Abstract
Genomic studies revealed the existence of health- and acne-associated P. acnes strains and suggested novel approaches for broadening understanding of acne vulgaris. However, clinical association of P. acnes with disease or health has yet to be corroborated experimentally. Current animal models of acne do not closely mimic human disease and have unclear translational value. We have developed a murine model of acne by combining P. acnes inoculation with topical application of a synthetic human sebum. We showed that human sebum promoted persistence of intradermally injected P. acnes with little loss of viability after 1 week and permitted use of more physiologic inoculums. Application of acne-associated P. acnes RT4/5 strains led to development of moderate to severe skin pathology compared with application of health-associated type II P. acnes strains (RT2/6). RT4/5 P. acnes strains uniformly induced higher levels of KC (IL-8), IL-1α, IL-1β, and IL-6 in vitro and in vivo compared with type II P. acnes strains. Overall, our data provide immunopathologic corroboration of health and disease association of clinical P. acnes strains and inform on a platform to query putative virulence factors uncovered by genomic studies.
Keywords: Dermatology, Microbiology
Keywords: Mouse models, Skin
Propionibacterium acnes strains with robust association with health or acne induce immunopathology in mice that is consistent with their human disease or health association.
Introduction
Acne vulgaris is an important disease affecting more than 80% of teenagers and young adults (1). It frequently results in permanent disfigurement, even with appropriate treatment. Common therapeutics include the use of antibiotics and antiinflammatory products, but a more targeted approach has eluded acne therapeutics. Although acne is a common disease, its etiology is still unclear. Acne is believed to be induced by multiple factors, including the abundant skin bacterium Propionibacterium acnes. Increased hormone levels and sebum production in puberty induce hyperkeratosis of the follicles and overgrowth of P. acnes, leading to skin inflammation.
Although P. acnes is thought to be important for the pathogenesis of acne vulgaris, it is found perplexingly to colonize both acne patients and healthy subjects. Recently, the enigma has been resolved through concerted effort by several groups: typing and genomic studies have identified various lineages of P. acnes with different degrees of health or acne association (2–4). Clade IA-2 strains (RT4 and RT5) have a particularly strong association with acne, while type II strains show a strong association with healthy human skin (3). Consistent with these clinical associations, genomic analyses revealed putative pathogenic factors encoded uniquely in acne-associated P. acnes strains and, therefore, suggest novel approaches to treat acne (3). In addition, a metagenomic study of the skin microbiome in acne suggests that the balance between health- and disease-associated microbes, rather than the presence of acne-associated strains, is an important determinant of acne (5). Altogether, the discovery of health- and acne-associated P. acnes strains suggests a novel approach to significantly broaden understanding of acne pathogenesis and further suggests that a targeted therapeutic approach should replace currently practiced nonspecific killing of P. acnes. However, transitioning from clinical association studies to pathogenesis and therapeutic studies requires first the corroboration of the clinical associations.
To date, studies of human monocyte stimulation have shown induction of higher IL-17 and IFN-γ and lower IL-10 by acne-associated P. acnes strains compared with health-associated P. acnes strains (6, 7), thereby providing a link between host adaptive inflammatory mechanisms and acne. However, other studies that explored the relationship between P. acnes strains and innate proinflammatory responses have suggested enhanced host innate immune responses in association with health- rather than acne-associated strains (8). There is no report to date of in vivo studies comparing immune response induced by health- and acne-associated P. acnes strains. Although many animal models of acne exist (9), no current animal model of acne recapitulates all features of the disease in humans, and the clinical significance and translational value of existing models are unclear. Several models employ unconventional animals or animals with limited immunologic competence (rhino mice, Mexican hairless dogs, and nude mice transplanted with human skin) (10–12). Bacterial infection is not needed for induction of acne in many models, which clearly limits the relevance of those models to the human condition. A frequently used platform induces skin inflammation on the back or ear of rodents using high intradermal inoculums of P. acnes (107–109 live or dead P. acnes) (13, 14).
Here, we established an improved murine model of acne that combines application of P. acnes and human sebum and studied, in vitro and in vivo, the immunopathologic properties of P. acnes with strong health and disease association.
Results
An acne murine model that combines P.
acnes and human sebum application. In initial experiments, we inoculated CD-1 mice in the back with 107P. acnes based on a modified published protocol (13). As shown in Figure 1, intradermal inoculation of the mice with 107 acne-associated P. acnes RT5 strain HL043PA1 in PBS resulted in minimal lesions and was associated with rapid clearance of the bacteria from the site of inoculation, consistent with reports of difficulty inducing skin pathology with P. acnes in immunocompetent mice (13). Borrowing from other pathogen models of infection (15), we washed and inoculated P. acnes in media instead of PBS. Use of media improved P. acnes recovery from lesions after 3 and 7 days but induced lesions remain modest.
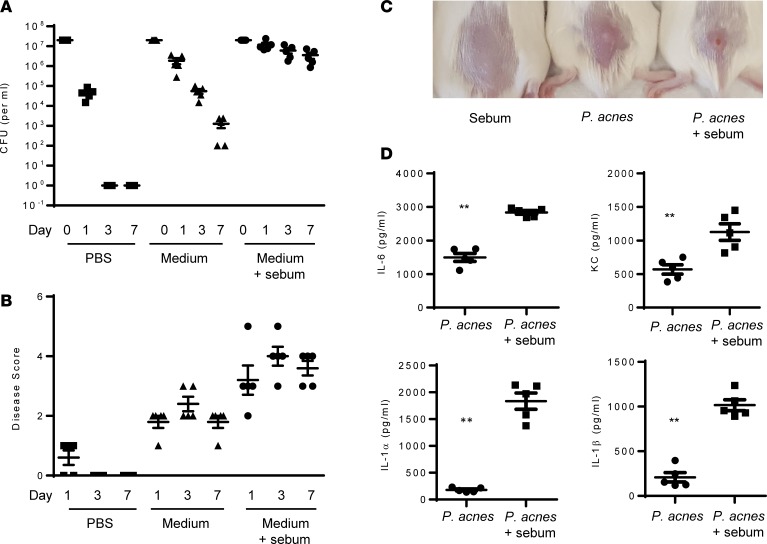
Figure 1. Sebum application enhances P.
acnessurvival in a murine model of acne immunopathology.P. acnes strain HL043PA1 (RT5, 107 CFU) was washed and resuspended in PBS or media prior to intradermal injection, with or without daily topical application of sebum (20 μl). (A) P. acnes recovery from skin lesions over 7 days. (B) Lesion sizes over 7 days and representative lesions on day 7. (C) Representative lesions induced with sebum alone, P. acnes alone, or both sebum and P. acnes. (D) Cytokines measured from skin homogenates, determined by ELISA. Data are shown as mean ± SEM, n = 5 mice per group for A, B, and D. Data in A and B were analyzed by ANOVA and showed significance at P < 0.01 at day 1, 3, and 7 between groups. Data in D were analyzed using Mann-Whitney. **P < 0.001.
Hormonally stimulated production of sebum at the onset of puberty is an important contributor to acne. Sebum is particularly abundant at anatomic sites with high concentration of P. acnes (16), and the sebum component oleic acid has been reported to promote growth of P. acnes in culture (17). Mouse sebum is different from human sebum, and current models of acne do not add human sebum to P. acnes as part of the models. We therefore tested if the combined use of human sebum and P. acnes would lead to enhanced pathology. We synthesized human sebum based on a published protocol (18). We then inoculated CD-1 mice with P. acnes and additionally applied freshly made sebum daily. As shown in Figure 1, addition of human sebum dramatically enhanced survival of P. acnes, with only modest loss of viability after 7 days. Although sebum alone had no effect on skin pathology, sebum when applied with P. acnes promoted reproducible and significant skin pathology, characterized by abscess formation, erythema, induration, skin necrosis, and scaling (Figure 1B and Supplemental Figure 1B; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.124687DS1). Accompanying the skin lesion, coapplication of human sebum and P. acnes induced higher levels of IL-6, KC, IL-1α, and IL-1β within the skin lesions compared with application of P. acnes alone. Sebum alone induced negligible levels of these cytokines (data not shown).
Up to 105–106 P. acnes could be routinely recovered from human body surfaces with high concentration of sebum (16). To determine the persistence of P. acnes with lower inoculums of P. acnes, we administered 105–107 CFU P. acnes intradermally with topical sebum. We showed that use of inoculums in the 105–106 range leads to more rapid clearance of P. acnes than use of 107 P. acnes (Supplemental Figure 1A).
Because the pathogenesis of acne vulgaris requires the presence of P. acnes in hair follicles, we also investigated if topical application of P. acnes along with topical sebum reproduced lesions that more closely mimic human disease. Although application of P. acnes with sebum, after tape stripping, induced skin erythema and plaques that were not observed with P. acnes or sebum application alone, individual lesions around hair follicles were not observed and bacteria were not always visualized within follicles. Therefore, the model was not perceived to be an improvement on the intradermal model (Supplemental Figure 2). As a consequence, we elected to investigate the pathogenic properties of health- and disease-associated P. acnes strains using the intradermal mouse model.
In vivo immunopathology induced by clinical P.
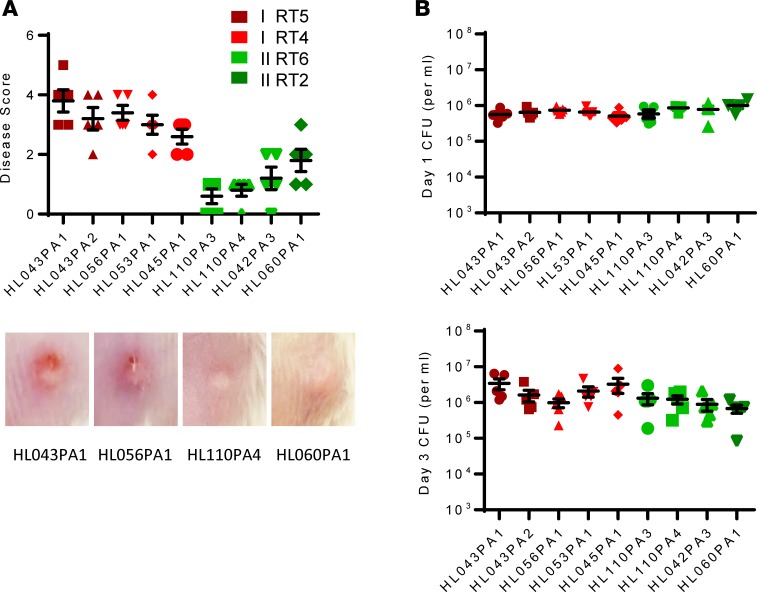
acnes strains with disease and heath association. MLST and microbiome studies have revealed that certain P. acnes strains are associated with acne, while others are associated with health (2–4). We have shown that clade IA-2 strains (RT4 and RT5) are strongly associated with acne, whereas type II strains (RT2/6) show strong association with healthy skin (3). We selected several strains belonging to these clades for our study: 2 RT5 strains, 3 RT4 strains, 3 RT6 strains, and 1 RT2 strain (Supplemental Table 1). We applied these strains intradermally along with topical sebum and measured disease score based on skin induration, erythema, dermonecrosis, and scaling. Additionally, we measured histology and cytokines that are suggested to be important in acne disease. Although use of lower inoculums was possible, we inoculated 107 P. acnes because that inoculum induces stable persistence of bacteria, which more closely mimics colonization.
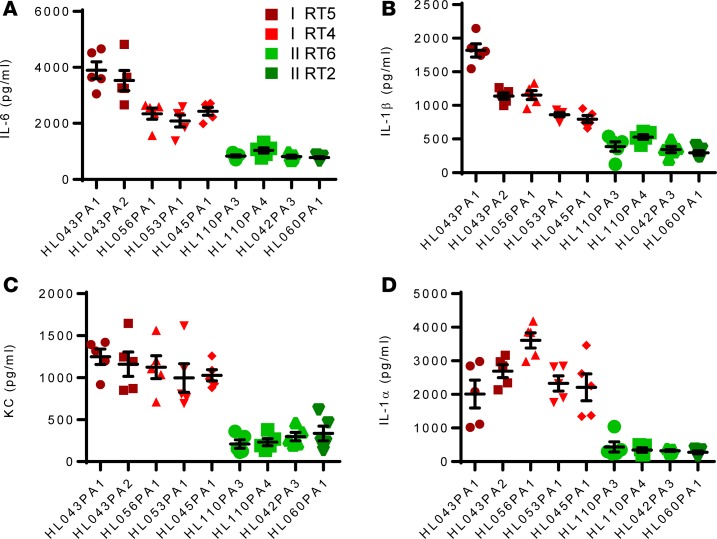
As shown in Figure 2, RT4/5 strains uniformly induced higher disease scores compared with RT2/6 strains, without significant differences in bacteria clearance from the lesions on day 1 and 3. Consistent with skin immunopathology, higher cytokine levels (IL-6, IL-1α, IL-1β) and chemokine levels (KC) were also recovered from the lesions on day 3 (Figure 3).
Figure 2. Induction of skin immunopathology by P.
acnesstrains associated with health and acne vulgaris.P. acnes strains (107 CFU) were injected intradermally, and sebum (20 μl) was applied topically daily. (A) After 3 days, skin lesions were scored. Representative lesions induced by health- and disease-associated P. acnes are shown. (B) Skin was excised, and bacteria burden on day 1 and 3 was determined. Data are shown as mean ± SEM, n = 5 mice per group. Data analysis compared cytokines induced by P. acnes RT4/5 (all 5 strains) versus RT2/6 (all 4 strains) using nonparametric Mann-Whitney U test. P < 0.001, comparing red and green strains.
Figure 3. In vivo proinflammatory activity of health- and acne-associated P.
acnesstrains. Mice were injected with 1 × 107 P. acnes, and sebum (20 μl) was applied topically daily. After 3 days the skin was excised and homogenized, and cytokines levels were determined by ELISA. Data are shown as mean ± SEM (n = 5 mice per group). Data analysis compared cytokines induced by P. acnes RT4/5 (all 5 strains) versus RT2/6 (all 4 strains) using nonparametric Mann-Whitney U test. P < 0.001, comparing red and green strains.
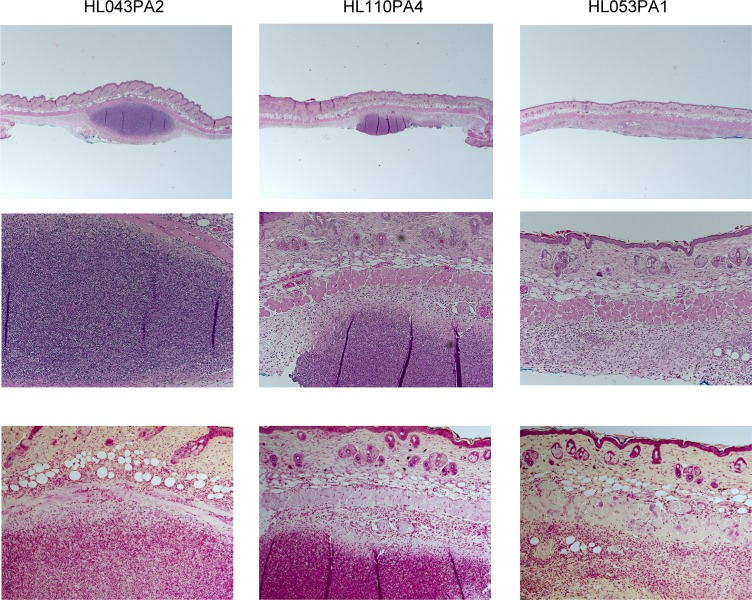
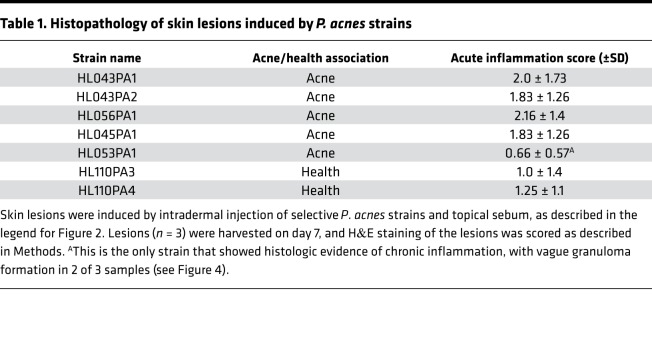
Histology of skin lesions was studied and overall showed immunopathology that reflected the disease score, although there was more variation between samples compared with measured cytokines, chemokines, and disease scores (Figure 4 and Table 1). In all lesions, there was no apparent acute and chronic inflammation involving hair follicles and sebaceous glands. Minimal hyper- and/or parakeratosis was noted focally in rare cases. Most cases showed acute inflammation, located in the deep soft tissue underneath skeletal muscle layer, either in a localized mass-like (abscess) pattern or a diffuse band-like pattern, with mild spill over to the subcutaneous layer. Few cases demonstrated a mixed localized and diffuse pattern. Overall evidence of chronic inflammation was minimal or mild and was mostly in a diffuse band-like pattern, with rare cases showing vague granulomatous change. Gram stain of the slides showed approximate colocalization of the bacteria and immune cells, with many aggregates of Gram-positive rods within cytoplasm of neutrophils (Figure 4).
Figure 4. Histology of skin lesions induced by health- and acne-associated strains.
Mice were injected intradermally with 1 × 107 P. acnes. Synthetic sebum (20 μl) was applied daily, and skin was excised. H&E and Gram staining was performed after 7 days. Shown are slides representing 3 histologic patterns. Corresponding blinded scores are shown in Table 1. Original magnification, ×20 (top, H&E); ×100 (middle, H&E; bottom, Gram).
Table 1. Histopathology of skin lesions induced by P. acnes strains.
In vitro host proinflammatory response to health- and disease-associated P.
acnes strains. The robust correlation between P. acnes health and disease association and immunopathology prompted us to evaluate the proinflammatory properties of the strains in vitro. We focused on few cytokines and chemokines that have been shown to be important in acne pathogenesis, including IL-1α, produced by keratinocytes and considered important for hyperkeratosis and comedo formation; IL-1β and IL-6, produced by monocyte/macrophages particularly in response to TLR2; and IL-8 (or mouse homolog KC), an important chemokine linked to acne (19). We challenged the human keratinocyte HaCaT cell line and mouse primary macrophages with either killed P. acnes strains or their supernatant to assess proinflammatory activity of cell-associated and secreted P. acnes factors.
Consistent with the findings from the murine acne model, P. acnes RT4/5 strains uniformly induced significantly higher levels of IL-8 and IL-6 from keratinocytes and IL-1α, IL-6, and KC from macrophages (Figures 5 and 6). Higher cytokine and chemokine levels were observed with killed bacteria and supernatants from P. acnes cultures, suggesting that both cell-associated and secreted factors from RT4/5 strains likely contribute to the severity of immunopathology. Minimal or no secreted IL-1β or IL-1α was detected in P. acnes–macrophage and P. acnes–keratinocyte coculture assays, respectively (data not shown). Combined with disease score and in vivo cytokines, these in vitro cytokine and chemokine data strongly corroborate the hypothesis that individual acne-associated P. acnes strains, through their background virulence genes, are sufficient to drive immunopathology associated with human acne disease.
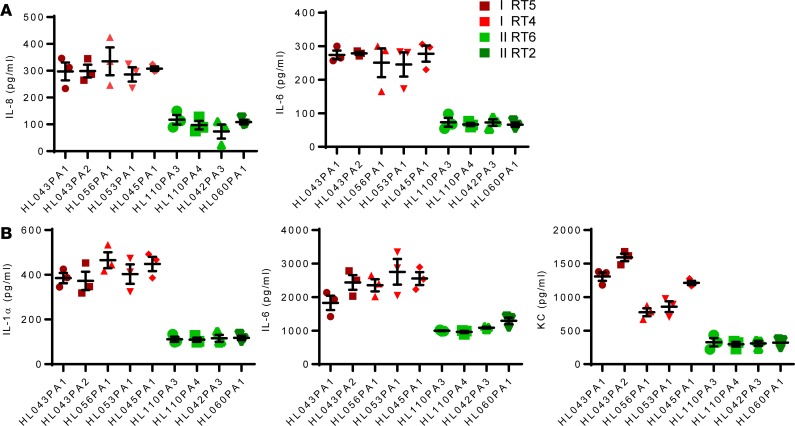
Figure 5. In vitro proinflammatory activity of heat-killed health- and acne-associated P.
acnesstrains. (A) HaCaT cells were stimulated for 12 hours with heat-killed P. acnes at an MOI of 10, and cytokines levels were determined by ELISA. (B) Bone marrow–derived macrophages were stimulated for 12 hours with heat-killed P. acnes at an MOI of 10, and cytokines were measured by ELISA. Data are shown as mean ± SEM (n = 3) and are representative of 3 experiments. Data analysis compared cytokines induced by P. acnes RT4/5 (all 5 strains) versus RT2/6 (all 4 strains) using nonparametric Mann-Whitney U test. P < 0.001, comparing red and green strains.
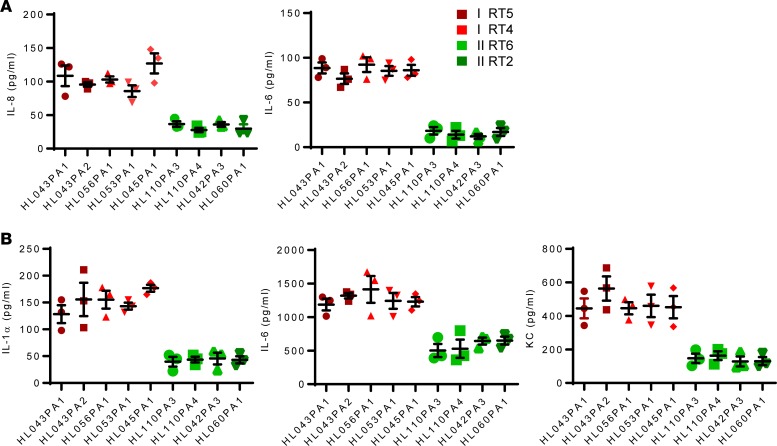
Figure 6. In vitro proinflammatory activity of supernatant from health- and acne-associated P.
acnesstrains. (A) HaCaT cells were stimulated for 12 hours with supernatant (10 μl) from overnight P. acnes cultures, and cytokines levels were determined by ELISA. (B) Bone marrow–derived macrophages were stimulated for 12 hours with supernatant (10 μl) from overnight P. acnes cultures, and cytokines were measured by ELISA. Data are shown as mean ± SEM (n = 3) and are representative of 3 experiments. Data analysis compared cytokines induced by P. acnes RT4/5 (all 5 strains) versus RT2/6 (all 4 strains) using nonparametric Mann-Whitney U test. P < 0.001, comparing red and green strains.
Discussion
Although decades of studies suggest that P. acnes is important for pathogenesis of acne vulgaris, remarkably little is understood of how this commensal bacterium affects acne. Contributing to this poor understanding are the relative difficulty to manipulate P. acnes and uncertainty of the clinical relevance of the animal models (9). In this study, we applied a synthetic human sebum to old murine models of acne and showed that survival of P. acnes is significantly enhanced with sebum. It isn’t clear how addition of human sebum improves survival of P. acnes, as both mice and human make sebum, albeit of different composition. The applied synthetic sebum is made of triglycerides, oleic acid, wax esters, and squalene and is simplified from the much more complex human sebum, made of squalene, cholesterol, cholesteryl esters, fatty acids, triglycerides, diglyceride, and wax esters (20, 21). Mouse sebum, compared with human sebum, has significantly lower triglycerides and squalene and higher wax esters (21). In a study that assessed P. acnes growth on the skin of laboratory animals (mice, rats, rabbits, sheep, guinea pigs, and dogs), only the sebaceous (perianal gland) regions of guinea pigs harbored a significant burden of P. acnes (22). The authors attributed growth of P. acnes in the guinea pig sebaceous gland to the higher concentration of triglyceride found in the region. Consistent with this report, oleic acid, a breakdown product of triglyceride, promotes growth of P. acnes in vitro (17, 23), although these findings have been disputed by another group (24). Squalene, which is also abundantly found in human sebum but not in mouse sebum (21), is found in higher concentrations in sebum of subjects with acne compared with controls without acne (25), but its role in supporting P. acnes growth is unclear. Additionally, the sheer abundance of sebum could also account for improved P. acnes survival, as human studies have shown that sebum concentration is highest on the face and back and correlates with the number of P. acnes isolated from those sites (11).
Irrespective of the mechanism whereby human sebum enhances the mouse model, application of human sebum permits use of lower inoculums of P. acnes and leads to more stable bacteria burden and robust immunopathology. Our model has several caveats, including the intradermal administration of P. acnes and the relative acute nature of infection, and the model is not improved by topical application of P. acnes and sebum. Dysregulation of host skin environment and hypercornification of sebaceous gland have been proposed to be initial events facilitating P. acnes induction of inflammation (26). We have been unable to model these initial events; therefore, further studies are clearly needed to refine the murine model. Nonetheless, when rigorously selected health- and disease-associated strains are applied, the P. acnes strains produced disease scores and immunopathology that correlated strongly with their clinical association. These findings suggest that the virulence properties of P. acnes that are important for enhancing human acne inflammation are also important for immunopathology in the murine model. Altogether, we suggest that our validated model will be able to more reliably predict human disease compared with published models.
Acne vulgaris is a multifactorial disease involving P. acnes and host and environmental factors. The extent of bacterial contribution to the disease has been unclear, since acne patients and healthy individuals both harbor significant numbers of P. acnes on their skin. Genomic and metatranscriptomic studies indicate that acne-associated P. acnes strains (2–4, 27, 28) may contribute to the disease through virulence genes and transcriptional and metabolic activities and further suggest that putative virulence factors encoded by acne-associated strains could be exploited for therapeutics. Our study provides strong laboratory corroboration of genomic and clinical findings by demonstrating remarkable and uniform correlation between health/disease association of P. acnes and immunopathology induced by the strains. These findings suggest that the contribution of P. acnes genetic background to acne disease must be substantial and that the investigation of putative virulence factors is likely to have translational and therapeutic value.
Currently, few P. acnes factors have been scrutinized for a potential role in acne pathogenesis. Huang and colleagues have shown that Christie-Atkins-Munch-Petersen (CAMP) factor contributes to inflammation and CAMP immunization ameliorates inflammation induced by P. acnes in the ear inflammation model (29, 30), although CAMP is expressed by both health- and acne-associated P. acnes strains/clades (6). We previously demonstrated that acne-associated P. acnes strains from type IA-2 RT4/5 strains produce higher levels of proinflammatory porphyrins compared with type II RT2/6 strains at baseline (27). In response to vitamin B12 supplementation, IA-2 RT4/5 strains further upregulate porphyrin production and promote inflammation, whereas type II RT6/2 strains are unresponsive to vitamin B12 supplementation (27, 28). Repressor gene deoR, which is present in type II strains but not in type IA-2 strains, is likely to be responsible for the modulation of porphyrin production (27, 28).
In addition to CAMP and porphyrin, comparative analysis of genomes from health- and acne-associated P. acnes strains has revealed other factors that could contribute to differences in immunopathology and induced inflammatory cytokines (6, 31–33). Specifically, IA-2 RT4/5 strains harbor two genomic islands and a linear plasmid with many unique genes, some of which are homologs to known virulence genes (3, 31). The linear plasmid encodes a tight adhesion (Tad) locus, which has demonstrated virulence functions in other microbes (34, 35). Within the genomic islands, a Sag gene cluster encodes proteins that have been shown to induce hemolysis in S. pyogenes and S. iniae with associated proinflammatory and pathogenic functions (36, 37). In addition, lipases and hyaluronidase are differentially expressed by acne- and health- associated strains and could also contribute to inflammation (31–33). At the proteome level, Kim and colleagues have shown that a cell wall hydrolase and several proteins of unknown function were expressed at significantly higher levels in acne-associated compared with health-associated strains (6). Characterization of these factors would significantly broaden our knowledge of acne pathogenesis and would likely be of clinical significance, given that P. acnes RT4/5 and RT2/6 strains have shown clear differences in pathology in our murine model.
Inflammation is an important driver of pathology in acne disease. Therefore, following the discovery of health- and acne-associated P. acnes strains and phylotypes, several groups have investigated the proinflammatory activity of P. acnes strains from diverse phylotypes. Nagy and colleagues showed that strains from type IA1 induced higher β-defensin from keratinocytes compared with a strain from type II (38). Conversely, the type II P. acnes induced higher levels of IL-8 (38). Jasson and colleagues challenged skin explant with lysates from P. acnes but showed higher expression of PAR-2, TIMP-2, MMP-13, and TNF-α by health- or non-acne-associated types (II and III) than by acne-associated strains (8). A caveat of these earlier studies is that the strains used were not well characterized for health and disease association and the studies looked mostly at single isolates from each phylotype. More recently, Kim and colleagues stimulated human monocytes with well-characterized health- and disease-associated P. acnes strains, including strains used in this study (6, 7). They concluded preferential induction of proinflammatory IL-17 by acne-associated P. acnes strains and enhanced induction of antiinflammatory IL-10 by health-associated P. acnes strains. Our in vitro studies further build on that study and demonstrate uniformly higher innate cytokine and chemokine induction by RT4/5 strains compared with type II strains. Increased IL-6 and Il-1β induction, which promote Th17 development, is consistent with the reported association between acne and Th17. Overall, these data support the hypothesis that acne-associated P. acnes strains promote acute and chronic inflammation through activation of the innate and adaptive branch of the immune system. Because of the close association of select cytokine secretion with disease, proinflammatory cytokines deserve to be further studied as potential markers for health and acne association when screening P. acnes strains. Identification and manipulation of health- or disease-associated strains could represent a novel strategy to modify risk of severe acne.
In summary, our study has revealed an unexpectedly strong laboratory correlation between P. acnes immunopathology in animals and clinical disease and health association. The validated model system will facilitate investigation of putative virulence factors identified through the genomic studies.
Methods
Bacterial strains and growth conditions.
P. acnes strains were isolated as stated previously (3). P. acnes strains were grown on blood agar plates and then isolated colonies were grown in BHI media at 37°C anaerobically until the stationary phase was reached. For supernatant samples, cultures were centrifuged at 3220 g for 5 minutes, and the supernatant was collected and filter sterilized. For heat-killed samples, overnight bacterial cultures were incubated at 65°C for 1 hour. For murine experiments, cultures were diluted 1:100 in fresh BHI and allowed to grow until an OD600 of 0.1–0.3 was reached. Cultures were centrifuged at 3220 g for 5 minutes and washed in BHI 3 times, and the resulting pellets were resuspended in media.
Synthetic sebum.
Synthetic sebum was prepared as previously described (18). Sebum was prepared on the day of experiment by mixing fatty acid (17% oleic acid, MilliporeSigma), triglyceride (45% triolein, Fisher), 25% wax monoester (25% jojoba oil), and squalene (13%, Fisher).
Murine model of acne immunopathology by intradermal injection of P.
acnes. Eight-week-old CD-1 female mice (Jackson Lab) were shaved before application of Nair (Church & Dwight). The next day, the mice were injected intradermally with approximately 1 × 10 7 CFU of P. acnes in 50 μl BHI media. Immediately following injection, 20 μl freshly made synthetic sebum was applied to the skin and reapplied daily. Lesions were aseptically harvested at specified time points, and CFU was determined on agar plates incubated anaerobically. In addition, homogenized lesions were centrifuged at 9300 g for 10 minutes, and the supernatants were stored at –80°C for cytokine analysis by ELISA. For histology, skin was fixed in 10% formalin (Medical Chemical Corporation), embedded in paraffin, and submitted to the Department of Pathology at Cedars-Sinai Medical Center for H&E and Gram staining.
Disease scoring.
Gross skin pathology was scored based on tabulation of the following: erythematous change (no = 0, mild = 1, and marked = 2); papule (flat = 0, small = 1, and large = 2); eschar (no = 0, mild = 1, and marked = 2); and xeroderma (no = 0, mild = 1, and marked = 2).
Histology slides were evaluated based on acute inflammatory changes in the intracorneal or subcutaneous/deep soft tissue (0 = normal, 1 = mild, 2 = moderate, 3 = severe).
Murine model of acne immunopathology by tape stripping and topical application of P.
acnes. Eight-week-old CD-1 female mice were shaved before application of Nair (Church & Dwight). The following day, mice were anesthetized and tape stripped 10 times with masking tape. Immediately after, 1 × 107 CFU of P. acnes in 20 μl was applied to the tape-stripped area with or without 20 μl freshly made synthetic sebum and allowed to absorb into the skin. Sebum was applied daily and lesions were measured on day 3.
Cell culture.
Bone marrow cells were isolated from the femurs and tibiae of 12-week-old C57BL/6 mice (Jackson Lab) and suspended in RPMI 1640 medium with 10% heat-inactivated fetal bovine serum. Ten percent supernatant from L929 cells (ATCC), containing M-CSF, was added to induce differentiation of bone marrow cells into macrophages (bone marrow–derived macrophages). The cells were cultured 5% CO2 at 37°C for 7 days prior to use.
HaCaT cells (ATCC), a human keratinocyte cell line, were maintained in DMEM (Invitrogen) media with the addition of 10% heat-inactivated fetal bovine serum, 4.5 g/l glucose, and 2 mM L-glutamine. Keratinocytes were seeded at an appropriate density in tissue culture plates in 5% CO2 at 37°C 1 day prior to stimulation.
ELISA.
Mouse IL1-α– (catalog 433404, Biolegend), IL1-β– (catalog 432601, Biolegend), IL-6– (catalog 431301, Biolegend), and KC/CXCL1- (DY453-05, R&D Systems) and human IL-6– (catalog 430504, Biolegend) and IL-8–specific (catalog no. 431501, Biolegend) ELISAs were performed according to the manufacturer’s instructions.
Statistics.
Data are expressed as mean ± SEM. Two-group analysis used unpaired t test (2 tailed) or nonparametric Mann-Whitney U test in the case of missing normality. Comparisons of multiple groups were performed using 1-way ANOVA and subsequent Bonferroni multiple comparisons. If normality or equal variance tests failed, then a Kruskal-Wallis test and subsequent Dunn’s multiple comparisons were used. All in vitro studies were done with at least 3 sets of independent experiments. GraphPad Prism was used for all analyses. A P value of less than 0.05 was considered significant.
Study approval.
All procedures were approved by the IACUC of Cedars-Sinai Medical Center and conducted in accordance with NIH guidelines for the care and use of laboratory animals.
Author contributions
SLK, GYL, JT, and HL conceived and designed the study. SLK and CMT performed the experiments. XF performed the pathological analyses. All authors contributed to data analyses. SLK and GYL wrote the manuscript. All authors contributed to manuscript editing.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R21AI103839 (to GYL) and R01GM099530 (to HL).
Version 1. 03/07/2019
Electronic publication
Footnotes
SLK’s present address is: C3J therapeutics, Los Angeles, California, USA.
Conflict of interest: The authors have declared that no conflict of interest exists.
License: Copyright 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(5):e124687. https://doi.org/10.1172/jci.insight.124687.
Contributor Information
Chih-Ming Tsai, Email: Chih-Ming.Tsai@cshs.org.
Juan Torres, Email: jacatltopiltzin@gmail.com.
Xuemo Fan, Email: Xuemo.Fan@cshs.org.
Huiying Li, Email: huiying@mednet.ucla.edu.
References
- 1.Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474–485. doi: 10.1111/bjd.12149. [DOI] [PubMed] [Google Scholar]
- 2.McDowell A, et al. An expanded multilocus sequence typing scheme for propionibacterium acnes: investigation of ‘pathogenic’, ‘commensal’ and antibiotic resistant strains. PLoS One. 2012;7(7):e41480. doi: 10.1371/journal.pone.0041480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitz-Gibbon S, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133(9):2152–2160. doi: 10.1038/jid.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS One. 2010;5(8):e12277. doi: 10.1371/journal.pone.0012277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnard E, Shi B, Kang D, Craft N, Li H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci Rep. 2016;6:39491. doi: 10.1038/srep39491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y, Champer J, Agak GW, Kao S, Modlin RL, Kim J. Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J Invest Dermatol. 2016;136(11):2221–2228. doi: 10.1016/j.jid.2016.06.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agak GW, et al. Phenotype and antimicrobial activity of Th17 cells induced by Propionibacterium acnes strains associated with healthy and acne skin. J Invest Dermatol. 2018;138(2):316–324. doi: 10.1016/j.jid.2017.07.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jasson F, Nagy I, Knol AC, Zuliani T, Khammari A, Dréno B. Different strains of Propionibacterium acnes modulate differently the cutaneous innate immunity. Exp Dermatol. 2013;22(9):587–592. doi: 10.1111/exd.12206. [DOI] [PubMed] [Google Scholar]
- 9.Mirshahpanah P, Maibach HI. Models in acnegenesis. Cutan Ocul Toxicol. 2007;26(3):195–202. doi: 10.1080/15569520701502815. [DOI] [PubMed] [Google Scholar]
- 10.Bernerd F, Ortonne JP, Bouclier M, Chatelus A, Hensby C. The rhino mouse model: the effects of topically applied all-trans retinoic acid and CD271 on the fine structure of the epidermis and utricle wall of pseudocomedones. Arch Dermatol Res. 1991;283(2):100–107. doi: 10.1007/BF00371617. [DOI] [PubMed] [Google Scholar]
- 11.Schwartzman RM, Kligman AM, Duclos DD. The Mexican hairless dog as a model for assessing the comedolytic and morphogenic activity of retinoids. Br J Dermatol. 1996;134(1):64–70. [PubMed] [Google Scholar]
- 12.Petersen MJ, Zone JJ, Krueger GG. Development of a nude mouse model to study human sebaceous gland physiology and pathophysiology. J Clin Invest. 1984;74(4):1358–1365. doi: 10.1172/JCI111546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang YH, Lee KC, Lee SJ, Kim DW, Lee WJ. HR-1 mice: a new inflammatory acne mouse model. Ann Dermatol. 2015;27(3):257–264. doi: 10.5021/ad.2015.27.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Young LM, Young JM, Ballaron SJ, Spires DA, Puhvel SM. Intradermal injection of Propionibacterium acnes: a model of inflammation relevant to acne. J Invest Dermatol. 1984;83(5):394–398. doi: 10.1111/1523-1747.ep12264715. [DOI] [PubMed] [Google Scholar]
- 15.Fortier AH, Mock BA, Meltzer MS, Nacy CA. Mycobacterium bovis BCG-induced protection against cutaneous and systemic Leishmania major infections of mice. Infect Immun. 1987;55(7):1707–1714. doi: 10.1128/iai.55.7.1707-1714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGinley KJ, Webster GF, Ruggieri MR, Leyden JJ. Regional variations in density of cutaneous propionibacteria: correlation of Propionibacterium acnes populations with sebaceous secretion. J Clin Microbiol. 1980;12(5):672–675. doi: 10.1128/jcm.12.5.672-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puhvel SM, Reisner RM. Effect of fatty acids on the growth of Corynebacterium acnes in vitro. J Invest Dermatol. 1970;54(1):48–52. doi: 10.1111/1523-1747.ep12551667. [DOI] [PubMed] [Google Scholar]
- 18.Wertz PW. Human synthetic sebum formulation and stability under conditions of use and storage. Int J Cosmet Sci. 2009;31(1):21–25. doi: 10.1111/j.1468-2494.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- 19.Dreno B, et al. Understanding innate immunity and inflammation in acne: implications for management. J Eur Acad Dermatol Venereol. 2015;29 Suppl 4:3–11. doi: 10.1111/jdv.13190. [DOI] [PubMed] [Google Scholar]
- 20.Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49(2):271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Nikkari T. Comparative chemistry of sebum. J Invest Dermatol. 1974;62(3):257–267. doi: 10.1111/1523-1747.ep12676800. [DOI] [PubMed] [Google Scholar]
- 22.Webster GF, Ruggieri MR, McGinley KJ. Correlation of Propionibacterium acnes populations with the presence of triglycerides on nonhuman skin. Appl Environ Microbiol. 1981;41(5):1269–1270. doi: 10.1128/aem.41.5.1269-1270.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson DA, Cummins CS. Nutritional requirements of anaerobic coryneforms. J Bacteriol. 1978;135(3):858–867. doi: 10.1128/jb.135.3.858-867.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gribbon EM, Cunliffe WJ, Holland KT. Interaction of Propionibacterium acnes with skin lipids in vitro. J Gen Microbiol. 1993;139(8):1745–1751. doi: 10.1099/00221287-139-8-1745. [DOI] [PubMed] [Google Scholar]
- 25.Pappas A, Johnsen S, Liu JC, Eisinger M. Sebum analysis of individuals with and without acne. Dermatoendocrinol. 2009;1(3):157–161. doi: 10.4161/derm.1.3.8473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suh DH, Kwon HH. What’s new in the physiopathology of acne? Br J Dermatol. 2015;172 Suppl 1:13–19. doi: 10.1111/bjd.13634. [DOI] [PubMed] [Google Scholar]
- 27.Johnson T, Kang D, Barnard E, Li H. Strain-level differences in porphyrin production and regulation in Propionibacterium acnes elucidate disease associations. mSphere. 2016;1(1):e00023-15. doi: 10.1128/mSphere.00023-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang D, Shi B, Erfe MC, Craft N, Li H. Vitamin B12 modulates the transcriptome of the skin microbiota in acne pathogenesis. Sci Transl Med. 2015;7(293):293ra103. doi: 10.1126/scitranslmed.aab2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, et al. The anti-inflammatory activities of Propionibacterium acnes CAMP factor-targeted acne vaccines. J Invest Dermatol. 2018;138(11):2355–2364. doi: 10.1016/j.jid.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Nakatsuji T, Tang DC, Zhang L, Gallo RL, Huang CM. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: potential targets for inflammatory acne treatment. PLoS One. 2011;6(4):e14797. doi: 10.1371/journal.pone.0014797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomida S, et al. Pan-genome and comparative genome analyses of propionibacterium acnes reveal its genomic diversity in the healthy and diseased human skin microbiome. MBio. 2013;4(3):e00003–e00013. doi: 10.1128/mBio.00003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nazipi S, Stødkilde-Jørgensen K, Scavenius C, Brüggemann H. The skin bacterium Propionibacterium acnes employs two variants of hyaluronate lyase with distinct properties. Microorganisms. 2017;5(3):57. doi: 10.3390/microorganisms5030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDowell A, Perry AL, Lambert PA, Patrick S. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008;57(Pt 2):218–224. doi: 10.1099/jmm.0.47489-0. [DOI] [PubMed] [Google Scholar]
- 34.Kachlany SC, et al. Nonspecific adherence by Actinobacillus actinomycetemcomitans requires genes widespread in bacteria and archaea. J Bacteriol. 2000;182(21):6169–6176. doi: 10.1128/JB.182.21.6169-6176.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreiner HC, et al. Tight-adherence genes of Actinobacillus actinomycetemcomitans are required for virulence in a rat model. Proc Natl Acad Sci USA. 2003;100(12):7295–7300. doi: 10.1073/pnas.1237223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuller JD, et al. Identification of a streptolysin S-associated gene cluster and its role in the pathogenesis of Streptococcus iniae disease. Infect Immun. 2002;70(10):5730–5739. doi: 10.1128/IAI.70.10.5730-5739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Humar D, Datta V, Bast DJ, Beall B, De Azavedo JC, Nizet V. Streptolysin S and necrotising infections produced by group G streptococcus. Lancet. 2002;359(9301):124–129. doi: 10.1016/S0140-6736(02)07371-3. [DOI] [PubMed] [Google Scholar]
- 38.Nagy I, Pivarcsi A, Koreck A, Széll M, Urbán E, Kemény L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124(5):931–938. doi: 10.1111/j.0022-202X.2005.23705.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.