Abstract
Background
People with stroke conventionally receive a substantial part of their rehabilitation in hospital. Services have now been developed that offer people in hospital an early discharge with rehabilitation at home (early supported discharge: ESD).
Objectives
To establish if, in comparison with conventional care, services that offer people in hospital with stroke a policy of early discharge with rehabilitation provided in the community (ESD) can: 1) accelerate return home, 2) provide equivalent or better patient and carer outcomes, 3) be acceptable satisfactory to patients and carers, and 4) have justifiable resource implications use.
Search methods
We searched the Cochrane Stroke Group Trials Register (January 2017), Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 1) in the Cochrane Library (searched January 2017), MEDLINE in Ovid (searched January 2017), Embase in Ovid (searched January 2017), CINAHL in EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to December 2016), and Web of Science (to January 2017). In an effort to identify further published, unpublished, and ongoing trials we searched six trial registries (March 2017). We also performed citation tracking of included studies, checked reference lists of relevant articles, and contacted trialists.
Selection criteria
Randomised controlled trials (RCTs) recruiting stroke patients in hospital to receive either conventional care or any service intervention that has provided rehabilitation and support in a community setting with an aim of reducing the duration of hospital care.
Data collection and analysis
The primary patient outcome was the composite end‐point of death or long‐term dependency recorded at the end of scheduled follow‐up. Two review authors scrutinised trials, categorised them on their eligibility and extracted data. Where possible we sought standardised data from the primary trialists. We analysed the results for all trials and for subgroups of patients and services, in particular whether the intervention was provided by a co‐ordinated multidisciplinary team (co‐ordinated ESD team) or not. We assessed risk of bias for the included trials and used GRADE to assess the quality of the body of evidence.
Main results
We included 17 trials, recruiting 2422 participants, for which outcome data are currently available. Participants tended to be a selected elderly group of stroke survivors with moderate disability. The ESD group showed reductions in the length of hospital stay equivalent to approximately six days (mean difference (MD) ‐5.5; 95% confidence interval (CI) ‐3 to ‐8 days; P < 0.0001; moderate‐grade evidence). The primary outcome was available for 16 trials (2359 participants). Overall, the odds ratios (OR) for the outcome of death or dependency at the end of scheduled follow‐up (median 6 months; range 3 to 12) was OR 0.80 (95% CI 0.67 to 0.95, P = 0.01, moderate‐grade evidence) which equates to five fewer adverse outcomes per 100 patients receiving ESD. The results for death (16 trials; 2116 participants) and death or requiring institutional care (12 trials; 1664 participants) were OR 1.04 (95% CI 0.77 to 1.40, P = 0.81, moderate‐grade evidence) and OR 0.75 (95% CI 0.59 to 0.96, P = 0.02, moderate‐grade evidence), respectively. Small improvements were also seen in participants' extended activities of daily living scores (standardised mean difference (SMD) 0.14, 95% CI 0.03 to 0.25, P = 0.01, low‐grade evidence) and satisfaction with services (OR 1.60, 95% CI 1.08 to 2.38, P = 0.02, low‐grade evidence). We saw no clear differences in participants' activities of daily living scores, patients subjective health status or mood, or the subjective health status, mood or satisfaction with services of carers. We found low‐quality evidence that the risk of readmission to hospital was similar in the ESD and conventional care group (OR 1.09, 95% CI 0.79 to 1.51, P = 0.59, low‐grade evidence). The evidence for the apparent benefits were weaker at one‐ and five‐year follow‐up. Estimated costs from six individual trials ranged from 23% lower to 15% greater for the ESD group in comparison to usual care.
In a series of pre‐planned analyses, the greatest reductions in death or dependency were seen in the trials evaluating a co‐ordinated ESD team with a suggestion of poorer results in those services without a co‐ordinated team (subgroup interaction at P = 0.06). Stroke patients with mild to moderate disability at baseline showed greater reductions in death or dependency than those with more severe stroke (subgroup interaction at P = 0.04).
Authors' conclusions
Appropriately resourced ESD services with co‐ordinated multidisciplinary team input provided for a selected group of stroke patients can reduce long‐term dependency and admission to institutional care as well as reducing the length of hospital stay. Results are inconclusive for services without co‐ordinated multidisciplinary team input. We observed no adverse impact on the mood or subjective health status of patients or carers, nor on readmission to hospital.
Plain language summary
Services for reducing duration of hospital care in people with acute stroke
Review question
We aimed to establish if Early Supported Discharge (ESD) services can result in a better patient recovery and if they are as acceptable and affordable as usual services.
Background
Services that try to offer stroke patients an earlier discharge from hospital with rehabilitation provided in the community have been termed Early Supported Discharge (ESD) services. ESD services are usually provided by multidisciplinary teams of therapists, nurses, and doctors who work in a co‐ordinated manner through regular meetings. They aim to allow patients to return home from hospital earlier than usual and also to receive more rehabilitation in the familiar environment of their own home.
Study characteristics
We identified 17 clinical trials recruiting 2422 stroke patients (searching completed to January 2017). Patients who were recruited tended to have a moderate degree of disability (able to walk with assistance) and be sufficiently well to consider returning home. We categorised services as those based on a multidisciplinary ESD team (with different levels of co‐ordination and delivery) and those with no multidisciplinary team co‐ordination (no ESD team).
Key results
The length of initial stay in hospital was reduced by approximately five days for the ESD group. At an average of six months after their stroke ESD patients were more likely to be living at home (an extra five patients living at home for every 100 receiving ESD services; moderate‐quality evidence). They were also more likely to be independent in daily activities (an extra six patients independent for every 100 receiving ESD services; moderate‐quality evidence). We identified no apparent hazards in terms of patient mood or quality of life, carer mood or quality of life, or the risk of readmission to hospital. The greatest reductions in disability seemed to be present in trials based around a co‐ordinated ESD team. When compared with usual care, costs of ESD services ranged from a reduction to a modest increase.
Quality of the evidence
The quality of the evidence was downgraded to 'moderate' for the main outcomes of death, discharge home or disability. This was because it was impossible to hide the treating service from participants or healthcare workers. These conclusions were not dependent on trials judged to be lower quality because of poor design or missing data. More information was missing for some of the other outcome measures, which we have downgraded to low‐quality evidence.
Conclusion
Appropriately resourced ESD services with co‐ordinated multidisciplinary team input can reduce disability and the length of time in hospital at least for a selected group of people with stroke. Results are unclear for services that are not based on a co‐ordinated multidisciplinary team input. We did not identify any substantial harmful effects.
Summary of findings
for the main comparison.
| ESD service compared with usual care for stroke | ||||||
|
Patient or population: people with stroke Settings: Hospital Intervention: Early supported discharge (ESD) service ‐ any type Comparison: Usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | ESD service | |||||
|
Death or dependency at end of scheduled follow‐up (median 6 months) |
Medium risk population | OR 0.80 (0.67 to 0.95) | 2359 (16) | ⊕⊕⊕⊝ moderate (a) | Assumed risk from baseline in included trials. Corresponding risk estimated from risk difference (95% CI). | |
| 450 per 1000 | 400 per 1000 (360 to 440) | |||||
|
Death at end of scheduled follow‐up (median 6 months) |
Medium risk population | OR 1.04 (0.77 to 1.40) | 2116 (16) | ⊕⊕⊕⊝ moderate (a) | As above | |
| 90 per 1000 | 90 per 1000 (70 to 120) | |||||
|
Death or institution care at end of scheduled follow‐up (median 6 months) |
Medium risk population |
OR 0.75 (0.59 to 0.96) |
1664 (12) |
⊕⊕⊕⊝ moderate (a) | As above | |
| 270 per 1000 | 220 per 1000 (190 to 260) | |||||
|
Extended activities of daily living (EADL) score at end of scheduled follow‐up (median 6 months) |
The mean EADL score ranged across control groups depending on the measure used (see Analysis 1.5) | The mean EADL score in the intervention groups was on average higher than usual care. | SMD 0.14 (0.03 to 0.25) | 1262 (11) | ⊕⊕⊝⊝ low (b) | Range of scores used to measure EADL (high score means better outcome) therefore comparison is within scores. |
|
Satisfaction with services at end of scheduled follow‐up (median 6 months) |
Medium risk population | OR 1.60 (1.08 to 2.38) | 513 (5) | ⊕⊕⊝⊝ low (b) | Stated satisfaction of patients with service received. | |
| 610 per 1000 | 690 per 1000 (620 to 770) | |||||
| Length of initial hospital stay (days) | The mean length of stay in hospital and/or institution ranged across control groups from 10 to 50 days. | The mean length of stay in the intervention groups was 5.5 (3 to 8) days shorter. |
MD ‐ 5.5 (2.9 to 8.2) days |
2161 (16) | ⊕⊕⊕⊝ moderate (c) | Length of stay in a hospital and/or institution. Most trials reported initial hospital stay. |
|
Readmission to hospital at end of scheduled follow‐up (median 6 months) |
Medium risk population | OR 1.09 (0.79 to 1.51) | 784 (6) | ⊕⊕⊝⊝ low (b) | ||
| 250 per 1000 | 270 per 1000 (230 to 350) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; OR: Odds Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
The trials on average focused on a middle band of stroke patients with moderate levels of disability.
a) Downgraded once for risk of performance bias. Sensitivity analyses indicate little risk from other potential biases.
b) Downgraded twice for risk of performance bias and potential risk of missing data.
c) Downgraded for risk of performance bias. Substantial heterogeneity of results are present but unlikely to alter direction of effect.
Background
Description of the condition
Stroke is a global healthcare problem and in most countries is one of the leading causes of death and acquired adult disability (Warlow 2008). Stroke is also expensive and consumes 5% of all health service resources within the UK National Health Service (Saka 2009). Despite major advances in the medical management of stroke, the majority of people with continue to rely on post‐stroke rehabilitation interventions (Langhorne 2011). Conventionally, rehabilitation after stroke is provided in hospital. Thus, in‐patient care of disabled stroke patients accounts for much of the substantial economic costs (Warlow 2008).
Rehabiliation in hospital can achieve good clinical outcomes. A recent updated systematic review evaluating in‐patient stroke care has indicated that organised in‐patient (stroke unit) care is effective in reducing death and disability (SUTC 2013). However, many important questions about stroke service provision remain unanswered. In particular, are there effective alternatives to in‐patient care and how can care be best provided after discharge from hospital?
Description of the intervention
A previous review focused on those systems of care which have been set up as complete alternatives to in‐patient care, that is, services such as 'hospital at home', which aim to prevent stroke patients being admitted to hospital (Langhorne 1999). A second approach has been to develop services that may accelerate the discharge of patients already admitted to hospital. These services have variously been termed 'early supported discharge (ESD) schemes', 'early home supported discharge services', 'accelerated discharge schemes' and 'post‐discharge support services', and form the basis of this review. This review focuses on the effectiveness of such early supported discharge services.
How the intervention might work
One of the main areas of concern to patients and carers is the organisation of discharge from hospital (Warlow 2008); moving from being cared for in hospital by a team of professionals, to being at home and the responsibility of themselves and their carers. ESD services were developed to try and improve the transition between hospital and community by accelerating discharge home from hospital but providing more continuity of rehabilitation in the home setting. Some arguments in favour of ESD services are summarised as better partnership between the patient and therapist, helping patient motivation by focusing on more realistic rehabilitation goals, providing rehabilitation in a more relevant context, encouraging more focus on self‐directed activities, and fostering a more realistic understanding of future recovery (Langhorne 2007).
Why it is important to do this review
Although arguments have been made for and against ESD services (Langhorne 2007), the basic question ‐ whether a policy of early hospital discharge with support is as effective and efficient as conventional hospital care, discharge planning, and post‐discharge care ‐ needs to be tested in rigorous trials and systematic reviews. This remains an area of great clinical interest that features in clinical practice guidelines (ESO 2008; RCP 2008), and is the subject of ongoing trials.
Objectives
To establish if, in comparison with conventional care, services that offer people in hospital with stroke a policy of early discharge with rehabilitation provided in the community (ESD) can: 1) accelerate return home, 2) provide equivalent or better patient and carer outcomes, 3) be satisfactory to patients and carers, and 4) have justifiable resource implications use.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised trials that allocated individual patients to either conventional hospital care and discharge procedures or alternative services that aimed to accelerate the patient's discharge from hospital. Therefore, randomisation will have taken place relatively early after hospital admission and before hospital discharge.
Types of participants
Any patient who has been admitted to hospital with a clinical diagnosis of stroke (defined as an acute focal neurological deficit caused by cerebrovascular disease). Where possible, we tried to record stroke severity (level of disability) at randomisation using activities of daily living (ADL) status.
Types of interventions
We included trials evaluating any intervention that aimed to accelerate discharge from hospital with the provision of support (with or without a 'therapeutic' rehabilitation intervention) in a community setting (ESD). We recorded the specific type of intervention, but this was not used as an exclusion criterion. We aimed to include trials that focused largely or entirely on stroke patients. We derived prespecified subgroups from recognised indicators of in‐patient stroke service quality, in particular whether care was planned and provided by a specialist team whose work was co‐ordinated through regular multidisciplinary team meetings.
Types of outcome measures
Primary outcomes
The main focus of the analysis was on the patient outcomes of: death, physical dependency (i.e. dependent on help for transfers, mobility, washing, dressing or toileting), and place of residence (home, residential home, nursing home, hospital).
The primary patient outcome was the composite end‐point of death or long‐term dependency recorded at the end of scheduled follow‐up.
We also analysed death or requiring institutional care (residential home, nursing home, hospital) at the end of scheduled follow‐up, and death at the end of scheduled follow‐up.
The main resource outcome was the length of the index hospital stay. We planned to record other resource outcomes (i.e. readmission to hospital, number of readmissions, number of readmission days, cost of in‐patient stay, total cost of service interventions), but in the end were limited to length of the index hospital stay, readmission to hospital, and total cost of service interventions.
Secondary outcomes
Activities of daily living (ADL) score.
Extended ADL score.
Subjective health status.
Mood (mood or depression score).
Carer outcomes (carer mood and subjective health status).
Patient and carer satisfaction and/or service preference.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of relevant papers where necessary.
Electronic searches
In collaboration with the Cochrane Stroke Group Information Specialist, we searched:
Cochrane Stroke Group Trials Register (to January 2017);
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) in the Cochrane Library (searched January 2017) (Appendix 1);
MEDLINE in Ovid (searched January 2017) (Appendix 2);
Embase in Ovid (searched January 2017) (Appendix 3);
CINAHL in EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to January 2017) (Appendix 4);
Web of Science (searched January 2017).
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress). We used the search strategy for MEDLINE with the assistance of the Cochrane Stroke Group Information Specialist and modified it to suit other databases (Appendix 2). To avoid duplication of effort, we restricted the searches of MEDLINE and Embase from January 2008 as these databases have already been searched to that date for all stroke trials and relevant trials added to the Cochrane Stroke Group Trials Register.
In March 2017, using the keywords 'stroke' and 'discharge', we searched:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (https://clinicaltrials.gov/);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/);
ISRCTN Registry (www.isrctn.com) (formerly Current Controlled Trials metaRegister of Controlled Trialls (mRCT)) active and archived registers (www.controlledtrials.com/mrct) and International Standard Randomised Controlled Trial Number Register (www.controlledtrials.com/isrctn/);
CenterWatch Clinical Trials Listing Service (http://www.centerwatch.com/);
Community Research & Development Information Service (of the European Union) (http://cordis.europa.eu/home_en.html);
Hong Kong clinical trials register (http://www.hkuctr.com/)
Searching other resources
In an effort to identify further published, unpublished, and ongoing trials we also performed citation tracking of included studies, checked reference lists of relevant articles, and contacted trialists.
Data collection and analysis
Selection of studies
One review author (PL) read the titles and abstracts of the records obtained from the electronic searches and excluded obviously irrelevant studies. We obtained the full copy of the remaining studies and two review authors (PL, SB) independently selected studies for inclusion based on the following eligibility criteria (ESD trialists 2012).
RCT.
Service intervention providing rehabilitation or physical support, or both, in a community setting.
Service aim is to accelerate discharge home from hospital (i.e. randomisation takes place during hospital admission).
Trial of stroke patients.
We previously contacted the trialists and invited them to join an individual patient data review of all comparable trials. This update is largely based on published trial data but we hope to include further individual patient data in future updates.
Data extraction and management
For the previous version of this review our primary aim was to obtain individual patient data from the trialists (ESD trialists 2012). We contacted the co‐ordinators of the eligible trials and invited them to join a collaborative group. We asked them to provide a detailed description of their intervention and control services and also to provide basic individual patient data particularly concerning the primary patient outcomes and pre‐planned subgroup analyses. Where these were not available in an appropriate format, we sought standardised (tabular) outcome data. Where data had to be taken from published sources, two review authors independently extracted the data using a standard data extraction form. We collected descriptive information about service characteristics using a standard questionnaire prior to the identification and analysis of outcome data.
For the current update two review authors (PL, SB) independently extracted the data using a standard data extraction form. We then cross‐checked our interpretation with the primary authors.
Assessment of risk of bias in included studies
We assessed risk of bias using Cochrane's 'Risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We identified the method of concealment of treatment allocation, the presence of an intention‐to‐treat analysis, and the presence of blinding of outcome assessment as potentially important factors for sensitivity analyses, but we did not use them as exclusion criteria.
Measures of treatment effect
The primary patient outcome was the composite end‐point of death or long‐term dependency recorded at the end of scheduled follow‐up. Where death, dependency or institutionalisation after the end of scheduled follow‐up were reported, we analysed these using the odds ratio (OR) and 95% confidence interval (CI).
We sought data on initial stroke severity using the most widely available marker of functional ability (Activities of Daily Living (ADL) score during the first week post stroke). Most trials could easily provide this as the Barthel Index at randomisation. However, in three trials randomisation frequently took place later (occasionally up to six weeks post stroke) (Adelaide 2000; Adelaide 2016; London 1997). Where possible, we estimated the baseline Barthel assuming a typical recovery of one Barthel point per week, e.g. Barthel of 14/20 at week four indicates an initial score of 10/20.
Many secondary outcomes were expressed as continuous outcome scores. We aimed to analyse these as the mean and standard deviation of the score. Where only medians were available we assumed these were approximate to the mean. Where only interquartile ranges (IQR) were reported we inferred the standard deviation as follows: the IQR will incorporate 50% of the distribution of data compared with standard deviation, which can be expected to include 70% (+ or ‐ 35%) of the distribution. Therefore, assuming a normal distribution then one standard deviation should equal the IQR/(2 x 0.7). Where no other data were provided with the mean value, we inferred the standard deviation as being at least as large as the comparable trials using the same measure. We used sensitivity analyses to check the impact of data assumptions.
Unit of analysis issues
For this update we planned to conduct all analyses at the level of the individual randomised participant. As a result of this modification, we removed one previously included cluster‐randomised trial from the analysis (Glostrup 2006).
Dealing with missing data
Where data were missing for the primary outcome, we assumed the patient to be alive, independent, and living at home. We explored the implications of this in a sensitivity analysis.
Assessment of heterogeneity
We planned to determine heterogeneity using the I2 statistic. We defined significant heterogeneity as an I2 of greater than 50%. Where significant heterogeneity occurred, we explored potential sources using pre‐planned sensitivity analyses and carried out funnel plots.
Assessment of reporting biases
We employed a comprehensive search strategy in an effort to avoid reporting biases. To identify unpublished studies, we searched trial registers and contacted trialists and other experts in the field.
Data synthesis
We checked all patient data for internal consistency and consistency with published reports. One review author entered data into Review Manager 5 (RevMan 2014), and a second review author checked the entries. We analysed binary outcome data using the OR and 95% CI. We used a fixed‐effect model first but replaced this with a random‐effects model if there was significant heterogeneity. If possible, we analysed continuous outcome data (e.g. ADL scores) using the mean difference (MD) and 95% CI for identical outcomes and the standard mean difference (SMD) where different measurement techniques were used to measure the same outcome domain. We used a fixed‐effect model first but replaced this with a random‐effects model if there was significant heterogeneity. We had to reverse several outcome scores (e.g. mood scores) to ensure all scores compared were operating in the same direction. This was done by subtracting the observed score from the maximum possible score. Where multi‐arm studies were identified we planned to combine the comparable groups. If this was not possible we planned to divide the control group and treat the individual arms as separate studies.
'Summary of findings' and GRADE
We included each of the main analyses in a 'Summary of findings' table and subjected them to a GRADE analysis (Table 1). These included the outcomes of death or dependency, death, death or institutional care, extended activities of daily living score, satisfaction with services, readmission to hospital (all recorded at the at the end of scheduled follow‐up), and length of initial stay in hospital.
Subgroup analysis and investigation of heterogeneity
Previous subgroup analyses were based on patient characteristics of age, gender, presence of carer, and stroke severity (Barthel Index in the first week). We based subgroup analyses of service characteristics on the ESD characteristics (whether based on a co‐ordinated multidisciplinary team), ESD service base (hospital out‐reach or community in‐reach), and the nature of the control service (based on a stroke unit or other service). We aimed to update these if the relevant data were available. We initially trichotomised stroke severity and age but subsequently collapsed these into two groups for simplicity and consistency with previous reviews (SUTC 2013).
Sensitivity analysis
We planned sensitivity analyses around the method of randomisation (concealment of treatment allocation), an intention‐to‐treat analysis (loss to follow‐up), and blinding of outcome assessment.
Results
Description of studies
(See: Characteristics of ongoing studies; Characteristics of studies awaiting classification; Characteristics of excluded studies)
Results of the search
The search strategy for the second version of this review (ESD trialists 2005) identified 29 potentially eligible trials of which three (Ayrshire 2000; Auckland 1999; Cumbria 2004) were in the early stages of planning but never started. The original assessors agreed on the inclusion of 10 trials, the exclusion of 14 trials and disagreed on two trials (Akershus 1998; New York 1986). After discussion and obtaining more information, both these trials were considered eligible but one was excluded (New York 1986) as no outcome information has ever been identified (see below). Therefore, the previous version of this review included 11 trials (ESD trialists 2005).
For the previous update (ESD trialists 2012), we identified three new trials (Copenhagen 2009; Glostrup 2006; Trondheim 2004) plus newly published data for three previously included trials (Montreal 2000; Stockholm 1998; Trondheim 2000). We required further information for two trials (ATTEND pilot 2015; Edirne 2001) to assess eligibility, and an additional five trials (Aveiro 2016; Bergen 2014; Hong Kong; Perth; West Denmark) did not yet have available outcome data. We asked the co‐ordinators of all eligible trials to provide a detailed description of their intervention and control services, which were collected using a standard questionnaire prior to the identification and analysis of outcome data.
For this current update we, identified 9872 titles and excluded 9618 as obviously irrelevant or duplicates (Figure 1). Of the 54 records reviewed as abstracts, 45 were duplicates or referred to previously identified studies. This left nine new reports (four included trials, one awaiting classification, two ongoing trials, two excluded trials), in addition to the 42 from the previous version (ESD trialists 2012); 13 included trials, two awaiting classification, five ongoing trials, and 22 excluded trials (including one cluster trial that was previously included). Therefore, for this update we had: 17 included trials (see Characteristics of included studies), three awaiting assessment (Edirne 2001; Shi 2014; Tian 2015), seven ongoing trials (ATTEND; Care4Stroke; Gothenburg; Hong Kong; Perth; RECOVER; West Denmark), and 24 excluded trials (see Characteristics of excluded studies).
1.
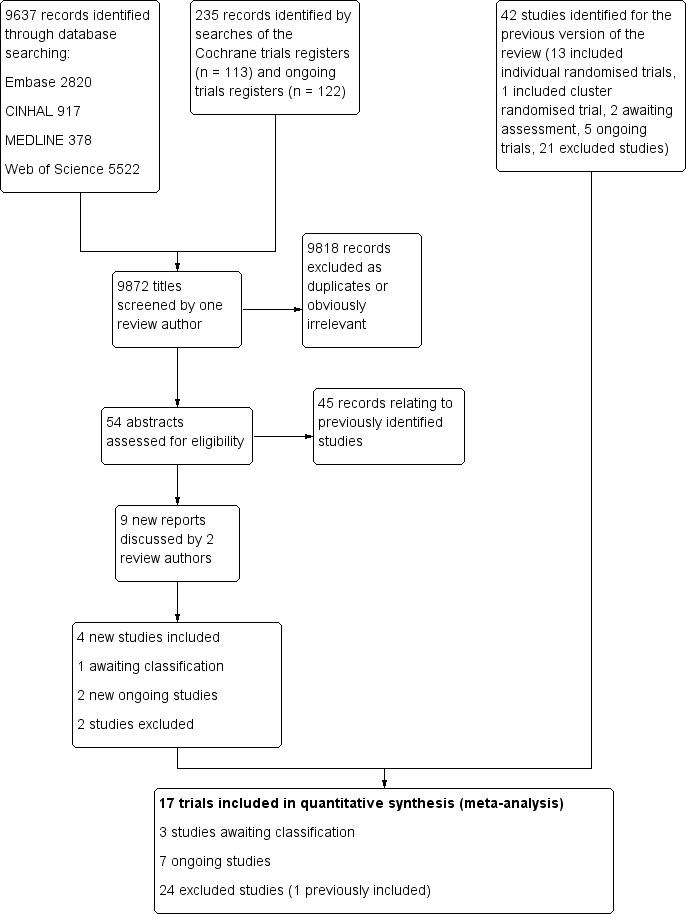
Flow diagram illustrating the results of the updated searches
Included studies
The services under comparison are outlined in detail (Characteristics of included studies). We were particularly interested in establishing the degree of co‐ordination and organisation of the community and hospital services (i.e. whether patients received care from a co‐ordinated multidisciplinary team with some specialist interest in stroke which met on a regular basis). By this definition the following classifications can be made.
Intervention services
Early supported discharge (ESD) team co‐ordination and delivery: in nine trials the ESD service comprised a multidisciplinary team which co‐ordinated discharge from hospital, post‐discharge care and provided rehabilitation and patient care at home or in a community setting (Adelaide 2000; Aveiro 2016; Belfast 2004; Copenhagen 2009; London 1997; Manchester 2001; Montreal 2000; Newcastle 1997; Stockholm 1998). The multidisciplinary team met on a regular basis to plan patient care.
ESD team co‐ordination: in four trials discharge home and the immediate post‐discharge care was planned and supervised by a co‐ordinated multidisciplinary team (Bergen 2014; Oslo 2000; Trondheim 2000; Trondheim 2004). However, care was subsequently handed over to existing community‐based agencies who provided continuing rehabilitation and support at home. These community‐based agencies did not always provide co‐ordinated multidisciplinary team care (i.e. input from a multidisciplinary team which met on a regular basis to plan patient care). However, in some trials the community teams were also multidisciplinary in nature and focused on working with stroke patients early after discharge (Bergen 2014; Oslo 2000). One recent trial randomised patients to one of two different forms of ESD service (Bergen 2014); based in a community day unit (Bergen 2014 ‐ Day unit) or in their homes with home‐visits from the community health team (Bergen 2014 ‐ Home care). Although both met the definition of an ESD service they have, where possible, been analysed separately to reflect this difference in design.
No ESD team: in four trials, patients had access to multidisciplinary team care in hospital, but this ended at hospital discharge (Adelaide 2016; Akershus 1998; ATTEND pilot 2015; Bangkok 2002). Their subsequent care was provided by a range of community stroke services which were; not planned or provided by a co‐ordinated team (Akershus 1998), were provided by trained healthcare volunteers (Bangkok 2002), or provided through supported training by a physiotherapist for patients and family (Adelaide 2016; ATTEND pilot 2015).
The boundary between groups 1 and 2 does not appear clear cut but indicates a spectrum of approaches where an ESD team plans and co‐ordinates discharge, provides early post‐discharge rehabilitation, and then hands over care to other community services.
ESD team structure, practices and procedures
Details of ESD team practices can best be obtained from the original trials. However, we previously developed a summary description of the services to indicate the type of service provided. From recorded staff contact time, we calculated standardised staffing levels (whole time equivalents (WTE) sufficient to manage a notional 100 new patients per year) (Adelaide 2000; Aveiro 2016; London 1997; Montreal 2000; Newcastle 1997; Stockholm 1998), or a typical team caseload (Belfast 2004; Trondheim 2000; Trondheim 2004). We assumed staff would have a 35‐hour working week with 20 hours direct contact time and 10 hours indirect contact time.
Typical ESD teams had approximately 3.1 WTE staff (range 2.6 to 4.6) as follows; medical 0.1, nursing (ranged from 0 to 1.2), physiotherapy 1.0, occupational therapy 1.0, speech and language therapy 0.3, assistant 0.4. Variable levels of social work (0 to 0.5 WTE) and secretarial support were also available (Table 2).
1. Characteristics and staffing of ESD trials.
| Trial | Setting | Key features | Control service base | ESD staffing (whole time equivalents for caseload of 100 patients/year; median and range) | |||||||
| Medical | Nursing | Physio | OT | SALT | Assistant | Other | Total | ||||
| ESD team co‐ordination and delivery | |||||||||||
| Adelaide 2000 | Urban | PHMR Goals documented |
Rehabilitation unit (stroke and neurological) | 0.06 | 0.06 | 0.7 | 1.6 | 0.25 |
0.4 |
Social work | 2.6 |
| Aveiro 2016 | Mixed | Tailored | Mixture (stroke unit, case managers in community‐based team) | 0.8 | 0 | 1.0 | 1.5 | 0 | 0 | Psychology | 3.2 |
| Belfast 2004 | Mixed | PHMR | Mixture (medical, geriatric, stroke unit) | 0.1 | 0 | 1.5 |
1.0 |
0.5 |
1.5 | Secretary Social work |
4.6 |
| Copenhagen 2009 | Urban | Tailored | Stroke unit | nd | nd | nd | nd | nd | nd | nd | nd |
| London 1997 | Urban | Equipment store | Mixture (medical, stroke unit) | 0.1 | 0 | 1 | 1 | 0.5 | 0.5 | ‐ | 3.1 |
| Manchester 2001 | Urban | Mixture (medical, stroke team or unit) | nd | nd | nd | nd | nd | nd | ‐ | nd | |
| Montreal 2000 | Urban | Mixture (medical neurology) | 0 | 0.4 | 1.0 | 0.7 | 0.4 | ‐ | Dietitian | 2.7 | |
| Newcastle 1997 | Urban | Envt visit Key worker 7‐day input PHMR |
Mixture (medical, geriatric) |
0 |
0 | 0.8 | 1.0 | 0.3 | 0.2 |
Secretary Social work Carers |
2.8 |
| Stockholm 1998 | Urban | Case manager Patient diary |
Stroke unit | 0.03 | 0 | 1.0 | 1.0 | 0.5 | ‐ | ‐ | 2.6 |
| West Denmark | Mixed | Tailored | Neurorehabilitation centres (3) | ? | 0 | ? | ? | 0 | 0 | 0 | ? |
| ESD team co‐ordination | |||||||||||
| Bergen 2014 | Urban | Day Unit ESD Home‐based ESD |
Stroke unit | nd | nd | nd | nd | nd | nd | nd | nd |
| Oslo 2000 | Urban | Key worker Community services |
Stroke unit | nd | nd | nd | nd | nd | nd | ‐ | nd |
| Trondheim 2000 | Urban | Key worker Team Community services |
Stroke unit | 0.12 | 1.2 | 1.2 | 1.2 | 0 | ‐ | ‐ | 3.7 |
| Trondheim 2004 | Rural | Stroke unit | 0.12 | 1.2 | 1.2 | 1.2 | 0 | ‐ | ‐ | 3.7 | |
|
10 urban 3 mixed 1 rural |
7 stroke unit 5 mixed service 2 neurorehabilitation unit |
0.10 (0 to 0.12) |
0 (0 to 1.2) |
1.0 (0.7 to 1.5) |
1.0 (0.7 to 1.6) |
0.3 (0 to 0.5) |
0.4 (0 to 1.5) |
‐ |
3.1 (2.6 to 4.6) |
||
| No ESD team | |||||||||||
| Adelaide 2016 | Urban | Caregiver‐mediated exercises combined with tele‐rehabilitation services | Stroke unit | nd | nd | nd? | nd | nd | nd | ‐ | nd |
| Akershus 1998 | Mixed | Range of community rehabilitation services | Stroke unit | nd | nd | nd | nd | nd | nd | ‐ | nd |
| ATTEND pilot 2015 | Mixed | Family‐mediated rehabilitation with mostly remote follow‐up | Stroke unit | nd | nd | < 1.0 | nd | nd | nd | ‐ | nd |
| Bangkok 2002 | Urban | Red Cross volunteers | Stroke unit | nd | nd | nd | nd | nd | nd | ‐ | nd |
MDT mtg: multidisciplinary team meeting N: number of participants nd: no comparable data OT: occupational therapy PHMR: patient‐held medical record physio: physiotherapy SALT: speech and language therapy
The ESD teams could either have a community (community in‐reach) or hospital base (hospital out‐reach) with experience in stroke rehabilitation/neurological rehabilitation (Adelaide 2000; Aveiro 2016; Belfast 2004; Bergen 2014; Copenhagen 2009; London 1997; Manchester 2001; Montreal 2000; Newcastle 1997; Oslo 2000; Stockholm 1998; Trondheim 2000; Trondheim 2004). All co‐ordinated their work through regular multidisciplinary team meetings. A typical approach would involve the early identification of the patient in hospital and a visit from the key worker (case manager) from the ESD team. Discharge was planned with the patient and carer, often involving a pre‐discharge home visit (attended by the patient) or environmental visit (not attended by the patient). Team input typically began on the day of discharge and could be provided as required. In practice this ranged from daily input to four to five days per week. Typically teams would agree recovery goals with the patient and negotiate the termination of services within three months (which would be tapered off as goals were achieved). Many teams used a patient‐held medical record and provided a formal discharge summary at the end of input.
Control services
These were categorised on whether organised stroke unit care was available to patients prior to discharge (Table 2). In 12 trials, all patients were recruited from a stroke unit or neurological rehabilitation unit staffed by a multidisciplinary team (Adelaide 2000; Adelaide 2016; Akershus 1998; ATTEND pilot 2015; Aveiro 2016; Bergen 2014; Copenhagen 2009; Oslo 2000; Stockholm 1998; Trondheim 2000; Trondheim 2004) or most patients (Belfast 2004). Five trials recruited a minority of patients from a multidisciplinary stroke unit setting (Bangkok 2002; London 1997; Manchester 2001; Montreal 2000; Newcastle 1997). Therefore, the control service was frequently provided in general wards. Discharge arrangements were variable in the control services with a minority undergoing a pre‐discharge home visit and variable follow‐up arrangements.
Settings of services
The trials identified come from nine countries (Australia, Canada, Denmark, India, Norway, Portugal, Sweden, Thailand, UK). Fourteen trials were established in city hospitals servicing largely urban areas while two (Aveiro 2016; Belfast 2004) covered a mixture of rural and urban areas. An additional trial recruited only patients from rural addresses who were admitted to a large urban hospital (Trondheim 2004).
Patient characteristics
Patients had a clinical diagnosis of stroke and the average patient age in the trials ranged from 60 to 80 years. There appeared to be a degree of selection of patients deemed suitable for the ESD services that was based on need (persisting disability), stability of their medical condition, and practicability (living within the local area). The average (mean or median) initial Barthel index (at the time of patient recruitment) in each study ranged from 10/20 to 19/20 with a lower IQR limit of 6 to 16/20 and an upper value of 14 to 19/20. Thus the typical patient population had an initial Barthel index of 14/20 with an IQR of 10 to 18.
We repeated this process to estimate the Barthel index (or equivalent score) at the time of discharge for those trials where the ADL score was recorded within one week prior to discharge (Adelaide 2000; Aveiro 2016; Belfast 2004; Bergen 2014; London 1997; Manchester 2001; Newcastle 1997; Trondheim 2000; Trondheim 2004). The average (mean or median) initial Barthel index (within one week prior to discharge) in each study ranged from 13/20 to 19/20 with a lower IQR limit of 10/20 to 16/20 and an upper value of 15/20 to 19/20. Thus the typical patient population prior to discharge had an initial Barthel index of 15/20 with an IQR of 11/20 to 17/20.
None of the trials recruited more than 70% of hospitalised stroke patients; a median of 33% (range 13% to 70%) of hospitalised stroke patients met the clinical criteria for the early discharge service (NB: in some trials, a further group of patients did not meet research criteria such as an ability to complete research assessments). We have summarised the inclusion and exclusion criteria of the individual trials in the Characteristics of included studies table.
Outcomes
Most trials included our main outcomes of death, residence (institutional care) and dependency (Barthel index, Rankin score or Functional Independence Measure), all recorded at the end of scheduled follow‐up, as well as our primary resource outcome length of initial hospital stay (Table 3). Missing data for the primary outcome are summarised in Table 4. Two trials subsequently reported further outcomes of death and dependency after scheduled follow‐up (at one year and five years) (Stockholm 1998; Trondheim 2000).
2. Plan and timing of primary analyses.
| Trial | Death | Institutional care | Dependency | Defined dependent | Length of stay |
| Adelaide 2000 | 6 months | 6 months | 6 months | Barthel index < 95/100 | Initial hospital discharge |
| Adelaide 2016 | 3 months | ‐ | 3 months | Barthel index | Initial hospital discharge and up to 12 months |
| Akershus 1998 | 7 months | 7 months | 7 months | Barthel index < 95/100 | Not used ‐ only available for acute hospital |
| ATTEND pilot 2015 | 6 months | ‐ | 6 months | Rankin score 3 to 5 | Initial hospital discharge (median, IQR) |
| Aveiro 2016 | 6 months | 6 months | 6 months | Functional Independence Measure < 60 points | Initial stroke unit stay (also stay in rehabilitation unit) |
| Bangkok 2002 | 6 months | 6 months | 6 months | Barthel index < 95/100 | Initial hospital discharge |
| Belfast 2004 | 12 months | 12 months | 12 months | Barthel index < 19/20 | Initial hospital discharge |
| Bergen 2014 | 6 months | 6 months | 6 months | Rankin score 3 to 5 | Initial hospital stay plus institution up to 6 months |
| Copenhagen 2009 | 5 months | 5 months | 3 months | Rankin score 3 to 5 | Initial hospital stay |
| London 1997 | 12 months | 12 months | 12 months | Barthel index < 19/20 | Initial hospital discharge |
| Manchester 2001 | 12 months | 12 months | 12 months | Barthel index < 19/20 | Initial hospital stay (acute and rehabilitation wards) |
| Montreal 2000 | 3 months | 3 months | 3 months | Barthel index < 95/100 | Initial hospital stay |
| Newcastle 1997 | 3 month | 3 month | 3 month | Rankin score 3 to 5 | Initial hospital stay |
| Oslo 2000 | 6 month | 6 month | 6 month | Rankin score 3 to 5 | Initial hospital stay |
| Stockholm 1998 | 6 month | 6 month | 6 month | Barthel index 95/100 | Initial hospital stay |
| Trondheim 2000 | 6 months | 6 months | 6 months | Barthel index 95/100 | Initial hospital stay |
| Trondheim 2004 | 12 months | 12 months | 12 months | Rankin score 3 to 5 | Initial hospital stay (acute and rehabilitation wards) |
IQR: interquartile range
3. Data missing for primary outcome.
| Trial | Recruited intervention |
Recruited control |
Recruited total |
Missing intervention |
Missing control | Available intervention |
Available control |
Available total |
Comments |
| ESD trialists 2012 | 885 | 874 | 1759 | 31 | 25 | 854 (96%) | 849 (97%) | 1703 (97%) | |
| Adelaide 2016 | 31 | 32 | 63 | 2 | 2 | 29 (94%) | 30 (94%) | 59 (94%) | Not available as dichotomous outcome |
| ATTEND pilot 2015 | 50 | 54 | 104 | 5 | 9 | 45 (90%) | 45 (83%) | 90 (87%) | |
| Aveiro 2016 | 95 | 95 | 190 | 19 | 17 | 76 (80%) | 78 (82%) | 154 (81%) | |
| Bergen 2014 | 207 | 99 | 306 | 44 | 33 | 163 (79%) | 66 (67%) | 229 (75%) | |
| Total | 1268 | 1154 | 2422 | 101 | 86 | 1166 (92%) | 1068 (93%) | 2234 (92%) |
Secondary outcomes included a range of measures, which are summarised in the Characteristics of included studies table and the sampling analysis schedule provided in Table 5 and Table 6.
4. Plan of secondary analyses: patient outcomes.
| Trial | Timing of outcome | ADL score | Extended ADL score | Subjective health | Mood | Service satisfaction | Hospital readmission |
| Adelaide 2000 | 6 months | Barthel index (median, IQR) | Adelaide Activities Profile | SF‐36 (General health perceptions) | SF‐36 (mental health) | Satisfied with rehabilitation programme | 6 months |
| Adelaide 2016 | 3 months | Barthel index (mean, 95% CI) | Nottingham extended ADL (mean, 95% CI) | Stroke Impact Scale | Hospital Anxiety and Depression Scale (HADS) | ‐ | 12 months |
| Akershus 1998 | 7 months | Barthel index (median, imputed SD) | ‐ | SF‐36 (general health perceptions) | SF‐36 (mental health) | ‐ | ‐ |
| ATTEND pilot 2015 | 6 months | ‐ | Nottingham Extended ADL | EQ‐5D | Hospital anxiety and depression scale (category) | ‐ | 6 months |
| Aveiro 2016 | 6 months | FIM (mean, SD) | Frenchay Activities Index | ‐ | ‐ | ‐ | ‐ |
| Bangkok 2002 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Belfast 2004 | 12 months | Barthel index | Nottingham extended ADL | SF‐36 (general health perceptions) | SF‐36 (mental health) | Satisfied with outpatient rehabilitation | 6 month |
| Bergen 2014 | 6 months | Barthel index (median, IQR) | ‐ | ‐ | ‐ | Satisfaction score (mean & SD) | ‐ |
| Copenhagen 2009 | 3 months | Barthel Index (median, imputed SD) | ‐ | EQ‐5D | ‐ | 5 months | |
| London 1997 | 12 months | Barthel index | Rivermead ADL score | Nottingham health profile (score reversed) | Number abnormal on hospital anxiety and depression scale | Satisfied with care in general | 12 month |
| Manchester 2001 | 12 months | Barthel index | Nottingham extended ADL score | Euroquol scale (0 to 100) | Hospital anxiety and depression scale (depression subscore, score reversed) | ‐ | ‐ |
| Montreal 2000 | 3 month | Barthel index | Instrumental ADL (OARS) scale | SF‐36 (general health perceptions) | SF‐36 (mental health) | ‐ | ‐ |
| Newcastle 1997 | 3 month | ‐ | Nottingham extended ADL score (median, IQR) | Dartmouth COOP chart overall health section (median, IQR; scale reversed) | Dartmouth COOP chart feelings section (median, IQR; scale reversed) | ‐ | 3 month |
| Oslo 2000 | 6 month | ‐ | Nottingham extended ADL score (median, IQR) | General Health Questionnaire (reversed score) | MADRS score | Satisfied with care in general | ‐ |
| Stockholm 1998 | 8 months | ‐ | Frenchay Activities index (median, IQR) | Sickness impact profile score (median, IQR) | ‐ | Satisfied with care received | 6 months |
| Trondheim 2000 | 12 months | ‐ | Frenchay social activity index | Nottingham Health Profile (average of sum 1 and 2) | MADRS | ‐ | ‐ |
| Trondheim 2004 | 12 months | Barthel Index | ‐ | Nottingham health profile | ‐ | ‐ | ‐ |
ADL: activities of daily living COOP: Care Cooperative Information Project GDS: Geriatric Depression Scale IQR: interquartile range MADRS: Montgomery‐Åsberg Depression Rating Scale OARS: Older Americans Resources and Services scale SD: standard deviation SF: short form
5. Plan of secondary analyses: carer outcomes.
| Trial | Timing of outcome | Subjective health | Mood | Service satisfaction |
| Adelaide 2000 | 6 months | SF‐36 general health perceptions | SF‐36 mental health | Satisfied with rehabilitation programme |
| Adelaide 2016 | 3 months | Caregiver Strain Index (score reversed) | Hospital Anxiety and Depression Scale (score reversed) | |
| Akershus 1998 | ‐ | ‐ | ‐ | ‐ |
| ATTEND pilot 2015 | 6 months | Caregiver Burden Scale (category) | ‐ | ‐ |
| Aveiro 2016 | ‐ | ‐ | ‐ | ‐ |
| Bangkok 2002 | ‐ | ‐ | ‐ | ‐ |
| Belfast 2004 | 6 months | Caregiver strain index (score reversed) | ‐ | Satisfied with outpatient services |
| Bergen 2014 | ‐ | ‐ | ‐ | ‐ |
| Copenhagen 2009 | 3 months | Satisfied with rehabilitation programme | ||
| London 1997 | 12 months | Caregiver strain index (score reversed) | ‐ | Satisfied with care in general |
| Manchester 2001 | 12 month | ‐ | Hospital Anxiety and Depression Scale (depression subscore, score reversed) | ‐ |
| Montreal 2000 | 3 months | Caregiver Burden Index | ‐ | ‐ |
| Newcastle 1997 | 3 months | General health questionnaire (median, range; score reversed) | ‐ | ‐ |
| Oslo 2000 | 6 months | General health questionnaire (score reversed) | ‐ | Satisfied with care in general |
| Stockholm 1998 | ‐ | ‐ | ‐ | ‐ |
| Trondheim 2000 | 12 months | Caregiver Burden score | ‐ | ‐ |
| Trondheim 2004 | 12 months | Caregiver strain index (score reversed) | ‐ | ‐ |
Excluded studies
See the Characteristics of excluded studies table.
Risk of bias in included studies
See the 'Risk of bias' graph (Figure 2), the 'Risk of bias' summary (Figure 3), and the Characteristics of included studies table.
2.
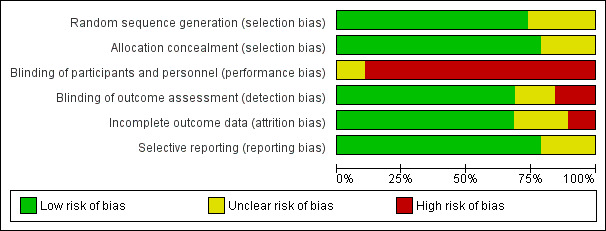
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
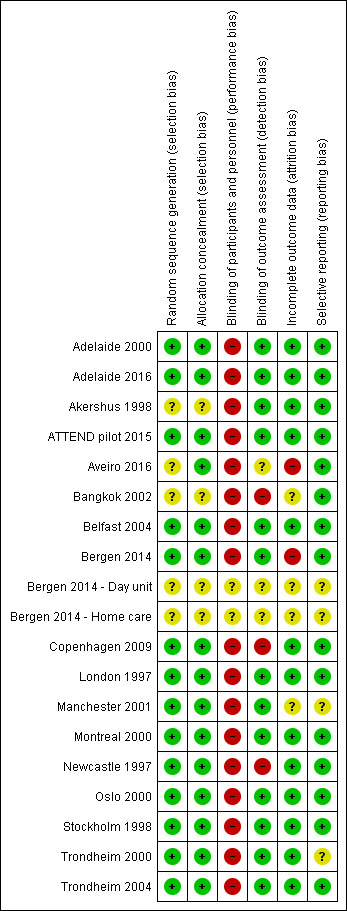
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Fifteen trials used a clearly concealed randomisation procedure (Adelaide 2000; Adelaide 2016; ATTEND pilot 2015; Aveiro 2016; Belfast 2004; Bergen 2014; Copenhagen 2009; London 1997; Manchester 2001; Montreal 2000; Newcastle 1997; Oslo 2000; Stockholm 1998; Trondheim 2000; Trondheim 2004).
Blinding
Performance bias was a potential risk in all included trials as blinding of participants or treating personnel was impossible due to the nature of the intervention.
Thirteen trials clearly reported using an independent (blinded) assessment of outcomes at a fixed time after recruitment (median six months; range three to 12 months) (Adelaide 2000; Adelaide 2016; Akershus 1998; ATTEND pilot 2015; Belfast 2004; Bergen 2014; London 1997; Manchester 2001; Montreal 2000; Oslo 2000; Stockholm 1998; Trondheim 2000; Trondheim 2004).
Incomplete outcome data
Those trials with published outcome data were generally complete, at least for the main outcomes of death, institutionalisation and dependency (see Results). For the primary outcome of death or dependency, data were missing for 101/1236 (8.2%) and 86/1122 (7.7%) of participants at the end of scheduled follow‐up (Table 4). However, one of these trials, which was missing two intervention patients and two controls, could not contribute to the primary analysis of the dichotomous outcome of death or dependency (Adelaide 2016) (Analysis 1.3).
1.3. Analysis.
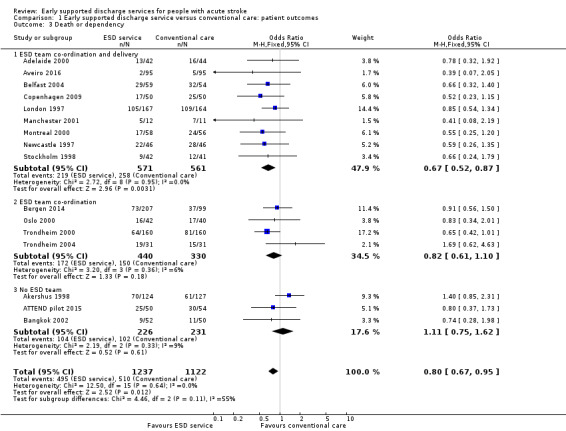
Comparison 1 Early supported discharge service versus conventional care: patient outcomes, Outcome 3 Death or dependency.
Selective reporting
We judged most trials to be at low risk of reporting bias, at least for the primary outcomes, as the outcomes were sought from, and provided by, the trialists. However, the completeness of reporting of secondary outcomes is less certain.
Other potential sources of bias
The trialists who participated in this review were, in general, the authors of the included trials. However, we ensured that trialists avoided making decisions on trial selection and data extraction for their own trial.
Effects of interventions
See: Table 1
We analysed results for all comparisons of ESD services (policy of early discharge with home‐based support and rehabilitation) versus conventional services (policy of hospital rehabilitation and conventional discharge arrangements) at the end of scheduled follow‐up (median six months; range three to 12 months). We divided services into three subgroups to reflect the pre‐specified view that effectiveness of ESD services may be influenced by the multidisciplinary teamwork of the ESD team responsible for post‐discharge care (see Description of studies). Therefore, we presented the analysis in the following subgroups:
ESD team co‐ordination and delivery: co‐ordinated multidisciplinary ESD team co‐ordinated and provided post‐discharge care;
ESD team co‐ordination: co‐ordinated multidisciplinary ESD team co‐ordinated supervised discharge and immediate post‐discharge care but then handed over to other services;
no ESD team: post‐discharge services were not provided by co‐ordinated multidisciplinary ESD team.
The interpretation, timing, and analysis of outcomes are shown in Table 3, Table 5 and Table 6.
1. Patient outcomes
1.1: Death
Outcome data were available for 16 trials (2116 participants). We assumed participants with missing data (57 intervention participants and 53 controls) were alive. Overall, there was no significant difference in case‐fatality between the ESD team and conventional services (odds ratio (OR) 1.04, 95% confidence interval (CI) 0.77 to 1.40, P = 0.81, moderate‐grade evidence). There was no significant degree of statistical heterogeneity but a statistical interaction (P = 0.01) between subgroups suggesting a higher case fatality in the subgroup without a co‐ordinated ESD team (Analysis 1.1).
1.1. Analysis.
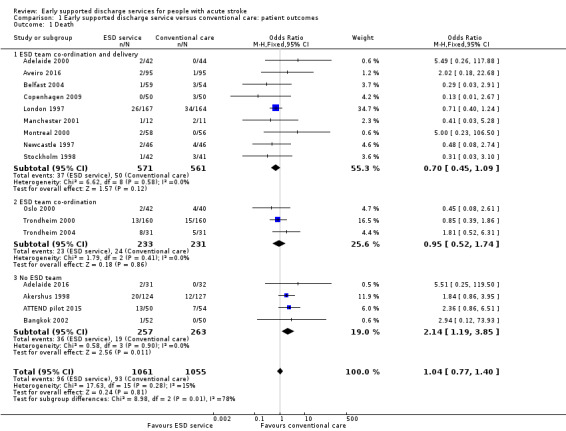
Comparison 1 Early supported discharge service versus conventional care: patient outcomes, Outcome 1 Death.
1.2: Death or requiring institutional care
Outcome data were available for 12 trials (1664 participants). We assumed participants with missing data (24 intervention participants and 19 controls) were alive and living at home. Overall, there was a significant reduction in the odds of patients dying or requiring long‐term institutional care (OR 0.75, 95% CI 0.59 to 0.96, P = 0.02, moderate‐grade evidence) with no significant heterogeneity. This equates to an extra five (one to eight) patients living at home for every 100 treated (Analysis 1.2).
1.2. Analysis.
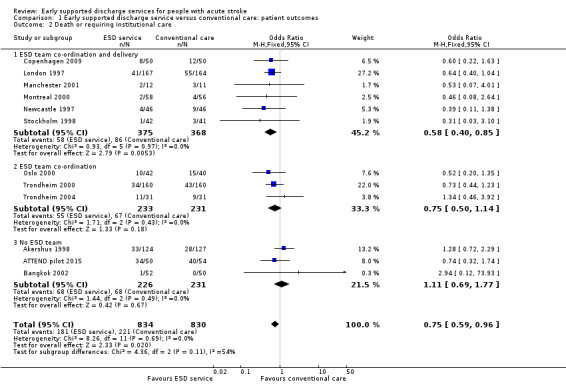
Comparison 1 Early supported discharge service versus conventional care: patient outcomes, Outcome 2 Death or requiring institutional care.
1.3: Death or dependency
Outcome data were available for 16 trials (2359 participants). We assumed participants with missing data (99 intervention participants and 84 controls) were alive and independent. Overall, there was a significant reduction in the odds of the combined adverse outcome of death or dependency (OR 0.80, 95% CI 0.67 to 0.95, P = 0.01, moderate‐grade evidence) with no significant heterogeneity. This equates to an extra five (one to nine) patients regaining independence for every 100 receiving ESD services. There was no substantial degree of statistical heterogeneity (Analysis 1.3).
1.4: Activities of daily living (ADL)
These data were available (in a variety of formats) for 12 trials (1449 participants). Overall, there was no apparent difference in the ADL scores of survivors for whom data were available with no significant heterogeneity (Analysis 1.4).
1.4. Analysis.
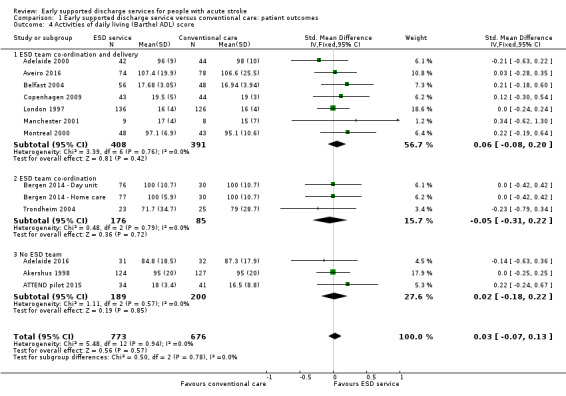
Comparison 1 Early supported discharge service versus conventional care: patient outcomes, Outcome 4 Activities of daily living (Barthel ADL) score.
1.5: Extended activities of daily living
These data were available (in a variety of formats) for 11 trials (1262 participants). Overall, there was an apparent increase in extended ADL scores among survivors receiving ESD services (standardised mean difference (SMD) 0.14, 95% CI 0.03 to 0.25, P = 0.01, low‐grade evidence). These results were largely dependent on data from the two subgroups of trials evaluating an ESD team (Analysis 1.5).
1.5. Analysis.
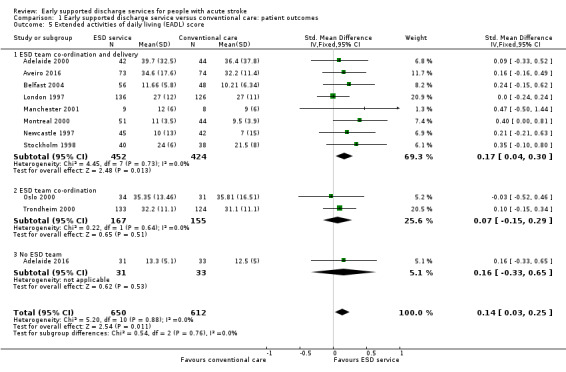
Comparison 1 Early supported discharge service versus conventional care: patient outcomes, Outcome 5 Extended activities of daily living (EADL) score.
1.6: Subjective health status
These data were available (in a variety of formats) from 11 trials (1202 participants). Overall, there was no apparent difference in the subjective health status scores of both groups. There was no significant degree of heterogeneity (Analysis 1.6).
1.6. Analysis.
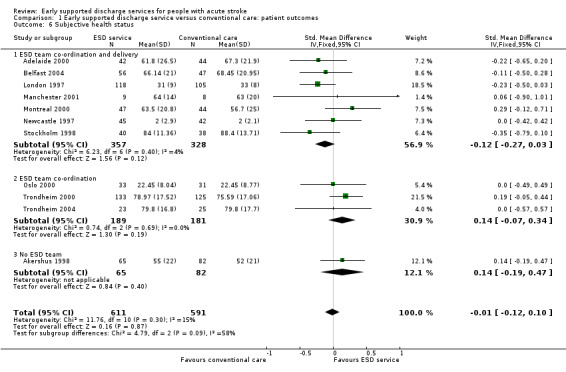
Comparison 1 Early supported discharge service versus conventional care: patient outcomes, Outcome 6 Subjective health status.
1.7: Mood status
These data were available (in a variety of formats) from nine trials (915 participants). Overall, there was no apparent difference in mood scores. There was no significant heterogeneity. Additional dichotomous data from one trial (London 1997) indicated that those people in the ESD service group were more likely to express anxiety (P = 0.02) and non‐significant trends towards higher levels of depression (Analysis 1.7).
1.7. Analysis.
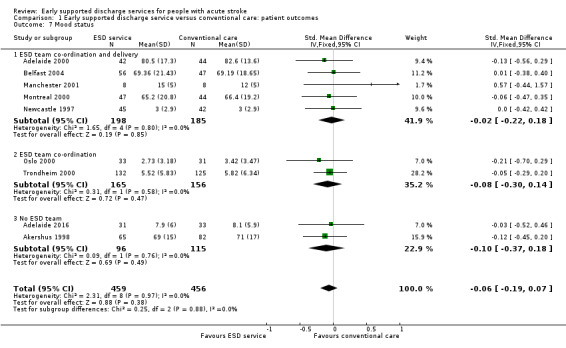
Comparison 1 Early supported discharge service versus conventional care: patient outcomes, Outcome 7 Mood status.
1.8: Patient satisfaction
These data were available (in a variety of formats) from five trials (513 participants). Overall, there was a pattern of ESD service patients being more likely to report satisfaction with outpatient services or services in general (OR 1.60, 95% CI 1.08 to 2.38, P = 0.02, low‐grade evidence). There was no significant heterogeneity (Analysis 1.8).
1.8. Analysis.
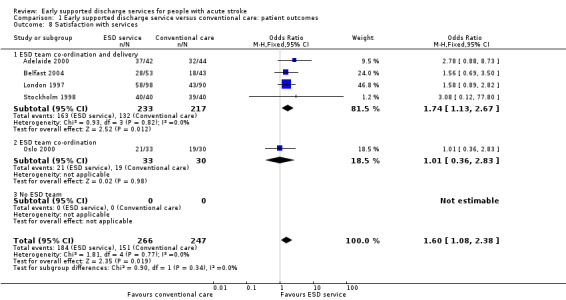
Comparison 1 Early supported discharge service versus conventional care: patient outcomes, Outcome 8 Satisfaction with services.
2. Duration of follow‐up
The primary outcome was recorded at the end of scheduled follow‐up (median six months; range three to 12 months). Two trials (403 participants) have reported extended outcome data subsequent to the end of scheduled follow‐up at one year and five years (Stockholm 1998; Trondheim 2000). There was a reduction in the odds of the combined adverse outcome of death or dependency censored by six months (OR 0.70, 95% CI 0.56 to 0.87). Overall, the pattern of a reduction in death or dependency appears to be sustained at one year and five years but included the possibility of no effect (OR 0.84, 95% CI 0.66 to 1.05 and OR 0.78, 95% CI 0.52 to 1.17, respectively) (Analysis 2.1; Analysis 2.2; Analysis 2.3).
2.1. Analysis.

Comparison 2 Early supported discharge service versus conventional care: duration of follow‐up, Outcome 1 Death or dependency: within 6 months.
2.2. Analysis.
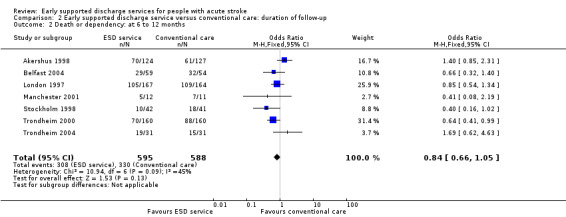
Comparison 2 Early supported discharge service versus conventional care: duration of follow‐up, Outcome 2 Death or dependency: at 6 to 12 months.
2.3. Analysis.
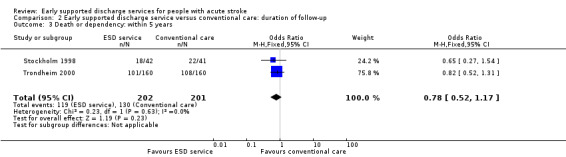
Comparison 2 Early supported discharge service versus conventional care: duration of follow‐up, Outcome 3 Death or dependency: within 5 years.
3. Carer outcomes
3.1: Subjective health status
These data were available (in a variety of formats) from nine trials (813 carers). Overall, there was no apparent difference in scores and no significant heterogeneity (Analysis 3.1).
3.1. Analysis.
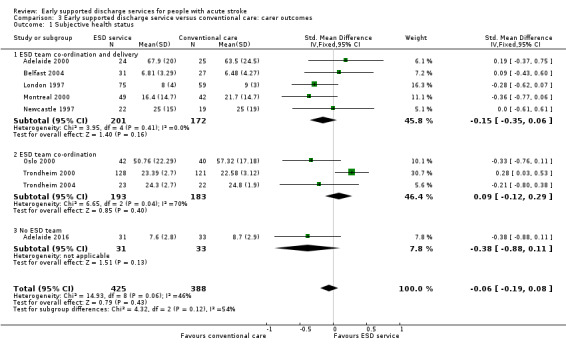
Comparison 3 Early supported discharge service versus conventional care: carer outcomes, Outcome 1 Subjective health status.
3.2: Mood status
These data were available from only three trials with 122 carers. Overall, there was no apparent reduction in the mood score of carers receiving ESD services, but significant heterogeneity was apparent between trials (Analysis 3.2).
3.2. Analysis.
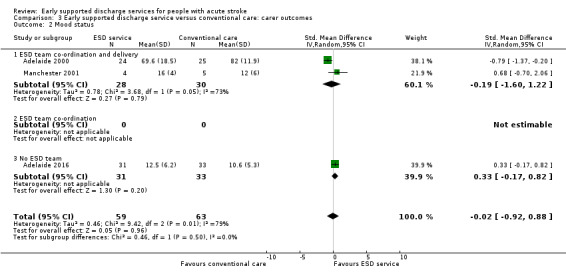
Comparison 3 Early supported discharge service versus conventional care: carer outcomes, Outcome 2 Mood status.
3.3: Carer satisfaction
These data were available (in a variety of formats) from four trials (279 carers). Overall, there was no convincing difference in the odds of carers who received ESD services expressing satisfaction with services (OR 1.56, 95% CI 0.87 to 2.81) (Analysis 3.3).
3.3. Analysis.
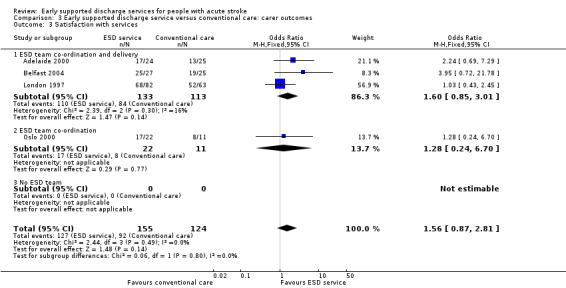
Comparison 3 Early supported discharge service versus conventional care: carer outcomes, Outcome 3 Satisfaction with services.
4. Resource use
(See: Analysis 4.1; Analysis 4.2)
4.1. Analysis.
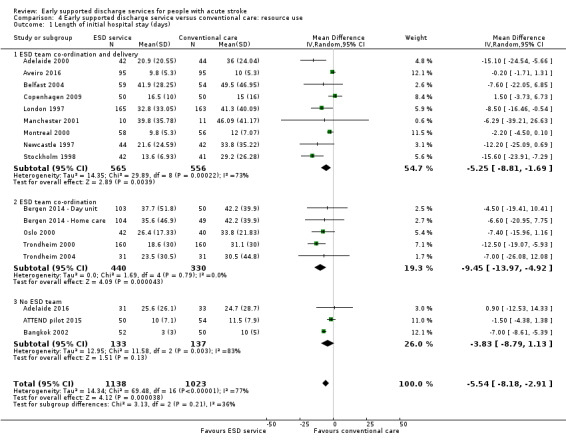
Comparison 4 Early supported discharge service versus conventional care: resource use, Outcome 1 Length of initial hospital stay (days).
4.2. Analysis.
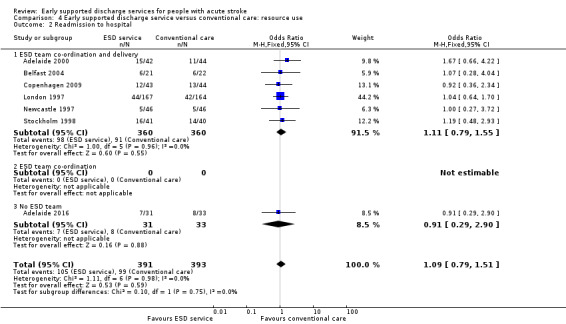
Comparison 4 Early supported discharge service versus conventional care: resource use, Outcome 2 Readmission to hospital.
4.1: Length of initial hospital stay
We were able to analyse data on length of initial hospital stay (using the longest available of acute care and rehabilitation for the index admission) for 16 trials (2161 patients) (Analysis 4.1). Across all trials, there was a reduction in the length of hospital stay (MD ‐ 5.5 days, 95% CI 2.9 to 8.2, P < 0.0001, moderate‐grade evidence), which is approximately equivalent to five days. There was, however, considerable heterogeneity, which reduces confidence in the estimates.
Data were incomplete for total length of stay including hospital readmissions. An analysis of the pattern of discharges based on six trials that could provide data (Adelaide 2000, Belfast 2004, London 1997, Manchester 2001, Oslo 2000, Stockholm 1998) is shown in Table 7.
6. Patterns of discharge from hospital in ESD and control groups.
| Time from randomisation | Number (%) discharged | Risk difference (95% CI) | Significance | |
| ESD service (364 patients) |
Control (354 patients) |
|||
| 2 weeks | 116 (32%) | 77 (22%) | 11 (‐3, 24) | 0.13 |
| 4 weeks | 236 (65%) | 179 (50%) | 19 (4, 35) | 0.01 |
| 6 weeks | 277 (76%) | 249 (70 %) | 8 (1, 15) | 0.02 |
| 8 weeks | 303 (83%) | 275 (78%) | 8 (3, 13) | 0.003 |
| 3 months | 345 (95%) | 324 (92%) | 2 (‐1, 6) | 0.21 |
| 6 months | 363 (100%) | 353 (100%) | 0 (‐2, 1) | 0.71 |
| Data are presented from six trials that could provide relevant data on 718 participants (Adelaide 2000; Belfast 2004; London 1997; Manchester 2001; Oslo 2000; Stockholm 1998). Discharges include deaths and do not include readmissions. The risk difference (95% confidence interval) is calculated taking into account variation between trials | ||||
4.2: Hospital readmissions
Seven trials (784 participants) provided data on the number of participants readmitted to hospital after the index admission. Readmission rates during scheduled follow‐up (27% versus 25%) were very similar (OR 1.09, 95% CI 0.79 to 1.51, P = 0.59, low‐grade evidence) between the ESD service and conventional care groups (Analysis 4.2).
Costs
Costing data are currently available from seven trials (Table 8), which estimated total costs up to three months (Montreal 2000), six months (Adelaide 2000; Newcastle 1997) or one year (London 1997; Stockholm 1998; Trondheim 2000) after randomisation. Estimated costs ranged from 23% less to 15% greater for the ESD group in comparison to controls. These estimates were reported to be stable in sensitivity analyses.
7. Service costs of individual trials.
| Trial | Items costed | ESD cost / patient | Control cost / pt | Percent difference |
| Adelaide 2000 | Cost minimisation. Direct and indirect | AUD 8040 | AUD 10,054 | ‐ 20% |
| London 1997 | Direct and indirect to 12 months | GBP 6800 | GBP 7432 | ‐ 9% |
| Montreal 2000 | Direct and indirect to 3 months | CAD 7784 | CAD 11,065 | ‐30% |
| Newcastle 1997 | Direct and indirect | GBP 7155 | GBP 7480 | ‐ 4% |
| Stockholm 1998 | Hospital, community, private costs | SEK 2806 | SEK 3475 | ‐ 19% |
| Trondheim 2000 | Direct costs to 12 months | EUR 5113 | EUR 6665 | ‐ 23% |
Sensitivity analyses
Analyses by methodological characteristics
Analysis of the primary outcome restricted to the 12 trials that reported concealed randomisation and blinded follow‐up showed a convincing reduction in death or dependency (OR 0.75, 95% CI 0.62 to 0.92, P = 0.005) with no heterogeneity (Adelaide 2000; ATTEND pilot 2015; Belfast 2004; Bergen 2014; Copenhagen 2009; London 1997; Manchester 2001; Montreal 2000; Oslo 2000; Stockholm 1998; Trondheim 2000; Trondheim 2004). Analysis restricted to the 10 trials that reported concealed randomisation and blinded follow‐up plus a very high rate of patient follow‐up for the primary outcome (1277/1318; 3.1% participants missing) showed similar results (OR 0.75, 95% CI 0.60 to 0.93, P = 0.01) with no heterogeneity (Adelaide 2000; ATTEND pilot 2015; Belfast 2004; London 1997; Manchester 2001; Montreal 2000; Oslo 2000; Stockholm 1998; Trondheim 2000; Trondheim 2004).
For the primary outcome of death or dependency, data were missing for 99 (8.0%) intervention participants and 84 controls (7.5%). Our primary analysis assumed they were alive and independent (OR 0.80, 95% CI 0.67 to 0.95) (Analysis 1.3). The result would be similar if all missing participants were assumed to be dead or dependent (OR 0.82, 0.70 to 0.97). The confidence intervals around the apparent effect of ESD services would only cross unity if there was a substantial imbalance in missing data outcomes favouring control services.
Subgroup analyses
Analyses by participant age and gender
Subgroup data for the primary outcome (death or dependency) were available for at least nine trials. Smaller amounts of data were available for death, death or institutionalisation, and length of stay. There was no significant association of participant age or gender with the apparent effect of the ESD service (Analysis 5.1; Analysis 5.2; Analysis 6.1; Analysis 6.2).
5.1. Analysis.
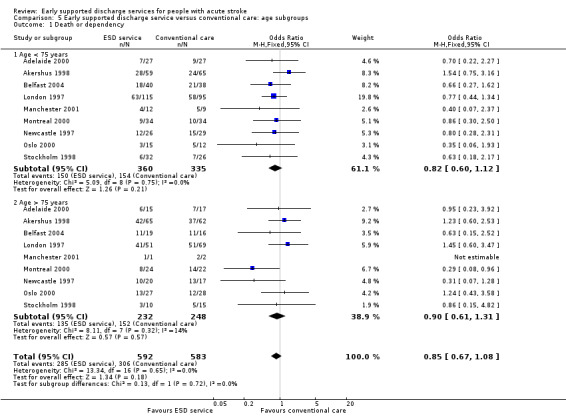
Comparison 5 Early supported discharge service versus conventional care: age subgroups, Outcome 1 Death or dependency.
5.2. Analysis.
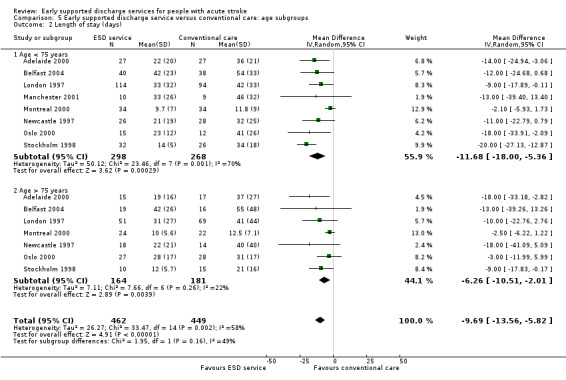
Comparison 5 Early supported discharge service versus conventional care: age subgroups, Outcome 2 Length of stay (days).
6.1. Analysis.
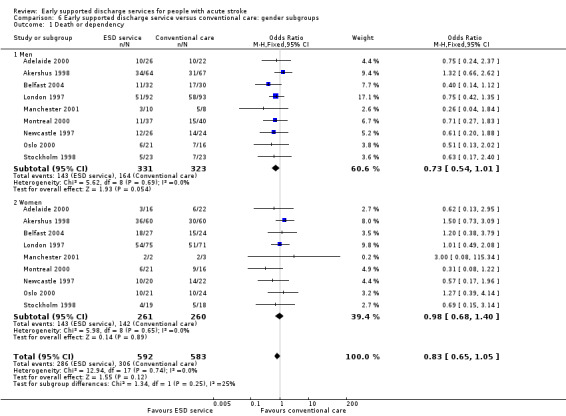
Comparison 6 Early supported discharge service versus conventional care: gender subgroups, Outcome 1 Death or dependency.
6.2. Analysis.
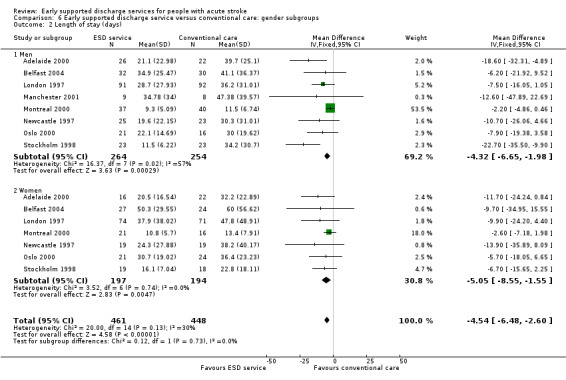
Comparison 6 Early supported discharge service versus conventional care: gender subgroups, Outcome 2 Length of stay (days).
Analyses by initial stroke severity
Data were available for 11 trials (1545 participants). Subgroup analysis by initial stroke severity revealed a differential effect in the odds of death or dependency between participants with moderate initial stroke severity (initial Barthel Index of > 9/20) and those in the severe subgroup (initial Barthel Index < 10/20). In the moderate subgroup there was a reduction (OR 0.77, 95% CI 0.61 to 0.98) as opposed to a non‐significant increase in the severe subgroup (OR 1.40, 95% CI 0.83 to 2.36); test for subgroup interaction P = 0.04. Similar patterns of results were seen for the outcome death or institutional care. The reduction in length of hospital stay was much greater (P < 0.0001) for the severe stroke subgroup (MD 28 days, 95% CI 17 to 40) than the moderate group (MD 3 days, 95% CI 1 to 7). Similar results were obtained if the Barthel index at randomisation was used from the two trials that randomised patients up to several weeks after stroke (Adelaide 2000; London 1997: Analysis 7.1; Analysis 7.2). Similar results were obtained if the three most recent trials for which we do not have individual patient data were included but analysed according to the mean Barthel index at randomisation (Adelaide 2016; Aveiro 2016; Bergen 2014).
7.1. Analysis.
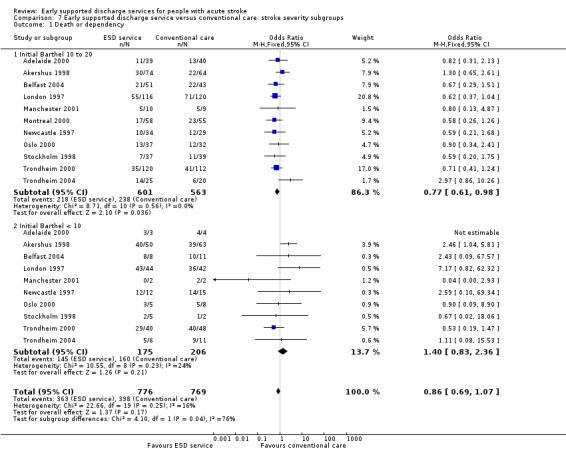
Comparison 7 Early supported discharge service versus conventional care: stroke severity subgroups, Outcome 1 Death or dependency.
7.2. Analysis.
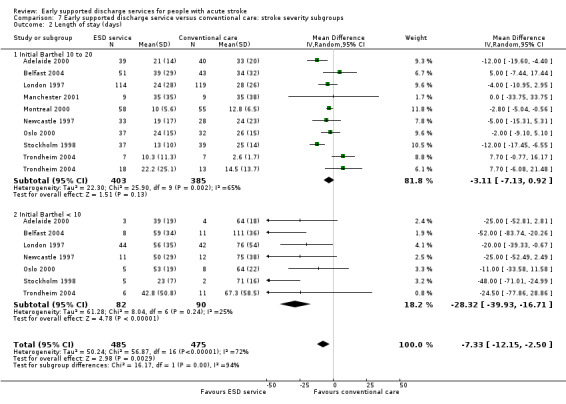
Comparison 7 Early supported discharge service versus conventional care: stroke severity subgroups, Outcome 2 Length of stay (days).
These results suggest that the greatest benefit in clinical outcomes was with the mild and moderate groups but the greatest reduction in hospital bed days was with the severe subgroup.
Analyses by carer availability
Eleven trials (1341 participants) could provide subgroup data on the availability of a carer. There was no apparent interaction of ESD service effect with the presence of a carer (Analysis 8.1; Analysis 8.2).
8.1. Analysis.
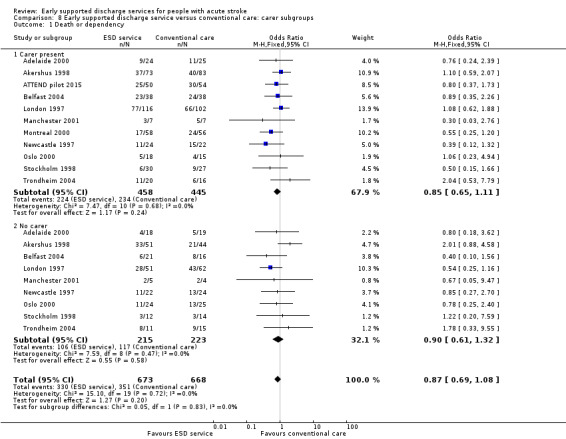
Comparison 8 Early supported discharge service versus conventional care: carer subgroups, Outcome 1 Death or dependency.
8.2. Analysis.
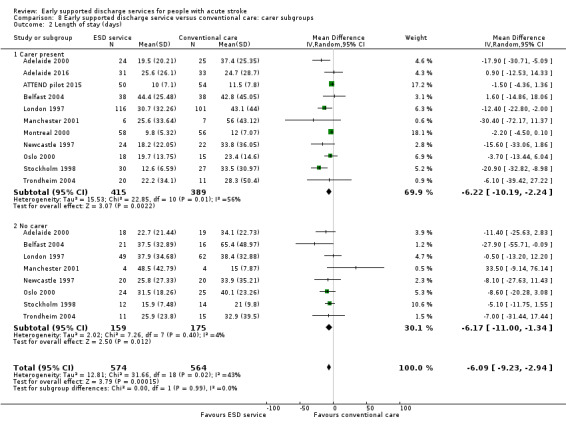
Comparison 8 Early supported discharge service versus conventional care: carer subgroups, Outcome 2 Length of stay (days).
Analyses by control service organisation
Subgroup analyses were carried out according to the background (control) service available; stroke unit or other ward. There were no apparent interactions with control service characteristics (Analysis 9.1; Analysis 9.2).
9.1. Analysis.
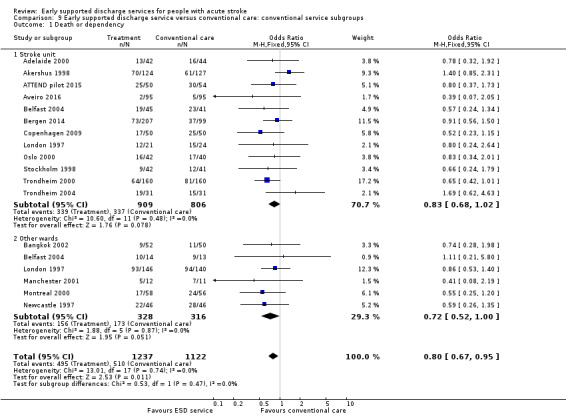
Comparison 9 Early supported discharge service versus conventional care: conventional service subgroups, Outcome 1 Death or dependency.
9.2. Analysis.
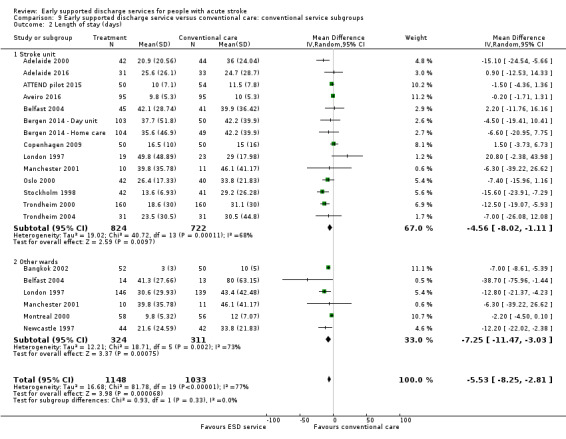
Comparison 9 Early supported discharge service versus conventional care: conventional service subgroups, Outcome 2 Length of stay (days).
Analyses by ESD service organisation
There was no significant interaction with the background service (stroke unit or other ward) or the base for the ESD team (community in‐reach or hospital out‐reach). The reduction in length of hospital stay was slightly greater in the hospital out‐reach group (MD 5 days, 95% CI 1 to 9) than the community in‐reach group (MD 4 days, 95% CI 1 to 7) but this was not statistically significant (P = 0.74) (Analysis 10.1; Analysis 10.2; Analysis 11.3; Analysis 11.4; Analysis 9.1; Analysis 9.2).
10.1. Analysis.
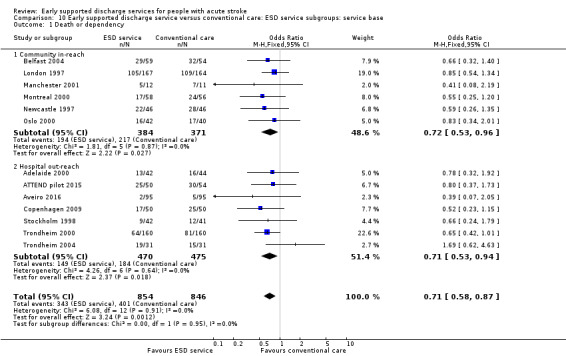
Comparison 10 Early supported discharge service versus conventional care: ESD service subgroups: service base, Outcome 1 Death or dependency.
10.2. Analysis.
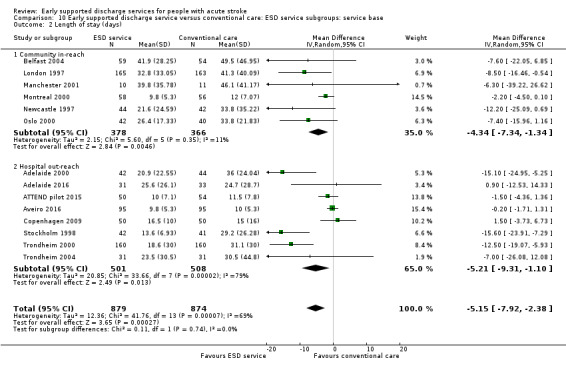
Comparison 10 Early supported discharge service versus conventional care: ESD service subgroups: service base, Outcome 2 Length of stay (days).
11.3. Analysis.
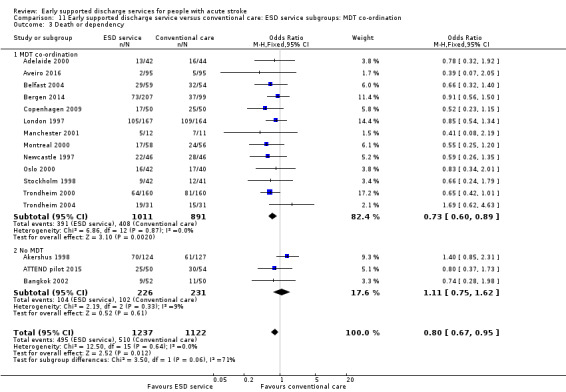
Comparison 11 Early supported discharge service versus conventional care: ESD service subgroups: MDT co‐ordination, Outcome 3 Death or dependency.
11.4. Analysis.
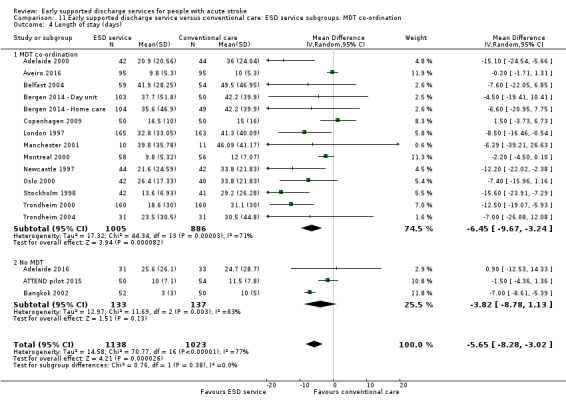
Comparison 11 Early supported discharge service versus conventional care: ESD service subgroups: MDT co‐ordination, Outcome 4 Length of stay (days).
The ESD services studied were classified according to the organisation of the multidisciplinary team (see Description of studies). Using this classification, there was a potential subgroup interaction (P = 0.06) by ESD characteristics. The trials with a co‐ordinated multidisciplinary ESD team (Analysis 11.3) showed an odds of death or dependency of OR 0.73 (95% CI 0.60 to 0.89) compared with OR 1.11 (95% CI 0.75 to 1.62) in those without an ESD team.
The staffing levels of each service did not differ sufficiently to allow meaningful subgroup analyses based on staff mix, service intensity, and supportive versus rehabilitative interventions.
Analysis of 'core' ESD services
Some commentators have criticised the original inclusion of trials that did not incorporate a robust multidisciplinary rehabilitation programme in the community (Akershus 1998; Adelaide 2016; ATTEND pilot 2015; Bangkok 2002). The remaining 13 trials are much more typical of what has become accepted as a 'core' ESD service (Fisher 2011). If the analyses are restricted to those 13 trials the results are more convincing for ESD services: death (OR 0.78, 95% CI 0.54 to 1.11; P = 0.17; Analysis 11.1), death or institutional care (OR 0.65, 95% CI 0.49 to 0.87; P = 0.003; Analysis 11.2), death or dependency (OR 0.73, 95% CI 0.60 to 0.89; P = 0.002; Analysis 11.3) and reduction in length of stay (MD 6 days; 95% CI 3 to 9; P < 0.0001; Analysis 11.4).
11.1. Analysis.
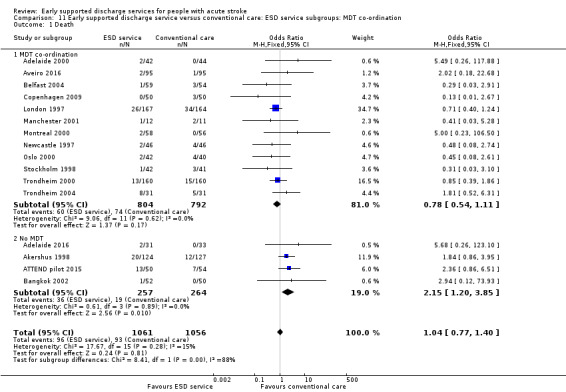
Comparison 11 Early supported discharge service versus conventional care: ESD service subgroups: MDT co‐ordination, Outcome 1 Death.
11.2. Analysis.
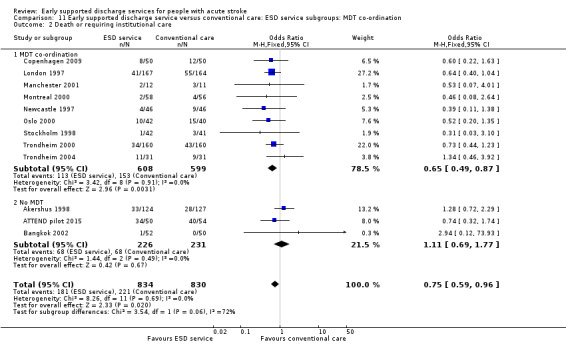
Comparison 11 Early supported discharge service versus conventional care: ESD service subgroups: MDT co‐ordination, Outcome 2 Death or requiring institutional care.
Discussion
Summary of main results
It is clear from this analysis of the randomised trials that services aiming to accelerate discharge from hospital can bring about a reduction in the length of hospital stay and that this reduction can be substantial. This updated analysis demonstrates that patients receiving ESD services were more likely to be independent and living at home six months after stroke than those who received conventional services. ESD patients scored better on extended ADL scores and were more likely to express satisfaction with services. Although we have limited information available, we have been unable to confirm earlier concerns about the impact of ESD services on the mood and well‐being of carers (in terms of subjective health score, mood, or satisfaction with services).
Economic analyses were carried out in six trials. Although the underlying costs and assumptions were different for each analysis, all concluded that the opportunity savings from hospital bed days released tended to be greater than, or similar to, the cost of the ESD service. Realising such cost savings in practice can be difficult but ESD services appear to offer one way to manage rising demand for a finite number of hospital beds.
The particular component of an ESD service responsible for the improvement in functional outcome seen remains unclear. Providing rehabilitation in the setting of the patients' own home is thought to be a significant contributing factor. It has also been suggested that patients receiving ESD services overall receive greater input from rehabilitation therapists and for a longer duration than those receiving conventional care. However, any potential increase in rehabilitation input does not appear to affect overall cost‐efficacy of ESD services in economic analyses.
In conclusion, appropriately resourced and co‐ordinated ESD teams can offer a further effective service option for a selected group of people with stroke and should be considered in addition to organised inpatient (stroke unit) care as part of a comprehensive stroke service.
Overall completeness and applicability of evidence
When interpreting the results of this review it is important to remember that the basic question addressed was whether a policy of early hospital discharge with support could be as effective and efficient as conventional care. Therefore, our inclusion criteria were broad and focused on trials that compared two policies of care for stroke patients in hospital: 1) conventional care, that is, the usual hospital care and discharge procedures; and 2) an alternative system of care that aimed to provide an earlier discharge with rehabilitation or support, or both, in a home‐based setting ('early supported discharge': ESD). Within this broad question we had anticipated that a 'core' group of trials would be testing a specialist multidisciplinary ESD team that had been established to provide this form of care to stroke patients. However, we also wished to retain the option of including other trials where a policy of early discharge was tested in other ways. The advantage of this broad approach is that it can allow us to examine both the effectiveness of a reasonably specific co‐ordinated ESD team 'package' of care, and also to explore the broader service factors (both inpatient and outpatient) that may influence patient outcomes. One potential hazard is that it is difficult to conduct such an exercise in a truly a priori and objective manner. The current update has maintained the original review structure.
In developing a clear question to guide this review, we have chosen to focus on the intention of the service intervention and to avoid terms such as 'hospital at home' which may have a different meaning to different people. However, we acknowledge that some services aim to both help avoid hospital admission and accelerate discharge (Wade 1985). We have not excluded any trials from the review solely on the basis of their service having this dual function. We have also focused the review on services for people with stroke. There are several potentially complementary trials that have recruited a mixed geriatric medical patient population. These have been reviewed (Shepperd 2009).
Quality of the evidence
This update identified four new trials (663 participants) and did not alter the main conclusions in comparison with the previous version of the review. While we acknowledge that the total amount of data available is limited (17 trials; 2422 participants), there do appear to be some general conclusions that can be drawn.
Most of the evidence of benefit of ESD services come from trials of a multidisciplinary ESD team whose work is co‐ordinated through regular meetings.
The typical multidisciplinary ESD team comprised physiotherapy, occupational therapy, and speech and language therapy staff with medical, nursing and social work support.
Such services appeared to be effective even in comparison with a standard service based on care in a stroke unit.
Although we could not find evidence that the setting of the service (hospital out‐reach or community in‐reach) influenced outcomes, all the ESD teams reported here had a specialist interest in stroke or rehabilitation, or both.
All trials recruited a selected subgroup (on average 33%) of people with stroke usually living in an urban setting. There is insufficient evidence to draw conclusions on ESD services for those living in a more dispersed rural setting.
Most of the evidence of ESD benefit appears to be for people with moderate disability (initial Barthel index of > 9/20), although the balance of cost and benefit is not clear for this subgroup. For people with more severe disability the substantial saving in bed‐days may well be outweighed by a risk of poorer patient outcomes. We, therefore, cannot exclude the possibility that the clinical benefits enjoyed by the moderate disability subgroup required a net increase in rehabilitation input while the main cost savings (in terms of bed days) came from the severe subgroup.
Although the quality of the evidence in general was good, many of the trials were completed over 10 years ago. In many countries the last decade has seen a significant overhaul of stroke services to enable greater access to hyperacute therapies (e.g. thrombolysis or thrombectomy). However, only a small proportion of people with stroke will be eligible for such therapies, with the great majority continuing to rely on post‐stroke rehabilitation to improve functional outcomes.
The conclusions about the potential benefit of ESD services appear to be robust. The results are strengthened if analyses focus on trials with clearly concealed randomisation, blinded outcome assessment, and near‐complete follow‐up (10 trials; 1318 participants), or on the 'core' group of trials (13 trials; 1902 participants) testing a co‐ordinated ESD team.
Potential biases in the review process
Through a thorough searching process and well‐established personal connections with researchers in this field we are confident that we should have identified all potentially relevant studies. However, for three studies we did not have sufficient information to carry out a preliminary classification according to our inclusion criteria (Edirne 2001; Shi 2014; Tian 2015). We realise the absence of data from these studies in our meta‐analysis may potentially have introduced bias.
As discussed, our inclusion criteria with respect to the service intervention were deliberately broad. We recognise that interpretation of patient and service characteristics raises the potential risk of a post‐hoc explanation of results. However, we tried as far as possible to plan analyses a priori.
A small proportion of patient data was missing for our dichotomous outcomes of death (57 intervention participants; 53 controls), death or institutionalisation (24 intervention participants; 19 controls), and death or dependency (99 intervention participants; 84 controls). In these instances we assumed the participants to be alive and independent. Similarly for continuous outcome data, where standard deviations were not reported they were inferred from the interquartile ranges (IQR) or alternatively estimated as being at least as large as the comparable trials using the same measure (see Measures of treatment effect). Whilst we recognise that this may have introduced potential bias to our results, we believe that including imputed and estimated data were preferable to excluding data from participants or studies.
Finally, the trialists who authored this review were in general the authors of the included trials. However, decisions on trial selection and data extraction were arranged to avoid trialists making decisions about their own trial.
Agreements and disagreements with other studies or reviews
Several systematic reviews have addressed the topic of how to improve the transition of discharge home for patients in hospital. A systematic review of discharge planning strategies for medical patients concluded that a discharge plan tailored to the individual patient may bring about reductions in length of hospital stay and readmission rates (Shepperd 2016). A systematic review of trials of generic (non‐stroke specific) early discharge hospital at home services concluded that such services could speed up discharge home, but commented on the limited evidence available (Shepperd 2009). A more recent stroke‐specific literature review on team co‐ordinated early supported discharge again concluded that this could be an effective approach for a selected patient group (Meyer 2016). None of these reviews have included such a comprehensive group of stroke‐specific trials.
Authors' conclusions
Implications for practice.
Selected stroke patients in hospital who received input from an early supported discharge (ESD) service returned home earlier than those receiving conventional care. They were also more likely to be independent and living at home six months after their stroke and to express satisfaction with the services they received. There were no apparent adverse effects in terms of hospital readmissions or on the subjective health status or mood of patients or carers. The apparent benefits of ESD services are largely derived from trials of services provided by co‐ordinated ESD teams and recruiting participants with less severe disability.
Although clarity around the specific models of ESD is required, the evidence summarised appears to be sufficient to encourage piloting of stroke ESD services as part of a comprehensive system of stroke care. A consensus on key elements of an ESD service has been developed by the original trialists to facilitate successful implementation at a national and international level (Fisher 2011).
Implications for research.
Our conclusions are based on a relatively modest number of trials of which only four have been published in the last decade. More research is required to define the important characteristics of effective ESD services and to define the balance of cost and benefit for different patient and service groups. Contemporary trials would provide data on resource use and functional outcome in an era with greater access to revascularisation therapies. Further research is required to establish if more generic ESD teams (e.g. services for a mixed elderly population) or those which shift tasks to families or support workers will obtain the same results as the stroke‐specific services reported here. The role of ESD services in poorer healthcare settings and in more dispersed rural communities has not really been adequately addressed.
Feedback
Clarification sought, 17 September 2012
Summary
We have two questions relating to forest plot 1.3 (Death or dependency).
For the London study, we cannot work out how the results were derived from the paper by Rudd et al (BMJ 1997). We calculate from Table 3 of the Rudd paper that there are 116 and 125 poor outcomes in the community and conventional arms respectively (by adding the numbers of deaths to Barhel 0‐14 + 15‐19). There were 45 versus 35 good outcomes, and 6 versus 4 unknown outcomes. Whatever you do with the unknowns, we think the result should be more extremely in favour of the treatment than that reported.
For the Newcastle study, the values in the forest plot seem to be the good outcome, instead of the poor outcome, and the treated and control arms have been swapped. The resulting odds ratio is actually correct (as in this case, two wrongs do make a right) but the numbers of events should really be 18/46 and 24/46.
Neither of these things would change the conclusions of the review. I have modified the conflict of interest statement to declare my interests: This issue was found as part of a methodological project funded by the UK MRC.
Contributors
Commenter: Steff Lewis
What's new
| Date | Event | Description |
|---|---|---|
| 10 April 2017 | Amended | The title was revised to 'Early supported discharge services for people with acute stroke' to better reflect the content of the review. |
| 10 April 2017 | New citation required but conclusions have not changed | We have updated the searches and added a new author. The conclusions of the review have not changed since the previous version was published in 2012. |
| 10 April 2017 | New search has been performed | We have restricted the updated analysis to individually‐randomised trials but have retained the original classification of Early Supported Discharge Services (three subgroups) to reflect the variety of trials being published. This updated review included four new trials (recruiting 663 participants) but not a previous cluster‐randomised trial (recruiting 198 participants). The review now incorporates data from 17 trials (recruiting 2422 participants). |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 30 May 2012 | New search has been performed | This updated review identified three new trials (360 patients) and now incorporates an individual patient data meta‐analysis of 14 trials (1957 patients). We have retained the modified classification of Early Supported Discharge Services (into three subgroups) to reflect the variety of trials being published. |
| 6 April 2012 | New citation required but conclusions have not changed | New authors. |
| 16 November 2004 | New search has been performed | This review (2004) incorporates an individual patient data meta‐analysis of 11 trials. This includes new data on more than double the number of patients included in the previous version. We have retained the modified classification of Early Supported Discharge Services (into three subgroups) to reflect the variety of trials being published. |
Acknowledgements
The Early Supported Discharge Trialists group consisted of: Craig Anderson (Auckland), Erik Bautz‐Holter (Oslo), Martin Dennis (Secretariat) Paola Dey (Manchester), Bent Indredavik (Trondheim), Birgitte Jepson (West Denmark), Peter Langhorne (Co‐ordinator), Nancy Mayo (Montreal), Paul Mogensen (West Denmark), Gordon Murray (Statistician), Michael Power (Belfast), Helen Rodgers (Newcastle), Ole Morten Ronning (Akershus), Anthony Rudd (London), Silvana Santana (Aviero), Nijasri Suwanwela (Bangkok), Gillian Taylor (Statistician), Lotta Widen‐Holmqvist (Stockholm) and Charles Wolfe (London).
More recent trial contacts have been: Maayken van den Berg (Adelaide 2016), Jeyaraj Pandian (ATTEND pilot), Hakon Hofstad (Bergen).
We are grateful to Ken Fullerton (Belfast), Sally Rubenach (Adelaide), and Jean Douglas (Administrator) who contributed to earlier versions of this review.
Appendices
Appendix 1. CENTRAL search strategy
IDSearchHits #1[mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "intracranial arterial diseases"] or [mh "intracranial arteriovenous malformations"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"stroke, lacunar"] or [mh ^"vasospasm, intracranial"] or [mh ^"vertebral artery dissection"] or [mh ^"brain injuries"] or [mh ^"brain injury, chronic"] #2(stroke or poststroke or post‐stroke or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH):ti,ab,kw (Word variations have been searched) #3((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab,kw (Word variations have been searched) #4((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab,kw (Word variations have been searched) #5[mh ^hemiplegia] or [mh paresis] #6{or #1‐#4} #7[mh ^"patient discharge"] #8[mh ^"progressive patient care"] #9[mh ^"home care services"] or [mh ^"home care services, hospital‐based"] or [mh ^"home nursing"] #10(early supported discharge or ESD):ti,ab,kw (Word variations have been searched) #11((early or earlier or prompt or accelerate* or acute or subacute or supported) near/5 discharg*):ti,ab,kw (Word variations have been searched) #12(reduce* near/5 (duration or length) near/5 (stay or hospital)):ti,ab,kw (Word variations have been searched) #13(reduce* near/5 (hospital or inpatient or in‐patient) near/5 (stay or care)):ti,ab,kw (Word variations have been searched) #14short‐term ward:ti,ab,kw (Word variations have been searched) #15((organi?ed or multidisciplinary) near/5 discharge near/5 team*):ti,ab,kw (Word variations have been searched) #16((early or earlier or prompt or accelerate* or supported) near/5 return* near/2 home*):ti,ab,kw (Word variations have been searched) #17(hospital* near/3 home*):ti,ab,kw (Word variations have been searched) #18hospital rehabilitation unit*:ti,ab,kw (Word variations have been searched) #19(rehabilitation near/3 home*):ti,ab,kw (Word variations have been searched) #20(intensive near/2 home near/5 (rehabilitation or support*)):ti,ab,kw (Word variations have been searched) #21(mobile near/2 team*):ti,ab,kw (Word variations have been searched) #22((extended stroke unit near/3 (service* or care)) or ESUS):ti,ab,kw (Word variations have been searched) #23((post‐discharge or home rehabilitation) near/5 (support* or care)):ti,ab,kw (Word variations have been searched) #24((early or earlier or acute or subacute or post‐discharge) near/5 (community or domiciliary or primary care or home or home‐based) near/5 (rehabilitation or support* or care)):ti,ab,kw (Word variations have been searched) #25{or #7‐#24} #26#6 and #24
Appendix 2. MEDLINE search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or stroke, lacunar/ or exp brain infarction/ or exp vertebral artery dissection/ 2. (stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$).tw. 3. ((brain$ or cerebr$ or cerebell$ or vertebrobasilar or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. 1 or 2 or 3 or 4 6. Patient Discharge/ 7. Progressive Patient Care/ 8. home care services/ or home care services, hospital‐based/ or home nursing/ 9. (early supported discharge or ESD).tw. 10. ((early or earlier or prompt or accelerate$ or acute or subacute or supported) adj5 discharg$).tw. 11. (reduce$ adj5 (duration or length) adj5 (stay or hospital)).tw. 12. (reduce$ adj5 (hospital or inpatient or in‐patient) adj5 (stay or care)).tw. 13. short‐term ward.tw. 14. ((organi?ed or multidisciplinary) adj5 discharge adj5 team$).tw. 15. ((early or earlier or prompt or accelerate$ or supported) adj5 return$ adj2 home$).tw. 16. (hospital$ adj3 home$).tw. 17. hospital rehabilitation unit$.tw. 18. (rehabilitation adj3 home$).tw. 19. (intensive adj2 home adj5 (rehabilitation or support$)).tw. 20. (mobile adj2 team$).tw. 21. organi?ed home care.tw. 22. ((extended stroke unit adj3 (service$ or care)) or ESUS).tw. 23. ((post‐discharge or home rehabilitation) adj5 (support$ or care)).tw. 24. ((early or earlier or acute or subacute or post‐discharge) adj5 (community or domiciliary or primary care or home or home‐based) adj5 (rehabilitation or support$ or care)).tw. 25. or/6‐24 26. 5 and 25 27. Randomized Controlled Trials as Topic/ 28. random allocation/ 29. Controlled Clinical Trials as Topic/ 30. control groups/ 31. clinical trials as topic/ 32. double‐blind method/ 33. single‐blind method/ 34. Research Design/ 35. Program Evaluation/ 36. randomised controlled trial.pt. 37. controlled clinical trial.pt. 38. clinical trial.pt. 39. random$.tw. 40. (controlled adj5 (trial$ or stud$)).tw. 41. (clinical$ adj5 trial$).tw. 42. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 43. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 44. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 45. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 46. (assign$ or allocat$).tw. 47. controls.tw. 48. trial.ti. 49. or/27‐48
Appendix 3. Embase search strategy
1. cerebrovascular disease/ or basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke/ 2. stroke patient/ or stroke unit/ 3. (stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$).tw. 4. ((brain$ or cerebr$ or cerebell$ or vertebrobasilar or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$)).tw. 5. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 6. 1 or 2 or 3 or 4 or 5 7. hospital discharge/ 8. early supported discharge/ 9. progressive patient care/ 10. home care/ or home physiotherapy/ or home rehabilitation/ 11. home environment/ 12. community based rehabilitation/ 13. (early supported discharge or ESD).tw. 14. ((early or earlier or prompt or accelerate$ or acute or subacute or supported) adj5 discharg$).tw. 15. (reduce$ adj5 (duration or length) adj5 (stay or hospital)).tw. 16. (reduce$ adj5 (hospital or inpatient or in‐patient) adj5 (stay or care)).tw. 17. short‐term ward.tw. 18. ((organi?ed or multidisciplinary) adj5 discharge adj5 team$).tw. 19. ((early or earlier or prompt or accelerate$ or supported) adj5 return$ adj2 home$).tw. 20. (hospital$ adj3 home$).tw. 21. hospital rehabilitation unit$.tw. 22. (rehabilitation adj3 home$).tw. 23. (intensive adj2 home adj5 (rehabilitation or support$)).tw. 24. (mobile adj2 team$).tw. 25. organi?ed home care.tw. 26. ((extended stroke unit adj3 (service$ or care)) or ESUS).tw. 27. ((post‐discharge or home rehabilitation) adj5 (support$ or care)).tw. 28. ((early or earlier or acute or subacute or post‐discharge) adj5 (community or domiciliary or primary care or home or home‐based) adj5 (rehabilitation or support$ or care)).tw. 29. or/7‐28 30. Randomized Controlled Trial/ 31. Randomization/ 32. Controlled Study/ 33. control group/ 34. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/ 35. Double Blind Procedure/ 36. Single Blind Procedure/ or triple blind procedure/ 37. Parallel Design/ 38. random$.tw. 39. (controlled adj5 (trial$ or stud$)).tw. 40. (clinical$ adj5 trial$).tw. 41. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 42. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 43. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 44. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 45. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 46. controls.tw. 47. trial.ti. 48. or/30‐47 49. 6 and 29 and 48.
Appendix 4. CINAHL search strategy
S1(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR ( (MH "Intracranial Embolism and Thrombosis") ) OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") OR (MH "Stroke Patients") OR (MH "Stroke Units") S2TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH) S3TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral) S4TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*) S5S3 AND S4 S6TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) S7TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*) S8S6 AND S7 S9S1 OR S2 OR S5 OR S8 S10(MH "Progressive Patient Care") OR (MH "Patient Discharge+") OR (MH "Multidisciplinary Care Team") S11(MH "Home Health Care") OR (MH "Home Rehabilitation+") OR (MH "Home Nursing") S12( TI ( (early or earlier or prompt or accelerate* or acute or subacute or supported) ) AND TI discharge* ) OR ( AB ( (early or earlier or prompt or accelerate* or acute or subacute or supported) ) AND AB discharge* ) S13( TI reduce* AND TI ( (duration or length) ) AND TI ( (stay or hospital) ) ) OR ( AB reduce* AND AB ( (duration or length) ) AND AB ( (stay or hospital) ) ) S14( TI reduc* AND TI ( (hospital or inpatient or in‐patient) ) AND TI ( (stay or care) ) ) OR ( AB reduc* AND AB ( (hospital or inpatient or in‐patient) ) AND AB ( (stay or care) ) ) S15TI short‐term ward OR AB short‐term ward S16TI ( (organi?ed or multidisciplinary) ) AND TI discharge AND TI team* S17( TI ( (organi?ed or multidisciplinary) ) AND TI discharge AND TI team* ) OR ( AB ( (organi?ed or multidisciplinary) ) AND AB discharge AND AB team* ) S18( TI ( (early or earlier or prompt or accelerate* or supported) ) AND TI return* AND TI home* ) OR ( AB ( (early or earlier or prompt or accelerate* or supported) ) AND AB return* AND AB home* ) S19TI ( (hospital* AND home*) ) OR AB ( (hospital* AND home*) ) S20TI hospital rehabilitation unit* OR AB hospital rehabilitation unit* S21TI ( (rehabilitation AND home*) ) OR AB ( (rehabilitation AND home*) ) S22( TI intensive AND TI home AND TI ( (rehabilitation or support*) ) ) OR ( AB intensive AND AB home AND AB ( (rehabilitation or support*) ) ) S23TI ( (mobile AND team*) ) OR AB ( (mobile AND team*) ) S24TI organi?ed home care OR AB organi?ed home care S25( TI extended stroke unit AND TI ( ((service* or care) or ESUS) ) ) OR ( AB extended stroke unit AND AB ( ((service* or care) or ESUS) ) ) S26( TI ( (post‐discharge or home rehabilitation) ) AND TI ( (support* or care) ) ) OR ( AB ( (post‐discharge or home rehabilitation) ) AND AB ( (support* or care) ) ) S27( TI ( (early or earlier or acute or subacute or post‐discharge) ) AND TI ( (community or domiciliary or primary care or home or home‐based) ) AND TI ( (rehabilitation or support* or care) ) ) OR ( AB ( (early or earlier or acute or subacute or post‐discharge) ) AND AB ( (community or domiciliary or primary care or home or home‐based) ) AND AB ( (rehabilitation or support* or care) ) ) S28S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 S29(MH "Randomized Controlled Trials") or (MH "Random Assignment") or (MH "Random Sample+") S30(MH "Clinical Trials") or (MH "Intervention Trials") or (MH "Therapeutic Trials") S31(MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies") S32(MH "Control (Research)") or (MH "Control Group") or (MH "Placebos") or (MH "Placebo Effect") S33(MH "Crossover Design") OR (MH "Quasi‐Experimental Studies") S34PT (clinical trial or randomized controlled trial) S35TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs) S36TI (controlled N5 (trial* or stud*)) or AB (controlled N5 (trial* or stud*)) S37TI (clinical* N5 trial*) or AB (clinical* N5 trial*) S38TI ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) or AB ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) S39((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) or AB ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) S40TI ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) or AB ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) S41TI (cross‐over or cross over or crossover) or AB (cross‐over or cross over or crossover) S42TI (placebo* or sham) or AB (placebo* or sham) S43TI trial S44TI (assign* or allocat*) or AB (assign* or allocat*) S45TI controls or AB controls S46TI (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) or AB (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) S47S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 S48S9 AND S28 AND S47
Appendix 5. Search strategy for trials registers
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov), World Health Organization (WHO) International Clinical Trials Registry Platform and additional trials registry search strategy "stroke" or "discharge".
Data and analyses
Comparison 1. Early supported discharge service versus conventional care: patient outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 16 | 2116 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.40] |
| 1.1 ESD team co‐ordination and delivery | 9 | 1132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.45, 1.09] |
| 1.2 ESD team co‐ordination | 3 | 464 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.52, 1.74] |
| 1.3 No ESD team | 4 | 520 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.19, 3.85] |
| 2 Death or requiring institutional care | 12 | 1664 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.96] |
| 2.1 ESD team co‐ordination and delivery | 6 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.40, 0.85] |
| 2.2 ESD team co‐ordination | 3 | 464 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.14] |
| 2.3 No ESD team | 3 | 457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.69, 1.77] |
| 3 Death or dependency | 16 | 2359 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.67, 0.95] |
| 3.1 ESD team co‐ordination and delivery | 9 | 1132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.52, 0.87] |
| 3.2 ESD team co‐ordination | 4 | 770 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.10] |
| 3.3 No ESD team | 3 | 457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.75, 1.62] |
| 4 Activities of daily living (Barthel ADL) score | 13 | 1449 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.07, 0.13] |
| 4.1 ESD team co‐ordination and delivery | 7 | 799 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.08, 0.20] |
| 4.2 ESD team co‐ordination | 3 | 261 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.31, 0.22] |
| 4.3 No ESD team | 3 | 389 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.18, 0.22] |
| 5 Extended activities of daily living (EADL) score | 11 | 1262 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [0.03, 0.25] |
| 5.1 ESD team co‐ordination and delivery | 8 | 876 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.04, 0.30] |
| 5.2 ESD team co‐ordination | 2 | 322 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.15, 0.29] |
| 5.3 No ESD team | 1 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.33, 0.65] |
| 6 Subjective health status | 11 | 1202 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.10] |
| 6.1 ESD team co‐ordination and delivery | 7 | 685 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.27, 0.03] |
| 6.2 ESD team co‐ordination | 3 | 370 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.07, 0.34] |
| 6.3 No ESD team | 1 | 147 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.19, 0.47] |
| 7 Mood status | 9 | 915 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.19, 0.07] |
| 7.1 ESD team co‐ordination and delivery | 5 | 383 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.22, 0.18] |
| 7.2 ESD team co‐ordination | 2 | 321 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.30, 0.14] |
| 7.3 No ESD team | 2 | 211 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.37, 0.18] |
| 8 Satisfaction with services | 5 | 513 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.08, 2.38] |
| 8.1 ESD team co‐ordination and delivery | 4 | 450 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.13, 2.67] |
| 8.2 ESD team co‐ordination | 1 | 63 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.36, 2.83] |
| 8.3 No ESD team | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Early supported discharge service versus conventional care: duration of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency: within 6 months | 10 | 1385 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.56, 0.87] |
| 2 Death or dependency: at 6 to 12 months | 7 | 1183 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.05] |
| 3 Death or dependency: within 5 years | 2 | 403 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.17] |
Comparison 3. Early supported discharge service versus conventional care: carer outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective health status | 9 | 813 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.19, 0.08] |
| 1.1 ESD team co‐ordination and delivery | 5 | 373 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.35, 0.06] |
| 1.2 ESD team co‐ordination | 3 | 376 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.12, 0.29] |
| 1.3 No ESD team | 1 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.88, 0.11] |
| 2 Mood status | 3 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.92, 0.88] |
| 2.1 ESD team co‐ordination and delivery | 2 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.60, 1.22] |
| 2.2 ESD team co‐ordination | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 No ESD team | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.17, 0.82] |
| 3 Satisfaction with services | 4 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.87, 2.81] |
| 3.1 ESD team co‐ordination and delivery | 3 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.85, 3.01] |
| 3.2 ESD team co‐ordination | 1 | 33 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.24, 6.70] |
| 3.3 No ESD team | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Early supported discharge service versus conventional care: resource use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of initial hospital stay (days) | 17 | 2161 | Mean Difference (IV, Random, 95% CI) | ‐5.54 [‐8.18, ‐2.91] |
| 1.1 ESD team co‐ordination and delivery | 9 | 1121 | Mean Difference (IV, Random, 95% CI) | ‐5.25 [‐8.81, ‐1.69] |
| 1.2 ESD team co‐ordination | 5 | 770 | Mean Difference (IV, Random, 95% CI) | ‐9.45 [‐13.97, ‐4.92] |
| 1.3 No ESD team | 3 | 270 | Mean Difference (IV, Random, 95% CI) | ‐3.83 [‐8.79, 1.13] |
| 2 Readmission to hospital | 7 | 784 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.79, 1.51] |
| 2.1 ESD team co‐ordination and delivery | 6 | 720 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.55] |
| 2.2 ESD team co‐ordination | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 No ESD team | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.29, 2.90] |
Comparison 5. Early supported discharge service versus conventional care: age subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency | 9 | 1175 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.08] |
| 1.1 Age < 75 years | 9 | 695 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.12] |
| 1.2 Age > 75 years | 9 | 480 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.61, 1.31] |
| 2 Length of stay (days) | 8 | 911 | Mean Difference (IV, Random, 95% CI) | ‐9.69 [‐13.56, ‐5.82] |
| 2.1 Age < 75 years | 8 | 566 | Mean Difference (IV, Random, 95% CI) | ‐11.68 [‐18.00, ‐5.36] |
| 2.2 Age > 75 years | 7 | 345 | Mean Difference (IV, Random, 95% CI) | ‐6.26 [‐10.51, ‐2.01] |
Comparison 6. Early supported discharge service versus conventional care: gender subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency | 9 | 1175 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.65, 1.05] |
| 1.1 Men | 9 | 654 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.54, 1.01] |
| 1.2 Women | 9 | 521 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.68, 1.40] |
| 2 Length of stay (days) | 8 | 909 | Mean Difference (IV, Fixed, 95% CI) | ‐4.54 [‐6.48, ‐2.60] |
| 2.1 Men | 8 | 518 | Mean Difference (IV, Fixed, 95% CI) | ‐4.32 [‐6.65, ‐1.98] |
| 2.2 Women | 7 | 391 | Mean Difference (IV, Fixed, 95% CI) | ‐5.05 [‐8.55, ‐1.55] |
Comparison 7. Early supported discharge service versus conventional care: stroke severity subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency | 11 | 1545 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.07] |
| 1.1 Initial Barthel 10 to 20 | 11 | 1164 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.98] |
| 1.2 Initial Barthel < 10 | 10 | 381 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.83, 2.36] |
| 2 Length of stay (days) | 9 | 960 | Mean Difference (IV, Random, 95% CI) | ‐7.33 [‐12.15, ‐2.50] |
| 2.1 Initial Barthel 10 to 20 | 9 | 788 | Mean Difference (IV, Random, 95% CI) | ‐3.11 [‐7.13, 0.92] |
| 2.2 Initial Barthel < 10 | 7 | 172 | Mean Difference (IV, Random, 95% CI) | ‐28.32 [‐39.93, ‐16.71] |
Comparison 8. Early supported discharge service versus conventional care: carer subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency | 11 | 1341 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.69, 1.08] |
| 1.1 Carer present | 11 | 903 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.65, 1.11] |
| 1.2 No carer | 9 | 438 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.61, 1.32] |
| 2 Length of stay (days) | 11 | 1138 | Mean Difference (IV, Random, 95% CI) | ‐6.09 [‐9.23, ‐2.94] |
| 2.1 Carer present | 11 | 804 | Mean Difference (IV, Random, 95% CI) | ‐6.22 [‐10.19, ‐2.24] |
| 2.2 No carer | 8 | 334 | Mean Difference (IV, Random, 95% CI) | ‐6.17 [‐9.00, ‐1.34] |
Comparison 9. Early supported discharge service versus conventional care: conventional service subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency | 16 | 2359 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.67, 0.95] |
| 1.1 Stroke unit | 12 | 1715 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.68, 1.02] |
| 1.2 Other wards | 6 | 644 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.52, 1.00] |
| 2 Length of stay (days) | 17 | 2181 | Mean Difference (IV, Random, 95% CI) | ‐5.53 [‐8.25, ‐2.81] |
| 2.1 Stroke unit | 14 | 1546 | Mean Difference (IV, Random, 95% CI) | ‐4.56 [‐8.02, ‐1.11] |
| 2.2 Other wards | 6 | 635 | Mean Difference (IV, Random, 95% CI) | ‐7.25 [‐11.47, ‐3.03] |
Comparison 10. Early supported discharge service versus conventional care: ESD service subgroups: service base.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency | 13 | 1700 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.58, 0.87] |
| 1.1 Community in‐reach | 6 | 755 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.96] |
| 1.2 Hospital out‐reach | 7 | 945 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.53, 0.94] |
| 2 Length of stay (days) | 14 | 1753 | Mean Difference (IV, Random, 95% CI) | ‐5.15 [‐7.92, ‐2.38] |
| 2.1 Community in‐reach | 6 | 744 | Mean Difference (IV, Random, 95% CI) | ‐4.34 [‐7.34, ‐1.34] |
| 2.2 Hospital out‐reach | 8 | 1009 | Mean Difference (IV, Random, 95% CI) | ‐5.21 [‐9.31, ‐1.10] |
Comparison 11. Early supported discharge service versus conventional care: ESD service subgroups: MDT co‐ordination.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 16 | 2117 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.40] |
| 1.1 MDT co‐ordination | 12 | 1596 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.11] |
| 1.2 No MDT | 4 | 521 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.20, 3.85] |
| 2 Death or requiring institutional care | 12 | 1664 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.96] |
| 2.1 MDT co‐ordination | 9 | 1207 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.49, 0.87] |
| 2.2 No MDT | 3 | 457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.69, 1.77] |
| 3 Death or dependency | 16 | 2359 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.67, 0.95] |
| 3.1 MDT co‐ordination | 13 | 1902 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.60, 0.89] |
| 3.2 No MDT | 3 | 457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.75, 1.62] |
| 4 Length of stay (days) | 17 | 2161 | Mean Difference (IV, Random, 95% CI) | ‐5.65 [‐8.28, ‐3.02] |
| 4.1 MDT co‐ordination | 14 | 1891 | Mean Difference (IV, Random, 95% CI) | ‐6.45 [‐9.67, ‐3.24] |
| 4.2 No MDT | 3 | 270 | Mean Difference (IV, Random, 95% CI) | ‐3.82 [‐8.78, 1.13] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adelaide 2000.
| Methods | RCT Randomisation using opaque sealed envelopes Independent (single‐blind) follow‐up | |
| Participants | 86 patients recruited from city hospital Inclusion criteria: clinical diagnosis of stroke in previous 6 months, requiring rehabilitation, needing light/moderate assistance with transfers, medically stable, living at a local address with adequate community support Characteristics: mean age 72 years (SD 11), median BI 85/100 (IQR 80 to 95). Trial included 86/398 (22%) of stroke patients admitted to hospital | |
| Interventions | Intervention: multidisciplinary community rehabilitation team, comprising medical, physiotherapy, occupational therapy, speech and language therapy and social work input. Combination of hospital out‐reach and community in‐reach services. Input initially intensive and then tapered off to stop when rehabilitation goals were met. Team had specialist interest in rehabilitation and their activities were co‐ordinated through weekly multidisciplinary meetings. Team co‐ordinated and delivered care
Control: these patients received conventional rehabilitation in a neurological rehabilitation unit with specialist interests in stroke and neurological disability. Controls received multidisciplinary care co‐ordinated through weekly meetings For both groups, discharge was frequently planned with pre‐discharge home visits |
|
| Outcomes | Outcomes recorded at 6 months: death, place of residence, dependency (modified BI, Adelaide Activities Profile), subjective health status (SF36), carer subjective health status (SF36, GHQ 28), patient and carer views (McMaster Family Assessment of recovery) | |
| Notes | Intervention focused on patient's own identified goals and received longer contact with the ESD therapy team | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "... contact by telephone for the allocation sequence which was computer generated" |
| Allocation concealment (selection bias) | Low risk | Quote "opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "independent of ... unaware of treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
Adelaide 2016.
| Methods | Proof‐of‐concept pragmatic pilot RCT | |
| Participants | 63 hospitalised stroke patients (and their carers) from 2 hospitals and a rehabilitation unit in metropolitan Adelaide, Australia (July 2013 to June 2014) Clinical diagnosis of stroke with mobility problems and MMSE > 18 Early in rehabilitation (1 day to 3 months). Actual recruitment was at approximately 16 days (range 4 to 43) post stroke Median age 68 years (range 19 to 94) years, 40 (63%) men Baseline BI 61/100 On average about 63/473 (13%) of surviving acute stroke patients were eligible |
|
| Interventions | An 8‐week programme of CME commenced in hospital combined with tele‐rehabilitation services compared with usual care Intervention: 8‐week caregiver‐mediated training programme with support using a customised exercise app loaded onto a tablet. In hospital, the patient and carer were provided with an iPad which was loaded with the CME application containing 37 standardised exercises aimed to improve gait and mobility (standing, turning, transfers). The patient and caregiver were asked to perform a selected set of exercises for 8 weeks (at least 5 times a week for 30 minutes) and had a weekly evaluation session with the physiotherapist. The programme continued at home with ongoing use of the exercise app, tele‐rehabilitation services provided a secure videoconferencing app to provide access to the treating therapists, and weekly home visits. Intervention group participants also wore a Fitbit activity monitor as a motivational tool. The decision to discharge patients home was made at the twice‐weekly multidisciplinary case conferences attended by medical, nursing, and allied health staff and made on the basis of clinical and psychosocial factors. Research clinicians did not attend these meetings By the 12‐week assessment 4/31 (13%) participants had withdrawn from the intervention but not from follow‐up Control: participants allocated to usual care received interdisciplinary rehabilitation following the standards outlined by the Australian clinical guidelines (which addressed mobility impairment, dysphagia or communication difficulties, upper limb activity, sensorimotor impairment, ADL, cognition) |
|
| Outcomes | Primary outcome was the Stroke Impact Scale mobility domain Secondary outcomes included length of stay, other Stroke Impact Scale domains, readmissions, motor impairment, strength, walking ability, balance, mobility, (extended) ADL, psychosocial functioning, self‐efficacy, quality of life, and fatigue. Additionally, caregiver's self‐reported fatigue, symptoms of anxiety, self‐efficacy, and strain were assessed |
|
| Notes | Proof‐of‐concept trial. Assessments were completed at baseline and at 8 and 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician external to the study generated the random sequence in random blocks of 2 to 6 using a computer software program |
| Allocation concealment (selection bias) | Low risk | A statistician external to the study generated the random sequence and created sequentially numbered, sealed opaque envelopes containing group allocation for participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and treating physiotherapists could not be masked to intervention group allocation. Physiotherapists who delivered usual care did not provide the CME training programme, and physiotherapists who delivered the CME training programme did not provide usual care to participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were reassessed at 8 and 12 weeks by an independent assessor blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 of 63 (5%) withdrew before randomisation |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
Akershus 1998.
| Methods | RCT (exact methods unclear) Independent (single‐blind) follow‐up | |
| Participants | 251 patients recruited from city hospital Inclusion criteria: clinical definition of stroke, age greater than or equal to 60 years of age, SSS 12 to 52, conscious and able to co‐operate with rehabilitation, living at private address Characteristics: mean age 75 (SD 6) years. Initial BI a median of 50/100 (IQR 30 to 70). A total of 238/550 (43%) of the patients screened were recruited | |
| Interventions | Intervention: community rehabilitation provided by a variety of municipality‐based rehabilitation services (41% admitted to nursing homes for rehabilitation, 25% received ambulatory physiotherapy, 4% speech therapy, 30% no treatment). Community rehabilitation services did not specialise in stroke and were not consistently co‐ordinated through regular multidisciplinary team meetings. Medical input from primary care physician with variable degree of nursing input Control: control patients received conventional inpatient rehabilitation in a 6‐bed bay of a rehabilitation unit. This comprised multidisciplinary rehabilitation provided by staff with a specialist interest in stroke rehabilitation and co‐ordinated through weekly team meetings | |
| Outcomes | Outcomes recorded at 7 months: death, place of residence, impairment (SSS), dependency (BI: in current analysis dependency = BI < 15/20), subjective health status (SF36), resource use (length of stay) | |
| Notes | This trial was set up as an evaluation of the stroke rehabilitation ward with municipality services acting as controls 7 intervention and 12 control patients could not be contacted at 7 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "patients were given a random number ... a person not involved in the study drew numbers for allocation" However, if the rehabilitation ward was full, patients randomised to this 'intervention' were assigned the control i.e. rehabilitation in the municipality (13 patients) |
| Allocation concealment (selection bias) | Unclear risk | "A person not involved in the study drew numbers for allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Who was unaware of where the patients had been treated" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
ATTEND pilot 2015.
| Methods | Prospective, randomised, open‐label, blinded outcome assessor, controlled trial design | |
| Participants | Stroke patients admitted to the Stroke Unit of the Department of Neurology at Christian Medical College and Hospital in Ludhiana, India Patients were > 18 years with residual disability (defined as requiring help from another person for everyday activities) and within 1 month of a clinically definite acute stroke (cerebral infarct or intracerebral haemorrhage). Low probability of death in the next 6 months and able to identify a suitable family‐nominated caregiver for training and subsequent delivery of care Recruited patients were: 60 (SD 13) years, 61 (59%) men, baseline NIHSS 7.8, baseline BI 48/100 On average 104/379 (27%) acute stroke patients were eligible |
|
| Interventions | This pilot study was to determine the feasibility of a multicentre, randomised, controlled trial in India of a family‐led, trained caregiver‐delivered, home‐based rehabilitation intervention vs routine care Intervention: these patients had their family‐nominated caregiver trained by a trial physiotherapist, using a structured assessment (cognition, language, function, and mobility) and recommended rehabilitation package. "The evidence‐based intervention package included: 1. information on stroke recovery trajectory, risk, identification and management of low mood, and the importance of repeated practice of task‐specific activities; 2. joint goal setting with patient, nominated family caregiver, and therapist (reviewed with the therapist as patient progresses and new goals set); 3. positioning, transfers, and mobility; 4. task‐orientated training (particularly walking, upper‐limb, and self‐care tasks); and 5. discharge planning The local team developed a culturally appropriate, simple, pictorial 'manual' covering key exercises relevant to ADL. In addition to the manual, training exercises were also chosen from the website http://www.physiotherapyexercises.com or as determined best for the patient by the therapist, all adhering to the intervention package". The caregiver training advised commencing in the hospital for approximately 60 min per day for about 3 days (with the intention of accelerating the patient's hospital discharge when safe). The caregiver would then continue the intervention when the patient was discharged home. The trial therapist could be contacted through telephone for support and guidance over the next 3 months Control: patients were free to access rehabilitation services provided on an in or outpatient basis after discharge from hospital but caregivers were not provided with trial‐specific training |
|
| Outcomes | Outcomes were as follows: Primary clinical outcome was good functional recovery defined by scores 0 to 2 on the mRS at 3 and 6 months Secondary clinical outcomes included: simple validated recovery and dependency questions, World Health Organization Quality of Life – BREF, Nottingham Extended Activities of Daily Living (13), HADS, Caregiver Burden Scale, and EuroQoL (EQ‐5D‐3L) and direct medical costs associated with healthcare utilisation |
|
| Notes | Professor Jeyaraj Pandian, Ludhiana, Punjab, India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized within seven‐days of hospital admission, using random allocation software" |
| Allocation concealment (selection bias) | Low risk | "This list was generated by a biostatistician and conveyed by telephone to the trial physiotherapist." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assessments were done by a psychologist who was blinded to the treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All key patient outcomes reported |
| Selective reporting (reporting bias) | Low risk | 15/104 (14%) missing at follow‐up (6 intervention, 9 control) |
Aveiro 2016.
| Methods | Prospective, randomised, open‐label, blinded‐endpoint trial | |
| Participants | Inclusion criteria: acute stroke patients (World Health Organization definition of stroke), aged 25 to 85 years, FIM no more than 100 who were admitted to the stroke unit with an initial and who gave informed consent Exclusion criteria: SAH, comorbidity, severe aphasia interfering seriously with the stroke rehabilitation, psychological and psychiatric problems or other severe illness interfering seriously with the stroke rehabilitation Actual recruitment: Mean age 67 years (range 35 to 84) Men 101 (53%) Baseline FIM 70 (range 24 to 100) On average 190/571 (33%) of screened acute stroke patients were eligible |
|
| Interventions | The main goal of this study was to adapt an 'early home‐supported discharge (EHSD)' service model to the conditions of Portugal, and then to evaluate the impact of this service Intervention: the intervention started in the stroke unit. The team co‐ordinator at the hospital identified potential patients for the study. After obtaining the informed consent, the patient would be randomised and the case manager contacted to schedule a visit to the patient. Community‐based multidisciplinary team comprising physiotherapist, occupational therapist, gerontologist (case manager), and psychologist ‐ all staff with previous experience in stroke care but no specialised training in stroke rehabilitation stroke care. Team co‐ordinate and deliver care. Team are co‐ordinated via weekly multidisciplinary meetings. The EHSD intervention started in the stroke unit, where the patient and informal caregiver were met by their assigned EHSD case manager. The assigned case manager was 1 of 2 gerontologists who were included to help negotiate the fragmented nature of the Portuguese health and social care systems. Input from the EHSD team of therapists (2 physiotherapists, 2 occupational therapists, and a psychologist) was selected according to the needs of a particular patient. For patients discharged to their homes, the intervention continued directly after discharge to provide a seamless transfer from the hospital to home (individual rehabilitation plan, provision of aids and modifications, providing information and tailored training to the patient and family). Rehabilitation was focused on daily activities valued by the patient. Caregivers were trained and made aware of the ability of the patient and were encouraged to follow their progress. The EHSD team worked with patients to provide approximately 8 home‐based training sessions for a maximum of 1 month. For patients discharged to an inpatient rehabilitation setting, contact with the EHSD team was reinitiated when discharge home was planned. For those patients discharged home while waiting for a place in a rehabilitation unit, the team provided rehabilitation at home to prevent loss of rehabilitation capability Control: patients in the usual care group were contacted in the stroke unit, introduced to the study, and assigned a case manager. They began their rehabilitation as part of standard care in the stroke unit and then accessed the standard rehabilitation available in the region following discharge They received information about services available in the community, but no further specific input was provided |
|
| Outcomes | The primary outcome of the study was independence in physical and cognitive activities as assessed by FIM at 2 and 6 months after randomisation. They proposed that a patient with 3 points in each variable (total score of less than 60) would require inpatient rehabilitation.This threshold value was used to further analyse the data. As it was not set a priori and there was no literature on which to base the decision, results were handled with care Secondary outcome measures included: the Frenchay Activity Index (FAI),the World Health Organization WHOQOLBREF quality of life assessment (WHOQOLBREF), Short Form‐6D, BI, and MMSE. Outcome measures were collected at the patients homes by the case managers. Length of stay at the stroke unit and the convalescence units was obtained from the clinical records |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospective, randomised, open label, blinded‐endpoint trial" |
| Allocation concealment (selection bias) | Low risk | "Allocation of patients to each group was done by taking one folded sheet of paper from a prefilled opaque envelope containing folded sheets of paper with either the letter H or the letter C written inside. This was done by a staff member not involved in the trial." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Outcome measures were collected at the patients homes by the case managers." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 36 (19%) lost to follow‐up (19 EHSD, 17 controls) |
| Selective reporting (reporting bias) | Low risk | All key patient outcomes reported. Recording of rehabilitation activities less complete. |
Bangkok 2002.
| Methods | RCT (exact methods unclear) Unblinded outcome assessments | |
| Participants | 102 acute stroke patients presenting to a city hospital Inclusion criteria: ischaemic stroke within 48 hours of onset; age 18 to 80 years Exclusion criteria: altered consciousness (NIHSS > 20), large infarct, embolic cause; aphasia | |
| Interventions | Intervention: discharge on 4th day to home care programme managed by Red Cross volunteers. Visit on day 3 then alternate day visits for 1 week, then visits on week 2, month 1, 3 and 6. Volunteers trained in stroke, simple rehabilitation and detection of complications. Volunteers reported back to nursing staff Control: managed in neurological or medical department for up to 10 days | |
| Outcomes | Outcomes recorded at 6 months: death, dependency (NIHSS 0 to 2, BI 75 to 100), patient satisfaction | |
| Notes | Same treatment during first 3 days Nadroparin given for 10 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessments were based on data from neurologist or Red Cross volunteer who were aware of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "102 patients were studied" No information is provided on withdrawals or those who did meet inclusion criteria, etc |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes have been reported |
Belfast 2004.
| Methods | RCT Central randomisation system using random number sequence Independent (single‐blind) follow‐up | |
| Participants | 113 hospitalised stroke patients within 3 weeks of onset Exclusion criteria: medically unstable, no rehabilitation needs Characteristics: age 68 (SD 12) years, men 55%, baseline BI 14/20 (SD 4) | |
| Interventions | Intervention: community rehabilitation in‐reach team with specialist interest in rehabilitation. Team consisted of physiotherapy, occupational therapy, speech and language therapy, support staff and medical input. Work was co‐ordinated through weekly team meetings. Planning often included pre‐discharge home visit. Team co‐ordinated and delivered care Control: conventional care comprised medical ward, geriatric medical ward, and stroke unit services. The majority of these patients were managed by a multidisciplinary team with a specialist interest in stroke and rehabilitation, which was co‐ordinated through weekly multidisciplinary team meetings and often included pre‐discharge home visits. Occasional day hospital follow‐up | |
| Outcomes | Outcomes recorded at 6 and 12 months: death, place of residence, dependency (modified Rankin score, Nottingham extended ADL score), subjective health status (SF36, Euroquol), carer health status (caregiver strain), patient and carer preference | |
| Notes | Main difference reported was that the intervention provided continuity of rehabilitation in community setting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomly assigned care options" |
| Allocation concealment (selection bias) | Low risk | "Administered solely by a named secretary. No research team member ... had access to this list" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Research nurses were blind at baseline to the particular group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
Bergen 2014.
| Methods | RCT comparing two different ESD models with treatment as usual | |
| Participants | Patients admitted to the stroke unit (Department of Neurology, Haukeland University Hospital, Bergen) who were living at home in the Municipality of Bergen prior to having a stroke, had a stroke within the previous 7 days, and were admitted to the stroke unit within the previous 5 days. NIHSS score of 2 to 26 of a 13 items Norwegian version (range 0 to 34). Patients with NIHSS score < 2 were included if the mRS score was > 1. The patients had to be awake and able to agree to participation in the study by signing an informed consent, either themselves or by their relatives At recruitment (2008 to 2011) characteristics were: Average 72 years (range 27 to 98), 169 (55%) men, baseline BI 95 (SD 40), baseline NIHSS 3 (SD 4) On average 306/1736 (18%) of screened patients were included |
|
| Interventions | Patients in 2 of the 3 study arms were treated according to the ESD concept. They were followed‐up by a designated multi‐disciplinary ambulatory team consisting of a nurse, a physiotherapist, and an occupational therapist from soon after admission to the stroke unit until shortly after discharge to home. This team originated from the rehabilitation department and served as a co‐ordinating link between the patient, relatives, hospital personnel, and the personnel in primary health care. The team was particularly important in the discharge process and co‐operated closely with the municipal health care in the planning and implementation of further treatment after discharge.
The two ESD arms differed by the location of treatment:
ESD 1 group received their treatment in a community day unit; whereas ESD 2 group patients stayed in their homes with home visits from the community health team Patients in the third study arm constituted a control group and were treated as usual without any intervention from the study, except outpatient appointments for testing. Treatment 'as usual' mainly comprised institutional stay if necessary and/or physiotherapy as needed in the municipality (0 to 2 hours per week). Patients in all 3 study arms received language therapy as needed, regardless of allocated arm The patients in the two ESD arms were discharged to their homes as soon as possible. Patients in need of a longer in‐patient treatment period than offered by the stroke unit were discharged to a municipal institution or rehabilitation department for a period before going home. All patients in the ESD arms were offered rehabilitative treatment by a multi‐disciplinary community health team, consisting of a nurse, a physiotherapist, and an occupational therapist The scheduled treatment period was 5 weeks and maximally 4 hours per day 5 days a week, but many patients did not comply with this |
|
| Outcomes | The primary study outcome was mRS at 6 months Secondary outcomes included mRS at 3 months, NIHSS, Barthel ADL Index, and patient satisfaction (5‐point Likert scale with 1 best) at 3 and 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomised according to a computer generated block randomisation list (six patients in each block; two for each study arm) and consecutively assigned to their groups in the same order as they were included into the study" |
| Allocation concealment (selection bias) | Low risk | "The randomisation list was kept by a study coordinator and was not known to any persons in the stroke unit" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The testers were blinded for study arm and the patients were instructed not to reveal this information" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 77/306 (25%) were not retested at 6 months (22 Day Unit; 22 Home group; 33 control) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes appear to have been reported |
Bergen 2014 ‐ Day unit.
| Methods | Subgroup of Bergen 2014 who received their treatment in a community day unit | |
| Participants | See Bergen 2014 | |
| Interventions | See Bergen 2014 | |
| Outcomes | See Bergen 2014 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Bergen 2014 |
| Allocation concealment (selection bias) | Unclear risk | See Bergen 2014 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | See Bergen 2014 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See Bergen 2014 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See Bergen 2014 |
| Selective reporting (reporting bias) | Unclear risk | See Bergen 2014 |
Bergen 2014 ‐ Home care.
| Methods | Subgroup of Bergen 2014 who stayed in their homes with home‐visits from the community health team | |
| Participants | See Bergen 2014 | |
| Interventions | See Bergen 2014 | |
| Outcomes | See Bergen 2014 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Bergen 2014 |
| Allocation concealment (selection bias) | Unclear risk | See Bergen 2014 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | See Bergen 2014 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See Bergen 2014 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See Bergen 2014 |
| Selective reporting (reporting bias) | Unclear risk | See Bergen 2014 |
Copenhagen 2009.
| Methods | RCT External list generated and managed by external person, blocks of 10 Opaque sealed envelopes |
|
| Participants | 100 patients recruited from stroke unit of 1 university hospital, 1 to 3 days post stroke Inclusion criteria: mRS 0 to 3 pre‐stroke, living at home Median age 81 (range 33 to 98) years, median BI 69 (0 to 100), median SSS 45 (11 to 58) |
|
| Interventions | Hospital out‐reach multidisciplinary team, based within stroke unit. Co‐ordinated and delivered low intensity (1 to 3 times per week) home based rehabilitation for a period of 1 month. All staff were skilled in stroke care and co‐ordinated via weekly multidisciplinary meetings Control: conventional discharge planning from combined acute/rehabilitation stroke unit and conventional after discharge care |
|
| Outcomes | At 90 days: dependency (mRS, BI, MAS, COPM), cognition (CT‐50), quality of life (EQ‐5D) At 150 days: mortality, use of municipal services, hospital contacts, cost, carer satisfaction |
|
| Notes | The published report excluded some mild stroke patients that were included in the original unpublished report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "In blocks of each ten patients" "Sealed envelopes containing a card with the word 'intervention' or 'control'" made by a research centre in the Capital Region of Denmark (Research Centre for Prevention and Health, Department of Planning Health and Quality) |
| Allocation concealment (selection bias) | Low risk | "Consecutively numbered and sealed envelopes containing a card with the word 'intervention' or 'control'" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Blinded investigators were not used in the trial and all tests were performed by members of the multidisciplinary team". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 patients in the intervention group and 3 control patients 'dropped out' prior to discharge and were not included in the final analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes appear to have been reported (unpublished) |
London 1997.
| Methods | RCT Permuted blocks of 10 provided in blank sealed opaque envelopes Final (12‐month assessment) was blinded to treatment allocation | |
| Participants | 331 patients recruited from 2 city hospitals Inclusion criteria: patients were medically stable, lived alone and were able to transfer independently (or could be transferred by a resident carer) Characteristics: mean age 71 years (range 27 to 103). Initial BI 15 to 19/20 in approximately 50% of patients 331 patients randomised out of over 660 screened (approximately 45% of patients were recruited) | |
| Interventions | Intervention: multidisciplinary community therapy team comprising physiotherapy, occupational therapy, speech and language therapy and medical input. The team had a special interest in neurology and stroke and were co‐ordinated through weekly multidisciplinary meetings. The community team liaised with hospital‐based rehabilitation staff and then provided a package of care after discharge. The maximum duration of the intervention was 3 months. Team co‐ordinated and delivered care Control: these patients received conventional care (less than 50% managed in co‐ordinated multidisciplinary stroke units) with conventional discharge planning and post discharge support | |
| Outcomes | Main outcomes recorded at 12 months (additional details at 2, 4 and 6 months): death, place of residence, dependency (BI, Frenchay activities index, Rivermead ADL score; in current analysis dependency = BI < 20/20), subjective health status (Nottingham Health Profile), patient mood (HADS), carer health status (caregiver strain), patient and carer satisfaction, resource use (hospital length of stay, place of residence, number of therapy sessions) | |
| Notes | Important characteristics were believed to be providing a co‐ordinated package of community rehabilitation 5 intervention and 4 control patients lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "permuted blocks of ten with random number tables" |
| Allocation concealment (selection bias) | Low risk | Quote: "blank opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "by a researcher blinded to which arm of the trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across groups (5 patients in intervention group and 4 control patients were lost to follow‐up) with similar reasons for withdrawal and proportionally unlikely to have impact |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
Manchester 2001.
| Methods | RCT of inpatient stroke team and home team Home team arm consists of early discharge trial Stratified randomisation conducted from offsite trials office Blinded outcome assessment | |
| Participants | 23 patients admitted to 2 city hospitals within 7 days of onset of clinical stroke Medically stable Characteristics: mean age 66 (SD 9) years. Men: 18 (77%). Initial BI: 15/20 (SD 6) | |
| Interventions | Intervention: community‐based, nurse‐led, stroke‐specific multidisciplinary team (nursing, physiotherapy, occupational therapy, speech and language therapy). Patients assessed pre‐discharge and allocated up to daily input at home for up to 3 months Control: conventional discharge planning by mobile stroke team or hospital stroke unit | |
| Outcomes | Outcomes at 12 months: death, place of residence, dependency (BI, Nottingham EADL score, Euroquol, Sickness Impact Profile 30, HADS, Carer HADS and caregiver burden scale) | |
| Notes | Trial terminated early after the withdrawal of 1 hospital and difficulty recruiting new staff 2 intervention and 1 control patient lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from protocol only: "The Centre for Cancer Epidemiology Trials Unit will generate the randomisation schedule" |
| Allocation concealment (selection bias) | Low risk | Quote from protocol only: "this schedule will be concealed from clinicians" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from protocol only: "will be concealed from ... therapists undertaking follow‐up assessments" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make a decision as to 'low‐risk' or 'high‐risk' |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a decision as to 'low‐risk' or 'high‐risk' |
Montreal 2000.
| Methods | RCT Telephone randomisation using opaque sealed envelopes held in a central office Single blinding of outcome assessment | |
| Participants | 114 patients recruited from 5 city hospitals Inclusion criteria: clinical diagnosis of stroke in the previous 28 days (mean delay 10 days), moderate disability, living with carer, medically stable Characteristics: mean age 70 (SD 13) years, mean BI 83/100 (SD 14). Trial included 164/1321 (13%) of patients screened | |
| Interventions | Intervention: community rehabilitation team providing intensive home rehabilitation. Team comprised nursing, physiotherapy, occupational therapy, speech therapy and dietitian input. Intervention was co‐ordinated and individualised. Intervention lasted 4 weeks with further care as required. Team co‐ordinated and delivered care Control: conventional care incorporated a variety of inpatient services (owing to health care cutbacks, only 27% of control patients received home care or rehabilitation centre care) | |
| Outcomes | Outcomes recorded at 3 months: death, place of residence, dependency (BI, instrumental ADL), subjective health status (SF36), service costs | |
| Notes | Health service changes during the study resulted in an increase in community services and reduction in inpatient facilities forcing earlier discharges on conventional care patients. As a result, the intervention group received an increased rehabilitation input | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block sizes that varied from 4 to 8 ... in the central office, group assignment was revealed over the telephone" |
| Allocation concealment (selection bias) | Low risk | "Opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Who were not informed about group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes reported |
Newcastle 1997.
| Methods | RCT Zelen randomisation procedure using a computerised randomisation system, accessed by telephone Independent (single‐blind) follow‐up of patients but security of blinding uncertain ITT analysis | |
| Participants | 92 stroke patients recruited from 3 city hospitals Inclusion criteria: within 3 days of stroke, BI 5 to 19, medically stable, living at private address Characteristics: median age 73 (44 to 93) years. Median BI 14/20 (range 2 to 20) at 1 week post‐stroke. 119/402 (30%) of patients screened were recruited | |
| Interventions | Intervention: community in‐reach multidisciplinary rehabilitation team with a specialist interest in stroke and co‐ordinated through weekly multidisciplinary meetings. Medical support by general practitioner and stroke physician. Rehabilitation team contacted patients and carers and carried out assessment of home circumstances prior to discharge. Following discharge, daily therapy and home care could be provided if required. Median duration of input was 9 weeks (range 1 to 44 weeks). Team co‐ordinated and delivered care Control: these patients received conventional hospital care, usually provided in general medical wards (less than half the patients received organised multidisciplinary stroke unit care) | |
| Outcomes | Outcomes recorded at 3, 6 and 12 months after randomisation: death, place of residence, dependency (Rankin score, Nottingham extended ADL; in current analysis dependency = Rankin score > 2, approximately equivalent to a BI < 19/20), subjective health status (COOP charts), mood status (Wakefield depression inventory), carer subjective health status (GHQ 30), patient and carer preferences (qualitative interviews), resource use (length of hospital stay, costing of services) | |
| Notes | Staff felt that continuity of care provided in the home environment were key elements 1 intervention and 3 control patients lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "central computerised randomisation service" |
| Allocation concealment (selection bias) | Low risk | Central allocation. Quote: "central computerised randomisation service" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "blinding to the randomisation group was not possible as it soon became apparent at the discharge interview" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals explained, ITT analysis followed |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome measures reported |
Oslo 2000.
| Methods | RCT Zelen's randomisation method (stratified for urinary incontinence) Concealed allocation Blinded outcome assessment | |
| Participants | 82 stroke patients admitted to an acute stroke unit in a city hospital Inclusion criteria: onset < 6 days, home dwelling, no prior disability, no major co‐morbidity, BI 5 to 19 at 72 hours after the stroke Exclusion criteria: SAH, cognitive or communication problems Characteristics: mean age 78 (SD 9) years, men 45%, baseline BI 14/20 (SD 5) | |
| Interventions | Intervention: multidisciplinary team , experienced in stroke rehabilitation (nurse, physiotherapist, occupational therapist) visited patient in hospital, prepared discharge and co‐ordinated rehabilitation. Rehabilitation at home provided by both the team and community services. Input as long as required Control: acute care and rehabilitation in co‐ordinated multidisciplinary stroke units | |
| Outcomes | Outcomes recorded at 6 months: death, residence, Nottingham extended ADL scale, GHQ, depression, resource use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "block randomised by computer generated numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes ... sequentially opened" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessments performed by a specially trained nurse "... who was neither informed about the intention nor the design or hypothesis of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 patients in the intervention group and 6 control patients were lost to follow‐up by 3 months; ITT analysis followed for all dichotomous variables |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes have been reported |
Stockholm 1998.
| Methods | RCT Opaque sealed envelopes Independent (single‐blind) outcome measurement | |
| Participants | 83 patients recruited from the neurology department of a city hospital Inclusion criteria: cerebral infarct or primary intracerebral haemorrhage, 5 to 7 days post stroke, continent and able to feed, residual impairment, medically stable, intact cognition Characteristics: median age 72 (range 49 to 89) years. Median Lindmark Motor Capacity scale 127/153 (IQR 100 to 138). Trial included 86/220 (38%) of patients screened (approximately 30% of all patients) | |
| Interventions | Intervention: multidisciplinary hospital out‐reach early supported discharge team, with special interest in rehabilitation and co‐ordinated through weekly meetings. This was a therapist‐based service (no nursing input) based in the hospital stroke unit. Pre‐discharge home visit carried out with the patient. Intervention provided on a less than daily basis for 3 to 4 months after discharge. Team co‐ordinated and delivered care Control: these patients received conventional hospital care involving co‐ ordinated multidisciplinary stroke unit care in a hospital stroke unit and conventional discharge procedures | |
| Outcomes | Outcomes measured at 3, 6 and 12 months: death, place of residence, dependency (Katz ADL, BI, Frenchay Activities Index; in the current analysis dependency = BI < 20/20), subjective health status (Sickness impact profile), carer subjective health status (Sickness impact profile), patient and carer satisfaction, resource use (length of stay and service costs) Outcome assessment was repeated again at 5 years ‐ including resource use |
|
| Notes | Team felt that co‐ordinated continuity of care provided at home was the key element 1 intervention and 1 control patient lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "blocks of two or four, ... by a computerized random procedure" |
| Allocation concealment (selection bias) | Low risk | "Sealed numbered envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assessors were blinded with respect to group assignment and were not involved in randomisation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals explained |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported at 1 year |
Trondheim 2000.
| Methods | RCT Opaque sealed envelopes | |
| Participants | 320 unselected acute stroke patients admitted to a stroke unit providing acute care and early rehabilitation Inclusion: acute stroke (< 7 days) patients screened within 3 days of admission Exclusion: coma (SSS < 2) or full recovery (SSS > 57) Characteristics: mean age 74 years, 53% men, mean BI 60/100, mean SSS 43/58. Trial included 320/468 (68%) of admissions | |
| Interventions | Intervention: hospital out‐reach stroke team (nurse, physiotherapy, occupational therapy) based in the stroke unit who made contact with patients in hospital, arranged discharge to home or rehabilitation unit, co‐ordinated rehabilitation and support services and provided follow‐up. Variable duration of input. Team co‐ordinated care which was largely delivered by other agencies Control: conventional procedures with acute care and early rehabilitation in a stroke unit, and discharge home or to a rehabilitation unit | |
| Outcomes | Outcomes measured at 6 weeks, 6 months and 12 months: death, place of residence, BI, Rankin score, Frenchay Activity Index, initial (stroke unit) length of stay, total (stroke unit + rehabilitation) length of stay Further outcomes at 12 months: Nottingham Health Profile, MMSE, Montgomery‐Asberg Depression Scale, Caregivers Strain index, cost analysis | |
| Notes | Outcomes repeated after 5 years: death, place of residence, Rankin score, BI, Frenchay Activity Index, SSS, MMSE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was restricted in permuted blocks with random number tables" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all assessments were blinded as far as is possible in such a trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All missing data are explained |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes reported |
Trondheim 2004.
| Methods | RCT Opaque sealed envelopes |
|
| Participants | 62 patients admitted to the stroke unit (acute care and early rehabilitation) who were resident in a rural community (30 to 90 minutes driving distance from hospital) Inclusion: acute stroke (< 7 days) patients screened within 3 days of admission Exclusion: coma (SSS < 2) or full recovery (SSS > 57) Characteristics: mean age 76 years, mean BI 56/100, mean SSS 43/58. Trial included 62/89 (70%) of admissions | |
| Interventions | Intervention: hospital out‐reach stroke team (physiotherapy, occupational therapy, nurse) based in the stroke unit who made contact with patients in hospital, arranged discharge to home or rehabilitation unit, co‐ordinated rehabilitation and support services and provided follow‐up. Team co‐ordinated care which was largely delivered by other agencies. Primary care provider assisted with co‐ordination of discharge home for patients living further than 45 minute driving distance from the hospital. ESD co‐ordination for 4 to 6 weeks, terminated by outpatient consultation (30 to 45 minutes driving distance) or home visit (> 45 minutes driving distance) Control: conventional procedures with acute care and early rehabilitation in a stroke unit, and discharge home or to a rehabilitation unit | |
| Outcomes | Outcomes measured at 6, 26 and 52 weeks: Modified Rankin Score, BI, Nottingham Health Profile, Caregiver Strain Index, death, initial (stroke unit) length of stay, total (stroke unit + rehabilitation) length of stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'patients ... were block randomised in blocks of four, six or eight .... The order of the blocks was randomly chosen' |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes by an external office |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but probably not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: 'An independent and blinded assessor' ... performed all outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals or missing data are explained |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes are reported |
ADL: activities of daily living BI: Barthel Index COOP: Care Cooperative Information Project COPM: Canadian Occupational Performance measure CME: caregiver‐mediated exercises EADL: extended activities of daily living ESD: early supported discharge EUROQOL / EQ‐5D: European Quality of Life instrument FIM: Functional Independence Measure GHQ: General Health Questionnaire HADS: Hospital Anxiety and Depression Scale hrs: hours IQR: interquartile range ITT: intention‐to‐treat MAS: Motor assessment scale MMSE: Mini Mental State Examination mRS: modified Rankin Scale NIHSS: National Institute of Health Stroke Scale RCT: randomised controlled trial SAH: subarachnoid haemorrhage SD: standard deviation SF36: Short Form 36 SSS: Scandinavian Stroke Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asplund 2000 | Participants had a variety of diagnoses |
| Auckland 1999 | Study was planned but did not commence recruitment |
| Ayrshire 2000 | Study was planned and funded but did not commence recruitment |
| Challis 1991 | Participants had a variety of diagnoses Non‐randomised trial |
| Cumbria 2004 | Study was planned but did not commence recruitment |
| Donald 1995 | Participants had a variety of diagnoses |
| Dunn 1994 | Participants had a variety of diagnoses |
| EXTRAS | Intervention after input from ESD service |
| Gladman 2001 | Participants had a variety of diagnoses |
| Glostrup 2006 | Cluster‐randomised trial |
| Grasel 2005 | Non‐randomised trial |
| Hirano 2012 | Inpatient intervention only |
| Kalra 2000 | Service to prevent admission to hospital |
| LHEC 1997 | Participants had a variety of diagnoses |
| Lincoln 2004 | Community setting |
| Mackay 1995 | Late rehabilitation intervention |
| Martin 1994 | Participants had a variety of diagnoses |
| New York 1986 | No outcome data available (unable to contact authors) |
| Ricauda 2004 | Service aimed to prevent hospital admission (patients did not leave hospital emergency room) |
| Shepperd 1998 | Service to prevent admission to hospital Participants had a variety of diagnoses |
| Townsend 1998 | Participants had a variety of diagnoses |
| Victor 1988 | Participants had a variety of diagnoses Non‐randomised trial |
| Wade 1985 | Service to prevent hospital admission as well as accelerate discharge Non‐randomised trial |
| Weiss 2004 | Non‐randomised trial |
ESD: early supported discharge
Characteristics of studies awaiting assessment [ordered by study ID]
Edirne 2001.
| Methods | RCT |
| Participants | Stroke patients in hospital |
| Interventions | In‐patient versus community rehabilitation |
| Outcomes | 2‐month outcomes |
| Notes | F Ozdemir, Trakya University School of Medicine, Edirne, Turkey |
Shi 2014.
| Methods | Hospital to community family transitional care model versus control in elderly hypertensive patients with complications (including stroke) |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tian 2015.
| Methods | Extended stroke unit service/ early supported discharge (ESUS) vs ordinary stroke unit service (OSUS) for 3 yr cost utility (Makov Model) |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ATTEND.
| Trial name or title | Early supported discharge with a family‐led caregiver‐delivered home‐based rehabilitation programme versus usual care post stroke (< 1 month from onset) |
| Methods | Multicentre RCT |
| Participants | |
| Interventions | Family‐led caregiver‐delivered home‐based rehabilitation programme |
| Outcomes | |
| Starting date | |
| Contact information | Richard Lindley |
| Notes | Clinical Trials Registry‐India (CTRI/2013/04/003557); Australian New Zealand Clinical Trials Registry (ACTRN12613000078752); and Universal Trial Number (U1111‐1138‐6707) |
Care4Stroke.
| Trial name or title | Caregiver‐mediated exercises with e‐healthsupport for early supported discharge after stroke (CARE4STROKE) |
| Methods | RCT |
| Participants | Stroke patients |
| Interventions | Caregiver‐mediated exercises with e‐healthsupport |
| Outcomes | |
| Starting date | |
| Contact information | Erwin van Wegen |
| Notes | Registered in the Dutch trial register as NTR4300. Uncertain if intention is to accelerate discharge. |
Gothenburg.
| Trial name or title | Very early supported discharge (VESD) vs ordinary discharge |
| Methods | RCT |
| Participants | Mild to moderate stroke patients |
| Interventions | |
| Outcomes | Anxiety, depression, independence, motor function |
| Starting date | |
| Contact information | Katharina S Sunnerhagen |
| Notes | ClinicalTrials.gov NCT01622205 |
Hong Kong.
| Trial name or title | Patient Engagement Program for Stroke (PEPS) |
| Methods | Unclear at present |
| Participants | Unclear at present |
| Interventions | Unclear at present |
| Outcomes | Unclear at present |
| Starting date | May 2010 |
| Contact information | Dr Fung Pui Man |
| Notes | Hong Kong |
Perth.
| Trial name or title | Establishing an effective and efficient early supported discharge rehabilitation program for stroke clients in Perth (Western Australia) |
| Methods | RCT |
| Participants | Unclear at present |
| Interventions | Unclear at present |
| Outcomes | Unclear at present |
| Starting date | 20 November 2011 |
| Contact information | Roslyn Jones |
| Notes | Main ID: ACTRN12611001243909 (anzctr.org.au) |
RECOVER.
| Trial name or title | The RECOVER trial |
| Methods | Multicentre RCT |
| Participants | First‐ever acute ischaemic/haemorrhagic/undifferentiated stroke (within 1 month) |
| Interventions | Family‐nominated caregiver trained in ESD and Electronic Data Capture (EDC) vs normal care |
| Outcomes | Physical functioning, quality of life, and caregiver burden |
| Starting date | |
| Contact information | Janet Prvu Bettger |
| Notes | The trial was registered in the clinicaltrials.gov database; registration number NCT02247921 |
West Denmark.
| Trial name or title | RCT Computer‐generated blocks of 10, opaque sealed envelopes |
| Methods | 198 acute stroke patients in second‐line neurological rehabilitation units within 4 centres (Brönderslev, Hammel, Ringe, Skive) screened on day 5 of admission |
| Participants | Intervention: hospital out‐reach multidisciplinary team comprising physiotherapy, occupational therapy, nursing and speech and language therapy (in hospital only). Co‐ordinate discharge planning, including pre‐assessment home visits and provide low‐intensity rehabilitation (maximum 8 sessions) in the community for a period of 1 month. Teams are co‐ordinated through twice‐weekly multidisciplinary meetings. Patients live between 0 to 70 km (average 30 km) of team base Control: conventional discharge planning from neurological rehabilitation unit with 1 pre‐assessment home visit and after care including home care, physiotherapy clinic and further inpatient rehabilitation if required |
| Interventions | Outomces at 6 months: FIM, Frenchay activity index, EUROQOL Mortality, institutionalisation, care requirements Patient and carer satisfaction |
| Outcomes | Unpublished information from authors |
| Starting date | 2009 |
| Contact information | Birgitte G Jepson, Poul Mogensen |
| Notes |
ESD: early supported discharge EUROQOL: European Quality of Life instrument FIM: functional independence measure RCT: randomised controlled trial
Differences between protocol and review
For the 2012 update some post‐hoc analyses were carried out. These are highlighted in the text. The 2012 update did not explicitly include or exclude cluster‐randomised trial design and one was included (Glostrup 2006). For the current update, we have clarified inclusion criteria to exclude cluster‐randomised trials because of: 1) difficulties in obtaining data for appropriate analysis, and 2) increasing focus on cluster‐randomised trial methodology for implementation rather than evaluation trials. This results in the loss of one trial of 198 participants with no change in the conclusions (Glostrup 2006). The title was revised in 2017 to 'Early supported discharge services for people with acute stroke' to better reflect the content of the review.
Contributions of authors
For this version of the review, Peter Langhorne updated and carried out the literature searches, reanalysed the data and redrafted the manuscript. Satu Baylan carried out trial selection and screening and helped redraft the review. The Early Supported Discharge Trialists group provided advice and input on data interpretation and redrafting of the manuscript. The new trialists contacts were; Maayken van den Berg (Adelaide 2016), Jeyaraj Pandian (ATTEND pilot 2015), Silvina Santana (Aveiro 2016) and Hakon Hofstad (Bergen 2014).
For the previous version of the review, Patricia Fearon updated and carried out the literature searches, reanalysed the data and redrafted the manuscript. Peter Langhorne supervised the update and revised the draft manuscript. The Early Supported Discharge Trialists group provided original data, data interpretation, and redrafted the manuscript (ESD trialists 2012).
For the initial version of the review, Peter Langhorne initiated the study, drafted the original protocol, co‐ordinated the project, and drafted the original manuscript (EDS Trialists 2001). For the 2005 version of the review, Peter Langhorne, Martin Dennis, and Gillian Taylor formed the writing committee. Gillian Taylor, Peter Langhorne, and Gordon Murray conducted the original statistical analyses. The Early Supported Discharge Trialists group provided original data, data interpretation, and redrafted the manuscript (ESD trialists 2005).
Early Supported Discharge Trialists group consisted of: Craig Anderson (Sydney), Erik Bautz‐Holter (Oslo), Martin Dennis (Secretariat) Paola Dey (Manchester), Bent Indredavik (Trondheim), Birgitte Jepson (West Denmark), Peter Langhorne (Co‐ordinator), Nancy Mayo (Montreal), Paul Mogensen (West Denmark), Gordon Murray (Stastician), Michael Power (Belfast), Helen Rodgers (Newcastle), Ole Morten Ronning (Akershus), Anthony Rudd (London), Silvana Santana (Aviero), Nijasri Suwanwela (Bangkok), Gillian Taylor (Statistician), Lotta Widen‐Holmqvist (Stockholm) and Charles Wolfe (London). All contributed to the study design, data collection, and analysis and revision of the manuscript.
Sources of support
Internal sources
University of Glasgow, UK.
University of Edinburgh, UK.
External sources
Stroke Association, UK.
Chest Heart and Stroke Scotland, UK.
Declarations of interest
Peter Langhorne co‐authored one trial and the ESD trialists conducted the original randomised trials (see 'Potential biases in the review process'). Otherwise no relevant conflicts are known for Peter Langhorne and Satu Baylan.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adelaide 2000 {published and unpublished data}
- Anderson C, Mhurchu CN, Rubenach S, Clark M, Spencer C, Winsor A. Home or hospital for stroke rehabilitation? Results of a randomized controlled trial. II: Cost minimization analysis at 6 months. Stroke 2000;31:1032‐7. [DOI] [PubMed] [Google Scholar]
- Anderson C, Mhurchu CNI, Hackett M, Rubenach S. Long‐term outcome in stroke patients with accelerated hospital discharge: a randomised controlled trial [Abstract]. Proceedings of the Consensus Conference on Stroke Treatment and Service Delivery. 7‐8 November 2000. UK, Edinburgh: Royal College of Physicians of Edinburgh. 2000:46 (Abst. PB18).
- Anderson C, Rubenach S, Mhurchu CN, Clark M, Spencer C, Winsor A. Home or hospital for stroke rehabilitation? Results of a randomized controlled trial. I: Health outcomes at 6 months. Stroke 2000;31:1024‐31. [DOI] [PubMed] [Google Scholar]
- Hackett M, Anderson C, Vandal A, Rubenach S. One year follow‐up of a RCT of accelerated hospital discharge and home‐based stroke rehabilitation. Stroke 2000;31:2817‐8. [Google Scholar]
- Hackett ML, Vandal AC, Anderson CS, Rubenach S. Long‐term outcome in stroke patients and caregivers following accelerated hospital discharge and home‐based rehabilitation. Stroke 2002;33(2):643‐5. [PubMed] [Google Scholar]
- Mhurchu CN, Anderson C, Rubenach S, Clark M, Spencer C. Home or hospital for stroke rehabilitation? Results of a randomised controlled trial. Cerebrovascular Diseases 2000;10 (Suppl 2):61. [Google Scholar]
- Rubenach S, Anderson C, Clark M, Russell M, Spencer C, Winsor A. Early supportive discharge and rehabilitation trial (ESPRIT) in stroke: preliminary results. Cerebrovascular Diseases 1998;8 (Suppl 4):P82. [Google Scholar]
- Rubenach S, Anderson C, Clark M, Russell M, Spencer CM, Winsor A. Early supportive discharge and rehabilitation trial (ESPRIT) in stroke: preliminary results [Abstract]. Australian and New Zealand Journal of Medicine 1998;28:498. [Google Scholar]
Adelaide 2016 {published data only}
- Berg M, Crotty M, Liu E, Killington M, Kwakkel G, Wegen E. Early supported discharge by caregiver‐mediated exercises and e‐Health support after stroke. A proof‐of‐concept trial. Stroke 2016;47:1885‐92. [DOI] [PubMed] [Google Scholar]
Akershus 1998 {published and unpublished data}
- Ronning OM, Guldvog B. Outcome of subacute stroke rehabilitation. A randomized controlled trial. Stroke 1998;29:779‐84. [DOI] [PubMed] [Google Scholar]
ATTEND pilot 2015 {published and unpublished data}
- Pandian JD. ATTEND ‐ family‐led rehabilitation after stroke in India. http://www.ClinicalTrials.gov 2014. [DOI] [PubMed]
- Pandian JD. Family‐led rehabilitation after stroke in India. http://ctri.nic.in 2014. [DOI] [PubMed]
- Pandian JD, Felix C, Kaur P, Sharma D, Julia L, Toor G, et al. Family‐led rehabilitation after stroke in India: the ATTEND pilot study. International Journal of Stroke 2015;10:609‐14. [DOI] [PubMed] [Google Scholar]
Aveiro 2016 {unpublished data only}
- Santana S, Rente J, Neves C, Redondo P, Szczygiel N, Larsen T, et al. Early home‐supported discharge for patients with stroke in Portugal: a randomised controlled trial. Clinical Rehabilitation 2016;31(2):197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bangkok 2002 {published data only}
- Suwanwela NC, Phanthumchinda K, Limtongkul S, Suvanprakorn P, Thai Red Cross Volunteers Bureau. Comparison of short (3‐day) hospitalization followed by home care treatment and conventional (10 day) hospitalization for acute ischaemic stroke. Cerebrovascular Diseases 2002;13:267‐71. [DOI] [PubMed] [Google Scholar]
Belfast 2004 {published data only}
- Donnelly M, Power M, Russell M, Fullerton K. Randomized controlled trial of an early discharge rehabilitation service: the Belfast community stroke trial. Stroke 2004;35(1):127‐33. [DOI] [PubMed] [Google Scholar]
Bergen 2014 {unpublished data only}
- Gjelsvik BEB, Hofstad H, Smedal T, Eide GE, Næss H, Skouen JS, et al. Balance and walking after three different models of stroke rehabilitation: early supported discharge in a day unit or at home, and traditional treatment (control). BMJ Open 2014;e004358:1136/bmjopen‐2013‐004358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjelsvik BEB, Smedal T, Hofstad H, Eide GE, Skouen JS, Frisk B, et al. Balance and walking outcome after stroke rehabilitation ‐ a randomised controlled trial comparing two early discharge models with treatment as usual. Cerebrovascular Diseases 2013;35 Suppl 3:95. [Google Scholar]
- Hofstad H, Eide GE, Moe‐Nilssen R, Naess H, Skouen JS. Early supported discharge after stroke in Bergen, Norway: no significant difference from in‐patient treatment, but home rehabilitation may be better. Stroke 2013;44:Abst.ATP313. [Google Scholar]
- Hofstad H, Gjelsvik BEB, Næss H, Eide GE, Skouen JS. Early supported discharge after stroke in Bergen (ESD Stroke Bergen): three and six months results of a randomised controlled trial comparing two early supported discharge schemes with treatment as usual. BMC Neurology 2014;14:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstad H, Naess H, Moe‐Nilssen R, Skouen JS. ESD Stroke Bergen ‐ an RCT comparing two different schemes of early supported discharge after stroke with ordinary treatment: results from 3 months follow‐up. Neurorehabilitation and Neural Repair 2012;26(6):748. [Google Scholar]
- Hofstad H, Naess H, Moe‐Nilssen R, Skouen JS. Early supported discharge after stroke in Bergen (ESD Stroke Bergen): a randomized controlled trial comparing rehabilitation in a day unit or in the patients' homes with conventional treatment. International Journal of Stroke 2013;8:582‐7. [DOI] [PubMed] [Google Scholar]
- Skouen JS. Early supported discharge after stroke in Bergen. http://www.ClinicalTrials.gov 2008.
- Taule T, Skouen JS, Raheim M. Life changed existentially. A qualitative study of experiences 6 months post stroke. Cerebrovascular Diseases 2013;35 Suppl 3:764 (Abst.791). [Google Scholar]
- Taule T, Strand LI, Assmus J, Skouen JS. Ability in daily activities after early supported discharge models of stroke rehabilitation. Scandinavian Journal of Occupational Therapy 2015;22(5):355‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergen 2014 ‐ Day unit {published data only}
- Hofstad H, Gjelsvik BEB, Næss H, Eide GE, Skouen JS. Early supported discharge after stroke in Bergen (ESD Stroke Bergen): three and six months results of a randomised controlled trial comparing two early supported discharge schemes with treatment as usual. BMC Neurology 2014;14:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergen 2014 ‐ Home care {published data only}
- Hofstad H, Gjelsvik BEB, Næss H, Eide GE, Skouen JS. Early supported discharge after stroke in Bergen (ESD Stroke Bergen): three and six months results of a randomised controlled trial comparing two early supported discharge schemes with treatment as usual. BMC Neurology 2014;14:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Copenhagen 2009 {unpublished data only}
- Kjaer P, Skerris A, Ostergaard A, Skou C, Christoffersen J, Seest LS, et al. Multidisciplinary hometraining of stroke patients. A randomised control intervention. Gentofte Hospital Report 2009.
- Rasmussen RS, Overgaard K, Ostergaard A, Kjaer P, Skerris A, Skou C, et al. Post‐stroke rehabilitation at home reduced disability and improved quality of life: a randomized controlled trial. [Abstract]. Cerebrovascular Diseases. 2013; Vol. 35(Suppl 3):94 (Abst.2).
- Rasmussen RS, Østergaard A, Kjær P, Skerris A, Skou C, Christoffersen J, et al. Stroke rehabilitation at home before and after discharge reduced disability and improved quality of life: a randomised controlled trial. Clinical Rehabilitation 2016;30(3):225‐36. [DOI] [PubMed] [Google Scholar]
London 1997 {published data only}
- Beech R, Rudd AG, Tilling K, Wolfe CDA. Economic consequences of early inpatient discharge to community‐based rehabilitation for stroke in an inner‐London teaching hospital. Stroke 1999;30:729‐35. [DOI] [PubMed] [Google Scholar]
- Rudd AG, Wolfe CDA, Tilling K, Beech R. Randomised controlled trial to evaluate early discharge scheme for patients with stroke. BMJ 1997;315:1039‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manchester 2001 {unpublished data only}
- Dey P, Woodman M, Gibbs A. Home team trial (North Manchester General and Stepping Hill Hospitals). Unpublished data.
Montreal 2000 {unpublished data only}
- Mayo N, Wood‐Dauphinee S, Cote R, Gayton D, Carlton J, Buttery J, et al. There's no place like home. An evaluation of early supported discharge for stroke. Stroke 2000;31:1016‐23. [DOI] [PubMed] [Google Scholar]
- Mayo N, Wood‐Dauphinee S, Tamblyn R, Cote R, Gayton D, Carlton J, et al. There's no place like home: a trial of early discharge and intensive home rehabilitation post‐stroke. Cerebrovascular Diseases 1998;8 (Suppl 4):94. [Google Scholar]
- Teng J, Mayo NE, Latimer E, Hanley J, Wood‐Dauphinee S, Cote R, et al. Costs and caregiver consequences of early supported discharge for stroke patients. Stroke 2003;34:528‐36. [DOI] [PubMed] [Google Scholar]
Newcastle 1997 {published data only (unpublished sought but not used)}
- McNamee P, Christensen J, Soutter J, Rodgers H, Craig N, Pearson P, et al. Cost analysis of early supported hospital discharge for stroke. Age and Ageing 1998;27:345‐51. [Google Scholar]
- Rodgers H, Soutter J, Kaiser W, Pearson P, Dobson R, Skilbeck C, et al. Early supported hospital discharge following acute stroke: pilot study results. Clinical Rehabilitation 1997;11:280‐7. [DOI] [PubMed] [Google Scholar]
- Soutter J, Rodgers H, Pearson P, Kaiser W, Skilbeck C, Bond J. Qualitatively: why an early supported discharge service for stroke patients? [Abstract]. Clinical Rehabilitation. 1998; Vol. 12:165.
Oslo 2000 {published and unpublished data}
- Bautz‐Holter E, Sveen U, Bruun Wyller T, Rygh J. Early supported discharge of patients with acute stroke. A randomised controlled trial. Cerebrovascular Diseases 2000;10 (Suppl 2):61. [DOI] [PubMed] [Google Scholar]
- Bautz‐Holter E, Sveen U, Rygh J, Rodgers H, Brunn Wyller T. Early supported discharge of patients with acute stroke: a randomized controlled trial. Disability and Rehabilitation 2002;24(7):348‐55. [DOI] [PubMed] [Google Scholar]
Stockholm 1998 {published and unpublished data}
- Thorsen AM, Holmqvist LW, Pedro‐Cuesta J, Koch L. A randomized controlled trial of early supported discharge and continued rehabilitation at home after stroke: Five year follow‐up of patient outcome. Stroke 2005;36:297‐303. [DOI] [PubMed] [Google Scholar]
- Thorsen AM, Holmqvist LW, Koch L. Early supported discharge and continued rehabilitation at home after stroke: 5‐year follow‐up of resource use. Journal of Stroke and Cerebrovascular Diseases 2006;15:139‐43. [DOI] [PubMed] [Google Scholar]
- Koch L, Pedro‐Cuesta J, Kostulas V, Almazan J, Widen Holmqvist L. Randomized controlled trial of rehabilitation at home after stroke: one‐year follow‐up of patient outcome, resource use and cost. Cerebrovascular Diseases 2001;12:131‐8. [DOI] [PubMed] [Google Scholar]
- Widen Holmqvist L, Koch L, Kostulas V, Holm M, Widsell G, Tegler H, et al. A randomised controlled trial of rehabilitation at home after stroke in southwest Stockholm. Stroke 1998;29:591‐7. [DOI] [PubMed] [Google Scholar]
- Widen Holmqvist L, Koch L, Pedro‐Cuesta J. A randomized controlled trial of early supported discharge and continued rehabilitation at home after stroke: one‐year follow‐up of patient outcome, resource use and cost [Abstract]. Proceedings of the Consensus Conference on Stroke Treatment and Service Delivery. 7‐8 November 2000. UK, Edinburgh: Royal College of Physicians of Edinburgh. 2000:45 (Abst. PB04).
- Widen Holmqvist L, Koch L, Pedro‐Cuesta J. Use of health care, impact on family caregivers and patient satisfaction of rehabilitation at home after stroke in southwest Sweden. Scandinavian Journal of Rehabilitation Medicine 2000;32:173‐9. [DOI] [PubMed] [Google Scholar]
- Ytterberg C, Thorsen AM, Liljedhal M, Holmqvist LW, Koch L. Changes in perceived health between one and five years after stroke: a randomized controlled trial of early supported discharge with continued rehabilitation at home versus conventional rehabilitation. Journal of the Neurological Sciences 2010;294:86‐8. [DOI] [PubMed] [Google Scholar]
Trondheim 2000 {published and unpublished data}
- Fjaeroft H, Indredavik B, Johnsen R, Lydersen S. Acute stroke unit care combined with early supported discharge. Long‐term effects on quality of life. A randomized controlled trial. Clinical Rehabilitation 2004;18:580‐6. [DOI] [PubMed] [Google Scholar]
- Fjaeroft H, Indredavik B, Magnussen J, Johnsen R. Early supported discharge for stroke patients improves clinical outcome. Does it also reduce use of health services and costs?. Cerebrovascular Diseases 2005;19:376‐83. [DOI] [PubMed] [Google Scholar]
- Fjaeroft H, Rohweder G, Indredavik B. Stroke unit care combined with early supported discharge improves 5‐year outcome. Stroke 2011;42:1707‐11. [DOI] [PubMed] [Google Scholar]
- Fjaertoft H, Ekeberg G, Loge AD, Morch B, Indredavik B. Extended stroke unit care with early supported discharge co‐ordinated by a stroke team improves outcome for stroke patients. European Journal of Neurology 1999;6(5):A8‐9. [Google Scholar]
- Fjaertoft H, Indredavik B, Ekeberg G, Loge AD, Morch B. Extended stroke unit service with early supported discharge co‐ordinated by a stroke team improves outcome for stroke patients [Abstract]. Proceedings of the Consensus Conference on Stroke Treatment and Service Delivery, 7‐8 November 2000, UK, Edinburgh: Royal College of Physicians of Edinburgh. 2000:49 (Abst. PB33).
- Fjaertoft H, Indredavik B, Lydersen S. Stroke unit care combined with early supported discharge. Long term follow‐up of a randomized controlled trial [Abstract]. Proceedings of the 12th Nordic Meeting on Cerebrovascular Diseases, 17‐20 September 2003, Norway, Oslo. 2003:16 (Abst. O‐004). [DOI] [PubMed]
- Fjaertoft H, Indredavik B, Lydersen S. Stroke unit care combined with early supported discharge. Long‐term follow‐up of a randomized controlled trial. Stroke 2003;34:2687‐92. [DOI] [PubMed] [Google Scholar]
- Indredavik B, Fjaertoft H, Ekeberg G, Loge AD, Morch B. Benefit of an extended stroke unit service with early supported discharge. A randomized, controlled trial. Stroke 2000;31:2989‐94. [DOI] [PubMed] [Google Scholar]
Trondheim 2004 {published and unpublished data}
- Askim T, Indredavik B, Lydersen S, Rohweder G. Early supported discharge for patients living in a rural community. Proceedings of the 12th Nordic Meeting on Cerebrovascular Diseases, 17‐20 September 2003, Oslo, Norway. 2003; Vol. 19:(Abst 0‐010).
- Askim T, Rohweder G, Lydersen S, Indredavik B. Evaluation of an extended stroke unit service with early supported discharge for patients living in a rural community. A randomized controlled trial. Cllinical Rehabilitation 2004;18:238‐48. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Asplund 2000 {published data only}
- Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, et al. Geriatric‐based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. Journal of the American Geriatrics Society 2000;48(11):1381‐8. [DOI] [PubMed] [Google Scholar]
Auckland 1999 {unpublished data only}
- Anderson C. Personal communication. Unpublished.
Ayrshire 2000 {unpublished data only}
- Walker A. Personal communication. Unpublished.
Challis 1991 {published data only}
- Challis D, Darton R, Johnson L, Stone M, Traske K. An evaluation of an alternative to long stay hospital care for frail elderly patients : 1. the model of care. Age and Ageing 1991;20:236‐44. [DOI] [PubMed] [Google Scholar]
Cumbria 2004 {unpublished data only}
- Orugun E. Personal communication. Unpublished.
Donald 1995 {published data only}
- Donald IP, Baldwin RN, Bannerjee M. Gloucester hospital at home: a randomised controlled study. Age and Ageing 1995;24:434‐9. [DOI] [PubMed] [Google Scholar]
Dunn 1994 {published data only}
- Dunn RB, Lewis PA, Vetter NJ, Guy PM, Hardman C, Jones RW. Health visitor intervention to reduce days of unplanned hospital readmission in patients recently discharged from geriatric wards: the results of a randomised controlled trial. Archives of Gerontology and Geriatrics 1994;18:15‐23. [DOI] [PubMed] [Google Scholar]
EXTRAS {published data only}
- Rodgers H, Shaw L, Cant R, Drummond A, Ford GA, Forster A, et al. Evaluating an extended rehabilitation service for stroke patients (EXTRAS): study protocol for a randomised controlled trial. Trials 2015;16:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gladman 2001 {published data only}
- Cunliffe A, Dewey M, Gladman J, Harwood R, Hubands S, Miller P. Evaluation of an early discharge scheme for elderly people: use of hospital beds at 3 months. Age and Ageing 2001;30 (Suppl 2):33. [Google Scholar]
Glostrup 2006 {published and unpublished data}
- Torp CR, Vinkler S, Pedersen KD, Hansen FR. Model of hospital‐supported discharge after stroke. Stroke 2006;37:1514‐20. [DOI] [PubMed] [Google Scholar]
Grasel 2005 {published data only}
- Grasel E, Biehler J. Intensification of the transition between inpatient neurological rehabilitation and home care of stroke patients. Controlled clinical trial with follow‐up assessment six months after discharge. Clinical Rehabilitation 2005;19:725‐36. [DOI] [PubMed] [Google Scholar]
Hirano 2012 {published data only}
- Hirano Y, Maeshima S, Osawa A, Nishio D, Takeda K, Baba M, et al. The effect of voluntary training with family participation on early home discharge in patients with severe stroke at a convalescent rehabilitation ward. European Neurology 2012;68(4):221–8. [DOI] [PubMed] [Google Scholar]
Kalra 2000 {published and unpublished data}
- Kalra L, Evans E, Perez I, Knapp M, Donaldson N, Swift C. Alternative strategies for stroke care: a prospective randomised controlled trial. Lancet 2000;356:894‐9. [DOI] [PubMed] [Google Scholar]
LHEC 1997 {published data only}
- London Health Economics Consortium. Evaluation of elderly ambulatory care project. Key results (short report). London School of Hygiene and Tropical Medicine 1997.
Lincoln 2004 {published data only}
- Lincoln NB, Walker MF, Dixon A, Knights P. Evaluation of a multiprofessional community stroke team: a randomised controlled trial. Clinical Rehabilitation 2004;18:40‐7. [DOI] [PubMed] [Google Scholar]
Mackay 1995 {published data only}
- Mackay S. A comparative study of motor and process skills in stroke patients receiving rehabilitation at home or in hospital. British Journal of Occupational Therapy 1995;58(8):342. [Google Scholar]
Martin 1994 {published data only}
- Martin F, Oyewole A, Moloney A. A randomised controlled trial of a high support hospital discharge team for elderly people. Age and Ageing 1994;23:228‐34. [DOI] [PubMed] [Google Scholar]
New York 1986 {published data only}
- Scheinberg L, Koren MJ, Bluestone M, McDowell FH. Effects of early hospital discharge to home care on the costs and outcome of care of stroke patients: a randomized trial in progress. In: Lechner H, Meyer JS, Ott E editor(s). Cerebrovascular Disease: Research and Clinical Management. 1. Vol. 1, Amsterdam: Elsevier, 1986:289‐96. [Google Scholar]
Ricauda 2004 {published data only}
- Ricauda NA, Bo M, Molaschi M, Massaia M, Salerno D, Amati D, et al. Home hospitalization service for acute uncomplicated first ischaemic stroke in elderly patients: a randomized trial. Journal of the American Geriatric Society 2004;52:278‐83. [DOI] [PubMed] [Google Scholar]
Shepperd 1998 {published and unpublished data}
- Shepperd S, Harwood D, Gray A, Vessey M, Morgan P. Randomised controlled trial comparing hospital at home with inpatient hospital care. II: cost minimisation analysis. BMJ 1998;316:1791‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Townsend 1998 {published data only}
- Townsend J, Piper M, Frank AO, Dyer S, North WRS, Meade TW. Reduction in hospital readmission stay of elderly patients by a community based hospital discharge scheme: a randomised controlled trial. BMJ 1988;297:544‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Victor 1988 {published data only}
- Victor CR, Vetter NJ. Rearranging the deck chairs on the Titanic: failure of an augmented home help scheme after discharge to reduce the length of stay in hospital. Archives of Gerontology and Geriatrics 1988;7:83‐91. [DOI] [PubMed] [Google Scholar]
Wade 1985 {published and unpublished data}
- Wade DT, Langton Hewer R, Skilbeck CE, Bainton D, Burns‐Cox C. Controlled trial of a home care service for acute stroke patients. Lancet 1985;i:323‐6. [DOI] [PubMed] [Google Scholar]
Weiss 2004 {published data only}
- Weiss Z, Snir D, Klein B, Avraham I, Shani R, Zetler H, et al. Effectiveness of home rehabilitation after stroke in Israel. International Journal of Rehabilitation Research 2004;27(2):119‐25. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Edirne 2001 {unpublished data only}
Shi 2014 {published data only}
- Shi Y. Construction of "Hospital‐Community‐Family" transitional care model for elderly hypertensive patients based on information platform. Chinese Clinical Trial Registry (ChiCTR). 2014; Vol. [http://www.chictr.org.cn/enIndex.aspx].
Tian 2015 {published data only}
- Tian J. Cost‐utility of analysis of different stroke rehabilitation model. Chinese Clinical Trial Registry (ChiCTR) 2015; Vol. [http://www.chictr.org.cn/enIndex.aspx].
References to ongoing studies
ATTEND {published data only}
- Alim M, Lindley R, Felix C, Gandhi DBC, Verma SJ, Tugnawat DK, et al. Family‐led rehabilitation after stroke in India: The ATTEND trial, study protocol for a randomized controlled trial. Trials 2016; Vol. 17:13. [DOI: 10.1186/s13063-015-1129-8] [DOI] [PMC free article] [PubMed]
- Billot L, Lindley RI, Harvey LA, Maulik PK, Hackett ML, Murthy GVS, et al. Statistical analysis plan for the family‐led rehabilitation after stroke in India (ATTEND) trial: A multicenter randomized controlled trial of a new model of stroke rehabilitation compared to usual care. International Journal of Stroke 2016. [DOI: 10.1177/1747493016674956] [DOI] [PubMed]
- Felix C, Pandian JD, Alim M, Gandhi DBC, Syrigapu A, Tugnawat DK, et al. Family‐led rehabilitation after stroke in India: ATTEND trial. Proceedings of the International Stroke Conference 2015. 11‐13 February 2015; Nashville, Tennesee, USA. (Abst. CT P26). 2015.
- Gandhi D, Pandian J, Lindley R, Alim M, Maulik P, Murthy GVS, et al. Family‐led rehabilitation after stroke in India: the ATTEND trial. International Journal of Stroke 2015;10 Suppl 2:174 (Abst.ESOC‐0373). [DOI] [PubMed] [Google Scholar]
- Lindley R, Pandian J, Felix C, Alim M, Gandhi DBC, Syrigapu A, et al. ATTEND (family led rehabilitation after stroke in India) trial: potential for better stroke rehabilitation access in India. Cerebrovascular Diseases 2016;42 Suppl 1:106 (Abst.P168). [Google Scholar]
- Lindley R, Pandian JD, Felix C, Alim M, Gandhi DBC, Verma SJ, et al. The ATTEND trial ‐ Family‐led rehabilitation after stroke in India: a modified version of early supported discharge with a caregiver delivered home based post‐stroke rehabilitation. [Abstract]. In: Proceedings of the European Stroke Conference 2014. 6‐9 May 2014; Nice, France. (Abst. OAID 51). 2014.
- Lindley R. . Australian New Zealand Clinical Trials Registry (ANZCTR). The ATTEND Trial: family‐led rehabilitation after stroke in India. Australian New Zealand Clinical Trials Registry (ANZCTR) 2013; Vol. [http://www.anzctr.org.au/].
- Lindley RI, Felix C, Pandian JD, Anderson CS, Mohammed A, Gandhi DB, et al. ATTEND (family led rehabilitation after stroke in India) trial: potential for health system change in India. Stroke 2016;47 Suppl 1:(Abst.TMP33). [Google Scholar]
- Liu H, Lindley R, Alim M, Felix C, Gandhi DBC, Verma SJ, et al. Protocol for process evaluation of a randomised controlled trial of family‐led rehabilitation post stroke (ATTEND) in India. BMJ Open. 2016;6:e012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A, Jeyaraj PD, Dorcas GBC, Shweta VJ, Cynthia F, Anuradha S, et al. Family‐led rehabilitation after stroke in India: the ATTEND trial. International Journal of Stroke 2015;10 Suppl 3:72. [Google Scholar]
- Pandian JD. A multicentre, randomized, blinded outcome assessor, controlled trial, whether a family‐led caregiver‐delivered home‐based rehabilitation intervention versus usual care is an effective, affordable Early Support Discharge strategy for those with disabling stroke in India. Clinical Trials Registry ‐ India (CTRI) 2013; Vol. [http://ctri.nic.in].
- Pandian JD, Felix C, Alim M, Gandhi DBC, Syrigapu A, Tugnawat DK, et al. The Attend trial‐family‐led rehabilitation after stroke in India: a modified version of early supported discharge with a caregiver delivered home based poststroke rehabilitation. International Journal of Stroke 2014;9 Suppl 3:252‐3 (Abst.WSC‐0944). [Google Scholar]
Care4Stroke {published data only}
- Vloothuis J, Mulder M, Nijland RHM, Konijnenbelt M, Mulder H, Hertogh CMPM, et al. Caregiver‐mediated exercises with e‐healthsupport for early supported discharge after stroke (CARE4STROKE): study protocol for a randomized controlled trial. BMC Neurology 2015;15:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gothenburg {published data only}
- Sunnerhagen KS, Danielsson A, Rafsten L, Bjorkdahl A, Axelsson AB, Nordin A, et al. Gothenburg very early supported discharge study (GOTVED) NCT01622205: a block randomized trial with superiority design of very early supported discharge for patients with stroke. BMC Neurology 2013;13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnerhagen KT. GOThenburg Very Early Supported Discharged (GOTVED). ClinicalTrials.gov 2012; Vol. [http://www.ClinicalTrials.gov].
Hong Kong {unpublished data only}
- Patient Engagement Program for Stroke (PEPS). Ongoing study May 2010.
Perth {unpublished data only}
- Jones R. Establishing an effective and efficient early supported discharge (ESD) rehabilitation program for stroke patients in Perth WA. Australian New Zealand Clinical Trials Registry (ANZCTR) 2011, issue [http://www.anzctr.org.au/].
RECOVER {published data only}
- Yan LL. Randomized controlled trial on rehabilitation through caregiver‐delivered nurse‐organised service programs for disabled stroke patients in rural China (RECOVER). George Institute for Global Health Neurological and Mental Health. 2016, issue http://www.georgeinstitute.org/units/neurological‐and‐mental‐health. [DOI] [PubMed]
- Yan LL. Rehabilitation for disabled stroke patients in rural China (RECOVER). ClinicalTrials.gov 2016; Vol. [http://www.ClinicalTrials.gov].
- Yan LL, Chen S, Zhou B, Zhang J, Xie B, Luo R, et al. A randomized controlled trial on rehabilitation through caregiver‐derived nurse‐organized service programs for disabled stroke patients in rural China (the RECOVER trial): design and rationale. International Journal of Stroke 2016;11(7):823‐30. [DOI] [PubMed] [Google Scholar]
West Denmark {unpublished data only}
- RCTComputer‐generated blocks of 10, opaque sealed envelopes. Ongoing study 2009.
Additional references
ESO 2008
- Ringleb PA, Bousser MG, Ford G, Bath P, Brainin M, Caso V, The European Stroke Organization (ESO) Executive Committee and the ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack. Cerebrovascular Diseases 2008;25:457‐507. [DOI] [PubMed] [Google Scholar]
Fisher 2011
- Fisher RJ, Gaynor C, Kerr M, Langhorne P, Anderson C, Bautz‐Holter E, et al. A consensus on stroke: early supported discharge. Stroke 2011;42(5):1392‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Langhorne 2007
- Langhorne P, Widen‐Holmqvist L. Early supported discharge after stroke. Journal of Rehabilitation Medicine 2007;39(2):103‐8. [DOI] [PubMed] [Google Scholar]
Langhorne 2011
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377:1693‐702. [DOI] [PubMed] [Google Scholar]
Meyer 2016
- Meyer MJ, Teasell R, Thind A, Koval J, Speechley M. A synthesis of peer‐reviewed literature on team‐coordinated and delivered early supported discharge after stroke. Canadian Journal of Neurological Sciences 2016;43(3):353‐9. [doi: 10.1017/cjn.2015.343. Epub 2016 Jan 8.] [DOI] [PubMed] [Google Scholar]
RCP 2008
- The Intercollegiate Working Party for Stroke. National clinical guidelines for stroke. Royal College of Physicians. 3rd Edition. Royal College of Physicians, London, 2008. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagan: The Nordic Cochrane Centre, 2014.
Saka 2009
- Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age and Ageing 2009;38:27‐32. [DOI] [PubMed] [Google Scholar]
Shepperd 2009
- Shepperd S, Doll H, Broad J, Gladman J, Iliffe S, Langhorne P, et al. Hospital at home early discharge. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000356.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepperd 2016
- Gonçalves‐Bradley DC, Lannin NA, Clemson LM, Cameron ID, Shepperd S. Discharge planning from hospital. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD000313.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
SUTC 2013
- Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD000197.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warlow 2008
- Warlow C, Gijn J, Dennis M, Wardlaw J, Bamford J, Hankey G, et al. Stroke: Practical Management. 3rd Edition. Oxford: Blackwell Publishing, 2008. [Google Scholar]
References to other published versions of this review
EDS Trialists 2001
- Early Supported Discharge Trialists. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000443] [DOI] [Google Scholar]
ESD trialists 2005
- Early Supported Discharge Trialists. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD000443.pub2] [DOI] [PubMed] [Google Scholar]
ESD trialists 2012
- Fearon P, Langhorne P, Early Supported Discharge Trialists. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000443.pub3] [DOI] [PubMed] [Google Scholar]
Langhorne 1999
- Langhorne P, Dennis M, Kalra L, Shepperd S, Wade D, Wolfe C. Services for helping acute stroke patients avoid hospital admission. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD000444] [DOI] [PubMed] [Google Scholar]
Langhorne 2001
- Langhorne P, on behalf of the Early Supported Discharge trialists. Early supported discharge services for stroke patients: a systematic review [Abstract]. Cerebrovascular Diseases 2001;11 (Suppl 4):44 (Abst.reha_3). [Google Scholar]
Langhorne 2003
- Langhorne P, for the Early Supported Discharge trialists. Early supported discharge services for stroke patients: an individual patient data meta‐analysis. Cerebrovascular Diseases 2003;16 (Suppl 4):96 (Abst.P407). [Google Scholar]


