Abstract
Introduction
Stereotactic body radiation therapy (SBRT) and Radiofrequency ablation (RFA) are two important treatment options for medically inoperable patients with stage I non-small cell lung cancer (NSCLC). Here we performed a systemic review and pooled analysis to compare clinical outcomes of these two techniques.
Methods
A comprehensive literature search for published trials from 2001 to 2012 was undertaken. Pooled analyses were performed to obtain overall survival (OS), local tumor control rates (LCR), and the adverse events. Regression analysis was conducted considering each study's proportions of stage IA and age.
Results
Thirty-one studies on SBRT (2767 patients) and 13 studies on RFA (328 patients) were eligible. LCR (95% confidence interval) at 1, 2, 3 and 5 years for RFA was 77% (70 – 85%), 48% (37 – 58%), 55% (47 – 62%), and 42% (30 – 54%) respectively, which was significantly lower than that for SBRT 97 % (96 – 98%), 92% (91 – 94%), 88% (86 – 90%), and 86% (85 – 88%) (P<.001). These differences remained significant after correcting for stage IA and age (P<.001 at 1 year, 2 years and 3 years; P=.04 at 5 years). The effect of RFA was not different from that of SBRT on OS (P>.05). The most frequent complication of RFA was pneumothorax, occurring in 31% of patients, while that for SBRT (≥grade 3) was radiation pneumonitis, occurring in 2% of patients.
Conclusions
Comparing to RFA, SBRT seems to have higher LCR, but similar OS. More studies with larger sample sizes are warranted to validate such findings.
Keywords: Non-small cell lung cancer, Stage I, Medically inoperable, Radiofrequency ablation, Stereotactic body radiation therapy
Introduction
Nearly 25% of patients with early stage or stage I non-small-cell lung cancer (NSCLC) are considered medically inoperable,[28,45] with five-year survivals only about 15% prior to the era of stereotactic body radiotherapy (SBRT), which are significantly poorer than those who receive surgery.[45,61] Local failure was the primary cause of failure[25,48]. SBRT, which is also referred to as stereotactic ablative radiotherapy (SABR), has emerged as an important treatment option for inoperable stage I NSCLC over the last 10 years. This radiation technology allows accurate delivery of a very high dose to the tumor, while sparing normal adjacent tissue. In prospective and large retrospective multicenter studies, when an adequate dose (BED≥100 Gy) is utilized, high local control rate (LCR) of over 90% has been reported, as well as promising overall survival (OS) advantages and good tolerance compared to conventional radiotherapy.[4,46,56] The recently published results from the Radiation Therapy Oncology Group (RTOG) 0236 trial demonstrated 3-year actuarial LCR of 98%, while altogether avoiding severe toxicity in more than 75% of treated patients.[56] With this and other evidence, SBRT is the current standard of care for medically inoperable stage I NSCLC.
Radiofrequency ablation (RFA) is another promising non-surgical therapeutic alternative for small, peripheral tumors. It can be performed percutaneously, under conscious sedation and image guidance, and as an outpatient or with a short hospital stay. The use of RFA to treat lung malignancies in human was first reported in 2000.[13] The largest prospective multicenter trial of RFA for lung tumors was the RAPTURE study published in 2008, which enrolled 106 inoperable patients with 183 lung tumors including 33 primary NSCLC patients.[30] A 75% 2-year OS has been shown in patients with stage I NSCLC (n=13), and a confirmed complete response lasting at least 1 year was 88%. RFA treatment was well tolerated. Major complications were symptomatic pneumothorax needing drainage (n=27) and pleural effusion needing drainage (n=4). Minor complications were pneumothorax (n=28) or pleural effusion (n=11) not needing treatment and self-limiting intrapulmonary haemorrhage (n=3). However, the majority of RFA studies in early stage NSCLC are small case series and the reported LCR ranged greatly from 58% to 95%. [18,19,37,40]
At present, no direct comparison of clinical outcomes between RFA and SBRT has been published. Furthermore, it is often challenging for tumor board to decide between RFA and SBRT, as many patients are candidates for both treatments and it is unknown which treatment would provide superior outcome. In addition, outcome data from randomized studies are lacking, and no such prospective trial is ongoing. Therefore, we performed this systemic review and pooled analysis to provide a comparison between RFA and SBRT, for treatment of medically inoperable stage I NSCLC, with a focus on LCR, OS, and treatment-related toxicities.
Methods
A comprehensive literature search was performed using MEDLINE, Embase and Cochrane Library from 2001 to 2012. Specific search terms used included radiofrequency ablation, thermal ablation, stereotactic radiotherapy, stereotactic radiation therapy, stereotactic ablative radiotherapy, non–small cell lung carcinoma, and NSCLC. Reference lists of obtained articles were searched as well.
Inclusion and exclusion criteria
The eligibility criteria included as follows: (1) stage I NSCLC diagnosis, (2) medically inoperability, and (3) reporting on the outcome of patients after RFA or SBRT. Outcome data included local tumor control rates (LCR) at 1, 2, 3 and 5 years, and overall survival (OS) rates at 1, 2, 3 and 5 years. Local control was assessed by follow-up radiological image and defined as no tumor recurrence/progression in the primary site. Procedure-related morbidity and mortality were also analyzed. Studies about RFA followed by immediate resection or radiotherapy, or SBRT with BED <100 Gy, fraction dose <8 Gy, or using more than 5 fractions, or with less than 5 patients in RFA group and 30 patients in SBRT group were excluded. We limited the SBRT to 5 fractions or less to make the patient population more comparable to that of RFA, i.e. peripheral location. Case reports, review articles, unpublished data, and publications in languages other than English were also excluded from further analysis. Studies and/or subgroups were included if reporting sufficient data on LCR, OS and treatment related morbidity. In case of potential duplication or overlapping studies only the largest and most complete data set was included, based on published information.
Data extraction
One investigator (N.B.) identified the articles for this topic. Two investigators (N.B., X.Z.) extracted the data on study characteristics, study population, technical details and outcome measurements using a standardized Excel file and reached consensus on all items. No attempt was made to get missing data from the authors. BED10 was calculated based on the following linear-quadratic equation: BED10 = nd*[1 + d/(a/b)], where a/b=10, n and d represent the number of fractions and the dose per fraction, respectively. The 1-, 2-, 3-, and 5-year LCR and OS were extracted from each study if available, as well as the occurrence of adverse events. In studies that survival rates were not explicitly stated but Kaplan-Meier survival curves were provided, survival data were extracted from survival curves.
In stage I NSCLC the occurrence of severe adverse events (CTCAE grade 3– 5) was infrequent for all treatment modalities. The majority of SBRT studies reported zero adverse events; therefore, we added up the adverse events occurring for each treatment modality instead of pooling the estimates using a regression. Proportions and 95% confidence intervals were calculated using a binomial distribution.
Data analysis
The primary endpoint of this study was LCR at 3 years. Secondary endpoints were OS and treatment related thoracic toxicity.
A pooled analysis was used to determine weighted summary statistics for each of the treatments. The results were presented as pooled proportions of patients surviving (or local-progression free surviving) 1-, 2-, 3-, and 5- years from diagnosis, with 95% confidence intervals (95%CI). Since standard errors for the surviving proportions of patients were not consistently reported, we inferred them from the reported median follow-up times using the parametric bootstrap[14]. Application of the parametric bootstrap to survival data requires models for both the survival and censoring processes. To obtain a model for the population survival process, for each study, a model was fit to the reported survival times (at 1-, 2-, 3-, and 5- years as reported). For studies with only one reported follow-up time, a one-parameter exponential model was used. For studies with two or more reported follow-up times, a two-parameter Weibull model was used. The right tail of the population survival curve was fit to the reported surviving patient fractions using least squares. The censoring process was modeled as an exponential distribution, with median equal to the reported median follow-up time. Simulation was then used to estimate the standard errors for estimating the surviving patient fractions, based on data sets of the reported sample size, from the estimated survival and censoring processes. Since differences between the characteristics of the study populations may influence the effectiveness of different treatment modalities, regression analysis was performed considering each study's proportions of Stage IA and patients’ age. All the analyses were done using R language (www.r-project.org).
Results
Literature search
The literature search performed in July 2012 yielded 1,270 articles (Figure 1). Among them, 478 articles were found to be pertinent. 306 studies were excluded as they failed to meet the eligibility criteria based on the screening of the titles and abstracts. Of the remaining 172 references the full text were retrieved and the data were analyzed. One additional reference on RFA was retrieved through manual searches of the reference lists. Ultimately, 44 articles were found to report data of interest and fulfilled the inclusion criteria of the present study: 13 studies including 328 patients on RFA [1,5,18,19,21,27,29,30,37,40,49,50,62] and 31 studies including 2767 patients on SBRT.[3,4,7–9,11,15–17,22–24,26,32,34–36,38,39,42,44,46,52–58,60,64] There were no RCTs or non-randomized studies directly comparing the differences between RFA and SBRT. All eligible studies were single arm observational studies or subgroup of comparative studies. Table 1 and Table 2 summarize the main characteristics of these studies.
Figure 1.
Flowchart of literature search and review.
Table 1.
Characteristics for RFA Studies (2001–2012) Included in Current Meta-analysis.
| First author | Year | Study. design |
Sample size |
Male (%) |
Median/ mean age (range) |
IA/I (%) |
Path. (%) |
Median F/U (mo) |
|---|---|---|---|---|---|---|---|---|
| Belfiore G16 | 2004 | R | 33 | 79 | 66 (44–75) | 39 | 100 | 12 |
| Pennathur A11 | 2007 | R | 19 | 42 | 78 (68–88) | 58 | 100 | 28 (9–52) |
| Simon CJ17 | 2007 | R | 75 | 57 | 69 (17–94) | 75 | NA | 21(3–74) |
| Lencioni R10 | 2008 | P | 33 | 76 | 67 (29–82) | 100 | 100 | 15 (1–30) |
| Okuma T12 | 2010 | R | 7 | 78 | 70 (31–94) | NA | NA | 12 (3–60) |
| Zemlyak A18 | 2010 | R | 12 | 56 | 74 (62–83) | NA | 100 | 33 |
| Ambrogi MC19 | 2011 | P | 59 | 79 | 74 (40–88) | 75 | 100 | 46 (12–82) |
| Hess A13 | 2011 | R | 15 | 60 | 64 (42–82) | 93 | 0 | 17.6 (2–31) |
| Hiraki T14 | 2011 | R | 50 | 58 | 75 (52–88) | 76 | 32 | 37 (2–88) |
| Lee H20 | 2011 | R | 16 | 75 | 73±8 | NA | 100 | 56 (6–64) |
| Sofocleous CT21 | 2011 | R | 12 | 67 | 65 (44–81) | 75 | 100 | 23 |
| Kim SR22 | 2012 | R | 8 | 88 | 72 (61–78) | 25 | 100 | 9 years |
| Lanuti M23 | 2012 | R | 45 | 40 | 70 (51–89) | 82 | 100 | 32 (2–75) |
Note: (%):F/U (mo): follow-up period in months;; Pts, patients with Stage I NSCLC; Path (%): percentage of disease with pathological confirmation; P: prospective; R: retrospective; N /A: not reported or not specified.
Table 2.
Characteristics of SBRT Studies (2001–2012) Included in Current Meta-analysis.
| First author | Year | Study. design |
Sampl e size |
Male (%) |
Median/ mean age |
IA/I (%) |
Path. (%) |
Dose range | BED10 | F/U (mo) |
|---|---|---|---|---|---|---|---|---|---|---|
| Nagata Y24 | 2005 | P | 45 | 74 | 77 (51–87) | 71 | 100.0 | 12Gy × 4 | 105.6 | 30 |
| Nyman J25 | 2006 | R | 45 | 57 | 74 (58–84) | 40 | 80.0 | 15Gy × 3 | 112.5 | 43 (24–74) |
| Timmerman R26 | 2006 | R | 70 | 74 | 70 (51–86) | 50 | / | 20Gy × 3 | 180 | 17.5 (0.6–44) |
| Zimmermann F27 | 2006 | R | 68 | 71 | 76 | 100 | 100.0 | 12.5Gy× 3; 7Gy× 5 (at 60%-isodose) | 84.4;59.5 | 17(3–44) |
| Koto M28 | 2007 | P | 31 | 81 | 77 (60–83) | 61 | 100.0 | 15Gy × 3; 7.5Gy × 8 | 112.5;105 | 32 (4–87) |
| Onishi H29 | 2007 | R | 257 | NA | 74 (39–92) | 64 | NA | 4.4~35/1-14F× | 117 (100–180) | 38 (2–128) |
| Fritz P30 | 2008 | R | 40 | 80 | 74 (59–82) | 55 | 100 | 30Gy×1 | 120Gy (isocenter) | 20 (6–61.5) |
| Lagerwaard FJ31 | 2008 | R | 206 | 57 | 73 | 59 | 31.0 | 20Gy × 3; 12Gy × 5; 7.5Gy × 8 | 132 | 12 |
| Onimaru R32 | 2008 | P | 41 | 69 | 76 (52–85) | 61.0 | NA | 10Gy × 4; 12Gy × 4 | 80;105.6 | 27 |
| Salazar OM33 | 2008 | R | 60 | NA | NA | 75 | NA | 13Gy × 4 | 119.6 | NA |
| Baumann P7 | 2009 | P | 57 | 44 | 75 (59–87) | 70.2 | NA | 15Gy × 3 | 112.5 | 35 (4–47) |
| Fakiris AJ34 | 2009 | P | 70 | NA | NA | 48.6 | NA | 20Gy × 3 (IA); 22Gy × 3 (IB) | 180; 211.2 | 50.2 |
| Kopek N35 | 2009 | R | 88 | 50 | 72 (47–88) | 58.6 | 100.0 | 15/22.5Gy × 3 | 112.5; 219.5 | 44 (1.6–96.5) |
| Stephans KL36 | 2009 | R | 56 | 52 | 72 (49–89) | 75.0 | NA | 10Gy × 5 | 100 | 19.8 |
| Takeda A37 | 2009 | R | 63 | 63 | 78 (56–91) | 60.3 | 82.5 | 10Gy × 5 | 100 | 31 (10–72) |
| Baba F38 | 2010 | R | 124 | 68 | 77 (29–89) | 70.2 | 91.9 | 11Gy × 4 (1.6%); 12Gy × 4; 13Gy × 4 | 92.4; 105.6; 119.6 | 26 (7–66) |
| Bradley JD39 | 2010 | P | 91 | 47 | 71 (31–93) | 72.5 | 86.3 | 18Gy × 3 | 151.2 | 18 (6–42) |
| Burdick MJ40 | 2010 | R | 72 | 52 | 74 (44–89) | 68.1 | 68.1 | 20Gy × 3; 10Gy × 5; 5Gy × 10 | 180; 100; 75 | 36 |
| Dunlap NE41 | 2010 | R | 40 | NA | 73 (54–87) | 67.5 | 100.0 | 42-60/3-5FX | 150 | 12.5 (2–35) |
| Hamamoto Y42 | 2010 | R | 52 | 70 | 78 (58–90) | 76.8 | 67.3 | 12Gy × 4 | 105.6 | 14 (3–34) |
| Ricardi U43 | 2010 | P | 62 | 84 | 73 (53–83) | 69.4 | 100.0 | 15Gy × 3 | 112.5 | 28 (9–60.7) |
| Timmerman R8 | 2010 | P | 59 | 38 | 72 (48–89) | 80.0 | 100.0 | 18Gy × 3 | 151.2 | 34.4 (4.8–49.9) |
| Van Der Voort Van Zyp NC44 | 2010 | P | 39 | NA | 77 (55–87) | 58.9 | NA | 20Gy × 3 | 180 | 17 |
| Bral S45 | 2011 | P | 40 | 83 | 73 (54–86) | 65 | 100 | 20Gy × 3; 15Gy × 4 | 180; 150 | 16 (5–33) |
| Lanni TB46 | 2011 | R | 45 | 40 | 76 (63–90) | 71 | 100 | 12Gy × 4; 12Gy × 5 | 105.6; 140 | 36 |
| Matsuo Y47 | 2011 | R | 101 | 73 | 77 (62–87) | 58.9 | 100.0 | 12Gy × 4 | 105.6 | 31.4 (4.2–118) |
| Nath SK48 | 2011 | R | 58 | 63 | 79 (60–88) | 58.9 | 100.0 | 10Gy × 5; 12Gy × 4; 13Gy × 4 | 100; 105.6; 119.6 | 17 (4–42) |
| Turzer M49 | 2011 | R | 36 | 28 | 74 (54–85) | 53 | 73.7 | 15Gy × 3 | 112.5 | 13.8 (0–21) |
| Widder50 | 2011 | P | 202 | 73 | 76 (46–93) | NA | 29 | 20Gy × 3; 12Gy × 5; 7.5Gy× 8 | 180; 140; 105 | 13 |
| Senthi S6 | 2012 | R | 676 | 61 | 73 (47–92) | 56 | 35 | 20Gy × 3 or 18Gy × 3; 12Gy × 5 or 11Gy × 5; 7.5Gy × 8 | 151·2; 115·5; 105 | 32.9(14.9–50.9) |
| Taremi M51 | 2012 | P | 108 | 49 | 73 (48–90) | 79.6 | 69.4 | 20Gy × 3; 18Gy × 3; 12Gy × 4; 7.5Gy× 8; 5Gy × 10 (9.6%) | 180; 151.2; 105. 6; 105; 75 | 19.1 (1–55.7) |
Note: BED10: biological equivalent dose with α/β=10; F/U (mo): follow-up period in months; Path (%): percentage of disease with pathological confirmation; P: prospective study; R: retrospective study; NA: not reported or not specified
Local control for medically inoperable Stage I NSCLC
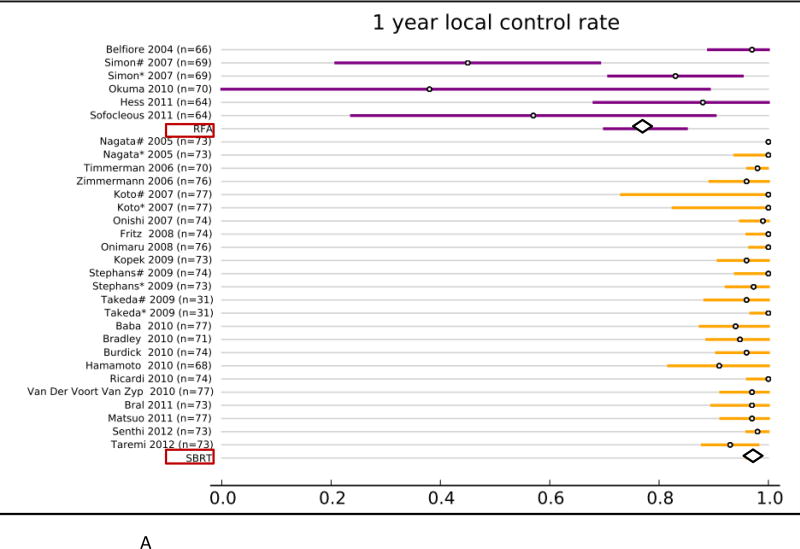
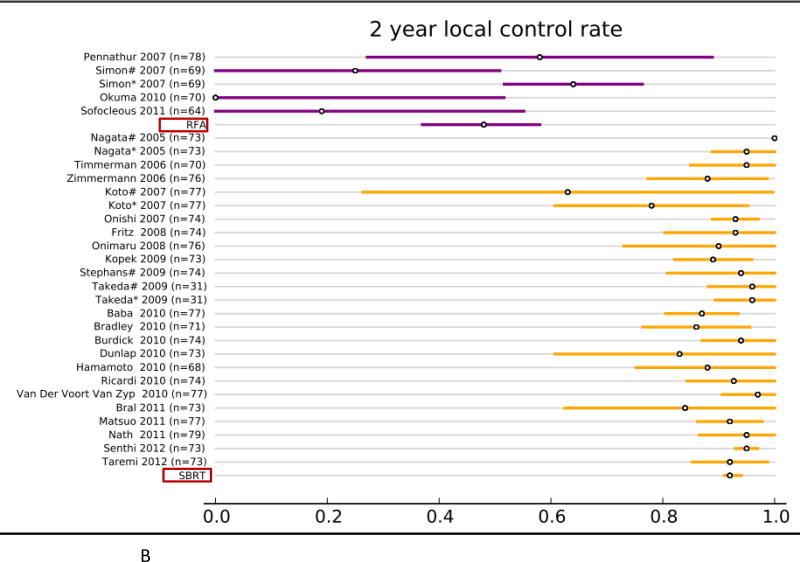
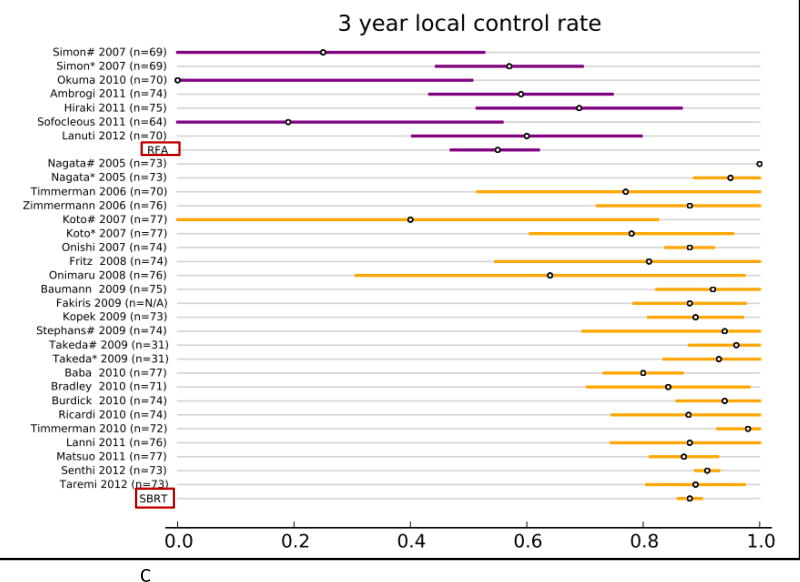
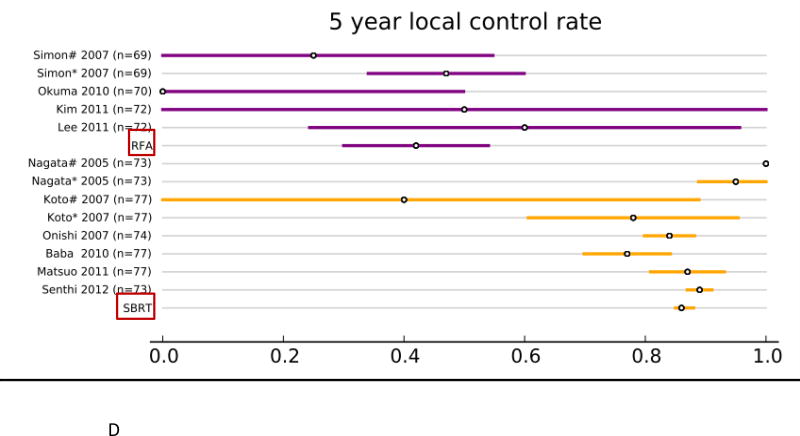
Forty studies reported local control rates (LCR, Fig. 1 and Table. 3). In the RFA group, 5 studies (142 patients) reported 1-year LCR, 4 studies (113 patients) reported 2-year LCR, 6 studies (248 patients) reported 3-year LCR, and 4 studies (106 patients) reported 5-year LCR. In the SBRT group, 20 studies (2,107 patients) reported 1-year LCR, 22 studies (2,137 patients) reported 2- year LCR, 21 studies (2,151 patients) reported 3-year LCR, and 6 studies (1,192 patients) reported 5-year LCR. The results of the fixed effect pooled analysis on LCR are presented in Table 3. The 1-, 2-y, 3-, 5-year LCR estimates and their 95%CI were 77% (70–85%), 48% (37–58%), 55% (47– 62%), 42% (30–54%) for RFA, and 97% (96–98%), 92% (91–94%), 88% (86– 90%), 86% (85–88%) for SBRT, respectively. The uncorrected pooled 1-, 2-, 3-, 5-year LCR for RFA were all significantly lower than that for SBRT (P-values all <.001).
Table 3.
Results of Meta-analysis for Local Control Rate (LCR)
| SBRT | RFA | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| No. of study |
LCR | 95% CI | No. of study |
LCR | 95% CI | P value | P value* | |
| 1 year | 20 | 0.97 | 0.96–0.98 | 5 | 0.77 | 0.70–0.85 | < .001 | < .001 |
| 2 year | 22 | 0.92 | 0.91–0.94 | 4 | 0.48 | 0.37–0.58 | < .001 | < .001 |
| 3 year | 21 | 0.88 | 0.86–0.90 | 6 | 0.55 | 0.47–0.62 | < .001 | < .001 |
| 5 year | 6 | 0.86 | 0.85–0.88 | 4 | 0.42 | 0.30–0.54 | < .001 | .04 |
Corrected by age and percentage of stage IA; LCR: local control rate; SBRT: stereotactic body radiation therapy; RFA: radiofrequency ablation; CI: confidence interval.
Overall survival for medically inoperable Stage I NSCLC
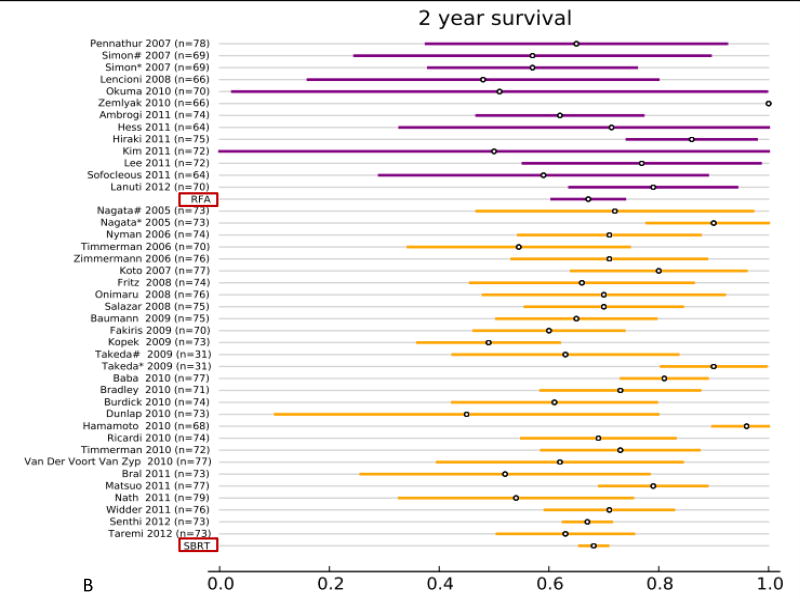
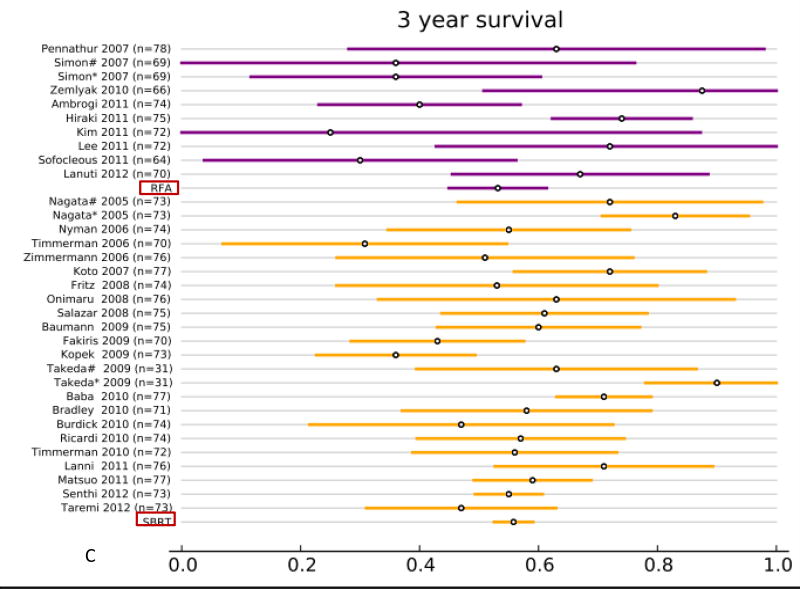
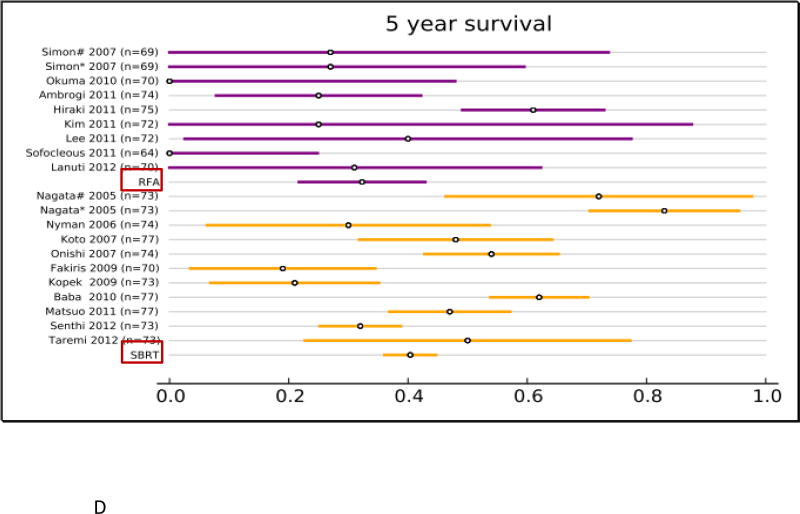
All studies reported overall survival (OS, Fig. 2 and supplemental Table. S1). In the RFA group, 12 studies (313 patients) reported 1-year OS, 12 studies (295 patients) reported 2-year OS, 9 studies (240 patients) reported 3-year OS, and 8 studies (216 patients) reported 5-year OS. In the SBRT group, 27 studies (2,467 patients) reported 1-year OS, 26 studies (2,377 patients) reported 2-year OS, 21 studies (2,003 patients) reported 3-year OS, and 10 studies (1,503 patients) reported 5-year OS. The results of the fixed effect pooled analysis on OS are presented in Table S1. The 1-, 2-y, 3-, 5-year OS estimates and their 95% CI were 85% (80–89%), 67% (61–74%), 53% (45– 61%), 32% (22–43%) for RFA, and 85% (84–87%), 68% (66–70%), 56% (53– 59%), 40% (36–45%) for SBRT. Neither treatment had significantly different uncorrected 1-, 2-, 3- and 5-year OS (P-values all >.05).
Figure 2.
Overview of local control rate (LCR) and 95% confidence intervals for all studies and pooled estimates. A. LCR at 1 year. B. LCR at 2 years. C LCR at 3 years. D. LCR at 5 years.
Comparison between RFA and SBRT: Regression analysis
Regression analysis was conducted to adjust for the different influence of clinical factors on effectiveness of different treatment modalities. After adjusting for age and percentage of stage IA, the corrected pooled 1-, 2-, 3-, and 5-year LCR for SBRT were significantly higher than that for RFA (P-values <.05, respectively; Table. 3). RFA and SBRT therapy did not have statistically significantly different 1-, 2-, 3-, and 5-year OS (Table. S1).
Occurrence of adverse events
The total occurrence of each adverse event per treatment modality, as well as the number of patients at risk, is listed in supplemental Table S2. Overall, both RFA and SBRT studies reported limited severe adverse events. The most frequent complication of RFA was pneumothorax (Grade ≥1), which reported in 31% (95%CI: 19–43%) patients. Severe pneumothorax that required intervention (Grade ≥3) occurred in 13% (95%CI: 0–27%) of patients. The most frequent grade 3 or greater toxicity for SBRT was radiation pneumonitis (RP), occurring in 2% of patients (95%CI: 1–4%). The second frequent toxicity was rib fracture, occurring in 2% of patients (95%CI: 1–3%). The incidence of severe (grade ≥3) acute esophageal toxicity, however, was uncommon. Several studies also reported cases of grade 3/4 adverse events, but because they did not specify the types of adverse events, these were not incorporated into the count.
Discussion
To our knowledge, this is the first systemic review and pooled analysis to compare the efficacy and morbidity of RFA and SBRT for medically inoperable early stage NSCLC. A total of 44 studies, 13 for RFA and 31 for SBRT, were used in the comparison. The 1-, 2-, 3-, and 5-year LCRs, corrected for differences in each study’s proportions of stage IA and age, were significantly higher after SBRT than that after RFA treatment. The OS rates for RFA and SBRT were comparable. Both SBRT and RFA reported limited risk of severe toxicity.
A number of literature reviews that separately addressed the effectiveness of SBRT and RFA were published recently.[10,33,41,51,63] However no actual pooled analysis has been published until now. Recently, Bial et al performed an English literature review to compare RFA with SBRT in patients with early stage medically inoperable NSCLC.[6] A total of 16 studies (9 studies for RFA and 7 studies for SBRT) were used to answer this question. They found that the OS at 1 year (68.2–95% vs. 81–85.7%) and 3 years (36–87.5% vs. 42.7– 56%) was similar between patients treated with RFA and SABR, while 5-year OS was higher in SBRT (47%) than RFA (20.1–27%). Local progression rates were lower in patients treated with SBRT (3.5–14.5% vs. 23.7–43%). These results were different from the pooled estimates presented in the current study, showing better local control without significant difference in 5-year survival.. The difference might be explained by two main factors. First, Bial et al. did not use a statistical approach to analyze all the data. Second, our analysis included more studies, and especially recently published studies. We utilized 8 studies for RFA and 10 studies for SBRT which reported 5-year OS results, while only 2 RFA studies and 1 SBRT study were included in Bial’s review. A cost-effectiveness analysis published by Sher et al. compared conventional RT, SBRT, and RFA for medically inoperable Stage I NSCLC.[47] They developed a Markov model to describe health states of 65-year-oldmen with medically inoperable NSCLC after treatment with 3D-CRT, SBRT, and RFA. They found that SBRT is the most cost-effective treatment for medically inoperable Stage I NSCLC. The incremental cost-effectiveness ratio for SBRT over 3D-CRT was $6,000/quality-adjusted life-year, and the incremental cost-effectiveness ratio for SBRT over RFA was $14,100/quality-adjusted life-year. However, these results were based on a wide range of assumptions, including the efficacy of each treatment modality. For example, the local recurrence rates they used for RFA treatment was only derived from one retrospective study published by Simon et al.[49] The 3-year LCR for RFA in that study was only 43%, which was significantly lower than pooled result (55%, 95%CI: 47–62%) from this study. The possible explanation of this difference is that the study by Simon et al. included not only primary early stage NSCLC but also metastatic lung cancer (more than 40%). Since the local recurrence risk of RFA is one key variable that could change the outcome of cost-effectiveness analysis, our pooled estimate results on SBRT and RFA might be helpful to improve the predictive accuracy of their model.
A strong correlation between the size of the targeted tumors and the RFA treatment results has been reported. Higher rates of relapse were noted in tumors larger than 2–4 cm. Simon et al. reported on 75 patients with stage I NSCLC that were treated with RFA and followed for 5 years; the 5-year progression-free survival (PFS) was significantly higher in tumors less than 3 cm (47%) compared to larger tumors (25%).[49] Okuma et al. identified that the significant risk factor for local progression after RFA was tumor size ≥ 2 cm (HR 1.5, 95% CI: 1.2–2.0),[37] while Huang et al. indicated that significant difference in the risk of local progression was found in tumors more than 4 cm.[20] A recent study including 55 ablations in 45 patients with stage I NSCLC reported more local failures in tumors more than 3 cm (80%), compared with lesions < 3cm (29%).[27] All these evidence suggested that the difference in the percentage of small tumor (<3cm, stage IA patients) between the study populations might significantly influence the outcome. Therefore, in the present study, we performed a regression analysis and found that after correcting for differences in tumor size (the percentage of stage IA patients), the local tumor control rates at 1-, 2-, 3- and 5-year were still significantly better for SBRT compared with RFA (P<.001 at 1 year, 2 years and 3 years; P=.04 at 5 years).
Although the local tumor control rate was significantly higher in patients treated with SBRT, the pooled estimates of OS between SBRT and RFA were similar. These results were consistent with a recent prospective study,[43] which included 116 patients treated with sublobar resection (n = 42), RFA (n = 25), or radiotherapy (n = 49). After adjusting for age and tumor size, a significant difference in the PR rate was observed, but no significant differences in OS. The comparison of long-term survival outcomes assessing alternate therapies for high-risk patients is challenging because the comorbidity and mortality rates are high. Evidence showed that even without any cancer, the median 5-year OS for patients with severe COPD was only about 40%.[59] As many as one-third of medically inoperable patients with NSCLC would die from comorbid conditions rather than cancer.[51] It has been suggested that for medically inoperable patients with NSCLC, local tumor control and relapse-free survival might be more valid endpoint than overall survival. [41]
Though our study demonstrated that the local tumor control was significantly better for SBRT than for RFA treatment, there are certainly a number of limitations. First, this is a systemic review and pooled analysis based on observational studies. When combining observational studies, heterogeneity of populations, design, and outcome are expected, and these differences may influence the pooled estimates. However, despite these challenges, when no RCTs or retrospective studies directly comparing RFA and SBRT are available, a systemic review and pooled analysis of observational studies might be a valid method for assessing efficacy and effectiveness, and could provide useful evidence to inform the decision whether more evidence is needed. Moreover, a regression analysis could correct for some potential differences between study populations or designs. Second, most RFA studies were small case series with relatively short follow up, which led to wide variance of LCR and OS estimated by formula. Four RFA studies were newly published.[2,12,31,43], while we were preparing this manuscript. A local progression rate of more than 20%, 1y-LCR 68.9%, and 2y-LCR 59.8% were reported, which were similar to our pooled results. Thirdly, definitions of local progression are not always consistent across the published reports. The wide range (3% to 50%) of local tumor progression after earlier RFA studies may be partially explained by these variations. Last, we did not look at trials presented only in their abstract form or at unpublished studies, nor at studies without separate data for early stage NSCLC patients. This emphasizes the importance that authors should fully document the characteristics of the study populations in their papers.
In conclusion, this systemic review and pooled analysis demonstrated that SBRT provided superior 1-, 2-, 3- and 5-year local tumor control over RFA, even when corrected for patients age and tumor size ≤ 3 cm. Therefore, at present, SBRT is still the most effective local treatment for inoperable Stage I NSCLC patients, and RFA should be offered only if patients are not candidates for SBRT. However, caution should be taken due to the relatively limited number of RFA trials and short follow-up time. More studies with larger sample sizes for RFA treatment are needed.
Supplementary Material
Figure 3.
Overview of overall survival (OS) and 95% confidence intervals for all studies and pooled estimates. A. OS at 1 year. B. OS at 2 years. C OS at 3 years. D. OS at 5 years.
Summary.
A systemic review and pooled analysis was performed to compare SBRT and RFA in inoperable stage I NSCLC. A comprehensive literature search for published trials from last 15 years was undertaken. LCR at 1, 2, 3 and 5 years for RFA was significantly lower than that for SBRT (P<.001). These differences remained significant after correcting for stage IA and age. However, caution is warranted due to the limited number of RFA studies.
Acknowledgments
Grant support: This work was funded in part by NIH-R21CA127057 and NIH-R01 CA142840.
We thank Jeffrey Campbell for English editing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceived and designed the experiments: NB FK. Performed the literature search: NB XZ. Analyzed the data: NB KS. Wrote the paper: NB KS FK.
This study was presented in part at the 54th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, Boston, MA, Oct 28–31, 2012.
References
- 1.Ambrogi MC, et al. Long-term results of radiofrequency ablation treatment of stage i non-small cell lung cancer: A prospective intention-to-treat study. J Thorac Oncol. 2011;6:2044–2051. doi: 10.1097/JTO.0b013e31822d538d. [DOI] [PubMed] [Google Scholar]
- 2.Ambrogi MC, et al. Wedge resection and radiofrequency ablation for stage i nonsmall cell lung cancer. Eur Respir J. 2015;45:1089–1097. doi: 10.1183/09031936.00188014. [DOI] [PubMed] [Google Scholar]
- 3.Baba F, et al. Clinical outcomes of stereotactic body radiotherapy for stage i non-small cell lung cancer using different doses depending on tumor size. Radiat Oncol. 2010;5:81. doi: 10.1186/1748-717X-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann P, et al. Outcome in a prospective phase ii trial of medically inoperable stage i non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 5.Belfiore G, et al. Ct-guided radiofrequency ablation: A potential complementary therapy for patients with unresectable primary lung cancer--a preliminary report of 33 patients. AJR Am J Roentgenol. 2004;183:1003–1011. doi: 10.2214/ajr.183.4.1831003. [DOI] [PubMed] [Google Scholar]
- 6.Bilal H, et al. Is radiofrequency ablation more effective than stereotactic ablative radiotherapy in patients with early stage medically inoperable non-small cell lung cancer? Interact Cardiovasc Thorac Surg. 2012;15:258–265. doi: 10.1093/icvts/ivs179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley JD, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: The pattern of failure is distant. Int J Radiat Oncol Biol Phys. 2010;77:1146–1150. doi: 10.1016/j.ijrobp.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Bral S, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: Results of a phase ii trial. Int J Radiat Oncol Biol Phys. 2011;80:1343–1349. doi: 10.1016/j.ijrobp.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 9.Burdick MJ, et al. Maximum standardized uptake value from staging fdg-pet/ct does not predict treatment outcome for early-stage non-small-cell lung cancer treated with stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1033–1039. doi: 10.1016/j.ijrobp.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 10.Das M, et al. Alternatives to surgery for early stage non-small cell lung cancer-ready for prime time? Current treatment options in oncology. 2010;11:24–35. doi: 10.1007/s11864-010-0119-z. [DOI] [PubMed] [Google Scholar]
- 11.Dunlap NE, et al. Size matters: A comparison of t1 and t2 peripheral non-small-cell lung cancers treated with stereotactic body radiation therapy (sbrt) J Thorac Cardiovasc Surg. 2010;140:583–589. doi: 10.1016/j.jtcvs.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 12.Dupuy DE, et al. Radiofrequency ablation of stage ia non-small cell lung cancer in medically inoperable patients: Results from the american college of surgeons oncology group z4033 (alliance) trial. Cancer. 2015;121:3491–3498. doi: 10.1002/cncr.29507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupuy DE, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–59. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- 14.Efron B. Bootstrap methods: Another look at the jackknife. The Annals of Statistics. 1979;7:1–26. [Google Scholar]
- 15.Fakiris AJ, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase ii study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 16.Fritz P, et al. Stereotactic, high single-dose irradiation of stage i non-small cell lung cancer (nsclc) using four-dimensional ct scans for treatment planning. Lung Cancer. 2008;60:193–199. doi: 10.1016/j.lungcan.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Hamamoto Y, et al. Local control of metastatic lung tumors treated with sbrt of 48 gy in four fractions: In comparison with primary lung cancer. Japanese journal of clinical oncology. 2010;40:125–129. doi: 10.1093/jjco/hyp129. [DOI] [PubMed] [Google Scholar]
- 18.Hess A, et al. Pulmonary radiofrequency ablation in patients with a single lung: Feasibility, efficacy, and tolerance. Radiology. 2011;258:635–642. doi: 10.1148/radiol.10100771. [DOI] [PubMed] [Google Scholar]
- 19.Hiraki T, et al. Percutaneous radiofrequency ablation of clinical stage i non-small cell lung cancer. J Thorac Cardiovasc Surg. 2011;142:24–30. doi: 10.1016/j.jtcvs.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, et al. Is radiofrequency thermal ablation a safe and effective procedure in the treatment of pulmonary malignancies? Eur J Cardiothorac Surg. 2011;39:348–351. doi: 10.1016/j.ejcts.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Kim SR, et al. Comparison between surgery and radiofrequency ablation for stage i non-small cell lung cancer. European journal of radiology. 2012;81:395–399. doi: 10.1016/j.ejrad.2010.12.091. [DOI] [PubMed] [Google Scholar]
- 22.Kopek N, et al. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;93:402–407. doi: 10.1016/j.radonc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Koto M, et al. A phase ii study on stereotactic body radiotherapy for stage i non-small cell lung cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2007;85:429–434. doi: 10.1016/j.radonc.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Lagerwaard FJ, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage i non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70:685–692. doi: 10.1016/j.ijrobp.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 25.Lagerwaard FJ, et al. Has 3-d conformal radiotherapy (3d crt) improved the local tumour control for stage i non-small cell lung cancer? Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2002;63:151–157. doi: 10.1016/s0167-8140(02)00009-9. [DOI] [PubMed] [Google Scholar]
- 26.Lanni TB, Jr, et al. Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. American journal of clinical oncology. 2011;34:494–498. doi: 10.1097/COC.0b013e3181ec63ae. [DOI] [PubMed] [Google Scholar]
- 27.Lanuti M, et al. Radiofrequency ablation for stage i non-small cell lung cancer: Management of locoregional recurrence. The Annals of thoracic surgery. 2012 doi: 10.1016/j.athoracsur.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 28.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 29.Lee H, et al. Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovascular and interventional radiology. 2011 doi: 10.1007/s00270-011-0194-y. [DOI] [PubMed] [Google Scholar]
- 30.Lencioni R, et al. Response to radiofrequency ablation of pulmonary tumours: A prospective, intention-to-treat, multicentre clinical trial (the rapture study) The lancet oncology. 2008;9:621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 31.Liu B, et al. Percutaneous radiofrequency ablation for medically inoperable patients with clinical stage i non-small cell lung cancer. Thorac Cancer. 2015;6:327–333. doi: 10.1111/1759-7714.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuo Y, et al. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:1104–1111. doi: 10.1016/j.ijrobp.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Mehta HJ, et al. Evaluation and treatment of high-risk patients with early-stage lung cancer. Clin Chest Med. 2011;32:783–797. doi: 10.1016/j.ccm.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Nagata Y, et al. Clinical outcomes of a phase i/ii study of 48 gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63:1427–1431. doi: 10.1016/j.ijrobp.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 35.Nath SK, et al. Locoregional and distant failure following image-guided stereotactic body radiation for early-stage primary lung cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2011;99:12–17. doi: 10.1016/j.radonc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Nyman J, Johansson KA, Hulten U. Stereotactic hypofractionated radiotherapy for stage i non-small cell lung cancer--mature results for medically inoperable patients. Lung Cancer. 2006;51:97–103. doi: 10.1016/j.lungcan.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Okuma T, et al. Determinants of local progression after computed tomography-guided percutaneous radiofrequency ablation for unresectable lung tumors: 9-year experience in a single institution. Cardiovascular and interventional radiology. 2010;33:787–793. doi: 10.1007/s00270-009-9770-9. [DOI] [PubMed] [Google Scholar]
- 38.Onimaru R, et al. Steep dose-response relationship for stage i non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:374–381. doi: 10.1016/j.ijrobp.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 39.Onishi H, et al. Hypofractionated stereotactic radiotherapy (hypofxsrt) for stage i non-small cell lung cancer: Updated results of 257 patients in a japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 40.Pennathur A, et al. Radiofrequency ablation for the treatment of stage i non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg. 2007;134:857–864. doi: 10.1016/j.jtcvs.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 41.Powell JW, et al. Treatment advances for medically inoperable non-small-cell lung cancer: Emphasis on prospective trials. The lancet oncology. 2009;10:885–894. doi: 10.1016/S1470-2045(09)70103-2. [DOI] [PubMed] [Google Scholar]
- 42.Ricardi U, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: Results of a prospective trial. Lung Cancer. 2010;68:72–77. doi: 10.1016/j.lungcan.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Safi S, et al. Sublobar resection, radiofrequency ablation or radiotherapy in stage i non-small cell lung cancer. Respiration. 2015;89:550–557. doi: 10.1159/000381555. [DOI] [PubMed] [Google Scholar]
- 44.Salazar OM, et al. Once-weekly, high-dose stereotactic body radiotherapy for lung cancer: 6-year analysis of 60 early-stage, 42 locally advanced, and 7 metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2008;72:707–715. doi: 10.1016/j.ijrobp.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 45.Scott WJ, et al. Treatment of non-small cell lung cancer stage i and stage ii: Accp evidence-based clinical practice guidelines. Chest. (2) 2007;132:234S–242S. doi: 10.1378/chest.07-1378. [DOI] [PubMed] [Google Scholar]
- 46.Senthi S, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: A retrospective analysis. The lancet oncology. 2012;13:802–809. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 47.Sher DJ, Wee JO, Punglia RS. Cost-effectiveness analysis of stereotactic body radiotherapy and radiofrequency ablation for medically inoperable, early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:e767–774. doi: 10.1016/j.ijrobp.2010.10.074. [DOI] [PubMed] [Google Scholar]
- 48.Sibley GS, et al. Radiotherapy alone for medically inoperable stage i non-small-cell lung cancer: The duke experience. Int J Radiat Oncol Biol Phys. 1998;40:149–154. doi: 10.1016/s0360-3016(97)00589-0. [DOI] [PubMed] [Google Scholar]
- 49.Simon CJ, et al. Pulmonary radiofrequency ablation: Long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 50.Sofocleous CT, et al. Pulmonary thermal ablation in patients with prior pneumonectomy. AJR Am J Roentgenol. 2011;196:W606–612. doi: 10.2214/AJR.10.5154. [DOI] [PubMed] [Google Scholar]
- 51.Stephans K. Stereotactic body radiotherapy for stage i non-small cell lung cancer. Cleve Clin J Med. 2012;79(Electronic Suppl 1):eS26–31. doi: 10.3949/ccjm.79.s2.06. [DOI] [PubMed] [Google Scholar]
- 52.Stephans KL, et al. A comparison of two stereotactic body radiation fractionation schedules for medically inoperable stage i non-small cell lung cancer: The cleveland clinic experience. J Thorac Oncol. 2009;4:976–982. doi: 10.1097/JTO.0b013e3181adf509. [DOI] [PubMed] [Google Scholar]
- 53.Takeda A, et al. Stereotactic body radiotherapy for primary lung cancer at a dose of 50 gy total in five fractions to the periphery of the planning target volume calculated using a superposition algorithm. Int J Radiat Oncol Biol Phys. 2009;73:442–448. doi: 10.1016/j.ijrobp.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 54.Taremi M, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: Prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys. 2012;82:967–973. doi: 10.1016/j.ijrobp.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 55.Timmerman R, et al. Excessive toxicity when treating central tumors in a phase ii study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 56.Timmerman R, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA : the journal of the American Medical Association. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turzer M, et al. Stereotactic body radiation therapy is effective and safe in patients with early-stage non-small cell lung cancer with low performance status and severe comorbidity. Case Rep Oncol. 2011;4:25–34. doi: 10.1159/000324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Voort van Zyp NC, et al. Quality of life after stereotactic radiotherapy for stage i non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2010;77:31–37. doi: 10.1016/j.ijrobp.2009.04.080. [DOI] [PubMed] [Google Scholar]
- 59.Vrdoljak E, et al. Survival analysis of untreated patients with non-small-cell lung cancer. Chest. 1994;106:1797–1800. doi: 10.1378/chest.106.6.1797. [DOI] [PubMed] [Google Scholar]
- 60.Widder J, et al. Survival and quality of life after stereotactic or 3d-conformal radiotherapy for inoperable early-stage lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:e291–297. doi: 10.1016/j.ijrobp.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 61.Wisnivesky JP, et al. Radiation therapy for the treatment of unresected stage i-ii non-small cell lung cancer. Chest. 2005;128:1461–1467. doi: 10.1378/chest.128.3.1461. [DOI] [PubMed] [Google Scholar]
- 62.Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage i non-small cell lung cancer. Journal of the American College of Surgeons. 2010;211:68–72. doi: 10.1016/j.jamcollsurg.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 63.Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Annals of surgical oncology. 2008;15:1765–1774. doi: 10.1245/s10434-008-9848-7. [DOI] [PubMed] [Google Scholar]
- 64.Zimmermann FB, et al. Stereotactic hypofractionated radiotherapy in stage i (t1–2 n0 m0) non-small-cell lung cancer (nsclc) Acta Oncol. 2006;45:796–801. doi: 10.1080/02841860600913210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.