Abstract
Background
Acetylcholinesterase inhibitors, such as neostigmine, have traditionally been used for reversal of non‐depolarizing neuromuscular blocking agents. However, these drugs have significant limitations, such as indirect mechanisms of reversal, limited and unpredictable efficacy, and undesirable autonomic responses. Sugammadex is a selective relaxant‐binding agent specifically developed for rapid reversal of non‐depolarizing neuromuscular blockade induced by rocuronium. Its potential clinical benefits include fast and predictable reversal of any degree of block, increased patient safety, reduced incidence of residual block on recovery, and more efficient use of healthcare resources.
Objectives
The main objective of this review was to compare the efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade caused by non‐depolarizing neuromuscular agents in adults.
Search methods
We searched the following databases on 2 May 2016: Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE (WebSPIRS Ovid SP), Embase (WebSPIRS Ovid SP), and the clinical trials registries www.controlled‐trials.com, clinicaltrials.gov, and www.centerwatch.com. We re‐ran the search on 10 May 2017.
Selection criteria
We included randomized controlled trials (RCTs) irrespective of publication status, date of publication, blinding status, outcomes published, or language. We included adults, classified as American Society of Anesthesiologists (ASA) I to IV, who received non‐depolarizing neuromuscular blocking agents for an elective in‐patient or day‐case surgical procedure. We included all trials comparing sugammadex versus neostigmine that reported recovery times or adverse events. We included any dose of sugammadex and neostigmine and any time point of study drug administration.
Data collection and analysis
Two review authors independently screened titles and abstracts to identify trials for eligibility, examined articles for eligibility, abstracted data, assessed the articles, and excluded obviously irrelevant reports. We resolved disagreements by discussion between review authors and further disagreements through consultation with the last review author. We assessed risk of bias in 10 methodological domains using the Cochrane risk of bias tool and examined risk of random error through trial sequential analysis. We used the principles of the GRADE approach to prepare an overall assessment of the quality of evidence. For our primary outcomes (recovery times to train‐of‐four ratio (TOFR) > 0.9), we presented data as mean differences (MDs) with 95 % confidence intervals (CIs), and for our secondary outcomes (risk of adverse events and risk of serious adverse events), we calculated risk ratios (RRs) with CIs.
Main results
We included 41 studies (4206 participants) in this updated review, 38 of which were new studies. Twelve trials were eligible for meta‐analysis of primary outcomes (n = 949), 28 trials were eligible for meta‐analysis of secondary outcomes (n = 2298), and 10 trials (n = 1647) were ineligible for meta‐analysis.
We compared sugammadex 2 mg/kg and neostigmine 0.05 mg/kg for reversal of rocuronium‐induced moderate neuromuscular blockade (NMB). Sugammadex 2 mg/kg was 10.22 minutes (6.6 times) faster then neostigmine 0.05 mg/kg (1.96 vs 12.87 minutes) in reversing NMB from the second twitch (T2) to TOFR > 0.9 (MD 10.22 minutes, 95% CI 8.48 to 11.96; I2 = 84%; 10 studies, n = 835; GRADE: moderate quality).
We compared sugammadex 4 mg/kg and neostigmine 0.07 mg/kg for reversal of rocuronium‐induced deep NMB. Sugammadex 4 mg/kg was 45.78 minutes (16.8 times) faster then neostigmine 0.07 mg/kg (2.9 vs 48.8 minutes) in reversing NMB from post‐tetanic count (PTC) 1 to 5 to TOFR > 0.9 (MD 45.78 minutes, 95% CI 39.41 to 52.15; I2 = 0%; two studies, n = 114; GRADE: low quality).
For our secondary outcomes, we compared sugammadex, any dose, and neostigmine, any dose, looking at risk of adverse and serious adverse events. We found significantly fewer composite adverse events in the sugammadex group compared with the neostigmine group (RR 0.60, 95% CI 0.49 to 0.74; I2 = 40%; 28 studies, n = 2298; GRADE: moderate quality). Risk of adverse events was 28% in the neostigmine group and 16% in the sugammadex group, resulting in a number needed to treat for an additional beneficial outcome (NNTB) of 8. When looking at specific adverse events, we noted significantly less risk of bradycardia (RR 0.16, 95% CI 0.07 to 0.34; I2= 0%; 11 studies, n = 1218; NNTB 14; GRADE: moderate quality), postoperative nausea and vomiting (PONV) (RR 0.52, 95% CI 0.28 to 0.97; I2 = 0%; six studies, n = 389; NNTB 16; GRADE: low quality) and overall signs of postoperative residual paralysis (RR 0.40, 95% CI 0.28 to 0.57; I2 = 0%; 15 studies, n = 1474; NNTB 13; GRADE: moderate quality) in the sugammadex group when compared with the neostigmine group. Finally, we found no significant differences between sugammadex and neostigmine regarding risk of serious adverse events (RR 0.54, 95% CI 0.13 to 2.25; I2= 0%; 10 studies, n = 959; GRADE: low quality).
Application of trial sequential analysis (TSA) indicates superiority of sugammadex for outcomes such as recovery time from T2 to TOFR > 0.9, adverse events, and overall signs of postoperative residual paralysis.
Authors' conclusions
Review results suggest that in comparison with neostigmine, sugammadex can more rapidly reverse rocuronium‐induced neuromuscular block regardless of the depth of the block. Sugammadex 2 mg/kg is 10.22 minutes (˜ 6.6 times) faster in reversing moderate neuromuscular blockade (T2) than neostigmine 0.05 mg/kg (GRADE: moderate quality), and sugammadex 4 mg/kg is 45.78 minutes (˜ 16.8 times) faster in reversing deep neuromuscular blockade (PTC 1 to 5) than neostigmine 0.07 mg/kg (GRADE: low quality). With an NNTB of 8 to avoid an adverse event, sugammadex appears to have a better safety profile than neostigmine. Patients receiving sugammadex had 40% fewer adverse events compared with those given neostigmine. Specifically, risks of bradycardia (RR 0.16, NNTB 14; GRADE: moderate quality), PONV (RR 0.52, NNTB 16; GRADE: low quality), and overall signs of postoperative residual paralysis (RR 0.40, NNTB 13; GRADE: moderate quality) were reduced. Both sugammadex and neostigmine were associated with serious adverse events in less than 1% of patients, and data showed no differences in risk of serious adverse events between groups (RR 0.54; GRADE: low quality).
Keywords: Adult, Humans, Neuromuscular Blockade, Androstanols, Androstanols/antagonists & inhibitors, Atracurium, Atracurium/analogs & derivatives, Atracurium/antagonists & inhibitors, Cholinesterase Inhibitors, Cholinesterase Inhibitors/administration & dosage, Cholinesterase Inhibitors/adverse effects, Cholinesterase Inhibitors/pharmacology, Neostigmine, Neostigmine/administration & dosage, Neostigmine/adverse effects, Neostigmine/pharmacology, Neuromuscular Nondepolarizing Agents, Neuromuscular Nondepolarizing Agents/antagonists & inhibitors, Randomized Controlled Trials as Topic, Rocuronium, Sugammadex, Time Factors, Vecuronium Bromide, Vecuronium Bromide/antagonists & inhibitors, gamma‐Cyclodextrins, gamma‐Cyclodextrins/administration & dosage, gamma‐Cyclodextrins/adverse effects, gamma‐Cyclodextrins/pharmacology
Plain language summary
Benefits and harms of sugammadex versus neostigmine in reversing induced paralysis
Background
Different levels of induced paralysis are sometimes necessary when patients are put to sleep or are prepared for operations. When the operation is finished, paralysis should be reversed in a fast, reliable, and safe way. Neostigmine is a medication that is traditionally used to reverse induced paralysis. However, its use can be associated with incomplete or slow reversal as well as changes in lung function, heart function, and vomiting and nausea. Sugammadex is a relatively new medication specifically designed to reverse rocuronium‐induced paralysis in a faster, more reliable, and safer way when compared with neostigmine.
Objective
This review systematically sets out to compare the benefits and harms of sugammadex and neostigmine. The evidence is current up to May 2017.
Study characteristics
We identified 41 randomized controlled trials comparing sugammadex with neostigmine that provided suitable data on efficacy and safety. All of these trials included adults undergoing surgery and involved a total of 4206 participants.
Key results
Data indicate that sugammadex was 10.22 minutes (6.6 times) faster than neostigmine (1.96 vs 12.87 minutes) in reversing moderate induced paralysis. Sugammadex was 45.78 minutes (16.8 times) faster than neostigmine (2.9 vs 48.8 minutes) in reversing deep induced paralysis. Participants receiving sugammadex appeared to have a 40% reduced risk of experiencing harmful events than those given neostigmine. Statistically, eight persons can be treated with sugammadex as opposed to neostigmine to avoid one person experiencing a single random harmful event. The occurrence of serious harmful events was nearly non‐existent and data show no differences between compared groups.
Conclusion
Sugammadex is more efficient and safer than neostigmine for reversing moderate and deep induced paralysis.
Quality of evidence
We consider our overall findings on benefits and harms to provide evidence of moderate quality in favour of sugammadex.
Summary of findings
Summary of findings for the main comparison. Sugammadex 2.0 mg/kg vs neostigmine 0.05 mg/kg.
| Sugammadex 2.0 mg/kg vs neostigmine 0.05 mg/kg | ||||||
| Patient or population: adult patients, ASA I to IV, who received non‐depolarizing NMBAs Setting: elective in‐patient or day‐case surgical procedures performed at centres across Europe and Asia Intervention: sugammadex 2.0 mg/kg Comparison: neostigmine 0.05 mg/kg | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Neostigmine 0.05 mg/kg | Sugammadex 2.0 mg/kg | |||||
| Recovery timea from second twitch (T2) to train‐of‐four ratio (TOFR) > 0.9 (moderate block) | Mean recovery time from T2 to TOFR > 0.9 was 12.87 minutes | Mean recovery time from T2 to TOFR > 0.9 was 1.96 minutes Mean recovery time from T2 to TOFR > 0.9 in the sugammadex group was10.22 minutes faster (8.48 to 11.96 minutes faster) than neostigmine |
‐ | 835 (10 studies) | ⊕⊕⊕⊝c Moderate |
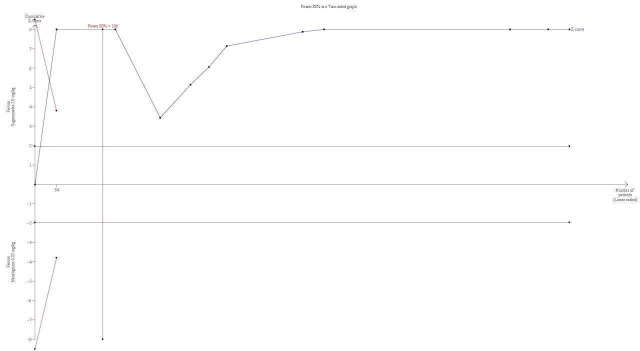
TSA alfa‐boundary adjusted MD is ‐10.22 (95% CI ‐12.11 to ‐8.33; diversity (D2) = 87%, I2 = 84%, random‐effects model, 80% power, alpha 0.05). Cumulative Z‐curve crosses the monitoring boundary (Figure 1) |
| Recovery timea from post‐tetanic count (PTC) 1 to 5 to train‐of‐four ratio (TOFR) > 0.9 (deep block) | Outcome not clinically relevant for this comparison | |||||
| Risks of adverse events and serious adverse eventsb, bradycardia, PONV, and signs of residual neuromuscular blockade | Outcome not analysed for this comparison | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality:We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aRecovery time was measured in minutes from administration of study drug to TOFR > 0.9 by TOF‐watch assessor using acceleromyography at the same monitoring site in all studies (ulnar nerve and adductor pollicis muscle)
bAdverse events and serious adverse events were defined by study authors and were observed and assessed by safety outcome assessors in the operating theatre, in post‐anaesthetic care unit, or up to seven days after surgery, depending on each study. Furthermore, overall clinical signs of postoperative residual paralysis reported by trials were regarded as adverse events in this review. Risk of adverse events was measured as number of adverse events per all participants and/or number of participants experiencing one or more adverse events per all participants, depending on the study. Only adverse events that were possibly, probably, or definitely related to study drug were included in risk assessments
cDowngraded one level owing to high risk of bias (evidence limited by inclusion of data from open‐label studies and studies with potential funding bias ‐ for details, see Figure 2 and Characteristics of included studies)
1.
TSA of all trials comparing sugammadex 2.0 mg/kg vs neostigmine 0.05 mg/kg; recovery time from T2 to TOFR > 0.9 minutes. With a required information size of 106, firm evidence in place favours sugammadex in a random‐effects model, with an alfa‐boundary adjusted MD of ‐10.22 (95% CI ‐12.11 to ‐8.33; diversity (D2) = 87%, I2 = 84%, random‐effects model). The cumulative Z‐curve crosses the monitoring boundary constructed for the required information size with 80% power and alpha of 0.05. However, none of the included trials had low risk of bias, and because TSA is ideally designed for trials with low risk of bias and cannot be adjusted for risk of bias, the precision of our findings has to be downgraded. Furthermore, the degree of diversity and heterogeneity is high, which once again raises questions about the reliability of the calculated required information size.
Summary of findings 2. Sugammadex 4.0 mg/kg vs neostigmine 0.07 mg/kg.
| Sugammadex 4.0 mg/kg vs neostigmine 0.07 mg/kg | ||||||
| Patient or population: adult patients, ASA I to IV, who received non‐depolarizing NMBAs Setting: elective in‐patient or day‐case surgical procedures performed in Italy and USA Intervention: sugammadex 4.0 mg/kg Comparison: neostigmine 0.07 mg/kg | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Neostigmine 0.07 mg/kg | Sugammadex 4.0 mg/kg | |||||
| Recovery timea from second twitch (T2) to train‐of‐four ratio (TOFR) > 0.9 (moderate block) | Outcome not clinically relevant for this comparison. | |||||
| Recovery timea from post‐tetanic count (PTC 1 to 5) to train‐of‐four ratio (TOFR) > 0.9 (deep block) | Mean recovery time from PTC 1 to 5 to TOFR > 0.9 was 48.8 minutes | Mean recovery time from PTC 1 to 5 to TOFR > 0.9 was 2.9 minutes Mean recovery time from PTC 1 to 5 to TOFR > 0.9 in the sugammadex group was 45.78 minutes faster (52.15 to 39.41 minutes faster) than in the neostigmine group |
‐ | 114 (2 studies) | ⊕⊕⊝⊝c Low |
|
| Risk of adverse events and serious adverse eventsb, bradycardia, PONV, and signs of residual neuromuscular blockade | Outcome not analysed for this comparison | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aRecovery time was measured in minutes from administration of study drug to TOFR > 0.9 by TOF‐watch assessor using acceleromyography at the same monitoring site in all studies (ulnar nerve and adductor pollicis muscle)
bAdverse events and serious adverse events were defined by study authors and were observed and assessed by safety outcome assessors in the operating theatre, in the post‐anaesthetic care unit, or up to seven days after surgery, depending on each study. Furthermore, overall clinical signs of postoperative residual paralysis reported by trials were regarded as adverse events in this review. Risk of adverse events was measured as number of adverse events per all participants and/or number of participants experiencing one or more adverse events per all participants, depending on the study. Only adverse events that were possibly, probably, or definitely related to study drug were included in risk assessments
cDowngraded one level owing to high risk of bias (evidence limited by inclusion of data from open‐label studies and studies with potential funding bias ‐ for details, see Figure 2 and Characteristics of included studies) and by one level owing to imprecision (small number of participants, n = 114)
Summary of findings 3. Sugammadex (any dose) vs neostigmine (any dose).
| Sugammadex (any dose) compared to Neostigmine (any dose) | ||||||
| Patient or population: Adult patients, ASA I‐IV, who received non‐depolarizing NMBAs Setting: Elective in‐patient or day‐case surgical procedures performed in centres across Europe, USA and Asia Intervention: Sugammadex (any dose) Comparison: Neostigmine (any dose) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with neostigmine (any dose) | Risk with sugammadex (any dose) | |||||
| Recovery timea from second twitch (T2) to train‐of‐four ratio (TOFR) > 0.9 (moderate block) | Outcome not clinically relevant for this comparison | |||||
| Recovery timea from post‐tetanic count (PTC) 1 to 5 to train‐of‐four ratio (TOFR) > 0.9 (deep block) | Outcome not clinically relevant for this comparison | |||||
| Risk of composite adverse eventsb | 283 per 1000 | 159 per 1000 (137 to 204) | RR 0.60 (0.49 to 0.74) | 2298 (28 studies) | ⊕⊕⊕⊝c Moderate |
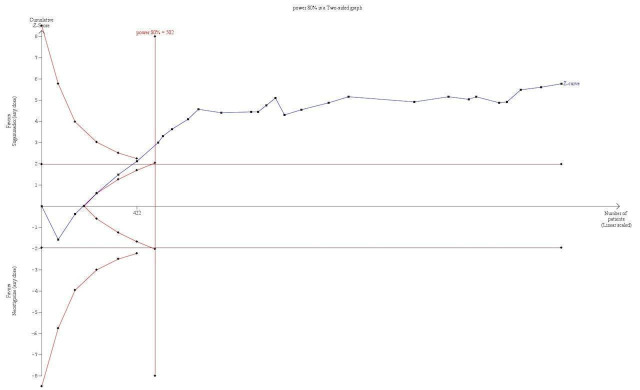
TSA with continuity adjustment for zero event trials (0.001 in each arm); alfa‐boundary adjusted RR 0.62 (95% CI 0.51 to 0.74; diversity (D2) = 34%, I2 = 14%, random‐effects model; 80% power, 0.05 alpha; Figure 3) |
| Bradycardia | 84 per 1000 | 13 per 1000 (6 to 28) | RR 0.16 (0.07 to 0.34) | 1218 (11 studies) | ⊕⊕⊕⊝d Moderate |
|
| PONV | 131 per 1000 | 68 per 1000 (33 to 115) | RR 0.52 (0.28 to 0.97) | 389 (6 studies) | ⊕⊕⊝⊝e Low |
|
| Overall signs of postoperative residual paralysis | 131 per 1000 | 52 per 1000 (37 to 75) | RR 0.40 (0.28 to 0.57) | 1474 (15 studies) | ⊕⊕⊕⊝f Moderate |
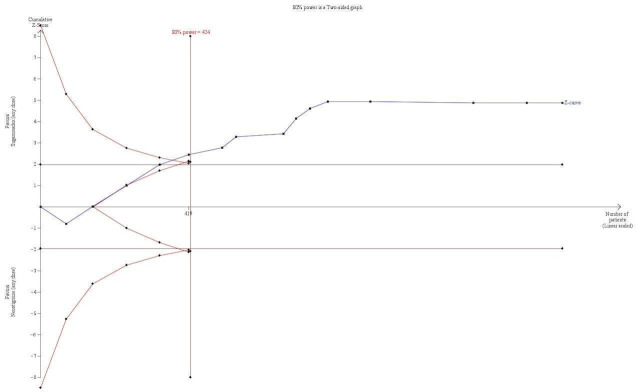
TSA with continuity adjustment for zero event trials (0.001 in each arm): alfa‐boundary adjusted RR 0.4 (95% CI 0.27 to 0.59; diversity (D2) = 0%, I2 = 0%, random‐effects model, 80% power, 0.05 alpha, Figure 4). Cumulative Z‐curve crosses the monitoring boundary constructed for a required information size of 424 participants indicating firm evidence in favour of sugammadex |
| Risk of serious adverse eventsb | 10 per 1000 | 6 per 1000 (1 to 23) | RR 0.54 (0.13 to 2.25) | 959 (10 studies) | ⊕⊕⊝⊝g Low |
TSA with continuity adjustment for zero event trials (0.001 in each arm): alfa‐boundary adjusted RR 0.35 (95% CI 0.00 to 3190; diversity (D2) = 0%, I2 = 0%, random‐effects model, 80% power, alpha 0.05), Cumulative Z‐curve does not cross the monitoring boundary constructed for a required information size of 8189 participants with 11.71% of the required information size included |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aRecovery time was measured in minutes from administration of study drug to TOFR > 0.9 by TOF‐watch assessor using acceleromyography at the same monitoring site in all studies (ulnar nerve and adductor pollicis muscle)
bAdverse events and serious adverse events were defined by study authors and were observed and assessed by safety outcome assessors in the operating theatre, in the post‐anaesthetic care unit or up to seven days after surgery, depending on each study. Furthermore, overall clinical signs of postoperative residual paralysis reported by trials were regarded as adverse events in this review. Risk of adverse events was measured as number of adverse events per all participants and/or number of participants experiencing one or more adverse events per all participants, depending on the study. Only adverse events that were possibly, probably, or definitely related to study drug were included in risk assessments
cDowngraded one level owing to high risk of bias (evidence limited by inclusion of data from open‐label studies and studies with potential funding bias ‐ for details, see Figure 2 and Characteristics of included studies)
dDowngraded one level owing to high risk of bias (evidence limited by inclusion of data from open‐label studies and studies with potential funding bias ‐ for details, see Figure 2 and Characteristics of included studies)
eDowngraded one level owing to high risk of bias (evidence limited by inclusion of data from open‐label studies and studies with potential funding bias ‐ for details, see Figure 2 and Characteristics of included studies) and by one level owing to imprecision (small number of participants‐ n = 389 ‐ and wide confidence interval (CI) ‐ 0.28 to 0.97)
fDowngraded one level owing to high risk of bias (evidence limited by inclusion of data from open‐label studies and studies with potential funding bias ‐ for details, see Figure 2 and Characteristics of included studies)
gDowngraded one level owing to high risk of bias (evidence limited by inclusion of data from open‐label studies and studies with potential funding bias ‐ for details, see Figure 2 and Characteristics of included studies) and by one level owing to imprecision (small number of events ‐ 10/1000 in the neostigmine group vs 6/1000 in the sugammadex group ‐ and wide confidence interval (CI) ‐ 0.13 to 2.25)
3.
TSA of dichotomous data on drug‐related risk of adverse events; sugammadex (any dose) vs neostigmine (any dose). This analyses includes continuity adjustment for zero event trials (0.001 in each arm) resulting in an alfa‐boundary adjusted RR of 0.62 (95% CI 0.51 to 0.74; diversity (D2) = 34%, I2 = 14%, random‐effects model), with a control event proportion of 27.97%. With the required information size of 502, analyses indicated firm evidence favouring sugammadex with 2298 participants included corresponding to a relative risk reduction (RRR) of 38% with 80% power and alpha of 0.05. Despite the fact that the cumulative Z‐curve does not cross the monitoring boundary directly, it is hard to imagine future trials radically changing the overall picture of this analysis. However, none of the included trials were at low risk of bias, and this does downgrade the reliability of our finding.
4.
TSA of dichotomous data on risk of signs of residual neuromuscular blockade; sugammadex (any dose) vs neostigmine (any dose). With continuity adjustment for zero event trials (0.001 in each arm), TSA resulted in an alfa‐boundary adjusted RR of 0.4 (95% CI 0.27 to 0.59; diversity (D2) = 0%, I2 = 0%, random‐effects model, with 80% power and alpha of 0.05), with a control event proportion of 13.08%. Cumulative Z‐curve crosses the monitoring boundary constructed for a required information size of 424 participants, indicating firm evidence in favour of sugammadex. However, none of the included trials had low risk of bias, and this equally diminishes the reliability and precision of our estimates.
Background
After several discussions with the editorial team, a decision was reached to split the original review (Abrishami 2009) into two reviews based on the very extensive number of publications (> 70) identified by the updated search along with various comparators, interventions, and outcome measures.
Description of the condition
Neuromuscular blockade
Neuromuscular blocking agents (NMBAs) are drugs that induce skeletal muscle relaxation primarily by causing a decreased response to the neurotransmitter acetylcholine (ACh) at the neuromuscular junction of skeletal muscle. At that site, ACh normally produces electrical depolarization of the postjunctional membrane of the motor end‐plate, which leads to conduction of muscle action potential and subsequently induces skeletal muscle contraction. Neuromuscular agents are classified as depolarizing or nondepolarizing (PubChem 2016). Non‐depolarizing NMBAs may be further subdivided into aminosteroidal and curariform types of agents.
Use of NMBAs during surgery facilitates tracheal intubation, protects patients from vocal cord injury, and improves surgical conditions by suppressing voluntary or reflex skeletal muscle movements (Bowman 2006; Keating 2016). Following surgery, relaxation is no longer needed, it is important that effects of the NMBA can be quickly and effectively terminated. Postoperative residual neuromuscular blockade and resulting muscle weakness caused by non‐depolarizing NMBAs have been shown to be associated with increased mortality and morbidity (Pedersen 1994; Shorten 1993). Residual neuromuscular blockade may result in pulmonary complications, for example, laboured breathing, low oxygen levels in the blood, lung infection, and entry of gastric contents into the lungs (Berg 1997; Bevan 1996; Eriksson 1993; Eriksson 1997; Murphy 2006; Murphy 2008; Sundman 2000). It can also lead to a postoperative decrease in muscle strength with associated complications, such as visual difficulties and delayed recovery and discharge time (Murphy 2011). Postoperative residual blockade frequently occurs after routine anaesthesia (Viby‐Mogensen 1979). Its incidence varies among trials depending on the type of NMBA used. Some studies have demonstrated a lower incidence of residual block following short‐acting or intermediate‐acting NMBAs in comparison with long‐acting agents (Bevan 1988; Brull 1991). However, postoperative residual neuromuscular blockade may still occur in the short‐acting or intermediate‐acting NMBA group, with incidence ranging from 16% to 60% (Appelbaum 2003; Baillard 2005; Bevan 1996; Debaene 2003; Fawcett 1995; Hayes 2001; Kim 2002; Maybauer 2007; McCaul 2002).
Monitoring of neuromuscular blockade
The degree of neuromuscular blockade is monitored by assessment of various patterns of electrical stimulation. The train‐of‐four (TOF) twitch stimulation was developed as a clinical tool that could be used to assess neuromuscular block in the anaesthetized patient (Ali 1970). This strategy involves stimulating the ulnar nerve with four supramaximal 200 microsecond stimuli separated by 0.5 seconds. This approach is repeated every 10 seconds. Twitches on a TOF pattern fade as relaxation increases. This enables the observer to compare T1 (first twitch of the TOF) versus T0 (control), as well as T4 (fourth twitch of the TOF) versus T1. This T1/T4 ratio is known as the TOF ratio (TOFR). Satisfactory recovery from neuromuscular block and clinical absence of residual curarization have not occurred until the TOFR is > 0.9 (Viby‐Mogensen 2000), contrary to TOFR > 0.7, as previously suggested (Ali 1971). During profound non‐depolarizing neuromuscular block, no response to TOF twitch stimulation may occur. In such circumstances, a post‐tetanic count (PTC) may be useful (Viby‐Mogensen 1981). If a 5 second tetanic stimulus at 50 Hz is administered, after no twitch response has been elicited, followed 3 seconds later by additional single twitches at 1 Hz, response to single twitch stimulation may occur. Although this pattern will not be seen during very profound block, a response will be seen in the early stages of recovery, before the TOF reappears. The number of post‐tetanic twitches is an indication of when the first twitch of the TOF will reappear.
The muscle response to peripheral nerve stimulation can be assessed by visual and tactile methods and by electromyography, acceleromyography, and mechanomyography. Visual observation and palpation of the contracting muscle group are the easiest but least accurate methods of assessing neuromuscular block. Acceleromyography was introduced for clinical use in 1988 (Jensen 1988; Viby‐Mogensen 1988). This technique measures acceleration of a distal digit, which is directly proportionate to the force of muscle contraction and therefore is inversely proportionate to the degree of neuromuscular block.
The monitor consists of an acceleration transducer (i.e. a piezo‐electric ceramic wafer with an electrode on each side) and a stimulation and computing unit. The transducer can be fastened to the thumb, and when the finger is moved in response to nerve stimulation, a voltage difference develops between the two electrodes. The voltage then is measured and is registered in the computing unit.
Description of the intervention
Reversal of neuromuscular blockade
The most commonly used NMBA reversal agents are neostigmine and edrophonium, both of which are cholinesterase inhibitors. They antagonize both aminosteroidal and curariform types of non‐depolarizing NMBAs by inhibiting the breakdown of ACh in the neuromuscular junction (NMJ), causing, ACh to bind the receptor and depolarize the muscle fibre and allowing greater transmission of nerve impulses. These medications, however, require that a muscarinic antagonist (e.g. glycopyrrolate, atropine) be used to compensate for their cholinergic side effects such as bradycardia, hypotension, bronchoconstriction, and postoperative nausea and vomiting (Tramer 1999). Adverse effects associated with the use of muscarinic antagonists include tachycardia, dry mouth, and urinary retention (Mirakhur 1985).
In contrast to cholinesterase inhibitors, the NMBA reversal agent sugammadex does not interfere with acetylcholinesterase receptor systems; therefore, it does not produce the muscarinic side effects associated with other reversal medications for NMBAs. Sugammadex is a synthetically modified ɣ‐cyclodextrin, a chemical structure with a hydrophilic exterior and a hydrophobic core. It was specifically designed to reverse rocuronium‐induced paralysis by encapsulating rocuronium; however, its inner cavity is large enough to encapsulate other aminosteroidal NMBAs such as vecuronium and, to a much lesser degree, pancuronium (Golembiewski 2016; Naguib 2009). Sugammadex does not bind nor does it reverse the neuromuscular blocking effects of curariform NMBAs. Upon binding, it creates a complex formation between the molecule and the aminosteroidal NMBA, which results in more rapid reversal of the neuromuscular blockade than is achieved by anticholinesterase drugs (Park 2015). Sugammadex does not bind to plasma proteins and is not metabolized. It is excreted unchanged in the urine by the kidneys. Renal clearance of sugammadex is rapid ‐ most of the dose (70%) is excreted within six hours (Golembiewski 2016).
How the intervention might work
The positively charged quaternary nitrogen of the aminosteroidal NMBA forms electrostatic bonds with negatively charged interior groups of sugammadex to encapsulate rocuronium and vecuronium (Golembiewski 2016). Sugammadex forms a stable, inactive 1:1 complex with rocuronium or vecuronium; this reduces the amount of free NMBA that is available to bind to nicotinic acetylcholine receptors at the neuromuscular junction, resulting in reversal of neuromuscular blockade (Keating 2016). Once the NMBA is removed from its site of action and is rendered inactive (by encapsulation within the sugammadex molecule in the plasma), neuromuscular transmission and muscle function are restored. By reversing aminosteroid‐induced neuromuscular blockade, one can avoid the associated risks caused by residual block, can shorten time in the operating room, and can improve the patient's quality of recovery and discharge time (Arbous 2005).
Why it is important to do this review
Residual neuromuscular block is a common complication in the post‐anaesthesia care unit, with approximately 40% of patients exhibiting a TOFR < 0.9 (Murphy 2010). The clinical safety and efficacy of sugammadex in reversing rocuronium‐induced neuromuscular blockade have been studied in several randomized controlled trials (RCTs) that compared this medication versus placebo or conventional reversal agents (de Boer 2007; Gijsenbergh 2005; Sacan 2007; Sorgenfrei 2006; Sparr 2007). The aim of our review was to update the best available evidence on this topic and to assess the efficacy and safety of sugammadex and neostigmine in reversal of neuromuscular blockade. We aimed to systematically review RCTs conducted to examine sugammadex and neostigmine administration.
Objectives
The main objective of this review was to compare the efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade caused by non‐depolarizing neuromuscular agents in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs irrespective of publication status, date of publication, blinding status, outcomes published, or language. We contacted trial investigators and study authors to ask for relevant data. We included unpublished trials only if trial data and methodological descriptions were provided in written form or could be retrieved from the trial authors. We excluded observational studies. We did not include studies using a non‐standard design, such as cross‐over trials and cluster‐randomized trials.
Types of participants
We included adults (> 18 years of age) classified as American Society of Anesthesiologists (ASA) I to IV who had received non‐depolarizing NMBAs for an elective in‐patient or day‐case surgical procedure, and who consented to be included in the study. We did not include paediatric participants, healthy volunteers, or participants not undergoing surgical procedures.
Types of interventions
We included all trials comparing sugammadex versus neostigmine in adults receiving non‐depolarizing NMBAs. We included any dose of sugammadex and neostigmine and any time point of administration of study drug.
We excluded trials that compared sugammadex and neostigmine versus only placebo or no intervention.
Types of outcome measures
Primary outcomes
Recovery time from second twitch (T2) to TOFR > 0.9
Recovery time from post‐tetanic count (PTC) 1 to 5 to TOFR > 0.9
For our first primary outcome "Recovery time from T2 to TOFR > 0.9", we compared sugammadex 2 mg/kg versus neostigmine 0.05 mg/kg. For our second primary outcome "Recovery time from PTC 1 to 5 to TOFR > 0.9", we compared sugammadex 4 mg/kg versus neostigmine 0.07 mg/kg. In all studies, the TOF‐watch assessor used acceleromyography to measure recovery time in minutes from administration of the study drug to TOFR > 0.9 at the same monitoring site (ulnar nerve and adductor pollicis muscle).
Secondary outcomes
Risk of adverse events
Risk of serious adverse events
Study authors defined and safety outcome assessors observed and assessed adverse events and serious adverse events in the operating theatre, in the post‐anaesthetic care unit, or up to seven days after surgery, depending on each study. Furthermore, this review regarded as adverse events overall clinical signs of postoperative residual paralysis reported by trial authors. We measured risk of adverse events as the number of adverse events per all participants and/or the number of participants experiencing one or more adverse events per all participants. We included in risk assessments only adverse events that were possibly, probably, or definitely related to study drug. We included in the analysis adverse events and serious adverse events observed following any administered dose of sugammadex and neostigmine and at any time point of study drug administration. Additionally, for the purposes of this review, we presented adverse events as specific adverse events as well as composite adverse events, defined as the combination of all adverse events.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4); MEDLINE (WebSPIRS Ovid SP, 1950 to 2 May 2016); and Embase (WebSPIRS Ovid SP, 1980 to 2 May 2016). We applied no language restrictions. We did a top‐up search in May 2017. For specific information regarding our search strategies and results, please see Appendix 1, Appendix 2, and Appendix 3.
Searching other resources
We searched for ongoing clinical trials and unpublished trials at the following Internet sites.
clinicaltrials.gov
We handsearched the reference lists of reviews, randomized and non‐randomized trials, and editorials for additional trials. We contacted the main authors of trials in this field to ask about missed, unreported, and ongoing trials. We applied no language restrictions to eligible reports.
We conducted the latest search on 2 May 2016, along with a top‐up search in May 2017.
Data collection and analysis
Two review authors (AMH, PD) independently screened and classified all citations as potential primary studies, review articles, or other; independently examined all potentially eligible primary trials and decided on their inclusion in the review; and furthermore independently extracted data from each trial and evaluated data on methods and outcomes in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We (AMH, PD) resolved disagreements by discussion and by consultation with the last review author (AA).
Selection of studies
We assessed articles identified via the described searches and excluded obviously irrelevant reports. Two review authors (AMH, PD) independently examined articles and screened titles and abstracts to identify eligible trials. We completed this process without blinding to study authors, institutions, journals of publication, or results. We resolved disagreements by reaching consensus among two review authors (AMH, PD) and by consultation with the last review author (AA). We listed all excluded trials along with reasons for their exclusion in the Characteristics of excluded studies table.
Data extraction and management
We independently extracted and collected data from each trial without blinding to study authors, source institutions, or publication sources of trials. We resolved disagreements by discussion and approached all first authors of included trials for additional information on risks of bias. For more detailed information, please see Contributions of authors.
Assessment of risk of bias in included studies
We evaluated the validity and design characteristics of each trial.
We evaluated trials for major potential sources of bias (random sequence generation, allocation concealment, blinding of participants, blinding of personnel, blinding of primary outcome assessor, blinding of secondary outcome assessor, incomplete outcome data, selective reporting, funding bias and other bias; see Appendix 4). We assessed each trial quality factor separately and defined trials as having low risk of bias only if they adequately fulfilled all of the criteria described below.
Measures of treatment effect
For our primary outcome (recovery time to TOFR > 0.9), we used mean differences (MDs) with 95% confidence intervals (CIs) because data were continuous and were measured in the same way by all trials. For our secondary outcomes (risks of adverse events and serious adverse events), we calculated risk ratios (RRs) with 95% CIs for dichotomous data (binary outcomes), which were measured in the same way between trials. We also presented data for primary and secondary outcomes as relative differences. (See Data collection and analysis section.)
Unit of analysis issues
Trials with multiple intervention groups
In accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we combined data for secondary outcomes extracted from trials with two or more groups receiving different doses of sugammadex or neostigmine. We excluded trials that compared only different doses of sugammadex or different doses of neostigmine, as well as trials without a control group.
Cross‐over trials
We planned to exclude cross‐over trials from our meta‐analyses because of potential risk for “carry‐over” of treatment effect. However, we identified no cross‐over trials through our search.
Dealing with missing data
We contacted the authors of trials with missing data to retrieve relevant information. For all included trials, we noted levels of attrition and any exclusions. In cases of missing data, we chose 'complete‐case analysis’ for our primary outcomes, which excludes from the analysis all participants for whom the outcome is missing.
Selective outcome reporting, which occurs when non‐significant results are selectively withheld from publication (Chan 2004), is defined as selection, on the basis of trial results, of a subset of the original variables recorded for inclusion in publication of trials (Hutton 2000). The most important types of selective outcome reporting include selective omission of outcomes from reports; selective choice of data for an outcome; selective reporting of different analyses using the same data; selective reporting of subsets of the data; and selective underreporting of data (Higgins 2011).
Assessment of heterogeneity
We explored heterogeneity using the I2 statistic and the Chi2 test. An I2 statistic above 50% represents substantial heterogeneity (Higgins 2011). In cases of substantial heterogeneity, we tried to determine the cause of heterogeneity by performing relevant subgroup and sensitivity analyses (excluding potential outliers to see visual impact of the overall value of the I2 statistic on forest plots). We used the Chi² test to provide an indication of heterogeneity between trials, with a P value ≤ 0.1 considered significant. However, in cases of presumed substantial clinical heterogeneity within an analysis, we planned to use the random‐effects model independent of I2 value.
Assessment of reporting biases
We included both published and unpublished studies during the selection process. We attempted to source published protocols for each of our included studies by using clinical trials registers. We compared published protocols versus published study results to assess the risk of selective reporting bias. Two review authors (AMH and PD) resolved disagreements by discussion and by consultation with the last review author (AA). As we included a sufficient number of studies (greater than 10), we assessed reporting biases (such as publication bias) by using funnel plots. We used the asymmetry of the funnel plot to assess risk of publication and other reporting bias (Higgins 2011). An asymmetrical funnel plot may indicate publication of only positive results (Egger 1997).
Data synthesis
Data analysis
We used Review Manager software (RevMan 5.3.5) and calculated MDs with 95% CIs for continuous outcomes, and RRs with 95% CIs for dichotomous variables. We used the Chi2 test to obtain an indication of heterogeneity between trials, with P ≤ 0.1 considered significant. We quantified the degree of heterogeneity observed in the results by using the I² statistic, which can be interpreted as the proportion of total variation observed between trials that is attributable to differences between trials rather than to sampling error (Higgins 2011). I² > 75% is considered as very heterogeneous. However, we chose a random‐effects model for all of our analyses because clinical heterogeneity was a considerable issue beside the inter‐study heterogeneity expressed by the I² statistic. Thus, we saw little rationale to carry out comparative analyses examining the impact of the choice between using a fixed‐effect versus a random‐effects model.
Trial sequential analysis
Risk of type 1 errors in meta‐analyses due to sparse data and repeated significance testing following updates with new trials remains a serious concern (Brok 2009; Thorlund 2009; Wetterslev 2008; Wetterslev 2009). As a result, spurious P values due to systematic errors from trials with high risk of bias, outcome reporting bias, publication bias, early stopping for benefit, and small trial bias may result in false conclusions. In a single trial, interim analysis increases the risk of type 1 errors. To avoid type 1 errors, group sequential monitoring boundaries (Lan 1983) are used to decide whether a trial could be terminated early because of a sufficiently small P value, with the cumulative Z‐curve crossing the monitoring boundary.
Sequential monitoring boundaries can be applied equally to meta‐analyses and are labelled 'trial sequential monitoring boundaries’. In 'trial sequential analysis’ (TSA), the addition of each new trial to a cumulative meta‐analysis is viewed as an interim meta‐analysis, which provides useful information on the need for additional trials (Wetterslev 2008).
It is appropriate and wise to adjust new meta‐analyses for multiple testing on accumulating data to control overall type 1 error risk in cumulative meta‐analysis (Pogue 1997; Pogue 1998; Thorlund 2009; Wetterslev 2009).
When TSA is performed, the cumulative Z‐curve crossing the boundary indicates that a sufficient level of evidence has been reached; as a consequence, one may conclude that no additional trials may be needed. However, evidence is insufficient to allow a conclusion if the Z‐curve does not cross the boundary or does not surpass the required information size.
To construct trial sequential monitoring boundaries (TSMBs), one needs a required information size, which is calculated as the least number of participants required in a well‐powered single trial with low risk of bias (Brok 2009; Pogue 1998; Wetterslev 2008).
In this updated review, we adjusted the required information size for heterogeneity by using the diversity adjustment factor (Wetterslev 2009). We applied TSA, as it prevents an increase in the risk of type 1 errors (20%). If the actual accrued information size was too small, we provided the required information size in the light of actual diversity (Wetterslev 2009).
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analyses.
-
Sugammadex 2.0 mg/kg versus neostigmine 0.05 mg/kg: recovery time from T2 to TOFR > 0.9
Total intravenous anaesthesia (TIVA) versus volatile anaesthetics
-
Sugammadex, any dose, versus neostigmine, any dose: adverse events
Composite adverse events: different dosages of sugammadex versus neostigmine
Composite adverse events: TIVA versus volatile anaesthetics
Bradycardia: atropine versus glycopyrrolate
Postoperative nausea and vomiting (PONV): TIVA versus volatile anaesthetics
If analyses of various subgroups were significant, we planned to perform a test of interaction (Altman 2003). We considered P values < 0.05 as indicating significant interaction between treatments and subgroup categories. However, because subgroup analyses showed no significant differences, we performed no tests of interaction.
Sensitivity analysis
We conducted the following sensitivity analyses.
Sugammadex 2.0 mg/kg versus neostigmine 0.05 mg/kg, recovery time from T2 to TOFR > 0.9, excluding meeting abstracts
Sugammadex, any dose, versus neostigmine, any dose, composite adverse events, excluding meeting abstracts
Summary of findings table and GRADE
We used the principles of the GRADE approach to perform an overall assessment of evidence related to all of our outcomes. We constructed a 'Summary of findings' table using GradePro software. As outcomes of clinical interest, we chose to present recovery time from T2 to TOFR > 0.9 (moderate block); recovery time from PTC 1 to 5 to TOFR > 0.9 (deep block); risks of adverse events, serious adverse events, bradycardia, and PONV; and signs of residual neuromuscular blockade (see Table 1; Table 2; and Table 3).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Results of the search
In May 2016, through electronic searches and searches of the references of potentially relevant articles, we identified 2502 publications. We excluded 2431 publications, as they were duplicates (n = 675), measured clearly irrelevant outcomes, or were not RCTs. We retrieved a total of 72 relevant publications for further assessment. Of these, 14 were ongoing trials, one trial was awaiting classification, and 16 were excluded with reasons. We reran the search in May 2017 and identified 513 citations (503 by searching databases and 10 by searching clinical trials). Upon reading titles/excluding duplicates, we found 11 studies of interest; of these, two are awaiting classification, six are ongoing, and three were excluded with explanation. In total, 41 RCTs (N = 4206) met our inclusion criteria. Of these, 31 trials (N = 2559) were eligible for meta‐analyses, 20 are ongoing, and three are awaiting classification. We have provided search results in a flow chart in Figure 5.
5.
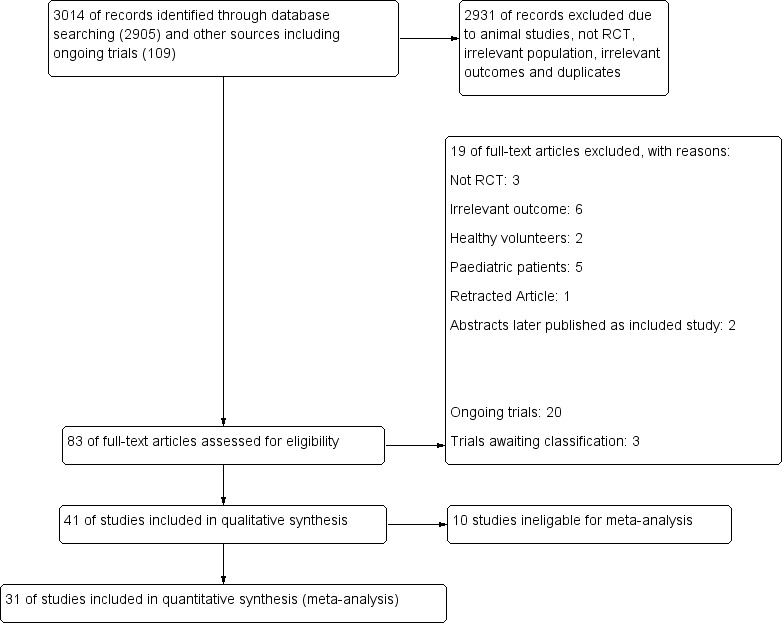
Study flow diagram.
Included studies
We included 41 trials (4206 participants) in our review.
Publication type
Of the 41 included trials, 29 (71%) were published as full‐text papers (Adamus 2011; Blobner 2010; Brueckmann 2015; Carron 2013; Castro 2014; Cheong 2015; Flockton 2008; Gaszynski 2011; Geldner 2012; Hakimoglu 2016; Illman 2011; Isik 2016; Jones 2008; Kaufhold 2016; Khuenl‐Brady 2010; Kizilay 2016; Koc 2015; Koyuncu 2015; Lemmens 2010; Martini 2014; Mekawy 2012; Pongracz 2013; Rahe‐Meyer 2014; Sabo 2011; Schaller 2010; Tas 2015; Woo 2013; Wu 2014; Yagan 2015). Twelve (29%) of the 41 trials were available only as meeting abstracts (Balaka 2011; Foletto 2014; Georgiou 2013; Grintescu 2009; Kogler 2012; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Raziel 2013; Riga 2014; Sherman 2014; Sustic 2012). All of the included trials were published in English, with the exception of one article that was published in Turkish (Koc 2015). We contacted all 41 trial authors for missing information; 12 (29%) replied and provided supplementary data.
Participants and settings
We reported full details of participants and settings in the Characteristics of included studies section.
Of the 41 included studies, 30 were single‐centre studies conducted in 15 countries: Turkey (seven studies: Hakimoglu 2016, Isik 2016, Kizilay 2016, Koc 2015, Koyuncu 2015, Tas 2015, Yagan 2015), Croatia (five studies: Kogler 2012, Kvolik 2012a, Kvolik 2012b, Kvolik 2013, Sustic 2012), Greece (three studies: Balaka 2011, Georgiou 2013, Riga 2014), Germany (two studies: Kaufhold 2016, Schaller 2010), Israel (two studies: Raziel 2013, Sherman 2014), Italy (two studies: Carron 2013, Foletto 2014) and one study each in Egypt (Mekawy 2012), Hungary (Pongracz 2013), Netherlands (Martini 2014), Czech Republic (Adamus 2011), Portugal (Castro 2014), Poland (Gaszynski 2011), Romania (Grintescu 2009), Korea (Cheong 2015), and USA (Brueckmann 2015). Eleven were multiple‐centre studies: 22 European centres in Rahe‐Meyer 2014, 13 European centres in Blobner 2010 and Khuenl‐Brady 2010, 10 European centres in Geldner 2012, nine US centres in Jones 2008 and Lemmens 2010, eight European centres in Flockton 2008, seven Korean centres in Woo 2013, six Chinese plus four European centres in Wu 2014, two Finnish centres in Illman 2011, and an unspecified number of US centres in Sabo 2011.
The sample size of included trials ranged from 22 to 1198 adults (aged > 18 years) with ASA status I to IV. Among studies reporting ASA status, the distribution of participants across groups was as follows: ASA I: 1003 participants (32%); ASA II: 1772 participants (56%); ASA III: 331 participants (11%); and ASA IV: 31 participants (1%).
Five trials included only morbidly obese (MOB) participants (Carron 2013; Castro 2014; Foletto 2014; Gaszynski 2011; Raziel 2013), and one trial focused on super‐obese (SO) patients (Georgiou 2013). One trial included participants classified as New York Heart Association (NYHA) II to III (Kizilay 2016), and one trial investigated participants with myasthenia gravis (Balaka 2011).
Participants underwent diverse elective surgical procedures under general anaesthesia: extreme lateral interbody fusion (Adamus 2011); trans‐sternal thymectomy (Balaka 2011); laparoscopic or open abdominal surgery (Brueckmann 2015); laparoscopic removal of adjustable gastric banding (Carron 2013); laparoscopic bariatric surgery (Castro 2014); laparoscopic sleeve gastrectomy (Foletto 2014; Raziel 2013; Sherman 2014); elective bariatric surgery (Gaszynski 2011); laparoscopic cholecystectomy or appendectomy (Geldner 2012); laparoscopic cholecystectomy (Grintescu 2009; Sustic 2012); open bariatric surgery (Georgiou 2013); arthroscopic surgery (Hakimoglu 2016); non‐cardiac surgery (Kizilay 2016); interventional bronchoscopy (Kogler 2012); extremity surgery (Koyuncu 2015); thyroidectomy (Kvolik 2012a; Kvolik 2012b); thyroidectomy or breast cancer surgery (Kvolik 2013); laparoscopic prostatectomy or nephrectomy (Martini 2014); endoscopic sinus surgery with or without septoplasty (Mekawy 2012); hip or knee joint replacement or hip fracture surgery (Rahe‐Meyer 2014); open abdominal and urogenital surgery (Sabo 2011); and septoplasty (Tas 2015).
Four studies combined participants who underwent diverse elective surgical procedures (Blobner 2010; Cheong 2015; Lemmens 2010; Woo 2013). Twelve studies provided no data on the type of elective surgical procedure performed (Flockton 2008; Illman 2011; Isik 2016; Jones 2008; Kaufhold 2016; Khuenl‐Brady 2010; Koc 2015; Pongracz 2013; Riga 2014; Schaller 2010; Wu 2014; Yagan 2015).
Investigators maintained anaesthesia with opioid most often in combination with volatile anaesthetics, specifically with sevoflurane in 15 trials (Adamus 2011; Blobner 2010; Cheong 2015; Grintescu 2009; Jones 2008; Khuenl‐Brady 2010; Kizilay 2016; Koc 2015; Lemmens 2010; Pongracz 2013; Riga 2014; Sabo 2011; Tas 2015; Woo 2013; Yagan 2015); desflurane in six trials (Carron 2013; Castro 2014; Gaszynski 2011; Hakimoglu 2016; Isik 2016; Koyuncu 2015); isoflurane in one trial (Mekawy 2012); and sevoflurane or desflurane in one trial (Illman 2011). Twelve trials used propofol for maintenance (Flockton 2008; Foletto 2014; Geldner 2012; Georgiou 2013; Kaufhold 2016; Kogler 2012; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Martini 2014; Schaller 2010; Wu 2014); and two trials used any anaesthetic, according to usual practice (Brueckmann 2015; Rahe‐Meyer 2014). Four trials provided no information on anaesthesia maintenance (Balaka 2011; Raziel 2013; Sherman 2014; Sustic 2012).
Most trials used rocuronium as a non‐depolarizing neuromuscular blocking‐agent (NMBA). However, Lemmens 2010 used vecuronium; Rahe‐Meyer 2014 used rocuronium or vecuronium, according to usual practice at the site; Flockton 2008 compared sugammadex following rocuronium versus neostigmine following cisatracurium; and Martini 2014 compared atracurium for induction and mivacurium for maintenance versus rocuronium for both induction and maintenance. Two studies provided no information on the NMBA agent used (Castro 2014; Sherman 2014).
Interventions
We summarized the interventions reported in included studies under Characteristics of included studies.
All studies compared sugammadex and neostigmine, but investigators administered these drugs in different doses: Adamus 2011 and Sustic 2012 compared sugammadex 2 mg/kg versus neostigmine 0.04 mg/kg; and 15 trials compared sugammadex 2 mg/kg versus neostigmine 0.05 mg/kg (Blobner 2010; Castro 2014; Cheong 2015; Flockton 2008; Foletto 2014, Grintescu 2009, Illman 2011; Kvolik 2012a, Kvolik 2012b, Khuenl‐Brady 2010; Koc 2015; Tas 2015; Woo 2013; Wu 2014; Yagan 2015). Two trials compared sugammadex 2 mg/kg versus neostigmine 0.07 mg/kg (Kogler 2012; Koyuncu 2015).
Three studies compared sugammadex 2 mg/kg versus neostigmine 2.5 mg (Balaka 2011; Raziel 2013; Sherman 2014). Kizilay 2016 compared sugammadex 3 mg/kg versus neostigmine 0.03 mg/kg, Isik 2016 compared sugammadex 4 mg/kg versus neostigmine 0.04 mg/kg. Four trials compared sugammadex 4 mg/kg versus neostigmine 0.05 mg/kg (Geldner 2012; Hakimoglu 2016; Mekawy 2012; Sabo 2011). Three trials compared sugammadex 4 mg/kg versus neostigmine 0.07 mg/kg (Carron 2013; Jones 2008; Lemmens 2010). Rahe‐Meyer 2014 compared sugammadex 4 mg/kg versus usual care (neostigmine with glycopyrrolate or atropine, no dose specified, or placebo/spontaneous recovery). Martini 2014 compared sugammadex 4 mg/kg versus neostigmine 1 to 2 mg, and Riga 2014 did not specify dose for sugammadex or neostigmine. Four trials compared several different doses of sugammadex versus several different doses of neostigmine (Brueckmann 2015; Kaufhold 2016; Pongracz 2013; Schaller 2010). Georgiou 2013 compared sugammadex 2 mg/kg ideal body weight versus sugammadex 2 mg/kg corrected body weight versus neostigmine 50 µg/kg ideal body weight versus neostigmine 50 µg/kg corrected body weight, Carron 2013 compared sugammadex 4 mg/kg total body weight versus neostigmine 70 μg/kg lean body weight, and Gaszynski 2011 compared sugammadex 2 mg/kg corrected body weight versus neostigmine 50 µg/kg corrected body weight.
Outcomes
Of the 41 RCTs that met our inclusion criteria, 12 trials (n = 949) were eligible for meta‐analysis of the primary outcome (recovery time > TOFR 0.9) (Blobner 2010; Carron 2013; Cheong 2015; Foletto 2014; Gaszynski 2011; Georgiou 2013; Grintescu 2009; Illman 2011; Jones 2008; Koc 2015; Woo 2013; Wu 2014).
Of the 41 trials, 28 (N = 2298) were eligible for meta‐analysis of secondary outcomes (adverse events and serious adverse events): Adamus 2011; Balaka 2011; Blobner 2010; Brueckmann 2015; Carron 2013; Castro 2014; Cheong 2015; Flockton 2008; Gaszynski 2011; Geldner 2012; Hakimoglu 2016; Illman 2011; Jones 2008; Kaufhold 2016; Khuenl‐Brady 2010; Kizilay 2016; Koc 2015; Kogler 2012; Koyuncu 2015; Kvolik 2012a; Lemmens 2010; Mekawy 2012; Pongracz 2013; Sabo 2011; Schaller 2010; Woo 2013; Wu 2014; Yagan 2015).
Ten RCTs (N = 1647) were ineligible for meta‐analysis (Isik 2016; Kvolik 2012a; Kvolik 2013; Martini 2014; Rahe‐Meyer 2014; Raziel 2013; Riga 2014; Sherman 2014; Sustic 2012; Tas 2015) for the reasons provided in Table 4 (table of studies ineligible for meta‐analysis).
1. Table of studies ineligible for meta‐analysis.
| Study ID | Reasons for ineligibility | Comparisons | Conclusions |
| Isik 2016 | Primary endpoint: acute effects of sugammadex and neostigmine on renal function | Sugammadex 4 mg /kg at reappearance of PTC 1 to 2 or T2 vs neostigmine 40 µg/kg + atropine 10 µg/kg at reappearance of T2 | We believe that the use of more specific and sensitive new‐generation markers such as Cystatin C to evaluate kidney function will provide better understanding and interpretation of our results. Sugammadex has more tolerable effects on kidney function than does neostigmine. However, when compared with preoperative values, negative alteration of postoperative values can be seen. Neostigmine and sugammadex do not cause renal failure but may affect kidney function |
| Kvolik 2012a | TOFR recovery data available only as mean, no data on standard deviation, study author has not replied | Sugammadex 2 mg/kg vs neostigmine 50 µg/kg | Recovery of cough reflexes was faster and respiration more efficient in patients receiving sugammadex. Safe extubation was determined by age, TOFR recovery, and effects of other anaesthetics |
| Kvolik 2013 | TOFR recovery data available only as mean, no data on standard deviation, study author has not replied | Sugammadex 2 mg/kg vs neostigmine 50 µg/kg + atropine 25 µg/kg | An increase in BIS Index registered after reversal of rocuronium effects was faster during the recovery period in patients who were given sugammadex as compared with neostigmine. Although rapid increase in BIS Indices was registered in sugammadex group, more sensitive measurements are needed to confirm clinical value of this observation |
| Martini 2014 | Primary endpoint: influence of depth of the NMB on SRS (surgical rating score) | Neostigmine 1 to 2 mg + atropine 0.5 to 1 mg (for reversal of moderate NMB) vs sugammadex 4 mg/kg (for reversal of deep NMB) | Application of 5‐point SRS showed that deep NMB results in improved quality of surgical conditions compared with moderate block in retroperitoneal laparoscopy, without compromise to patients’ perioperative and postoperative cardiorespiratory conditions |
| Rahe‐Meyer 2014 | Comparison: sugammadex 4 mg/kg vs usual care (neostigmine with glycopyrrolate or atropine, or placebo/spontaneous recovery). Study author has not replied with separate data on neostigmine with glycopyrrolate or atropine or placebo/spontaneous recovery. | Sugammadex 4 mg/kg vs usual care (neostigmine with glycopyrrolate or atropine, or placebo/spontaneous recovery) |
Sugammadex produced limited, transient (< 1 hour) increases in activated partial thromboplastin time and prothrombin time but was not associated with increased risk of bleeding vs usual care |
| Raziel 2013 | No useable data available for quantitative meta‐analysis on recovery time or risk of adverse events | Sugammadex 2 mg/kg vs neostigmine 50 µg/kg + atropine 10 µg/kg | Sugammadex facilitates reversal of neuromuscular blockade after bariatric surgery, depending on the depth of neuromuscular blockade induced |
| Riga 2014 | Primary outcome: cognitive function assessed by change in Mini‐Mental State Evaluation test (MMSE), Clock Drawing Test, and Isaacs Set Test, performed preoperatively, 1 hour postoperatively, and at discharge (1 to 15 days postoperatively) | Sugammadex vs neostigmine/atropine | No significant difference was observed regarding cognitive function after neostigmine/atropine combination or sugammadex was received for reversal of rocuronium‐induced neuromuscular blockade for elective surgery |
| Sherman 2014 | Primary outcome: postoperative complications, data not available in useful format | Sugammadex 2 mg/kg vs neostigmine 2.5 mg/kg | Use of sugammadex (compared with neostigmine) as reversal agent following laparoscopic sleeve gastrectomy; surgery was associated with higher postoperative oxygen saturation despite lower TOF count before administration of reversal agent. Lack of differences in other measured variables may stem from the small size of patient groups studied |
| Sustic 2012 | Outcome: gastric emptying evaluated by paracetamol absorption test | Sugammadex 2 mg/kg vs neostigmine 40 µg/kg + atropine group 15 µg/kg | Although study results show a tendency toward faster gastric emptying in sugammadex group, this difference is not significant in most, possibly owing to small sample size in this study |
| Tas 2015 | Aim: to evaluate effects of sugammadex on postoperative nausea‐vomiting, pain, coagulation parameters, and quantity of postoperative bleeding. Data not available in useful format | Neostigmine 0.05 mg/kg + atropine 0.02 mg/kg vs sugammadex 2 mg/kg | Sugammadex was associated with greater postoperative bleeding than neostigmine in septoplasty patients. For surgical procedures with high risk of bleeding, the safety of sugammadex needs to be verified |
Acronyms:
BIS ‐ Bispectral Index
MMSE ‐ Mini‐Mental State Examination
NMB ‐ neuromuscular blockade
T2 ‐ second twitch in train‐of‐four stimulation
TOFR ‐ train‐of‐four ratio
PTC ‐ post‐tetanic count
SRS ‐ surgical rating score
See Characteristics of included studies for further information on the included studies.
Excluded studies
Among 83 identified relevant trials, we excluded 19 publications (Aho 2012; Baysal 2013; Dahaba 2012; Gaona 2012; Ghoneim 2015; Harazim 2014; Kakinuma 2013; Kara 2014; Kzlay 2013; Nagy 2014; Ozgun 2014; Pecek 2013; Sacan 2007; Schepens 2015; Stourac 2016; Veiga Ruiz 2011; Nagashima 2016; Nemes 2016; NCT03111121).
We have explained reasons for exclusion of each trial in the Characteristics of excluded studies table.
Ongoing studies
We identified 20 ongoing and unpublished trials by searching www.controlled‐trials.com, clinicaltrials.gov, and www.centerwatch.com. The following five trials have been completed but to the best of our knowledge, no data from these trials have yet been published: NCT01539044; NCT01748643; NCT02160223; NCT02330172; NCT02414880). Six trials are currently recruiting participants (NCT02256280; NCT02361060; NCT02454504; NCT02666014; NCT02698969; NCT02860507). Six trials are classified as ongoing (NCT02909439; NCT02697929; NCT03108989; NCT03116997; NCT02939430; NCT03144453) and three trials are not yet open for recruiting participants (NCT02648503; NCT02845375; NCT02861131).
See Characteristics of ongoing studies for details.
Studies awaiting classification
We reran the search in May 2017 and found three trials (NCT02243943; Kim 2016; Sen 2016) that published data after we had completed our main search in May 2016; we will include these trials in the next updated version of this review.
Risk of bias in included studies
We assessed the risk of bias of included studies using the 'Risk of bias' tool developed by Cochrane. The first review author (AMH) and the second review author (PD) independently assessed risk of bias for each study and resolved disagreements by discussion or by consultation with the last review author (AA). We have presented the various bias domains in Figure 2 ‐ Risk of bias graph ‐ and Figure 6 ‐ Risk of bias summary
2.
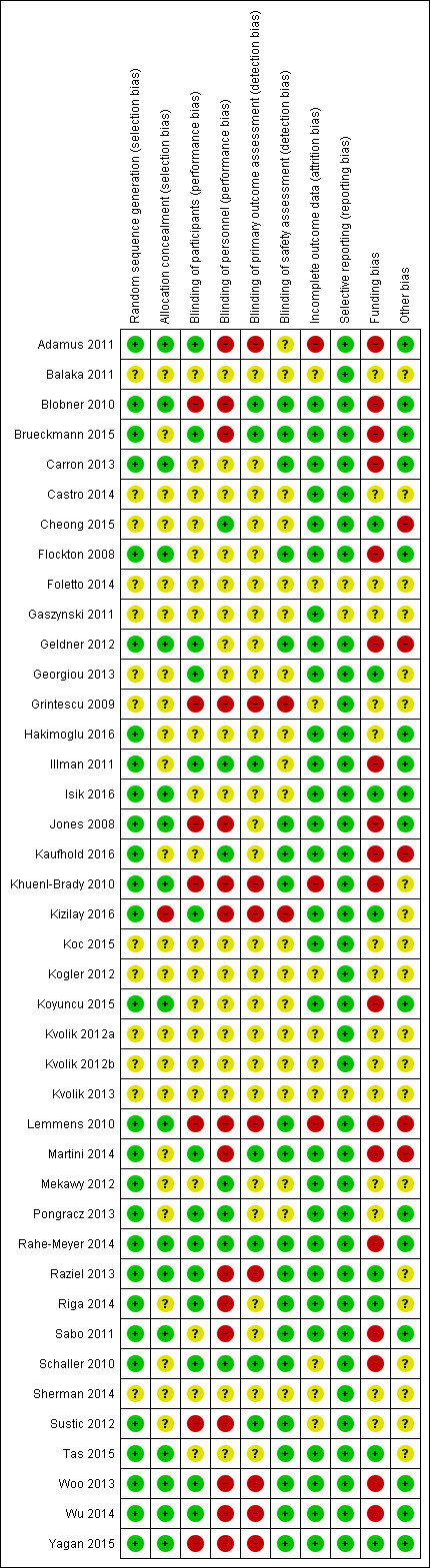
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
6.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation (selection bias)
Twenty‐seven trials (66%) reported adequate generation of random sequence that was computer‐based (Adamus 2011; Brueckmann 2015; Carron 2013; Hakimoglu 2016; Illman 2011; Isik 2016; Jones 2008; Kaufhold 2016; Martini 2014; Mekawy 2012; Pongracz 2013; Raziel 2013; Riga 2014; Schaller 2010; Sustic 2012; Tas 2015; Yagan 2015); or was performed by using a central randomization system (Blobner 2010; Flockton 2008; Geldner 2012; Khuenl‐Brady 2010; Koyuncu 2015; Lemmens 2010; Rahe‐Meyer 2014; Sabo 2011; Woo 2013; Wu 2014).
Furthermore, one trial (2%) reported randomization by lots (Kizilay 2016). Thirteen trials (32%) did not report sufficient information for assessment of risk of bias(Balaka 2011; Castro 2014; Cheong 2015; Foletto 2014; Gaszynski 2011; Georgiou 2013; Grintescu 2009; Koc 2015; Kogler 2012; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Sherman 2014).
Allocation concealment (selection bias)
Eighteen trials (44%) reported adequate allocation concealment performed by using sequentially numbered opaque sealed envelopes (SNORES) (Adamus 2011; Carron 2013; Isik 2016; Jones 2008; Martini 2014; Tas 2015; Yagan 2015); or secondary to a central randomization system (Blobner 2010; Flockton 2008; Geldner 2012; Khuenl‐Brady 2010; Koyuncu 2015; Lemmens 2010; Rahe‐Meyer 2014; Raziel 2013; Sabo 2011; Woo 2013; Wu 2014).
One trial (2%) reported using no allocation concealment (Kizilay 2016). Twenty‐two trials (54%) did not describe their method of allocation concealment (Balaka 2011; Brueckmann 2015; Castro 2014; Cheong 2015; Foletto 2014; Gaszynski 2011; Georgiou 2013; Grintescu 2009; Hakimoglu 2016; Illman 2011; Kaufhold 2016; Koc 2015; Kogler 2012; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Mekawy 2012; Pongracz 2013; Riga 2014; Schaller 2010; Sherman 2014; Sustic 2012).
Blinding
Blinding of participants (performance bias)
Fourteen trials (34%) adequately blinded participants and therefore had low risk of performance bias (Adamus 2011; Brueckmann 2015; Geldner 2012; Georgiou 2013; Illman 2011; Kizilay 2016; Martini 2014; Pongracz 2013; Rahe‐Meyer 2014; Raziel 2013; Riga 2014; Schaller 2010; Woo 2013; Wu 2014).
Eight trials (20%) did not adequately blind participants and therefore had high risk of performance bias; two of these specifically reported that participants were not blinded (Sustic 2012; Yagan 2015), and six were marked as “open‐label” trials (Blobner 2010; Flockton 2008; Grintescu 2009; Jones 2008; Khuenl‐Brady 2010; Lemmens 2010).
The remaining 19 trials (46%) did not provide sufficient data on participant blinding and we assigned risk of performance bias as unclear(Balaka 2011; Carron 2013; Castro 2014; Cheong 2015; Foletto 2014; Gaszynski 2011; Hakimoglu 2016; Isik 2016; Kaufhold 2016; Koc 2015; Kogler 2012; Koyuncu 2015; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Mekawy 2012; Sabo 2011; Sherman 2014; Tas 2015).
Blinding of personnel (performance bias)
Seven trials (17%) reported adequate blinding of the anaesthesiologist and therefore had low risk of performance bias (Cheong 2015; Illman 2011; Kaufhold 2016; Mekawy 2012; Pongracz 2013; Rahe‐Meyer 2014; Schaller 2010).
Seventeen trials (41%) did not report adequate blinding of anaesthesiologists and therefore had high risk of performance bias; 11 of these specifically reported that the anaesthesiologist was not blinded: (Adamus 2011; Brueckmann 2015; Kizilay 2016; Martini 2014; Raziel 2013; Riga 2014; Sabo 2011; Sustic 2012; Woo 2013; Wu 2014; Yagan 2015), and six trials were marked as “open‐label” trials (Blobner 2010; Flockton 2008; Grintescu 2009; Jones 2008; Khuenl‐Brady 2010; Lemmens 2010).
The remaining 17 trials (41%) did not provide sufficient data on anaesthesiologist blinding and therefore had unclear risk of performance bias (Balaka 2011; Carron 2013; Castro 2014; Foletto 2014; Gaszynski 2011; Geldner 2012; Georgiou 2013; Hakimoglu 2016; Isik 2016; Koc 2015; Kogler 2012; Koyuncu 2015; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Sherman 2014; Tas 2015).
Blinding of TOF‐watch assessment (detection bias)
Two trials (5%) specifically reported that the anaesthesiologist was also the TOF‐watch assessor: (Adamus 2011; Illman 2011). Four trials (10%) reported adequate blinding of the TOF‐watch assessor and therefore had low risk of performance bias (Brueckmann 2015; Illman 2011; Martini 2014; Schaller 2010).
Twelve trials (29%) did not provide adequate blinding of the TOF‐watch assessor and therefore had high risk of detection bias; six of these trials specifically reported that the anaesthesiologist was not blinded (Adamus 2011; Kizilay 2016; Raziel 2013; Woo 2013; Wu 2014; Yagan 2015), and six trials were marked as “open‐label” trials (Blobner 2010; Flockton 2008; Grintescu 2009; Jones 2008; Khuenl‐Brady 2010; Lemmens 2010).
For two trials (5%), risk of bias assessment was of no relevance, as trial authors presented no TOF‐watch data (Rahe‐Meyer 2014; Sustic 2012).
The remaining 23 trials (56%) did not provide sufficient data on TOF‐watch assessor blinding and had unclear risk of detection bias (Balaka 2011; Carron 2013; Castro 2014; Cheong 2015; Foletto 2014; Gaszynski 2011; Geldner 2012; Georgiou 2013; Hakimoglu 2016; Isik 2016; Kaufhold 2016; Koc 2015; Kogler 2012; Koyuncu 2015; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Mekawy 2012; Pongracz 2013; Riga 2014; Sabo 2011; Sherman 2014; Tas 2015).
Blinding of safety assessment (detection bias)
Twenty trials (49%) reported adequate blinding of the safety assessor and therefore had low risk of detection bias (Blobner 2010; Brueckmann 2015; Carron 2013; Flockton 2008; Geldner 2012; Jones 2008; Kaufhold 2016; Khuenl‐Brady 2010; Lemmens 2010; Martini 2014; Rahe‐Meyer 2014; Raziel 2013; Riga 2014; Sabo 2011; Schaller 2010; Sustic 2012; Tas 2015; Woo 2013; Wu 2014; Yagan 2015).
Two trials (5%) did not adequately blind the safety assessor and therefore had high risk of detection bias; one of these specifically reported that the safety assessor was not blinded (Kizilay 2016), and the other trial was marked as an “open‐label” study (Grintescu 2009).
The remaining 19 trials (46%) did not provide sufficient data on safety assessor blinding and had unclear risk of detection bias (Adamus 2011; Balaka 2011; Castro 2014; Cheong 2015; Foletto 2014; Gaszynski 2011; Georgiou 2013; Hakimoglu 2016; Illman 2011; Isik 2016; Koc 2015; Kogler 2012; Koyuncu 2015; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Mekawy 2012; Pongracz 2013; Sherman 2014).
Incomplete outcome data
The following 28 trials (68%) had low risk of attrition bias as either all participants were accounted for, or missing outcome data were properly balanced among groups: Adamus 2011; Blobner 2010; Brueckmann 2015; Carron 2013; Castro 2014; Cheong 2015; Flockton 2008; Gaszynski 2011; Geldner 2012; Hakimoglu 2016; Illman 2011; Isik 2016; Jones 2008; Kaufhold 2016; Kizilay 2016; Koc 2015; Koyuncu 2015; Martini 2014; Mekawy 2012; Pongracz 2013; Rahe‐Meyer 2014; Raziel 2013; Riga 2014; Sabo 2011; Tas 2015; Woo 2013; Wu 2014; Yagan 2015.
For three trials (7%), missing outcome data were not balanced across intervention groups (Khuenl‐Brady 2010; Lemmens 2010; Schaller 2010); these studies therefore had high risk of attrition bias.
The remaining 10 trials (24%) did not provide sufficient data on incomplete outcomes and had unclear risk of attrition bias (Balaka 2011; Foletto 2014; Georgiou 2013; Grintescu 2009; Kogler 2012; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Sherman 2014; Sustic 2012).
Selective reporting
Twenty trials (49%) had low risk of reporting bias, as they were registered online: 16 on clinicaltrials.gov (Blobner 2010 – NCT00451217; Brueckmann 2015 – NCT01479764; Flockton 2008 ‐ NTC00451100; Geldner 2012 – NCT00724932; Georgiou 2013 ‐ NCT01629394; Jones 2008 ‐ NCT00473694; Khuenl‐Brady 2010 – NCT00451217; Lemmens 2010 – NCT00473694; Martini 2014 – NCT 01631149; Rahe‐Meyer 2014 – NCT01422304; Raziel 2013 – NCT01631396; Riga 2014 – NCT02419352; Schaller 2010 – NCT00895609; Woo 2013 – NCT01050543; Wu 2014 – NCT00825812; Yagan 2015 – NCT02215382); one on SYNABA – The Polish Clinical Trials authorization (Gaszynski 2011 – 252922); one on ANZCTR ‐ Australian New Zealand Clinical Trials Registry (Hakimoglu 2016 ‐ ACTRN12614000651684); and finally two on Eudra‐CT (Illman 2011 ‐ 2009‐013537‐22; Pongracz 2013 ‐ 2011‐001683‐22).
The remaining 20 trials (49%) were not registered online, but it is clear that the published article or meeting abstract includes all expected outcomes (Adamus 2011; Balaka 2011; Carron 2013; Castro 2014; Cheong 2015; Grintescu 2009; Isik 2016; Kaufhold 2016; Kizilay 2016; Koc 2015; Kogler 2012; Koyuncu 2015; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Mekawy 2012; Sabo 2011; Sherman 2014; Sustic 2012; Tas 2015). Therefore, these trials had low risk of reporting bias.
One trial (2%) did not provide sufficient information for assessment of risk of bias and had unclear risk of reporting bias (Foletto 2014).
Other potential sources of bias
Funding bias
Merck, Sharp and Dohme or Schering‐Plough provided financial support for 11 trials (27%), indicating high risk of funding bias (Blobner 2010; Geldner 2012; Illman 2011; Jones 2008; Khuenl‐Brady 2010; Lemmens 2010; Martini 2014; Rahe‐Meyer 2014; Sabo 2011; Woo 2013; Wu 2014). Authors of the following trials were former employees, current employees, or members of advisory boards of Merck, Sharp and Dohme/Schering‐Plough, or had received honoraria for lectures, consultancy, or advisory board membership, or travel grants from Merck, Sharp and Dohme/Schering‐Plough: Adamus 2011; Blobner 2010; Brueckmann 2015; Carron 2013; Flockton 2008; Gaszynski 2011; Geldner 2012; Illman 2011; Kaufhold 2016; Khuenl‐Brady 2010; Koyuncu 2015; Lemmens 2010; Martini 2014; Rahe‐Meyer 2014,Schaller 2010; Woo 2013; Wu 2014). These studies had high risk of funding bias.
We could not assess funding risk of bias for the following 14 trials (34%) owing to insufficient information: Balaka 2011; Castro 2014; Foletto 2014; Grintescu 2009; Hakimoglu 2016; Koc 2015; Kogler 2012; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Mekawy 2012; Pongracz 2013; Sherman 2014; Sustic 2012; these studies had unclear risk of funding bias.
Eight trials (20%) had low risk of funding bias, as they were funded by departmental sources (Georgiou 2013; Isik 2016; Kaufhold 2016; Koyuncu 2015; Raziel 2013; Riga 2014; Schaller 2010; Tas 2015). Trial authors funded two trials (5%) (Kizilay 2016; Yagan 2015), and in two cases (5%), study authors received research grants (Gaszynski 2011; Polish Government grant; and Cheong 2015; Inje University research grant).
Other bias
Twenty‐one trials (51%) had low risk of other bias, as they reported specific information on sample size calculation (Adamus 2011; Blobner 2010; Brueckmann 2015; Carron 2013; Cheong 2015; Flockton 2008; Geldner 2012; Hakimoglu 2016; Illman 2011; Isik 2016; Jones 2008; Kaufhold 2016; Koyuncu 2015; Lemmens 2010; Martini 2014; Pongracz 2013; Rahe‐Meyer 2014; Sabo 2011; Woo 2013; Wu 2014; Yagan 2015).
Of these 21 trials, 12 (29%) were powered to address this review’s primary outcome (Adamus 2011; Blobner 2010; Carron 2013; Cheong 2015; Flockton 2008; Illman 2011; Jones 2008; Lemmens 2010; Pongracz 2013; Sabo 2011; Woo 2013; Wu 2014), and seven trials (17%) were powered to address this review’s secondary outcome (Brueckmann 2015; Geldner 2012; Hakimoglu 2016; Isik 2016; Koyuncu 2015; Rahe‐Meyer 2014; Yagan 2015). Twenty trials (49%) did not provide information on sample size calculation (Balaka 2011; Castro 2014; Foletto 2014; Gaszynski 2011; Georgiou 2013; Grintescu 2009; Khuenl‐Brady 2010; Kizilay 2016; Koc 2015; Kogler 2012; Kvolik 2012a; Kvolik 2012b; Kvolik 2013; Mekawy 2012; Raziel 2013; Riga 2014; Schaller 2010; Sherman 2014; Sustic 2012; Tas 2015).
Treatment groups were generally comparable with respect to baseline characteristics, except Cheong 2015, which described significant differences in body weight between groups that might have influenced the dosage of administered drugs; and Flockton 2008, which reported a higher proportion of women, higher mean age, and a higher percentage of ASA II to III participants in the sugammadex group. Furthemore, Lemmens 2010 discontinued one intervention group owing to a marked difference in efficacy between groups after interim analysis. Therefore, these trials had high risk of other bias.
All trials used the same method (acceleromyography) and at the same monitor site (ulnar nerve, adductor pollicis muscle). We analysed quality variables of neuromuscular recording methods among full‐text trials have provided a summary in Table 5 ‐ Quality variables of neuromuscular monitoring methods among included trials.
2. Quality variables of neuromuscular monitoring methods among included trials.
| Study ID | Method of recording | Monitor site | Arm fixation | Supramaximal stimulation | Temperature maintained and recorded | Initial signal stabilization | Twich height calibration | Preload used |
| Adamus 2011 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Yes | Yes | Yes | Not mentioned | Yes | Not mentioned |
| Blobner 2010 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Yes | Yes | Yes | Not mentioned | Yes | Not mentioned |
| Brueckmann 2015 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Not mentioned | Not mentioned | Not mentioned | Yes | Not mentioned |
| Carron 2013 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Yes | Not mentioned | Not mentioned | Yes | Yes | No |
| Castro 2014 | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Cheong 2015 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Not mentioned | Yes | Not mentioned | Not mentioned | Not mentioned |
| Flockton 2008 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Yes | Yes | Yes | Yes | Not mentioned |
| Gaszynski 2011 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Yes | Yes | Yes | Not mentioned | Not mentioned | Not mentioned |
| Geldner 2012 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Not mentioned | Not mentioned | Yes | Yes | Not mentioned |
| Hakimoglu 2016 | Acceleromyography | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Illman 2011 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Yes | Yes | Yes | Yes | No | |
| Isik 2016 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Yes | Not mentioned | Yes | Not mentioned | Not mentioned | Not mentioned |
| Jones 2008 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Yes | Yes | Yes | Yes | Not mentioned |
| Kaufhold 2016 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Yes | Yes | Yes | Yes | Yes | Not mentioned |
| Khuenl‐Brady 2010 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Not mentioned | Yes | Yes | Yes | Not mentioned |
| Kizilay 2016 | Acceleromyography | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Koc 2015 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Not mentioned | Yes | Not mentioned | Yes | Not mentioned |
| Koyuncu 2015 | Acceleromyography | N. ulnaris, M. adductor pollicis |
No | Yes | No | No | Yes | Not mentioned |
| Lemmens 2010 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Yes | Yes | Yes | Yes | Not mentioned |
| Martini 2014 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Yes | Not mentioned | Yes | Yes | Yes |
| Mekawy 2012 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Not mentioned | Not mentioned | Not mentioned | Yes | Not mentioned |
| Pongracz 2013 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Yes | Yes | Yes | Yes | Yes | Yes |
| Sabo 2011 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Schaller 2010 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Yes | Yes | Yes | Yes | Yes | Not mentioned |
| Tas 2015 | Acceleromyography | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Woo 2013 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Yes * | No * | Yes * | Yes | Yes | No * |
| Wu 2014 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Yes | Not mentioned | Yes | Yes | Not mentioned |
| Yagan 2015 | Acceleromyography | N. ulnaris, M. adductor pollicis |
Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
Studies with only abstracts were not included in this table because they did not document information regarding neuromuscular monitoring
List of abbreviations:
N. ulnaris ‐ ulnar nerve
M. adductor pollicis ‐ adductor pollicis muscle
Effects of interventions
See: Table 1; Table 2; Table 3
See Table 1; Table 2; and Table 3.
Comparison 1. Sugammadex 2 mg/kg versus neostigmine 0.05 mg/kg for rocuronium reversal
1.1 Primary outcome 1: recovery time from T2 to TOFR > 0.9
Ten trials were included in this category (Blobner 2010; Cheong 2015; Foletto 2014; Gaszynski 2011; Georgiou 2013; Grintescu 2009; Illman 2011; Koc 2015; Woo 2013; Wu 2014).
All trials used rocuronium for intubation and maintenance. The intubating dose of rocuronium was 0.6 mg/kg in five trials (Blobner 2010; Cheong 2015; Koc 2015; Woo 2013; Wu 2014), 0.6 to 1 mg/kg in Illman 2011, and 1 mg/kg in Gaszynski 2011. The maintenance dose of rocuronium was 0.1 to 0.2 mg/kg in four trials (Blobner 2010; Koc 2015; Woo 2013; Wu 2014), 0.06 mg/kg corrected body weight (CBW) with maximum two additional doses in Gaszynski 2011, and 5 to 10 mg in two trials (Cheong 2015; Illman 2011). No information on rocuronium dosage was available for three trials (Foletto 2014; Georgiou 2013; Grintescu 2009).
Meta‐analysis of results showed that sugammadex 2 mg/kg reversed neuromuscular blockade from T2 to TOFR > 0.9 in 1.96 minutes, and neostigmine 0.05 mg/kg reversed neuromuscular blockade from T2 to TOFR > 0.9 in 12.87 minutes. Therefore, sugammadex 2 mg/kg was on average 10.22 minutes (6.6 times) faster than neostigmine 0.05 mg/kg in reversing neuromuscular blockade at T2 reappearance (MD 10.22 minutes, 95% CI 8.48 to 11.96; I2 = 84%; 10 studies; n = 835; random‐effects model; Analysis 1.1; GRADE quality of evidence: moderate; Table 1). We downgraded the GRADE quality of evidence by one owing to high risk of bias.
1.1. Analysis.
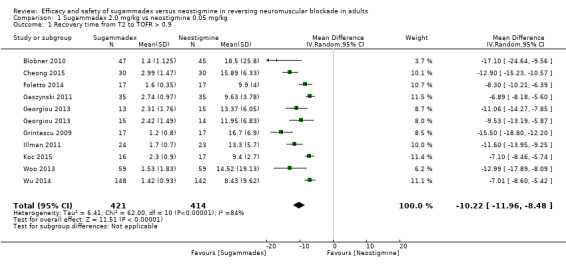
Comparison 1 Sugammadex 2.0 mg/kg vs neostigmine 0.05 mg/kg, Outcome 1 Recovery time from T2 to TOFR > 0.9.
The following trials used NMBAs other than rocuronium and therefore were not included in the meta‐analysis.
Flockton 2008 compared rocuronium‐sugammadex 2 mg/kg versus cisatracurium‐neostigmine 0.05 mg/kg and found that reversal with sugammadex was 4.7 times faster than with neostigmine (geometric mean recovery time of 1.9 vs 9.0; P < 0.0001).
Khuenl‐Brady 2010 investigated the effect of sugammadex 2 mg/kg versus neostigmine 0.05 mg/kg in reversing vecuronium‐induced neuromuscular blockade (induction 0.1 mg/kg, maintenance 0.03 to 0.03 mg/kg) and described that the geometric mean time of recovery to TOFR > 0.9 was significantly faster with sugammadex than with neostigmine (2.7 minutes, 95% CI 2.2 to 3‐3 vs 17.9, 95% CI 13.1 to 24.3, respectively; P < 0.0001; n = 93).
Other trials did not provide enough information or compared doses of sugammadex and neostigmine other than those previously mentioned and as such could not be included in the meta‐analysis: Kvolik 2012a compared sugammadex 2 mg/kg versus neostigmine 0.05 mg/kg and reported T2 to TOFR > 0.9 recovery time of 2.5 minutes versus 8.5 minutes, respectively (P = 0.045, n = 38), but these data could not be included in the meta‐analysis, as standard deviation (SD) data were not reported in the paper and could not be obtained. Mekawy 2012 examined recovery time from T2 to TOFR > 0.9 comparing sugammadex 4 mg/kg (n = 20) versus neostigmine 0.05 mg/kg plus atropine 0.02 mg/kg (n = 20) and reported that mean reversal time (SD) was 2.47 (0.51) versus 24.21 (4.7) minutes, respectively.
Subgroup analysis
1.2 TIVA versus volatile anaesthetics
Seven trials maintained anaesthesia with volatile anaesthetic (Blobner 2010; Cheong 2015; Gaszynski 2011; Grintescu 2009; Illman 2011; Koc 2015; Woo 2013), and three trials used TIVA for maintenance (Foletto 2014; Georgiou 2013; Wu 2014). Subgroup analysis of results showed no significant subgroup differences in recovery time to TOFR > 0.9 (Analysis 1.2).
1.2. Analysis.
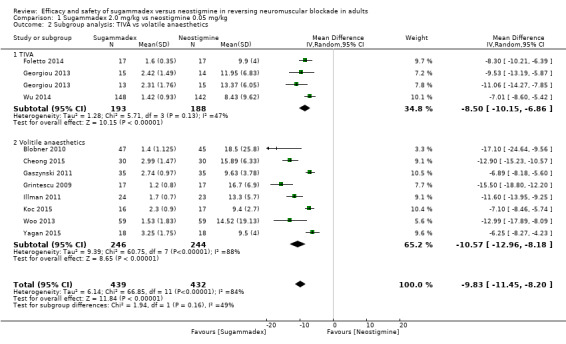
Comparison 1 Sugammadex 2.0 mg/kg vs neostigmine 0.05 mg/kg, Outcome 2 Subgroup analysis: TIVA vs volatile anaesthetics.
Sensitivity analysis
1.3. Excluding meeting abstracts
Sensitivity analysis that excluded data from meeting abstracts (MD 9.27 minutes, 95% CI 7.40 to 11.14; I2 = 82%; n = 767; random‐effects model; Analysis 1.3) did not change overall results regarding significance.
1.3. Analysis.
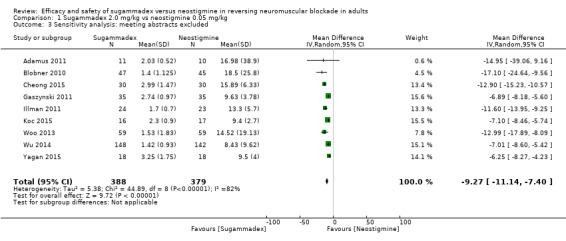
Comparison 1 Sugammadex 2.0 mg/kg vs neostigmine 0.05 mg/kg, Outcome 3 Sensitivity analysis: meeting abstracts excluded.
Primary outcome 2: recovery time from PTC 1 to 5 to TOFR > 0.9
This outcome is not clinically relevant as dosages of sugammadex 2 mg/kg and neostigmine 0.05 mg/kg are too low to reverse the deep rocuronium‐induced neuromuscular blockade seen at PTC 1 to 5.
Secondary outcomes: risk of adverse events and risk of serious adverse events
We have described these outcomes in detail under Comparison 3 (Analysis 3.2).
3.2. Analysis.
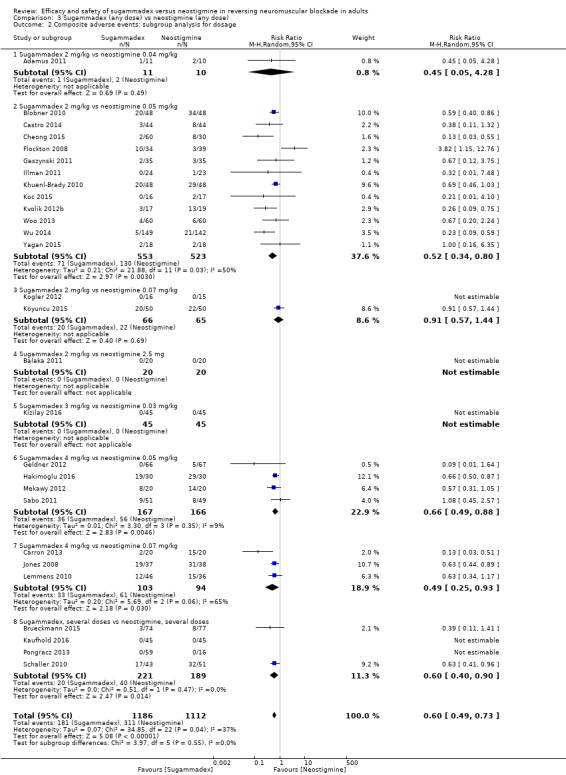
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 2 Composite adverse events: subgroup analysis for dosage.
Comparison 2. Sugammadex 4 mg/kg versus neostigmine 0.07 mg/kg for rocuronium reversal
Primary outcome 1. Recovery time from T2 to TOFR > 0.9
This outcome is not clinically relevant as dosages of sugammadex 4 mg/kg and neostigmine 0.07 mg/kg are too high to reverse the moderate rocuronium‐induced neuromuscular blockade seen at T2.
2.1 Primary outcome 2: recovery time from PTC 1 to 5 to TOFR > 0.9
We combined two trials in this category (Carron 2013; Jones 2008). Both trials used rocuronium 0.6 mg/kg as a single intubating dose and rocuronium 0.15 mg/kg for maintenance. Carron 2013 combined neostigmine with atropine 0.01 mg/kg, and Jones 2008 combined neostigmine with glycopyrrolate 0.014 mg/kg. Carron 2013 administered sugammadex or neostigmine at reappearance of PTC 1 to 5, and Jones 2008 at reappearance of PTC 1 to 2. Carron 2013 included morbidly obese female participants. Carron 2013 maintained anaesthesia with desflurane, and Jones 2008 with sevoflurane.
Meta‐analysis of trial results showed that sugammadex 4 mg/kg reversed neuromuscular blockade from PTC 1 to 5 to TOFR > 0.9 in 2.9 minutes, and neostigmine 0.07 mg/kg reversed neuromuscular blockade from PTC 1 to 5 to TOFR > 0.9 in 48.8 minutes. Sugammadex 4 mg/kg was therefore on average 45.78 minutes (16.8 times) faster than neostigmine 0.07 mg/kg in reversing neuromuscular blockade at reappearance of PTC 1 to 5 (MD 45.78 minutes, 95% CI 39.41 to 52.15; I2 = 0%; two studies; n = 114; random‐effects model; Analysis 2.1; GRADE quality of evidence: low; Table 2). We downgraded GRADE quality of evidence two levels owing to high risk of bias and imprecision.
2.1. Analysis.
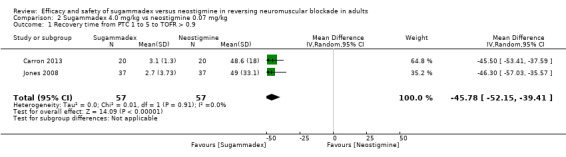
Comparison 2 Sugammadex 4.0 mg/kg vs neostigmine 0.07 mg/kg, Outcome 1 Recovery time from PTC 1 to 5 to TOFR > 0.9.
The following trials used NMBAs other than rocuronium, gave a dose of neostigmine different from the one described above, or had missing SD values and were not included in the meta‐analysis. Lemmens 2010 investigated the effect of sugammadex 4 mg/kg versus neostigmine 0.07 mg/kg in reversing vecuronium‐induced neuromuscular blockade (induction 0.1 mg/kg, maintenance 0.015 mg/kg) and described that the geometric mean time of recovery to TOFR > 0.9 was 15‐fold faster with sugammadex than with neostigmine (4.5 vs 66.2 minutes, respectively; P < 0.0001; n = 83). Geldner 2012 reported that participants receiving sugammadex 4 mg/kg administered at PTC 1 to 2 recovered 3.4 times faster than those given neostigmine 0.05 mg/kg plus atropine 0.01 mg/kg (geometric mean recovery time of 2.4 (2.1 to 2.7) vs 8.4 (7.2 to 9.8) minutes, respectively; P < 0.0001). Kogler 2012, reported that median recovery time from PTC 1 to 2 to TOFR > 0.9 after sugammadex 2 mg/kg was 1.1 minutes versus 10.13 minutes for neostigmine 0.07 mg/kg (P < 0.001; n = 31; no SD value reported).
Secondary outcomes: risk of adverse events and risk of serious adverse events
We have described these outcomes in detail under Comparison 3 (Analysis 3.2).
Other recovery times
Some trials measured recovery times other than those described in the comparisons above. Only single trials measured these data; therefore, we could not include them in the meta‐analysis, but we can describe the qualitative data as follows.
Balaka 2011 reported mean recovery time from TOFR of 50% to > 90% as 9.7 minutes after administration of neostigmine 2.5 mg and 2.8 minutes after administration of sugammadex 4 mg/kg (P < 0.05; n = 40). Yagan 2015 compared sugammadex 2 mg/kg versus neostigmine 0.05 mg/kg administered at T4/T1 20% and found that extubation time (defined as time to TOFR > 0.9) was seven minutes in the neostigmine group and two minutes in the sugammadex group (P > 0.05; n = 36). Martini 2014 compared moderate NMB (T1 to 2) induced by atracurium/mivacurium reversed by neostigmine 1 to 2 mg plus atropine 0.5 to 1 mg (n = 12) versus deep NMB (PTC 1 to 2) induced by high‐dose rocuronium and reversed by sugammadex 4 mg/kg (n = 12). Recovery times to TOFR > 0.9 expressed as mean (SD) were, respectively, 10.9 (4.9) versus 5.1 (2.4) (P < 0.01). Pongracz 2013 investigated adequate doses for reversal of reappearance of four twitches of TOF and discovered that sugammadex 1 mg/kg, unlike neostigmine, rapidly and effectively reverses rocuronium‐induced block that has recovered spontaneously to threshold TOF count four. Furthermore, sugammadex 0.5 mg/kg reverses a similar block within eight minutes. Sabo 2011 compared sugammadex 4.0 mg/kg versus neostigmine 0.05 mg/kg plus glycopyrrolate 0.01 mg/kg administered when the TOF‐blinded anaesthesiologist considered the patient ready for reversal of NMB. The anaesthesiologist could ask the TOF‐watch operator whether the patient had recovered to at least 1 to 2 PTC before administering the reversal agent. This trial demonstrated significantly faster recovery to TOFR > 0.9 ratio within two minutes (95% CI 1.8 to 2.5) in the sugammadex group versus eight minutes (95% CI 3.8 to 16.5 minutes) in the neostigmine group. Schaller 2010 investigated the efficacy of sugammadex (0.0625, 0.125, 0.25, 0.5, or 1.0 mg/kg), neostigmine (5, 8, 15, 25, or 40 µg/kg), and saline, and by using a bi‐exponential model and regression analysis concluded that sugammadex 0.22 mg/kg and neostigmine 34 µg/kg effectively and comparably reverse a rocuronium‐induced shallow residual neuromuscular block at TOFR = 0.5 (n = 99). Kaufhold 2016 investigated several different doses of sugammadex or neostigmine as well as placebo administered at TOFR ≥ 0.2 and found that residual neuromuscular block of TOFR = 0.2 cannot be reversed reliably with neostigmine within 10 minutes. However, substantially lower doses of sugammadex than the approved dose of 2.0 mg/kg may be sufficient to reverse residual rocuronium‐induced neuromuscular block at recovery of TOFR ≥ 0.2. Koyuncu 2015 looked at the effects of sugammadex 2 mg/kg (n = 50) versus neostigmine 70 µg/kg + atropine 0.4 mg per 1 mg neostigmine administered when four twitches of TOF were visible with fade and found that sugammadex speeds recovery of neuromuscular strength but only slightly (P > 0.01; n = 100).
Comparison 3. Sugammadex (any dose) versus neostigmine (any dose)
Primary outcome 1: recovery time from T2 to TOFR > 0.9
This outcome was not clinically relevant as doses for sugammadex and neostigmine used are specific to the depth of the neuromuscular blockade.
Primary outcome 2: recovery time from PTC 1 to 5 to TOFR > 0.9
This outcome was not clinically relevant as doses for sugammadex and neostigmine used are specific to the depth of the neuromuscular blockade.
3.1. Secondary outcomes: risks of adverse events and serious adverse events
The following 28 trials investigated adverse events possibly, probably, or definitely related to study drug: Adamus 2011; Balaka 2011; Blobner 2010; Brueckmann 2015; Carron 2013; Castro 2014; Cheong 2015; Flockton 2008; Gaszynski 2011; Geldner 2012; Hakimoglu 2016; Illman 2011; Jones 2008; Kaufhold 2016; Khuenl‐Brady 2010; Kizilay 2016; Koc 2015; Kogler 2012; Koyuncu 2015; Kvolik 2012a; Lemmens 2010; Mekawy 2012; Pongracz 2013; Sabo 2011; Schaller 2010; Woo 2013; Wu 2014; Yagan 2015.
Meta‐analysis of trial results showed significantly fewer adverse events in the sugammadex group than in the neostigmine group (RR 0.60, 95% CI 0.49 to 0.74; I2 = 40%; 28 studies, n = 2298; random‐effects model; Analysis 3.1; GRADE quality of data: moderate; Table 3; quality of evidence downgraded one level owing to high risk of bias). Specifically, the risk of composite adverse events was 283/1000 in the neostigmine group and 159/1000 in the sugammadex group. With number needed to treat for an additional beneficial outcome (NNTB) of eight to avoid an adverse event, sugammadex appears to have a stronger safety profile than neostigmine. Furthermore, data show significantly fewer participants with one or more adverse events (RR 0.62, 95% CI 0.48 to 0.81; I2 = 0%; n = 1766; random‐effects model; Analysis 3.5; GRADE quality of data: moderate; Table 3) in the sugammadex group than in the neostigmine group.
3.1. Analysis.
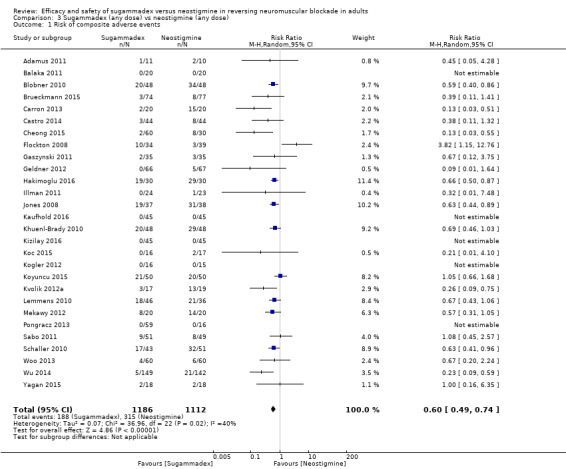
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 1 Risk of composite adverse events.
3.5. Analysis.
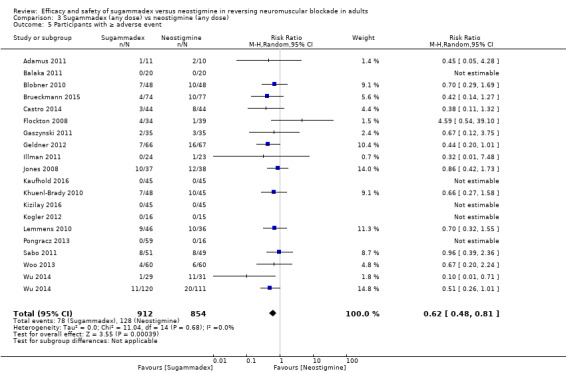
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 5 Participants with ≥ adverse event.
Data on specific adverse events show significantly less risk of the following adverse events in the sugammadex group than in the neostigmine group: bradycardia (RR 0.16, 95% CI 0.07 to 0.34; I2 = 0%; n = 1218; random‐effects model; Analysis 3.6; NNTB 14; GRADE quality of data: moderate; Table 3; downgraded one level owing to high risk of bias), PONV (RR 0.52, 95% CI 0.28 to 0.97; I2 = 0%; n = 389; random‐effects model; Analysis 3.7; NNTB 16; GRADE quality of data: low; Table 3; downgraded two levels owing to high risk of bias and imprecision), desaturation (RR 0.23, 95% CI 0.06 to 0.83; I2 = 0%; n = 134; random‐effects model; Analysis 3.8), need for transitory oxygen supplementation (RR 0.24, 95% CI 0.09 to 0.66; I2 = 0%; n = 76; random‐effects model; Analysis 3.10), and procedural complications (RR 0.12, 95% CI 0.02 to 0.97; n = 168; I2 = 0%; random‐effects model; Analysis 3.9). Also, significantly fewer participants were unable to perform 5 seconds of sustained head‐lift at extubation (RR 0.34, 95% CI 0.15 to 0.78; I2 = 0%; n = 395; random‐effects model; Analysis 3.11) in the sugammadex group than in the neostigmine group.
3.6. Analysis.
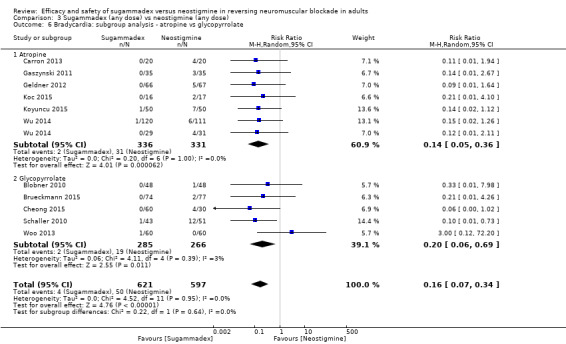
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 6 Bradycardia: subgroup analysis ‐ atropine vs glycopyrrolate.
3.7. Analysis.
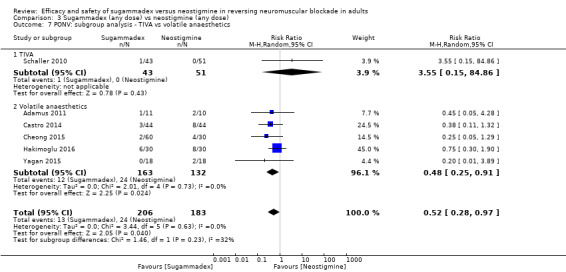
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 7 PONV: subgroup analysis ‐ TIVA vs volatile anaesthetics.
3.8. Analysis.
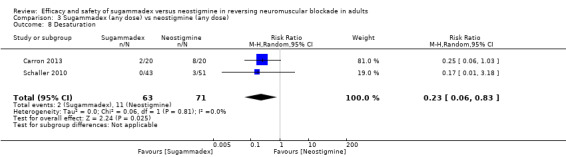
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 8 Desaturation.
3.10. Analysis.
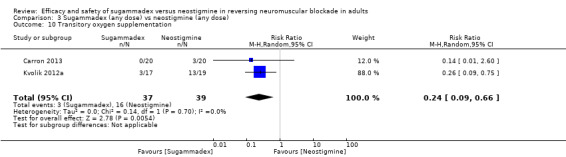
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 10 Transitory oxygen supplementation.
3.9. Analysis.
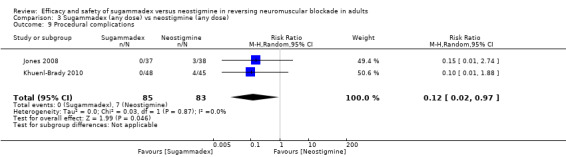
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 9 Procedural complications.
3.11. Analysis.
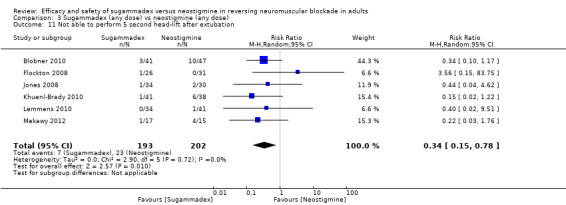
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 11 Not able to perform 5 second head‐lift after extubation.
Data show no significant differences between sugammadex and neostigmine with regard to nausea (RR 0.83, 95% CI 0.44 to 1.56; I2 = 0%; n = 719; Analysis 3.13), vomiting (RR 2.05, 95% CI 0.50 to 8.48; I2 = 0%; n = 297; Analysis 3.14), postprocedural nausea (RR 1.39, 95% CI 0.27 to 7.12; I2 = 0%; n = 168; Analysis 3.15), headache (RR 1.02, 95% CI 0.48 to 2.18; I2 = 0%; n = 388; Analysis 3.16), hypertension (RR 1.45, 95% CI 0.23 to 9.05; I2 = 0%; n = 287; Analysis 3.17), hypotension (RR 1.23, 95% CI 0.38 to 3.96; I2 = 0%; n = 465; Analysis 3.18), cough (RR 1.42, 95% CI 0.42 to 4.81; I2 = 65%; n = 200; Analysis 3.19), dry mouth (RR 0.44, 95% CI 0.10 to 1.87; I2 = 17%; n = 289; Analysis 3.20), dizziness (RR 0.98, 95% CI 0.10 to 9.23; I2 = 0%; n = 168; Analysis 3.21), tachycardia (RR 0.44, 95% CI 0.09 to 2.22; I2 = 0%; n = 338; Analysis 3.22), pruritus (RR 1.62, 95% CI 0.20 to 12.88; I2 = 0%; n = 175; Analysis 3.23), pyrexia (RR 1.43, 95% CI 0.23 to 8.91; I2 = 0%; n = 264; Analysis 3.24), shivering (RR 0.75, 95% CI 0.40 to 1.43; I2 = 0%; n = 190; Analysis 3.25), chills (RR 4.04, 95% CI 0.46 to 35.85; I2 = 0%; n = 166; Analysis 3.26), rash (RR 0.83, 95% CI 0.17 to 3.96; I2 = 0%; n = 701; Analysis 3.27), supraventricular extrasystoles (RR 0.32, 95% CI 0.03 to 3.05; I2 = 0%; n = 189; Analysis 3.28), laryngospasm (RR 0.34, 95% CI 0.07 to 1.65; I2 = 0%; n = 100; Analysis 3.29), increased upper airway secretion (RR 0.37, 95% CI 0.09 to 1.59; I2 = 0%; n = 442; Analysis 3.30), procedural complications (RR 0.12, 95% CI 0.02 to 0.97; I2 = 0%; n = 168; Analysis 3.9), procedural hypertension (RR 1.65, 95% CI 0.33 to 8.21; I2 = 0%; n = 267; Analysis 3.31), procedural hypotension (RR 0.49, 95% CI 0.02 to 14.15; I2 = 60%; n = 391; Analysis 3.32), abdominal pain (RR 0.98, 95% CI 0.10 to 9.27; I2 = 0%; n = 196; Analysis 3.33). Furthermore, data show no significant differences in reported clinical signs of residual NMB (RR 1.0; n = 646; Analysis 3.34), inadequate reversal of NMB (RR 0.11, 95% CI 0.01 to 2.02; n = 368; Analysis 3.35), and recurrence of NMB (RR 0.74, 95% CI 0.05 to 10.74; I2 = 33; n = 1289; Analysis 3.36). Clinical tests revealed no significant differences in the number of participants reporting general muscle weakness at extubation (RR 0.61, 95% CI 0.31 to 1.18; I2 = 0%; n = 288; Analysis 3.12), at PACU discharge (RR 0.49, 95% CI 0.12 to1.90; I2 = 0%; n = 410; Analysis 3.37), or in the number of participants unable to perform five seconds of sustained head‐lift at PACU discharge (RR 1.0; n = 399; Analysis 3.38).
3.13. Analysis.
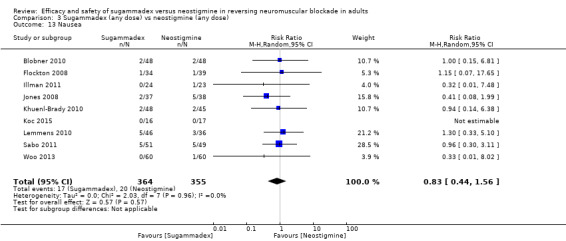
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 13 Nausea.
3.14. Analysis.
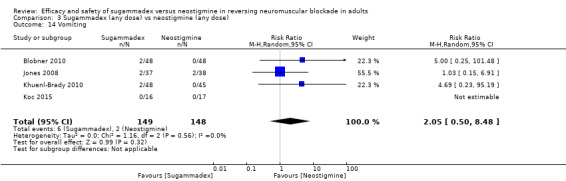
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 14 Vomiting.
3.15. Analysis.
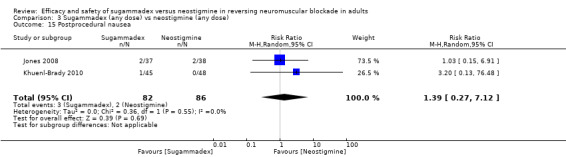
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 15 Postprocedural nausea.
3.16. Analysis.
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 16 Headache.
3.17. Analysis.
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 17 Hypertension.
3.18. Analysis.
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 18 Hypotension.
3.19. Analysis.
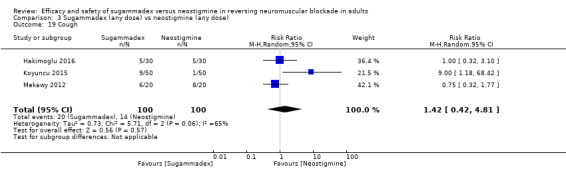
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 19 Cough.
3.20. Analysis.
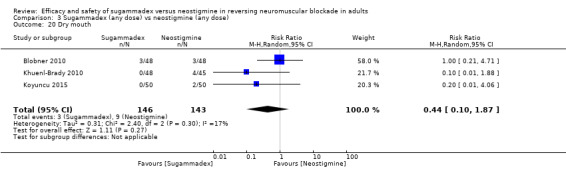
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 20 Dry mouth.
3.21. Analysis.
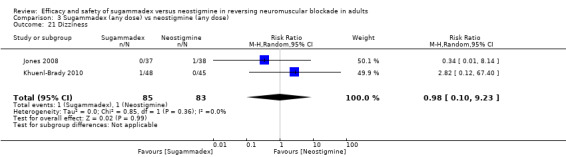
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 21 Dizziness.
3.22. Analysis.
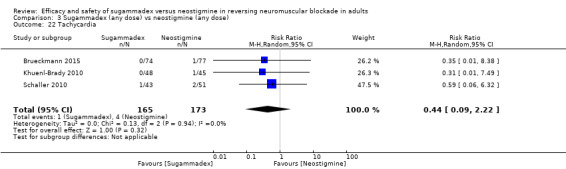
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 22 Tachycardia.
3.23. Analysis.
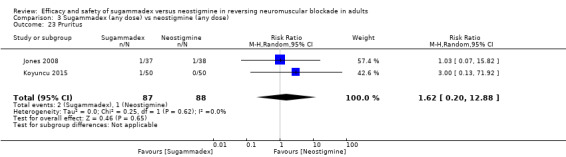
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 23 Pruritus.
3.24. Analysis.
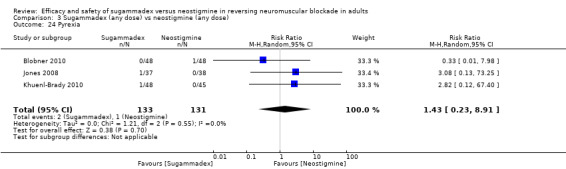
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 24 Pyrexia.
3.25. Analysis.
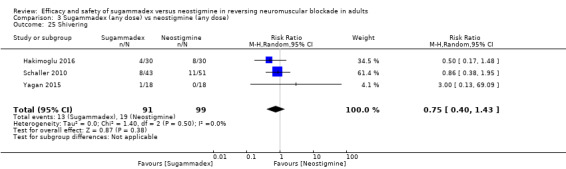
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 25 Shivering.
3.26. Analysis.
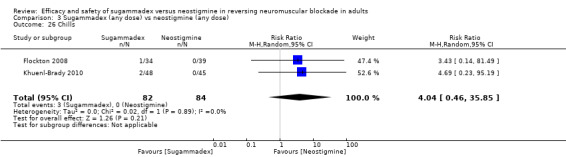
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 26 Chills.
3.27. Analysis.
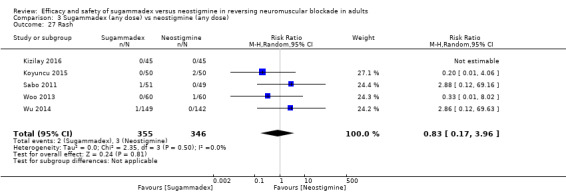
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 27 Rash.
3.28. Analysis.
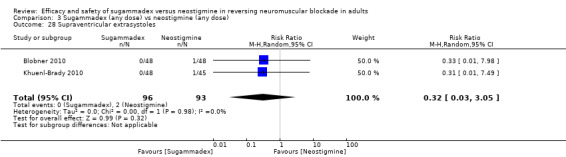
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 28 Supraventricular extrasystoles.
3.29. Analysis.
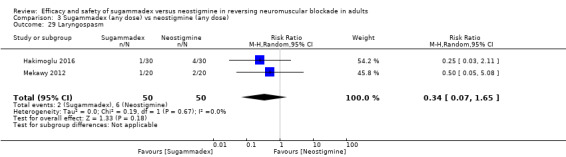
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 29 Laryngospasm.
3.30. Analysis.
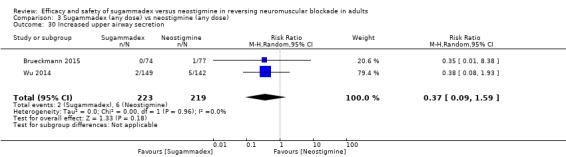
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 30 Increased upper airway secretion.
3.31. Analysis.
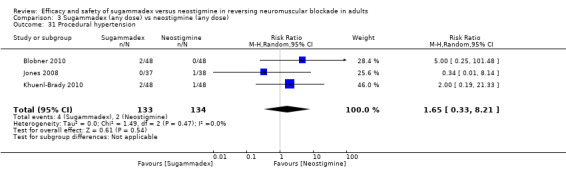
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 31 Procedural hypertension.
3.32. Analysis.
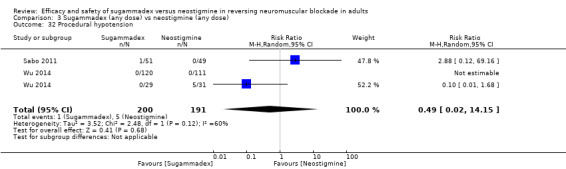
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 32 Procedural hypotension.
3.33. Analysis.
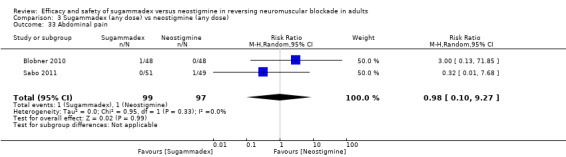
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 33 Abdominal pain.
3.34. Analysis.
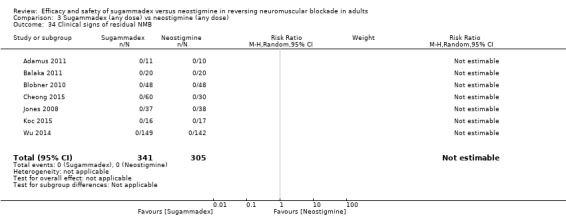
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 34 Clinical signs of residual NMB.
3.35. Analysis.
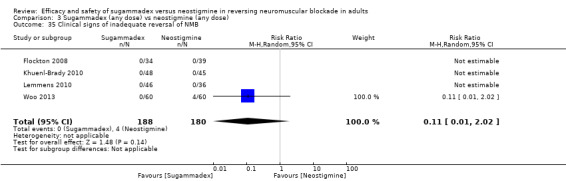
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 35 Clinical signs of inadequate reversal of NMB.
3.36. Analysis.
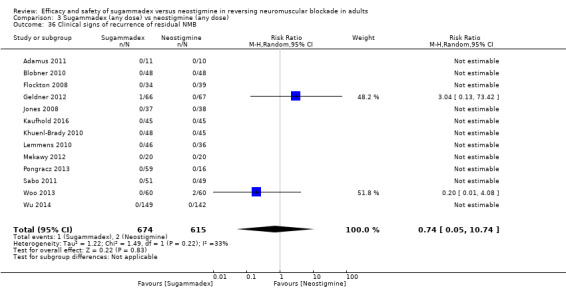
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 36 Clinical signs of recurrence of residual NMB.
3.12. Analysis.
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 12 General muscle weakness after extubation.
3.37. Analysis.
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 37 General muscle weakness at PACU discharge.
3.38. Analysis.
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 38 Not able to perform 5 second head‐lift at PACU discharge.
A single trial observed some drug‐related adverse events; therefore, we could not include them in a meta‐analysis of specific adverse events, but we used the data to calculate overall risk of adverse events. The following isolated adverse events were observed in the sugammadex group: three cases of breath‐hold (10%) in Hakimoglu 2016, two cases of strange taste in the mouth (6%) in Gaszynski 2011, two cases of increased beta‐N‐acetyl‐D‐glucosaminidase (6%) in Flockton 2008, two cases of bronchospasm (4%) in Koyuncu 2015, and one case of each of the following: severe abdominal pain (2%), pharyngolaryngeal pain (2%), diarrhoea (2%), and tinnitus (2%) in Blobner 2010; decreased hematocrit (1%) and procedural haemorrhage (1%) in Brueckmann 2015; tremor (3%) and altered facial sensation (3%) in Flockton 2008; postprocedural hypertension (3%), paraesthesia (3%), and increased blood creatinine phosphokinase (3%) in Jones 2008; retching (2%), airway complication to anaesthesia (2%), and hot flush (2%) in Khuenl‐Brady 2010; procedural pain (2%) in Sabo 2011; leukocytosis (2%) in Lemmens 2010; mild hypoventilation (1%) in Wu 2014; and finally one case of intraoperative movement (2%) in Schaller 2010.
In the neostigmine group, the following isolated drug‐related adverse events were reported: four cases of breath‐hold (13%) in Hakimoglu 2016; two cases of albumin present in the urine (4%) in Blobner 2010; two cases of leukocytosis (5%) in Lemmens 2010; and one case of each of the following: involuntary muscle contractions (2%), visual accommodation disorder (2%), increased urine beta‐2 microglobulin (2%), severe bradycardia (2%), and productive cough (2%) in Blobner 2010; respiratory distress (1%) and delayed recovery from anaesthesia (1%) in Brueckmann 2015; hyperhidrosis (3%), decreased blood protein (3%), restlessness (3%), chest discomfort (3%), incision site complication (3%), and postprocedural complication (3%) in Jones 2008; ventricular extrasystoles (2%), sleep disorder (2%), and increased gamma‐glutamyltransferase (2%) in Khuenl‐Brady 2010; anxiety (3%), depression (3%), and fatigue (3%) in Lemmens 2010; dyspepsia (2%) and somnolence (2%) in Sabo 2011; severe muscle weakness (1%) in Wu 2014; and finally one case of intraoperative movement (2%) in Schaller 2010.
We have described in Table 6 each observed adverse event possibly, probably, or definitely related to sugammadex or neostigmine. This table also presents risk of adverse events in descending order, as well as the number of studies observing each adverse event.
3. Table of adverse events.
| Sugammadex | Neostigmine | ||||||||
| Specific adverse events | Number of AEs | Number of participants | Risk of AEs, % | Number of AEs | Number of participants | Risk of AEs, % | RR (95% CI) | Number of studies | Total number of participants |
| Cough | 20 | 100 | 20,0 | 14 | 100 | 14,0 | 1.42 (0.42‐4.81) | 3 | 200 |
| Shivering | 13 | 91 | 14,3 | 19 | 99 | 19,2 | 0.75 (0.40‐1.43) | 3 | 190 |
| Desaturation | 2 | 63 | 3,2 | 11 | 71 | 15,5 | 0.23 (0.06‐0.83) | 2 | 134 |
| General muscle weakness after extubation | 13 | 142 | 9,2 | 22 | 146 | 15,1 | 0.61 (0.31‐1.18) | 4 | 288 |
| Breath‐hold | 3 | 30 | 10,0 | 4 | 30 | 13,3 | ‐ | 1 | 60 |
| PONV | 13 | 206 | 6,3 | 24 | 183 | 13,1 | 0.52 (0.28‐0.97) | 6 | 389 |
| Laryngospasm | 2 | 50 | 4,0 | 6 | 50 | 12,0 | 0.34 (0.07‐1.65) | 2 | 100 |
| Not able to perform 5 second head‐lift after extubation | 7 | 193 | 3,6 | 23 | 202 | 11,4 | 0.34 (0.15‐0.78) | 6 | 395 |
| Bradycardia | 4 | 621 | 0,6 | 50 | 597 | 8,4 | 0.16 (0.07‐0.34) | 11 | 1218 |
| Procedural complications | 0 | 85 | 0,0 | 7 | 83 | 8,4 | 0.12 (0.02‐0.97) | 2 | 168 |
| Postprocedural nausea | 8 | 128 | 6,3 | 5 | 122 | 4,1 | 1.34 (0.47‐3.81) | 3 | 250 |
| Dry mouth | 3 | 146 | 2,1 | 9 | 143 | 6,3 | 0.44 (0.10‐1.87) | 3 | 289 |
| Headache | 12 | 195 | 6,2 | 11 | 193 | 5,7 | 1.02 (0.48‐2.18) | 4 | 388 |
| Increased beta‐N‐acetyl‐D‐glucosaminidase | 2 | 34 | 5,9 | 0 | 39 | 0,0 | ‐ | 1 | 73 |
| Strange taste in mouth | 2 | 35 | 5,7 | 0 | 35 | 0,0 | ‐ | 1 | 70 |
| Nausea | 17 | 364 | 4,7 | 20 | 355 | 5,6 | 0.83 (0.44‐1.56) | 9 | 719 |
| Leukocytosis | 1 | 46 | 2,2 | 2 | 36 | 5,6 | ‐ | 1 | 82 |
| Albumin present in the urine | 0 | 48 | 0,0 | 2 | 48 | 4,2 | ‐ | 1 | 96 |
| Vomiting | 6 | 149 | 4,0 | 2 | 148 | 1,4 | 2.05 (0.50‐8.48) | 4 | 297 |
| Bronchospasm | 2 | 50 | 4,0 | 1 | 50 | 2,0 | ‐ | 1 | 100 |
| Chills | 3 | 82 | 3,7 | 0 | 84 | 0,0 | 4.04 (0.46‐35.85) | 2 | 166 |
| General muscle weakness at PACU discharge | 3 | 208 | 1,4 | 6 | 202 | 3,0 | 0.49 (0.12‐1.90) | 5 | 410 |
| Procedural hypertension | 4 | 133 | 3,0 | 2 | 134 | 1,5 | 1.65 (0.33‐8.21) | 3 | 267 |
| Tremor | 1 | 34 | 2,9 | 0 | 39 | 0,0 | ‐ | 1 | 73 |
| Altered facial sensation | 1 | 34 | 2,9 | 0 | 39 | 0,0 | ‐ | 1 | 73 |
| Postprocedural hypertension | 1 | 37 | 2,7 | 0 | 38 | 0,0 | ‐ | 1 | 75 |
| Paraesthesia | 1 | 37 | 2,7 | 0 | 38 | 0,0 | ‐ | 1 | 75 |
| Increased blood PK | 1 | 37 | 2,7 | 0 | 38 | 0,0 | ‐ | 1 | 75 |
| Increased upper airway secretions | 2 | 223 | 0,9 | 6 | 219 | 2,7 | 0.37 (0.09‐1.59) | 2 | 442 |
| Hyperhidrosis | 0 | 37 | 0 | 1 | 38 | 2,6 | ‐ | 1 | 75 |
| Decreased blood protein | 0 | 37 | 0 | 1 | 38 | 2,6 | ‐ | 1 | 75 |
| Restlessness | 0 | 37 | 0 | 1 | 38 | 2,6 | ‐ | 1 | 75 |
| Chest discomfort | 0 | 37 | 0 | 1 | 38 | 2,6 | ‐ | 1 | 75 |
| Incision site complication | 0 | 37 | 0 | 1 | 38 | 2,6 | ‐ | 1 | 75 |
| Procedural hypotension | 1 | 200 | 0,5 | 5 | 191 | 2,6 | 0.49 (0.02‐14.1) | 2 | 391 |
| Postprocedural complication | 0 | 37 | 0,0 | 1 | 38 | 2,6 | ‐ | 1 | 75 |
| Tachycardia | 1 | 165 | 0,6 | 4 | 173 | 2,3 | 0.44 (0.09‐2.22) | 3 | 338 |
| Pruritus | 2 | 87 | 2,3 | 1 | 88 | 1,1 | 1.62 (0.20‐12.88) | 2 | 175 |
| Intraoperative movement | 1 | 43 | 2,3 | 1 | 51 | 2,0 | ‐ | 1 | 94 |
| Anxiety | 0 | 46 | 0 | 1 | 46 | 2,2 | ‐ | 1 | 92 |
| Depression | 0 | 46 | 0 | 1 | 46 | 2,2 | ‐ | 1 | 92 |
| Fatigue | 0 | 46 | 0 | 1 | 46 | 2,2 | ‐ | 1 | 92 |
| Hypotension | 5 | 227 | 2,2 | 5 | 238 | 2,1 | 1.23 (0.38‐3.96) | 4 | 465 |
| Supraventricular extrasystoles | 0 | 96 | 0,0 | 2 | 93 | 2,2 | 0.32 (0.03‐3.05) | 2 | 189 |
| Clinical signs of inadequate reversal of NMB | 0 | 188 | 0,0 | 4 | 180 | 2,2 | 0.11 (0.01‐2.02) | 4 | 368 |
| Leukocytosis | 1 | 46 | 2,2 | 0 | 36 | 0,0 | ‐ | 1 | 82 |
| Ventricular extrasystoles | 0 | 48 | 0,0 | 1 | 45 | 2,2 | ‐ | 1 | 93 |
| Sleep disorder | 0 | 48 | 0,0 | 1 | 45 | 2,2 | ‐ | 1 | 93 |
| Increased gamma‐glutamyl‐transferase | 0 | 48 | 0,0 | 1 | 45 | 2,2 | ‐ | 1 | 93 |
| Retching | 1 | 48 | 2,1 | 0 | 45 | 0,0 | ‐ | 1 | 93 |
| Airway complication to anaesthesia | 1 | 48 | 2,1 | 0 | 45 | 0,0 | ‐ | 1 | 93 |
| Hot flush | 1 | 48 | 2,1 | 0 | 45 | 0,0 | ‐ | 1 | 93 |
| Abdominal pain | 1 | 48 | 2,1 | 0 | 48 | 0,0 | ‐ | 1 | 96 |
| Severe abdominal pain | 1 | 48 | 2,1 | 0 | 48 | 0,0 | ‐ | 1 | 96 |
| Pharyngolaryngeal pain | 1 | 48 | 2,1 | 0 | 48 | 0,0 | ‐ | 1 | 96 |
| Diarrhoea | 1 | 48 | 2,1 | 0 | 48 | 0,0 | ‐ | 1 | 96 |
| Tinnitus | 1 | 48 | 2,1 | 0 | 48 | 0,0 | ‐ | 1 | 96 |
| Involontary muscle contractions | 0 | 48 | 0,0 | 1 | 48 | 2,1 | ‐ | 1 | 96 |
| Visual accomodation disorder | 0 | 48 | 0,0 | 1 | 48 | 2,1 | ‐ | 1 | 96 |
| Increased B2‐microglobulin | 0 | 48 | 0,0 | 1 | 48 | 2,1 | ‐ | 1 | 96 |
| Severe bradycardia | 0 | 48 | 0,0 | 1 | 48 | 2,1 | ‐ | 1 | 96 |
| Productive cough | 0 | 48 | 0,0 | 1 | 48 | 2,1 | ‐ | 1 | 96 |
| Pyrexia | 2 | 133 | 1,5 | 1 | 131 | 0,8 | 1.43 (0.23‐8.91) | 3 | 264 |
| Hypertension | 2 | 143 | 1,4 | 1 | 144 | 0,7 | 1.45 (0.23‐9.05) | 3 | 287 |
| Decreased hematocrit | 1 | 74 | 1,4 | 0 | 77 | 0,0 | ‐ | 1 | 151 |
| Procedural haemorrhage | 1 | 74 | 1,4 | 0 | 77 | 0,0 | ‐ | 1 | 151 |
| Delayed recovery from anaesthesia | 0 | 74 | 0,0 | 1 | 77 | 1,3 | ‐ | 1 | 151 |
| Respiratory distress | 0 | 74 | 0,0 | 1 | 77 | 1,3 | ‐ | 1 | 151 |
| Dizziness | 1 | 85 | 1,2 | 1 | 83 | 1,2 | 0.98 (0.10‐9.23) | 2 | 168 |
| Abdominal pain | 1 | 99 | 1,0 | 1 | 97 | 1,0 | 0.98 (0.10‐9.27) | 2 | 196 |
| Rash | 2 | 355 | 0,6 | 3 | 346 | 0,9 | 0.83 (0.17‐3.96) | 5 | 701 |
| Severe muscle weakness | 0 | 149 | 0,0 | 1 | 142 | 0,7 | ‐ | 1 | 291 |
| Mild hypoventilation | 1 | 149 | 0,7 | 0 | 142 | 0,0 | ‐ | 1 | 291 |
| Clinical signs of recurrence of residual NMB | 1 | 674 | 0,1 | 2 | 615 | 0,3 | 0.74 (0.05‐10.7) | 13 | 1289 |
| Clinical signs of residual NMB | 0 | 341 | 0,0 | 0 | 305 | 0,0 | ‐ | 7 | 646 |
| Not able to perform 5 second head‐lift at PACU discharge | 0 | 205 | 0,0 | 0 | 194 | 0,0 | ‐ | 5 | 399 |
| Redness at injection site | 0 | 50 | 0,0 | 0 | 50 | 0,0 | ‐ | 1 | 100 |
| Hypersensitivity | 0 | 60 | 0,0 | 0 | 30 | 0,0 | ‐ | 1 | 90 |
Table of reported adverse events possibly, probably, or definitely related to sugammadex or neostigmine, listed in descending order according to risk of adverse events. Furthermore, the number of studies observing for each adverse event is presented
List of abbreviations:
NMB ‐ neuromuscular blockade
PACU ‐ post‐anaesthesia care unit
The largest trial in this review (Rahe‐Meyer 2014) randomized 1198 participants and reported that 64 out of 596 participants (10.7%) in the sugammadex group and 72 out of 588 (12.2 %) in the usual care group had at least one drug‐related adverse event. Unfortunately, we could not include these data in our meta‐analysis, as the "usual care" group combined participants who received either neostigmine or placebo, and we were not able to obtain data from the neostigmine group.
Subgroup analysis of composite adverse events
3.2 Different dosages of sugammadex and neostigmine
Different trials used different dosages of sugammadex and neostigmine.
Adamus 2011 compared sugammadex 2 mg/kg versus neostigmine 0.04 mg/kg. Twelve trials compared sugammadex 2 mg/kg versus neostigmine 0.05 mg/kg (Blobner 2010; Castro 2014; Cheong 2015; Flockton 2008; Gaszynski 2011; Illman 2011; Khuenl‐Brady 2010; Koc 2015; Kvolik 2012b; Woo 2013; Wu 2014; Yagan 2015). Two trials compared sugammadex 2 mg/kg versus neostigmine 0.07 mg/kg (Kogler 2012; Koyuncu 2015). Balaka 2011 compared sugammadex 2 mg/kg versus neostigmine 2.5 mg. Kizilay 2016 compared sugammadex 3 mg/kg versus neostigmine 0.03 mg/kg. Four trials compared sugammadex 4 mg/kg versus neostigmine 0.05 mg/kg (Geldner 2012; Hakimoglu 2016; Mekawy 2012; Sabo 2011). Three trials compared sugammadex 4 mg/kg versus neostigmine 0.07 mg/kg (Carron 2013; Jones 2008; Lemmens 2010). Four trials compared several different doses of sugammadex versus several different doses of neostigmine (Brueckmann 2015; Kaufhold 2016; Pongracz 2013; Schaller 2010). Subgroup analysis of data showed no significant subgroup differences in RR for composite adverse events (Analysis 3.2).
3.3. TIVA versus volatile anaesthetics
Twenty trials maintained anaesthesia with volatile anaesthetic (Adamus 2011; Blobner 2010; Brueckmann 2015; Carron 2013; Castro 2014; Cheong 2015; Gaszynski 2011; Hakimoglu 2016; Illman 2011; Jones 2008; Khuenl‐Brady 2010; Kizilay 2016; Koc 2015; Koyuncu 2015; Lemmens 2010; Mekawy 2012; Pongracz 2013; Sabo 2011; Woo 2013; Yagan 2015). Seven trials used TIVA for maintenance (Flockton 2008; Geldner 2012; Kaufhold 2016; Kogler 2012; Kvolik 2012b; Schaller 2010; Wu 2014). One trial provided insufficient information (Balaka 2011). Subgroup analysis of trial results showed no significant subgroup differences in RR for composite adverse events (Analysis 3.3).
3.3. Analysis.
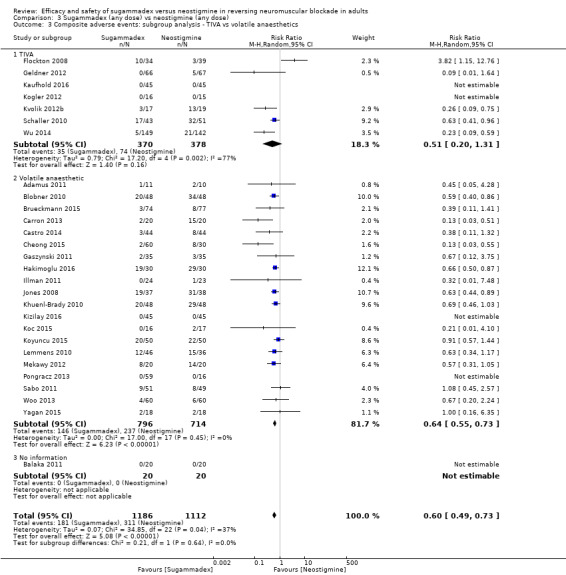
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 3 Composite adverse events: subgroup analysis ‐ TIVA vs volatile anaesthetics.
Sensitivity analysis of composite adverse events
3.4 Excluding meeting abstracts
Sensitivity analysis excluding data from meeting abstracts (RR 0.60, 95% CI 0.49 to 0.74; I2 = 35%; n = 2091; random‐effects model; Analysis 3.4) did not change overall results regarding significance.
3.4. Analysis.
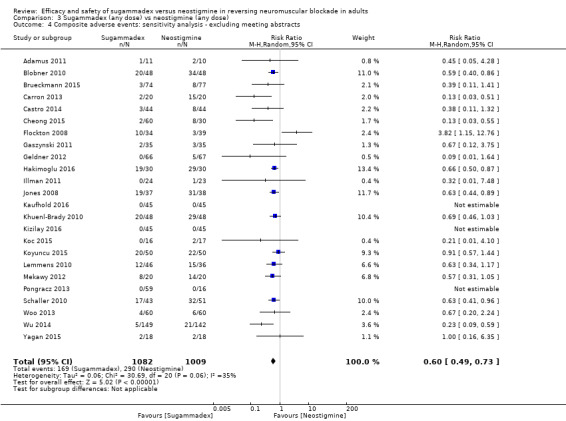
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 4 Composite adverse events: sensitivity analysis ‐ excluding meeting abstracts.
Subgroup analysis of bradycardia
3.7 Atropine versus glycopyrrolate
All trials reporting bradycardia combined neostigmine with an antimuscarinic drug. Six trials used atropine (Carron 2013; Gaszynski 2011; Geldner 2012; Koc 2015; Koyuncu 2015; Wu 2014). Five trials used glycopyrrolate (Blobner 2010; Brueckmann 2015; Cheong 2015; Schaller 2010; Woo 2013). Subgroup analysis of trial results showed no significant subgroup differences in RR for bradycardia (Analysis 3.6).
Subgroup analysis of PONV
3.9 TIVA versus volatile anaesthetics
Five trials maintained anaesthesia with volatile anaesthetic (Adamus 2011; Castro 2014; Cheong 2015; Hakimoglu 2016; Yagan 2015), One trial used TIVA for maintenance (Schaller 2010). Subgroup analysis of trial results showed no significant subgroup differences in RR for PONV (Analysis 3.7).
Qualitative data
Investigators reported effects of sugammadex and neostigmine on the following parameters in data format that was ineligible for meta‐analysis.
Intraocular pressure (IOP)
Hakimoglu 2016 described that post‐extubation intraocular pressures (IOPs) were similar between sugammadex and neostigmine groups (P > 0.05; n = 60); Yagan 2015 reported lower end‐extubation IOPs when sugammadex 2 mg/kg was used in comparison with neostigmine 0.05 mg/kg ‐ atropine 0.02 mg/kg (P < 0.05; n = 36), suggesting that sugammadex may be a better option for reversal of neuromuscular blockade in conditions for which an increase in IOP is not desired, such as glaucoma and penetrating eye injury.
Haemodynamic effects
Kizilay 2016 (n = 90) examined the haemodynamic effects of sugammadex and neostigmine in cardiac participants undergoing non‐cardiac surgery. Investigators found that the sugammadex group had lower systolic, diastolic, and mean blood pressures and heart rate when compared with the neostigmine group (P < 0.05). They reported no significant differences between and within groups in terms of QTc interval values. Study authors suggest that sugammadex might be preferred to neostigmine‐atropine combination for reversal of rocuronium‐induced neuromuscular blockade in cardiac patients undergoing non‐cardiac surgery,
Bleeding events
The largest trial in this review(Rahe‐Meyer 2014; n = 1198) included participants undergoing hip/knee surgery or hip fracture surgery and compared sugammadex 4 mg/kg versus usual care (neostigmine or spontaneous recovery). Investigators reported bleeding events within 24 hours in 17 (2.9%) sugammadex and 24 (4.1%) usual care participants (RR 0.70, 95% CI 0.38 to 1.29). Compared with usual care, increases of 5.5% in activated partial thromboplastin time (aPTT; P < 0.001) and 3.0% in prothrombin time (P < 0.001) from baseline occurred with sugammadex 10 minutes after administration and resolved within 60 minutes. Data show no significant differences between sugammadex and usual care for other blood loss measures (transfusion, 24‐hour drain volume, drop in haemoglobin, and anaemia) or for risk of venous thromboembolism, and trials reported no cases of anaphylaxis. Sugammadex induced limited (< 8% at 10 minutes) and transient (< 1 hour) increases in aPTT and prothrombin time but was not associated with increased risk of bleeding or increased severity of bleeding. A much smaller trial (Tas 2015; n = 50) investigated effects of sugammadex and neostigmine on postoperative coagulation parameters and bleeding after seroplasty with sugammadex, increasing postoperative bleeding measured by nasal tip dressings (4.1 ± 2.7 mL in the sugammadex group vs 2.5 ± 2.7 mL in the neostigmine group; P = 0.013) without significantly affecting prothrombin time (PT) (P = 0.953), aPTT values (P = 0.734), or international normalized ratio (INR) values (P = 0.612).
Mekawy 2012 reported no differences in intraoperative blood loss between sugammadex 4 mg/kg (n = 20) and neostigmine 0.05 mg/kg plus atropine 0.02 mg/kg groups (104.6 ± 13.2 vs 111.2 ± 9.8 mL, respectively; P = 0.060)
Renal function
Isik 2016 (n = 50) investigated effects of neostigmine and sugammadex on kidney function and found that both drugs may affect kidney function but sugammadex has more tolerable effects than neostigmine.
Gastric emptying
Sustic 2012 measured gastric emptying by using the paracetamol absorption test. Values of plasma paracetamol concentration (PPC) immediately after arrival of participants in the recovery room (T0) were significantly higher between the sugammadex 2 mg/kg group (1.2 ± 0.9) and the neostigmine 0.04 mg/kg/atropine 0.015 mg/kg group (0.4 ± 0.4) (P < 0.01). Values of PPC at 15, 30, 60, 120, and 150 minutes were higher without reaching statistical difference: T15, 2.1 ± 1.5 vs 1.5 ± 1.4; T30, 3.7 ± 3.8 vs 2.9 ± 2.2; T60, 4.2 ± 2.8 vs 3.5 ± 2.7; T120, 5.0 ± 3.4 vs 4.6 ± 3.6; and T150, 5.9 ± 3.4 vs 4.9 ± 3.2.
Values for PPC at 90 minutes were minimally higher in the neostigmine‐atropine group: time 90, 4.6 ± 3.4 vs 4.7 ± 3.4 (P = NS). Study authors concluded that although results show a tendency toward faster gastric emptying in the sugammadex group, this difference did not reach statistical difference, possibly owing to the small sample size of the study .
Thyroid function
Kvolik 2012a (n = 24) investigated effects on thyroid function and observed a significant increase in T4 levels compared with baseline one hour after anaesthesia (from 13.3 to 17.5 in the neostigmine group, and from 12.6 to 16.2 pmol/L in the sugammadex group; P < 0.05) that returned to baseline after 24 hours in both groups. T3 decreased in both groups postoperatively (from 5.2 to 3.5 in the neostigmine group, and from 4.9 to 3.3 pmol/L in the sugammadex group), with no intergroup differences noted (P > 0.05). Mean thyroid‐stimulating hormone (TSH) after 24 hours was not different between groups (1.32 in the neostigmine group vs 1.27 pmol/L in the sugammadex group; P = 0.49). In conclusion, sugammadex treatment did not change the levels of thyroid hormones and may be used safely in patients undergoing total thyroidectomy.
Cognitive function
Riga 2014 (n = 114) investigated cognitive function in patients receiving sugammadex or neostigmine and found no significant differences between groups when measuring cognitive function with the mini‐mental state evaluation test (P = 0.25), as described in Tombaugh 1992, and the Clock Drawing test (P = 0.06), as described in Agrell 1998.
Postoperative vomiting and nausea (PONV)
Carron 2013 reported higher PONV scores in the neostigmine group than in the sugammadex group (3.2 ± 1.5 vs 1.9 ± 1.3; P = 0.015; n = 40) with no significant difference in antiemetic supplement (7 (35%) vs 3 (15%); P = 0.10).
Tas 2015 compared sugammadex 2 mg/kg (n = 24) versus neostigmine 0.05 mg/kg plus atropine 0.02 mg/kg (n = 26) and reported no differences regarding nausea/vomiting between groups (P = 0.512).
Raziel 2013 (n = 40) observed no differences between sugammadex 2 mg/kg and neostigmine 0.05 mg/kg in nausea/vomiting among morbidly obese participants undergoing bariatric surgery.
Pain
Martini 2014 compared moderate NMB (T1 to T2) induced by atracurium/mivacurium reversed by neostigmine 1 to 2 mg plus atropine 0.5 to 1 mg (n = 12) versus deep NMB (PTC 1 to 2) induced by high‐dose rocuronium and reversed by sugammadex 4 mg/kg (n = 12) and found no significant differences in pain score as measured by a 10‐point scale (2.6 ± 1.6 vs 2.1 ± 2.2, respectively).
Tas 2015 compared sugammadex 2 mg/kg (n = 24) versus neostigmine 0.05 mg/kg plus atropine 0.02 mg/kg (n = 26) and reported no differences regarding postoperative pain between groups (P = 0.280).
Overall signs of postoperative residual paralysis
We chose the following parameters as overall signs of postoperative residual paralysis: inability to perform 5 second head‐lift test and general muscle weakness after extubation and at PACU discharge, amblyopia, asthenia, desaturation < 90%, transitory oxygen supplementation, respiratory distress, respiratory depression, postoperative respiratory complications (evaluated by PRSES – postoperative system evaluation score), moderate dyspnoea, pneumonia, acute lung failure, or symptoms of residual NMB or recurrence of NMB if specifically reported by study authors. The following 15 studies reported any of these adverse events: Balaka 2011; Blobner 2010; Brueckmann 2015; Carron 2013; Flockton 2008; Geldner 2012; Jones 2008; Khuenl‐Brady 2010; Koyuncu 2015; Kvolik 2012b; Lemmens 2010; Mekawy 2012; Schaller 2010; Woo 2013; Wu 2014).
Meta‐analysis of trial results showed significantly reduced risk of overall signs of postoperative residual paralysis (RR 0.40, 95% CI 0.28 to 0.57; I2 = 0%; n = 1474; random‐effects model; NNTB 13; Analysis 3.39; GRADE quality of evidence: moderate; Table 3) in the sugammadex group when compared with the neostigmine group. We downgraded GRADE quality of evidence one level owing to high risk of bias.
3.39. Analysis.
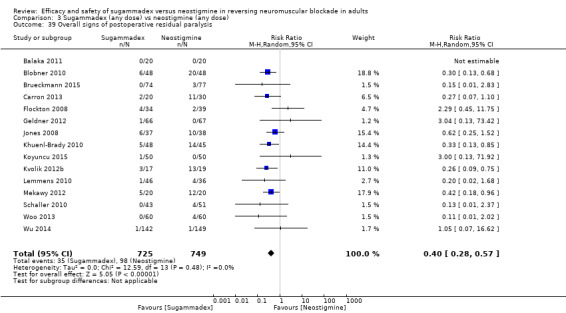
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 39 Overall signs of postoperative residual paralysis.
Investigators reported the following data on overall events of postoperative residual paralysis, which were ineligible for meta‐analysis. Carron 2013 (n = 40) found higher peripheral oxygen saturation levels (SpO2) levels at recovery admission in the sugammadex group (97 ± 2.3% vs 94.4 ± 4%; P = 0.018), along with faster ability to swallow after extubation (7.1 ± 1.8 minutes vs 12.2 ± 6 minutes; P = 0.0027), and faster ability to get into bed independently (24 ± 9 minutes vs 33.4 ± 12 minutes; P = 0.022) when compared with the neostigmine group.
Foletto 2014 (n = 34) reported that respiratory function was restored more quickly in morbidly obese (MOB) participants who received sugammadex when measured by postoperative forced vital capacity (1.6 ± 0.7 vs 2.41 ± 0.8 L; P < 0.05), forced expiratory volume in one second (1.37 ± 0.7 vs 2.05 ± 0.6 L/s; P < 0.05), and peak expiratory flow 30 minutes postoperatively (2.55 ± 1.7 vs 3.75 ± 1.4 L/s; P < 0.05), but observed no significant differences in spirometry performed 15 minutes postoperatively.
Raziel 2013 (n = 40) observed no differences between sugammadex 2 mg/kg and neostigmine 0.05 mg/kg in respiratory function among morbidly obese participants undergoing bariatric surgery.
Martini 2014 compared moderate NMB (T1 to T2) induced by atracurium/mivacurium reversed by neostigmine 1 to 2 mg plus atropine 0.5 to 1 mg (n = 12) with deep NMB (PTC 1 to 2) induced by high‐dose rocuronium and reversed by sugammadex 4 mg/kg (n = 12), and found no significant difference in saturation in PACU (98.6 ± 1.8 vs 98.2 ± 1.4, respectively) or breathing rate in PACU (14.5 ± 2.2 vs 14.5 ± 2.2, respectively).
Sherman 2014 found lower saturation levels (95.8 ± 0.014 vs 96.72 ± 0.01; P < 0.02), lower minimal saturation (93% vs 94%), and no difference in respiratory complications when comparing neostigmine 2.5 mg (n = 25) versus sugammadex 2 mg/kg (n = 32).
Tas 2015 compared sugammadex 2 mg/kg (n = 24) versus neostigmine 0.05 mg/kg plus atropine 0.02 mg/kg (n = 26) and reported no differences between groups regarding saturation levels after extubation (97.6 ± 0.2 vs 98.0 ± 0.2, respectively; P = 0.280).
Furthermore, several trials conducted postoperative neuromuscular monitoring to quantify the risk of residual neuromuscular blockade, defined as TOFR < 0.9: Brueckmann 2015 found that zero out of 74 (0%) sugammadex participants and 33 out of 76 (43.4%) neostigmine participants had TOFR > 0.9 at PACU admission (odds ratio (OR) 0.0, 95% CI 0.0 to 0.6; P < 0.0001). Of the 33 neostigmine participants, 2 also had clinical evidence of residual NMB.
Sabo 2011 described that 2 out of 50 participants (4%) in the sugammadex group had residual NMB (TOFR < 0.9) at the time of extubation compared with 26 out of 43 participants (60.5) in the neostigmine group, although data provided no clinical evidence (i.e. respiratory problems) of residual NMB in either group.
Gaszynski 2011 described that TOF at PACU was 109.8% versus 85.5% (P < 0.05; n = 70) in the sugammadex and neostigmine groups, respectively, and reached > 90% in every case in the sugammadex group but not in the neostigmine group.
No participants experienced recurrence of neuromuscular blockade based on neuromuscular monitoring in Geldner 2012 (n = 133).
Sugammadex (any dose) versus neostigmine (any dose), drug‐related serious adverse events (SAEs)
Fourteen trials reported serious adverse events (SAEs) possibly, probably, or definitely related to study drug (Adamus 2011; Blobner 2010; Brueckmann 2015; Flockton 2008; Geldner 2012; Hakimoglu 2016; Jones 2008; Kaufhold 2016; Khuenl‐Brady 2010; Koyuncu 2015; Lemmens 2010; Schaller 2010; Woo 2013; Wu 2014). Meta‐analysis of trial results showed no significant differences between sugammadex and neostigmine regarding participants with one or more serious adverse events or for composite adverse events (RR 0.54, 95% CI 0.13 to 2.25; I2 = 0%; ten studies; n = 959; random‐effects model; Analysis 3.40; GRADE quality of evidence: low; Table 3). We downgraded GRADE quality of evidence two levels owing to high risk of bias and imprecision.
3.40. Analysis.
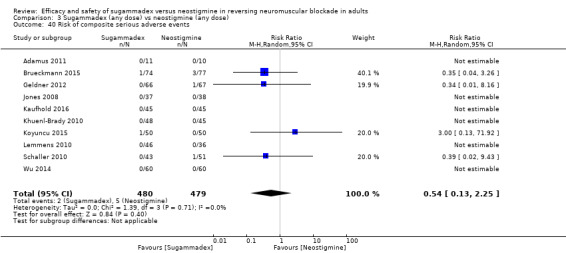
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 40 Risk of composite serious adverse events.
Clearly reported drug‐related serious adverse events included one case of acute myocardial infarction, pneumonia, and inadequate NMB reversal in the neostigmine group (Brueckmann 2015), one case of acute lung failure in the neostigmine group (Schaller 2010), one case of postoperative upper abdominal pain in the neostigmine group (Geldner 2012), one case of postprocedural haemorrhage in the sugammadex group (Brueckmann 2015), and finally one case of respiratory depression in the sugammadex group (Koyuncu 2015).
Trial sequential analysis (TSA)
We applied TSA to several outcome data as described in Table 1, Table 2, and Table 3.
TSA of all trials comparing neostigmine 0.05 mg/kg versus sugammadex 2.0 mg/kg with regard to recovery time from T2 to TOFR > 0.9 minutes indicates that with a required information size of 106, firm evidence sugammadex in a random‐effects model, with an alfa‐boundary adjusted MD of ‐10.22 (95% CI ‐12.11 to ‐8.33; diversity (D2) = 87%; I2 = 84%; random‐effects model; Figure 1). The cumulative Z‐curve crossed the monitoring boundary constructed for the required information size with 80% power and alpha of 0.05. However, none of the included trials had low risk of bias, and given that TSA is ideally designed for trials with low risk of bias and cannot be adjusted for risk of bias, the precision of our findings has to be downgraded. Furthermore, we found a high degree of diversity and heterogeneity, which once again raises questions about the reliability of the calculated required information size.
TSA of dichotomous data on drug‐related risk of adverse events when neostigmine (any dose) was compared with sugammadex (any dose) with continuity adjustment for zero event trials (0.001 in each arm) resulted in an alfa‐boundary adjusted RR of 0.62 (95% CI 0.51 to 0.74; diversity (D2) = 34%; I2 = 14%; random‐effects model; Figure 3), with a control event proportion of 27.97%. With the required information size of 502, analyses provided firm evidence in favour of sugammadex, with 2298 participants included, corresponding to a relative risk reduction (RRR) of 38% with 80% power and alpha of 0.05. Despite the fact that the cumulative Z‐curve does not cross the monitoring boundary directly, it is hard to imagine future trials radically changing the overall picture of this analysis. Once again, none of the included trials had low risk of bias and this does downgrade the reliability of our finding.
TSA of dichotomous data on risk of serious adverse events when neostigmine (any dose) was compared with sugammadex (any dose) with continuity adjustment for zero event trials (0.001 in each arm) resulted in an alfa‐boundary adjusted RR of 0.35 (95% CI 0.00 to 3190; diversity (D2) = 0%; I2 = 0%; random‐effects model), with a control event proportion of 1.04%. The cumulative Z‐curve does not cross the monitoring boundary constructed for a required information size of 8189 participants, with 11.71% of the required information size included across included trials so far with 80% power and alpha of 0.05. Once again, none of the included trials had low risk of bias and this affects the reliability and precision of our estimates.
TSA of dichotomous data on risk of signs of residual neuromuscular blockade when neostigmine (any dose) was compared with sugammadex (any dose) with continuity adjustment for zero event trials (0.001 in each arm) resulted in an alfa‐boundary adjusted RR of 0.4 (95% CI 0.27 to 0.59; diversity (D2) = 0%; I2 = 0%; random‐effects model), with 80% power and alpha of 0.05 (Figure 4), with a control event proportion of 13.08%. The cumulative Z‐curve crosses the monitoring boundary constructed for a required information size of 424 participants, indicating firm evidence in favour of sugammadex. However, as previously described, none of the included trials had low risk of bias and this equally diminishes the reliability and precision of our estimates.
Finally, owing to overall high risks of bias, imprecision, and indirectness involved in assessment of GRADE for the above analysis, one could easily argue that the required power should be 90% ‐ not 80% ‐ by which the required information size would be increased; nevertheless we cannot rule out the direction of results in favour of sugammadex, despite the absence of large trials with low risk of bias.
Discussion
Summary of main results
In this systematic review of 41 randomized controlled trials (RCTs; 4206 participants) comparing the efficacy and safety of sugammadex versus neostigmine in reversing rocuronium‐induced neuromuscular blockade (NMB), we found a large and significant difference in reversal time favouring sugammadex. For meta‐analyses of primary outcomes, 12 studies (n = 949) were eligible.
Meta‐analysis of trial results showed that sugammadex 2 mg/kg reversed NMB from second twitch (T2) to train‐of‐four ratio (TOFR) > 0.9 in 1.96 minutes, and neostigmine 0.05 mg/kg reversed NMB from T2 to TOFR > 0.9 in 12.87 minutes. Sugammadex 2 mg/kg was therefore on average 10.22 minutes (6.6 times) faster than neostigmine 0.05 mg/kg in reversing NMB at T2 reappearance (mean difference (MD) 10.22 minutes, 95% confidence interval (CI) 8.48 to 11.96; I2 = 84%; ten studies; n = 835; random‐effects model; GRADE quality of evidence: moderate; Analysis 1.1). Reversal time from post‐tetanic count (PTC) 1 to 5 to TOFR > 0.9 was not investigated; this was considered clinically irrelevant owing to the doses of sugammadex and neostigmine used for this comparison.
Sugammadex 4 mg/kg reversed NMB from PTC 1 to 5 to TOFR > 0.9 in 2.9 minutes, and neostigmine 0.07 mg/kg reversed NMB from PTC 1 to 5 to TOFR > 0.9 in 48.8 minutes. Sugammadex 4 mg/kg was therefore on average 45.78 minutes (16.8 times) faster than neostigmine 0.07 mg/kg in reversing NMB at PTC 1 to 5 reappearance (MD 45.78 minutes, 95% CI 39.41 to 52.15; I2 = 0%; n = 114; random‐effects model; GRADE quality of evidence: low; Analysis 2.1). Reversal time from T2 to TOFR > 0.9 was not investigated since it was deemed clinically irrelevant owing to the doses of sugammadex and neostigmine used for this comparison.
We found 28 trials (n = 2298) eligible for meta‐analysis of the secondary outcomes (risks of adverse events and serious adverse events). We found significantly fewer composite adverse events in the sugammadex group than in the neostigmine group (risk ratio (RR) 0.60, 95% CI 0.49 to 0.74; I2 = 40%; 28 studies; n = 2298; random‐effects model; GRADE quality of data: moderate; Analysis 3.1). Specifically, the risk of composite adverse events was 283/1000 in the neostigmine group and 159/1000 in the sugammadex group. Analysis of number needed to treat for an additional beneficial outcome (NNTB) revealed that eight patients should be treated with sugammadex rather then neostigmine to avoid one patient experiencing a single random adverse event. Furthermore, significantly fewer participants had one or more adverse events (RR 0.62, 95% CI 0.48 to 0.81; I2 = 0%; n = 1766; random‐effects model; GRADE quality of data: moderate; Analysis 3.5) in the sugammadex group than in the neostigmine group. Review of specific adverse events in the sugammadex group compared with the neostigmine group revealed significantly less risk of the following adverse events: bradycardia (Analysis 3.6), postoperative nausea and vomiting (PONV) (Analysis 3.7), desaturation (Analysis 3.8), and need for transitory oxygen supplementation (Analysis 3.10). Also, a significantly lower number of participants in the sugammadex group were not able to perform 5 second sustained head‐lift at extubation (Analysis 3.11). Data showed no significant differences between sugammadex and neostigmine regarding participants with one or more serious adverse events, nor in composite adverse events (RR 0.54, 95% CI 0.13 to 2.25; I2 = 0%; ten studies; n = 959; random‐effects model; GRADE quality of evidence: low; Analysis 3.40). Reversal time from T2 and PTC 1 to 5 to TOFR > 0.9 was not investigated, as it is clinically irrelevant owing to the doses of sugammadex and neostigmine used for this comparison.
Overall completeness and applicability of evidence
For our primary outcome, we performed a comparison of the effects of sugammadex and neostigmine at two depths of NMB: moderate block as indicated by reappearance of T2, and deep block as indicated by reappearance of PTC 1 to 5 on neuromuscular monitoring. However, administration of neostigmine is not recommended for reversal of deep block and absence of any signs of neuromuscular recovery due to the ceiling effect (Caldwell 2009; Plaud 2010), which is seen when maximal acetylcholine concentration is not sufficient to adequately compete with the muscle relaxant. According to the current prescribing information, this is an off‐label indication (www.fda.gov). Nevertheless, our search identified two trials (Carron 2013, Jones 2008) in which sugammadex and neostigmine were used to reverse rocuronium‐induced deep NMB, and one trial (Lemmens 2010) in which sugammadex and neostigmine were used to reverse vecuronium‐induced deep block. As this was not an exclusion criterion in the original protocol and the data were available, we chose to include these three studies in our review. However, for reasons explained above, the clinical importance of these comparatory findings aside from the obvious faster reversal due to sugammadex remains questionable.
In this context, one could argue that a comparison between sugammadex and neostigmine for reversing a shallow NMB would be more relevant. However, this was not a predefined outcome in the original protocol. Furthermore, our search identified five trials in which some degree of shallow block was indicated (Kaufhold 2016; Koyuncu 2015; Pongracz 2013; Schaller 2010; Yagan 2015), but none of these trials obtained comparable data on recovery time to TOFR > 0.9.
The overall quantity of data on which our conclusions can be based is large, and data were drawn from 41 randomized controlled trials with 4206 participants. According to GRADE, the quality of evidence for most of our meta‐analyses is moderate. Most trial participants were adults classified as American Society of Anesthesiologists (ASA) I to III who were undergoing elective surgery, and reported outcomes were relevant in a clinical setting. Primary and secondary outcomes, recovery time to TOFR > 0.9, and adverse effects, were generally well reported. Therefore, on basis of the large number of identified studies and participants, available evidence seems to be applicable to adult patients of ASA classification I to III who are undergoing elective surgery.
According to our meta‐analyses, sugammadex 2 mg/kg given at T2 reverses the NMB within 1.96 minutes and 6.6 times (10.22 minutes) faster than neostigmine 0.05 mg/kg (12.87 minutes). Furthermore, sugammadex 4 mg/kg, given to deep NMB at PTC 1 to 5 reappearance, reverses the block in 2.9 minutes and 16.8 times (45.78 minutes) faster than neostigmine 0.07 mg/kg (48.8 minutes).
The time difference offers several potential advantages in that a patient who is paralysed with a neuromuscular blocking agent has to be out of the NMB with TOFR > 0.9 before undergoing tracheal extubation, to avoid adverse effects due to residual paralysis (Eikermann 2006; Murphy 2008; Murphy 2013).
Sugammadex rapidly reverses NMB. This appears favourable because it reduces required anaesthesia time for the patient. Additionally, unlike neostigmine, sugammadex can be administered at any stage during a surgical procedure and independent of the depth of blockade. A reduced duration of anaesthesia not only may improve recovery time for the patient but could potentially reduce costs by saving the time needed for a prolonged awakening and potentially enabling smoother flow of patients through the operating theatre.
The cost‐effectiveness of sugammadex was not a predefined outcome of this review. To demonstrate cost‐effectiveness of sugammadex, two issues must be established: reduced patient recovery time perioperatively, and translation of any such reduction into resource utilization in terms of freeing up staff to work on productive alternative activities such as caring for other patients. This outcome is very difficult to assess owing to various confounders, such as the organizational structure of each hospital (Dexter 1995), procedural flow, variability of NMB, monitoring and extubation practices, turnover times between procedures, frequency of emergency procedures, operating room overtime resource use, staff payments, productive alternative use of freed resources (Fuchs‐Buder 2012; Paton 2010), and finally the cost of available drugs in each country. Furthermore, it is difficult to calculate whether any reduction in adverse events associated with sugammadex, besides improved quality of care, can readily be translated into cost‐effectiveness.
One systematic review (Paton 2010) compared the cost‐effectiveness of sugammadex versus neostigmine/glycopyrrolate for routine reversal of moderate or profound muscle relaxation produced by rocuronium and vecuronium. Results from included trials (Flockton 2008, Blobner 2010, Lemmens 2010, Jones 2008) indicate that sugammadex 2 mg/kg (4 mg/kg) produces more rapid recovery from moderate NMB than is achieved with neostigmine/glycopyrrolate. Economic assessment indicated that if the reductions in recovery time associated with sugammadex in these trials were replicated in routine clinical practice, sugammadex would be cost‐effective if those reductions were achieved in the operating theatre, but not if they were achieved in the recovery room. Review authors went on to conclude that further research is required to evaluate the effects of sugammadex on patient safety, predictability of recovery from NMB, patient outcomes, and efficient use of resources. A recent Canadian study (Insinga 2016) used a discrete model‐event simulation to investigate the potential impact of substituting sugammadex for neostigmine on operating room efficiency and incidence of residual NMB. Study authors concluded that the principal impact for patients managed by moderate NMB is likely to be seen as a reduction in the risk of residual NMB and associated complications. For patients maintained at a deep level of block until the procedure is completed, sugammadex was likely to both enhance operating room efficiency and reduce residual block complications. Last but not least, the cost per anaesthetic case might increase in case of unrestricted use of sugammadex, as shown in a retrospective observational audit (Ledowski 2012).
In conclusion, considerable uncertainties remain regarding the cost‐effectiveness of sugammadex, and further investigation is needed. Currently, the cost of sugammadex is relatively high as the result of proprietary rights. The price for the smallest vial (100 mg/mL, 2 mL) in Denmark is around 117 euros. In addition, drug patents are set to expire on 27 January 2021 (Drugs.com). How this will affect the price and clinical usage of sugammadex remains to be established.
Another important clinical consideration in the choice of reversing agent is the risk of adverse effects.
The decision to use a drug is based on an overall assessment of its benefits and harms. Monitoring and reporting of adverse events during a clinical trial constitutes a cumbersome and complex task involving many assumptions and choices, such as adequate blinding of study participants and investigators, distinction between adverse and serious adverse events, causality of adverse events to study drugs, reporting by patients, and finally consistent and transparent monitoring, coding, and reporting by investigators.
Trials included in this review defined, monitored, and reported adverse events in many different ways. Some trials (Blobner 2010; Jones 2008; Lemmens 2010) coded all adverse events and serious adverse events described by the investigator in a systematic way using the Medical Dictionary for Regulatory Activities (MeDRA). Other trials reported symptoms related to study drug administration without necessarily defining them as adverse events (Adamus 2011; Mekawy 2012) ‐ an issue most often seen in meeting abstracts (Balaka 2011; Georgiou 2013; Kvolik 2012b) that is probably due to word count restriction. Furthermore, some included trials specifically addressed causality between adverse events and study drugs by presenting not only adverse events observed regardless of relation to study drug but also adverse events possibly, probably, or definitely related to study drug (Blobner 2010; Jones 2008; Lemmens 2010; Woo 2013), although others did not specifically address this issue (Adamus 2011; Castro 2014; Yagan 2015). Smaller trials with few observed adverse events usually presented all observed adverse events (Balaka 2011; Koc 2015; Koyuncu 2015; Yagan 2015), while bigger trials presented the most frequently occurring adverse events (Brueckmann 2015; Jones 2008; Lemmens 2010; Woo 2013). Additionally, some trials used blinded safety outcome assessors (Blobner 2010; Brueckmann 2015; Carron 2013; Flockton 2008; Woo 2013) in contrast to others (Grintescu 2009; Kizilay 2016). Last but not least, very few of the included trials were designed and powered to address safety as a primary outcome (Brueckmann 2015; Rahe‐Meyer 2014).
As explained earlier in the Methods and Results sections, overall clinical signs of postoperative residual paralysis such as inability to perform 5 second head‐lift and general muscle weakness observed in some trials (Blobner 2010; Flockton 2008; Jones 2008; Khuenl‐Brady 2010; Lemmens 2010) were regarded as adverse events in this review. Furthermore, we decided to include reported symptoms related to drug administration as adverse events, even though they were not specifically defined as adverse events, to avoid potentially dismissing good quality data because of lack of correct phrasing. We have addressed and explained under the notes section in Characteristics of included studies any discrepancy in adverse events presented in the original article and in this review due to definitions of adverse events or additional data about adverse events supplied through email correspondence with trial authors. Readers of medical journals and of this review need to be aware of these issues as they appraise this review and the literature critically.
Included trials provided sparse data regarding which body weight dose calculations were based upon (i.e. ideal, correlated, or lean body weight), and we were unable to retrieve additional data that would shed light on this. As a consequence, we have regarded the weight data provided as total body weight.
Our results show an overall significantly lower risk of adverse events in the sugammadex group than in the neostigmine group (Analysis 3.1; Analysis 3.5), along with an NNTB of eight for avoidance of an adverse event.
Data show significantly less risk of the clinically important adverse effect PONV (Analysis 3.7) and less risk of overall signs of postoperative residual paralysis in the sugammadex group (Analysis 3.39), making this treatment preferable because residual blockade increases the risk of serious adverse effects such as acute respiratory failure (Murphy 2008; Sauer 2011). Data also show reduced risk of bradycardia (Analysis 3.6) in the sugammadex group. However, the two groups reported many adverse reactions similarly, as presented in the Results section. Results show that no cases of anaphylaxis were reported.
Our results may not be directly applicable to all groups of patients because sugammadex may have different outcomes for patients with higher ASA classes and for patients with special comorbidities or systemic dysfunction.
These patients are not represented well in the trials included in our meta‐analyses, but lower risk of adverse effects as well as sufficient reversal from neuromuscular blockade may be even more beneficial for this group of patients, and inclusion of these more fragile patients in future trials could potentially reduce the NNTB for avoidance of adverse events. However, this might not be applicable to all patient groups (e.g.. severe renal impairment has been discussed as a possible contraindication to treatment. Sugammadex is excreted unchanged in the urine by the kidneys. Renal clearance of sugammadex is rapid, with most of the dose (70%) excreted within six hours (Golembiewski 2016). None of the included trials enrolled participants with renal dysfunction. However, Isik 2016 (n = 50) investigated the effects of neostigmine and sugammadex on adults of ASA I to II with normal renal function and found that both drugs may impair renal function, but sugammadex was more tolerable than neostigmine. A pharmacokinetic study (Staals 2010) investigated the pharmacokinetics of sugammadex 2 mg/kg and of rocuronium 0,6 mg/kg in 15 participants with renal failure and in 15 healthy controls. Investigators found that urinary excretion of sugammadex was reduced among participants with renal failure. The median quantity of sugammadex excreted in the urine within 72 hours among participants with renal failure was 29%, and 73% in controls. Nevertheless, one has to conclude on the basis of existing evidence that studies on the use of sugammadex for patients with renal impairment are needed to examine safety, preferably with longer follow‐up than 72 hours, because late renal impairment has to be addressed equally.
Sugammadex has been suspected of increasing the risk of specific adverse effects such as QTc prolongation and bleeding events (Bridion 2014). However, we found limited data from few trials in our systematic review on these variables, and presented data were ineligible for meta‐analysis.
The summary of product characteristics provided by Bridion states that the "administration of 4 and 16 mg/kg of Sugammadex in healthy volunteers resulted in maximum and mean prolongations of the aPTT by 17% and 22%, respectively, and PT by 11% and 22%, respectively. These mean aPTT and PT prolongations were limited and of short duration < 30 min" (Bridion 2014). Rahe‐Meyer 2014 (n = 1198) included participants undergoing hip or knee surgery and compared sugammadex 4 mg/kg versus usual care (neostigmine or spontaneous recovery). Study findings indicate that sugammadex induced limited (< 8% at 10 minutes) and transient (< 1 hour) increases in activated partial thromboplastin time (aPTT) and prothrombin time (PT) but was not associated with increased risk or severity of bleeding. Tas 2015 (n = 50) investigated the effects of sugammadex and neostigmine on postoperative coagulation parameters and bleeding after seroplasty and demonstrated that sugammadex increased postoperative bleeding without significantly affecting PT and aPTT values. An RCT of healthy adults reported that after administration of sugammadex at doses of 4 mg/kg and 16 mg/kg, a dose‐dependent, limited, temporary, and clinically irrelevant prolongation in PT and aPTT was observed (De Kam 2014). A one‐year retrospective study (n = 193) performed in participants with high risk of postoperative bleeding (laparotomy for cancer surgery requiring suction drains) did not find sugammadex at doses of 2 and 4 mg·kg–1 to be associated with increased bleeding as measured by the amount of blood found in suction drains and dressings (Raft 2011).
However, upon review of the published literature, we are unable to refute or reject any safety concern with regard to sugammadex for patients at high risk of bleeding due to existing severe coagulopathy or due to the nature of procedures associated with high risk of transfusion because evidence is inadequate to support or withhold any use of sugammadex.
We found limited evidence with regard to haemodynamic implications of sugammadex use, but Kizilay 2016 compared the haemodynamic effects of sugammadex and neostigmine in participants with cardiac disease undergoing non‐cardiac surgery. Haemodynamic parameters were more prominently increased among participants receiving neostigmine, and cardiac function was noted to be more stable among those given sugammadex. Data show no significant differences between and within groups in terms of QTc values.
Morbidly obese patients make up a high‐risk group (Gaszynski 2011), and because of their often compromised respiratory function, they are considered especially vulnerable to residual curarization in the postoperative period influencing respiratory function (Gaszynski 2011). Three trials (n = 161) investigated the optimal sugammadex dose per kilogram body weight; total body weight (TBW) (Foletto 2014), corrected body weight (CBW) (Gaszynski 2011; Georgiou 2013), and ideal body weight (IBW) (Georgiou 2013). All three studies found sugammadex 2 mg/kg to be significantly faster than neostigmine 0.04 to 0.05 mg/kg in reversing neuromuscular blockade at T2 reappearance, and in reducing the risk of postoperative residual curarization (Foletto 2014; Gaszynski 2011).
Researchers have speculated about the influence of volatile anaesthetics and recovery times when neuromuscular blocking agents (NMBAs) are used (Reid 2001). However, we found no significant differences in recovery time to TOFR > 0.9 when anaesthesia maintained with volatile anaesthetic (eight trials; n = 490) was compared with total intravenous anaesthetic (TIVA) (four trials; n = 381) (Analysis 1.2).
Sugammadex was specifically designed to reverse rocuronium as a non‐depolarizing NMBA, as is demonstrated by most of the trials included in this review. However, two of the included trials (Lemmens 2010; Rahe‐Meyer 2014) used sugammadex to revert vecuronium. Furthermore, Flockton 2008 compared sugammadex following rocuronium versus neostigmine following cisatracurium, and Martini 2014 compared atracurium for induction and mivacurium for maintenance versus rocuronium for both induction and maintenance. Two studies (Castro 2014; Sherman 2014) provided no information on the NMBA used.
Quality of the evidence
This systematic review provides a robust assessment of the efficacy of sugammadex because it includes a large number of trials with large numbers of participants showing a consistent direction of results across all trials and additional confirmation through various exploratory analyses favouring the intervention for our primary outcome.
However, this review also has several potential limitations, as our findings and interpretations are limited by the quality and quantity of available evidence from included RCTs. The RCT is considered the most rigorous method of determining whether a cause‐effect relationship exists between an intervention and an outcome. The strength of the RCT lies in the process of randomization, but several potential risks of bias in trial methods can affect results.
The review authors have judged the risk of bias for each included study by using the recommended risk of bias assessment in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). All of our studies had at least one "high or unclear risk of bias", and we considered the risk that trials may overestimate or underestimate the true intervention effect a serious limitation for all trials. In particular, judgements of performance risk of bias and funding risk of bias were overall high. We judged none of the included studies as having low risk of bias.
Application of the GRADE approach enables us to incorporate risk of bias, directness of evidence, heterogeneity, precision of effect estimate, and risk of publication bias.
The GRADE quality of our findings ranks as moderate for our primary outcome, and from low to moderate across different outcomes. The main limiting factors that accounted for decreased quality of evidence included high risk of bias and imprecision (Table 1; Table 2; Table 3).
We mainly assessed the risk of bias of included trials using published data, which ultimately may not reflect the truth. We contacted all trial authors; 12 (33.3%) responded and provided further information. Lack of reporting of some of the data may have affected our judgement on risk of bias in either direction.
We applied several statistical methods to explore and reduce the extent of bias, such as complete case analysis, trial sequential analysis (TSA), overall methodological bias assessment, and analyses of various relevant clinical and physiological outcomes.
Application of TSA to our primary outcome indicates that at this stage, sugammadex appears superior to neostigmine. TSA provided firm evidence in favour of sugammadex for outcomes such as recovery time from T2 to TOFR > 0.9 minutes, adverse events, and overall signs of postoperative residual paralysis. However, none of the included trials were at low risk of bias, and as TSA cannot be adjusted for risk of bias, we did not calculate the low risk of bias adjusted information size, which ultimately affects the reliability and precision of our findings.
Evaluated outcomes consistently favoured sugammadex. However, we graded the quality of evidence as moderate because of the high proportion of trials at high risk of bias, large clinical and statistical heterogeneity, and small sample sizes, but we upgraded the level of evidence in favour of sugammadex as indicated by TSA analyses.
On the basis of the criteria mentioned above, we deemed the overall GRADE quality of evidence in this review to be moderate.
Sugammadex was specifically designed to reverse rocuronium as a non‐depolarizing NMBA, as most of the trials included in this review demonstrated this. However, two of the included trials (Lemmens 2010; Rahe‐Meyer 2014) used sugammadex to revert vecuronium.
Potential biases in the review process
We have followed the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions as this official guide describes in detail the process of preparing and maintaining Cochrane systematic reviews on the effects of healthcare interventions (Higgins 2011). We have adhered to this handbook in handling the included RCTs.
Meta‐analyses are limited by the quality and quantity of available evidence. Even though our meta‐analyses are based on a large quantity of data, results and methods for some of the included studies were not thoroughly described. Furthermore, some of the included trials were not specifically designed to address the primary or secondary outcomes of this review, leading to possibly biased data. We have addressed this problem by labelling studies with high risk of "other bias", as is shown in Characteristics of included studies, Figure 2, and Figure 6, and by downgrading the GRADE quality of evidence (Table 1, Table 2, and Table 3). Additionally, we are aware that as we have performed many analyses of specific adverse events, the probability of achieving significant results by chance is high.
We used the same search strategy as was used in the original version of this review (Abrishami 2009), and we found 41 eligible studies for inclusion. We cannot exclude the possibility that we may have missed some of the published literature beyond the electronic databases searched for this review. However, the 41 trials with 4206 participants included in this review appear to provide sufficient data for meta‐analyses, and our TSAs indicate a better safety profile and clinical superiority of sugammadex compared with neostigmine for the population included in this trial.
We found 20 relevant ongoing trials registered at https://clinicaltrials.gov and three trials awaiting classification, but none of these studies have published data within our main search update from 2008 to 2 May 2016. When published, these trials may change the results and conclusions of this review. However, the main strength of this update consists of the quantity of data comparing sugammadex versus neostigmine in reversing NMB. The new search added eight years of research and 38 new trials to the review that was originally published (Abrishami 2009), which comprised three trials comparing sugammadex and neostigmine. Additionally, we have substantially updated and revised the methods of this review compared with methods of the previous one.
As a consequence, this review diverges from intended adherence to the Cochrane Handbook for Systematic Reviews of Interventions by not following the original protocol (Abrishami 2009) prepared for the first version of this review (Abrishami 2009). After several discussions with the editorial team, we made the decision to split the original review in two based on the extensive number of publications using various comparators, interventions, and outcome measures. Therefore, it seemed more appropriate to take the original review in a different direction and place more emphasis on safety issues and efficacy. Although this may be perceived as introduction of post hoc analyses, review authors selected outcomes and subgroup and sensitivity analyses for this review before identifying included trials (search) and extracting data to minimize the risk of bias.
Agreements and disagreements with other studies or reviews
The original published review (Abrishami 2009) found no difference with regard to adverse effects between sugammadex and neostigmine. This review found that sugammadex reduced the risk of adverse events when compared with neostigmine. We updated this review as of 2 May 2016 with regard to the search, adding eight years of research and 38 new trials; the original review (Abrishami 2009) comprised three trials. We re‐ran the search on 10 May 2017. Currently three trials are awaiting classification and 20 studies are ongoing.
Our results on the primary outcome, recovery time, are in accordance with the findings of all RCTs included in the meta‐analyses, as they reflect superiority for sugammadex as a reversing agent over neostigmine. With regard to our secondary outcomes ‐ risks of adverse and serious adverse events ‐ we found more diverging results among the included trials, although overall risk of adverse events was reduced in the sugammadex group (Analysis 3.1; Analysis 3.5). No previous publication has addressed this issue with the same rigour.
A recent systematic review of sugammadex versus neostigmine for reversal of NMB (Abad‐Gurumeta 2015) included 1553 participants across 17 RCTs (all are included in this review).
Abad‐Gurumeta 2015 focused mainly on postoperative residual paralysis and drug‐related adverse events (Abad‐Gurumeta 2015). Review authors found that sugammadex reduced all signs of residual postoperative paralysis (RR 0.46, 95% CI 0.29 to 0.71; P = 0.0004) and risk of minor respiratory events (RR 0.51, 95% CI 0.32 to 0.80; P = 0.0034). However, they reported no differences in critical respiratory events (RR 0.13, 95% CI 0.02 to 1.06; P = 0.06). Sugammadex reduced drug‐related adverse effects (RR 0.72, 95% CI 0.54 to 0.95; P = 0.02) but data show no differences in the rate of postoperative nausea or the rate of postoperative vomiting, Findings of this review were generally in line with the results of our updated review with regard to adverse and serious adverse events.
Another systematic review (Paton 2010), which included four trials (n = 606), compared sugammadex versus neostigmine/glycopyrrolate for routine reversal of NMB with economics evaluation. Researchers found that sugammadex was beneficial in terms of enhanced patient safety and increased predictability of recovery from rocuronium‐induced NMB, with more efficient use of theatre time and staff. Conclusions of review authors on recovery time, adverse events, and cost‐benefit considerations are in line with those of our updated review.
Authors' conclusions
Implications for practice.
In conclusion, results of this systematic review suggest that, in comparison with neostigmine, sugammadex can more rapidly reverse rocuronium‐induced neuromuscular block (NMB) regardless of the depth of the block. Sugammadex 2 mg/kg is 10.22 minutes (˜ 6.6 times) faster in reversing moderate NMB (second twitch (T2)) than neostigmine 0.05 mg/kg (1.96 vs 12.87 minutes), and sugammadex 4 mg/kg is 45.78 minutes (˜ 16.8 times) faster in reversing deep NMB (post‐tetanic count (PTC) 1 to 5) when compared with neostigmine 0.07 mg/kg (2.9 vs 48.8 minutes). With number needed to treat for an additional beneficial outcome (NNTB) of eight to avoid an adverse event, sugammadex appears to have a better safety profile than neostigmine when reversing NMB. Patients receiving sugammadex had 40% fewer adverse events than those given neostigmine (risk ratio (RR), specifically risk of bradycardia (RR 0.16, NNTB 14), postoperative nausea and vomiting (RR 0.52, NNTB 16), and overall signs of postoperative residual paralysis (RR 0.40, NNTB 13) were reduced. Both sugammadex and neostigmine were associated with serious adverse events in < 1% of patients, and data show no difference in risk of serious adverse events between groups.
Implications for research.
We suggest future trials should include large and adequate sample sizes and low risk of bias to confirm the findings mentioned above, specifically to evaluate the effect of sugammadex on risks of adverse events and serious adverse events, as well as on patient‐related outcomes, such as risk of residual NMB and other complications after NMB. More trials are needed to directly establish the efficacy and safety of sugammadex when used in situations such as "cannot intubate, cannot ventilate" and failed intubation during rapid sequence inducing with rocuronium.
What's new
| Date | Event | Description |
|---|---|---|
| 29 September 2017 | Amended | We corrected a typo in the Plain language summary. We changed the sentence: "Participants receiving sugammadex appeared to have a 40% reduced risk of experiencing harmful events than those given sugammadex", to "Participants receiving sugammadex appeared to have a 40% reduced risk of experiencing harmful events than those givenn eostigmine" in Key results under Plain language summary section. |
History
Review first published: Issue 8, 2017
| Date | Event | Description |
|---|---|---|
| 10 May 2017 | New citation required and conclusions have changed | The original published review (Abrishami 2009) concluded that trials found no difference in the instance of unwanted effects between sugammadex and neostigmine. Our updated review concludes that sugammadex reduces the risk of adverse events when compared with neostigmine. |
| 10 May 2017 | New search has been performed | The original published review (Abrishami 2009) has been updated by new review authors and split into two reviews, one review comparing sugammadex and neostigmine, and the other comparing sugammadex and placebo, as well as different doses of sugammadex. This review compares sugammadex and neostigmine and has been updated as of 10 May 2017 with regard to the search. The new search added eight years of research and 38 new trials to this review, including three trials that compared sugammadex and neostigmine. In total, this review comprises 41 included studies as well as 3 studies awaiting classification and 20 ongoing studies. Furthermore, review authors have completely revised the current review methodologically in accordance with the latest recommendations from Cochrane, with incorporation of full risk of bias tables, summary of finding tables, and trial sequential analysis. For more details, see Differences between protocol and review and Published notes. |
Notes
July 2017
After several discussions with the editorial team, a decision was reached to split the original review (Abrishami 2009), into two reviews based on the very extensive number of publications (>70) identified by the updated search with various comparators, interventions and different outcome measures. This updated review therefore does not follow the protocol (Abrishami 2008) made for the original version of the review (Abrishami 2009). In the updated review, we decided to only focus on Sugammadex and Neostimgine and compare their efficacy and safety.
Acknowledgements
We would like to thank Andrew Smith (Content Editor); Vibeke E Horstmann (Statistical Editor); Jan‐Uwe Schreiber and Jeffrey K Lu (Peer Reviewers); Roy Buffery (Consumer Referee); and Jane Cracknell (Managing Editor) for help and editorial advice provided during preparation of this systematic review. We would like to thank Janne Vendt (Information Specialist) and Karen Hovhannisyan (former Trials Search Co‐ordinator) for undertaking the electronic searches. We would also like to acknowledge Merck, Sharp and Dohme/Schering‐Plough, the manufacturer of sugammadex, and all trial authors who generously provided us with detailed information about trials included in this published systematic review.
Appendices
Appendix 1. MEDLINE (Ovid) 1950 to May 2017
#1 "sugammadex".mp.
#2 "selective relaxant binding agent".mp.
#3 "SRBA".mp.
#4 "org 25969".mp.
#5 "bridion".mp.
#6 or/1‐5
Appendix 2. Embase (Ovid) 1980 to May 2017
#1 "sugammadex".mp.
#2 "selective relaxant binding agent".mp.
#3 "SRBA".mp.
#4 "org 25969".mp.
#5 "bridion".mp.
#6 or/1‐5
Appendix 3. CENTRAL (The Cochrane Library; 2017, Issue 4)
#1 "sugammadex"
#2 "selective relaxant binding agent"
#3 "SRBA"
#4 "org 25969"
#5 "bridion"
#6 or/1‐5
Appendix 4. Assessment of risk of bias in included studies
1. Random sequence generation
Assessment of selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence.
Low risk: any truly random process based on computer‐generated random numbers, random number table, coin tossing, shuffling of cards, shuffling of envelopes, throwing of dice, or drawing of lots.
High risk: any non‐random process based on date of birth, date of admission, hospital record number, clinic record number, results of laboratory tests, or allocation by availability of the intervention, judgment of clinician, or preference of participant.
Unclear risk: insufficient information.
2. Allocation concealment
Assessment of allocation bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment.
Low risk: central allocation including telephone, Web‐based, and pharmacy‐controlled randomization, use of sequentially numbered opaque sealed envelopes (SNOSE) or sequentially numbered drug containers of identical appearance.
High risk: open random allocation schedule, assignment envelopes without appropriate safeguards, alteration or rotation, date of birth, case control number, or any other explicitly unconcealed procedure.
Unclear risk: insufficient information.
3. Blinding
Assessment of performance bias due to knowledge of allocated interventions by participants and personnel during the study.
PERFORMANCE BIAS: blinding of participants
Low risk: blinding of participants ensured and unlikely to have been broken.
High risk: no blinding or incomplete blinding of participants, and outcome likely to be influenced by lack of blinding, appropriate blinding of participants likely to have been broken or study categorized as ”open‐label”.
Unclear risk: insufficient information.
PERFORMANCE BIAS: blinding of key personnel (anaesthesiologist and surgeon)
Low risk: blinding of key personnel ensured and unlikely to have been broken.
High risk: no blinding or incomplete blinding of key personnel and outcome likely to be influenced by lack of blinding, appropriate blinding of key personnel likely to have been broken or study categorized as ”open‐label”.
Unclear risk: insufficient information.
DETECTION BIAS: blinding of primary outcome (TOF‐watch) assessment
Low risk: blinding of TOF‐watch assessor ensured and unlikely to have been broken.
High risk: no blinding or incomplete blinding of TOF‐watch assessor and outcome likely to be influenced by lack of blinding, appropriate blinding of TOF‐watch assessor likely to have been broken, study categorized as ”open‐label”.
Unclear risk: insufficient information.
DETECTION BIAS: blinding of secondary outcome (safety) assessment
Low risk: blinding of safety assessor ensured and unlikely to have been broken.
High risk: no blinding or incomplete blinding of safety assessor and outcome likely to be influenced by lack of blinding, appropriate blinding of safety assessor likely to have been broken or study categorized as ”open‐label”.
Unclear risk: insufficient information.
4. Incomplete outcome data
Assessment of attrition bias due to quantity, nature, or handling of incomplete outcome data.
Low risk: no missing outcome data, missing outcome data described (numbers and reasons for drop‐puts and withdrawals) and balanced in numbers across intervention groups with similar reasons for missing data across groups, or missing data have been imputed by appropriate methods.
High risk: missing outcome data, missing outcome data not described (numbers and reasons for drop‐puts and withdrawals) or not balanced in numbers across intervention groups with similar reasons for missing data across groups, or missing data have not been imputed by appropriate methods.
Unclear risk: insufficient information.
5. Selective reporting
Assesment of reporting bias due to selective outcome reporting.
Low risk: Study protocol is available, and all of the study’s prespecified (primary and secondary) outcomes of interest to the review have been reported in the prespecified way, or the study protocol is not available but it is clear that the published report includes all expected outcomes, including those that were prespecified.
High risk: Not all of the study’s prespecified primary outcomes have been reported, one or more of the primary outcomes are reported using measurements, analysis methods, or subsets of data that were not prespecified, one or more primary outcomes were not prespecified, one or more outcomes of interest to the review are reported incompletely so that they cannot be entered into the meta‐analysis, or the study report fails to include results for a key outcome that would be expected to be reported in such a study.
Unclear risk: insufficient information.
6. Funding bias
Assesment of any possible funding bias.
Low risk: reported no funding or funding by trial authors themselves, funding from Universities and other public institutions.
High risk: funding from private investor, pharmaceutical companies, or any trial investigator employed by or receiving grants, travel funding, or honoraria from a pharmaceutical company
Unclear risk: insufficient information.
7. Other bias
Assessment of any possible sources of bias not addressed in domains one to six.
Low risk: Report appears to be free of bias due to problems not covered elsewhere in the table.
High risk: At least one important bias is present that is related to study design, sample size calculation, early stopping because of some data‐dependent process, extreme baseline imbalance, academic bias, claimed fraudulence, or other problems.
Unclear risk: Information is insufficient for assessment of whether an important risk of bias exists, or the rationale or evidence is insufficient to suggest that an identified problem will introduce bias.
Appendix 5. All abbreviations
ACh ‐ Acetylcholine
AE – Adverse event
AEs – Adverse events
aPTT ‐ Activated partial thromboplastin time
ASA ‐ American Society of Anaesthesiologists
AST ‐ Aspartate aminotransferase
BIS ‐ Bispectral Index
BMI ‐ Body mass index
BP ‐ Blood pressure
BUN ‐ Blood urea nitrogen
CBW ‐ Corrected body weight
CI – Confidence interval
CIs – Confidence intervals
CNS ‐ Central nervous system
COPD ‐ Chronic obstructive pulmonary disease
Cr ‐ Creatinine
CrCL ‐ Creatinine clearance
Cys ‐ Cysteine
DEF – Dynamic end‐tidal forcing
DL – Diffusion lung capacity
DLCO/VA – Diffusion lung capacity for carbon monoxide/alveolar volume ratio
dNMB – deep neuromuscular blockage
ECG ‐ electrocardiography
eGFR – estimated glomerular filtration rate
EMGdi ‐ diaphragmatic electromyogram
ENT – Ear–nose‐throat
FOC ‐ Free of charge
FEV1 – Forced expiratory volume in one second
FVC – Functional vital capacity
GCS – Glasgow Coma Scale
GOLD – Global Initiative for Cronic Obstructive Lung Disease
GRADE – Grades of Recommendation Assessment, Development and Evaluation
H ‐ Hour
Hb ‐ Haemoglobin
HR ‐ Heart rate
ICU ‐ Intensive care unit
INR ‐ International normalized ratio
IOP ‐ Intraocular pressure
ITT ‐ Intention to treat
IV ‐ Intravenous
kg ‐ Kilograms
LBW ‐ Lean body weight
M2 ‐ Meters squared
mA ‐ Miliamperes
MAC ‐ Minimal alveolar concentration
MAP ‐ Mean arterial blood pressure
MedDRA ‐ Medical Dictionary for Regulatory Activities
MELD ‐ Model for End‐Stage Liver Disease
METS ‐ Metabolic equivalent of tasks
MG ‐ Myasthenia gravis
Mg ‐ Milligrams
MHR ‐ Mean heart rate
Min ‐ Minutes
Mmol/L ‐ Milimol/litre
MMSE ‐ Mini‐Mental State Examination
MO ‐ Morbidly obese
NM ‐ Neuromuscular
NMB ‐ Neuromuscular blockade
NMBA ‐ Neuromuscular blocking agent
NMBAs – Neuromuscular blocking agents
NMJ – Neuromuscular Junction
NMT ‐ Neuromuscular technique
NNTB ‐ Number needed to treat for an additional beneficial outcome
NS ‐ Not significant
NSQIP ‐ National Surgical Quality Improvement Program
NYHA ‐ New York Heart Assosiation
PACU ‐ Post‐anaesthesia care unit
PADSS ‐ Post‐anaesthesia discharge score system
PEF ‐ Peak expiratory flow
PONV ‐ Postoperative nausea and vomiting
PORC ‐ Postoperative residual curarization
PPC ‐ Plasma paracetamol concentration
PQRS ‐ Postoperative Quality Recovery Scale
PRSES ‐ Postoperative Respiratory System Evaluation Score
PT ‐ Prothrombin time
PTC ‐ Post‐tetanic count
QTc ‐ QTc interval
RBW – Real body weight
RCT – Randomized controlled trial
RCTs – Randomized controlled trials
RPONB ‐ Residual postoperative neuromuscular blockade
RR – Risk ratio
SAE ‐ Serious adverse event
SAEs – Serious adverse events
SAS ‐ SAS Institute
SD ‐ Standard deviation
Sec ‐ Seconds
SEVO ‐ Sevoflurane
SO ‐ Super obese
Sq ‐ Square
SRS ‐ Surgical Rating Scale
SX ‐ Symptoms
T – Twitch in train‐of‐four stimulation
T2 ‐ second twitch in train‐of‐four stimulation
TBW – Total body weight
TIVA – Total intravenous anaesthesia
TOF ‐ Train of four
TOFR ‐ Train‐of‐four ratio
TSA – Trial sequential analysis
TSH ‐ Thyroid‐stimulating hormone
µg ‐ Micrograms
VAS ‐ Visual Analogue Scale
Vs ‐ Versus
XLIF ‐ Extreme lateral interbody fusion
Yr ‐ Years
Data and analyses
Comparison 1. Sugammadex 2.0 mg/kg vs neostigmine 0.05 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovery time from T2 to TOFR > 0.9 | 10 | 835 | Mean Difference (IV, Random, 95% CI) | ‐10.22 [‐11.96, ‐8.48] |
| 2 Subgroup analysis: TIVA vs volatile anaesthetics | 11 | 871 | Mean Difference (IV, Random, 95% CI) | ‐9.83 [‐11.45, ‐8.20] |
| 2.1 TIVA | 3 | 381 | Mean Difference (IV, Random, 95% CI) | ‐8.50 [‐10.15, ‐6.86] |
| 2.2 Volitile anaesthetics | 8 | 490 | Mean Difference (IV, Random, 95% CI) | ‐10.57 [‐12.96, ‐8.18] |
| 3 Sensitivity analysis: meeting abstracts excluded | 9 | 767 | Mean Difference (IV, Random, 95% CI) | ‐9.27 [‐11.14, ‐7.40] |
Comparison 2. Sugammadex 4.0 mg/kg vs neostigmine 0.07 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovery time from PTC 1 to 5 to TOFR > 0.9 | 2 | 114 | Mean Difference (IV, Random, 95% CI) | ‐45.78 [‐52.15, ‐39.41] |
Comparison 3. Sugammadex (any dose) vs neostigmine (any dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of composite adverse events | 28 | 2298 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.49, 0.74] |
| 2 Composite adverse events: subgroup analysis for dosage | 28 | 2298 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.49, 0.73] |
| 2.1 Sugammadex 2 mg/kg vs neostigmine 0.04 mg/kg | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.05, 4.28] |
| 2.2 Sugammadex 2 mg/kg vs neostigmine 0.05 mg/kg | 12 | 1076 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.34, 0.80] |
| 2.3 Sugammadex 2 mg/kg vs neostigmine 0.07 mg/kg | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.44] |
| 2.4 Sugammadex 2 mg/kg vs neostigmine 2.5 mg | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Sugammadex 3 mg/kg vs neostigmine 0.03 mg/kg | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Sugammadex 4 mg/kg vs neostigmine 0.05 mg/kg | 4 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.49, 0.88] |
| 2.7 Sugammadex 4 mg/kg vs neostigmine 0.07 mg/kg | 3 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.93] |
| 2.8 Sugammadex, several doses vs neostigmine, several doses | 4 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.40, 0.90] |
| 3 Composite adverse events: subgroup analysis ‐ TIVA vs volatile anaesthetics | 28 | 2298 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.49, 0.73] |
| 3.1 TIVA | 7 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.20, 1.31] |
| 3.2 Volatile anaesthetic | 20 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.55, 0.73] |
| 3.3 No information | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Composite adverse events: sensitivity analysis ‐ excluding meeting abstracts | 24 | 2091 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.49, 0.73] |
| 5 Participants with ≥ adverse event | 19 | 1766 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.48, 0.81] |
| 6 Bradycardia: subgroup analysis ‐ atropine vs glycopyrrolate | 11 | 1218 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.07, 0.34] |
| 6.1 Atropine | 6 | 667 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.05, 0.36] |
| 6.2 Glycopyrrolate | 5 | 551 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.06, 0.69] |
| 7 PONV: subgroup analysis ‐ TIVA vs volatile anaesthetics | 6 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.97] |
| 7.1 TIVA | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 3.55 [0.15, 84.86] |
| 7.2 Volatile anaesthetics | 5 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.25, 0.91] |
| 8 Desaturation | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.06, 0.83] |
| 9 Procedural complications | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.97] |
| 10 Transitory oxygen supplementation | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.09, 0.66] |
| 11 Not able to perform 5 second head‐lift after extubation | 6 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.15, 0.78] |
| 12 General muscle weakness after extubation | 4 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.31, 1.18] |
| 13 Nausea | 9 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.44, 1.56] |
| 14 Vomiting | 4 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [0.50, 8.48] |
| 15 Postprocedural nausea | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.27, 7.12] |
| 16 Headache | 4 | 388 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.48, 2.18] |
| 17 Hypertension | 3 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.23, 9.05] |
| 18 Hypotension | 4 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.38, 3.96] |
| 19 Cough | 3 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.42, 4.81] |
| 20 Dry mouth | 3 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.10, 1.87] |
| 21 Dizziness | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.10, 9.23] |
| 22 Tachycardia | 3 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.09, 2.22] |
| 23 Pruritus | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.20, 12.88] |
| 24 Pyrexia | 3 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.23, 8.91] |
| 25 Shivering | 3 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.40, 1.43] |
| 26 Chills | 2 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 4.04 [0.46, 35.85] |
| 27 Rash | 5 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.17, 3.96] |
| 28 Supraventricular extrasystoles | 2 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.03, 3.05] |
| 29 Laryngospasm | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.07, 1.65] |
| 30 Increased upper airway secretion | 2 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.09, 1.59] |
| 31 Procedural hypertension | 3 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.33, 8.21] |
| 32 Procedural hypotension | 2 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.02, 14.15] |
| 33 Abdominal pain | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.10, 9.27] |
| 34 Clinical signs of residual NMB | 7 | 646 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Clinical signs of inadequate reversal of NMB | 4 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.02] |
| 36 Clinical signs of recurrence of residual NMB | 13 | 1289 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.05, 10.74] |
| 37 General muscle weakness at PACU discharge | 5 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 1.90] |
| 38 Not able to perform 5 second head‐lift at PACU discharge | 5 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Overall signs of postoperative residual paralysis | 15 | 1474 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.28, 0.57] |
| 40 Risk of composite serious adverse events | 10 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.13, 2.25] |
| 41 Participants with ≥ 1 serious adverse event | 10 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.13, 2.25] |
3.41. Analysis.
Comparison 3 Sugammadex (any dose) vs neostigmine (any dose), Outcome 41 Participants with ≥ 1 serious adverse event.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adamus 2011.
| Methods |
Study design: randomized, controlled trial Sample size calculation: powered to detect a significant difference of 6 minutes or longer in recovery time from injection of sugammadex or neostigmine to TOFR > 0.9 |
|
| Participants |
Number of randomized participants: 22 Inclusion criteria: patients scheduled for XLIF (extreme lateral interbody fusion) under general anaesthesia requiring tracheal intubation Exclusion criteria: ASA > II; expected difficult tracheal intubation and contraindication to drugs used in the study; using medication known to interfere with NMBAs; having severe renal, hepatic, metabolic, or neuromuscular disease |
|
| Interventions |
Anaesthesia: induction with midazolam (1 to 2 mg), sufentanil (0.2 to 0.3 µg/kg), and propofol (2 mg/kg); anaesthesia maintained with SEVO to MAC 1. Boluses of sufentanil 5 to 10 µg administered when necessary NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 2.5 mg Comparison: sugammadex 2 mg/kg (n = 11) vs neostigmine 0.04 mg/kg + atropine 0.02 mg/kg (n = 11) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes |
Main objective: To determine the extent to which NMB must be reversed for reliable identification of lumbar nerve roots Secondary objective: time course of reversal after sugammadex or neostigmine |
|
| Notes |
Publication type: peer‐reviewed article Country: Czech Republic Conversions: Median + Range to Mean + SD following guidelines from Hozo 2005 Handling of adverse events: no discrepancy between AEs presented in the original article and AEs presented in this review Authors' conclusions: Intraoperative reversal of shallow rocuronium‐induced block with sugammadex or neostigmine is an efficient method. For reliable detection of lumbar nerve roots with a stimulating current of 10 mA, the block should be reversed to a TOFR ≥ 0.70. For current intensity of 5 mA, TOFR should reach 0.90 Contact: first trial author Milan Adamus contacted by email: milan.adamus@seznam.cz; replied * Indicates unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers with block‐wise randomization |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque, sealed envelopes* |
| Blinding of participants (performance bias) | Low risk | Participants were under general anaesthesia and therefore were blinded* |
| Blinding of personnel (performance bias) | High risk | Surgeon was blinded to the reversal drug used for a particular participant at the beginning of the study; however, because differences in the onset of effect between sugammadex and neostigmine were substantial, he/she gradually learned to guess which was injected. Anaesthesiologist was not blinded |
| Blinding of primary outcome assessment (detection bias) | High risk | Anaesthesiologist was the TOF‐watch assessor and was not blinded |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22 patients were enrolled in the study, and reliable NMT monitoring was set up for all of them. However, for 1 patient in the neostigmine group, the appropriate lumbar nerve roots were not identified despite full recovery from NMB (TOFR = 0.99). This patient was excluded from the study. Resulting groups consisted of 11 and 10 participants in the sugammadex and neostigmine groups, respectively |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published article clearly includes all expected outcomes |
| Funding bias | High risk | Conflict of interest: Milan Adamus is a member of the advisory board of MSD (Schering‐Plough, s.r.o., a subsidiary of Merck & Co., Inc.) and has received lecture honoraria from MSD. This study received no financial support from MSD |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's primary outcome. No significant differences between groups regarding age, gender, weight, height, BMI, and ASA scores |
Balaka 2011.
| Methods |
Study design: randomized, prospective study Sample‐size calculation: no information available |
|
| Participants |
Number of randomized participants: 40 Inclusion criteria: aged 18 to 63, with myasthenia gravis (MG) ‐ Osserman's classification I to III and Leventhal score < 10 points, ASA physical status I to III, undergoing transsternal thymectomy Exclusion criteria: no information available |
|
| Interventions |
Anaesthesia: no information available NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.15 mg/kg Comparison: sugammadex 4.0 mg/kg (n = 20) vs neostigmine 2.5 mg (n = 20) Administration time of sugammadex or neostigmine: TOF∼ 50% |
|
| Outcomes | Recovery time from TOF ∼ 50% to TOF > 90%, signs of residual NMB | |
| Notes |
Publication type: meeting abstract Country: Greece Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and in this review Authors' conclusions: Sugammadex seems to be superior to neostigmine as a reversal agent of rocuronium‐induced intense NMB, leading to a more rapid reappearance of normal muscle activity in these patients with their highly increased sensitivity to non‐depolarizing neuromuscular blocking drugs Contact: first trial author Christina Balaka contacted by email: christinabalaka@yahoo.com on 30.09.2015; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Divided randomly"; no further information available |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess owing to insufficient information |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published meeting abstract clearly includes all expected outcomes |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | Unable to assess owing to insufficient information |
Blobner 2010.
| Methods |
Study design: phase 3A, European, 13‐centre, randomized, parallel‐group, comparative, active‐controlled, safety assessor‐blinded trial (the AURORA trial) Sample size calculation: powered to detect a difference ≥ 5 minutes from start of administration of sugammadex/neostigmine to TOFR > 0.9 between treatment groups |
|
| Participants |
Number of randomized participants: 98 Inclusion criteria: ASA I to III, age ≥ 18 and of any body weight, scheduled for an elective surgical procedure under general anaesthesia Exclusion criteria: expected difficult intubation; receiving medication known to interact with rocuronium or vecuronium; having neuromuscular or significant renal disease, a history of malignant hyperthermia, an allergy, or other contraindication to medications used during the study; pregnant, potentially pregnant, or breastfeeding |
|
| Interventions |
Anaesthesia: induced with propofol and maintained with sevoflurane NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.1 to 0.2 mg/kg Comparison: sugammadex 2.0 mg/kg (n = 49) vs neostigmine 50 µg/kg plus glycopyrrolate 10 µg/kg (n = 49) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes |
Primary endpoint: time from start of administration of sugammadex/neostigmine to TOFR > 0.9 Secondary endpoint: time from start of administration of sugammadex/neostigmine to TOFR > 0.8 and 0.7 Other efficacy analysis: assessment of clinical signs of recovery (level of consciousness, 5 second head‐lift, general muscle weakness) Safety analysis: adverse events, serious adverse events, heart rate, blood pressure |
|
| Notes |
Publication type: peer‐reviewed article Country: European study, 13 centres Conversions: Median + Range to Mean + SD following guidelines from Hozo 2005 Handling of adverse events: Data presented in Table 2 ‐ "Summary of the clinical signs of recovery and level of consciousness" ‐ regarding number of participants with muscle weakness and number of participants not able to perform 5 second head‐lift were considered to be adverse events in this review and were counted as such. Furthermore, study authors provided more detailed information regarding adverse events through email correspondence Authors' conclusions: Recovery of neuromuscular function after rocuronium to a TOFR = 0.9 is on average about 13 times faster with 2 mg/kg sugammadex than with 50 µg/kg neostigmine. Even more important, 98% of participants were sufficiently recovered within 5 minutes after sugammadex but 100 minutes after neostigmine before 98% of participants were sufficiently recovered. The safety profile did not differ between sugammadex‐treated and neostigmine‐treated patients Contact: First trial author Manfred Blobner contacted by email: blobner@lrz.tum.de; replied to questions regarding blinding of outcome assessor and referred to Tiffany Woo about questions regarding adverse events; replied 29.03.16 * Indicates unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization codes were entered into a central randomization system ‐ part of a secured trial website. Enrolled participants were given a number in sequence of their enrolment and received a treatment code using the randomization system |
| Allocation concealment (selection bias) | Low risk | Central allocation (secondary to central randomization) |
| Blinding of participants (performance bias) | High risk | Open‐label study |
| Blinding of personnel (performance bias) | High risk | Open‐label study |
| Blinding of primary outcome assessment (detection bias) | Low risk | TOF‐watch assessor was blinded to treatment assignment* |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessor was blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: 98 participants were enrolled ‐ 49 in the sugammadex group and 49 in the neostigmine group. One participant in each group did not receive study drug, and the all‐patients‐treated population included 48 participants in each group. All of these had ≥ 1 postbaseline efficacy measurement and, therefore, made up the intention‐to‐treat population |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT00451217); all of the study's prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | This work was supported by MSD, Oss, The Netherlands. M.B. and J.S. have received honoraria and travel grants from MSD within the past 3 years. L.I.E. is a scientific adviser to MSD and Abbott Scandinavia AB; his institution has received an institutional grant from MSD. M.E.P. is an employee of MSD. J.M. and G.D.R. have no conflicts of interest |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's primary outcome. Treatment groups were mostly comparable in terms of their baseline characteristics and distribution of surgery types |
Brueckmann 2015.
| Methods |
Study design: randomized, controlled study Sample size calculation: powered to detect a significant difference in the incidence of TOFR < 0.9 between groups |
|
| Participants |
Number of randomized participants: 154 Inclusion criteria: > 18 years of age, ASA I to III, scheduled to undergo an elective abdominal surgical procedure under general anaesthesia, and expected to undergo neuromuscular relaxation with rocuronium for endotracheal intubation Exclusion criteria: suspected difficult intubation; neuromuscular disorder(s); known or suspected severe renal insufficiency (defined as estimated creatinine clearance < 30 mL/min) or significant hepatic dysfunction; history or family history of malignant hyperthermia; allergies to sugammadex, opioids, NMBs, or other medication(s) used during general anaesthesia; toremifene application 24 hours before or within 24 hours after study drug administration; planned ICU admission after surgery or overnight (> 12 hours); stay in the PACU; cardiac pacemaker; pregnancy and breastfeeding; use of any other investigational drugs within 30 days of randomization; or participation in another clinical trial within 30 days |
|
| Interventions |
Anaesthesia: induced and maintained according to clinical need of the participant, and per usual centre practice, with IV induction agents, IV opioids, inhaled anaesthetics, and other agent(s), most commonly a combination of fentanyl, propofol, and sevoflurane NMBA: single intubating dose: rocuronium ˜ 0.6 mg/kg; maintenance dose: rocuronium ˜ 0.15 mg/kg Comparison: sugammadex 2 mg/kg or 4 mg/kg (n = 76) vs neostigmine + glycopyrrolate (n = 78) (dosing per usual clinical practice; maximum dose 5 mg) Administration time of sugammadex or neostigmine: moderate neuromuscular blockade: TOF 1 to 3 or deep neuromuscular blockade: PTC ≥ 1 |
|
| Outcomes |
Primary endpoint: presence of residual neuromuscular blockade at PACU admission, defined as TOFR < 0.9 on arrival to PACU Key secondary endpoint: time from start of study medication administration to time patient was ready for discharge from the operating room, defined as time point deemed by the providing anaesthesiologist as medically appropriate for the patient to leave the operating room Exploratory endpoints: Those related to surgical efficiency parameters were also measured Safety assessments: physical examination at screening and at postanaesthetic visit, vital signs at screening, continuous ECG, oxygen saturation throughout anaesthesia and postoperatively, vital signs at PACU, signs of partial neuromuscular blockade, adverse events and serious adverse events |
|
| Notes |
Publication type: peer‐reviewed article Country: USA, Massachusetts General Hospital Conversions: PACU time ‐ range to SD following guidelines from Hozo 2005 Handling of adverse events: More detailed information regarding adverse events possibly, probably, or definitely related to study drug was provided by study authors through email correspondence Authors’ conclusions: After abdominal surgery, sugammadex reversal eliminated residual neuromuscular blockade in the PACU and shortened time from start of study medication administration to time patient was ready for discharge from the operating room Contact: corresponding trial author M. EIkermann contacted by email: meikermann@partners.org; has replied * Indicates unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomization* |
| Allocation concealment (selection bias) | Unclear risk | Sample of 200 sealed envelopes were prepared by the sponsor: 100 for the sugammadex group and 100 for the neostigmine/glycopyrrolate group; however, no information on whether envelopes were sequentially numbered and opaque |
| Blinding of participants (performance bias) | Low risk | Participants were blinded* |
| Blinding of personnel (performance bias) | High risk | Anaesthesiologist was unblinded to study drug, as he/she needed to be able to adjust anaesthesia and neuromuscular blockade according to treatment group, and to assess effects of sugammadex on patient flow through the operating room |
| Blinding of primary outcome assessment (detection bias) | Low risk | TOF‐watch assessors were blinded to treatment group, did not observe preparation of trial medications and were not involved in randomization or preparation of study drug, or were not allowed in the operating room during surgery |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessors were blinded to treatment group, did not observe preparation of trial medications and were not involved in randomization or preparation of study drug, or were not allowed in the operating room during surgery |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: 154 participants were randomized (2 were excluded owing to adverse events, 1 withdrew consent), 151 participants received study drug (1 participant was excluded owing to unplanned admission to intensive care unit), resulting in 150 participants who had available primary endpoint (sugammadex group n = 74, neostigmine group n = 76) |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT01479764), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | Declaration of interest: M.K.L. and T.W. are employees of Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA. P.G. is an employee of MSD Oss, The Netherlands. All may own stock and/or hold stock options in the Company. J.de B. was formerly an employee of Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA. M. E., B.B., M.M., J.L., J.K., A.S.S., F.McG., N.S., and R.P. work for institutions that received research funding for conduct of the study from Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's secondary outcome. No statistically significant differences regarding baseline characteristics between participant groups |
Carron 2013.
| Methods |
Study design: randomized clinical trial Sample size calculation: based on study's primary and secondary endpoints, powered to detect significant intergroup differences |
|
| Participants |
Number of enrolled participants: 40 female morbidly obese patients Inclusion criteria: morbidly obese with BMI ≥ 40 kg/m2, age ≥18 years, scheduled for laparoscopic removal of adjustable gastric banding under general anaesthesia using rocuronium for tracheal intubation and maintenance of NMB, presence of 1 to 5 post‐tetanic counts (PTCs) at completion of surgery Exclusion criteria: ASA > III, difficult tracheal intubation, known or suspected disorder affecting NMB, renal and/or hepatic dysfunction, malignant hyperthermia, pregnancy, breastfeeding, and allergy or contraindication to narcotics, NMBAs, sugammadex, neostigmine, or other medications used during anaesthesia |
|
| Interventions |
Anaesthesia: induced with fentanyl 3.5 μg/kg lean body weight (LBW) and propofol 3 mg/kg LBW, maintained with desflurane and remifentanil 0.05 to 0.1 μg/kg/min titrated to a target state entropy value of 35 ± 5 NMBA: single intubating dose: rocuronium 0.9 mg/kg ideal body weight (IBW); maintenance dose: rocuronium 0.15 mg/kg Comparison: sugammadex 4 mg/kg total body weight (n = 20) vs neostigmine 70 μg/kg LBW + atropine 10 μg/kg (n = 20) Administration time of sugammadex or neostigmine: presence of PTC 1 to 5 |
|
| Outcomes |
Primary endpoint: difference in anaesthesia time between groups: (1) anaesthesia: time from preoxygenation of participant to tracheal extubation, (2) induction: time from end of preoxygenation to tracheal intubation, (3) maintenance: time from tracheal intubation to beginning of reversal of NMB, (4) reversal: time from reversal of drug administration to TOFR ≥ 0.9, and (5) extubation: time from cessation of desflurane Secondary endpoints: differences in oxygen saturation levels and TOFR upon PACU admission and ability to swallow after extubation Other considerations: postoperative complications, analgesic and antiemetic requirements, ability to get into bed independently, time to discharge from PACU |
|
| Notes |
Publication type: peer‐reviewed article Country: Italy Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors' conclusions: Sugammadex allowed safer and faster recovery from profound rocuronium‐induced NMB when compared with neostigmine in participants with MO. Sugammadex may play an important role in fast‐track bariatric anaesthesia Contact: first trial author Michele Carron contacted by email: micarron@libero.it on 30.09.2015; no reply received. Last author Carlo Ori contacted by email: carloori@unipd.it on 25.10.2015; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | "Opening a sealed opaque envelope immediately before surgery by one investigator" |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | No specific information on identity or blinding of TOF‐watch assessor |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessor was not involved in the randomization process and was not present during anaesthesia |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 40 patients recruited into the study ‐ 20 allocated to neostigmine group and 20 to sugammadex group |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published article clearly includes all expected outcomes |
| Funding bias | High risk | Conflict of Interest: Michele Carron has received a payment for lecture from MSD; Mirto Foletto has received a payment for consultancy from Johnson & Johnson Medical; Carlo Ori has received payments and travel funding for lectures and as a member of MSD Advisory Board |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's primary outcome. No statistically significant differences in participants' demographic characteristics |
Castro 2014.
| Methods |
Study design: prospective, controlled, randomized study Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 88 Inclusion criteria: morbidly obese (MO) and scheduled for laparoscopic bariatric surgery under general anaesthesia Exclusion criteria: lack of consent, followed in chronic pain consultation, already enrolled in another study conducted at our institution (pregabalin effect as preemptive analgesia for surgery in the obese), and previous LBS in the same patient |
|
| Interventions |
Anaesthesia: propofol 1.5 to 2.0 mg/kg CBW, analgesia maintained with remifentanil 0.15 to 0.30 mg/kg CBW, anaesthesia maintained with mixture of oxygen, air, and desflurane in vol % NMBA: no information available Comparison: sugammadex 2 mg/kg (n = 44) vs neostigmine 0.05 µg/kg + atropine 20 µg/kg (n = 44) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes | Pain using the visual analogue scale at 4 different moments: arrival to PACU, 30 minutes after arrival, 60 minutes after arrival, and immediately before leaving PACU; presence of postoperative nausea and vomits (PONV); and duration of PACU stay before discharge to the ward | |
| Notes |
Publication type: peer‐reviewed article Country: Portugal Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: Sugammadex is associated with less pain in the PACU. This “opioid‐sparing” effect, combined with less PONV and faster discharge from the PACU, makes sugammadex an indispensable drug for this type of patient and allows fast‐track surgery in the MO Contact: first trial author Diogo S. Castro contacted by email: diogosousacastro@hotmail.com on 15.05.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization was performed by the investigator using previously prepared envelopes |
| Allocation concealment (selection bias) | Unclear risk | Randomization was performed by the investigator using previously prepared envelopes |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 88 eligible participants were randomized into 2 groups of 44; no patients were excluded |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published article clearly includes all expected outcomes |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | No apparent other type of bias, except no information on sample size calculation. No differences in participant characteristics between groups |
Cheong 2015.
| Methods |
Study design: randomized, controlled study Sample size calculation: calculated under the presumption that the difference in time to 90% recovery of TOFR between groups was not longer than 30 seconds |
|
| Participants |
Number of randomized participants: 120 Inclusion criteria: age between 18 and 65 years, ASA I to II, scheduled for elective surgery Exclusion criteria: expected to have difficult intubation owing to anatomical abnormality or limited neck mobility at preoperative evaluation; neuromuscular abnormality; cardiovascular disease; kidney function disorder; liver function disorder; pregnancy; and history of side effects with aesthetics and analgesics. Experiment withdrawal criteria were unexpected massive haemorrhage; unrecovered electrocardiograph (ECG) abnormality; profound hypotension; respiratory abnormality; and TOF device error during experiment |
|
| Interventions |
Anaesthesia: induced with propofol 1.5 to 2.5 mg/kg and maintained with sevoflurane 1.5 to 2.5 vol % and 50% N2O. NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 5 to 10 mg Comparison: sugammadex 2 mg/kg (S2) (n = 30), sugammadex 1 mg/kg (S1) (n = 30), sugammadex 1 mg/kg + neostigmine 0.05 mg/kg + glycopyrrolate 0.01 mg/kg (SN) (n = 30), and neostigmine 0.05 mg/kg + glycopyrrolate 0.01 mg/kg (N) (n = 30) Administration time of sugammadex, sugammadex + neostigmine, or neostigmine: reappearance of T1 to 2 |
|
| Outcomes | Time to 90% recovery of TOFR, adverse events: PONV score, signs of residual blockade, BP, oxygen saturation | |
| Notes |
Publication type: peer‐reviewed article Country: Korea Conversions: sugammadex time Mean + SD from seconds to minutes Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: For reversal from rocuronium‐induced moderate neuromuscular blockade, combined use of sugammadex and neostigmine may be helpful to decrease recovery time and can reduce the required dosage of sugammadex. However, the increased incidence of systemic muscarinic side effects must be considered Contact: first trial author Wonjin Lee contacted by email: 2wonjin@hanmail.net on 15.05.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects randomly assigned"; no further information available |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Low risk | To minimize observer bias, drugs were prepared in syringes labelled "reverse" by a third party |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs: 120 participants were enrolled and randomized, resulting in 4 groups of 30 participants |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published article clearly includes all expected outcomes |
| Funding bias | Low risk | This work was supported by the 2011 Inje University research grant |
| Other bias | High risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's primary outcome. Baseline characteristics showed significant differences (P = 0.035) between body weight in 2 groups, which influences the dosage of administered drug and therefore can influence time to recovery of TOFR, MBP, HR, and PONV score |
Flockton 2008.
| Methods |
Study design: multi‐centre, randomized, safety assessor‐blinded, parallel‐group, phase 3a study (CRYSTAL trial) Sample size calculation: powered to detect a difference ≥ 3 minutes in mean time to recovery of TOFR = 0.9 between sugammadex and neostigmine groups |
|
| Participants |
Number of randomized participants: 84 Inclusion criteria: aged ≥ 18 years, ASA class I to III, undergoing surgery in the supine position under general anaesthesia requiring muscle relaxation Exclusion criteria: expected to have a difficult intubation for anatomical reasons; neuromuscular disorder or significant renal dysfunction; history or family history of malignant hyperthermia; or known allergy to narcotics, NMBAs, or other medication used during general anaesthesia; receiving antibiotics, anticonvulsants, or magnesium at a time likely to interfere with neuromuscular block; already participated in a previous sugammadex study or any other study within 30 days of entering this study; pregnant, breastfeeding, or of childbearing potential, and not using an adequate method of contraception |
|
| Interventions |
Anaesthesia: induced with IV propofol and remifentanil, fentanyl, or sufentanil; maintained by a continuous infusion of propofol and further increments or infusions of analgesic as needed NMBA: single intubating dose: rocuronium 0.6 mg/kg or cisatracurium 0.1 to 0.2 mg/kg; maintenance dose: rocuronium 0.15 to 3 mg/kg or cisatracurium 0.3 mg/kg, up to a maximum of 2 doses Comparison: sugammadex 2.0 mg/kg following rocuronium (n = 40) vs neostigmine 50 µg/kg + glycopyrrolate 10 µg/kg following cisatracurium (n = 44) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes |
Primary efficacy variable: time from start of administration of study drug to recovery of TOFR > 0.9 Secondary efficacy variables: time from start of administration of study drug to recovery of TOFR > 0.7 or 0.8 and clinical signs of recovery after extubation, but before transfer to the recovery room and before discharge from the recovery room; time from administration of the intubating dose of rocuronium or cisatracurium to occurrence of maximum block (onset time) Safety assessments: adverse events, serious adverse events, monitoring of incidents related to use of the TOF‐watch, laboratory variables, physical examination, vital signs |
|
| Notes |
Publication type: peer‐reviewed article Country: 8 centres in Europe Conversions: Median + Range to Mean + SD following guidelines from Hozo 2005 Recovery time to TOFR > 0.9, Mean + SD, from seconds to minutes Handling of adverse events: Data presented in Table 3 ‐ "Assessement of clinical signs of recovery" ‐ regarding number of participants with general muscle weakness and number of participants not able to perform 5 second head‐lift were considered to be adverse events in this review and were counted as such Authors’ conclusions: Sugammadex 2 mg/kg administered at reappearance of T2 was significantly faster in reversing rocuronium‐induced blockade than neostigmine was in reversing cisatracurium‐induced block Contact: corresponding trial author Elizabeth Flockton contacted by email: Elizabeth.Flockton@rlbuht.nhs.uk on 10.10.2015; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization system |
| Allocation concealment (selection bias) | Low risk | Central allocation (secondary to central randomization system) |
| Blinding of participants (performance bias) | Unclear risk | Open‐label; no further information available |
| Blinding of personnel (performance bias) | Unclear risk | Open‐label; no further information available |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Open‐label; no further information available |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessor was blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: 84 participants were randomized to treatment (rocuronium – sugammadex n = 4 0, cisatracurium – neostigmine n = 44), 6 participants did not receive sugammadex (inability to record a stable baseline TOFR in 4 participants, withdrawal of consent in 1, and study medication unavailable in 1), 5 participants did not receive neostigmine (inability to record a stable baseline TOFR in 4 participants, and postponement of surgery in 1), leading to their exclusion from the AST group (n = 73). All treated participants had ≥ 1 efficacy assessment carried out and therefore constituted the ITT population (sugammadex n = 34, neostigmine n = 39) |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT00451100), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | Declaration of interest: M.E.P. is an employee of N.V. Organon, a part of Schering‐Plough Corporation, Oss, The Netherlands. R.K.M. is a member of the Scientific Advisory Board of N.V. Organon, a part of Schering‐Plough Corporation |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's primary outcome. No clinically relevant differences in baseline characteristics, although the sugammadex group included a higher proportion of women, higher mean age, and a higher percentage of ASA II to III patients compared with the neostigmine group |
Foletto 2014.
| Methods |
Study design: randomized, controlled trial Sample‐size calculation: No information available |
|
| Participants |
Number of randomized patients: 34 morbidly obese (MO) patients Inclusion criteria: morbidly obese and undergoing laparoscopic‐sleeve gastrectomy Exclusion criteria: no information available |
|
| Interventions |
Anaesthesia: propofol and remifentanil anaesthesia; no further information available NMBA: rocuronium; no further information available Comparison: sugammadex 2 mg/kg (n = 17) vs neostigmine 50 µg/kg (n = 17) Administration time of sugammadex or neostigmine: reappearance of T1 to 2 |
|
| Outcomes | Recovery time to TOFR > 0.9, spirometry 15 minutes postoperative (postoperative forced vital capacity, forced expiratory volume in 1 second, peak expiratory flow) | |
| Notes |
Publication type: meeting abstract Country: Italy Conversions: none Authors’ conclusions: Respiratory function was restored more quickly in morbidly obese participants who received sugammadex to reverse rocuronium‐induced NMB Contact: first trial author Mirto Foletto contacted by email: mirto.foletto@unipd.it on 07.10.2015; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random"; no further information |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess owing to insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess owing to insufficient information |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | No differences in participant characteristics, anaesthetic drugs, and baseline spirometry were observed between groups. No information on sample size calculation was provided |
Gaszynski 2011.
| Methods |
Study design: prospective, randomized study Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 70 Inclusion criteria: morbidly obese (BMI > 40 kg/m2) and undergoing elective bariatric surgery Exclusion criteria: lack of consent, coexisting muscular disease, severe cardiovascular disease (NYHA > II) |
|
| Interventions |
Anaesthesia: Induction with propofol 1.5 to 2 mg/kg CBW (corrected body weight), fentanyl 0.05 mg/kg CBW for intraoperative analgesia. Maintainance with desflurane NMBA: single intubating dose: rocuronium 1 mg/kg CBW; maintenance dose: rocuronium 0.06 mg/kg CBW, maximum 2 additional doses Comparison: sugammadex 2 mg/kg CBW (n = 35) vs neostigmine 50 µg/kg CBW + atropine 20 µg/kg CBW (n = 35) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes | Neuromuscular function was recorded and time to achieve 90% of TOF (safe extubation) was measured. PORC (postoperative residual curarization) was measured using TOF stimulation. Neuromuscular monitoring in the PACU | |
| Notes |
Publication type: peer‐reviewed article Country: Poland Conversions: recovery time to TOFR > 0.9, Mean + SD, from seconds to minutes Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: Administration of sugammadex provides fast recovery of neuromuscular function and prevents postoperative residual curarization (PORC) in the morbidly obese; however, neostigmine does not Contact: first trial author T. Gaszynski contacted by email: tomgaszyn@poczta.onet.pl on 07.10.2015; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Previously prepared envelopes"; no further information |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | No specific information on identity or blinding of TOF‐watch assessor. Study investigator measuring PORC using TOF stimulation was blinded |
| Blinding of safety assessment (detection bias) | Unclear risk | No specific information on identity or blinding of safety assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70 participants were enrolled and randomized ‐ 35 in each group. All participants are accounted for, and no outcome data are missing |
| Selective reporting (reporting bias) | Unclear risk | Study was registered with SYNABA ‐ The Polish Clinical Trials Authorization, ref nr. 252922. Study's primary and secondary outcomes/efficacy endpoints are not clearly stated in the published paper |
| Funding bias | Unclear risk | T.G. is a member of the national advisory committee on introduction of sugammadex into clinical practice. T.G. has received an honorarium from MSD Company for lectures during scientific meetings on use of neuromuscular blocking agents in general anaesthesia. Study was sponsored by government grant no. N N403 3755 33 |
| Other bias | Unclear risk | No apparent other type of bias, except no information on sample size calculation. No difference in participant characteristic data and total dose of rocuronium between groups |
Geldner 2012.
| Methods |
Study design: randomized, active‐controlled, parallel‐group, multi‐centre, safety assessor‐blinded trial Sample size calculation: powered to detect a difference with respect to length of stay in theatre and post‐anaesthesia recovery unit between the 2 treatments of half a standard deviation |
|
| Participants |
Number of randomized participants: 140 Inclusion criteria: age ≥ 18 years; ASA physical status I to III; scheduled laparoscopic cholecystectomy or appendectomy under general anaesthesia; and written, informed consent Exclusion criteria: suspected difficult tracheal intubation; disorder affecting neuromuscular blockade; known or suspected significant renal dysfunction; known or suspected severe hepatic dysfunction; history of malignant hyperthermia; allergy to opioids, neuromuscular blocking drugs, or other medications used during general anaesthesia; contraindication to neostigmine and ⁄or atropine; pregnancy (excluded both by medical history and by a human chorionic gonadotropin test within 24 hours of surgery in women of childbearing age) and breastfeeding; already participated in another sugammadex study or participated in another clinical study not preapproved by the sponsor within 30 days |
|
| Interventions |
Anaesthesia: induced and maintained using intravenous propofol and opioids (most frequently fentanyl) as required; choice and dose of which were decided by the responsible anaesthetist NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.1 to 0.2 mg/kg Comparison: sugammadex 4 mg/kg (n = 70) vs neostigmine 50 µg/kg + atropine 10 µg/kg (n = 70) Administration time of sugammadex or neostigmine: PTC 1 to 2 for sugammadex and reappearance of T2 for neostigmine + atropine |
|
| Outcomes |
Primary efficacy parameter: time from start of sugammadex or neostigmine administration to TOFR > 0.9 Secondary outcome parameters: safety and length of stay in the operating room and the PACU following administration of study drug Safety assessments: adverse events, vital signs, physical examination findings |
|
| Notes |
Publication type: peer‐reviewed article Country: European study ‐ 3 centres in Russia, 4 in Germany, 2 in Finland, and 1 in UK Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: In participants undergoing laparoscopic surgery under propofol anaesthesia, neuromuscular blockade reversal with sugammadex administered at a PTC of 1 to 2 (deep neuromuscular blockade) after rocuronium was well tolerated and resulted in faster recovery of the TOFR to 0.9 compared with neostigmine administered at reappearance of T2 (moderate neuromuscular blockade) (P < 0.0001). Sugammadex therefore may allow rapid reversal of deep neuromuscular blockade at completion of surgery without a delay in recovery Contact: first trial author G. Geldner contacted by email: goetz.geldner@kliniken‐lb.de; has replied |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online randomization list created by Orcapharma (Heesch, The Netherlands) using the software package SAS̀ (SAS Institute, Cary, NC, USA) in compliance with international protocols |
| Allocation concealment (selection bias) | Low risk | Central allocation (secondary to web randomization) |
| Blinding of participants (performance bias) | Low risk | Participants were blinded |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | No specific information on identity or blinding of the TOF‐watch assessor |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessor was blinded to treatment assignment, was not involved in the randomization process, was not present during anaesthesia, and was not involved in preparation of the trial medication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: 140 participants were assigned to sugammadex (70) or neostigmine (70); 4 participants in the sugammadex group and three in the neostigmine group did not receive study drug. Two participants in the neostigmine group were not included in the efficacy analysis because of failure of the neuromuscular monitoring device. Data were imputed by a conservative approach towards sugammadex for 3 participants in the sugammadex group and 5 in the neostigmine group, because time to recovery of the TOFR to 0.9 was not available. Out of these, for 2 in the sugammadex group and 2 in the neostigmine group, the TOFR did not reach 0.9; for the remaining 2 participants, times were considered unreliable owing to an unstable trace (neostigmine group) or unsuccessful calibration (sugammadex group) |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT00724932), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | Study sponsor, MSD, was involved in both study design and analysis of the data. The overall design and conduct of the study, as well as final analysis of study data and opinions, conclusions, and interpretation of the data, are the responsibility of the study authors. Medical writing assistance was provided by Neil Venn, PhD, of Prime Medica Ltd (Knutsford, UK); this assistance was funded by Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ. The study sponsor was allowed to review the manuscript before submission, but final decisions on content remained the responsibility of trial authors, and all trial authors approved the final text of the manuscript before submission Götz Geldner has acted as a scientific advisor to MSD (formerly Organon) and GlaxoSmithKline and has delivered lectures for and received research funding from both companies. Henk Rietbergen is an employee of MSD. The other study authors declare no competing interests |
| Other bias | High risk | |
Georgiou 2013.
| Methods |
Study design: randomized controlled trial Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 57 Inclusion criteria: super‐obese (SO) (BMI > 50 kg/m2) scheduled for open bariatric surgery Exclusion criteria: cardiovascular disease (NYHA > 2); refusal to participate in the study; contraindication to epidural catheter placement (e.g. anticoagulation, anti‐platelet medication); coexisting neuromuscular disease; history of allergic reaction to neuromuscular blocking agents; history of difficult intubation; creatinine levels > 159 mmol/L |
|
| Interventions |
Anaesthesia: propofol and remifentanil NMBA: single intubating dose: rocuronium, dose not available; maintenance dose: not specified Comparison: sugammadex 2 mg/kg ideal body weight (n = 15) vs sugammadex 2 mg/kg corrected body weight (n = 13) vs neostigmine 50 µg/kg ideal body weight (n = 14) vs neostigmine 50 µg/kg corrected body weight (n = 15) Administration time of sugammadex or neostigmine or placebo: reappearance of T2 |
|
| Outcomes |
Primary endpoint: full decurarization Secondary endpoint: ability to get into bed independently on arrival to the PACU and clinical signs of residual paralysis |
|
| Notes |
Publication type: meeting abstract Country: Greece Conversions: recovery time to TOFR > 0.9, Mean + SD, from seconds to minutes Authors’ conclusions: Although transfer times to wards in neostigmine groups were ˜ 53 minutes longer than those in sugammadex groups, the cost of Sugammadex was > 400 times higher than the cost of neostigmine. Under current economic crisis conditions, one should take this seriously into consideration Contact: first trial author P. Georgiou contacted by email: prgeorg@yahoo.gr: 09.10.2015; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned"; no further information |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Low risk | Participants blinded |
| Blinding of personnel (performance bias) | Unclear risk | Investigator blinded; no further information available |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Investigator blinded; no further information available |
| Blinding of safety assessment (detection bias) | Unclear risk | Investigator blinded; no further information available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT01629394), and all of the study's prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | Low risk | University of Patras |
| Other bias | Unclear risk | Unable to assess owing to insufficient information |
Grintescu 2009.
| Methods |
Study design: open randomized trial Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 34 Inclusion criteria: undergoing laparoscopic cholecystectomy Exclusion criteria: no information available |
|
| Interventions |
Anaesthesia: propofol, remifentanil, and sevoflurane NMBA: rocuronium; no further information available Comparison: sugammadex 2 mg/kg (n = 17) vs neostigmine 50 µg/kg (n = 17) Administration time of sugammadex or neostigmine: moderate residual block |
|
| Outcomes | Total time spent by participant in the operating theatre complex, surgical procedure time, and time between reversal agent administration and extubation (recovery time) | |
| Notes |
Publication type: meeting abstract Country: Romania Conversions: none Authors’ conclusions: Sugammadex reduces total time spent in the operating theatre by providing fast and reliable recovery from neuromuscular block with no risk of postoperative residual curarization. In daily practice, this could improve the use of operating theatre facilities and could lower the total cost of a surgical procedure Contact: first trial author Ioana Grintescu contacted by email: ioana.grintescu@rospen.ro on 11.10.2015; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | High risk | Open randomized trial; no further information available |
| Blinding of personnel (performance bias) | High risk | Open randomized trial; no further information available |
| Blinding of primary outcome assessment (detection bias) | High risk | Open randomized trial, no further information available |
| Blinding of safety assessment (detection bias) | High risk | Open randomized trial; no further information available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess owing to insufficient information |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published meeting abstract clearly includes all expected outcomes |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | Unable to assess owing to insufficient information |
Hakimoglu 2016.
| Methods |
Study design: randomized, controlled trial Sample size calculation: powered to detect a 15% change in IOP (intraocular pressure) between groups |
|
| Participants |
Number of randomized participants: 60 Inclusion criteria: age 18 to 65 years, ASA I to II, undergoing arthroscopic surgery under general anaesthesia Exclusion criteria: chronic diseases other than hypertension; previous ocular disease or ocular surgery, allergy to tetracaine or other agents used in anaesthesia |
|
| Interventions |
Anaesthesia: induced using propofol 2.5 mg/kg and fentanyl 1.0 µg/kg, maintained using desflurane 4% to 6% (3 L/min) in a 50:50% oxygen/air mixture NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: no information available Comparison: sugammadex 4 mg/kg (n = 30) vs neostigmine 0.05 mg/kg + atropine 0.015 mg/kg (n = 30) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes |
Primary outcome: evaluation of intraocular pressure changes with sugammadex and neostigmine + atropine with a Tono‐Pen XL applanation tonometer, measured before induction and at 30 seconds and 2 and 10 minutes after extubation Secondary outcomes: investigation of the effects of sugammadex and neostigmine on haemodynamic parameters (heart rate, mean arterial pressure, peripheral arterial oxygen saturation), measured by electrocardiography, non‐invasive oscillometry method, and pulse oximetry. Also investigation of effects of sugammadex and neostigmine on complications (gagging, nausea, vomiting, breath holding, laryngospasm, and tremors) after extubation |
|
| Notes |
Publication type: peer‐reviewed article Country: Turkey Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: Postextubation IOP values for the sugammadex group were similar to those for the neostigmine‐atropine group. Additionally, in agreement with previous studies, extubation time in our study was found to be shorter in the sugammadex group than in the neostigmine‐atropine group. Additional studies that include more participants are needed Contact: first trial author Sedat Hakimoglu contacted by email: sedathakimoglu@gmail.com on 15.05.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomized by "computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs: 60 patients were enrolled and randomized, resulting in 30 participants in each group |
| Selective reporting (reporting bias) | Low risk | Study protocol was retrospectively registered on ANZCTR ‐ Australian New Zealand Clinical Trials Registry (ACTRN12614000651684), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | Unclear risk | All trial authors declare no conflicts of interest; no information on funding provided |
| Other bias | Low risk | Study sample size calculation designed to address this review's secondary outcome. No significant differences detected between groups when demographic data and anaesthesia time were compared |
Illman 2011.
| Methods |
Study design: double‐blinded, randomized, multi‐centre study Sample size calculation: powered to detect a 7‐minute difference in time from administration of neostigmine or sugammadex to achievement of TOFR of 0.9 |
|
| Participants |
Number of randomized participants: 50 Inclusion criteria: both genders, age 18 to 70, BMI < 32.5, scheduled for elective surgery requiring general anaesthesia Exclusion criteria: clinically significant renal, hepatic, or ventilatory dysfunction; increased intracranial pressure; pregnancy or lactation; muscular dystrophies, myopathy, or cerebral palsy; history of intolerance to any of the study drugs; taking medication known to interfere with neuromuscular transmission; simultaneous participation in other studies |
|
| Interventions |
Anaesthesia: induced by propofol and an opioid, according to routine of the study centre, maintained by a volatile anaesthetic (sevoflurane or desflurane), together with opioids NMBA: single intubating dose: rocuronium 0.6 to 1 mg/kg; maintenance dose: rocuronium 5 to 10 mg Comparison: sugammadex 2 mg/kg (n = 25) vs neostigmine 50 µg/kg + glycopyrrolate 10 µg/kg (n = 25) Administration time of sugammadex or neostigmine: reappearance of T1 to 2 |
|
| Outcomes |
Main objective: time gap between loss of visual fade to return of TOFR = 0.9, i.e. potentially unsafe period of recovery Secondary endpoints: times for return of TOFR to 0.70, 0.80, 0.90 after reversal; TOFR at loss of visual fade; time of tracheal extubation Safety assessment: any adverse events, time, severity, and duration |
|
| Notes |
Publication type: peer‐reviewed article Country: Finland, 2 centres Conversions: none Handling of adverse events: No discrepancy exists between AE presented in the original article and those reported in this review Authors’ conclusions: A significant time gap occurs between visual loss of fade and return of TOFR > 0.9 after reversal of a rocuronium block by neostigmine. Sugammadex in comparison with neostigmine allows safer reversal of a moderate NMB when relying on visual evaluation of the TOF response Contact: first trial author Hanna Illman contacted by email: hanna.illman@tyks.fi on 12.10.2015; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes containing written instructions to prepare neostigmine or sugammadex, no specific information on whether envelopes were opaque and sequentially numbered |
| Blinding of participants (performance bias) | Low risk | Participants were blinded to treatment groups as randomization occurred while participants were under general anaesthesia |
| Blinding of personnel (performance bias) | Low risk | Anaesthesiologist was blinded to reversal drug throughout anaesthesia |
| Blinding of primary outcome assessment (detection bias) | Low risk | Anaesthesiologist, who also was the TOF‐watch assessor, was blinded |
| Blinding of safety assessment (detection bias) | Unclear risk | No specific information on identity or blinding of safety assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: All enrolled participants completed the study, but 2 participants from the neostigmine group and 1 from the sugammadex group were excluded. Reasons for exclusion included technical failure of TOF‐watch in 2 participants (1 each from the neostigmine and sugammadex groups), and 1 participant (from the neostigmine group) awoke from anaesthesia before TOFR = 0.90 was established. Accordingly, 23 participants in the neostigmine group and 24 in the sugammadex group were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrialsregister.eu (Eudra CT 2009‐013537‐22), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | Study was supported by Finnish MSD (Finnish Schering‐Plough, Inc.). Disclosure: Klaus T. Olkkola, Olli A. Meretoja, and Seppo Alahuhta are members of the advisory board of Finnish MSD and have received lecture honoraria from Finnish MSD. Hanna Illman has received lecture honoraria from Finnish MSD and MSD Inc. (Schering‐Plough Inc.) |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's primary outcome. No significant differences between patient groups and in conduct of anaesthesia |
Isik 2016.
| Methods |
Study design: randomized, controlled study Sample size calculation: powered to detect a minimum difference of 10% in values of Cystatin C between the 2 groups |
|
| Participants |
Number of randomized participants: 50 Inclusion criteria: between the ages of 18 and 65 years, ASA I to II, scheduled for elective surgery under general anaesthesia with normal renal function (serum Cr < 1.5 mg/dL) Exclusion criteria: liver failure, kidney failure, neuromuscular disorders, pregnant or breastfeeding, treated with corticosteroids or oral contraceptives, contraindication to study drugs, allergy to study drugs, BMI > 30 kg/m2, receiving medication known to interfere with the action of rocuronium (e.g. amino glycoside antibiotics and anticonvulsants), or did not wish to participate |
|
| Interventions |
Anaesthesia: induced with fentanyl 2 μg/kg and propofol 2 mg/kg, Maintainance: 60% N2O‐O2 and 4% to 6% desflurane NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.15 mg/kg Comparison: sugammadex 4 mg /kg at reappearance of PTC 1 to 2 or T2 (n = 25) vs neostigmine 40 µg/kg + 10 at reappearance of T2 (n = 25) Administration time of sugammadex or neostigmine: reappearance of PTC 1 to 2 or T2 |
|
| Outcomes |
Primary endpoint: acute effects of sugammadex or neostigmine on renal function Serum Cys C, Cr urea, blood urea nitrogen (BUN), sodium (Na), potassium (K), and calcium (Ca) levels and urine a1μg, b2μg, and μA levels were preoperatively and postoperatively determined |
|
| Notes |
Publication type: peer‐reviewed article Country: Turkey Conversions: none Authors’ conclusions: We believe that the use of more specific and sensitive new‐generation markers like cystatin C to evaluate kidney function will result in better understanding and interpretation of our results. Sugammadex has more tolerable effects on kidney function than does neostigmine. However, comparison with preoperative values yields a negative alteration of postoperative values. Neostigmine and sugammadex do not cause renal failure but may affect kidney function Contact: first trial author Isik Yasemin contacted by email: yaseminmd@yahoo.com on 24.05.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was generated by using computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Randomziation was performed by one of the review authors, who used previously prepared, sealed, opaque envelopes |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published article clearly includes all expected outcomes |
| Funding bias | Low risk | Study supported by Yuzuncu Yil University, Department of Scientific Research Projects; study authors have no conflicts of interest |
| Other bias | Low risk | Study sample size calculation designed to address this review's secondary outcome. Baseline characteristics data and total rocuronium doses were comparable in both groups |
Jones 2008.
| Methods |
Study design: phase 3, multi‐centre, randomized, parallel‐group, safety assessor‐blinded study (SIGNAL study) Sample size calculation: powered to detect a difference of 5 minutes or greater from start of administration of Org 25969/neostigmine to recovery T4/T1 ratio to 0.9 between treatment groups |
|
| Participants |
Number of randomized participants: 88 Inclusion criteria: ASA I to IV, > 18 years, scheduled to undergo elective surgery during general anaesthesia in supine position. Exclusion criteria: expected difficult airway; known or suspected neuromuscular disorders that might impair neuromuscular blockade; significant renal dysfunction; a (family) history of malignant hyperthermia; allergy to narcotics, muscle relaxants, or other medications used during anaesthesia; receiving medication at a dose and/or time known to interfere with NMBAs (e.g. antibiotics, anticonvulsants, magnesium salts); use of neostigmine and/or glycopyrrolate was contraindicated; female patients who were pregnant, breastfeeding, or of childbearing age and were not using reliable birth control; already participated in another clinical trial within 30 days of entering this study |
|
| Interventions |
Anaesthesia; induced with IV opioid and propofol, maintained with intravenous opioid and sevoflurane NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.15 mg/kg Comparison: sugammadex 4.0 mg/kg (n = 48) vs neostigmine 70 µg/kg + 14 µg/kg glycopyrrolate (n = 40) Administration time of sugammadex or neostigmine: reappearance of PTC 1 to 2 |
|
| Outcomes |
Primary efficacy parameter: time from start of administration of Org 25969/neostigmine to recovery T4/T1 ratio to 0.9 Secondary efficacy variables: time from start of administration of Org 25969/neostigmine to recovery T4/T1 ratio to 0.7 and 0.8; assessment of clinical signs of recovery (level of consciousness, 5 second head‐lift, general muscle weakness) Safety assessment: adverse events, serious adverse events, physical examination, vital signs, blood samples, urine samples |
|
| Notes |
Publication type: peer‐reviewed article Country: USA, 9 centres Conversion: Median + Range to Mean + SD following guidelines from Hozo 2005 Handling of adverse events: Data presented in the "Efficacy results" section (page 821, paragraph 2) regarding number of participants with general muscle weakness and number not able to perform 5 second head‐lift were considered to show adverse events in this review and were counted as such Authors' conclusions: Recovery from profound rocuronium‐induced neuromuscular blockade was significantly faster with sugammadex than with neostigmine, suggesting that sugammadex has a unique ability to rapidly reverse profound rocuronium neuromuscular blockade Contact: first trial author R. Kevin Jones contacted by email: kevinjones@accurateclinicaltrials.net on 23.09.2015; no reply received * Indicates unpublished data collected by authors of the previous review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated" * Participants were randomly assigned to treatment groups according to a randomization schedule card prepared in advance by Schering‐Plough |
| Allocation concealment (selection bias) | Low risk | Randomization lists were kept by the person who was responsible for preparing the medication (or placebo). This person was not involved in administering the medication to participants, nor in participants' care or data collection * |
| Blinding of participants (performance bias) | High risk | Open‐label study; no further information available |
| Blinding of personnel (performance bias) | High risk | Open‐label study; no further information available |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | No specific information on identity or blinding of TOF‐watch assessor |
| Blinding of safety assessment (detection bias) | Low risk | Blinded safety assessor (who was not involved in randomization of participants nor in preparation or administration of trial medication or allowed in the operating room during surgery) performed a physical examination before surgery and during the postanaesthetic visit, as well as monitored all participants for adverse and serious adverse events |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for in the article, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: 88 participants were randomized in the rocuronium arm of the study ‐ 48 to sugammadex and 40 to neostigmine. 14 (sugammadex n = 11, neostigmine n = 3) discontinued the study. 13 of these discontinued before receiving rocuronium or study drug, primarily for surgery‐related reasons; 1 participant in the sugammadex group discontinued after receiving rocuronium prematurely. Therefore, the all‐subjects‐treated group comprised 75 participants (sugammadex n = 37, neostigmine n = 38), and the intent‐to‐treat group comprised 74 participants (n = 37 in each group) |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT00473694), and all of the study's prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | Supported by Schering‐Plough, Roseland, New Jersey |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's primary outcome. Treatment groups generally comparable with respect to baseline characteristics |
Kaufhold 2016.
| Methods |
Study design: Single‐centre, randomized, parallel‐group, double‐blinded study (SUNDRO20) Sample size calculation: powered to detect doses necessary to accelerate time between study drug administration at a TOFR ≥ 0.2 to a TOFR ≥ 0.9 in 50% of participants within 2 minutes |
|
| Participants |
Number of randomized participants: 99 Inclusion criteria: age > 18 years; ASA physical status I to III; undergoing elective surgery under general anaesthesia with rocuronium for tracheal intubation; written informed consent Exclusion criteria: expected to have a difficult airway or with known neuromuscular disease, significant hepatic or renal dysfunction, family history of malignant hyperthermia, known allergy to one of the drugs used in this protocol; or intake of any medication that might interact with muscle relaxants; pregnant women or women who were breastfeeding; individuals who have participated in another clinical study in the past 30 days |
|
| Interventions |
Anaesthesia: induced with propofol 2 to 3 mg/kg IV and fentanyl 0.1 to 0.2 μg/kg IV and maintained with propofol and remifentanil according to clinical need and preference for the anaesthetist NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.1 to 0.2 mg/kg Comparison: sugammadex 0.25 mg/kg (n = 9), 0.5 mg/kg (n = 9), 0.75 mg/kg (n = 9), 1.0 mg/kg (n = 9), and 1.25 mg/kg (n = 9), neostigmine 10 μg/kg (n = 9), 25 μg/kg (n = 9), 40 μg/kg (n = 9), 55 μg/kg (n = 9), and 70 μg/kg (n = 9) in a mixture with 1 μg glycopyrrolate per 5 μg neostigmine, or saline (n = 9) Administration time of sugammadex or neostigmine or placebo: TOFR ≥ 0.2 |
|
| Outcomes |
Primary endpoints: doses necessary to achieve this effect in 50% of patients within 2 minutes or in 95% of patients within 5 minutes Secondary endpoints: doses for less advanced acceleration (i.e. in 50% of participants within 5 minutes or in 95% of participants within 10 minutes) Safety assessment: heart rate, blood pressure, and clinical muscle test function (eye opening, head‐lift test, arm‐lift test, swallowing a bolus of 20 mL of water, test for general muscle weakness) |
|
| Notes |
Publication type: peer‐reviewed article Country: Germany Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: A residual neuromuscular block for a TOFR = 0.2 cannot be reversed reliably with neostigmine within 10 minutes. In the conditions studied, substantially lower doses of sugammadex than the approved dose of 2.0 mg/kg may be sufficient to reverse residual rocuronium‐induced neuromuscular block at recovery of TOFR ≥ 0.2 Contact: first trial author S. Schaller contacted by email: s.schaller@tum.de on 07.06.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization list"; every participants received a consecutive number |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Low risk | In the operating room, unblinded study staff attending anaesthetist, who was the only person with access to the randomization list, prepared the study drug corresponding to the randomization number in an unlabelled syringe. Upon request of the blinded anaesthetist, responsible for the participant (without access to the randomization list and study medication); unlabelled study drug was injected |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: A total of 99 participants were initially enrolled after 109 had been screened. One participant, who had received neostigmine 70 μg/kg, withdrew his written informed consent after surgery. Therefore, 98 participants were included in statistical analysis. No protocol violations occurred |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT01006720), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | Declaration of interest: N.K. has received a travel grant from MSD Sharpe & Dohme. S.J.S. holds stocks for the following companies in the healthcare sector in small amounts: Bayer AG, Siemens AG, GE, Merck & Co. Inc., Rhoen‐Klinikum AG, and Fresenius SE; however, these holdings did not influence any decisions regarding the study. C.G.S. has received honoraria and a travel grant from MSD Sharpe & Dohme. H.F. has received honoraria and travel grants from the following companies: MSD Sharp & Dohme, Essex, Baxter, Care Fusion, and GE Healthcare. M.B. has received honoraria and travel grants from MSD Sharp & Dohme and GlaxoSmithKline. E.B. and K.U. have declared no conflicts Funding: Klinik für Anaesthesiologie, Klinikum rechts der Isar der Technischen Universität München, Munich, Germany |
| Other bias | High risk | Study sample size calculation not designed to address this review's primary or secondary outcome. Groups did not differ regarding age, weight, height, sex, and ASA physical status |
Khuenl‐Brady 2010.
| Methods |
Study design: multi‐centre, randomized, active control, safety assessor‐blinded trial Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 100 Inclusion criteria: age ≥ 18 years, ASA I to III, scheduled for a surgical procedure under general anaesthesia in a supine position requiring tracheal intubation Exclusion criteria: anticipated difficult airway; known or suspected neuromuscular disorders; significant renal dysfunction; known or suspected family history of malignant hyperthermia; allergy to narcotics, muscle relaxants, or other medication used during general anaesthesia; receiving medication at a dose and/or time point likely to interfere with NMBDs and for whom use of neostigmine and/or glycopyrrolate could be contraindicated; participated in a previous sugammadex trial, pregnant, breastfeeding, or female of childbearing age using only hormonal contraception or no means of birth control |
|
| Interventions |
Anaesthesia: induced with IV opioid (at the discretion of the investigator) and IV propofol, maintained with sevoflurane MAC 1 to 2 and opioids, according to each participant's needs NMBA: single intubating dose: vecuronium 0.1 mg/kg; maintenance dose: vecuronium 0.02 to 0.03 mg/kg Comparison: sugammadex 2 mg/kg (n = 51) vs neostigmine 50 µg/kg + glycopyrrolate 10 µg/kg (n = 49) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes |
Primary efficacy variable: time from start of administration of sugammadex or neostigmine to recovery of TOFR to 0.9 Secondary efficacy variables: time from start of administration of sugammadex or neostigmine to recovery of TOFR to 0.7, time to recovery of TOFR to 0.8, and assessments of clinical signs of recovery (level of consciousness, 5 second head‐lift test, and general muscle weakness) before transfer to the recovery room after tracheal extubation and before discharge from the recovery room Safety assessments: pretreatment events; serious trial procedure‐related events (up to 7 days post dose); vital signs, blood samples, urinalysis, adverse events, and serious adverse events; physical examination findings; clinical signs of possible residual paralysis or recurrence of neuromuscular block |
|
| Notes |
Publication type: peer‐reviewed article Country: 13 centres in Europe: Austria, Belgium, Germany, Italy, Spain, Sweden, and United Kingdom Conversions: Median + Range to Mean + SD following guidelines from Hozo 2005 Handling of adverse events: Data presented in the "Efficacy results" section (last paragraph on page 68 and first paragraph on page 69) regarding number of participants with general muscle weakness and not able to perform 5 second head‐lift, which were considered adverse events in this review and were counted as such Authors’ conclusions: Sugammadex provided significantly faster reversal of vecuronium‐induced neuromuscular blockade compared with neostigmine Contact: first trial author Karin S. Khuenl‐Brady contacted by email: karin.khuenl‐brady@i‐med.ac.at on 15.10.2010; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization system, part of a secure trial website |
| Allocation concealment (selection bias) | Low risk | All enrolled participants were allocated a subject number in sequential order of their enrolment into the trial and received a treatment code using the central randomization system |
| Blinding of participants (performance bias) | High risk | Open‐label; no further information available |
| Blinding of personnel (performance bias) | High risk | Open‐label; no further information available |
| Blinding of primary outcome assessment (detection bias) | High risk | Open‐label; no further information available |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessor was blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All participants are accounted for, but missing outcome data are not balanced in numbers across intervention groups, as 25% fewer participants are included in the neostigmine group compared with the sugammadex group: 100 participants were enrolled in the study: 51 randomized to the sugammadex group and 49 to the neostigmine group. Three participants in the sugammadex group and 4 in the neostigmine group did not receive study drug. Reasons for discontinuation in the sugammadex group were refusal of surgical procedure (n = 1) and TOF‐watch SX problems (n = 2). In the neostigmine group, participants were discontinued because of unavailability of site staff to perform the protocol (n = 1), randomization failure (n = 1), surgeon’s withdrawal of consent for operating room time for the research team (n = 1), and a TOF‐watch SX problem (n = 1). Hence, 48 participants in the sugammadex group and 45 in the neostigmine group were treated (representing the all‐subjects‐treated population). Data were excluded for 2 participants in the sugammadex group as TOF data to 0.9, 0.8, and 0.7 were considered unreliable because of unstable TOF baseline Data were excluded for 11 participants in the neostigmine group because TOF data to 0.9 were missing (8 participants failed to achieve a TOFR of 0.9; 1 participant did not have recovery time measured for TOFR 0.9, and in 2 participants, TOFR data to 0.9 were considered unreliable because of unstable TOFR baseline) |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT00451217), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | Supported by Schering‐Plough, Oss, The Netherlands. Henk Rietbergen is employed by Schering‐Plough |
| Other bias | Unclear risk | No apparent other type of bias, except no information on sample size calculation. Treatment groups had similar baseline characteristics |
Kizilay 2016.
| Methods |
Study design: prospective, randomized study Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 90 Inclusion criteria: aged 18 to 75 with grade 2 or 3 cardiovascular disease according to New York Heart Association classification undergoing non‐cardiac surgery; free of any clinical infection; chronic alcohol use or substance abuse history; free of contraindications to atropine, neostigmine, or sugammadex Exclusion criteria: did not give written consent;respiratory or cardiac arrest, cerebral bleeding, ischaemia, infarct, or hypersensitivity reaction to any of the study medications |
|
| Interventions |
Anaesthesia: induction with 5 mg/kg IV thiopental sodium; maintenance: sevoflurane, 70% N2O and 30% O2 to MAC 1 NMBA: single intubating dose: rocuronium 0.8 mg/kg; maintenance dose: no information available Comparison: sugammadex 3 mg/kg (n = 45) vs neostigmine 30 µg/kg (n = 45) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes | Heart rate, mean systolic and diastolic blood pressures, and electrocardiographic alterations including QTc (QT Fredericia and QT Bazett) were recorded | |
| Notes |
Publication type: peer‐reviewed article Country: Turkey Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: We suggest that sugammadex might be preferred, as it provides greater haemodynamics stability than is provided by the neostigmine‐atropine combination to reverse rocuronium‐induced neuromuscular blockade in cardiac patients undergoing non‐cardiac surgery Contact: first trial author Deniz Kizilay contacted by email: denizkizilay@yahoo.com on 24.05.2016; replied 29.05 * Indicates unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by lots * |
| Allocation concealment (selection bias) | High risk | No allocation concealment * |
| Blinding of participants (performance bias) | Low risk | Participants were blinded * |
| Blinding of personnel (performance bias) | High risk | Personnel were not blinded * |
| Blinding of primary outcome assessment (detection bias) | High risk | TOF‐watch assessor was not blinded * |
| Blinding of safety assessment (detection bias) | High risk | Safety assessor was not blinded * |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs: 90 participants were randomized, 45 to each group |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published article clearly includes all expected outcomes |
| Funding bias | Low risk | Study was funded by the first trial author * |
| Other bias | Unclear risk | No apparent other type of bias, except no information on sample size calculation. No significant differences between groups in terms of age, sex, weight, ASA‐, NYHA‐ classification, or comorbid disorders, except coronary disease |
Koc 2015.
| Methods |
Study design: randomized, prospective, controlled trial Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 33 Inclusion criteria: aged 18 to 65, ASA I to III, undergoing short‐term (< 90 minutes) elective abdominal surgery (colectomy, incisional and umbilical hernia) Exclusion criteria: expected difficult intubation; receiving medication known to interact with rocuronium; neuromuscular disease, significant renal or liver disease, an allergy or other contraindication to medication used during the study; pregnancy;morbid obesity |
|
| Interventions |
Anaesthesia: induced with 1 to 2 µg/kg fentanyl, 5 to 7 mg/kg thiopental; maintained with 50% O2‐N2O and 1% sevoflurane NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.1 to 0.2 mg/kg Comparison: sugammadex 2 mg/kg (n = 16) vs neostigmine 50 µg/kg + atropine 20 µg/kg (n = 17) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes | Time to recovery of TOFR > 0.9; efficacy and cost‐effectiveness of sugammadex vs neostigmine | |
| Notes |
Publication type: peer‐reviewed article Country: Turkey, 1 centre Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: Recovery of neuromuscular function after rocuronium to TOFR of 0.9 was faster with 2 mg/kg sugammadex than with 50 μg/kg neostigmine; sugammadex was more expensive than neostigmine Contact: corresponding trial author Guldem Turan contacted by e‐mail: gturanmd@yahoo.com on 12.10.2015; no reply received Language: article in Turkish |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly divided"; no further information available |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published article clearly includes all expected outcomes |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | Unable to assess owing to insufficient information |
Kogler 2012.
| Methods |
Study design: prospective, randomized study Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 31 Inclusion criteria: adult; ASA IV; scheduled for procedures in interventional bronchoscopy Exclusion criteria: no information available |
|
| Interventions |
Anaesthesia: induced with midazolam, propofol, and sufentanil; maintained with increments of propofol NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.15 mg/kg Comparison: sugammadex 2.0 mg/kg (n = 16) vs neostigmine 70 µg/kg (n = 15) Administration time of sugammadex or neostigmine: PTC 1 to 2 |
|
| Outcomes |
Primary efficacy parameter: time to recovery of TOFR to 0.9 Other parameters: time from beginning of anaesthesia to time of patient discharge to the PACU and blood gas analysis at time of discharge, adverse events |
|
| Notes |
Publication type: meeting abstract Country: Croatia Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: Sugammadex provided significantly faster recovery time from rocuronium‐induced profound neuromuscular block in comparison with neostigmine, and shorter duration from beginning of anaesthesia to patient discharge to PACU with lower values of PaCO2 Contact: third trial author Maja Karaman Ilic contacted: mkilic@inet.hr on 13.10.2015; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized"; no further information |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess owing to insufficient information |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published meeting abstract clearly includes all expected outcomes |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | Unable to assess owing to insufficient information |
Koyuncu 2015.
| Methods |
Study design: single‐centre, randomized, double‐blinded trial Sample size calculation: powered to detect a 0.5 difference between groups on 4‐point PONV scale |
|
| Participants |
Number of randomized participants: 100 Inclusion criteria: ASA I to II, scheduled for extremity surgery (tendon repair and skin graft surgery) during general anaesthesia Exclusion criteria: any contraindication to sugammadex or neostigmine administration; emergency or urgent procedures; BMI ≥27 kg/m2, hepatic impairment (alanine aminotransferase or aspartate aminotransferase > 2 times normal), renal impairment (serum creatinine > 2 mg/dL) |
|
| Interventions |
Anaesthesia: induced with propofol 2 to 2.5 mg/kg and fentanyl 1 μg/kg, maintained with 5% to 6% desflurane in 66% nitrous oxide in oxygen NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.15 mg/kg Comparison: sugammadex 2 mg/kg (n = 50) vs neostigmine 70 µg/kg + atropine 0.4 mg per 1 mg neostigmine (n = 50) Administration time of sugammadex or neostigmine: 4 twitches of TOF visible with fade |
|
| Outcomes | PONV, postoperative pain on VAS, clinical recovery parameters (extubation time, first eye opening, head‐lift time, first flatus, first oral intake, ambulation), heart rate, non‐invasive blood pressure, oxygen saturation, antiemetic consumption and side effects; bradycardia (heart rate < 60/min), hypotension (decrease in systolic arterial pressure < 10 mmHg from baseline), itching, headache, respiratory depression (respiratory rate < 10), cough, bronchospasm, irritation at injection site, abnormally increased oral secretions | |
| Notes |
Publication type: peer‐reviewed article Country: Turkey Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: Non‐depolarizing neuromuscular blocking antagonism with sugammadex speeds recovery of neuromuscular strength but only slightly and transiently reduces PONV compared with neostigmine and atropine Contact: first trial author Onur Koyuncu contacted by e‐mail: onurko@yahoo.com on 25.05.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomization; participants were "randomly assigned 1:1 without stratification" |
| Allocation concealment (selection bias) | Low risk | Central allocation (secondary to web‐based randomization) |
| Blinding of participants (performance bias) | Unclear risk | "Double‐blind study"; no further information available |
| Blinding of personnel (performance bias) | Unclear risk | "Double‐blind study"; no further information available |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | "Double‐blind study"; no further information available |
| Blinding of safety assessment (detection bias) | Unclear risk | An anaesthetist blinded to treatment queried participants about postoperative pain using VAS; no further information available about blinding of assessor of other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs: 100 consenting patients who fulfilled entry criteria were enrolled; all completed the entire study and were included in the final analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published article clearly includes all expected outcomes |
| Funding bias | High risk | Trial authors have no financial relationship with any organization. Supported by internal funds only. Department of Outcomes Research is supported by grants from Merck, and Dr Sessler has served on a Merck advisory board |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's secondary outcome. Participants assigned to each medication comparable with respect to age, height, body weight, ASA physical status, Apfel score, duration of surgery, and duration of anaesthesia |
Kvolik 2012a.
| Methods |
Study design: prospective, randomized study Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 36 Inclusion criteria: undergoing thyroidectomy Exclusion criteria: no information available |
|
| Interventions |
Anaesthesia: propofol and fentanyl for both induction and maintenance NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.1 mg/kg Comparison: sugammadex 2 mg/kg (n = 17) vs neostigmine 50 µg/kg (n = 19) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes | Recovery of TOFR > 90% of baseline, recovery of cough reflexes enabling safe extubation, spontaneous minute volume at the time of extubation | |
| Notes |
Publication type: meeting abstract Country: Croatia Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the meeting abstract and those reported in this review Authors’ conclusions: Recovery of cough reflexes was faster and respiration more efficient in patients receiving sugammadex. A safe extubation was determined by age, TOF recovery, and effects of other anaesthetics Contact: first trial author Slavica Kvolik contacted by email: slavica.kvolik@os.t‐com.hr on 14.10.2015; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized"; no further information available |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess owing to insufficient information |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published meeting abstract clearly includes all expected outcomes |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | No information on sample size calculation. No differences regarding participant characteristics and preparative FT4, FT3, and TSH levels between groups |
Kvolik 2012b.
| Methods |
Study design: prospective, randomized study Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 24 Inclusion criteria: euthyroid, undergoing general anaesthesia for thyroidectomy Exclusion criteria: no information available |
|
| Interventions |
Anaesthesia: propofol and fentanyl NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.1 mg/kg Comparison: neostigmine 50 µg/kg vs sugammadex 2 mg/kg Administration time of sugammadex or neostigmine: end of surgery |
|
| Outcomes | Thyroid hormones (FT3, FT4, and TSH) measured before surgery, 1 hour after reversal, and 24 hours after surgery | |
| Notes |
Publication type: meeting abstract Country: Croatia Conversions: none Authors’ conclusions: Sugammadex treatment did not change levels of thyroid hormones and may be safely used in patients undergoing total thyroidectomy Contact: first trial author Slavica Kvolik contacted by e‐mail: slavica.kvolik@os.t‐com.hr on 24.05.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized study"; no further information available |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess owing to insufficient information |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published meeting abstract clearly includes all expected outcomes |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | No information on sample size calculation. No differences regarding participant characteristics and drug consumption between groups |
Kvolik 2013.
| Methods |
Study design: prospective randomized study Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 44 Inclusion criteria: adults, ASA I to III, undergoing thyroidectomy or breast cancer surgery Exclusion criteria: no information available |
|
| Interventions |
Anaesthesia: induced with propofol 2 mg/kg and fentanyl 5 µg/kg; maintenance: no information available NMBA: single intubating dose: rocuronium 0.6 mg/kg Comparison: sugammadex 2 mg/kg (n = 20) vs neostigmine 50 µg/kg + atropine 25 µg/kg (n = 24) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes | Time to recovery of TOF 90% and mean increase in BIS indices per each minute after reversal | |
| Notes |
Publication type: meeting abstract Country: Croatia Conversions: none Authors’ conclusions: An increase in BIS index registered after reversal of rocuronium effects was faster during the recovery period among patients who were given sugammadex rather than neostigmine. Although a rapid increase in BIS indices was registered in the sugammadex group, more sensitive measurements are needed to confirm the clinical value of this observation Contact: first trial author Slavica Kvolik contacted by e‐mail: slavica.kvolik@os.t‐com.hr on 14.10.2015; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess owing to insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available, but published meeting abstract clearly includes all expected outcomes |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | Unable to assess owing to insufficient information |
Lemmens 2010.
| Methods |
Study design: multi‐centre, randomized, parallel‐group, safety assessor‐blinded, phase 3a trial (SIGNAL study) Sample size calculation: powered to detect a difference of 5 minutes in time to recovery of TOFR to 0.9 |
|
| Participants |
Number of randomized participants: 94 Inclusion criteria: adults > 18 years, ASA I to IV, scheduled to undergo elective surgery in the supine position under general anaesthesia requiring use of a neuromuscular blocking agent for tracheal intubation and maintenance of neuromuscular block Exclusion criteria: neuromuscular disorder; history of malignant hyperthermia; significant renal dysfunction; allergy to narcotics, muscle relaxants, or other medication used during general anaesthesia; using medication known to interfere with neuromuscular blocking agents (e.g. antibiotics, anticonvulsants, magnesium); or pregnant, breastfeeding, or of childbearing potential and not using an adequate method of contraception |
|
| Interventions |
Anaesthesia: induced with intravenous opioid and propofol; maintained with intravenous opioid and sevoflurane NMBA: single intubating dose: vecuronium 0.1 mg/kg; maintenance dose: vecuronium 0.015 mg/kg Comparison: sugammadex 4.0 mg/kg (n = 52) vs neostigmine 70 µg/kg + 14 µg/kg glycopyrrolate (n = 42) Administration time of sugammadex or neostigmine: reappearance of PTC 1 to 2 |
|
| Outcomes |
Primary efficacy variable: time from start of administration of Org 25969/neostigmine to recovery T4/T1 ratio to 0.9 Secondary efficacy variables: time from start of administration of Org 25969/neostigmine to recovery T4/T1 ratio to 0.7 and 0.8; assessment of clinical signs of recovery (level of consciousness, 5 second head‐lift, general muscle weakness) Safety analysis: adverse events, serious adverse events, laboratory data, vital signs |
|
| Notes |
Publication type: peer‐reviewed article Country: USA, 9 centres Handling of adverse events: Data presented in "Clinical signs of recovery" section (page 7) regarding number of participants with general muscle weakness and number not able to perform 5 second head‐lift were considered to show adverse events in this review and were counted as such Conversions: none Authors’ conclusions: Sugammadex provided effective and rapid reversal of profound neuromuscular block induced by vecuronium under sevoflurane anaesthesia Contact: first trial author Hendrikus JM Lemmens contacted by email: hlemmens@stanford.edu; replied referring to Merck, but did not supply contact email address at Merck |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization schedule prepared centrally by the study sponsor" |
| Allocation concealment (selection bias) | Low risk | Central allocation (secondary to central randomization) |
| Blinding of participants (performance bias) | High risk | Open‐label study; no further information available |
| Blinding of personnel (performance bias) | High risk | Open‐label study; no further information available |
| Blinding of primary outcome assessment (detection bias) | High risk | Open‐label study; no further information available |
| Blinding of safety assessment (detection bias) | Low risk | Only safety assessor was blinded. Drugs were prepared by an investigator who was not involved in safety assessments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in distribution: After interim analysis and recommendation by the Data and Safety Monitoring Board, the neostigmine group was discontinued because of marked differences in efficacy between treatments, although by this time, 42 participants had already been randomized into the neostigmine group. A total of 11 participants (5 sugammadex and 6 neostigmine) discontinued the trial before receiving the study drug. In addition, 1 participant randomized to vecuronium and sugammadex received rocuronium plus neostigmine and was excluded from the all‐subjects‐treated population, but was included in the intent‐to‐treat population according to the randomization schedule. Therefore, the all‐subjects‐treated population consisted of 46 participants treated with sugammadex and 36 treated with neostigmine, and the intent‐to‐treat population consisted of 47 participants randomized to sugammadex and 36 randomized to neostigmine |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT00473694), and all of the study's prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | Study was funded by Merck Research Laboratories, Summit, New Jersey, USA. Hendrikus Lemmens has participated in a Merck advisory board. Jovino Ben Morte is an employee of Merck Research Laboratories, Summit, New Jersey, USA. Mohammad El‐Orbany has received research funding from Merck. James Berry and Gavin Martin declare that they have no other competing interests |
| Other bias | High risk | Study sample size calculation designed to address this review's primary outcome. One intervention group was discontinued owing to marked differences in efficacy between groups after interim analysis |
Martini 2014.
| Methods |
Study design: randomized blinded study (BLISS trial) Sample size calculation: based on the expectation of the surgeon for distribution of surgical ratings between moderate and deep neuromuscular block |
|
| Participants |
Number of randomized participants: 26 Inclusion criteria: scheduled to undergo an elective laparoscopic prostatectomy or nephrectomy (partial or total) who have given written consent Exclusion criteria: ASA class > III, age < 18 years, inability to give informed consent, known or suspected neuromuscular disease, allergy to medication to be used during anaesthesia, (family) history of malignant hyperthermia, renal insufficiency (serum creatinine > 2 times normal, urine output < 0.5 mL/kg/h, glomerular filtration rate < 60 mL/h, or proteinuria), previous retroperitoneal surgery, body mass index ≥ 35 kg/m2 |
|
| Interventions |
Anaesthesia: propofol and sufentanil NMBA: single intubating dose: atracurium 0.5 mg/kg (for moderate NMB) or rocuronium 1.0 mg/kg (for deep NMB); maintenance dose: mivacurium 0.5 mg/kg/h (for moderate NMB) or rocuronium 0.6 mg/kg/h (for deep NMB) Comparison: neostigmine 1 to 2 mg + atropine 0.5 to 1 mg (for reversal of moderate NMB) (n = 12) vs sugammadex 4 mg/kg (for reversal of deep NMB) (n = 12) Administration time of neostigmine or sugammadex: reappearance of T2 or PTC 1 to 2 |
|
| Outcomes |
Primary endpoint: influence of the depth of NMB on the SRS (surgical rating score) Secondary endpoints: (1) assessment of the level of agreement between anaesthetists and surgeon in terms of their rating of surgical conditions, (2) effects of level of NMB on haemodynamic variables during surgery, time to TOFR.= 0.9, and relevant variables in the PACU (pain rating, sedation levels, and cardiorespiratory variables) |
|
| Notes |
Publication type: peer‐reviewed article Country: Netherlands Conversions: none Authors’ conclusions: Application of the 5‐point SRS showed that deep NMB results in improved quality of surgical conditions compared with moderate block in retroperitoneal laparoscopies, without compromise to patients’ perioperative and postoperative cardiorespiratory conditions Contact: first trial author A. Dahan contacted by e‐mail: a.dahan@lumc.nl on 27.05.2016; replied on 27.05.16 * Indicates unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using a computer‐generated randomization code |
| Allocation concealment (selection bias) | Unclear risk | Sealed, opaque, and sequentially numbered envelopes with codes were presented to the attending anaesthetist who prepared the medication and took care of participant dosing during anaesthesia * |
| Blinding of participants (performance bias) | Low risk | Participants were under general anaesthesia and therefore were blinded |
| Blinding of personnel (performance bias) | High risk | Attending anaesthesiologist was not blinded; the surgical team, the research team, and the anaesthetist who scored the video were all blinded |
| Blinding of primary outcome assessment (detection bias) | Low risk | TOF measurements were performed by a fully blinded researcher or research nurse * |
| Blinding of safety assessment (detection bias) | Low risk | PACU evaluation (pain, sedation, cardiovascular variables) was performed by a fully blinded researcher or research nurse * |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: A total of 30 patients were screened. Four patients met 1 or more exclusion criteria. The others were randomized. Two patients withdrew consent before treatment, resulting in 12 participants in each group |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT01631149), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | L.P.A. and A.D. received speaker fees from Merck BV, Oss, The Netherlands. This study is supported in part by Merck BV, Oss, The Netherlands, and by institutional funds from the Department of Anaesthesiology, Leiden University Medical Centre, Leiden, The Netherlands. Merck was not involved in the design and conduct of the study, data analysis, and production of the manuscript. Merck’s statistician Hein Fennema assisted with sample size analysis |
| Other bias | High risk | Study sample size calculation not designed to address this review's primary or secondary outcomes. The 2 treatment groups were similar in physical characteristics, gender, types of surgery, and haemodynamic variables |
Mekawy 2012.
| Methods |
Study design: randomized, controlled study Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 40 Inclusion criteria: age 20 to 45, ASA I to II, with chronic sinuitis undergoing endoscopic sinus surgery with or without seroplasty Exclusion criteria: cardiovascular system pathology, coagulation defects, bronchial asthma, COPD, muscle disease or neuromuscular disorder, renal or hepatic disease, taking any drugs that affect renal function or blood coagulation, history of difficult intubation or suspected to be difficult |
|
| Interventions |
Anaesthesia: induced with: propofol 2 to 2.5 mg/kg and fentanyl 1 µg/kg. Maintained with 50% oxygen in air and isoflurane to MAC 1.5. Hypotensive anaesthesia was used to maintain MAP 50 to 60 mmHg NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.10 to 0.15 mg/kg Comparison: sugammadex 4 mg/kg (n = 20) vs neostigmine 50 µg/kg + atropine 20 µg/kg (n = 20) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes | Time from administration of study drug until TOFR of 0.9, assessment of postoperative respiratory complications using the Postoperative Respiratory System Evaluation Score (PRSES) at 1 and 5 minutes after extubation | |
| Notes |
Publication type: peer‐reviewed article Country: Egypt Conversions: none Handling of adverse events: Data presented in Table 4 ‐ "Incidence of postoperative respiratory complications using PRSES score: PRESES 2 and PRSES 3‐5 at 1 minute" ‐ as well as data regarding number of participants not able to perform 5 second head‐lift (presented on page 177) were considered as adverse events in this review and were counted as such Authors’ conclusions: Use of sugammadex in reversing rocuronium‐induced neuromuscular block among patients undergoing functional endoscopic surgery is superior to use of neostigmine. Additional studies are required to weigh the cost‐benefit relationship of the use of sugammadex in routine clinical practice Contact: corresponding trial author E.A. Fouad Ali contacted by e‐mail: Mhz_home@hotmail.com on 16.10.2015; has not replied |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated system" |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Low risk | Study drugs were prepared in identical 10 mL syringes and were injected by a resident who was blinded to the drug injected |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: A total of 54 participants consented to participate in this study, and all were ASA I and II. Nine patients were excluded because they did not meet the inclusion criteria, and 5 were withdrawn from the study owing to inability to apply the study protocol; these 5 patients had BIS readings higher than 60 before the reversal drug injection that mandates reopening of inhalational agents; this violates the study protocol |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published article clearly includes all expected outcomes |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | No apparent other types of bias, except no information on sample size calculation. No significant differences regarding demographic characteristics, surgery, isoflurane consumption, nitroglycerin requirements, rocuronium supplemented, and intraoperative blood loss among the 2 groups |
Pongracz 2013.
| Methods |
Study design: single‐centre, randomized, controlled, double‐blind, 4 groups parallel‐arm study Sample size calculation: powered to detect a 300 second decrease in time of recovery to TOFR > 0.9 |
|
| Participants |
Number of randomized participants: 80 Inclusion criteria: aged 18 to 65 years, body mass index 18.5 to 25.0 kg/m2, ASA I to III, scheduled for elective surgery with an expected duration > 50 minutes under general anaesthesia with intubation of the trachea Exclusion criteria: participated in another clinical trial within 1 month, suspected difficult airway, bronchial asthma, chronic obstructive pulmonary disease, known NM disease, suspected malignant hyperthermia, hepatic or renal dysfunction, glaucoma, allergy to medication used in this trial, taking medicaments that might influence the effect of NMB agents, pregnant or breastfeeding |
|
| Interventions |
Anaesthesia: induced with intravenous propofol (1.5 to 2.5 mg/kg) and fentanyl (2 μg/kg) and maintained with inhaled sevoflurane (1.1 to 1.8 vol %) in air–oxygen mixture and intravenous fentanyl according to clinical need NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.1 to 0.15 mg/kg Comparison: sugammadex 0.5 mg/kg (n = 19), 1.0 mg/kg (n = 20), 2.0 mg/kg (n = 20), and neostigmine 0.05 mg/kg + atropine 0.015 mg/kg (n = 16) Administration time of sugammadex or neostigmine: reappearance of T4 at 3 consecutive TOF measurements |
|
| Outcomes |
Primary endpoint: rapid reversal (≤ 2.0 minutes average, upper limit of 5.0 minutes) Secondary endpoint: slower reversal (≤ 5.0 minutes average, upper limit of 10 minutes) |
|
| Notes |
Publication type: peer‐reviewed article Country: Hungary Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: Sugammadex 1.0 mg/kg rapidly and effectively reverses rocuronium‐induced block that has recovered spontaneously to a threshold TOF count four. A dose of 0.5 mg/kg was equally effective, but satisfactory antagonism took as long as 8 minutes to take place Contact: corresponding trial author Bela Fülesdi contacted by email: fulesdi@dote.hu on 28.02.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted‐block randomization. Ten numbers of 1 to 4 were prepared 20 times each and were placed into an envelope; each number identified 1 of the 4 study groups |
| Allocation concealment (selection bias) | Unclear risk | Envelopes were used; no information on whether they were sealed, opaque, and sequentially numbered |
| Blinding of participants (performance bias) | Low risk | In the operating room, a different anaesthesiologist prepared the study drug in an unlabelled syringe according to randomization and injected it upon request of the blinded anaesthesiologist who was responsible for the participant |
| Blinding of personnel (performance bias) | Low risk | In the operating room, a different anaesthesiologist prepared the study drug in an unlabelled syringe according to randomization and injected it upon request of the blinded anaesthesiologist who was responsible for the participant |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | No information on identity of blinding or of TOF‐watch assessor |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: Study drugs were injected in 80 participants; 5 were excluded. In 4 participants, the TOFR did not reach 1.0 within 15 minutes after injection of neostigmine; therefore 2 mg/kg of sugammadex was given as rescue medication to prevent RPONB. In 1 patient (0.5 mg/kg sugammadex group), the study drug was injected at a TOFR of 0.6 (minor protocol violation). With 5 participants excluded from the final efficacy analysis, 75 participants were finally analysed for TOF recovery |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrialsregister.eu (EudraCT Number: 2011‐001683‐22), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's primary outcome |
Rahe‐Meyer 2014.
| Methods |
Study design: randomized, parallel‐group, double‐blind trial Sample size calculation: powered to address the primary endpoint (bleeding events) |
|
| Participants |
Number of randomized participants: 1198 Inclusion criteria: adults (≥ 18 years of age) of ASA class I to III undergoing joint (hip or knee) replacement surgery/revision or intracapsular or extracapsular hip fracture surgery, and planned to receive thrombose‐prophylaxis and neuromuscular blockade with rocuronium or vecuronium Exclusion criteria: suspected anatomical malformations that could make endotracheal intubation more difficult; neuromuscular disorders that might affect neuromuscular blockade; medical history of coagulation disorder, bleeding diathesis, systemic lupus erythematosus, or antiphospholipid syndrome; history or evidence of active abnormal bleeding or blood clotting (e.g. thrombosis) within 30 days before screening; severe hepatic dysfunction; active hip or knee infection scheduled for revision surgery; known or suspected severe renal insufficiency (estimated creatinine clearance < 30 mL/min); family history of malignant hyperthermia; morbid obesity (body mass index > 35); hypersensitivity to or conditions that would contraindicate the use of sugammadex, muscle relaxants or their excipients, or other medication(s) used during general anaesthesia; receiving treatment with toremifene and/or fusidic acid intravenously within 24 hours before or after study medication administration because of potential drug–drug interaction; previously treated with sugammadex, participated in a previous sugammadex trial, or participated in another clinical trial within 30 days of this trial; pregnant or breast‐feeding |
|
| Interventions |
Anaesthesia: induction and maintenance according to usual practice at the site NMBA: rocuronium or vecuronium, according to usual practice at the site Comparison: sugammadex 4 mg/kg (n = 596) vs usual care (neostigmine with glycopyrrolate or atropine, or placebo/spontaneous recovery) (n = 588) Administration time of sugammadex, neostigmine, or placebo: not stated |
|
| Outcomes |
Primary endpoint: proportion of participants with ≥ 1 adjudicated event of bleeding that occurred within 24 hours after trial medication administration Key secondary endpoints: change from baseline in aPTT at 10 and 60 minutes after trial medication administration Additional endpoints: postoperative drainage volumes within first 24 hours after trial medication administration; rates of postoperative transfusion (initiated after sugammadex or placebo/neostigmine was given) and respective transfusion volumes; postoperative changes in haemoglobin based on the bleeding index; incidence of anaemia with onset within 72 hours after administration of trial medication Safety assessment: adverse events and serious adverse events |
|
| Notes |
Publication type: peer‐reviewed article Country: Austria, Belgium, and Germany (22 centres) Conversions: none Authors’ conclusions: Sugammadex produced limited, transient (< 1 hour) increases in aPTT and PT but was not associated with increased risk of bleeding vs usual care Contact: first trial author Niels Rahe‐Meyer contacted by email: rahe‐meyer.niels@mh‐hannover.de on 28.03.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized interactive voice and Web response system |
| Allocation concealment (selection bias) | Low risk | Central allocation (secondary to central randomization) |
| Blinding of participants (performance bias) | Low risk | Participants were blinded |
| Blinding of personnel (performance bias) | Low risk | Trial medication was administered in a blinded manner by the anaesthesiologist after preparation in an unblinded manner by the pharmacist. To further maintain blinding, opaque, coloured syringes were used to mask potential differences in the tint of study treatments |
| Blinding of primary outcome assessment (detection bias) | Low risk | Not relevant as TOF‐watch assessment was not reported in this study |
| Blinding of safety assessment (detection bias) | Low risk | Initial determination was made by a blinded safety assessor on site, who was a medically qualified member of the surgical team. For all bleeding events thus identified, available medical information was submitted for adjudication to the independent, blinded Primary Adjudication Committee, consisting of external experts in the field |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: Of 1198 participants randomized, 1184 were treated (sugammadex n = 596, usual care n = 588) from October 2011 to September 2012. A total of 52% of usual care participants received neostigmine, and 48% underwent spontaneous recovery. Overall, 1137 participants completed the trial: 575 (96.5%) in the sugammadex group and 562 (95.6%) in the usual care group |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT01422304), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | Authors Fennema, Speek, McCrary Sisk, Williams‐Herman, Woo, and Szegedi are current or former employees of subsidiaries of Merck & Co., Inc. (Whitehouse Station, New Jersey), and may own stock or hold stock options in the company. Dr. Rahe‐Meyer reports receiving honoraria for advisory board membership, lectures, and/or consultancy for CSL‐Behring (King of Prussia, Pennsylvania) and Merck (Whitehouse Station, New Jersey). His institution received a grant for the study. Dr. Wulf reports receiving honoraria for advisory board memberships from Boehringer Ingelheim (Ingelheim, Germany), Sintectica (Canton Ticino, Switzerland), and Carefusion (San Diego, California), and for lectures or consultancy from Teleflex (Wayne, Pennsylvania), Sintetica (Canton Ticino, Switzerland), Vygon (Landsdale, Pennsylvania), B. Braun Medical Inc. (Melsungen, Germany), Pajunk GmbH (Geisingen, Germany), SonoSite Inc. (Bothell, Washington), and Merck (Whitehouse Station, New Jersey). Dr. Blobner reports receiving fees from Merck (Whitehouse Station, New Jersey) for consulting, lectures, advisory board membership, and participation in reviews and committees. He reports that his institution received grants and money for travel related to the study from Merck (Whitehouse Station, New Jersey). Dr. Schulman reports receiving travel support from Merck (Whitehouse Station, New Jersey) for investigators’ meetings and an honorarium for work on the Adjudication Committee. Dr. Przemeck reports receiving travel support from Merck (Whitehouse Station, New Jersey) for investigators’ meetings. His institution received a grant for patient visits and other costs associated with the study. Dr. Klimscha reports his institution received funds from Merck (Whitehouse Station, New Jersey) associated with the study |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's secondary outcome. Baseline characteristics similar across treatment groups |
Raziel 2013.
| Methods |
Study design: prospective, single‐centre, double‐arm study Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 40 Inclusion criteria: morbidly obese male or female patients aged 20 to 65 years who are candidates for bariatric surgery, can read and understand the fundamental nature of the clinical protocol, and must sign the Informed Consent Form Exclusion criteria: treated with drugs that might interact with rocuronium; history of malignant hyperthermia or significant renal disease; known allergy to one of the drugs used during anaesthesia; known muscular disease; severe cardiovascular disease (NYHA > 2); breastfeeding; refusing to follow the clinical protocol; participating in a different clinical trial; refusing to sign the Informed Consent Form; physician's objection |
|
| Interventions |
Anaesthesia: no information available NMBA: single intubating dose: rocuronium 0.4 mg/kg; maintenance dose: rocuronium 0.1 to 0.2 mg/kg (not more than × 2) Comparison: sugammadex 2 mg/kg (n = 21) vs neostigmine 50 µg/kg + atropine 10 µg/kg (n = 19) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes |
Primary outcome measures: safety of sugammadex reversal ‐ number of drug‐related adverse events with sugammadex vs neostigmine, monitoring of neuromuscular reaction from end of anaesthesia recovery (in the OR) until participant is released from hospital (48 to 72 hours post surgery) Secondary outcome measures: use of sugammadex for neuromuscular anaesthesia reversal; higher patient satisfaction compared with neostigmine, monitoring of neuromuscular reaction from end of anaesthesia recovery (in the OR) until participant is released from hospital (48 to 72 hours post surgery) |
|
| Notes |
Publication type: meeting abstract Country: Israel Conversions: none Authors’ conclusions: Sugammadex facilitates reversal of neuromuscular blockade after bariatric surgery, depending on depth of neuromuscular blockade induced Contact: first trial author Asnat Raziel contacted by e‐mail: drraziel@zahav.net.il * Indicates unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done by the anaesthesiologist at the end of surgery and when 2 responses were achieved on TOF stimulation with computer randomization software, when 1 of the study drugs was administered * |
| Allocation concealment (selection bias) | Low risk | Adeqaute allocation concealment secondary to the randomization method |
| Blinding of participants (performance bias) | Low risk | Participants were blinded * |
| Blinding of personnel (performance bias) | High risk | Anaesthesiologists were not blinded; surgeons were blinded * |
| Blinding of primary outcome assessment (detection bias) | High risk | TOF‐watch assessor was not blinded * |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessor was blinded * |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs * |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT01631396), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | Low risk | Study was funded by internal sources for the hospital. Sugammadex was received FOC by manufacturer. Manufacturer was not involved at any stage of the study * |
| Other bias | Unclear risk | No apparent other type of bias, except no information on sample size calculation |
Riga 2014.
| Methods |
Study design: prospective, randomized, double‐blinded trial Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 114 Inclusion criteria: age > 40, ASA I to III, received general anaesthesia for elective surgery, with written consent Exclusion criteria: neurological, vascular, orthopaedic, or cardiac surgery; known psychiatric disorder or disease of the CNS; history of craniotomy; receiving tranquillisers or antidepressants on a regular basis preoperatively; alcoholism or drug dependence; history of stroke; refusal of patient; inability to read or write; MMSE < 22 preoperatively |
|
| Interventions |
Anaesthesia: induced and maintained with propofol, fentanyl/remifentanil, and sevoflurane NMBA: rocuronium; no information available on dose Comparison: sugammadex vs neostigmine/atropine; no information available on dose Administration time of sugammadex or neostigmine: reappearance of T2 in TOF sequence |
|
| Outcomes | Primary outcome: cognitive function assessed by change in MMSE, clock drawing test, and Isaac's set test, performed preoperatively, 1 hour postoperatively, and at discharge (1 to 15 days postoperatively) | |
| Notes |
Publication type: meeting abstract Country: Greece Conversions: none Sample size calculation: no information available Authors’ conclusions: No significant difference was observed regarding cognitive function after neostigmine/atropine combination or sugammadex was received for reversal of rocuronium‐induced neuromuscular blockade for elective surgery Contact: corresponding trial author Chrysanthi Batistaki contacted by email: chrysabatistaki@yahoo.gr; replied 17.05.2016 * Indicates unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐based randomization" * |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Low risk | Participants were blinded |
| Blinding of personnel (performance bias) | High risk | Other personnel were not blinded |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Low risk | Outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five drop‐outs due to intensive sedation postoperatively and inability to perform the MMSE 1 hour postoperatively * |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT02419352), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | Low risk | Study was not funded * |
| Other bias | Unclear risk | No apparent other type of bias, except no information on sample size calculation |
Sabo 2011.
| Methods |
Study design: multi‐centre, randomized, parallel‐group, safety assessor‐blinded and anaesthesiologist TOF‐watch‐blinded phase 4 study (Lightspeed study) Sample size calculation: based on anticipated difference in time to recovery to TOFR > 0.9, assuming that tracheal extubation would occur 2 to 3 minutes after sugammadex administration and 2 to 12 minutes after neostigmine administration |
|
| Participants |
Number of randomized participants: 106 Inclusion criteria: adults aged ≥ 18 years and ≤ 65 years, ASA class I to III and scheduled to undergo elective open abdominal surgery under general anaesthesia, requiring use of an NMBA, in a position that would not interfere with use of the TOF‐watch SX Exclusion criteria: neuromuscular disorder that complicated NMB assessment; history of malignant hyperthermia; significant renal (creatinine clearance < 30 mL/min) or hepatic dysfunction; allergy to opioids, muscle relaxants, or other medications used during general anaesthesia; pregnant, breastfeeding, or of childbearing potential and not using an adequate method of contraception |
|
| Interventions |
Anaesthesia: induced with intravenous (IV) propofol, opioids, and/or nitrous oxide, and maintained with sevoflurane, IV opioids, and/or nitrous oxide with oxygen NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.15 mg/kg Comparison: sugammadex 4.0 mg/kg (n = 54) vs neostigmine 0.05 mg/kg + glycopyrrolate 0.01 mg/kg (n = 52) Administration time of sugammadex or neostigmine: time when the TOF‐blinded anaesthesiologist considered participant ready for NMB reversal, but could ask the TOF‐watch operator whether the participant recovered to at least PTC 1 to 2 |
|
| Outcomes |
Primary efficacy variable: incidence of residual NMB at time of tracheal extubation Secondary efficacy variables: time from study drug administration to recovery of TOFR to 0.7, 0.8, and 0.9 Safety assessment: all adverse events (AEs), serious adverse events (SAEs), vital signs |
|
| Notes |
Publication type: peer‐reviewed article Country: United States Conversions: none Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: Significantly more sugammadex‐treated participants recovered to a TOFR ≥ 0.9 at extubation and did so significantly faster than neostigmine‐treated participants. This study confirms that sugammadex is more effective than neostigmine in reducing potential for residual blockade in the absence of objective NMB monitoring Contact: corresponding author Daniel Sabo contacted by email: sabodp@anes.upmc.edu on 01.10.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web randomization system prepared centrally by study sponsor, whereby participants were randomly allocated to receive sugammadex or neostigmine in 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Central allocation (secondary to central randomization) |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | High risk | Anaesthesiologists were not blinded to study drug, as they needed to be able to adjust the anaesthetic regimen according to treatment group, but they were blinded to the specific depth of NMB based on TOF‐watch recording at administration of reversal agent and degree of neuromuscular recovery at tracheal extubation |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessor was blinded to treatment group and did not observe preparation of trial medication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients are accounted for, and missing outcome data are balanced in numbers across intervention groups (Figure 1): A total of 106 participants were randomized (54 in the sugammadex group and 52 in the neostigmine group), of whom 100 received treatment (sugammadex n = 51, neostigmine n = 49). Three participants from the sugammadex group were discontinued for the following reasons: pretreatment adverse event n = 1; participant withdrew consent n = 1; and did not fulfil inclusion/exclusion criteria n = 1. Three participants were excluded from the neostigmine group for the following reasons: did not fulfil inclusion/exclusion criteria n = 1; TOF‐watch difficulties n = 1; participant discharged before assessments n = 1. Three participants in the neostigmine group did not undergo efficacy assessments, thus the ITT group comprised 97 participants in total |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published article clearly includes all expected outcomes |
| Funding bias | High risk | Study was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's primary outcome. Participant demographics well balanced between treatment groups |
Schaller 2010.
| Methods |
Study design: single‐centre, randomized, parallel‐group, double‐blinded study (SUNDRO) Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 99 Inclusion criteria: informed written consent, age 18 to 65 years, ASA I to III, scheduled for elective surgery under general anaesthesia with rocuronium for tracheal intubation Exclusion criteria: expected to have a difficult airway; known neuromuscular disease; significant hepatic or renal dysfunction; family history of malignant hyperthermia; known allergy to 1 of the drugs used in this protocol; intake of any medication that might interact with muscle relaxants; pregnant or breastfeeding; participated in another clinical study in the past 30 days |
|
| Interventions |
Anaesthesia: induced with propofol (2 to 3 mg/kg) and fentanyl (0.1 to 0.2 µg/kg), maintained with propofol and remifentanil according to clinical need and anaesthesiologist preference NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.1 to 0.2 mg/kg Comparison: sugammadex 0.0625 mg/kg (n = 9), 0.125 mg/kg (n = 9), 0.25 mg/kg (n = 9), 0.5 mg/kg (n = 9), or 1.0 mg/kg (n = 9); or neostigmine 5 µg/kg (n = 9), 8 µg/kg (n = 9), 15 µg/kg (n = 9), 25 µg/kg (n = 9), or 40 µg/kg (n = 9) in a mixture with 1 µg glycopyrrolate/5 µg neostigmine or saline (n = 9) Administration time of sugammadex, neostigmine, or placebo: TOFR = 0.5 |
|
| Outcomes |
Primary endpoint: dose of sugammadex or neostigmine to accelerate time between start of administration of the respective study drug at a TOFR of 0.5 to a TOFR ≥ 0.9 in an average of 2 minutes, with an upper limit of 5 minutes for 95% of participants Secondary endpoints: doses of sugammadex and neostigmine for slower acceleration of reversal (i.e. average time of 5 minutes with upper limit of 10 minutes for 95% of participants) Safety assessment: adverse events and severe adverse events |
|
| Notes |
Publication type: peer‐reviewed article Country: Germany Conversions: none Handling of adverse events: More detailed information regarding number of adverse events possibly, probably, or definitely related to study drug was provided by trial authors through email correspondence; we used these updated numbers in the review Authors’ conclusions: Sugammadex 0.22 mg/kg can reverse a TOFR of 0.5 to 0.9 or higher in an average time of 2 minutes. Within 5 minutes, 95% of patients reach this TOFR. Neostigmine 34 µg/kg can reverse a TOFR of 0.5 Contact: corresponding trial author Manferd Blobner contacted by email: blobner@lrz.tu‐muenchen.de on 15.03.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Low risk | Participants were blinded |
| Blinding of personnel (performance bias) | Low risk | In the operating room, an additional anaesthesiologist prepared study drug according to participant number on the randomization list in an unlabelled syringe. Upon request of the blinded anaesthesiologist responsible for the participant, study drug was injected |
| Blinding of primary outcome assessment (detection bias) | Low risk | Outcome assessor was blinded |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants are accounted for, but missing outcome data are not balanced in numbers across intervention groups, and it remains unclear whether missing outcome data are due to attrition or exclusion: Study drug was injected in 99 participants. In 5 participants, major protocol violations occurred: in 1 participant, neostigmine was incompletely injected as a result of a leaking venous cannula; and in 4 participants, electromyographic response was unstable (1 each in 5, 8, and 40 µg/kg neostigmine groups; 2 in 0.125 mg/kg sugammadex group). Because these violations might have affected primary and secondary endpoints, respective participant data were omitted, resulting in a per‐protocol population of 94 participants |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT00895609) and EudraCT (2008‐008239‐27); all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | Support was provided solely from institutional and/or departmental sources. Drs. Blobner and Fink have received honoraria and travel grants from Schering‐Plough, Inc. (Kenilworth, New Jersey), although this work was not sponsored by Schering‐Plough in any way |
| Other bias | Unclear risk | No apparent other type of bias, except no information on sample size calculation. Groups did not differ significantly regarding sex, age, weight, height, and ASA physical status |
Sherman 2014.
| Methods |
Study design: prospective, randomized study Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 57 Inclusion criteria: undergoing laparoscopic sleeve gastrectomy Exclusion criteria: no information available |
|
| Interventions |
Anaesthesia: no information available NMBA: no information available Comparison: sugammadex 2 mg/kg (n = 32) vs neostigmine 2.5 mg (n = 25) Administration time of sugammadex or neostigmine: completion of surgery |
|
| Outcomes | Postoperative complications: critical respiratory events, pulmonary complications, minimum SpO2 values in the PACU, airway and pulmonary morbidity, unexpected ICU admission, incidence of reintubation, and duration of hospitalizations | |
| Notes |
Publication type: meeting abstract Country: Israel Conversions: none Authors’ conclusions: Use of sugammadex (compared with neostigmine) as a reversal agent following laparoscopic sleeve gastrectomy surgery was associated with higher postoperative oxygen saturation despite lower TOF count before administration of reversal agent. Lack of differences in other measured variables may stem from the small patient groups studied Contact: first trial author Tiberiu Ezri contacted by email: tezri@netvision.net.il on 26.05.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized study"; no further information available |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess owing to insufficient information |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published meeting abstract clearly includes all expected outcomes |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | Unable to assess owing to insufficient information. Demographic data were similar between groups |
Sustic 2012.
| Methods |
Study design: prospective, randomized clinical study Sample size calculation: no information available |
|
| Participants |
Number of randomized participants: 42 Inclusion criteria: adults undergoing laparoscopic cholecystectomy Exclusion criteria: no information available |
|
| Interventions |
Anaesthesia: no information available NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.15 to 3 mg/kg Comparison: sugammadex 2 mg/kg (n = 21) vs neostigmine 40 µg/kg + atropine group 15 µg/kg (n = 21) Administration time of sugammadex or neostigmine: no information available |
|
| Outcomes | Gastric emptying evaluated by paracetamol absorption test. Paracetamol absorption was assessed from the plasma paracetamol concentration (PPC) | |
| Notes |
Publication type: meeting abstract Country: Croatia Conversions: none Authors’ conclusions: Although study results show a tendency toward faster gastric emptying in the sugammadex group, this difference is not significant in most, possibly owing to small sample size Contact: first author Alan Sustic contacted by email: alan.sustic@uniri.hr on 24.05.2016; replied 25.05 * Indicates unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomization * |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of participants (performance bias) | High risk | Participants not blinded * |
| Blinding of personnel (performance bias) | High risk | Participants not blinded * |
| Blinding of primary outcome assessment (detection bias) | Low risk | Not relevant as TOF measurement not performed in this study |
| Blinding of safety assessment (detection bias) | Low risk | Outcomes assessor was blinded * |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess owing to insufficient information |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published meeting abstract clearly includes all expected outcomes |
| Funding bias | Unclear risk | Unable to assess owing to insufficient information |
| Other bias | Unclear risk | Unable to assess owing to insufficient information |
Tas 2015.
| Methods |
Study design: randomized, prospective study Sample size calculation: performed, but no specific information available |
|
| Participants |
Number of randomized participants: 52 Inclusion criteria: ASA I and II, age 18 to 65 years, scheduled for septoplasty operation Exclusion criteria: taking antiaggregant/anticoagulant treatment, history of bleeding disorder, abnormal complete blood count and coagulation tests (prothrombin time (PT), activated partial thromboplastin time (aPTT), and international normalized ratio (INR)) |
|
| Interventions |
Anaesthesia: induced with propofol 2 to 2.5 mg/kg, fentanyl 0.5 μg/kg, maintained with sevoflurane 2%, remifentanil 0.25 μg/kg/min infusion NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: no information available Comparison: neostigmine 0.05 mg/kg + atropine 0.02 mg/kg (n = 26) vs sugammadex 2 mg/kg (n = 26) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes | Amount of bleeding measured by evaluating the blood leak on the nasal tip dressing over 3 hours postoperatively at 30 minute intervals during first hour, then every hour during the next 2 hours Blood samples were taken 120 minutes after administration of sugammadex or neostigmine for PT (seconds) and aPTT (seconds) measurements Mean arterial pressure (MAP; mmHg), mean heart rate (MHR; beats/min), peripheral oxygen saturation (SpO2; %), and presence of nausea/vomiting (Likert scale) and pain (visual analogue scale) |
|
| Notes |
Publication type: peer‐reviewed article Country: Turkey Conversions: none Authors’ conclusions: Sugammadex was associated with greater postoperative bleeding than neostigmine in patients undergoing septoplasty. For surgical procedures having high risk of bleeding, the safety of sugammadex needs to be verified Contact: first author Nilay Tas contacted by email: drnil.anest@hotmail.com on 26.05.2016; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization sequence was generated by using computer generated random numbers" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed using the previously prepared, sealed opaque envelopes" |
| Blinding of participants (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of primary outcome assessment (detection bias) | Unclear risk | Unable to assess owing to insufficient information |
| Blinding of safety assessment (detection bias) | Low risk | Measurements of quantity of bleeding done by the surgeon without knowledge of which drug was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: 52 envelopes prepared for probable sample loss (26 for neostigmine, 26 for sugammadex). Two participants in Group S were discarded (1 participant did not come to surgery, and for another participant, surgery was postponed because of recent upper respiratory tract infection). So study population included 26 participants in the neostigmine group and 24 in the sugammadex group |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but published article clearly includes all expected outcomes |
| Funding bias | Low risk | Source of support: departmental sources |
| Other bias | Unclear risk | No apparent other type of bias, except no information on sample size calculation. No differences between participant characteristics such as age, gender, surgery duration, and ASA classification |
Woo 2013.
| Methods |
Study design: randomized, parallel‐group, active‐controlled, safety assessor‐blinded, phase 4 study Sample size calculation: powered to detect whether geometric mean recovery time to TOFR > 0.9 with sugammadex is ≥ 5 times faster than geometric mean time with neostigmine, and whether geometric mean recovery time to TOFR > 0.9 with sugammadex is < 3 minutes |
|
| Participants |
Number of randomized participants: 128 Inclusion criteria: ASA I to III, either sex, aged > 18 years, of Korean descent, born in Korea, never having emigrated out of Korea and with a Korean home address, scheduled for elective surgery under general anaesthesia Exclusion criteria: any anatomical malformation that might cause difficult intubation; transferred to the ICU after surgery; neuromuscular disorders that could affect the NMB; significant renal or hepatic dysfunction; requirement of a pneumatic tourniquet during surgery; (family) history of malignant hyperthermia; allergy to opioids/opiates, cyclodextrins including sugammadex, muscle relaxants and their excipients, or other medications used during general anaesthesia; administration of toremifene and/or fusidic acid within 24 hours of study drug administration (or plan to administer these drugs within 24 hours after study drug administration); any condition contraindicating neostigmine and/or glycopyrrolate; pregnant females; participation in a previous sugammadex study; participation in another clinical drug study within 30 days inclusive of signing consent for the current study; or a member of, or related to, the investigational staff or sponsor staff |
|
| Interventions |
Anaesthesia: induced with intravenous propofol and maintained with inhalational sevoflurane. Opioids were administered according to local practice when clinically required NMBA: single intubation dose: rocuronium 0.6 mg/kg; maintenance dose: 0.1 to 0.2 mg/kg rocuronium as clinically required Comparison: sugammadex 2.0 mg/kg (n = 64) vs neostigmine 50 µg/kg plus glycopyrrolate 10 µg/kg (n = 64) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes |
Primary efficacy endpoint: time from start of administration of sugammadex to recovery of TOFR to 0.9 Secondary efficacy endpoints: time to recovery of TOFR to 0.7 and 0.8; time to reappearance of T2 after last dose of rocuronium Safety assessment: adverse events, serious adverse events, vital signs, physical examination findings, clinical evidence of residual NMB and recurrence of NMB |
|
| Notes |
Publication type: peer‐reviewed article Country: 7 sites in the Republic of Korea Conversions: range to SD following guidelines from Hozo 2005 Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors' conclusions: Sugammadex was well tolerated and provided rapid reversal of moderate rocuronium‐induced NMB in Korean patients, with recovery time 8.1 times faster than that of neostigmine. These results are consistent with those reported for Caucasian patients Contact: first trial author Tiffany Woo contacted first time by email: tiffany.woo@merck.com on 22.09.2015; has replied * Indicates unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomized on a 1:1 basis A centralized computer‐generated randomization schedule was used * |
| Allocation concealment (selection bias) | Low risk | Electronic interactive Web‐based system, so randomization codes were located inside the system and could not be accessed until a participant was registered in the system and 1 code was assigned per participant * |
| Blinding of participants (performance bias) | Low risk | Participants were considered to be blinded, as they did not participate in the randomization procedure and were under general anaesthesia * |
| Blinding of personnel (performance bias) | High risk | The anaesthesiologist administering anaesthesia during the surgical procedure was not blinded to the randomized study drug, but was not allowed to reveal the assigned treatment group to the safety assessor |
| Blinding of primary outcome assessment (detection bias) | High risk | TOF‐watch assessor was not blinded * |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessors were blinded to the treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: 128 participants randomized, all‐subjects treated population: 120 (n = 60 in each group), intention to treat population: 118 (n = 59 in each group), per‐protocol population: 116 (n = 59 in the sugammadex group, n = 57 in the neostigmine group). Two participants had major protocol violations (received neostigmine more than 2 minutes after reappearance of T2). All efficacy data for these participants were excluded from the per‐protocol analysis set. Imputed data in both groups were due to loss of calibration of TOF watch during the course of the trial and inability to recalibrate the TOF watch to collect efficacy data * |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT01050543), and all of the study's prespecified primary and secondary outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. Disclosures: Tiffany Woo and Phillip Phiri are employees of Merck Sharp & Dohme Corp., Whitehouse Station, NJ |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's primary outcome. Participant demographics well balanced between treatment groups; types of elective surgical procedures performed are comparable |
Wu 2014.
| Methods |
Study design: randomized, parallel‐group, multi‐centre, safety assessor‐blinded study Sample size calculation: powered to demonstrate that recovery of the TOFR to 0.9 after sugammadex 2 mg/kg is ≥ 2 times faster than after neostigmine 50 μg/kg |
|
| Participants |
Number of randomized participants: Chinese 247, Caucasian 61, all in all 308 Inclusion criteria: age 18 to 64 years, ASA I to III, scheduled for elective surgery under general anaesthesia, allowing stable neuromuscular monitoring, which requires neuromuscular blockade using rocuronium; compliant with dose/visit schedules, and used an accepted method of contraception (if applicable). Chinese participants had to be born in China, to have never emigrated out of China, and to have a Chinese home address. Similarly, Caucasian participants had to be born in Europe, to have never emigrated out of Europe, and to have a European home address Exclusion criteria: anatomical malformations expected to lead to difficult tracheal intubation; neuromuscular disorders affecting NMB; significant renal/hepatic dysfunction (as determined by the investigator); (family) history of malignant hyperthermia; allergy to general anaesthesia medications; contraindication to study drugs; or clinically significant condition that may interfere with the trial (as determined by the investigator) |
|
| Interventions |
Anaesthesia: Anaesthesia was induced and maintained with IV propofol according to clinical needs of the participant. Opioids could be administered according to local practice NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.1 to 0.2 mg/kg Comparison: sugammadex 2 mg/kg (Chinese n = 126, Caucasian n = 29) vs neostigmine 50 μg/kg plus atropine 10 to 20 μg/kg (Chinese n = 121, Caucasian n = 32) Administration time of sugammadex or neostigmine: reappearance of T2 |
|
| Outcomes |
Primary efficacy variable: time from start of administration of sugammadex or neostigmine/atropine
to recovery of TOFR to 0.9 Secondary efficacy variable: time to recovery of the TOFR to 0.7 and 0.8 Safety assessments: adverse events, serious adverse events, vital signs, and physical examination findings |
|
| Notes |
Publication type: peer‐reviewed article Country: 6 sites in China and 4 sites in Europe (2 sites in Denmark and 1 site each in Belgium and Norway) Conversions: Median + Range to Mean + SD following guidelines from Hozo 2005 Handling of adverse events: More detailed information regarding number of adverse events possibly, probably, or definitely related to study drug was provided by the authors through email correspondence, and we used these updated numbers in the review Authors’ conclusions: Both Chinese and Caucasian participants recovered from NMB significantly faster after sugammadex 2 mg/kg than after neostigmine 50 μg/kg, with recovery that was ˜ 5.7 times (P < 0.0001) faster with sugammadex than with neostigmine in Chinese participants. Sugammadex was generally well tolerated Contact: first trial author Xinmin Wu contacted by email: xmwu2784@hotmail.com on 15.04.2016; no reply received; email sent to last author Woo 15.05.2016; replied 21.07.2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomized via a central randomization system. Sponsor produced a computer‐generated randomization schedule with treatment codes in blocks, using a validated SAS‐based application. The schedule associated each treatment code with a participant number, and participants were randomized in a 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Central allocation (secondary to central randomization) |
| Blinding of participants (performance bias) | Low risk | Participants were blinded * |
| Blinding of personnel (performance bias) | High risk | Personnel in the OR were not blinded |
| Blinding of primary outcome assessment (detection bias) | High risk | TOF‐watch assessor was not blinded |
| Blinding of safety assessment (detection bias) | Low risk | Safety assessments were performed by a safety assessor who was blinded to the treatment administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: Of 247 randomized Chinese participants, 16 discontinued the study, and one who completed the study had missing efficacy data. Hence, 231 Chinese participants received study treatment and were included in the safety analysis (AST group), and 230 Chinese subjects with evaluable data were included in the efficacy analysis (full analysis set; sugammadex n = 119, neostigmine n = 111). In total, 61 Caucasian participants were randomized, 60 of whom received treatment (AST group) and 59 who had evaluable data (full analysis set; sugammadex n = 29, neostigmine n = 30) |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT00825812), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way |
| Funding bias | High risk | Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA,. provided financial support to the study. Medical writing support was provided by Melanie More of Prime Medica Ltd., Knutsford, Cheshire, UK. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. HR is an employee of MSD, Oss, The Netherlands, and TW is an employee of Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA, both of whom may own stock and/or hold stock options in the Company. EA was formerly an employee of MSD, Oss, The Netherlands. XW, SY, JL, BV, LX, CC, VD, YY, HO, and YH work for institutions that received research funding from Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA. BV and VD have also received research funding from Merck & Co., Inc. for previous studies |
| Other bias | Low risk | Appears free of other sources of bias. Study sample size calculation designed to address this review's primary outcome. Baseline characteristics comparable within participant groups |
Yagan 2015.
| Methods |
Study design: single‐blind, prospective, randomized, controlled study Sample size calculation: powered to detect 3 mmHg change in intraocular pressure |
|
| Participants |
Number of randomized participants: 36 Inclusion criteria: ASA I to II, between 18 and 65 years of age, scheduled to have general anaesthesia with endotracheal intubation for elective surgery, written informed consent Exclusion criteria: undergoing laparoscopic surgery, ophthalmic surgery, predicted difficult tracheal intubation (Mallampati III/IV); history of glaucoma, uncontrolled hypertension, and cardiovascular disease; body mass index (BMI) > 30 kg/m2; increased intracranial pressure; using drugs affecting IOP; surgical positions except supine position |
|
| Interventions |
Anaesthesia: induced by fentanyl 1 µg/kg and propofol 2.5 mg/kg. Maintained with 2% sevoflurane in 50% O2/air mixture and 0.2 to 0.7 µg/kg/min remifentanil infusion NMBA: single intubating dose: rocuronium 0.6 mg/kg; maintenance dose: rocuronium 0.1 to 0.2 mg/kg Comparison: sugammadex 2 mg/kg (n = 18) or neostigmine 0.05 mg/kg + atropine 0.02 mg/kg (n = 18) Administration time of sugammadex or neostigmine: TOF response T4/T1 at 20% |
|
| Outcomes | Heart rate (HR), mean arterial pressure (MAP), and intraocular pressure (IOP) were measured as baseline before the induction (T1), after application of reversal agent (T2), and at 1 (T3), 3 (T4), 5 (T5). and 10 (T6) minutes after extubation. Extubation time (time to TOFR of 90% after administration of reversal agent), amount of rocuronium and remifentanil consumption during surgery, and type and duration of surgery were recorded. Complications after surgery such as nausea, vomiting, and shivering | |
| Notes |
Publication type: peer‐reviewed article Country: Turkey Conversions: Median + Range to Mean + SD following guidelines from Hozo 2005 Handling of adverse events: No discrepancy exists between AEs presented in the original article and those reported in this review Authors’ conclusions: Lower end‐extubation intraocular pressure levels were obtained when sugammadex was used as a neuromuscular block reversal agent in comparison with the neostigmine‐atropine combination. Sugammadex may be a better option for reversal of neuromuscular blockade, and intraocular pressure increase should be avoided in patients with glaucoma or penetrating eye injury Contact: first trial author Ozgur Yagan contacted by email: ozguryagan@hotmail.com on 15.05.2016; replied 18.05.2016 * Indicates unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated sequence of numbers and an opaque * sealed envelope technique, participants were randomly divided into 2 groups |
| Allocation concealment (selection bias) | Low risk | Using a computer‐generated sequence of numbers and an opaque * sealed envelope technique, participants were randomly divided into 2 groups |
| Blinding of participants (performance bias) | High risk | Participants not blinded * |
| Blinding of personnel (performance bias) | High risk | Personnel not blinded * |
| Blinding of primary outcome assessment (detection bias) | High risk | TOF‐watch assessor not blinded * |
| Blinding of safety assessment (detection bias) | Low risk | IOP measuring researcher and assessor of the quality of extubation were blinded * |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, and missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups: Of 49 patients approached, 13 had to be excluded (BMI > 30 kg/m2 n = 6; uncontrolled hypertension n = 4; ASA III and above, n = 3) and 36 represented the final sample, which was randomly divided into 2 groups of 18 each |
| Selective reporting (reporting bias) | Low risk | Study protocol is available on clinicaltrials.gov (NCT02215382), and all of the study’s prespecified outcomes of interest to the review have been reported in the prespecified way * |
| Funding bias | Low risk | Study funded by trial authors themselves * |
| Other bias | Low risk | Study sample size calculation designed to address this review's secondary outcome. No significant differences between groups regarding age, gender, BMI, and ASA scores |
List of acronyms and abbreviations used in these tables
AE ‐ adverse events; aPTT ‐ activated partial thromboplastin time; ASA ‐ American Society of Anesthesiologists; AST ‐ aspartate aminotransferase; BIS ‐ Bispectral index; BMI ‐ body mass index; BP ‐ blood pressure; BUN ‐ blood urea nitrogen; C ‐ clearance; CBW ‐ corrected body weight; CNS ‐ central nervous system; COPD ‐ chronic obstructive pulmonary disease; Cr ‐ creatinine; Cys ‐ cysteine; ECG ‐ electrocardiography; FOC ‐ free of charge; FT3 ‐ free triiodothyronine; FT4 ‐ free thyroxine; Hg ‐ haemoglobin; HR ‐ heart rate; IBW ‐ ideal body weight; ICU ‐ intensive care unit; INR ‐ international normalized ratio; IOP ‐ intraocular pressure; ITT ‐ intention to treat; IV ‐ intravenous; LBS ‐ Laparoscopic Bariatric Surgery; LBW ‐ lean body weight; MAC ‐ minimal alveolar concentration; MAP ‐ mean arterial blood pressure; MBP ‐ mean blood pressure; MG ‐ myasthenia gravis; Mg ‐ magnesium; MHR ‐ mean heart rate; min ‐ minimum; MMSE ‐ Mini‐Mental State Examination; MO ‐ morbidly obese; NM ‐ neuromuscular; NMB ‐ neuromuscular blockade; NMBA ‐ neuromuscular blocking agents; NMT ‐ neuromuscular technique; NYHA ‐ New York Heart Association; PaCO2 ‐ partial pressure of carbon dioxide; PACU ‐ post‐anaesthesia care unit; PONV ‐ postoperative nausea and vomiting; PORC ‐ postoperative residual curarization; PPC ‐ plasma paracetamol concentration; PRSES ‐ postoperative respiratory system evaluation score; PT ‐ prothrombin time; PTC ‐ post‐tetanic count; QTc ‐ QTc interval; RPONB ‐ residual postoperative neuromuscular blockade; SAE ‐ serious adverse event; SAS ‐ SAS institute; SD ‐ standard deviation; SEVO ‐ sevoflurane; SO ‐ super obese; SpO2 ‐ peripheral oxygen saturation; SRS ‐ surgical rating scale; SX ‐ symptoms; T2 ‐ second twitch; TOF ‐ train of four; TOFR ‐ train‐of‐four ratio; TSH ‐ thyroid‐stimulating hormone; XLIF ‐ extreme lateral interbody fusion
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aho 2012 | Study outcomes not of interest to our review RCT investigating elevated BIS and entropy values after reversal with sugammadex 200 mg vs neostigmine 2.5 mg following rocuronium 0.6 mg kg‐1 |
| Baysal 2013 | Study outcomes not of interest to our review RCT investigating use of sugammadex 1 mg kg‐1 for reversal of residual blockade after administration of neostigmine 0.07 mg kg‐1 and atropine 0.02 mg kg‐1 |
| Dahaba 2012 | Study outcomes not of interest to our review RCT investigating effects of sugammadex 4 mg kg‐1 vs neostigmine 0.05 mg kg‐1/glycopyrrolate 0.01 mg kg‐1 neuromuscular block reversal on bispectral index monitoring |
| Gaona 2012 | Study included 30 paediatric patients, aged 2 to 11 years RCT comparing efficacy and safety of reversal with sugammadex 4 mg kg‐1 vs neostigmine 0.05 mg kg‐1/atropine 0.025 mg kg‐1 in paediatric patients with deep blockade induced by rocuronium 0.6 mg kg‐1 |
| Ghoneim 2015 | RCT investigating use of sugammadex and neostigmine for reversing profound NMB in paediatric neurosurgical patients who have undergone posterior fossa tumour excision |
| Harazim 2014 | Same meeting abstract data later published in peer‐reviewed article (Stourac 2016) |
| Kakinuma 2013 | Study comparison not relevant to our review. RCT comparing sugammadex 1 mg/kg vs sugammadex 0.5 mg/kg + neostigmine 0.04 mg/kg, examining the cost of reversal and recovery time |
| Kara 2014 | RCT in paediatric population, comparing efficacy of sugammadex and neostigmine for reversing NMB in 80 paediatric patients, aged 2 to 12 years, undergoing outpatient surgical procedures |
| Kzlay 2013 | Same meeting abstract data later published in peer‐reviewed article (Kizilay 2016) |
| Nagashima 2016 | Study outcomes not of interest to our review Effects of neostigmine and sugammadex on QT interval and QT dispersion Participants received a combination of neostigmine and atropine or sugammadex (2 mg/kg) for reversal of neuromuscular blockade |
| Nagy 2014 | Study retracted owing to changes in protocol made by trial authors, after the protocol was submitted to the Ethics Comittee of the Department of Anesthesiology, Faculty of Medicine, Cairo University, Egypt |
| NCT03111121 | Study outcomes not of interest to our review Trial examines use of sugammadex for reversal of paralysis in microlaryngoscopy |
| Nemes 2016 | Not an RCT. Trial is a prospective, partially randomised, placebo‐controlled, double‐blind, four‐group parallel‐arm study. Participants received nothing (recover spontaneously), sugammadex, neostigmine, or placebo at the preference of each anaesthesiologist |
| Ozgun 2014 | Study included paediatric patients and compared clinical effects of sugammadex vs combination of anticholinergic‐anticholinesterase agents for reversing of non‐depolarizing neuromuscular block |
| Pecek 2013 | Prospective, observational study. Participants received sugammadex or neostigmine at the preference of each anaesthesiologist |
| Sacan 2007 | Not a truly randomized process, as participants could choose to not be included in the sugammadex group |
| Schepens 2015 | Study included healthy volunteers and compared electromyographic activity of the diaphragm (EMGdi) during recovery from neuromuscular blockade using neostigmine and sugammadex |
| Stourac 2016 | Study comparison not relevant to our review RCT comparing muscle relaxation induced with rocuronium 1 mg/kg, reversal with sugammadex 2 to 4 mg/kg with succinylcholine 1 mg/kg for induction, rocuronium 0.3 mg/kg for maintenance, and neostigmine 0.03 mg/kg for reversal of neuromuscular blockade |
| Veiga Ruiz 2011 | Study performed on 24 paediatric patients, aged 2 to 9 Aim of the RCT was to compare the efficacy and security of sugammadex 2 mg kg‐1 vs neostigmine 0.05 mg kg‐1/atropine 0.025 mg kg‐1 in reversing moderate blockade with rocuronium 0.45 mg kg‐1 |
List of acronyms and abbreviations used in these tables
BIS ‐ Bispectral Index; EMGd ‐ diaphragmatic electromyogram; NMB ‐ neuromuscular blockade; RCT ‐ randomized controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Kim 2016.
| Methods |
Allocation: computer‐generated randomization Intervention model: parallel assignment Masking: state blinded, no additional details Primary purpose: treatment |
| Participants |
Enrolment: 80 adult patients Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Control group: neostigmine 50 mg/kg with glycopyrrolate 10 mg/kg after operation Intervention group: sugammadex 2.0 mg/kg after operation |
| Outcomes | Primary objective was to determine recovery time and response after sugammadex or neostigmine administration of first twitch (T1) and train‐of‐four ratio (TOFR) to 0.9 during rocuronium‐induced moderate neuromuscular blockade |
| Notes | This study has been completed. Data will be published in the next updated version of this review |
NCT02243943.
| Methods |
Allocation: randomized Intervention model: parallel assignment Masking: double‐blind (participant, investigator) Primary purpose: treatment |
| Participants | Enrolment: 100 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sugammadex 2 to 4 mg/kg Neostigmine 1.0 to 2.5 mg and atropine 0.5 to 1.0 mg |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Notes | This study has been completed. Based on personal correspondence with the last trial author, we became aware of preliminary results, published as a letter (September 2016), after our last search (2 May 2016). These data will be published in the next updated version of this review |
Sen 2016.
| Methods |
Allocation: randomized Intervention model: parallel assignment Masking: double‐blind (participant, investigator) Primary purpose: safety/efficacy study |
| Participants | 72 patients with American Society of Anesthesiologists (ASA) physical status I or II, scheduled for total thyroid surgery Inclusion criteria:
Exclusion criteria:
|
| Interventions | When 4 twitches were observed on train‐of‐four stimulation, neuromuscular block was reversed conservatively in the control group (neostigmine 0.04 mg/kg + atropine 0.015 mg/kg) and with sugammadex (sugammadex 2 mg/kg) in the study group |
| Outcomes |
Primary outcome measures: Median time of first flatus Secondary outcome measures: Occurrences of nausea, vomiting, diarrhoea, or constipation. |
| Notes | This study has been completed. These data will be published in the next updated version of this review |
Characteristics of ongoing studies [ordered by study ID]
NCT01539044.
| Trial name or title | Optimal relaxation technique for laparotomies with rocuronium infusion followed by sugammadex reversal (ProjectO5Rs) |
| Methods |
Allocation: randomized Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, caregiver, outcomes assessor) Primary purpose: treatment |
| Participants |
Enrollment: 49 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Active comparator: IB‐neostigmine Participant will be given intermittent bolus of rocuronium during surgery and reversal of neostigmine at completion of surgery at TOFR 2 Experimental: CI‐sugammadex Participant will be given continuous infusion of rocuronium and reversal of sugammadex at completion of surgery at PTC 1 to 2 |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | February 2012 |
| Contact information | Principal investigator: Dr. Maria HS lee, MMed(Anaes), Clinical Research Centre, Johor, Malaysia |
| Notes | This study has been completed. No study data have been published to the best of our knowledge |
NCT01748643.
| Trial name or title | CURES: The effect of deep curarization and reversal with sugammadex on surgical conditions and perioperative morbidity (CURES) |
| Methods |
Allocation: randomized Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, investigator, outcomes assessor) Primary purpose: supportive care |
| Participants |
Enrolment: 60 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Drug: deep neuromuscular blockade with rocuronium, reversal with sugammadex after induction of anaesthesia, a rocuronium infusion (0.6 mg/kg (lean body mass)/h) is started and titrated to a post‐tetanic count of 1 to 2 twitches. At completion of surgery, neuromuscular blockade will be reversed with sugammadex 4 mg/kg. Participants are extubated when TOFR > 0.9 Drug: normal neuromuscular blockade reversal with rocuronium, reversal with neostigmine After induction of anaesthesia, top‐ups of rocuronium (10 mg) are given as needed to maintain a TOF count of 1 to 2. At completion of surgery, neuromuscular blockade will be reversed with neostigmine 50 μg/kg and glycopyrrolate 10 μg/kg (lean body mass). Patients are extubated when TOFR > 0.9 |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | April 2013 |
| Contact information | Pascal Vanelderen, MD, Principal Investigator, Ziekenhuis Oost‐Limburg |
| Notes | This study has been completed. No data have yet been published to the best of our knowledge |
NCT02160223.
| Trial name or title | Sugammadex compared with neostigmine/atropine for neuromuscular block reversal in patients with obstructive sleep apnoea |
| Methods |
Allocation: randomized Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, outcomes assessor) Primary purpose: treatment |
| Participants |
Inclusion criteria: ASA I to III scheduled for surgery for obstructive sleep apnoea Exclusion criteria: Neuromuscular disorders, hepatic or renal dysfunction, allergy to study drugs, using medication that could interfere with NMBAs, pregnancy or breastfeeding |
| Interventions | Group S participants will receive 2 mg kg‐1 sugammadex at completion of surgery Group N participants will receive 50 µg kg‐1 neostigmine and 0.5 mg atropine at completion of surgery |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
Other outcome measures:
|
| Starting date | January 2012 |
| Contact information | Principal Investigator: Dilek Yazicioglu, Dişkapı Yildirim Beyazit Teaching and Research Hospital |
| Notes | This study has been completed. No study results have been published yet to the best of our knowledge |
NCT02256280.
| Trial name or title | A randomized double blind controlled trial comparing sugammadex and neostigmine after thoracic anaesthesia (DATA) |
| Methods |
Allocation: randomized Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, caregiver, investigator) |
| Participants |
Estimated enrolment: 266 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sugammadex 2 or 4 mg/kg IV once at completion of surgery Neostigmine 0.05 or 0.07 mg/kg (+ atropine 0.02 mg/kg) IV once at completion of surgery |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
Other outcome measures:
|
| Starting date | January 2015 |
| Contact information | Contact: Federico Piccioni, MD, +39223902282: federico.piccioni@istitutotumori.mi.it Fondazione IRCCS Istituto Nazionale dei Tumori, Milano |
| Notes | This study is currently recruiting participants. Estimated Study Completion Date: July 2017 |
NCT02330172.
| Trial name or title | Sugammadex provides better surgical condition compared with neostigmine in laryngeal microsurgery |
| Methods |
Allocation: randomized Endpoint classification: efficacy study Intervention model: parallel assignment Masking: single‐blind (outcomes assessor) Primary purpose: treatment |
| Participants |
Estimated enrolment: 80 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Active comparator: rocuronium 0.45 ‐ neostigmine when anaesthetic induction in rocuronium 0.45 mg/kg will be administered for muscle relaxation At completion of operation, injection of neostigmine or sugammadex will be administered Active comparator: rocuronium 0.9 ‐ sugammadex When anaesthetic induction, rocuronium 0.9 mg/kg will be injected to rocuronium 0.9 ‐ sugammadex group for muscle relaxation At completion of operation, an injection of neostigmine or sugammadex will be administered |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | December 2014 |
| Contact information | Principal Investigator: Ah Young Oh, Seoul National University Hospital |
| Notes | Study completion date: August 2016. This study has been completed. No study results have been published yet to the best of our knowledge |
NCT02361060.
| Trial name or title | Effects of neuromuscular block reversal with sugammadex vs neostigmine on postoperative respiratory outcomes after major abdominal surgery |
| Methods |
Allocation: randomized Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants |
Estimated enrolment: 130 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sugammadex 4 mg/kg Neostigmine 40 µg/kg in combination with atropine 10 µg/kg. |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | February 2015 |
| Contact information | Anesthesiology Service. Hospital Universitario La Princesa Enrique EAM Alday, MD +34 91 5202476; kikealday@hotmail.com Principal Investigator: Enrique EAM Alday, MD Sub‐Investigator: Antonio APR Planas, MD Sub‐Investigator: Manuel MMM Muñoz, MD Sub‐Investigator: Esperanza EML Mata, MD Sub‐Investigator: Carlos CAZ Álvarez, MD |
| Notes | This study is currently recruiting participants. Estimated Study Completion Date: December 2016 |
NCT02414880.
| Trial name or title | Sugammadex versus neostigmine in patients with liver cirrhosis undergoing liver resection |
| Methods |
Allocation: randomized Endpoint classification: pharmacodynamics study Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants |
Estimated enrolment: 60 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sugammadex 2 mg/kg ‐ normal liver Neostigmine 50 micrograms/kg combined with atropine 20 micrograms/kg ‐ normal liver Sugammadex 2 mg/kg ‐ liver cirrhosis Neostigmine 50 micro‐grams/kg combined with atropine 20 micro‐grams/kg ‐ liver cirrhosis |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | December 2014 |
| Contact information | Mohamed Abdulatif Mohamed, MD, Cairo University |
| Notes | This study has been completed. No study results have been published yet to the best of our knowledge |
NCT02454504.
| Trial name or title | Quality of awakening and impact on cognitive function after administration of sugammadex in robotic radical cystectomy |
| Methods |
Allocation: randomized Intervention model: parallel assignment Masking: open‐label |
| Participants |
Estimated enrolment: 60 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sugammadex at completion of surgery Neostigmine at completion of surgery |
| Outcomes |
Primary outcome measures:
|
| Starting date | March 2014 |
| Contact information | Ester Forastiere, Dr, 0039 06 52662995: forastiere@ifo.it Regina Elena Cancer Institute Rome, RM, Italy, 00144 Sub‐Investigator: Claudia Claroni, MD |
| Notes | This study is currently recruiting participants. Estimated Primary Completion Date: December 2016 |
NCT02648503.
| Trial name or title | Deep neuromuscular block and sugammadex versus standard of care on quality of recovery in patient undergoing elective laparoscopic cholecystectomy |
| Methods |
Allocation: randomized Endpoint classification: efficacy study Intervention model: parallel assignment Masking: single‐blind (outcomes assessor) Primary purpose: treatment |
| Participants |
Estimated enrolment: 120 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Deep neuromuscular block using rocuronium and reversal with sugammadex Moderate neuromuscular block using rocuronium and reversal with neostigmine (1 to 2 mg) and atropine (0.5 to 1 mg) |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
Other outcome measures:
|
| Starting date | March 2016 |
| Contact information | Vu TN Phan, PhD. MD +84‐908883458: vuphan682003@yahoo.com Ho Chi Minh City University of Medicine and Pharmacy |
| Notes | This study is not yet open for participant recruitment |
NCT02666014.
| Trial name or title | Sugammadex versus neostigmine for postoperative nausea and vomiting after laparoscopic gynaecological surgery |
| Methods |
Allocation: randomized Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, investigator) Primary purpose: prevention |
| Participants |
Estimated enrolment: 300 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sugammadex 2 mg/kg, to be given as a single dose via intravenous injection upon completion of surgery and guided by peripheral nerve stimulation Neostigmine 0.040 mg/kg, along with atropine 0.015 mg/kg, diluted in normal saline to make up 5 mL total volume to maintain blinding |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | June 2015 |
| Contact information | KK Women's and Children's Hospital, Singapore, Jing Wen Ng: Ng.Jing.Wen@kkh.com.sg Principal Investigator: Deepak Mathur Sub‐Investigator: Ban Leong Sng |
| Notes | Currently recruiting participants. Estimated Study Completion Date: July 2017 |
NCT02697929.
| Trial name or title | Sugammadex/neostigmine and liver transplantation |
| Methods |
Allocation: randomized Intervention model: parallel assignment Masking: no masking Primary purpose: prevention |
| Participants |
Estimated enrolment: 40 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Control group: Neostigmine at completion of surgery: administration of 50 mcg/kg of neostigmine after third T2 twitch at TOF stimulation Intervention group: Sugammadex: at completion of surgery; administration of 2 mg/kg of sugammadex after third T2 twitch at TOF stimulation |
| Outcomes |
Primary outcome measures: Recovery time from moderate neuromuscular block to TOFR > 0.9 after administration of sugammadex or neostigmine [Time Frame: 30 minutes] Secondary outcome measures: TOFR < 0.9 within 20 minutes after extubation [Time Frame: 20 minutes] |
| Starting date | January 2014 |
| Contact information | Azienda Ospedaliera S. Maria della Misericordia, Udine, Italy, Principal Investigator: Livia Pompei; livia.pompei@uniud.it Study Director: Giorgio Della Rocca; giorgio.dellarocca@uniud.it |
| Notes | Currently recruiting participants. Estimated Study Completion Date: March 2017 |
NCT02698969.
| Trial name or title | Recovery of muscle function after deep neuromuscular block by means of diaphragm ultrasonography |
| Methods |
Allocation: randomized Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, caregiver) Primary purpose: treatment |
| Participants |
Estimated enrolment: 58 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sugammadex 2 mg*kg‐1 at completion of surgery Neostigmine 50 mcg*kg‐1 and atropine 15 mcg*kg‐1 at completion of surgery |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | November 2014 |
| Contact information | Chiara Adembri, MD, +390554271101: chiara.adembri@unifi.it Azienda Ospdaliero Universitaria Careggi Principal Investigator: Chiara Adembri, MD Sub‐Investigator: Iacopo Cappellini, MD Sub‐Investigator: Daniele Ostento, MD Sub‐Investigator: Fabio Picciafuochi, MD |
| Notes | This study is currently recruiting participants. Estimated Study Completion Date: July 2017 |
NCT02845375.
| Trial name or title | Effect of neuromuscular blockade and reversal on breathing (BREATH) |
| Methods |
Allocation: randomized Endpoint classification: pharmacodynamics study Intervention model: parallel assignment Masking: double‐blind (participant, investigator, outcomes assessor) Primary purpose: treatment |
| Participants |
Estimated enrolment: 30 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Placebo will be administered following a period of muscle relaxation after which respiratory measurements will be obtained Neostigmine will be administered following a period of muscle relaxation after which respiratory measurements will be obtained Sugammadex will be administered following a period of muscle relaxation after which respiratory measurements will be obtained |
| Outcomes |
Primary outcome measures:
|
| Starting date | September 2016 |
| Contact information | Leiden University Medical Center, Leiden, ZH, Netherlands, 2333 ZA |
| Notes | This study is not yet open for participant recruitment |
NCT02860507.
| Trial name or title | Study to determine if administration of sugammadex impacts hospital efficiency |
| Methods |
Allocation: randomized Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, caregiver, investigator, outcomes assessor) Primary purpose: treatment |
| Participants |
Estimated enrolment: 50 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Neostigmine 0.06 mg/kg and glycopyrrolate 0.04 mg/kg IV Sugammadex 4 mg/kg |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | August 2016 |
| Contact information | Enrico Camporesi, Attending Anesthesiologist & Director of Research, SE, University of South Florida |
| Notes | This study is enrolling participants by invitation only. Estimated Primary Completion Date: May 2017 |
NCT02861131.
| Trial name or title | The effect of sugammadex versus neostigmine on postoperative pulmonary complications |
| Methods |
Allocation: randomized Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: prevention |
| Participants |
Estimated enrolment: 200 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sugammadex 2 mg/kg IV once at completion of surgery Neostigmine 0.07 mg/kg to maximum of 5 mg (+ glycopyrrolate 0.1 to 0.2 mg per 1 mg of neostigmine administered) IV once at completion of surgery |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
Other outcome measures:
|
| Starting date | Study Start Date: November 2016 Estimated Study Completion Date: May 2018 |
| Contact information | Contact: Miriam Treggiari, MD, PhD, MPH; 503‐494‐7229 treggiar@ohsu.edu Contact: Nabil J Alkayed, MD; 503‐494‐7229 alkayedn@ohsu.edu Principal Investigator: Brandon M Togioka, MD Sub‐Investigator: Michael Aziz, MD Sub‐Investigator: Miriam Treggiari, MD, PhD, MPH Oregon Health and Science University |
| Notes | This study is not yet open for participant recruitment |
NCT02909439.
| Trial name or title | Quality of recovery after reversal with neostigmine or sugammadex |
| Methods |
Allocation: randomized Intervention model: parallel assignment Masking: outcomes assessor Primary purpose: treatment |
| Participants |
Estimated enrolment: 80 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Control group: Participants in this arm will receive neostigmine for reversal of neuromuscular blockade. No further details Intervention group: Participants in this arm will receive sugammadex for reversal of neuromuscular blockade. No further details. |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | November 2016 |
| Contact information | Stony Brook University Hospital,Stony Brook, New York, United States Contact: Sabeen Rizwan: sabeen.rizwan@stonybrookmedicine.edu Principal Investigator: Ramon Abola |
| Notes | Currently recruiting participants. Estimated Study Completion Date: September 2017 |
NCT02939430.
| Trial name or title | Sugammadex reversal of neuromuscular blockade and postoperative bleeding (Suga_bleeding) |
| Methods |
Allocation: randomized Intervention model: parallel assignment Masking: participant, investigator Primary purpose: diagnostic |
| Participants |
Estimated enrolment: 40 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Control group: Reversal of neuromuscular blockade will be performed using classic drugs (neostigmine 80 mg/kg and atropine 40 mic/kg) Intervention group: Reversal of neuromuscular blockade will be performed using sugammadex 2 mg/kg |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | November 2016 |
| Contact information | Mansoura University, Mansoura, Dkahleya, Egypt Contact: Alreafey Kandeel: refa3ey2@yahoo.com Contact: Amr M Yassen: amryassen@hotmail.com |
| Notes | Currently recruiting participants. Estimated Study Completion Date: October 2017 |
NCT03108989.
| Trial name or title | Comparison of the postoperative quality of recovery between neostigmine and sugammadex in elderly patients undergoing trans pars plana vitrectomy with general anesthesia |
| Methods |
Allocation: randomized Intervention model: parallel assignment Masking: participant, outcomes assessor Primary purpose: prevention |
| Participants |
Estimated enrolment: 40 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Control group: After completion of surgery, neostigmine will be administered to reverse neuromuscular blockade. Intervention group: After completion of surgery, sugammadex 2 mg/kg will be administered to reverse neuromuscular blockade. |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 8 February 2017 |
| Contact information | Republic of Korea, Seoul, Yonsei University, Department of Anesthesiology and Pain Medicine. Contact: Sun Joon Bai: SJBAE@yuhs.ac |
| Notes | Currently recruiting participants. Estimated Study Completion Date: 31 October 2017 |
NCT03116997.
| Trial name or title | Study of recovery of strength after surgery comparing two different medications for reversal of muscle relaxant |
| Methods |
Allocation: randomized Intervention model: parallel assignment Masking: participant, outcomes assessor Primary purpose: treatment |
| Participants |
Estimated enrolment: 202 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Control group: At conclusion of surgery, neuromuscular blockade reversed with neostigmine/glycopyrrolate Intervention group: At conclusion of surgery, neuromuscular blockade reversed with sugammadex |
| Outcomes |
Primary outcome measures:
|
| Starting date | 7 April 2017 |
| Contact information | United States, New Jersey Memoral Sloan Kettering Basking Ridge, Basking Ridge, New Jersey, United States United States, New York Memorial Sloan Kettering Cancer Center, Commack, New York, United StatesMemoral Sloan Kettering Westchester, Harrison, New York, United States Memorial Sloan Kettering Cancer Center, New York, New York, United States Contact: German Echeverry: echeverg@mskcc.org |
| Notes | Currently recruiting participants. Estimated Study Completion Date: April 2019 |
NCT03144453.
| Trial name or title | Recovery from anesthesia after robotic assisted radical cystectomy |
| Methods |
Allocation: randomized Intervention model: parallel assignment Masking: participant, care provider, investigator, outcomes assessor Primary purpose: treatment |
| Participants |
Estimated enrolment: 50 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Control group: Participants received neostigmine + atropine as neuromuscular blockade reversal Interventions group: Participants received sugammadex as neuromuscular blockade reversal |
| Outcomes |
Primary outcome measures:
|
| Starting date | 2 May 2017 |
| Contact information | Ester Forastierem Rome, Italy Contact: Ester Forastiere: ester.forastiere@ifo.gov.it Regina Elena Cancer Institute, Rome,Italy Contact: Forastiere Ester: forastiere@ifo.it Sub‐Investigator: Claudia Claroni |
| Notes | Currently recruiting participants. Estimated Study Completion Date: November 2017 |
List of acronyms and abbreviations used in these tables
ASA ‐ American Society of Anesthesiology; BMI ‐ body mass index; CI ‐ confidence interval; CNS ‐ central nervous system; COPD ‐ chronic obstructive pulmonary disease; CrCL ‐ creatinine clearance; DEF – dynamic end‐tidal forcing; DL – diffusion lung capacity; DLCO/VA – diffusion lung capacity for carbon monoxide/alveolar volume ratio; dNMB – deep neuromuscular blockage; eGFR ‐ estimated glomerular filtration rate;
ENT – ear, nose, and throat; FEV1 – forced expiratory volume in one second; FiO2 ‐ fraction of inspired oxygen; FVC – functional vital capacity; GCS – Glasgow Coma Scale; GOLD – Global initiative for Chronic Obstructive Lung Disease; Hb ‐ haemoglobin; IU/L – international unit/litre; ICU ‐ intensive care unit; IU/L ‐ international units/litre; IV ‐ intravenous; MedDRA ‐ Medical Dictionary for Regulatory Activities; MELD ‐ model for end‐stage liver disease; METS ‐ metabolic equivalent of tasks; NMB ‐ neuromuscular blocking; NMBA ‐ neuromuscular blocking agent; NSQIP ‐ national surgical quality improvement program; OR ‐ operations room; PaCO2 ‐ partial pressure of carbon dioxide; PACU ‐ post anaesthesia care unit; PADSS ‐ post anaesthesia discharge score system; PaO2 ‐ partial pressure of oxygen in blood; PEF ‐ peak expiratory flow; pO2 ‐ partial pressure of oxygen; PONV ‐ postoperative nausea and vomiting; PTC ‐ post‐tetanic count; PQRS ‐ postoperative quality recovery scale; SpO2 ‐ peripheral oxygen saturation; Sq ‐ square; SRS ‐ surgical rating score; TOF ‐ train of four; TOFR ‐ train‐of‐four ratio; VA ‐ alveolar volume; VAS ‐ visual analogue scale
Differences between protocol and review
2017
This updated review does not follow the protocol (Abrishami 2008)) prepared for the original version of this review. This is because after several discussions with the editorial team, we made the decision to split the original review (Abrishami 2009) into two reviews based on the extensive number of publications (> 70) identified by the updated search using various comparators, interventions, and outcome measures. In this updated review, we decided to focus only on sugammadex and neostigmine and to compare their efficacy and safety.
Contributions of authors
Updated review
Ana‐Marija Hristovska (AMH), Patricia Duch (PD), Mikkel Allingstrup (MA), Arash Afshari (AA).
Conceiving the review: AMH, AA, PD.
Co‐ordinating the review: AMH.
Undertaking manual searches: AMH, PD; MA.
Screening search results: AMH, PD.
Organizing retrieval of papers: AMH, PD, MA.
Screening retrieved papers against inclusion criteria: AMH, PD.
Appraising quality of papers: AMH, PD, AA.
Abstracting data from papers: AMH, PD.
Writing to authors of papers for additional information: AMH.
Providing additional data about papers: AMH.
Obtaining and screening data from unpublished trials: AMH, PD.
Managing data for the review: AMH.
Entering data into Review Manager (RevMan 5.3): AMH.
Analysing RevMan statistical data: AMH.
Conducting other statistical analysis not using RevMan: AA.
Performing double entry of data (data entered by person one: AMH; data entered by person two: PD).
Interpreting data: AMH, AA.
Making statistical inferences: AA.
Writing the review: AMH (abstract, methods, results, discussion, conclusions), PD (discussion), MA (background, methods), AA (abstract, methods, results, discussion, conclusions).
Securing funding for the review: This review was not funded.
Performing previous work that was the foundation of the present study: none of the review authors.
Taking responsibility for reading and checking the review before submission: AMH, AA.
Declarations of interest
Ana‐Marija Hristovska declares no conflict of interest.
Patricia Duch declares no conflict of interest.
Mikkel Allingstrup declares no conflict of interest.
Arash Afshari declares no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Adamus 2011 {published and unpublished data}
- Adamus M, Hrabalek L, Wanek T, Gabrhelik T, Zapletalova J. Intraoperative reversal of neuromuscular block with sugammadex or neostigmine during extreme lateral interbody fusion, a novel technique for spine surgery. Journal of Anesthesia 2011;25(5):716‐20. [PUBMED: 21842171] [DOI] [PubMed] [Google Scholar]
Balaka 2011 {published data only (unpublished sought but not used)}
- Balaka C, Poulida S, Miliatou M, Roussakis A, Lavranou V, Moshaki M, et al. Comparison of sugammadex to neostigmine reversal of neuromuscular blockade in patients with myasthenia gravis. Journal of Cardiothoracic and Vascular Anesthesia. 2011; Vol. 25:S22‐3. [3275631; http://www.jcvaonline.com/article/S1053‐0770(11)00191‐1/pdf]
Blobner 2010 {published and unpublished data}
- Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium‐induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomised, controlled trial. European Journal of Anaesthesiology 2010;27:874‐81. [PUBMED: 20683334] [DOI] [PubMed] [Google Scholar]
Brueckmann 2015 {published and unpublished data}
- Brueckmann B, Sasaki N, Grobara P, Li MK, Woo T, Bie J, et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: a randomized,controlled study. British Journal of Anaesthesia 2015;115(5):743‐51. [PUBMED: 25935840] [DOI] [PubMed] [Google Scholar]
Carron 2013 {published data only (unpublished sought but not used)}
- Carron M, Veronese S, Foletto M, Ori C. Sugammadex allows fast‐track bariatric surgery. Obesity Surgery 2013;23(10):1558‐63. [PUBMED: 23519634] [DOI] [PubMed] [Google Scholar]
Castro 2014 {published data only (unpublished sought but not used)}
- Castro DS Jr, Leão P, Borges S, Gomes L, Pacheco M, Figueiredo P. Sugammadex reduces postoperative pain after laparoscopic bariatric surgery: a randomized trial. Surgical Laparoscopy Endoscopy & Percutaneous Techniques 2014;24(5):420‐3. [PUBMED: 24752165] [DOI] [PubMed] [Google Scholar]
Cheong 2015 {published data only (unpublished sought but not used)}
- Cheong SH, Ki S, Lee J, Lee JH, Kim MH, Hur D, et al. The combination of sugammadex and neostigmine can reduce the dosage of sugammadex during recovery from the moderate neuromuscular blockade. Korean Journal of Anesthesiology 2015;68(6):547‐55. [PUBMED: 26634077] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flockton 2008 {published data only (unpublished sought but not used)}
- Flockton EA, Mastronardi P, Hunter JM, Gomar C, Mirakhur RK, Aguilera L, et al. Reversal of rocuronium‐induced neuromuscular block with sugammadex is faster than reversal of cisatracurium‐induced block with neostigmine. British Journal of Anaesthesia 2008;100(5):622‐30. [PUBMED: 18385265] [DOI] [PubMed] [Google Scholar]
Foletto 2014 {published data only (unpublished sought but not used)}
- Foletto M, Carron M, Zarantonello F, Prevedello L. Respiratory function after sugammadex and neostigmine administration for reversal of moderate rocuronium‐induced neuromuscular blockade in morbidly obese patients. 8 ed. Springer New York. Montreal, Canada: 19th World Congress of the International Federation for the Surgery of Obesity and Metabolic Disorders, IFSO 2014, 2014; Vol. 24:1139.
Gaszynski 2011 {published data only (unpublished sought but not used)}
- Gaszynski T, Szewczyk T, Gaszynski W. Randomized comparison of sugammadex and neostigmine for reversal of rocuronium‐induced muscle relaxation in morbidly obese undergoing general anaesthesia. British Journal of Anaesthesia 2012;108(2):236‐9. [PUBMED: 22012861] [DOI] [PubMed] [Google Scholar]
Geldner 2012 {published and unpublished data}
- Geldner G, Niskanen M, Laurila P, Mizikov V, Hubler M, Beck G, et al. A randomised controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparoscopic surgery. Anaesthesia 2012;67(9):991‐8. [PUBMED: 22698066] [DOI] [PubMed] [Google Scholar]
Georgiou 2013 {published data only (unpublished sought but not used)}
- Georgiou P, Zotou A, Siampalioti A, Tagari P, Tsiotsi P, Filos KS. Clinical and cost‐effectiveness of Sugammadex versus Neostigmine reversal of Rocuronium‐induced neuromuscular block in super obese patients undergoing open laparotomy for bariatric surgery. A randomized controlled trial. European Journal of Anaesthesiology. Barcelona: Euroanesthesia 2013, 2013; Vol. 30:141.
Grintescu 2009 {published data only (unpublished sought but not used)}
- Grintescu I, Mirea L, Ologoiu D, Ungureanu R, MekauvarS, Vasilescu M. Comparison of the cost effectiveness of sugammadex and neostigmine during general anaesthesia for laparoscopic cholecystectomy. British Journal of Anaesthesia. 2009; Vol. 103, issue 6:917.
Hakimoglu 2016 {published data only (unpublished sought but not used)}
- Hakimoğlu S, Tuzcu K, Davarcı I, Karcıoğlu M, Ayhan Tuzcu E, Hancı V, et al. Comparison of sugammadex and neostigmine‐atropine on intraocular pressure and postoperative effects. Kaohsiung Journal of Medical Sciences 2016;32(2):80‐5. [PUBMED: 26944326] [DOI] [PMC free article] [PubMed] [Google Scholar]
Illman 2011 {published data only (unpublished sought but not used)}
- Illman HL, Laurila P, Antila H, Meretoja OA, Alahuhta S, Olkkola KT. The duration of residual neuromuscular block after administration of neostigmine or sugammadex at two visible twitches during train‐of‐four monitoring. Anesthesia and Analgesia 2011;112(1):63‐8. [PUBMED: 20978247] [DOI] [PubMed] [Google Scholar]
Isik 2016 {published data only (unpublished sought but not used)}
- Isik Y, Palabiyik O, Cegin BM, Goktas U, Kati I. Effects of sugammadex and neostigmine on renal biomarkers. Medical Science Monitor 2016;22:803‐9. [PUBMED: 26963316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2008 {published and unpublished data}
- Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium‐induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology 2008;109(5):816–24. [PUBMED: 18946293] [DOI] [PubMed] [Google Scholar]
Kaufhold 2016 {published data only (unpublished sought but not used)}
- Kaufhold N, Schaller SJ, Stäuble CG Baumüller E, Ulm K, Blobner M, et al. Sugammadex and Neostigmine dose‐finding study for reversal of residual neuromuscular block at a train‐of‐four ratio of 0.2 (SUNDRO20). British Journal of Anaesthesia 2016;116(2):233‐40. [PUBMED: 26787792] [DOI] [PubMed] [Google Scholar]
Khuenl‐Brady 2010 {published data only (unpublished sought but not used)}
- Khuenl‐Brady KS, Wattwil M, Vanacker BF, Lora‐Tamayo JI, Rietbergen H, Alvarez‐Gómez JA. Sugammadex provides faster reversal of vecuronium‐induced neuromuscular blockade compared with neostigmine: a multicenter, randomized, controlled trial. Anesthesia and Analgesia 2010;110(1):64‐73. [PUBMED: 19713265] [DOI] [PubMed] [Google Scholar]
Kizilay 2016 {published and unpublished data}
- Kizilay D, Dal D, Saracoglu KT, Eti Z, Gogus FY. Comparison of neostigmine and sugammadex for hemodynamic parameters in cardiac patients undergoing noncardiac surgery. Journal of Clinical Anesthesia 2016;28:30‐5. [PUBMED: 26796612] [DOI] [PubMed] [Google Scholar]
Koc 2015 {published data only (unpublished sought but not used)}
- Koç F, Turan G, Subaşı D, Ekinci O. Comparison of sugammadex and neostigmine in short term surgery [Turkish: Kısa süreli cerrahide sugammadeks ile neostigminin karşılaştırılması]. Journal of Clinical and Analytical Medicine 2015;6(1):41‐5. [3275673; http://www.jcam.com.tr/files/KATD‐1694.pdf] [Google Scholar]
Kogler 2012 {published data only (unpublished sought but not used)}
- KoglerJ, Chalfe N, Karaman Ilić M, Hodoba N. Sugammadex reversal of rocuronium‐induced neuromuscular block in interventional bronchoscopy procedures: a comparison with neostigmine. European Journal of Anaesthesiology. 2012; Vol. 29:146.
Koyuncu 2015 {published data only (unpublished sought but not used)}
- Koyuncu O, Turhanoglu S, Ozbakis Akkurt C, Karcıoglu M, Ozkan M, Ozer C, et al. Comparison of sugammadex and conventional reversal on postoperative nausea and vomiting: a randomized, blinded trial. Journal of Clinical Anesthesia 2015;27(1):51‐6. [PUBMED: 25544263] [DOI] [PubMed] [Google Scholar]
Kvolik 2012a {published data only (unpublished sought but not used)}
- Kvolik S, Mraovic B, Kristek J, Stefanic M, Mihaljevic I. Reversal with sugammadex hastens recovery of tracheal reflexes and extubation compared with neostigmine in patients undergoing thyroidectomy. European Journal of Anaesthesiology. 2012; Vol. 29:142.
Kvolik 2012b {published data only (unpublished sought but not used)}
- Kvolik S, Mraovic B, Kristek J, Stefanic M, Mihaljevic I. Sugammadex does not influence thyroid hormones in the patients undergoing thyroidectomy in propofol anesthesia. European Journal of Anaesthesiology. 2012; Vol. 29:141.
Kvolik 2013 {published data only (unpublished sought but not used)}
- Kvolik S, Rakipovic A, Mraovic B, Kristek J, Ikic V, Kristic M. Faster increase of BIS registered after the reversal of neuromuscular blockade with sugammadex vs. neostigmine. European Journal of Anaesthesiology. 2013; Vol. 30:40.
Lemmens 2010 {published data only (unpublished sought but not used)}
- Lemmens HJ, El‐Orbany MI, Berry J, Morte JB Jr, Martin G. Reversal of profound vecuronium‐induced neuromuscular block under sevofluraneanesthesia: sugammadex versus neostigmine. BMC Anesthesiology 2010;10:15. [PUBMED: 20809967] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martini 2014 {published and unpublished data}
- Martini CH, Boon M, Bevers RF, Aarts LP, Dahan A. Evaluation of surgical conditions during laparoscopic surgery in patients with moderate vs deep neuromuscular block. British Journal of Anaesthesia 2014;112(3):498‐505. [PUBMED: 24240315] [DOI] [PubMed] [Google Scholar]
Mekawy 2012 {published data only (unpublished sought but not used)}
- Mekawy N, Eman A, Ali F. Improved recovery profiles in sinonasal surgery sugammadex: does it have a role?. Egyptian Journal of Anaesthesia 2012;28(3):175‐8. [DOI: 10.1016/j.egja.2011.12.007] [DOI] [Google Scholar]
Pongracz 2013 {published data only (unpublished sought but not used)}
- Pongrácz A, Szatmári S, Nemes R, Fülesdi B, Tassonyi E. Reversal of neuromuscular blockade with sugammadex at the reappearance of four twitches to train‐of‐four stimulation. Anesthesiology 2013;119(1):36‐42. [PUBMED: 23665915] [DOI] [PubMed] [Google Scholar]
Rahe‐Meyer 2014 {published data only (unpublished sought but not used)}
- Rahe‐Meyer N, Fennema H, Schulman S, Klimscha W, Przemeck M, Blobner M, et al. Effect of reversal of neuromuscular blockade with sugammadex versus usual care on bleeding risk in a randomized study of surgical patients. Anesthesiology 2014;121(5):969‐77. [PUBMED: 25208233] [DOI] [PubMed] [Google Scholar]
Raziel 2013 {published and unpublished data}
- Raziel A, Messinger G, Sakran N, Szold A, Goitein D. Comparison of two neuromuscular anesthetics reversal in obese patients undergoing bariatric surgery ‐ a prospective study. 21st International Congress of the European Association for Endoscopic Surgery, EAES. Vienna, Austria, 2013:31.
Riga 2014 {published and unpublished data}
- Riga M, Batistaki C, Matsota P, Lyrakos G, Zafeiropoulou F, Kostopanagiotou G. Effect of sugammadex versus neostigmine/atropine combination on cognitive function of adult patients after elective surgery: preliminary results. European Journal of Anaesthesiology. 2014; Vol. 31:157‐8.
Sabo 2011 {published data only (unpublished sought but not used)}
- Sabo D, Jones RK, Berry J, Sloan T, Chen JY, Groudine S. Residual neuromuscular blockade at extubation: a randomized comparison of sugammadex and neostigmine reversal of rocuronium‐induced blockade in patients undergoing abdominal surgery. Anesthesia and Clinical Research Journals 2011;2(6):Open assess journal, no pages. [Google Scholar]
Schaller 2010 {published data only (unpublished sought but not used)}
- Schaller SJ, Fink H, Ulm K, Blobner M. Sugammadex and neostigmine dose‐finding study for reversal of shallow residual neuromuscular block. Anesthesiology 2010;113(5):1054‐60. [DOI: 10.1097/ALN.0b013e3181f4182a; PUBMED: 20885293] [DOI] [PubMed] [Google Scholar]
Sherman 2014 {published data only (unpublished sought but not used)}
- Sherman A, Abelansky Y, Evron S, Ezri T. The effect of sugammadex vs. neostigmine on the postoperative respiratory complications following laparoscopic sleeve gastrectomy. European Journal of Anaesthesiology. 2014; Vol. 31:152.
Sustic 2012 {published data only}
- Sustic A, Dijana D. Early postoperative gastric emptying in patients undergoing laparoscopic cholecystectomy: sugammadex vs. neostigmine/atropine neuromuscular blockade reversal agents. European Anaesthesiology Congress, EUROANAESTHESIA. Lippincott Williams and Wilkins, 2012; Vol. 29:140.
Tas 2015 {published data only (unpublished sought but not used)}
- Taş N, Korkmaz H, Yağan Ö, Korkmaz M. Effect of sugammadex on postoperative bleeding and coagulation parameters after septoplasty: a randomized prospective study. Medical Science Monitor 2015;14:2382‐6. [PUBMED: 26271275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woo 2013 {published and unpublished data}
- Woo T, Kim KS, Shim YH, Kim MK, Yoon SM, Lim YJ, et al. Sugammadex versus neostigmine reversal of moderate rocuronium‐induced neuromuscular blockade in Korean patients. Korean Journal of Anesthesiololgy 2013;65(6):501‐7. [PUBMED: 24427455] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2014 {published data only (unpublished sought but not used)}
- Wu X, Oerding H, Liu J, Vanacker B, Yao S, Dahl V, et al. Rocuronium blockade reversal with sugammadex vs. neostigmine: randomized study in Chinese and Caucasian subjects. BMC Anesthesiology 2014;14(53):eCollection. [DOI: 10.1186/1471-2253-14-53; PUBMED: 25187755] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yagan 2015 {published and unpublished data}
- Yagan O, Karakahya RH, Tas N, Canakci E, Hanci V, Yurtlu BS. Intraocular pressure changes associated with tracheal extubation: comparison of sugammadex with conventional reversal of neuromuscular blockade. Journal of the Pakistan Medical Assosication 2015;65(11):1219‐25. [PUBMED: 26564297] [PubMed] [Google Scholar]
References to studies excluded from this review
Aho 2012 {published data only}
- Aho Aj, Kamata K, Yli‐Hankala A, Lyytikäinen L‐P, Kulkas A, Jäntti V. Elevated BIS and entropy values after sugammadex or neostigmine: an electroencephalographicor electromyographic phenomenon?. Acta Anaesthesiologica Scandinavica 2012;56(4):465‐73. [PUBMED: 22289106] [DOI] [PubMed] [Google Scholar]
Baysal 2013 {published data only}
- Baysal A, Dogukan M, Toman H, Sagiroglu G, Kocak T. The use of sugammadex for reversal of residual blockade after administration of neostigmine and atropine. European Anaesthesiology Congress, EUROANAESTHESIA. Lippincott Williams and Wilkins, 2013; Vol. 30:142.
Dahaba 2012 {published data only}
- Dahaba AA, Bornemann H, Hopfgartner E, Ohran M, Kocher K, Liebmann M, et al. Effect of sugammadex or neostigmine neuromuscular block reversal on bispectral index monitoring of propofol/remifentanil anaesthesia. British Journal of Anaesthesia 2012;108(4):602‐6. [PUBMED: 22315331] [DOI] [PubMed] [Google Scholar]
Gaona 2012 {published data only}
- Gaona D, Carceles MD, Veiga G, Tedesco M, Motta P. Efficacy and safety of the reversal with sugammadex in deep neuromuscular blockade induced by rocuronium in pediatrics. 15th WFSA World Congress of Anaesthesiologists Predio Ferial de Buenos Aires Argentina. Oxford University Press, 2012; Vol. 108:308‐9.
Ghoneim 2015 {published data only}
- Ghoneim AA, Beltagy MA. Comparative study between sugammadex and neostigmine in neurosurgical anesthesia in pediatric patients. Saudi Journal of Anaesthesia 2015;9(3):247‐52. [PUBMED: 26240540] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harazim 2014 {published data only}
- Harazim H, Stourac P, Seidlova D, Adamus M, Krikava I, Pavlik T. Use of rocuronium and active reversal of neuromuscular blockade with sugammadex in anaesthesia for caesarean section led to reduction of myalgia incidence in early postoperative period: prospective randomised interventional multicentric trial. European Journal of Anaesthesiology. 2014; Vol. 31:194‐5.
Kakinuma 2013 {published data only (unpublished sought but not used)}
- Kakinuma A, Nagatani H, Yasuda A, Yoshimura T, Sawai J, Nakata Y. Combined use of sugammadex and neostigmine for the reversal of rocuronium‐induced profound neuromuscular blockade. Anesthesia and Clinical Research Journal 2013;4(7):337. [DOI: 10.4172/2155-6148.1000337] [DOI] [Google Scholar]
Kara 2014 {published data only}
- Kara T, Ozbagriacik O, Turk HS, Isil CT, Gokuc O, Unsal O, et al. Sugammadex versus neostigmine in pediatric patients: a prospective randomized study. Revista Brasileira de Anestesiologia 2014;64(6):400‐5. [PUBMED: 25437696] [DOI] [PubMed] [Google Scholar]
Kzlay 2013 {published data only (unpublished sought but not used)}
- Kzlay D, Dal D, Saraçoğlu T, Eti Z, Göğüş Y. The effects of sugammadex and neostigmine‐atropine administration on hemodynamic parameters in cardiac patients undergoing non‐cardiac surgery. European Journal of Anaesthesiology. 2013; Vol. 30:75.
Nagashima 2016 {published and unpublished data}
- Nagashima S, Takasusuki T, Yamaguchi S, Hamaguchi S. Effects of neostigmine and sugammadex on QT interval and QT dispersion. Dokkyo Journal of Medical Sciences 2016;43:15‐22. [Google Scholar]
Nagy 2014 {published data only}
- Nagy HIA, Elkadi HW. Can sugammadex improve the reversal profile of atracurium under sevoflurane anesthesia?. Egyptian Journal of Anaesthesia 2014;30(1):95‐9. [DOI: 10.1016/j.egja.2013.09.007] [DOI] [Google Scholar]
NCT03111121 {unpublished data only}
- NCT03111121. Use of Sugammadex for Reversal of Paralysis in Microlaryngoscopy. https://clinicaltrials.gov/show/NCT03111121, March 23, 2017.
Nemes 2016 {published and unpublished data}
- Nemes R, Fülesdi B, Pongrácz A, Asztalos L, Szabó‐Maák Z, Lengyel S, et al. Impact of reversal strategies on the incidence of postoperative residual paralysis after rocuronium relaxation without neuromuscular monitoring: a partially randomised placebo controlled trial. European Journal of Anaesthesiology 2016;1:1. [PUBMED: 28030444] [DOI] [PubMed] [Google Scholar]
Ozgun 2014 {published data only}
- Ozgün C, Cakan T, Baltacı B, Başar H. Comparison of reversal and adverse effects of sugammadex and combination of anticholinergic‐anticholinesterase agents in pediatric patients. Journal of Research in Medical Sciences 2014;19(8):762‐8. [PUBMED: 25422663] [PMC free article] [PubMed] [Google Scholar]
Pecek 2013 {published data only}
- Pecek B, Hollan J, Priman T, Tokic Crnic N, Stankovic V, Polh D. New way of dosing sugammadex for termination of vecuronium induced neuromuscular block. Zdravniski Vestnik 2015;84:439–46. [Google Scholar]
Sacan 2007 {published data only}
- Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium‐induced neuromuscular blockade: a comparison with neostigmine‐glycopyrrolate and edrophonium‐atropine. Anesthesia and Analgesia 2007;104(3):569‐74. [PUBMED: 17312210 ] [DOI] [PubMed] [Google Scholar]
Schepens 2015 {published data only}
- Schepens T, Cammu G, Saldien V, Neve N, Jorens PG, Foubert L, et al. Electromyographic activity of the diaphragm during neostigmine or sugammadex‐enhanced recovery after neuromuscular blockade with rocuronium: a randomised controlled study in healthy volunteers. European Journal of Anaesthesiology 2015;32(1):49‐57. [PUBMED: 25111539] [DOI] [PubMed] [Google Scholar]
Stourac 2016 {published and unpublished data}
- Stourac P, Adamus M, Seidlova D, Pavlik T, Janku P, Krikava I, et al. Low‐dose or high‐dose rocuronium reversed with neostigmine or sugammadex for cesarean delivery anesthesia: a randomized controlled noninferiority trial of time to tracheal intubation and extubation. Anesthesia and Analgesia 2016;122(5):1536‐45. [DOI: 10.1213/ANE.0000000000001197; PUBMED: 26974018] [DOI] [PubMed] [Google Scholar]
Veiga Ruiz 2011 {published data only}
- Veiga Ruiz G, Carceles Baron MD, Dominguez Serrano N, Lopez Fuentes L, Orozco Montes J, Alvarez‐Gomez JA. Sugammadex reversal efficacy and security vs neostigmine in the rocuronium‐induced neuromuscular blockade in paediatric patients. European Journal of Anaesthesiology. Abstracts and Programme: EUROANAESTHESIA 2011: The European Anaesthesiology Congress: Paediatric Anaesthesia and Intensive Care, 2011; Vol. 28:153.
References to studies awaiting assessment
Kim 2016 {published data only}
- Kim K, Oh Y, Kim T, Oh S, Sin Y. Relationship between first‐twitch depression and train‐of‐four ratio during sugammadex reversal of rocuronium‐induced neuromuscular blockade. Korean Journal of Anesthesiology 2016;1:1. [PUBMED: 27274368] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02243943 {unpublished data only}
- NCT02243943. Effect of Neuromuscular Reversal With Sugammadex on Postoperative Recovery Profile (Neuropa). https://clinicaltrials.gov/show/NCT02243943, September 12, 2014.
Sen 2016 {unpublished data only}
- Sen A, Erdivanli B, Tomak Y, Pergel A. Reversal of neuromuscular blockade with sugammadex or neostigmine/atropine: effect on postoperative gastrointestinal motility. Journal of Clinical Anesthesia 2016;1:1. [PUBMED: 27290978] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01539044 {unpublished data only}
- NCT01539044. Optimal Relaxation Technique for Laparotomies With Rocuronium Infusion Followed by Sugammadex Reversal (ProjectO5Rs). https://clinicaltrials.gov/show/NCT01539044, February 15, 2012.
NCT01748643 {unpublished data only}
- NCT01748643. CURES: The Effect of Deep Curarisation and Reversal With Sugammadex on Surgical Conditions and Perioperative Morbidity (CURES). https://clinicaltrials.gov/show/NCT01748643, December 6, 2012.
NCT02160223 {unpublished data only}
- NCT02160223. Sugammadex Compared With Neostigmin/Atropin for Neuromuscular Block Reversal in Patients With Obstructive Sleep Apnea. https://clinicaltrials.gov/show/NCT02160223, June 8, 2014.
NCT02256280 {unpublished data only}
- NCT02256280. A Randomized Double Blind Controlled Trial Comparing Sugammadex and Neostigmine After Thoracic Anesthesia (DATA). https://clinicaltrials.gov/show/NCT02256280, September 24, 2014.
NCT02330172 {unpublished data only}
- NCT02330172. Sugammadex Provide Better Surgical Condition Compared With Neostigmine in Laryngeal Microsurgery. https://clinicaltrials.gov/show/NCT02330172, December 9, 2014.
NCT02361060 {unpublished data only}
- NCT02361060. Effects of Neuromuscular Block Reversal With Sugammadex vs Neostigmine on Postoperative Respiratory Outcomes After Major Abdominal Surgery. https://clinicaltrials.gov/show/NCT02361060, December 23, 2014.
NCT02414880 {unpublished data only}
- NCT02414880. Sugammadex Versus Neostigmine in Patients With Liver Cirrhosis Undergoing Liver Resection. https://clinicaltrials.gov/show/NCT02414880, April 5, 2015.
NCT02454504 {unpublished data only}
- NCT02454504. Quality of Awakening and Impact on Cognitive Function After Administration of Sugammadex in Robotic Radical Cystectomy. https://clinicaltrials.gov/show/NCT02454504, May 6, 2015.
NCT02648503 {unpublished data only}
- NCT02648503. Deep Neuromuscular Block and Sugammadex Versus Standard of Care on Quality of Recovery in Patients Undergoing Elective Laparoscopic Cholecystectomy. https://clinicaltrials.gov/show/NCT02648503, December 7, 2015.
NCT02666014 {unpublished data only}
- NCT02666014. Sugammadex Versus Neostigmine for Postoperative Nausea and Vomiting After Laparoscopic Gynaecological Surgery. https://clinicaltrials.gov/show/NCT02666014, February 2, 2015.
NCT02697929 {unpublished data only}
- NCT02697929. Sugammadex/Neostigmine and Liver Transplantation. https://clinicaltrials.gov/show/NCT02697929, February 13, 2016.
NCT02698969 {unpublished data only}
- NCT02698969. Recovery of Muscle Function After Deep Neuromuscular Block by Means of Diaphragm Ultrasonography. https://clinicaltrials.gov/show/NCT02698969, February 15, 2016.
NCT02845375 {unpublished data only}
- NCT02845375. Effect of Neuromuscular Blockade and Reversal on Breathing (BREATH). https://clinicaltrials.gov/show/NCT02845375, July 14, 2016.
NCT02860507 {unpublished data only}
- NCT02860507. Study to Determine if Administration of Sugammadex Impacts Hospital Efficiency. https://clinicaltrials.gov/show/NCT02860507, August 2, 2016.
NCT02861131 {unpublished data only}
- NCT02861131. The Effect of Sugammadex Versus Neostigmine on Postoperative Pulmonary Complications. https://clinicaltrials.gov/show/NCT02861131, July 14, 2016.
NCT02909439 {unpublished data only}
- NCT02909439. Quality of Recovery After Reversal With Neostigmine or Sugammadex. https://clinicaltrials.gov/show/NCT02909439, August 31, 2016.
NCT02939430 {unpublished data only}
- NCT02939430. Sugammadex Reversal of Neuromuscular Blockade and Postoperative Bleeding (Suga_bleeding). https://clinicaltrials.gov/show/NCT02939430, October 17, 2016.
NCT03108989 {unpublished data only}
- NCT03108989. Comparison the Postoperative Quality of Recovery Between Neostigmine and Sugammadex in Elderly Patients Undergoing Trans Pars Plana Vitrectomy With General Anesthesia ‐ Randomized Controlled Trial. https://clinicaltrials.gov/show/NCT03108989, April 6, 2017.
NCT03116997 {unpublished data only}
- NCT03116997. Study of Recovery of Strength After Surgery Comparing Two Different Medications for Reversal of Muscle Relaxant. https://clinicaltrials.gov/show/NCT03116997, April 12, 2017.
NCT03144453 {unpublished data only}
- NCT03144453. Recovery From Anesthesia After Robotic Assisted Radical Cystectomy. https://clinicaltrials.gov/show/NCT03144453, May 3, 2017.
Additional references
Abad‐Gurumeta 2015
- Abad‐Gurumeta A, Ripollés‐Melchor J, Casans‐Francés R, Espinosa A, Martínez‐Hurtado E, Fernández‐Pérez C, et al. A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade. Anaesthesia 2015;70(12):1441‐52. [PUBMED: 26558858] [DOI] [PubMed] [Google Scholar]
Agrell 1998
- Agrell B, Dehlin O. The clock‐drawing test. Age and Aging 1998;27:399‐403. [PUBMED: 23144287] [DOI] [PubMed] [Google Scholar]
Ali 1970
- Ali HH, Utting JE, Gray C. Stimulus frequency in the detection of neuromuscular block in humans. British Journal of Anaesthesia 1970;42(11):967‐78. [PUBMED: 5488360] [DOI] [PubMed] [Google Scholar]
Ali 1971
- Ali HH, Utting JE, Gray TC. Quantitative assessment of residual antidepolarizing block II. British Journal of Anaesthesia 1971;43(5):478‐85. [PUBMED: 4254032] [DOI] [PubMed] [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [PUBMED: 12543843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Appelbaum 2003
- Appelbaum R, Mulder R, Saddler J, Kopman AF, McShane AJ. Atracurium associated with postoperative residual curarization (multiple letters). British Journal of Anaesthesia 2003;90(4):523‐4. [PubMed] [Google Scholar]
Arbous 2005
- Arbous MS, Meursing AE, Kleef JW, Lange JJ, Spoormans HH, Touw P, et al. Impact of anaesthesia management characteristics on severe morbidity and mortality. Anesthesiology 2005;102(2):257‐68. [PUBMED: 15681938] [DOI] [PubMed] [Google Scholar]
Baillard 2005
- Baillard C, Clec'h C, Catineau J, Salhi F, Gehan G, Cupa M, et al. Postoperative residual neuromuscular block: a survey of management. British Journal of Anaesthesia 2005;95(5):622‐6. [PUBMED: 16183681 ] [DOI] [PubMed] [Google Scholar]
Berg 1997
- Berg H, Roed J, Viby‐Mogensen J, Mortensen CR, Engbaek J, Skovgaard LT, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiologica Scandinavica 1997;41(9):1095‐103. [PUBMED: 9366929 ] [DOI] [PubMed] [Google Scholar]
Bevan 1988
- Bevan DR, Smith CE, Donati F. Postoperative neuromuscular blockade: a comparison between atracurium, vecuronium and pancuronium. Anesthesiology 1988;69(2):272‐6. [PUBMED: 2900612 ] [PubMed] [Google Scholar]
Bevan 1996
- Bevan DR, Kahwaji R, Ansermino JM, Reimer E, Smith MF, O'Connar GA, et al. Residual block after mivacurium with or without edrophonium reversal in adults and children. Anesthesiology 1996;84(2):362‐7. [PUBMED: 8602667 ] [DOI] [PubMed] [Google Scholar]
Bowman 2006
- Bowman WC. Neuromuscular block. British Journal of Pharmacology 2006;147(Suppl 1):S277‐86. [PUBMED: 16402115] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bridion 2014
- http://www.ema.europa.eu/. Summary of Product Characteristics of BRIDION. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000885/WC500052310.pdf.
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. Journal of Clinical Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Brull 1991
- Brull SJ, Ehrenwerth J, Connelly NR, Silverman DG. Assessment of residual curarization using low‐current stimulation. Canadian Journal of Anaesthesia 1991;38(2):164‐8. [PUBMED: 1673643 ] [DOI] [PubMed] [Google Scholar]
Caldwell 2009
- Caldwell JE. Clinical limitations of acetylcholinesterase antagonists. Journal of Critical Care 2009;24(1):21‐8. [PUBMED: 19272535] [DOI] [PubMed] [Google Scholar]
Chan 2004
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [PUBMED: 15161896] [DOI] [PubMed] [Google Scholar]
de Boer 2007
- Boer HD, Driessen JJ, Marcus MA, Kerkkamp H, Heeringa M, Klimek M. Reversal of rocuronium‐induced (1.2mg/kg) profound neuromuscular block by sugammadex: a multicenter, dose‐finding and safety study. Anesthesiology 2007;107(2):239‐44. [PUBMED: 17667567] [DOI] [PubMed] [Google Scholar]
De Kam 2014
- Kam PJ, Kruithof AC, Lierop MJ, Moerland M, Dennie J, Troyer MD, et al. Lack of a clinically relevant effect of sugammadex on anti‐Xa activity or activated partial thromboplastin time following pretreatment with either unfractionated or low‐molecular‐weight heparin in healthy subjects. International Journal of Clinical Pharmacology and Therapeutics 2014;52(8):631‐41. [PUBMED: 24800921] [DOI] [PubMed] [Google Scholar]
Debaene 2003
- Debaene B, Plaud B, Dilly MP, Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology 2003;98:1042‐8. [PUBMED: 12717123] [DOI] [PubMed] [Google Scholar]
Dexter 1995
- Dexter F, Coffin S, Tinker JH. Decreases in anesthesia‐controlled time cannot permit one additional surgical operation to be reliably scheduled during the workday. [Decreases in anesthesia‐controlled time cannot permit one additional surgical operation to be reliably scheduled during the workday]. Anesthesia & Analgesia 1995;81(6):1263‐8. [PUBMED: 7486114] [DOI] [PubMed] [Google Scholar]
Drugs.com
- Generic Bridion availability. www.drugs.com. [www.drugs.com/availability/generic‐bridion.html]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eikermann 2006
- Eikermann M, Blobner M, Groeben H, Rex C, Grote T, Neuhäuser M, et al. Postoperative upper airway obstruction after recovery of the train of four ratio of the adductor pollicis muscle from neuromuscular blockade. Anesthesia and Analgesia 2006;102(3):937‐42. [PUBMED: 16492855] [DOI] [PubMed] [Google Scholar]
Eriksson 1993
- Eriksson LI, Sato M, Severinghaus JW. Effect of vecuronium‐induced partial neuromuscular block on hypoxic ventilatory response. Anesthesiology 1993;78(4):693‐9. [PUBMED: 8096684 ] [DOI] [PubMed] [Google Scholar]
Eriksson 1997
- Eriksson LI, Sundman E, Olsson R, Nilsson L, Witt H, Ekberg O, et al. Functional assessment of the pharynx at rest and during swallowing in partially paralysed humans. Simultaneous video manometry and mechanomyography of awake human volunteers. Anesthesiology 1997;87(5):1035‐43. [PUBMED: 9366453 ] [DOI] [PubMed] [Google Scholar]
Fawcett 1995
- Fawcett WJ, Dash A, Francis GA, Liban JB, Cashman JN. Recovery from neuromuscular blockade: residual curarization following atracurium or vecuronium by bolus dosing or infusions. Acta Anaesthesiologica Scandinavica 1995;39(3):288‐93. [PUBMED: 7793202] [DOI] [PubMed] [Google Scholar]
Fuchs‐Buder 2012
- Fuchs‐Buder T, Meistelman C, Schreiber JU. Is sugammadex economically viable for routine use. Current Opinions in Anesthesiology 2012;25(2):217‐20. [PUBMED: 22157200] [DOI] [PubMed] [Google Scholar]
Gijsenbergh 2005
- Gijsenberg F, Ramael S, Houwing N, Lersel T. First human exposure of Org 25969, a novel agent to reverse the action of rocuronium bromide. Anesthesiology 2007;103(4):695‐703. [PUBMED: 16192761] [DOI] [PubMed] [Google Scholar]
Golembiewski 2016
- Golembiewski J. Sugammadex. Journal of PeriAnesthesia Nursing 2016;31(4):354‐7. [PUBMED: 27444769] [DOI] [PubMed] [Google Scholar]
GradePro software [Computer program]
- gradepro.org. GRADEpro GDT. Version Accessed December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hayes 2001
- Hayes AH, Mirakyr RK, Brestin DS, Reid JE, McCourt KC. Postoperative residual block after intermediate acting neuromuscular blocking drugs. Anaesthesia 2001;56(4):312‐8. [PUBMED: 11284816 ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. handbook.cochrane.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. British Medical Research Methodology 2005;5(13):1‐10. [DOI: 10.1186/1471-2288-5-13; PUBMED: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2000
- Hutton JL, Williamson PR. Bias in meta‐analysis due to outcome variable selection within studies. Journal of the Royal Statistical Society Series C 2000;49:359‐70. [DOI: 10.1111/1467-9876.00197] [DOI] [Google Scholar]
Insinga 2016
- Insinga RP, Joyal C, Goyette A, Galarneau A. A discrete event simulation model of clinical and operating room efficiency outcomes of sugammadex versus neostigmine for neuromuscular block reversal in Canada. BMC Anesthesiology 2016;16(1):114. [PUBMED: 7852231] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 1988
- Jensen E, Viby‐Mogensen J, Bang U. The Accelograph: a new neuromuscular transmission monitor. Acta Anaesthesiologica Scandinavica 1988;32(1):49‐52. [PUBMED: 2830761] [DOI] [PubMed] [Google Scholar]
Keating 2016
- Keating GM. Sugammadex: a review of neuromuscular blockade reversal. Drugs 2016;76(10):1041‐52. [PUBMED: 27324403] [DOI] [PubMed] [Google Scholar]
Kim 2002
- Kim KS, Lew SH, Cho HY, Cheong MA. Residual paralysis induced by either vecuronium or rocuronium after reversal with pyridostigmine. Anesthesia and Analgesia 2002;95(6):1656‐60. [PUBMED: 12456433 ] [DOI] [PubMed] [Google Scholar]
Lan 1983
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70(3):659‐63. [DOI: 10.1093/biomet/70.3.659] [DOI] [Google Scholar]
Ledowski 2012
- Ledowski T, Hillyard S, Kozman A, Johnston F, Gillies E, Greenaway M, et al. Unrestricted access to sugammadex: impact on neuromuscular blocking agent choice, reversal practice and associated healthcare costs. Anesthesia and Intensive Care Journal 2012;40(2):340‐3. [PUBMED: 22417031] [DOI] [PubMed] [Google Scholar]
Maybauer 2007
- Maybauer DM, Geldner G, Blobner M, Puhringer F, Hofmockel R, Rex C, et al. Incidence and duration of residual paralysis at the end of surgery after multiple administrations of cisatracurium and rocuronium. Anaesthesia 2007;62(1):12‐7. [PUBMED: 17156221] [DOI] [PubMed] [Google Scholar]
McCaul 2002
- McCaul C, Tobin E, Boylan JF, McShane AJ. Atracurium is associated with postoperative residual curarization. British Journal of Anaesthesia 2002;89(5):766‐9. [PUBMED: 12393778] [PubMed] [Google Scholar]
Mirakhur 1985
- Mirakhur RK. Antagonism of the muscarinic effects of edrophonium with atropine or glycopyrrolate. A comparative study. British Journal of Anaesthesia 1985;57(12):1213‐6. [PUBMED: 4084429 ] [DOI] [PubMed] [Google Scholar]
Murphy 2006
- Murphy GS. Residual neuromuscular blockade: incidence, assessment, and relevance in the postoperative period. Minerva Anestesiologica 2006;72(3):97‐109. [PUBMED: 16493386] [PubMed] [Google Scholar]
Murphy 2008
- Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesthesia and Analgesia 2008;107(1):130‐7. [PUBMED: 18635478] [DOI] [PubMed] [Google Scholar]
Murphy 2010
- Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I. Definitions, Incidence, and adverse physiologic effects of residual neuromuscular block. Anesthesia and Analgesia 2010;111(1):120‐8. [PUBMED: 20442260] [DOI] [PubMed] [Google Scholar]
Murphy 2011
- Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Marymont JH, Vender JS, et al. Intraoperative acceleromyography monitoring reduces symptoms of muscle weakness and improves quality of recovery in the early postoperative period. Anesthesiology 2011;115(5):946‐54. [PUBMED: 21946094] [DOI] [PubMed] [Google Scholar]
Murphy 2013
- Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear T, Vender JS, et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesthesia and Analgesia 2013;117(1):133‐41. [PUBMED: 23337416] [DOI] [PubMed] [Google Scholar]
Naguib 2009
- Naguib M, Brull SJ. Sugammadex: a novel selective relaxant binding agent. Expert Review of Clinical Pharmacology 2009;2(1):37‐53. [PUBMED: 24422770] [DOI] [PubMed] [Google Scholar]
Park 2015
- Park JY. Benefits and risks of sugammadex. Korean Journal of Anesthesiology 2015;68(1):1‐2. [PUBMED: 25664147] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paton 2010
- Paton F, Paulden M, Chambers D, Heirs M, Duffy S, Hunter JM, et al. Sugammadex compared with neostigmine/glycopyrrolate for routine reversal of neuromuscular block: a systematic review and economic evaluation. British Journal of Anaesthesia 2010;105(5):558‐67. [PUBMED: 20935005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedersen 1994
- Pedersen T. Complications and death following anaesthesia. A prospective study with special reference to the influence of patient‐, anaesthesia‐, and surgery‐related risk factors. Danish Medical Bulletin 1994;41(3):319‐31. [PUBMED: 7924461] [PubMed] [Google Scholar]
Plaud 2010
- Plaud B, Debaene B, Donati F, Marty J. Residual paralysis after emergence from anesthesia. Anesthesiology 2010;112(4):1013‐22. [PUBMED: 20234315] [DOI] [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93. [PUBMED: 9408720] [DOI] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):45‐52. [PUBMED: 9433436] [DOI] [PubMed] [Google Scholar]
PubChem 2016
- National Center for Biotechnology Information. Rocuronium. PubChem Compound Database 2016. [https://pubchem.ncbi.nlm.nih.gov/compound/441290]
Raft 2011
- Raft J, Betala Belinga JF, Jurkolow G, Desandes E, Longrois D, Meistelman C. Clinical evaluation of post‐surgical bleeding after a sugammadex injection. Annales Françaises d'Anesthésie et de Réanimation 2011;30(10):714‐7. [PUBMED: 21741200] [DOI] [PubMed] [Google Scholar]
Reid 2001
- Reid JE, Breslin DS, Mirakhur RK, Hayes AH. Neostigmine antagonism of rocuronium block during anesthesia with sevoflurane, isoflurane or propofol. Canadian Journal of Anesthesia 2001;48(4):351‐5. [PUBMED: 11339776] [DOI] [PubMed] [Google Scholar]
RevMan 5.3.5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Sauer 2011
- Sauer M, Stahn A, Soltesz S, Noeldge‐Schomburg G, Mencke T. The influence of residual neuromuscular block on the incidence of critical respiratory events. A randomised, prospective, placebo‐controlled trial. European Journal of Anaesthesiology 2011;28(12):842‐8. [PUBMED: 21455074] [DOI] [PubMed] [Google Scholar]
Shorten 1993
- Shorten GD. Postoperative residual curarization: incidence, aetiology and associated morbidity. Anaesthesia and Intensive Care 1993;21(6):782‐9. [PUBMED: 8122734 ] [DOI] [PubMed] [Google Scholar]
Sorgenfrei 2006
- Sorgenfrei IF, Norrild K, Larsen PB, Stensballe J, Ostergaard D, Prins ME, et al. Reversal of rocuronium‐induced neuromuscular block by the selective relaxant binding agent sugammadex, a dose‐finding and safety study. Anesthesiology 2006;104(4):667‐74. [PUBMED: 16571960] [DOI] [PubMed] [Google Scholar]
Sparr 2007
- Sparr HJ, Vermeyen KM, Beaufort AM, Rietbergen H, Proost JH, Saldien V, et al. Early reversal of profound rocuronium‐induced neuromuscular blockade by sugammadex in a randomised multicenter study: efficacy, safety, and pharmacokinetics. Anesthesiology 2007;106(5):935‐43. [PUBMED: 17457124 ] [DOI] [PubMed] [Google Scholar]
Staals 2010
- Staals LM, Snoeck MM, Driessen JJ, Hamersvelt HW, Flockton EA, Heuvel MW, et al. Reduced clearance of rocuronium and sugammadex in patients with severe to end‐stage renal failure: a pharmacokinetic study. British Journal of Anaesthesia 2010;104(1):31‐9. [PUBMED: 20007792 ] [DOI] [PubMed] [Google Scholar]
Sundman 2000
- Sundman E, Witt H, Olsson R, Ekberg O, Kuylenstierna R, Eriksson LI. The incidence and mechanisms of pharyngeal and upper oesophageal dysfunction in partially paralysed humans. Pharyngeal video radiography and simultaneous manometry after atracurium. Anesthesiology 2000;92(4):977‐84. [PUBMED: 10754616 ] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [PUBMED: 18824467] [DOI] [PubMed] [Google Scholar]
Tombaugh 1992
- Tombaugh TN, McIntyre NJ. The mini‐mental state examination: a comprehensive review. Journal of the American Geriatric Society 1992;40(9):922‐35. [PUBMED: 1512391] [DOI] [PubMed] [Google Scholar]
Tramer 1999
- Tramer MR, Fuchs‐Buder T. Omitting antagonism of neuromuscular block: effect on postoperative nausea and vomiting and risk of residual paralysis. A systematic review. British Journal of Anaesthesia 1999;82(3):379‐86. [PUBMED: 10434820] [DOI] [PubMed] [Google Scholar]
Viby‐Mogensen 1979
- Viby‐Mogensen J, Jørgensen BC, Ording H. Residual curarization in the recovery room. Anesthesiology 1979;50(6):539‐41. [PUBMED: 156513] [DOI] [PubMed] [Google Scholar]
Viby‐Mogensen 1981
- Viby‐Mogensen J, Howardy‐Hansen P, Chraemmer‐Jørgensen B, Ording H, Engbaek J, Nielsen A. Posttetanic count (PTC): a new method of evaluating an intense nondepolarizing neuromuscular blockade. Anesthesiology 1981;55(4):458‐61. [PUBMED: 7294384] [PubMed] [Google Scholar]
Viby‐Mogensen 1988
- Viby‐Mogensen J, Jensen E, Werner M, Nielsen HK. Measurement of acceleration: a new method of monitoring neuromuscular function. Acta Anaesthesiologica Scandinavica 1988;32(1):45‐8. [PUBMED: 2830760] [DOI] [PubMed] [Google Scholar]
Viby‐Mogensen 2000
- Viby‐Mogensen J. Postoperative residual curarization and evidence‐based anaesthesia. British Journal of Anaesthesia 2000;84(3):301‐3. [PUBMED: 10793585] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
www.fda.gov
- Highligts of prescribing information: BLOXIVERZ™ (neostigmine methylsulfate). www.fda.gov. [https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204078s000lbl.pdf]
References to other published versions of this review
Abrishami 2008
- Abrishami A, Ho J, Wong J, Yin L, Chung F. Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007362] [DOI] [PubMed] [Google Scholar]
Abrishami 2009
- Abrishami A, Ho J, Wong J, Yin L, Chung F. Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007362.pub2] [DOI] [PubMed] [Google Scholar]