Abstract
Pseudo-oligosaccharides are microbial-derived secondary metabolites whose chemical structures contain pseudosugars (glycomimetics). Due to their high resemblance to the molecules of life (carbohydrates), most pseudo-oligosaccharides show significant biological activities. Some of them have been used as drugs to treat human and plant diseases. Because of their significant economic value, efforts have been put into understanding their biosynthesis, optimizing their fermentation conditions, and engineering their metabolic pathways to obtain better production yields. A number of unusual enzymes participating in diverse biosynthetic pathways to pseudo-oligosaccharides have been reported. Various methods and conditions to improve the production yields of the target compounds and eliminate byproducts have also been developed. This review article describes recent studies on the biosynthesis, fermentation optimization, and metabolic engineering of high-value pseudo-oligosaccharides.
Introduction
Among the myriad of naturally occurring compounds, sugars play many important roles in biological systems. They are involved in both the morphology and physiological processes in all organisms, including bacteria, as well as viruses. Sugar association in biological systems is limitless, from components of bacterial cell walls (e.g., peptidoglycans and lipopolysaccharides), to signaling molecules for cellular functions (e.g., glycoproteins, glycolipids), to the genetic materials DNA and RNA. Sugars are involved in cell-cell adhesion (the basis for inflammation and cancer metastasis), cell recognition (e.g., viral entry), biofilm formation, energy generation, and many other processes.
In nature, many bacteria, particularly the actinomycetes, produce secondary metabolites whose chemical structures closely resemble sugars (glycomimetics) (1). These natural products normally contain one or more six-membered ring structures similar to pyrano-sugars (glucomimetics), or five-membered ring structures similar to furano-sugars (ribomimetics). The natural glycomimetics, also known as pseudosugars or cyclitols, include the aminoglycoside antibiotics (e.g., kanamycin, neomycin, and gentamicin) (2), the pseudo-oligosaccharides (e.g., acarbose, validamycins, amylostatins, and trestatins) (1), and the aminocyclopentitol pactamycin (3). As sugars are important components of living organisms, sugar mimetics such as these usually show significant biological activities.
The pseudo-oligosaccharides are oligosaccharides in which one or more of their sugar moieties have been replaced with pseudosugar (cyclitol) units. These pseudosugars include valienamine, an unsaturated aminocyclitol, and its epoxy, hydroxy, or dihydro forms. While the function(s) of pseudo-oligosaccharides in nature remain elusive, a number of them have been used to treat human and plant diseases. For example, the pseudo-tetrasaccharide acarbose, a potent α-glucosidase inhibitor produced by an Actinoplanes sp. soil bacterium, is clinically used to treat type 2 non-insulin-dependent diabetes mellitus (NIDDM) (4). This pseudosugar-containing oligosaccharide reduces the blood glucose level by slowing down the release of glucose from polysaccharides or more complex carbohydrates in the small intestines.
Another pseudo-oligosaccharide, validamycin A, is one of the most successful bacterial-derived antifungal agents used to treat sheath blight disease in rice plants. It is produced by Streptomyces hygroscopicus spp. and has strong inhibitory activity toward trehalase enzymes (5, 6). Fungi rely on trehalose (α-d-glucopyranosyl-(1→1)-α-d-glucopyranoside) for energy storage and use trehalase to hydrolyze trehalose to two units of glucose for growth and other cellular functions. In addition to its use as a crop protectant, validamycin A is the precursor of the antidiabetic drug voglibose (7). Thus, validamycin A has been an important commodity in both the pharmaceutical and agricultural industries. In China alone, about 40,000 tons of validamycin are produced annually (8).
Following the commercial success of acarbose and validamycin A, significant efforts have been put into screening for new pseudosugar-producing microorganisms, developing synthetic strategies to produce the compounds, optimizing fermentation conditions, and engineering the biosynthesis or metabolic pathways in the producing bacteria to obtain better production yields. A number of review articles have been published to describe their discovery, biological activity, chemical synthesis, and biosynthesis (1, 9–13). Here, we focus on more recent reports on the biosynthesis, fermentation optimization, and metabolic engineering of pseudo-oligosaccharides, particularly those with significant economic value.
Pseudo-oligosaccharide natural products
Since the discovery of acarbose in the early 1970s, research on this class of natural products has continued to grow. As a result, many acarbose analogues have been identified (1). Among them are the amylostatins, the trestatins, and the oligostatins, which were isolated from the fermentation broths of Streptomyces diastaticus subsp. amylostaticus (14, 15), Streptomyces dimorphogenes (16), and Streptomyces myxogenes (17), respectively. Similar to acarbose, these compounds contain an acarviosin core residue (valienamine and 6-deoxyglucose) or its derivatives (Figure 1), which are responsible for their α-glucosidase inhibitory activity. Other acarbose analogues include the adiposins (produced by Streptomyces calvus) (18), salbostatin (produced by Streptomyces albus ATCC 21838) (19, 20), the acarviostatins (produced by Streptomyces coelicoflavus ZG0656) (21), and the oxirane pseudo-oligosaccharides (e.g., NS complex, W-46 complex, CK-4416, CKD-711, and CKD-711a) (1). Some of them have also been isolated as butyl or isovaleryl esters (22, 23). While the exact function(s) of pseudo-oligosaccharides in nature remains unclear, some reports have suggested that acarbose plays a role as a “carbophore”, which acts as a sugar transport vehicle by cycling in and out between intra and extracellular spaces of bacteria (Figure 2) (24, 25). Due to its strong glycosidase inhibitory activity, it may also inhibit the degradation of starch or maltooligosaccharides by other microorganisms and provide a competitive advantage for the slow-growing acarbose-producing bacteria.
Figure 1.

Chemical structures of select natural pseudo-oligosaccharides. m = 0~1; n = 0~2; p = 0~3; q = 1~3; r = 0~8; s = 0~5; t = 1~5.
Figure 2.
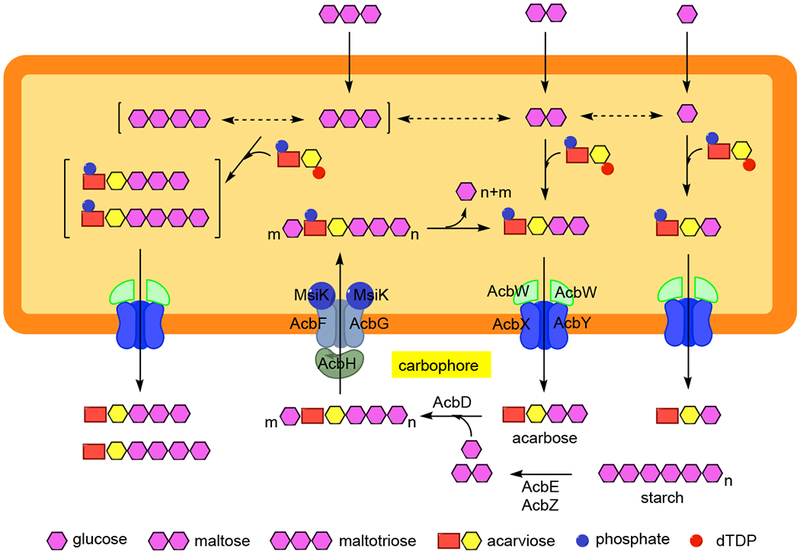
Carbon sources for acarbose biosynthesis and proposed acarbose carbophore cycle in the acarbose producers Actinoplanes sp. and Streptomyces glaucescens GLA.O. In this model, acarbose is produced in the cell as acarbose 7-phosphate and exported by the AcbWXY (GacWXY) ABC transport system as free acarbose. Outside the cell, the free unbound acarbose is used as an acceptor molecule for the uptake of glucose or oligosaccharides catalyzed by the glycosyltransferase AcbD. The resulting pseudooligosaccharides are transported into the cell through the AcbHFG importer, presumably in complex with an ATPase subunit (MsiK). In the cell, glucose units are then released and shuttled into metabolism. The free acarbose is rephosphorylated and reexported for a new round of carbophore cycle. Dashed arrow indicates suboptimal conversion. (Modified from (25–27))
In contrast to acarbose, validamycin A contains two pseudosugars (valienamine and validamine) that form a core structure, validoxylamine A (Figure 1). The chemical structure of validoxylamine A has a high resemblance to trehalose, making it a strong competitive inhibitor for the trehalase enzymes. While the production of validamycin A and its congeners has traditionally been observed only in certain variants of Streptomyces hygroscopicus, recent genome mining and metabolomic studies have revealed validoxylamine A production in other bacterial genera, e.g. Actinosynnema mirum DSM 43827 (28).
Biosynthesis of pseudo-oligosaccharides
The C7-pseudosugar (valienamine and/or validamine) units in acarbose, validamycin A, and some other pseudo-oligosaccharides are derived from 2-epi-5-epi-valiolone, a cyclization product of sedoheptulose 7-phosphate (a pentose phosphate pathway intermediate) (20, 29, 30). The enzyme 2-epi-5-epi-valiolone synthase (EEVS) is highly homologous to dehydroquinate synthases (DHQS) from the shikimate pathway (31, 32). Interestingly, in contrast to DHQS, which is absent in vertebrates, EEVS is present in fish, amphibians, reptiles, and birds, and is involved in the biosynthesis of the sunscreen compound gadusol (33, 34). On the other hand, not all C7-pseudosugars are derived from 2-epi-5-epi-valiolone. In A. mirum DSM 43827, the valienamine and validamine moieties are derived from 2-epi-valiolone, which is also a cyclization product of sedoheptulose 7-phosphate but its formation is catalyzed by a slightly different enzyme, 2-epi-valiolone synthase (EVS) (28).
The biosynthetic gene clusters for acarbose (acarviostatins) have so far been identified in three different strains of bacteria, Actinoplanes sp. SE50/110 (the acb cluster), Streptomyces glaucescens GLA.O (the gac cluster), and S. coelicoflavus ZG0656 (the sct cluster), with some genetic distinctions between the acb cluster and the gac/sct clusters, predominantly in the early steps of the pathways (Figure 3A-3C) (11, 26, 35). The lack of genes homologous to acbL and acbN in the gac cluster also suggests that the pathways to the pseudosugar moiety in Actinoplanes sp. SE50/110 and S. glaucescens GLA.O are different. However, besides the activity of GacC and GacK (26, 34), no enzymes in the Gac pathway have been biochemically characterized, leaving the exact pathway to acarbose in this organism unclear.
Figure 3.

Biosynthetic gene clusters for acarbose and validamycin A in various bacteria. (A)-(C) Acarbose/acarviostatin gene clusters in Actinoplanes sp. SE50/110 (11), S. glaucescens GLA.O (26), and S. coelicoflavus (35). (D)-(F) Validamycin/validoxylamine gene clusters in S. hygroscopicus subsp. limoneus KCCM 11405 (36), S. hygroscopicus subsp. jinggangensis 5008 (37), and A. mirum DSM 43827 (28). Yellow arrows, acarbose structural genes; dark brown arrows, transport proteins; blue arrows, glycosidase/glycosyltransfase genes; green arrows, acarbose 7-kinase; orange arrows, validamycin structural genes, red arrows, validamycin tailoring genes; black arrows, unknown/putative/other genes; asterisk, EVS (instead of EEVS); double asterisk, a bifunctional cyclitol kinase/epimerase.
In Actinoplanes sp. SE50/110, 2-epi-5-epi-valiolone is phosphorylated by the ATP-dependent kinase AcbM, followed by epimerization at C2 by the epimerase AcbO to yield 5-epi-valiolone 7-phosphate (Figure 4) (38). It has been suggested that the product is subsequently reduced by the cyclitol dehydrogenase AcbL to give 5-epi-valiolol 7-phosphate (11), but no report describing the detailed characterization of this enzyme has been published. What lies further downstream of the pathway is largely unknown at the moment, leaving speculation as to how this high-value pseudo-oligosaccharide is synthesized in nature.
Figure 4.
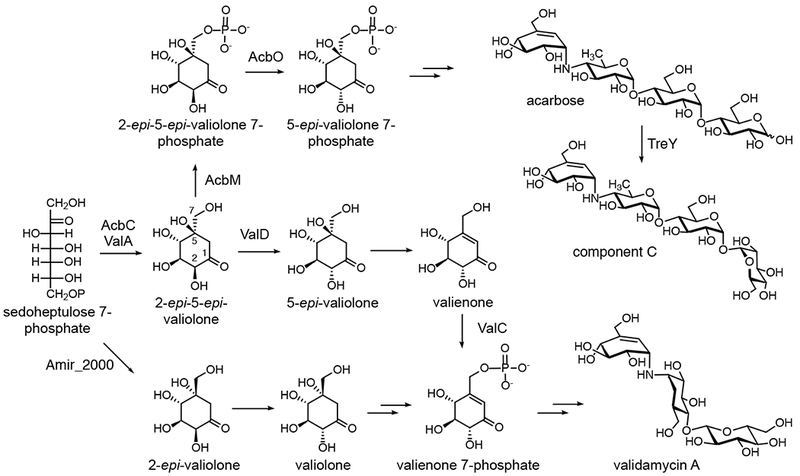
Biosynthetic pathways to acarbose and validamycin A. Although both acarbose and validamycin A contain a valienamine moiety, their biosynthetic pathways are different.
While acarbose and validamycin A share a pseudosugar (valienamine) moiety in their structures, the pathways leading to valienamine in these compounds are somewhat different. In contrast to what is seen in the acarbose pathway, in the validamycin producer S. hygroscopicus, 2-epi-5-epi-valiolone is first epimerized by the epimerase enzyme ValD to give 5-epi-valiolone (39), and then undergoes dehydration to give valienone (Figure 4) (40). So far, the enzyme that is responsible for this dehydration reaction has not been identified. Phosphorylation of valienone by the kinase ValC gives valienone 7-phosphate (41). Interestingly, in A. mirum, valienone 7-phosphate is formed through a different route involving 2-epi-valiolone (42) (Figure 4). Valienone 7-phosphate serves as a common precursor for both the unsaturated (valienamine) and the saturated (validamine) pseudosugar units (30), where the saturated pseudosugar is derived from the unsaturated one by the zinc-dependent dehydrogenase ValN (43).
One of the most intriguing questions related to the biosynthesis of pseudo-oligosaccharides asks how the pseudosugar is attached to the acceptor to form a C-N bond in those compounds. Detailed bioinformatic and biochemical studies on the validamycin pathway revealed that a glycosyltransferase-like protein (VldE/ValL) is responsible for the formation of the nonglycosidic C-N bond in validoxylamine A using GDP-valienol as a donor pseudosugar and validamine 7-phosphate as an acceptor pseudosugar (Figure 5) (44, 45). The product, validoxylamine A 7´-phosphate, is then converted to validoxylamine A by the phosphatase VldH. These reactions are homologous to those of trehalose 6-phosphate synthase (TPS) and trehalose 6-phosphate phosphatase (TPP) enzymes in trehalose biosynthesis. X-ray crystal structures of VldE/ValL and OtsA, a TPS from Escherichia coli, show high similarity between the two proteins (46, 47). Equally interesting is the presence of a nucleotidyltransferase that can convert valienol 1-phosphate to GDP-valienol. The enzyme, VldB/ValB, is a sugar 1-phosphate nucleotidyltransferase homologue similar to the glucose 1-phosphate adenylyltransferase GlgC (48). Finally, the transformation of validoxylamine A to validamycin A is catalyzed by the glycosyltransferase ValG (49).
Figure 5.
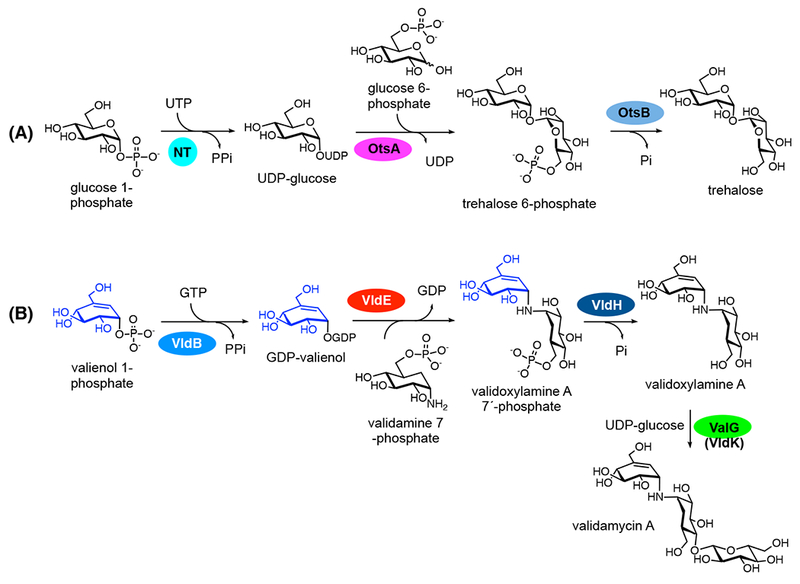
The biosynthetic pathway to validamycin A is highly similar to that to trehalose. (A) Biosynthetic pathway to trehalose. NT, nucleotidyltransferase. OtsA, a trehalose 6-phosphate synthase. OtsB, a trehalose 6-phosphate phosphatase. (B) Biosynthetic pathway to validamycin A. VldB, a cyclitol nucleotidyltransferase. VldE, a pseudoglycosyltransferase similar to OtsA. VldH, a phosphatase similar to OtsB. ValG or VldK, a glycosyltransferase.
Effects of medium and growth condition on pseudo-oligosaccharide production
Most pseudo-oligosaccharides are produced as a mixture of compounds varying in the length of the oligosaccharides and the type of anomeric bonding (1→4 or 1→1) (1). For example, the acarbose-producing Actinoplanes spp. strains also produce a number of acarbose analogues (e.g., acarviosyl-glucose, acarviosyl-maltotriose, and acarviosyl-maltotetraose) and a significant amount of component C, an acarbose byproduct in which the terminal maltose (α-d-glucopyranosyl-(1→4)-α-d-glucopyranoside) unit has been converted to trehalose (α-d-glucopyranosyl-(1→1)-α-d-glucopyranoside) (Figure 4). The conversion of acarbose into component C is catalyzed by an intramolecular glycosyltransferase (also known as maltooligosyltrehalose synthase, TreY) that in addition to converting acarbose into component C, can also convert maltose into trehalose and maltooligosaccharides into maltooligosyl trehaloses (50).
As the production of byproducts is undesirable and cost-ineffective, efforts to increase acarbose production and eliminate side pathways have been actively pursued. The production of pseudo-oligosaccharides of various lengths by acarbose-producing bacteria appears to be dictated by the carbon source available in the production broth (27, 51, 52). A high concentration of maltose in the medium has been found to be necessary to achieve efficient production of acarbose (51, 52), and the use of other carbohydrates as carbon sources, such as glucose and galactose, can negatively affect acarbose production (27). Actinoplanes sp. SE50/110 grown in a minimal medium supplemented with glucose produced acarviosyl-glucose as the major product with acarbose as a minor product, whereas those supplemented with galactose mainly produced acarbose, albeit in low yield (~5% of that in maltose-containing media) (27).
A genome-wide transcriptomic analysis of Actinoplanes sp. SE50/110 between cells grown in maltose- or glucose-containing media showed that the acarbose biosynthetic genes were highly expressed in maltose-containing media and almost silent in the glucose-containing medium (53). This lack of expression of the acarbose genes, many of which are necessary for the formation of the acarviosyl moiety, is surprising and inconsistent with the high production of acarviosyl-glucose in the glucose-containing medium (27). Although one could argue that the determination of the production yield is an integral measurement over a period of culturing time whereas gene transcription only reflects a snapshot of the cells (53), it is not clear if the timing of the gene expression would differ significantly between cultures containing mannose and glucose.
The transcriptomic study also revealed low expression levels of acbHFG which are proposed to code for an acarbose importer in the carbophore model (Figure 2), in all media (53). This is consistent with the results of binding assays and crystallographic studies that show AcbH exclusively binds d-galactose and not acarbose (54), which suggests that AcbH is not likely to be involved in acarbose transport. Interestingly, similar studies with GacH, a homologue of AcbH from S. glaucescens GLA.O, showed that it does bind acarbose as well as other oligosaccharides, but the pseudosugar moiety partially protrudes from the GacH surface (25). This suggests that GacH is not specific for acarbose or its derivatives. On the other hand, analysis by 2D protein gel electrophoresis of the cytosolic and extracellular proteome of Actinoplanes sp. SE50/110 grown in a maltose minimal medium revealed that some of the acarbose gene cluster proteins (AcbD, AcbE, and AcbZ) were found both inside and outside of the cells, which is consistent with the proposed roles of these proteins in the context of the carbophore model (Figure 2) (55).
Increased osmolality in the production broths also has a positive effect on acarbose production (51). Some acarbose producers, e.g., Actinoplanes utahensis ZJB-08196, have been reported to be osmosis resistant, with a broad osmolality optimum between 309 mOsm/kg and 719 mOsm/kg (52). However, as component C is derived from acarbose, its production also increases with the increase of osmolality. Interestingly, valienamine was found to be a potent inhibitor of this conversion, resulting in a >90% reduction in component C formation (50, 51). The addition of validamine to the culture broths of A. utahensis ZJB-08196 also increased acarbose production from 3.5 g/L to 5 g/L and reduced the production of component C byproduct from 289 mg/L to 107 mg/L (52). Moreover, the addition of validamine to the fermentation medium prior to inoculation followed by two supplementations with a mixture of glucose (6 g/L), maltose (14 g/L), and soybean flour (9 g/L) at 72 h and 96 h of cultivation further increased the production of acarbose to 6.6 g/L while maintaining low production of component C at 212 mg/L at 168 h of cultivation. Besides the carbon source and osmolality, pH and dissolved oxygen have also been shown to have an effect on acarbose production (56).
The use of d-glucose, d-xylose, and l-arabinose as carbon sources in S. hygroscopicus 5008 cultures was found to improve the production of validamycin A in a concentration-dependent manner, where d-xylose appears to be the most efficient carbon source (57). Remarkably, increased validamycin A and decreased validoxylamine A production were also observed when a high concentration of UDP-glucose was used to supplement the fermentation broth of S. hygroscopicus TL01 (58). It remains unclear, however, how UDP-glucose could penetrate or be transported into the cells, or whether it would merely be used by an extracellular glycosyltransferase to transform validoxylamine A to validamycin A.
In addition to the carbon source, which plays a direct role in pseudo-oligosaccharide biosynthesis, nitrogen concentration (59), temperature (60, 61), alkaline pH shock (62), and intracellular reactive oxygen species (ROS) (63–65) can affect validamycin production in S. hygroscopicus 5008. In contrast to the normal growth and sporulation temperature of actinomycetes, the optimal temperature for validamycin A production is 35-37 °C. When grown at this temperature, transcription levels of all eight necessary structural genes, which are organized in three operons (valABC, valKLMN and valG), increased. Similarly, the activity of the glucose 6-phosphate dehydrogenase (G6PDH) of the pentose-phosphate pathway was also elevated (60). In fact, the expression of 7.5% of coding sequences in the S. hygroscopicus 5008 genome, including the validamycin biosynthetic genes, increased at 37 °C. This thermo-controlled transcription appears to be regulated by a SARP-family regulatory gene (66).
A sudden change of pH can also increase validamycin production. An alkaline pH shock using an NaOH solution (pH 8) at 20 h of incubation was found to increase validamycin A production by 27% (62). This change in pH also increased the sugar consumption rate and the cell weight after 96 h of incubation. Under these conditions, the transcription level of most validamycin A biosynthetic genes also increased, except for the glycosyltransferase gene valG. Furthermore, the addition of ethanol (63), hydrogen peroxide (64), or liquid paraffin (65), as well as a temperature shift (61), appears to increase the medium dissolved oxygen and/or the intracellular oxidative stress level. Intracellular ROS was found to act as the molecular signal for the transcription of validamycin A biosynthetic genes.
Metabolic engineering of pseudo-oligosaccharides
Due to the lack of a suitable genetic tool to manipulate the acarbose biosynthetic pathway in Actinoplanes sp. SE50/110, earlier studies were limited to isotopic incorporation experiments (29, 67–71) and biochemical characterizations of the putative enzymes in the pathway (31, 38, 72, 73). However, the identification of other acarbose producers (26, 74) as well as the recent development of a genetic method to engineer Actinoplanes sp. SE50/110 strains have opened up new opportunities for the metabolic engineering of acarbose in these bacteria (75). For instance, the formation of the byproduct component C can now be eliminated by inactivating the maltooligosyl-trehalose synthase gene (treY) in Actinoplanes sp. SE50/110, which slightly increases the overall yield of acarbose from 2.7 g/L to 3 g/L (75).
In contrast to Actinoplanes sp. SE50/110, the validamycin A producer S. hygroscopicus 5008 is more susceptible to genetic manipulations. This has greatly aided the elucidation of the validamycin biosynthetic pathway through genetic approaches (32, 37, 41, 48, 49). In addition, metabolic engineering in S. hygroscopicus 5008 has led to the production of two new pseudo-oligosaccharides, 1,1´-bis-valienamine and validienamycin (43).
More recent metabolic engineering studies have been directed toward improving the production yield of validamycin A. This includes amplification of the validamycin gene cluster in S. hygroscopicus 5008 (76), elimination of endogenous linear plasmids in the producing strain (77), and modulation of the regulatory systems for validamycin biosynthesis (78–80). Using the zouA-mediated amplification system (81, 82), mutant strains containing multiple (3–5) copies of the validamycin gene cluster have been generated. However, despite having multiple copies of the gene cluster, validamycin synthesis increased only moderately (~34%) compared to that in the wild type strain. Also, the downside of this approach is that the copy number of the gene cluster gradually decreased in the absence of selection pressure, and was completely lost after sporulation (76).
The 10,383,684-bp genome of S. hygroscopicus 5008 consists of a linear chromosome, a 164.57-kb linear plasmid (pSHJG1), and a 73.28-kb circular plasmid (66). Interestingly, eliminating the linear plasmid pSHJG1 in S. hygroscopicus 5008 increased the production of validamycin A up to 43%, or 12.5% in the high-yielding TL01 strain (from 19.16 ± 1.93 to 21.56 ± 2.25 g/L) (77). However, mutants lacking the linear plasmid grow more slowly, indicating the presence of genes that support cellular growth in pSHJG1.
The addition of exogenous 1,4-butyrolactone has also been shown to stimulate A-factor-like cascades and increase validamycin biosynthesis by 30% in S. hygroscopicus 5008 (78). However, a high concentration of this compound (10 mM) is necessary to enhance the biosynthetic pathway. Although γ-butyrolactones (e.g., 2-isocapryloyl-3R-hydroxymethyl-γ-butyrolactone or A-factor) have been known to play an important role in the regulation of sporulation and secondary metabolism in Streptomyces spp., 1,4-butyrolactone is not likely to be a natural inducer of validamycin biosynthesis. Nevertheless, a significant increase in the transcription of the pleiotropic regulatory genes adpA-H (transcriptional activator genes) and the validamycin biosynthetic genes were observed upon the addition of this compound.
Bioinformatic studies have revealed that there are three pairs of afsA/arpA homologues in the S. hygroscopicus 5008 genome (79). AfsA catalyzes the biosynthesis of A-factor and ArpA (A-factor receptor protein) is an adpA-H transcriptional repressor (83, 84). Deletion of the afsA homologues (shbA1, shbA2, and shbA3) significantly decreased the biosynthesis of validamycin (61-90%), whereas deletion of the arpA homologues shbR1 and shbR3 (but not shbR2) increased validamycin production by 26% and 20%, respectively (79). When deleted together, the mutant gave a 55% increase in validamycin production. Among these arpA homologues, only shbR3 appears to encode the receptor for the exogenous 1,4-butyrolactone (78).
A global regulator in nitrogen metabolism (GlnR) has been found to be involved in validamycin biosynthesis through its specific association with the valK-valA intergenic promoter region (80). Internal deletion of glnR in S. hygroscopicus 5008 increased the transcription of certain validamycin biosynthetic genes, but it reduced validamycin production by 80%. It also impaired aerial hyphae formation and sporulation. It appears that GlnR has two binding sites in the valK-valA-intergenic promoter region (Figure 6). Site-specific mutagenesis of GlnR binding site I enhanced validamycin A production by 2.5-fold, whereas mutation of GlnR binding site II resulted in a >50% reduction in validamycin A production. It is proposed that GlnR serves as both an inhibitor and an activator by binding sites I and II, respectively (Figure 6) (80).
Figure 6.

Proposed regulatory model for GlnR roles in validamycin biosynthesis in S. hygroscopicus 5008. It is speculated that GlnR first binds to site II and then to site I. The binding of GlnR to site II positively enhances the expression of val genes and validamycin A production. When the concentration of GlnR reaches a critical level, it would bind to site I. This would block the interaction between the two bound AdpA dimers, resulting in a decrease in val gene expression and validamycin A production. When the GlnR binding site I is mutated, the production of validamycin A increased. (Modified from (80))
Conclusion
Due to their high resemblance to carbohydrates, pseudo-oligosaccharides show significant biological activities. In nature, they may play a role as carbophores, which shuttle sugars in and out between intra and extracellular spaces of bacteria, and/or are used for niche protection by inhibiting carbohydrate-degrading enzymes of other organisms. Based on this second function, acarbose has been developed as an antidiabetic drug, whereas validamycin A has been used widely as a crop protectant. Due to this significant economic value, recent efforts have been focused on understanding their biosyntheses, optimizing their fermentation conditions, and engineering their metabolic pathways to obtain better production yields.
The pseudo-oligosaccharides are uniquely assembled via a number of different metabolic pathways. The existence of multiple pathways to the same pseudosugar unit in various bacteria is fascinating and may indicate that it is a privileged chemical scaffold in living organisms. The biosynthetic pathways involve many unusual enzymes such as cyclitol kinases, aminotransferases, oxidoreductases, cyclitol nucleotidyltransferases, and many others. While these enzymes are similar to those commonly found in carbohydrate biosynthesis, they have obtained new capabilities that recognize non-sugar compounds as substrates. For instance, the pseudoglycosyltransferase VldE/ValL can catalyze the formation of a non-glycosidic C-N bond in validamycin biosynthesis. While the X-ray crystal structures and detailed biochemical studies of this enzyme have been reported, the mechanism underlying the C-N bond formation remains unclear.
While the carbon source is one of the most important factors that dictates the production of pseudo-oligosaccharides, other conditions such as osmolality, pH, temperature, and oxidative stress can also affect their production yields. In addition, the use of small molecules to modulate biosynthetic gene expression and/or byproduct reduction has resulted in increased overall yields of acarbose and validamycin A. Modulation of the regulatory systems in validamycin biosynthesis has also been achieved through metabolic engineering with some meaningful success. This area holds great promise and needs further investigation, particularly with the acarbose-producing bacteria. Also, there remain many gaps in our knowledge of the biosynthesis of these high-value natural products. For example, with the exception of the first few steps, the acarbose pathway is largely unknown at the moment. This is also the case with other pseudo-oligosaccharides. Therefore, further studies are needed to uncover the modes of formation of this promising class of natural products and how they are regulated.
Acknowledgement
The authors thank Philip J. Proteau for critical reading of this manuscript. This work was supported by grants GM112068 and AI129957 from the National Institute of General Medical Sciences and the National Institute of Allergy And Infectious Diseases, respectively. The content is solely the responsibility of the authors and does not represent the official views of the National Institute of General Medical Sciences, the National Institute of Allergy And Infectious Diseases, or the National Institutes of Health (NIH).
References
- 1.Mahmud T (2003) The C7N aminocyclitol family of natural products. Nat. Prod. Rep 20, 137–166. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi Y, Igarashi M (2017) Destination of aminoglycoside antibiotics in the ‘post-antibiotic era’. J. Antibiot doi: 10.1038/ja.2017.117 [DOI] [PubMed] [Google Scholar]
- 3.Bhuyan BK (1962) Pactamycin production by Streptomyces pactum. Appl. Microbiol 10, 302–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laube H, Fouladfar M, Aubell R, Schmitz H (1980) [Effect of glucosidase inhibitor, Bay g 5421 (acarbose), on the blood glucose in obese diabetic patients ty pe 2 (NIDDM) (author’s transl)]. Arzneimittelforschung 30, 1154–1157. [PubMed] [Google Scholar]
- 5.Iwasa T, Higashide E, Yamamoto H, Shibata M (1971) Studies on validamycins, new antibiotics. II. Production and biological properties of validamycins A and B. J. Antibiot 24, 107–113. [DOI] [PubMed] [Google Scholar]
- 6.Shibata M, Uyeda M, Mori K (1981) Stimulation of the extension of validamycin-inhibited hyphae by the hyphal extension factor present in Rhizoctonia solani. J. Antibiot 34, 447–451. [DOI] [PubMed] [Google Scholar]
- 7.Horii S, Fukase H, Matsuo T, Kameda Y, Asano N, Matsui K (1986) Synthesis and alpha-D-glucosidase inhibitory activity of N-substituted valiolamine derivatives as potential oral antidiabetic agents. J. Med. Chem 29, 1038–1046. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Ji X, Kan S, Qiao H, Jiang M, Lu D, Wang J, Huang H, Jia H, Ouyuang P, Ying H (2010) Past, present, and future industrial biotechnology in China. Adv. Biochem. Eng. Biotechnol 122, 1–42. [DOI] [PubMed] [Google Scholar]
- 9.Flatt PM, Mahmud T (2007) Biosynthesis of aminocyclitol-aminoglycoside antibiotics and related compounds. Nat. Prod. Rep 24, 358–392. [DOI] [PubMed] [Google Scholar]
- 10.McCranie EK, Bachmann BO (2014) Bioactive oligosaccharide natural products. Nat. Prod. Rep 31, 1026–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wehmeier UF, Piepersberg W (2004) Biotechnology and molecular biology of the alpha-glucosidase inhibitor acarbose. Appl. Microbiol. Biotechnol 63, 613–625. [DOI] [PubMed] [Google Scholar]
- 12.Mahmud T, Lee S, Floss HG (2001) The biosynthesis of acarbose and validamycin. Chem. Rec 1, 300–310. [DOI] [PubMed] [Google Scholar]
- 13.Mahmud T, Flatt PM, Wu X (2007) Biosynthesis of unusual aminocyclitol-containing natural products. J. Nat. Prod 70, 1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuhara K, Murai H, Murao S (1982) Amylostatins, Other Amylase Inhibitors Produced by Streptomyces diastaticus subsp. Amylostaticus No. 2476. Agric. Biol. Chem 48, 2021–2030. [Google Scholar]
- 15.Murao S, Ohyama K, Ogura S (1977) Isolation of Amylase Inhibitor-producing Microorganism. Agric. Biol. Chem 41, 919–924. [Google Scholar]
- 16.Yokose K, Ogawa K, Sano T, Watanabe K, Maruyama HB, Suhara Y (1983) New alpha-amylase inhibitor, trestatins. I. Isolation, characterization and biological activities of trestatins A, B and C. J. Antibiot 36, 1157–1165. [DOI] [PubMed] [Google Scholar]
- 17.Itoh J, Omoto S, Shomura T, Ogino H, Iwamatsu K, Inouye S, Hidaka H (1981) Oligostatins, new antibiotics with amylase inhibitory activity. I. Production, isolation and characterization. J. Antibiot 34, 1424–1428. [DOI] [PubMed] [Google Scholar]
- 18.Namiki S, Kangouri K, Nagate T, Hara H, Sugita K, Omura S (1982) Studies on the alpha-glucoside hydrolase inhibitor, adiposin. I. Isolation and physicochemical properties. J. Antibiot 35, 1234–1236. [DOI] [PubMed] [Google Scholar]
- 19.Vértesy L, Fehlhaber H-W, Schulz A (1994) The Trehalase Inhibitor Salbostatin, a Novel Metabolite from Streptomyces albus, ATCC21838. Angew. Chem., Int. Ed. Engl 33, 1844–1846. [Google Scholar]
- 20.Choi WS, Wu X, Choeng YH, Mahmud T, Jeong BC, Lee SH, Chang YK, Kim CJ, Hong SK (2008) Genetic organization of the putative salbostatin biosynthetic gene cluster including the 2-epi-5-epi-valiolone synthase gene in Streptomyces albus ATCC 21838. Appl. Microbiol. Biotechnol 80, 637–645. [DOI] [PubMed] [Google Scholar]
- 21.Geng P, Sun T, Zhong Q, Li X, Shi L, Bai F, Bai G (2013) Two novel potent alpha-amylase inhibitors from the family of acarviostatins isolated from the culture of Streptomyces coelicoflavus ZG0656. Chem. Biodivers 10, 452–459. [DOI] [PubMed] [Google Scholar]
- 22.Si D, Zhong D, Xu Q (2001) Two butylated aminooligosaccharides isolated from the culture filtrate of Streptomyces luteogriseus. Carbohydr. Res 335, 127–132. [DOI] [PubMed] [Google Scholar]
- 23.Si D, Zhong D, Chen X (2001) Profiling of isovalertatin family aminooligosaccharides extracted from the culture of Streptomyces luteogriseus by using liquid chromatography/electrospray ionization mass spectrometry. Anal. Chem 73, 3808–3815. [DOI] [PubMed] [Google Scholar]
- 24.Brunkhorst C, Wehmeier UF, Piepersberg W, Schneider E (2005) The acbH gene of Actinoplanes sp. encodes a solute receptor with binding activities for acarbose and longer homologs. Res. Microbiol 156, 322–327. [DOI] [PubMed] [Google Scholar]
- 25.Vahedi-Faridi A, Licht A, Bulut H, Scheffel F, Keller S, Wehmeier UF, Saenger W, Schneider E (2010) Crystal structures of the solute receptor GacH of Streptomyces glaucescens in complex with acarbose and an acarbose homolog: comparison with the acarbose-loaded maltose-binding protein of Salmonella typhimurium. J. Mol. Biol 397, 709–723. [DOI] [PubMed] [Google Scholar]
- 26.Rockser Y, Wehmeier UF (2009) The gac-gene cluster for the production of acarbose from Streptomyces glaucescens GLA.O: identification, isolation and characterization. J. Biotechnol 140, 114–123. [DOI] [PubMed] [Google Scholar]
- 27.Wendler S, Ortseifen V, Persicke M, Klein A, Neshat A, Niehaus K, Schneiker-Bekel S, Walter F, Wehmeier UF, Kalinowski J, Puhler A (2014) Carbon source dependent biosynthesis of acarviose metabolites in Actinoplanes sp. SE50/110. J. Biotechnol 191, 113–120. [DOI] [PubMed] [Google Scholar]
- 28.Asamizu S, Abugreen M, Mahmud T (2013) Comparative Metabolomic Analysis of an Alternative Biosynthetic Pathway to Pseudosugars in Actinosynnema mirum DSM 43827. ChemBioChem 14, 1548–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmud T, Tornus I, Egelkrout E, Wolf E, Uy C, Floss HG, Lee S (1999) Biosynthetic studies on the alpha-glucosidase inhibitor acarbose in Actinoplanes sp.: 2-epi-5-epi-valiolone is the direct precursor of the valienamine moiety. J. Am. Chem. Soc 121, 6973–6983. [Google Scholar]
- 30.Dong H, Mahmud T, Tornus I, Lee S, Floss HG (2001) Biosynthesis of the validamycins: identification of intermediates in the biosynthesis of validamycin A by Streptomyces hygroscopicus var. limoneus. J. Am. Chem. Soc 123, 2733–2742. [DOI] [PubMed] [Google Scholar]
- 31.Stratmann A, Mahmud T, Lee S, Distler J, Floss HG, Piepersberg W (1999) The AcbC protein from Actinoplanes species is a C7-cyclitol synthase related to 3-dehydroquinate synthases and is involved in the biosynthesis of the alpha-glucosidase inhibitor acarbose. J. Biol. Chem 274, 10889–10896. [DOI] [PubMed] [Google Scholar]
- 32.Yu Y, Bai L, Minagawa K, Jian X, Li L, Li J, Chen S, Cao E, Mahmud T, Floss HG, Zhou X, Deng Z (2005) Gene cluster responsible for validamycin biosynthesis in Streptomyces hygroscopicus subsp. jinggangensis 5008. Appl. Environ. Microbiol 71, 5066–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osborn AR, Almabruk KH, Holzwarth G, Asamizu S, LaDu J, Kean KM, Karplus PA, Tanguay RL, Bakalinsky AT, Mahmud T (2015) De novo synthesis of a sunscreen compound in vertebrates. Elife 4, e05919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osborn AR, Kean KM, Alseud KM, Almabruk KH, Asamizu S, Lee JA, Karplus PA, Mahmud T (2017) Evolution and Distribution of C7-Cyclitol Synthases in Prokaryotes and Eukaryotes. ACS Chem. Biol 12, 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo X, Geng P, Bai F, Bai G, Sun T, Li X, Shi L, Zhong Q (2012) Draft genome sequence of Streptomyces coelicoflavus ZG0656 reveals the putative biosynthetic gene cluster of acarviostatin family alpha-amylase inhibitors. Lett. Appl. Microbiol 55, 162–169. [DOI] [PubMed] [Google Scholar]
- 36.Singh D, Seo MJ, Kwon HJ, Rajkarnikar A, Kim KR, Kim SO, Suh JW (2006) Genetic localization and heterologous expression of validamycin biosynthetic gene cluster isolated from Streptomyces hygroscopicus var. limoneus KCCM 11405 (IFO 12704). Gene 376, 13–23. [DOI] [PubMed] [Google Scholar]
- 37.Bai L, Li L, Xu H, Minagawa K, Yu Y, Zhang Y, Zhou X, Floss HG, Mahmud T, Deng Z (2006) Functional analysis of the validamycin biosynthetic gene cluster and engineered production of validoxylamine A. Chem. Biol 13, 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang CS, Stratmann A, Block O, Bruckner R, Podeschwa M, Altenbach HJ, Wehmeier UF, Piepersberg W (2002) Biosynthesis of the C7-cyclitol moiety of acarbose in Actinoplanes species SE50/110. 7-O-phosphorylation of the initial cyclitol precursor leads to proposal of a new biosynthetic pathway. J. Biol. Chem 277, 22853–22862. [DOI] [PubMed] [Google Scholar]
- 39.Xu H, Zhang Y, Yang J, Mahmud T, Bai L, Deng Z (2009) Alternative epimerization in C7N-aminocyclitol biosynthesis is catalyzed by ValD, a large protein of the vicinal oxygen chelate superfamily. Chem. Biol 16, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahmud T, Xu J, Choi YU (2001) Synthesis of 5-epi-[6-2H2]valiolone and stereospecifically monodeuterated 5-epi-valiolones: exploring the steric course of 5-epi-valiolone dehydratase in validamycin A biosynthesis. J. Org. Chem 66, 5066–5073. [DOI] [PubMed] [Google Scholar]
- 41.Minagawa K, Zhang Y, Ito T, Bai L, Deng Z, Mahmud T (2007) ValC, a New Type of C7-Cyclitol Kinase Involved in the Biosynthesis of the Antifungal Agent Validamycin A. ChemBioChem 8, 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asamizu S, Xie P, Brumsted CJ, Flatt PM, Mahmud T (2012) Evolutionary divergence of sedoheptulose 7-phosphate cyclases leads to several distinct cyclic products. J. Am. Chem. Soc 134, 12219–12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H, Yang J, Bai L, Deng Z, Mahmud T (2009) Genetically engineered production of 1,1′-bis-valienamine and validienamycin in Streptomyces hygroscopicus and their conversion to valienamine. Appl. Microbiol. Biotechnol 81, 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asamizu S, Yang J, Almabruk KH, Mahmud T (2011) Pseudoglycosyltransferase catalyzes nonglycosidic C-N coupling in validamycin a biosynthesis. J. Am. Chem. Soc 133, 12124–12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abuelizz HA, Mahmud T (2015) Distinct Substrate Specificity and Catalytic Activity of the Pseudoglycosyltransferase VldE. Chem. Biol 22, 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavalier MC, Yim YS, Asamizu S, Neau D, Almabruk KH, Mahmud T, Lee YH (2012) Mechanistic insights into validoxylamine A 7′-phosphate synthesis by VldE using the structure of the entire product complex. PLoS One 7, e44934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng L, Zhou X, Zhang H, Ji X, Li L, Huang L, Bai L, Zhang H (2012) Structural and functional analysis of validoxylamine A 7′-phosphate synthase ValL involved in validamycin A biosynthesis. PLoS One 7, e32033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang J, Xu H, Zhang Y, Bai L, Deng Z, Mahmud T (2011) Nucleotidylation of unsaturated carbasugar in validamycin biosynthesis. Org. Biomol. Chem 9, 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H, Minagawa K, Bai L, Deng Z, Mahmud T (2008) Catalytic analysis of the validamycin glycosyltransferase (ValG) and enzymatic production of 4″-epi-validamycin A. J. Nat. Prod 71, 1233–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi BT, Shin CS (2004) Isolation and characterization of a novel intracellular glucosyltransferase from the acarbose producer Actinoplanes sp. CKD485–16. Appl. Microbiol. Biotechnol 65, 273–280. [DOI] [PubMed] [Google Scholar]
- 51.Choi BT, Shin CS (2003) Reduced formation of byproduct component C in acarbose fermentation by Actinoplanes sp. CKD485–16. Biotechnol. Prog 19, 1677–1682. [DOI] [PubMed] [Google Scholar]
- 52.Wang YJ, Liu LL, Wang YS, Xue YP, Zheng YG, Shen YC (2012) Actinoplanes utahensis ZJB-08196 fed-batch fermentation at elevated osmolality for enhancing acarbose production. Bioresour. Technol 103, 337–342. [DOI] [PubMed] [Google Scholar]
- 53.Schwientek P, Wendler S, Neshat A, Eirich C, Ruckert C, Klein A, Wehmeier UF, Kalinowski J, Stoye J, Puhler A (2013) Comparative RNA-sequencing of the acarbose producer Actinoplanes sp. SE50/110 cultivated in different growth media. J. Biotechnol 167, 166–177. [DOI] [PubMed] [Google Scholar]
- 54.Licht A, Bulut H, Scheffel F, Daumke O, Wehmeier UF, Saenger W, Schneider E, Vahedi-Faridi A (2011) Crystal structures of the bacterial solute receptor AcbH displaying an exclusive substrate preference for beta-D-galactopyranose. J. Mol. Biol 406, 92–105. [DOI] [PubMed] [Google Scholar]
- 55.Wendler S, Hurtgen D, Kalinowski J, Klein A, Niehaus K, Schulte F, Schwientek P, Wehlmann H, Wehmeier UF, Puhler A (2013) The cytosolic and extracellular proteomes of Actinoplanes sp. SE50/110 led to the identification of gene products involved in acarbose metabolism. J. Biotechnol 167, 178–189. [DOI] [PubMed] [Google Scholar]
- 56.Li KT, Zhou J, Wei SJ, Cheng X (2012) An optimized industrial fermentation processes for acarbose production by Actinoplanes sp. A56. Bioresour. Technol 118, 580–583. [DOI] [PubMed] [Google Scholar]
- 57.Zhou TC, Zhong JJ (2015) Production of validamycin A from hemicellulose hydrolysate by Streptomyces hygroscopicus 5008. Bioresour. Technol 175, 160–166. [DOI] [PubMed] [Google Scholar]
- 58.Zhou X, Wu H, Li Z, Zhou X, Bai L, Deng Z (2011) Over-expression of UDP-glucose pyrophosphorylase increases validamycin A but decreases validoxylamine A production in Streptomyces hygroscopicus var. jinggangensis 5008. Metab. Eng 13, 768–776. [DOI] [PubMed] [Google Scholar]
- 59.Wei ZH, Bai L, Deng Z, Zhong JJ (2012) Impact of nitrogen concentration on validamycin A production and related gene transcription in fermentation of Streptomyces hygroscopicus 5008. Bioprocess. Biosyst. Eng 35, 1201–1208. [DOI] [PubMed] [Google Scholar]
- 60.Liao Y, Wei ZH, Bai L, Deng Z, Zhong JJ (2009) Effect of fermentation temperature on validamycin A production by Streptomyces hygroscopicus 5008. J. Biotechnol 142, 271–274. [DOI] [PubMed] [Google Scholar]
- 61.Wei ZH, Wu H, Bai L, Deng Z, Zhong JJ (2012) Temperature shift-induced reactive oxygen species enhanced validamycin A production in fermentation of Streptomyces hygroscopicus 5008. Bioprocess. Biosyst. Eng 35, 1309–1316. [DOI] [PubMed] [Google Scholar]
- 62.Jiang J, Sun YF, Tang X, He CN, Shao YL, Tang YJ, Zhou WW (2018) Alkaline pH shock enhanced production of validamycin A in fermentation of Streptomyces hygroscopicus. Bioresour. Technol 249, 234–240. [DOI] [PubMed] [Google Scholar]
- 63.Zhou WW, Ma B, Tang YJ, Zhong JJ, Zheng X (2012) Enhancement of validamycin A production by addition of ethanol in fermentation of Streptomyces hygroscopicus 5008. Bioresour. Technol 114, 616–621. [DOI] [PubMed] [Google Scholar]
- 64.Wei ZH, Bai L, Deng Z, Zhong JJ (2011) Enhanced production of validamycin A by H2O2-induced reactive oxygen species in fermentation of Streptomyces hygroscopicus 5008. Bioresour. Technol 102, 1783–1787. [DOI] [PubMed] [Google Scholar]
- 65.Feng J, Jiang J, Liu Y, Li W, Azat R, Zheng X, Zhou WW (2016) Significance of oxygen carriers and role of liquid paraffin in improving validamycin A production. J. Ind. Microbiol. Biotechnol 43, 1365–1372. [DOI] [PubMed] [Google Scholar]
- 66.Wu H, Qu S, Lu C, Zheng H, Zhou X, Bai L, Deng Z (2012) Genomic and transcriptomic insights into the thermo-regulated biosynthesis of validamycin in Streptomyces hygroscopicus 5008. BMC Genomics 13, 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Degwert U, van Hulst R, Pape H, Herrold RE, Beale JM, Keller PJ, Lee JP, Floss HG (1987) Studies on the biosynthesis of the alpha-glucosidase inhibitor acarbose: valienamine, a m-C7N unit not derived from the shikimate pathway. J. Antibiot 40, 855–861. [DOI] [PubMed] [Google Scholar]
- 68.Lee S, Egelkrout E (1998) Biosynthetic studies on the alpha-glucosidase inhibitor acarbose in Actinoplanes sp.: glutamate is the primary source of the nitrogen in acarbose. J. Antibiot 51, 225–227. [DOI] [PubMed] [Google Scholar]
- 69.Lee S, Sauerbrei B, Niggemann J, Egelkrout E (1997) Biosynthetic studies on the alpha-glucosidase inhibitor acarbose in Actinoplanes sp.: source of the maltose unit. J. Antibiot 50, 954–960. [DOI] [PubMed] [Google Scholar]
- 70.Arakawa K, Bowers SG, Michels B, Trin V, Mahmud T (2003) Biosynthetic studies on the alpha-glucosidase inhibitor acarbose: the chemical synthesis of isotopically labeled 2-epi-5-epi-valiolone analogs. Carbohydr. Res 338, 2075–2082. [DOI] [PubMed] [Google Scholar]
- 71.Mahmud T (2007) Isotope tracer investigations of natural products biosynthesis: The discovery of novel metabolic pathways. J. Labelled Comp. Radiophar 50, 1039–1051. [Google Scholar]
- 72.Zhang CS, Podeschwa M, Block O, Altenbach HJ, Piepersberg W, Wehmeier UF (2003) Identification of a 1-epi-valienol 7-kinase activity in the producer of acarbose, Actinoplanes sp. SE50/110. FEBS Lett 540, 53–57. [DOI] [PubMed] [Google Scholar]
- 73.Zhang CS, Podeschwa M, Altenbach HJ, Piepersberg W, Wehmeier UF (2003) The acarbose-biosynthetic enzyme AcbO from Actinoplanes sp. SE 50/110 is a 2-epi-5-epi-valiolone-7-phosphate 2-epimerase. FEBS Lett 540, 47–52. [DOI] [PubMed] [Google Scholar]
- 74.Geng P, Bai G (2008) Two novel aminooligosaccharides isolated from the culture of Streptomyces coelicoflavus ZG0656 as potent inhibitors of alpha-amylase. Carbohydr. Res 343, 470–476. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Q, Xie H, Peng Y, Wang X, Bai L (2017) Improving acarbose production and eliminating the by-product component C with an efficient genetic manipulation system of Actinoplanes sp. SE50/110. Synth. Syst. Biotechnol 2, 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou TC, Kim BG, Zhong JJ (2014) Enhanced production of validamycin A in Streptomyces hygroscopicus 5008 by engineering validamycin biosynthetic gene cluster. Appl. Microbiol. Biotechnol 98, 7911–7922. [DOI] [PubMed] [Google Scholar]
- 77.Lu C, Wu H, Su X, Bai L (2017) Elimination of indigenous linear plasmids in Streptomyces hygroscopicus var. jinggangensis and Streptomyces sp. FR008 to increase validamycin A and candicidin productivities. Appl. Microbiol. Biotechnol 101, 4247–4257. [DOI] [PubMed] [Google Scholar]
- 78.Tan GY, Bai L, Zhong JJ (2013) Exogenous 1,4-butyrolactone stimulates A-factor-like cascade and validamycin biosynthesis in Streptomyces hygroscopicus 5008. Biotechnol. Bioeng 110, 2984–2993. [DOI] [PubMed] [Google Scholar]
- 79.Tan GY, Peng Y, Lu C, Bai L, Zhong JJ (2015) Engineering validamycin production by tandem deletion of gamma-butyrolactone receptor genes in Streptomyces hygroscopicus 5008. Metab. Eng 28, 74–81. [DOI] [PubMed] [Google Scholar]
- 80.Qu S, Kang Q, Wu H, Wang L, Bai L (2015) Positive and negative regulation of GlnR in validamycin A biosynthesis by binding to different loci in promoter region. Appl. Microbiol. Biotechnol 99, 4771–4783. [DOI] [PubMed] [Google Scholar]
- 81.Murakami T, Burian J, Yanai K, Bibb MJ, Thompson CJ (2011) A system for the targeted amplification of bacterial gene clusters multiplies antibiotic yield in Streptomyces coelicolor. Proc. Natl. Acad. Sci. U.S.A 108, 16020–16025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murakami T, Sumida N, Bibb M, Yanai K (2011) ZouA, a putative relaxase, is essential for dna amplification in Streptomyces kanamyceticus. J. Bacteriol 193, 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kato JY, Funa N, Watanabe H, Ohnishi Y, Horinouchi S (2007) Biosynthesis of gamma-butyrolactone autoregulators that switch on secondary metabolism and morphological development in Streptomyces. Proc. Natl. Acad. Sci. U.S.A 104, 2378–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohnishi Y, Yamazaki H, Kato JY, Tomono A, Horinouchi S (2005) AdpA, a central transcriptional regulator in the A-factor regulatory cascade that leads to morphological development and secondary metabolism in Streptomyces griseus. Biosci. Biotechnol. Biochem 69, 431–439. [DOI] [PubMed] [Google Scholar]


