Abstract
Inflammatory arthritis encompasses a set of common diseases characterized by immune-mediated attack on joint tissues. Most but not all affected patients manifest circulating autoantibodies. Decades of study in human and animal arthritis have identified key roles for autoantibodies in immune complexes and through direct modulation of articular biology. However, joint inflammation can arise because of pathogenic T cells and other pathways that are antibody-independent. Here we review the evidence for these parallel tracks, in animal models and in humans, to explore the range of mechanisms engaged in the pathophysiology of arthritis and to highlight opportunities for targeted therapeutic intervention.
Introduction
Inflammatory arthritis affects approximately 1% to 2% of the population (1). Although dwarfed numerically by osteoarthritis, its collective impact remains substantial because of the potential for severe disability and because patients are often young. The most common adult form of inflammatory arthritis, rheumatoid arthritis (RA), can begin in teenagers, whereas juvenile idiopathic arthritis (JIA) peaks before the age of 6 years. This family of diseases therefore disproportionately threatens a period of life typically characterized by good health, leading to impaired quality of life as well as loss of productivity and medical expenses.
Inflammatory arthritis is not a single disease. Broadly, inflammatory arthritis may be divided into conditions focused on the synovium and those that also affect the entheses, the insertion zones of tendons, ligaments, and joint capsules into bone. This division is far from absolute but usefully reflects a clinical and pathophysiologic spectrum that ranges from RA, characterized by an aggressive synovitis affecting peripheral joints, to ankylosing spondylitis, characterized by enthesitis and new bone formation in the axial skeleton. Ankylosing spondylitis, reactive arthritis, and psoriatic arthritis form part of a family of enthesis-focused diseases termed “spondyloarthritis.” Arthritis beginning in childhood was traditionally regarded as a distinct disease family, although it is increasingly clear that most forms of JIA resemble their adult counterparts (2). This Review will focus on synovitis and its genesis and perpetuation by antibodies and antibody-independent mechanisms. Mechanisms of spondyloarthritis have been recently reviewed (3).
Autoantibodies in inflammatory arthritis
In common with other autoimmune diseases, many forms of inflammatory arthritis are associated with circulating autoantibodies (Table 1 and refs. 4–10). In 1939, serum from a patient with RA was noted to aggregate sheep red blood cells opsonized with rabbit IgG (11). This serologic capacity was subsequently found in many but not all RA patients and determined to reflect the presence of RF, an antibody directed against the fragment crystallizable (Fc) region of IgG (12, 13). Although detectable in other disease states, RFs associated with RA exhibit affinity maturation of the antibody complementarity–determining region, potentially implicating a history of T cell help that is uncommon for RF generated outside of RA (14–18). Clinically, RA accompanied by RF — termed “seropositive RA” and representing 40% to 80% of all RA — is characterized by a tendency toward greater disease severity (19–22). RFs are a heterologous group of antibodies, most commonly IgM but also IgG or IgA. Although RF of all isotypes is associated with more aggressive disease and bone erosions, IgA RF is particularly correlated with extra-articular manifestations, such as interstitial lung disease, nodule formation, and rheumatoid vasculitis (23–27).
Table 1. Autoantibodies in RA.
The identification of RF spurred the search for other RA-associated autoantibodies. One of the first antigen-specific autoantibodies was discovered in 1964, when serum from some RA patients was shown to bind in a perinuclear pattern to human epithelial cells cultured from buccal mucosa. This autoantibody was named “antiperinuclear factor” (28). Reactivity was subsequently noted against the keratin layer of rat esophagus (29). These antibodies were directed against peptides modified posttranslationally through conversion of arginine to citrulline, an enzymatic reaction executed by the peptidyl arginine deiminases (PADs) that neutralizes the positive charge of the arginine side chain (30–32). Such ACPAs are highly specific for RA and are present in approximately two-thirds of patients (33). ACPAs recognize citrullinated fibrinogen, vimentin, enolase, and collagen peptides, among other antigens (Table 1). Indeed, ACPAs often recognize multiple citrullinated targets because affinity is driven by the citrulline residue itself, modulated only modestly by peptide context (34). For reasons still to be determined, RF+ and ACPA+ patient subsets largely overlap, with ACPA reactivity often preceding the appearance of RF (35). Intriguingly, the citrullination pathway is also targeted by other RA autoantibodies, with approximately 20% to 40% of RA patients manifesting antibodies against PAD4 (36–38). These patients may exhibit more joint erosion, while RA patients with anti-PAD4/PAD3 antibodies demonstrate more interstitial lung disease (37, 39, 40).
Both RF and ACPAs can be generated within the synovium, as evidenced both by higher levels in the synovium and synovial fluid than in paired blood and by the presence of corresponding plasma cells in joint tissue (41–43). Local autoantibody production is likely fostered by a recently identified lymphocyte, the T peripheral helper (TPH) cell, which provides help for B cells outside of lymph nodes (44). ACPAs can also be generated in the lung, supporting the possibility that RA can begin as an immune reaction in the pulmonary epithelium that extends to affect the joints (45).
Other autoantibodies have been associated with inflammatory arthritis. Approximately 45% of RA patients exhibit antibodies against peptides that have undergone another posttranslational modification, carbamylation (5). Antibodies may be formed against proteins modified through acetylation, oxidation, or malondialdehyde-acetaldehyde adducts (46–48). IgG isolated from RA joints exhibits reactivity against histones (49). ANA is common and in RA is associated with concomitant Sjogren’s syndrome (50). In JIA, the presence of ANA correlates with early disease onset and risk for chronic anterior uveitis, especially when anti-histone antibodies are also present (7, 8, 51, 52). Oligoarticular JIA with uveitis has also been associated with antibodies against the chemoattractant nuclear protein DEK (9).
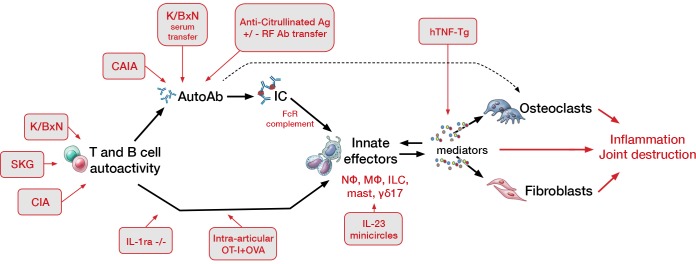
The pathogenic relevance of antibodies is amply confirmed in animals. The 2 best-studied experimental arthritis models, K/BxN arthritis and collagen-induced arthritis (CIA), are both mediated primarily through IgG. The K/BxN mouse is a cross (x) between mice bearing the KRN-transgenic T cell receptor in a C57BL/6 background (K/B) and the nonobese diabetic (NOD) mouse (N). The KRN receptor recognizes bovine ribonuclease as presented by the class II major histocompatibility complex (MHC) molecule Ak. However, in the context of the NOD MHC II, termed I-Ag7, this receptor instead recognizes a peptide from the glycolytic enzyme glucose-6-phosphate isomerase (GPI). Resulting anti-GPI antibodies engender arthritis with clinical and histopathologic similarities to RA, including symmetry, a distal-to-proximal gradient of severity, formation of erosive pannus, and accumulation of neutrophils in the joint. In CIA, mice immunized with allogenic type II collagen produce antibodies reactive against articular cartilage. In both K/BxN and CIA systems, joint disease can be induced in naive wild-type mice via antibody transfer, without additional contribution from adaptive immunity (Figure 1 and refs. 53–60). Adoptive transfer of ACPAs can further intensify synovitis, supporting a pathogenic role for these autoantibodies (61, 62).
Figure 1. Intersection of murine arthritis models with the pathogenic sequence of inflammatory arthritis.
Inflammation in murine arthritis can arise through immune dysfunction arising at a wide variety of levels, ranging from errant T and B cell–driven antigen targeting to overexpression of downstream inflammatory mediators. Autoimmunity can translate into arthritis via antibody-dependent pathways, typically through IgG and immune complexes (ICs), but also independent of autoantibodies through the action primarily of pathogenic T lymphocytes. Murine models (red-lined gray boxes) illustrating each respective mechanism are indicated. CAIA, collagen antibody–induced arthritis; FcR, Fcγ receptor; C′, complement; Cit, citrullinated peptide; OVA, ovalbumin; Nɸ, neutrophil; Mɸ, monocyte/macrophage; ILC, innate lymphocyte; mast, mast cell; hTNF-Tg, human TNF–transgenic. Modified from Monach et al. (184). Illustrated by Mao Miyamoto.
Mechanisms of antibody-mediated arthritis
Arthritis mediated by IgG arises via distinct pathways that can be divided into 2 categories based on the role of ICs (Figure 2). We will review each category in turn, recognizing that individual patients with arthritis may proceed simultaneously via both mechanisms as well as through IgG-independent pathways, discussed subsequently.
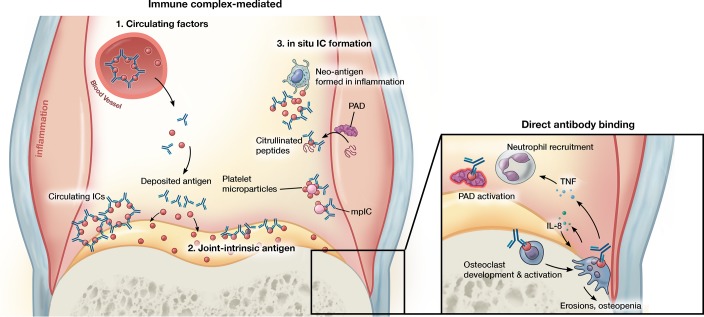
Figure 2. Antibody-mediated mechanisms of inflammatory arthritis.
Joint inflammation mediated by antibodies can proceed via distinct and not mutually exclusive pathways. ICs formed in circulation can precipitate in joint tissue and trigger enhanced vascular permeability that enables entry of pathogenic antibodies into the joint. ICs/antibody clusters can form within the joint through binding to joint-intrinsic antigens such as collagen, blood-borne antigen deposited on cartilage, or antigens formed de novo in the inflamed milieu such as citrullinated peptides and platelet microparticles, the latter generating microparticle-associated immune complexes (mpICs). Independent of IC formation, antibodies can mediate pathology via binding to specific targets. For example, antibodies promote citrullination by altering the activation threshold of PADs and stimulate the development or activation of osteoclasts. In addition to promotion of bone erosions and local osteopenia, osteoclasts may promote further osteoclastogenesis and contribute to neutrophil recruitment via IL-8 and other mediators. Illustrated by Mao Miyamoto.
IC-mediated arthritis.
ICs are aggregates of antibodies around a multivalent target and may contain IgG, IgM, and sometimes IgA. Antibody clustering engages low-affinity IgG Fc receptors and induces conformational changes in the Fc region that permit IgG to activate complement, a process termed “complement fixation.” The complement system was originally identified as a soluble component of serum able to “complement” antibody-induced lysis of bacteria and consists of a set of proteins with multiple immune functions. These proteins are the anaphylatoxins (C3a and C5a) that mediate inflammation, vasodilation, and chemotaxis; opsonins (C3b and C4b) that can bind to ICs to facilitate clearance by cells bearing complement receptors; and the membrane attack complex (MAC, C5b–C9) that forms transmembrane pores in target cells to induce osmotic lysis. Complement can be activated through 3 pathways. The classical pathway is initiated by the Fc portions of antigen-bound IgG, IgM, and sometimes IgA through complement proteins C1q/r/s, C4, and C2. The alternative pathway relies upon the spontaneous hydrolysis of complement component C3 into C3b, which binds to and cleaves factor B to generate a C3 convertase that is key to completing the complement cascade and forming the MAC. The lectin pathway is homologous to the classical pathway but is activated by lectins, carbohydrate-binding proteins that recognize specific glycans, linking to the C3 convertase and its downstream effector pathways via mannose-binding lectin-associated serine proteases (MASPs) (63).
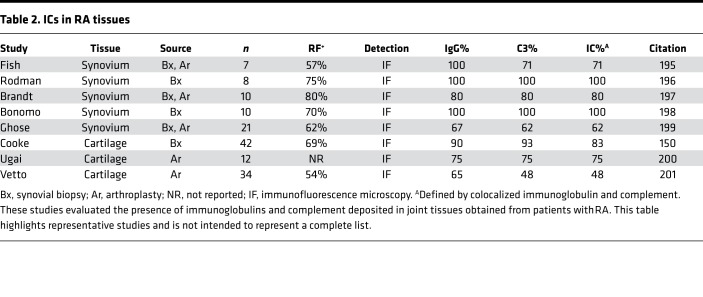
Accumulation of ICs in joints occurs in 3 ways (Figure 2). Circulating ICs may deposit directly in joint tissue. Alternately, antibodies against clustered joint-intrinsic antigens may form ICs locally. Finally, antibodies may encounter antigen deposited in the joint from the circulation or generated within the joint itself, again resulting in local IC formation. ICs are likely a major route to human joint inflammation, particularly in seropositive RA, as suggested by the high prevalence of synovial fluid complement fixation, ICs within synovial fluid neutrophils, and ICs in joint explant tissue from this RA subset (refs. 64–66 and Table 2).
Table 2. ICs in RA tissues.
The capacity of RF to bind multiple IgG molecules at once can promote IC formation. Self-aggregated RF as well RF bound to IgG activates complement (67–70). Correspondingly, RF is common in ICs isolated from RA joints (68, 71–75). RF inhibits the ability of complement to break up ICs and can link smaller complexes together into larger, less soluble ones, potentially accounting for the observations that RF is found in most RA patients with vasculitis and depressed serum complement and that synovial fluid neutrophils containing ICs are observed primarily in seropositive patients (66, 76–78). RF amplifies cell activation by ICs containing ACPAs and citrullinated target peptides, and patients with both RF and ACPAs exhibit higher disease activity and circulating proinflammatory mediators than those with either autoreactivity alone (20, 22, 79). The nucleating antigen may also play a key role. Citrullinated fibrinogen is a common component of circulating ICs in ACPA-positive RA patients. Although ICs can activate macrophages by cross-linking Fcγ receptors, synovial macrophages also bear Toll-like receptor 4 (TLR4), an innate immune receptor that recognizes conserved microbial products but also fibrinogen. Therefore, ICs containing citrullinated fibrinogen synergistically activate Fcγ receptor and TLR4 pathways to trigger an inflammatory response (80). Of note, transfusion of RF-containing plasma from patients with RA into nonarthritic human recipients (!) failed to yield joint inflammation (81). Further, RF may appear years before the onset of clinical symptoms, confirming that RF alone is not sufficient to develop RA (35, 82).
IC-mediated arthritis is among the best-understood arthritis pathways because of the availability of tractable animal models. Both CIA and K/BxN arthritis arise via ICs. In CIA, clusters of IgG form on type II collagen in the joint (56–58). In K/BxN arthritis, joint specificity despite the ubiquity of the autoantigen is thought to arise through deposition of positively charged GPI onto the negatively charged cartilage surface, followed by in situ IC formation, likely with ICs deposited from the circulation (53–55, 83, 84). The requirement for ICs is clear from the fact that multiple antibodies recognizing nonoverlapping epitopes are generally required (59, 60, 85). Entry of autoantibodies is facilitated by vascular leakiness of the synovial vasculature induced by the binding of ICs to circulating neutrophils and platelets as well as perivascular mast cells, leading to the release of vasoactive mediators, such as TNF-α, histamine, and platelet-derived serotonin (86–88). Inflammation then proceeds through activation of cells via low-affinity IC receptors (in mouse, receptors FcγRIII and FcγRIV) together with the receptor for anaphylatoxin C5a (19, 89–94). Cells implicated in immune sensing include neutrophils, mast cells, and potentially macrophages, with C5a serving to sensitize cells to FcγR-mediated activation and to arrest neutrophils on the synovial endothelium (95–99). Inflammation is then mediated by infiltrating neutrophils and monocytes together with local cells, including fibroblasts and macrophages (86, 100–105).
Despite the key role of the classical pathway in IC recognition and clearance, classical pathway components C1q, C2, and C4 are not required for IgG-mediated arthritis in the mouse. Rather, the alternative pathway is dominant, and murine arthritis is markedly attenuated in the absence of C3 or factor B (89, 106–108). The mechanistic basis for this observation is incompletely understood. IgG is not canonically considered to activate the alternative pathway, although in fact such activation has been reported for murine IgG, human ACPAs, and human IgA (109–111). Murine studies reveal at least 2 related mechanisms. IgG immobilized on cartilage triggers cleavage of alternative pathway component factor D via lectin pathway MASP-1/3 (MASP-1 and MASP-3 are splice variants of a single gene and so difficult to distinguish genetically) (112, 113). Correspondingly, mice lacking MASP-1/3 are resistant to CIA (114, 115). Another lectin pathway protease, MASP-2, activates C3 via the “C4/C2 bypass pathway,” a poorly defined pathway whereby C3 is cleaved without requiring C2 and C4, components of the typical lectin pathway C3 convertase. Mice deficient in MASP-2 are partially resistant to CIA (116). How pathogenic IgG engages MASPs remains to be defined, but deposition of ICs likely represents a key step. The role of complement in arthritis has recently been comprehensively reviewed (63).
A further line of evidence for the role of ICs and complement in arthritis arises from studies of cartilage. Clinical observation shows that arthritis often recurs in RA joints subjected to synovectomy but tends to abate in joints from which all cartilage has been resected, even if synovium remains (117–119). The articulating surface of cartilage lacks a bilipid membrane, and as a result complement inhibitory proteins such as CD59 are absent. Complement fixation is constrained by less efficient chemical mechanisms, such as surface sialic acids, and by the maintenance of very low levels of complement in normal synovial fluid (64, 120). As complement factors enter the joint from the blood in the context of inflammation-mediated vascular leak, the cartilage surface becomes the focus of poorly controlled complement fixation, including via the alternative pathway (63, 113). Further, ICs deposited or formed on cartilage must be cleared by inflammatory cells recruited into the joint, and neutrophils encountering ICs can disgorge their granule enzymes directly into the cartilage surface in a potentially injurious process termed “frustrated phagocytosis” (121). Consistent with this biology, in murine arthritis, antibody arthritogenicity correlates with cartilage binding (62, 122). It is therefore likely that the cartilage surface explains why IC-mediated inflammation in RA manifests predominantly in the joints — in other words — why RA is an arthritis at all.
Indeed, ongoing formation of ICs within the joint likely represents a key mechanism of disease chronicity. In the transient arthritis of serum sickness, joint inflammation resolves once the shower of ICs subsides. In chronic inflammatory arthritis, autoantibodies recognizing antigens that are formed or released in the inflamed joint, such as citrullinated peptides and DEK, generate an amplification loop whereby joint inflammation begets ICs that in turn perpetuate joint inflammation. Feeding into this cycle, IC-mediated MAC deposition on synovial fluid neutrophils hyperactivates intracellular PADs to generate citrullinated RA autoantigens (123–125). Microparticles are another source of locally generated ICs. Murine and human studies implicate CD41+ (GP1b+) microparticles released by platelets and potentially megakaryocytes as important mediators of joint inflammation, at least in part through IL-1α and IL-1β (126, 127). Platelet microparticles feature citrullinated surface proteins recognized as RA autoantigens, including vimentin and fibrinogen. These microparticles nucleate many of the ICs found in RA synovial fluid, creating microparticle-associated ICs (mpICs) (Figure 2 and ref. 128).
Glycosylation as a modulator of IgG effector function.
The ability of IgG to bind Fc receptors and fix complement depends on IgG Fc glycosylation. A short biantennary glycan, typically 7–13 monosaccharides in length, is attached to asparagine 297 in the CH2 region of each IgG Fc heavy chain. These 2 glycans interact with each other and with the protein backbone of the opposite heavy chain to modulate the structure of the Fc region. IgG lacking Fc glycans loses much of its effector capacity, and in vivo enzymatic removal abrogates antibody-mediated arthritis (129). Variation among the greater than 30 possible Fc glycoforms fine-tunes the ability of IgG to interact with ligands such as C1q, the human IC receptor FcγRIIA, and mannose-binding lectin. Precise structure-to-function correlation remains controversial, both because experimental findings diverge and because murine and human systems overlap incompletely (130). In general, glycoforms with reduced galactose and sialic acid are thought to confer enhanced proinflammatory capacity, whereas highly galactosylated and sialylated IgG engage antiinflammatory mechanisms to skew IgG toward immunomodulatory function. Loss of sialylation represents a “molecular switch” that converts innocuous autoantibodies into antibodies capable of initiating murine arthritis (109, 131). Desialylation enables ICs to activate osteoclasts, thereby contributing to local and generalized bone loss (132). Patients with RA and JIA exhibit reduced galactosylation with more modest changes in sialylation, sometimes predating overt disease by months or years (133–136). ACPAs accompanying seropositive RA exhibit even more marked hypogalactosylation, as well as striking fragment antigen-binding (Fab) glycosylation of unknown functional significance (136–138).
Correlative evidence for an etiologic role of IgG Fc glycosylation in human RA comes from pregnancy. Pregnancy is accompanied by marked increase in circulating estrogen, the only factor known to modulate human IgG glycans in vivo through its capacity to enhance Fc galactosylation (139). Pregnancy is accompanied by a marked decrease in hypogalactosylated IgG, often corresponding temporally with an amelioration of RA disease activity. IgG glycosylation normalizes after parturition, again often coincident with flaring RA (140, 141). These intriguing correlations suggest that RA improvement in pregnancy may be mediated in part through IgG Fc glycosylation, although glycan shifts could still represent either an epiphenomenon or a result of reduced inflammation. Indeed, in the mouse, estrogen attenuates arthritis flare in postpartum mice with only modest changes in IgG galactosylation (142). It is possible, and even probable, that glycan changes both reflect the inflammatory milieu and alter IgG function to potentiate further inflammation. Further study will be required to define causality in the relationship between IgG glycan changes and arthritis in the human context.
Arthritis mediated by IgG independent of ICs.
IgG can engender joint inflammation through the molecules that they target, without formation of ICs. ACPA-mediated activation of osteoclasts and antibodies that enhance PAD function are 2 examples (Figure 2).
ACPAs are among the strongest predictors of a destructive arthritis course (143, 144). Anti–citrullinated vimentin antibodies bind to osteoclasts, stimulating osteoclastogenesis and leading to increased bone resorption (145). The introduction of anti–citrullinated vimentin antibodies is sufficient to cause periarticular bone loss in wild-type mice as well as generalized osteopenia in lymphocyte-deficient Rag-1–/– mice (145, 146). How ACPAs stimulate osteoclastogenesis remains unclear. Studies have implicated both direct binding to citrullinated vimentin on osteoclasts and desialylation-dependent engagement of low-affinity Fc receptors (132, 145). Other work implicated IL-8 elaborated by ACPA-stimulated osteoclasts, but the antibodies used were later found to lack citrulline specificity, implicating citrulline-independent stimulatory mechanisms (34, 147). IL-8 not only promotes further osteoclastogenesis but also is an important neutrophil chemoattractant, raising the possibility that osteoclasts play a role early in antibody-mediated inflammation as well as in later bone erosion, including but not limited to disease mediated by ACPAs.
Antibodies directed against PADs can also be pathogenic. PAD enzymes require supraphysiologic concentrations of calcium (5–10 mM in vitro) for optimal citrullination. Intracellular calcium concentrations rarely exceed 0.1 mM even with activation, while typical extracellular calcium concentrations approximate 1 mM (148). The binding of anti-PAD4 antibody increases the sensitivity of PAD4 to calcium, effectively increasing enzymatic activity 10-fold. The anti-PAD3/PAD4 cross-reactive antibody further stabilizes PAD4 enzymatic activity, resulting in a 400-fold increase of histone citrullination at low calcium concentrations (148). Both anti-PAD4 and anti-PAD3/PAD4 are associated with more erosive arthritis, with the severity of joint erosion observed to correlate with PAD4 activity, suggesting that these autoantibodies may accelerate RA (148).
Mechanisms of antibody-independent arthritis
IgG is not the only pathway to inflammatory arthritis. Many patients lack any detectable arthritis-associated autoantibodies, and evidence for IgG-mediated inflammation in the joints is common but not uniform. In particular, fixation of complement is a feature of seropositive RA but is largely absent in seronegative RA, psoriatic arthritis, and reactive arthritis (64, 65, 67, 149–152). Neutrophils containing IgG ICs are observed almost exclusively in seropositive disease (66). The presence of ICs in surgically excised arthritic joint tissues is not ubiquitous but rather correlates strikingly with seropositivity (Table 2). Circulating ICs are more prevalent in seropositive disease, though methodologic challenges render this finding less clear-cut than in synovial fluid (153, 154). These studies may fail to detect arthritis induced by IgG independent of ICs but are consistent with the supposition that a sizable minority of human inflammatory arthritis arises independently of autoantibodies, a suggestion further supported by the observation that B cell–fostering TPH cells are found in much greater abundance in synovium from seropositive than seronegative patients (44).
Animal studies confirm that arthritis can arise independent of immunoglobulins (Figure 1). For example, arthritis may be engendered by an excess of TNF induced by overexpression or by mRNA dysregulation, reflecting engagement of pathogenic effector pathways without requirement for an inciting immune trigger (155, 156). Arthritis can be mediated directly by pathogenic T cells. Examples include SKG (Sakaguchi, after its discoverers) arthritis resulting from mutation in Zap70 and arthritis due to deficiency of IL-1 receptor antagonist (IL-1ra), discussed further below (157, 158). In all these systems, IgG-independent arthritis clinically resembles IgG-dependent arthritis, illustrating how phenotype represents an imperfect guide to pathophysiology.
Compared with IgG-dependent arthritis (Figure 2), antibody-independent arthritis is technically more difficult to explore experimentally, but several pathways to disease have been dissected mechanistically in the mouse (Figures 1 and 3).
Figure 3. Antibody-independent processes in the initiation and perpetuation of inflammatory arthritis.
Multiple cell lineages contribute to the pathogenesis of inflammatory arthritis independent of antibodies. Murine studies implicate multiple lymphocyte subtypes in specific contexts, including CD4+ T cells, exFoxP3-converted Tregs, entheseal-resident T cells, and γδ T cells. CD8+ T cells and B cells/plasma cells are abundant in synovium but their role is unclear. Evidence for the presence and function of synovial TRM cells is preliminary. Fibroblasts within the synovium are heterogeneous and contribute via mediator production and direct attachment and invasion, including into cartilage. Myeloid cells of multiple types participate actively in disease. Neutrophils appear essential for the normal evolution of chronic inflammatory arthritis in most contexts. Local resident macrophages, recruited monocytes, and newly differentiated macrophages are implicated both in propagation and in resolution of synovitis. Mast cells can initiate arthritis and are abundant in chronic synovitis, but their role in established arthritis is not well defined. Osteoclasts mediate bone erosion and can potentially amplify or even initiate joint inflammation. Downstream effector pathways include cytokines (e.g., TNF, IL-1, IL-6, IL-8, IL-17), chemokines such as ligands for the chemokine receptors CCR1 and CXCR2, lipid mediators such as leukotriene B4, proteases, and direct tissue injury. Illustrated by Mao Miyamoto.
CD4+ T cells.
In SKG arthritis, a point mutation in the T cell receptor–signaling molecule Zap70 leads to failure of thymic negative selection and escape of autoreactive T cells (157). Like human patients with RA, these mice express RF and other RA-associated autoantibodies and develop erosive arthritis and lung inflammation. Despite the presence of RF, disease is transferable by CD4+ T cells rather than serum. Transfer of SKG thymocytes, but not T cell–depleted bone marrow, into T and B cell–deficient mice is sufficient to engender arthritis, excluding an obligate role for IgG (157). CD4+ T cells directly infiltrate into the synovium, where joint inflammation is mediated by TNF-α, IL-1, and IL-6, reminiscent of RA (157, 159).
γδ T cells.
A second example of IgG-independent joint inflammation results from deficiency of IL-1ra (158). Despite the hallmark role of IL-1 in innate immunity, arthritis in mice lacking this endogenous antagonist for both IL-1α and IL-1β is strictly dependent on T cells (160). Excess IL-1 signaling promotes the development of IL-17–producing γδ T cells that mediate arthritis dependent on IL-1β, IL-17A, IL-6, and IL-23R (the latter 2 required for development of Th17-like γδ T cells), as well as on pathogenic CD4+ T cells (161–163). γδ T cells are a subpopulation of T cells that express a limited diversity of T cell receptor rearrangements and exhibit a generally tendency toward autoreactivity. They have been described in the synovium and synovial fluid of RA patients, albeit expressing IFN-γ rather than IL-17A (164). The IL-1ra–deficient mouse may mimic arthritis arising in systemic JIA and its adult equivalent, adult-onset Still’s disease, which both exhibit a key role for IL-1 early in the disease course (165).
RORγt+CD3+CD4–CD8– entheseal-resident lymphocytes and exFoxP3 cells.
Murine arthritis driven by overexpression of IL-23 begins as an enthesitis, initiated through activation of IL-23R–expressing RORγt+CD3+CD4–CD8– entheseal-resident lymphocytes (166). A newly discovered lymphocyte population, these cells respond to IL-23 signaling to produce inflammatory mediators. In CIA, a population of pathogenic T cells has been identified that appear to be Tregs that have converted to Th17 cells (exFoxP3 cells) (167). These cells lose their FoxP3 expression and immunosuppressive function in an arthritic environment, subsequently becoming autoreactive T cells that produce IL-17 and promote more severe arthritis. Whether these processes are replicated in human disease is unclear, although enthesitis in spondyloarthritis patients exhibits a primarily lymphocytic infiltrate (168, 169).
T resident memory cells.
Lymphocytes resident within joint tissues may play a key role in chronic/recurrent arthritis. Studies have identified T resident memory (TRM) cells as key drivers of recurrent site-specific inflammation in skin (170–172). Inflammatory arthritis can display similar “joint-specific memory,” with the same joints flaring again and again in a pattern characteristic of each individual (173). Preliminary evidence suggests that both human and murine synovitis feature TRM cells that could nucleate joint-specific flares (174, 175).
Multiple other IgG-independent mechanisms also contribute to inflammatory arthritis (Figure 3). For example, synovial fibroblasts are key effectors of joint inflammation, and epigenetic changes affecting these cells during the course of arthritis could convert them into autonomous agents that perpetuate local disease through mediator release and tissue invasion (102, 176, 177). Generation of endogenous TLR agonists within the inflamed joint, such as heat shock proteins, high-mobility group B1 protein, hyaluronan breakdown products, citrullinated fibrinogen, RNA, and ribosomal DNA, could supply ongoing IgG-independent stimuli to macrophages and other cells within the joint (178–183). Autoinflammatory diseases, such as familial Mediterranean fever, Blau syndrome, and PAPA (pyogenic arthritis, pyoderma gangrenosum, and acne), feature transient or persistent arthritis that is thought to occur without adaptive immune involvement via innate lineages, such as neutrophils and macrophages. Even in arthritis triggered initially by IgG, such antibody-independent mechanisms likely contribute to the persistence of inflammation.
Summary and synthesis
Inflammatory arthritis is a pathogenically complex disease. Disease can arise through either antibody-dependent or antibody-independent pathways (Figures 2 and 3). Long-standing arthritis can engage additional mechanisms, including antibody-independent pathways of disease perpetuation, even in disease originally sparked by autoantibodies.
Correspondingly, studies in murine models should be understood as testing specific mechanisms within arthritis, rather than arthritis biology in its entirety. This caveat is particularly important for models mediated by autoantibody transfer, which are experimentally highly tractable but cannot be expected to mirror all aspects of chronic long-standing RA. Mice and humans differ immunologically in important respects, including Fc receptor biology and IgG Fc glycosylation. A nuanced approach to animal data will restrain overly optimistic extrapolation to human disease while avoiding paralyzing skepticism about experimental models, which still represent an irreplaceable tool to interrogate disease pathogenesis in vivo.
Each mechanism outlined here represents a disease target. For autoimmune arthritis, whether mediated through antibodies or other routes, restoration of immune tolerance remains the “holy grail.” Short of this goal, numerous links in the pathogenic chain are susceptible to intervention, including antigen presentation, antibody generation, IgG antigen–binding domain and Fc glycosylation, and cytokines and cellular actors that mediate inflammation and tissue injury. Relevant targets will vary with disease category and chronicity. Divergent and partial treatment responses likely reflect the failure to address all active pathways. In aspiring to understand arthritis mechanisms better, we seek the ability to provide more specific and effective interventions and thereby attain superior clinical outcomes.
Acknowledgments
We are grateful to the following for comments on the manuscript: Julia F. Charles, Paul A. Monach, and Deepak A. Rao (Brigham and Women’s Hospital). MHC is supported by a Scientist Development Award from the Rheumatology Research Foundation. PAN is funded by NIH awards R01 AR065538, R01 AR073201, and P30 AR070253; by an Innovative Research Award from the Rheumatology Research Foundation; and by the Fundación Bechara and the Arbuckle Family Fund for Arthritis Research.
Version 1. 03/07/2019
Electronic publication
Footnotes
Conflict of interest: PAN has received research grants from Novartis, Sobi, and AbbVie and consulting income from Novartis, Sobi, AbbVie, Pfizer, and Bristol-Myers Squibb.
Published: March 7, 2019
Reference information: JCI Insight. 2019;4(5):e125278. https://doi.org/10.1172/jci.insight.125278.
References
- 1.Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Nigrovic PA, Raychaudhuri S, Thompson SD. Review: Genetics and the classification of arthritis in adults and children. Arthritis Rheumatol. 2018;70(1):7–17. doi: 10.1002/art.40350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374(26):2563–2574. doi: 10.1056/NEJMra1406182. [DOI] [PubMed] [Google Scholar]
- 4.Wegner N, et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev. 2010;233(1):34–54. doi: 10.1111/j.0105-2896.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 5.Shi J, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A. 2011;108(42):17372–17377. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darrah E, Andrade F. Editorial: citrullination, and carbamylation, and malondialdehyde-acetaldehyde! Oh my! Entering the forest of autoantigen modifications in rheumatoid arthritis. Arthritis Rheumatol. 2015;67(3):604–608. doi: 10.1002/art.38970. [DOI] [PubMed] [Google Scholar]
- 7.Monestier M, et al. Antihistone antibodies in antinuclear antibody-positive juvenile arthritis. Arthritis Rheum. 1990;33(12):1836–1841. doi: 10.1002/art.1780331212. [DOI] [PubMed] [Google Scholar]
- 8.Nordal EB, Songstad NT, Berntson L, Moen T, Straume B, Rygg M. Biomarkers of chronic uveitis in juvenile idiopathic arthritis: predictive value of antihistone antibodies and antinuclear antibodies. J Rheumatol. 2009;36(8):1737–1743. doi: 10.3899/jrheum.081318. [DOI] [PubMed] [Google Scholar]
- 9.Murray KJ, et al. Antibodies to the 45 kDa DEK nuclear antigen in pauciarticular onset juvenile rheumatoid arthritis and iridocyclitis: selective association with MHC gene. J Rheumatol. 1997;24(3):560–567. [PubMed] [Google Scholar]
- 10.Konig MF, Giles JT, Nigrovic PA, Andrade F. Antibodies to native and citrullinated RA33 (hnRNP A2/B1) challenge citrullination as the inciting principle underlying loss of tolerance in rheumatoid arthritis. Ann Rheum Dis. 2016;75(11):2022–2028. doi: 10.1136/annrheumdis-2015-208529. [DOI] [PubMed] [Google Scholar]
- 11.Waaler E. On the occurrence of a factor in human serum activating the specific agglutintion of sheep blood corpuscles. 1939. APMIS. 2007;115(5):422–438. doi: 10.1111/j.1600-0463.2007.apm_682a.x. [DOI] [PubMed] [Google Scholar]
- 12.Rose HM, Ragan C. Differential agglutination of normal and sensitized sheep erythrocytes by sera of patients with rheumatoid arthritis. Proc Soc Exp Biol Med. 1948;68(1):1–6. doi: 10.3181/00379727-68-16375. [DOI] [PubMed] [Google Scholar]
- 13.Christian CL. The discovery of the rheumatoid factor. II. Rose, Ragan, Pearce & Lipman. 1948. Clin Exp Rheumatol. 1998;16(3):345–349. [PubMed] [Google Scholar]
- 14.Burastero SE, Casali P, Wilder RL, Notkins AL. Monoreactive high affinity and polyreactive low affinity rheumatoid factors are produced by CD5+ B cells from patients with rheumatoid arthritis. J Exp Med. 1988;168(6):1979–1992. doi: 10.1084/jem.168.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randen I, et al. Clonally related IgM rheumatoid factors undergo affinity maturation in the rheumatoid synovial tissue. J Immunol. 1992;148(10):3296–3301. [PubMed] [Google Scholar]
- 16.Mageed RA, Børretzen M, Moyes SP, Thompson KM, Natvig JB. Rheumatoid factor autoantibodies in health and disease. Ann N Y Acad Sci. 1997;815:296–311. doi: 10.1111/j.1749-6632.1997.tb52071.x. [DOI] [PubMed] [Google Scholar]
- 17.Tighe H, et al. Peripheral deletion of rheumatoid factor B cells after abortive activation by IgG. Proc Natl Acad Sci U S A. 1997;94(2):646–651. doi: 10.1073/pnas.94.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu DR, et al. T cell-dependent affinity maturation and innate immune pathways differentially drive autoreactive B cell responses in rheumatoid Arthritis. Arthritis Rheumatol. 2018;70(11):1732–1744. doi: 10.1002/art.40578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nell VP, et al. Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Ann Rheum Dis. 2005;64(12):1731–1736. doi: 10.1136/ard.2005.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokolove J, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813–821. doi: 10.1002/art.38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajeganova S, et al. Anticitrullinated protein antibodies and rheumatoid factor are associated with increased mortality but with different causes of death in patients with rheumatoid arthritis: a longitudinal study in three European cohorts. Ann Rheum Dis. 2016;75(11):1924–1932. doi: 10.1136/annrheumdis-2015-208579. [DOI] [PubMed] [Google Scholar]
- 22.Hecht C, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA. Ann Rheum Dis. 2015;74(12):2151–2156. doi: 10.1136/annrheumdis-2014-205428. [DOI] [PubMed] [Google Scholar]
- 23.Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med. 2012;106(11):1591–1599. doi: 10.1016/j.rmed.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Nyhäll-Wåhlin BM, et al. The presence of rheumatoid nodules at early rheumatoid arthritis diagnosis is a sign of extra-articular disease and predicts radiographic progression of joint destruction over 5 years. Scand J Rheumatol. 2011;40(2):81–87. doi: 10.3109/03009742.2010.509103. [DOI] [PubMed] [Google Scholar]
- 25.Voskuyl AE, et al. Diagnostic strategy for the assessment of rheumatoid vasculitis. Ann Rheum Dis. 2003;62(5):407–413. doi: 10.1136/ard.62.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westedt ML, et al. Rheumatoid factors in rheumatoid arthritis and vasculitis. Rheumatol Int. 1985;5(5):209–214. doi: 10.1007/BF00541338. [DOI] [PubMed] [Google Scholar]
- 27.Jónsson T, Valdimarsson H. What about IgA rheumatoid factor in rheumatoid arthritis? Ann Rheum Dis. 1998;57(1):63–64. doi: 10.1136/ard.57.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nienhuis RL, Mandema E. A new serum factor in patients with rheumatoid arthritis; the antiperinuclear factor. Ann Rheum Dis. 1964;23:302–305. doi: 10.1136/ard.23.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young BJ, Mallya RK, Leslie RD, Clark CJ, Hamblin TJ. Anti-keratin antibodies in rheumatoid arthritis. Br Med J. 1979;2(6182):97–99. doi: 10.1136/bmj.2.6182.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girbal-Neuhauser E, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999;162(1):585–594. [PubMed] [Google Scholar]
- 31.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101(1):273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sebbag M, et al. The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1995;95(6):2672–2679. doi: 10.1172/JCI117969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ménard HA. “ACPA” in rheumatoid arthritis: from population-based data to personalized medicine. J Rheumatol. 2015;42(5):733–735. doi: 10.3899/jrheum.150281. [DOI] [PubMed] [Google Scholar]
- 34. doi: 10.1002/art.40698. Structural basis of cross-reactivity of anti-citrullinated protein antibodies [published online ahead of print August 27, 2018]. Arthritis Rheumatol. doi: 10.1002/art.40698. [DOI] [PubMed] [Google Scholar]
- 35.Nielen MM, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50(2):380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 36.Kolfenbach JR, et al. Autoimmunity to peptidyl arginine deiminase type 4 precedes clinical onset of rheumatoid arthritis. Arthritis Rheum. 2010;62(9):2633–2639. doi: 10.1002/art.27570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris ML, et al. Association of autoimmunity to peptidyl arginine deiminase type 4 with genotype and disease severity in rheumatoid arthritis. Arthritis Rheum. 2008;58(7):1958–1967. doi: 10.1002/art.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyes-Castillo Z, et al. Comparative analysis of autoantibodies targeting peptidylarginine deiminase type 4, mutated citrullinated vimentin and cyclic citrullinated peptides in rheumatoid arthritis: associations with cytokine profiles, clinical and genetic features. Clin Exp Immunol. 2015;182(2):119–131. doi: 10.1111/cei.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navarro-Millán I, et al. Association of anti-peptidyl arginine deiminase antibodies with radiographic severity of rheumatoid arthritis in African Americans. Arthritis Res Ther. 2016;18(1):241. doi: 10.1186/s13075-016-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giles JT, et al. Association of cross-reactive antibodies targeting peptidyl-arginine deiminase 3 and 4 with rheumatoid arthritis-associated interstitial lung disease. PLoS One. 2014;9(6):e98794. doi: 10.1371/journal.pone.0098794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones VE, Jacoby RK, Cowley PJ, Warren C. Immune complexes in early arthritis. II. Immune complex constituents are synthesized in the synovium before rheumatoid factors. Clin Exp Immunol. 1982;49(1):31–40. [PMC free article] [PubMed] [Google Scholar]
- 42.Natvig JB, Munthe E. Self-associating IgG rheumatoid factor represents a major response of plasma cells in rheumatoid inflammatory tissue. Ann N Y Acad Sci. 1975;256:88–95. doi: 10.1111/j.1749-6632.1975.tb36038.x. [DOI] [PubMed] [Google Scholar]
- 43.Jones V, Taylor PC, Jacoby RK, Wallington TB. Synovial synthesis of rheumatoid factors and immune complex constituents in early arthritis. Ann Rheum Dis. 1984;43(2):235–239. doi: 10.1136/ard.43.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao DA, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542(7639):110–114. doi: 10.1038/nature20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holers VM, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol. 2018;14(9):542–557. doi: 10.1038/s41584-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juarez M, et al. Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis. Ann Rheum Dis. 2016;75(6):1099–1107. doi: 10.1136/annrheumdis-2014-206785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nissim A, et al. Generation of neoantigenic epitopes after posttranslational modification of type II collagen by factors present within the inflamed joint. Arthritis Rheum. 2005;52(12):3829–3838. doi: 10.1002/art.21479. [DOI] [PubMed] [Google Scholar]
- 48.Thiele GM, et al. Malondialdehyde-acetaldehyde adducts and anti-malondialdehyde-acetaldehyde antibodies in rheumatoid arthritis. Arthritis Rheumatol. 2015;67(3):645–655. doi: 10.1002/art.38969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monach PA, et al. A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106(37):15867–15872. doi: 10.1073/pnas.0908032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao KP, et al. Associations of autoantibodies, autoimmune risk alleles, and clinical diagnoses from the electronic medical records in rheumatoid arthritis cases and non-rheumatoid arthritis controls. Arthritis Rheum. 2013;65(3):571–581. doi: 10.1002/art.37801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nordal E, et al. Incidence and predictors of Uveitis in juvenile idiopathic arthritis in a Nordic long-term cohort study. Pediatr Rheumatol Online J. 2017;15(1):66. doi: 10.1186/s12969-017-0195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravelli A, et al. Patients with antinuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheum. 2005;52(3):826–832. doi: 10.1002/art.20945. [DOI] [PubMed] [Google Scholar]
- 53.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87(5):811–822. doi: 10.1016/S0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 54.Korganow AS, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10(4):451–461. doi: 10.1016/S1074-7613(00)80045-X. [DOI] [PubMed] [Google Scholar]
- 55.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286(5445):1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 56.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 58.Nandakumar KS, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am J Pathol. 2003;163(5):1827–1837. doi: 10.1016/S0002-9440(10)63542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148(7):2103–2108. [PubMed] [Google Scholar]
- 60.Nandakumar KS, Holmdahl R. Efficient promotion of collagen antibody induced arthritis (CAIA) using four monoclonal antibodies specific for the major epitopes recognized in both collagen induced arthritis and rheumatoid arthritis. J Immunol Methods. 2005;304(1–2):126–136. doi: 10.1016/j.jim.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Kuhn KA, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116(4):961–973. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uysal H, et al. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J Exp Med. 2009;206(2):449–462. doi: 10.1084/jem.20081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holers VM, Banda NK. Complement in the initiation and evolution of rheumatoid arthritis. Front Immunol. 2018;9:1057. doi: 10.3389/fimmu.2018.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pekin TJ. Zvaifler NJ: Synovial fluid findings in systemic lupus erythematosus (SLE) Arthritis Rheum. 1970;13(6):777–785. doi: 10.1002/art.1780130607. [DOI] [PubMed] [Google Scholar]
- 65.Ruddy S, Austen KF. The complement system in rheumatoid synovitis. I. An analysis of complement component activities in rheumatoid synovial fluids. Arthritis Rheum. 1970;13(6):713–723. doi: 10.1002/art.1780130601. [DOI] [PubMed] [Google Scholar]
- 66.Britton MC, Schur PH. The complement system in rheumatoid synovitis. II. Intracytoplasmic inclusions of immunoglobulins and complement. Arthritis Rheum. 1971;14(1):87–95. doi: 10.1002/art.1780140111. [DOI] [PubMed] [Google Scholar]
- 67.Zvaifler NJ. Breakdown products of C 3 in human synovial fluids. J Clin Invest. 1969;48(8):1532–1542. doi: 10.1172/JCI106119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mannik M, Nardella FA. IgG rheumatoid factors and self-association of these antibodies. Clin Rheum Dis. 1985;11(3):551–572. [PubMed] [Google Scholar]
- 69.Sabharwal UK, Vaughan JH, Fong S, Bennett PH, Carson DA, Curd JG. Activation of the classical pathway of complement by rheumatoid factors. Assessment by radioimmunoassay for C4. Arthritis Rheum. 1982;25(2):161–167. doi: 10.1002/art.1780250208. [DOI] [PubMed] [Google Scholar]
- 70.Gale RJ, Nikoloutsopoulos A, Bradley J, Roberts-Thomson PJ. Immune complex activation of neutrophils and enhancement of the activation by rheumatoid factor and complement. J Rheumatol. 1985;12(1):21–26. [PubMed] [Google Scholar]
- 71.Winchester RJ, Agnello V, Kunkel HG. Gamma globulin complexes in synovial fluids of patients with rheumatoid arthritis. Partial characterization and relationship to lowered complement levels. Clin Exp Immunol. 1970;6(5):689–706. [PMC free article] [PubMed] [Google Scholar]
- 72.Munthe E, Natvig JB. Characterization of IgG complexes in eluates from rheumatoid tissue. Clin Exp Immunol. 1971;8(2):249–262. [PMC free article] [PubMed] [Google Scholar]
- 73.Winchester RJ. Characterization of IgG complexes in patients with rheumatoid arthritis. Ann N Y Acad Sci. 1975;256:73–81. doi: 10.1111/j.1749-6632.1975.tb36036.x. [DOI] [PubMed] [Google Scholar]
- 74.Male D, Roitt IM, Hay FC. Analysis of immune complexes in synovial effusions of patients with rheumatoid arthritis. Clin Exp Immunol. 1980;39(2):297–306. [PMC free article] [PubMed] [Google Scholar]
- 75.Male DK, Roitt IM. Molecular analysis of complement-fixing rheumatoid synovial fluid immune complexes. Clin Exp Immunol. 1981;46(3):521–529. [PMC free article] [PubMed] [Google Scholar]
- 76.Mitchell WS, Naama JK, Veitch J, Whaley K. IgM-RF prevents complement-mediated inhibition of immune precipitation. Immunology. 1984;52(3):445–448. [PMC free article] [PubMed] [Google Scholar]
- 77.Balestrieri G, Tincani A, Migliorini P, Ferri C, Cattaneo R, Bombardieri S. Inhibitory effect of IgM rheumatoid factor on immune complex solubilization capacity and inhibition of immune precipitation. Arthritis Rheum. 1984;27(10):1130–1136. doi: 10.1002/art.1780271008. [DOI] [PubMed] [Google Scholar]
- 78.Mongan ES, Cass RM, Jacox RF, Vaughen JH. A study of the relation of seronegative and seropositive rheumatoid arthritis to each other and to necrotizing vasculitis. Am J Med. 1969;47(1):23–35. doi: 10.1016/0002-9343(69)90238-1. [DOI] [PubMed] [Google Scholar]
- 79.Laurent L, et al. IgM rheumatoid factor amplifies the inflammatory response of macrophages induced by the rheumatoid arthritis-specific immune complexes containing anticitrullinated protein antibodies. Ann Rheum Dis. 2015;74(7):1425–1431. doi: 10.1136/annrheumdis-2013-204543. [DOI] [PubMed] [Google Scholar]
- 80.Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum. 2011;63(1):53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harris J, Vaughan JH. Transfusion studies in rheumatoid arthritis. Arthritis Rheum. 1961;4:47–55. doi: 10.1002/art.1780040105. [DOI] [PubMed] [Google Scholar]
- 82.de Hair MJ, et al. Features of the synovium of individuals at risk of developing rheumatoid arthritis: implications for understanding preclinical rheumatoid arthritis. Arthritis Rheumatol. 2014;66(3):513–522. doi: 10.1002/art.38273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsumoto I, et al. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol. 2002;3(4):360–365. doi: 10.1038/ni772. [DOI] [PubMed] [Google Scholar]
- 84.Wipke BT, Wang Z, Kim J, McCarthy TJ, Allen PM. Dynamic visualization of a joint-specific autoimmune response through positron emission tomography. Nat Immunol. 2002;3(4):366–372. doi: 10.1038/ni775. [DOI] [PubMed] [Google Scholar]
- 85.Maccioni M, et al. Arthritogenic monoclonal antibodies from K/BxN mice. J Exp Med. 2002;195(8):1071–1077. doi: 10.1084/jem.20011941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wipke BT, Wang Z, Nagengast W, Reichert DE, Allen PM. Staging the initiation of autoantibody-induced arthritis: a critical role for immune complexes. J Immunol. 2004;172(12):7694–7702. doi: 10.4049/jimmunol.172.12.7694. [DOI] [PubMed] [Google Scholar]
- 87.Binstadt BA, et al. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7(3):284–292. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 88.Cloutier N, et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc Natl Acad Sci U S A. 2018;115(7):E1550–E1559. doi: 10.1073/pnas.1720553115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ji H, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157–168. doi: 10.1016/S1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 90.Mancardi DA, et al. Cutting Edge: The murine high-affinity IgG receptor FcγRIV is sufficient for autoantibody-induced arthritis. J Immunol. 2011;186(4):1899–1903. doi: 10.4049/jimmunol.1003642. [DOI] [PubMed] [Google Scholar]
- 91.Díaz de Ståhl T, Andrén M, Martinsson P, Verbeek JS, Kleinau S. Expression of FcgammaRIII is required for development of collagen-induced arthritis. Eur J Immunol. 2002;32(10):2915–2922. doi: 10.1002/1521-4141(2002010)32:10<2915::AID-IMMU2915>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 92.Monach PA, et al. Neutrophils in a mouse model of autoantibody-mediated arthritis: critical producers of Fc receptor gamma, the receptor for C5a, and lymphocyte function-associated antigen 1. Arthritis Rheum. 2010;62(3):753–764. doi: 10.1002/art.27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sadik CD, Kim ND, Iwakura Y, Luster AD. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcγR signaling. Proc Natl Acad Sci U S A. 2012;109(46):E3177–E3185. doi: 10.1073/pnas.1213797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tanaka D, Kagari T, Doi H, Shimozato T. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis. Immunology. 2006;119(2):195–202. doi: 10.1111/j.1365-2567.2006.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297(5587):1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 96.Nigrovic PA, et al. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc Natl Acad Sci U S A. 2007;104(7):2325–2330. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nigrovic PA, et al. C5a receptor enables participation of mast cells in immune complex arthritis independently of Fcγ receptor modulation. Arthritis Rheum. 2010;62(11):3322–3333. doi: 10.1002/art.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miyabe Y, et al. Complement C5a receptor is the key initiator of neutrophil adhesion igniting immune complex-induced arthritis. Sci Immunol. 2017;2(7):eaaj2195. doi: 10.1126/sciimmunol.aaj2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shushakova N, et al. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcγRs in immune complex-induced lung disease. J Clin Invest. 2002;110(12):1823–1830. doi: 10.1172/JCI16577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen M, et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203(4):837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203(4):829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee DM, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315(5814):1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 103.Wang JX, et al. Ly6G ligation blocks recruitment of neutrophils via a β2-integrin-dependent mechanism. Blood. 2012;120(7):1489–1498. doi: 10.1182/blood-2012-01-404046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chou RC, et al. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33(2):266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Misharin AV, et al. Nonclassical Ly6C(–) monocytes drive the development of inflammatory arthritis in mice. Cell Rep. 2014;9(2):591–604. doi: 10.1016/j.celrep.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Banda NK, Takahashi K, Wood AK, Holers VM, Arend WP. Pathogenic complement activation in collagen antibody-induced arthritis in mice requires amplification by the alternative pathway. J Immunol. 2007;179(6):4101–4109. doi: 10.4049/jimmunol.179.6.4101. [DOI] [PubMed] [Google Scholar]
- 107.Banda NK, et al. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J Immunol. 2006;177(3):1904–1912. doi: 10.4049/jimmunol.177.3.1904. [DOI] [PubMed] [Google Scholar]
- 108.Hietala MA, Jonsson IM, Tarkowski A, Kleinau S, Pekna M. Complement deficiency ameliorates collagen-induced arthritis in mice. J Immunol. 2002;169(1):454–459. doi: 10.4049/jimmunol.169.1.454. [DOI] [PubMed] [Google Scholar]
- 109.Banda NK, et al. Initiation of the alternative pathway of murine complement by immune complexes is dependent on N-glycans in IgG antibodies. Arthritis Rheum. 2008;58(10):3081–3089. doi: 10.1002/art.23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trouw LA, et al. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum. 2009;60(7):1923–1931. doi: 10.1002/art.24622. [DOI] [PubMed] [Google Scholar]
- 111.Hiemstra PS, et al. Activation of complement by human serum IgA, secretory IgA and IgA1 fragments. Mol Immunol. 1988;25(6):527–533. doi: 10.1016/0161-5890(88)90074-0. [DOI] [PubMed] [Google Scholar]
- 112.Takahashi M, et al. Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J Exp Med. 2010;207(1):29–37. doi: 10.1084/jem.20090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arend WP, et al. Roles of adipocytes and fibroblasts in activation of the alternative pathway of complement in inflammatory arthritis in mice. J Immunol. 2013;190(12):6423–6433. doi: 10.4049/jimmunol.1300580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Banda NK, et al. Essential role of complement mannose-binding lectin-associated serine proteases-1/3 in the murine collagen antibody-induced model of inflammatory arthritis. J Immunol. 2010;185(9):5598–5606. doi: 10.4049/jimmunol.1001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Banda NK, et al. Mannan-binding lectin-associated serine protease 1/3 cleavage of pro-factor D into factor D in vivo and attenuation of collagen antibody-induced arthritis through their targeted inhibition by rna interference-mediated gene silencing. J Immunol. 2016;197(9):3680–3694. doi: 10.4049/jimmunol.1600719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Banda NK, et al. Deconstructing the lectin pathway in the pathogenesis of experimental inflammatory arthritis: essential role of the lectin ficolin b and mannose-binding protein-associated serine protease 2. J Immunol. 2017;199(5):1835–1845. doi: 10.4049/jimmunol.1700119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ellison MR, Kelly KJ, Flatt AE. The results of surgical synovectomy of the digital joints in rheumatoid disease. J Bone Joint Surg Am. 1971;53(6):1041–1060. doi: 10.2106/00004623-197153060-00001. [DOI] [PubMed] [Google Scholar]
- 118.Patzakis MJ, Mills DM, Bartholomew BA, Clayton ML, Smyth CJ. A visual, histological, and enzymatic study of regenerating rheumatoid synovium in the synovectomized knee. J Bone Joint Surg Am. 1973;55(2):287–300. doi: 10.2106/00004623-197355020-00004. [DOI] [PubMed] [Google Scholar]
- 119.Bryan RS, Peterson LF, Combs JJ., Jr Polycentric knee arthroplasty. A review of 84 patients with more than one year follow-up. Clin Orthop Relat Res. 1973;(94):136–139. doi: 10.1097/00003086-197307000-00017. [DOI] [PubMed] [Google Scholar]
- 120.Fearon DT. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Henson PM. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971;107(6):1547–1557. [PubMed] [Google Scholar]
- 122.Ge C, et al. Anti-citrullinated protein antibodies cause arthritis by cross-reactivity to joint cartilage. JCI Insight. 2017;2(13):e93688. doi: 10.1172/jci.insight.93688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Romero V, et al. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med. 2013;5(209):209ra150. doi: 10.1126/scitranslmed.3006869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carmona-Rivera C, et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol. 2017;2(10):eaag3358. doi: 10.1126/sciimmunol.aag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khandpur R, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boilard E, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327(5965):580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cunin P, et al. Megakaryocytes compensate for Kit insufficiency in murine arthritis. J Clin Invest. 2017;127(5):1714–1724. doi: 10.1172/JCI84598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cloutier N, et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med. 2013;5(2):235–249. doi: 10.1002/emmm.201201846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc Natl Acad Sci U S A. 2008;105(39):15005–15009. doi: 10.1073/pnas.0808248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dekkers G, Rispens T, Vidarsson G. Novel concepts of altered immunoglobulin G galactosylation in autoimmune diseases. Front Immunol. 2018;9:553. doi: 10.3389/fimmu.2018.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pfeifle R, et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol. 2017;18(1):104–113. doi: 10.1038/ni.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harre U, et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun. 2015;6:6651. doi: 10.1038/ncomms7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parekh RB, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 134.Ercan A, et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2010;62(8):2239–2248. doi: 10.1002/art.27533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ercan A, et al. Multiple juvenile idiopathic arthritis subtypes demonstrate pro-inflammatory IgG glycosylation. Arthritis Rheum. 2012;64(9):3025–3033. doi: 10.1002/art.34507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rombouts Y, et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis. 2015;74(1):234–241. doi: 10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- 137.Scherer HU, et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62(6):1620–1629. doi: 10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- 138.Rombouts Y, et al. Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann Rheum Dis. 2016;75(3):578–585. doi: 10.1136/annrheumdis-2014-206598. [DOI] [PubMed] [Google Scholar]
- 139.Ercan A, et al. Estrogens regulate glycosylation of IgG in women and men. JCI Insight. 2017;2(4):e89703. doi: 10.1172/jci.insight.89703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.van de Geijn FE, et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther. 2009;11(6):R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alavi A, Arden N, Spector TD, Axford JS. Immunoglobulin G glycosylation and clinical outcome in rheumatoid arthritis during pregnancy. J Rheumatol. 2000;27(6):1379–1385. [PubMed] [Google Scholar]
- 142.Mattsson R, Mattsson A, Holmdahl R, Whyte A, Rook GA. Maintained pregnancy levels of oestrogen afford complete protection from post-partum exacerbation of collagen-induced arthritis. Clin Exp Immunol. 1991;85(1):41–47. doi: 10.1111/j.1365-2249.1991.tb05679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van der Linden MP, et al. Value of anti-modified citrullinated vimentin and third-generation anti-cyclic citrullinated peptide compared with second-generation anti-cyclic citrullinated peptide and rheumatoid factor in predicting disease outcome in undifferentiated arthritis and rheumatoid arthritis. Arthritis Rheum. 2009;60(8):2232–2241. doi: 10.1002/art.24716. [DOI] [PubMed] [Google Scholar]
- 144.van Gaalen FA, et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum. 2004;50(3):709–715. doi: 10.1002/art.20044. [DOI] [PubMed] [Google Scholar]
- 145.Harre U, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122(5):1791–1802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Engdahl C, Bang H, Dietel K, Lang SC, Harre U, Schett G. Periarticular bone loss in arthritis is induced by autoantibodies against citrullinated vimentin. J Bone Miner Res. 2017;32(8):1681–1691. doi: 10.1002/jbmr.3158. [DOI] [PubMed] [Google Scholar]
- 147.Krishnamurthy A, et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis. 2016;75(4):721–729. doi: 10.1136/annrheumdis-2015-208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Darrah E, Giles JT, Ols ML, Bull HG, Andrade F, Rosen A. Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Sci Transl Med. 2013;5(186):186ra65. doi: 10.1126/scitranslmed.3005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ruddy S, Fearon DT, Austen KF. Depressed synovial fluid levels of properdin and properdin factor B in patients with rheumatoid arthritis. Arthritis Rheum. 1975;18(4):289–295. doi: 10.1002/art.1780180401. [DOI] [PubMed] [Google Scholar]
- 150.Cooke TD, Hurd ER, Jasin HE, Bienenstock J, Ziff M. Identification of immunoglobulins and complement in rheumatoid articular collagenous tissues. Arthritis Rheum. 1975;18(6):541–551. doi: 10.1002/art.1780180603. [DOI] [PubMed] [Google Scholar]
- 151.Ruddy S, Britton MC, Schur PH, Austen KF. Complement components in synovial fluid: activation and fixation in seropositive rheumatoid arthritis. Ann N Y Acad Sci. 1969;168(1):161–172. doi: 10.1111/j.1749-6632.1969.tb43105.x. [DOI] [PubMed] [Google Scholar]
- 152.Vaughan JH, Barrnett EV, Sobel MV, Jacox RF. Intracytoplasmic inclusions of immunoglobulins in rheumatoid arthritis and other diseases. Arthritis Rheum. 1968;11(2):125–134. doi: 10.1002/art.1780110202. [DOI] [PubMed] [Google Scholar]
- 153.Bluestone R, Goldberg LS, Cracchiolo A. Hidden rheumatoid factor in seronegative nodular rheumatoid arthritis. Lancet. 1969;2(7626):878–879. doi: 10.1016/s0140-6736(69)92331-9. [DOI] [PubMed] [Google Scholar]
- 154.Halla JT, Volanakis JE, Schrohenloher RE. Immune complexes in rheumatoid arthritis sera and synovial fluids: a comparison of three methods. Arthritis Rheum. 1979;22(5):440–448. doi: 10.1002/art.1780220502. [DOI] [PubMed] [Google Scholar]
- 155.Keffer J, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10(13):4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Phillips K, Kedersha N, Shen L, Blackshear PJ, Anderson P. Arthritis suppressor genes TIA-1 and TTP dampen the expression of tumor necrosis factor alpha, cyclooxygenase 2, and inflammatory arthritis. Proc Natl Acad Sci U S A. 2004;101(7):2011–2016. doi: 10.1073/pnas.0400148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sakaguchi N, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426(6965):454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 158.Horai R, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191(2):313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hata H, et al. Distinct contribution of IL-6, TNF-α, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114(4):582–588. doi: 10.1172/JCI21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kotani M, et al. CD28-dependent differentiation into the effector/memory phenotype is essential for induction of arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 2006;54(2):473–481. doi: 10.1002/art.21769. [DOI] [PubMed] [Google Scholar]
- 161.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100(10):5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Akitsu A, et al. IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2(+)Vγ6(+)γδ T cells. Nat Commun. 2015;6:7464. doi: 10.1038/ncomms8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cho ML, et al. STAT3 and NF-κB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176(9):5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 164.Ito Y, et al. γ/δ T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009;60(8):2294–2303. doi: 10.1002/art.24687. [DOI] [PubMed] [Google Scholar]
- 165.Nigrovic PA. Review: is there a window of opportunity for treatment of systemic juvenile idiopathic arthritis? Arthritis Rheumatol. 2014;66(6):1405–1413. doi: 10.1002/art.38615. [DOI] [PubMed] [Google Scholar]
- 166.Sherlock JP, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4–CD8– entheseal resident T cells. Nat Med. 2012;18(7):1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 167.Komatsu N, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20(1):62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 168.Tuttle KS, Vargas SO, Callahan MJ, Bae DS, Nigrovic PA. Enthesitis as a component of dactylitis in psoriatic juvenile idiopathic arthritis: histology of an established clinical entity. Pediatr Rheumatol Online J. 2015;13:7. doi: 10.1186/s12969-015-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Watad A, Cuthbert RJ, Amital H, McGonagle D. Enthesitis: much more than focal insertion point inflammation. Curr Rheumatol Rep. 2018;20(7):41. doi: 10.1007/s11926-018-0751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483(7388):227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mizukawa Y, et al. Direct evidence for interferon-gamma production by effector-memory-type intraepidermal T cells residing at an effector site of immunopathology in fixed drug eruption. Am J Pathol. 2002;161(4):1337–1347. doi: 10.1016/S0002-9440(10)64410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med. 2004;199(5):731–736. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Roberts WN, Daltroy LH, Anderson RJ. Stability of normal joint findings in persistent classic rheumatoid arthritis. Arthritis Rheum. 1988;31(2):267–271. doi: 10.1002/art.1780310215. [DOI] [PubMed] [Google Scholar]
- 174. Henderson L, et al. 3-D explant method facilitates the study of lymphocytes in synovium and reveals a population of resident memory-like t cells in rheumatoid arthritis [abstract]. Arthritis Rheumatol. 2017;69(suppl 4). https://acrabstracts.org/abstract/3-d-explant-method-facilitates-the-study-of-lymphocytes-in-synovium-and-reveals-a-population-of-resident-memory-like-t-cells-in-rheumatoid-arthritis-2/ Accessed January 29, 2019.
- 175. Chang MH, Levescot A, Morris A, Nelson-Maney N, Fuhlbrigge R, Nigrovic PA. Murine model of arthritis flare identifies CD8+ tissue resident memory T cells in recurrent synovitis [abstract]. Arthritis Rheumatol. 2017;69(suppl 4). https://acrabstracts.org/abstract/murine-model-of-arthritis-flare-identifies-cd8-tissue-resident-memory-t-cells-in-recurrent-synovitis/ Accessed January 29, 2019.
- 176.Turner JD, Filer A. The role of the synovial fibroblast in rheumatoid arthritis pathogenesis. Curr Opin Rheumatol. 2015;27(2):175–182. doi: 10.1097/BOR.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 177.Hammaker D, Firestein GS. Epigenetics of inflammatory arthritis. Curr Opin Rheumatol. 2018;30(2):188–196. doi: 10.1097/BOR.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brentano F, Kyburz D, Schorr O, Gay R, Gay S. The role of Toll-like receptor signalling in the pathogenesis of arthritis. Cell Immunol. 2005;233(2):90–96. doi: 10.1016/j.cellimm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 179.Choe JY, Crain B, Wu SR, Corr M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. J Exp Med. 2003;197(4):537–542. doi: 10.1084/jem.20021850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Duffau P, et al. Promotion of inflammatory arthritis by interferon regulatory factor 5 in a mouse model. Arthritis Rheumatol. 2015;67(12):3146–3157. doi: 10.1002/art.39321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maciejewska Rodrigues H, Jüngel A, Gay RE, Gay S. Innate immunity, epigenetics and autoimmunity in rheumatoid arthritis. Mol Immunol. 2009;47(1):12–18. doi: 10.1016/j.molimm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 182.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11(1):91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 183.Mandelin AM, et al. Transcriptional profiling of synovial macrophages using minimally invasive ultrasound-guided synovial biopsies in rheumatoid arthritis. Arthritis Rheumatol. 2018;70(6):841–854. doi: 10.1002/art.40453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Monach PA, Benoist C, Mathis D. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv Immunol. 2004;82:217–248. doi: 10.1016/S0065-2776(04)82005-4. [DOI] [PubMed] [Google Scholar]
- 185.Manivel VA, et al. Anticollagen type II antibodies are associated with an acute onset rheumatoid arthritis phenotype and prognosticate lower degree of inflammation during 5 years follow-up. Ann Rheum Dis. 2017;76(9):1529–1536. doi: 10.1136/annrheumdis-2016-210873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gilliam BE, Chauhan AK, Moore TL. Evaluation of anti-citrullinated type II collagen and anti-citrullinated vimentin antibodies in patients with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2013;11(1):31. doi: 10.1186/1546-0096-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Othman MA, Ghazali WSW, Hamid WZWA, Wong KK, Yahya NK. Anti-carbamylated protein antibodies in rheumatoid arthritis patients and their association with rheumatoid factor. Saudi Med J. 2017;38(9):934–941. doi: 10.15537/smj.2017.9.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nakabo S, et al. Carbamylated albumin is one of the target antigens of anti-carbamylated protein antibodies. Rheumatology (Oxford) 2017;56(7):1217–1226. doi: 10.1093/rheumatology/kex088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shi J, et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis. 2014;73(4):780–783. doi: 10.1136/annrheumdis-2013-204154. [DOI] [PubMed] [Google Scholar]
- 190.Jiang X, et al. Anti-CarP antibodies in two large cohorts of patients with rheumatoid arthritis and their relationship to genetic risk factors, cigarette smoking and other autoantibodies. Ann Rheum Dis. 2014;73(10):1761–1768. doi: 10.1136/annrheumdis-2013-205109. [DOI] [PubMed] [Google Scholar]
- 191.Verheul MK, et al. Identification of carbamylated alpha 1 anti-trypsin (A1AT) as an antigenic target of anti-CarP antibodies in patients with rheumatoid arthritis. J Autoimmun. 2017;80:77–84. doi: 10.1016/j.jaut.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 192.Jasin HE. Autoantibody specificities of immune complexes sequestered in articular cartilage of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1985;28(3):241–248. doi: 10.1002/art.1780280302. [DOI] [PubMed] [Google Scholar]
- 193.Clague RB, Moore LJ. IgG and IgM antibody to native type II collagen in rheumatoid arthritis serum and synovial fluid. Evidence for the presence of collagen-anticollagen immune complexes in synovial fluid. Arthritis Rheum. 1984;27(12):1370–1377. doi: 10.1002/art.1780271207. [DOI] [PubMed] [Google Scholar]
- 194.Matsumoto I, et al. Low prevalence of antibodies to glucose-6-phosphate isomerase in patients with rheumatoid arthritis and a spectrum of other chronic autoimmune disorders. Arthritis Rheum. 2003;48(4):944–954. doi: 10.1002/art.10898. [DOI] [PubMed] [Google Scholar]
- 195.Fish AJ, Michael AF, Gewurz H, Good RA. Immunopathologic changes in rheumatoid arthritis synovium. Arthritis Rheum. 1966;9:267. doi: 10.1002/art.1780090202. [DOI] [Google Scholar]
- 196.Rodman WS, Williams RC, Bilka PJ, Müller-Eberhard HJ. Immunofluorescent localization of the third and the fourth component of complement in synovial tissue from patients with rheumatoid arthritis. J Lab Clin Med. 1967;69(1):141–150. [PubMed] [Google Scholar]
- 197.Brandt KD, Cathcart ES, Cohen AS. Studies of immune deposits in synovial membranes and corresponding synovial fluids. J Lab Clin Med. 1968;72(4):631–647. [PubMed] [Google Scholar]
- 198.Bonomo L, Tursi A, Trizio D, Gillardi U, Dammacco F. Immune complexes in rheumatoid synovitis: a mixed staining immunofluorescence study. Immunology. 1970;18(4):557–563. [PMC free article] [PubMed] [Google Scholar]
- 199.Ghose T, Woodbury JF, Ahmad S, Stevenson B. Immunopathological changes in rheumatoid arthritis and other joint diseases. J Clin Pathol. 1975;28(2):109–117. doi: 10.1136/jcp.28.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ugai K, Ishikawa H, Hirohata K, Shirane H. Interaction of polymorphonuclear leukocytes with immune complexes trapped in rheumatoid articular cartilage. Arthritis Rheum. 1983;26(12):1434–1441. doi: 10.1002/art.1780261204. [DOI] [PubMed] [Google Scholar]
- 201.Vetto AA, Mannik M, Zatarain-Rios E, Wener MH. Immune deposits in articular cartilage of patients with rheumatoid arthritis have a granular pattern not seen in osteoarthritis. Rheumatol Int. 1990;10(1):13–19. doi: 10.1007/BF02274776. [DOI] [PubMed] [Google Scholar]