrinology 2014;30(3):205‐8. [DOI] [PubMed] [Google Scholar]
- Raffone E, Rizzo P, Benedetto V. Insulin sensitiser agents alone and in co‐treatment with r‐FSH for ovulation induction in PCOS women. Gynecological Endocrinology 2010;26(4):275‐80. [DOI] [PubMed] [Google Scholar]
- Ruder EH, Hartman TJ, Reindollar RH, Goldman MB. Female dietary antioxidant intake and time to pregnancy among couples treated for unexplained infertility. Fertility and Sterility. United States: Elsevier Inc. (360 Park Avenue South, New York NY 10010, United States), 2014; Vol. 101, issue 3:759‐66. [DOI] [PMC free article] [PubMed]
- Salem HT, Ismail A. L‐carnitin as a treatment in clomiphene resistant PCO for improving quality of ovulation and pregnancy outcome; a novel treatment. Human Reproduction2012; Vol. 27 Suppl 2:ii302‐ii337: Abstract number: P‐529.
- Santanam N, Kavtaradze N, Dominguez C, Rock J, Partasarathy S, Murphy A. Antioxidant supplementation reduces total chemokines and inflammatory cytokines in women with endometriosis. Fertility & Sterility2003; Vol. 80, issue Suppl 3:S32‐3. Abstract no: O‐85.
- Taheri M, Modarres M, Abdollahi A. The effect of vitamin d supplementation on anti‐mullerian hormone levels in reproductive‐age women. Reproduction, Fertility and Development 2015;27:185‐6. [Google Scholar]
- Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. Journal of Pineal Research 2008;44(3):280‐7. [DOI] [PubMed] [Google Scholar]
- Twigt JM, Hammiche F, Sinclair KD, Beckers NG, Visser JA, Lindemans J, et al. Preconception folic acid use modulates estradiol and follicular responses to ovarian stimulation. Journal of Clinical Endocrinology and Metabolism 2011;96(2):E322‐9. [DOI] [PubMed] [Google Scholar]
- Vargas ML, Almario RU, Buchan W, Kim K, Karakas SE. Metabolic and endocrine effects of long‐chain versus essential omega‐3 polyunsaturated fatty acids in polycystic ovary syndrome. Metabolism 2011;60(12):1711‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JW, Kim CH, Ahn JW, Kang HJ, Song BM. Effect of omega‐3‐polyunsaturated fatty acids and metformin on ovarian morphology and intraovarian blood flow in patients with polycystic ovary syndrome. Fertility & Sterility. 2010; Vol. 94/4 Abstract no. P‐346:S194. [ISSN: 0015‐0282] [Google Scholar]
References to ongoing studies
- CTRI/2012/08/002943. Nutritional supplement for women with polycystic ovary syndrome or subfertility. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=5002&EncHid=&modid=&compid=%27%27,%27%275002det%27%27 31st August 2012.
- Fernando S, Osianlis T, Vollenhoven B, Wallace E, Rombauts L. A pilot double‐blind randomised placebo‐controlled dose‐response trial assessing the effects of melatonin on infertility treatment (MIART): study protocol. BMJ Open 2014;4(8):e005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRCT201112148408N1. Evaluation of the effects of Calcium‐ Vitamine D supplementary on ovulation and fertility outcomes of patients with poly cystic ovary syndrome referring to Infertility Clinic of Imam Khomeini Hospital in 2011 and 2012 for in vitro fertilization. Iranian Registry of Clinical Trials2012.
- NCT01019785. Vitamin D during in vitro fertilisation (IVF) ‐ a prospective randomised trial (delivery). clinicaltrials.gov/show/NCT01019785 23 November 2009. [ClinicalTrials.gov : NCT01019785]
- NCT01267604. Improving oocyte retrieval using a combined therapy of recombinant follicle stimulating hormone (rFSH) and inositol and melatonin. clinicaltrials.gov/ct2/show/NCT01267604 23rd December 2010. [NCT01267604]
- NCT01782911. Effect of Resveratol on metabolic parameters and oocyte quality in PCOS patients (RES‐IVF). clinicaltrials.gov/ct2/show/NCT01782911 28th January 2013.
- NCT02058212. Use of antioxidant in endometriotic women to improve intracytoplasmic sperm injection (ICSI). clinicaltrials.gov/ct2/show/NCT02058212 5th February 2014.
- NCT03023514. Lipoic acid supplementation in IVF. clinicaltrials.gov/ct2/show/NCT03023514 13th January 2017.
Additional references
- Agarwal A, Aponte‐Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reproductive Biology and Endocrinology 2012;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A, State O, Abdelgawad S. N‐acetyl cysteine and clomiphene citrate for induction of ovulation in polycystic ovary syndrome: a cross‐over trial. Acta Obstetricia et Gynecologica Scandinavica 2007;86(2):218‐22. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD004183.pub3] [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD007176.pub2] [DOI] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment‐seeking: potential need and demand for infertility medical care. Human Reproduction (Oxford, England) 2007;22(6):1506‐12. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Rich‐Edwards JW, Rosner BA, Willett WC. Use of multivitamins, intake of B vitamins, and risk of ovulatory infertility. Fertility and Sterility 2008;89:668‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J, Rumste M, Farquhar C, Johnson N, Mol B, Herbison P. Measuring outcomes in fertility trials ‐ can we rely on clinical pregnancy rates?. Fertility and Sterility 2010;94(5):1647‐51. [DOI] [PubMed] [Google Scholar]
- Dias S, McNamee R, Vail A. Evidence for improving quality of reporting randomized controlled trials in subfertility. Human Reproduction 2006;21(10):2617‐27. [DOI] [PubMed] [Google Scholar]
- Drugs.com. Pentoxifylline official FDA information, side effects and uses. www.drugs.com/pro/pentoxifylline.html (Accessed 26.06.13).
- Ebisch I, Thomas C, Peters W, Braat D, Steegers‐Theunissen R. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Human Reproduction Update 2007;13(2):163‐74. [DOI] [PubMed] [Google Scholar]
- Evers J. Female subfertility. Lancet 2002;360(9327):151‐9. [DOI] [PubMed] [Google Scholar]
- 2016 Global Supplement report. www.marketresearch.com/Nutrition‐Business‐Journal‐v2520/Global‐Supplement‐10063772/2016.
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 28 February 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
- Grajecki D, Zyriax B‐C, Buhling KJ. The effect of micronutrient supplements on female fertility: a systematic review. Archives of Gynecology and Obstetrics 2012;285(5):1463‐71. [DOI] [PubMed] [Google Scholar]
- Gupta S, Agarwal A, Banerjee J, Alvarez JG. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: a systematic review. Obstetrical and Gynecological Survey 2007;62(5):335‐47. [DOI] [PubMed] [Google Scholar]
- Haggarty P, McCallum H, McBain H, Andrews K, Duthie S, McNeill G, et al. Effect of B vitamins and genetics on success of in‐vitro fertilisation: prospective cohort study. Lancet 2006;367(9521):1513‐9. [DOI] [PubMed] [Google Scholar]
- Hart R. Unexplained infertility, endometriosis, and fibroids. BMJ 2003;327(7417):721‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.handbook.cochrane.org.
- Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertility and Sterility 2014;102(2):460‐8.e3. [DOI] [PubMed] [Google Scholar]
- Ko EY, Sabanegh ES Jr. The role of over‐the‐counter supplements for the treatment of male infertility‐‐fact or fiction?. Journal of Andrology 2012;33(3):292‐308. [DOI] [PubMed] [Google Scholar]
- Ledee‐Bataille N, Olivennes F, Lefaix JL, Chaouat G, Frydman R, Delanian S. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Human Reproduction 2002;17:1249‐53. [DOI] [PubMed] [Google Scholar]
- Levin I, Almog B, Shwartz T, Gold V, Ben‐Yosef D, Shaubi M, et al. Effects of laser polar‐body biopsy on embryo quality. Fertility and Sterility 2012;97(5):1085‐8. [DOI] [PubMed] [Google Scholar]
- Lu D, Song H, Li Y, Clarke J, Shi G. Pentoxifylline for endometriosis. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD007677.pub3] [DOI] [PubMed] [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Medicine 2012;9(12):e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintel. Vitamin and supplements market in good health: 46% of all Brits are daily users. www.mintel.com/press‐centre/social‐and‐lifestyle/vitamin‐and‐supplements‐market‐in‐good‐health‐46‐of‐all‐brits‐are‐daily‐users 21st September 2016.
- Nestler JE. Myo‐inositolphosphoglycans (IPGs) as mediators of insulin’s steroidogenic actions. Journal of BasicClinical Physiological Pharmacology 1998;9(2‐4):197‐204. [DOI] [PubMed] [Google Scholar]
- NHS. Supplements: who needs them? NHS choices. www.nhs.uk/news/2011/05May/Documents/BtH_supplements.pdf (Accessed 25th June 2017).
- O'Reilly E, Sevigny M, Sabarre K‐A, Phillips KP. Perspectives of complementary and alternative medicine (CAM) practitioners in the support and treatment of infertility. BMC Complementary and Alternative Medicine 2014;14:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A, Dotta A, Multigner L. Pentoxifylline and antioxidants improve sperm quality in male patients with varicocoele. Fertility & Sterility 2009;91 Suppl 4:1536‐9. [DOI] [PubMed] [Google Scholar]
- Pacis MM, Fortin CN, Zarek SM, Mumford SL, Segars JH. Vitamin D and assisted reproduction: should vitamin D be routinely screened and repleted prior to ART? A systematic review. Journal of Assisted Reproduction and Genetics 2015;32(3):323‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reportlinker.com. Vitamin and supplement industry: market research reports, statistics and analysis, 2010. www.reportlinker.com/ci02037/Vitamin‐and‐Supplement.html (Accessed 26.06.10).
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Human Reproduction Update 2008;14(4):345‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon L, Gupta S, Kim Y, Agarwal A. Female infertility and antioxidants. Current Women’s Health Reviews 2010;6:84‐95. [Google Scholar]
- Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD007411.pub2] [DOI] [PubMed] [Google Scholar]
- Showell MG, Mackenzie‐Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD007411.pub3] [DOI] [PubMed] [Google Scholar]
- Showell MG, Mackenzie‐Proctor R, Jordan V, Hodgson R, Brown J, Farquhar C. Inositol for subfertile women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD012378; CD012378] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz M, Smith C, Alino H, Norman R. The use of complementary medicine and therapies by patients attending a reproductive medicine unit in South Australia: a prospective survey. Australian and New Zealand Journal of Obstetrics and Gynaecology 2007;47(2):145‐9. [DOI: 10.1111/j.1479-828X.2007.00702.x] [DOI] [PubMed] [Google Scholar]
- Takasaki A, Tamura H, Taniguchi K, Asada H, Taketani T, Matsuoka A, et al. Luteal blood flow and luteal function. Journal of Ovarian Research 2009;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson RL, Spedding S, Buckley JD. Vitamin D in the aetiology and management of polycystic ovary syndrome. Clinical Endocrinology 2012;77(3):343‐50. [DOI] [PubMed] [Google Scholar]
- Tremellen K. Oxidative stress and male infertility ‐ a clinical perspective. Human Reproduction Update 2008;14(3):243‐58. [DOI] [PubMed] [Google Scholar]
- Unfer V, Nestler JE, Kamenov ZA, Prapas N, Facchinetti F. Effects of inositol(s) in women with PCOS: a systematic review of randomized controlled trials. International Journal of Endocrinology 2016;2016:1849162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vircheva S, Alexandrova A, Georgieva A, Mateeva P, Zamfirova R, Kubera M. In vivo effects of pentoxifylline on enzyme and non‐enzyme antioxidant levels in rat liver after carrageenan‐induced paw inflammation.. Cell Biochemistry & Function 2010;28(8):668‐72. [DOI] [PubMed] [Google Scholar]
- Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biology of Reproduction 2007;77(2):190‐201. [DOI] [PubMed] [Google Scholar]
- Zegers‐Hochschild F, Adamson GD, Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertility and Sterility 2009;92(5):1520‐4. [DOI] [PubMed] [Google Scholar]
- Zhao W, Mosley BS, Cleves MA, Melnyk S, James SJ, Hobbs CA. Neural tube defects and maternal bio markers of folate, homocysteine, and glutathione metabolism. Birth Defects Research Part A: Clinical and Molecular Teratology 2006;76(4):230‐6. [DOI: 10.1002/bdra.20240] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Clarke J, Showell MG, Hart RJ, Agarwal A, Gupta S. Antioxidants for female subfertility. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007807] [DOI] [PubMed] [Google Scholar]
- Showell MG, Brown J, Clarke J, Hart RJ. Antioxidants for female subfertility. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD007807.pub2] [DOI] [PubMed] [Google Scholar]


