Abstract
OBJECTIVES
The recurrence rate of sudden sensorineural hearing loss (SSNHL) varies from 0.8% to 40%. However, to the best of our knowledge, no data on long-term hearing variations are present in the literature. The aim of this observational study was to analyze long-term variations of the hearing threshold in unilateral SSNHL without recurrence.
MATERIALS and METHODS
A total of 50 patients affected by unilateral SSNHL were evaluated. Patients underwent a treatment consisting of intravenous corticosteroids. Clinical and audiometric features were recorded. Patients underwent pure tone audiometry at a mean follow-up of 5.26±2.28 years. Differences between the affected and unaffected ear were analyzed.
RESULTS
Comparing the post-treatment and follow-up audiograms, there was a worsening of hearing in the unaffected ear. On the contrary, no significant difference over time was found for the affected ear. 54% of patients showed no changes over time, 26% showed worsening, and 20% showed an improvement in hearing. The variation correlated with alcohol consumption and the presence of vasculopathies. An average improvement of hearing over time was observed at low frequencies.
CONCLUSION
The time evolution in SSNHL is not predictable on the basis of the clinical and audiometric data. The majority the patients shows no changes in hearing loss in the affected ear. Patients who consume alcohol or have vasculopathies also have a higher risk of worsening of hearing. Further prospective studies are mandatory to better assess variations over time and their relationship with the effect of aging on hearing.
Keywords: Sudden sensorineural hearing loss, pure tone audiometry, risk factors, long-term outcomes, autoimmune disorders
INTRODUCTION
Sudden sensorineural hearing loss (SSNHL) is defined as a 30 dB or greater sensorineural hearing loss over at least three consecutive frequencies occurring within 72 hours. The etiology is, in most cases, unknown and may include infections, vascular accidents, traumas, tumors, endolymphatic hydrops, and autoimmune disorders [1]. Recovery depends on several factors, including age, associated symptoms such as tinnitus and vertigo, severity of hearing impairment, and the time lapse between the onset and treatment [1].
Concerning its recurrence, the literature is very heterogeneous: the incidence varies from 0.8% to 47% [2–6]. A higher recurrence rate was reported at low frequencies SSNHL. Approximately 9% of cases with SSNHL develops Menière’s disease, but only some cases of SSNHL involving low frequencies develop this disease [6]. The evolution of hearing loss over time was studied in endolymphatic hydrops [7], but not in SSNHL.
When assessing the evolution of hearing loss over time, it is important to consider the effect of aging on the cochlear function. There is a lack of literature regarding the effect of aging on affected and unaffected ear of subjects with SSNHL. Moreover, no study analyzed the correlation between the hearing evolution over time and clinical features. Guidelines did not report any recommendations about a long-term follow-up of patients with SSNHL [1].
The aim of this observational study was to analyze long-term variations of the hearing threshold in unilateral SSNHL without recurrence. Correlations with clinical characteristics were assessed.
MATERIALS AND METHODS
A total of 50 patients observed for unilateral SSNHL between 2007 and 2015 were included in this observational study. Exclusion criteria were as follows: vertigo at onset, Menière’s disease, trauma, ototoxicity, vestibular schwannoma, Cogan syndrome, concomitant neurological symptoms, and recurrence of SSNHL. Clinical and audiometric data were recorded. All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval by the Institutional Review Board was not needed because of the retrospective nature of the study. Informed consent was obtained from all participants included in the study.
After diagnosis, each patient was treated with intravenous corticosteroids (betamethasone 4 mg per day: Bentelan, Alfasigma, Milano, Italy) and mannitol (18% 250 mL per day: Mannitolo, Fresenius Kabi Italia, Verona, Italy) for 5 days. Eighteen patients (36%) underwent a second cycle of intravenous therapy. Five patients (10%) underwent hyperbaric oxygen therapy in addition to medical treatment (three patients before and two after it). Intratympanic corticosteroids were administered to four patients after intravenous therapy (8%). Seven patients (14%) continued with oral corticosteroids for 8 days.
Pre-, post-treatment, and follow-up pure tone audiometry (PTA) was recorded analyzing the severity of hearing loss, audiogram curve morphology, and hearing recovery. Each patient underwent PTA at the end of treatment, 1 and 6 months after treatment, and then once a year. For this study we considered the first and the last audiogram after the treatment. Average PTA at 0.5, 1, 2, and 4 kHz (speech frequencies) was calculated. The severity of hearing loss was based on the PTA threshold as follows: mild (25–40 dB HL), moderate (41–55 dB HL), moderately severe (56–70 dB HL), severe (71–90 dB HL), and profound (>90 dB HL) [1]. Audiometric curve morphology was classified as upward-sloping curves, downward-sloping curves, flat loss, or trough shaped curves [1]. Guidelines criteria for hearing recovery were used [1]. Audiograms variation over time during the follow-up was classified as follows: improvement (improved hearing thresholds ≥10 dB HL on all the frequencies, compared to post-treatment audiogram); worsening (worsened hearing thresholds ≥10 dB HL on all the frequencies, compared to post-treatment audiogram); and no change (improved or worsened hearing thresholds within 10 dB HL, compared to post-treatment audiogram).
The mean age of the study group was 53.02±13.84 years (range, 17–88 years). Nine patients (18%) were older than 60 years. The mean age at the onset of SSNHL was 47.36±14.63 years (range, 11–82 years). Table 1 reports patient characteristics. Alcohol consumption was defined as the consumption of more than one drink per day. Vasculopathies included diseases of the arteries of the heart, brain, neck, and lower limbs. The mean follow-up was 5.26±2.28 years (range, 1–9 years). Forty-three patients (86%) had at least a 2-year follow-up, while 27 (54%) had a 5-year follow-up. Encephalic magnetic resonance imaging scans with contrast medium did not identify any vestibular schwannomas.
Table 1.
Patient characteristics
| Sex | |
| Male | 23 (46%) |
| Female | 27 (54%) |
| Smoker | 11 (22%) |
| Alcohol consumption | 29 (58%) |
| Affected ear | |
| Right | 21 (42%) |
| Left | 29 (58%) |
| Tinnitus on onset | 30 (60%) |
| Tinnitus in the next years | 25 (50%) |
| Systemic hypertension | 11 (22%) |
| Diabetes mellitus | 0 (0%) |
| Dyslipidemia | 13 (26%) |
| Vasculopathies | 11 (22%) |
| Previous chemotherapy | 3 (6%) |
Statistical Analysis
All statistical analyses were carried out using the Statistical Package for Social Sciences, version 20.0 (IBM Corp., Armonk, NY, USA). A descriptive analysis of all data was performed, and they were reported as means or percentages and standard deviations. The Kolmogorov–Smirnov test demonstrated a non-Gaussian distribution of variables, so non-parametric tests were used. The Friedman test was used to assess differences among more than two paired groups in the mean of continuous variables. Post-hoc testing involved the Wilcoxon signed-rank test. The Bonferroni method was used to have a stricter criterion on whether to accept an effect as significant. The Mann-Whitney U test was used to assess differences between two independent groups in the mean of continuous variables. The chi-squared test was used for categorical variables. A p<0.05 was considered statistically significant.
RESULTS
The distribution of audiogram patterns and degree of hearing loss in our sample are reported in Table 2. Most patients had a downward-sloping or flat hearing loss at audiogram. Hearing loss was mainly mild (56% of cases), and 14 (28%) patients were affected by a severe/profound hearing loss.
Table 2.
Audiograms characteristics
| Audiogram morphology | |
| Upward-sloping | 10 (20%) |
| Downward-sloping | 24 (48%) |
| Flat loss | 16 (32%) |
| Trough shaped | 0 (0%) |
| Hearing loss (based on average PTA) | |
| Mild (25–40 dB HL) | 28 (56%) |
| Moderate (41–55 dB HL) | 6 (12%) |
| Moderate-severe (56–70 dB HL) | 2 (4%) |
| Severe (71–90 dB HL) | 9 (18%) |
| Profound (>90 dB HL) | 5 (10%) |
PTA: pure tone audiometry
The mean time between the onset and therapy was 11.52±14.58 days (range, 0–60 days). The treatment was administered seven or more days after the appearance SSNHL in 17 patients (34%). Complete recovery occurred in 13 patients (26%), partial recovery in 19 (38%), and no recovery in 18 (36%).
Average PTAs at 0.5, 1, 2, and 4 kHz at diagnosis, after treatment, and at follow-up are reported in Table 3. Differences between the first post-treatment control and follow-up evaluation were significant at the Wilcoxon signed-rank test only for the unaffected ear (p<0.05). After the follow-up, the mean data showed a slight non-significant improvement of the PTA threshold of the affected ear and a significant worsening of the hearing threshold of the unaffected ear (Table 3). This pattern is better evidenced in Figures 1 and 2. Figure 1 compares the mean PTA values before treatment, immediately after treatment, and at the last follow-up, while in Figure 2 the mean audiograms are reported, on the basis of the value at each frequency tested. Figure 2 and Table 3 show how the threshold difference between the affected and the unaffected ear decreased after a long follow-up.
Table 3.
PTA values (dB HL), as mean±standard deviation, with p values between the post-treatment and follow-up evaluations
| Affected ear | ||||
|---|---|---|---|---|
| Pre-treatment | Post-treatment | Follow-up | p | |
| 250 Hz | 45.90±28.73 | 34.40±25.77 | 30.70±24.91 | 0.092 |
| 500 Hz | 46.90±29.74 | 35.30±28.04 | 31.40±24.83 | 0.051 |
| 1000 Hz | 45.90±31.47 | 34.30±27.55 | 33.60±26.63 | 0.509 |
| 2000 Hz | 44.20±29.28 | 34.80±27.29 | 35.50±27.47 | 0.778 |
| 3000 Hz | 50.70±28.39 | 39.30±26.59 | 40.80±28.06 | 0.873 |
| 4000 Hz | 53.70±29.10 | 43.90±27.50 | 46.80±28.24 | 0.329 |
| 6000 Hz | 60.80±26.71 | 51.00±28.77 | 53.70±28.66 | 0.433 |
| 8000 Hz | 62.30±28.98 | 53.10±30.07 | 54.59±31.17 | 0.438 |
| Average PTAa | 47.68±27.99 | 37.08±26.22 | 39.70±25.75 | 0.850 |
| Unaffected ear | ||||
| Pre-treatment | Post-treatment | Follow-up | p | |
| 250 Hz | 18.40±9.66 | 18.10±9.58 | 20.70±11.16 | <0.001 |
| 500 Hz | 18.30±11.94 | 18.30±12.06 | 20.50±12.17 | 0.001 |
| 1000 Hz | 18.80±12.10 | 18.60±11.78 | 20.70±13.05 | 0.001 |
| 2000 Hz | 20.20±14.10 | 19.60±12.73 | 23.30±16.65 | <0.001 |
| 3000 Hz | 22.40±15.72 | 21.90±14.46 | 24.60±17.20 | 0.001 |
| 4000 Hz | 24.10±17.13 | 23.60±16.41 | 27.30±19.36 | 0.001 |
| 6000 Hz | 30.00±21.04 | 29.00±20.87 | 32.50±21.91 | 0.016 |
| 8000 Hz | 32.40±23.95 | 32.00±23.58 | 36.60±25.80 | 0.006 |
| Average PTAa | 20.35±12.98 | 20.03±12.40 | 25.11±15.36 | <0.001 |
| Difference between the affected and unaffected ear | ||||
| Pre-treatment | Post-treatment | Follow-up | ||
| 250 Hz | 28.80±28.10 | 17.50±26.09 | 11.50±25.32 | |
| 500 Hz | 30.30±29.06 | 18.70±28.30 | 12.60±24.56 | |
| 1000 Hz | 28.80±30.45 | 17.30±27.32 | 14.70±25.98 | |
| 2000 Hz | 25.90±27.64 | 16.80±26.60 | 14.50±26.64 | |
| 3000 Hz | 29.90±26.41 | 18.80±26.10 | 18.20±26.65 | |
| 4000 Hz | 31.30±26.26 | 21.80±26.55 | 21.60±25.52 | |
| 6000 Hz | 32.80±24.44 | 24.00±28.05 | 23.10±26.51 | |
| 8000 Hz | 32.00±26.24 | 23.00±28.84 | 19.20±28.42 | |
| Average PTAa | 29.16±26.39 | 18.73±25.78 | 15.93±24.19 | |
PTA: pure tone audiometry;
Average PTA on 0.5, 1, 2, and 4 kHz
Figure 1.
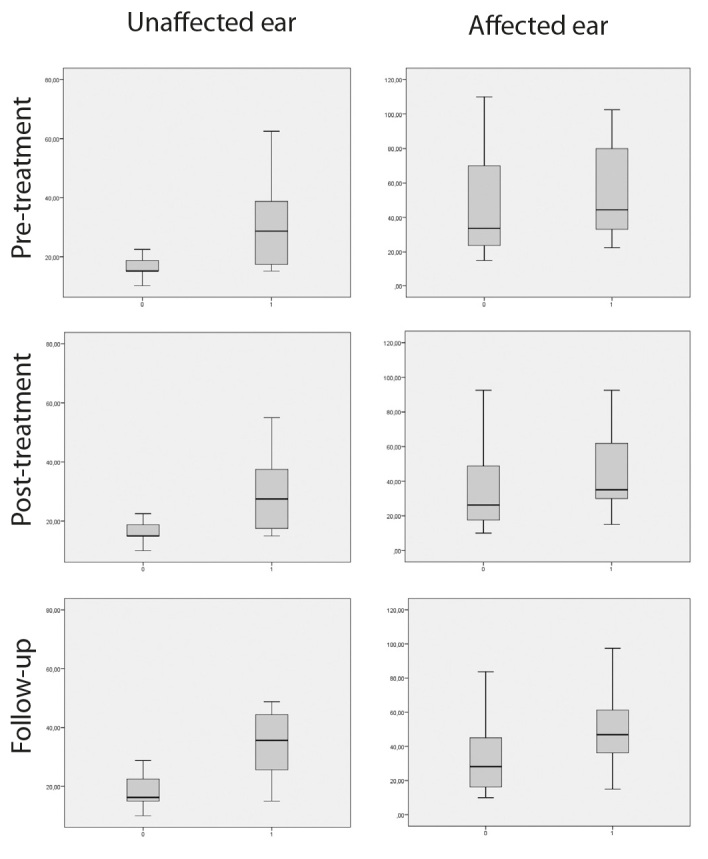
Average PTA on 0.5, 1, 2, and 4 kHz (unaffected and affected ear) at the pre-treatment, post-treatment, and follow-up evaluation. 0=age <60 years; 1=age >60 years.
Figure 2.
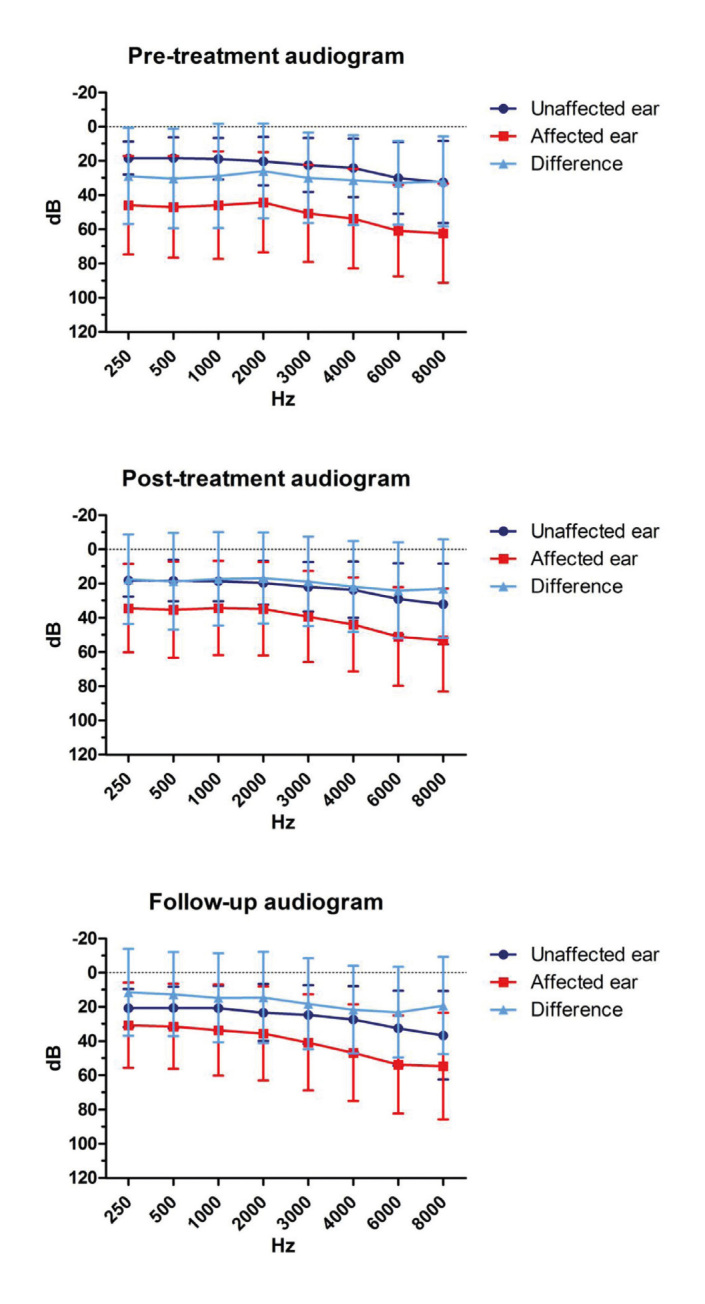
Average audiograms at the pre-treatment, post-treatment, and follow-up evaluation.
An average PTA correlated with aging at the pre-treatment, post-treatment, and follow-up evaluation (p<0.05 at the Mann-Whitney U test). Figure 1 highlights that the difference between patients aged <60 and >60 years was more evident for the unaffected ear, compared to the affected one. In particular, older patients showed a higher PTA threshold of the unaffected ear, while the PTA threshold of the affected ear was similar to younger patients.
Comparing differences over time (post-treatment vs. follow-up) between the unaffected and affected ear at each frequency, they were significant only at 250 and 500 Hz (p<0.05 at the Wilcoxon signed-rank test; Figure 3a). It was more evident in the cases of upward-sloping curves and flat loss (Figures 3b, 3c, and 3d).
Figure 3.
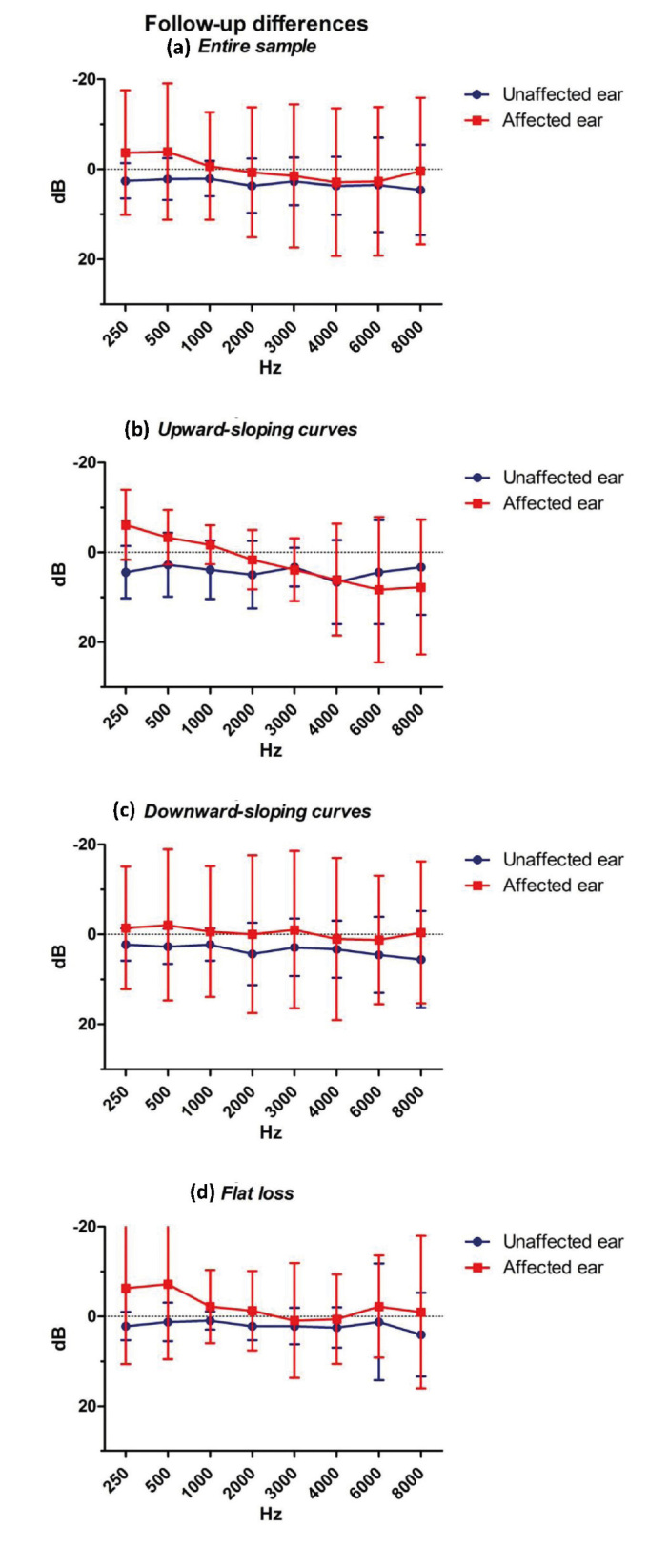
Differences between the post-treatment and follow-up audiograms. Data on the entire sample and according to audiogram morphology are shown (10 cases with upward-sloping curves, 24 with downward-sloping curves, and 16 with flat loss).
The analysis of the differences over time (post-treatment vs. follow-up) for the unaffected and affected ear did not show any significance comparing patients with and without 5-year follow-up (p>0.05 at the Mann-Whitney U test). Therefore, the passage of time after the SSNHL seemed to not affect the difference in the PTA threshold in both ears. However, a great standard deviation (as noticeable in Figure 3) was present and could be the reason for the absence of significance. Moreover, there was no correlation between the degree of recovery and differences over time (p>0.05 at the Mann-Whitney U test), neither in the unaffected ear nor in the affected one.
For the affected ear, 27 patients (54%) showed no changes over time, 13 cases (26%) showed worsening, and 10 cases (20%) showed an improvement in hearing, according to our classification of audiograms variation over time (Figure 4). The variation was correlated with alcohol consumption and the presence of vasculopathies (p<0.05 at the chi-squared test) (Table 4). Patients who consume alcohol or have vasculopathies have a higher risk of worsening of hearing. On the contrary, there was no correlation between the audiograms variation over time and the degree of recovery after treatment (p<0.05 at the chi-squared test).
Figure 4.
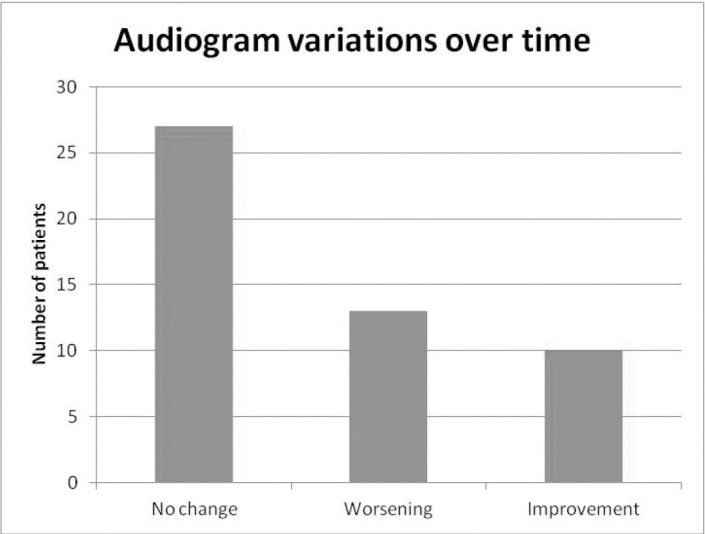
Audiograms variation over time.
Table 4.
Correlation tests for audiogram variation over time
| p | |
|---|---|
| Sex | 0.573 |
| Age >60 years | 0.312 |
| Smoking | 0.580 |
| Alcohol | 0.022 |
| Systemic hypertension | 0.236 |
| Dyslipidemia | 0.156 |
| Vasculopathies | 0.048 |
| Previous chemotherapy | 0.476 |
| Tinnitus on onset | 0.710 |
| Time before treatment >7 days | 0.886 |
| Audiogram morphology | 0.412 |
| Hearing loss >70 dB | 0.086 |
| Difference between the affected and unaffected ear >30 dB | 0.125 |
| Recovery | 0.222 |
DISCUSSION
SSNHL has controversial etiologies, treatments, and prognosis. The cause can be identified only in a small percentage of cases (approximately 10%) [1]. The literature indicates several risk factors for SSNHL, in particular cardiovascular factors [8–10]. The treatment is generally based on corticosteroids, and prognosis varies from absent to complete recovery [1].
Previous studies focused on post-treatment auditory recovery and recurrences [2–6, 11, 12]. Approximately 25%–33% of cases had complete recovery of the auditory function 2 weeks after the event, a similar percentage had no recovery, while a partial recovery could affect up to 50% of patients [1, 11]. Subjective feelings for residual tinnitus are almost the same at 6 and 24 months after the SSNHL treatment [12]. However, no study analyzed long-term audiometric outcomes in SSNHL. Not even the clinical practice guidelines report recommendations about a long-term follow-up [1].
Many factors can affect the post-treatment auditory recovery. Age, downward-sloping curves, the severity of hearing loss, and the time lapse between the onset and treatment seems to be a negative factor on recovery [11, 14–15]. Concerning the relapse, the literature is very heterogeneous: the incidence varies from 0.8% to 47% [2–6]. A higher recurrence rate was reported for low frequencies SSNHL [6]. Approximately 9% of cases with SSNHL develops Menière’s disease, but not all SSNHLs involving low frequencies develop to this disease (approximately 23%) [16–18]. Contrary to the literature, in our study, no patient with the SSNHL involving low tones developed Menière’s disease.
The effect of aging on the cochlear function in subjects without ear disease and without occupational exposure to noise is well known [19]. A mathematical model based on a population without the occupational exposure to noise allows for the prediction of a PTA threshold at different frequencies in relation to age (ISO 7029–2000) [20]. In particular, it is known that aging mainly affects high frequencies. Our study analyzed the audiometric evolution over time in patients with previous SSNHL, comparing the affected and unaffected ear. Basing on our criteria, 54% of subjects showed no changes over time, 26% showed worsening, and 20% an improvement of hearing over time, after a mean follow-up of 5.26±2.28 years. Interestingly, aging seemed to effect on average the unaffected ear but not the ears with SSNHL. We can speculate that aging begins to influence the ear with previous SSNHL only when the other ear reaches the audiometric threshold of the affected ear [19]. Thus, the effects of presbycusis and SSNHL seems to be non-additive. The reason could be the possible common damage on inner hairy cells and/or auditory nerve fibers. Generally, we can say that, after SSNHL, the affected ear shows a stability in the hearing loss over time, compared to the unaffected one.
Our analysis of the hearing variation during the follow-up showed that its evolution over time was not influenced by the degree of post-treatment recovery. However, patients who recovered completely after the initial treatment did not improve further, and few got worse.
The difference between the threshold values of the affected and unaffected ear at the follow-up audiogram could be considered the effect of SSNHL on the cochlear function over time. An average improvement of hearing over time was observed at low frequencies. It was more evident in cases of upward-sloping curves and flat loss. However, a great standard deviation was present. Therefore, further studies with larger samples are needed to correctly evaluate this time trend. Moreover, a specific focus on low-frequency SSNHL should be set to better assess the possibility of an endolymphatic hydrops in such cases. Because of the fluctuating nature of hearing loss in the initial phase of endolymphatic hydrops, prospective studies are required to analyze when the improvement of hearing at low frequencies occurs in patients with SSNHL.
The presence of vasculopathy and alcohol consumption was associated with a worse long-term prognosis. This is in compliance with the studies that identified vascular risk factors, like smoking, that affect hearing [21].
The limits of our study include the presence of heterogeneous treatments and a small number of patients. Therefore, this retrospective study may be considered a pilot study, with the need of further prospective studies on larger samples.
CONCLUSION
The evolution over time of SSNHL is not predictable on the basis of the clinical and audiometric data. However, the majority of cases shows a stability of hearing in the affected ear, compared to the unaffected one. Further prospective studies are mandatory to better assess the variations over time, prognostic factors, and the relationship with the effect of aging on hearing.
Footnotes
Ethics Committee Approval: All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval by the Institutional Review Board was not needed because of the retrospective nature of the study.
Informed Consent: Informed consent was obtained from all participants included in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – G.P., G.R., R.A.; Design - G.P., G.R.,R.A.; Supervision - G.P., G.R.; Resource - G.P., G.R.; Materials - G.R., N.N., G.B.; Data Collection and/or Processing - G.R., N.N., G.B., M.N.; Analysis and/or Interpretation – G.R., R.A.; Literature Search - G.R., N.N., G.B., M.N.; Writing - G.R., N.N., G.B., R.A.; Critical Reviews – R.A., G.P.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Stachler RJ, 1, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146:S1–35. doi: 10.1177/0194599812436449. [DOI] [PubMed] [Google Scholar]
- 2.Furuhashi A, Matsuda K, Asahi K, Nakashima T. Sudden deafness: long-term follow-up and recurrence. Clin Otolaryngol. 2002;27:458–63. doi: 10.1046/j.1365-2273.2002.00612.x. [DOI] [PubMed] [Google Scholar]
- 3.Psifidis AD, Psillas GK, Daniilidis JC. Sudden sensorineural hearing loss: long-term follow-up results. Otolaryngol Head Neck Surg. 2006;134:809–15. doi: 10.1016/j.otohns.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Park I, Kim YB, Choi SH, Hong SM. Clinical Analysis of Recurrent Sudden Sensorineural Hearing Loss. ORL J Otorhinolaryngol Relat Spec. 2013;75:245–9. doi: 10.1159/000353552. [DOI] [PubMed] [Google Scholar]
- 5.Wu CM. Recurrence of idiopathic sudden sensorineural hearing loss: a retrospective cohort study. Otol Neurotol. 2014;35:1736–41. doi: 10.1097/MAO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 6.Fushiki H, Junicho M, Aso S, Watanabe W. Recurrence rate of idiopathic sudden low-tone sensorineural hearing loss without vertigo: a long-term follow-up study. Otol Neurotol. 2009;30:295–8. doi: 10.1097/MAO.0b013e31819d3496. [DOI] [PubMed] [Google Scholar]
- 7.Albera R, Canale A, Cassandro C, Albera A, Sammartano AM, Dagna F. Relationship between hearing threshold at the affected and unaffected ear in unilateral Meniere’s disease. Eur Arch Otorhinolaryngol. 2016;273:51–6. doi: 10.1007/s00405-014-3466-8. [DOI] [PubMed] [Google Scholar]
- 8.Lin RJ, Krall R, Westerberg BD, Chadha NK, Chau JK. Systematic review and meta-analysis of the risk factors for sudden sensorineural hearing loss in adults. Laryngoscope. 2012;122:624–635. doi: 10.1002/lary.22480. [DOI] [PubMed] [Google Scholar]
- 9.Chau JK, Lin JR, Atashband S, Irvine RA, Westerberg BD. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope. 2010;120:1011–21. doi: 10.1002/lary.20873. [DOI] [PubMed] [Google Scholar]
- 10.Lasagni A, Giordano P, Lacilla M, Raviolo A, Trento M, Camussi E, et al. Cochlear, auditory brainstem responses in Type 1 diabetes: relationship with metabolic variables and diabetic complications. Diabet Med. 2016;33:1260–7. doi: 10.1111/dme.13039. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn M, Heman-Ackah SE, Shaikh JA, Roehm PC. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif. 2011;15:91–105. doi: 10.1177/1084713811408349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michiba T, Kitahara T, Hikita-Watanabe N, Fukushima M, Ozono Y, Imai R, et al. Residual tinnitus after the medical treatment of sudden deafness. Auris Nasus Larynx. 2013;40:162–6. doi: 10.1016/j.anl.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Pecorari G, Riva G, Naqe N, Nardo M, Bruno G, Albera R. Sudden sensorineural hearing loss: risk factors and comorbidities. Otolaryngol. 2019;69:9–14. doi: 10.23736/S0392-6621.18.02203-8. [DOI] [Google Scholar]
- 14.Fetterman BL, Saunders JE, Luxford WM. Prognosis and treatment of sudden sensorineural hearing loss. Am J Otol. 1996;17:529–36. [PubMed] [Google Scholar]
- 15.Bogaz EA, Maranhão ASA, Inoue DP, Suzuki FAB, Penido NO. Variables with prognostic value in the onset of idiopathic sudden sensorineural hearing loss. Braz J Otorhinolaryngol. 2015;81:520–6. doi: 10.1016/j.bjorl.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junicho M, Aso S, Fujisaka M, Watanabe Y. Prognosis of low-tone sudden deafness - does it inevitably progress to Meniere’s disease? Acta Otolaryngol. 2008;128:304–8. doi: 10.1080/00016480601002096. [DOI] [PubMed] [Google Scholar]
- 17.Fushiki H, Junicho M, Kanazawa Y, Aso S, Watanabe Y. Prognosis of sudden low-tone loss other than acute low-tone sensorineural hearing loss. Acta Otolaryngol. 2010;130:559–64. doi: 10.3109/00016480903311245. [DOI] [PubMed] [Google Scholar]
- 18.Albera A, Albera R, Canale A, Caranzano F, Gervasio CF. Short-term result of mannitol administration on hearing loss improvement in Menière’s disease and in sensorineural low-frequency fluctuating hearing loss without vertigo. Otorhinolaryngol. 2018;68:2–5. [Google Scholar]
- 19.Albera R, Lacilla M, Piumetto E, Canale A. Noise-induced hearing loss evolution: influence of age and exposure to noise. Eur Arch Otorhinolaryngol. 2010;267:665–71. doi: 10.1007/s00405-009-1096-3. [DOI] [PubMed] [Google Scholar]
- 20.International Organization for Standard: Acoustics. ISO 7029. Geneva: International Organization for Standard. ISO 7029; 2000. Statistical distribution of hearing thresholds as a function of age. [Google Scholar]
- 21.Pezzoli M, Lofaro D, Oliva A, Orione M, Cupi D, Albera A, et al. Effects of smoking on Eustachian tube and hearing. Int Tinnitus J. 2017;21:98–103. doi: 10.5935/0946-5448.20170019. [DOI] [PubMed] [Google Scholar]


