Abstract
Objective
An important clinical question is how many patients with acute schizophrenia do not respond to antipsychotics despite being treated for adequate time and with an effective dose. However, up to date, the exact extent of the phenomenon remains unclear.
Methods
We calculated the nonresponse and nonremission percentages using individual patient data from 16 randomized controlled trials (RCTs). Six thousand two hundred twenty-one patients were assigned to one antipsychotic (amisulpride, flupenthixol, haloperidol, olanzapine, quetiapine, risperidone, or ziprasidone) at an adequate dose; the response was assessed at 4–6 weeks. As various definitions of nonresponse have been used in the literature, we applied 4 different cut-offs covering the whole range of percent Positive and Negative Syndrome Scale (PANSS)/Brief Psychiatric Rating Scale (BPRS) reduction (≤0%, <25%, <50%, <75%).For symptomatic remission, we used the definition proposed by Andreasen without employing the time criterion.
Results
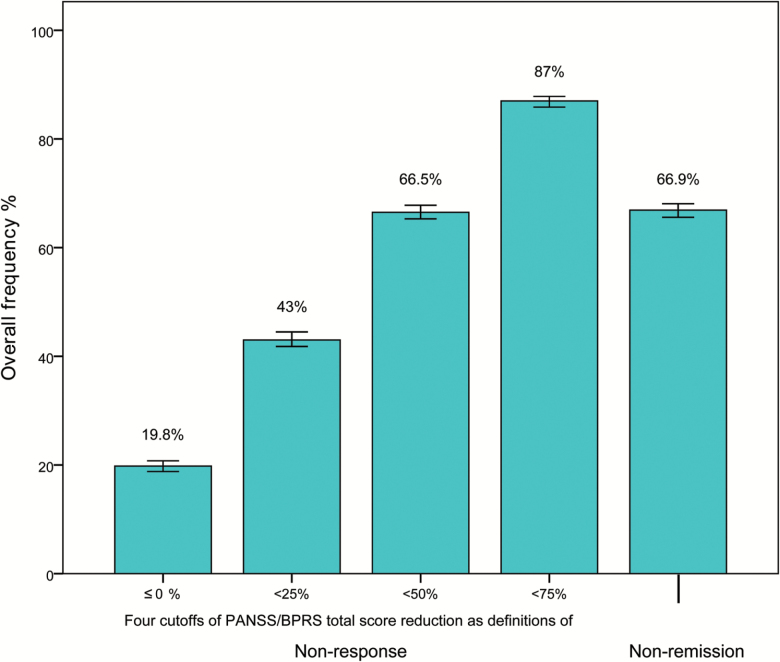
The overall nonresponse for the cut-off of ≤0% PANSS/BPRS reduction was 19.8% (18.8%–20.8%); for the cut-off of <25% reduction it was 43% (41.7%–44.3%); for the cut-off of <50% reduction it was 66.5% (65.3%–67.8%); and for the cut-off of <75% reduction it was 87% (86%–87.9%). The overall percentage of no symptomatic remission was 66.9% (65.7%–68.1%). Earlier onset of illness, lower baseline severity and the antipsychotic used were significantly associated with higher nonresponse percentages.
Conclusions
Nonresponse and nonremission percentages were notably high. Nevertheless, the patients in our analysis could represent a negative selection since they came from short-term RCTs and could have been treated before study inclusion; thus, further response may not have been observed. Observational studies on this important question are needed.
Keywords: nonresponse, nonremission, unresponsive, neuroleptic
Introduction
A considerable number of patients with schizophrenia do not respond to antipsychotic drugs. Nevertheless, the exact epidemiology of the phenomenon remains unclear. Estimates regarding the percentage of patients that do not respond or respond partially to treatment vary a lot. For example, the treatment guidelines from the American Psychiatric Association mention that “about 10%–30% of patients have little or no response to antipsychotic medications, and up to an additional 30% of patients have partial responses to treatment” (Lehman et al.1), but the authors fail to provide any reference. Similarly, vague statements can be found in other reports and textbooks such as “most controlled trials continue to find a subgroup of 10 to 20% of patients who derive little benefit from typical neuroleptic drug therapy,”2 “the general consensus is that from 5 to 25% of schizophrenic patients are partially or totally unresponsive to antipsychotic drug therapy,”3 “up to 30% of patients fail to respond adequately to conventional neuroleptic treatment,”4 or “it is generally accepted that between 10 and 60% of patients with schizophrenia respond poorly or only partially to antipsychotic treatment”5 but all of these statements are not based on firm evidence.
An important reason for the uncertainty about the percentage of patients not responding to antipsychotics is that definitions of response vary. Response to treatment can be described as a clinically meaningful amelioration of patient’s symptoms. Usually, it is estimated based on a specific percentage reduction of the initial total score in symptom rating scales such as the Positive and Negative Syndrome Scale (PANSS)6 or the Brief Psychiatric Rating Scale (BPRS).7 Nevertheless, different cut-offs of percentage reduction have been applied in the literature (20%, 30%, 40%, and 50% have all been used), making it difficult to provide a review of this question. The situation has somewhat improved by the introduction of the remission criteria according to Andreasen et al,8 but their limitation is that they depend a lot on the baseline symptoms severity of the patients. For example, if most patients are mildly ill at baseline, many will be in remission at the end although they have improved only little.9
Having a more precise estimate about how many patients do not respond to antipsychotic drugs would be very important to inform patients, clinicians, and designers of clinical trials about which rates of failure must be expected from antipsychotic drug trials. We, therefore, computed the nonresponse and nonremission percentages in a large dataset of randomized multicenter trials and presented them in a systematic way. In addition, we attempted to identify clinically useful baseline predictors of later nonresponse to treatment.
Methods
The Database
We used individual patient data from 16 randomized controlled trials (RCTs) that compared the efficacy of olanzapine or amisulpride with other antipsychotics or placebo for the treatment of patients with acute exacerbation of schizophrenia. Treatment efficacy was measured using the PANSS scale in 8 studies and the BPRS scale in the other 8 studies. The 16 RCTs were sponsored by the pharmaceutical industry and have already been published.10–25 All trials were randomized, and all, but one open-label,19 were double-blind. One study included only first-episode patients21 and one study patients with predominant negative symptoms.25 Important characteristics of the included studies are presented in the supplementary eTable 1.
As our research question was how many patients do not respond to antipsychotic medication after adequate time of treatment, we defined a period of 4–6 weeks (preferably 6) as follow-up time to assess response26 and we excluded patients who received placebo or an antipsychotic drug at an ineffective dose, ie, outside the target dose ranges according to the International Consensus of Antipsychotic Dosing published by Gardner et al.27 Six thousand two hundred twenty-one patients who received amisulpride (N = 1092), flupenthixol (N = 62), haloperidol (N = 1421), olanzapine (N = 2604), quetiapine (N = 175), risperidone (N = 596), and ziprasidone (N = 271) were included in the analysis. The mean age of the included patients was 37.2 years (CI: 36.9–37.5), the mean duration of illness was 13.6 years (CI: 13.4–3.9), and most of them were males (65.8%, N = 4093).
Response Criteria
The primary objective of our analysis was to identify how many patients do not respond to antipsychotic drugs. Our primary criterion was therefore defined as ≤0% reduction of the total score of the PANSS or the BPRS. This is the most stringent definition of absolute nonresponse which was also used for the predictor analysis. Moreover, we applied various degrees of nonresponse as suggested by Leucht et al.9,28,29 This means that we provided the percentage of nonresponders in 25% steps, ie, how many patients had less than 25% BPRS/PANSS reduction, less than 50% BPRS/PANSS reduction, and less than 75% BPRS/PANSS reduction. The advantage of this display is that it illustrates the full distribution of nonresponse rather than one arbitrary cut-off. Notably, studies have shown that a cut-off of at least 25% PANSS/BPRS score reduction roughly corresponds to “minimally improved” according to the Clinical Global Impression (CGI-I)30 of the raters while a cut-off of 50% reduction to a CGI-I of “much improved.”31–35 In addition, we assessed treatment nonresponse defined as less than 20% PANSS/BPRS reduction since this definition has been frequently applied in the literature.36–42 For remission, we used the consensus criteria proposed by Andreasen et al.8,43 According to these criteria, a patient is in remission if 8 key items of the PANSS are rated as “mildly present” or better for at least 6 months. As we used results from short-term clinical trials, the time criterion for assessing remission was not employed. In a secondary analysis, we examined nonresponse and nonremission rates in positive symptoms applying the same cut-offs as above (eg, ≤0%, <25%, <50%, and <75%). For illustration reasons, we also calculated nonresponse and nonremission percentages across treatments within the same study and the respective percentages of patients receiving placebo.
According to the definitions above, nonresponse and nonremission represent 2 inherently different measurements of treatment efficacy; response shows how much a patient improves, irrespectively of the presence or not of symptoms, whereas remission whether a patient has still symptoms, irrespectively of the magnitude of the improvement.
Statistical Analysis
For the calculation of the PANSS/BPRS percentage reduction from baseline to endpoint, we subtracted the 18/30 minimum score.44,45 All analyses were based on the last observation carried forward method (LOCF), where missing cases were replaced by the last available observation (“once randomized, analyzed”).46 In a sensitivity analysis, we analyzed only the observed cases at 6 (or 4) weeks. In a second sensitivity analysis, we excluded studies examining special populations such as first-episode patients or patients with predominant negative symptoms.
We calculated the nonresponse and nonremission frequencies for each study and synthesized them using the weighted average of their logit transformations; weights were assigned according to the inverse of their variance. A 95% confidence interval (CI) was derived assuming a normal distribution. To test whether important study and patient characteristics could independently predict nonresponse defined as ≤0% PANSS/BPRS reduction at 6 weeks, a mixed-effects logistic regression model was used. The fixed effects were gender, age, duration of illness, age at onset, weight, total symptom severity score at baseline, positive and negative symptom subscores at baseline (for items see below), and antipsychotic drug used. The study was used as a random effect indicating that there is an extra variation in the model according to the study in which patients were enrolled. Continuous predictor variables such as total, positive, and negative symptom severity scores at baseline need to be expressed in the same scale; thus, whenever necessary, we converted the BPRS total scores into PANSS using an established algorithm.47 Positive and negative symptom subscores were expressed as BPRS subscores (BPRS items 4, 11, 12, and 15 for positive and 3, 13, and 16 for negative subscore) for which all required items were available even when a patient was assessed only by PANSS (PANSS items 2, 3, 6, and 23 for positive and 8, 9, and 21 for negative subscore).48–50 The analyses were made using Stata Release 12.51
Results
Nonresponse and Nonremission Rates
The overall nonresponse for the cut-off of ≤0% PANSS/BPRS reduction was 19.8% (CI: 18.8%–20.8%); for the cut-off of less than 25% reduction it was 43% (CI: 41.7%–44.3%); for the cut-off of 50% reduction it was 66.5% (CI: 65.3%–67.8%); and for the cut-off of 75% reduction it was 87% (CI: 86%–87.9%) (figure 1). For the frequently used cut-off of less than 20% PANSS/BPRS reduction, the overall nonresponse was 38% (CI: 36.7%–39.2%). The pooled percentage of no symptomatic remission was 66.9% (CI: 65.7%–68.1%). Table 1 presents the nonresponse and nonremission rates per study, supplementary eTables 2 and 3 present the rates across treatments within each study and in placebo arms, and supplementary eFigures 1 and 2 show the distribution of percent total score reduction with antipsychotic drug treatment and placebo, respectively. Nonresponse and nonremission rates in positive symptoms did not differ much from the rates based on total scores (supplementary eTable 4).
Figure 1.
Overall frequency of nonresponse and nonremission. The first 4 bars display the overall frequency of nonresponse according to the following criteria of nonresponse: ≤0%, <25%, <50%, and <75% PANSS/BPRS total score reduction. The fifth bar displays the overall frequency of nonremission according to Andreasen criteria. The error bars display the 95% confidence intervals.
Table 1.
Nonresponse and Nonremission Rates (LOCF)
| Study | N | ≤0% | <25% | <50% | <75% | Nonremission |
|---|---|---|---|---|---|---|
| Beasley et al11 | 50 | 15 (30%) |
32 (64%) |
43 (86%) |
48 (96%) |
43 (86%) |
| Beasley et al22 | 202 | 53 (26.2%) |
89 (44.1%) |
133 (65.8%) |
178 (88.1%) |
— |
| Beasley et al10 | 252 | 60 (23.5%) |
105 (41.2%) |
183 (71.8%) |
238 (93.3%) |
193 (76%a) |
| Breier et al24 | 548 | 75 (13.7%) |
201 (36.7%) |
352 (64.2%) |
481 (87.8%) |
360 (66.5%a) |
| Carrière et al18 | 202 | 15 (7.4%) |
52 (25.7%) |
105 (52%) |
173 (85.6%) |
144 (71.3%) |
| Colonna et al19 | 486 | 62 (12.8%) |
173 (35.6%) |
309 (63.6%) |
436 (89.7%) |
345 (71%) |
| Keefe et al23 | 414 | 106 (25.6%) |
274 (66.2%) |
377 (91.1%) |
410 (99%) |
328 (82%a) |
| Kinon et al25 | 346 | 98 (28.3%) |
212 (61.3%) |
306 (88.4%) |
339 (98%) |
272 (78.8%a) |
| Lieberman et al21 | 263 | 52 (19.8%) |
115 (43.7%) |
183 (69.6%) |
244 (92.8%) |
167 (63.7%a) |
| Möller et al12 | 191 | 38 (17.3%) |
60 (31.4%) |
98 (51.3%) |
138 (72.3%) |
114 (59.7%) |
| Peuskens et al17 | 228 | 32 (14%) |
66 (29%) |
116 (50.9%) |
189 (82.9%) |
138 (60.5%) |
| Puech et al16 | 258 | 39 (15.1%) |
63 (24.4%) |
99 (38.4%) |
183 (70.9%) |
152 (58.9%) |
| Sechter et al20 | 310 | 29 (9.4%) |
70 (22.6%) |
155 (50%) |
247 (79.7%) |
129 (41.9%a) |
| Tollefson et al13 | 1996 | 448 (22.4%) |
993 (49.8%) |
1493 (74.8%) |
1871 (93.7%) |
1351 (70.5%a) |
| Tran et al14 | 339 | 49 (14.5%) |
110 (32.5%) |
235 (69.3%) |
310 (91.5%) |
223 (66.4%a) |
| Wetzel et al15 | 133 | 17 (12.8%) |
32 (24.1%) |
48 (36.1%) |
80 (60.2%) |
56 (42.1%) |
| Weighted mean (CI) | 6221 | 19.8% (18.8%–20.8%) |
43% (41.7%–44.3%) |
66.5% (65.3%–67.8%) |
87% (86%–87.9%) |
67.5% (66.2%–68.7%) |
Note: To obtain response rates, percentages should be subtracted from 100%. That means that the cumulative response rate for the cut-off >0% is 80.2%, for the cut-off ≥25% it is 57%, for the cut-off ≥50% it is 33.5%, for the cut-off ≥75% it is 13%, and for remission, it is 32.5%. CI, 95% confidence interval; LOCF, last observation carried forward method.
aSlightly different total number of patients (N) for remission because those in remission at baseline were removed from the analysis: 254; 541; 400; 345; 262; 308; 1916; 336.
Sensitivity Analyses
When only observed cases were examined, the overall nonresponse for the cut-off of ≤0% PANSS/BPRS reduction was 8.8% (CI: 7.9%–9.7%); for the cut-off of less than 25% reduction, it was 29.3% (CI: 27.9%–30.8%); for the cut-off of 50% reduction, it was 56.9% (CI: 55.4%–58.5%); and for the cut-off of 75% reduction, it was 83.1% (CI: 81.9%–84.3%). The pooled percentage of no symptomatic remission was 58.2% (CI: 56.7%–59.7%). The nonresponse and nonremission rates of observed cases per study and intervention, and in the positive symptoms are presented in the Supplementary appendix (supplementary eTables 5–8 and supplementary eFigures 3 and 4).
When studies in specific populations were excluded (ie, one in first-episode patients21 and one in patients with prominent negative symptoms25), the overall nonresponse for the cut-off of ≤0% reduction was 19.2% (CI: 18.1%–20.2%); for the cut-off of less than 25% reduction, it was 41.8% (CI: 40.5%–43.1%); for the cut-off of 50% reduction, it was 65.5% (CI: 64.1%–66.8%); and for the cut-off of 75% reduction, it was 86.4% (CI: 85.4%–87.4%). The pooled percentage of no symptomatic remission was 66.4% (CI: 65.1%–67.6%).
Mixed-Effects Logistic Regression Analysis
Earlier onset of illness and lower baseline severity in terms of PANSS total score were significantly associated with higher nonresponse rates whereas treatment with amisulpride, olanzapine, or risperidone was significantly associated with lower nonresponse rates (table 2). The initial investigation of significant variables had pointed out baseline severity of positive and negative symptom subscores as well (supplementary eTable 9). However, after including baseline total scores in the same model with baseline positive and negative subscores, the effects of the latter 2 variables were no longer significant. Results of the regression analysis of observed cases are similar (supplementary eTable 10).
Table 2.
Mixed-Effects Logistic Regression Analyses for Predicting Nonresponse Defined as ≤0% PANSS/BPRS Reduction (LOCF)
| Coefficient | SE | z | P | Upper limit | Lower limit | |
|---|---|---|---|---|---|---|
| Sex | −0.112 | 0.0703 | −1.60 | .110 | −0.250 | 0.026 |
| Age | −0.002 | 0.0031 | −0.80 | .426 | −0.009 | 0.004 |
| Duration of illness | 0.006 | 0.0034 | 1.64 | .102 | −0.001 | 0.012 |
| Age of onseta | −0.014 | 0.0046 | −3.12 | .002 | −0.023 | −0.005 |
| Weight | 0.001 | 0.0019 | 0.77 | .443 | −0.002 | 0.005 |
| PANSS total score at baselinea | −0.010 | 0.0019 | −5.44 | .000 | −0.014 | −0.007 |
| BPRS positive subscore at baselineb | 0.006 | 0.0138 | 0.45 | .649 | −0.021 | 0.033 |
| BPRS negative subscore at baselineb | −0.007 | 0.0139 | −0.49 | .625 | −0.034 | 0.020 |
| Antipsychotic drugs (haloperidol is the reference group) | ||||||
| Amisulpride | −0.389 | 0.1559 | −2.49 | .013 | −0.694 | −0.083 |
| Flupentixol | −0.256 | 0.4683 | −0.55 | .585 | −1.174 | 0.662 |
| Olanzapine | −0.279 | 0.0876 | −3.19 | .001 | −0.451 | −0.108 |
| Quetiapine | 0.176 | 0.2496 | 0.70 | .481 | −0.313 | 0.665 |
| Risperidone | −0.387 | 0.1636 | −2.36 | .018 | −0.708 | −0.066 |
| Ziprasidone | 0.346 | 0.2533 | 1.37 | .172 | −0.151 | 0.842 |
Note: For the continuous predictor variables (eg, age of onset, PANSS total score at baseline, etc.), positive coefficients (>0) indicate that the nonresponse rates increase as the predictor variable increases while negative coefficients (<0) indicate that the nonresponse rates increase as the predictor variable decreases. For categorical predictor variables (eg, gender, antipsychotic drug), positive coefficients indicate that the displayed categories (eg, quetiapine, ziprasidone) are associated with higher nonresponse rates while negative coefficients indicate that the displayed categories (eg, female gender; amisulpride, olanzapine, and risperidone, etc.) are associated with lower nonresponse rates. LOCF, last observation carried forward method; PANSS, Positive and Negative Syndrome Scale; BPRS, Brief Psychiatric Rating Scale.
aAge of onset and PANSS total score at baseline were included in the same model.
bAge of onset, PANSS total score at baseline, BPRS positive subscore and BPRS negative subscore were included in the same model.
Discussion
Our analysis was based on 16 studies including 6221 participants randomly assigned to 7 different antipsychotics. The results of our study could provide an important insight in the frequency of nonresponse and nonremission in schizophrenia. Approximately 2 out of 10 patients (19.8%) starting treatment with an antipsychotic drug did not show any symptom improvement after 4–6 weeks of therapy (≤0% PANSS/BPRS reduction) whereas for the response criterion of at least 25% PANSS/BPRS reduction, approximately 4 out of 10 patients (43%) did not respond to antipsychotic treatment. Considering that less than 0% PANSS/BPRS score reduction basically means symptom worsening and less than 25% score reduction means that patients are at best minimally improved compared with baseline,31–35 these criteria of nonresponse are extremely stringent and do not meet clinicians’ expectations of treatment efficacy for the typical patient presented with acute schizophrenia and treated with an adequate dosage of an antipsychotic drug. However, nonresponse rates according to these criteria were very high. When more demanding definitions of response such as at least 50% or 75% PANSS/BPRS reduction were applied, most of the patients suffering from schizophrenia did not respond to acute treatment (66.5% and 87%, respectively). Similar results to the 50% cut-off were obtained for no symptomatic remission (66.9%).
Up to date and despite numerous conflicting statements,2,3,5,52 the belief that about one third of patients show inadequate response to antipsychotic medication has prevailed among psychiatrists and in medical literature.1,4,53,54 Our results, based on a large sample, map the full range of response to antipsychotic treatment and provide the first solid evidence that a considerable number of patients in clinical trials do not respond at all to antipsychotic treatment.
Higher rates of nonresponse were observed in patients with earlier onset of illness, lower baseline total symptom severity score and in patients not treated with amisulpiride, olanzapine, or risperidone. Early onset of illness has already been identified as a predictor of poor response to antipsychotic treatment55,56 although there is recent evidence that the effect of earlier onset may be mediated by the duration of untreated psychosis and the number of relapses57 rather than predicting response itself.58 Thus, achieving and maintaining remission early during the course of schizophrenia is critical since it may improve longer-term outcomes.59 In line with previous reports,60,61 our study also associated lower baseline symptom severity with poorer response to treatment. As for amisulpride, olanzapine, and risperidone, these antipsychotics are proven to be more efficacious when compared with the other major antipsychotics,62 but, since the power of the comparisons between the included antipsychotics varied a lot, no firm conclusion can be driven based on our data.
Strengths and Limitations
The main strength of our analysis is the inclusion of a large sample of patients which makes our results rather robust. Further, the use of individual patient data enabled us to apply uniform definitions of nonresponse and nonremission and to provide a pooled estimate for all studies, surpassing the lack of consensus regarding response definition among single studies. Moreover, although not all patients responding to antipsychotics should be attributed to treatment, the included RCTs are from years ago, before the placebo response became alarmingly high.63 Drug response, which is the interest of the present analysis, remained stable over the years in placebo-controlled trials63 or even decreased in head-to-head comparisons of antipsychotics,64 which form the majority of our included trials. Thus, had we a set of older trials, the response could have been higher.
Nevertheless, a number of limitations should be addressed. The patients in our analysis were highly selected since they came from RCTs with strict inclusion and exclusion criteria. For example, for ethical and safety reasons, suicidal patients and other highly symptomatic patients who might respond particularly well to antipsychotic drugs are usually excluded from RCTs. Highly symptomatic patients cannot be included, because they have so many positive symptoms that they are unable to sign the often several pages long informed consent forms and need immediate treatment. But, we have shown that such patients benefit the most.61 In addition, pharmaceutical companies are trying to conduct large trials to assure statistical significance which leads to more recruitment pressure; the “patient clock” is running down, thus patients are recruited quickly by professional centers; most of them are improved and stabilized on antipsychotics and enter an RCT after a short wash-out phase of a few days. As most of the antipsychotic effect occurs early on,65–67 further response may not be observed which could, at least partly, explain the relatively low number of responders. The increased “relapse” rates on placebo (supplementary eTable 3, patients with ≤0%) also point to the direction that previous antipsychotics were beneficial. Moreover, since many antipsychotics are nowadays available, patients think twice before consenting to an RCT and those who do consent can create negative selection; samples usually include chronic patients, either partial nonresponders who consent to an RCT hoping for an improvement with the new drug or the so-called “professional patients” who may benefit from a free trial by answering a newspaper advertisement. And, although there is some evidence that patients treated in RCTs do not differ much from those in epidemiological studies, at least in first-episode psychosis trials,68 in our analysis there was just one study that was restricted to first-episode patients.21 Therefore, the results are not necessarily generalizable to routine care and replications in large naturalistic studies would be useful; pharma critical readers should take this situation into account.
Furthermore, results are based on short-term data (4–6 weeks), and remission is only symptomatic; although most patients with schizophrenia improve considerably during the first 2 weeks of treatment,67 more time may be needed until antipsychotics develop their full effects.66,69–71 Moreover, our sample was large (>6000 patients) and included many antipsychotics, but it is still a “convenience” sample in that we used the data obtained from just 2 companies; there are more antipsychotics and many more RCTs to be examined. In addition, despite the use of the same criteria for nonresponse and nonremission, the heterogeneity of the rates among single studies remained very high, which could be attributed to clinical characteristics,72 challenging the statistical grounds of the pooled estimates. Finally, it should be noticed that the true nonresponse rates are somewhere in between results in table 1 (LOCF) and in supplementary eTable 5 (observed cases). Table 1 shows larger nonresponse rates as these participants spent less time under treatment. These results are valid if patients dropped out because of lack of improvement; but, if they dropped out due to side-effects, then table 1 may exaggerate the nonresponse rates as these patients could have improved had they stayed in the trial. Nevertheless, in RCTs in patients with schizophrenia, more participants withdraw because of inefficacy.62
Notwithstanding these limitations, our results provide a unique insight in the question how many patients do not respond to antipsychotic treatment. Future studies should consistently report their results in steps of 25% PANSS/BPRS score reduction from baseline to endpoint (unimproved or worse, up to 25% PANSS/BPRS reduction from baseline, up to 50%, up to 75%) as well as remission rates; in such a way, we would soon have estimates based on larger patient samples on this important question.
Supplementary Material
Acknowledgments
In the last 36 months, Stefan Leucht has received honoraria for lectures from EliLilly, Lundbeck (Institute), Pfizer, Janssen, BMS, Johnson and Johnson, Otsuka, Roche, SanofiAventis, ICON, Abbvie, AOP Orphan, Servier; for consulting/advisory boards from Roche, Janssen, Lundbeck, EliLilly, Otsuka, TEVA; for the preparation of educational material and publications from Lundbeck Institute and Roche. The other authors have no conflict of interest to declare.
References
- 1. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1–56. [PubMed] [Google Scholar]
- 2. Kane JM, Honigfeld G, Singer J, Meltzer H. Clozapine in treatment-resistant schizophrenics. Psychopharmacol Bull. 1988;24:62–67. [PubMed] [Google Scholar]
- 3. Brenner HD, Dencker SJ, Goldstein MJ, et al. Defining treatment refractoriness in schizophrenia. Schizophr Bull. 1990;16:551–561. [DOI] [PubMed] [Google Scholar]
- 4. Remington G, Chong SA. Conventional versus novel antipsychotics: changing concepts and clinical implications. J Psychiatry Neurosci. 1999;24:431–441. [PMC free article] [PubMed] [Google Scholar]
- 5. Lindenmayer JP, Khan A. Assessment of therapy-resistant schizophrenia. In: Elkis H, Meltzer HY, eds. Therapy-Resistant Schizophrenia. Vol 26 Basel: Karger; 2010:9–32. [Google Scholar]
- 6. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 7. Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 8. Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. [DOI] [PubMed] [Google Scholar]
- 9. Leucht S. Measurements of response, remission, and recovery in schizophrenia and examples for their clinical application. J Clin Psychiatry. 2014;75(suppl 1):8–14. [DOI] [PubMed] [Google Scholar]
- 10. Beasley CM Jr, Hamilton SH, Crawford AM, et al. Olanzapine versus haloperidol: acute phase results of the international double-blind olanzapine trial. Eur Neuropsychopharmacol. 1997;7:125–137. [DOI] [PubMed] [Google Scholar]
- 11. Beasley CM Jr, Sanger T, Satterlee W, Tollefson G, Tran P, Hamilton S. Olanzapine versus placebo: results of a double-blind, fixed-dose olanzapine trial. Psychopharmacology (Berl). 1996;124:159–167. [DOI] [PubMed] [Google Scholar]
- 12. Möller HJ, Boyer P, Fleurot O, Rein W. Improvement of acute exacerbations of schizophrenia with amisulpride: a comparison with haloperidol. PROD-ASLP Study Group. Psychopharmacology (Berl). 1997;132:396–401. [DOI] [PubMed] [Google Scholar]
- 13. Tollefson GD, Beasley CM Jr, Tran PV, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry. 1997;154:457–465. [DOI] [PubMed] [Google Scholar]
- 14. Tran PV, Hamilton SH, Kuntz AJ, et al. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol. 1997;17:407–418. [DOI] [PubMed] [Google Scholar]
- 15. Wetzel H, Gründer G, Hillert A, et al. Amisulpride versus flupentixol in schizophrenia with predominantly positive symptomatology—a double-blind controlled study comparing a selective D2-like antagonist to a mixed D1-/D2-like antagonist. The Amisulpride Study Group. Psychopharmacology (Berl). 1998;137:223–232. [DOI] [PubMed] [Google Scholar]
- 16. Puech A, Fleurot O, Rein W. Amisulpride, and atypical antipsychotic, in the treatment of acute episodes of schizophrenia: a dose-ranging study vs. haloperidol. The Amisulpride Study Group. Acta Psychiatr Scand. 1998;98:65–72. [DOI] [PubMed] [Google Scholar]
- 17. Peuskens J, Bech P, Möller HJ, Bale R, Fleurot O, Rein W. Amisulpride vs. risperidone in the treatment of acute exacerbations of schizophrenia. Amisulpride Study Group. Psychiatry Res. 1999;88:107–117. [DOI] [PubMed] [Google Scholar]
- 18. Carrière P, Bonhomme D, Lempérière T. Amisulpride has a superior benefit/risk profile to haloperidol in schizophrenia: results of a multicentre, double-blind study (the Amisulpride Study Group). Eur Psychiatry. 2000;15:321–329. [DOI] [PubMed] [Google Scholar]
- 19. Colonna L, Saleem P, Dondey-Nouvel L, Rein W. Long-term safety and efficacy of amisulpride in subchronic or chronic schizophrenia. Amisulpride Study Group. Int Clin Psychopharmacol. 2000;15:13–22. [DOI] [PubMed] [Google Scholar]
- 20. Sechter D, Peuskens J, Fleurot O, Rein W, Lecrubier Y; Amisulpride Study Group Amisulpride vs. risperidone in chronic schizophrenia: results of a 6-month double-blind study. Neuropsychopharmacology. 2002;27:1071–1081. [DOI] [PubMed] [Google Scholar]
- 21. Lieberman JA, Tollefson G, Tohen M, et al. ; HGDH Study Group. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160:1396–1404. [DOI] [PubMed] [Google Scholar]
- 22. Beasley CM Jr, Tollefson G, Tran P, Satterlee W, Sanger T, Hamilton S. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14:111–123. [DOI] [PubMed] [Google Scholar]
- 23. Keefe RS, Young CA, Rock SL, Purdon SE, Gold JM, Breier A; HGGN Study Group One-year double-blind study of the neurocognitive efficacy of olanzapine, risperidone, and haloperidol in schizophrenia. Schizophr Res. 2006;81:1–15. [DOI] [PubMed] [Google Scholar]
- 24. Breier A, Berg PH, Thakore JH, et al. Olanzapine versus ziprasidone: results of a 28-week double-blind study in patients with schizophrenia. Am J Psychiatry. 2005;162:1879–1887. [DOI] [PubMed] [Google Scholar]
- 25. Kinon BJ, Noordsy DL, Liu-Seifert H, Gulliver AH, Ascher-Svanum H, Kollack-Walker S. Randomized, double-blind 6-month comparison of olanzapine and quetiapine in patients with schizophrenia or schizoaffective disorder with prominent negative symptoms and poor functioning. J Clin Psychopharmacol. 2006;26:453–461. [DOI] [PubMed] [Google Scholar]
- 26. Kane JM, Marder SR. Psychopharmacologic treatment of schizophrenia. Schizophr Bull. 1993;19:287–302. [DOI] [PubMed] [Google Scholar]
- 27. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. [DOI] [PubMed] [Google Scholar]
- 28. Leucht S, Davis JM, Engel RR, Kane JM, Wagenpfeil S. Defining ‘response’ in antipsychotic drug trials: recommendations for the use of scale-derived cutoffs. Neuropsychopharmacology. 2007;32:1903–1910. [DOI] [PubMed] [Google Scholar]
- 29. Leucht S, Davis JM, Engel RR, Kissling W, Kane JM. Definitions of response and remission in schizophrenia: recommendations for their use and their presentation. Acta Psychiatr Scand Suppl. 2009(438):7–14. [DOI] [PubMed] [Google Scholar]
- 30. Guy W. ECDEU Assessment Manual for Psychopharmacology —Revised. Rockville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 31. Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. Br J Psychiatry. 2005;187:366–371. [DOI] [PubMed] [Google Scholar]
- 32. Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?Schizophr Res. 2005;79:231–238. [DOI] [PubMed] [Google Scholar]
- 33. Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology. 2006;31:2318–2325. [DOI] [PubMed] [Google Scholar]
- 34. Leucht S, Kane JM. Measurement-based psychiatry: definitions of response, remission, stability, and relapse in schizophrenia. J Clin Psychiatry. 2006;67:1813–1814. [DOI] [PubMed] [Google Scholar]
- 35. Levine SZ, Rabinowitz J, Engel R, Etschel E, Leucht S. Extrapolation between measures of symptom severity and change: an examination of the PANSS and CGI. Schizophr Res. 2008;98:318–322. [DOI] [PubMed] [Google Scholar]
- 36. Chouinard G, Jones B, Remington G, et al. A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. J Clin Psychopharmacol. 1993;13:25–40. [PubMed] [Google Scholar]
- 37. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. [DOI] [PubMed] [Google Scholar]
- 38. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151:825–835. [DOI] [PubMed] [Google Scholar]
- 39. Peuskens J. Risperidone in the treatment of patients with chronic schizophrenia: a multi-national, multi-centre, double-blind, parallel-group study versus haloperidol. Risperidone Study Group. Br J Psychiatry. 1995;166:712–726; discussion 727–733. [DOI] [PubMed] [Google Scholar]
- 40. Claus A, Bollen J, De Cuyper H, et al. Risperidone versus haloperidol in the treatment of chronic schizophrenic inpatients: a multicentre double-blind comparative study. Acta Psychiatr Scand. 1992;85:295–305. [DOI] [PubMed] [Google Scholar]
- 41. Høyberg OJ, Fensbo C, Remvig J, Lingjaerde O, Sloth-Nielsen M, Salvesen I. Risperidone versus perphenazine in the treatment of chronic schizophrenic patients with acute exacerbations. Acta Psychiatr Scand. 1993;88:395–402. [DOI] [PubMed] [Google Scholar]
- 42. Davis JM, Chen N. Clinical profile of an atypical antipsychotic: risperidone. Schizophr Bull. 2002;28:43–61. [DOI] [PubMed] [Google Scholar]
- 43. van Os J, Burns T, Cavallaro R, et al. Standardized remission criteria in schizophrenia. Acta Psychiatr Scand. 2006;113:91–95. [DOI] [PubMed] [Google Scholar]
- 44. Obermeier M, Mayr A, Schennach-Wolff R, Seemüller F, Möller HJ, Riedel M. Should the PANSS be rescaled?Schizophr Bull. 2010;36:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leucht S, Kissling W, Davis JM. The PANSS should be rescaled. Schizophr Bull. 2010;36:461–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adams CE, Coutinho E, Duggan L, Leucht S, Srisurapanont M, Tharyan P; The Cochrane Schizophrenia Group The Cochrane Library. Chichester, UK: John Wiley & Sons Ltd; 2006. [Google Scholar]
- 47. Leucht S, Rothe P, Davis JM, Engel RR. Equipercentile linking of the BPRS and the PANSS. Eur Neuropsychopharmacol. 2013;23:956–959. [DOI] [PubMed] [Google Scholar]
- 48. McCreadie RG, Todd N, Livingston M, et al. A double-blind comparative study of remoxipride and thioridazine in the acute phase of schizophrenia. Acta Psychiatr Scand Suppl. 1990;358:136–137. [DOI] [PubMed] [Google Scholar]
- 49. Nicholson IR, Chapman JE, Neufeld RW. Variability in BPRS definitions of positive and negative symptoms. Schizophr Res. 1995;17:177–185. [DOI] [PubMed] [Google Scholar]
- 50. Prosser ES, Csernansky JG, Kaplan J, Thiemann S, Becker TJ, Hollister LE. Depression, Parkinsonian symptoms, and negative symptoms in schizophrenics treated with neuroleptics. J Nerv Ment Dis. 1987;175:100–105. [DOI] [PubMed] [Google Scholar]
- 51. StataCorp. Stata Statistical Software: Release 13 [computer program]. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 52. Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169:1203–1210. [DOI] [PubMed] [Google Scholar]
- 53. Meltzer HY. Treatment-resistant schizophrenia—the role of clozapine. Curr Med Res Opin. 1997;14:1–20. [DOI] [PubMed] [Google Scholar]
- 54. Conley RR, Kelly DL.. Pharmacologic Treatment of Schizophrenia. 3rd ed. Durant, OK: Professional Communications, INC; 2007. [Google Scholar]
- 55. Meltzer HY, Rabinowitz J, Lee MA, et al. Age at onset and gender of schizophrenic patients in relation to neuroleptic resistance. Am J Psychiatry. 1997;154:475–482. [DOI] [PubMed] [Google Scholar]
- 56. Dernovsek MZ, Tavcar R. Age at onset of schizophrenia and neuroleptic dosage. Soc Psychiatry Psychiatr Epidemiol. 1999;34:622–626. [DOI] [PubMed] [Google Scholar]
- 57. Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16:505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rabinowitz J, Werbeloff N, Caers I, et al. Determinants of antipsychotic response in schizophrenia: implications for practice and future clinical trials. J Clin Psychiatry. 2014;75:e308–e316. [DOI] [PubMed] [Google Scholar]
- 59. Goff DC, Falkai P, Fleischhacker WW, et al. The long-term effects of antipsychotic medication on clinical course in schizophrenia. Am J Psychiatry. 2017;174:840–849. [DOI] [PubMed] [Google Scholar]
- 60. Stern RG, Kahn RS, Davidson M. Predictors of response to neuroleptic treatment in schizophrenia. Psychiatr Clin North Am. 1993;16:313–338. [PubMed] [Google Scholar]
- 61. Furukawa TA, Levine SZ, Tanaka S, et al. Initial severity of schizophrenia and efficacy of antipsychotics: participant-level meta-analysis of 6 placebo-controlled studies. JAMA Psychiatry. 2015;72:14–21. [DOI] [PubMed] [Google Scholar]
- 62. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. [DOI] [PubMed] [Google Scholar]
- 63. Leucht S, Leucht C, Huhn M, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174:927–942. [DOI] [PubMed] [Google Scholar]
- 64. Rutherford BR, Pott E, Tandler JM, Wall MM, Roose SP, Lieberman JA. Placebo response in antipsychotic clinical trials: a meta-analysis. JAMA Psychiatry. 2014;71:1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60:1228–1235. [DOI] [PubMed] [Google Scholar]
- 66. Leucht S, Busch R, Hamann J, Kissling W, Kane JM. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry. 2005;57:1543–1549. [DOI] [PubMed] [Google Scholar]
- 67. Samara MT, Leucht C, Leeflang MM, et al. Early improvement as a predictor of later response to antipsychotics in schizophrenia: a diagnostic test review. Am J Psychiatry. 2015;172:617–629. [DOI] [PubMed] [Google Scholar]
- 68. Rabinowitz J, Bromet EJ, Davidson M. Are patients enrolled in first episode psychosis drug trials representative of patients treated in routine clinical practice?Schizophr Res. 2003;61:149–155. [DOI] [PubMed] [Google Scholar]
- 69. National Institute of Mental Health Psychopharmacology Research Branch Collaborative Study Group. Differences in clinical effects of three phenothiazines in “acute” schizophrenia. Dis Nerv Syst. 1967;28:369–383. [PubMed] [Google Scholar]
- 70. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28:995–1003. [DOI] [PubMed] [Google Scholar]
- 71. Robinson DG, Woerner MG, Alvir JM, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 1999;156:544–549. [DOI] [PubMed] [Google Scholar]
- 72. Levine SZ, Leucht S. Elaboration on the early-onset hypothesis of antipsychotic drug action: treatment response trajectories. Biol Psychiatry. 2010;68:86–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.