Abstract
Background
Seborrhoeic dermatitis is a chronic inflammatory skin disorder affecting primarily the skin of the scalp, face, chest, and intertriginous areas, causing scaling and redness of the skin. Current treatment options include antifungal, anti‐inflammatory, and keratolytic agents, as well as phototherapy.
Objectives
To assess the effects of topical pharmacological interventions with established anti‐inflammatory action for seborrhoeic dermatitis occurring in adolescents and adults.
Search methods
We searched the following databases up to September 2013: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library (2013, Issue 9), MEDLINE (from 1946), Embase (from 1974), LILACS (from 1982), and the GREAT database. We searched five trials databases and checked the reference lists of included studies for further references to relevant randomised controlled trials (RCTs).
Selection criteria
We included RCTs in adults or adolescents (> 16 years) with diagnosed seborrhoeic dermatitis of the scalp or face, comparing topical anti‐inflammatory treatments (steroids, calcineurin inhibitors, and lithium salts) with other treatments.
Data collection and analysis
Pairs of authors independently assessed eligibility for inclusion, extracted data, and evaluated the risk of bias. We performed meta‐analyses if feasible.
Main results
We included 36 RCTs (2706 participants), of which 31 examined topical steroids; seven, calcineurin inhibitors; and three, lithium salts. The comparative interventions included placebo, azoles, calcipotriol, a non‐steroidal anti‐inflammatory compound, and zinc, as well as different anti‐inflammatory treatments compared against each other. Our outcomes of interest were total clearance of symptoms, erythema, scaling or pruritus scores, and adverse effects. The risk of bias in studies was most frequently classified as unclear, due to unclear reporting of methods.
Steroid treatment resulted in total clearance more often than placebo in short‐term trials (four weeks or less) (relative risk (RR) 3.76, 95% confidence interval (CI) 1.22 to 11.56, three RCTs, 313 participants) and in one long‐term trial (lasting 12 weeks). Steroids were also more effective in reducing erythema, scaling, and pruritus. Adverse effects were similar in both groups.
There may be no difference between steroids and calcineurin inhibitors in total clearance in the short‐term (RR 1.08, 95% 0.88 to 1.32, two RCTs, 60 participants, low‐quality evidence). Steroids and calcineurin inhibitors were found comparable in all other assessed efficacy outcomes as well (five RCTs, 237 participants). Adverse events were less common in the steroid group compared with the calcineurin group in the short‐term (RR 0.22, 95% CI 0.05 to 0.89, two RCTs, 60 participants).
There were comparable rates of total clearance in the steroid and azole groups (RR 1.11, 95% CI 0.94 to 1.32, eight RCTs, 464 participants, moderate‐quality evidence) as well as of adverse effects in the short‐term, but less erythema or scaling with steroids.
We found mild (class I and II) and strong (class III and IV) steroids comparable in the assessed outcomes, including adverse events. The only exception was total clearance in long‐term use, which occurred more often with a mild steroid (RR 0.79, 95% CI 0.63 to 0.98, one RCT, 117 participants, low‐quality evidence).
In one study, calcineurin inhibitor was more effective than placebo in reducing erythema and scaling, but there were similar rates in total clearance or adverse events for short‐term treatment. In another study, calcineurin inhibitor was comparable with azole when erythema, scaling, or adverse effects were measured for longer‐term treatment.
Lithium was more effective than placebo with regard to total clearance (RR 8.59, 95% CI 2.08 to 35.52, one RCT, 129 participants) with a comparable safety profile. Compared with azole, lithium resulted in total clearance more often (RR 1.79, 95% CI 1.10 to 2.90 in short‐term treatment, one RCT, 288 participants, low‐quality evidence).
Authors' conclusions
Topical steroids are an effective treatment for seborrhoeic dermatitis of the face and scalp in adolescents and adults, with no differences between mild and strong steroids in the short‐term. There is some evidence of the benefit of topical calcineurin inhibitor or lithium salt treatment. Treatment with azoles seems as effective as steroids concerning short‐term total clearance, but in other outcomes, strong steroids were more effective. Calcineurin inhibitor and azole treatment appeared comparable. Lithium salts were more effective than azoles in producing total clearance.
Steroids are similarly effective to calcineurin inhibitors but with less adverse effects.
Most of the included studies were small and short, lasting four weeks or less. Future trials should be appropriately blinded; include more than 200 to 300 participants; and compare steroids to calcineurin inhibitors or lithium salts, and calcineurin inhibitors to azoles or lithium salts. The follow‐up time should be at least one year, and quality of life should be addressed. There is also a need for the development of well‐validated outcome measures.
Plain language summary
Topical anti‐inflammatory agents for seborrhoeic dermatitis of the face or scalp
Seborrhoeic dermatitis is an inflammation of the skin that most often affects areas of the body that have a lot of sebaceous glands. These include the skin of the scalp; face; chest; and flexure areas such as the armpits, groin, and abdominal folds. The most typical symptoms of seborrhoeic dermatitis are scaling of the skin and reddish patches. Seborrhoeic dermatitis is fairly common: one to three in 100 people have seborrhoeic dermatitis. The disease is more common in men than in women. Anti‐inflammatory, antifungal, and antikeratolytic treatments can be used to treat seborrhoeic dermatitis. The treatment does not cure the disease but relieves the symptoms.
We included 36 randomised controlled trials with 2706 participants, examining the effect of anti‐inflammatory treatments on seborrhoeic dermatitis. These trials were short‐term; most of them lasting four weeks or less.
Topical steroid treatment (such as hydrocortisone and betamethasone), topical calcineurin inhibitor treatment (such as tacrolimus and pimecrolimus), and topical lithium salts all reduced the symptoms of seborrhoeic dermatitis when compared with placebo treatment. Mild (such as hydrocortisone 1%) and strong (such as betamethasone) steroid compounds were comparable in short‐term follow up. Short‐term total clearance was achieved with antifungal azole treatment (such as ketoconazole and miconazole), as well as with steroids. Strong steroids were better than azole treatment in reducing erythema, scaling, and pruritus, and were comparable in terms of safety. Steroids were also as effective as calcineurin inhibitors, but side‐effects occurred more often with calcineurin inhibitors. We found no differences between calcineurin inhibitors and azole treatments in effectiveness or side‐effects. Lithium was more effective than azoles but had a similar frequency of side‐effects (one study).
The most common side‐effects were burning, itching, erythema, and dryness in all treatment groups.
Topical anti‐inflammatory agents are useful in treating seborrhoeic dermatitis. Steroids are the most investigated anti‐inflammatories. We still do not know the effects and safety of topical anti‐inflammatory treatments in long‐term or continuous use. This is regrettable as the disease is chronic in nature. Furthermore, there are no data concerning the effects of different treatments on quality of life.
Summary of findings
Summary of findings for the main comparison. Calcineurin inhibitor compared with steroid for seborrhoeic dermatitis of the scalp or face.
| Steroid compared with calcineurin inhibitor for seborrhoeic dermatitis of the scalp or face | ||||||
| Patient or population: people with seborrhoeic dermatitis Settings: community setting implied from context but not stated Intervention: steroid Comparison: calcineurin inhibitor | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Calcineurin inhibitor | Steroid | |||||
| Total clearance (at 4 weeks or less) Investigator's assessment Follow up: ≦ 2 weeks | 839 per 1000 | 906 per 1000 (738 to 1000) | RR 1.08 (0.88 to 1.32) | 60 (2 studies) | ⊕⊕⊝⊝ low¹,² | ‐ |
| *The basis for the assumed risk is the risk in the control groups of the relevant trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Participants were not blinded. ²Small number of participants in studies.
Summary of findings 2. Steroid compared with azole for seborrhoeic dermatitis of the scalp or face.
| Steroid compared with azole for seborrhoeic dermatitis of the scalp or face | ||||||
| Patient or population: people with seborrhoeic dermatitis of the scalp or face Settings: community setting implied from context but not stated Intervention: steroid Comparison: azole | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Azole | Steroid | |||||
| Total clearance (at 4 weeks or less) Investigator's assessment Follow up: 3 to 4 weeks | 474 per 1000 | 526 per 1000 (445 to 625) | RR 1.11 (0.94 to 1.32) | 464 (8 studies) | ⊕⊕⊕⊝ moderate¹ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Risk of bias considerable in all studies.
Summary of findings 3. Strong steroid (class III or IV) compared with mild steroid (class I or II) for seborrhoeic dermatitis of the scalp or face.
| Strong steroid (class III or IV) compared with mild steroid (class I or II) for seborrhoeic dermatitis | ||||||
| Patient or population: people with seborrhoeic dermatitis Settings: community setting implied from context but not stated Intervention: strong steroid (class III or IV) Comparison: mild steroid (class I or II) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mild steroid (class I or II) | Strong steroid (class III or IV) | |||||
| Total clearance (at 4 weeks or less) Investigator's assessment Follow up: 3 to 4 weeks | 413 per 1000 | 397 per 1000 (268 to 578) | RR 0.96 (0.65 to 1.4) | 93 (2 studies) | ⊕⊕⊕⊝ moderate¹ | ‐ |
| Total clearance (more than 4 weeks) Investigator's assessment Follow up: 6 weeks | 644 per 1000 | 509 per 1000 (406 to 631) | RR 0.79 (0.63 to 0.98) | 117 (1 study) | ⊕⊕⊝⊝ low² | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Imprecision (two small studies). ²One study that was not blinded (patient and care provider not blinded; blinding of outcome assessment not reported).
Summary of findings 4. Lithium compared with azole for seborrhoeic dermatitis of the scalp or face.
| Lithium compared with azole for seborrhoeic dermatitis | ||||||
| Patient or population: people with seborrhoeic dermatitis Settings: community setting Intervention: lithium Comparison: azole | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Azole | Lithium | |||||
| Total clearance Investigator's assessment Follow up: 4 weeks | 147 per 1000 | 263 per 1000 (162 to 426) | RR 1.79 (1.1 to 2.9) | 288 (1 study) | ⊕⊕⊝⊝ low¹ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹One study in which participants were not blinded and blinding of others was not reported.
Background
Seborrhoeic dermatitis or eczema is a chronic inflammatory skin disorder primarily affecting areas rich in sebaceous glands (Kim 2010). Such areas include, for example, skin of the scalp, face, chest, and intertriginous areas (areas where folds of skin are touching each other, such as the armpits, groin, and abdominal folds). These areas are liable to irritation from sweating and infection (Naldi 2009). Typical symptoms of the disease are scaling of the skin and erythematous (reddish) patches (Schwartz 2006).
Description of the condition
The specific cause of seborrhoeic dermatitis (SeD) is not known in detail. Despite its name and affected areas, this disease is not always associated with excessive sebum secretion (Burton 1983). It has been suggested that many endogenous and exogenous factors are associated with the course and severity of this disorder. These include hormonal factors; comorbidities (associated diseases); individual immunological features; and nutritional, environmental, and lifestyle factors (Gupta 2004; Schwartz 2006), but the mechanism of action of each of these factors has not been determined. A causative role has been suggested for Malassezia yeasts because SeD responds to antifungal treatments when a concurrent decrease of the number of the yeasts on the skin is seen (Gupta 2004). However, the overall evidence is still somewhat unclear.
The diagnosis of this disease is largely clinical and based on affected areas and the type of rash. Ill‐defined erythematous patches with fine scaling on the sides of the nose, eyebrows, and scalp are seen most often in adult patients. Pruritus (itch) is often present in an affected scalp (Del Rosso 2011). In dark‐skinned people, SeD can present as postinflammatory changes, such as hypopigmentation (Halder 2003). A skin biopsy is rarely needed for diagnosis, but it can be useful for excluding other less common conditions, such as lupus (Naldi 2009; Schwartz 2006). Dandruff is a commonly used term for any scalp condition that produces fine scales, but it has also been used in the context of mild SeD (Naldi 2009; Schwartz 2006). The disease has a chronic nature with occasional relapses. The severity of SeD varies from mild flaking to severe oily scaling. The distribution of lesions is generally symmetrical (Gupta 2004). Although the disease affects the skin of the scalp, it does not normally cause baldness.
Seborrhoeic dermatitis is a fairly common skin disorder. The prevalence is not known precisely as there are no validated criteria for diagnosis of the condition (Naldi 2009). An infantile form (cradle cap) has been reported to affect as many as 70% of newborns during the first three months of life, but this quickly resolves (Foley 2003). So, the overall prevalence of seborrhoeic dermatitis is 10% in children five years of age or younger (Foley 2003). In the adult population, prevalence is between 1% to 3%, and occurrence is more common in adolescents and young adults than those in middle age (Gupta 2004). The incidence increases again in people over 50 years of age (Gupta 2004). Seborrhoeic dermatitis affects men more frequently than women, and some diseases, such as Parkinson's disease and HIV (human immunodeficiency virus)/AIDS, are known to increase the risk of the disease (Naldi 2009).
Description of the intervention
The standard treatments for seborrhoeic dermatitis include topical anti‐inflammatory (immunomodulatory) agents, such as corticosteroids and calcineurin inhibitors, to reduce inflammation; topical antifungals, such as azoles, ciclopirox olamine, and zinc pyrithione, to reduce Malassezia; and topical keratolytic agents, such as salicylic acid, tar, selenium sulphide, and zinc pyrithione, to soften and remove thick hardened crusts. Many agents have multiple mechanisms of action, and in some, the exact mechanism is not known (Gupta 2004; Naldi 2009; Schwartz 2006).
How the intervention might work
Topical corticosteroids (e.g. hydrocortisone, betamethasone, clobetasol, and desonide) have traditionally been used in the treatment of SeD. They reduce inflammation and relieve erythema and itching. Calcineurin inhibitors (e.g. pimecrolimus and tacrolimus) have also been used for their anti‐inflammatory effects. It has been suggested that lithium salts, lithium succinate (often in combination with zinc sulphate), and lithium gluconate have anti‐inflammatory effects, but they may also have antifungal properties (Gupta 2004; Naldi 2009; Schwartz 2006).
Why it is important to do this review
Seborrhoeic dermatitis is a fairly common skin disorder that affects a considerable number of children and adults. There are many available treatment options, but it is unclear which should be preferred. It is important to evaluate the efficacy of these options in order to improve the outcome of the therapy. This review is one of two Cochrane systematic reviews on this topic and will focus on treatment options with an established anti‐inflammatory mechanism. The other Cochrane review is focused on drugs with an antifungal mechanism (Okokon 2011).
Objectives
To assess the effects of topical pharmacological interventions with established anti‐inflammatory action for seborrhoeic dermatitis occurring in adolescents and adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials and cross‐over randomised controlled trials (including within‐patient studies).
We excluded cluster randomised trials.
Types of participants
We included studies of adults or adolescents (> 16 years) with diagnosed seborrhoeic dermatitis of the scalp or face. At least 75% of the study participants had to be over 10 years of age to fulfil the age criterion.
We excluded studies of people having other skin diseases or seborrhoeic dermatitis occurring solely in areas other than the scalp or face.
Types of interventions
We included the following topically administered drugs with an established anti‐inflammatory mechanism of action: corticosteroids and calcineurin inhibitors. We also included lithium salts in the review as it has been suggested that their effect is based on anti‐inflammatory properties.
We excluded studies in which the anti‐inflammatory intervention had been combined with a non‐anti‐inflammatory agent in preparation. We examined all clinically relevant comparisons between treatments.
Types of outcome measures
Primary outcomes
Percentage of treated persons with total resolution of symptoms as evaluated by the outcome assessor (total clearance).
Disease severity scores for scaling, pruritus, or erythema at the end of treatment as evaluated by participant self‐report, outcome assessor, or both.
Percentage of persons treated who develop side‐effects or intolerance to treatment.
Secondary outcomes
Improvement in quality of life.
Timing of outcomes
We defined the timing of outcomes using the following categories:
When the treatment period lasted for four weeks or less, we defined these outcomes as short‐term effects.
When the treatment period lasted for more than four weeks, we defined these outcomes as long‐term effects.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 18 September 2013:
the Cochrane Skin Group Specialised Register using the following terms: "seborrh* dermatitis" or "scalp dermatos*" or "scalp dermatitis" or "scalp eczema" or "cradle cap" or dandruff or malassezia or "seborrh* eczema";
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library using the search strategy in Appendix 1;
MEDLINE via OVID (from 1946) using the strategy in Appendix 2;
Embase via OVID (from 1974) using the strategy in Appendix 3;
the Global Resource of EczemA Trials (GREAT, Centre of Evidence Based Dermatology, accessed at http://www.greatdatabase.org.uk on 18 September 2013) using the same search terms as for the Skin Group Specialised Register above; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 4.
Trials databases
We searched the following trials registers on 22 October 2013, using the following search terms: seborrheic dermatitis or seborrhoeic or dandruff or cradle cap or malassezia or scalp dermatoses.
The metaRegister of Controlled Trials (www.controlled‐trials.com).
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch).
The EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
References from included studies
We checked the bibliographies of included studies for further references to relevant trials.
Adverse effects
We did not perform a separate search for adverse effects of the target interventions. We examined data on adverse effects from the included studies we identified.
Data collection and analysis
Selection of studies
Three authors (TOk, HK, and JJ) independently identified relevant articles retrieved from the literature searches by assessing their titles and abstracts. Where we had differing views, we retained the article for full‐text assessment.
The same three authors and one additional author (TOr) independently assessed the full‐text papers using study eligibility forms in order to determine which studies satisfied the inclusion criteria. Where there were differing views that could not be resolved between the review authors, a third author (PP) made the decision of inclusion or exclusion.
Data extraction and management
The same authors (TOk, HK, and JJ) carried out data extraction independently using data extraction forms. A third researcher (PP) resolved discrepancies if consensus could not be found between the primary authors. TOk and HK managed the data including entering it into Review Manager (RevMan). HK checked the entered data for accuracy. We requested any further information needed from the original authors by email and included any relevant information obtained in this manner in the review.
Assessment of risk of bias in included studies
The assessment of the risk of bias included an evaluation of the following components for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
(a) selection bias ‐ we considered whether the methods of randomisation were adequate and whether the treatment allocation was concealed in the included studies. As there was some overlap between the clinical spectrum of seborrhoeic eczema and dandruff, we paid attention to the presence of the diagnosis of seborrhoeic dermatitis in participants and to the baseline severity of the disease in study groups; (b) performance bias ‐ we assessed whether the participants and the caregivers were blinded to the interventions and whether cointerventions and other treatments were similar in study groups; (c) detection bias ‐ we evaluated whether the outcome assessors were blinded to the interventions; (d) attrition bias ‐ we assessed whether the trial described dropout rates and whether they were acceptable, whether compliance was acceptable in all groups, and whether the study reports used intention‐to‐treat analysis (we used the number of randomised participants in our analyses, where available); (e) reporting bias ‐ we evaluated whether there were signs of selective reporting in the studies; and (f) other bias ‐ we evaluated whether there might have been other sources of bias, for example, relating to particular study designs.
We assessed the study quality without blinding to authorship or journal.
We have summarised the information in the 'Risk of bias' table for each included study.
Measures of treatment effect
For dichotomous outcomes, we expressed the combined estimate of effects as risk ratios (RR) and their 95% confidence intervals (Cl). For the main outcome (total clearance), we expressed summary estimates also as number needed to treat (NNT) for statistically significant findings, with a 95% CI and the baseline risk to which it applies.
For continuous outcomes, we used the mean difference with a 95% CI for summarising results. Where similar outcomes were measured differently across studies but measured the same concept, we used the standardised mean difference and a 95% confidence interval.
Unit of analysis issues
When there was intrapatient correlation in studies that had randomised body parts of the same participant and the study authors had not adjusted for this clustering effect, we did this adjustment according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We analysed studies with multiple treatment groups using pair‐wise comparisons. We avoided counting the control group of multiple treatment studies twice, by dividing the number of control participants over the number of comparisons in the same meta‐analysis, excluding the outcome of any adverse effects.
Dealing with missing data
We applied the intention‐to‐treat (ITT) principle by considering dropouts as non‐responders (conservative approach). If data necessary for meta‐analysis (such as standard deviations) were missing in the trial reports, we asked study authors for additional information. If they could not be reached, we calculated the necessary data from other statistics, if such information was available, or approximated them from information (e.g. graphics) given in the reports.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining types of participants, interventions, and outcomes in each study. We assessed statistical heterogeneity using the I² statistic. We interpreted heterogeneity in effect estimates as considerable when the I² statistic was greater than 50%.
Assessment of reporting biases
We assessed reporting bias as within‐study reporting bias (selective outcome reporting) and as publication bias. We did not perform funnel plot analyses as the number of studies was small in our meta‐analyses. To avoid language bias, we imposed no language restrictions.
Data synthesis
For studies judged to be clinically and statistically homogenous with an I² statistic < 50%, we pooled the measures of treatment effect using their weighted average for the treatment effect (using a fixed‐effect meta‐analysis method, as implemented in Review Manager). For studies deemed to be heterogeneous (I² statistic ≥ 50%), we performed a random‐effects meta‐analysis. For studies with I² statistics more than 80%, we did not perform a meta‐analysis, but described the results individually.
Subgroup analysis and investigation of heterogeneity
We planned to explore heterogeneity by examining age (less than 65 or over 65 years), gender (male or female), and dose (frequency) distributions of the studies. We aimed to conduct subgroup analyses if significant heterogeneity between the studies for the primary outcomes in a comparison appeared. The number of studies was small in most comparisons; therefore, performing subgroup analyses was not reasonable with the exception of comparison between mild and strong steroids.
Sensitivity analysis
We aimed to but did not perform sensitivity analyses to examine the effects of risk of bias as there were few studies in each comparison. Furthermore, the overall risk of bias was at least moderate in most studies.
Results
Description of studies
Results of the search
The database searches yielded 1019 records. We identified a further seven records:
five from handsearching the references of our included studies;
one from a related Cochrane review (Okokon 2011); and
one published study from a trials register (Ortonne 2011).
We screened 1026 records, of which we excluded 912 based on the title and abstract or because they were duplicates.
We screened 114 full‐text articles. We excluded 76 records (see the 'Characteristics of excluded studies' tables).
Altogether, we included 36 studies (see the 'Characteristics of included studies' tables). We assigned two studies to the Studies awaiting classification section on the grounds that it was unclear whether they measured the outcomes of interest.
We present our screening process in Figure 1.
1.
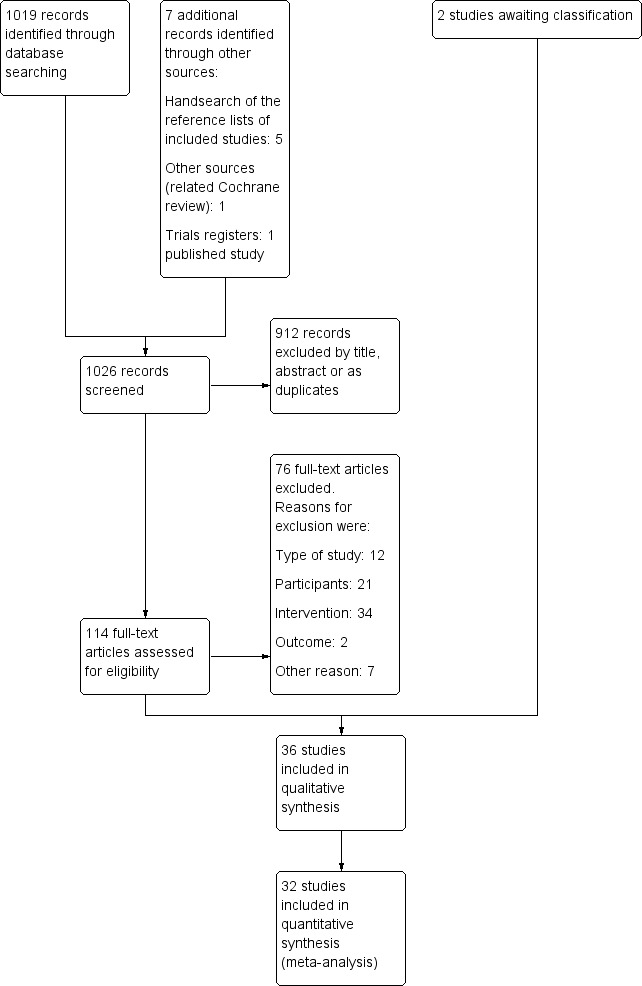
Study flow diagram
Included studies
Study design
All 36 included studies, with 2706 participants, were reported as randomised controlled trials, with three comparing body parts.
Year of study
The 36 included studies were carried out between 1970 and 2012 with 13 studies before the year 1990, 10 studies between 1990 and 2000, and 13 studies after the year 2000.
Participants
In seven studies, a physician explicitly diagnosed participants with seborrhoeic dermatitis (SeD), and in 29 studies, this was unclear (implied from context but not clearly stated). The definition of SeD was given in one study only (Cicek 2009). The studied area was the scalp only in 16 reports; the face only in 10 reports; the face and scalp in one report; and the face, scalp, or both with other areas in seven reports. Two studies did not specify the affected and investigated areas, but it could be concluded that they included facial or scalp involvement based on the assessed body areas.
Six studies included participants under 18 years of age (from ages 12, 14, or 15 upwards). Four reports did not state the age of the participants. In three studies, there was an upper limit of age (65 years in two and 55 years in one study). All studies but one included both men and women (Langtry 1997 included only homosexual men with HIV). Frequent exclusion criteria were pregnancy, lactation state, other dermatoses or interventions, too severe or mild disease, or HIV. The older studies often did not report the exclusion criteria.
The number of participants in individual studies varied between 12 and 303, resulting in a median of 64.
Geography
The geographical variation of studies was as follows: USA (10 studies), France (four studies), Greece (four studies), Sweden (four studies), Turkey (three studies), Finland (two studies), UK (two studies), Iran (one study), Denmark (one study), Korea (one study), India (one study), Canada (one study), and Netherlands (one study). One study was multicentre (Belgium, France, Germany, Mexico, and South Korea).
Interventions
The included studies used the following drugs (doses and mode of delivery) for seborrhoeic dermatitis.
Mild steroids (class I or class II, classification according to the ATC (Anatomical Therapeutic Chemical) classification by the World Health Organization (WHO))
hydrocortisone (cream 1%, liniment 1%, lotion 0.1%, ointment 1.0%, and solution 1%)
alclometasone (ointment 0.05%)
desonide (cream 0.05%)
Strong steroids (class III or class IV, classification according to ATC classification by the WHO)
methylprednisolone (cream 1%)
betamethasone (lotion 0.1%, lotion 0.05%, cream 0.1%, and solution 1 mg/ml)
clobetasol (shampoo 0.05% and cream 0.05%)
amcinonide (lotion 0.1%)
mometasone (solution 0.1% or cream 0.1%)
fluocinolone acetonide (solution 0.01%, shampoo 0.01%)
Calcineurin inhibitors
pimecrolimus (cream 1%)
tacrolimus (ointment 0.1%)
Azoles
ketoconazole (cream 2%, foaming gel 2%, shampoo 2%, shampoo 1%, and hydrogel 20 mg/g)
metronidazole (gel 0.75%)
miconazole (base 2%)
Lithium (gluconate ointment 8% and succinate ointment 8%)
Zinc pyrithione (shampoo 1%)
Calcipotriol (solution 50 μg/ml)
Promiseb® (cream)
Placebo or propylene glycol
We decided to pool together all steroid studies as there were so many different steroidal compounds studied and often only one study on one compound. We also decided to pool together all calcineurin inhibitors and all azoles as the number of studies was limited. This enabled us to make the following direct comparisons.
Steroids compared with placebo (six trials)
Steroids compared with calcineurin inhibitors (five trials)
Steroids compared with azoles (12 trials)
Steroids compared with other compounds (calcipotriol, zinc pyrithione, Promiseb®) (three trials)
Mild steroids compared with strong steroids (five trials)
Calcineurin inhibitors compared with placebo (one trial)
Calcineurin inhibitors compared with azoles (two trials)
Calcineurin inhibitors compared with other compounds (zinc pyrithione) (one trial)
Lithium salts compared with placebo (two trials)
Lithium salts compared with azoles (one trial)
We also identified two studies comparing a mild steroid with another mild steroid (Cornell 1986; Cornell 1993) and one study comparing a strong steroid with another strong steroid (Cornell 1989). We do not display the results for these comparisons, however, as the focus of this review is to compare anti‐inflammatory treatments with placebo and comparisons between different anti‐inflammatory treatment classes.
We contacted three authors for additional data, which we received from two.
Outcomes
Twenty‐three of the 36 included studies used total clearance as a measure of outcome. Resolution of symptoms was measured either on scale or as resolution of that specific symptom as follows: scaling in 19 studies, erythema in 17 studies, pruritus in 15 studies. The validation of the scales used was not reported in any of the articles. In four studies, adverse events were the only outcome we could use in this review (Cicek 2009; Ortonne 1992; Ortonne 2011). Seven studies (19%) did not report the side‐effects.
Excluded studies
We excluded 76 studies. The most frequent reason for exclusion was that the intervention in the study was not anti‐inflammatory or it was a combination of two drugs. Another common reason for excluding a study was that the proportion of people with SeD was unclear or that the proportion of them was too small. We identified two studies that did not report outcomes relevant for this review or did not report them in numerical form. We excluded them as they had no useful data to add to the analyses (Kim 2012; Marks 1974).
We present detailed reasons for exclusions in the 'Characteristics of excluded studies' tables.
Studies awaiting classification and ongoing studies
We assigned two studies to the Studies awaiting classification section as we had no evidence that they measured the outcomes of interest. We will reconsider these studies in the next update of the review. See the 'Characteristics of studies awaiting classification' tables for details. We identified seven studies from trials registers that are either ongoing or not yet published. See the 'Characteristics of ongoing studies' tables for details.
Risk of bias in included studies
We assessed the risk of bias in the included studies as described above (Assessment of risk of bias in included studies). The most frequent classification of risk of bias in studies was unclear. This was especially because of unclear reporting of methods, such as reporting studies to be double‐blind without specifying who was blinded. Figure 2 displays the overall percentages of risk of bias for the studies included in the review. Figure 3 displays the risk of bias judged for each included study.
2.
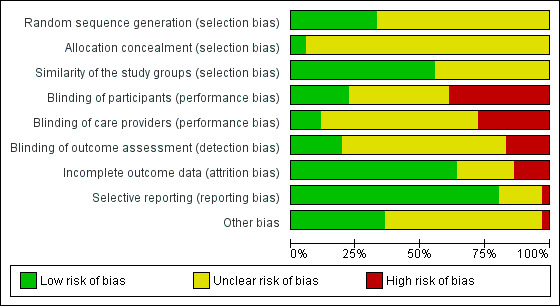
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.
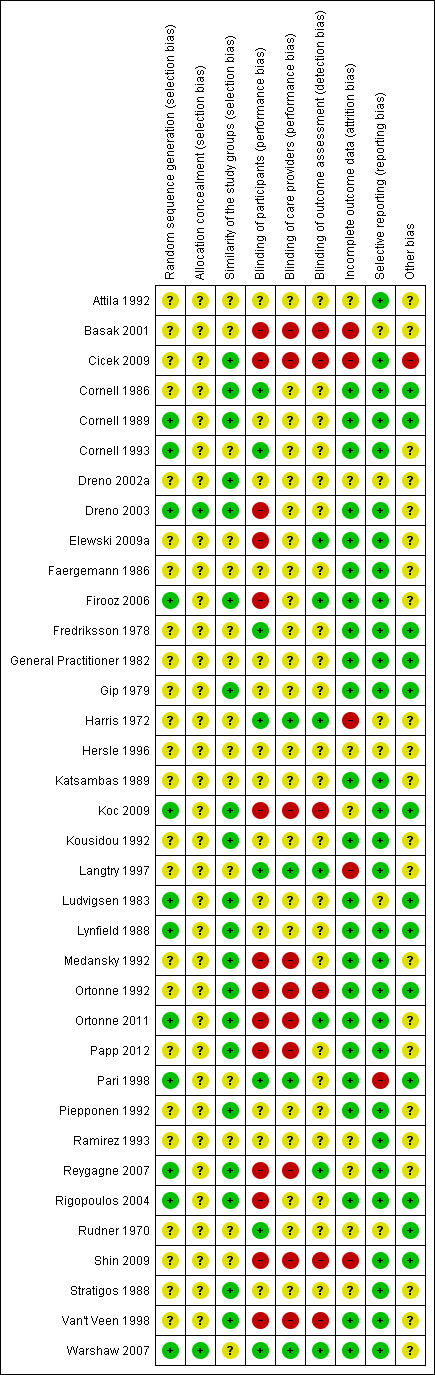
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
Most reports classified selection bias as unclear. They often stated that participants were randomly allocated but did not report the methods of randomisation or allocation sequence concealment in detail. Twelve studies reported the generation of the randomisation sequence, and most commonly, it was computer‐based. Only two studies described the allocation concealment method; otherwise, the studies did not mention it at all, and therefore we classified them as unclear risk.
Blinding
Most often the studies were reported to be double‐blind, but it was not clear which two of the three parties (the participants, the caregivers, or the outcome assessors) were blinded. In these cases, we evaluated the risk of bias as unclear. Seven reports stated that the whole study was outcome assessor blinded. Six of the included studies were completely open‐label or did not mention blinding, which we rated as at high risk of detection bias.
Incomplete outcome data
We evaluated attrition bias to be low in 23 of the studies. The reason we classified a study with a high risk for attrition bias was most often because of a considerable dropout rate (over 20%). We evaluated attrition bias to be unclear in eight studies when it was unclear whether the results given in percentages were calculated using the randomised or the completed participant numbers.
Selective reporting
We classified the risk for reporting bias as low in 29 of the studies. One study did not prespecify the outcomes in the report, but the outcomes reported were those commonly used in SeD studies. In seven studies, there was no mention of side‐effects at all, which we consider a serious omission. However, we did not assess the lack of information concerning side‐effects as a reporting bias unless the measurement of adverse effects was part of the predefined outcome measures, but had not been reported.
Other potential sources of bias
We sought other potential sources of bias. One study (Cicek 2009) evaluated both the efficacy outcome and side‐effects using the same symptoms (erythema and pruritus were both an efficacy outcome and a side‐effect); therefore, it was impossible for a reader to evaluate when or why these symptoms were classified as an outcome or a side‐effect. Therefore, we classified the risk of bias as high for this trial. One study (Koc 2009) did not report the affected area (although we could conclude from the report that facial involvement was an inclusion criterion); therefore, we were not sure that the efficacy or the intervention on facial or scalp SeD was the same as reported in the article. We judged the risk of bias as low regarding this study. One study (Langtry 1997) only included male HIV participants, which may limit the ability to generalise the results into other populations. (We judged risk of bias as unclear.)
The most common circumstance in studies classified as having unclear risk was author affiliation to the pharmaceutical industry or interventions sponsored or provided by the pharmaceutical industry (N = 21 for studies having some kind of affiliations to the pharmaceutical industry). This classification was done categorically, and it does not imply that in the opinion of the review authors, these studies have increased risk of bias. In 13 studies, we were unable to identify other potential sources of bias, so we classed these at low risk of bias.
Similarity of study groups (selection bias)
Most of the included studies described adequately the similarity of study groups, and the risk of bias was low in 20 studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We have addressed the comparisons under the following headings.
Steroids versus comparators
Steroids versus placebo
Steroids versus calcineurin inhibitors
Steroids versus azoles
Mild steroids versus strong steroids
Other comparisons for steroids
Calcineurin inhibitors versus comparators
Calcineurin inhibitors versus placebo
Calcineurin inhibitors versus azoles
Calcineurin inhibitors versus zinc pyrithione
Lithium versus comparators
Lithium salts versus placebo
Lithium salts versus azoles
For each of these comparisons, we addressed our prespecified outcomes. Our primary outcomes were as follows.
Percentage of treated persons with total resolution of symptoms as evaluated by the outcome assessor (total clearance).
Disease severity scores for scaling, pruritus, or erythema at the end of treatment as evaluated by participant self‐report, outcome assessor, or both.
Percentage of persons treated who develop side‐effects or intolerance to treatment.
With regard to our primary outcome 'Total clearance', we considered this to be present where the terms "complete" or "total" resolution of symptoms or cure or clearance were used, whereas we did not accept as total clearance the term "excellent" without any definition of its meaning. Total clearance was the investigator's assessment unless otherwise stated. The included studies reported our other primary outcomes 'Disease severity' and 'Adverse events'.
None of the included studies assessed our secondary outcome 'Quality of life'.
Steroids versus comparators
We identified six studies comparing steroids with placebo or vehicle, five studies comparing steroids with calcineurin inhibitors, and 12 studies comparing steroids with azoles. Of these studies, one compared steroid with placebo and azole, one compared steroid with calcineurin inhibitor and azole, and one compared steroid with calcineurin inhibitor and zinc pyrithione. Additionally, we identified one study that compared steroid with Promiseb®, which is a non‐steroidal compound, and one study that compared steroid with calcipotriol (vitamin D).
We performed subgroup analyses using the strength of the steroid compound as classification criterion. We classified class I and II steroids as mild steroids, and we classified class III and IV steroids as strong steroids. Five identified studies compared strong steroids with mild steroids. Furthermore, we identified two studies comparing a mild steroid with another mild steroid (Cornell 1986; Cornell 1993), and one study comparing a strong steroid with another strong steroid (Cornell 1989). We did not display the results for these three comparisons, as the focus of this review is to compare anti‐inflammatory treatments with placebo and comparisons between different anti‐inflammatory treatment classes. We also included analyses comparing mild steroids with strong steroids. We consider these comparisons to be most important from the clinical decision‐making point of view.
Steroids versus placebo
In our analyses, steroids displayed a stronger effect on the studied outcomes than placebo with a comparable safety profile.
Total clearance (at four weeks or less of treatment)
Three studies, with a total of 303 participants, investigated this outcome. Participants achieved 'Total clearance' with steroids more often than with placebo (risk ratio (RR) 3.76, 95% confidence interval (CI) 1.22 to 11.56) when pooling steroids together (Analysis 1.1) (number needed to treat (NNT) 4, 95% CI 3 to 6). Two studies not included in this meta‐analysis (Harris 1972; Reygagne 2007; Table 5) further supported this finding. However, there were indications that only a strong steroid is more effective than placebo (RR 5.92, 95% CI 0.99 to 35.52) in Analysis 1.1.
1.1. Analysis.
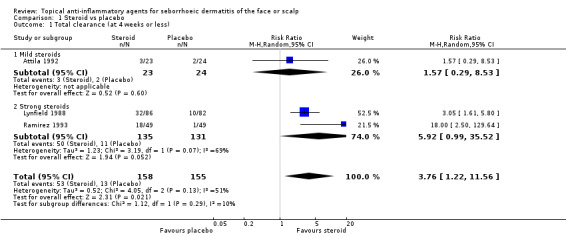
Comparison 1 Steroid vs placebo, Outcome 1 Total clearance (at 4 weeks or less).
1. Reygagne 2007.
| The study investigated clobetasol propionate shampoo (0.05%) with three different application times (2.5 minutes, 5 minutes, and 10 minutes) and the comparisons included ketoconazole and vehicle. Each group included 11 participants. Some of the results are unobtainable from the figures in the report, and only results stated in the text could be used. The study lasted 4 weeks. The study has not been included in the meta‐analyses as the mode of application was different from all other studies. | |||
| Steroid | Vehicle | Azole | |
| Total clearance | 18.2% to 45.5% (in different application groups) | 9.1% | 9.1% |
| Erythema scores¹ | 0.1 in clobetasol 5‐minute group; otherwise, not reported (P value = 0.024 for comparison with vehicle) | 0.7 | 0.1 |
| Scaling scores (loose desquamation)² | 0.3 in clobetasol 10‐minute group, and 0.4 in clobetasol 5‐minute group; otherwise, not reported (P value = 0.027 for comparison between clobetasol 10‐minute group and vehicle group) | 1.0 | Not reported |
| Pruritus score | ‐ 4.8 mm in clobetasol 5‐minute group; otherwise, not reported (P value = 0.007 for comparison with vehicle) | ‐ 34 mm | ‐ not reported in text (8.9 mm approximated from figure) |
| Any adverse effects | 1 participant (9%) in all groups experienced burning. 1 participant in clobetasol 10‐minute group reported dry skin. 1 participant in clobetasol 5‐minute group reported folliculitis | 1 participant (9%) experienced burning. Eczema was reported in 1 person | 1 participant (9%) experienced burning |
¹Outcome "erythema scores" refers to erythema scores at end of study. A lower score relates to a better treatment effect. ²Outcome "scaling scores" refers to scaling scores at end of study. A lower score relates to a better treatment effect.
Total clearance (at four weeks or more)
One study with 43 participants examined the effect of a strong steroid on total clearance compared with placebo and found that participants achieved total clearance more often with the steroid than with placebo (RR 2.24, 95% CI 1.10 to 4.56) (Analysis 1.2) (NNT 3, 95% 1 to 11).
1.2. Analysis.
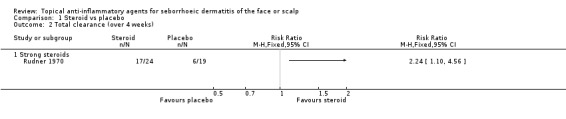
Comparison 1 Steroid vs placebo, Outcome 2 Total clearance (over 4 weeks).
Erythema (at four weeks or less of treatment)
One study with 134 participants examined the mean change in erythema scores. There was a greater reduction in erythema score with a strong steroid (in favour of steroid) than with placebo (mean difference (MD) 0.53, 95% CI 0.27 to 0.79) (Analysis 1.3).
1.3. Analysis.
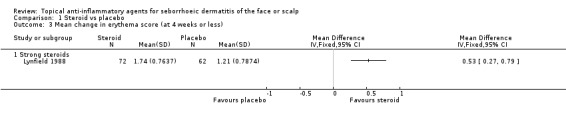
Comparison 1 Steroid vs placebo, Outcome 3 Mean change in erythema score (at 4 weeks or less).
This finding is furthermore supported by another study (44 participants) (Reygagne 2007; Table 5). A study with 98 participants examined the level of erythema scores at the end of treatment and found that with strong steroid the erythema score was lower (in favour of steroid) when compared with placebo (MD ‐0.79, 95% CI ‐1.07 to ‐0.51) (Analysis 1.4).
1.4. Analysis.
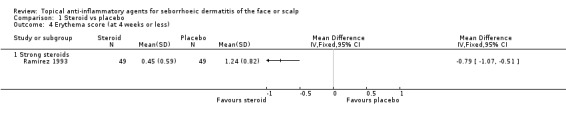
Comparison 1 Steroid vs placebo, Outcome 4 Erythema score (at 4 weeks or less).
Scaling (at four weeks or less of treatment)
One study with 136 participants examined the mean change in scaling scores. There was a greater reduction in scaling score with strong steroid (in favour of steroid) than with placebo (MD 0.77, 95% CI 0.49 to 1.05) (Analysis 1.5). Another study with 98 participants reported the level of scaling scores at the end of treatment, and here also, a strong steroid was more effective than placebo because the scaling score was lower with steroid (MD ‐0.80, 95% CI ‐1.10 to ‐0.50) (Analysis 1.6).
1.5. Analysis.
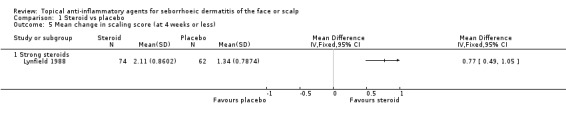
Comparison 1 Steroid vs placebo, Outcome 5 Mean change in scaling score (at 4 weeks or less).
1.6. Analysis.
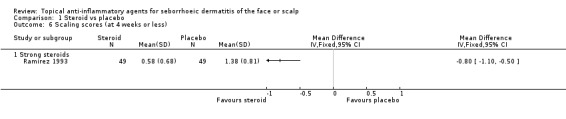
Comparison 1 Steroid vs placebo, Outcome 6 Scaling scores (at 4 weeks or less).
One study with 44 participants (Reygagne 2007; Table 5) further supported this finding.
Pruritus (at four weeks or less of treatment)
One study with 116 participants examined the mean change in pruritus scores and found that there were no statistically significant differences between a strong steroid and placebo (MD 0.27, 95% CI ‐0.04 to 0.58) (Analysis 1.7). Another study with 98 participants reported the level of pruritus scores at the end of treatment. In this study, a strong steroid proved to be more effective than placebo (MD ‐0.41, 95% CI ‐0.69 to ‐0.13) (Analysis 1.8).
1.7. Analysis.
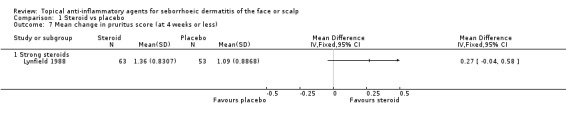
Comparison 1 Steroid vs placebo, Outcome 7 Mean change in pruritus score (at 4 weeks or less).
1.8. Analysis.
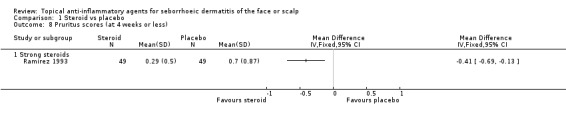
Comparison 1 Steroid vs placebo, Outcome 8 Pruritus scores (at 4 weeks or less).
Another study with 44 participants found a strong steroid to be more effective in this outcome when compared with placebo (Reygagne 2007; Table 5).
Any adverse effects
One study compared a mild steroid and placebo, and three studies compared a strong steroid and placebo. As a whole, there were 606 participants in these trials. We found no statistically significant differences between steroid treatment and placebo regardless of the strength of the steroid (pooled RR 0.89, 95% CI 0.29 to 2.72) (Analysis 1.9). One study with 44 participants (Reygagne 2007; Table 5) supported this finding. We could not use in meta‐analysis one study with 100 randomised participants, which reported that there were no adverse effects (Ramirez 1993), as the effect estimate was inestimable.
1.9. Analysis.
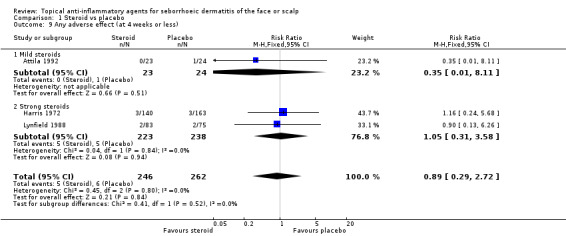
Comparison 1 Steroid vs placebo, Outcome 9 Any adverse effect (at 4 weeks or less).
The most commonly reported adverse effects were burning and itching in both steroid and placebo treatment. The proportion of participants experiencing any adverse effect was mostly low, approximately two to three per cent of the total study population.
Steroids versus calcineurin inhibitors
In our analyses, there were no statistically significant differences between steroids and calcineurin inhibitors in terms of the assessed outcomes in three studies. In two studies, only the adverse events outcomes were of relevance to this review (Cicek 2009; Papp 2012). There were implications that adverse events may be more common in calcineurin inhibitor treatment when compared with steroids.
Total clearance (at four weeks or less of treatment)
There was no statistically significant difference between steroids and calcineurin inhibitors for this outcome in two studies with a combined total of 60 participants (RR 1.08, 95% CI 0.88 to 1.32) (Analysis 2.1), and there were no conclusive statistical differences between a strong and a mild steroid when compared with calcineurin inhibitor. We rated the quality of the evidence as low (Table 1).
2.1. Analysis.
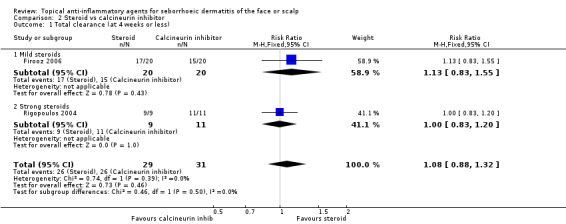
Comparison 2 Steroid vs calcineurin inhibitor, Outcome 1 Total clearance (at 4 weeks or less).
Erythema (at four weeks or less of treatment)
One study with 37 participants examined the erythema scores at the end of treatment and found that there was no statistically significant difference between a mild steroid and calcineurin inhibitor (MD ‐0.05, 95% CI ‐0.22 to 0.12) (Analysis 2.2).
2.2. Analysis.

Comparison 2 Steroid vs calcineurin inhibitor, Outcome 2 Erythema score (at 4 weeks or less).
Scaling (at four weeks or less of treatment)
One study with 38 participants examined the scaling scores at the end of treatment and found that there were no statistically significant differences between steroids and calcineurin inhibitors (MD 0.00, 95% CI ‐0.24 to 0.24) (Analysis 2.3). Another study with 32 participants examined the mean change in dandruff scores, and the findings of this study were similar (MD ‐0.20, 95% CI ‐0.73 to 0.33) (Analysis 2.4).
2.3. Analysis.
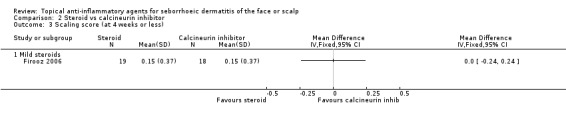
Comparison 2 Steroid vs calcineurin inhibitor, Outcome 3 Scaling score (at 4 weeks or less).
2.4. Analysis.
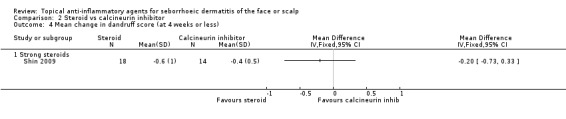
Comparison 2 Steroid vs calcineurin inhibitor, Outcome 4 Mean change in dandruff score (at 4 weeks or less).
Pruritus (at four weeks or less of treatment)
One study with 37 participants examined pruritus scores and found that there were no statistically significant differences between a mild steroid and calcineurin inhibitor (Firooz 2006). We could not use the results of the trial in analyses for statistical reasons. (The standard deviations were 0.00 in the other treatment arm.)
Any adverse effects
Two studies with a combined total of 60 participants examined the incidence of adverse events when comparing calcineurin inhibitors with steroids for short‐term treatment. Adverse events were found to be less common with steroid treatment (RR 0.22, 95% CI 0.05 to 0.89) (Analysis 2.5). The most commonly reported adverse effects were erythema, burning, and prickling sensations.
2.5. Analysis.
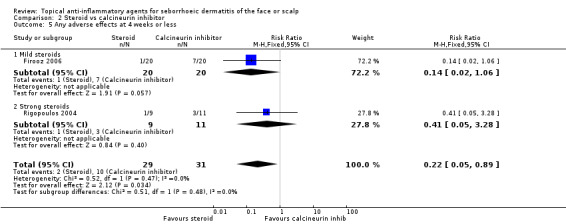
Comparison 2 Steroid vs calcineurin inhibitor, Outcome 5 Any adverse effects at 4 weeks or less.
Two studies with a combined total of 72 participants examined the incidence of adverse effects for long‐term treatment. There was no statistically significant difference between steroid and calcineurin‐inhibitor treatment (RR 0.62, 95% CI 0.26 to 1.47) (Analysis 2.6). One study with 54 participants (Shin 2009) did not report adverse effects with sufficient detail.
2.6. Analysis.
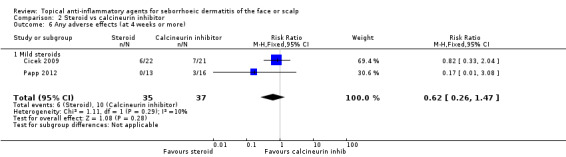
Comparison 2 Steroid vs calcineurin inhibitor, Outcome 6 Any adverse effects (at 4 weeks or more).
Steroids versus azoles
No statistically significant differences were found between steroids and azoles in their efficacy in producing total clearance when evaluated by the investigator for short‐term treatment, whereas when evaluated by the participant, azole treatment was found to be more effective than a mild steroid. For short‐term treatment, the effect of azoles was milder than that of (at least strong) steroids on erythema, scaling, or pruritus. When long‐term treatment was given, an azole compound was found to be more effective than a steroid compound in producing total clearance. There seemed to be no differences between steroids and azoles for adverse effects; however, in one study of long‐term use, there were more adverse effects with a strong steroid than with an azole compound.
Total clearance (at four weeks or less of treatment)
A total of eight studies with a combined number of 464 participants assessed the comparative effectiveness of steroids and azoles in producing total clearance and found that there were no statistically significant differences between them (RR 1.11, 95% CI 0.94 to 1.32) when judged by the investigator (Analysis 3.1). The finding was similar in studies investigating mild and strong steroids. We rated the quality of the evidence as moderate (Table 2).
3.1. Analysis.
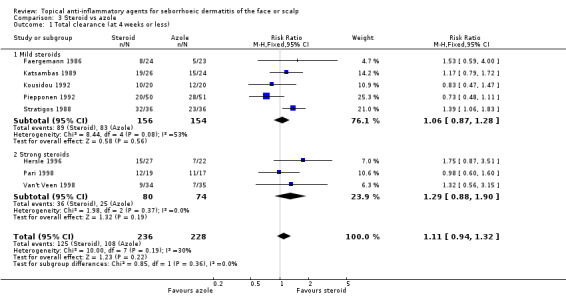
Comparison 3 Steroid vs azole, Outcome 1 Total clearance (at 4 weeks or less).
One study with 44 participants, which we did not include in the meta‐analysis, reached inconclusive results (Reygagne 2007; Table 5). There was also one study (62 participants) with conflicting results where azole treatment was more effective than steroid treatment in producing an excellent (this trial did not use total clearance as an outcome) response (Ortonne 1992).
Two studies assessed the comparative effectiveness of steroids and azoles in producing total clearance when judged by the participant. In one study with 101 participants, azole treatment was more effective in producing total clearance when compared with a mild steroid (RR 1.55, 95% CI 1.09 to 2.21) (Piepponen 1992), whereas in another study with 69 participants, there were no statistically significant differences between a strong steroid and an azole treatment (RR 0.99, 95% CI 0.80 to 1.23) (Van't Veen 1998) (Analysis 3.2).
3.2. Analysis.
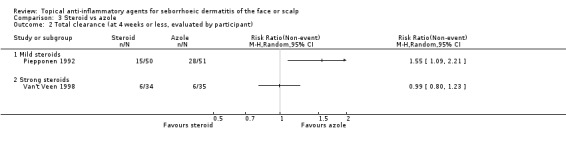
Comparison 3 Steroid vs azole, Outcome 2 Total clearance (at 4 weeks or less, evaluated by participant).
Erythema (at four weeks or less of treatment)
Three studies with a combined total of 160 participants addressed the effect of steroids and azoles on erythema evaluated by erythema scores at the end of treatment. One study (49 participants) comparing a strong steroid with azole found steroid to be more effective (MD ‐0.19, 95% CI ‐0.26 to ‐0.12) (Analysis 3.3). In two studies comparing mild steroids with azoles (111 participants), the results were inconsistent (Kousidou 1992; Stratigos 1988). We could not use these trials in the analysis because of missing statistical data.
3.3. Analysis.
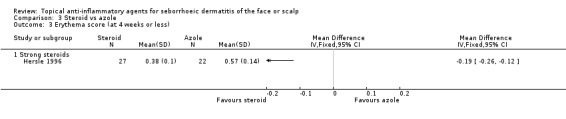
Comparison 3 Steroid vs azole, Outcome 3 Erythema score (at 4 weeks or less).
One study (101 participants, mild steroid) assessed the mean change in erythema scores. There was no statistically significant difference between the two treatments (MD 0.12, 95% CI ‐0.27 to 0.51) (Analysis 3.4).
3.4. Analysis.
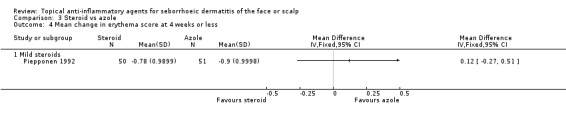
Comparison 3 Steroid vs azole, Outcome 4 Mean change in erythema score at 4 weeks or less.
Scaling (at four weeks or less of treatment)
Two studies with a combined total of 118 participants addressed the effect of steroids when compared with azoles on scaling evaluated by scaling scores at the end of treatment. Strong steroids were associated with statistically significantly lower scaling scores (in favour of steroids) at the end of treatment (standardised mean difference (SMD) ‐2.72, 95% CI ‐3.24 to ‐2.21 for strong steroids, two studies) (Analysis 3.5).
3.5. Analysis.
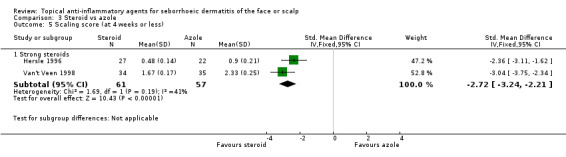
Comparison 3 Steroid vs azole, Outcome 5 Scaling score (at 4 weeks or less).
By contrast, there was a high degree of heterogeneity (I² statistic of 90%) between the results for the two trials comparing mild steroids with azoles (Kousidou 1992; Stratigos 1988, altogether 111 participants). The first mentioned trial found azole treatment to be more effective when compared with steroid (MD 0.92, 95% CI 0.26 to 1.59, 39 participants), whereas the results of the latter trial displayed no statistically significant difference between steroid and azole treatment (MD ‐0.38, 95% CI ‐0.85 to 0.08, 72 participants).
One study with 101 participants addressed the mean change in scaling scores and found that the treatments were equally effective (MD ‐0.05, 95% CI ‐0.40 to 0.30) (Analysis 3.6).
3.6. Analysis.
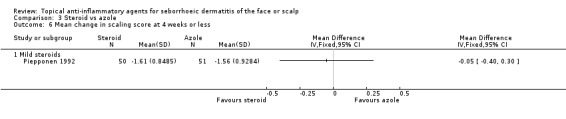
Comparison 3 Steroid vs azole, Outcome 6 Mean change in scaling score at 4 weeks or less.
Pruritus (at four weeks or less of treatment)
Five studies with a combined total of 260 participants assessed the effect of steroids and azoles on pruritus evaluated by pruritus scores at the end of treatment. One trial with mild steroids (Stratigos 1988, 72 participants) noted no statistically significant difference in pruritus at four weeks (72 participants). We could not use the results of this trial in the meta‐analysis because of lack of statistical data. In the other trial with mild steroids, the treatments seemed comparable as well (MD 0.06, 95% CI ‐0.02 to 0.14, 39 participants) (Analysis 3.7).
3.7. Analysis.
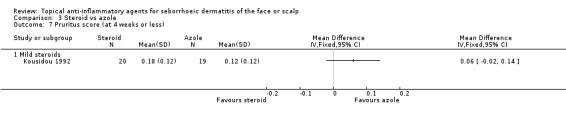
Comparison 3 Steroid vs azole, Outcome 7 Pruritus score (at 4 weeks or less).
The results of three studies with strong steroids displayed a high degree of heterogeneity (I² statistic of 83%), and therefore we could not pool them together in a meta‐analysis. However, in two of these trials, there were indications that strong steroids are more effective than azoles in reducing pruritus (MD ‐1.52, 95% CI ‐2.17 to ‐0.88, 49 participants in Hersle 1996) (MD ‐1.81, 95% CI ‐2.38 to ‐1.25, 69 participants in Van't Veen 1998), whereas in the third study, there were no statistically significant differences between a strong steroid and azole treatment (MD ‐0.28, 95% CI ‐0.99 to 0.43, 31 participants) (Pari 1998).
One study with 101 participants evaluated the mean change in pruritus scores and found that the treatments were equally effective (MD 0.03, 95% CI ‐0.36 to 0.42) (Analysis 3.8).
3.8. Analysis.
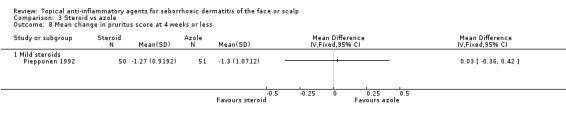
Comparison 3 Steroid vs azole, Outcome 8 Mean change in pruritus score at 4 weeks or less.
Total clearance (at more than four weeks of treatment)
Only one study (Ortonne 1992), with 62 participants lasting four months, assessed clearance as a long‐term outcome. However, this trial did not measure total clearance; instead, it evaluated excellent clearance. We did not predefine excellent clearance as an outcome of interest.
Any adverse effects
Three studies did not report adverse effects at all (Faergemann 1986; Fredriksson 1978; Pari 1998).
Altogether, six studies with a combined total of 381 participants reported the occurrence of any adverse effects at four weeks or less of treatment, and there was no statistically significant difference between steroid and azole treatment (RR 1.45, 95% CI 0.74 to 2.85) (Analysis 3.9). Adverse effects most often reported were dryness of skin, burning, and dandruff. Dryness of skin was more often associated with steroid treatment than with azole treatment.
3.9. Analysis.
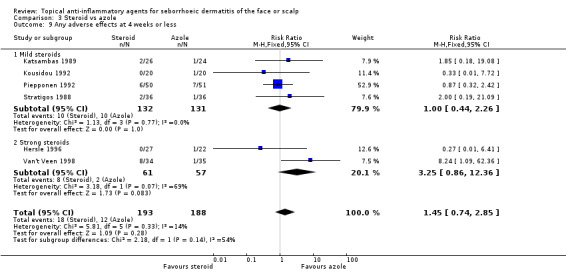
Comparison 3 Steroid vs azole, Outcome 9 Any adverse effects at 4 weeks or less.
In the long‐term studies (four weeks or more of treatment) comparing steroid and azole treatment, strong steroids seemed to produce adverse effects more often than azoles (RR 3.20, 95% CI 1.34 to 7.65, one study with 62 participants), whereas there were no statistically significant differences between mild steroid and azole treatment in one study with 43 participants (RR 0.48, 95% CI 0.22 to 1.04) (Analysis 3.10).
3.10. Analysis.
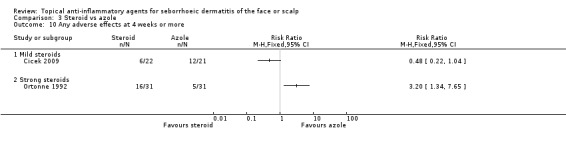
Comparison 3 Steroid vs azole, Outcome 10 Any adverse effects at 4 weeks or more.
Mild steroids versus strong steroids
We compared mild steroids (class I and II steroids) with strong steroids (class III or IV) in three studies. In general, there were no differences between mild and strong steroids with regard to the assessed outcomes including adverse effects.
Total clearance (at four weeks or less of treatment)
Two studies lasting four weeks or less, with 93 participants, assessed total clearance. We found that there were no statistically significant differences between mild or strong steroids whether total clearance was evaluated by the investigator (RR 0.96, 95% CI 0.65 to 1.40) (Analysis 4.1; Table 3) or by the participant (one study, 29 participants) (RR 1.03, 95% CI 0.65 to 1.61) (Analysis 4.2). We rated the quality of the evidence as moderate.
4.1. Analysis.
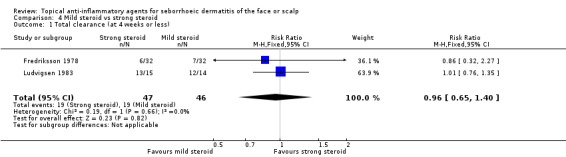
Comparison 4 Mild steroid vs strong steroid, Outcome 1 Total clearance (at 4 weeks or less).
4.2. Analysis.
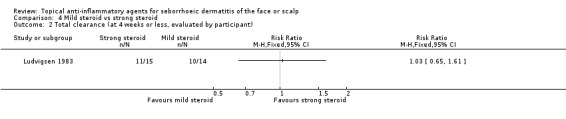
Comparison 4 Mild steroid vs strong steroid, Outcome 2 Total clearance (at 4 weeks or less, evaluated by participant).
Total clearance (at more than four weeks of treatment)
One study with 117 participants assessed total clearance at four weeks or more. In this study, we found a mild steroid to be more effective than a strong steroid (RR 0.79, 95% CI 0.63 to 0.98) (NNT 6, 95% CI 3 to 59) (Analysis 4.3). We rated the quality of the evidence as low (Table 4).
4.3. Analysis.
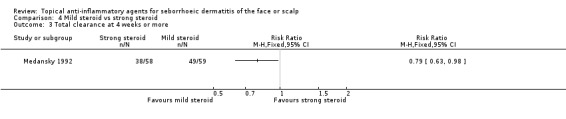
Comparison 4 Mild steroid vs strong steroid, Outcome 3 Total clearance at 4 weeks or more.
Erythema (at four weeks or less of treatment)
Two studies with a combined total of 55 participants assessed the effect of mild or strong steroids on erythema evaluated with erythema scores. We found that there was no statistically significant difference between mild and strong steroids (MD 0.10, 95% CI ‐0.34 to 0.54, one study, 35 participants) (Analysis 4.4). Another trial (Ludvigsen 1983) with 20 participants supported this finding. We could not use the results of the latter trial in the analysis because of a lack of statistical data.
4.4. Analysis.
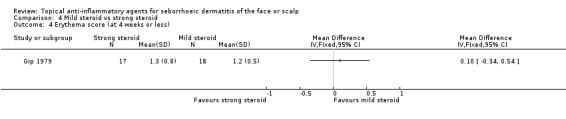
Comparison 4 Mild steroid vs strong steroid, Outcome 4 Erythema score (at 4 weeks or less).
Scaling (at four weeks or less of treatment)
Two studies with a combined total of 63 participants assessed the effect of mild or strong steroids on scaling evaluated with scaling scores, and we found that there was no statistically significant difference (SMD ‐0.05, 95% CI ‐0.55 to 0.45) (Analysis 4.5).
4.5. Analysis.
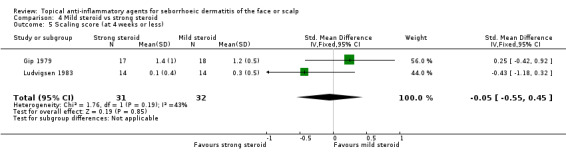
Comparison 4 Mild steroid vs strong steroid, Outcome 5 Scaling score (at 4 weeks or less).
Pruritus (at four weeks or less of treatment)
Three studies with a combined total of 114 participants assessed the effect of mild or strong steroids on pruritus evaluated with pruritus scores, and we found that there was no statistically significant difference (SMD 0.13, 95% CI ‐0.24 to 0.50) (Analysis 4.6).
4.6. Analysis.
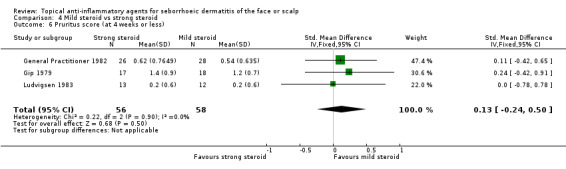
Comparison 4 Mild steroid vs strong steroid, Outcome 6 Pruritus score (at 4 weeks or less).
Any adverse effects
When used in the short‐term, there was no statistically significant difference between mild and strong steroids with regard to rate of adverse effects (RR 1.37, 95% CI 0.32 to 5.93) in three studies with a combined total of 118 participants (Analysis 4.7), and in long‐term use, the finding was similar (RR 5.90, 95% CI 0.73 to 47.49) in one study with 117 participants (Analysis 4.8). The reported adverse effects were scalp dryness or appearance of papules or other kinds of rash.
4.7. Analysis.
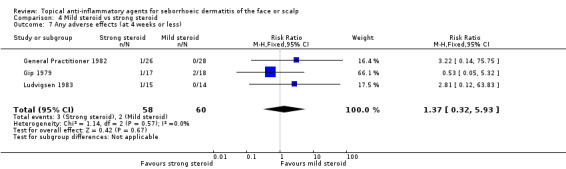
Comparison 4 Mild steroid vs strong steroid, Outcome 7 Any adverse effects (at 4 weeks or less).
4.8. Analysis.
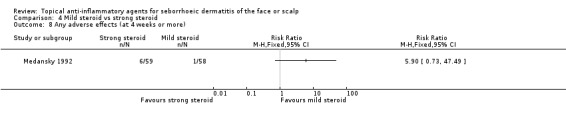
Comparison 4 Mild steroid vs strong steroid, Outcome 8 Any adverse effects (at 4 weeks or more).
Other comparisons for steroids
There were two additional studies that compared a mild steroid with another mild steroid (Cornell 1986; Cornell 1993), and one study compared a strong steroid with another strong steroid (Cornell 1989). We did not perform analyses on these studies as we were focusing on differences between different classes of drugs.
One study with 56 participants compared a strong steroid (betamethasone) with zinc pyrithione, and we found no statistically significant differences in their effect on scaling (MD ‐0.40, 95% CI ‐0.92 to 0.12) (Analysis 5.1), but this study (Shin 2009) did not report adverse effects.
5.1. Analysis.

Comparison 5 Steroid vs zinc pyrithione, Outcome 1 Scaling score (< 4 weeks).
One study with 77 participants compared a mild steroid (desonide) with non‐steroidal cream, Promiseb® (Elewski 2009a). There was no statistically significant difference in the effect on total clearance (RR 1.83, 95% CI 0.88 to 3.80) (Analysis 6.1). In the same study, there were no statistically significant differences between the two drugs with regard to their ability to reduce erythema, scaling, or pruritus or to produce adverse effects.
6.1. Analysis.
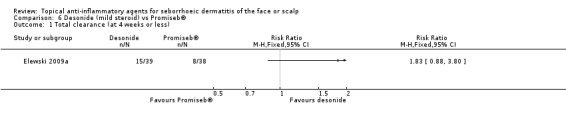
Comparison 6 Desonide (mild steroid) vs Promiseb®, Outcome 1 Total clearance (at 4 weeks or less).
One study with 60 participants compared steroid with calcipotriol (vitamin D compound) (Basak 2001). In this study, steroid proved to be more effective in accomplishing total clearance when compared with calcipotriol (RR 2.86, 95% CI 1.42 to 5.73) (Analysis 7.1). Furthermore, the incidence of adverse effects was lower with steroid treatment than with calcipotriol treatment (RR 0.12, 95% CI 0.03 to 0.47) (Analysis 7.2).
7.1. Analysis.
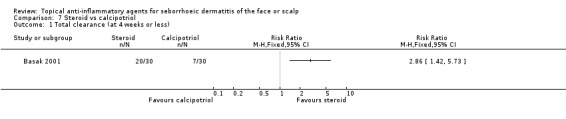
Comparison 7 Steroid vs calcipotriol, Outcome 1 Total clearance (at 4 weeks or less).
7.2. Analysis.
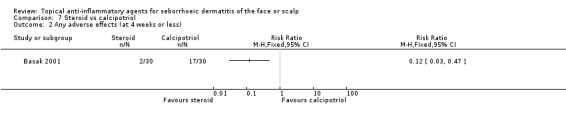
Comparison 7 Steroid vs calcipotriol, Outcome 2 Any adverse effects (at 4 weeks or less).
Calcineurin inhibitors versus comparators
We identified four studies comparing calcineurin inhibitors to steroids as described above. Of these, one study compared calcineurin inhibitor with azole and steroid, one compared calcineurin inhibitor with steroid and zinc pyrithione, one compared calcineurin inhibitor with placebo, and one compared calcineurin inhibitor with azole only.
Calcineurin inhibitors versus placebo
One study with 96 participants found calcineurin inhibitors to be more effective in reducing erythema and scaling when compared with placebo. There were no differences in total clearance or adverse effects between calcineurin inhibitors and placebo.
Total clearance (at four weeks or less of treatment)
We identified only one study (96 participants) assessing the effect of calcineurin inhibitors on total clearance when compared with placebo; there was no statistically significant difference in the effect on total clearance (RR 1.41, 95% CI 0.81 to 2.48) (Analysis 8.1).
8.1. Analysis.
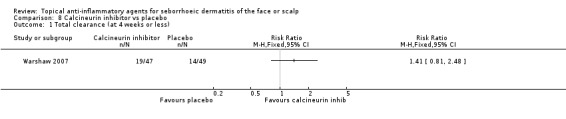
Comparison 8 Calcineurin inhibitor vs placebo, Outcome 1 Total clearance (at 4 weeks or less).
Erythema (at four weeks or less of treatment)
This study (results available for 86 participants) compared the effect of calcineurin inhibitors on erythema with placebo, and we found that calcineurin inhibitor was more effective in reducing erythema when evaluated by mean change in erythema scores (MD 0.40, 95% CI 0.06 to 0.74) (Analysis 8.2).
8.2. Analysis.
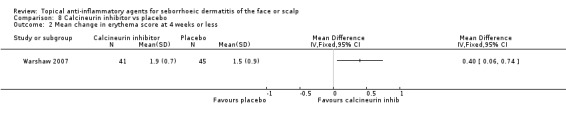
Comparison 8 Calcineurin inhibitor vs placebo, Outcome 2 Mean change in erythema score at 4 weeks or less.
Scaling (at four weeks or less of treatment)
This study (results available for 86 participants) compared the effect of calcineurin inhibitor on scaling with placebo. We found that calcineurin inhibitor was more effective in reducing scaling as evaluated by mean change in scaling scores (MD 0.30, 95% CI 0.00 to 0.60) (Analysis 8.3).
8.3. Analysis.
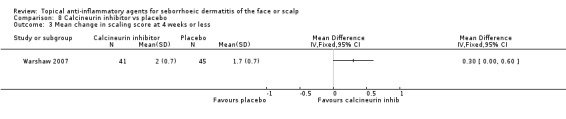
Comparison 8 Calcineurin inhibitor vs placebo, Outcome 3 Mean change in scaling score at 4 weeks or less.
Any adverse effects
In this study (results available for 86 participants), the proportion of participants experiencing adverse effects (at four weeks or less of treatment) was not statistically significantly different between the calcineurin inhibitor group and the placebo group (RR 1.43, 95% CI 0.87 to 2.37) (Analysis 8.4). The nature of these adverse events was not reported.
8.4. Analysis.
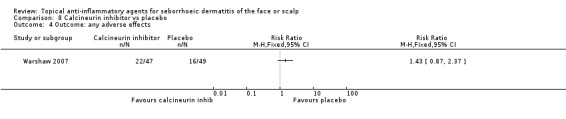
Comparison 8 Calcineurin inhibitor vs placebo, Outcome 4 Outcome: any adverse effects.
Calcineurin inhibitors versus azoles
We identified two studies with a combined total of 90 participants assessing the effect of calcineurin inhibitors when compared with azoles. Of these, in one study, there were data concerning adverse effects relevant for this review. These two studies did not assess total clearance. With regard to efficacy outcomes, we identified no statistically significant differences between calcineurin inhibitors and azoles. Evidence concerning adverse effects was not conclusive.
Erythema (at four weeks or more of treatment)
In one study with 38 participants, we found no statistically significant differences between a calcineurin inhibitor and an azole in their effect on erythema evaluated with erythema scores at the end of treatment (MD 0.17, 95% CI ‐0.24 to 0.58) (Analysis 9.1).
9.1. Analysis.
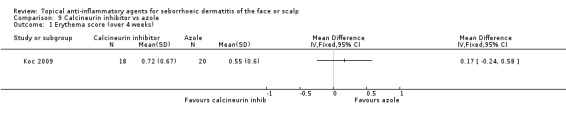
Comparison 9 Calcineurin inhibitor vs azole, Outcome 1 Erythema score (over 4 weeks).
Scaling (at four weeks or more of treatment)
In the same study, we found the calcineurin inhibitor to be comparable to azole treatment in its effect on scaling evaluated with scaling scores at the end of treatment (MD ‐0.02, 95% CI ‐0.33 to 0.29) (Analysis 9.2).
9.2. Analysis.
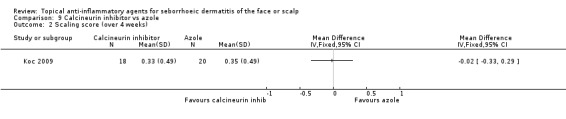
Comparison 9 Calcineurin inhibitor vs azole, Outcome 2 Scaling score (over 4 weeks).
Any adverse effects
Two studies with a combined total of 90 participants addressed the incidence of adverse effects (at four weeks or more of treatment) (Analysis 9.3). Their results were conflicting with a heterogeneity (I² statistic) of over 80%; therefore, we did not use their results in a meta‐analysis. In a study with 42 participants, there were no statistically significant differences in adverse effect rate between calcineurin inhibitor treatment and azole treatment (Cicek 2009). In another trial with 58 participants, there were more adverse events in calcineurin inhibitor treatment when compared with azole treatment (Koc 2009). The trial reported burning, pruritus, and irritation as adverse effects.
9.3. Analysis.
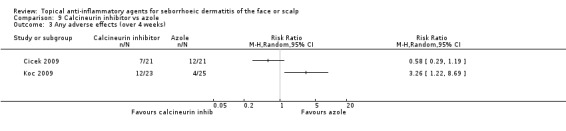
Comparison 9 Calcineurin inhibitor vs azole, Outcome 3 Any adverse effects (over 4 weeks).
Calcineurin inhibitors versus zinc pyrithione
One study compared calcineurin inhibitor (tacrolimus) with zinc pyrithione. It also included a comparison with a steroid. We found that when compared with zinc pyrithione, calcineurin inhibitor was more effective in reducing the dandruff scores (MD ‐0.60, 95% CI ‐1.01 to ‐0.19) (Analysis 10.1). The study did not report adverse effects in sufficient detail (Shin 2009).
10.1. Analysis.
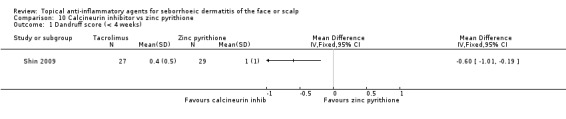
Comparison 10 Calcineurin inhibitor vs zinc pyrithione, Outcome 1 Dandruff score (< 4 weeks).
Lithium versus comparators
We identified two studies comparing lithium salts with placebo (Dreno 2002a; Langtry 1997) and one study comparing lithium salt with azole treatment (Dreno 2003). Lithium seemed to be more effective than placebo with regard to total clearance, but concerning erythema or scaling, there were no statistically significant differences between lithium and placebo. Lithium was also more effective when compared with azole with regard to total clearance. The differences between lithium and its comparators were ambiguous with regard to adverse effects, which were most often burning, erythema, dryness, and pruritus.
Lithium salts versus placebo
Lithium seems to be more effective when compared with placebo with regard to total clearance, with a comparable safety profile.
Total clearance (at four weeks or more of treatment)
Only one study with 129 participants assessed total clearance. We found that lithium was more effective than placebo (RR 8.59, 95% CI 2.08 to 35.52) (NNT 4, 95% CI 3 to 9) (Analysis 11.1).
11.1. Analysis.
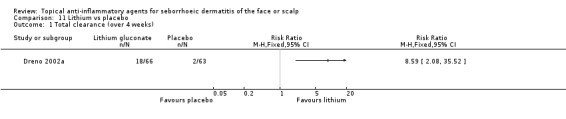
Comparison 11 Lithium vs placebo, Outcome 1 Total clearance (over 4 weeks).
Erythema (at four weeks or less of treatment)
One study with 12 participants assessed the effect of lithium salts on erythema, comparing it to placebo (Langtry 1997). This study was a body‐part study on HIV‐positive male participants. At two weeks of treatment, It was found that there were no statistically significant differences in the percentage change in erythema scores between the lithium compound (30.7% of the baseline value) and placebo treatment (47.1% of the baseline value) (P = 0.055). However, at this point, 50% of the participants had already dropped out.
Scaling (at four weeks or less of treatment)
One study assessed the effect of lithium salts on scaling, comparing it to placebo. This study was a body‐part randomisation study on 12 HIV‐positive male participants (Langtry 1997). At two weeks of treatment, it was found that there were no statistically significant differences in the percentage change in erythema scores between the lithium compound (19.5% of the baseline value) and the placebo treatment (33.8% of the baseline value) (P = 0.76). However, at this point, 50% of the participants had already dropped out.
Any adverse effects
In one study lasting for eight weeks, there was no statistically significant difference between lithium and placebo in the occurrence of adverse effects (RR 0.72, 95% CI 0.31 to 1.66, 123 participants) (Analysis 11.2). In this study, the adverse effects reported most often were burning, erythema, and pruritus. The report of the other study (Langtry 1997) comparing lithium to placebo was imprecise regarding adverse effects.
11.2. Analysis.
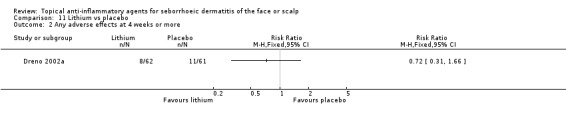
Comparison 11 Lithium vs placebo, Outcome 2 Any adverse effects at 4 weeks or more.
Lithium salts versus azoles
Total clearance (at four weeks or less of treatment)
One study with 288 participants compared the effect of lithium salt with azole on total clearance of SeD. In this study, we found that lithium salt was more effective (RR 1.79, 95% CI 1.10 to 2.90) (Analysis 12.1) in terms of short‐term results (four weeks). We rated the quality of the evidence as low (Table 4).
12.1. Analysis.
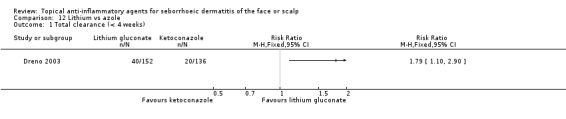
Comparison 12 Lithium vs azole, Outcome 1 Total clearance (< 4 weeks).
Total clearance (at four weeks or more of treatment)
The results were similar at eight weeks (RR 1.79, 95% CI 1.32 to 2.43) (Analysis 12.2).
12.2. Analysis.
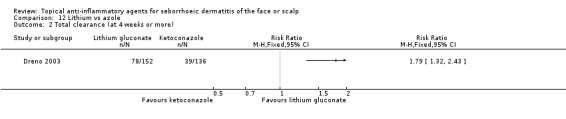
Comparison 12 Lithium vs azole, Outcome 2 Total clearance (at 4 weeks or more).
Any adverse effects
This study reported adverse events in 26% of participants using topical lithium and in 25% of participants using topical azole treatment. Most commonly reported adverse events included erythema, burning, and dryness.
Discussion
Summary of main results
We located 36 studies, of which 31 studies examined steroid as one intervention; seven examined calcineurin inhibitor as one intervention; and three examined lithium as one intervention.
Based on four studies, steroids increased the total clearance of all symptoms when compared with placebo, but with a similar safety profile both at four weeks and 12 weeks of follow‐up. Steroids and calcineurin inhibitors had similar effects, but there were more often adverse effects with calcineurin inhibitor treatment than with steroid treatment. Compared with azoles, the effect varied between different outcomes. There were no differences between azole and steroid treatment regarding short‐term total clearance. The effect of azoles on erythema, scaling, and pruritus was weaker than that of strong steroids. The rate of adverse effects was similar, at least in short‐term use. In general, for short‐term use, there were no differences between mild and strong steroids for outcomes including adverse effects.
Calcineurin inhibitors were more effective in reducing erythema and scaling when compared with placebo. Calcineurin inhibitors and azoles had similar effects and a similar rate of adverse effects.
Lithium was more effective than placebo with regard to total clearance, but there were no differences in erythema or scaling. The safety of these two was comparable. Lithium was also more effective than azoles with regard to total clearance with a similar safety profile.
The median rate of adverse effects was 7% in active treatment groups. Across treatments, the most commonly reported adverse effects were burning, itching, erythema, and dandruff. Some of these symptoms are similar to the symptoms of seborrhoeic dermatitis itself.
Overall completeness and applicability of evidence
We performed the literature searches without language restrictions up until September 2013. These searches provide assurance that we located the majority of studies on topical anti‐inflammatory treatments for seborrhoeic dermatitis. Most studies included both men and women, and the age range was wide. Therefore, we consider the results to be applicable to both adult men and women. However, as pregnancy was a widely used exclusion criterion, it is unclear whether those who have seborrhoeic dermatitis when they are pregnant should use anti‐inflammatory treatments. The studies rarely reported compliance rates. There were also multiple modes of delivering the interventions, which may account for some variation in the results. The trials covered several races including Caucasian and Asian participants, but not many people of African origin. This is important as seborrhoeic dermatitis may have a different pattern of symptoms in those with dark skin.
We only included trials investigating face or scalp involvement, and therefore the results of this review may not be applicable to people with seborrhoeic dermatitis affecting other parts of the body. It is also questionable whether the results can be generalised to people with dandruff but without the diagnosis of seborrhoeic dermatitis, as in most of the trials, the diagnosis of seborrhoeic dermatitis was an inclusion criteria. The available evidence does not allow us to determine whether there are differences in the effects of the assessed agents in different areas of the body or to make comparisons between the treatments in this regard.
The overwhelming majority of the trials were of short duration, whereas the disease itself is chronic in nature. Relapses often occur, sometimes triggered by environmental or individual stimuli. The available evidence does not cover the treatment effects (including side‐effects) of repeated, long‐term, or continuous use of anti‐inflammatory agents. Seborrhoeic dermatitis cannot be cured, but the symptoms can be relieved.
This review did not include combination treatments in which an anti‐inflammatory agent had been combined with an agent having another mechanism of action, such as an antimycotic or antiproliferative effect. There are also other topical treatments that may have anti‐inflammatory effects (such as coal tar, selenium sulfide, and zinc). We did not include these treatments in the review however as their suggested modes of action have been classified as unclear or included additional mechanisms, such as antiproliferative, bacteriostatic, or fungistatic properties.
Quality of the evidence
For the main outcome (total clearance) and for the main comparisons, we assessed the quality of the evidence using the Grade Profiler software (Table 1; Table 2; Table 4). For this outcome, the quality of the evidence was low to moderate.
For other outcomes, we also considered the quality of evidence as low to moderate on the basis of small sample sizes; short follow up; limitations in study design and reporting; and uncertainties in, or lack of blinding of, the participants, caregivers, or outcome assessors. In general, the level of evidence remains limited, and it is possible that further research may change the effect estimates substantially. The pharmaceutical industry sponsored most (21 trials, 58%) of the trials, or the study authors had considerable affiliations.
Potential biases in the review process
Two independent assessors assured the eligibility of the identified articles based on title or abstract. If either author regarded an article as possibly relevant, they retained the article for full‐text assignment. Two independent authors also assessed the eligibility of the identified articles based on full text. We consider that the literature search was adequate and comprehensive.
We excluded reports that did not contain enough data (e.g. posters) if they gave results in a form not eligible for the purposes of the review (e.g. symptom scores were given as pooled, not separate, for each symptom), or if they only stated outcomes not relevant for this review (e.g. microbial growth indicators). We considered that this approach did not introduce bias.
If there were insufficient data in the included reports, we excluded the studies from the meta‐analyses. Nevertheless, we described the results qualitatively when the reports used prespecified outcomes.
Agreements and disagreements with other studies or reviews
We identified a recently published systematic review on pimecrolimus cream for the treatment of seborrhoeic dermatitis (Ang‐Tiu 2012). We have considered all the trials included in that review (Cicek 2009; Firooz 2006; Koc 2009; Warshaw 2007) in our Cochrane review as well. The conclusions of the authors concerning clinical efficacy are consistent with our conclusions.
Authors' conclusions
Implications for practice.
Topical steroids and lithium salts are more effective than placebo in achieving total clearance in seborrhoeic dermatitis of the face or scalp. Calcineurin inhibitors show benefit over placebo in reducing erythema and scaling. Azoles may be comparable to steroids in achieving total clearance, but there are implications that strong steroids are more efficient with symptoms like erythema, scaling, and pruritus. Furthermore, adverse effects occur at a similar rate at four weeks' follow‐up. Calcineurin inhibitors seem to be comparable with azoles and steroids concerning efficacy. Lithium is more effective than azole with regard to total clearance. Mild and strong steroids seemed to be comparable with regard to efficacy and adverse effects in up to six weeks' follow‐up. However, there are no data regarding the efficacy or safety of repeated, long‐term (such as more than one year), or continuous use of any of the assessed medicines.
The median rate of achieving total clearance was 53% with anti‐inflammatory treatments across studies. This is an indication of the need for further research to identify optimal treatment agents, possibly treatment combinations, regimens, and length.
Implications for research.
To prevent reporting bias, authors should first publish a protocol of their study and register this in a trials registration database. To further increase the quality of evidence of topical anti‐inflammatory treatments for seborrhoeic dermatitis, future trials should deal with the following issues.
Quality of methods: Trials should properly conduct and report random sequence allocation, as well as allocation concealment and the method of blinding.
Quality of reporting: Trials should report results in numbers, preferably in tables, instead of graphs, as well as reporting standard deviations and exact P values.
Outcomes: There is an urgent need for one or more validated outcome measures for seborrhoeic dermatitis that should at least cover erythema, scaling, pruritis, and the area of the body and the amount of skin affected. Trials should also examine patient‐centered outcomes, such as quality‐adjusted life measures, as well as compliance. Trials should measure outcomes at long‐term follow up, such as one year after starting treatment, in order to assess the relapse rate or the efficacy in long‐term use. For adverse effects, we need measurements at several years of follow up.
Economic evaluations: Trials should put the therapeutic value of a treatment into context with its economic value in order to be able to use treatments rationally.
What's new
| Date | Event | Description |
|---|---|---|
| 22 August 2017 | Amended | Typos corrected (two instances of vitamin C should be vitamin D) |
History
Protocol first published: Issue 11, 2011 Review first published: Issue 5, 2014
| Date | Event | Description |
|---|---|---|
| 9 July 2015 | Amended | A search of MEDLINE and Embase in June 2015 found studies that were, in the main, looking at single interventions, which are unlikely to alter the overall conclusion. There are a few small studies on sertaconazole, but not quite enough to merit an update, so an update has not been considered necessary at this time. Our Trials Search Co‐ordinator will run a new search in summer 2016 to re‐assess whether an update is needed. |
Notes
A search of MEDLINE and Embase in June 2015 found studies that were, in the main, looking at single interventions, which are unlikely to alter the overall conclusion. There are a few small studies on sertaconazole, but not quite enough to merit an update, so an update has not been considered necessary at this time. Our Trials Search Co‐ordinator will run a new search in summer 2016 to re‐assess whether an update is needed.
Acknowledgements
We would like to thank the Cochrane Occupational Safety and Health Review Group for their training and support.
The Cochrane Skin Group editorial base wishes to thank Hywel Williams who was the Cochrane Dermatology Editor for this review; Matthew Grainge and Ching‐Chi Chi who were the Statistical and Methods Editors, respectively; the clinical referees, Charlene U Ang‐Tiu and Ana Luisa Sampaio; and the consumer referee, Jack Tweed.
We are grateful to Mr Jong‐Myon Bae, MD, PhD, and Miss Quan Yang, PhD, for their help in translating articles.
Appendices
Appendix 1. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Malassezia] this term only #2 ("scalp dermatoses" or "scalp dermatosis" or "scalp dermatitis" or "scalp eczema"):ti,ab,kw #3 ("seborrheic dermatitis" or "seborrhoeic dermatitis" or malassezia or "cradle cap" or dandruff or "seborrheic eczema" or "seborrhoeic eczema"):ti,ab,kw #4 MeSH descriptor: [Dermatitis, Seborrheic] this term only #5 MeSH descriptor: [Scalp Dermatoses] this term only #6 #1 or #2 or #3 or #4 or #5
Appendix 2. MEDLINE (OVID) search strategy
1. exp Dermatitis, Seborrheic/ 2. seborrh$ dermatitis.mp. 3. scalp dermatos$.mp. 4. exp Scalp Dermatoses/ 5. scalp dermatitis.mp. 6. scalp eczema.mp. 7. dandruff.mp. 8. Malassezia.mp. or exp Malassezia/ 9. cradle cap.mp. 10. seborrh$ eczema.mp. 11. or/1‐10 12. randomized controlled trial.pt. 13. controlled clinical trial.pt. 14. randomized.ab. 15. placebo.ab. 16. clinical trials as topic.sh. 17. randomly.ab. 18. trial.ti. 19. 12 or 13 or 14 or 15 or 16 or 17 or 18 20. exp animals/ not humans.sh. 21. 19 not 20 22. 11 and 21
Appendix 3. Embase (OVID) search strategy
1. random$.mp. 2. factorial$.mp. 3. (crossover$ or cross‐over$).mp. 4. placebo$.mp. or PLACEBO/ 5. (doubl$ adj blind$).mp. 6. (singl$ adj blind$).mp. 7. (assign$ or allocat$).mp. 8. volunteer$.mp. or VOLUNTEER/ 9. Crossover Procedure/ 10. Double Blind Procedure/ 11. Randomized Controlled Trial/ 12. Single Blind Procedure/ 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. Seborrh$ dermatitis.ti,ab. 15. scalp dermatitis.ti,ab. 16. scalp eczema.ti,ab. 17. cradle cap.ti,ab. 18. exp *dandruff/ 19. exp *Malassezia/ 20. dandruff.ti,ab. 21. malassezia.ti,ab. 22. exp *seborrheic dermatitis/ 23. scalp dermatos$.ti,ab. 24. seborrh$ eczema.ti,ab. 25. or/14‐24 26. 13 and 25
Appendix 4. LILACS search strategy
((Pt RANDOMIZED CONTROLLED TRIAL OR Pt CONTROLLED CLINICAL TRIAL OR Mh RANDOMIZED CONTROLLED TRIALS OR Mh RANDOM ALLOCATION OR Mh DOUBLE‐BLIND METHOD OR Mh SINGLE‐BLIND METHOD OR Pt MULTICENTER STUDY) OR ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((CT ANIMALS OR MH ANIMALS OR CT RABBITS OR CT MICE OR MH RATS OR MH PRIMATES OR MH DOGS OR MH RABBITS OR MH SWINE) AND NOT (CT HUMAN AND CT ANIMALS)) [Words] and “seborrh$ dermatitis” or seborreico or dandruff or caspa or “cradle cap” or “costra lactea” or malassezia or “scalp dermatos$” or “eczema seborreico” or “dermatitis seborreica” [Words]
Data and analyses
Comparison 1. Steroid vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total clearance (at 4 weeks or less) | 3 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 3.76 [1.22, 11.56] |
| 1.1 Mild steroids | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.29, 8.53] |
| 1.2 Strong steroids | 2 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 5.92 [0.99, 35.52] |
| 2 Total clearance (over 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Strong steroids | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean change in erythema score (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Strong steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Erythema score (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Strong steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Mean change in scaling score (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Strong steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Scaling scores (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Strong steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Mean change in pruritus score (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Strong steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Pruritus scores (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Strong steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Any adverse effect (at 4 weeks or less) | 3 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.29, 2.72] |
| 9.1 Mild steroids | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.11] |
| 9.2 Strong steroids | 2 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.31, 3.58] |
Comparison 2. Steroid vs calcineurin inhibitor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total clearance (at 4 weeks or less) | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.32] |
| 1.1 Mild steroids | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.83, 1.55] |
| 1.2 Strong steroids | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.83, 1.20] |
| 2 Erythema score (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Mild steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Scaling score (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mild steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mean change in dandruff score (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Strong steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Any adverse effects at 4 weeks or less | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.89] |
| 5.1 Mild steroids | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.06] |
| 5.2 Strong steroids | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.05, 3.28] |
| 6 Any adverse effects (at 4 weeks or more) | 2 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.26, 1.47] |
| 6.1 Mild steroids | 2 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.26, 1.47] |
Comparison 3. Steroid vs azole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total clearance (at 4 weeks or less) | 8 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.94, 1.32] |
| 1.1 Mild steroids | 5 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.28] |
| 1.2 Strong steroids | 3 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.88, 1.90] |
| 2 Total clearance (at 4 weeks or less, evaluated by participant) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Mild steroids | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Strong steroids | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Erythema score (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Strong steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mean change in erythema score at 4 weeks or less | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Mild steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Scaling score (at 4 weeks or less) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Strong steroids | 2 | 118 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐2.72 [‐3.24, ‐2.21] |
| 6 Mean change in scaling score at 4 weeks or less | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Mild steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Pruritus score (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Mild steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Mean change in pruritus score at 4 weeks or less | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Mild steroids | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Any adverse effects at 4 weeks or less | 6 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.74, 2.85] |
| 9.1 Mild steroids | 4 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.44, 2.26] |
| 9.2 Strong steroids | 2 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [0.86, 12.36] |
| 10 Any adverse effects at 4 weeks or more | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Mild steroids | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Strong steroids | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Mild steroid vs strong steroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total clearance (at 4 weeks or less) | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.40] |
| 2 Total clearance (at 4 weeks or less, evaluated by participant) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Total clearance at 4 weeks or more | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Erythema score (at 4 weeks or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Scaling score (at 4 weeks or less) | 2 | 63 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.55, 0.45] |
| 6 Pruritus score (at 4 weeks or less) | 3 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.24, 0.50] |
| 7 Any adverse effects (at 4 weeks or less) | 3 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.32, 5.93] |
| 8 Any adverse effects (at 4 weeks or more) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Steroid vs zinc pyrithione.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Scaling score (< 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Desonide (mild steroid) vs Promiseb®.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total clearance (at 4 weeks or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Steroid vs calcipotriol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total clearance (at 4 weeks or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Any adverse effects (at 4 weeks or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Calcineurin inhibitor vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total clearance (at 4 weeks or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mean change in erythema score at 4 weeks or less | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in scaling score at 4 weeks or less | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Outcome: any adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. Calcineurin inhibitor vs azole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Erythema score (over 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Scaling score (over 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Any adverse effects (over 4 weeks) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Calcineurin inhibitor vs zinc pyrithione.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dandruff score (< 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 11. Lithium vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total clearance (over 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Any adverse effects at 4 weeks or more | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 12. Lithium vs azole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total clearance (< 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Total clearance (at 4 weeks or more) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Attila 1992.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 72 in the whole study. We only included participants in the hydrocortisone group (N = 23) and in the placebo group (N = 24) in this Cochrane review Number of dropouts: 8 in the whole study (not reported separately in different intervention groups) Sex: 49 male, 23 female Mean age (range): 35 (14 to 79) years Country: Finland |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | Standard deviations were not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Similarity of the study groups (selection bias) | Unclear risk | Quote: "There were no significant differences between the treatment groups" Differences in the severity of symptoms was not reported |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate was not reported separately for the intervention groups |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes as stated in the article were reported |
| Other bias | Unclear risk | 2 authors were affiliated to the pharmaceutical industry |
Basak 2001.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: not reported Intention‐to‐treat analysis used: not reported |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 60 in total (betamethasone N = 30, calcipotriol N = 30) Number of dropouts: 7, all from 1 treatment arm (23%) Sex: not reported Age: not reported Country: Turkey |
|
| Interventions |
Treatment
Comparator/s
If there was only slight improvement at the end of 4 weeks, treatment was continued for another 4‐week period After cessation of treatment, participants entered a follow‐up period for 4 weeks Before the start of treatment, there was a 1‐week wash‐out period, during which only a mild non‐medicated shampoo was used |
|
| Outcomes |
|
|
| Notes | Results were reported mainly at 4 weeks. The dropout rate was unbalanced and considerable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was not reported in detail Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Unclear risk | This was not reported in sufficient detail |
| Blinding of participants (performance bias) | High risk | There was no mention of blinding in the report |
| Blinding of care providers (performance bias) | High risk | There was no mention of blinding in the report |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no mention of blinding in the report |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 20% of participants withdrew from the calcipotriol arm |
| Selective reporting (reporting bias) | Unclear risk | Not all results related to predefined outcomes were reported in sufficient detail |
| Other bias | Unclear risk | No other bias was identified |
Cicek 2009.
| Methods | Study type: individual RCT Randomisation method: not reported sufficiently Blinding: not reported Intention‐to‐treat analysis used: not fully |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 64 in total (pimecrolimus N = 21, methylprednisolone N = 22, metronidazole N = 21) Number of dropouts: 4 (6%) Sex: 32 males, 32 females Mean age (range): pimecrolimus arm = 31.6 (19 to 56) years, methylprednisolone arm = 34.2 (17 to 65) years, metronidazole arm = 30.7 (17 to 44) years Country: Turkey |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | Erythema and pruritus were used to assess both efficacy and side‐effects. It was not reported how the judgement between a lack of efficacy and a side‐effect was done. Erythema, scaling, and pruritus scores were not reported separately but as part of mean clinical severity score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were divided into three randomized groups" |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Similarity of the study groups (selection bias) | Low risk | Differences were not statistically significant |
| Blinding of participants (performance bias) | High risk | No information was provided |
| Blinding of care providers (performance bias) | High risk | No information was provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate was 20% in 1 group only and reported to be due to side‐effects; these participants were excluded from the study. The side‐effect rate was reported for the remaining participants only |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias was identified |
| Other bias | High risk | Erythema and pruritus were used both as efficacy and adverse effect elements. How the judgement between efficacy effect and adverse effect classification was conducted was not reported |
Cornell 1986.
| Methods | Study type: RCT of body parts Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 51 in total Number of dropouts: 6 (12%) Sex: 23 males, 28 females Age (range): 19 to 82 years Country: USA |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | The location of seborrhoeic dermatitis lesions was not mentioned as inclusion criteria. However, the outcomes were evaluated on the face/neck, retroauricular areas, and scalp. The primary objective of the study was to compare atrophogenic potential of the products, but their efficacy was also evaluated. This is 1 of 2 included studies that compared 2 mild steroids with each other. The results concerning side‐effects are controversial. Score data can not be used because standard deviations and P values are lacking | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Study was a randomized" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Low risk | Pretreatment symptom scores were identical between groups |
| Blinding of participants (performance bias) | Low risk | Double‐blind: Colour‐coded side‐labelled tubes were used |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None were identified |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Cornell 1989.
| Methods | Study type: individual RCT Randomisation method: computer‐generated randomisation Blinding: blinded Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 54 in total (amcinonide N = 26, betamethasone N = 28) Number of dropouts: 0 Sex: 30 males, 24 females Mean age (range): amcinonide arm = 42 (26 to 81), betamethasone arm = 45 (23 to 73) years Country: USA |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | This was the only included study comparing 2 strong steroids with each other. Necessary data were calculated from other statistics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation list was used |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Similarity of the study groups (selection bias) | Low risk | There were no statistically significant differences between groups |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was small |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Cornell 1993.
| Methods | Study type: RCT of body parts Randomisation method: computerised randomisation Blinding: double‐blind Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 30 in total Number of dropouts: 1 (3%) Sex: 14 males, 16 females Age (mean): 37.7 years Country: USA |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | 1 of 2 included studies comparing mild steroids with each other. The adverse events rate was calculated from a table | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation was used |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Similarity of the study groups (selection bias) | Unclear risk | Bilateral disease severity was not reported separately |
| Blinding of participants (performance bias) | Low risk | Colour‐coded side‐labelled tubes were used |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of dropouts was small |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias was identified |
| Other bias | Unclear risk | The pharmaceutical industry supported the study, and the corresponding author was affiliated with the pharmaceutical industry |
Dreno 2002a.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: included all randomised participants who had at least 1 efficacy evaluation after baseline |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 129 in total (lithium N = 66, placebo N = 63; however, there were follow‐up data available only for 63 participants in the lithium group and 61 participants in the placebo group, and 1 participant was left out of the analyses as he did not fulfil the inclusion criteria) Number of dropouts: 22 (17%) Sex: 85 males, 44 females Mean age (range): lithium arm = 38.6 (19 to 69) years, placebo arm = 40.2 (19 to 73) years Country: France |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | For symptoms scores (erythema and desquamation), results were presented only in figures without numerical data. Results concerning burning, pruritus, and stretching, as well as skin oiliness, were mentioned very briefly in the text without numerical or visual data given. For this reason, these data could not be used in the meta‐analysis in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Low risk | There were no statistically significant differences between groups |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether the percentages were calculated using ITT or per‐protocol analysis |
| Selective reporting (reporting bias) | Unclear risk | Most results were not reported in sufficient detail |
| Other bias | Unclear risk | The pharmaceutical industry supported the study |
Dreno 2003.
| Methods | Study type: individual RCT Randomisation method: computer‐generated Blinding: Investigators were blinded until the first follow‐up visit (not reported for participant or care provider) Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 288 in total (lithium N = 152, ketoconazole N = 136) Number of dropouts: 34 withdrawn and 19 excluded from analyses because of major protocol deviation (18%) Sex: 183 males, 105 females Age (mean): lithium arm = 29.2 years, ketoconazole arm = 41.3 years Country: France |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | This was the only included study comparing lithium with azole | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated |
| Allocation concealment (selection bias) | Low risk | This was probably low risk Quote: "computer‐generated blocks and the randomisation code was concealed in sealed envelopes" |
| Similarity of the study groups (selection bias) | Low risk | There were no statistically significant differences between groups |
| Blinding of participants (performance bias) | High risk | Participants were not blinded |
| Blinding of care providers (performance bias) | Unclear risk | This was not reported in detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was not reported in detail. The investigators were blinded at the allocation period |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of dropouts was less than 20% and comparable between groups |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias was identified |
| Other bias | Unclear risk | The pharmaceutical industry organised and sponsored the study |
Elewski 2009a.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: investigator‐blinded Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 77 in total (desonide N = 39, Promiseb® N = 38) Number of dropouts: 5 (7%) Sex: 56 males, 21 females Mean age (range): 51.9 (21.1 to 84.5) years Country: USA |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | No standard deviations were given for symptom scores. Additional data were requested (results in numerical form with standard deviations and P values), but could not be received. This was the only included study comparing steroid with Promiseb® topical cream | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Similarity of the study groups (selection bias) | Unclear risk | This was not reported sufficiently |
| Blinding of participants (performance bias) | High risk | The study was "investigator blind" |
| Blinding of care providers (performance bias) | Unclear risk | The study was "investigator blind". It was not clear whether the care providers were investigators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigator was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of dropouts was small |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias was identified |
| Other bias | Unclear risk | The pharmaceutical industry supported the study, and the author was affiliated to the pharmaceutical industry. The pharmaceutical industry provided the comparator treatment |
Faergemann 1986.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: not reported |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 70 participants in the whole study. In this review, we only considered the participants in the hydrocortisone (N = 24) and miconazole (N = 23) arms Number of dropouts: 3 (5%) Sex: 36 males, 34 females Mean age (range): 38 (21 to 69) years Country: Sweden |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | The combination treatment arm of the trial was not included in this review. Adverse events were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Unclear risk | Baseline data were not reported |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of dropouts was small |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Unclear risk | The pharmaceutical industry provided the intervention solutions |
Firooz 2006.
| Methods | Study type: individual RCT Randomisation method: computer‐generated randomisation list Blinding: investigator‐blind Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 40 in total (pimecrolimus N = 20, hydrocortisone N = 20) Number of dropouts: 3 (8%) Sex: 28 males, 12 females Age (mean): pimecrolimus arm = 28.65 years, hydrocortisone arm = 37.45 years Country: Iran |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Low risk | There were no statistically significant differences between groups |
| Blinding of participants (performance bias) | High risk | Only investigators were blinded |
| Blinding of care providers (performance bias) | Unclear risk | Quote: "investigator blind" This probably refers to outcome assessment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigator was blinded. We assume this refers to outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of dropouts was acceptable |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Unclear risk | The pharmaceutical industry provided ‐ free of charge ‐ the intervention creams |
Fredriksson 1978.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: not clearly reported; at least the participants were blinded Intention‐to‐treat analysis used: no |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 64 in total (betamethasone N = 32, hydrocortisone N = 32) Number of dropouts: 2 (3%) Sex: 35 males, 27 females Age (range): 15 to 61 years Country: Sweden |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | Dosing frequency was not reported. Adverse events were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported in detail |
| Similarity of the study groups (selection bias) | Unclear risk | No information was provided |
| Blinding of participants (performance bias) | Low risk | Quote: "Patients...received the lotion in identical, coded bottles" |
| Blinding of care providers (performance bias) | Unclear risk | Quote: "Patients...received the lotion in identical, coded bottles" This was not reported in sufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Patients...received the lotion in identical, coded bottles" This was not reported in sufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was acceptable |
| Selective reporting (reporting bias) | Low risk | There were limitations in reporting, but results were displayed for all predefined outcomes |
| Other bias | Low risk | No other bias was identified |
General Practitioner 1982.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: no |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 55 in total (hydrocortisone 17‐butyrate N = 28, betamethasone N = 28) Number of dropouts: 1 (2%) Sex: 33 males, 22 females Mean age (range): 40.4 (15 to 75) years Country: United Kingdom |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | Dosing frequency was not reported. The location of the SeD lesions was not mentioned as inclusion criteria. It was however reported that the outcomes were evaluated in the head and neck | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was not reported in detail Quote: "in a random manner" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Unclear risk | Quote: "The two groups matched one another in relation to these various factors, except that there was a higher proportion of male patients in the Betnovate group and minor differences in relation to age, duration and treatment given during the previous two weeks...with the exception of the eyelids, which were relatively more severely involved in the Locoid group than in the Betnovate group" |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was small |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Gip 1979.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: probably yes, as there were no dropouts |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 35 in total (betamethasone N = 17, hydrocortisone 17‐butyrate N = 18) Number of dropouts: 0 Sex: 17 males, 18 females Age (mean): betamethasone arm = 49.9 years, hydrocortisone arm = 46.8 years Country: Sweden |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" The randomisation method was not reported |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Similarity of the study groups (selection bias) | Low risk | Baseline characteristics were comparable |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported in sufficient detail |
| Other bias | Low risk | No other bias was identified |
Harris 1972.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: no |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 391 in total (initial assignment numbers were not reported; at 2 weeks, 140 participants were using betamethasone, and 163 participants were using placebo) Number of dropouts: 88 (23%) Sex: 171 males, 132 females Age: not reported Country: USA |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | Response was regarded as excellent with clearance of 75% or more. This is less than in other included studies, and therefore we excluded the results from the meta‐analysis. For scores, no standard deviations or exact P values were given. The actual number of participants randomised to each group was unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The test preparations were supplied...in identical packages, code labelled for blind randomized assignment to patients...Master codes for each study were maintained separately from the investigators..." |
| Similarity of the study groups (selection bias) | Unclear risk | No information was provided |
| Blinding of participants (performance bias) | Low risk | Quote: "double‐blind" Quote: "Neither patient nor physician was aware of which of the two was being used" |
| Blinding of care providers (performance bias) | Low risk | Quote: "double‐blind" Quote: "Neither patient nor physician was aware of which of the two was being used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" The codes were kept separately from the investigators |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The dropout rate was 23%. The actual number of participants randomised to each group was not reported. There is considerable uncertainty in the reporting of the therapeutic response Quote: "Patients who did not return due to a successful response were included in the excellent group and patients who did not return because of treatment failure were included in the poor group" |
| Selective reporting (reporting bias) | Unclear risk | Predefined outcomes were reported, but not in sufficient detail. For example, the standard deviations were not given |
| Other bias | Unclear risk | The author was affiliated to a pharmaceutical company |
Hersle 1996.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: no |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 54 in total (the initial assignment numbers were not reported by treatment group; at 4 weeks, 27 participants were treated with mometasone, and 22 participants were treated with ketoconazole) Number of dropouts: 5 (9%) Sex: 40 males, 14 females Mean age (range): 58 (22 to 85) years Country: Sweden |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | Participants and investigators evaluated reduction of pruritus, scaling, and erythema scores, but numerical information of these was not reported. We approximated the numbers from figures. The actual number of participants randomised to each group was unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but not reported in sufficient detail |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Similarity of the study groups (selection bias) | Unclear risk | No information was provided |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The actual number of participants randomised to each group was unknown. The dropout rate was 10%, but the study did not report if this was balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | The primary outcomes were not prespecified in detail, yet the outcomes reported were those that are usually used in such studies |
| Other bias | Unclear risk | The pharmaceutical industry supported the study |
Katsambas 1989.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 50 in total (hydrocortisone N = 26, ketoconazole N = 24) Number of dropouts: 0 Sex: not reported Age: not reported Country: Greece |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized fashion" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomized fashion" There was no mention of the allocation concealment |
| Similarity of the study groups (selection bias) | Unclear risk | This was not reported |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Unclear risk | 1 author was affiliated to the pharmaceutical industry |
Koc 2009.
| Methods | Study type: individual RCT Randomisation method: participants were randomised according to a random digit table Blinding: no (open‐label) Intention‐to‐treat analysis used: no |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 48 in total (pimecrolimus N = 23, ketoconazole N = 25) Number of dropouts: 10 (21%) Sex: 34 males, 4 females Mean age (range): pimecrolimus arm = 32.3 (21 to 50), ketoconazole arm = 29.8 (20 to 47) Country: Turkey |
|
| Interventions |
Treatment
Comparator/s
The total follow‐up time was 12 weeks |
|
| Outcomes |
|
|
| Notes | We received additional data from the first author The affected area or site of SeD lesions as inclusion criteria were not reported. However, coexistent dermatoses involving the face or other affected areas were mentioned as exclusion criteria, suggesting that facial SeD was an inclusion criterion |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random digits table" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "random digits table" |
| Similarity of the study groups (selection bias) | Low risk | Quote: "The treatment groups were not statistically significantly different at baseline" |
| Blinding of participants (performance bias) | High risk | This was an open‐label study |
| Blinding of care providers (performance bias) | High risk | This was an open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was an open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The dropout rate over was 20%; reasons were not given in detail. There was discrepancy between text and figure 1 with regard to distribution of dropouts |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes as stated in the article were reported |
| Other bias | Low risk | Note: The affected area was not reported |
Kousidou 1992.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: not reported |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 40 in total (hydrocortisone N = 20, ketoconazole N = 20) Number of dropouts: 1 (3%) Sex: 21 males, 19 females Age (mean): 33.7 years Country: Greece |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | No standard deviations nor exact P values were given in the report. We have approximated the actual numbers from figures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomized" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Low risk | Quote: "At the start of treatment, erythema, scaling, and pruritus were present in all patients of both groups without any statistically significant difference in the mean scores" |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was small |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Unclear risk | 2 authors were affiliated to the pharmaceutical industry |
Langtry 1997.
| Methods | Study type: RCT of body parts Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: not used |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 12 in total Number of dropouts: 6 participants (50%) by 2 weeks; 7 participants by end of study at 8 weeks Sex: only male participants Age: not reported Country: UK |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | 50% of participants had dropped out by 2 weeks, and only 5 participants out of 12 completed the study. However, the last visit occurred at 47 +/‐ 15 days (standard deviation) giving a wide variation there. We decided to use the results obtained at 2 weeks. It was not reported whether the paired t‐test was used, and the available data did not allow recalculations. Therefore, the results have been presented qualitatively. The trial included only men with HIV as participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each was randomly assigned to be applied" |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Similarity of the study groups (selection bias) | Unclear risk | Bilateral disease severity was not reported in detail |
| Blinding of participants (performance bias) | Low risk | Quote: "Both doctor and patient were blinded" |
| Blinding of care providers (performance bias) | Low risk | Quote: "Both doctor and patient were blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both doctor and patient were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The dropout rate was 50% by 2 weeks and 58% by the end of the study at 8 weeks |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Unclear risk | The pharmaceutical industry supported the study. 2 authors were affiliated to the pharmaceutical industry |
Ludvigsen 1983.
| Methods | Study type: individual RCT Randomisation method: computer‐based randomisation Blinding: double‐blind Intention‐to‐treat analysis used: not used |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 30 in total (betamethasone N = 15, hydrocortisone N = 15, but 1 of the participants in the latter group proved to be too young to be included, and the results were not used) Number of dropouts: 1 participant was excluded after randomisation because of their young age (not considered a dropout); 1 participant was lost to follow up (considered a dropout, 3%) Sex: 17 males, 12 females Mean age (range): 49.9 (19 to 82) years Country: Denmark |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | Participants used drugs for 3 weeks or until complete healing. Outcomes were assessed at 3 weeks. Scores were calculated only for those who had the symptom | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer programme was used to give the randomization code, which was stratified into blocks of 10 with a restriction against more than three successive patients receiving the same therapy" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomization code, which was stratified into blocks of 10 with a restriction against more than three successive patients receiving the same therapy" |
| Similarity of the study groups (selection bias) | Low risk | Quote: "No statistically significant difference in patient classification of mean age, sex distribution and initial symptom score distribution was found between the two treatment groups but there was a numerically lower mean age in the HCB‐treated group" 44 years versus 56 years |
| Blinding of participants (performance bias) | Unclear risk | The study was "double‐blind"; this was not reported in detail. The participants were given Locoid® or Diproderm®, and it was not reported if the packages were blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was small |
| Selective reporting (reporting bias) | Unclear risk | Predefined outcomes were reported, but they were assessed as scores only for those who had the symptom |
| Other bias | Low risk | No other bias was identified |
Lynfield 1988.
| Methods | Study type: individual RCT Randomisation method: computer‐based randomisation Blinding: double‐blind Intention‐to‐treat analysis used: not used |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 168 in total (amcinonide N = 86, placebo N = 82) Number of dropouts: 10 (6%) Sex: 121 males, 47 females Mean age (range): amcinonide arm = 51.2 (20 to 88) years, placebo arm = 50.9 (18 to 87) years Country: USA |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | Final evaluations were performed as soon as the participant had cleared completely if this occurred before week 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated randomization list designed to produce approximately equal numbers of patients in each study arm" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported in detail |
| Similarity of the study groups (selection bias) | Low risk | Quote: "There were no statistically significant differences between the two treatment groups for any of the demographic variables...for severity of the objective signs of erythema and scaling and the subjective symptom of pruritus" |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was acceptable |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Medansky 1992.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: "third‐party‐blind"; it is not specified who was blinded Intention‐to‐treat analysis used: not reported, but probably was; no dropouts during the treatment phase |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 117 in total (mometasone N = 59, hydrocortisone N = 58) Number of dropouts: no dropouts during the treatment phase Sex: 68 males, 49 females Mean age (range): mometasone arm = 45 (15 to 70) years, hydrocortisone arm = 43 (13 to 70) years Country: not reported, probably USA |
|
| Interventions |
Treatment
Comparator/s
All antiseborrhoeic agents were prohibited for at least 2 weeks prior to the initiation of treatment, and systemic corticosteroids were prohibited for at least 4 weeks |
|
| Outcomes |
|
|
| Notes | Any medications that might have affected the course of the disease were not allowed during the course of the study. The last evaluation was made at 2 weeks post‐treatment. 1 area on the face of each participant was selected for evaluation of treatment effectiveness | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was not reported in detail Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Low risk | Quote: "The two treatment groups were comparable for all comparisons" |
| Blinding of participants (performance bias) | High risk | Quote: "Third‐party‐blind" ‐ obviously not referring to the participant |
| Blinding of care providers (performance bias) | High risk | Quote: "Third‐party‐blind" ‐ obviously not referring to the care‐giver |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Third‐party‐blind" ‐ possibly referring to the outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | Results concerning predefined outcomes were reported, with the exception of participants' own assessment where the results were not given in a numerical form |
| Other bias | Unclear risk | At least 4 out of 8 authors were affiliated to the pharmaceutical industry |
Ortonne 1992.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: "a single blind" Intention‐to‐treat analysis used: not used |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 62 in total (betamethasone N = 31, ketoconazole N = 31) Number of dropouts: 9 (15%) Sex: 39 males, 23 females Mean age (range): betamethasone arm = 41 (23 to 68) years, ketoconazole arm = 35 (18 to 65) years Country: France |
|
| Interventions |
Treatment
Phase 1: once daily for the first week, every other day in the second week, twice weekly until the end of the first month of treatment Phase 2: once weekly for 3 months Comparator/s
Phase 1: twice weekly for 1 month Phase 2: once weekly for 3 months Phase 3: This was a wash‐out phase for both treatment arms (1 month) |
|
| Outcomes |
|
|
| Notes | Total clearance is not evaluated in the report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was not reported in detail Quote: "randomized fashion" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Low risk | Quote: "The treatment groups were comparable for all the patient characteristics, as well as for all symptoms and localizations" |
| Blinding of participants (performance bias) | High risk | This was a single‐blind study; it did not specify which party was blinded |
| Blinding of care providers (performance bias) | High risk | This was a single‐blind study; it did not specify who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was a single‐blind study; it did not specify who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was acceptable |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Ortonne 2011.
| Methods | Study type: individual RCT Randomisation method: "central computed randomization list, block‐size of 4" Blinding: "blinded investigators" Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 326 in the whole study; we used only the results of the clobetasol group (N = 82) and the ketoconazole group (N = 80) Number of dropouts: 12 (7%) at "end of study" Sex: 88 males, 74 females Age (mean): clobetasol arm = 44.9 years, ketoconazole arm = 44.7 years Country: Belgium, France, Germany, Mexico, South Korea |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | Only the results for the clobetasol propionate only, and the ketoconazole only, and for the treatment phase are used in the analyses The results for the outcomes were expressed in a way not relevant for the review; therefore, we could use only information for adverse events |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized in a 1:1:1:1 ratio by a designated statistician (using a central computed randomization list that generated treatment numbers in a block‐size of 4)" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported clearly |
| Similarity of the study groups (selection bias) | Low risk | Quote: "The demographic and baseline disease characteristics were similar among the four groups" |
| Blinding of participants (performance bias) | High risk | The participants were not blinded |
| Blinding of care providers (performance bias) | High risk | The care providers were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigator was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was acceptable |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Unclear risk | 4 out of 8 authors were affiliated to the pharmaceutical industry |
Papp 2012.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: "The primary investigator was blinded to treatment" Intention‐to‐treat analysis used: Only those who completed at least 4 weeks of treatment were included in the efficacy analyses on an intent‐to‐treat basis |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 30 in total (tacrolimus N = 16, hydrocortisone N = 14) Number of dropouts: 1 (3%) Sex: 24 males, 6 females Mean age (range): tacrolimus arm = 52.8 (25 to 70) years, hydrocortisone arm = 52.9 (20 to 80) years Country: Canada |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | The only outcomes relevant for this review were adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" This was not reported in sufficient detail |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Low risk | Quote: "The two treatment groups were well balanced for baseline demographics" |
| Blinding of participants (performance bias) | High risk | For participants, the study was open‐label |
| Blinding of care providers (performance bias) | High risk | Only the primary investigator was blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The primary investigator was blinded to treatment, but the participant was not. Therefore, outcomes evaluated and reported by the participant or a care provider other than the primary investigator were subject to detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was acceptable |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Unclear risk | Quote: "This study was an investigator‐initiated research project funded by Astellas Pharma Canada Inc" 1 author was financially supported by Astellas Pharma Canada Inc. The primary investigator reported receiving grants and honoraria from Astellas Pharma Canada Inc |
Pari 1998.
| Methods | Study type: individual RCT Randomisation method: "stratified blocked random method" Blinding: double‐blind Intention‐to‐treat analysis used: at least partly |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 36 in total (clobetasol N = 19, ketoconazole N = 17) Number of dropouts: 5 (14%) Sex: not reported Age: not reported Country: India |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | Results were not reported separately for face and trunk. Adverse events were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A stratified blocked random method was used to allocate the recruited patients into two groups according to severity" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported in detail |
| Similarity of the study groups (selection bias) | Unclear risk | This was not reported in detail |
| Blinding of participants (performance bias) | Low risk | Quote: "Neither the doctor nor the patient knew" |
| Blinding of care providers (performance bias) | Low risk | Quote: "Neither the doctor nor the patient knew" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study did not report whether the outcome was assessed by a third party |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was acceptable |
| Selective reporting (reporting bias) | High risk | The predefined outcomes included side‐effects, but they were not reported |
| Other bias | Low risk | No other bias was identified |
Piepponen 1992.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 101 in total (hydrocortisone N = 50, ketoconazole N = 51) Number of dropouts: 4 (4%) Sex: 38 males, 63 females Age (mean): 52.9 years Country: Finland |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | The participants were diagnosed with either SeD or dandruff. The proportion of participants with dandruff was 37%. Location of SeD lesions was not mentioned as inclusion criteria, but the interventions were used on the skin of the scalp. Necessary data were calculated from other statistics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This randomized...study" This was not reported in sufficient detail |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Low risk | Quote: "There were no significant differences between the treatment groups" |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was acceptable |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Unclear risk | The first author was affiliated to the pharmaceutical industry, and the pharmaceutical industry provided medication |
Ramirez 1993.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: not reported |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 100 in total (fluocinolone N = 50, vehicle N = 50) Number of dropouts: 2 (2%) Sex: 75 males, 25 females Age (range): 16 to 83 years Country: USA |
|
| Interventions |
Treatment
Comparator/s
After 2 weeks, the participants were asked to discontinue use of the test product and were re‐evaluated 7 days post‐treatment |
|
| Outcomes |
|
|
| Notes | Non‐medicated shampoo to be used when necessary | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was not reported in detail Quote: "The patients...were each assigned a number and randomly allocated...according to a schedule known only to the sponsor" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Unclear risk | This was not reported. According to information in Table 1 of the report, there were no significant differences between the groups in erythema, scaling, and pruritus scores |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The dropout rate was acceptable |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Unclear risk | The first author was affiliated to industry, and the sponsor provided non‐medicated shampoos |
Reygagne 2007.
| Methods | Study type: individual RCT Randomisation method: computer‐based randomisation Blinding: investigator‐blinded Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 55 in total (11 participants in each group) Number of dropouts: 4 (7%) Sex: 30 males, 25 females Mean age (range): 36.9 (18 to 64) years Country: France |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | The design of this trial was different from any other included study. The treatments were applied for different times: clobetasol propionate shampoo 0.05% for 2.5, 5, or 10 minutes; clobetasol propionate vehicle for 10 minutes; or ketoconazole foaming gel 2% for 5 minutes. After that, they were to be rinsed off. These kind of application methods were not used in any other included studies; therefore, we did not use the results in the meta‐analysis. Results were not obtainable for all groups from the printed article. The actual symptom scores relevant for this review were not given in the text. Itching scores were given in a figure, but the figure displays only the results for the placebo (vehicle) and the azole group, which was irrelevant for this review. Only the results for complete clearance were given in the text | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "were randomized according to a computerized randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Low risk | According to the report, there were no significant differences between the treatment groups for any of the symptoms, race, age, and gender distribution |
| Blinding of participants (performance bias) | High risk | Quote: "investigator blinded" |
| Blinding of care providers (performance bias) | High risk | Quote: "investigator blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "investigator‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The overall dropout rate was acceptable, but varied between 0%, 9%, and 18% in different treatment arms |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported |
| Other bias | Unclear risk | Some authors were affiliated to a pharmaceutical company |
Rigopoulos 2004.
| Methods | Study type: individual RCT Randomisation method: computer‐based randomisation Blinding: no (open‐label) Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 20 in total (pimecrolimus N = 11, betamethasone N = 9) Number of dropouts: 0 Sex: 16 males, 4 females Mean age (range): pimecrolimus arm = 36.4 (24 to 45) years, betamethasone arm = 37.2 (24 to 47) years Country: Greece |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | The participants were instructed to discontinue use of the medicine as soon as symptoms were absent. All participants stopped treatment by day 9 because symptoms had disappeared We could not use erythema, pruritus, and scaling scores in the meta‐analysis because standard deviations or exact P values were not given in the report The location of SeD lesions were not mentioned as inclusion criteria, but other dermatoses of the face were reported as exclusion criteria suggesting that the skin of the face was a site of interest in the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to treatment...using a program that allocated every consecutive group of two patients to one patient in each group. The random numbers were generated by a computer and were assigned to the patients by the investigator's assistant" |
| Allocation concealment (selection bias) | Unclear risk | The randomisation and allocation program allocated every consecutive group of 2 participants to 1 participant in each group, so the assistant would have known the latter participant's group in advance. However, the same assistant enrolled and assigned the treatment of the participants, whereas the investigator was masked |
| Similarity of the study groups (selection bias) | Low risk | Quote: "The mean baseline score for erythema, pruritus and scaling did not differ significantly between the two treatment groups" |
| Blinding of participants (performance bias) | High risk | The study was not blinded |
| Blinding of care providers (performance bias) | Unclear risk | This was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "in an attempt to make the assessments investigator masked" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None were identified |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Rudner 1970.
| Methods | Study type: individual RCT Randomisation method: a standard randomisation sheet was used Blinding: double‐blind Intention‐to‐treat analysis used: no |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 50 in total (the initial group assignment numbers have not been reported. By the end of the study, there were 24 participants in the fluocinolone group and 19 participants in the vehicle group) Number of dropouts: 7 (14%) Sex of those who completed (baseline was not reported): 21 males and 22 females Age: not reported as mean, median, or range (reported as number of participants in 6 different age groups) Country: USA |
|
| Interventions |
Treatment
Comparator/s
Each participant was instructed to shampoo the scalp once weekly with Drytergent® |
|
| Outcomes |
|
|
| Notes | Only inpatients were included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was not reported in detail Quote: "standard randomization sheet" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Unclear risk | This was not reported |
| Blinding of participants (performance bias) | Low risk | The study was double‐blind, but it was not reported specifically which parties were blinded. Nevertheless, the participants received the intervention and the comparison in identical containers |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The dropout rate was 24% in the control group. The initial group assignment numbers were not reported |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Shin 2009.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: no (open‐label) Intention‐to‐treat analysis used: no |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 83 in total (tacrolimus N = 27, betamethasone N = 27, zinc pyrithione N = 29) Number of dropouts: altogether, 27 at 8 weeks (33%). At week 4, the dropout rate was 23% in the treatment arm that received treatment for 4 weeks only. The dropout rate at 4 weeks was not reported for the group that continued the treatment for 8 weeks. The dropout rate for this group was 38% at week 8 Sex: not reported Age (mean): tacrolimus arm = 38.0 years, betamethasone arm = 39.0 years, zinc pyrithione arm = 34.7 years Country: Korea |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | "At week 4, 53 patients continued the same treatment for an additional 4 weeks, but the other 30 patients stopped the treatments and were followed up at week 8." We only used the results from week 4 in this review because the only efficacy outcome that we could use was dandruff score, and the results for dandruff score were given at 4 weeks only. The dropout rate for those that used the interventions for 8 weeks was 38%. We requested and received additional data from the contact author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly allocated" |
| Similarity of the study groups (selection bias) | Unclear risk | The differences in baseline clinical severity scores and dandruff scores were evaluated only for those who completed the 8‐week follow‐up‐study. We used the results at 4 weeks |
| Blinding of participants (performance bias) | High risk | This was an open‐label study |
| Blinding of care providers (performance bias) | High risk | This was an open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was an open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The dropout rate was 23% in the treatment arm that received treatment for 4 weeks only, but there was considerable variation between the groups (dropout rate was 50% in the betamethasone group, 0% in the tacrolimus group, and 20% in the zinc pyrithione group). The dropout rate at 4 weeks was not reported for the group that continued the treatment for 8 weeks. At 8 weeks, the dropout rates varied between 16% in the zinc pyrithione group, 24% in the betamethasone group, and 76% in the tacrolimus group. There were no intention‐to‐treat analyses |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias was identified |
| Other bias | Low risk | No other bias was identified |
Stratigos 1988.
| Methods | Study type: individual RCT Randomisation method: not reported Blinding: double‐blind Intention‐to‐treat analysis used: no |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 78 in total (6 participants were excluded because of a lack of treatment data. Finally, there were 36 participants in both groups.). The initial assignment numbers in each group were not reported Number of dropouts: 6 (8%) Sex: not reported Mean age (range): hydrocortisone arm = 32.0 (18 to 73) years, ketoconazole arm = 34 (18 to 78 years) Country: Greece |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | Most of the results were not given in numerical form. We have approximated the numbers from figures, where applicable. The location of SeD lesions was not mentioned as inclusion criteria, but the sites of interest were reported to include the scalp, retroauricular area, eyebrows, hairline, nasolabial folds, sternum, external ear canal, and bridge of the nose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Similarity of the study groups (selection bias) | Low risk | Quote: "Both groups were comparable for age, weight, height, sex distribution, and duration of the infection" |
| Blinding of participants (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of care providers (performance bias) | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whilst the study was reported to be double‐blind, it was not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The dropout rate was acceptable. The initial number of participants in each group was not reported |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes were reported |
| Other bias | Unclear risk | Some of the authors were affiliated to the pharmaceutical industry |
Van't Veen 1998.
| Methods | Study type: individual RCT Randomisation method: not mentioned Blinding: no (open‐label trial) Intention‐to‐treat analysis used: yes |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 69 in total (betamethasone N = 34, ketoconazole N = 35) Number of dropouts: 0 Sex: 33 males, 36 females Mean age (range): betamethasone arm = 45.6 (20 to 75) years, ketoconazole arm = 40.1 (18 to 73) years Country: the Netherlands |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | "72 patients gave written informed consent and entered the wash‐out period, but 2 had spontaneous remission and 1 withdrew for a non‐study‐related reason leaving 69 patients randomized". The results were given in figures and not in exact numbers. We approximated the numbers from figures, where feasible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was not reported in detail Quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | This was not reported in detail Quote: "randomly allocated" |
| Similarity of the study groups (selection bias) | Low risk | Quote: "The groups were very well matched for demography and clinical characteristics" |
| Blinding of participants (performance bias) | High risk | This was an open‐label study |
| Blinding of care providers (performance bias) | High risk | This was an open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was an open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was acceptable |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported, albeit not sufficiently enough for use in the meta‐analysis |
| Other bias | Unclear risk | 1 author was affiliated to Glaxo‐Wellcome (The Netherlands) BV, and Glaxo‐Wellcome provided all products Quote: "Financial support for the study was generously provided by Glaxo‐Wellcome (The Netherlands) BV" |
Warshaw 2007.
| Methods | Study type: individual RCT Randomisation method: computer‐generated blocks of 4 Blinding: double‐blind Intention‐to‐treat analysis used: Both ITT and PP analyses were used |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Number of randomised participants: 96 in total (pimecrolimus N = 47, vehicle N = 49) Number of dropouts: 2 (2%) Sex: 85 males, 11 females Mean age (range): pimecrolimus arm = 59.5 (27 to 84) years, placebo arm = 59.6 (20 to 88) years Country: USA |
|
| Interventions |
Treatment
Comparator/s
|
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The computer‐generated randomization assignment (blocks of 4) was only accessible to the research pharmacist during the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "The computer‐generated randomization assignment (blocks of 4) was only accessible to the research pharmacist during the study" |
| Similarity of the study groups (selection bias) | Unclear risk | Quote: "At baseline, both groups had similar demographics...with the exception that a higher percentage of participants in the pimecrolimus group (38%) had previously used medication to treat their seborrhoeic dermatitis, compared with participants in the vehicle group (29%). In addition, participants in the vehicle group had milder disease at baseline compared with those in the pimecrolimus group with regard to mean scale target area score...and with regard to mean facial IGA" |
| Blinding of participants (performance bias) | Low risk | The study was double‐blind Quote: "The two creams were packaged in identical tubes" |
| Blinding of care providers (performance bias) | Low risk | Quote: "double‐blind" Blinded parties were not specified. Nevertheless, the research pharmacist was the only person that knew the participants' assignments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Blinded parties were not specified. Nevertheless, the research pharmacist was the only person that knew the participants' assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was acceptable |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Unclear risk | Quote: "This investigator‐initiated study was supported by Novartis Pharmaceuticals Corporation" Novartis Pharmaceuticals Corporation employed at least 2 of the authors |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aertgeerts 1985 | Only 1 of 161 participants had SeD |
| Albrecht 1986 | Only 9 of 383 participants had SeD |
| Alebiosu 2003 | Only 2 of the participants had dandruff; there was no mention about the proportion of SeD participants |
| Alexander 1967 | The intervention was not anti‐inflammatory (tar) |
| Amos 1994 | The interventions were not anti‐inflammatory (tar and ketoconazole) |
| Anonymous 1994a | This study was not a RCT, and the interventions were not anti‐inflammatory |
| Anonymous 1994b | This was a review |
| Arenas 1999 | The intervention was not anti‐inflammatory (ichthyol/octopirox/salicylic acid) |
| Attarzadeh 2013 | Allocation of treatment (emu oil or either clotrimazole or hyrocortisone) was not randomised as treatment 1 was always used on the right side, and treatment 2 was used on the left side of the face |
| Banerjee 1975 | The interventions were a combinations of drugs (a combination of nitrofurazone and hydrocortisone acetate compared with a combination of framycetin sulfate and dexamethasone acetate and a combination of neomycin, bacitracin, polymyxin B sulfate, and hydrocortisone) |
| Barbanoj 2005 | The intervention was not anti‐inflammatory (eberconazole), and participants were healthy volunteers |
| Basak 1999 | This was a poster. The efficacy and safety results were not given in numerical form, and it was not possible to ensure that efficacy was assessed in ways relevant for the review |
| Bertamino 1975 | Less than 75% of participants had seborrhoeic dermatitis; randomisation was not explained clearly |
| Binder 1972 | Less than 75% of participants had seborrhoeic dermatitis; the affected area was not reported |
| Boyle 1986 | The intervention was a combination of lithium succinate and zinc |
| Camarasa 1975 | Only 1 participant out of 37 had SeD |
| Carboni 1982 | The comparison was between 2 formulas of clobetasol |
| Christodoulou 1983 | The intervention was in peroral form |
| Cuelenaere 1992 | The interventions was a combination of lithium succinate and zinc sulphate |
| Curley 1990 | The diagnosis of the participants was mainly psoriasis or eczema; only a few had SeD |
| Davies 1999 | The intervention was tar or ciclopirox olamine, which are not relevant for this review |
| de la Brassine 1984 | Randomisation, site, and age were unclear |
| Dobrev 2003 | 1 intervention is a combination of drugs (salicylic acid, plant tar, and green microalgae), and others were not anti‐inflammatory (selenium sulphide, zinc pyrithione, and a combination of ketoconazole, metronidazole, and sulphur) |
| Elewski 2009b | This was a review |
| Elie 1983 | Only 17 out of 40 participants had SeD |
| Eun 2009 | This was a poster, which did not contain enough data |
| Franz 2000 | Participants had psoriasis not SeD |
| Fredriksson 1975a | The study was not randomised |
| Fredriksson 1985 | The intervention was not anti‐inflammatory (tar) |
| Freeman 2002 | Less than 75% of participants had seborrhoic dermatitis in the desonide group |
| Fritz 1995 | The intervention was a combination of lithium and zinc sulphate |
| Futterer 1981 | The comparison was irrelevant (piroctone olamine and zinc pyrithione) |
| Gayko 2006 | The intervention was a combination of ichthyol and ketoconazole |
| Gentry 1973 | Age and affected area are unknown |
| Goffin 1996 | The interventions were not anti‐inflammatory (econazole nitrate, piroctone olamine, senium sulphide, and zinc pyrithione) |
| Gould 1988 | The reference was a summary of a paper. The used efficacy measures were not reported in detail. The results were not reported in numerical form |
| Grossman 1997 | The interventions were not anti‐inflammatory (zinc pyrithione and ketoconazole) |
| High 2006 | The study was not randomised or controlled |
| Hochman 1988 | The interventions were combinations of non‐anti‐inflammatory agents (sulphur + salicylic acid) |
| Humke 2002 | The nature of the intervention was unclear: a new shampoo free of ketoconazole versus a ketoconazole‐containing shampoo |
| Jacksonville 1969 | The age of the participants was unknown; randomisation was unclear; and the time‐in‐between was not reported |
| Jafferany 2008 | This was a review |
| Jaramillo 1992 | The intervention was not anti‐inflammatory (zinc pyrithione) |
| Jensen 2009 | This was a poster. The age of the participants was not reported. The outcomes used were not in the interest of this review |
| Jensen 2010 | This was a poster. There was no information on the age of the participants or the affected/investigated site. The used outcomes were not reported, and the results of interest in this review were not reported in numerical form |
| Kaminester 2002 | The intervention was not anti‐inflammatory (sulphacetamide) |
| Karsono 2010 | The intervention was not anti‐inflammatory (zinc pyrithione) |
| Kim 2012 | This was a poster. The results of interest for this review were not reported in detail or in numerical form. No useful data could be added to the analyses |
| Kim 2013 | This study included an induction phase with an active treatment only (not controlled) and thereafter a controlled maintenance phase |
| Kircik 2009 | Participants were healthy volunteers, and the intervention was not anti‐inflammatory |
| Levy 1974 | There was only 1 participant with SeD |
| Li 2000 | The interventions were irrelevant for the review (Triatop®, which is a ketoconazole‐containing compound, and tar) |
| Lin 2010 | This was a review |
| Luo 1993 | The intervention was antifungal (bifonazole) |
| López Padilla 1996 | The interventions were not anti‐inflammatory (ketoconazole and climbazole) |
| Marks 1974 | The outcomes used in the study were not relevant for the review. There were no useful data to be added to the analyses |
| Mensing 2008 | Only 10 out of 27 participants had SeD, and there was no control treatment |
| Nolting 1983 | Less than 75% of participants had SeD, and results were not reported separately for SeD participants |
| Nolting 1985 | Only 1 out of 80 participants had the diagnosis of SeD |
| Pierard‐Franchimont 1995 | The interventions were not anti‐inflammatory (econazole, ketoconazole, piroctone olamine, and selenium sulphide) |
| Pierard‐Franchimont 1999 | The intervention was not anti‐inflammatory (tar). This was a poster |
| Pierard‐Franchimont 2000 | The intervention was not anti‐inflammatory (tar) |
| Pierard‐Franchimont 2002a | The interventions were a combination of non‐anti‐inflammatory agents (ketoconazole, piroctone olamine, and zinc pyrithione formulations) |
| Pierard‐Franchimont 2002b | The intervention was a combination of antifungal and anti‐inflammatory drugs (ketoconazole and desonide combination) |
| Pierard‐Franchimont 2002c | The interventions were not anti‐inflammatory (ketoconazole and zinc pyrithione) |
| Reiffenstuhl 1973 | Only 3 out of 54 participants had SeD, and there was no control intervention |
| Reinhard 1974 | Only 5 out of 122 participants had SeD |
| Sohn 1978 | This was a non‐randomised study |
| Tomoka 1973 | Only 2 out of 84 participants had SeD |
| Turnbull 1982 | Less than 75% of participants had seborrhoeic dermatitis |
| Veien 1980 | The intervention was a combination of 2 non‐anti‐inflammatory agents (coal tar and zinc pyrithione) |
| Wacker 1989 | Randomisation and proportion of SeD participants was unclear |
| Weiss 2011 | The intervention was not anti‐inflammatory (ketoconazole) |
| Wollina 2006 | This was a review |
| Wollina 2007 | This was a review |
| Yawalkar 1983 | Less than 75% of participants had SeD |
Characteristics of studies awaiting assessment [ordered by study ID]
Fredriksson 1975b.
| Methods | Randomised, controlled, double‐blind study Duration: 44 days with 3 phases (control, treatment, and follow‐up period) |
| Participants |
|
| Interventions |
|
| Outcomes | Scored degree of seborrheic involvement (summary scores) |
| Notes | The participants were not blinded. Only the evaluating physician was blinded (single‐blind). Side‐effects were not reported |
Snyder 1969.
| Methods | Randomised, controlled, double‐blind study Duration: not reported |
| Participants |
|
| Interventions |
|
| Outcomes | Clinical effectiveness defined as test drug preferred and identified; improvement (either partial or complete decrease of redness, scaling and itching), side effects |
| Notes | ‐ |
Characteristics of ongoing studies [ordered by study ID]
EudraCT 2005‐006208‐21.
| Trial name or title | Clinical efficacy of pimecrolimus cream in seborrheic dermatitis. Efficacy of pimecrolimus in normalizing clinical symptoms, explorative study of barrier function, hydration, lipid content and differentiation in seborrheic dermatitis: a randomized, double‐blind study in adults with seborrheic dermatitis treated with 1% pimecrolimus cream versus 2% ketoconazole cream as control |
| Methods | This is a randomised, controlled, double‐blind study Duration: 4 weeks |
| Participants |
|
| Interventions |
|
| Outcomes |
Primary outcome/s of the trial
Secondary outcome/s of the trial
|
| Starting date | Entered into database: August 2006 |
| Contact information | Department of Dermatology, University of Kiel, Germany |
| Notes | Ongoing. Database accessed on 17 December 2012 |
EudraCT 2006‐003984‐30.
| Trial name or title | A multicenter, randomized, double‐blind, two‐arm, vehicle‐controlled, parallel‐group, two stage study to evaluate and demonstrate the efficacy and to evaluate the safety of pimecrolimus 1% cream in the treatment of seborrhoeic dermatitis in patients 12 years of age and older |
| Methods | This is a randomised, double‐blind, parallel group, 2‐stage study Initial estimate of the duration of the trial: 9 months |
| Participants |
|
| Interventions |
|
| Outcomes |
Primary outcome/s of the trial
Secondary outcome/s of the trial
|
| Starting date | Entered into database: October 2006 |
| Contact information | Novartis Pharma Services AG, Switzerland |
| Notes | Completed, but no publications provided in searched databases. In the title, the age of the participants is limited to 12 or older whereas in the inclusion criteria, the age limit is 18 or older. The trial has 2 stages, but these are not defined clearly. Database accessed on 17 December 2012 |
EudraCT 2007‐007088‐25.
| Trial name or title | Efficacy and tolerance of V0071 GM 01A in inflammatory seborrhoeic dermatitis of the scalp |
| Methods | This is a randomised, open‐label (investigator‐masked in initiation therapy), parallel group study (phase II) Initial estimate of the duration of the trial: 11 months |
| Participants |
|
| Interventions |
|
| Outcomes |
Primary outcome/s of the trial
Secondary outcome/s of the trial
|
| Starting date | Entered into database: January 2008 |
| Contact information | Pierre Fabre Dermatologie, France |
| Notes | Ongoing study. Database accessed on 17 December 2012 |
EudraCT 2009‐013120‐23.
| Trial name or title | Efficacité et tolérance du LBC 45 dans la dermite séborrhéique du cuir chevelu |
| Methods | This is a randomised, controlled, double‐blind, parallel group, phase II study Initial estimate of the duration of the trial: 70 days |
| Participants |
|
| Interventions |
|
| Outcomes |
Primary outcome/s of the trial
Secondary outcome/s of the trial
|
| Starting date | Entered into database: August 2009 |
| Contact information | LABCATAL, France |
| Notes | Ongoing study. Database accessed on 17 December 2012 |
EudraCT 2010‐022861‐93.
| Trial name or title | Confirmation de l'efficacité et de la tolérance du LBC 45 dans la dermite séborrhéique du cuir chevelu |
| Methods | This is a randomised, controlled, single‐blind, parallel group, phase II study Initial estimate of the duration of the trial: 70 days |
| Participants |
|
| Interventions |
|
| Outcomes |
Primary outcome/s of the trial
Secondary outcome/s of the trial
|
| Starting date | Entered into database: October 2010 |
| Contact information | Laboratoire LABCATAL, France |
| Notes | Ongoing study. Database accessed on 17 December 2012 |
NCT00403559.
| Trial name or title | A 4 week randomized double‐blind parallel group active comparator controlled study of Elidel for the treatment of seborrheic dermatitis |
| Methods | This is a randomised, double‐blind, parallel group study |
| Participants |
|
| Interventions |
|
| Outcomes |
Primary outcome/s of the trial
Secondary outcome/s of the trial
|
| Starting date | January 2007 |
| Contact information | Joseph F Fowler Jr, Dermatology Specialists Research |
| Notes | Completed in January 2009, but no publications provided. Database accessed on 17 December 2012 |
NCT01011621.
| Trial name or title | Comparative evaluation of the efficacy and tolerability of prednisolone acetate 0.5% cream versus betamethasone valerate 0.1% cream in the treatment of pediatric and adult dermatosis |
| Methods | This is a randomised, open‐label, parallel group phase III study |
| Participants |
|
| Interventions |
|
| Outcomes |
Primary outcome/s of the trial
Secondary outcome/s of the trial
|
| Starting date | February 2010 |
| Contact information | Cláudia Domingues cdomingues@mantecorp.com |
| Notes | The study is not yet open for participant recruitment. At this point, it is impossible to know if this study will be relevant for this review. Database accessed on 17 December 2012 |
Differences between protocol and review
In the protocol under Types of participants, we stated that we would include studies of adults or adolescents (> 16 years) with SeD. When assessing the eligibility of the trials, we used the percentage of 75 or more as a measure to judge whether the trial fulfilled this inclusion criterion. We made the decision that at least 75% of the study participants had to be over 10 years of age to fulfil the age criterion.
We did not explore heterogeneity other than regarding the strength of steroid treatment where feasible. This is reasoned by the small number of studies in each comparison. In the protocol, we planned to possibly explore age, gender, and dose (frequency) distributions as a cause for heterogeneity.
Two additional authors (JJ and TOr) were added to the people undertaking the data collection and analysis. An additional resource was added to electronic searches (GREAT).
Contributions of authors
HK was the contact person with the editorial base. HK co‐ordinated the contributions from the co‐authors. HK, TOk, PP, TOr, and JJ screened papers against eligibility criteria. HK and TOk obtained data on ongoing and unpublished studies. HK and TOk appraised the quality of papers. HK, TOk, and JJ extracted data for the review and sought additional information about papers. TOk and HK entered data into RevMan. HK and VK analysed and interpreted data. VK, HK, and TOk worked on the methods sections. HK, EO, and TOk drafted the clinical sections of the background and responded to the clinical comments of the referees. KA commented on the drafts (protocol and review). JV was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. JV is the guarantor of the review.
Disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health, UK, or the Finnish Medicines Agency Fimea.
Sources of support
Internal sources
Finnish Medicines Agency (FIMEA), Finland.
The Finnish Institute of Occupational Health, Finland.
The Cochrane Occupational Safety and Health Review Group, Finland.
The Nigerian Branch of the South African Cochrane Centre, Nigeria.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
PP has received a research grant from AstraZeneca and consultancy fees from ESiOR Ltd (a health economy consultancy that provides research and consulting services to the pharmaceutical industry). These projects have not been related to treatment for seborrhoeic dermatitis.
None of the other authors involved in this review have declared any interests.
Edited (no change to conclusions)
References
References to studies included in this review
Attila 1992 {published data only}
- Attila P, Förström L, Hannuksela M, Karvonen J, Lehtonen L, Salo OP. Clotrimazole‐hydrocortisone, hydrocortisone and propylene glycol liniment in the treatment of seborrhoeic dermatitis of the scalp. Journal of Dermatological Treatment 1992;3(4):185‐8. [EMBASE: 1993020255] [Google Scholar]
Basak 2001 {published data only}
- Basak PY, Ergin S. Comparative effects of calcipotriol and betamethasone 17‐valerate solution in the treatment of seborrhoeic dermatitis of the scalp. Journal of the European Academy of Dermatology & Venereology 2001;15(1):86‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cicek 2009 {published data only}
- Cicek D, Kandi B, Bakar S, Turgut D. Pimecrolimus 1% cream, methylprednisolone aceponate 0.1% cream and metronidazole 0.75% gel in the treatment of seborrhoeic dermatitis: a randomized clinical study. Journal of Dermatological Treatment 2009;20(6):344‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cornell 1986 {published data only}
- Cornell RC. Atrophogenic potential of alclometasone dipropionate ointment 0.05% vs hydrocortisone ointment 1.0%. Current Therapeutic Research Clinical & Experimental 1986;39(2):260‐8. [EMBASE: 1987091141] [Google Scholar]
Cornell 1989 {published data only}
- Cornell RC, Ellis CN. Comparison of the efficacy and safety of amcinonide lotion 0.1% with those of betamethasone valerate lotion 0.1% in patients with seborrheic dermatitis. Current Therapeutic Research Clinical & Experimental 1989;46(3):577‐86. [EMBASE: 1989232568] [Google Scholar]
Cornell 1993 {published data only}
- Cornell RC, Baker MD. Dermal safety comparison of 0.05% desonide cream and 1.0% hydrocortisone cream. Current Therapeutic Research Clinical & Experimental 1993;53(4):356‐9. [EMBASE: 1993234707] [Google Scholar]
Dreno 2002a {published data only}
- Dreno B, Moyse D. Lithium gluconate in the treatment of seborrhoeic dermatitis: a multicenter, randomised, double‐blind study versus placebo. European Journal of Dermatology 2002;12(6):549‐52. [EMBASE: 2002448306] [PubMed] [Google Scholar]
- Dreno B, Revuz J, Chosidow O, Bayrou O, Beylot C, Bonnetblanc JM, et al. Lithium gluconate 8% ointment in the treatment of moderate to severe seborrhoeic dermatitis. Journal of the European Academy of Dermatology & Venereology 2002;16(Suppl 1):153. [Google Scholar]
Dreno 2003 {published data only}
- Dreno B, Chosidow O, Revuz J. Lithium vs ketoconazole in seborrheic dermatitis: a multicenter, randomized, controlled study. Annales of Dermatology & Venereology 2002;129(1):S696. [Google Scholar]
- Dreno B, Chosidow O, Revuz J, Moyse D, Study Investigator Group. Lithium gluconate 8% vs. ketoconazole 2% in the treatment of seborrhoeic dermatitis: a multicentre, randomized study. British Journal of Dermatology 2003;148(6):1230‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elewski 2009a {published data only}
- Elewski B. An investigator‐blind, randomized, 4‐week, parallel‐group, multicenter pilot study to compare the safety and efficacy of a nonsteroidal cream (Promiseb Topical Cream) and desonide cream 0.05% in the twice‐daily treatment of mild to moderate seborrheic dermatitis of the face. Clinics in Dermatology 2009;27(6 Suppl):S48‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Faergemann 1986 {published data only}
- Faergemann J. Seborrhoeic dermatitis and Pityrosporum orbiculare: treatment of seborrhoeic dermatitis of the scalp with miconazole‐hydrocortisone (Daktacort), miconazole and hydrocortisone. British Journal of Dermatology 1986;114(6):695‐700. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Firooz 2006 {published data only}
- Firooz A, Solhpour A, Gorouhi F, Daneshpazhooh M, Balighi K, Farsinejad K, et al. Pimecrolimus cream, 1%, vs hydrocortisone acetate cream, 1%, in the treatment of facial seborrheic dermatitis: a randomized, investigator‐blind, clinical trial. Archives of Dermatology 2006;142(8):1066‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fredriksson 1978 {published data only}
- Fredriksson T. A comparison between hydrocortisone 17‐butyrate and betamethasone 17α‐valerate in seborrhoeic dermatitis of the scalp. IRCS Medical Science: Clinical Medicine; Clinical Pharmacology and Therapeutics; Connective Tissue, Skin and Bone 1978;6(2):70. [EMBASE: 0979014893] [Google Scholar]
General Practitioner 1982 {published data only}
- General Practitioner Research Group. Topical corticosteroids in seborrhoeic dermatitis. A report from the general practitioner research group. Practitioner 1982;226(1368):1178‐9. [EMBASE: 1982217563] [PubMed] [Google Scholar]
Gip 1979 {published data only}
- Gip L. A study to compare the efficacy of hydrocortisone 17‐butyrate (Locoid) 0.1% scalp lotion and betamethasone 17‐valerage (Betnovate) 0.1% lotion in the treatment of seborrhoeic dermatitis of the scalp. Current Therapeutic Research Clinical & Experimental 1979;26(5):607‐10. [EMBASE: 1980112442] [Google Scholar]
Harris 1972 {published data only}
- Harris JJ. A national double‐blind clinical trial of a new corticosteroid lotion: a 12‐investigator cooperative analysis. Current therapeutic research, clinical & experimental 1972;14(9):638‐46. [EMBASE: 4263888] [PubMed] [Google Scholar]
Hersle 1996 {published data only}
- Hersle K, Mobacken H, Nordin P. Mometasone furoate solution 0.1% compared with ketoconazole shampoo 2% for seborrheic dermatitis of the scalp. Current Therapeutic Research Clinical & Experimental 1996;57(7):516‐22. [EMBASE: 1996233065] [Google Scholar]
Katsambas 1989 {published data only}
- Katsambas A, Antoniou C, Frangouli E, Avgerinou G, Michailidis D, Stratigos J. A double‐blind trial of treatment of seborrheic dermatitis with 2% ketoconazole cream compared with 1% hydrocortisone cream. British Journal of Dermatology 1989;121(3):353‐7. [EMBASE: 1989222189] [DOI] [PubMed] [Google Scholar]
Koc 2009 {published data only}
- Koc E, Arca E, Kose O, Akar A. An open, randomized, prospective, comparative study of topical pimecrolimus 1% cream and topical ketoconazole 2% cream in the treatment of seborrheic dermatitis. Journal of Dermatological Treatment 2009;20(1):4‐9. [EMBASE: 2009111157] [DOI] [PubMed] [Google Scholar]
Kousidou 1992 {published data only}
- Kousidou T, Panagiotidou D, Boutli F, Mourellou O, Ioannidis D, Fotidou D, et al. A double‐blind comparison of 2% ketoconazole cream and 1% hydrocortisone cream in the treatment of seborrheic dermatitis. Current Therapeutic Research, Clinical & Experimental 1992;51(5):723‐9. [EMBASE: 1992159176] [Google Scholar]
Langtry 1997 {published data only}
- Langtry J, Rowland C, Staughton R, Stewart J, Horrobin D. Topical lithium succinate ointment (Efalith) in the treatment of AIDS‐related seborrhoeic dermatitis. Clinical & Experimental Dermatology 1997;22(5):216‐9. [EMBASE: 1998045600] [PubMed] [Google Scholar]
Ludvigsen 1983 {published data only}
- Ludvigsen K. Treatment of seborrhoeic dermatitis of the scalp. Report of a double‐blind comparative trial of hydrocortisone 17‐butyrate 0.1% lotion (Locoid) and betamethasone 17,21‐Dipropionate 0.05% Lotion (Diproderm). Clinical Trials Journal 1983;20(6):313‐8. [EMBASE: 1984018112] [Google Scholar]
Lynfield 1988 {published data only}
- Lynfield Y, Cornell RC, Horwitz SN, Huntley AC, Millikan LE. Amcinonide lotion 0.1% in the treatment of patients with seborrheic dermatitis of the scalp and/or other hairy areas. Current Therapeutic Research Clinical & Experimental 1988;44(2):304‐14. [EMBASE: 1989002094] [Google Scholar]
Medansky 1992 {published data only}
- Medansky RS, Lepaw MI, Shavin JS, Zimmerman EH, Jones ML, Peets EA, et al. Mometasone furoate cream 0.1% vs. hydrocortisone cream 1% in the treatment of seborrhoeic dermatitis. Journal of Dermatological Treatment 1992;3(3):125‐8. [EMBASE: 1992296411] [Google Scholar]
Ortonne 1992 {published data only}
- Ortonne J‐P, Lacour J‐P, Vitetta A, Fichoux Y. Comparative Study of Ketoconazole 2% Foaming Gel and Betamethasone Dipropionate 0,05% Lotion in the Treatment of Seborrhoeic Dermatitis in Adults. Dermatology 1992;184(4):275‐80. [EMBASE: 1992177939] [DOI] [PubMed] [Google Scholar]
Ortonne 2011 {published data only}
- Ortonne JP, Nikkels AF, Reich K, Ponce Olivera RM, Lee JH, Kerrouche N, et al. Efficacious and safe management of moderate to severe scalp seborrhoeic dermatitis using clobetasol propionate shampoo 0.05% combined with ketoconazole shampoo 2%: a randomized, controlled study. British Journal of Dermatology 2011;165(1):171‐6. [EMBASE: 2011355146] [DOI] [PubMed] [Google Scholar]
Papp 2012 {published data only}
- Papp KA, Papp A, Dahmer B, Clark CS. Single‐blind, randomized controlled trial evaluating the treatment of facial seborrheic dermatitis with hydrocortisone 1% ointment compared with tacrolimus 0.1% ointment in adults. Journal of the American Academy of Dermatology 2012;67(1):e11‐5. [EMBASE: 2012342025] [DOI] [PubMed] [Google Scholar]
Pari 1998 {published data only}
- Pari T, Pulimood S, Jacob M, George S, Jeyaseelan L, Thomas K. Randomized double blind controlled trial 2% ketoconazole cream versus 0,05% clobetasol 17‐butyrate cream in seborrhoeic dermatitis. Journal of the European Academy of Dermatology & Venereology 1998; Vol. 10, issue 1:89‐90. [EMBASE: 1998052808] [DOI] [PubMed]
Piepponen 1992 {published data only}
- Piepponen T, Suhonen R, Rantanen T, Blomqvist K, Pajarre R, Lehtonen L. Treatment of dandruff with a ketoconazole 2% shampoo. Journal of Dermatological Treatment 1992;3(3):119‐23. [EMBASE: 1992296410] [Google Scholar]
Ramirez 1993 {published data only}
- Ramirez RG, Dorton D. Double‐blind placebo‐controlled multicentre study of fluocinolone acetonide shampoo (FS Shampoo) in scalp seborrhoeic dermatitis. Journal of Dermatological Treatment 1993;4(3):135‐7. [EMBASE: 1993311639] [Google Scholar]
Reygagne 2007 {published data only}
- Reygagne P, Poncet M, Sidou F, Soto P. Clobetasol propionate shampoo 0.05% in the treatment of seborrheic dermatitis of the scalp: results of a pilot study. Cutis 2007;79(5):397‐403. [EMBASE: 17569404] [PubMed] [Google Scholar]
Rigopoulos 2004 {published data only}
- Rigopoulos D, Ioannides D, Kalogeromitros D, Gregoriou S, Katsambas A. Pimecrolimus cream 1% vs. betamethasone 17‐valerate 0.1% cream in the treatment of seborrhoeic dermatitis. A randomized open‐label clinical trial. British Journal of Dermatology 2004;151(5):1071‐5. [EMBASE: 2004512755] [DOI] [PubMed] [Google Scholar]
Rudner 1970 {published data only}
- Rudner EJ, Samovitz M. Seborrheic Dermatitis of the Face ‐ Treated with Fluocinolone Acetonide Solution: A Double Blind Study. Cutis 1970;6:320‐2. [Google Scholar]
Shin 2009 {published data only}
- Shin H, Kwon OS, Won CH, Kim BJ, Lee YW, Choe YB, et al. Clinical efficacies of topical agents for the treatment of seborrheic dermatitis of the scalp: a comparative study. Journal of Dermatology 2009;36(3):131‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stratigos 1988 {published data only}
- Stratigos J, Antoniou C, Katsambas A, Böhler K, Fritsch P, Schmölz A, et al. Ketoconazole 2% cream versus hydrocortisone 1% cream in the treatment of seborrheic dermatitis. A double‐blind comparative study. Journal of the American Academy of Dermatology 1988;19(5 Pt 1):850‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van't Veen 1998 {published data only}
- Van't Veen AJ, Prevoo RLMA, Velders AJ, Jagtman BA, Niel JCG, Stolz E. Betamethasone‐17‐valerate compared with ketoconazole for topical treatment of seborrhoeic dermatitis of the scalp in adults. Results of a Dutch multicenter study. Journal of Dermatological Treatment 1998;9(4):239‐45. [EMBASE: 1999025626] [Google Scholar]
Warshaw 2007 {published data only}
- Warshaw EM, Wohlhuter RJ, Liu A, Zeller SA, Wenner RA, Bowers S, et al. Results of a randomized, double‐blind, vehicle‐controlled efficacy trial of pimecrolimus cream 1% for the treatment of moderate to severe facial seborrheic dermatitis. Journal of the American Academy of Dermatology 2007;57(2):257‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aertgeerts 1985 {published data only}
- Aertgeerts P, Albring M, Klaschka F, Nasemann T, Patzelt‐Wenczler R, Rauhut K, et al. Comparative testing of Kamillosan cream and steroidal (0.25% hydrocortisone, 0.75% fluocortin butyl ester) and non‐steroidal (5% bufexamac) dermatologic agents in maintenance therapy of eczematous diseases [Vergleichende Prufung von Kamillosan Creme gegenuber steroidalen (0,25% Hydrocortison, 0,75% Fluocortinbutylester) und nichtsteroidalen (5% Bufexamac) Externa in der Erhaltungstherapie von Ekzemerkrankungen]. Zeitschrift für Hautkrankheiten 1985;60(3):270‐7. [EMBASE: 3158124] [PubMed] [Google Scholar]
Albrecht 1986 {published data only}
- Albrecht G. Berlin Multicenter Study on Prednicarbate Preparations in Dermatologic Practices [Das Berliner Modell. Multicenterstudie mit Prednicarbat in verschiedenen Vehikeln]. Zeitschrift für Hautkrankheiten 1986;61(Suppl 1):88‐96. [MEDLINE: ] [PubMed] [Google Scholar]
Alebiosu 2003 {published data only}
- Alebiosu CO, Ogunledun A, Ogunleye DS. A report of clinical trial conducted on Toto ointment and soap products. Journal of the National Medical Association 2003;95(1):95‐105. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Alexander 1967 {published data only}
- Alexander S. Do shampoos affect dandruff?. British Journal of Dermatology 1967;79(2):92‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amos 1994 {published data only}
- Amos HE, MacLennan AI, Boorman GC. Clinical efficacy of Polytar AF (Fongitar) and Nizoral scalp treatments in patients with dandruff/seborrhoeic dermatitis. Journal of Dermatological Treatment 1994;5(3):127‐30. [EMBASE: 1994316061] [Google Scholar]
Anonymous 1994a {published data only}
- Anonymous. Management of seborrhoic dermatitis [Therapie der seborrhoischen Dermatitis]. Der Hautarzt 1994;45(4 Suppl):1‐4. [EMBASE: 9218049] [PubMed] [Google Scholar]
Anonymous 1994b {published data only}
- Anonymous. Seborrheic dermatitis: Lithium deprives the growth basis of yeast fungus [Seborrhoische Dermatitis: Lithium entzieht Hefepilz die Wachstumsgrundlage]. Therapiewoche 1994;44(18):1032‐3. [EMBASE: 1994176542] [Google Scholar]
Arenas 1999 {published data only}
- Arenas R, Monroy E, Farrera RF, Abiega CD. Double blind and aleatory study to evaluate efficacy of icthiol/octopirox/salicilic acid in seborrehic dermatitis [Estudio doble ciego, al azar, para evaluar la eficacia de ictiol/octopirox/acido salicilico en dermatitis seborreica de la piel cabelluda]. Dermatologia Revista Mexicana 1999;43(4):157‐63. [EMBASE: 1999365285] [Google Scholar]
Attarzadeh 2013 {published data only}
- Attarzadeh Y, Asilian A, Shahmoradi Z, Adibi N. Comparing the efficacy of emu oil with clotrimazole and hydrocortisone in the treatment of seborrheic dermatitis: a clinical trial. Journal of Research in Medical Sciences 2013;18(6):477‐81. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Banerjee 1975 {published data only}
- Banerjee BN, Mandal SB, Dutta AK. Evaluation of furacin‐s (nitrofurazone and hydrocortisone acetate) in the treatment of different dermatoses. Indian Journal of Dermatology, Venerology & Leprology 1975;41(6):209‐14. [EMBASE: 0977052085] [Google Scholar]
Barbanoj 2005 {published data only}
- Barbanoj MJ, Antonijoan R, Garcia‐Gea C, Puntes M, Gich I, Jane F. Eberconazole cream: topical and general tolerability, sensitisation potential, and systemic availability. Methods & Findings in Experimental & Clinical Pharmacology 2005;27(4):227‐34. [EMBASE: 2005220616] [DOI] [PubMed] [Google Scholar]
Basak 1999 {published data only}
- Başak PY, Ergin Ş. Comparative effects of calcipotriol and betamethasone 17‐valerate in seborrhoeic dermatitis of the scalp [Abstract P‐547]. The 8th Congress of the European Academy of Dermatology and Venereology. Amsterdam, The Netherlands 29thSept‐3rd October 1999. Journal of European Adacemy of Dermatology and Venereology 1999;12(Suppl 2):S295. [DOI] [PubMed] [Google Scholar]
Bertamino 1975 {published data only}
- Bertamino R. A new topical corticosteroid. Its evaluation in a double‐blind trial [Un nuovo Corticosteroide per uso topico. Sua valutazione in condizioni di doppia cecità]. Riforma Medica 1975;89(15):706‐13. [EMBASE: 0976142422] [Google Scholar]
Binder 1972 {published data only}
- Binder R, McCleary J. Comparison of fluocinonide in a double‐blind study with betamethasone valerate. Current Therapeutic Research 1972;14(1):35‐8. [EMBASE: 4257862] [PubMed] [Google Scholar]
Boyle 1986 {published data only}
- Boyle J, Burton JL, Faergemann J. Use of topical lithium succinate for seborrhoeic dermatitis. British Medical Journal 1986;292(6512):28. [EMBASE: 1986074945] [DOI] [PMC free article] [PubMed] [Google Scholar]
Camarasa 1975 {published data only}
- Camarasa G. Clinical testing of a new local corticoid: desoxymethasone [Klinische Erprobung eines neuen lokalen Kortikoids: Desoximetason]. Zeitschrift für Hautkrankheiten 1975;50(Suppl 2):21‐4. [EMBASE: 132780] [PubMed] [Google Scholar]
Carboni 1982 {published data only}
- Carboni G, Longhi‐Gelati M, Pierfelice V. Clinical observations on two new liquid forms of clobetasol propionate in seborrhoeic eczema [Osservazioni cliniche su due nuove forme liquide di clobetasolo propionato sull'eczema seborroico]. Giornale Italiano di Dermatologia e Venereologia 1982;117(1):V‐X. [EMBASE: 1982225929] [PubMed] [Google Scholar]
Christodoulou 1983 {published data only}
- Christodoulou GN, Georgala S, Vareltzides A, Catsarou A. Lithium in seborrheic dermatitis. Psychiatric Journal of the University of Ottawa 1983;8(1):27‐9. [EMBASE: 1983187066] [PubMed] [Google Scholar]
Cuelenaere 1992 {published data only}
- Cuelenaere C, Bersaques J, Kint A. Use of topical lithium succinate in the treatment of seborrhoeic dermatitis. Dermatology 1992;184(3):194‐7. [EMBASE: 1992148393] [DOI] [PubMed] [Google Scholar]
Curley 1990 {published data only}
- Curley RK, Vickers CF, Norris T, Glover DR. A comparative study of betamethasone dipropionate with salicylic acid and betamethasone valerate for the treatment of steroid‐responsive dermatoses of the scalp. Journal of Dermatological Treatment 1990;1(4):203‐6. [EMBASE: 1990343409] [Google Scholar]
Davies 1999 {published data only}
- Davies DB, Boorman GC, Shuttleworth D. Comparative efficacy of shampoos containing coal tar (4.0% w/W;TarmedTM), coal tar (4.0% w/w) plus ciclopirox olamine (1.0% w/w; TarmedTM AF) and ketoconazole (2.0% w/w; Nizoral®) for the treatment of dandruff/seborrhoeic dermatitis. Journal of Dermatological Treatment 1999;10(3):177‐83. [EMBASE: 1999324981] [Google Scholar]
de la Brassine 1984 {published data only}
- Brassine M, Kint A, Lachapelle JM, Tennstedt D. Halomethasone (C 48.401‐Ba) for the Topical Treatment of Common Dermatoses. Journal of International Medical Research 1984;12(5):307‐9. [EMBASE: 1984244422] [DOI] [PubMed] [Google Scholar]
Dobrev 2003 {published data only}
- Dobrev H, Zissova L, Yankova R. Study of four antidandruff shampoos therapeutic effectiveness [Abstract P25‐7]. The 12th Congress of the European Academy of Dermatology and Venereology Barcelona, Spain 15‐18th October 2003. Journal of the European Academy of Dermatology & Venereology 2003;17(Suppl 3):342. [Google Scholar]
Elewski 2009b {published data only}
- Elewski BE. Safe and effective treatment of seborrheic dermatitis. Cutis 2009;83(6):333‐8. [EMBASE: 19681345] [PubMed] [Google Scholar]
Elie 1983 {published data only}
- Elie R, Durocher LP, Kavalec EC. Effect of salicylic acid on the activity of betamethasone‐17,21‐dipropionate in the treatment of erythematous squamous dermatoses. Journal of International Medical Research 1983;11(2):108‐12. [EMBASE: 1983129354] [DOI] [PubMed] [Google Scholar]
Eun 2009 {published data only}
- Eun HC. Clinical efficacies of topical agents for the treatment of seborrheic dermatitis on the scalp. 67th Annual Meeting of the American Academy of Dermatology, AAD San Francisco, CA United States. Conference Start: 20090306 Conference End: 20090310. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB47. [EMBASE: 70141908] [Google Scholar]
Franz 2000 {published data only}
- Franz TJ, Parsell DA, Myers JA, Hannigan JF. Clobetasol propionate foam 0.05%: A novel vehicle with enhanced delivery. International Journal of Dermatology 2000;39(7):535‐8. [EMBASE: 2000277868] [DOI] [PubMed] [Google Scholar]
Fredriksson 1975a {published data only}
- Fredriksson T, Gip L, Hamfelt A. Investigations of a new synthetic steroid, betamethasone‐17, 21‐dipropionate, in alcoholic solution. Current Therapeutic Research, Clinical & Experimental 1975;18(2):324‐31. [EMBASE: 0976132342] [PubMed] [Google Scholar]
Fredriksson 1985 {published data only}
- Fredriksson T. Controlled comparison of Clinitar Shampoo and Selsun Shampoo in the treatment of seborrhoeic dermatitis of the scalp. British Journal of Clinical Practice 1985;39(1):25‐8. [EMBASE: 1985059997] [PubMed] [Google Scholar]
Freeman 2002 {published data only}
- Freeman S, Howard A, Foley P, Rosen R, Wood G, See J‐A, et al. Efficacy, cutaneous tolerance and cosmetic acceptability of desonide 0.05% lotion (Desowen®) versus vehicle in the short‐term treatment of facial atopic or seborrhoeic dermatitis. Australasian Journal of Dermatology 2002;43(3):186‐9. [EMBASE: 2002259552] [DOI] [PubMed] [Google Scholar]
Fritz 1995 {published data only}
- Fritz K, Itschert G. Topical application of 8% Lithiumsuccinat and 0.05% Zincsulfate in seborrhoic Dermatitis. [Lokaltherapie des seborrhoischen Ekzems mit 0.05% Zinksulfat und 8% Lithiumsuccinat]. H + G Zeitschrift Fur Hautkrankheiten 1995;70:155‐6. [Google Scholar]
Futterer 1981 {published data only}
- Futterer E. Evaluation of efficacy of antidandruff agents. Journal of the Society of Cosmetic Chemists of Japan 1981;32(6):327‐38. [EMBASE: 1982133716] [Google Scholar]
Gayko 2006 {published data only}
- Gayko G, Warnecke J, Zelenkova H. The use of a pale type of Ichthyol in cosmetic dermatology. Cosmetic, single‐centre, controlled and randomized double blind study for proof of efficacy and tolerance of a combination of Ichthyol Pale 0.5% and ketoconazole 0.5% vs. ketoconazole 1.0% shampoo in treatment of moderate to severe dandruff. Dermatologia Kliniczna 2006;8(4):243‐7. [EMBASE: 2007006167] [Google Scholar]
Gentry 1973 {published data only}
- Gentry WC Jr, Rosenberg EW, Goltz RW. A Clinical Evaluation of 0.05% Desonide Cream. A New Nonfluorinated Topical Corticosteroid. Archives in Dermatology 1973;107(6):870‐1. [MEDLINE: ] [PubMed] [Google Scholar]
Goffin 1996 {published data only}
- Goffin V, Pierard‐Franchimont C, Pierard GE. Antidandruff shampoos and the stratum corneum. Journal of Dermatological Treatment 1996;7(4):215‐8. [EMBASE: 1997028593] [Google Scholar]
Gould 1988 {published data only}
- Gould DJ, Davies MG, Kersey PJW, Kenicer K, Green C, Strong A, et al. Topical lithium succinate ‐ a safe and effective treatment for seborrhoeic dermatitis in adults. International Pharmaceutical Abstracts British Journal of Dermatology 1988;119:27‐8. [Google Scholar]
Grossman 1997 {published data only}
- Grossman R, Gisoldi E, Phillips S, Cauwenbergh G. A comparative efficacy study of two dandruff shampoos [Abstract 1206]. The 19th World Congress of Dermatology 15‐20 June, 1997 Sydney, Australia. Australasian Journal of Dermatology 1997;38(Suppl 2):94. [Google Scholar]
High 2006 {published data only}
- High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. Journal of the American Academy of Dermatology 2006;54(6):1083‐8. [EMBASE: 2006227424] [DOI] [PubMed] [Google Scholar]
Hochman 1988 {published data only}
- Hochman HA, Schwartz SR. A comparison of two sulfur and salicylic acid shampoos in the treatment of dandruff. Current Therapeutic Research Clinical & Experimental 1988;44(6):1071‐5. [EMBASE: 1989023984] [Google Scholar]
Humke 2002 {published data only}
- Humke S, Budde MA, Richard A, Rougier A, Krutmann J. Efficacy and tolerability of a new shampoo in the treatment of dandruff [Abstract PO425]. 20th World Congress of Dermatology Paris 1st to 5th July 2002. Annales de Dermatologie et de Venereologie 2002;129:1S454. [Google Scholar]
Jacksonville 1969 {published data only}
- Jacksonville Dermatology Society. Hydrocortisone Alcohol in the Treatment of Seborrheic Dermatitis. Cutis 1969;5(9):1130‐2. [Google Scholar]
Jafferany 2008 {published data only}
Jaramillo 1992 {published data only}
- Jaramillo Antillón O, Hernández López J, Koon Rodríguez S, Rodríguez Vindas J, Freer Bustamante E. Comparative double‐blind, randomized zinc pyrithion 1.5 shampoo in the treatment of dandruff [Estudio comparativo a doble ciego y aleatoria del zinc pyrithion al 1.5% en champu en el tratamiento de la caspa]. Revista Costarricense de Ciencias Medicas 1992;13(1/2):33‐6. [Google Scholar]
Jensen 2009 {published data only}
- Jensen J, Clausen C, Folster‐Holst R, Proksch E, Poblete‐Gutierrez P. Seborrheic eczema responds more quickly to treatment with pimecrolimus cream than treatment with ketoconazole cream. 36th Annual Meeting of the Arbeitsgemeinschaft Dermatologische Forschung, ADF Heidelberg Germany. Conference Start: 20090305 Conference End: 20090307. Experimental Dermatology 2009;18(3):302. [EMBASE: 70202963] [Google Scholar]
Jensen 2010 {published data only}
- Jensen J‐M, Clausen C, Brasch J, Brautigam M, Glaser R, Schwarz T, et al. Pimecrolimus Cream Treatment Normalizes Clinical Symptoms, Skin Barrier Function and Antimicrobial Protein Expression in Seborrheic Eczema. 40th Annual Meeting of the European Society for Dermatological Research Helsinki Finland. Conference Start: 20100908 Conference End: 20100911. Journal of Investigative Dermatology 2010;130(Issue S2):S69. [EMBASE: 70263800] [Google Scholar]
Kaminester 2002 {published data only}
- Kaminester L, Bhagwat D. Clinical efficacy of a unique 10% sulfacetamide lotion in patients with seborrheic dermatitis of the scalp [Abstract P1982]. 20th World Congress of Dermatology Paris 1st to 5th July 2002. Annales de Dermatologie et de Venereologie 2002;129:1S758. [Google Scholar]
Karsono 2010 {published data only}
- Karsono JK, Kerr K, Darcy T, Henry J, Mizoguchi H, Schwartz J, et al. New innovations in anti‐dandruff technology: Proteomics application [Abstract]. 1st Eastern Asia Dermatology Congress, EADC2010 Fukuoka Japan. Conference Start: 20100930 Conference End: 20101003. Journal of Dermatology 2010;37:97. [EMBASE: 70284716] [Google Scholar]
Kim 2012 {published data only}
- Kim TW, Jwa SW, Song M, Kim HS, Ko HC, Kim MB, et al. How frequently should 0.1% tacrolimus ointment be used to reduce exacerbation of stabilised adult facial seborrhoeic dermatitis? Results from a randomised, double‐blind, vehicle‐controlled, multi‐centre trial [Abstract]. 2nd Eastern Asia Dermatology Congress Beijing China. Conference Start: 20120613 Conference End: 20120615. Journal of Dermatology 2012;39(Suppl):19. [EMBASE: 70800535] [Google Scholar]
Kim 2013 {published data only}
- Kim TW, Mun JH, Jwa SW, Song M, Kim HS, Ko HC, et al. Proactive treatment of adult facial seborrhoeic dermatitis with 0.1% tacrolimus ointment: randomized, double‐blind, vehicle‐controlled, multi‐centre trial. Acta Dermato‐Venereologica 2013;93(5):557‐561. [EMBASE: 2013558915] [DOI] [PubMed] [Google Scholar]
Kircik 2009 {published data only}
- Kircik L. An open‐label, single‐center pilot study to determine the antifungal activity of a new nonsteroidal cream (Promiseb Topical Cream) after 7 days of use in healthy volunteers. Clinics in Dermatology 2009;27(6 Suppl):S44‐7. [EMBASE: 19878780] [DOI] [PubMed] [Google Scholar]
Levy 1974 {published data only}
- Levy J, Highly FM Jr. Double‐Blind Trial of a New Topical Corticosteroid in a US Naval Hospital. Military Medicine 1974;139(9):728‐30. [EMBASE: 0975135470] [Google Scholar]
Li 2000 {published data only}
- Li FC, Liu F, Wang XL. Observations to the curative effects in the treatment of 120 cases of seborrhoeic dermatitis and pityriasis capitis with triatop and coal tar lotion. Journal of Clinical Dermatology 2000;29:290. [Google Scholar]
Lin 2010 {published data only}
- Lin AN. Innovative use of topical calcineurin inhibitors. Dermatologic Clinics 2010;28(3):535‐45. [EMBASE: 2010324521] [DOI] [PubMed] [Google Scholar]
López Padilla 1996 {published data only}
- López Padilla S, Carvajal A. Ketoconazole 1% shampoo vs climbazole shampoo on the treatment of seborrhoeic dermatitis in scalp skin [Ketoconazol al 1 por ciento champú vs. climbazole champú en el tratamiento de la dermatitis seborreica en piel cabelluda]. Dermatología Revista Mexina 1996;40(3):190‐5. [Google Scholar]
Luo 1993 {published data only}
- Luo QL, Shun GX, Wu XG, Li S, Tang Z. The clinical observation of 901 cases multi‐effective lotion for scalp steatorrhea dermatitis (Chinese). Chinese Journal of Dermatovenereology 1993;7(2):98‐9. [Google Scholar]
Marks 1974 {published data only}
- Marks R, Bhogal B, Wilson L. The effect of betamethasone valerate on seborrhoeic dermatitis of the scalp. A clinical, histopathological and cell kinetic study. Acta Dermato‐Venereologica 1974;54(5):373‐5. [EMBASE: 0975122569] [PubMed] [Google Scholar]
Mensing 2008 {published data only}
Nolting 1983 {published data only}
- Nolting S, Hagemeier HH. Betamethasone‐17,21‐dipropionate plus salicylic acid compared with betamethasone‐dipropionate solution in the therapy of erythemato‐squamous dermatoses [Therapie erythrosquamoser Dermatosen. Betamethason‐Dipropionat plus Salizylsaure im Vergleich zu Betamethason‐Dipropionat‐Losung]. Fortschritte der Medizin 1983;101(37):1679‐83. [EMBASE: 6227542] [PubMed] [Google Scholar]
Nolting 1985 {published data only}
- Nolting S. Treatment with topical corticosteroids in severe or resistant dermatoses [Behandlung mit Kortikosteroid‐Externa bei schweren oder resistenten Dermatosen]. Dermatosen in Beruf und Umwelt 1985;33(4):140‐4. [EMBASE: 1985211204] [PubMed] [Google Scholar]
Pierard‐Franchimont 1995 {published data only}
- Pierard‐Franchimont C, Pierard P. Subjects using anti‐dandruff shampoos. Journal of the European Academy of Dermatology & Venereology 1995;5(Suppl 1):S153. [Google Scholar]
Pierard‐Franchimont 1999 {published data only}
- Pierard‐Franchimont C, Henry F, Uhoda I, Pierard GE. Comparative anti‐dandruff efficacy between a tar and a non‐tar shampoo. Journal of the European Academy of Dermatology & Venereology 1999;12:S305. [DOI] [PubMed] [Google Scholar]
Pierard‐Franchimont 2000 {published data only}
- Pierard‐Franchimont C, Pierard GE, Vroome V, Lin GC, Appa Y. Comparative anti‐dandruff efficacy between a tar and a non‐tar shampoo. Dermatology 2000;200(2):181‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pierard‐Franchimont 2002a {published data only}
- Pierard‐Franchimont C, Goffin V, Henry F, Uhoda I, Braham C, Pierard GE. Nudging hair shedding by antidandruff shampoos. A comparison of 1% ketoconazole, 1% piroctone olamine and 1% zinc pyrithione formulations. International Journal of Cosmetic Science 2002;24(5):249‐56. [EMBASE: 2002427181] [DOI] [PubMed] [Google Scholar]
Pierard‐Franchimont 2002b {published data only}
- Pierard‐Franchimont C, Pierard GE. A double‐blind placebo‐controlled study of ketoconazole + desonide gel combination in the treatment of facial seborrheic dermatitis. Dermatology 2002;204(4):344‐7. [EMBASE: 2002222909] [DOI] [PubMed] [Google Scholar]
Pierard‐Franchimont 2002c {published data only}
- Pierard‐Franchimont C, Goffin V, Decroix J, Pierard GE. A multicenter randomized trial of ketoconazole 2% and zinc pyrithione 1% shampoos in severe dandruff and seborrheic dermatitis. Skin Pharmacology & Applied Skin Physiology 2002;15(6):434‐41. [EMBASE: 2003001937] [DOI] [PubMed] [Google Scholar]
Reiffenstuhl 1973 {published data only}
- Reiffenstuhl B, Wolf D. Clinical experiences with fluocinonide FAPG [Klinische Erfahrungen mit Fluocinonid FAPG]. Wiener Medizinische Wochenschrift 1973;123(38):555‐6. [EMBASE: 4270843] [PubMed] [Google Scholar]
Reinhard 1974 {published data only}
- Reinhard W, Weber G, Petres J. Comparative studies with halcinonide and fluocortolone in local administration [Vergleichende Untersuchungen mit Halcinonid und Fluocortolon bei lokaler Applikation]. Zeitschrift für Hautkrankheiten 1974;49(7):267‐72. [EMBASE: 4275779] [PubMed] [Google Scholar]
Sohn 1978 {published data only}
- Sohn HS, Kim YP. A Study on the Effectiveness of Clocortolone Pivalate (Purantix®) Cream on Certain Common Skin diseases. Korean Journal of Dermatology 1978;16(4):311‐7. [EMBASE: 0979011221] [Google Scholar]
Tomoka 1973 {published data only}
- Tomoka MT, Ochoa AG. Clinical effectiveness of fluocinonide in several skin diseases [Klinische Wirksamkeit des Fluocinonid bei verschiedenen Dermatosen]. Wiener medizinische Wochenschrift 1973;123(15):247‐8. [EMBASE: 4267190] [PubMed] [Google Scholar]
Turnbull 1982 {published data only}
- Turnbull BC. Locoid vs Betnovate lotion in the treatment of seborrhoeic and atopic dermatitis of the scalp. New Zealand Medical Journal 1982;95(718):738‐40. [EMBASE: 1983057047] [PubMed] [Google Scholar]
Veien 1980 {published data only}
- Veien NK, Pilgaard CE, Gade M. Seborrhoeic dermatitis of the scalp treated with a tar/zinc pyrithione shampoo. Clinical & Experimental Dermatology 1980;5(1):53‐6. [EMBASE: 1980099261] [DOI] [PubMed] [Google Scholar]
Wacker 1989 {published data only}
- Wacker KH. Treatment of seborrhoeic dermatitis and other disorders of the scalp requiring corticosteroids using alclometasone dipropionate 0.05% solution. Results of a double‐blind‐study [DELONAL LOSUNG (ALCLOMETASONDIPROPIONAT 0,05%) BEI SEBORRHOISCHEM KOPFEKZEM UND ANDEREN KORTIKOIDBEDURFTIGEN KOPFHAUTERKRAMKUNGEN. ERGEBNISSE EINER DOPPELBLINDSTUDIE]. Aktuelle Dermatologie 1989;15(3):84‐7. [EMBASE: 1989080414] [Google Scholar]
Weiss 2011 {published data only}
- Weiss S, Raton B, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions [Abstract]. 69th Annual Meeting of the American Academy of Dermatology New Orleans, LA United States. Conference Start: 20110204 Conference End: 20110208. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB50. [EMBASE: 70331516] [Google Scholar]
Wollina 2006 {published data only}
- Wollina U, Hansel G, Koch A, Abdel‐Naser MB. Topical pimecrolimus for skin disease other than atopic dermatitis. Expert Opinion on Pharmacotherapy 2006;7(14):1967‐75. [EMBASE: 2006483409] [DOI] [PubMed] [Google Scholar]
Wollina 2007 {published data only}
- Wollina U. The role of topical calcineurin inhibitors for skin diseases other than atopic dermatitis. American Journal of Clinical Dermatology 2007;8(3):157‐73. [EMBASE: 2007314620] [DOI] [PubMed] [Google Scholar]
Yawalkar 1983 {published data only}
- Yawalkar SJ, Macarol V, Montanari C. An Overview of International Clinical Trials with Halometasone Cream. Journal of International Medical Research 1983;11(Suppl 1):1‐7. [EMBASE: 1983071917] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fredriksson 1975b {published data only}
- Fredriksson T. Treatment of seborrheic dermatitis: double‐blind studies comparing dexamethasone 0.012% and fluocinolone acetonide 0.01%. Cutis 1975;15(5):765‐8. [EMBASE: 0976065942] [Google Scholar]
Snyder 1969 {published data only}
- Snyder BL. A double blind evaluation of fluocinolone acetonide solution in the treatment of seborrheic dermatitis of the face. Pennsylvania Medicine 1969;72(6):68‐9. [EMBASE: 4239005] [PubMed] [Google Scholar]
References to ongoing studies
EudraCT 2005‐006208‐21 {published data only}
- 2005‐006208‐21. Clinical Efficacy of Pimecrolimus Cream in Seborrheic Dermatitis. Efficacy of pimecrolimus in normalizing clinical symptoms, explorative study of barrier function, hydration, lipid content and differentiation in seborrheic dermatitis: a randomized, double‐blind study in adults with seborrheic dermatitis treated with 1 % pimecrolimus cream versus 2 % ketoconazole cream as control. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2005‐006208‐21 (accessed 28 May 2013).
EudraCT 2006‐003984‐30 {published data only}
- 2006‐003984‐30. A multicenter, randomized, double‐blind, two‐arm, vehicle‐controlled, parallel‐group, two stage study to evaluate and demonstrate the efficacy and to evaluate the safety of pimecrolimus 1% cream in the treatment of seborrhoeic dermatitis in patients 12 years of age and older. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2006‐003984‐30 (accessed 28 May 2013).
EudraCT 2007‐007088‐25 {published data only}
- 2007‐007088‐25. Inflammatory seborrhoeic dermatitis of the scalp in adult. www.clinicaltrialsregister.eu/ctr‐search/search?query=2007‐007088‐25 (accessed 28 May 2013).
EudraCT 2009‐013120‐23 {published data only}
- 2009‐013120‐23. Efficacy and safety of LBC 45 (lithium gluconate) for the treatment of seborrhoeic dermatitis of the scalp [Efficacité et tolérance du LBC 45 dans la dermite séborrhéique du cuir chevelu]. www.clinicaltrialsregister.eu/ctr‐search/search?query=2009‐013120‐23 (accessed 28 May 2013).
EudraCT 2010‐022861‐93 {published data only}
- 2010‐022861‐93. Confirmation of th efficacy and safety of LBC 45 (lithium gluconate) for the treatment of seborrhoeic dermatitis of the scalp [Confirmation de l'efficacité et de la tolérance du LBC 45 dans la dermite séborrhéique du cuir chevelu]. www.clinicaltrialsregister.eu/ctr‐search/search?query=2010‐022861‐93 (accessed 28 May 2013).
NCT00403559 {published data only}
- NCT00403559. A 4 Week Study of Elidel for the Treatment of Seborrheic Dermatitis. clinicaltrials.gov/ct2/show/NCT00403559 (accessed 28 May 2013).
NCT01011621 {published data only}
- NCT01011621. Efficacy and Tolerability of Prednisolone Acetate 0.5% Cream Versus Betamethasone Valerate 0.1% Cream in Cortisosensitive Dermatosis. clinicaltrials.gov/ct2/show/study/NCT01011621 (accessed 28 May 2013).
Additional references
Ang‐Tiu 2012
- Ang‐Tiu C, Maghrajani C, Maano C. Pimecrolimus 1% cream for the treatment of seborrheic dermatitis: a systematic review of randomized controlled trials.. Expert Review of Clinical Pharmacology 2012;5(1):91‐7. [EMBASE: 2011676752] [DOI] [PubMed] [Google Scholar]
Burton 1983
- Burton JL, Pye RJ. Seborrhoea is not a feature of seborrhoeic dermatitis. British Medical Journal 1983;286:1169‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Rosso 2011
- Rosso JQ. Adult Seborrheic Dermatitis: A Status Report on Practical Topical Management. The Journal of Clinical and Aesthetic Dermatology 2011;4(5):32‐8. [PUBMED: 21607192 ] [PMC free article] [PubMed] [Google Scholar]
Foley 2003
- Foley P, Zuo Y, Plunkett A, Merlin K, Marks R. The frequency of common skin conditions in pre‐school‐aged children in Australia: seborrheic dermatitis and pityriasis capitis (cradle cap). Archives of Dermatology 2003;139(3):318‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gupta 2004
- Gupta A, Bluhm R. Seborrheic dermatitis. Journal of European Academy of Dermatology & Venereology 2004;18(1):13‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Halder 2003
- Halder RM, Nootheti PK. Ethnic skin disorders overview. Journal of the American Academy of Dermatology 2003;48:143‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org.
Kim 2010
- Kim D, Lockey R. Dermatology for the allergist. World Allergy Organization Journal 2010;3(6):202‐15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naldi 2009
- Naldi L, Rebora A. Clinical practice. Seborrheic dermatitis. The New England Journal of Medicine 2009;360(4):387‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Okokon 2011
- Okokon EO, Oyo‐Ita A, Chosidow O. Interventions for seborrhoeic dermatitis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008138] [DOI] [Google Scholar]
Schwartz 2006
- Schwartz RA, Januz CA, Janniger CK. Seborrheic dermatitis: an overview. American Family Physician 2006;74(1):125‐30. [MEDLINE: ] [PubMed] [Google Scholar]


