Abstract
Background
Multiparametric magnetic resonance imaging (MRI), with or without MRI‐targeted biopsy, is an alternative test to systematic transrectal ultrasonography‐guided biopsy in men suspected of having prostate cancer. At present, evidence on which test to use is insufficient to inform detailed evidence‐based decision‐making.
Objectives
To determine the diagnostic accuracy of the index tests MRI only, MRI‐targeted biopsy, the MRI pathway (MRI with or without MRI‐targeted biopsy) and systematic biopsy as compared to template‐guided biopsy as the reference standard in detecting clinically significant prostate cancer as the target condition, defined as International Society of Urological Pathology (ISUP) grade 2 or higher. Secondary target conditions were the detection of grade 1 and grade 3 or higher‐grade prostate cancer, and a potential change in the number of biopsy procedures.
Search methods
We performed a comprehensive systematic literature search up to 31 July 2018. We searched CENTRAL, MEDLINE, Embase, eight other databases and one trials register.
Selection criteria
We considered for inclusion any cross‐sectional study if it investigated one or more index tests verified by the reference standard, or if it investigated the agreement between the MRI pathway and systematic biopsy, both performed in the same men. We included only studies on men who were biopsy naïve or who previously had a negative biopsy (or a mix of both). Studies involving MRI had to report on both MRI‐positive and MRI‐negative men. All studies had to report on the primary target condition.
Data collection and analysis
Two reviewers independently extracted data and assessed the risk of bias using the QUADAS‐2 tool. To estimate test accuracy, we calculated sensitivity and specificity using the bivariate model. To estimate agreement between the MRI pathway and systematic biopsy, we synthesised detection ratios by performing random‐effects meta‐analyses. To estimate the proportions of participants with prostate cancer detected by only one of the index tests, we used random‐effects multinomial or binary logistic regression models. For the main comparisions, we assessed the certainty of evidence using GRADE.
Main results
The test accuracy analyses included 18 studies overall.
MRI compared to template‐guided biopsy: Based on a pooled sensitivity of 0.91 (95% confidence interval (CI): 0.83 to 0.95; 12 studies; low certainty of evidence) and a pooled specificity of 0.37 (95% CI: 0.29 to 0.46; 12 studies; low certainty of evidence) using a baseline prevalence of 30%, MRI may result in 273 (95% CI: 249 to 285) true positives, 441 false positives (95% CI: 378 to 497), 259 true negatives (95% CI: 203 to 322) and 27 (95% CI: 15 to 51) false negatives per 1000 men. We downgraded the certainty of evidence for study limitations and inconsistency.
MRI‐targeted biopsy compared to template‐guided biopsy: Based on a pooled sensitivity of 0.80 (95% CI: 0.69 to 0.87; 8 studies; low certainty of evidence) and a pooled specificity of 0.94 (95% CI: 0.90 to 0.97; 8 studies; low certainty of evidence) using a baseline prevalence of 30%, MRI‐targeted biopsy may result in 240 (95% CI: 207 to 261) true positives, 42 (95% CI: 21 to 70) false positives, 658 (95% CI: 630 to 679) true negatives and 60 (95% CI: 39 to 93) false negatives per 1000 men. We downgraded the certainty of evidence for study limitations and inconsistency.
The MRI pathway compared to template‐guided biopsy: Based on a pooled sensitivity of 0.72 (95% CI: 0.60 to 0.82; 8 studies; low certainty of evidence) and a pooled specificity of 0.96 (95% CI: 0.94 to 0.98; 8 studies; low certainty of evidence) using a baseline prevalence of 30%, the MRI pathway may result in 216 (95% CI: 180 to 246) true positives, 28 (95% CI: 14 to 42) false positives, 672 (95% CI: 658 to 686) true negatives and 84 (95% CI: 54 to 120) false negatives per 1000 men. We downgraded the certainty of evidence for study limitations, inconsistency and imprecision.
Systemic biopsy compared to template‐guided biopsy: Based on a pooled sensitivity of 0.63 (95% CI: 0.19 to 0.93; 4 studies; low certainty of evidence) and a pooled specificity of 1.00 (95% CI: 0.91 to 1.00; 4 studies; low certainty of evidence) using a baseline prevalence of 30%, systematic biopsy may result in 189 (95% CI: 57 to 279) true positives, 0 (95% CI: 0 to 63) false positives, 700 (95% CI: 637 to 700) true negatives and 111 (95% CI: 21 to 243) false negatives per 1000 men. We downgraded the certainty of evidence for study limitations and inconsistency.
Agreement analyses: In a mixed population of both biopsy‐naïve and prior‐negative biopsy men comparing the MRI pathway to systematic biopsy, we found a pooled detection ratio of 1.12 (95% CI: 1.02 to 1.23; 25 studies). We found pooled detection ratios of 1.44 (95% CI 1.19 to 1.75; 10 studies) in prior‐negative biopsy men and 1.05 (95% CI: 0.95 to 1.16; 20 studies) in biopsy‐naïve men.
Authors' conclusions
Among the diagnostic strategies considered, the MRI pathway has the most favourable diagnostic accuracy in clinically significant prostate cancer detection. Compared to systematic biopsy, it increases the number of significant cancer detected while reducing the number of insignificant cancer diagnosed. The certainty in our findings was reduced by study limitations, specifically issues surrounding selection bias, as well as inconsistency. Based on these findings, further improvement of prostate cancer diagnostic pathways should be pursued.
Plain language summary
Is prostate MRI, with or without MRI‐targeted biopsy, better than systematic biopsy for detecting prostate cancer in men?
Background
Many prostate cancers are slow growing and may not have any harmful effects during a man's lifetime. Meanwhile, clinically significant cancers can cause problems such as blockage of the urinary tract, painful bone lesions and death. The prostate‐specific antigen (PSA) test followed by tissue samples of the prostate with ultrasound guidance is often used to detect these cancers early. More recently, magnetic resonance imaging (MRI) has also been used to help make the diagnosis.
What is the aim of this review?
The aim of this review was to compare MRI alone, MRI together with a biopsy, and a pathway that uses MRI to help decide whether to do a biopsy or not (hereinafter named ‘the MRI pathway’) with the standard ultrasound guided biopsy (hereinafter called ‘systematic biopsy’) in reference to template‐guided biopsy.
What are the main results?
We examined evidence up to July 2018. The review included 43 studies, mainly from Western countries, of men aged 61 to 73 years.
In a population of 1000 men at risk for prostate cancer, where 300 men actually have clinically significant prostate cancer, MRI will correctly identify 273 men as having clinically significant prostate cancer but miss the remaining 27 men; for the 700 men that do not have clinically significant prostate cancer, MRI will correctly identify 259 as not having prostate cancer but will misclassify 441 men as having clinically significant prostate cancer.
In the same population, MRI‐targeted biopsy will correctly identify 240 of 300 men as having clinically significant prostate cancer but miss the remaining 60 men; for the 700 men that do not have clinically significant prostate cancer, MRI will correctly identify 658 as not having prostate cancer but misclassify 42 men as having clinically significant prostate cancer.
The MRI pathway will correctly identify 216 of 300 men as having clinically significant prostate cancer but miss the remaining 84 men; for the 700 men that do not have clinically significant prostate cancer, MRI pathway will correctly identify 672 as not having prostate cancer but will misclassify 28 men as having clinically significant prostate cancer.
Systematic biopsies will correctly identify 189 of 300 men as having clinically significant prostate cancer but miss the remaining 111 men; for the 700 men that do not have clinically significant prostate cancer, systematic biopsies may correctly identify all 700 as not having prostate cancer and will not misclassify any men as having clinically significant prostate cancer.
When comparing the MRI pathway to systematic biopsy in a mixed group of men who may or may not have had a prior biopsy, we found that MRI pathway is 12% more likely to make the correct diagnosis. In men without a prior biopsy, the MRI pathway is 5% more likely to make the correct diagnosis, whereas in men who have had a negative biospy, it is 44% more likely to make the correct diagnosis.
How reliable is the evidence?
We rated the quality of evidence for the main findings of this review as low. Additional high‐quality research is likely to change these findings.
What are the implications of this review?
The findings of this Cochrane review suggest that the MRI pathway is better than systematic biopsies in making a correct diagnosis of clinically significant prostate cancer. However, the MRI pathway still misses some men with clinically significant prostate cancer. Therefore, further research in this area is important.
Summary of findings
Background
Target condition being diagnosed
Prostate cancer is the most frequently diagnosed solid cancer among men in high‐income countries (Torre 2015). Prostate cancer is the sixth leading cause of cancer death (7.4% of deaths) among men worldwide (Center 2012). A large proportion of prostate cancer, however, is indolent and will not lead to any complaints or death if left undetected (Bell 2015). When indolent prostate cancer is detected, it can be managed by active surveillance and does not necessarily need direct treatment. In contrast, clinically significant prostate cancer has direct therapeutic implications as it may progress, metastasise and lead to prostate cancer‐specific mortality.
Next to the psychological burden of becoming a cancer patient, the harm of overdiagnosing indolent prostate cancer mainly lies in overtreatment, as many men are still offered radical prostatectomy or radiotherapy. Given the sharp increase in prostate‐specific antigen (PSA)‐testing, prostate cancer diagnoses and the increasing concerns of overdiagnosis and overtreatment, the distinction between indolent and clinically significant prostate cancer has become more important (Ilic 2013). Defining clinically significant prostate cancer, however, remains difficult with varying definitions in the world literature (Moore 2013a). Established definitions are based on histologic parameters scored by the Gleason grading (Epstein 2010), or the International Society of Urological Pathology (ISUP) grade systems (Epstein 2016), with some using additional parameters like PSA, familial history, race or volume of cancer (Epstein 1994; Goto 1996; Harnden 2008; Wolters 2011). Moreover, other clinical parameters such as age and comorbidity may also influence the potential for progression and mortality of the individual with prostate cancer.
Clinical pathway
Opportunistic PSA‐based screening is practised worldwide and men considered to be at risk of clinically significant prostate cancer (elevated PSA level, abnormal digital rectal examination, African‐American origin and positive family history) are generally advised to have a systematic biopsy (Carter 2013; Carroll 2016; Mottet 2017). Prediction models and clinical risk calculators, using a variety of clinical parameters and biomarkers, are being investigated and implemented to help select patients for biopsy (Alberts 2019; Ankerst 2018; Ferro 2016; Foley 2016; Radtke 2017). The systematic biopsy may be repeated several times in the case of persistent suspicion of clinically significant prostate cancer after a prior‐negative biopsy or during active surveillance of indolent prostate cancer.
Any prostate biopsy is associated with a risk of infection (1% to 8%) and an increased risk of life‐threatening sepsis (1% to 4%), as a consequence of increasing antibiotic resistance (Borghesi 2017; Loeb 2013). Other associated morbidities include dysuria, hematospermia, haematuria, rectal bleeding, vasovagal episodes and urinary retention (Djavan 2001; Loeb 2013). These drawbacks of prostate biopsy limit the willingness of physicians and patients to perform and undergo potentially unnecessary biopsies.
In contrast with systematic biopsy, magnetic resonance imaging (MRI)‐targeted biopsy is only performed when suspected lesions for clinically significant prostate cancer are detected on MRI. Due to the selective performance of targeted biopsies, the MRI, with MRI‐targeted biopsy, is able to more accurately detect clinically significant prostate cancer while purposefully detecting less indolent prostate cancer (Schoots 2015; Siddiqui 2015). Therefore, MRI and MRI‐targeted biopsy are increasingly investigated in addition to or as a replacement for systematic biopsy, either in the setting of prior‐negative biopsy, initial biopsy or during active surveillance. Studies have shown that MRI and MRI‐targeted biopsy significantly improved the detection rate in the prior‐negative biopsy men, but not in biopsy‐naïve men (Schoots 2015; Valerio 2015). Moreover, randomised controlled trials performed in biopsy‐naïve men provide contradictory findings as to whether or not MRI with MRI‐targeted biopsy has a higher detection rate for clinically significant prostate cancer as compared to systematic biopsy (Baco 2016; Kasivisvanathan 2018; Panebianco 2015; Porpiglia 2017; Tonttilla 2016). Consequently, international guidelines recommend considering the use of MRI and MRI‐targeted biopsy, if available, in the setting of persistent clinical suspicion of prostate cancer after prior‐negative biopsy (AUA Guideline 2018; EAU Guideline 2018). However, international guidelines do not recommend a pre‐biopsy MRI or upfront MRI‐directed biopsy management in biopsy‐naïve men, let alone MRI‐directed biopsy management as an alternative to systematic biopsy. Figure 1 illustrates the clinical pathway and design of this review.
1.

Clinical pathway flow diagram and study design
Index tests
MRI
MRI is used to identify and locate suspicious lesions for clinically significant prostate cancer. Different MRI techniques and MRI systems from different vendors are used worldwide. The multiparametric pulse sequences are T2‐weighted imaging (T2W), diffusion‐weighted imaging (DWI), dynamic contrast‐enhanced (DCE) imaging and spectroscopy. Furthermore, different MRI magnets on different platforms from different vendors exist.
In addition, several scoring systems for the suspicion of prostate cancer on MRI have been developed. Radiologists use multi‐level scoring systems according to the Likert scale principle; where the presence of clinically significant prostate cancer in a lesion can be subjectively categorised as highly unlikely to highly likely, with a varying number of subdivisions. The 1 to 5 scale according to the Prostate Imaging ‐ Reporting and Data System (PI‐RADS) version 2 (Weinreb 2016), provides guidance for radiologists with more objective criteria and is currently most often used.
MRI‐targeted biopsy
MRI‐targeted biopsy in men with a positive MRI can either be performed with MRI‐guidance within the MRI scanner (in‐bore), or by ultrasound guidance with the use of computer‐based software that overlays the target identified on MRI onto the ultrasound image, 'software registration', or without the use of software, 'visual registration'. No significant differences in clinically significant prostate cancer detection appear to exist between these navigational approaches (Moore 2013a; Schoots 2015; Wegelin 2017).
MRI pathway
The MRI pathway (MRI with or without MRI‐targeted biopsy) comprises the performance of an MRI and subsequent performance of MRI‐targeted biopsies if a suspicious lesion is seen. Therefore, men with a negative MRI do not receive MRI‐targeted biopsy.
Systematic biopsy
Systematic transrectal ultrasound (TRUS)‐guided biopsy is a biopsy technique in which the peripheral zone of the prostate is sampled by 8 to 12 cores (with a maximum of 19), depending on the size of the prostate. TRUS is performed primarily for anatomic guidance, as suspicious lesions for prostate cancer, in general, cannot be visualised by ultrasound. This approach may, therefore, result in random and systematic errors, which can lead to hitting insignificant lesions while missing significant lesions (El‐Shater Bosaily 2015). The estimated false‐negative rate of systematic biopsy for any cancer is 25% to 40% (Hu 2012). Also, misclassification occurs by not hitting the cancer lesion at its greatest diameter or highest grade, shown by reclassification in almost half of men when a more accurate biopsy test is applied (Barzell 2007; Barzell 2012; Taira 2010; Taira 2013).
Alternative test(s)
Different biopsy approaches, such as transrectal or transperineal, with different numbers of biopsy cores are used. Transrectal saturation biopsy (defined as more than 20 biopsies of the prostate) aims comprehensively to sample the prostate (Kuru 2013b). However, most transrectal biopsy approaches do not sample the anterior zones of the prostate and therefore lack accuracy. In addition, such an intensified biopsy approach is less frequently used in daily clinical practice as it is widely seen as being a high burden to patients, having an increased complication rate and contributing to overdiagnosing insignificant prostate cancer (Jiang 2013). Furthermore, different ultrasound imaging techniques for localizing suspicious lesions in the prostate are also being developed and evaluated, including contrast‐enhanced ultrasound, computer‐assisted TRUS, sonoelastography and histoscanning. However, these techniques need further development before considering a potential application in daily clinical care (Kuru 2015).
Rationale
To reduce overdiagnosis and overtreatment of indolent prostate cancer, while improving the detection of clinically significant prostate cancer and reducing the number of biopsy procedures, we need more accurate diagnostic methods and better risk‐stratification (Alberts 2015). In a recent international multicentre randomised controlled trial, MRI in combination with MRI‐targeted biopsy (the MRI pathway) detected 12% more clinically significant prostate cancer and 13% less indolent prostate cancer than systematic biopsy in biopsy‐naïve men, and achieved a 28% reduction of biopsies, because men with a negative MRI did not receive prostate biopsy (Kasivisvanathan 2018). These results indicate that a pre‐biopsy MRI and MRI‐targeted biopsy in the presence of an MRI‐suspicious lesion would be superior to a systematic biopsy. If that is confirmed by other studies and longer follow‐up of those men not biopsied, it may initiate a change to the guidelines.
Previous systematic reviews on diagnostic performances of the MRI pathway or the pre‐biopsy MRI approach written by De Rooij 2014a, Futterer 2015, Gayet 2016, Hamoen 2015, Moore 2013b, Schoots 2015, Valerio 2015 and Van Hove 2014 have been based on study designs that did not accurately capture target conditions and index or reference test definitions, leading to a number of biases and inaccurate findings. Studies in these reviews included mainly men with a positive MRI, and disregarded men with a negative MRI, inevitably leading to inaccurate true‐negative and false‐negative values of the MRI pathway. In addition, they used systematic biopsy or radical whole‐mount surgical specimens as reference standards, which inherently have a number of biases: systematic biopsy may miss clinically significant prostate cancer caused by both random and systematic errors, whereas radical whole‐mount surgical specimens are only available for men with a positive biopsy who opted for surgery. Furthermore, the established definitions of clinically significant prostate cancer, based on histology from systematic biopsy and possibly additional non‐histological parameters, cannot be applied to results from the MRI pathway (Robertson 2014). The intention of the MRI pathway is to oversample areas of high suspicion, with the result that MRI‐targeted biopsies tend to show longer cancer core length and higher Gleason grading than systematic biopsies (Haffner 2011). This results in a drift towards higher risk classification, which is an artefact of the MRI‐targeted sampling method and may prompt men and physicians to more radical treatment. Based on these observations, the International Working Group on Standards of Reporting for MRI‐targeted biopsy studies (START) agreed that definitions of clinical significance in MRI‐targeted biopsy studies should solely focus on histologic definitions, that is, Gleason grade and maximum cancer core length (Moore 2013a).
Considering the above information, we performed a systematic review and meta‐analysis of the literature. We only included studies with data on both MRI‐positive and ‐negative men, that reported histologically confirmed target conditions only. Furthermore, we only included studies that used an appropriate reference standard (described in Reference standards) for the test accuracy analyses. To provide additional evidence where test accuracy evidence was limited, we selected from the agreement evidence only those studies that investigated the MRI pathway and systematic biopsy in the same men according to the above‐stated criteria.
We aimed to assess the diagnostic accuracy of the four index tests (MRI, MRI‐targeted biopsy, the MRI pathway and systematic biopsy) and the agreement between the two main index tests (the MRI pathway versus systematic biopsy) for detecting prostate cancer.
Objectives
Primary objective
To determine the diagnostic accuracy of the index tests MRI only, MRI‐targeted biopsy, MRI pathway (MRI with or without MRI‐targeted biopsy) and systematic biopsy as compared to template‐guided biopsy as the reference standard in detecting ISUP grade 2 or higher, grade 3 or higher and grade 1 prostate cancer.
Secondary objectives
To compare the diagnostic accuracy between the index tests MRI only, MRI‐targeted biopsy, MRI pathway (MRI with or without MRI‐targeted biopsy) and systematic biopsy in detecting grade 2 or higher, grade 3 or higher and grade 1 prostate cancer.
To determine the agreement between the two index tests, the MRI pathway and systematic biopsy, for detecting grade 2 or higher, grade 3 or higher and grade 1 prostate cancer.
To determine the proportion of prostate cancer not detected by systematic biopsy but only by the MRI pathway (added value MRI pathway) and the proportion of prostate cancer not detected by the MRI pathway but only by systematic biopsy (added value systematic biopsy) for grade 2 or higher, grade 3 or higher and grade 1 prostate cancer.
To determine the potential change in the number of biopsy procedures between the MRI pathway and systematic biopsy in the test accuracy and the agreement analyses.
To investigate what clinical and methodological sources of heterogeneity affect the index tests, including type of population (prior‐negative biopsy or biopsy‐naïve), MRI pulse sequences (mpMRI or bpMRI or additional spectroscopy), MRI scoring system, MRI suspicion score threshold for MRI‐targeted biopsy, navigational approach of MRI‐targeted biopsy, MRI lesion location, number of biopsy cores (or biopsy density) and core distribution in the reference standard.
Methods
Criteria for considering studies for this review
Types of studies
We considered any cross‐sectional study, if it investigated:
the diagnostic accuracy of one or more of the index tests (MRI, MRI pathway (including MRI‐targeted biopsy) or systematic biopsy) verified by the reference standard (template‐guided biopsy), with each index test and reference standard performed in the same men or compared as in a randomised trial of test accuracy; or
agreement evidence between the MRI pathway and systematic biopsy, with each test performed in the same men.
Studies involving MRI had to report on both MRI‐positive and MRI‐negative men.
We excluded studies when we could not extract a complete two‐by‐two table on a per‐participant basis for the primary target condition, even after contacting the study authors.
We did not apply any language or other restrictions.
Participants
The study population consisted of men with a clinical suspicion of prostate cancer (based on PSA or digital rectal exam (DRE) outcome) in the biopsy‐naïve or prior‐negative biopsy setting (or a mix of both). We excluded men with a previous diagnosis of prostate cancer.
Index tests
MRI
MRI was comprised of at least T2‐weighted imaging and one functional imaging technique (DWI or DCE), reported according to any MRI‐scoring system. The assessment categories for prostate MRI are based on a 5‐point scale (Likert or PI‐RADS), defined as very low (1), low (2), intermediate (3), high (4) and very high (5) (Dickinson 2011; Weinreb 2016). We defined the default threshold for MRI‐positivity as 3/5 or more where possible. We categorised thresholds from related assessment scores such as 2/4 or more, 6/10 or more and 5/15 or more as low, intermediate and high, based on expert opinion, for the purpose of heterogeneity analyses. We performed sensitivity analyses with studies that used a threshold of 3/5 or more. We performed additional analyses by increasing or decreasing the MRI‐positivity threshold, categorizing the MRI scores into 4/5 or more and 2/5 or more. We based all the analyses on per‐participant analysis and not on per‐lesion analysis, therefore, we did not take into account spatial concordance between MRI findings and biopsy findings.
MRI‐targeted biopsy
MRI‐targeted biopsy included only MRI‐positive men. We included all methods for MRI‐targeted biopsy (direct in‐bore, visual‐registration or software‐registration). We extracted data for this index test from studies reporting on the MRI pathway verified by the reference standard. We defined a positive MRI‐targeted biopsy as a histopathological confirmation of one of the target conditions in the MRI‐targeted biopsy cores.
The MRI pathway
The MRI pathway included MRI‐positive men (in whom MRI‐targeted biopsy was performed) and MRI‐negative men (in whom no MRI‐targeted biopsy was performed), reflecting the complete spectrum of men in the clinical population. We defined a positive MRI pathway as a histopathological confirmation of one of the target conditions by MRI‐targeted biopsy in MRI‐positive men. Therefore, we defined a negative MRI pathway as a negative MRI or a negative MRI‐targeted biopsy Appendix 1.
Systematic biopsy
Systematic biopsy included either systematic transrectal or transperineal ultrasound‐guided biopsies, with generally 8 to 12 cores dedicated to the peripheral zone of the prostate; we excluded studies on additional ultrasound imaging techniques. We defined a positive systematic biopsy as a histopathological confirmation of one of the target conditions in the biopsy cores.
Target conditions
The primary target condition was clinically significant prostate cancer, defined as ISUP grade 2 or higher, based on histopathology findings and scored as Gleason score (GS) 3 + 4 or higher (Epstein 2016). Secondary target conditions were grade 1 (GS 3 + 3, indolent prostate cancer) and grade 3 or higher (GS 4 + 3 or higher). We based all target conditions on ISUP grade only, without cancer volume criteria, in order to overcome differences between definitions and biopsy methods, according to START guidelines (Moore 2013a).
Reference standards
Template‐guided biopsy served as the reference standard. In general, two different techniques are used: the transperineal template‐guided mapping biopsy (TTMB) and the template‐guided saturation biopsy (TSB). TTMB is defined as “transperineal TRUS‐guided biopsies of the prostate performed with the patient in lithotomy position using a 5‐mm brachytherapy grid, with at least one biopsy from each hole”. TSB is defined as “20 or more transperineal or transrectal TRUS‐guided biopsies of the prostate performed with the intention to comprehensively sample the whole prostate, according to a predefined core distribution pattern” (Kuru 2013b; Sivaraman 2015). Template‐guided biopsies using a uniform grid and taken at 5 mm intervals can technically only miss those tumours that are smaller than the distance between the adjacent cores (Ahmed 2011; Sivaraman 2015). The sensitivity and negative predictive value of this technique for detecting grade 2 or higher prostate cancer 0.5 cm3 or greater in volume have both been shown to be 95%, with a sensitivity of 76% for detecting all cancers (Ahmed 2011; Crawford 2013; Simmons 2014). Although the template‐guided biopsy is not perfect, owing to the fact that the test accuracy depends on the intensity of cores taken and core trajectory (Huo 2012; Pham 2015; Valerio 2015), it is the optimal reference standard, as it avoids the biases of other reference standards that have been used as described in the Rationale. An alternative approach could be to use template‐guided biopsy in combination with other biopsy methods (a ‘composite’ reference standard) to overcome the inadequacy of template‐guided biopsy only; however, this would introduce incorporation bias.
Therefore, in this analysis, we used only template‐guided biopsy as the reference standard. Template‐guided biopsy had to comprehensively sample all (including the anterior) zones of the prostate, with a minimum of 20 biopsy cores. We defined a positive template‐guided biopsy as histopathological confirmation of one of the target conditions within the biopsy cores. We used the alternative composite reference standard in the sensitivity analyses.
Search methods for identification of studies
Electronic searches
We performed a comprehensive search, with no restriction on language of publication or publication status, in the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 7) in the Cochrane Library (searched 31 July 2018), including ClinicalTrials;
MEDLINE Ovid, including electronic publications ahead of print (from inception to 31 July 2018);
Embase.com (from inception to 31 July 2018);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; from inception to 31 July 2018);
Web of Science (Core Collection) (from inception to 31 July 2018);
Scopus (from inception to 31 July 2018);
Google.com (31 July 2018);
Google Scholar (31 July 2018);
WorldCat (31 July 2018);
ProQuest (ProQuest Dissertations & Theses; 31 July 2018);
OpenGrey (31 July 2018).
The search strategies are provided in Appendix 2.
Searching other resources
We searched for additional references in the Science Citation Index of Web of Science and by manually searching the references of relevant articles.
We also searched the following trials registers for planned or ongoing studies:
ClinicalTrials.gov (31 July 2018);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 31 July 2018);
Open trials (https://opentrials.net/, searched 31 July 2018).
We searched Embase and Web of Science for conference proceedings.
Data collection and analysis
Selection of studies
We checked the primary search results for overlapping content and Cochrane Urology's Information Specialist deduplicated the search results (Bramer 2016). Two reviewers (FD, DO) independently screened all abstracts and full‐text articles for eligibility according to the Criteria for considering studies for this review. We contacted study authors to obtain additional information when reported data were insufficient. When more than one publication on the same cohort was found, we selected the most complete publication. We resolved disagreements by consensus (FD, DO and IS).
Data extraction and management
Two review authors (FD, DO) extracted data using a predefined data‐extraction form. FD and DO extracted variables on study methodology, patient characteristics, test characteristics, the definition of target conditions and results. We constructed two‐by‐two tables for cross‐classification of the index tests versus reference standard for test accuracy data, and the MRI pathway versus systematic biopsy for agreement data, based on per‐participant data (Appendix 1). We contacted study authors to obtain additional information when necessary. We resolved any data extraction disagreements by consensus (FD, DO, IS).
Assessment of methodological quality
Two review authors (FD, DO) independently assessed all included studies for methodological quality using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool (Whiting 2011), tailored to this review (Table 7). We resolved any discrepancies by discussion (FD, DO, IS).
1. QUADAS‐2 tool for assessing methodological quality of included studies.
| Domain 1: Participant selection | |
| SQ 1: Was a consecutive or random sample of participants enrolled? | Yes: if stated that participants were consecutively or randomly selected No: if one of these criteria was not met Unclear: if insufficient information to make a judgement |
| SQ 2: Did the study avoid inappropriate exclusions? | Yes: if stated that the study did not exclude men 1) aged between 50 and 70 years, 2) with PSA values between 4 and 10 ng/mL, or 3) with an abnormal DRE No: if one of these criteria was not met Unclear: insufficient information to make a judgement |
|
Risk of bias Could the selection of participants have introduced bias? |
Low risk: if ‘Yes’ for all SQ's High risk: if ‘No’ for at least 1 SQ Unclear risk: if 'Unclear' for at least 1 SQ |
|
Concerns for applicability Are there concerns that the included participants and setting do not match the review question? |
Low concern: the participants were referred because of a suspicion of prostate cancer. High concern: the participants were not referred because of a suspicion of prostate cancer, e.g. PSA‐screening trials are less applicable to the current clinical practice. Unclear concern: insufficient information to make a judgement |
| Domain 2: Index texts | |
| SQ 1: If applicable, was the MRI assessed without knowledge of the results of the reference (or other index) biopsies? | Yes: if stated that the radiologist was unaware of all biopsy results; or, if the order of testing was MRI before all biopsies for every participant No: if stated that the radiologist was aware of any biopsy results during MRI assessment Unclear: insufficient information to make a judgement |
| SQ 2: If applicable, were the MRI‐targeted biopsies performed independently of the performance and the results of the reference (or other index) biopsies? | Yes: if stated that the performance of MRI‐targeted biopsies was not influenced by the performance or trajectory of reference (or other index) biopsies No: if stated that MRI‐targeted biopsies were not, or differently, taken from locations already hit by the reference (or other index) biopsies; or, if the performance of MRI‐targeted biopsies was dependent on the judgement of the same operator that also performed the reference (or other index) biopsies without blinding Unclear: insufficient information to make a judgement |
| SQ 3: If applicable, were the systematic biopsies taken independently of the performance and the results of the reference (of other index) biopsies? | Yes: if stated that the systematic biopsies were taken blinded for
No: if stated that the systematic biopsy operator was not blinded for MRI results, or was the same operator that also performed the reference (or other index) biopsies without blinding Unclear: insufficient information to make a judgement |
|
Risk of bias Could the conduct or interpretation of the index test have introduced bias? |
Low risk: ‘Yes’ for all applicable SQs High risk: ‘No’ for at least one applicable SQ Unclear risk: ‘Unclear’ for at least one applicable SQ |
|
Concerns for applicability Are there concerns that the index tests, their conduct or their interpretation differ from the review question? |
Low concern: if stated that, when applicable,
High concern: the index test did not meet the criteria above Unclear concern: insufficient information to make a judgement |
| Domain 3: Reference standard | |
| SQ1: Is the reference standard likely to correctly classify the target condition? (i.e. Is histological diagnosis made from appropriately sampled tissue?) | Yes: if stated that the whole prostate was comprehensively sampled by a full 5‐mm transperineal TTMB, or by a equivalently well described transperineal template‐guided biopsy method with a prostate volume based median of ≤ 20 biopsy cores. No: one of these criteria was not met (i.e. in‐house transperineal saturation biopsy or transrectal saturation biopsy are less likely to appropriately sample the whole prostate). Unclear: insufficient information to make a judgement |
| SQ2: Was the reference standard performed independent of the index test? | Yes: if stated that the reference biopsies were taken without knowledge of the MRI‐score and location of target lesions; and, if incorporation was avoided (i.e. the index test was not part of the reference standard). No: one of these criteria was not met Unclear: insufficient information to make a judgement |
|
Risk of bias Could the reference standard, its conduct, or its interpretation have introduced bias? |
Low risk: 'Yes’ for all SQs High risk: ’No’ for at least 1 of the 3 SQs Unclear risk: ’Unclear’ for at least 1 SQ |
|
Concerns for applicability Are there concerns that the target condition as defined by the reference standard does not match the question? |
Low concern: data were presented for GS ≥ 3+4 without any volume criteria (ISUP grade ≥ 2), if necessary after requesting additional data from study authors High concern: data were presented for an alternative target condition definition and study authors did not provide additional data. Unclear: insufficient information to make a judgement |
| Domain 4: Flow and timing | |
| SQ1: Did all participants receive the same biopsy methods (i.e. was differential verification avoided)? | Yes: if stated that all participants received the same type of index test(s) and reference standard, prostate volume dependency was allowed. No: if one of these criteria was not met Unclear: if insufficient information to make a judgement |
| SQ2: Were all enrolled participants included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes: if stated that all eligible participants were enrolled and included in the final analyses; or, if reasons to excluded participants did not cause a relevant bias (e.g. participants with claustrophobia who refused MRI). No: one of these criteria was not met. Unclear: if insufficient information to make a judgement |
|
Risk of bias Could the participant flow have introduced bias? |
Low risk: ’Yes’ for all SQs High risk: ’No’ for at least 1 SQ Unclear risk: ’Unclear’, for at least 1 SQ |
| DCE: dynamic contrast‐enhanced; DRE: digital rectal examination; DWI: diffusion‐weighted imaging; MRI: magnetic resonance imaging; PSA: prostate‐specific antigen; QUADAS: Quality Assessment of Diagnostic Accuracy Studies; SQ: signalling question; TTMB: template‐guided mapping biopsy; ISUP: International Society of Urological Pathology | |
Statistical analysis and data synthesis
For the test accuracy analyses (MRI, MRI‐targeted biopsy, MRI pathway, systematic biopsy versus reference standard (template‐guided biopsy)), we calculated pooled estimates of sensitivity and specificity using the bivariate model, in accordance with the Cochrane Handbook for Diagnostic Test Accuracy Reviews (Macaskill 2010). Furthermore, we assessed heterogeneity graphically using paired forest plots of sensitivity and specificity (Macaskill 2010). If we observed little or no heterogeneity, we considered simplifications of the bivariate models by dropping the correlation between sensitivity and specificity. We compared index tests by combining all the studies that investigated the index test of interest and adding a covariate to the bivariate model for the type of index test. We used likelihood ratio tests to assess whether the pooled sensitivity and specificity differed significantly between index tests. We based prevalences on the number of prostate cancers detected by the reference standard.
For the agreement analysis (MRI pathway versus systematic biopsy), we focused on the number of target conditions identified (concordance and discordance of test results) because neither test is a valid reference test. We calculated the proportion of detected cases (total number of cancers) as the number of concordant positive results plus the number of discordant positive results of both tests (Appendix 1). We calculated the detection rate of either test as the number of positive results of that test divided by the total number of cancers detected. We synthesised pooled estimates of detection ratios (detection rate of the MRI pathway:detection rate of systematic biopsy) by performing random‐effects meta‐analyses. We calculated the variance of the detection ratio taking into account the paired data in the analysis. We pooled the detection ratio on a log‐scale and used the delta method to estimate the standard error of the detection ratio on the log scale.
To calculate pooled proportions of prostate cancer not detected by systematic biopsy but only by the MRI pathway (added value MRI pathway) and pooled proportions of prostate cancer not detected by the MRI pathway but only by systematic biopsy (added value systematic biopsy), we used mixed models (multinomial logistic regression models with a random intercept for study effects). To calculate the pooled proportions of participants with prostate cancer and a negative MRI, we performed a random‐effects meta‐analysis on these proportions after transformation to the log‐odds scale. The added‐value data were constructed such that we assessed the tests as add‐on tests (i.e. considering reclassification by each test) (Appendix 3). We based post‐test probability estimates (negative predictive values (NPV) and positive predictive values (PPV)) on Bayes’ theorem, using the point estimates and 95% confidence intervals of the pooled positive and negative likelihood ratio, with prevalences based on the test accuracy data and given clinically useful percentages (10% (low) to 50% (high)). We used Statistical Analysis Software (SAS) version 9.3 for Windows and R version 3.5.0 to perform all statistical analyses.
Investigations of heterogeneity
To explore sources of heterogeneity, we assessed the following covariates by adding them one by one in our bivariate model: population setting (biopsy naïve versus prior negative biopsy); MRI magnet strength (3 versus 1.5 T); MRI sequence (multiparametric MRI versus biparametric MRI); MRI positivity threshold (4/5 or more (high) versus 3/5 or more (intermediate) versus 2/5 or more (low)); use of endorectal coil; MRI‐targeted biopsy method (software versus visual registration); biopsy approach (transperineal versus transrectal); and radiologist experience (high versus little or unclear). We scored radiologist experience in studies as high when the radiologist was 'experienced', 'dedicated', a 'uro‐' or 'mpMRI‐radiologist', or when radiologists had prostate MRI training, more than one year's or more than 100 cases' experience in reading prostate MRI. We scored radiologist experience as 'little' when studies reported a lack of experience. We tested the same covariates using meta‐regression techniques for the detection ratio. To ensure adequate data for the analyses, we applied an arbitrary threshold of five studies for each subgroup of a covariate investigated in the analyses of heterogeneity.
Sensitivity analyses
To examine the robustness of our findings, we performed several sensitivity analyses, limited to studies meeting certain quality or additional criteria. The quality criteria comprised low risk of bias and no applicability concerns in the QUADAS‐2 domains. The additional criteria comprised:
using an MRI positivity threshold of 3/5 of more;
tests with head‐to‐head comparative data only (MRI versus the MRI pathway; MRI positivity threshold effect (3/5 or more to 4/5 or more));
comparison within the same study (biopsy naïve versus prior negative biopsy);
a reference standard with template‐guided biopsy via the transperineal approach;
a composite reference standard (template‐guided biopsy and MRI‐targeted biopsy); and
highly experienced radiologist(s).
Assessment of reporting bias
We did not assess reporting bias, since there is no evidence of reporting bias in test accuracy reviews nor is there a reliable method to detect this (Deeks 2005).
Certainty of evidence and summary of findings tables
We rated the certainty of evidence on a per‐outcome basis according to Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidance for studies of diagnostic accuracy (Schünemann 2008). GRADE takes into account five criteria related not only to internal validity (study limitations or risk of bias, inconsistency, imprecision, publication bias), but also to external validity (directness of results). We applied the following methods:
Study limitations and risk of bias: We used QUADAS‐2 to assess risk of bias.
Indirectness: We considered indirectness from the perspective of test accuracy. We used QUADAS‐2 for concerns of applicability and looked for important differences between the populations studied (for example, in the spectrum of disease) and the setting.
Inconsistency: We assessed pooled sensitivity and specificity estimates for clinically important inconsistency and downgraded if this remained unexplained by prespecified secondary analyses.
Imprecision: We used a contextualized approach and considered a precise estimate to be one that would allow a clinically meaningful decision. When assessing the need to downgrade for imprecision, we assessed whether an effect size taken from the upper or lower boundary of the confidence intervals for our projected true positives, false negatives, true negatives and false positives for a given prevalence would have changed these clinical judgments about the usefulness of a given test.
Publication bias: See above.
For the four main comparisons, we rated the certainty of evidence for true positives and false negatives as well as true negatives and false positives as 'high', 'moderate', 'low', or 'very low' using GRADEpro GDT. We present summaries of the evidence in 'Summary of findings' tables (Table 3; Table 4; Table 5; Table 6), which provide key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the confidence in effect estimates.
Summary of findings 3. Should MRI be used to diagnose ISUP grade ≥ 2 prostate cancer in men suspected of having clinically significant prostate cancer?
| Question: Should MRI be used to diagnose ISUP grade 2 or higher prostate cancer in men suspected of having clinically significant prostate cancer? | |||||
| Population: men suspected of having clinically significant prostate cancer undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||
| Setting: university hospitals and specialized care centers | |||||
| New test: MRI only | Cut‐off value: MRI score ≥ 3 out of 5 | |||||
| Reference test: template‐guided biopsy, which comprehensively samples all zones of the prostate | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Pooled sensitivity: 0.91 (95% CI: 0.83 to 0.95) | Pooled specificity: 0.37 (95% CI: 0.29 to 0.46) | |||||
| Test result | Number of results per 1,000 men tested (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence 10% | Prevalence 30% | Prevalence 40% | |||
| True positives | 9 (83 to 95) | 273 (249 to 285) | 364 (332 to 380) | 3091 (12) | ⊕⊕○○ LOWa, b |
| False negatives | 9 (5 to 17) | 27 (15 to 51) | 36 (20 to 68) | ||
| True negatives | 333 (261 to 414) | 259 (203 to 322) | 222 (174 to 276) | 3091 (12) | ⊕⊕○○ LOWa, b |
| False positives | 567 (486 to 639) | 441 (378 to 497) | 378 (324 to 426) | ||
|
MRI: magnetic resonance imaging; ISUP: International Society of Urological Pathology; CI: confidence interval aA considerable number of studies had a high or unclear risk of bias, mainly in the participant selection and reference standard domains. bA considerable, clinically relevant heterogeneity was observed across pooled study results. | |||||
Summary of findings 4. Should MRI‐targeted biopsy be used to diagnose ISUP grade ≥ 2 prostate cancer in men suspected of having clinically significant prostate cancer?
| Question: Should MRI‐targeted biopsy be used to diagnose ISUP grade 2 or higher prostate cancer in men suspected of having clinically significant prostate cancer? | |||||
| Population: men with a positive MRI suspected of having clinically significant prostate cancer undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||
| Setting: university hospitals and specialized care centers | |||||
| New test: MRI‐targeted biopsy | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Reference test: template‐guided biopsy, which comprehensively samples all zones of the prostate | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Pooled sensitivity: 0.80 (95% CI: 0.69 to 0.87) | Pooled specificity: 0.94 (95% CI: 0.90 to 0.97) | |||||
| Test result | Number of results per 1,000 men tested (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence 10% | Prevalence 30% | Prevalence 40% | |||
| True positives | 80 (69 to 87) | 240 (207 to 261) | 320 (276 to 348) | 1553 (8) | ⊕⊕○○ LOWa, b |
| False negatives | 20 (13 to 31) | 60 (39 to 93) | 80 (52 to 124) | ||
| True negatives | 846 (810 to 873) | 658 (630 to 679) | 564 (540 to 582) | 1553 (8) | ⊕⊕○○ LOWa, b |
| False positives | 54 (27 to 90) | 42 (21 to 70) | 36 (18 to 60) | ||
|
MRI: magnetic resonance imaging; ISUP: International Society of Urological Pathology; CI: confidence interval aA considerable number of studies had a high or unclear risk of bias, mainly in the participant selection and reference standard domains. bA considerable, clinically relevant heterogeneity was observed across pooled study results. | |||||
Summary of findings 5. Should an MRI‐pathway be used to diagnose ISUP grade ≥ 2 prostate cancer in men suspected of having clinically significant prostate cancer?
| Question: Should an MRI pathway be used to diagnose ISUP grade 2 or higher prostate cancer in men suspected of having clinically significant prostate cancer? | |||||
| Population: men suspected of having clinically significant prostate cancer undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||
| Setting: university hospitals and specialized care centers | |||||
| New test: MRI pathway | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Reference test: template‐guided biopsy, which comprehensively samples all zones of the prostate | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Pooled sensitivity: 0.72 (95% CI: 0.60 to 0.82) | Pooled specificity: 0.96 (95% CI: 0.94 to 0.98) | |||||
| Test result | Number of results per 1,000 men tested (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence 10% | Prevalence 30% | Prevalence 40% | |||
| True positives | 72 (60 to 82) | 216 (180 to 246) | 288 (240 to 328) | 2257 (8) | ⊕⊕○○ LOWa, b |
| False negatives | 28 (18 to 40) | 84 (54 to 120) | 112 (72 to 160) | ||
| True negatives | 864 (846 to 882) | 672 (658 to 686) | 576 (564 to 588) | 2257 (8) | ⊕⊕○○ LOWa, b |
| False positives | 36 (18 to 54) | 28 (14 to 42) | 24 (12 to 36) | ||
| MRI pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; ISUP: International Society of Urological Pathology; CI: confidence interval aA considerable number of studies had a high or unclear risk of bias, mainly in the participant selection and reference standard domains. bA considerable, clinically relevant heterogeneity was observed across pooled study results. | |||||
Summary of findings 6. Should systematic biopsy be used to diagnose ISUP grade ≥ 2 prostate cancer in men suspected of having clinically significant prostate cancer?
| Question: Should systematic biopsy be used to diagnose ISUP grade 2 or higher prostate cancer in men suspected of having clinically significant prostate cancer? | |||||
| Population: men suspected of having clinically significant prostate cancer undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||
| Setting: university hospitals and specialized care centers | |||||
| New test: systematic biopsy | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Reference test: template‐guided biopsy, which comprehensively samples all zones of the prostate | Threshold: ISUP grade 2 or higher prostate cancer | |||||
| Pooled sensitivity: 0.63 (95% CI: 0.19 to 0.93) | Pooled specificity: 1.00 (95% CI: 0.91 to 1.00) | |||||
| Test result | Number of results per 1,000 men tested (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence 10% | Prevalence 30% | Prevalence 40% | |||
| True positives | 63 (19 to 93) | 189 (57 to 279) | 252 (76 to 372) | 3421 (4) | ⊕⊕⊕○ MODERATEa, b, c |
| False negatives | 37 (7 to 81) | 111 (21 to 243) | 148 (28 to 324) | ||
| True negatives | 900 (819 to 900) | 700 (637 to 700) | 600 (546 to 600) | 3421 (4) | ⊕⊕○○ LOWa, b, c |
| False positives | 0 (0 to 81) | 0 (0 to 63) | 0 (0 to 54) | ||
|
ISUP: International Society of Urological Pathology; CI: confidence interval aA considerable number of studies had a high or unclear risk of bias, mainly in the participant selection and reference standard domains. bA considerable, clinically relevant heterogeneity was observed across pooled study results. cImportant imprecision was noted, which contributed to decision to downgrade for inconsistency. | |||||
Results
Results of the search
Of the 18,286 records found through the search strategy, we assessed 551 full‐text articles for eligibility (Figure 2). A total of 43 studies were eligible for inclusion in this review and provided data for multiple tests. We present study and patient baseline characteristics per test in Table 8 and Table 9 for the test accuracy analysis and Table 10 and Table 11 for the agreement analysis (and Appendix 4).
2.
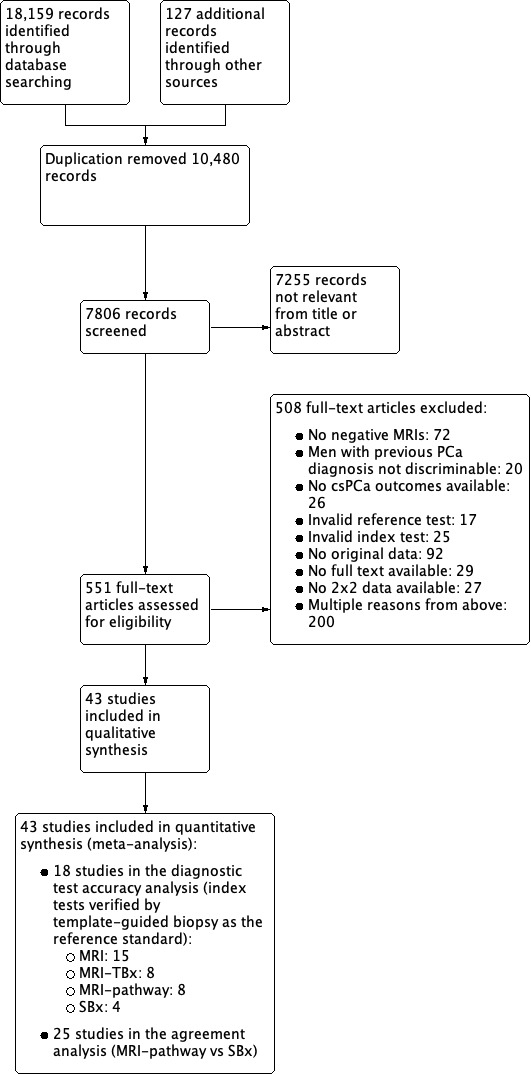
Study flow chart csPCa: clinically significant prostate cancer; MRI: magnetic resonance imaging; MRI pathway: magnetic resonance imaging with subsequent magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; SBx: systematic biopsy
2. Study characteristics of the diagnostic test accuracy analyses studies.
| Study | MRI | Index biopsy | Reference standard | Target conditions | |||||
| Study | Consecutive enrolment (study designa) | N of participants | Index test(s) | MRI‐scale; threshold | MRI‐TBx Technique/route | Technique | Median N cores (range) | Independence | ISUP grade (G) |
| Abd‐Alazeez 2014 | No (retrospective) | 54 | MRI | 1‐5; ≥ 3 | Cognitive/transperineal | TTMB | 45 (21‐137) | No | G = 1 G ≥ 2 G ≥ 3 |
| Ahmed 2017 | Yes (prospective) | 576 | MRI, SBx | 1‐5; ≥ 3 | NA/transrectal | TTMB | > 40b | Yes | G = 1 G ≥ 2 G ≥ 3 |
| Dal Moro 2019 | Yes (prospective) | 123 | MRI, MRI‐TBx, MRI‐pathway | 1‐5; ≥ 3 | Cognitive/transrectal | TSBc | 24d | Yes | G = 1 G ≥ 2 G ≥ 3 |
| Distler 2017 | Yes (prospective) | Bx‐naïve: 597 Prior‐negative Bx: 443 | MRI, MRI‐TBx, MRI‐pathway | 1‐5; ≥ 3 | Software/transperineal | TSBe | 24 (22‐25) | No | G ≥ 2 |
| Grey 2015 | Yes (prospective) | Bx‐naïve: 83 Prior‐negative Bx: 103 | MRI | 1‐5; ≥ 3 | Cognitive/transperineal | TSBe | (24‐40) | No | G = 1 G ≥ 2 G ≥ 3 |
| Hansen 2016a | Yes (prospective) | 295 | MRI, MRI‐TBx, MRI‐pathway | 1‐5; ≥ 3 | Software/transperineal | TSBe | (18‐24) | Unclear | G = 1 G ≥ 2 G ≥ 3 |
| Hansen 2018 | Yes (prospective) | Centre 1: 163 Centre 3: 242 | MRI | 1‐5; ≥ 3 | Software, cognitive/transperineal | TSBe | 24 (22‐26f), 20 (20‐21f) | No | G = 1 G ≥ 2 G ≥ 3 |
| Hansen 2017 | Unclear (prospective) | 287 | MRI, MRI‐TBx, MRI‐pathway | 1‐5; ≥ 3 | Software/transperineal | TSBe | 24 (24‐25) | Unclear | G ≥ 2 |
| Kesch 2017 | Unclear (prospective) | Bx‐naïve: 95 Prior‐negative Bx: 51 | MRI, MRI‐TBx, MRI‐pathway | 1‐5; ≥ 3 | Software/transperineal | TSBg | 24 (23‐27f) | Yes | G = 1 G ≥ 2 G ≥ 3 |
| Lawrence 2014 | No (retrospective) | 39 | MRI, MRI‐TBx, MRI‐pathway | 1‐4; ≥2 | Software/transperineal | TSBe | 24 (14‐34) | No | G = 1 G ≥ 2 |
| Mortezavi 2018 | Yes (retrospective) | 163 86 | MRI, MRI‐TBx, MRI‐pathway | 1‐5; ≥ 3 | Software/Transrectal | TSB | 40 (30‐55) | No | G = 1 G ≥ 2 G ≥ 3 |
| Muthuveloe 2016 | Unclear (retrospective) | 9 162 | MRI | 1‐5; ≥ 3 | NA | TSBh | 24 (24–28) | Unclear | G = 1 G ≥ 2 G ≥ 3 |
| Pepe 2013 | Unclear (prospective) | 78 | MRI, MRI‐TBx, MRI‐pathway | 0‐1: ≥1 | Cognitive/transrectal | TSBh | 28 (26‐32) | No | G = 1 G ≥ 2 |
| Thompson 2016 | Yes (prospective) | 344 | MRI | 1‐5; ≥ 3 | Software, cognitive/transperineal | TTMB | 30 | No | G = 1 G ≥ 2 G ≥ 3 |
| Tsivian 2017 | Unclear (retrospective) | 33 | MRI | 1‐5; ≥ 3 | NA | TTMB | 55 (42‐63f) | Yes | G = 1 G ≥ 2 G ≥ 3 |
| Nafie 2014 | Unclear (prospective) | 50 | SBx | NA | NA/transrectal | TSBh | 36 | Yes | G = 1 G ≥ 2 G ≥ 3 |
| Nafie 2017 | Unclear (prospective) | 42 | SBx | NA | NA/transrectal | TSBh | 36 | Yes | G = 1 G ≥ 2 |
| Ploussard 2014 | Yes (prospective) | 2753 | SBx | NA | NA/transrectal | TSBc | 21 | No | G = 1 G ≥ 2 |
| Bx: biopsy; ISUP G : International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; PI‐RADS v1, v2: Prostate Imaging Reporting Data System version 1 or 2; SBx: systematic biopsy; TSB: transperineal saturation biopsy; TTMB: transperineal template mapping biopsy | |||||||||
aIncluded participants were part of the same study cohort (no randomised populations were included). bNot reported but estimated. cTransrectal. dMean value (as opposed to median). eGinsburg biopsies. fInterquartile range (as opposed to range). gTransperineal optimised prostate biopsy (TOP). hIn‐house transperineal saturation biopsy
3. Patient characteristics of the diagnostic test accuracy studies.
| Patient characteristics of the included diagnostic test accuracy studies | ||||
| Study | Population | Median age (range/SD) | Median PSA in ng/mL (range) | Median prostate volume in cm3 (range) |
| Abd‐Alazeez 2014 | Prior‐negative Bx | 64 (39‐75) | 10 (2‐23) | 53 (19‐136) |
| Ahmed 2017 | Bx‐naïve | 63 (7.6)a | 7.1 (2.9)a | NR |
| Dal Moro 2019 | Prior‐negative Bx | 62 (57‐68b) | 6.3 (4,8‐8,9b) | 55 (20‐149)a |
| Distler 2017 | Mixedc | 65 (60‐71b) | 7.2 (5.3‐10.4b) | 45 (34‐64b) |
| Grey 2015 | Mixedc | 64 (6.8)a 65 (7.6)a | 13.3 (12,1)a 12.6 (13.7)a | 68 (35)a 54 (31)a |
| Hansen 2016a | Prior‐negative Bx | 65 (59‐69b) | 7.8 (6.0‐12b) | 65 (44‐83b) |
| Hansen 2018 | Bx‐naïve | 64 (57‐69b) 65 (60‐70b) | 6.6 (4.6‐9.0b) 5.9 (4.6‐8.0b) | 44 (33‐55b) 25 (24‐47b) |
| Hansen 2017 | Prior‐negative Bx | 66 (61‐72b) | 9.7 (7.1‐13.9b) | 52 (36‐75b) |
| Kesch 2017 | Mixedc | 65 (58‐71b) | 7.2 (5.4‐10.2b) | 46 (36‐60b) |
| Lawrence 2014 | Prior‐negative Bx | 64 (47‐77)a | 10 (1.2‐36) | NR |
| Mortezavi 2018 | Bx‐naïve Prior‐negative Bx | 63 (57‐68b) 64 (60‐69b) | 5.8 (4.4‐8.9b) 8.6 (5.7‐13b) | 44 (34‐60b) 54 (41‐70b) |
| Muthuveloe 2016 | Bx‐naïve Prior‐negative Bx | 68 (46‐81) 65 (47‐78)d | 11.5 (1.2‐92.5) 10 (2.7‐61)d |
NR |
| Pepe 2013 | Prior‐negative Bx | 63 (49‐72) | 11 (3.7‐45) | NR |
| Thompson 2016 | Bx‐naïve | 63 (56‐67b) | 5.2 (3.7‐7.1b) | 40 (30‐54b) |
| Tsivian 2017 | Prior‐negative Bx | 65 (61‐69b) | 7.1 (5.1‐13.6b) | 44 (32‐65b) |
| Nafie 2014 | Bx‐naïve | 67 (54‐84)a | 8 (4‐18)a | 58 (19‐165)a |
| Nafie 2017 | Prior‐negative Bx | 65 (50‐75)a | 8.3 (4.4‐19)a | 59 (21‐152)a |
| Ploussard 2014 | Bx‐naïve | 64 (8)a | 12.5 (7.2)a | 46 (25)a |
| Bx: biopsy; NR: not reported; PSA: prostate specific antigen | ||||
aMean (standard deviation or range) (as opposed to median (range)). bInterquartile range (as opposed to range). cResults not reported per population type. dReported per transperineal saturation biopsy‐positive (n = 71) and transperineal saturation biopsy‐negative men (n = 103), respectively.
4. Study characteristics of the agreement analyses studies.
| Study | MRI | Index biopsy | Target conditions | ||||||
| Study | Consecutive enrolment (study designa) | N of participants | Index tests | MRI‐scale; threshold | MRI‐TBx | SBx | MRI‐TBx & SBx | ISUP grade (G) | |
| Technique | Median N cores (range) | Independence | Route | ||||||
| Alberts 2017 | Yes (prospective) | Bx‐naïve: 74 Prior‐negative Bx: 84 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Software | 12 (12‐12b) |
Yes | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Boesen 2017a | Unclear (prospective) | 206 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Software | 10 (10‐10) |
Yes | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Boesen 2018 | Yes (prospective) | 1020 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Software | 10c | Yes | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Castellucci 2017 | Yes (prospective) | 168 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Cognitive | (8‐19) | Unclear | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Chang 2017 | Yes (retrospective) | 65 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Cognitive | 18 (16.2‐19.8b) | No | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Chen 2015 | Yes (prospective) | 420 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Cognitive | 12d | Yes | Transperineal | G ≥ 2 |
| Cool 2016 | Unclear (prospective) | Bx‐naïve: 50 Prior‐negative Bx: 50 | MRI‐pathway vs. SBx | Other | Software | 12‐14e | Unclear | Transrectal | G = 1 G ≥ 2 |
| Costa 2013 | No (retrospective) | 38 | MRI‐pathway vs. SBx | 1‐5; ≥4 | Cognitive | NR | No | Transrectal | G ≥ 2 G ≥ 3 |
| Delongchamps 2013 | Yes (prospective) | 391 | MRI‐pathway vs. SBx | TZ: 0‐4; ≥2 PZ: 0‐10; ≥6 | Software Cognitive |
12 (10‐12) |
Unclear | Transrectal | G ≥ 2 |
| Filson 2016 | Yes (prospective) | Bx‐naïve: 329 Prior‐negative Bx: 324 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Software | 12 | Unclear | Transrectal | G ≥ 2 G ≥ 3 |
| Garcia Bennett 2017 | Unclear (prospective) | 60 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Cognitive | 12 | Yes | Transperineal | G = 1 G ≥ 2 G ≥ 3 |
| Grönberg 2018 | Yes (prospective) | Bx‐naïve: 387 Prior‐negative Bx: 145 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Software | 11 (10‐12) |
No | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Jambor 2015 | Unclear (unclear) | 53 | MRI‐pathway vs. SBx | 1‐5; ≥4 | Cognitive | 12 | Yes | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Jambor 2017 | Unclear (prospective) | Bx‐naïve: 134 Prior‐negative Bx: 27 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Cognitive | 12c | No | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Kim 2017 | Unclear (retrospective) | Bx‐naïve: 183 Prior‐negative Bx: 154 | MRI‐pathway vs. SBx | 1‐5; ≥4 | Software Cognitive | 14c | No | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Lee 2016 | Unclear (retrospective) | 76 | MRI‐pathway vs. SBx | 1‐4; ≥2 | Cognitive | 12 (12‐12) |
No | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Lee 2017 | Unclear (retrospective) | 123 | MRI‐pathway vs. SBx | 1‐4; ≥2 | Cognitive | 12 | No | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Okcelik 2016 | Unclear (prospective) | 52 | MRI‐pathway vs. SBx | 0‐1: ≥1 | Cognitive | NR | Unclear | Transrectal | G = 1 G ≥ 2 |
| Panebianco 2015 | Yes (prospective) | Bx‐naïve: 570 Prior‐negative Bx: 355 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Cognitive | 10, 14 or 45f | Unclear | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Peltier 2015 | Yes (prospective) | 110 | MRI‐pathway vs. SBx | 1‐4; ≥2 | Software | 15 (12‐18) | No | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Pokorny 2014 | Yes (prospective) | 223 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | In‐bore | 12 | Unclear | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Rouvière 2019a | Yes (prospective) | 251 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | Software Cognitive |
12.2c | Yes | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Say 2016 | Yes (retrospective) | 143 | MRI‐pathway vs. SBx | 1‐4; ≥2 | Software | 12c | Unclear | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Tonttilla 2016 | Yes (prospective) | 53 | MRI‐pathway vs. SBx | 1‐4; ≥2 | Cognitive | 12 (12‐14) |
Yes | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Van der Leest 2018 | Yes (prospective) | 626 | MRI‐pathway vs. SBx | 1‐5; ≥ 3 | In‐bore | 12c | Yes | Transrectal | G = 1 G ≥ 2 G ≥ 3 |
| Bx: biopsy; ISUP G : International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; PI‐RADS v1, v2: Prostate Imaging Reporting Data System version 1 or 2; PZ: peripheral zone; SBx: systematic biopsy; TSB: transperineal saturation biopsy; TTMB: transperineal template mapping biopsy; TZ: transition zone | |||||||||
aIncluded participants were part of the same study cohort (no randomised populations were included). bInterquartile range (as opposed to range). cMean value (as opposed to median value). d10 cores in peripheral zone, two cores in transition zone. e2 additional cores in transitional zone in prior‐negative Bx men. f10 and 14 in Bx‐naïve men with positive and negative MRI, respectively; 10 and 45 in prior‐negative Bx men with a positive and negative MRI, respectively.
5. Patient characteristics of the agreement analyses studies.
| Study | Population | Median age (range) | Median PSA in ng/mL (range) | Median prostate volume in cm3 (range) |
| Alberts 2017a | Bx‐naïve Prior‐negative Bx | 73 (72‐74b) | 4.2 (3.4–5.8b) | 53 (37‐71b) |
| Boesen 2017a | Prior‐negative Bx | 65 (58‐68b) | 12.8 (8.9‐19.6b) | NR |
| Boesen 2018 | Bx‐naïve | 67 (61‐71b) | 8 (5.7‐13b) | 53 (40‐72b) |
| Castellucci 2017 | Bx‐naïve | 61 (8)c | 8.3 (6.1)c | 49 (7)c |
| Chang 2017 | Prior‐negative Bx | 64 (60‐68b) | 10.9 (7.2‐14.7b) | 48 (34‐63b) |
| Chen 2015 | Bx‐naïve | 67 (45‐91) | 9.7 (2.4‐35.7) | 45 (21‐83) |
| Cool 2016 | Bx‐naïve Prior‐negative Bx | 59 (8)c 62 (7)c | 6.0 (3.5)c 7.9 (3.9)c | 38 (18)c 56 (27)c |
| Costa 2013 | Prior‐negative Bx | 64 (48‐77)c | 14.4 (1.8‐33.1)c | NR |
| Delongchamps 2013 | Bx‐naïve | 64 (7)c | 8.5 (3.9)c | 56 (30)c |
| Filson 2016 | Bx‐naïve Prior‐negative Bx | 64 (59‐69b) 66 (59‐70b) | 5.8 (4.4‐8.1b) 7.6 (5‐11.5b) | 45(33‐62b) 58 (40‐84b) |
| Garcia Bennett 2017 | Bx‐naïve | 64 (6.7)c | 7.2 (6‐9.4b) | 48 (35‐63b) |
| Grönberg 2018a | Bx‐naïve Prior‐negative Bx | 64 (45–74)c | 6.3 (4.4b) | (32‐70)d |
| Jambor 2015 | Bx‐naïve | 66 (47‐76) | 7.4 (4‐14) | 42 (17‐107) |
| Jambor 2017a | Mixed | 65 (6)c | 7.5 (5.7‐9.6b) | 37 (28‐49b) |
| Kim 2017 | Bx‐naïve Prior‐negative Bx | 64 (7)c | 10.2 (15.1)c | NR |
| Lee 2016 | Bx‐naïve | 66 (43‐83) | 6.4 (3.3‐9.8) | 39 (17‐127) |
| Lee 2017 | Bx‐naïve | 62 (10)c | 6.4 (1.8)c | 40 (18)c |
| Okcelik 2016 | Bx‐naïve | 62 (43‐79) | 5 (3‐8.9) | 45 (17‐93) |
| Panebianco 2015a | Bx‐naïve Prior‐negative Bx | 64 (51‐82) | NR | NR |
| Peltier 2015 | Bx‐naïve | 65 (7)c | 8.4 (6.3)c | 49 (22)c |
| Pokorny 2014 | Bx‐naïve | 63 (57‐68b) | 5.3 (4.1‐6.6b) | 41 (30‐59b) |
| Rouvière 2019a | Bx‐naïve | 64 (59‐68b) | 6.5 (5.6‐9.6b) | 50 (38‐63b) |
| Say 2016 | Prior‐negative Bx | 64 (47‐82)c | 11.59 (0.4‐96.9)c | 69 (17‐309)c |
| Tonttilla 2016 | Bx‐naïve | 63 (60‐66b) | 6.1 (4.2‐9.9b) | 28 (24‐37b) |
| Van der Leest 2018 | Bx‐naïve | 65 (59‐68b) | 6.4 (4.6‐8.2b) | 55 (41‐77b) |
| Bx: biopsy; NR: not reported; PSA: prostate specific antigen | ||||
aResults not reported per population type. bInterquartile range (as apposed to range). cMean (SD or range) (as opposed to median (range)). dRange of interquartile ranges across three centres.
Eighteen studies addressed the test accuracy analysis (index tests versus reference standard (template‐guided biopsy)): 15 studies on MRI (Abd‐Alazeez 2014; Ahmed 2017; Dal Moro 2019; Distler 2017; Grey 2015; Hansen 2016a; Hansen 2018; Hansen 2017; Kesch 2017; Lawrence 2014; Mortezavi 2018; Muthuveloe 2016; Pepe 2013; Thompson 2016; Tsivian 2017); eight studies on MRI, MRI‐targeted biopsy and the MRI pathway in the same men (Dal Moro 2019; Distler 2017; Hansen 2016a; Hansen 2017; Kesch 2017; Lawrence 2014; Mortezavi 2018; Pepe 2013); and four studies on systematic biopsy (Ahmed 2017; Nafie 2014; Nafie 2017; Ploussard 2014). These studies included 6871 men, of whom 5075 were biopsy naïve and 1796 had a history of at least one prior negative biopsy. We did not find any studies that investigated both the MRI pathway and systematic biopsy verified by the reference standard in the same men.
Twenty‐five studies addressed the agreement analysis between the MRI pathway and systematic biopsy in detecting prostate cancer (Alberts 2017; Boesen 2017a; Boesen 2018; Castellucci 2017; Chang 2017; Chen 2015; Cool 2016; Costa 2013; Delongchamps 2013; Filson 2016; Garcia Bennett 2017; Grönberg 2018; Jambor 2015; Jambor 2017; Kim 2017; Lee 2016; Lee 2017; Okcelik 2016; Panebianco 2015; Peltier 2015; Pokorny 2014; Rouvière 2019a; Say 2016; Tonttilla 2016; Van der Leest 2018), with 6944 men, of whom 5353 were biopsy naïve and 1591 had a history of at least one prior negative biopsy.
Methodological quality of included studies
Test accuracy studies
Thirteen out of 18 test accuracy studies used a prospective study design, while the remaining studies used a retrospective design (Table 8). According to our QUADAS‐2 assessment (Table 7), the studies assessed and presented results per index test (MRI (Figure 3); MRI‐targeted biopsy (Figure 4); the MRI pathway (Figure 5); and systematic biopsy (Figure 6)). A considerable number of studies had a high or unclear risk of bias in the participant selection (n = 9/18) and reference standard domains (n = 12/18). Almost no risk of bias was present in the index test (n = 1/18) and flow and timing domains (n = 3/18). Furthermore, only three out of 18 studies had applicability concerns because either they had selected an explicitly high‐risk population or had used an alternative MRI‐scale or MRI‐positivity threshold (other than the default 5‐point scale with an MRI‐positivity threshold of 3/5 or more).
3.

Diagnostic test accuracy of magnetic resonance imaging (MRI) verified by template‐guided biopsy: risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
4.
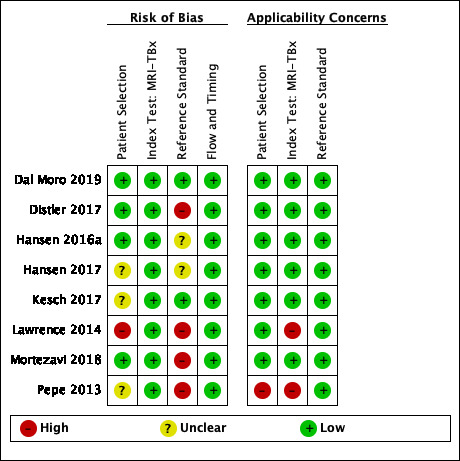
Diagnostic test accuracy of magnetic resonance imaging‐targeted biopsy (MRI‐TBx) in MRI‐positive men: risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
5.
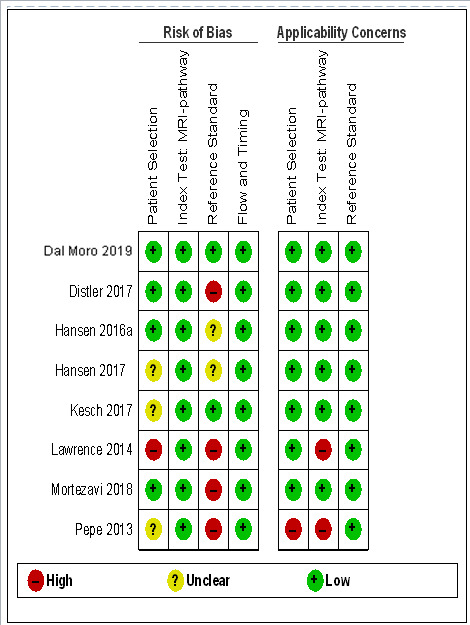
Diagnostic test accuracy of the MRI pathway: risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
6.
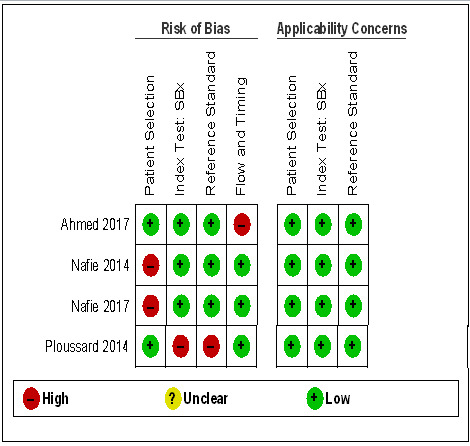
Diagnostic test accuracy of systematic biopsy (SBx): risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Agreement studies
Eighteen out of 25 agreement studies used a prospective study design, while the remaining studies used a retrospective design (Table 10). A considerable number of studies (n = 13/25) had a high or unclear risk of bias in the participant selection domain (Figure 7). In the index test domain, a considerable number of studies (n = 15/25) had a high or unclear risk of bias in the performance of systematic biopsy but almost no risk of bias was present in the performance of the MRI pathway (n = 1/18). Few studies had a high or unclear risk of bias in the flow and timing domain (n = 8/25). Furthermore, applicability concerns were present in 15 out of 25 studies, mainly because they used an alternative method to perform one of the index tests (other than that defined in Table 7).
7.
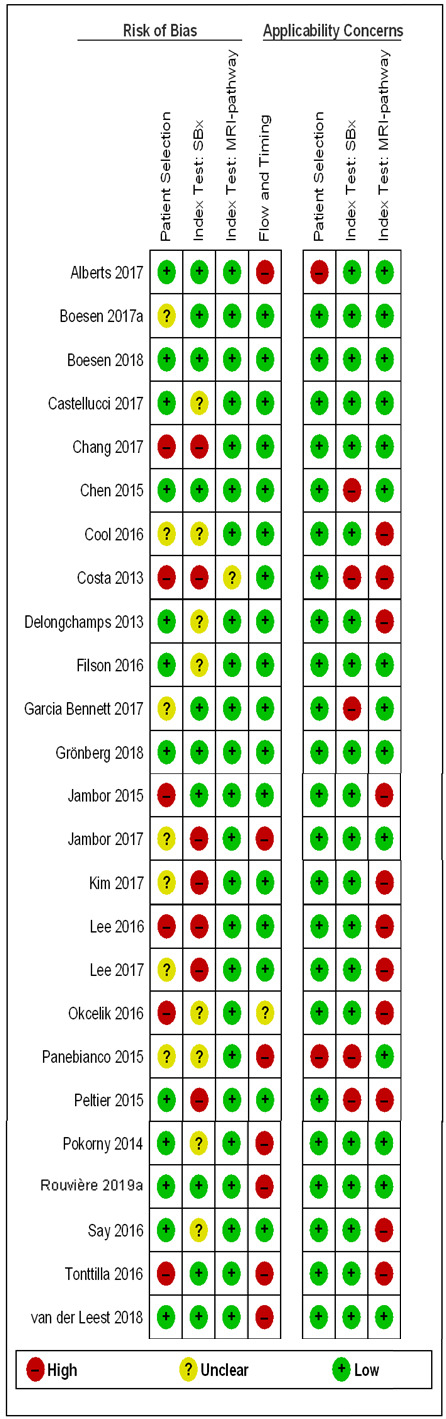
Agreement analyses between the MRI pathway and systematic biopsy (SBx): risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Overall, we acknowledge concerns about the independence and applicability of tests in both test accuracy and agreement analyses, for which we performed sensitivity analyses to exclude studies with such quality concerns.
Findings
Test accuracy: index tests verified by the reference standard, template‐guided biopsy
In this section, we quantified the test accuracy of the different index tests for detecting grade 2 or higher, grade 3 or higher and grade 1 prostate cancer, in mixed populations of men with first and repeat biopsies, using sensitivity, specificity and predictive values.
Sensitivity and specificity
Detection of grade 2 or higher prostate cancer
1. MRI compared with template‐guided biopsy
For grade 2 or higher prostate cancer, the pooled sensitivity and specificity of prostate MRI was 0.91 (95% CI 0.83 to 0.95) and 0.37 (95% CI 0.29 to 0.46), respectively (12 studies, 3091 men; prevalence 29% (95% CI 22% to 38%); Table 12; Figure 8). Hence, 9% of men with grade 2 or higher prostate cancer were not identified as such by MRI. In other words, at the assumptive prevalence of 30%, MRI may result in 273 (95% CI: 249 to 285) true positives, 441 false positives (95% CI: 378 to 497), 259 true negatives (95% CI: 203 to 322) and 27 (95% CI: 15 to 51) false negatives per 1000 men (Table 3).
6. Diagnostic accuracy of the index tests.
| Diagnostic accuracy of the index tests verified by template‐guided biopsy as the reference standard | |||||||
| Index test | MRI populationa | Target condition | N participants (studies) | Proportion negative MRI (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | P value |
| MRI | Positive + negative | G = 1 | 1764 (10) | 0.28 (0.20 to 0.38) | 0.70 (0.59 to 0.80) | 0.27 (0.19 to 0.37) | P < 0.01b |
| G ≥ 1 | 1764 (10) | 0.39 (0.30 to 0.50) | 0.84 (0.74 to 0.90) | 0.39 (0.30 to 0.50) | NA | ||
| G ≥ 2 | 3091 (12) | 0.29 (0.22 to 0.37) | 0.91 (0.83 to 0.95) | 0.37 (0.29 to 0.46) | P < 0.01b | ||
| G ≥ 3 | 1438 (7) | 0.31 (0.21 to 0.42) | 0.95 (0.87 to 0.99) | 0.35 (0.26 to 0.46) | ID | ||
| MRI‐TBx | Positive | G = 1 | 497 (5) | NA | 0.51 (0.21 to 0.81) | 1.00 (0.77 to 1.00) | NA |
| G ≥ 1 | 611 (6) | NA | 0.71 (0.61 to 0.80) | 0.93 (0.87 to 0.96) | NA | ||
| G ≥ 2 | 1553 (8) | NA | 0.80 (0.69 to 0.87) | 0.94 (0.90 to 0.97) | NA | ||
| G ≥ 3 | 428 (3) | NA | ID | ID | ID | ||
| MRI‐pathway | Positive + negative | G = 1 | 681 (5) | 0.24 (0.16 to 0.36) | 0.34 (0.19 to 0.53) | 1.00 (0.90 to 1.00) | P = 0.52c |
| G ≥ 1 | 844 (6) | 0.28 (0.21 to 0.35) | 0.58 (0.52 to 0.65) | 0.96 (0.92 to 0.98) | NA | ||
| G ≥ 2 | 2257 (8) | 0.29 (0.24 to 0.35) | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | P = 0.06c | ||
| G ≥ 3 | 604 (3) | 0.29 (0.26 to 0.33) | ID | ID | ID | ||
| SBx | NA | G = 1 | 3421 (4) | NA | 0.55 (0.25 to 0.83) | 0.99 (0.81 to 1.00) | NA |
| G ≥ 1 | 3421 (4) | NA | 0.65 (0.31 to 0.88) | 1.00 (0.88 to 1.00) | NA | ||
| G ≥ 2 | 3421 (4) | NA | 0.63 (0.19 to 0.93) | 1.00 (0.91 to 1.00) | NA | ||
| G ≥ 3 | 626 (2) | NA | ID | ID | ID | ||
| CI: confidence interval; G: International Society of Urological Pathology grade; ID: inadequate data; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; SBx: systematic biopsy | |||||||
aData did not allow differentiation between the mix of included participants (biopsy‐naïve and prior‐negative biopsy men). bComparing sensitivity between MRI and the MRI‐pathway. cComparing sensitivity between the MRI‐pathway and SBx.
8.
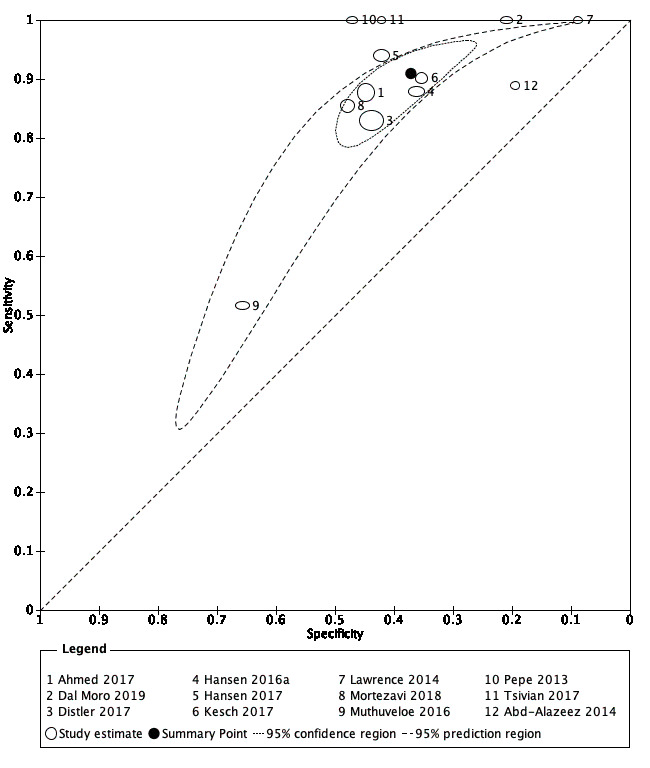
Diagnostic test accuracy of MRI for indicating grade 2 and higher prostate cancer.
Summary ROC plot of MRI verified by template‐guided biopsy. The 95% confidence region illustrates the uncertainty around the pooled summary point; the 95% prediction region illustrates the heterogeneity MRI: magnetic resonance imaging
2. MRI‐targeted biopsy compared with template‐guided biopsy
For grade 2 or higher prostate cancer, the pooled sensitivity and specificity of MRI‐targeted biopsy (in men with a positive MRI) were 0.80 (95% CI 0.69 to 0.87) and 0.94 (95% CI 0.90 to 0.97), respectively (8 studies, 1553 men; prevalence 34% (95% CI 24% to 46%); Table 12; Figure 9). Hence, MRI‐targeted biopsy in men with a positive MRI missed 20% of men with grade 2 or higher prostate cancer. At the assumptive prevalence of 30%, MRI‐targeted biopsy may result in 240 (95% CI: 207 to 261) true positives, 42 (95% CI: 21 to 70) false positives, 658 (95% CI: 630 to 669) true negatives and 60 (95% CI: 39 to 93) false negatives per 1000 men biopsied (Table 4).
9.
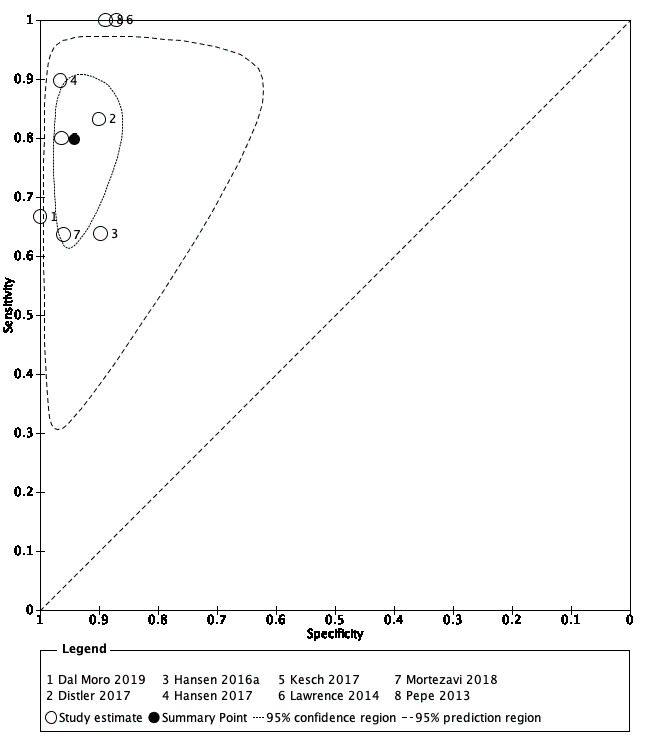
Diagnostic test accuracy of MRI‐targeted biopsy for detecting grade 2 and higher prostate cancer
Summary ROC plot of MRI‐targeted biopsy (in an MRI‐positive population) verified by template‐guided biopsy. The 95% confidence region illustrates the uncertainty around the pooled summary point; the 95% prediction region illustrates the heterogeneity MRI: magnetic resonance imaging
3. MRI pathway (MRI with or without MRI‐targeted biopsy) compared with template‐guided biopsy
For grade 2 or higher prostate cancer, the pooled sensitivity and specificity of MRI pathway were 0.72 (95% CI 0.60 to 0.82) and 0.96 (95% CI 0.94 to 0.98), respectively (8 studies, 2257 men; prevalence 26% (95% CI 18% to 36%); Table 12; Figure 10). Hence, the MRI pathway missed 28% of men with grade 2 or higher prostate cancer. At the assumptive prevalence of 30%, the MRI pathway may result in 216 (95% CI: 180 to 246) true positives, 28 (95% CI: 14 to 42) false positives, 672 (95% CI: 658 to 686) true negatives and 84 (95% CI: 54 to 120) false negatives per 1000 men (Table 5). The implications of these results, taking into account each step in the MRI pathway (MRI with subsequent MRI‐targeted biopsy in MRI‐positive men only), are shown in Figure 11.
10.
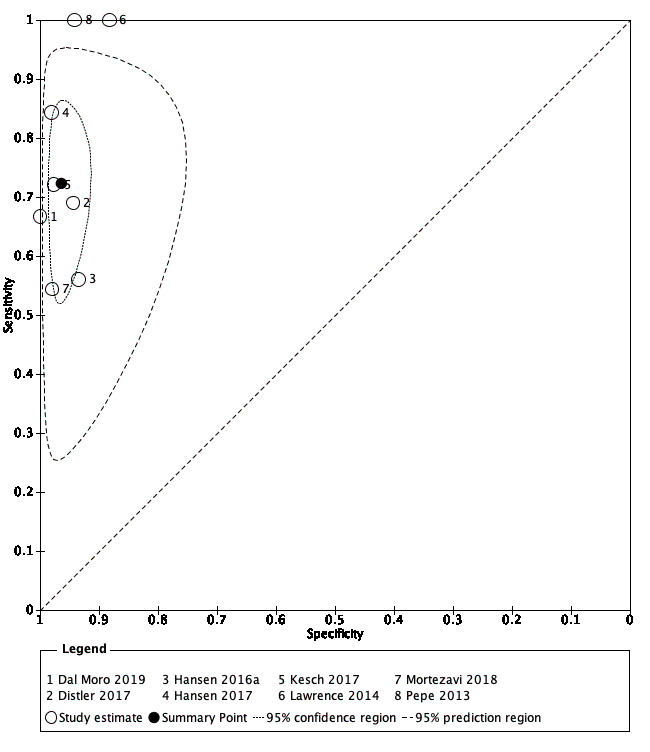
Diagnostic test accuracy of the MRI pathway for detecting grade 2 and higher prostate cancer
Summary ROC plot of the MRI pathway verified by template‐guided biopsy. The 95% confidence region illustrates the uncertainty around the pooled summary point; the 95% prediction region illustrates the heterogeneity MRI: magnetic resonance imaging; MRI pathway: MRI with or without MRI‐targeted biopsy
11.

Test consequence graphic showing results that would be obtained if a hypothetical cohort of 1000 men were tested for prostate cancer using the MRI pathway.
4. Systematic biopsy compared with template‐guided biopsy
For grade 2 or higher prostate cancer, the pooled sensitivity and specificity of systematic biopsy were 0.63 (95% CI 0.19 to 0.93) and 1.00 (95% CI 0.91 to 1.00), respectively (4 studies, 3421 men; prevalence 34% (95% CI 21% to 51%); Table 12; Figure 12). This analysis included the large and high‐quality PROMIS‐study, Ahmed 2017 (sensitivity 0.48 (95% CI 0.43 to 0.54); specificity 0.99 (95% CI 0.97 to 1.00); 576 men; prevalence 53%). Hence, the systematic biopsy approach missed approximately 37% of men with grade 2 or higher prostate cancer. At the assumptive prevalence of 30%, systematic biopsy may result in 189 (95% CI: 57 to 279) true positives, 0 (95% CI: 0 to 63) false positives, 700 (95% CI: 637 to 700) true negatives and 111 (95% CI: 21 to 243) false negatives per 1000 men (Table 6, Figure 13).
12.
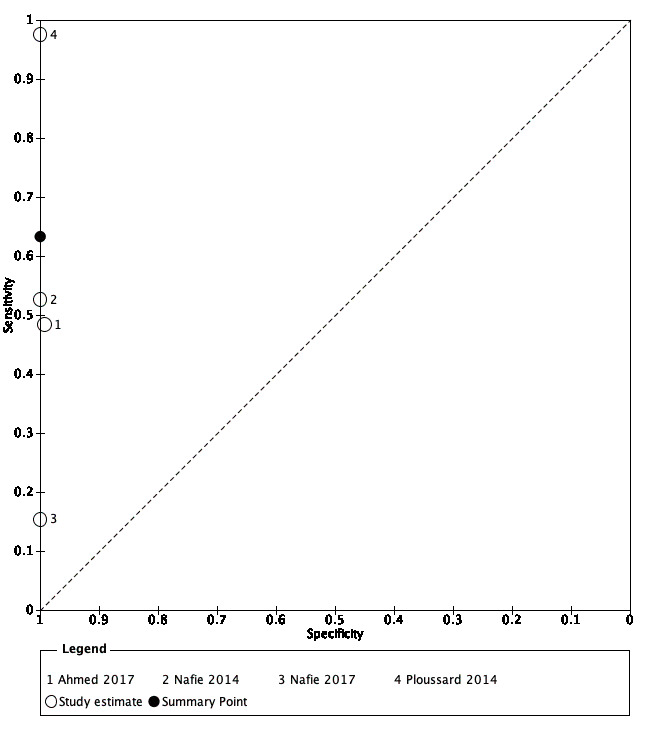
Diagnostic test accuracy of systematic biopsy for detecting grade 2 and higher prostate cancer
Summary ROC plot of systematic biopsy verified by template‐guided biopsy
13.
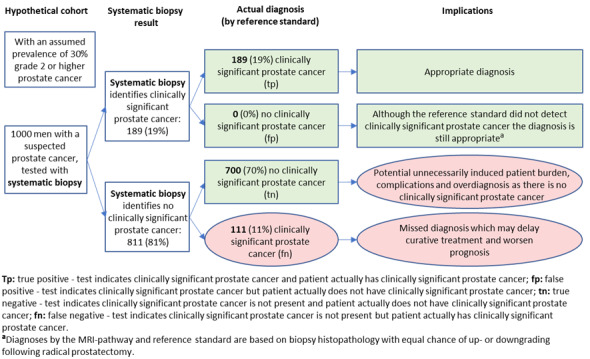
Test consequence graphic showing results that would be obtained if a hypothetical cohort of 1000 men were tested for prostate cancer using systematic biopsy.
5. Comparison of diagnostic accuracy between the index tests
Comparing the accuracy of the MRI with the accuracy of the MRI pathway showed a substantial decrease in sensitivity (0.91 versus 0.72) and increase in specificity (0.37 versus 0.96), which were both statistically significant (P < 0.01; Figure 14). Comparing the accuracy of the MRI pathway with the accuracy of systematic biopsy showed a substantial decrease in sensitivity (0.72 versus 0.63; P = 0.06) and similar specificities (Figure 15).
14.

Comparison of diagnostic test accuracy between MRI and the MRI pathway for detecting grade 2 and higher prostate cancer.
Summary ROC plot of MRI and the MRI pathway verified by template‐guided biopsy G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI pathway: MRI with or without MRI‐targeted biopsy
15.
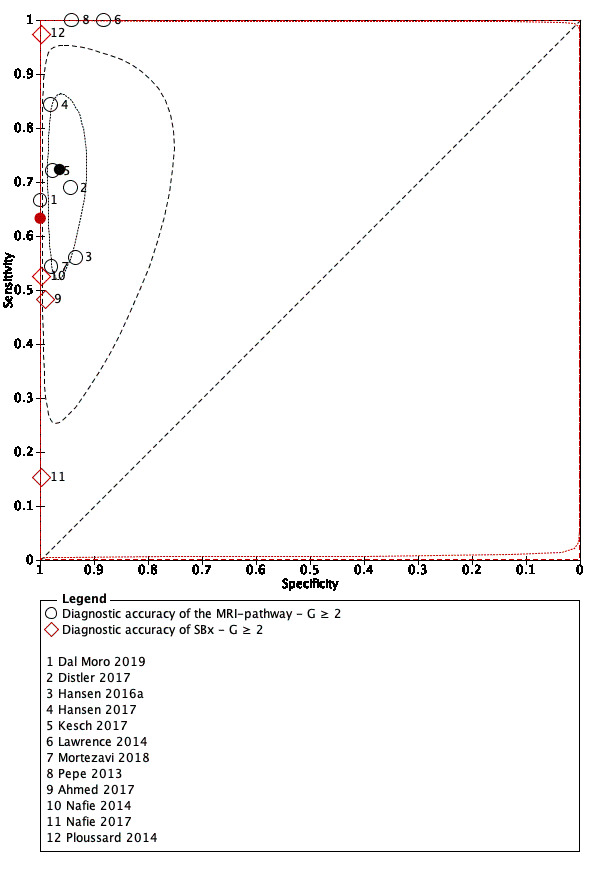
Comparison of diagnostic test accuracy between the MRI pathway and systematic biopsy for detecting grade 2 and higher prostate cancer.
Summary ROC plot of the MRI pathway versus systematic biopsy, verified by template‐guided biopsy G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI pathway: MRI with or without MRI‐targeted biopsy; SBx: systematic biopsy
Detection of grade 3 or higher prostate cancer
1. MRI compared with template‐guided biopsy
The pooled sensitivity and specificity of MRI were 0.95 (95% CI 0.87 to 0.99) and 0.35 (95% CI 0.26 to 0.46), respectively (7 studies, 1438 men; prevalence 14% (95% CI 8% to 23%); Table 12). Hence, 5% of men with grade 3 or higher prostate cancer were not identified by MRI. At the assumptive prevalence of 14%, MRI may result in 133 (95% CI: 122 to 139) true positives, 559 (95% CI: 464 to 636) false positives, 301 (95% CI: 244 to 396) true negatives and 7 (95% CI: 1 to 18) false negatives per 1000 men.
2. MRI‐targeted biopsy, MRI pathway and systematic biopsy compared with template‐guided biopsy
For MRI‐targeted biopsy, the MRI pathway and systematic biopsy, insufficient data on grade 3 or higher prostate cancer were available to perform meta‐analyses; individual study results are presented in the Data table 19, Data table 23 and Data table 27, respectively.
19. Test.
Diagnostic accuracy of TBx ‐ G ≥ 3.
23. Test.
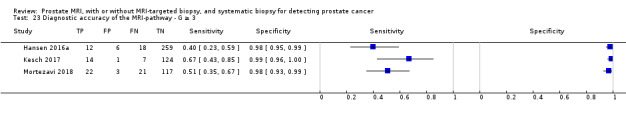
Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 3.
27. Test.

Diagnostic accuracy of SBx ‐ G ≥ 3.
Detection of grade 1 prostate cancer
The sensitivities and specificities for grade 1 prostate cancer were as follows:
1. MRI: 0.70 (95% CI 0.59 to 0.80) and 0.27 (95% CI 0.19 to 0.37), respectively (10 studies, 1764 men; prevalence 20% (95% CI 17% to 23%); Table 12);
2. MRI‐targeted biopsy: 0.51 (95% CI 0.21 to 0.81) and 1.00 (95% CI 0.77 to 1.00), respectively (5 studies, 497 men; prevalence 22% (95% CI 19% to 26%); Table 12);
3. MRI pathway: 0.34 (95% CI 0.19 to 0.53) and 1.00 (95% CI 0.90 to 1.00), respectively (5 studies, 681 men; prevalence 21% (95% CI 18% to 24%); Table 12);
4. systematic biopsy: 0.55 (95% CI 0.25 to 0.83) and 0.99 (95% CI 0.81 to 1.00), respectively (4 studies, 3421 men; prevalence 20% (95% CI 16% to 25%); Table 12).
Hence, comparing the sensitivity of the MRI pathway and systematic biopsy, the MRI pathway potentially avoided the detection of 66% of men with indolent prostate cancer, whereas systematic biopsy potentially avoided detection of 45% of men with indolent prostate cancer (P = 0.52).
Predictive values
The pooled prevalences of grade 2 or higher prostate cancer in the accuracy studies that assessed MRI, MRI‐targeted biopsy, MRI pathway and systematic biopsy, were 29% (95% CI 22% to 38%), 34% (95% CI 24% to 46%), 26% (95% CI 18% to 36%), and 34% (95% CI 21% to 51%), respectively (Table 13). Obviously, the prevalence of grade 2 or higher prostate cancer for MRI‐targeted biopsy is higher than that for the other index tests, due to the 'enriched' population resulting from the selection of only MRI‐positive men.
7. Predictive values of the index tests and prevalences.
| Predictive values of the index tests and prostate cancer prevalences | ||||||
| Test | MRI populationa | Target condition | N participants (studies) | Prevalenceb (95% CI) | NPVc (95% CI) | PPVc (95% CI) |
| MRI | Positive + negative | G = 1 | 1764 (10) | 0.20 (0.17 to 0.23) | 0.79 (0.74 to 0.82) | 0.20 (0.18 to 0.21) |
| G ≥ 2 | 3091 (12) | 0.29 (0.22 to 0.38) | 0.91 (0.86 to 0.94) | 0.37 (0.35 to 0.39) | ||
| G ≥ 3 | 1438 (7) | 0.14 (0.08 to 0.23) | 0.98 (0.95 to 0.99) | 0.19 (0.17 to 0.21) | ||
| MRI‐TBx | Positive | G = 1 | 497 (5) | 0.22 (0.19 to 0.26) | 0.88 (0.78 to 0.94) | 0.98 (0.23 to 1.00) |
| G ≥ 2 | 1553 (8) | 0.34 (0.24 to 0.46) | 0.90 (0.85 to 0.93) | 0.88 (0.80 to 0.92) | ||
| G ≥ 3 | 428 (3) | 0.21 (0.12 to 0.35) | ID | ID | ||
| MRI‐pathway | Positive + negative | G = 1 | 681 (5) | 0.21 (0.18 to 0.24) | 0.85 (0.81 to 0.88) | 0.95 (0.38 to 1.00) |
| G ≥ 2 | 2257 (8) | 0.26 (0.18 to 0.36) | 0.91 (0.87 to 0.94) | 0.88 (0.80 to 0.92) | ||
| G ≥ 3 | 604 (3) | 0.16 (0.09 to 0.27) | ID | ID | ||
| SBx | NA | G = 1 | 3421 (4) | 0.20 (0.16 to 0.25) | 0.90 (0.81 to 0.95) | 0.94 (0.37 to 1.00) |
| G ≥ 2 | 3421 (4) | 0.34 (0.21 to 0.51) | 0.84 (0.60 to 0.95) | 1.00 (0.76 to 1.00) | ||
| G ≥ 3 | 626 (2) | 0.10 (0.08 to 0.12) | ID | ID | ||
| CI: confidence interval; G: International Society of Urological Pathology grade; ID: inadequate data; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; NA: not applicable; NPV: negative predictive value; PPV: positive predictive value; SBx: systematic biopsy | ||||||
aData did not allow differentiation between the mix of included participants (biopsy‐naïve and prior‐negative biopsy men). bPrevalence is pooled estimate of all detected cancer by template‐guided biopsy. cBased on the Bayes’ theorem using the point estimates and 95% confidence intervals of the pooled positive and negative likelihood ratio and the point estimate of the prevalence.
The NPVs and PPVs of the index tests as a function of the pooled grade 2 or higher, grade 3 or higher and grade 1 prostate cancer prevalences are presented in Table 13. We are only able to compare these predictive values for the index tests at a prespecified prevalence. At a prespecified prevalence of 30% grade 2 or higher prostate cancer (based on the prevalence findings in the test accuracy analysis), the NPVs for MRI, MRI‐targeted biopsy, the MRI pathway and systematic biopsy are 91% (95% CI 86 to 94%), 92% (95% CI 88 to 94%), 89% (95% CI 85 to 92%) and 86% (95% CI 65 to 95%), respectively (Appendix 5). Consequently, in the MRI pathway, a negative MRI falsely predicts the absence of grade 2 or higher prostate cancer in 9% of men (Figure 9), while a negative systematic biopsy falsely predicts the absence of grade 2 or higher prostate cancer in 14% of men (Figure 13).
Sensitivity and specificity at a higher MRI‐positive threshold
In clinical practice, lesions with an MRI suspicion score of 3 (likelihood for clinically significant cancer is equivocal (Barentsz 2012)) might or might not be targeted with biopsies. By increasing the threshold of MRI‐positivity from 3/5 to 4/5, the proportion of negative MRI increased from 30% (95% CI 23% to 38%) to 59% (95% CI 43% to 74%) (Table 14). The pooled sensitivity of MRI for detecting grade 2 or higher prostate cancer decreased from 0.89 (95% CI 0.82 to 0.94) to 0.72 (95% CI 0.52 to 0.86). The pooled specificity increased from 0.39 (95% CI 0.32 to 0.47) to 0.78 (95% CI 0.68 to 0.86), indicating that with a threshold 4/5 for MRI positivity, a negative MRI failed to identify 28% of men with grade 2 or higher prostate cancer.
8. MRI‐positivity threshold effect.
| MRI‐positivity threshold effect, verified by template‐guided biopsy as the reference standard, with threshold ≥ 3and ≥ 4 out of 5 for identifying prostate cancer | |||||
| MRI threshold | Target condition | N participants (studies)a | Proportion negative MRI (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
| ≥ 3/5 | G = 1 | 1647 (8) | 0.29 (0.21 to 0.40) | 0.68 (0.57 to 0.77) | 0.28 (0.19 to 0.39) |
| G ≥ 2 | 2974 (10) | 0.30 (0.23 to 0.38) | 0.89 (0.82 to 0.94) | 0.39 (0.32 to 0.47) | |
| G ≥ 3 | 1438 (7) | 0.31 (0.21 to 0.42) | 0.96 (0.87 to 0.99) | 0.35 (0.26 to 0.46) | |
| ≥ 4/5 | G = 1 | 834 (4) | 0.60 (0.38 to 0.78) | 0.26 (0.16 to 0.40) | 0.57 (0.36 to 0.76) |
| G ≥ 2 | 1083 (5) | 0.59 (0.43 to 0.74) | 0.72 (0.52 to 0.86) | 0.78 (0.68 to 0.86) | |
| G ≥ 3 | 834 (4) | 0.60 (0.38 to 0.78) | 0.86 (0.51 to 0.97) | 0.68 (0.51 to 0.81) | |
| CI: confidence interval; G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; N: number | |||||
aData did not allow differentiation between the mix of included participants (biopsy‐naïve and prior‐negative biopsy men).
Furthermore, the pooled sensitivity of MRI for detecting grade 3 or higher prostate cancer at a threshold of 4/5 is 0.86 (95% CI 0.51 to 0.97), indicating that a positive MRI missed 14% of men with grade 3 or higher prostate cancer. The MRI‐threshold dependency (3/5 versus 4/5) for detecting grade 2 or higher and grade 3 or higher prostate cancer is depicted by ROC plots in Figure 16 and Figure 17, respectively.
16.
MRI‐positivity threshold effect for indicating grade 2 and higher prostate cancer.
Summary ROC plot of MRI verified by template‐guided biopsy, with different thresholds for positivity: intermediate (3/5) vs high (4/5) G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging
17.
MRI‐positivity threshold effect for indicating grade 3 and higher prostate cancer.
Summary ROC plot of MRI verified by template‐guided biopsy, with different thresholds for positivity: intermediate (3/5) vs high (4/5) G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging
Agreement between the MRI pathway and systematic biopsy
In this section, we focused on agreement and disagreement (concordance and discordance) in the number of target conditions identified by the MRI pathway and systematic biopsy. In addition, we have presented the proportions of participants with prostate cancer detected only by the MRI pathway and only by systematic biopsy (added values).
Prostate cancer detection in the MRI pathway and systematic biopsy
Detection ratios for grade 2 or higher prostate cancer
In a mixed population (of biopsy‐naïve and prior‐negative biopsy men), the pooled detection ratio of grade 2 or higher prostate cancer was 1.12 (95% CI 1.02 to 1.23; 25 studies, 6944 men; Table 15; Figure 18), meaning that the MRI pathway increased the grade 2 or higher prostate cancer detection rate by 12% over systematic biopsy.
9. Agreement analysis: detection ratio MRI‐pathway versus systematic biopsy.
| Population |
Target condition |
N participants (studies) |
Proportion prostate cancer detected in % (95% CI) | Detection ratiob (95% CI) | Difference between populations, P valuec | ||||
| Biopsy status | MRI, proportion in % (95% CI)a | MRI‐pathway and SBx combined (total cancer detected) | MRI‐pathway | SBx | MRI‐pathway versus SBx | P value | |||
| Mixedd | Positive + negative 100 (100 to 100) | G = 1 | 5442 (21) | 25.6 (22.8 to 28.8) | 12.3 (10.1 to 15.1) | 20.8 (18.0 to 24.1) | 0.61 (0.52 to 0.71) | P < 0.01 | NA |
| G ≥ 1 | 6524 (24) | 50.2 (46.4 to 54.3) | 37.9 (33.4 to 42.6) | 43.3 (39.1 to 47.8) | 0.88 (0.81 to 0.95) | P < 0.01 | NA | ||
| G ≥ 2 | 6944 (25) | 26.7 (23.3 to 30.7) | 22.9 (19.5 to 26.8) | 19.4 (15.9 to 23.5) | 1.12 (1.02 to 1.23) | P = 0.01 | NA | ||
| G ≥ 3 | 5981 (21) | 15.0 (12.7 to 18.0) | 12.7 (10.5 to 15.6) | 9.7 (7.5 to 12.7) | 1.20 (1.06 to 1.36) | P < 0.01 | NA | ||
| Positive 67.6 (60.2 to 74.3) | G = 1 | 3460 (19) | 29.5 (26.0 to 33.8) | 18.8 (15.2 to 23.4) | 22.4 (18.9 to 26.9) | 0.85 (0.75 to 0.97) | P = 0.01 | NA | |
| G ≥ 1 | 3998 (20) | 68.0 (62.3 to 73.5) | 61.1 (54.1 to 67.7) | 58.9 (51.5 to 65.9) | 1.03 (0.95 to 1.10) | P = 0.52 | NA | ||
| G ≥ 2 | 3998 (20) | 42.6 (37.6 to 48.1) | 37.9 (32.7 to 43.7) | 31.6 (26.2 to 37.9) | 1.17 (1.07 to 1.28) | P < 0.01 | NA | ||
| G ≥ 3 | 3902 (18) | 24.2 (20.9 to 28.1) | 21.0 (17.8 to 24.8) | 16.3 (13.1 to 20.3) | 1.24 (1.11 to 1.38) | P < 0.01 | NA | ||
| Biopsy‐naïve | Positive + negative 100 (100 to 100) | G = 1 | 4079 (17) | 27.2 (23.9 to 31.1) | 13.5 (10.7 to 17.2) | 22.4 (19.1 to 26.3) | 0.63 (0.54 to 0.74) | P < 0.01 | P = 0.91 |
| G ≥ 1 | 4799 (19) | 53.2 (48.7 to 57.9) | 41.0 (35.8 to 46.4) | 47.8 (42.8 to 52.9) | 0.85 (0.77 to 0.93) | P < 0.01 | P = 0.12 | ||
| G ≥ 2 | 5219 (20) | 27.7 (23.7 to 32.6) | 23.4 (19.3 to 28.1) | 21.4 (17.2 to 26.5) | 1.05 (0.95 to 1.16) | P = 0.35 | P < 0.01 | ||
| G ≥ 3 | 4306 (16) | 15.5 (12.6 to 19.5) | 12.7 (9.9 to 16.5) | 10.8 (8.0 to 14.8) | 1.09 (0.94 to 1.26) | P = 0.27 | P < 0.01 | ||
| Positive 67.0 (58.7 to 74.4) | G = 1 | 2682 (16) | 31.8 (27.7 to 36.9) | 21.3 (17.0 to 26.9) | 23.7 (19.6 to 29.1) | 0.85 (0.74 to 0.98) | P = 0.03 | P = 0.35 | |
| G ≥ 1 | 2955 (17) | 70.9 (65.0 to 76.6) | 63.7 (56.3 to 70.6) | 63.8 (56.2 to 70.7) | 0.99 (0.92 to 1.08) | P = 0.88 | P = 0.05 | ||
| G ≥ 2 | 2955 (17) | 44.2 (38.6 to 50.4) | 39.2 (33.3 to 45.7) | 34.4 (28.3 to 41.3) | 1.12 (1.01 to 1.23) | P = 0.03 | P < 0.01 | ||
| G ≥ 3 | 2899 (15) | 24.8 (21.0 to 29.6) | 21.2 (17.4 to 25.7) | 17.5 (13.8 to 22.3) | 1.16 (1.02 to 1.31) | P = 0.02 | P < 0.01 | ||
|
Prior‐negative biopsy |
Positive + negative 100 (100 to 100) | G = 1 | 1202 (8) | 23.0 (18.0 to 30.2) | 10.9 (7.9 to 15.3) | 17.8 (12.7 to 25.2) | 0.62 (0.44 to 0.88) | P < 0.01 | P = 0.91 |
| G ≥ 1 | 1564 (10) | 40.7 (35.1 to 47.2) | 30.0 (24.1 to 37.0) | 30.3 (24.3 to 37.5) | 0.97 (0.85 to 1.11) | P = 0.70 | P = 0.12 | ||
| G ≥ 2 | 1564 (10) | 22.8 (20.0 to 26.2) | 20.5 (17.7 to 23.5) | 13.2 (10.8 to 16.4) | 1.44 (1.19 to 1.75) | P < 0.01 | P < 0.01 | ||
| G ≥ 3 | 1514 (9) | 12.6 (10.5 to 15.6) | 11.5 (9.4 to 14.2) | 6.3 (4.4 to 9.1) | 1.64 (1.27 to 2.11) | P < 0.01 | P < 0.01 | ||
| Positive 69.6 (54.7 to 81.3) | G = 1 | 655 (7) | 27.9 (22.1 to 36.2) | 18.2 (12.8 to 26.7) | 18.9 (13.3 to 27.5) | 1.03 (0.89 to 1.18) | P = 0.71 | P = 0.35 | |
| G ≥ 1 | 920 (8) | 54.8 (44.6 to 66.4) | 48.5 (37.0 to 61.5) | 39.4 (27.1 to 53.9) | 1.16 (1.02 to 1.32) | P = 0.02 | P = 0.05 | ||
| G ≥ 2 | 920 (8) | 31.3 (27.4 to 36.1) | 28.6 (24.7 to 33.1) | 18.3 (15.1 to 22.5) | 1.49 (1.22 to 1.82) | P < 0.01 | P < 0.01 | ||
| G ≥ 3 | 880 (7) | 17.9 (14.3 to 22.9) | 16.7 (13.1 to 21.5) | 9.4 (6.4 to 14.2) | 1.65 (1.30 to 2.09) | P < 0.01 | P < 0.01 | ||
| CI: confidence interval; G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; SBx: systematic biopsy | |||||||||
aProportion of participants with a positive or negative magnetic resonance imaging result, based on the studies reporting grade 2 or higher. bDetection ratio is detection rate of magnetic resonance imaging‐pathway divided by detection rate of systematic biopsy; the detection rate is the pooled number of positive results of the test divided by the pooled total number of positive results from both tests. cEvaluating the difference in detection ratio's between the populations (biopsy‐naïve men versus prior‐negative biopsy) for each target condition. dMixed: biopsy‐naïve and prior‐negative biopsy men.
18.

Forest plots of the agreement analysis (MRI pathway vs systematic biopsy) for detecting grade 2 and higher prostate cancer
For men in the biopsy‐naïve setting, cancer proportion (total prostate cancer detected by both tests) was 27.7% (95% CI 23.7 to 32.6%; 20 studies, 5219 men), versus prior‐negative biopsy setting 22.8% (95% CI 20.0 to 26.2%; 10 studies, 1564 men). The pooled detection ratios for grade 2 or higher prostate cancer were 1.05 (95% CI 0.95 to 1.16) versus 1.44 (95% CI 1.19 to 1.75), respectively (P < 0.01; Table 15, Figure 18).
When focusing on only MRI‐positive men in both subgroups, the pooled detection ratio increased from 1.05 to 1.12 (95% CI 1.01 to 1.23) and from 1.44 to 1.49 (95% CI 1.22 to 1.82), respectively (Figure 18).
Detection ratios for grade 3 or higher prostate cancer
For men in the biopsy‐naïve setting, cancer proportion was 15.5% (95% CI 12.6 to 19.5%; 16 studies, 4306 men), and in the prior‐negative biopsy setting cancer proportion was 12.6% (95% CI 10.5 to 15.6%; 9 studies; 1514 men). The pooled detection ratio of grade 3 or higher prostate cancer was 1.09 (95% CI 0.94 to 1.26) and 1.64 (95% CI 1.27 to 2.11), respectively (Table 15). When focusing on only MRI‐positive men in both subgroups, the pooled detection ratio increased from 1.09 to 1.16 (95% CI 1.02 to 1.31) and from 1.64 to 1.65 (95% CI 1.30 to 2.09), respectively.
Detection ratios for grade 1 prostate cancer
For men in the biopsy‐naïve setting, cancer proportion was 27.2% (95% CI 23.9 to 31.1%; 17 studies, 4079 men), and in the prior‐negative biopsy setting, cancer proportion was 23.0% (95% CI 18.0 to 30.2%; 8 studies; 1202 men). The pooled detection ratio of grade 1 prostate cancer was 0.63 (95% CI 0.54 to 0.74) and 0.62 (95% CI 0.44 to 0.88), respectively (Table 15).
The agreement data results based on meta‐analysis with mixed modelling (multinomial logistic regression models) are presented in Table 15; the results based on direct random‐effects meta‐analysis are presented in Appendix 6.
Added values of the MRI pathway and systematic biopsy in prostate cancer detection
Added values in grade 2 or higher prostate cancer detection
Per 100 biopsy‐naïve men, the MRI pathway detected approximately 23 men with grade 2 or higher prostate cancer (23.4%, 95% CI 19.4 to 28.2%; 20 studies, 5219 men; Table 16). In addition to the MRI pathway, systematic biopsy detected four additional men (4.3%, 95% CI 2.6% to 6.9%) (Table 16). The total number of detected cases was 27 (27.7%, 95% CI 23.7% to 32.6%). Conversely, systematic biopsy detected 21 men (21.4%, 95% CI 17.2% to 26.5%), and the MRI pathway detected six additional men (6.3%, 95% CI 4.8% to 8.2%).
10. Agreement analysis: added values of MRI‐pathway and systematic biopsy.
| Population | Target condition | N participants (studies) | Proportion prostate cancer detected in % (95% CI) | ||||||
| Biopsy status | MRI, proportion in % (95% CI)a | MRI‐pathway and SBx combined (total cancer detected) | MRI‐pathway | SBx | Both MRI‐pathway and SBx | Only MRI‐pathway (added valueb) | Only SBx (added valueb) | ||
| Mixedc | Positive + negative 100 (100 to 100) | G = 1d | 5442 (21) | 19.5 (16.9 to 22.7) | 10.3 (8.1 to 13.1) | 16.8 (14.2 to 19.9) | 7.6 (5.5 to 10.2) | 2.7 (1.8 to 4.0) | 9.2 (7.4 to 11.4) |
| G ≥ 1 | 6524 (24) | 50.2 (46.4 to 54.3) | 37.9 (33.4 to 42.6) | 43.3 (39.1 to 47.8) | 30.9 (26.3 to 36.0) | 6.9 (5.2 to 9.2) | 12.4 (10.2 to 14.9) | ||
| G ≥ 2 | 6944 (25) | 26.7 (23.3 to 30.7) | 22.9 (19.5 to 26.9) | 19.4 (15.9 to 23.6) | 15.6 (12.2 to 19.6) | 7.3 (5.9 to 9.0) | 3.8 (2.5 to 5.7) | ||
| G ≥ 3 | 5981 (21) | 15.0 (12.7 to 18.0) | 12.7 (10.5 to 15.6) | 9.7 (7.5 to 12.7) | 7.4 (5.3 to 10.2) | 5.3 (4.3 to 6.5) | 2.3 (1.4 to 3.7) | ||
| Positive 67.6 (60.2 to 74.3) | G = 1d | 3460 (19) | 19.7 (15.9 to 24.7) | 15.8 (12.2 to 20.7) | 15.8 (12 to 20.8) | 12.0 (8.4 to 16.8) | 3.9 (2.6 to 5.7) | 3.8 (2.3 to 6.2) | |
| G ≥ 1 | 3998 (20) | 68.0 (62.3 to 73.5) | 61.1 (54.1 to 67.7) | 58.9 (51.5 to 65.9) | 52.0 (43.6 to 59.9) | 9.1 (5.9 to 13.5) | 6.9 (4.6 to 10.1) | ||
| G ≥ 2 | 3998 (20) | 42.6 (37.6 to 48.1) | 37.9 (32.7 to 43.7) | 31.6 (26.2 to 37.9) | 27.0 (21.4 to 33.4) | 10.9 (8.5 to 13.9) | 4.6 (2.9 to 7.2) | ||
| G ≥ 3 | 3902 (18) | 24.2 (20.9 to 28.1) | 21 (17.8 to 24.8) | 16.3 (13.1 to 20.3) | 13.2 (10.1 to 16.9) | 7.9 (6.3 to 9.7) | 3.1 (1.9 to 5.2) | ||
| Negative 32.4 (25.7 to 39.8) | G = 1d | 1666 (19) | 16.8 (12.9 to 21.6) | NA | 16.8 (12.9 to 21.6) | NA | NA | 16.8 (12.9 to 21.6) | |
| G ≥ 1 | 1781 (20) | 23.1 (19.7 to 26.9) | NA | 23.1 (19.7 to 26.9) | NA | NA | 23.1 (19.7 to 26.9) | ||
| G ≥ 2 | 1781 (20) | 7.2 (5.3 to 9.8) | NA | 7.2 (5.3 to 9.8) | NA | NA | 7.2 (5.3 to 9.8) | ||
| G ≥ 3 | 1725 (18) | 2.7 (1.6 to 4.6) | NA | 2.7 (1.6 to 4.6) | NA | NA | 2.7 (1.6 to 4.6) | ||
| Biopsy‐naïve | Positive + negative 100 (100 to 100) | G = 1d | 4079 (17) | 20.9 (18.0 to 24.7) | 11.2 (8.4 to 14.9) | 18.5 (15.6 to 22.2) | 8.8 (6.2 to 12.3) | 2.4 (1.4 to 4.0) | 9.8 (8.0 to 11.8) |
| G ≥ 1 | 4799 (19) | 53.2 (48.7 to 57.9) | 41.0 (35.8 to 46.4) | 47.8 (42.8 to 52.9) | 35.6 (30.2 to 41.2) | 5.4 (3.6 to 8.0) | 12.2 (8.7 to 16.7) | ||
| G ≥ 2 | 5219 (20) | 27.7 (23.7 to 32.6) | 23.4 (19.4 to 28.2) | 21.4 (17.2 to 26.5) | 17.1 (13.0 to 22) | 6.3 (4.8 to 8.2) | 4.3 (2.6 to 6.9) | ||
| G ≥ 3 | 4306 (16) | 15.5 (12.6 to 19.5) | 12.7 (9.9 to 16.5) | 10.8 (8.0 to 14.8) | 8.0 (5.4 to 11.6) | 4.7 (3.5 to 6.3) | 2.8 (1.7 to 4.8) | ||
| Positive 67.0 (58.7 to 74.4) | G = 1d | 2682 (16) | 21.1 (16.7 to 27.1) | 17.0 (12.6 to 22.9) | 17.7 (13.3 to 23.8) | 13.6 (9.3 to 19.5) | 3.4 (2.1 to 5.3) | 4.1 (2.5 to 6.7) | |
| G ≥ 1 | 2955 (17) | 70.9 (65.0 to 76.6) | 63.7 (56.3 to 70.6) | 63.8 (56.2 to 70.7) | 56.6 (47.7 to 64.6) | 7.1 (4.2 to 11.9) | 7.2 (4.7 to 10.8) | ||
| G ≥ 2 | 2955 (17) | 44.2 (38.6 to 50.4) | 39.2 (33.3 to 45.7) | 34.4 (28.3 to 41.3) | 29.5 (23.2 to 36.5) | 9.8 (7.1 to 13.2) | 4.9 (2.8 to 8.3) | ||
| G ≥ 3 | 2899 (15) | 24.8 (21.0 to 29.6) | 21.2 (17.4 to 25.7) | 17.5 (13.8 to 22.3) | 13.9 (10.3 to 18.3) | 7.3 (5.4 to 9.7) | 3.7 (2.2 to 6.1) | ||
| Negative 33.0 (25.6 to 41.3) | G = 1 | 1287 (16) | 18.4 (14.2 to 23.7) | NA | 18.4 (14.2 to 23.7) | NA | NA | 18.4 (14.2 to 23.7) | |
| G ≥ 1 | 1343 (17) | 25.5 (20.7 to 30.9) | NA | 25.5 (20.7 to 30.9) | NA | NA | 25.5 (20.7 to 30.9) | ||
| G ≥ 2 | 1343 (17) | 8.1 (5.6 to 11.6) | NA | 8.1 (5.6 to 11.6) | NA | NA | 8.1 (5.6 to 11.6) | ||
| G ≥ 3 | 1297 (15) | 3.0 (1.6 to 5.5) | NA | 3.0 (1.6 to 5.5) | NA | NA | 3.0 (1.6 to 5.5) | ||
|
Prior‐negative biopsy |
Positive + negative 100 (100 to 100) | G = 1d | 1202 (8) | 17.6 (13.0 to 25.0) | 9.8 (6.9 to 14.3) | 13.5 (8.9 to 21.0) | 5.8 (3.2 to 10.0) | 4.1 (2.6 to 6.2) | 7.7 (3.9 to 14.8) |
| G ≥ 1 | 1564 (10) | 40.7 (35.1 to 47.2) | 30.0 (24.1 to 37.0) | 30.3 (24.3 to 37.5) | 19.6 (13.7 to 27.1) | 10.3 (7.5 to 13.9) | 10.7 (7.4 to 15) | ||
| G ≥ 2 | 1564 (10) | 22.8 (20.0 to 26.2) | 20.5 (17.7 to 23.5) | 13.2 (10.8 to 16.4) | 10.9 (8.7 to 13.5) | 9.6 (7.7 to 11.8) | 2.3 (1.2 to 4.5) | ||
| G ≥ 3 | 1514 (9) | 12.6 (10.5 to 15.6) | 11.5 (9.4 to 14.2) | 6.3 (4.4 to 9.1) | 5.1 (3.4 to 7.7) | 6.3 (5.2 to 7.7) | 1.1 (0.5 to 2.6) | ||
| Positive 69.6 (54.7 to 81.3) | G = 1d | 655 (7) | 19.5 (13.9 to 28.8) | 16.5 (11.0 to 25.2) | 12.4 (7.2 to 21.6) | 9.4 (4.6 to 17.9) | 7.1 (4.1 to 11.8) | 3.0 (1.0 to 8.0) | |
| G ≥ 1 | 920 (8) | 54.8 (44.6 to 66.4) | 48.5 (37.0 to 61.5) | 39.4 (27.1 to 53.9) | 33.1 (20.1 to 48.7) | 15.4 (8.2 to 26.4) | 6.3 (3.8 to 9.8) | ||
| G ≥ 2 | 920 (8) | 31.3 (27.4 to 36.1) | 28.6 (24.7 to 33.1) | 18.3 (15.1 to 22.5) | 15.7 (12.7 to 19.1) | 13.0 (9.7 to 17.0) | 2.7 (1.2 to 5.7) | ||
| G ≥ 3 | 880 (7) | 17.9 (14.3 to 22.9) | 16.7 (13.1 to 21.5) | 9.4 (6.4 to 14.2) | 8.2 (5.2 to 12.6) | 8.5 (6.1 to 11.5) | 1.2 (0.4 to 3.2) | ||
| Negative 30.4 (18.7 to 45.3) | G = 1 | 341 (7) | 14.2 (5.9 to 30.2) | NA | 14.2 (5.9 to 30.2) | NA | NA | 14.2 (5.9 to 30.2) | |
| G ≥ 1 | 400 (8) | 19.5 (12.9 to 28.3) | NA | 19.5 (12.9 to 28.3) | NA | NA | 19.5 (12.9 to 28.3) | ||
| G ≥ 2 | 400 (8) | 5.3 (3.1 to 8.9) | NA | 5.3 (3.1 to 8.9) | NA | NA | 5.3 (3.1 to 8.9) | ||
| G ≥ 3 | 390 (7) | 3.3 (1.7 to 6.3) | NA | 3.3 (1.7 to 6.3) | NA | NA | 3.3 (1.7 to 6.3) | ||
| CI: confidence interval; G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy: N: number; NA: not applicable; SBx: systematic biopsy | |||||||||
aProportion of participants with a positive or negative MRI result, based on the studies reporting grade 2 or higher. bAdded value MRI‐pathway is the proportion of prostate cancer not detected by systematic biopsy but only by the MRI‐pathway; added value of systematic biopsy is the proportion of prostate cancer not detected by the MRI‐pathway but only by systematic biopsy. cMixed: biopsy‐naïve and prior‐negative biopsy men. dThe tests are considered as 'add‐on tests', taking into account grade reclassification by each test (Appendix 3). Therefore, G = 1 results differ from results in Table 15, where the tests are considered as 'replacement tests', not taking into account grade reclassification.
Per 100 men with prior negative biopsy, the MRI pathway detected 21 men with grade 2 or higher prostate cancer (20.5%, 95% CI 17.7% to 23.5%; 10 studies, 1564 men; Table 16). In addition to the MRI pathway, systematic biopsy detected two additional men (2.3%, 95% CI 1.2% to 4.5%). The total number of detected cases was 23 (22.8%, 95% CI 20.0% to 26.2%). Conversely, systematic biopsy detected 13 men (13.2%, 95% CI 10.8% to 16.4%), and the MRI pathway detected 10 additional men (9.6%, 95% CI 7.7% to 11.8%).
Figure 19 shows the point estimates of the added values with their 95% confidence region and 95% prediction region. The 95% confidence region illustrates the uncertainty around the point estimate; the 95% prediction region illustrates the heterogeneity. Although the uncertainty of the point estimates was reasonably small, the heterogeneity was large, especially in the direction of systematic biopsy. This indicates that future individual studies might find considerable divergent results, especially for the added value of systematic biopsy. Furthermore, the heterogeneity appeared to be larger in biopsy‐naïve men than in prior‐negative men.
19.
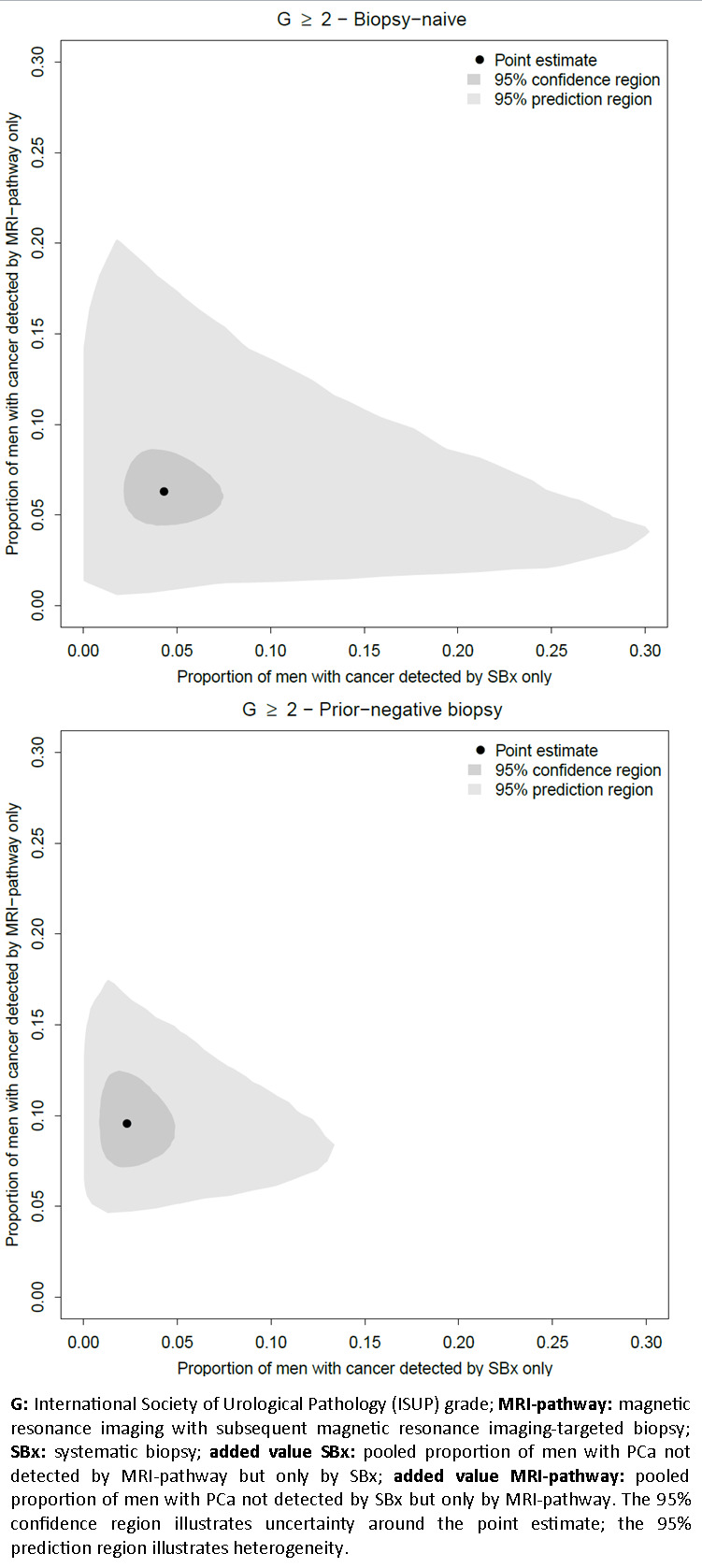
Added value of systematic biopsy plotted against the added value of the MRI pathway per population type in the agreement analysis, for detecting grade 2 and higher prostate cancer
Added values in grade 3 or higher prostate cancer detection
Per 100 biopsy‐naïve men, the MRI pathway detected approximately 13 men with grade 3 or higher prostate cancer (12.7%, 95% CI 9.9% to 16.5%; 16 studies, 4306 men; Table 10). In addition to the MRI pathway, systematic biopsy detected three additional men (2.8%, 95% CI 1.7% to 4.8%; Table 16). The total number of detected cases was 16 (15.5%, 95% CI 12.6% to 19.5%). Conversely, systematic biopsy detected 11 men (10.8%, 95% CI 8.0% to 14.8%) and the MRI pathway detected five additional men (4.7%, 95% CI 3.5% to 6.3%).
Per 100 men with prior negative biopsy, the MRI pathway detected 12 men with grade 3 or higher prostate cancer (11.5%, 95% CI 9.4% to 14.2%; 9 studies, 1514 men; Table 16). In addition to the MRI pathway, systematic biopsy detected one additional man (1.1%, 95% CI 0.5% to 2.6%). The total number of detected cases was 13 (12.6%, 95% CI 10.5% to 15.6%). Conversely, systematic biopsy detected six men (6.3%, 95% CI 4.4% to 9.1%), and the MRI pathway detected six additional men (6.3%, 95% CI 5.2% to 7.7%).
Added values in grade 1 prostate cancer detection
Per 100 biopsy‐naïve men, the MRI pathway detected approximately 11 men with grade 1 prostate cancer (11.2%, 95% CI 8.4% to 14.9%; 17 studies, 4079 men; Table 16). In addition to the MRI pathway, systematic biopsy detected 10 additional men (9.8%, 95% CI 8.0% to 11.8%). The total number of detected cases was 21 (20.9%, 95% CI 18.0% to 24.7%). Conversely, systematic biopsy detected 19 men (18.5%, 95% CI 15.6% to 22.2%) and the MRI pathway detected two additional men (2.4%, 95% CI 1.4% to 4.0%).
Per 100 men with prior negative biopsy, the MRI pathway detected 10 men with grade 1 prostate cancer (9.8%, 95% CI 6.9% to 14.3%; 8 studies, 1202 men; Table 16). In addition to the MRI pathway, systematic biopsy detected eight additional men (7.7%, 95% CI 3.9% to 14.8%). The total number of detected cases was 18 (17.6%, 95% CI 13.0% to 25.0%). Conversely, systematic biopsy detected 14 men (13.5%, 95% CI 8.9% to 21.0%), and the MRI pathway detected four additional men (4.1%, 95% CI 2.6% to 6.2%).
Added values of the MRI pathway and systematic biopsy in MRI‐positive and MRI‐negative men
Stratifying men further into MRI positive and MRI negative aids in interpreting the added value in each of these categories. The pooled proportions of positive and negative MRI were respectively 67.0% (95% CI 58.7% to 74.4%) and 33.0% (95% CI 25.6% to 41.3%) in the biopsy‐naïve setting and were equivalent in the prior negative biopsy setting (Table 16).
Per 100 biopsy‐naïve men with a positive MRI, the MRI pathway detected approximately 39 men with grade 2 or higher prostate cancer (39.2%, 95% CI 33.3% to 45.7%; 17 studies, 2955 men; Table 16). In addition to the MRI pathway, systematic biopsy detected five men (4.9%, 95% CI 2.8% to 8.3%). The total number of detected cases was 44 (44.2%, 95% CI 38.6% to 50.4%). Conversely, systematic biopsy detected 34 men (34.4%, 95% CI 28.3% to 41.3%) and the MRI pathway detected 10 additional men (9.8%, 95% CI 7.1% to 13.2%).
Per 100 biopsy‐naïve men with a negative MRI, systematic biopsy detected eight additional men with grade 2 or higher prostate cancer (8.1%, 95% CI 5.6% to 11.6%; 17 studies, 1343 men) and 18 additional men with grade 1 prostate cancer (18.4%, 95% CI 14.2% to 23.7%; 16 studies, 1287 men).
Per 100 men with a prior negative biopsy and a positive MRI, the MRI pathway detected approximately 29 men with grade 2 or higher prostate cancer (28.6%, 95% CI 24.7% to 33.1%; 8 studies, 920 men). In addition to the MRI pathway, systematic biopsy detected three men (2.7%, 95% CI 1.2% to 5.7%). The total number of detected cases was 31 (31.3%, 95% CI 27.4% to 36.1%). Conversely, systematic biopsy detected 18 men (18.3%, 95% CI 15.1% to 22.5%) and the MRI pathway detected an extra 13 men (13.0%, 95% CI 9.7% to 17.0%).
Per 100 men with a prior negative biopsy and a negative MRI, systematic biopsy detected five men with grade 2 or higher prostate cancer (5.3%, 95% CI 3.1% to 8.9%; 8 studies, 400 men) and an 14 additional men with grade 1 prostate cancer (14.2%, 95% CI 5.9% to 30.2%; 7 studies, 341 men).
Number needed to biopsy by systematic biopsy in addition to the MRI pathway
In biopsy‐naïve men with a positive MRI, the number needed to biopsy (NNB) for systematic biopsy in addition to MRI‐targeted biopsy for grade 2 or higher prostate cancer detection was 20 (95% CI 12 to 36; Table 17). In other words, to detect one additional man with grade 2 or higher prostate cancer, 20 men need to be biopsied by systematic biopsy in addition to MRI‐targeted biopsy. The NNB for detecting grade 3 or higher prostate cancer was 27 (95% CI 16 to 45).
11. Agreement analysis: number needed to biopsy.
| Agreement analysis: number needed to biopsy by systematic biopsy to detect one extra prostate cancer not detected by the MRI‐pathway | |||
| Population | Target condition | NNBa (95% CI) | |
| Biopsy status | MRI | ||
| Biopsy‐naïve | Positive | G = 1 | 24 (15 to 40) |
| G ≥ 2 | 20 (12 to 36) | ||
| G ≥ 3 | 27 (16 to 45) | ||
| Negative | G = 1 | 5 (4 to 7) | |
| G ≥ 2 | 13 (9 to 18) | ||
| G ≥ 3 | 33 (18 to 63) | ||
| Prior‐negative biopsy | Positive | G = 1 | 33 (13 to 100) |
| G ≥ 2 | 37 (18 to 83) | ||
| G ≥ 3 | 83 (31 to 250) | ||
| Negative | G = 1 | 7 (3 to 17) | |
| G ≥ 2 | 19 (11 to 32) | ||
| G ≥ 3 | 31 (16 to 63) | ||
| CI: confidence interval; G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; NNB: number needed to biopsy; SBx: systematic biopsy | |||
aNumber needed to biopsy by systematic biopsy is 100 divided by the added value of systematic biopsy.
In biopsy‐naïve men with a negative MRI, the NNB for grade 2 or higher prostate cancer detection was 13 (95% CI 9 to 18). The NNB for detecting grade 3 or higher was 33 (95% CI 18 to 63), considerably higher than for detecting grade 2 or higher prostate cancer.
In men with a prior negative biopsy and a positive MRI, the NNBs for grade 2 or higher and grade 3 or higher prostate cancer were 37 (95% CI 18 to 83) and 83 (95% CI 31 to 250), respectively. The NNBs in MRI‐negative men were 19 (95% CI 11 to 32) and 31 (95% CI 16 to 63), respectively.
Heterogeneity analyses
For the test accuracy analyses (index tests versus reference standard (template‐guided biopsy)), the heterogeneity is illustrated by the 95% prediction region around the pooled estimates, as shown in Figure 8 (MRI), Figure 9 (MRI‐targeted biopsy), Figure 10 (MRI pathway) and Figure 12 (systematic biopsy). We observed considerable heterogeneity in all index tests. Due to limited data, we were unable to explore heterogeneity for these tests.
For the agreement analyses (MRI pathway versus systematic biopsy), the heterogeneity (total τ2 = 0.03) is illustrated in Figure 18. Due to limited data, exploration of heterogeneity was only possible by independent analyses of different population types, endorectal coil use, MRI pulse sequences, MRI risk thresholds and MRI‐targeted biopsy techniques (Table 18). We found a statistically significant difference in the detection ratio of the MRI pathway versus systematic biopsy between the subgroups of population (prior negative biopsy versus biopsy naïve) and endorectal coil use (‘yes’ versus ‘no’), suggesting that they may be sources of heterogeneity. There was no statistically significant difference in the detection ratio of the MRI pathway versus systematic biopsy, between studies using mpMRI or bpMRI, between studies with a low or intermediate MRI risk threshold, and between studies using a software or a cognitive MRI‐targeted biopsy technique.
12. Heterogeneity exploration in the agreement analysis.
| Heterogeneity exploration in the agreement analysis: detection ratio MRI‐pathway vs systematic biopsy for G ≥ 2 prostate cancer | ||||
| Covariate | Category | N participants (studies) | Detection ratio for G ≥ 2 PCa (95% CI)a | P value |
| Population | Biopsy‐naïve | 5219 (20) | 1.05 (0.95 to 1.16) | 0.002 |
| Prior to negative biopsy | 1564 (10) | 1.44 (1.19 to 1.75) | ||
| Field strength | 3T | 5407 (19) | ID | ID |
| 1.5T | 1143 (4) | ID | ID | |
| Endorectal coil | Yes | 1815 (6) | 1.42 (1.07 to 1.88) | 0.008 |
| No | 4082 (14) | 1.03 (0.94 to 1.12) | ||
| MRI pulse sequence | mpMRI | 4941 (16) | 1.18 (1.05 to 1.33) | 0.233 |
| bpMRI | 1775 (6) | 1.03 (0.91 to 1.17) | ||
| mpMRI + spectroscopy | 105 (2) | ID | ID | |
| MRI risk threshold | Low | 605 (6) | 1.18 (1.03 to 1.35) | 0.556 |
| Intermediate | 5859 (15) | 1.14 (1.03 to 1.26) | ||
| High | 428 (3) | ID | ID | |
| MRI‐TBx technique | Software | 3313 (9) | 1.15 (0.99 to 1.33) | 0.483 |
| Cognitive | 2194 (12) | 1.17 (1.00 to 1.36) | ||
| In‐bore | 849 (2) | ID | ID | |
| Route index test | Transrectal | 6464 (23) | ID | ID |
| Transperineal | 480 (2) | ID | ID | |
| bpMRI: biparametric magnetic resonance imaging; CI: confidence interval; G: International Society of Urological Pathology grade; ID: inadequate data; mpMRI: multiparametric magnetic resonance imaging; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; SBx: systematic biopsy | ||||
aDetection ratio is the detection rate of MRI‐pathway divided by detection rate of systematic biopsy; the detection rate = the pooled number of positive results of the test divided by the pooled total number of positive results from both tests.
Sensitivity analyses
We performed sensitivity analyses for the detection of grade 2 or higher prostate cancer by excluding studies based on certain quality and additional criteria.
Test accuracy analyses
Excluding studies with a high or unclear risk of bias or applicability concern in one of the four QUADAS‐2 domains did not substantially change the accuracy results of MRI, MRI‐targeted biopsy and the MRI pathway (Table 19), although we were unable to confirm this for applicability concerns in MRI‐targeted biopsy and the MRI pathway analyses because of a limited number of studies. We could not perform any sensitivity analyses for systematic biopsy due to the limited number of studies.
13. Sensitivity analysis of the diagnostic test accuracy analyses.
| Sensitivity analyses of the diagnostic test accuracy of MRI and the MRI‐pathway for detecting G ≥ 2 prostate cancer, verified by template‐guided biopsy as the reference standard | ||||||||
| Covariate | Category | MRI | MRI‐pathwaya | |||||
| N studies | Sensitivity (95% CI) | Specificity (95% CI) | N studies | Sensitivity (95% CI) | Specificity (95% CI) | |||
| Main analyses (as reference) | No selection | 12 | 0.91 (0.83 to 0.95) | 0.37 (0.29 to 0.46) | 8 | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | |
| QUADAS domains | Participant selection | Only low risk of bias | 5 | 0.86 (0.83 to 0.88) | 0.39 (0.31 to 0.47) | 4 | 0.61 (0.54 to 0.69) | 0.97 (0.92 to 0.99) |
| Only low applicability concern | 11 | 0.91 (0.83 to 0.96) | 0.36 (0.28 to 0.46) | 7 | 0.69 (0.60 to 0.77) | 0.97 (0.94 to 0.98) | ||
| Index test | Only low risk of bias | 12 | 0.91 (0.83 to 0.95) | 0.37 (0.29 to 0.46) | 8 | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | |
| Only low applicability concern | 9 | 0.90 (0.85 to 0.94) | 0.37 (0.31 to 0.43) | 6 | 0.68 (0.59 to 0.77) | 0.97 (0.94 to 0.99) | ||
| Reference standard | Only low risk of bias | 4 | 0.93 (0.82 to 0.98) | 0.34 (0.24 to 0.45) | 2 | ID | ID | |
| Only low applicability concern | 12 | 0.91 (0.83 to 0.95) | 0.37 (0.29 to 0.46) | 8 | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | ||
| Flow and timing | Only low risk of bias | 11 | 0.91 (0.83 to 0.96) | 0.36 (0.28 to 0.46) | 8 | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | |
| Additional analyses | MRI positivity threshold | Only threshold 3/5 | 10 | 0.89 (0.82 to 0.94) | 0.39 (0.32 to 0.47) | 6 | 0.68 (0.59 to 0.77) | 0.97 (0.94 to 0.98) |
| MRI positivity threshold effect | MRI positivity threshold 3/5 (only studies with also 4/5) | 5 | 0.87 (0.73 to 0.94) | 0.45 (0.33 to 0.57) | 0 | ID | ID | |
| MRI positivity threshold 4/5 (only studies with also 3/5) | 5 | 0.72 (0.52 to 0.86) | 0.78 (0.68 to 0.86) | 0 | ID | ID | ||
| MRI vs MRI‐pathway | Only MRI and MRI‐pathway in the same men (paired data) | 8 | 0.92 (0.83 to 0.96) | 0.35 (0.27 to 0.44) | 8 | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | |
| Reference standard | Only TTMB, TSB or TOP | 9 | 0.90 (0.84 to 0.93) | 0.36 (0.29 to 0.44) | 6 | 0.69 (0.58 to 0.78) | 0.96 (0.93 to 0.97) | |
| Template‐guided biopsy + MRI‐TBx (composite reference standard) | 11 | 0.94 (0.91 to 0.96) | 1.00 (1.00 to 1.00) | 8 | 0.72 (0.63 to 0.80) | 1.00 (1.00 to 1.00) | ||
| Experience of radiologist | Only high experience | 10 | 0.91 (0.85 to 0.95) | 0.34 (0.27 to 0.42) | 7 | 0.69 (0.60 to 0.77) | 0.97 (0.94 to 0.98) | |
| CI: confidence interval; G: International Society of Urological Pathology grade; ID: inadequate data; MRI: magnetic resonance imaging; MRI‐pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; QUADAS: Quality Assessment of Diagnostic Accuracy Studies; SBx: systematic biopsy; TOP: transperineal optimised prostate biopsy;TSB: Ginsburg transperineal saturation biopsy; TTMB: transperineal template mapping biopsy | ||||||||
aThe diagnostic test accuracy analyses of magnetic resonance imaging‐targeted biopsy are based on the same studies as the MRI‐pathway.
To further assess the reliability of our results, we performed additional sensitivity analyses. In particular, excluding studies with MRI‐positivity thresholds other than threshold 3/5 did not substantially change the accuracy results of all MRI‐involved tests. Furthermore, the accuracy of MRI and the MRI pathway did not substantially change when assessed only in studies that had performed both tests in the same men (paired data), indicating no selection bias in the analysis comparing MRI with the MRI pathway (Figure 14). Similarly, the accuracy of MRI did not substantially change when assessed only in studies that had investigated multiple MRI‐positivity thresholds in the same men (paired data), indicating no selection bias in the MRI‐positivity threshold effect analyses (Figure 16). Regarding our choice of reference standard, excluding studies with an in‐house TSB or a transrectal TSB (potentially less accurate techniques than TTMB, with biopsies at every 5 mm) did not substantially change the accuracy of MRI and the MRI pathway. In addition, using a composite reference standard (template‐guided biopsy + MRI‐targeted biopsy), thus regarding the additional prostate cancer detected by MRI‐targeted biopsy as ‘true’ positives instead of ‘false’ positives, did not substantially change the accuracy of MRI, MRI‐targeted biopsy and the MRI pathway. Excluding studies in which the radiologist had little or unclear experience did not change the accuracy results of MRI, MRI‐targeted biopsy and the MRI pathway.
Agreement analyses
Excluding studies with a high or unclear risk of bias or applicability concern in three of the four QUADAS‐2 domains (participant selection, index test (MRI pathway), flow & timing) did not substantially change the detection ratio between the MRI pathway and systematic biopsy (Table 20). Excluding studies with a high or unclear risk of bias and applicability concern in the index test (systematic biopsy) domain, however, did result in an equal detection rate of both index tests instead of a higher detection rate of the MRI pathway. Furthermore, excluding studies with MRI‐positivity thresholds other than threshold 3/5 did not substantially change the detection ratio between the MRI pathway and systematic biopsy. The difference in the detection ratios between population types did not notably change when we analysed only studies that compared biopsy‐naïve and prior‐negative biopsy men in the same study. Excluding studies in which the radiologist had little or unclear experience did not change the detection ratio between the MRI pathway and systematic biopsy.
14. Sensitivity analysis of the agreement analyses.
| Sensitivity analyses of the agreement between the MRI‐pathway vs systematic biopsy for detecting G ≥ 2 prostate cancer | ||||
| Covariate | Category | N studies | Detection ratio (95% CI)a | |
| Main analyses (as reference) | Mixed population | 25 | 1.12 (1.02 to 1.23) | |
| QUADAS domains | Patient selection | Only low risk of bias | 12 | 1.08 (1.00 to 1.17) |
| Only low applicability concern | 23 | 1.09 (1.01 to 1.17) | ||
| Index test (MRI‐pathway) | Only low risk of bias | 24 | 1.11 (1.02 to 1.22) | |
| Only low applicability concern | 14 | 1.13 (1.01 to 1.26) | ||
| Index test (SBx) | Only low risk of bias | 10 | 1.04 (0.94 to 1.15) | |
| Only low applicability concern | 20 | 1.07 (0.99 to 1.15) | ||
| Flow and timing | Only low risk of bias | 17 | 1.10 (1.00 to 1.22) | |
| Additional analyses | MRI positivity threshold | Only threshold 3/5 | 15 | 1.14 (1.03 to 1.26) |
| Population | Biopsy‐naïve (only studies with also prior‐negative biopsy men) | 6 | 0.98 (0.76 to 1.28)b | |
| Prior‐negative biopsy (only studies with also biopsy‐naïve men) | 6 | 1.42 (1.03 to 1.95)b | ||
| Experience of radiologist | Only high experience | 21 | 1.13 (1.03 to 1.24) | |
| CI: confidence interval; G: International Society of Urological Pathology grade; MRI‐pathway: magnetic resonance imaging (MRI) with or without MRI‐targeted biopsy; N: number; QUADAS: Quality Assessment of Diagnostic Accuracy Studies; SBx: systematic biopsy | ||||
aDetection ratio is the detection rate of the MRI‐pathway divided by detection rate of systematic biopsy; the detection rate is the pooled number of positive results of the test divided by the pooled total number of positive results from both tests. bThe reference detection ratio for these categories are 1.05 (95% CI 0.95 to 1.16) for the biopsy‐naïve men and 1.44 (95% CI 1.19 to 1.75) for the prior‐negative biopsy men (Table 15).
Discussion
Summary of main results
This systematic review presents the test accuracy of the MRI, MRI‐targeted biopsy, the MRI pathway (MRI with or without MRI‐targeted biopsy) and the current standard testing with systematic biopsies in prostate cancer diagnosis, using template‐guided biopsy sampling of the whole prostate as the reference standard (Figure 1). Although the results of the MRI pathway represent the complete MRI‐informed clinical pathway, the diagnostic test accuracy results of the MRI and MRI‐targeted biopsy inform us on each diagnostic step in between (Figure 9). The MRI test alone indicates the presence of disease without MRI‐targeted biopsy results. The MRI‐targeted biopsy refers to only MRI‐positive men with targeted biopsy results.
We carried out two types of analyses:
test accuracy analyses of four index tests in prostate cancer diagnosis, providing evidence to determine their discriminative value in current clinical practice; and
agreement analyses for detecting prostate cancer between two index tests (the MRI pathway and the current practice of systematic biopsy), providing additional evidence for biopsy decision making.
Quantity and quality of evidence
A considerable number of studies in both the diagnostic accuracy (n = 9/18) and agreement analyses (n = 13/25) had a high or unclear risk of bias or applicability concern in one of the QUADAS‐2 domains. These issues, in addition to concerns over inconsistency and imprecision, prompted us to downgrade the certainty of evidence to low for all four main comparisons and outcomes. Overall, we acknowledge concerns about the independent performance and applicability of tests in both test accuracy and agreement analyses, for which we performed sensitivity analyses to exclude studies with such quality concerns. Furthermore, a considerable amount of heterogeneity was present in both diagnostic accuracy and agreement analyses, but only limited exploration was possible due to the paucity of studies in each subgroup. Only population type (biopsy‐naïve versus prior‐negative biopsy men) and the usage of an endorectal coil (‘yes ’ versus ‘no ’) may have explained some of the heterogeneity in the agreement analyses.
Test accuracy analysis of MRI, MRI‐targeted biopsy, MRI pathway and systematic biopsy, verified by the reference standard, template‐guided biopsy
The MRI missed the identification of 9% of men with grade 2 or higher prostate cancer (pooled sensitivity 0.91, 95% CI 0.83 to 0.95; specificity 0.37, 95% CI 0.29 to 0.46; Table 3); MRI‐targeted biopsy in MRI‐positive men missed the diagnosis in 20% of men with grade 2 or higher prostate cancer (pooled sensitivity of 0.80, 95% CI 0.69 to 0.87; specificity 0.94, 95% CI 0.90 to 0.97; Table 4); whereas the MRI pathway (in both MRI‐positive and MRI‐negative men) missed the diagnosis in 28% (pooled sensitivity 0.72, 95% CI 0.60 to 0.82; specificity 0.96, 95% CI 0.94 to 0.98; Table 5). Systematic biopsy missed 37% of men with grade 2 or higher prostate cancer (pooled sensitivity 0.63, 95% CI 0.19 to 0.93; specificity 1.00, 95% CI 0.91 to 1.00; Table 6). Hence, systematic biopsy had a substantially lower sensitivity than the MRI pathway (P = 0.06; Figure 15; Table 1).
Summary of findings 1. Detecting ISUP grade 2 or higher prostate cancer by MRI, MRI‐targeted biopsy, MRI‐pathway and systematic biopsy.
| Detecting ISUP grade 2 or higher prostate cancer by MRI, MRI‐targeted biopsy, MRI pathway and systematic biopsy | ||||||||||
| Population | 13,770 men with a suspicion of prostate cancer (PSA‐ or DRE‐based) undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||||||
| Setting | University hospitals and specialized care centers | |||||||||
| Index tests | MRI; MRI‐targeted biopsy (MRI‐TBx) in men with a positive MRI; the MRI pathway (MRI with or without MRI‐TBx); and systematic biopsy (SBx) | |||||||||
| Reference standard | Template‐guided biopsy, which comprehensively samples all zones of the prostate | |||||||||
| Tests | Population type (biopsy‐naïve, prior‐negative biopsy, or mixed) | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Detection ratio (95% CI) | Missed grade 2 or higher prostate cancer per 1000 men (95% CI)a | Number of participants (studies) | Number of studies with a high or unclear risk of bias | |||
| Participant selection | Index test(s) | Reference standard | Flow and timing | |||||||
| MRI | Mixed | 0.91 (0.83 to 0.95) | 0.37 (0.29 to 0.46) | NA | 27 (15 to 51) | 3091 (12) | 7 | 0 | 11 | 2 |
| MRI‐TBx | Mixed | 0.80 (0.69 to 0.87) | 0.94 (0.90 to 0.97) | NA | 60 (39 to 93) | 1553 (8) | 4 | 0 | 6 | 0 |
| MRI pathway | Mixed | 0.72 (0.60 to 0.82) | 0.96 (0.94 to 0.98) | NA | 84 (54 to 120) | 2257 (8) | 4 | 0 | 6 | 0 |
| SBx | Mixed | 0.63 (0.19 to 0.93) | 1.00 (0.91 to 1.00) | NA | 111 (21 to 243) | 3421 (4) | 2 | 1 | 1 | 1 |
| MRI pathwayvs SBx | Mixed | NA | NA | 1.12 (1.02 to 1.23) | MRI pathway missed 12% (2 to 23) less than SBx | 6944 (25) | 13 | 15 | NA | 8 |
| Biopsy‐naïve | NA | NA | 1.05 (0.95 to 1.16) | MRI pathway missed 5% (−5 to 16) less than SBx | 5219 (20) | 9 | 12 | NA | 7 | |
| Prior‐negative biopsy | NA | NA | 1.44 (1.19 to 1.75) | MRI pathway missed 44% (19 to 75) less than SBx | 1564 (10) | 5 | 6 | NA | 1 | |
| DRE: digital rectal exam; ISUP: International Society of Urological Pathology; MRI: magnetic resonance imaging; MRI‐TBx: MRI‐targeted biopsy; MRI pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; PSA: prostate‐specific antigen; SBx: systematic biopsy. aAt the representative pre‐test probability of 30% of having grade 2 or higher prostate cancer, based on prevalence findings in the test accuracy analysis (proportion missed = [prevalence*1000]*[1‐sensitivity]). | ||||||||||
The MRI pathway beneficially avoided the detection of 66% of grade 1 prostate cancer (pooled sensitivity 0.34, 95% CI 0.19 to 0.53) and reduced 29% of biopsies all in MRI‐negative men (pooled percentage negative MRI 29%, 95% CI 24% to 35%; Table 2). In contrast, the systematic biopsy approach avoided 45% of grade 1 prostate cancer (pooled sensitivity 0.55, 95% CI 0.25 to 0.83) and a biopsy procedure was performed in all men (100%).
Summary of findings 2. Detecting ISUP grade 1 prostate cancer by MRI, MRI‐targeted biopsy, MRI‐pathway and systematic biopsy.
| Detecting ISUP grade 1 prostate cancer by MRI, MRI‐targeted biopsy, MRI pathway and systematic biopsy | ||||||||||
| Population | 10,051 men with a suspicion of prostate cancer (PSA‐ or DRE‐based) undergoing their first biopsy (biopsy‐naïve men) or a repeat biopsy (prior‐negative biopsy men) | |||||||||
| Setting | University hospitals and specialized care centers | |||||||||
| Index tests | MRI; MRI‐targeted biopsy (MRI‐TBx) in men with a positive MRI; the MRI pathway (MRI with or without MRI‐TBx); and systematic biopsy (SBx) | |||||||||
| Reference standard | Template‐guided biopsy, which comprehensively samples all zones of the prostate | |||||||||
| Tests | Population type (biopsy‐naïve, prior‐negative biopsy, or mixed) | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Detection ratio (95% CI) | Avoided overdiagnosis per 1000 men (95% CI)a | Number of participants (studies) | Number of studies with a high or unclear risk of bias | |||
| Participant selection | Index test(s) | Reference standard | Flow and timing | |||||||
| MRI | Mixed | 0.70 (0.59‐0.80) | 0.27 (0.19‐0.37) | NA | 63 (42‐86) | 1764 (10) | 5 | 0 | 5 | 1 |
| MRI‐TBx | Mixed | 0.51 (0.21‐0.81) | 1.00 (0.77‐1.00) | NA | 103 (40‐166) | 497 (5) | 3 | 0 | 3 | 0 |
| MRI pathway | Mixed | 0.34 (0.19‐0.53) | 1.00 (0.90‐1.00) | NA | 139 (99‐170) | 681 (5) | 3 | 0 | 3 | 0 |
| SBx | Mixed | 0.55 (0.25‐0.83) | 0.99 (0.81‐1.00) | NA | 95 (36‐158) | 3421 (4) | 2 | 1 | 1 | 1 |
| MRI pathwayvs SBx | Mixed | NA | NA | 0.61 (0.52‐0.71) | MRI pathway avoided more overdiagnosis (and biopsy proceduresb) than SBx | 5442 (21) | 11 | 11 | NA | 8 |
| Biopsy‐naïve | NA | NA | 0.63 (0.54‐0.74) | 4079 (17) | 9 | 9 | NA | 7 | ||
| Prior‐negative biopsy | NA | NA | 0.62 (0.44‐0.88) | 1202 (8) | 5 | 5 | NA | 2 | ||
|
DRE: digital rectal exam; ISUP: International Society of Urological Pathology; MRI: magnetic resonance imaging; MRI‐TBx: MRI‐targeted biopsy; MRI pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; N: number; NA: not applicable; PSA: prostate‐specific antigen; SBx: systematic biopsy. aAt the representative pre‐test probability of 21% of having grade 1 prostate cancer, based on prevalence findings in the test accuracy analysis (proportion avoided = [prevalence*1000]*[1‐sensitivity]). bMRI‐TBx is not performed in 29% (24‐35) of men with a negative MRI, whereas SBx is performed in 100% of men. | ||||||||||
Agreement analyses between the MRI pathway and systematic biopsy
The MRI pathway significantly outperformed systematic biopsy by detecting 12% more grade 2 or higher prostate cancer (pooled detection ratio 1.12, 95% CI 1.02 to 1.23), irrespective of population type (Table 1). This percentage increased in men with prior negative biopsies to 44% (pooled detection ratio 1.44, 95% CI 1.19 to 1.75) but decreased in biopsy‐naïve men to 5% (pooled detection ratio 1.05, 95% CI 0.95 to 1.16). We observed similar outcomes for the detection of grade 3 or higher prostate cancer.
The MRI pathway beneficially detected less grade 1 prostate cancer than systematic biopsy, with a reduction of 37% in biopsy‐naïve men (pooled detection ratio 0.63, 95% CI 0.54 to 0.74) and 38% in men with prior negative biopsy (pooled detection ratio 0.62, 95% CI 0.44 to 0.88; Table 2). The MRI pathway beneficially reduced a third of biopsies, all in MRI‐negative men (pooled percentage negative MRI 33%, 95% CI 26% to 41%; and 30%, 95% CI 19% to 44%; in biopsy‐naïve and prior‐negative biopsy men, respectively).
Strengths and weaknesses of the review
Strengths and weaknesses of included studies.
Strengths included that the test accuracy studies investigated one or more index tests verified by template‐guided biopsy in the same men, comprehensively sampling all zones of the prostate with a minimum of 20 biopsy cores (reference standard). The studies in the agreement analysis investigated the MRI pathway and systematic biopsy in the same men. We included only studies involving MRI for both test accuracy and agreement analyses that investigated men with positive and negative MRIs. These criteria ensured that we avoided a number of biases and inaccurate findings, as stated in the Rationale. This systematic review contains many large studies, including the appraised PROMIS study (Ahmed 2017) and others (Distler 2017; Hansen 2016a; Hansen 2017; Kesch 2017; Mortezavi 2018) that showed results very consistent with the pooled accuracy estimates from our meta‐analyses. We have summarised the limitations of the included studies with reference to each of the four, quality domains, as assessed by our QUADAS‐2 tool:
Participant selection: In both test accuracy and agreement analyses, multiple studies showed an unclear or high risk of bias in this domain. Retrospective and nonconsecutive inclusion of participants might have led to manipulation of data.
Index tests: In the test accuracy studies, we identified almost no high or unclear risk of bias in the performance of index tests. In the agreement analysis, however, multiple studies did not perform the MRI pathway and systematic biopsy blinded from each other. This could possibly have led to MRI‐informed systematic biopsy in some studies, with (sub‐)conscious over‐ or underperformance of systematic biopsies.
Reference test: Similar concerns exist for the reference standard in the diagnostic accuracy analyses, because multiple studies showed an unclear or high risk of bias regarding the independent performance of template‐guided biopsies or appropriate sampling of the whole prostate. Both factors possibly led to (sub‐)conscious under‐ or overestimation of index test accuracy in some studies. Because template‐guided biopsy is performed mostly in the context of scientific research and is not performerd regularly in most clinical practices, the possibility of selection bias should be taken into consideration. However, investigators responsible for the largest test accuracy studies included in this review do perform template‐guided biopsy in regular practice.
Flow and timing: Only a limited number of studies showed a high or unclear risk of bias, indicating that most studies performed the tests in a similar manner in all participants and did not exclude any participants for reasons that could cause bias.
Despite the risks of biases as described in the above domains, the sensitivity analyses, which excluded studies with a high or unclear risk of bias, demonstrated the robustness of the main results (Table 19; Table 20).
Strengths and weaknesses of the review process
Quality assessment and data extraction
We selected the included studies from the available literature using a very sensitive method, without restrictions, and two review authors independently extracted data, according to the Cochrane DTA principles (Higgins 2011). We successfully requested additional data from study authors to enable accurate extraction of two‐by‐two contingency tables, which otherwise we would have had to exclude from this review. Similarly, in order to minimise heterogeneity, extensive effort was undertaken to retrieve data for the target condition solely based on Gleason Score grading. Regardless, we had to exclude several eligible studies due to insufficient reported data. Limited reporting of methodological details resulted in multiple ‘unclear’ assessments of methodological quality items and limited heterogeneity explorations.
Review analyses
The use of template‐guided biopsy to verify the index tests ensured that the absence or presence of the disease was accurately investigated in the whole population referred for biopsy. This approach excluded all the inherent biases of other reference standards (i.e. systematic biopsies and radical prostatectomies) used in previous systematic reviews. However, it should be noted that template‐guided biopsy is not a perfect test, as its diagnostic accuracy is dependent on the intensity and trajectory of cores taken. This is reflected by the pooled specificity of MRI‐targeted biopsy (0.94, 95% CI 0.90 to 0.97), which indicates that MRI‐targeted biopsy detected 6% grade 2 or higher prostate cancer in addition to those detected by the reference standard. These ‘false’ positives, however, would likely be regarded as ‘true’ positives in clinical practice. Because the results of both tests are based on the same histopathological diagnosis, either positive result will be considered in subsequent decision making. Sensitivity analyses with a composite reference standard (template‐guided biopsy + MRI‐targeted biopsy), thus regarding these ‘false’ positives as ‘true’ positives, however, showed no substantial difference in the accuracy of MRI and the MRI pathway. Nevertheless, underestimation of the specificity and PPV of both MRI‐targeted biopsy and MRI pathway should be considered accordingly. Furthermore, the inherent chance of up‐ or downgrading of prostate cancer of any biopsy result following radical prostatectomy should be taken into account (Epstein 2012).
It should also be taken into consideration that the results are based on per‐participant analyses and not on per‐lesion analyses. Therefore, spatial concordance between (multiple) MRI findings and biopsy findings are not taken into account. For example, when a suspicious MRI lesion is identified in the right apex, while cancer is detected by template‐guided biopsy in the left apex, the MRI is regarded as a true positive in the per‐participant analyses; in reality, however, the MRI reading is a false positive in the right apex and false negative in the left apex. The underlying cause could be both interpretative problems with MRI, such as original misreading or truly invisible tumours (Borofsky 2018; Rosenkrantz 2017; Schouten 2017), and inaccurate MRI‐targeted biopsy, due to technical or mechanical flaws or intralesional heterogeneity (Cash 2016; Coker 2018; Gold 2019). As a consequence, the sensitivity of the MRI might be overestimated. Unfortunately, no data were available to assess the individual contributions of these factors in this review.
We analysed the test accuracy of MRI, MRI‐targeted biopsy and MRI pathway separately to provide insight into the accuracy of different steps in the MRI‐informed clinical pathway. MRI‐targeted biopsy is only performed in MRI‐positive men, and therefore its results disregard men with false‐negative MRIs. Caution must be taken when applying the results of only MRI or MRI‐targeted biopsy to the clinical practice in which the MRI pathway applies, as suggested in previous studies and reviews (De Rooij 2014a; Futterer 2015; Gayet 2016; Hamoen 2015; Moore 2013b; Schoots 2015; Valerio 2015;Van Hove 2014). The diagnostic accuracy analyses of the MRI pathway in this review overcome the above‐discussed difficulties of MRI and MRI‐targeted biopsy by presenting histological findings of the whole population.
In addition to the assessment of test accuracy, this review also analysed the agreement of prostate cancer detection between the MRI pathway and systematic biopsy in studies that performed both tests in the same men. Agreement evidence focuses on the number of target conditions identified (concordance and discordance of test results) because neither test is a valid reference test. Consequently, agreement analysis does not provide diagnostic accuracy measures like sensitivity and specificity but rather a detection ratio that indicates which test detects more of the target condition. These analyses enabled us to provide evidence in clinical scenarios in addition to evidence from test accuracy data.
Despite strict inclusion criteria, we still included a relatively large number of studies in the test accuracy analyses of MRI (n = 15), MRI‐targeted biopsy (n = 8) and the MRI pathway (n = 8)—and an even larger number of studies in the agreement analyses between the MRI pathway and systematic biopsy (n = 25) —resulting in reliable analyses regarding the primary objectives. However, a relatively limited number of studies was available to assess the diagnostic accuracy of systematic biopsy (n = 4), with the consequence that the pooled sensitivity estimate of systematic biopsy was imprecise. The small number of studies per covariate precluded us form performing subgroup analyses for test accuracy analyses. Similarly, a relatively limited number of agreement studies resulted in large 95% confidence intervals around some of the pooled detection ratio estimates in the subgroup analyses.
Regarding the heterogeneity exploration in the agreement analyses, only population type (prior‐negative biopsy versus biopsy‐naïve men) and endorectal coil use (‘yes’ versus ‘no’) were statistically significant factors that may have explained some of the heterogeneity. A sensitivity analysis suggested population type to be a significant factor. However, we were not able to rule out the possibility that the statistically significant difference between studies with and without the use of an endorectal coil is caused by dependence on other factors, such as period of investigation (most prior to 2015) or risk of bias and applicability concerns in the performance of the tests. Furthermore, heterogeneity exploration suggested that MRI pulse sequences (mpMRI versus bpMRI) or MRI‐targeted biopsy techniques (software versus cognitive) were not significant sources of heterogeneity. Although we could not perform any reliable heterogeneity exploration in the test accuracy analyses, it should be considered that the test accuracy estimates were based on studies with (a mix of) different population types and methods of index tests.
Furthermore, we evaluated several test accuracy measures to inform both policymakers and clinical physicians. These measures are related to two categories:
differentiation between men with and without clinically significant prostate cancer (discrimination); and
estimation of the post‐test probability of clinically significant prostate cancer (prediction).
While discrimination purposes are mainly of concern in health‐policy decisions, predictive measures are most useful in daily practice for predicting the probability of clinically significant prostate cancer in a man suspected of having prostate cancer, once the test result is known.
Within‐ and between‐study comparisons
We compared the test accuracy of MRI and the MRI pathway with a mix of within‐ and between‐study evidence. We confirmed the findings in sensitivity analyses with only within‐study data; however, we could only compare test accuracy between the MRI pathway and systematic biopsy with between‐study data. Although the agreement analyses between MRI pathway and systematic biopsy do not provide diagnostic test accuracy estimates, we investigated it only in within‐study data, in which individual studies performed both tests in the same population.
Diagnostic test accuracy analysis versus agreement analysis
In the test accuracy analysis in a mixed population, the pooled sensitivity for detecting grade 2 or higher prostate cancer was 0.72 (95% CI 0.60 to 0.82) for the MRI pathway and 0.63 (95% CI 0.19 to 0.93) for systematic biopsy—substantially in favour of the MRI pathway (P = 0.06). Similarly, in the agreement analysis between MRI pathway and systematic biopsy in the mixed population, the pooled detection ratio for detecting grade 2 or higher prostate cancer was 1.12 (95% CI 1.02 to 1.23; P = 0.01), statistically significantly in favour of the MRI pathway. Furthermore, the results of both analyses regarding grade 1 prostate cancer show that the MRI pathway beneficially detected less than systematic biopsy. Therefore, the results and conclusions from the test accuracy analysis and agreement analysis are consistent, despite the numerous differences between the two types of analyses.
Comparison with previous research
Previously published reviews on test accuracy of the MRI pathway or the prebiopsy MRI approach have been based on study designs that did not accurately capture target conditions and index or reference test definitions, leading to a number of biases and inaccurate findings, as described in the Rationale (De Rooij 2014a; Futterer 2015; Gayet 2016; Hamoen 2015; Moore 2013b; Schoots 2015; Valerio 2015; Van Hove 2014; Wegelin 2017; Woo 2018). These reviews included studies that reported only on men with a positive MRI, thereby disregarding men with a negative MRI, inevitably leading to inaccurate true‐negative and false‐negative values for the MRI pathway. In addition, they used systematic biopsy or radical whole‐mount surgical specimens as reference standards.
Distinguishing between biopsy‐naïve men and men with prior‐negative biopsy is paramount in daily practice. Several international prostate cancer guidelines recently started to recommend prebiopsy MRI in prior‐negative biopsy men, based on a beneficial prostate cancer detection by the MRI pathway over systematic biopsy (EAU Guideline 2018, NCCN Guideline 2018). However, international guidelines have not made any such recommendations in biopsy‐naïve men. High‐level evidence of prostate cancer detection by the MRI pathway as compared to systematic biopsy in biopsy‐naïve men has been scarce. Single‐centre, randomised controlled trials provided contradictory findings as to whether or not the MRI pathway has a higher detection rate for clinically significant prostate cancer compared to systematic biopsy (Baco 2016; Panebianco 2015; Tonttilla 2016).
Two multicentre randomised controlled trials (Kasivisvanathan 2018; Porpiglia 2017) investigated the MRI pathway and systematic biopsy in biopsy‐naïve men. Furthermore, two high‐quality prospective multicentre cohort studies (Rouvière 2019a; Van der Leest 2018) investigated the agreement of prostate cancer detection between the MRI pathway and systematic biopsy. We did not include the randomised controlled trials in this review, as they did not meet the inclusion criteria of performing the index tests and/or reference standard in the same men. Both randomised controlled trials showed that the MRI pathway detected significantly more grade 2 or higher prostate cancer than systematic biopsy, in contrast to the results from the agreement analyses in this review, including the two cohort studies. The data can be compared as follows:
Kasivisvanathan 2018: The MRI pathway avoided 28% of biopsy procedures. The MRI pathway detected 37.7% (95% CI 31.7% to 43.7%; 95/252) men with grade 2 or higher prostate cancer versus 25.8% (95% CI 20.4% to 31.3%; 64/248) by systematic biopsy. The MRI pathway detected 9.1% (95% CI 5.6% to 12.7%; 23/252) men with grade 1 prostate cancer versus 22.2% (95% CI 17.0% to 27.3%; 55/248) by systematic biopsy. The MRI pathway detected significantly more men with grade 2 or higher prostate cancer (absolute difference 11.9%, 95% CI 3.8% to 20.0%) and beneficially reduced the detection of grade 1 prostate cancer (absolute difference 13.1%, 95% CI 6.8% to 19.3%).
Porpiglia 2017: The MRI pathway avoided 24% of biopsy procedures. The MRI pathway detected 41.1% (95% CI 31.8% to 50.4%; 44/107) men with grade 2 or higher prostate cancer versus 13.3% (95% CI 6.8% to 19.8%; 14/105) by systematic biopsy. The MRI pathway detected 4.7% (95% CI 0.7% to 8.7%; 5/107) men with grade 1 prostate cancer versus 16.2% (95% CI 9.1% to 23.2%; 17/105) by systematic biopsy. The MRI pathway detected significantly more men with grade 2 or higher prostate cancer (absolute difference 27.8%, 95% CI 16.4% to 39.2%) and beneficially reduced the detection of grade 1 prostate cancer (absolute difference 11.5%, 95% CI 3.4% to 19.6%).
Rouvière 2019a: The total proportion of detected men with grade 2 or higher prostate cancer was 37.5% (95% CI 31.4% to 43.8%; 94/251). The MRI pathway could have avoided 17.9% (45/251) of biopsy procedures. The MRI pathway detected 32.3% (95% CI 26.5% to 38.1%; 81/251) men with grade 2 or higher prostate cancer versus 29.9% (95% CI 24.2% to 35.5%; 75/251) by systematic biopsy. The MRI pathway detected 9.2% (95% CI 5.6 to 12.7%; 23/251) men with grade 1 versus 22.3% (95% CI 17.2% to 27.5%; 56/251) by systematic biopsy. The MRI pathway detected an equivalent proportion of grade 2 or higher prostate cancer (absolute difference 2.4%, 95% CI −5.7% to 10.5%) and beneficially reduced the detection of grade 1 prostate cancer (absolute difference 13.1%, 95% CI 6.9% to 19.4%).
Van der Leest 2018: The total proportion of detected men with grade 2 or higher prostate cancer was 32.0% (95% CI 28% to 36%; 200/626). The MRI pathway could have avoided 49.4% of biopsy procedures. The MRI pathway detected 25.4% (95% CI 22% to 29%; 159/626) men with grade 2 or higher prostate cancer versus 23.3% (95% CI 20% to 27%; 146/626) by systematic biopsy. The MRI pathway detected 14.1% (95% CI 11% to 17%; 88/626) men with grade 1 versus 24.8% (95% CI 21% to 28%; 155/626) by systematic biopsy. The MRI pathway detected an equivalent proportion of men with grade 2 or higher prostate cancer (absolute difference 2.1%, 95% CI to 2.7% to 6.8%) and beneficially reduced the detection of grade 1 prostate cancer (absolute difference 10.7%, 95% CI 6.4% to 15.0%).
This Cochrane review, Drost 2019: The total proportion of detected grade 2 or higher prostate cancer in biopsy‐naïve men was 27.7% (95% CI 23.7% to 32.6%; Table 15). The MRI pathway could have avoided 33% of biopsy procedures. The MRI pathway detected 23.4% (95% CI 19.3% to 28.1%) men with grade 2 or higher prostate cancer versus 21.4% (95% CI 17.2% to 26.5%) by systematic biopsy. The MRI pathway detected 13.5% (95% CI 10.7% to 17.2%) men with grade 1 versus 22.4% (95% CI 19.1% to 26.3%) by systematic biopsy. The MRI pathway detected an equivalent proportion of men with grade 2 or higher prostate cancer (absolute difference 2.0%, 95% CI 1.1% to 4.6%) and beneficially reduced the detection of grade 1 prostate cancer (absolute difference 8.2%, 95% CI 6.0% to 10.3%).
The most remarkable differences are the following:
In this Cochrane review, the proportion of negative MRIs was 33% (95% CI 26 to 41%), with similar rates in both the randomised controlled trials, while it was 49.4% in the cohort study Van der Leest 2018. This study classified only 6.4% of MRIs as PI‐RADS assessment score 3. Although in this Cochrane review most included studies used experienced radiologists, obviously a dedication to limit PI‐RADS assessment score 3, as strived for by Van der Leest 2018, may safely increase the proportion of negative MRIs and may avoid more biopsies.
In this Cochrane review, an equivalent proportion of men with grade 2 or higher prostate cancer was detected by the MRI pathway and systematic biopsy, consistent with the two agreement studies of Van der Leest 2018 and Rouvière 2019a. In contrast, the MRI pathway detected considerably more men with grade 2 or higher prostate cancer than systematic biopsy in the two randomised controlled trials: Kasivisvanathan 2018 (absolute difference 11.9%, 95% CI 3.8% to 20.0%), and Porpiglia 2017 (absolute difference 27.8%, 95% CI 16.4% to 39.2%). Hence, while the randomised controlled trials showed a superiority of the MRI pathway over systematic biopsy, the agreement studies did not. Despite these inconsistencies, none of the studies showed an inferiority of the MRI pathway over systematic biopsy in detecting grade 2 or higher prostate cancer.
In this Cochrane review, the proportion of men with grade 2 or higher prostate cancer detected by the MRI pathway was 23.4%, 95% CI 19.3 to 28.1%), significantly higher in the two randomised controlled trials (Kasivisvanathan 2018: 37.7%, 95% CI 31.7 to 43.7%; Porpiglia 2017: 41.1%, 95% CI 31.8 to 50.4%).
In this Cochrane review, the MRI pathway detected 13.5% (95% CI 10.7 to 17.2%) of men with grade 1 prostate cancer, while the MRI pathway detected 9.1% (95% CI 5.6 to 12.7%) in Kasivisvanathan 2018 and 4.7% (95% CI 0.7 to 8.7%) in Porpiglia 2017.
Explanatory reasons for these inconsistencies might be multiple. With the published information and data in this review, we could not clarify these inconsistencies. However, we may discuss some general exploratory findings within the context of this review:
The quality and methodology of the tests might influence results, as investigated by our heterogeneity analyses (Table 18). However, we could not objectify the influence of many quality and methodology covariates due to limited numbers in the subgroups and shortcomings in study focus.
Although the systematic biopsy is suggested to be a standardised test and has a systematic approach, we still observed a remarkably large variance in detection rates in the included studies. We observed a similar large variance in detection rates for the MRI pathway. Next to differences in the proportion (and severity) of detected prostate cancer, this might also suggest differences in the quality of biopsy procedures. The introduction of software registration for MRI‐targeted biopsy and the visual feedback it provides during the performance of biopsy procedures might, in fact, train operators (i.e. urologists and radiologists) to distribute systematic biopsy cores more evenly throughout the prostate according to the standardised systematic biopsy protocol. This may lead to an improved prostate cancer detection rate by systematic biopsy. Furthermore, systematic biopsy protocols in a study may outperform daily clinical practice. Another explanation for equivalent outcome could be the lack of blinding for MRI results during the performance of systematic biopsy, which may influence systematic biopsy positively. In this review, however, a sensitivity analysis with only studies with a low risk of such bias resulted in an equal detection rate of both tests. Moreover, both the cohort studies, Rouvière 2019a and Van der Leest 2018, followed strict standardised biopsy protocols for systematic biopsy and results of both index tests were blinded but they observed no significant difference in detection rates between the MRI pathway and systematic biopsy.
The number of MRI‐targeted biopsy cores may influence the outcome of the MRI pathway, owing to the fact that diagnostic accuracy depends on the intensity and trajectory of cores taken due to the potential presence of considerable tumour heterogeneity (Huo 2012; Pham 2015; Valerio 2015). Therefore, a high number of MRI‐targeted biopsy cores per suspicious lesion may benefit the diagnostic yield. In this review, the included studies showed a large variation in the number of MRI‐targeted biopsy cores per lesions or per participant (Appendix 4), and we could not perform a heterogeneity analysis. Although the biopsy protocols differed between the two randomised and two cohort studies, we could not draw any explanatory conclusions. Kasivisvanathan 2018 used a maximal four cores per target; Porpiglia 2017 used three to six cores per target; Van der Leest 2018 obtained two to four cores per target; and Rouvière 2019a obtained up to three cores.
The proportion (and severity) of detected prostate cancer within a population may influence the final outcome of the test (Rouvière 2019a). In a high‐prevalence or high‐risk (large volume clinically significant prostate cancer) population, both tests are likely to detect more grade 2 or higher prostate cancer; a high pre‐test probability will result in a high post‐test probability. Hypothetically, in a high‐risk population, systematic biopsy might more easily detect an equivalent proportion of grade 2 or higher prostate cancer compared to the MRI pathway. This may influence the added value of the MRI pathway and systematic biopsy either way. Therefore, the population at risk (either biopsy‐naïve or prior‐negative biopsy men) may influence the diagnostic yield of either test. In the agreement analysis, the proportion of detected grade 2 or higher prostate cancer was 27.7% (95% CI 23.7% to 32.6%) in biopsy‐naïve men and 22.8% (20.0 to 26.2%) in prior‐negative biopsy men. We were unable to investigate within this review whether this difference explained the difference in detection ratios between the two population groups.
Applicability of findings to the review question
Participant selection
Inclusion criteria allowed a broad spectrum of men with a suspicion of prostate cancer and an indication for prostate biopsy to be investigated, in accordance with most clinical practices. We excluded from our analyses only men with a previous diagnosis of prostate cancer.
We made a clear distinction between different types of population (biopsy naïve, prior‐negative biopsy or mixed). Importantly, in the test accuracy analysis, we could not perform a subgroup analysis between biopsy‐naïve and prior‐negative biopsy men for the MRI pathway and systematic biopsy because most studies presented data only as a mixed population, not per population type. This limits the extrapolation of the results to daily practice, in which distinguishing between both populations is critical. In the MRI pathway analysis, the number of men with prior‐negative biopsy (n = 1402) dominated the number of biopsy‐naïve men (n = 855). In contrast, in the systematic biopsy analysis, the number of biopsy‐naïve men (n = 3379) dominated the number of men with prior‐negative biopsy (n = 42). Therefore, caution is advised when extrapolating these results from a mixed population to populations of only biopsy‐naïve men or prior‐negative biopsy men. In the agreement analyses between MRI pathway and systematic biopsy, on the other hand, subgroup analysis showed a substantial difference in population type. In prior‐negative biopsy men, the pooled detection ratio for detecting grade 2 or higher prostate cancer was 1.44 (95% CI 1.19 to 1.75) in favour of the MRI pathway. However, in biopsy‐naïve men, the pooled detection ratio for detecting grade 2 or higher prostate cancer was only 1.05 (95% CI 0.95 to 1.16), not favouring one test over the other.
We included very few studies with applicability concerns regarding the indication for biopsy (e.g. prostate cancer screening studies with a very low threshold for biopsy). However, studies may have used considerably different thresholds for the indication of a biopsy.
Sensitivity and specificity are often regarded as independent of disease prevalence and results from one setting are transferred to another setting with a different prevalence of prostate cancer in the population. However, it should be acknowledged that sensitivity and specificity do depend on the spectrum of the disease (e.g. a more severe cancer is more easily recognised on MRI and diagnosed by biopsy). Furthermore, positive and negative predictive values are heavily dependent on disease prevalence and can, therefore, not be applied in settings with disease prevalence differing from that of the evaluated population (Rouvière 2018).
The prevalences and proportions of detected grade 2 or higher prostate cancer in the included studies in this review were rather high (Table 13; Table 16) compared to the setting of most clinical practices. These prevalences were based on template‐guided biopsy, and the proportions were based on the combined use of the MRI pathway and systematic biopsy. Moreover, it should be taken into account that the populations studied were mostly from referral (tertiary), high‐volume and expert centres, with the advantages of state‐of‐the‐art equipment, optimised protocols, and highly experienced subspecialised radiologists. Consequently, it is critical to consider the prevalence (and severity) of the disease and the setting of the population to be evaluated before applying the results of this review.
The issues of prostate cancer diagnosis are global, but the current analysis is highly focused on Western populations. The literature shows an incomplete picture of other populations where the advantages of MRI may not be forthcoming because of the higher prevalence of advanced cancers. Prevalence differences have been investigated in subpopulations within the same country (Rodger 2015) and between different populations and races (Feletto 2015; Kamangar 2006; Kelly 2017). These differences may influence the potential benefit of an MRI‐directed biopsy management in those populations.
Index tests
All techniques for the performance of the MRI pathway (including MRI and MRI‐targeted biopsy) were eligible, with the only criteria being the use of T2‐weighted imaging and one functional imaging technique (DWI or DCE). The included studies used 1.5 or 3 Tesla MRI magnets and cognitive‐ or software‐guided MRI‐targeted biopsy via transrectal or transperineal routes, among other variations in methodology. These variations are likely to explain some amount of heterogeneity in the results, but we could not reliably investigate them as sources of heterogeneity in the diagnostic accuracy analyses and could only partially investigate them in the agreement analyses.
Differences in MRI‐scoring system and thresholds for MRI positivity (and for MRI‐targeted biopsy) are likely to influence results. Applicability assessment showed multiple studies with alternative MRI scoring systems and lower or higher positivity thresholds than the default (defined as 3/5 or more) in both test accuracy and agreement analyses. The pooled estimates from both main analyses, however, did not change importantly after excluding studies with alternative MRI scoring systems and thresholds in the sensitivity analysis. This shows the robustness of the main pooled estimates.
For systematic biopsy in the test accuracy and agreement analyses, there were almost no concerns of applicability, as systematic biopsy was mainly performed with 8 to 12 cores directed at the peripheral zone of the prostate in all studies.
Reference standard
There were no applicability concerns regarding the reference standard (template‐guided biopsy), as the target conditions were based on histopathology findings according to the Gleason scoring system and the ISUP grade without any volume criteria. Although in clinical practice other definitions are being used, our target condition definitions enable and simplify comparison between tests and literature.
Authors' conclusions
Implications for practice.
MRI‐directed biopsy management
The diagnostic workup of prostate cancer may benefit from including prostate MRI prior to biopsy. We found evidence that both the MRI pathway and systematic biopsy missed considerable proportions of grade 2 or higher prostate cancer but that the MRI pathway missed less than systematic biopsy. The difference between the detection rates of the MRI pathway and systematic biopsy was largest in men with a prior negative biopsy and insignificant in biopsy‐naïve men. Evidence further suggested that the MRI pathway beneficially missed more grade 1 prostate cancer than systematic biopsy in both population types. Therefore, the MRI pathway could potentially reduce the amount of overdiagnosis, and harms related to surveillance and overtreatment.
The benefits of MRI—a reduction in the number of biopsy procedures performed and the frequency of overdiagnosis of grade 1 prostate cancer, combined with an improvement in the detection of grade 2 and higher prostate cancer—are greatest when MRI has a direct impact on biopsy decision management and shared decision making. In other words, the MRI before any biopsy and the MRI pathway as the replacement for systematic biopsy, thus omitting systematic biopsy in specified circumstances, might provide the most favourable diagnostic strategy.
MRI‐negative men and systematic biopsy
This meta‐analysis showed that approximately one‐third of all men had a negative MRI. The added value of performing systematic biopsy in MRI‐negative men for the detection of grade 2 or higher prostate cancer could be considered as limited with regard to total detection and additional harms. As a prostate biopsy is associated with patient burden, overdiagnosis and related overtreatment, infection and morbidity, it should be avoided when possible (Borghesi 2017; Loeb 2013). Omitting systematic biopsy in men with a negative MRI might be considered acceptable in some clinical situations. However, benefits and harms are difficult to balance on an individual basis. Therefore, men with a negative MRI could be counselled to pursue clinical and biochemical monitoring as a reasonable alternative for systematic biopsy, as also argued by Moldovan 2017, Padhani 2019 and Panebianco 2018.
MRI‐positive men and systematic biopsy
Men with a positive MRI have a clear indication for MRI‐targeted biopsy and can opt for additional systematic biopsy. The added value of performing systematic biopsy in MRI‐positive men for the detection of grade 2 or higher prostate cancer, however, could be considered as limited with regard to total detection and additional harms. The conditions under which systematic biopsy could be safely avoided in men with a positive MRI remain to be defined (Richenberg 2019; Padhani 2019; Rouvière 2018). When in this risk population the MRI pathway fails to detect significant prostate cancer, a monitoring approach based on clinical, biochemical and imaging parameters could be introduced in the place of of systematic biopsy and would result in a ‘safety net’ that could be easily adopted in the shared decision‐making of the current diagnostic workup—as already recommended in international guidelines (AUA Guideline 2018; EAU Guideline 2018; NCCN Guideline 2018).
MRI‐positivity threshold
Data suggest that the use of an MRI‐positivity threshold of MRI suspicion score 3 out of 5 would be most beneficial in the detection of grade 2 or higher prostate cancer. Any higher threshold would result in unacceptably missing a substantial proportion of men with grade 2 or higher and grade 3 or higher prostate cancer. Therefore, the threshold should only be increased in the context of shared decision‐making with the patient after a thorough discussion of the potential risks. Further research is warranted to decrease the grade 2 or higher prostate cancer detection in these ‘equivocal’ or ‘indeterminate’ MRI lesions assessed as score 3 (Schoots 2018).
Costs and availability
The potential benefit of MRI within the diagnostic workup will have implications on economic metrics. Although cost‐effectiveness was not part of our analyses, this review may contribute to assumptions made in such analyses (Barnett 2018; De Rooij 2014b; Faria 2018; Pahwa 2017; Venderink 2017). A recent cost‐effectiveness study was performed by Brown 2018 based on a study included in our review (the PROMIS study (Ahmed 2017)). They found that the most cost‐effective strategy involved testing all men with prostate MRI, followed by an MRI‐directed biopsy in those men with suspected clinically significant prostate cancer (the MRI pathway), followed by rebiopsy if clinically significant prostate cancer was not detected. This strategy was cost‐effective and detected 95% (95% CI 92% to 98%) of clinically significant prostate cancer. However, in the study on which these findings were based, the diagnostic workup did not take any MRI‐targeted biopsies of MRI suspicious lesions. The investigators made the assumption that MRI‐targeted biopsy was as accurate as MRI. As shown by the results of our meta‐analysis, this assumption may be incorrect. The sensitivity for grade 2 or higher prostate cancer decreased substantially when comparing MRI with MRI‐targeted biopsy and MRI pathway. Hence, as cost‐effectiveness analyses heavily rely on assumed input parameters and, in addition, depend on regional differences in the healthcare system, readers should interpret these cost‐effectiveness results carefully.
Final considerations
Balancing the potential disadvantages (missing some grade 2 or higher prostate cancer) against the potential benefits (reduction of biopsies and a decrease of grade 1 prostate cancer overdiagnosis) and without taking into accounteconomic metrics (availability and costs), we conclude that the results show that MRI pathway may represent a more favourable diagnostic test than systematic biopsy. Our certainty in our findings was reduced by study limitations, specifically issues surrounding the selection bias, as well as inconsistency. Furthermore, the MRI pathway relies on experience and skills in reading MRI and targeting biopsy and on the use of high‐end MRI equipment and biopsy hardware and software—elements that are not yet widely available. This diagnostic chain is only as strong as its weakest link (Rouvière 2019b). Based on these considerations, further improvement of the prostate cancer diagnostic pathways should be pursued.
Implications for research.
This systematic review provides diagnostic accuracy evidence of MRI, MRI‐targeted biopsy, the MRI pathway and systematic biopsy, with additional evidence by agreement analyses. To improve the clinical utility of MRI‐driven tests, several factors should be further investigated.
The number of well‐performed studies investigating the index tests verified by template‐guided biopsy, as in our test accuracy analyses, should be increased where the burden of testing allows. Studies should be performed according to the START (Moore 2013a) and STARD (Cohen 2016) criteria to ensure clear and complete description of interchangeable methods that increase comparability between study results. Special effort should be taken to differentiate possible subgroups, methodology and definitions of target conditions. The quality and applicability of evidence greatly depend on the criteria described in our QUADAS‐2 tool (Table 7). This also applies to studies that investigate the agreement between the MRI pathway and systematic biopsy. With an increased number of well‐performed and well‐presented studies, subgroup analyses will be more reliable and more details can be elucidated.
The considerable reduction in grade 2 or higher prostate cancer detection between MRI and the MRI pathway should be assessed with per‐lesion–based data to overcome the lack of spatial concordance between MRI findings and biopsy findings, thereby investigating what factors influence the underlying MRI reading problems and inaccurate MRI‐targeted biopsy. Furthermore, quality control in the MRI pathway should be employed to improve MRI reading and MRI‐targeted biopsy methods. Education, training, procedural standardisation and better imaging and biopsy equipment require a multidisciplinary approach in the management of men with suspected prostate cancer (Moore 2013b; Moore 2017; Puech 2015; Weinreb 2016). The urologist, radiologist and pathologist must collaborate from the moment of clinical suspicion through the process of prostate biopsy and afterwards to accurately make a diagnosis.
Future studies may consider assessing different MRI‐positivity thresholds for MRI‐targeted biopsy, as men with MRI suspicion scores 2, 4 or 5 might have a different pretest risk profile than men with MRI suspicion score 3 (Schoots 2018). In addition, improved MRI interpretation with the reduced number of equivocal or indeterminate lesions (PI‐RADS assessment score 3) may decrease overdiagnosis as demonstrated by Van der Leest 2018.
Whether the number of MRI‐targeted biopsy cores influence athe outcome of the MRI pathway should be investigated, because its diagnostic accuracy could depend on the relation between tumour heterogeneity and the intensity and trajectories of cores taken (Huo 2012; Pham 2015; Valerio 2015). The fact that a high number of MRI‐targeted biopsy cores per suspicious lesion may benefit diagnostic yield may be an argument for focal saturation biopsy (Bryk 2017; Padhani 2019; Rouvière 2019a; Van der Leest 2018), although none of the studies included in this review described or investigated such a strategy.
Most risk classification criteria are still based on systematic biopsy sampling. The potential of risk migration towards higher‐risk categories by an MRI‐directed biopsy management could lead to overtreatment. MRI‐targeted biopsy of suspected lesions on MRI might find higher‐rated risk features than when the prostate is sampled by systematic biopsy. Moreover, traditional risk criteria, including tumour volume measures, cannot be applied to MRI‐targeted biopsy findings. This could result in so‐called ‘risk inflation’, and patients and physicians may be erroneously encouraged to pursue more active treatment because of an apparent increase in risk (reclassification) rather than a true change in their cancer (Robertson 2014). Appropriate risk classification is not yet fully understood when MRI‐targeted biopsy is used. Therefore, the results of MRI‐targeted biopsy must be regarded with caution and future research on risk migration needs to be encouraged.
Risk calculators may aid in balancing harms and benefits by further refining the selection of those men that are at risk of potentially life‐threatening disease. Research should be initiated with recently introduced multivariable risk prediction models, including the MRI suspicion score as an extra input variable, to better identify who would benefit from MRI and subsequent MRI‐targeted biopsy or additional systematic biopsy or both (Alberts 2019; Ankerst 2018; Foley 2016; Mehralivand 2018; Radtke 2017). We have not included risk calculators in this review, however, and their impact cannot be assessed through meta‐analyses of literature because individual participant data would be needed. Similarly, whether clinical parameters and biomarkers can predict which patients may benefit from the MRI pathway (or systematic biopsy) remains outside the scope of this review and should be a subject of future research. Decision‐curve analyses, cost‐effectiveness and the feasibility of obtaining prebiopsy MRI in all patients referred for biopsy were also beyond the scope of this review and should be a subject of future research.
Acknowledgements
We thank Mr Wichor M Bramer, Information Specialist, Medical Library, Erasmus University Medical Centre, Rotterdam, for conducting the systematic literature search. We thank Myriam MG Hunink for critically evaluating the protocol. We thank Jan Verbeek for his thoughts and input in discussions. We thank Caroline M Moore, Anwar R Padhani, and Olivier Rouviere for their extensive review. We wish to acknowledge the support of the Cochrane Collaboration’s Diagnostic Test Accuracy editorial team, the Cochrane Urology editorial team and the peer referees for their assistance. We thank Philipp Dahm as Coordinating Editor of Cochrane Urology (and member of the US GRADE Network) for his assistance with generating the GRADE summary of findings tables.
Appendices
Appendix 1. Template two‐by‐two contingency tables
Diagnostic test accuracy analyses: MRI vs reference standarda
| Histopathology by | |||||
| MRI threshold |
MRI‐TBx outcome |
Template‐guided biopsies | Total | ||
| + | ‐ | ||||
| MRI | + | x | TP | FP | |
| ‐ | x | FN | TN | ||
| Total | |||||
Diagnostic test accuracy analyses: MRI ± MRI‐TBx (MRI pathway) vs reference standardb
| Histopathology by | |||||
| MRI threshold |
MRI‐TBx outcome |
Template‐guided biopsies | Total | ||
| + | ‐ | ||||
| MRI±MRI‐TBx (MRI pathway) |
+ | + | TP | FP | |
| + | ‐ | FN/TP | TN/FP | ||
| ‐ | x | FN | TN | ||
| Total | |||||
The 3x2 table above converts to the 2x2 table below:
| Histopathology by | |||||
| MRI threshold |
MRI‐TBx outcome |
Template‐guided biopsies | Total | ||
| + | ‐ | ||||
| MRI ± MRI‐TBx (MRI pathway) |
+ | + | TP | FP | |
| +/‐ | ‐ | FN | TN | ||
| Total | |||||
Diagnostic test accuracy analyses: SBx vs reference standard
| Histopathology by | ||||
| SBx outcome |
Template‐guided biopsies | Totals | ||
| + | ‐ | |||
| SBx | + | TP | FP | |
| ‐ | FN | TN | ||
| Totals | ||||
Agreement analyses: MRI pathway vs SBxc
| Histopathology by | |||||
| MRI threshold |
MRI‐TBx outcome |
SBx | Total | ||
| + | ‐ | ||||
| MRI pathway | + | + | Concordant positive | Discordant (Dposneg) | |
| + | ‐ | Discordant (Dnegpos) | Concordant negative | ||
| ‐ | x | Discordant (Dnegpos) | Concordant negative | ||
| Total | |||||
The 3x2 table above converts to the 2x2 table below:
| Histopathology by | |||||
| MRI threshold |
MRI‐TBx outcome |
SBx | Total | ||
| + | ‐ | ||||
| MRI pathway | + | + | Concordant positive | Discordant (Dposneg) | |
| +/‐ | ‐ | Discordant (Dnegpos) | Concordant negative | ||
| Total | |||||
aReference standard is template‐guided biopsy. bFor MRI ± MRI‐TBx and the MRI pathway a negative test can result in two ways:
a negative MRI (thus no (x) MRI‐TBx are taken)
a positive MRI but negative MRI‐TBx result.
Both negative outcomes should be merged, creating a two‐by‐two from a three‐by‐two contingency table. cIn the agreement analyses (MRI pathway vs SBx) we have focused on the number of cancers identified and the concordance and discordance between both index tests.
Dnegpos: discordant MRI‐positive/negative + MRI‐targeted biopsy‐negative and systematic biopsy‐positive; Dposneg: discordant MRI‐positive + MRI‐targeted biopsy‐positive and systematic biopsy‐negative; FN: false‐negative; FP: false‐positive; G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; PCa: prostate cancer; SBx: systematic biopsy; TN: true‐negative; TP: true‐positive
Appendix 2. Search strategies
CENTRAL: ((prostat*):ab,ti) AND ((biops*):ab,ti) AND (('magnetic resonance' OR mri OR ((mr OR nmr OR perfusion OR multiparamet* OR multimodal*) NEAR/6 imag*) OR template* OR saturat* OR mapping OR T2‐weighted OR t2w OR Diffusion‐weighted OR dwi OR dynamic‐contrast‐enhanced OR dce OR Spectroscop*) :ab,ti)
MEDLINE ovid: ("Prostatic Neoplasms"/ OR prostate/ OR (prostat*).ab,ti,kf.) AND (exp biopsy/ OR (biops*).ab,ti.) AND ("Magnetic Resonance Imaging"/ OR "Diffusion Magnetic Resonance Imaging"/ OR Magnetic resonance spectroscopy/ OR Image guided biopsy/ OR ("magnetic resonance" OR mri OR ((mr OR nmr OR perfusion OR multiparamet* OR multimodal*) ADJ6 imag*) OR template* OR saturat* OR mapping OR T2‐weighted OR t2w OR Diffusion‐weighted OR dwi OR dynamic‐contrast‐enhanced OR dce OR Spectroscop*).ab,ti,kf.)
Embase.com: ('prostate tumor'/exp OR prostate/de OR 'prostate biopsy'/de OR (prostat*):ab,ti) AND (biopsy/exp OR 'biopsy device'/exp OR (biops*):ab,ti) AND ('nuclear magnetic resonance imaging'/exp OR 'nuclear magnetic resonance'/exp OR 'image guided biopsy'/de OR ('magnetic resonance' OR mri OR ((mr OR nmr OR perfusion OR multiparamet* OR multimodal*) NEAR/6 imag*) OR template* OR saturat* OR mapping OR T2‐weighted OR t2w OR Diffusion‐weighted OR dwi OR dynamic‐contrast‐enhanced OR dce OR Spectroscop*):ab,ti) NOT (conference abstract)/lim
CINAHL ebsco: (MH "Prostatic Neoplasms+" OR MH prostate+ OR (prostat*)) AND (MH biopsy+ OR (biops*)) AND (MH "Magnetic Resonance Imaging" OR ("magnetic resonance" OR mri OR ((mr OR nmr OR perfusion OR multiparamet* OR multimodal*) N5 imag*) OR template* OR saturat* OR mapping OR T2‐weighted OR t2w OR Diffusion‐weighted OR dwi OR dynamic‐contrast‐enhanced OR dce OR Spectroscop*))
Web‐of‐science: TS=(((prostat*)) AND ((biops*)) AND (("magnetic resonance" OR mri OR ((mr OR nmr OR perfusion OR multiparamet* OR multimodal*) NEAR/5 imag*) OR template* OR saturat* OR mapping OR T2‐weighted OR t2w OR Diffusion‐weighted OR dwi OR dynamic‐contrast‐enhanced OR dce OR Spectroscop*) ) ) AND DT=(article)
Scopus: TITLE‐ABS‐KEY(((prostat*)) AND ((biops*)) AND (("magnetic resonance" OR mri OR ((mr OR nmr OR perfusion OR multiparamet* OR multimodal*) W/5 imag*) OR template* OR saturat* OR mapping OR T2‐weighted OR t2w OR Diffusion‐weighted OR dwi OR dynamic‐contrast‐enhanced OR dce OR Spectroscop*) ) ) AND DOCTYPE(ar)
Google.com: "prostate|prostatic biopsy|biopsies" "magnetic resonance"|mri|"mr|nmr|perfusion|multiparametric|multimodal imaging|image|images"|template|templates|saturation|saturated|mapping filetype:pdf
Google scholar: "prostate|prostatic biopsy|biopsies" "magnetic resonance"|mri|"mr|nmr|perfusion|multiparametric|multimodal imaging|image|images"|template|templates|saturation|saturated|mapping
worldcat.org: Ti:(Prostate* AND biops* AND ("magnetic resonance" OR mri OR template* OR saturat* OR mapping OR T2‐weighted OR t2w OR Diffusion‐weighted OR dwi OR dynamic‐contrast‐enhanced OR dce OR Spectroscop*) )
ProQuest (incl. Dissertations and theses): (ti(Prostate*) OR ab(Prostate*)) AND (ti(biops*) OR ab(biops*)) AND (ti("magnetic resonance" OR mri OR template* OR saturat* OR mapping OR T2‐weighted OR t2w OR Diffusion‐weighted OR dwi OR dynamic‐contrast‐enhanced OR dce OR Spectroscop*) OR ab("magnetic resonance" OR mri OR template* OR saturat* OR mapping OR T2‐weighted OR t2w OR Diffusion‐weighted OR dwi OR dynamic‐contrast‐enhanced OR dce OR Spectroscop*) )
OpenGrey: Prostate* AND biops* AND ("magnetic resonance" OR mri OR template* OR saturat* OR mapping OR T2‐weighted OR t2w OR Diffusion‐weighted OR dwi OR dynamic‐contrast‐enhanced OR dce OR Spectroscop*)
Appendix 3. Added value calculation in the agreement analyses (MRI‐pathway vs systematic biopsy)
For grade 2 or higher prostate cancer, the input for the two‐by‐two contingency tables is constructed as shown in the table belowa.
| Systematic biopsy (SBx) | |||||
| No PCa | G = 1 | G ≥ 2 | |||
| MRI | MRI‐negative | No MRI‐TBx | Concordant negative |
Concordant negative |
Discordant (Dnegpos) |
| MRI‐positive + TBx | No PCa | Concordant negative |
Concordant negative |
Discordant (Dnegpos) |
|
| G = 1 | Concordant negative |
Concordant negative |
Discordant (Dnegpos) |
||
| G ≥ 2 |
Discordant (Dposneg) |
Discordant (Dposneg) |
Concordant positive |
||
For grade 1 prostate cancer, the input for the two‐by‐two contingency tables is constructed as shown in the table belowb.
| Systematic biopsy (SBx) | |||||
| No PCa | G = 1 | G ≥ 2 | |||
| MRI | MRI‐negative | No MRI‐TBx | Concordant negative |
Discordant (Dnegpos) |
Concordant negative |
| MRI‐positive + TBx | No PCa | Concordant negative |
Discordant (Dnegpos) |
Concordant negative |
|
| G = 1 |
Discordant (Dposneg) |
Concordant positive |
Concordant negative |
||
| G ≥ 2 | Concordant negative |
Concordant negative |
Concordant negative |
||
aThe construction of input for the two‐by‐two tables (shown in Appendix 1) for grade 2 or higher and grade 3 or higher prostate cancer is similar. bThe construction of input for the two‐by‐two tables for grade 1 prostate cancer needs to consider grade reclassification by each test in order to assess the tests and their added values as add‐on tests as in Table 16 (in contrast to assessment of detection ratio's where the tests are considered as 'replacement tests', not taking into account grade reclassification (Table 15)). The grey boxes with 'Dposneg' represent the number of cancers detected only by the MRI pathway; the grey boxes with 'Dnegpos' represent the number of cancers detected only by systematic biopsy.
Dnegpos: discordant MRI‐positive/negative + MRI‐targeted biopsy‐negative and systematic biopsy‐positive; Dposneg: discordant MRI‐positive + MRI‐targeted biopsy‐positive and systematic biopsy‐negative; FN: false‐negative; FP: false‐positive; G: International Society of Urological Pathology grade; MRI: multiparametric magnetic resonance imaging; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; PCa: prostate cancer; SBx: systematic biopsy; TN: true‐negative; TP: true‐positive
Appendix 4. MRI and MRI‐targeted biopsy characteristics of included studies
| Study | MRI technique | MRI reading | MRI‐TBx | ||||||
| Study | Inclusion period | Machine |
Magnetic field strength |
Pulse sequences |
Endorectal coil |
MRI score system | Experience / consensus reading | Technique | N cores |
| Diagnostic test accuracy analyses studies | |||||||||
| Abd‐Alazeez 2014 | < Apr 2013 | Achieva, Philips/Avanto, Siemens | 1.5 & 3 | T2, DWI, DCE | No | PI‐RADS v1 | Experienced / NR | Cognitive | NR |
| Ahmed 2017 | May 2012 Nov 2015 | NR | 1.5 | T2, DWI, DCE | No | PI‐RADS v1a | Experienced / NR | NA | NA |
| Dal Moro 2019 | Jan 2013 Dec 2016 | NR | 1.5 | T2, DWI | NR | PI‐RADS v1 | Experienced / NR | Cognitive | 1/lesion |
| Distler 2017 | Oct 2012 Dec 2015 | Magnetom Prisma, Siemens/Biograph mMR, Siemens | 3 | T2, DWI, DCE | No | PI‐RADS v1 | Experienced / No | BiopSee, Pi Medical/MedCom (rigid) | 3/lesion |
| Grey 2015 | Jul 2012 Nov 2013 | Signa Excite, GE/Magnetom Symphony, Siemens | 1.5 | T2, DWI | No | PI‐RADS v1 | Experienced / No | Cognitive | NR |
| Hansen 2016a | Mar 2013 Oct 2015 | NR | 1.5 & 3 | T2, DWI | No | PI‐RADS v1 | Experienced / No | BiopSee, Pi Medical/MedCom (rigid) | 2/lesion |
| Hansen 2018 | Oct 2012 May 2016 | Discovery MR450/MR750 HDx, GE/Magnetom, Siemens | 1.5 & 3 | T2, DWI, DCE | NR | PI‐RADS v1, v2 | Experienced / Yes | BiopSee, Pi Medical/MedCom (rigid) | 2‐4/pt (IQR 2‐5) |
| Hansen 2017 | Oct 2013 Nov 2015 | Magnetom, Siemens | 3 | T2, DWI, DCE | No | PI‐RADS v1 | Experienced / NR | BiopSee, Pi Medical/MedCom (rigid) | 2/pt (IQR 2‐4) |
| Kesch 2017 | Oct 2013 Mar 2014 | Magnetom, Siemens | 3 | T2, DWI, DCE | NR | PI‐RADS v1 | Experienced / NR | BiopSee, Pi Medical/MedCom (rigid) | 2/lesion (range 2‐3) |
| Lawrence 2014 | Feb 2012 Jun 2012 | MR450, GE | 1.5 & 3 | T2, DWI | No | PI‐RADS v1 | Experienced / Yes | BiopSee, Pi Medical/MedCom (rigid) | 7/pt (range 0‐14) |
| Mortezavi 2018 | Nov 2014 Sep 2016 | Magnetom Skyra, Siemens | 3 | T2, DWI, DCE | Yes/No | PI‐RADS v1 | Experienced / Yes and No | BiopSee, Pi (Medical)/MedCom (non‐rigid) | 2‐4/lesion |
| Muthuveloe 2016 | Mar 2013 Dec 2014 | NR | NR | T2, DWI, DCE | NR | PI‐RADS v1 | Unclear / NR | NA | NA |
| Pepe 2013 | Jun 2011 Dec 2012 | Achieva, Philips | 3 | T2, DWI, DCE, spectroscopy | No | In‐house | Unclear / No | Cognitive | 3,5/pt (range 3‐4) |
| Thompson 2016 | Apr 2012 Mar 2014 | NR | 1.5 & 3 | T2, DWI, DCE | No | PI‐RADS v1 | Experienced / Yes | BioJet, Geoscan (rigid) | NR |
| Tsivian 2017 | 2011 2014 | Signa HDx, GE/Skyra, Siemens | 3 | T2, DWI, DCE | Yes/No | PI‐RADS v1a | Experienced / No | NA | NA |
| Agreement analyses studies | |||||||||
| Alberts 2017 | Oct 2013 Apr 2016 | Discovery MR750, GE | 3 | T2, DWI, DCE | No | PI‐RADS v2 | Experienced / Yes | Urostation, Koelis (elastic) | 2‐3/pt |
| Boesen 2017a | Sep 2012 Sep 2013 | Ingenia, Philips | 3 | T2, DWI, DCE | No | PI‐RADS v1 | Experienced / No | Real‐Time Virtual Sonography, Hitachi (rigid) | 1‐2/lesion |
| Boesen 2018 | Nov 2015 Jun 2017 | Philips Healthcare | 3 | T2, DWI | No | PI‐RADS v2a | Experienced / No | HI‐RVS (Hitachi; n=877), Uro‐Nav system (Invivo; n=143) | 1‐2/lesion |
| Castellucci 2017 | Jul 2011 Jul 2014 | Achieva, Philips | 1.5 | T2, DWI | NR | PI‐RADS v1 | Experienced / Yes | Cognitive | 2/lesion |
| Chang 2017 | Mar 2012 Dec2014 | Signa HDx, GE | 3 | T2, DWI, DCE | NR | PI‐RADS v1, v2 | Experienced / NR | Cognitive | ≥ 2/lesion |
| Chen 2015 | Jun 2008 Dec 2013 | Achieva, Philips | 3 | T2, DWI | No | In‐house | Experienced / NR | Cognitive | 1‐2/lesion |
| Cool 2016 | Sep 2011 Mar 2014 | NR, GE | 3 | T2, DWI, DCE | Yes/No | In‐house | Experienced / No | Artemis, Eigen (elastic) | 1.9/lesion |
| Costa 2013 | Aug 2003 Aug 2008 | Genesis Signa LX Excite, GE | 3 | T2, DCE | Yes | In‐house | Experienced / NR | Cognitive | NR |
| Delongchamps 2013 | Jan 2011 Mar 2012 | NR | 1.5 | T2, DWI, DCE | Yes/No | In‐house | Experienced / Yes | Urostation, Koelis (elastic)/Virtual Navigator, Esaote (rigid)/Cognitive | 4/pt (range 2‐10) |
| Filson 2016 | Sep 2009 Feb 2015 | TrioTim Somatom, Siemens | 3 | T2, DWI, DCE | No | In‐house | Experienced / No | Artemis, Eigen (elastic) | 1 per 3 mm. lesion diameter |
| Garcia Bennett 2017 | Oct 2014 Apr 2016 | Signa, GE | 3 | T2, DWI | No | PI‐RADS v1 | Experienced / Yes | Cognitive | NR |
| Grönberg 2018 | May 2016 May 2017 | Magnetom Avanto, Siemens/Magnetom Aera, Siemens | 1.5 | T2, DWI | No | PI‐RADS v2 | Experienced / Yes | Urostation, Koelis/Artemis, Eigen/BioJet, D&K Technologies | NR |
| Jambor 2015 | Apr 2011 Mar 2013 | Magnetom Verio, Siemens | 3 | T2, DWI, DCE, spectroscopy | No | In‐house | Unclear / NR | Cognitive | NR |
| Jambor 2017 | Mar 2013 Feb 2015 | Magnetom Verio, Siemens | 3 | T2, DWI | NR | In‐house | Experienced/ No | Cognitive | 2/index lesion |
| Kim 2017 | Jan 2012 Dec 2015 | Magnetom Trio/Skyra, Siemens | 3 | T2, DWI, DCE | No | In‐house, PI‐RADS v1, v2 | Experienced / No | UroNav, Invivo (rigid) | 6.7/pt |
| Lee 2016 | Jan 2014 Dec 2014 | Intera Achieva, Philips | 3 | T2, DWI | No | In‐house | Experienced / NR | Cognitive | 2.4/pt |
| Lee 2017 | 2016 | Intera Achieva, Philips | 3 | T2, DWI, (DCE 55 pts) T2, DWI (68 pts) | No | PI‐RADS v2a | Experienced / NR | Cognitive | NR |
| Okcelik 2016 | Feb 2013 Mar 2014 | Avanto, Siemens | 1.5 | T2, DWI, DCE, spectroscopy | NR | In‐house | Unclear / NR | Cognitive | NR |
| Panebianco 2015 | Oct 2011 Mar 2014 | Discovery MR750, GE/Magnetom Verio, Siemens | 3 | T2, DWI, DCE | Yes/No | PI‐RADS v1 | Experienced / Yes | Cognitive | 2/pt |
| Peltier 2015 | Mar 2012 Sep 2013 | Magnetom Verio, Siemens | 3 | T2, DWI, DCE | Yes/No | PI‐RADS v1a | Experienced / No | Urostation, Koelis (elastic) | 2,4/lesion (range 1‐4) |
| Pokorny 2014 | Jul 2012 Jan 2013 | Magnetom Skyra, Siemens | 3 | T2, DWI, DCE | No | PI‐RADS v1 | Experienced / Yes | In‐bore | 2/pt (range 2‐3) |
| Rouvière 2019a | Jul 2015 Aug2016 | MR 750, GE/MR 450, GE/Ingenia, Philips/Avanto, Siemens/Intera, Philips/Aera, Siemens/Achieva, Philips/Skyra, Siemens/Priesma, Siemens | 1.5 & 3 | T2, DWI, DCE | Yes/No | PI‐RADS v1/v2a | Experienced / No | Urostation, Koelis/Smart Fusion, Toshiba/Percunav, Philips | 3/lesion |
| Say 2016 | Dec 2012 Jun 2015 | NR | NR | T2, DWI, DCE | NR | In‐house, PI‐RADS v1 | Unclear / NR | Artemis, Eigen (elastic) | NR |
| Tonttilla 2016 | Apr 2011 Dec 2014 | Magnetom Skyra, Siemens | 3 | T2, DWI, DCE | No | In‐house | No experience / Unclear | Cognitive | 2/pt (range 2‐3) |
| Van der Leest 2018 | Feb 2015 Feb 2017 | Magnetom Skyra, Siemens | 3 | T2, DWI, DCE | NR | PI‐RADS v2 | Experienced / Yes | In‐bore, Invivo | 2‐4/lesion |
|
abased on the PI‐RADS v1/v2 guidelines but either before official publication or practically identical. DCE: dynamic contrast‐enhanced imaging; DWI: diffusion‐weighted imaging; MRI: magnetic resonance imaging; NA: not applicable; NR: not reported; PI‐RADS v1, v2: Prostate Imaging Reporting Data System version 1 or 2; pt(s): participant(s); SBx: systematic biopsy; T2: T2‐weighted imaging | |||||||||
Appendix 5. Predictive values of the index tests at prespecified prevalences of prostate cancer
| Index test | MRI populationa | Target condition | Prevalence | NPV (95% CI)b | PPV (95% CI)b |
| MRI | Positive + Negative | G = 1 | 0.10 | 0.89 (0.87 to 0.91) | 0.10 (0.09 to 0.11) |
| 0.20 | 0.79 (0.74 to 0.82) | 0.20 (0.18 to 0.21) | |||
| 0.30 | 0.68 (0.63 to 0.73) | 0.29 (0.27 to 0.31) | |||
| 0.40 | 0.58 (0.52 to 0.64) | 0.39 (0.37 to 0.42) | |||
| 0.50 | 0.48 (0.42 to 0.54) | 0.49 (0.47 to 0.52) | |||
| G ≥ 2 | 0.10 | 0.97 (0.96 to 0.98) | 0.14 (0.13 to 0.15) | ||
| 0.20 | 0.94 (0.91 to 0.96) | 0.27 (0.25 to 0.28) | |||
| 0.30 | 0.91 (0.86 to 0.94) | 0.38 (0.36 to 0.40) | |||
| 0.40 | 0.86 (0.79 to 0.91) | 0.49 (0.47 to 0.51) | |||
| 0.50 | 0.81 (0.72 to 0.87) | 0.59 (0.57 to 0.61) | |||
| G ≥ 3 | 0.05 | 0.99 (0.98 to 1.00) | 0.07 (0.06 to 0.08) | ||
| 0.10 | 0.99 (0.97 to 0.99) | 0.14 (0.13 to 0.16) | |||
| 0.15 | 0.98 (0.95 to 0.99) | 0.21 (0.19 to 0.23) | |||
| 0.20 | 0.97 (0.93 to 0.99) | 0.27 (0.25 to 0.29) | |||
| 0.25 | 0.96 (0.90 to 0.98) | 0.33 (0.30 to 0.36) | |||
| MRI‐TBx | Positive | G = 1 | 0.10 | 0.95 (0.90 to 0.97) | 0.94 (0.11 to 1.00) |
| 0.20 | 0.89 (0.80 to 0.94) | 0.97 (0.21 to 1.00) | |||
| 0.30 | 0.83 (0.70 to 0.91) | 0.98 (0.32 to 1.00) | |||
| 0.40 | 0.75 (0.60 to 0.86) | 0.99 (0.42 to 1.00) | |||
| 0.50 | 0.67 (0.50 to 0.81) | 0.99 (0.52 to 1.00) | |||
| G ≥ 2 | 0.10 | 0.98 (0.96 to 0.98) | 0.60 (0.47 to 0.72) | ||
| 0.20 | 0.95 (0.92 to 0.97) | 0.77 (0.67 to 0.86) | |||
| 0.30 | 0.92 (0.88 to 0.94) | 0.85 (0.77 to 0.91) | |||
| 0.40 | 0.88 (0.82 to 0.91) | 0.90 (0.84 to 0.94) | |||
| 0.50 | 0.83 (0.75 to 0.88) | 0.93 (0.89 to 0.96) | |||
| G ≥ 3 | 0.05 | ID | ID | ||
| 0.10 | ID | ID | |||
| 0.15 | ID | ID | |||
| 0.20 | ID | ID | |||
| 0.25 | ID | ID | |||
| MRI pathway | Positive + Negative | G = 1 | 0.10 | 0.93 (0.91 to 0.95) | 0.89 (0.21 to 1.00) |
| 0.20 | 0.86 (0.82 to 0.89) | 0.95 (0.37 to 1.00) | |||
| 0.30 | 0.78 (0.73 to 0.82) | 0.97 (0.50 to 1.00) | |||
| 0.40 | 0.69 (0.63 to 0.75) | 0.98 (0.61 to 1.00) | |||
| 0.50 | 0.60 (0.53 to 0.66) | 0.99 (0.70 to 1.00) | |||
| G ≥ 2 | 0.10 | 0.97 (0.96 to 0.98) | 0.69 (0.56 to 0.79) | ||
| 0.20 | 0.93 (0.90 to 0.95) | 0.83 (0.74 to 0.90) | |||
| 0.30 | 0.89 (0.85 to 0.92) | 0.90 (0.83 to 0.94) | |||
| 0.40 | 0.84 (0.78 to 0.89) | 0.93 (0.88 to 0.96) | |||
| 0.50 | 0.78 (0.70 to 0.84) | 0.95 (0.92 to 0.97) | |||
| G ≥ 3 | 0.05 | ID | ID | ||
| 0.10 | ID | ID | |||
| 0.15 | ID | ID | |||
| 0.20 | ID | ID | |||
| 0.25 | ID | ID | |||
| SBx | NA | G = 1 | 0.10 | 0.95 (0.91 to 0.98) | 0.87 (0.20 to 0.99) |
| 0.20 | 0.90 (0.81 to 0.95) | 0.94 (0.37 to 1.00) | |||
| 0.30 | 0.84 (0.71 to 0.92) | 0.96 (0.50 to 1.00) | |||
| 0.40 | 0.77 (0.61 to 0.88) | 0.98 (0.61 to 1.00) | |||
| 0.50 | 0.69 (0.52 to 0.83) | 0.98 (0.70 to 1.00) | |||
| G ≥ 2 | 0.10 | 0.96 (0.88 to 0.99) | 1.00 (0.41 to 1.00) | ||
| 0.20 | 0.92 (0.76 to 0.97) | 1.00 (0.61 to 1.00) | |||
| 0.30 | 0.86 (0.65 to 0.95) | 1.00 (0.73 to 1.00) | |||
| 0.40 | 0.80 (0.54 to 0.93) | 1.00 (0.81 to 1.00) | |||
| 0.50 | 0.73 (0.44 to 0.90) | 1.00 (0.86 to 1.00) | |||
| G ≥ 3 | 0.05 | ID | ID | ||
| 0.10 | ID | ID | |||
| 0.15 | ID | ID | |||
| 0.20 | ID | ID | |||
| 0.25 | ID | ID | |||
| aData did not allow differentiation between the mix of included participants (biopsy‐naïve and prior‐negative biopsy men). bNPV and PPV are based on the Bayes’ theorem, using the point estimates and 95% confidence intervals of the pooled positive and negative likelihood ratio and prespecified prevalences. CI: confidence interval; G: International Society of Urological Pathology grade; ID: inadequate data; MRI: magnetic resonance imaging; MRI pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; NA: not applicable; NPV: negative predictive value; PPV: positive predictive value; SBx: systematic biopsy | |||||
Appendix 6. Agreement analysis of MRI‐pathway versus systematic biopsy with random‐effects meta‐analysis
| Population | Target condition | N participants (studies) | Proportion prostate cancer detected in % (95% CI) a | Detection ratio (95% CI) b | P‐value | Difference between populations, P valued | |||
| Biopsy status | MRI in % (95% CI) c | MRI pathwayand SBx combined (total cancer detected) | MRI pathway | SBx | MRI pathwayversus SBx | ||||
| Mixed population e | Positive + negative (100 (100 to 100)) | G=1 | 5442 (21) | 26.9 (22.9 to 31.2) | 12.9 (10.1 to 16.2) | 22.0 (18.9 to 25.4) | 0.608 (0.521 to 0.711) | 0.000 | NA |
| G=1f | 5442 (21) | 21.2 (17.7 to 25.2) | 10.9 (8.2 to 14.4) | 18.2 (15.5 to 21.2) | 0.622 (0.506 to 0.764) | 0.000 | NA | ||
| G≥1 | 6524 (24) | 51.7 (47.3 to 56.1) | 38.6 (34.0 to 43.4) | 43.9 (39.4 to 48.5) | 0.877 (0.807 to 0.954) | 0.002 | NA | ||
| G≥2 | 6944 (25) | 28.5 (24.2 to 33.4) | 23.8 (20.1 to 28.0) | 20.5 (16.7 to 25.0) | 1.120 (1.024 to 1.225) | 0.013 | NA | ||
| G≥3 | 5981 (21) | 16.4 (13.3 to 20.2) | 13.3 (10.5 to 16.7) | 11.3 (8.9 to 14.3) | 1.201 (1.059 to 1.363) | 0.004 | NA | ||
| Positive (67.6 (60.2 to 74.3)) | G=1 | 3460 (19) | 31.2 (26.4 to 36.4) | 19.9 (15.4 to 25.4) | 23.9 (19.9 to 28.4) | 0.853 (0.753 to 0.967) | 0.013 | NA | |
| G=1f | 3460 (19) | 22.9 (18.4 to 28.2) | 16.8 (12.1 to 22.8) | 18.5 (14.9 to 22.7) | 0.938 (0.812 to 1.083) | 0.381 | NA | ||
| G≥1 | 3998 (20) | 69.1 (62.2 to 75.2) | 60.7 (52.7 to 68.1) | 57.9 (51.9 to 63.7) | 1.025 (0.951 to 1.104) | 0.522 | NA | ||
| G≥2 | 3998 (20) | 43.9 (37.1 to 51.0) | 38.2 (32.2 to 44.6) | 32.4 (26.4 to 38.9) | 1.171 (1.073 to 1.277) | 0.000 | NA | ||
| G≥3 | 3902 (18) | 25.6 (21.2 to 30.6) | 21.5 (17.5 to 26.0) | 17.7 (14.3 to 21.7) | 1.238 (1.109 to 1.382) | 0.000 | NA | ||
| Biopsy‐naïve men | Positive + negative (100 (100 to 100)) | G=1 | 4079 (17) | 28.7 (24.1 to 33.8) | 14.3 (10.8 to 18.6) | 23.7 (20.1 to 27.8) | 0.630 (0.535 to 0.742) | 0.000 | 0.905 |
| G=1f | 4079 (17) | 23.1 (19.1 to 27.7) | 11.9 (8.4 to 16.7) | 20.1 (17.1 to 23.4) | 0.611 (0.485 to 0.769) | 0.000 | |||
| G≥1 | 4799 (19) | 55.2 (50.1 to 60.1) | 40.9 (35.2 to 46.8) | 48.5 (43.8 to 53.3) | 0.845 (0.767 to 0.930) | 0.001 | 0.121 | ||
| G≥2 | 5219 (20) | 29.5 (24.3 to 35.3) | 24.0 (19.7 to 29.0) | 22.6 (17.9 to 28.2) | 1.050 (0.948 to 1.162) | 0.349 | 0.002 | ||
| G≥3 | 4306 (16) | 17.1 (13.1 to 21.9) | 13.2 (9.8 to 17.5) | 13.0 (10.0 to 16.6) | 1.087 (0.937 to 1.261) | 0.269 | 0.004 | ||
| Positive (67.0 (58.7 to 74.4)) | G=1 | 2682 (16) | 32.7 (27.3 to 38.6) | 21.4 (16.1 to 28.0) | 25.6 (20.9 to 31.0) | 0.854 (0.743 to 0.982) | 0.026 | 0.347 | |
| G=1f | 2682 (16) | 24.4 (19.1 to 30.7) | 17.9 (12.4 to 25.2) | 20.5 (16.2 to 25.6) | 0.911 (0.782 to 1.062) | 0.233 | |||
| G≥1 | 2955 (17) | 71.9 (64.8 to 78.1) | 63.4 (54.7 to 71.3) | 62.5 (56.9 to 67.8) | 0.994 (0.915 to 1.079) | 0.881 | 0.053 | ||
| G≥2 | 2955 (17) | 45.6 (38.2 to 53.2) | 39.5 (33.1 to 46.2) | 35.1 (28.5 to 42.4) | 1.119 (1.014 to 1.234) | 0.025 | 0.005 | ||
| G≥3 | 2899 (15) | 26.4 (21.3 to 32.2) | 21.6 (17.1 to 26.9) | 19.1 (15.2 to 23.7) | 1.158 (1.024 to 1.310) | 0.020 | 0.007 | ||
| Prior‐negative biopsy men | Positive + negative (100 (100 to 100)) | G=1 | 1202 (8) | 24.6 (17.8 to 33.0) | 11.2 (7.5 to 16.4) | 19.4 (13.3 to 27.3) | 0.624 (0.444 to 0.878) | 0.007 | 0.905 |
| G=1f | 1202 (8) | 19.5 (13.2 to 27.9) | 10.1 (6.6 to 15.2) | 15.5 (10.1 to 22.9) | 0.720 (0.507 to 1.023) | 0.067 | |||
| G≥1 | 1564 (10) | 42.6 (36.5 to 48.9) | 30.8 (24.1 to 38.4) | 32.5 (27.3 to 38.2) | 0.974 (0.854 to 1.111) | 0.696 | 0.121 | ||
| G≥2 | 1564 (10) | 24.3 (22.2 to 26.6) | 21.2 (18.5 to 24.3) | 14.2 (11.5 to 17.4) | 1.441 (1.190 to 1.745) | 0.000 | 0.002 | ||
| G≥3 | 1514 (9) | 13.7 (11.9 to 15.7) | 12.4 (10.5 to 14.4) | 7.1 (4.9 to 10.1) | 1.637 (1.270 to 2.112) | 0.000 | 0.004 | ||
| Positive (69.6 (54.7 to 81.3)) | G=1 | 655 (7) | 30.2 (21.0 to 41.5) | 19.3 (11.6 to 30.5) | 21.0 (13.7 to 30.8) | 1.027 (0.892 to 1.183) | 0.707 | 0.347 | |
| G=1f | 655 (7) | 23.2 (14.9 to 34.2) | 17.5 (9.9 to 29.1) | 15.8 (9.9 to 24.2) | 1.212 (1.036 to 1.418) | 0.016 | |||
| G≥1 | 920 (8) | 56.9 (42.3 to 70.5) | 49.2 (32.4 to 66.1) | 40.2 (32.0 to 49.0) | 1.163 (1.023 to 1.322) | 0.021 | 0.053 | ||
| G≥2 | 920 (8) | 32.2 (26.0 to 39.1) | 28.5 (22.2 to 35.8) | 19.3 (16.0 to 23.2) | 1.492 (1.223 to 1.822) | 0.000 | 0.005 | ||
| G≥3 | 880 (7) | 18.9 (14.5 to 24.3) | 17.4 (12.7 to 23.4) | 10.4 (7.0 to 15.1) | 1.648 (1.298 to 2.093) | 0.000 | 0.007 | ||
| CI: confidence interval; G: International Society of Urological Pathology grade; MRI: magnetic resonance imaging; MRI pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; N: number; SBx: systematic biopsy | |||||||||
Footnotes
aResults are based on direct random‐effects meta‐analysis. Results that are based on meta‐analysis with mixed modelling (multinomial logistic regression models) are presented in Table 15. Results may slightly differ between both statistical methods. bDetection ratio is detection rate of magnetic resonance imaging‐pathway divided by detection rate of systematic biopsy; the detection rate is the pooled number of positive results of the test divided by the pooled total number of positive results from both tests. cProportion of participants with a positive or negative magnetic resonance imaging result, based on the studies reporting grade 2 or higher. dEvaluating the difference in detection ratio's between the populations (biopsy‐naïve men versus prior‐negative biopsy) for each target condition. eMixed: biopsy‐naïve and prior‐negative biopsy men. fTaking into account grade reclassification by each test (Appendix 3). Therefore, G = 1f results (with reclassification) differ from G =1 results (without reclassification).
Appendix 7. Glossary and abbreviations
| Added value MRI pathway: pooled proportion of participants with prostate cancer not detected by systematic biopsy but only detected by the MRI pathway |
| Added value systematic biopsy: pooled proportion of participants with prostate cancer not detected by the magnetic resonance imaging‐pathway but only detected by systematic biopsy |
| Agreement analysis: provides pooled estimates of detection ratios (detection rate magnetic resonance imaging‐pathway/detection rate systematic biopsy) |
| csPCa: clinically significant prostate cancer, defined in this review as grade 2 and higher prostate cancer |
| DCE imaging: dynamic contrast‐enhanced imaging |
| Detection rate: the pooled number of positive results of the test divided by the pooled total number of positive results from both tests |
| Detection ratio: the detection rate of the magnetic resonance imaging‐pathway divided by the detection rate of systematic biopsy |
| Diagnostic test accuracy analysis: provides pooled estimates of sensitivity and specificity |
| DRE: digital rectal exam |
| DWI or DW‐MRI: diffusion‐weighted magnetic resonance imaging |
| G: prostate cancer grade as scored by the International Society of Urological Pathology system |
| ISUP grade: International Society of Urological Pathology grade |
| Mixed population: mix of biopsy‐naïve and prior‐negative biopsy men |
| MRI: magnetic resonance imaging |
| MRI pathwayMRI pathway: magnetic resonance imaging with or without magnetic resonance imaging‐targeted biopsy |
| MRI‐TBx: magnetic resonance imaging‐targeted biopsy |
| NA: not applicable |
| ID: inadequate data |
| NPV: negative predictive value (proportion of negative results that are true‐negative) |
| PCa: Prostate cancer |
| PI‐RADS v1, v2: Prostate Imaging Reporting Data System version 1 or 2 |
| PPV: positive predictive value (proportions of positive results that are true‐positive) |
| PSA: prostate‐specific antigen |
| QUADAS‐2: a tool for the Quality Assessment of Diagnostic Accuracy Studies |
| Reference standard: template‐guided biopsy (comprehensively sampling all zones of the prostate) by a transperineal template mapping biopsy or transperineal or transrectal saturation biopsy technique |
| START: International Working Group on Standards of Reporting for MRI‐targeted biopsy studies |
| SBx: systematic biopsy |
| T2W imaging: T2‐weighted magnetic resonance imaging |
| TRUS: transrectal ultrasound |
| TSB: transperineal or transrectal saturation biopsy (sampling all zones of the prostate with > 20 cores, according to a predefined core distribution pattern) |
| TTMB: transperineal template mapping biopsy (using a 5‐mm brachytherapy grid, with ≥ 1 biopsy from each hole) |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
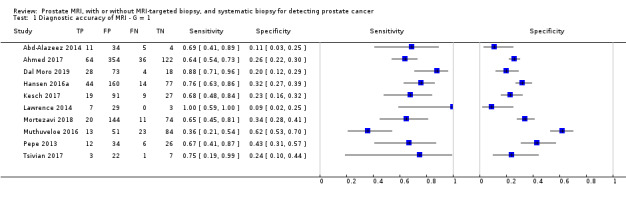
Diagnostic accuracy of MRI ‐ G = 1.
2. Test.
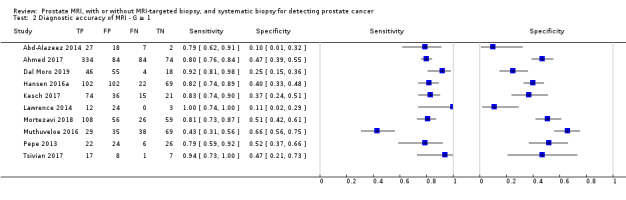
Diagnostic accuracy of MRI ‐ G ≥ 1.
3. Test.
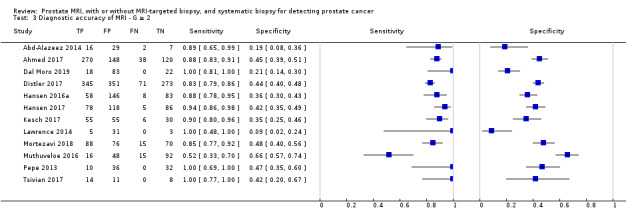
Diagnostic accuracy of MRI ‐ G ≥ 2.
4. Test.
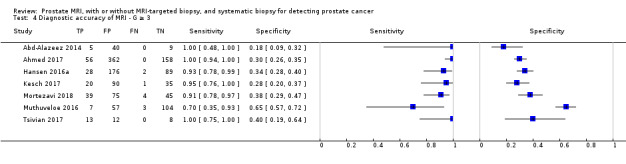
Diagnostic accuracy of MRI ‐ G ≥ 3.
5. Test.
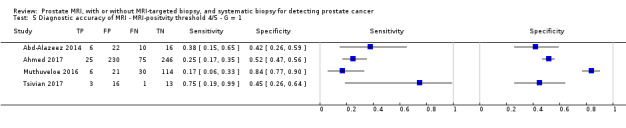
Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G = 1.
6. Test.
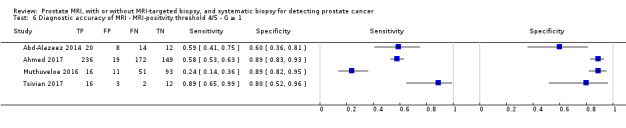
Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 1.
7. Test.
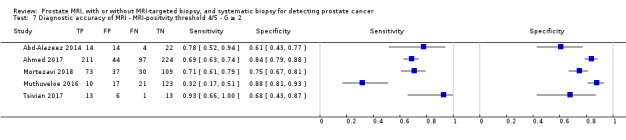
Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 2.
8. Test.
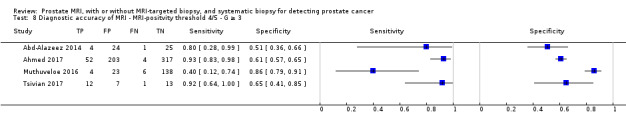
Diagnostic accuracy of MRI ‐ MRI‐positvity threshold 4/5 ‐ G ≥ 3.
9. Test.
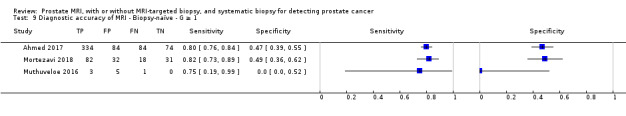
Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 1.
10. Test.

Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 2.
11. Test.
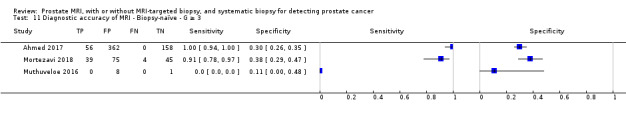
Diagnostic accuracy of MRI ‐ Biopsy‐naïve ‐ G ≥ 3.
12. Test.
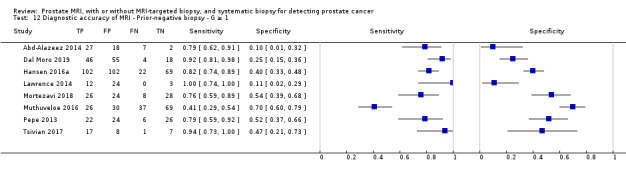
Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 1.
13. Test.
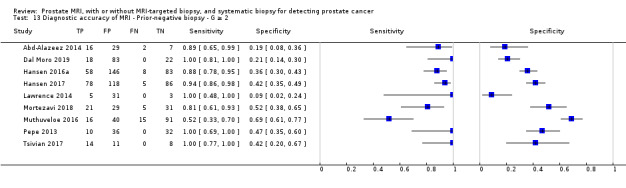
Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 2.
14. Test.
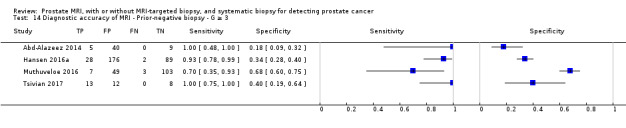
Diagnostic accuracy of MRI ‐ Prior‐negative biopsy ‐ G ≥ 3.
15. Test.
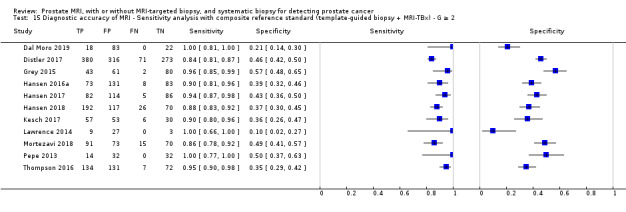
Diagnostic accuracy of MRI ‐ Sensitivity analysis with composite reference standard (template‐guided biopsy + MRI‐TBx) ‐ G ≥ 2.
16. Test.
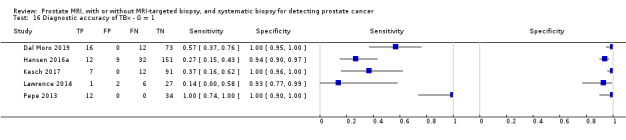
Diagnostic accuracy of TBx ‐ G = 1.
17. Test.
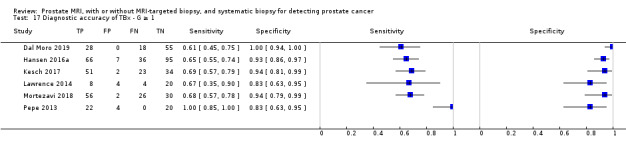
Diagnostic accuracy of TBx ‐ G ≥ 1.
18. Test.
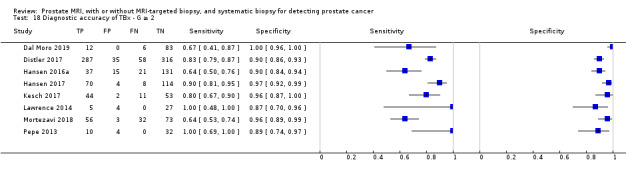
Diagnostic accuracy of TBx ‐ G ≥ 2.
20. Test.
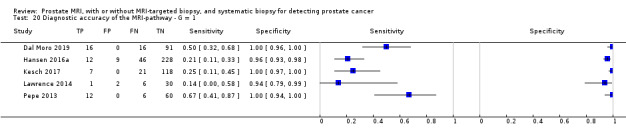
Diagnostic accuracy of the MRI‐pathway ‐ G = 1.
21. Test.

Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 1.
22. Test.
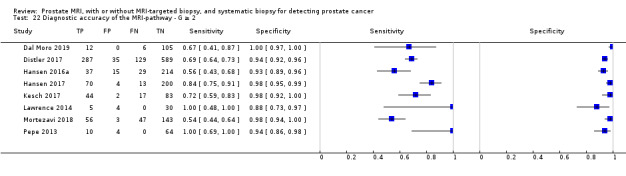
Diagnostic accuracy of the MRI‐pathway ‐ G ≥ 2.
24. Test.
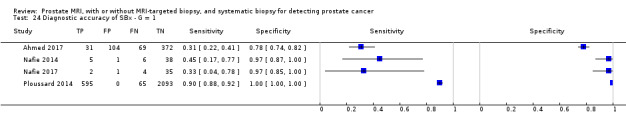
Diagnostic accuracy of SBx ‐ G = 1.
25. Test.
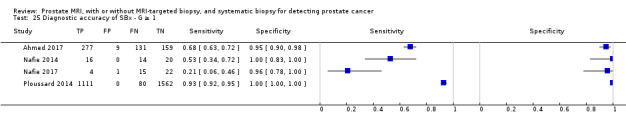
Diagnostic accuracy of SBx ‐ G ≥ 1.
26. Test.
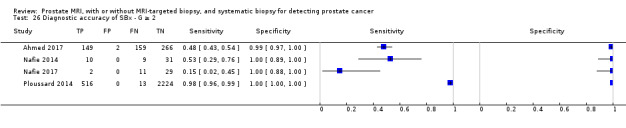
Diagnostic accuracy of SBx ‐ G ≥ 2.
28. Test.
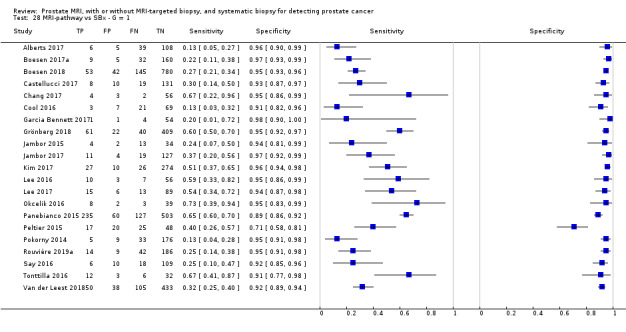
MRI‐pathway vs SBx ‐ G = 1.
29. Test.
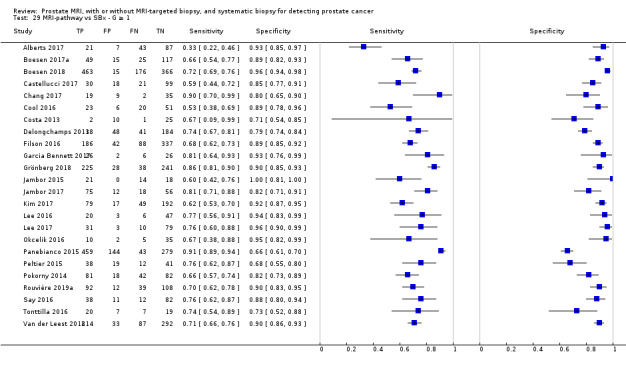
MRI‐pathway vs SBx ‐ G ≥ 1.
30. Test.
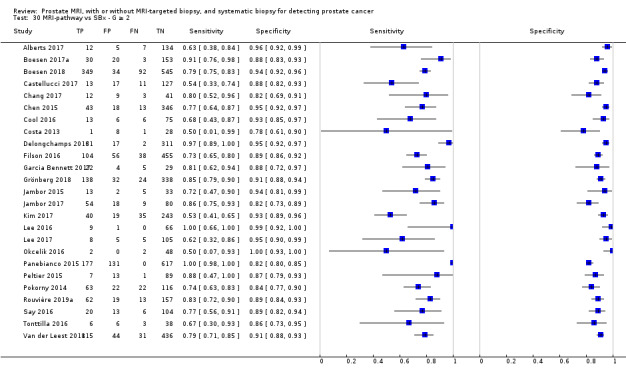
MRI‐pathway vs SBx ‐ G ≥ 2.
31. Test.

MRI‐pathway vs SBx ‐ G ≥ 3.
32. Test.
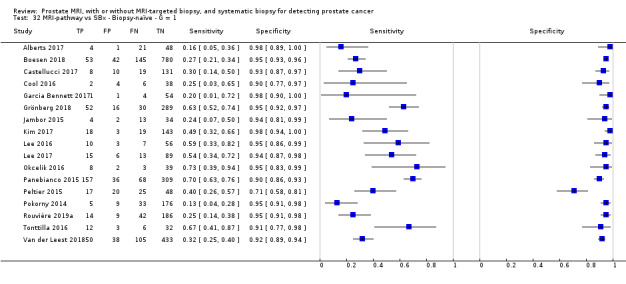
MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G = 1.
33. Test.
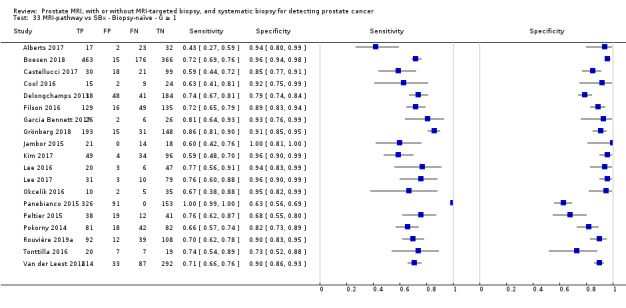
MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 1.
34. Test.
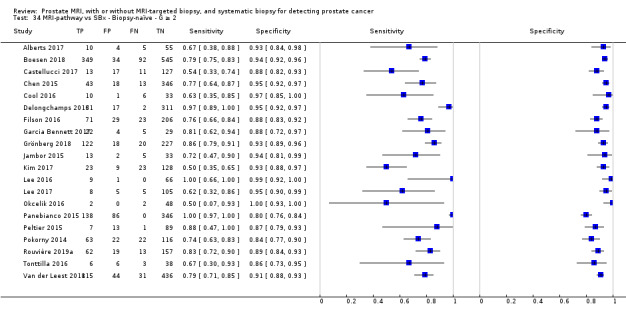
MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 2.
35. Test.
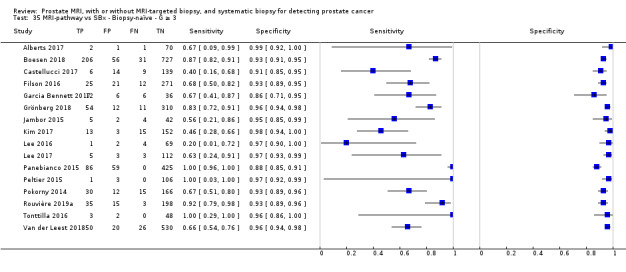
MRI‐pathway vs SBx ‐ Biopsy‐naïve ‐ G ≥ 3.
36. Test.
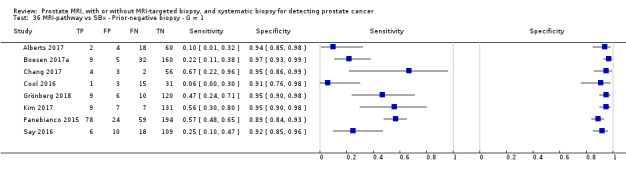
MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G = 1.
37. Test.
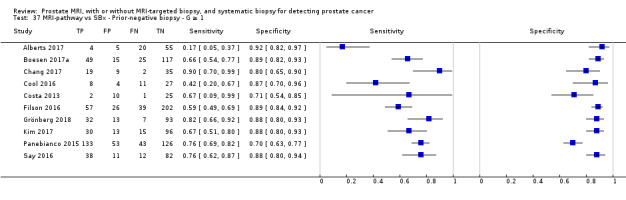
MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 1.
38. Test.
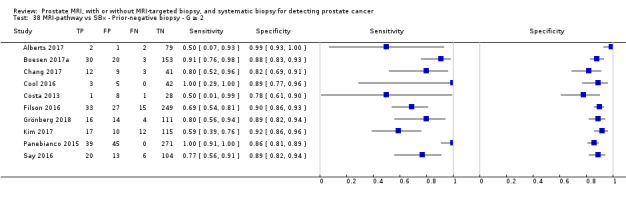
MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 2.
39. Test.

MRI‐pathway vs SBx ‐ Prior‐negative biopsy ‐ G ≥ 3.
40. Test.

MRI‐pathway vs SBx ‐ Positive MRI ‐ G = 1.
41. Test.
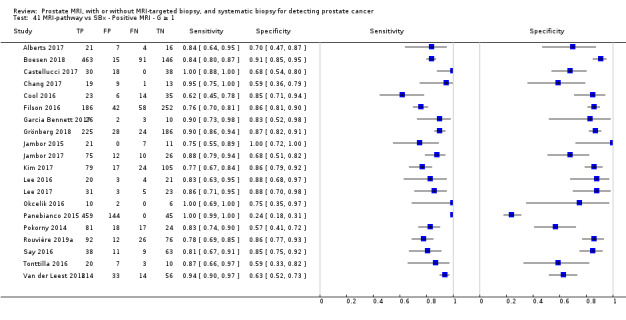
MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 1.
42. Test.
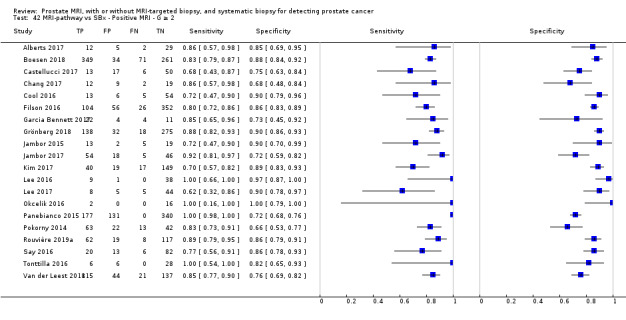
MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 2.
43. Test.
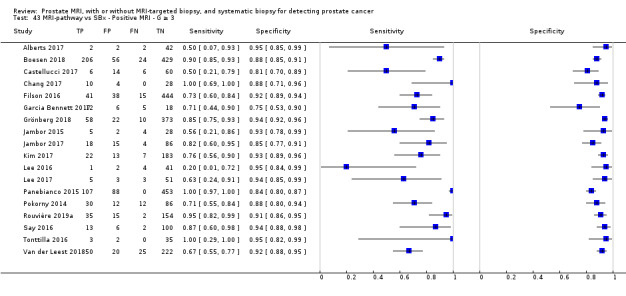
MRI‐pathway vs SBx ‐ Positive MRI ‐ G ≥ 3.
44. Test.
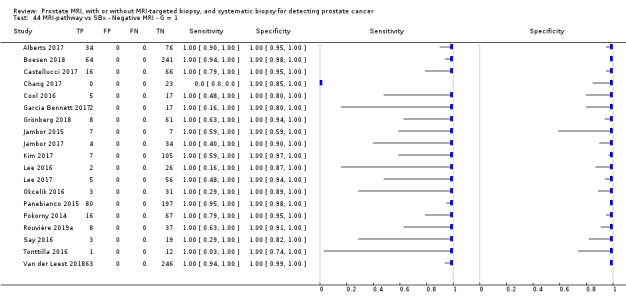
MRI‐pathway vs SBx ‐ Negative MRI ‐ G = 1.
45. Test.
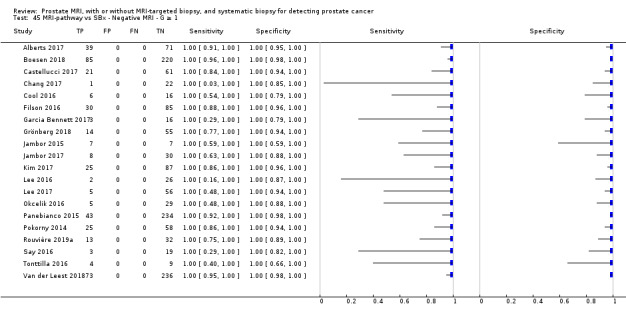
MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 1.
46. Test.
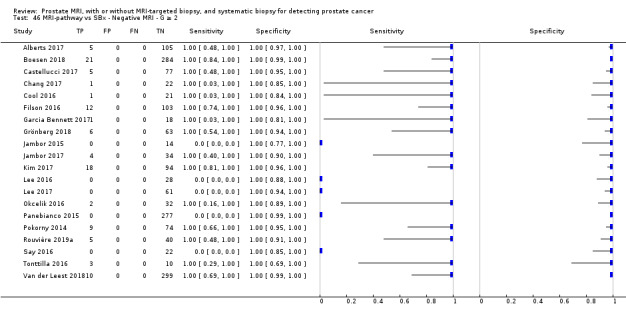
MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 2.
47. Test.
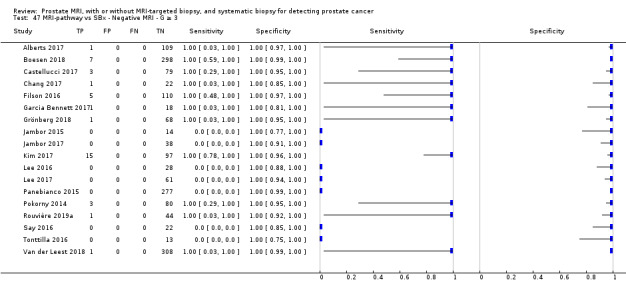
MRI‐pathway vs SBx ‐ Negative MRI ‐ G ≥ 3.
48. Test.
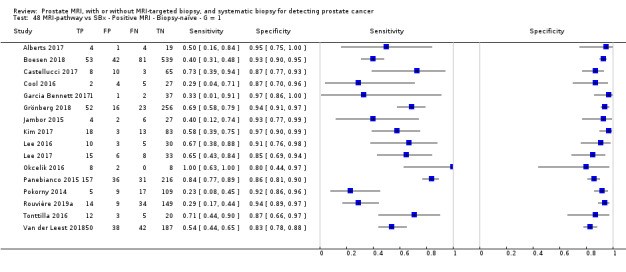
MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G = 1.
49. Test.
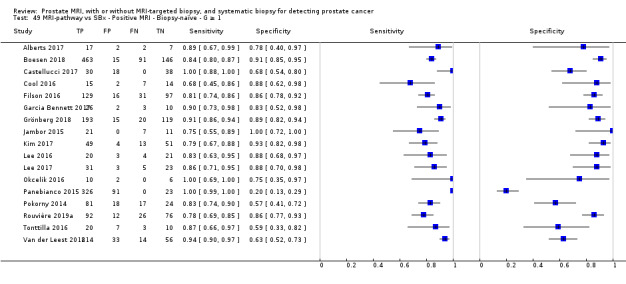
MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 1.
50. Test.
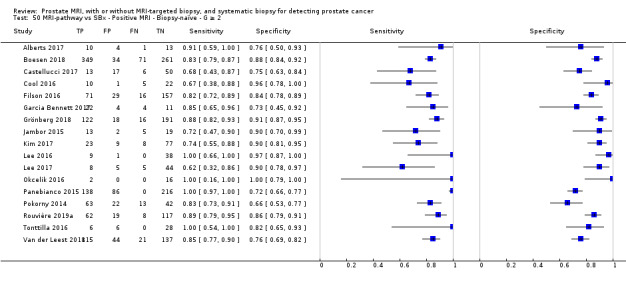
MRI‐pathway vs SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 2.
51. Test.
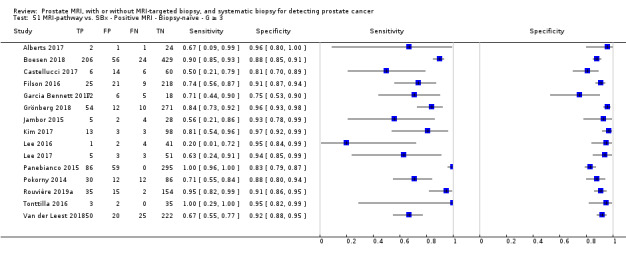
MRI‐pathway vs. SBx ‐ Positive MRI ‐ Biopsy‐naïve ‐ G ≥ 3.
52. Test.
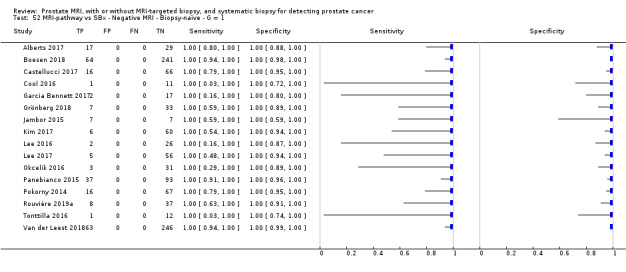
MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G = 1.
53. Test.
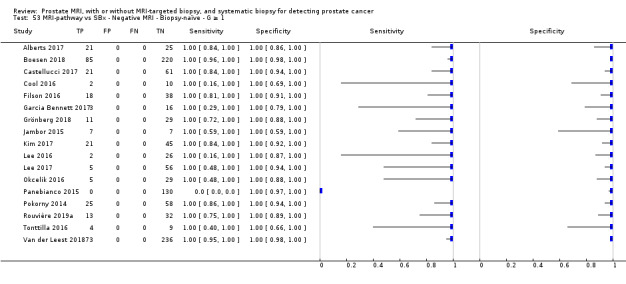
MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 1.
54. Test.
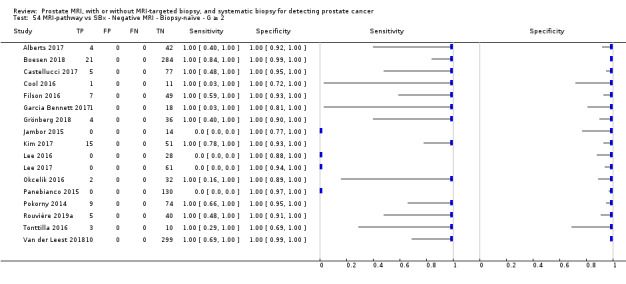
MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 2.
55. Test.
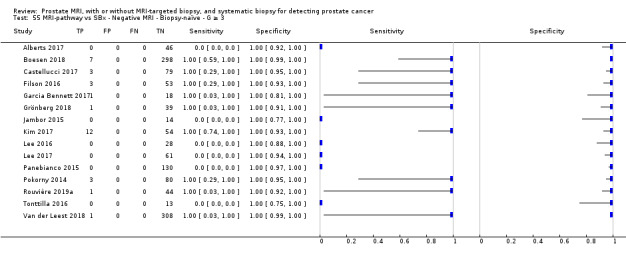
MRI‐pathway vs SBx ‐ Negative MRI ‐ Biopsy‐naïve ‐ G ≥ 3.
56. Test.
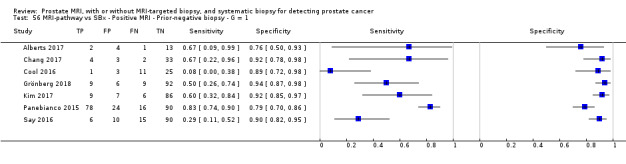
MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G = 1.
57. Test.
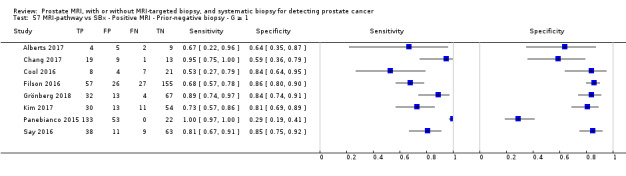
MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 1.
58. Test.
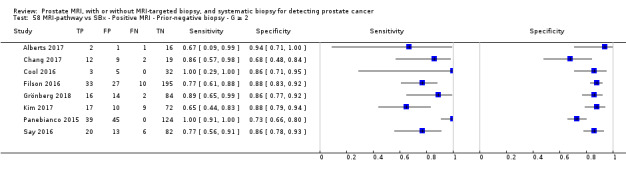
MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 2.
59. Test.
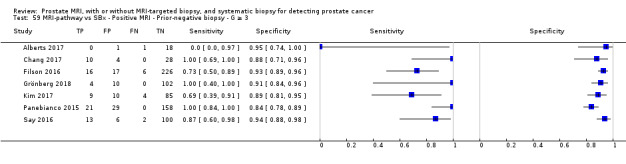
MRI‐pathway vs SBx ‐ Positive MRI ‐ Prior‐negative biopsy ‐ G ≥ 3.
60. Test.
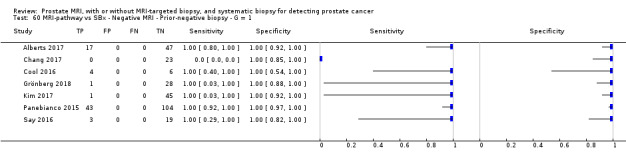
MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G = 1.
61. Test.
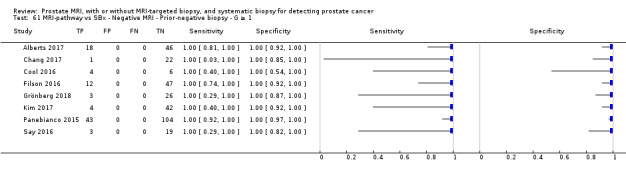
MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 1.
62. Test.
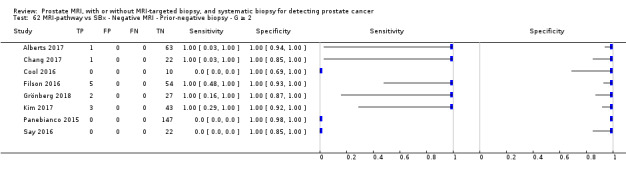
MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 2.
63. Test.
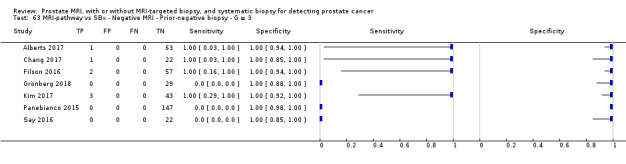
MRI‐pathway vs SBx ‐ Negative MRI ‐ Prior‐negative biopsy ‐ G ≥ 3.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abd‐Alazeez 2014.
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the performance of mpMRI in men with prior‐negative SBx Type of study: retrospective cohort Selection: unclearly reported Enrolled/eligible: 54/58 Inclusion period: not reported, but before April 2013 |
||
| Patient characteristics and setting | Inclusion criteria: men who had ≥ 1 negative SBx and underwent mpMRI (index test) followed by TTMB (reference standard). All men included in the study had either increasing or persistently high PSA level Exclusion criteria: 4 men were excluded from the study as they received limited TTMB (< 20 cores were taken) Setting: London, UK. University hospital Age: median 64 years (range 39‐75) PSA: 10 ng/mL (range 2‐23) Prostate volume: 53 mL (range 19‐136) Previous number of negative Bx: 33 men had 1, 16 had 2, 5 had 3 |
||
| Index tests | Index tests: MRI only, with an MRI‐score 1‐5 with threshold ≥ 3 for positivity. A 1.5 Tesla (Philips Achiva) and 3.0 Tesla (Siemens Avanto) MRI machine, with T2, DWI and DCE sequences were used. Index test performed first, then the reference test. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4, GS ≥ 4+3 and others. Pathology grading before ISUP 2005: GS was based upon most frequent pattern instead of highest grade detected. Reference standard: systematic TTMB with the use of a brachytherapy grid under general anaesthesia, as described by Barzell. Basal and apical cores were obtained routinely, and the minimum number of samples was 20. MRI results were available during TTMB. |
||
| Flow and timing | All men underwent the same reference test. No men were excluded from analysis | ||
| Comparative | |||
| Notes | Study authors provided additional data Although MRI‐TBx were taken in a subset of 15 men, their results are not reported nor are they taken into account in our analysis. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Ahmed 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to test diagnostic accuracy of mpMRI and SBx against a reference test, TTMB Type of study: multicentre, paired‐cohort, prospective study Selection: consecutive Enrolled/eligible: 576/740 Inclusion period: May 2012‐November 2015 |
||
| Patient characteristics and setting | Inclusion criteria: PSA ≤ 15 ng/mL within previous 3 months, organ confined disease on DRE Exclusion criteria: previous history of PBx, prostate surgery or treatment for PCa (interventions for benign prostatic hyperplasia/bladder outflow obstruction were accepted. Evidence of a urinary tract infection or history of acute prostatitis within the last 3 months. Contraindication to MRI (e.g. claustrophobia, pacemaker, estimated GFR = 50). Previous history of hip replacement surgery, metallic hip replacement or extensive pelvic orthopedic metal work. Treated using 5‐alpha‐reductase inhibitors at time of registration or during the prior 6 months. Setting: London, UK. University and peripheral hospitals Age: mean 63.4 years (SD 7.6) PSA: mean 7.1 ng/mL (SD 2.9) Prostate volume: not reported |
||
| Index tests | Index test 1: MRI only: at multiple sites, 1.5 Tesla MRI scanners (T1, T2, DWI and DCE sequences) were used. Radiologists were provided with clinical details. A 5‐point Likert radiology reporting scale was used, with score of ≥ 3 designated a suspicious scan. Radiologist had undergone additional centralised training. Index test 2: 10–12 core transrectal SBx. Participants and physicians remained blinded to the mpMRI images and report. Participants first underwent the TTMB, followed by the SBx |
||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4, GS ≥ 4+3 and others Reference standard: TTMB, blinded for MRI report, with cores taken from every hole in the 5‐mm sampling frame. |
||
| Flow and timing | All men underwent the same reference test. Participants left the study for various reasons: 4 were ineligible, 2 were unblinded, 69 had large prostates (> 100 mL), 5 had T4 or nodal disease, 21 had clinical reasons, 52 did not want to proceed, 11 had other reasons |
||
| Comparative | |||
| Notes | Study authors provided additional data. Number of TTMB cores not reported but estimated | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
Alberts 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the potential of a risk‐based strategy including MRI to selectively identify men aged ≥ 70 years with high‐grade PCa Type of study: prospective, 2‐arm, PSA‐screening study: 179 men received 6 core SBx only; 158 received MRI+/‐MRI‐TBx and SBx Selection: consecutive selection based on invitation to participate in a population‐based PSA screening trial Enrolled/eligible: 337/406 (69 participants refused Bx) In the current analysis, only the 158 men in the group receiving MRI and MRI‐TBx are included, of which 85 had a prior‐negative Bx and 74 were Bx‐naïve Inclusion period: Octobr 2013‐April 2016 |
||
| Patient characteristics and setting | Inclusion criteria: PSA ≥ 3.0 ng/mL Exclusion criteria: none Setting: PSA‐screening study. Rotterdam, the Netherlands. University hospital Age: median 73.1 years (IQR 72.4‐73.8)* PSA: median 4.2 ng/mL (IQR 3.4–5.8)* Prostate volume: median 52.9 (IQR 36.8‐70.9)* DRE positive: 14 participants* *of the 158 prior‐negative‐ and Bx‐naïve participants taken together |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (Discovery MR750, General Electric Healthcare) was used, with T2, DWI, and DCE sequences. PI‐RADS version 2 was used, with score 1‐5 and score ≥ 3 for positivity. The Koelis Urostation was used for software fused transrectal MRI‐TBx from all MRI‐positive lesions Index test 2: transrectal extended sextant SBx were taken, blinded for MRI results, before taking the MRI‐TBx |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses (MRI‐pathway vs SBx) study, therefore the reference standard domain is not applicable and disregarded | ||
| Flow and timing | All participants underwent the same reference test. During the study, 69 participants refused Bx. |
||
| Comparative | |||
| Notes | Only the 158 participants in the group receiving MRI and MRI‐TBx are included in the current analysis; the 179 participants with sextant Bx only are excluded from our analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
Boesen 2017a.
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare the PCa detection rate of SBx and mpMRI‐TBx Type of study: prospective cohort Selection: unclear Enrolled/eligible: 206/213 Inclusion period: September 2012‐September 2013 |
||
| Patient characteristics and setting | Inclusion criteria: ≥ 1 prior‐negative SBx session (10–12 cores) and a persistent clinical suspicion of PCa (elevated PSA, an abnormal DRE, or a previous abnormal TRUS image) that warranted a repeat SBx Exclusion criteria: a prior PCa diagnosis, prior prostate mpMRI, or presence of general contraindications for MRI Setting: Herlev, Denmark. University hospital Age: median 65 years (IQR 58‐68) PSA: median 12.8 ng/mL (IQR 8.9‐19.6) Prostate volume: not reported. Instead, PSA‐density: median 0.20 (IQR 0.13‐0.29) DRE positive: 18 men |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Ingenia, Philips) was used, with T2, DWI and DCE sequencing. PI‐RADS version 1 with a Likert 1‐5 score and threshold for positivity of ≥ 2 was used*. Software fusion (Hitachi Ltd, HI‐RVS‐system) MRI‐TBx were taken of all MRI‐positive lesions Index test 2: a 10‐core transrectal SBx. Abnormalities on TRUS were sampled using the standard core for the relevant segment. This was performed first and blinded for MRI results, afterwards the MRI‐TBx were taken |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded | ||
| Flow and timing | All participants underwent the same reference test. During the study, 7 participants were excluded because of technical problems or claustrophobia |
||
| Comparative | |||
| Notes | *Although MRI‐TBx scores 2‐5 were taken, the results for a threshold of ≥ 3 can be distinguished. In our analysis, therefore, we used the threshold of ≥ 3 for a positive MRI and MRI‐TBx. We contacted study authors but they could not provide additional data. Other comparisons were not possible to the lack of detailed data. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Boesen 2018.
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the diagnostic accuracy and negative predictive value of a novel bpMRI method in Bx‐naïve men in detecting and ruling out significant PCa. Type of study: prospective, single‐institutional, paired diagnostic study Selection: consecutive selection Enrolled/eligible: 1020/1063 (43 participants were excluded for various reasons) Inclusion period: November 2015–June 2017 |
||
| Patient characteristics and setting | Inclusion criteria: Bx‐naïve men with a clinical suspicion of PCa (PSA ≥ 4 ng/mL and/or abnormal DRE results) Exclusion criteria: prior PBx, evidence of acute urinary tract infections, acute prostatitis, general contraindications for MRI, and prior hip replacement surgery or other metallic implants in the pelvic area Setting: Herlev, Denmark, University Hospital Age: median 67 years (IQR 61‐71) PSA: median 8 ng/mL (IQR 5.7‐13) Prostate volume: median 53 mL (IQR 40‐72) DRE positive: 377/1020 (37%) participants |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (Philips) with a pelvic‐phased‐array coil was used with T2, DWI sequences, without DCE (bpMRI). An in‐house modified PI‐RADS version 2 was used with score 1‐5 and score ≥ 3 for positivity. Transrectal software fused MRI‐TBx were performed from all MRI‐positive lesions, using Hitachi (n = 877) and Invivo (n = 143) systems. Index test 2: transrectal 10‐core SBx were taken before the MRI‐TBx, the performers were blinded for the MRI results |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same type of tests. The minimal exclusions were sufficiently explained not leading to relevant bias. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Castellucci 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the diagnostic efficacy of cognitive mpMRI‐TBx compared to SBx in Bx‐naïve men Type of study: prospective single‐centre cohort study Selection: consecutive Enrolled/eligible: 168/168 Inclusion period: July 2011‐July 2014 |
||
| Patient characteristics and setting | Inclusion criteria: Bx‐naïve men, with a clinical suspicion of PCa because of elevated PSA levels and/or an abnormal DRE Setting: Madrid, Spain. University Hospital Age: mean 61.4 years (± 7.6) PSA: mean 8.3 ng/mL (± 6.1) Prostate volume: mean 48.9 mL (± 6.7) |
||
| Index tests | Index test 1: MRI‐pathway: mpMRI was performed with a 1.5 Tesla machine (Achieva, Philips Healtcare, Best, the Netherlands) with surface coil, using T1, T2 and DWI. PI‐RADS version 1 was used to assess the MRI by 2 readers independently, with a 1‐5 score and score ≥ 3 for positivity. All PI‐RADS ≥ 3 lesions were targeted cognitively with 2 MRI‐TBx cores. Index test 2: all men underwent transrectal SBx based on the Vienna nomogram (8‐19 biopsy cores depended on age and prostate volume), before MRI‐TBx were taken, by the same urologist. Blinding of MRI results during SBx was not reported |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded | ||
| Flow and timing | All participants underwent the same type of tests No participants were excluded |
||
| Comparative | |||
| Notes | Study authors provided additional data | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Unclear | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Chang 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to investigate the overall and clinically significant PCa detection rates of MRI‐TBx and SBx in prior‐negative Bx men Type of study: retrospective study Selection: consecutive selection, but performance of MRI according to the physicians’ clinical considerations Enrolled/eligible: 185/185 (65 men underwent MRI and Bx, 120 men underwent only Bx without prior MRI) Inclusion period: March 2012–December 2014 |
||
| Patient characteristics and setting | Inclusion criteria: men with prior‐negative Bx, persistently elevated serum PSA level and normal DRE Exclusion criteria: positive DRE Setting: Taichung, Taiwan. University hospital Age: median 64 years (IQR 60.3‐67.8) PSA: median 10.9 ng/mL (IQR 7.2‐14.7) Prostate volume: median 48 mL (IQR 33.5‐62.5) DRE positive: none |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (Signa HDx, General Electric Healthcare) was used with T2, DWI, and DCE sequences. PI‐RADS version 1 was converted to PI‐RADS version 2, with score 1‐5 and score ≥ 3 for positivity. Transrectal cognitive MRI‐TBx were performed from all MRI‐positive lesions with ≥ 2 cores Index test 2: transrectal SBx were taken with ≥ 16 cores from the peripheral zone and transitional zone, after the MRI‐TBx were taken by the same operator. |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same reference test. No participants were excluded. | ||
| Comparative | |||
| Notes | The 120 participants in the control group who underwent only SBx without prior MRI were excluded from our analysis. Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | No | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Chen 2015.
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine the detection rate of 3‐Tesla MRI and MRI‐TBx compared to SBx Type of study: prospective cohort Selection: consecutive selection of participants who presented with a suspicion of PCa Enrolled/eligible: 420/429 Inclusion period: June 2008‐December 2013 |
||
| Patient characteristics and setting | Inclusion criteria: abnormal DRE findings and/or persistently elevated PSA levels Exclusion criteria: not reported Setting: Shanghai, China. University hospital Age: median 67 years (range 45‐91) PSA: median 9.7 ng/mL (range 2.4‐35.7) Prostate volume: median 44.8 mL (range 21.2‐83.2) DRE positive: 52 participants |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (Philips Achieva) was used, with T1, T2, T2 spectral presaturation attenuated inversion recovery (SPAIR) and DWI sequences. An in‐house MRI score 1‐5 with threshold ≥ 3 for positivity were used. Cognitive transperineal MRI‐TBx were performed from all MRI‐positive lesions. Index test 2: a 10‐core fan‐shaped transperineal SBx from the peripheral zone with 2‐cores from transition zone was performed, blinded for MRI results, before taking the MRI‐TBx |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same reference test Except for the 9 excluded participants (DWI artifacts due to movement of the participant) all participants were included in the analysis. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Cool 2016.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the clinical benefit of MRI‐TBx over SBx between first‐time and repeat SBx patients with prior (ASAP) Type of study: prospective cohort Selection: unclear Enrolled/eligible: 100/unclear (50 participants with prior‐negative Bx, 50 Bx‐naïve men) Inclusion period: September 2011‐March 2014 |
||
| Patient characteristics and setting | Inclusion criteria: PSA 2‒20 ng/L or DRE abnormalities. Bx‐naïve or ≥ 1 prior Bx with ASAP and ongoing clinical concern for malignancy Exclusion criteria: known PCa diagnosis, previous prostate MRI or contraindication to MRI or SBx Setting: Ontario, Canada. University hospital Age*: mean (SD) 59.4 (7.7); 61.9 (6.5) PSA*: mean (SD) 6.0 ng/mL (3.5); 7.9 (3.9) Prostate volume*: mean (SD) 38 g (18); 56 (27) *for Bx‐naïve men; and previous ASAP men, respectively |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (GE Healthcare) was used, with T2, DWI and DCE sequences. A binary MRI suspicion score was used with a low threshold set to initiate software fusion (Artemis system) transrectal MRI‐TBx from all MRI‐positive lesions. Index test 2: a standard 12‐core transrectal SBx was performed in Bx‐naïve men, 2 additional cores were taken from the transition zone in previous ASAP men. No blinding of MRI results is reported. MRI‐TBx were taken prior to SBx |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants (with the same indication) underwent the same type of tests All participants were included in the analysis |
||
| Comparative | |||
| Notes | Retrospectively, MRI was reassessed using PI‐RADS version 2. The presented Bx data, however, are based on the prospective binary MRI‐score | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Unclear | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Costa 2013.
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the value of MRI and MRI‐TBx added to standard SBx for detecting clinical relevant PCa Type of study: retrospective analysis Selection: retrospective selection of participants meeting inclusion criteria Enrolled/eligible: 38/1053 (of the 1053 participants who had had an MRI, 38 participants met the inclusion criteria) Inclusion period: August 2003‐August 2008 |
||
| Patient characteristics and setting | Inclusion criteria: men with ≥ 2 prior‐negative biopsies who underwent MRI and subsequent SBx complemented with MRI‐TBx of MRI‐suspicious lesions. All men were referred for MRI because of PSA > 4 ng/mL, PSA velocity > 0.75 ng/mL/year or equivocal histopathology from previous Bx Setting: Boston, USA. University hospital Age: mean 64 (range 48‐77) PSA: mean 14.4 (range 1.8‐33.1) Prostate volume: not reported |
||
| Index tests | Index test 1 : MRI‐pathway: a 3 Tesla MRI machine (Genesis Signa LX Excite, GE Healthcare) was used, with T1, T2 and DCE sequences. An in‐house MRI Likert 1‐5 scale was used, grouping score 1‐3 negative and 4‐5 positive. Cognitive MRI‐TBx from MRI‐positive lesions were taken, depending on judgement of urologist Index test 2: transrectal SBx was performed. Sequence of tests and number of cores were dependent on judgement of urologist. A total median of 19 (range 8‐28) cores (MRI‐TBx + SBx) were taken. MRI results were known at time of SBx performance |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same type of tests. All participants were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | No | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| High | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Unclear | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Dal Moro 2019.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate whether adding 1.5 T magnetic field mpMRI‐TBx improves PCa detection in men undergoing blind 24‐core saturation PBx Type of study: prospective collected data Selection: consecutive selection Enrolled/eligible: 123/123 Inclusion period: January 2013–December 2016 |
||
| Patient characteristics and setting | Inclusion criteria: men who had already undergone a first 10/12‐core PBx with suspected PCa due to an increased PSA level and/or positive DRE Exclusion criteria: > 1 set of 10/12‐core Bx, TURP or other lower urinary tract endoscopic procedures Setting: Padua, Italy. University hospital Age: median 62 years (IQR 57‐68) PSA: median 6.27 ng/mL (IQR 4.75‐8.9) Prostate volume: mean 54.59 mL (range 20‐149) DRE positive: 8.9% (11/123) of the participants |
||
| Index tests | Index tests: MRI only + MRI‐TBx + MRI‐pathway: a 1.5 Tesla MRI machine was used with T2 and DWI sequences. PI‐RADS version 1 was used with score 1‐5 and score ≥ 3 for positivity. Transrectal cognitive MRI‐TBx were performed from all MRI‐positive lesions | ||
| Target condition and reference standard(s) | Target conditions: GS 3+3 = 6, GS ≥ 3+3, GS ≥ 3+4 Reference standard: transrectal 24‐core saturation Bx including 8 anterior biopsies, blinded for MRI results. When a suspicious lesion was present, the operator performed first the MRI‐TBx and then the saturation biopsies (unblinded) |
||
| Flow and timing | All participants underwent the same reference test. No participants were excluded | ||
| Comparative | |||
| Notes | Study authors provided additional data | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Delongchamps 2013.
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare the accuracy of visual MRI‐TBx versus software MRI‐TBx using a rigid or elastic approach Type of study: prospective cohort Selection: consecutive selection, divided into 3 groups: the first 127 participants received visual MRI‐TBx, the next 131 participants had the rigid fusion MRI‐TBx and the last 133 participants had the elastic fusion MRI‐TBx Enrolled/eligible: 391/391 Inclusion period: January 2011‐March 2012 |
||
| Patient characteristics and setting | Inclusion criteria: PSA > 4 ng/mL, and/or suspicious DRE and no previous PBx Exclusion criteria: none Setting: Paris, France. University hospital Age*: mean 62.7 years (SD 7.4); 64.5 (7.9); 64.6 (6.7) PSA*: mean (SD) 8.1 ng/mL (3.7); 9.0 (3.9); 8.3 (4.1) Prostate volume* (SD): 53 mL (25); 58.3 (28.6); 55.7 (35.1) DRE positive*: 20; 16; 16 *For the 3 groups, respectively: visual‐; elastic‐; rigid fusion |
||
| Index tests | Index test 1: MRI‐pathway: a 1.5 Tesla MRI machine was used, with T2, DWI and DCE sequences. An in‐house MRI‐score: 0‐4 score in transitional zone and 0‐10 in peripheral zone were used, with threshold ≥ 2 and ≥ 6 for positivity, respectively. Either cognitive MRI‐TBx or software fusion MRI‐TBx (Koelis, elastic MRI‐TRUS image registration System; Esaote, rigid navigation system) were taken from all positive lesions. Index test 2: 10‐12 core transrectal SBx was performed first. Blinding for MRI results was not reported. Subsequently, MRI‐TBx were taken of suspicious lesions |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same type of tests. All participants were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Distler 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to analyse the negative predictive value of MRI and PSA density to rule out significant PCa Type of study: prospective cohort Selection: consecutive selection of men with a suspicion of PCa (PSA >4.0 ng/ml and/or suspicious digital rectal examination (DRE)) who were either biopsy‐naïve or after previous negative biopsy. Enrolled/eligible: 1040/1040 (597 Bx‐naïve + 443 prior‐negative Bx men) Inclusion period: October 2012‐December 2015 |
||
| Patient characteristics and setting | Inclusion criteria: suspicion of PCa: PSA > 4.0 ng/mL and/or suspicious DRE, and who were Bx‐naïve or had undergone a prior‐negative Bx Exclusion criteria: none Setting: Heidelberg, Germany. University hospital Age: median 65 years (IQR 60‐71) PSA: median 7.2 ng/mL (IQR 5.3‐10.4) Prostate volume: median 45 mL (IQR 34‐64) DRE positive: 291 |
||
| Index tests | Index tests: MRI only + MRI‐TBx + MRI‐pathway: a 3 Tesla MRI machine (Magnetrom Prisma or Biograph mMR (Siemens Healthcare) was used, with T2, DWI and DCE sequences. The PI‐RADS version 1 Likert 1‐5 score was used, with threshold ≥ 3 for positivity. Transperineal MRI‐TBx were taken from all positive lesions with the Biopsee system (rigid software registration). First MRI‐TBx were taken, subsequently the reference biopsies | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+4 Reference standard: volume‐based systematic transperineal grid‐directed Bx with a median of 24 cores according to the Ginsburg protocol. Bx operators first performed the MRI‐TBx and had access to MRI data during whole procedure. |
||
| Flow and timing | All participants underwent the same reference test. All participants were included in the analysis | ||
| Comparative | |||
| Notes | Results not reported separately for the two participant groups | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Filson 2016.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the performance of MRI‐TBx in diagnosing clinically significant PCa Type of study: prospective cohort Selection: consecutive selection Enrolled/eligible: 1042/1042 (328 Bx‐naïve‐, 324 prior‐negative Bx‐ and 390 active surveillance men) Inclusion period: September 2009‐February 2015 |
||
| Patient characteristics and setting | Inclusion criteria: elevated PSA level or abnormal DRE or 2) confirmation of low‐risk PCa for men considering active surveillance Exclusion criteria: none reported Setting: Los Angeles, USA. University hospital Age*: median (IQR) 64.4 years (58.5‐69.4); 65.7 (59.3‐70.2) PSA*: median (IQR) 5.8 ng/mL (4.4‐8.1); 7,6 (5‐11.5) Prostate volume*: median (IQR) 45 mL (33‐61.5); 57.7 (39.8‐83.5) *respectively, for the Bx‐naïve‐ and prior‐negative Bx participant groups |
||
| Index tests | Index tests 1: MRI‐pathway: a 3 Tesla MRI machine (Trio Trim/Somatom, Philips) was used, with T2, DWI and DCE sequences. An in‐house Likert 1‐5 score was used, with threshold ≥ 3 for positivity. MRI‐TBx (Artemis fusion device (Eigen, Grass Valley, Calif) were taken first in case of a suspicious lesion, then SBx were taken Index test 2: transrectal 12‐core SBx were taken in all participants, after MRI‐TBx. No blinding for MRI is reported |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same reference test. All participants were included in the analysis. | ||
| Comparative | |||
| Notes | Participants on active surveillance (n = 390) were excluded from our analysis. Although in text 328 participants are reported in the biopsy‐naïve group, in the data tables 329 participants are reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Garcia Bennett 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the differences in PCa detection rate and Bx effectiveness between MRI‐TBx and transperineal standard SBx in Bx‐naïve men Type of study: prospective cohort Selection: not explicitly reported Enrolled/eligible: 60/unclear Inclusion period: October 2014‐April 2016 |
||
| Patient characteristics and setting | Inclusion criteria: PSA > 4 ng/mL, a PSA density > 0.18 ng/mL/mL, a PSA velocity > 0.75 ng/mL/year or a pathological DRE Exclusion criteria: previous history of prostate biopsies, prostate surgery or radiotherapy or medical treatment for benign prostate hyperplasia Setting: Reus, Spain. University hospital Age: mean 64.1 years (SD 6.7). PSA: median 7.2 ng/mL (IQR 6‐9.4) Prostate volume: median 47.8 mL (IQR 34.6‐63.2) |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI machine (Signa, GE) was used, with T1, T2 and DWI sequences. The PI‐RADS version 1 Likert 1‐5 score was used, with threshold ≥ 4 for positivity (if no PI‐RADS ≥ 4 lesions were present, also PIRADS 2 and 3 were targeted). (Because study authors provided additional data we were able to use the results for a MRI‐threshold of ≥ 3.) The MRI targets were discussed with radiologist and cognitive fusion transperineal MRI‐TBx on target lesions was performed. Index test 2: 12‐core transperineal SBx in all men: two cores were directed towards the medial segments of the peripheral zone, two towards the lateral segments of the peripheral and two towards the transition zone for each lobe, with blinding for MRI results. Subsequently, MRI‐TBx were taken. |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same reference test. All participants were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. In our analysis we were therefore able to use the results for MRI‐threshold ≥ 3 for positivity. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Grey 2015.
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine the sensitivity and specificity of mpMRI for significant PCa with transperineal sector Bx as the reference standard Type of study: prospective cohort Selection: consecutive patients Enrolled/eligible: 201/205 (83 Bx‐naïve‐, 103 prior‐negative Bx‐, 15 active surveillance participants; 4 participants were excluded due to contraindications to MRI) Inclusion period: July 2012‐November 2013 |
||
| Patient characteristics and setting | Inclusion criteria: a prior‐negative PBx with ongoing suspicion of PCa because of rising PSA levels (n = 103); those undergoing a primary PBx because of raised PSA level or abnormal DRE (n = 83) Exclusion criteria: previous history of PBx, prostate surgery or radiotherapy or medical treatment for benign prostate hyperplasia Setting: London, UK. University hospital Age*: mean (SD) 65 years (7.6); 64.1 (6.8). PSA*: mean (SD) 12.6 ng/mL (13.7); 13.3 (12.1) Prostate volume*: mean (SD) 54 mL (31); 68 (35) *Although test results are reported only for the mix of the 2 participant groups, these basic characteristics are reported for the 2 groups separately (103 prior‐negative Bx‐; Bx‐naïve patients, respectively) |
||
| Index tests | Index test: MRI only. A 1.5 Tesla MRI machine (Signa Excite, GE Healthcare) with T2 and DWI sequences was used. The PI‐RADS version 1 Likert 1‐5 score was used, with threshold ≥ 3 for positivity | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: transperineal sector Bx, with 24‐40 cores (depending on prostate size) with a brachytherapy grid. MRI‐positive lesions were targeted by cognitive registrated MRI‐TBx, but results were not reported separately |
||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | All active surveillance participants (n = 15) were excluded from our analysis after additional information was received from study authors | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Grönberg 2018.
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the performance of combining a blood‐based biomarker panel and MRI‐TBx for PCa detection Type of study: prospective, multicentre, paired diagnostic study Selection: consecutive selection Enrolled/eligible: 532/727 (195 participants were excluded due to incomplete data) Inclusion period: May 2016–May 2017 |
||
| Patient characteristics and setting | Inclusion criteria: men aged 45‐75 years, no previous PCa, referral for PCa work‐up Exclusion criteria: previous diagnosis of PCa Setting: Stockholm, Sweden; Oslo, Norway; and Tonsberg, Norway. University and peripheral hospitals (cancer centre) Age: Stockholm (n = 160): mean 63 years (6.2); Oslo (n = 236): mean 65 years (7.8); Tonsberg (n = 136): mean 64 years (6.8) PSA: Stockholm (n = 160): median 6.2 ng/mL (IQR 4.8‐8,2) Oslo (n = 236): median 6 ng/mL (IQR 4‐9) Tonsberg (n = 136): median 7.1 ng/mL (IQR 4.7‐11) Prostate volume: Stockholm (n = 160): median 51 mL (IQR 38‐70) Oslo (n = 236): median 42 mL (IQR 32‐54) Tonsberg (n = 136): median 44 mL (IQR 33‐55) DRE positive: not reported |
||
| Index tests | Index test 1: MRI‐pathway, a 1.5 Tesla MRI machine (Avanto and Aera, Siemens) was used with T2, DWI sequences, without DCE. PI‐RADS version 2 was used with score 1‐5 and score ≥ 3 for positivity. Transrectal software fused MRI‐TBx were performed from all MRI‐positive lesions, using several machines. Index test 2: transrectal extended sextant SBx were taken after the MRI‐TBx and therefore not blinded for MRI results. |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. Men with incomplete data were explained and excluded from the analysis, not leading to relevant bias. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Hansen 2016a.
| Study characteristics | |||
| Patient sampling | Aim of the study: to describe the Ginsburg protocol for transperineal MRI‐TBx supported by mpMRI and TRUS image fusion, and report biopsy results Type of study: prospective cohort study Selection: consecutive patients Enrolled/eligible: 571/571 (107 Bx‐naïve‐, 295 prior‐negative Bx‐ and 169 active surveillance men) Inclusion period: March 2013‐October 2015 |
||
| Patient characteristics and setting | Inclusion criteria: indication for repeat Bx: either rising PSA or ASAP or multifocal high‐grade prostatic intraepithelial neoplasia on a previous Bx Exclusion criteria: previous prostate MRI or a transperineal Bx Setting: Cambridge, UK. University hospital Age: median 65 years (IQR 59‐69) PSA: median 7.8 ng/mL (IQR 60‐12) Prostate volume: median 65 mL (IQR 44‐83) |
||
| Index tests | Index test: MRI only, MRI‐TBx and MRI‐pathway. A 1.5 Tesla (MR450) or a 3 Tesla (Discovery MR750 HDx) machine of GE Healthcare was used with T2, DWI and DCE sequences. The PI‐RADS version 1, Likert 1‐5 score was used, with threshold ≥ 3 for positivity. First transperineal software fusion MRI‐TBx cores were taken (BiopSee platform, Medcom) of every suspicious lesion. Then the reference standard was performed. |
||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: an 18‐24 core systematic transperineal Bx according to the Ginsburg protocol, with 1‐2 cores from each of the 12 sectors, using the BiopSee MRI‐TRUS fusion platform with a brachytherapy grid for guidance. Blinding of MRI results not reported |
||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. In our analysis we excluded the 169 active surveillance participants. Furthermore, we excluded the 106 Bx‐naïve participants because of overlapping data with Hansen 2018. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Hansen 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the detection rates of transperineal MRI‐TBx and SBx for men with previous benign transrectal SBx in 2 high‐volume centres Type of study: prospective cohort study Selection: unclear Enrolled/eligible: 487/487 (200 from centre 1, 287 from centre 2) Inclusion period: October 2013‐November 2015 |
||
| Patient characteristics and setting | Inclusion criteria: indication for repeat Bx: rising PSA or a previous SBx specimen showing suspicion of cancer (ASAP) or multifocal high‐grade prostatic intraepithelial neoplasia Exclusion criteria: none reported Setting: Heidelberg, Germany. University hospital Age: median 66 years (IQR 61‐72) PSA: median 9.7 ng/mL (IQR 7.1‐13.9) Prostate volume: median 52 mL (IQR 36‐75) |
||
| Index tests | Index tests: MRI only,MRI‐TBx and MRI‐pathway. A 3 Tesla MRI machine (Magnetron, Siemens) with T2, DWI and DCE sequences was used. The PI‐RADS version 2, Likert 1‐5 score was used, with threshold ≥ 3 for positivity. First transperineal software fusion MRI‐TBx cores were taken (BiopSee platform, Medcom) of every suspicious lesion. Then template Ginsburg Bx was performed. |
||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: a volume‐based, transperineal template Bx scheme, with a median of 24 cores, according to Ginsburg protocol was performed, using the BiopSee MRI‐TRUS fusion platform with brachytherapy grid for guidance. Blinding of MRI results not reported |
||
| Flow and timing | All participants underwent the same reference test. All participants were included in the analysis. |
||
| Comparative | |||
| Notes | Only participants from centre 1 (Heidelberg, Germany) were included in our analysis, due to overlap with patients of centre 2 (Cambridge, UK) in Hansen 2016b. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Hansen 2018.
| Study characteristics | |||
| Patient sampling | Aim of the study: to analyse the detection rates of primary MRI‐fusion transperineal PBx using combined targeted and systematic core distribution in 3 tertiary referral centres Type of study: prospective cohort Selection: consecutive patients Enrolled/eligible: 856/807 (163 participants from centre 1, 402 from centre 2* and 242 from centre 3; 49 participants did not comply with the inclusion criteria) Inclusion period: October 2012‐May 2016 |
||
| Patient characteristics and setting | Inclusion criteria: first suspicion of PCa, based on raised PSA levels above age‐related normal range, a suspicious DRE, or other including family history Exclusion criteria: age > 79 years, PSA level > 30 ng/mL, prior‐negative Bx or previous diagnosis or treatment of PCa Setting Centre 1: Cambridge UK, tertiary care hospital Age: median 64 years (IQR 57‐69) PSA: 6.6 ng/mL (IQR 4.6‐9.0) Prostate volume: 44 mL (IQR 33‐55) Positive DRE: 39 participants Setting Centre 2: Heidelberg, Germany, University Hospital (participants from centre 2 in this study were excluded from analyses in this review to prevent overlapping data with the included study Distler 2017*) Age: median 65 years (IQR 60‐70) PSA: 6.9 ng/mL (IQR 5.2‐9.1) Prosate volume: 47 mL (IQR 32‐62) Postive DRE: 94 participants Setting Centre 3: Melbourne, Australia, tertiary care hospital Age: median 65 years (IQR 60‐70) PSA: 5.9 ng/mL (IQR 4.6‐8,0) Prostate volume: 25 mL (IQR 24‐47) Positive DRE: 54 participants |
||
| Index tests | Centre 1: index test: MRI only, a 1.5 Tesla (MR450) or a 3 Tesla (Discovery MR750 HDx) machine of GE Healthcare was used with T2, DWI and DCE sequences. The PI‐RADS version 1 (until 2015) and version 2 (onwards) with a Likert 1‐5 score were used, with threshold ≥ 3 for positivity. Transperineal software fusion MRI‐TBx cores were taken (BiopSee system, Medcom) of every suspicious lesion, followed by template Bx. However, MRI‐TBx results were not reported separately. Centre 3: index test: MRI only, a 3 Tesla Magnetom (Siemens) was used with T2, DWI and DCE sequences. The PI‐RADS version 1 (until 2015) and version 2 (onwards) with a Likert 1‐5 score were used, with threshold ≥ 3 for positivity. Transperineal cognitive MRI‐TBx cores were taken of every suspicious lesion, followed by template Bx. However, MRI‐TBx results were not reported separately. |
||
| Target condition and reference standard(s) | Target condition in both centres: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard in both centres: volume‐based transperineal template Bx with a median of 24 cores according to the Ginsburg protocol. Bx operators had access to MRI data during whole procedure. MRI‐TBx were taken in addition to the template Ginsburg biopsies and included in the reference standard results. Centre 1 used the Biopsee system (Medcom) with a 5‐mm spacing brachytherapy grid Centre 3 used a 5‐mm spacing brachytherapy grid (BK Ultrasound) and a transrectal probe mounted on a stepper |
||
| Flow and timing | All participants underwent same reference standard. No participants were excluded for analysis. | ||
| Comparative | |||
| Notes | *Only the 163 participants from centre 1 and the 242 patients from centre 3 are included in our analysis; we excluded the 402 patients from centre 2 because they are also reported in Distler 2017 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Jambor 2015.
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the diagnostic accuracy of MRI and MRI‐TBx using visual registration Type of study: multicentre study, unclear design Selection: unclear Enrolled/eligible: 55/unclear Inclusion period: April 2011‐March 2013 |
||
| Patient characteristics and setting | Inclusion criteria: PSA > 4 ng/mL on 2 consecutive measurements in the last 6 months Exclusion criteria were:
Setting: Turku, Finland/Bratislava, Slovakia. University hospitals Age: median 66 years (range 47–76) PSA: median 7.4 ng/mL (range 4–14) Prostate volume: median 42 mL (range 17–107) |
||
| Index tests | Index test 1: MRI‐pathway, a 3 Tesla machine (Magnetom Verio 3T, Siemens) was used with T2, DWI, DCE and spectroscopy sequences. An in‐house MRI Likert 1‐5 scale was used, with threshold ≥ 4 for positivity and MRI‐TBx (but small discrete lesions (maximum diameter of 7–9 mm on mpMRI) were also targeted. Cognitive transrectal MRI‐TBx were taken of all suspicious lesions, after SBx Index test 2: transrectal extended sextant SBx were taken, blinded for MRI results |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. All participants were included in the analysis; except for 2 participants who did not receive MRI‐TBx due to technical problems, which in our current analysis had to be excluded. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Jambor 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the role of a MRI combined with MRI‐TBx for improving risk stratification of men with elevated PSA Type of study: prospective cohort Selection: unclear selection Enrolled/eligible: 161/175 (134 Bx‐naïve, 27 prior‐negative Bx participants and 14 exclusions) Inclusion period: March 2013‐February 2015 |
||
| Patient characteristics and setting | Inclusion criteria: 2 repeated measurements of PSA in the range 2.5–20.0 ng/mL and/or abnormal DRE Exclusion criteria: previous PCa diagnosis, previous Bx within 6 months, prostate surgery, clinical infection or MRI contraindication Setting: Turku, Finland. University hospital Age: mean 64.7 years (SD 6.4) PSA: median 7.5 (IQR 5.7‐9.6). Prostate volume: median 37 (IQR 27.5‐49) |
||
| Index tests | Index tests 1: MRI‐pathway: a 3 Tesla machine (Magnetom Verio 3T, Siemens) was used with T2 and DWI sequences. An in‐house MRI Likert 1‐5 scale was used, with threshold ≥ 3 for positivity and MRI‐TBx. Cognitive transrectal MRI‐TBx were taken of all index lesions, prior to SBx. Index test 2: 12‐core transrectal SBx, without blinding for MRI results (although strictly following the SBx scheme). |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. Not all participants were included in analysis. 4 withdrew consent before and 7 after MRI, 1 had a non‐diagnostic MRI, 2 had a PSA < 2.5 or > 20 | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
Kesch 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate a volume‐based, computer‐assisted method for TOP‐Bx Type of study: prospective cohort Selection: unclear selection Enrolled/eligible: 172/unclear (mix of 95 Bx‐naïve‐, 51 prior‐negative‐ and 26 active surveillance participants) Inclusion period: October 2013‐March 2014 |
||
| Patient characteristics and setting | Inclusion criteria: abnormal PSA or suspicious DRE, persistent suspicion of PCa after prior‐negative Bx Exclusion criteria: none reported Setting: Darmstadt, Germany. University hospital Age*: median 65 years (IQR 58‐71) PSA*: median 7.2 ng/mL (IQR 5.4‐10.2) Prostate volume*: median 46 mL (IQR 36‐60) Positive DRE*: 37 participants |
||
| Index tests | Index tests: MRI only + MRI‐TBx + MRI‐pathway. A 3 Tesla machine (Magnetom, Siemens) was used with T1, T2, DWI and DCE sequences. The PI‐RADS version 1, Likert 1‐5 scale was used, with threshold ≥ 3 for positivity and MRI‐TBx. Software fusion transperineal MRI‐TBx were taken of all index lesions independently of the TOP‐Bx, using the BiopSee MRI‐TRUS fusion platform (Medcom). | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: Novel, volume‐based, automated core‐placement method for TOP‐Bx placement was performed with a needle distribution sampling each conceivable tumour lesion ≥ 0.5 mL in the complete prostate (100%), with a median of 24 (IQR 23‐27) cores, independent of MRI results. |
||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. We excluded the 26 active surveillance participants from our analysis. *However, the basic characteristics are based on all participants (including the active surveillance participants). |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Kim 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine the added value of prostate MRI to the Prostate Cancer Prevention Trial risk calculator Type of study: retrospective study of prospective database Selection: consecutive patients who received prostate MRI prior to Bx Enrolled/eligible: 421/unclear (185 Bx‐naïve‐, 154 prior‐negative Bx and 82 active surveillance participants). Inclusion period: January 2012‐December 2015 |
||
| Patient characteristics and setting | Inclusion criteria: indication for MRI and Bx, no details reported Exclusion criteria not reported Setting: St. Louis, MO, USA. University hospital Age*: mean 63.9 years (SD 7.6) PSA*: mean 10.2 ng/mL (SD 15.1) Prostate volume: not reported Positive DRE*: 48 participants *only reported for the whole group (Bx‐naïve and prior‐negative Bx participants combined) |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla machine (Siemens) was used with T2, DWI and DCE sequences. 2 MRI‐scoring systems were used: in the first 205 participants a binary in‐house score, in the last 194 participants a PI‐RADS version 1 and version 2 Likert 1‐5 score. The MRI‐TBx thresholds for positivity and MRI‐TBx were a comparable triple suspicious (on T2, DWI, DCE) or a PIRADS version 2 4/5 lesion. MRI‐TBx was performed prior to SBx: 70 participants received cognitive MRI‐TBx using the TargetScan system (Best Nomos); 129 with software fusion MRI‐TBx (UroNav system, Invivo). Index test 2: 12‐core transrectal SBx, without blinding for MRI results |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same type of tests and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. We excluded from our analysis the 82 active surveillance participants. Furthermore we excluded 2 Bx‐naïve participants because only the highest GS was recorded (not differentiating between Bx methods). The remaining 337 (183 Bx‐naïve‐ and 154 prior‐negative Bx‐) participants were included. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Lawrence 2014.
| Study characteristics | |||
| Patient sampling | Aim of the study: to measure the performance characteristics of the MRI suspicion score prior to MRI‐TRUS fusion template transperineal repeat Bx Type of study: retrospective study of prospective data Selection: preselected patients in a MRI‐TRUS fusion template transperineal prostate repeat Bx programme Enrolled/eligible: 39/unclear Inclusion period: February 2012‐June 2012 |
||
| Patient characteristics and setting | Inclusion criteria:
Exclusion criteria: none Setting: Cambridge, UK. University hospital Age: mean 64 (range 47‐77) PSA: median 10 ng/mL (range 1.2‐36) Prostate volume: not reported |
||
| Index tests | Index test: MRI only, MRI‐TBx and MRI‐pathway. A 1.5 or 3 Tesla MRI (MR450, GE healthcare) were used, with T1, T2 and DWI. A PI‐RADS version 1 adapted sum score 1‐10 was used, with a score < 6 = no suspicion, 6 = low suspicion, 7‐8 = intermediate suspicion and 9‐10 = high suspicion, with threshold ≥ 6 for positivity and MRI‐TBx. Transperineal software fused MRI‐TBx were taken of all positive lesions, using the Biopsee system (Medcom), prior to the Ginsburg‐Bx | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 Reference standard: 24‐36 volume‐based transperineal biopsies were taken according to the Ginsburg protocol, without resampling MRI‐TBx trajectories, using the Biopsee system. MRI‐TBx were taken prior to the template biopsies. |
||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | For comparison 1a, we assume that the results of the MRI‐TBx that corresponded to the trajectory of the reference Bx (and thus were not resampled) are also considered results for the template Bx, as it seems it is reported as such. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Lee 2016.
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare PCa detection rates between SBx and mpMRI‐TBx for men with PSA < 10 ng/mL Type of study: retrospective analysis of prospectively collected data Selection: before PBx decision making, mpMRI‐TBx was explained to the participants. Those participants who agreed to the MRI‐TBx pathway (instead of standard SBx) were consecutively selected. Enrolled/eligible: 76/unclear Inclusion period: January 2014‐December 2014 |
||
| Patient characteristics and setting | Inclusion criteria: PSA level < 10 ng/mL, normal DRE and no previous PBx Setting: Yangsan, Korea. University hospital Age: median 65.8 years (range 43‐83) PSA: median 6.4 ng/mL (range 3.3‐9.8) Prostate volume: median 38.8 mL (range 17‐127) |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Intera Achieva, Phillips) was used, with T2 and DWI sequences. A modified 1‐4‐point MRI score was used:
Threshold for positive MRI and MRI‐TBx was score ≥ 2. Cognitive transrectal MRI‐TBx was performed of all positive lesions, prior to SBx. Index test 2: transrectal extended sextant SBx, without blinding for MRI results |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Lee 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine the efficacy of cognitive MRI‐TBx using biparametric MRI for men with PSA levels < 10 ng/mL Type of study: retrospective analysis Selection: before PBx, each urologist explained the MRI‐TBx technique to the participants; the final choice regarding the use of the technique (MRI‐TBx or standard SBx) was left to each participant. Hence, all consecutive participants who chose MRI‐TBx were selected. Enrolled/eligible: 123/464 (464 participants underwent PBx. Excluded were: 126 participants with a PSA > 10 ng/mL, 207 participants who chose SBx only, and 8 participants who had a prior‐negative Bx) Inclusion period: 2016 |
||
| Patient characteristics and setting | Inclusion criteria: Bx indication by elevated PSA and choice for MRI‐pathway Exclusion criteria: PSA > 10 ng/mL, previous PBx Setting: Yangsan, Korea. University hospital Age*: mean (SD) 61.8 years (11.7); 62 (7.8) PSA*: mean (SD) 6.7 ng/mL (1.67); 6.19 (1.82) Prostate volume* (SD): 38.6 mL (18.6); 40.2 (18.1) *reported for the mpMRI participants (n = 55) and bpMRI‐participants (n = 68), respectively |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Intera Achieva, Phillips) was used. In 68 participants only T2 and DWI sequences were used, in 55 DCE was also used. A modified 1‐4‐point MRI score was used, based on PI‐RADS version 2:
Threshold for positive MRI and MRI‐TBx was score ≥ 2. Cognitive transrectal MRI‐TBx was performed of all positive lesions, prior to SBx. Index test 2: transrectal extended sextant SBx, without blinding for MRI results |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Mortezavi 2018.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the diagnostic accuracy of mpMRI and mpMRI /TRUS fusion‐guided MRI‐TBx against transperineal TSB for the detection of PCa Type of study: retrospective analysis Selection: consecutive selection Enrolled/eligible: 415/415 (163 Bx‐naïve, 86 prior‐negative Bx, 166 previous positive Bx men) Inclusion period: November 2014–September 2016 |
||
| Patient characteristics and setting | Inclusion criteria: men who underwent mpMRI ± MRI‐TBx followed by template Bx Exclusion criteria: previously treated for PCa Setting: Zurich, Switzerland. University hospital Age: Bx‐naïve men: median 63 years (IQR 57‐68) Repeat‐Bx men: median 64 years (IQR 60‐69) PSA: Bx‐naïve men: median 5.8 ng/mL (IQR 4.4‐8.9) Repeat‐Bx men: median 8.6 ng/mL (IQR 5.7‐13) Prostate volume: Bx‐naïve men: median 44.6 mL (IQR 34‐60.1) Repeat‐Bx men: median 53.6 mL (IQR 41‐70) DRE positive: not reported |
||
| Index tests | Index test: MRI only, MRI‐TBx and MRI‐pathway. A 3 Tesla MRI machine (Magnetom Skyra, Siemens) was used with T2, DWI, and DCE sequences. In 16% of participants mpMRI was performed elsewhere. MRI was performed without an endorectal coil in 84% of participants. A Likert score analogous to PI‐RADS version 1 was used, with score 1‐5 and score ≥ 3 for positivity. The Biopsee Pi Medical/MedCom was used for software fused transrectal MRI‐TBx from all MRI‐positive lesions, with 2‐4 cores, after completing the TSB. | ||
| Target condition and reference standard(s) | Target conditions: Bx‐naïve men: GS 3+3 = 6, GS ≥ 3+3, GS ≥ 3+4, GS ≥ 4+3 Repeat‐Bx men: GS 3+3 = 6, GS ≥ 3+3, GS ≥ 3+4 Reference standard: transperineal template saturation prostate biopsies were taken according to the 20 Barzell zones (median 40 cores), not blinded for MRI results. |
||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | The 166 participants with previous positive Bx were excluded from our analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Muthuveloe 2016.
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the detection rate of significant PCa by transperineal template‐guided Bx Type of study: partial prospective and retrospective analysis Selection: all men who received MRI prior to template Bx were selected, no criteria reported for performing MRI or template Bx Enrolled/eligible: 200/unclear (9 Bx‐naïve‐, 162 prior‐negative Bx and 29 active surveillance participants). Inclusion period: March 2013‐December 2014 |
||
| Patient characteristics and setting | Inclusion criteria: transperineal template‐guided PBx and MRI prior to Bx Exclusion criteria: previous brachytherapy, previous template biopsies for anorectal abnormalities Setting: Birmingham, UK. Tertiary referral centre Age*: median (range) 68 years (46‐81); 65 (47‐78) PSA*: median (range) 11.5 ng/mL (1.2‐92.5); 10 (2.7‐61). Prostate volume: not reported *reported for template Bx positive (n = 71) and template Bx negative (n = 103) participants, respectively |
||
| Index tests | Index test: MRI only, assessed prior to template Bx. No details for MRI‐acquisition are reported. The PI‐RADS version 1 was used with a 1‐5 score and threshold ≥ 3 for positivity. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: a minimum of 24 sector transperineal prostatic Bx cores were taken in a systematic fashion using a 5 mm brachytherapy template grid, prostate volume depended. Blinding for MRI results was not reported. |
||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. We excluded the 29 active surveillance or other indication participants from this current analysis. The remaining 9 Bx‐naïve‐ and 162 prior‐negative participants were included. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Nafie 2014.
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare PCa detection rates between SBx and transperineal template PBx, in Bx‐naïve men Type of study: prospective cohort Selection: unclear Enrolled/eligible: 50/unclear Inclusion period: August 2012‐August 2013 |
||
| Patient characteristics and setting | Inclusion criteria: benign DRE, elevated PSA < 20 ng/mL, > 10 years' life expectancy Exclusion criteria: previous PBx Setting: Leicester, UK. University hospital Age: mean 67 years (range 54‐84) PSA: mean 8 ng/mL (range 4‐18) Prostate volume: mean 58 mL (range 19‐165) |
||
| Index tests | Index test: transrectal 12‐core SBx were taken from the right and left peripheral zones. Index test was taken first, then the reference test, in the same setting. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: 36‐core transperineal template PBx using a brachytherapy grid, after the performance of the SBx |
||
| Flow and timing | All participants underwent the same reference test and were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Nafie 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to determine whether transperineal template PBx is superior to SBx in the detection of PCa Type of study: prospective Selection: not reported Enrolled/eligible: 42/unclear Inclusion period: August 2012‐August 2014 |
||
| Patient characteristics and setting | Inclusion criteria: a history of 1 prior‐negative SBx with benign pathology, benign‐feeling prostate on DRE and a persistently elevated serum PSA more than the age‐specific range but < 20 ng/mL Exclusion criteria: none reported Setting: Leicester, UK. University hospital Age: median 65 years (range 50‐75) PSA: 8.3 ng/mL (range 4.4‐19) Prostate volume: 59 mL (range 21‐152) |
||
| Index tests | Index tests: 12 core transrectal SBx. Index test was taken first, then the reference test, in the same setting. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 Reference standard: 36‐cores transperineal template PBx using a brachytherapy grid |
||
| Flow and timing | All participants underwent the same reference test and were excluded from analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Okcelik 2016.
| Study characteristics | |||
| Patient sampling | Aim of the study: to analyse the contribution of MRI and PCA3 in detecting PCa Type of study: prospective cohort Selection: unclear Enrolled/eligible: 53/unclear Inclusion period: February 2013‐March 2014 |
||
| Patient characteristics and setting | Inclusion criteria: serum PSA level 3‐10 ng/mL participants with normal DRE scheduled for initial PBx Exclusion criteria: none reported Setting: Ankara, Turkey. Single‐centre, university hospital Age: median 62 years (IQR 43‐79) PSA: 5 ng/mL (range 3‐8.9) Prostate volume: median 45 mL (range 17‐93) |
||
| Index tests | Index tests 1: MRI‐pathway: a 1.5 Tesla MRI (Avanto, Siemens) was used, with T2, DWI, DCE and spectroscopy sequencing. A binary MRI score was reported, with additional cognitive transrectal MRI‐TBx taken from all positive lesions. Index test 2: transrectal extended sextant SBx with a mean number of 12.7 cores (including the additional MRI‐TBx only in MRI‐positive men), no further details reported |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. 1 participant did not undergo MRI for unclear reasons and was not included in analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Unclear | ||
| Unclear | |||
Panebianco 2015.
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess whether the proportion of men with clinically significant PCa is higher among men randomised to MRI‐Bx vs those randomised to SBx Type of study: prospective, 2‐armed RCT. Arm 1: MRI +/‐ MRI‐TBx and SBx; arm 2: SBx only. Participants from the SBx‐only arm with a negative Bx result subsequently received MRI +/‐ MRI‐TBx and SBx (with a standard scheme if MRI was positive and a saturation scheme if MRI was negative), therefore we regarded these participants as prior‐negative Bx participants. Selection: consecutive patients meeting the inclusion criteria Enrolled/eligible: 1040/1040 (570 participants in arm 1 and 570 participants in arm 2) Inclusion period: October 2011‐March 2014 |
||
| Patient characteristics and setting | Inclusion criteria: PSA level > 4 ng/mL, PSA density > 0.15, PSA velocity > 0.75 ng/mL/year, free/total PSA ratio < 0.10 when total PSA was 4‐10 ng/mL. The participants needed to meet all 4 inclusion criteria. Exclusion criteria: previous PBx The prior‐negative Bx participants (in arm 2) were not referred in a common clinical way, but selected on the basis of the prior‐negative SBx within the randomised population of Bx‐naïve participants. Setting: Rome, Italy. University hospital Age: median 64 years (range 51‐82) for all 1040 participants PSA: not reported Prostate volume: not reported |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Discovery MR750, GE Healthcare or MAGNETOM Verio, Siemens) was used with T2, DWI and DCE sequencing. PI‐RADS version 1 was used resulting in a Likert 1‐5 scale with threshold ≥ 3 for positivity and MRI‐TBx. All MRI suspicious lesions were cognitively targeted with 2 transrectal MRI‐TBx cores. Index test 2:
Order of index tests unclear, no blinding for MRI results during the Bx procedure reported. |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | In arm 1 (Bx‐naïve participants) all participants received the same tests. In arm 2 (prior‐negative Bx participants) participants received a significantly different type of SBx, depending on MRI‐result. All participants were included in the analysis. |
||
| Comparative | |||
| Notes | Study authors provided additional data. For our analysis, the 115 participants in arm 2 who had an initial positive SBx result were excluded. The remaining 355 participants of arm 2 contributed to our analysis as prior‐negative Bx participants. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| High | |||
Peltier 2015.
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare the detection of clinically significant disease by standard SBx vs MRI‐TBx Type of study: prospective Selection: consecutive Enrolled/eligible: 110/129 (14 men with previous Bx and 5 men with contraindications for MRI were excluded) Inclusion period: March 2012‐September 2013 |
||
| Patient characteristics and setting | Inclusion criteria: clinical suspicion of PCa due to an abnormal PSA and/or DRE Exclusion criteria: previous PBx, MRI contraindications Setting: Brussels, Belgium. Tertiary care hospital Age: median 65.8 years (IQR 59.5‐70.7) PSA: median 6.9 ng/mL (IQR 4.6‐9.6) Prostate volume: median 44 mL (IQR 35‐59) |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Verio, Siemens) was used, with T2, DWI and DCE sequences. An in‐house MRI score was used resulting in a 1‐4‐point scale (assessment based on PI‐RADS version 1 recommendations): 1 = no suspicious lesions, 2 = low suspicion (0‐1 parameter positive), 3 = moderate suspicion (2 parameters positive, including DWI), 4 = high suspicion (3‐4 parameters positive), with threshold score ≥ 2 for positivity and MRI‐TBx. Transrectal MRI‐TBx were taken with software fusion (Urostation, Koelis), after the performance of SBx. Index test 2: transrectal standard 12 core SBx + 2‐4 additional cores from the transitional zone according to the volume of the prostate. The operator performing SBx was not blinded to MRI results. |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent same tests and were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Pepe 2013.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate MRI accuracy in PCa diagnosis in men submitted to saturation PBx Type of study: prospective, single‐centre, multi‐departmental study Selection: unclear. Men were selected from a PCa case‐finding protocol (including 14,453 patients) if meeting the inclusion criteria and when having an indication for saturation Bx Enrolled/eligible: 78/unclear Inclusion period: June 2011‐December 2012 |
||
| Patient characteristics and setting | Inclusion criteria: 1 single prior‐negative Bx > 6 months before. Indications for saturation Bx: a persistently high or increasing PSA value, abnormal DRE and PSA > 10 ng/mL or PSA values 4.1‐10 or 2.6‐4 ng/mL with free/total PSA ≤ 25% and ≤ 20%, respectively Setting: Catania, Italy. University Hospital Age: median 63 years (range 49‐72) PSA: median 11 ng/mL (range 3.7‐45) Prostate volume: not reported |
||
| Index tests | Index test: MRI only, MRI‐TBx and MRI‐pathway. A 3 Tesla MRI (Achieva, Philips) was used, with T2, DWI, DCE and spectroscopy sequences. An in‐house binary MRI score was used, with positive lesions cognitively targeted by MRI‐TBx, after the performance of saturation Bx. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 Reference standard: transperineal TSB with a median of 28 cores (range 26‐32) including 4‐6 cores in the transition and anterior zone. MRI results were not blinded during Bx procedure. |
||
| Flow and timing | All participants underwent same reference standard and were included in the analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test MRI‐TBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Ploussard 2014.
| Study characteristics | |||
| Patient sampling | Aim of the study: comparison of the PCa detection rate between SBx versus template Bx Type of study: prospective cohort Selection: consecutive Enrolled/eligible: 2753/2753 Inclusion period: December 2001‐December 2011 |
||
| Patient characteristics and setting | Inclusion criteria: suspicious for PCa, by
Exclusion criteria: none Setting: Créteil, France. Tertiary care hospital Age: mean 64.2 years (SD 7.8) PSA: mean 12.5 ng/mL (SD 7.2) Prostate volume: mean 46.4 mL (SD 25.3) Positive DRE: 318 participants |
||
| Index tests | Index test: transrectal extended sextant 12‐cores SBx, as part of a 21‐core transrectal Bx protocol | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 Reference standard: 21‐core transrectal Bx protocol: first 6 sextant biopsies (standard 45° angle), then 3 Bx in each peripheral zone (80° angle), then 3 Bx in each transition zone, and finally 3 Bx in the midline peripheral zone. The SBx were part of the 21‐core saturation Bx protocol, and therefore the reference standard is not independent of the index test. |
||
| Flow and timing | All participants underwent the same 21‐core Bx protocol. No participants were excluded for analysis. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Pokorny 2014.
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare the diagnostic efficacy of the MRI‐pathway with SBx Selection: prospective cohort, consecutive series of Bx‐naïve men suspected of having PCa Enrolled/eligible: 223/229 Inclusion period: July 2012‐January 2013 |
||
| Patient characteristics and setting | Inclusion criteria: Bx‐naïve men with concerning PSA levels and/or an abnormal DRE, referred from urologists Exclusion criteria: not reported Setting: prospective single‐centre diagnostic study. Brisbane, Australia, University hospital Age: median 63 years (IQR 57‐68) PSA: median 5.3 ng/mL (IQR 4.1‐6.6) Prostate volume: median 41 mL (IQR 30‐59) |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Skyra, Siemens) was used. PI‐RADS version 1 was used, with score ≥ 3 as threshold for MRI‐TBx MRI was reported before the Bx procedure. In‐bore transrectal MRI‐TBx was performed, independently of reference test as first the MRI‐TBx in case of lesion were taken and subsequently the 12‐core SBx. Index test 2: standard transrectal 12‐core SBx, performed after the MRI‐TBx. The urologist performing 12‐core SBx was blinded to MRI findings and MRI‐TBx procedure. However, the order of the 2 Bx sessions might have made it possible for the urologist to identify the MRI‐TBx tracks and thereby take SBx from the suspicious lesion. |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. 6 participants were excluded because their PSA normalised, or they refused the MRI or Bx. | ||
| Comparative | |||
| Notes | Study authors provided additional information | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
Rouvière 2019a.
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare in the same Bx‐naïve patients the detection rates of ISUP grade group ≥ 2 cancers obtained by 12‐14 core SBx and 3‐6 core MRI‐TBx Type of study: prospective multicentre study Selection: consecutive selection Enrolled/eligible: 251/275 (only participants included with central pathology reading; specimens of 24 participants did not have central reading) Inclusion period: July 2015–August 2016 |
||
| Patient characteristics and setting | Inclusion criteria: primary suspicion of PCa based on elevated PSA, abnormal DRE and/or family history of PCa Exclusion criteria: prior Bx, PSA > 20 ng/mL, T3 disease on DRE, PCa diagnosis, history of hip prosthesis, pelvic radiation Setting: 16 centres in France (11 university hospitals, 2 cancer centres and 3 private hospitals) Age: median 64 years (IQR 59‐68) PSA: median 6.5 ng/mL (IQR 5.6‐9.6) Prostate volume: median 50 mL (IQR 38‐63) DRE positive: 31% (77/251) of the participants |
||
| Index tests | Index test 1: MRI‐pathway: several 1.5 and 3 Tesla MRI machines were used, with or without an endorectal coil, using T2, DWI, and DCE sequences. Both a Likert score based on PI‐RADS version 1, and PI‐RADS version 2 were used with score 1‐5 and score ≥ 3 for positivity. Transrectal cognitive or software fused MRI‐TBx were performed from all MRI‐positive lesions, within 3 months after performing MRI, after taking the SBx. Several machines were used for software fused MRI‐TBx. Index test 2: transrectal extended sextant SBx were taken, blinded for MRI results. |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same reference test. | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
Say 2016.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate mpMRI and MRI‐TRUS fusion MRI‐TBx as a means of detecting clinically significant cancer as well as a potential indicator for avoiding repeat Bx Type of study: retrospective Selection: consecutive Enrolled/eligible: 143/374 (231 participants did not comply with inclusion criteria) Inclusion period: December 2012–June 2015 |
||
| Patient characteristics and setting | Inclusion criteria: indication for repeat PBx Exclusion criteria: Bx‐naïve men, or previous diagnosis of PCa Setting: New Haven, USA. Tertiary care hospital Age: mean 64.1 years (range 47‐82) PSA: mean 11.6 ng/mL (range 0.4‐96.9) Prostate volume: 68.5 mL (range 16.5‐309) |
||
| Index tests | Index test 1: MRI‐pathway: no details reported about the acquisition of the MRI. An in‐house MRI 4‐point suspicion score was used: negative MRI (1), low (2), moderate (3) and high (4) suspicion, with threshold ≥ 2 for positivity and MRI‐TBx. All MRI‐TBx were performed using the Artemis/Pro‐Fuse™ system (Eigen, Grass Valley, California), after the performance of SBx. Index test 2: extended sextant SBx. Blinding of MRI results during the performance of SBx is not reported |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent same reference standard. All participants were included in the analysis. | ||
| Comparative | |||
| Notes | 4 participants were unable to tolerate the complete MRI exam of whom 2 participants had no suspicious lesions during the part of the exam that was completed. The other 2 participants were not specified. We were unable to differentiate and exclude these 4 men from the Bx results. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Thompson 2016.
| Study characteristics | |||
| Patient sampling | Aim of the study: to assess the accuracy of mpMRI for significant PCa detection before diagnostic Bx in men with an abnormal PSA/DRE Type of study: prospective cohort Selection: consecutive Enrolled/eligible: 344/388 (44 participants were excluded due to refusing informed consent, MRI or Bx) Inclusion period: April 2012‐March 2014 |
||
| Patient characteristics and setting | Inclusion criteria: men > 40 years, scheduled to undergo Bx for abnormal PSA or DRE, with a life expectancy > 10 years and no previous prostate MRI or Bx Exclusion criteria: none Setting: Sydney, Australia, University hospital Age: median 62.9 years (IQR 55.9‐67.1) PSA: median 5.2 ng/mL (IQR 3.7‐7.1) Prostate volume: median 40 mL (IQR 30‐54) |
||
| Index tests | Index test: MRI only. A 1.5 and 3 Tesla MRI (vendor unknown) was used in 2 centres. PI‐RADS version 1 was used, with score ≥ 3 considered positive. MRI was reported before the Bx procedure. Cognitive‐fusion transperineal MRI‐TBx were performed, independent of the reference test. However, the MRI‐TBx results are not reported separately from template Bx results. Therefore the MRI‐TBx could not be assessed as an index test. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: transperineal mapping biopsies (median 30 cores, using a brachytherapy grid, with relative periurethral zone sparing) from 18 template locations. MRI outcomes were known at time of Bx. MRI‐TBx were taken in addition to the TTMB and could not be disaggregated from the TTMB results and were therefore included in the reference standard results. |
||
| Flow and timing | All participants underwent same TTMB technique. 44 participants were excluded: 16 refused consent, 8 refused MRI and 10 refused Bx |
||
| Comparative | |||
| Notes | Study authors provided additional information | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
Tonttilla 2016.
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare (TRUS)‐fusion mpMRI‐TBx with routine SBx for overall and clinically significant PCa detection among men with suspected PCa based on PSA values Type of study: prospective RCT, with randomisation 1:1 to the mpMRI or control group. Participants in the mpMRI group underwent pre‐Bx mpMRI followed by SBx and MRI‐TBx; the control group underwent SBx alone. For our current analysis only the mpMRI group is used. Selection: consecutive Enrolled/eligible: 53/65 (of the mpMRI group; 12 participants were excluded because of PSA normalisation, Bx protocol violation or MRI could not be performed) Inclusion period: April 2011‐December 2014 |
||
| Patient characteristics and setting | Inclusion criteria:
Exclusion criteria:
Setting: Oulu, Finland, University hospital Age: median 63 years (IQR 60‐66) PSA: median 6.1 ng/mL (IQR 4.2‐9.9) Prostate volume: median 27.8 mL (IQR 23.5‐36.6) |
||
| Index tests | Index test 1: MRI‐pathway: a 3 Tesla MRI (Skyra, Siemens) was used. An in‐house MRI score on a 1‐4‐point scale was used:
With score ≥ 3 considered a positive MRI and threshold for MRI‐TBx. MRI was reported before the Bx procedure. Cognitive‐fusion transrectal MRI‐TBx were performed independent from the SBx. Index test 2: standard transrectal 12‐core SBx were performed with blinding for the MRI results and before the performance of the MRI‐TBx |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent same tests. Participants with normalised PSA, Bx protocol violation or in which MRI could not be performed were excluded from analysis (n = 12). | ||
| Comparative | |||
| Notes | Study authors provided additional data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
Tsivian 2017.
| Study characteristics | |||
| Patient sampling | Aim of the study: to evaluate the diagnostic properties of mpMRI in the detection, localisation and characterisation of PCa using 3‐D transperineal TTMB histopathology as the comparator. Selection: retrospective chart review of consecutive men who underwent mpMRI followed by 3‐D TTMB. Enrolled/eligible: 50/unclear Inclusion period: 2011‐2014 |
||
| Patient characteristics and setting | Inclusion criteria: indication for TTMB was either evaluation of elevated PSA with prior‐negative conventional office‐based SBx or restaging of potential candidates for active surveillance of focal therapy. Exclusion criteria: men with prior PCa treatment were excluded Setting: Durham, USA, University hospital Age: median 65 years (range 61‐69) PSA: median 7.1 ng/mL (range 5.1‐13.6) Prostate volume: median 43.9 mL (range 31.8‐64.7) |
||
| Index tests | Index test: MRI only. A 3 Tesla MRI (Signa HDx GE Healthcare of Skyra Siemens) was used. An in‐house MRI 1‐5 Likert score was used, with score ≥ 3 considered positive. MRI was reported before the Bx procedure. No MRI‐TBx were performed. | ||
| Target condition and reference standard(s) | Target condition: GS ≥ 3+3, GS ≥ 3+4 and GS ≥ 4+3 Reference standard: TTMB technique, 26 regions were sampled using a 5‐mm grid, independent of MRI results. |
||
| Flow and timing | All participants underwent same tests. No participants were excluded for analysis. | ||
| Comparative | |||
| Notes | Study authors provided additional information. We were able to exclude the 17 active surveillance participants for our current analysis; the remaining 33 participants with a prior‐negative Bx were included. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test MRI | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | Yes | ||
| Low | |||
Van der Leest 2018.
| Study characteristics | |||
| Patient sampling | Aim of the study: to compare the detection rates of clinically significant PCa and insignificant PCa in Bx‐naïve men with PSA levels ≥ 3 ng/mL for an MRI‐pathway and SBx‐pathway; to evaluate the total number of men with a non‐suspicious mpMRI, and the total number of Bx needles needed per pathway Type of study: prospective, multicentre, powered, comparative effectiveness study Selection: consecutive Enrolled/eligible: 626/699 Inclusion period: February 2015‐February 2017 |
||
| Patient characteristics and setting | Inclusion criteria: Bx‐naïve men, aged 50‐75 years with a PSA > 3 ng/mL Exclusion criteria: age < 50 or > 75 years, history of previous PBx or PCa, general contraindications for MRI, use of medications or hormones that are known to affect serum PSA levels, symptoms of urinary tract infection, and a history of invasive treatments for prostate benign hyperplasia Setting: 4 medical centres in the Netherlands (1 university and 3 non‐university centres) Age: median 65 years (IQR 59‐68) PSA: median 6.4 ng/mL (IQR 4.6‐8.2) Prostate volume: mean 55 mL (IQR 41‐77) DRE positive: 176 (28%) clinically significant PCa and insignificant PCa |
||
| Index tests | Index test 1: MRI‐pathway: mpMRI was performed with a 3‐Tesla machine (Magnetom Skyra, Siemens Healthineers, Erlangen, Germany), using T2, DWI and DCE. PI‐RADS version 2 was used to assess the MRI, with a 1‐5 score scale and score ≥ 3 for positivity, co‐read by multiple experienced radiologists. Transrectal in‐bore MRI‐TBx were taken from all positive lesions, with 2‐4 cores per lesion. Index test 2: all men underwent 12‐core transrectalSBx, after MRI‐TBx were taken, by a urologist who was blinded to the imaging results and not informed if a MRI‐TBx procedure was performed. |
||
| Target condition and reference standard(s) | No reference standard is used in this agreement analyses study (MRI‐pathway vs SBx), therefore the reference standard domain is not applicable and disregarded. | ||
| Flow and timing | All participants underwent the same tests. A total of 73 participants were excluded due to several reasons (personal reasons, Bx refusal), possibly leading to verification bias. | ||
| Comparative | |||
| Notes | Study authors provided additional data | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test SBx | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | |||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | |||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test MRI‐pathway | |||
| Was the MRI assessed without knowledge of the results of the (reference or other index) biopsies? | Yes | ||
| Were the MRI‐TBx performed independent of the (reference or other index) biopsies? | Yes | ||
| Was the performance of the SBx not influenced by the performance of the (reference or other index) biopsies? | |||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was the reference standard performed independent from the index test? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all enrolled patients included in the analysis, or were exclusions explained and not leading to a relevant bias? | No | ||
| High | |||
3‐D: three‐dimensional; ASAP: atypical small acinar proliferation; bpMRI: biparametric magnetic resonance imaging; Bx: biopsy; Bx‐naïve: biopsy‐naïve; DCE: dynamic contrast‐enhanced; DRE: digital rectal exam; DWI: diffusion‐weighted imaging; GFR: glomerular filtration rate; GS: Gleason score; IQR: interquartile range; ISUP: International Society of Urological Pathology; mpMRI: multi‐parametric magnetic resonance imaging; MRI: magnetic resonance imaging; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; PBx: prostate biopsy; PCa: prostate cancer; PCA3: prostate cancer antigen 3; PI‐RADS: Prostate Imaging ‐ Reporting and Data System; PSA: prostate‐specific antigen; RCT: randomised controlled trial; SBx: systematic transrectal ultrasound‐guided biopsy; SD: standard deviation; TBx: target biopsy; TOP‐Bx: transperineal optimised prostate biopsy; TRUS: transrectal ultrasound; TTMB: template‐guided mapping biopsy; TSB: template‐guided saturation biopsy; TURP: transurethral resection of the prostate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arsov 2015 | In group B within this RCT participants received MRI‐TBx and SBx. However, only MRI‐positive participants were investigated thereby not reporting on MRI‐negative participants. |
| Baco 2016 | The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. |
| Boesen 2017b | As for other studies from this author, this study reported on overlapping data with Boesen 2017a, which presented more complete data. |
| Brock 2015 | The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. |
| Fiard 2013 | The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. |
| Haffner 2011 | The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. |
| Hansen 2016b | Overlapping data with Hansen 2018. |
| Kasivisvanathan 2018 | This RCT did not perform the index tests in the same men but in 2 separate groups, therefore no 2x2 tables could be derived. |
| Komai 2013 | The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. |
| Kuru 2013a | The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. |
| Numao 2013 | The reference standard did not comply with our criteria: participants before 2008 underwent 3‐D, 26‐core (transperineal 14 cores plus transrectal 12 cores (n = 203 men); however after 2008, 3‐D, 14‐core Bx (transperineal 8‐core plus transrectal 6 cores) were performed in 102 men. Furthermore, men aged > 75 years or significant comorbidity (n = 46) received a transperineal 14‐core Bx. |
| Pepe 2015 | Overlapping data with Pepe 2013, which presents more complete data. |
| Pepe 2017 | The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. |
| Porpiglia 2017 | This RCT did not perform the index tests in the same men but in 2 separate groups, therefore no 2x2 tables could be derived. |
| Radtke 2015 | Overlapping data with Distler 2017, which presents more complete data. |
| Simmons 2018 | The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4, differentiating between participants with and without a prior PCa diagnosis. |
| Sonn 2014 | The study authors did not present or provide additional data such that 2x2 tables could be derived for our primary target condition GS ≥ 3+4. |
| Thompson 2014 | Overlapping data with Thompson 2016. |
| Weaver 2016 | The reference standard (12‐region, 48‐core template TRUS‐guided Bx using the TargetScan system) did not sample the whole prostate in all participants. The transition and anterior zones were often only sampled when a MRI lesion was present, often only by MRI‐TBx. Study authors provided additional data. |
| Winther 2017 | Pilot study of Boesen 2018. Therefore Boesen 2018 is included, which is more recent and more complete. |
3‐D: three‐dimensional; Bx: biopsy; GS: Gleason score; MRI: magnetic resonance imaging; MRI‐TBx: magnetic resonance imaging‐targeted biopsy; PCa: prostate cancer; RCT: randomised controlled trial; SBx: systematic biopsy; TRUS: transrectal ultrasound
Differences between protocol and review
The following methodological changes when comparing the actual review and its published protocol deserve consideration:
We changed the title of the review from ‘MRI pathway and TRUS‐guided biopsy for detecting clinically significant prostate cancer’ to ‘Prostate MRI, with or without MRI‐targeted biopsy, and systematic biopsy for detecting prostate cancer’ (Drost 2017) to better reflect the main objectives of the review.
In order to provide comprehensive results, we reorganised our index tests and added MRI‐targeted biopsy in men with a positive MRI as a specific subset, extracted from the previously defined MRI pathway.
The objectives were refined to meet Cochrane standards and to offer additional details regarding the aim of this review. Specifically, we articulated that the need to include agreement data for the analyses of the MRI pathway versus systematic biopsy was to provide important clinical evidence where diagnostic accuracy evidence was lacking.
Due to limited data, source exploration of heterogeneity in the test accuracy evidence was not possible. Subgroup analyses using the agreement data appeared possible that were not specified as such in the protocol.
We refined our tailored QUADAS‐2 in accordance with feedback from the Cochrane DTA group.
Myriam Hunink did not contribute to the final review and resigned as co‐author; nevertheless, we thank her for her contributions to the protocol (see also acknowledgements).
The initial protocol did not plan for the use of the GRADE approach for rating the certainty of evidence. GRADE summary of findings tables were added for clarity when presenting the main review findings.
Contributions of authors
Frank‐Jan H Drost (FD), Ivo G Schoots (IS) and Monique J Roobol (MR) all initiated the review and wrote the protocol. FD and Daniël F Osses (DO) conducted the literature search, reviewed abstracts and full‐text studies for eligibility, and performed the quality assessment and data extraction. IS assisted with the inclusion of studies, quality assessment and resolving disagreements. Daan Nieboer (DN) and FD performed the analyses. FD, IS, DN, MR and DO interpreted the analyses. FD and IS drafted the final review. MR contributed to the writing of the review. Chris H Bangma and Ewout W Steyerberg critically evaluated the protocol and provided general advice on the review.
Sources of support
Internal sources
-
Erasmus University Medical Center, Netherlands.
Evidence based research Grant 2014 (project 2014‐14103).
External sources
None, Other.
Declarations of interest
Frank‐Jan H Drost: none known
Daniel F Osses: none known
Daan Nieboer: none known
Ewout W Steyerberg reports the following relevant financial activities outside the submitted work: receives royalties from Springer for the textbook entitled Clinical Prediction Models
Chris H Bangma: none known
Monique J Roobol: none known
Ivo G Schoots reports the following relevant activities related to the submitted work: a guideline associate panel member of the EAU–ESTRO–ESUR–SIOG Guidelines on Prostate Cancer
New
References
References to studies included in this review
Abd‐Alazeez 2014 {published and unpublished data}
- Abd‐Alazeez, M, Arya HU, Charman SC, Anastasiadis E, Freeman A, Emberton M, et al. The accuracy of multiparametric MRI in men with negative biopsy and elevated PSA level‐Can it rule out clinically significant prostate cancer?. Urologic Oncology 2014;32(1):45.e17‐45.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2017 {published and unpublished data}
- Ahmed HU, El‐Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi‐parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389(10071):815‐22. [DOI] [PubMed] [Google Scholar]
Alberts 2017 {published data only}
- Alberts AR, Schoots IG, Bokhorst LP, Drost FH, Leenders GJ, Krestin GP, et al. Characteristics of prostate cancer found at fifth screening in the European Randomized Study of Screening for Prostate Cancer Rotterdam: can we selectively detect high‐grade prostate cancer with upfront multivariable risk stratification and magnetic resonance imaging?. European Urology 19 June 2017 [Epub ahead of print];S0302‐2838(17):30514‐6. [DOI] [PubMed] [Google Scholar]
Boesen 2017a {published data only (unpublished sought but not used)}
- Boesen L, Nørgaard N, Løgager V, Balslev I, Thomsen HS. A prospective comparison of selective multiparametric magnetic resonance imaging fusion‐targeted and systematic transrectal ultrasound‐guided biopsies for detecting prostate cancer in men undergoing repeated biopsies. Urologia Internationalis 2017;99(4):384‐91. [DOI] [PubMed] [Google Scholar]
Boesen 2018 {published and unpublished data}
- Boesen L, Nørgaard N, Løgager V, Balslev I, Bisbjerg R, Thestrup K‐C, Winther, et al. Assessment of the diagnostic accuracy of biparametric magnetic resonance imaging for prostate cancer in biopsy‐naïve men the Biparametric MRI for Detection of Prostate Cancer (BIDOC) Study. JAMA Network Open 2018;1(2):e180219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castellucci 2017 {published and unpublished data}
- Castellucci R, Linares Quevedo AI, Sánchez Gómez FJ, Díez Rodríguez J, Cogorno L, Cogollos Acuña I, et al. Prospective nonrandomized study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound‐guided biopsy to magnetic resonance imaging with subsequent MRI‐guided biopsy in biopsy‐naïve patients. Minerva Urologica e Nefrologica 2017;69(6):589‐95. [DOI] [PubMed] [Google Scholar]
Chang 2017 {published and unpublished data}
- Chang CH, Chiu HC, Lin WC, Ho TL, Chang H, Chang YH, et al. The influence of serum prostate‐specific antigen on the accuracy of magnetic resonance imaging targeted biopsy versus saturation biopsy in patients with previous negative biopsy. BioMed Research International 2017;2017:7617148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen J, Yi XL, Jiang LX, Wang R, Zhao JG, Li YH, et al. 3‐tesla magnetic resonance imaging improves the prostate cancer detection rate in transrectral ultrasound‑guided biopsy. Experimental and Therapeutic Medicine 2015;9(1):207‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cool 2016 {published data only}
- Cool DW, Romagnoli C, Izawa JI, Chin J, Gardi L, Tessier D, et al. Comparison of prostate MRI‐3D transrectal ultrasound fusion biopsy for first‐time and repeat biopsy patients with previous atypical small acinar proliferation. Canadian Urological Association Journal 2016;10(9‐10):342‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 2013 {published data only}
- Costa DN, Bloch BN, Yao DF, Sanda MG, Ngo L, Genega EM, et al. Diagnosis of relevant prostate cancer using supplementary cores from magnetic resonance imaging‐prompted areas following multiple failed biopsies. Magnetic Resonance Imaging 2013;31(6):947‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dal Moro 2019 {published and unpublished data}
- Dal Moro F, Zecchini G, Morlacco A, Gardiman MP, Lacognata CS, Lauro A, et al. Does 1.5 T mpMRI play a definite role in detection of clinically significant prostate cancer? Findings from a prospective study comparing blind 24‐core saturation and targeted biopsies with a novel data remodeling model. Aging Clinical and Experimental Research 2019;31(1):115‐23. [DOI] [PubMed] [Google Scholar]
Delongchamps 2013 {published data only}
- Delongchamps NB, Peyromaure M, Schull A, Beuvon F, Bouazza N, Flam T, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. The Journal of Urology 2013;189(2):493‐9. [DOI] [PubMed] [Google Scholar]
Distler 2017 {published data only}
- Distler FA, Radtke JP, Bonekamp D, Kesch C, Schlemmer HP, Wieczorek K, et al. The value of PSA density in combination with PI‐RADS for the accuracy of prostate cancer prediction. The Journal of Urology 2017;198(3):575‐82. [DOI] [PubMed] [Google Scholar]
Filson 2016 {published data only}
- Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ, et al. Prostate cancer detection with magnetic resonance‐ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer 2016;122(6):884‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia Bennett 2017 {published and unpublished data}
- Garcia Bennett J, Vilanova JC, Guma Padro J, Parada D, Conejero A. Evaluation of MR imaging‐targeted biopsies of the prostate in biopsy naïve patients. A single centre study. Diagnostic and Interventional Imaging 2017;98(10):677‐84. [DOI] [PubMed] [Google Scholar]
Grey 2015 {published and unpublished data}
- Grey AD, Chana MS, Popert R, Wolfe K, Liyanage SH, Acher PL. Diagnostic accuracy of magnetic resonance imaging (MRI) prostate imaging reporting and data system (PI‐RADS) scoring in a transperineal prostate biopsy setting. BJU International 2015;115(5):728‐35. [DOI] [PubMed] [Google Scholar]
Grönberg 2018 {published and unpublished data}
- Grönberg H, Eklund M, Picker W, Aly M, Jäderling F, Adolfsson J, et al. Prostate cancer diagnostics using a combination of the Stockholm3 blood test and multiparametric magnetic resonance imaging. European Urology 2018;74(6):722‐8. [DOI] [PubMed] [Google Scholar]
Hansen 2016a {published and unpublished data}
- Hansen N, Patruno G, Wadhwa K, Gaziev G, Miano R, Barrett T, et al. Magnetic resonance and ultrasound image fusion supported transperineal prostate biopsy using the Ginsburg protocol: technique, learning points, and biopsy results. Eurpean Urology 2016;70(2):332‐40. [DOI] [PubMed] [Google Scholar]
Hansen 2017 {published data only}
- Hansen NL, Kesch C, Barrett T, Koo B, Radtke JP, Bonekamp D, et al. Multicentre evaluation of targeted and systematic biopsies using magnetic resonance and ultrasound image‐fusion guided transperineal prostate biopsy in patients with a previous negative biopsy. BJU International 2017;120(5):631‐8. [DOI] [PubMed] [Google Scholar]
Hansen 2018 {published data only}
- Hansen NL, Barrett T, Kesch C, Pepdjonovic L, Bonekamp D, O'Sullivan R, et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy naïve men with suspicion of prostate cancer. BJU International 2018;122(1):40‐9. [DOI] [PubMed] [Google Scholar]
Jambor 2015 {published data only}
- Jambor I, Kähkönen E, Taimen P, Merisaari H, Saunavaara J, Alanen K, et al. Prebiopsy multiparametric 3T prostate MRI in patients with elevated PSA, normal digital rectal examination, and no previous biopsy. Journal of Magnetic Resonance Imaging 2015;41(5):1394‐404. [DOI] [PubMed] [Google Scholar]
Jambor 2017 {published data only}
- Jambor I, Boström PJ, Taimen P, Syvänen K, Kähkönen E, Kallajoki M, et al. Novel biparametric MRI and targeted biopsy improves risk stratification in men with a clinical suspicion of prostate cancer (IMPROD Trial). Journal of Magnetic Resonance Imaging 2017;46(4):1089‐95. [DOI] [PubMed] [Google Scholar]
Kesch 2017 {published and unpublished data}
- Kesch C, Radtke JP, Popeneciu IV, Gasch C, Dieffenbacher SC, Klein T, et al. TOP: prospective evaluation of a volume based, computer assisted method for transperineal optimized prostate biopsy. Urologia Internationalis 2017;99(2):149‐55. [DOI] [PubMed] [Google Scholar]
Kim 2017 {published and unpublished data}
- Kim EH, Weaver JK, Shetty AS, Vetter JM, Andriole GL, Strope SA. Magnetic resonance imaging provides added value to the prostate cancer prevention trial risk calculator for patients with estimated risk of high‐grade prostate cancer less than or equal to 10%. Urology 2017;102:183‐9. [DOI] [PubMed] [Google Scholar]
Lawrence 2014 {published data only}
- Lawrence EM, Tang SY, Barrett T, Koo B, Goldman DA, Warren AY, et al. Prostate cancer: performance characteristics of combined T2W and DW‐MRI scoring in the setting of template transperineal re‐biopsy using MR‐TRUS fusion. European Radiology 2014;24(7):1497‐505. [DOI] [PubMed] [Google Scholar]
Lee 2016 {published and unpublished data}
- Lee DH, Nam JK, Park SW, Lee SS, Han JY, Lee SD, et al. Visually estimated MRI targeted prostate biopsy could improve the detection of significant prostate cancer in patients with a PSA level. Yonsei Medical Journal 2016;57(3):565‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017 {published and unpublished data}
- Lee DH, Nam JK, Lee SS, Han JY, Lee JW, Chung MK, et al. Comparison of multiparametric and biparametric MRI in first round cognitive targeted prostate biopsy in patients with PSA levels under 10 ng/mL. Yonsei Medical Journal 2017;58(5):994‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mortezavi 2018 {published data only}
- Mortezavi A, Märzendorfer O, Donati OF, Rizzi G, Rupp NJ, Wettstein MS, et al. Diagnostic accuracy of multiparametric magnetic resonance imaging and fusion guided targeted biopsy evaluated by transperineal template saturation prostate biopsy for the detection and characterization of prostate cancer. Journal of Urology 2018;200(2):309‐18. [DOI] [PubMed] [Google Scholar]
Muthuveloe 2016 {published and unpublished data}
- Muthuveloe D, Telford R, Viney R, Patel P. The detection and upgrade rates of prostate adenocarcinoma following transperineal template‐guided prostate biopsy – a tertiary referral centre experience. Central European Journal of Urology 2016;69(1):42‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nafie 2014 {published data only}
- Nafie S, Mellon JK, Dormer JP, Khan MA. The role of transperineal template prostate biopsies in prostate cancer diagnosis in biopsy naïve men with PSA less than 20 ng mL‐1. Prostate Cancer and Prostatic Diseases 2014;17(2):170‐3. [DOI] [PubMed] [Google Scholar]
Nafie 2017 {published data only}
- Nafie S, Wanis M, Khan M. The efficacy of transrectal ultrasound guided biopsy versus transperineal template biopsy of the prostate in diagnosing prostate cancer in men with previous negative transrectal ultrasound guided biopsy. Urology Journal 2017;14(2):3008‐12. [PubMed] [Google Scholar]
Okcelik 2016 {published and unpublished data}
- Okcelik S, Soydan H, Ates F, Berber U, Saygin H, Sonmez G, et al. Evaluation of PCA3 and multiparametric MRI's: collective benefits before deciding initial prostate biopsy for patients with PSA level between 3‐10ng/mL. International Brazillian Journal of Urology 2016;42(3):449‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Panebianco 2015 {published and unpublished data}
- Panebianco V, Barchetti F, Sciarra A, Ciardi A, Indino EL, Papalia R, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urologic Oncology 2015;33(1):17.e1‐17.e7. [DOI] [PubMed] [Google Scholar]
Peltier 2015 {published data only}
- Peltier A, Aoun F, Lemort M, Kwizera F, Paesmans M, Velthoven R. MRI‐targeted biopsies versus systematic transrectal ultrasound guided biopsies for the diagnosis of localized prostate cancer in biopsy naïve men. Biomed Research International 2015;2015:571708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pepe 2013 {published data only (unpublished sought but not used)}
- Pepe P, Garufi A, Priolo G, Candiano G, Pietropaolo F, Pennisi M, et al. Prostate cancer detection at repeat biopsy: can pelvic phased array multiparametric MRI replace saturation biopsy?. Anticancer Research 2013;33(3):1195‐200. [PubMed] [Google Scholar]
Ploussard 2014 {published data only}
- Ploussard G, Nicolaiew N, Marchand C, Terry S, Vacherot F, Vordos D, et al. Prospective evaluation of an extended 21‐core biopsy scheme as initial prostate cancer diagnostic strategy. European Urology 2014;65(1):154‐61. [DOI] [PubMed] [Google Scholar]
Pokorny 2014 {published and unpublished data}
- Pokorny MR, Rooij M, Duncan E, Schröder FH, Parkinson R, Barentsz JO, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound‐guided biopsy versus magnetic resonance (MR) imaging with subsequent MR‐guided biopsy in men without previous prostate biopsies. European Urology 2014;66(1):22‐9. [DOI] [PubMed] [Google Scholar]
Rouvière 2019a {published data only}
- Rouvière O, Puech P, Renard‐Penna R, Claudon M, Roy C, Mège‐Lechavallier F, et al. MRI‐FIRST Investigators. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy‐naïve patients (MRI‐FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncology 2019;20(1):100‐9. [DOI] [PubMed] [Google Scholar]
Say 2016 {published data only}
- Say RK. MRI‐ultrasound fusion targeted biopsy in men with prior negative prostate biopsy for prostate cancer [PhD thesis]. Vol. 2078, New Haven, CT: Yale Medicine Thesis Digital Library, 2016. [elischolar.library.yale.edu/ymtdl/2078] [Google Scholar]
Thompson 2016 {published and unpublished data}
- Thompson JE, Leeuwen PJ, Moses D, Shnier R, Brenner P, Delprado W, et al. The diagnostic performance of multiparametric magnetic resonance imaging to detect significant prostate cancer. Journal of Urology 2016;195(5):1428‐35. [DOI] [PubMed] [Google Scholar]
Tonttilla 2016 {published and unpublished data}
- Tonttila PP, Lantto J, Pääkkö E, Piippo U, Kauppila S, Lammentausta E, et al. Prebiopsy multiparametric magnetic resonance imaging for prostate cancer diagnosis in biopsy‐naïve men with suspected prostate cancer based on elevated prostate‐specific antigen values: results from a randomized prospective blinded controlled trial. European Urology 2016;69(3):419‐25. [DOI] [PubMed] [Google Scholar]
Tsivian 2017 {published and unpublished data}
- Tsivian M, Gupta RT, Tsivian E, Qi P, Mendez MH, Abern MR, et al. Assessing clinically significant prostate cancer: diagnostic properties of multiparametric magnetic resonance imaging compared to three‐dimensional transperineal template mapping histopathology. International Journal of Urology 2017;24(2):137‐43. [DOI] [PubMed] [Google Scholar]
Van der Leest 2018 {published data only}
- Leest MM, Cornel EB, Israël B, Hendriks RJ, Padhani AR, Hoogenboom M, et al. Head‐to‐head comparison of transrectal ultrasound‐guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance‐guided biopsy in biopsy‐naïve men with elevated prostate‐specific antigen: a large prospective multicenter clinical study. European Urology 23 November 2018 [Epub ahead of print];S0302‐2838(18):30880‐7. [DOI: 10.1016/j.eururo.2018.11.023] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arsov 2015 {published data only}
- Arsov C, Rabenalt R, Blondin D, Quentin M, Hiester A, Godehardt E, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)‐guided in‐bore biopsy to MRI‐ultrasound fusion and transrectal ultrasound‐guided prostate biopsy in patients with prior negative biopsies. European Urology 2015;68(4):713‐20. [DOI] [PubMed] [Google Scholar]
Baco 2016 {published data only}
- Baco E, Rud E, Eri LM, Moen G, Vlatkovic L, Svindland A, et al. A randomized controlled trial to assess and compare the outcomes of two‐core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12‐core systematic biopsy. European Urology 2016;69(1):149‐56. [DOI] [PubMed] [Google Scholar]
Boesen 2017b {published data only}
- Boesen L, Norgaard N, Logager V, Balslev I, Thomsen HS. Multiparametric MRI in men with clinical suspicion of prostate cancer undergoing repeat biopsy: a prospective comparison with clinical findings and histopathology. Acta Radiologica 2017;59(3):371‐80. [DOI] [PubMed] [Google Scholar]
Brock 2015 {published data only}
- Brock M, Bodman C, Palisaar J, Becker W, Martin‐Seidel P, Noldus J. Detecting prostate cancer a prospective comparison of systematic prostate biopsy with targeted biopsy guided by fused MRI and transrectal ultrasound. Deutsches Ärzteblatt International 2015;112(37):605‐U13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiard 2013 {published data only}
- Fiard G, Hohn N, Descotes JL, Rambeaud JJ, Troccaz J, Long JA. Targeted MRI‐guided prostate biopsies for the detection of prostate cancer: initial clinical experience with real‐time 3‐dimensional transrectal ultrasound guidance and magnetic resonance/transrectal ultrasound image fusion. Urology 2013;81(6):1372‐8. [DOI] [PubMed] [Google Scholar]
Haffner 2011 {published data only}
- Haffner J, Lemaitre L, Puech P, Haber GP, Leroy X, Jones JS, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging‐targeted and systematic biopsy for significant prostate cancer detection. BJU International 2011;108(8 B):E171‐E8. [DOI] [PubMed] [Google Scholar]
Hansen 2016b {published data only}
- Hansen N, Patruno G, Wadhwa K, Gaziev G, Miano R, Barrett T, et al. Magnetic resonance and ultrasound image fusion supported transperineal prostate biopsy using the Ginsburg protocol: technique, learning points, and biopsy results. European Urology 2016;70(2):332‐40. [DOI] [PubMed] [Google Scholar]
Kasivisvanathan 2018 {published data only}
- Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI‐targeted or standard biopsy for prostate‐cancer diagnosis. New England Journal of Medicine 2018;378:1767‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Komai 2013 {published data only}
- Komai Y, Numao N, Yoshida S, Matsuoka Y, Nakanishi Y, Ishii C, et al. High diagnostic ability of multiparametric magnetic resonance imaging to detect anterior prostate cancer missed by transrectal 12‐core biopsy. Journal of Urology 2013;190(3):867‐73. [DOI] [PubMed] [Google Scholar]
Kuru 2013a {published data only}
- Kuru TH, Roethke MC, Seidenader J, Simpfendörfer T, Boxler S, Alammar K, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. Journal of Urology 2013;190(4):1380‐6. [DOI] [PubMed] [Google Scholar]
Numao 2013 {published data only}
- Numao N, Yoshida S, Komai Y, Ishii C, Kagawa M, Kijima T, et al. Usefulness of pre‐biopsy multiparametric magnetic resonance imaging and clinical variables to reduce initial prostate biopsy in men with suspected clinically localized prostate cancer. Journal of Urology 2013;190(2):502‐8. [DOI] [PubMed] [Google Scholar]
Pepe 2015 {published data only}
- Pepe P, Garufi A, Priolo G, Pennisi M. Can 3‐tesla pelvic phased‐array multiparametric MRI avoid unnecessary repeat prostate biopsy in patients with PSA < 10 ng/mL?. Clinical Genitourinary Cancer 2015;13(1):e27‐e30. [DOI] [PubMed] [Google Scholar]
Pepe 2017 {published data only}
- Pepe P, Garufi A, Priolo G, Pennisi M. Transperineal versus transrectal MRI/TRUS fusion targeted biopsy: detection rate of clinically significant prostate cancer. Clinical Genitourinary Cancer 2017;15(1):e33‐e6. [DOI] [PubMed] [Google Scholar]
Porpiglia 2017 {published data only}
- Porpiglia F, Manfredi M, Mele F, Cossu M, Bollito E, Veltri A, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy‐naïve patients with suspected prostate cancer. European Urology 2017;72(2):282‐8. [DOI] [PubMed] [Google Scholar]
Radtke 2015 {published data only}
- Radtke JP, Kuru TH, Boxler S, Alt CD, Popeneciu IV, Huettenbrink C, et al. Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging‐ultrasound fusion guidance. Journal of Urology 2015;193(1):87‐94. [DOI] [PubMed] [Google Scholar]
Simmons 2018 {published data only (unpublished sought but not used)}
- Simmons LA, Kanthabalan A, Arya M, Briggs T, Barratt D, Charman SC. Accuracy of transperineal targeted prostate biopsies, both visual‐estimation and image‐fusion for men needing a repeat biopsy in the PICTURE trial. Journal of Urology 2018;200(6):1227‐34. [DOI] [PubMed] [Google Scholar]
Sonn 2014 {published data only}
- Sonn GA, Chang E, Natarajan S, Margolis DJ, MacAiran M, Lieu P, et al. Value of targeted prostate biopsy using magnetic resonance‐ultrasound fusion in men with prior negative biopsy and elevated prostate‐specific antigen. European of Urology 2014;65(4):809‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2014 {published data only}
- Thompson JE, Moses D, Shnier R, Brenner P, Delprado W, Ponsky L, et al. Multiparametric magnetic resonance imaging guided diagnostic biopsy detects significant prostate cancer and could reduce unnecessary biopsies and over detection: a prospective study. Journal of Urology 2014;192(1):67‐74. [DOI] [PubMed] [Google Scholar]
Weaver 2016 {published data only}
- Weaver JK, Kim EH, Vetter JM, Fowler KJ, Siegel CL, Andriole GL. Presence of magnetic resonance imaging suspicious lesion predicts Gleason 7 or greater prostate cancer in biopsy naïve patients. Urology 2016;88:119‐24. [DOI] [PubMed] [Google Scholar]
Winther 2017 {published data only}
- Winther MD, Balslev I, Boesen L, Logager V, Noergaard N, Thestrup KC, et al. Magnetic resonance imaging‐guided biopsies may improve diagnosis in biopsy naïve men with suspicion of prostate cancer. Danish Medical Journal 2017;64(5):A5355. [PubMed] [Google Scholar]
Additional references
Ahmed 2011
- Ahmed HU, Hu Y, Carter T, Arumainayagam N, Lecornet E, Freeman A, et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. Journal of Urology 2011;186(2):458‐64. [DOI] [PubMed] [Google Scholar]
Alberts 2015
- Alberts AR, Schoots IG, Roobol MJ. Prostate‐specific antigen‐based prostate cancer screening: past and future. International Journal of Urology 2015;22(6):524‐32. [DOI] [PubMed] [Google Scholar]
Alberts 2019
- Alberts AR, Roobol MJ, Verbeek JF, Schoots IG, Chiu PK, Osses DF, et al. Prediction of high‐grade prostate cancer following multiparametric magnetic resonance imaging: improving the Rotterdam European Randomized Study of Screening for Prostate Cancer risk calculators. European Urology 2019;75(2):310‐8. [DOI] [PubMed] [Google Scholar]
Ankerst 2018
- Ankerst DP, Straubinger J, Selig K, Guerrios L, Hoedt A, Hernandez J, et al. A contemporary prostate biopsy risk calculator based on multiple heterogeneous cohorts. European Urology 2018;74(2):197‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
AUA Guideline 2018
- Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, et al. American Urological Associations Guideline Panel. Early detection of prostate cancer: AUA guideline. www.auanet.org/guidelines/prostate‐cancer‐early‐detection‐(2013‐reviewed‐for‐currency‐2018)#x2637 2018 (accessed on 15‐01‐2019).
Barentsz 2012
- Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. European Radiology 2012;22(4):746‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnett 2018
- Barnett CL, Davenport MS, Montgomery JS, Wei JT, Montie JE, Denton BT. Cost‐effectiveness of magnetic resonance imaging and targeted fusion biopsy for early detection of prostate cancer. BJU International 2018;122(1):50‐8. [DOI] [PubMed] [Google Scholar]
Barzell 2007
- Barzell WE, Melamed MR. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3‐dimensional pathologic mapping of the prostate ‐‐ a 4‐year experience. Urology 2007;70(6 Suppl):27‐35. [DOI] [PubMed] [Google Scholar]
Barzell 2012
- Barzell WE, Melamed MR, Cathcart P, Moore CM, Ahmed HU, Emberton M. Identifying candidates for active surveillance: an evaluation of the repeat biopsy strategy for men with favorable risk prostate cancer. Journal of Urology 2012;188(3):762‐7. [DOI] [PubMed] [Google Scholar]
Bell 2015
- Bell KJ, Mar C, Wright G, Dickinson J, Glasziou P. Prevalence of incidental prostate cancer: a systematic review of autopsy studies. International Journal of Cancer 2015;137(7):1749‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borghesi 2017
- Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja E, et al. Complications after systematic, random, and image‐guided prostate biopsy. European Urology 2017;71(3):353–65. [DOI] [PubMed] [Google Scholar]
Borofsky 2018
- Borofsky S, George AK, Gaur S, Bernardo M, Greer MD, Mertan FV, et al. What are we missing? False‐negative cancers at multiparametric MR imaging of the prostate. Radiology 2018;286(1):186‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bramer 2016
- Bramer WM, Giustini D, Jonge GB, Holland L, Bekhuis T. De‐duplication of database search results for systematic reviews in EndNote. Journal of the Medical Library Association: JMLA 2016;104(3):240‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2018
- Brown LC, Ahmed HU, Faria R, El‐Shater Bosaily A, Gabe R, Kaplan RS, et al. Multiparametric MRI to improve detection of prostate cancer compared with transrectal ultrasound‐guided prostate biopsy alone: the PROMIS study. Health Technology Assessment 2018;22(39):1‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryk 2017
- Bryk DJ, Llukani E, Taneja SS, Rosenkrantz AB, Huang WC, Lepor H. The role of ipsilateral and contralateral transrectal ultrasound‐guided systematic prostate biopsy in men with unilateral magnetic resonance imaging lesion undergoing magnetic resonance imaging‐ultrasound fusion‐targeted prostate biopsy. Journal of Urology 2017;102:178‐82. [DOI] [PubMed] [Google Scholar]
Carroll 2016
- Carroll PR, Parsons JK, Andriole G, Bahnson RR, Castle EP, Catalona WJ, et al. NCCN guidelines insights: prostate cancer early detection, version 2. Journal of the National Comprehensive Cancer Network 2016;14(5):509‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2013
- Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, et al. Early detection of prostate cancer: AUA Guideline. Journal of Urology 2013;190(2):419‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cash 2016
- Cash H, Gunzel K, Maxeiner A, Stephan C, Fischer T, Durmus T, et al. Prostate cancer detection on transrectal ultrasonography‐guided random biopsy despite negative real‐time magnetic resonance imaging/ultrasonography fusion‐guided targeted biopsy: reasons for targeted biopsy failure. BJU International 2016;118(1):35‐43. [DOI] [PubMed] [Google Scholar]
Center 2012
- Center MM, Jemal A, Lortet‐Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. European Urology 2012;61(6):1079‐92. [DOI] [PubMed] [Google Scholar]
Cohen 2016
- Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016;6(11):e012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coker 2018
- Coker MA, Glaser ZA, Gordetsky JB, Thomas JV, Rais‐Bahrami S. Targets missed: predictors of MRI‐targeted biopsy failing to accurately localize prostate cancer found on systematic biopsy. Prostate Cancer and Prostatic Diseases 2018;21(4):549‐55. [DOI] [PubMed] [Google Scholar]
Crawford 2013
- Crawford ED, Rove KO, Barqawi AB, Maroni PD, Werahera PN, Baer CA, et al. Clinical‐pathologic correlation between transperineal mapping biopsies of the prostate and three‐dimensional reconstruction of prostatectomy specimens. Prostate 2013;73(7):778‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Rooij 2014a
- Rooij M, Hamoen EH, Futterer JJ, Barentsz JO, Rovers MM. Accuracy of multiparametric MRI for prostate cancer detection: a meta‐analysis. American Journal of Roentgenology 2014;202(2):343‐51. [DOI] [PubMed] [Google Scholar]
De Rooij 2014b
- Rooij M, Crienen S, Witjes JA, Barentsz JO, Rovers MM, Grutters JP. Cost‐effectiveness of magnetic resonance (MR) imaging and MR‐guided targeted biopsy versus systematic transrectal ultrasound‐guided biopsy in diagnosing prostate cancer: a modelling study from a health care perspective. European Urology 2014;66(3):430‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882‐93. [DOI] [PubMed] [Google Scholar]
Dickinson 2011
- Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. European Urology 2011;59(4):477‐94. [DOI] [PubMed] [Google Scholar]
Djavan 2001
- Djavan B, Waldert M, Zlotta A, Dobronski P, Seitz C, Remzi M, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. Journal of Urology 2001;166(3):856‐60. [PubMed] [Google Scholar]
EAU Guideline 2018
- Mottet N, Bergh RCN, Briers E, Bourke L, Cornford P, Santis M, et al. European Association of Urology: guideline on prostate cancer. uroweb.org/guideline/prostate‐cancer/ 2018 (accessed on 15‐01‐2019).
El‐Shater Bosaily 2015
- El‐Shater Bosaily A, Parker C, Brown LC, Gabe R, Hindley RG, Kaplan R, et al. PROMIS ‐ Prostate MR imaging study: a paired validating cohort study evaluating the role of multi‐parametric MRI in men with clinical suspicion of prostate cancer. Contemporary Clinical Trials 2015;42:26‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epstein 1994
- Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994;271(5):368‐74. [PubMed] [Google Scholar]
Epstein 2010
- Epstein JI. An update of the Gleason grading system. Journal of Urology 2010;183(2):433‐40. [DOI] [PubMed] [Google Scholar]
Epstein 2012
- Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. European Urology 2012;61(5):1019‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epstein 2016
- Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. American Journal of Surgical Pathology 2016;40(2):244‐52. [DOI] [PubMed] [Google Scholar]
Faria 2018
- Faria R, Soares MO, Spackman E, Ahmed HU, Brown LC, Kaplan R, et al. Optimising the diagnosis of prostate cancer in the era of multiparametric magnetic resonance imaging: a cost‐effectiveness analysis based on the Prostate MR Imaging Study (PROMIS). European Urology 2018;73(1):23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feletto 2015
- Feletto E, Bang A, Cole‐Clark D, Chalasani V, Rasiah K, Smith DP. An examination of prostate cancer trends in Australia, England, Canada and USA: is the Australian death rate too high?. World Journal of Urology 2015;33(11):1677‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferro 2016
- Ferro M, Buonerba C, Terracciano C, Lucarelli G, Cosimato V, Bottero D, et al. Biomarkers in localized prostate cancer. Future Oncology 2016;12(3):399‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foley 2016
- Foley RW, Maweni RM, Gorman L, Murphy K, Lundon DJ, Durkan G, et al. European Randomised Study of Screening for Prostate Cancer (ERSPC) risk calculators significantly outperform the Prostate Cancer Prevention Trial (PCPT) 2.0 in the prediction of prostate cancer: a multi‐institutional study. BJU International 2016;118(5):706‐13. [DOI] [PubMed] [Google Scholar]
Futterer 2015
- Futterer JJ, Briganti A, Visschere P, Emberton M, Giannarini G, Kirkham A, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. European Urology 2015;68(6):1045‐53. [DOI] [PubMed] [Google Scholar]
Gayet 2016
- Gayet M, Aa A, Beerlage HP, Schrier BP, Mulders PF, Wijkstra H. The value of magnetic resonance imaging and ultrasonography (MRI/US)‐fusion biopsy platforms in prostate cancer detection: a systematic review. BJU International 2016;117(3):392‐400. [DOI] [PubMed] [Google Scholar]
Gold 2019
- Gold SA, Hale GR, Bloom JB, Smith CP, Rayn KN, Valera V, et al. Follow‐up of negative MRI‐targeted prostate biopsies: when are we missing cancer?. World Journal of Urology 2019;37(2):235‐41. [DOI] [PubMed] [Google Scholar]
Goto 1996
- Goto Y, Ohori M, Arakawa A, Kattan MW, Wheeler TM, Scardino PT. Distinguishing clinically important from unimportant prostate cancers before treatment: value of systematic biopsies. Journal of Urology 1996;156(3):1059‐63. [PubMed] [Google Scholar]
Hamoen 2015
- Hamoen EH, Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the prostate imaging reporting and data system (PI‐RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta‐analysis. European Urology 2015;67(6):1112‐21. [DOI] [PubMed] [Google Scholar]
Harnden 2008
- Harnden P, Naylor B, Shelley MD, Clements H, Coles B, Mason MD. The clinical management of patients with a small volume of prostatic cancer on biopsy: what are the risks of progression? A systematic review and meta‐analysis. Cancer 2008;112(5):971‐81. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. Available from www.handbook.cochrane.org, 2011. [Google Scholar]
Hu 2012
- Hu Y, Ahmed HU, Carter T, Arumainayagam N, Lecornet E, Barzell W, et al. A biopsy simulation study to assess the accuracy of several transrectal ultrasonography (TRUS)‐biopsy strategies compared with template prostate mapping biopsies in patients who have undergone radical prostatectomy. BJU International 2012;110(6):812‐20. [DOI] [PubMed] [Google Scholar]
Huo 2012
- Huo AS, Hossack T, Symons JL, PeBenito R, Delprado WJ, Brenner P, et al. Accuracy of primary systematic template guided transperineal biopsy of the prostate for locating prostate cancer: a comparison with radical prostatectomy specimens. Journal of Urology 2012;187(6):2044‐9. [DOI] [PubMed] [Google Scholar]
Ilic 2013
- Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD004720.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2013
- Jiang X, Zhu S, Feng G, Zhang Z, Li C, Li H, et al. Is an initial saturation prostate biopsy scheme better than an extended scheme for detection of prostate cancer? A systematic review and meta‐analysis. European Urology 2013;63(6):1031‐9. [DOI] [PubMed] [Google Scholar]
Kamangar 2006
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. Journal of Clinical Oncology 2006;24(14):2137‐50. [DOI] [PubMed] [Google Scholar]
Kelly 2017
- Kelly SP, Rosenberg PS, Anderson WF, Andreotti G, Younes N, Cleary SD, et al. Trends in the incidence of fatal prostate cancer in the United States by race. European Urolgy 2017;71(2):195‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuru 2013b
- Kuru TH, Wadhwa K, Chang RT, Echeverria LM, Roethke M, Polson A, et al. Definitions of terms, processes and a minimum dataset for transperineal prostate biopsies: a standardization approach of the Ginsburg Study Group for Enhanced Prostate Diagnostics. BJU International 2013;112(5):568‐77. [DOI] [PubMed] [Google Scholar]
Kuru 2015
- Kuru TH, Fütterer JJ, Schiffmann J, Porres D, Salomon G, Rastinehad AR. Transrectal ultrasound (US), contrast‐enhanced US, real‐time elastography, histoscanning, magnetic resonance imaging (MRI), and MRI‐US fusion biopsy in the diagnosis of prostate cancer. European Urology Focus 2015;1(2):117‐26. [DOI] [PubMed] [Google Scholar]
Loeb 2013
- Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. European Urology 2013;64(6):876‐92. [DOI] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors), Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available from: srdta.cochrane.org/.
Mehralivand 2018
- Mehralivand S, Shih JH, Rais‐Bahrami S, Oto A, Bednarova S, Nix JW, et al. A magnetic resonance imaging‐based prediction model for prostate biopsy risk stratification. JAMA Oncology 2018;4(5):678‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moldovan 2017
- Moldovan PC, Broeck T, Sylvester R, Marconi L, Bellmunt J, Bergh RC, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta‐analysis from the European Association of Urology Prostate Cancer Guidelines Panel. European Urology 2017;72(2):250‐66. [DOI] [PubMed] [Google Scholar]
Moore 2013a
- Moore CM, Kasivisvanathan V, Eggener S, Emberton M, Futterer JJ, Gill IS, et al. Standards of reporting for MRI‐targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. European Urology 2013;64(4):544‐52. [DOI] [PubMed] [Google Scholar]
Moore 2013b
- Moore CM, Robertson NL, Arsanious N, Middleton T, Villers A, Klotz L, et al. Image‐guided prostate biopsy using magnetic resonance imaging‐derived targets: a systematic review. European Urology 2013;63(1):125‐40. [DOI] [PubMed] [Google Scholar]
Moore 2017
- Moore CM, Giganti F, Albertsen P, Allen C, Bangma C, Briganti A, et al. Reporting magnetic resonance imaging in men on active surveillance for prostate cancer: the precise recommendations‐a report of a European School of Oncology task force. European Urology 2017;71(4):648‐55. [DOI] [PubMed] [Google Scholar]
Mottet 2017
- Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, Santis M, et al. EAU‐ESTRO‐SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. European Urology 2017;71(4):618‐29. [DOI] [PubMed] [Google Scholar]
NCCN Guideline 2018
- National Complrehensive Cancer Network (NCCN) Guidelines on Prostate Cancer: 2018 update. www.nccn.org/professionals/physician_gls/default.aspx 2018 (accessed on 15‐01‐2019).
Padhani 2019
- Padhani AR, Weinreb J, Rosenkrantz AB, Villeirs G, Turkbey B, Barentsz J. Prostate Imaging‐Reporting and Data System Steering Committee: PI‐RADS v2 status update and future directions. European Urology 2019;75(3):358‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pahwa 2017
- Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost‐effectiveness of MR imaging‐guided strategies for detection of prostate cancer in biopsy‐naïve men. Radiology 2017;285(1):157‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Panebianco 2018
- Panebianco V, Barchetti G, Simone G, Monte M, Ciardi A, Grompone MD, et al. Negative multiparametric magnetic resonance imaging for prostate cancer: what's next?. European Urology 2018;74(1):48‐54. [DOI] [PubMed] [Google Scholar]
Pham 2015
- Pham KN, Porter CR, Odem‐Davis K, Wolff EM, Jeldres C, Wei JT, et al. Transperineal template guided prostate biopsy selects candidates for active surveillance‐‐how many cores are enough?. Journal of Urology 2015;194(3):674‐9. [DOI] [PubMed] [Google Scholar]
Puech 2015
- Puech P, Randazzo M, Ouzzane A, Gaillard V, Rastinehad A, Lemaitre L, et al. How are we going to train a generation of radiologists (and urologists) to read prostate MRI?. Current Opinion in Urology 2015;25(6):522‐35. [DOI] [PubMed] [Google Scholar]
Radtke 2017
- Radtke JP, Wiesenfarth M, Kesch C, Freitag MT, Alt CD, Celik K, et al. Combined clinical parameters and multiparametric magnetic resonance imaging for advanced risk modeling of prostate cancer‐patient‐tailored risk stratification can reduce unnecessary biopsies. European Urology 2017;72(6):888‐96. [DOI] [PubMed] [Google Scholar]
Richenberg 2019
- Richenberg J, Logager V, Panebianco V, Rouviere O, Villeirs G, Schoots IG. The primacy of multiparametric MRI in men with suspected prostate cancer. European Radiology 2019 (in press). [DOI] [PMC free article] [PubMed]
Robertson 2014
- Robertson NL, Hu Y, Ahmed HU, Freeman A, Barratt D, Emberton M. Prostate cancer risk inflation as a consequence of image‐targeted biopsy of the prostate: a computer simulation study. European Urology 2014;65:628‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodger 2015
- Rodger JC, Supramaniam R, Gibberd AJ, Smith DP, Armstrong BK, Dillon A, et al. Prostate cancer mortality outcomes and patterns of primary treatment for Aboriginal men in New South Wales, Australia. BJU International 2015;115 Suppl 5:16‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenkrantz 2017
- Rosenkrantz AB, Babb JS, Taneja SS, Ream JM. Proposed adjustments to PI‐RADS version 2 decision rules: impact on prostate cancer detection. Radiology 2017;283(1):119‐29. [DOI] [PubMed] [Google Scholar]
Rouvière 2018
- Rouvière O, Souchon R, Melodelima C. Pitfalls in interpreting positive and negative predictive values: application to prostate multiparametric magnetic resonance imaging. Diagnostic and Interventional Imaging 2018;99(9):515‐8. [DOI] [PubMed] [Google Scholar]
Rouvière 2019b
- Rouvière O, Schoots IG, Mottet N, EAU‐EANM‐ESTRO‐ESUR‐SIOG Prostate Cancer Guidelines Panel. Multiparametric magnetic resonance imaging before prostate biopsy: a chain is only as strong as its weakest link. European Urology 2019 (Epub ahead of print). [DOI: 10.1016/j.eururo.2019.03.023] [DOI] [PubMed]
Schoots 2015
- Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging‐targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound‐guided biopsy: a systematic review and meta‐analysis. European Urology 2015;68(3):438‐50. [DOI] [PubMed] [Google Scholar]
Schoots 2018
- Schoots IG. MRI in early prostate cancer detection: how to manage indeterminate or equivocal PI‐RADS 3 lesions?. Translational Andrology and Urology 2018;7(1):70‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schouten 2017
- Schouten MG, Leest M, Pokorny M, Hoogenboom M, Barentsz JO, Thompson LC, et al. Why and where do we miss significant prostate cancer with multi‐parametric magnetic resonance imaging followed by magnetic resonance‐guided and transrectal ultrasound‐guided biopsy in biopsy‐naïve men?. European Urology 2017;71(6):896‐903. [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. British Medical Journal 2008;336(7653):1106‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siddiqui 2015
- Siddiqui MM, Rais‐Bahrami S, Turkbey B. Comparison of MR/ultrasound fusion–guided biopsy with ultrasound‐guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313(4):390‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simmons 2014
- Simmons LA, Ahmed HU, Moore CM, Punwani S, Freeman A, Hu Y, et al. The PICTURE study ‐‐ prostate imaging (multi‐parametric MRI and Prostate HistoScanning) compared to transperineal ultrasound guided biopsy for significant prostate cancer risk evaluation. Contemporary Clinical Trials 2014;37(1):69‐83. [DOI] [PubMed] [Google Scholar]
Sivaraman 2015
- Sivaraman A, Sanchez‐Salas R, Barret E, Ahallal Y, Rozet F, Galiano M, et al. Transperineal template‐guided mapping biopsy of the prostate. International Journal of Urology 2015;22(2):146‐51. [DOI] [PubMed] [Google Scholar]
Taira 2010
- Taira AV, Merrick GS, Galbreath RW, Andreini H, Taubenslag W, Curtis R, et al. Performance of transperineal template‐guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer and Prostatic Diseases 2010;13(1):71‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taira 2013
- Taira AV, Merrick GS, Bennett A, Andreini H, Taubenslag W, Galbreath RW, et al. Transperineal template‐guided mapping biopsy as a staging procedure to select patients best suited for active surveillance. American Journal of Clinical Oncology 2013;36(2):116‐20. [DOI] [PubMed] [Google Scholar]
Torre 2015
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a Cancer Journal for Clinicians 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
Valerio 2015
- Valerio M, Donaldson I, Emberton M, Ehdaie B, Hadaschik BA, Marks LS, et al. Detection of clinically significant prostate cancer using magnetic resonance imaging‐ultrasound fusion targeted biopsy: a systematic review. European Urology 2015;68(1):8‐19. [DOI] [PubMed] [Google Scholar]
Van Hove 2014
- Hove A, Savoie PH, Maurin C, Brunelle S, Gravis G, Salem N, et al. Comparison of image‐guided targeted biopsies versus systematic randomized biopsies in the detection of prostate cancer: a systematic literature review of well‐designed studies. World Journal of Urology 2014;32(4):847‐58. [DOI] [PubMed] [Google Scholar]
Venderink 2017
- Venderink W, Govers TM, Rooij M, Fütterer JJ, Sedelaar JP. Cost‐effectiveness comparison of imaging‐guided prostate biopsy techniques: systematic transrectal ultrasound, direct in‐bore MRI, and image fusion. American Journal of Roentgenology 2017;208(5):1058‐63. [DOI] [PubMed] [Google Scholar]
Wegelin 2017
- Wegelin O, Melick HH, Hooft L, Bosch JL, Reitsma HB, Barentsz JO, et al. Comparing three different techniques for magnetic resonance imaging‐targeted prostate biopsies: a systematic review of in‐bore versus magnetic resonance imaging‐transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique?. European Urology 2017;71(4):517‐31. [DOI] [PubMed] [Google Scholar]
Weinreb 2016
- Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI‐RADS prostate imaging ‐ reporting and data system: 2015, version 2. European Urology 2016;69(1):16‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
Wolters 2011
- Wolters T, Roobol MJ, Leeuwen PJ, Bergh RC, Hoedemaeker RF, Leenders GJ, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. Journal of Urology 2011;185(1):121‐5. [DOI] [PubMed] [Google Scholar]
Woo 2018
- Woo S, Suh CH, Kim SY, Cho JY, Kim SH, Moon MH. Head‐to‐head comparison between biparametric and multiparametric MRI for the diagnosis of prostate cancer: a systematic review and meta‐analysis. ARJ. American Journal of Roentgenology 2018;211(5):W226‐41. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Drost 2017
- Drost FJ, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG, et al. MRI pathway and TRUS‐guided biopsy for detecting clinically significant prostate cancer. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD012663] [DOI] [Google Scholar]


