Abstract
Background
Non‐invasive ventilation (NIV) with bi‐level positive airway pressure (BiPAP) is commonly used to treat patients admitted to hospital with acute hypercapnic respiratory failure (AHRF) secondary to an acute exacerbation of chronic obstructive pulmonary disease (AECOPD).
Objectives
To compare the efficacy of NIV applied in conjunction with usual care versus usual care involving no mechanical ventilation alone in adults with AHRF due to AECOPD. The aim of this review is to update the evidence base with the goals of supporting clinical practice and providing recommendations for future evaluation and research.
Search methods
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED), and PsycINFO, and through handsearching of respiratory journals and meeting abstracts. This update to the original review incorporates the results of database searches up to January 2017.
Selection criteria
All randomised controlled trials that compared usual care plus NIV (BiPAP) versus usual care alone in an acute hospital setting for patients with AECOPD due to AHRF were eligible for inclusion. AHRF was defined by a mean admission pH < 7.35 and mean partial pressure of carbon dioxide (PaCO2) > 45 mmHg (6 kPa). Primary review outcomes were mortality during hospital admission and need for endotracheal intubation. Secondary outcomes included hospital length of stay, treatment intolerance, complications, changes in symptoms, and changes in arterial blood gases.
Data collection and analysis
Two review authors independently applied the selection criteria to determine study eligibility, performed data extraction, and determined risk of bias in accordance with Cochrane guidelines. Review authors undertook meta‐analysis for data that were both clinically and statistically homogenous, and analysed data as both one overall pooled sample and according to two predefined subgroups related to exacerbation severity (admission pH between 7.35 and 7.30 vs below 7.30) and NIV treatment setting (intensive care unit‐based vs ward‐based). We reported results for mortality, need for endotracheal intubation, and hospital length of stay in a 'Summary of findings' table and rated their quality in accordance with GRADE criteria.
Main results
We included in the review 17 randomised controlled trials involving 1264 participants. Available data indicate that mean age at recruitment was 66.8 years (range 57.7 to 70.5 years) and that most participants (65%) were male. Most studies (12/17) were at risk of performance bias, and for most (14/17), the risk of detection bias was uncertain. These risks may have affected subjective patient‐reported outcome measures (e.g. dyspnoea) and secondary review outcomes, respectively.
Use of NIV decreased the risk of mortality by 46% (risk ratio (RR) 0.54, 95% confidence interval (CI) 0.38 to 0.76; N = 12 studies; number needed to treat for an additional beneficial outcome (NNTB) 12, 95% CI 9 to 23) and decreased the risk of needing endotracheal intubation by 65% (RR 0.36, 95% CI 0.28 to 0.46; N = 17 studies; NNTB 5, 95% CI 5 to 6). We graded both outcomes as 'moderate' quality owing to uncertainty regarding risk of bias for several studies. Inspection of the funnel plot related to need for endotracheal intubation raised the possibility of some publication bias pertaining to this outcome. NIV use was also associated with reduced length of hospital stay (mean difference (MD) ‐3.39 days, 95% CI ‐5.93 to ‐0.85; N = 10 studies), reduced incidence of complications (unrelated to NIV) (RR 0.26, 95% CI 0.13 to 0.53; N = 2 studies), and improvement in pH (MD 0.05, 95% CI 0.02 to 0.07; N = 8 studies) and in partial pressure of oxygen (PaO2) (MD 7.47 mmHg, 95% CI 0.78 to 14.16 mmHg; N = 8 studies) at one hour. A trend towards improvement in PaCO2 was observed, but this finding was not statistically significant (MD ‐4.62 mmHg, 95% CI ‐11.05 to 1.80 mmHg; N = 8 studies). Post hoc analysis revealed that this lack of benefit was due to the fact that data from two studies at high risk of bias showed baseline imbalance for this outcome (worse in the NIV group than in the usual care group). Sensitivity analysis revealed that exclusion of these two studies resulted in a statistically significant positive effect of NIV on PaCO2. Treatment intolerance was significantly greater in the NIV group than in the usual care group (risk difference (RD) 0.11, 95% CI 0.04 to 0.17; N = 6 studies). Results of analysis showed a non‐significant trend towards reduction in dyspnoea with NIV compared with usual care (standardised mean difference (SMD) ‐0.16, 95% CI ‐0.34 to 0.02; N = 4 studies). Subgroup analyses revealed no significant between‐group differences.
Authors' conclusions
Data from good quality randomised controlled trials show that NIV is beneficial as a first‐line intervention in conjunction with usual care for reducing the likelihood of mortality and endotracheal intubation in patients admitted with acute hypercapnic respiratory failure secondary to an acute exacerbation of chronic obstructive pulmonary disease (COPD). The magnitude of benefit for these outcomes appears similar for patients with acidosis of a mild (pH 7.30 to 7.35) versus a more severe nature (pH < 7.30), and when NIV is applied within the intensive care unit (ICU) or ward setting.
Plain language summary
Non‐invasive ventilation for people with respiratory failure due to exacerbation of chronic obstructive pulmonary disease (COPD)
Why is this question important?
When people have a severe attack of COPD, their breathing becomes very difficult. This can turn into breathing failure (acute hypercapnic respiratory failure (AHRF)) that often requires urgent hospital‐based medical care. One of the treatments that may be given is breathing support (intubation and mechanical ventilation). This involves delivery of air and/or oxygen via a ventilator connected to a tube inserted down the throat and into the lungs. This is undoubtedly a lifesaving procedure for patients with severe life‐threatening exacerbations of COPD, but it is associated with several possible unwanted side effects.
Non‐invasive ventilation (NIV) involves delivery of breathing support via a ventilator connected to a nose mask or a face mask. NIV is used more frequently nowadays to help such patients in many hospitals. This review aimed to determine the effectiveness of adding NIV to usual care for this patient group.
How did we answer the question?
We reviewed all available evidence up to January 2017 regarding effects of NIV combined with usual care compared with usual care alone (involving no ventilation). Because up to 20% of people with COPD who have respiratory failure can die from it, we looked at the number of deaths as the primary outcome. We also looked at need for intubation and time spent in hospital.
What did we find?
We included information from 17 clinical trials involving a total of 1264 patients. Compared with usual care in this patient group, we found that NIV was more beneficial for reducing deaths and the number of patients who needed to be intubated. On average, risk of dying was reduced by 46% and risk of needing intubation was reduced by 65%. Reviewers rated the quality of evidence for both of these findings as 'moderate' (according to GRADE criteria). People who had NIV spent an average of three and a half days less in hospital than those who did not.
Conclusion
This review provides convincing evidence to support the use of NIV as an effective treatment strategy for patients admitted to hospital for acute exacerbations of COPD and respiratory failure.
Summary of findings
Summary of findings for the main comparison. Non‐invasive ventilation versus usual medical care for management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease (overall effects).
| Non‐invasive ventilation versus usual medical care for management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease (overall effects) | ||||||
| Patient or population: Patients admitted to hospital with acute hypercapnic respiratory failure due to an exacerbation of chronic obstructive pulmonary disease (COPD) Setting: Acute inpatient Intervention: Non‐invasive ventilation Comparison: Usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care ‐ Overall | Risk with NIV | |||||
| Mortality | 183 per 1000 | 99 per 1000 (70 to 139) | RR 0.54 (0.38 to 0.76) | 854 (12 RCTs) | ⊕⊕⊕⊝ MODERATEa | Downgraded owing to risk of bias for some included studies |
| Need for endotracheal intubation | 341 per 1000 | 123 per 1000 (95 to 157) | RR 0.36 (0.28 to 0.46) | 1105 (17 RCTs) | ⊕⊕⊕⊝ MODERATEa | Downgraded owing to risk of bias for some included studies |
| Length of hospital stay (days) | Mean length of hospital stay (days) was 17.5 | MD 3.39 lower (5.93 lower to 0.85 lower) | ‐ | 888 (10 RCTs) | ⊕⊕⊕⊝ MODERATEa,b | Downgraded owing to risk of bias and inconsistency of findings for some included studies |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aSeveral risk of bias items rated 'unclear'
bOne study reported an effect estimate that favoured usual medical care (non‐significant); significant statistical heterogeneity identified within the intensive care unit subgroup was unable to be resolved
Background
Individuals with chronic obstructive pulmonary disease (COPD), particularly those with more severe disease, are prone to exacerbations that frequently result in admission to hospital. Severe acute exacerbations of COPD (AECOPDs) are commonly characterised by development of acute respiratory acidaemia due to prolonged hypercapnia (elevated levels of carbon dioxide). This clinical state is known as acute hypercapnic respiratory failure (AHRF). Between one fifth and one third of patients with COPD admitted to hospital with AHRF die in hospital despite the use of mechanical ventilation support strategies (Ambrosino 1995; Bott 1993; Brochard 1995; Foglio 1992; Jeffrey 1992; Roberts 2011).
Description of the condition
In severe COPD, hyperinflation places the respiratory muscles at a mechanical disadvantage, and they function close to their maximum capacity (Macklem 1984; Tobin 1986). During acute exacerbations, elastic and resistive loads on the respiratory muscles increase, and this may lead to ventilatory failure. Ensuing tissue acidosis further impairs ventilatory muscle function, which leads to the downward spiral of ventilatory failure (Jaun 1984).
Various methods of ventilatory support are available for the compromised patient. Conventional therapy aims to facilitate adequate oxygenation while treating the cause of the exacerbation. This is usually achieved with the use of bronchodilators, corticosteroids, antibiotics, and controlled oxygen. Traditionally, patients who do not respond to conventional treatment would receive invasive mechanical ventilation. This mode of ventilation involves sedation, intubation (insertion of a tube into the airway for breathing), attachment to a mechanical ventilator, and transfer to an intensive care unit (ICU). This treatment strategy has been commonly used in clinical practice for some years and is associated with successful reversal of hypercapnic acidaemia and recovery of breathing function in some individuals. However, it is also associated with significant risks. The intubation process may cause damage to local tissue structures, and the course of ventilation may be complicated by factors such as ventilator‐associated pneumonia and sinusitis (Fagon 1993; Koenig 2006; Waters 2015). Invasive mechanical ventilation in patients with COPD is also associated with high morbidity and difficulty weaning from ventilatory support (Brochard 1994; Esteban 1995). Prolonged length of ICU stay is therefore not uncommon for this patient group.
Description of the intervention
Non‐invasive ventilation (NIV) is an alternative management option for AHRF secondary to AECOPD (Bott 1993; Fagon 1993; Kramer 1995; Meduri 1989). NIV allows provision of positive pressure ventilation; however unlike invasive ventilation, NIV is performed without the need for sedation and intubation. Instead, ventilatory support is provided by a flow generator connected to NIV via a full face or nasal mask. Advantages of NIV over invasive ventilation include the ability to apply it for short, intermittent periods (which may be sufficient to reverse ventilatory failure);lack of sedation and its potential adverse secondary effects (e.g. ventilatory suppression); maintenance of the ability to eat, drink, and converse; and the consequent opportunity for individuals to have continued involvement in decisions regarding their care. It is important to note that the incidence of nosocomial pneumonia observed with NIV use is less than that seen among intubated patients (Guerin 1997; Kramer 1999; Nourdine 1999). NIV is increasingly used as adjunctive therapy in the management of acute exacerbations of COPD. Therefore, it is essential that the effectiveness of NIV as a primary management option is accurately determined to verify its use in patients with AECOPD previously characterised by greater reliance on invasive ventilation.
How the intervention might work
The mechanisms underpinning effects of NIV among patients with AHRF are fundamentally similar to those supporting mechanical ventilation, that is, NIV works to enhance ventilation by providing pressure‐supported airflow to unload fatigued ventilatory muscles. This enables recovery of function of respiratory muscles of ventilation and facilitates normalisation of, or improvement in, lung volumes and lung mechanics to reverse acidaemia (Appendi 1994). Clinical improvement is most commonly determined via analysis of arterial blood gas samples and overall clinical state. NIV is used increasingly in clinical practice and is an established form of treatment for patients with a variety of chronic hypoventilatory syndromes (Moloney 1999).
Why it is important to do this review
Use of NIV in AHRF due to AECOPD has been supported by a number of case series (Brochard 1990; Foglio 1992; Meduri 1989) and randomised controlled trials (RCTs; Bott 1993; Celikel 1998, Plant 2001). Despite this fact, NIV is not more successful than usual care in all cases of AHRF due to AECOPD (Barbe 1996), and failure rates of between 9% and 50% have been reported (Kramer 1995; Soo Hoo 1994). Factors that may relate to this include patient‐ventilator dyssynchrony, the impact of coadjuvant polypharmacy for AECOPDs such as anxiolytics or respiratory suppressants, and factors related to staff (e.g. time, expertise) and individual patients (e.g. claustrophobia). A matter of concern is that NIV, particularly when applied unsuccessfully, may delay the start of endotracheal intubation and mechanical ventilation, thereby potentially resulting in poorer health outcomes (Ambrosino 1996; Wood 1998). This may be influenced by the common clinical situation whereby patients with AECOPD find tight‐fitting NIV masks (whether nasal or full face) uncomfortable or claustrophobic. Intolerance may result in poor treatment adherence and, ultimately, in NIV ineffectiveness. This is an update of a Cochrane Review (Ram 2004).
Objectives
To compare the efficacy of NIV applied in conjunction with usual care versus usual care involving no mechanical ventilation alone in adults with AHRF due to AECOPD. The aim of this review is to update the evidence base with the goals of supporting clinical practice and providing recommendations for future evaluation and research.
Methods
Criteria for considering studies for this review
Types of studies
We considered only RCTs for inclusion in this review. We did not exclude studies described as 'randomised' but lacking sufficient information to reveal the adequacy of such methods. Cross‐over studies were not eligible for inclusion in the review.
Types of participants
Studies must have been conducted on adult patients admitted to hospital with AHRF due to AECOPD. Studies of patients who commenced NIV before hospital admission were not eligible for inclusion. We defined AHRF by a mean admission pH < 7.35 and mean baseline admission partial pressure of carbon dioxide (PaCO2) greater than 45 mmHg (6 kPa). If we could not verify mean baseline pH data, we accepted studies if investigators stated within their inclusion criteria that participants needed to have had an admission pH < 7.35. Studies of participants with a primary diagnosis of pneumonia and of those with other underlying pathologies were not eligible for inclusion. Studies involving a mixed group of participant pathologies (e.g. some with COPD, some with congestive cardiac failure (CCF)) were eligible if data specifically pertaining to those with COPD were available or could be obtained. We excluded no studies on the basis of the presence of concurrent respiratory comorbidities such as obesity, obstructive sleep apnoea, obesity, and hypoventilation syndrome.
Types of interventions
Studies must have compared effects of NIV versus usual care. Non‐invasive ventilation was defined as delivery of BiPAP when inspiratory positive airway pressure (IPAP) was greater than expiratory positive airway pressure (EPAP). NIV may have been delivered via any type of interface (e.g. full face mask, nasal mask, helmet). Usual care was defined according to trial authors' definitions, which typically involveda combination of supplemental oxygen, antibiotics, bronchodilators, steroids, respiratory stimulants, and/or other suitable medical interventions (e.g. diuretics, methylxanthines). However, usual care could not include any form of 'usual' NIV or invasive ventilation. Studies that involved participants who had already received a form of invasive or non‐invasive ventilation (including continuous positive airway pressure (CPAP)) before enrolment, including studies of NIV weaning, were not eligible for inclusion.
Types of outcome measures
Primary outcomes
Mortality during hospital episode of respiratory failure
Need for endotracheal intubation (qualification for intubation and mechanical ventilation criteria, as defined by study investigators. If criteria regarding the need for endotracheal intubation were not specified or could not be accurately evaluated, actual incidence of intubation was accepted)
Secondary outcomes
Length of hospital stay
Length of ICU stay
Symptom scores (e.g. ratings of dyspnoea)
Treatment intolerance (e.g. participant unable or unwilling to adhere to treatment owing to undesirable treatment effects)
Complications (NIV‐related and those not related to NIV)
Arterial blood gas tensions one hour following commencement of NIV (pH, PaCO2, partial pressure of oxygen (PaO2))
Search methods for identification of studies
Electronic searches
For this review update, we identified trials from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org).
Weekly searches of MEDLINE Ovid SP 1946 to date.
Weekly searches of Embase Ovid SP 1974 to date.
Monthly searches of PsycINFO Ovid SP.
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature).
Monthly searches of AMED EBSCO (Allied and Complementary Medicine).
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of the Cochrane Airways Review Group. Details of these strategies, as well as a list of handsearched conference proceedings, can be found in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We conducted searches with no restriction on language or type of publication. This review update included searches conducted in November 2013, July 2015, and January 2017. We performed additional searches of three online clinical trials registries: ClinicalTrials.gov (www.ClinicalTrials.gov), controlled‐trials (www.controlled‐trials.com), and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/) (refer Appendix 3). For search methods used before 2004, see Appendix 4.
Searching other resources
We searched the reference lists of included RCTs for additional papers that might be eligible for inclusion in the review. We contacted authors of included RCTs to ask about other published and unpublished studies.
Data collection and analysis
Selection of studies
Two review authors independently assessed studies yielded by electronic searches for inclusion in the review. We coded studies as include, unclear, or exclude, according to the following criteria.
INCLUDE: Study clearly met all review criteria.
UNCLEAR: Study met some review criteria but available information is insufficient to confirm eligibility.
EXCLUDE: Study clearly did not meet review criteria.
We determined final study inclusion by obtaining consensus of two review authors using full‐text copies of studies identified as INCLUDE and UNCLEAR. We resolved discordance between review authors through consultation with a third review author.
Data extraction and management
Two review authors independently extracted data from included studies using a standardised template designed specifically for this review. When data were missing, or when we were uncertain about data presented in included studies, we contacted original authors by email to attempt to obtain data or resolve uncertainty. We included these data only if we obtained confirmation from trial authors. Two review authors entered data into Revman 5.3.5 and randomly checked accuracy. No review authors handled data from clinical trials on which they were a named investigator.
Assessment of risk of bias in included studies
We assessed risk of bias of included studies using the Cochrane 'Risk of bias' tool (Higgins 2008). This tool evaluates potential for study bias according to six domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and 'other issues'). Within this approach, we specified the following four additional items considered relevant to the context of the present review: imbalance among outcome measures at baseline, comparability of intervention and control group characteristics at baseline, protection against contamination, and selective recruitment of participants. We rated risk of bias as low, high, or unclear for all domains and presented our assessment in a 'Risk of bias' table within the review.
Measures of treatment effect
We pooled for meta‐analysis outcome data that were clinically homogenous. For continuous variables, we calculated mean differences (MDs) or standardised mean differences (SMDs) and 95% confidence intervals (95% CIs). For dichotomous variables, we calculated risk ratios (RRs) with 95% CIs, as well as the number needed to treat for an additional beneficial outcome (NNTB) using the formula NNTB = 1/ [CER * (1 ‐ RR)] (where CER = control event rate and RR = risk ratio).
Unit of analysis issues
We analysed mortality, need for endotracheal intubation, treatment intolerance, and complications as dichotomous data. We reported all other variables as continuous data. We analysed measures of blood gas tensions for PaCO2 and PaO2 as mmHg, and we converted data presented as kPa using the formula: mmHg = kPa*7.5. As we anticipated the risk of treatment intolerance to be very low in the usual care group, we evaluated data related to this outcome as risk differences.
Dealing with missing data
We attempted to contact authors of included studies if data were not readily available for analysis. We reported unpublished data obtained from study authors in characteristics of studies tables. We included only data on participants with COPD from studies that comprised mixed patient conditions (e.g. COPD and heart failure), if we could obtain the data.
Assessment of heterogeneity
We performed meta‐analyses using a fixed‐effect model when possible. When outcome data demonstrated a 'greater than moderate' risk of statistical heterogeneity, indicated by an I2 statistic > 60% (Higgins 2008), we undertook analysis using a random‐effects model.
Assessment of reporting biases
We explored the potential for publication bias in the meta‐analysis by generating funnel plots for the outcomes of mortality, need for endotracheal intubation, and hospital length of stay, assuming that five or more studies were included.
Data synthesis
We collated and analysed data from all trials using Review Manager 5.3.5. We evaluated data related to primary review outcomes as well as hospital length of stay according to the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) and presented this information in a 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
We investigated the cause of any significant statistical heterogeneity (I2 > 60%) for any outcome on the basis of duration of NIV, type of mask used to administer NIV, and risk of bias.
As management of AHRF may differ according to the severity of the presenting condition and the hospital setting in which treatment is provided, we specified the following two subgroup analyses a priori and conducted these analyses for the primary outcomes of mortality and need for endotracheal intubation.
pH: We compared studies of participants with initial mean presentation pH < 7.30 (i.e. worse) versus studies of participants with initial mean presentation pH between 7.30 and 7.35 (i.e. better); and
Hospital setting for delivery of intervention: We compared studies that applied NIV on a general ward (or in an emergency department (ED)) versus studies that applied NIV in the ICU. We defined hospital location in accordance with study author descriptions (i.e. we employed no review‐specific operationalised definitions).
Sensitivity analysis
We performed a sensitivity analysis for our primary review outcomes to evaluate the impact of studies that did not report outcome data on an intention‐to‐treat basis. We believed this was necessary, as anecdotal evidence suggests that some patients drop out or withdraw from studies of NIV after randomisation and/or upon initiation of NIV owing to discomfort.
Results
Description of studies
Refer to Characteristics of included studies and Characteristics of excluded studies for complete details of studies included or excluded from the review. This is an update of a Cochrane Review (Ram 2004).
Results of the search
Refer to Figure 1 for the PRISMA flow chart.
1.
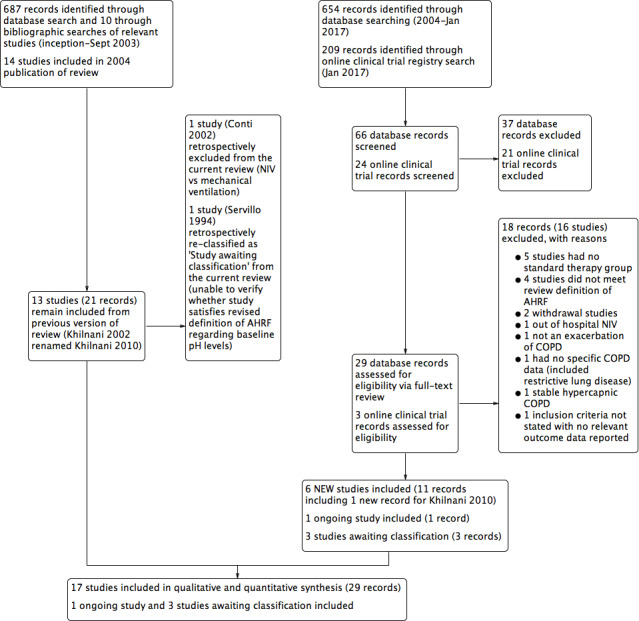
Study flow diagram for 2004‐2017 literature searches.
An electronic search conducted in September 2003 yielded 697 citations: 602 from the Cochrane Airways Trials Register and 85 from Embase, MEDLINE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and online respiratory journal databases. We obtained 10 additional references through bibliographic searching of relevant articles. On the basis of review of 697 abstracts, we identified 160 studies as potentially suitable for inclusion. Full‐text review resulted in exclusion of 138 studies and preparation of a complete list of reasons for exclusion provided under Characteristics of excluded studies. We included the remaining 22 records from 14 original studies after identifying duplicate records for Brochard 1995 (single), Dikensoy 2002 (single), and Plant 2001 (six duplicate records).
We updated the review in April 2004 with exclusion of one further study (Potena 2003).
An updated search conducted in September 2013 resulted in identification of six additional appropriate studies for inclusion (Carrera 2009; Collaborative 2005; Khilnani 2010; Liu 2005; Matuska 2006; Samaria 2009), one of which was a more complete version of the original abstract study of Khilnani 2002 (study ID changed to Khilnani 2010). We identified three Studies awaiting classification because of uncertainty regarding randomisation (Samaria 2013) and baseline pH status required to confirm the presence of AHRF (Liao 2004; Servillo 1994). We attempted to contact authors of these studies for clarification, without reply. We identified one ongoing study (Ongoing studies) via the clinical trials registry search (Duan 2011). At this time, we removed a posteriori from the review one study (Conti 2002) because we noted that it clearly failed to meet one eligibility criterion (comparison of NIV vs mechanical ventilation). The most recent updates (July 2015 and January 2017) yielded no additional included studies but one excluded study (Kong 2015).
Included studies
Seventeen studies met review inclusion criteria: Avdeev 1998; Barbe 1996; Bott 1993; Brochard 1995; Carrera 2009; Celikel 1998; Collaborative 2005; del Castillo 2003; Dikensoy 2002; Khilnani 2010; Kramer 1995; Liu 2005; Matuska 2006; Plant 2001; Samaria 2009; Thys 2002; Zhou 2001. We provide full methodological details of these studies under Characteristics of included studies and summary details below.
Design
All studies were RCTs using a parallel‐group design. We found no cross‐over studies. Some studies reported on participants crossing from the control group to receive the NIV intervention as 'rescue therapy', but we did not include such data in meta‐analyses.
Population
The included studies spanned various regions of the world including Belgium (Thys 2002), China (Collaborative 2005; Liu 2005; Zhou 2001), Czech Republic (Matuska 2006), France (Brochard 1995), India (Khilnani 2010; Samaria 2009), Italy (Brochard 1995), Russia (Avdeev 1998), Spain (Barbe 1996; Brochard 1995; Carrera 2009; del Castillo 2003), Turkey (Celikel 1998; Dikensoy 2002), the United Kingdom (Bott 1993; Plant 2001), and the United States of America (Kramer 1995). Six of the included studies were multi‐centric, including Brochard 1995 (the only international multi‐centric study, conducted in France, Spain, and Italy), Carrera 2009 (conducted in seven hospitals in Spain), Kramer 1995 (conducted in two hospitals in the USA), Bott 1993 (conducted in three centres in the UK), Plant 2001 (conducted in 14 centres in the UK), and Collaborative 2005 (conducted across 19 hospitals in China). All trials included patients who had AHRF due to AECOPD, but two studies also included patients with other diagnoses. Kramer 1995 included patients with AECOPD, heart failure, pneumonia, asthma, and pulmonary embolus, and Thys 2002 included patients with acute respiratory failure due to AECOPD and acute pulmonary oedema. For both studies, we included in the review only data related to patients with AECOPD. It is likely that participants in included studies did not represent the full spectrum of patients with AHRF due to AECOPD observed in clinical practice, as those requiring immediate intubation were typically ineligible for inclusion in the clinical trials of this review.
The number of participants in each included study ranged from 20 to 342 (median 41), with an aggregate total of 1264 (at the time of randomisation) in the review. We could not determine the precise number of participants who completed clinical trials. All trials recruited similar numbers of patients for both study groups. Available data show that mean age at recruitment was 66.8 (range 57.7 to 70.5) years, and males and females accounted for 65% and 35% of participants, respectively.
Interventions
All included studies compared NIV plus usual care versus usual care alone. The precise nature of usual care varied slightly between studies, but it typically included combinations of pharmacological therapies such as oxygen therapy, bronchodilators, corticosteroids, theophylline, antibiotics, mucolytics, doxapram, diuretics, and heparin. Variability in care was most likely attributable to differences in the years studies were conducted and/or local practices within specific regions or hospitals. Six trials were conducted in hospital respiratory/medical wards (Barbe 1996; Bott 1993; Carrera 2009; del Castillo 2003; Dikensoy 2002; Plant 2001), seven in ICU or critical care settings (Brochard 1995; Celikel 1998; Khilnani 2010; Kramer 1995; Liu 2005; Matuska 2006; Samaria 2009), one on an 'intermediate care ward' (Avdeev 1998), and one (Thys 2002) primarily in a hospital ED. We did not include in location subgroup analyses data from this latter study (Thys 2002), and we could not determine the setting for two studies (Collaborative 2005; Zhou 2001).
Investigators most commonly delivered NIV via pressure‐cycled ventilation (N = 21 studies). One study (Bott 1993) used volume‐cycled nasal NIV. Mean (range) inspiratory positive airway pressure (IPAP) values used when NIV was commenced were 10.7 (3 to 20) cmH2O, but IPAP levels were frequently titrated during early phases, according to the maximum level tolerated by the patient or a target respiratory rate. The mean (range) expiratory positive airway pressure (EPAP) value used upon NIV initiation was 4 (0 to 5) cmH2O.
Nine studies delivered NIV via a face mask interface (Brochard 1995; Carrera 2009; Celikel 1998; Collaborative 2005; del Castillo 2003; Dikensoy 2002; Liu 2005; Matuska 2006; Thys 2002), and three used nasal masks only (Barbe 1996; Bott 1993; Khilnani 2010). Four studies allowed optional use of a face mask and/or a nasal mask (Avdeev 1998; Kramer 1995; Plant 2001; Zhou 2001). We could not determine the type of interface used in the remaining included studies.
The duration of total NIV use was highly variable across included studies. Studies typically implemented NIV according to protocols that aimed to achieve a target number of hours of NIV use per day (reduced from early to late admission), but the total number of hours of NIV use was almost always individualised according to the time needed for AHRF to resolve.
Outcomes
The most commonly reported outcomes of relevance for this review were mortality (N = 12 studies), need for endotracheal intubation (N = 17 studies), and hospital length of stay (N = 10 studies). The outcome of treatment intolerance rarely included adverse events in the control group, hence results clearly appeared to favour usual care over NIV. One should consider this when interpreting the quantitative findings derived from this analysis. The extent of outcome data retrieved upon request from study authors is provided under Characteristics of included studies.
Excluded studies
We have provided a full list of reasons for study exclusion under Characteristics of excluded studies. The most common reasons for exclusion were lack of a suitable control group (N = 5 studies) and failure to meet the review definition of AHRF (N = 4 studies).
Risk of bias in included studies
We have provided in Figure 2 a summary of risk of bias for all included studies.
2.
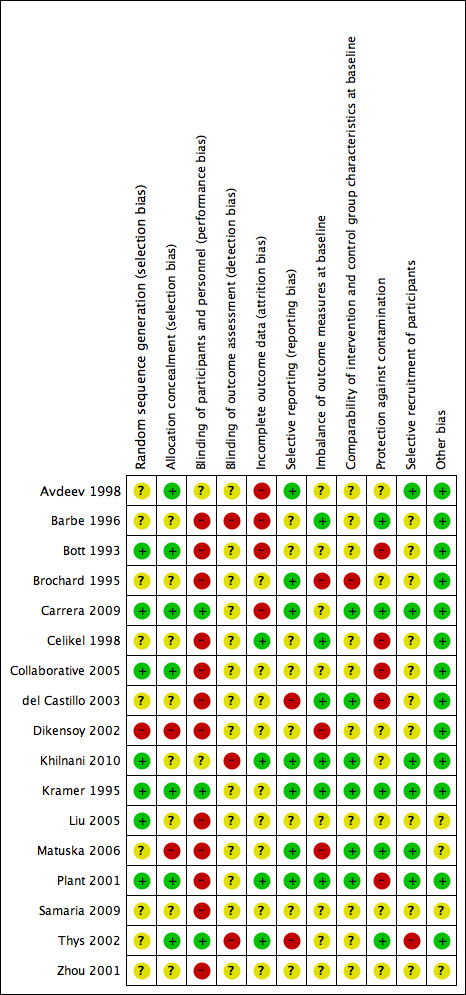
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Risk of bias due to selection procedures (random sequence generation and/or allocation concealment) was low or unclear for most studies, and we rated only Dikensoy 2002 and Matuska 2006 as high risk.
Blinding
Adequate blinding of participants to reduce knowledge of the received intervention was rare and occurred in only three studies (Carrera 2009; Kramer 1995; Thys 2002). Adequate blinding is inherently difficult to achieve in clinical trials of NIV interventions, as delivery of placebo care is challenging, and differences between active and inactive treatments are easily detectable. Knowledge of the intervention group may have affected subjective patient‐reported outcome measures such as ratings of dyspnoea, but it is likely that most other outcomes were not affected. Much uncertainty surrounds the adequacy of assessor blinding across included studies. Lack of outcome assessor blinding may have affected results related to several of the secondary review outcomes.
Incomplete outcome data
Four studies had low risk of bias owing to adequate completeness of outcome data (Celikel 1998; Khilnani 2010; Plant 2001; Thys 2002). Four studies demonstrated high risk of bias for this item owing to attrition related to primary or secondary outcomes and/or failure to adopt an intention‐to‐treat approach for analysis (Avdeev 1998; Barbe 1996; Bott 1993; Carrera 2009).
Selective reporting
For many studies (N = 10), risk of bias due to selective reporting of outcome data was unclear. We rated two studies as having high risk of bias (del Castillo 2003; Thys 2002) and the rest as having low risk.
Other potential sources of bias
We specified additional risk of bias items related to (a) imbalance of outcome measures at baseline; (b) comparability of group characteristics at baseline; (c) protection against contamination; and (d) selective recruitment of participants, owing to their potential to impact outcomes in the context of this review question. Studies at high risk of bias for these items, respectively, were (a) Brochard 1995; Dikensoy 2002; and Matuska 2006; (b) Brochard 1995; (c) Bott 1993; Celikel 1998; Collaborative 2005; del Castillo 2003; and Plant 2001; and (d) Thys 2002.
For most studies, we identified no other sources of bias and therefore determined that they were at low risk of other bias. The risk of other sources of bias was uncertain for the remaining four studies owing to insufficient information by which to judge this (Liu 2005; Matuska 2006; Samaria 2009; Zhou 2001).
Effects of interventions
See: Table 1
Mortality during the hospital episode of respiratory failure
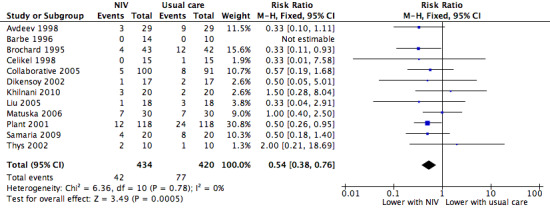
Twelve studies including 854 participants (Avdeev 1998; Barbe 1996; Brochard 1995; Celikel 1998; Collaborative 2005; Dikensoy 2002; Khilnani 2010; Liu 2005; Matuska 2006; Plant 2001; Samaria 2009; Thys 2002) contributed data towards this outcome. The overall pooled analysis shows a significantly lower incidence of mortality among participants who received NIV compared with those who received usual care. Investigators observed a 46% risk reduction (RR 0.54, 95% CI 0.38 to 0.76; participants = 854; studies = 12; I2 = 0%) (Analysis 1.1; Figure 3), yielding an NNTB of 12 (95% CI 9 to 23; Figure 4). No publication bias was evident in the funnel plot (Figure 5). One study (Barbe 1996) reported no events in either group. Another study (Bott 1993) reported mortality incidence as 3/30 for the NIV group and 9/30 for the usual care group (not significant for between‐group analysis); these data refer to 30‐day mortality. One study (Khilnani 2010) demonstrated an effect estimate that tended to favour usual care (not statistically significant); this study was unusual as participants were characterised by very severe hypercapnia upon presentation to hospital (PaCO2 > 80 mmHg in both groups). For this outcome, we included from Collaborative 2005 only data related to subgroups with pH < 7.35. We rated findings for this outcome as showing 'moderate' quality according to GRADE owing to an 'unclear' risk of bias rating for several items (Table 1).
1.1. Analysis.
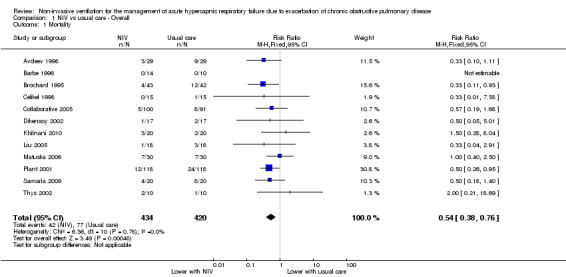
Comparison 1 NIV vs usual care ‐ Overall, Outcome 1 Mortality.
3.
NIV vs usual care (overall) ‐ Mortality
4.
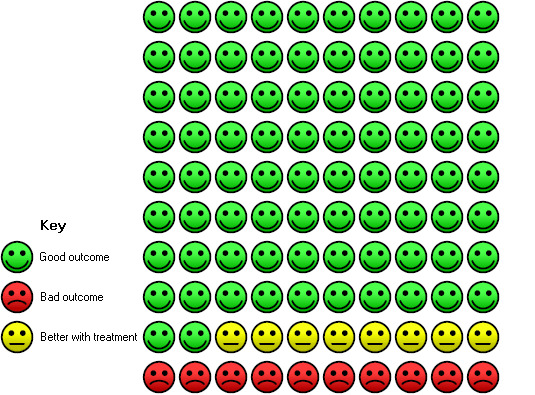
Cates plot Analysis 1.1 (mortality), NIV group: In the usual care group, 18 of 100 people died during the period of hospitalisation, compared with 10 (95% CI 7 to 14) of 100 in the NIV group.
5.
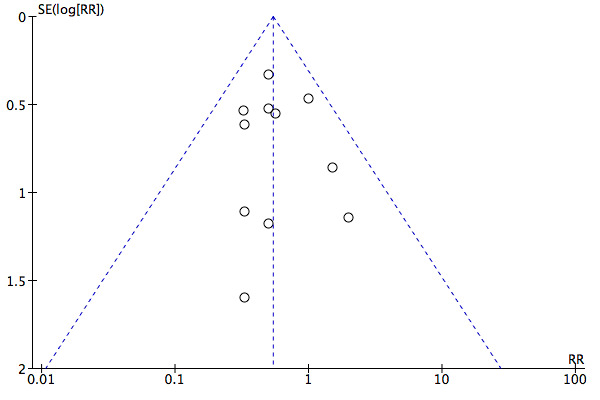
Funnel plot of comparison: 1 NIV vs usual care ‐ Overall, outcome: 1.1 Mortality.
Admission pH subgroups
Results for admission pH subgroups ranging from 7.35 to 7.30 (Barbe 1996; Collaborative 2005; Liu 2005; Plant 2001; Samaria 2009) and below 7.30 (Avdeev 1998; Brochard 1995; Celikel 1998; Collaborative 2005; Dikensoy 2002; Khilnani 2010; Matuska 2006; Thys 2002) were significantly lower with NIV use (RR 0.50, 95% CI 0.30 to 0.84; participants = 454; studies = 5; I2 = 0%; and RR 0.57, 95% CI 0.35 to 0.90; participants = 400; studies = 8; I2 = 0%, respectively). Differences between the two pH subgroups were not statistically significant according to the test for subgroup differences (Analysis 2.1).
2.1. Analysis.
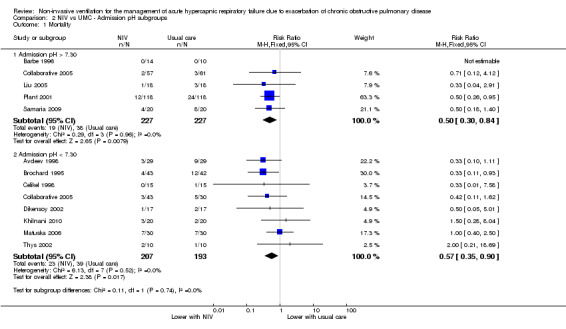
Comparison 2 NIV vs UMC ‐ Admission pH subgroups, Outcome 1 Mortality.
Study location subgroups
Mortality was significantly reduced in the NIV group based on use of a ward setting (Avdeev 1998; Bott 1993; Collaborative 2005; Dikensoy 2002; Plant 2001) (RR 0.48, 95% CI 0.29 to 0.78; participants = 543; studies = 5; I2 = 0%). Barbe 1996 (ward‐based) reported no events in either group. Data from studies conducted in the ICU (Brochard 1995; Celikel 1998; Khilnani 2010; Liu 2005; Matuska 2006) showed a trend towards reduced mortality that did not reach statistical significance (RR 0.60, 95% CI 0.34 to 1.07; participants = 251; studies = 5; I2 = 1%). Results showed no significant differences between the two locations (ICU vs ward) for the outcome of mortality (Analysis 3.1).
3.1. Analysis.
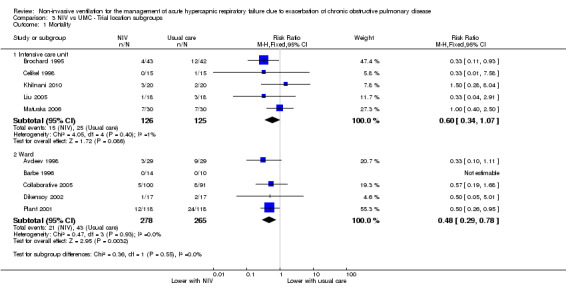
Comparison 3 NIV vs UMC ‐ Trial location subgroups, Outcome 1 Mortality.
Need for endotracheal intubation
A total of 17 studies including 1105 participants (Avdeev 1998; Barbe 1996; Bott 1993; Brochard 1995; Carrera 2009; Celikel 1998; Collaborative 2005; del Castillo 2003; Dikensoy 2002; Khilnani 2010; Kramer 1995; Liu 2005; Matuska 2006; Plant 2001; Samaria 2009; Thys 2002; Zhou 2001) contributed data towards this outcome. Results showed a significant reduction in the risk of intubation of approximately two‐thirds (64%) in the NIV group compared with the usual care group (RR 0.36, 95% CI 0.28 to 0.46; participants = 1105; studies = 17; I2 = 0%; Analysis 1.2; Figure 6) with an NNTB of 5 (95% CI 5 to 6; Figure 7). Visual inspection of the funnel plot for this outcome raised some potential for publication bias, evident by a relative lack of study data in the lower right‐hand quadrant of the plot (area signifying lack of clinical benefit in studies of small sample sizes) (Figure 8). Barbe 1996 reported no events in either group. For this outcome, we included from Collaborative 2005 only data related to subgroups with a pH < 7.35. We rated evidence as showing 'moderate' quality according to GRADE owing to ratings of 'unclear' risk of bias for several items (Table 1).
1.2. Analysis.
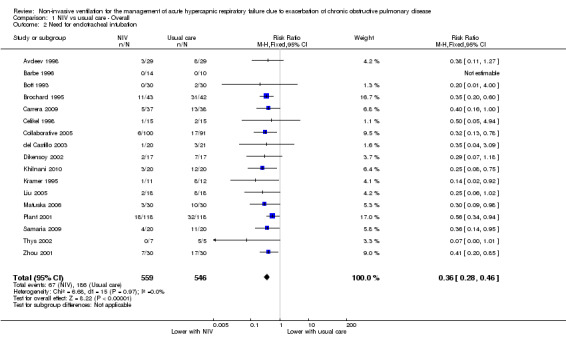
Comparison 1 NIV vs usual care ‐ Overall, Outcome 2 Need for endotracheal intubation.
6.
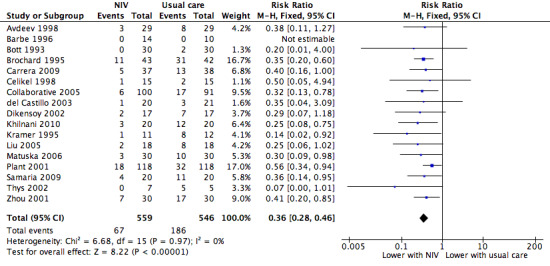
NIV vs usual care (overall) ‐ Need for endotracheal intubation
7.
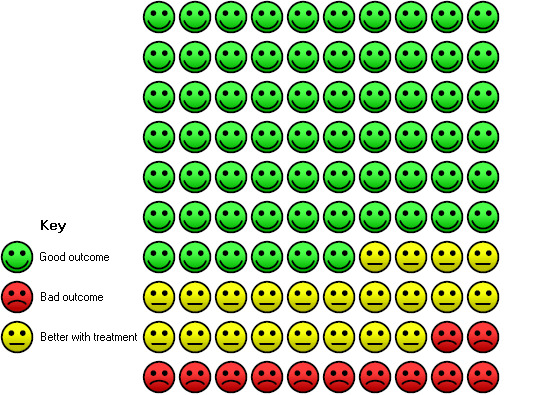
Cates plot Analysis 1.2 (need for endotracheal intubation), NIV group: In the usual care group, 34 of 100 people experienced the need for endotracheal intubation during the period of hospitalisation, compared with 12 (95% CI 10 to 16) of 100 in the NIV group.
8.
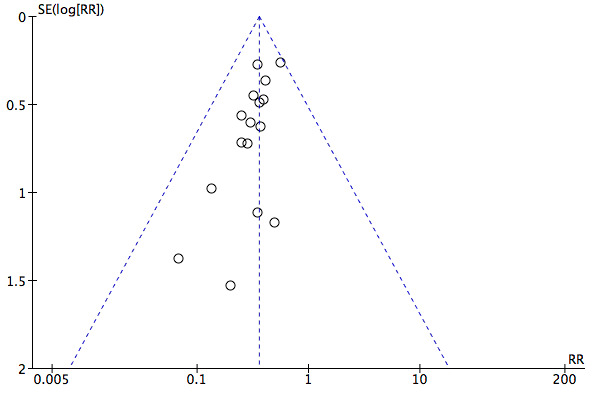
Funnel plot of comparison: 1 NIV vs usual care ‐ Overall, outcome: 1.2 Need for endotracheal intubation.
Admission pH subgroups
Need for intubation among admission subgroups with pH between 7.35 and 7.30 (Bott 1993; Carrera 2009; Collaborative 2005; Liu 2005; Plant 2001; Samaria 2009) and below 7.30 (Avdeev 1998; Brochard 1995; Celikel 1998; del Castillo 2003; Dikensoy 2002; Khilnani 2010; Kramer 1995; Matuska 2006; Thys 2002; Zhou 2001) was significantly less with NIV use (RR 0.44, 95% CI 0.30 to 0.63; participants = 589; studies = 7; I2 = 0%; and RR 0.31, 95% CI 0.22 to 0.42; participants = 516; studies = 11; I2 = 0%; test for subgroup differences; P = 0.16; Analysis 2.2). Barbe 1996 (pH > 7.30) reported no events in either group. Collaborative 2005 is represented in both subgroups, as researchers reported specific data separately for each pH cutoff threshold.
2.2. Analysis.
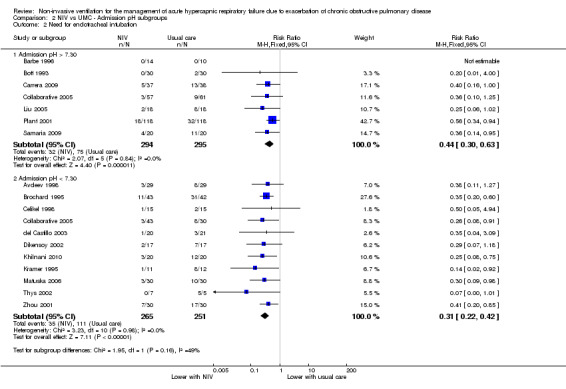
Comparison 2 NIV vs UMC ‐ Admission pH subgroups, Outcome 2 Need for endotracheal intubation.
Study location subgroups
Among location subgroups, risk of intubation was significantly reduced by NIV in both ICU‐based (Brochard 1995; Carrera 2009; Celikel 1998; Khilnani 2010; Kramer 1995; Liu 2005; Matuska 2006; Samaria 2009; Thys 2002) and ward‐based subgroups (Barbe 1996; Bott 1993; Carrera 2009; Collaborative 2005; del Castillo 2003; Dikensoy 2002; Plant 2001; Zhou 2001) (RR 0.30, 95% CI 0.21 to 0.43; participants = 401; studies = 9; I2 = 0%; and RR 0.43, 95% CI 0.31 to 0.60; participants = 721; studies = 8; I2 = 0%, respectively). No significant differences were noted between the two subgroups (studies based in the ICU or on the ward) regarding need for intubation (test for subgroup differences; P = 0.15; Analysis 3.2). Barbe 1996 (ward‐based study) reported no events in either group. Samaria 2009 involved delivery of NIV in the ICU setting but usual care on the ward.
3.2. Analysis.
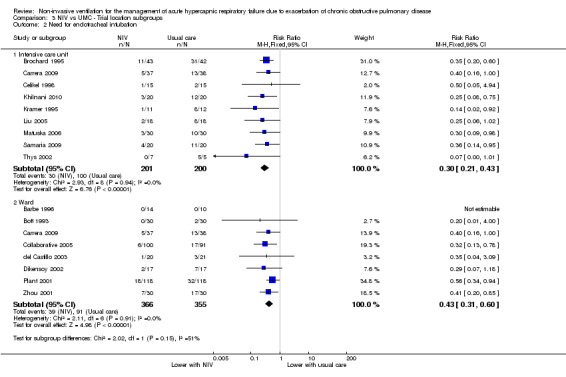
Comparison 3 NIV vs UMC ‐ Trial location subgroups, Outcome 2 Need for endotracheal intubation.
Length of hospital stay
Ten studies involving 888 participants (Avdeev 1998; Barbe 1996; Brochard 1995; Celikel 1998; Collaborative 2005; Dikensoy 2002; Khilnani 2010; Kramer 1995; Plant 2001; Thys 2002) revealed length of hospital stay to be significantly shorter for participants who received NIV compared with those who did not (MD ‐3.39, 95% CI ‐5.93 to ‐0.85; participants = 888; studies = 10; I2 = 84%). We used a random‐effects model for this analysis owing to significant statistical heterogeneity. Step‐by‐step removal of studies suggested that these results were most heavily affected by data from Collaborative 2005 (which included data pertaining to participants with admission pH ≥ 7.35) and Khilnani 2010. Additionally, Bott 1993 reported the same median length of stay for both groups (nine days). Despite several 'unclear' ratings of items for this outcome and modest inconsistency of findings related to Collaborative 2005 (which tended to favour usual care, albeit non‐significant), we believe the impact of these factors in the large review sample equated to downgrading of only one level according to GRADE criteria, resulting in an overall evidence rating of 'moderate' quality (Table 1).
Length of ICU stay
One study (Thys 2002) involving 20 participants provided data for length of ICU stay. Although a non‐significant effect favoured a reduction in ICU length of stay in the NIV group (MD ‐2.70 days, 95% CI ‐6.79 to 1.39), this finding should be interpreted with caution, as data were skewed but non‐parametric data were not available for analysis.
Symptom scores
Four studies measured dyspnoea via three different metrics (Borg scale used by Avdeev 1998 and Barbe 1996; visual analogue scale used by Bott 1993; and custom scale used by Collaborative 2005). Data from Avdeev 1998 and Collaborative 2005 represent endpoint dyspnoea ratings (at 1 and 24 hours, respectively), and data from Bott 1993 represent median symptoms over the first three days of admission (not included within the meta‐analysis). Data from Barbe 1996 represent the magnitude of symptom change over time. Pooled meta‐analysis of these data via SMD revealed a non‐significant trend towards favourable reductions in dyspnoea with NIV compared with usual care (SMD ‐0.16, 95% CI ‐0.34 to 0.02; participants = 484; studies = 4; I2 = 70%). This finding was heavily influenced by Collaborative 2005 (71.8% weighting). Plant 2001 reported a statistically significant reduction in time to resolution of dyspnoea (median time 4 days in NIV group vs 7 days in control group; P = 0.025); however these data were not suitable for inclusion in the meta‐analysis.
Treatment intolerance
Six studies involving 346 participants (Avdeev 1998; Barbe 1996; Dikensoy 2002; Khilnani 2010; Liu 2005; Matuska 2006) demonstrated significantly greater (11%) risk of treatment intolerance in the NIV group compared with the usual care group (risk difference (RD) 0.11, 95% CI 0.04 to 0.17; participants = 252; studies = 6; I2 = 0%; Analysis 1.6). Plant 2001 noted that some participants were intolerant of NIV treatment, but we could not ascertain the magnitude of this estimate nor the direction of effect relative to participants in the usual care group. Owing to the clear difference between the nature of NIV and that of usual care interventions, we expected treatment intolerance to be higher in the NIV group than in the usual care group.
1.6. Analysis.
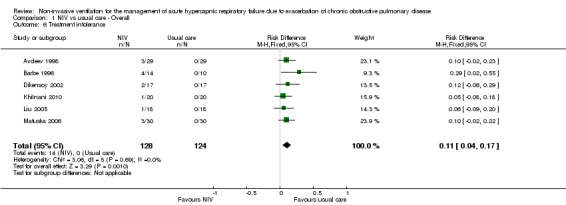
Comparison 1 NIV vs usual care ‐ Overall, Outcome 6 Treatment intolerance.
Complications of treatment
Six studies involving 567 participants (Brochard 1995; Celikel 1998; Collaborative 2005; Dikensoy 2002; Khilnani 2010; Liu 2005) contributed data towards this outcome. Analysis showed that usual care had a significantly lower risk of NIV‐related treatment complications compared with NIV (RR 29.60, 95%CI 9.47, 92.51; participants = 567; studies = 6; I2 =24%). Owing to the nature of this outcome, this result is to be expected. Two studies evaluated effects of interventions on treatment complications unrelated to NIV and found significantly lower risks of complications with NIV compared with usual care (RR 0.26, 95%CI 0.13 to 0.53; participants = 125; studies = 2; I2 =29%).
Arterial blood gas tensions one hour following commencement of NIV
pH one hour post intervention
Eight studies involving 585 participants provided pH data one hour after initiation of treatment (Avdeev 1998; Brochard 1995; Carrera 2009; Celikel 1998; Dikensoy 2002; Khilnani 2010; Matuska 2006; Plant 2001). Data revealed a significant improvement in pH with NIV compared with usual care (MD 0.05, 95% CI 0.02 to 0.07; participants = 585; studies = 8; I2 = 73%). As we detected significant statistical heterogeneity, we performed step‐by‐step elimination of each study, which revealed that Avdeev 1998 contributed the most to this heterogeneity. Exclusion of this study from the analysis reduced heterogeneity but did not meaningfully alter the pooled effect estimate. Data from Dikensoy 2002 were associated with very large confidence intervals for reasons that were not clear from the original article. Caution is recommended regarding interpretation of the data from this particular study.
PaCO2 one hour post intervention (mmHg)
Eight studies involving 585 participants provided data on PaCO2 one hour after the start of treatment (Avdeev 1998; Brochard 1995; Carrera 2009; Celikel 1998; Dikensoy 2002; Khilnani 2010; Matuska 2006; Plant 2001). The overall result tended to favour use of NIV, but this difference was not statistically significant and statistical heterogeneity was high (MD ‐4.62, 95% CI ‐11.05 to 1.08; participants = 585; studies = 8; I2 = 84%). Neither use of a random‐effects model nor previously defined criteria resolved the heterogeneity. Step‐by‐step elimination of studies from the meta‐analysis revealed that Avdeev 1998 was making the greatest contribution to this heterogeneity. Removal of this study reduced the I2 statistic to 60% but did not fundamentally affect the pooled effect estimate. We observed statistically significant improvement in the magnitude of change in PaCO2 in Bott 1993 (MD 9.0, 95% CI 3.38 to 15.23; P < 0.01), but insufficient study information was available for incorporation into the meta‐analysis. Post hoc examination of findings related to this outcome revealed that two studies (Dikensoy 2002 and Matuska 2006) had inconsistent overall mean effect estimates relative to the others, and that each study was at high risk of bias owing to imbalance of outcome measures related specifically to this outcome. Exploratory (unplanned) sensitivity analysis involving removal of these two studies from the meta‐analysis resulted in an overall effect estimate that became statistically significant in favour of NIV use (MD ‐8.35 units, 95% CI ‐14.84 to ‐1.86; participants = 491; studies = 6; I2 = 81%). We have provided in the Discussion section of this review additional details regarding these studies.
PaO2 one hour post intervention (mmHg)
Eight studies involving 585 participants provided data on PaO2 one hour after the start of treatment (Avdeev 1998; Brochard 1995; Carrera 2009; Celikel 1998; Dikensoy 2002; Khilnani 2010; Matuska 2006; Plant 2001). The overall result revealed a statistically significant improvement in PaO2 favouring NIV compared with usual care, but significant statistical heterogeneity was present (MD 7.47, 95% CI 0.78 to 14.16; participants = 585; studies = 8; I2 = 80%). Removal of Avdeev 1998 resolved this issue but resulted in loss of statistical significance for the final pooled effect estimate (MD 4.71, 95% CI ‐0.25 to 9.66; participants = 527; studies = 7; I2 = 50%).
Sensitivity analysis
We identified four studies as being at high risk of bias owing to attrition and/or failure to adopt an intention‐to‐treat approach for analysis (Avdeev 1998; Barbe 1996; Bott 1993; Carrera 2009). As described earlier, we found that Avdeev 1998 influenced the extent of observed statistical heterogeneity in several analyses. Removal of these studies had little effect on most of the primary outcomes, but removal of Avdeev 1998 resulted in loss of statistical significance for the outcome of mortality in the pH < 7.30 subgroup, with a revised risk estimate of RR 0.63, 95% CI 0.38 to 1.06. Barbe 1996 delayed initiation of NIV for 12 to 48 hours, and other studies started NIV as soon as possible. Removal of this study from relevant meta‐analyses had a negligible effect on outcome effect estimates.
Discussion
Summary of main results
Addition of non‐invasive ventilation (NIV) to usual care for management of acute hypercapnic respiratory failure due to acute exacerbation of chronic obstructive pulmonary disease (COPD) significantly reduces risks of mortality and endotracheal intubation. Treatment with NIV is associated with a significant reduction in hospital length of stay on average, but this finding relates most often to patients with prolonged admissions. NIV appears to improve acidosis within one hour of initiation. Results appear generally consistent across both intensive care unit (ICU) and ward settings, and for patients admitted with more severe (pH < 7.30) or less severe (7.30 to 7.35) acidaemia.
Interpretation of main findings
We included in the review 17 randomised controlled trials (RCTs) involving 1264 participants. This represents a substantial increase from the original review in the number of studies and number of participants included. Trial results demonstrate clear benefits associated with use of NIV as adjunctive therapy to usual care (compared with usual care alone) for treatment of patients admitted to hospital with acute hypercapnic respiratory failure (AHRF) secondary to acute exacerbation of COPD (AECOPD). Benefits were consistent across a range of outcomes considered to be clinically important. Compared with usual care, the overall pooled effect of NIV across included studies included significant reductions in risk of mortality and need for endotracheal intubation, with the average number of patients required to be treated to derive benefit in the magnitude of 12 and 5, respectively. Although no clinical consensus has been reached regarding the most acceptable number needed to treat for an additional beneficial outcome (NNTB) for such outcomes, this approach appears to represent good return upon investment with respect to the importance of these clinical outcomes and the generally low incidence of adverse events reported in studies included in this review. Effects of NIV + usual care versus usual care alone across secondary outcomes were derived from a significantly smaller pool than derived across primary outcomes and were less consistent. The magnitude of effect observed among subgroups defined on the basis of admission pH (< 7.30 or from 7.35 to 7.30) or clinical setting (ICU vs ward) did not significantly differ for most outcomes, with the exception of hospital length of stay, which demonstrated significantly greater benefit for those with more severe acidosis (pH < 7.30) than for those with milder acidosis (pH 7.35 to 7.30). This suggests that benefits derived from NIV use are likely to extend across a range of differing clinical scenarios.
Researchers have reported a significant 46% relative reduction in risk of mortality with NIV compared with usual care. This equates to potential avoidance of one death for every 12 patients treated with NIV (a slight increase from the original review (NNTB = 10)). This mortality benefit across the large number of included studies is of considerable clinical importance. Debate has surrounded the issue of whether NIV would delay necessary endotracheal intubation and therefore increase mortality. However, this position is not supported by the findings of this review. Mortality with NIV was reduced overall and across almost all of the pH and location subgroups, inferring generally similar responses irrespective of such factors. The only subgroup that did not reach statistical significance for this outcome was the ICU subgroup (risk ratio (RR) 0.60, 95% confidence interval (CI) 0.34 to 1.07). It is noteworthy to mention that this subgroup included the Khilnani 2010 study, which was unique compared with any other included study, as the mean admission partial pressure of carbon dioxide (PaCO2) across both groups was in excess of 80 mmHg. This would be considered an indicator for intubation at many hospitals. Although this study demonstrated significant improvement in arterial blood gases and need for intubation, the mortality effect was small and non‐significant (two deaths in NIV group due to septicaemia and acute coronary event vs one death in the control group due to septicaemia). In translating findings related to this outcome into clinical practice, it may be worth considering that the potential for mortality in clinical practice could be greater than that observed in clinical trials owing to factors related to patient suitability for mechanical ventilation. Individuals who are poor candidates for intubation and ventilation, including those with a very poor prognosis or a low likelihood of satisfactory quality of life or prespecified end‐of‐life choices (e.g. not for resuscitation/intubation wishes), are unlikely to feature in clinical trials such as those included within this review, yet may be appropriate candidates for NIV.
Need for endotracheal intubation was reduced by approximately two‐thirds (64%) relative to usual care, equating to just five patients needing to be treated with NIV to potentially avoid intubation of one patient. The magnitude of benefit was clear and statistically significant for all subgroups, and no significant differences were observed for this outcome across subgroups related to admission pH or treatment location. These data further demonstrate the clearly important clinical benefits associated with NIV. It is worth noting that findings related to this outcome could be considered a conservative underestimation of the true effect of NIV due to inherent challenges in evaluating and reporting this outcome in clinical trials. Whereas failure of treatment for patients enrolled into an NIV treatment arm commonly results in progression to intubation and mechanical ventilation or withdrawal of treatment, failure in a usual care arm typically results in escalation of medical management to 'off‐protocol' NIV, followed by potential subsequent intubation and mechanical ventilation (or treatment withdrawal). The precise incidence of 'actual intubation' therefore is likely to be less than the 'need for intubation' in usual care groups. Additionally, actual intubation rates may be influenced by the availability of beds in ICU settings. Therefore we considered the need for intubation as our principal definition for this outcome, as we believed this more accurately evaluated treatment 'success' versus 'failure'. Studies that reported only actual intubation rates were not rated as having high risk of bias due to data contamination, as we believed this phenomenon was representative of clinical care and was unlikely to overinflate effect estimates related to this outcome.
Need for endotracheal intubation is not always considered a 'negative' outcome, particularly in the context of treatment failure and concerns regarding the timeliness of 'essential' intubation and mechanical ventilation. Although this review did not set out to answer specific questions related to such examples, some inferences may be drawn from the present data (with due caution related to indirectness of the data for answering such questions) on the basis of lack of significant differences in beneficial effect estimates for the need for intubation (and mortality) in subgroups defined according to baseline pH levels. Although time to intubation was not specifically examined within this review, the data should alleviate some concerns regarding the safety of NIV as a first‐line therapy option (or trial of therapy for some) for patients who may present later in the course of their AHRF (when concerns regarding timeliness of invasive ventilation may be greater).
NIV use was not associated with exclusively positive outcomes. Data from our review demonstrate the need for intubation criteria was met by 66 of the 559 participants in the intervention group (12% incidence; Analysis 1.2). Rapid access to teams and resources capable of delivering invasive ventilation would therefore appear advisable for individuals considered appropriate for escalation of care when NIV is used. However, it is essential that evaluations and judgements regarding end‐of‐life decisions are made for all patients with severe AECOPDs characterised by AHRF on an individual needs basis. Although some patients with COPD may not be candidates for invasive ventilation, a considerable proportion of those who present to hospital with severe exacerbations requiring NIV have greater underlying disease severity and, in the setting of NIV treatment failure, may be more appropriate for attempted continuation/titration of NIV, conservative management, or treatment withdrawal. For example, patients who may have received prolonged (> 7 days) intubation/ventilation in the past owing to respiratory muscle atrophy may be characterised by reduced ventilatory reserve and impaired capacity to clear pulmonary secretions ‐ features that are likely to recur during subsequent exacerbations (Coakley 1992, Helliwell 1991; Le Bourdelles 1994). Other risks associated with invasive positive pressure ventilation such as barotrauma, cardiac output impairment, increased work of breathing related to dead space ventilation (due to length of the endotracheal tube), and the potential for prolonged or difficult weaning (Shapiro 1986) are relevant factors for consideration when treatment plans for such patients are determined.
The findings of this review are intended to be interpreted with respect to initial management of AHRF secondary to AECOPD. Several studies have been conducted to examine the effectiveness of NIV in patients who are weaning from invasive ventilation; we explicitly excluded these from this review and believe that our results should not be extrapolated to such contexts.
It is noteworthy to reflect upon Barbe 1996, which was one of the only studies to conclude that addition of NIV to usual care was not beneficial. This trial adopted a less common approach to delivery of NIV, as investigators delayed initiation of nasal NIV by 12 to 48 hours after hospital admission (a period longer than most other included studies) and administered it in two fixed sessions (three hours per day). Most other clinical trials in this field, however, adopted flexible prescription practices, allowing quicker initiation and longer duration of treatment in accordance with participant responses (e.g. change in clinical condition). This latter approach is more likely to reflect current clinical practice in many countries where NIV is common. Removal of this study from analyses did not meaningfully impact results, most likely because of the relatively low weighting attributed to this (or any) study, in the light of the large quantity of pooled data for most review outcomes. This small study (N = 24) was also characterised by a mild baseline level of acidosis (mean admission pH of 7.33) at which significant mortality may not be expected to occur.
NIV significantly reduced length of hospital stay by more than three days. It should be noted that the (weighted) mean length of hospital stay for the usual care group was very high (17.5 days). This duration of admission is far in excess of that commonly observed in clinical practice in many countries (Chandra 2012). It remains to be seen whether the magnitude of benefit would be the same for patients admitted to clinical settings in which shorter admissions are more common. This could be an important area of future research. Only one study (Thys 2002) contributed data to the outcome of ICU length of stay, limiting wider applicability of this non‐significant finding that tended to favour use of NIV.
The high incidence of intolerance in the NIV group was not surprising, given that this finding is clearly related to the potential discomfort of NIV in the NIV group and the absence of such discomfort in the usual care group. Although this finding indicates that NIV is not well tolerated by all patients with AHRF due to AECOPD, care should be taken not to interpret this finding as indicative of harm or a reason to deny a patient the opportunity to receive NIV when indicated. When review authors explored the incidence of complications, it became clear that most complications were related to delivery of NIV (e.g. mask‐related facial pressure areas, bloating) but were of a generally mild nature with little long‐term clinical consequence (Analysis 1.7). The two studies that reported data unrelated to NIV use described significant benefits favouring NIV use; however, additional confirmatory data appear necessary to verify this finding.
1.7. Analysis.
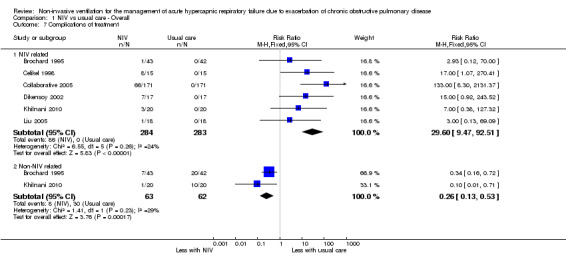
Comparison 1 NIV vs usual care ‐ Overall, Outcome 7 Complications of treatment.
Four studies provided data regarding patient‐reported ratings of perceived breathlessness. Investigators used three different measurement instruments (Visual Analogue Scale (VAS), Borg score, and verbal rating score) at various times after admission. Therefore, pooling of these data was difficult. Although we noted statistical significance for the single study (N = 60) using VAS, we believe this does not represent any preferable sensitivity to detect changes associated with NIV use. It makes clinical sense to expect that resolution of AHRF would be associated with reduced perception of dyspnoea. Hence, although data for this outcome appear limited in the extent to which they may apply beyond this review, we believe that additional research is not strongly indicated to vigorously pursue data collection for this outcome.
Acidosis has been shown to be an important prognostic factor of survival from respiratory failure in COPD; thus early correction of acidosis is an essential goal of therapy (Jeffrey 1992). This review has shown that NIV (compared with usual care) achieves more rapid correction of acidosis within the first hour. The collective observed benefits of NIV for pH, PaO2, PaCO2 (not statistically significant), and symptoms of dyspnoea suggest a picture of overall clinical improvement in respiratory failure status. Although data presently available are not confirmatory, it is intuitive to hypothesise that this clinical improvement may be a logical mechanism underpinning observed beneficial effects of NIV on need for endotracheal intubation and mortality. A previous study using NIV in respiratory failure secondary to exacerbations of COPD (Brochard 1990), which was not included in this review (not an RCT), reported reductions in respiratory rate and transdiaphragmatic activity with increases in tidal volume and minute ventilation. These findings support the mechanism that NIV not only rapidly improves gas exchange but also allows respiratory muscles to rest, thereby reducing respiratory muscle work in respiratory failure. NIV appears to optimise the window of opportunity for respiratory muscle recovery and for other conventional treatments (bronchodilators, oxygen, corticosteroids, antibiotics, etc.) to take maximal effect. It is interesting to note that in the current review, the outcome for PaCO2 did not reach statistical significance; however, our post hoc exploration of this finding showed that it was heavily influenced by data derived from Dikensoy 2002 and Matuska 2006, both of which were rated as having high risk of bias owing to imbalance of outcomes at baseline. In each instance, participants in the NIV group happened to have statistically significantly worse baseline PaCO2 levels before commencing the intervention (despite randomisation). Endpoint data recorded at one hour were used for meta‐analysis, yet these values appeared numerically worse in the NIV group than in the usual care group. Inspection of the degree of change in both studies showed NIV to be superior to usual care in both instances (NIV group improved and usual care group deteriorated in both studies). This finding may need to be interpreted with due diligence.
A specific point to consider regarding observed changes in blood acidity levels relates to the unit of measurement for the pH outcome. It is perhaps ideal to express changes in this outcome in units of hydrogen ion concentrations (H+) rather than pH; however the latter clearly is used more widely and is easier to interpret in the clinical domain than the former. pH is the negative log of the hydrogen ion concentration; because of the logarithmic nature of pH, one cannot assume that differences in hydrogen ion concentrations between (for example) a pH of 7.26 versus 7.27 compared with 7.27 versus 7.28 are linear. Descriptions of mean changes in acidity of 0.01 of a pH unit can therefore risk becoming meaningless. As reporting of pH in clinical trials and clinical practice regarding NIV use is far more common than reporting of use of H+, we opted to report data in this more conventional manner and do not advocate change. Rather, we encourage appropriate due diligence in implementation of findings related to this outcome, especially when stratification according to acidosis severity may be pertinent.
Overall completeness and applicability of evidence
We attempted to contact the authors of all included studies to verify study quality and/or obtain additional data (as required). Authors of five studies (Avdeev 1998; Bott 1993; Kramer 1995; Plant 2001; Thys 2002) supplied requested information.
Quality of the evidence
We rated evidence for the primary review outcomes of mortality and need for endotracheal intubation as showing 'moderate' quality according to GRADE criteria. This rating was largely due to uncertainty regarding risk of bias judgements for several included studies. In the light of the relatively small weighting of any individual trial in this large meta‐analysis, the impact of such issues could be considered potentially small, especially given the nature of robust outcomes such as mortality, for which the threat of issues such as performance or detection bias could be considered less. Further research may impact the magnitude and our confidence in treatment effect estimates, particularly for several of the secondary review outcomes.
Potential biases in the review process
One should consider some important factors when interpreting the findings of this review. One issue affecting data across numerous secondary outcomes is the systematic bias associated with treatment success/failure in favour of the intervention. Treatment failure typically would result in loss of data collection for some outcomes (e.g. arterial blood gas parameters measured after the first hour of treatment), hence it is reasonable to suspect that observed effect estimates may overestimate the true effectiveness of NIV in management of respiratory failure for some outcomes.
It is also well accepted that no definition of an intensive care unit has been universally accepted, and this has resulted in widespread variability in the scope and context of such settings across the world. The applicability of our subgroup analyses comparing effectiveness of NIV in ICU and ward environments was therefore constrained by the definitions proposed by study authors. We believe this is the most appropriate approach for handling this issue.
Many of the studies included in the pH subgroup < 7.30 (indicative of more severe acidaemia) examined NIV delivered within the ICU environment. Similarly, most studies conducted in the pH subgroup 7.30 to 7.35 took place in the ward environment. Risk ratios for primary outcomes for subgroup analyses were not significantly different. Therefore it is challenging to distinguish between the relative impact of the severity of presenting acidaemia versus NIV hospital setting on these outcomes. One could speculate the null hypothesis that little difference would likely exist between subgroups, and that ICU‐based studies and studies with lower mean admission pH would do better with NIV.
Potentially relevant research findings might always be presented in works that are not available for inclusion within a review or have not been published owing to factors such as 'negative' outcomes. We believe our comprehensive search strategy was sufficient for this purpose, as it included electronic searching of databases regularly updated through the Cochrane Airways Group Specialised Register of trials (CAGR), including handsearching of respiratory journals and meeting abstracts. Selection bias from the review team was minimised by systematic extraction of data by two independent members of the review team using standardised templates.
The extent to which bias related to clinical decision making for study participants influenced clinical outcomes is difficult to accurately ascertain. No study in this review could be described as 'double‐blinded' owing to the practical challenges associated with such procedures; however Thys 2002 employed 'sham' NIV. Other studies ensured that research personnel responsible for making clinical management decisions were unaware of which treatment arm a participant was assigned to, until NIV had commenced (Bott 1993; Brochard 1995), or were not involved in decisions to intubate (Celikel 1998; Kramer 1995), or employed a priori criteria for decisions regarding intubation (del Castillo 2003; Plant 2001; Thys 2002) or treatment failure (Dikensoy 2002; Plant 2001). It is clearly important that interventions associated with a real potential for serious adverse events (such as NIV) should have sufficient monitoring in place and should provide appropriate rescue therapy in the event of clinical deterioration. Although we would like to assume that all patients who 'fail' NIV would be offered immediate alternative management (e.g. intubation and mechanical ventilation), it remains possible that, in an unblinded trial, management could be delayed to prolong the window of opportunity for clinical benefit associated with NIV. Variability in the criteria used to define such treatment failure therefore has significant potential to influence such outcomes. For example, Khilnani 2010 enrolled patients presenting to hospital with severe AHRF characterised by PaCO2 levels over 80 mmHg in both groups. This would warrant immediate intubation at many hospitals.
Finally, NIV itself has limitations. Some 13% to 29% of patients are unable to tolerate the mask (Foglio 1992; Wood 1998), and facial skin ulcers can be caused by mask pressure (Stauffer 1982). Other limitations include lack of direct access to the airways, which could promote mucus plugging and/or atelectasis in patients with excessive secretions, thereby increasing the risk of aspiration. NIV initiation requires a conscious and co‐operative patient and cannot be done in patients with haemodynamic instability or life‐threatening hypoxaemia. No conclusions can be drawn regarding NIV as an alternative to intubation owing to eligibility requirements of this review.
Agreements and disagreements with other studies or reviews
A previous review evaluated effects of NIV added to standard treatment for management of acute respiratory failure (Keenan 1997). The Keenan 1997 review, which analysed participants with COPD separately, showed a strong survival benefit (odds ratio (OR) = 0.29) and a reduced need for intubation (OR = 0.12) in favour of NIV. Although our systematic review derived similar conclusions as those presented by Keenan and coworkers, some important limitations of this previous review led to the need for the present review. First, our review included 17 RCTs, but we included only three of the seven studies included in the Keenan 1997 review and excluded the remaining four studies for various reasons. Ahmed 1992 did not compare NIV with usual care but compared NIV with doxapram; Daskalopoulou 1993 used an inadequate method for randomisation (alternation); Martin 1995 included only a subgroup of participants with COPD and not all participants met the inclusion criterion of PaCO2 > 45 mmHg; and Wysocki 1995 excluded all patients who had COPD.
Second, we sought to implement a more comprehensive search strategy compared with the one used by Keenan 1997, as indexing of MEDLINE alone is likely to miss potentially relevant studies. The strategy used in this review included electronic database searches, handsearching, and inclusion of non‐English studies.
Our current review includes only studies that were primarily set up to compare NIV with usual care for management of respiratory failure secondary to an acute exacerbation of COPD. The Keenan 1997 review set out to investigate NIV versus usual care in acute respiratory failure due to various causes, including cor pulmonale, respiratory distress, COPD, and pneumonia. The Keenan 1997 review conducted a post hoc analysis of the COPD subgroup.
Authors' conclusions
Implications for practice.
Available data from a large number of good quality randomised controlled trials included in this review offer convincing evidence supporting the use of NIV as a first‐line adjunct intervention to usual medical care for patients admitted with AHRF secondary to AECOPD. This review had some risks of bias (e.g. performance bias); however review authors believe that these conferred a relatively minor impact upon primary review outcomes, particularly with due consideration given to challenges associated with 'shamming' NIV interventions. Although it makes clinical sense to consider implementing NIV early in the course of respiratory failure (before acidosis progresses to a more severe nature), our data related to pH subgroups suggest that the intervention is no less effective as a means of reducing risk of endotracheal intubation and mortality among these patients. We regarded the evidence supporting a likely reduction in hospital length of stay due to NIV to be of moderate quality according to GRADE criteria, and we believe the magnitude of the impact related to this outcome is of high clinical interest (particularly to healthcare administrators).
Previous research has shown NIV use to be no more costly than management via intubation and/or mechanical ventilation, no more time‐consuming in terms of nursing involvement (Bott 1993; Kramer 1995; Nava 1997), and no more costly to deliver within or outside the ICU setting (Kramer 1995), mainly as the result of cost benefits associated with reductions in ICU resources (Plant 2001). Data in this review support the use of NIV in the ward setting; however decisions regarding implementation of such therapy in different settings should be made with careful consideration of factors specific to individual institutions that may impact patient safety, including degree of illness severity and/or acuity;availability and expertise of appropriately trained medical, nursing, and allied health staff for safe implementation and sufficient monitoring of patients (e.g. frequent observations); adequate staff‐to‐patient ratios; and appropriate access to and understanding of different NIV interfaces and equipment (e.g. arterial blood gas analysers) to accurately evaluate the impact of NIV upon a diverse range of clinical outcomes.
Implications for research.
An important issue emerging from the present review concerns the feasibility of conducting future studies of NIV versus no NIV. This is due in part to the clear benefit of NIV for a highly important clinical outcome such as mortality and to increasing implementation of this intervention as part of routine clinical care for patients with AHRF secondary to AECOPD. Therefore, it will become increasingly difficult to define 'usual medical care' without NIV as a therapy consistent with evidence‐based practice. This may pose particular challenges in obtaining future human ethical committee approval to conduct parallel‐group clinical trials such as those included in this review. For example, although future research may reasonably impact and/or refine the precise estimate of effect related to hospital length of stay, we do not believe this warrants future research employing control groups that receive usual medical care without NIV.
One should interpret study findings within the context of the specific questions posed by this review ‐ which were limited to issues of clinical effectiveness in the hospital setting. Additional research would enhance our ability to more accurately select the right patients and the right levels of ventilation (e.g. dosage, duration, mode). Studies are also needed to assess the feasibility, safety, and effectiveness of NIV applied in different settings (e.g. out‐of‐hospital, high‐dependency units, non‐specialist departments/wards) and to evaluate the feasibility (if any) of using NIV as an alternative to endotracheal intubation. Fewer data were available for several of the secondary outcomes of this review (e.g. symptoms, length of ICU stay), suggesting that further confirmatory evidence could still be needed to determine the impact of NIV on these outcomes. We contend, however, that targeting the primary outcomes of this review should remain the primary focus of future research related to clinical effectiveness. Outcomes not considered within the present review include those related to quality of life or to the diverse longer‐term impact of critical (or near‐critical) illness survival known to significantly affect morbidity in the time post discharge.
Finally, it would be clinically advantageous to identify optimal monitoring strategies for patients with differing acuity levels (e.g. extent of acidaemia) to guide evidence‐based treatment decisions for such patients. This may be particularly relevant for environments outside the ICU setting, where access to resources for monitoring clinical progress (e.g. blood gas analysis) may be challenging. It would also seem worthwhile for researchers to explore the utility of emerging practices, such as expanded development of device interfaces (e.g. helmets) and the increasing popularity of methods such as transcutaneous carbon dioxide analysis or venous blood gas analysis for patient evaluation owing to their relative simplicity compared with arterial sampling. Research in these fields is still in its relative infancy.
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2017 | New search has been performed | New search run and results fully incorporated |
| 18 January 2017 | New citation required and conclusions have changed | A new review author team. Six new studies added plus one ongoing study and three studies awaiting classification. Outcomes have changed. Methods updated to reflect Cochrane MECIR standards, including updating risk of bias across all included studies and adding a summary of findings table and GRADE rating. Much of the review text was redrafted |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 2 July 2008 | Amended | Converted to new review format |
| 31 March 2004 | New citation required and conclusions have changed | Substantive amendments |
Acknowledgements
We would like to acknowledge all authors involved in earlier versions of this Cochrane review (Felix SF Ram, Josephine Lightowler, and Jadwiga Wedzicha) for their substantial contributions to the text and content of this review. We wish to thank authors of studies who responded to requests for clarification or further data regarding their trials (Avdeev 1998; Bott 1993; Khilnani 2010; Kramer 1995; Plant 2001; Thys 2002). We would like to thank the following colleagues for providing assistance with data extraction and translation of articles published in languages other than English: Alieksei Seniukovich, Yi Liu, Claudio Bravo, Petra Kristyna Matejkova, Hong Fan, Zongwang Zhang, and Jan Strojil.
Rebecca Normansell was the Editor for this review and commented critically on the review.
The Methods sections of this review were based on a standard template used by the Cochrane Airways Review Group.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 MeSH DESCRIPTOR Positive‐Pressure Respiration Explode All
#8 (nasal* OR mechanical*) NEAR ventilat*
#9 non‐invasive or "non invasive"
#10 "positive pressure" or positive‐pressure
#11 "pressure support"
#12 "positive airway"
#13 "intermittent positive pressure"
#14 airway* NEAR pressure
#15 pressure‐control*
#16 volume‐control*
#17 bi‐level
#18 ventilat* NEAR support
#19 (NIPPV OR OR NIV):TI,AB,KW
#20 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
#21 #6 AND #20
#22 (#21) AND (INREGISTER)
[Note: In search line #4, MISC1 denotes the field in which the reference has been coded for condition, in this case, COPD]
Appendix 3. Sources and search strategy for online clinical trial registry
Three clinical trial registries were searched on the 28th of September 2015 and 18th January 2017. These were ClinicalTrials.gov (www.ClinicalTrials.gov), Controlled trials (www.controlled‐trials.com) and the WHO trials portal (www.who.int/ictrp/en/). Keywords for the searches were as follows: Non invasive ventilation AND COPD
Clinicaltrials.gov search: https://clinicaltrials.gov/ct2/results?term=non+invasive+ventilation+AND+COPD&pg=1
Controlled‐trials search: http://www.controlled‐trials.com/search?q=non+invasive+ventilation+AND+COPD
International Clinical Trials Registry Platform (ICTRP – WHO) search: http://apps.who.int/trialsearch/default.aspx
Appendix 4. Search methods up to 2004
An initial search was carried out using the Cochrane Airways Group RCT register up to and including September 2003. This Cochrane Airways RCT register contains records downloaded from MEDLINE, CINAHL, EMBASE and UK Research Register as well as records identified through hand searching of key respiratory journals and abstracts from meetings of the American Thoracic Society, British Thoracic Society and European Respiratory Society. Randomised controlled trials are identified for this RCT register using the following search strategy: (placebo* OR trial* OR random* OR double‐blind OR double blind OR single‐blind OR single blind OR controlled study OR comparative study).
The following search terms were used in the above mentioned Cochrane Airways RCT register to search for trials for this review: (nasal OR mechanical OR non‐invasive or non invasive or positive pressure OR intermittent positive pressure OR airway* pressure OR pressure‐controlled OR volume‐controlled AND ventilat*) OR positive pressure OR bi‐level positive pressure OR ventilation support OR NIPPV OR NIV OR NIAV OR NIV
Data and analyses
Comparison 1. NIV vs usual care ‐ Overall.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 12 | 854 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.38, 0.76] |
| 2 Need for endotracheal intubation | 17 | 1105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.28, 0.46] |
| 3 Length of hospital stay (days) | 10 | 888 | Mean Difference (IV, Random, 95% CI) | ‐3.39 [‐5.93, ‐0.85] |
| 4 Length of ICU stay (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Symptom scores (higher score means more dyspnoea) | 4 | 484 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.34, 0.02] |
| 5.1 Borg score | 2 | 82 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.79, 0.08] |
| 5.2 Visual analogue scale | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐1.40, ‐0.34] |
| 5.3 Dyspnoea score at 24 hours | 1 | 342 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.21, 0.21] |
| 6 Treatment intolerance | 6 | 252 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.17] |
| 7 Complications of treatment | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 NIV related | 6 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 29.60 [9.47, 92.51] |
| 7.2 Non‐NIV related | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.53] |
| 8 pH 1 hour post intervention | 8 | 585 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.02, 0.07] |
| 9 PaCO2 mmHg ‐ 1 hour post intervention | 8 | 585 | Mean Difference (IV, Random, 95% CI) | ‐4.62 [‐11.05, 1.80] |
| 10 PaO2 mmHg ‐ 1 hour post intervention | 8 | 585 | Mean Difference (IV, Random, 95% CI) | 7.47 [0.78, 14.16] |
1.3. Analysis.
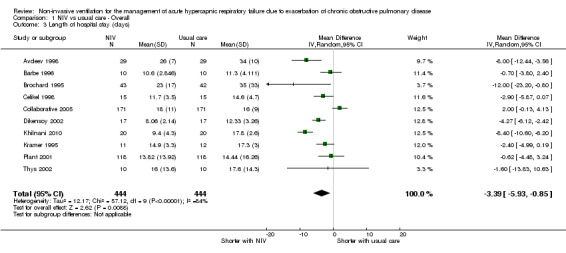
Comparison 1 NIV vs usual care ‐ Overall, Outcome 3 Length of hospital stay (days).
1.4. Analysis.
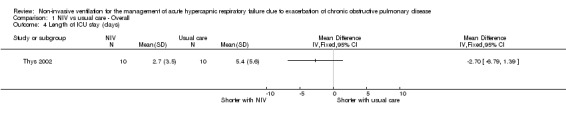
Comparison 1 NIV vs usual care ‐ Overall, Outcome 4 Length of ICU stay (days).
1.5. Analysis.
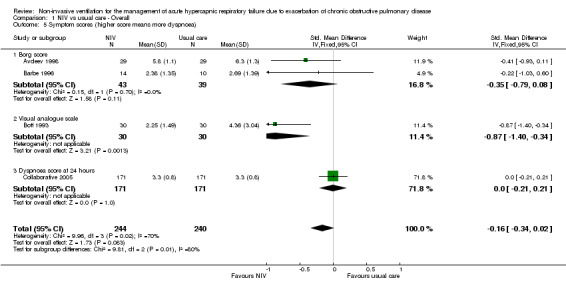
Comparison 1 NIV vs usual care ‐ Overall, Outcome 5 Symptom scores (higher score means more dyspnoea).
1.8. Analysis.
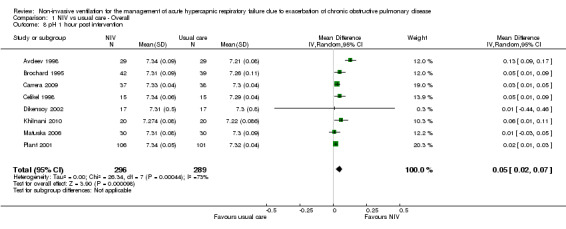
Comparison 1 NIV vs usual care ‐ Overall, Outcome 8 pH 1 hour post intervention.
1.9. Analysis.
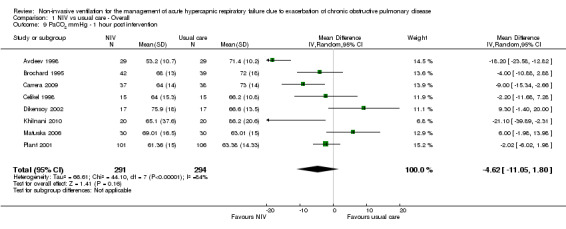
Comparison 1 NIV vs usual care ‐ Overall, Outcome 9 PaCO2 mmHg ‐ 1 hour post intervention.
1.10. Analysis.
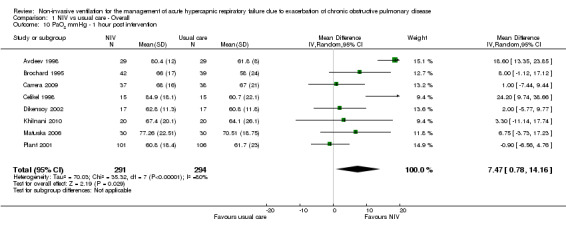
Comparison 1 NIV vs usual care ‐ Overall, Outcome 10 PaO2 mmHg ‐ 1 hour post intervention.
Comparison 2. NIV vs UMC ‐ Admission pH subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Admission pH > 7.30 | 5 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.30, 0.84] |
| 1.2 Admission pH < 7.30 | 8 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.35, 0.90] |
| 2 Need for endotracheal intubation | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Admission pH > 7.30 | 7 | 589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.63] |
| 2.2 Admission pH < 7.30 | 11 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.22, 0.42] |
Comparison 3. NIV vs UMC ‐ Trial location subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Intensive care unit | 5 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.07] |
| 1.2 Ward | 5 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.29, 0.78] |
| 2 Need for endotracheal intubation | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Intensive care unit | 9 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.21, 0.43] |
| 2.2 Ward | 8 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.31, 0.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Avdeev 1998.
| Methods |
Country: Russia Design: Randomised controlled parallel trial. Participants were matched for demographic and physiological norm values Study site: One hospital in Moscow, conducted between September 1995 and March 1997 Method of analysis: Unclear Aim: To determine the effect of NIV on need for endotracheal intubation, mortality rate, length of hospital stay, and incidence of complications in patients with acute respiratory failure caused by AECOPD |
|
| Participants |
Eligible for study: Not stated Recruited: 58 adult patients with acute respiratory failure due to AECOPD (29 in each group) Completed: Not stated Age: NIV group: mean (SD) age = 63.4 (5.5) years; usual care group: mean (SD) age = 66.2 (7.1) years Gender: NIV group (M:F) = 26:3, usual care group (M:F) = 22:7 Criteria used to define COPD: Not stated Inclusion criteria: Insufficient information available Exclusion criteria: Insufficient information available |
|
| Interventions |
Intervention description: NIV plus usual care. BiPAP ventilators (Respironics, Inc., Murrysville, PA, USA) used with inspiratory pressure titrated to 30 cmH2O and expiratory pressure of 4 to 6 cmH2O. Both face masks and nasal masks were used Control description: Oxygen, bronchodilators, steroids, and theophylline Duration of intervention: Mean (SD) duration of NIV was 29 (25) hours Intervention delivery by: Insufficient information available Setting: Intermediate care unit |
|
| Outcomes |
Method of outcome data collection: Not clear Prespecified primary outcomes: Not clear which outcomes deemed primary. Specified outcomes were need for intubation, mortality rate, length of hospital stay, and incidence of complications. No clinical trial registry to confirm Prespecified secondary outcomes: Other outcomes reported included breathlessness score (Borg) and ABGs Follow‐up period: Data collected until hospital discharge |
|
| Notes | Trial was published in Russian Study author was contacted and provided additional information Funder: Unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were used for treatment allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Owing to nature of the intervention, blinding was not possible. No sham NIV used; however, unlikely to have affected primary outcomes. May have affected subjective ratings of dyspnoea |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unsure whether investigators were involved in participant treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants from the NIV group were excluded owing to NIV intolerance. Insufficient information available to determine reasons or potential impact |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Imbalance of outcome measures at baseline All outcomes | Unclear risk | Insufficient information available |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Participants in the NIV group had lower age at baseline. Unclear whether statistically significant. Insufficient information available for other outcomes |
| Protection against contamination All outcomes | Unclear risk | Insufficient information available |
| Selective recruitment of participants | Low risk | N‐values and methods of recruitment were similar |
| Other bias | Low risk | No other sources of bias identified |
Barbe 1996.
| Methods |
Country: Spain Design: Randomised controlled parallel trial Study site: Single site, University hospital in Palma de Mallorca, Spain Method of analysis: Unpaired t‐tests; 2‐way ANOVA with Tukey adjustment Aim: To determine whether NIV support with BiPAP facilitates recovery from acute respiratory failure in patients with COPD |
|
| Participants |
Eligible for study: Not stated Recruited: 24 patients recruited: 14 in the NIV group; 10 in the usual care group Completed: 20 participants completed the study (10 in each group), as 4 participants in the NIV group were unable to tolerate the procedure Age: NIV group: mean (SD) age = 70 (9) years; usual care group: mean (SD) age = 65 (13) years Gender: All male Criteria used to define COPD: Not stated Inclusion criteria: Attendance at emergency department for acute respiratory failure due to AECOPD Exclusion criteria: Clinical or radiological evidence of bacterial pneumonia, pleural effusion, left ventricular failure, or nasal deformity |
|
| Interventions |
Intervention description: NIV plus usual care. BiPAP ventilators were used with a mean (SD) inspiratory pressure of 14.8 (2.18) cmH2O, and expiratory pressure set at 5 cm H2O. Nasal masks were used Control description: Aerosolised salbutamol (5 mg four times per day), IV prednisolone (40 mg three times per day, later tapered on an individual basis), and controlled oxygen via Venturi mask to keep SpO2 > 90%, delivered during first 3 days of arrival on the ward (within 12 to 48 hours of hospitalisation) Duration of intervention: NIV was given for two 3‐hour periods (am and pm) for 3 days on a hospital ward. Patients were recruited to the study within 12 to 48 hours of hospitalisation Intervention delivery by: Adaptation to BiPAP was supervised by one of the study authors Setting: Conventional hospital respiratory ward (non‐ICU setting) |
|
| Outcomes |
Method of outcome data collection: Bedside measurement of lung function tests, ABGs; hospital data Prespecified primary outcomes: Not explicitly stated Prespecified secondary outcomes: All outcomes listed (in order) as shortness of breath (Borg scale), ventilatory pattern, occlusion pressure, ABGs, peak flow, MIP, and MEP. Data also reported on mortality and intubation Follow‐up period: Measured 30 minutes before and after cessation of oxygen and/or BiPAP support on days 1 and 3 of hospitalisation. Nil follow‐up beyond day 3 |
|
| Notes | Study author contacted and additional information requested, without reply Funder: Supported, in part, by Fondo de Investi‐gaciones Sanitarias de la Seguridad Social(FIS 93/0860), ABEMAR, and CarburosMetalicos SA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned only that participants were randomised. Method was not described |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to nature of the intervention, blinding was not possible. No sham NIV used; however, outcomes reported were objective outcomes and were unlikely to be affected |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Adaptation to BiPAP was supervised at the bedside by one of the study authors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete data were excluded from analysis. Compared only 2 groups, each consisting of 10 participants |
| Selective reporting (reporting bias) | Unclear risk | Data were collected 30 minutes before and after oxygen/NIV cessation on days 1 and 3, yet data were presented only as a single time point at 24, 32, 72, and 80 hours after emergency department presentation and upon hospital discharge. FVC at discharge not reported. Unlikely to have affected mortality or intubation outcomes |
| Imbalance of outcome measures at baseline All outcomes | Low risk | Baseline values not considered in analysis, but no baseline differences reported |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Only age and BMI were mentioned as baseline characteristics. Unsure if both groups had similar baseline characteristics |
| Protection against contamination All outcomes | Low risk | Low owing to the nature of the intervention |
| Selective recruitment of participants | Unclear risk | Insufficient information available. No details provided on the number of patients screened. Unclear why imbalance of participants in intervention groups (10:14, control:NIV) |
| Other bias | Low risk | No other sources of bias identified |
Bott 1993.
| Methods |
Country: England, United Kingdom Design: Prospective multi‐centre randomised controlled trial Study site: Two centres in London (Kings College and London Chest Hospitals) and 1 centre in Southampton Method of analysis: Paired t‐test/Mann‐Whitney U tests with exploration of differences at baseline Aim: To determine the effectiveness of NIV vs conventional treatment for patients admitted to hospital with ventilatory failure due to AECOPD |
|
| Participants |
Eligible for study: Not stated Recruited: 60 adult patients ("approximately 10 in each group at each of the 3 centres") admitted to respiratory emergency department or ward Completed: Data reported from 60 participants for some outcomes. Four participants in the NIV group did not receive NIV: 2 were confused and unco‐operative, 1 was unable to breathe through his nose, and 1 had all active treatment withdrawn upon request Age: Not stated. Participants just described as "less than or equal to 80 years" Gender: Not stated Criteria used to define COPD: Not stated Inclusion criteria: AECOPD, aged ≤ 80, PaO2 < 7.5 kPa, PaCO2 > 6 kPa Exclusion criteria: Severe disease not attributable to chronic respiratory disease, severe psychiatric illness, use of NIV at home |
|
| Interventions |
Intervention description: NIV plus usual care. Volume‐cycled nasal positive pressure ventilation was started as soon as possible in the NIV group. Ventilation was provided through a silicon nasal mask (Respironics, Inc., Murrysville, PA, USA) Control description: “Conventional treatment was that deemed appropriate by the clinicians responsible: oxygen at 24 to 28%; inhaled bronchodilators; and all, or a combination of, antibiotics, diuretics, respiratory stimulants, intravenous or oral corticosteroids, and bronchodilators. Patients were assessed and treated as necessary by a physiotherapist” Duration of intervention: Participants in the NIV group received 7.63 hours (range 1 to 23 hours) of ventilation per day over 6 days (range 2 to 9 days). Control intervention was provided until discharge: median (IQR) 9 (1 to 39) days Intervention delivery by: NIV set up by a member of the research team who was not involved in patient care. Usual medical team treated participants Setting: Insufficient information to be certain. NIV appears to have been conducted on respiratory wards, but may have been started in emergency department at some sites |
|
| Outcomes |
Method of outcome data collection: Direct observation/participant data collection. Uncertain how 30‐day survival was measured Prespecified primary outcomes: Not clearly defined. Appears to be ABGs: "Arterial blood gases were measured on admission, after 1 hour on allocated treatment, on day 3, and day 7, while breathing room air (except at 1 h after admission, when the patient was using either oxygen or NIV" Prespecified secondary outcomes: “VAS scores for shortness of breath, well‐being, and quality of sleep were obtained from the patients; and nursing care requirements from a senior nurse, daily until day 3, and then on day 7. FEV1, FVC, and peak expiratory flow rate were measured during the hospital stay". Mortality and intubation were also reported Follow‐up period: "At least 30 days" |
|
| Notes | Study author contacted and additional information supplied Funder: Supported by a grant from the British Lung Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Manuscript states only "patients were randomly allocated to..." Additional information from study authors ‐ "randomisation was performed by a third party using computer generated random tables, stratified for each of the 3 centres to ensure 10 patients in each group" |
| Allocation concealment (selection bias) | Low risk | Manuscript states only "patients were randomly allocated to..." Additional information from study authors ‐ "numbered sealed envelopes were used for treatment allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to nature of the intervention, blinding was not possible. No sham NIV was used. Subjective ratings of dyspnoea could have been affected |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Ventilation was started and VAS measured by physiotherapists and medical researchers not otherwise involved in the management of the patients...The study investigators [who set up the ventilators] were not involved in the clinical management of patients" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | An intention‐to‐treat analysis was performed for mortality/survival only. All other analyses excluded the 4 participants who did not receive NIV and anyone who did not have complete data (n not described for each outcome) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported on, except PaO2. Day 3 and day 7 ABG data were not provided but were cited as showing no significant difference |
| Imbalance of outcome measures at baseline All outcomes | Unclear risk | Insufficient data provided. Differences between sites acknowledged but not accounted for in analysis. Unclear whether differences existed between intervention groups |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Insufficient data provided. Differences between sites acknowledged but not accounted for in analysis. Unclear whether differences existed between intervention groups |
| Protection against contamination All outcomes | High risk | Five participants in the control group were ventilated (3 with NIV); 4 participants in the NIV group did not receive NIV |
| Selective recruitment of participants | Unclear risk | Insufficient information available |
| Other bias | Low risk | No other sources of bias identified |
Brochard 1995.
| Methods |
Country: Multi‐national study conducted in France, Spain, and Italy between September 1990 and November 1991 Design: Multi‐national multi‐centre prospective randomised controlled parallel‐group trial Study site: Five intensive care units: 3 in Paris (France), 1 in Barcelona (Spain), and 1 in Rome (Italy) Method of analysis: t‐tests; multiple comparisons were performed via repeated measures ANOVA and pairwise comparisons (Fisher's exact test). Qualitative data were compared with the Chi2 test. Influence of endotracheal intubation on mortality was analysed via extended Mantel–Haenszel test Aim: To compare the efficacy of NIV delivered through a face mask vs standard medical treatment in patients admitted because of AECOPD |
|
| Participants |
Eligible for study: 275 (190 not included, with reasons stated) Recruited: 85 adult patients (42 in usual care; 43 in NIV group) Completed: 85 participants (42 in usual care; 43 in NIV group) contributed data for most outcomes Age: NIV group: mean (SD) age = 71 (9) years; usual care group: mean (SD) age = 69 (10) years Gender: Not stated Criteria used to define COPD: Not stated Inclusion criteria: Known or highly probable COPD (on the basis of clinical history, physical examination, and chest film), with respiratory acidosis and elevated bicarbonate level. Patients also had 'an exacerbation of dyspnoea' lasting less than 2 weeks and at least 2 of the following: respiratory rate > 30 breaths/min, PaO2 < 45 mmHg, and pH < 7.35 after breathing room air for ≥ 10 minutes Exclusion criteria: Presence of any of the following: RR < 12 breaths/min; need for immediate intubation; tracheotomy or endotracheal intubation performed before admission; sedative drugs administered in previous 12 hours; central nervous system disorder unrelated to hypercapnic encephalopathy or hypoxaemia; cardiac arrest in past 5 days; cardiogenic pulmonary oedema; chronic respiratory failure caused by kyphoscoliosis or neuromuscular disorder; upper airway obstruction or asthma; a clear cause of decompensation requiring specific treatment (e.g. peritonitis, septic shock, acute myocardial infarction, pulmonary thromboembolism, pneumothorax, haemoptysis, severe pneumonia, recent surgery or trauma); facial deformity; or enrolment in other investigative protocols. In addition, patients who refused to undergo endotracheal intubation, whatever the initial therapeutic approach, were excluded from the study |
|
| Interventions |
Intervention description: usual care plus NIV via ARM 25 (Taema, Antony, France). This flow‐triggered system provides constant pressure during inspiration and a rapid pressurisation rate (flow rates 10 to 35 L/min). Pressure support was initiated at 20 cmH2O, but no EPAP/PEEP (atmospheric only). Pressure support was initiated at 20 cm H2O, but no EPAP/PEEP (atmospheric only). Oxygen was incorporated to maintain saturations > 90% Investigators used specially developed face masks that included a foam internal lining to decrease dead space Control description: Oxygen (max 5 L/min via nasal prongs) to achieve arterial oxygen saturation > 90%; subcutaneous heparin, antibiotic agents, and bronchodilators (subcutaneous terbutaline, aerosolised and intravenous albuterol, and corticosteroids or intravenous aminophylline or both), plus correction of electrolyte abnormalities Duration of intervention: Participants underwent NIV for at least 6 hours each day. The period could be lengthened, depending on patient tolerance. Participants were allowed to breathe spontaneously (with oxygen but with no assistance) each day for 2 hours. Overall NIV duration was based upon clinical criteria and ABG levels at the discretion of the physician in charge Intervention delivery by: Not stated Setting: NIV was conducted in ICU |
|
| Outcomes |
Method of outcome data collection: Not stated Prespecified primary outcomes: Need for endotracheal intubation and mechanical ventilation at any time during the study Prespecified secondary outcomes: Hospital length of stay, complications not present upon admission (e.g. pneumonia, barotrauma, gastrointestinal haemorrhage, renal insufficiency, neurological events, and pulmonary embolism), duration of ventilatory assistance, and in‐hospital mortality. Data also reported on encephalopathy score and loss to follow‐up Follow‐up period: Respiratory rate, encephalopathy score, and ABG analyses were performed 1, 3, and 12 hours after start of treatment, then daily until ICU discharge. SAPS was calculated at 24 hours. PFTs were performed before discharge, when possible, or within 3 months after discharge |
|
| Notes | Study author contacted and additional information requested, without reply Funder: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information available. "Patients were randomly assigned to..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available. "Random assignments were made with sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to nature of the intervention, blinding was not possible. No sham NIV was used. However, outcomes reported were objective outcomes and were unlikely to be affected |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned who delivered NIV |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unsure that data analysed were completed data |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Imbalance of outcome measures at baseline All outcomes | High risk | SAPS and encephalopathy scores were significantly different between intervention groups at baseline |
| Comparability of intervention and control group characteristics at baseline | High risk | SAPS and encephalopathy scores were significantly different between intervention groups at baseline and were not factored into statistical analysis. No analyses were conducted to explore differences between sites/clusters |
| Protection against contamination All outcomes | Unclear risk | Four participants in the NIV group started the intervention, then it was ceased and subsequently re‐started. A large proportion of participants in the control group were intubated, does not appear they received NIV before intubation |
| Selective recruitment of participants | Unclear risk | Appears low risk, but full breakdown of reasons for exclusion not provided |
| Other bias | Low risk | No other sources of bias identified |
Carrera 2009.
| Methods |
Country: Spain Design: Prospective multi‐centre double‐blind randomised controlled trial Study site: 7 tertiary hospitals Method of analysis: Results are shown as mean ± SD. Continuous and categorical variables were compared by Student t‐test and Chi2 test. Two‐way analysis of variance (ANOVA) was used to determine the statistical significance of differences between groups and over time. A P value < 0.05 was considered statistically significant. Length of hospital stay data were not normally distributed (Kolmogorov‐Smirnov test), thus Mann‐Whitney U test was performed, and median to represent central measurement of the sample and dispersion was represented as percentiles Aim: To determine whether NIV reduces the need for endotracheal intubation and enhances recovery in patients hospitalised owing to AECOPD |
|
| Participants |
Eligible for study: Not reported Recruited: 75 patients with AECOPD were recruited from emergency department of 7 tertiary hospitals in Spain: 37 were randomised to NIV group and 38 to control group with sham NIV Completed: 32 participants in NIV group completed study and 5 met intubation criteria (3 were intubated and 2 were continued on NIV off protocol), whereas 25 participants in control group completed the study and 13 met intubation criteria (4 were intubated, 7 were offered NIV, and 2 continued with medical therapy) Age: NIV group: mean (SD) age = 72 (10) years; usual care group: mean (SD) age = 69 (7) years Gender: Not stated Criteria used to define COPD: Not mentioned Inclusion criteria: previous known diagnosis of COPD (not defined by study authors), with symptoms of increasing dyspnoea, cough, and/or sputum production of recent onset (last 2 weeks) in the absence of an alternate diagnosis that leads the attending physician at the emergency department to diagnose AECOPD of sufficient severity as to require hospitalisation according to the following criteria: arterial pH < 7.35 and PaCO2 > 50 mmHg 30 to 60 minutes after intensive medical management (bronchodilator, steroids, oxygen). Recruitment occurred within 24 hours after admission Exclusion criteria: Respiratory rate < 12 bpm or need for immediate intubation for cardiopulmonary resuscitation; arterial pH < 7.25; GCS > 8; administration of sedative drugs within previous 12 hours; neuromuscular disorders; thoracoplasty or kyphoscoliosis; known cause of decompensation requiring specific treatment (pneumothorax, haemoptysis, pneumonia, etc.); medical history of sleep apnoea, asthma, or any severe systemic disease; BMI > 40; facial deformity; history of acute episodes that required NIV treatment; long‐term NIV treatment; history of drug and alcohol abuse or refusal to participate |
|
| Interventions |
Intervention description: Intervention delivered via NIV; all centres used same BiPAP and facial mask models (Respironics, Inc., Murrysville, PA, USA). EPAP was set at 4 cmH2O, whereas IPAP was adjusted individually to the maximum tolerated (to achieve alleviation of dyspnoea, decreased respiratory rate, and good patient‐ventilator synchrony) in assisted/controlled mode. All participants received conventional treatment with supplementary oxygen to maintain SpO2 ≥ 90%, bronchodilators, steroids, and antibiotics Control description: Sham NIV was delivered via a modified commercially available BiPAP (Respironics, Inc., Murrysville, PA, USA) to provide only controlled oxygen therapy without inspiratory pressure support. To dissipate pressure generated by the machine, investigators drilled a hole in the tube that connects the pressure generator to the patient mask and controlled FiO2 with the oxygen line through this hole, connected to a facial mask of the standard Venturi regulator (Kendall Proclinics, Barcelona, Spain). Sham device was validated and ABGs were indistinguishable from those obtained when a standard Venturi mask was used. All participants received conventional treatment with bronchodilators, steroids, and antibiotics Duration of intervention: First 3 days of hospitalisation for as much time as possible between 3 pm and 8 am for both NIV and sham NIV Intervention delivery by: In the morning (8 am to 3 pm), a respiratory specialist recruited participants and prescribed standard treatment and oxygen. The same physician visited the participant every morning (while patient was off ventilator), decided on treatment modifications, and set the timing for discharge. This physician was not involved in participant care after 3 pm From 3 pm to 8 am, a study investigator (respiratory physician) with experience in NIV, familiar with the BiPAP, who was totally independent of participant care, added BiPAP or sham BiPAP according to the randomisation scheme, and removed device from the room before 8 am the next morning Both NIVs were discontinued on day 4 of hospitalisation Setting: Respiratory ward |
|
| Outcomes |
Method of outcome data collection: ABG, GCS, force spirometry at discharge, respiratory rate, heart rate, dyspnoea (visual analogue dyspnoea scale). These variables were recorded at inclusion, 1 hour after NIV, and at day 1, day 2, and day 3 Prespecified primary outcomes: Need for endotracheal intubation with presence of 1 or more of the following criteria: cardiorespiratory arrest, arterial pH < 7.20 after 30 minutes on optimal medical treatment or pH between 7.20 and 7.25 on 2 occasions 1 hour apart, pO2 < 45 mmHg despite maximum tolerated oxygen therapy, hypercapnic coma (GCS < 8). Patient fulfilling these criteria were considered failures and were excluded from the study and managed on an open‐label basis and off protocol Prespecified secondary outcomes: Speed of recovery of ABG and length of hospital stay Follow‐up period: From admission until discharge; precise number of follow‐up time points not clearly mentioned |
|
| Notes | Primary outcome was need for intubation, not actual intubation. Some participants provided with successful rescue therapy Funder: Supported in part by ABEMAR, Fundación CAUBET‐CIMERA, Programa I3SNS (Línea de Intensificación de la Investigación), SEPAR, Red Respira (ISCII,RTCI 03/11) grants, and CIBERes. These institutions were not involved in study design; in collection, analysis, and interpretation of data; in writing of the manuscript; and in the decision to submit the manuscript for publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization scheme was generated by a computer in the coordinating center (HUSD) and sent to the participating centers using sequentially numbered, sealed, opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | "The randomization scheme was generated by a computer in the coordinating center (HUSD) and sent to the participating centers using sequentially numbered, sealed, opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled intervention (sham NIV) used in control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcomes assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data analysis included off‐protocol patients; however, anyone who fulfilled the criteria for need for intubation (i.e. 'treatment failure') was excluded from the study |
| Selective reporting (reporting bias) | Low risk | All specified outcomes were reported |
| Imbalance of outcome measures at baseline All outcomes | Unclear risk | NIV group had slightly poorer PaO2 (P = 0.05), but the effect of this on study outcomes was not formally evaluated |
| Comparability of intervention and control group characteristics at baseline | Low risk | No statistically significant differences observed at baseline, but PaO2 levels were slightly worse (lower) in the NIV group than in the control group (P = 0.05) |
| Protection against contamination All outcomes | Low risk | Control group may have received the intervention, but these individuals were excluded from analyses |
| Selective recruitment of participants | Low risk | "Consecutive patients" were recruited |
| Other bias | Low risk | No other sources of bias identified |
Celikel 1998.
| Methods |
Country: Turkey. Design: Single‐centre prospective randomised controlled parallel‐group trial conducted between March 1993 and November 1996 Study site: Single university hospital in Istanbul, Turkey Method of analysis: Mann‐Whitney U test, ANOVA, log rank test, Chi2 test Aim: To compare the efficacy of standard medical therapy and NIV in patients with AHRF due to AECOPD |
|
| Participants |
Eligible for study: Not stated Recruited: 30 adult patients (15 in each group) with AHRF due to AECOPD Completed: Data from 30 participants available for some outcomes Age: Not stated Gender: Not stated Criteria used to define COPD: Previous PFTs (FEV/FVC < 75% and < 12% bronchodilator response) or clinical history, physical examination, chest radiography, and ABGs (arterial CO2 retention, elevated bicarbonate) Inclusion criteria: Known to have COPD diagnosed on the basis of previous PFTs (FEV/FVC < 75% and < 12% bronchodilator response) or clinical history, physical examination, chest radiography, and ABGs (arterial CO2 retention, elevated bicarbonate), as well as (1) PaCO2 > 45 mmHg and pH < 7.35; and (2) evidence of respiratory muscle fatigue (RR > 22 breaths/min, accessory muscle use, and respiratory distress via direct observation of ICU staff) Exclusion criteria: Need for urgent intubation due to respiratory arrest, haemodynamic instability (systolic BP < 90 mmHg), severe cardiac arrhythmia, abundant secretions, myocardial infarction or cardiac arrest within last 3 months, and unwillingness to participate in the study |
|
| Interventions |
Intervention description: Usual care plus continuous NIV. Pressure support ventilation (PSV) was delivered via mechanical ventilator (Model 720; Puritan‐ Bennett; Carlsbad, CA) and full face mask (Dryden, Clear Comfort Face Mask; Gibeck Respiration; Uplandsvasby, Sweden; and 9000; Vital Signs Corp; Totowa, NJ). Initial settings: PSV 15 cmH2O; PEEP 5 cmH2O; sensitivity 1 cmH2O; FiO2 0.5; active apnoea backup. Setting adjustments: PS to achieve 5 to ‐7 mL/kg expired TV, FiO2 to maintain SpO2 90% to 92%, and sensitivity as low as possible with no auto‐triggering Control description: Oxygen (min 1 L/min to keep SpO2 90% to 2%), aminophylline infusion (to keep serum theophylline levels 8 to 15 mg/L), atropine (1 mg 4‐hourly), salbutamol nebuliser (2.5 mg 4‐hourly), IV methylprednisolone (40 mg 6‐hourly), antibiotics if indicated (cefuroxime or sulbactam‐ampicillin until culture results available) Duration of intervention: Mean duration of NIV was 26.7 hours (SD 16.1) Intervention delivery by: Not clear Setting: Pulmonary medicine directed critical care unit at a university hospital |
|
| Outcomes |
Method of outcome data collection: Systolic and diastolic BP, heart rate, RR, ABGs (on room air), and PaO2/FiO2 ratio measured upon admission; at 30, 60, 90, 120, and 180 minutes; then every 3 hours thereafter Prespecified primary outcomes: Not clearly defined Prespecified secondary outcomes: Systolic and diastolic BP, heart rate, RR, ABGs (on room air), PaO2/FiO2 ratio, complications (abdominal distension, nasal bridge abrasion, aspiration), duration of mechanical ventilation, expired tidal volume, and minute ventilation. Mortality, treatment failure, and intubation were also reported Follow‐up period: Until hospital discharge |
|
| Notes | Study author contacted and additional information requested, without reply Funder: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information available |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomised ... by the envelope method". Unsure if opaque or clear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to nature of the intervention, blinding was not possible. No sham NIV was used. However, outcomes reported were objective outcomes and were unlikely to be affected "This prospective, randomized, controlled but unblinded study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | An ICU physician on call, who was not participating in the study, assessed treatment failure according to participant progress. Effects of outcome blinding on other study outcomes less clear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants' outcome data appear to be reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. Unclear which outcomes were prespecified |
| Imbalance of outcome measures at baseline All outcomes | Low risk | No evidence of statistically significant differences at baseline; however PFT data not available for all participants |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | No consideration of baseline factors in statistical analyses but no significant baseline differences reported |
| Protection against contamination All outcomes | High risk | Mortality data very likely to have been influenced by rescue cross‐over to NIV intervention. Participants in the standard therapy group with treatment failure were switched to NIV, then to mechanical ventilation if needed |
| Selective recruitment of participants | Unclear risk | Insufficient information available. Difficult to tell how many other potentially eligible participants may have been excluded from the study |
| Other bias | Low risk | No other sources of bias identified |
Collaborative 2005.
| Methods |
Country: China Design: Multi‐centre prospective randomised controlled trial. Randomisation via a centralised interactive voice system Study site: 19 teaching hospitals in China; general ward setting Methods of analysis: A 2‐tailed unpaired test with P = 0.05. Results are given as mean ± standard deviation (SD). Means were compared by unpaired t‐test or 1‐way analysis of variance. Chi2 test was used for rate of intubation and in‐hospital mortality. Analyses were done by SPSS 10.0 Aim: To evaluate outcomes of AECOPD if NIV is administered within 24 to 48 hours of admission to patients with respiratory muscle fatigue and mild respiratory insufficiency, especially those not fulfilling the conventional criterion of mechanical ventilatory support |
|
| Participants |
Eligible for study: 342 patients with AECOPD, age < 85 years, pH > 7.25, and PCO2 > 45 mmHg on arrival to the general ward were enrolled within 24 to 48 hours of admission Recruited: N = 342; n = 171 for intervention; n = 171 for control Completed: Intervention group: All completed the study: 8 participants required intubation (including 5 deaths) and 161 were discharged (2 deaths). Control group: 26 required intubation (including 12 deaths) and 145 were discharged Age: Intervention group: 69 ± 10 years. Control group: 70 ± 8 years Gender: Intervention group: 113 males, 58 females. Control group: 99 males, 72 females Criteria used to define COPD: Definitive or highly probable COPD based on clinical history, physical examination, CXR, spirometry, and ABGs. AECOPD was characterised by an exacerbation of dyspnoea, cough and increased sputum production, and changes in CXR Exclusion criteria: refused to receive NIV, pH < 7.25, GCS < 8, airway or facial deformity, pneumothorax/pneumomediastinum, unable to spontaneously clear secretions from the airway, SBP < 90 mmHg, uncontrolled cardiac arrhythmias, unable to co‐operate with application of NIV, severe organ dysfunction (APO, GI bleed, DIC, hepatic and renal dysfunction) |
|
| Interventions |
Intervention description: All centres used the same apparatus to deliver NIV (Harmony, Respironics, Inc., or BiBAP S/T30, Respironics, Inc., Murrysville, PA, USA) with an oronasal mask. Pressure support ventilation was initially delivered with EPAP of 2 to 4 cmH2O and IPAP of 6 to 8 cmH2O. EPAP was increased gradually to 4 to 6 cm H2O. IPAP was adjusted in increments of 2 cm H2O every 5 to 6 minutes to obtain a satisfactory spontaneous breathing pattern, or with maximal tolerated value for each participant. FiO2 was set to achieve SpO2 90% to 95%. All participants also received usual medical care, which included oxygen via nasal cannula, to maintain SpO2 90% to 95%, steroids, beta‐2 agonists, theophylline, mucolytics, respiratory stimulants, and antibiotics Control description: Usual medical care for management of AECOPD, which included oxygen via nasal cannula to maintain SpO2 90% to 95%, steroids, beta2‐agonists, theophylline, mucolytics, respiratory stimulants, and antibiotics Duration of intervention: Ventilatory support was initiated within 2 hours, for at least 12 hours for initial 3 days, 8 hours for next 2 days. At least 5 days of continuous ventilatory support was provided for all participants; 7 to 10 days was recommended Intervention delivered by: Not mentioned |
|
| Outcomes |
Method of outcome data collection: Data were collected in the general ward setting at baseline and throughout hospital stay Prespecified primary outcome: Protocol not available. In text outcomes: Need for intubation, in‐hospital mortality, length of hospital stay Prespecified secondary outcome: Protocol not available. In text outcomes: ABG, physiological parameters Validation: RR, HR and BP, APACHE II score, GCS, ABG, spirometry (bedside), dyspnoea score (grade 1 to 4), accessory muscle use score (0 to 5), ventilatory setting (IPAP, EPAP) and duration of NIV, adverse effects of NIV Follow‐up period: Throughout admission, until discharge Number of follow‐up periods reported on during study: Not reported Indications for intubation: Endotracheal intubation was considered if any of the following criteria were met: pH < 7.20 with progressive increase in PaCO2 or hypoxaemia PaO2 < 50 mmHg despite adequate FiO2 supplied; severe obtundation or loss of consciousness; cardiac or respiratory arrest; respiratory rate < 8/min or > 40/min. Once patients met intubation criteria, they were offered to continue intervention (NIV or M + usual care) or to introduce NIV or intubation according to patients' or first‐degree relatives' desire |
|
| Notes | Funder: Beijing Science and Technology Committee (No. 9555102600) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised interactive voice system |
| Allocation concealment (selection bias) | Low risk | Centralised interactive voice system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to nature of the intervention, blinding was not possible. No sham NIV was used. However, outcomes reported were objective outcomes and were unlikely to be affected |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned who collected the data and who initiated and adjusted NIV. Unsure whether investigator intervened in participant treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unsure whether enrolled numbers were same as recruited numbers. Unsure who managed the NIV for those who continued NIV after intubation criteria were met |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available, but all prespecified outcomes were reported in the Results section |
| Imbalance of outcome measures at baseline All outcomes | Unclear risk | Insufficient information reported |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Insufficient information reported |
| Protection against contamination All outcomes | High risk | 7 participants who met criteria for intubation received NIV |
| Selective recruitment of participants | Unclear risk | Insufficient information reported |
| Other bias | Low risk | No other sources of bias identified |
del Castillo 2003.
| Methods |
Country: Spain Design: Single‐centre prospective randomised controlled parallel‐group trial Study site: Single tertiary university hospital in Seville, Spain, between March 1998 and December 2000 Method of analysis: t‐test/Mann‐Whitney U, ANOVA/Friedman's test, Chi2 test, and Fisher's exact test Aim: To evaluate possible benefits of NIV plus standard therapy vs standard therapy alone in patients admitted with AHRF to the respiratory unit of a tertiary hospital |
|
| Participants |
Eligible for study: Not stated Recruited: 41 patients (20 in NIV group, 21 in usual care group) Completed: Not stated Age: NIV group: mean (SD) age = 66 (9) years; usual care group: mean (SD) age = 69 (7) years Gender: M:F, NIV group = 19:1, control group = 19:2 Criteria used to define COPD: Criteria used by the Spanish Respiratory Society (SEPAR) and, for those without diagnostically confirmed COPD, diagnosis based on a smoking history and clinical data (radiological and gasometric compatibility with chronic airflow obstruction) Inclusion criteria: AHRF (PaO2 < 60 mmHg, PaCO2 ≥ 55 mmHg, pH < 7.35) with clinical evidence of respiratory muscle fatigue (RR > 25 breaths/min and accessory muscle use) Exclusion criteria: Any of the following: suspected pulmonary embolism, malignancy, or pneumonia; previous diagnosis of obstructive sleep apnoea syndrome; severe ischaemic heart disease (unstable angina or acute myocardial infarction in the past 3 months); haemodynamic instability (systolic blood pressure < 90 mmHg) and uncontrolled co‐existent serious arrhythmia; severe bronchospasm; immediate need for intubation; lack of patient co‐operation or refusal to participate in the study |
|
| Interventions |
Intervention description: Usual care plus NIV with a standard mask connected to a BiPAP ventilatory assist device (Respironics, Inc., Murrysville, PA, USA). Initial settings: IPAP 10 cmH2O, EPAP 4 cmH2O, S‐T mode, RR 12 breaths/min. IPAP was progressively increased in the first minute up to 20 cmH2O (max), guided by patient tolerance and oxygen saturation levels. EPAP remained at 4 cmH2O. Oxygen (1 to 2 L/min) was supplied through a cannula connected to the mask. Participants started with a nasal mask but changed to the oronasal route if mouth leaking was observed Control description: Oxygen through Venturi mask (FiO2 = 0.24) or 1.5 L/min via nasal prongs if intolerant to mask or ABG improvement, methylprednisolone (40 mg/12 h), and antibiotics (cefuroxime 750 mg/8 h) intravenously, nebulised salbutamol and ipratropium bromide, ranitidine (gastric protection), heparin (low molecular weight as prophylaxis of venous thromboembolism), and respiratory physiotherapy Duration of intervention: Patients received NIV during the night (at least 7 hours/night), daily and throughout hospital stay Intervention delivery by: After initial stabilisation, NIV monitoring was provided by nursing staff, who were properly trained and familiar with the NIV device Setting: Respiratory unit of a tertiary hospital |
|
| Outcomes |
Method of outcome data collection: RR, BP, degree of encephalopathy, and ABGs were taken at 2, 6, 24, 48, and 72 hours after the start of treatment. PFTs were measured at discharge Prespecified primary outcomes: Not clearly defined Prespecified secondary outcomes: Duration of NIV, pressure levels, NIV complications and tolerance, hospital length of stay, intubation, and ABG analyses Follow‐up period: Hospital discharge |
|
| Notes | Funder: Unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information available. Described only as randomised |
| Allocation concealment (selection bias) | Unclear risk | Information not reported in publication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No sham NIV used. Blinding of participants did not occur |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants were initially monitored by a physician who participated in the study, to ensure adaptation to NIV. Nurses monitored NIV care after this time. Changes in medical management occurred at the judgement of the clinician responsible for the participant. Unclear whether personnel were involved in data collection |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Blood pressure was reported to have been collected, but results were not presented |
| Selective reporting (reporting bias) | High risk | Insufficient information available; blood pressure was reported to have been collected but results were not presented |
| Imbalance of outcome measures at baseline All outcomes | Low risk | "Both groups had similar characteristics upon their admission in the hospital" |
| Comparability of intervention and control group characteristics at baseline | Low risk | "Both groups had similar characteristics upon their admission in the hospital" |
| Protection against contamination All outcomes | High risk | Three participants in the control group received NIV owing to deterioration during early hours of the day. Mortality and intubation outcomes were likely affected |
| Selective recruitment of participants | Unclear risk | Insufficient information available |
| Other bias | Low risk | No other sources of bias identified |
Dikensoy 2002.
| Methods |
Country: Turkey Design: Single‐centre prospective randomised controlled parallel‐group trial Study site: One tertiary health centre in South East Turkey Method of analysis: Statistical analysis done using SPSS 9.0 for Windows. Results expressed as mean ± SD. Wilcoxon's rank‐sum test; Mann‐Whitney U test. P < 0.05 was considered statistically significant Aim: To compare the effectiveness of NIV plus usual care vs usual care alone in patients with AHRF due to AECOPD |
|
| Participants |
Eligible for study: Not stated Recruited: 34 adult male patients (17 in NIV group; 17 in usual care group) recruited immediately after presentation to emergency department with AECOPD Completed: 32 (15 in NIV group; 17 in usual care group ‐ 2 participants were unable to tolerate the intervention and were excluded) Age: NIV group: mean (SD) age = 65.1 (6.1) years; usual care group: mean (SD) age = 64.2 (7.5) years Gender: Not stated Criteria used to define COPD: ATS criteria Inclusion criteria: Unclear Exclusion criteria: Unclear |
|
| Interventions |
Intervention description: Usual care plus NIV. BiPAP ventilators were used with IPAP of 9 cmH2O and fixed EPAP of 3 cmH2O, via full face mask. Mean IPAP level was 15.3 cmH2O (SD 4.3) Control description: Oxygen, salbutamol 2.5 mg 4‐hourly, ipratropium bromide 500 mcg 4‐hourly by nebulisation, prednisolone 1 mg/kg/d IV, aminophylline infusion 0.5 mg/kg/d IV, enoxaparin sodium 20 mg/d subcutaneously, and antibiotics if indicated Duration of intervention: Mean duration of NIV was 11.2 (SD 9.5) hours. NIV was continued until the respiratory rate was < 25/min, pH > 7.35, and SaO2 > 88% (during oxygen inhalation) Intervention delivery by: Not clearly stated Setting: General medical ward |
|
| Outcomes |
Prespecified primary outcomes: Unclear Prespecified secondary outcomes: Mortality, intubation, pH, PCO2, PO2, respiratory rate, heart rate, systolic blood pressure, HCO3 , treatment failure, and complications Follow‐up period: Until discharge |
|
| Notes | Additional details requested from study authors (no reply received) Funder: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation by direct numeration |
| Allocation concealment (selection bias) | High risk | Randomisation, then continued sequentially with the next patient admitted to the clinic |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, blinding was not possible. No sham NIV was used. However, outcomes reported were objective outcomes and were unlikely to be affected |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unsure whether investigators were involved in participants' care at any time |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned how missing variables, if any, were handled. Unsure whether all participants completed the study. Data were analysed via intention‐to‐treat |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. Unclear which outcomes were prespecified |
| Imbalance of outcome measures at baseline All outcomes | High risk | Study authors reported that by chance baseline PaCO2 and HCO3 were significantly different between the 2 study groups |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Baseline differences were noted in PaCO2 and HCO3 but unclear if these were considered in statistical analysis |
| Protection against contamination All outcomes | Unclear risk | Insufficient information available |
| Selective recruitment of participants | Unclear risk | Insufficient information available |
| Other bias | Low risk | No other sources of bias identified |
Khilnani 2010.
| Methods |
Country: India Design: Randomised, non‐blinded, non‐placebo‐controlled trial Study site: Single‐centre ICU setting at All India Institute of Medical Sciences, New Delhi, from March 1999 to March 2001 Method of analysis: Categorical variables were described in proportions and Chi2 test was used for comparison between baseline data and post‐admission data within 2 groups and between 2 groups. Continuous variables were described by mean ± SD, independent t‐test was used to compare 2 groups, and paired t‐test was used for intragroup comparison. Multiple comparisons were performed using ANOVA. Significance was considered at P < 0.05 (2‐tailed) Aim: To determine the safety and efficacy of NIPPV in the subgroup of patients with most severe acute exacerbations of COPD admitted to medical intensive care unit |
|
| Participants |
Recruited: 62 patients with AECOPD admitted to ICU screened for study inclusion; 40 recruited (20 in NIV group, 20 in usual care group) Completed: Unclear Age: Intervention group – mean (SD) age = 55.3 (10.1) years. Control group – mean (SD) age = 60 (11.1) years Gender: Intervention group – 15 male and 5 female. Control group – 16 male and 4 female Inclusion criteria: Patients with AECOPD leading to hypoxaemia and respiratory acidosis with pH < 7.35 and PaCO2 > 45 mmHg admitted to the ICU Exclusion criteria: Respiratory arrest, haemodynamic instability, altered sensorium, copious secretion, unco‐operative Criteria used to define COPD: COPD diagnosed according to characteristic findings on history and examination with typical radiographic abnormalities. AECOPD defined by presence of hypoxaemia and respiratory acidosis (pH < 7.35 and PaCO2 > 45 mmHg) |
|
| Interventions |
Intervention descriptions: BiPAP (Nellcor Puritan Bennett, Boulder, CO, USA) with adjustable pressure limits; participant was ventilated as per predefined inspiratory and expiratory airway pressure settings, with each inspiration triggered by a spontaneous breath. The interface used was a well‐fitting nasal mask (moderate to large size). Participant was propped up to a 45‐degree angle. Usually initiated on IPAP 8 cmH2O and EPAP 4 cmH2O; subsequent adjustments were carried out according to participant needs and results of blood gas analysis. The protocol was to augment IPAP and EPAP by 2 cmH2O every 5 to 10 minutes, participant comfort and arterial oxygen saturation permitting. Each participant was encouraged to use NIV up to 16 hours/d including night, and duration of ventilation was recorded Control descriptions: 3 to 4 L/min oxygen to maintain SpO2 > 90% and pharmacological treatment with bronchodilators (inhaled salbutamol, ipratropium, subcutaneous terbutaline), IV steroids, and IV antibiotics Duration of intervention: Weaning if sustained clinical improvement noted with reduction of RR < 24/min, as well as HR 100/min, normal pH, and PaCO2 < 55 mmHg and SpO2 > 90% Intervention delivery by: Investigators (GCK and NS) in all cases Setting: ICU |
|
| Outcomes |
Method of outcome data collection: Closely monitored for participant's discomfort and intolerance, accessory muscle used, increase or decrease in dyspnoea, appearance and disappearance of cyanosis, heart rate, respiratory rate, blood pressure, and level of consciousness. ABG at 1 hour, 6 hours, 24 hours, 48 hours, 72 hours, fifth day, and anytime if participant's condition required Prespecified primary outcomes: Incidence of need for endotracheal intubation with the following criteria: worsening gas exchange parameters (rising PaCO2 and/or worsening pH), GCS < 8, mean arterial pressure (MAP) < 60 mmHg, copious secretions, and intolerance for face mask Prespecified secondary outcomes: Hospital mortality Duration of hospital stay Change in clinical and blood gas parameters Complications – safety variables (aspiration, bloating and skin ulcers, development of ventilator‐associated pneumonia, HD instability) Follow‐up period: until end of admission |
|
| Notes | Mean IPAP was 15.5 ± 3.4 cmH2O and EPAP 9.9 ± 1.9 cmH2O. Maximum IPAP was 18 cmH2O and EPAP 11 cmH2O Mean admission PaCO2 very high in both groups (NIV ‐ 85.4 ± 14.9 mmHg; usual care ‐ 81.1±11.7 mmHg) Funder: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation using random number table was utilised for group allocation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of intervention described. Unlikely to have affected primary outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Nil evidence of outcome assessor blinding. All NIV was initiated by investigators. May have influenced decisions re intubation (process not objectively described) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not mentioned how missing variables, if any, were handled. Unsure whether all participants completed the study |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available but all prespecified outcome data were reported in the Results |
| Imbalance of outcome measures at baseline All outcomes | Low risk | No baseline differences apparent, and no statistical adjustments required |
| Comparability of intervention and control group characteristics at baseline | Low risk | Groups not significantly different at baseline |
| Protection against contamination All outcomes | Unclear risk | Not clear how complications in the control group (e.g. pneumonia, pneumothorax) were managed |
| Selective recruitment of participants | Low risk | Number of participants and methods of recruitment similar for both groups |
| Other bias | Low risk | No other sources of bias identified |
Kramer 1995.
| Methods |
Country: United States Design: Randomised controlled trial, prospective design Study site: Conducted between October 1992 and June 1993 at Rhode Island Hospital and Roger Williams Medical Centre ‐ both teaching affiliates of Brown University School of Medicine (Providence, RI, USA) Methods of analysis: Demographic and baseline data and continuous variables were compared between groups using unpaired t‐tests. Need for intubation and mortality rates were compared using Chi2 test with continuity correction for 2 by 2 tables. Differences were considered significant at P < 0.05. Data were presented as mean ± SE Aim: To test the hypotheses that, in patients with acute respiratory failure who are otherwise stable, NIV reduces the need for endotracheal intubation; more rapidly improves respiratory frequency, heart rate, and sense of dyspnoea; and shortens length of hospitalisation in comparison with standard therapy alone |
|
| Participants |
Eligible for study: 31 patients with acute respiratory failure: 16 in the NIV group and 15 in the control group. Included patients with COPD, heart failure, pneumonia, asthma, and pulmonary embolus Recruited: 23 patients with COPD were recruited: 11 in the NIV group and 12 in the usual care group Completed: Not clearly described Age: No COPD‐specific data available Gender: No COPD‐specific data available Criteria to define COPD: Not stated Inclusion criteria: Patients with COPD in respiratory distress: moderate to severe dyspnoea, accessory muscle use or abdominal paradox and acute respiratory failure with pH < 7.35, PaCO2 > 45 mmHg, and RR > 24 bpm Exclusion criteria: respiratory arrest or need for immediate intubation; hypotension (SBP < 90 mmHg); uncontrolled arrhythmias; upper airway obstruction or facial trauma; inability to clear secretions; inability to co‐operate or fit mask |
|
| Interventions |
Intervention description: All participants were first fitted with a nasal mask, however this was substituted with an oronasal mask if participant intolerant or excessive air leak. NIV was administered using a BiPAP ventilator assist system (Respironics, Inc,, Murrysville, PA, USA), a pressure‐limited device that cycles between adjustable (up to 20 cmH2O) inspiratory and expiratory pressure using S or T modes. The S/T mode was used for this study. Ventilation was initiated with a backup rate of 12 breaths/min. IPAP was set at 8 cmH2O initially, and EPAP was set at the lowest possible setting (˜2 cmH2O). Oxygen was blended in via a mask port to maintain SpO2 ≥ 90%. IPAP was increased by 1 cmH2O every 15 to 30 minutes or as tolerated. Subsequent adjustment in IPAP if ABG showed persistent respiratory acidosis or clinical evidence of continued respiratory distress All participants also received corticosteroids, frequent respiratory treatments, supplemental oxygen, and antibiotics Control description: Corticosteroids, frequent respiratory treatments, supplemental oxygen, and antibiotics. Control participants who declined intubation were offered a trial of NIV if their condition deteriorated sufficiently to warrant intubation and if they were considered to have needed intubation for purposes of analysis Duration of intervention: Encouraged to use NIV as long as tolerated, aiming for at least 8 hours per day. Weaning no sooner than 6 hours after initiation of NIV once clinical stability was achieved (RR < 24 bpm, HR < 110 bpm, pH > 7.35, and SpO2 > 90%; no more than 3 L/min oxygen flow) achieved. Intervention delivery by: Not reported |
|
| Outcomes |
Method of outcome data collection: Data were collected upon admission at baseline and throughout hospital stay Prespecified primary outcome: Protocol not available. In text outcome: Need for intubation Prespecified secondary outcomes: Protocol not available. In text outcomes as below: heart rate; respiratory rate; ABG; oxygen supplementation; self‐assessment of dyspnoea based on visual analogue scale (0 to 10, with 10 greatest degree of dyspnoea); nursing and respiratory therapy time consumption; level of care; total hospital length of stay; mortality; and charges for total hospital stay and respiratory services Validation: Vitals, ABG, visual analogue score Follow‐up period: Participants were assessed at 1, 2, 3, 6, 12, 24, 48, and 72 hours Intubation criteria: Worsening mental status, dyspnoea or tachypnoea, hypotension (SBP < 90 mmHg), rise in PaCO2 of 5 to 10 mmHg, or fall in pH of 0.05 to 0.1 units Setting: ICU or step‐down unit |
|
| Notes |
Complications: 11 in NIV group Average duration for NIV: 3.8 ± 1.4 days (0.2 to 23 days) Average usage during first 24 hours: 20.1 ± 0.4 hours Average IPAP: 11.3 ± 0.9 cmH2O Average EPAP: 2.6 ± 0.3 cmH2O Study included patients with diagnoses other than COPD; however, only data from patients with COPD were included, when available Additional study information obtained through email contact with study author Funder: Partially supported by Respironics, Inc., Murrysville, PA, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomised scheme |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used for treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Owing to nature of the intervention, blinding was not possible. No sham NIV was used. May have affected self‐reported levels of dyspnoea, but unlikely to have adversely affected other objective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unsure who delivered NIV and whether investigator was involved in participants' care. Decisions to intubate were made by participant's primary physician. Several secondary outcomes could have been affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned how missing variables, if any, were handled. Unsure whether all participants completed the study |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but all prespecified outcomes were reported |
| Imbalance of outcome measures at baseline All outcomes | Low risk | No baseline differences required to be adjusted in analysis |
| Comparability of intervention and control group characteristics at baseline | Low risk | Baseline data reported and similar across groups |
| Protection against contamination All outcomes | Low risk | Some participants in the control group received the intervention as 'rescue therapy'; however, these data were clearly reported and distinguished |
| Selective recruitment of participants | Low risk | All patients who met study eligibility criteria were referred by the primary physician to study investigators and were offered entry into the study. Unclear how many patients were screened for study eligibility |
| Other bias | Low risk | No other sources of bias identified |
Liu 2005.
| Methods |
Country: China, Nanjing Design: Randomised controlled trial. Randomisation method was not reported Study site: Single centre in Nanjing, from December 2001 to December 2003 Setting: ICU Methods of analysis: SPSS 11.5 for analysis. P < 0.05 statistically significant Aim: To evaluate effects of early use of non‐invasive positive pressure ventilation on gas exchange, rate of endotracheal intubation, and in‐hospital mortality among patients with acute exacerbations of COPD |
|
| Participants |
Eligible for study: Not mentioned Recruited: 36 patients with acute exacerbations of COPD with pH from 7.25 to 7.35 and PaCO2 >45 mmHg were enrolled. 18 participants were randomised to NIV group and 18 to standard therapy group. Baseline characteristics were similar in both groups Completed: 18 participants in NIV group and 18 in usual care group Age: NIV group: 70.8 ± 5.1 years. Usual care group: 68.4 ± 6.0 years Gender: NIV group: 15 male and 3 female. Usual care group: 14 male and 4 female Criteria used to define COPD: COPD as defined in 1997 Chinese Association of Respiratory Physician COPD plan Inclusion criteria: Acute exacerbation of COPD according to history and physical examination with radiological findings; pH 7.23 to 7.35 and PaCO2 > 45 mmHg Exclusion criteria: Refusal for NIV; GCS < 8; pneumothorax; respiratory arrest; arrhythmia; multi‐organ failure; severe abdominal distension, bowel perforation or bleeding, recent bowel surgery; face trauma; face irregularities |
|
| Interventions |
Intervention description: NIV provided by BiPAP vision using full face mask, with initial PEEP 4 cmH2O and pressure support 2 to 4 cmH2O, titrated by 2 cmH2O until RR < 28 bpm and SpO2 > 90%. All participants also received bronchodilator, antibiotics, mucolytics, and supplementary oxygen to maintain SpO2 > 90% Control description: Bronchodilator, antibiotics, mucolytics, supplementary oxygen to maintain SpO2 > 90% Duration of intervention: At least 3 days. Initial NIV maintained over 2 hours with aim of at least 8 hours per day Intervention delivery by: Not reported in text |
|
| Outcomes |
Method of outcome data collection: Data were collected at baseline and throughout hospital stay Prespecified primary outcome: Protocol not available. In text outcomes: Endotracheal intubation rate, in‐hospital mortality rate, ABG changes Prespecified secondary outcome: Protocol not available. In text outcomes: NIV complications; RR and HR Validation: ABG, HR, RR Follow‐up period: Throughout admission, until discharged or end point reached Number of follow‐up periods reported on during study: Not reported Indications for intubation: pH < 7.25, increasing PCO2, PO2 < 45 mmHg, GCS < 8, cardiopulmonary arrest, RR < 8/min, or RR > 40/min Complications: NIV was not tolerated in 1 participant. One had a face pressure ulcer, which resolved after NIV was stopped |
|
| Notes |
Mean duration: 6.1 ± 1.9 days; 53.7 ± 26.6 hours; mean 8.8 ± 3.6 hours per day Maximum PS 14.3 ± 2.8 cmH2O; maximum PEEP 4.3 ± 0.8 cmH2O Paper in Chinese, with limited translation from translator. Attempted to contact study authors for more information, but no reply Funder: Great Topic Foundation of Health Bureau Jiangsu Province, China (No. H200102) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used for randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, blinding was not possible. No sham NIV was used. However, outcomes reported were objective outcomes and were unlikely to be affected |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned who delivered the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned how missing variables, if any, were handled. Unsure whether all participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes not clearly explained |
| Imbalance of outcome measures at baseline All outcomes | Unclear risk | Insufficient information reported |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Insufficient information reported |
| Protection against contamination All outcomes | Unclear risk | Insufficient information reported |
| Selective recruitment of participants | Unclear risk | Insufficient information reported |
| Other bias | Unclear risk | Limited information available to assess whether free of other sources of bias |
Matuska 2006.
| Methods |
Country: Czech Republic Design: Prospective randomised controlled trial. Randomisation method not mentioned Study site: Single centre from 2002 to 2004 Setting: Respiratory department ICU Methods of analysis: Chi2test was used to compare the sex ratio among groups. The non‐parametric Wilcoxon test was used to compare figures at the beginning vs at the end. The Friedman ANOVA test was used to compare data at baseline, after 1 hour, and at the end of the trial. All data were on the level of significance of α = 5%, and all used tests were mutual Aim: To verify that use of NIV support in AECOPD leads to a decrease in the number of endotracheal intubations |
|
| Participants |
Eligible for study: Not mentioned Recruited: 60 were recruited. 30 participants were randomised to the NIV group and 30 to the control group Completed: 23 of 30 participants in the NIV group and 18 of 30 in the control group completed the study Age: NIV group – mean age 65.6 years; usual care group – mean age 68.4 years Gender: Not mentioned in each arm. Overall 43 males and 17 females Criteria used to define COPD: FEV1/FVC < 75% and improvement in FEV1 after bronchodilators of < 12%; physical examination, known retention of CO2, and elevated bicarbonate Inclusion criteria: Acute exacerbation of previously diagnosed COPD by PFT (FEV1/FVC < 75% and improvement in FEV1 after bronchodilators of < 12%; physical examination, known retention of CO2, and elevated bicarbonate. Participants had acute exacerbations of COPD, pH < 7.35, PaCO2 > 6 kPa, respiratory rate > 25/min Exclusion criteria: Pulmonary arrest; reduced consciousness; hypotension < 90 mmHg of systolic pressure; acute myocardial infarction; severe cardiac arrhythmia |
|
| Interventions |
Intervention description: NIV was provided by BiPAP Respironics T using a full face mask, PEEP 4 cmH2O, inspiratory pressure 10 cmH2O gradually increased to maximum tolerated by participant. Intermittent ventilation, participants ventilated with pauses for meals, inhalations, and cough. NIV was terminated at pH > 7.35 and respiratory frequency was reduced to < 25 /min. All participants also received oxygen by nasal cannula or mask with flow set to maintain saturation at 90%, continuous aminophylline at 0.6 mg/kg/h to maintain range between 10 and 20 mg/L, methylprednisolone 40 to 80 mg IV every 8 hours, antibiotics, expectorants, and nebulised bronchodilators (salbutamol 1 mL three times a day) Control description: Oxygen by nasal cannula or mask with flow set to maintain saturation at 90%, continuous aminophylline at 0.6 mg/kg/h to maintain range between 10 and 20 mg/L, methylprednisolone 40 to 0 mg IV every 8 hours, antibiotics, expectorants, and nebulised bronchodilators (salbutamol 1 mL three times a day) Duration of intervention: NIV was terminated at pH > 7.35 and reduction of respiratory frequency < 25 /min Intervention delivery by: Not reported in text |
|
| Outcomes |
Method of outcome data collection: Data were collected at baseline and throughout the hospital stay Prespecified primary outcome: Protocol not available. In text outcomes: Mortality, duration of stay in ICU, number of intubated participants Prespecified secondary outcome: Protocol not available. In text outcomes: Faster reduction in respiratory rate and heart rate; reduction in PaCO2; increase in PaO2; spirometry parameters, subjective dyspnoea score Validation: ABG during ICU admission; after 1, 3, 6, 12, 48, and 72 hours; and during ICU discharge. Respiratory rate, heart rate, and dyspnoea score (1 to 10 score with 10 as the worst dyspnoea) were observed at the same intervals. FEV1 and FVC were measured with portable spirometry at baseline and at the end of the trial Follow‐up period: Throughout admission, until discharged or endpoint reached Number of follow‐up periods reported on during study: Not mentioned in text Indications for intubation: Pulmonary arrest, reduced consciousness, hypotension < 90 mmHg of systolic pressure, acute myocardial infarction, severe cardiac arrhythmia Complications: Three participants (10%) did not tolerate NIV (unsure whether participants discontinued); 1 had a nose decubitus |
|
| Notes | Mean NIV duration was 15.7 hours Paper in Czech. Acknowledged Jan Strojil and Kristyna Matejkova for translation. Attempted to contact study authors for more information, but no reply Funder: Grant IGA MY 7717‐3/2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information available |
| Allocation concealment (selection bias) | High risk | Sealed envelopes were used; not mentioned whether opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to nature of the intervention, blinding was not possible. No sham NIV was used. However, outcomes reported were objective outcomes and were unlikely to be affected |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unsure who delivered the NIV and whether investigators were involved in participants' care |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some reasons for excluded participants were explained, but some outcomes excluded those who died (i.e. respiratory rate) |
| Selective reporting (reporting bias) | Low risk | Published reports included all prespecified and expected outcomes |
| Imbalance of outcome measures at baseline All outcomes | High risk | PCO2 statistically higher in NIV group than in control group at baseline |
| Comparability of intervention and control group characteristics at baseline | Low risk | No imbalance in baseline characteristics |
| Protection against contamination All outcomes | Low risk | No rescue NIV given to control group |
| Selective recruitment of participants | Low risk | Participant flow chart as described in graph 1, with n values the same in both groups |
| Other bias | Unclear risk | Unsure whether free of other biases |
Plant 2001.
| Methods |
Country: United Kingdom Design: Multi‐centre prospective randomised controlled trial. Randomisation via a blocked design to each centre and generated by an independent statistician who used random numbers Study site: 14 tertiary hospitals in the UK between November 1996 and September 1998 Setting: General medical/respiratory wards with no invasive monitoring. 22 wards had no experience of NIV, and only 1 was fully experienced. The mean amount of formal training given over the first 3 months after a ward was opened by the research doctor and nurse was 7.6 hours (SD 3.6) Methods of analysis: Aimed to recruit 236 patients, which gave the study 80% power for detecting a clinically significant difference in the proportion of patients experiencing treatment failure at the 5% level of significance, on the assumption that 30% of the standard group would fulfil the criteria for intubation, and that a 15% reduction in the NIV group would be clinically relevant. Results given as means (SD) for normally distributed data and as medians with 5th and 95th centiles for non‐normally distributed variables. All tests and P values are 2‐tailed and were analysed on an intention‐to‐treat basis. Group means were compared by t‐test, and medians by Mann‐Whitney U test. Bonferroni’s correction to multiple comparisons. 2 × 2 tables were analysed by Fisher’s exact test. Kaplan‐Meier curves for time data and log‐rank test for comparison. Analyses were done by SPSS version 7 Aim: To evaluate whether NIV was feasible on the ward in non‐specialist units, and whether it was effective in reducing the need for intubation and in‐hospital mortality, compared with standard treatment, in patients admitted with mild to moderate acidosis due to an exacerbation of COPD |
|
| Participants |
Eligible for study: Patients eligible if admitted as an emergency with AECOPD (on the basis of clinical history, physical examination, and CXR), with tachypnoea with RR ≥ 23/min and pH 7.25 to 7.35 with PaCO2 > 6kPa on arrival to the general respiratory ward within maximum of 12 hours from admission Recruited: 236 randomised: 118 in intervention group and 118 in control group Completed: Intervention group: 12 died and 106 survived. Control group: 24 died and 94 survived Age: Intervention group: 69 ± 7 years. Control group: 69 ± 8 years Gender: Intervention group: 54 males, 64 females. Control group: 63 males, 55 females. More males in control group Criteria used to define COPD: Based on clinical history, physical examination, and CXR Exclusion criteria: pH < 7.25, GCS < 8, or active treatment deemed inappropriate |
|
| Interventions |
Intervention description: NIV was initiated by the nurse at 13 centres and by the physiotherapist at 1, with a standardised protocol. All centres used the same bi‐level assist‐mode ventilator (VPAP‐II, ResMed, UK) and were supplied with identical sets of masks. Two face masks (Aircraft mask (Friday Medical, UK) and a small full‐face mask (Respironics, Inc., Murraysville, PA, USA) plus 2 nasal masks (small and medium Bubblie Cushion Series 3 (ResMed, UK) were supplied with accompanying headgear. EPAP was set at 4 cmH2O. IPAP was initially set at 10 cmH2O, then was increased in increments of 5 cmH2O to 20 cmH2O or the maximum tolerated over 1 hour. All participants also received usual care, which included oxygen to maintain a target oxygen saturation of 85% to 90% via pulse oximetry. The standard drug protocol consisted of nebulised salbutamol (5 mg every 4 hours) or terbutaline, nebulised ipratropium bromide (500 mcg every 6 hours), corticosteroids (prednisolone 30 mg every day for minimum of 5 days), and an antibiotic. Aminophylline and doxapram could be used at the discretion of the attending medical staff Control description: Received controlled oxygen with fixed percentage masks (or nasal cannulae if masks not tolerated) to maintain a target oxygen saturation of 85% to 90% via pulse oximetry Standard drug protocol consisted of nebulised salbutamol (5 mg every 4 hours) or terbutaline, nebulised ipratropium bromide (500 mcg every 6 hours), corticosteroids (prednisolone 30 mg every day for minimum of 5 days), and an antibiotic. Aminophylline and doxapram could be used at the discretion of the attending medical staff Duration of intervention: Encouraged to use NIV as much as possible on day 1, for 16 hours on day 2, and for 12 hours on day 3. Routinely discontinued on day 4, although an option to continue was available if clinically indicated Intervention delivery by: NIV was initiated by the nurse at 13 centres and by the physiotherapist at 1, with a standardised protocol |
|
| Outcomes |
Method of outcome data collection: Data were collected in the general ward setting at baseline and throughout hospital stay Prespecified primary outcome: Protocol not available. In text outcome: Need for intubation, if participants met any criteria within 14 days of admission. After criteria were met, attending physicians were able to offer any of the following: continued standard treatment, NIV off protocol by a more sophisticated NIV, or intubation and mechanical ventilation Prespecified secondary outcome: Protocol not available. In text outcomes: Mortality; respiratory rate; ABG at enrolment, 1 hour and 4 hours after randomisation, and on day 3; hospital length of stay. Mask comfort and breathlessness were assessed by 5‐point verbal rating. Mobility and nutritional status assessed by nursing staff and nursing workload. ABG at room air and spirometry measured when possible at discharge or within 3 months Validation: Respiratory rate and ABG at enrolment, 1 hour and 4 hours after randomisation, and on day 3. Mask comfort and breathlessness were assessed by 5‐point verbal rating scales – comfortable/mildly uncomfortable/moderately uncomfortable/very uncomfortable/intolerable; not breathless/mildly breathless/moderately breathless/very breathless/most breathless I have ever been. ABG at room air and spirometry measured when possible at discharge or within 3 months Follow‐up period: Throughout admission, until discharge Number of follow‐up periods reported on during study: 4 (at enrolment, 1 hour and 4 hours after randomisation, and on day 3) Indications for intubation: pH < 7.20; pH 7.2 to 7.25 on 2 occasions 1 hour apart; hypercapnic coma (GCS < 8 and PaCO2 > 8 kPa); PaO2 < 6 kPa despite maximum tolerated FiO2; cardiorespiratory arrest |
|
| Notes | The number of participants intolerant of NIV was not stated; however, 7.2% of participants used NIV < 1 hour on first day, rising to 23.6% on day 2, and 32.3% on day 3. Those assigned to NIV used it for a median duration of 3 days (range 0 to 26). Median NIV use was 8 hours on day 1, 7 hours on day 2, and 5 hours on day 3. Median comfort score on first 3 days for NIV group was 2 – mildly uncomfortable Funder: Northern and Yorkshire NHS Executive |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule had a blocked design for each centre and was generated by an independent statistician, who used random numbers |
| Allocation concealment (selection bias) | Low risk | Individual assignments were made by using opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to nature of the intervention, blinding was not possible. No sham NIV was used. However, outcomes reported were objective outcomes and were unlikely to be affected |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | In all cases NIV was initiated by nurses or physiotherapist. Unsure whether this involved investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were analysed on intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but all prespecified outcomes were reported in the Results section |
| Imbalance of outcome measures at baseline All outcomes | Low risk | Similar baseline ABG in 2 groups |
| Comparability of intervention and control group characteristics at baseline | Low risk | Baseline characteristics similar between the 2 groups |
| Protection against contamination All outcomes | High risk | 15 participants in control group received NIV after treatment failure |
| Selective recruitment of participants | Low risk | As explained in trial profile in Figure 1 |
| Other bias | Low risk | No other sources of bias identified |
Samaria 2009.
| Methods |
Country: India Design: Randomised controlled trial but no information about randomisation method or allocation concealment Study site: Not stated in abstract Setting: ICU and ward. NIV was conducted in ICU, and usual care was conducted on the ward Methods of analysis: Not stated in abstract Aim: Not stated in abstract |
|
| Participants |
Eligible for study: Not stated in abstract Recruited: 40 were recruited: 20 participants in NIV group and 20 in usual care group Completed: Not stated in abstract Age: No data available in abstract Gender: No data available in abstract Criteria used to define COPD: Not stated in abstract Inclusion criteria: Not stated in abstract Exclusion criteria: Not stated in abstract |
|
| Interventions |
Intervention description: Participant received NIV in ICU through BiPAP machine in S/T mode using a backup respiratory rate of 12 bpm with an initial setting of IPAP 12 cmH2O and EPAP 5 cmH2O Participants received the same appropriate pharmacological therapy and oxygen supplementation Control description: Stated only appropriate pharmacological therapy and oxygen supplementation in abstract. No specific therapy reported Duration of intervention: Participants underwent NIV for at least 10 hours each day. The period could be lengthened as required Intervention delivery by: Not reported in text |
|
| Outcomes |
Method of outcome data collection: Not stated in abstract Prespecified primary outcome: Protocol not available. In abstract outcomes: Intubation rate, mortality rate Prespecified secondary outcome: Protocol not available. In text outcome: Intubation rate Validation: Not stated in abstract Follow‐up period: Not stated in abstract Number of follow‐up periods reported on during study: Not stated in abstract Indications for intubation: Not stated in abstract |
|
| Notes | Abstract(s) only. Usual care provided on a respiratory ward, whilst NIV provided in an ICU setting ABG results obtained 2 hours post treatment Emailed study author to obtain study protocol and additional data, without reply Funder: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information available |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to nature of the intervention, blinding was not possible. No sham NIV was used. However, objective outcomes were unlikely to be adversely affected |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information available |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available |
| Imbalance of outcome measures at baseline All outcomes | Unclear risk | Insufficient information available |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Insufficient information available |
| Protection against contamination All outcomes | Unclear risk | Insufficient information available |
| Selective recruitment of participants | Unclear risk | Insufficient information available |
| Other bias | Unclear risk | Insufficient information available |
Thys 2002.
| Methods |
Country: Belgium Design: Single‐centre randomised controlled parallel‐group trial conducted between 1999 and 2000 Study site: One urban university teaching hospital in Brussels, Belgium Method of analysis: t‐tests, ANOVA Aim: To determine whether (1) benefits of NIV on need for intubation, length of stay, and mortality are noted when NIV is performed in an emergency department (ED) very early after admission; and (2) early NIV has a real, rather than placebo, effect on objectively measured parameters and clinical status |
|
| Participants |
Eligible for study: 187 (of either COPD or APO diagnosis) patients admitted to emergency department with acute respiratory failure Recruited: 20 adult patients (10 in NIV group; 10 in control group) with acute respiratory failure secondary to AECOPD (n = 12) or APO (n = 8). Of those with AECOPD, 7 were in NIV group; 5 in control group Completed: 20 participants (10 in each group) Age: Unable to ascertain for COPD‐only participants. Total (combined diagnoses) for NIV group: mean (SD) age = 71 (9) years; usual care group: mean (SD) age = 76 (7) years Gender: Unable to ascertain for COPD‐only participants. Total (combined diagnoses) gender (M:F) = 7:3 (NIV group); 4:6 (control group) Criteria used to define COPD: AECOPD defined as acute respiratory distress in a cigarette smoker with known history of long‐lasting dyspnoea on exertion with frequent exacerbations and cough, and mucus hyperproduction, without symptoms or signs of other specific causes (absence of pneumothorax, pneumonia, pleural effusion, no reason to suspect an episode of pulmonary embolism) Inclusion criteria: Aged 18 years or older with evidence of ARF (3 of the following criteria: acute onset of moderate to severe dyspnoea; respiratory rate > 30 (or < 10) breaths/min; hypoxaemia (PaO2 < 7.3 kPa (55 mmHg) on room air) or need for oxygen supplementation; respiratory acidosis (pH < 7.33) Exclusion criteria: immediate indication for endotracheal intubation (respiratory and/or cardiac arrest); major unrest; haemodynamic instability despite a fluid challenge; facial or thoracic trauma; lack of co‐operation; difficult adaptation of a facial mask to a patient’s facial anatomy; clinical suspicion of pulmonary embolism; retrosternal pain suggestive of a myocardial ischaemia even with normal admission electrocardiogram (ECG) |
|
| Interventions |
Intervention description: Usual care (no sham NIV) plus bi‐level NIV (BiPAP ST/D 30; Respironics, Inc., Murrysville, PA, USA). BiPAP was used with inspiratory PS initially set at 10 cmH2O and EPAP at 4 cmH2O, used in assist‐control mode with a backup frequency of 10 breaths/min, via face mask (Bird, Bird Corp., Riverside, CA, USA). IPAP was increased by 2 cmH2O steps, until signs of discomfort (increasing sensation of dyspnoea), observation of air leaks, or pressure of 20 cmH2O was reached. EPAP was similarly increased until discomfort. Supplemental oxygen included via nasal catheter as required to maintain oxygen saturation > 90% Control description: Supplemental oxygen, bronchodilator aerosol therapy (fenoterol 1500 mg and ipratropium bromide 0.4 mg) repeated every 20 minutes and IV glucocorticoids (methylprednisolone 80 mg). Sham NIV employed, involving same setup as NIV group but a modified T‐connector piece (several holes created) between mask and tubing to enable IPAP = EPAP = 0 cmH2O. Oxygen was added with a nasal catheter inside the mask as needed to obtain saturation of 90% Treatment failure and success were defined in advance. Treatment success led to study end, treatment failure led to intubation in the active NIV group. Failure in the placebo NIV group led first to active NIV (rescue protocol) Duration of intervention: Until treatment success or failure Intervention delivery by: An attending physician delivered initial care and referred eligible patients. NIV or sham NIV was delivered by 2 study investigators. The attending physician remained present for the duration of the study (to decide treatment success or failure at any time point) Setting: Emergency department |
|
| Outcomes |
Prespecified primary outcomes: Treatment failure (defined by all as worsened dyspnoea, respiratory and/or heart frequency, sweating and agitation, or deterioration in blood gases and/or haemodynamic status). For NIV group, this represented intubation; for control group, this represented cross‐over to NIV intervention (before potential subsequent intubation) Prespecified secondary outcomes: Hospital mortality, ICU admission, LOS (of ED, ICU, and hospital), NIV complications (skin damage, gastric dilatation, vomiting) Effect of NIV on: ‐ Dyspnoea (VAS), ABGs (at baseline, 20 minutes after Rx and at end of Rx) ‐ Continuous trace ECG, HR, SpO2, BP, EMG (of SCM muscle), respiratory inductive plethysmography (thoracoabdominal movements) Follow‐up period: Until hospital discharge |
|
| Notes | Study included patients with a primary diagnosis of COPD or APO; however, only data from participants with COPD are described (kindly supplied by study author following email request) Funder: Partly supported by a grant to F. Thys from the "Fondation Saint‐Luc" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information available. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed with opaque, sealed envelopes in batches of 20 that were opened at the time of inclusion |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham/placebo NIV used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Attending physician was not blinded to group allocation, which may have affected decisions re failure/success, although a priori criteria were defined. Potential for secondary outcomes to be biased owing to knowledge of intervention, except length of stay. Study authors report the study as 'single‐blind' on page 546 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of data loss or attrition |
| Selective reporting (reporting bias) | High risk | No study protocol available for cross‐referencing. Primary study outcomes appear to be reported, but data for several secondary outcomes were not reported (e.g. SpO2, BP, HR, complications) |
| Imbalance of outcome measures at baseline All outcomes | Unclear risk | Appears balanced at baseline, but participants with COPD not discernible from those with APO |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Groups appear evenly matched at baseline, but participants with COPD not discernible from those with APO. No adjustments evident in analyses |
| Protection against contamination All outcomes | Low risk | All participants in control group received cross‐over rescue NIV, but data gathered before cross‐over were reported |
| Selective recruitment of participants | High risk | A large number of potentially eligible patients were not referred by local emergency medical teams "A total of 187 of these patients had a diagnosis of APO or acute exacerbation of COPD. The investigators were contacted for this study in 65 cases (37 acute exacerbations of COPD and 28 APO)” |
| Other bias | Low risk | No other sources of bias identified |
Zhou 2001.
| Methods |
Country: China Design: Randomised controlled trial, but no information about randomisation method or allocation concealment provided Study site: Single centre at the Second Xiangya Hospital, Central South University, Changsha, China Setting: Not stated whether conducted in ICU or on ward Methods of analysis: Means for before and after intervention were compared by t‐test. Chi2 analysis was used for differences in rates of intubation between 2 groups Aim: To observe effects of NIV on gas exchange and on patients’ transformation, and to evaluate clinical value |
|
| Participants |
Eligible for study: Not mentioned Recruited: Total of 60 participants with COPD were randomised to 2 groups: 30 to NIV group and 30 to usual care group. No differences in baseline characteristics between 2 groups; P > 0.05 Completed: Unsure, not mentioned in text or table. Presumed all 60 participants completed study. Intention‐to‐treat data were analysed with 30 participants on each arm Age: NIV group: 63.5 ± 9.1 years. Usual care group: 64.3 ± 9.4 years Gender: NIV group: 22 male and 8 female. Usual care group: 24 male and 6 female Criteria used to define COPD: No details available Inclusion criteria: Patients with COPD admitted to hospital with respiratory failure of PaCO2 > 50 mmHg Exclusion criteria: Patients who were hypotensive with SBP < 90 mmHg, with cardiac arrhythmias, or comatose |
|
| Interventions |
Intervention description: NIV was provided via Respironics, Inc. BiPAP ST‐D model with nasal/face mask. S/T mode was selected with IPAP 8 to 14 cmH2O , EPAP 2 to 6 cmH2O. All participants also received oxygen FiO2 22% to 33%, antibiotics, mucolytics, bronchodilator, glucocorticoids, and nutrients that required improving treatments and respiratory stimulants for patients with pulmonary encephalopathy Control description: Oxygen FiO2 22% to 33%, antibiotics, mucolytics, bronchodilator, glucocorticoids, and nutrients that required improving treatments and respiratory stimulants for patients with pulmonary encephalopathy Duration of intervention: 2 days with ≥ 4 hours of NIV each day Intervention delivery by: Not reported in text |
|
| Outcomes |
Method of outcome data collection: Data were collected at baseline and throughout hospital stay Prespecified primary outcome: Protocol not available. In text outcomes: ABG, heart rate and respiratory rate changes Prespecified secondary outcome: Protocol not available. In text outcome: Intubation rate Validation: ABG, HR, RR Follow‐up period: Throughout admission, until discharged or endpoint reached Number of follow‐up periods reported on during study: Not mentioned in text Indications for intubation: PaCO2 > 70 mmHg or PaCO2 increased by 5 to 10 mmHg, pH decreased by 0.05 to 0.1, reduced GCS or PaO2 < 45 mmHg |
|
| Notes | ABGs and vital signs changes were compared from baseline to 2 days post treatment. No data available 1 hour post NIV. Hence data not included in meta‐analysis Paper written in Chinese, with limited translation by translator. Attempts to contact study authors for more information were met with no reply Funder: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned in the paper; stated only that participants were randomly assigned to control vs intervention group |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the paper; stated only that participants were randomly assigned to control vs intervention group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No sham NIV used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in paper who delivered NIV and who collected data |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information available |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available |
| Imbalance of outcome measures at baseline All outcomes | Unclear risk | Insufficient information available |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Insufficient information available |
| Protection against contamination All outcomes | Unclear risk | Insufficient information available |
| Selective recruitment of participants | Unclear risk | Insufficient information available |
| Other bias | Unclear risk | Insufficient information reported in paper. Attempts to contact study authors were met with no response |
ABG: Arterial blood gases; AECOPD: Acute exacerbation of chronic obstructive pulmonary disease; AHRF: Acute hypercapnic respiratory failure; APO: Acute pulmonary oedema; BiPAP: bi‐level positive airway pressure; BMI: Body mass index; BP: blood pressure; CHF: Congestive heart failure; cmH2O: centimetres of water; COPD: Chronic obstructive pulmonary disease; CXR: chest x‐ray; DIC: disseminated intravascular coagulation; ECG: electrocardiography; ED: Emergency department; EMG: electromyography; EPAP: Expiratory positive pressure; FEV1: Forced expiratory volume in one second; FiO2: Fraction of inspired oxygen; GCS: Glasgow Coma Scale; GI: gastrointestinal; HR: Heart rate; ICU: Intensive care unit; IV: intravenously; IPAP: Inspiratory positive pressure; LOS: length of stay; mmHg: millimetres of mercury; NIPPV: non‐invasive positive pressure ventilation; NIV: Non‐invasive ventilation; PE: Pulmonary embolism; PaO2: Partial pressure of oxygen (arterial); PaCO2: Partial pressure of carbon dioxide (arterial); PEEP: Positive expiratory end pressure; PFT: pulmonary function test; PS: pressure support; RR: Respiratory rate; SaO2: arterial oxygen saturation; SAPS: simplified acute physiology score; SCM: sternocleidomastoid; SD: Standard deviation; SE: standard error; SpO2: peripheral oxygen saturation; SPSS: Statistical Package for the Social Sciences; TV: tidal volume; VAS: Visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 1992 | Study does not compare NIV and standard therapy. All participants received standard therapy and were then randomised to either NIV or doxapram |
| Ambrosino 1992 | Not an RCT. Participants have stable COPD. Comparison of 2 modes of ventilation |
| Ambrosino 1995 | Not an RCT. Retrospective review of case notes |
| Ambrosino 1997 | Review article |
| Angus 1996 | Study compares NIV with doxapram. Cohort of patients from the Ahmed study is included |
| Anton 2000 | Not an RCT |
| Antonelli 1998 | Study specifically excluded patients with COPD |
| Bardi 2000 | Not an RCT. Participants assigned to NIV or standard therapy on the basis of availability of ventilators |
| Benhamou 1992 | Not an RCT. Participants received NIV on the basis of family or clinician wishes |
| Boix 1995 | Compares NIV vs external high‐frequency oscillation around a negative baseline |
| Brijker 1999 | Not an RCT |
| Brochard 1990 | Not an RCT |
| Brochard 2002 | Review article |
| Carlucci 2001 | Trial of post‐extubation initiation of NIV |
| Casanova 2000 | Participants had stable COPD |
| Caubel 2001 | Not an RCT, but a retrospective analysis of data from previous years |
| Chen 1992a | Not an RCT; an historical controlled study |
| Chen 1992b | Not an RCT |
| Chen 2000 | Comparison of different masks ‐ not NIV vs standard treatment |
| Christensen 1990 | Stable COPD. Does not compare NIV vs standard treatment |
| Ciuffreda 2011 | NIV group vs NIV group. No standard treatment group |
| Clini 2002 | Participants had stable COPD |
| Confalonieri 1994 | Not an RCT, but a case‐control study |
| Confalonieri 1996 | Not an RCT, but a case‐control study with historical controls |
| Confalonieri 1998 | Not an RCT |
| Confalonieri 1999 | Primary diagnosis of severe community‐acquired pneumonia, not COPD. Participants with COPD were included in a post hoc analysis. Trial used CPAP as the intervention |
| Conti 2002 | NIV vs mechanical ventilation |
| Conway 1993 | Not an RCT |
| Corrado 2002 | Not an RCT. Retrospective case‐controlled study |
| Criner 1994 | Not an RCT. Comparison of different types of face masks in patients with stable COPD |
| Criner 1999 | Not an RCT. Stable COPD |
| Da Porto 2000 | Not an RCT |
| Daskalopoulou 1993 | Inadequate randomisation procedure (alternation). Abstract data only |
| De Rosa 2002 | Not an RCT. Retrospective study |
| Desideri 2004 | No standard medical treatment group |
| Diaz 1999 | Not an RCT, but a before‐and‐after study |
| Diaz 2002 | Participants had stable COPD |
| Duiverman 2008 | Participants with stable hypercapnic COPD |
| Elliot 2002 | Review article |
| Elliott 1990 | Not an RCT |
| Elliott 1997 | Not an RCT |
| Elston 2001 | Not an RCT; no control group in trial |
| Fernandez 1993 | Not an RCT |
| Ferrer 2002 | Weaning study |
| Foglio 1992 | Not an RCT |
| Foglio 1994 | Not an RCT. Summary of 2 papers published elsewhere |
| Gali 2003 | Review article |
| Garrod 2000 | Participants with stable COPD; NIV added to exercise training |
| Gibbons 2002 | Review article |
| Girault 1997a | Study compares 2 modes of NIV, with no control group |
| Girault 1997b | Study compares 2 modes of NIV, with no control group |
| Gorini 2001 | Not an RCT; no control group in trial |
| Gorini 2001b | All interventions involved negative pressure, not positive pressure, ventilation |
| Gorini 2002 | Not an RCT. Investigation of trigger sensitivity levels in NIV |
| Guerin 2002 | Invasive ventilation |
| Hawker 1996 | Not an RCT; letter only. Examines invasive ventilation |
| Heindl 1997 | Not an RCT |
| Hilbert 2000 | Not an RCT |
| Holanda 2001 | Not an RCT; no control group in trial |
| Hui 2001 | Not an RCT, but a retrospective analysis based on participant notes |
| Hui 2001a | Not an RCT; no control group in trial |
| IPPBT Group 1983 | Stable COPD |
| Jaber 2000 | Comparison of 2 gas mixtures |
| Johnson 2002 | Participants with stable COPD |
| Jones 1998 | Stable COPD. Not an RCT |
| Kaminski 1999 | Stable COPD |
| Katz‐P 2000 | Comparison of BiPAP and CPAP; no control group |
| Kaya 2000 | Not an RCT |
| Keenan 1997 | Meta‐analysis |
| Keenan 2000 | Economic evaluation of a previously published meta‐analysis |
| Keenan 2003 | Systematic review |
| Keenan 2005 | Mean pH > 7.35 |
| Khouaja 2012 | Mean pH > 7.35 |
| Kikawada 2001 | Case study. No COPD |
| Klein 1981 | Stable COPD. Comparison of NIV vs home oxygen |
| Koehnlein 2014 | Participants with stable COPD |
| Kong 2015 | No exacerbation of COPD |
| Kossler 2000 | Participants witth stable COPD |
| Laier‐Groenveld 1991 | Not an RCT. Stable COPD |
| Laier‐Groenveld 1995 | Review article |
| Leger 1994 | Not an RCT |
| Lien 1993 | Stable COPD. Comparison of NIV vs iron lung ventilation |
| Lien 1996 | Not an RCT. Does not compare NIV vs standard treatment |
| Lien 2000 | Stable COPD. Comparison of BiPAP and pressure‐controlled ventilation |
| Lukyanov 2013 | Inclusion criteria not stated. Outcome is aortic pulse wave velocity; no data available for mortality or intubation rate |
| Lun 2013 | NIV vs NIV. No standard treatment group |
| Maggiore 2010 | NIV with heliox vs NIV only. No standard treatment group |
| Martin 1995 | Subgroup of participants in the study had COPD (< 50%); however, not all participants in the control group met the inclusion criteria for PaCO2 |
| Martin 2000 | Subgroup of participants in the study had COPD (< 50%); however, not all participants in the control group met the inclusion criteria for PaCO2. Cohort of participants from Martin 1995 included |
| Meduri 1989 | Not an RCT |
| Meecham Jones 1995 | Stable COPD. Comparison of NIV vs long‐term oxygen therapy |
| Meechan Jones 1994 | Stable COPD. Comparison of 4 different modes of ventilation |
| Mega 2012 | NIV vs NIV. No standard treatment group |
| Moretti 2001 | Not an RCT, but a retrospective analysis based on participants' notes |
| Nava 1997 | Not an RCT. Compares NIV vs invasive ventilation after failed NIV |
| Nava 2001 | Participants with stable COPD |
| Nava 2011 | No COPD‐specific data despite contact with study author. Kyphoscoliosis and restrictive lung disease included |
| NCT01869387 | Participants with stable hypercapnic COPD |
| Oliveria 2001 | Not an RCT, but a retrospective analysis |
| Pankow 2001 | Not an RCT; participants with stable COPD |
| Pastaka 2007 | Mean pH > 7.35 |
| Peigang 2002 | Review article |
| Perrin 2000 | Not an RCT |
| Pinheiro 2001 | Not an RCT; no control group in trial |
| Plant 2000a | Not an RCT; study undertaken to find out the prevalence of acidosis in patients with COPD admitted to hospital |
| Polese 2000 | Participants with stable COPD |
| Pollack 1996 | Feasibility study with no control group |
| Poponick 1999 | Not an RCT |
| Porta 2000 | Review article |
| Porta 2002 | Participants with stable COPD or restrictive chest wall disease. No control group |
| Potena 2003 | Not an RCT, but an observational study on patients admitted to a respiratory ward treated with or without NIV. No randomisation was reported |
| Putinati 2000 | Not an RCT |
| Rappaport 1994 | No control group |
| Reissmann 2000 | Weaning study |
| Rizvi 2001 | Not an RCT; reported as a prospective non‐randomised study |
| Robino 2003 | Retrospective study comparing NIV in 2 different patient populations |
| Roessler 2012 | Out‐of‐hospital NIV vs standard treatment |
| Scala 2001 | Not an RCT, but a retrospective analysis/review of experience on a respiratory ward |
| Scandroglio 2002 | Review article |
| Schonhofer 2001 | Not an RCT. Stable COPD |
| Seith 1976 | Review article |
| Sellares 2012 | Withdrawal study. No standard treatment group |
| Servera 1995a | Not an RCT |
| Servera 1995b | Not an RCT |
| Shang 2001 | Not an RCT |
| Shang 2014 | Mean pH > 7.35 |
| Sidhu 2000 | Review article |
| Simonds 1995 | Not an RCT |
| Sinuff 2003 | Review article |
| Soo Hoo 1994 | Not an RCT |
| Summers 2002 | Two‐part study: 1 part with healthy volunteers, and the other a retrospective observational study of patients with COPD |
| Tarrega 2000 | Not an RCT |
| Teba 1996 | Not an RCT |
| Thys 1999 | Report describes a non‐randomised trial |
| Todisco 2001 | Not an RCT |
| Tsuboi 1999 | Review article |
| Vanpee 2001 | Review article |
| Vanpee 2002 | Not an RCT; stable participants |
| Vanpee 2002b | Questionnaire survey of NIV use |
| Vanpee 2002c | Not an RCT; stable participants |
| Vitacca 1993 | Randomised trial compares 2 modes of ventilation. Comparision of NIV vs standard treatment is not randomised |
| Vitacca 2000 | Not an RCT |
| Vitacca 2000b | Stable COPD |
| Vitacca 2002 | Stable COPD and other diseases |
| Wedzicha 1996a | Review article |
| Wedzicha 1996b | Review article |
| Wedzicha 2002 | Review article |
| Windisch 2002 | Weaning study; no COPD |
| Windisch 2002b | Not an RCT; participants with stable COPD |
| Wong 2007 | Pilot study. NIV during resolution phase of acute hypercapnic respiratory failure (withdrawal study) |
| Wood 1998 | Mixed population of patients. Data on participants with COPD not presented separately ‐ email contact with study authors attempted, without reply |
| Wysocki 1995 | No patients with COPD |
| Xue 2000 | No patients with acute exacerbation. Assessment of postoperative NIV vs standard medical treatment |
| Yang 2002 | Invasive ventilation |
| Ye 2000 | Not an RCT; compares PAV vs PSV ventilation |
| Ye 2002 | Weaning study |
COPD: chronic obstructive pulmonary disease; CPAP: continuous positive airway pressure; NIV: non‐invasive ventilation; PaCO2: partial pressure of carbon dioxide; PAV: proportional assist ventilation; PSV: pressure support ventilation; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Liao 2004.
| Methods |
Country: China Design: Single‐centre prospective randomised controlled trial. Randomisation method was not reported Study site: Xinqiao Hospital, Third Military Medical University, Chongqing, from August 2002 to February 2003 Setting: Not mentioned in text whether conducted in ICU or ward setting Methods of analysis: Chi2 and t‐test analysis. Statistical analysis performed with SPSS10.0 Aim: To investigate the role of NIV in management of respiratory failure secondary to acute exacerbations of COPD |
| Participants |
Eligible for study: Not stated. Patients with AECOPD and admission ABG pH > 7.25, PaCO2 > 45 mmHg Recruited: 40 patients: 20 in NIV group and 20 in standard therapy group. Baseline characteristics in both groups were similar with P > 0.05 Completed: 37 completed, 3 from intervention group dropped out (2 owing to financial difficulty and 1 for incompatibility with NIV). None from the control group were withdrawn Age: Intervention group – 69.7 ± 8.7 years. Control group – 66.4 ± 9.2 years Gender: Intervention group – 16 male and 4 female. Control group – 12 male and 8 female Criteria used to define COPD: As defined in 1997 Chinese Association of Respiratory Physician COPD plan. AECOPD correlates with history, physical examination, and radiological findings Exclusion criteria: Pneumothorax, multi‐organ failure, intolerant to NIPPV |
| Interventions |
Intervention description: NIV provided by BiPAP vision (Respironics, Inc., Murrysville, PA, USA) in spontaneous mode via nasal mask. IPAP initiated at 8 to 10 cmH2O increased progressively until 12 to 22 cmH2O at 30 minutes; EPAP set to 4 to 6 cmH2O. FiO2 30 to 60%. All participants also received oxygen 2 to 4 L/min, nebulised bronchodilators, antibiotics, and mucolytics Control description: All participants received oxygen 2 to 4 L/min, nebulised bronchodilators, antibiotics, and mucolytics Duration of intervention: Aimed ≥ 3 days BiPAP with minimum 12 hours per day and weaning as clinical state with 5 to 10 days' total duration Intervention delivery by: Not reported in text |
| Outcomes |
Method of outcome data collection: Data were collected at baseline and throughout hospital stay Prespecified primary outcome: Protocol not available. In text outcomes: Intubation rate, mortality, duration of hospitalisation, cost for hospitalisation Prespecified secondary outcome: Protocol not available. No secondary outcomes mentioned in text Validation: ABG at baseline, at 2 hours, at 72 hours, and at discharge. RR, HR, MAP, and dyspnoea score (SAARM) Follow‐up period: Throughout admission, until discharged or endpoint reached Number of follow‐up periods reported on during study: Not mentioned in text Indications for intubation: Any of the following criteria met: pH < 7.20 with worsening PaCO2 despite intervention, or PaO2 < 50 mmHg; reduced conscious state; cardiopulmonary arrest; RR < 8 or > 40 bpm Discharge criteria: Discharged if no evidence of dyspnoea with meals and sleep with ability to mobilise around the bed, reduce sputum production; clinically stable for 24 hours and no signs of other organ failure |
| Notes | Paper in Chinese, with limited translation from translator. Attempted to contact study authors for more informations, but no reply Unable to determine whether mean pH > 7.35 or mean PaCO2 > 45 mmHg Funder: Not stated |
Samaria 2013.
| Methods | Unclear whether study was randomised |
| Participants | 100 with AECOPD |
| Interventions | 50 on NIV with conventional therapy (oxygen, bronchodilator, systemic steroids and antibiotics), and 50 on usual care alone |
| Outcomes | 25% of participants in NIV group required endotracheal intubation as compared with 55% in usual care group. Hospital stay was significantly longer in usual care group (15.9 ± 7.4 days) vs NIV group (10.2 ± 5.2 days); P < 0.001 |
| Notes | Abstract only Attempted to contact study authors were met with no reply. Unable to determine relationship between this abstract and abstract of Samaria 2009 |
Servillo 1994.
| Methods | Randomised controlled parallel‐group trial conducted in Naples, Italy Country: Italy Design: Randomised controlled parallel‐group trial Study site: Naples Method of analysis: Not stated Aim: "To evaluate the efficacy of mask pressure support ventilation in COPD patients admitted to ICU for acute respiratory failure" |
| Participants |
Eligible for study: Not stated Recruited: 10 adult patients with COPD in acute respiratory failure (number in each group not stated) Completed: Not stated Age: Not stated Gender: Not stated. Baseline characteristics described only as "very much alike" Criteria used to define COPD: Not stated Inclusion criteria: Not stated Exclusion criteria: Not stated |
| Interventions |
Intervention description: Usual care plus NIV (mask pressure support ventilation). A Puritan Bennett 7200 was used with the inspiratory pressure support initially set at 15 (SD 4) cmH2O, PEEP at 4 (SD 2), and FiO2 at 60% Control description: Oxygen and bronchodilators Duration of intervention: Not stated. Only length of ICU stay (days) provided for each group Intervention delivery by: Not stated Setting: ICU |
| Outcomes |
Method of outcome data collection: Not stated Prespecified outcomes: Not stated. Data reported for length of ICU stay (days), need for intubation, and mortality. Unclear which were primary vs secondary Follow‐up period: Not stated. Appears restricted to ICU admission |
| Notes | Conference abstract only Unable to determine whether mean pH > 7.35 or mean PaCO2 > 45 mmHg Funder: Not stated |
ABGs: arterial blood gases; AECOPD: acute exacerbation of chronic obstructive pulmonary disease; BiPAP: bi‐level positive airway pressure; COPD: chronic obstructive pulmonary disease; EPAP: expiratory positive airway pressure; FiO2: fraction of inspired oxygen; HR: heart rate; ICU: intensive care unit; IPAP: inspiratory positive airway pressure; MAP: mean arterial pressure; NIV: non‐invasive ventilation; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen; RR: risk ratio; SAARM: scores of the activity accessory respiratory muscle; SPSS: Statistical Package for the Social Sciences.
Characteristics of ongoing studies [ordered by study ID]
Duan 2011.
| Trial name or title | Observation of non‐invasive positive ventilation united inhalation treatment therapeutic effect on COPD with type II respiratory failure |
| Methods |
Country: China Design: Randomised controlled trial Study site: Zhongshan Hospital, Qingpu Branch, Shanghai Aim: To investigate the clinical value of non‐invasive bi‐level positive airway pressure (BiPAP) ventilation treatment in chronic obstructive pulmonary disease (COPD) with type II respiratory failure |
| Participants |
Eligible for study: Not stated Recruited: 68 patients with COPD and type II respiratory failure. Unclear how many in NIV or usual care group Completed: Not stated Age: Not stated Gender: Not stated Criteria used to define COPD: Not stated Inclusion criteria: Not stated Exclusion criteria: Not stated |
| Interventions |
Intervention description: Usual care plus NIV. "Respirometer parameters were: S/T model, respiratory frequency 15/minutes, and oxygen concentration 40%. The respiratory pressure was raised gradually from 12 to 25 cmH2O twice a day 4 hours at a time, over a 5‐day period" Control description: "...anti‐infection, eliminating phlegm, relieving asthma and inhaling low concentration oxygen" Duration of intervention: 5 days Intervention delivery by: Not stated Setting: Unclear |
| Outcomes |
Method of outcome data collection: Not stated Pre specified outcomes: Arterial pH, partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2), respiratory muscle fatigue, heart rate, respiratory rate, dyspnoea (method not stated). Unclear which outcomes deemed primary vs secondary Follow‐up period: Not stated. Study appears restricted to inpatient hospital admission setting |
| Starting date | Not stated |
| Contact information | Duan Y. Department of Respiratory Medicine, Zhongshan Hospital, Qingpu Branch, Shanghai, China |
| Notes | Abstract only. Nil data available for use in meta‐analysis. Attempts to contact study authors were met with no response |
Differences between protocol and review
Updates undertaken since publication of the original review resulted in the following changes to the protocol.
Significant updates to the methods to bring the review in line with current Cochrane (MECIR) standards. This included insertion of a 'Summary of findings' table and updates to the risk of bias methods (removal of the Jadad scale). This process included evaluation of the following additional domains of potential bias: imbalance of outcome measures at baseline, comparability of intervention and control group characteristics at baseline, protection against contamination, and selective recruitment of participants.
Following critical feedback regarding this review, we amended the definition of AHRF from the initial review to now include reference to pH < 7.35. We deemed this essential to enhance its consistency with clinical practice.
Amendment of primary review outcomes. In the initial version, the primary outcome was 'treatment failure', defined according to any combination of intubation, mortality, or treatment failure. To remove ambiguity regarding overlap between these definitions, the current version lists treatment intolerance as an independent secondary review outcome. We also made clarifications to the definition of our primary outcome 'need for endotracheal intubation' to minimise ambiguity.
Exclusion of one study that was included in the original review (Conti 2002) due to inclusion of mechanical ventilation as a comparator intervention; and reclassification of one study originally included in the review (Servillo 1994) to 'Awaiting classification' due to lack of sufficient information pertaining to mean baseline pH to determine eligibility for inclusion in the review in accordance with our revised definition of AHRF.
Replacement of one abstract included in the original review with a full‐text version in the update (Khilnani 2002 is now Khilnani 2010).
Minor edits to wording of two eligibility criteria to explicitly clarify that (a) studies of patients who commenced NIV before hospital admission are ineligible for inclusion in the review; and (b) 'usual medical care' may not include any form of positive pressure ventilation considered to be 'usual' for that study centre.
Thys 2002 was originally included as an 'ICU' study in the subgroup analysis of ward versus ICU care. As this study was based in an emergency department (potentially more intensive than a ward, but not an ICU setting), in keeping with the primary aim of the review, we excluded this study from the ward versus ICU subgroup analysis for the present (and subsequent) updates.
Data from Bott 1993 were originally included in some of the meta‐analyses related to blood gas tensions; however owing to lack of sufficient study information regarding the number of participants included in these outcome data, this study was subsequently removed from the meta‐analysis and was reported separately. Additionally, mortality data were removed from the meta‐analysis, as they were identified as relevant to the post‐discharge period, not to in‐hospital mortality.
We conducted subgroup analyses of admission pH and location only for the primary outcomes of interest for this review (mortality and need for endotracheal intubation), rather than for all outcomes.
One additional table (percentage change in PaCO2 at one hour post intervention) included in the earlier review was removed.
Type of NIV for subgroup analysis was removed from the investigation of heterogeneity section of the protocol, as all studies except one used pressure‐cycled NIV.
Contributions of authors
CO and VT updated the latest version of the review, including protocol revision, literature screening, data extraction, data analysis, and write‐up of results. KC provided assistance with all of these activities. JP and JW contributed to the previous publication of this review (April 2004) and assisted in protocol design and review of the draft for the latest version of the review. Felix SF Ram, Josephine Lightowler, and JW completed the original review.
Sources of support
Internal sources
Respiratory Medicine Department, The Queen Elizabeth Hospital, Australia.
External sources
The authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
CO: none known.
VT: none known.
KC: none known.
JP: none known.
JW: none known.
BS: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Avdeev 1998 {published and unpublished data}
- Avdeev SN, Tretyakov AV, Grigoryants RA, Kutsenko MA, Chuchalin AG. Noninvasive positive airway pressure ventilation: role in treating acute respiratory failure caused by chronic obstructive pulmonary disease. Anesteziologita Reanimatologia 1998;3:45‐51. [Google Scholar]
Barbe 1996 {published data only}
- Barbe R, Togores B, Rubi M, Pons S, Maimo A, Agusti AGN. Noninvasive ventilatory support does not facilitate recovery from acute respiratory failure in chronic obstructive pulmonary disease. European Respiratory Journal 1996;9:1240‐5. [DOI] [PubMed] [Google Scholar]
Bott 1993 {published and unpublished data}
- Bott J, Carroll MP, Conway JH, Keilty SEJ, Ward EM, Brown AM, et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet 1993;341(8860):1555‐7. [DOI] [PubMed] [Google Scholar]
Brochard 1995 {published data only}
- Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. New England Journal of Medicine 1995;333(13):817‐22. [DOI] [PubMed] [Google Scholar]
- Mancebo J, Benito S, Net A. The effects of face mask ventilation with pressure support ventilation in patients with chronic obstructive respiratory failure in acute decompensation [Efectos de la ventilacion con presion de soporte con mascara facial en pacientes con insuficiencia respiratoria cronica en descompensacion aguda]. Medicina Clinica 1994;102(17):641‐6. [PubMed] [Google Scholar]
Carrera 2009 {published data only}
- Carrera M, Marin JM, Anton A, Chiner E, Alonso ML, Masa JF, et al. A controlled trial of noninvasive ventilation for chronic obstructive pulmonary disease exacerbations. Journal of Critical Care 2009;24(3):473.e7‐473.e14. [DOI] [PubMed] [Google Scholar]
Celikel 1998 {published data only}
- Celikel T, Sungur M, Ceyhan B, Karakurt S. Comparison of noninvasive positive pressure ventilation with standard medical therapy in hypercapnic acute respiratory failure. Chest 1998;114:1636‐42. [DOI] [PubMed] [Google Scholar]
Collaborative 2005 {published data only}
- Collaborative Research Group of Noninvasive Mechanical Ventilation for Chronic Obstructive Pulmonary Disease. Early use of non‐invasive positive pressure ventilation for acute exacerbations of chronic obstructive pulmonary disease: a multicentre randomized controlled trial. Chinese Medical Journal 2005;118(24):2034‐40. [PubMed] [Google Scholar]
- Collaborative Research Group of Noninvasive Mechanical Ventilation for Chronic Obstructive Pulmonary Disease. [Early use of noninvasive positive pressure ventilation for patients with acute exacerbations of chronic obstructive pulmonary disease: a multicentre randomized controlled trial]. [Chinese]. Chung‐Hua Chieh Ho Ho Hu Hsi Tsa Chih Chinese Journal of Tuberculosis & Respiratory Diseases 2005;28(10):680‐4. [PubMed] [Google Scholar]
del Castillo 2003 {published data only}
- Castillo D, Barrot E, Laserna E, Otero R, Cayuela A, Castillo Gomez J. Noninvasive positive pressure ventilation for acute respiratory failure in chronic obstructive pulmonary disease in a general respiratory ward. Medicina Clinica (Barc) 2003;120(17):647‐51. [DOI] [PubMed] [Google Scholar]
Dikensoy 2002 {published data only}
- Dikensoy O, Ikidag B, Bayram N, Filiiz A. A randomized controlled trial of noninvasive ventilation in acute hypercapnic respiratory failure. European Respiratory Journal 2001;18(33):28s. [Google Scholar]
- Dikensoy O, Ikidag B, Filiiz A, Bayram N. Comparison of non‐invasive ventilation and standard medical therapy in acute hypercapnic respiratory failure: a randomised controlled trial at a tertiary health centre in SE Turkey. International Journal of Clinical Practice 2002;56(2):85‐8. [PMID: 11926711] [PubMed] [Google Scholar]
Khilnani 2010 {published data only}
- Khilnani GC, Saikia N, Banga A, Sharma SK. Non‐invasive ventilation for acute exacerbation of COPD with very high PaCO2: a randomized controlled trial. Lung India 2010;27(3):125‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khilnani GC, Saikia N, Sharma SK, Pande JN, Malhotra OP. Efficacy of non‐invasive positive pressure ventilation (NPPV) for management of COPD with acute or acute on chronic respiratory failure: a randomised controlled trial. American Thoracic Society 98th International Conference; 2002 May 17‐22; Georgia. 2002.
Kramer 1995 {published and unpublished data}
- Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure [see comments]. American Journal of Respiratory and Critical Care Medicine 1995;151(6):1799‐1806. [DOI] [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu L, Qiu HB, Zheng RQ, Yang Y. [Prospective randomized controlled clinical study of early use of noninvasive positive pressure ventilation in the treatment for acute exacerbation of chronic obstructive pulmonary disease]. Zhongguo wei zhong bing ji jiu yi xue 2005;17(8):477‐80. [PubMed] [Google Scholar]
Matuska 2006 {published data only}
- Matuska P, Pilarova O, Merta Z, Skrickova J. Non‐invasive ventilation support in patients with acute exacerbation of chronic obstructive pulmonary disease (COPD). [Czech]. Vnitrni Lekarstvi 2006;52(3):241‐8. [PubMed] [Google Scholar]
Plant 2001 {published and unpublished data}
- Plant PK, Owen J L, Elliott MW. Non‐invasive ventilation (NIV) in acute exacerbations of COPD ‐ the Yorkshire non‐invasive ventilation trial. Thorax 1998;53(Suppl 4):A11. [Google Scholar]
- Plant PK, Owen JL, Elliott MW. A cost‐effectiveness analysis of non‐invasive ventilation (NIV) in acute exacerbations of COPD. Thorax 1999;54(Suppl 3):S43. [Google Scholar]
- Plant PK, Owen JL, Elliott MW. Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet 2000;355(9219):1931‐5. [MEDLINE: ; UI: 20315569] [DOI] [PubMed] [Google Scholar]
- Plant PK, Owen JL, Elliott MW. Non‐invasive ventilation in acute exacerbations of chronic obstructive pulmonary disease: long term survival and predictors of in‐hospital outcome. Thorax 2001;56(9):708‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant PK, Owen JL, Elliott MW, Robinson A. Longterm survival following an exacerbation of COPD treated with and without non‐invasive ventilation. Thorax 1999;54(Suppl 3):S44. [Google Scholar]
Samaria 2009 {published data only}
- Samaria JK. Need for mechanical ventilation and in‐hospital mortality in patients of acute exacerbation of COPD (AECOPD) on conventional treatment versus non invasive positive airway pressure (NIPPV) [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A1515. [Google Scholar]
- Samaria JK. Nosocomial pneumonia in patients with acute exacerbation of COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A1516. [Google Scholar]
- Samaria JK, Srivastava SK. Profile of nosocomial pneumonia in patients with acute exacerbation of COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine 2009:A1501 [Poster #K53]. [Google Scholar]
- Samaria JK, Srivastava SK. Study of pH changes in patients of acute exacerbation of COPD on conventional treatment versus on NIPPV [Abstract]. American Journal of Respiratory and Critical Care Medicine 2009;179:A1499 [Poster #K51]. [Google Scholar]
- Samaria JK, Srivastava SK, Mathur SK. Mechanical ventilation and in‐hospital mortality in patients of acute exacerbation of COPD (AECOPD) on conventional treatment versus non invasive positive pressure (NIPPV) [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20 San Diego. 2009; Vol. 179:A1500 [Poster #K52].
Thys 2002 {published data only}
- Thys F, Roeseler J, Reynaert M, Liistro G, Rodenstein DO. Noninvasive ventilation for acute respiratory failure: a prospective randomised placebo‐controlled trial. European Respiratory Journal 2002;20(3):545‐55. [DOI] [PubMed] [Google Scholar]
Zhou 2001 {published data only}
- Zhou R, Chen P, Luo H, Xiang XD. Effects of noninvasive positive pressure ventilation on gas exchange and patients' transformation in chronic obstructive pulmonary disease and respiratory failure. Bulletin of Hunan Medical University 2001;26(3):261‐2. [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmed 1992 {published data only}
- Ahmed AH, Fenwick L, Angus RM, Peacock AJ. Nasal ventilation versus doxapram in the treatment of type II respiratory failure complicating chronic airflow obstruction. Thorax 1992;47:858. [Google Scholar]
Ambrosino 1992 {published data only}
- Ambrosino N, Nava S, Bertone P, Fracchia C, Rampulla C. Physiologic evaluation of pressure support ventilation by nasal mask in patients with stable COPD. Chest 1992;101:385‐91. [DOI] [PubMed] [Google Scholar]
Ambrosino 1995 {published data only}
- Ambrosino N, Foglio K, Rubini F, Clini E, Nava S, Vitacca M. Non‐invasive mechanical ventilation in acute respiratory failure due to chronic obstructive pulmonary disease: correlates for success. Thorax 1995;50:755‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ambrosino 1997 {published data only}
- Ambrosino N. Noninvasive mechanical ventilation in acute on chronic respiratory failure: determinants of success or failure. Monaldi Archives for Chest Disease 1997;52(1):73‐5. [PubMed] [Google Scholar]
Angus 1996 {published data only}
- Angus RM, Ahmed AA, Fenwick LJ, Peacock AJ. Comparison of the acute effects on gas exchange of nasal ventilation and doxapram in exacerbations of chronic obstructive pulmonary disease. Thorax 1996;51:1048‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anton 2000 {published data only}
- Anton A, Guell R, Gomez J, Serrano J, Castellano A, Carrasco JL, et al. Predicting the result of noninvasive ventilation in severe acute exacerbations of patients with chronic airflow limitation. Chest 2000;117:828‐33. [DOI] [PubMed] [Google Scholar]
Antonelli 1998 {published data only}
- Antonelli M, Conti G, Rocco M, Bufi M, Blasi RA, Vivino G, et al. A comparison of noninvasive positive‐pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. New England Journal of Medicine 1998;339:429‐35. [DOI] [PubMed] [Google Scholar]
Bardi 2000 {published data only}
- Bardi G, Pierotello R, Desideri M, Valdisseri L, Bottai M, Palla A. Nasal ventilation in COPD exacerbations: early and late results of a prospective, controlled study. European Respiratory Journal 2000;15:98‐104. [DOI] [PubMed] [Google Scholar]
Benhamou 1992 {published data only}
- Benhamou D, Girault C, Faure C, Portier F, Muir J‐F. Nasal mask ventilation in acute respiratory failure. Chest 1992;102:912‐7. [DOI] [PubMed] [Google Scholar]
Boix 1995 {published data only}
- Boix J H, Tejeda M, Alvarez F, Ernesto E, Bertomeu F, Bano M. Non‐invasive ventilatory support in patients with chronic obstructive pulmonary disease. Comparison of two methods. [Apoyo ventilatorio no invasivo en pacientes con enfermedad pulmonar obstructiva cronica. Comparacion de dos metodos.]. Revista Clinica Espana 1995;195:678‐83. [PubMed] [Google Scholar]
Brijker 1999 {published data only}
- Brijker F, Elshout FJ, Rijk A, Folgering HT, Bosch FH. Use of noninvasive mechanical ventilation to avoid intubation during acute respiratory insufficiency [Niet‐invasieve beademing ter voorkoming van intubatie tijdens acute respiratoire insufficientie]. Nederlands Tijdschrift Voor Geneeskunde 1999;146(36):1819‐23. [PubMed] [Google Scholar]
Brochard 1990 {published data only}
- Brochard L, Isabey D, Piquet J, Amaro P, Mancebo J, Messadi AA, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. New England Journal of Medicine 1990;323(22):1523‐30. [DOI] [PubMed] [Google Scholar]
Brochard 2002 {published data only}
- Brochard L, Mancebo J, Elliott MW. Noninvasive ventilation for acute respiratory failure. [Review]. European Respiratory Journal 2002;19(4):712‐21. [DOI] [PubMed] [Google Scholar]
Carlucci 2001 {published data only}
- Carlucci A, Gregoretti C, Squadrone V, Navalesi P, Delmastro M, Nava S. Preventive use of non‐invasive mechanical ventilation (NIMV) to avoid post‐extubation respiratory failure: a randomized controlled trial. European Respiratory Journal 2001;18(33):29s. [Google Scholar]
Casanova 2000 {published data only}
- Casanova C, Celli BR, Tost L, Soriano E, Abreu J, Velasco V, Santolaria F. Long‐term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000;118(6):1582‐90. [DOI] [PubMed] [Google Scholar]
Caubel 2001 {published data only}
- Caubel A, Roche N, Lefebvre A, Rabbat A, Huchon GJ. Trends in the use of non‐invasive mechanical ventilation (NIMV) in a respiratory intensive care unit (ICU) between 1996 and 2000. European Respiratory Journal 2001;18(33):27s. [Google Scholar]
Chen 1992a {published data only}
- Chen RC, Zeng YX, Li YM, Zhong NS. Mask ventilation with bi‐level positive airway pressure (BiPAP) ventilator in COPD patients with acute respiratory failure. European Respiratory Journal 1992;5(Suppl 15):515s. [Google Scholar]
Chen 1992b {published data only}
- Chen RC. Facial or nasal mask pressure support ventilation in managing acute exacerbation of chronic respiratory failure in chronic obstructive pulmonary diseases. Chinese Journal of Tuberculosis and Respiratory Diseases 1992;15(5):285‐7. [PubMed] [Google Scholar]
Chen 2000 {published data only}
- Chen R, Zhang X, He G. Modification of facial mask on the dead space effect in non‐invasive mask ventilation. [Chinese]. Chung‐Hua Chieh Ho Ho Hu Hsi Tsa Chih Chinese Journal of Tuberculosis & Respiratory Diseases 2000;23(12):734‐6. [PubMed] [Google Scholar]
- Chen R, Zhang X, He G. Modification of facial mask on the dead space effect in non‐invasive mask ventilation. [Chinese]. Chung‐Hua Chieh Ho Ho Hu Hsi Tsa Chih Chinese Journal of Tuberculosis & Respiratory Diseases 2000;23(12):734‐6. [PubMed] [Google Scholar]
Christensen 1990 {published data only}
- Christensen HR, Simonsen K, Lange P, Clementsen P, Kampmann JP, Viskum K, et al. PEEP‐masks in patients with severe obstructive pulmonary disease: a negative report. European Respiratory Journal 1990;3:267‐72. [PubMed] [Google Scholar]
Ciuffreda 2011 {published data only}
- Ciuffreda M, Sarni A, Mozzillo M, Zotti M, Aiuti M, Alessandri C. Home versus intensive care ventilators providing noninvasive ventilation (NIV): a clinical comparison during acute respiratory failure due to COPD exacerbation [Abstract]. European Respiratory Journal 2011;38:683s [P3782]. [Google Scholar]
Clini 2002 {published data only}
- Clini E, Sturani C, Rossi A, Viaggi S, Corrado A, Donner CF, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. European Respiratory Journal 2002;20(3):529‐38. [DOI] [PubMed] [Google Scholar]
Confalonieri 1994 {published data only}
- Confalonieri M, Aiolfi S, Gandola L, Scartabellati A, Della Porta R, Parigi P. Severe exacerbations of chronic obstructive pulmonary disease treated with BiPAP by nasal mask. Respiration 1994;61(6):310‐6. [PMID: 7824810] [DOI] [PubMed] [Google Scholar]
Confalonieri 1996 {published data only}
- Confalonieri M, Parigi P, Scartabellati A, Aiolfi S, Scorsetti S, Nava S, et al. Noninvasive mechanical ventilation improves the immediate and long‐term outcome of COPD patients with acute respiratory failure. European Respiratory Journal 1996;9(3):422‐30. [PMID: 8729999] [DOI] [PubMed] [Google Scholar]
Confalonieri 1998 {published data only}
- Confalonieri M, Gazzaniga P, Gandola L, Aiolfi S, Della Porta R, Frisinghelli A, et al. Haemodynamic response during initiation of non‐invasive positive pressure ventilation in COPD patients with acute ventilatory failure. Respiratory Medicine 1998;92:331‐7. [DOI] [PubMed] [Google Scholar]
Confalonieri 1999 {published data only}
- Confalonieri M, Potena A, Carbone G, Della Porta R, Tolley EA, Meduri GU. Acute respiratory failure in patients with severe community‐acquired pneumonia. American Journal of Respiratory and Critical Care Medicine 1999;160:1585‐91. [DOI] [PubMed] [Google Scholar]
Conti 2002 {published data only}
- Conti G, Antonelli M, Navalesi P, Rocco M, Bufi M, Spadetta G, et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Medicine 2002;28(12):1701‐7. [DOI] [PubMed] [Google Scholar]
Conway 1993 {published data only}
- Conway JH, Hitchcock RA, Godfrey RC, Carroll MP. Nasal intermittent positive pressure ventilation in acute exacerbations of chronic obstructive pulmonary disease ‐ a preliminary study. Respiratory Medicine 1993;87:387‐94. [DOI] [PubMed] [Google Scholar]
Corrado 2002 {published data only}
- Corrado A, Confalonieri M, Marchese S, Mollica C, Villella G, Gorini M, et al. Iron lung vs mask ventilation in the treatment of acute on chronic respiratory failure in COPD patients: a multicenter study. Chest 2002;121(1):189‐95. [DOI] [PubMed] [Google Scholar]
Criner 1994 {published data only}
- Criner GJ, Travaline JM, Brennan KJ, Kreimer DT. Efficacy of a new full face mask for noninvasive positive pressure ventilation. Chest 1994;106:1109‐15. [DOI] [PubMed] [Google Scholar]
Criner 1999 {published data only}
- Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest 1999;116:667‐75. [DOI] [PubMed] [Google Scholar]
Da Porto 2000 {published data only}
- Porto R, Monacci A, Bongiorni E, Giovannetti P, Petrone A, Siclari C, et al. Non invasive assist control mechanical ventilation with volume control in severe COPD patiens with acute respiratory failure. European Respiratory Society 10th Annual Congress; 2000 Aug 30‐Sep 3; Florence. 2000:Abstract 1803.
Daskalopoulou 1993 {published data only}
- Daskalopoulou E, Tsara V, Fekete K, Koutsantsas V, Christaki P. Treatment of acute respiratory failure in COPD patients with positive airway pressure via nasal mask (NIPPV). Chest 1993;103(3 (Suppl)):271S. [Google Scholar]
De Rosa 2002 {published data only}
- Rosa M, Triolo L, Reale G, Montanari A, Sanguinetti CM. Analysis of outcome measures in COPD patients requiring NIPPV. American Thoracic Society 98th International Conference; 2002 May 17‐22; Georgia. 2002.
Desideri 2004 {published data only}
- Desideri M, Gregori G, Marchetti G, Marrelli F, Pulera N, Tempestini F, et al. Outcomes of NPPV in patients with ARF due to AECOPD and associated obesity. Respiratory Journal 2004;24(Suppl 48):313s. [Google Scholar]
Diaz 1999 {published data only}
- Diaz P, Iglesia R, Ferrer M, Zavala E, Santos C, Wagner PD, et al. Effects of noninvasive ventilation on pulmonary gas exchange and haemodynamics during acute hypercapnic exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1997;156(6):1840‐5. [PMID: 9412564] [DOI] [PubMed] [Google Scholar]
Diaz 2002 {published data only}
- Diaz O, Begin P, Torrealba B, Jover E, Lisboa C. Effects of noninvasive ventilation on lung hyperinflation in stable hypercapnic COPD. European Respiratory Journal 2002;20(6):1490‐8. [DOI] [PubMed] [Google Scholar]
Duiverman 2008 {published data only}
- Duiverman ML, Wempe JB, Bladder G, Jansen DF, Kerstjens HA, Zijlstra JG, et al. Nocturnal non‐invasive ventilation in addition to rehabilitation in hypercapnic patients with COPD. Thorax 2008;93:1052‐7. [DOI] [PubMed] [Google Scholar]
Elliot 2002 {published data only}
- Elliott MW, Confalonieri M, Nava S. Where to perform noninvasive ventilation?. European Respiratory Journal 2002;19(6):1159‐66. [DOI] [PubMed] [Google Scholar]
Elliott 1990 {published data only}
- Elliott MW, Steven MH, Phillips GD, Branthwaite MA. Non‐invasive mechanical ventilation for acute respiratory failure. BMJ 1990;300(6721):358‐60. [PMID: 2106984] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 1997 {published data only}
- Elliott MW. Non‐invasive ventilation in chronic obstructive pulmonary disease. British Journal of Hospital Medicine 57;3:83‐6. [PubMed] [Google Scholar]
Elston 2001 {published data only}
- Elston CM, Haque AS, Eraut D, Davison AG, Ward LM. Can non‐invasive ventilation (NIV) for COPD be used on a respiratory ward in a district general ward. European Respiratory Journal 2001;18(33):28s. [Google Scholar]
Fernandez 1993 {published data only}
- Fernandez R, Blanch L, Valles J, Baigorri F, Artigas A. Pressure support ventilation via face mask in acute respiratory failure in hypercapnic COPD patients. Intensive Care Medicine 1993;19:456‐61. [DOI] [PubMed] [Google Scholar]
Ferrer 2002 {published data only}
- Ferrer M, Iglesia R, Roca J, Burgos F, Torres A, Rodriguez‐Roisin R. Pulmonary gas exchange response to weaning with pressure‐support ventilation in exacerbated chronic obstructive pulmonary disease patients. Intensive Care Medicine 2002;28(11):1595‐9. [DOI] [PubMed] [Google Scholar]
Foglio 1992 {published data only}
- Foglio C, Vitacca M, Quadri A, Scalvini S, Marangoni S, Ambrosino N. Acute exacerbations in severe COLD patients. Treatment using positive pressure ventilation by nasal mask. Chest 1992;101(6):1533‐8. [DOI] [PubMed] [Google Scholar]
Foglio 1994 {published data only}
- Foglio K, Clini E, Vitacca M. Different modes of noninvasive intermittent positive pressure ventilation (NIPPV) in acute exacerbations of COLD patients. Monaldi Archives for Chest Disease 1994;49(6):556‐7. [PubMed] [Google Scholar]
Gali 2003 {published data only}
- Gali B, Goyal DG. Positive pressure mechanical ventilation. Emergency Medicine Clinics of North America 2003;21(2):453‐73. [DOI] [PubMed] [Google Scholar]
Garrod 2000 {published data only}
- Garrod R, Mikelsons C, Paul EA, Wedzicha JA. Randomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;162(4 Pt 1):1335‐41. [DOI] [PubMed] [Google Scholar]
Gibbons 2002 {published data only}
- Gibbons D, Milner P. Non‐invasive positive pressure ventilation for COPD patients. Professional Nurse 2002;17(7):405‐8. [PubMed] [Google Scholar]
Girault 1997a {published data only}
- Girault C, Richard JC, Chevron V, Tamion F, Pasquis P, Leroy J, et al. Comparative physiologic effects of noninvasive assist‐control and pressure support ventilation in acute hypercapnic respiratory failure. Chest 1997;111(6):1639‐48. [DOI] [PubMed] [Google Scholar]
Girault 1997b {published data only}
- Girault C, Chevron V, Richard JC, Daudenthun I, Pasquis P, Leroy J, et al. Physiological effects and optimisation of nasal assist‐control ventilation for patients with chronic obstructive pulmonary disease in respiratory failure. Thorax 1997;52(8):690‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorini 2001 {published data only}
- Gorini M, Corrado A, Ginanni R, Villela G, Augustynen A, Tozzi D. The role of invasive and noninvasive mechanical ventilation in the treatment of acute on chronic respiratory failure: a prospective cohort study. European Respiratory Journal 2001;18(33):28s. [Google Scholar]
Gorini 2001b {published data only}
- Gorini M, Corrado A, Villella G, Ginanni R, Augustynen A, Tozzi D. Physiologic effects of negative pressure ventilation in acute exacerbation of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1614‐8. [DOI] [PubMed] [Google Scholar]
Gorini 2002 {published data only}
- Gorini M, Villella G, Ginanni R, Augustynen A, Tozzi D, Corrado A. Effect of assist negative pressure ventilation by microprocessor based iron lung on breathing effort. Thorax 2002;57(3):258‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guerin 2002 {published data only}
- Guerin C, Lemasson S, Cara MF, Fournier G. Physiological effects of constant versus decelerating inflation flow in patients with chronic obstructive pulmonary disease under controlled mechanical ventilation. Intensive Care Medicine 2002;28(2):164‐9. [DOI] [PubMed] [Google Scholar]
Hawker 1996 {published data only}
- Hawker F, Breen D, Torzillo P, Herkes R. Randomized prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. American Journal of Respiratory and Critical Care Medicine 1996;153:1188. [DOI] [PubMed] [Google Scholar]
Heindl 1997 {published data only}
- Heindl S, Karg O, Bullemer F, Kroworsch P, Pahnke J. Noninvasive ventilation in acute respiratory failure [Nichtinvasive Beatmung bei akuter respiratorischer Insuffizienz]. Medizinische Klinik 1997;92(1):114‐8. [PubMed] [Google Scholar]
Hilbert 2000 {published data only}
- Hilbert G, Gruson D, Vargas F, Valentino R, Portel L, Gbikpi‐Benissan G, et al. Noninvasive ventilation for acute respiratory failure. Quite low time consumption for nurses. European Respiratory Journal 2000;16(4):710‐6. [DOI] [PubMed] [Google Scholar]
Holanda 2001 {published data only}
- Holanda MA, Rocha EM, Farias MS, Farias MF, Paiva MA, Silva AM, et al. Non‐invasive positive pressure ventilation in patients with acute respiratory failure: factors associated with failure or success. European Respiratory Journal 2001;18(33):30s. [Google Scholar]
Hui 2001 {published data only}
- Hui DS, Li ST, Ko FW, Chan D, Chan AT, Tong MW, et al. Non‐invasive positive pressure ventilation (NIPPV) on the medical wards: a retrospective study of patients with advanced COPD with acute exacerbations in hypercapnic respiratory failure. European Respiratory Journal 2001;18(33):27s. [Google Scholar]
Hui 2001a {published data only}
- Hui KP. Non‐invasive positive pressure ventilation (NIPPV) improves outcomes in a district general hospital. European Respiratory Journal 2001;18(33):28s. [Google Scholar]
IPPBT Group 1983 {published data only}
- [no authors listed]. Intermittent positive pressure breathing therapy of chronic obstructive pulmonary disease. Annals of Internal Medicine 1983;99(5):612‐20. [DOI] [PubMed] [Google Scholar]
Jaber 2000 {published data only}
- Jaber S, Fodil R, Carlucci A, Boussarsar M, Pigeot J, Lemaire F, et al. Noninvasive ventilation with helium‐oxygen in acute exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(4 Pt 1):1191‐200. [DOI] [PubMed] [Google Scholar]
Johnson 2002 {published data only}
- Johnson JE, Gavin DJ, Adams‐Dramiga S. Effects of training with heliox and noninvasive positive pressure ventilation on exercise ability in patients with severe COPD. Chest 2002;122(2):464‐72. [DOI] [PubMed] [Google Scholar]
Jones 1998 {published data only}
- Jones SE, Packham S, Hebden M, Smith AP. Domiciliary nocturnal intermittent positive pressure ventilation in patients with respiratory failure due to severe COPD: long term follow up and effect on survival. Thorax 1998;53:495‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaminski 1999 {published data only}
- Kaminski D, Sliwinski P, Bielen P, Zielinski J. Noninvasive positive pressure ventilation (NIPPV) in COPD patients with hypercapnic respiratory failure [Nieinwazyjne wspomaganie wentylacji dodatnim cisnieniem u chorych na pochp w okresie hiperkapnicznej niewydolnosci oddychania]. Pneunomologia i Alergologia Polska 1999;67(1‐2):45‐52. [PubMed] [Google Scholar]
Katz‐P 2000 {published data only}
- Katz‐Papatheophilou E, Heindl W, Gelbmann H, Hollaus P, Neumann M. Effects of biphasic positive airway pressure in patients with chronic obstructive pulmonary disease. European Respiratory Journal 2000;15(3):498‐504. [DOI] [PubMed] [Google Scholar]
Kaya 2000 {published data only}
- Kaya A, Celik G, Ural O, Ozdemir O, Acican T, Saryal S. Nasal noninvasive mechanical ventilation for hypercapnic respiratory failure. European Respiratory Society 10th Annual Congress; 2000 Aug 30‐Sep 3; Florence. 2000:Abstract 1793.
Keenan 1997 {published data only}
- Keenan SP, Kernerman PD, Cook DJ, Martin CM, McCormack D, Sibbald WJ. Effect of noninvasive positive pressure ventilation on mortality in patients admitted with acute respiratory failure: a meta‐analysis. Critical Care Medicine 25;10:1685‐92. [DOI] [PubMed] [Google Scholar]
Keenan 2000 {published data only}
- Keenan SP, Gregor J, Sibbald WJ, Cook D, Gafni A. Noninvasive positive pressure ventilation in the setting of severe, acute exacerbations of chronic obstructive pulmonary disease: More effective and less expensive. Critical Care Medicine 2000;28(6):2094‐102. [DOI] [PubMed] [Google Scholar]
Keenan 2003 {published data only}
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive‐pressure ventilation? A systematic review of the literature. Annals of Internal Medicine 2003;138(11):861‐70. [DOI] [PubMed] [Google Scholar]
Keenan 2005 {published data only}
- Keenan SP, Powers CE, McCormack DG. Noninvasive positive‐pressure ventilation in patients with milder chronic obstructive pulmonary disease exacerbations: a randomized controlled trial. Respiratory Care 2005;50(5):610‐6. [PubMed] [Google Scholar]
Khouaja 2012 {published data only}
- Khouaja I, Ghedira H. Nasal non‐invasive positive pressure ventilation for moderate exacerbation of chronic obstructive pulmonary disease (COPD) treated in a Tunisian medical ward [Abstract]. European Respiratory Journal 2012;40:356s [P2032]. [Google Scholar]
Kikawada 2001 {published data only}
- Kikawada M, Oyama T, Ogawa K, Fukutomi A, Arai H, Katsunuma H, et al. Noninvasive positive‐pressure ventilation in an elderly woman with acute respiratory failure caused by influenza a virus pneumonia. [Japanese]. Nippon Ronen Igakkai Zasshi ‐ Japanese Journal of Geriatrics 2001;38(3):409‐13. [DOI] [PubMed] [Google Scholar]
Klein 1981 {published data only}
- Klein G, Matthys H, Costabel U. [Oxygen therapy vs. intermittent positive pressure respiration in the long‐term treatment of chronic obstructive pulmonary disease (author's transl)] [German]. Praxis und Klinik der Pneumologie 1981;35(11):528‐31. [PubMed] [Google Scholar]
Koehnlein 2014 {published data only}
- Köhnlein T, Windisch W, Köhler D, Drabik A, Geiseler J, Hartl S, et al. Non‐invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respiratory Medicine 2014;2(9):698‐705. [DOI] [PubMed] [Google Scholar]
Kong 2015 {published data only}
- Kong WX. Efficacy of noninvasive positive pressure ventilation in the patients with acute respiratory failure due to COPD complicated with multi‐pneumatocele. Journal of the American Geriatrics Society 2015;63:S327. [Google Scholar]
Kossler 2000 {published data only}
- Kossler W, Lahrmann H, Brath H, Wei T, Frank W, Wild M, et al. Feedback‐controlled negative pressure ventilation in patients with stable severe hypercapnic chronic obstructive pulmonary disease. Respiration 2000;67(4):362‐6. [DOI] [PubMed] [Google Scholar]
- Kossler W, Lahrmann H, Brath H, Wei T, Frank W, Wild M, et al. Feedback‐controlled negative pressure ventilation in patients with stable severe hypercapnic chronic obstructive pulmonary disease. Respiration 2000;67(4):362‐6. [DOI] [PubMed] [Google Scholar]
Laier‐Groenveld 1991 {published data only}
- Laier‐Groenveld G, Huttemann U, Criee P‐C. NIPPV in chronic ventilatory failure [Die nichtinvasive intermittierende Selbtstbeatmung (ISB) als Therapie der chronischen Ateminsuffizienz]. Medizinische Klinik 1991;86(5):229‐33. [PubMed] [Google Scholar]
Laier‐Groenveld 1995 {published data only}
- Laier‐Groenveld G, Criee CP. Long term effects and life expectancy after 6 years intermittent self ventilation [Langzeiteffekte und Lebenserwartung nach sechs Jahren unter intermittierender Selbstbeatmung]. Medizinische Klinik 1995;90(1):62‐3. [PubMed] [Google Scholar]
Leger 1994 {published data only}
- Leger P, Bedicam JM, Cornette A, Reybet‐Degat O, Langevin B, Polu JM, et al. Nasal intermittent positive pressure ventilation ‐ long‐term follow‐up in patients with severe chronic respiratory insufficiency. Chest 1994;105:100‐5. [DOI] [PubMed] [Google Scholar]
Lien 1993 {published data only}
- Lien TC, Wang JH, Chang MT, Kuo CD. Comparison of BiPAP nasal ventilation and ventilation via iron lung in severe stable COPD. Chest 1993;104(2):460‐6. [DOI] [PubMed] [Google Scholar]
Lien 1996 {published data only}
- Lien T‐C, Wang J‐H, Wu T‐C. Short‐term effects of nasal pressure support ventilation in acute exacerbation of hypercapnic COPD. Chinese Medical Journal 1996;57:335‐42. [PubMed] [Google Scholar]
Lien 2000 {published data only}
- Lien T‐C, Wang J‐H, Huang S‐H, Chen S‐D. Comparison of bilevel positive airway pressure and volume ventilation via nasal or facial masks in patients with severe stable COPD. Chinese Medical Journal 2000;63:542‐51. [PubMed] [Google Scholar]
Lukyanov 2013 {published data only}
- Lukyanov S, Gorbunov V, Alexeev S, Lazutkin M, Aksenova T. The influence of NIV on the pulse wave velocity in patients with the exacerbation of COPD [Abstract]. European Respiratory Journal 2013;42:759s [P3675]. [Google Scholar]
Lun 2013 {published data only}
- Lun C‐T, Chan VL, Leung W‐S, Cheung APS, Cheng S‐L, Tsui MSN, et al. A pilot randomized study comparing two methods of non‐invasive. Respirology 2013;18(5):814‐9. [DOI] [PubMed] [Google Scholar]
- Lun CT, Chu CM, Chan VL, Leung WS, Cheung PS, Cheng SL, et al. A randomised controlled trial comparing stepwise versus immediate withdrawal from non‐invasive ventilation in chronic obstructive pulmonary disease patients recovering from acute hypercapnic respiratory failure [Abstract]. European Respiratory Journal 2012;40:364s [P2062]. [Google Scholar]
Maggiore 2010 {published data only}
- Maggiore SM, Richard JC, Abroug F, Diehl JL, Antonelli M, Sauder P, et al. A multicentre, randomised trial of noninvasive ventilation with helium‐oxygen mixture in exacerbations of chronic obstructive lung disease. Critical Care Medicine 2010;38(1):145‐51. [DOI] [PubMed] [Google Scholar]
Martin 1995 {published data only}
- Martin TJ, Sanders MH, Bierman MI, Hovis JD. Non‐invasive application of bi‐level positive airway pressure to prevent endotracheal intubation in acute respiratory failure. Critical Care Medicine 1995;23(1 (Suppl)):A129. [Google Scholar]
Martin 2000 {published data only}
- Martin TJ, Hovis JD, Costantino JP, Bierman MI, Donahoe MP, Rogers RM, et al. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Pt 1):807‐13. [DOI] [PubMed] [Google Scholar]
Meduri 1989 {published data only}
- Meduri GU, Conoscenti CC, Menashe P, Nair S. Noninvasive face mask ventilation in patients with acute respiratory failure. Chest 1989;95(4):865‐70. [DOI] [PubMed] [Google Scholar]
Meecham Jones 1995 {published data only}
- Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. American Journal of Respiratory and Critical Care Medicine 1995;152:538‐44. [DOI] [PubMed] [Google Scholar]
Meechan Jones 1994 {published data only}
- Meecham Jones DJ, Paul EA, Grahame‐Clarke C, Wedzicha JA. Nasal ventilation in acute exacerbations of chronic obstructive pulmonary disease: effect of ventilator mode on arterial blood gas tensions. Thorax 1994;49:1222‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mega 2012 {published data only}
- Mega C, Pisani L, Navalesi P, Bellone A, Scala R, Repetto V, et al. Noninvasive ventilation (NIV) for acute hypercapnic respiratory failure (AHRF): is the helmet an effective interface? A pilot RCT [Abstract]. European Respiratory Journal 2012;40:356s [P2025]. [Google Scholar]
Moretti 2001 {published data only}
- Moretti M, Girardis M, Marchioni A, Guglielmo M, Lombardini C, Rinaldi L, et al. Non‐invasive mechanical ventilation vs invasive mechanical ventilation in COPD patients with comatose acute respiratory failure. European Respiratory Journal 2001;18(33):29s. [Google Scholar]
Nava 1997 {published data only}
- Nava S, Evangelisti I, Rampulla C, Compagnoni ML, Fracchia C, Rubini F. Human and financial costs of noninvasive mechanical ventilation in patients affected by COPD and acute respiratory failure. Chest 1997;111(6):1631‐8. [DOI] [PubMed] [Google Scholar]
Nava 2001 {published data only}
- Nava S, Fanfulla F, Frigerio P, Navalesi P. Physiologic evaluation of 4 weeks of nocturnal nasal positive pressure ventilation in stable hypercapnic patients with chronic obstructive pulmonary disease. Respiration 2001;68(6):573‐83. [DOI] [PubMed] [Google Scholar]
Nava 2011 {published data only}
- Nava S, Grassi M, Fanfulla F, Domenighetti G, Carlucci A, Perren A, et al. Non‐invasive ventilation in elderly patients with acute hypercapnic respiratory failure: a randomised controlled trial. Age and Ageing 2011;40(4):444‐50. [DOI] [PubMed] [Google Scholar]
NCT01869387 {unpublished data only}
- NCT01869387. Respiratory muscle function in COPD exacerbations [Improvement of respiratory muscle function with noninvasive ventilation in exacerbated COPD patients presenting hypercapnic respiratory failure without acidosis]. clinicaltrials.gov/ct2/show/NCT01869387 (first received 27 May 2013). [Clinicaltrials.gov # NCT01869387]
Oliveria 2001 {published data only}
- Oliveira F, Mota L, Santos C, Paula F, Ferreira L, Leitao MC, et al. Non‐invasive mechanical ventilation (NIMV) for acute exacerbations (AE) in chronic obstructive insufficiency (CRI). Experience on a general ward. European Respiratory Journal 2001;18(33):27s. [Google Scholar]
Pankow 2001 {published data only}
- Pankow W, Becker H, Kohler U, Schneider H, Penzel T, Peter JH. Patient‐ventilator interaction during noninvasive pressure supported spontaneous respiration in patients with hypercapnic COPD. Pneumologie 2001;55(1):7‐12. [DOI] [PubMed] [Google Scholar]
Pastaka 2007 {published data only}
- Kostikas KT, Karetsi E, Pastaka C, Tsolaki V, Gourgoulianis KI. Non invasive ventilation in hypercapnic respiratory failure due to COPD exacerbations that require hospital admission [Abstract]. American Journal of Respiratory and Critical Care Medicine 2006:A643 [Poster J44]. [Google Scholar]
- Pastaka C, Kostikas K, Karetsi E, Tsolaki V, Antoniadou I, Gourgoulianis KI. Non‐invasive ventilation in chronic hypercapnic COPD patients with exacerbation and a pH of 7.35 or higher. European Journal of Internal Medicine 2007;18(7):524‐30. [DOI] [PubMed] [Google Scholar]
Peigang 2002 {published data only}
- Peigang Y, Marini JJ. Ventilation of patients with asthma and chronic obstructive pulmonary disease. Current Opinion in Critical Care 2002;8(1):70‐6. [DOI] [PubMed] [Google Scholar]
Perrin 2000 {published data only}
- Perrin C, Vandenbos F, Tamisier R, Lemoigne F, Blaive B. Impact of acute respiratory failure on survival of COPD patients managed with long‐term non‐invasive ventilation and oxygen therapy [Impact de la décompensation respiratoire aiguë sur la survie des BPCO prises en charge au long cours par ventilation non invasive et oxygénothérapie]. Revue des Maladies Respiratoires 2000;17(1):91‐7. [PubMed] [Google Scholar]
Pinheiro 2001 {published data only}
- Pinheiro BV, Carvalho EV, Oliveira JC, Rocha EM, Carmeiro PS, Holanda MA. Non invasive positive‐pressure ventilation (NIPPV) in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD): factors associated with early and late failures. European Respiratory Journal 2001;18(33):27s. [Google Scholar]
Plant 2000a {published data only}
- Plant PK, Owen JL, Elliott MW. One year period prevalence study of respiratory acidosis in acute exacerbations of COPD: implications for the provision of non‐invasive ventilation and oxygen administration. Thorax 2000;55(7):550‐4. [MEDLINE: ; UID: 20316216] [DOI] [PMC free article] [PubMed] [Google Scholar]
Polese 2000 {published data only}
- Polese G, Vitacca M, Bianchi L, Rossi A, Ambrosino N. Nasal proportional assist ventilation unloads the inspiratory muscles of stable patients with hypercapnia due to COPD. European Respiratory Journal 2000;16(3):491‐8. [DOI] [PubMed] [Google Scholar]
Pollack 1996 {published data only}
- Pollack CJ, Torres MT, Alexander L. Feasibility study of the use of bilevel positive airway pressure for respiratory support in emergency department. Annals of Emergency Medicine 1996;27(2):189‐92. [PMID: 8629750] [DOI] [PubMed] [Google Scholar]
Poponick 1999 {published data only}
- Poponick JM, Renston JP, Bennett RP, Emerman CL. Use of a ventilatory support system (BiPAP) for acute respiratory failure in the emergency department. Chest 1999;116(1):166‐71. [DOI] [PubMed] [Google Scholar]
Porta 2000 {published data only}
- Porta R, Ambrosino N. Noninvasive positive pressure ventilation in COPD patients with chronic respiratory insufficiency. Monaldi Archives for Chest Disease 2000;55(6):509‐10. [PubMed] [Google Scholar]
Porta 2002 {published data only}
- Porta R, Appendini L, Vitacca M, Bianchi L, Donner CF, Poggi R, et al. Mask proportional assist vs pressure support ventilation in patients in clinically stable condition with chronic ventilatory failure. Chest 2002;122(2):479‐88. [DOI] [PubMed] [Google Scholar]
Potena 2003 {published data only}
- Potena A, Putinati S, Ballerin L, Ritrovato L, Piattella M, Zabini F. Non‐invasive mechanical ventilation in acute respiratory failure due to chronic obstructive pulmonary disease: prognostic factors and long term survival. Minerva Pneumologica 2003;42(4):201‐10. [Google Scholar]
Putinati 2000 {published data only}
- Putinati S, Ballerin L, Piattella M, Panella GL, Potena A. Is it possible to predict the success of non‐invasive positive pressure ventilation in acute respiratory failure due to COPD?. Respiratory Medicine 2000;94(10):997‐1001. [DOI] [PubMed] [Google Scholar]
Rappaport 1994 {published data only}
- Rappaport SH, Shpiner R, Yoshihara G, Wright J, Chang P, Abraham E. Randomized, prospective trial of pressure‐limited versus volume‐controlled ventilation in severe respiratory failure. Critical Care Medicine 1994;22(1):22‐32. [DOI] [PubMed] [Google Scholar]
Reissmann 2000 {published data only}
- Reissmann HK, Ranieri VM, Goldberg P, Gottfried SB. Continuous positive airway pressure facilitates spontaneous breathing in weaning chronic obstructive pulmonary disease patients by improving breathing pattern and gas exchange. Intensive Care Medicine 2000;26(12):1764‐72. [DOI] [PubMed] [Google Scholar]
Rizvi 2001 {published data only}
- Rizvi N, Mehmood N. Role of Bi‐Pap in acute respiratory failure due to acute exacerbation of COPD. European Respiratory Society 10th Annual Congress; 2000 Aug 30‐Sep 3; Florence. 2000; Vol. Abstract 1794.
Robino 2003 {published data only}
- Robino C, Faisy C, Diehl JL, Rezgui N, Labrousse J, Guerot E. Effectiveness of non‐invasive positive pressure ventilation differs between decompensated chronic restrictive and obstructive pulmonary disease patients. Intensive Care Medicine 2003;29(4):603‐10. [DOI] [PubMed] [Google Scholar]
Roessler 2012 {published data only}
- Roessler MS, Schmid DS, Michels P, Schmid O, Jung K, Stober J, et al. Early out‐of‐hospital non‐invasive ventilation is superior to standard medical treatment in patients with acute respiratory failure: a pilot study. Emergency Medicine Journal 2012;29(5):409‐14. [DOI] [PubMed] [Google Scholar]
Scala 2001 {published data only}
- Scala R, Bartolucci S, Fabianelli F, Rossi M. Non invasive positive pressure mechanical ventilation (NIV) in acute respiratory failure (ARF): 5 years' experience in a respiratory ward (RW). European Respiratory Journal 2001;18(33):27s. [Google Scholar]
Scandroglio 2002 {published data only}
- Scandroglio M, Piccolo U, Mazzone E, Agrati P, Aspesi M, Gamberoni C, et al. Use and nursing of the helmet in delivering non invasive ventilation. Minerva Anestesiologica 2002;68(5):475‐80. [PubMed] [Google Scholar]
Schonhofer 2001 {published data only}
- Schonhofer B, Barchfeld T, Wenzel M, Kohler D. Long term effects of non‐invasive mechanical ventilation on pulmonary haemodynamics in patients with chronic respiratory failure. Thorax 2001;56(7):524‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seith 1976 {published data only}
- Seith U. Evaluation of therapeutic results of intermittent positive pressure respiration (author's translation) [Uber die Schwierigkeit, die Behandlungsergebnisse der Inhalation mit intermittierendem Uberdruck (IPPB) zu verifizieren]. Praxis Der Pneumologie Vereinigt Mit Der Tuberkulosearzt 1976;30(8):509‐10. [PubMed] [Google Scholar]
Sellares 2012 {published data only}
- Sellares J, Ferrer M, Bencosme C, Loureiro H, Martinez P, Pajares V, et al. Assessment of two methods to withdraw non‐invasive ventilation in acute hypercapnic respiratory failure [Abstract]. Euroepan Respiratory Journal 2012;40:833s [4564]. [Google Scholar]
Servera 1995a {published data only}
- Servera E, Perez M, Marin J, Vergara P, Castano R. Noninvasive nasal mask ventilation beyond the ICU for an exacerbation of chronic respiratory insufficiency. Chest 1995;108:1572‐6. [DOI] [PubMed] [Google Scholar]
Servera 1995b {published data only}
- Servera E, Vergara P, Marin J, Perez M, Castano R, Mora H. Assisted ventilation via nasal mask in patients hospitalized in a pneumology ward for decompensation of their chronic air‐flow obstruction [Ventilacion asistida via mascara nasal en pacientes hospitalizados en una sala de neumologia por descompensacion de su obstruccionn cronica al flujo aereo]. Archivos de Bronconeumologia 1995;31(8):399‐402. [DOI] [PubMed] [Google Scholar]
Shang 2001 {published data only}
- Shang M, Wang C, Dai H. Changes in respiratory and circulatory function during sequential invasive‐noninvasive mechanical ventilation [I]. Chung‐Hua Chieh Ho Ho Hu Hsi Tsa Chih Chinese Journal of Tuberculosis and Respiratory Diseases 2001;24(8):487‐9. [PubMed] [Google Scholar]
Shang 2014 {published data only}
- Shang D, Dang X‐M, Yang L, Han J‐F, Sun Z‐M, Feng S‐F, et al. Treatment of respiratory muscle fatigue in patients with AECOPD by non‐invasive positive pressure ventilation. [Chinese]. Journal of Xi'an Jiaotong University (Medical Sciences) 2014;35(6):824‐27, 847. [Google Scholar]
Sidhu 2000 {published data only}
- Sidhu US, Behera D. Non invasive ventilation in COPD. Indian Journal of Chest Diseases and Allied Sciences 2000;42(2):105‐14. [PubMed] [Google Scholar]
Simonds 1995 {published data only}
- Simonds AK, Elliott MW. Outcome of domiciliary nasal intermittent positive pressure ventilation in restrictive and obstructive disorders. Thorax 1995;50:604‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinuff 2003 {published data only}
- Sinuff T, Cook DJ. Health technology assessment in the ICU: noninvasive positive pressure ventilation for acute respiratory failure. Journal of Critical Care 2003;18(1):59‐67. [DOI] [PubMed] [Google Scholar]
Soo Hoo 1994 {published data only}
- Soo Hoo GW, Santiago S, Williams AJ. Nasal mechanical ventilation for hypercapnic respiratory failure in chronic obstructive pulmonary disease: determinants of success and failure. Critical Care Medicine 1994;22(8):1253‐61. [DOI] [PubMed] [Google Scholar]
Summers 2002 {published data only}
- Summers RL, Patch J, Kolb JC. Effect of the initiation of noninvasive bi‐level positive airway pressure on haemodynamic stability. European Journal of Emergency Medicine 2002;9(1):37‐41. [DOI] [PubMed] [Google Scholar]
Tarrega 2000 {published data only}
- Tarrega J, Anton A, Guell R, Sanchis J. Survival in COPD patients after noninvasive ventilation for acute exacerbation. European Respiratory Society 10th Annual Congress; 2000 Aug 30‐Sep 3; Florence. 2000; Vol. Abstract 2760.
Teba 1996 {published data only}
- Teba L, Marks P, Benzo R. Non‐invasive mechanical ventilation: the benefits of the BiPAP system. West Virginia Medical Journal 1996;92:18‐21. [PubMed] [Google Scholar]
Thys 1999 {published data only}
- Thys F, Roeseler J, Delaere S, Palavecino L, Gariani A, Marion E, et al. Two‐level non‐invasive positive pressure ventilation in the initial treatment of acute respiratory failure in an emergency department. European Journal of Emergency Medicine 1999;6(3):207‐14. [PubMed] [Google Scholar]
Todisco 2001 {published data only}
- Todisco T, Baglioni S, Eslami A. Application of non invasive ventilation in patients with acute hypercapnic exacerbation of chronic respiratory failure: our experience on use of negative pressure ventilation and non invasive intermittent positive pressure ventilation. European Respiratory Journal 2001;18(33):29s. [Google Scholar]
Tsuboi 1999 {published data only}
- Tsuboi T. Noninvasive positive pressure ventilation in patients with COPD. Japanese Journal of Clinical Medicine 1999;57(9):2074‐82. [PubMed] [Google Scholar]
Vanpee 2001 {published data only}
- Vanpee D, Delaunois L, Gillet JB. Non‐invasive positive pressure ventilation for exacerbation of chronic obstructive pulmonary patients in the emergency department. European Journal of Emergency Medicine 2001;8(1):21‐5. [DOI] [PubMed] [Google Scholar]
Vanpee 2002 {published data only}
- Vanpee D, Khawand C, Rousseau L, Jamart J, Delaunois L. Effects of nasal pressure support on ventilation and inspiratory work in normocapnic and hypercapnic patients with stable COPD. Chest 2002;122(1):75‐83. [DOI] [PubMed] [Google Scholar]
Vanpee 2002b {published data only}
- Vanpee D, Delaunois L, Lheureux P, Thys F, Sabbe M, Meulemans A, et al. Survey of non‐invasive ventilation for acute exacerbation of chronic obstructive pulmonary disease patients in emergency departments in Belgium. European Journal of Emergency Medicine 2002;9(3):217‐24. [DOI] [PubMed] [Google Scholar]
Vanpee 2002c {published data only}
- Vanpee D, Khawand C, Rousseau L, Jamart J, Delaunois L. Does inspiratory behaviour affect the efficiency of non‐invasive ventilation in COPD patients?. Respiratory Medicine 2002;96(9):709‐15. [DOI] [PubMed] [Google Scholar]
Vitacca 1993 {published data only}
- Vitacca M, Rubini F, Foglio K, Scalvini S, Nava S, Ambrosino N. Non‐invasive modalities of positive pressure ventilation improve the outcome of acute exacerbations in COLD patients [see comments]. Intensive Care Medicine 1993;19(8):450‐5. [DOI] [PubMed] [Google Scholar]
Vitacca 2000 {published data only}
- Vitacca M, Clini E, Pagani M, Bianchi L, Rossi A, Ambrosino N. Physiologic effects of early administered mask proportional assist ventilation in patients with chronic obstructive pulmonary disease and acute respiratory failure. Critical Care Medicine 2000;28(6):1791‐7. [DOI] [PubMed] [Google Scholar]
Vitacca 2000b {published data only}
- Vitacca M, Nava S, Confalonieri M, Bianchi L, Porta R, Clini E, Ambrosino N. The appropriate setting of noninvasive pressure support ventilation in stable COPD patients. Chest 2000;118(5):1286‐93. [DOI] [PubMed] [Google Scholar]
Vitacca 2002 {published data only}
- Vitacca M, Barbano L, D'Anna S, Porta R, Bianchi L, Ambrosino N. Comparison of five bilevel pressure ventilators in patients with chronic ventilatory failure: a physiologic study. Chest 2002;122(6):2105‐14. [DOI] [PubMed] [Google Scholar]
Wedzicha 1996a {published data only}
- Wedzicha JA. Nasal positive pressure ventilation in COPD. Monaldi Archives for Chest Disease 1996;51(1):81‐2. [PubMed] [Google Scholar]
Wedzicha 1996b {published data only}
- Wedzicha JA. Non‐invasive ventilation for exacerbations of respiratory failure in chronic obstructive pulmonary disease. Thorax 1996;51(Suppl 2):S35‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wedzicha 2002 {published data only}
- Wedzicha JA, Muir JF. Noninvasive ventilation in chronic obstructive pulmonary disease, bronchiectasis and cystic fibrosis. European Respiratory Journal 2002;20(3):777‐84. [DOI] [PubMed] [Google Scholar]
Windisch 2002 {published data only}
- Windisch W, Storre JH, Matthys H, Sorichter S, Virchow JC Jr. Weaning from mechanical ventilation by long‐term nasal positive pressure ventilation in two patients with acute respiratory distress syndrome associated with pneumococcal sepsis. Respiration 2002;69(5):464‐7. [DOI] [PubMed] [Google Scholar]
Windisch 2002b {published data only}
- Windisch W, Vogel M, Sorichter S, Hennings E, Bremer H, Hamm H, et al. Normocapnia during NIPPV in chronic hypercapnic COPD reduces subsequent spontaneous PaCO2. Respiratory Medicine 2002;96(8):572‐9. [DOI] [PubMed] [Google Scholar]
Wong 2007 {published data only}
- Wong P, Tee A, Eritaia J, Goldin J, Irving L. NIV during the resolution phase of acute hypercapnic respiratory failure secondary to COPD in improving sleep quality and recovery. Respirology 2007;12(Suppl 4):A207. [Google Scholar]
Wood 1998 {published data only}
- Wood KA, Lewis L, Harz B, Kollef MH. The use of noninvasive positive pressure ventilation in the emergency department. Chest 1998;113(5):1339‐46. [DOI] [PubMed] [Google Scholar]
Wysocki 1995 {published data only}
- Wysocki M, Tric L, Wolff MA, Millet H, Herman B. Noninvasive pressure support ventilation in patients with acute respiratory failure: a randomized comparison with conventional therapy. Chest 1995;107(3):761‐8. [DOI] [PubMed] [Google Scholar]
Xue 2000 {published data only}
- Xue Z, Bai L, Ma Q. Noninvasive positive pressure ventilation for patients with chronic obstructive pulmonary disease after upper abdominal and thoracic surgery. Chinese Journal of Anesthesiology 2000;03:145‐8. [Google Scholar]
Yang 2002 {published data only}
- Yang SC, Yang SP. Effects of inspiratory flow waveforms on lung mechanics, gas exchange, and respiratory metabolism in COPD patients during mechanical ventilation. Chest 2002;122(6):2096‐104. [DOI] [PubMed] [Google Scholar]
Ye 2000 {published data only}
- Ye Q, Wang C, Tong Z. Proportional assist ventilation: methodology therapeutics on COPD patients compared with pressure support ventilation. Chung‐Hua Chieh Ho Ho Hu Hsi Tsa Chih Chinese Journal of Tuberculosis and Respiratory Diseases 2000;23(4):228‐31. [PubMed] [Google Scholar]
Ye 2002 {published data only}
- Ye Q, Wang C, Tong Z, Huang K, Jiang C, Weng X. Proportional assist ventilation: methodology and therapeutics on COPD patients compared with pressure support ventilation. Chinese Medical Journal 2002;115(2):179‐83. [PubMed] [Google Scholar]
References to studies awaiting assessment
Liao 2004 {published data only}
- Liao XQ, Li Q, Lin KX. Non‐invasive positive pressure ventilation for early treatment of respiratory failure due to exacerbation of chronic obstructive pulmonary disease: a random controlled trial. Acta Academic Medicine Militaris Tertiae 2004;26(8):739‐41. [Google Scholar]
Samaria 2013 {published data only}
- Samaria JK. Comparison of conventional therapy with non‐invasive positive ventilation in patients of acute exacerbation of COPD. American Journal of Respiratory and Critical Care Medicine 2013;187:A1453. [Google Scholar]
Servillo 1994 {published data only}
- Servillo G, Ughi L, Rossano F, Leone D. Non invasive mask pressure support ventilation in COPD patients. Intensive Care Medicine 1994;20 Suppl:S54. [Google Scholar]
References to ongoing studies
Duan 2011 {published data only}
- Duan Y. Observation of non‐invasive positive ventilation united inhalation treatment therapeutic effect on chronic obstructive pulmonary disease with type II respiratory failure [Abstract]. Respirology 2011;16:93 [187]. [Google Scholar]
Additional references
Ambrosino 1996
- Ambrosino, N. Noninvasive mechanical ventilation in acute respiratory failure. European Respiratory Journal 1996;9:795‐807. [DOI] [PubMed] [Google Scholar]
Appendi 1994
- Appendini L, Patessio A, Zanaboni S, Carone M, Gukov B, Donner CF, et al. Physiologic effects of positive end‐expiratory pressure and mask pressure support during exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1994;149(5):1069‐76. [PUBMED: 8173743] [DOI] [PubMed] [Google Scholar]
Brochard 1994
- Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1994;150:896‐903. [DOI] [PubMed] [Google Scholar]
Chandra 2012
- Chandra D, Stamm JA, Taylor B, Ramos RM, Satterwhite L, Krishnan JA, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. American Journal of Respiratory and Critical Care Medicine 2012;185(2):152‐9. [PUBMED: PMC3297087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coakley 1992
- Coakley JH, Nagendran K, Ormerod IE, Ferguson CN, Hinds CJ. Prolonged neurogenic weakness in patients requiring mechanical ventilation for acute airflow limitation. Chest 1992;101:1413‐6. [DOI] [PubMed] [Google Scholar]
Esteban 1995
- Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. New England Journal of Medicine 1995;332:345‐50. [DOI] [PubMed] [Google Scholar]
Fagon 1993
- Fagon JY, Chastre J, Hance A, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. American Journal of Medicine 1993;94:281‐7. [DOI] [PubMed] [Google Scholar]
Guerin 1997
- Guerin C, Girard R, Chemorin C, Varax R, Fournier G. Facial mask noninvasive mechanical ventilation reduces the incidence of nosocomial pneumonia. A prospective epidemiological survey from a single ICU. Intensive Care Medicine 1997;23:1024‐32. [DOI] [PubMed] [Google Scholar]
Helliwell 1991
- Helliwell TR, Coakley JH, Wagenmakers AJ, Griffiths RD, Campbell IT, Green CJ, et al. Necrotizing myopathy in critically ill patients. Journal of Pathology 1991;164(4):307‐14. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. London, UK: The Cochrane Collaboration,, 2011. [Google Scholar]
Jaun 1984
- Jaun G, Calverley P, Talamo C, Schnader J, Roussos C. Effect of carbon dioxide on diaphragmatic function in human beings. New England Journal of Medicine 1984;310:874‐9. [DOI] [PubMed] [Google Scholar]
Jeffrey 1992
- Jeffrey AA, Warren PM, Flenley DC. Acute hypercapnic respiratory failure in patients with chronic obstructive lung disease: risk factors and use of guidelines for management. Thorax 1992;47(1):34‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koenig 2006
- Koenig SM, Truwit JD. Ventilator‐associated pneumonia: diagnosis, treatment, and prevention. Clinical Microbiology Reviews 2006;19(4):637‐57. [PUBMED: 17041138] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 1999
- Kramer B. Ventilator‐associated pneumonia in critically ill patients. Annals of Internal Medicine 1999;130:1027‐8. [DOI] [PubMed] [Google Scholar]
Le Bourdelles 1994
- Bourdelles G, Vires N, Bockzowki SN, Pavolovic D, Aubier M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. American Journal of Respiratory and Critical Care Medicine 1994;149:1539‐44. [DOI] [PubMed] [Google Scholar]
Macklem 1984
- Macklem PT. Hyperinflation. American Review of Respiratory Disease 1984;129:1‐2. [DOI] [PubMed] [Google Scholar]
Moloney 1999
- Moloney E, Kiely JL, McDonnell T, McNicholas WT. Nocturnal nasal intermittent positive pressure ventilation (NIPPV) therapy for chronic respiratory failure: long‐term effects. Irish Medical Journal 1999;92(6):401‐3. [PubMed] [Google Scholar]
Nourdine 1999
- Nourdine K, Combes P, Carton M‐J, Beuret P, Cannamela A, Ducreux J‐C. Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Medicine 1999;25:567‐73. [DOI] [PubMed] [Google Scholar]
Roberts 2011
- Roberts CM, Stone RA, Buckingham RJ, Pursey NA, Lowe D. Acidosis, non‐invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax 2011;66(1):43‐8. [PUBMED: 21075776] [DOI] [PubMed] [Google Scholar]
Shapiro 1986
- Shapiro M, Wilson RK, Casar G, Bloom K, Teague RB. Work of breathing through different sized endotracheal tubes. Critical Care Medicine 1986;14:1028‐31. [DOI] [PubMed] [Google Scholar]
Stauffer 1982
- Stauffer JL, Silvestri RC. Complications of endotracheal intubation, tracheostomy and artificial airways. Respiratory Care 1982;27:417‐34. [Google Scholar]
Tobin 1986
- Tobin MJ, Perez W, Guenther SM, Semmes BJ, Madar MJ, Allen SJ, et al. The pattern of breathing during successful and unsuccessful trials of weaning from mechanical ventilation. American Review of Respiratory Disease 1986;134(6):1111‐8. [DOI] [PubMed] [Google Scholar]
Waters 2015
- Waters B, Muscedere J. A 2015 update on ventilator‐associated pneumonia: new insights on its prevention, diagnosis, and treatment. Current Infectious Disease Reports 2015;17(8):496. [PUBMED: 26115700] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ram 2004
- Ram FSF, Picot J, Lightowler J, Wedzicha JA. Non‐invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004104.pub3] [DOI] [Google Scholar]


