Abstract
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 7, 2014) on 'Felbamate as an add‐on therapy for refractory epilepsy'. Epilepsy is a chronic and disabling neurologic disorder, affecting approximately 1% of the population. Up to 30% of people with epilepsy have seizures that are resistant to currently available drugs. Felbamate is one of the second‐generation antiepileptic drugs and we have assessed its effects as an add‐on therapy to standard drugs in this review.
Objectives
To evaluate the efficacy and tolerability of felbamate versus placebo when used as an add‐on treatment for people with refractory partial‐onset epilepsy.
Search methods
For the latest update we searched the Cochrane Epilepsy Specialized Register, CENTRAL, MEDLINE, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform, up to 20 October 2016. There were no language and time restrictions. We reviewed the reference lists of retrieved studies to search for additional reports of relevant studies. We also contacted the manufacturers of felbamate and experts in the field for information about any unpublished or ongoing studies.
Selection criteria
Randomised placebo‐controlled add‐on studies of people of any age with refractory partial‐onset seizures. The studies could be double‐blind, single‐blind or unblinded and could be of parallel or cross‐over design.
Data collection and analysis
Two review authors independently selected studies for inclusion and extracted information. We resolved disagreements by discussion. If disagreements persisted, the third review author arbitrated. We assessed the following outcomes: 50% or greater reduction in seizure frequency; absolute or percentage reduction in seizure frequency; treatment withdrawal; adverse effects; quality of life.
Main results
We included four randomised controlled trials with a total of 236 participants. Two trials were parallel design, the third had a two‐period cross‐over design, and the fourth had a three‐period cross‐over design. Two studies were at an unclear risk of bias for random sequence generation and allocation concealment. These two studies did not include any description of their methods for outcome assessment and performance blinding (i.e. participants or doctors). Two studies were at high risk of bias for incomplete outcome data. Due to significant methodological heterogeneity, clinical heterogeneity and differences in outcome measures, it was not possible to perform a meta‐analysis of the results. Only one study reported 50% or greater reduction in seizure frequency. One study reported absolute and percentage reduction in seizure frequency compared to placebo, P values were 0.046 and 0.018, respectively. One study reported percentage reduction in seizure frequency compared to placebo, but there were no P values. Adverse effects rates were higher during the felbamate period than the placebo period, particularly headache, nausea and dizziness.
Authors' conclusions
In view of the methodological deficiencies, limited number of individual studies and differences in outcome measures, we have found no reliable evidence to support the use of felbamate as an add‐on therapy in people with refractory partial‐onset epilepsy. A large‐scale, randomised controlled trial conducted over a longer period of time is required to inform clinical practice.
Keywords: Humans; Anticonvulsants; Anticonvulsants/adverse effects; Anticonvulsants/therapeutic use; Drug Resistance; Epilepsies, Partial; Epilepsies, Partial/drug therapy; Phenylcarbamates; Phenylcarbamates/adverse effects; Phenylcarbamates/therapeutic use; Propylene Glycols; Propylene Glycols/adverse effects; Propylene Glycols/therapeutic use; Randomized Controlled Trials as Topic
Felbamate as an add‐on therapy for refractory partial epilepsy
Review question
This review aimed to assess whether felbamate has an impact on people with refractory partial‐onset epilepsy when used with other antiepileptic drugs.
Background
Up to 30% people with epilepsy still suffer epileptic seizures despite the use of multiple treatments. Felbamate is a second‐generation antiepileptic drug. However, it is not clear whether felbamate is efficient when used with other antiepileptic drugs.
Study characteristics
We conducted a review of all available, relevant evidence about the impact of felbamate on epilepsy when used with other antiepileptic drugs. This review examined four randomised controlled trials.
Key results
We found no clear evidence to suggest that felbamate was better than placebo at reducing the seizure frequency of 50% or more, nor reducing seizure frequency. Additionally, there was no clear evidence that participants discontinued taking felbamate for intolerable adverse effects.
Certainty of the evidence
It is important to note that the four trials in this review are small and the quality of the evidence is low. The evidence is current to 20 October 2016.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 7, 2014) on 'Felbamate as an add‐on therapy for refractory epilepsy'.
Description of the condition
Epilepsy is a chronic and disabling neurologic disorder characterised by seizures of various types and frequency. It affects approximately 1% of the population (French 1999). Although up to two‐thirds of people with epilepsy will become seizure‐free on a single antiepileptic drug, up to 30% are considered refractory and are not seizure‐free despite multiple medications (Granata 2009). Various criteria have been used to define refractory epilepsy. The consensus definition of refractory epilepsy proposed by the Task Force of the International League Against Epilepsy (ILAE) is now, "failure of adequate trials of two tolerated and appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom" (Kwan 2010).
Over the past 15 to 20 years, numerous second‐generation antiepileptic drugs have become available, since standard drugs (e.g. carbamazepine, phenytoin, valproate) do not control all seizures in all people. Felbamate, one of these antiepileptic drugs, is the subject of this review.
Description of the intervention
Felbamate is an antiepileptic drug that can be taken orally. It is thought to be a broad spectrum drug that is effective for a number of seizure types (Pellock 1999). The use of felbamate has been limited following reports of aplastic anaemia and hepatic failure (Pellock 1999).
How the intervention might work
The exact mechanism of action is unclear. The following possible mechanisms have been suggested: the inhibition of N‐methyl‐D‐aspartate (NMDA) receptor‐related sodium currents; the potentiation of ϒ‐aminobutyric acid (GABA)‐ergic activity; and the inhibition of voltage‐gated sodium channels (Meldrum 1996; Rho 1994).
Why it is important to do this review
Felbamate is marketed in a number of countries as an add‐on treatment. A summary of data regarding its efficacy and tolerability from randomised controlled trials will help inform treatment decisions.
Objectives
To evaluate the efficacy and tolerability of felbamate versus placebo when used as an add‐on treatment for people with refractory partial‐onset epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised placebo‐controlled studies. We included parallel‐group or cross‐over design studies. The studies were double‐blind, single‐blind or unblinded.
Types of participants
Participants of any age with refractory partial‐onset seizures (simple partial, complex partial or secondarily generalised tonic‐clonic seizures).
Types of interventions
The active treatment group received felbamate in addition to conventional antiepileptic drug treatment.
The control group received matching placebo in addition to conventional antiepileptic drug treatment.
Types of outcome measures
Primary outcomes
Reduction in seizure frequency of 50% or more
We compared the proportion of participants with a 50% or greater reduction in seizure frequency in the treatment period with the proportion in the pre‐randomisation baseline period.
Secondary outcomes
Absolute or percentage reduction in seizure frequency
Absolute reduction in seizure frequency is the seizure frequency in the baseline period minus the seizure frequency in the treatment period.
Percentage reduction in seizure frequency is the absolute reduction in seizure frequency divided by the seizure frequency in the baseline period, all multiplied by 100.
Treatment withdrawal
We used the proportion of participants having treatment withdrawn during the course of the treatment period as a measure of 'global effectiveness'. The treatment may have been withdrawn due to adverse effects, lack of efficacy or a combination of both.
Adverse effects
The proportion of participants experiencing the following seven adverse effects:
aplastic anaemia;
hepatic failure;
ataxia;
dizziness;
fatigue;
nausea;and
somnolence.
We chose aplastic anaemia and hepatic failure as they had been reported as the potential risks of felbamate. We chose the others as we considered them to be common and important side effects of antiepileptic drugs.
The proportion of participants experiencing the ten most common adverse effects if different from above.
Quality of life
Since there is lack of consensus on how quality of life should be measured, we summarised data qualitatively.
Search methods for identification of studies
We ran the search for the original review on 20 May 2010. Subsequent searches were run on 24 July 2013, 4 Aug 2015, and 20 October 2016.
Electronic searches
For the latest update we searched the following databases. There were no language and time restrictions.
The Cochrane Epilepsy Specialized Register (20 October 2016) using the search strategy outlined in Appendix 1.
The Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 20 October 2016) using the search strategy outlined in Appendix 2.
MEDLINE (Ovid, 1946 to 20 October 2016) using the search strategy outlined in Appendix 3. (This strategy was based on the Cochrane highly sensitive search strategy for identifying randomised trials (Lefebvre 2011).
ClinicalTrials.gov (20 October 2016) using the search strategy: felbamate AND epilepsy | Studies received on or after 08 April 2015.
WHO International Clinical Trials Registry Platform (ICTRP, 20 October 2016) using the search strategy: felbamate AND epilepsy NOT NCT* (we manually selected trials not found by previous searches).
Searching other resources
We reviewed the reference lists of retrieved studies to search for additional reports of relevant studies.
We contacted the manufacturers of felbamate and experts in the field for information about any unpublished or ongoing studies.
Data collection and analysis
Selection of studies
Two review authors (Shi and Geng) independently assessed studies for inclusion. We resolved disagreements by discussion.
Data extraction and management
We planned to extract data from the trials or make calculations from the primary data supplied by the authors and the manufactures of felbamate. The same two review authors (Shi and Geng) extracted the following information from the included studies. We resolved disagreements by discussion. If disagreements persisted, a third review author (Wu) arbitrated.
Methodological/trial design
Method of randomisation and concealment of randomisation
Method of blinding
Duration of baseline period
Duration of treatment period
Duration of 'wash‐out' period in cross‐over studies
Dose(s) of felbamate tested
Description of withdrawals and drop‐outs
Participant demographic information
Total number of participants allocated to each treatment group
Age/sex
Types of seizure
Mean baseline seizure frequency
Number of background drugs
Outcomes
We recorded the number of participants experiencing each outcome (see Types of outcome measures) per randomised group.
Assessment of risk of bias in included studies
Two review authors (Shi and Geng) independently assessed the methodological quality of the included studies and recorded their findings according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We noted four domains of risk of bias that were random sequence generation and allocation concealment attributed to selection bias, blinding of participants and personnel attributed to performance bias, blinding of outcome assessment attributed to detection bias and incomplete outcome data attributed to attrition bias. We described supporting information and our judgement of each study. We resolved disagreements by discussion. If disagreements persisted, a third review author (Wu) arbitrated.
Measures of treatment effect
We planned to express relative treatment effects as risk ratios with 95% confidence intervals for dichotomous data, and mean differences with 95% confidence intervals for continuous data. A P value of less than or equal to 0.05 would be taken as statistically significant.
Dealing with missing data
We planned to carry out intention‐to‐treat analysis according to the treatment allocation regardless of the final treatments. Where possible, we analysed participants according to seizure type and assumed participants not completing follow‐up or with inadequate seizure data to be non‐responders.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important participant factors between included studies (age, predominant seizure type, duration of epilepsy, number of antiepileptic drugs taken at time of randomisation). We assessed methodological heterogeneity by comparing included trials (randomisation, concealment, blinding, losses to follow‐up).
Assessment of reporting biases
We planned to assess the reporting bias according to Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). We planned to assess potential publication bias using the funnel plot if more than nine studies were to be included.
Data synthesis
We planned to analyse data using Review Manager 5.3, if data were similar enough (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We planned subgroup analysis according to age, seizure type, duration of epilepsy, and number of antiepileptic drugs taken at the time of randomization, but due to insufficient data, these were not possible.
Sensitivity analysis
We planned to perform a sensitivity analysis to investigate the robustness of the meta‐analysis by removing studies with low methodological quality, or excluding studies with large effect size.
Results
Description of studies
Results of the search
The initial search yielded 142 references after duplicates were removed. Thirty‐five references were potentially related after title or abstract screening. We excluded four of these (Li 1996; Sachdeo 1990; Theodore 1990; Wilder 1991) and listed the reasons for exclusion in Characteristics of excluded studies.
See Figure 1 for study flow selection diagram.
Figure 1.
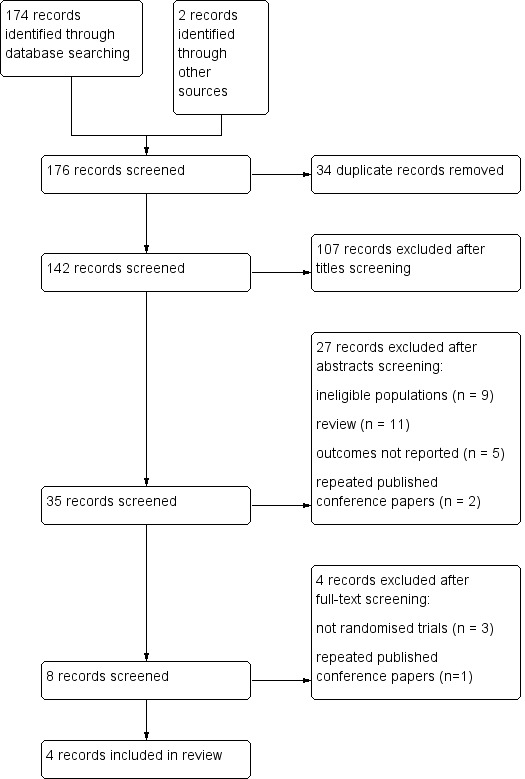
Flow chart of study selection
Included studies
See Characteristics of included studies
Four studies met our inclusion criteria, with a total of 236 participants (Binelli 1999; Bourgeois 1993; Leppik 1991; Theodore 1991). All four studies were randomised, double‐blind, placebo‐controlled trials. Two were parallel‐group trials, the third was a three‐period cross‐over trial, and the fourth was a two‐period cross‐over trial. They utilised varying felbamate doses, varying treatment periods, differing baseline antiepileptic drugs, and differing methodology for assessment of efficacy. Participants randomised in the four studies had differing seizure frequency during baseline.
Binelli 1999 was a parallel group trial including 8‐week baseline period, a period of gradual increase of the drug to the maximum tolerated dose, and 8‐week maintenance phase. The inclusion criteria and exclusion criteria were not mentioned in the trial. Concomitant antiepileptic therapy could be made up of no more than two of the following drugs: carbamazepine, γ‐vinyl‐GABA, lamotrigine, gabapentin, benzodiazepine. But there was no description of which drugs the felbamate group or the placebo group received. During the 8‐week baseline period, more than eight seizures were required.
Bourgeois 1993 was a parallel‐group trial including 4‐week treatment period included 3‐day titration period. The study utilised strict diagnosis, inclusion and exclusion criteria. The diagnosis criteria were video/electroencephalogram (EEG)‐confirmed partial‐onset seizures. The inclusion criteria were seizure frequency not exceeding an average of four complex partial‐onset seizures per day or more than one secondarily generalised seizure per day during the last three days of the surgical evaluation; interictal duration of greater than two hours; minimum average of one seizure per day for the last three days of the surgical evaluation period; previous CT or MRI to confirm the absence of a progressive lesion; age at least 18 years and body weight at least 40 kg; and ECG and chest X‐ray without significant findings during the previous year. Exclusion criteria were status epilepticus in the last three months, significant medical disorders, recent history of psychiatric disorder, poor compliance with prior antiepileptic drug therapy, serious antiepileptic drug complication in the past, change in benzodiazepine dosing during the surgical evaluation phase, drug or alcohol abuse and seizure aetiology considered treatable or progressive. At the end of the four‐week outpatient baseline period, the participants underwent a routine evaluation for epilepsy surgery. The treatment period immediately followed the surgical evaluation period and consisted of eight hospital days and 21 outpatient days. The mean number of other antiepileptic drugs was 5.7 in the felbamate group and 5.5 in the placebo group. During the evaluation period, standard antiepileptic drugs were reduced or discontinued. Throughout the hospitalisation period, the participants continued the same antiepileptic drug regimen present on the last day of the surgical evaluation period. During the outpatient days, participants were on less than the full dosage of standard antiepileptic drug(s). One of the antiepileptic drugs or standard antiepileptic drug (if participants received one antiepileptic drug during their presurgical evaluation period) was returned to their presurgical evaluation dosage. All participants in the felbamate group were treated at the maximum dosage. There was no description of which baseline antiepileptic drugs were being taken, or which participants on which antiepileptic drugs were the best responders to felbamate.
Leppik 1991 was a two‐period cross‐over study which took place at the Epilepsy Research Center, University of Minnesota (UMN) and the University of Virginia Health Sciences Center (UVA). The trial was supported by a research grant from Carter‐Wallace, Inc., but the drug patent is now owned by Meda Pharmaceuticals. The trial included 8 to 10‐day titration period and 10‐week treatment period with 3‐week wash‐out period. Diagnosis of epilepsy was based on observation of at least one ictal event by trained personnel and supported by EEG. The inclusion criteria (requiring four or more partial seizures per month with no seizure‐free interval of more than 20 days despite stable plasma concentrations of both phenytoin and carbamazepine) and exclusion criteria (people with medical conditions other than epilepsy, non compliant or unable to accurately report seizures) were well described. The other antiepileptic drugs were phenytoin and carbamazepine. The final eight weeks of seizure data from the first and second treatment periods were used in the analyses of seizure frequency reduction (SFR), seizure frequency percentage reduction (SFPR), and truncated seizure frequency percentage reduction (TSFPR).
Theodore 1991 was a three‐period cross‐over study. There were four treatment sequences: felbamate‐placebo‐felbamate; felbamate‐placebo‐placebo; placebo‐felbamate‐placebo; placebo‐felbamate‐felbamate. The treatments were administered over the course of alternating titration and analysis periods, each lasting two weeks. The diagnosis was based on clinical (partial seizures with or without secondary generalisation), EEG (onset in one cortical region), and imaging (either focal imaging abnormality or normal scan) criteria. People who had at least six seizures and one or more seizures in every week were included. The exclusion criteria, acquired by contacting the first study author, included people with treatable causes of seizures, metastatic tumours except skin cancer, progressive neurological disorders, other serious medical or psychiatric disorders, gastrointestinal abnormalities, history of generalised tonic‐clonic status epilepticus within baseline and poor compliance, abusers of other drugs, people having received an investigational antiepileptic drug within three months prior to baseline or chronic therapy with non antiepileptic drugs, abnormal laboratory values. There was no description of mean baseline seizure frequencies. All but two participants received felbamate 3000 mg a day. The two exceptions received 2400 mg a day. One of the two was the participant who left the study owing to seizure exacerbation. Nine of the 30 participants randomised had the other antiepileptic drug carbamazepine decreased or increased. The mean seizure frequencies during baseline were not reported and were unavailable through contacting the first author of the study. During the first titration period, the participant received gradually increasing doses of felbamate or placebo. The target felbamate dosage was 3000 mg a day. During the analysis period, the participant was observed with a steady dose. The other antiepileptic drug was carbamazepine.
Risk of bias in included studies
In Binelli 1999, there was no description of the randomisation schedule and the method of allocation concealment. Whether the medication schedule was blinded to participants or personnel or assessor was not mentioned in the study. Seventy‐one out of 83 participants completed the maintenance phase.
Bourgeois 1993 was randomised by permuted block and blinded by using identical packages. The randomisation was done at Wallace Laboratories and no one at the clinical sites was involved in the allocation. Three out of 64 participants were excluded from the analysis of the mean rank of seizure frequency. All 64 participants were evaluated for the analyses of time to the fourth seizure and adverse effects.
Leppik 1991 reported that all medications were pre‐packed under the supervision of the unblinded pharmacist. However, neither the method of allocation concealment nor the method of generating the random sequence was described. Fifty‐six out of 67 participants completed the trial and were included in the analysis.
In Theodore 1991 the randomisation schedule was generated by the statistician of the National Institutes of Health (NIH) and administered by the pharmacy. None of the participants, physicians, nurses, social workers, or technicians interacting with the participants knew what treatment they were being given, felbamate or placebo, at any time. Forty‐seven individuals were enrolled, 17 were not randomised, two of the 30 randomised participants left the study after randomisation.
Effects of interventions
Due to methodological and clinical heterogeneity, it was not possible to perform meta‐analysis of the study results. We have therefore presented a summary of the included studies with regard to our outcome measures: 50% or greater reduction in seizure frequency, absolute or percentage reduction in seizure frequency, treatment withdrawal, adverse effects and quality of life. As the clinical characteristics of the participants in the four included studies were heterogeneous, we were also unable to carry out our planned subgroup analysis.
Reduction in seizure frequency of 50% or more
Binelli 1999 reported that in the group treated with felbamate, 38% of participants had a greater than 50% reduction in seizures and of these 11% were completely controlled.
Absolute or percentage reduction in seizure frequency
Binelli 1999 reported that, in the felbamate group, there was a reduction in seizures of 35.8% in the maintenance period, and in the placebo group there was an increase in seizures of 3.3%.
Leppik 1991 reported that the mean seizure frequency was 34.4 seizures per eight weeks during felbamate treatment and 40.2 seizures per eight weeks during placebo treatment. Felbamate was statistically significantly better than placebo for absolute reduction in seizure frequency (felbamate: 4.95 ± 24.55, placebo: ‐0.36 ± 27.19, P = 0.046), and for percentage reduction in seizure frequency (felbamate: 4.24 ± 55.61, placebo: ‐19.14 ± 79.70, P = 0.018). The analyses described in the study were based on the 56 participants who completed the study. The other three participants who dropped out of the study were included by considering their felbamate period seizure frequencies as eight‐week frequencies and placebo period seizure frequency as zero. The resulting P values were identical. An additional analysis was done using the data from the last 10 weeks of each treatment period, with similar results.
Neither Bourgeois 1993 nor Theodore 1991 presented these results.
Treatment withdrawal
Binelli 1999 reported that four participants receiving felbamate withdrew from the study. In two cases treatment discontinuation was caused by adverse events (diplopia in one case, asthenia and collapse in the other). One participant died from the consequences of a seizure, the fourth withdrew consent to continue the trial. Eight participants treated with placebo did not complete the study.
Bourgeois 1993 reported that two participants in the felbamate group dropped out due to adverse clinical events. One participant in the placebo group withdrew consent.
Leppik 1991 reported that three participants discontinued the trial during the felbamate period because of diplopia, nausea and vomiting, and fever with malaise, respectively.
Theodore 1991 reported that two participants left the study during the felbamate analysis period, one owing to seizure exacerbation and the other owing to hyponatraemia, which might be related to carbamazepine therapy.
Adverse effects
Binelli 1999 reported 26 adverse events in the group of participants treated with felbamate. The adverse events were central nervous system events such as headache, dizziness, ataxia, diplopia, confusion, depression, sedation, and paraesthesia, and gastrointestinal tract events such as stomach pain, nausea, vomiting and loss of appetite. Only 4.9% (2/41) discontinued the treatment for the occurrence of side effects. In the group of participants treated with felbamate significant weight loss occurred (mean reduction from 72.9 kg to 71.4 kg) and in 19.5% (8/41), the decrease in weight was of 5 kg to 7 kg, justifiable in two cases because of gastrointestinal side effects. 2.4% (1/41) suffered a modest and transient reduction in the value of leukocytes. There was no description of adverse effects in the group of participants treated with placebo.
Bourgeois 1993 reported that the most commonly occurring adverse experience in both treatment groups was headache (40% felbamate and 12% placebo). Other commonly occurring adverse experiences in the felbamate group were insomnia (37%), nausea (37%), dizziness (23%), fatigue (20%), constipation (20%), anorexia (20%), dyspepsia (17%), anxiety (13%), and vomiting (13%). The most common adverse experiences in the placebo group were dizziness (15%), dyspepsia (9%), somnolence (9%), insomnia (6%), fatigue (6%), anxiety (6%), nausea (3%), constipation (3%), and vomiting (3%). Only one participant in the felbamate group had a severe adverse experience: stupor and confusion. Two participants in the felbamate group failed to complete the trial due to adverse experiences.
Leppik 1991 reported that the most frequent adverse effects were in the central nervous system and gastrointestinal tract, of which headache (36% felbamate and 3% placebo), dizziness (36% felbamate and 5% placebo), diplopia (36% felbamate and 2% placebo), blurred vision (22% felbamate and 5% placebo), ataxia (32% felbamate and 2% placebo), nausea (39% felbamate and 7% placebo), and vomiting (25% felbamate and 3% placebo) were noted.
Theodore 1991 reported that 87% (26/30) suffered headache, 57% (17/30) suffered nausea, 50% (15/30) suffered dizziness, 33% (10/30) suffered diplopia, 33% (10/30) suffered vomiting, 30% (9/30) suffered blurred vision, 17% (5/30) suffered fatigue, and 10% (3/30) suffered poor balance. Nausea, double vision and blurred vision were significantly associated with periods during which felbamate was administered. Felbamate led to a decrease in both blood urea nitrogen and white blood count.
Quality of life
Bourgeois 1993 was the only study to describe quality of life. During the four‐week outpatient baseline period each participant's vital signs were obtained and the Short Neuropsychological Test was administered. For those who had the Short Neuropsychological Test and completed the treatment period, motor skills and memory skills remained the same or were improved. However, no detailed data were provided.
Theodore 1991 failed to detect a significant effect of felbamate on seizure frequency at the 0.05 level. In Bourgeois 1993, the primary efficacy analysis was the mean rank according to seizure frequency. The difference between felbamate and placebo was statistically significant in favour of felbamate. The secondary analysis was the time to the fourth seizure, it was indicated in the fourth seizure survival curves for the two treatments that participants in the placebo group experienced a fourth seizure more frequently and earlier than participants in the felbamate group.
Discussion
Summary of main results
Four studies representing 236 recruited participants met the inclusion criteria for this review (Binelli 1999; Bourgeois 1993; Leppik 1991; Theodore 1991). Among them, two were randomised, double‐blind, placebo‐controlled, parallel‐group trials, one was a randomised, double‐blind, placebo‐controlled, two‐period cross‐over trial, and the fourth was a randomised, double‐blind, placebo‐controlled, three‐period cross‐over trial. Bourgeois 1993 was supported by a research grant from Carter‐Wallace which was the manufacturer of felbamate at that time.
The risk of bias in two included studies (Bourgeois 1993; Theodore 1991) was low. The reporting of important methodological factors, such as the method of randomisation, allocation concealment, double‐blinding, and incomplete outcome data was poor in Binelli 1999 and Leppik 1991.
Due to differences in the study methodology, choice of outcomes and inadequate reporting of outcome data, it was not possible to summarise data in a meta‐analysis. We therefore summarised data from studies narratively in this review. The current data do not provide convincing evidence to support the use of felbamate in people with drug‐refractory partial‐onset epilepsy, when used as an add‐on therapy. Felbamate may reduce seizure frequency. The impact of felbamate on quality of life has not been adequately assessed.
Overall completeness and applicability of evidence
Among the included studies, the felbamate doses and the length of the treatment periods were variable, as summarised in the Characteristics of included studies table.
Adverse effects rates were higher during the felbamate period than the placebo period, particularly headache, nausea and dizziness. The serious adverse effects aplastic anaemia and hepatic failure, which were reviewed in 1999 (Pellock 1999), were not reported in the four included studies. One reason for this might be that these two severe adverse effects are small probability events. The most likely incidence of felbamate‐associated aplastic anaemia is 127 per million (Pellock 1999). A total of 18 cases of hepatic failure had been reported in people receiving felbamate (Pellock 1999), the rate of which was lower than the incidence of aplastic anaemia. Another reason might be that the duration of the four included trials was not long enough for the occurrence of the two severe adverse effects (the longest exposure time in felbamate was 10 weeks in Leppik 1991). All cases of aplastic anaemia presented after two and a half to six months of felbamate therapy (Pennell 1995). The mean time to hepatic failure presentation was 217 days (range 25 to 939 days) (Pellock 1999).
Authors' conclusions
Since the last version of this review we found one new study. For people with drug‐resistant focal epilepsy, when used as an add‐on therapy, felbamate may reduce seizure frequency, but there is no convincing evidence. The quality of existing data is poor and it is not possible to define the size of treatment effect. The most commonly reported adverse effects in these short‐term studies were headache, nausea and dizziness. Evidence to recommend the use of felbamate as an antiepileptic drug is insufficient.
A large‐scale, randomised controlled trial conducted over a longer period of time (at least one year) is required to inform clinical practice. The trial should recruit a heterogeneous population with well‐defined seizure and epilepsy types to allow the identification of patient factors, pathology, seizure types and baseline antiepileptic drugs associated with the greatest benefit or harm. In addition, research is increasingly being undertaken into epilepsy genetics, with regard to the factors contributing to refractory epilepsy, to identify the people in which antiepileptic drugs will achieve the greatest efficacy. Such investigation should be included in future research into felbamate.
Acknowledgements
We would like to acknowledge Cochrane Epilepsy, Ellen Dougan, Rachael Kelly, and Tony Marson for their technical support; Kathy Mahan for her help with translation; authors of included trials, William Theodore and Blaise Bourgeois for unreported data they supplied; Larry Gever, Meda Pharmaceuticals, for his paper support; Ilo E Leppik and Brandy Fureman for their efforts.
Appendices
Appendix 1. Cochrane Epilepsy Specialized Register search strategy
#1 (felba* or taloxa) AND INREGISTER
#2 #1 AND >04/08/2015:CRSCREATED
Appendix 2. CENTRAL via CRSO search strategy
#1 (felba* or taloxa or "ADD‐03055" or "W‐554" or "ADD 03055" or "W 554"):TI,AB,KY
#2 (epilep* OR seizure* OR convuls*):TI,AB,KY
#3 MESH DESCRIPTOR Epilepsy EXPLODE ALL TREES
#4 MESH DESCRIPTOR Seizures EXPLODE ALL TREES
#5 #2 OR #3 OR #4
#6 #1 AND #5
#7 * NOT INMEDLINE AND 04/08/2015 TO 20/10/2016:CD
#8 #6 AND #7
Appendix 3. MEDLINE search strategy
This strategy was based on the Cochrane highly sensitive search strategy for identifying randomised trials (Lefebvre 2011).
1. felbamate.nm. or (felba* or taloxa or "ADD‐03055" or "W‐554" or "ADD 03055" or "W 554").tw.
2. exp Epilepsy/
3. exp Seizures/
4. (epilep$ or seizure$ or convuls$).tw.
5. 2 or 3 or 4
6. exp *Pre‐Eclampsia/ or exp *Eclampsia/
7. 5 not 6
8. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
9. clinical trials as topic.sh.
10. trial.ti.
11. 8 or 9 or 10
12. exp animals/ not humans.sh.
13. 11 not 12
14. 1 and 7 and 13
15. (monotherap$ not (adjunct$ or "add‐on" or "add on" or adjuvant$ or combination$ or polytherap$)).ti.
16. 14 not 15
17. remove duplicates from 16
18. limit 17 to ed=20150804‐20161020
Data and analyses
This review has no analyses.
What's new
Last assessed as up‐to‐date: 20 October 2016.
| Date | Event | Description |
|---|---|---|
| 20 October 2016 | New search has been performed | Searches updated 20 October 2016. |
| 20 October 2016 | New citation required but conclusions have not changed | One new study (Binelli 1999) has been included in this update. The conclusions remain unchanged. |
Differences between protocol and review
In the protocol, we defined refractory epilepsy as "continued seizures despite antiepileptic drug treatment" (French 2006), and in the review, we used the definition proposed by the Task Force of the International League Against Epilepsy (ILAE) "failure of adequate trials of two tolerated and appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom" (Kwan 2010).
In the protocol, we planned to summarise data in a meta‐analysis, assess the reporting biases, and do sensitivity analyses. In the review, due to the clinical and methodological heterogeneity in the four included trials, we summarised data narratively.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double‐blind, placebo‐controlled trial 8‐week baseline period, a period of gradual increase of the drug to the maximum tolerated dose, and 8‐week maintenance phase |
|
| Participants | Multicenter study 83 participants (mean age 33.5 years) were enrolled and randomised, 45 to felbamate and 38 to placebo The average monthly seizure frequency in baseline period:
41 participants taking felbamate and 30 participants taking placebo completed the maintenance period. |
|
| Interventions | Add‐on felbamate or placebo A period of gradual increase of the drug to the maximum tolerated dose, but not higher than 3600 mg/d, and then an 8‐week maintenance phase |
|
| Outcomes |
|
|
| Notes | 4 participants receiving felbamate withdrew from the study. In two cases treatment discontinuation was caused by adverse events (diplopia in one case, asthenia and collapse in the other). 1 participant died from the consequences of a seizure, the fourth withdrew consent to continue the trial. 8 participants treated with placebo did not complete the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selective reporting (reporting bias) | Unclear risk | There was no description |
| Random sequence generation (selection bias) | Unclear risk | There was no description |
| Allocation concealment (selection bias) | Unclear risk | There was no description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no description |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no description |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 71/83 completed the maintenance period |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study Randomisation was by permuted block. The randomisation was done at Wallace Laboratories and no‐one at the clinical sites was involved in the allocation. Medication (or placebo) was provided in identical packages. 4‐week treatment period included 3‐day titration period. |
|
| Participants | Multicenter study 64 participants were randomised (38 male), aged 17 to 51 years. 30 participants were randomised to felbamate and 34 to placebo. Mean 4‐weekly baseline seizure frequency:
Number of other AEDs: felbamate group = 3‐9; placebo group = 2‐9 |
|
| Interventions | Add‐on felbamate or placebo Felbamate was titrated from 1600 mg/d to 3600 mg/d over a period of 3 days and maintained on 3600 mg/d or the maximum tolerated dose, not to exceed 3600 mg/d |
|
| Outcomes |
|
|
| Notes | Of the 64 participants randomised to the double‐blind phase, 3 were excluded from the analysis of the mean rank of seizure frequency. All 64 participants were evaluated for the analyses of time to 4th seizure and adverse effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the methods were reported. |
| Random sequence generation (selection bias) | Low risk | Quote: "they were randomised to the felbamate or placebo treatment groups". We contacted the study author and the reply was as follows: "I think that randomisation was by permuted block, but I do not remember for sure". |
| Allocation concealment (selection bias) | Low risk | We contacted the study author and the reply was as follows: "The randomisation was done at Wallace Laboratories and no one at the clinical sites was involved in the allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We contacted the study author and the reply was as follows: "Medication (or placebo) was provided by Wallace Laboratories to the clinical sites in identical packages. The study was double‐blind. The patients as well as the doctors and nurses did not know whether the treatment was felbamate or placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/64 were excluded from the analysis of the mean rank of seizure frequency. All 64 participants were evaluated for the analyses of time to 4th seizure and adverse effects |
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over study 2 treatment sequences: felbamate‐placebo and placebo‐felbamate 8 to 10‐day titration period and 10‐week treatment period with 3‐week wash‐out period |
|
| Participants | The Epilepsy Research Center, University of Minnesota (UMN) and the University of Virginia Health Sciences Center (UVA). A total of 59 participants were randomised, aged 18‐55 years. 56 participants (32 male) completed the trial. 31 participants were randomised to felbamate‐placebo sequence and 28 to placebo‐felbamate. Mean 8‐weekly baseline seizure frequencies of the 56 participants who completed the trial:
Other AEDs were phenytoin and carbamazepine Simple partial seizures, complex partial seizures, secondary generalised seizures |
|
| Interventions | Add‐on placebo or felbamate In the initial 8 to 10‐day treatment, the dosage was increased daily to 3000 mg/d. Due to reports of nausea and vomiting, the dosage was reduced to 2600 mg/d. The mean felbamate dosage was 2300 mg/d |
|
| Outcomes |
|
|
| Notes | Of the 59 participants randomised, 3 dropped out. 2 of them completed the placebo period, and the 3rd did not begin the placebo period. All 3 received felbamate treatment for short periods. The primary efficacy analyses, such as SFR, SFPR, TSFPR (TSFPR = SFPR except that SFPR values less than ‐ 100 are truncated to ‐ 100), were based on 56 participants who completed both treatment periods. Adverse effects were analysed based on 59 participants randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the methods were reported. |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomised ... clinical trial. Table 1 summarizes the baseline characteristics of these 56 patients by center and randomised treatment sequence." Comment: not stated how random sequence generated |
| Allocation concealment (selection bias) | Unclear risk | There was no description of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...double‐blind treatment periods. ...All medications were pre‐packed under the supervision of the unblinded pharmacist." Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no description. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 56/67 completed the trial |
| Methods | Randomised, double‐blinded, three‐period cross‐over study The randomization schedule was generated by the NIH statistician and administered by pharmacy None of the participants, physicians, nurses, social workers, or technicians interacting with the participants knew what treatment they were being given, felbamate or placebo, at any time. 4 treatment sequence: felbamate‐placebo‐felbamate; felbamate‐placebo‐placebo; placebo‐felbamate‐placebo; placebo‐felbamate‐felbamate The treatments were administered over the course of alternating titration and analysis periods, each lasting 2 weeks. |
|
| Participants | 30 participants were randomised (10 male), aged 19‐50 years. 8 participants were randomised to felbamate‐placebo‐felbamate sequence, 8 participants were randomised to felbamate‐placebo‐placebo sequence, 7 participants were randomised to placebo‐felbamate‐placebo sequence, 7 participants were randomised to placebo‐felbamate‐felbamate sequence. Simple partial seizures, complex partial seizures, generalised tonic‐clonic seizures Mean baseline seizure frequencies of the participants were unavailable. The other AED was carbamazepine. |
|
| Interventions | Add‐on placebo or felbamate 28 participants who completed the study received felbamate dosage of 3000 mg/d. The 2 exceptions who left the study received felbamate dosage of 2400 mg/d. |
|
| Outcomes |
|
|
| Notes | 2 participants left the study after randomisation; 1 owing to seizure exacerbation and the other owing to hyponatraemia possibly related to carbamazepine. Both were included in the data analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the methods were reported. |
| Random sequence generation (selection bias) | Low risk | We contacted the study author and the reply was as follows: "The randomisation schedule was generated by the NIH statistician and administered by the pharmacy." |
| Allocation concealment (selection bias) | Low risk | We contacted the study author and the reply was as follows: "The randomisation schedule was generated by the NIH statistician and administered by the pharmacy." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We contacted the study author and the reply was as follows: "None of the physicians or nurses or patients knew what drug they were being given, felbamate or placebo, at any time." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "47 individuals were enrolled, 17 were not randomised, 2 of the 30 randomisation left the study after randomisation." |
AED: antiepileptic drug d: day NIH: National Institutes of Health
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Li 1996 | Open‐label add‐on study, but not a RCT |
| Sachdeo 1990 | Open‐label blinded study, but not a RCT |
| Theodore 1990 | repeated published conference paper |
| Wilder 1991 | Not a RCT |
RCT: randomised controlled trial
Contributions of authors
Li Li Shi ‐ all correspondence; drafting protocol and review versions; search for trials; selection of trials for inclusion/exclusion; extraction of data; interpretation of data analyses; updating review. JianCheng Dong ‐ search for trials; drafting review versions; updating review. HengJian Ni ‐ obtaining copies of trial reports. JinSong Geng ‐ selection of trials for inclusion/exclusion; extraction of data. Taixiang Wu ‐ methodology expert; arbiter of selection of trials for inclusion/exclusion.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Epilepsy. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Li Li Shi: no conflicts of interest JianCheng Dong: no conflicts of interest HengJian Ni: no conflicts of interest JinSong Geng: no conflicts of interest Taixiang Wu: no conflicts of interest
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
- Binelli S, Canafoglia L, Mamoli D, Avanzini G, Guidolin L, Canger R, et al. Felbamate as add‐on therapy in adult patients with partial drug‐ resistant epilepsy. Bollettino ‐ Lega Italiana Contro L'Epilessia 1999;106‐107:317‐8. [Google Scholar]
- Bourgeois B, Leppik IE, Sackellares JC, Laxer K, Lesser R, Messenheimer JA, et al. Felbamate: a double‐blind controlled trial in patients undergoing presurgical evaluation of partial seizures. Neurology 1993;43(4):693‐6. [DOI] [PubMed] [Google Scholar]
- Leppik IE, Dreifuss FE, Pledger GW, Graves NM, Santilli N, Drury I, et al. Felbamate for partial seizures: results of a controlled clinical trial. Neurology 1991;41(11):1785‐9. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Raubertas RF, Porter RJ, Nice F, Devinsky O, Reeves P, et al. Felbamate: a clinical trial for complex partial seizures. Epilepsia 1991;32(3):392‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Li LM, Nashef L, Moriarty J, Duncan JS, Sander JW. Felbamate as add‐on therapy. European Neurology 1996;36(3):146‐8. [DOI] [PubMed] [Google Scholar]
- Sachdeo RC, Sachdeo SN. Long‐term felbamate therapy. Epilepsia 1990;31(5):619. [Google Scholar]
- Theodore WH, Reeves P, Porter RJ, Nice FJ, Devinsky O, Broomfield E, et al. Long‐term follow‐up of felbamate therapy in patients with complex partial seizures. Epilepsia 1990;31(5):619. [Google Scholar]
- Wilder BJ, Campbell KW, Uthman BM, Gilmore RL, Barber CP, Hargrove SC. Felbamate for intractable epilepsy: a long‐term study. Epilepsia 1991;32(Suppl 3):59. 1985831 [Google Scholar]
Additional references
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005‐2009. Epilepsia 2010;51(4):676‐85. [DOI] [PubMed] [Google Scholar]
- French J, Smith M, Faught E, Brown L. Practice advisory: the use of felbamate in the treatment of patients with intractable epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 1999;52(8):1540‐5. [DOI] [PubMed] [Google Scholar]
- French JA. Refractory epilepsy: one size does not fit all. Epilepsy Currents 2006;6(6):177‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata T, Marchi N, Carlton E, Ghosh C, Gonzalez‐Martinez J, Alexopoulos AV, et al. Management of the patient with medically refractory epilepsy. Expert Review of Neurotherapeutics 2009;9(12):1791‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Kwan P, Arzimanoglou A, Berg A, Brodie M, Hauser WA, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51(6):1069‐77. [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Meldrum BS. Update on the mechanism of action of antiepileptic drugs. Epilepsia 1996;37(Suppl 6):S4‐11. [DOI] [PubMed] [Google Scholar]
- Pellock JM. Felbamate. Epilepsia 1999;40(Suppl 5):S57‐62. [DOI] [PubMed] [Google Scholar]
- Pennell PB, Ogaily MS, Macdonald RL. Aplastic anemia in a patient receiving felbamate for complex partial seizures. Neurology 1995;45:456‐60. [DOI] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rho JM, Donevan SD, Rogawski MA. Mechanism of action of the anticonvulsant felbamate: opposing effects on N‐methyl‐D‐aspartate and gamma‐aminobutyric acidA receptors. Annals of Neurology 1994;35(2):229‐34. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
References to other published versions of this review
- Shi LL, Dong J, Ni H, Geng J, Wu T. Felbamate as an add‐on therapy for refractory epilepsy. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008295] [DOI] [Google Scholar]
- Shi LL, Dong J, Ni H, Geng J, Wu T. Felbamate as an add‐on therapy for refractory epilepsy. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008295.pub2] [DOI] [PubMed] [Google Scholar]
- Shi LL, Dong J, Ni H, Geng J, Wu T. Felbamate as an add‐on therapy for refractory epilepsy. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD008295.pub3] [DOI] [PubMed] [Google Scholar]


