Abstract
Non-typhoidal Salmonella (NTS) bacteremia is a significant cause of morbidity and mortality worldwide. It is considered to be an emerging and neglected tropical disease in Africa. We studied this in two tertiary hospitals–Al Farwaniya and Al Amiri–in Kuwait, a subtropical country, from April 2013-May 2016. NTS bacteremia was present in 30 of 53,860 (0.75%) and 31 of 290,36 (1.33%) blood cultures in the two hospitals respectively. In Al Farwaniya hospital, one-third of the patients were from some tropical developing countries of Asia. About 66% of all patients (40/61) had diarrhea, and of these, 65% had the corresponding blood serovar isolated from stool culture. A few patients had Salmonella cultured from urine. Patients were either young or old. Most of the patients had co-morbidities affecting the immune system. Two patients each died in both hospitals. The number of different serovars cultured in each hospital was 13, and most infections were due to S. Enteritidis (all sequence type [ST]) 11) and S. Typhimurium (all ST19) except in a subgroup of expatriate patients from tropical developing countries in Al Farwaniya hospital. About a quarter of the isolates were multidrug-resistant. Most patients were treated with a cephalosporin with or without other antibiotics. S. Enteritidis and S. Typhimurium isolates were typed by pulsed field-gel electrophoresis (PFGE) and a selected number of isolates were whole-genome sequenced. Up to four different clades were present by PFGE in either species. Whole-genome sequenced isolates showed antibiotic-resistance genes that showed phenotypic correlation, and in some cases, phenotypes showed absence of specific genes. Whole-genome sequenced isolates showed presence of genes that contributed to blood-stream infection. Phylogeny by core genome analysis showed a close relationship with S. Typhimurium and S. Enteritidis from other parts of the world. The uniqueness of our study included the finding of a low prevalence of infection, mortality and multidrug-resistance, a relatively high prevalence of gastrointestinal infection in patients, and the characterization of selected isolates of S. Typhimurium and S. Enteritidis serovars by whole-genome sequencing that shed light on phylogeny, virulence and resistance. Similarities with studies from developing countries especially Africa included infection in patients with co-morbidities affecting the immune system, predominance of S. Typhimurium and S. Enteritidis serovars and presence of drug-resistance in isolates.
Author summary
Salmonella organisms are classified into typhoidal Salmonella (causing enteric fever) and non-typhoidal Salmonella (NTS) (causing infections other than enteric fever). Apart from causing other infections, NTS causes blood-stream infection (bacteremia and septicemia). NTS blood stream infection (NTS-BI) is considered to be an emerging and neglected tropical disease in Africa. It causes a very high morbidity and mortality in Africa. The individuals affected in Africa are children, malnourished people, patients with malaria or HIV etc. These conditions affect the immune system and make them vulnerable to infection with NTS. In these patients, diarrheal disease due to NTS is rare. The majority of infections are due to two types of NTS: Typhimurium and Enteritidis. There is a very high prevalence of multidrug-resistance in NTS making the infection difficult to treat. NTS-BI is also present in other parts of the world including developed countries albeit at a lower prevalence. Kuwait is a high-income, subtropical country in transition (from a developing to developed country), located in the Middle East. We studied NTS-BI in Al Farwaniya and Al Amiri hospitals in Kuwait during April 2013 to May 2016. Out of nearly 30,000 to more than 50,000 blood cultures done in these hospitals, NTS was present in 0. 75 to 1.33% of blood cultures, representing a very small proportion of blood cultures, unlike in Africa. This showed that 31 patients in Al Farwaniya hospital and 30 patients in Al Amari hospital had NTS-BI. Most of these patients had underlying illnesses such as diabetes, lung infection, cancer etc. that affect the immune system, as in Africa. Many patients also had diarrheal disease caused by the same NTS that caused blood stream infection, unlike in Africa. Only two patients in each hospital died, a low mortality, unlike in Africa. The majority of the isolates belonged to Typhimurium and Enteritidis as in Africa. Even though resistance to drugs was a problem, about quarter of the isolates only were multidrug-resistant, a lower prevalence compared to in Africa. In Kuwait, we performed a detailed genetic study of a selected number of Typhimurium and Enteritidis isolates by a modern technique called whole genome sequencing. This revealed genetic determinants encoding drug-resistance and virulence causing blood-stream infection. This type of study was not performed in African isolates. Thus, our study revealed similarities and differences with studies of NTS-BI in Africa.
Introduction
Salmonella enterica subspecies enterica serovar Typhi and Salmonella Paratyphi A, B and C cause enteric fever, a systemic febrile illness that occurs only in humans. There are more than 2500 serovars of non-typhoidal Salmonella (NTS). NTS infects a variety of hosts and are frequently zoonotic in origin [1]. It mainly causes self-limiting gastroenteritis in humans. NTS has also been recognized as a major cause of extra-intestinal invasive bacterial infection in young children and immunocompromised patients worldwide [2,3]. It is a cause of severe bacteremia [1]. It has been estimated that globally about 3.4 million cases of bacteremia due to NTS occur every year [4]. The estimated worldwide mortality from NTS infection is 155,000 per year [1]. Invasive NTS disease is considered to be an emerging and neglected tropical disease in Africa [1].
Kuwait is a high-income subtropical country situated in the Middle East. It is neither a developing nor a developed country, but is a country in transition. There are no systemic studies of bacteremia due to NTS in Kuwait even though NTS is a major cause of diarrhea in Kuwait [5,6]. Antimicrobial resistance is also a problem among diarrheal stool isolates of NTS in Kuwait [7]. Also, there are no data on serovars of NTS causing infection in Kuwait. Therefore, the primary objective of the study was to assess the case-fraction of NTS bacteremia among bacteremia cases in two tertiary hospitals in Kuwait. The secondary objective was to determine the serovars of NTS isolates and their antibiotic susceptibilities. A study was conducted in two tertiary hospitals in Kuwait. We determined the serovars of NTS isolates and their antibiotic susceptibilities. Moreover, all isolates of S. Enteritidis and S. Typhimurium serovars were typed by pulsed-field gel electrophoresis (PFGE) to study their relatedness. In addition, selected isolates were subjected to whole genome sequencing for further characterization. We also compared our results with those from Africa. The results are presented in this communication.
Materials and methods
Patients. The only criterion for inclusion in the study was the isolation of a NTS from blood culture irrespective of any accompanying conditions and age. The indication for doing a blood culture was a suspicion of sepsis. Isolates were collected from Al Farwaniya hospital (which has pediatric and adult wards) from July 2013 until May 2016. The period of collection of isolates from Al Amiri hospital (which has neonatal, pediatric and adult wards) was from April 2013 until December 2015. The distance between the two hospitals is 26 km (16.2 miles). Al Farwaniya hospital is a 900-bed tertiary hospital which serves a population of 1.2 million people. Even though it serves both Kuwaitis and non-Kuwaitis, about 80% of the population served are non-Kuwaitis. Al Amiri hospital is a 418-bed tertiary hospital and it serves a population of 50,000 people. Although the hospital caters to both Kuwaitis and non-Kuwaitis, most of the people treated are Kuwaitis. All patients including diabetic patients and cancer patients have access to these hospitals, although for cancer treatment itself, there is a separate hospital. The patients included in our study were admitted in these hospitals, because of gastrointestinal symptoms and/or fever. Even though there are general guidelines for antimicrobial therapy, the antimicrobial therapy administered to patients in both hospitals was left to the discretion of attending physician. Antibiotics were given based on severity of infection, comorbidities and immune status of the patients.
Since typhoid is not endemic in Kuwait, typhoid vaccine is not offered to its residents. However, the vaccine is offered to residents travelling to high-risk areas.
Blood culture. Blood culture was done using BD BACTEC system (Becton Dickinson, MD, USA). From adults, 20 ml blood was drawn and 10 ml was inoculated into an aerobic bottle and 10 ml into an anaerobic bottle. From children, 1–5 ml of blood was drawn and inoculated into the aerobic bottle only. After inoculation of the bottle in the wards, they were immediately transported at ambient temperature to the hospital microbiology laboratory. Aerobic bottle signalling growth was subcultured on sheep blood agar, MacConkey agar and chocolate agar. The first two plates were incubated aerobically and the last plate in 5% CO2 atmosphere at 37°C for 24 h. Anaerobic bottle signaling growth was subcultured on brucella agar plate and the plate was incubated anaerobically at 37°C for 48 h. Identification of Salmonella and the initial antibiotic susceptibility were done from lactose non-fermenting colonies on MacConkey agar by Vitek 2 system (BioMerieux, Marcy l'Etoile, France). For grouping of Salmonella, the colony was inoculated into triple sugar iron agar and if the reaction was suggestive of Salmonella, the growth was used in a slide agglutination test against Salmonella polyvalent and group-specific antisera (Denka Seiken, Tokyo, Japan). As soon as Salmonella was detected in blood culture, empirical antimicrobial therapy for the patient was initiated. Further identification of Salmonella and antibiotic susceptibility testing were done as described below.
Stool was cultured for Salmonella from all Salmonella blood culture-positive patients. When warranted, urine culture was done. Stool was inoculated onto MacConkey agar and Salmonella-Shigella agar (SSA) and enriched in selenite F broth. Selenite F broth was subcultured onto SSA after 20 h of incubation. When urinary tract infection was suspected, urine was cultured on CLED (cysteine, lysine, electrolyte-deficient) agar and blood agar. All plates were incubated for 20–24 h at 37°C. Suspected Salmonella colonies were subjected to identification as above. Subcultures of Salmonella isolates on MacConkey agar plates from the hospital microbiology laboratories were sent to the reference laboratory at the Faculty of Medicine, Kuwait University, where further tests, listed below, were arranged or carried out.
Typing of Salmonella isolates. Internal fragments of seven housekeeping genes (thrA, purE, sucA, hisD, aroC, hemD, dnaN) were amplified by PCR [8]. Sequences of the genes were trimmed to the required lengths (399–501 bps). Sequence types were assigned in accordance with the Salmonella enterica database, http://mlst.ucc.ie/mlst/dbs/Senterica. From sequence types, serovars were assigned according to the scheme of Achtman et al [9]. Isolates belonging to S. Tyhimurium and S. Enteritidis serovars were further confirmed by specific PCR assays [10].
Antibiogram. Antibiotic susceptibility test was carried out by E test (Biomerieux) and interpreted by the criteria laid down by Clinical and Laboratory Standards Institute (CLSI) [11]. The breakpoints (μg/ml) (intermediate resistance, resistance) for various antibiotics were as follows: ciprofloxacin (0.12 to 0.5, ≥1), chloramphenicol (16, ≥32), trimethoprim/sulfamethoxazole (not applicable, ≥4), gentamicin (8, ≥16), tetracycline (8, ≥16), ampicillin (16, ≥32), ceftazidime (8, ≥16), cefotaxime (2, ≥4), ceftriaxone (2, ≥4), imipenem (2, ≥4), meropenem (2, ≥4) and piperacillin/tazobactam (32 to 64, ≥128). Resistant and intermediate-resistant minimum inhibitory concentrations were categorized as resistant in accordance with clinical practice.
When there was a necessity of testing resistance to other antibiotics (streptomycin, neomycin, carbenicillin, trimethoprim and sulfonamide), this was done by Kirby-Bauer disc diffusion test [12].
Results were interpreted according to the criteria of CLSI [13]. Escherichia coli ATCC 25922 strain was used as a quality control strain.
Extended spectrum β-lactamase (ESBL) production
E test. Isolates suspected of producing ESBL were tested for clavulanic-inhibitable ESBL production with E test ESBL strips–ESBL CT/CTL 16/1, ESBL TZ/TZL 32/4, ESBL PM/PML- as per manufacturer’s instructions (BioMerieux).
Vitek 2 test. The Vitek 2 ESBL test (bioMérieux) is based on the simultaneous assessment of the antibacterial activity of cefepime, cefotaxime and ceftazidime, measured either alone or in the presence of clavulanate. This test relies on card wells containing 1.0 mg/L of cefepime, or 0.5 mg/L of cefotaxime or ceftazidime, either alone or associated with 10 or 4 mg/L of clavulanate, respectively. After inoculation, cards were introduced into the Vitek 2 machine, and for each antibiotic tested, turbidity was measured at regular intervals. The proportional reduction of growth in wells containing a cephalosporin combined with clavulanate was then compared with that achieved by the cephalosporin alone and was interpreted as ESBL- positive or–negative through a computerized expert system (Advanced Expert System) [14].
Detection of genes encoding ESBL. PCR assays were performed to detect genes encoding blaCTX-M, blaTEM and blaSHV [7] and blaPSE-1 [15].
AmpC disk test and modified three dimensional test (M3DT) for detection of AmpC β-latcamases. This test was performed as described by Coskun et al [16]. Enhanced growth of bacteria around blank disks and AmpC disk or intersected growth in the zone of inhibition was considered positive.
Pulsed-field gel electrophoresis (PFGE). PFGE was performed according to the CDC protocol (http://www.cdc.gov/pulsenet/protocols.htm) using XbaI restriction enzyme (Roche, Mannheim, Germany) and a CHEF-DR III PFGE system (Bio-Rad, Munich, Germany). Salmonella Braenderup strain H9812(ATCC BAA 664) was used as a reference strain. The bands were visualized under UV light with Gel DOC(Bio-Rad) and analyzed with Bionumerics software (Applied Maths NV, Belgium). Pairwise similarities between patterns were calculated by DICE’s similarity coefficient. Clustering was based on unweighted pair-wise group method with averages (UPGMA) setting tolerance and optimization each at 1.5%.
Whole genome sequencing (WGS). Based on differences in banding patterns, S. Enteritidis and S. Typhimurium isolates were selected for whole genome sequencing. Sequencing libraries were prepared using the Nextera XT DNA sample preparation kit (Illumina, San Diego, CA, USA) and the sequence read data were produced on the Illumina NextSeq instrument (paired end, 150 base reads). Read data were submitted to the sequence read archive under project number PRJNA363099 (between 70x and 140x read depth coverage for each isolate). De novo assembly of the read data from each isolate was performed using MegaHit [17]. The resulting draft genome sequences were used to derive MLST (MLST:https://github.com/tseemann/mlst PubMLST: https://pubmlst.org/).
Abricate (https://github.com/tseemann/abricate) was used to detect virulence genes ([VFDB]: [18]). Antibiotic resistance gene profile was determined using Abricate and the Resfinder database [19].
Data management and analysis. Data on NTS bacteremia were obtained from laboratory records. Patient charts were traced, and relevant information was extracted. Results of bacterial analysis including serovars, antibiogram and WGS data were linked to patient data and tabulated in the reference laboratory. This dataset was used for analysis as appropriate.
Statistics. The difference in the prevalence of resistance to antibiotics in the two hospitals was calculated by Chi square test. A P value of ≤0.05 was considered significant.
Ethical approval. Bacterial isolates studied were a part of the routine collection at the Enteric Microbiology Reference Laboratory, Kuwait University, for further studies and archiving. It was not possible to get informed consent of patients as the clinical data were retrospectively collected. No additional specimens were collected for this study and patient identity was kept anonymous. Therefore, a waiver for informed consent, and approval for the study were granted by the Ethics Committee of Ministry of Health, State of Kuwait (permit number 898/2018).
Results
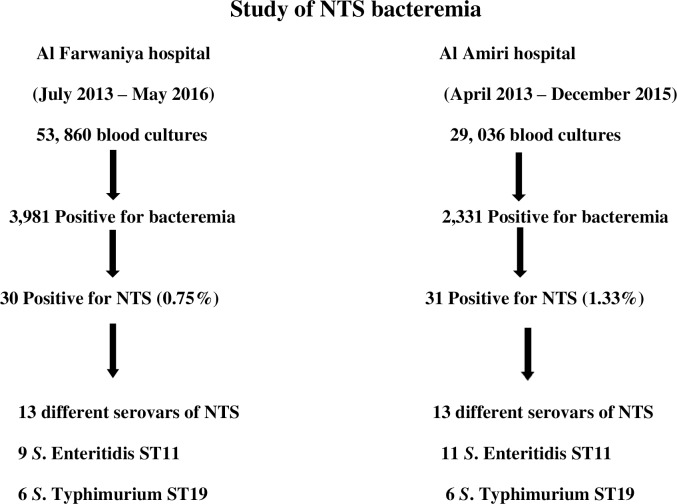
NTS from blood culture. The isolation of NTS from blood cultures of both hospitals is shown in Fig 1. In Al Farwaniya hospital, 53,860 blood cultures were done and 3981 were positive for microorganisms. Of the positive cultures, 30 were positive for a non-typhoidal Salmonella serovar (0.75%). There were 13 different Salmonella serovars, but 50% of them belonged to S. Enteritidis (all sequence type [ST]11) and S. Typhimurium (all ST19). In Al Amiri hospital, 29, 036 blood cultures were done and 2,331 were positive for microorganisms. Of the positive cultures, 31 were positive for a non-typhoidal serovar (1.33%). There were 13 different serovars of Salmonella infecting the patients, but 19 isolates (61.3%) belonged to S. Enteritidis (all ST11) and S. Typhimurium (all ST19).
Fig 1. Non-typhoidal Salmonella isolation from blood cultures of patients in Al Farwaniya and Al Amiri hospitals.
A fraction (0.75–1.33%) belonged to non-typhoidal Salmonella (NTS).
Patient characteristics, Salmonella serovars isolated, response to therapy and patient outcome in Al Farwaniya and Al Amiri hospitals are presented in Table 1. Isolate numbers with suffix F are from Al Farwaniya hospital and those with suffix A are from Al Amiri hospital. Patients were both Kuwaitis and expatriates of many nationalities in both hospitals. Median age for all 61 patients was 58 y and 62.3% of patients were >50 y old. Eight patients (13.1%) were children <5 y old. Approximately 67% of patients had chronic diseases such as diabetes mellitus, cancer, blood disorder, kidney disease or lung disease. Forty patients (65.6%) had diarrhea and 26 of them (65%) had a Salmonella (identical to the corresponding blood isolate) cultured from the stool. Four of these 26 patients had also the corresponding Salmonella serovar cultured from the urine. One patient who had diarrhea did not have Salmonella cultured from the stool, but urine culture was positive for the corresponding blood isolate. None of the non-diarrheal patients had Salmonella cultured from the stool. Most patients responded to antibiotic therapy and were discharged. Many classes of antibiotics were used, but many received a cephalosporin. In addition, six patients (22A, 36A, 51A, 59A, 60A, 70A) received steroids. Only four patients died.
Table 1. Details of patients from Al Farwaniya hospital and Al Amiri hospital from whom Salmonella species were isolated from blood cultures.
| Isolate No. | Age | Gender | Nationality | Diagnosis | Salmonella species from blood | Diarrhea present | Isolation of Salmonella from sites other than blood |
Antibiotics given | Response to antibiotics | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1F | 41 | M | Indian | Abdominal pain | Livingston | No | No | Cefotaxime, Metronidazole | +ve | Discharged |
| 2F | 81 | M | Kuwaiti | Cerebrovascular accident | Unspeciated type 2641 | No | No | Ceftriaxone | Uncertain | Died |
| 3F | 38 | F | Kuwaiti | Diarrhea | Enteritidis | Yes | No | Cefotaxime | +ve | Discharged |
| 5F | 58 | M | Indian | Abdominal pain, fever, vomiting | Infantis | No | No | Piperacillin/ tazobactam | +ve | Discharged |
| 7F | 2.5 | M | Kuwaiti | Intestinal obstruction, diarrhea | Enteritidis | Yes | No | Ceftriaxone | Uncertain | Transferred to surgical unit |
| 9F | 5 | M | Saudi | Abdominal pain, sickle cell anemia | Typhimurium | No | No | Cefotaxime | +ve | Discharged |
| 10F | 27 | M | Indian | Pancytopenia | Enteritidis | No | No | Ceftriaxone | Uncertain | Transferred to Infect Dis Hospital for? HIV infection |
| 11F | <1 | F | Egyptian | Fever, bronchiolitis, diarrhea | Typhimurium | Yes | No | Cefotaxime, gentamicin | +ve | Discharged |
| 12 F | 72 | M | Kuwaiti | Diabetes mellitus, ischemic heart disease, diarrhea, vomiting, fever | Enteritidis | Yes | No | Piperacillin/ tazobactam, metronidazole | +ve | Discharged |
| 13F | 30 | M | Filipino | Fever, diarrhea | Enteritidis | Yes | No | Cefotaxime, metronidazole | +ve | Discharged |
| 14F | 45 | M | Egyptian | Diabetes mellitus, chronic renal failure, fever | Livingston | No | No | Cefotaxime | +ve | Discharged |
| 15F | 65 | M | Pakistani | Diabetes mellitus, fever, jaundice | Livingston | No | No | Cefotaxime, clarithromycin | +ve | Discharged |
| 16F | 2 | F | Kuwaiti | Diarrhea | Minnesota | Yes | No | None, oral rehydration only | +ve | Discharged |
| 18F | 56 | M | Bangladeshi | Diabetes, eczema, diarrhea | Kentucky | Yes | No | Ciprofloxacin | +ve | Discharged |
| 19F | 54 | F | Iraqi | Diabetes, diarrhea | Typhimurium | Yes | No | Ciprofloxacin, metronidazole | +ve | Discharged |
| 46F | 73 | M | Kuwaiti | Diabetes mellitus, ischemic heart disease, fever, vomiting, diarrhea | Typhimurium | Yes | No | Meropenem | +ve | Discharged |
| 47F | 76 | M | Kuwaiti | Diabetes mellitus, colon cancer, acute kidney injury, loss of appetite | Bareilly | Yes | No | Ceftriaxone | +ve | Discharged |
| 48F | 47 | F | Kuwaiti | Sickle cell anemia, fever | Enteritidis | No | No | Ciprofloxacin | +ve | Discharged |
| 52F | 52 | M | Egyptian | Colon cancer, weight loss | Typhimurium | No | No | Ciprofloxacin, amoxicillin/ clavulanic acid | +ve | Discharged |
| 53F | 70 | F | Jordanian | Ischemic heart disease, chronic obstructive pulmonary disease, sinusitis | Enteritidis | No | No | Ceftriaxone | Uncertain | Transferred to neurosurgery for suspected brain abscess |
| 55F | 24 | F | Kuwaiti | Fever, vomiting, diarrhea | Braenderup | Yes | Stool | None, oral rehydration only | +ve | Discharged |
| 57F | 47 | F | Kuwaiti | Thalassemia, sickle cell anemia, upper respiratory infection, abdominal pain, chills | Poona | No | No | Ceftriaxone, azithromycin | +ve | Discharged |
| 58F | 46 | M | Indian | Fever, thrombocytopenia, diarrhea | Telaviv | Yes | Stool | Piperacillin/ tazobactam, metronidazole | Uncertain | Discharged against recommendation |
| 62F | 65 | M | Kuwaiti | Acute kidney injury, dehydration | Typhimurium | No | No | Meropenem, ceftriaxone | +ve | Discharged |
| 64F | 1 | M | Kuwaiti | Diarrhea, fever | Enteritidis | Yes | Stool | Cefotaxime | +ve | Discharged |
| 66F | 59 | M | Non- Kuwaiti* | Diabetes mellitus, end-stage renal disease, sepsis | Poona | No | No | No information | No information | No information |
| 68F | 1 | M | Non- Kuwaiti* | Diarrhea | Anatum | Yes | Stool | None, oral rehydration only | +ve | Discharged |
| 69F | 66 | M | Pakistani | Diabetes mellitus, cellulitis of thigh, diarrhea | Minnesota | Yes | Stool | Piperacillin/ tazobactam, cefotaxime, clindamycin | +ve | Discharged |
| 71F | 59 | M | Indian | Hyponatremia, ruptured aortic valve | Enteritidis | No | No | Cefotaxime | -ve | Died |
| 74F | 1 | M | Kuwaiti | Diarrhea | Uganda | Yes | No | None, oral rehydration only | +ve | Discharged |
| 21A | 79 | F | Kuwaiti | Chronic kidney disease, liver chirrosis | Typhimurium | Yes | No | Ciprofloxacin | -ve | Died |
| 22A | 75 | F | Kuwaiti | Diabetes mellitus, thrombocytopenia, Darriers disease | Enteritidis | No | No | Ceftriaxone | +ve | Discharged |
| 23A | 62 | M | Syrian | Diabetes mellitus, ischemic heart disease | Enteritidis | Yes | Stool, urine | Ceftriaxone Metronidazole | +ve | Discharged |
| 24A | 54 | M | Kuwaiti | Diabetes mellitus, Ormond's disease, periaertitis | Typhimurium | Yes | No | Ciprofloxacin Ceftriaxone | +ve | Discharged |
| 25A | 63 | M | Kuwaiti | Acquired hemophilia | ST516 | Yes | Stool | Ceftriaxone | +ve | Discharged |
| 26A | 70 | F | Kuwaiti | Diabetes mellitus | Enteritidis | Yes | Stool | Ciprofloxacin | +ve | Discharged |
| 27A | 81 | M | Kuwaiti | Diabetes mellitus, chronic kidney disease, liver cirrhosis | Enteritidis | Yes | Stool | Ceftriaxone, Meropenem | +ve | Discharged |
| 28A | 85 | M | Kuwaiti | Chronic kidney disease, ischemic heart disease, dyslipidemia | ST27 | Yes | Stool | Ceftriaxone | +ve | Discharged |
| 29A | 72 | M | Kuwaiti | Diabetes mellitus | Typhimurium | Yes | Stool | Ceftriaxone | +ve | Discharged |
| 30A | 32 | F | Kuwaiti | Ankylosing spondylitis, psoriasis | Enteritidis | No | No | Ceftriaxone Clindamycin | +ve | Discharged |
| 31A | 83 | M | Kuwaiti | Diarrhea | New type | Yes | No | Ceftriaxone | +ve | Discharged |
| 32A | 33 | F | Egyptian | Sickle cell anemia, hepatitis C virus infection | Enteritidis | Yes | Stool | No | Uncertain | Died within hours |
| 33A | 69 | M | Kuwaiti | Diabetes mellitus | Enteritidis | Yes | Stool | Ciprofloxacin | +ve | Discharged |
| 34A | 65 | F | Kuwaiti | Chronic kidney disease | Typhimurium | No | No | Ceftriaxone | +ve | Discharged |
| 35A | 53 | M | Pakistani | Acute kidney injury | Agona | Yes | Stool | No | +ve | Discharged |
| 36A | 52 | M | Iranian | Chronic autoimmune skin disease, sepsis, acute kidney injury | Typhimuriam | Yes | Stool | Ciprofloxacin Meropenem Metronidazole | +ve | Discharged |
| 37A | 69 | M | Kuwaiti | Diabetes mellitus, chronic kidney disease | Anatum | Yes | Stool | Ciprofloxacin Meropenem | +ve | Discharged |
| 38A | 2 | M | Kuwaiti | Diarrhea | Enteritidis | Yes | Stool | Ceftriaxone | +ve | Discharged |
| 39A | 73 | M | Kuwaiti | Diabetes mellitus, skin disease, acute renal injury, sepsis | Typhimurium | No | No | Ceftriaxone Clindamycin | +ve | Discharged |
| 40A | 54 | M | Indian | Diabetes mellitus, ulcerative colitis | Enteritidis | Yes | Stool | Ciprofloxacin | +ve | Discharged |
| 41A | 80 | F | Kuwaiti | Ischemic heart disease, toxic megacolon, dyslipidemia | Albany | Yes | Stool | Ceftriaxone | +ve | Discharged |
| 42A | 58 | F | Kuwaiti | Diabetes mellitus, chronic kidney disease | Typhimurium | Yes | Stool | Ceftriaxone | +ve | Discharged |
| 43A | 73 | M | Kuwaiti | Diabetes mellitus, dyslipidemia | Newport | Yes | Stool | Ciprofloxacin | +ve | Discharged |
| 44A | 75 | M | Kuwaiti | Liver cirrhosis | Enteritidis | Yes | Stool | Ciprofloxacin Ceftriaxone | +ve | Discharged |
| 45A | 70 | M | Kuwaiti | Diabetes mellitus, chronic kidney disease, fever | ST324/ST2606 | No | No | Ciprofloxacin | +ve | Discharged |
| 49A | 60 | F | Kuwaiti | Chronic kidney disease | Minnesota | Yes | Stool, urine | Ciprofloxacin Ceftriaxone | +ve | Discharged |
| 51A | 45 | M | Kuwaiti | Diabetes mellitus, Addison's disease, asthma | Typhimurium | No | Stool, urine | Ceftriaxone Clarithromycin | +ve | Discharged |
| 59A | 36 | M | Kuwaiti | Endstage renal disease | Bareilly | Yes | Stool, urine | Ciprofloxacin | +ve | Discharged |
| 60A | 30 | M | Kuwaiti | Lupus nephritis | Enteritidis | Yes | Urine | Ciprofloxacin Metronidazole Amphotericin B | +ve | Discharged |
| 61A | 78 | F | Kuwaiti | Diabetes mellitus, dyslipidemia, pneumonia, vomiting | Give | No | No | Ciprofloxacin Cefotaxime | +ve | Discharged |
| 70A | 36 | F | Kuwaiti | Multiple sclerosis | Typhimurium | Yes | No | piperacillin/ Tazobactam | +ve | Discharged |
*Non-Kuwaiti, but nationality uncertain
Antibiogram. The prevalence of resistance to all antibiotics was similar in both hospitals (P>0.05 for all comparisons) (S1A & S1B Table). Therefore, resistance for isolates from both hospitals was combined and is presented in Table 2. The prevalence of resistance to ampicillin, ciprofloxacin and tetracycline ranged between 36.1 to 50.8%. The prevalence of resistance to other antibiotics was either negligible or absent.
Table 2. Antibiotic susceptibility of non-typhoidal Salmonella isolated from blood cultures of 61 patients admitted in Al Farwaniya and Al Amiri hospitals, Kuwait.
| Antibiotic | Range (μg/ml) | MIC50 | MIC90 | No. (%) resistant |
|---|---|---|---|---|
| Ampicillin (AMP) | 0.125 - ›256 | 2 | ›256 | 22 (36.1) |
| Ceftazidime (CAZ) | 0.094–32 | 0.38 | 2 | 5 (8.2) |
| Cefotaxime (CTX) | 0.094–128 | 0.125 | 0.5 | 6 (9.8) |
| Ceftriaxone (CRO) | 0.032–24 | 0.094 | 1.5 | 5 (8.2) |
| Imipenem (IPM) | 0.032–1.5 | 0.19 | 0.5 | 0 (0.0) |
| Meropenem(MEM) | 0.004–0.38 | 0.032 | 0.125 | 0 (0.0) |
| Piperacillin/Tazobactam (TZP) | 1.0–8 | 2 | 4 | 0 (0.0) |
| Tetracycline (TET) | 1.5–256 | 8 | 256 | 31 (50.8) |
| Gentamicin (GM) | 0.38–48 | 0.5 | 0.75 | 2 (3.3) |
| Trimethoprim—Sulfamethoxazole (SXT) | 0.047–32 | 0.25 | 32 | 9 (14.8) |
Chloramphenicol (CHL) |
0.032–256 | 6 | 16 | 7 (11.5) |
| Ciprofloxacin (CIP) | 0.016–1.5 | 0.047 | 0.5 | 24 (39.3) |
The resistance phenotypes in both hospitals are shown in Table 3. A total of 52 of 61 isolates (85. 2%) were resistant to one or more antibiotics tested. Of the resistant isolates, 30(60%) were either S. Typhimurium or S. Enteritidis. Among the 16 multi-resistant (resistant to three or more classes of antibiotics) isolates, 8 (50%) were either S. Typhimurium or S. Enteritidis. Fourteen isolates (23%) were resistant to a cephalosporin.
Table 3. Resistance phenotypes of non-typhoidal Salmonella isolates.
| Resistance phenotype | Number of isolates |
|---|---|
| Cip | 1 |
| Amp | 2 |
| Tet | 16 |
| Cip, Tet | 7 |
| Amp, Tet | 3 |
| Tet, Chl | 2 |
| Tet, Cro | 1 |
| Tet, Ctx | 1 |
| Cip, Sxt, Tet | 2 |
| Cip, Tet, Ctx | 1 |
| Cip, Chl, Tet | 1 |
| Amp, Caz, Ctx | 2 |
| Amp, Sxt, Tet | 2 |
| Amp, Tet, Caz | 1 |
| Amp, Ctx, Cro | 1 |
| Cip, Amp, Tet | 1 |
| Cip, Amp, Chl, Tet | 1 |
| Chl, Tet, Caz, Ctx, Cro | 1 |
| Cip, Amp, Chl, Sxt, Gent, Tet | 1 |
| Cip, Amp, Chl, Sxt, Tet, Caz | 1 |
| Cip, Chl, Tet, Caz, Ctx, Cro | 1 |
| Cip, Amp, Chl, Tet, Caz, Ctx, Cro | 2 |
| Cip, Amp, Chl, Sxt, Gent, Tet, Ctx, Cro | 1 |
Cip: Ciprofloxacin, Amp: Ampicillin, Chl:Chloramphenicol, Cro: Ceftriaxone, Ctx: Cefotaxime
Sxt = Trimethoprim-sulphamethoxazole, Gen = Gentamicin, Tet = Tetracycline, Caz = Ceftazidime
ESBL production. One isolate (69F) from Al Farwaniya hospital was resistant to all three cephalosporins tested. Two isolates (45A and 49A) from Al Amiri hospital were resistant to all three cephalosporins. All three isolates were negative for clavulanic acid-inhibitable ESBL production by E test and for specific genes encoding ESBL, but were positive for ESBL production by both Vitek 2 test and E test. Two isolates (69F and 49A) were positive for AmpC test.
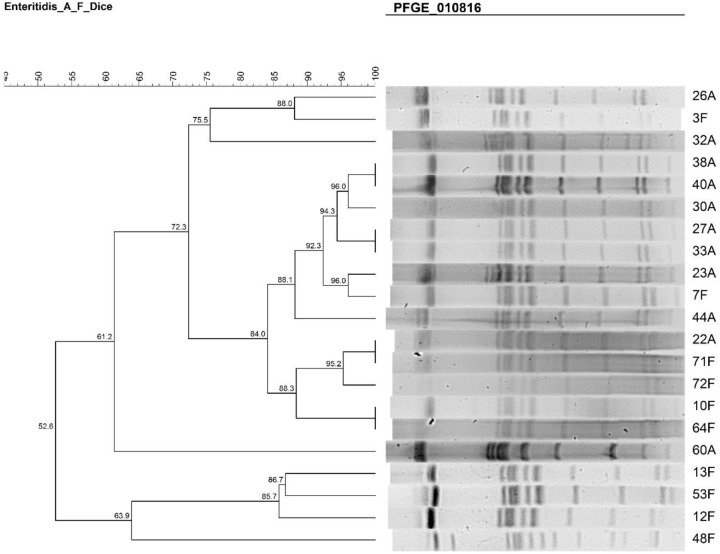
PFGE. The dendrogram of S. Enteritidis isolates from both Al Amiri and Al Farwaniya hospitals is shown in Fig 2. There were three clusters–cluster 1 comprised isolates 12F, 53F, and 13F; cluster 2 comprised isolates 64F, 10F, 72F, 71F, 22A, 44A, 7F, 23A, 33A, 27A, 30A, 40A, and 38A; and cluster 3 comprised isolates 32A, 3F and 26A. Isolates 48F and 60A were outliers.
Fig 2. PFGE dendrogram showing the relationship among S. Enteritidis.
The similarities between isolates were evaluated using Dice coefficient and UPGMA clustering method.
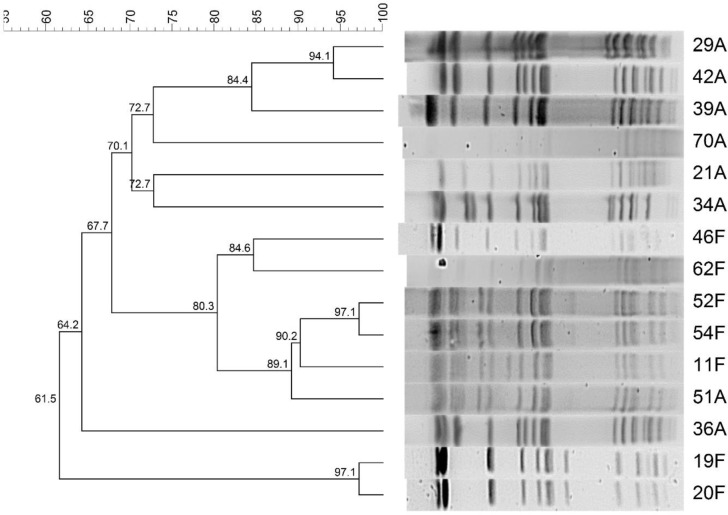
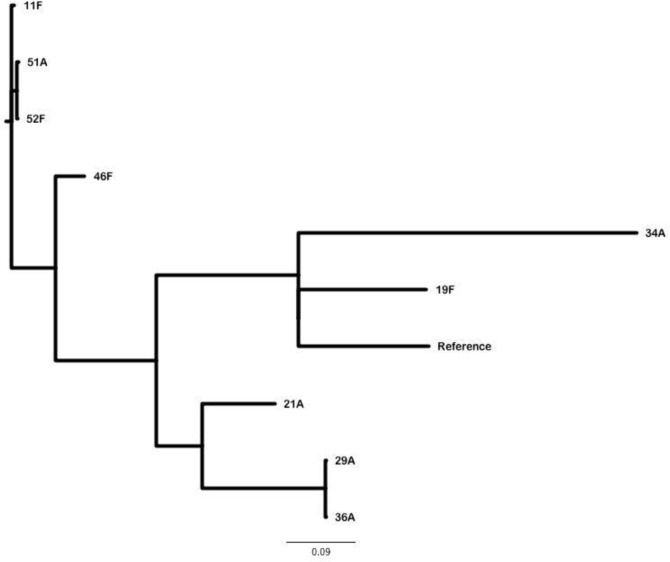
The dendrogram of S. Typhimurium isolates from both Al Amiri and Al Farwaniya hospitals is shown in Fig 3. There were three clusters–cluster 1 comprised of isolates 20F and 19F; cluster 2 comprised isolate 36A; cluster 3 comprised isolates 51A, 11F, 54F, 52F, 62F, and 46F; cluster 4 comprised isolates 34A, 21A, 70A, 39A, 42A and 29A. Isolate 36A was an outlier.
Fig 3. PFGE dendrogram showing the relationship among S. Typhimurium.
The similarities between isolates were evaluated using Dice coefficient and UPGMA clustering method.
WGS. Four S. Enteritidis isolates from Al Amiri hospital (23A, 32A, 38A, 60A) and two S. Enteritidis isolates from Al Farwaniya hospital (12F, 48F) were sequenced. Five S. Typhimurium isolates from Al Amiri hospital (21A, 51A, 34A, 29A, 36A) and four S. Typhimurium isolates from Al Farwaniya hospital (46F, 52F, 11F, 19F) were sequenced. These isolates belonged to different clusters by PFGE.
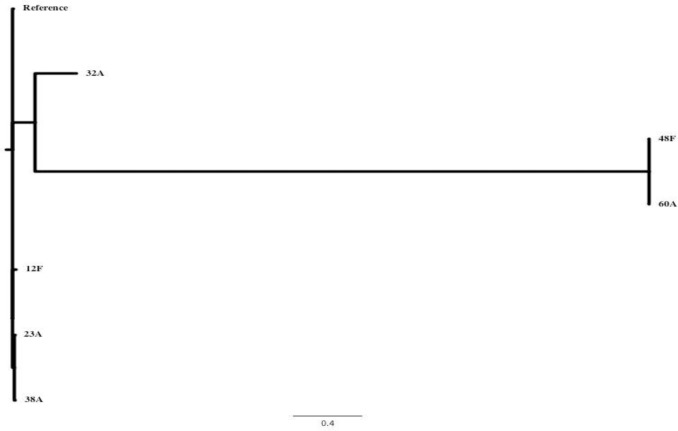
The phylogenetic relationship among the six S. Enteritidis isolates based on core genome sequence is shown in Fig 4. The genome of S. Enteritidis strain P125109 was used as the reference genome sequence. More than 97% of the reference genome was present in the genomes of the six clinical S. Enteritidis isolates. The core genome contained 1999 sites that varied in the six clinical isolates. The pairwise distance was greatest (1670 SNPs [single nucleotide polymorphisms]) between isolates 60A and12F. There were three clusters formed by 12F, 23A & 38A; 32A; and 48F & 60A.
Fig 4. An unrooted tree showing the inferred relationship between the six S. Enteritidis clinical isolates.
The S. Enteritidis strain P125109 was used as the reference genome sequence for read mapping. More than 97% of the reference genome was included in the core genome derived for this set of isolates. The core genome contained 1,999 sites that varied in one or more of the clinical isolates. The tree was inferred using Fast Tree and the greatest pairwise distance between isolates is 1670 SNPs, e.g. between isolates 60A and 12F.
The phylogenetic relationship based on the core genomes of the six clinical S. Enteritidis isolates in relation to core genomes of 59 S. Enteritidis strains from different parts of the world for which there are closed genome sequences, is shown in S1 Fig. The greatest pairwise distance of 1833 SNPs was found between isolate 48F and OLF-SE9-10012, a clam isolate of 2010 from Canada. There were a total of seven clusters and Kuwaiti isolates exhibited three clusters: 12F, 23A & 38A; 32A; and 48F & 60A. The details of genomes of 60 S. Enteritidis strains which were used for comparative analysis are given in S2 Table.
The phylogenetic relationship of the nine S. Typhimurium isolates based on their core genome sequence is shown in Fig 5. The genome of S. Typhimurium strain LT2 was used as the reference genome. More than 95% of the genome of the reference strain was contained in the genomes of the nine clinical S. Typhimurium isolates. The core genome contained 1979 sites that varied among the isolates. The greatest pairwise distance of 1017 SNPs was found between isolates 36A and 34A. Kuwaiti isolates formed five clusters: 11F, 51A &52F; 46F; 34A & 19F; 21A; 29A & 36A.
Fig 5. An unrooted tree showing the inferred relationship between the nine S. Typhimurium clinical isolates.
The S. Typhimurium strain LT2 was used as the reference genome sequence for read mapping. More than 95% of the reference genome was included in the core genome derived for this set of isolates. The core genome contained 1,979 sites that varied in one or more of the clinical isolates. The tree was inferred using Fast Tree and the greatest pairwise distance between isolates is 1,017 SNPs between isolates 36A and 34A.
The phylogenetic relationship of the nine S. Typhimurium isolates based on their core genomes in relation to 21 S. Typhimurium strains from different parts of the world whose closed genome sequences are known, is shown in S2 Fig. The greatest pairwise distance of 1333 SNPs was found between isolates 22792 (a cormorant isolate of 2008 from Canada) and RM10961 (an isolate from an agricultural produce in the USA, whose isolation details are not known). There were a total of 10 clusters and Kuwait isolates formed four clusters: 19F; 34A; 21A, 29A & 36A; 46F, 11F, 51A & 52F. The details of genomes of 21 S. Typhimurium strains which were used for comparative analysis are shown in S2 Table.
Virulence genes. The catalog of virulence genes identified in the whole genome-sequenced S. Enteritidis and S. Typhimurium is shown in S3 Table. The organisms possessed genes encoding a variety of type III secretion system proteins that manipulate host cell transduction pathways and cellular processes to pathogen’s advantage. Other genes included were those for chemotaxis and different fimbriae; anti- inflammatory effector genes for enhancing colonization; genes for resistance to antimicrobial peptides, mouse cecal colonization and prolonged shedding, cellular invasion, enteritis, fluid secretion etc. Of note are: ste gene (for spread and survival in host tissue), sse gene (for proliferation inside the macrophage and systemic infection in mice), sodC1 gene (for resistance to phagocytosis), rck gene (for serum resistance) and spv genes (for proliferation inside the macrophages and late apoptosis and spread of infection).
Antibiotic resistance genes. Antimicrobial resistance genes were found in some of the whole genome- sequenced S. Typhimurium and S. Enteritidis isolates. These are shown in Table 4. Based on the presence of an antimicrobial resistance gene, resistance to the corresponding antibiotic was found in the isolate as indicated in the footnote to the Table. Even though S. Typhimurium isolate 34 A possessed blaCARB-2 gene, it was susceptible to imipenem and meropenem. S. Typhimurium isolate 34A resistant to ampicillin, chloramphenicol and tetracycline had corresponding resistance genes. The same isolate was ciprofloxacin- intermediate resistant and had a substitution of asparagine (N) for aspartic acid (D) at position 87 in the gyrA gene. Multi-resistant S. Typhimurium isolate 51A was ciprofloxacin-intermediate resistant and had a substitution of tyrosine (Y) for serine (S) at position 83 in the gyrA gene [20]. S. Enteritidis isolates, 23A and 38A carried the ESBL gene, blaTEM-1B and were resistant to ampicillin [21]. Cephalosporin-intermediate-resistant isolates were not among the isolates that were subjected to whole genome sequencing.
Table 4. Antibiotic resistance genes in sequenced S. Enteritidis and S. Typhimurium isolates.
| Genea | S. Enteritidis Isolates | S. Typhimurium Isolates | GenBank | Resistance | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12F | 23A | 32A | 38A | 48F | 60A | 11F | 19F | 21A | 29A | 34A | 36A | 46F | 51A | 52F | |||
| aac(6')-Iaa_1 | 100b | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | NC_003197 | Aminoglycoside resistance |
| aph(3'')-Ib_5 | -c | - | - | - | - | - | - | - | 100 | - | - | - | 98.1 | 100 | 100 | AF321551 | Aminoglycoside resistance |
| aph(3')-Ia_1 | - | - | - | - | - | - | - | - | 100 | - | - | - | - | - | - | V00359 | Aminoglycoside resistance |
| aph(6)-Id_1 | - | - | - | - | - | - | - | - | 100 | - | - | - | 100 | 100 | 100 | M28829 | Aminoglycoside resistance |
| blaCARB-2_1 d | - | - | - | - | - | - | - | - | - | - | 100 | - | - | - | - | M69058 | Beta-lactam resistance |
| blaTEM-1B_1 e | - | 100 | - | 100 | - | - | - | - | 100 | - | - | - | - | - | - | JF910132 | Beta-lactam resistance |
| dfrA5_1 | - | - | - | - | - | - | - | - | 100 | - | - | - | - | - | - | X12868 | Trimethoprim resistance |
| floR_2 | - | - | - | - | - | - | - | - | - | - | 100 | - | - | - | - | AF118107 | Phenicol resistance |
| sul2_2 | - | - | - | - | - | - | - | - | 100 | - | - | - | 100 | 100 | 100 | AY034138 | Sulphonamide resistance |
| tet(A)_6 | - | - | - | - | - | - | - | - | 97.8 | - | - | - | 100 | 97.8 | 97.8 | AF534183 | Tetracycline resistance |
| tet(G)_2 | - | - | - | - | - | - | - | - | - | - | 100 | - | - | - | - | AF133140 | Tetracycline resistance |
| gyrA mutation | - | - | - | - | - | - | - | - | - | - | D87N | - | - | S83Y | - | X78977 | Fluoroquinolone resistance |
aGene symbol as per ResFinder database
bPercent gene coverage
cNegative for presence
dAlternate name: PSE-1, blaP1b
eAlternate name: Rbla TEM-1
Resistance to trimethoprim, sulphonamide and carbenicillin was tested by disk diffusion method. Resistance to other antibiotics was tested by E test
Discussion
A small fraction (0.75–1.33%) of blood culture-positive isolates only accounted for NTS bacteremia in Kuwait. The prevalence of NTS bacteremia as a proportion of community-acquired bacteremia varies widely according to geographic areas. It was 8% in Southern Africa, 25% in Central Africa, 27% in East Africa, and 18% in Western Africa [22]. In a multicenter study covering Indonesia, Thailand and Vietnam, the prevalence of NTS in individuals positive for any blood-stream bacterial infection was 27.5% among children, and 11.7% among adults [23]. In a study in Bangladesh, the prevalence of NTS in blood culture was 0.16% [24]. A Malaysian study found a prevalence rate of 16.2% with most of the cases occurring in children below 1 y of age [25]. The risk factors contributing to NTS bacteremia are extremes of age, immunosuppressive therapy, and underlying comorbidities such as diabetes mellitus, cancer, cardiovascular diseases etc. that affect the immune system [26]. In Africa, the risk factors for children are: sickle cell disease and malnutrition, and the risk factors not associated with age are malaria, anemia and human immunodeficiency virus infection [22, 26–32]. In Kuwait too, the affected patients were either old people or children. The patients also suffered from comorbidities such as diabetes mellitus, cancer, blood disorders, lung diseases and kidney diseases. In our case series, 4 out of 61 patients died with a case- fatality rate (CFR) of 6.6%. This low CFR in our study may be attributed to prompt and appropriate therapy of our patients including better management of underlying diseases. CFR was 20.6% in Sub-Saharan Africa [22]; 25% in Bangladesh [24]; 33% in Israel [33]; and 8.7% in Taiwan [34]. A higher CFR of 40.5% was seen among NTS bacteremic patients with malignancy compared to 17.7% among NTS bacteremic patients without malignancy in Taiwan [35]. In Al Farwaniya hospital, 16 patients (53.3%) had diarrhea, and of these, 5 patients (31.3%) had the same serovar of Salmonella as the one in blood culture isolated from the stool. There was an interval of 1–2 days between blood culture and stool culture. If blood culture of a patient was positive for a NTS, the patient was empirically treated with antibiotics during this interval. This antibiotic treatment would have affected the recovery of Salmonella in the stool culture of the patient. Approximately 5% of individuals with gastrointestinal illness caused by NTS will develop bacteremia [36]. However, in primary NTS bacteremia, most of the patients do not develop diarrhea and NTS is also not cultured from stool [37]. This suggested that a higher proportion of Kuwaiti patients with NTS bacteremia had gastrointestinal illness with the isolation of corresponding blood isolates from stools. Many different NTS serovars caused blood stream infection in Kuwaiti patients. However, 50–60% of the infections were due to S. Enteritidis and S. Typhimurium species in the two hospitals. Nonetheless, in Al Farwaniya hospital, most of the expatriate patients from South Asia and Southeast Asia had infection with neither of these serovars.
Although many serovars of NTS can cause blood stream infection, S. Enteritidis and S. Typhimurium serovars are the predominant serovars causing blood stream infection in many parts of the world [22, 29, 38–40]. NTS infection is usually zoonotic in origin contracted by contact with animals or consumption of contaminated water or food of animal origin [41]. However, there is also evidence of person- to- person transmission [42, 43]. In the largely urban environment, coupled with the cultural context of Kuwait where pet animals such as dogs are a religious taboo, contact with animals is less likely, and the most likely routes are consumption of contaminated food and person- to- person contact.
Patients were treated with many antibiotic classes, but most were treated with a third-generation cephalosporin–ceftriaxone, cefotaxime or ceftazidime. Most patients responded to these antibiotics. The antibiotic response concurs with the susceptibility data (Tables 2 and 3). ESBL production has been reported in NTS worldwide including in Kuwait [7, 44, 45]. In the current study, three out of 13 cephalosporin- resistant isolates showed ESBL production. In two isolates, ESBL production may be mediated by AmpC. All the 13 resistant isolates were negative for specific genes. Resistance may also be due to other mechanisms such as an altered porin with decreased entry of the antibiotic into bacterial cell, with the bacteria showing intermediate susceptibility [46, 47]. A combination of tests needs to be done for the detection of ESBL production [48]. Isolate 34A possessed the carbapenemase resistant gene, blaCARB-2_1, yet, it was susceptible to carbapenem. This concurs with a previous report [15]. Most of the isolates were resistant to one or more antibiotics, and 26.2% were multidrug-resistant. Half of the multidrug-resistant isolates belonged to S. Typhimurium or S. Enteritidis. Drug resistance is a problem among NTS isolates in many parts of the world [39, 27, 28, 49, 50, 51, 52]. Studies in African countries showed a higher prevalence of multidrug-resistant bacteria (40 to 100%) [28, 49–52] than in our study.
We typed all NTS isolates by PFGE and some selected isolates by WGS. There were discrepancies between the two typing methods. This is not unexpected as approaches to typing are different in the two methods. Nevertheless, studies have shown that WGS is more discriminatory than traditional typing methods including PFGE [53,54]. Moreover, WGS gives a near thorough sequence information of all genes present in the bacteria. In our series, the MLST of S. Typhimurium was ST19 and that of S. Enteritidis was ST11.
In Sub- Saharan Africa, the predominant ST of S. Typhimurium was ST313 [55, 56]. ST 19 was the predominant type found in both Europe and North America [57]. ST11 and ST19 were the predominant types causing gastroenteritis in Qatar, another Middle Eastern country [58]. ST19 was the predominant type in Iran being isolated from blood, urine and stool specimens [59]. It was also the predominant ST found in China [60]. S. Enteritidis ST11 causes diarrhea and blood stream infection world-wide. It caused blood stream infections from Mozambique [61], Kenya [55], and Vietnam [62].
Sequencing of our NTS isolates showed that they carried a full complement of virulence genes. Of note are the presence of genes that contribute to systemic spread and survival in the blood stream—ste gene for spread and survival in the host tissue through multiplication in a membrane-bound compartment, SCV [63], sse gene for survival and replication inside the macrophages via a type III secretory system[64], sodC1 gene for protection of bacteria against superoxide generated within phagocytes [65], rck gene for serum resistance [66], and spv genes for virulence of NTS to cause extra-intestinal disease by cytotoxicity and apoptosis of macrophages [67].
Thus, our data showed that unlike in sub-Saharan Africa and some parts of Asia, there was only a low-case fraction of NTS isolated from blood cultures done at these two hospitals in Kuwait. From a public health point of view, these patients need to be protected from contracting NTS infection by provision of thoroughly cooked foods of animal origin, and a high standard of personal hygiene of caregivers. A significant proportion of our patients had gastrointestinal illness, and mortality was negligible. Similarities with other studies included the following: the patients affected were young or old; most patients had immunocompromising co- morbidities; an array of serovars of Salmonella caused blood stream infection, but most of the isolates were S. Typhimurium and S. Enteritidis; and drug resistance was a problem in the isolates, but most infections were treated with a third-generation cephalosporin with or without other antibiotics. Unlike other studies, we performed phylogenetic analysis of all S. Typhimurium or S. Enteritidis isolates by PFGE and some selected PFGE typed isolates by WGS. These two typing methods showed that the isolates showed closely related clusters. Phylogeny by core genome analysis showed a close relationship with S. Typhimurium and S. Enteritidis from other parts of the world. WGS showed that S. Typhimurium and S. Enteritidis had a complement of virulence genes mediating extra-intestinal infection and antibiotic resistance genes mostly corresponding to the resistance phenotypes.
Our study has several limitations since it is a hospital-based study and cases were diagnosed by blood culture. For hospitalization, there is a selection bias towards more severe cases and associated conditions. Hospitalized cases are not truly representative of community cases where a spectrum of severity of cases can occur, and because of this, community population at risk cannot be properly defined. Therefore, our findings cannot be extrapolated to a general population. Hospital records are not primarily designed for research purposes because of incomplete and unstandardized information and diagnostic variability between hospitals. Retrospective studies may have inferior level of evidence compared with prospective studies, and may be subject to confounding variables that may be present, but not measured. Moreover, temporal relationships are difficult to assess in retrospective studies. The concentration of NTS in blood is about 1 cfu per ml [68]. Therefore, conventional blood culture and even polymerase chain reaction (PCR) method may not be sensitive enough to detect all true positive cases [69]. This is prompting investigators to design more sensitive methods [70].
Supporting information
(TIF)
(TIF)
A. Prevalence of antimicrobial resistance in non-typhoidal Salmonella from blood cultures of 30 patients in Al Farwaniya hospital. B. Prevalence of antimicrobial resistance in non-typhoidal Salmonella from blood cultures of 31 patients in Al Amiri hospital.
(DOCX)
(XLSX)
(XLSX)
Data Availability
All relevant data are contained within the manuscript and/or supporting information files.
Funding Statement
The authors received no specific funding for this work
References
- 1.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012; 379: 2489–2499. 10.1016/S0140-6736(11)61752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, et al. The global burden of nontyphoidal salmonella gastroenteritis. Clin Infect Dis. 2010; 50: 882–889. 10.1086/650733 [DOI] [PubMed] [Google Scholar]
- 3.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010; 10: 417–432. 10.1016/S1473-3099(10)70072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal salmonella disease, 2010. Emerg Infect Dis. 2015; 21: 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi SK, Khuffash FA, Al-Nakib W. Microbial etiology of acute gastroenteritis in hospitalized children in Kuwait. Pediatr Infect Dis. 1989; 8: 593–597. [DOI] [PubMed] [Google Scholar]
- 6.Albert MJ, Rotimi VO, Iqbal J, Chehadeh W. Evaluation of the xTAG gastrointestinal pathogen panel assay for the detection of enteric pathogens in Kuwait. Med Princ Pract. 2016; 25: 472–476. 10.1159/000447698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotimi VO, Jamal W, Pal T, Sovenned A, Albert MJ. Emergence of CTX-M-15 type extended-spectrum b-lactamase-producing Salmonella spp. in Kuwait and the United Arab Emirates. J Med Microbiol. 2008; 57: 881–886. 10.1099/jmm.0.47509-0 [DOI] [PubMed] [Google Scholar]
- 8.Kidgell C, Reichard U, Wain J, Linz B, Torydahl M, Dougan G, et al. Salmonella Typhi, the causative agent of typhoid fever, is approximately 50,000 years old. Infect Genet Evol. 2002; 2: 39–45. [DOI] [PubMed] [Google Scholar]
- 9.Achtman M, Wain J, Weill F-X, Nair S, Zhou Z, Sangal V, et al. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog. 2012; 8: e1002776 10.1371/journal.ppat.1002776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soumet C, Ermel G, Rose V, Rose N, Drouin P, Salvat G, et al. Identification by a multiplex PCR-based assay of Salmonella Typhimurium and Salmonella Enteritidis strains from environmental swabs of poultry houses. Lett Appl Microbiol. 1999; 29: 1–6. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute (CLSI): M100-S26. Performance standards for antimicrobial susceptibility testing; 26th informational supplement. Wayne, PA: CLSI, 2016. [Google Scholar]
- 12.Reller LB, Weinstein M, Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin infect Dis. 2009; 49: 1749–1755. [DOI] [PubMed]
- 13.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 25th informal supplement, Jan 2015, M100-S25, 35, no. 3, Wayne, PA, USA.
- 14.Drieux L, Brossier F, Sougakoff W, Jarlier V. Phenotypic detection of extended-spectrum beta- lactamase production in Enterobacteriaceae: review and bench guide. Clin Microbiol Infect. 2008; 14(Suppl 1): 90–103. [DOI] [PubMed] [Google Scholar]
- 15.Poirel L, Guibert M, Bellais S, Naas T, Nordmann P. Integron- and carbenicillinase-mediated reduced susceptibility to amoxicillin-clavulanic acid in isolates of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 from French patients. Antimicrob Agents Chemother. 1999; 43: 1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coskun S, Altanlar N, Keyvan E. Detection of plasmid mediated AmpC beta-lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae. Sch J App Med Sci. 2016; 4: 444–450. [Google Scholar]
- 17.Li D, Liu CM, Luo R, Sadakane K, Lam TW. An ultrafast single node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015; 31: 1674–1676. 10.1093/bioinformatics/btv033 [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Zheng D, Liu B, Yang J, Jin Q. Hierarchical and refined dataset for big data analysis-10 years on. Nucleic Acids Res. 2016; 44: D694–D697. 10.1093/nar/gkv1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zankari E, Hasman H, Cosento S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012; 67: 2640–2644. 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karunakaran R, Tay ST, Rahim FF, Lim BB, Puthucheary SD. Molecular analysis of ciprofloxacin resistance among nontyphoidal Salmonella with reduced susceptibility to ciprofloxacin isolated from patients at a tertiary care hospital in Kuala Lumpur, Malaysia. Jpn J Infect Dis. 2014; 67: 157–162. [DOI] [PubMed] [Google Scholar]
- 21.Delmani F-A, Jaran AS, Al Tarazi Y, Masaadeh H, Zaki O. Characterization of ampicillin resistant gene (blaTEM-1) isolated from Escherichia coli in northern Jordan. Asian J Biomed Pharmaceut Sci. 2017; 7: 11–15. [Google Scholar]
- 22.Uche IV, MacLennan CA, Saul A. A systematic review of the incidence, risk factors and case fatality rates of invasive nontyphoidal Salmonella (iNTS) disease in Africa (1966 to 2014). PLoS Negl Trop Dis. 2017; 11: e0005118 10.1371/journal.pntd.0005118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Southeast Asia Infectious Disease Clinical Research Network (SAIDCRN). Causes and outcomes of sepsis in southeast Asia: A multinational multicenter cross-sectional study. Lancet Glob Health. 2017; 5: e157–e167. 10.1016/S2214-109X(17)30007-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahunja KM, Leung DT, Ahmed T, Bardhan PK, Ahmed D, Qadri F, et al. Factors associated with non typhoidal Salmonella bacteremia versus typhoidal Salmonella bacteremia in patients presenting for care in an urban diarrheal disease hospital in Bangladesh. PLoS Negl Trop Dis. 2015; 9: e0004066 10.1371/journal.pntd.0004066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nor Azizah A, Fadzilah MN, Mariam M, Anis Siham ZA, Ariza A, Noor Shafina MN, et al. Community acquired bacteremia in paediatrics: epidemiology, aetiology and patterns of antimicrobial resistance in a tertiary care centre, Malaysia. Med J Malaysia. 2016; 71:117–121. [PubMed] [Google Scholar]
- 26.Turgeon P, Murray R, Nesbitt A. Hospitalizations associated with salmonellosis among seniors in Canada, 2000–2010. Epidemiol Infect. 2017; 145:1527–1534. 10.1017/S0950268817000292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahon BE, Fields PI. Invasive infections with nontyphoidal Salmonella in Sub-Saharan Africa. Microbiol Spectr. 2016; 4:0015–2016. [DOI] [PubMed] [Google Scholar]
- 28.Crump JA, Heyderman RS. A perspective on invasive Salmonella disease in Africa. Clin Infect Dis. 2015; 1: S235–S240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crump JA, Sjolund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev. 2015; 28:901–937. 10.1128/CMR.00002-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SE, Pak GD, Aaby P, Aaby P, Adu-Sarkodie Y, Ali M, et al. Incidence of invasive salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study. Lancet Global Health.2017; 5(3): e310–e323. 10.1016/S2214-109X(17)30022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kariuki S, Onsare RS. Epidemiology and genomics of invasive nontyphoidal Salmonella infections in Kenya. Clin Infect Dis. 2015; 1: S317–S324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mastroeni P, Rossi O. Immunology, epidemiology and mathematical modelling towards a better understanding of invasive nontyphoidal Salmonella disease and rational vaccination approaches. Expert Rev Vaccines. 2016; 15:1545–1555. 10.1080/14760584.2016.1189330 [DOI] [PubMed] [Google Scholar]
- 33.Shimoni Z, Pitlik S, Leibovici L, Samra Z, Konigsberger H, Drucker M, et al. Nontyphoid Salmonella bacteremia: Age-related differences in clinical presentation, bacteriology, and outcome. Clin Infect Dis. 1999; 28:822–7. 10.1086/515186 [DOI] [PubMed] [Google Scholar]
- 34.Huang C-F, Chen P-L, Liu M-F, Lee C-C, Lee N-Y, Chang C-M, et al. Nontyphoidal Salmonella bacteremia in patients with connective tissue diseases. J Microbiol Immunol Infect. 2012; 45: 350–355. 10.1016/j.jmii.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 35.Li C-W, Chen P-L, Lee N-Y, Lee H-C, Chang C-M, Lee C-C, et al. Non-typhoidal Salmonella bacteremia among adults: An adverse prognosis in patients with malignancy. J Microbiol Immunol Infect. 2012; 45:343–349. 41. 10.1016/j.jmii.2011.12.015 [DOI] [PubMed] [Google Scholar]
- 36.Hohmann L. Nontyphoidal salmonellosis. Clin Infect Dis. 2001; 32: 263–269. 10.1086/318457 [DOI] [PubMed] [Google Scholar]
- 37.Tennant SM, MacLennan CA, Levine MM. Invasive nontyphoidal Salmonella disease in Africa: current status. Expert Rev in Anti Infect Ther. 2013; 11: 443–446. [DOI] [PubMed] [Google Scholar]
- 38.Eibach D, Al-Emran HM, Dekker DM, Krumkamp R, Adu-Sarkodie Y, Espinoza MC, et al. The emergence of reduced ciprofloxacin susceptibility in Salmonella enterica causing bloodstream infections in rural Ghana. Clin Infect Dis. 2016; 62 (S1): S32–S36. [DOI] [PubMed] [Google Scholar]
- 39.Angelo KM, Reynolds J, Karp BE, Hoekstra RM, Scheel CM, Friedman C. Antimicrobial resistance among nontyphoidal Salmonella isolated from blood in the United States, 2003–2013. J Infect Dis. 2016; 214: 1565–1570. 10.1093/infdis/jiw415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhanoa A, Fatt QK. Non-typhoidal Salmonella bacteraemia: Epidemiology, clinical characteristics and its association with severe immunosuppression. Ann Clin Microbiol Antimicrob. 2009; 8: 15 10.1186/1476-0711-8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Painter JA, Hoekstra RM, Ayers T, Tauxe RV, Braden CR, Angulo FJ, et al. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998 2008. Emerg Infect Dis. 2013; 19:407–415. 10.3201/eid1903.111866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacLennan CA, Simon R, Martin LB, Khan MI. Nontyphoidal salmonella disease: Current status of vaccine research and development. Vaccine. 2016; 34: 2907–2910. 10.1016/j.vaccine.2016.03.072 [DOI] [PubMed] [Google Scholar]
- 43.Wiksvo ME, Kambhampati A, Shioda K, Walsh KA, Bowen A. Outbreaks of acute gastroenteritis transmitted by person to person contact, environmental contamination, and unknown modes of Transmission-United States. MMWR. 2015; 64: 1–16. [DOI] [PubMed] [Google Scholar]
- 44.Yates C, Amyes S. Extended-spectrum β-lactamases in non-typhoidal Salmonella spp. isolated in the UK are now a reality: why the late arrival? J Antimicrob Chemother. 2005; 56: 262–264. 10.1093/jac/dki237 [DOI] [PubMed] [Google Scholar]
- 45.Kim S, Hu J, Gautom R, Kim J, Lee B, Boyle DS. CTX-M extended-spectrum b-lactamases, Washington State. Emerg Infect Dis. 2007; 13: 513–514. 10.3201/eid1303.060479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thenmozhi S, Moorthy K, Sureshkumar BT, Suresh M. Antibiotic resistance mechanism of ESBL producing Enterobacteriaceae in clinical field. Int J Pure App Biosci. 2014; 2: 207–226. [Google Scholar]
- 47.Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016; 4(2): VMBF-0016- 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robin F, Delmas J, Schweitzer C, Bonnet R. Evaluation of Vitek-2 extended-spectrum beta-lactamase test against non-duplicate strains of Enterobacteriaceae producing a broad diversity of well-characterised beta-lactamases. Clin Microbiol Infect. 2008; 14: 148–154. 10.1111/j.1469-0691.2007.01893.x [DOI] [PubMed] [Google Scholar]
- 49.Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, Molyneux, et al. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008; 46: 963–969. 10.1086/529146 [DOI] [PubMed] [Google Scholar]
- 50.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Hart CA. Characterisation of community acquired nontyphoidal Salmonella from bacteremia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol. 2006; 6: 101 10.1186/1471-2180-6-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lunguya O, Lejon V, Phoba M-F, Bertrand S, Vanhoof R, Glupczynski Y, et al. Antimicrobial resistance in invasive nontyphoid Salmonella from the Democratic Republic of the Congo: emergence of decreased fluoroquinolone susceptibility and extended-spectrum beta lactamases. PLos Negl Trop Dis. 2013; 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oneko M, Kariuki S, Muturi-Kioi V, Oteino K, Oteino VO, Williamson JM, et al. Emergence of community- acquired, multidrug-resistant invasive nontyphoidal Salmonella disease in rural Western Kenya, 2009–2013. Clin Infect Dis. 2015; 1: S310–S316. [DOI] [PubMed] [Google Scholar]
- 53.Leekitcharoenphon P, Nielsen EM, Kaas RS, Lund O, Aarestrup FM. Evaluation of whole genome sequencing for outbreak detection of Salmonella enterica. PLOS ONE. 9(2): e87991 10.1371/journal.pone.0087991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng X, Shariat N, Driebe EM, Roe CC, Tolar B, Trees E, et al. Comparative analysis of subtyping methods against a whole-genome-sequencing standard for Salmonella enterica serotype Enteritidis. J Clin Microbiol. 2015; 53: 212–218. 10.1128/JCM.02332-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akullian A, Montgomery JM, John-Stewart G, Miller SI, Hayden HS, Radey MC, et al. Multi-drug resistant non-typhoidal Salmonella associated with invasive disease in western Kenya. PLOS Negl Trop Dis. 2018; 12(1): e0006156 10.1371/journal.pntd.0006156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feasey NA, Cain AK, Msefula CL, Pickard D, Alaerts M, Aslett M, et al. Drug Resistance in Salmonella enterica ser. Typhimurium bloodstream infection, Malawi. Emerg Infect Dis. 2014; 20: 1957–1959. 10.3201/eid2011.141175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haselbeck AH, Panzner U, Im J, Baker S, Myer CG, Marks F. Current perspectives on invasive nontyphoidal Salmonella disease. Curr Opin Infect Dis. 2017; 30: 498–503. 10.1097/QCO.0000000000000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang YC, Scaria J, Ibraham M, Doiphode S, Chang Y-F, Sultan A, et al. Distribution and factors associated with Salmonella enterica genotypes in a diverse population of humans and animals in Qatar using multi locus sequence typing (MLST). J Infect Pub Health. 2016; 9, 315–323. [DOI] [PubMed] [Google Scholar]
- 59.Ranjbar R, Elhagi P, Shokoohizadeh L. Multilocus sequence typing of the clinical isolates of Salmonella enterica serovar Typhimurium in Tehran Hospitals. Iran J Med Sci. 2017; 42: 443–448. [PMC free article] [PubMed] [Google Scholar]
- 60.Sun J, Ke B, Huang Y, He D, Li X, Liang Z, et al. The molecular epidemiological characteristics and genetic diversity of Salmonella Typhimurium in Guangdong, China, 2007–2011. PLOS ONE. 9(11): e113145 10.1371/journal.pone.0113145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.García V, Mandomando I, Ruiz J, Herrera-León S, Alonso PL, Rodicio MR. Salmonella enterica serovars Typhimurium and Enteritidis causing mixed infections in febrile children in Mozambique. Infect Drug Resist.2018; 11: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lan NPH, Phuong TLT, Huu HN, Le Thuy L, Mather AE, Park SE, et al. Invasive non- typhoidal Salmonella infections in Asia: Clinical observations, disease outcome and dominant serovars from an infectious disease hospital in Vietnam. PLOS Negl Trop Dis. 2016; 10(8): e0004857 10.1371/journal.pntd.0004857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Domingues L, Holden DW, Mota LJ. The Salmonella effector SteA contributes to the control of membrane dynamics of Salmonella-containing vacuoles. Infect Immun. 2014; 82: 2923–2934. 10.1128/IAI.01385-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nikolaus T, Deiwick J, Rappl C, Freeman JA, Schröder W, Miller SI, et al. SseBCD proteins are secreted by the Type III secretion system of Salmonella pathogenicity island 2 and function as a translocon. J Bacteriol. 2001;183: 6036–6045. 10.1128/JB.183.20.6036-6045.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ammendola S, Ajello M, Pasquali P, Kroll JS, Langford PR, Rotilio G, et al. Differential contribution of sodC1 and sodC2 to intracellular survival and pathogenicity of Salmonella enterica serovar Choleraesuis. Microbes Infect. 2005; 7: 698–707. 10.1016/j.micinf.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 66.Heffernan EJ, Harwood J, Fierer J, Guiney D. The Salmonella typhimurium virulence plasmid complement resistance gene rck is homologous to a family of virulence-related outer membrane protein genes, including pagC and ail. J Bacteriol. 1992; 174: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guiney DG, Fierer J. The role of the spv genes in Salmonella pathogenesis. Front Microbiol. 2011; 2:129 10.3389/fmicb.2011.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gordon MA, Kaukwatira AMK, Mwafulirwa G, Walsh AL, Hopkins MJ, Parry CM, et al. Invasive non-typhoid salmonellae establish systemic intracellular infection in HIV-infected adults: an emerging disease pathogenesis. Cin Infect Dis. 2010; 50: 953–962. [DOI] [PubMed] [Google Scholar]
- 69.Gordon MA. Invasive nontyphoidal Salmonella disease: epidemiology, pathogenesis and diagnosis. Curr Opin Infect Dis. 2011; 24: 484–489. 10.1097/QCO.0b013e32834a9980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tennant SM, Zhang Y, Galen JE, Geddes CD, Levine MM. Ultra-fast and sensitive detection of non-typhoidal Salmonella using microwave-accelerated metal-enhanced fluorescence. PLOS ONE. 2011; 6: e18700 10.1371/journal.pone.0018700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
A. Prevalence of antimicrobial resistance in non-typhoidal Salmonella from blood cultures of 30 patients in Al Farwaniya hospital. B. Prevalence of antimicrobial resistance in non-typhoidal Salmonella from blood cultures of 31 patients in Al Amiri hospital.
(DOCX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are contained within the manuscript and/or supporting information files.