Abstract
OBJECTIVES
There is limited literature regarding the objective estimation of auditory attention in healthy individuals who regularly practice dance. This study attempted to evaluate the contralateral suppression of otoacoustic emissions (OAE) in Bharatanatyam dancers and non-dancers.
MATERIALS and METHODS
The study included40 adults (20 dancers and 20 non-dancers) with normal hearing. The differences in the contralateral suppression of distortion product OAE between the groups were compared.
RESULTS
The results of the present study revealed that there was an increased amount of suppression of OAE in dancers compared with non-dancers. It suggests that dance practice enhances sensory perception and improves auditory attention. The constant practice of dance could have led to plasticity of the efferent auditory system.
CONCLUSION
Thus, dance training may be used to strengthen efferent auditory system functioning. However, further studies with a larger sample size are essential for better generalization of the results.
Keywords: Dancers, non-dancers, otoacousticemissions, contralateral suppression
INTRODUCTION
The organ of Corti at the basilar membrane is the sensory organ for hearing and is made up of sensory hair cells along with accessory cells and structures. Sensory hair cells are of two types, including flask-shaped inner hair cells (IHC) and tube-like outer hair cells (OHC). The afferent nerve supply has sensory neurons that are ascending and carry electrical inputs from the cochlea to the auditory nervous system. The efferent auditory nerve supply has descending neurons that carry electrical signals to the cochlea from the nervous system. Efferent signals through the olivocochlear bundle or Rasmussen’s bundle reach the cochlea [1]. The efferent neuron directly synapses with the OHC. However, in the case of IHC, it synapses with the afferent neuron associated with the IHC.
Functioning of the efferent auditory system is vital for human auditory perception. The medial efferent olivocochlear bundle plays a major role in auditory attention [1–3]. The efferent system causes inhibition of responses that are not important and thus enhances attention [4]. The functioning of the medial efferent auditory system is assessed through contralateral suppression of otoacoustic emissions (OAE), which is reported to be modulated by auditory attention [5, 6]. Dance is a form of art that requires constant auditory attention to pitch, rhythm, and tempo of the music [7, 8].
Bharatanatyam is a traditional Indian classical dance form, mainly practiced in South India. The word Bharatanatyam connotes a dance form that would harmoniously express the bhava, raga, and tala. Thus, it is a culmination of the perception of music and expression through body movements appropriate to the rhythm of the music. The dance form requires auditory attention to perform and act appropriately. Hence, improved attention because of practicing dance may induce plasticity in the neural system important for attention. The efferent auditory system is also crucial in improving auditory attention [1, 2]. Research also shows that there is enhanced activity of the medial efferent auditory system in musicians when compared with non-musicians because of higher auditory attention [9–11]. Walsh et al. [12] reported that the auditory efferent system was more active during selective attention for both visual and auditory tasks.
Thus, there is evidence to show that dance enhances attention [7] and auditory attention modulates olivocochlear efferent functioning [6]. However, there is a paucity of literature in the objective estimation of auditory attention in healthy individuals who regularly practice dance. Thus, the present study investigates differences in the olivocochlear activity between individuals who practice and do not practice Bharatanatyam dance regularly. The difference in the amount of suppression of transient evoked otoacoustic emissions (TEOAE) and distortion product otoacoustic emissions (DPOAE) between the two groups was determined. Thus, the study aimed to evaluate auditory efferent system functioning between dancers and non-dancers by comparing the TEOAE and DPOAE amplitudes in the presence and absence of noise.
MATERIALS and METHODS
Participants
Forty individuals (age, 18–25 years) with normal hearing participated in the study. They were divided into two groups depending upon whether they practice Bharatanatyam (20 participants; Mean age, 21.66 years; SD, 3.2) or not (20 participants; Mean age, 20.98 years; SD, 2.9). Individuals in the dancers group were practicing for at least five to 10 years. For this study, the participants were only females as studies have reported that the amplitude of DPOAE varies across gender. All the participants of the study had no historyofotologic symptoms, noise exposure, familial hearing loss, and use of ototoxic drugs. All the participants had normal hearing sensitivity with a mean pure tone average of 8.25 (SD=4.16) in the control group and 7.75 (SD=5.13) in the experimental group. All the participants in both groups were from the same region and ethnicity.
Procedure
Air and bone conduction thresholds for pure tones and speech identification scores were obtained using a two-channel diagnostic audiometer using the modified version of the Hughson and Westlake procedure [13]. Phonemically balanced words in Kannada were used to obtain speech identification scores using headphones. The middle ear status of the participants was examined using the GrasonStadler Inc. Tympstar (GSI-TS) immittance meter. Tympanogram and acoustic reflexes were obtained for both the ears with a probe tone frequency of 226 Hz.
All the OAE measurements were performedon both ears. The recordings were conducted using the Mimosa Acoustics OAE equipment. TEOAE was measured using non-linear click trains at 80 dB peSPL ensuring appropriate probe fit. TEOAE amplitude (dB SPL) was noted and recorded at frequencies of 1000, 1500, 2000, 3000, and 4000 Hz. It was recorded with and without contralateral white noise presented at an intensity of 50 dB SPL. Similarly, distortion product signal amplitudes across the frequencies of 1000 Hz, 2000 Hz, 3000 Hz, 4000 Hz, 5000 Hz, and 6000 Hz were noted. It was also recorded with and without contralateral white noise presented at an intensity of 50 dB SPL.
For the study, non-invasive testing procedures adhered to the conditions of the ethical approval committee of the institute.
RESULTS
The amount of suppression was calculated by subtracting the amplitude of the TEOAE and DPOAE with noise from the TEOAE and DPOAE amplitude without noise. The results of the study showed an increase in the amount of suppression for Bharatanatyam dancers compared with non-dancers. The mean and SD of the amount of TEOAE suppression across the frequency for both the groups are shown in figure 1. The mean and SD of the amount of DPOAE suppression across frequency for both the groups are shown in Figure 2.
Figure 1.
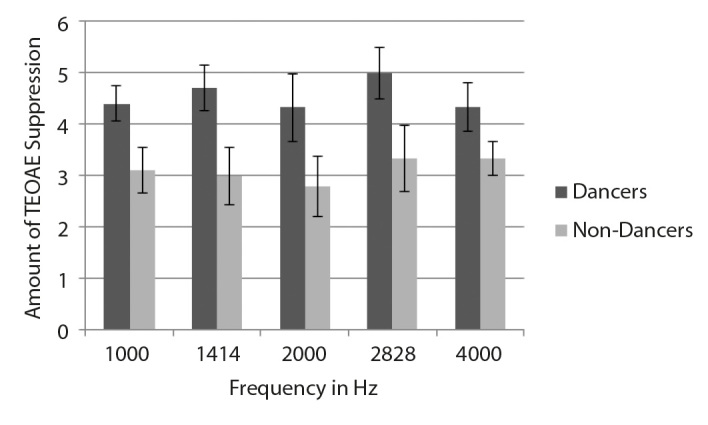
Mean and SD of the amount of TEOAE suppression across frequencies in dancers and non-dancers.
Figure 2.
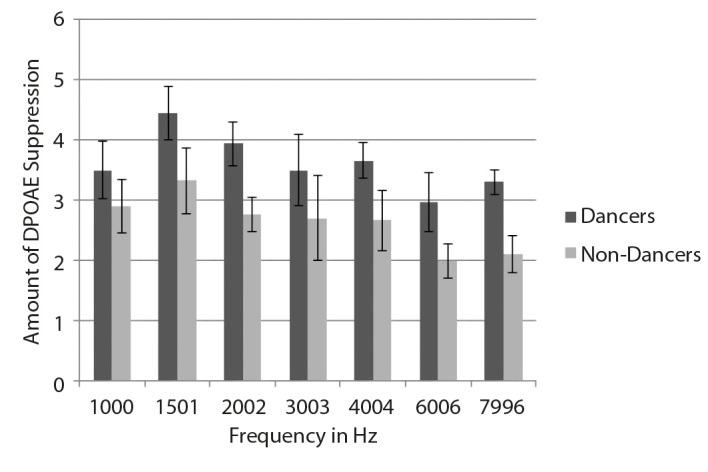
Mean and SD of the amount of DPOAE suppression across frequencies in dancers and non-dancers.
The Shapiro–Wilk test of normality revealed that the data did not fit a normal distribution. Thus, non-parametric inferential statistics were analyzed. Mann–Whitney U-tests were performed to compare the differences in the amount of suppression across the two groups for TEOAE and DPOAE. The results of the Mann–Whitney U-test revealed a significant increase (p<0.05) in the amount of suppression for dancers compared with non-dancers for both TEOAE and DPOAE. The amount of suppression was averaged across all frequencies for each participant, and the mean differences were also analyzed. The results revealed a significant increase (p<0.05) in the amount of suppression (~2 dB for TEOAE and ~3 dB for DPOAE) in individuals who were Bharatanatyam dancers.
DISCUSSION
The results of the study indicated increased activation of medial olivocochlear bundle functioning in Bharatanatyam dancers compared with non-dancers. The increased contralateral suppression of OAE suggests that there is an enhancement of auditory attention in dancers. Poikonen et al. [7] reported that early auditory processing is significantly enhanced in contemporary dancers compared with non-dancers using electrophysiological studies. Studies have revealed that musicians have significantly more suppression. This was reasoned to be due to the constant dose of low-level noise exposure in the form of music which may have a conditioning effect on the musician’s ears, thereby increasing the ability to suppress otoacoustic emissions [14]. Because music is similarly processed in both dancers and musicians, our results corroborate with those of previous studies. Silva et al. [8] reported that temporal resolution and auditory figure-ground perception is enhanced in dancers compared with non-dancers. The results supplement the above findings and suggest that auditory attention is improved in dancers than in non-dancers. This study provides objective evidence of enhanced auditory attention in Bharatanatyam dancers. Thus, the study indicates increased plasticity of the efferent auditory system with regular dance practice and the use of dance as a tool to improve auditory attention. However, further studies using other sensory modalities on a larger sample are necessary for better generalization.
CONCLUSION
The present study evaluated contralateral suppression of OAE in Bharatanatyam dancers and non-dancers. The results indicated that there was an increased amount of suppression of OAE among dancers compared with non-dancers. The results of the study suggest that practicing dance enhances sensory perception and improves auditory attention. The constant practice of dance could have led to plasticity of the efferent auditory system. Thus, dance training may be used to strengthen efferent auditory system functioning. To generalize these findings, further studies on a larger sample size are warranted.
Acknowledgements
The authors would thank the Director, All India Institute of Speech and Hearing for permitting us to carry out the study. The authors would also thank all the participants of the study.
Footnotes
This study was presented at the “Indian Speech and Hearing Association Conference”, January, 2018, Mysuru, India.
Ethics Committee Approval: Ethical approval was obtained from the Institutional Ethical Review Board of All India Institute of Speech and Hearing, Mysuru.
Informed Consent: Informed consent was obtained from all the participants of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – J.J., A.S., G.K.J., P.P.; Design – J.J., A.S., G.K.J., P.P.; Supervision – P.P.; Resource – J.J., A.S., G.K.J., P.P.; Materials – J.J., A.S., G.K.J., P.P.; Data Collection and/or Processing – J.J., A.S., G.K.J., P.P.; Analysis and/or Interpretation – J.J., A.S., G.K.J., P.P.; Literature Search – J.J., A.S., G.K.J., P.P.; Writing – J.J., A.S., G.K.J., P.P.; Critical Reviews – J.J., A.S., G.K.J., P.P.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Giard MH, Collet L, Bouchet P, Pernier J. Auditory selective attention in the human cochlea. Brain Res. 1994;633:353–6. doi: 10.1016/0006-8993(94)91561-X. [DOI] [PubMed] [Google Scholar]
- 2.de Boer J, Thornton AR. Neural correlates of perceptual learning in the auditory brainstem: efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J Neurosci. 2008;28:4929–37. doi: 10.1523/JNEUROSCI.0902-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill JC, Prasher DK, Luxon LM. Latency of contralateral sound-evoked auditory efferent suppression of otoacousticemissions. Acta Otolaryngol. 1997;117:343–51. doi: 10.3109/00016489709113405. [DOI] [PubMed] [Google Scholar]
- 4.Scharf B, Magnan J, Collet L, Ulmer E, Chays A. On the role of the olivocochlear bundle in hearing: A case study. Hear Res. 1994;75:11–26. doi: 10.1016/0378-5955(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 5.Froehlich P, Collet L, Morgon A. Transiently evoked otoacoustic emission amplitudes change with changes of directed attention. Physiol Behav. 1993;53:679–82. doi: 10.1016/0031-9384(93)90173-D. [DOI] [PubMed] [Google Scholar]
- 6.Berlin CI, Hood LJ, Wen H, Szabo P, Cecola RP, Rigby P, et al. Contralateral suppression of non-linear click-evoked otoacoustic emissions. Hear Res. 1993;71:1–11. doi: 10.1016/0378-5955(93)90015-S. [DOI] [PubMed] [Google Scholar]
- 7.Poikonen H, Toiviainen P, Tervaniemi M. Early auditory processing in musicians and dancers during a contemporary dance piece. Sci Rep. 2016;6:33056. doi: 10.1038/srep33056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva MRD, Dias KZ, Pereira LD. Study of the auditory processes of temporal resolution and auditory figure-ground in dancers. Revista CEFAC. 2015;17:1033–41. doi: 10.1590/1982-0216201517413514. [DOI] [Google Scholar]
- 9.Brashears SM, Morlet TG, Berlin CI, Hood LJ. Olivocochlear efferent suppression in classical musicians. J Am Acad Audiol. 2003;14:314–24. [PubMed] [Google Scholar]
- 10.Micheyl C, Khalfa S, Perrot X, Collet L. Difference in cochlear efferent activity between musicians and non-musicians. Neuroreport. 1997;8:1047–50. doi: 10.1097/00001756-199703030-00046. [DOI] [PubMed] [Google Scholar]
- 11.Perrot X, Micheyl C, Khalfa S, Collet L. Stronger bilateral efferent influences on cochlear biomechanical activity in musicians than in non-musicians. Neurosci Lett. 1999;262:167–70. doi: 10.1016/S0304-3940(99)00044-0. [DOI] [PubMed] [Google Scholar]
- 12.Walsh KP, Pasanen EG, McFadden D. Changes in otoacoustic emissions during selective auditory and visual attention. J Acoust Soc Am. 2015;137:2737–57. doi: 10.1121/1.4919350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carhart R, Jerger JF. Preferred method for clinical determination of pure-tone thresholds. J Speech Hear Disord. 1959;24:330–45. doi: 10.1044/jshd.2404.330. [DOI] [Google Scholar]
- 14.Kumar P, Sanju HK, Nikhil J. Temporal resolution and active auditory discrimination skill in vocal musicians. Int Arch Otorhinolaryngol. 2016;20:310–4. doi: 10.1055/s-0035-1570312. [DOI] [PMC free article] [PubMed] [Google Scholar]


