Abstract
Background
Anticholinergic agents such as ipratropium bromide are sometimes used in the treatment of chronic asthma. They effect bronchodilation and have also been used in combination with ß2 ‐agonists in the management of chronic asthma.
Objectives
To examine the effectiveness of anticholinergic agents versus placebo and in comparison with ß2 ‐agonists or as adjunctive therapy to ß2 ‐agonists.
Search methods
The Cochrane Airways Group asthma and wheeze database was searched with a pre‐defined search strategy. Searches were current as of August 2008. Reference lists of articles were also examined.
Selection criteria
Randomised trials or quasi‐randomised trials were considered for inclusion. Studies assessing an anticholinergic agent versus placebo or in combination/comparison with ß2 ‐agonists were included. In practice, all ß2 ‐agonists were short acting. Short‐term (less than 24 hours duration) were not considered for this review.
Data collection and analysis
Two reviewers independently assessed abstracts for retrieval of full text articles. Papers were then assessed for suitability for inclusion in the review. Data from included studies were extracted by two reviewers and entered into the software package (RevMan 4.2). We contacted authors for missing data and some responded. Adverse effect data were analysed if reported in the included studies.
Main results
The studies analysed were in two groups: those comparing anticholinergics with placebo and those comparing the combination of anticholinergics with short acting ß2 ‐agonists versus short acting ß2 ‐agonists alone. The former group had 13 studies involving 205 participants included in this review, and the latter 9 studies involving 440 patients. Generally methodological quality was poorly reported, and there were some reservations with respect to the quality of the studies. Despite the limited number of studies that could be combined, anticholinergic agents in comparison with placebo resulted in more favourable symptom scores particularly in respect of daytime dyspnoea (WMD ‐0.09 (95%CI ‐0.14, ‐0.04, 3 studies, 59 patients). Daily peak flow measurements also showed a statistically significant improvement for the anticholinergic (e.g. morning PEF: WMD =14.38 litres/min (95%CI 7.69, 21.08; 3 studies, 59 patients). However the clinical significance is small and in terms of peak flow measurements equates to approximately a 7% increase over placebo. The more clinically relevant comparison of a combination of anticholinergic plus short acting ß2 ‐agonist versus short acting ß2 ‐agonist alone gave no evidence in respect of symptom scores or peak flow rates of any significant differences between the two regimes. Again there are reservations with respect to the quality of the information from which these conclusions are drawn. An update search in August 2004 did not identify any new studies.
Authors' conclusions
Overall this review provides no justification for routinely introducing anticholinergics as part of add‐on treatment for patients whose asthma is not well controlled on standard therapies. This does not exclude the possibility that there may be a sub‐group of patients who derive some benefit and a trial of treatment in individual patients may still be justified. The role of long term anticholinergics such as tiotropium bromide has yet to be established in patients with asthma and any future trials might draw on the messages derived from this review.
Plain language summary
Anticholinergic agents for chronic asthma in adults
Anticholinergic agents such as Atrovent are sometimes used to treat people with asthma as a bronchodilator that opens up the airways in the lungs. This review found that although this treatment was better than placebo, the size of the effect was rather small. When the drug was used in combination with more widely used bronchodilators (beta‐agonists such as fenoterol), it did not appear to add much benefit. However, there are concerns about the quality of the studies that have been analysed. It could be that there are some adults with chronic asthma who respond to treatment with anticholinergic drugs, but the review has not been able to identify their common characteristics.
Background
Asthma is the commonest chronic disease affecting all age groups in developed countries (Harrison 1998), and has been recognised and treated in the East for centuries (Gross 1984). The anticholinergic agent, atropine was probably the first treatment used for asthma and was introduced in the West in the early 1800s (Gross 1984). Atropine acts as a bronchodilator, but has adverse effects, even at doses close to those used for bronchodilation (Gross 1988), and, as a result, fell from favour following the commercial development of ephedrine and adrenaline ‐ beta‐agonists ‐ in the 1920s and, later, the methylxanthines. However, in the 1970s a quaternary ammonium synthetic analogue of atropine, ipratropium bromide, was developed, which gave few systemic side effects and revived interest in the anticholinergics (Gross 1988). A recent study in the USA (Taylor 1999) showed that anticholinergics were prescribed for asthma in 7 of 85 cases (8%) ‐ age range 18‐54 years, with a range of disease severities. Data from a UK general practice database on new use bronchodilators in severe asthma showed that, of 14657 patients, 20% received ipratropium bromide (Meier 1997).
Anticholinergic agents act as bronchodilators. The rationale for using them in asthma is based on the assumption that activation of the parasympathetic (cholinergic) nerve pathway is an important mechanism for producing airway obstruction. Anticholinergics work by competing with acetylcholine for receptor sites at the vagus nerve‐nerve or nerve‐muscle junctions. This prevents transmission of reflexes induced by asthma stimuli (Gross 1997, Beakes 1997).
Until the early 1980s, the treatment for asthma was regular bronchodilator agents (especially short‐acting selective ß2 ‐agonists ‐ the successors to adrenaline and ephedrine) and other agents were added only when symptoms reached an unacceptable level of frequency or severity (O'Connor 1998). Treatment was based around relieving the symptoms (airway constriction) and patients were often encouraged to tolerate continuing symptoms, treating them when necessary with repeated doses of ß2 ‐agonists. However, in the mid 1980s, opinions on asthma changed rather strikingly, because asthma was recognised as a chronic inflammatory disease (Barnes 1996). The therapeutic emphasis in chronic asthma in adults changed to embrace agents that suppress the underlying inflammation, notably inhaled corticosteroids (BTS 1993, Bousquet 2000).
Asthma guidelines have been available since the late 1980s and are updated regularly (Harrison 1998, Kemp 2000, Creer 1999). Initially they were arrived at by consensus, based on discussion and state‐of‐the‐art literature reviews. More recent guidelines such as the BTS/SIGN guidelines (BTS/SIGN 2004) have adhered to systematic electronic searching and standardised guideline methodology. The grades of recommendation are derived from levels of evidence. According to current guidelines, the early introduction of anti‐inflammatory treatment in the form of inhaled steroids is indicated for all but the mildest forms of asthma. Bronchodilators are adjuvant treatments used in one of two ways. Firstly, short‐acting bronchodilators in the form of ß2 ‐agonists are used as‐needed to relieve episodic symptoms. Secondly, in patients whose asthma is inadequately controlled despite regular inhaled steroids, the addition of long‐acting ß2 ‐agonists has been shown to improve lung function and symptoms, and to decrease exacerbations (Ni Chroinin 2005). A small proportion of patients have asthma which is not adequately controlled despite moderate doses of inhaled steroid and a long‐acting ß2 ‐agonist. There are few clinical trials in this specific patient group and possible options include an increase in the dose of inhaled steroid or additional treatment, which might include leukotriene receptor antagonists, theophyllines or anticholinergics.
It is a widely held view that anticholinergics are less effective than ß2 ‐agonists in the symptomatic treatment of chronic asthma (Cazzola 1998), although there is considerable variation in treatment effect amongst patients. Attempts to identify subgroups that respond better to anticholinergics have not been very successful. In general, though, anticholinergics may be better in the following: older patients ‐ the ß2 ‐agonist responsiveness apparently declines with age (Cazzola 1998, Connolly 1993); patients intolerant to ß2 ‐agonists (Boulet 1999); patients with nocturnal asthma (Beakes 1997); patients with chronic asthma and concurrent fixed airway obstruction (Cazzola 1998), patients with intrinsic asthma and those with longer duration of asthma (Partridge 1981). Patients with high serum immunoglobulin‐E levels may be better treated with anticholinergic agents (Cazzola 1998), although earlier studies show that atopic patients respond less well to anticholinergics than those with non‐atopic disease (Jolobe 1984).
The possible differences in responsiveness to anticholinergics between different patient groups illustrates the heterogeneous nature of asthma. This is further compounded when taking into account the overlap of asthma and COPD. At one end of the spectrum are patients whose airflow limitation shows marked spontaneous fluctuations and improves considerably with treatment (asthma). At the other end of the spectrum are patients whose disease fluctuates to a very limited extent and is "irreversible". Whilst an improvement of greater than 15% after inhaling a short‐acting ß2 ‐agonist indicates a degree of reversibility, any such figure is inevitably arbitrary when there is a spectrum of airway disease ranging from "reversible" to "irreversible". Clinical features such as age, nature of symptoms, atopic status and smoking history are important factors in terms of diagnosis. This blurring of the boundaries becomes important when considering anticholinergics since it is generally regarded that they may have a small but proportionately greater effect than ß2 ‐agonists in patients with COPD.
The other factor that needs to be taken into consideration is the relative safety of ß2 ‐agonists and anticholinergics ‐ the former can cause an increased heart rate and have also been associated with increased bronchial activity and increased mortality (Emilien 1998, Spitzer 1992). In comparison, the anticholinergics have relatively few side effects. Finally, anticholinergics are also used in combination with ß2 ‐agonists (Gross 1984). In severe asthma, the main treatment is based on inhaled steroids, but the benefit : harm ratio of inhaled steroids appears to be reduced once the total daily dose exceeds 1000 mg (O'Connor 1998), with adverse effects leading to increased risk of osteoporosis, skin thinning and adrenal problems (van Schayck 1995). As an alternative to high doses of inhaled steroid, moderate levels of inhaled steroid have been used in conjunction with oral steroids, leukotriene‐receptor antagonists, steroid sparing alternatives, theophylline, anticholinergics or long‐acting ß2 ‐agonists (O'Connor 1998). Oral steroids and some of the alternatives, including long‐acting beta‐agonists, have adverse effects too (Barnes 1997, Hougardy 2000), which might indicate a place for the relatively safe anticholinergics.
Objectives
The objectives of this review, as defined in the original protocol ("Anticholinergic agents for chronic asthma in adults"), were to investigate the efficacy and safety of the various anticholinergic agents in chronic asthma in adults in two modes of treatment:
Anticholinergics for symptom relief (as‐needed use), either alone or in combination with ß2 ‐agonists, in comparison with ß2 ‐agonists or placebo. This included both acute (short‐term) bronchodilating effects and the longer‐term effectiveness of bronchodilators for symptom relief.
Anticholinergics as regular, longer‐term treatment, including their use as adjuncts to inhaled steroids, in comparison with ß2 ‐agonists (long‐ or short‐ acting) or placebo.
Protocol splitting It was decided to split the original review in line with the two objectives because there was so much data, in an attempt to make the reviews more comprehensible. In practice there were no prn (as‐needed) studies in the longer term, so that the two reviews focussed on (A) short‐term bronchodilator effects (up to 24 hours) and (B) longer‐term regular treatment (for periods from 2 days to 3 years). The latter focus is reported in this review and the former in the review, "Anticholinergic agents for chronic asthma in adults short term".
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials and quasi‐randomised trials (e.g. allocation to treatment by alternation or date of birth) were analysed in the review. If a study did not report allocation details, but the allocation could have been randomised or quasi‐randomised, further information was sought from the authors and the study was included if randomisation could be confirmed. Included studies reported the measurement of at least one of the outcome variables.
Types of participants
Adult patients with stable asthma: stability was defined as an absence of exacerbations, and stable medication prior to the study. Asthma therapy used during the study was also required to be stable.
The studies were required to report that the patients had asthma ‐ preferably this was defined according to reliable criteria, such as those of the ATS, but less exactly, the patients had to display a reversibility in their lung function of at least 15% following administration of a short term ß2 ‐agonist (reversibility shown using anticholinergics was considered confounded and such studies were excluded). Studies of patients with serious, non‐respiratory diseases (e.g. cancer, heart disease) were excluded, as were those of patients with cystic fibrosis or irreversible airways disease. Studies involving a mixed group of patients having either asthma or COPD were included if separate results were given (or could be obtained from trialists) for the patients with asthma.
Types of interventions
Studies were included if they used anticholinergic agents (including ipratropium bromide, oxitropium bromide, tiotropium bromide, atropine methonitrate and glycopyrrolate) used as a bronchodilator, in comparison with a ß2 ‐agonist or placebo. Delivery systems included nebulisers, metered dose inhalers and powder inhalers, with and without a spacer device.
Studies were included that compared:
Anticholinergics versus ß2 ‐agonist
Anticholinergics versus placebo
The combination of ß2 ‐agonist and anticholinergic versus ß2 ‐agonist alone
Different doses of anticholinergics
One anticholinergic versus another
Analysis was carried out only for comparisons 2 and 3 for the longer‐term studies.
It was decided not to analyse trials that had atropine sulphate as the sole anticholinergic agent, because this has a different mode of action to the quaternary salts of atropine, has adverse effects and is therefore not routinely used as a bronchodilator in chronic asthma. Similarly, studies using non‐selective ß‐agonists such as isoprenaline were included, but not analysed.
Types of outcome measures
All possible outcome measures were considered, but the main focus was as follows:
Daytime and night‐time asthma symptom scores (primary outcome)
Bronchodilator use for symptom relief (i.e. 'rescue medication')
Daily peak flow (PEF; morning and evening)
Patient preference
Quality of life score
Asthma exacerbation rates/ hospital admissions ‐ number of patients, not exacerbations
Adverse events and effects, e.g. tachycardia
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as 'asthma' were searched using the following terms:
Anticholinergic* OR cholinergic* OR ipratropium OR Muscarinic* OR ipratropium OR N‐isopropylatropine OR oxitropium OR tiotropium OR atropine OR glycopyrrolate OR Atrovent OR Sch1000 OR Oxivent
The most recent search was conducted in August 2008.
Other sources
Bibliographies from retrieved trials, meta‐analyses and narrative reviews were checked to identify relevant cross‐references. Attempts were made to obtain additional data from the authors and from the drug company, Boehringer Ingelheim (Bracknell UK); largely these were fruitless because many of the studies were about 20 years old and the data not available or we were unable to contact the authors.
Data collection and analysis
Step I. Trials that appeared potentially relevant were identified independently by two reviewers (MW and MB) from the search citation abstracts.
Step II. Using the full text of each study, two reviewers (MB and MW) independently selected trials for inclusion in the review. Agreement was measured using simple agreement and differences were resolved by discussion.
Step III. After a preliminary review of all studies to confirm the basic requirements, two reviewers independently assessed the methodological quality of the included trials with particular emphasis on the concealment of allocation, which was ranked using Cochrane criteria (grade A: adequate concealment; grade B: uncertain; grade C: clearly inadequate concealment). Any differences were resolved by discussion. The Jadad scale (Jadad 1996), described in the protocol, was not used for additional analysis because it and other scales are no longer thought to be the best approach to quality assessment (Clarke 2003).
Step IV. Two reviewers (MB and MW) independently extracted data from the included trials and one reviewer (MW) entered results into the Cochrane Collaboration software program (Review Manager, version 4.2). These data were checked by MB. A pilot for the data extraction form was carried out on a small sample of studies and was revised appropriately. In practice this led to the use of two forms, the first stage involving details of participants, the interventions, the type of outcomes and whether variability measures (e.g. standard errors, p‐values) were reported. The second form contained results if available (e.g. mean and standard deviations for continuous outcomes). In some cases, information regarding outcomes was estimated from graphs; this was performed independently by the two reviewers.
Data extraction included the following items:
Participants: age, gender, smoking status, type of asthma (intrinsic, extrinsic), allergic status (atopic, non‐atopic), presence of co‐existing COPD, severity of asthma (rated by MB based on baseline lung function, maintenance therapy and descriptions of the authors), history of asthma, symptom status at trial entry (symptomatic/asymptomatic)
Intervention: anticholinergic agent, dose, schedule, spacer device, concurrent ß2 ‐agonist
Control: ß2 ‐agonist, placebo
Outcomes: self‐rated daily symptom score/symptoms ‐ daytime and night‐time, pulmonary function measures (baseline and daily peak flow rates), bronchodilator use for symptom relief, hospital admissions/rate of acute exacerbations, quality‐of‐life instruments, adverse events and effects.
Design: method of randomisation, allocation concealment, blinding, presence and type of run‐in period, study design (parallel, cross‐over), concurrent background therapy, duration of study and, for crossover studies, the duration of any washout period and/or the time between successive crossover arms
Funding and multi‐centre trials
STATISTICAL CONSIDERATIONS
The results for particular outcomes for each trial were combined using RevMan (Version 4.2). An intention‐to‐treat analysis was completed where possible. Most of the studies in this review had a crossover design, that is, the patients were given each intervention in a random order. This means that there are within‐patient correlations, which, ideally, should be treated using a paired analysis (Elbourne 2002). Where possible, this was carried out for continuous outcomes according to the guidance given by Elbourne 2002. The standard error for the difference of means was calculated, either from individual patient data or from p‐values, and the between‐period correlation parameter, ρ, was calculated. For some studies the standard error was imputed using the lowest value of ρ from the other studies. For parallel studies the standard error was calculated as the square root of the sum of the variances for each arm. All similar parallel and crossover studies with sufficient data were pooled using the fixed effects generic inverse variance method in RevMan Version 4.2 to give a weighted mean difference and 95% confidence intervals.
In the absence of paired data the trials were analysed by the conventional approach of treating the two arms of the crossover as if they were from a parallel trial with separate groups (i.e. setting ρ=0). If this was the case, parallel and crossover trials were analysed separately.
For non‐paired data and continuous variables, a fixed effects weighted mean difference (WMD) or standardised mean difference (SMD) and 95% confidence interval (CI) were calculated for each study. All similar studies were pooled using fixed effects WMD/SMD and 95% CIs.
For dichotomous variables, a fixed effects odds ratio (OR) with 95% confidence intervals (95% CI) was calculated for individual studies. All similar studies were pooled using fixed effects OR and 95% CIs.
For pooled effects, heterogeneity was tested using the Breslow‐Day test; p < 0.1 was considered statistically significant. The statistical term I2 (which is a measure of the inconsistency among results) was also used to monitor the variability among studies.
Funnel plots were not constructed because of the paucity of included studies.
SUB‐GROUP/SENSITIVITY ANALYSES:
If there had been sufficient studies sub‐group analyses would have been carried out. A‐priori defined subgroups were:
Concurrent therapy with inhaled corticosteroid or not
Asthma severity (mild, moderate or severe)
Concurrent COPD or not
Smoking status
Age (<40; 40‐69; 70+)
Symptom status at trial entry (symptomatic/asymptomatic)
Asthma type (intrinsic, extrinsic)
Allergic status (atopic, non‐atopic)
Duration of asthma
Dose, duration and delivery method of therapy
Type of anticholinergic agent
Sensitivity analyses had been proposed using the following domains:
Methodological quality
Random effects versus fixed effects modelling
However, there was insufficient data to examine these.
Rationale for the presentation of studies in the review The original review had a very large number of included studies, separated into two modes of treatment (short‐term bronchodilation and longer‐term regular treatment). To manage the data we used a Microsoft Access database and prioritised the comparisons to be studied. This prioritisation took place before data extraction. For the longer‐term studies, the clinical question of particular interest was whether a combination of anticholinergic with ß2 ‐agonist would give additional symptom relief compared to that obtained with ß2 ‐agonist alone, so this was the main focus. It was decided to set aside the comparison anticholinergic versus ß2 ‐agonist because it was of little practical interest, and to look at the comparison anticholinergic versus placebo only if no difference was found between the combination and the ß2 ‐agonist. This would clarify whether the anticholinergic had any effect (relative to placebo) alone. In reality, two types of comparison were analysed for the longer term studies:
Anticholinergic versus placebo
Anticholinergic plus ß2 ‐agonist versus ß2 ‐agonist alone.
It was felt appropriate to separate the studies according to duration and the following definitions were provided by MB prior to data extraction: short (2 days to 2 weeks), medium (more than 2 weeks to 2 months) and long (more than 2 months).
Results
Description of studies
Results of the search
From electronic searches conducted for the original review, a total of 623 references were identified. From the abstracts of the study reports, 210 references were marked for further scrutiny, and the remaining 413 were excluded because they clearly did not meet the inclusion criteria (for example, the patients were children or were treated for acute asthma). A further 103 possible studies were identified from other sources (e.g. references in included studies and reviews). This gave 302 possible references for which the full papers were obtained where possible. 26 reports were in non‐English languages, of which 19 were translated. 239 studies were included in the original review. Of these, 45 studies had only longer‐term data, 187 had only short‐term data and 7 had both. The longer‐term studies were all concerned with the regular addition of anticholinergics and there were no studies of longer‐term as‐needed use. For details of the studies that were excluded and the studies included in the short term review see the Table of Excluded Studies. Two longer‐term studies were later excluded and deserve particular mention: Tarlo 1982, which is a crossover comparison of anticholinergic and placebo over 90 days was excluded because the patients were instructed to reduce gradually their dose of bronchodilators ‐ thus their concurrent medication was not stable, which we considered to be confounding. Secondly, Brand 1992 (and its 7 accompanying papers) was a large parallel 3 year comparison of the combination of ipratropium bromide and terbutaline versus terbutaline alone. Unfortunately the study did not report separate results for asthma, although these were recorded, and contact with the authors has failed to produce any data. Four other studies did not state if the patients were randomised to treatments, and no further information was obtained from the authors. This left 39 reports of randomised or quasi randomised trials. Of these 39 longer‐term study reports, 9 were additional reports of trials already reported, leaving 30 trials.
The 30 longer‐term trials sometimes included more than two randomised interventions, giving rise to more than one comparison per trial; thus 23 trials had one comparison, 6 two comparisons and 1 trial had 3 comparisons. The number of trials reporting the different comparisons was:
Anticholinergic versus placebo in 13 trials (analysed in this review)
Anticholinergic plus ß2 ‐agonist versus ß2 ‐agonist alone in 9 trials (analysed in this review)
Anticholinergic versus ß2 ‐agonist compared in 12 trials (not analysed)
Anticholinergic dose comparison in 4 trials (not analysed)
Different anticholinergics were compared in 0 trials (not analysed).
Thus, 22 trials were included for the two types of comparison prioritised here. The flow of studies is shown in figure 1 (Figure 1).
1.
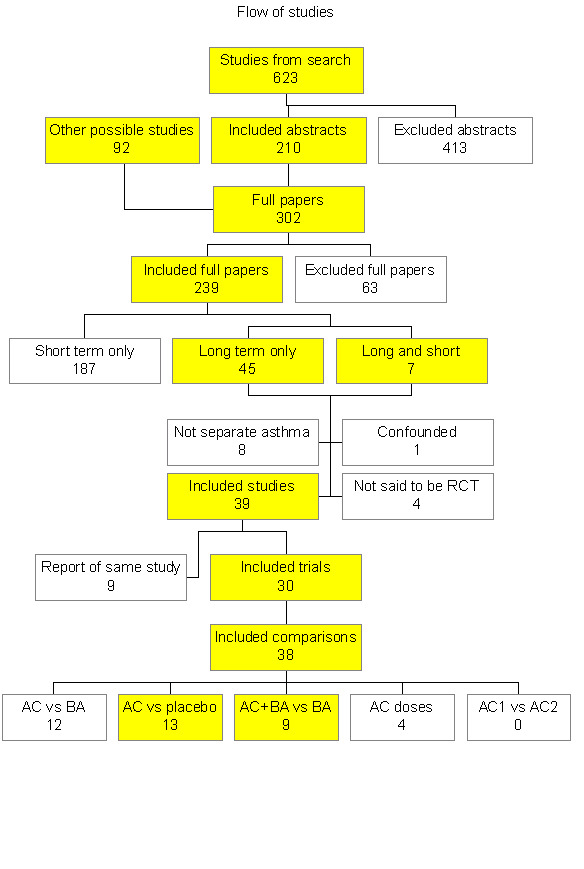
Fig 1 Study flow.
Update searches to August 2008 identified two studies which we excluded as they were in children (Dutt 1990; Li 2003).
Included studies
Study size
In the placebo comparison the study size of the crossover studies ranged from 6 to 39 patients and all but two studies (Vaughan 1988, Wheeler 1987) had fewer than 20 patients with asthma. In the combination comparison there were two parallel trials each with over 100 patients and the remaining crossover trials had a study size ranging from 12 to 28, with only two studies having more than 20 participants (Rebuck 1983, Tammivaara 1993).
Design
In the placebo comparison all the trials had a crossover design; for the combination versus ß2 ‐agonist comparison there were 2 parallel trials and 7 with a crossover design. For the placebo comparison, 1 trial had a quasi‐randomised design (Light 1977), but all other included trials reported randomisation.
Duration
In the placebo comparison the duration ranged from 4 days to 1 month per phase; for the combination versus ß2 ‐agonist comparison the parallel trials had durations ranging from 8 ‐12 weeks, and the crossover trials' phases ranged from 2 weeks to 3 months.
Participants
In the anticholinergic‐placebo comparison, one study (Taytard 1984) had patients with perennial asthma that became worse in spring and summer and improved over the course of the study. The authors found an order effect and for this reason only first period results were used for this study, and the individual patient data analysed as a parallel trial.
One study did not give any details about concurrent medication (Ruffin 1990), one was unclear but had no oral steroids (Coe 1986), two had no concurrent medication (Ahonen 1978, Taytard 1984), one had sodium cromoglycate only (Bellia 1988), three had inhaled steroids only (Pavia 1989, Tormey 1995, Wheeler 1987), one had unspecified steroids (Kreisman 1981) and the others had oral and inhaled steroids (Light 1977, Salorinne 1988, Taylor 1986, Vaughan 1988).
Four studies were assessed to have patients with mild asthma (Ahonen 1978, Bellia 1988, Ruffin 1990, Taytard 1984), 5 with moderate asthma (Kreisman 1981, Pavia 1989, Salorinne 1988, Tormey 1995, Vaughan 1988) and 4 with heterogeneous patient populations (Coe 1986, Light 1977, Taylor 1986, Wheeler 1987). No study claimed to have patients with concurrent COPD.
In the combination comparison, one study did not give any details about concurrent medication (Waite 1987), one had aminophylline but did not say if steroids were used (Macaluso 1986), one had no steroids (Tammivaara 1993), one had only inhaled steroids and sodium cromoglycate (Haahtela 1991), two had unspecified steroids (Mazzei 1985, Philip‐Joet 1990) and the other three had oral and inhaled steroids (Pierce 1982, Rebuck 1983, Wolstenholme 1989). Most of the studies also used methylxanthines as concurrent medication. Four studies were assessed to have patients with moderate asthma (Haahtela 1991, Pierce 1982, Tammivaara 1993, Wolstenholme 1989), two gave no information on severity (Macaluso 1986, Waite 1987) and three had heterogeneous patient populations (Mazzei 1985, Rebuck 1983, Philip‐Joet 1990). One study (Pierce 1982) had patients with concurrent bronchitis.
Interventions
For the anticholinergic‐placebo comparisons, there were three main variables with respect to the anticholinergic. The anticholinergic drugs included ipratropium bromide (7 studies), oxitropium bromide (8), nebulised atropine methonitrate (1) and inhaled atropine sulphate (Light 1977). The latter study was not analysed. The dose of ipratropium bromide ranged from 40 µg to 200 µg delivered either by metered dose inhaler or powder inhaler. The frequency of administration ranged from twice daily to five times daily. For further details see table of included studies.
All of the studies comparing a combination of ß2 ‐agonist plus anticholinergic versus ß2 ‐agonist alone used ipratropium bromide as the anticholinergic agent at either 40 or 80 µg, three or four times per day. In all studies the ß2 ‐agonist was short acting. In eight studies fenoterol was combined with ipratropium as Duovent (40 µg ipratropium, 100 µg fenoterol), Berodual (20 µg ipratropium, 50 µg fenoterol) or using two separate inhalers. In the ß2 comparison arm, three studies used fenoterol and the remaining studies used salbutamol. Three further studies used terbutaline as the ß2 ‐agonist, both in combination and as the comparator. However one of these studies (Tammivaara 1993) used oral controlled‐release tablets and this study was analysed separately because of the different pharmacokinetics of the oral formulation. For further details see table of included studies.
Outcome measures
Outcome measures chosen were those generally accepted to reflect the primary outcome of asthma control. Daytime and night time asthma symptom scores were derived from daily diaries. Objective measurements of lung function were daily peak flow measurements, recorded morning and evening, before and after use of inhaler. Additional outcomes were treatment related withdrawal from the study, patient preference, bronchodilator use as rescue medication (number of patients requiring this), number of patients with exacerbations and adverse effects. Clinical and physiological assessments at the beginning and end of each trial period were regarded as being less valuable, given the recognised spontaneous fluctuations which can occur in asthma.
Risk of bias in included studies
The methodological quality was poorly reported in all 22 studies, but the authors provided further details for five studies (Philip‐Joet 1990, Pierce 1982, Vaughan 1988, Wheeler 1987, Wolstenholme 1989). All but one of these (Philip‐Joet 1990) still did not give an adequate description of allocation concealment, with only partial details provided (for example using sealed envelopes). One additional study (Light 1977) reported an inadequate method of allocation concealment. Generally the reporting of blinding was vague, with all but two being described as double blind or blind (1 study). The Philip‐Joet study stated that it did not have a double blind design and the comparison we used for the Salorinne study (inhaled Ipratropium bromide versus powder placebo) could not have been blinded. The Macaluso study, despite stating it was double blinded, was not blinded because the combination arm used three inhalations and the ß2 ‐agonist four. The Pavia study reported that some patients could distinguish by taste oxitropium bromide from placebo, and it is likely that the blinding was broken in some of the other placebo studies too. This may also be true of the combination comparison, because ipratropium bromide and salbutamol, for instance, taste quite different. Nine of the 22 studies had intention to treat analyses carried out and for two other studies it was unclear what was done. Six studies (Haahtela, Philip‐Joet, Tammivaara, Wolstenholme, Ruffin and Wheeler) had at least 20% of the patients' results missing for some outcomes, and nearly 40% of the patients were not analysed for the 8‐week PEF outcome in the Philip‐Joet study.
Effects of interventions
Duration
Although the original intention was to separate the study durations as described in the methods section, in practice there were too few studies to make this feasible, so all durations were considered together.
A) Anticholinergic versus placebo
There were 13 studies comparing anticholinergic with placebo. Of these, one was not included in our analysis because it used atropine sulphate (Light 1977).
Seven studies were also not included in the initial analysis: 6 studies did not record means for the continuous outcomes and 1 other study did not report standard deviations or p‐values. The authors of these studies were contacted and Boehringer Ingelheim provided individual patient data for Wheeler 1987; some other authors offered data, but to date these had not arrived. Thus, there were only 4 studies that provided sufficient data for analysis of the continuous outcomes, although 7 studies gave data for the dichotomous outcomes.
Ahonen 1978 compared two doses of anticholinergic and, in order to clarify the main analysis, we decided to choose one dose of ipratropium bromide, based on the head‐to‐head comparison. It was found that for both symptom scores and peak flow measurements the summary statistics were in favour of the lower dose (40µg), although none was statistically significant (see additional Table 1 and forest plots). Therefore for this study the lower dose was used in comparison with placebo in the main analyses.
1. Comparison of Ahonen study (IB 40 vs IB 80).
| Outcome | Mean difference | % MD of IB 80 | 95% CI |
| Daily Symptom score ‐ daytime dyspnoea | ‐0.30 | 17% decrease | ‐0.85, 0.25; not statistically significant |
| Daily Symptom score ‐ daytime cough | ‐0.50 | 22% decrease | ‐1.12, 0.12; not statistically significant |
| Daily Symptom score ‐ night dyspnoea | ‐0.20 | 11% decrease | ‐0.91, 0.51; not statistically significant |
| Daily PEFR ‐ am pre‐dose | 34 litres/min | 11% increase | ‐4.06, 74.06; not statistically significant |
| Daily PEFR ‐ pm pre‐dose | 24 litres/min | 6% increase | ‐19.16, 67.16; not statistically significant |
| Daily PEFR ‐ am post‐dose | 29 litres/min | 7% increase | ‐11.21, 69.21; not statistically significant |
The results for various outcomes for the comparison of anticholinergic with placebo are summarised in additional tables 2‐5. Where there was more than one similar study, the results were combined and the summary statistics reported in the additional table and shown in a forest plot. Otherwise the results in the table are for single studies.
For some outcomes, the percentage increase or decrease over placebo was calculated. This is an approximation used to give an estimate of the magnitude of the effect in clinical terms. It was done using as denominator the simple average, over the individual studies, of the placebo means; the numerator was the weighted mean difference from the meta‐analysis (or single study). It should be noted that the studies involved had a range of asthma severities, so that patients with more severe disease might derive greater benefit than represented by the mean percentage change, but there is insufficient information to look at subgroups, either for peak flows or symptom scores.
1. Symptom scores (Table 2) The data were analysed by calculating the difference in means and the paired difference standard error and pooling the data, where appropriate, using the generic inverse variance method. The Wheeler study gave individual patient data (IPD) and the Ahonen study reported p‐values for the comparison. The scales for the studies were different (Ahonen 1978: 1‐4; Wheeler 1987; Salorinne 1988: 0‐3 and Taytard 1984 0‐13), but the first two scales were linearly equivalent and so could be combined in the analysis. Taytard 1984 was analysed separately because the trialists reported a combined 'clinical score', which was a composite of day‐time and night‐time symptom scores and drug consumption for symptomatic relief. For this study, only first period results were used (because the authors reported an order effect); the study reported individual patient data and because of large baseline differences, the change scores were used.
2. Anticholinergic versus placebo ‐ symptom scores.
| Outcome | Scale | Studies | Pooled summary stats | Heterogeneity | Single study results | Stat sig? | % change on placebo | Comments |
| Symptom score ‐ dyspnoea daytime | Ahonen 1978 Salorinne 1988 Wheeler 1987 | Paired mean difference ‐0.09 (95%CI ‐0.14, ‐0.04) | p = 0.45, I‐squared = 0% | Y | 15% decrease (i.e., favours anticholinergic) | Wheeler: rho = 0.817 | ||
| Symptom score ‐ dyspnoea daytime (no Salorinne) | 1‐4 0‐3 | Ahonen 1978 Wheeler 1987 | Paired mean difference ‐0.07 (95%CI ‐0.13, ‐0.02) | p = 0.97, I‐squared = 0% | Y | 12% decrease | Wheeler: rho = 0.817 | |
| Symptom score ‐ cough daytime | 1‐4 0‐3 | Ahonen 1978 Salorinne 1988 | Mean difference Aho ‐0.05 (95%CI ‐0.16, 0.06) Sal ‐0.05 (no SDs) | N | 10% decrease 11% decrease | |||
| Symptom score ‐ dyspnoea night‐time | 1‐4 0‐3 | Ahonen 1978 Salorinne 1988 | Mean difference Aho ‐0.13 (95%CI ‐0.27, 0.01) Sal ‐0.09 (no SDs) | N | 24% decrease 26% decrease | |||
| Symptom score ‐ cough night‐time | 0‐3 | Salorinne 1988 | Mean difference +0.04 (no SDs) | N | 14% Increase (i.e. anticholinergic worse than placebo) | |||
| Symptom score ‐ night‐time overall score | 0‐3 | Wheeler 1987 | Paired mean difference +0.09 (95%CI ‐0.09, 0.26) | N | 28% Increase | Wheeler: rho = 0.611 | ||
| Clinical score ‐ day and night (change score) | 0‐13 | Taytard 1984 | Mean difference (parallel trial) ‐0.72 (95%CI ‐1.37, ‐0.07) | Y | 71% decrease |
The four studies had treatment periods ranging from 4 days to 4 weeks and it was decided to combine these different durations, by calculating the mean symptom score per day.
It should be noted that Wheeler 1987 (which provided individual patient data) had a number of patients who failed to report their scores every day, so we calculated symptom scores based only on the patients who completed their diary cards for more than 20 days (28/30 patients). The study reported symptom scores over the whole period for each patient, so we calculated their daily symptom score (for the number of days they reported), and then took the mean for all patients included. Ahonen 1978 reported placebo values averaged over two placebo arms.
The night‐time symptom scores could not be combined because the Ahonen study measured only dyspnoea and the Wheeler study gave an overall measure of cough, breathlessness and wheeze; Salorinne 1988 gave no standard deviations, although it measured cough and dyspnoea separately. Thus there was only one symptom score outcome for which data could be pooled ‐ daytime dyspnoea. In order to use the generic inverse variance method, we calculated the interaction parameter, ρ, for the Wheeler study (ρ=0.817) and used this in the Ahonen study to impute the standard error. Salorinne 1988 gave no standard deviations so for each arm we used the mean of the standard deviations from Ahonen 1978 and Wheeler 1987 and then calculated the standard error for the difference using the Wheeler value of ρ. Although not ideal, we feel that this gives the best representation of the data. This approximation for the Salorinne 1988 was examined by a sensitivity analysis and it was found that the addition of the Salorinne 1988 made little difference to the summary statistics.
The results are summarised in additional Table 2; Table 3; Table 4 Daytime dyspnoea: the summary statistics were: WMD ‐0.09 (95%CI ‐0.14, ‐0.04, 3 studies, 59 patients), i.e. the anticholinergic agent gave more favourable symptom scores than the placebo, and the result was statistically significant. There was little heterogeneity (I2=0%, p =0.45). This corresponds to a 15% decrease in symptom score over placebo. The other symptom scores could not be pooled and only the Taytard study gave a statistically significant result on its own ‐ which was in favour of the anticholinergic agent. Otherwise the results of single studies indicated that, relative to placebo, the anticholinergic showed some improvement in daytime cough and night‐time dyspnoea, but some worsening in night‐time cough.
3. Anticholinergic versus placebo ‐ daily peak flows.
| Outcome | Time (post dose) | Studies | Pooled summary stats | Heterogeneity | Single study results | Stat sig? | % on placebo mean | Comments |
| Daily PEFR am pre‐bronchodilator | 07.00 (6h) 08.00 (10h) "on rising" (12h) | Ahonen 1978 Salorinne 1988 Wheeler 1987 | Paired mean difference 14.38 L/min (95%CI 7.69, 21.08) | p = 0.22 I‐squared = 34% | Y | 7% increase (i.e. favours Antichol) | Wheeler: rho = 0.919 | |
| Daily PEFR am pre‐bronchodilator (no Salorinne) | 07.00 (6h) "on rising" (12h) | Ahonen 1978 Wheeler 1987 | Paired mean difference 16.26 l/min (95%CI 9.21, 23.31) | p = 0.65 I‐squared = 0% | Y | 5% increase | ||
| Daily PEFR pm pre‐bronchodilator | 22.00 (3h) 22.00 (4h) "on retiring" (12h) | Ahonen 1978 Salorinne 1988 Wheeler 1987 | Paired mean difference 23.48 l/min (95%CI 12.32, 34.65) | p = 0.01 I‐squared = 78% | Y | 7% increase | Wheeler: rho = 0.911 | |
| Daily PEFR am post‐bronchodilator | 10.00 (3h) 10.00 (2h) | Ahonen 1978 Salorinne 1988 | Paired mean difference 34.91 l/min (95%CI 20.13, 49.70) | p = 0.06 I‐squared = 71% | Y | 9% increase | ||
| Daily PEFR pm post‐bronchodilator | 00.00 (2h) | Salorinne 1988 | Paired mean diff. 23.94 L/min (95%CI 5.20, 42.68) | Y | 7% increase | |||
4. Anticholinergic versus placebo ‐ other outcomes.
| Outcome | Studies | Pooled summary stats | Heterogeneity | Single study results | Stat sig? | Interpretation |
| Number of patients withdrawing | Pavia 1987 Wheeler 1987 | OR 0.70 (95%CI 0.13, 3.72) | p = 0.25 I‐squared = 23% | N | About two thirds the odds for withdrawal with anticholinergic as placebo | |
| Patient preference (crossover studies) | Salorinne 1988 Wheeler 1987 | OR 1.64 (95%CI 0.68, 3.94) | p = 0.82 I‐squared = 0% | N | About 1.5 times the odds for patients preferring anticholinergic as placebo | |
| No. patients using rescue medication | Wheeler | Daytime OR 0.66 (95%CI 0.18, 2.36) Night OR 0.58 (95%CI 0.21, 1.62) | N N | About half the odds for using rescue medication with anticholinergic as placebo |
In addition, Kreisman 1981 reported that patients coughed more on the ipratropium regimes. Tormey 1995 reported that there were no significant differences in symptom scores (unspecified) between placebo and oxitropium bromide over 28 days; and Vaughan 1988 reported that 'total symptom scores' (cough and wheeze) were no different when using nebulised atropine methonitrate compared with placebo over 2 weeks.
2. PEF measurements (Table 3) Four studies reported daily peak flow measurements, but one of these gave no p‐values or standard deviations; the Ahonen and Salorinne studies reported some p‐values, and IPD were given for the Wheeler 1987. ρ values for this study (although not used) were respectively 0.919 and 0.911 for the am and pm peak flows. The outcomes were daily peak flow rates measured before and after the bronchodilator dose.
Summary statistics were calculated using the generic inverse variance method. The Ahonen study gave an upper bound for the p‐value for the ipratropium‐placebo comparison, which allowed calculation of the standard error for the mean of the difference (AC ‐ placebo). The Salorinne study gave an upper bound for the p‐value for the evening PEF, but for the morning value the standard deviation for the Wheeler study was taken and the standard error calculated from this (Ahonen 1978 was not used for this purpose because of a discrepancy between the graph and table values).
The following results were found: a) Morning PEF The PEF daily average weighted mean difference (WMD) was: WMD =14.38 litres/min (95%CI 7.69, 21.08; 3 studies, 59 patients) i.e., statistically significantly higher for the anticholinergic; this showed some heterogeneity (p = 0.22, I2 = 34%) and corresponds to an increase of about 7% over placebo. We tested the assumptions for Salorinne 1988 using a sensitivity analysis, and found that the heterogeneity was removed (p=0.65, I2 = 0%), but there was little effect on the WMD (16.26 L/min; 95%CI 9.21, 23.31). Post‐bronchodilator morning PEF measurements were available for 2 studies, and were taken 2 and 3h after the dose. The WMD was 34.91 litres/minute (95%CI 20.13, 49.70, 2 studies, 31 patients), a statistically significant difference, corresponding to an increase of 9% over placebo.
b) Evening PEF The evening (about 10 pm) PEF daily average WMD was: 23.48 L/m (95%CI 12.32, 34.65, 3 studies, 60 patients) i.e., statistically significantly larger peak flow rate for the anticholinergic, but there was heterogeneity (p=0.01, I2=78%). The WMD represents an 7% increase over placebo. Post‐bronchodilator evening PEF measurements were available only for Salorinne 1988: WMD 23.94 L/m (95%CI 5.20, 42.68, 1 study, 18 patients), giving a statistically significant increase of 7%.
Vaughan 1988 used nebulised atropine methonitrate over 2 weeks, and reported that the peak flow measurements were not different between placebo and Atropine methonitrate either in the morning or at 5pm.
3. Use of rescue medication (Table 4) Three studies reported that they recorded the use of rescue medication, but relative numbers of patients were given only in the Wheeler 1987. Taytard 1984 (3 weeks) reported that the 'mean consumption of salbutamol was essentially identical for placebo and oxitropium bromide' and Salorinne 1988 (1 week) reported that 'the number of additional treatments did not differ between the various treatments'. For the Wheeler 1987 (4 weeks) 23 of 30 patients taking oxitropium and 25/30 taking placebo required rescue medication during the day (OR 0.66 ; 95%CI 0.18, 2.36 ‐ not statistically significant) and the corresponding night time ratios were 15/30 and 19/30 (OR 0.58 ;95%CI 0.21, 1.62). 4. Patient preference (Table 4) The number of patients preferring anticholinergic was compared with the number preferring placebo in two studies (Salorinne 1988 and Wheeler 1987) ‐ in the latter case , some patients did not have a preference. In the former study patients chose between 40 µg, 200 µg (inhaled) and 200 µg powder ipratropium bromide as well as placebo (which was a powder); the odds ratio for patient preference was: OR 1.64 (95%CI 0.68, 3.94) i.e. non‐significantly in favour of the anticholinergic, with little heterogeneity.
5. Drug‐related withdrawal (Table 4) Two studies gave data on the number of asthma patients withdrawing while taking one of the study drugs. The pooled odds ratio was 0.70 (95%CI 0.13, 3.72) ‐ non significantly in favour of anticholinergic, with some heterogeneity.
6. Adverse effects (Table 5) Six studies gave some information on adverse effects, but of these Kreisman 1981 and Bellia 1988 only gave a descriptive account, and Ahonen 1978 and Salorinne 1988 reported the number of effects or symptom scores, rather than the number of patients with those effects. Kreisman 1981 stated that the side effects were equally reported, and Bellia 1988 reported that no significant side effects were recorded for oxitropium bromide. For the studies that gave numerical data, individual side effects (e.g. mouth dryness) gave similar results for anticholinergic and placebo, with no statistically significant differences observed at the lower doses used, and for overall side effects, the pooled odds ratio was OR 1.87 (95%CI 0.51, 6.81) ‐ not statistically significant.
5. Anticholinergic vs placebo ‐ Adverse effects.
| Effect | Outcome | Studies | Pooled summary stats | Single study results | Stat sig? | Comments | |
| Overall adverse effects | Number of patients | Vaughan 1988 Wheeler 1987 | OR 1.87 (95%CI 0.51, 6.81) p = 0.81, I‐squared = 0% | N | About twice the odds for patients having adverse effects with AC as with placebo | ||
| Gastric irritation Irritation | Number of patients Number of days/pt Symptom scores | Wheeler 1987 Ahonen 1978 Salorinne 1988 | OR 3.10 (95%CI 0.12, 79.23) anticholinergic = 1; placebo =2.5 anticholinergic = 8; placebo = 4 | N "N" | Authors: "no major differences" | ||
| Headache Unpleasant taste | Symptom scores Number of patients | Salorinne 1988 Wheeler 1987 | anticholinergic = 7; placebo = 14 OR 2.07 (95%CI 0.18, 24.15) | N | |||
| Mouth dryness | Number of patients Symptom scores | Vaughan 1988 Salorinne | OR 1.00 (95%CI 0.13, 7.81) anticholinerg = 44; placebo = 19 | N "N" | Authors; "not statistically significantly different" | ||
| Weight gain Tremor Tremor | Number of patients Number of days/pt Symptom scores | Wheeler 1987 Ahonen 1978 Salorinne 1988 | OR 3.10 (95%CI 0.12, 79.23) anticholinergic = 0; placebo =6 anticholinergic = 0; placebo = 6 | N "N" | Authors: "no major differences" | ||
| Anxiety / fear Palpitations | Number of days/pt Symptom scores | Ahonen 1978 Salorinne 1988 | anticholinergic = 2; placebo =0 anticholinerg = 10; placebo = 11 | "N" | Authors: "no major differences" | ||
| Urinary hesitancy | Number of patients | Vaughan 1988 | OR 3.14 (95%CI 0.12, 81.35) | N |
B) Combination of anticholinergic and ß2 ‐agonist versus ß2 ‐agonist alone
There were 9 trials comparing the combination of anticholinergic and ß2‐agonist with ß2 ‐agonist alone. One of the studies (Tammivaara 1993) used oral CR terbutaline as the ß2 ‐agonist and we analysed this study separately because of the different pharmacokinetics. One study could not be analysed because it did not give any results and 2 studies did not report standard deviations or P‐values. The authors of these studies were contacted and some offered data, but to date these had not arrived. Additional information, was however provided by Wolstenholme 1989 and Philip‐Joet 1990. This left only six studies that provided sufficient data for analysis of the continuous outcomes, and six studies gave data for the dichotomous outcomes. For all studies the final values of the outcome measures were used, rather than adopting change scores.
In Haahtela 1991, the peak flows and symptom scores were reported as a daily average for weeks 1‐4 and, separately, 9‐12 weeks; we used the mean of 1‐4 and 9‐12 weeks as a measure of the response. Philip‐Joet 1990 reported the weekly mean peak flows and standard deviations for weeks 1 to 8. However, many patients dropped out (48/196 overall) and the PEF results showed such a large number of missing patients (e.g., at 8 weeks, 37 of 101 from the combination arm and 40 of 95 from the ß2 ‐agonist arm) that we decided to include only the results for the first week as being the least confounded (still 11/101 and 12/95 missing).
Unlike the anticholinergic‐placebo comparison, these studies differed from one another in various important ways, making pooling questionable. Firstly, there were differences in study duration, but we decided it was reasonable to combine all durations.
Secondly, there was a mixture of study designs, 2 parallel and 7 crossover trials. It was not possible to analyse the crossover studies using the generic inverse variance method because none reported IPD or P‐values, and consequently we had to analyse the crossover studies as if they were parallel studies, which meant that the crossover and (true) parallel studies could not be combined in an analysis.
Thirdly, the ß2 ‐agonist in the two arms differed in both composition and dose. We decided to treat all the ß2 ‐agonists as having the same equivalence and recorded the difference in doses in a single variable, the ratio of ß2 ‐agonist in the combination arm to that in the single arm. Two ratios were analysed 1:1 and 1:2 ‐ the latter can be considered as the substitution of anticholinergic for some of the ß2 ‐agonist in the single arm and the former as the addition of anticholinergic to the same level of ß2 ‐agonist. Thus the breakdown of studies is:
Substitution of anticholinergic for ß2 ‐agonist (ratio 1:2 combination ß2 ‐agonist:single ß2 ‐agonist): Haahtela 1991, two comparisons (Berodual versus 200 µg salbutamol and Duovent versus 400 µg salbutamol) and Philip‐Joet 1990
Addition of anticholinergic to ß2 ‐agonist (ratio 1:1): Haahtela 1991 (Duovent versus 200 µg salbutamol), all other studies
The differing types of design and interventions meant that, in general, the study data could not be pooled, and the results are shown in additional tables 6‐9. Where there was more than one similar study, the results were combined and the summary statistics reported in the additional table and shown in a forest plot. Otherwise the results in the table are for single studies.
1. Symptom scores (Table 6) Daily symptom scores were measured in six studies and all used a scale of 0‐3. Mazzei 1985 only reported symptom scores at a clinic visit, not daily (thus not meeting our criteria). No results were reported for 2 studies and no standard deviations were given for one. Macaluso 1986 reported graphically a distribution of symptom scores, but the exact nature of the representation was unclear; despite these uncertainties, we calculated a mean symptom score and standard deviation from these data. Of the remaining studies, one reported the weekly symptom scores and two reported daily scores: the former values were adjusted to daily scores. The only possible studies that were sufficiently similar to be combined were Macaluso 1986 and Wolstenholme 1989, but in view of the uncertainties about the Macaluso 1986 symptom score data, we decided to analyse these as single studies.
6. Anticholinergic‐Beta agonist combination versus BA alone ‐ Symptom scores.
| Studies | Study design | BA Ratio (comb:sing) | Outcome | Single study results | Stat sig? | % change on BA |
| Haahtela 1991 | Parallel 12 weeks | 1:2 (Berodual/Sal) 1:2 (Duovent/Sal ) | Daytime Dyspnoea Daytime Cough Daytime Dyspnoea Daytime Cough | Mean difference ‐0.04 (minus = favours AC/BA) +0.03 (NB all symptom scores include large baseline differences) +0.26 +0.40 | "N" (authors) "N" (authors) | 6% decrease 5% increase 26% increase 80% increase |
| Haahtela 1991 | Parallel 12 weeks | 1:1 (Duovent/Sal) | Daytime Dyspnoea Daytime Cough | +0.26 (NB all symptom scores include large baseline differences) +0.30 | "N" (authors) "N" (authors) | 27% increase 50% increase |
| Macaluso 1986 | Crossover 2 weeks | 1:1 (Duovent/Sal) | Daytime Dyspnoea Daytime Cough Night Dyspnoea Night Cough | WMD ‐0.13 (95%CI ‐0.55, +0.29) WMD ‐0.06 (95%CI ‐0.54, +0.42) WMD ‐0.10 (95%CI ‐0.54, +0.34) WMD ‐0.03 (95%CI ‐0.46, +0.40) | N N N N | 37% decrease relative to BA 12% decrease 27% decrease 7% decease |
| Tammivaara | Crossover 3 weeks | 1:1 (IB+oral CR terbutaline/ oral Ter) | Daytime Dyspnoea Night Dyspnoea | WMD +0.11 (95%CI ‐0.24, +0.46) WMD +0.01 (95%CI ‐0.28, +0.30) | N N | 19% increase 2% increase |
| Wolstenholme | Crossover 4 weeks | 1:1 (Duovent/Sal) | Night Dyspnoea Night Cough Night Wheeze | WMD +0.16 (95%CI ‐0.30, +0.62) WMD +0.12 (95%CI ‐0.24, +0.48) WMD +0.01 (95%CI ‐0.54, +0.56) | N N N | 22% increase 28% increase 2% increase |
Generally there is no evidence, from the single studies analysed, for a significant difference in any symptom score between the combination and the single arm, regardless of the trial design, time duration or ratio of ß2 ‐agonist. The authors of Haahtela 1991 also stated that, for all symptom score measurements, there were no significant differences among any of the 4 regimens, even when the subgroup of more severe patients was analysed. The authors of Macaluso 1986 stated that there was a statistically significant difference in favour of Duovent for daytime dyspnoea, but our analysis of their available data did not confirm this. The Pierce study stated that 'symptom score cards (measuring daytime and nocturnal dyspnoea, wheeze and cough) failed to reveal any significant differences between the combination and either drug alone'. Waite 1987 stated that 'symptom scores recorded in the morning and evening for wheeze, cough and restrictions in activity showed no significant differences between the two treatments'.
2. Lung function measurements (Table 7) Daily peak flow measurements were measured in 5 studies. An additional study, Pierce 1982 measured daily mean flow using an airflowmeter so this study was analysed separately. Rebuck 1983 reported no data (but stated that there was a similar improvement for each arm) and Haahtela 1991 gave no standard deviations for the daily peak flow measurement.
7. AC/BA Combination versus BA alone ‐ daily peak flows.
| Studies | Design&Duration | Ratio BA | Outcome | Time (post dose) | Single study results | Stat sig? | %change on BA |
| Philip‐Joet 1990 | Parallel design 1 week measurements only used (see text) | 1:2 Comb : single | PEFR am PEFR pm PEFR am post dose PEFR pm post dose | NS (NS but qid, so guess 6h) Not Stated (NS) NS (20 mins) NS (20 mins) | Mean difference 4.65 l/m (95%CI ‐26.24, 35.54) MD 8.17 l/m (95%CI ‐24.08, 40.42) MD 3.31 l/m (95%CI ‐29.78, 36.40) MD ‐4.61 l/m (95%CI ‐37.93, 28.71) | N N N N | 2% increase (i.e. in favour of combination) 3% increase 1% increase 1% decrease |
| Haahtela 1991 | Parallel design 12 weeks | 1:2 Comb : Single | PEFR am PEFR pm PEFR am post dose PEFR pm post dose | 07.00h (NS but qid so guess 6h) 19.00h (NS) 07.00h (1h post dose) 19.00h (1h post dose) | Mean difference +2 (Duovent/Sal) Mean difference ‐4 l/m (Berodual/Sal) Mean difference 6 l/m (Duovent/Sal) Mean difference 2 l/m (Berodual/Sal) Mean difference 10.5 l/m (Duo/Sal) Mean difference ‐2 l/m (Bero/Sal) Mean difference 10 l/m (Duo/Sal) Mean difference ‐2.5 l/m (Bero/Sal) | "N" authors "N" authors "N" authors "N" authors | 1% increase 1% decrease 1% increase 1% increase 2% increase 0.5% decrease 2% increase 1% decrease |
| Haahtela 1991 | Parallel design 12 weeks | 1:1 Comb : single | PEFR am PEFR pm PEFR am post dose PEFR pm post dose | 07.00h (NS but qid so guess 6h) 19.00h (NS) 07.00h (1h post dose) 19.00h (1h post dose) | Mean difference +1.5 litres/min Mean difference 9.5 l/m Mean difference 15 l/m Mean difference 14 l/m | "N" authors "N" authors "N" authors "N" authors | 0.5% increase 2% increase 3% increase 3% increase |
| Wolstenholme 1989 | Crossover design 4 weeks | 1:1 Comb : single | PEFR am PEFR pm PEFR am post dose PEFR pm post dose | NS (NS but qid so guess 6h) NS (NS) NS (30mins) NS (30 mins) | Mean difference ‐8.70 l/m (95%CI ‐83.66, 66.26) Mean difference ‐1.70 l/m (95%CI ‐89.35, 85.95) Mean difference ‐3.00 l/m (95%CI ‐78.77, 72.77 ) Mean difference ‐4.20 l/m (95%CI ‐76.47, 68.07 ) | N N N N | 3% decrease 0.5% decrease 1% decrease 1% decrease |
| Tammivaara 1993 | Crossover design 3 weeks beta‐agonist was oral CR terbutaline | 1:1 Comb : single | PEFR am PEFR pm | 08.00h (12h post dose) 20.00h (4h post ipratropium, 12h after oral terbutaline) | Mean difference ‐5.00 l/m (95%CI ‐50.72, 40.72 ) Mean difference 0.00 l/m (95%CI ‐47.48, 47.48 ) | N N | 1% decrease no difference |
| Pierce 1982 FLOW not peak flow | Crossover design 4 weeks | 1:1 Comb : single | Flow am Flow pm Flow pm post dose | 08.00h (6h post dose) 19.00h (NS; probably 5‐6h post dose) 20.00h (1h post dose) | Mean difference 3.40 litres (95%CI ‐4.18, 10.98 ) Mean difference 6.86 litres (95%CI ‐5.03, 18.75 ) Mean difference 11.59 litres (95%CI ‐4.61, 27.79 ) | N N N | 48% increase 48% increase 57% increase |
The different characteristics of the studies (parallel/crossover, PEF/flow and oral/inhaled beta‐agonist) meant that no studies were sufficiently similar to be combined. However, the general trend is that there is very little difference between interventions with no single study achieving statistical significance. Haahtela 1991 also reported that 'analysis did not reveal any significant differences, even when the subgroup of more severe patients was analysed'. Post‐bronchodilator measurements were made for three studies and were taken after 20m, 30m and 1h. There was little difference between the interventions.
3. Patient preference (Table 8) Only two crossover studies reported the preference of the patients (Tammivaara 1993; Wolstenholme 1989), but the former used oral beta‐agonist. In Tammivaara 1993 the patients preferred the combination, but in Wolstenholme 1989 the reverse was the case; neither study showed statistical significance. Rebuck 1983 reported no significant difference in patient preference. Macaluso 1986 reported that the 'patient's opinion was more favourable with Duovent than salbutamol', but this may have been because the patients received respectively 3 and 4 administrations of the test drug.
8. AC/BA combination vs BA ‐ other outcomes.
| Study | Design&Duration | Comb:BA ratio | Outcomes | Pooled summary stats | SIngle study results | Stat sig? | Comment |
| Wolstenholme 1989 Tammivaara 1993 | Crossover trial 4 weeks Crossover trial 3 weeks | 1:1 1:1 ORAL CR terbutaline | Patient preference | OR 0.79 95%CI 0.20, 3.06 OR 1.70 95%CI 0.40, 7.20 | N N | 1.3 times worse with AC/BA 1.7 times better with AC/BA | |
| Philip Joet 1990 | Parallel design 8 weeks | 1:2 | Number of patients with exacerbations | OR 1.70 95%CI 0.64, 4.51 | N | 1.7 times worse with AC/BA | |
| Tammivaara 1993 | Crossover trial 3 weeks | 1:1 CR ORAL terbutaline | Number of patients with exacerbations No. withdrawing due to treatment failure | OR 0.32 95%CI 0.01, 8.24 OR 0.32 95%CI 0.01, 8.24 | N N | 3 times better with AC/BA 3 times better with AC/BA | |
| Rebuck 1983 | Crossover trial 4 weeks | 1:1 | No. withdrawing due to treatment failure | OR 2.10 95%CI 0.18, 25.01 | N | 2 times worse with AC/BA | |
| Haahtela 1991 | Parallel trial 12 weeks | 1:1 Duovent / Sal | No. withdrawing due to treatment failure | OR 5.09 95%CI 0.98, 26.43 | N | 5 times worse with AC/BA | |
| Haahtela 1991 Philip‐Joet 1990 | Parallel trial 12 weeks Parallel trial 8 weeks | 1:2 Berod/Sal 1:2 Berod/Sal | Number of patients withdrawing due to treatment failure | OR 1.40 95%CI 0.69, 2.84 2 studies, 256 patients; Heterogen. p=0.22, I‐squared =32.2% | N | 1.4 times worse with AC/BA | |
4. Drug‐related withdrawal (Table 8) Four studies reported the number of patients withdrawing while taking one of the study drugs, but only 2 studies could be combined. The pooled odds ratio for the parallel study substitutional‐anticholinergic was: 1.40 (95%CI 0.69, 2.84, 2, studies, using the Berodual‐salbutamol comparison in Haahtela 1991, 161 patients), i.e. in favour of the ß2 ‐agonist, but not statistically significant and with some heterogeneity (p=0.22, I2=32%). Overall, there were fewer withdrawals from the ß2 ‐agonist arm, regardless of the time duration, ratio of ß2 ‐agonist levels and trial design, but no result was statistically significant. The exception was the oral beta‐agonist crossover study, Tammivaara 1993, which showed fewer withdrawals for the combination (not statistically significant).
5. Number of patients with exacerbations (Table 8) Two studies reported the number of patients with exacerbations (or worsening), Philip‐Joet 1990 and Tammivaara 1993, the former shows fewer patients with exacerbations for the ß2 ‐agonist arm and the latter shows the reverse, neither is statistically significant, and the results have not been combined because of the use of the oral ß2 ‐agonist in Tammivaara 1993.
6. Adverse effects (Table 9) Six studies gave some information on adverse effects, but of these Haahtela 1991 and Mazzei 1985 reported the number of effects or symptom scores, rather than the number of patients with those effects and Philip‐Joet 1990 had too much missing data to be included without confounding. Two studies gave a descriptive account only: Wolstenholme 1989 stated that there was no difference in side effects reported for the two periods and Macaluso 1986 found that side effects (tremor, palpitations and tachycardia) occurred in negligible percentages for both treatments. The remaining studies were not pooled because they had different ratios of ß2 ‐agonist and different designs (see additional table 9). Generally, there is little evidence for a difference in adverse effects between the combination and single arm ß2 ‐agonist, and no result is statistically significant.
9. AC/BA combination vs BA ‐ adverse effects.
| Effect | Outcome | Study | Details | Single study stats | Stat sig? | Comments |
| Overall side effects | Number of patients Number of patients | Rebuck 1983 Tammivaara 1993 | Crossover 1:1 Crossover 1:1 Oral terbutaline | OR 1.35 (95%CI 0.30, 6.13) OR 0.81 (95%CI 0.23, 2.88) | N N | Combination worse Combination better |
| Overall side effects | No. of pts withdrawn for side effects Number of side effects | Haahtela 1991 | Parallel 1:1 | OR 3.10 (95%CI 0.12, 79.23) Combination 39; BA 25 | N | Combination worse Combination worse |
| Overall side effects | Number of patients withdrawn for side effects No. of side effects | Haahtela 1991 | Parallel 1:2 Duovent:Salbutamol Berodual:Salbutamol Duovent:Salbutamol Berodual:Salbutamol | OR 1.00 (95%CI 0.06, 16.76) OR 5.35 (95%CI 0.25, 116.31) Duovent 39; Salbutamol 34 Berodual 28; Salbutamol 25 | N | No difference Combination worse Similar Similar |
| Tremor Palpitation Bad taste Drying Paresthesia Somnolence | Incidence | Mazzei 1985 | Crossover; 1:1 | Combination 4; BA 1 Combination 4; BA 8 Combination 3; BA 3 Combination 3; BA 1 Combination 1; BA 0 Combination 0; BA 2 cont. | Mixed effects | |
| Insommnia Excitation | Combination 2; BA 0 Combination 2; BA 3 |
Discussion
As a chronic disease, with no known cure, the accepted goals of management in chronic asthma are to minimise the adverse impact of the disease on the patient's physical and mental well‐being, to prevent long term damage with fixed airflow obstruction and to reduce mortality. In the majority of patients treatment is therefore directed at improving physiological end‐points and patient‐perceived physical and mental health. Although anticholinergics are frequently used in patients with more severe forms of asthma, the evidence justifying its use remains limited. Both short‐term and long‐term anticholinergics are of some benefit in patients with COPD, their role in asthma is less clearly defined. The recent BTS guidelines include anticholinergics as one of the options for short term bronchodilator relief at Step 1, and in the management of acute severe asthma (BTS/SIGN 2004). They receive no mention as regular add‐on treatment for use in combination with inhaled steroids and long‐acting ß2 ‐agonists.
The acute bronchodilator effect of an anticholinergic is usually demonstrated by making physiological measurements before and after inhaling the drug. The most commonly used measurements include the FEV1 and vital capacity, although other end‐points such as the ability to modify bronchial challenge tests have also been widely studied. It is on this basis that anticholinergics are sometimes used to relieve symptoms although the majority of acute studies do not include symptom scores as part of their evaluation.
This review was specifically directed at examining the role of anticholinergics used on a regular basis, especially when used as an adjunct to a ß2 ‐agonist. The comparison between a regular anticholinergic and placebo is of limited clinical relevance but was undertaken to determine whether there was a detectable subjective or objective improvement. Despite the limited number of studies, anticholinergic agents resulted in more favourable symptom scores particularly in respect of daytime dyspnoea. Daily peak flow measurements also showed a statistically significant improvement for the anticholinergic. However, the clinical significance is small and in terms of peak flow measurements equates to approximately a 7% increase over placebo. Secondary outcome measures such as use of rescue medication, patient preference and drug‐related withdrawals showed no significant differences although these conclusions were based on very limited objective data. The same reservations applied to adverse effects where again there was no evidence for a systematic difference between anticholinergic and placebo.
The second part of the review is more clinically relevant and addresses the question as to whether a combination of anticholinergic plus ß2 ‐agonist confers significant advantage when compared with ß2 ‐agonist alone. There was no evidence in respect of primary outcome measures, symptom scores or peak flow rates of any significant differences between the two regimes. Similarly, there were no significant benefits shown in respect of secondary outcome measures. There are however major reservations with respect to the quality of the information from which these conclusions are drawn. The total number of studies suitable for analysis was small and involved limited numbers of patients. A number of studies were excluded from analysis because of methodological flaws, incomplete data or because they included patients with COPD. Even for those studies included, differences in methodology meant that they could not easily be combined. The main differences which might affect analysis have already been identified but merit re‐iteration.
Patient characteristics
All the included studies define the patients as having asthma or reversible airflow obstruction. A 15% improvement of FEV1 after an inhaled ß2 ‐agonist was the most frequently used physiological test although some studies included spontaneous variability. Criteria as defined by the American Thoracic Society were used in a significant number of studies. Patient characteristics varied considerably both between and within studies. There was a wide range of patient age, variable numbers of atopic patients and variable numbers of smokers with symptoms of chronic bronchitis. Despite this heterogeneity, it seems likely that most studies focused on patients at the more reversible end of the spectrum of airway disease.
Severity of the disease
Again there was considerable heterogeneity both between and within studies with patients ranging from having mild to severe asthma. Defining criteria were often not stated. Factors taken into consideration in trying to assess the severity of the disease included patient symptoms, baseline lung function and maintenance treatment. In addition, studies were only included if the asthma was regarded as being "stable". It is theoretically possible that patients with more severe disease might derive greater symptomatic benefit from small improvements in lung function. There were insufficient data to provide sub‐group analysis.
Maintenance treatment
Once again there were significant variations both between and within studies. These ranged from low maintenance treatment to patients who were on regular treatment with theophylline, inhaled or oral steroids. Additional ß2 ‐agonists used as rescue treatment were included in a number of studies and used as a secondary outcome measure.
Methodological differences: different drug combinations
There were significant differences between studies with respect to the different drug regimes studied. These included a variety of anticholinergics using different doses. The ß2 ‐agonist used also varied and in some studies the comparator ß2 ‐agonist was different from that used in the combination.
Methodological differences: outcome measures
Although diary cards were used in a number of studies, data was often incomplete and the scoring system varied. Studies using regular peak flow measurements were similar although the timing of the measurements with respect to the preceding dose may have influenced the outcome measurement.
The failure to demonstrate any subjective or objective improvement by the addition of anticholinergic to a regular ß2 ‐agonist may have various explanations which merit further consideration. As indicated, it may simply be that the studies are insufficiently powered to detect what may be small but significant differences. Patient selection is also likely to be critically important. Patients with mild disease will have little potential for further improvement over and above that achieved by inhaled steroids and ß2 ‐agonists. Conversely, patients with more intractable disease may well have airway obstruction with only limited reversibility. There were insufficient data to permit subgroup analyses, looking specifically at defined patient groups.
None of the studies specifically identified those patients whose asthma was inadequately controlled despite being on regular ß2 ‐agonists and inhaled steroids. In part this reflects the fact that the studies analysed have been undertaken over the past 20 years and guidelines have evolved during this period.
Overall this review provides no justification for routinely introducing anticholinergics as part of add‐on treatment for patients whose asthma is not well controlled on standard therapies. This does not exclude the possibility that there may be a sub‐group of patients who derive some benefit and a trial of treatment in individual patients may still be justified. The review does highlight the need for studies of sufficient power using standardised criteria and methodology in order to answer specific questions. It is unlikely that such studies will be undertaken using the currently available short term anticholinergics. The role of long term anticholinergics such as tiotropium bromide has yet to be established in patients with asthma and any future trials might draw on the messages derived from this review.
Authors' conclusions
Implications for practice.
Inhaled anticholinergics have a bronchodilator effect in patients with asthma. In clinical and physiological terms this effect is small. The addition of anticholinergics to regular inhaled bronchodilators has not been shown to confer significant benefits, so the current evidence does not justify their routine use. The study does not exclude the fact that there may be sub‐groups of patients who derive some symptomatic benefit.
Implications for research.
Future studies need to be sufficiently powered to answer specific questions, which need to be clearly formulated. The role of long term anticholinergics in the management of asthma is a potential case in point. Patient characteristics need to be clearly defined and particular focus directed at those patients with more severe disease that is inadequately controlled on a combination of inhaled steroids and long acting ß2 ‐agonists. In addition, outcome measures need to be standardised and to include patient‐derived symptom scores and quality of life data in addition to physiological measurements.
What's new
| Date | Event | Description |
|---|---|---|
| 11 July 2017 | Amended | The previous what's new event was deleted. This review is no longer being updated. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 19 September 2008 | New search has been performed | Literature search re‐run between August 2005 and August 2008; no new studies found. |
| 30 June 2008 | Amended | Converted to new review format. |
| 14 May 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Margaret Westby acknowledges the award of a Daphne Jackson research Fellowship and financial support from the Leverhulme Trust.
We would also like to thank the Airways Group for support with literature searches and retrieval of studies (namely Karen Blackhall, Liz Arnold, Sarah Tracy and Bettina Reuben) and the people who translated articles from non‐English languages: Jane Dennis, Toby Lasserson, Johannes van der Wouden, Derek Scoins and Makiko Meguro.
Data and analyses
Comparison 1. Longer term AC+BA vs BA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawals due to treatment failure/side effects | 2 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.69, 2.84] |
| 1.1 Medium/long term, parallel, BA alone dose = 2 x comb dose | 2 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.69, 2.84] |
| 2 No of patients with side effects | 2 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.51 [0.96, 21.27] |
| 2.1 Parallel studies, overall side effects, BA alone dose = 2 x comb dose | 2 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.51 [0.96, 21.27] |
1.1. Analysis.
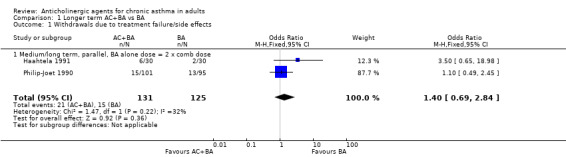
Comparison 1 Longer term AC+BA vs BA, Outcome 1 Withdrawals due to treatment failure/side effects.
1.2. Analysis.
Comparison 1 Longer term AC+BA vs BA, Outcome 2 No of patients with side effects.
Comparison 2. Longer Term AC vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom score paired mean difference (Scale ‐1 to +1) | 3 | Symptom score diff (Fixed, 95% CI) | Subtotals only | |
| 1.1 Symptom score (daily average) up to 28 days dyspnoea, daytime, IB 40 | 3 | Symptom score diff (Fixed, 95% CI) | ‐0.09 [‐0.14, ‐0.04] | |
| 1.2 Symptom score (daily) <28 days dyspnoea, daytime, IB 40; no Salorinne | 2 | Symptom score diff (Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.02] | |
| 1.3 Symptom score (daily average) medium term overall score, night | 1 | Symptom score diff (Fixed, 95% CI) | 0.09 [‐0.09, 0.26] | |
| 2 PEF paired analysis (scale ‐100 to +100 l/min) | 3 | Mean Difference L/mn (Fixed, 95% CI) | Subtotals only | |
| 2.1 PEF am | 3 | Mean Difference L/mn (Fixed, 95% CI) | 14.38 [7.69, 21.08] | |
| 2.2 PEF am (without Salorinne) | 2 | Mean Difference L/mn (Fixed, 95% CI) | 16.26 [9.21, 23.31] | |
| 2.3 PEF pm, short term | 3 | Mean Difference L/mn (Fixed, 95% CI) | 23.48 [12.32, 34.65] | |
| 2.4 PEF am, short term, post bronchodilator | 2 | Mean Difference L/mn (Fixed, 95% CI) | 34.91 [20.13, 49.70] | |
| 2.5 PEF pm, short term, post bronchodilator | 1 | Mean Difference L/mn (Fixed, 95% CI) | 23.94 [5.20, 42.68] | |
| 3 Patient preference | 2 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.68, 3.94] |
| 4 Number of patients dropping out | 2 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.72] |
| 5 Adverse effects ‐ number of patients | 2 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.51, 6.81] |
| 5.1 overall | 2 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.51, 6.81] |
2.1. Analysis.
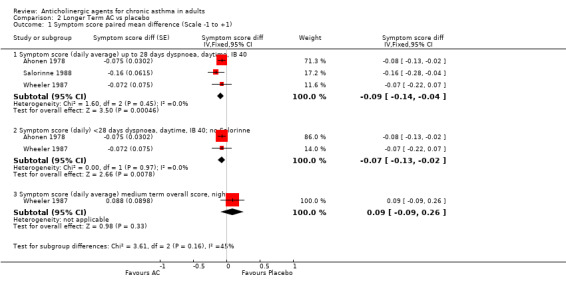
Comparison 2 Longer Term AC vs placebo, Outcome 1 Symptom score paired mean difference (Scale ‐1 to +1).
2.2. Analysis.
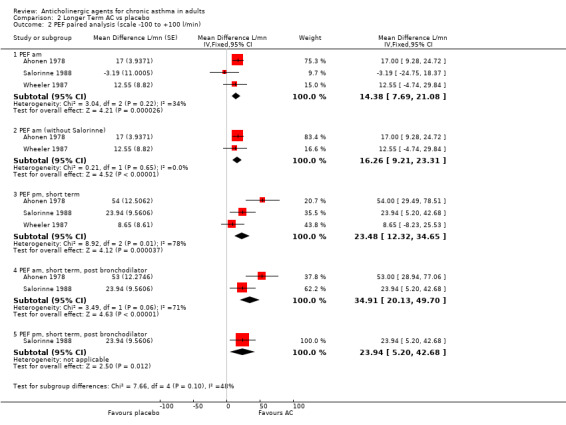
Comparison 2 Longer Term AC vs placebo, Outcome 2 PEF paired analysis (scale ‐100 to +100 l/min).
2.3. Analysis.
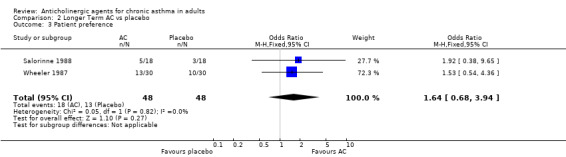
Comparison 2 Longer Term AC vs placebo, Outcome 3 Patient preference.
2.4. Analysis.

Comparison 2 Longer Term AC vs placebo, Outcome 4 Number of patients dropping out.
2.5. Analysis.
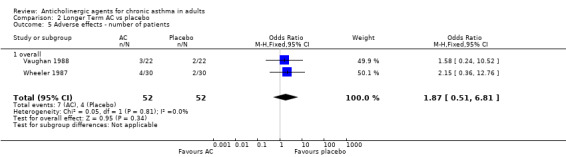
Comparison 2 Longer Term AC vs placebo, Outcome 5 Adverse effects ‐ number of patients.
Comparison 3. IB 40 vs IB 80.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom score (scale ‐4 to +4) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Dyspnoea daytime | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.85, 0.25] |
| 1.2 Cough daytime | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐1.12, 0.12] |
| 1.3 Dyspnoea night | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.91, 0.51] |
| 2 PEF (scale ‐100 to +100 l/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 PEF am | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 34.0 [‐4.06, 72.06] |
| 2.2 PEF pm | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 24.0 [‐19.16, 67.16] |
| 2.3 PEF post dose, am | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 29.00 [‐11.21, 69.21] |
3.1. Analysis.

Comparison 3 IB 40 vs IB 80, Outcome 1 Symptom score (scale ‐4 to +4).
3.2. Analysis.
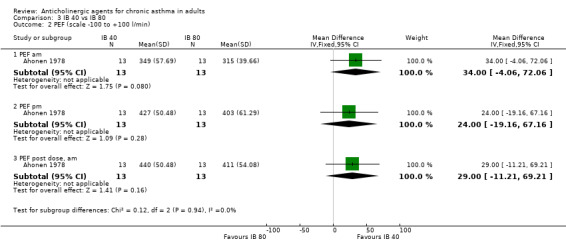
Comparison 3 IB 40 vs IB 80, Outcome 2 PEF (scale ‐100 to +100 l/min).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahonen 1978.
| Methods | Crossover design; each phase 4 days. All patients completed the study correctly. Said to be double blind, but no details. No other concurrent asthma medication. | |
| Participants | Inclusion criteria: patients had asthma ‐ diagnosis according to Ciba Guest symposium criteria; stable asthma medication. Patient details: 13 participants (M/F 7/6); mean age 37; mild asthma (severity homogeneous); 5/8 atopy; no information on concurrent COPD. | |
| Interventions | 5 interventions: ipratropium bromide 40 and 80µg, fenoterol 400µg, placebo (twice) by pressurised inhaler; all interventions 5 times per day (01.00, 07.00, 13.00, 19.00, 22.00h). | |
| Outcomes | Daily PEF (measured before dose and at 10am; 7am and 10pm (pre‐bronchodilator) and 10am (3h post‐bronchodilator) data used in analysis. Daily symptom scores (day and night). Adverse effects. | |
| Notes | Placebo is the mean of 2 periods. The graph recording PEFR did not agree with the table for the SEM ‐ likely that table incorrect (see text). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Ashutosh 1980.
| Methods | Crossover design, each phase 7 days. | |
| Participants | ||
| Interventions | atropine sulphate and isoprenaline. | |
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Bellia 1988.
| Methods | Crossover design; each phase 7 days. All patients completed the study correctly. Said to be double blind, with placebos used, but no other details. Not double blind for dose comparison. Concurrent asthma medication ‐ sodium cromoglycate. | |
| Participants | Inclusion criteria: patients had asthma ‐ diagnosis according to ACCP‐ATS criteria; stable asthma medication; recent nocturnal asthma. Exclusions: CV, ocular & genito‐urinary diseases; history of intolerance to theophylline or anti‐muscarinic agents. Patient details: 12 participants; age 18‐60; mild asthma; no information on concurrent COPD or atopy. | |
| Interventions | 2 interventions: oxitropium bromide 400/600µg, placebo by MDI; all interventions t.i.d. (unclear when). | |
| Outcomes | % change daily PEF(morning/ max PEF; not analysed), symptom scores (night or morning ‐ no numbers); % predicted FEV1 at end of period (not analysed). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Chapman 1983.
| Methods | See Rebuck 1983 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Same trial as Rebuck 1983 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Coe 1986.
| Methods | Crossover design; each phase 14 days. All patients completed the study correctly. Said to be double blind (vs placebo), but no details. Concurrent asthma medication unclear, but none had oral steroids or anticholinergics; 200µg salbutamol given each morning and evening as part of test procedure. | |
| Participants | Inclusion criteria: patients had documented nocturnal asthma ‐ difference of >20% in PEFR between evening and morning readings; nocturnal asthma. Patient details: 18 participants (M/F 7/11); age 18‐76; mild‐severe asthma (heterogeneous severity); 10:8 atopy; no information on concurrent COPD. | |
| Interventions | 3 interventions: oxitropium bromide 200 and 400µg, placebo ; all interventions taken once at night. | |
| Outcomes | Daily PEF, morning and evening (no results or SDs); difference in PEFR (evening‐morning) ‐ not analysed; rescue medication ‐ no results; daily symptom scores ‐ no data. | |
| Notes | 200µg salbutamol given each morning and evening as part of test procedure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Dompeling 1992.
| Methods | See van Schayck 1991a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Dompeling 1993.
| Methods | See van Schayck 1991a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Haahtela 1991.
| Methods | Parallel design; duration 12 weeks. Overall, 24/120 patients withdrew from study. Said to be double blind, but no details. Concurrent asthma medication: inhaled steroids, cromoglycate, methylxanthines; none had oral steroids; inhaled bronchodilators withdrawn. | |
| Participants | Inclusion criteria: patients had asthma ‐ reversible (>15% increase in FEV1) after beta‐agonist. Exclusions: patients taking oral steroids. Patient details: 120 participants, but 96 completed ‐ patient details for 96: M/F 35/61; age 46 (mean); moderate asthma (homogeneous severity); 56:40 atopy; no information on concurrent COPD (but all non‐smokers). | |
| Interventions | 4 interventions: Berodual, Duovent, Fenoterol 200 and 400µg ; all interventions q.i.d. ( 07.00 and 19.00h and two other unspecified times). | |
| Outcomes | PEF (daily) for 1‐4weeks and 8‐12w ; symptom scores ; extra inhaler use (not number of patients); heart rate, side effects score; number of patient withdrawals; no standard deviations/p values for continuous outcomes. | |
| Notes | Large baseline differences for symptom scores. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Kreisman 1981.
| Methods | Crossover design; each phase 14 days. 3/15 patients withdrew from study. Said to be double blind (vs placebo), but no other details. Concurrent asthma medication: theophylline (study drug), maintenance beta‐agonists and steroids kept constant. | |
| Participants | Inclusion criteria: patients had asthma ‐ diagnosis according to ATS criteria; stable asthma medication. Exclusions: no history of chronic bronchitis, cardiac, renal or hepatic disease. Patient details: 15 participants (M/F 7/5); age 21‐54 (mean 34); mild‐moderate asthma; atopy not stated. No concurrent COPD. | |
| Interventions | 4 interventions: ipratropium bromide 40µg and placebo, each with/without theophylline; all interventions q.i.d. (unclear when). | |
| Outcomes | FEV1 at 1 and 2 weeks; symptom scores (wheeze breathlessness, chest tightness, ‐ no numbers); side effects only in words. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Laitinen 1979.
| Methods | Crossover design; 1st phase 3 weeks, 2nd phase 2 weeks. | |
| Participants | ||
| Interventions | Ipratropium bromide 80µg and salbutamol 400µg; both interventions q.i.d. | |
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Light 1977.
| Methods | Crossover design; each phase 2 weeks. All patients completed the study correctly. Said to be double blind (vs placebo), but no other details. Concurrent asthma medication various, some had oral steroids. | |
| Participants | Inclusion criteria: patients had asthma ‐ reversible after isoprenaline and atropine; stable asthma medication; continuous symptoms for 3 months. Patient details: 6 participants; age 28‐42 (mean 35); moderate asthma (heterogeneous severity). | |
| Interventions | 2 interventions: oral atropine sulphate 5mg and placebo; both interventions q.i.d. | |
| Outcomes | FEV1, side effects. | |
| Notes | Oral atropine ‐ not included in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Investigators not blinded to randomisation sequence (Cochrane Grade C) |
Lightbody 1977.
| Methods | See Lightbody 1978 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Lightbody 1978.
| Methods | Crossover design; each phase 3 days. | |
| Participants | ||
| Interventions | 2 interventions: Ipratropium bromide 40µg and Salbutamol 200µg, q.i.d. | |
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Macaluso 1986.
| Methods | Crossover design; each phase 2 weeks. All patients completed the study. Said to be double blind, but could not be because of different number of doses (Duovent 3; Salbutamol 4 per day). Concurrent asthma medication: all patients received aminophylline (long acting), no further details; no information on withdrawal of bronchodilators. | |
| Participants | Inclusion criteria: patients had "partially reversible chronic bronchospasm". Patient details: 15 participants (M/F 7/8); age 32‐65; no information on severity of asthma, atopy or concurrent COPD. | |
| Interventions | 2 interventions: Duovent t.i.d. (times not stated), Salbutamol 200µg q.i.d (times not stated). | |
| Outcomes | Daily symptom scores (but unclear what is reported; standard deviation calculated from graph), use of extra aerosol. | |
| Notes | Unblinded study ‐ different number of inhalations; unclear reporting of results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Mazzei 1985.
| Methods | Crossover design; each phase 2 weeks. All patients completed the study. Said to be 'blind', but no details. Concurrent asthma medication: steroids (unspecified), methylxanthines; usual bronchodilators withdrawn. | |
| Participants | Inclusion criteria: patients had asthma ‐ diagnosis according to ATS criteria; exclusions: serious cardiac pathology, arrhymias, hepatic/renal failure, COPD, other pulmonary diseases, neoplasias, pregnancy. Patient details: 20 participants (M/F 7/13); age 16‐72 (mean 46); moderate asthma (heterogeneous severity); atopy not stated; no concurrent COPD. | |
| Interventions | 2 interventions: Berodual, Salbutamol 200µg; all interventions t.i.d. (every 8h, unclear when). | |
| Outcomes | FEV1, %FEV1/FVC, FVC, symptom scores at 1 and 2 weeks; side effects; patient & physician assessment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
O'Driscoll 1990.
| Methods | Crossover design; each phase 1 month. | |
| Participants | ||
| Interventions | 2 interventions: Ipratropium bromide 500µg (nebuliser) and salbutamol 5mg (neb); q.i.d. | |
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. Different trial to O'Driscoll 1992. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
O'Driscoll 1992.
| Methods | Crossover design; each phase 1 month. | |
| Participants | ||
| Interventions | Ipratropium bromide 500µg (nebuliser) and salbutamol 5mg (neb); q.i.d. | |
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. Different trial to O'Driscoll 1990. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Pavia 1987.
| Methods | As Pavia 1989 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Same trial as Pavia 1989 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Pavia 1989.
| Methods | Crossover design; each phase 28 days. 1/11 asthma patients withdrew from study. Said to be double blind (vs placebo), but some patients could distinguish the OxBr by its taste, no other details. Concurrent asthma medication: no oral steroids; inhaled steroids and oral bronchodilators. | |
| Participants | Inclusion criteria: patients had asthma ‐ reversible (>15% increase in FEV1) after beta‐agonist; stable asthma medication. Patient details: 22 participants (11 with asthma(M/F for 10: 4/6), 11 with bronchitis ‐ not separated for most outcomes); age (asthmatics) mean 59; moderate asthma; atopy not stated. | |
| Interventions | 2 interventions: oxitropium bromide 200µg and placebo; both interventions t.i.d. (on rising, early pm and on retiring). | |
| Outcomes | PEF (am, early pm, retiring ‐ paired difference +SEM for am); FEV1 at 4 weeks; rescue medication (not for placebo) ‐ all these for mixed asthma/COPD; number of dropouts (asthma only). | |
| Notes | Most outcomes for athma and COPD combined; 1 author worked for Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Petersen 1979.
| Methods | Crossover design; each phase 3 days | |
| Participants | ||
| Interventions | 2 interventions: ipratropium bromide 125µg (nebuliser) and terbutaline 5mg (neb); q.i.d. | |
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Philip‐Joet 1990.
| Methods | Parallel design; duration 8 weeks. Overall, 48/196 patients withdrew from study, but 77/196 were not analysed at 8w. "A double blind design was not used"; not stated if outcome assessor blinded. Concurrent asthma medication: steroids (unspecified), methylxanthines; inhaled bronchodilators withdrawn. | |
| Participants | Inclusion criteria: patients had asthma ‐ diagnosis according to ATS criteria and >15% reversibility after beta‐agonist; exclusions: pregnancy, respiratory failure, diabetes, contraindications to study drugs. Patient details: 196 participants (M/F group B 56% M; group S 57% M); age: Group B 45 (mean), Group S 48; moderate asthma ( fairly heterogeneous severity); atopy not stated; no information on concurrent COPD. | |
| Interventions | 2 interventions: Berodual, Salbutamol 200µg; both interventions q.i.d. (times not stated). | |
| Outcomes | PEF (daily) over 7 days (1,2,3,4,5,6,7,8w), symptom scores (not daily), FEV1, PEFR, FVC at 30 and 60 days; adverse effects (but too much data missing); standard deviations. | |
| Notes | Many withdrawals and missing patient data, therefore only used week 1 data for PEFR; concealment: numbered boxes, serially allocated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Investigators blinde to randomisation sequence (Cochrane Grade A) |
Pierce 1982.
| Methods | Crossover design; each phase 4 weeks. 1/10 patients not analysed because of changes in medication. Said to be double blind, with placebo inhalers, but no other details. Concurrent asthma medication: theophylline & steroids (oral/inhaled); withdrawal of inhaled bronchodilators unclear. | |
| Participants | Inclusion criteria: patients had asthma ‐ reversibility >25% after beta‐agonist. Patient details: 10 participants (all male); age 52‐67 (mean 59); moderate asthma; 7/10 atopy; 8/10 concurrent COPD. | |
| Interventions | 3 interventions: ipratropium bromide (40µg)+terbutaline (500µg), terbutaline (500µg), ipratropium bromide (40µg); all interventions q.i.d. (times not clear). | |
| Outcomes | Flow ‐ not PEF ‐ (daily) over 4weeks; symptom scores (no results) over 4 weeks; FEV1 at 2 & 4 weeks (mean); adverse effects. | |
| Notes | Partial allocation concealment ‐ opaque sealed envelopes opened after patient enrollment (not serially numbered). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Rebuck 1983.
| Methods | Crossover design; each phase 30 days. 4/22 patients withdrawn and 17 patients' results were analysed. Said to be double blind, with placebos and the code was not broken until the study was complete. Concurrent asthma medication: steroids (oral and inhaled), cromoglycate, theophylline (study drug in all arms); withdrawal of bronchodilators unclear. | |
| Participants | Inclusion criteria: patients had asthma ‐ diagnosis according to ATS criteria and reversibility >15% after beta‐agonist; all patients clinically stable for >1 month. Exclusions: cough and sputum during clinically stable periods, recent respiratory tract infections, cardiac, hepatic, renal, metabolic disease, and others; hypersensitivity to atropinic compounds. Patient details: 22 participants (M/F 9/13); age 17‐75 (mean for completers 47); moderate asthma (heterogeneous severity); 13/22 atopy; unclear if concurrent COPD. | |
| Interventions | 3 interventions: ipratropium bromide (40µg)+fenoterol (200µg) ‐ may be separate inhalers, fenoterol (200µg), ipratropium bromide (40µg); all interventions q.i.d. (times not clear) and all arms had oral theophylline (200mg) q.i.d. | |
| Outcomes | PEF (daily) over 30d (no results); FEV1 at 30days; patient preference and number of exacerbations (descriptive); adverse effects. | |
| Notes | Authors unable to provide any more data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Ruffin 1990.
| Methods | Crossover design; phase length unclear ‐ could be 8 weeks. 5/21 patients withdrew from study. Said to be double blind (vs placebo), but no other details. Concurrent medication: not stated. | |
| Participants | Inclusion criteria: patients had asthma ‐ diagnosis reasons not stated. Patient details: 16 remaining participants (M/F 9/7); mean age 56; mild asthma; no information about atopy or concurrent COPD. | |
| Interventions | 2 interventions: oxitropium bromide 200µg and placebo; both interventions t.i.d. (not stated when). | |
| Outcomes | PEF (am and pm, probably at end of period); FEV1 at end of period. | |
| Notes | Abstract; funded by Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Salorinne 1988.
| Methods | Crossover design; each phase 7 days. All patients completed the study correctly. Not blinded for the comparison IB (40) vs placebo (powder); no details re outcome assessor. Concurrent medication: 5/18 had oral steroids; 17 had theophylline. | |
| Participants | Inclusion criteria: patients had asthma ‐ improvement in FEV1 of 16‐100% (not stated how). Patient details: 18 participants (M/F 10/8); age 22‐70 (mean 48); moderate‐severe asthma; atopy 5/18, no information on concurrent COPD. | |
| Interventions | 4 interventions: ipratropium bromide 40 and 200µg (aerosol) and 200µg powder, and placebo powder ; all interventions q.i.d (08.00, 12.00, 18.00, 22.00h). | |
| Outcomes | PEF (8, 10am, midday, 2, 6, 8, 10pm & midnight); Symptom scores (dyspnoea day and night, cough); rescue medication (words); patient preference. | |
| Notes | Placebo was powder. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Storms 1986.
| Methods | Parallel design; 90 days. | |
| Participants | ||
| Interventions | 2 interventions: ipratropium bromide 40µg and metaproterenol 1500µg; q i.d | |
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Tammivaara 1993.
| Methods | Crossover design; each phase 3 weeks. 7/28 patients withdrawn. Said to be double blind and double dummy, but no other details. Concurrent medication: theophylline, none had steroids; patients allowed to use inhaled bronchodilators when needed (NB study drug oral). | |
| Participants | Inclusion criteria: patients had asthma ‐ nocturnal decline in PEFR of at least 20%. Patient details: 28 participants (M/F 10/18); age 18‐59 (mean 39); moderate asthma (homogeneous severity); no information on atopy or concurrent COPD. | |
| Interventions | 3 interventions: ipratropium bromide 40µg+oral controlled‐release terbutaline (10mg), oral CR terbutaline (10mg)+aerosol placebo and 200µg powder, and ipratropium bromide 40µg+oral placebo; aerosols q.i.d (08.00, 12.00, 16.00, 20.00h); oral tablets b.i.d. (08.00 and 20.00h). | |
| Outcomes | PEF (daily), symptom scores over last 2 weeks of treatment period (standard deviations); number of patients with exacerbations, number needing rescue medications (words), patient preference, adverse effects. | |
| Notes | Oral CR terbutaline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Taylor 1986.
| Methods | Crossover design; each phase 4 weeks. 8/23 (of all patients) failed to complete the study. Double blind (identical inhalers), but no other details. Concurrent asthma medication: mixed asthma/COPD study ‐ medication not distinguished for asthma (3/all patients had oral steroids, 7 had aminophylline). | |
| Participants | Inclusion criteria: patients had asthma ‐ reversibility of >20% with conventional bronchodilators; stable asthma medication implied. Patient details: 23 participants (asthma 7, COPD 8, no details 8) (overall M/F 12/3); asthma & COPD age 27‐71 (median 60); mild‐severe asthma (severity heterogeneous); no information on concurrent COPD. | |
| Interventions | 2 interventions: ipratropium bromide 200µg and placebo; all interventions t.i.d. (not stated when). | |
| Outcomes | Daily symptom scores (breathlessness, cough, wheeze), PEFR and FEV1 at end of period, number of patients with exacerabations (all mixed asthma/COPD); treatment related withdrawals (mixed asthma/COPD) | |
| Notes | Mixed asthma/COPD mainly; Boehringer Ingelheim financial support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Taytard 1984.
| Methods | Crossover design, but analysed as 1st period only; each phase 21 days. All patients completed the study (although there is no patient number 2). Said to be double blind (with identical treatments), but no details about the assessor. Concurrent asthma medication: no oral or inhaled steroids, no regular treatment but 8 had theophylline for relief. | |
| Participants | Inclusion criteria: patients had asthma ‐ diagnosis according to ATS criteria; presenting an allergic diathesis and consulting an allergology unit; perennial asthma. Patient details: 16 participants (M/F 9/7); age 14‐48 (mean 31); mild asthma; all atopic, no information on concurrent COPD. | |
| Interventions | 2 interventions: oxitropium bromide 200µg and placebo; all interventions t.i.d. (08.00, 14.00, 20.00h). | |
| Outcomes | Clinical scores (IPD); rescue medication; patient rating. | |
| Notes | Significant order effect, so used 1st period data only from IPD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Tormey 1995.
| Methods | Crossover design; each phase 28 days. Not clear if intention to treat. Said to be double blind (vs placebo), but no other details. Concurrent asthma medication: inhaled steroids. | |
| Participants | Inclusion criteria: patients had asthma ‐ no diagnostic evidence; stable asthma medication implied. Patient details: 12 participants; age not stated; moderate asthma; no information on atopy or concurrent COPD. | |
| Interventions | 3 interventions: oxitropium bromide 200µg, salbutamol 200µg and placebo; all interventions t.i.d. (not stated when). | |
| Outcomes | Daily symptom scores (unspecified, words only); FEV1 after 4 weeks. | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
van Schayck 1991a.
| Methods | Crossover design; each phase 1 year | |
| Participants | ||
| Interventions | 4 interventions: ipratropium bromide 40µg q.i.d., salbutamol 400µg q.i.d., ipratropium bromide on demand, salbutamol on demand | |
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
van Schayck 1991b.
| Methods | See van Schayck 1991a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
van Schayck 1994.
| Methods | See van Schayck 1991a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
van Schayck 1995.
| Methods | See van Schayck 1991a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | This comparison not analysed, so details not extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Vaughan 1988.
| Methods | Crossover design; each phase 2 weeks. All patients assumed to have completed the study. Double blind study (code in sealed envelope). Concurrent asthma medication: 2 groups of patients ‐ 12 had inhaled & oral steroids; 10 had no steroids. | |
| Participants | Inclusion criteria: patients had asthma ‐ diagnosis ATS criteria and >15% reversibiliy. Patient details: 22 participants; age 44‐77; moderate‐severe asthma (2 homogeneous groups); no information on atopy or concurrent COPD. | |
| Interventions | 2 interventions: atropine methonitrate 2mg and placebo, nebulised; both interventions q.i.d. (unclear when). | |
| Outcomes | FEV at 2 weeks; daily symptom scores and PEF not reported in results (but 'no difference'). | |
| Notes | Authors stated that the allocation schedule was sealed in envelopes until the study was completed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Waite 1987.
| Methods | Crossover design; each phase 12 weeks. No information on patient withdrawal nor on concurrent asthma medication; no information on withdrawal of bronchodilators. Said to be double blind, but no details. | |
| Participants | Inclusion criteria: patients had asthma ‐ reversibiliy >15% following beta‐agonist. Patient details: 12 participants; no further details. | |
| Interventions | 2 interventions: Duovent and salbutamol; no further details. | |
| Outcomes | Daily symptom scores; use of inhaled bronchodilator; PEFR am (only descriptive). | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Wheeler 1987.
| Methods | Crossover design; each phase 4 weeks. 8/39 failed to complete the study and 9/39 patients' results were not analysed. Double blind (identical, masked containers). Concurrent asthma medication: none had oral steroids; 26/31 had inhaled steroids; 22 had methylxanthines. | |
| Participants | Inclusion criteria: patients had asthma ‐ diagnosis >15% reversibiliy (28 had asthma, 2 had COAD). Patient details: 39 participants; age 17‐76; mild‐severe asthma (severity heterogeneous); no information on concurrent COPD. | |
| Interventions | 2 interventions: oxitropium bromide 200µg, and placebo; all interventions b.i.d. (on rising and on retiring). | |
| Outcomes | Daily PEF (am, pm); Symptom scores (day, breathlessness; night, overall); rescue medication (day & night); patient preference; side effects. | |
| Notes | Boehringer Ingelheim study; IPD given but some patients' diary notes were incomplete; partial concealment (randomisation carried out by BI, but list supplied). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Wolstenholme 1989.
| Methods | Crossover design; each phase 4 weeks. All patients completed the study; 13/17 patients' results analysed for PEF. Said to be double blind and double dummy, but no other details. Concurrent asthma medication: steroids (oral and inhaled), cromoglycate; all bronchodilators withdrawn. | |
| Participants | Inclusion criteria: patients had asthma ‐ >15% reversibiliy following beta‐agonist; morning dip of >15% for half of the 2 week run‐in. Patient details: 17 participants; age 19‐39 (median 25); moderate asthma (severity homogeneous); 100% atopy; no information on concurrent COPD (but all were non‐smokers). | |
| Interventions | 2 interventions: Duovent+placebo and salbutamol 200µg+placebo separate inhalers; both interventions q.i.d. (times not stated). | |
| Outcomes | PEF (daily), daily symptom scores, FEV1, FVC, PEF ( at 2 and 4 weeks), nocturnal waking, patient preference, adverse effects (words); standard deviations given. | |
| Notes | Partial allocation concealment ‐ extra communication from the author stated that sealed envelopes were used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abboud 1987 | Wrong comparison |
| Adinoff 1998 | Wrong comparison |
| Advenier 1984 | Review |
| Alanko 1983 | Short term study <24h ‐ included in short term review |
| Allen 1980a | Short term study <24h ‐ included in short term review |
| Allen 1980b | Short term study <24h ‐ included in short term review |
| Alliott 1972 | Short term study <24h ‐ included in short term review |
| Altounyan 1964 | Short term study <24h ‐ included in short term review |
| Andersen 1986 | Short term study <24h ‐ included in short term review |
| Anderson 1980 | Short term study <24h ‐ included in short term review |
| Anderson 1981 | Short term study <24h ‐ included in short term review |
| Anderson 1983 | Short term study <24h ‐ included in short term review |
| Aquilina 1986 | Not randomised |
| Ariano 1981 | COPD |
| Baigelman 1977 | Short term study <24h ‐ included in short term review |
| Banos 1983 | Short term study <24h ‐ included in short term review |
| Barber 1975 | Short term study <24h ‐ included in short term review |
| Barber 1977 | Short term study <24h ‐ included in short term review |
| Bauer 1973 | Short term study <24h ‐ included in short term review |
| Beasley 1987 | Confounded ‐ patients selected who responded to Ipratropium |
| Bell 1982 | Confounded ‐ additional salbutamol given to both arms on a regular basis |
| Bellia 1987 | Not said to be randomised; no further data from authors |
| Bernabeu 1987 | Wrong comparison |
| Bezel 1987 | Short term study <24h ‐ included in short term review |
| Bish 1997 | Confounded ‐ patients selected who responded to Ipratropium |
| Bohadana 1980 | Short term study <24h ‐ included in short term review |
| Bonsignore 1986 | wrong comparison; patients had ongoing attack |
| Botts 1985 | Short term study <24h ‐ included in short term review |
| Brady 1979 | Short term study <24h ‐ included in short term review |
| Brand 1992 | Long term study ‐ mixed asthma and COPD patients; separate data not available for asthma despite request to authors |
| Bremner 1992 | Short term study <24h ‐ included in short term review |
| Britton 1988 | Short term study <24h ‐ included in short term review |
| Brom 1985 | Not anticholinergic trial |
| Bruderman 1983 | Short term study <24h ‐ included in short term review |
| Brumby 1980 | Long term study ‐ not stated if patients in long term study were randomised; no response from authors |
| Bryant 1990a | Short term study <24h ‐ included in short term review |
| Bryant 1990b | Short term study <24h ‐ included in short term review |
| Bryant 1992 | Short term study <24h ‐ included in short term review |
| Burge 1980 | Short term study <24h ‐ included in short term review |
| Burki 1997 | Short term study <24h ‐ included in short term review |
| Capel 1964 | Short term study <24h ‐ included in short term review |
| Carpentiere 1986 | Wrong comparison Duovent vs placebo |
| Carratu 1983 | Short term study <24h ‐ included in short term review |
| Casali 1979 | Short term study <24h ‐ included in short term review |
| Catterall 1988 | Short term study <24h ‐ included in short term review |
| Cañete 1991 | Asthmatic crisis |
| Chakravarti 1987 | Confounded ‐ patients selected who responded to Ipratropium |
| Chervinsky 1977 | Short term study <24h ‐ included in short term review |
| Chhabra 2002 | Short term study <24h ‐ included in short term review |
| Chick 1977 | Short term study <24h ‐ included in short term review |
| Chuchalin 1985 | Short term study <24h ‐ included in short term review |
| Ciappi 1986 | Short term study <24h ‐ included in short term review |
| Clauzel 1987 | Short term study <24h ‐ included in short term review |
| Cockcroft 1977 | Challenge study |
| Cox 1984 | Not randomised and wrong comparison |
| Crane 1986a | Short term study <24h ‐ included in short term review |
| Crane 1986b | Short term study <24h ‐ included in short term review |
| Crane 1987 | Short term study <24h ‐ included in short term review |
| del Buffalo 1982 | Short term study <24h ‐ included in short term review |
| Donkers 1998 | Short term study <24h ‐ included in short term review |
| Donner 1980 | Wrong comparator |
| Dutt 1990 | Study conducted in children |
| Elwood 1982 | Short term study <24h ‐ included in short term review |
| Emirgil 1975 | Short term study <24h ‐ included in short term review |
| Endoh 1998 | Not asthma |
| Filiz 1994 | Not randomised |
| Firstater 1981 | Short term study <24h ‐ included in short term review |
| Fischbacher 1984 | Short term study <24h ‐ included in short term review |
| Flint 1983a | Short term study <24h ‐ included in short term review |
| Flint 1983b | Short term study <24h ‐ included in short term review |
| Flint 1984 | Short term study <24h ‐ included in short term review |
| Foster 1983 | Short term study <24h ‐ included in short term review |
| Freeman 1992 | Challenge study |
| Frith 1987 | Short term study <24h ‐ included in short term review |
| Gaba 1987 | Short term study <24h ‐ included in short term review |
| Gillies 1987 | Challenge study |
| Ginesu 1983 | Short term study <24h ‐ included in short term review |
| Gotz 1983 | Short term study <24h ‐ included in short term review |
| Grandordy 1988 | Short term study <24h ‐ included in short term review |
| Gross 1975a | Short term study <24h ‐ included in short term review |
| Gross 1975b | Short term study <24h ‐ included in short term review |
| Gross 1984 | Review |
| Gross 1987 | Not asthma |
| Gutersohn 1975 | Confounded ‐ patients selected who responded to Ipratropium |
| Guyatt 1986 | Asthma not stable |
| Higgins 1991 | Short term study <24h ‐ included in short term review |
| Hockley 1985 | Short term study <24h ‐ included in short term review |
| Hoffstein 1994 | Short term study <24h ‐ included in short term review |
| Holst 1976 | Not stated to be randomised (abstract) ‐ too long ago to write to authors |
| Huhti 1986 | Short term study <24h ‐ included in short term review |
| Hunt 1983 | Short term study <24h ‐ included in short term review |
| Irsigler 1975 | Short term study <24h ‐ included in short term review |
| Jenkins 1981 | Short term study <24h ‐ included in short term review |
| Jenkins 1982 | Short term study <24h ‐ included in short term review |
| Jindal 1989 | Not randomised |
| Johnson 1989 | Short term study <24h ‐ included in short term review |
| Jolobe 1981 | Not randomised |
| Kaik 1975 | COPD |
| Kaik 1976 | Wrong comparator |
| Kaik 1979 | COPD |
| Kamburoff 1975 | Short term study <24h ‐ included in short term review |
| Kaptein 1993 | As Brand 1992 |
| Kennedy 1964 | Short term study <24h ‐ included in short term review |
| Kerstjens 1992 | As Brand 1992 |
| Kerstjens 1993a | As Brand 1992 |
| Kerstjens 1993b | As Brand 1992 |
| Kerstjens 1994 | As Brand 1992 |
| Kerstjens 1995 | As Brand 1992 |
| Kok‐Jensen 1979 | Not randomised |
| Kradjan 1992 | Short term study <24h ‐ included in short term review |
| Kreisman 1984 | Short term study <24h ‐ included in short term review |
| Krivoy 1987 | Short term study <24h ‐ included in short term review |
| Kunkel 1976 | COPD |
| Kunkel 2000 | Wrong comparison |
| Lahdensuo 1975 | Short term study <24h ‐ included in short term review |
| Laitinen 1986 | Short term study <24h ‐ included in short term review |
| Lanser 1975 | COPD |
| Larsson 1987 | Challenge study |
| Lavandier 1987 | Short term study <24h ‐ included in short term review |
| Lawford 1983 | Short term study <24h ‐ included in short term review |
| Lefcoe 1982 | Short term study <24h ‐ included in short term review |
| Lehrer 1993 | Short term study <24h ‐ included in short term review |
| Lehrer 1994 | Short term study <24h ‐ included in short term review |
| Li 2003 | |
| Lichterfeld 1979 | Review |
| Linehan 1975 | Short term study <24h ‐ included in short term review |
| Maesen 1975a | Short term study <24h ‐ included in short term review |
| Maesen 1975b | Short term study <24h ‐ included in short term review |
| Maesen 1982 | Short term study <24h ‐ included in short term review |
| Maesen 1985 | Short term study <24h ‐ included in short term review |
| Mann 1984 | Confounded ‐ patients selected who responded to Ipratropium |
| Marcatili 1984 | Short term study <24h ‐ included in short term review |
| Marin 1987 | Short term study <24h ‐ included in short term review |
| Marlin 1978 | Short term study <24h ‐ included in short term review |
| Marlin 1979 | Short term study <24h ‐ included in short term review |
| Mattila 1966 | Short term study <24h ‐ included in short term review |
| McIntosh 1986a | Short term study <24h ‐ included in short term review |
| McIntosh 1986b | Short term study <24h ‐ included in short term review |
| Millar 1990 | Short term study <24h ‐ included in short term review |
| Minette 1979 | Short term study <24h ‐ included in short term review |
| Minette 1985 | Short term study <24h ‐ included in short term review |
| Morrison 1988 | Short term study <24h ‐ included in short term review |
| Morrison 1989 | Short term study <24h ‐ included in short term review |
| Morrison 1991 | Challenge study |
| Morton 1984 | Not randomised |
| Muittari 1968 | Short term study <24h ‐ included in short term review |
| Nishi 1993 | Short term study <24h ‐ included in short term review |
| Nishimura 1999 | Wrong comparison oxitropium vs nothing (long term study) |
| Nolte 1978 | Short term study <24h ‐ included in short term review |
| Osterwalder 1991 | Short term study <24h ‐ included in short term review |
| Pavia 1979 | COPD; only 2 patients with asthma |
| Peel 1984 | Short term study <24h ‐ included in short term review |
| Peerless 1983 | Short term study <24h ‐ included in short term review |
| Petrie 1975a | Short term study <24h ‐ included in short term review |
| Petrie 1975b | Short term study <24h ‐ included in short term review |
| Petro 1981 | COPD |
| Philip‐Joet 1986 | Acute asthma |
| Pierce 1979 | Short term study <24h ‐ included in short term review |
| Pounsford 1983 | Short term study <24h ‐ included in short term review |
| Pounsford 2001 | Short term study <24h ‐ included in short term review |
| Rafferty 1987 | Short term study <24h ‐ included in short term review |
| Rebuck 1979 | Short term study <24h ‐ included in short term review |
| Riding 1970 | Short term study <24h ‐ included in short term review |
| Ruffin 1977 | Short term study <24h ‐ included in short term review |
| Ruffin 1978 | Short term study <24h ‐ included in short term review |
| Ruffin 1982 | Short term study <24h ‐ included in short term review |
| Ruffin 1987b | Short term study <24h ‐ included in short term review |
| Rutkowski 1994 | Not reported to be randomised; no response from authors |
| Rutten‐van‐Molken 19 | As Brand 1992 |
| Sahay 1980 | As Bell 1982 |
| Sahlstrom 1986 | Short term study <24h ‐ included in short term review |
| Salome 1988 | Challenge study |
| Schindl 1973 | COPD |
| Schlueter 1978 | Short term study <24h ‐ included in short term review |
| Schroeckenstein 1988 | Short term study <24h ‐ included in short term review |
| Schultze‐Werninghaus | Challenge study |
| Shneerson 1986 | Short term study <24h ‐ included in short term review |
| Simonsson 1979 | Review |
| Smith 1988 | Challenge study |
| Snow 1979 | Short term study <24h ‐ included in short term review |
| Spector 1975 | Short term study <24h ‐ included in short term review |
| Speirs 1987 | Short term study <24h ‐ included in short term review |
| Stewart 1987 | Short term study <24h ‐ included in short term review |
| Storms 1975a | Short term study <24h ‐ included in short term review |
| Storms 1975b | Short term study <24h ‐ included in short term review |
| Stresemann 1975 | Short term study <24h ‐ included in short term review |
| Stresemann 1976a | Short term study <24h ‐ included in short term review |
| Stresemann1976b | Short term study <24h ‐ included in short term review |
| Sur 1990 | Short term study <24h ‐ included in short term review |
| Tang 1984 | Short term study <24h ‐ included in short term review |
| Tarlo 1982 | Long term study ‐ asthma maintenance medication not stable |
| Taube 2002 | Short term study <24h ‐ included in short term review |
| Thiessen 1979 | Short term study <24h ‐ included in short term review |
| Trinquet 1975 | Short term study <24h ‐ included in short term review |
| Tukiainen 1985 | Short term study <24h ‐ included in short term review |
| Uffholtz 1975 | Short term study <24h ‐ included in short term review |
| Ullah 1980 | Short term study <24h ‐ included in short term review |
| Ullah 1981 | Short term study <24h ‐ included in short term review |
| Ulmer 1979 | One study COPD; other study not randomised (before and after) |
| Vakil 1985 | No comparison |
| Verstraeten 1974a | Short term study <24h ‐ included in short term review |
| Verstraeten 1974b | COPD |
| Verstraeten 1975 | Short term study <24h ‐ included in short term review |
| Villate 1980 | Short term study <24h ‐ included in short term review |
| Vlagopoulos 1975 | Short term study <24h ‐ included in short term review |
| Vlagopoulos 1976 | Short term study <24h ‐ included in short term review |
| Vogt 1974 | Short term study <24h ‐ included in short term review |
| Walker 1987 | Short term study <24h ‐ included in short term review |
| Ward 1987 | Acute asthma |
| Watson 1992 | Not asthma |
| Wegener 1987a | Short term study <24h ‐ included in short term review |
| Wegener 1987b | Short term study <24h ‐ included in short term review |
| Wempe 1992 | Short term study <24h ‐ included in short term review |
| Wesolowski 1985 | Children |
| Wildbolz 1977 | Short term study <24h ‐ included in short term review |
| Wilson 1978 | Not randomised |
| Windom 1990 | Short term study <24h ‐ included in short term review |
| Yanai 1991 | Short term study <24h ‐ included in short term review |
| Yang 1989 | Short term study <24h ‐ included in short term review |
| Yeager 1976 | Short term study <24h ‐ included in short term review |
| Zanen 1995 | Not randomised and wrong comparator |
| Zaritsky 1999 | Children; acute asthma |
| Zuck 1987 | Confounded ‐ patients selected who responded to Ipratropium |
Contributions of authors
Airways group conceived the idea for the review and MW and MB formulated the question and wrote the protocol, with additional input from PG. Decisions about prioritisation in the review and splitting of the original protocol into two reviews were made by MW and MB in consultation with PG. MW devised the MS Access database, managed the studies and wrote to the authors for missing data. MW and MB carried out the inclusion of studies, quality assessment and data extraction procedures. MW entered the data into RevMan and conducted the analysis. MW and MB wrote the review, with additional input from PG.
Sources of support
Internal sources
No sources of support supplied
External sources
Daphne Jackson Trust, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Ahonen 1978 {published data only}
- Ahonen A, Alanko K, Mattson K. Bronchodilator action of two different doses of ipratropium bromide (SCH 1000) compared with fenoterol and placebo. Current therapeutic research, clinical and experimental. 1978;24:65. [Google Scholar]
Ashutosh 1980 {published data only}
- Ashutosh K, Mead G, Dickey JC Jr, Berman P, Kuppinger M. Density dependence of expiratory flow and bronchodilator response in asthma. Chest 1980;77:68. [DOI] [PubMed] [Google Scholar]
Bellia 1988 {published data only}
- Bellia V, Ferrara G, Cibella F, Cuttitta G, Visconti A, Insalaco G, et al. Comparison of the effect of oxitropium bromide and of slow‐release theophylline on nocturnal asthma. Postgraduate Medical Journal 1988;64:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 1983 {published data only}
- Chapman KR, Rebuck AS. Anticholinergic and sympathomimetic combination therapy of asthma. Annals of allergy 1983;50:350. [DOI] [PubMed] [Google Scholar]
Coe 1986 {published data only}
- Coe CI, Barnes PJ. Reduction of nocturnal asthma by an inhaled anticholinergic drug. Chest 1986;90:485. [DOI] [PubMed] [Google Scholar]
Dompeling 1992 {published data only}
- Dompeling E, Schayck CP, Molema J, Folgering H, Grunsven PM, Weel C. Inhaled beclomethasone improves the course of asthma and COPD. European Respiratory Journal 1992;5:945. [PubMed] [Google Scholar]
Dompeling 1993 {published data only}
- Dompeling E, Schayck C, Grunsven P, et al. Slowing the deterioration of asthma and chronic obstructive pulmonary disease observed during bronchodilator therapy by adding inhaled. Annals of Internal Medicine 1993;118:770. [DOI] [PubMed] [Google Scholar]
Haahtela 1991 {published data only}
- Haahtela T, Ahokas I, Ahonen A, Assendelft A, Havu M, Havu K, et al. Inhaled bronchodilator's in asthma: low or high dose?. Annals of Allergy 1991;66:175. [PubMed] [Google Scholar]
Kreisman 1981 {published data only}
- Kreisman H, Frank H, Wolkove N, Gent M. Synergism between ipratropium and theophylline in asthma. Thorax 1981;36:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laitinen 1979 {published data only}
- Laitinen LA, Poppius H, Haahtela T. Comparison of ipratropium bromide and salbutamol in a long‐term trial in asthmatic and bronchitis patients in a cold climate. Scandinavian Journal of Respiratory Diseases‐Supplementum 1979;103:163‐9. [PubMed] [Google Scholar]
Light 1977 {published data only}
- Light RW, George RB. Oral atropine in the treatment of chronic asthma. Annals of allergy 1977;38(1):58‐61. [PubMed] [Google Scholar]
Lightbody 1977 {published data only}
- Lightbody I, Ingram C, Legge JS, Johnston RN. Ipratropium bromide, salbutamol, and prednisolone in bronchial asthma and chronic bronchitis. Thorax 1977;32:645. [DOI] [PubMed] [Google Scholar]
Lightbody 1978 {published data only}
- Lightbody IM, Ingram CG, Legge JS, Johnston RN. Ipratropium bromide, salbutamol and prednisolone in bronchial asthma and chronic bronchitis. British Journal of Diseases of the Chest 1978;72:181. [DOI] [PubMed] [Google Scholar]
Macaluso 1986 {published data only}
- Macaluso S, Torre L. Efficacy and compliance to prolonged treatment of bronchospasm. Respiration 1986;50(Suppl 2):222‐5. [DOI] [PubMed] [Google Scholar]
Mazzei 1985 {published data only}
- Mazzei JA, Torres J. Blind randomized cross‐over comparative study of salbutamol and the combination fenoterol‐ipratropium IK6 in patients with bronchial asthma. Respiration 1986;50(Suppl 2):313. [DOI] [PubMed] [Google Scholar]
- Mazzei JA, Torres J. Comparative randomized blind cross over study between salbutamol and the fenoterol‐ipratropium association (IK 6) in patients with bronchial asthma. Prensa medica Argentina 1985;72:329. [Google Scholar]
O'Driscoll 1990 {published data only}
- O'Driscoll BR, Kay EA, Taylor RJ, Bernstein A. Home nebulizers: can optimal therapy be predicted by laboratory studies?. Respiratory Medicine 1990;84:471. [DOI] [PubMed] [Google Scholar]
O'Driscoll 1992 {published data only}
- O'Driscoll BR, Kay EA, Taylor RJ, Weatherby H, Chetty MC, Bernstein A. A long‐term prospective assessment of home nebulizer treatment. Respiratory Medicine 1992;86:317. [DOI] [PubMed] [Google Scholar]
Pavia 1987 {published data only}
- Pavia D, Lopez‐Vidriero MT, Agnew JE, Taylor RG, Eyre‐Brook A, Lawton WA, et al. Effect of four weeks' oxitropium bromide treatment on lung mucociliary clearance in patients with chronic bronchitis or asthma. Postgraduate Medical Journal 1987;63(Suppl 1):15a. [DOI] [PubMed] [Google Scholar]
Pavia 1989 {published data only}
- Pavia D, Lopez‐Vidriero MT, Agnew JE, Taylor RG, Eyre‐Brook A, Lawton WA, et al. Effect of four‐week treatment with oxitropium bromide on lung mucociliary clearance in patients with chronic bronchitis or asthma. Respiration 1989;55:33. [DOI] [PubMed] [Google Scholar]
Petersen 1979 {published data only}
- Petersen BN, Weeke E. A comparative study of the bronchodilatating effects of ipratropium and terbutalin inhaled with Monaghan IPPB‐M 515 by 19 patients with chronic asthma. Scandinavian Journal of Respiratory Diseases 1979;103:178. [PubMed] [Google Scholar]
Philip‐Joet 1990 {published and unpublished data}
- Philip‐Joet F, Reynaud‐Gaubert M, Jirou‐Najou JL, Arnaud A. Comparison of Berodual and salbutamol in asthma: a multicenter evaluation. Respiration 1990;57:379. [DOI] [PubMed] [Google Scholar]
Pierce 1982 {published and unpublished data}
- Pierce RJ Holmes PW Campbell AH. Use of ipratropium bromide in patients with severe airways obstruction. Australian and New Zealand journal of medicine 1982;12:38. [DOI] [PubMed] [Google Scholar]
Rebuck 1983 {published data only}
- Rebuck AS, Gent M, Chapman KR. Anticholinergic and sympathomimetic combination therapy of asthma. The Journal of Allergy and Clinical Immunology 1983;71(3):317‐23. [DOI] [PubMed] [Google Scholar]
Ruffin 1990 {published data only}
- Ruffin R, Blight M. Effect of inhaled oxitropium bromide in asthmatics. Australian and New Zealand Journal of Medicine 1990;20:525. [Google Scholar]
Salorinne 1988 {published data only}
- Salorinne Y, Ahonen A. Comparison of the bronchodilator effect of ipratropium bromide powder and two doses of ipratropium bromide aerosol in the treatment of asthma. Current therapeutic research, clinical and experimental. 1988;43(6):1073‐80. [Google Scholar]
Storms 1986 {published data only}
- Storms WW, Bodman SF, Nathan RA, Busse WW, Bush RK, Falliers CJ, O'Hollaren JD, et al. Use of ipratropium bromide in asthma Results of a multi‐clinic study. The American Journal of Medicine 1986;81:61. [DOI] [PubMed] [Google Scholar]
Tammivaara 1993 {published data only}
- Tammivaara R, Elo J, Mansury L. Terbutaline controlled‐release tablets and ipratropium aerosol in nocturnal asthma. Allergy 1993;48:45. [DOI] [PubMed] [Google Scholar]
Taylor 1986 {published data only}
- Taylor RG, Pavia D, Agnew J, Lopez‐Vidriero M, Newman S, Lennard‐Jones T, Clarke S. Effect of four weeks' high dose ipratropium bromide treatment on lung mucociliary clearance. Thorax 1986;41:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taytard 1984 {published data only}
- Taytard A, Auzerie J, Vergeret J, Tozon N, Freour P. Treatment of adult asthma: controlled double‐blind clinical trial of oxitropium bromide. European Journal of Clinical Pharmacology 1984;26:429. [DOI] [PubMed] [Google Scholar]
Tormey 1995 {published data only}
- Tormey VJ, Pathmakanthan S, Faul J, Leonard C, Poulter LW, Burke CM. A comparison of the effects of inhaled salbutamol or oxitropium bromide on bronchial hyperresponsiveness in asthmatic patients treated with. Irish Journal of Medical Science 1995;164:172. [Google Scholar]
van Schayck 1991a {published data only}
- Schayck CP, Dompeling E, Herwaarden CL, Folgering H, Verbeek AL, Hoogen HJ, et al. Bronchodilator treatment in moderate asthma or chronic bronchitis: continuous or on demand? A randomised controlled study. BMJ 1991;303:1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Schayck 1991b {published data only}
- Schayck CP, Folgering H, Harbers H, Maas KL, Weel C. Effects of allergy and age on responses to salbutamol and ipratropium bromide in moderate asthma and chronic bronchitis. Thorax 1991;46:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Schayck 1994 {published data only}
- Schayck CP, Folgering H, Otter JJ, Tirimanna P, Weel C. Does continuous use of bronchodilators disguise the progression of chronic nonspecific lung diseases?. Tijdschrift voor geneeskunde 1994;50:787. [Google Scholar]
van Schayck 1995 {published data only}
- Schayck CP, Dompeling E, Herwaarden CL, Folgering H, Akkermans RP, Broek PJ, et al. Continuous and on demand use of bronchodilators in patients with non‐steroid dependent asthma and chronic bronchitis: four‐year follow‐up randomized controlled study. British Journal of General Practice 1995;45:239. [PMC free article] [PubMed] [Google Scholar]
Vaughan 1988 {published and unpublished data}
- Vaughan TR, Bowen RE, Goodman DL, Weber RW, Nelson HS. The development of subsensitivity to atropine methylnitrate A double‐blind, placebo‐controlled crossover study. The American Review of Respiratory Disease 1988;138:771. [DOI] [PubMed] [Google Scholar]
Waite 1987 {published data only}
- Waite DA. Comparison of Duovent with salbutamol in chronic asthmatics ‐ a long term study in community practice. Postgraduate Medical Journal 1987;63(Suppl 1):4a. [Google Scholar]
Wheeler 1987 {published and unpublished data}
- Wheeler AW, Guest PG, Britton MG. A three month study of oxitropium bromide in patients with bronchial asthma. Postgraduate Medical Journal 1987;63:8. [Google Scholar]
Wolstenholme 1989 {published and unpublished data}
- Wolstenholme RJ, Shettar SP. Comparison of a combination of fenoterol with ipratropium bromide (Duovent) and salbutamol in young adults with nocturnal asthma. Respiration 1989;55:152. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abboud 1987 {published data only}
- Abboud R, Gibson N, Vickerson F, Mukherjee J. Bronchodilating effect of increasing doses of fenoterol (Berotec) given in combination with a standard dose of ipratropium (Atrovent) in asthma. Postgraduate Medical Journal 1987;63(Suppl 1):5. [Google Scholar]
Adinoff 1998 {published data only}
- Adinoff AD, Schwartz HJ, Rickard KA, Yancey SW, Swearingen BE. Salmeterol compared with current therapies in chronic asthma. Journal of Family Practice 1998;47:278. [PubMed] [Google Scholar]
Advenier 1984 {published data only}
- Advenier C, Cerrina J, Duroux P. Bronchodilators. Semaine Des Hopitaux 1984;60:179. [PubMed] [Google Scholar]
Alanko 1983 {published data only}
- Alanko K, Sahlstrom K, Harkonen R. Combination of fenoterol and ipratropium bromide in the treatment of bronchial asthma. European Journal of Respiratory Diseases‐Supplement 1983;128(Pt 2):546. [PubMed] [Google Scholar]
Allen 1980a {published data only}
- Allen CJ, Campbell AH. Comparison of inhaled atropine sulphate and atropine methonitrate. Thorax 1980;35:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 1980b {published data only}
- Allen CJ, Campbell AH. Dose response of ipratropium bromide assessed by two methods. Thorax 1980;35:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alliott 1972 {published data only}
- Alliott RJ, Lang BD, Rawson DR, Leckie WJ. Effects of salbutamol and isoprenaline‐phenylephrine in reversible airways obstruction. BMJ 1972;1:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altounyan 1964 {published data only}
- Altounyan RE. Variation of drug action on airway obstruction in man. Thorax 1964;19:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen 1986 {published data only}
- Andersen L. Pulmonary function and acid application in the esophagus. Chest 1986;90:358. [DOI] [PubMed] [Google Scholar]
Anderson 1980 {published data only}
- Anderson G, Jariwalla AG, Turnbull P. The effects of fenoterol, ipratropium bromide and their combination in asthma and chronic bronchitis. British Journal of Diseases of the Chest 1980;74:416. [Google Scholar]
Anderson 1981 {published data only}
- Anderson G, Jariwalla AG, Turnbull P, Veale J. The effects of fenoterol, ipratroprium bromide and their combination in asthma and chronic bronchitis. IRCS (International Research Communications System) Medical Science 1981;9:251. [Google Scholar]
Anderson 1983 {published data only}
- Anderson G. Anticholinergic drugs in airways obstruction. Postgraduate Medical Journal 1983;59(Suppl 3):64. [PubMed] [Google Scholar]
Aquilina 1986 {published data only}
- Aquilina R, Bergero F, Noceti P, Mirabelli S, Luciani G. Protective effect of Duovent versus salbutamol in long‐term treatment. Respiration 1986;50(Suppl 2):240. [DOI] [PubMed] [Google Scholar]
Ariano 1981 {published data only}
- Ariano R, Giacca S. Variations of individual susceptibility to beta‐adrenergic and anticholinergic bronchodilator drugs. Minerva Pneumologica 1981;20:141. [Google Scholar]
Baigelman 1977 {published data only}
- Baigelman W, Chodosh S. Bronchodilator action of the anticholinergic drug, ipratropium bromide (Sch 1000), as an aerosol in chronic bronchitis and asthma. Chest 1977;71:324. [DOI] [PubMed] [Google Scholar]
Banos 1983 {published data only}
- Banos Hidalgo P, Ramos Martos A, Cabrera Moreno R, Luque Leal J, Palacios Giner A. Comparative double blind study of the aerosol association of ipratropium bromide and salbutamol with its individual effect in chronic bronchitis. Revista Clinica Espanola 1983;171:265. [PubMed] [Google Scholar]
Barber 1975 {published data only}
- Barber P, Chatterjee SS, Scott R. The effect of two anticholinergic bronchodilators on ventilatory function in patients with chronic bronchitis or asthma as measured by spirometry. Postgraduate Medical Journal 1975;51(Suppl):112. [Google Scholar]
Barber 1977 {published data only}
- Barber PV, Chatterjee SS, Scott R. A comparison of ipratropium bromide, deptropine citrate and placebo in asthma and chronic bronchitis. British Journal of Diseases of the Chest 1977;71:101. [DOI] [PubMed] [Google Scholar]
Bauer 1973 {published data only}
- Bauer P, Kummer F. Double‐blind study for the comparison of the broncholytic effect of a new tropic acid derivative with isoprenaline. International Journal of Clinical Pharmacology and Therapeutics 1973;8:135. [PubMed] [Google Scholar]
Beasley 1987 {published data only}
- Beasley CR, Rafferty P, Holgate ST. Bronchoconstrictor properties of preservatives in ipratropium bromide (Atrovent) nebuliser solution. BMJ 1987;294:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 1982 {published data only}
- Bell R, Sahay JN, Barber PV, Chatterjee SS, Cox G. A therapeutic comparison of ipratropium bromide and salbutamol in asthmatic patients. Current Medical Research & Opinion 1982;8:242. [DOI] [PubMed] [Google Scholar]
Bellia 1987 {published data only}
- Bellia V, Cibella F, Insalaco G, Mirto M, Peralta G, Visconti A, Schiassi M. Comparison of the effectiveness of oxitropium bromide and of a slow‐release theophylline in the prevention of 'morning dip' in asthmatics. Postgraduate Medical Journal 1987;63:9. [Google Scholar]
Bernabeu 1987 {published data only}
- Bernabeu L, Bourcereau J, Delacenserie R, Prosper M, Salen JM, Vincent J, Jirou‐Najou JL. Ipratropium bromide ‐fenoterol combination: optimum dosage ratio in asthma. Postgraduate Medical Journal 1987;63(Suppl 1):6. [Google Scholar]
Bezel 1987 {published data only}
- Bezel R, Brandli O, Braun P, Haegi V, Villiger B. Additive effect of sustained‐release theophylline combined with anticholinergic and sympathomimetic therapy in COPD. Postgraduate Medical Journal 1987;63(Suppl 1):22. [Google Scholar]
Bish 1997 {published data only}
- Bish R, Thien F, Whitehead F, Walters J, Walters EH. The use of regular ipratropium bromide to reduce requirements for beta‐2 agonists in symptomatic asthmatic subjects [abstract]. Australian and New Zealand Journal of Medicine 1997;27:246. [Google Scholar]
Bohadana 1980 {published data only}
- Bohadana AB, Peslin R, Teculescu D, Polu JM, et al. The bronchodilator action of theophylline aerosol in subjects with chronic airflow obstruction. Clinical Respiratory Physiology 1980;16:13. [PubMed] [Google Scholar]
Bonsignore 1986 {published data only}
- Bonsignore G, Bellia V, Peralta G, Alessi N, Migliara G. The combination of fenoterol and ipratropium bromide in bronchial asthma: comparison of the acute effects of two different dosages. Respiration 1986;50(Suppl 2):148. [DOI] [PubMed] [Google Scholar]
Botts 1985 {published data only}
- Botts LD, Pingleton SK, Schroeder CE, Robinson RG, Hurwitz A. Prolongation of gastric emptying by aerosolized atropine. The American Review of Respiratory Disease 1985;131:725. [DOI] [PubMed] [Google Scholar]
Brady 1979 {published data only}
- Brady RE, Easton JG. The value of atropine in the documentation of reversible airways obstruction. Annals of Allergy 1979;42:211. [PubMed] [Google Scholar]
Brand 1992 {published data only}
- Brand PL, Kerstjens HA, Postma DS, Sterk PJ, Quanjer PH, Sluiter HJ, et al. Long‐term multicentre trial in chronic nonspecific lung disease: methodology and baseline assessment in adult patients Dutch CNSLD Study Group. European Respiratory Journal 1992;5:21. [PubMed] [Google Scholar]
Bremner 1992 {published data only}
- Bremner P, Burgess C, Beasley R, Woodman K, Marshall S, Crane J, Pearce N. Nebulized fenoterol causes greater cardiovascular and hypokalaemic effects than equivalent bronchodilator doses of salbutamol in asthmatics. Respiratory Medicine 1992;86:419. [DOI] [PubMed] [Google Scholar]
Britton 1988 {published data only}
- Britton J, Hanley SP, Garrett HV, Hadfield JW, Tattersfield AE. Dose related effects of salbutamol and ipratropium bromide on airway calibre and reactivity in subjects with asthma. Thorax 1988;43:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brom 1985 {published data only}
- Brom AL. Prophylaxis of bronchial asthma in adults with the use of ketotifen : Prolongation of gastric emptying by aerosolized atropine. The American review of respiratory disease 1985;8:725. [DOI] [PubMed] [Google Scholar]
Bruderman 1983 {published data only}
- Bruderman I, Cohen‐Aronovski R, Smorzik J. A comparative study of various combinations of ipratropium bromide and metaproterenol in allergic asthmatic patients. Chest 1983;83:208. [DOI] [PubMed] [Google Scholar]
Brumby 1980 {published data only}
- Brumby KH. Comparative clinical study of the efficacy of Atrovent and Berotec, singly and combined, with comparison of placebo. Therapiewoche 1980;30:2796. [Google Scholar]
Bryant 1990a {published data only}
- Bryant DH Rogers P. Oxitropium bromide: an acute dose response study of a new anticholinergic drug in combination with fenoterol in asthma and chronic bronchitis. Pulmonary Pharmacology 1990;3:55. [DOI] [PubMed] [Google Scholar]
Bryant 1990b {published data only}
- Bryant D, Rogers P. Comparison of preservative‐free ipratropium bromide and atrovent nebuliser solution in treatment of asthma [abstract]. Australian and New Zealand Journal of Medicine 1990;20:525. [Google Scholar]
Bryant 1992 {published data only}
- Bryant DH, Rogers P. Effects of ipratropium bromide nebulizer solution with and without preservatives in the treatment of acute and stable asthma. Chest 1992;102:742. [DOI] [PubMed] [Google Scholar]
Burge 1980 {published data only}
- Burge PS, Harries MG, l'Anson E. Comparison of atropine with ipratropium bromide in patients with reversible airways obstruction unresponsive to salbutamol. British Journal of Diseases of the Chest 1980;74:259. [DOI] [PubMed] [Google Scholar]
Burki 1997 {published data only}
- Burki NK. The effects of the combination of inhaled ipratropium and oral theophylline in asthma. Chest 1997;111:1509. [DOI] [PubMed] [Google Scholar]
Cañete 1991 {published data only}
- Cañete CE. Treatment with anticholinergic drugs in asthmatic crisis. Archivos De Bronconeumología Suppl 1991;27:32. [Google Scholar]
Capel 1964 {published data only}
- Capel LH, Fletcher EC. Spirometric integrals after isoprenaline and atropine in asthma. British Journal of Diseases of the Chest 1964;58:174. [DOI] [PubMed] [Google Scholar]
Carpentiere 1986 {published data only}
- Carpentiere G, Marino S, Castello F, Bonanno CT. Comparison between morning and afternoon effects of inhaling a combined preparation of fenoterol with ipratropium bromide. Current Therapeutic Research 1986;40:265. [Google Scholar]
Carratu 1983 {published data only}
- Carratu L, Sofia M. Comparison of the effects of inhalation of a single dose of a ipratropium bromide‐fenoterol combination with a single dose of fenoterol. Archivio Monaldi Per La Tisiologia e Le Malattie Dell Apparato Respiratorio 1983;38:263. [PubMed] [Google Scholar]
Casali 1979 {published data only}
- Casali L, Grassi C, Rampulla C, Rossi A. Clinical pharmacology of a combination of bronchodilators. International Journal of Clinical Pharmacology and Biopharmacy 1979;17:277. [PubMed] [Google Scholar]
Catterall 1988 {published data only}
- Catterall JR, Rhind GB, Whyte KF, Shapiro CM, Douglas NJ. Is nocturnal asthma caused by changes in airway cholinergic activity?. Thorax 1988;43:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chakravarti 1987 {published data only}
- Chakravarti A, Pratley M, Barcharb J, Musiosky M, McDonald F, Vickerson F, et al. Ipratropium‐fenoterol interaction study in asthmatic adults. Postgraduate Medical Journal 1987;63(Suppl 1):5. [Google Scholar]
Chervinsky 1977 {published data only}
- Chervinsky P. Double‐blind study of ipratropium bromide, a new anticholinergic bronchodilator. Journal of Allergy and Clinical Immunology 1977;59:22. [DOI] [PubMed] [Google Scholar]
Chhabra 2002 {published data only}
- Chhabra SK, Pandey KK. Comparison of acute bronchodilator effects of inhaled ipratropium bromide and salbutamol in bronchial asthma. Journal of Asthma 2002;39:375. [DOI] [PubMed] [Google Scholar]
Chick 1977 {published data only}
- Chick TW, Jenne JW. Comparative bronchodilator responses to atropine and terbutaline in asthma and chronic bronchitis. Chest 1977;72:719. [DOI] [PubMed] [Google Scholar]
Chuchalin 1985 {published data only}
- Chuchalin AG, Konganova NA, Pashkova TL, Treskunov VK, Apul'tsina ID. Berodual in the treatment of bronchial asthma. Sovetskaia Meditsina 1985;11:81. [PubMed] [Google Scholar]
Ciappi 1986 {published data only}
- Ciappi G, Magnini P, Valente S, Toscano L, Corbo GM, Culla G, Patalano F. Dose‐response relationship: fenoterol, ipratropium bromide and their combination. Respiration 1986;50(Suppl 2):140. [DOI] [PubMed] [Google Scholar]
Clauzel 1987 {published data only}
- Clauzel A, Michel F, Jirou‐Najou J. Study of dose response curves of fenoterol, ipratropium bromide and Berodual. Postgraduate Medical Journal 1987;63(Suppl 1):13. [Google Scholar]
Cockcroft 1977 {published data only}
- Cockcroft DW, Killian DN, Mellon JJ, Hargreave FE. Protective effect of drugs on histamine‐induced asthma. Thorax 1977;32:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 1984 {published data only}
- Cox ID, Hughes DT, McDonnell KA. Ipratropium bromide in patients with nocturnal asthma. Postgraduate Medical Journal 1984;60:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crane 1986a {published data only}
- Crane J, Gamble S. Single dose comparison of salbutamol and a fenoterol/ipratropium combination from metered aerosols in patients with asthma. New Zealand Medical Journal 1986;99:420. [PubMed] [Google Scholar]
Crane 1986b {published data only}
- Crane J. Single‐dose comparison of salbutamol and Duovent‐Berodual in asthma. Respiration 1986;50(Suppl 2):285. [DOI] [PubMed] [Google Scholar]
Crane 1987 {published data only}
- Crane J, Town G, O'donnell T. Duovent in obstructive airways disease: comparison with salbutamol by metered aerosol in respect of degree and duration of bronchodilation in. Postgraduate Medical Journal 1987;63(Suppl 1):3. [Google Scholar]
del Buffalo 1982 {published data only}
- Bufalo DC, Fabbri M, Gunella G, Longi R. Effetti sulla funzione ventilatoria dell'associazione fenoterolo‐bromuro di ipratropium. Rivista di Patologia e Clinica della Tubercolosi e di Pneumologia 1982;53:637. [Google Scholar]
Donkers 1998 {published data only}
- Donkers JM, Schayck CP, Akkermans RP. The difference in bronchodilation effect of a beta two‐mimetic and an anticholinergic agent in patients with asthma or chronic obstructive lung. Ned Tijdschr Geneeskd 1998;142:1419. [Google Scholar]
Donner 1980 {published data only}
- Donner CF. [IS there a placebo ie beclo plus (IB vs placebo?)] Bronchospasm management by means of ipratropium, beclomethasone and their combination. Giornale Italiano della Tubercolosi e Delle Malattie del Torace 1980;34:307. [Google Scholar]
Dutt 1990 {published data only}
- Dutt NS, Reddy TC, Raghuveer TS, Yeshwanth M, Sunil Dutt N, Chinnapa Reddy T, et al. A comparative study of atropine sulphate, terbutaline and their combination in asthmatic children. Lung India 1990;8(2):73‐5. [Google Scholar]
Elwood 1982 {published data only}
- Elwood RK, Abboud RT. The short‐term bronchodilator effects of fenoterol and ipratropium in asthma. Journal of Allergy and Clinical Immunology 1982;69:467. [DOI] [PubMed] [Google Scholar]
Emirgil 1975 {published data only}
- Emirgil C, Dwyer K, Baskette P, Sobol BJ. A new parasympatholytic bronchodilator: a study of its onset of effect after inhalation. Current Therapeutic Research 1975;17:215. [PubMed] [Google Scholar]
Endoh 1998 {published data only}
- Endoh N, Ichinose M, Takahashi T, Miura M, Kageyama N, Mashito Y, et al. Relationship between cholinergic airway tone and serum immunoglobulin E in human subjects. European Respiratory Journal 1998;12:71. [DOI] [PubMed] [Google Scholar]
Filiz 1994 {published data only}
- Filiz A, Ekinci E, Dikensoy O, Bulgur D, Oz M. Comparison of a single dose of aerosol salbutamol and fenoterol/ipratropium on bronchial asthmatic patients. Delaware Medical Journal 1994;66:549. [PubMed] [Google Scholar]
Firstater 1981 {published data only}
- Firstater E, Mizrachi E, Topilsky B. The effect of vagolytic drugs on airway obstruction in patients with bronchial asthma. Annals of Allergy 1981;46:332. [PubMed] [Google Scholar]
Fischbacher 1984 {published data only}
- Fischbacher CM, Milroy R, Giannini D, Monie RD. Comparison of Duovent and salbutamol inhalers in chronic stable asthma. Postgraduate Medical Journal 1984;60(Suppl 1):28. [PubMed] [Google Scholar]
Flint 1983a {published data only}
- Flint KC, Hockley B, Johnson NM. A comparison between a combination of ipratropium bromide plus fenoterol in a single metered dose inhaler (Duovent) and salbutamol in asthma. Postgraduate Medical Journal 1983;59:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flint 1983b {published data only}
- Flint K, Hockley B, Johnson NM. Salbutamol versus Duovent (a combination of fenoterol and ipratropium bromide) in asthma. European Journal of Respiratory Diseases‐Suppl 1983;128(Pt 2):548. [PubMed] [Google Scholar]
Flint 1984 {published data only}
- Flint K, Hockley B, Johnson NM. A comparison between a combination of ipratropium bromide plus fenoterol in a single metered dose inhaler (Duovent) and salbutamol in asthma. Postgraduate Medical Journal 1984;60(Suppl 1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 1983 {published data only}
- Foster WM, Langenback EG, Glaser ML, Bergofsky EH. Acute effect of ipratropium bromide at therapeutic dose on mucus transport of adult asthmatics. European Journal of Respiratory Diseases‐Suppl 1983;128(Pt 2):554. [PubMed] [Google Scholar]
Freeman 1992 {published data only}
- Freeman W, Javaid A, Cayton RM. The effect of ipratropium bromide on maximal exercise capacity in asthmatic and non‐asthmatic men. Respiratory Medicine 1992;86:151. [DOI] [PubMed] [Google Scholar]
Frith 1987 {published data only}
- Frith PA, Atkinson J. Airway anticholinergic effects of oxitropium. Postgraduate Medical Journal 1987;63(Suppl 1):5. [Google Scholar]
Gaba 1987 {published data only}
- Gaba S, Trigui F, Michel FB, Jirou‐Najou JL. Circadian rhythms in asthmatics: comparison of Berotec, Atrovent and Berodual bronchodilating action. Postgraduate Medical Journal 1987;63(Suppl 1):13. [Google Scholar]
Gillies 1987 {published data only}
- Gillies AJ, Stewart D. A dose response study comparing Duovent with salbutamol. Postgraduate Medical Journal 1987;63(Suppl 1):19. [PubMed] [Google Scholar]
Ginesu 1983 {published data only}
- Ginesu F, Ortu AR, Schena GP, Deiola G, Polo F. Controlled clinical study of the effects of a single aerosol administration of a fenoterol‐ipratropium bromide combination in comparison with. Archivio Monaldi Per La Tisiologia e Le Malattie Dell Apparato Respiratorio 1983;38:241. [PubMed] [Google Scholar]
Gotz 1983 {published data only}
- Gotz RS. Effect of the inhalation of an atropine derivative (ipratropium bromide) in powder form on respiratory mechanics in obstructive airway. Praxis und Klinik der Pneumologie 1983;37(Suppl 1):927. [PubMed] [Google Scholar]
Grandordy 1988 {published data only}
- Grandordy BM, Thomas V, Lauture D, Marsac J. Cumulative dose‐response curves for assessing combined effects of salbutamol and ipratropium bromide in chronic asthma. European Respiratory Journal 1988;1:531. [PubMed] [Google Scholar]
Gross 1975a {published data only}
- Gross NJ. Sch 1000: a new anticholinergic bronchodilator. The American Review of Respiratory Disease 1975;112:823. [DOI] [PubMed] [Google Scholar]
Gross 1975b {published data only}
- Gross HJ. Dose‐response study of the effect of Sch 1000 MDI on forced vital capacity (FVC), FEV1.0, maximal mid‐expiratory flow (MMEF) and heart rate, ECG. Postgraduate Medical Journal 1975;51(Suppl 7):95. [Google Scholar]
Gross 1984 {published data only}
- Gross NJ, Skorodin MS. Anticholinergic, antimuscarinic bronchodilators[Review] [243 refs]. The American review of respiratory disease 1984;129:856. [DOI] [PubMed] [Google Scholar]
Gross 1987 {published data only}
- Gross NJ, Bankwala Z. Effects of an anticholinergic bronchodilator on arterial blood gases of hypoxemic patients with chronic obstructive pulmonary disease;. The American review of respiratory disease 1987;136:1091. [DOI] [PubMed] [Google Scholar]
Gutersohn 1975 {published data only}
- Gutersohn. The effect of Raw on Sch1000 MDI or fenoterol MDI and the combined administration of subthreshold dosages of both compounds. Postgraduate Medical Journal 1975;51(Suppl 7):113. [Google Scholar]
Guyatt 1986 {published data only}
- Guyatt G, Sackett D, Taylor DW, Chong J, Roberts R, Pugsley S. Determining optimal therapy Randomized trials in individual patients. New England Journal of Medicine 1986;314:889. [DOI] [PubMed] [Google Scholar]
Higgins 1991 {published data only}
- Higgins BG, Powell RM, Cooper S, Tattersfield AE. Effect of salbutamol and ipratropium bromide on airway calibre and bronchial reactivity in asthma and chronic bronchitis. European Respiratory Journal 1991;4:415. [PubMed] [Google Scholar]
Hockley 1985 {published data only}
- Hockley B, Johnson NM. A comparison of three high doses of ipratropium bromide in chronic asthma. British Journal of Diseases of the Chest 1985;79:379. [DOI] [PubMed] [Google Scholar]
Hoffstein 1994 {published data only}
- Hoffstein V, Zamel N, McClean P, Chapman KR. Changes in pulmonary function and cross‐sectional area of trachea and bronchi in asthmatics following inhalation of procaterol hydrochloride and. American Journal of Respiratory and Critical Care Medicine 1994;149:81. [DOI] [PubMed] [Google Scholar]
Holst 1976 {published data only}
- Holst PE, Gebbie T, Martin PD, O'Donnell TV. A long term trial of ipratropium in asthmatic patients. Australian and New Zealand Journal of Medicine 1976;11:367. [Google Scholar]
Huhti 1986 {published data only}
- Huhti E, Poukkula A. Comparison of fenoterol, ipratropium bromide, and their combination in patients with asthma or chronic airflow obstruction. Respiration 1986;50(Suppl 2):298. [DOI] [PubMed] [Google Scholar]
Hunt 1983 {published data only}
- Hunt D, MacDonald GF, Reilly P, Plumley D, Kazim F. Bronchodilator response to several doses of ipratropium bromide in asthmatics and bronchitics. Current Therapeutic Research, Clinical and Experimental. 1983;33:651. [Google Scholar]
Irsigler 1975 {published data only}
- Irsigler G. Flow volume curves and changes in FEV1.0, forced vital capacity (FVC), mid and slow vital capacity (Mid VC and SVC) in patients with bronchial. Postgraduate medical journal Suppl 7 1975;51:96. [Google Scholar]
Jenkins 1981 {published data only}
- Jenkins CR, Chow CM, Fisher BL, Marlin GE. Comparison of ipratropium bromide and salbutamol by aerosolized solution. Australian and New Zealand Journal of Medicine 1981;11:513. [DOI] [PubMed] [Google Scholar]
Jenkins 1982 {published data only}
- Jenkins CR, Chow CM, Fisher BL, Marlin GE. Ipratropium bromide and fenoterol by aerosolized solution. British Journal of Clinical Pharmacology 1982;14:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jindal 1989 {published data only}
- Jindal SK, Kaur SJ. Relative bronchodilatory responsiveness attributable to sympathetic and parasympathetic activity in bronchial asthma. Respiration 1989;56:16. [DOI] [PubMed] [Google Scholar]
Johnson 1989 {published data only}
- Johnson MA, Newman SP, Bloom R, Talaee N, Clarke SW. Delivery of albuterol and ipratropium bromide from two nebulizer systems in chronic stable asthma‐ Efficacy and pulmonary deposition. Chest 1989;96:6. [DOI] [PubMed] [Google Scholar]
Jolobe 1981 {published data only}
- Jolobe OM, Lane DJ. Atropine responsiveness in asthma in relation to steroid aerosol therapy. British Journal of Diseases of the Chest 1981;75:413. [DOI] [PubMed] [Google Scholar]
Kaik 1975 {published data only}
- Kaik G. Untersuchungen mit dem neuen Bronchospamolytikum Ipratropiumbromid (Sch 1000, Atrovent). Therapiewoche 1975;25:2428. [Google Scholar]
Kaik 1976 {published data only}
- Kaik G. [Whole‐body plethysmographic studies on the bronchospasmolytic effect of 2 antiasthmatic drug combinations and ipratropium bromide]. Wiener Medizinische Wochenschrift 1976;126:127. [PubMed] [Google Scholar]
Kaik 1979 {published data only}
- Kaik G. [Lung function studies with the bronchodilator terbutaline]. Acta medica Austriaca 1979;6:1. [PubMed] [Google Scholar]
Kamburoff 1975 {published data only}
- Kamburoff PL, Gunthner W. Dose‐dependent effects of Sch 1000 MDI on the airways resistance indices in patients with chronic airways obstruction. Postgraduate Medical Journal 1975;51(Suppl 7):97. [Google Scholar]
Kaptein 1993 {published data only}
- Kaptein AA, Brand PL, Dekker FW, Kerstjens HA, Postma DS, Sluiter HJ. Quality‐of‐life in a long‐term multicentre trial in chronic nonspecific lung disease: Assessment at baseline. European Respiratory Journal 1993;6:1479. [PubMed] [Google Scholar]
Kennedy 1964 {published data only}
- Kennedy MC, Thursby‐Pelham DC. Some adrenergic drugs and atropine methonitrate given by inhalation for asthma: a comparative study. BMJ 1964;1:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerstjens 1992 {published data only}
- Kerstjens HA, Brand PL, Hughes MD, Robinson NJ, Postma DS, Sluiter HJ, et al. A comparison of bronchodilator therapy with or without inhaled corticosteroid therapy for obstructive airways disease. New England Journal of Medicine 1992;327:1413. [DOI] [PubMed] [Google Scholar]
Kerstjens 1993a {published data only}
- Kerstjens HA, Brand PL, Quanjer PH, Bruggen‐Bogaarts Koeter GH, Postma DS. Variability of bronchodilator response and effects of inhaled corticosteroid treatment in obstructive airways disease. Dutch CNSLD Study Group. Thorax 1993;48:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerstjens 1993b {published data only}
- Kerstjens HA. Airways hyperresponsiveness, bronchodilator response, allergy and smoking predict improvement in FEV1 during long‐term inhaled corticosteroid. European respiratory journal 1993;6:868. [PubMed] [Google Scholar]
Kerstjens 1994 {published data only}
- Kerstjens HA, Brand PL, Jong PM, Koeter GH, Postma DS. Influence of treatment on peak expiratory flow and its relation to airway hyperresponsiveness and symptoms. Thorax 1994;49:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerstjens 1995 {published data only}
- Kerstjens HA, Schouten JP, Brand PLP, Schoonbrood DF, Sterk PJ, Postma DS, et al. Importance of total serum IgE for improvement in airways hyperresponsiveness with inhaled corticosteroids in asthma and chronic obstructive. American Journal of Respiratory & Critical Care Medicine 1995;151:360. [DOI] [PubMed] [Google Scholar]
Kok‐Jensen 1979 {published data only}
- Kok‐Jensen AA. Combined inhalation of salbutamol and ipratropin in chronic bronchial obstruction [Kombineret inhalation af salbutamol og ipratropin ved kronisk bronkial obstruktion]. Ugeskrift for Laeger 1979;141(30):2039‐41. [PubMed] [Google Scholar]
Kradjan 1992 {published data only}
- Kradjan WA, Driesner NK, Abuan TH, Emmick G, Schoene RB. Effect of age on bronchodilator response. Chest 1992;101:1545. [DOI] [PubMed] [Google Scholar]
Kreisman 1984 {published data only}
- Kreisman H, Cohen C, Ghezzo H, Vickerson F, Frank H, Wolkove N. Combined therapy with ipratropium and theophylline in asthma. Annals of Allergy 1984;52:90. [PubMed] [Google Scholar]
Krivoy 1987 {published data only}
- Krivoy N, Jubarin A, Gaitini L, Rubin A, Alroy G. Combination therapy of ipratropium bromide, terbutaline and slow release theophylline in asthma and chronic airways obstruction. Postgraduate Medical Journal 1987;63(Suppl 1):21. [Google Scholar]
Kunkel 1976 {published data only}
- Kunkel GS. The importance of nervus vagus for bronchial obstruction of allergic and non‐allergic origin and the therapeutic consequences. Praxis der Pneumologie 1976;30:459. [PubMed] [Google Scholar]
Kunkel 2000 {published data only}
- Kunkel G, Magnussen H, Bergmann KC, Juergens UR, Mey C, Freund E, Hinzmann R, Beckers B. Respimat (a new soft mist inhaler) delivering fenoterol plus ipratropium bromide provides equivalent bronchodilation at half the cumulative dose. Respiration 2000;67:306. [DOI] [PubMed] [Google Scholar]
Lahdensuo 1975 {published data only}
- Lahdensuo A, Viljanen A, Muittari A. A comparative study on the effect of Sch1000 MDI, salbutamol and placebo MDI in bronchial asthma. Postgraduate Medical Journal 1975;51(Suppl 7):116. [Google Scholar]
Laitinen 1986 {published data only}
- Laitinen LA, Poppius H. Combination of oxitropium bromide and salbutamol in the treatment of asthma with pressurized aerosols. British Journal of Diseases of the Chest 1986;80:179. [DOI] [PubMed] [Google Scholar]
Lanser 1975 {published data only}
- Lanser KG, Foldi M, Sill V. The effect of Tropaacidester Sch 1000 on respiratory resistance [Therapie der obstruktiven Atemwegerkrankung mit einem Atropinderivat (Sch 1000)]. Medizinische Klinik 1975;70(9):380‐4. [PubMed] [Google Scholar]
Larsson 1987 {published data only}
- Larsson K. Ipratropium bromide: bronchodilator action and effect on methacholine‐induced bronchoconstriction. Journal of Asthma 1987;24:29. [DOI] [PubMed] [Google Scholar]
Lavandier 1987 {published data only}
- Lavandier M, Bourcereau J, Jirou‐Najou J. Comparison of bronchodilating onset of action of Berotec, Atrovent and Berodual. Postgraduate Medical Journal 1987;63(Suppl 1):13. [Google Scholar]
Lawford 1983 {published data only}
- Lawford P, Palmer KN. A single dose comparison of a combination of fenoterol and ipratropium aerosols in bronchial asthma. Postgraduate Medical Journal 1983;59:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefcoe 1982 {published data only}
- Lefcoe NM, Toogood JH, Blennerhassett G, Baskerville J, Paterson NA. The addition of an aerosol anticholinergic to an oral beta agonist plus theophylline in asthma and bronchitis A double‐blind single dose study. Chest 1982;82:300. [DOI] [PubMed] [Google Scholar]
Lehrer 1993 {published data only}
- Lehrer PM, Hochron SM, Isenberg S, Rausch L, Carr R. The Asthma Symptom Profile: a psychophysically based scale for assessment of asthma symptoms. Journal of Psychosomatic Research 1993;37:515. [DOI] [PubMed] [Google Scholar]
Lehrer 1994 {published data only}
- Lehrer PM, Hochron SM, Rausch L, Carr R. Effects of aerosol ipratropium bromide on cardiac vagal tone. Chest 1994;105:1701. [DOI] [PubMed] [Google Scholar]
Li 2003 {published data only}
- Li SF, Yao WJ. Combined application of salbutamol and ipratropium bromide in the treatment of bronchial asthma in children. Herald of Medicine 2003;22(2):96‐7. [Google Scholar]
Lichterfeld 1979 {published data only}
- Lichterfeld A. Safety of Atrovent. Scandinavian journal of respiratory diseases Suppl 1979;103:143. [PubMed] [Google Scholar]
Linehan 1975 {published data only}
- Linehan WD. The effect of Sch1000 and terbutaline given as MDI on ventilatory function in patients with asthma as measured by spirometry. Postgraduate medical journal Suppl 7 1975;51:116. [Google Scholar]
Maesen 1975a {published data only}
- Maesen F, Buytendijk HJ. Dose‐ and time‐response curves of Sch 1000 MDI and placebo. Differential response in asthmatics and bronchitics as neasured by FEV1.0, vital. Postgraduate Medical Journal 1975;51(Suppl 7):97. [Google Scholar]
Maesen 1975b {published data only}
- Maesen FP. Comparison of the effects of Sch 1000 MDI and fenoterol MDI in patients with bronchial asthma and chronic bronchitis. Postgraduate Medical Journal 1975;51(Suppl 7):116. [Google Scholar]
Maesen 1982 {published data only}
- Maesen FP, Smeets JJ, Rubingh G. Vergelijking van het bronchospasmolytisch effect van druk‐aerosolen met fenoterol (Berotec) en van een combinatie van fenoterol en een. Tijdschrift voor geneeskunde 1982;7:1184. [Google Scholar]
Maesen 1985 {published data only}
- Maesen FP, Smeets JJ, Pover GM. Comparison of the effects of a combination inhaler containing salbutamol and atropine methonitrate By comparison with each component given. Tijdschrift voor Therapie Geneesmiddel en Onderzoek 1985;10:1000. [Google Scholar]
Mann 1984 {published data only}
- Mann JS, Howarth PH, Holgate ST. Bronchoconstriction induced by ipratropium bromide in asthma: relation to hypotonicity. BMJ 1984;289:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marcatili 1984 {published data only}
- Marcatili S, Maselli R. Effects of administration of a single dose of iprafen (l puff) compared with those of a single dose of ipratropium bromide (40 mcg ). Archivio Monaldi Per La Tisiologia e Le Malattie Dell Apparato Respiratorio 1984;39:37. [PubMed] [Google Scholar]
Marin 1987 {published data only}
- Marin J, Servera E, Verara P. Efficacy devoid of side effects in the combination of an atropine‐like bronchodilator and a low dose of a beta2‐mimetic. Postgraduate Medical Journal 1987;63(Suppl 1):18. [Google Scholar]
Marlin 1978 {published data only}
- Marlin GE, Bush DE, Berend N. Comparison of ipratropium bromide and fenoterol in asthma and chronic bronchitis. British Journal of Clinical Pharmacology 1978;6:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marlin 1979 {published data only}
- Marlin GE, Berend N, Harrison AC. Combined cholinergic antagonist and beta 2‐adrenoceptor agonist bronchodilator therapy by inhalation. Australian and New Zealand Journal of Medicine 1979;9:511. [DOI] [PubMed] [Google Scholar]
Mattila 1966 {published data only}
- Mattila M, Muittari A. The effect of bronchodilator aerosols on the peak expiratory flow rate in asthmatic patients. Acta Medica Scandinavica 1966;180:421. [DOI] [PubMed] [Google Scholar]
McIntosh 1986a {published data only}
- McIntosh D. A comparison between fenoterol/ipratropium bromide combinations and salbutamol administered as nebulizer solutions assessing dose‐response. Pharmatherapeutica 1986;4:416. [PubMed] [Google Scholar]
McIntosh 1986b {published data only}
- McIntosh D. Comparison between fenoterol/ipratropium bromide combinations and salbutamol administered as nebulizer solution assessing dose response and duration of action. Respiration 1986;50(Suppl 2):310‐2. [DOI] [PubMed] [Google Scholar]
Millar 1990 {published data only}
- Millar AB, Bush A, Al‐Hillawi H, Goldman J, Denison DM. Ipratropium bromide: are patients treated with optimal therapy?. Postgraduate Medical Journal 1990;66:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Minette 1979 {published data only}
- Minette A, Marcq M. Oxitropium bromide (Ba 253), an advance in the field of anticholinergic bronchodilating treatments Preliminary results. Revue De l Institut d Hygiene Des Mines 1979;34:115. [PubMed] [Google Scholar]
Minette 1985 {published data only}
- Minette P, Dubois P, Delwiche JP. Validity of air‐helium DVmax measurements in trials of bronchodilators. Bulletin Europeen de Physiopathologie Respiratoire 1985;21:357. [PubMed] [Google Scholar]
Morrison 1988 {published data only}
- Morrison JF Pearson SB Dean HG. Parasympathetic nervous system in nocturnal asthma. BMJ 1988;296:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 1989 {published data only}
- Morrison JF, Pearson SB. The effect of the circadian rhythm of vagal activity on bronchomotor tone in asthma. British Journal of Clinical Pharmacology 1989;28:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 1991 {published data only}
- Morrison JF, Pearson S. The parasympathetic nervous system and the diurnal variation of lung mechanics in asthma. Respiratory Medicine 1991;85:285. [DOI] [PubMed] [Google Scholar]
Morton 1984 {published data only}
- Morton O. Response to Duovent of chronic reversible airways obstruction‐‐a controlled trial in general practice. Postgraduate Medical Journal 1984;60(Suppl 1):32. [PubMed] [Google Scholar]
Muittari 1968 {published data only}
- Muittari A. Atropine methonitrate in bronchial asthma. Annales Medicinae Internae Fenniae 1968;57:193. [PubMed] [Google Scholar]
Nishi 1993 {published data only}
- Nishi K, Fujimura M, Myou S, Ooka T, Sakamoto S, Saitou M, et al. Comparison of the bronchodilator activities of oxitropium bromide, fenoterol, and their combination in patients with chronic obstructive. Clinical Autonomic Research 1993;3:41. [DOI] [PubMed] [Google Scholar]
Nishimura 1999 {published data only}
- Nishimura K, Koyama H, Ishihara K, Hasegawa T, Katayama S, Nakashima A, Izumi T. Additive effect of oxitropium bromide in combination with inhaled corticosteroids in the treatment of elderly patients with chronic asthma. Allergology International 1999;48:85. [Google Scholar]
Nolte 1978 {published data only}
- Nolte D. Double‐blind crossover comparison between a beta‐adrenergic agent and a new anticholinergic agent by metered dose inhaler. Respiration 1978;36:32. [DOI] [PubMed] [Google Scholar]
Osterwalder 1991 {published data only}
- Osterwalder JJ. Randomized and controlled single case‐study ‐ A scientific approach to difficult therapeutic decisions A practical example for use of. Schweizerische Rundschau Fuer Medizin Praxis 1991;80:1168. [PubMed] [Google Scholar]
Pavia 1979 {published data only}
- Pavia D, Bateman JR, Sheahan NF, Clarke SW. Effect of ipratropium bromide on mucociliary clearance and pulmonary function in reversible airways obstruction. Thorax 1979;34:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peel 1984 {published data only}
- Peel ET, Anderson G, Cheong B, Broderick N. A comparison of oxitropium bromide and ipratropium bromide in asthma. European Journal of Respiratory Diseases 1984;65:106. [PubMed] [Google Scholar]
Peerless 1983 {published data only}
- Peerless AG, Rachelefsky GS, Mickey MR, Katz RM, Siegel SC. Bronchodilator characteristics of nebulized metaproterenol sulfate, isoetharine, and atropine in chronic asthma. Journal of Allergy and Clinical Immunology 1983;72:702. [DOI] [PubMed] [Google Scholar]
Petrie 1975a {published data only}
- Petrie GR, Palmer KN. Comparison of aerosol ipratropium bromide and salbutamol in chronic bronchitis and asthma. BMJ 1975;1:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrie 1975b {published data only}
- Petrie GR, Palmer K. Comparative trial of Sch1000 MDI and salbutamol MDI in chronic bronchitis and bronchial asthma. Postgraduate Medical Journal 1975;51(Suppl 7):117. [Google Scholar]
Petro 1981 {published data only}
- Petro W. Bronchodilator effect of a combination of a beta‐adrenergic agonist and a cholinergic antagonist [Broncholyse unter Kombinierter anwendun]. Praxis und Klinik der Pneumologie 1981;35(1 Spec):779. [Google Scholar]
Philip‐Joet 1986 {published data only}
- Philip‐Joet F Herkert A Vervloet D Arnaud A. [Comparative study of anticholinergic and betamimetic agents in the treatment of asthma attacks]. Revue De Pneumologie Clinique 1986;42:292. [PubMed] [Google Scholar]
Pierce 1979 {published data only}
- Pierce RJ, Allen CJ, Campbell AH. A comparative study of atropine methonitrate, salbutamol, and their combination in airways obstruction. Thorax 1979;34:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pounsford 1983 {published data only}
- Pounsford JC, Fuller RW, Saunders KB. A cumulative dose response study of oxitropium bromide (Ba 253) in asthmatics. European Journal of Respiratory Diseases‐Supplement 1983;128(Pt 2):536‐9. [PubMed] [Google Scholar]
Pounsford 2001 {published data only}
- Pounsford JC. What are the relative places of ipratropium, salmeterol and montelukast in maintenance therapy asthma in the elderly? [ongoing trial]. National Research Register (UK) 2001. [N0234093764]
Rafferty 1987 {published data only}
- Rafferty P, Howarth P, Mann JS, Holgate S. Bronchoconstriction induced by nebulized ipratropium bromide: relation to the bromide ion. Postgraduate Medical Journal 1987;63(Suppl 1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rebuck 1979 {published data only}
- Rebuck AS, Marcus HI. SCH 1000 in psychogenic asthma. Scandinavian Journal of Respiratory Diseases 1979;103(Suppl):186. [PubMed] [Google Scholar]
Riding 1970 {published data only}
- Riding WD, Dinda P, Chatterjee SS. The bronchodilator and cardiac effects of five pressure‐packed aerosols in asthma. British Journal of Diseases of the Chest 1970;64:37. [DOI] [PubMed] [Google Scholar]
Ruffin 1977 {published data only}
- Ruffin RE, Fitzgerald JD, Rebuck AS. A comparison of the bronchodilator activity of Sch 1000 and salbutamol. Journal of Allergy and Clinical Immunology 1977;59:136. [DOI] [PubMed] [Google Scholar]
Ruffin 1978 {published data only}
- Ruffin RE, Newhouse MT. Ipratropium bromide (Sch 1000) monohydrate aerosol: bronchodilator effect of three dose levels in asthmatics. Lung 1978;155:141. [DOI] [PubMed] [Google Scholar]
Ruffin 1982 {published data only}
- Ruffin RE, McIntyre E, Crockett AJ, Zielonka K, Alpers JH. Combination bronchodilator therapy in asthma. Journal of Allergy and Clinical Immunology 1982;69:60. [DOI] [PubMed] [Google Scholar]
Ruffin 1987b {published data only}
- Ruffin RE, Meki M, Alpers JH. Combined bronchodilator protection against histamine‐induced bronchoconstriction. Postgraduate Medical Journal 1987;63(Suppl 1):10. [DOI] [PubMed] [Google Scholar]
Rutkowski 1994 {published data only}
- Rutkowski R, Kazberuk M, Siergiejko Z, Chyrek‐Borowska S. Effect of fenoterol, ipratropium bromide and combination drug‐berodual‐on selected clinical parameters and lung function. Pneumonologia i Alergologia Polska 1994;62:358. [PubMed] [Google Scholar]
Rutten‐van‐Molken 19 {published data only}
- Rutten‐van‐Molken MP, van‐Doorslaer EK, Jansen MC, Kerstjens HA, Rutten FF. Costs and effects of inhaled corticosteroids and bronchodilators in asthma and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1995;151:975. [DOI] [PubMed] [Google Scholar]
Sahay 1980 {published data only}
- Sahay JN, Bell R, Barber PV, Chatterjee SS, Cox GA. Comparison of ipratropium bromide and salbutamol therapy by inhalation in asthma. British Journal of Diseases of the Chest 1980;75:323. [Google Scholar]
Sahlstrom 1986 {published data only}
- Sahlstrom K, Alanko K, Harkonen R. Treatment of asthma bronchiale with a combination of ipratropium bromide and fenoterol. Respiration 1986;50(Suppl 2):302. [DOI] [PubMed] [Google Scholar]
Salome 1988 {published data only}
- Salome CM, Wright W, Sedgwick CJ, Woolcock AJ. Acute effects of fenoterol (Berotec) and ipratropium bromide (Atrovent) alone and in combination on bronchial hyperresponsiveness in asthmatic. Progress in Clinical & Biological Research 1988;263:405. [PubMed] [Google Scholar]
Schindl 1973 {published data only}
- Schindl‐R. [Atropine derivatives as dose aerosol in inhalation therapy of bronchial asthma]. Medizinische Welt 1973;24:603. [PubMed] [Google Scholar]
Schlueter 1978 {published data only}
- Schlueter DP, Neumann JL. Double blind comparison of acute bronchial and ventilation‐perfusion changes to atrovent and isoproterenol. Chest 1978;73(Suppl 6):982. [DOI] [PubMed] [Google Scholar]
Schroeckenstein 1988 {published data only}
- Schroeckenstein DC, Bush RK, Chervinsky P, Busse WW. Twelve‐hour bronchodilation in asthma with a single aerosol dose of the anticholinergic compound glycopyrrolate. Journal of Allergy and Clinical Immunology 1988;82:115. [DOI] [PubMed] [Google Scholar]
Schultze‐Werninghaus {published data only}
- Schultze‐Werninghaus G. Anticholinergic versus beta 2‐adrenergic therapy in allergic airways obstruction: double‐blind trials on bronchodilator effect and antiallergic. Respiration 1981;41:239. [DOI] [PubMed] [Google Scholar]
Shneerson 1986 {published data only}
- Shneerson JM. Comparison of the bronchodilation of ipratropium bromide and fenoterol nebuliser solutions administered alone and in combination. Respiration 1986;50(Suppl 2):274. [DOI] [PubMed] [Google Scholar]
Simonsson 1979 {published data only}
- Simonsson BG. Acute effects of ipratropium bromide (ITBR, Sch 1000, Atrovent): a review of previous studies. Scandinavian Journal of Respiratory Diseases 1979;103(Suppl):130. [PubMed] [Google Scholar]
Smith 1988 {published data only}
- Smith CM, Anderson SD, Seale JP. The duration of action of the combination of fenoterol hydrobromide and ipratropium bromide in protecting against asthma provoked by hyperpnea. Chest 1988;94:709. [DOI] [PubMed] [Google Scholar]
Snow 1979 {published data only}
- Snow RM, Miller WC, Blair HT, Rice DL. Inhaled atropine in asthma. Annals of Allergy 1979;42:286. [PubMed] [Google Scholar]
Spector 1975 {published data only}
- Spector S, Ball RE. Bronchodilating effects of aerosolized Sch 1000 and atropine sulfate in asthmatics. Chest 1975;68:426. [Google Scholar]
Speirs 1987 {published data only}
- Speirs D, Hill L, Kirkbride P. The dose response study to compare Atrovent metered dose inhalers with and without an extension tube. Postgraduate Medical Journal 1987;63(Suppl 1):6. [Google Scholar]
Stewart 1987 {published data only}
- Stewart DE, Gillies AJ. A dose response study comparing Duovent vs salbutamol. New Zealand Medical Journal 1987;100:742. [PubMed] [Google Scholar]
Storms 1975a {published data only}
- Storms WW, DoPico G, Reed C. Aerosol Sch 1000. The American Review of Respiratory Disease 1975;111:419. [DOI] [PubMed] [Google Scholar]
Storms 1975b {published data only}
- Storms WW, Reed CE. Dose‐titration study of Sch 1000 MDI in comparison with isoprenaline. Postgraduate Medical Journal 1975;51(Suppl 7):99. [Google Scholar]
Stresemann 1975 {published data only}
- Stresemann E. Time‐response curves of Sch 1000 and isoprenaline as metered dose inhalers (MDI) as assessed by measurements of total airways resistance (Rt). Postgraduate Medical Journal 1975;51(Suppl 7):119. [Google Scholar]
Stresemann 1976a {published data only}
- Stresemann E. Time response determinations from analysis of pulmonary function following ipratropiumbromide and isoprenaline. Arzneimittel Forschung 1976;26(5A):1015. [Google Scholar]
Stresemann1976b {published data only}
- Stresemann‐E. Short Communication: Rotation‐viscosimetric determinations of the effect of ipratropiumbromide on bronchial excretion/a double blind study. Arzneimittel Forschung 1976;26(5A):1013. [PubMed] [Google Scholar]
Sur 1990 {published data only}
- Sur S, Mohiuddin AA, Vichyanond P, Nelson HS. A random double‐blind trial of the combination of nebulized atropine methylnitrate and albuterol in nocturnal asthma. Annals of Allergy 1990;65:384. [PubMed] [Google Scholar]
Tang 1984 {published data only}
- Tang OT, Flatley M. A comparison of effects of inhaling a combined preparation of fenoterol with ipratropium bromide (Duovent) with those of fenoterol and. Postgraduate Medical Journal 1984;60(Suppl 1):24. [PubMed] [Google Scholar]
Tarlo 1982 {published data only}
- Tarlo SM, Broder I, Corey P. Six‐month double‐blind cross‐over trial comparing an inhaled anticholinergic agent and placebo in the treatment of asthma. Current Therapeutic Research, Clinical and Experimental. 1982;32:265. [Google Scholar]
Taube 2002 {published data only}
- Taube C, Kanniess F, Gronke L, Richter K, Mucke M, Paasch K, et al. Reproducibility of forced inspiratory and expiratory volumes after bronchodilation in patients with COPD or asthma. Respiratory Medicine 2002;97:568. [DOI] [PubMed] [Google Scholar]
Thiessen 1979 {published data only}
- Thiessen B, Pedersen OF. A double blind cross‐over study of maximal expiratory flows and arterial blood gas tensions in normals, asthmatics and bronchitics after. Scandinavian Journal of Respiratory Diseases 1979;103(Suppl):170. [PubMed] [Google Scholar]
Trinquet 1975 {published data only}
- Trinquet G, Duchier J, Jacquot C. The bronchodilating effect of Sch 1000 MDI and inhalations of atropine sulphate in patients suffering from bronchial asthma or from chronic. Postgraduate Medical Journal 1975;51(Suppl):119. [Google Scholar]
Tukiainen 1985 {published data only}
- Tukiainen P, Salorinne Y. Oxitropium, salbutamol and their combination in asthma. European Journal of Respiratory Diseases 1985;67:31. [PubMed] [Google Scholar]
Uffholtz 1975 {published data only}
- Uffholtz H. The effects of Sch 1000 MDI on specific airways resistance in comparison with salbutamol in patients with asthma and chronic bronchitis. Postgraduate Medical Journal 1975;51(Suppl 7):120. [Google Scholar]
Ullah 1980 {published data only}
- Ullah M, Saunders KB. Influence of age on response to ipratropium bromide and salbutamol in asthmatic patients. Progress in Respiratory Research 1980;14:150. [Google Scholar]
Ullah 1981 {published data only}
- Ullah MI, Newman GB, Saunders KB. Influence of age on response to ipratropium and salbutamol in asthma. Thorax 1981;36:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ulmer 1979 {published data only}
- Ulmer WT. Treatment of obstructive disease with fenoterol‐ipratropiumbromid dosage aerosol (IK 6). Short and long‐terms results [Die Behandlung obstruktiver Atemwegserkrankungen mit fenoterol‐ipratropiumbromidgemisch‐Dosieraerosol (IK6)]. Medizinische Klinik 1979;74(42):1548‐52. [PubMed] [Google Scholar]
Vakil 1985 {published data only}
- Vakil DV, Ayiomamitis A, Nizami RM. Use of ipratropium aerosol in the long‐term management of asthma. Journal of Asthma 1985;22:165. [DOI] [PubMed] [Google Scholar]
Verstraeten 1974a {published data only}
- Verstraeten JM. [Can the association of an atropinic (SCH 1000) and a sympathicomimetic improve the bronchodilatation obtained by a sympathicomimetic alone]. Acta tuberculosea et pneumologica Belgica 1974;65:395. [PubMed] [Google Scholar]
Verstraeten 1974b {published data only}
- Verstraeten JM. [Comparative study of the bronchodilating effect of SCH 1000, berotec and of the association of the two drugs]. Acta tuberculosea et pneumologica Belgica 1974;65:534. [PubMed] [Google Scholar]
Verstraeten 1975 {published data only}
- Verstraeten JM. Bronchial response of asthmatic and bronchitic patients to fenoterol MI with or without subsequent Sch 1000 MDI. Postgraduate Medical Journal 1975;51(Suppl 7):120. [Google Scholar]
Villate 1980 {published data only}
- Villate Navarro JI, Sobradillo Pena V, Atxotequi Iaraoligoitia V, Salaverri Nalda A, Orive Martinez C. [Comparative effects of terbutaline sulphate and ipratropium bromide on the respiratory system]. Medecine Clinique et Experimentale 1980;74:275. [PubMed] [Google Scholar]
Vlagopoulos 1975 {published data only}
- Vlagopoulos T, Townley R, Ghazanshahi S, Bewtra A, Burke K. Comparison of the onset of action and bronchodilation effects of the anticholinergic agent SCH 1000 with isoproterenol. Journal of Allergy and Clinical Immunology 1975;55:99. [Google Scholar]
Vlagopoulos 1976 {published data only}
- Vlagopoulos T, Townley R, Ghazanshahi S, Bewtra A, Burke K. Comparison of the bronchodilating effects of Sch 1000 with isoproterenol in patients with bronchial asthma. Annals of Allergy 1976;36:223. [PubMed] [Google Scholar]
Vogt 1974 {published data only}
- Vogt R, Rychel F. Acute test comparing ipratropium bromide (Sch 1000), fenoterol and placebo, by measured dose aerosol, in obstructive lung disease. Therapiewoche 1974;24:4228. [Google Scholar]
Walker 1987 {published data only}
- Walker FB, Kaiser DL, Kowal MB, Suratt PM. Prolonged effect of inhaled glycopyrrolate in asthma. Chest 1987;91:49. [DOI] [PubMed] [Google Scholar]
Ward 1987 {published data only}
- Ward MJ, MacFarlane JT, Davies D. Anticholinergics in severe asthma. Postgraduate Medical Journal 1987;63(Suppl 1):2. [Google Scholar]
Watson 1992 {published data only}
- Watson A, Lim TK, Joyce H, Pride NB. Failure of inhaled corticosteroids to modify bronchoconstrictor or bronchodilator responsiveness in middle‐aged smokers with mild airflow. Chest 1992;101:350. [DOI] [PubMed] [Google Scholar]
Wegener 1987a {published data only}
- Wegener T, Hedenstrom H. Effect of nebulized ipratropium bromide on lung function in nonallergic bronchial asthma. Respiration 1987;52:1. [DOI] [PubMed] [Google Scholar]
Wegener 1987b {published data only}
- Wegener T, Hedenstrom R. Effect of nebulized ipratropium bromide on lung function in non‐allergic bronchial asthma. Postgraduate Medical Journal 1987;63(Suppl 1):6. [DOI] [PubMed] [Google Scholar]
Wempe 1992 {published data only}
- Wempe JB, Postma DS, Breederveld N, Alting‐Hebing D, Mark TW, Koeter GH. Separate and combined effects of corticosteroids and bronchodilators on airflow obstruction and airway hyperresponsiveness in asthma. Journal of Allergy and Clinical Immunology 1992;89:679. [DOI] [PubMed] [Google Scholar]
Wesolowski 1985 {published data only}
- Wesolowski S. [The role of anticholinergic drugs in the treatment of bronchospastic conditions]. Pneumonologia Polska 1985;53:49. [PubMed] [Google Scholar]
Wildbolz 1977 {published data only}
- Wildbolz U, Kyd K, Scherrer M. Ipratropium in addition to theophylline‐sustained betastimulation. Lung 1977;154:141. [Google Scholar]
Wilson 1978 {published data only}
- Wilson RH, Battaglia PJ, Wilson NL. Crossover study with nebulized bronchodilators and atropine. Chest 1978;73(Suppl 6):998. [DOI] [PubMed] [Google Scholar]
Windom 1990 {published data only}
- Windom HH, Burgess CD, Siebers RW, Purdie G, Pearce N, Crane J, Beasley R. The pulmonary and extrapulmonary effects of inhaled beta‐agonists in patients with asthma. Clinical Pharmacology and Therapeutics 1990;48:296. [DOI] [PubMed] [Google Scholar]
Yanai 1991 {published data only}
- Yanai M, Ohrui T, Sekizawa K, Shimizu Y, Sasaki H, Takishima T. Effective site of bronchodilation by antiasthma drugs in subjects with asthma. Journal of Allergy and Clinical Immunology 1991;87:1080. [DOI] [PubMed] [Google Scholar]
Yang 1989 {published data only}
- Yang SC, Wu MC. The response pattern of patients with chronic airway obstruction to bronchodilators. Journal of the Formosan Medical Association of Taiwan 1989;88:700. [PubMed] [Google Scholar]
Yeager 1976 {published data only}
- Yeager H, Weinberg RM, Kaufman LV, Katz S. Asthma: comparative bronchodilator effects of ipratropium bromide and isoproterenol. Journal of Clinical Pharmacology 1976;16:198. [DOI] [PubMed] [Google Scholar]
Zanen 1995 {published data only}
- Zanen P, Go LT, Lammers JW. The optimal particle size for parasympathicolytic aerosols in mild asthmatics. International Journal of Clinical Pharmacology and Therapeutics 1995;114:111. [Google Scholar]
Zaritsky 1999 {published data only}
- Zaritsky A, Qureshi F. Ipratropium does indeed reduce admissions to hospital with severe asthma. BMJ 1999;318:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zuck 1987 {published data only}
- Zuck P, Peiffert G, Jirou‐Najou JL. Study of fenoterol and ipratropium bromide additive action in asthmatic patients. Postgraduate Medical Journal 1987;63(Suppl 1):14. [Google Scholar]
Additional references
Barnes 1996
- Barnes PJ. Pathophysiology of asthma. British Journal of Clinical Pharmacology 1996;42:3‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 1997
- Barnes PJ. Current therapies for asthma. Chest 1997;111(Suppl.):17S‐26S. [DOI] [PubMed] [Google Scholar]
Beakes 1997
- Beakes DE. The use of anticholinergics in asthma. Journal of Asthma 1997;34:357‐68. [DOI] [PubMed] [Google Scholar]
Boulet 1999
- Boulet L‐P, Becker A, Berube D, Beveridge R, Ernst P. Summary of recommendations from the Canadian Asthma Consensus Report 1999. Canadian Medical Association Journal 1999;161(11 Suppl):S1‐S12. [PMC free article] [PubMed] [Google Scholar]
Bousquet 2000
- Bousquet J. Global initiative for asthma (GINA) and its objectives. Clinical & Experimental Allergy 2000;30(Suppl.1):2‐5. [DOI] [PubMed] [Google Scholar]
BTS 1993
- British Thoracic Society. Guidelines on the management of asthma. Thorax 1993;48(Suppl.):s1‐s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS/SIGN 2004
- Scottish Intercollegiate Guidelines Network. Clinical Guidelines. http://www.sign.ac.uk (accessed February 2004).
Cazzola 1998
- Cazzola M, Centanni S, Donner CF. Anticholinergic agents. Pulmonary Pharmacology & Therapeutics 1998;11:381‐92. [DOI] [PubMed] [Google Scholar]
Clarke 2003
- Clarke M, Oxman AD (editors). Cochrane Reviewers' Handbook 4.2.0 [updated March 2003]. Cochrane Collaboration, 2003. [Google Scholar]
Connolly 1993
- Connolly MJ. Ageing, late‐onset asthma and the beta‐adrenoceptor. Pharmacology & Therapeutics 1993;60:389‐404. [DOI] [PubMed] [Google Scholar]
Creer 1999
- Creer TL, Winder JA, Tinkelman D. Guidelines for the diagnosis and management of asthma: accepting the challenge. Journal of Asthma 1999;36:391‐407. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Emilien 1998
- Emilien G, Maloteaux J‐M. Current therapeutic uses and potential of beta‐adrenoceptor antagonists. European Journal of Clinical Pharmacology 1998;53:389‐404. [DOI] [PubMed] [Google Scholar]
Gross 1988
- Gross NJ. Ipratropium bromide. New England Journal of Medicine 1988;319:486‐94. [DOI] [PubMed] [Google Scholar]
Gross 1997
- Gross NJ. Anticholinergic drugs. In: Barnes PJ, Grunstein MM editor(s). Asthma. Philadelphia: Lippincott‐Raven, 1997:1555‐68. [Google Scholar]
Harrison 1998
- Harrison B. Towards evidence‐based guidelines for asthma: should we? can we? how will it affect our practice?. Clinical Asthma Reviews 1998;2:139‐46. [Google Scholar]
Hougardy 2000
- Hougardy DM, Peterson GM, Bleasel MD, Randall CT. Is enough attention being given to the adverse effects of corticosteroid therapy. Journal of Clinical Pharmacology & Therapeutics 2000;25:227‐34. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jolobe 1984
- Jolobe OM. Asthma vs. non‐specific reversible airflow obstruction: clinical features and responsiveness to anticholinergic drugs. Respiration 1984;45:237‐42. [DOI] [PubMed] [Google Scholar]
Kemp 2000
- Kemp JP. Guidelines Update. Drugs 2000;59(Suppl.1):23‐28. [DOI] [PubMed] [Google Scholar]
Meier 1997
- Meier CR, Hershel J. Drug use and pulmonary death rates in increasingly symptomatic asthma patients in the UK. Thorax 1997;52:612‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ni Chroinin 2005
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, Ducharme FM. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005535] [DOI] [PubMed] [Google Scholar]
O'Connor 1998
- O'Connor BJ. Combination therapy. Pulmonary Pharmacology & Therapeutics 1998;11:397‐9. [DOI] [PubMed] [Google Scholar]
Partridge 1981
- Partridge MR, Saunders KB. Site of action of ipratropium bromide and clinical and physiological determinants of response in patients with asthma. Thorax 1981;36:530‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spitzer 1992
- Spitzer WO, Suissa S, Ernst P, Horwitz R, Habbick B, Cockcroft D, et al. The use of beta‐agonists and the risk of death and near death from asthma. New England Journal of Medicine 1992;326:501‐6. [DOI] [PubMed] [Google Scholar]
Taylor 1999
- Taylor DM, Auble TE, Calhoun WJ, Mosesso VN. Current outpatient management of asthma shows poor compliance with international consensus guidelines. Chest 1999;116:1638‐45. [DOI] [PubMed] [Google Scholar]


