Abstract
Readily-available small-diameter arterial grafts require a great combination of materials properties, including high strength, compliance, suturability, blood sealing and anti-thrombogenicity, as well as anti-kinking property for those used in challenging anatomical situations. We have constructed grafts composed of coaxially-structured polycaprolactone (PCL)/gelatin nanofibres, and tailored the material structures to achieve high strength, compliance and kink resistance, as well as excellent water sealing and anti-thrombogenicity. Coaxially-structured fibres in the grafts provided mechanical stability through the core, while flexibility and cell adhesion through the sheath. Results showed that graft compliance increased while strength decreased with the concentration ratio between core and sheath polymers. Compared to pure PCL fibrous surfaces, coaxial PCL/gelatin fibrous surfaces potently inhibited platelet adhesion and activation, providing excellent anti-thrombogenicity. To render sufficient burst strength and suturability, an additional layer of pure PCL was necessary to cap the layer of coaxial PCL/gelatin fibres. The two-layered grafts with the wall thickness comparable to native arteries demonstrated artery-like compliance and kink resistance, properties important to arteries under complex mechanical loading. The in vivo evaluation was performed using the interposition carotid artery graft model in rabbits for three months. Interestingly, results from ultrasonic imaging and histological analysis demonstrated that the two-layered grafts with a thinner outer PCL layer, which possessed higher compliance and kink resistance, showed increased blood flow, minimal lumen reduction and fibrosis. All vascular grafts exhibited patency and induced limited cell infiltration. Together, we presented a facile and useful approach to fabricate vascular grafts with superior graft performances, biomechanical properties, and blood compatibility. Grafts with artery-like compliance and flexibility have demonstrated improved implantation outcomes.
Keywords: Coaxial electrospinning, gelatin, nanofibres, vascular tissue, engineered scaffolds
1. Introduction
Vascular diseases are widely characterized by narrowing or occlusion of arteries and veins, constituting a major health and socio-economic burden in most countries [1]. Bypass surgery or vascular reconstruction is the only course of correction in some cases [2]. Autologous vascular grafts are primarily used for the rectification, but the availability of usable vessels imperates the need for a synthetic vascular graft for long-term treatment. Synthetic vascular grafts of Dacron and polytetrafluoroethylene have been demonstrated to achieve a wide range of success with large diameter vascular replacements. However, the utility of the same materials for vascular replacement of diameters < 6mm proves to be a large-scale failure due to its low patency and intermediate thrombosis [3]. An ideal synthetic conduit for vascular implants should be mechanical stabile to withstand physiological hemodynamic pressures and highly flexible to sustain the opening under complex loading environments, which mimic the properties of extracellular matrix (ECM) [4]. In the past two decades, impressive progress in the area of vascular tissue engineering has demonstrated the possibility of using a cell-seeded living biograft as a blood vessel substitute [5]. However, the in vitro process of creating engineered vascular tissues often depends on culture environments that stimulate cells to synthesize matrix components which enhance graft strength and flexibility. Also, the clinical potential of these approaches could be tempered by time-consuming, labor-intensive preparations, and rigorous culture regulation. In clinical settings, ideal vascular replacements need scaffold technologies to not only provide suitable cellular environments, but also consider the reproducibility and availability, ideally “off-the-shelf’, to vascular surgeons [6]. These require that grafts possess physical and mechanical properties for immediate grafting and biodegradable environments for tissue remodeling in vivo. Additionally, the surface of vascular implants is critical to determine their interactions with platelets and cells for short-term and long-term implant performances [7–10].
A long-standing challenge in the development of a readily-implantable, synthetic vascular graft is to achieve a balance between required mechanical strength that withstands physiological stresses and artery-like compliance that avoids adverse hemodynamic environments for cells [11]. On one hand, robust physical and mechanical properties, including burst strength, water sealing and suture strength, are required for grafting. On the other hand, artery-like compliance is critical, because the compliance mismatch between a vascular graft and neighboring arteries at the site of anastomosis is a major cause for graft failure. At the junction of mismatched compliance, disturbed blood flow led to adverse cell responses and/or pro-inflammatory milieu [12, 13]. Compliance mismatch generally results from the stiffness of graft materials, and/or the lack of optimal pore sizes. Pore sizes, however, also alter water permeability, cell penetration and tissue remodeling [14, 15].
Considering the availability to surgery, the requirement for physical and mechanical properties, and the potential for vascular remodeling, we aim to design a readily-available, strong and compliant graft, consisting of biodegradable materials that are hemocompatible and allow cell adhesion and tissue remodeling. Electrospinning is one of the few techniques with great versatility for modulating the architecture and mechanical properties of resultant structures to mimic the ECM proteins [11, 15, 16]. Biocompatible and biodegradable hydrophobic polymers polycaprolactone (PCL) and polyurethane (PU) have been used in myriad tissue engineering applications including vascular regeneration [6, 11, 15]. Despite being mechanically strong and stable, these polymers lack the innate reactive sites for cell adhesion. The hydrophobic nature of these polymers also tends to attract platelet and plasma protein adhesion, leading to the platelet aggregation and intimal hyperplasia of the artificial blood vessels. Additionally, these polymer materials are very stiff. Collagen is one of the most predominant ECM proteins found in the vasculature. However, electrospinning with the use of strong organic solvent and high voltage always causes the denaturation of collagen into gelatin [17]. Gelatin possesses competent biodegradable properties along with excellent biocompatibility, anti-thrombogenicity and non-antigenicity comparable to collagen. Nascent physical blending of polymers during electrospinning led to a compromise in mechanical or biocompatible properties when compared to the individual polymers [18]. Coaxial electrospinning, on the other hand, can be more pronounced in retaining both properties [19]. In addition, the interaction between the core and the sheath was found to be a determining factor of mechanical properties rather than the individual properties of the core polymer themselves [6].
Our previous work has developed three types of coaxially-structured materials composed of PU/gelatin, PCL/gelatin or polylactic acid/gelatin fibres composed of a hydrophobic core and a gelatin sheath with a fixed ratio concentration of 1:5 [6]. The addition of gelatin around the hydrophobic polymer nanofibre led to the formation of hydrogel-like materials, showing tissue-like viscoelasticity, compliance, and swelling capability. In particular, among the three systems, the PCL/gelatin and PU/gelatin fibres exhibiting relatively thicker gelatin-sheathed fibres demonstrated increased elasticity and water swelling. Utilizing these new hybrid, biodegradable fibrous biomaterials with interactive core-sheath structure, we aim to evaluate their use for vascular grafts. The present work aims at developing implantable vascular grafts from coaxially electrospun materials by modulating the core and sheath polymer concentrations, forming multilayer structure, and then evaluating their potential as a small diameter vascular scaffold in a rabbit model. The rabbit model has been widely used for the study of atherosclerosis, because this species shares several aspects of lipoprotein metabolism with human beings, and has reasonable handling size and relatively low cost [20,21].
2. Materials and Methods
2.1. Materials
Unless specified otherwise, all polymers and chemicals, including poly-ε-caprolactone or PCL (Mn = 80,000), polyurethane or PU, gelatin, 2,2,2 trifluroethanol and 1,1,1,3,3,3 hexafluoro-2-propanaol (HFP), were purchased from Sigma Aldrich Inc. (St Louis, MO).
2.2. Electrospinning of polymer fibres
The apparatus used for obtaining coaxial fibres was developed in house. The core hydrophobic polymer solution was passed through the inner needle of 22 G (0.71 mm in internal diameter) and the sheath gelatin solution was passed through the outer needle of 16 G (1.65 mm in internal diameter). A dual syringe holder was used to place the syringes loaded with polymer solutions. This design allows the solutions to be extruded simultaneously. Because our result with 1:5 (weight ratio) PCL/gelatin fibres showed the material was very compliant but not strong enough as grafts, the PCL content was then increased in the PCL/gelatin coaxial fibres. Polymer solutions with 1%, 2%, or 3.5% (w/v) concentration of PCL, and 5% (w/v) of gelatin were prepared by dissolving the predetermined amount of PCL or gelatin in HFP. For comparisons, PU/gelatin materials were also prepared. As PU/gelatin of 1:5 ratio showed low elasticity but high strength, the gelatin was increased in the PU/gelatin coaxial fibres. Polymer solutions with 2% of PU, 5%, 8% or 10% (w/v) of gelatin were prepared in HFP. Blending of synthetic polymers like PCL and PU with natural polymers such as collagen, gelatin and chitosan requires a miscible solvent system with good electrical conductivity, such as HFP which possesses strong acidity, which assists electrospinning and reduces surface tension in polymer solutions enabling the use of lower concentration of polymers for electrospinning. The solutions obtained after stirring for 6 to 8 hours were loaded in 5 mL syringes connected to the positive terminal of a high voltage ES30P 10 W power supply (Gamma High Voltage Research, Ormond Beach, FL). The polymer solutions were extruded at 1 mL/h using syringe pumps (Pump 11 Plus, Harvard Apparatus, Boston, MA) and was subjected to an electric potential of 1kV/cm. The fibres were deposited onto a grounded static aluminum substrate or cylindrical aluminum rod of 2mm in diameter rotating at 150rpm and placed at a distance of 10 cm perpendicular to the needle. The aluminum rod was coupled to a BMU230AP-A-3 brushless DC motor (Oriental Motor Corp, USA).
The three types of grafts for the study of vascular graft performances, labeled as “fibre gel”, “thin cap” and “thick cap”, were prepared using the same PCL/gelatin (1:5 ratio) coaxial fibres as inner layer while changing the thickness of an outer layer. The “fibre gel” grafts have no outer layer, while both “thin cap” and “thick cap” have an extra coating “cap” layer of 6% (w/v) PCL spun at different time intervals. The solution of 2,2,2 trifluroethanol was used as the solvent to electrospin the capping polymers. The other electrospinning parameters remained the same as those for the coaxial electrospinning. The obtained samples were stored at the room temperature until further use.
Genipin (Wako Chemicals, Japan) was used to crosslink electrospun fibres. The scaffolds composed of coaxially electrospun fibres were subjected to crosslinking for 48 hr by immersion in solution containing 0.25 % (w/v) genipin dissolved in 100% ethanol. [22] Immediately after the crosslinking process, fibrous scaffolds were rinsed with phosphate buffered saline (pH 7.4) and used as such or air dried overnight.
2.3. Material structure characterization with transmission electron spectroscopy
The nanostructure of the coaxial fibres was observed using H7650 transmission electron microscope (TEM; Hitachi Ltd, Tokyo, Japan) operated at 80 kV. The electrospun fibre samples for TEM observation were prepared by directly depositing the as-spun fibres on carbon-coated TEM grids.
2.4. Mass swelling ratio
To study swelling behavior of hydrogels, hydrogels were incubated in DI water at room temperature for 24 h to remove un-cross-linked species. Hydrogels were removed from the DI water, blotted the hydrogel surfaces with Kimwipe tissue, and weighted to obtained swollen weights (Wswollen). Hydrogels were then dried and weighted to obtain dried polymer weights (Wdry). Hydrogel mass swelling ratios (q) were defined as: q = Wswollen / Wdry
2.5. Vascular graft functional characterizations - Physical and mechanical characterizations
The water permeability, burst strength, compliance, and suture retention strength of vascular grafts were determined according to the methods prescribed in the industrial standard—ANSI/AAMI/ISO 7198 (Cardiovascular Implants: Tubular Vascular Prostheses). A custom-made apparatus designed for the determination of water permeability, burst pressure and compliance was used as previously described [27].
2.5.1. Graft permeability
Tube adapters were inserted onto both ends of the multilayer graft. Paraffin films were used to secure the graft on the adapters. Then, the graft was cannulated by connecting one end of the graft to a water reservoir with a flow valve and the other end was connected to the other end of the water reservoir. Water pressurized at 16 kPa (120 mmHg), was imposed using a pulsatile flow pump. Once the flow became steady, the permeated fluid through the graft due to the pressure was collected in a beaker and the water volume collected within 1 min was measured. The graft permeability (units: mL/cm2/min) was determined using the equation:
where S is the graft permeability, Q is the fluid volume passing through the graft, and A is the cross-sectional area of the aperture in the sample holder.
2.5.2. Burst strength
Tube adapters were inserted on both ends of the vascular grafts. Paraffin films were used to secure the graft onto the latex tube near the adapters. The graft was then loaded onto a custom-made fixture and connected to a custom flow system via pressure sensors on both ends of the graft. Pressure sensors were obtained from Omega Engineering (PX209-015G15V, Omega Engineering Inc., Norwalk, CT) and fitted with nylon (polyamine) tee-joints. Pressure sensors were placed close to the graft terminals to reduce head loss from connector tubing. Data could be sampled with the frequency up to 225 Hz and recorded on a real-time target board (sb-RIO 9392, National Instruments, Austin, TX). Water was pressurized through the graft at an increment of 10mmHg. The increasing pressure led to an expansion of the graft diameter and surface area. The expansion was allowed until graft failure occurred. The pressure at which this failure of the vascular graft occurred was recorded as the burst strength or burst pressure of the graft.
2.5.3. Graft compliance
The graft compliance was measured by similar method adopted for burst pressure. The graft was then loaded onto a custom-made fixture and connected to the flow system via pressure sensors on both ends of the graft, as described in 2.4.2. The pressure in the graft was controlled using varying speed of a water pump, to obtain a steady pressure from 10 mmHg to 200 mmHg, with an increment of 10 mmHg. At each pressure, images of the graft dilatation were taken using a camera (Canon EOS 450D, Japan). These images were analyzed using a customized script in MatlabVR (MathWorks, USA) to obtain the graft diameter at each pressure point. Then, the compliance of the graft (% per 100 mmHg) was determined with the following equation:
Where C is compliance (%), R0 is the original graft diameter, R1 is the changed graft diameter, Pinlet is the inlet pressure and Poutlet is the outlet pressure.
2.5.4. Graft suturability tests: Suture retention and multi-stitching strength
Suture retention was measured by cutting the 3D vascular graft into rectangular strips with dimensions of 10 mm × 5 mm. A suture was inserted 2 mm from the end of the stretched strip of the graft through the graft wall to form a half loop and the other end of the graft was attached to the lower clamp of the MTS electromechanical tensile testing system (MTS Systems Corp., Eden Prairie, MN). The other end of the suture was attached to a 2 kN load cell (MTS Systems Corp). The suture was pulled at the rate of 10 mm/min. The force required to pull the suture through the graft or to rupture the graft wall was recorded as suture retention.
Though suture retention test reflects the industrial standard, the suturability during the actual implantation practice, particularly for soft material grafts, may be better predicted by simulating the stitching and knotting techniques during implantation. After our initial failure of graft implantation attempts, we developed and utilized multi-stitching strength test, which involved a similar stitching technique as performed during the implantation. Briefly, the strength measurement was obtained from a 1 cm long sample of graft material stitched to a carotid artery explant with the same length. The graft was cut longitudinally and measured for the thickness. The sample was then trimmed to a uniform width of 1 cm. The artery sample was cut to the same width. Five stitches were used to bind the two samples together using implantation sutures (Monosof black MV-135-4 taper, Medtronic Inc, Minneapolis, MN). Each suture was placed roughly 1.5 mm apart to spread evenly across the suturing line. Once sutures were in place, a tensile test was performed on the combined samples by pulling apart perpendicular to the sutured surface using a MTS system until the two samples were separated. The tensile test was performed with a 5 N load cell with the initial speed of 0.2 in/min and data were acquired at 10.0 Hz.
2.5.5. Kink radius
Measurement of kink radius was performed by first administering the flow of water through a 3 cm section of graft conduit at a pressure of 100mmHg. The grafts were then bent around until a kink occurred, which was signaled by visually detectable reduction in the conduit outer diameter at the point of bending as well as the occlusion of fluid flow through the graft marked by a significant increase in fluid pressure upstream from the graft using a pressure transducer. At the point just prior to kink formation, images were obtained on the bent graft conduit. The radius of the inner loop formed by the conduit was measured using NIH ImageJ software, and the corresponding internal loop diameter was calculated from the perimeter, assuming a circular loop.
2.6. Platelet adhesion assay
To obtain platelet rich plasma (PRP), whole blood from adult rats was obtained using multiple 1 ml syringes containing 0.1ml of 10% sodium citrate solution in sterile PBS. Blood was transferred to blood tubes and centrifuged at 160 × g for 20 min at room temperature. Two fractions form after centrifugation, and the blood from 1.4 mm below the dividing line was transferred to new blood tube and centrifuged again at 400 × g for 15 minutes at room temperature. The bottom 0.35 ml volume was saved as PRP and the volume above is platelet poor plasma (PPP). To obtain the exact working concentration of 1 × 108 platelets per ml, PRP was mixed with PPP. Confirmation of platelets was performed by dilution of working concentration of PRP by 1 to 6 with PBS, which was then added to equal parts 10mM mepacrine solution in PBS and incubated at room temperature for 90 min. The solution was then centrifuged at 500 × g for 5 min. The pellet of platelets was then re-suspended in fresh PBS and imaged with a florescent microscope. For the platelet assay, 4mm square pieces of graft were cut and placed in 96-well culture plate. 150 μl of PRP with 1 × 108 platelets per ml were transferred to each sample well and allowed to incubate under gentle shaking at 37°C for 1 hour. Samples were then washed three times with 200 μl PBS in order to remove unattached platelets. The samples were then bathed in 2.5 wt % glutaraldehyde (200 μl per well) at 4°C for 2 hours and then rinsed three times with PBS. For fluorescent staining, samples were incubated in 10 mM mepacrine solution in PBS for 90 min at room temperature then washed 3 times with PBS. Quantitative measurement of adhered platelets to graft samples was obtained using a ZEISS LSM 710. For each sample, the number of platelets in eight randomly selected fields was counted for statistical analysis. Confocal images were obtained from a BioRad Radiance2100 Multi-Photon. Samples were then dehydrated in gradient alcohol and observed under scanning electron microscope (SEM; JEOL JSM 6480LV).
2.7. Cell viability assay
To evaluate the material effects on cell viability, we cultured rat pulmonary artery endothelial cells on the PCL/gelatin coaxial fibre material, PCL fibre material and tissue culture plate (control) on multi-well plates in the culture medium for 24 hrs. Four separate samples were used for each type of materials. The LIVE/DEAD viability/cytotoxicity assay kit (Invitrogen Corp, Carlsbad, CA) was used for the evaluation. The kit contains two components, calcein AM and ethidium homodimer (EthD-1). Calcein AM is well retained within live cells, producing an intense uniform green fluorescence in live cells (EX/EM ~495 nm/~515 nm), while EthD-1 enters cells with damaged membranes and produces a bright red fluorescence upon binding to nucleic acids in dead cells (EX/EM ~495 nm/~635 nm). S-Figure 1 shows the representative images from the viability assays on different materials. No significant difference in the cell viability percentage was found among the materials; all had an average above 95%.
2.8. Graft implantation for in vivo studies
Prior to implantation, the cleaning and sterilization of grafts were performed by immersing them in 100 % ethanol for 24 hrs, and then 70% ethanol for 0.5hr before transferring to 1x concentration of Pen/Strep (CellGro products from Corning Life Science, Coming, NY) in PBS for 24 hrs. Eight New Zealand white rabbits, four for each type of graft, were premedicated with subcutaneous injections of medetomidin (210 mcg/kg), midazolam (0.5 mg/kg), and morphine (1 mg/kg). The age of rabbits on the day of implantation was about 1 year old, and the weight of the rabbits was roughly 4.5 kg. An intravenous catheter was placed in a vein of the ear and propofol (5–8 mg/kg) was used to induce anesthesia. After intubation, anesthesia was maintained with Isoflurane in 100% oxygen. At the time of induction, rabbits receive enrofloxacin at a dose of 5 mg/kg. The right anterior neck was clipped with a #40 blade and prepared with chlorhexidine for surgery. After draping the head and neck with sterile surgical drape, an incision was performed. They were ligated with polypropylene sutures. Heparin was administered at the dose of 200 U/kg intravenously. After dissection of the right carotid artery, vascular clamps were applied to isolate the carotid artery. The carotid artery was transected between the clamps. The graft with a 1.5 mm diameter was anastomosed in an end-to-end fashion with two continuous 7-0 polypropylene sutures. After completion of the anastomosis, the clamps were removed and the anastomosis inspected for leakage and patency. The incision was then closed in a routine fashion. The animals received buprenorphine (0.05 mg/kg) and meloxicam (0.3mg/kg) after the surgery. Explantation of the grafts was performed at 13 weeks post implant. Plasma and serum samples were obtained. The rabbits were euthanized by overdose of pentobarbital. The animal experiments were approved and performed according to the Institutional Animal Care and Use Committee (IACUC) at the Colorado State University and complied with the NIH’s Guidelines for the Care and Use of Laboratory Animals.
2.8. Histology
Samples of the graft, upstream vessel, downstream vessel and carotid arteries were sectioned and stored in 10% formalin. Formalin fixed samples were embedded in paraffin blocks. Sections with 5 micron thickness were cut through the transverse plane and collected onto glass slides. Samples slides were stained using the protocol outlined from the Russell-Movat Pentachrome Staining kit (American MasterTech Inc, Lodi, CA), and imaged using a Zeiss Axioskop 40. Pentachrome staining was used because of its capability of all basic information for rough histological evaluation, including the matrix composition (collagen, elastin and ground substances) and cell density.
2.9. Ultrasonic imaging
After 12 weeks of implantation, ultrasound imaging and measurements were taken with Antares ultrasound system (Siemens Corp, Munich, Germany) to obtain flow patterns and flow rates through the vascular grafts. The linear array ultrasound transducer (Siemens vfx9-4), which is often used for peripheral vascular applications, was used to probe graft morphology and flow in the rabbits. Prior to graft explantation, animals were anesthetized and shaved around the area of implant with ultrasound gel applied. Graft sections were located using color doppler methods and transverse sectional images of flow were taken.
2.10. Data and statistics analysis
Unless otherwise specified, all the data presented were the mean values quantified from at least three independent experiments. Error bars on all the graphs represented the standard deviation of the mean based on the total number of the samples. Prizm software was used for performing statistics and plotting graph. Data were statistically analyzed using the one-way analysis of variance test. Student’s t test was then used to compare the means of each individual group. The level of statistical significance was set at a α = 0.05 for 95% confidence. For each experiment for microscopy images, at least four sampling regions were analyzed.
3. Results
3.1. Transmission electron microscopy imaging of fibre nanostructure: the effect of the core polymer concentration on the core-to-sheath ratio in the fibres
Figures 1A-C showed the TEM images of the fibres in various scaffolds produced by coaxial electrospinning of PCL/gelatin. S-Figures 2A-C in the supporting information showed the TEM images of PU/gelatin fibres. TEM images of coaxial PCL/gelatin fibres with a constant sheath concentration of gelatin (5%) but varied core concentrations of PCL (i.e. 1%, 2.5%, and 3.5%) showed that the core diameter increased while the overall fibre diameter decreased with increasing PCL concentration, resulting in increased core-to-sheath ratio (Figure 1A-B). S-Figure 2A–1C and S-Table 1 (in the supplementary information) show the TEM images of PU coaxial systems with varied concentration ratios between core (PU) and sheath (gelatin) polymers, showing similar trends in terms of the correlation between the core-to-sheath ratio and the core polymer concentration.
Fig. 1.
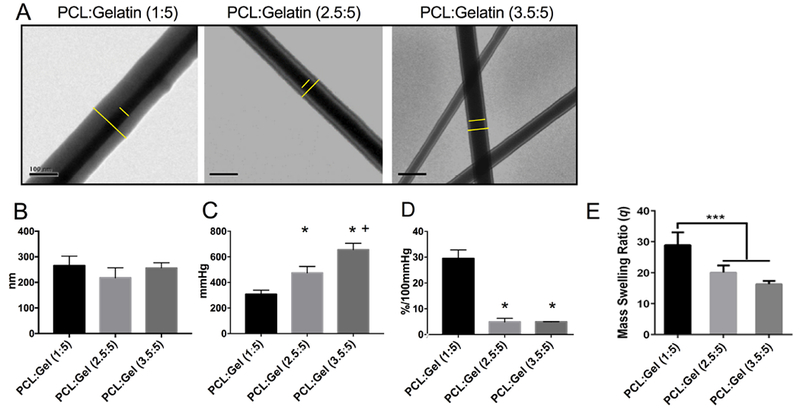
Morphological and physical properties of coxially structured PCL/gelatin fibres. (A) TEM images of PCL/gelatin fibres with a PCL-to-gelatin ratio of 1:5, 2.5:5 or 3.5:5. Clear core and sheath boundaries were identified. All scale bar shows 100 nm. (B) Quantitative analysis of fibre diameters. (C) Burst pressure for the tubular grafts consisting of coaxial PCL/gelatin fibres. (D) Compliance of these grafts. Graft compliance measurement shows the percent of vascular graft diameter change per 100 mm Hg. (E) Mass swelling ratio of PCL/gelatin fibres showing the hydrogel nature of the materials. Data represent mean ± SEM. n = 3–4 samples. * p < 0.05; *** p < 0.01, vs PCL:Gel (1:5). + p < 0.05, vs PCL:Gel (2.5:5).
3.2. Mechanical behaviors of coaxially structured micro/nano-fibre graft
To further assess the effects of core-to-sheath ratio on mechanical properties associated with tubular graft performances, the burst strength, compliance and water permeability of coaxial PCL/gelatin fibre system were measured according to the industrial standards for vascular grafts. As shown in Figure 1C, the average burst pressure increased with the core-to-sheath ratio, from 380 mmHg for PCL/gelatin fibre (1:5) to 656 mmHg for PCL/gelatin fibre (3.5:5). Conversely, the compliance drastically decreased with increasing core-to-sheath ratio from 29.7% per 100 mmHg for PCL/gelatin fibre (1:5) to 4.9% for PCL/gelatin fibre (3.5:5), as shown in Figure 1D. The observations were likely due to nascent mechanical properties of PCL and gelatin – higher strength and lower elasticity for PCL compared to gelatin. To demonstrate the hydrogel nature of the fibrous materials, their swelling ratios were evaluated by measuring the changes of material weight before and after hydration. The result (Figure 1E) showed that PCL/gelatin fibre (1:5), characterized by the highest gelatin-to-PCL ratio or lowest core-to-sheath ratio among the three coaxial fibres, displayed highest swelling ratio. Additionally, results from the water permeability tests showed that all the coaxially-structured fibrous materials exhibited no water leakage (<0.1 ml/min mL/cm2/min) under a flow pressure of 120 mmHg, which further indicated the hydrogel nature of these coaxially-structured fibres. As the entire surfaces of coaxial PCL/gelatin fibres were covered by gelatin that effectively retained the water content, excellent sealing function of grafts were achieved. This characteristic of coaxial fibres stay in contrast with porous, hydrophobic PCL materials which were known to have high water permeability [27].
3.3. PCL outer layer strengthened coaxial PCL/gelatin fibrous graft
An ideal vascular graft should be able to achieve excellent burst pressure (>1000 mmHg) with artery-like compliance, which is about 12% - 16% per 100 mmHg for human carotid artery in young healthy subjects [23]. However, the compliances of most polymeric grafts fall in the range for native vein grafts such as saphenous vein (~4.5%) and umbilical vein (~3.7%). The 1:5 PCL/gelatin fibre-based grafts possessed a compliance of 29.7%, which was much greater than arterial compliance, but the burst pressure was much lower than the targeted value. Increasing concentration of PCL in the core resulted in increased burst pressure; however, the burst strength was still not sufficiently high, while the expense of that increase was a significantly decreased compliance. Therefore, we sought to further improve the burst strength while maintaining the compliance of PCL/gelatin fibre within the range for arterial compliance [23]. To this end, a sheet of fibrous PCL layer was added outside the PCL/gelatin graft.
A thin or thick layer of pure PCL fibre were electrospun outside the PCL/gelatin (1:5) fibrous graft. These bilayered grafts consist of an inner PCL/gelatin layer and an outer PCL layer at varied thicknesses. The total thicknesses of thin-cap and thick-cap were 180 μm and 320 μm, respectively (Figure 2A). Interestingly, the introduction of outer layer resulted in a significant increase in the burst pressure to ≥ 1000 mmHg for both thin-cap and thick-cap grafts (Figure 2B). This made the grafts fall in a highly safe range and mimicked mechanical performance of an artery whose burst pressure is greater than 1000 mmHg to withstand blood pressure (<200 mmHg). Nevertheless, the outer layer decreased the graft compliance down to 16.2% for thin-cap grafts or to 7.8% for thick-cap grafts (Figure 2C). S-Figure 3 demonstrated representative images illustrating the difference in the graft compliance among varied grafts under physiological pressure. Together, the thin-cap graft displayed similar thickness and compliance to the normal carotid artery, and offered high burst strength for grafting.
Fig. 2.
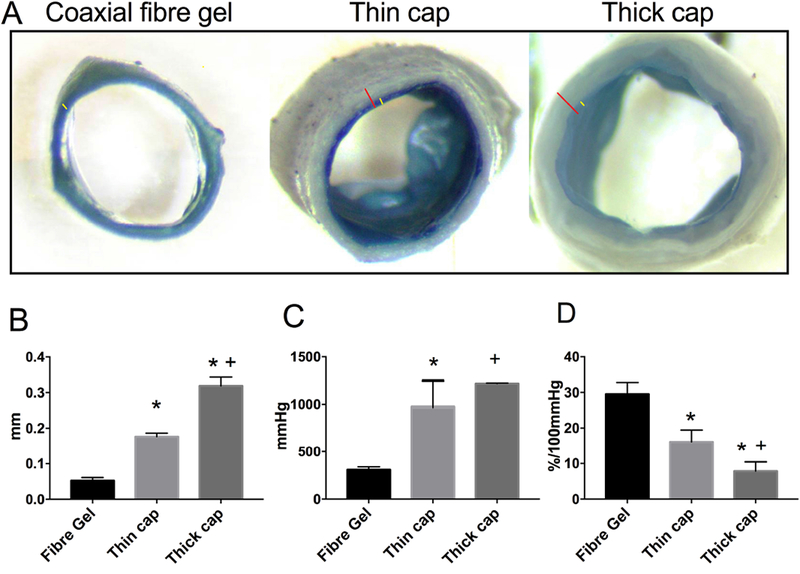
Structure and mechanical behaviors of tubular grafts with varied designs. (A) Representative images of tubular grafts showing varied graft wall structures. The layer of coaxial PCL/gelatin fibres is identified with yellow line while the overall wall thickness shown with red. The images were taken with the same magnification. (B) Average graft wall thickness. (C) Burst strength of grafts. (D) Graft compliance. Data represent mean ± SEM. n = 4; * p < 0.05, vs PCL:Gel (1:5). + p < 0.05, vs PCL:Gel (2.5:5). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. PCL cap layer improved suturability and anti-kink property
The suture retention of grafts was evaluated to assess the suturability of grafts for in vivo implantation. The thick-cap graft showed much higher suture retention, when compared to fibre gel (no-cap) or thin-cap grafts (Figure 3 A), indicating that graft suturability significantly increased with the thickness of hydrophobic PCL layer. Interestingly, though there was little difference in the suture retention results between fibre-gel and thin-cap grafts, we found that grafts with no outer layer were easily torn by suture stitches when actually suturing the graft to neighboring arteries during implantation, while thin-cap grafts were always sutured to arteries without any leakage or graft damage. This showed that fibre gel (no-cap) grafts did not possess sufficient strength to withstand tension imposed by multiple stitches. Therefore, besides conventional suture retention test which measures graft strength under the tension of a single suture stitch, we performed a surgically-relevant test involving similar suture stitching and knotting techniques as done during implantation. This test, named as multi-stitching strength test, better predicted the success of suturability grafts to the blood vessel during implantation. Results from the suture stitch strength showed significant differences between fibre gel grafts and thin-cap grafts showing a two-fold increase that reflects actual surgical situation (Figure 3B). Therefore, the addition of a PCL layer to the grafts was critical to the success of vascular grafts during implantation surgery by ensuring adequate suture retention and graft integrity.
Fig. 3.
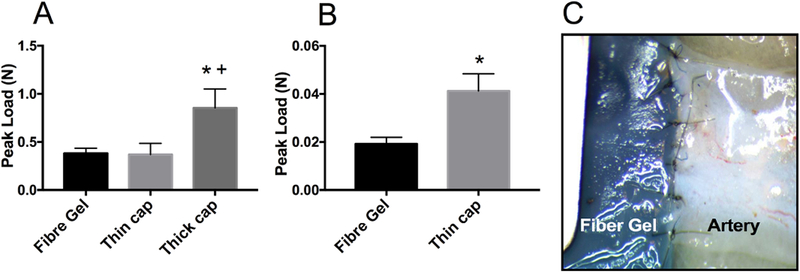
Graft suturability evaluation. (A) Suture retention values resulted from the standard test employing single suture; (B) Multi-stitching strength results using similar surgical stitching and knotting techniques as performed during the implantation; (C) A representative illustration showing multi-stitching strength test. * p < 0.05, vs PCL:Gel (1:5). + p < 0.05, vs PCL:Gel (2.5:5).
Vascular grafts can be twisted inadvertently during implantation. If twisted excessively, they may kink and obstruct flow. Excellent flexibility with kink resistance is often needed for a number of grafting conditions, in particular to resist crush under physiological stresses, or to withstand the traction of moving parts. When current vascular grafts are used in the angulated and tortuous anatomy or in the anatomic location where graft motion is expected, external spiral support is often needed to provide resistance to kink and compression. Thus, kink radius measurement was performed to demonstrate the potential of graft materials in anatomical areas prone to kink, such as bypass or interpositional graft for a carotid artery, where continuous neck bending and moving cause the graft to kink. In this study, kink radius was defined as the minimal radius of mandrel, at which a graft can be bent around without kinking to narrow or occlude the lumen and drop the pressure in the flow circulation. Our results showed that the radius of graft mandrel increased from 2 mm to 5 mm and 9 mm after the addition of thin and thick outer PCL layers, respectively, outside the pristine PCL/gelatin fibrous graft (Figure 4). Thus the thin-cap graft and the pristine PCL/gelatin fibrous grafts possessed an excellent kink resistance.
Fig. 4.
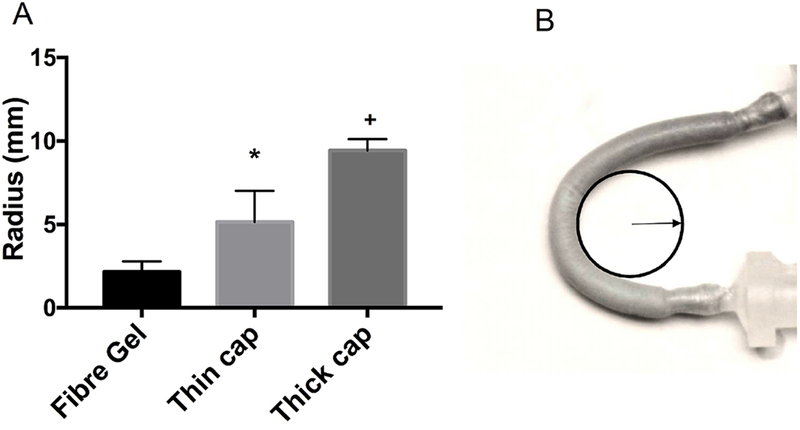
Kink resistance of the grafts. (A) Comparison of kink radius among different grafts; * p < 0.05; vs PCL:Gel (1:5). + p < 0.05, vs PCL:Gel (2.5:5). (B) A representative illustration of kink radius measurement.
3.5. Coaxial PCL/gelatin fibres inhibited platelet adhesion and activation
Platelet adhesion assay was performed to evaluate the thrombogenicity of the coaxial PCL/gelatin as the luminal surfaces of grafts, when compared to the PCL used as a positive control. Confocal images (Figure 5A-B) and SEM images (Figure 5D-E) both showed that coaxial PCL/gelatin fibres significantly reduced platelet adhesion, using similar platelet-rich plasma (Figure 5C). The number of adhered platelets on coaxial PCL/gelatin fibres decreased by 75% when compared to PCL fibres (Figure 5F). Moreover, SEM images showed that platelets adhered to coaxial PCL/gelatin fibres were still round without obvious dendritic structures and spreading, indicating unactivated states. These findings indicated that coaxial PCL/gelatin fibres significantly inhibited platelet adhesion and activation, revealing a strong anti-thrombogenicity for implantation.
Fig. 5.
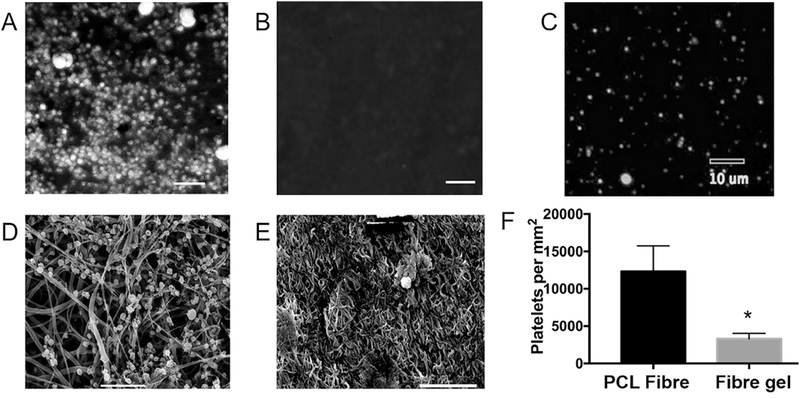
Platelet adhesion assay comparing two luminal graft surfaces. (A–C) Representative confocal images showing platelet adhesions on the PCL fibre surface (A) and coaxial PCL/gel fibre surface (B), as well as platelets in the testing solution (C). (D–E) Representative SEM images confirming that the coaxial PCL/gel surface (E) significantly decreased the adhesion and activation of platelets, when compared to the PCL surface (D). (F) Quantitative results from the platelet adhesion assay. All scale bars show 10 μm.
3.6. In vivo evaluation of vascular grafts using a rabbit model
The potential of developed coaxially-electrospun tubular scaffolds as vascular grafts were evaluated in vivo using a carotid artery implantation model in rabbits, where small vascular grafts were used to replace carotid arteries in rabbits (Figure 6A and 6B). After one day, reperfusion with pulsatile blood flow in all implanted grafts was found, indicating no acute thrombosis occurs (Supplementary video 1). After three months, all eight rabbits survived the surgeries and their grafts were all successfully explanted. Prior to graft explantation, ultrasonic doppler imaging and measurement were performed on the interposition vascular grafts replacing right carotid arteries together with left carotid arteries which served as the control, to analyze the characteristics of blood flow through the grafts. Notably, a high speed blood flow was observed inside thin-cap grafts (Figure 6C). Thick-cap grafts induced a significant decrease in blood flow compared to thin-cap grafts (Figure 6D). Also, a profound decrease in the size of the graft lumen, approximately 50% reduction in the area, was noted in thick-cap grafts compared to thin-cap ones. This result was likely caused by cell growth and migration during healing and remodeling around grafts, which was more active in the thick-cap grafts leading to 50% luminal reduction. To confirm this, histological studies were performed.
Fig. 6.
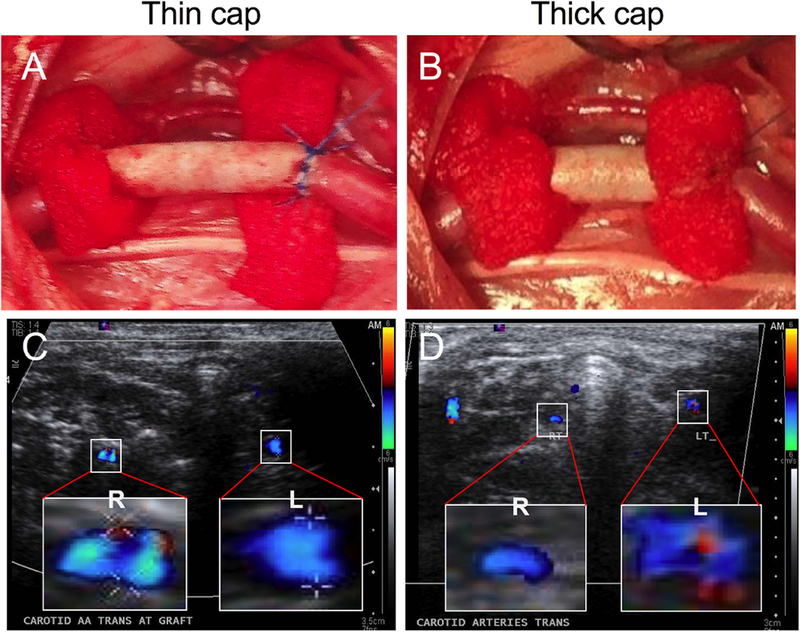
Interpositional grafting for right carotid artery in a rabbit model. (A–B) Implantation of vascular graft with a thin cap (A) or thick cap (B). Hemo-pads were used to cover the graft-artery junction after stitching. (C–D) Representative ultrasonic doppler images, showing the flow velocity profile in the transverse section of the graft with thin capping (C) versus that with thick capping (D). Only the right carotid artery (R) was reperfused with synthetic grafts, while the left artery (L) was used as a control during the detection.
After three-month implantation period, grafts were harvested for histological evaluations. Explanted multilayered coaxially electrospun grafts showed no blood clots or platelet adhesions. There were also no unfavorable systemic inflammation or thrombogenic reactions produced by the grafts in all the experimented animals. Serial cross-sections at various positions along the graft were assessed using pentachrome stain for overall evaluation (Figure 7). As expected, a separate layer of coaxial PCL/gelatin fibres from the pure PCL layer was not found on the wall of all grafts, likely due to the degradation of gelatin. The layer composed of the PCL fibres, however, was not completely absorbed. The pentachrome staining provided histological information about cells with nuclei in black and muscle tissue in red, and about ECM with collagen fibres in yellow, ground substance in blue, and elastic fibres in black. Interestingly, the cell infiltration inside the PCL layer, though few, was found for both types of grafts, which could be important to healing of graft implantation. Additionally, the accumulation of cells and extracelular matrices was found around the PCL layer for both types of grafts, extending to luminal and abluminal areas with approximately similar thickness on both sides. The ECM content, such as ground substance (blue), collagen (yellow) or their mixture (green), was found more abundant in regions away from the PCL layer, while close to PCL was more cellular tissues showing mixed black (nuclei) and red (smooth muscle) colors. Such ECM content simulates that of the native arterial wall, which is composed of a large amount of collagen in ground substance. Ground substance provides lubrication for collagen fibers. However, the elastin was lacking in surrounding tissues, suggesting the limitation in regeneration. The substantial cell presence and infiltration, even into PCL, as well as the ECM production suggest the advantages provided by the gelatin sheath, because PCL-based vascular grafts usually showed limited cellular regeneration, even with the addition of cell-adhesive RGD molecules [24]. Other stiff grafting materials also shows similar bioinertness. A more significant reduction in the lumen size was found for thick-cap grafts, which resulted mostly from the deposition of thicker tissue layers in the luminal area. Intriguingly, a high collagen content (yellow) in the thick tissues was found to form in the region close to the lumen of thick-cap grafts (Figure 7B), suggesting the occurrence of fibrosis. This was likely caused by flow disturbance due to artery-graft compliance mismatch and/or motion-caused graft kinking. Thus, highly compliant, flexible, kink-resistant thin-cap grafts were more advantageous for cellular and tissue remodeling during post-implantation in vivo. Together, animal studies suggest the migration and proliferation of host cells into and around the grafts, which could potentially maintain graft strength after the full degradation of PCL (~1.5 year).
Fig. 7.
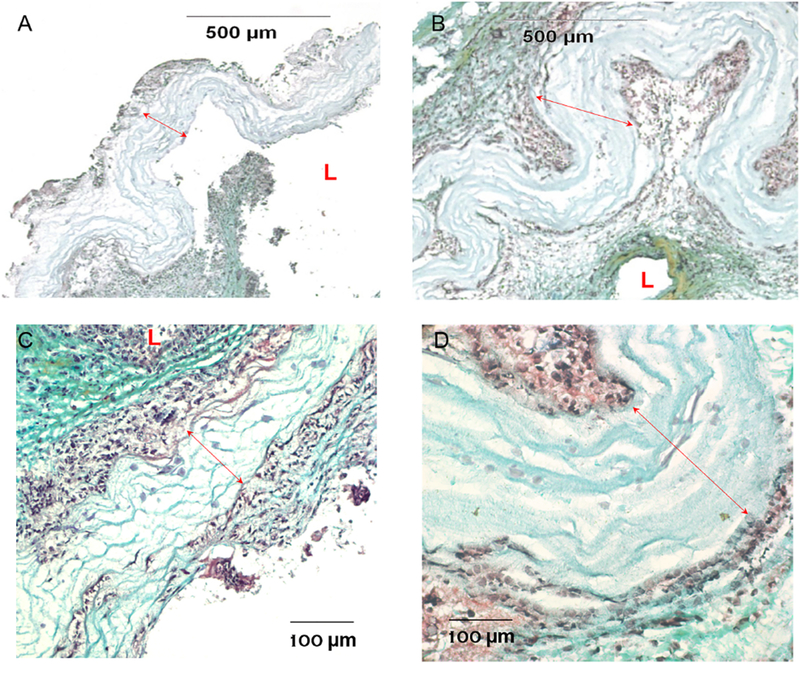
Histological analysis of grafts with pentachrome stain. (A–B) Representative images of thin-cap implant (A) versus thick-cap implant (B) with a 10× objective. The luminal area was significantly reduced in those with thick cap, which was accompanied by more significant wall remodeling in both luminal and abluminal areas. (C–D) Representaive images of thin-cap implant (C) versus thick-cap (D) with a higher magnification (40×), showing cell infiltration in the graft wall. “L” shows the luminen, and the red lines show the PCL material region. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This study has demonstrated a unique graft manufacturing technique, producing grafts with a great combination of compliance, strength, surface softness, sealing and suturability. The material structure was optimized in order to improve graft performances. The resultant coaxially-structured fibre grafts, demonstrating hydrogel characteristics, were strong, soft and flexible. Such properties facilitated easy manipulation and implantation, allowing excellent sealing or fluid retention (i.e. water permeability of < 0.1 ml/cm2/min at 120mmHg maximum pressure), suturability (as demonstrated by the structural integrity at the anastomosis and easy passage of sutures through the graft wall), as well as kink resistance. The design of mimetic arterial grafts requires both high strength and artery-like compliance. The PCL core in coaxial PCL/gelatin micro/nano-fibres ensured sufficient mechanical strength, while the sheath of gelatin, crosslinked into a gel network, offered elasticity, high water content, as well as cell adhesive sites and softness over the entire surfaces. In addition to enhanced physico-chemical properties, the coaxial design is important to the stability of PCL/gelatin hybrid materials. Due to the drastic differences in material mechanics, surface property and hydrophilicity, the interaction between PCL and gelatin is extremely weak. Simple blending or layering of two polymeric materials yielded instable structure; layer delamination occurred even with minimal handling in an aqueous solution (data not shown). To improve the material compatibility, the interfacial interaction was enhanced by increasing their interactive surface. The coaxial fibre structure offered large interactive surfaces for the two materials to form stable structure. Our study also showed the tunablity of coaxial PCL/gelatin fibre mechanics through modifying the PCL-to-gelatin ratio. Furthermore, the crosslinked coaxial fibres formed more stable interaction with PCL, when compared to gelatin alone, which was likely due to increased stiffness and decreased swelling. As shown in the study, a thin PCL outer layer was required to render sufficient strength for implantation and retain high compliance, i.e. 16.2 %, which closely resembled mechanical properties of human carotid artery [23].
Flexibility and kink resistance are important graft design consideration. For PTFE or other existing grafts, external spiral support is often needed in angulated or tortuous anatomic sites, or in anatomic locations with high mobility, in order to provide resistance to compression and kinking [25]. In these challenging anatomical situations such as carotid artery, the kink and buckling resistance is particularly critical for arterial implants, because they are under complex mechanical loads including lumen pressure, outer pressure, axial tension due to tissue tethering, and twisting and bending due to body movement. From an engineering perspective, blood vessels can be considered cylindrical structures subject to combined loads including tension, bending, and torsion. The tubular arteries may buckle under these loads and deform from their normally straight cylindrical shape. The external support used in existing grafts, however, often covered entire length of grafts, compromising the mechanical properties and radiopaque for graft visualization. The flexibility and hydrogel nature of gelatin as the sheath layer offered good anti-kinking property of coaxial PCL/gelatin grafts. With the use of flow bending test, graft samples were tested here in a large range of bending angles; the radius was determined as the mininal angle for a graft to achieve without reducing flow. Coaxial PCL/gelatin grafts with excellent anti-kinking property thus provide promising applications for those with challenging conditions or in high mobility locations such as carotid artery used as the animal model here. To futher ensure graft implantability prior to animal studies, we have modified some conventional testing approaches that determine graft performances, including burst strength, compliance and suture retention. A wide range of methods have been used to measure burst pressure and compliance, but many likely overestimate material performance. Examples include calculating these values based on the tensile testing results of materials [26], or inserting a nonporous sleeve into the graft lumen during direct measurement of 3D tubular samples under flow conditions, which removes the impact of structural defects presented by non-uniform pores [27, 28]. In this study, the evaluation of grafts was directly performed under flow, which closely reflected physiological stresses that vascular grafts would experience in vivo. We also used two methods for suture strength evaluation, one of which simulated actual surgical grafting practice and mechanical environments.
In addition to all structural and mechanical properties needed for vascular grafting, anti-thrombogenicity is required for successful implantation, which was assessed by platelet adhesion assay in vitro. The surface free energies of hydrophobic PCL are considerably higher than the natural hydrogel polymers like gelatin [29, 30], which encourages the adsorption of blood proteins like fibrinogen and von Willerbrand factor onto hydrophobic PCL, leading to a significant amount of platelet adhesion. The addition of gelatin sheath in coaxial PCL/gelatin fibres potently inhibited platelet adhesion and activation, which reduced the possibility of acute coagulation at an early stage of implantation. The superior anti-thrombogenicity of coaxial PCL/gelatin fibres was confirmed by reperfusion and maintenance of pulsatile blood flow in implanted grafts in three-month implantation.
During the three-month implantation period, normal blood flow and limited narrowing were found in thin-cap coaxial PCL/gelatin fibrous grafts. However, the velocity of blood flow and the lumen size was significantly reduced in thick-cap grafts, which was apparently due to thick tissue formation on luminal and abluminal sides, leading to lumen narrowing and wall stiffening. The difference in in vivo evaluation results between thin-cap and thick-cap grafts might be related to the differences in physical and mechanical properties of the grafts, in particular, higher compliance and improved kink resistance of thin-cap grafts, both of which could significantly alter the flow environments including hemodynamics-induced shear stress and circumferential stress throughout the graft and anastomoses [4]. Nevertheless, the mechanism by which low compliance of thick PCL cap induces restenosis needs further study. The rapid re-endotheliazation is critical to the success of vascular grafts since vascular endothelium plays important roles in preventing blood coagulation and over-proliferation of smooth muscle cells [31]. The luminal surface of thin-cap fibrous grafts should be further improved to allow rapid endothelialization.
Overall, the innovation here includes the use of a hybrid fiber and demonstration of compliance effect for a mechano-active arterial graft in vivo. Though copolymers and simple blends of two or more polymers have been used to make grafts, our core-shell microfibers, showing well-defined nanostructure with a combination of hydrophobic synthetic polymer and hydrophilic natural polymer, have never been explored for arterial or other grafting in vivo. In another innovation aspect, limited is yet known about how the compliance of inherently elastic grafts influences cell infiltration and matrix production. Our results suggest that a higher compliance of mechano-active bioengineered graft can stimulate matrix production and cell infiltration, but limiting such remodeling efforts to a level more approaching to mechano-biological homeostasis.
5. Conclusion
In this study, coaxial PCL/gelatin fibrous grafts were fabricated by co-eletrospinning technique and the mechanical behaviors for graft performances were optimized by varying concentration ratio of PCL-to-gelatin and introduction of PCL “cap” layer outside the PCL/gelatin coaxial layer. The thin-cap grafts possess superior mechanical properties for grafting in terms of high burst strength (within a safe range of >8 times of the normal systolic pressure of human), arterial compliance, good suturability and anti-kinking capability, resembling biomechanics of native artery. In addition, coaxial PCL/gelatin fibrous surfaces significantly inhibited platelet adhesion and activation. Moreover, the thin-cap grafts composed of coaxial PCL/gelatin fibre show enhanced patency and cell infiltration into fibrous grafts after three-month implantation in a rabbit model. Our approach and findings here provide useful guidance on testing and design of future readily-available vascular grafts.
Supplementary Material
Highlights:
A facile approach was developed to fabricate readily-available vascular grafts
Coaxially nanostructured fibres render grafts’ strength, compliance and flexibility
Hydrogel sheath of fibers offer excellent water sealing and blood compatibility
Grafts with artery-like mechanics demonstrated improved implantation outcomes
Acknowledgement
The work was financially supported by (NHLBI R01HL119371 to W. Tan). The authors wish to thank the Department of Radiology in the Veterinarian Teaching Hospital for help with imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].WHO. Cardiovascular Diseases. http://wwwwhoint/mediacentre/factsheets/fs317/en/ 2017.
- [2].Seifu DG, Purnama A, Mequanint K, Mantovani D. Small-diameter vascular tissue engineering. Nature Reviews Cardiology 2013;10:410–21. [DOI] [PubMed] [Google Scholar]
- [3].Mattix B, Poole T, Casco M, Uvarov O, Visconti R. Small diameter vascular grafts: replacement strategies, maturation techniques and challenges. J Tissue Sci Eng 2013;4. [Google Scholar]
- [4].Sarkar S, Schmitz-Rixen T, Hamilton G, Seifalian AM. Achieving the ideal properties for vascular bypass grafts using a tissue engineered approach: a review. Medical & Biological Engineering & Computing 2007;45:327–36. [DOI] [PubMed] [Google Scholar]
- [5].Ravi S, Chaikof EL. Biomaterials for vascular tissue engineering. Regenerative Medicine 2010;5:107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nagiah N, Johnson R, Anderson R, Elliott W, Tan W. Highly compliant vascular grafts with gelatin-sheathed coaxially structured nanofibres. Langmuir 2015;31:12993–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fedel M, Motta A, Maniglio D, igliaresi C. Surface properties and blood compatibility of commercially available DLC coatings for cardiovascular applications. J. Biomed. Mater. Res. Part B 2009; 90B: 338–349. [DOI] [PubMed] [Google Scholar]
- [8].Nuhn H, Blanco CE, Desai TA. Nanoengineered stent surface to reduce in-stent restenosis in vivo. ACS Appl. Mater. Interfaces 2017; 9: 19677–19686 [DOI] [PubMed] [Google Scholar]
- [9].Fan Y, Luo R, Han H, Weng Y, Wang H, Li J, Yang P, Wang Y, Huang N. Platelet adhesion and activation on chiral surfaces: The Influence of Protein Adsorption. Langmuir 2017; 33:10402–10410. [DOI] [PubMed] [Google Scholar]
- [10].Li J, Zhang K, Huang N. Engineering cardiovascular implant surfaces to create a vascular endothelial growth microenvironment. Biotechnol J 2017; 2: Epub 2017. [DOI] [PubMed] [Google Scholar]
- [11].Wise SG, Byrom MJ, Waterhouse A, Bannon PG, Ng MK, Weiss AS. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomaterialia 2011;7:295–303. [DOI] [PubMed] [Google Scholar]
- [12].Prim DA, Zhou B, Hartstone-Rose A, Uline MJ, Shazly T, Eberth JF. A mechanical argument for the differential performance of coronary artery grafts. Journal of the Mechanical Behavior of Biomedical Materials 2016;54:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nappi F, Carotenuto AR, Cutolo A, Fouret P, Acar C, Chachques JC, et al. Compliance mismatch and compressive wall stresses drive anomalous remodelling of pulmonary trunks reinforced with Dacron grafts. Journal of the Mechanical Behavior of Biomedical Materials 2016;63:287–302. [DOI] [PubMed] [Google Scholar]
- [14].Bashur CA, Eagleton MJ, Ramamurthi A. Impact of electrospun conduit fibre diameter and enclosing pouch pore size on vascular constructs grown within rat peritoneal cavities. Tissue Engineering Part A 2012;19:809–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Z, Cui Y, Wang J, Yang X, Wu Y, Wang K, et al. The effect of thick fibres and large pores of electrospun poly (ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 2014;35:5700–10. [DOI] [PubMed] [Google Scholar]
- [16].Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, et al. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomaterialia 2014;10:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zeugolis DI, Khew ST, Yew ES, Ekaputra AK, Tong YW, Yung L-YL, et al. Electro-spinning of pure collagen nano-fibres-just an expensive way to make gelatin? Biomaterials 2008;29:2293–305. [DOI] [PubMed] [Google Scholar]
- [18].Han J, Lazarovici P, Pomerantz C, Chen X, Wei Y, Lelkes PI. Co-electrospun blends of PLGA, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules 2010;12:399–408. [DOI] [PubMed] [Google Scholar]
- [19].Pakravan M, Heuzey M-C, Ajji A. Core-shell structured PEO-chitosan nanofibres by coaxial electrospinning. Biomacromolecules 2012;13:412–21. [DOI] [PubMed] [Google Scholar]
- [20].Cutiongco MA, Kukumberg M, Peneyra JL, Yeo MS, Yao JY, Rufaihah AJ, Visage CL, Ho JP, Yim EK. Submillimeter Diameter Poly(Vinyl Alcohol) Vascular Graft Patency in Rabbit Model. Front Bioeng Biotechnol. 2016; 4: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vase Biol. 2012; 32(5): 1104–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Madhavan K, Belchenko D, Tan W. Roles of genipin crosslinking and biomolecule conditioning in collagen-based biopolymer: Potential for vascular media regeneration. J Biomed Mater Res A. 2011; 97(1): 16–26. [DOI] [PubMed] [Google Scholar]
- [23].Hansen F, Mangell P, Sonesson B, Lanne T. Diameter and compliance in the human common carotid artery—variations with age and sex. Ultrasound in Medicine & Biology 1995;21:1–9. [DOI] [PubMed] [Google Scholar]
- [24].Wang Z, Zheng W, Wu Y, Wang J, Zhang X, Wang K, Zhao Q, Kong D, Ke T, Li C. Differences in the performance of PCL-based vascular grafts as abdominal aorta substitutes in healthy and diabetic rats. Biomater Sci. 2016;4: 1485–92 [DOI] [PubMed] [Google Scholar]
- [25].Hung Y-N, Ko P-J, Ng Y-Y, Wu S-C. The longevity of arteriovenous graft for hemodialysis patients—externally supported or nonsupported. Clinical Journal of the American Society of Nephrology 2010:CJN. 08181109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nieponice A, Soletti L, Guan J, Deasy BM, Huard J, Wagner WR, et al. Development of a tissue-engineered vascular graft combining a biodegradable scaffold, muscle-derived stem cells and a rotational vacuum seeding technique. Biomaterials 2008;29:825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Drilling S, Gaumer J, Lannutti J. Fabrication of burst pressure competent vascular grafts via electrospinning: effects of microstructure. Journal of Biomedical Materials Research Part A 2009;88:923–34. [DOI] [PubMed] [Google Scholar]
- [28].Madhavan K, Elliott WH, Bonani W, Monnet E, Tan W. Mechanical and biocompatible characterizations of a readily available multilayer vascular graft. J Biomed Mater Res B Appl Biomater. 2013;101(4): 506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Biresaw G, Carriere CJ. Surface energy parameters of polymers from directly measured interfacial tension with probe polymers, J Adhes Sci Tech 2004, 18:1675–1685 [Google Scholar]
- [30].Li J, Sha Z, Zhang W, Tao F, Yang P. Preparation and antibacterial properties of gelatin grafted with an epoxy silicone quaternary ammonium salt. J Biomat Sci Polymer Ed 2016, 27: 1017–1028. [DOI] [PubMed] [Google Scholar]
- [31].Ding Y, Yang M, Yang Z, Luo R, Lu X, Huang N, et al. Cooperative control of blood compatibility and re-endothelialization by immobilized heparin and substrate topography. Acta Biomaterialia 2015;15:150–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


