Abstract
Background
This is an updated version of the original Cochrane review published in 2014, Issue 4. Cervical intraepithelial neoplasia (CIN) precedes the development of invasive carcinoma of the cervix. Current treatment of CIN is quite effective, but there is morbidity for the patient related to pain, bleeding, infection, cervical stenosis and premature birth in a subsequent pregnancy. Effective treatment with medications, rather than surgery, would be beneficial.
Objectives
To evaluate the effectiveness and safety of non‐steroidal anti‐inflammatory agents (NSAIDs), including cyclooxygenase‐2 (COX‐2) inhibitors, to induce regression and prevent the progression of CIN.
Search methods
Previously, we searched the Cochrane Gynaecological Cancer Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 11), MEDLINE (November, 2013) and Embase (November week 48, 2013). An updated search was performed in August 2017 for CENTRAL (2017, Issue 8), MEDLINE (July, week 3, 2017) and Embase (July week 31, 2017). Trial registries and journals were also searched as part of the update.
Selection criteria
Randomised controlled trials (RCTs) or controlled trials of NSAIDs in the treatment of CIN.
Data collection and analysis
Three review authors independently abstracted data and assessed risks of bias in accordance with Cochrane methodology. Outcome data were pooled using fixed‐effect meta‐analyses.
Main results
In three RCTs, 171 women over the age of 18 years were randomised to receive celecoxib 400 mg daily for 14 to 18 weeks versus placebo (one study, 130 participants), celecoxib 200 mg twice daily by mouth for six months versus placebo (one study, 25 participants), or rofecoxib 25 mg once daily by mouth for three months versus placebo (one study, 16 participants). The study with rofecoxib was discontinued when the medicine was withdrawn from the market in 2004. The trials ran from June 2005 to April 2012, June 2002 to October 2003, and May to October 2004, respectively. We have chosen to include the data from the rofecoxib study as outcomes may be similar when other such NSAIDs are utilised.
Partial or complete regression of CIN 2 or CIN 3 occurred in 31 out of 70 (44%) in the treatment arms and 19 of 62 (31%) in the placebo arms (risk ratio (RR) 1.45, 95% confidence interval (CI) 0.93 to 2.27; P value 0.10), three studies, 132 participants; moderate‐certainty evidence). Complete regression of CIN 2 or CIN 3 occurred in 15 of 62 (24%) of those receiving celecoxib versus 10 of 54 (19%) of those receiving placebo (RR 1.31, 95% CI 0.65 to 2.67; P value 0.45, two studies, 116 participants; moderate‐certainty evidence). Partial regression of CIN 2 or CIN 3 occurred in 14 of 62 (23%) of those receiving celecoxib versus 8 of 54 (15%) of those receiving placebo (RR 1.56, 95% CI 0.72 to 3.4; P value 0.26), two studies, 116 participants; moderate‐certainty evidence).
Progression to a higher grade of CIN, but not to invasive cancer, occurred in one of 12 (8%) of those receiving celecoxib and two of 13 (15%) receiving placebo (RR 0.54, 95% CI 0.05 to 5.24; P value 0.60, one study, 25 participants; very low‐certainty evidence). Two studies reported no cases of progression to invasive cancer within the timeframe of the study. No toxicity was reported in the two original articles. The trial added in this update had one Grade 3 gastrointestinal adverse effect in the treatment arm, but otherwise had similar Grade 1 to 2 side effects between treatment and placebo groups. Although the studies were well‐conducted and randomised, some risk of bias was detected in all studies. Furthermore, the duration of the studies was short, which may mask identifying progression to cancer.
The addition of the trial in this update quadrupled the number of patients in the original review and was a well‐designed multicentre trial thus, increasing the overall certainty of evidence from very low to moderate for this review.
Authors' conclusions
There are currently no convincing data to support a benefit for NSAIDs in the treatment of CIN. With the addition of this new, larger randomised trial we would rate this as overall moderate‐certainty evidence by the GRADE criteria.
Plain language summary
The treatment of cervical pre‐cancer (CIN) with anti‐inflammatory agents to induce regression and prevent the progression to cervical cancer
Background This review is an update of a previously published review in The Cochrane Database of Systematic Reviews 2014, Issue 4 on non‐steroidal anti‐inflammatory agents (NSAIDs) to induce regression and prevent the progression of cervical intraepithelial neoplasia (CIN). Cervical intraepithelial neoplasia (CIN) is a common pre‐cancerous condition of the cervix associated with HPV (the human papillomavirus), which can occur in anyone but is commonly found in younger women who wish to maintain their fertility and treatment often involves surgical excision. CIN can progress to invasive cancer of the cervix. CIN is identified by screening and can be treated with surgery to the cervix either by removal with surgical excision or destruction of the cells covering the cervix such as with laser therapy, heating, or freezing. While this is effective in the majority of cases, the surgery can cause immediate unwanted effects, such as bleeding and infection, or later complications including difficulty with menses due to scarring of the cervix and early (premature) labour.
NSAIDs have been found to prevent the development of cancer of the large bowel and other organs, but with some unwanted side effects especially on the heart and blood vessels. Although rofecoxib, used in one of these studies, was withdrawn from the market in 2004, it may shed light on the feasibility of treatment with other NSAIDs.
We wanted to discover whether the use of NSAIDs for women with CIN could promote regression or prevent progression to cervical cancer without undue risk or side effects.
The aim of the review To identify the utility of treating CIN with non‐steroidal anti‐inflammatory drugs (NSAIDs) such as celecoxib to cause regression of the abnormal findings and avoid surgical procedures.
Study characteristics We identified three randomised studies up to August 2017, including 171 women over the age of 18 years, with moderate or severe CIN. The trials ran from June 2005 to April 2012, June 2002 to October 2003, and May to October 2004. One of them was discontinued before it was completed. The women were given either celecoxib or rofecoxib versus a placebo (sugar tablet) daily by mouth for a period of three to six months.
Key Results With the addition of the third trial to this review, there is now a sufficient number of patients in the review to conclude that NSAIDs have minimal effect over placebo in causing regression of CIN. No patients progressed to invasive cervical cancer, and overall, the drug was well‐tolerated compared to placebo.
Quality of the evidence The studies appear to have been well‐conducted. There are some questions related to the quality of evidence in relation to concealment and women dropping out of the study before completion of assigned medications. We therefore concluded that the certainty (quality) of the evidence was moderate. There was insufficient information to assess accuracy of the reporting of information. It is possible that there are other incomplete and unreported studies that have not been identified.
Conclusion The literature available at this time suggests that there are no convincing data to suggest NSAIDs as a treatment for CIN.
Summary of findings
for the main comparison.
| Non‐steroidal anti‐inflammatory agents (NSAIDs) compared with placebo for CIN 2 or CIN 3 | ||||||
|
Patient or population: women with CIN 2 or CIN 3 Settings: outpatient Intervention: celecoxib 400 mg by mouth daily for 14‐18 weeks, celecoxib 200 mg by mouth twice daily for six months or rofecoxib 40 mg by mouth daily for three months Comparison: placebo tablet by mouth, daily for three to six months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Progression of CIN to a higher grade of CIN | 77 per 10001 | 42 per 1000 | RR 0.54 (0.06 to 5.24) | 25 (one study) | ⊕⊝⊝⊝3 very low | |
| Partial or complete regression of CIN 2 or CIN 3 | 308 per 10002 | 447 per 1000 | RR 1.45 (0.93 to 2.27) | 132 (three studies) | ⊕⊕⊕⊝3 moderate | |
| Complete regression of CIN 2 or CIN 3 | 174 per 10001 | 228 per 1000 | RR 1.31 (0.65 to 2.67) | 116 (two studies) | ⊕⊕⊕⊝3 moderate | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CIN: cervical intraepithelial neoplasia; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1The basis for the assumed risk is from the spontaneous complete regression rate in the placebo arm of Farley 2006 and Rader 2017
2 The basis for the assumed risk is from the combined spontaneous partial or complete regression rates in the placebo arms of Farley 2006; Hefler 2006; Rader 2017
3Given the increased sample size with the addition of Rader 2017, we have upgraded the certainty to high other than the Progression analysis as it is based on one small study and thus remained very low certainty.
Background
Description of the condition
This review is an update of a previously published review in The Cochrane Database of Systematic Reviews 2014, Issue 4 on 'Non‐steroidal anti‐inflammatory agents (NSAIDs) to induce regression and prevent the progression of cervical intraepithelial neoplasia' (Grabosch 2014).
Invasive carcinoma of the cervix is the most common cancer in women in developing countries with more than 500,000 cases diagnosed worldwide each year (Ferlay 2010). It is preceded by cervical intraepithelial neoplasia (CIN), which can be detected on cervical cytology screens (Papanicolaou test or Pap smear). Treatment of CIN is effective at reducing, but not eliminating, the risk of subsequent invasive carcinoma (Soutter 1997). However, the cost of treatment of CIN and follow‐up is high, and the surgery involved can have short‐ and long‐term adverse effects (Arbyn 2008; Martin‐Hirsch 2010).
Description of the intervention
The family of agents called non‐steroidal anti‐inflammatory agents (NSAIDs), including aspirin, ibuprofen, indomethacin, naproxen, piroxicam, and sulindac (Fischer 2011), are able to block both cyclooxygenase (COX) ‐1 and ‐2 enzymes. Selective COX‐2 inhibitors such as celecoxib, rofecoxib and valdecoxib specifically interfere with COX‐2. These agents can be taken orally.
How the intervention might work
COX enzymes catalyse the rate‐limiting step in the conversion of arachidonic acid to prostaglandins and other eicosanoids (DeWitt 1991). COX‐1 is involved in homeostatic functions such as gastrointestinal cytoprotection and is constitutively expressed in most tissues, whereas COX‐2 is rapidly induced after stimulation of quiescent cells by growth factors and also by oncogenes, carcinogens and tumour‐promoters (Hia 1992; Hershmann 1996).
Over‐expression of COX‐2 is thought to be a key factor in malignant transformation in many tissues (Mohammed 1999; Lim 2000; Shamma 2000; Shariat 2003; Shirahama 2000; Surh 2005; Tan 2005). Increased levels of COX‐2 protein expression have been reported in malignancies arising in various sites such as the stomach (Lim 2000), breast (Hwang 1998), oesophagus (Shamma 2000), ovary Munkarah 2005, cervix (Ferrandina 2000; Ferrandina 2002; Kulkarni 2001; Ryu 2000), and colon (Eberhart 1994; Soslow 2000).
COX‐2 may play a role in malignant transformation in the cervix. Increased COX‐2 expression is found with higher grades of CIN (Farley 2004; Mitchell 2007). There is an increased risk of persistent or recurrent disease when resection margins (following excisional procedures) are positive for the enzyme (Farley 2004). COX‐2 is present in invasive carcinoma (Dursun 2007; Farley 2004; Kim 2005; Kulkarni 2001; Mitchell 2007). Similar to other cancers (Cao 2002; Sobolewski 2010), high levels of expression are associated with poorer prognostic features such as shorter time to first recurrence, decreased survival (Farley 2004; Ferrandina 2000; Ferrandina 2002; Gaffney 2001), increased lymph node metastasis or parametrial invasion (Gaffney 2003; Kim 2003; Ryu 2000), and chemotherapy or radiation resistance (Ferrandina 2000; Kim 2002).
The mechanism of the effect on the development of tumours is still being investigated, but it has already been shown that increased expression of COX‐2 will inhibit apoptosis (Tsujii 1995), promote angiogenesis (Tsujii 1998), and enhance the invasiveness of malignant cells (Tsujii 1997). It has been hypothesised that over‐expression of COX‐2 could impair host immune responses (Huang 1998; Pockaj 2004), partly because COX‐2 inhibitors reverse tumour‐associated immunosuppression (Huang 1998; Stolina 2000).
The inhibition of the prostaglandin biosynthetic cascade by NSAIDs has been demonstrated to modulate carcinogenesis in both human and animal epithelial tissues (Kelloff 1996). Selective COX‐2 inhibitors suppress tumorigenesis in experimental models of colon, bladder, breast, prostate, stomach, skin, and lung cancers (Elmets 2010; Fischer 1999; Fischer 2011; Harris 2000; Kawamori 1998; Liu 2000; Narisawa 1981; Okajima 1998; Reddy 2000.
One of the proposed benefits of selective COX‐2 inhibitors was a reduction of significant gastrointestinal toxicity related to blocking of COX‐1 from non‐selective NSAIDs (Psaty 2006). While this is true, COX‐2 inhibition is unfortunately associated with other adverse effects, principally related to inhibition of the production of prostacyclin in the arterial endothelial and smooth muscle cells (Grosser 2006). In two large randomised controlled trials (RCTs) investigating celecoxib for the prevention of colorectal adenomas, cardiovascular events (myocardial infarction, stroke, congestive heart failure, and death) were increased 12.7% in patients taking celecoxib, and reduced by 4.4% in those taking aspirin (Arber 2006; Bertagnolli 2006; Psaty 2006). These patients had a median age of 59 to 61, were treated for relatively long periods of time (one to three years) and many had pre‐existing cardiovascular disease. While these patients represent a different population than most who would be treated for CIN, cardiovascular events remain a concern with the use of COX‐2 inhibitors. Rofecoxib, a COX‐2 inhibitor, which was used by one of the studies in this review, was withdrawn from the market in 2004 for such concerns.
It should be noted that many women may experience regression of CIN without treatment: CIN 2 regression is estimated in around 50% of women within six months (Bleecker 2014) and CIN 3 regression in approximately 30% of women within six months. In a RCT investigating medical treatment for CIN 2/CIN 3, 31% in the placebo arm had complete histologic regression in six months (Follen 2001), whilst in another similar study 32% of CIN 2/CIN 3 lesions regressed to a lower level of CIN in three months without treatment (Alvarez 2003). Differences in regression may be related to the subtype of high risk human papillomavirus (HPV) as HPV‐16 and HPV‐33 are less likely to be spontaneously cleared and more likely to progress to higher CIN (Jaisamrarn 2013).
Why it is important to do this review
The potential use of NSAIDs, including COX‐2 inhibitors, to induce regression or prevent the progression of CIN to invasive carcinoma is an exciting prospect. To our knowledge, no recent systematic review has been published that specifically addresses this subject.
Objectives
To evaluate the effectiveness and safety of non‐steroidal anti‐inflammatory agents (NSAIDs), including cyclooxygenases inhibitors, to induce regression and prevent the progression of cervical intraepithelial neoplasia (CIN).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or non‐RCTs.
Studies were considered only if they included details of how the grading of CIN was determined. The gold standard for follow‐up of CIN includes cervical cytology, colposcopy and biopsy, where indicated. However, studies were accepted for review with cytologic assessment only. Progression was defined as elevation of the grade of dysplasia by at least one grade (CIN 1 to CIN 2, CIN 2 to CIN 3 and CIN 3 to invasive disease). Regression was defined as reduction in the grade of dysplasia by at least one grade (CIN 3 to CIN 2, CIN 2 to CIN 1, and CIN 1 to no dysplasia).
Types of participants
Women 18 years of age and over with an initial diagnosis of CIN.
Types of interventions
Treatment with non‐steroidal anti‐inflammatory agents (NSAIDs), including cyclooxygenase‐2 (COX‐2) inhibitors alone, by any route in an outpatient setting.
Types of outcome measures
Primary outcomes
Progression of CIN to higher grades of CIN
Progression of CIN to invasive carcinoma
Regression of CIN
Secondary outcomes
Adverse events related to treatment.
Search methods for identification of studies
We searched for papers in all languages and planned to have them translated as necessary.
Electronic searches
For the original query, we searched the Specialised Register of the Cochrane Gynaecological Cancer Review Group (CGCRG), the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library 2013, Issue 11) (see Appendix 1); MEDLINE (1946 up to Novemeber, 2013) (see Appendix 2); and Embase (1980 up to 2013, week 48) (see Appendix 3). An updated literature search was performed in August 2017 through CENTRAL (2017, Issue 8), MEDLINE (July week 3, 2017) and Embase (July week 31, 2017).
Searching other resources
We carried out an electronic search of abstracts presented to the annual or biennial meetings of the following organisations: Society of Gynecologic Oncologists, International Gynecological Cancer Society, European Society of Gynecologic Cancer, American Society of Colposcopy and Cervical Pathology, and the American Society of Clinical Oncology by reviewing their respective online journals of Gynecologic Oncology, The International Journal of Gynecological Cancer, Journal of Lower Genital Tract Diseases and Journal of Clinical Oncology.
We searched the following databases for ongoing trials.
Metaregister (http://www.controlled‐trials.com/rct).
Physicians Data Query (http://www.nci.nih.gov).
http://www.clinicaltrials.gov.
http://www.cancer.gov/clinicaltrials.
Handsearching
We checked the references from published studies to identify additional trials.
Data collection and analysis
The methodology of the review was based on the guidelines stated in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011).
We downloaded all titles and abstracts retrieved by electronic searching and removed duplicates. Three review authors (originally OMS and CWH, and recently SMG and CWH) examined the remaining references independently. We excluded those studies which clearly did not meet the inclusion criteria and obtained copies of the full text of potentially relevant references. Three review authors (originally OMS and CWH, and recently SMG and CWH) independently assessed the eligibility of retrieved papers. The review authors resolved disagreements by discussion. There was no disagreement that required resolution from a third review author. We documented reasons for exclusion.
Selection of studies
Three review authors (originally OMS and CWH, and recently SMG and CWH) searched the titles and abstracts from the initial computerised searches for potential trials to include. The review authors then independently assessed the full text of these provisionally included studies to determine whether a study met the inclusion criteria. We resolved disagreement by consensus.
Data extraction and management
We analysed the included studies for the following data.
Author, year of publication and journal citation (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology
-
Study population
Total number enrolled
Patient characteristics
Age
Co‐morbidities
Other baseline characteristics
-
Intervention details
Patients receiving NSAIDs of any type or route for the purposes of CIN treatment were considered in the intervention group
-
Comparison
Patients used as the controls in the selected studies (not receiving NSAIDs) were used as the comparison
Risk of bias
Duration of accrual
Compliance
Duration of follow‐up
Outcomes: For each outcome we extracted the outcome definition
Results: We extracted the number of participants allocated to each intervention group, the total number analysed for each outcome, and the missing participants
If reported, we extracted both unadjusted and adjusted statistics.
Where possible, all data extracted were relevant to an intention‐to‐treat analysis, in which participants are analysed in the groups to which they were assigned.
Three review authors (initially OMS and CWH and later SMG and CWH) independently extracted data onto a data abstraction form specially designed for the review. Differences between review authors were resolved by discussion.
Assessment of risk of bias in included studies
We assessed the risk of bias in included RCTs using Cochrane's 'Risk of bias' tool and the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included assessment of the following domains.
Selection bias: Random sequence generation and allocation concealment
Performance bias: Blinding of participants and personnel (patients and treatment providers)
Detection bias: Blinding of outcome assessment
Attrition bias: Incomplete outcome data. We recorded the proportion of participants whose outcomes were not reported at the end of the study and considered greater than 20% attrition to be at a high risk of bias
Reporting bias: Selective reporting of outcomes
Other possible sources of bias
Three review authors (initially OMS and CWH and later SMG and CWH) applied the 'Risk of bias' tool (Appendix 4) independently and differences were resolved by discussion. We have presented results in a 'Risk of bias' graph and 'Risk of bias' summary and interpreted the results of the meta‐analyses in light of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment.
For time to event data, we planned to use the hazard ratio (HR), if possible.
For dichotomous outcomes, we used the risk ratio (RR).
Dealing with missing data
We did not impute missing outcome data for the primary outcome.
Assessment of reporting biases
Given the description of outcomes reported in the studies, we did not examine funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects such as publication bias.
Data synthesis
Statistical analysis was performed using Review Manager 5.3 software (RevMan 2014). Where appropriate, we pooled results of comparable trials using a fixed‐effect model. We reported results as RRs with their corresponding 95% confidence intervals (CIs). We performed the meta‐analysis using Review Manager 5.3 (RevMan 2014).
Results
Description of studies
Results of the search
For the previous version of this review (Grabosch 2014), the search strategy identified references that we screened by title and abstract in order to identify studies as potentially eligible for the review. From 232 unique references, we identified six articles as potentially eligible for the review. Following the full‐text screening of these six articles, we excluded four for the reasons described in the table 'Characteristics of excluded studies'. The remaining two RCTs met the inclusion criteria and are described in the table 'Characteristics of included studies'. We did not identify any additional relevant studies fro the ancillary searches.
With the updated search, we identified 35 new references with one article meeting the criteria for inclusion (Figure 1).
1.
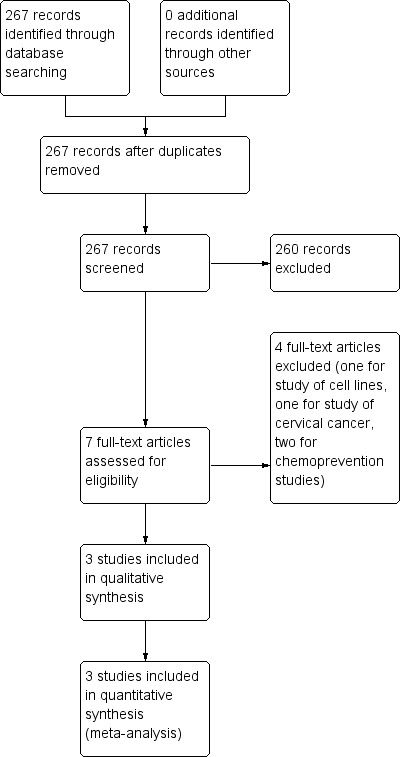
Study flow diagram.
Included studies
The initial two included RCTs randomised a total of 41 women; one study included 25 women (Farley 2006), and the other study 16 women (Hefler 2006). The new study included in this version of the review (Rader 2017) involved 130 women resulting in a total of 171 women included in this review. All three studies used cyclooxygenase‐2 (COX‐2) inhibitors.
The studies were performed as a multi‐institution United States trial (Rader 2017), and two single‐institution trials at Tripler United States Army Medical Center in Hawaii, USA (Farley 2006), and Medical University of Vienna, Austria (Hefler 2006), respectively. The Austrian study was closed early when rofecoxib was withdrawn from the market, due to potential toxicity (Hefler 2006). The mean age of the treatment and placebo groups was 23 years ± 4 years and 22.5 years ± 3 years (overall range 19 to 27 years) (Farley 2006), and 27.1 years ± 3.3 years and 31.2 years ± 5.5 years (overall range 24 to 36 years) (Hefler 2006). The multi‐institution trial did not include a median age for the groups; however, overall age range was 18 to 49 years (Rader 2017). Thirty‐one patients had CIN 2, 25 patients had CIN 2‐3, and 76 had CIN 3. The treatment groups received celecoxib 400 mg once daily by mouth for 14 to 18 weeks (Rader 2017), celecoxib 200 mg twice daily by mouth for six months (Farley 2006), and rofecoxib 25 mg by mouth daily (Hefler 2006). In all studies the comparison arm received placebo tablets (Characteristics of included studies).
Excluded studies
We excluded four studies because they were reviews of chemoprevention (Mitchell 2007; Vlastos 2003), a clinical study of celecoxib together with chemoradiation for the treatment of cervical cancer (Herrera 2007), and a laboratory investigation of celecoxib in cervical cancer cell lines (Ferrandina 2003) (Characteristics of excluded studies).
Risk of bias in included studies
We assessed the risk of bias as being lower in the completed study (Farley 2006), as the randomisation code in the other study (Hefler 2006) was broken when rofecoxib could no longer be given to patients (Characteristics of included studies), (Figure 2; Figure 3).
2.
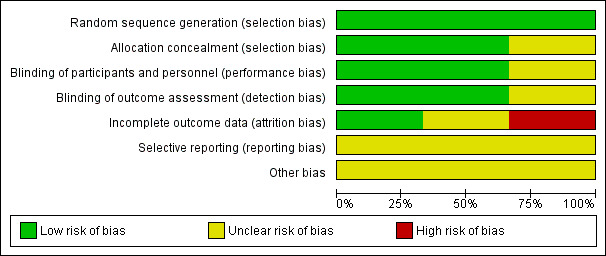
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
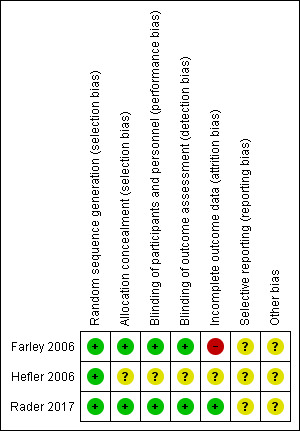
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Overall, the multi‐institution trial (Rader 2017) had a low risk of bias, but it should be noted that 91 of 130 (70%) enrolled completed the study with evaluable tissue thus contributing to possible attrition bias.
Allocation
Sequence generation
Adequacy of randomisation was confirmed in all three studies (Farley 2006; Hefler 2006; Rader 2017).
Allocation
Concealment of allocation was low risk in Farley 2006, and Rader 2017, but was unclear for Hefler 2006. (Characteristics of included studies)
Blinding
Performance bias was assessed as being at low risk of bias in Farley 2006 and Rader 2017 where both patients and the examining physicians were blinded to the treatment arm to which the patients were randomised, and unclear in Hefler 2006, since the statistician and pathologist were blinded at all times, but the randomisation code was broken (Characteristics of included studies).
Detection bias was assessed as low risk of bias with physicians, patients and pathologist blinded at all times in Farley 2006 and Rader 2017, but unclear in Hefler 2006 because of the broken code (Characteristics of included studies).
Incomplete outcome data
Attrition bias was assessed as high risk in Farley 2006 because "five patients (20%) discontinued the study early but were included in the final statistical analysis" and unclear in Hefler 2006 (Characteristics of included studies). For Rader 2017, we have assessed this as low risk given that the higher number of patients included in the study decreases the potential for bias even with a high loss of eligible/evaluable patients and that the analysis was performed on both the patients completing the trial and as an intent‐to‐treat analysis.
Selective reporting
There was insufficient information to assess in the studies.
Other potential sources of bias
One study closed early (Farley 2006), but otherwise, there are insufficient data to identify whether other sources of bias exist.
Effects of interventions
See: Table 1
Efficacy
Primary outcomes
1. Progression of CIN to higher grades of cervical intraepithelial neoplasia (CIN)
Progression to a higher grade of CIN occurred in one out of 12 (8%) of those receiving celecoxib and two out of 13 (15%) receiving placebo (risk ratio (RR) 0.54, 95% confidence interval (CI) 0.06 to 5.24; P value 0.60) (Analysis 1.1, one study, 25 participants; very low‐certainty evidence).
1.1. Analysis.
Comparison 1 Progression of CIN, Outcome 1 Progression of CIN to higher grade of CIN.
The Heffler study did not comment on progression (Hefler 2006). There was no progression of disease in Rader 2017.
2. Progression of CIN to invasive carcinoma
Neither single‐institution study reported on progression to invasive cancer (Farley 2006; Hefler 2006). There was no progression of disease in Rader 2017.
3. Regression of CIN
Partial or complete regression of CIN 2 or CIN 3 occurred in 31 out of 70 (44%) in the treatment arms and 19 of 62 (31%) in the placebo arms (RR 1.45, 95% CI 0.93 to 2.27; P value 0.10) (Analysis 2.1: three studies, 132 participants; moderate‐certainty evidence).
2.1. Analysis.
Comparison 2 Regression of CIN 2 and 3, Outcome 1 Partial or complete regression of CIN 2 or CIN 3.
Complete regression of CIN 2 or CIN 3 occurred in 15 of 62 (24%) of those receiving celecoxib versus 10 of 54 (19%) of those receiving placebo (RR 1.31, 95% CI 0.65 to 2.67; P value 0.45 (Analysis 2.2; two studies, 116 participants; moderate‐certainty evidence).
2.2. Analysis.
Comparison 2 Regression of CIN 2 and 3, Outcome 2 Complete regression of CIN 2 or CIN 3.
Partial regression of CIN 2 or CIN 3 occurred in 14 of 62 (23%) of those receiving celecoxib versus 8 of 54 (15%) of those receiving placebo (RR 1.56, 95% CI 0.72 to 3.40; P value 0.26) (Analysis 2.3, two studies, 116 participants; moderate certainty evidence).
2.3. Analysis.
Comparison 2 Regression of CIN 2 and 3, Outcome 3 Partial regression of CIN 2 or CIN 3.
Secondary outcome
Adverse events related to treatment were not reported in either single‐institution study (Farley 2006; Hefler 2006). Other than one Grade 3 adverse event in the treatment group of Rader 2017, the grade 1 and 2 toxicities were similar between the treatment and placebo arms.
Discussion
The pursuit of non‐invasive methods to prevent the progression of cervical intraepithelial neoplasia (CIN) remains important in order to allow women to avoid the potentially serious morbidities of invasive treatments, including pain, bleeding, infection, cervical stenosis and premature birth in a subsequent pregnancy, and to reduce the healthcare costs involved in the prevention of invasive carcinoma of the cervix. Although there are sound theoretical reasons why non‐steroidal anti‐inflammatory agents (NSAIDs) might have an effect on prevention of progression of CIN (as detailed in the Background), the findings of this review are not surprising in view of the small size and quality of the published data that were identified. The questions can only be resolved through the performance of appropriately designed clinical trials; such trials are difficult to perform in this setting.
It is important to note that any therapeutic benefit demonstrated as a result of further clinical trials will need to be weighed against the possible adverse effects of both NSAIDs and cyclooxygenase‐2 (COX‐2) inhibitors that have been identified in large chemoprevention studies for colorectal adenomas.
In light of the causal association of high‐risk human papilloma virus (HPV) infection with the development and progression of CIN (Munoz 2006), and the high negative predictive value of clearance of high‐risk HPV infection following treatment, such testing might be an important factor to incorporate in future studies (Paraskevaidis 2004). However, HPV‐16 status did not seem to contribute to celecoxib response with 36.2% and 38.5% responding with and without the virus in the newly reviewed trial (Rader 2017).
Summary of main results
We found no convincing evidence to support the use of NSAIDS in CIN 2/3 to induce complete or partial regression, or to prevent progression to higher grades.
Overall completeness and applicability of evidence
This update includes recently published data which adds to the body of literature that unfortunately, while well‐tolerated, NSAIDs do not prove effective in the treatment of CIN significantly more than the disease is naturally cleared while on placebo. With the addition of this larger trial, the authors feel that this topic has been adequately explored.
Quality of the evidence
The trials in our original review appear to have been well‐conducted although they were of small size and one was closed early due to withdrawal of the study agent. With the addition of the multi‐institution randomised trial in this update (Rader 2017), we have upgraded the evidence to moderate certainty according to GRADE criteria given that the addition of the new trial quadrupled the number of patients in our original review and was a well‐designed multi‐centre trial (Table 1). Given that the natural history of CIN lends to some regression without intervention and no convincing evidence that NSAIDs have a significant impact over placebo, we do not feel that further research would alter the conclusions.
Potential biases in the review process
We undertook a comprehensive search to identify eligible studies. Three review authors extracted data independently. It is possible that there are other incomplete and unreported studies that have not been identified. We chose to retain data from the use of rofecoxib (removed from the market in 2004) in the review as findings may be similar to other COX‐2 inhibitors.
Agreements and disagreements with other studies or reviews
We did not identify any relevant studies or reviews.
Authors' conclusions
Implications for practice.
In this updated review, there is sufficient evidence to determine that, while overall safe and well‐tolerated, non‐steroidal anti‐inflammatory agents (NSAIDs) are not efficacious in the treatment of cervical intraepithelial neoplasia (CIN). The addition of the multi‐centre, randomised trial in this review to the two small short‐term studies reviewed previously now provides high‐quality support for these conclusions. For those patients strongly opposed to surgery, an NSAID trial would not be unreasonable but close surveillance should be implemented with surgical intervention if no response is achieved.
Implications for research.
Further understanding of the molecular mechanisms and pathways involved in cancer development and the interactions of NSAIDs with these pathways could lead to more effective chemo‐preventive agents without substantial toxicity, and should be pursued. Although there is currently insufficient evidence to promote use of NSAIDs for treatment of CIN, further studies may support a medical management approach, and save women (especially in their child‐bearing years) unnecessary procedures, particularly as personalised, targeted treatment is being emphasised in medicine. Biomarker analysis prior to, and following treatment, could lead to further understanding of regression of CIN seen with NSAIDs.
What's new
| Date | Event | Description |
|---|---|---|
| 24 January 2019 | Review declared as stable | A new study was identified with a search in August 2017 but this new information did not change the conclusions of the review. The conclusions of this Cochrane review are considered to be up to date for this topic. A scoping search of the literature will be reviewed in 2022. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 4, 2014
| Date | Event | Description |
|---|---|---|
| 13 November 2017 | New search has been performed | Literature searches updated in August 2017. |
| 13 November 2017 | New citation required but conclusions have not changed | One additional randomised controlled trial (Rader 2017) included but conclusions remain unchanged. |
Acknowledgements
We would like to acknowledge Judith Wulff for her help. We thank Aaron Howell, Administrative Assistant, Division of Gynecologic Oncology; James Graham, Brown Cancer Center, University of Louisville, USA for assistance with the first phase of this review; Jo Morrison for clinical and editorial advice; Jo Platt for designing and running the searches and Gail Quinn, Clare Jess and Tracey Harrison for their contribution to the editorial process.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Uterine Cervical Neoplasms] this term only # 2 MeSH descriptor: [Uterine Cervical Dysplasia] this term only #3 MeSH descriptor: [Cervical Intraepithelial Neoplasia] this term only #4 (cervi* near/5 (neoplas* or tumor* or tumour* or malignan* or carcinoma* or cancer* or dysplas* or intraepithel* or intra‐epithel*)) #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Anti‐Inflammatory Agents] explode all trees #7 (anti‐inflammatory or antiinflammatory) #8 NSAID* #9 (cyclo‐oxygenase or cyclooxygenase) #10 #6 or #7 or #8 or #9 #11 #5 and #10
Appendix 2. MEDLINE search strategy
1 Uterine Cervical Neoplasms/ 2 Uterine Cervical Dysplasia/ 3 Cervical Intraepithelial Neoplasia/ 4 (cervi* adj5 (neoplas* or tumour* or tumour* or malignan* or carcinoma* or cancer* or dysplas* or intraepithel* or intra‐epithel*)).mp. 5 1 or 2 or 3 or 4 6 exp Anti‐Inflammatory Agents/ 7 (anti‐inflammatory or antiinflammatory).mp. 8 NSAID*.mp. 9 (cyclo‐oxygenase or cyclooxygenase).mp. 10 6 or 7 or 8 or 9 11 5 and 10 12 randomised controlled trial.pt. 13 controlled clinical trial.pt. 14 randomized.ab. 15 placebo.ab. 16 drug therapy.fs. 17 randomly.ab. 18 trial.ab. 19 groups.ab. 20 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21 11 and 20 22 exp animals/ not humans.sh. 23 21 not 22 key: mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt=publication type ab=abstract sh=subject heading ti=title
Appendix 3. Embase search strategy
1 exp uterine cervix tumour/ 2 uterine cervix dysplasia/ 3 uterine cervix carcinoma in situ/ 4 (cervi* adj5 (neoplas* or tumour* or tumour* or malignan* or carcinoma* or cancer* or dysplas* or intraepithel* or intra‐epithel*)).mp. 5 1 or 2 or 3 or 4 6 exp antiinflammatory agent/ 7 (anti‐inflammatory or antiinflammatory).mp. 8 NSAID*.mp. 9 (cyclo‐oxygenase or cyclooxygenase).mp. 10 6 or 7 or 8 or 9 11 5 and 10 12 exp controlled clinical trial/ 13 crossover procedure/ 14 double‐blind procedure/ 15 randomised controlled trial/ 16 single‐blind procedure/ 17 random*.mp. 18 factorial*.mp. 19 (crossover* or cross over* or cross‐over*).mp. 20 placebo*.mp. 21 (double* adj blind*).mp. 22 (singl* adj blind*).mp. 23 assign*.mp. 24 allocat*.mp. 25 volunteer*.mp. 26 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27 11 and 26 28 (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 29 27 not 28
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Appendix 4. Risk of bias tool
We applied this tool to included studies to assess the risk of bias.
1. Random sequence generation
Low risk of bias e.g. participants assigned to treatments on basis of a computer‐generated random sequence or a table of random numbers.
High risk of bias e.g. participants assigned to treatments on basis of date of birth, clinic ID number or surname, or no attempt to randomise participants.
Unclear risk of bias e.g. not reported, information not available.
2. Allocation concealment
Low risk of bias e.g. where the allocation sequence could not be foretold.
High risk of bias e.g. allocation sequence could be foretold by patients, investigators or treatment providers.
Unclear risk of bias e.g. not reported.
3. Blinding of participants and personnel
Low risk of bias if participants and personnel were adequately blinded.
High risk of bias if participants were not blinded to the intervention that the participant received.
Unclear risk of bias if this was not reported or unclear.
4. Blinding of outcomes assessors
Low risk of bias if outcome assessors were adequately blinded.
High risk of bias if outcome assessors were not blinded to the intervention that the participant received.
Unclear risk of bias if this was not reported or unclear.
5. Incomplete outcome data
Low risk of bias, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms.
High risk of bias, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms.
Unclear risk of bias, if loss to follow‐up was not reported.
6. Selective reporting of outcomes
Low risk of bias e.g. reports all outcomes specified in the protocol.
High risk of bias e.g. it is suspected that outcomes have been selectively reported.
Unclear risk of bias e.g. if it is unclear whether outcomes have been selectively reported.
7. Other bias
Low risk of bias if you do not suspect any other source of bias and the trial appears to be methodologically sound.
High risk of bias if you suspect that the trial was prone to an additional bias.
Unclear risk of bias if it is uncertain whether an additional bias may have been present.
Data and analyses
Comparison 1. Progression of CIN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progression of CIN to higher grade of CIN | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.06, 5.24] |
Comparison 2. Regression of CIN 2 and 3.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Partial or complete regression of CIN 2 or CIN 3 | 3 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.93, 2.27] |
| 2 Complete regression of CIN 2 or CIN 3 | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.65, 2.67] |
| 3 Partial regression of CIN 2 or CIN 3 | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.72, 3.40] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Farley 2006.
| Methods | True randomisation with allocation by computer‐generated blocks. Parallel design without cross‐over. Patient, provider, pathologist blinded. All pathologic specimens were reviewed by a blinded central pathologist. Enrollment June 2002 to October 2003. Analysis was by ITT | |
| Participants | 25 women 18 years or older with biopsy proven CIN 2 or CIN 3. Exclusions included: pregnancy or breast feeding, history of stomach bleeding, NSAID allergy, severe kidney disease (such as creatinine greater than 1.2 mg/dL) or liver problems (such as AST/ALT greater than 80 U/L), immunosuppression, NSAIDs use for other medical conditions, or NSAID‐associated asthma. Also women with positive dysplasia on endocervical curettage or cervical cytology diagnosis of atypical glandular cells, carcinoma in situ, adenocarcinoma in situ, or invasive carcinoma. Age range 19 to 27 years. Race distribution not stated. Study performed at Tripler Army Medical Center, Hawaii, USA. | |
| Interventions | Celecoxib 200 mg or placebo by mouth twice daily with meals for six months or until progression of dysplasia. All patients underwent initial colposcopy, thin‐prep liquid‐based cervical cytology, reflex HPV testing, and colpography of lesion with specimens reviewed by a blinded pathologist. Patients were seen at eight‐week intervals for six months with thin‐prep liquid‐based cervical cytology, colposcopy, photography of the cervix and biopsy. If colposcopy was normal, representative biopsy was taken from the area of previous dysplastic abnormality. Participants were removed from the study and underwent loop electrosurgical excision procedure (LEEP) for any increase in severity of CIN on cytology or histology. After six months, persistent CIN 3 required removal from study and treatment with LEEP. Participants missing an eight‐week follow‐up were contacted and asked to return for evaluation as soon as possible. Anyone not attending follow‐up was removed from the study and treated with LEEP | |
| Outcomes | Primary objectives were to determine response rate to treatment and toxicity of treatment. Toxicity was assessed at each eight‐week follow‐up visit by the treating pharmacist by direct questioning about the most common side effects of celecoxib. No toxicity was reported by participants.
Response was defined as any decrease in severity of CIN on histology. A complete response was defined as a complete visual resolution of the lesion on colposcopy and normal thin‐prep liquid‐based cytology and tissue histology. Partial response was defined as a decrease in severity of CIN on histology with no cytologic change in severity of CIN. Stable disease defined as no change in histologic degree of CIN from entry cervical biopsy. Progression was defined as any increase in severity of CIN on cytology or histology. Of the 25 participants 13 were randomised to the placebo and 12 to the treatment arm. Five participants (two in the treatment and three in the placebo arm) discontinued the study early because they desired definitive treatment. Overall response was N = 4 (31%) placebo versus N = 9 (75%) treatment. Mean time to response was 72 (+/‐ 38) days in the placebo arm and 73 (+/‐ 26) days in the treatment arm (P value 0.39). Complete response N = 2 (15%) placebo versus N = 4 (33%) treatment. Partial response was N = 2 (15%) placebo versus N = 5 (42%) treatment. Progression to higher degree of CIN was N = 2 (15%) placebo and N = 1 (8%) treatment arm with a mean time to progression of 65 days. No patients progressed to invasive carcinoma. The treatment arm was 2.5 x more likely to have regression of their cervical dysplasia |
|
| Notes | 100% of treatment arm women were positive for high‐risk types of HPV by Hybrid Capture II in comparison with 85% in the placebo arm. Eleven of 12 (92%) women in the treatment arm and 11 of 13 (85%) in the placebo arm had CIN 2 with 1 of 12 (8%) in the treatment arm and 2 of 13 (15%) in the placebo arm having CIN 3. Two women in the treatment arm regressed from CIN 2 to CIN 1 in eight weeks, but wished then to be treated with LEEP, having final diagnoses of moderate dysplasia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer generator Random Allocation Software, which randomised each participant to a single treatment, by using the method of randomly permuted blocks |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was accomplished by the Department of Pharmacy. All physicians who participated in the implementation of the treatments, medication or placebo, were blinded to the randomisation process. Only the Pharmacy co‐ordinator (E.G.) was aware of the allocation of the medications. Participants obtained their medications from E.G. in a separate encounter to their clinical follow‐up |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the patients and the examining physicians were blinded to the treatment option to which the patients were randomised |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both the patients and the examining physicians were blinded to the treatment option to which the patients were randomised |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Five patients (20%) discontinued the study early but were included in the final statistical analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Hefler 2006.
| Methods | True randomisation with allocation by computer‐generated blocks. Parallel design without cross‐over. Patient and physicians were blinded. No mention of pathologist. Analysis by ITT. No mention of toxicity assessment. Enrollment May to October 2005. Study was closed after 87 days due to withdrawal of rofecoxib from the market. Randomisation code broken at this point | |
| Participants | 16 women with histological evidence of CIN 2 or 3, a fully visible transformation zone and lesion margin, compliant patient, and safe contraception.
Exclusions included presence of (micro‐)invasive cancer, endocervical lesion, upper margin of lesion not visible on colposcopy, non‐compliant patient, prior history of an adverse gastrointestinal event (ulcer, haemorrhage), age greater than 60 years; concurrent use of glucocorticoids, or NSAID allergy. Age: range 24 to 36 years, mean 27.1 years for treatment arm and 31.2 years for placebo arm |
|
| Interventions | Rofecoxib 25 mg or placebo, by mouth, daily for three months. After the screening visit a physical examination was performed, a questionnaire was answered and study medication was distributed. At initial visit all patients meeting inclusion criteria were evaluated for HPV status via Hybrid Capture (Digene Corp., Gaithersburg, MD, USA). Follow‐up examinations performed at three and six months and included gynaecological examination with ecto‐ and endocervical cytology smears, colposcopy and biopsy, HPV test and pregnancy test. These modalities were used for the evaluation of regression, persistence, or progression of cervical dysplasia |
|
| Outcomes | Primary outcomes assessed were regression and remission of CIN over a 3‐month treatment period, and secondary were safety and side effects. 16 women participated: eight in the treatment arm (four with CIN 2; four with CIN 3) and eight in the placebo arm (five with CIN 2; three with CIN 3). Regression occurred in two of eight (25%) in the treatment arm and one of eight (12.5%) in the placebo arm (P value 0.9), after a mean of 87 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a random number sequence with permuted block size of 12 |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Randomisation code broken when rofecoxib withdrawn from the market. Statistician and pathologist blinded at all times |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Randomisation code broken when rofecoxib withdrawn from the market. Statistician and pathologist blinded at all times |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Early closure of study. Insufficient information to assess whether an important risk of bias exists |
Rader 2017.
| Methods | Randomised 1:1 with stratification by lesion size (coverage of ≤ 50% of cervix or >50%) and severity (CIN 3 versus CIN 2/3). Enrollment June 2005 through April 2012. | |
| Participants | 130 woman 18 years or older with CIN 3 verified on central pathology review and a visible lesion present by colposcopy after initial biopsy. Exclusion criteria included adenocarcinoma in situ, pregnancy, allergy to sulphonamides, history of cardiovascular disease or uncontrolled hypertension, and renal or hepatic disorders. Multicentre trial. | |
| Interventions | Celecoxib 400 mg daily or placebo. Baseline exam with colposcopy, serum sample, and cervical swabs were obtained. Interval colposcopy repeated at 8 weeks. Treatment continued 14‐18 weeks. | |
| Outcomes | Primary endpoints were regression to CIN 1 or less and estimation of toxicity. Complete response was regression to normal tissue or squamous metaplasia. Partial response was regression to CIN 1. Progressive disease was development of squamous carcinoma and persistent disease was presence of CIN 2 or 3 or squamous carcinoma in‐situ after treatment. 67 patients were randomised to celecoxib with 63 receiving treatment and 50 having evaluable tissue. 63 were randomised to placebo with 58 receiving treatment and 41 having evaluable tissue. One grade 3 gastrointestinal adverse event was noted in the celecoxib group; otherwise, toxicity was similar between arms. Histological responses were present in 20/50 (40%) of the celecoxib arm versus 14/41 (34.1%) in the placebo group. With an ITT analysis, response rates were 20/67 (29.9%) and 14/63 (22.2%) for treatment and placebo. 31/81 (38%) response was noted if the lesion was less than 50% of the cervix versus 3/10 (30%) if greater than 50%. Response rates were similar if HPV‐16 was present or absent at 36.2% and 38.5%. Patients with high serum VEGF levels were more like to response to celecoxib versus placebo at 47.4% and 14.3%. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised in 1:1 fashion. |
| Allocation concealment (selection bias) | Low risk | Celecoxib and placebo supplied by Biologics Inc. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both physicians and patients blinded to treatment group. Translational biological sample analysis was also blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both physicians and patients blinded to treatment group. Translational biological sample analysis was also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a loss of 30% of the patients; however, the larger number of women enrolled reduces the risk for attrition bias. Data were analysed both on patients completing the study and as ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
ALT: alanine aminotransferase AST: aspartate aminotransferase CIN: cervical intraepithelial neoplasia HPV: human papillomavirus ITT: intention‐to‐treat LEEP: loop electrosurgical excision procedure NSAID: non‐steroidal anti‐inflammatory agent VEGF: vascular endothelial growth factor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ferrandina 2003 | Laboratory study of celecoxib in cervical cancer cell lines |
| Herrera 2007 | Phase I‐II study of celecoxib with chemoradiation in patients with cervical cancer |
| Mitchell 1995 | Review of chemoprevention trials and surrogate end point biomarkers in the cervix |
| Vlastos 2003 | Review of biomarkers and their use in cervical cancer chemoprevention |
Differences between protocol and review
Shannon Grabosch MD joined the review group. The search strategy was reworked to make it more effective and an up‐to‐date search was performed. The background was extensively edited and updated. The manuscript was edited to reflect the search and literature information and the updated Cochrane review requirements and guidelines.
Contributions of authors
Link with Cochrane Review Group: CW Helm. Drafted the protocol: CW Helm. Searched for trials: OM Shariff, SM Grabosch, CW Helm. Abstraction of study data: SM Grabosch, OMS Shariff, CW Helm. Writing of the review: SM Grabosch, CW Helm.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Farley 2006 {published data only}
- Farley JH, Truong V, Goo E, Uyehara C, Belnap C, Larsen WI. A randomized double‐blind placebo‐controlled phase II trial of the cyclooxygenase‐2 inhibitor Celecoxib in the treatment of cervical neoplasia. Gynecologic Oncology 2006;103(2):425‐30. [PUBMED: 16677697] [DOI] [PubMed] [Google Scholar]
Hefler 2006 {published data only}
- Hefler LA, Grimm C, Speiser P, Sliutz G, Reinthaller A. The cyclooxygenase‐2 inhibitor rofecoxib (Vioxx) in the treatment of cervical dysplasia grade II‐III. A phase II trial. European Journal of Obstetrics and Gynecology and Reproductive Biology 2005;125(2):251‐4. [PUBMED: 16188370] [DOI] [PubMed] [Google Scholar]
Rader 2017 {published data only}
- Rader JS, Sill MW, Beumer JH, Lankes HA, Benbrook DM, Garcia F, et al. A stratified randomized double‐blind phase II trial of celecoxib for treating patients with cervical intraepithelial neoplasia: The potential predictive value of VEGF serum levels: An NRG Oncology/Gynecologic Oncology Group study. Gynecologic Oncology 2017;145(2):291‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ferrandina 2003 {published data only}
- Ferrandina G, Ranelletti FO, Legge F, Lauriola L, Salutari V, Gessi M, et al. Celecoxib modulates the expression of cyclooxygenase‐2, ki67, apoptosis‐related marker, and microvessel density in human cervical cancer: a pilot study. Clinical Cancer Research 2003;9(12):4324‐31. [PUBMED: 14555502] [PubMed] [Google Scholar]
Herrera 2007 {published data only}
- Herrera FG, Chan P, Doll C, Milosevic M, Oza A, Syed A, et al. A prospective phase I‐II trial of the cyclooxygenase‐2 inhibitor celecoxib in patients with carcinoma of the cervix with biomarker assessment of the tumor microenvironment. International Journal of Radiation Oncology, Biology and Physics 2006;67(1):97‐103. [PUBMED: 17056201] [DOI] [PubMed] [Google Scholar]
Mitchell 1995 {published data only}
- Mitchell MF, Hittelman WK, Lotan R, Nishioka K, Tortolero‐Luna G, Richards‐Kortum R, et al. Chemoprevention trials and surrogate end point biomarkers in the cervix. Cancer 1995;76(10 Suppl):1956‐77. [PUBMED: 8634987] [DOI] [PubMed] [Google Scholar]
Vlastos 2003 {published data only}
- Vlastos AT, Schottenfeld D, Follen M. Biomarkers and their use in cervical cancer chemoprevention. Critical Reviews in Oncology Hematology 2003;46(3):261‐73. [PUBMED: 12791426] [DOI] [PubMed] [Google Scholar]
Additional references
Alvarez 2003
- Alvarez RD, Conner MG, Weiss H, Klug PM, Niwas S, Manne U, et al. The efficacy of 9‐cis retinoic acid (aliretinoin) as a chemopreventive agent for cervical dysplasia: results of a randomized double blind clinical trial. Cancer, Epidemiology, Biomarkers & Prevention 2003;12(2):114‐9. [PUBMED: 12582020] [PubMed] [Google Scholar]
Arber 2006
- Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, et al. PreSAP Trial Investigators. Celecoxib for the prevention of colorectal adenomatous polyps. New England Journal of Medicine 2006;355:885‐95. [PUBMED: 16943401] [DOI] [PubMed] [Google Scholar]
Arbyn 2008
- Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin‐Hirsch P, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta‐analysis. BMJ 2008;337:a1284. [PUBMED: 18801868] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bertagnolli 2006
- Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. APC Study Investigators. Celecoxib for the prevention of sporadic colorectal adenomas. New England Journal of Medicine 2006;355(9):873‐84. [PUBMED: 16943400] [DOI] [PubMed] [Google Scholar]
Bleecker 2014
- Bleecker E, Koehler E, Smith J, Budwit D, Rahangdale L. Outcomes after management of young women with cervical intraepithelial neoplasia 2 with a 6‐month observation protocol. Journal of Lower Genital Tract Disease 2014;18(1):46‐9. [PUBMED: 23959297] [DOI] [PubMed] [Google Scholar]
Cao 2002
- Cao Y, Prescott SM. Many actions of cyclooxygenase‐2 in cellular dynamics and in cancer. Journal of Cell Physiology 2002;190(3):279‐86. [PUBMED: 11857443] [DOI] [PubMed] [Google Scholar]
DeWitt 1991
- DeWitt DL. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochimica et Biophysica Acta 1991;1083(2):121‐34. [PUBMED: 1903657] [DOI] [PubMed] [Google Scholar]
Dursun 2007
- Dursun P, Yuce K, Usubutun A, Ayhan A. Cyclooxygenase‐2 expression in cervical intraepithelial neoplasia III and squamous cell cervical carcinoma, and its correlation with clinicopathologic variables. International Journal of Gynecological Cancer 2007;17(1):164‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eberhart 1994
- Eberhart CE, Coffey RJ, Radhik A. Up‐regulation of cyclooxygenase‐2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994;107(4):1183‐8. [PUBMED: 7926468] [DOI] [PubMed] [Google Scholar]
Elmets 2010
- Elmets CA, Viner JL, Pentland AP, Cantrell W, Lin HY, Bailey H, et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double‐blind, placebo‐controlled trial. Journal of the National Cancer Institute 2010;102(24):1835‐44. [PUBMED: 21115882] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farley 2004
- Farley J, Uyehara C, Hashiro G, Belnap C, Birrer M, Salminen E. Cyclooxygenase‐2 expression predicts recurrence of cervical dysplasia following loop electrosurgical excision procedure. Gynecologic Oncology 2004;92(2):596‐602. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferlay 2010
- Ferlay J, Shin H‐R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010;127(12):2893‐917. [PubMed: : 21351269] [DOI] [PubMed] [Google Scholar]
Ferrandina 2000
- Ferrandina G, Lauriola L, Zannoni GF, Distefano MG, Legge F, Salutari V, et al. Expression of cyclooxygenase‐2 (COX‐2) in tumour and stroma compartments in cervical cancer: clinical implications. Cancer 2002;87(10):1145‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrandina 2002
- Ferrandina G, Lauriola L, Distefano MF, Zannoni GF, Gessi M, Legge F, et al. Increased cyclooxygenase‐2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. Journal of Clinical Oncology 2002;20(4):973‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fischer 1999
- Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubert RA, et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase‐2 inhibitor, and indomethacin against ultraviolet‐light induced skin carcinogenesis. Molecular Carcinogenesis 1999;25(4):231‐40. [PUBMED: 10449029] [PubMed] [Google Scholar]
Fischer 2011
- Fischer SM, Hawk ET, Lubet RA. Coxibs and other nonsteroidal anti‐inflammatory drugs in animal models of cancer prevention. Cancer Prevention Research 2011;4(11):1728‐35. [PUBMED: 21778329] [DOI] [PMC free article] [PubMed] [Google Scholar]
Follen 2001
- Follen M, Atkinson EN, Schottenfeld D, Malpica A, West L, Lippman S, et al. A randomized clinical trial of 4‐hydroxyphenylretinamide for high‐grade squamous intraepithelial lesions of the cervix. Clinical Cancer Research 2001;7(11):3356‐65. [PUBMED: 11705848] [PubMed] [Google Scholar]
Gaffney 2001
- Gaffney DK, Holden J, Davies M. Elevated cyclooxygenase‐2 expression correlates with diminished survival in carcinoma of the cervix treated with radiotherapy. International Journal of Radiation, Oncology, Biology and Physics 2001;49(5):1213‐7. [PUBMED: 11286825] [DOI] [PubMed] [Google Scholar]
Gaffney 2003
- Gaffney DK, Haslam D, Tsodikov A, Hammond E, Seaman J, Holden J, et al. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) negatively affect overall survival in carcinoma of the cervix treated with radiotherapy. International Journal of Radiation Oncology Biology Physics 2003;56(4):922‐8. [PUBMED: 12829126] [DOI] [PubMed] [Google Scholar]
Grosser 2006
- Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX‐2 inhibition: therapeutic challenges and opportunities. Journal of Clinical Investigation 2006;116(1):4‐15. [PUBMED: 16395396] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2000
- Harris RE, Alshafie GA, Abou‐Isaa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Research 2000;60(8):2101‐3. [PUBMED: 10786667] [PubMed] [Google Scholar]
Hershmann 1996
- Herschman HR. Prostaglandin synthase 2. Biochimica et Biophysica Acta 1996;1299(1):125‐40. [PUBMED: 8555245] [DOI] [PubMed] [Google Scholar]
Hia 1992
- Hia T, Neilson K. Human cyclooxygenase‐2 cDNA. Proceedings of National Academy of Science USA 1992;89(16):7384‐8. [PUBMED: 1380156] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huang 1998
- Huang D, Skollard D, Sharma S. Non‐small cell lung cancer cyclooxygenase‐2‐dependent regulation of cytokine balance in lymphocytes and macrophages: up regulation of interleukins 10 and down regulation of interleukins 12 production. Cancer Research 1998;58(6):1208‐16. [MEDLINE: ] [PubMed] [Google Scholar]
Hwang 1998
- Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase‐1 and cyclooxygenase‐2 in human breast cancer. Journal of the National Cancer Institute 1998;90(6):455‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jaisamrarn 2013
- Jaisamrarn U, Castellsagué X, Garland SM, Naud P, Palmroth J, Rosario‐Raymundo MR, et al. HPV PATRICIA Study Group. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PLOS One 2013;8(11):e79260. [PUBMED: 24260180] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawamori 1998
- Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase‐2 inhibitor against colon carcinogenesis. Cancer Research 1998;58(3):409‐12. [PUBMED: 9458081] [PubMed] [Google Scholar]
Kelloff 1996
- Kelloff GJ, Boone CW, Crowell JA, Steele VE, Lubet RA, Doody LA. New agents for cancer chemoprevention. Journal of Cellular Biochemistry 1996;26S:1‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2002
- Kim YB, Kim GE, Cho NH, Pyo HR, Shim SJ, Chang SK, et al. Overexpression of cyclooxygenase‐2 is associated with a poor prognosis in patients with squamous cell carcinoma of the uterine cervix treated with radiation and concurrent chemotherapy. Cancer 2002;95(3):531‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2003
- Kim MH, Seo SS, Song YS, Kang DH, Park IA, Kang SB, et al. Expression of cyclooxygenase‐1 and ‐2 associated with expression of VEGF in primary cervical cancer and at metastatic lymph nodes. Gynecologic Oncology 2003;90(1):83‐90. [PUBMED: PMID: 12821346] [DOI] [PubMed] [Google Scholar]
Kim 2005
- Kim JY, Lim SJ, Park K, Lee CM, Kim J. Cyclooxygenase‐2 and c‐erbB‐2 expression in uterine cervical neoplasm assessed using tissue microarrays. Gynecologic Oncology 2005;97(2):337‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kulkarni 2001
- Kulkarni S, Rader JS, Zhang F, Liapis H, Koki AT, Masferrer JL, et al. Cyclooxygenase‐2 is overexpressed in human cervical cancer. Clinical Cancer Research 2001;7:429‐34. [MEDLINE: ] [PubMed] [Google Scholar]
Lim 2000
- Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, et al. Increased expression of cyclooxygenase‐2 protein in human gastric carcinoma. Clinical Cancer Research 2000;6(2):519‐25. [MEDLINE: ] [PubMed] [Google Scholar]
Liu 2000
- Liu XH, Kirschenbaum A, Yao S, Lee R, Holland JF, Levine AC. Inhibition of cyclooxygenase‐2 suppresses angiogenesis and the growth of prostate cancer in vivo. Journal of Urology 2000;164(3):820‐5. [PUBMED: 10953162] [DOI] [PubMed] [Google Scholar]
Martin‐Hirsch 2010
- Martin‐Hirsch PPL, Paraskevaidis E, Bryant A, Dickinson HO, Keep SL. Surgery for cervical intraepithelial neoplasia. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD001318.pub3; PUBMED: 20556751] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2007
- Mitchell A, Newton JM, Brite K, Einspahr J, Ellis M, Davis J, et al. Cyclooxygenase 2 expression in cervical intraepithelial neoplasia and vulvar cancer. Journal of Lower Genital Tract Disease 2007;11(2):80‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mohammed 1999
- Mohammed SI, Knapp DW, Bostwick DG, Foster RS, Khan KN, Masferrer JL, et al. Expression of cyclooxygenase‐2 (COX‐2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Research 1999;59(22):5647‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Munkarah 2005
- Munkarah A, Ali‐Fehmi R. COX‐2: a protein with an active role in gynecological cancers. Current Opinion in Obstetrics and Gynecology 2005;17(1):49‐53. [PUBMED: 15711411] [DOI] [PubMed] [Google Scholar]
Munoz 2006
- Munoz N, Castellsague X, Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006;24(Suppl 3):S1‐S10. [PUBMED: 16949995] [DOI] [PubMed] [Google Scholar]
Narisawa 1981
- Narisawa T, Sato M, Tani M, Kudo T, Takahashi T, Goto A. Inhibition of development of methylnitrosourea‐induced rat colon tumors by indomethacin treatment. Cancer Research 1981;41(5):1954‐7. [PUBMED: 7214363] [PubMed] [Google Scholar]
Okajima 1998
- Okajima E, Denda A, Ozono S, Takahama M, Akai H, Sasaki Y, et al. Chemopreventive effects of nimesulide, a selective cyclooxygenase‐2 inhibitor, on the development of rat urinary bladder carcinomas initiated by N‐butyl‐N‐(4‐hydroxybutyl) nitrosamine. Cancer Research 1998;58(14):3028‐31. [PUBMED: 9679967] [PubMed] [Google Scholar]
Paraskevaidis 2004
- Paraskevaidis E, Arbyn M, Sotiriadis A, Diakomanolis E, Martin‐Hirsch P, Koliopoulos G, et al. The role of HPV DNA testing in the follow‐up period after treatment for CIN: a systematic review of the literature. Cancer Treatment Reviews 2004;30:205‐11. [PUBMED: 15023438] [DOI] [PubMed] [Google Scholar]
Pockaj 2004
- Pockaj BA, Basu GD, Pathangey LB, Gray RJ, Hernandez JL, Gendler SJ, et al. Reduced T‐cell and dendritic cell function is related to cyclooxygenase‐2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Annals of Surgical Oncology 2004;11(3):328‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Psaty 2006
- Psaty BM, Potter JD. Risks and benefits of celecoxib to prevent recurrent adenomas. New England Journal of Medicine 2006;355(9):950‐2. [PUBMED: 16943408] [DOI] [PubMed] [Google Scholar]
Reddy 2000
- Reddy BS, Hirose Y, Lubet R, Steele V, Kelloff G, Paulson S, et al. Chemoprevention of colon cancer by specific cyclooxygenase‐2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Research 2000;60(2):293‐7. [PUBMED: 10667579] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryu 2000
- Ryu HS, Chang KH, Yang HW, Kim MS, Kwon HC, Oh KS. High cyclooxygenase‐2 expression in stage 1B cervical cancer with lymph node metastasis or parametrial invasion. Gynecologic Oncology 2000;76(3):320‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shamma 2000
- Shamma A, Yamamoto H, Doki Y, Okami J, Kondo M, Fujiwara Y, et al. Up‐regulation of cyclooxygenase‐2 in squamous carcinogenesis of the oesophagus. Clinical Cancer Research 2000;6(4):1229‐38. [MEDLINE: ] [PubMed] [Google Scholar]
Shariat 2003
- Shariat SF, Kim JH, Ayala GE, Kho K, Wheeler TM, Lerner SP. Cyclooxygenase‐2 is highly expressed in carcinoma in situ and T1 transitional cell carcinoma of the bladder. Journal of Urology 2003;169(3):938‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shirahama 2000
- Shirahama T. Cyclooxygenase‐2 expression is up‐regulated in transitional cell carcinoma and its preneoplastic lesions in the human urinary bladder. Clinical Cancer Research 2000;6(6):2424‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Sobolewski 2010
- Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase‐2 in cell proliferation and cell death in human malignancies. International Journal of Cell Biology 2010 Mar 17 [Epub ahead of print]. [PUBMED: 20339581] [DOI] [PMC free article] [PubMed]
Soslow 2000
- Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, et al. COX‐2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 2000;89(12):2637‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Soutter 1997
- Soutter WP, Barros Lopes A, Fletcher A, Monaghan JM, Duncan ID, Paraskevaidis E, et al. Invasive cervical cancer after conservative therapy for cervical intraepithelial neoplasia. Lancet 1997;349(9057):978‐80. [PUBMED: 9100623] [DOI] [PubMed] [Google Scholar]
Stolina 2000
- Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, et al. Specific inhibition of cyclooxygenase‐2 restores tumor reactivity by altering the balance of IL‐10 and IL‐12 synthesis. Journal of Immunology 2000;164(1):361‐70. [PUBMED: 10605031] [DOI] [PubMed] [Google Scholar]
Surh 2005
- Surh Y‐J, Kundu JM. Signal transduction network leading to COX‐2 induction: a road map in search of cancer chemopreventives. Archives of Pharmacal Research 2005;28(1):1‐15. [PUBMED: 15742801] [DOI] [PubMed] [Google Scholar]
Tan 2005
- Tan KB, Putti TC. Cyclooxygenase 2 expression in nasopharyngeal carcinoma: immunohistochemical findings and potential implications. Journal of Clinical Pathology 2005;58(5):535‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsujii 1995
- Tsujii M, Dubois RN. Alterations in cellular adhesion and apoptosis in epithelial cells over expressing prostaglandin endoperoxide synthase 2. Cell 1995;83(3):493‐501. [PUBMED: 8521479] [DOI] [PubMed] [Google Scholar]
Tsujii 1997
- Tsujii M, Kawano S, DuBois RN. Cyclooxygenase‐2 expression in human colon cancer cells increases metastatic potential. Proceedings of National Academy of Science USA 1997;94(7):3336‐40. [PUBMED: 9096394] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsujii 1998
- Tsujii M, Kawano S, Tsujii S, Sawaoka H, Hori M, Dubois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998;93(5):705‐16. [PUBMED: 9630216] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Grabosch 2014
- Grabosch SM, Shariff OM, Wulf JL, Helm CW. Non‐steroidal anti‐inflammatory agents to induce regression and prevent the progression of cervical intraepithelial neoplasia. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD004121.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Helm 2003
- Helm CW, Meyer NJ. Anti‐inflammatory agents for preventing the progression of cervical intraepithelial neoplasia. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004121] [DOI] [Google Scholar]
Shariff 2008
- Shariff OM, Wulff JL, Helm CW. Anti‐inflammatory agents for preventing the progression of cervical intraepithelial neoplasia. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD004121.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


