Abstract
Background
Hypertension is an important risk factor for adverse cardiovascular events including stroke, myocardial infarction, heart failure and renal failure. The main goal of treatment is to reduce these events. Systematic reviews have shown proven benefit of antihypertensive drug therapy in reducing cardiovascular morbidity and mortality but most of the evidence is in people 60 years of age and older. We wanted to know what the effects of therapy are in people 18 to 59 years of age.
Objectives
To quantify antihypertensive drug effects on all‐cause mortality in adults aged 18 to 59 years with mild to moderate primary hypertension. To quantify effects on cardiovascular mortality plus morbidity (including cerebrovascular and coronary heart disease mortality plus morbidity), withdrawal due adverse events and estimate magnitude of systolic blood pressure (SBP) and diastolic blood pressure (DBP) lowering at one year.
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomized controlled trials up to January 2017: the Cochrane Hypertension Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (from 1946), Embase (from 1974), the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. We contacted authors of relevant papers regarding further published and unpublished work.
Selection criteria
Randomized trials of at least one year' duration comparing antihypertensive pharmacotherapy with a placebo or no treatment in adults aged 18 to 59 years with mild to moderate primary hypertension defined as SBP 140 mmHg or greater or DBP 90 mmHg or greater at baseline, or both.
Data collection and analysis
The outcomes assessed were all‐cause mortality, total cardiovascular (CVS) mortality plus morbidity, withdrawals due to adverse events, and decrease in SBP and DBP. For dichotomous outcomes, we used risk ratio (RR) with 95% confidence interval (CI) and a fixed‐effect model to combine outcomes across trials. For continuous outcomes, we used mean difference (MD) with 95% CI and a random‐effects model as there was significant heterogeneity.
Main results
The population in the seven included studies (17,327 participants) were predominantly healthy adults with mild to moderate primary hypertension. The Medical Research Council Trial of Mild Hypertension contributed 14,541 (84%) of total randomized participants, with mean age of 50 years and mean baseline blood pressure of 160/98 mmHg and a mean duration of follow‐up of five years. Treatments used in this study were bendrofluazide 10 mg daily or propranolol 80 mg to 240 mg daily with addition of methyldopa if required. The risk of bias in the studies was high or unclear for a number of domains and led us to downgrade the quality of evidence for all outcomes.
Based on five studies, antihypertensive drug therapy as compared to placebo or untreated control may have little or no effect on all‐cause mortality (2.4% with control vs 2.3% with treatment; low quality evidence; RR 0.94, 95% CI 0.77 to 1.13). Based on 4 studies, the effects on coronary heart disease were uncertain due to low quality evidence (RR 0.99, 95% CI 0.82 to 1.19). Low quality evidence from six studies showed that drug therapy may reduce total cardiovascular mortality and morbidity from 4.1% to 3.2% over five years (RR 0.78, 95% CI 0.67 to 0.91) due to reduction in cerebrovascular mortality and morbidity (1.3% with control vs 0.6% with treatment; RR 0.46, 95% CI 0.34 to 0.64). Very low quality evidence from three studies showed that withdrawals due to adverse events were higher with drug therapy from 0.7% to 3.0% (RR 4.82, 95% CI 1.67 to 13.92). The effects on blood pressure varied between the studies and we are uncertain as to how much of a difference treatment makes on average.
Authors' conclusions
Antihypertensive drugs used to treat predominantly healthy adults aged 18 to 59 years with mild to moderate primary hypertension have a small absolute effect to reduce cardiovascular mortality and morbidity primarily due to reduction in cerebrovascular mortality and morbidity. All‐cause mortality and coronary heart disease were not reduced. There is lack of good evidence on withdrawal due to adverse events. Future trials in this age group should be at least 10 years in duration and should compare different first‐line drug classes and strategies.
Keywords: Adult, Humans, Middle Aged, Young Adult, Antihypertensive Agents, Antihypertensive Agents/adverse effects, Antihypertensive Agents/therapeutic use, Bendroflumethiazide, Bendroflumethiazide/therapeutic use, Blood Pressure, Blood Pressure/drug effects, Cause of Death, Coronary Disease, Coronary Disease/mortality, Coronary Disease/prevention & control, Hypertension, Hypertension/drug therapy, Hypertension/mortality, Methyldopa, Methyldopa/therapeutic use, Myocardial Infarction, Myocardial Infarction/mortality, Myocardial Infarction/prevention & control, Patient Dropouts, Patient Dropouts/statistics & numerical data, Propranolol, Propranolol/therapeutic use, Randomized Controlled Trials as Topic, Stroke, Stroke/mortality, Stroke/prevention & control
Plain language summary
Treatment for hypertension in adults aged 18 to 59 years
Review question
We wanted to study the benefits and harms of using blood pressure lowering (antihypertensive) medicines in adults aged 18 to 59 years with raised blood pressure (hypertension).
We searched the available medical literature to find all the trials that had assessed this question. The data included in this review is up to date as of January 2017.
Background
Hypertension increases the risk of stroke, heart attacks and heart failure; therefore, the main goal of treatment with antihypertensive medicines is to reduce this risk. There is substantial evidence mostly in people older than 60 years that antihypertensive therapy reduces these outcomes.
Study characteristics
We found seven studies that randomly assigned 17,327 people aged 18 to 59 years with hypertension to either antihypertensive medicines or placebo (pretend treatment)/no treatment. The average duration of treatment was five years. Medicine classes studied in most people included medicines called thiazide diuretics or beta‐blockers.
Key results
Treatment may have little or no effect on death from any cause compared with placebo or no treatment (2.4% with placebo/no treatment versus 2.3% with treatment; low quality evidence) and it may reduce the number of people experiencing heart disease or death from heart disease from 4.1% to 3.2% (low quality evidence). It may reduce stroke by a small amount from 1.3% to 0.6% (low quality evidence). We are not certain about the effects of treatment on the number of people who had blocked arteries (low quality evidence). Withdrawal due to side effects increased from 0.7% to 3.0% although the quality of evidence for this result was very low. The effects of treatment on blood pressure varied between the studies and we are uncertain as to how much of a difference treatment makes on average.
Conclusions
Antihypertensive medicines for adults aged 18 to 59 years with raised blood pressure have a small beneficial effect to reduce stroke. However, death due to all‐causes and heart attack were not reduced and withdrawals due to side effects were increased.
Quality of evidence
The overall evidence was graded as low or very low quality.
Summary of findings
Summary of findings for the main comparison. Antihypertensive drug therapy compared to control for hypertension in adults aged 18 to 59 years.
| Antihypertensive drug therapy compared to control for hypertension in adults aged 18 to 59 years | ||||||
| Patient or population: adults aged 18‐59 years with primary hypertension (mild to moderate systolic or diastolic hypertension) Setting: outpatient Intervention: antihypertensive drug therapy (mean duration: 5 years) Comparison: control (placebo or untreated) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with antihypertensive drug therapy | |||||
| All‐cause mortality | 24 per 1000 | 23 per 1000 (19 to 28) | RR 0.94 (0.77 to 1.13) | 16,776 (5 RCTs) | ⊕⊕⊝⊝ Low 1,2 | ‐ |
| Total cardiovascular mortality + morbidity | 41 per 1000 | 32 per 1000 (27 to 37) | RR 0.78 (0.67 to 0.91) | 17,278 (6 RCTs) | ⊕⊕⊝⊝ Low 1,3 | ARR 0.9%, NNTB = 112 |
| Cerebrovascular mortality + morbidity | 13 per 1000 | 6 per 1000 (5 to 9) | RR 0.46 (0.34 to 0.64) | 17,278 (6 RCTs) | ⊕⊕⊝⊝ Low 1,3 | ARR 0.7%, NNTB 143 |
| Coronary heart disease mortality + morbidity | 26 per 1000 | 26 per 1000 (21 to 31) | RR 0.99 (0.82 to 1.19) | 16,241 (4 RCTs) | ⊕⊕⊝⊝ Low 1,3 | ‐ |
| Withdrawal due to adverse events | 7 per 1000 | 32 per 1000 (11 to 93) | RR 4.82 (1.67 to 13.92) | 1,223 (3 RCTs) | ⊕⊝⊝⊝ Very low 1,2,4 | ARI 3.8%, NNTH 27 |
|
Decrease in SBP5 at end of 1 year |
The mean SBP was 0 | MD 14.98 lower (20.44 lower to 9.52 lower) | ‐ | 14,845 (3 studies) | ⊕⊝⊝⊝ Very low 1,3,7 | ‐ |
|
Decrease in DBP6 at end of 1 year |
The mean DBP was 0 | MD 7.62 lower (10.55 blower to 4.69 lower) | ‐ | 15,857 (4 studies) | ⊕⊝⊝⊝ Very low 1,3,7 | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARI: absolute risk increase; ARR: absolute risk reduction; CI: confidence interval; DBP: diastolic blood pressure; MD: mean difference; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat to for an additional harmful outcome; RCT: randomized controlled trial; RR: risk ratio; SBP: systolic blood pressure. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Several additional trials met the inclusion criteria but did not report data in the 18‐ to 59‐year‐old subgroup of participants (which are listed under Characteristics of excluded studies table).
2Imprecision due to wide confidence interval.
3High risk of bias due to lack of blinding of physician and participants as well as incomplete outcome data reporting in the MRC‐TMH trial.
4Only 3 out of 7 included trials reported this outcome. The data from MRC‐TMH 1985 for this subgroup were not available. High risk of attrition bias and unclear risk of reporting bias of the USPHSHCSG 1977 study which contributes 100% weight to the effect size.
5The range of change in SBP in the control group ranged from increase by 1.5 mmHg to decrease from 9 to 14 mmHg.
6The range of decrease in DBP in the control group ranged from 0.6 mmHg to 7 mmHg.
7Significant heterogeneity (I2 > 95%).
Background
Description of the condition
Elevated blood pressure (BP), commonly called hypertension, is an important healthcare problem internationally. Hypertension has been defined as a systolic blood pressure (SBP) of at least 140 mmHg or a diastolic blood pressure (DBP) of at least 90 mmHg, or both. The worldwide prevalence of elevated BP is about 26% of the adult population, and the prevalence increases with age (Kearney 2005). Elevated blood pressure is a major contributor to adverse cardiovascular events (cardiovascular disease; CVD) and has been estimated to contribute 4.5% to the global disease burden (WHO 2003). People of younger age (less than 60 years) have a lower prevalence of hypertension than older people.
The main goal of antihypertensive treatment is to reduce strokes, myocardial infarctions (MI) and heart failure. Several systematic reviews have shown benefit of antihypertensive drug therapy in reducing cardiovascular mortality and morbidity primarily in people over 60 years of age (Collins 1990; Thijs 1992; Psaty 1997; Gueyffier 1999; Psaty 2003; Musini 2009; Wright 2009).
Does benefit due to antihypertensive therapy differ in different age groups? There are various claims made in the literature regarding the benefits of antihypertensive therapy in different age groups. One meta‐analysis of 31 trials with 190,606 participants by the Blood Pressure Lowering Treatment Trialists' Collaboration concluded that reduction of BP produces benefits in younger (aged less than 65 years) and older (aged greater than 65 years) adults, with no strong evidence that protection against major vascular events afforded by different drug classes varies substantially with age (BPLTTC 2008). In contrast, the results from the Prospective Studies Collaboration meta‐analysis by analyzing individual data of one million adults from 61 prospective observational studies of BP and mortality showed that at ages 40 to 69 years, each difference of 20 mmHg usual SBP (approximately 10 mmHg usual DBP) was associated with more than a two‐fold difference in the stroke death rate, and with two‐fold differences in the death rates from ischaemic heart disease (IHD) and other vascular causes. The annual absolute estimated differences in risk are greater in old age (Prospective Studies Collaboration 2002).
Although reduction in BP is believed to result in reduction in clinically important outcomes such as stroke or MIs, it a poor surrogate outcome measure for all‐cause mortality and morbidity. Similar or greater reduction in BP by different classes of antihypertensive drugs may not necessarily result in similar or greater magnitude of reduction in clinically relevant outcomes (Wright 2009).
Description of the intervention
High BP should be managed first by changing life style (eating a healthy diet with less salt, exercising regularly, quitting smoking and maintaining a healthy weight). When these lifestyle changes are not sufficient, treatment with antihypertensive drugs is recommended. Several different classes of medications are available to reduce BP (thiazide diuretics, beta‐blockers, angiotensin‐converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB), calcium channel blockers and alpha‐blockers) (NICE 2016).
How the intervention might work
Different classes of antihypertensive drugs have different mechanisms of action. The mechanism of action by which thiazide diuretics lower BP in the long term is not fully understood. After chronic use, thiazides lower peripheral resistance. The mechanism of these effects is uncertain as it may involve effects on 'whole body,' renal autoregulation or direct vasodilator actions (Hughes 2004; Longo 2012). Thiazides act on the kidney to inhibit reabsorption of sodium (Na+) and chloride (Cl‐) ions from the distal convoluted tubules in the kidneys by blocking the thiazide‐sensitive Na+‐Cl‐ symporter (Duarte 2010). They also increase calcium reabsorption at the distal tubule and increase the reabsorption of calcium ions (Ca2+) by a mechanism involving the reabsorption of sodium and calcium in the proximal tubule in response to sodium depletion.
Alpha‐blockers (α1 adrenergic receptor blockers) inhibit the binding of norepinephrine (noradrenaline) to the α1 receptors causing vasodilation.
Beta‐blockers are competitive antagonists that block the receptor sites for epinephrine (adrenaline) and norepinephrine on adrenergic beta‐receptors. Some block activation of all types of beta‐adrenergic receptors (β1, β2 and β3) and others are selective for one of the three types of beta receptors (Frishman 2005).
Calcium channel blockers reduce BP through various mechanisms: by vasodilation, reduction in the force of contraction of the heart, by slowing the heart beat and by directly reducing aldosterone production.
ACE inhibitors block the conversion of angiotensin I (AI) to angiotensin II (AII). They thereby lower arteriolar resistance and increase venous capacity. They decrease cardiac output, cardiac index, stroke work and volume, and lower resistance in blood vessels in the kidneys. As a result of negative feedback of conversion of AI to AII, renin and AI increase in concentration in the blood. Bradykinin increases because of less inactivation by ACE.
ARBs block the activation of angiotensin II AT1 receptors. Blockage of AT1 receptors directly causes vasodilation, reduces secretion of vasopressin, and reduces production and secretion of aldosterone.
Why it is important to do this review
Clinical trials in adults with mild to moderate hypertension have included people over a wide age range from 18 to 104 years of age. There are two Cochrane Reviews evaluating the effectiveness of antihypertensive drug therapy in people with primary hypertension compared to placebo or no treatment: "First line drugs for hypertension" in adults aged 18 years or older (Wright 2009) and an updated review "Pharmacotherapy of hypertension in the elderly" (aged 60 years or older) with a subgroup meta‐analysis in very elderly people (aged 80 years or older) (Musini 2009).
Wright 2009 found that despite a similar magnitude of reduction in BP, a surrogate outcome measure, the clinical effectiveness in terms of all‐cause mortality and cardiovascular mortality and morbidity was different for different drug classes. First‐line low‐dose thiazides reduced all morbidity and mortality outcomes. First‐line ACE inhibitors and calcium channel blockers may have been similarly effective but the evidence was less robust. First‐line high‐dose thiazides and first‐line beta‐blockers were inferior for some outcomes to first‐line low‐dose thiazides. However, the review did not provide data on clinical effectiveness of antihypertensive drug therapy in different age groups.
Musini 2009 concluded that all‐cause mortality as well as cardiovascular mortality and morbidity were modestly reduced in elderly people (aged 60 years or older). However, the decrease in all‐cause mortality was limited to people 60 to 79 years of age.
Therefore, it is important to know the relative and absolute magnitude of the effect of antihypertensive drugs in different age groups of people with primary hypertension. This systematic review aimed to find the relative and absolute magnitude of the reduction in all‐cause mortality as well as cardiovascular mortality and morbidity in adults aged 18 to 59 years with primary hypertension (mild to moderate systolic or diastolic hypertension).
Objectives
To quantify antihypertensive drug effects on all‐cause mortality in adults aged 18 to 59 years with mild to moderate primary hypertension. To quantify effects on cardiovascular mortality plus morbidity (including cerebrovascular and coronary heart disease mortality plus morbidity), withdrawal due adverse events and estimate magnitude of systolic and diastolic blood pressure lowering at one year.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCT) of at least one year' duration. Trials must have included a control group that either received a placebo or received no antihypertensive therapy.
We excluded trials using non‐randomized allocation methods such as alternate allocation, week of presentation or retrospective controls. Trials that compared two specific antihypertensive first‐line therapies without a placebo or untreated control were also excluded.
Types of participants
Trials included only people aged 18 to 59 years or separately report outcomes for people in this age group. To maximize data inclusion, if trials included at least 90% of participants in the specified age group (18 to 59 years) but did not report outcomes data separately for this specific age group, we included the overall data in all randomized participants provided in these trials.
Participants must have had primary hypertension with a SBP of at least 140 mmHg or a DBP of at least 90 mmHg, or both at baseline.
Types of interventions
Acceptable antihypertensive drug therapies included: diuretics, ACE inhibitors, ARBs, beta‐blockers, combined alpha‐ and beta‐blockers, calcium‐channel blockers, alpha‐blockers, central sympatholytics, direct vasodilators or peripheral adrenergic antagonists. Drugs could have been administered alone or in combination, and in fixed or stepped care regimens. The control group must have received a placebo or no anti‐hypertensive therapy.
Types of outcome measures
Primary outcomes
All‐cause mortality (death from all causes).
Secondary outcomes
Cardiovascular mortality plus morbidity (included fatal and non‐fatal stroke, fatal and non‐fatal MI, sudden death, hospitalization or death from congestive heart failure and other significant vascular deaths such as ruptured aneurysms). It did not include angina, transient ischaemic attacks, surgical or other procedures, or accelerated hypertension.
Cerebrovascular mortality plus morbidity including fatal and non‐fatal stroke.
Coronary heart disease mortality plus morbidity including fatal and non‐fatal MI, and sudden or rapid cardiac death.
Withdrawal due to adverse events.
Decrease in SBP and DBP.
When the primary trial did not report on outcomes that fitted the above definitions, decisions were made based on maximizing the inclusion of the data as defined in each included study and maintaining concordance with how the data were classified in previous reviews (Musini 2009; Wright 2009).
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist (DS) conducted systematic searches in the following databases for RCTs without language, publication year or publication status restrictions:
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 23 January 2017);
the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web) (searched 23 January 2017);
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 23 January 2017);
Embase Ovid (searched 23 January 2017);
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 23 January 2017);
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch) (searched 23 January 2017).
The search strategy used in this review was identical to the Cochrane Review on "First line drugs for hypertension" (Wright 2009) and "Pharmacotherapy for hypertension in elderly patients" (Musini 2009). The Cochrane Hypertension Information Specialist (DS) modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomized controlled (as described in the Cochrane Handbook for Systematic Reviews of Interventions (Box 6.4.b; Higgins 2011a). Search strategies for major databases are provided in Appendix 1. Search was not done for regulatory documents from the Food and Drug Administration (FDA) or European Medicine Agency (EMA).
There were no language restrictions.
Searching other resources
We carefully checked the bibliographies of previously published meta‐analyses on the treatment of hypertension to help identify references to trials (Collins 1990; Thijs 1992; MacMahon 1993; Insua 1994; Mulrow 1994; Pearce 1995; Gueyffier 1996; Psaty 1997; Mulrow 1998; Gueyffier 1999; Quan 1999; Nikolaus 2000; Psaty 2003; Turnbull 2003; Kang 2004; BBLTTC 2005; BPLTTC 2008; Law 2009; Musini 2009; Wright 2009; Thomopoulos 2014; Sundström 2015; Zanchetti 2015; Parsons 2016; Tan 2016; Thomopoulos 2016; Kızılırmak 2017; Wiysonge 2017).
We contacted experts in the field to identify any other trials missed in our search. We checked reference lists of included studies and contacted relevant trialists for information about unpublished or ongoing studies.
Data collection and analysis
Selection of studies
One review author (VM or LP or DS) screened the titles and abstracts of citations from the search. Articles were rejected on initial screen if they were not reports of an RCT or there was no possibility that the trial could fit the requirements of this review. Of the articles selected for further review, at least two review authors (VM, LP or JMW) independently assessed whether they met the inclusion criteria.
Data extraction and management
Two review authors (VM and JMW) independently performed data abstraction, cross‐checked and compared whenever possible to data from previously published meta‐analyses. The data abstraction form included details of study design, randomization, blinding, duration of treatment, baseline characteristics, number of participants lost to follow‐up, outcomes, intervention, statistical analysis and reporting. Trial characteristics are detailed in the Characteristics of included studies table. One review author (FG) provided data in the 18‐ to 59‐year‐old subgroup from the INDANA (Gueyffier 1995) database for the MRC‐TMH 1985 trial.
Assessment of risk of bias in included studies
Two review authors (VM and JMW) independently assessed risk of bias of each included trial and resolved any disagreements by adjudication or discussion with a third review author (AT or LP or FG). Risk of bias assessment was done according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We assessed seven domains: randomization and allocation concealment to assess selection bias; blinding of the participants and physicians to assess performance bias; blinding of outcome assessor to assess detection bias; incomplete outcome reporting to assess attrition bias; selective reporting of outcomes to assess selective reporting bias and other bias to assess whether the study was funded by a drug manufacturer (in which case it was assessed as high risk of bias).
'Summary of findings' table
We used GRADEpro software to present the 'Summary of findings' table (GRADEpro). We decided to include all clinically relevant primary and secondary outcomes such as all‐cause mortality, total cardiovascular mortality and morbidity, cerebrovascular mortality and morbidity, coronary heart disease mortality and morbidity, withdrawal due to adverse events and magnitude of reduction in SBP and DBP.
The five factors considered in grading overall quality of evidence were: limitations in study design and implementation, indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision in results and high probability of publication bias. This approach specifies four levels of quality: high, moderate, low and very low quality evidence. The highest quality rating is for randomized trial evidence. Quality rating is downgraded by one level for each factor, up to a maximum of three levels for all factors. If there are severe problems for any one factor (when assessing limitations in study design and implementation, in concealment of allocation, loss of blinding or attrition over 50% of participants during follow‐up), randomized trial evidence may fall by two levels due to that factor alone.
Measures of treatment effect
We used Review Manager 5 for data synthesis and analyses (RevMan 2014). Quantitative analyses of outcomes were based on intention‐to‐treat results. We used risk ratios (RR) with 95% confidence intervals (CI) to combine outcomes across trials. If there was a significant difference in any outcome measure, we presented an absolute risk reduction (ARR) and number needed to treat for an additional beneficial (NNTB) or harmful (NNTH) outcome in the 'Summary of findings' table. For continuous outcomes (SBP and DBP), we calculated the mean difference (MD) with 95% CI to combine outcomes across studies.
Unit of analysis issues
For all outcomes measures reported, we used data from each trial at the end of the follow‐up period mentioned in each trial, which varied from two to 10 years.
Dealing with missing data
When participants were lost to follow‐up, we used data as reported for participants who were followed until end of the study in the analyses. Refer to how data was accounted for and included in each study under assessment of attrition bias in the Risk of bias in included studies section.
For example, in the MRC‐TMH 1985 study, events such as non‐fatal stroke or MI terminated participation in the study and follow‐up of such participants to the end of the study was not done. In such instances, data available up to the time point during which participants were followed were included in the analyses.
Assessment of heterogeneity
We tested heterogeneity of treatment effect between the trials using a standard Chi² statistic for heterogeneity. We used the fixed‐effect model to obtain summary statistics of pooled trials, unless there was significant between‐study heterogeneity, in which case we used the random‐effects model to test statistical significance. When heterogeneity was estimated to be significant (I² greater than 50%), we explored the factors contributing to heterogeneity.
Assessment of reporting biases
We planned to assess publication bias if at least 10 studies met the inclusion criteria using a funnel plot. However, in this review, only seven studies met the inclusion criteria and provided data for quantitative analyses, so funnel plot analyses were not performed.
Data synthesis
We used Review Manager 5 to perform data synthesis and analyses (RevMan 2014). We presented dichotomous outcomes as RR with 95% CI using a fixed‐effect model and continuous outcomes (SBP and DBP) as MD with 95% CI.
Subgroup analysis and investigation of heterogeneity
Since most participants included in this review were subgroups of participants from four of the seven trials in adults aged 18 years or older, further dividing participants into various other subgroup and analysing outcomes would lead to an increase in alpha error. Multiple comparisons within further subgroups would increase the chances of finding significant differences and therefore was not done.
When heterogeneity was significant (I² greater than 50%), we attempted to identify trials that would contribute to heterogeneity and explore their population characteristics, baseline BP, blinded or open‐label study design, use of antihypertensive drugs as fixed dose or stepped up therapy or response to placebo that would possibly explain the reason for heterogeneity. As the decrease in SBP and DBP showed significant heterogeneity, we presented results as MD with 95% CI using a random‐effects model instead of a fixed‐effect model.
Sensitivity analysis
To test for robustness of results, we conducted the following sensitivity analyses:
placebo controlled trials versus untreated trials;
double blind trials versus open‐label or single‐blind trials;
trials using combined starting drugs as stepped up therapy versus fixed‐dose therapy.
Results
Description of studies
Results of the search
Since the search strategy used in this review was identical to the Cochrane Review "First line drugs for hypertension" (Wright 2009) and "Pharmacotherapy for hypertension in elderly patients" (Musini 2009), we included search findings to 2008 from these reviews. The PRISMA diagram for search to 2008 is not shown.
The Wright 2009 review search from 1946 to 2008 resulted the identification of 6232 citations. Of these, 5985 articles were rejected on initial screen as they were not reports of an RCT or there was no possibility that the trial could fit the requirements of this review. A total of 247 citations were retrieved for detailed evaluation by two review authors (JMW and VM) of which 13 were review articles. Of the remaining 234 citations, 59 were excluded upon detailed reading as they did not meet minimum inclusion criteria. A total of 175 reports of 57 potentially first‐line trials were evaluated. Forty‐eight reports of 33 trials were excluded and were listed in the 'Characteristics of excluded studies' table in the Wright 2009 review. Finally, 127 reports of 24 trials were included in the Wright 2009 review. Not all trials included in the Wright 2009 review pertained to the age group 18 to 59 years old therefore we excluded 17 of the 24 studies included in the Wright 2009 review. These are listed in the Characteristics of excluded studies table.
The search strategy from 2008 to January 2017 resulted in 11,855 citations after removing duplicates. We screened the titles and abstracts and excluded 11,500 citations. Articles were rejected on this initial screen if the article was not a report of a RCT or there was no possibility that the trial could fit the requirements of this review. At leat two review authors (LP, VM or JMW) assessed the remaining articles to determine if they met the inclusion criteria. Three hundred and fifty‐five full‐text articles were retrieved of which all 355 articles were excluded on detailed reading as they did not meet the minimum inclusion criteria (Figure 1).
1.
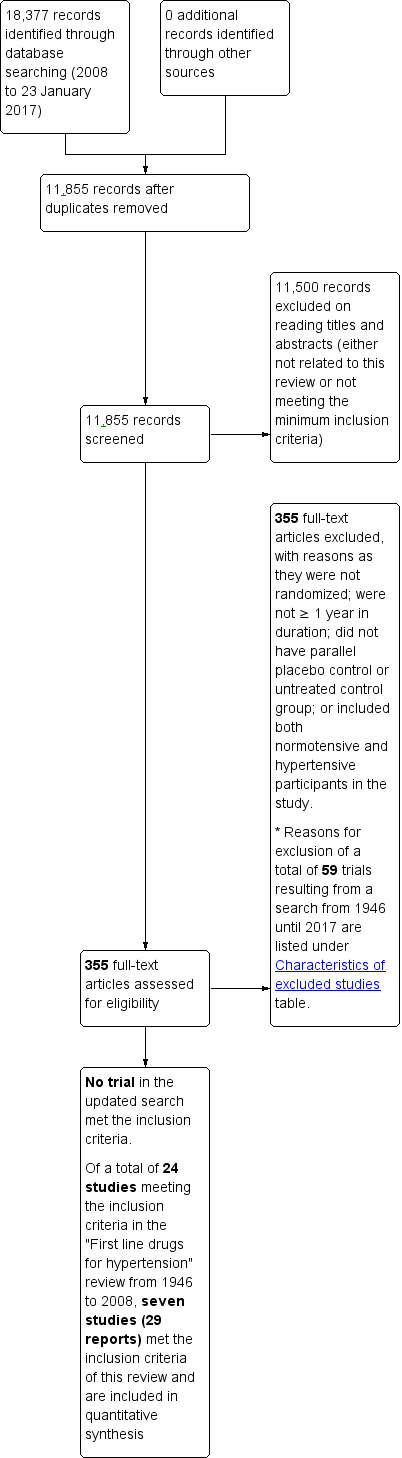
This is a partial study flow diagram and shows search findings from 2008 until 2017. The search done from 1946 to 2008 was identical to the "First line drugs for hypertension" review in which 24 trials met the inclusion criteria of which 17 had to be excluded and only seven studies provided data for quantitative synthesis in 18‐ to 59‐year‐old participants.
A total of seven studies (in 27 reports) identified previously in the "First line drugs for hypertension" review (Wright 2009) met the inclusion criteria and provide information in 18‐ to 59‐year‐old participants and were therefore included in the quantitative analyses.
Please note that 59 trials including 17 trials from the "First line drugs for hypertension" review (Wright 2009) resulting from literature search from 1946 until 2017 were excluded and reasons for exclusion were listed under the Characteristics of excluded studies table.
Included studies
Seven trials met the inclusion criteria (Carter 1970; VA‐II 1970; HSCSG 1974; USPHSHCSG 1977; VA‐NHLBI 1977; MRC‐TMH 1985; Oslo 1986).
This review included 17,327 (88%) participants aged 18 to 59 years from a total of 19,684 randomized participants from the seven included studies in adults with hypertension. In the MRC‐TMH 1985 trial, we were able to include only the people aged 18 to 59 years. The MRC‐TMH study contributed 14,541 (84%) of the total randomized participants with a mean age of 50 years and mean baseline BP of 160/98 mmHg and a mean duration of follow‐up of five years. Treatment used in this study was bendrofluazide 10 mg daily or propranolol 80 mg to 240 mg daily and with methyldopa added if required.
Refer to the Characteristics of included studies table and Table 2 for details regarding baseline characteristics for all included participants. No data regarding baseline characteristics was available for the specific 18‐ to 59‐year‐old subgroup of participants in four trials (Carter 1970; VA‐II 1970; HSCSG 1974; MRC‐TMH 1985). Three trials included only 18‐ to 59‐year‐old participants (USPHSHCSG 1977; VA‐NHLBI 1977; Oslo 1986). VA‐II 1970 did not provide the age range of included participants. The mean age of participants included in these four trials was 41.3 years.
1. Baseline characteristics of included studies in all randomized participants aged 18 years or older.
|
Study Study design |
Number of 18‐ to 59‐year‐old participants/total randomized participants (%) Characteristics of all randomized participants in each study |
Mean baseline SBP/DBP (mmHg) |
Mean age (range) | Control group | Treatment used | Outcomes reported in 18‐ to 59‐year‐old participants |
|
Carter 1970 Open label |
49/97 (50.5%); included all hypertensive survivors aged < 80 years of ischaemic type major strokes admitted to Ashford Hospital. Men: 57% |
165/101 | Not reported (40 to 79 years) |
Untreated | Stepped‐up therapy Bendrofluazide (93%), methyldopa and debrisoquine FU 4.0 years |
All‐cause mortality |
|
HSCSG 1974 Double blind |
252/452 (55.7%). 80% of participants had completed stroke in year before randomization; 16% completed stroke + TIA and 4% TIA only. Severe neurological disability in 9.9% of treated group and 5% of control group. Black: 80% Men: 60% |
167/100 77% of the drug treated and 74% of the placebo treated participants had SBP < 180 mmHg |
59 years (< 75 years) |
Placebo | Fixed‐dose therapy Deserpidine 1 mg + methyclothiazide 10 mg FU 3.0 years |
Cardiovascular mortality + morbidity Cerebrovascular mortality + morbidity CHD mortality + morbidity |
|
MRC‐TMH 1985 Single blind |
14,541/17,354 (83.8%). Men: 52%. Primary prevention participants. Refer to exclusion criteria listed in Characteristics of included studies table. |
161/98. | 52 years (35‐64 years) |
Placebo 288/12,375 (0.02%) participants randomly assigned to observation taking no tablets and merged with placebo |
Stepped‐up therapy Bendrofluazide 10 mg daily (71% monotherapy), propranolol 80‐240 mg daily (78% monotherapy), methyldopa added if required FU 4.9 years |
All‐cause mortality Cardiovascular mortality + morbidity Cerebrovascular mortality + morbidity CHD mortality + morbidity SBP DBP |
|
Oslo 1986 Open label |
785/785 (100%) Men: 100% Primary prevention participants. Refer to exclusion criteria listed in Characteristics of included studies table |
156/97 | 45 years (40‐49 years) |
Untreated | Stepped‐up therapy Hydrochlorothiazide (95%), methyldopa and propranolol (26%). At 5‐year follow‐up, 36.7% were on hydrochlorothiazide alone, 26% were on hydrochlorothiazide + propranolol, 20% were on hydrochlorothiazide + methyldopa and 18% participants were on other drugs. FU 5.5 years |
All‐cause mortality Cardiovascular mortality + morbidity Cerebrovascular mortality + morbidity CHD mortality + morbidity SBP DBP |
|
USPHSHCSG 1977 Double blind |
389/389 (100%) Men: 80% Primary prevention participants. Refer to exclusion criteria listed in Characteristics of included studies table |
147/99 | 44 years (21‐55 years) |
Placebo | Fixed‐dose therapy Chlorothiazide 500 mg twice daily + Rauwolfia serpentina 100 mg twice daily FU 7.0 years |
All‐cause mortality Cardiovascular mortality + morbidity Cerebrovascular mortality + morbidity CHD mortality + morbidity SBP DBP |
|
VA‐II 1970 Double blind |
299/380 (78.7%) Men: 100% Primary prevention participants. Refer to exclusion criteria listed in Characteristics of included studies table |
176/103 | 51 years (range not reported) |
Placebo | Stepped‐up therapy First line: hydrochlorothiazide 100 mg + reserpine 0.2 mg Second line: hydralazine 75‐150 mg FU 3.3 years |
Cardiovascular mortality + morbidity Cerebrovascular mortality + morbidity CHD mortality + morbidity |
|
VA‐NHLBI 1977 Double blind |
1012/1012 (100%) Men: 100% Primary prevention participants. Refer to exclusion criteria listed in Characteristics of included studies table |
SBP not reported/93 | 38 years (range 21‐50 years) |
Placebo | Stepped‐up therapy Chlorthalidone 50 mg, 100 mg (53% chlorthalidone alone) Reserpine 0.25 mg FU 1.5 years |
All‐cause mortality Cardiovascular mortality + morbidity Cerebrovascular mortality + morbidity CHD mortality + morbidity DBP |
| Total of 7 studies published from 1970 to 1986 | 17,327/19,684 (88% of total randomized participants in the 7 studies were 18‐59 years of age) of which only 301/1,7327 (0.02%) participants were secondary prevention | SBP 147‐176/DBP 99‐103 mmHg | Mean age 37.5‐45 years | 5 RCTs were placebo controlled and 2 RCTS had untreated group as control |
Mostly high‐dose thiazide and beta‐blockers Mean follow‐up 1.5‐7 years. |
5/7 RCTs report on all clinically important outcomes |
CHD: coronary heart disease; DBP: diastolic blood pressure; FU: follow‐up; SBP: systolic blood pressure; TIA: transient ischaemic attack.
All seven trials used first‐line high‐dose thiazide drugs for lowering BP. Two trials evaluated beta‐blockers versus placebo (MRC‐TMH 1985; Oslo 1986). Three of the seven trials used methyldopa (Carter 1970; MRC‐TMH 1985; Oslo 1986). Two trials used reserpine (VA‐II 1970; VA‐NHLBI 1977). Five trials used a stepped approach to antihypertensive drug administration (Carter 1970; VA‐II 1970; VA‐NHLBI 1977; MRC‐TMH 1985; Oslo 1986). The remaining two trials used a standard fixed dose of drug in the intervention arm.
Two trials included only men (Oslo 1986; VA‐II 1970; Oslo 1986). Three trials did not report ethnicity (Carter 1970; MRC‐TMH 1985; Oslo 1986). The four remaining trials reported ethnicity for all included participants. African‐Americans comprised the following percentages in these trials: HSCSG 1974 80%; VA‐II 1970 42%; VA‐NHLBI 1977 25%; and USPHSHCSG 1977 28%. Three trials based entry on DBP (VA‐II 1970; USPHSHCSG 1977; VA‐NHLBI 1977). Four trials based entry on either SBP or DBP (Carter 1970; HSCSG 1974; MRC‐TMH 1985; Oslo 1986).
Definition of stroke differed across trials. In more recent trials, it was defined as the presence of neurological deficit lasting more than 24 hours. Older trials (such as HSCSG 1974) defined stroke as a neurological deficit lasting more than 24 hours or a marked increase in transient ischaemic attacks (twice the weekly prerandomization level of occurrence, more than four per week or deterioration of more than 8 points in neurological score). VA‐NHLBI 1977 defined stroke as typical weakness or paralysis. Some trials did not define stroke. In our opinion combining all strokes (including reversible ischaemic neurological deficit (RIND)), into one outcome is not optimal. More clinically relevant interpretations could be made if strokes were subdivided into three groups: strokes with no disability, strokes with mild disability and strokes with severe disability. The definition of MI and sudden death was consistent across most trials. MI was defined as typical chest pain with ECG changes or increased cardiac enzymes; sudden death was defined as death within 24 hours of first evidence of acute CVD or unrelated to other known pre‐existing diseases.
It was possible in most trials to determine which participants were treated for primary or secondary prevention. All trials excluded people with angina and congestive heart failure, as these conditions would require use of antihypertensive drugs for reasons independent of their antihypertensive action. Some trials allowed people with prior MI or stroke if they were not recent (e.g. within the previous three months). Thus, by determining the baseline prevalence of stroke and MI it was possible to calculate the percentage that represented secondary prevention. In six trials, the study population consisted of predominantly ambulatory participants recruited from the community, primary care centres or hospital‐based clinics (VA‐II 1970; HSCSG 1974; USPHSHCSG 1977; VA‐NHLBI 1977; MRC‐TMH 1985; Oslo 1986). Two trials were secondary prevention and included stroke survivors who were ambulatory and contributed 301 (0.02%) of total randomized participants(Carter 1970; HSCSG 1974). One trial did not report prevalence of stroke or MI (VA‐II 1970). Three trials were 100% primary prevention (USPHSHCSG 1977; VA‐NHLBI 1977; Oslo 1986).
One trial reported baseline prevalence of diabetes as 0% (USPHSHCSG 1977). Three trials reported baseline prevalence of smoking (USPHSHCSG 1977 46.7%; MRC‐TMH 1985 29.7%; Oslo 1986 41.7%;).
Mean baseline BP for all the trials ranged from 147 mmHg to 176 mmHg for SBP and from 99 mmHg to 103 mmHg for DBP. One trial did not report baseline SBP level (VA‐NHLBI 1977). For complete description of the BP inclusion criteria for each study see the Characteristics of included studies table. One trial that contributed the most weight to effect size in this review included mostly people receiving primary prevention followed up for a mean duration of five years with mean baseline BP of 160/98 mmHg (MRC‐TMH 1985). The mean duration of follow‐up across the seven included studies ranged from two to 10 years.
Excluded studies
Fifty‐nine trials resulting from the search from 1946 to 2017 were excluded from this review and reasons for exclusion are provided in the Characteristics of excluded studies table. Twenty‐four trials met the inclusion criteria in the "First line drugs for hypertension" review by Wright 2009 and "Pharmacotherapy for hypertension in the elderly review" by Musini 2009, but 17 were excluded from this review. The reasons for excluding them were mostly because they did not report outcomes separately for the 18 to 59 years age group or were conducted in people 60 years or older.
Risk of bias in included studies
We assessed risk of bias for each included study using the Cochrane 'Risk of bias' tool for RCTs as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Potential parameters of methodological quality listed in the 'Risk of bias' table include: method used to randomize participants, whether randomization was completed in an appropriate and blinded manner; whether participants, providers, outcome assessors, or a combination of these were blinded to assigned therapy; whether the control group received a placebo or no treatment; percent of participants who did not complete follow‐up (dropouts); percent of participants not on assigned active or placebo therapy at study completion; selective reporting of outcomes and other bias in terms of funding of the trial by the manufacturer.
Refer to Figure 2 for the 'Risk of bias' graph which provides review authors' judgements about each risk of bias item presented as percentages across all included studies. Refer to Figure 3 for the 'Risk of bias' summary which provides review authors' judgements about each risk of bias item for each included study.
2.
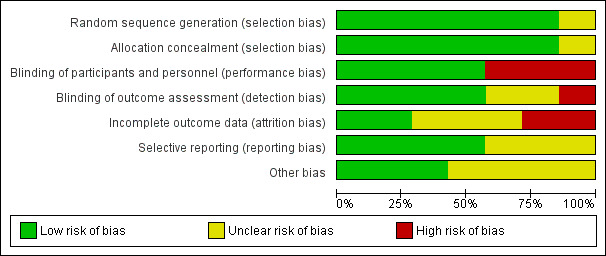
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomization was at low risk of bias in six trials (Carter 1970; VA‐II 1970; HSCSG 1974; USPHSHCSG 1977; MRC‐TMH 1985; Oslo 1986). It was judged as unclear in VA‐NHLBI 1977.
Allocation concealment was at low risk of bias in six trials (Carter 1970; VA‐II 1970; HSCSG 1974; USPHSHCSG 1977; MRC‐TMH 1985; Oslo 1986), and unclear in VA‐NHLBI 1977.
Blinding
Blinding of participant and personnel was at low risk of bias in four trials (VA‐II 1970; HSCSG 1974; USPHSHCSG 1977; VA‐NHLBI 1977). It was at high risk of bias in three trials; two as treating physicians were aware of the treatment being prescribed (Carter 1970; MRC‐TMH 1985), and one as both participants and treating physicians were aware of the treatment being prescribed (Oslo 1986).
Blinding of outcome assessors was at low risk of bias in four trials (VA‐II 1970; HSCSG 1974; MRC‐TMH 1985; Oslo 1986). It was at unclear risk of bias in two trials (USPHSHCSG 1977; VA‐NHLBI 1977), and at high risk of bias in one trial as study did not mention blinding of physician or outcome assessor (Carter 1970).
Incomplete outcome data
Incomplete outcome data was at low risk of bias in two trials (Carter 1970; Oslo 1986), at unclear risk of bias in three trials (VA‐II 1970; HSCSG 1974; VA‐NHLBI 1977), and at high risk of bias in two trials (USPHSHCSG 1977; MRC‐TMH 1985). In the MRC‐TMH 1985 trial, participant participation was terminated in the event of non‐fatal stroke or MI. In the USPHSHCSG 1977 trial, attrition rate was high due to dropouts or occurrence of morbid events. Vital statistics information was unknown in 26 participants who dropped out.
Selective reporting
Selective reporting was at low risk of bias in four trials (Carter 1970; HSCSG 1974; MRC‐TMH 1985; Oslo 1986), and at unclear risk of bias in three trials (VA‐II 1970; USPHSHCSG 1977; VA‐NHLBI 1977).
Other potential sources of bias
Other potential bias in terms of funding by the manufacturer was at low risk of bias in three trials (Carter 1970; HSCSG 1974; VA‐NHLBI 1977), and at unclear risk of bias in four trials (VA‐II 1970; USPHSHCSG 1977; MRC‐TMH 1985; Oslo 1986).
Effects of interventions
See: Table 1
See Table 1.
All‐cause mortality
Antihypertensive drug therapy as compared to placebo or untreated control had no effect on all‐cause mortality (RR 0.94, 95% CI 0.77 to 1.13; participants = 16,776; studies = 5; I² = 43% Analysis 1.1).
1.1. Analysis.
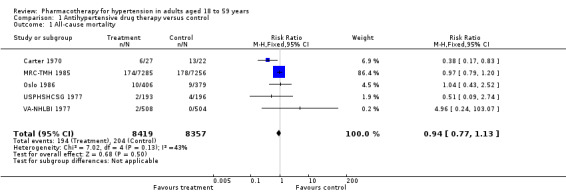
Comparison 1 Antihypertensive drug therapy versus control, Outcome 1 All‐cause mortality.
Data from two studies (VA‐II 1970; HSCSG 1974) were not provided in the original Mulrow 1998 review for the 18‐ to 59‐year‐old subgroup. These two studies contributed 551 (3.2%) of total randomized participants included.
Cardiovascular mortality plus morbidity
Antihypertensive drug therapy as compared to placebo or untreated control reduced cardiovascular mortality plus morbidity (RR 0.78, 95% CI 0.67 to 0.91; participants = 17,278; studies = 6; I² = 13%; Analysis 1.2).
1.2. Analysis.
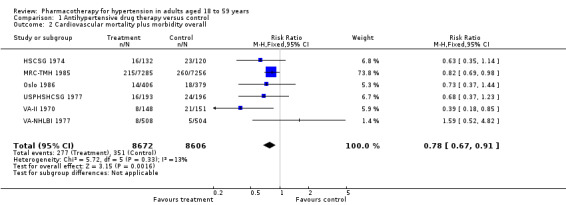
Comparison 1 Antihypertensive drug therapy versus control, Outcome 2 Cardiovascular mortality plus morbidity overall.
The original Mulrow 1998 review and the Carter 1970 study did not provide total cardiovascular mortality plus morbidity data in 29 (0.2%) of total randomized participants.
Cerebrovascular mortality plus morbidity
Antihypertensive drug therapy as compared to placebo or untreated control reduced stroke (RR 0.46, 95% CI 0.34 to 0.64; participants = 17,278; studies = 6; I² = 40%; Analysis 1.3).
1.3. Analysis.
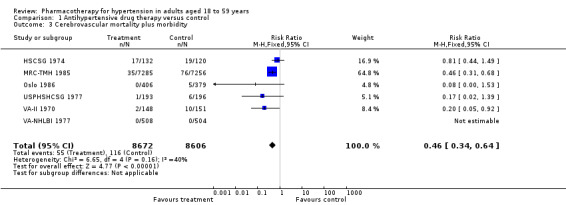
Comparison 1 Antihypertensive drug therapy versus control, Outcome 3 Cerebrovascular mortality plus morbidity.
Coronary heart disease mortality plus morbidity
Antihypertensive drug therapy as compared to placebo or untreated control did not reduce coronary heart disease (RR 0.99, 95% CI 0.82 to 1.19; participants = 16,241; studies = 4; I² = 0%; Analysis 1.4).
1.4. Analysis.
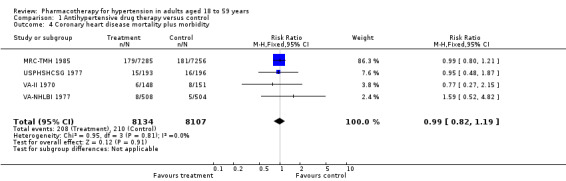
Comparison 1 Antihypertensive drug therapy versus control, Outcome 4 Coronary heart disease mortality plus morbidity.
Withdrawal due to adverse events
Data for withdrawal due to adverse events in the 18‐ to 59‐year‐old subgroup were not available in four trials (VA‐II 1970; HSCSG 1974; VA‐NHLBI 1977; MRC‐TMH 1985). Three trials report this outcome (Carter 1970; USPHSHCSG 1977; Oslo 1986). However, two trials reported no adverse events in either the treatment and the control groups (Carter 1970; Oslo 1986). The RR was based on one study (RR 4.82, 95% CI 1.67 to 13.92; participants = 1223; study = 1; Analysis 1.5) (USPHSHCSG 1977).
1.5. Analysis.
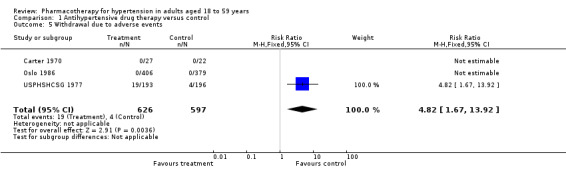
Comparison 1 Antihypertensive drug therapy versus control, Outcome 5 Withdrawal due to adverse events.
Withdrawal due to adverse events was not available in the 18‐ to 59‐year‐old subgroup in one trial (MRC‐TMH 1985).
Decrease in systolic and diastolic blood pressure
Four trials reported data for DBP in 18‐ to 59‐year‐old subgroup of participants at end of 1 year (USPHSHCSG 1977; VA‐NHLBI 1977; MRC‐TMH 1985; Oslo 1986), and three studies reported data for SBP (USPHSHCSG 1977; MRC‐TMH 1985; Oslo 1986). Therefore, we added this outcome to the list of secondary outcomes in the review.
Antihypertensive drug therapy significantly lowered both SBP and DBP as compared to the control group. Since heterogeneity was significant for BP data results were presented using a random‐effects model (SBP: MD ‐14.98, 95% CI ‐20.44 to ‐9.52; participants = 14,845; studies = 3; I² = 95%; Analysis 1.6; DBP: MD ‐7.62, 95% CI ‐10.55 to ‐4.69; participants = 15,857; studies = 4; I² = 96%; Analysis 1.7).
1.6. Analysis.
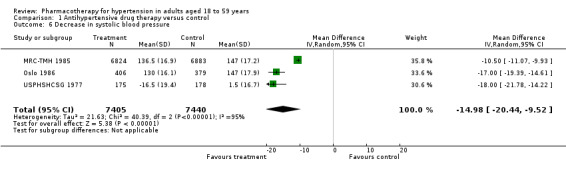
Comparison 1 Antihypertensive drug therapy versus control, Outcome 6 Decrease in systolic blood pressure.
1.7. Analysis.
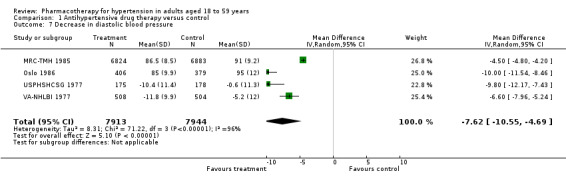
Comparison 1 Antihypertensive drug therapy versus control, Outcome 7 Decrease in diastolic blood pressure.
Sensitivity analysis
In a sensitivity analyses to test the robustness of the overall treatment effect size, we deselected trials based on placebo controlled versus untreated group; double‐blind versus open‐label or single‐blind trials; trials using combined starting drugs as stepped up therapy versus fixed dose therapy; and secondary prevention versus mostly primary prevention. In all these instances, there were no clinically important changes in the treatment effect.
Discussion
Summary of main results
This review summarizes the effects of antihypertensive drug therapy for elevated BP in adults aged 18 to 59 years. The pooled data showed that using antihypertensive drug therapy (mostly high‐dose thiazide diuretics) reduced cardiovascular mortality and morbidity primarily due to a reduction in cerebrovascular mortality and morbidity, but had no effect on all‐cause mortality or coronary heart disease. Withdrawals due to adverse events were increased with drug therapy. The mean decrease in SBP/DBP at end of 1 year was 15/8 mmHg.
Overall completeness and applicability of evidence
The subgroup of people 18 to 59 years of age from the MRC‐TMH 1985 study represented 84% of total randomized participants and thus contributed the most to the treatment effect size for all mortality and morbidity outcomes. Therefore, the evidence from this review is most applicable to people who were eligible for this study. The mean age of participants was 50 years and the mean baseline BP was 160/98 mmHg. This means that about half of the participants would be in the mild hypertension range and half would be in the moderate hypertension range. Most of the participants (97.8%) were being treated for primary prevention. The mean duration of follow‐up was five years. Treatment used in this study was first‐line bendrofluazide 10 mg daily or first‐line propranolol 80 mg to 240 mg daily and methyldopa was added if required.
In this review, in five trials, about 40% of the participants did not achieve the DBP goal of less than 90 mmHg although three trials used stepped care therapy (VA‐II 1970; MRC‐TMH 1985; Oslo 1986), and two trials used fixed‐dose combination therapy (HSCSG 1974; USPHSHCSG 1977). This observation is important to note because it implies that in routine practice a substantial proportion of people with elevated BP are unlikely to be able to achieve BP targets. This does not mean these people will not benefit as BP reduction is only partly responsible for the risk reduction of antihypertensive treatment (Boissel 2005).
The data on withdrawals due to adverse events was incomplete as only three out of seven trials reported this outcome, with two trials (total participants = 834) reporting zero events in both arms. MRC‐TMH 1985 was not included in the withdrawals due to adverse events outcome as that outcome was not available separately for the 18‐ to 59‐year age group. However, withdrawals due to adverse events were increased by drug treatment in the full MRC‐TMH 1985 trial (RR 4.81, 95% CI 4.15 to 5.58; absolute risk increase 8.9%). This is very similar to the estimate from the present review (RR 4.82; absolute risk increase 3.8%), which primarily represents the results of the USPHSHCSG 1977 trial (participants = 389). Even though we have graded this outcome as very low quality evidence, it is likely a reasonably robust estimate.
The thiazide arm of the MRC‐TMH 1985 trial was classified as high‐dose thiazide in the "First line drugs for hypertension" review (Wright 2009). That review concluded that using low‐dose thiazide was beneficial in terms of reducing all‐cause mortality, total cardiovascular events, coronary heart disease and stroke. This benefit for coronary heart disease and all‐cause mortality was not observed in first‐line high‐dose thiazide trials. Thus, first‐line low‐dose thiazides are preferred, even though we do not have any evidence for low‐dose thiazides in the age group studied here.
We calculated the absolute risk reduction (ARR) and NNTB for clinical outcomes that were significantly reduced with antihypertensive therapy compared to placebo or untreated control groups. For cardiovascular mortality plus morbidity, the relative risk reduction was 22%, ARR was 0.9% and NNTB was 112. For cerebrovascular events (fatal and non‐fatal stroke) the relative risk reduction was 64%, ARR was 0.7%, and NNTB was 143. This means that approximately 112 people need to be treated for about five years to prevent one adverse cardiovascular event and most of this benefit comes from a reduction in stroke (cerebrovascular mortality and morbidity).
Sensitivity analyses did not show any clinically important changes in the treatment effect.
MRC‐TMH 1985 also contributed more than 90% of the weight to the estimate of BP reduction in this review. Therefore, the mean BP reduction found in this review was close to that seen in the MRC‐TMH 1985 trial. The BP lowering effect was greater in the other trials, which resulted in high heterogeneity. This is likely because the design and number of drugs used in the trials differed.
Quality of the evidence
The overall quality of the evidence was graded using online GRADEpro software (GRADEpro). The 'Summary of findings' table provided the grading of the quality of evidence for each clinically important outcome. All‐cause mortality, cardiovascular mortality plus morbidity, cerebrovascular mortality plus morbidity and coronary heart disease mortality plus morbidity were downgraded to low quality evidence because of high risk of performance and attrition bias as well as lack of reporting of data from several trials that had to be excluded (Table 1). Low quality evidence means our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Withdrawal due to adverse events was downgraded to very low quality evidence as it was only reported in three of the seven trials meeting the inclusion criteria. Very low quality evidence means we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Since one study with high risk of performance and attrition bias provided most of the data to the estimated effect size, it is likely to be an overestimate of the true effect (MRC‐TMH 1985).
Potential biases in the review process
Reporting bias is possible as eight studies ( met the inclusion criteria but did not report data in the 18‐ to 59‐year‐old subgroup of participants separately and thus were excluded from this review (HOPE HYP 2000; TEST 1995; UKPDS 1998; VA‐I 1962; Wolff 1966; Anon 1973; ATTMH 1984; PATS 1995). Availability of data for all clinically relevant outcomes from all randomized studies meeting the inclusion criteria in this age group is needed.
Three trials combined a reserpine derivative with thiazide as first‐line drug therapy (VA‐II 1970; HSCSG 1974; USPHSHCSG 1977). The MRC‐TMH 1985 trial, which contributed the most weight in this review, added methyldopa if needed to first‐line high‐dose thiazide or beta‐blocker therapy. Therefore, results of this review are mostly applicable to those treatment approaches.
Since all seven included studies used high‐dose thiazides, at present we do not have evidence for low‐dose antihypertensive drug therapy as compared to placebo or untreated control in adults aged 18‐ to 59‐years with primary hypertension. Given the findings and conclusions of the first‐line drug review regarding all‐cause mortality and morbidity benefits of using low‐dose thiazide (Wright 2009), we recommend future RCTs compare first‐line low‐dose thiazide with other first‐line classes of antihypertensive drugs in young adults aged 18 to 59 years.
Agreements and disagreements with other studies or reviews
This is the first systematic review of drug therapy in adults aged 18 to 59 years with elevated BP. The ARR in adults aged 18‐ to 59‐years in this review for cardiovascular mortality and morbidity was small (0.9%) with an NNTB of 112 over five years. It is important for clinicians and participants to know the approximate magnitude of benefit in this setting. For people aged 60‐ to 79‐years the absolute reductions over five years were greater, total cardiovascular events, ARR 3.8% with an NNTB 26 over five years (Musini 2009).
Several guidelines to treat people with hypertension have been published. The eighth revision of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC) mainly based their recommendations on evidence from RCTs. However, five out of 10 recommendations are based on expert opinion (James 2014).
They recommended the following:
thiazide‐type diuretics, ACE inhibitors, ARBs and calcium channel blockers as the initial therapy of choice. Although all these drug classes have comparable outcome benefits they concluded that thiazide‐type diuretics are superior to the other three classes in terms of heart failure;
specific recommendation in people aged less than 60 years, treatment goal is SBP less than 140 mmHg (expert opinion ‐ grade E evidence);
in the general population aged less than 60 years, the target should be DBP less than 90 mmHg and treatment should be initiated above this value (for ages 30 to 59 years, strong recommendation grade A; for ages 18 to 29 years, expert opinion ‐ grade E);
in the general population excluding black people (including people with diabetes), initial antihypertensive treatment should include a thiazide‐type diuretic, calcium channel blocker, ACE inhibitor or ARB (general population moderate recommendation ‐ grade B and for black people with diabetes weak recommendation ‐ grade C);
beta blockers are no longer considered as an initial therapy option.
The European Society of hypertension (ESH) 2013 guidelines recommend diuretics, beta‐blockers, calcium channel blockers, ACE inhibitors and ARBs are included as viable options for initial hypertension therapy. Goal BP in people with diabetes mellitus is less than 140/85 mmHg. Recommendation for specific age group less than 60 years is SBP/DBP less than 140/90 mmHg (Mancia 2013).
The American Society of Hypertension/International Society of Hypertension (ASH/ISH) recommend ACE inhibitors or ARB as initial therapy in non‐black people under 60 years of age; a calcium channel blocker or thiazide diuretic is recommended for black people (Weber 2014).
Authors' conclusions
Implications for practice.
Antihypertensive drugs used to treat predominantly healthy adults aged 18 to 59 years with mild to moderate primary hypertension have a small absolute effect to reduce cardiovascular mortality and morbidity (0.9% over a five‐year period) primarily due to reduction in cerebrovascular mortality and morbidity (0.7%). All‐cause mortality and coronary heart disease were not reduced. There is lack of good evidence on withdrawal due to adverse events.
Implications for research.
Long‐term randomized controlled trials of at least 10 years' duration are needed to investigate which first‐line drug is best in people in this age group as they will potentially be taking these drugs over a long duration. Future randomized controlled head to head studies should compare first‐line low‐dose thiazides versus other first‐line classes and measure all‐cause mortality, total serious adverse events and total cardiovascular serious adverse events. Long‐term high‐quality observational studies may also provide valuable additional information.
Acknowledgements
We would like to acknowledge the contributions of the editorial team of the Cochrane Hypertension Group. We would like to thank Stephen Adams for retrieving references.
Appendices
Appendix 1. Search strategy used in various databases
1 MEDLINE search strategy
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 23 January 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp thiazides/ (15913) 2 exp sodium chloride symporter inhibitors/ (14603) 3 exp sodium potassium chloride symporter inhibitors/ (14205) 4 ((ceiling or loop) adj diuretic?).tw. (2564) 5 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).tw. (34947) 6 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw. (2353) 7 or/1‐6 [THZ] (50922) 8 exp angiotensin‐converting enzyme inhibitors/ (44001) 9 angiotensin converting enzyme inhibit$.tw. (18514) 10 (ace adj2 inhibit$).tw. (18317) 11 acei.tw. (2857) 12 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or enalaprilat or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw. (27811) 13 or/8‐12 [ACEI] (59794) 14 exp Angiotensin Receptor Antagonists/ (22055) 15 (angiotensin adj3 (receptor antagon$ or receptor block$)).tw. (11889) 16 arb?.tw. (5159) 17 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten).tw. (15972) 18 or/14‐17 [ARB] (31152) 19 exp calcium channel blockers/ (83029) 20 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw. (62158) 21 (calcium adj2 (antagonist? or block$ or inhibit$)).tw. (39065) 22 or/19‐21 [CCB] (111557) 23 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. (16154) 24 (reserpine or serpentina or rauwolfia or serpasil).mp. (20757) 25 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. (19858) 26 exp hydralazine/ (4964) 27 (hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).tw. (4648) 28 or/23‐27 [CNS] (59745) 29 exp adrenergic beta‐antagonists/ (84772) 30 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw. (62363) 31 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. (101145) 32 or/29‐31 [BB] (159886) 33 exp adrenergic alpha antagonists/ (51279) 34 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw. (14099) 35 (adrenergic adj2 (alpha or antagonist?)).tw. (20625) 36 ((adrenergic or alpha or receptor?) adj2 block$).tw. (58854) 37 or/33‐36 [AB] (116499) 38 hypertension/ (236571) 39 hypertens$.tw. (375255) 40 ((high or elevat$ or rais$) adj2 blood pressure).tw. (26803) 41 or/38‐40 (441992) 42 randomized controlled trial.pt. (484850) 43 controlled clinical trial.pt. (97360) 44 randomized.ab. (371359) 45 placebo.ab. (182081) 46 clinical trials as topic/ (194591) 47 randomly.ab. (255305) 48 trial.ti. (167150) 49 or/42‐48 (1091343) 50 animals/ not (humans/ and animals/) (4782114) 51 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ (908496) 52 (pregnancy‐induced or ocular hypertens$ or preeclampsia or pre‐eclampsia).ti. (14675) 53 49 not (50 or 51 or 52) (963798) 54 (7 or 13 or 18 or 22 or 28 or 32 or 37) and 41 and 53 (16902) 55 54 and (2016$ or 2017$).ed. (350) 56 remove duplicates from 55 (262)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Hypertension Register Segment via Cochrane Register of Studies (CRS‐Web) Search Date: 23 January 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 hypertension AND antihypertensive* AND (Meta‐analysis OR Review):MISC2 AND INSEGMENT (481)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Central Register of Controlled Trials on Wiley <2017, Issue 1> via Cochrane Register of Studies Online Search Date: 23 January 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1MeSH descriptor Thiazides explode all trees2264 #2MeSH descriptor Sodium Chloride Symporter Inhibitors explode all trees2727 #3MeSH descriptor Sodium Potassium Chloride Symporter Inhibitors explode all trees978 #4(loop or ceiling) next diuretic*:ti,ab352 #5(amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide*):ti,ab4989 #6(chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide):ti,ab922 #7#1 OR #2 OR #3 OR #4 OR #5 OR #66548 #8MeSH descriptor Angiotensin‐Converting Enzyme Inhibitors explode all trees5579 #9"angiotensin converting enzyme" next inhibit*:ti,ab2953 #10ace near3 inhibit*:ti,ab2920 #11acei:ti,ab594 #12(alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or saralasin or s nitrosocaptopril or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril*):ti,ab7539 #13#8 OR #9 OR #10 OR #11 OR #1210315 #14MeSH descriptor Angiotensin Receptor Antagonists explode all trees2489 #15angiotensin near3 (receptor next antagon* or receptor next block*):ti,ab2114 #16arbs:ti,ab367 #17(abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan):ti,ab4793 #18#14 OR #15 OR #16 OR #175695 #19MeSH descriptor Calcium Channel Blockers explode all trees8037 #20(amlodipine or amrinone or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil):ti,ab11368 #21calcium near2 (antagonist* or block* or inhibit*):ti,ab4225 #22#19 OR #20 OR #2113977 #23(methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa):ti,ab,kw572 #24(reserpine or serpentina or rauwolfia or serpasil):ti,ab,kw236 #25(clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets):ti,ab,kw2740 #26MeSH descriptor Hydralazine explode all trees300 #27(hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat):ti,ab,kw435 #28#23 OR #24 OR #25 OR #26 OR #273942 #29MeSH descriptor Adrenergic beta‐Antagonists explode all trees9495 #30(acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol):ti,ab13874 #31beta near2 (adrenergic* or antagonist* or block* or receptor*):ti,ab8680 #32#29 OR #30 OR #3118044 #33MeSH descriptor Adrenergic alpha‐Antagonists explode all trees2990 #34(alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin):ti,ab2066 #35adrenergic near2 (alpha or antagonist*):ti,ab417 #36(adrenergic or alpha or receptor*) near2 block*:ti,ab5740 #37#33 OR #34 OR #35 OR #369205 #38MeSH descriptor Hypertension13998 #39hypertens*:ti,ab33338 #40(elevat* or high* or raise*) near2 blood pressure:ti,ab2119 #41#38 OR #39 OR #4035759 #42#7 OR #13 OR #18 OR #22 OR #28 OR #32 OR #3748936 #43#41 AND #4216773 #4415/07/2016 TO 23/01/2017:CD48785 #45#43 AND #44282
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Embase <1974 to 2017 January 20> Search Date: 23 January 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp thiazide diuretic agent/ (51659) 2 exp loop diuretic agent/ (65822) 3 ((loop or ceiling) adj diuretic?).tw. (3851) 4 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).tw. (41498) 5 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw. (3678) 6 or/1‐5 [THZ] (119840) 7 exp dipeptidyl carboxypeptidase inhibitor/ (156912) 8 angiotensin converting enzyme inhibit$.tw. (22618) 9 (ace adj2 inhibit$).tw. (26647) 10 acei.tw. (5851) 11 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or enalaprilat or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw. (36182) 12 or/7‐11 [ACEI] (164401) 13 exp angiotensin receptor antagonist/ (76376) 14 (angiotensin adj3 (receptor antagon$ or receptor block$)).tw. (17419) 15 arb?.tw. (10600) 16 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten).tw. (24341) 17 or/13‐16 [ARB] (82029) 18 calcium channel blocking agent/ (56888) 19 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw. (77978) 20 (calcium adj2 (antagonist? or block$ or inhibit$)).tw. (46895) 21 or/18‐20 [CCB] (139374) 22 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. (28804) 23 (reserpine or serpentina or rauwolfia or serpasil).mp. (29001) 24 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. (45436) 25 hydralazine/ (18117) 26 (hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).tw. (6288) 27 or/22‐26 [CNS] (106569) 28 exp beta adrenergic receptor blocking agent/ (269004) 29 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw. (79345) 30 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. (116005) 31 or/28‐30 [BB] (322790) 32 exp alpha adrenergic receptor blocking agent/ (130668) 33 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw. (16607) 34 (adrenergic adj2 (alpha or antagonist?)).tw. (18171) 35 ((adrenergic or alpha or receptor?) adj2 block$).tw. (71101) 36 or/32‐35 [AB] (197581) 37 exp hypertension/ (630228) 38 (hypertens$ or antihypertens$).tw. (545187) 39 ((high or elevat$ or rais$) adj2 blood pressure).tw. (35380) 40 or/37‐39 (797400) 41 double blind$.mp. (218473) 42 placebo$.tw. (252570) 43 blind$.tw. (339409) 44 or/41‐43 (491232) 45 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5877496) 46 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ (699940) 47 (pregnancy‐induced or ocular hypertens$ or preeclampsia or pre‐eclampsia).ti. (19926) 48 44 not (45 or 46 or 47) (456527) 49 (6 or 12 or 17 or 21 or 27 or 31 or 36) and 40 and 48 (13447) 50 49 and (2016$ or 2017$).dc. (346) 51 remove duplicates from 50 (342)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: ClinicalTrials.gov Search Date: 23 January 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Search terms: randomized Study type: Interventional Studies Conditions: hypertension Interventions: antihypertensives First Received: From 07/15/2016 to 01/23/2017 (40)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: WHO International Clinical Trials Registry Platform (ICTRP) Search Date: 23 January 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ antihypertensive AND hypertension AND randomized (92) antihypertensive AND high blood pressure AND randomized (32)
Data and analyses
Comparison 1. Antihypertensive drug therapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 5 | 16776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.77, 1.13] |
| 2 Cardiovascular mortality plus morbidity overall | 6 | 17278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.67, 0.91] |
| 3 Cerebrovascular mortality plus morbidity | 6 | 17278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.34, 0.64] |
| 4 Coronary heart disease mortality plus morbidity | 4 | 16241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.19] |
| 5 Withdrawal due to adverse events | 3 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.82 [1.67, 13.92] |
| 6 Decrease in systolic blood pressure | 3 | 14845 | Mean Difference (IV, Random, 95% CI) | ‐14.98 [‐20.44, ‐9.52] |
| 7 Decrease in diastolic blood pressure | 4 | 15857 | Mean Difference (IV, Random, 95% CI) | ‐7.62 [‐10.55, ‐4.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carter 1970.
| Methods | Randomized, single site study conducted in UK. Participants were stroke survivors admitted to hospital and followed in clinics. | |
| Participants | 99 participants of which 71 were aged 18 to 59 years; 54% men; age range: 40‐79; mean: 69 years; race/ethnicity: not reported.
Mean BP at entry: not reported. Pre‐existing factors: stroke: 100%; BP entry criteria: SBP > 160 mmHg and DBP < 110 mmHg) or DBP ≥ 110 mmHg irrespective of SBP. Exclusion criteria: cerebral haemorrhage; embolism; tumour; accelerated hypertension; "those with an obvious need for hypotensive therapy;" left ventricular failure; congestive cardiac failure; gross radiological cardiac enlargement; various cardiac arrhythmias or evidence of renal failure. Mean follow‐up: 4.0 years. |
|
| Interventions |
Treatment: first choice: thiazide diuretic (dose or type of thiazide not specified; assumed to be high‐dose thiazide); second choice: methyldopa; third choice: bethanidine, debrisoquine or guanethidine. Control: observation without placebo. |
|
| Outcomes | All‐cause mortality; stroke; CHD; CHF not reported for 18‐ to 59‐year‐old subgroup. | |
| Notes | Definition of total cardiovascular outcomes: not reported. Dropouts due to adverse events: not reported. Quality of life or functional status outcomes: not reported. Percentage of participants not on assigned therapy at study end: not reported. Difference in BP at study end: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Placed at random into treated (50) or control (49) groups. The two groups matched reasonably closely with regard to numbers, age, sex, and severity of hypertension." Comment: method of randomization not described. Probably randomization achieved as groups matched at baseline. |
| Allocation concealment (selection bias) | Low risk | Method for allocation concealment not mentioned. Probably achieved as groups matched well at baseline. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Did not state blinding of participants or personnel. Treating physicians aware of treatment being prescribed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 out of 99 patients (2%) have been lost to follow‐up, a treated man aged 65 and untreated women of 70 ‐ so results are available for 49 treated and 48 untreated patients." Comment: attrition rate was low and although reason for loss to follow‐up not mentioned, it could not have affected the outcome analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol not available to confirm reporting bias. Mortality rate and recurrence rate of strokes mentioned as study objectives were reported in results section. Quote: "Figures for minor strokes or transient cerebral ischaemic attacks are not available." |
| Other bias | Low risk | "Part of the expenses of this research project was covered by a grant from the clinical research subcommittee of the North West Metropolitan Regional Hospital Board." |
HSCSG 1974.
| Methods | Randomized, double‐blind, placebo‐controlled trial conducted in USA with a 6‐week drug run‐in phase. | |
| Participants | Ambulatory stroke survivors with mild to moderate hypertension, 80% African‐Americans, mean age 59 years, range < 75 years, 60% men. Baseline SBP/DBP 167/100 mmHg, pulse pressure 67 mmHg. 80% of participants had completed stroke in year before randomization. 16% had mixtures of completed stroke and TIA, and 4% had only TIAs. Inclusion criteria: SBP ≥ 140‐220 mmHg and DBP 90‐115 mmHg and stroke or TIA or both in previous year. Ambulatory, capable of long‐term attendance at treatment clinic, < 75 years of age and no concomitant disease that might be influenced adversely by prolonged treatment with drug or placebo. Follow‐up: 3 years. |
|
| Interventions | Treatment: deserpidine 1 mg + methyclothiazide 10 mg. Control: no treatment. |
|
| Outcomes | All‐cause mortality, stroke, CHD, CHF, SBP and DBP. | |
| Notes | Stroke endpoint defined by same criteria for entry into study. Also confirmed by a majority of a committee consisting of 2 members outside of the study and the Central Registry neurologist. Marked increase in frequency of TIAs (twice the weekly prerandomization level of occurrence and > 4 per week), or a deterioration of more than 8 points in the neurological score also qualified as a stroke endpoint. Scoring system of residual deficits by neurologist was based on total of 100 points, allowed a maximum of 35 points for level of consciousness and mentation, 9 points for cranial nerve function, 30 points for motor system, 3 points for reflexes, 3 points for sensory function and 20 points for "health and performance" function. Quote: "The study was terminated earlier than planned when it became evident that further follow‐up would not significantly affect the results. All patients without endpoints were under observation for at least one year; the mean follow‐up period for all individuals including those with end points was 27.4 months, and for those not having endpoints, it was 38.6 months." Mulrow 1998 provided data for cardiovascular mortality and morbidity and cerebrovascular mortality and morbidity in people aged ≥ 60 years for this study which is referenced as HTN COOP 1974. Data on all‐cause mortality not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: "A prospective double blind cooperative study was undertaken to determine the influence of treatment on the prognosis in stroke survivors with mild to moderate hypertension. If no intolerable side effects occurred, the patient was placed on a regimen of two tablets daily of randomized medication." "To ensure that drug and placebo were balanced among groups with characteristics of possible prognostic importance, patients were divided into cells based on these characteristics, and drug or placebo was prescribed to maintain a balance within these cells. The characteristics for which this randomization was conducted were sex, race, diastolic blood pressure above or below 100 mm Hg, and the four stroke categories." "Although no effort was made to assure an equal distribution of drug‐treated and placebo‐treated patients within each clinic, the drug‐placebo ratio differed appreciably in only two of the ten clinics." "No statistically significant differences were noted in the frequency of abnormalities in the laboratory findings, ECGs, and chest X ray films between the drug and placebo groups." Comment: randomization probably done. However, method for random sequence generation not mentioned. However, baseline characteristics well matched. |
| Allocation concealment (selection bias) | Low risk | Quotes: "The bio‐statistical section was responsible for assignment of patients to drug or placebo regimens, distribution of medication by mail to the individual clinics, data preparation, coding, and analysis." "For use in an emergency, a sealed envelope held by a disinterested person at the local clinic identified the type of medication the patient was receiving" (page 410). Comment: method of allocation concealment not reported; however, baseline characteristics well matched. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "A prospective double blind cooperative study." "Neither the doctor nor the patient was aware of whether placebo or drug had been supplied. For use in an emergency, a sealed envelope held by a disinterested person at the local clinic identified the type of medication the patient was receiving." Comment: participants and treating physicians blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "the report of the stroke event and the neurological findings were submitted to Central Registry for confirmation. A stroke endpoint was defined by the same criteria for entry into the study. It also was confirmed by a majority of a committee consisting of two members outside of the study and the Central Registry neurologist." "Similarly, any medical event justifying removal of the patient from the study was carefully reviewed and classified into cardiovascular and non‐cardiovascular categories. The events of a cardiovascular nature were confirmed by an outside cardiologist." "Patient records without information concerning blood pressure or type of medication were given to both cerebrovascular and cardiovascular reviewers" (page 410). Comment: outcome assessor blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Five‐hundred one patients were exposed to pre randomization drug trial. Forty‐nine who entered the drug trial were not subsequently randomized." Comment: of the 452 participants randomized, total withdrawals not reported. Quote: "The study was terminated earlier than planned (3 years follow up) when it became evident that further follow‐up would not significantly affect the results. All patients without endpoints were under observation for at least one year; the mean follow‐up period for all individuals including those with endpoints was 27.4 months, and for those not having endpoints, it was 38.6 months." Comment: attrition rate not mentioned and how data were analyzed in these participants not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not available. Cerebrovascular and cardiovascular outcomes, BP measurements, drug intolerance and laboratory measurements were reported. |
| Other bias | Low risk | Investigation supported by grants from the National Institute of Neurological Diseases and Stroke. |
MRC‐TMH 1985.
| Methods | Randomized, single‐blind trial comparing 2 treatments and placebo in ambulatory people in England, Scotland and Wales. | |
| Participants |
Overall randomized participants: 17,354 (treatment 8700; control 8654); 8306 men and 9048 women; mean age 52 years, range 35‐64 years. Ethnicity not reported. Men (49%). Baseline mean SBP/DBP 161.4/98.2 mmHg, pulse pressure 63 mmHg. Subgroup 18‐ to 59‐year age group: 14,541 (83.8%) participants (bendrofluazide 3611; propranolol 3674; placebo 7256); men 7863 (54.1%); SBP 160 ± 16.9 mmHg; DBP 94.5 ± 5.8 mmHg; smokers 4321 (29.7%). Inclusion criteria: SBP < 200 mmHg and DBP 90‐109 mmHg. Exclusion criteria: secondary hypertension; taking antihypertensive treatment; normally accepted indications for antihypertensive treatment (such as congestive cardiac failure) present; myocardial infarction or stroke within the previous 3 months; presence of angina, intermittent claudication, diabetes, gout, bronchial asthma, serious intercurrent disease or pregnancy. Follow‐up: 5 years. |
|
| Interventions |
Treatment: bendrofluazide 10 mg daily, propranolol 80‐240 mg daily, + methyldopa if required. Control: placebo. Note: 288 participants were randomly assigned to observation only taking no tablets and were merged with placebo. |
|
| Outcomes | All‐cause mortality, stroke, CHD, total cardiovascular events, SBP and DBP. | |
| Notes | For age group 18 to 59 years in this trial, data were provided by author FG from the INDANA database. CHF and withdrawal due to adverse event data not available. Note that CHF data were not included in total cardiovascular outcomes in 18‐ to 59‐year‐old subgroup. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated at entry... Randomisation was in stratified blocks of eight within each sex, 10 year age group, and clinic." Comment: no information provided for sequence generation. Random sequence generation achieved properly and baseline characteristics were matching. |
| Allocation concealment (selection bias) | Low risk | No description of method for allocation concealment provided; however, baseline characteristics well matched. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "four treatments: the thiazide diuretic bendrofluazide; placebo tablets that looked like bendrofluazide; the beta blocker propranolol; and placebo tablets that looked like propranolol. The two placebo groups were treated as one in all analyses." Quote: "When the protocol was written, it was judged unreasonable to ask general practitioners to undertake such adjustments in a double blind study, and the trial was therefore single blind only." Comment: participants blinded but not the physicians. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The evidence on which the diagnosis of each terminating event was based was assessed by an arbitrator ignorant of the treatment regimen... The arbitrator used WHO [World Health Organization] criteria for classification." "All events were assessed by an independent arbiter who was blind to the treatment regimen." "Each electrocardiogram tracing was read by two observers who were blind to the treatment regimen; the second reader was also blind to the first reader's coding. If these two readers disagreed, a third reader was used." Comment: adjudication was independent and blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotes: "All analyses presented here are based on randomised treatment ("intention to treat") categories. Thus data for all participants are presented as if the individual was still in the treatment group to which he was originally randomised, although substantial percentages of patients (see below) were in fact withdrawn from their randomly allocated regimen during follow up." "The total five and a half year cumulative percentages of men who stopped taking their randomised treatment, including both those withdrawn from their randomly allocated regimen but continuing on follow up and those lapsing from the trial, were 43% of the bendrofluazide group, 42% of the propranolol group, and 47% of the placebo group. For women the figures were 33%, 40%, and 40% respectively. The cumulative percentages of people not taking either primary active drug by five and a half years were smaller: 33% of men originally randomised to bendrofluazide and 34% of men randomised to propranolol and 28% and 31% respectively of women." "Events terminating a patient’s participation were: stroke, whether fatal or non‐fatal; coronary events, including sudden death thought to be due to a coronary cause, death known to be due to myocardial infarction, and non‐fatal myocardial infarction; other cardiovascular events, including deaths due to hypertension (ICD [International Classification of Diseases] 400‐404) and to rupture or dissection of an aortic aneurysm; and death from any other cause. Clinic staff reported these events to the coordinating centre. The records of all patients who suffered non‐fatal terminating events and of any others who lapsed from the trial, whatever the reason, were "flagged" at the Southport NHS [National Health Service] central register to ensure notification of death). Comment: myocardial infarction and stroke were reasons for terminating the study follow‐up, except for death flagging. This induces a censoring attrition bias, limited to the occurrence non‐fatal events myocardial infarction or stroke. |
| Selective reporting (reporting bias) | Low risk | No information about prespecified outcomes available on which to make this assessment. However, aim was to study mortality and morbidity, which were reported. |
| Other bias | Unclear risk | Conflict of interest was not reported. "The working party thanks the general practitioners and nurses collaborating in the trial; the staff at the coordinating centre; the staff of the Wolfson Research Laboratories, Queen Elizabeth Medical Centre, Birmingham, for carrying out the biochemical analyses; Duncan, Flockhart and Co Ltd for tablets of bendrofluazide and placebo; Imperial Chemical Industries Ltd for financial support and for tablets of propranolol and placebo; Ciba Laboratories for supplies of guanethidine; and Merck Sharp and Dohme Ltd for a mobile screening unit, funds for its staffing, and supplies of methyldopa." |
Oslo 1986.
| Methods | Open, randomized trial conducted in ambulatory young men randomized to treatment or no treatment in Norway. | |
| Participants | 785 men, mean age 45.3 years, range 40‐49 years. Ethnicity not reported. Baseline mean SBP/DBP 156.2/97 mmHg, pulse pressure 59 mmHg. Inclusion criteria: SBP 150‐179 mmHg and DBP < 110 mmHg. Followed for 5‐6 years. Target BP < 140/90 mmHg. Exclusion criteria: new or previous CHD; cardiovascular disease; intermittent claudication; CHF or valvular heart disease; drug‐treated hypertension during the last year; diabetes mellitus (fasting blood sugar ≥ 8.3 mmol/L); retinopathy (Keith‐Wagener grade 3 and 4); renal disease (proteinuria, haematuria, creatinine ≥ 123.8 mmol/L, chronic nephritis); hepatic disease; psychosis; severe neurosis; people regularly treated with psychopharmacological drugs; malignant disease; chronic disease such as rheumatoid arthritis; endocrine disorders; obvious alcohol abuse and social maladjustment; secondary hypertension; electrocardiographic changes at rest: left bundle branch block, atrial fibrillation, S‐T segment depressions; marked left ventricular hypertrophy; Rmax + Smax in precordial leads ≥ 45 mm and simultaneous ST‐T changes (Minnesota code 4‐l) S‐T segment depression and T‐wave flattening or inversion. Mean follow‐up: 5 years. |
|
| Interventions |
Treatment: hydrochlorothiazide (95%), methyldopa, and propranolol (26%). At 5‐year follow‐up, 36.7% were taking hydrochlorothiazide alone, 26% were taking hydrochlorothiazide + propranolol, 20% were taking hydrochlorothiazide + methyldopa and 18% were taking other drugs. Control: no treatment. |
|
| Outcomes | Stroke, CHD, all‐cause mortality, CHF, SBP and DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "During 1973, 785 men, aged 40 to 49, with mild, symptom‐free hypertension were randomly assigned for a five‐year controlled drug treatment study, 406 men in the treatment group and 379 in the control group." "The randomization was performed by a "random number table"." Comment: participants were randomly allocated using random number tables and the baseline characteristics were similar. |
| Allocation concealment (selection bias) | Low risk | Method used for allocation concealment not mentioned. Participants and physicians aware of treatment given; however, baseline characteristics were similar. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Drug treatment was started with hydrochlorothiazide. If SBP remained above 140 mm Hg and/or DBP above 90 mm Hg, alpha methyldopa was added. If there were side effects, methyldopa was replaced with propranolol. The control group was not given a placebo." Comment: no mention of blinding, both participants and physicians aware of treatment provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Possible and definite coronary events and other cardiac complications were also evaluated by a "blind" diagnostic board of two independent cardiologists." Comment: blinding of outcome assessors probably done, though it was not clearly stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: "In this study the patients have seen the same physicians and the same paramedical staff during 4 years and the drop‐out rate has been small, 0.6 per cent and the same in both groups." "Three men refused the drugs, and 13 (1.7%) dropped out of the study, three in the treatment group and 10 in the control group." "The mean observation time was 66 months (range: 60 to 76). Only 13 (1.7%) men failed to report for regular examinations. However, these men were followed for possible cardiovascular events at the end of the study." Comment: number of dropouts was low and participants were followed until end of study to account for all outcomes. |
| Selective reporting (reporting bias) | Low risk | Quote: "Each patient with cardiovascular events has been counted once, i.e., the number of events is identical with the number of patients with events. If a patient had more than one event, the most serious was counted. Nobody had both coronary heart disease and a cerebrovascular event." Comment: all outcomes (coronary, cerebrovascular and other events) properly reported and accounted for in results section. |
| Other bias | Unclear risk | Conflict of interest not reported. Source of funding not stated. |
USPHSHCSG 1977.
| Methods | Randomized, double‐blind, placebo‐controlled trial conducted in ambulatory young people in USA. | |
| Participants | 389 participants. Mean age 44.3 years, range 21‐55 years. 28% African‐Americans. 80% men. Baseline mean SBP/DBP 146.9/99 mmHg and pulse pressure 48 mmHg. Inclusion criterion: DBP 90‐115 mmHg. Target: none (medication was not titrated). Exclusion criteria: diabetes mellitus, renal insufficiency, or hypercholesterolaemia; abnormal ECG including single or double Master test; radiographic cardiomegaly; grade III or IV retinopathy; clinical history or findings of previous arterial thrombosis or vascular insufficiency, whether coronary, cerebral or peripheral; CHF; angina pectoris; valvular heart disease; or secondary or correctable hypertension; and known sensitivities to the intervention agents. Follow‐up: 10 years. |
|
| Interventions |
Treatment: chlorothiazide 500 mg twice daily + rauwolfia serpentina 100 mg twice daily. Control: placebo. |
|
| Outcomes | All‐cause mortality, CHD, stroke, CHF, SBP and DBP. | |
| Notes | Study carried out in middle‐aged population with mild hypertension which is itself a low‐risk factor for studying mortality and morbidity data in such a population. Study terminated if participant had a stroke. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: "Subjects were randomly assigned to treatment or placebo and then matched by race and sex for two broad age groups (under 46 and 46‐55). The randomization was carried out within each of the six participating clinics" (page 100). "The distribution of all pre‐treatment characteristics into the active drug and placebo groups was uniform." Comment: randomization carried out, but method used for generation of random numbers not specified. |
| Allocation concealment (selection bias) | Low risk | Quote: "At the conclusion of the trial period, subjects were randomly assigned either active or placebo treatment, and this medication was substituted for the identical placebo of the trial period and administered in double‐blind fashion. Active therapy consisted of chlorothiazide, 500 mg, plus rauwolfia serpentina, 100 mg, in one tablet taken twice daily. There was no intervention on diet or smoking or other behavioral factors." Comment: allocation carried out randomly but methodology used to conceal allocation not mentioned; however, baseline characteristics well matched. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "At the conclusion of the trial period, subjects were randomly assigned either active or placebo treatment, and this medication was substituted for the identical placebo of the trial period and administered in double‐blind fashion." "The complications were also classified in terms of those events considered likely to be the consequence of elevated pressure per se and those which are predominantly associated with vascular sclerosis (Table 2). All such events were reviewed by two consultants otherwise unassociated with the trial, who were provided with all pertinent information except knowledge of the treatment regimen." Comment: participants and treating physicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: "administered in double‐blind fashion." "Follow‐up continued for another 4 months, the last 2 weeks of which included home blood pressures again. At that point the annual examination procedures were repeated. Thereafter, the regimens were unblinded and investigators were at liberty to treat as clinically indicated." "Of the complications observed, only stroke required termination from the regimen to which they were randomized. For myocardial infarction it was elective, depending on the clinical circumstances. Thus, by design, most subjects continued on the same double‐blind follow‐up after their first morbid event and were at risk for additional subsequent events. Others were followed on known medication." Comment: blinding of outcome assessor not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotes: "Of importance in considering the morbidity and side effects data in this report is the fact that there was no differential dropout rate between the treatment and control groups (33.2% vs. 34.7%). This applies to those who simply failed to return ("Lost to Follow‐up") as well as those who voluntarily "withdrew" from assigned therapy but remained under follow‐up. The number for whom vital status is unknown is also similar in the two groups (14 vs.12)." "During that time, 206 (52.9%) were terminated from their assigned regimen (Table 5). Dropouts accounted for 132 (33.9%), of whom 75 have been lost to regular follow‐up. The vital status of 26 of the dropouts is unknown. Drug intolerance necessitated terminations in 23 cases and major morbid events in 27, four of which were deaths. The remainder of those terminated have continued under regular follow‐up on known medications, including 24 who were terminated as treatment failures on the basis of progressive elevation of blood pressure to above a predetermined level." "The dropout rate of 33.9% overall is within that allowed for in the calculation of sample size (5% per year of follow‐up). At the beginning of closeout, one‐half remained on their assigned coded medication." Comment: attrition rate high. Attrition rates per year not mentioned as cut‐off was kept at 5% lost to follow‐up, and if > 5% the outcome measures will be affected. |
| Selective reporting (reporting bias) | Unclear risk | Comment: secondary and tertiary outcomes as mentioned in the objectives not mentioned in results section. |
| Other bias | Unclear risk | Comment: conflict of interest not reported. Source of funding not stated. |
VA‐II 1970.
| Methods | Randomized, double‐blind, placebo‐controlled study conducted in ambulatory men in USA. | |
| Participants | 380 men, mean age 52 years, range not reported. 42% participants were African‐Americans. 100% men. Baseline mean SBP/DBP 162/104 mmHg and pulse pressure 58 mmHg. Inclusion criterion: DBP 90‐114 mmHg. Exclusion criteria: history of severe hypertensive complication such as a cerebral or subarachnoid haemorrhage, hypertensive neuroretinopathy, dissecting aneurysm or renal failure, but did not include atherosclerotic complications such as coronary artery disease or cerebrovascular thrombosis; people with surgically curable hypertension, unrelated fatal diseases such as malignant tumours, people unwilling or unable to return to clinic and poorly motivated or otherwise unco‐operative or unreliable people. Follow‐up: 3.7 years. |
|
| Interventions |
Treatment: step 1: hydrochlorothiazide 100 mg + reserpine 0.2 mg;
step 2: hydralazine 75‐150 mg. Control: placebo. |
|
| Outcomes | All‐cause mortality, CHD, stroke, CHF, SBP and DBP. | |
| Notes | Study is referenced as VA COOP in Mulrow 1998 review. The review provides data on total cardiovascular mortality + morbidity, cerebrovascular mortality + morbidity, and CHD mortality + morbidity in people aged ≥ 60 years. However, data on all‐cause mortality were not provided in Mulrow 1998 review. Quotes: "The study was terminated in the subgroup of 143 patients whose diastolic blood pressures averaged 115 through 129 mm Hg prior to randomization." "Many uncooperative and unreliable patients were identified and eliminated from the trial on the basis of pill counts, urine fluorescence test results, and irregularity of clinic attendance during a pre randomization observation period. Treatment obviously would not have been as effective in a group of patients less carefully selected with regard to their desire to cooperate. The population was further limited in that it excluded female patients and patients with labile hypertension whose diastolic blood pressures averaged lower than 90 mm Hg during the fourth through the sixth day of hospitalization." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Three hundred and eighty male hypertensive patients with diastolic blood pressures averaging 90 to 114 mm Hg were randomly assigned to either active antihypertensive agents or placebos." Comment: although method used for random sequence generation not stated it was probably done. Tables 1 and 2 indicated that the two groups were comparable according to the indicated variables. |
| Allocation concealment (selection bias) | Low risk | Quote: "Accepted patients were then randomly assigned double‐blind to either active drugs or placebos." Comment: method used for allocation concealment not reported; however, baseline characteristics well matched. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "Accepted patients were then randomly assigned double‐blind to either active drugs or placebos." "Active drugs consisted of two types of tablets, one being a combination tablet containing 50 mg hydrochlorothiazide and 0.1 mg reserpine which was given twice daily. The other was 25 mg of hydralazine hydrochloride given three times daily. The latter medication was raised to 50 mg three times daily if the diastolic blood pressure remained at 90 mm Hg or higher. Obviously, practically all of the patients in the placebo group had their "doses" raised to this level." "Patients in the control group received placebos identical in taste and appearance to the active drugs." "In order to avoid losses to protocol because of side effects presumably caused by one or the other of the two agents, provision was made to permit substitution of a tablet which contained either reserpine or hydrochlorothiazide alone and omitted the offending medication. These special tablets were made available on request of a participating physician. Similar appearing placebo tablets were made available for the control patients and the physician did not know whether the substitution represented active drugs or placebos." Comment: trial was double‐blind where participants and physicians were not aware of treatment allocated to either group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The records of the patients reported as having assessable morbid events were reviewed by two consulting physicians who had not participated in the trial." "All available data pertaining to each organic complication, except the type of protocol treatment and the level of blood pressure, were presented to the reviewers and their decisions regarding the occurrence and classification of an event according to the definitions given in the protocol (see list of assessable events at the end of the communication) were accepted as final." Comment: outcome assessors probably blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quotes: "Fifty‐six or 15% of the 380 randomized patients were classified as drop‐outs during the course of the trial. Of this number 27 had been randomized to receive placebos and 29 to receive active drugs. The average period of follow up prior to dropping out was 17.6 months with a range from less than 1 month to 49 months." "Thus, the earliest entrants were observed for 5.5 years and the latest entrants for a minimum of 1 year. The average potential duration of observation, disregarding losses and terminations, was 3.9 years for the control group and 3.7 years for the treated patients. However, because of the losses and terminations due to elevated diastolic blood pressure described below, the actual duration of post randomization observation was 3.3 years for the control group and 3.2 years for the treated patients." Comment: reasons for dropouts mentioned; though reasons were not given separately for the 2 groups. How data were analyzed in these participants not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not available. Mortality (various causes of death) and morbidity (various terminating morbid events other than death) data reported. |
| Other bias | Unclear risk | Conflicts of interest not reported. |
VA‐NHLBI 1977.
| Methods | Randomized, double‐blind, placebo‐controlled trial conducted in ambulatory people in USA. | |
| Participants | Mean age 37.5 years, range 21‐50 years. 74% white and 25% African‐American and 1% other. 81% men. Baseline mean DBP 93.3 mmHg with 66% having mean DBP 85‐95 mmHg at time of randomization. Inclusion criterion: DBP 85‐105 mmHg. Target < 85 mmHg. Exclusion criteria: significant cardiovascular renal abnormalities, insulin‐requiring diabetes, treatment with vasoactive drugs, concomitant "fatal" disease, history of depression or of recent (within last 2 years) gout or peptic ulcer, and any conditions felt to make non‐compliance likely. Follow‐up: 2 years. |
|
| Interventions |
Treatment: step 1: chlorthalidone 50 mg, step 2 100 mg (53% chlorthalidone alone), step 3: chlorthalidone 100 mg + reserpine 0.25 mg. Control: placebo. |
|
| Outcomes | All‐cause mortality, stroke, CHD, CHF and DBP. | |
| Notes | No intervention on diet or smoking or other behavioural factors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the conclusion of the trial period, subjects were randomly assigned either active or placebo treatment, and this medication was substituted for the identical placebo of the trial period and administered in double‐blind fashion." Comment: method for random sequence generation not specified. Table for baseline characteristics of the 2 groups not provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment not mentioned. Baseline characteristics of the 2 groups could not be assessed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "The Cooperative Studies Program Central Research Pharmacy was responsible for distribution of coded double‐blind study drug to each clinical center and for assuring proper handling of these drugs when they were distributed to the individual subjects." "The blinded active and placebo drugs were both designated by small letters in parentheses; whereas the known drugs were designated by underlined capital letters: C, 2C, 1/2C and R. The protocol defined three standard successive therapeutic steps: (c), (2c), and (2c)+(r). Each subject began (c) when he was randomized." "The study biostatistician supervised data management and reporting. The data center supplied each clinical center with instruction manuals, data forms, randomization numbers, and individual subject identification labels for all forms, drug bottles, sample containers, electrocardiograms, and x‐rays." Comment: trial stated as double‐blind and probably participants and physicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessor not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "By preliminary count there were 98 losses to study in the active group and 104 in the placebo group, making the total cumulative dropout rate equal to 20% with a “dropout” being defined as a subject with an appointment overdue for more than 60 days." Comment: dropout rates 19.3% (98/508 participants) in treatment group and 20.6% (104/504 participants) in placebo group. Reasons for loss to follow‐up not stated separately for teach group. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Comment: major and minor morbid events reported with adverse events for each group. |
| Other bias | Low risk | Quotes: "This project was jointly supported by the Cooperative Studies Program of the Medical Research Service of the Veterans Administration and by an Interagency Agreement (2 Y01‐HV‐40012‐04) awarded by the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health, Education, and Welfare." "A feasibility trial to investigate the practicality of determining the advantages and disadvantages of prompt pharmacologic treatment for mild hypertension was jointly funded by the Veterans Administration and the National Heart, Lung and Blood Institute" (page 286). |
BP: blood pressure; CHD: coronary heart disease; CHF: congestive heart failure; DBP: diastolic blood pressure; ECG: electrocardiogram; SBP: systolic blood pressure; TIA: transient ischaemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ADVANCE 2007 | Randomi zed controlled trial; 215 collaborating centres in 20 countries from Asia, Australasia, Europe and North America. After 6‐week active run‐in period, 11,140 pa rticipants with type 2 diabetes aged ≥ 55 years were randomi zed to treatment with fixed combination of perindopril + indapamide or matching placebo, in addition to current therapy. No BP criteria for inclusion. All other treatments continued at discretion of responsible physician, with exception of ACE inhibitors. Participants taking ACE inhibitor other than perindopril had this treatment withdrawn and were offered substitution with open‐label perindopril at 2 mg or 4 mg a day. Control group included non‐specific antihypertensive therapy. |
| ALLHAT 1996 | Prospective, randomiz ed, double‐blind, active‐controlled clinical trial. Drug‐drug comparison of different drug classes with no placebo or untreated control group. |
| Anon 1973 | Single ‐blind, randomi zed, placebo‐ controlled trial conducted in the UK in 116 men and women aged 45 ‐69 years with 2 casual, sitting DBP of 100 ‐120 mmHg on each of 2 occasions separated by an interval of ≥ 2 weeks. Untreated control group received calcium lactate tablets. Treatment group received any combination of bendrofluazide with potassium supplement, methyldopa or debrisoquine ; choice of treatment at discretion of physician. However, data in 45‐ to 59 ‐year ‐old pa rticipants not reported separately. |
| ATTMH 1984 | Australian National Blood Pressure Study, a controlled therapeutic trial of antihypertensive drug treatment in 3427 men and women with mild hypertension. Pa rticipants were aged 30 ‐69 years and randomi zed to active treatment or placebo. Active group had chlorothiazide 500 mg daily as first‐order drug. Thereafter, if blood pressure control was not achieved, dose was increased to 500 mg twice daily or second‐order drug (methyldopa, propranolol or pindolol) was added (or both). Subsequently, third‐order drugs (hydralazine or clonidine) were given if necessary. Initial objective was to reduce DBP to ≤ 90 mmHg but after 2 years the goal was lowered to 80 mmHg. Of 1721 pa rticipants randomi zed to treatment, 1428 were aged 30 ‐59 years and of 1706 randomi zed to placebo, 1417 were aged 30 to 59 years. Data in the age group 30‐ to 59 ‐years were not reported separately. |
| Berglund 1981 | Drug‐drug comparison of bendrofluazide 2.5 mg vs propranolol 160 mg with no placebo or untreated control group. |
| CASTEL 1994 | Drug‐drug comparison with no placebo or untreated control group. Control group included non‐specific antihypertensive therapy. |
| Coope 1986 | Randomized multisite study comparing antihypertensive treatment to control (observation without placebo) in p eople aged 60‐ to 79‐ years. |
| Dutch TIA 1993 | Randomiz ed, double‐blind, placebo‐controlled trial in 1473 pa rticipants aged > 65 years with TIA or disabling stroke were randomized to atenolol or placebo. Only p eople aged ≥ 65 years were included in this trial. |
| EWPHBPE 1988 | Randomi zed, placebo ‐controlled, double‐blind trial conducted in Europe. Only pa rticipants aged ≥ 60 years were included in this trial. |
| Fuchs 2011 | Randomized, double‐blind, clinical trial, controlled by placebo in pa rticipants aged 30‐70 years with prehypertension. |
| GENERIC 2010 | Single‐centre, randomiz ed, double‐blind, placebo‐controlled, cross‐over trial comparing effects of moexipril and placebo on insulin sensitivity and 24‐hour BP control in postmenopausal women with essential hypertension. Only an 8‐ week trial. |
| GLANT 1995 | Employed alternate allocation (i.e . not random allocation). Drug‐drug comparison of delapril 30‐120 mg vs several dihydropyridine calcium channel blockers with no placebo or untreated control group. |
| HANE 1997 | Morbidity and mortality outcomes not reported. |
| HAPPHY 1987 | 6569 men aged 40‐64 years with mild to moderate hypertension randomized to diuretic or beta ‐blocker therapy. No placebo control group. |
| HDFP 1982 | Treated group included various lifestyle measures in addition to antihypertensive drug therapy. Control group was usual care and participants were not necessarily untreated controls. |
| HOPE HYP 2000 | Double‐blind, randomized trial in pa rticipants aged ≥ 55 years with previous coronary artery disease, cerebrovascular disease, peripheral vascular disease or diabetes + 1 additional risk factor (high BP > 160 mmHg or > 90 mmHg, total cholesterol > 5.2 mmol/L, high‐density lipoprotein cholesterol < 0.9 mmol/L, current cigarette smoking or known microalbuminuria). Pa rticipants randomized to ramipril 2.5 mg titrating to 10 mg or placebo. Other factor was v itamin E 400 IU/day. Data in 55‐ to 59 ‐year‐ old pa rticipants were not reported separately. |
| HOT 1995 | Evaluated the effects of achieving prespecified levels of DBP control with all pa rticipants receiving antihypertensive treatment. No placebo control group. |
| HYVET 2003 | Randomized, open ‐label trial conducted in Europe. Only included participa nts aged ≥ 80 years. |
| HYVET 2008 | Randomiz ed, double‐blind, placebo ‐controlled trial. Only included participa nts aged ≥ 80 years. |
| IDM 2001 | Multinational, randomized, double‐blind, placebo‐controlled study . 590 participants aged 30‐70 years with hypertension with type 2 diabetes and microalbuminuria . Participants randomized to irbesartan 150 mg daily or 300 mg daily, and followed for 2 years. Other antihypertensive drugs were prescribed to 56% of the placebo group. |
| INSIGHT 1996 | Prospective, randomiz ed, double‐blind trial in Europe and Israel with 6321 participa nts aged 55‐ 80 years with hypertension ( BP 150/95 mmHg, or SBP 160 mmHg). Participa nts had ≥ 1 additional cardiovascular risk factor. Participa nts randomly assigned to nifedipine 30 mg in a long‐acting gastrointestinal‐transport system formulation (3157 participa nts), or co‐amilozide hydrochlorothiazide 25 g + amiloride 2 .5 mg ( 3164 participants). Dose titration by dose doubling, and addition of atenolol 25‐ 50 mg or enalapril 5‐ 10 mg. No placebo or untreated control group. |
| IPPPSH 1985 | Randomized, double ‐blind study in 6357 men and women aged 40‐64 years with uncomplicated hypertension. All participa nts received beta ‐blocker oxprenolol. Participa nts then randomized to either continuing treatment with oxprenolol or receiving placebo. Study medications could be increased or other non ‐beta‐blocker antihypertensive drugs could be added as necessary in both groups with aim of reducing DBP to ≤ 95 mmHg. |
| Jikei 2007 | Did not truly randomiz e participants to treatment arms and control group included non‐specific antihypertensive therapy. |
| Kuramoto 1981 | Randomi zed, double‐blind, placebo ‐controlled trial conducted in Japan. Included participa nts aged 60‐90 years. |
| Kuramoto 1994 | Head‐to‐h ead comparison of different drug therapies (nicardipine vs trichlormethiazide) without placebo or untreated control group. |
| Lewis 2001 | Prospective, randomiz ed, double‐blind clinical trial. Included participa nts aged 30 ‐70 years, a documented diagnosis of type 2 diabetes mellitus, hypertension or documented treatment with antihypertensive agents, and proteinuria, with urinary protein excretion ≥ 900 mg per 24 hours. 1715 hypertensive participa nts randomly assigned by a central office to 1 of 3 treatment regimens: irbesartan, amlodipine or placebo. Control group also received antihypertensive medications. |
| MAPHY 1988 | 3234 white men aged 40 ‐64 years randomized to metoprolol or thiazide diuretic. No placebo control group. |
| Materson 1993 | Randomized, double ‐blind study of 1292 men aged ≥ 18 years with hypertension (DBP 95 ‐109 mmHg). M ean age 58 ± 10 years. Participa nts randomized to placebo or 1 of 6 drugs: hydrochlorothiazide, atenolol, captopril, clonidine, diltiazem sustained release or prazosin. 546 participa nts were age d < 60 years but results not reported separately . |
| MIDAS 1996 | Drug‐drug comparison of hydrochlorothiazide 25 mg vs isradipine 5 mg with no placebo or untreated control group. |
| Morgan 1980 | Allocation to groups not random, based on week of presentation at clinic. |
| MRCO 1992 | Prospective, randomi zed, placebo‐controlled, single‐blind trial. 4396 participa nts in the UK aged 65‐74 years with hypertension (SBP 160‐209 mmHg; DBP < 115 mmHg). Trial only included participa nts aged 65‐74 years. |
| NORDIL 2000 | Prospective, randomiz ed, open, blinded endpoint study. E nrolled 10,881 participa nts, aged 50‐ 74 years, at health centres in Norway and Sweden, who had DBP ≥ 100 mmHg. Participa nts randomly assigned to diltiazem or diuretics or both. No placebo or untreated control group. |
| PATS 1995 | Randomi zed, double‐blind, placebo ‐controlled trial conducted in China. 5665 Chinese men (72%) and women (28%) with history of TIA, minor stroke or major stroke without severe disability. Mean age 60 ± 8 years. Baseline BP 154/93 mmHg. 16% of participa nts were not hypertensive with BP < 140/90 mmHg. Mean follow‐up 2 years. After single‐ blind run‐in phase on placebo, eligible participa nts were randomi zed to indapamide 2.5 mg treatment or placebo. Data o n 18‐ to 59‐ year‐ old participa nts not reported separately. |
| PROGRESS 2001 | < 50% of participa nts had elevated BP and about 50% of participa nts were receiving other antihypertensive therapy at baseline and throughout trial. |
| QUIET 2001 | Most participa nts did not have elevated BP. 25% of participa nts receiv ed a beta‐blocker. |
| SCAT 2000 | > 60% did not have hypertension and about 50% received beta‐blockers at baseline and throughout. |
| Schmieder 2009 | Randomized, double‐blind, multicentre, placebo‐ controlled study. After 2‐ to 4‐week placebo run‐in, 1124 participa nts were randomized to aliskiren 150 mg, hydrochlorothiazide 12.5 mg or placebo once daily. Forced titration (to aliskiren 300 mg or hydrochlorothiazide 25 mg) occurred at week 3; at week 6, participa nts receiving placebo were reassigned (1:1 ratio) to aliskiren 300 mg or hydrochlorothiazide 25 mg. From week 12, amlodipine 5 mg was added and titrated to 10 mg from week 18 for participa nts whose BP remained uncontrolled. Not a placebo‐ controlled study of 52 weeks' duration. |
| SCOPE 2003 | 4964 participa nts aged 70 ‐89 years, with SBP 160 ‐179 mmHg or DBP 90‐ 99 mmHg, and a Mini Mental State Examination test score > 24. Participa nts randomly assigned to receive the angiotensin receptor blocker candesartan or placebo, with open‐label active antihypertensive therapy added as needed. As a consequence, active antihypertensive therapy was extensively used in control group (84% of participa nts). Mean follow‐up 3.7 years. No true placebo control group. Both groups allowed some participa nts to take hydrochlorothiazide at baseline and most of control group received antihypertensive therapy. |
| SHELL 1994 | Head‐to‐head comparison of different drug therapies with no placebo or untreated control group. |
| SHEP ‐ Pilot 1985 | Randomi zed, double‐blind, placebo ‐controlled trial conducted in USA. Participa nts aged > 60 years. |
| SHEP 1991 | Randomi zed, double‐blind, placebo ‐controlled trial conducted in USA. Participa nts aged > 60 years. |
| Sprackling 1981 | Randomi zed, open ‐label study in welfare home for elderly people. Age range not reported. Mean age 80.7 years. Participa nts randomized to methyldopa 250 mg twice daily or observation without placebo. Data in 18‐ to 59‐ year old participants not reported separately. |
| STONE 1996 | Employed alternate allocation (i.e . not random allocation). 4 weeks after group assignment, attending physicians reallocated participants from placebo group to treatment group if their DBP was ≥ 110 mmHg. |
| STOP 1991 | Randomi zed, double ‐blind, placebo ‐controlled multisite study in Sweden. Participa nts aged 70‐84 years. |
| STOP‐2 1993 | Prospective, randomiz ed, double‐blind, intervention study compar ing effects of active antihypertensive therapy and placebo on frequency of fatal and non‐fatal stroke and myocardial infarction and other cardiovascular death in hypertensive Swedish men and women aged 70‐84 years. |
| Strandberg 1991 | 5‐ year long, multifactorial, randomized, controlled primary prevention trial in 1222 middle‐ aged healthy men. Age range not reported. Mean age 48 years. Treatment group had multiple interventions. Drug treatment (mainly the beta‐blockers, propranolol hydrochloride or pindolol, or diuretics, hydrochlorothiazide alone or combined with amiloride hydrochloride for hypertension, or both; probucol mainly for type IIA and clofibrate for type IIB and IV hyperlipidaemias) used if target levels were not reached by advice alone. Control group was usual treatment not untreated control. |
| Syst China 1993 | Allocation to treatment and control groups not randomized (alternate allocation employed). |
| Syst‐Eur 1991 | Randomized, double‐blind, placebo‐ controlled trial conducted in Europe. Participa nts aged > 60 years. |
| TEST 1995 | Randomiz ed, double‐blind, placebo‐ controlled trial conducted in Sweden. 720 Swedish participa nts aged > 40 years, within 3 weeks of a stroke or TIA with SBP > 140 mmHg. Participa nts randomized to atenolol or placebo. Data in 40‐ to 59‐ year‐ old pa rti cipants not reported separately. |
| TOMHS 1995 | 4‐ year, multicentre, randomized, double ‐blind study of 902 men and women aged 45 ‐69 years. All participants took part in a lifestyle intervention programme to reduce weight, decrease sodium and alcohol intake, and increase leisure physical activity. Participa nts randomized to placebo or 1 of 5 antihypertensive medications. However, data in 45‐ to 59 ‐year‐ old participa nts not reported separately. |
| UKPDS 1998 | Randomiz ed, controlled, open ‐label trial conducted in the UK. Newly diagnosed participa nts aged 25 ‐65 years with type 2 diabetes mellitus and hypertension (SBP ≥ 160 or DBP ≥ 90 mmHg (or both) in participa nts not on antihypertensive therapy and SBP ≥ 150 or ≥ 85 mmHg in participa nts on antihypertensive therapy). Tight BP group received captopril 25‐50 mg twice daily or atenolol 50‐100 mg once daily . Supplemental drugs added were frusemide 20‐40 mg twice daily, slow‐ release nifedipine 10‐40 mg twice daily, methyldopa 250‐500 mg twice daily, prazosin 1‐5 mg 3 times daily given sequentially to achieve target BP. Control group were given treatment if SBP ≥ 200 or DBP ≥ 105 mmHg (or both) (e.g. frusemide, long‐ acting nifedipine, methyldopa, prazosin given sequentially to control BP). If possible ACE inhibitor s and beta‐blockers avoided. Data in 25‐ to 59‐ year‐ old participa nts not reported separately. |
| VA Coop 1960 | 425 participa nts (255 participa nts aged < 60 years) with mild, moderately severe or severe hypertension randomized to treatment with placebo or reserpine both alone and in combination with hydralazine in mild cases; in severe cases to 3 blocking agents, pentolinium tartrate, mecamylamine hydrochloride and chlorisondamine chloride each in combination with reserpine. In moderately severe group, both series of therapeutic regimens were utilized to compare ganglionic blocking agents with the less drastic forms of antihypertensive drug therapy. Data in 18‐ to 59‐ year‐ old participa nts not reported separately. |
| VA‐I 1962 | Randomiz ed, double‐blind, placebo‐ controlled trial conducted in USA. Participa nts were 30‐ to 70‐ year ‐old men. Participa nts were randomized to treatment: step 1 : hydrochlorothiazide 100 mg + reserpine 0.2 mg + hydralazine 75 mg; step 2: hydralazine 150 mg. Data in 18‐ to 59‐ year‐ old participa nts not reported separately. |
| VACS 1982 | Prospective, multicentre, randomiz ed, double‐blind trial. 394 m en aged 21‐65 years with hypertension (DBP 95‐114 mmHg). Propranolol 80‐640 mg daily vs hydrochlorothiazide 50‐200 mg daily for 12 months. No placebo control group. |
| VHAS 1997 | Prospective, multicentre, randomiz ed, parallel‐group trial. 1414 participa nts in Italy aged 40‐65 years with hypertension (mean seated SBP ≥ 160 mmHg and mean seated DBP ≥ 95 mmHg). Drug‐drug comparison of chlorthalidone 25 mg vs verapamil 240 mg with no placebo or untreated control group. |
| White 1995 | Not a randomiz ed controlled study. No placebo group and participa nts not randomly allocated to moexipril or moexipril + hydrochlorothiazide. |
| Wolff 1966 | Double ‐blind randomized study of 87 outpatients aged 21‐70 years . Randomized to antihypertensive drug therapy (reserpine 0.25 mg 3 times daily, chlorothiazide 0.5 g twice daily or hydrochlorothiazide 25 mg 4 times daily, and guanethidine by titration given, as needed, on successive visits until standing DBP fell < 90 mmHg or placebo. Data in 21‐ to 59‐ year‐ old participa nts not reported separately. |
ACE: angiotensin‐converting enzyme ; BP: blood pressure ; DBP: diastolic blood pressure ; SBP: systolic blood pressure ; T IA : transient isch a emic attack .
Differences between protocol and review
We changed the title of the review from "Pharmacotherapy for hypertension in non‐elderly adults" to define the age group of eligible participants (Musini 2010).
Although the protocol stated in the objectives "To quantify antihypertensive drug effect on all‐cause mortality in people 18 to 59 years with mild to moderate systolic or diastolic hypertension" we have now further clarified that it included only participants with primary hypertension similar to the systematic review "First line drugs for hypertension" in which participants with secondary hypertension were excluded.
The protocol was revised to include an additional outcome measure Decrease in systolic and diastolic blood pressure. Since there was significant heterogeneity (I2 greater than 90%) in the mean difference in both systolic and diastolic blood pressure we used a random‐effects model to present the overall effect size instead of the fixed‐effect model.
Total cardiovascular mortality plus morbidity was identified as a secondary outcome under objectives in the protocol. Since total cardiovascular mortality plus morbidity includes cerebrovascular mortality plus morbidity and coronary heart disease mortality plus morbidity, we decided to show data for these two outcomes separately in addition to total cardiovascular mortality plus morbidity outcome.
Following the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), we presented a 'Summary of findings' table.
Contributions of authors
VM and JMW were responsible for the design of the protocol.
VM, LP, and DS screened titles and abstracts, and assessed all retrieved trials for inclusion or exclusion.
VM, JMW and FG extracted data independently and verified data entry.
VM and JMW assessed risk of bias, performed data analysis and graded evidence overall.
All authors contributed to the final draft of the review.
Sources of support
Internal sources
-
Department of Anesthesiology, Pharmacology and Therapeutics, Faculty of Medicine, University of British Columbia, Canada.
Office space.
External sources
-
CIHR grant to the Hypertension Review group, Vancouver, Canada.
Infrastructure.
Declarations of interest
VM: none.
FG: none.
LP: none.
DS: none.
JMW: none.
New
References
References to studies included in this review
Carter 1970 {published data only}
- Carter AB. Hypotensive therapy in stroke survivors. Lancet 1970;1:485‐89. [DOI] [PubMed] [Google Scholar]
HSCSG 1974 {published data only}
- Hypertension‐Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence. JAMA 1974;229:409‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MRC‐TMH 1985 {published data only}
- Anonymous. Adverse reactions to bendrofluazide and propranolol for the treatment of mild hypertension. Report of Medical Research Council Working Party on Mild to Moderate Hypertension. Lancet 1981;2(8246):539‐43. [MEDLINE: ] [PubMed] [Google Scholar]
- Anonymous. Comparison of the antihypertensive efficacy and adverse reactions of two doses of bendrofluazide and hydrochlorothiazide and the effect of potassium supplementation on the hypotensive action of bendrofluazide: sub‐studies of the Medical Research Council's trials of treatment of mild hypertension: Medical Research Council Working Party. Journal of Clinical Pharmacology 1987;27(4):271‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Anonymous. Comparison of the antihypertensive efficacy and adverse reactions to two doses of bendrofluazide and hydrochlorothiazide and the effect of potassium supplementation on the hypotensive action of bendrofluazide: substudies of the Medical Research Council's trials of treatment of hypertension: Medical Research Council Working Party. Journal of Clinical Pharmacology 1987;27(4):271‐7. [MEDLINE: ; CRSREF: 3042232] [DOI] [PubMed] [Google Scholar]
- Anonymous. Coronary heart disease in Medical Research Council trial of mild hypertension. Medical Research Council Working Party on Mild Hypertension. British Heart Journal 1988;59(3):364‐78. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Randomised controlled trial of treatment of mild hypertension: design and pilot trial. Report of the Medical Research Council Working party on Mild to Moderate Hypertension. BMJ 1977;1(6074):1437‐40. [MEDLINE: ; CRSREF: 3042228] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Stroke and coronary heart disease in mild hypertension: risk factors and the value of treatment. Medical Research Council Working Party on Mild Hypertension. BMJ 1988;296(6636):1565‐70. [MEDLINE: ; CRSREF: 3042229] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. BMJ 1985;291(6488):97‐104. [MEDLINE: ; CRSREF: 3042230] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall WE, Brennan PJ, Mann AH. Medical Research Council's Treatment Trial for Mild Hypertension: an interim report. Clinical Science and Molecular Medicine 1976;51:563s‐5s. [MEDLINE: ; 3042231] [DOI] [PubMed] [Google Scholar]
- Peart S. Results of the MRC (UK) trial of drug therapy for mild hypertension. Clinical and Investigative Medicine. Medecine Clinique et Experimentale 1987;10(6):616‐20. [MEDLINE: ; CRSREF: 3042233] [PubMed] [Google Scholar]
Oslo 1986 {published data only}
- Helgeland A. Treatment of mild hypertension: a five year controlled drug trial. The OSLO study. American Journal of Medicine 1980;69:725‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Helgeland A, Baksaas IA, Leren P. Mild hypertension ‐ early drug treatment or follow‐up only? the Oslo study. Acta Medica Scandinavica 1979;626:34‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Helgeland A, Hjermann I, Holme I, Liren P. Serum triglycerides and serum uric acid in untreated and thiazide‐treated patients with mild hypertension. American Journal of Medicine 1978;64:34‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Helgeland A, Leren P. Oslo study: treatment of mild hypertension. Nephron 1987;47(Suppl 1):108‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Helgeland A, Leren P, Foss OP, Hjermann I, Holme I, Lund‐Larsen PG. Serum glucose levels during long‐term observation of treated and untreated men with mild hypertension. American Journal of Medicine 1984;76:802‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holme I. Coronary risk factors and their possible causal role in the development of coronary heart disease. Journal Oslo City Hospital 1982;32:79‐105. [MEDLINE: ] [PubMed] [Google Scholar]
- Holme I, Helgeland A, Hjermann I, Leren P, Mogensen SB. Correlates of blood pressure change in middle‐aged male mild hypertensives: results from the untreated control group in the Oslo hypertension trial. American Journal of Epidemiology 1988;127(4):742‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leren P, Eide I, Foss OP, Helgeland A, Hjermann I, Holme I, et al. Blood lipids and antihypertensive drugs. Journal of Pharmacology (Paris) 1983;14(Suppl II):217‐20. [MEDLINE: ] [PubMed] [Google Scholar]
- Leren P, Helgeland A. Coronary heart disease and treatment of hypertension. Some Oslo data. American Journal of Medicine 1986;80(Suppl 2A):306. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leren P, Helgeland A. Oslo hypertension study. Drugs 1986;31(Suppl 1):41‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leren P, Helgeland A, Hjermann I, Holme I. The Oslo study: CHD risk factors, socioeconomic influences, and interventions. American Heart Journal 1983;106:1200‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
USPHSHCSG 1977 {published data only}
- Anonymous. Morbidity and mortality in mild essential hypertension. Circulation Research 1972;30/31(Suppl II):110‐20. [PubMed] [Google Scholar]
- Smith WM, Johnson WP, Bromer L. Intervention trial in mild hypertension, U.S. Public Health Service Hospitals Cooperative Study Group. Miami (FL): Symposia Specialists: In Epidemiology and Control of Hypertension, 1975. [Google Scholar]
- US Public Health Service Hospital Cooperative Study Group (USPHSHCSG). Treatment of mild hypertension. Results of a ten‐year intervention trial. Circulation Research 1977;40(Suppl 1):98‐105. [MEDLINE: ] [PubMed] [Google Scholar]
VA‐II 1970 {published data only}
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA 1970;213:1143‐52. [MEDLINE: ] [PubMed] [Google Scholar]
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension: influence of age, diastolic pressure, and prior cardiovascular disease: further analysis of side effects. Circulation 1972;45:991‐1004. [DOI] [PubMed] [Google Scholar]
VA‐NHLBI 1977 {published data only}
- Anonymous. Evaluation of drug treatment in mild hypertension: VA‐NHLBI feasibility trial. Annals of the New York Academy of Sciences 1978;304:267‐88. [MEDLINE: ; CRSREF: 3042309] [DOI] [PubMed] [Google Scholar]
- Perry HM Jr. Treatment of mild hypertension. Preliminary results of a two year feasibility trial. Circulation Research 1977;40 Suppl:1180‐7. [MEDLINE: ; CRSREF: 3042310] [PubMed] [Google Scholar]
References to studies excluded from this review
ADVANCE 2007 {published data only}
- The ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370:829‐40. [DOI: 10.1016/S0140-6736(07)61303-8] [DOI] [PubMed] [Google Scholar]
ALLHAT 1996 {published data only}
- ALLHAT Group. Diuretic versus alpha‐blocker as first‐step antihypertensive therapy: final results from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2003;42:239‐46. [DOI] [PubMed] [Google Scholar]
- Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT Jr, Cushman WC, et al. for the ALLHAT Research Group. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). American Journal of Hypertension 1996;9:342‐60. [DOI] [PubMed] [Google Scholar]
Anon 1973 {published data only}
- Anonymous. Control of moderately raised blood pressure: report of a co‐operative randomized controlled trial. British Medical Journal 1973;III:434‐6. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
ATTMH 1984 {published data only}
- Abernethy JD. The Australian Therapeutic Trial in Mild Hypertension. Hypertension 1984;6(5):774‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Australian National Blood Pressure Management Committee. The Australian Therapeutic Trial in Mild Hypertension. Lancet 1980;1:1261‐7. [PubMed] [Google Scholar]
- The Management Committee. Treatment of mild hypertension in the elderly. A study initiated and administered by the National Heart Foundation of Australia. Medical Journal of Australia 1981;2:398‐402. [PubMed] [Google Scholar]
Berglund 1981 {published data only}
- Berglund G, Andersson O. Beta‐blockers or diuretics in hypertension? A six year follow up of blood pressure and metabolic side effects. Lancet 1981;I:744‐7. [DOI] [PubMed] [Google Scholar]
CASTEL 1994 {published data only}
- Casiglia E, Spolaore P, Mazza A, Ginocchio G, Colangeli G, Onesto C, et al. Effect of two different therapeutic approaches on total and cardiovascular mortality in a Cardiovascular Study in the Elderly (CASTEL). Japanese Heart Journal 1994;35(5):589‐600. [DOI] [PubMed] [Google Scholar]
- Casiglia E, Spolaore P, Mormino P, Maschio O, Colangeli G, Celegon L. The CASTEL project (CArdiovascular STudy in the ELderly): protocol, study design, and preliminary results of the initial survey. Cardiologia 1991;36:569‐76. [PubMed] [Google Scholar]
Coope 1986 {published data only}
- Coope J. Randomised trial of treatment of hypertension in elderly patients in primary care. BMJ 1986;293:1145‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dutch TIA 1993 {published data only}
- Dutch TIA Trial Study Group. Trial of secondary prevention with atenolol after transient ischemic attack or non‐disabling ischemic stroke. Stroke 1993;24:543‐8. [DOI] [PubMed] [Google Scholar]
EWPHBPE 1988 {published data only}
- European Working Party on High Blood Pressure in the Elderly (EWPHE). An international trial of antihypertensive therapy in elderly patients. Objectives, protocol and organization. Archives Internationales de Pharmacodynamie et de Therapie 1985;275:300‐34. [PubMed] [Google Scholar]
- O'Malley KO, McCormack P, O'Brien ET. Isolated systolic hypertension: data from the European Working Party on High Blood Pressure in the Elderly. Journal of Hypertension 1988;6(Suppl 1):S105‐8. [PubMed] [Google Scholar]
Fuchs 2011 {published data only}
- Fuchs FD, Fuchs SC, Moreira LB, Gus M, Nobrega AC, Poli‐de‐Figueiredo CE, et al. Prevention of hypertension in patients with pre‐hypertension: protocol for the PREVER‐prevention trial. Trials 2011;12(65):1‐7. [DOI: 10.1186/1745-6215-12-65] [DOI] [PMC free article] [PubMed] [Google Scholar]
GENERIC 2010 {published data only}
- Punzi HA, Lewin AJ, Lukic T, Goodin T, Chen W. Efficacy and safety of nebivolol monotherapy in Hispanics with stage I‐II hypertension (Abstract). Journal of Clinical Hypertension 2010;12:536. [DOI] [PubMed] [Google Scholar]
GLANT 1995 {published data only}
- Study Group on Long‐term Antihypertensive Therapy. A 12‐month comparison of ACE inhibitor and CA antagonist therapy in mild to moderate essential hypertension ‐ the GLANT Study. Hypertension Research 1995;18:235‐44. [PubMed] [Google Scholar]
HANE 1997 {published data only}
- Phillip T, Anlauf M, Distler A, Holzgreve H, Michaelis J, Wellek S. Randomised, double blind, multicenter comparison of hydrochlorothiazide, atenolol, nitrendipine, and enalapril in antihypertensive treatment: results of the HANE study. BMJ 1997;315:154‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
HAPPHY 1987 {published data only}
- Wilhelmsen L, Berglund G, Elmfeldt D, Fitzsimons T, Holzgreve H, Hosie J, et al. Beta‐blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. Journal of Hypertension 1987;5(5):561‐72. [DOI] [PubMed] [Google Scholar]
HDFP 1982 {published data only}
- Curb JD, Borhani NO, Schnaper H, Kass E, Entwisle G, Williams W, et al. Detection and treatment of hypertension in older individuals. American Journal of Epidemiology 1985;121(3):371‐6. [DOI] [PubMed] [Google Scholar]
- Hypertension Detection and Follow‐up Program Cooperative Group. Five‐year findings of the Hypertension Detection and Follow‐up Program: reduction in mortality in persons with high blood pressure, including mild hypertension. JAMA 1979;242:2562‐71. [PubMed] [Google Scholar]
HOPE HYP 2000 {published data only}
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Outcomes Study Investigators. New England Journal of Medicine 2000;342(3):145‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HOT 1995 {published data only}
- Hansson L, Zanchetti A. The Hypertension Optimal Treatment (HOT) Study: 12‐month data on blood pressure and tolerability. With special reference to age and gender. Blood Pressure 1995;4:313‐9. [DOI] [PubMed] [Google Scholar]
HYVET 2003 {published data only}
- Bulpitt CJ, Beckett NS, Cooke J, Dumiktrascu DL, Gil‐Extremera B, Nachev C, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. Journal of Hypertension 2003;21:2409‐17. [DOI] [PubMed] [Google Scholar]
HYVET 2008 {published data only}
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. New England Journal of Medicine 2008;358(18):1887‐98. [DOI] [PubMed] [Google Scholar]
- Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET‐COG): a double‐blind, placebo controlled trial. Lancet Neurology 2008;7(8):683‐9. [DOI] [PubMed] [Google Scholar]
IDM 2001 {published data only}
- Parving HH, Lehnert H, Brochner‐Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. New England Journal of Medicine 2001;345:870‐8. [DOI] [PubMed] [Google Scholar]
INSIGHT 1996 {published data only}
- Brown MJ, Castaigne A, Ruilope LM, Mancia G, Rosenthal T, Leeuw PW, et al. INSIGHT: International Nifedipine GITS study: intervention as a goal in hypertension treatment. Journal of Human Hypertension 1996;10(Suppl 13):S157‐60. [MEDLINE: ] [PubMed] [Google Scholar]
- Brown MJ, Castaigne A, Leeuw PW, Mancia G, Rosenthal T, Ruilope LM. Study population and treatment titration in the International Nifedepine GITS study: intervention as a goal in hypertension treatment (INSIGHT). Journal of Hypertension 1998;16:2113‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Brown MJ, Palmer CR, Castaigne A, Leeuw PW, Mancia G, Rosenthal T, et al. Morbidity and mortality in patients randomised to double‐blind treatment with long‐acting calcium‐channel blocker or diuretic in the International Nifedipine GITS study. Lancet 2000;356:366‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IPPPSH 1985 {published data only}
- IPPPSH Collaborative Group. Cardiovascular risk and risk factors in a randomized trial of treatment based on the beta‐blocker oxprenolol: the International Prospective Primary Prevention Study in Hypertension (IPPPSH). Journal of Hypertension 1985;3(4):379‐92. [DOI] [PubMed] [Google Scholar]
Jikei 2007 {published data only}
- Mochizuki S, Dahlof B, Shimizu M, Ikewaki K, Yoshikawa M, Taniguchi I, et al. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open‐label, blinded end point morbidity‐mortality study. Lancet 2007;369:1431‐9. [DOI] [PubMed] [Google Scholar]
Kuramoto 1981 {published data only}
- Kuramoto K, Matsushita S, Kuwajima T, Murakami M. Prospective study on the treatment of mild hypertension in the aged. Japanese Heart Journal 1981;22(1):75‐85. [DOI] [PubMed] [Google Scholar]
Kuramoto 1994 {published data only}
- Kuramoto K. Treatment of the elderly hypertensives in Japan: National Intervention Cooperative Study in Elderly Hypertensives. The National Intervention Cooperative Study Group. Journal of Hypertension 1994;12(6):S35‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Lewis 2001 {published data only}
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. New England Journal of Medicine 2001;345:851‐60. [DOI] [PubMed] [Google Scholar]
MAPHY 1988 {published data only}
- Wikstrand J, Warnold I, Olsson G, Tuomilehto J, Elmfeldt D, Berglund G. Primary prevention with metoprolol in patients with hypertension. Mortality results from the MAPHY study. JAMA 1988;259(13):1976‐82. [PubMed] [Google Scholar]
Materson 1993 {published data only}
- Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single‐drug therapy for hypertension in men: a comparison of six antihypertensive agents with placebo. New England Journal of Medicine 1993;328(13):914‐21. [DOI] [PubMed] [Google Scholar]
- Materson BJ. Reda DJ, Cushman WC. Department of Veterans Affairs single‐drug therapy of hypertension study. Revised figures and new data. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. American Journal of Hypertension 1995;8(2):189‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MIDAS 1996 {published data only}
- Borhani NO, Mercuri M, Borhani PA, Buckalew VM, Canossa‐Terris M, Carr AA, et al. Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS). JAMA 1996;276(10):785‐91. [PubMed] [Google Scholar]
Morgan 1980 {published data only}
- Morgan TO, Adams WR, Hodgson M, Gibberd RW. Failure of therapy to improve prognosis in elderly males with hypertension. Medical Journal of Australia 1980;2(1):27‐31. [PubMed] [Google Scholar]
MRCO 1992 {published data only}
- MRC Working Party. Medical Research Council Trial of Treatment of Hypertension in Older Adults: principal results. BMJ 1992;304(6824):405‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
NORDIL 2000 {published data only}
- Hansson L, Hedner T, Lund‐Johansen P, Kjeldsen SE, Lindholm LH, Syvertsen JO, et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta‐blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000;356(9227):359‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PATS 1995 {published data only}
- PATS Collaborating Group. Post‐stroke antihypertensive treatment study. A preliminary result. Chinese Medical Journal 1995;108(9):710‐7. [PubMed] [Google Scholar]
PROGRESS 2001 {published data only}
- PROGRESS Collaborative Group. Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6105 individuals with previous stroke or transient ischemic attack. Lancet 2001;358:1033‐41. [DOI] [PubMed] [Google Scholar]
QUIET 2001 {published data only}
- Pitt B, O'Neill B, Feldman R, Ferrari R, Schwartz L, Judra H, et al. The QUinapril Ischemic Event Trial (QUIET): evaluation of chronic ACE inhibitor therapy in patients with ischemic heart disease and preserved left ventricular function. American Journal of Cardiology 2001;87:1058‐63. [DOI] [PubMed] [Google Scholar]
SCAT 2000 {published data only}
- Teo KK, Burton JR, Buller CE, Plante S, Catellier D, Tymchak W, et al. Long‐term effects of cholesterol lowering and angiotensin converting enzyme inhibition on coronary atherosclerosis. Circulation 2000;102:1748‐54. [DOI] [PubMed] [Google Scholar]
Schmieder 2009 {published data only}
- Schmieder RE, Philipp T, Guerediaga J, Gorostidi M, Smith B, Weissbach N, et al. Long‐term antihypertensive efficacy and safety of the oral direct renin Inhibitor aliskiren. A 12‐month randomized, double‐blind comparator trial with hydrochlorothiazide. Circulation 2009;119(3):417‐25. [DOI: 10.1161/CIRCULATIONAHA.107.750745] [DOI] [PubMed] [Google Scholar]
SCOPE 2003 {published data only}
- Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double‐blind intervention trial. Journal of Hypertension 2003;21:875‐86. [DOI: 10.1097/01.hjh.0000059028.82022.89] [DOI] [PubMed] [Google Scholar]
SHELL 1994 {published data only}
- Malacco E, Gnemmi AE, Romagnoli A, Coppini A. Systolic hypertension in the elderly: long‐term lacidipine treatment. Objective, protocol, and organization. SHELL Study Group. Journal of Cardiovascular Pharmacology 1994;23(5):S6206. [PubMed] [Google Scholar]
SHEP 1991 {published data only}
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265(24):3255‐64. [PubMed] [Google Scholar]
- SHEP Cooperative Research Group. Rationale and design of a randomized clinical trial on prevention of stroke in isolated systolic hypertension. Journal of Clinical Epidemiology 1988;41(12):1197‐208. [DOI] [PubMed] [Google Scholar]
SHEP ‐ Pilot 1985 {published data only}
- Perry HM, Smith WM, McDonald RH, Black D, Cutler JA, Furberg CD, et al. Morbidity and mortality in the Systolic Hypertension in the Elderly Program (SHEP) Pilot Study. Stroke 1989;20(1):4‐13. [DOI] [PubMed] [Google Scholar]
Sprackling 1981 {published data only}
- Sprackling ME, Mitchell JRA, Short AH, Watt G. Blood pressure reduction in the elderly: a randomised controlled trial of methyldopa. BMJ 1981;283:1151‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
STONE 1996 {published data only}
- Gong L, Zwang W, Zhu Y, Hamet P. Shanghai Trial of Nifedipine in the Elderly (STONE) study. Journal of Hypertension 1996;14:1237‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
STOP 1991 {published data only}
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOPHypertension). Lancet 1991;338:1281‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
STOP‐2 1993 {published data only}
- Dahlöf B, Hansson L, Lindholm LH, Scherstén B, Wester PO, Ekbom T. STOP‐Hypertension‐2: a prospective intervention trial of "newer" versus "older" treatment alternatives in old patients with hypertension. Blood Pressure 1993;2:136‐41. [DOI] [PubMed] [Google Scholar]
Strandberg 1991 {published data only}
- Strandberg TE, Salomaa VV, Naukkarinen VA, Vanhanen HT, Sarna SJ, Miettinen TA. Long‐term mortality after 5‐year multifactorial primary prevention of cardiovascular diseases in middle‐aged men. JAMA 1991;266(9):1255‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Syst China 1993 {published data only}
- Anonymous. Systolic hypertension in the elderly: Chinese trial (Syst‐China). Interim report. Zhonghua Xin Xue Guan Bing za Zhi 1992;20(5):270‐5, 323. [PubMed] [Google Scholar]
Syst‐Eur 1991 {published data only}
- Amery A, Birkenhager W, Bulpitt CJ, Clément D, Leeuw P, Dollery CT, et al. Syst‐Eur: a multicenter trial on the treatment of isolated systolic hypertension in the elderly; objectives, protocol and organisation. Aging 1991;3:287‐302. [DOI] [PubMed] [Google Scholar]
TEST 1995 {published data only}
- Eriksson S, Olofsson BO, Wesley PO, for the TEST study group. Atenolol for secondary prevention after stroke. Cerebrovascular Diseases (Basel, Switzerland) 1995;5:21‐5. [Google Scholar]
TOMHS 1995 {published data only}
- Elmer PJ, Grimm R, Laing B, Grandits G, Svedsen K, Heel N, et al. Lifestyle intervention: results of the Treatment of Mild Hypertension Study (TOMHS). Preventive Medicine 1995;24:378‐88. [DOI] [PubMed] [Google Scholar]
UKPDS 1998 {published data only}
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998;317:713‐20. [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703‐13. [PMC free article] [PubMed] [Google Scholar]
VA Coop 1960 {published data only}
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. A double‐blind control study of antihypertensive agents. I. Comparative effect of reserpine, reserpine and hydralazine, and three ganglionic blocking agents, clorisondamine, mecamylamine, and pentolinium tartrate. Archives of Internal Medicine 1960;106:133‐48. [PubMed] [Google Scholar]
VACS 1982 {published data only}
- Veterans Administrative Cooperative Study Group on Antihypertensive Agents. Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. II Results of long term therapy. JAMA 1982;248:2004‐11. [MEDLINE: ] [PubMed] [Google Scholar]
VA‐I 1962 {published data only}
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. A double‐blind control study of antihypertensive agents. III. Chlorothiazide alone and in combination with other agents: preliminary results. Archives of Internal Medicine 1962;110:230‐5. [Google Scholar]
VHAS 1997 {published data only}
- Agabiti RE, Dal Palu D, Leonetti G, Magnani G, Pessina A, Zanchetti A. Clinical results of the verapamil in hypertension and atherosclerosis study. Journal of Hypertension 1997;15:1337‐44. [DOI] [PubMed] [Google Scholar]
White 1995 {published data only}
- White WB, Stimpel M. Long‐term safety and efficacy of moexipril alone and in combination with hydrochlorothiazide in elderly patients with hypertension. Journal of Human Hypertension 1995;9(11):879‐84. [PubMed] [Google Scholar]
Wolff 1966 {published data only}
- Wolff FW, Lindeman RD. Effects of treatment in hypertension. Results of a controlled study. Journal of Chronic Diseases 1966;19:227‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
BBLTTC 2005
- Turnbull F, Neal B, Albert C, Chalmers J, Chapman N, Cutler J, et al. Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood pressure‐lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Archives of Internal Medicine 2005;165(12):1410‐9. [DOI] [PubMed] [Google Scholar]
Boissel 2005
- Boissel JP, Gueyffier F, Boutitie F, Pocock S, Fagard R. Apparent effect on blood pressure is only partly responsible for the risk reduction due to antihypertensive treatments. Fundamental & Clinical Pharmacology 2005;19:579‐84. [DOI] [PubMed] [Google Scholar]
BPLTTC 2008
- Blood Pressure Lowering Treatment Trialists' Collaboration, Turnbull F, Neal B, Ninomiya T, Algert C, Arima H, Barzi F, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta‐analysis of randomised trials. BMJ 2008;336(7653):1121‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1990
- Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease ‐ Part 2, short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827‐38. [DOI] [PubMed] [Google Scholar]
Duarte 2010
- Duarte JD, Cooper‐DeHoff RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide‐like diuretics. Expert Review of Cardiovascular Therapy 2010;8(6):793‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frishman 2005
- Frishman WH, Cheng‐Lai A, Nawarskas J. Current Cardiovascular Drugs. 4th Edition. Philadelphia, PA 19106: Springer Science & Business Media, 2005. [Google Scholar]
GRADEpro
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook, Updated October 2013. [Google Scholar]
Gueyffier 1995
- Gueyffier F, Boutitie F, Boissel JP, Coope J, Cutler J, Ekbom T, et al. INDANA: a meta‐analysis on individual patient data in hypertension. Protocol and preliminary results. Therapie 1995;50:353‐62. [PubMed] [Google Scholar]
Gueyffier 1996
- Gueyffier F, Froment A, Gouton M. New meta‐analysis of treatment trials of hypertension: improving the estimate of therapeutic benefit. Journal of Human Hypertension 1996;10:1‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Gueyffier 1999
- Gueyffier F, Bulpitt C, Boissel JP, Schron E, Ekbom T, Fagard R, et al. for the INDANA Group. Antihypertensive drugs in very old people: a subgroup meta‐analysis of randomised controlled trials. Lancet 1999;353:793‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hughes 2004
- Hughes AD. How do thiazide and thiazide‐like diuretics lower blood pressure?". Journal of the Renin‐Angiotensin‐Aldosterone System 2004;5(4):155‐60. [DOI] [PubMed] [Google Scholar]
Insua 1994
- Insua JT, Sacks HS, Lau TS, Lau J, Reitman D, Pagano D, et al. Drug treatment of hypertension in the elderly: a meta‐analysis. Annals of Internal Medicine 1994;121:355‐62. [DOI] [PubMed] [Google Scholar]
James 2014
- James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, et al. Evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311(5):507‐20. [DOI] [PubMed] [Google Scholar]
Kang 2004
- Kang S, Wu FY, An N, Ren M. A systematic review and meta‐analysis of the efficacy and safety of a fixed low‐dose perindopril‐indapamide combination as first‐line treatment of hypertension. Clinical Therapeutics 2004;26(2):257‐70. [DOI] [PubMed] [Google Scholar]
Kearney 2005
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217‐23. [DOI] [PubMed] [Google Scholar]
Kızılırmak 2017
- Kızılırmak P, Uresin Y, Ozdemir O, Kilickiran B, Tokgözoğlu L, Öngen Z. Renin‐angiotensin‐aldosterone system blockers and cardiovascular outcomes: a meta‐analysis of randomized clinical trials [Renin‐anjiyotensin‐aldosteron sistemi blokerleri ve kardiyovasküler sonuçları: Randomize klinik çalışmaların meta‐analizi]. Turk Kardiyoloji Dernegi Arsivi 2017;45(1):49‐66. [DOI] [PubMed] [Google Scholar]
Law 2009
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Longo 2012
- Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson J, Loscalzo J. Harrison's Principals of Internal Medicine. Vol. 2, New York (NY): McGraw‐Hill, 2012. [ISBN 978‐0‐07‐174887‐2] [Google Scholar]
MacMahon 1993
- MacMahon S, Rogers A. The effects of blood pressure reduction in older patients: an overview of five randomized controlled trials in elderly hypertensives. Clinical and Experimental Hypertension 1993;15(6):967‐78. [DOI] [PubMed] [Google Scholar]
Mancia 2013
- Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. Task Force Members, 2013. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European Heart Journal 2013;34:2159‐219. [DOI] [PubMed] [Google Scholar]
Mulrow 1994
- Mulrow CD, Cornell JA, Herrera CR, Kadri A, Farnett L, Aguilar C. Hypertension in the elderly: Implications and generalizability of randomized trials. JAMA 1994;272:1932‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Mulrow 1998
- Mulrow C, Lau J, Cornell J, Brand M. Pharmacotherapy for hypertension in the elderly. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000028] [DOI] [PubMed] [Google Scholar]
Musini 2009
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD000028.pub2] [DOI] [PubMed] [Google Scholar]
NICE 2016
- National Institute for Health and Care Excellence. Treating high blood pressure with lifestyle changes, August 2011, updated November 2016. www.nice.org.uk/guidance/cg127/ifp/chapter/treating‐high‐blood‐pressure‐with‐lifestyle‐changes (accessed prior to 28 July 2017).
Nikolaus 2000
- Nikolaus T, Sommer N, Becker C. Treatment of arterial hypertension with diuretics, beta‐ and calcium channel blockers in old patients. Zeitschrift fur Gerontologie und Geriatrie 2000;33:427‐32. [DOI] [PubMed] [Google Scholar]
Parsons 2016
- Parsons C, Murad MH, Andersen S, Mookadam F, Labonte H. The effect of antihypertensive treatment on the incidence of stroke and cognitive decline in the elderly: a meta‐analysis. Future Cardiology 2016;12(2):237‐48. [DOI] [PubMed] [Google Scholar]
Pearce 1995
- Pearce KA, Furberg CD, Rushing J. Does antihypertensive treatment of the elderly prevent cardiovascular events or prolong life? A meta‐analysis of hypertension treatment trials. Archives of Family Medicine 1995;4:943‐50. [DOI] [PubMed] [Google Scholar]
Prospective Studies Collaboration 2002
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360(9349):1903‐13. [DOI] [PubMed] [Google Scholar]
Psaty 1997
- Psaty BM, Smith NL, Siscovick DS, Koepsell TD, Weiss NS, Heckbert SR, et al. Health outcomes associated with antihypertensive therapies used as first‐line agents. A systematic review and meta‐analysis. JAMA 1997;277(9):739‐45. [PubMed] [Google Scholar]
Psaty 2003
- Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, et al. Health outcomes associated with various antihypertensive therapies used as first‐line agents: a network meta‐analysis. JAMA 2003;289:2534‐44. [DOI] [PubMed] [Google Scholar]
Quan 1999
- Quan A, Kerlikowske K, Gueyffier F, Boissel JP, INDANA investigators. Efficacy of treating hypertension in women. Journal of General Internal Medicine 1999;14:718‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sundström 2015
- Sundström J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, et al. Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of blood pressure reduction in mild hypertension. A systematic review and meta‐analysis. Annals of Internal Medicine 2015;162:184‐91. [DOI] [PubMed] [Google Scholar]
Tan 2016
- Tan XY, Hu JB. ACEIs/ARBs for the prevention of type 2 diabetes in patients with cardiovascular diseases: a systematic review and meta‐analysis. International Journal of Clinical and Experimental Medicine 2016;9(5):7624‐37. [Google Scholar]
Thijs 1992
- Thijs L, Fagard R, Lijnen P, Staessen J, Hoof R, Amery A. A meta‐analysis of outcome trials in elderly hypertensives. Journal of Hypertension 1992;10:1103‐9. [DOI] [PubMed] [Google Scholar]
Thomopoulos 2014
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta‐analyses, and meta‐regression analyses of randomized trials. Journal of Hypertension 2014;32(12):2285‐95. [DOI] [PubMed] [Google Scholar]
Thomopoulos 2016
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure‐lowering treatment. 6. Prevention of heart failure and new‐onset heart failure ‐ meta‐analyses of randomized trials. Journal of Hypertension 2016;34(3):373‐84. [DOI] [PubMed] [Google Scholar]
Turnbull 2003
- Turnbull F, Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet 2003;362:1527‐35. [DOI] [PubMed] [Google Scholar]
Weber 2014
- Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community. A statement by the American Society of Hypertension and the International Society of Hypertension. Journal of Hypertension 2014;32(1):3‐15. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization/International Society of Hypertension (ISH) statement on management of hypertension. Journal of Hypertension 2003;21:1983‐92. [DOI] [PubMed] [Google Scholar]
Wiysonge 2017
- Wiysonge CS, Bradley H, Mayosi BM, Maroney R, Mbewu A, Opie LH, et al. Beta‐blockers for hypertension. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD002003.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2009
- Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001841.pub2] [DOI] [PubMed] [Google Scholar]
Zanchetti 2015
- Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: a critical reappraisal. Circulation Research 2015;116(6):1058‐73. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Musini 2010
- Musini VM, Tejani AM, Labeit AM, Salzwedel DM, Wright JM. Pharmacotherapy for hypertension in non‐elderly adults. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008276] [DOI] [Google Scholar]


