Abstract
Background
We aimed to determine whether late adolescent visual impairment is associated with later psychosis.
Methods
We conducted a longitudinal cohort study of Swedish male military conscripts aged 18 or 19 years from January 1, 1974, through December 31, 1997 (N = 1140710). At conscription, uncorrected and optometry-lens-corrected distance visual acuity was measured. Participants were then followed up to see if they received an inpatient diagnosis of non-affective psychotic disorder, including schizophrenia (N = 10769). Multivariable Cox modeling was used to estimate differences between groups.
Results
After adjustment for confounders, those with severe impairment before optical correction in their best eye (decimal fraction <0.3) had an increased psychosis rate compared to those with normal uncorrected vision (decimal fraction 1.0) (hazard ratio [HR] 1.26, 95% CI 1.16–1.37). Larger interocular visual acuity difference was associated with an increased psychosis rate (adjusted HR 1.49, 95% CI 1.37–1.63 in those with differences >0.5 compared to those with no between eye acuity difference). Individuals with impaired vision that could not be corrected to normal with lenses had highest rates of psychosis (best eye adjusted HR 1.56; 95% CI 1.33–1.82), those with imperfect, but correctable vision also had elevated rates (best eye adjusted HR 1.21; 95% CI 1.15–1.28). Individuals with visual impairment had higher rates of psychosis than their full siblings with normal vision (adjusted HR 1.20, 95% CI 1.07–1.35).
Conclusions
Impaired visual acuity is associated with non-affective psychosis. Visual impairment as a phenotype in psychosis requires further consideration.
Keywords: eyesight, vision, schizophrenia, sibling-design
Introduction
Visual deficits have been identified at all clinical stages of psychotic illness in increasing severity, from high risk,1,2 and prodromal states,3 through first episode,4 to chronic schizophrenia.5,6 As such, it has been suggested that visual defects represent a phenotype which could contribute to more reliable diagnostic criteria for schizophrenia.7
One important measure of visual function is acuity. Visual acuity is a composite measure of the capacity for the eye to accurately focus light on the retina, the integrity of the retina, and the brains ability to interpret the information provided.8 While neurological abnormalities have long been a focus of interest in the aetiology of psychosis, it is only more recently via techniques such as electroretinography and optical coherence tomography, that cross-sectional associations between retinal anomalies and schizophrenia have been identified.6 Myopia is the most common cause of reduced distance acuity and affects over 20% of the world’s population.9 Despite the high prevalence of abnormal acuity and the apparent increase in visual deficits as psychotic illness progresses,4,5,10 very little research has examined the potential relationship between visual acuity and psychosis longitudinally, or on a population-level. One study in offspring of mothers with psychosis found that visual dysfunction aged 4 was associated with schizophrenia-spectrum disorders at 22.1 In another cohort with family history of psychosis, visual dysfunction before age 13 was associated with psychosis aged 33.2 Both of these studies were small and did not account for any potential confounders. The largest study of which we are aware, found a negative association between corrected refractive errors detected during conscription interviews for the Israeli army and future risk of schizophrenia.11 This result is at odds with the rest of the literature.7
The limited evidence-base is surprising given the large number of studies examining the relationship between hearing loss and psychosis, in which an association is consistently found.12 Similarly, a number of theoretical models for psychosis suggest that impaired vision could be a risk factor. Computational models suggest that psychosis can be understood in terms of defective Bayesian processes; ie, abnormal interactions between perceptual inputs and prior beliefs which lead to experiences of false concepts (delusions) or percepts (hallucinations).13,14 Other models, such as the social defeat hypothesis,15 impaired theory of mind,16 and source monitoring errors17 are equally applicable to hearing and visual deficits. In particular, the “protection against schizophrenia” model hypotheses that there will be an inverted U relationship between visual impairment and psychosis risk,7 as normal vision and absent vision are associated with decreased psychosis risk.18 However, to our knowledge, this has not been investigated in a general population cohort.
We used data from Swedish conscript Snellen eye chart tests to examine whether distance visual acuity is associated with future risk of psychotic illness.
Methods
Study Design and Setting
Data were extracted from linked national registers, which include data on all people officially resident in Sweden after January 1, 1932, anonymized by Statistics Sweden for research purposes. Eyesight data were derived from the Military Service Conscript Register and the Military Archives of Sweden. Assessments were conducted with nearly all male members of the Swedish population from January 1, 1974, through December 31, 1997, when aged 18 or 19 years. Individuals could only be exempted from assessment by their General Practitioner because of severe disability. This register also includes IQ test results, reviews of physical and mental health, and sociodemographic data. The diagnosis of psychotic illness was extracted from the National Patient Register for all inpatient treatment episodes in Sweden from January 1, 1974, through December 31, 2011. We also extracted data on the total number of psychiatric admissions for each person diagnosed with psychosis, to act as a proxy for illness severity. The Migration and Cause of Death registers were used to define when people left the cohort. All individuals were censored at the earliest of psychotic illness diagnosis date, migration, death date or December 31, 2011. Individuals who had admissions for serious mental illness (SMI) including bipolar disorder, schizoaffective disorder, schizophrenia or other psychotic illness, before their conscription interview were excluded from the analysis, as were individuals who were assessed as having psychosis at the conscription interview. Parents of conscripts were identified in the Multi-Generational Register and linked to the National Patient Register to identify parental SMI. Parental socioeconomic status (SES) data were derived from census information from 1970 through 1990, and linked through the Multi-Generational Register. Full siblings were also identified through the Multi-Generational Register and their exposure, outcome and covariate information was extracted from the other registers. The study was approved by the Regional Ethical Review Board, Stockholm, Sweden, which waived the need for informed consent for these publicly available data.
Distance Visual Acuity
Uncorrected and corrected visual acuity assessment was completed by an optometrist using the Snellen eye chart placed at 6 meters. The upper testing limit was 1.0. Corrected acuity was assessed using lenses offered by the optometrist (ie, optimal visual acuity). Results were recorded as decimal fractions. In line with previous research, and World Health Organisation guidance,19,20 we categorized uncorrected acuity as “normal” (1.0), “mild visual impairment” (<1.0 to >0.5), “moderately impaired” (≤0.5 to ≥0.3), and “severely impaired” (scores <0.3). We categorized optimal visual acuity as “impaired following correction” (scores <1.0) and “normal following correction” (1.0). We assessed the association with psychosis in both eyes separately. Binocular visual acuity is considered to be approximately equal to the better-seeing eye.21 We also considered uncorrected visual acuity as a continuous measure. In addition, we categorized interocular acuity difference as no difference, >0.0 to 0.2, >0.2 to 0.5, and >0.5 decimal fractions. Registered blind individuals were exempted from conscription.
Non-affective Psychotic Illness
Schizophrenia or other non-affective nonorganic psychoses diagnoses were based on Swedish versions of the International Classification of Diseases (ICD-8 to ICD-10). Schizophrenia ICD-8: 295.0-.4, .6, .8, .9; ICD-9: 295.A-.G, .W, .X; ICD-10: F20.0-.3, .5, .6, .8, .9. Other non-affective psychoses ICD-8: 297.0, .1, .9; 298.1-.3, .9; 299.9; ICD-9: 297.B-.D, .W, .X; 298.B-.E, .W, .X; ICD-10: F21; F22; F23; F24, F28, F29. Diagnosis date was defined as the date of the first psychiatric inpatient record. Schizophrenia and non-schizophrenia psychosis diagnoses were also examine separately as outcomes. To account for the varying follow-up time we defined psychiatric admission frequency as the number of admissions per 5 years of follow-up (categorized as <1.0, 1.0 to <2.0, 2.0 to <5.0, ≥5.0).
Other Covariates
Potential confounding factors were included, based on previous research. Covariates were: age, year of conscription interview, SES (defined by highest parental employment using the Swedish Occupational Class schema and categorized as unemployed, worker, white-collar-workers or business owners),22,23 IQ (categorized as <74, 74–81, 82–89, 90–95, 96–104, 105–110, 111–118, 119–126, >126),24,25 history of common mental disorder (CMD), including depression or anxiety disorders (from conscription assessment and medical records),26,27 parental history SMI history,28 alcohol use disorder and substance use disorder (from conscription assessment).29,30
Statistical Analysis
Cox proportional hazards regression analyses were conducted comparing the relative hazards of developing psychotic illness in different categories of uncorrected and corrected visual impairment. We adjusted for potential confounders described above. Analysis of Schoenfeld residuals was completed to test the proportional hazards assumption.31 We tested for acuity-IQ and acuity-parental SMI interactions using likelihood ratio tests. We repeated the uncorrected acuity analysis using acuity as a continuous variable and fitted a fractional polynomial model. We ran all models after removing participants who developed psychosis within 5 years of their conscription interview, to assess whether any association was likely to be the result of prodromal psychosis. We carried out similar analyses with the exposure defined as the interocular acuity difference. We conducted a multivariable ordinal logistic regression to assess the association between acuity and frequency of psychiatric hospitalization.
We identified full sibling dyads in the cohort, selecting the nearest-born sibling if there were multiple. We tested whether impaired acuity in the sibling was associated with psychosis in the index participant. This analysis should test for shared genetic liability or common environmental influences for impaired acuity and psychosis. We then identified dyads discordant for normal vs impaired acuity in their best eye. We carried out a multivariable Cox regression adjusting for confounders that could differ between siblings (age, year of conscription interview, birth order, IQ, CMD, alcohol, and other substance use disorders) and accounting for the effect of family using a clustered sandwich estimator. This analysis should partially account for unmeasured genetic, social and lifestyle confounders that are shared within families, as full siblings share up to half their genetic makeup and generally share their early environment.32 All analyses were conducted with Stata software (Version 14; StataCorp).33
Results
Of the men attending conscription interviews (N = 1229862), 1140710 had interpretable visual acuity tests. Normal acuity (1.0) in the best eye was found in 903227 men (79.18%). Individuals with severe visual impairment were most likely to have parents who were white-collar workers, more likely to have a higher IQ and less likely to have a history of alcohol or substance use disorder (table 1). The mean follow-up for individuals in the cohort was 24.75 years (SD 8.32) There were 10769 new cases of psychosis (including 5522 with schizophrenia) within the follow-up time of 28.46 million person-years (3.78 per 10000 person-years at risk [PYAR]; 95% CI 3.71–3.86). The mean age of first diagnosis of psychotic illness was 29.53 (SD 8.25 y).
Table 1.
Baseline Characteristics by Uncorrected Visual Acuity in Best Eye
| Normal Acuity (1.0) | Mild Impairment (<1.0 to >0.5) | Moderate Impairment (≤0.5 to ≥0.3) | Severe Impairment (<0.3) | |
|---|---|---|---|---|
| Total, N (%) | 903227 (79.18) | 84663 (7.42) | 62678 (5.49) | 90142 (7.90) |
| Age, mean (SD) | 18.30 (0.79) | 18.32 (0.85) | 18.32 (0.79) | 18.30 (0.68) |
| SES, N (%) | ||||
| Unemployed | 25950 (2.87) | 2746 (3.24) | 1864 (2.97) | 2467 (2.74) |
| Worker | 307377 (34.03) | 27495 (32.48) | 19131 (30.52) | 25379 (28.15) |
| White-collar | 414902 (45.94) | 40930 (48.34) | 32070 (51.17) | 49259 (54.65) |
| Business owner | 122880 (13.60) | 10167 (12.01) | 7280 (11.61) | 10005 (11.10) |
| Missing | 32118 (3.56) | 3325 (3.93) | 2333 (3.72) | 3032 (3.36) |
| IQ group, median | 96–104 | 96–104 | 96–104 | 105–110 |
| CMD, N (%) | 17202 (1.90) | 1608 (1.90) | 1025 (1.64) | 1093 (1.21) |
| Parental SMI, N (%) | 31280 (3.46) | 3030 (3.58) | 2252 (3.59) | 2997 (3.32) |
| Alcohol misuse N (%) | 1424 (0.16) | 112 (0.13) | 49 (0.08) | 33 (0.04) |
| Substance misuse N (%) | 2068 (0.23) | 142 (0.17) | 72 (0.11) | 49 (0.05) |
Note: SES, socioeconomic status; CMD, common mental disorder; SMI, serious mental illness.
Uncorrected Vision
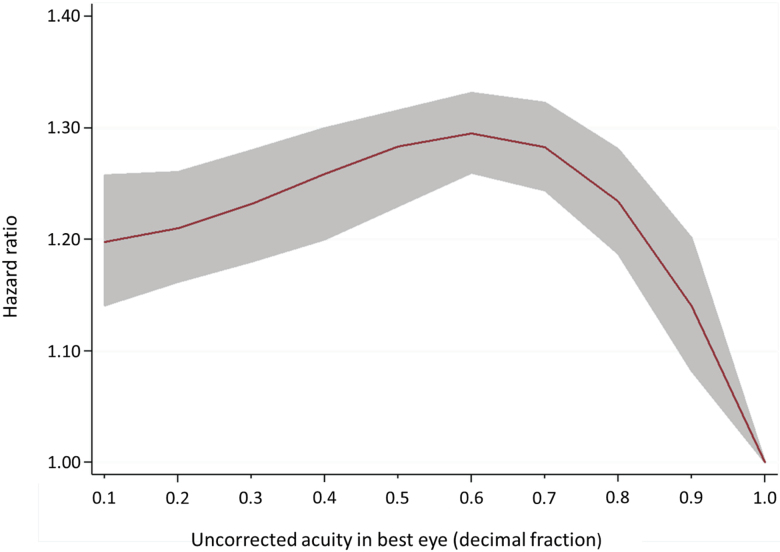
Impaired acuity in either eye was associated with increased psychotic illness rates (table 2). When using acuity as a continuous measure, for each decimal reduction in acuity there was an increase in rate of psychosis until 0.6 (figure 1). There was no evidence of interaction between acuity and IQ (P = .88) or acuity and parental SMI (P = .76). Removing individuals who developed psychosis within 5 years of their conscription interview (N = 2511) had little effect on the association (best eye fully adjusted hazard ratio [HR] 1.19, 95% CI 1.09–1.28; HR 1.21, 95% CI 1.10–1.34; HR 1.25, 95% CI 1.15–1.37 for mild, moderate, and severe impairment, respectively compared to normal vision). Impaired acuity (<1.0) was associated with both increased rates of schizophrenia diagnoses (adjusted HR 1.31, 95% CI 1.22–1.41) and of other psychoses (adjusted HR 1.17, 95% CI 1.08–1.26). Increasing interocular acuity difference was associated with an increased psychosis rate (table 3). Impaired acuity in the best eye was associated with an odds ratio of 1.13, 95% CI 1.03–1.24 per group increase in 5-year admission rate, after adjustment, with groups defined as <1.0 (N = 4906), 1.0 to <2.0 (N = 2258), 2.0 to <5.0 (N = 2375), ≥5.0 (N = 1230) admissions per 5 years. There was no evidence this model violated the parallel regression assumption.
Table 2.
Association Between Visual Acuity and Non-affective Psychosis
| Best eye | Worst Eye | |||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Events/PYAR | HR (95% CI) | N (%) | Events/PYAR | HR (95% CI) | |||
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |||||
| Uncorrected acuity | ||||||||
| Normal (1.0) | 903227 (79.18) | 8441/22.97 × 106 | 1 (Reference) | 1 (Reference) | 795250 (69.72) | 7138/20.14 × 106 | 1 (Reference) | 1 (Reference) |
| Mild impairment (<1.0 to >0.5) | 84663 (7.42) | 946/20.27 × 105 | 1.24 (1.16–1.33) | 1.25 (1.16–1.34) | 107476 (9.42) | 1190/25.97 × 105 | 1.27 (1.20–1.35) | 1.22 (1.14–1.31) |
| Moderate impairment (≤0.5 to ≥0.3) | 62678 (5.49) | 609/15.17 × 105 | 1.07 (0.99–1.17) | 1.19 (1.09–1.31) | 86659 (7.60) | 952/21.42 × 105 | 1.24 (1.16–1.33) | 1.34 (1.24–1.44) |
| Severe impairment (<0.3) | 90142 (7.90) | 773/19.48 × 105 | 1.02 (0.94–1.09) | 1.26 (1.16–1.37) | 151325 (13.27) | 1489/35.74 × 105 | 1.15 (1.09–1.21) | 1.38 (1.30–1.47) |
| Corrected acuity | ||||||||
| Normal uncorrected (1.0) | 903227 (79.18) | 8441/22.97 × 106 | 1 (Reference) | 1 (Reference) | 795250 (69.72) | 7138/20.14 × 106 | 1 (Reference) | 1 (Reference) |
| Corrected to normal (1.0) | 225896 (19.80) | 2123/52.12 × 105 | 1.06 (1.02–1.12) | 1.21 (1.15–1.28) | 290790 (25.49) | 2869/69.38 × 105 | 1.14 (1.09–1.19) | 1.30 (1.24–1.36) |
| Imperfectly corrected (<1.0) | 11511 (1.01) | 205/27.75 × 104 | 1.98 (1.72–2.27) | 1.56 (1.33–1.82) | 54670 (4.79) | 762/13.74 × 105 | 1.56 (1.44–1.68) | 1.38 (1.28–1.50) |
Note: PYAR, person-years at risk; HR, hazard ratio.
aAdjusted for age at conscription, calendar year, IQ, socioeconomic status, common mental disorder, parental serious mental illness, alcohol use disorder and substance use disorder.
Fig. 1.
Uncorrected visual acuity and psychosis diagnosis from fully adjusted fractional polynomial model. Hazard ratio and 95% CI.
Table 3.
Rate of Psychosis by Interocular Difference in Acuity
| N (%) | Events/PYAR | HR (95% CI) | Adjusteda | |
|---|---|---|---|---|
| Unadjusted | ||||
| Interocular difference (decimal fractions) | ||||
| 0 | 922448 (80.87) | 8320/22.96 × 106 | 1 (Reference) | 1 (Reference) |
| >0.0 to 0.2 | 114946 (10.08) | 1186/27.37 × 105 | 1.18 (1.11–1.25) | 1.19 (1.12–1.28) |
| >0.2 to 0.5 | 56059 (4.91) | 638/13.80 × 105 | 1.27 (1.17–1.38) | 1.30 (1.19–1.41) |
| >0.5 | 47257 (4.14) | 625/13.18 × 105 | 1.37 (1.26–1.48) | 1.49 (1.37–1.63) |
Note: PYAR; person-years at risk, HR; hazard ratio.
aAdjusted for age at conscription, calendar year, IQ, socioeconomic status, common mental disorder, parental serious mental illness, alcohol use disorder, and substance use disorder.
We conducted additional analyses in which we compared full sibling dyads (table 4). Sibling acuity was not associated with index participant psychosis (adjusted HR 1.00, 95% CI 0.91–1.10). In discordant sibling-pairs there were elevated psychosis rates in siblings with impaired visual acuity (adjusted HR 1.23, 95% CI 1.07–1.43).
Table 4.
Sibling Dyad Analyses
| N (%) | Events/PYAR | HR (95% CI) | Adjusteda | |
|---|---|---|---|---|
| Unadjusted | ||||
| Acuity of sibling of index participant | ||||
| 1.0 | 289683 (79.80) | 2536/72.86 × 105 | 1 (Reference) | 1 (Reference) |
| <1.0 | 73341 (20.20) | 606/17.34 × 105 | 0.98 (0.90–1.07) | 1.00 (0.91–1.10) |
| Discordant sibling pairs | ||||
| 1.0 | 49045 (50.00) | 393/11.95 × 105 | 1 (Reference) | 1 (Reference) |
| <1.0 | 49045 (50.00) | 446/11.63 × 105 | 1.16 (1.01–1.32) | 1.23 (1.07–1.43) |
Note: PYAR; person-years at risk, HR; hazard ratio.
aSibling acuity analysis adjusted for index participant acuity age at conscription, calendar year, IQ, common mental disorder, alcohol use disorder and substance use disorder, discordant sibling pair analysis adjusted for age at conscription, calendar year, IQ, common mental disorder, alcohol use disorder and substance use disorder. All models account for the effect of family using a clustered sandwich estimator.
Corrected Vision
There were increased psychosis rates in individuals who had impaired eyesight corrected to normal (best eye adjusted HR 1.21, 95% CI 1.15–1.28), and higher rates in those who had their eyesight imperfectly corrected (best eye adjusted HR 1.56, 95% CI 1.33–1.82) compared to those with normal vision without correction (table 2). Removal of individuals potentially in the prodromal phase of psychotic illness (ie, psychosis within 5 y) had minimal effect (corrected to normal best eye adjusted HR 1.23, 95% CI 1.18–1.30; corrected to imperfect best eye adjusted HR 1.49, 95% CI 1.33–1.67).
Discussion
As far as we are aware, this is the largest study of the association between visual acuity in late adolescence and future risk of psychotic illness. We studied over 1 million men with up to 38 years of follow-up. We found a gradient in the relationship between uncorrected distance acuity and psychotic illness, which was not explained by age, calendar period, SES, IQ, CMD, parental SMI, alcohol or substance misuse. Correction of acuity to normal did not reduce the risk of psychosis to baseline. Exclusion of individuals developing psychosis within 5 years of their conscription interview, to remove potential influence of prodromal psychotic symptoms on test performance, did not affect our results. Increase in the interocular difference in acuity was associated with psychosis. Impaired acuity was also associated with increases in hospitalization frequency in the group with psychosis, suggesting illness severity may additionally be linked to acuity. We found a similar association in siblings discordant for visual acuity impairment, reducing the possibility that the observed association is due to unmeasured or residual confounding. Sibling acuity was not associated with index participant psychosis, again suggesting that the association is not through a shared genetic liability or shared environment. Our results support the “protection against schizophrenia” model developed by Landgraf and Osterheider.7
Despite studies of psychotic symptoms in old age,34 and cross-sectional studies of visual impairment in patients with psychosis,4,5,10 there is relatively little previous literature clarifying the temporal relationship between acuity and psychosis. The study most similar to ours, which used an Israeli military service cohort, found that a binary definition of corrected refractive error at aged 17 was related to reduced, rather than increased, risk of schizophrenia.11 This study examined schizophrenia specifically, and found a very low prevalence of refractive errors (5% in the “control” group and 0.9% in the schizophrenia group). This is at odds with a study using the same Israeli conscription data, which reports approximately 20% myopia.35 Rates of schizophrenia diagnosis are not presented, and the paper appears to use a combination of case-control and cohort approaches, making comparison with our study challenging. The other cohort studies of which we are aware, were small in comparison, but found a positive association between visual disturbance earlier in childhood and adult psychosis.1,2
That the association between impaired acuity and psychosis/schizophrenia may be causal accords with many models for psychosis.13–17 However, our data do not permit the nature of this association to be specified or whether a third, as yet unknown variable, might be related to the association. People with impaired acuity have impairments in reading, face recognition, stereoacuity, and performance of everyday vision-related tasks.36 Possible routes for the impact of impaired acuity are through impaired schema development and impaired social cognition. It has been hypothesized that visual perceptual anomalies can interrupt continuity in the experience of self, in particular theory of mind and aspects of self-monitoring, leading to psychosis.37 This is supported by research in experimental psychopathology and cognitive neuroscience.38 Social perception has been found to mediate the relationship between visual processing and functional status in schizophrenia.39 One argument against the causal effect of visual impairment is that individuals with a correctable impairment remain at increased risk. However, we do not know how long individuals lived with uncorrected visual impairment before detection and correction.
Alternately, there may be common causes that poor acuity and psychosis share. Schubert et al., measured visual disturbance in 4-year olds, and found that all children with visual dysfunction had neurological abnormalities aged 6, and suggested that their results supported a neurodevelopmental aetiology hypothesis.1 In our study, it seems unlikely that impaired visual acuity at 18 reflects quantifiable neurological abnormalities in 20% of the population. The majority of impaired acuity at this age is likely to be because of myopia.40 Therefore, there may be particular vulnerability to psychosis in young children with early visual impairment, related to neurodevelopmental abnormalities, but potentially other pathways in later life related to myopia. For example, experimental and epidemiological evidence suggests that myopia risk is inversely associated with outdoor light intensity and time spend outdoors.40 It has been postulated that increased light might protect against myopia because of increased transmitter dopamine41 or increased vitamin D.42 Schizophrenia and other non-affective psychosis has long recognized to be associated with increased latitude and reduced sunlight exposure.43 Similarly, vitamin D has been implicated as playing a role.43 That dopamine dysfunction is the final pathway to psychosis is one of the most enduring hypotheses in psychiatry.44 The argument against myopia and psychosis sharing a common cause such as this is that the association remained when we adjusted for a number of confounders, and when we compared siblings discordant for impaired acuity; these siblings are likely to have experienced similar environmental exposures. It is notable that countries that countries with increasing incidence of myopia45 also report increasing psychosis rates.46 Scandinavian rates of myopia and psychosis are likely have been stable over our study period.47,48
Strengths and Limitations
Beyond the size and long follow-up of this cohort, its strength is it represents nearly all men in Sweden. Conscription was mandatory until 2000, and only 3%–5% were exempt from assessment due to severe disability (including blindness).49 The prevalence of impaired visual acuity (20%) and the incidence of psychosis (4 per 10000 PYAR) reflect rates in other European countries,9,50,51 suggesting our findings may generalize to other populations. The relatively young age at which acuity was assessed reduces the risk of reverse causation. In addition, we were able to control for a number of potential confounding factors.
Our study has a number of limitations. A one-off measure using a Snellen chart is a potentially imperfect reflection of visual acuity. It is a subjective test, reliant on the individual’s engagement with the task, so there is potential for fabrication of results.52 However, any fabrication is likely to be non-differential with regards to the psychosis outcome. In addition, the test used at conscription had an upper limit of 1.0, whereas some extend to 2.0, potentially providing further insights into our hypothesis. Conversely, the strength of the Snellen test is that it is simple to administer, used worldwide, and is considered the primary indicator of the magnitude of functional impairment due to vision loss.53 The age at which individuals developed acuity problems and how quickly they were detected and corrected is unknown. If there were long periods with uncorrected vision, this could potentially explain why even individuals with vision that could be corrected to normal at 18 remained at increased risk of psychosis. Visual correction via glasses wearing has been associated with bullying,54 which in turn is associated with psychosis.55
Outcome misclassification because of the use of hospital records is unlikely in schizophrenia, because more than 90% will be admitted to hospital at some point during their illness.56,57 However, there is potentially more variability in other psychosis diagnoses. For example, in Stockholm 75% of incident psychosis cases were treated in inpatient care by the end of the follow-up period of this study, before this the percentage was higher due to limited community services.58 If this misclassification were non-differential with respect to acuity, our results would represent an underestimate of the true association. Similarly, there may be issues with using hospitalization frequency as a proxy for severity, because of changes in service provision. SES was the only variable with missing data (<5%) and therefore a complete case analysis should produce unbiased results.59
It is possible that our results contain residual or unmeasured confounding. Generally, factors which increase the risk of psychosis will be associated with low SES and lower IQ, but this is not the case for visual acuity. Unmeasured confounders that may increase both myopia and psychosis are urbanicity9,60,61 and latitude.62,63 However, siblings are likely to share both these exposures and our discordant sibling analysis produced similar results suggesting that unmeasured shared genetic and environmental exposures do not explain our findings. To rule out shared genetic architecture between acuity and psychosis would require genome-wide association data on all individuals. In addition, our analysis may still be limited by unmeasured time-varying or sibling-varying covariates within families. Finally, our population only included men and it is not clear if we can extrapolate our findings to women; however, sex generally does not modify the effect of other psychosis risk factors.64
Conclusion
Impaired visual acuity in men aged 18 is a precursor to future risk of psychosis. This does not appear to be related to effects of psychosis prodrome, and is not explained by genetic or familial environmental factors. Given the extensive hunt for phenotypes and biological markers, impaired vision has been relatively unexplored in the aetiology of psychosis. Further exploration of timing and trajectories of visual acuity changes, along with clarification of the association of psychosis with specific subtypes of acuity deficit (ocular vs neurological)6 may shed light on the phenomenology and symptomology of psychotic illnesses.
Funding
This study was supported by grant MR/K021362/1 from the Medical Research Council and grant 523-2010-1052 from the Swedish Research Council. J.F.H., G.L., and D.P.J.O. are supported by the UCLH NIHR Biomedical Research Centre. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgment
The authors would like to thank Victoria Vickerstaff for her statistical advice. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Schubert EW, Henriksson KM, McNeil TF. A prospective study of offspring of women with psychosis: visual dysfunction in early childhood predicts schizophrenia-spectrum disorders in adulthood. Acta Psychiatr Scand. 2005;112:385–393. [DOI] [PubMed] [Google Scholar]
- 2. Schiffman J, Maeda JA, Hayashi K, et al. . Premorbid childhood ocular alignment abnormalities and adult schizophrenia-spectrum disorder. Schizophr Res. 2006;81:253–260. [DOI] [PubMed] [Google Scholar]
- 3. Nieman D, Becker H, van de Fliert R, et al. . Antisaccade task performance in patients at ultra high risk for developing psychosis. Schizophr Res. 2007;95:54–60. [DOI] [PubMed] [Google Scholar]
- 4. Kiss I, Fábián A, Benedek G, Kéri S. When doors of perception open: visual contrast sensitivity in never-medicated, first-episode schizophrenia. J Abnorm Psychol. 2010;119:586–593. [DOI] [PubMed] [Google Scholar]
- 5. Kimhy D, Corcoran C, Harkavy-Friedman JM, Ritzler B, Javitt DC, Malaspina D. Visual form perception: a comparison of individuals at high risk for psychosis, recent onset schizophrenia and chronic schizophrenia. Schizophr Res. 2007;97:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adams SA, Nasrallah HA. Multiple retinal anomalies in schizophrenia. Schizophr Res. 2018;195:3–12. [DOI] [PubMed] [Google Scholar]
- 7. Landgraf S, Osterheider M. “To see or not to see: that is the question.” The “Protection-Against-Schizophrenia” (PaSZ) model: evidence from congenital blindness and visuo-cognitive aberrations. Front Psychol. 2013;4:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cline D, Hofstetter HW, Griffin JR.. Dictionary of Visual Science. Oxford, UK: Butterworth-Heinemann; 1997. [Google Scholar]
- 9. Foster PJ, Jiang Y. Epidemiology of myopia. Eye (Lond). 2014;28:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viertiö S, Laitinen A, Perälä J, et al. . Visual impairment in persons with psychotic disorder. Soc Psychiatry Psychiatr Epidemiol. 2007;42:902–908. [DOI] [PubMed] [Google Scholar]
- 11. Caspi A, Vishne T, Reichenberg A, et al. . Refractive errors and schizophrenia. Schizophr Res. 2009;107:238–241. [DOI] [PubMed] [Google Scholar]
- 12. Linszen MM, Brouwer RM, Heringa SM, Sommer IE. Increased risk of psychosis in patients with hearing impairment: review and meta-analyses. Neurosci Biobehav Rev. 2016;62:1–20. [DOI] [PubMed] [Google Scholar]
- 13. O’Regan JK, Noë A. A sensorimotor account of vision and visual consciousness. Behav Brain Sci. 2001;24:939–973; discussion 973. [DOI] [PubMed] [Google Scholar]
- 14. Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. The computational anatomy of psychosis. Front Psychiatry. 2013;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Selten JP, van der Ven E, Rutten BP, Cantor-Graae E. The social defeat hypothesis of schizophrenia: an update. Schizophr Bull. 2013;39:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31:21–42. [DOI] [PubMed] [Google Scholar]
- 17. Nelson B, Whitford TJ, Lavoie S, Sass LA. What are the neurocognitive correlates of basic self-disturbance in schizophrenia?: integrating phenomenology and neurocognition. Part 1 (Source monitoring deficits). Schizophr Res. 2014;152:12–19. [DOI] [PubMed] [Google Scholar]
- 18. Silverstein SM, Wang Y, Keane BP. Cognitive and neuroplasticity mechanisms by which congenital or early blindness may confer a protective effect against schizophrenia. Front Psychol. 2012;3:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Visual Impairment and Blindness. Fact Sheet No. 282. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 20. Bourne RR, Flaxman SR, Braithwaite T, et al. . Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e888–e897. [DOI] [PubMed] [Google Scholar]
- 21. Rubin GS, Muñoz B, Bandeen-Roche K, West SK. Monocular versus binocular visual acuity as measures of vision impairment and predictors of visual disability. Invest Ophthalmol Vis Sci. 2000;41:3327–3334. [PubMed] [Google Scholar]
- 22. Robaei D, Rose K, Ojaimi E, Kifley A, Huynh S, Mitchell P. Visual acuity and the causes of visual loss in a population-based sample of 6-year-old Australian children. Ophthalmology. 2005;112:1275–1282. [DOI] [PubMed] [Google Scholar]
- 23. Werner S, Malaspina D, Rabinowitz J. Socioeconomic status at birth is associated with risk of schizophrenia: population-based multilevel study. Schizophr Bull. 2007;33:1373–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saw SM, Tan SB, Fung D, et al. . IQ and the association with myopia in children. Invest Ophthalmol Vis Sci. 2004;45:2943–2948. [DOI] [PubMed] [Google Scholar]
- 25. Zammit S, Allebeck P, David AS, et al. . A longitudinal study of premorbid IQ Score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry. 2004;61:354–360. [DOI] [PubMed] [Google Scholar]
- 26. Rees G, Tee HW, Marella M, Fenwick E, Dirani M, Lamoureux EL. Vision-specific distress and depressive symptoms in people with vision impairment. Invest Ophthalmol Vis Sci. 2010;51:2891–2896. [DOI] [PubMed] [Google Scholar]
- 27. Yung AR, Phillips LJ, Yuen HP, et al. . Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60:21–32. [DOI] [PubMed] [Google Scholar]
- 28. Rasic D, Hajek T, Alda M, Uher R. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophr Bull. 2014;40:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tien AY, Anthony JC. Epidemiological analysis of alcohol and drug use as risk factors for psychotic experiences. J Nerv Ment Dis. 1990;178:473–480. [PubMed] [Google Scholar]
- 30. Brown B, Adams AJ, Haegerstrom-Portnoy G, Jones RT, Flom MC. Effects of alcohol and marijuana on dynamic visual acuity: I. Threshold measurements. Atten Percept Psychophys. 1975;18(6):441–446. [Google Scholar]
- 31. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 32. Donovan SJ, Susser E. Commentary: advent of sibling designs. Int J Epidemiol. 2011;40:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. StataCorp. Stata Statistical Software: Release 14 College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 34. Vannorsdall TD, Schretlen DJ. Late-onset schizophrenia. In: Ravdin LD, Katzen HL, eds. Handbook on the Neuropsychology of Aging and Dementia. New York, NY: Springer; 2013:487–500. [Google Scholar]
- 35. Bar Dayan Y, Levin A, Morad Y, et al. . The changing prevalence of myopia in young adults: a 13-year series of population-based prevalence surveys. Invest Ophthalmol Vis Sci. 2005;46:2760–2765. [DOI] [PubMed] [Google Scholar]
- 36. Rubin GS, Muñoz B, Bandeen-Roche K, West SK. Monocular versus binocular visual acuity as measures of vision impairment and predictors of visual disability. Invest Ophthalmol Vis Sci. 2000;41:3327–3334. [PubMed] [Google Scholar]
- 37. Uhlhaas PJ, Mishara AL. Perceptual anomalies in schizophrenia: integrating phenomenology and cognitive neuroscience. Schizophr Bull. 2007;33:142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Postmes L, Sno HN, Goedhart S, van der Stel J, Heering HD, de Haan L. Schizophrenia as a self-disorder due to perceptual incoherence. Schizophr Res. 2014;152:41–50. [DOI] [PubMed] [Google Scholar]
- 39. Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739–1748. [DOI] [PubMed] [Google Scholar]
- 41. Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013;114:106–119. [DOI] [PubMed] [Google Scholar]
- 42. Yazar S, Hewitt AW, Black LJ, et al. . Myopia is associated with lower vitamin D status in young adults. Invest Ophthalmol Vis Sci. 2014;55:4552–4559. [DOI] [PubMed] [Google Scholar]
- 43. Kinney DK, Teixeira P, Hsu D, et al. . Relation of schizophrenia prevalence to latitude, climate, fish consumption, infant mortality, and skin color: a role for prenatal vitamin d deficiency and infections?Schizophr Bull. 2009;35:582–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32:3–16. [DOI] [PubMed] [Google Scholar]
- 46. Chan KY, Zhao FF, Meng S, et al. ; Global Health Epidemiology Reference Group (GHERG) Prevalence of schizophrenia in China between 1990 and 2010. J Glob Health. 2015;5:010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rose K, Smith W, Morgan I, Mitchell P. The increasing prevalence of myopia: implications for Australia. Clin Exp Ophthalmol. 2001;29:116–120. [DOI] [PubMed] [Google Scholar]
- 48. McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guttormsson U. Drogutvecklingen i Sverige. Stockholm, Sweden: Centralförbundet för alkohol-och narkotikaupplysning (CAN); 2006. [Google Scholar]
- 50. Jobke S, Kasten E, Vorwerk C. The prevalence rates of refractive errors among children, adolescents, and adults in Germany. Clin Ophthalmol. 2008;2:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kirkbride JB, Errazuriz A, Croudace TJ, et al. . Incidence of schizophrenia and other psychoses in England, 1950-2009: a systematic review and meta-analyses. PLoS One. 2012;7:e31660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pandit JC. Testing acuity of vision in general practice: reaching recommended standard. BMJ. 1994;309:1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Council NR. Visual Impairments: Determining Eligibility for Social Security Benefits. Washington, DC: National Academies Press; 2002. [PubMed] [Google Scholar]
- 54. Horwood J, Waylen A, Herrick D, Williams C, Wolke D. Common visual defects and peer victimization in children. Invest Ophthalmol Vis Sci. 2005;46:1177–1181. [DOI] [PubMed] [Google Scholar]
- 55. Catone G, Marwaha S, Kuipers E, et al. . Bullying victimisation and risk of psychotic phenomena: analyses of British national survey data. Lancet Psychiatry. 2015;2:618–624. [DOI] [PubMed] [Google Scholar]
- 56. Geddes JR, Kendell RE. Schizophrenic subjects with no history of admission to hospital. Psychol Med. 1995;25:859–868. [DOI] [PubMed] [Google Scholar]
- 57. Weiser M, Werbeloff N, Dohrenwend BP, Levav I, Yoffe R, Davidson M. Do psychiatric registries include all persons with schizophrenia in the general population? A population-based longitudinal study. Schizophr Res. 2012;135:187–191. [DOI] [PubMed] [Google Scholar]
- 58. Jörgensen L, Ahlbom A, Allebeck P, Dalman C. The Stockholm non-affective psychoses study (snaps): the importance of including out-patient data in incidence studies. Acta Psychiatr Scand. 2010;121:389–392. [DOI] [PubMed] [Google Scholar]
- 59. Dong Y, Peng CY. Principled missing data methods for researchers. Springerplus. 2013;2:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Castillejos MC, Martín-Pérez C, Moreno-Küstner B. A systematic review and meta-analysis of the incidence of psychotic disorders: the distribution of rates and the influence of gender, urbanicity, immigration and socio-economic level. Psychol Med. 2018:1–5. [DOI] [PubMed] [Google Scholar]
- 61. Ip JM, Rose KA, Morgan IG, Burlutsky G, Mitchell P. Myopia and the urban environment: findings in a sample of 12-year-old Australian school children. Invest Ophthalmol Vis Sci. 2008;49:3858–3863. [DOI] [PubMed] [Google Scholar]
- 62. Saha S, Chant DC, Welham JL, McGrath JJ. The incidence and prevalence of schizophrenia varies with latitude. Acta Psychiatr Scand. 2006;114:36–39. [DOI] [PubMed] [Google Scholar]
- 63. Vannas AE, Ying GS, Stone RA, Maguire MG, Jormanainen V, Tervo T. Myopia and natural lighting extremes: risk factors in Finnish army conscripts. Acta Ophthalmol Scand. 2003;81:588–595. [DOI] [PubMed] [Google Scholar]
- 64. Laurens KR, Luo L, Matheson SL, et al. . Common or distinct pathways to psychosis? A systematic review of evidence from prospective studies for developmental risk factors and antecedents of the schizophrenia spectrum disorders and affective psychoses. BMC Psychiatry. 2015;15:205. [DOI] [PMC free article] [PubMed] [Google Scholar]