Abstract
Meroterpenoid natural products are important bioactive molecules with broad distribution throughout nature. In Streptomyces bacteria, naphthoquinone-based meroterpenoids comprise a simple yet structurally fascinating group of natural product antibiotics that are enzymatically constructed through a series of asymmetric alkene and arene halofunctionalization reactions. This account article highlights our discovery and characterization of a group of vanadium-dependent chloroperoxidase enzymes that catalyze halogen-assisted cyclization and rearrangement reactions and have inspired biomimetic syntheses of numerous meroterpenoid natural products.
Keywords: biomimetic, haloperoxidase, α-hydroxyketone rearrangement, meroterpenoid, natural product biosynthesis
Graphical Abstract

1. Introduction
The architectural beauty of nature’s chemistry continues to motivate scientists to explore the logic of how exactly complex natural product molecules are constructed.1 Such biosynthetic studies have unraveled many of the remarkable mysteries behind how natural compounds are made2 and led to numerous discoveries and applications in chemistry and biology.3 The discovery of biosynthetic enzymes and pathways has inspired the development of new chemical reactions and reagents that have expanded the tools available for chemists and engineers to bring nature’s chemistry to life in a flask.
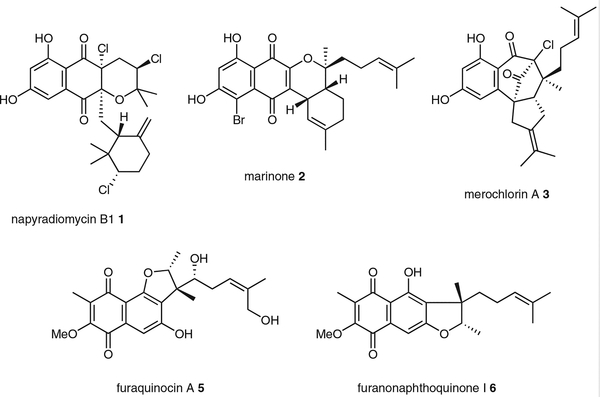
A group of molecules that has intrigued me for over a decade are simple polyketide naphthoquinone–terpenoid hybrid compounds, or meroterpenoids, produced by a variety of Streptomyces bacteria.4 These gene-encoded small molecules, such as the antimicrobial agents napyradiomycin B1 1,5 marinone 2,6 and merochlorin A 3,7 often harbor fascinating structural features suggestive of halofunctionalization reactions involving alkene and arene substrates (Figure 1). I’ve always marveled at the different strategies that nature invented to install halogens into natural products8 and wondered if these meroterpenoid molecules could teach us something new. Little did I know that after 10 years of working on the biosynthesis of these molecules that my laboratory would still be unraveling their secrets that have shown us that halogenating enzymes don’t simply just perform straightforward substitution reactions but also initiate remarkable bond-forming construction reactions. In this account article, I highlight several intertwining biosynthetic stories that have helped identify vanadium-dependent haloperoxidases (VHPOs) as a new group of bacterial halogenating enzymes9 and guided the logic of natural product total synthesis through biomimetic approaches.
Figure 1.
Structures of representative naphthoquinone-based meroterpenoids produced by Streptomyces bacteria
2. Early Biosynthetic Insights and the Characterization of Alkene Halofunctionalization in Napyradiomycin Biosynthesis
Amongst the first naphthoquinone-based meroterpenoid natural product groups that my laboratory interrogated were the napyradiomycins. These streptomycete compounds were first characterized in the mid 1980s,5 and new members continue to be reported from terrestrial soil and marine sediment strains because of their distinct chemical structures and biological properties in antimicrobial and anticancer assays.10 What caught our attention with this compound series were the cyclized terpene groups that were often halogenated with chlorine atoms. The placement of the halogen adjacent to the gem-dimethyl group of the precursor isoprene unit was suggestive of an oxidative halogenation reaction of the isoprene olefin resulting in an intramolecular ring cyclization with a nearby electron-rich oxygen or alkene. At that time in 2007, while there was no biosynthetic precedence in a bacterial system, an example enzymatic reaction had been established by the Butler group for the formation of the brominated snyderol terpenes in red algae by vanadium-dependent bromoperoxidases.11
To explore the route of napyradiomycin biosynthesis, we set out to identify the corresponding biosynthetic gene cluster (BGC) in the marine isolate Streptomyces sp. CNQ525 that my Scripps colleagues Bill Fenical and Paul Jensen had discovered.10c Earlier isotope labeling experiments by Shiomi et al. established the biosynthetic building blocks of the napyradiomycins being derived from the symmetrical polyketide 1,3,6,8-tetrahydroxynaphthalene (THN, 4) and two isoprene chains assembled by the mevalonate pathway (Scheme 1).12 We thus PCR-amplified THN synthase (THNS, a type III polyketide synthase) and prenyltransferase (PTase) gene fragments and identified a cosmid clone that fortuitously contained the majority of the 43-kb napyradiomycin BGC (nap).13 At that time in 2007, only two other THN meroterpenoid BGCs had been reported a year earlier by the Dairi and Heide groups for the structurally related furaquinocin A 514 and furanonaphthoquinone I 615, respectively. While all three THN meroterpenoid BGCs similarly contained genes encoding THNS, PTase, and a full complement of enzymes for mevalonate isoprene diphosphate biosynthesis, the nap BGC uniquely encoded three VHPOs. The co-clustering of VHPO genes within a natural product BGC was a novel observation at the time and would become the key to understanding the myriad of alkene and arene halofunctionalization reactions in napyradiomycin as well as many other meroterpenoid natural products.
Scheme 1.
Biosynthesis of THN 4 and the NapH1-catalyzed alkene halofunctionalization of SF2415B1 7 to napyradiomycin analogue SF2415B3 8
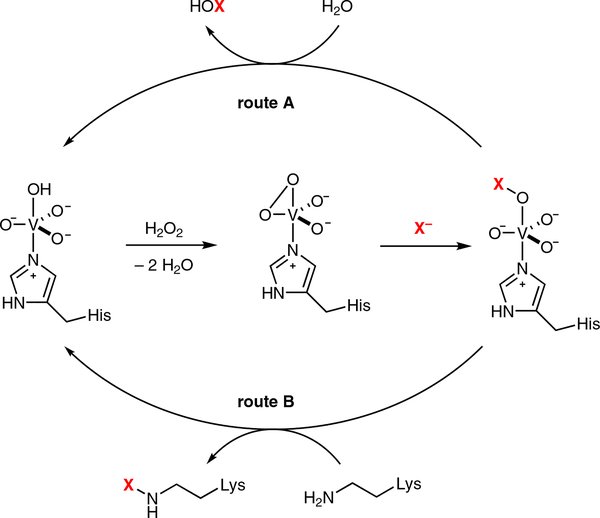
We validated the nap locus by heterologous production of the napyradiomycins in the host Streptomyces albus, which allowed us to begin investigating the biosynthetic mechanism of halogenation.13 The first nap-encoded VHPO we characterized was NapH1 in 2011.16 We confirmed that NapH1 behaved like a eukaryotic VHPO in its requirement of vanadate and H2O2 for catalysis, and demonstrated its unique specificity and catalytic efficiency in the asymmetric halogen-assisted cyclization of SF2415B1 7 to the napyradiomycin analogue SF2415B3 8 (Scheme 1). This selective and efficient alkene halofunctionalization enzymatic reaction preferred chloride as the halogenating reagent, as bromide additionally yielded nonspecific byproducts and, moreover, deactivated the enzyme over time. Our discovery and characterization of the NapH1 vanadium-dependent chloroperoxidase (VCPO) as the first highly substrate-selective VHPO in natural product asymmetric biosynthesis prompted us to explore its substrate binding and catalytic mechanism. Through strategic mutagenesis experiments of the NapH1 protein, we showed that substrate binding and haloperoxidase activities could be decoupled, thereby establishing that NapH1 must selectively bind its substrate to ensure the strategic delivery of the oxidized chlorine reagent (proposed either as HOX or an N-halo lysine residue) to the substrate olefin (Scheme 2). However, many unsolved questions still remain about the exact delivery mechanism that we hoped would be solved with a high-resolution structure of the NapH1 protein. Unfortunately, although we solved the three-dimensional structures of the homodimeric NapH1 with and without vanadate several years ago (PDB codes 3W36 and 3W35), we have yet to visualize how this enzyme positions its substrate prior to the delivery of the halogen.
Scheme 2.
Proposed catalytic scheme for the oxidation of halide ions by VHPO enzymes (adapted from references 9 and 31a). Hydrogen peroxide in the first step reacts with the histidine-ligated vanadate center to form a vanadium–peroxo complex that reacts in the second step with a halide to give a vanadium-OX species in which X = Cl, Br, and I. VHPO enzymes are widely proposed to generate a reactive HOX species upon reacting with water to restore the vanadate active site (route A). The stereoselective Streptomyces VHPOs, like NapH1 and Mcl24, are suspected to generate an enzyme-bound halogen species (route B).
3. Discovery of the Merochlorin Natural Products and Enzymatic Aryl Halofunctionalization
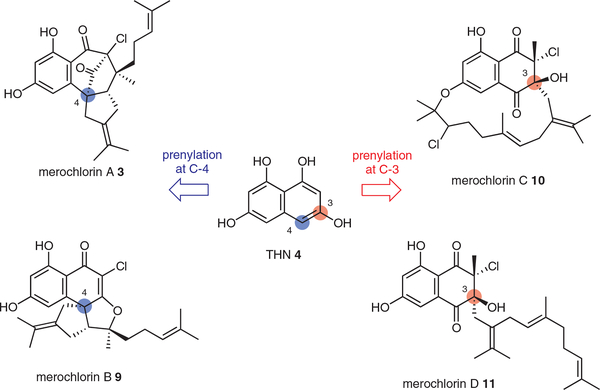
The discovery of the napyradiomycin VCPO NapH1 motivated us to explore other biosynthetic systems for new examples of VHPOs in biosynthesis. Such an opportunity presented itself the following year in 2012. My Scripps colleague Bill Fenical and his team were in the process of isolating a series of very unusual meroterpenoid antibiotics called the merochlorins from the marine sediment strain Streptomyces sp. CNH-189.7,17 Over the last dozen years since my move to the Scripps Institution of Oceanography at UC San Diego, the close physical proximity of our laboratories has allowed our research groups to share their emerging results and pursue joint projects merging our small-molecule discovery and biosynthesis programs.18 So with the discovery of merochlorin A 3, B 9, C 10, and D 11 as highly rearranged, chlorinated members of the naphthoquinone meroterpenoid family, we had the chance to discover and characterize new halogen-assisted cyclization reactions.
We quickly identified the merochlorin BGC (mcl) by genome scanning, which revealed that Streptomyces sp. CNH189 harbored a THN meroterpenoid gene locus with a single PTase and two VHPO-encoding genes.7 Through mutagenesis and heterologous expression, we confirmed that the mcl BGC controlled the biosynthesis of all merochlorin molecules, thereby establishing that despite their different carbon skeletons they all shared the same biogenesis. The merochlorins can first be divided into two structure groups in which the unusually branched C15 isoprene moiety is attached at either C-4 (3 and 9) or at C-3 (10 and 11) of the THN precursor (Figure 2). To account for the different terpene attachment sites in the two products, we proposed at the time, albeit incorrectly, that the lone PTase Mcl23 must have relaxed substrate specificity and deliver the terpene substrate to adjacent carbons of an oxidized THN molecule. While we later experimentally corrected this original theoretical proposal, we did show at the time of our original publication7 that the VHPO encoding gene mcl40 participates in the macrocyclization of 11 to 10. The asymmetric construction of the massive 15-membered cyclic ether ring in 10 represents, to the best of our knowledge, the largest natural olefin halofunctionalization reaction to result in a cyclized product, a remarkable feat in substrate control and stereoselectivity.
Figure 2.
Structures and biosynthetic grouping of merochlorins A–D
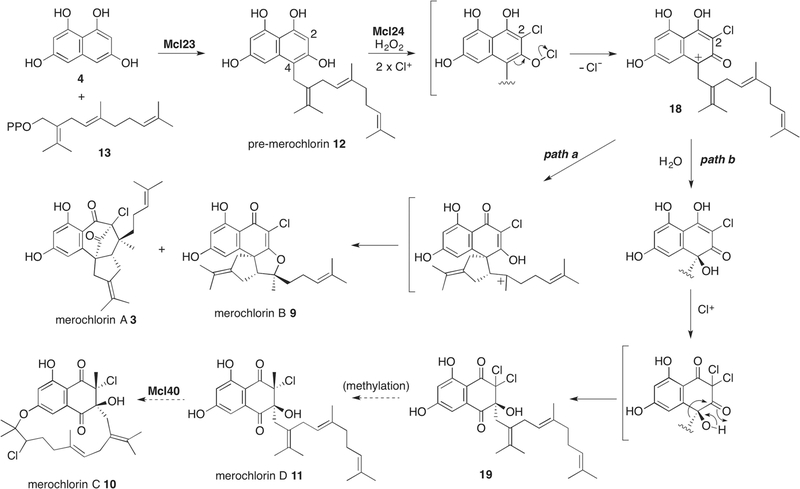
While we have not yet been able to functionally express the Mcl40 VCPO enzyme, as we did earlier with the napyradiomycin NapH1 VCPO,16 we did successfully characterize the second merochlorin biosynthesis VHPO encoded by mcl24, which resulted in an unanticipated observation.19 We originally hypothesized that Mcl24 would be a VCPO that would oxidatively chlorinate an alkene group of the precursor “pre-merochlorin” 12 intermediate to facilitate a series of cyclization reactions to give the multicyclic merochlorin A and B products.7 While somewhat true, what transpired turned out much more interesting.19
To interrogate the function of the Mcl24 VCPO, we fully recapitulated the biosynthesis of the merochlorins using recombinant enzymes in a one-pot enzymatic synthesis using just four enzymes (Scheme 3).19a We showed that premerochlorin 12 is biosynthesized by the coupling of the substrates THN 4 and the unprecedented sesquiterpene isosesquilavandulyl diphosphate 13 by the PTase Mcl23. Needing both 12 and 13 for our biochemical studies, we teamed up with Phil Baran and his student Matthew Villaume from the nearby Scripps Research Institute. Having access to those novel synthetic standards allowed us not only to confirm their biosynthetic intermediacy, but also provided valuable material to interrogate the enzymatic reactivity of these labile molecules.
Scheme 3.
Biosynthesis of the merochlorins. The conversion of 19 to merochlorin D 11 is proposed and that from 11 to merochlorin C 10 has not yet been confirmed enzymatically.
Remarkably, incubation of 12 with the VCPO Mcl24 directly gave 3 and 9 through a series of unprecedented biosynthetic transformations involving a site-specific naphthol chlorination followed by an oxidative dearomatization/terpene cyclization (Scheme 3).19 This sequence of reactions resulted in the construction of the stereochemically complex carbon framework of the merochlorin molecules in a single step. At the outset, we had not anticipated that the Mcl24 VCPO would be able to wholly catalyze this transformation as there was no literature precedent for secondary metabolic oxidative cyclization reactions involving an aromatic core. Yet despite identifying numerous oxidative enzyme-encoding genes in the merochlorin BGC, including an iron–sulfur cluster-containing protein, we clearly demonstrated that the Mcl24 VCPO solely performs both the oxidative cyclization and halogenation reactions.
We were curious if we could further develop a parallel chemical protocol to induce the oxidative cyclization of 12 through a chlorination event.19b We interrogated a number of standard chlorination protocols that mostly failed until we added an amine to the reaction mixture. Using N-chlorosuccinimide in the presence of either two equivalents of iPr2NH or NaH gave 30 or 22% yield, respectively, of the merochlorin product mixture (Scheme 4). Interestingly, although 3 and 9 were synthetically generated, they were in the minority. Rather, the primary reaction products were four new merochlorin molecules – deschloro-merochlorin B 14, isochloro-merochlorin B 15, deschloro-merochlorin A 16, and minor amounts of dichloro-merochlorin B 17. All these synthetic merochlorin molecules were also produced enzymatically from Mcl24 and naturally by Streptomyces sp. CNH-189, albeit in those cases, they were minor products and were thus not originally characterized due to sample limitations. The inverted reaction profiles suggested that while the enzyme appears to preferentially chlorinate at C-2 in pre-merochlorin prior to cyclization, the synthetic treatment of pre-merochlorin primarily leads to oxidative cyclization followed by C-7 chlorination. In fact, we could exclusively produce the deschloro-merochlorin A 16 and B 14 derivatives by conducting the chemical chlorination reaction at low temperature (–78 °C). Although we have yet to solve the molecular basis for the reactivity profile of the Mcl24 enzyme, we recently solved the high-resolution crystal structure of Mcl24 that we hope will shed light on its selective reactivity (V. Agarwal, S. Diethelm, B. S. Moore, unpublished).
Scheme 4.
Oxidative dearomatization through halofunctionalization of pre-merochlorin to merochlorins A, B, and analogues. NCS = N-chlorosuccinimide
The mechanistic rationale for the formation of merochlorin A, B and their derivatives is suggestive of the in situ generation of a chloramine intermediate as the active oxidant, both chemically with iPr2NH and biochemically with a nearby lysine residue.19b In the case of Mcl24, the product profiles suggest two halogenation events resulting in C-2 chlorination and an aromatic hypochlorite that upon loss of chloride would yield a benzylic carbocation intermediate 18 (Scheme 3). Finally, terpene cyclization of the carbocation via a common intermediate would give the merochlorin A and B scaffolds. Elegant total syntheses of 3 and 9 by the George20 and Trauner21 laboratories, respectively, similarly took advantage of biomimetic approaches and the inherent reactivity of this molecular class.
4. Discovery and Development of Unifying THN-Based Meroterpenoid Biosynthesis and Synthesis Approaches
One of the more perplexing and unsolved questions with many of the THN-based meroterpenoids was the common placement of the isoprene unit on electron-poor C-3 carbon centers of the THN precursor. For instance in the merochlorin series, while 3 and 9 have their isoprenes attached to the electron-rich C-4 of THN, in 10 the same isoprene group is rather attached at C-3 (Figure 2).7 This same C-3 attachment is observed in most other meroterpenoid natural products, including the napyradiomycins,5 marinone,6 and naphterpin.22 We recently organized the THN meroterpenoids into two structural classes depending on the attachment site of the isoprene chain to the THNderived naphthoquinone core with class I THN meroterpenoids having electron-rich C-2/C-4 isoprene attachments and class II’s with electron-poor C-3 attachments.23
A major breakthrough in solving the riddle of how electrophilic isoprene diphosphates are appended to the electron-deficient C-3 position of THN came with a key observation of an additional minor product of the VCPO Mcl24 enzymatic reaction. We observed that under basic reaction conditions a new Mcl24 product increased in amount, and that upon its isolation and characterization, we identified its structure as a novel C-3 prenylated derivative 19 that exhibited dichlorination at C-2.23 This unexpected finding suggested that the isoprene chain attached at C-4 in 12 migrates to the C-3 position (Scheme 2). This mechanism would necessitate the trapping of the previously proposed benzylic carbocation intermediate 18 with water followed by a second chlorination reaction at C-2 to give the geminal dichloride intermediate 19. To provide evidence for this mechanism, we performed the reaction in 18OH2 and showed that the new oxygen atom in the product was indeed water-derived. Finally, the C-4 prenylated intermediate is perfectly set up to undergo an α-hydroxyketone rearrangement to produce the C-3 substituted product.
The consequence that Mcl24 can additionally catalyze a halogenation-mediated α-hydroxyketone rearrangement, the first of its kind in nature, was that this remarkable enzyme is singularly responsible for generating the majority of the structural complexity and diversity in the entire merochlorin structural family (Scheme 3). Mcl24’s ability to bombard the prenylated THN nucleus with successive halogen atoms ultimately is what is responsible for exposing pre-merochlorin to the observed series of carbocationic cascade reactions. This biochemical discovery opened our eyes to halogenating enzymes performing not just simple substitution reactions but also supporting diverse bond construction reactions, much like how a chemist would use halogens to direct chemical reactivity. This discovery also suggested that this α-hydroxyketone rearrangement might be universally responsible for the C-3 attachment of isoprenes in all class II THN meroterpenoids.
Around this time we had another pleasant surprise. Synthetic chemist Jonathan George from the University of Adelaide, whose lab had earlier completed the biomimetic synthesis of merochlorin A,20 contacted me about a proposal to jointly explore napyradiomycin biosynthesis. His group had at the time recently established a new biomimetic synthesis of naphthomevalin 20 and predicted that their synthetic intermediates could be useful as chemical probes in our biosynthetic system.23 Remarkably, they invoked an α-hydroxyketone rearrangement reaction as a key step in their synthesis and wondered if such a reaction could be catalyzed by an enzyme. What a coincidence of timing! Our groups enthusiastically shared ideas and materials to explore whether the geranyl group in napyradiomycin does indeed result from an α-hydroxyketone rearrangement from C-4 to C-3 and whether one of the remaining and uncharacterized napyradiomycin VHPOs catalyzes the reaction like we had just observed with Mcl24.
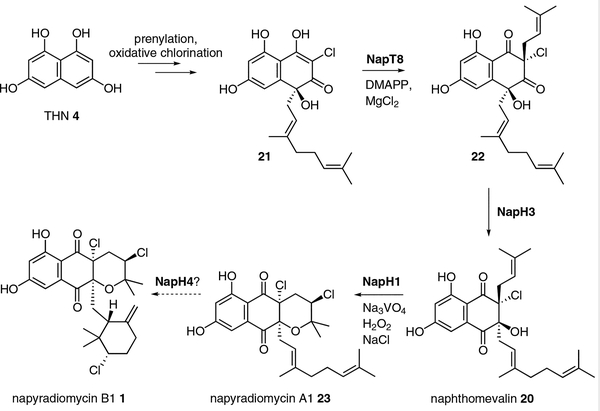
Thus armed with the knowledge of George’s naphthomevalin biomimetic synthesis and access to their unprotected synthetic intermediates, we set out to interrogate whether naphthomevalin is indeed biosynthesized through an α-hydroxyketone rearrangement reaction.23 The napyradiomycin BGC encodes two PTases, presumably for the selection and attachment of the two isoprene units. We thus first explored whether the C-4 geranylated substrate 21, a deprotected synthetic intermediate of the naphthomevalin synthetic pathway, was a substrate for one of the uncharacterized nap PTases. We showed that 21 was a substrate for the NapT8 PTase, which catalyzed the stereoselective addition of dimethylallyl pyrophosphate to the C-2 position of 21 to produce 22 (Scheme 5). We next evaluated whether one of the orphan nap VHPO homologues could catalyze an analogous α-hydroxyketone rearrangement of 22 to give the naphthomevalin 20 natural product. Such a reaction, however, would not require halogenation like in the merochlorin example as the naphthomevalin precursor 22 is already halogenated. We thus generated recombinant NapH3, a VCPO homologue with about 57% identity with the previously characterized NapH1, and showed that it efficiently supported the C-4 to C-3 α-hydroxyketone rearrangement to 20. Moreover, this enzyme did not require H2O2 or Na3VO4 for activity, nor was it active in standard haloperoxidase assays.
Scheme 5.
Enzymatic biosynthesis of naphthomevalin 20 and other napyradiomycins through an α-hydroxyketone rearrangement catalyzed by NapH3. The conversions of 4 to 21 and 23 to 1 have not yet been biochemically established. DMAPP = dimethylallyl pyrophosphate
These observations suggest that the merochlorin and napyradiomycin biosynthetic pathways share a central VCPO-assisted α-hydroxyketone rearrangement reaction but differ in how this reaction is catalyzed. In merochlorin, just a single VHPO (Mcl24) is needed to catalyze both the halogen-mediated oxidative dearomatization reaction and the α-hydroxyketone rearrangement reaction, while in napyradiomycin biosynthesis, this two-step, single enzyme process is more complex.23 While we have yet to report the halogenating enzyme responsible for the construction of the chlorinated intermediate 21, we have been able to show that the PTase NapT8 and the VHPO-homologue NapH3 are together required for its rearrangement. This observation was consistent with theoretical studies that showed that geminal disubstitution of C-2 is required for the α-hydroxyketone rearrangement to be thermodynamically favored. In contrast, the monochlorinated 21 was unreactive toward the 1,2-shift, as it possesses a conjugated π system, and hence the requirement of the PTase NapT8 to break the sp2 character of C-2 with the addition of the dimethyl allyl group and thus set up the 1,2-suprafacial shift mechanism for the α-hydroxyketone rearrangement.
A number of biosynthetic questions still remain about napyradiomycin, however, that involve additional halofunctionalization reactions. First, what halogenating enzyme is responsible for generating 21, and second, what enzyme catalyzes the asymmetric cyclization of the cyclohexyl ring in napyradiomycin B1 1 from the linear geranyl diene precursor, napyradiomycin A1 23? Both of these reactions have hallmarks of VHPO biochemistry, and with one additional nap VHPO still uncharacterized, at least one of these reactions is bound to be catalyzed by NapH4. We are actively pursuing these remaining questions and hope to soon report the total enzymatic synthesis of napyradiomycin B1.
5. Insights into Naphterpin and Marinone Biosynthesis Involving Cryptic Aryl Halofunctionalization Reactions
The merochlorin and napyradiomycin α-hydroxyketone rearrangement reactions also suggested that a similar 1,2-suprafacial shift mechanism may take place in the naphterpin 24 and marinone 2 natural products. These molecules have long intrigued me for their asymmetry about the THNderived naphthoquinone core.24 Years ago when I first encountered these compounds, I initially questioned whether their structures had been misassigned, as I assumed based on straightforward biosynthetic logic that they were derived directly from flaviolin or another similarly oxidized THN molecule. If so, this would simply necessitate the swapping of the C-2 and C-3 substituents and thereby place the ether oxygen atom at C-3 (instead of at C-2) where it is in all other THN-derived meroterpenoids. NMR spectroscopy would have a difficult time differentiating between those two structure types. The structure and absolute configuration of naphterpin 24, the maiden structure in this natural product series, was rigorously established by X-ray crystallography and is correct as originally published.22 Thus another more exotic biosynthetic explanation is needed.
Early isotope labeling experiments established the symmetrical nature of the biosynthetic intermediate leading to the common naphthoquinone core in naphterpin24 and marinone25 and suggested the intermediacy of THN. Unfortunately, the BGCs for both molecules have not yet been reported, although three genes from the naphterpin BGC were described in 2005 by Kuzuyama and colleagues, including a THN synthase and a PTase.26 The naphterpin PTase Orf2 (now renamed NphB27) was examined and shown to have relaxed substrate specificity toward various naphthols, flavonoids, and other phenols, yet THN was never evaluated as a substrate.26 In the case of marinone, the draft genome of the marinone-producing bacterium Streptomyces sp. CNQ-509 was recently reported28 and shown to contain a BGC with a THN synthase, PTase and, significantly, genes encoding VHPOs homologous to Mcl24 and NapH3 that catalyze α-hydroxyketone rearrangements.
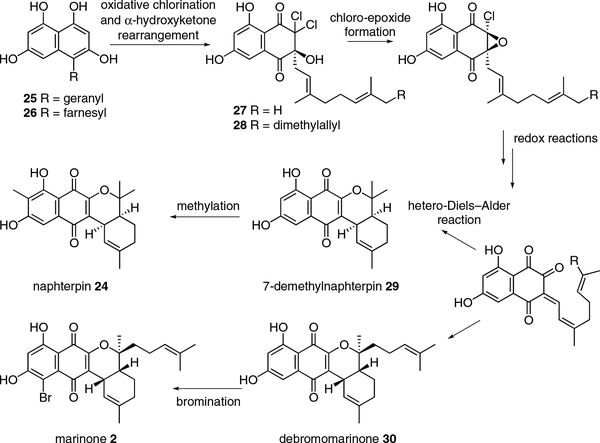
Thus, based on the established biochemistry of the merochlorin Mcl24 VCPO, we propose a similar reaction in the naphterpin and marinone biosynthetic pathways that only differ initially on the length of the terpene chain (Scheme 6). C-4 prenylation of THN with geranyl or farnesyl groups, like that in the construction of pre-merochlorin 12,23 would give intermediates 25 and 26 leading, respectively, to naphterpin and marinone. We propose that VCPO-catalyzed oxidative dearomatization and dichlorination at C-2 followed by α-hydroxyketone rearrangement would give the merochlorin analogues 27 and 28. We anticipate at this point a series of rearrangement and redox reactions to effectively remove both chlorine atoms and replace them with an enone would set up an intramolecular hetero-Diels–Alder reaction to construct the tetracyclic nucleus of 7-demethylnaphterpin 29 and debromomarinone 30.
Scheme 6.
Proposed biosynthesis of naphterpin and marinone
Recently, the George lab completed a simple, biomimetic synthesis of (±)-7-demethylnaphterpin 29 following this biosynthetic proposal.29 We are now actively working to-gether to interrogate the naphterpin and marinone biosynthetic systems to explore if VHPOs are indeed central to their biosyntheses and, if so, how many are involved in making the putative Diels–Alder substrate.
6. Closing Thoughts
Significant progress has been made over the past few years in establishing the biosynthetic logic of how the structurally diverse family of THN meroterpenoids is constructed at the genetic and biochemical levels. This work has taught us the central importance of VHPO enzymes in catalyzing a diversity of asymmetric alkene and arene halofunctionalization reactions involving cyclizations and rearrangements. Such reactions are presently difficult to control synthetically to achieve enantioselectivity and thus serve as guiding models for the synthetic and bioengineering communities. A major synthetic advance in asymmetric halofunctionalization of alkenes has taken place in recent years,30 thus promising new strategies and opportunities in the controlled addition of hyperreactive oxidized halogen atoms.
Little is known, however, about how meroterpenoid VHPO enzymes control the precise stereochemical addition of halonium ions to their electrophilic, organic substrates. These enzymes were once considered nonselective for their indiscriminate production and delivery of hypohalous acid to electron-rich substrates,9,31 yet their discovery from Streptomyces meroterpenoid BGCs changed that perception for their asymmetric properties.16 We suspect that VHPOs will eventually fall into two groups based on their ability to control or not control the stereoselective addition of reactive halogen ions to their substrates (Scheme 2, routes A and B). We anticipate that the Streptomyces VHPO enzymes have evolved a specialized mechanism to trap “Cl+” species once generated by the vanadate/H2O2 oxidant, perhaps as a chloroamine attached to an active site lysine residue, to facilitate its controlled delivery to a bound substrate. How this biochemical tango takes place and whether it can be hijacked to allow for the creation of engineered VHPOs as broad-use, asymmetric halogenating biocatalysts remains to be seen. This field is only just starting to take shape.
Acknowledgment
I am grateful to the many graduate students, postdocs, visiting scientists, and collaborators with whom I have had the pleasure of working with over the years on characterizing and exploiting the biosynthesis of meroterpenoid antibiotics. Their published work is cited throughout this article.
Funding Information
Research in B.S.M.’s laboratory on meroterpenoid natural products has been generously supported by NIH grant R01-AI047818.
Biography

Bradley S. Moore is Professor of Marine Chemical Biology at the Scripps Institution of Oceanography and Chair and Professor of Pharmaceutical Chemistry at the Skaggs School of Pharmacy and Pharmaceutical Sciences at UC San Diego. He holds degrees in chemistry from the University of Hawaii (B.S. 1988) and Washington (Ph.D. 1994), was a postdoc at the University of Zurich (1994–95), and held prior faculty appointments at the University of Washington (1996–99) and Arizona (1999–2005). Dr. Moore has published over 180 papers on the chemistry, biochemistry, and genetics of natural product drug leads and toxins from (primarily) marine microbes. His marine pharmaceutical biosynthesis and genomics research has been recognized through numerous international lectureships, awards, and recognitions, including Chair of the Natural Product Reports Editorial Board (since 2011), President of the American Society for Pharmacognosy (2013–14), and the Arthur C. Cope Scholar prize from the American Chemical Society (2013).
References
- (1).Walsh CT; Fischbach MA J. Am. Chem. Soc 2010, 132, 2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Li B;Yu JP; Brunzelle JS;Moll GN; van der Donk WA; Nair SK Science 2006, 311, 1464. [DOI] [PubMed] [Google Scholar]; (b) Freeman MF; Gurgui C; Helf MJ; Morinaka BI; Uria AR; Oldham NJ; Sahl HG; Masunaga S; Piel J Science 2012, 338, 387. [DOI] [PubMed] [Google Scholar]; (c) Teufel R; Miyanaga A; Michaudel Q; Stull F; Louie G; Noel JP; Baran PS; Palfey B; Moore BS Nature 2013, 503, 552. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Dutta S; Whicher JR; Hansen DA; Hale WA; Chemler JA; Congdon GR; Narayan AR; Hakansson K; Sherman DH; Smith JL; Skiniotis G Nature 2014, 510, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Gaudelli NM; Long DH; Townsend CA Nature 2015, 520, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zou Y; Garcia-Borras M; Tang MC; Hirayama Y; Li DH; Li L; Watanabe K; Houk KN; Tang Y Nat. Chem. Biol 2017, 13, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Nakamura H, Schultz EE, Balskus EP; Nat. Chem. Biol; 2017, in press; DOI: 10 1038/nchembio.2421 [DOI] [PubMed] [Google Scholar]
- (3).(a) Gao X; Xie X; Pashkov I; Sawaya MR; Laidman J; Zhang W; Cacho R; Yeates TO; Tang Y Chem. Biol 2009, 16, 1064. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Laureti L; Song L; Huang S; Corre C; Leblond P; Challis GL; Aigle B Proc. Natl. Acad. Sci. U.S.A 2011, 108, 6258. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mo S; Kim DH; Lee JH; Park JW; Basnet DB; Ban YH; Yoo YJ; Chen SW; Park SR; Choi EA; Kim E; Jin YY; Lee SK; Park JY; Liu Y; Lee MO; Lee KS; Kim SJ; Kim D; Park BC; Lee SG; Kwon HJ; Suh JW; Moore BS; Lim SK; Yoon YJ J. Am. Chem. Soc 2011, 133, 976. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Coelho PS; Brustad EM; Kannan A; Arnold FH Science 2013, 339, 307. [DOI] [PubMed] [Google Scholar]; (e) Eustáquio AS; Chang LP; Steele GL; O’Donnell CJ; Koehn FE Metab. Eng 2016, 33, 67. [DOI] [PubMed] [Google Scholar]
- (4).Kuzuyama T; Seto H Nat. Prod. Rep 2003, 20, 171. [DOI] [PubMed] [Google Scholar]
- (5).(a) Shiomi K; Nakamura H; Iinuma H; Naganawa H; Isshiki K; Takeuchi T; Umezawa HJ Antibiot. 1986, 39, 494. [DOI] [PubMed] [Google Scholar]; (b) Shiomi K; Nakamura H; Iinuma H; Naganawa H; Takeuchi T; Umezawa HJ Antibiot. 1987, 40, 1213. [DOI] [PubMed] [Google Scholar]
- (6).(a) Pathirana C; Jensen PR; Fenical W Tetrahedron Lett. 1992, 33, 7663. [Google Scholar]; (b) Hardt IH; Jensen PR; Fenical W Tetrahedron Lett. 2000, 41, 2073. [Google Scholar]
- (7).Kaysser L; Bernhardt P; Nam SJ; Loesgen S; Ruby JG; Skewes-Cox P; Jensen PR; Fenical W; Moore BS J. Am. Chem. Soc 2014, 136, 14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Agarwal V; Miles ZD; Winter JM; Eustáquio AS; El Gamal AA; Moore BS Chem. Rev 2017, 117, 5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Winter JM; Moore BS J. Biol. Chem 2009, 284, 18577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Gomi S; Ohuchi S; Sasaki T; Itoh J; Sezaki MJ Antibiot. 1987, 40, 740. [DOI] [PubMed] [Google Scholar]; (b) Fukuda DS; Mynderse JS; Baker PJ; Berry DM; Boeck LD J. Antibiot 1989, 43, 623. [Google Scholar]; (c) Soria-Mercado IE; Prieto-Davo A; Jensen PR; Fenical WJ Nat. Prod 2005, 68, 904. [DOI] [PubMed] [Google Scholar]; (d) Motohashi K; Sue M; Furihata K; Ito S; Seto HJ Nat. Prod 2008, 71, 595. [DOI] [PubMed] [Google Scholar]; (e) Wu Z; Li S; Li J; Chen Y; Saurav K; Zhang Q; Zhang H; Zhang W; Zhang S; Zhang C Mar. Drugs 2013, 11, 2113. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Farnaes L; Coufal NG; Kauffman CA; Rheingold AL; DiPasquale AG; Jensen PR; Fenical WJ Nat. Prod 2014, 77, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Cheng YB; Jensen PR; Fenical W Eur. J. Org. Chem 2013, 3751. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Lacret R; PérezVictoria I; Oves-Costales D; de la Cruz M; Domingo E; Martin J; Díaz C; Vicente F; Genilloud O; Reyes F Mar. Drugs 2016, 14, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Carter JN; Butler AJ Am. Chem. Soc 2004, 126, 15060. [DOI] [PubMed] [Google Scholar]
- (12).Shiomi K; Iinuma H; Naganawa H; Isshiki K; Takeuchi T; Umezawa HJ Antibiot. 1987, 40, 1740. [DOI] [PubMed] [Google Scholar]
- (13).Winter JM; Moffitt MC; Zazopoulos E; McAlpine JB; Dorrestein PC; Moore BS J. Biol. Chem 2007, 282, 16362. [DOI] [PubMed] [Google Scholar]
- (14).Kawasaki T; Hayashi Y; Kuzuyama T; Furihata K; Itoh N; Seto H; Dairi T J. Bacteriol. 2006, 188, 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Haagen Y; Glück K; Fay K; Kammerer B; Gust B; Heide L ChemBioChem 2006, 7, 2016. [DOI] [PubMed] [Google Scholar]
- (16).Bernhardt P; Okino T; Winter JM; Miyanaga A; Moore BS J. Am. Chem. Soc 2011, 133, 4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Sakoulas G; Nam SJ; Loesgen S; Fenical W; Jensen PR; Nizet V; Hensler M PLoS One 2012, 7, e29439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).(a) Udwary DW; Zeigler L; Asolkar RN; Singan V; Lapidus A; Fenical W; Jensen PR; Moore BS Proc. Natl. Acad. Sci. U.S.A 2007, 104, 10376. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Schultz AW; Oh DC; Carney JR; Williamson RT; Udwary DW; Jensen PR; Gould SJ; Fenical W; Moore BS J. Am. Chem. Soc 2008, 130, 4507. [DOI] [PubMed] [Google Scholar]; (c) McGlinchey RP; Nett M; Eustáquio AS; Asolkar RN; Fenical W; Moore BS J. Am. Chem. Soc 2008, 130, 7822. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Eustáquio AS; Nam SJ; Penn K; Lechner A; Wilson MC; Fenical W; Jensen PR; Moore BS ChemBioChem 2011, 12, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wilson MC; Nam SJ; Gulder TA; Kauffman CA; Jensen PR; Fenical W; Moore BS J. Am. Chem. Soc 2011, 133, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Kersten RD; Yang YL; Xu Y; Cimermancic P; Nam SJ; Fenical W; Fischbach MA; Moore BS; Dorrestein PC Nat. Chem. Biol 2011, 7, 794. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Xu Y; Kersten RD; Nam SJ; Lu L; Al-Suwailem AM; Zheng H; Fenical W; Dorrestein PC; Moore BS; Qian PY J. Am. Chem. Soc 2012, 134, 8625. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Lane AL; Nam SJ; Fukuda T; Yamanaka K; Kauffman CA; Jensen PR; Fenical W; Moore BS J. Am. Chem. Soc 2013, 135, 4171. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Awakawa T; Crüsemann M; Munguia J; Ziemert N; Nizet V; Fenical W; Moore BS ChemBioChem 2015, 16, 1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Diethelm S; Teufel R; Kaysser L; Moore BS Angew. Chem. Int. Ed 2014, 53, 11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (b).Teufel R; Kaysser L; Villaume MT; Diethelm S; Carbullido MK; Baran PS; Moore BS Angew. Chem. Int. Ed 2014, 53, 11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).(a) Pepper HP; George JH Angew. Chem. Int. Ed 2013, 52, 12170. [DOI] [PubMed] [Google Scholar]; (b) Pepper HP; George JH Synlett 2015, 26, 2485. [Google Scholar]
- (21).Meier R; Strych S; Trauner D Org. Lett 2014, 16, 2634. [DOI] [PubMed] [Google Scholar]
- (22).Shin-ya K; Imai S; Furihata K; Hayakawa Y; Kato Y; Vanduyne GD; Clardy J; Seto HJ Antibiot. 1990, 43, 444. [DOI] [PubMed] [Google Scholar]
- (23).Miles ZD, Diethelm S, Pepper HP, Huang DM, George JH, Moore BS; Nat. Chem; 2017, in press; doi: 10 1038/nchem.2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Shin-ya K; Furihata K; Hayakawa Y; Seto H Tetrahedron Lett. 1990, 42, 6025. [Google Scholar]
- (25).Kalaitzis JA; Hamano Y; Nilsen G; Moore BS Org. Lett 2003, 5, 4449. [DOI] [PubMed] [Google Scholar]
- (26).Kuzuyama T; Noel JP; Richard SB Nature 2005, 435, 983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kuzuyama TJ Antibiot. 2017, 70, 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).(a) Rückert C; Leipoldt F; Zeyhle P; Fenical W; Jensen PR; Kalinowski J; Heide L; Kaysser LJ Biotechnol. 2015, 216. [DOI] [PubMed] [Google Scholar]; (b) Leipoldt F; Zeyhle P; Kulik A; Kalinowski J; Heide L; Kaysser L PLoS One 2015, 10, e0143237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Murray LAM, Cruickshank MC, López-Pérez B, Sassnink SA, Pepper HP, Sumby CJ, Moore BS, George JH, unpublished.
- (30).(a) Denmark SE; Kuester WE; Burk MT Angew. Chem. Int. Ed 2012, 51, 10938. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hu DX; Shibuya GM; Burns NZ J. Am. Chem. Soc 2013, 135, 12960. [DOI] [PubMed] [Google Scholar]; (c) Soltanzadeh B; Jaganathan A; Staples RJ; Borhan B Angew. Chem. Int. Ed 2015, 54, 9517. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Landry ML; Hu DX; McKenna GM; Burns NZ J. Am. Chem. Soc 2016, 138, 5150. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Denmark SE; Ryabchuk P; Burk MT; Gilbert BB J. Org. Chem 2016, 81, 10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).(a) Weyand M; Hecht H-J; Kiess M; Liaud M-F; Vilter H; Schomburg D J. Mol. Biol 1999, 293, 595. [DOI] [PubMed] [Google Scholar]; (b) Dong JJ; Fernández-Fueyo E; Li J; Guo Z; Renirie R; Wever R; Hollmann F Chem. Commun 2017, 53, 6207. [DOI] [PubMed] [Google Scholar]