Abstract
Background
Developmental co‐ordination disorder (DCD) is a common childhood disorder, which can persist into adolescence and adulthood. Children with DCD have difficulties in performing the essential motor tasks required for self‐care, academic, social and recreational activities.
Objectives
To assess the effectiveness of task‐oriented interventions on movement performance, psychosocial functions, activity, and participation for children with DCD and to examine differential intervention effects as a factor of age, sex, severity of DCD, intervention intensity, and type of intervention.
Search methods
In March 2017, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, 13 other databases, and five trials registers. We also searched reference lists, and contacted members of the mailing list of the International Conference on DCD to identify additional studies.
Selection criteria
We included all randomised controlled trials (RCTs) and quasi‐RCTs that compared the task‐oriented intervention with either an inactive control intervention or an active control intervention in children and adolescents aged four to 18 years with a diagnosis of DCD.
Types of outcome measures included changes in motor function, as assessed by standardised performance outcome tests and questionnaires; adverse events; and measures of participation.
Data collection and analysis
All review authors participated in study selection, data extraction, and assessments of risk of bias and quality, and two review authors independently performed all tasks. Specifically, two review authors independently screened titles and abstracts to eliminate irrelevant studies, extracted data from the included studies, assessed risk of bias, and rated the quality of the evidence using the GRADE approach. In cases of ambiguity or information missing from the paper, one review author contacted trial authors.
Main results
This review included 15 studies (eight RCTs and seven quasi‐RCTs).
Study characteristics
The trials included 649 participants of both sexes, ranging in age from five to 12 years.
The participants were from Australia, Canada, China, Sweden, Taiwan, and the UK.
Trials were conducted in hospital settings; at a university‐based clinic, laboratory, or centre; in community centres; at home or school, or both at home and school.
The durations of task‐oriented interventions were mostly short term (less than six months), with the total number of sessions ranging from five to 50. The length of each session ranged from 30 to 90 minutes, and the frequencies ranged from once to seven times per week.
We judged the risk of bias as moderate to high across the studies. Some elements were impossible to achieve (such as blinding of administering personnel or participants).
Key results: primary outcomes
A meta‐analysis of two RCTs and four quasi‐RCTs found in favour of task‐oriented interventions for improved motor performance compared to no intervention (mean difference (MD) ‐3.63, 95% confidence interval (CI) ‐5.88 to ‐1.39; P = 0.002; I2 = 43%; 6 trials, 169 children; very low‐quality evidence).
A meta‐analysis of two RCTs found no effect of task‐oriented interventions for improved motor performance compared to no intervention (MD ‐2.34, 95% CI ‐7.50 to 2.83; P = 0.38; I2 = 42%; 2 trials, 51 children; low‐quality evidence).
Two studies reported no adverse effects or events. Through personal correspondence, the authors of nine studies indicated that no injuries had occurred.
Key results: secondary outcomes
Due to the limited number of studies with complete and consistent data, we were unable to perform any meta‐analyses on our secondary measures or any subgroup analysis on age, sex, severity of DCD, and intervention intensity.
Authors' conclusions
We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. The conclusions drawn from previous reviews, which unanimously reported beneficial effects of intervention, are inconsistent with our conclusions. This review highlights the need for carefully designed and executed RCTs to investigate the effect of interventions for children with DCD.
Keywords: Child; Child, Preschool; Female; Humans; Male; Activities of Daily Living; Motor Skills Disorders; Motor Skills Disorders/therapy; Movement Disorders; Movement Disorders/therapy; Non‐Randomized Controlled Trials as Topic; Non‐Randomized Controlled Trials as Topic/statistics & numerical data; Randomized Controlled Trials as Topic; Randomized Controlled Trials as Topic/statistics & numerical data; Social Skills; Task Performance and Analysis
Plain language summary
Task‐oriented intervention for children with developmental co‐ordination disorder
Review question
We reviewed the evidence for the effects of interventions that aim to practise real‐life tasks on the movement skills of children with developmental co‐ordination disorder (DCD).
Background
DCD is a common childhood disorder characterised by difficulties in performing essential movement‐based activities. DCD can make it difficult for children to take care of themselves at home, do well at school, or participate in sport and leisure activities because they find it difficult to move their hands and body effectively. Their movement problems can affect their confidence and social life. Task‐oriented interventions use specific activities that are meaningful to the children and provide them with an opportunity to practise these activities to improve corresponding motor skills. This review investigated how effective task‐oriented interventions are for the movement performance, psychosocial functions, activity, and participation for children with DCD.
Study characteristics
We systematically searched for studies that examined the effect of task‐oriented interventions for children with DCD. We found 15 appropriate studies involving 649 children from five to 12 years of age with a diagnosis of DCD. The participants were from Australia, Canada, China, Sweden, Taiwan, and the UK. Trials were conducted in hospital settings; at a university‐based clinic, laboratory, or centre; in community centres; at home or school, or both at home and school. Most trials were small and of poor quality. The duration of the intervention was often short (i.e. less than six months).
Key results
We were only able to combine the results from six studies in a meta‐analysis, a statistical method to summarise the results from several independent studies. Together these studies suggest that task‐oriented interventions have a moderately positive effect on movement problems. However, the finding from the two strongest studies alone indicated that task‐oriented interventions do not improve movement problems.
We were unable to use the remaining nine included studies in a meta‐analysis because of insufficient data, or because the interventions used in the control groups (without a task‐oriented intervention) were too different to combine. As a result, we were unable to perform any meta‐analyses on many of our intended outcome measures or look at the effects of age, sex, severity of DCD, or how much intervention was received.
Two studies reported no side effects. Through email correspondence, the authors of nine studies indicated that no injuries had occurred.
Quality of the evidence
The quality of the evidence was generally low, meaning we are very uncertain about the findings of this review.
Conclusions
At the moment, task‐oriented interventions may be useful for children with DCD in improving their performance on movement tests. We cannot be sure about benefits in other areas. Higher‐quality research is needed to investigate and establish the effect of task‐oriented intervention for children with DCD.
Summary of findings
Summary of findings for the main comparison. Task‐oriented interventions versus no intervention for children with developmental co‐ordination disorder (DCD).
| Task‐oriented interventions versus no intervention for children with developmental co‐ordination disorder (DCD) | ||||||
|
Participant or population: children with DCD Settings: hospital settings; university‐based clinic, laboratory, or centre; community centres; home; and school Intervention: task‐oriented interventions Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risks with no intervention | Risks with task‐oriented interventions | |||||
|
MABC,Total Impairment Score, RCTs and quasi‐RCTs.
Scale from: 0 to 40. Follow‐up: range 6 weeks to 6 months. |
The mean MABC, Total Impairment Score in the inactive control groups ranged from 5.5 to 27.0 | The mean MABC, Total Impairment Score in the intervention group was 3.63 lower (5.88 lower to 1.39 lower) | ‐ | 169 (6 RCTs) | ⊕⊝⊝⊝ Very low1 |
Green 2008; Hillier 2010; Pless 2000b; Tsai 2009; Tsai 2012 Analysis conducted on the total impairment scores of the MABC; the higher the score, the more impaired. |
|
MABC, Total Impairment Score, RCTs only.
Scale from: 0 to 40. Follow‐up: range 6 weeks to 20 weeks. |
The mean MABC, Total Impairment Score in the inactive control groups ranged from 17.13 to 20.80 | The mean MABC, Total Impairment Score in the intervention group was 2.34 lower (7.50 lower to 2.83 higher) | ‐ | 51 (2 RCTs) | ⊕⊕⊝⊝ Low2 |
Green 2008; Hillier 2010 Analysis conducted on the total impairment scores of the MABC; the higher the score, the more impaired. |
| Adverse events | 0 events reported in both the intervention and control group. | Not estimable | 340 (11 RCTs) |
‐ | ACTRN12614000106639; Fong 2016; Green 2008; Hillier 2010; Hung 2010; Miller 2001; Pless 2000b; Sugden 2003; Thornton 2016; Tsai 2009; Tsai 2012 | |
| Changes in motor co‐ordination, as measured by standardised rating scales | 1 study used the DCDQ (Green 2008), and 2 studies used the MABC Checklist (Pless 2000b; Sugden 2003). Green 2008 reported no data on the questionnaire to be used for a randomised comparison. Pless 2000b found no significant intervention effect on the MABC Checklist, and Sugden 2003 used the MABC Checklist at pre‐intervention only, not at postintervention. | Not estimable | 111 (3 RCTs) |
⊕⊝⊝⊝ Very low3 | Important outcome to accumulate data for future evidence synthesis. | |
| Measures of impairment (e.g. sensation, physical fitness) | See comment. | ‐ | ‐ | ‐ | Not reported. Important outcome to accumulate data for future evidence synthesis. | |
| Measures of psychosocial factors | 3 studies used perceived competence scales (ACTRN12614000106639; Hillier 2010; Miller 2001). ACTRN12614000106639 used the PSPCSA and found a significant intervention effect on perceived physical competence, but no differential intervention effect between intervention setting and provider. Hillier 2010 also used the PSPCSA and found no evidence of an effect on perceived physical competence as a result of intervention. Miller 2001 used the Self Perception Profile for Children (Harter 1985) and found no significant intervention effect on the scale. It was impossible to estimate the anticipated absolute effects of the 3 studies for 2 reasons: 1. Hillier 2010 reported median and range, whereas ACTRN12614000106639 and Miller 2001 reported means and SDs; 2. Hillier 2010 and Miller 2001 used inactive controls, whereas ACTRN12614000106639 used active controls. | Not estimable | 126 (3 RCTs) |
⊕⊝⊝⊝ Very low3 | Important outcome to accumulate data for future evidence synthesis. | |
| Measures of occupational and task performance | 2 studies used the COPM and reported improved performance and satisfaction as a result of intervention (Miller 2001; Thornton 2016). Miller 2001 reported that the differential intervention effect between the CO‐OP and the contemporary treatment approach (defined as a variety of approaches) was significant on the satisfaction subscale only, which had a considerable baseline difference between the 2 groups. Thornton 2016 reported the significant pre‐post improvement in the CO‐OP group only; the change in the control group is not reported. | Not estimable | 40 (2 RCT) |
⊕⊝⊝⊝ Very low3 | Important outcome to accumulate data for future evidence synthesis. | |
| Measures of participation | 2 trials measured participation in physical activities by self‐made questionnaires only after the intervention (Hillier 2010; Pless 2000b). Hillier 2010 administered a participation questionnaire after the intervention and found no group difference between the intervention and the control group. Pless 2000b found no group difference in practising motor tasks at home. | Not estimable | 49 (2 RCTs) |
⊕⊝⊝⊝ Very low3 | Important outcome to accumulate data for future evidence synthesis. | |
| * The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CO‐OP: Cognitive Orientation to daily Occupational Performance (Missiuna 2001); COPM: Canadian Occupation Performance Measure (Law 1998); DCDQ: Developmental Coordination Disorder Questionnaire (Wilson 2009); MABC: Movement Assessment Battery for Children (Henderson 2007); PSPCSA: Pictorial Scale of Perceived Competence and Social Acceptance (Harter 1984); RCT: randomised controlled trial; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded two levels for very serious imprecision (small sample size) and one level for study limitations (concerns with allocation concealment in Green 2008, Pless 2000b, Tsai 2009, Tsai 2012, and Wilson 2016, which comprise 92.90% of the total number of participants included in the meta‐analysis). 2 Downgraded two levels for very serious imprecision (small sample size). 3 Downgraded two levels for very serious imprecision (small sample size) and one level for very serious study limitations.
Background
Description of the condition
Children with developmental co‐ordination disorder (DCD) have significant difficulty in acquiring and executing the essential, co‐ordinated motor skills required for self‐care (e.g. dressing), social and recreational activities (e.g. riding a bicycle), and academic achievement (e.g. handwriting) as compared with typically developing children of the same age (APA 2013). Additionally, the disturbance in motor skills is not explained by a lack of opportunity for skill learning and use, or by any known medical conditions, including intellectual disability or visual impairment (APA 2013).
A diagnosis of DCD is made if the child satisfies the diagnostic criteria from the Diagnostic and Statistical Manual for Mental Disorders Fifth Edition (DSM‐5; APA 2013). The assessment involves taking a developmental history, performing a clinical examination to rule out possible medical conditions, assessing the child's functional motor skills (usually through parent or teacher report), and objectively assessing the child's motor competence using a performance‐based motor assessment (Blank 2012). DCD is usually diagnosed between the ages of five and 16 years (Blank 2012), with its symptoms being recognised in the early developmental period. By definition, children with suspected DCD should be free from definite neurological conditions (Gibbs 2007); however, minor neurological dysfunctions are frequently reported in children with DCD, suggesting that early brain lesions might be causative (Hadders‐Algra 2003). Moreover, studies using brain imaging report differences in neural networks and brain activation patterns between children with DCD and children without DCD (Kashiwagi 2009; Zwicker 2010).
The prevalence of DCD has been cited as 6% of school‐aged children (APA 2013), and the male‐to‐female ratio has been reported as 1.9:1 in one UK study of seven‐year‐old children (Lingam 2009). DCD may also be referred to as clumsy child syndrome, dyspraxia (Miyahara 2000), or specific developmental disorder of motor function (WHO 2010). Currently, the DSM‐5 criteria accept comorbidities of DCD with attention‐deficit or hyperactivity disorder (or both), communication disorders, intellectual disability, and specific learning disorders (APA 2013).
DCD is included in the manual of mental disorders because of its consequential avoidance behaviours and psychosocial impacts (Spitzer 1994). The self‐esteem of children with DCD, in terms of physical competence, is diminished to a greater extent than that of children with severe physical disabilities (Miyahara 2006). They are likely to be onlookers in playgrounds, and isolated and solitary in the school yard (Smyth 2000). Rejection by their peers (Lingam 2012) can lead to children with DCD missing out on important socialisation experiences, resulting in sub‐optimal social skills (Cummins 2005). They may be easy targets for bullies (Lingam 2012; Piek 2005). Their levels of depressive symptoms and anxiety are higher than typically developing children (Schoemaker 1994; Skinner 2001), and adolescents (Cantell 1994; Skinner 2001). DCD influences children's physical functions and health status, as well as their emotional life and social participation, not only during childhood but also throughout adolescence (Losse 1991) and adulthood (Cousins 2003; Missiuna 2008). Their reduced levels of participation in physical activity (Cairney 2005) have secondary consequences, such as reduced cardiorespiratory fitness (Cairney 2006), and increased risk for obesity and coronary vascular disease (Cairney 2007). While the motor difficulties of children with DCD may appear to be less debilitating than those experienced by children with severe physical disabilities (e.g. cerebral palsy), it is the high prevalence of DCD, and its impact on children's socio‐emotional well‐being and future health status (Miyahara 2016a), that makes DCD a significant condition in need of appropriate intervention.
DCD is often measured using performance‐based and impairment‐based motor outcomes. Performance‐based measures of fine and gross motor function, such as the Movement Assessment Battery for Children (MABC; Henderson 1992; Henderson 2007) and the Bruininks Oseretsky Test of Motor Performance (BOTMP; Bruininks 1978; Bruininks 2005), assess general motor ability, which underpins activities of daily living and academic performance. These measures employ neutral tasks that vary slightly from real‐life functional tasks to avoid item bias. They are also standardised, objective, and sensitive to change. Some measures of task performance, such as the Canadian Occupational Performance Measure (COPM; Law 1990) or the Goal Attainment Scaling (GAS; Kiresuk 1968), offer a self‐report perspective of task‐related outcomes and are used to complement objective measures of task performance. Impairment‐based measures, an historic way of approaching intervention and assessment, investigate specific manifestations such as strength or sensation. It is important to cover the spectrum of theInternational Classification of Functioning, Disability and Health (ICF; WHO 2001) (impairment, activity limitations, participation restrictions) in any assessment schedule.
Description of the intervention
Existing interventions range from movement‐based therapies and education (usually provided by physiotherapists, occupational therapists, and physical educators) to pharmacology, dietary supplements, and psychological interventions such as those that address self‐concept via counselling or cognitive behavioural therapy. Traditionally, the movement‐based approaches have been classified in accordance with the emphasis of the intervention; that is, task‐oriented versus process‐oriented. Interventions that focus on the performance of specific movement tasks or 'occupations', such as tying shoelaces, ball catching, and handwriting, are collectively called task‐oriented approaches. Within the task‐oriented approaches are task‐specific training (Revie 1993), cognitive motor approach (Henderson 1992), Cognitive Orientation to daily Occupational Performance (CO‐OP; Missiuna 2001), neuromotor task training (NTT; Schoemaker 2003), and ecological intervention (Sugden 2007). The common theme of task‐oriented approaches resides in the employment of specific tasks in an attempt to improve corresponding skills. The differences between task‐oriented approaches depend on where the relative emphasis is placed, such as task‐specificity in motor skill learning (Revie 1993); the interaction between cognitive, affective, and motor competence (Henderson 2007); child‐centred cognitive strategies (Missiuna 2001); analysis of neuromotor processes underlying motor control (Schoemaker 2003); and making the task relevant and ecologically valid (Sugden 2007). In contrast, process‐oriented approaches work on the principle that there is an underlying deficit, which must be remediated before functional change can take place. One of the most popular approaches in this category is sensory integration therapy, first devised by Ayres in the 1960s, which aims to improve the effectiveness and efficiency of processing and co‐ordinating sensory information input to improve motor performance (Ayres 1979). However, there is more evidence against the effectiveness of this approach than in favour of it (Zimmer 2012). In this review, we evaluated existing research on the more recently proposed task‐oriented approaches in comparison to other process‐oriented approaches, so that consumers and professionals can make informed decisions.
How the intervention might work
Based on principles of motor control and learning, task‐oriented approaches involve concentration on the task, or group of tasks, to be mastered. In essence, they capitalise on the assumption that learning and skill acquisition is strongest when the learner understands the meaning of the training, and finds the task to be useful or relevant to his or her life. Thus, aspects of motivation and engagement are catered for, as well as the current understanding around brain plasticity, which supports the idea that learning effectiveness is enhanced when the person perceives the goal, or likely reward, as functional and beneficial (Hoerzer 2014). At the behavioural level, the intervention effects are explained in terms of the variables involved in motor learning, such as repetition, duration, intensity, frequency of practice, and the types of feedback given (Henderson 1992; Keogh 1985; Revie 1993; Schoemaker 2003). At the cognitive level, the improvement of motor skills is explained in terms of intellectual understanding of motor tasks and verbal mediation, or talking through movements in the process of perceiving stimuli, and preparing and executing movements (Cratty 1989; Henderson 1992; Missiuna 2001). The impact of incorporating ecological aspects involves adapting or manipulating the environment and context to reproduce, as closely as possible, the actual learning task environment. This ensures contextual relevance and meaning, and thus is ecologically valid to the child with the support of significant others such as parents and teachers (Sugden 2007).
Why it is important to do this review
Parents of children with DCD need a readily understandable review to help them make informed decisions about the best available interventions, as do service providers. Since the publication of earlier systematic (Hillier 2007) and meta‐analytic (Pless 2000a) reviews of the intervention effects for children with DCD, new evidence has accumulated. More recent systematic and meta‐analytic reviews included relatively new trials only and did not evaluate these data together with older evidence (Smits‐Engelsman 2013; Wilson 2013). Meta‐analytic studies considered the intervention effects of the foregoing studies altogether, rather than examining the differential intervention effects of the children's age, the environment of intervention, interventionist (Hillier 2007), and the quality of research (Miyahara 2016b). Likewise, the latest systematic and meta‐analytic review reviewed new trials only, and took no account of the quality of evidence to draw conclusions (Preston 2016). The identification of differential intervention effects, particularly the effects of high‐quality evidence derived from randomised controlled trials (RCTs), would allow service providers and consumers to make better informed decisions. It is of clinical and theoretical interest whether the intervention effects are transferred from specific intervened tasks to general motor ability.
Objectives
To assess the effectiveness of task‐oriented interventions on movement performance, psychosocial functions, activity, and participation for children with DCD and to examine differential intervention effects as a factor of age, sex, severity of DCD, intervention intensity, and type of intervention.
Methods
Criteria for considering studies for this review
Types of studies
RCTs and quasi‐RCTs.
Types of participants
Children aged four to 18 years, diagnosed with DCD, as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM), Fourth Edition (DSM‐IV; APA 1994) and Fifth Edition (DSM‐5; APA 2013), or children referred to as clumsy, physically awkward, or with dyspraxia who otherwise meet the criteria.
Types of interventions
We included studies where the intervention was described as task‐oriented and formally required practise of a specific task or occupation as the principal form of intervention. This included task‐specific training, cognitive motor approach, ecological Intervention, NTT, and CO‐OP. If a trial intervention appeared to be task‐oriented but was not formally labelled as such, we included it if all review authors agreed that it complied with the definition as stated.
We included studies that compared the task‐oriented intervention with either an inactive control intervention (e.g. usual care or a waiting‐list control), or an active control intervention (e.g. a process‐oriented approach such as sensory integration therapy (Ayres 1979), pharmacology, counselling, or dietary advice).
Types of outcome measures
We considered both movement performance and impairment‐based measures to examine changes in fine and gross motor function following intervention.
Primary outcomes
-
Changes in fine and gross motor function following intervention as measured by standardised performance outcome tests such as the following.
Bruininks Oseretsky Test of Motor Performance (BOTMP; Bruininks 1978; Bruininks 2005).
McCarron Assessment of Neuromuscular Development (MAND; McCarron 1997).
Movement Assessment Battery for Children Test (MABC; Henderson 1992).
Test of Gross Motor Development (TGMD; Ulrich 1985; Ulrich 2000).
Adverse effects or events: By the very nature of task‐oriented interventions, everyday tasks are performed under closer than usual scrutiny or supervision, or both, and therefore are assumed to be safer than those encountered in everyday life. However, we searched for any reports of adverse events that conceivably could include musculoskeletal injury, falls, or pain.
Secondary outcomes
-
Changes in fine and gross motor function following intervention as assessed by the following.
Changes in motor co‐ordination, as measured by standardised rating scales based on parent and teacher report such as the Developmental Coordination Disorder Questionnaire (DCDQ; Wilson 2009), or the Movement Assessment Battery for Children ‐ Checklist (MABC‐C; Henderson 2007).
Measures of impairment (e.g. sensation as measured by tests such as stereognosis or pressure detection; muscle strength as measured by tests such as one repetition maximum; or co‐ordination as measured by tests such as the Purdue Pegboard).
Measures of psychosocial factors (self‐esteem, self‐concept) such as the Perceived Competence Scale for Children (PCSC; Harter 1982), the Pictorial Scale for Perceived Competence and Social Acceptance for Young Children (PSPCSA; Harter 1984).
Measures of occupational and task performance such as the Canadian Occupational Performance Measure (COPM; Law 1998), and the Goal Attainment Scaling (GAS; McDougall 1999).
Measures of participation (academic level, sporting participation, recreation) such as the Assessment of Life Habits Scale (LIFE‐H; Fougeyrollas 1998), or teacher and family reports of level of participation.
Search methods for identification of studies
Electronic searches
We searched the electronic databases and trials registers listed below in August 2014, applying no language or date limits, and re‐ran the searches in April 2016 and March 2017. The search strategies for each database are reported in Appendix 1. Additional search details, including the exact search dates for each source, are reported in Appendix 2.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 3) in the Cochrane Library, which contains the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 5 April 2017).
MEDLINE Ovid (1946 to March Week 4 2017).
MEDLINE Epub Ahead of Print Ovid (3 April 2017).
MEDLINE In‐Process & Other Non‐Indexed Citations (3 April 2017).
Embase Ovid (1974 to 2017 Week 14).
ERIC ProQuest (Education Resources Information Center; 1966 onwards; searched 5 April 2017).
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to current; searched 4 April 2017).
PsycINFO Ovid (1806 to April Week 2 2017).
Science Citation Index ‐ Expanded Web of Science (SCI‐Expanded; 1970 to 31 March 2017; searched 4 April 2017).
Social Sciences Citation Index Web of Science (SSCI; 1970 to 31 March 2017; searched 4 April 2017).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1990 to 31 March 2017; searched 4 April 2017).
Conference Proceedings Citation Index ‐ Social Science & Humanities Web of Science (CPCI‐SS&H; 1990 to 31 March 2017; searched 4 April 2017).
Cochrane Database of Systematic Reviews (CDSR; 2017, Issue 4), in the Cochrane Library (searched 5 April 2017).
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2), in the Cochrane Library (searched 5 April 2017).
ProQuest Dissertations & Theses: UK & Ireland (searched 5 April 2017).
WorldCat (worldcat.org; searched 4 April 2017).
ClinicalTrials.gov (clinicaltrials.gov; searched 31 March 2017).
metaRegister of Controlled Trials (www.controlled‐trials.com; searched 18 June 2015). This service was under review in 2016 and 2017.
ISRCTN registry (isrctn.com; searched 31 March 2017).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch; searched 31 March 2017).
Australian New Zealand Clinical Trials Registry (ANZCTR; anzctr.org.au; searched 31 March 2017).
Searching other resources
We distributed an email to members of the International Society for Research on DCD and asked them to provide any unpublished studies (including studies written in languages other than English) that met our inclusion criteria (see Criteria for considering studies for this review). We also searched the reference lists of relevant papers found by the literature search. We further searched relevant websites identified by international experts, such as advocacy groups or education resource listings, that may have identified unpublished trials.
Data collection and analysis
Two review authors (MM and SLH) independently assessed all identified studies for inclusion, extracted data, and assessed risk of bias. Both review authors resolved disagreements by discussion or with mediation with the third (LP) and the fourth (SN) review authors.
Selection of studies
Two review authors (MM and SLH) screened the titles and abstracts of all records retrieved by the search, and excluded those that were clearly irrelevant. We retrieved the full‐text papers for the remaining records and determined final inclusion. Any disagreements were resolved by discussion. We recorded our decisions in a PRISMA diagram (Moher 2009; Figure 1).
1.
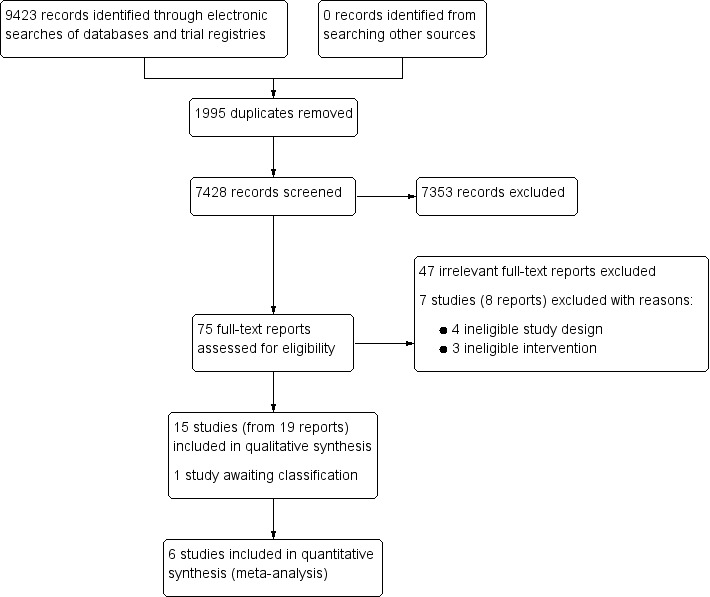
Study flow diagram.
Data extraction and management
Using the ERC data collection form (Version 3, April 2014), two review authors (MM and SLH) independently extracted data from included trials on the following.
Methods: including aim, design, and unit of allocation.
Participants: including inclusion or exclusion criteria (or both), number randomised, withdrawals and exclusion, and sample characteristics.
Intervention: type of intervention (e.g. NTT or CO‐OP), mode of delivery (individual or group), personnel (health, education, or non‐trained staff), location (clinic, hospital, school, home), duration, frequency, and intensity.
Outcomes: including time points measured, unit of measurement, and power.
Risk of bias assessment: including details of sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, and selective outcome reporting.
Data and analysis: including length of follow‐up, loss to follow‐up, unit of analysis, and statistical methods used.
Other: source of funding and possible conflicts of interest.
To assess the effects of the intervention, we extracted data for outcomes of interest (means and standard deviations for continuous outcomes), where available in the published reports. If unavailable, the first review author (MM) contacted the authors of the original studies to request the data. Two review authors (MM and SN) entered and verified the data extracted from each study into Review Manager 5 (Review Manager 2014), resolving any inconsistencies by discussion.
Assessment of risk of bias in included studies
Two review authors (MM and SLH for the initial search in August 2014 and MM and LP for the second and third search in April 2016 and March 2017) independently assessed the risk of bias for each study and overall risk of bias, using Cochrane's 'Risk of bias' tool (Higgins 2011a). We assessed the risk of bias for each included study against key criteria: random sequence generation, allocation concealment, blinding of participants and personnel (impossible given the nature of the intervention), blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias. We also contacted study authors if no outcome data relevant to the primary or secondary outcomes of the review had been published for a trial (Kirkham 2010). We explicitly judged each of the foregoing domains as low risk of bias, high risk of bias, or unclear risk of bias (either lack of information or uncertainty over the potential for bias). We reported the full 'Risk of bias' assessment and whether or not it reduced our confidence in the analysed effects. When the risk of bias of the individual included studies was high, we interpreted the results of the studies with caution.
Measures of treatment effect
We performed all statistical analyses using Review Manager 5 (Review Manager 2014).
Continuous outcome data
We analysed continuous outcomes measured on the same scale between trials (e.g. MABC; Henderson 1992) using the mean difference (MD) with 95% confidence intervals (CIs). In the case of no relevant information being available, we requested information from the authors of the original studies.
See our protocol, Miyahara 2014, and Differences between protocol and review for methods to analyse outcomes measured on different scales archived for use in future updates of this review.
Multiple outcome data
For studies with postintervention data at multiple time points, we extracted the MD from both postintervention and follow‐up phases. We considered the immediate postintervention data as the primary outcome data.
Dichotomous data
Only one trial reported dichotomous data (Pless 2000b), which were divided into definite motor difficulties (MABC scores < 5th percentile) and borderline motor difficulties (MABC scores between 5th and 15th percentile).
See our protocol, Miyahara 2014, and Differences between protocol and review section for methods to handle dichotomous data archived for use in future updates of this review.
Unit of analysis issues
For cross‐over trials (i.e. Green 2008), we extracted outcome data from the first phase of the intervention period only, to avoid carry‐over effects (Higgins 2011b). See Differences between protocol and review.
See our protocol, Miyahara 2014, and Differences between protocol and review for methods to analyse cluster‐randomised trials archived for use in future updates of this review.
Dealing with missing data
When we found any missing, inconsistent, or incomplete data (e.g. missing outcomes, missing summary data, missing study characteristics), the first review author (MM) contacted the authors of the original studies to request the data. We recorded all relevant information on missing data and dropouts for each study as part of our 'Risk of bias' assessment. We also examined the reasons for missing data and dropouts, and took those reasons into account when drawing conclusions.
Assessment of heterogeneity
MM, SLH, and LP (each of whom has clinical experience) assessed clinical heterogeneity, evaluating the variability across study participants; for example, age, sex, severity of DCD, interventions (frequency, duration, types), and outcomes (types).
SLH and SN assessed methodological heterogeneity by evaluating variability in research designs and risk of bias. We examined differences in effect size between studies that used adequate randomisation, allocation concealment, and blinding, and the studies that did not perform them adequately. We also grouped the reviewed studies into high and low risk of bias groups, and evaluated the difference in effect sizes.
We identified statistical heterogeneity by visual inspection of the forest plots, and by using the Chi2 test and I2 statistic. We also reported Tau2, which is an estimate of between‐study variability, when reporting the results of the random‐effects meta‐analysis. We used a P value of 0.10 to determine statistical significance of the Chi2 test for a small sample size (Deeks 2011). We evaluated the importance of the I2 statistic by an observed I2 value greater than 40%, the magnitude and direction of effects, and the evidence for heterogeneity from the Chi2 test.
Assessment of reporting biases
We were unable to conduct visual assessment of funnel plot asymmetry to identify possible publication bias (Sterne 2011), because our meta‐analysis contained fewer than 10 studies. See Miyahara 2014 and Differences between protocol and review.
Data synthesis
We used random‐effects meta‐analyses to combine MDs using Review Manager 5 (Review Manager 2014). We conducted sensitivity analyses using a fixed‐effect model (Sensitivity analysis). See Differences between protocol and review.
'Summary of findings' table
We presented our results for the following outcomes in a 'Summary of findings' table (Schünemann 2011), constructed by one review author (MM): changes in fine and gross motor function, changes in motor co‐ordination, psychosocial factors, occupation and task performance, and adverse effects or events (see Types of outcome measures).
Using the GRADE approach (Schünemann 2011), two review authors (MM and SLH) independently assessed the quality of the evidence for all but two studies (ACTRN12614000106639 and Hillier 2010, which were assessed by MM and LP), resolving any discrepancies by discussion.
Subgroup analysis and investigation of heterogeneity
We were unable to find sufficient studies to perform any subgroup analysis (see Miyahara 2014; Differences between protocol and review).
Sensitivity analysis
Due to the small number of eligible studies, we were unable to conduct our preplanned sensitivity analyses, which have been archived for use in future updates of this review (see Miyahara 2014). However, we did conduct the following post hoc sensitivity analyses to:
compare two sets of task‐oriented intervention studies (one set combining RCT and quasi‐RCT studies and the second set of RCT studies only); and
test the robustness of the results from the random‐effects model compared to the fixed‐effect model.
Results
Description of studies
For more information, see Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification tables.
Results of the search
We ran the searches for this review in August 2014, April 2016, and March 2017. Our searches yielded a total of 9423 records. After removing 1995 duplicates, we screened 7428 records for eligibility (see Criteria for considering studies for this review). We excluded 7353 irrelevant records based on their titles and abstracts. We retrieved the full‐text reports of the remaining 75 records and excluded 55. We provided reasons for exclusion for seven studies (from eight reports), as they appeared initially to meet our criteria (Excluded studies). We identified 15 studies (from 19 reports), which met our criteria, and one study awaiting classification. See Figure 1.
Included studies
This review included 15 studies, of which eight were RCTs (ACTRN12614000106639; Au 2014; Fong 2016; Green 2008; Hillier 2010; Hung 2010; Miller 2001; Sugden 2003), and seven were quasi‐RCTs (Fong 2012; Pless 2000b; Tsai 2009; Tsai 2012; Thornton 2016; Wilson 2002; Wilson 2016). See Characteristics of included studies table.
We contacted all 15 authors of the included studies requesting further information, and 10 responded (ACTRN12614000106639; Green 2008; Hillier 2010; Hung 2010; Miller 2001; Pless 2000b; Sugden 2003; Tsai 2009; Tsai 2012; Thornton 2016).
Duration
All trials were short term (less than six months in duration). The length of each session ranged from 30 minutes (Hillier 2010) to 90 minutes (Fong 2016). The frequency of sessions ranged from one (ACTRN12614000106639; Au 2014; Fong 2012; Green 2008; Hillier 2010; Hung 2010; Pless 2000b; Thornton 2016; Wilson 2002; Wilson 2016) to seven (Sugden 2003) sessions per week. The total number of sessions ranged from five (Wilson 2002; Wilson 2016) to 50 (Tsai 2012).
Location
Five trials were conducted in Australia (ACTRN12614000106639; Hillier 2010; Thornton 2016; Wilson 2002; Wilson 2016), four in China (Au 2014; Fong 2012; Fong 2016; Hung 2010), two in Taiwan (Tsai 2009; Tsai 2012), two in the UK (Green 2008; Sugden 2003), and one trial each in Canada (Miller 2001) and Sweden (Pless 2000b).
Setting
Four trials were conducted in hospital settings (Au 2014; Fong 2012; Hillier 2010; Hung 2010), five at a university‐based clinic, laboratory, or centre (ACTRN12614000106639; Fong 2016; Miller 2001; Pless 2000b; Wilson 2002), two in community centres (Fong 2012; Green 2008), and four at home or school, or both (ACTRN12614000106639; Sugden 2003; Tsai 2009; Tsai 2012). Two trials provided no information on the setting (Thornton 2016; Wilson 2016).
Participants
The eight RCTs included 332 children (with the reported percentages of girls ranging from 14% to 32% (mean 27%), equivalent to a boy:girl ratio of 3:1). All children were aged between five and 12 years.
The seven quasi‐RCTs included 317 children (with the reported percentages of girls ranging from 0% to 55% (mean 24%), equivalent to a boy:girl ratio of 3:1). All participants were aged between five and 10 years. Note, two studies (Wilson 2002, Wilson 2016) did not provide a breakdown by sex, hence the boy:girl ratio is based on five studies only.
Nine of the 15 studies performed sample power calculations (ACTRN12614000106639; Au 2014; Fong 2012; Fong 2016; Green 2008; Hillier 2010; Hung 2010; Thornton 2016; Wilson 2016).
Interventions
Eight trials used a motor skill intervention (ACTRN12614000106639; Au 2014; Fong 2016; Hung 2010; Pless 2000b; Sugden 2003; Wilson 2002; Wilson 2016), four trials used a specific sport training (Fong 2012; Hillier 2010; Tsai 2009; Tsai 2012), and three trials used the CO‐OP (Green 2008; Miller 2001; Thornton 2016).
Ten trials used inactive controls (Fong 2012; Fong 2016; Green 2008; Hillier 2010; Pless 2000b; Thornton 2016; Tsai 2009; Tsai 2012; Wilson 2002; Wilson 2016), and seven trials used active controls (ACTRN12614000106639; Au 2014; Hung 2010; Miller 2001; Sugden 2003; Wilson 2002; Wilson 2016).
Outcomes
Primary outcomes
The most common measure was the MABC, used in 10 trials (Fong 2016; Green 2008; Hillier 2010; Hung 2010; Pless 2000b; Sugden 2003; Tsai 2009; Tsai 2012; Wilson 2002; Wilson 2016). Two trials used the MABC‐2; one used it as a screening tool but not as an outcome measure (Fong 2012) and the other used it as an outcome measure (Thornton 2016). One trial used the BOTMP (Miller 2001), and one trial used the BOTMP, Second Edition (Au 2014). Two trials (Green 2008; Miller 2001) used the Development Test of Visual‐Motor Integration ‐ Revised (Beery 1997).
Two studies reported no adverse effects or events (Fong 2016; Hung 2010). When we asked the authors of the other included studies whether any injuries had occurred during the intervention, nine authors responded and reported that no injuries had occurred (ACTRN12614000106639; Green 2008; Hillier 2010; Miller 2001; Pless 2000b; Sugden 2003; Thornton 2016; Tsai 2009; Tsai 2012).
Secondary outcomes
Three trials measured psychosocial factors using perceived competence scales (Hillier 2010; Miller 2001; Pless 2000b), two trials used the MABC‐C (Sugden 2003; Wilson 2002), and two trials used the COPM (Miller 2001; Thornton 2016).
Two trials measured participation in physical activities (Hillier 2010; Pless 2000b).
Excluded studies
We read 75 full‐text reports and excluded 47 as clearly irrelevant. We formally excluded seven studies (eight reports); four on the basis of ineligible study type and three on the basis of ineligible intervention. See Characteristics of excluded studies for further details.
Studies awaiting classification
We identified one study in which both the participants and the intervention met the criteria for this review, but the method of randomisation was unclear (Farhat 2016). We contacted the first author of the study for clarification but received no response at the time of publication of this review. See Characteristics of studies awaiting classification.
Risk of bias in included studies
We assessed the risk of bias of each included trial using Cochrane's 'Risk of bias' tool (Higgins 2011b). A summary of our assessment is shown in Figure 2 and Figure 3.
2.
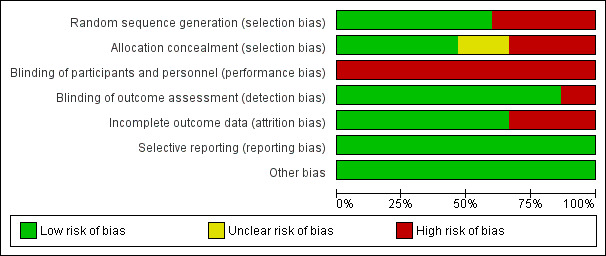
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
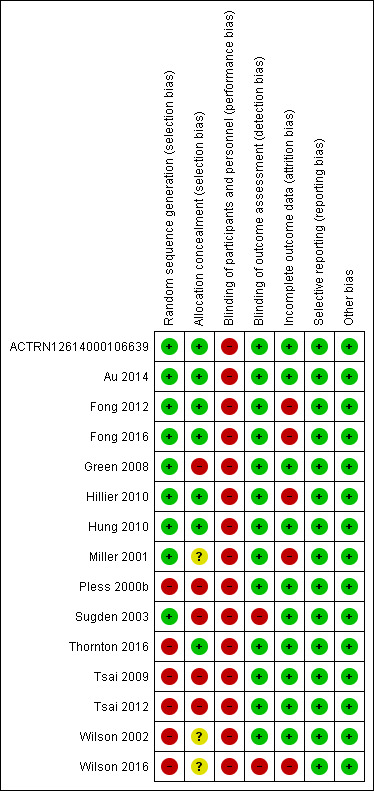
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Overall, we judged the included studies to have moderate to high risk of bias. We rated the RCTs by ACTRN12614000106639, Au 2014, and Hung 2010 to be at low risk of bias in all domains except for performance bias. We judged the remaining RCTs (Fong 2016; Green 2008; Hillier 2010; Miller 2001; Sugden 2003) to be at lower risk of bias than the seven quasi‐RCTs (Fong 2012; Pless 2000b; Thornton 2016; Tsai 2009; Tsai 2012; Wilson 2002; Wilson 2016), which had higher risk of bias due to issues in their blinding and randomisation.
Allocation
Random sequence generation
We judged nine trials, which specified acceptable methods of random sequence generation, to be at low risk bias for random sequence generation (ACTRN12614000106639; Au 2014; Fong 2012; Fong 2016; Green 2008; Hillier 2010; Hung 2010; Miller 2001; Sugden 2003). We judged six trials to be at high risk of bias: four (unblinded) trials used blocked randomisation (Pless 2000b; Thornton 2016; Wilson 2002; Wilson 2016) and two trials broke the generated sequences (Tsai 2009; Tsai 2012).
Allocation concealment
We rated seven trials, which specified the method of allocation concealment, to be at low risk of bias for allocation concealment (ACTRN12614000106639; Au 2014; Fong 2012; Fong 2016; Hillier 2010; Hung 2010; Thornton 2016), five trials, which did not conceal allocation appropriately to be at high risk of bias (Green 2008; Pless 2000b; Sugden 2003; Tsai 2009; Tsai 2012), and three trials to be at unclear risk of bias because the authors did not respond to our inquiries about allocation concealment (Miller 2001; Wilson 2002; Wilson 2016).
Blinding
Performance bias
We considered all trials to be at high risk of performance bias due to the nature of movement‐based interventions (ACTRN12614000106639; Au 2014; Fong 2012; Fong 2016; Green 2008; Hillier 2010; Hung 2010; Miller 2001; Pless 2000b; Sugden 2003; Thornton 2016; Tsai 2009; Tsai 2012; Wilson 2002; Wilson 2016). A movement‐based intervention is obvious to participants who are receiving it, and so the blinding of participants is impossible. The blinding of personnel may be feasible if participants with DCD are mixed with participants without DCD.
Detection bias
We considered 13 trials, which employed independent assessors, to be at low risk of detection bias (ACTRN12614000106639; Au 2014; Fong 2012; Fong 2016; Green 2008; Hillier 2010; Hung 2010; Miller 2001; Pless 2000b; Thornton 2016; Tsai 2009; Tsai 2012; Wilson 2002), and two trials, which used interventionists to conduct the assessment, to be at high risk of detection bias (Sugden 2003; Wilson 2016).
Incomplete outcome data
We considered attrition bias to be high in five trials as the attrition rate was larger than 10% or the intervention period was extended to complete the planned number of intervention sessions (Fong 2012; Fong 2016; Hillier 2010; Miller 2001; Wilson 2016), and low (less than 10% attrition rate) in all remaining trials (ACTRN12614000106639; Au 2014; Green 2008; Hung 2010; Pless 2000b; Sugden 2003; Thornton 2016; Tsai 2009; Tsai 2012; Wilson 2002).
Selective reporting
There were protocols for three trials (Au 2014 (NCT01207544); ACTRN12614000106639 (ACTRN12614000106639); and Fong 2016 (NCT02393404)). We compared the outcomes listed in the protocols with those described in the Results section of the reports. For the remaining studies, we compared outcomes listed in the Methods section of the reports with those described in the Results section. Eleven studies reported all outcomes listed in the Methods section, and we assumed that the reports included all prespecified variables (Fong 2012; Hillier 2010; Hung 2010; Miller 2001; Pless 2000b; Sugden 2003; Thornton 2016; Tsai 2009; Tsai 2012; Wilson 2002; Wilson 2016). One study did not report several secondary outcomes listed in the Methods section (Green 2008), such as Matrix Analogies Test and DCDQ, but the unreported outcomes were mentioned adequately. Consequently, we judged all trials to be at low risk of reporting bias.
Other potential sources of bias
We identified no other sources of bias and so judged all trials to be at low risk of bias on this domain (ACTRN12614000106639; Au 2014; Fong 2012; Fong 2016; Green 2008; Hillier 2010; Hung 2010; Miller 2001; Pless 2000b; Sugden 2003; Thornton 2016; Tsai 2009; Tsai 2012; Wilson 2002; Wilson 2016).
Effects of interventions
See: Table 1
We assessed all 15 trials for methodological quality and of these included six trials in a meta‐analysis (Green 2008; Hillier 2010; Pless 2000b; Tsai 2009; Tsai 2012; Wilson 2016). As the trial by Green 2008 was a cross‐over trial, we used data from the first phase only, treating it as an RCT with inactive control. We were unable to use the remaining nine studies in a meta‐analysis for the following reasons: five had active controls (ACTRN12614000106639; Au 2014; Hung 2010; Miller 2001; Sugden 2003); one had no primary outcome data (Fong 2012); and three reported no mean or standard deviations, or both (Fong 2016; Thornton 2016; Wilson 2002). Only Thornton 2016 responded to our requests for information.
In the following, we present the results of meta‐analyses performed for one primary outcome: MABC total score. For a summary of key results, see Summary of main results.
Comparison 1: task‐oriented intervention versus no intervention
Primary outcomes
We were able to combine data on MABC scores from six trials (Green 2008; Hillier 2010; Pless 2000b; Tsai 2009; Tsai 2012; Wilson 2016). We presented the results of the random‐effects model only, and tested the robustness of the results by conducting sensitivity analysis with the fixed‐effect model.
A random‐effects meta‐analysis of both RCTs and quasi‐RCTs suggested that task‐oriented interventions significantly improved motor co‐ordination compared to no intervention (MD ‐3.63, 95% CI ‐5.88 to ‐1.39; P = 0.002; I2 = 43%, Tau2 = 3.24, 6 studies, 169 participants; Analysis 1.1). We downgraded the quality of evidence from high to very low due to high risk of bias, including broken randomisation, and imprecision (small number of participants). Although the I2 was 43%, the directions of the effects were consistently positive, and the P value from the Chi2 test was not significant, meaning high uncertainty for the I2 value. Therefore, we did not downgrade for inconsistency. See Table 1.
1.1. Analysis.
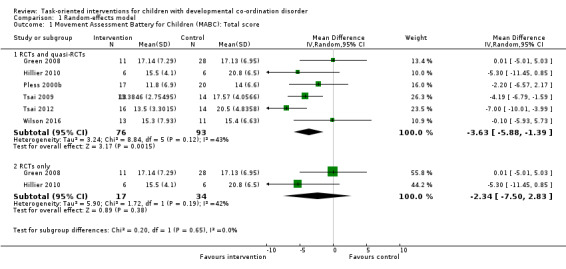
Comparison 1 Random‐effects model, Outcome 1 Movement Assessment Battery for Children (MABC): Total score.
For a sensitivity analysis, we excluded four quasi‐RCTs and conducted a meta‐analysis of the two RCTs only. The meta‐analysis of RCTs alone did not find in favour of task‐oriented interventions to significantly improve motor co‐ordination compared to no intervention (MD ‐2.34, 95% CI ‐7.50 to 2.83; P = 0.38; I2 = 42%, Tau2 = 5.90; 2 trials, 51 participants; Analysis 1.1). We downgraded the quality of evidence from high to low due to imprecision (small number of participants). Although the I2 was 42%, the directions of the effects were consistently positive, and the P value from the Chi2 test was not significant, meaning high uncertainty for the I2 value. Therefore, we did not downgrade for inconsistency. See Table 1.
Secondary outcomes
The included studies assessed some secondary outcomes, such as the MABC‐C assessed in three studies (Pless 2000b; Sugden 2003; Wilson 2002), perceived competence assessed in two studies (Hillier 2010; Miller 2001), and the COPM assessed in three studies (Green 2008; Miller 2001; Thornton 2016). However, there was a high level of methodological heterogeneity or insufficient reporting across these studies, or both, and therefore quantitative synthesis could not be undertaken. For example, only Pless 2000b reported MABC‐C data in forms of medians and ranges, which yielded no significant change after intervention. We did not perform a meta‐analysis on the data of perceived competence by Hillier 2010 and Miller 2001 because one study had an active control (Miller 2001) and the other had an inactive control group (Hillier 2010). Miller 2001 found no intervention effect on perceived competence and Hillier 2010 reported no evidence of an effect on perceived physical competence in the intervention group as compared to the inactive control group. With regard to the COPM, Thornton 2016 reported no mean or standard deviations. Miller 2001 reported improved performance and satisfaction as a result of intervention, but the differential intervention effect between the CO‐OP and the contemporary treatment approach (CTA; defined as a variety of approaches) was significant on satisfaction only. Green 2008 reported no data on the COPM. In summary, none of the secondary outcomes detected significant improvement after the task‐oriented intervention.
Comparison 2: task‐oriented intervention versus active control intervention
Five trials employed active control interventions (ACTRN12614000106639; Au 2014; Hung 2010; Miller 2001; Sugden 2003). Four studies used task‐oriented intervention for active controls but delivered in different ways (CO‐OP versus CTA in Miller 2001; home versus school in Sugden 2003; individual versus group in Hung 2010; school versus health clinic in ACTRN12614000106639). Au 2014 used a task‐oriented motor programme as an active control for a core stability programme. None of the 15 included studies employed a process‐oriented approach, to examine the relative effect of task‐oriented intervention versus non‐task‐oriented intervention.
Subgroup and sensitivity analyses
There were insufficient data to carry out our planned subgroup analyses (see Subgroup analysis and investigation of heterogeneity), or our planned sensitivity analyses to explore the impact of study quality (Sensitivity analysis). See Differences between protocol and review. Instead, we performed limited sensitivity analyses by 1. using the fixed‐effect model for pooling; 2. comparing a combination of two RCTs (Green 2008; Hillier 2010) and four quasi‐RCTs (Pless 2000b; Tsai 2009; Tsai 2012; Wilson 2016) versus RCTs only (i.e. excluding the quasi‐RCTs).
The results were similar whether the effect was analysed by random‐effects model (Analysis 1.1) or by fixed‐effect model (Analysis 2.1). As in the case of the analysis by random‐effects model (Analysis 1.1; Figure 4), the fixed‐effect meta‐analysis of both RCTs and quasi‐RCTs indicated that the intervention effect remained statistically significant (MD ‐4.06, 95% CI ‐5.63 to ‐2.50; P < 0.001; I2 = 43%; 6 trials, 169 participants; Analysis 2.1; Figure 5). However, when the four quasi‐RCTs were excluded, the fixed‐effect meta‐analysis indicated that the effect of the task‐oriented intervention was not statistically significant (MD ‐2.11, 95% CI ‐6.00 to 1.78; P = 0.29; I2 = 42%; 2 trials, 51 participants; Analysis 2.1; Figure 5), in keeping with the results of the random‐effects model (Analysis 1.1; Figure 4).
2.1. Analysis.
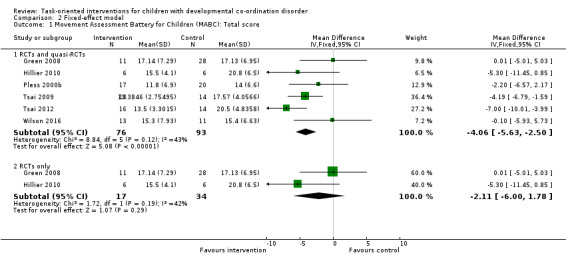
Comparison 2 Fixed‐effect model, Outcome 1 Movement Assessment Battery for Children (MABC): Total score.
4.
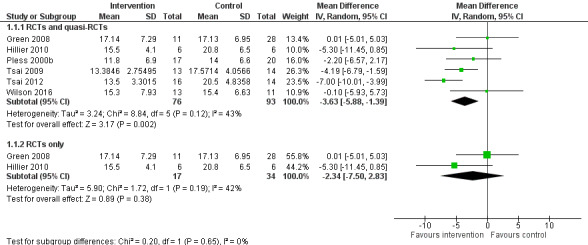
Forest plot of comparison: 1 Random‐effects model, outcome: 1.1 Movement Assessment Battery for Children (MABC): Total score.
5.
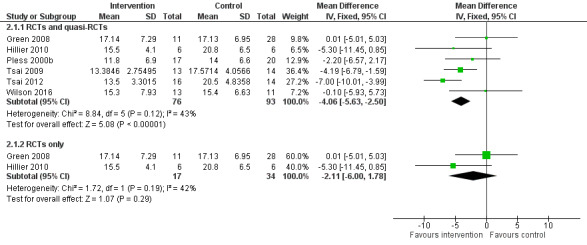
Forest plot of comparison: 3 Fixed model, outcome: 2.1 Movement Assessment Battery for Children Total.
In summary, the intervention effect from a combination of RCTs and quasi‐RCTs differed from the intervention effect from a group of RCTs only in that the former effect was statistically significant, whereas the latter effect was not significant.
Discussion
Summary of main results
The present systematic review included 15 studies (eight RCTs and seven quasi RCTs) that assessed the effectiveness of task‐oriented interventions for children with DCD. Of the 74 full‐text reports deemed potentially relevant for inclusion in this review, we excluded 54 because they did not meet the selection criteria of RCTs or quasi‐RCTs, task‐oriented interventions, or outcome measures (i.e. use of movement performance or impairment‐based measures) (see Criteria for considering studies for this review). Of the 15 studies included in the review, we were able to combine data from six studies (two RCTs and four quasi‐RCTs) in a meta‐analysis of the primary outcome: MABC scores. We found an effect of four points in favour of the task‐oriented interventions on the MABC raw score. This may represent a clinically significant difference.
We found similar results whether the effects were computed using a random‐effects model or a fixed‐effect model. When we conducted the analysis using only the two RCTs, we detected little evidence for a difference between task‐oriented intervention and inactive control, and the 95% CIs for the MD from both types of models were very large. We judged the risk of bias to be moderate to high and the quality of the evidence to be low to very low. Therefore, we are very uncertain about the findings of this review.
The included studies reported the secondary outcomes of the MABC‐C, perceived competence, and the COPM. However, the small numbers of studies were reported in ways that did not allow a quantitative synthesis. A narrative review detected no significant effect of task‐oriented interventions on the MABC‐C, perceived competence, and the COPM.
Overall completeness and applicability of evidence
This review highlights two major issues regarding the overall completeness and applicability of the evidence for the effects of task‐oriented intervention for children with DCD:
the small number of included trials did not allow subgroup analysis on age, sex, severity of DCD, intervention intensity, and type of intervention; and
the small number of included trials reporting the secondary outcome measures of psychosocial functions, activity, and participation for children with DCD.
Taken together, the identified studies are not sufficient to definitively address the objectives of the review. The implication from the results of the review for current practice is that task‐oriented intervention may or may not improve motor skills more than an inactive control, as assessed by the MABC. Although there are intervention studies that report benefits for psychosocial functions, activity, and participation, the quality of evidence is very low.
The generalisability of these findings to adolescents and adults with DCD and to non‐research settings is limited because the included studies recruited participants aged only between five and 12 years and task‐oriented interventions were conducted mostly in research settings.
Quality of the evidence
Using the GRADE approach (Schünemann 2011), we assessed the quality of evidence from the RCTs only as low, because of high risk of bias (including lack of blinding due to impossibility of blinding participants) and imprecision (small number of participants), and the evidence from the combination of two RCTs and four quasi‐RCTs as very low, because of high risk of bias (broken randomisation, loss of blinding due to impossibility of blinding participants), and imprecision (small number of participants). These ratings imply a low degree of confidence and uncertainty in the intervention effects.
We also rated the quality of evidence for the measures of psychosocial functions, occupational and task performance, and participation as very low due to the floor effect of the GRADE approach (Schünemann 2011). In fact, the quality of evidence for these outcomes was far more limited than that of the four quasi‐RCTs, in terms of higher risk of bias due to problems with the research design (non‐RCT) and imprecision (smaller number of participants).
See Table 1.
Potential biases in the review process
To minimise bias, we developed a protocol for the review (see Miyahara 2014), according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). We conducted extensive searches of relevant databases and websites, and requested unpublished studies from the International Conference on DCD. Two review authors independently selected studies, extracted data, assessed the risk of bias of each included trial using Cochrane's 'Risk of bias' tool (Higgins 2011a), and graded the quality of the evidence using the GRADE approach (Schünemann 2011).
Trial reporting was often minimal; we attempted to clarify points that were unclear or absent, and obtain any missing, inconsistent or incomplete data by contacting study authors. We excluded one study, Fong 2016, from the meta‐analysis as we were unable to contact the author to obtain the necessary data.
One review author (SLH) was involved in two of the included trials (ACTRN12614000106639; Hillier 2010). In order to reduce the potential for bias, MM and SN reviewed these studies.
Agreements and disagreements with other studies or reviews
Over the past 20 years, five systematic or meta‐analytic reviews (or both) that summarised intervention effects for children with DCD have been published (Hillier 2007; Miyahara 1996; Pless 2000a; Preston 2016; Smits‐Engelsman 2013). As described in the Why it is important to do this review section, these reviews were limited in several ways, including the year ranges covered and the outcome variables analysed by the primary studies. Although our screening process covered a wider range of years, only several intervention studies met our methodological inclusion criteria of RCT and quasi‐RCT (see Criteria for considering studies for this review), and the small number of studies with limited independent variables (e.g. age, the environment of intervention, interventionist) were insufficient for us to conduct subgroup analyses or to test transfer effects between outcome variables.
The foregoing reviews have several shortcomings (Miyahara 2016b). The most serious concerns are inclusion of non‐randomised studies in a meta‐analysis (Miyahara 1996), and inclusion of a mixture of non‐randomised studies with RCTs in systematic reviews and meta‐analyses (Hillier 2010; Pless 2000a; Preston 2016; Smits‐Engelsman 2013). The present review agrees with the past reviews in that primary intervention studies vary in their methodological quality. What differentiates our review from the foregoing reviews is that we excluded non‐randomised studies, and searched all years since inception of the databases. Meta‐analysis performed on biased primary studies cannot generate unbiased conclusions (Chalmers 2001); the conclusions drawn from the previous reviews, which unanimously reported beneficial effects of intervention, are inconsistent with ours.
Authors' conclusions
Implications for practice.
The results of this review are necessarily limited, due to the small numbers of participants in the small numbers of included studies with low quality evidence, substantial clinical heterogeneity across the studies, and a mixture of coherence and incoherence between the outcomes and the interventions. When we analysed the effect of task‐oriented interventions with a combination of high‐quality (i.e. RCT) and lower‐quality (i.e. quasi‐RCT) studies, the overall intervention effect was statistically significant (see Figure 4; Figure 5). We found no statistically significant beneficial effect from the two high‐quality studies alone (see Figure 4; Figure 5). Taken together, there is some evidence in favour of the relative effects of task‐oriented intervention versus inactive control, and no evidence to inform on the relative effects of task‐oriented intervention versus any other forms of intervention for children with DCD. We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. The decision to use a task‐oriented intervention may be further based on the children's and their care providers' preferences and costs.
Implications for research.
This review demonstrates a scarcity of high‐quality RCTs that have examined the effectiveness of task‐oriented interventions for children with DCD, and reported sufficient data to allow meta‐analysis. Although practical difficulties exist with double‐blinding and with ethical issues for an inactive control group, there are ways to lower the risk of bias. Using as exemplars the studies with lower risk of bias included in this review, researchers in the field are encouraged to conduct well‐designed and executed RCTs. Future intervention studies also need to report their results fully, in terms of group means and standard deviations, the number of participants in clinical terms (probable and definite DCD), socio‐demographic data, ethnicity, setting of intervention, adverse effects, and number of withdrawals, which will allow meta‐analysis for both statistical and clinical significance. Finally, researchers are encouraged to remain open and transparent by sharing study information and data.
What's new
| Date | Event | Description |
|---|---|---|
| 1 August 2017 | Amended | Correcting error in PLS |
Acknowledgements
We thank the following for their assistance.
The University of Otago, the University of South Australia, and the University of New South Wales: the development and publication of the protocol (Miyahara 2014), and this review, was made possible thanks to a salary from each author's university.
Cochrane Developmental, Psychosocial and Learning Problems (CDPLP): provided advice and assistance in producing this review.
Richard German (Librarian), Health Sciences Library (Medical and Dental) and Justin Farquhar (Librarian), Science Library, University of Otago, and Anthea Worley (Research Associate), University of South Australia: assisted with searching strategies.
Margaret Anderson, CDPLP: conducted the final search.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, which contains the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register
#1[mh "motor skills disorders"] #2[mh "psychomotor Disorders"] #3(coordination or co‐ordination) near/3 disorder* #4(motor next skill* near/3 disorder*) #5(motor next function* near/3 disorder*) #6DCD:ti,ab #7(clumsy or clumsiness) #8(physical* near/1 awkward*) #9(incoordination or in next coordination) #10(motor near/1 (competence or impair* or difficulty or difficulties or proficiency)) #11(movement* near/1 (difficulty or difficulties)) #12[mh Apraxias] #13dyspraxi* #14{or #1‐#13} #15[mh "Developmental Disabilities"] #16(co next ordination or coordination or motor*) #17#15 and #16 #18#14 or #17 #19[mh ^child] #20[mh adolescent] #21(child* or preschool* or pre next school* or boy* or girl* or teen* or adolescen* or young next people* or youth*) #22{or #19‐#21} #23#18 and #22 in Trials
Ovid MEDLINE
1 Motor Skills Disorders/ 2 Psychomotor Disorders/ 3 ((coordination or co‐ordination) adj3 disorder$).tw. 4 (motor skill$ adj3 disorder$).tw. 5 (motor function$ adj3 disorder$).tw. 6 DCD.tw. 7 (clumsy or clumsiness).tw. 8 (physical$ adj1 awkward$).tw. 9 (inco?ordination or in‐co?ordination).tw. 10 (motor adj1 (competence or impair$ or difficulty or difficulties or proficiency)).tw. 11 (movement$ adj1 (difficulty or difficulties)).tw. 12 exp Apraxias/ 13 dyspraxi$.tw. 14 or/1‐13 15 Developmental Disabilities/ 16 (co‐ordination or coordination or motor$).tw. 17 15 and 16 18 14 or 17 19 exp child/ 20 Adolescent/ 21 (child$ or preschool$ or pre‐school$ or boy$ or Girl$ or teen$ or adolescen$ or young people$ or youth$).tw. 22 or/19‐21 23 18 and 22 24 randomized controlled trial.pt. 25 controlled clinical trial.pt. 26 randomi#ed.ab. 27 placebo$.ab. 28 drug therapy.fs. 29 randomly.ab. 30 trial.ab. 31 groups.ab. 32 or/24‐31 33 exp animals/ not humans.sh. 34 32 not 33 35 23 and 34 36 remove duplicates from 35
Ovid MEDLINE(R) Epub Ahead of Print
1 ((coordination or co‐ordination) adj3 disorder$).tw. 2 (motor skill$ adj3 disorder$).tw. 3 (motor function$ adj3 disorder$).tw. 4 DCD.tw. 5 (clumsy or clumsiness).tw. 6 (physical$ adj1 awkward$).tw. 7 (inco?ordination or in‐co?ordination).tw. 8 (motor adj1 (competence or impair$ or difficulty or difficulties or proficiency)).tw. 9 (movement$ adj1 (difficulty or difficulties)).tw. 10 dyspraxi$.tw. 11 (developmental disorder$ and (co‐ordination or coordination or motor$)).tw. 12 or/1‐11 13 (child$ or preschool$ or pre‐school$ or boy$ or girl$ or teen$ or adolescen$ or young people$ or youth$).tw. 14 12 and 13 15 (random$ or control$ or group$ or cluster$ or placebo$ or trial$ or assign$ or prospectiv$ or meta‐analysis or systematic review or longitudinal$).tw. 16 14 and 15
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations
1 ((coordination or co‐ordination) adj3 disorder$).tw. 2 (motor skill$ adj3 disorder$).tw. 3 (motor function$ adj3 disorder$).tw. 4 DCD.tw. 5 (clumsy or clumsiness).tw. 6 (physical$ adj1 awkward$).tw. 7 (inco?ordination or in‐co?ordination).tw. 8 (motor adj1 (competence or impair$ or difficulty or difficulties or proficiency)).tw. 9 (movement$ adj1 (difficulty or difficulties)).tw. 10 dyspraxi$.tw. 11 (developmental disorder$ and (co‐ordination or coordination or motor$)).tw. 12 or/1‐11 13 (child$ or preschool$ or pre‐school$ or boy$ or girl$ or teen$ or adolescen$ or young people$ or youth$).tw. 14 12 and 13 15 (random$ or control$ or group$ or cluster$ or placebo$ or trial$ or assign$ or prospectiv$ or meta‐analysis or systematic review or longitudinal$).tw. 16 14 and 15
Embase Ovid
1 developmental coordination disorder/ 2 psychomotor disorder/ 3 motor dysfunction/ 4 ((coordination or co‐ordination) adj3 disorder$).tw. 5 (motor skill$ adj3 disorder$).tw. 6 (motor function$ adj3 disorder$).tw. 7 DCD.tw. 8 (clumsy or clumsiness).tw. 9 (physical$ adj1 awkward$).tw. 10 (inco?ordination or in‐co?ordination).tw. 11 (motor adj1 (competence or impair$ or difficulty or difficulties or proficiency)).tw. 12 (movement$ adj1 (difficulty or difficulties)).tw. 13 exp apraxia/ 14 dyspraxi$.tw. 15 or/1‐14 16 developmental disorder/ 17 (co‐ordination or coordination or motor$).tw. 18 16 and 17 19 15 or 18 20 juvenile/ or exp adolescent/ or exp child/ 21 (child$ or preschool$ or pre‐school$ or boy$ or Girl$ or teen$ or adolescen$ or young people$ or youth$).tw. 22 20 or 21 23 19 and 22 24 Randomized controlled trial/ 25 controlled clinical trial/ 26 Single blind procedure/ 27 Double blind procedure/ 28 triple blind procedure/ 29 Crossover procedure/ 30 (crossover or cross‐over).tw. 31 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 32 Placebo/ 33 placebo.tw. 34 prospective.tw. 35 factorial$.tw. 36 random$.tw. 37 assign$.ab. 38 allocat$.tw. 39 volunteer$.ab. 40 or/24‐39 41 23 and 40 42 remove duplicates from 41 43 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 44 human/ or normal human/ or human cell/ 45 43 and 44 46 43 not 45 47 42 not 46
ERIC ProQuest (Education Resources Information Center)
S21 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 S20 S16 OR S17 OR S18 OR S19 S19 random or randomly OR intervention* OR experiment* OR trial* S18 ((compar* OR control*) N2 group*) S17 ((evaluat* OR comparative) N2 (study OR studies OR research)) S16 DE "Longitudinal Studies" OR DE "Control Groups" OR DE "Program S15 S13 OR S14 S14 (child* or preschool* or pre‐school* or boy* or Girl* or teen* or adolescen* or young people* or youth*) S13 DE "Adolescents" OR DE "Children" OR DE "Early Adolescents" OR DE "Late Adolescents" OR DE "Youth" S12 dyspraxi* S11 (movement* N1 (difficulty or difficulties)) S10 (motor N1 (competence or impair* or difficulty or difficulties or proficiency)) S9 incoordination or "inco‐ordination" S8 (physical* N1 awkward*) S7 (clumsy or clumsiness) S6 DCD S5 (motor function* N3 disorder*) S4 (motor skill* N3 disorder*) S3 ((coordination or co‐ordination) N3 disorder*) S2 DE "Psychomotor Skills" S1 DE "Perceptual Motor Coordination"
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S35 S23 AND S34 S34 S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 S33 (MH "Quantitative Studies") S32 TX placebo* S31 (MH "Placebos") S30 (MH "Random Assignment") S29 TX (random* N3 (allocat* or assign*)) S28 TX (randomi* control* trial*) S27 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) S26 TX (clinic* n1 trial*) S25 PT Clinical trial S24 (MH "Clinical Trials+") S23 S18 AND S22 S22 S19 OR S20 OR S21 S21 (child* or preschool* or pre‐school* or boy* or Girl* or teen* or adolescen* or young people* or youth*) S20 (MH "Adolescence+") S19 (MH "Child+") S18 S14 OR S17 S17 S15 AND S16 S16 (co‐ordination or coordination or motor*) S15 (MH "Developmental Disabilities") S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S13 dyspraxi* S12 (MH "Apraxia+") S11 (movement* N1 (difficulty or difficulties)) S10 (motor N1 (competence or impair* or difficulty or difficulties or proficiency)) S9 incoordination or in‐coordination) S8 (physical* N1 awkward*) S7 (clumsy or clumsiness) S6 DCD S5 (motor function* N3 disorder*) S4 (motor skill* N3 disorder*) S3 ((coordination or co‐ordination) N3 disorder*) S2 (MH "Psychomotor Disorders") S1 (MH "Motor Skills Disorders")
PsycINFO Ovid
1 movement disorders/ 2 motor coordination/ 3 dyspraxia/ 4 ((coordination or co‐ordination) adj3 disorder$).tw. 5 (motor skill$ adj3 disorder$).tw. 6 (motor function$ adj3 disorder$).tw. 7 DCD.tw. 8 (clumsy or clumsiness).tw. 9 (physical$ adj1 awkward$).tw. 10 (inco?ordination or in‐co?ordination).tw. 11 (motor adj1 (competence or impair$ or difficulty or difficulties or proficiency)).tw. 12 (movement$ adj1 (difficulty or difficulties)).tw. 13 dyspraxi$.tw. 14 developmental disabilities/ 15 (co‐ordination or coordination or motor$).tw. 16 14 and 15 17 or/1‐13 18 16 or 17 19 (adolescence 13 17 yrs or childhood birth 12 yrs or infancy 2 23 mo or preschool age 2 5 yrs or school age 6 12 yrs).ag. 20 (child$ or preschool$ or pre‐school$ or boy$ or Girl$ or teen$ or adolescen$ or young people$ or youth$).tw. 21 19 or 20 22 18 and 21 23 clinical trials/ 24 random$.tw. 25 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).tw. 26 (crossover$ or "cross over$").tw. 27 trial$.tw. 28 group$.ab. 29 exp program evaluation/ 30 treatment effectiveness evaluation/ 31 treatment outcome clinical trial.md. 32 ((effectiveness or evaluat$) adj2 (stud$ or research$)).tw. 33 (allocat$ or assign$).tw. 34 placebo.ab. 35 or/23‐34 36 22 and 35
Science Citation Index ‐ Expanded (SCI‐EXPANDED), Social Sciences Citation Index (SSCI), Conference Proceedings Citation Index ‐ Science (CPCI‐S), Conference Proceedings Citation Index ‐ Social Science & Humanities (CPCI‐SS&H); Web of Science
# 14 #13 AND #12 # 13 TS=(random* or control* or trial* or blind* or placebo* ) # 12 #11 AND #10 # 11 TS=(child* or preschool* or "pre school*" or boy*or Girl* or teen* or adolescen* or "young people" or youth*) # 10 #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 9 TS= (dysprax*) #8 TS= (movement* near/1 (difficulty or difficulties)) # 7 TS= (motor near/1 (competence or impair* or difficulty or difficulties or proficiency)) # 6 TS= (incoordination) # 5 TS=((physical* near/1 awkward*)) # 4 TS=(clumsy or clumsiness) # 3 TS=("motor function* " near/3 disorder*) # 2 TS=("motor skill*" near/3 disorder*) # 1 TS=((coordination or co‐ordination) near/3 disorder* )
Cochrane Database of Systematic Reviews (CDSR), in the Cochrane Library
#1[mh "motor skills disorders"] #2[mh "psychomotor Disorders"] #3((coordination or co‐ordination) near/3 disorder*):ti,ab #4(motor next skill* near/3 disorder*):ti,ab #5(motor next function* near/3 disorder*) :ti,ab #6DCD:ti,ab #7(clumsy or clumsiness):ti,ab #8(physical* near/1 awkward*):ti,ab #9(incoordination or in next coordination):ti,ab #10(motor near/1 (competence or impair* or difficulty or difficulties or proficiency)):ti,ab #11(movement* near/1 (difficulty or difficulties)) #12[mh Apraxias] #13dyspraxi*:ti,ab #14{or #1‐#13} #15[mh "Developmental Disabilities"] #16(co next ordination or coordination or motor*):ti,ab #17#15 and #16 #18#14 or #17 #19[mh ^child] #20[mh adolescent] #21(child* or preschool* or pre next school* or boy*or Girl* or teen* or adolescen* or young next people* or youth*):ti,ab #22{or #19‐#21} #23#18 and #22 in Cochrane Reviews (Reviews and Protocols)
Database of Abstracts of Reviews of Effectiveness (DARE), in the Cochrane Library
#1[mh "motor skills disorders"] #2[mh "psychomotor Disorders"] #3((coordination or co‐ordination) near/3 disorder*):ti,ab #4(motor next skill* near/3 disorder*):ti,ab #5(motor next function* near/3 disorder*) :ti,ab #6DCD:ti,ab #7(clumsy or clumsiness):ti,ab #8(physical* near/1 awkward*):ti,ab #9(incoordination or in next coordination):ti,ab #10(motor near/1 (competence or impair* or difficulty or difficulties or proficiency)):ti,ab #11(movement* near/1 (difficulty or difficulties)) #12[mh Apraxias] #13dyspraxi*:ti,ab #14{or #1‐#13} #15[mh "Developmental Disabilities"] #16(co next ordination or coordination or motor*):ti,ab #17#15 and #16 #18#14 or #17 #19[mh ^child] #20[mh adolescent] #21(child* or preschool* or pre next school* or boy*or Girl* or teen* or adolescen* or young next people* or youth*):ti,ab #22{or #19‐#21} #23#18 and #22 in Other Reviews
Proquest Dissertations & Theses: UK & Ireland
TI("developmental coordination disorder" OR "developmental co‐ordination disorder" OR DCD OR "motor impair*" OR "motor disorder*" OR incoordination OR dyspraxia* OR clumsy)
WorldCat
TI:(developmental coordination disorder OR developmental co‐ordination disorder OR DCD OR motor impair* OR motor disorder* OR incoordination OR dyspraxia* OR clumsy) AND kw:(child* OR adolescen* OR teen* OR infant*) AND KW:(intervention* OR random* OR effectiveness OR evaluat* OR treat*)
ClinicalTrials.gov
(clinicaltrials.gov)
"developmental co‐ordination disorder" AND children
metaRegister of Controlled Trials
"developmental co‐ordination disorder" AND children
ISRCTN Registry
"developmental co‐ordination disorder" AND children
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)
(apps.who.int/trialsearch)
developmental co‐ordination disorder AND children
Australian New Zealand Clinical Trials Registry
(anzctr.org.au)
"developmental co‐ordination disorder" AND children
Appendix 2. Search summary
| Database | Search date | Database date range/issue/version | Number of records |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | 21 August 2014 | July 2014 (Issue 7) | 506 |
| 20 April 2016 | April 2016 (Issue 4) | 77 | |
| 5 April 2017 | March 2017 (Issue 3) | 36 | |
| Ovid MEDLINE | 20 August 2014 | 1946 to August Week 1 2014 | 1740 |
| 18 April 2016 | 1946 to April Week 1 2016 | 221 | |
| 4 April 2017 | 1946 to March Week 4 2017 | 116 | |
| Ovid MEDLINE Epub Ahead of Print | 4 April 2017 | 3 April 2017 | 49 |
| Ovid MEDLINE In‐Process & Other Non‐Indexed Citations | 4 April 2017 | 3 April 2017 | 143 |
| Embase (Ovid) | 20 August 2014 | 1980 to 2014 Week 33 | 1508 |
| 18 April 2016 | 1980 to 2016 Week 16 | 298 | |
| 4 April 2017 | 1974 to 2017 Week 14 | 208 | |
| ERIC ProQuest (Education Resources Information Center) | 20 August 2014 | 1966 to current | 1047 |
| 20 April 2016 | 1966 to current | 62 | |
| 5 April 2017 | 1966 to current | 73 | |
| CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature) | 21 August 2014 | 1937 to current | 604 |
| 18 April 2016 | 1937 to current | 66 | |
| 4 April 2017 | 1937 to current | 54 | |
| PsycINFO (Ovid) | 20 August 2014 | 1806 to August Week 2 2014 | 359 |
| 18 April 2016 | 1806 to April Week 2 2016 | 68 | |
| 4 April 2017 | 1806 to April Week 2 2017 | 31 | |
| Science Citation Index ‐ Expanded and Social Sciences Citation Index Web of Science (SCI‐Expanded; SSCI, respectively) | 22 August 2014 | 1970 to 21 August 2014 | 1306 |
| 20 April 2016 | 1970 to 18 April 2016 | 342 | |
| 4 April 2017 | 1970 to 31 March 2017 | 156 | |
| Conference Proceedings Citation Index ‐ Science and Conference Proceedings Citation Index ‐ Social Science & Humanities Web of Science (CPCI‐S; CPCI‐SS&H, respectively) | 22 August 2014 | 1990 to 21 August 2014 | 112 |
| 20 April 2016 | 1990 to 18 April 2016 | 5 | |
| 4 April 2017 | 1990 to 31 March 2017 | 1 | |
| Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library | 21 August 2014 | August 2014 (Issue 8 of 12) | 10 |
| 20 April 2016 | April 2016 (Issue 4 of 12) | 7 | |
| 5 April 2017 | April 2017 (Issue 4 of 12) | 2 | |
| Database of Reviews of Abstracts of Effects (DARE) in the Cochrane Library | 21 August 2014 | July 2014 (Issue 3 of 4) | 14 |
| 20 April 2016 | April 2015 (Issue 2 of 4) | 0 | |
| ProQuest Dissertations & Theses: UK & Ireland | 22 August 2014 | Current issue | 49 |
| 20 April 2016 | Current issue | 4 | |
| 5 April 2017 | Current issue | 0 | |
| WorldCat (worldcat.org) | 22 August 2014 | Current issue | 36 |
| 20 April 2016 | Current issue | 7 | |
| 4 April 2017 | Current issue | 0 | |
| ClinicalTrials.gov (clinicaltrials.gov) | 18 June 2015 | Current issue | 9 |
| 22 April 2016 | After 6 January 2015 | 16 | |
| 31 March 2017 | Current issue | 20 | |
| metaRegister of Controlled Trials (mRCT; www.controlled‐trials.com) This service was under review in 2016 and 2017. | 18 June 2015 | Current issue | 1 |
| ISRCTN registry (www.isrctn.com) | 22 April 2016 | Current issue | 8 |
| 31 March 2017 | Current issue | 6 | |
| World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch) | 18 June 2015 | Current issue | 12 |
| 22 April 2016 | Current issue | 6 | |
| 31 March 2017 | Current issue | 18 | |
| Australian New Zealand Clinical Trials Registry (ANZCTR; anzctr.org.au/trialSearch.aspx) | 18 June 2015 | Current issue | 2 |
| 22 April 2016 | Current issue | 4 | |
| 31 March 2017 | Current issue | 4 |
Data and analyses
Comparison 1. Random‐effects model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Movement Assessment Battery for Children (MABC): Total score | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 RCTs and quasi‐RCTs | 6 | 169 | Mean Difference (IV, Random, 95% CI) | ‐3.63 [‐5.88, ‐1.39] |
| 1.2 RCTs only | 2 | 51 | Mean Difference (IV, Random, 95% CI) | ‐2.34 [‐7.50, 2.83] |
Comparison 2. Fixed‐effect model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Movement Assessment Battery for Children (MABC): Total score | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 RCTs and quasi‐RCTs | 6 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐4.06 [‐5.63, ‐2.50] |
| 1.2 RCTs only | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐2.11 [‐6.00, 1.78] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACTRN12614000106639.
| Methods | Single‐blind RCT. | |
| Participants | Number screened: 1814. Number included: 93. Number followed up: 92. Number of withdrawals: 1. Diagnosis of DCD: DSM‐IV‐TR. Presence and absence of comorbid conditions: children were excluded if they had global developmental delay, an intellectual disability or other medical condition that is known to cause motor impairment. Regarding participants completing the study Age: in school with a school assistant (mean 6 years 3 months, SD 11 months), in school with a physiotherapist (mean 6 years 7 months, SD 11 months), in a clinic with a physiotherapist (mean 6 years 1 months, SD 13 months). Sex: in school with a school assistant (25 boys, 12 girls), in school with a physiotherapist (23 boys, 7 girls), in a clinic with a physiotherapist (18 boys, 8 girls). Ethnicity: majority were white.* Country: Australia. Setting: school and clinic. Sociodemographics: a mixture of ranks for the School Index for Disadvantage in each mode which is based on socioeconomic status. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: in school with a school assistant vs in school with a physiotherapist vs in a clinic with a physiotherapist. Intervention schedule: 45 min/wk. Duration of intervention: 8 wk. Mode of delivery: face‐to‐face small group (4 to 6 children). Intervention material: all groups used predominantly task‐oriented therapy consisting of age‐appropriate activities and the principles of skill mastery. The intervention used meta‐themes (repetition of skills, heightened feedback about performance and experience at success). The warm‐up activities for each session addressed issues, such as strength, motor planning, and proprioception that are more consistent with the perceptual‐motor approach to intervention. Intervention procedure: warm‐up activities followed by functional activities. Each session had a theme (e.g. going to the beach, on the farm). Intervention provider: physiotherapist with paediatric experience (2 to 6 years) or school assistant with 3 hr DCD/intervention training and previous experience running gross motor groups, supervised by a physiotherapist. Place of intervention: school or outpatient clinic. Intervention compliance: overall attendance rates for participants at intervention sessions were 92% for group 1 (school/school assistant), 93% group 2 (school/physiotherapist) and 88% group 3 (clinic/physiotherapist). |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: none recorded.* |
|
| Notes | Study start date: 14 July 2006 ‐ actual date of first participant enrolment. Study completion date: 5 March 2007 ‐ actual date last participant enrolled. Sample calculation: yes. Ethics approval: yes. Comments from study authors Limitations:
Key conclusions of study authors Group intervention programmes for DCD can be run by either a health professional or school assistant (supported by physiotherapist) in either the school or clinic environment and provide successful outcomes. Comment from review authors Active control only. * Email correspondence with study authors: February 2016. We contacted the first study author and obtained raw data and supplementary information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation generated from a computer random numbers table. |
| Allocation concealment (selection bias) | Low risk | Allocated by a third party independent of the study and stratified according to school socioeconomic status. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 dropped out (moved overseas). |
| Selective reporting (reporting bias) | Low risk | Protocol identified (ACTRN12614000106639). |
| Other bias | Low risk | Funding: authors acknowledged the Volunteer Service for Flinders Medical Centre Inc. for funding some equipment in this study. Conflicts of interest: study authors, who worked for School of Health Sciences and School of Exercise and Health Sciences at universities, indicated no conflicts of interest. |
Au 2014.
| Methods | Single‐blind RCT. | |
| Participants | Number screened: 26. Number included: 22. Number followed up: 20. Number of withdrawals: 2. Diagnosis of DCD: DSM‐IV and MABC test < 15th percentile or BOTMP < 1.5 SD. Presence and absence of comorbid conditions: children were excluded if they had major comorbid medical problems, such as moderate‐to‐severe mental disability, profound visual or hearing impairment, or any major behavioural problems. 3 children had attention deficit hyperactive disorder and 4 children had dyslexia. However, there was no significant difference in the numbers allocated to each group. Regarding participants completing the study Age: 6 to 12 years. Sex: 15 boys, 7 girls. Ethnicity: no information. Country: China. Setting: outpatient unit in a hospital. Sociodemographics: no information. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Comparison: core stability programme vs task‐oriented motor programme (active control). Intervention schedule: 1 hr once/wk. Duration of intervention: 8 wk. Mode of delivery: face‐to‐face individual or group (not explicitly stated) sessions with daily home programme. Intervention material: Core stability programme: physio ball. Core stability exercises (supine, prone, sitting, and standing positions with instructions to focus on co‐contracting the abdominal and back muscles to maintain the spine in a neutral position. Task‐oriented motor programme: focus on training functional tasks, which included those that involved mainly body stability (e.g. standing) and those that required body transport (e.g. walking, running, jumping, hopping, skipping, and galloping). Intervention procedure: both groups commenced and ended with 5‐min warm‐up and cool‐down involving stretching. Both groups increased over time the complexity of the tasks, number of repetitions, and duration of each exercise/activity and reduced the level of assistance or guidance. Intervention provider: physiotherapist (10 years' paediatric experience). Place of intervention: paediatric physiotherapy outpatient unit, local hospital. Compliance: attendance rate not significantly different between groups (mean (± SD): core stability 6.2 (± 1.2) sessions; task‐oriented 6.8 (± 1.0) sessions). Compliance with the home exercise programme was also not significantly different between groups (mean (± SD): core stability 2.9 (± 2.2) days/wk and task‐oriented 1.8 (± 0.6) days/wk). |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: no information. Measures of participation: no information. |
|
| Notes | Study start date: April 2010. Study completion date: December 2011. Sample calculation: yes. Ethics approval: yes, from the ethics committee of the local University and the Institutional Review Board of the local hospital. Comments from study authors Limitations:
Key conclusions of study authors The core stability exercise program is as effective as task‐oriented training in improving motor proficiency among children with DCD. Comment from review authors Email correspondence with study authors: April and May 2016. We wrote to the authors twice to request supplementary information on data but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing lots. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 withdrawal from each group for not being able to commit the time. |
| Selective reporting (reporting bias) | Low risk | Protocol identified (ClinicalTrials.gov; NCT01207544). |
| Other bias | Low risk | Funding: research not funded by any specific grant from funding agency. Conflicts of interest: study authors, who worked for Physiotherapy Departments of hospitals and Department of Rehabilitation Sciences at Polytechnic University, indicated no conflicts of interest. |
Fong 2012.
| Methods | Single‐blind quasi‐RCT. | |
| Participants | Number screened: 91. Number included: 62 (44 children with DCD who were randomised + 18 children in non‐DCD control group). Number followed up: 39 (29 children with DCD, 10 children in non‐DCD control group). Number of withdrawals: 23 (15 children with DCD, 8 children in non‐DCD control group). Diagnosis of DCD: DSM‐IV‐TR. Presence and absence of comorbid conditions: relevant comorbid conditions were excluded (e.g. a formal diagnosis of emotional, neurological, or other movement disorders; significant congenital, musculoskeletal, or cardiopulmonary condition; excessive disruptive behaviour). Regarding participants completing the study Age (mean ± SD): DCD‐TKD group: 7.7 ± 1.3 years; DCD inactive control: 7.4 ± 1.2 years; non‐DCD control: 7.2 ± 1.0 years. Sex: DCD‐TKD group: 17 boys, 4 girls; DCD inactive control: 18 boys, 5 girls; non‐DCD control: 14 boys, 4 girls. Ethnicity: no information. Country: China. Setting: child assessment centres, hospitals, and community. Sociodemographics: no information. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Comparison: DCD TKD training vs DCD inactive control vs non‐DCD control. Intervention schedule: 1 hr/wk. Duration of intervention: 12 wk. Mode of intervention delivery: face‐to‐face group sessions plus home exercises from face‐to‐face sessions. Intervention material: log books. Intervention procedure: based on typical TKD syllabus; protocol outlined in report. Intervention provider: syllabus modified by experienced physiotherapist and skilled TKD practitioner, sessions conducted by World TKD Federation 4th and 2nd dan black belt instructors. Place of Intervention: Hong Kong Polytechnic University. Intervention compliance: checked by daily log books of participants and parents. |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: no information. Measures of participation: no information. |
|
| Notes | Study start date: not available. Study completion date: not available. Sample calculation: yes. Ethics approval: yes. Comments from study authors Limitations:
Key conclusions of study authors: TKD training can remedy unilateral standing balance and vestibular function impairments in children with DCD. Comment from review authors Effect of TKD training on DCD is unknown because primary outcome of DCD was not assessed. Email correspondence with study authors: February 2016. We wrote to the authors twice to request supplementary information on data but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via drawing lots. |
| Allocation concealment (selection bias) | Low risk | Independent to study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent to study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for dropout accounted for, but 37% dropout rate was too high. |
| Selective reporting (reporting bias) | Low risk | Unable to get study protocol. MABC‐2 not used for diagnosis, but for exclusion of control children. |
| Other bias | Low risk | Funding: research not funded by any specific grant from funding agency. Conflicts of interest: study authors, who were affiliated with Department of Rehabilitation Sciences, indicated no conflicts of interest. |
Fong 2016.
| Methods | Single‐blind RCT. | |
| Participants | Number screened: 178. Number included: 108. Number followed up: 88. Number of withdrawals: 20. Diagnosis of DCD: DSM IV and MABC test < 5th percentile or BOTMP gross motor composite score ≤ 42. Presence and absence of comorbid conditions: the following comorbid conditions were identified in the participants: attention deficit hyperactivity disorder: intervention 10 (21.3%) and control 10 (24.4%), dyslexia: intervention 5 (10.6%) and control 7 (17.1%), and suspected autism‐spectrum disorder: intervention 12 (25.5%) and control 13 (31.7%). Children were excluded with a diagnosis of emotional, neurological, or other movement disorders; significant congenital, musculoskeletal, cardiopulmonary, or sensorimotor disorders capable of affecting balance performance; disruptive behaviour; or an inability to follow instructions accurately. Regarding participants completing the study Age: 6 to 10 years. Sex: 61 boys, 27 girls. Ethnicity: no information. Country: China. Setting: university laboratory. Sociodemographics: no information. Inclusion criteria
Exclusion criteria
Disruptive behaviour or an inability to follow instructions accurately. |
|
| Interventions |
Comparison: task‐specific balance training (functional movement training) vs inactive control. Intervention schedule: 1.5 hr 2/wk. Duration of intervention: 12 wk. Mode of delivery: face‐to‐face (?) individual/group. Intervention material: NeuroTrac MyoPlus 4 machine; Stability Trainer; balance board; peg board and tennis ball. Intervention procedure: specific balance training with electromyographic feedback. Schedule: 2‐leg balance on foam with electromyographic biofeedback (10 min); 1 leg balance on ground (alternating feet) (5 min); walking in a straight line with heels raised (5 min); double leg hops (5 min); ball balance while walking (5 min). Schedule practised repeatedly during 1.5‐hr session and challenge was increased over duration of the intervention. Verbal feedback at end of each training session. Intervention provider: trained research assistant with sports coaching qualification (supervised by a physiotherapist). Place of intervention: University of Hong Kong Health and Physical Activity Laboratory. Intervention compliance: no information. |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: no major adverse events. During training, transient muscle soreness (2 children) and non‐injurious fall (1 child) reported in the journal article. Measures of participation: no information. |
|
| Notes | Study start date: March 2014 given as start date in protocol. Study completion date: March 2017 given as estimated study completion date in protocol. Sample calculation: yes. Ethics approval: yes from the Human Research Ethics Committee of the University of Hong Kong. Comments from study authors Limitations:
Key conclusions of study authors Task‐specific balance training marginally improved the somatosensory function and somewhat improved the functional balance performance of children with DCD. However, it did not improve vestibular and visual contributions to postural control in this group of children. Comment from review authors Email correspondence with study authors: May 2016 and May 2017. We wrote to the authors twice to request supplementary information on data but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Testers were blind to the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All reasons reported, but 18.5% was too high. |
| Selective reporting (reporting bias) | Low risk | Protocol identified (ClinicalTrials.gov; NCT02393404). |
| Other bias | Low risk | Funding: research funded by the Research Grants Council (RGC) of Hong Kong (27100614) and the University of Hong Kong Merit Award for projects funded by the RGC's General Research Fund/Early Career Scheme. Conflicts of interest: study authors, who were affiliated with Institute of Human Performance, Department of Rehabilitation Sciences, School of Science and Health (Occupational Therapy), Health, Physical Education and Recreation Department, and Department of Physical Education of universities, indicated no conflicts of financial interest. |
Green 2008.
| Methods | Unblinded RCT with 4 groups. | |
| Participants | Number screened: 78. Number included: 43. Number followed up: 43. Number of withdrawals: 0. Diagnosis of DCD: DSM IV. Presence and absence of comorbid conditions: children excluded if they had a severe learning difficulty or neuromotor or significant medical problem. Regarding participants completing the study Age: mean 97.7 months, range 62 to 128 months. Sex: 37 boys and 6 girls. Ethnicity: data collected but not analysed.* Country: UK. Setting: community adult education centre/leisure centre. Sociodemographics: mean socioeconomic status ‐0.55, range from ‐5 to 7. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Comparison: CO‐OP intervention (group 1) vs inactive control (group 2) vs inactive control (group 3) vs inactive control (group 4). Phases: each of the 4 groups received the intervention once during 1 of the 4 phases. Intervention schedule: 1 hr/wk. Duration of intervention: 20 wk. Mode of delivery: face‐to‐face group sessions. Intervention material: global strategy (GPDC) and strategies specific to the child and activity. Intervention procedure: introduction of the global cognitive strategy, practise and implementation of domain‐specific strategies, consolidation strategies, and consolidation. Intervention provider: senior therapist and a junior assistant therapist trained in the CO‐OP approach. Place of intervention: local adult education centre. Intervention compliance: all participants attended > 50%, a majority attended 16 sessions out of 20. |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: none recorded.* |
|
| Notes | Study start date: not available. Study completion date: not available. Sample calculation: yes. Ethics approval: yes. Comments from study authors Limitation:
Key conclusions of study authors Children with severe DCD were likely to have continuing difficulties despite progress following intervention. Comment from review authors Only the data from the first phase were used for meta‐analysis. * Email correspondence with study authors: June 2015, January 2016 and February 2016. We contacted the first study author several times and obtained raw data for meta‐analysis and supplementary information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation into treatment groups of 6 children done according to age bands (6 to 8 years and 9 to 10.8 years) using the random sample selection function of the Statistical Package for Social Sciences (SPSS Inc., 1999). |
| Allocation concealment (selection bias) | High risk | High as the author knew the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | High as the author stated single blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Testers, who recorded scores, were blinded to each child's intervention group status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All accounted for. |
| Selective reporting (reporting bias) | Low risk | No protocol obtained. All primary outcomes and 1 secondary outcome reported. |
| Other bias | Low risk | Funding: no information available. Conflicts of interest: no information available. Baseline imbalance. |
Hillier 2010.
| Methods | Single‐blind RCT. | |
| Participants | Number screened: 47. Number included: 13. Number followed up: 12. Number of withdrawals: 1. Diagnosis of DCD: DSM‐IV. Presence and absence of comorbid conditions: children were excluded if they had comorbid conditions that may have impacted on the tests or intervention. Regarding participants completing the study Age: aquatic physiotherapy: median 7 years 3 months, range 5 years 8 months to 8 years; control group: median 6 years 10 months, range 5 years 5 months to 8 years 9 months. Sex: 5 boys and 1 girl in both groups. Ethnicity: white. Country: Australia. Setting: hospital‐based hydrotherapy pool. Sociodemographics: no information. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Comparison: aquatic physiotherapy vs inactive control. Intervention schedule: 30 min/wk for 6 sessions. Duration of intervention: 6 to 12 wk. Mode of delivery: face‐to‐face 1:1 sessions. Intervention material: indoor pool, various equipment items for pool use. Intervention procedure: graded task‐specific training (ball skills, standing balance, and walking/running) with feedback in multi‐sensory pool environment. A full manual of potential exercises and progressions was produced and each child's individual programme was selected from this template. Intervention provider: physiotherapist with experience in aquatic therapy. Place of Intervention: hospital hydrotherapy pool. Compliance: yes, by attendance.* |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: none recorded.* |
|
| Notes | Study start date: not available. Study completion date: not available. Sample calculation: yes. Ethics approval: yes. Comments from study authors Limitations:
Key conclusions of study authors Aquatic therapy was a feasible intervention for children with DCD and may be effective in improving their gross motor skills. Comment from review authors Task‐specific training was integrated. * Email correspondence with study authors: February 2016. We contacted the first study author several times and obtained supplementary information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation process completed by a person independent to the study, using a computer program, which randomly allocated intervention or control to each participant. |
| Allocation concealment (selection bias) | Low risk | Concealed envelope method. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcome group: all participants knew the group affiliation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An assessor blinded to participant group conducted all assessments. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 child withdrew and the scheduled 6 sessions took 12 wk. |
| Selective reporting (reporting bias) | Low risk | All reported. |
| Other bias | Low risk | Funding: no information available. Conflicts of interest: study authors, who worked for Centre for Allied Health Evidence and the School of Health Sciences of a university and a senior clinician at a hospital, indicated no conflicts of interest. Small numbers. |
Hung 2010.
| Methods | Single‐blind RCT. | |
| Participants | Number screened: not reported. Number included: 23. Number followed up: 23. Number of withdrawals: 0. Diagnosis of DCD: DSM‐IV‐TR. Presence and absence of comorbid conditions: children were excluded if they had disruptive behaviour or sensory issues (visual or hearing). other comorbidities not reported. Regarding participants completing the study Age (mean ± SD): group‐based motor skill training (8 years 4 months ± 1 year 2 months), individual‐based motor skill training (7 years 8 months ± 1 year 2 months). Sex: group‐based motor skill training (10 boys, 2 girls), individual‐based motor skill training (9 boys, 2 girls). Ethnicity: no information. Country: China. Setting: Pediatric Physiotherapy Outpatient Department of Kowloon Hospital. Sociodemographics: urban residents of Kaohsiung.* Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Comparison: group‐based motor skill training vs individual‐based motor skill training. Intervention schedule: 45 min/wk. Duration of intervention: 8 wk. Mode of delivery: face‐to‐face individual (1:1) versus group (4:1 to 6:1) training plus home exercises to reinforce the session. Intervention material: trampoline, rope, obstacles, balance beam, uneven surfaces, therapy ball, balls, beanbags. Intervention procedure: motor training sessions in either group had same structure and components (agility and balance, core stability, bilateral co‐ordination, eye‐hand and eye‐foot co‐ordination). Intervention provider: therapist. Place of intervention: Pediatric Physiotherapy Outpatient Department of Kowloon Hospital. Intervention compliance: 100% attendance, home exercise compliance using a logbook. |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: none. Measures of participation: no information. |
|
| Notes | Study start date: not available. Study completion date: not available. Sample calculation: yes. Ethics approval: yes. Comments from study authors Limitations:
Key conclusions of study authors Group‐based training and individual‐based training had a similar effect on motor performance. Group‐based training may be more cost‐effective than individual‐based training. Comment from review authors Active control only. Email correspondence with study authors: February 2014 and February 2016. We wrote to the authors and obtained raw data in 2014. In 2016, we wrote to the authors twice to request supplementary information on data, but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed envelope. |
| Allocation concealment (selection bias) | Low risk | Independent to study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind, as double‐blind was impossible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All completed and reported. |
| Selective reporting (reporting bias) | Low risk | No protocol obtained. All prespecified outcomes of interest reported. |
| Other bias | Low risk | Funding: research funded by Hong Kong Polytechnic University. Conflicts of interest: no information available. Small numbers. |
Miller 2001.
| Methods | Single‐blind RCT. | |
| Participants | Number screened: 29. Number included: 20. Number followed up: 20. Number of withdrawals: 2 from treatment, 1 did not complete all testing. Diagnosis of DCD: by a physician. Presence and absence of comorbid conditions: excluded children with medical diagnosis of a specific neurological disorder or a physical or sensory deficit causing the motor problem. Regarding participants completing the study Age (mean ± SD): CO‐OP: 8.90 ± 1.52 years (range 7 to 12 years), CTA: 9.20 ± 0.92 years (range 7 to 12 years). Sex: 14 boys, 6 girls. Ethnicity: no information. Country: Canada. Setting: Cloverleaf Research and Treatment Clinic in School of Occupational Therapy at University of Western Ontario. Sociodemographics: no information. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Comparison: CO‐OP vs CTA. Intervention schedule: 50 min for 10 sessions, no frequency information. Duration of intervention: no information. Mode of delivery: face‐to‐face individual sessions. Intervention material: CO‐OP: GPDC strategy and its application to target tasks; CTA: target tasks practise under skill direction and corrections. Intervention procedure: CO‐OP: self‐talk and problem‐solving strategies are used to solve motor problems and applied to new situations; CTA: children were instructed in the performance of the task and the therapist actively provided skill direction and corrective instructions for problematic motor components. Intervention provider: 1 therapist experienced in the administration of CO‐OP and 2 therapists experienced in the administration of CTA. Place of intervention: Cloverleaf Research and Treatment Clinic in School of Occupational Therapy at University of Western Ontario. Intervention compliance: monitored by investigators. 1 mid‐point treatment session was video‐taped to check adherence of treatment protocol. |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: no adverse effect.* Measures of participation: not reported. |
|
| Notes | Study start date: not available. Study completion date: not available. Sample calculation: to be conducted based on the pilot study. Ethics approval: no information. Comments from study authors Limitations:
Key conclusions of study authors CO‐OP more effective than CTA. Comment from review authors A high dropout rate (> 10%) with no explanation as to reasons and group affiliations. * Email correspondence with study authors: May 2016. We wrote to the study authors twice to request supplementary information on data and obtained supplementary information. Follow‐up data obtained for 8 participants in CO‐OP group and 7 in CTA group. Follow‐up intervals varied from 7.5 months to 13 months. No inactive control group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | System of random envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 3 independent assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 dropouts from intervention, 1 did not complete all required testing. |
| Selective reporting (reporting bias) | Low risk | No protocol obtained. All prespecified outcomes of interest reported. |
| Other bias | Low risk | Funding: research funded by a research grant from the Hospital for Sick Children Foundation Toronto, Ontario. Conflicts of interest: no information available. Small numbers. |
Pless 2000b.
| Methods | Single‐blind, quasi‐RCT. | |
| Participants | Number screened: 100. Number included: 37. Number followed up: 37. Number of withdrawals: 0. Diagnosis of DCD: DSM IV and MABC test < 15th percentile. Presence and absence of comorbid conditions: children with a neurological or behavioural disorder were excluded. Regarding participants completing the study Age: 5 to 6 years. Sex: 26 boys, 11 girls. Ethnicity: no information. Country: Sweden. Setting: 3 different centres in Uppsala Primary Health Care and a room at the University hospital. Sociodemographics: no information. Inclusion criteria
Exclusion criteria None stated. |
|
| Interventions |
Comparison: motor skill intervention vs inactive control. Intervention schedule: 45‐min session/wk for 10 wk, no information on the length of each session. Duration of intervention: 6 months. Mode of delivery: face‐to‐face group sessions. Intervention material: wall bars, a horizontal bar, a box horse, carpets, flying rings, skipping ropes, balls in an obstacle course. Intervention procedure: children practised on gymnastic apparatus in an obstacle course. At the end of a session they played a game together. Intervention provider: physical educator. Place of intervention: gymnastic hall at a school. Intervention compliance: physical educator in the group motor skill intervention noted on a score sheet at each session whether the child had attended the session and to what degree each child had participated in the activities during the session (we used a simple scale stating 0 = did not participate in the activities, 1 = participated in < 10% of the activities, 2 = partly participated in 10% to 100% of the session, 3 = participated in all the activities). The sessions were very popular and since the number of children were few in the group and the parents also attended and helped all children who were rated as 3 in all sessions.* |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: none.* |
|
| Notes | Study start date: not available. Study completion date: not available. Sample calculation: no. Ethics approval: yes, from the Ethics Committee of the Faculty of Medicine at the University of Uppsala (No 219/95).* Comments from study authors Limitation:
Key conclusions of study authors Group motor skill intervention may have positive effects in children with DCD with borderline motor difficulties. For children with definite motor difficulties, more specific and individualised intervention is required in a 1‐to‐1 setting. Comment from review authors A 4‐month follow‐up assessment was initially planned but cancelled. * Email correspondence with study authors: January 2016. We wrote to the first author, who provided supplementary information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | First boy and first girl randomised to either group and then allocated in turn to other group. |
| Allocation concealment (selection bias) | High risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated and also not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | MABC motor test carried out by an independent rater (MP); the first author (MP) assessed the children before any intervention, and again after 6 months. In response to our inquiry, the author wrote: "I did not know at any time whether they were attending the Group Motor Skill Intervention or not." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates not stated. |
| Selective reporting (reporting bias) | Low risk | No protocol obtained. All prespecified outcomes of interest reported. |
| Other bias | Low risk | Funding: research partly funded by Uppsala University, the Vårdal Foundation in Stockholm, and the Gillberg Foundation in Uppsala, all in Sweden. Conflicts of interest: no information available. Noted small numbers and slight imbalance. Only 6‐month follow‐up assessment was analysed because most of the children in the intervention group had not finished the 10‐wk intervention. |
Sugden 2003.
| Methods | Unblind RCT. | |
| Participants | Number screened: 41.* Number included: 31. Number followed up: 31. Number of withdrawals: 0. Diagnosis of DCD: DSM‐IV. Presence and absence of comorbid conditions: no child had a generic learning difficulty or a medical condition such as cerebral palsy. Regarding participants completing the study Age (mean ± SD): 8.04 ± 0.7 years (range 7.01 to 9.06 years). Sex: 22 boys and 9 girls. Ethnicity: all white.* Country: UK. Setting: home and school. Sociodemographics: mixture of inner city and rural schools. Mixed socioeconomic status.* Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: parent intervention vs teacher intervention. Intervention schedule: frequency school (1 to 5 times/wk), home (2 to 7 times/wk). Duration of intervention: 7 wk. Mode of delivery: teachers and parents delivered intervention under the weekly guidance of the researcher. Intervention material: functional tasks in settings which are as near as possible to everyday life, such as handwriting. Intervention procedure: profile of the child prepared, priorities identified which were then translated into weekly practises parents and teachers could use. Intervention provider: teachers and parents. Place of intervention: school and home. Intervention compliance: monitored by a questionnaire and informal contact. Teachers kept a record.* |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: none reported.* Measures of participation: not reported. |
|
| Notes | Study start date: not available. Study completion date: not available. Sample calculation: no.* Ethics approval: yes. Comments from study authors Limitations:
Key conclusions of study authors Both teachers and parents provided effective intervention for children with DCD. Comment from review authors Cross‐over design, but the first phase could be considered an RCT with active control. * Email correspondence with study authors: April and May 2014 and January and February 2016. We contacted the first study author several times and obtained supplementary information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing lots 1 or 2.* |
| Allocation concealment (selection bias) | High risk | Not attempted. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not attempted and not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor knew children received parent intervention or parent intervention.* |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition from intervention. |
| Selective reporting (reporting bias) | Low risk | No protocol obtained. All prespecified outcomes of interest reported. |
| Other bias | Low risk | Funding: research funded by a medical/health based charity. Conflict of interest: no information available. Carry‐over effect in cross‐over trials, but only the first phase considered. |
Thornton 2016.
| Methods | Blocked RCT in an unblinded trial. | |
| Participants | Number screened: not stated. Number included: 20. Number followed up: 20. Number of withdrawals: no information. Diagnosis of DCD: no information. Presence and absence of comorbid conditions: no information. Regarding participants completing the study Age: 8 to 10 years. Sex: All boys. Ethnicity: no information. Country: Australia. Setting: no information. Sociodemographics: no information. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Comparison: CO‐OP intervention vs inactive control. Intervention schedule: 60 min at 1/wk for 10 wk plus 15 min/day home activities. Duration of intervention: 10 wk. Mode of delivery: group sessions, with each group consisting 3 to 4 children. Intervention material: global problem solving strategy (GODC method) to achieve handwriting speed and legibility and other fine motor goals. Intervention procedure: the group program was developed to address at least 2 to 3 goals for each child who was encouraged to develop and modify individual plans, while evaluating which strategies were successful and which were not successful in the group. Intervention provider: 2 occupational therapists trained and experienced in the use of the CO‐OP intervention. Place of intervention: no information. Intervention compliance: no information. |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: none.* |
|
| Notes | Study start date: not available. Study completion date: not available. Sample calculation: yes. Ethics approval: yes. Comments from study authors Limitations:
Key conclusions of study authors The CO‐OP group intervention, targeted towards individualised goal attainments, can lead to improvements across impairment, activity and participation. Comment from review authors The author calls the design "quasi‐experimental, pre‐post test study", and "block randomised" each participant to two groups individually matched for age (within six months). Email correspondence with study authors: May 2017. We contacted the first study author several times and obtained supplementary information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Blocked randomisation to match for age in an unblinded trial. An independent party used a simple computerised random number generator.* |
| Allocation concealment (selection bias) | Low risk | ID number unique to each child matched to a corresponding name after randomisation.* |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blind, but impossible to achieve double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Testers were blind to the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After enquiry, the first author stated no attrition. |
| Selective reporting (reporting bias) | Low risk | No protocol published.* All prespecified outcomes of interest reported. |
| Other bias | Low risk | Funding: PhD budget allocated by the University of Western Australia.* Conflicts of interest: the authors reported no conflicts of interest. |
Tsai 2009.
| Methods | Quasi‐RCT. | |
| Participants | Number screened: 286. Number included: 43. Number followed up: 43. Number of withdrawals: 0. Diagnosis of DCD: based on the symptomatic information by parents and special education teachers and MABC test. Presence and absence of comorbid conditions: children were excluded if they had comorbid conditions that may impact on the tests or intervention. Regarding participants completing the study Age: 9 to 10 years. Sex: DCD table‐tennis training (6 boys, 7 girls),* DCD inactive control (6 boys, 8 girls),* non‐DCD inactive control (13 boys and 16 girls). Ethnicity: Taiwanese. Potentially very low rate of aborigine.* Country: Taiwan. Setting: Primary schools.* Sociodemographics: urban residents of Kaohsiung.* Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Comparison: DCD table‐tennis training vs DCD inactive control vs non‐DCD inactive control. Intervention schedule: 50 min at 3/wk for 10 wk. Duration of intervention: 10 wk.* Mode of delivery: face‐to‐face sessions. Intervention material: table tennis table and equipment, ball‐projection machine. Intervention procedure: warm‐up, main part of table tennis training, playing a game, cool down. Emphasis first on general skills and then on task‐specific skills. Intervention provider: special education and physical education teacher. Place of intervention: school. Intervention compliance: no absence. All enjoyed participation.* |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: none reported by the instructor.* Measures of participation: not reported. |
|
| Notes | Study start date: not available. Study completion date: not available. Sample calculation: no. Ethics approval: yes. Comments from study authors Limitations:*
Key conclusions of study authors Group table tennis training intervention seemed to offer promising results for motor ability and inhibitory control. Comment from review authors A larger effect size of intervention outcome than other studies. This may be due to the quality and the high frequency of training or quasi‐RCT design. * Email correspondence with study authors: April 2014 to February 2016. We contacted the first study author several times and obtained raw data for meta‐analysis and supplementary information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 28 children with DCD, with the Total Impairment Score at or below the 5th percentile cutoff point of the MABC test, were assigned quasi‐randomly to the table tennis DCD‐training group or DCD non‐training group. However, if the child and their parents' consent was not forthcoming in the DCD‐training group, then the child was moved to the DCD non‐training group. |
| Allocation concealment (selection bias) | High risk | Not true randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blind, but impossible to achieve double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After enquiry, the first author stated that blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After enquiry, the first author stated no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol obtained. All prespecified outcomes of interest reported. |
| Other bias | Low risk | Funding: research funded by a grant from the National Science Council of the Republic of China, Taiwan (NSC 97‐2410‐H‐006‐085). Conflicts of interest: no information available. Small numbers. |
Tsai 2012.
| Methods | Quasi‐RCT. | |
| Participants | Number screened: 368. Number included: 52 (30 children with DCD who were quasi‐randomised plus 22 typically developing children). Number followed up: 52. Number of withdrawals: 0. Diagnosis of DCD: DSM‐IV. Presence and absence of comorbid conditions: children were excluded if they had comorbid conditions that may impact on the tests or intervention. Regarding participants completing the study Age: overall range 9 to 10 years: DCD soccer training (mean ± SD): 116.81 ± 5.37 months; DCD inactive control (mean ± SD): 114.00 ± 3.68 months). Sex: DCD soccer training (9 boys and 7 girls); DCD inactive control (9 boys and 5 girls); non‐DCD control (12 boys and 10 girls). Ethnicity: Taiwanese. Potentially very low rate of aborigine.* Country: Taiwan. Setting: Primary schools.* Sociodemographics: urban residents of Kaohsiung.* Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: DCD soccer training vs DCD inactive control vs non‐DCD inactive control. Intervention schedule: 50 min at 5/wk for 10 wk. Duration of intervention: 10 wk.* Mode of delivery: face‐to‐face group sessions. Intervention material: soccer balls, obstacles, goal posts. Intervention procedure: warm‐up, main part of soccer training, playing a game, cool down. emphasis first on general skills and then on task‐specific skills. Intervention provider: trained soccer coach. Place of intervention: school. Intervention compliance: no absence. All enjoyed participation.* |
|
| Outcomes |
Primary
Secondary
Adverse effects or events: none reported by the instructor.* Measures of participation: not reported. |
|
| Notes | Study start date: not available. Study end date: not available. Sample calculation: no. Ethics approval: yes. Comments from study authors Limitations:*
Key conclusions of study authors Soccer training could significantly facilitate the development of motor skills and improve inhibitory control and neuroelectric indices of attention networks. Comment from review authors A larger effect size of intervention outcome than other studies. This may be due to the quality and the high frequency of training or quasi‐RCT design. * Email correspondence with study authors: May 2014 and February 2016. We contacted the first study author several times and obtained raw data for meta‐analysis and supplementary information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomly subdivided. If consent was not forthcoming then the child was moved to the non‐training group. This is going against the randomisation process. |
| Allocation concealment (selection bias) | High risk | Randomisation was broken. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blind, but impossible to achieve double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After enquiry, the first author stated that blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After enquiry, the first author stated no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol obtained. All prespecified outcomes of interest reported. |
| Other bias | Low risk | Funding: research funded by a grant from the National Science Council in Taiwan (NSC 99‐2314‐B‐006‐006‐MY2 and NSC 98‐2410‐H‐006‐106‐MY2). Conflicts of interest: no information available. Small numbers. |
Wilson 2002.
| Methods | Blocked RCT in an unblinded trial. | |
| Participants | Number screened: not stated. Number included: 54. Number followed up: 54. Number of withdrawals: no information. Diagnosis of DCD: no information. Presence and absence of comorbid conditions: children excluded if they had current or history of neurological disease, including head injury, psychiatric disorders, or attention‐deficit disorder. Regarding participants completing the study Age: 7 to 12 years. Sex: no information. Ethnicity: no information. Country: Australia. Setting: assessment conducted at schools, and training interventions provided in the Centre for Movement Education and Research, Griffith University. Sociodemographics: no information. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: image training vs traditional perceptual‐motor training vs inactive control. Intervention schedule of traditional perceptual‐motor training programme: 60 min once/wk. Duration of intervention: 5 wk. Mode of delivery: face‐to‐face individual sessions. Intervention material: image training: a selection of fundamental motor skills (catching a tennis ball, throwing a tennis ball, striking a softball, jumping to a target using a 2‐leg take‐off, balancing a ball on a bat while walking, and placing objects using a formboard); traditional perceptual‐motor training: combination of gross‐motor, fine‐motor, and perceptual‐motor activities (hopping, skipping, jumping, climbing, and marching activities with hoops, ropes, ladders, and a trampoline; throwing, catching, and striking balls and quoits; scooter boards; balance beams, boards, and balls; fitball aerobics; pegwork; origami; and handwriting activities). Intervention procedure: image training: observation of video‐audio sequences of a selection of fundamental motor skills, mental exercise, and physical practise; traditional perceptual‐motor training: approach was client centred, with sufficient scope for children to engage in activities of interest while at the same time developing areas of weakness. Intervention provider: 2 research assistants with experience in conventional approaches to perceptual‐motor therapy. Place of intervention: Centre for Movement Education and Research, Griffith University. Intervention compliance: no information. |
|
| Outcomes |
Primary
Adverse effects or events: no information. Measures of participation: no information. |
|
| Notes | Study start date: not available. Study completion date: not available. Sample calculation: no information. Ethics approval: yes. Comments from study authors Limitations:
Key conclusions of study authors
Comment from review authors Not all children below the 50th percentile have DCD. Email correspondence with study authors: January 2016. We wrote to the authors twice to request supplementary information on data but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not stated. No reply to enquiry. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. No reply to enquiry. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, but impossible anyway. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent evaluator blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | No protocol obtained. All prespecified outcomes of interest reported. |
| Other bias | Low risk | Funding: research funded by an Australian Research Council grant awarded by Griffith University. Conflict of interest: no information available. Small numbers. |
Wilson 2016.
| Methods | Blocked RCT in an unblinded trial. | |
| Participants | Number screened: not stated. Number included: 42. Number followed up: 36. Number of withdrawals: 6. Diagnosis of DCD: no information. Presence and absence of comorbid conditions: children excluded if they had current or history of neurological disease, including head injury, psychiatric disorders, or attention‐deficit disorder. Regarding participants completing the study Age: 7 to 12 years. Sex: no information. Ethnicity: no information. Country: Australia. Setting: assessment conducted at schools, and training interventions provided in the Centre for Movement Education and Research, Griffith University. Sociodemographics: no information. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Comparison: image training vs traditional perceptual‐motor training vs inactive control. Intervention schedule of traditional perceptual‐motor training programme: 60 min once/wk. Duration of intervention: 5 wk. Mode of delivery: face‐to‐face individual sessions. Intervention material: Image training: six fundamental motor skills: catching and throwing a tennis ball, striking a softball, jumping, balancing a ball on a bat while walking, and placing objects on a form board. Traditional perceptual‐motor training: various static and dynamic balance activities using hoops, low beams, fit balls, ropes, and mini trampoline, minor ball games, pegboard games,origami, and drawing activities. Intervention procedure: Image training: visual imagery exercises, relaxation protocol and mental preparation, mental rehearsal of skills from external and internal perspectives, mental rehearsal displayed on a LCD monitor. Traditional perceptual‐motor training: approach was collaborative and child centred. Intervention provider: trained research assistant/therapist Place of intervention: no information. Compliance: no information. |
|
| Outcomes |
Primary
Adverse effects or events: no information. Measures of participation: no information. |
|
| Notes | Study start date: not available. Study completion date: not available. Sample calculation: yes. Ethics approval: yes. Comments from study authors Limitations:
Key conclusions of study authors
Comment from review authors Email correspondence with study authors: May 2017. We wrote to the authors twice to request supplementary information on data but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not stated. No reply to enquiry. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. No reply to enquiry. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | All children were assessed and trained individually under one of the three training conditions. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Six of 42 children (14%) from unspecified groups dropped out of the study due to competing family commitments. |
| Selective reporting (reporting bias) | Low risk | No protocol obtained. However, this study is a replication of Wilson 2002, meaning that the method of the previous study served as a protocol. |
| Other bias | Low risk | Funding: no information available. Conflicts of interest: no information available. Baseline imbalance. |
BOTMP: Bruininks‐Oseretsky Test of Motor Proficiency; COPM: Canadian Occupational Performance Measure; CO‐OP: Cognitive Orientation to daily Occupational Performance; CTA: contemporary treatment approach; DCD: developmental co‐ordination disorder; DCDQ: Developmental Coordination Disorder Questionnaire; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; GPDC: Goal‐Plan‐Do‐Check; hr: hour; ID: identifier; LCD: liquid crystal display; MABC: Movement Assessment Battery for Children; min: minute; RCT: randomised controlled trial; SD: standard deviation; SOT: Sensory Organization Test; TGMD: Test of Gross Motor Development; TKD: Taekwondo; wk: week.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Caçola 2015 | Study design: not a randomised controlled trial. |
| De Milander 2015 | Study design: not a cluster‐randomised controlled trial, but a quasi‐experimental study, which lacked an element of random assignment. |
| Ferguson 2013 | Study design: not a randomised controlled trial; participants not randomised but allocated according to schools that were not sufficiently similar. |
| Hammond 2014 | Intervention: Wii Fit and active control 'Jump Ahead' may or may not be task‐oriented interventions. |
| Peens 2008 | Intervention: motor‐based intervention programme involved both task‐specific and process‐oriented (kinaesthetic and sensory integration) treatment. |
| Tsai 2014 | Intervention: exercise intervention not considered to be a task‐specific intervention. |
| Yu 2016 | Study design: two clusters only and intra‐cluster correlation coefficient not clear. |
Characteristics of studies awaiting assessment [ordered by study ID]
Farhat 2016.
| Methods | Unclear if randomised. |
| Participants | DCD. |
| Interventions | Task specific based. |
| Outcomes | Includes MABC. |
| Notes |
Comment from review authors Email correspondence with study authors: May 2017. We wrote to the authors twice, to request supplementary information on data, but received no reply. |
DCD: developmental co‐ordination disorder; MABC: Movement Assessment Battery for Children.
Differences between protocol and review
Please see our protocol (Miyahara 2014).
Methods section
Types of outcomes
We moved adverse effects from our list of secondary outcomes to our primary outcomes in keeping with MECIR standards (Chandler 2013).
Search methods for identification of studies
We added WorldCat (worldcat.org) as an international source of theses, and searched for relevant systematic reviews in the Cochrane Database of Systematic Reviews (CDSR) and Database of Abstracts of Reviews of Effects (DARE). We also searched Ovid MEDLINE Epub Ahead of Print and Ovid MEDLINE In‐Process & Other Non‐Indexed Citations to find the most current MEDLINE content. Since the protocol for this review was published (Miyahara 2014), Current Controlled Trials has been replaced by the ISTRCN Registry (isrctn.com), and the metaRegister of Controlled Trials is under review and no longer in service. We did not search SportDiscus because the database was no longer available to the editorial base or review team.
Data collection and analysis
We specified in our protocol (Miyahara 2014), that " Two authors (SH and MM) will independently assess all studies identified by the search strategy for inclusion." However, this task was performed by MM and LP for two studies on which SLH is an author (ACTRN12614000106639; Hillier 2010). See also: Declarations of interest.
Data extraction and management
We specified that MM and SN would independently extract and manage data, but this task was performed by MM and SLH.
Assessment of risk of bias in included studies
We specified that we also assessed the blinding of participants and personnel (even though this was impossible given the nature of the intervention), and other potential sources of bias.
Measures of treatment effect
Continuous outcome data
Because all the studies that were included in meta‐analyses employed the MABC, we used the mean difference (MD) instead of the standardised mean difference (SMD). In future updates of this review, should we encounter studies that use different measures for the same outcome, we will use the standardised mean difference (SMD) (Borenstein 2009).
Multiple outcome data
As there was an insufficient number of included studies with postintervention data at multiple time points, we were unable to conduct a separate meta‐analysis using the SMD from both postintervention and follow‐up phases, as planned. We used immediate postintervention data only for meta‐analyses.
Dichotomous data
We found one trial, Pless 2000b, which reported dichotomous data. Pless 2000b divided participants into definite motor difficulties (MABC scores < 5th percentile) and borderline motor difficulties (MABC scores between 5th and 15th percentile) because no participant improved beyond the 15th percentile after intervention. It should be noted that in future studies, there is a possibility that some participants may improve beyond the 15th percentile, which would create trichotomous data. In this circumstance a future review would need a consensus to be reached as to whether the diagnostic cutoff should be the 5th or 15th percentile; this would enable dichotomous data to be extracted. Should we find such data in future updates of this review, we will calculate the odds ratio and convert them to SMDs (Borenstein 2009).
We used the latest version of Review Manager 5.3 to perform the analyses (Review Manager 2014).
Unit of analysis issues
For cross‐over trials, we decided to extract the outcome data from the first phase of the intervention period only, to avoid carry‐over effects. This is a change from the protocol where we said we would account for the carry‐over effect by obtaining (inter‐individual) correlation coefficients between pre‐ and postintervention periods (Miyahara 2014).
We were unable to include cluster‐randomised trials in the meta‐analyses. In future updates of this review, we will endeavour to obtain the intra‐cluster correlation coefficients from the study authors, as necessary, so we may appropriately integrate the effect from cluster‐randomised trials when calculating the SMDs and corresponding variances.
Dealing with missing data.
As expected, we did not have any missing data that required multiple imputation. Should we encounter such data in future updates of this review, we will conduct a sensitivity analysis using a multiple imputation technique for missing subgroup information, assuming the data are missing at random (Pigott 2012).
Assessment of heterogeneity
As we used the random‐effects model, we also reported Tau2, which is an estimate of between‐study variability.
In future updates of this review, where we encounter moderate to high heterogeneity, we will conduct a random‐effects meta‐analysis and explore the causes of heterogeneity by conducting subgroup analyses and meta‐regression, providing we have sufficient studies.
Assessment of reporting bias
We did not assess small study effects as we only included six studies in meta‐analyses. In future updates of this review, should we include sufficient studies, we will conduct a visual assessment of funnel plot asymmetry to identify possible publication bias and other small study effects (Sterne 2011). If funnel asymmetry is due to a lack of data points in the non‐statistically significant region of a funnel plot, we will interpret the funnel plot asymmetry as an indicator of possible publication bias; that is, multiple or singular publication of research findings, depending on the nature and direction of the results. We will only create a funnel plot for a meta‐analysis that contains at least 10 studies (SMDs).
Data synthesis
We decided to present the results of the random‐effects model only. We conducted a sensitivity analysis using the fixed‐effect model to assess the robustness of the results.
In future updates of this review, we will conduct subsequent meta‐analyses on subgroups according to the criteria described in the 'Subgroup analysis and investigation of heterogeneity' section, should we find sufficient studies. Also, where studies are unsuitable for meta‐analysis, we will provide narrative descriptions of their results.
Summary of findings tables
We included a new section on 'Summary of findings' tables in the Methods, at the request of the editorial base.
Subgroup analysis and investigation of heterogeneity
We could not conduct any subgroup analyses due to the small number of included studies with limited independent variables. In future updates of this review, if we find sufficient studies, we will perform the following subgroup analyses.
Age (preschool versus junior; primary versus senior; primary versus secondary or high school).
Sex (male versus female).
Severity of DCD in terms of cutoffs used for standard performance outcome tests and questionnaires (for example, second percentile; fifth percentile; 15th percentile).
Intervention intensity calculated as a combination of frequency and duration (for example, < 3 times a week versus ≧ 3 times a week; or < 6 weeks versus ≧ 6 weeks).
Type of intervention (for example, NTT versus CO‐OP).
Sensitivity analysis
Due to the small number of eligible studies, we were unable to conduct our preplanned sensitivity analyses. In future updates of this review, we will conduct sensitivity analyses to explore the impact of all three aspects of study quality (risk of bias) associated with issues of blinding of outcome assessment, completeness of data, and sequence generation and allocation concealment.
We tested the robustness of the results by conducting the following, post hoc sensitivity analyses.
Fixed‐effect model, combining MDs (not SMDs) for the reason explained above, versus random‐effects model.
Meta‐analysis combining two RCTs and three quasi‐RCTs versus meta‐analysis of two RCTs only.
Contributions of authors
Motohide Miyahara (MM) drafted the protocol and all authors contributed advice.
Susan L Hillier (SLH) and Liz Pridham (LP) performed second review author roles with MM, from inclusion through to risk of bias and data extraction stages.
Shinichi Nakagawa provided statistical advice.
MM, SLH, and LP wrote the final review text.
Sources of support
Internal sources
-
University of Otago, New Zealand.
In the form of a salary for Motohide Miyahara and Shinichi Nakagawa (to March 2015)
-
University of South Australia, Australia.
In the form of a salary for Susan Hillier and Liz Pridham
-
University of New South Wales, Australia.
In the form of salary for Shinichi Nakagawa (from 1 March 2015)
External sources
None, Other.
Declarations of interest
Motohide Miyahara (MM): none known.
Susan L Hillier (SLH) is an author of two included studies: Hillier 2010 and ACTRN12614000106639. SLH was not involved in assessing the risk of bias or the quality of the evidence from these studies. These tasks were performed by two independent review authors (MM and LP).
Liz Pridham (LP): none known.
Shinichi Nakagawa: none known.
Edited (no change to conclusions)
References
References to studies included in this review
ACTRN12614000106639 {published and unpublished data}
- Miyahara M. Request for supplementary information [personal communication]. Email to: E Ward 24 February 2016.
- Miyahara M. Request for supplementary information [personal communication]. Email to: E Ward 25 February 2016.
- Ward E. Adverse effects or events [personal communication]. Email to: M Miyahara 26 February 2016.
- Ward E. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 25 February 2016.
- Ward E. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 26 February 2016.
- Ward E, Hillier S, Raynor A, Petkov J. A range of service delivery modes for children with developmental coordination disorder are effective: a randomised controlled trial, 2014. www.anzctr.org.au/TrialSearch.aspx?searchTxt=ACTRN12614000106639 (accessed 30 April 2017). [DOI] [PubMed]
Au 2014 {published data only}
- Au MK, Chan WM, Lee L, Chen TM, Chau RM, Pang MY. Core stability exercise is as effective as task‐oriented motor training in improving motor proficiency in children with developmental coordination disorder: a randomized controlled pilot study. Clinical Rehabilitation 2014;28(10):992‐1003. [DOI: 10.1177/0269215514527596; PUBMED: 24668358] [DOI] [PubMed] [Google Scholar]
- Miyahara M. Request for supplementary information [personal communication]. Email to: M Pang 10 May 2016.
- Miyahara M. Request for supplementary information [personal communication]. Email to: M Pang 27 April 2016.
Fong 2012 {published data only (unpublished sought but not used)}
- Fong SM. Effect of Taekwando Training on Sensori‐Motor Performance and Postural Control in Children With and Without Developmental Coordination Disorder [PhD thesis]. Hong Kong: Hong Kong Polytechnic University, 2012. [Google Scholar]
- Fong SS, Chung JW, Chow LP, Ma AW, Tsang WW. Differential effect of Taekwondo training on knee muscle strength and reactive and static balance control in children with developmental coordination disorder: a randomized controlled trial. Research in Developmental Disabilities 2013;34(5):1446‐55. [DOI: 10.1016/j.ridd.2013.01.025; PUBMED: 23474997] [DOI] [PubMed] [Google Scholar]
- Miyahara M. Request for supplementary information [personal communication]. Email to: S Fong and W Tsang 25 February 2016.
- Miyahara M. Request for supplementary information [personal communication]. Email to: S Fong and W Tsang 4 February 2016.
Fong 2016 {published data only}
- Fong SS, Guo X, Liu KP, Ki WY, Louie LH, Chung RCK, et al. Task‐specific balance training improves the sensory organisation of balance control in children with developmental coordination disorder: a randomised controlled trial. Scientific Reports 2016;6:20945. [DOI: 10.1038/srep20945; PMC4750073] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara M. Request for supplementary data [personal communication]. Email to: S Fong 11 May 2016.
- Miyahara M. Request for supplementary data [personal communication]. Email to: S Fong 2 May 2017.
Green 2008 {published and unpublished data}
- Green D. Adverse effects or events [personal communication]. Email to: M Miyahara 1 February 2016.
- Green D. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 1 February 2016.
- Green D. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 29 June 2015.
- Green D. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 5 January 2016.
- Green D, Chambers ME, Sugden DA. Does subtype of developmental coordination disorder count: is there a differential effect on outcome following intervention?. Human Movement Science 2008;27(2):363‐82. [DOI: 10.1016/j.humov.2008.02.009; PUBMED: 18400322] [DOI] [PubMed] [Google Scholar]
- Miyahara M. Request for supplementary data [personal communication]. Email to: D Green 29 June 2015.
- Miyahara M. Request for supplementary information [personal communication]. Email to: D Green 31 January 2016.
- Miyahara M. Request for supplementary information [personal communication]. Email to: D Green 5 January 2016.
Hillier 2010 {published and unpublished data}
- Hillier S. Adverse effects or events [personal communication]. Email to: M Miyahara 3 February 2016.
- Hillier S. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 3 February 2016.
- Hillier S. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 4 February 2016.
- Hillier S, McIntyre A, Plummer L. Aquatic physical therapy for children with developmental coordination disorder: a pilot randomized controlled trial. Physical and Occupational Therapy in Pediatrics 2010;30(2):111‐24. [DOI: 10.3109/01942630903543575; PUBMED: 20367516] [DOI] [PubMed] [Google Scholar]
- Miyahara M. Request for supplementary data [personal communication]. Email to: S Hillier 3 February 2016.
- Miyahara M. Request for supplementary data [personal communication]. Email to: S Hillier 4 February 2016.
Hung 2010 {published data only}
- Hung WW, Pang MY. Effects of group‐based versus individual‐based exercise training on motor performance in children with developmental coordination disorder: a randomized controlled study. Journal of Rehabilitation Medicine 2010;42(2):122‐8. [DOI: 10.2340/16501977-0496; PUBMED: 20140407] [DOI] [PubMed] [Google Scholar]
- M Pang. Reply to request for supplementary data [personal communication]. Email to: M Miyahara 13 February 2014.
- Miyahara M. Request for supplementary data [personal communication]. Email to: M Pang 13 February 2014.
- Miyahara M. Request for supplementary information [personal communication]. Email to: M Pang 25 February 2016.
- Miyahara M. Request for supplementary information [personal communication]. Email to: M Pang 3 February 2016.
Miller 2001 {published data only (unpublished sought but not used)}
- H Polatajko. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 4 May 2016.
- Miller LT, Polatajko HJ, Missiuna C, Mandich AD, Macnab JJ. A pilot trial of a cognitive treatment for children with developmental coordination disorder. Human Movement Science 2001;20(1‐2):183‐210. [PUBMED: 11471396] [DOI] [PubMed] [Google Scholar]
- Miyahara M. Request for supplementary information [personal communication]. Email to: H Polatajko 10 May 2016.
- Miyahara M. Request for supplementary information [personal communication]. Email to: H Polatajko 3 May 2016.
- Polatajko H. Adverse effects or events [personal communication]. Email to: M Miyahara 4 May 2016.
- Polatajko H. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 12 May 2016.
- Polatajko HJ, Mandich AD, Miller LT, Macnab JJ. Cognitive Orientation to daily Occupational Performance (CO‐OP): part II ‐ the evidence. Physical and Occupational Therapy in Pediatrics 2001;20(2‐3):83‐106. [PUBMED: 11345514] [PubMed] [Google Scholar]
Pless 2000b {published and unpublished data}
- Miyahara M. Request for supplementary information [personal communication]. Email to: M Pless 21 January 2016.
- Miyahara M. Request for supplementary information [personal communication]. Email to: M Pless 31 January 2016.
- Pless M. Adverse effects or events [personal communication]. Email to: M Miyahara 31 January 2016.
- Pless M. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 25 January 2016.
- Pless M. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 31 January 2016.
- Pless M, Carlsson M, Sundelin C, Persson K. Effects of group motor skill intervention on five‐ to six‐year old children with developmental coordination disorder. Pediatric Physical Therapy 2000;12(4):183‐9. [PUBMED: 17091030] [PubMed] [Google Scholar]
- Pless M, Carlsson M, Sundelin C, Persson K. Pre‐school children with developmental co‐ordination disorder: self‐perceived competence and group motor skill intervention. Acta Paediatrica 2001;90(5):532‐8. [PUBMED: 11430713] [PubMed] [Google Scholar]
Sugden 2003 {published and unpublished data}
- Miyahara M. Request for supplementary information [personal communication]. Email to: D Sugden 11 January 2016.
- Miyahara M. Request for supplementary information [personal communication]. Email to: D Sugden 12 February 2016.
- Miyahara M. Request for supplementary information [personal communication]. Email to: D Sugden 16 April 2014.
- Miyahara M. Request for supplementary information [personal communication]. Email to: D Sugden 2 February 2016.
- Miyahara M. Request for supplementary information [personal communication]. Email to: D Sugden 5 January 2016.
- Sugden D. Adverse effects or events [personal communication]. Email to: M Miyahara 12 February 2016.
- Sugden D. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 1 May 2014.
- Sugden D. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 12 February 2016.
- Sugden D. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 12 January 2016.
- Sugden DA, Chambers ME. Intervention in children with Developmental Coordination Disorder: the role of parents and teachers. British Journal of Educational Psychology 2003;73(4):545‐61. [DOI: 10.1348/000709903322591235; PUBMED: 14713377] [DOI] [PubMed] [Google Scholar]
- Sugden DA, Chambers ME. Stability and change in children with developmental coordination disorder. Child: Care, Health and Development 2007;33(5):520‐8. [DOI: 10.1111/j.1365-2214.2006.00707.x] [DOI] [PubMed] [Google Scholar]
Thornton 2016 {published data only (unpublished sought but not used)}
- Miyahara M. Request for further details [personal communication]. Email to: A Thornton 16 May 2017.
- Miyahara M. Request for further details [personal communication]. Email to: A Thornton 3 May 2017.
- Miyahara M. Request for further details [personal communication]. Email to: A Thornton 9 May 2017.
- Thornton A. Adverse effects or events [personal communication]. Email to: M Miyahara 5 May 2017.
- Thornton A. Clarification of some study details [personal communication]. Email to: M Miyahara 9 May 2017.
- Thornton A. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 20 May 2017.
- Thornton A. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 9 May 2017.
- Thornton A, Licari M, Reid S, Armstrong J, Fallows R, Elliott C. Cognitive Orientation to (Daily) Occupational Performance intervention leads to improvements in impairments, activity and participation in children with Developmental Coordination Disorder. Disability and Rehabilitation 2016;38(10):979‐86. [DOI: 10.3109/09638288.2015.1070298; PUBMED: 26213242] [DOI] [PubMed] [Google Scholar]
Tsai 2009 {published and unpublished data}
- Miyahara M. Request for supplementary data [personal communication]. Email to: C Tsai 2 April 2014.
- Miyahara M. Request for supplementary information [personal communication]. Email to: C Tsai 1 May 2015.
- Tsai CL. Adverse effects or events [personal communication]. Email to: M Miyahara 8 February 2016.
- Tsai CL. Reply to request for supplementary data [personal communication]. Email to: M Miyahara 3 April 2014.
- Tsai CL. Reply to request for supplementary information [personal communication]. Email to: M Miyahara 4 May 2015.
- Tsai CL. The effectiveness of exercise intervention on inhibitory control in children with developmental coordination disorder: using a visuospatial attention paradigms a model. Research in Developmental Disabilities 2009;30(6):1268‐80. [DOI: 10.1016/j.ridd.2009.05.001; PUBMED: 19497707] [DOI] [PubMed] [Google Scholar]
Tsai 2012 {published and unpublished data}
- Miyahara M. Request for supplementary data [personal communication]. Email to: C Tsai 3 May 2014.
- Tsai CL. Adverse effects or events [personal communication]. Email to: M Miyahara 8 February 2016.
- Tsai CL. Reply to request for supplementary data [personal communication]. Email to: M Miyahara 4 May 2014.
- Tsai CL, Wang CH, Tseng YT. Effects of exercise intervention on event‐related potential and task performance indices of attention networks in children with developmental coordination disorder. Brain and Cognition 2012;79(1):12‐22. [DOI: 10.1016/j.bandc.2012.02.004; PUBMED: 22387276] [DOI] [PubMed] [Google Scholar]
Wilson 2002 {published data only (unpublished sought but not used)}
- Miyahara M. Request for supplementary data [personal communication]. Email to: P Wilson 12 January 2016.
- Miyahara M. Request for supplementary data [personal communication]. Email to: P Wilson 25 January 2016.
- Wilson PH, Thomas PR, Maruff P. Motor imagery training ameliorates motor clumsiness in children. Journal of Child Neurology 2002;17(7):491‐8. [DOI: 10.1177/088307380201700704; PUBMED: 12269727] [DOI] [PubMed] [Google Scholar]
Wilson 2016 {published data only (unpublished sought but not used)}
- Miyahara M. Request for supplementary data [personal communication]. Email to: P Wilson 16 May 2017.
- Miyahara M. Request for supplementary data [personal communication]. Email to: P Wilson 3 May 2017.
- Wilson PH, Adms ILJ, Caeyenberghs K, Thomas P, Smits‐Engelsman B, Steenbergen B. Motor imagery training enhances motor skill in children with DCD: A replication study. Research in Developmental Disabilities 2016;57:54‐62. [DOI: 10.1016/j.ridd.2016.06.014; PUBMED: 27388492] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Caçola 2015 {published data only}
- Caçola P, Romero M, Ibana M, Chuang J. Effects of two distinct group motor skill interventions in psychological and motor skills of children with Developmental Coordination Disorder: a pilot study. Disability and Health Journal 2015;40(2):165‐75. [DOI: 10.1016/j.dhjo.2015.07.007; PUBMED: 26329699] [DOI] [PubMed] [Google Scholar]
De Milander 2015 {published and unpublished data}
- Milander M, Coetzee FF, Venter A. Perceptual‐motor intervention for developmental coordination disorder in grade 1 children. South African Journal for Research in Sport, Physical Education and Recreation 2015;37(2):15‐32. [Google Scholar]
- Wilson PH, Adams IL, Caeyenberghs K, Thomas P, Smits‐Engelsman B, Steenbergen B. Motor imagery training enhances motor skill in children with DCD: a replication study. Research in Developmental Disabilities 2016;57:54‐62. [DOI: 10.1016/j.ridd.2016.06.014; PUBMED: 27388492] [DOI] [PubMed] [Google Scholar]
Ferguson 2013 {published data only}
- Ferguson GD, Jelsma D, Jelsma J, Smits‐Engelsman BC. The efficacy of two task‐orientated interventions for children with developmental coordination disorder: Neuromotor task training and Nintendo Wii Fit training. Research in Developmental Disabilities 2013;34(9):2449‐61. [DOI: 10.1016/j.ridd.2013.05.007; PUBMED: 23747936] [DOI] [PubMed] [Google Scholar]
Hammond 2014 {published data only}
- Hammond J, Jones V, Hill EL, Green D, Male I. An investigation of the impact of regular use of the Wii Fit to improve motor and psychosocial outcomes in children with movement difficulties: a pilot study. Child: Care, Health and Development 2014;40(2):165‐75. [DOI: 10.1111/cch.12029; PUBMED: 23363371] [DOI] [PubMed] [Google Scholar]
Peens 2008 {published data only}
- Peens A, Pienaar AE, Nienaber AW. The effect of different intervention programmes on the self‐concept and motor proficiency of 7‐ to 9‐year‐old children with DCD. Child: Care, Health and Development 2008;34(3):316‐28. [DOI: 10.1111/j.1365-2214.2007.00803.x; PUBMED: 18294260] [DOI] [PubMed] [Google Scholar]
Tsai 2014 {published data only}
- Tsai CL, Chang YK, Chen FC, Hung TM, Pan CY, Wang CH. Effects of cardiorespiratory fitness enhancement on deficits in visuospatial working memory in children with developmental coordination disorder: a cognitive electrophysiological study. Archives of Clinical Neuropsychology 2014; Vol. 29, issue 2:173‐85. [DOI: 10.1093/arclin/act081; PUBMED: 24172937] [DOI] [PubMed]
Yu 2016 {published data only}
- Yu J, Sit CH, Burnett A, Capio CM, Ha AS, Huang WY. Effects of fundamental movement skills training on children with developmental coordination disorder. Adapted Physical Activity Quarterly 2016;33(2):134‐55. [DOI: 10.1123/APAQ.2015-0008; PUBMED: 27078269] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Farhat 2016 {published data only}
- Farhat F, Hsairi I, Baati H, Smits‐Engelsman BCM, Masmoudi K, McHirgui R, et al. The effect of a motor skills training program in the improvement of practiced and non‐practiced tasks performance in children with developmental coordination disorder (DCD). Human Movement Science 2016;46:10‐22. [DOI: 10.1016/j.humov.2015.12.001; PUBMED: 26703915] [DOI] [PubMed] [Google Scholar]
- Miyahara M. Request for supplementary data [personal communication]. Email to: F Farhat 19 May 2017.
- Miyahara M. Request for supplementary data [personal communication]. Email to: F Farhat 3 May 2017.
Additional references
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
Ayres 1979
- Ayres J. Sensory Integration and the Child. Los Angeles (CA): Western Psychological Services, 1979. [Google Scholar]
Beery 1997
- Beery KE. The Developmental Test of Visual‐Motor Integration ‐ Revised. Parsippany (NJ): Modern Curriculum Press, 1997. [Google Scholar]
Blank 2012
- Blank R. European Academy of Childhood Disability (EACD): recommendations on the definition, diagnosis and intervention of developmental coordination disorder (pocket version). German‐Swiss interdisciplinary clinical practice guideline S3‐standard according to the Association of the Scientific Medical Societies in Germany. Pocket version. Definition, diagnosis, assessment, and intervention of developmental coordination disorder (DCD). Developmental Medicine and Child Neurology 2012;54(11):e1‐7. [DOI: 10.1111/j.1469-8749.2011.04175.x; PUBMED: 22320659] [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. Chichester: John Wiley & Sons, 2009. [Google Scholar]
Bruininks 1978
- Bruininks RH. The Bruininks‐Oseretsky Test of Motor Proficiency. Circle Pines (MN): American Guidance Service, 1978. [Google Scholar]
Bruininks 2005
- Bruininks RH, Bruininks BD. The Bruininks‐Oseretsky Test of Motor Proficiency. 2nd Edition. Circle Pines (MN): American Guidance Service, 2005. [Google Scholar]
Cairney 2005
- Cairney J, Hay JA, Faught BE, Wade TJ, Corna L, Flouris A. Developmental coordination disorder, generalized self‐efficacy toward physical activity, and participation in organized and free play activities. Journal of Pediatrics 2005;147(4):515‐20. [DOI: 10.1016/j.jpeds.2005.05.013; PUBMED: 16227039] [DOI] [PubMed] [Google Scholar]
Cairney 2006
- Cairney J, Hay JA, Wade TJ, Faught BE, Flouris A. Developmental coordination disorder and aerobic fitness: is it all in their heads or is measurement still the problem?. American Journal of Human Biology 2006;18(1):66‐70. [PUBMED: 16378337] [DOI] [PubMed] [Google Scholar]
Cairney 2007
- Cairney J, Hay JA, Faught BE, Flouris A, Klentrou P. Developmental coordination disorder and cardiorespiratory fitness in children. Pediatric Exercise Science 2007;19(1):20‐8. [PUBMED: 17554154] [DOI] [PubMed] [Google Scholar]
Cantell 1994
- Cantell MH, Smyth MM, Ahonen TP. Clumsiness in adolescence: educational, motor, and social outcomes of motor delay detected at 5 years. Adapted Physical Activity Quarterly 1994;11(2):115‐29. [DOI: 10.1123/apaq.11.2.115] [DOI] [Google Scholar]
Chalmers 2001
- Chalmers I. Forward. In: Egger M, Smith GD, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. London: BMJ Publishing, 2001:xiii‐xviii. [Google Scholar]
Chandler 2013
- Chandler J, Churchill R, Higgins J, Lasserson T, Tovey D. Methodological standards for the conduct of new Cochrane Intervention Reviews. editorial‐unit.cochrane.org/sites/editorial‐unit.cochrane.org/files/uploads/MECIR_conduct_standards%202.3%2002122013.pdf (accessed 31 May 2017).
Cousins 2003
- Cousins M, Smyth MM. Developmental coordination impairments in adulthood. Human Movement Science 2003;22(4‐5):433‐59. [PUBMED: 14624827] [DOI] [PubMed] [Google Scholar]
Cratty 1989
- Cratty BJ. Adapted Physical Education in the Mainstream. Denver (CO): Love Publishing Company, 1989. [Google Scholar]
Cummins 2005
- Cummins A, Piek JP, Dyck MJ. Motor coordination, empathy, and social behaviour in school‐aged children. Developmental Medicine and Child Neurology 2005;47(7):437‐42. [PUBMED: 15991862] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Fougeyrollas 1998
- Fougeyrollas P, Noreau L, Bergeron H, Cloutier R, Dion SA, St‐Michel G. Social consequences of long term impairments and disabilities: conceptual approach and assessment of handicap. International Journal of Rehabilitation Research 1998;21(2):127‐42. [PUBMED: 9924676] [DOI] [PubMed] [Google Scholar]
Gibbs 2007
- Gibbs J, Appleton J, Appleton R. Dyspraxia or developmental coordination disorder? Unravelling the enigma. Archives of Disease in Childhood 2007;92(6):534‐9. [DOI: 10.1136/adc.2005.088054; PMC2066137] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadders‐Algra 2003
- Hadders‐Algra M. Developmental coordination disorder: is clumsy motor behaviour caused by a lesion of the brain at early age?. Neural Plasticity 2003;10(1‐2):39‐50. [DOI: 10.1155/NP.2003.39; PMC2565415; PUBMED: 14640306] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harter 1982
- Harter S. The Perceived Competence Scale for Children. Child Development 1982;53(1):87‐97. [DOI: 10.2307/1129640] [DOI] [Google Scholar]
Harter 1984
- Harter S, Pike R. The Pictorial Scale of Perceived Competence and Social Acceptance for young children. Child Development 1984;55(6):1969‐82. [PUBMED: 6525886] [PubMed] [Google Scholar]
Harter 1985
- Harter S. Self Perception Profile for Children. Denver (CO): University of Denver, 1985. [Google Scholar]
Henderson 1992
- Henderson SE, Sugden DA. Movement Assessment Battery for Children. London (UK): Psychological Corporation, 1992. [Google Scholar]
Henderson 2007
- Henderson SE, Sugden DA, Barnett A. Movement Assessment Battery for Children. 2nd Edition. London (UK): Pearson, 2007. [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI: 10.1136/bmj.d5928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hillier 2007
- Hillier SL. Intervention for children with developmental coordination disorder: a systematic review. Internet Journal of Allied Health Science and Practice 2007;5(3):1‐11. [Google Scholar]
Hoerzer 2014
Kashiwagi 2009
- Kashiwagi M, Iwaki S, Narumi Y, Tamai H, Suzuki S. Parietal dysfunction in developmental coordination disorder: a functional MRI study. NeuroReport 2009;20(15):1319‐24. [DOI: 10.1097/WNR.0b013e32832f4d87; PUBMED: 19730138] [DOI] [PubMed] [Google Scholar]
Keogh 1985
- Keogh J, Sugden D. Movement Skill Development. New York (NY): Macmillan, 1985. [Google Scholar]
Kiresuk 1968
- Kiresuk TJ, Sherman RE. Goal Attainment Scaling: a general method for evaluating comprehensive community mental health programs. Community Mental Health Journal 1968;4(6):443‐53. [DOI: 10.1007/BF01530764; PUBMED: 24185570] [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [PUBMED: 20156912] [DOI] [PubMed] [Google Scholar]
Law 1990
- Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian Occupational Performance Measure: an outcome measure for occupational therapy. Canadian Journal of Occupational Therapy 1990;57(2):82‐7. [DOI: 10.1177/000841749005700207; PUBMED: 10104738] [DOI] [PubMed] [Google Scholar]
Law 1998
- Law M, Baptiste S, Carswell A, McColl MA, Polatajko H, Pollock N. Canadian Occupational Performance Measure. 2nd Edition. Toronto (ON): CAOT Publications ACE, 1998. [Google Scholar]
Lingam 2009
- Lingam R, Hunt L, Golding J, Jongmans M, Emond A. Prevalence of developmental coordination disorder using the DSM‐IV at 7 years of age: a UK population‐based study. Pediatrics 2009;123(4):e693‐700. [DOI: 10.1542/peds.2008-1770; PUBMED: 19336359] [DOI] [PubMed] [Google Scholar]
Lingam 2012
- Lingam R, Jongmans MJ, Ellis M, Hunt LP, Golding J, Emond A. Mental health difficulties in children with developmental coordination disorder. Pediatrics 2012;129(4):e882‐91. [DOI: 10.1542/peds.2011-1556; PUBMED: 22451706] [DOI] [PubMed] [Google Scholar]
Losse 1991
- Losse A, Henderson SE, Elliman D, Hall D, Knight E, Jongmans M. Clumsiness in children ‐ do they grow out of it? A 10‐year follow‐up study. Developmental Medicine and Child Neurology 1991;33(1):55‐68. [PUBMED: 1704864] [DOI] [PubMed] [Google Scholar]
McCarron 1997
- McCarron LT. McCarron Assessment of Neuromuscular Development. Dallas (TX): Common Market Press, 1997. [Google Scholar]
McDougall 1999
- McDougall J, King GA. Goal Attainment Scaling: Description, Utility, and Applications in Pediatric Therapy Services. London (ON): Thames Valley Children's Centre, 1999. [Google Scholar]
Missiuna 2001
- Missiuna C, Mandich AD, Polatajko HJ, Malloy‐Miller T. Cognitive Orientation to daily Occupational Performance (CO‐OP). Physical & Occupational Therapy in Pediatrics 2001;20(2‐3):69‐81. [DOI: 10.1080/J006v20n02_05] [DOI] [PubMed] [Google Scholar]
Missiuna 2008
- Missiuna C, Moll S, King G, Stewart D, Macdonald K. Life experiences of young adults who have coordination difficulties. Canadian Journal of Occupational Therapy 2008;75(3):157‐66. [DOI: 10.1177/000841740807500307; PUBMED: 18615927] [DOI] [PubMed] [Google Scholar]
Miyahara 1996
- Miyahara M. A meta‐analysis of intervention studies on children with developmental coordination disorder. Corpus, Psyche et Societas 1996;3:11‐8. [Google Scholar]
Miyahara 2000
- Miyahara M, Register C. Perceptions of three terms to describe physical awkwardness in children. Research in Developmental Disabilities 2000;21(5):367‐76. [PUBMED: 11100800] [DOI] [PubMed] [Google Scholar]
Miyahara 2006
- Miyahara M, Piek J. Self‐esteem of children and adolescents with physical disabilities: quantitative evidence from meta‐analysis. Journal of Developmental and Physical Disabilities 2006;18(3):219‐34. [DOI: 10.1007/s10882-006-9014-8] [DOI] [Google Scholar]
Miyahara 2016a
- Miyahara M, Rigoli D. Piek, JP. Physical disability and self‐esteem. In: Levesque RJR editor(s). Encyclopedia of Adolescence. 2nd Edition. Cham, Switzerland: Springer International Publishing, 2016:1‐8. [Google Scholar]
Miyahara 2016b
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piek 2005
- Piek JP, Barrett NC, Allen LS, Jones A, Louise M. The relationship between bullying and self‐worth in children with movement coordination problems. British Journal of Educational Psychology 2005;75(Pt 3):453‐63. [DOI: 10.1348/000709904X24573; PUBMED: 16238876] [DOI] [PubMed] [Google Scholar]
Pigott 2012
- Pigott TD. Advances in Meta‐Analysis. New York (NY): Springer, 2012. [Google Scholar]
Pless 2000a
- Pless M, Carlsson M. Effects of motor skill intervention on developmental coordination disorder: a meta‐analysis. Adapted Physical Activity Quarterly 2000;17(4):381‐401. [DOI: ] [Google Scholar]
Preston 2016
- Preston N, Magallón S, Hill LJ, Andrews E, Ahern SM, Mon‐Williams M. A systematic review of high quality randomized controlled trials investigating motor skill programmes for children with developmental coordination disorder. Clinical Rehabilitation 2016 Aug 1 [Epub ahead of print]:1‐14. [DOI: 10.1177/0269215516661014; PUBMED: 27481937] [DOI] [PMC free article] [PubMed]
Revie 1993
- Revie G, Larkin D. Task‐specific intervention with children reduces movement problems. Adapted Physical Activity Quarterly 1993;10(1):29‐41. [DOI: 10.1123/apaq.10.1.29] [DOI] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schoemaker 1994
- Schoemaker MM, Kalverboer AF. Social and affective problems of children who are clumsy: how early do they begin?. Adapted Physical Activity Quarterly 1994;11(2):130‐40. [DOI: 10.1123/apaq.11.2.130] [DOI] [Google Scholar]
Schoemaker 2003
- Schoemaker MM, Niemeijer AS, Reynders K, Smits‐Engelsman BC. Effectiveness of neuromotor task training for children with developmental coordination disorder: a pilot study. Neural Plasticity 2003;10(1‐2):155‐63. [DOI: 10.1155/NP.2003.155; PMC2565416] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Skinner 2001
- Skinner RA, Piek JP. Psychosocial implications of poor motor coordination in children and adolescents. Human Movement Science 2001;20(1‐2):73‐94. [PUBMED: 11471399] [DOI] [PubMed] [Google Scholar]
Smits‐Engelsman 2013
- Smits‐Engelsman BC, Blank R, Kaay A, Mosterd‐van der Meijs R, Vlugt‐van den Brand E, Polatajko HJ, et al. Efficacy of interventions to improve motor performance in children with developmental coordination disorder: a combined systematic review and meta‐analysis. Developmental Medicine and Child Neurology 2013;55(3):229‐37. [DOI: 10.1111/dmcn.12008; PUBMED: 23106530] [DOI] [PubMed] [Google Scholar]
Smyth 2000
- Smyth MM, Anderson HI. Coping with clumsiness in the school playground: social and physical play in children with coordination impairments. British Journal of Developmental Psychology 2000;18(3):389‐413. [DOI: 10.1348/026151000165760] [DOI] [Google Scholar]
Spitzer 1994
- Spitzer RL, Gibbon M, Skodol AE, Williams JBW, First MB. DSM‐IV Casebook: A Learning Companion to the Diagnostic and Statistical Manual of Mental Disorders. Washington (DC): American Psychiatric Press, Inc, 1994. [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sugden 2007
- Sugden D, Henderson SE. Ecological Intervention for Children with Movement Difficulties. London (UK): Harcourt Assessment, 2007. [Google Scholar]
Ulrich 1985
- Ulrich D. Test of Gross Motor Development. Austin (TX): Pro‐Ed, 1985. [Google Scholar]
Ulrich 2000
- Ulrich D. Test of Gross Motor Development. 2nd Edition. Austin (TX): Pro‐Ed, 2000. [Google Scholar]
WHO 2001
- World Health Organization. International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: WHO, 2001. [Google Scholar]
WHO 2010
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision. Geneva, Switzerland: WHO, 2010. [Google Scholar]
Wilson 2009
- Wilson BN, Crawford SG, Green D, Roberts G, Aylott A, Kaplan B. Psychometric properties of the Revised Developmental Coordination Disorder Questionnaire. Physical & Occupational Therapy in Pediatrics 2009;29(2):182‐202. [DOI: 10.1080/01942630902784761; PUBMED: 19401931] [DOI] [PubMed] [Google Scholar]
Wilson 2013
- Wilson PH, Ruddock S, Smits‐Engelsman BC, Polatajko HJ, Blank R. Understanding performance deficits in developmental coordination disorder: a meta‐analysis of recent research. Developmental Medicine and Child Neurology 2013;55(3):217‐28. [DOI: 10.1111/j.1469-8749.2012.04436.x; PUBMED: 23106668] [DOI] [PubMed] [Google Scholar]
Zimmer 2012
- Zimmer M, Desch L. Sensory integration therapies for children with developmental and behavioral disorders. Pediatrics 2012;129(6):1186‐9. [DOI: 10.1542/peds.2012-0876; PUBMED: 22641765] [DOI] [PubMed] [Google Scholar]
Zwicker 2010
- Zwicker JG, Missiuna C, Harris SR, Boyd LA. Brain activation of children with developmental coordination disorder is different than peers. Pediatrics 2010;126(3):e678‐86. [DOI: 10.1542/peds.2010-0059; PUBMED: 20713484] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Miyahara 2014
- Miyahara M, Hillier SL, Pridham L, Nakagawa S. Task‐oriented interventions for children with developmental co‐ordination disorder. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010914] [DOI] [PMC free article] [PubMed] [Google Scholar]


