Abstract
Background
A large proportion of people with advanced cancer will experience moderate to severe pain. Tapentadol is a novel, centrally acting analgesic medicine acting at the μ‐opioid receptor and inhibiting noradrenaline reuptake. The efficacy of tapentadol is stated to be comparable to morphine and oxycodone.
Objectives
To assess the analgesic efficacy of tapentadol for the relief of cancer pain in adults, and the adverse events associated with its use in clinical trials.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and EMBASE from January 2005 to July 2015, together with reference lists of retrieved papers and review articles, and two clinical trial registries. Searches started from 2005 because this covered the period during which clinical trials were conducted. We contacted the manufacturer of tapentadol in the UK to find additional trials not identified by electronic searches. We did not restrict searches by language.
Selection criteria
We included randomised controlled trials (RCTs) of tapentadol compared with placebo or active controls in adults with moderate to severe cancer pain. Pain had to be measured using a validated assessment tool, and studies had to include at least 10 participants per treatment arm.
Data collection and analysis
Two review authors independently extracted data using a standard form and assessed risk of bias. We extracted available data on study design, participant details, interventions, and outcomes, including analgesic outcome measures, withdrawals, and adverse events.
Main results
We included four studies with 1029 participants. All the studies used a parallel‐group design, and included an initial titration phase to determine the maximum effective and tolerated dose, followed by a maintenance phase. Tapentadol medication was taken twice daily and doses ranged from 50 to 500 mg per day. Rescue medication (morphine or oxycodone immediate‐release) was available to participants in all studies.
Overall, 440 participants were randomised in classically designed RCTs, and 589 participants were enrolled in enriched‐enrolment, randomised‐withdrawal (EERW) trials. A total of 476 participants were randomised to titration with tapentadol and 338 participants took tapentadol throughout the maintenance phase of their trial.
All studies used numerical rating scores, Patient Global Impression of Change scores, and use of rescue medication as measures of efficacy, and all reported on adverse events and withdrawals.
All studies enrolled fewer than 200 participants per treatment arm and were therefore at risk of overestimating efficacy. One study was terminated early due to problems with supply of rescue medication, with fewer than 20 participants enrolled per treatment arm in the maintenance phase of the trial. We judged another study at high risk of bias due to an open‐label design.
There were insufficient data for pooling and statistical analysis. Response rates for pain intensity were comparable across treatment groups in each study. In one EERW study, response rates were high across both treatment and placebo arms during the maintenance phase (62% tapentadol, 69% morphine, 50% placebo). For pain relief, tapentadol is no more and no less effective than oxycodone or morphine (low quality evidence).
Treatment emergent adverse event rates were high, approximately 50% to 90%. The most common adverse events were gastrointestinal (nausea, vomiting, constipation) (low quality evidence). There was no advantage of tapentadol over morphine or oxycodone in terms of serious adverse events. The number of people experiencing effects on consciousness, appetite, or thirst was low.
Authors' conclusions
Information from RCTs on the effectiveness and tolerability of tapentadol was limited. The available studies were of moderate or small size and used different designs, which prevented pooling of data. Pain relief and adverse events were comparable between the tapentadol and morphine and oxycodone groups.
Plain language summary
Oral tapentadol for cancer pain
Tapentadol taken by mouth produced good pain relief for people with moderate to severe cancer pain, similar to morphine or oxycodone.
One person in two or three who gets cancer will experience moderate to severe pain. As the cancer advances, the pain may get worse. Morphine has been used since the 1950s for controlling cancer pain. Since then, a number of medications with morphine‐like actions have been developed for controlling pain, one of which is tapentadol. Tapentadol has been studied in clinical trials since 2005, but has only been used in the UK since 2011. It is available in tablets of different strengths, and is normally taken twice a day. In this review, we set out to estimate how well tapentadol worked and how many people had side effects, including serious effects or those that stopped people from taking the medication.
We searched medical databases for clinical trials in adults with moderate to severe cancer pain that compared tapentadol with placebo (dummy medicine) or other pain‐relieving medicines, and measured pain using recognised assessment methods.
We found four studies with 1029 participants. All four studies compared participants taking tapentadol to participants taking similar medicine, such as morphine or oxycodone. All studies gave participants a period of time to find the best dose to take, before continuing on the medication and comparing their pain levels.
All the studies were small or medium sized, so the results are at risk of being influenced by random fluctuations rather than real differences, and they may also overestimate any effects. One trial allowed participants to know what medication they were taking, and one trial was stopped early due administrative problems, so they did not have enough people in the study. We have to be cautious interpreting results from these studies.
Because the studies all used different designs, we could not compare the results from one with another. However, each study showed that there was not much difference between the pain levels of people taking tapentadol and people taking morphine and oxycodone. Pain levels were generally well controlled. The studies also showed there was no measurable difference in how many adverse effects people had while taking tapentadol, morphine, or oxycodone.
Therefore, we can conclude only that the studies to date show tapentadol was no more or less effective and no more or less well tolerated than morphine and oxycodone.
Background
Description of the condition
Cancer is a common disease. There is a greater than 1 in 3 risk of developing cancer over a person's lifetime. In 2011 (the latest year for which statistics are available), approximately 331,000 people were diagnosed with cancer in the United Kingdom (UK), and the incidence is rising (Cancer Research UK). Worldwide there were estimated to be around 14.1 million new cases of cancer in 2012, with incidence rates varying across the world (Cancer Research UK). One review of the pharmacological management of cancer pain reported that 24% to 62% of adults have pain at the time of cancer diagnosis, and almost all patients will be in pain in the terminal stages of the disease (Cleary 2007). Pain can be debilitating and have a serious impact on the quality of life.
Description of the intervention
Tapentadol is an opioid used to treat moderate to severe pain. It was first marketed in December 2010 in Australia, and in 2011 in the UK and United States of America (USA). Internationally, it is available as 25 mg, 50 mg, 100 mg, 150 mg, 200 mg, and 250 mg prolonged‐release tablets given 12‐hourly to a maximum of 500 mg daily for maintenance treatment; 50 mg and 75 mg immediate‐release (IR) tablets given four‐ to six‐hourly are available for acute pain and dose titration (UKMI 2011). Tapentadol is a novel, centrally acting analgesic medicine, with an analgesic efficacy stated to be comparable to that of strong opioids such as oxycodone and morphine. It is both a μ‐opioid receptor agonist and noradrenaline reuptake inhibitor. It is a controlled drug under the Misuse of Drugs regulations in the UK, and a Schedule II controlled substance in the USA. A separate Cochrane review of tapentadol for chronic musculoskeletal pain is available (Santos 2015).
How the intervention might work
Tapentadol is purported to work by two different mechanisms: at opioid receptors and by inhibiting noradrenaline reuptake. Both mechanisms of action contribute to the analgesic activity and produce analgesia in a synergistic manner, such that relatively moderate activity at the two target sites is sufficient to produce a strong analgesic action (Tzschentke 2014).
Why it is important to do this review
This review is one of a number of Cochrane reviews on the efficacy of strong analgesics for the relief of cancer‐related pain.
Objectives
To assess the analgesic efficacy of tapentadol for the relief of cancer pain in adults, and the adverse events associated with its use in clinical trials.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs)
Double‐blind studies, but we included single‐blind and open studies for inclusion as secondary evidence
Placebo or active controls, or both
Minimum of 10 participants per treatment arm
Types of participants
Adult men and women aged 18 years and over, with cancer pain of moderate to severe intensity.
Types of interventions
Oral tapentadol compared to placebo or active controls, in any dose, frequency, or duration of treatment.
Types of outcome measures
Pain had to be measured using a validated assessment tool. For pain intensity, for example, this could be a 100 mm visual analogue scale (VAS) (no pain to worst pain imaginable) or a four‐point categorical scale (none, mild, moderate, severe), and for pain relief a 100 mm VAS (no relief to complete relief), or five‐point categorical scale (none, a little, some, a lot, complete or words to that effect). Measures of at least 30% (moderate) and at least 50% (substantial) reduction of pain over baseline are recommended outcomes for chronic pain studies from the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) (Dworkin 2008). When considering Patient Global Impression of Change (PGIC), at least 30% reduction of pain over baseline equates to much improved or very much improved, and at least 50% to very much improved.
Primary outcomes
Number of participants with pain reduction of ≥ 30% from baseline
Number of participants with pain reduction of ≥ 50% from baseline
Number of participants with pain no worse than mild
Number of participants with PGIC of much improved or very much improved (or equivalent wording)
Secondary outcomes
Quality of life measures
Use of rescue medication
Participant satisfaction or preference
Adverse events: any, serious
Attrition: withdrawals due to lack of efficacy or adverse events (including death)
Search methods for identification of studies
Electronic searches
We searched the following databases.
The Cochrane Central Register of Controlled Trials (CENTRAL, via CRSO; to 28 July 2015).
MEDLINE (via Ovid; 2005 to 28 July 2015).
EMBASE (via Ovid; 2005 to 28 July 2015).
Searches started from 2005 because this covered the period during which clinical trials were conducted.
Searching other resources
We searched the reference lists of all included studies and any relevant reviews. We also searched two clinical trial registers (ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/)) to identify additional published or unpublished data.
We contacted the manufacturer of tapentadol in the UK to find additional trials not identified by electronic searches.
Language
We did not restrict searches or inclusion by language.
Data collection and analysis
Selection of studies
Two review authors independently read the titles and abstracts of all studies identified by the searches, and excluded those that clearly did not meet the inclusion criteria. For the remaining studies, we read the full manuscripts to assess whether they should be included. We resolved any discrepancies between review authors by discussion; where necessary, we consulted a third review author. We did not anonymise studies before selection.
Data extraction and management
Two review authors independently extracted data using a standard form and agreed on the data before entry into Review Manager 5 software (RevMan 2014). We extracted information about the number of participants treated and demographic details, type of cancer, drug and dosing regimen, study design (placebo or active control) and methods, study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group. We resolved any disagreements by discussion. We assessed the following for each included study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, for example random number table; computer random number generator); unclear risk of bias (when the method used to generate sequence was not clearly stated). We excluded studies at high risk of bias that used a non‐random process (for example, odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (for example, telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (when the method was not clearly stated). We excluded studies that did not conceal allocation and were therefore at a high risk of bias (for example, open list).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, for example, identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved); high risk of bias (study was not double‐blind).
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (fewer than 10% of participants did not complete the study or used 'baseline observation carried forward' (BOCF) analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); high risk of bias (used 'completer' analysis).
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm)
Measures of treatment effect
We planned to use dichotomous data to calculate risk ratios (RR) with 95% confidence intervals (CI), and calculated numbers needed to treat for an additional beneficial outcome (NNT) and the number needed to treat for an additional harmful outcome (NNH) as the reciprocal of the absolute risk reduction (McQuay 1998). In the event, there were insufficient data for statistical analysis.
If there had been sufficient data, we would have used the following terms to describe adverse outcomes in terms of harm or prevention of harm.
When significantly fewer adverse outcomes occurred with tapentadol compared with control (placebo or active), we planned to use the term 'number needed to treat to prevent one event' (NNTp).
When significantly more adverse outcomes occurred with tapentadol compared with control (placebo or active), we planned to use the term 'number needed to harm or cause one event' (NNH).
We did not plan to use continuous data for the primary outcome because it is inappropriate where there is an underlying skewed distribution, as is usually the case with analgesic response.
Dealing with missing data
We planned to use intention‐to‐treat (ITT) analysis, including participants who were randomised, took the study medication, and gave a minimum of one post‐baseline assessment. Where there were missing participants or information, we planned to assign them to a zero improvement category where possible. We also looked for information about how data from withdrawals and drop‐outs were handled. In original studies, participants may have been analysed using LOCF (that is, their level of pain when stopping the medication) or BOCF (that is, returned to their baseline observation).
Where there were substantial numbers (> 10%) of participants missing from analyses, we have commented.
Assessment of heterogeneity
We planned to assess statistical heterogeneity using L'Abbé plots, a visual method for assessing differences in results of individual studies (L'Abbé 1987), and by using the I2 statistic. We anticipated that there could be an effect of differences between participants, environment (inpatient versus outpatient), and outcome measures. We planned to explore these with sensitivity analyses where there were sufficient data. In the event, there were insufficient data for statistical analysis.
Assessment of reporting biases
The aim of this review was to use dichotomous data of known utility (Moore 2010; Moore 2013). The review did not depend on what authors of the original studies chose to report or not.
We planned to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean an NNT of 10 or higher) (Moore 2008). In the event, there were insufficient data for statistical analysis.
Data synthesis
We planned to carry out data synthesis and statistical analysis using Review Manager 5 software (RevMan 2014). Where appropriate, we would have pooled data for each dichotomous outcome and calculated RRs with 95% CIs using the fixed‐effect model (Morris 1995), together with NNTs with 95% CIs (Cook 1995). We would have assumed a statistically significant benefit of active treatment over control when the lower limit of the 95% CI of the RR was greater than one, and of control over active treatment when the upper limit of the 95% CI was less than one. We planned to calculate RR and NNH for adverse outcomes in the same way.
We planned to analyse studies carried out under double‐blind conditions separately from those that were not, and not to carry out pooled analysis where there were fewer than 200 participants in the comparison (Moore 1998). In the event, there were insufficient data for statistical analysis.
We intended to test for statistically significant differences between subgroups using the z test (Tramèr 1997); however, data were insufficient.
Subgroup analysis and investigation of heterogeneity
We did not plan to carry out subgroup analysis because were aware that the evidence base was small.
Different doses were not considered because participants were titrated to effective dose.
Sensitivity analysis
We planned to carry out sensitivity analyses for duration of study, age of participants (< 18 years versus ≥ 18 years), and setting (inpatient versus outpatient), but there were insufficient data for analysis.
Results
Description of studies
Results of the search
Searches identified 60 potentially relevant studies in CENTRAL, 16 in MEDLINE, and 133 in EMBASE. We also identified six studies in ClinicalTrials.gov. After screening titles and abstracts, we read three studies and four clinical trial records in full (Figure 1). The records in ClinicalTrials.gov provided useful data on adverse events that were not available in the published studies.
1.
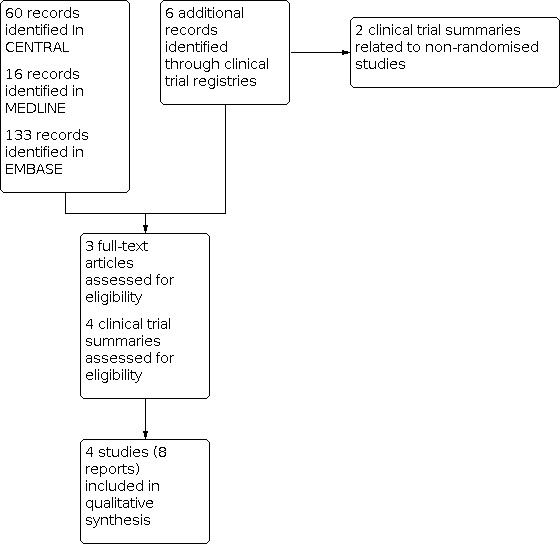
Study flow diagram.
We identified no additional studies through contacting the manufacturer.
Included studies
We included four studies (eight reports) with 1029 participants (Imanaka 2013; Imanaka 2014; Kress 2014; NCT00505414). All the studies enrolled participants with chronic malignant tumour‐related pain who were experiencing pain intensities of at least 4/10 with current treatment. The mean age was 60 to 65 years, with most participants aged 50 to 75 years.
All the studies included an initial titration phase to determine the maximum effective and tolerated dose. Medication was taken twice daily. Starting doses were dependent on previous opioid exposure and were as low as 25 mg twice daily for participants who had not taken opioids in the previous 28 days in Imanaka 2013, but 100 mg twice daily in opioid naïve participants in Kress 2014 and NCT00505414. Maximum doses were 200 to 250 mg twice daily.
Rescue medication was available to participants in all studies; three studies used IR morphine as rescue medication for all treatment groups, and one study used an IR form of either morphine or oxycodone, with doses varying from morphine IR 5 mg or oxycodone IR 2.5 mg to a dose equivalent to one‐sixth of total daily opioid dose. All studies used pain intensity numerical rating scores (NRS), PGIC scores, and use of rescue medication as primary outcome measures.
All the studies were randomised and used a parallel‐group design, but otherwise their methods were different. Study size varied from 93 to 496 participants.
No studies reported on quality of life, quantities of rescue medication used, or participant satisfaction or preference.
Imanaka 2013 was a four‐week (including titration) double‐blind, active‐controlled study of tapentadol extended‐release (ER) with oxycodone controlled‐release (CR).
Imanaka 2014 was a four‐week open‐label, active‐controlled, dose‐titration study using tapentadol ER and morphine sustained‐release (SR).
Kress 2014 was an enriched‐enrolment, randomised‐withdrawal, active‐ and placebo‐controlled, double‐blind efficacy trial. It had a two‐week titration phase (tapentadol ER or morphine SR) and four‐week maintenance phase (tapentadol ER or placebo, or morphine SR).
NCT00505414 used a similar design to Kress 2014. This study was terminated early due to a recall of the morphine rescue medication and issues regarding supply of an alternative. Only 93 of the planned 573 participants were available for analysis.
Full details are in the Characteristics of included studies table. CR, SR, and ER are equivalent terms for formulations designed to provide prolonged effects while maintaining relatively constant drug levels that are within a 'safe' therapeutic window.
Excluded studies
We did not exclude any studies after reading the full text. Two of the clinical trial reports related to non‐randomised studies.
Risk of bias in included studies
Allocation
All the studies stated that they were randomised, and three reported adequate methods of sequence generation and allocation concealment (Imanaka 2013; Imanaka 2014; Kress 2014). The online clinical trial summary of the remaining study did not report details of the sequence generation or allocation process, although it is likely that this was adequate (NCT00505414).
Blinding
Two studies adequately reported the methods used to maintain blinding of participants and study personnel (Kress 2014; NCT00505414), and one did not report details of the method (Imanaka 2013). The remaining study was an open‐label study during which participants were switched from one treatment to another (Imanaka 2014); we judged this study at high risk of bias.
Incomplete outcome data
There were several minor discrepancies between the text and the tables or figures in the published studies, and between published results and the online clinical trial summaries. We considered that these were unlikely to change our conclusions substantially, given the already small amount of data and uncertainties about any treatment effects.
Other potential sources of bias
None of the included studies enrolled 200 or more participants per treatment arm, which we consider is the minimum required to give confidence in the results. Three studies enrolled between 50 and 199 participants per treatment arm (Imanaka 2013; Imanaka 2014; Kress 2014), where the effects of size are unknown. One study was terminated early and enrolled fewer than 50 participants per treatment arm (NCT00505414). We judged this study to be potentially at high risk of bias.
Figure 2 and Figure 3 show a graphical representation.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Effect on pain
Imanaka 2013 was a four‐week (including titration) double‐blind study of 340 Japanese and Korean participants. The majority of participants (> 92%) had metastatic cancers. Those recruited were not satisfied with their current pain management and after screening were randomised to receive either tapentadol ER at 25 to 200 mg twice daily or oxycodone CR at 5 to 40 mg daily.
The study did not report our primary outcome of no worse than mild pain.
Mean pain intensity was assessed once daily using an 11‐point NRS. For tapentadol 63/168, and for oxycodone 59/172, participants achieved a reduction in pain intensity of at least 50%. For tapentadol 80/168 and for oxycodone 82/172, participants achieved a reduction in pain intensity of at least 30%. Using PGIC at the level of 'much or very much improved', this was achieved by 74/168 participants in the tapentadol group and 70/172 participants in the oxycodone group, and for 'very much improved' the numbers were 22/168 for tapentadol and 17/172 for oxycodone.
Imanaka 2014 was a four‐week open‐label, active‐controlled, dose‐titration study in 100 Japanese participants with chronic malignant tumour‐related pain. In this case, participants were converted from other opioid medicines (oxycodone, morphine, or fentanyl) using a ratio of 10:2 for oxycodone, 10:3 for morphine, and 10:0.03 for fentanyl. Participants were randomised to receive either tapentadol ER at 100 to 500 mg daily, or morphine CR at 20 to 140 mg daily.
The study did not report our primary outcomes of ≥ 30% and ≥ 50% pain reduction, or no worse than mild pain.
Pain intensity was assessed as an average score once a day using an 11‐point NRS. For tapentadol 42/50, and for morphine 49/50, participants maintained pain control comparable with pre‐study levels. Investigators also assessed PGIC, but as these participants started the study with good pain relief, the results were low and not meaningful.
Kress 2014 was an enriched‐enrolment study conducted in 16 countries. A total of 496 participants were either opioid naive or dissatisfied with their current opioid therapy and presented with a pain intensity of > 5/10. Participants were titrated to an optimal dose using either tapentadol ER or morphine CR over two weeks. Participants who completed this phase and were stable with a mean pain intensity of < 5/10 were moved into the maintenance phase (a period of stable dose after titration). Participants receiving morphine continued on the same dose as before while participants receiving tapentadol were randomised to either tapentadol or placebo. Oral morphine sulphate IR was available as a rescue medication with no maximum dose.
The study did not report our primary outcomes of ≥ 30% and ≥ 50% pain reduction and no worse than mild pain. PGIC was reported only in the ClinicalTrials.gov record (NCT00472303, see Kress 2014). PGIC much and very much improved at the end of the maintenance phase was reported by 33/94 participants with tapentadol, 29/97 with morphine, and 37/103 with placebo. PGIC very much improved at the end of the maintenance phase was reported by 4/94 participants with tapentadol, 6/97 with morphine, and 6/103 with placebo.
Response was assessed by pain intensity, but also a measure of rescue medication use and a measure of treatment adherence linked to tolerability. Responders had pain intensity < 5/10 (≤ moderate pain), used a mean morphine dose of < 20 mg daily, and remained in the study for the whole 28 days. Due to the complex design of this study and because we were unable to perform a meta‐analysis, we have reported per‐protocol data. In the titration phase, 174/229 participants with tapentadol and 83/100 with morphine achieved a pain intensity score of < 5. In the maintenance phase, the numbers of responders were 65/105 participants with tapentadol, 75/109 with morphine, and 55/111 with placebo.
NCT00505414 used a similar design to Kress 2014. This study was terminated early but limited results were available on the ClinicalTrials.gov website. Only 93 of the planned 573 participants were available for analysis.
The study did not report our primary outcomes of ≥ 30% and ≥ 50% pain reduction, or no worse than mild pain. PGIC much and very much improved at the end of the maintenance phase was reported by 8/15 participants with tapentadol, 4/13 with morphine, and 2/9 with placebo. PGIC very much improved at the end of the maintenance phase was reported by 0/15 participants with tapentadol, 1/13 with morphine, and 0/9 with placebo.
Compared with Kress 2014, responders could have up to 30 mg morphine IR rescue medication per day on average. The maintenance phase enrolled fewer than 20 participants per group. Responders in the maintenance phase were recorded as 8/15 participants with tapentadol, 6/18 with morphine, and 3/14 with placebo.
Adverse events
Imanaka 2013 reported that treatment‐emergent adverse events were similar in both groups with approximately 90% of participants experiencing such an event. The most common events were gastrointestinal: nausea, vomiting, or constipation. The rates were 55% (93/168) with tapentadol and 67% (116/172) with oxycodone. These events were most common in week one and were similar irrespective of gender, age, or initial pain intensity. Adverse events leading to treatment withdrawal were experienced by 13% (22/168) of participants with tapentadol and 17% (29/172) with oxycodone. Somnolence occurred in 17% (29/168) of participants with tapentadol and 20% (36/172) with oxycodone. Delirium was reported by 6/168 participants with tapentadol and 6/172 with oxycodone based on the ClinicalTrials.gov record for this study.
In addition, 21/168 participants taking tapentadol and 24/172 taking oxycodone reported a decrease in appetite. Just one participant in each group reported dehydration (based on ClinicalTrials.gov record).
Imanaka 2014 reported that treatment‐emergent adverse events were similar in both groups with approximately 90% of participants experiencing such an event, the percentage was slightly higher in the cohort aged over 65 years. The most common events were gastrointestinal: nausea, vomiting, or constipation. The rates were 38% (19/50) with tapentadol and 54% (27/50) with morphine. These events were most common in week one. Adverse events leading to treatment withdrawal were experienced by 28% (14/50) of participants with tapentadol and 38% (19/50) with morphine. Somnolence occurred in 16% (8/50) of participants with tapentadol and delirium was recorded for 1/50 participants with morphine.
One participant in the morphine group reported weight loss, one participant in each group reported a decrease in appetite, and 2/50 participants with tapentadol and 1/50 with morphine reported stomatitis.
Kress 2014 reported that treatment‐emergent adverse events were recorded in 50% of the tapentadol group and 64% of the morphine group during the titration phase. The most common events were gastrointestinal: nausea, vomiting, or constipation. The rates were 30% (100/338) with tapentadol and 47% (74/158) with morphine. During the maintenance phase, the number of participants experiencing treatment‐emergent adverse events remained high, at about 60% for all groups: 66/106 participants with tapentadol, 68/109 with morphine, and 63/112 with placebo. The number experiencing gastrointestinal events was 25% to 30% (24/106 participants with tapentadol, 25/109 with morphine, and 28/112 with placebo). Adverse events leading to treatment withdrawal occurred in about 8% of participants in the titration phase for both tapentadol (28/338) and morphine (12/158), and 5% in the maintenance phase for all three treatment groups (5/106 participants with tapentadol, 6/109 with morphine, and 6/112 with placebo). Somnolence occurred in 3% (3/106) of participants with tapentadol and 5% (3/109) with morphine, and delirium was not reported.
In the maintenance phase, dry mouth was reported by 3/106 participants with tapentadol, 1/109 with morphine, and 2/112 with placebo. A decreased appetite was reported for 8/106 participants with tapentadol, 6/109 with morphine, and 6/112 with placebo.
NCT00505414 reported that treatment‐emergent adverse events were recorded in 63% of the tapentadol group and 80% of the morphine group. The most common events were gastrointestinal: nausea, vomiting, or constipation. The rates were 48% (29/62) participants with tapentadol and 61% (19/31) with morphine. Adverse events leading to treatment withdrawal were low in all groups. Somnolence occurred in 3% (2/62) of participants with tapentadol and 6% (2/31) with morphine, and delirium was not reported.
Dry mouth was reported for one participant in the placebo group and thirst by one in the morphine group. There were three cases of anorexia: two in the morphine group and one in the placebo group.
Withdrawals
Withdrawals due to adverse events, lack of efficacy, death, or other reasons are described in Appendix 1. There was no evidence of a difference between tapentadol, oxycodone, morphine, or placebo for any cause of withdrawal.
Discussion
Summary of main results
For pain relief these studies show that tapentadol is as effective as oxycodone or morphine in the comparator studies. However, there is no evidence that tapentadol is superior (low quality evidence). There were two enriched‐enrolment randomised‐withdrawal studies (Kress 2014; NCT00505414), the second of which was terminated early due to supply problems with the rescue medication. In the Kress 2014 study, the response rates during the double‐blind withdrawal phase were high in all groups including the placebo arm. We note that entry criteria included a pain intensity of greater than 5/10 and that for response, a pain intensity of less than 5/10 was required. We anticipate that such a small improvement is readily obtainable.
There was no advantage of tapentadol over morphine or oxycodone in terms of serious adverse events in these studies (very low quality evidence).
In a previous review we examined the impact of four opioids on patient consciousness, appetite, and thirst (Wiffen 2014). These symptoms have raised concern relating to the management of people who are nearing the end of life. In this review, the numbers of participants experiencing these symptoms were low.
Overall completeness and applicability of evidence
Tapentadol was first licensed for use in the UK and USA in 2011. To date, there are just three completed studies published worldwide, with approximately 1000 participants. We anticipate that more trials will be undertaken. The current published trials give some indication of the place of this analgesic in managing cancer pain.
Quality of the evidence
The included studies were generally of good quality, although one study was an open‐label study that could have been blinded (Imanaka 2014), and all studies had fewer than 200 participants per treatment arm. Clinically useful outcomes were reported for both efficacy and harm in all studies.
Potential biases in the review process
We carried out a thorough search for studies and feel it is unlikely that we have missed any large body of research. The different study designs precluded any pooling of data, so we reported results in a narrative manner.
Agreements and disagreements with other studies or reviews
We are not aware of any other up‐to‐date reviews of oral tapentadol for cancer pain.
Authors' conclusions
Implications for practice.
For people with cancer
There is little in this review to suggest that tapentadol should be considered above other opioids for the treatment of cancer‐related pain in terms of benefits or of harms.
For clinicians
Current policies on the use of opioids, particularly morphine, do not need to be amended.
For policy‐makers and funders
There is no persuasive evidence to amend existing cancer pain guidance to include tapentadol. Tapentadol may be worth trying if other medications have failed.
Implications for research.
General
All opioids present challenges in terms of the risk:benefit ratio. Research should focus on finding better analgesics to manage cancer‐related pain.
Design
Randomised controlled trials of large size, ideally at least 200 participants per treatment arm, using robust methods, and reporting clinically useful outcomes are needed to distinguish between different opioids to treat cancer‐related pain in terms of benefit, and particularly for adverse events.
Measurement (endpoints)
A reduction of pain intensity of at least 50% should be included alongside other endpoints such as Patient Global Impression of Change for determining efficacy, and adverse event outcomes should take into account concerns about safety and serious adverse events. Data should be presented as the number of participants who respond. Mean data for pain relief are not acceptable.
What's new
| Date | Event | Description |
|---|---|---|
| 14 October 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 1, 2015 Review first published: Issue 9, 2015
| Date | Event | Description |
|---|---|---|
| 18 February 2020 | Amended | Clarification added to Declarations of interest. |
| 12 September 2019 | Review declared as stable | See Published notes. |
| 28 May 2019 | Amended | Contact details updated. |
| 3 July 2017 | Review declared as stable | See Published notes. |
Notes
Assessed for updating in 2017
A restricted search in June 2017 did not identify any potentially relevant studies likely to change the conclusions. Tapentadol is a new drug (first marketed in December 2010 in Australia, and in 2011 in the UK and United States of America (USA)) and there may be ongoing studies not recorded on trials registries. This review has now been stabilised for two years following discussion with the authors and editors, and we will reassess the review for updating in 2019. If appropriate, we will update the review if new evidence likely to change the conclusions is published before this date, or if standards change substantially which necessitate major revisions.
Assessed for updating in 2019
The authors and editors are not aware of any new studies with the potential to change the conclusions. This review has now been stabilised again and we will reassess the review for updating in 2020. If appropriate, we will update the review if new evidence likely to change the conclusions is published before this date, or if standards change substantially which necessitate major revisions.
Assessed for updating in 2020
At September 2020 we did not identify any potentially relevant studies likely to change the conclusions, although we did find a post hoc subgroup analysis for Kress 2014 (Kress 2016). Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Summary of outcomes: adverse events and withdrawals
| Study ID | Treatment | Any AEs | SAEs | Withdrawals |
| Imanaka 2013 | Tapentadol ER (25 to 200 mg twice daily), n = 168 Oxycodone CR (5 to 40 mg twice daily), n = 172 |
Tapentadol 147/168 Oxycodone 155/172 | Tapentadol 78/168 Oxycodone 69/172 Most SAEs due to disease progression (tapentadol 40, oxycodone 36) and judged unrelated to study medication | AE Tapentadol 12/168 Oxycodone 14/172 Deaths Tapentadol 3/168 Oxycodone 4/172 LoE Tapentadol 4/168 Oxycodone 1/172 Disease progression Tapentadol 10/168 Oxycodone 14/172 Other Tapentadol 29/168 Oxycodone 18/172 |
| Imanaka 2014 | Tapentadol ER titrated to maximum 500 mg daily, n = 50 Morphine SR titrated to maximum 140 mg daily, n = 50 |
Tapentadol 45/50 Morphine 47/50 | Tapentadol 16/50
Morphine 16/50 Deaths Tapentadol 6/50 (disease progression 5, GI perforation 1) Oxycodone 4/50 (disease progression) |
AE Tapentadol 5/50 Morphine 8/50 Disease progression Tapentadol 9/50 Morphine 11/50 LoE Tapentadol 3/50 Morphine 1/50 Other Tapentadol 5/50 Morphine 1/50 |
| Kress 2014 | Tapentadol PR 100 to 250 mg twice daily, n = 338 (titration) and n = 106 (maintenance) Morphine CR 40 to 100 mg twice daily, n = 158 (titration) and n = 109 (maintenance) Placebo n = 112 (maintenance) |
During titration
Tapentadol 169/338
Morphine 101/158 During maintenance Tapentadol 66/106 Morphine 68/109 Placebo 63/112 |
During titration
Tapentadol 25/338
Morphine 6/158
Most common (7/338, 2/158) were neoplasms During maintenance Tapentadol 12/106 Morphine 6/109 Placebo 10/112 (Neoplasms 5/106, 1/109, 2/112) |
During and after titration AE Tapentadol 29/338 Morphine 11/158 LoE Tapentadol 58/338 Morphine 17/158 Other Tapentadol 30/338 Morphine 18/158 Maintenance AE Tapentadol 5/106 Morphine 7/109 Placebo 5/112 LoE Tapentadol 2/106 Morphine 2/109 Placebo 4/112 Other Tapentadol 7/106 Morphine 8/109 Placebo 5/112 |
| NCT00505414 | Tapentadol PR 100 to 250 mg twice daily (titration and maintenance), n = 62 (titration) and n = 15 (maintenance)
Morphine sulphate CR 45 to 90 mg twice daily (titration and maintenance), n = 31 (titration) and n = 18 (maintenance) Placebo, n = 14 (maintenance) |
Over entire treatment period
Tapentadol 39/62
Morphine 25/31 Placebo 6/14 |
Tapentadol 13/62
Morphine 8/31 Placebo 4/14 Deaths During titration Tapentadol 2/62 Morphine 0/31 During maintenance Tapentadol 0/15 Morphine 2/18 Placebo 1/14 |
Titration AE Tapentadol 8/62 Morphine 3/31 LoE Tapentadol 10/62 Morphine 1/31 Other Tapentadol 10/62 Morphine 8/31 Maintenance AE Tapentadol 2/15 Morphine 3/18 Placebo 2/14 LoE Tapentadol 1/15 Morphine 2/18 Placebo 1/14 Other Tapentadol 2/15 Morphine 0/18 Placebo 3/14 |
| AE: adverse event; CR: controlled release; ER: extended release; GI: gastrointestinal; LoE: lack of efficacy; n: number of participants in treatment arm; PR: prolonged release; SAE: serious adverse event; SR: sustained release. | ||||
Appendix 2. Search strategy for MEDLINE (via Ovid)
(tapentadol or Nucynta or Palexia).mp. (172)
exp Neoplasms/ (2618893)
cancer.mp (999207)
exp Pain/ (310902)
pain*.mp. (499161)
2 or 3 (2754707)
4 or 5 (575504)
1 and 6 and 7 (16)
Limit 8 to yr="2005‐Current" (16)
Appendix 3. Search strategy for EMBASE (via Ovid)
(tapentadol or Nucynta or Palexia).mp. (710)
exp Neoplasm/ (3386153)
cancer.mp (2247602)
exp Pain/ (310902)
pain*.mp. (865079)
2 or 3 (3720917)
4 or 5 (1115949)
1 and 6 and 7 (133)
Limit 8 to yr="2005‐Current" (133)
Appendix 4. Summary of outcomes: efficacy
| Study ID | Treatment | Clinical response | Other response | Participants using rescue medication |
| Imanaka 2013 | Tapentadol ER (25 to 200 mg twice daily), n = 168 Oxycodone CR (5 to 40 mg twice daily), n = 172 |
Using ITT denominators ≥ 50% red in PI Tapentadol 63/168 Oxycodone 59/172 ≥ 30% red in PI Tapentadol 80/168 Oxycodone 82/172 |
Using ITT denominators PGIC very much improved Tapentadol 22/168 Oxycodone 17/172 PGIC much and very much improved Tapentadol 74/168 Oxycodone 70/172 |
PP population Used: Tapentadol 94/126 Oxycodone 103/139 Days of use: Tapentadol 7.6 (SD 7.7) Oxycodone 7.2 (SD 7.8) Doses/day: Tapentadol 1.4 (SD 0.5) Oxycodone 1.4 (SD 0.4) Mean TDD for double‐blind period (in those using it) Tapentadol 6.95 (SD 2.30) (n = 94) Oxycodone 6.73 (SD 2.15) (n = 103) |
| Imanaka 2014 | Tapentadol ER titrated to maximum 500 mg daily, n = 50 Morphine SR titrated to maximum 140 mg daily, n = 50 |
Maintained control Tapentadol 42/50 Morphine 49/50 PGIC ≥ not changed (PP population) Tapentadol 37/48 (week 1), 26/28 (week 8) Morphine 42/48 (week 1), 22/28 (week 8) |
PGIC much or very much improved (PP population)
Tapentadol 2/48 (week 1), 3/28 (week 8) 9 participants discontinued due to disease progression Morphine 6/48 (week 1), 5/28 (week 8) 11 participants discontinued due to disease progression |
Mean doses per day at baseline, and during week 8
Tapentadol 0.4 (SD 0.6), 0.5 (SD 0.8)
Morphine 0.4 (SD 0.6), 0.4 (SD 0.6) Mean days of use (over 8 weeks) Tapentadol 15.9 (SD 20) Morphine 9.2 (SD 13) |
| Kress 2014 | Tapentadol PR 100 to 250 mg twice daily, n = 338 (titration) and n = 106 (maintenance) Morphine CR 40 to 100 mg twice daily, n = 158 (titration) and n = 109 (maintenance) Placebo n = 112 (maintenance) |
Responder ‐ titration (PP population)
Tapentadol 174/229
Morphine 83/100 Responder ‐ maintenance Tapentadol 65/105 Morphine 75/109 Placebo 55/111 |
PGIC very much improved at end of maintenance phase
Tapentadol 4/94
Morphine 6/97 Placebo 6/103 PGIC much and very much improved at end of maintenance phase Tapentadol 33/94 Morphine 29/97 Placebo 37/103 |
Used rescue medication
During titration
Tapentadol 241/335
Morphine 91/157 During maintenance Tapentadol 75/105 Morphine 67/109 Placebo 80/111 |
| NCT00505414 | Tapentadol PR 100 to 250 mg twice daily (titration and maintenance), n = 62 (titration) and n = 15 (maintenance)
Morphine sulphate CR 45 to 90 mg twice daily (titration and maintenance), n = 31 (titration) and n = 18 (maintenance) Placebo, n = 14 (maintenance) |
Responder ‐ maintenance
Tapentadol 8/15
Morphine 6/18 Placebo 3/14 |
PGIC very much improved at end of maintenance phase
Tapentadol 0/15
Morphine 1/13 Placebo 0/9 PGIC much and very much improved at end of maintenance phase Tapentadol 8/15 Morphine 4/13 Placebo 2/9 |
No data |
| CR: controlled release; ER: extended release; ITT: intention to treat; n: number of participants in treatment arm; PGIC: Patient Global Impression of Change; PI: pain intensity; PP: per protocol; PR: prolonged release; red: reduction; SD: standard deviation; SR: sustained release; TDD: total daily dose. | ||||
Appendix 5. Search strategy for CENTRAL (via CRSO)
(tapentadol or Nucynta or Palexia):TI,AB,KW (60)
MESH DESCRIPTOR Neoplasms EXPLODE ALL TREES (41649)
cancer:TI,AB,KW (53548)
MESH DESCRIPTOR pain EXPLODE ALL TREES (29947)
pain*:TI,AB,KW (69524)
2 or 3 (71098)
4 or 5 (74835)
1 and 6 and 7 (60)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Imanaka 2013.
| Study characteristics | ||
| Methods | Randomised, double‐blind, parallel‐group, active control study of tapentadol ER with oxycodone CR | |
| Participants | Chronic malignant tumour‐related pain with PI ≥ 4 on 11‐point numerical rating scale, requiring opioid treatment (investigator assessment) but not having taken an opioid apart from codeine or dihydrocodeine as antitussive over last 28 days Setting: multicentre, Japan and Korea N = 340 Mean age 65 years (SD 11 years) 56% men |
|
| Interventions | Tapentadol ER (25 to 200 mg twice daily), n = 168 Oxycodone CR (5 to 40 mg twice daily), n = 172 Rescue medication: oral morphine IR 5 mg Duration of treatment: 4 weeks |
|
| Outcomes | "Responder" defined as either ≥ 50% reduction from baseline in mean daily PI or ≥ 30% reduction PGIC (7‐point) assessed weekly or early withdrawal (probably LOCF) Rescue medication use Adverse events Withdrawals |
|
| Notes | Funding: Janssen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details of blinding method not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of blinding method not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT responder analysis can be calculated |
| Size | Unclear risk | 50 to 199 participants per treatment arm |
Imanaka 2014.
| Study characteristics | ||
| Methods | Randomised, open‐label, parallel‐group dose titration study | |
| Participants | Chronic malignant tumour‐related pain using opioid analgesia regularly (defined maximum dose and type of opioid), with PI ≤ 4 on 11‐point numerical scale over 3 days prior to randomisation Setting: outpatient, multicentre, Japan N = 100 Mean age 65 years (SD 10 years) 52% men |
|
| Interventions | Tapentadol ER titrated to equivalent of previous total daily opioid dose (maximum 500 mg daily), n = 50 Morphine SR titrated to equivalent of previous total daily opioid dose (maximum 140 mg daily), n = 50 Rescue medication: oral morphine or oxycodone, one‐sixth of the total daily dose of the round‐the‐clock opioid analgesic Duration of treatment: 4 weeks |
|
| Outcomes | Maintenance of control defined as < 1.5 point increase in mean 24‐hour PI and ≤ 2 doses rescue medication per day on 3 consecutive days during first week of treatment PGIC (7‐point) assessed weekly or early withdrawal (probably LOCF) Rescue medication use Adverse events Withdrawals |
|
| Notes | Funding: Janssen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Interactive voice responder system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT responder analysis can be calculated |
| Size | Unclear risk | 50 to 199 participants per treatment arm |
Kress 2014.
| Study characteristics | ||
| Methods | enriched‐enrolment, randomised‐withdrawal, parallel‐group, active‐ and placebo‐controlled, double‐blind efficacy trial Two‐week titration phase (tapentadol or morphine) and 4‐week maintenance phase (tapentadol or morphine or placebo) |
|
| Participants | Chronic malignant tumour‐related pain. PI ≥ 5 on 11‐point numerical rating scale Setting: multicentre, 16 countries, presumed outpatient setting N = 496 randomised into titration phase, 327 entered maintenance phase, 217 completed protocol Mean age 60 years (SD 10 years) 53% men |
|
| Interventions | Tapentadol PR 100 to 250 mg twice daily, n = 338 (titration) and n = 106 (maintenance) Morphine CR 40 to 100 mg twice daily, n = 158 (titration) and n = 109 (maintenance) Placebo n = 112 (maintenance) Rescue medication: morphine sulphate IR 10 mg Duration of treatment: 2‐week titration phase and 4‐week maintenance phase |
|
| Outcomes | "Initial responder" defined as participant who remained in study with PI < 5/10 and mean daily dose of rescue morphine ≤ 20 mg during last 3 days "Prolonged responder" defined as participant who completed 28‐day maintenance period with mean PI ≤ 5/10 and mean daily dose of rescue morphine ≤ 20 mg PGIC (7‐point) at end of maintenance period Use of rescue medication Adverse events Withdrawals |
|
| Notes | Funding: Janssen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Interactive voice recognition system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT responder analysis can be calculated for most outcomes |
| Size | Unclear risk | 50 to 199 participants per treatment arm |
NCT00505414.
| Study characteristics | ||
| Methods | enriched‐enrolment, randomised‐withdrawal, parallel‐group, active‐ and placebo‐controlled, double‐blind efficacy trial Two‐week titration phase (morphine or tapentadol) and 4‐week maintenance phase (morphine or tapentadol or placebo) |
|
| Participants | Chronic malignant tumour‐related pain. Opioid naïve or previously treated with up to 160 mg/day oral morphine equivalent, without satisfactory control. PI ≥ 5 on 11‐point numerical rating scale N = 93 Mean age 62 years (SD 12 years) 49% men |
|
| Interventions | Tapentadol PR 100 to 250 mg twice daily (titration and maintenance)
Morphine sulphate CR 45 to 90 mg twice daily (titration and maintenance) Placebo (maintenance) Rescue medication: morphine sulphate IR Duration of treatment: 2‐week titration phase and 4‐week maintenance phase |
|
| Outcomes | "Responder" defined as participant who completed 28‐day maintenance period with PI ≤ 5/10 and mean daily dose of rescue morphine ≤ 20 mg PGIC: 7‐point scale at end of maintenance phase Adverse events Withdrawals |
|
| Notes | Early termination, due to a recall of the morphine rescue medication and issues regarding supply of an alternative, lead to only 93 participants out of the 573 planned (16%) being available for analysis. The data should be interpreted with caution Funding: Janssen |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of sequence allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "matching placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "matching placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Early study termination, but reason should not lead to bias |
| Size | High risk | < 50 participants per treatment arm |
CR: controlled release; ER: extended release; IR: immediate release; ITT: intention to treat; LOCF: last observation carried forward; N: number of participants in study; n: number of participants in treatment arm; PGIC: Patient Global Impression of Change; PI: pain intensity; PR: prolonged release; SD: standard deviation.
Contributions of authors
PW, SD, and RFB searched for studies and selected studies for inclusion.
KN and SD carried out data extraction and analysis.
SD and PW wrote the first draft, and all authors contributed to writing the full review.
PW will be responsible for the update.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK
General institutional support
External sources
-
The National Institute for Health Research (NIHR), UK
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain.
Declarations of interest
PW, SD, and KN declare no relevant interests.
RFB was a consultant for Pfizer Norway A/S and Grunenthal A/S in a limited capacity, but this was not related to this review.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Imanaka 2013 {published data only}
- Imanaka K, Tominaga Y, Etropolski M, Van Hove I, Ohsaka M, Wanibe M, et al. Adverse event reporting in the recent study by Imanaka et al. describing the efficacy and safety of tapentadol extended release for tumor-related pain. Current Medical Research and Opinion 2014;30(9):1909-10. [DOI: 10.1185/03007995.2014.919909] [DOI] [PubMed] [Google Scholar]
- *.Imanaka K, Tominaga Y, Etropolski M, Hove I, Ohsaka M, Wanibe M, et al. Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain. Current Medical Research and Opinion 2013;29(10):1399-409. [DOI: 10.1185/03007995.2013.831816] [DOI] [PubMed] [Google Scholar]
- Janssen Research and Development, LLC. A safety and efficacy study of JNS024 extended release (ER) in Japanese and Korean patients with chronic malignant tumor-related cancer pain. www.clinicaltrials.gov/ct2/show/NCT01165281 (accessed 14 January 2015) 2013. [CTG: ]
Imanaka 2014 {published data only}
- *.Imanaka K, Tominaga Y, Etropolski M, Ohashi H, Hirose K, Matsumura T. Ready conversion of patients with well-controlled, moderate to severe, chronic malignant tumor-related pain on other opioids to tapentadol extended release. Clinical Drug Investigation 2014;34(7):501-11. [DOI: 10.1007/s40261-014-0204-3] [Clinicaltrials.gove identifier: NCT01309386] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen Pharmaceutical KK. A safety and efficacy study of oral tapentadol extended-release in Japanese participants. www.clinicaltrials.gov/ct2/show/NCT01309386 (accessed 14 January 2015) 2013. [CTG: ]
Kress 2014 {published data only}
- Kress HG (principal investigator). A study to evaluate tapentadol (CG5503) in the treatment of chronic tumor related pain compared with placebo and morphine. www.clinicaltrials.gov/ct2/show/NCT00472303 (accessed 14 January 2015) 2014. [CTG: ]
- Kress HG, Koch ED, Kosturski H, Steup A, Karcher K, Dogan C, et al. Direct conversion from tramadol to tapentadol prolonged release for moderate to severe, chronic malignant tumour-related pain. European Journal of Pain 2016;20(9):1513-8. [DOI: 10.1002/ejp.875] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kress HG, Koch ED, Kosturski H, Steup A, Karcher K, Lange B, et al. Tapentadol prolonged release for managing moderate to severe, chronic malignant tumor-related pain. Pain Physician 2014;17(4):329-43. [PMID: ] [PubMed] [Google Scholar]
NCT00505414 {unpublished data only}
- Poulin P (principal investigator). A study to evaluate the effectiveness and safety of CG5503 (tapentadol) in the treatment of chronic tumor-related pain compared with placebo and morphine. www.clinicaltrials.gov/ct2/show/NCT00505414 (accessed 14 January 2015) 2010. [CTG: ]
Additional references
Cancer Research UK
- Cancer Research UK . Cancer incidence for all cancers combined. info.cancerresearchuk.org/cancerstats/incidence/all-cancers-combined/(accessed 7 September 2015).
Cleary 2007
- Cleary JF. The pharmacologic management of cancer pain. Journal of Palliative Medicine 2007;10(6):1369-94. [DOI: 10.1089/jpm.2007.9842] [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105-21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine 1987;107(2):224-33. [DOI] [PubMed] [Google Scholar]
McQuay 1998
- McQuay H, Moore R. An Evidence-based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0-19-263048-2] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything - large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209-16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: Moore RA, Kalso E, McQuay HJ, editors(s). Systematic Reviews in Pain Research: Methodology Refined. 1st edition. Seattle: IASP Press, 2008:15-24. [ISBN: 978-0931092695] [Google Scholar]
Moore 2010
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374-9. [DOI: 10.1136/ard.2009.107805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2013
- Moore RA, Straube S, Aldington D. Pain measures and cut-offs - 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400-12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG, editors(s). Statistics with Confidence - Confidence Intervals and Statistical Guidelines. London: BMJ Books, 1995:50-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Santos 2015
- Santos J, Alarcão J, Fareleira F, Vaz-Carneiro A, Costa J. Tapentadol for chronic musculoskeletal pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD009923. [DOI: 10.1002/14651858.CD009923.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tramèr 1997
- Tramèr MR, Reynolds DJ, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta-analysis: a case study. BMJ 1997;315(7109):635-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tzschentke 2014
- Tzschentke TM, Christoph T, Kögel BY. The mu-opioid receptor agonist/noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia: the case of tapentadol. CNS Drugs 2014;29:319-29. [DOI: 10.1007/s40263-014-0151-9] [DOI] [PubMed] [Google Scholar]
UKMI 2011
- Davis H. Tapentadol prolonged release. UKMI New Medicine Profile 2011;(3):1-5.
Wiffen 2014
- Wiffen PJ, Derry S, Moore RA. Impact of morphine, fentanyl, oxycodone or codeine on patient consciousness, appetite and thirst when used to treat cancer pain. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD011056. [DOI: 10.1002/14651858.CD011056.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


