Abstract
Prominent conceptual models characterize schizophrenia as a dysconnectivity syndrome, with recent research focusing on the contributions of the cerebellum in this framework. The present study examined the role of the cerebellum and its effective connectivity to the cerebrum during sensorimotor synchronization in schizophrenia. Specifically, the role of the cerebellum in temporally coordinating cerebral motor activity was examined through path analysis. Thirty-one individuals diagnosed with schizophrenia and 40 healthy controls completed a finger-tapping fMRI task including tone-paced synchronization and self-paced continuation tapping at a 500 ms intertap interval (ITI). Behavioral data revealed shorter and more variable ITIs during self-paced continuation, greater clock (vs motor) variance, and greater force of tapping in the schizophrenia group. In a whole-brain analysis, groups showed robust activation of the cerebellum during self-paced continuation but not during tone-paced synchronization. However, effective connectivity analysis revealed decreased connectivity in individuals with schizophrenia between the cerebellum and primary motor cortex but increased connectivity between cerebellum and thalamus during self-paced continuation compared with healthy controls. These findings in schizophrenia indicate diminished temporal coordination of cerebral motor activity by cerebellum during the continuation tapping portion of sensorimotor synchronization. Taken together with the behavioral finding of greater temporal variability in schizophrenia, these effective connectivity results are consistent with structural and temporal models of dysconnectivity in the disorder.
Keywords: psychosis, cerebellum, finger tapping, effective connectivity, basal ganglia, fMRI
Introduction
Schizophrenia has long been identified as a dysconnectivity syndrome, beginning with Bleuler’s conceptualization of a “fragmented phrene”1 and Stransky’s intrapsychic ataxia theory,2 both of which theorized a dyscoordination of the motor and cognitive processes in the disorder. Andreasen’s3 cognitive dysmetria theory extended these conceptualizations by suggesting a neural mechanism by which such processes occur, namely disruption to the cortico-cerebellar-thalamic-cortical circuit (CCTCC). In this circuit, the cerebellar node plays a primary coordinative role.4 Explicit examination of the function of the cerebellum in this circuit is important because many large-scale studies of neural connectivity exclude the cerebellum,5,6 which is known to be interconnected with cerebrum7,8 and contains an upward estimate of 80% of the brain’s total neurons.9 Calls from the NIMH to add a “motor systems” domain to the Research Domain Criteria (RDoC) matrix underscore the importance of understanding neural mechanisms contributing to motor impairment in psychopathology.10 The present study assessed neural dysconnectivity in schizophrenia within the CCTCC during a sensorimotor task.
Sensorimotor timing tasks are well suited to investigations of neural dyscoordination, and the processing circuits involved have been well characterized.11,12 Cerebellum, cortical (primary motor [M1], supplementary motor, and prefrontal cortices), and subcortical (caudate, putamen, and thalamus) structures have been implicated broadly in motor and perceptual timing and integration.13–15 Lesion studies point to critical contributions of the cerebellum and basal ganglia14 as a distributed timing network for subsecond, discrete, rhythmic timing. Moreover, basal ganglia and cerebellar projections converge on subregions of the thalamus, before projecting to M1.16
In sensorimotor synchronization finger-tapping—wherein participants synchronize tapping with a tone and then attempt to maintain the same pace after the tone discontinues—the cerebellum is more activated during the self-paced continuation portion compared to the stimulus-cued synchronization portion. Such findings demonstrate the cerebellum’s function as a timekeeper or internal “clock,”17 in which cerebellar-generated temporal representations putatively drive cerebral motor areas to maintain internal temporal representations, thereby maximizing task performance. Research on the neural substrates of this form of sensorimotor synchronization has shown predominant cerebellar involvement, and the cortico-cerebellar circuit, in tasks of automatic, subsecond timing.18–21
The basal ganglia, specifically the putamen, is also a key structure in interval timing tasks, including sensorimotor synchronization,11,13 and is heavily integrated with the cerebellum during time estimation and motor output in both the subsecond and second range.11,22 The thalamus is also crucial for sensorimotor integration.23 Involvement of subcortical structures in this timing circuit has been likened to a “coincidence detection” system24 that integrates sensory inputs and motor outputs and facilitates coordinated communication between cerebellar and cerebral structures.
In addition to their critical roles in the timing circuit, deficits in the cerebellum, basal ganglia, and thalamus, and the interconnectivity between these regions have been identified in schizophrenia. Cerebellar volume, symmetry, and function are abnormal in schizophrenia.4,25–29 Cerebellar soft signs have been reported in schizophrenia,30 including medication naïve participants,26,31 suggesting these deficits are features of the underlying disorder rather than effects of medication. Moreover, neurological soft signs predict abnormal white matter development in the cerebellar-thalamic tract in individuals at ultra-high risk for schizophrenia.32 Findings of dopamine-dysregulation and motor dysfunction in schizophrenia also implicate dysfunction in the basal ganglia and its connections.33 In schizophrenia, studies have revealed decreased basal ganglia activation that was associated with both positive symptoms and motor deficits.34 Altered functional connectivity during motor tasks, where decreased posterior putamen activation was associated with decreased thalamic activation, has also been reported.35 During time estimation and frequency discrimination tasks, thalamic and putamen activation was decreased in individuals with schizophrenia, with hypoactivity in thalamic and striatal regions observed with increased task difficulty.36 Finally, disturbances in thalamocortical connectivity have been reported between the motor and sensory regions in individuals with schizophrenia37,38 and those at high risk for the disorder.39
Taken together, there is evidence that sensorimotor synchronization and continuation finger tapping engages discrete, rhythmic timing processes involving cerebellum, basal ganglia, and thalamus13,14,40 and that these same regions are implicated in schizophrenia. However, despite evidence of robust behavioral impairments in schizophrenia on this sensorimotor synchronization task, the neural correlates of these deficits have not been investigated.
The present study utilized a sensorimotor synchronization task to, for the first time, determine the neural correlates of sensorimotor timing deficits in schizophrenia, including the connectivity of the cerebellum to the cerebrum. The following predictions were made: First, that individuals with schizophrenia would exhibit increased tapping variability and a shorter ITI, consistent with previous findings.41 Second, that the task itself would activate M1, cerebellum, basal ganglia, and thalamus, as evidenced by blood-oxygen-level dependent (BOLD) response.13,14 Third, that individuals with schizophrenia would show decreased cerebellar activation compared with the healthy control group, which would further be correlated with their task performance. Fourth, the schizophrenia group would exhibit impairments in effective connectivity during the task within the theorized timing circuit, with hypoconnectivity between the cerebellum and cerebral motor regions and subcortical structures (ie, thalamus and basal ganglia), suggesting temporal dyscoordination. Finally, exploratory analyses examined neural, cognitive, and symptom correlates.
Materials and Methods
Participants
Participants were recruited from local community and inpatient clinics in Bloomington and Indianapolis, IN. All procedures were approved by the Indiana University Institutional Review Board. Written, informed consent was provided by 42 healthy controls and 33 individuals with schizophrenia or schizoaffective disorder (SZ) as diagnosed by DSM-IV. Forty controls and 31 SZ participants were retained for analyses (see table 1 and exclusion information below).
Table 1.
Values for Sex and Ethnicity Reflect Frequency; Values for Age, WASI, and WAIS Represent Mean and SD
| Participant Demographics | ||||
|---|---|---|---|---|
| Healthy (n = 40) | Schizophrenia (n = 31) | Statistics (t or χ2) | P-value | |
| Sex (male/female) | 19/21 | 20/11 | 2.043 | .153 |
| Ethnicity (C/A/H/O) | 33/5/0/2 | 13/15/1/2 | 13.884 | .003 |
| Age (years) | 38.9 (9.4) | 36.7 (10.7) | 1.073 | .340 |
| WASI42 | ||||
| FSIQ | 115.2 (10.9) | 100.3 (16.8) | 4.222 | <.001 |
| Vocabulary | 59.0 (7.7) | 48.9 (12.7) | 3.820 | <.001 |
| Matrix reasoning | 59.5 (12.7) | 51.3 (9.7) | 2.951 | .004 |
| WAIS43 | ||||
| Digit-symbol44 | 12.5 (2.8) | 7.8 (2.6) | 7.122 | <.001 |
Note: WASI, Wechsler Abbreviated Scale of Intelligence; FSIQ, Full Scale Intelligent Quotient; WAIS, Wechsler Adult Intelligence Scale; C, Caucasian; A, African American; H, Hispanic; O, Other. Italics indicate P-values that met a significance threshold of P < 0.05.
Clinical Assessment
Participants were administered Structured Clinical Interviews for DSM-IV Criteria for Axis I and Axis II disorders (SCID-I Patient45 or Non-Patient Version46 and SCID-II47) and other clinical measures to establish the diagnosis and clinical state (supplementary material). A urine drug screen confirmed that participants were not using illicit substances. Participants completed cognitive measures shown in table 1.
Sensorimotor Synchronization Finger-Tapping Task
Participants underwent three consecutive sessions of a 6-minute functional magnetic resonance imaging (fMRI) scan during a sensorimotor synchronization finger-tapping task, including 6 blocks of the following sequence: 6-second synchronization tapping, 20-second continuation tapping, 6-second listen, and 15-second rest periods. Participants tapped with their right index finger (SZ: 7 left-handed, 2 ambidextrous; HC: 2 left-handed, 3 ambidextrous) on a handheld tapping pad with a 1.5 cm diameter force sensor. Handedness did not impact behavioral or neuroimaging findings (supplementary material).
Behavioral Analyses
Mean and variability of consecutive tapping intervals, and tapping force were analyzed. Variability was analyzed as the coefficient of variation (ie, SD divided by the mean within subjects). Participants with at least 8 synchronization ITIs and 30 continuation ITIs were included in the analysis to ensure adequate statistical power. Accordingly, 2 SZ and 18 controls were excluded, for a final sample of 31 SZ and 24 controls for these analyses.
Wing–Kristofferson48 (W-K; cf,41) analysis was performed to parse clock timing variance and motor execution variance from total behavioral tapping variance (supplementary material). A threshold of at least 10 consecutive error-free taps in 10 consecutive trials within the continuation-tapping block resulted in 6 SZ and 8 controls being excluded from this analysis.
Magnetic Resonance Imaging (MRI) Acquisition
Data acquisition was carried out on a Siemens 3T Magnetom Trio-Tim Scanner. Functional scans were acquired using a single-shot echo-planar-imaging (SS-EPI) sequence with a 12-channel head coil [repetition time (TR) = 2500 ms; echo time (TE) = 30 ms; 40 transverse slices; slice thickness 3.2 mm; field of view (FOV) = 220 × 220 mm2; imaging matrix = 96 × 96; in-plane voxel size = 2.3 × 2.3 mm2]. T1-weight anatomical scans were acquired with a 32-channel head coil, using an 8-minute magnetization prepared rapid gradient echo (MP-RAGE) sequence [TR = 1800 ms; TE = 2.67 ms; TI = 900 ms; FOV = 256 × 256 mm2; 160 slices in sagittal plane; flip angle 9°; voxel size = 1 × 1 × 1 mm3].
MRI Preprocessing and Analysis
Preprocessing of T1-weighted MRI was performed using FSL toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki). T1-weighted MRIs were normalized to Montreal Neurological Institute (MNI) space, in which the linear transformation was conducted using FLIRT49,50 and nonlinear transformation using FNIRT.51,52 Structural scans were resampled to MNI152 space using FSL (function applywarp). Data preprocessing and analysis of functional data was done with SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12). Preprocessing included slice timing correction, motion correction, coregistration, segmentation, spatial normalization, and spatial smoothing [6 mm full width at half maximum (FWHM)].
First-level, whole-brain analysis followed a block-design general linear model (GLM) including 36 regressors–5 block (eg, synchronization, continuation, rest) and 6 motion parameters for each scan session plus 3 total session parameters. Covariates of age and sex were added to this model because of known effects on the cerebellum and brain size. The output of this model was a BOLD map. Activation was defined as the t-values of this BOLD map. Two main contrasts were computed: Synchronization (Tone-Paced Tapping minus Rest) and Continuation (Self-Paced Tapping minus Rest). An additional 2 post hoc contrasts were also computed (supplementary material). Generated contrast maps were used to compute 2-sample t-tests (control vs SZ) for second level analyses. Motion was assessed using methods described by Power and colleagues53; a threshold of frame-wise displacement of 2 mm in 10% or more scans was set, and 2 subjects per group were excluded for a final sample of 31 SZ and 40 controls.
Contrast weights, or beta weights from the continuation contrast, were extracted for individual subjects in regions of interest (ROIs) defined by a 3 mm sphere around maximum peak voxels of interest (VOIs) in cerebellar lobule V, thalamus, putamen, and M1 (coordinates in table 3). ROIs were selected based on (1) previous findings and (2) theoretical models of timing and sensorimotor synchronization. Specifically, data suggest the critical role of the cerebellum in generating self-paced, subsecond timing cues17–20 and M1 serves as a primary generator of the output response.13,14 Crucial for the synchronization of these cues is the thalamus, through which cerebellum and M1 are anatomically connected.16,23,24 Finally, putamen serves as a relay station within this circuit to modulate thalamic signals.11,13,54,55 Relevance of these structures in the schizophrenia literature4,26,33,36,38,39,56–58 and observed activations from the whole brain analysis further confirmed the suitability of these ROIs for these analyses. Extracted weights were used in correlational and path analyses, performed using SPSS (24.0, IBM Corporation) and AMOS (23.0, IBM Corporation).
Table 3.
Whole Brain Analysis Activation Coordinates and Cluster Size
| Healthy Control (N = 40) | Schizophrenia Spectrum (N = 31) | |||||||
|---|---|---|---|---|---|---|---|---|
| x a | y | z | k E b | x a | y | z | k E b | |
| Synchronization | ||||||||
| Primary motor cortex | −32 | −26 | 56 | 862 | −32 | −30 | 58 | 508 |
| Supplementary motor area | −6 | −2 | 54 | 84 | ||||
| Premotor cortex | −58 | 6 | 30 | 26 | ||||
| Auditory cortex | −44 | −28 | 8 | 58 | ||||
| Occipital cortex | −32 | −88 | −10 | 30 | ||||
| 38 | −84 | −8 | 61 | |||||
| Striatum | −24 | 10 | −6 | 102 | ||||
| Continuation | ||||||||
| Primary motor cortex | −34 | −26 | 56 | 4163 | −40 | −22 | 46 | 2112 |
| Finger | 60 | 8 | 20 | 100 | ||||
| Supplementary motor area | −4 | −8 | 50 | 431 | ||||
| 4 | 0 | 64 | 725 | |||||
| Premotor cortex | −10 | −22 | 48 | 47 | −56 | 4 | 26 | 156 |
| 66 | −30 | 18 | 69 | 60 | −30 | 20 | 47 | |
| 54 | −2 | 46 | 160 | 54 | 2 | 44 | 113 | |
| 56 | 8 | 24 | 88 | |||||
| Somatosensory | −50 | −24 | 18 | 703 | ||||
| 56 | −16 | 20 | 22 | 54 | −18 | 20 | 59 | |
| Thalamus | −14 | −20 | 2 | — | −14 | −20 | 6 | 394 |
| Putamen | 22 | 6 | 0 | 202 | 24 | 0 | 0 | 103 |
| Cerebellum | −28 | −60 | −22 | 128 | −24 | −58 | −20 | 49 |
| 12 | −52 | −18 | 1965 | 18 | −52 | −18 | 1300 | |
| 12 | −64 | −46 | 392 | |||||
| Insula | −28 | 24 | 10 | 34 | −40 | 0 | 2 | 1135 |
| 48 | 12 | 0 | 453 | 40 | 2 | 8 | 203 | |
| Occipital cortex | −16 | −98 | −2 | 377 | ||||
| 26 | −96 | 10 | 334 | 32 | −92 | 8 | 80 | |
| 10 | −88 | −10 | 44 | 16 | −88 | −16 | 63 | |
| Cingulate | 6 | 18 | 30 | 33 | ||||
Note: Bold coordinate values indicate extracted voxel of interest (VOI) coordinates for path analysis; SMA, supplementary motor area.
a x-Coordinate differentiates the left and right hemispheres of the brain, with positive values indicating the right (ipsilateral to the tapping hand) hemisphere and negative values indicating left (contralateral to the tapping hand) hemisphere.
b K E is the cluster size, or number of voxels contributing to the area of activation meeting FWE P <.05 threshold criteria.
Effective Connectivity Models, Analyses, and Correlations
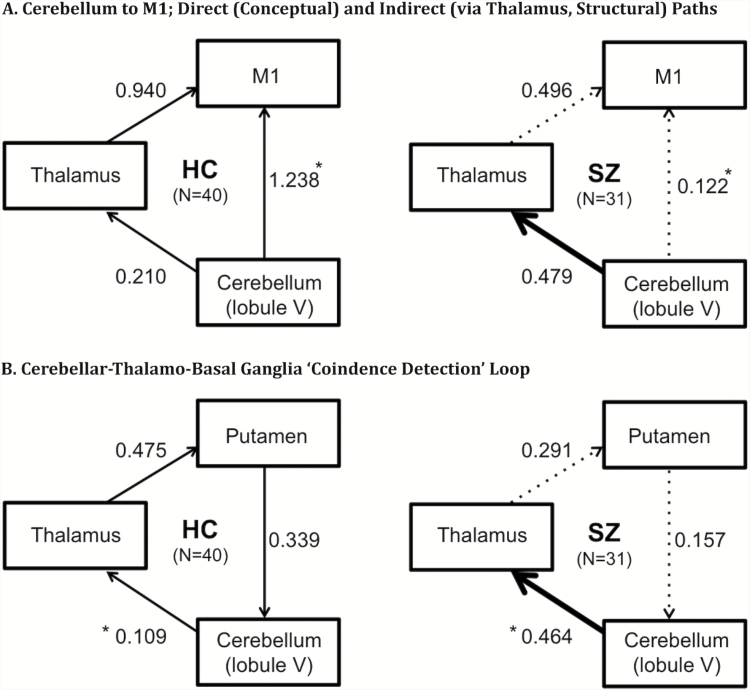
Extracted continuation contrast weights, as defined above, were used to assess effective connectivity. Effective connectivity was defined, according to Lindquist,59 as the change in activity in one ROI as influenced by another ROI, averaged across a given time interval. Though these models indicate a causal relationship between these ROIs, the directionality is determined a priori and is not derived from the data itself. Individual subjects’ beta weights from these ROIs were input into a predefined model (figure 2). Multigroup path analysis was used to generate path coefficients, or “connectivity,” between ROIs of the tested models and to compare models between groups. Two models were evaluated: a conceptual, causal model of covariation between (A) cerebellum, M1, and thalamus and (B) cerebellum, thalamus, and putamen. The directionality of model A was determined based on the functional conceptualization of the cerebellum as an oscillatory pacemaker, which prompts M1 with timing cues during continuation tapping.11,13,18,60 This model allowed us to test the hypothesis that cerebellar activation during continuation tapping would be more closely related to the intended target of the timing signal (M1) than to the relay node (thalamus), for these signals as they travel to cortex61 in healthy compared to schizophrenia participants. Model B allowed us to test the hypothesis that in healthy controls compared to the schizophrenia group cerebellar activation during continuation tapping would be more closely related to activation within the “coincidence detection loop,” thalamus and putamen, hypothesized to regulate the integration of motor and clock processes and facilitate error detection.13,14,60,62 Exploratory correlational analyses were conducted between the following 6 variables given their centrality to the critical constructs of interest in this article: cerebellar activation; tapping variability and force; Digit-symbol as a measure of visuomotor coordination and processing speed (ie, Digit-symbol task); and the PANSS standard negative subscore and disorganized factor score63 to assess severity of psychopathology.
Fig. 2.
Unstandardized path coefficients for 2 models of interest (thick arrow = increased effective connectivity in SZ [schizophrenia spectrum; right side] compared to HC [healthy control; left side]; dotted arrow = decreased effective connectivity in SZ compared to HC). HC, healthy control; SZ, schizophrenia spectrum. *Significant group difference for indicated path.
Results
Behavioral Findings
Individuals with schizophrenia exhibited shorter ITIs [t(46.396) = 2.151, P = .037, d = 0.562] and more variable tapping [t(53) = −2.414, P = .019, d = 0.655] during continuation only compared with controls (table 2). The schizophrenia group tapped with greater force in the synchronization [t(52) = −3.667, P < .005, d = 0.972] and continuation [t(51.518) = −3.720, P < .005, d = 0.985) portions of the task. No group differences were observed in variability or coefficient of variation of tapping force (table 2).
Table 2.
Behavioral Analyses
| ITI | Mean (ms) | CV | ||
| Synchronization | Continuation a | Synchronization | Continuation a | |
| HC (N = 24) | 478.41 (26.77) | 509.22 (26.85) | 0.1036 (0.034) | 0.080 (0.046) |
| SZ (N = 31) | 476.09 (37.10) | 485.50 (53.29) | 0.1173 (0.042) | 0.110 (0.045) |
| Force | Mean | CV | ||
| Synchronization a | Continuation a | Synchronization | Continuation | |
| HC (N = 24) | 703.3 (357.7) | 666.2 (355.9) | 0.481 (0.148) | 0.487 (0.142) |
| SZ (N = 31) | 1146.4 (536.0) | 1123.0 (551.3) | 0.500 (0.197) | 0.462 (0.162) |
| W-K | Mean | |||
| Motor a | Clock b | Total a | Ratio (motor/clock) | |
| HC (N = 16) | 1359.9 (783.1) | 4215.6 (2457.1) | 5575.5 (3239.7) | 0.756 |
| SZ (N = 25) | 2179.0 (1468.1) | 6671.9 (4450.2) | 8850.9 (5917.9) | 0.754 |
Note: Coefficient of variation (CV) = standard deviation/mean; ITI, intertap interval; W-K, Wing-Kristofferson; HC, healthy control; SZ, schizophrenia spectrum.
aSignificant difference between groups.
bTrending difference between groups.
Increased total tapping variance was observed in schizophrenia compared with controls [t(39) = −2.022, P = .050]. When total variance was decomposed into clock and motor variance using the W-K model the schizophrenia group had significantly higher motor variance [t(39) = −2.321, P = .026, d = 0.696) and a trend toward higher clock variance [t(39) = −2.014, P = .051, d = 0.683] compared with controls. Clock variance was higher than motor variance for both groups; the ratio of clock to motor variance did not statistically differ between diagnostic groups, with clock variance accounting for approximately 75% of the total (table 2).
Functional MRI Findings
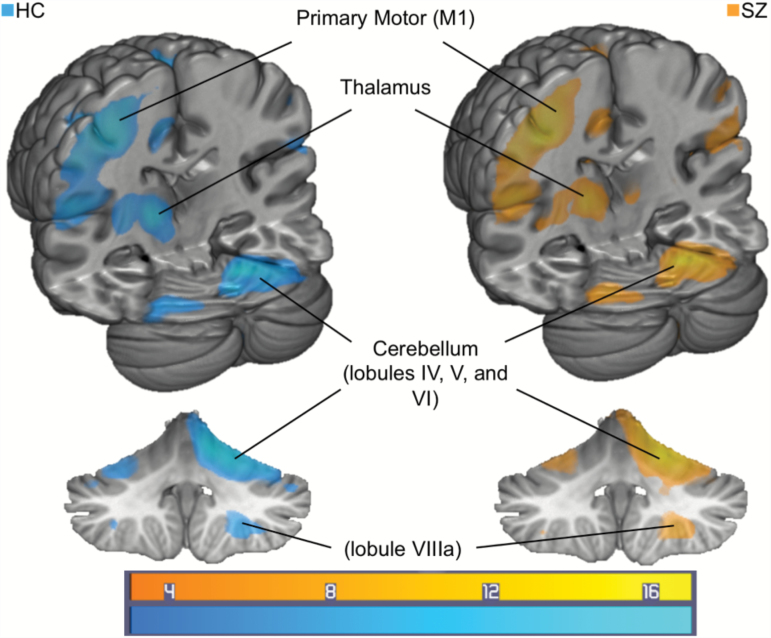
Whole brain analyses were corrected to family-wise error (FWE) rate P <.05 with an extent threshold of 20 voxels (2 × 2 × 2 mm3). Whole brain analysis revealed significant BOLD activation of M1 during the synchronization compared to rest portion of the task (figure 1; table 3). During continuation, the cerebellum, thalamus, supplementary motor area,27 putamen, and inferior parietal cortex were also significantly activated compared to rest periods. Significant activation in the cerebellum was observed in lobules identified in both “motor” (IV, V, and VIIIa) and “cognitive” (VI) cerebellar regions (cf,40,64). No group differences were found between SZ and HC regarding whole-brain activation.
Fig. 1.
Task activation for a contrast of continuation tapping minus rest periods for HC (left, N = 40) and SZ (right, N = 31). Color bar represents t-value for activation (FWE corrected P < .05, extent threshold 20 voxels (2 × 2 × 2 mm3). FWE, family-wise error; HC, healthy control; SZ, schizophrenia spectrum.
Effective Connectivity
In the first path analysis model (figure 2A) evaluating connections between the cerebellum and M1 directly or through the thalamus, the path fit between the cerebellum and M1 was significantly decreased in the SZ group (χ2 = 7.262, P = .007). There were no significant differences in cerebellum–thalamus or thalamus–M1 paths between groups (figure 2A). A model of the groups in which the nonsignificant cerebellum–thalamus and thalamus–M1 paths are constrained (ie, set equal) between groups exhibits good fit of the data (χ2 = 3.464, P = .177, comparative fit index [CFI] = 0.963, Bollen’s parsimonious fit index [BFI] or incremental index of fit [IFI] = 0.966). The second model (figure 2B) evaluating a loop between cerebellum and thalamus and putamen revealed significantly increased connectivity between cerebellum and thalamus in the schizophrenia group (χ2 = 4.042, P = .044). No significant group differences were observed in thalamus–putamen or putamen–cerebellum paths. Good model fit was observed when nonsignificant thalamus–putamen and putamen–cerebellum paths are constrained between groups (χ2 = 1.280, P = .527, CFI = 1.000, BFI/IFI = 1.020).
Exploratory Correlations of Neural Activation With Cognitive and Symptom Measures
In the schizophrenia sample, PANSS negative symptom scores were inversely correlated with tapping force during synchronization (r = −.454, P = .010) and continuation (r = −.434, P = .015). PANSS disorganized factor scores were inversely correlated with continuation tapping force (r = −.385, P = .043). Digit-symbol scores negatively correlated with cerebellar activation (continuation-rest contrast) (r = −.487, P = .022) in the nonpatient control group only.
Discussion
The present study was the first to examine the cerebellum’s role as a temporal pacemaker driving M1-generated motor behavior during tapping continuation of a cerebellar-dependent sensorimotor synchronization task, using effective connectivity analysis in persons with schizophrenia spectrum disorders. As predicted, SZ was associated with aberrant connectivity in the cerebellar-thalamocortical and cerebellar-thalamo-basal ganglia loops. Moreover, individuals with schizophrenia exhibited temporal dysfunction indicated by faster and more variable tapping. Contrary to predictions, no differences in whole-brain activation during the task were observed, suggesting aberrant functional connectivity as a source of the group differences in sensorimotor timing.
Consistent with the only other previous report of sensorimotor synchronization in schizophrenia,41 impaired timing was observed in SZ. Specifically, shorter and more variable ITIs were observed during the continuation phase (table 2). These findings are consistent with suggestions that individuals with SZ have a sped up internal clock, which has been associated with hyper-dopaminergic states.65 Moreover, timing deficits in schizophrenia may be associated with, and contribute to, widely observed impairments of learning, memory, and perception in the disorder.66 For example, a meta-analysis showed that select neural regions—putamen, inferior parietal cortex, insula—were activated during both interval timing and cognitive (ie, working memory, executive functioning) tasks.67 Finally, timing deficits have been associated with errors in misattribution, self-monitoring, and top-down processing, leading to hypotheses that timing deficits may underlie hallucinations.68
The major new finding from the current study was that individuals with schizophrenia showed aberrant effective connectivity between cerebellum and M1 (figure 2A) during task performance compared with controls. In this statistical path model, allowing cerebellum to functionally co-vary with M1 and thalamus, it is interesting that functional covariation in controls shows increased cerebellum–M1 association compared with cerebellum–thalamus given that anatomically these regions are connected via thalamus. Assuming cerebellum provides a pacemaker signal, the functional co-variation should be highest between cerebellum (source of temporal coordination signal) and M1 (source of motor tapping execution), where activation may be more relevant to cerebellar oscillations than modulatory processes occurring elsewhere in the circuit. Aberrations in this path in the SZ group point to impairments synchronizing the cerebellar-generated pacemaker signal and motor output processed in M1 (evidenced by a weaker cerebellum–M1 relationship in SZ), thereby producing inaccurately timed behavioral responses. Alternatively, the cerebellum may be sending improper timing signals to M1 via the thalamus. Accordingly, a second possible explanation is that connectivity deficits within the basal ganglia (figure 2B) are structurally hindering cerebello-cerebral associations (evident from weaker thalamus–putamen and putamen–cerebellum connections in schizophrenia), disrupting coincidence detection. Increased activation in the SZ group between cerebellum and thalamus (figure 2B) may represent an attempt to compensate for the failure of the thalamus to engage in other regulatory processes, thereby increasing its association with the cerebellum during this active, highly cerebellar task.
Broadly, these findings are consistent with the theory that in SZ the cerebellum and its associated temporal processing regions (ie, basal ganglia) fail to play their usual coordinative roles within the CCTCC. These results complement recent findings of aberrant cerebello-cortical and cortico-striatal connectivity in schizophrenia. For example, abnormalities in cerebellar-cortical connectivity were recently found in clinical high-risk and early schizophrenia groups,39,69 unaffected siblings,70 and individuals with schizophrenia during a working memory task.71 Further, disrupted cerebellar-thalamic functional connectivity and fractional anisotropy relationships in schizophrenia are associated with decreased cerebellar-cortical function connectivity and integrity.72 Moreover, abnormal resting-state functional connectivity between the cerebellum and motor cortex in schizophrenia was recently linked to abnormal spontaneous motor activity,73 which together with the present findings may suggest a mechanism underpinning increased motor variability observed during sensorimotor synchronization tasks.
The fact that the schizophrenia and control groups did not differ in whole-brain BOLD activation suggests that it is not the nodes in the network that are impaired per se; rather it is the connectivity between those nodes that are impaired during task performance. For example, as predicted, whole-brain analysis exhibited significant activation of M1 during synchronization in both groups as well as activation of M1, the cerebellum, thalamus, putamen, and other regions (figure 1, table 3) known to be associated with motor timing.11–13,42,74 Activation of these regions during continuation tapping suggests that substantial cross-region communication is necessary for integrating the tapping “motor” component (ie, M1 identified during synchronization) and the timing “clock” component (ie, recruitment of cerebellum, thalamus, and basal ganglia during continuation) of the task. However, as shown by Kim and colleagues,28 schizophrenia is associated with a disruption of the modular architecture of the cerebellum, which could result in unusual patterns of cerebello-cerebral connectivity. Taken together, this suggests aberrant organization and connectivity between cerebellum and cerebrum in schizophrenia, rather than impaired cerebellar functional integrity alone, during sensorimotor synchronization.
Increased tapping force in the SZ group may indicate deficits in sensorimotor feedback (ie, decreased integration of proprioceptive input) or differences in mechanical tap execution in the schizophrenia group, both of which may suggest further impairment within the CCTCC. A negative correlation, accounting for 21% of the variance between tapping force and negative symptoms assessed by the PANSS, points to a testable hypothesis for future studies. Specifically, decreased motor engagement (ie, force) in this task is associated with increased negative symptoms. In fact, motor dysfunction in the form of akinesia is significantly positively correlated with negative symptoms in schizophrenia,67 including drug-naïve patients.43,44 Likewise, force in the continuation condition was correlated with PANSS disorganized symptom factor scores. Such relationships between motor75 or sensorimotor76 abnormalities and PANSS disorganized factor scores have been previously observed in schizophrenia patients. Moreover, though not observed in the current study, it has been shown that accelerated tapping is negatively correlated with PANSS negative symptoms.15 Interestingly, studies have observed a positive correlation between negative symptoms and increased M1-striatal connectivity.77 Altogether, these findings underscore the association of motor aberrations and symptomology in schizophrenia.
Other exploratory analyses indicated that cerebellar activation was positively correlated with Digit-symbol performance in the HC group only (accounting for 24% of shared variance in controls vs 7% in SZ). Due to their exploratory nature, these correlations should be interpreted with caution, but suggest the testable hypotheses that the cerebellum plays a coordinative role in visuomotor performance, which is impaired in SZ.78
There are limitations and potential confounds that warrant consideration and further study. First, the use of antipsychotic medications, most of which alter dopamine signaling, in the schizophrenia group may have affected the current findings. It is well-established that increases in dopamine, particularly within basal ganglia, can alter movement and timing.13 However, any anticipated effects of dopamine blockers would have been to slow the “internal clock,”79,80 rather than accelerate it; thus, the present study may have underestimated clock speed. Alternatively, antipsychotic use could be responsible for connectivity or downstream neural signaling aberrations within dopamine-rich subcortical structures (cf,81). Medication assessed by chlorpromazine-equivalent doses82 was not correlated with behavioral or neuroimaging measures in the current study (supplementary material). Nonetheless, assessing sensorimotor synchronization in a medication-naïve population of individuals with schizophrenia or unaffected first-degree relatives would be informative. Second, groups significantly differed on cognitive functioning (table 1). Though cognitive deficits are common in schizophrenia, intelligence alone has been shown to account for upwards of 15% of the variance on a sensorimotor synchronization task in healthy individuals.83,84 Differences in IQ may be associated with core differences in timing ability or strategies, which may be reflected in these group differences independent of diagnostic status. Third, the effective connectivity models evaluated in this study were limited by their unidirectionality. Anatomical tract tracing studies indicate that many of these regions are bidirectionally connected; ie, they form closed-loops as opposed to sending information exclusively in one direction.22 Fourth, the correlational analyses, while theoretically guided, were exploratory and must be replicated in subsequent studies. Finally, sample size limited the number of variables that could be included in the path analysis and precluded the use of other analyses (ie, dynamic causal modeling); therefore, it was not possible to study a larger, more realistic functional-anatomical circuit of the behavior. Larger sample sizes would allow more power to detect relationships between multiple brain regions simultaneously, and more extensive examination of symptom correlates.
Conclusions
This is the first study to investigate the potential neural source of sensorimotor synchronization behavioral deficits in a schizophrenia population. As predicted, individuals with schizophrenia exhibited accelerated, more variable timing during sensorimotor synchronization. These deficits appeared to be associated with aberrations in cerebellar-cortical (M1) and cerebellar-subcortical (basal ganglia and thalamus) effective connectivity. These findings are consistent with conceptualizations of schizophrenia as a dysconnectivity syndrome, and they directly implicate the cerebellum in a fundamental coordinative process that has been hypothesized to be a cardinal feature of schizophrenia (cf, Andreasen’s cognitive dysmetria model and Bleuler’s “fragmented phrene”1,3).
Funding
This work was supported by the National Institutes of Health (T32 MH103213 to W.P.H., A.B.M., and J.R.P.; R01 MH074983 to W.P.H.), Indiana Clinical and Translational Sciences Institute award (TL1 TR001107 and UL1 TR001108 to A.B.M. and J.R.P. and R21 MH091774 to B.F.O.), and National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award to A.R.B.
Supplementary Material
Acknowledgments
We wish to thank the patients and their families for participation as well as support from the staff and administration of Larue D. Carter Memorial Hospital. We also thank the IUB Imaging Research Facility staff and Psychological and Brain Sciences technical support group, especially Jeffrey Sturgeon and Alex Shroyer. All authors declare that they have no conflicts of interest.
References
- 1. Bleuler E. Dementia Praecox or the Group of Schizophrenias, translated by J. Zinkin. New York: International Universities Press, Inc.; 1911/1950. [Google Scholar]
- 2. Stransky E. Zur Lehre der dementia praecox. Zbl Nervenheilk. 1904;27:1–19. [Google Scholar]
- 3. Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–920. [DOI] [PubMed] [Google Scholar]
- 4. Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griffa A, Baumann PS, Ferrari C et al. . Characterizing the connectome in schizophrenia with diffusion spectrum imaging. Hum Brain Mapp. 2015;36:354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van den Heuvel MP, Fornito A. Brain networks in schizophrenia. Neuropsychol Rev. 2014;24:32–48. [DOI] [PubMed] [Google Scholar]
- 7. Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–198. [DOI] [PubMed] [Google Scholar]
- 8. Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815. [DOI] [PubMed] [Google Scholar]
- 9. Herculano-Houzel S. Not all brains are made the same: new views on brain scaling in evolution. Brain Behav Evol. 2011;78:22–36. [DOI] [PubMed] [Google Scholar]
- 10. Garvey MA, Cuthbert BN. Developing a motor systems domain for the NIMH RDoC program. Schizophr Bull. 2017;43:935–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rubia K, Smith A. The neural correlates of cognitive time management: a review. Acta Neurobiol Exp (Wars). 2004;64:329–340. [DOI] [PubMed] [Google Scholar]
- 12. Repp BH. Sensorimotor synchronization: a review of the tapping literature. Psychon Bull Rev. 2005;12:969–992. [DOI] [PubMed] [Google Scholar]
- 13. Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. [DOI] [PubMed] [Google Scholar]
- 15. Lošák J, Hüttlová J, Lipová P et al. . Predictive motor timing and the cerebellar vermis in schizophrenia: an fMRI study. Schizophr Bull. 2016;42:1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pelzer EA, Melzer C, Timmermann L, von Cramon DY, Tittgemeyer M. Basal ganglia and cerebellar interconnectivity within the human thalamus. Brain Struct Funct. 2017;222:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawashima R, Okuda J, Umetsu A et al. . Human cerebellum plays an important role in memory-timed finger movement: an fMRI study. J Neurophysiol. 2000;83:1079–1087. [DOI] [PubMed] [Google Scholar]
- 18. Del Olmo MF, Cheeran B, Koch G, Rothwell JC. Role of the cerebellum in externally paced rhythmic finger movements. J Neurophysiol. 2007;98:145–152. [DOI] [PubMed] [Google Scholar]
- 19. Molinari M, Leggio MG, Thaut MH. The cerebellum and neural networks for rhythmic sensorimotor synchronization in the human brain. Cerebellum. 2007;6:18–23. [DOI] [PubMed] [Google Scholar]
- 20. Thaut MH, Stephan KM, Wunderlich G et al. . Distinct cortico-cerebellar activations in rhythmic auditory motor synchronization. Cortex. 2009;45:44–53. [DOI] [PubMed] [Google Scholar]
- 21. Valera EM, Spencer RM, Zeffiro TA et al. . Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dhamala M, Pagnoni G, Wiesenfeld K, Zink CF, Martin M, Berns GS. Neural correlates of the complexity of rhythmic finger tapping. Neuroimage. 2003;20:918–926. [DOI] [PubMed] [Google Scholar]
- 24. Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. [DOI] [PubMed] [Google Scholar]
- 25. Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry. 2004;55:1146–1153. [DOI] [PubMed] [Google Scholar]
- 27. Edwards CR, Newman S, Bismark A et al. . Cerebellum volume and eyeblink conditioning in schizophrenia. Psychiatry Res. 2008;162:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim DJ, Kent JS, Bolbecker AR et al. . Disrupted modular architecture of cerebellum in schizophrenia: a graph theoretic analysis. Schizophr Bull. 2014;40:1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bernard JA, Mittal VA. Dysfunctional activation of the cerebellum in schizophrenia: a functional neuroimaging meta-analysis. Clin Psychol Sci. 2015;3:545–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Picard H, Amado I, Mouchet-Mages S, Olié JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull. 2008;34:155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varambally S, Venkatasubramanian G, Thirthalli J, Janakiramaiah N, Gangadhar BN. Cerebellar and other neurological soft signs in antipsychotic-naïve schizophrenia. Acta Psychiatr Scand. 2006;114:352–356. [DOI] [PubMed] [Google Scholar]
- 32. Mittal VA, Dean DJ, Bernard JA et al. . Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophr Bull. 2014;40:1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perez-Costas E, Melendez-Ferro M, Roberts RC. Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J Neurochem. 2010;113:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bernard JA, Russell CE, Newberry RE, Goen JR, Mittal VA. Patients with schizophrenia show aberrant patterns of basal ganglia activation: evidence from ALE meta-analysis. Neuroimage Clin. 2017;14:450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menon V, Anagnoson RT, Glover GH, Pfefferbaum A. Functional magnetic resonance imaging evidence for disrupted basal ganglia function in schizophrenia. Am J Psychiatry. 2001;158:646–649. [DOI] [PubMed] [Google Scholar]
- 36. Volz HP, Nenadic I, Gaser C, Rammsayer T, Häger F, Sauer H. Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. Neuroreport. 2001;12:313–316. [DOI] [PubMed] [Google Scholar]
- 37. Anticevic A, Cole MW, Repovs G et al. . Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24:3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anticevic A, Haut K, Murray JD et al. . Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015;72:882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59:1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009;71:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage. 2008;42:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bermanzohn PC, Siris SG. Akinesia: a syndrome common to parkinsonism, retarded depression, and negative symptoms of schizophrenia. Compr Psychiatry. 1992;33:221–232. [DOI] [PubMed] [Google Scholar]
- 44. Peralta V, Campos MS, De Jalón EG, Cuesta MJ. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov Disord. 2010;25:1068–1076. [DOI] [PubMed] [Google Scholar]
- 45. First M, Spitzer R, Gibbon M, Williams J.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York: Biometrics Research; 2002. [Google Scholar]
- 46. Spitzer M, Robert L, Gibbon M, Williams J.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 47. First MB, Benjamin LS, Gibbon M, Spitzer RL, Williams JBW.. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- 48. Wing AM, Kristofferson AB. Response delays and the timing of discrete motor responses. Atten Percept Psychophys. 1973;14:5–12. [Google Scholar]
- 49. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 50. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. [DOI] [PubMed] [Google Scholar]
- 51. Andersson JLR, Jenkinson M, Smith S.. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. Oxford: FMRIB Analysis Group of the University of Oxford; 2007:2. [Google Scholar]
- 52. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 53. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010;20:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Andreasen NC, Arndt S, Swayze V II et al. . Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. [DOI] [PubMed] [Google Scholar]
- 57. Bernard JA, Orr JM, Mittal VA. Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. Neuroimage Clin. 2017;14:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol. 2009;117:347–368. [DOI] [PubMed] [Google Scholar]
- 59. Lindquist MA. The statistical analysis of fMRI data. Stat Sci. 2008;23:439–464. [Google Scholar]
- 60. Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philos Trans R Soc Lond B Biol Sci. 2009;364:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Res Cogn Brain Res. 2004;21:139–170. [DOI] [PubMed] [Google Scholar]
- 63. van der Gaag M, Hoffman T, Remijsen M et al. . The five-factor model of the positive and negative syndrome scale II: a ten-fold cross-validation of a revised model. Schizophr Res. 2006;85:280–287. [DOI] [PubMed] [Google Scholar]
- 64. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Allman MJ, Meck WH. Pathophysiological distortions in time perception and timed performance. Brain. 2012;135:656–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gómez J, Jesús Marín-Méndez J, Molero P, Atakan Z, Ortuño F. Time perception networks and cognition in schizophrenia: a review and a proposal. Psychiatry Res. 2014;220:737–744. [DOI] [PubMed] [Google Scholar]
- 67. Radua J, Del Pozo NO, Gómez J, Guillen-Grima F, Ortuño F. Meta-analysis of functional neuroimaging studies indicates that an increase of cognitive difficulty during executive tasks engages brain regions associated with time perception. Neuropsychologia. 2014;58:14–22. [DOI] [PubMed] [Google Scholar]
- 68. Waters F. Time perception and discrimination in individuals suffering from hallucinations. In: Jardri R et al., ed. The neuroscience of hallucinations. New York, NY: Springer; 2013: Chapter 11. [Google Scholar]
- 69. Du Y, Fryer SL, Fu Z et al. . Dynamic functional connectivity impairments in early schizophrenia and clinical high-risk for psychosis (published online ahead of print October 14 2017). Neuroimage. 2017. doi: 10.1016/j.neuroimage.2017.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Collin G, Hulshoff Pol HE, Haijma SV, Cahn W, Kahn RS, van den Heuvel MP. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry. 2011;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Meyer-Lindenberg A, Poline JB, Kohn PD et al. . Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–1817. [DOI] [PubMed] [Google Scholar]
- 72. Liu H, Fan G, Xu K, Wang F. Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: a combined resting-state functional MRI and diffusion tensor imaging study. J Magn Reson Imaging. 2011;34:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull. 2017;43:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Repp BH, Su YH. Sensorimotor synchronization: a review of recent research (2006–2012). Psychon Bull Rev. 2013;20:403–452. [DOI] [PubMed] [Google Scholar]
- 75. Walther S, Ramseyer F, Horn H, Strik W, Tschacher W. Less structured movement patterns predict severity of positive syndrome, excitement, and disorganization. Schizophr Bull. 2014;40:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Giersch A, Wilquin H, Capa RL, Delevoye-Turrell YN. Combined visual and motor disorganization in patients with schizophrenia. Front Psychol. 2013;4:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bernard JA, Goen JRM, Maldonado T. A case for motor network contributions to schizophrenia symptoms: evidence from resting-state connectivity. Hum Brain Mapp. 2017;38:4535–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. [DOI] [PubMed] [Google Scholar]
- 79. Rammsayer TH. On dopaminergic modulation of temporal information processing. Biol Psychol. 1993;36:209–222. [DOI] [PubMed] [Google Scholar]
- 80. MacDonald CJ, Meck WH. Differential effects of clozapine and haloperidol on interval timing in the supraseconds range. Psychopharmacology (Berl). 2005;182:232–244. [DOI] [PubMed] [Google Scholar]
- 81. Hutcheson NL, Sreenivasan KR, Deshpande G et al. . Effective connectivity during episodic memory retrieval in schizophrenia participants before and after antipsychotic medication. Hum Brain Mapp. 2015;36:1442–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Holm L, Ullén F, Madison G. Intelligence and temporal accuracy of behaviour: unique and shared associations with reaction time and motor timing. Exp Brain Res. 2011;214:175–183. [DOI] [PubMed] [Google Scholar]
- 84. Madison G, Forsman L, Blom Ö, Karabanov A, Ullén F. Correlations between intelligence and components of serial timing variability. Intelligence. 2009;37:68–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.