Abstract
Inhibitory failure represents a core dysfunction in patients with schizophrenia (SP), which has predominantly been tested in the literature using reactive (ie, altering behavior after a stimulus) rather than proactive (ie, purposefully changing behavior before a stimulus) response inhibition tasks. The current study replicates/extends our previous findings of SP exhibiting sensorimotor cortex (SMC) hyperactivity and connectivity abnormalities in independent samples of patients and controls. Specifically, 49 clinically well-characterized SP and 54 matched healthy controls (HC) performed a proactive response inhibition task while undergoing functional magnetic resonance imaging and resting-state data collection. Results indicated that the majority of SP (84%) and HC (88%) successfully inhibited all overt motor responses following a cue, eliminating behavioral confounds frequently present in this population. Observations of left SMC hyperactivity during proactive response inhibition, reduced cortical connectivity with left SMC, and increased connectivity between left SMC and ventrolateral thalamus were replicated for SP relative to HC in the current study. Similarly, negative symptoms (eg, motor retardation) were again associated with SMC functional and connectivity abnormalities. In contrast, findings of a negative blood oxygenation level-dependent response in the SMC of HC did not replicate. Collectively, current and previous findings suggest that SMC connectivity abnormalities may be more robust relative to evoked hemodynamic signals during proactive response inhibition. In addition, there is strong support that these SMC abnormalities are a key component of SP pathology, along with dysfunction within other sensory cortices, and may be associated with certain clinical deficits such as negative symptoms.
Keywords: connectivity, fMRI, motor, response inhibition
Introduction
Inhibitory deficits represent a core dysfunction in patients with schizophrenia (SP), and have been characterized through a variety of cognitive tasks1–3 as well as by postmortem studies (ie, reduced dendritic GABAergic interneuron projections in lateral prefrontal cortex [PFC]).4,5 Previous studies have reported reactive response inhibition deficits in SP on classic tasks such as stop-signal and go/no go, in which inhibition occurs on a trial-by-trial basis following the presentation of a stimulus.1,3,6 In contrast, fewer studies have explored deficits in proactive response inhibition in SP, in which motor programs are inhibited in a planned and purposeful manner such as occurs following a cue.2 Recent findings suggest that proactive processes may be more impaired in SP than reactive processes in other cognitive control domains,7–9 potentially due to reliance on different neural networks.
Specifically, several studies suggest that the motor network and/or posterior parietal lobes may serve as a potential epicenter of proactive inhibitory deficits in SP2,10,11 rather than lateral PFC regions observed in reactive inhibitory tasks1,6,12,13 or proactive cognitive control tasks with high working memory demands.7,8 During classic reactive response inhibition tasks, a positive hemodynamic response is typically observed in the presupplementary motor area (pre-SMA), with individual task requirements and complexity (typically attention or working memory) driving activity in lateral prefrontal/posterior parietal areas.14–17 Proactive response inhibition tasks elicit a similar positive blood oxygenation level-dependent (BOLD) response in prefrontal and parietal cortex in conjunction with a negative or suppressed BOLD response in sensorimotor cortices (SMC).2,9,18 Preclinical studies have linked the negative BOLD response results with neuronal inhibition/suppression in deep cortical layers,19–21 suggesting that it can be used as an in vivo marker of neuronal inhibition.
Increased motor evoked potentials in SP relative to reduced potentials in healthy controls (HC) have also been observed during repetitive transcranial magnetic stimulation of the premotor cortex,11 with in vivo documentation of impaired intracortical inhibition in both medicated and unmedicated patients.11,22,23 Similarly, opposing patterns of hemodynamic activation (SP = positive BOLD; HC = negative BOLD) have been observed within bilateral premotor cortex/SMC during proactive response inhibition.2 Proactive inhibitory control has been associated with striatal, inferior frontal, and temporoparietal junction abnormalities in SP during a stop-signal task that included an anticipatory component.9 Finally, SP have also been shown to exhibit abnormal SMC connectivity, both intrinsically2,10,24,25 and distally with thalamus and other sensory cortices.2,10,25–35 Thus, establishing that motor network dysfunction underlies proactive inhibitory deficits in SP would allow inhibitory deficits to be reliably assessed and potentially appropriately targeted for treatment.36
To achieve this goal, the current study replicated our prior findings2 of SMC activation deficits underlying proactive inhibition in SP in a new, larger, more representative sample, utilizing new image acquisition and analyses techniques. A proactive response inhibition task was selected to both reduce trial-by-trial response uncertainty associated with reactive inhibition14 and behavioral confounds (ie, performance deficits) associated with more difficult tasks.37 We predicted null differences in task performance between groups in conjunction with hyperactivation of the SMC for SP relative to HC. Second, we predicted that SP would exhibit reduced connectivity during rest within the SMC network relative to HC and that measures of motor retardation/negative symptoms would account for a significant amount of variance in both SMC activity and reduced connectivity.
Methods
Participants
Sixty-seven clinically stable SP and 59 HC between the ages of 18 and 50 years were enrolled. There was no overlap in participants between our current sample and our previously reported sample.2 All diagnoses were confirmed by a board-certified psychiatrist based on the Structured Clinical Interview for DSM-IV-TR (SCID-II).38 Several individuals (supplementary methods) did not complete all study procedures (8 SP and 3 HC). Others were excluded for poor behavioral performance (8 SP and 2 HC) or excessive head motion (2 SP). The final task sample therefore consisted of 49 SP (32 males; 32.7 ± 8.6 years old) and 54 HC (36 males; 33.1 ± 7.7 years old), with 47 SP (31 males; 32.5 ± 8.6 years old) and 53 HC (36 males; 33.1 ± 7.8 years old) included in resting state analyses (supplementary methods). All participants provided informed consent according to institutional guidelines at the University of New Mexico School of Medicine.
Exclusion criteria consisted of a history of (1) severe neurological incidents or diagnoses (including head injury with greater than 30 min loss of consciousness); (2) developmental disorders (autism or intellectual disability); (3) contraindications for magnetic resonance imaging (MRI; including pregnancy); (4) electroconvulsive therapy within the previous month; and (5) recent history of substance abuse disorders (with the exception of marijuana use—see supplementary analysis). Urine-based drug screens were conducted for all participants, with positive results leading to study exclusion or postponed enrollment. Exclusion criteria for HC were identical with the addition of (1) a history of Axis 1 disorder; (2) a history of substance abuse (except for nicotine); (3) first-degree relative with a diagnosis of a psychotic disorder; and (4) a score of more than 29 on the Beck Depression Inventory (BDI).
Clinical and Neuropsychological Assessments
All participants completed the Wechsler Test of Adult Reading (WTAR) as an estimate of premorbid intelligence. Participants were also administered the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB), the Edinburgh Handedness Inventory (EHI), the UCSD Performance-Based Skills Assessment Brief Version (UPSA-B), the Quality of Life Questionnaire in Schizophrenia 18 (S-QoL 18), the Fagerstrom Test for Nicotine Dependence (FTND), and a questionnaire detailing cigarette, alcohol, and caffeine consumption as well as amount of sleep for the last 24 hours. Additional clinical assessments for SP included the Positive and Negative Syndrome Scale (PANSS), measures of extrapyramidal symptoms, and a review of medical history by a physician. Finally, an olanzapine equivalence measure was calculated to determine current antipsychotic medication dose.39,40 Full details on assessments are presented in supplementary methods.
Task Descriptions
Our multisensory attention/response inhibition task has been previously described.2 Each block began with a multisensory (audio-visual) cue indicating the sensory modality for focused attention (“HEAR” = attend-auditory; “LOOK” = attend-visual; “NONE” = proactive response inhibition; 300 ms duration). After 1000 ms, cues were followed by a string of congruent or incongruent multisensory numeric stimuli (target words = “ONE”, “TWO”, or “THREE”; 300 ms duration) at either low (0.33 Hz; 3 trials per block) or higher (0.83 Hz; 6 trials per block) rates of stimulus frequency over a 6300 ms duration (entire block = 8700 ms). All multisensory stimuli (cues and targets) were presented foveally and binaurally via headphones (head-centered). During the NONE trials (7 blocks of 6 trials each, over 3 imaging runs), participants were instructed to proactively inhibit their motor response to targets while maintaining constant head and eye positioning (visual fixation on a centrally presented cross). Participants in the attend-auditory and attend-visual conditions responded to target numbers with a right-handed button press while ignoring simultaneously presented numbers in the opposite sensory modality (results presented separately). The inter-block interval varied between 3900 and 5740 ms. Relative to the prior version of the task,2 the current version contained no low-frequency NONE trials, a faster rate of stimulus delivery during high-frequency trials (0.83 Hz as opposed to 0.66 Hz), and shorter inter-block intervals to increase design efficiency. For the resting state scan, participants stared at a foveally presented fixation cross for approximately 5 minutes.
MRI and Statistical Analyses
MRI data were collected on a Siemens 3T Tim Trio scanner with a 32-channel head coil (supplementary methods). A high-resolution structural scan was collected with 5-echo multi-echo Magnetization Prepared Rapid Acquisition Gradient Echo T1-weighted (1 mm × 1 mm × 1 mm voxels) images. Functional data were collected with a single-shot, gradient-echo echoplanar pulse sequence with simultaneous multi-slice technology (repetition time [TR] = 460 ms; multiband acceleration factor = 8; 3.02 mm × 3.02 mm × 3.00 mm voxels) for both the proactive response inhibition trials and resting state data. The more rapid TR (460 ms) provided improved modeling of the hemodynamic response function (HRF) relative to our initial article. The first 3 images of each run were eliminated to account for T1 equilibrium effects, resulting 1731 images for the proactive task and 649 images for resting state task. A single band reference image (SBREF) and 2 distortion mapping prescan sequences were also collected for image preprocessing.
For both task and resting state data, anomalous time-series data associated with motion and other artifacts were identified and replaced based on values from the previous and subsequent image using AFNI’s despiking protocol.41 All time-series data were then temporally interpolated to the first slice to account for differences in slice acquisition and spatially registered in 2- and 3-dimensional space to a reference image to reduce the effects of head motion followed by the calculation of mean framewise displacement (FD). Susceptibility-induced field distortion was estimated and corrected using FSL Topup.42,43 Data were converted to standard stereotaxic coordinate space44 using a nonlinear algorithm (AFNI 3dQwarp) and spatially blurred using a 6-mm Gaussian full-width half-maximum filter.
A voxel-wise deconvolution analysis generated a single HRF for each trial-type relative to the baseline state (visual fixation plus gradient noise) based on the first 22.54 seconds poststimulus onset. Separate regressors were modeled for the 6 motion parameters, their derivatives, and error trials to remove variance related to motion or false-positive responses.45 The beta coefficients were divided by the model intercept to get an estimate of percent signal change, which was then summed to get separate estimates of early peak activity (3.68 and 8.28 s) and late peak activity (8.28 and 12.88 s). The current study examined multiple hemodynamic epochs based on previous results indicating both magnitude and HRF shape abnormalities in SP.46–48
Analysis
A priori ROIs were identified for the left and right SMC based on results from our previous study.2 A 2 × 2 [Group (SP vs HC) x Peak (early vs late)] mixed-measures ANCOVA (FD as a covariate) was conducted for each SMC ROI to evaluate our primary hypothesis regarding inhibitory deficits in SP. A whole-brain analysis was also conducted to determine whether any other regions demonstrated group differences. Functional connectivity maps were calculated by first regressing motion parameters, their derivatives, and estimates of physiological noise (eroded white matter and cerebral spinal fluid masks), and then bandpass filtering the data (0.01–0.1 Hz). The a priori SMC cluster served as an empirical seed region (see “Results” section) with resulting correlation coefficients converted to Fisher z-scores. Task (P < .001 and minimum cluster size of 547 µl) and connectivity (P < .001 and minimum cluster size of 793 µl) results were corrected for false positives at P < .05 based on 10000 Monte-Carlo simulations and individual estimates of data smoothness using spherical autocorrelation (AFNI 3dClust).
Multiple regressions examined the relationship between activation in sensorimotor areas and either clinical symptoms (independent variables: PANSS positive/negative symptom scores and PANSS motor retardation) or neuropsychological performance (independent variables: MCCB working memory, MCCB processing speed and declarative memory). Multiple regressions were also used to examine relationships between the 2 observed patterns of connectivity abnormalities (connectivity where HC > SP and where SP > HC) using the same clinical and neuropsychological variables.
Results
Clinical and Behavioral Results
Groups did not differ in age, sex, or handedness (table 1). As expected, HC had significantly higher education (t101 = −3.16 P = .002), premorbid intelligence estimate (t78.8 = −3.06, P = .003), and a trend for less nicotine dependence (U = 1083.5; Z = −1.92; P = .055) relative to SP. In addition, SP exhibited lower scores on working memory (t101 = −4.11, P < .001), processing speed (t101 = −4.31, P < .001), declarative memory (t101 = −5.21, P < .001), overall neuropsychological functioning (t101 = −5.90, P < .001), everyday functioning (t101 = −3.31, P < .001) and quality of life (t86 = −4.41, P < .001). Six (12.2%) patients were unmedicated, 11 (22.4%) were on monotherapy (only one antipsychotic medication), and 32 (65.3%) were on polytherapy (more than 2 types of antipsychotic or a combination of antipsychotic and nonantipsychotic psychotropic medications).
Table 1.
Summary of Participant Measures
| Characteristic | SP (N = 49) | HC (N = 54) | P value | Cohen’s d | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Demographics | ||||||
| Sex (females/males) | 17/32 | 18/36 | .884 | |||
| Age (years) | 32.71 | 8.61 | 33.13 | 7.70 | .797 | −0.05 |
| Handedness (EHI) | 65.21 | 59.05 | 81.09 | 40.74 | .122 | −0.31 |
| Education (years) | 13.35 | 2.59 | 14.87 | 2.30 | .002 | −0.62 |
| FTND | 1.40 | 2.45 | 0.54 | 1.49 | .055 | |
| Functioning | ||||||
| UPSA-B total | 70.23 | 12.34 | 78.12 | 11.80 | <.001 | −0.65 |
| S-QoL 18 | 55.90 | 13.91 | 66.52 | 9.98 | <.001 | −0.88 |
| Neuropsychological functioning | ||||||
| WTAR | 50.56 | 11.40 | 56.36 | 7.09 | .003 | −0.61 |
| MCCB overall | 35.71 | 11.35 | 47.93 | 9.64 | <.001 | −1.16 |
| MCCB working memory | 39.14 | 10.10 | 47.20 | 9.78 | <.001 | −0.81 |
| MCCB processing speed | 41.41 | 11.47 | 50.54 | 10.05 | <.001 | −0.85 |
| MCCB declarative memory | 38.40 | 9.92 | 47.85 | 8.50 | <.001 | −1.02 |
| Clinical (patient only) | ||||||
| Age of onset (years) | 21.40 | 6.42 | ||||
| Illness duration (years) | 11.35 | 8.40 | ||||
| PANSS positive | 14.43 | 5.13 | ||||
| PANSS negative | 13.96 | 4.55 | ||||
| PANSS total | 55.82 | 13.77 | ||||
| Medication symptoms | ||||||
| AIMS | 0.57 | 0.87 | ||||
| BAS | 0.47 | 0.96 | ||||
| SAS | 1.02 | 1.95 | ||||
| Olanzapine equivalent | 13.42 | 10.38 | ||||
Note: SP, patients with schizophrenia; HC, healthy controls; EHI, Edinburgh Handedness Inventory; FTND, Fagerstrom Test for Nicotine Dependence; UPSA-B, UCSD Performance Based Skills Assessment Brief Version; S-QoL 18, Quality of Life Questionnaire in Schizophrenia 18; WTAR, Wechsler Test of Adult Reading; MCCB, Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery; PANSS, Positive and Negative Syndrome Scale; AIMS, Abnormal Involuntary Movements Scale; BAS, Barnes Akathisia Scale; SAS, Simpson Angus Scale. FTND is highly skewed, thus Cohen’s d is not provided.
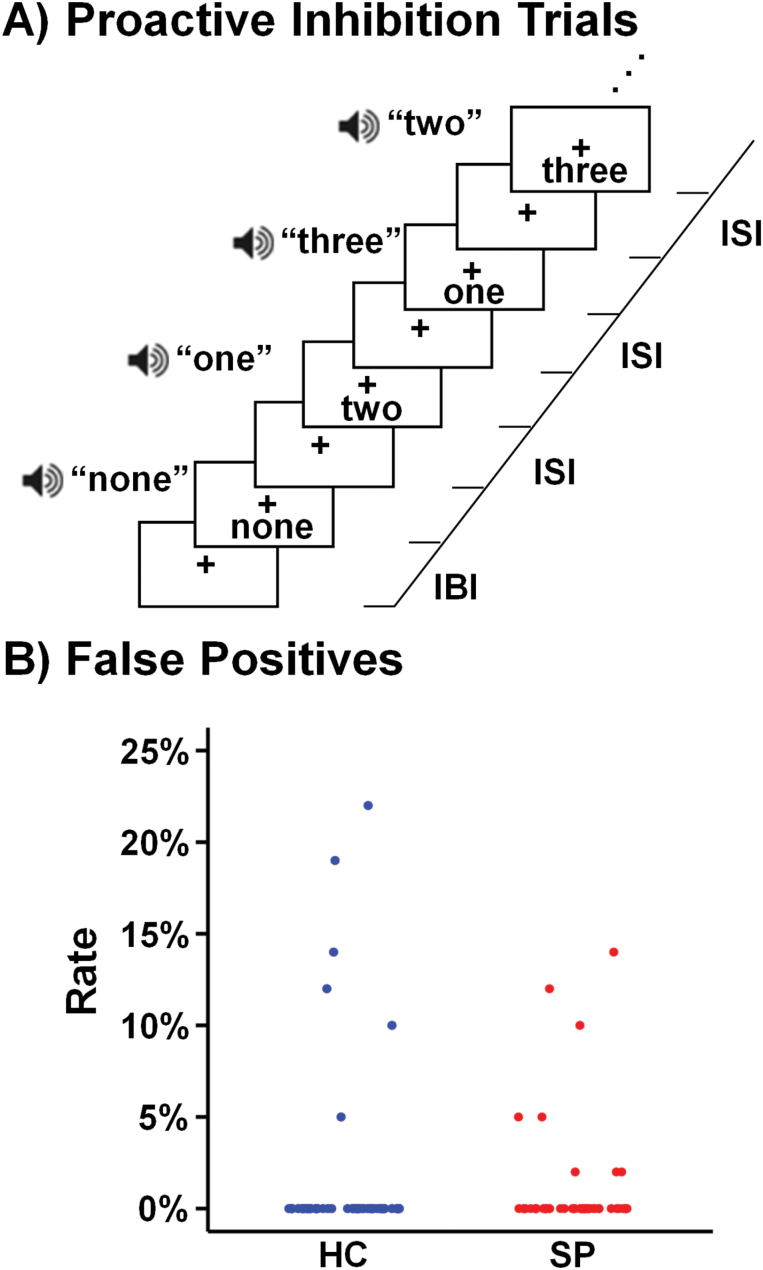
The number of individuals with 100% accuracy in inhibiting all motor trials did not (P = .441) vary across HC (88%; 48/54) and SP (84%; 41/49; figure 1) following exclusions for poor behavioral performance. Among participants who made errors, HC made more false positive errors than SP (U = 8.5, P = .043).
Fig. 1.
Proactive response inhibition trials utilized in the current experiment (A). The right side of each panel indicates the interstimulus interval (ISI) as well as the interblock interval (IBI). The scatterplot (B) presents the percentage of false-positive response rates across all blocks for healthy controls (HC; blue) and patients with schizophrenia (SP; red).
Imaging Results
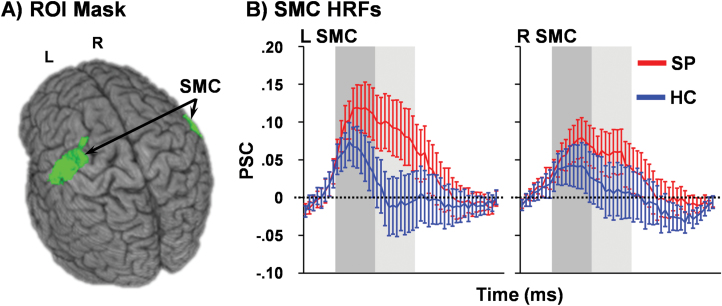
SP exhibited increased head motion (t86 = 3.41, P = .001) during the response inhibition task relative to HC but not during resting state (P = .225). Mean FD was therefore used as a covariate for task-related analyses. One HC was identified as having abnormally high activation (more than 3 times the interquartile range) relative to their cohort in the left SMC and was therefore excluded from that analysis. A significant main effect of Group (F1,99 = 5.26, P = .024; SP > HC) and Group × Peak interaction (F1,99 = 4.14, P = .044) were observed in the left but not right (Group: F1,100 = 1.26, P = .265; Interaction: F1,100 = 2.05, P = .155) SMC (figure 2). Simple effects testing indicated that left SMC hyperactivation was significantly greater (SP > HC) during the late peak phase (P = .016) whereas the early peak activation was only a trend (P = .065). These results did not change when SP and HC without 100% accuracy were excluded from the analyses (supplementary analysis).
Fig. 2.
Left (L) and right (R) sensorimotor cortices (SMC; green) derived from a previous publication2 were used as regions of interest (ROI) for the current analysis (A). In B, percent signal change (PSC) data are presented for patients with schizophrenia (SP; red line) and healthy controls (HC; blue line) for the entire hemodynamic response function (HRF), with shaded bars indicating peak (dark gray) and late peak (light gray) phases. Error bars represent standard error of the mean.
A whole brain voxel-wise 2 × 2 [Group (SP vs HC) × Peak (early vs late)] ANCOVA was not significant following multiple comparisons correction.
Connectivity Results
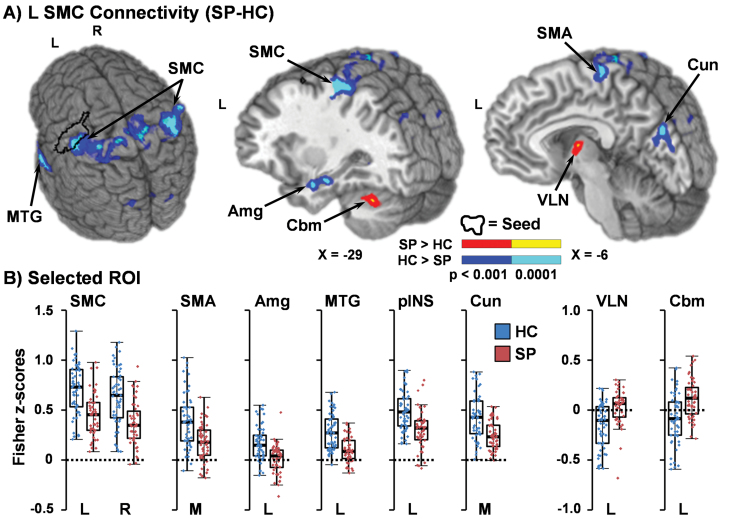
The left SMC ROI was therefore used as an empirical seed for resting-state connectivity analyses. Results indicated widespread differences in functional connectivity, primarily characterized by 2 separate patterns (figure 3). In the first and more predominant pattern, HC demonstrated increased connectivity relative to SP between the left SMC and right (7470 µl; BAs 1/2/3/4/5/6/40) and left (7885 µl; BAs 1/2/3/4/5/40) SMC, bilateral SMA extending into the paracentral lobule (4182 µl; BAs b. 3/4/6, r. 5/7), the left middle and superior temporal gyrus (STG; 3319 µl; BAs 20/21/22/38/41/42), left posterior insula/STG (1019 µl; BAs 13/22/41), right (3294 µl) and left (1485 µl) lingual gyrus (BAs 18/19), bilateral cuneus (4644 µl; BAs b. 18/19), and left amygdala/hippocampus/medial temporal lobe (1007 µl). In the second pattern, SP exhibited a positive correlation (ie, increased connectivity) between the left SMC and left ventral lateral nucleus of the thalamus (1096 µl) and left Lobules VI and VIIa of the cerebellum (854 µl), whereas these regions of motor circuitry were anticorrelated in HC.
Fig. 3.
A depicts results from connectivity analysis performed with the left sensorimotor cortex (SMC; black outline) as an empirically derived seed from the Group × Peak interaction during the proactive response inhibition trials. Regions are color coded based on whether patients with schizophrenia (SP) exhibited either decreased (cool colors: P < .001 = blue; P < .0001 = cyan) or increased (warm colors: P < .001= red; P < .0001 = yellow) connectivity relative to healthy controls (HC). Locations of sagittal (X) slices are given according to the Talairach atlas. Left (L), right (R), and midline (M) regions of interest (ROI) showing decreased functional connectivity for SP included the SMC, supplementary motor area (SMA), amygdala (Amg), middle temporal gyrus (MTG), posterior insula (pINS; not pictured) and cuneus (Cun; B). ROI where SP exhibited increased connectivity included ventral lateral nucleus (VLN) of the thalamus, and Lobules VI/VIIa of the cerebellum (Cbm). All box and scatter plots present Fisher z-scores within selected ROI for HC (blue) and SP (red).
Clinical Relevance of SMC Abnormalities
Two multiple regression analyses investigated the association between hemodynamic activity in the left SMC with either clinical symptoms (PANSS positive, negative, and motor retardation symptom scores) or cognitive performance (MCCB processing speed and working memory domain scores, and declarative memory index) for SP only. Neither the clinical symptoms model (F3,48 = 1.28, P = .294) nor the cognitive performance model (F3,48 = 0.79, P = .505) was significant. However, the degree of negative symptoms was positively associated with the magnitude of SMC activity at a trend level (β = 0.29, t = 1.81, P = .077).
Connectivity coefficients were first averaged to form separate indices for the regions exhibiting either decreased (predominant cortical pattern) or increased (thalamus and Lobules VI and VIIa cerebellum) connectivity abnormalities in SP. Four multiple regression analyses were then conducted to investigate potential associations between decreased or increased connectivity with clinical symptoms or cognitive deficits. Neither cognitive deficits (F3,46 = 0.25, P = .860) nor clinical symptoms (F3,46 = 0.70, P = .559) were associated with the general pattern of decreased connectivity, and cognitive deficits were also not associated with increased connectivity (F3,46 = 0.73, P = .540). However, clinical symptoms were associated with the pattern of increased connectivity abnormalities at a trend level (F3,43 = 2.38, P = .083), with significant variance accounted for by motor retardation (β = 0.39, t = 2.48, P = .017) and a trend association with negative symptoms (β = −0.31, t = −1.96, P = .056).
A binary logistic regression determined whether SMC hyperactivation, SMC to cortical hypoconnectivity or SMC to thalamic/cerebellar hyperconnectivity reliably differentiated SP from HC. Results indicated that SMC to cortical hypoconnectivity was the best predictor of diagnosis (Wald = 8.58, P = .003), with non-negligible predictive value provided by other variables (SMC hyperactivation: Wald = 2.61, P = .106; hyperconnectivity: Wald = 2.52, P = .113). Overall model classification accuracy was high (78.8%), with better prediction of SP diagnosis (83.0%) than HC (75.0%).
Finally, medication-induced motor signs (eg, extrapyramidal, akathisia) and medication load (olanzapine equivalents) were not related to hyperactivation of the left SMC (F4,48 = 0.75, P = .566), nor to abnormal patterns of increased (F4,46 = 0.23, P = .920) or decreased (F4,46 = 0.59, P = .672) connectivity. In addition, SP who tested positive for marijuana at time of data collection also did not significantly influence these findings (supplementary analyses).
Discussion
The “replication crisis” represents a frequently discussed but rarely addressed issue within the behavioral sciences.49 A primary goal of the current study was to replicate our previous findings of proactive inhibition deficits and SMC connectivity abnormalities in an independent, larger sample of SP.2 As expected, the majority of SP and HC successfully inhibited overt motor responses following a cue. We replicated hyperactivation of the left but not right SMC in SP during proactive response inhibition, as well as decreased connectivity between left SMC and other sensory areas (motor, auditory, and visual), and increased connectivity between left SMC and subcortical motor areas (thalamus and cerebellum). In addition, we extended our previous findings to indicate differences in the shape of the left SMC HRF for SP (Group × Peak interaction). However, we were unable to replicate a negative BOLD response within the SMC of HC during our proactive inhibition task. Second, negative symptoms were again associated with both increased SMC-thalamic/cerebellum connectivity during rest and increased activity within left SMC during proactive response inhibition.
The lateralization of motor control to the dominant left hemisphere (regardless of handedness) provides one potential explanation for the more consistent finding of left-lateralized SMC deficits in SP.50–52 Specifically, the left hemisphere is thought to specialize in predictive motor control and the planning and coordinating of motor actions, whereas the right hemisphere is involved in updating ongoing motor actions.50 Thus, the increased positive BOLD response in left SMC in SP during proactive inhibition may reflect an abnormally heightened need for predictive (ie, proactive) inhibitory motor control for both right and left motor neural networks. Evidence of motor inhibition deficits in SP are also present across multiple techniques, as previous repetitive transcranial magnetic stimulation studies of the left premotor cortex reported hyperactivation (ie, increased motor evoked potentials) in SP11 regardless of medication status.23
Sustained hyperactivation in the left SMC during proactive inhibition may result from abnormal connectivity, as dense excitatory and inhibitory cortico-cortico connections exist between the premotor and within the primary motor cortex.53 Both current and previous studies2 in independent samples of SP observed reduced connectivity between SMC and motor (ie, primary motor cortex, SMA, posterior SMC), auditory (ie, STG) and visual (ie, lingual gyrus and cuneus) processing areas relative to HC during rest. Similarly, we also replicated2 findings of increased connectivity between the SMC and core nodes of the motor learning network (thalamus and cerebellum). Importantly, these abnormal SMC connectivity patterns have been reported from multiple independent labs using a variety of different (ie, seed-based, graph, independent component analyses) connectivity methodologies (supplementary table 1). SP spectrum disorders (including risk for psychosis) consistently exhibit aberrant connectivity within the SMC (reduced),10,24,25 between the SMC and sensory cortices (reduced),10,33 as well as between the SMC and thalamus (increased).10,25–35 Although abnormal connectivity may underlie SMC’s hyperactivation during proactive inhibition, it is important to note that current connectivity findings were limited to a resting state rather than task-based scan.
There is also much evidence in the literature for thalamic connectivity deficits (supplementary table 1). The thalamus exhibits hypoconnectivity focally25,30 and with the PFC, striatum, and cerebellum,26–28,30,32 and exhibits hyperconnectivity with auditory, and visual sensory cortices.25,26,28,33,34 Strikingly, 5 separate studies examining SP spectrum disorders and those at risk26–28,30,32 found similar results—increased connectivity between the thalamus and sensory motor areas and decreased connectivity between thalamus and prefrontal and cerebellar areas. Interestingly, abnormal thalamic connectivity has been posited to be caused by arrested brain maturation during adolescence, ceasing normal development of prefrontal-thalamic connections (resulting in hypoconnectivity26–28,30,32), as well as the pruning of somatomotor-thalamic connections (resulting in hyperconnectivity10,25–35).
In current and previous studies, negative symptoms were associated with these motor circuit abnormalities. Specifically, motor retardation was associated with increased SMC-thalamic/cerebellum connectivity during rest, whereas increased negative symptoms rather than motor retardation predict a heightened use of left SMC during proactive inhibition. Previously, motor retardation was related to right and left SMC hyperactivity during proactive response inhibition.2 The relationship between SMC-thalamic hyperconnectivity and increased negative symptoms has been reported previously in other studies as well (supplementary table 1).25,28,29 Importantly, negative symptom severity predicts cognitive performance and may further mediate the relationship between neurocognition and functional outcome.54,55 Our power to detect weaker/more variable relationships between motor circuit connectivity/SMC hyperactivation abnormalities and negative symptoms was limited in both the current and previous studies, an important issue for future studies in validating the importance of this potential biomarker.
Baseline activity was observed rather than a negative hemodynamic response in the SMC of HC.2 Negative BOLD responses are posited to result from inhibitory neural activity in deep cortical layers, and are opposite to the physiology occurring during the positive BOLD response.19,20,56 The lack of negative BOLD replication across current and previous studies could possibly be attributed to differences in task design (ie, fewer trials, faster frequency of target stimulus delivery and shorter inter-block interval), potentially altering the cognitive strategies that were adopted to perform the task. Future research on proactive inhibition studies may benefit from a task that more reliably results in neural inhibition, such as transcallosal inhibition.57 Conversely, the failure in replication could result from random sampling differences, as the current HC cohort exhibited worse performance than SP (ie, a greater number of false positive errors) even though more SP were eliminated from all analyses. Regardless, current and previous studies suggest that connectivity abnormalities (full replication) may be more reproducible than evoked BOLD results using the current task (partial replication).
There are several limitations to the current study. First, similar to resting state data, proactive response inhibition tasks are limited for quantifying task performance (ie, false positive rate only), making it impossible to ensure that participants were actively inhibiting vs passively viewing the stimuli. Second, hyperactivation of the left SMC could possibly result from threshold or subthreshold right-handed motor responses. This is unlikely as the majority of final participants in both groups inhibited all trials, and the remaining HC exhibited increased false positives in conjunction with lower activity. Third, SP exhibited more overall motion than HC. Although FD was used as a covariate for task analyses, we cannot fully rule out the contribution of head motion to observed group differences. Fourth, antipsychotic medications are known to have deleterious side-effects on motor functioning. However, neither current nor past2 task/connectivity findings were related to extrapyramidal side effects or medication dose.
In summary, current findings provide partial replication of our previous study2 and establish SMC abnormalities as important to SP pathology.10 The left SMC is reliably hyperactive during proactive response inhibition in SP, potentially as a heightened need for predictive motor control. Our central observation was replicated: the SMC is involved in proactive inhibition in SP. Thus, we provide important evidence for mechanisms underlying proactive response inhibition. In addition, we noted reduced connectivity within the SMC and between the SMC and other sensory areas, in conjunction with increased connectivity between SMC and subcortical regions of motor circuitry. Collectively, current results support a growing literature of abnormal sensory, motor, and thalamic functional connectivity in SP, which has replicable associations with negative clinical symptoms.
Funding
This work was supported by the National Institutes of Health (1R01MH101512-03 to A.R.M.).
Supplementary Material
Acknowledgments
We would like to thank Diana South and Catherine Smith for their assistance with data collection.
References
- 1. Hughes ME, Fulham WR, Johnston PJ, Michie PT. Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biol Psychol. 2012;89:220–231. [DOI] [PubMed] [Google Scholar]
- 2. Mayer AR, Hanlon FM, Dodd AB, et al. Proactive response inhibition abnormalities in the sensorimotor cortex of patients with schizophrenia. J Psychiatry Neurosci. 2016;41:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thakkar KN, Schall JD, Logan GD, Park S. Response inhibition and response monitoring in a saccadic double-step task in schizophrenia. Brain Cogn. 2015;95:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry. 1999;46:616–626. [DOI] [PubMed] [Google Scholar]
- 5. Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. [DOI] [PubMed] [Google Scholar]
- 6. Kaladjian A, Jeanningros R, Azorin JM, Grimault S, Anton JL, Mazzola-Pomietto P. Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. Schizophr Res. 2007;97:184–193. [DOI] [PubMed] [Google Scholar]
- 7. Edwards BG, Barch DM, Braver TS. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Front Hum Neurosci. 2010;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lesh TA, Westphal AJ, Niendam TA, et al. Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. Neuroimage Clin. 2013;2:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zandbelt BB, van Buuren M, Kahn RS, Vink M. Reduced proactive inhibition in schizophrenia is related to corticostriatal dysfunction and poor working memory. Biol Psychiatry. 2011;70:1151–1158. [DOI] [PubMed] [Google Scholar]
- 10. Kaufmann T, Skåtun KC, Alnæs D, et al. Disintegration of sensorimotor brain networks in schizophrenia. Schizophr Bull. 2015;41:1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oxley T, Fitzgerald PB, Brown TL, de Castella A, Daskalakis ZJ, Kulkarni J. Repetitive transcranial magnetic stimulation reveals abnormal plastic response to premotor cortex stimulation in schizophrenia. Biol Psychiatry. 2004;56:628–633. [DOI] [PubMed] [Google Scholar]
- 12. Nishimura Y, Takizawa R, Muroi M, Marumo K, Kinou M, Kasai K. Prefrontal cortex activity during response inhibition associated with excitement symptoms in schizophrenia. Brain Res. 2011;1370:194–203. [DOI] [PubMed] [Google Scholar]
- 13. Rubia K, Russell T, Bullmore ET, et al. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res. 2001;52:47–55. [DOI] [PubMed] [Google Scholar]
- 14. Criaud M, Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci Biobehav Rev. 2013;37:11–23. [DOI] [PubMed] [Google Scholar]
- 15. Mostofsky SH, Schafer JG, Abrams MT, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Res Cogn Brain Res. 2003;17:419–430. [DOI] [PubMed] [Google Scholar]
- 16. Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of go/no-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. [DOI] [PubMed] [Google Scholar]
- 18. Li JY, Lai PH, Chen R. Transcallosal inhibition in patients with callosal infarction. J Neurophysiol. 2013;109:659–665. [DOI] [PubMed] [Google Scholar]
- 19. Boorman L, Kennerley AJ, Johnston D, et al. Negative blood oxygen level dependence in the rat: a model for investigating the role of suppression in neurovascular coupling. J Neurosci. 2010;30:4285–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Devor A, Trevelyan A, Kleinfeld D. Is there a common origin to surround-inhibition as seen through electrical activity versus hemodynamic changes? Focus on “Duration-dependent response in SI to vibrotactile stimulation in squirrel monkey”. J Neurophysiol. 2007;97:1880–1882. [DOI] [PubMed] [Google Scholar]
- 21. Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. [DOI] [PubMed] [Google Scholar]
- 22. Fitzgerald PB, Brown TL, Daskalakis ZJ, Kulkarni J. A transcranial magnetic stimulation study of inhibitory deficits in the motor cortex in patients with schizophrenia. Psychiatry Res. 2002;114:11–22. [DOI] [PubMed] [Google Scholar]
- 23. Fitzgerald PB, Brown TL, Marston NA, et al. Reduced plastic brain responses in schizophrenia: a transcranial magnetic stimulation study. Schizophr Res. 2004;71:17–26. [DOI] [PubMed] [Google Scholar]
- 24. Sheffield JM, Kandala S, Tamminga CA, et al. Transdiagnostic associations between functional brain network integrity and cognition. JAMA Psychiatry. 2017;74:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernard JA, Goen JRM, Maldonado T. A case for motor network contributions to schizophrenia symptoms: evidence from resting-state connectivity. Hum Brain Mapp. 2017;38:4535–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anticevic A, Cole MW, Repovs G, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24:3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anticevic A, Haut K, Murray JD, et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015;72:882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng W, Palaniyappan L, Li M, et al. Voxel-based, brain-wide association study of aberrant functional connectivity in schizophrenia implicates thalamocortical circuitry. NPJ Schizophr. 2015;1:15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martino M, Magioncalda P, Yu H, et al. Abnormal resting-state connectivity in a substantia nigra-related striato-thalamo-cortical network in a large sample of first-episode drug-naïve patients with schizophrenia. Schizophr Bull. 2017;44:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skatun KC, Kaufmann T, Brandt CL, et al. Thalamo-cortical functional connectivity in schizophrenia and bipolar disorder. Brain Imaging Behav. 2017;12:640–652. [DOI] [PubMed] [Google Scholar]
- 31. Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull. 2017;43:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Damaraju E, Allen EA, Belger A, et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 2014;5:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Çetin MS, Christensen F, Abbott CC, et al. Thalamus and posterior temporal lobe show greater inter-network connectivity at rest and across sensory paradigms in schizophrenia. Neuroimage. 2014;97:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaufmann T, Alnæs D, Brandt CL, et al. Task modulations and clinical manifestations in the brain functional connectome in 1615 fMRI datasets. Neuroimage. 2017;147:243–252. [DOI] [PubMed] [Google Scholar]
- 36. Meyer HC, Bucci DJ. Neural and behavioral mechanisms of proactive and reactive inhibition. Learn Mem. 2016;23:504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497–510. [DOI] [PubMed] [Google Scholar]
- 38. First MB, Spitzer RL, Gibbon M, Williams JB.. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. Arlington, VA: American Psychiatric Pub; 2012. [Google Scholar]
- 39. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. [DOI] [PubMed] [Google Scholar]
- 40. Meltzer HY. Illuminating the molecular basis for some antipsychotic drug-induced metabolic burden. Proc Natl Acad Sci USA. 2007;104:3019–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 42. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20:870–888. [DOI] [PubMed] [Google Scholar]
- 43. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 44. Talairach J, Tournoux P.. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 45. Mayer AR, Teshiba TM, Franco AR, et al. Modeling conflict and error in the medial frontal cortex. Hum Brain Mapp. 2012;33:2843–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hanlon FM, Shaff NA, Dodd AB, et al. Hemodynamic response function abnormalities in schizophrenia during a multisensory detection task. Hum Brain Mapp. 2016;37:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dyckman KA, Lee AK, Agam Y, et al. Abnormally persistent fMRI activation during antisaccades in schizophrenia: a neural correlate of perseveration?Schizophr Res. 2011;132: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mayer AR, Ruhl D, Merideth F, et al. Functional imaging of the hemodynamic sensory gating response in schizophrenia. Hum Brain Mapp. 2013;34:2302–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Open Science Collaboration. Estimating the reproducibility of psychological science. Psychobiology. 2015;349: aac4716. [DOI] [PubMed] [Google Scholar]
- 50. Mutha PK, Haaland KY, Sainburg RL. The effects of brain lateralization on motor control and adaptation. J Mot Behav. 2012;44:455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Serrien DJ, Ivry RB, Swinnen SP. Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci. 2006;7:160–166. [DOI] [PubMed] [Google Scholar]
- 52. Netz J, Ziemann U, Hömberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res. 1995;104:527–533. [DOI] [PubMed] [Google Scholar]
- 53. Kraskov A, Prabhu G, Quallo MM, Lemon RN, Brochier T. Ventral premotor-motor cortex interactions in the macaque monkey during grasp: response of single neurons to intracortical microstimulation. J Neurosci. 2011;31:8812–8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin CH, Huang CL, Chang YC, et al. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr Res. 2013;146:231–237. [DOI] [PubMed] [Google Scholar]
- 55. Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr Res. 2009;113:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mullinger KJ, Mayhew SD, Bagshaw AP, Bowtell R, Francis ST. Poststimulus undershoots in cerebral blood flow and BOLD fMRI responses are modulated by poststimulus neuronal activity. Proc Natl Acad Sci USA. 2013;110:13636–13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brass M, Zysset S, von Cramon DY. The inhibition of imitative response tendencies. Neuroimage. 2001;14:1416–1423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.